Endocrine Views



Also in this issue New ESE Clinical Guidelines Preparing for Prague: ECE 2026
Editor: Marek Bolanowski, Poland
Email: marek.bolanowski@umw.edu.pl
Deputy Editor: Eleni Armeni, Greece/UK
Email: eleniarmeni@hotmail.com
Co-Editor: Wiebke Arlt, UK
Email: ese.president@ese-hormones.org
Editorial Board:
Faisal Ahmed, UK
Bjørn Olav Åsvold, Norway
Juan Manuel Jiménez Vacas, UK
Niki Karavitaki, UK
Karim Meeran, UK
Stavroula Paschou, Greece
Victoria Withy, ESE Office
Maria Chiara Zatelli, Italy
Karin Zibar Tomšić, Croatia
Managing Editor: Caroline Brewser
Design: Qube Design Associates
Website: www.ese-hormones.org
©2025 European Society of Endocrinology
Cover Image: The future of medicine: image generated using DeepAI.org
The views expressed by the contributors are not necessarily those of ESE. This document is available on the ESE website, www.ese-hormones.org
The addresses used to email this issue of Endocrine Views were supplied by the members of ESE. If you do not wish to receive further mailings, you can update your mailing preferences in your Member Portal or email info@ese-hormones.org
ESE thanks the following for their support.
Premium Corporate Members: Ascendis Pharma, Esteve, Novo Nordisk, Recordati Rare Diseases
Corporate Members: Alexion, BridgeBio, Camurus Pharmaceuticals, Crinetics Pharmaceuticals, Inozyme Pharma, Ipsen, Kyowa Kirin, Neurocrine Biosciences, Rhythm Pharmaceuticals, Soleno Therapeutics, Uni-Pharma Supporter: Amgen, Immunovant Inc.

Excitingly, the recent publication of EndoCompass has provided us all with an incredible resource. This valuable tool, crafted by many expert colleagues, will help us shape research projects, funding proposals, clinical trials, career planning and more. Turn to page 5 to take full advantage of it today.
Among the eight endocrine specialties and five cross-cutting themes covered in EndoCompass is ‘Artificial intelligence in endocrinology’. We all know that AI is set to transform the way we work. In this issue, two articles shine a light on the reality of incorporating AI into our practice. On page 7, Jowita Halupczok-Żyła looks at how we can facilitate sharing of clinical data and so unblock bottlenecks in developing AI. Then, on page 8, Antoan Stefan Šojat and Guillaume Assié examine how we, as endocrinologists, can influence the development of AI in our discipline.
This issue also welcomes new Clinical Guidelines on a wide range of topics. On page 9, we consider the recent ESE and Endocrine Society Joint Clinical Practice Guideline on Pre-existing Diabetes and Pregnancy, with recommendations spanning screening, timing of delivery, medications, diabetes technology and contraception.
Mary Ann Lumsden and Leonie van Hulsteijn introduce the new ESE Clinical Practice Guideline for Evaluation and Management of Menopause on page 10. It provides information and advice for clinicians who might see women with menopausal problems, covering diagnosis and a range of approaches to management.
On page 11, Leonie van Hulsteijn and Jens Bollerslev discuss the newly updated ESE Clinical Practice Guideline on Management of Chronic Hypoparathyroidism in Adults, including recommendations for diagnosis, management and monitoring of patients with this two-hormone deficiency syndrome.
Meanwhile, our Editor’s Selection column highlights two papers from the new ESE journal Obesity and Endocrinology, chosen and commented on by Editor-in-Chief Melania Manco (page 12). We also have an important opportunity to get to know ESE’s President-Elect Mirjam Christ-Crain, in our interview on page 6
But first of all, on page 3, you will discover the plans for our forthcoming Congress in Prague in May. Don’t miss ECE 2026 in ESE’s 20th Anniversary

Areas of interest in this issue:
and Nutrition

Don’t miss the 28th European Congress of Endocrinology on 9−12 May 2026.
The largest endocrine event in Europe is coming to Prague in May. Attracting over 3000 delegates from more than 100 countries, ECE 2026 will also take place during ESE’s 20th Anniversary year – a cause for celebration!
ECE 2026 offers a unique opportunity for collaborations, networking, making new friends and reuniting with friends and colleagues. Delegates can either attend in person at the Prague Congress Centre or online via ECE@HOME.
The diverse programme includes streamlined content for basic scientists, nurses, clinicians, early careers, patients, allied health professionals and women in endocrinology.
• 8 award lectures
• 8 plenary lectures
• 16 symposia
• 1 ‘How do I?’ session
• 17 Meet the Expert sessions
• 3 debates
• 12 oral and rapid communications sessions
• Guideline sessions
• Joint sessions with partner endocrine societies
• Industry satellites
• Patients’ Voice sessions
your abstracts
Promote your latest research at ECE 2026. Accepted abstracts are presented as Posters, ePosters, Rapid Communications or Oral Communications, and published open access and fully citable in a supplement to European Journal of Endocrinology
Abstract deadline 20 January 2026
Find out more and submit EXTERNAL-LINK-ALT
Local and Programme Organising Committee Chairs Michal Kršek (Czech Republic), Peter Igaz (Hungary) and Sander Kooijman (The Netherlands) talk to Cynthia Andoniadou (UK), ESE Congress Committee Chair.

• Androgen Excess and PCOS Society Update Meeting
• European Academy of Andrology Course
• Growth Hormone Research Society Congress • Nurses’ Course
• Thyroid Ultrasound Course
World Hormone Day returns on 24 April 2026. This global awareness day is a chance to highlight the vital role hormones play in our health and well-being. The 2026 campaign focuses on raising public awareness of what hormones are, why hormones matter and the steps we can all take to promote good hormone health.
You can choose the themes and activities that are most relevant to your community, uniting behind the #BecauseHormonesMatter message.
We hope you’ll join us in marking the day. You can take part online or hold in-person events with your community. ESE will provide materials and resources to support your activities, including new social media graphics, videos, posters and templates. You’ll be able to join the conversation on social media using the hashtags #BecauseHormonesMatter and #WorldHormoneDay.
A new ‘Because Hormones Matter’ Facebook page EXTERNAL-LINK-ALT also provides a hub to help us amplify each other’s activities.
Find out more by visiting www.worldhormoneday.org. Start thinking about how you can get involved: could you book screens in your institution or clinic to share the slideshow on the day, or book a venue for an event?


Have you seen the new section of the ESE website promoting current jobs in endocrinology and endocrine-related research?
• Do you have a vacancy? If you’d like to advertise any clinical jobs/research positions or PhD opportunities on the ESE job board, please send us the details.
• Are you seeking a new opportunity? New positions will be added when they are received. There are also links to useful sites to help you in your search.
Submit, search or find out more EXTERNAL-LINK-ALT
You can find the EndoCompass Research Roadmap: Directions for the Future of Endocrine Science as an open access supplement to European Journal of Endocrinology EXTERNAL-LINK-ALT and shortly also Hormone Research in Paediatrics EXTERNAL-LINK-ALT.
What is EndoCompass?
The Roadmap sets out priorities to strengthen endocrine research across Europe. It aims to inform policy and funding decisions, while supporting the endocrine community in shaping research projects, funding proposals, clinical trials and career planning. Through an expert-led analysis of the gaps and opportunities, EndoCompass

The ESE-EYES Observership Programme provides opportunities for early-career investigators to gain experience and knowledge by spending time in a leading endocrine centre away from their home institution. The programme includes clinical (COP), research (ROP) and advanced research (AdROP) exchange placements in Europe, as well as the bilateral programme (BOP) offering placements in Brazil. Placements are between one and three months and can include funding from ESE of between €1000 and €3000.
Submit your application from early December EXTERNAL-LINK-ALT
provides a reference point for the endocrine community, with the ultimate goal of improving hormone health.
Who’s involved?
Jointly led by ESE and the European Society for Paediatric Endocrinology (ESPE), the Roadmap was developed by more than 228 experts across Europe, covering all major endocrine fields, with contributions from 10 specialist partners and nine patient advocacy groups.
What does it cover?
It spans eight endocrine specialties and five cross-cutting themes, alongside an analysis of endocrine science in Horizon 2020. The recommendations highlight where research and investment can make the greatest difference.
How can I use it?
• Cite the Roadmap in funding proposals and papers
Your guide to the future of endocrine research is now available to support your work EXTERNAL-LINK-ALT. Find the Roadmap at www.ese-hormones.org/ endocompass
• Include it in presentations and strategies
• Share it with your teams and institutions
• Advocate for national policy support and funding

The 12th Annual Meeting of ESE Young Endocrinologists and Scientists (EYES) took place in Milan, Italy, in September.

IFCAH is an organisation founded by parents to promote research into congenital adrenal hyperplasia (CAH). A total fund of €350,000 is currently available for relevant projects, and up to €150,000 will be assigned to an individual project. The first step in applying is to submit a letter of intention by 15 January 2026.
Find out more now EXTERNAL-LINK-ALT
The many delegates enjoyed keynote lectures by Constantine Stratakis (Greece/USA), Sadaf Farooqi (UK), Guillaume Assié (France) and Roberto Vettor (Italy). The award for best presentation was made to Nesrine Benanteur (France), with runner up Niklas Geiger (Germany).
Nesrine commented, ‘Having the opportunity to present our work, and having valuable feedback and enlightening debates around it, was truly
motivating.’ The programme also included diverse opportunities to promote networking.
Next year’s event will take place in Belgrade, Serbia, on 4−6 September 2026. Watch out for more information

Mirjam Christ-Crain became the new President-Elect of ESE during the Joint Congress in Copenhagen in May. We took the opportunity to ask her a few questions and get to know her better.
Please tell us about yourself I went to medical school and did my MD at the University Hospital Basel in Switzerland, before doing my PhD in Ashley Grossman and Márta Korbonits’ research group at St Bartholomew’s Hospital in London, UK. When I returned from London in 2008, I built my own research group, with the help of a research professorship of the Swiss National Science Foundation. Today, I am a clinician− scientist and Head of the Clinic for Endocrinology at the University Hospital Basel.
In my clinical work, I see patients with endocrine and metabolic diseases ranging from diabetes and obesity to thyroid, adrenal and pituitary disorders. My research focuses on pituitary disorders, especially the posterior pituitary, and water electrolyte disorders. I teach endocrinology to medical students at the University of Basel. I have a long-standing relationship with ESE, and served as Chair of the Education Committee from 2020 to 2024, helping to develop the new ESE Curriculum EXTERNAL-LINK-ALT and setting up the European Board Exam EXTERNAL-LINK-ALT
Why are you excited to be President-Elect?
It is a great honour. ESE is a great society, an incredible success story for almost 20 years, with an excellent, professional ESE Team, a great Executive Committee, a brilliant strategy and a growing membership. It excites me that I can be part of this success story! And I look forward to serving the Society to achieve its aims. What particularly excites me is the opportunity to help shape the future of endocrinology to reflect our increasingly interconnected world. ESE is uniquely positioned to build bridges across Europe and beyond, connecting clinicians, basic and clinical researchers, educators and policymakers to tackle shared challenges. I strongly believe that we can only advance the field though collaborations, with every voice heard.
What main challenges face endocrinology?
We face a dual challenge: the rising burden of endocrine diseases, and the fragmentation of healthcare systems and research efforts. Disparities in access to education, care and funding continue to divide countries and communities. Tackling these issues will require strong international collaboration, more harmonised standards if possible, and a firm commitment to bridging gaps between regions, professions and generations in our field.
Which issues should be addressed first?
First, a more general answer: one urgent priority is strengthening the visibility and voice of endocrinology within European health policy. We must make sure that endocrine health is recognised as a public health priority. We must also address inequalities in education and care, particularly in under-resourced areas. That means expanding our network of endocrine centres, supporting cross-border educational initiatives, and listening more closely to the voices of patients and early-career professionals.
More specifically, EndoCompass now highlights areas which need more research. I would like ESE to facilitate more pan-European investigator-initiated clinical studies, to increase the possibility of funding, and also to foster basic research in these areas, and thus attract more basic scientists to the Society.
As part of our work to increase the number of young ESE members, I would like to strengthen the existing exchange programmes (observerships), as collaboration between European countries is increasingly important. The revised ESE Curriculum and new European Board Exam also need to be implemented and expanded.
Why should people join ESE? ESE is more than a professional society − it is the voice of endocrinology in Europe. It

unites our community, provides a platform for scientific excellence, and advocates for our specialty at national and international levels. Whether you’re a clinician, researcher or trainee, ESE offers the tools, networks and inspiration to grow. Especially in today’s world, being part of ESE means joining a community committed to building bridges, sharing knowledge and advancing health together.
How can ESE support clinician−researchers?
This is a common and very real challenge. ESE can play a pivotal role by offering flexible, high-quality research training and international exchange programmes; fostering collaborative and interdisciplinary networks and research consortia; and increasing access and awareness to funding opportunities tailored for clinician−scientists.
What next steps are needed in education?
As a former Education Committee Chair, I know that education is at the heart of our mission. I believe we should continue developing digital learning pathways that can be adapted to different career stages and national systems. Implementing the ESE Curriculum and building more joint educational programmes with national societies would also help bridge educational
gaps. It would be great to see more countries using the European Board Exam as their final exit exam. Importantly, I’d also like to see more patient engagement in education, to foster a truly integrated patientcentred approach to care.
What is your advice to people setting out in the field?
My main advice is stay curious, stay connected, never give up and support each other. Build your career step by step, with strong foundations, but also with flexibility to adapt. Seek good mentors (this is particularly important) and be generous with your peers. When you build your own group, be a good and motivating mentor yourself, but overall, don’t be afraid to fail. ESE offers an amazing platform to learn, connect, contribute and grow − make the most of it!
What else would you like to say?
Now, more than ever, we must build bridges − across disciplines, professional sectors and countries − to ensure that endocrinology remains innovative, inclusive and impactful. I look forward to working with our incredible community to shape a healthy future for all. I really look forward to these coming four years, to serve the Society, and to work with all the wonderful people in and around it!
The sharing of clinical data is a bottleneck in developing artificial intelligence (AI) to benefit endocrinology. Challenges include security, interoperability, accessibility, property and confidentiality.
We are witnessing a digital transformation in the healthcare industry. The sector is undergoing exceptional changes with the emergence of AI. The development of AI may revolutionise endocrinology in terms of managing endocrine diseases − including accelerating diagnosis, enabling initiation of the appropriate treatment, improving patient monitoring, and providing more accurate prognoses. All these aspects contribute to creating personalised medicine, tailored to a patient’s needs. Discovering new drugs and analysing multi-drug therapies before their administration are also among the many benefits that AI can bring.1
Taking these issues into account, the question is no longer whether we want to use AI tools but, rather, how we can use them wisely and safely. Despite growing awareness of the potential of AI in medicine, there are still limitations to its implementation. Identifying and addressing these bottlenecks will allow for more efficient and effective deployment of AI in endocrinology.
Data protection
In healthcare, including endocrinology, medical data are at the heart of discussions about the future of AI. Handling clinical data requires a balance between ensuring confidentiality and security, and facilitating data-driven research that further enhances the potential of AI.
Clinical data are highly sensitive, and are protected by various legal regulations, including the General Data Protection Regulation in Europe and the Health Insurance Portability and Accountability Act in the United States.2 Medical institutions are concerned about unauthorised and uncontrolled access to patient data, as well as their potential misuse, without providing any collective benefit. As a result, hospital representatives are among the key players who can potentially block the implementation of AI-based solutions.3
To break the ‘data fortress’ and unlock access to valuable information, there is a fundamental requirement to establish a data-sharing framework. Designing a comprehensive and reliable system of laws and best practice will enable a secure and ethical exchange of data between stakeholders, ensuring data integrity, privacy and legal compliance.
Data intermediaries can help manage and facilitate the use of data. Privacy-enhancing technologies, such as federated learning, secure multiparty computation and differential privacy, may also be promising options for dealing with privacy protection, at the same time enabling data sharing.2
Some doctors are also skeptical about AI, and block access to clinical data.3 One reason may be the lack of transparency and explainability regarding how AI algorithms are created and whether the results obtained with AI tools can be translated into clinical practice. The ‘black box’ effect imposes the need to develop explainable AI.4
Adopting change is always difficult at the beginning, and requires extra effort to make things better in the future. In a study conducted by the European Network for the Study of Adrenal Tumors (ENS@T), insufficient IT tools and staff shortages were identified as the most significant obstacles to adrenal tumour data collection, despite concerns about ethics and law. Data sharing and use are effectively limited by the lack of funding for retrospective clinical research. Academic institutions should focus on providing financial support and agreements between participating centres.4
AI is one of the key research areas discussed in the new EndoCompass Research Roadmap. Find out more EXTERNAL-LINK-ALT

We have reached a time when working with datasets and their quality are no longer just concerns for scientists and academic teachers, but for the entire medical staff. Raising awareness that research results can be used to improve diagnostics, expand therapeutic propositions, and ultimately improve the quality of patient care, is fundamental to encouraging co-operation between the medical community and government institutions. The issue is particularly important in the context of rare diseases, where multi-centre research is crucial. Several strategies aim to facilitate the processing of diverse medical data in an appropriate framework for clinical data sharing in Europe. The European Health Data Space is intended to represent a trustworthy option.3 However, determining ownership continues to be a hot topic in debates. Developing transparent and consistent guidelines and policies can help overcome this barrier.
Another hurdle to using big data is the data’s differing origins and unstructured form. Integrating various data sources is a unique challenge. Survey participants from ENS@T centres reported that the main source of data is electronic health record (EHR) systems. However, significant data variability between different EHR providers was also demonstrated. To organise data, IT professionals, justification for interoperability and support for the process of collecting and sharing data are needed.3 Interoperability should be viewed as the exchange of information in a structured, understandable and secure manner, encompassing not only technical aspects but also syntactic, semantic and organisational levels. It is important to emphasise that interoperability reduces operating costs and improves the co-ordination of patient care between healthcare providers.5 Widespread adoption of standards such as Fast Healthcare Interoperability Resources is pivotal for building a cohesive health data ecosystem.6
Endocrinologists should actively engage in the process of determining a reasonable direction for AI development, creating guidelines and recommendations together with other professionals. There is a lot of work to do, but together we can deliver effective strategies and responsible technologies to overcome data sharing bottlenecks in the AI landscape.
Jowita Halupczok-Żyła Head of AI and Innovative Technological Solutions in Healthcare Group, Lower Silesian Medical
Chamber, Wrocław, Poland
REFERENCES
1. Oikonomakos et al. 2024 Exploration of Endocrine & Metabolic Diseses https://doi.org/10.37349/eemd.2023.00004
2. Nwachukwu et al. 2025 Data Privacy and Security Concerns in AI-Driven Healthcare https://www.researchgate.net/publication/394275058_Data_ Privacy_and_Security_Concerns_in_AI-Driven_Healthcare
3. Šojat et al. 2025 European Journal of Endocrinology https://doi.org/10.1093/ejendo/lvaf005
4. Toro-Tobon et al. 2023 Thyroid https://doi.org/10.1089/thy.2023.0132
5. Pronovost et al. (Eds) 2018 Procuring Interoperability: Achieving High-Quality, Connected, and Person-Centered Care, chapter II. Washington, DC: National Academies Press https://www.ncbi.nlm.nih.gov/books/NBK594847
6. Pereira et al. 2021 et al https://doi.org/10.3390/healthcare9070827
Medicine is far more than just a job, because the moment you stop answering difficult questions, they inevitably grow into obstacles that can no longer be overcome.
There is no real debate on the use of artificial intelligence (AI) and large language models (LLMs) in essential fields like medicine. By the time you read this article, AI and LLMs will probably already be shaping the thought processes of everyone you work with. These processes are everywhere: from a patient consulting an LLM to search for a differential diagnosis, to their data being generated and processed; from AI-driven therapeutic solutions in endocrinology, such as closedloop insulin pumps, all the way to a researcher using it to draft an abstract for a conference. And we’re not even talking about medical students passing their degree with the hidden help of an LLM...
All these cases see replacement of the medical workers’ skill, which so far has been exclusively human. But how do we navigate something this exponential? How do we manage its use and its ethics? And whose responsibility is it?
First, it is important to acknowledge that most everyday AI use still resembles an enriched real-time search engine. However, as fields narrow in expertise, the true possibilities and drawbacks of these systems become clearer. Endocrinologists stand at the intersection between traditional approaches to science and treatment, and a new era of AI integration. It would be easy to write yet another article about how this integration will improve diagnostic accuracy, treatment pathways and individualised patient care. That, however, has been said many times.
The real issue is that we are now standing at this crossroads with insufficient training and knowledge to guide the transition. Too often, we are led by trends rather than taking the lead ourselves. On one hand, the rise of big data has made the prospect of impactful, large-scale studies more realistic than ever. On the other hand, new and variable regulations on data sharing create significant barriers, especially for retrospective data.1 In Europe, frameworks such as GDPR, the European AI Act, and the European Health Data Space provide structure, but their implementation often bypasses medical professionals, who are neither integrated into their development nor properly trained and supported to navigate them.
AI is undoubtedly a powerful engine for progress, yet the healthcare ecosystem remains one of the most complex environments for its integration, between heterogeneous data sources, fragmented infrastructures and the delicate balance of clinical judgment. Data transactions represent a hot topic: should we pursue centralised data repositories to maximise algorithmic performance, or favour federated models that preserve decentralisation and promote a more efficient and ‘natural’ model for democratic data use and sharing?
Education in place of deskilling
Another hot issue is preventing the deskilling of experts due to overreliance on AI systems, and ensuring that AI and LLM training becomes a routine part of medical education. While general AI courses exist, curricula tailored for endocrinologists are still limited. An effective programme should combine technical foundations with clinical, ethical and societal perspectives.
Endocrinologists must gain a working knowledge of algorithms, programming and data handling to critically evaluate AI tools and collaborate effectively with data scientists.
2 Training should also expose clinicians to real-world applications − predictive models in diabetes, thyroid risk stratification or support for rare endocrine disorders − through case-based learning with clinical datasets. Ethical and regulatory aspects are just as essential, given concerns about bias, transparency, privacy and the business models driving AI in healthcare. Finally, clinicians must understand the broader societal impact of AI, including its effects on public health, equity and patient trust.

The empowerment of individuals through LLMs and more globally generative AI represents a profound shift that compels physicians to reconsider their role. The era of traditional paternalism in medicine is fading; at the same time, new forces are shaping public perceptions of health, including social media, misinformation, and the influence of commercial interests often driven more by profit than by the welfare of patients. A potential dystopian scenario emerges in which a patient consults an LLM for a diagnosis, selects therapy online, self-manages treatment, and ultimately evaluates the system with a ‘five-star rating’, entirely bypassing established scientific evidence and quality controls. Perhaps the most concerning risk is the reduction of the physician to a mere technician of the body, perceived as someone whose expertise can be replaced by automated systems. Yet when confronted with personal suffering or life-threatening illness, we must ask, ‘Can humans truly rely on their own rationality alone, or does the physician’s role as guide, interpreter and advocate remain irreplaceable?’
In this context, innovative teaching approaches are needed, from simulation-based workshops and interdisciplinary projects to faculty development in collaboration with computer scientists and industry experts. Lifelong learning opportunities, from short courses to advanced degrees, will ensure continuous adaptation as technology evolves. In this way, endocrinologists can become not only users of AI, but also active shapers of its applications, translating innovation into better care and research. Alongside technical training, equal emphasis must be placed on the moral and ethical dimensions of AI. Bias, transparency and accountability must form the backbone of every curriculum, enabling clinicians to recognise and manage risks to fairness and care quality. Ethical instruction cannot be left to technology itself; educators remain the key gatekeepers, adapting established biomedical ethics to new challenges. Authority in defining and enforcing these standards should rest on collaboration between clinicians, ethicists, lawyers and technologists. Only through such interdisciplinary oversight can AI tools be implemented responsibly. Embedding rigorous ethical education into AI training safeguards patient rights, sustains trust and upholds professional integrity.
The rapid expansion of medical data generation presents both opportunities and challenges for early-career investigators. While young clinicians remain the backbone of patient care, they face uneven preparedness in handling data. This problem is amplified by both a projected decline in the endocrinology workforce3 and the lack of structured AI and data science education in medical schools. Initiatives such as the COST Action Harmonisation (CA20122) illustrate the value of collaborative networks for early-career investigators, particularly in adrenal endocrinology. Expanding these initiatives to include
AI-focused training could close key gaps in technical, clinical and regulatory knowledge. With sustainable funding and a bottom-up approach, earlycareer investigators could learn practical distinctions in data collection and governance while building long-term capacity for responsible data sharing. Beyond system-level data, the increasing flow of unstandardised information from wearable devices adds new risks. Without proper education, patients may misinterpret isolated metrics, undermining clinical care. Future endocrinologists will therefore need to act not only as clinicians and scientists, but also as communicators, capable of translating complex digital health data into clear, actionable insights for patients and peers alike.
In conclusion
The future of AI in endocrinology is unpredictable, with potential for both breakthroughs and pitfalls. A bottom-up approach is crucial; groundwork and regulation must originate from clinicians and scientists, not solely from external regulatory bodies or IT experts outside the field. At the same time, we must accept that the classical model of science − long reviews, textbooks, ex cathedra lectures − may be losing ground in efficiency to AI. This does not signal the end of science, but
a call to adapt. By combining rigorous technical and ethical training, embracing collaboration and asserting leadership, endocrinologists can ensure that AI serves medicine rather than the other way around.
Antoan Stefan Šojat
Clinic for Endocrinology, Diabetes and Metabolic Diseases, University Clinical Centre of Serbia, Belgrade, Serbia
Guillaume Assié
Service d’Endocrinologie APHP, Université Paris Cité− Hôpital Cochin−Port-Royal, Paris, France
REFERENCES
1. Šojat et al. 2025 European Journal of Endocrinology
https://doi.org/10.1093/ejendo/lvaf005
2. Assié & Allassonnière 2024 Journal of Clinical Endocrinology & Metabolism
https://doi.org/10.1210/clinem/dgae154
3. Vigersky et al. 2014 Journal of Clinical Endocrinology & Metabolism
https://doi.org/10.1210/jc.2014-2257
The second joint ESE and Endocrine Society guideline has recently been published on pregnancy in diabetes.1
Adverse pregnancy outcomes such as miscarriages or birth defects are common in individuals with pre-existing diabetes and are often related to modifiable factors such as maternal high blood sugar and body mass index.
The new guideline therefore recommends that women with diabetes receive proper preconception care and access to emerging diabetes technology and therapeutics to manage their blood sugar before, during and after pregnancy. Screening women of reproductive age who have diabetes for intent to conceive at every reproductive, diabetes and primary care visit would help ensure that they get the appropriate preconception care and reduce health risks.
A summary of the guideline’s key recommendations include: Screening: ask all women with diabetes of reproductive age about their intent to conceive at every reproductive, diabetes and primary care visit.
Delivery timing: aim for early delivery before 39 weeks for pregnant individuals with diabetes, as the risks associated with continued pregnancy may outweigh those of early delivery.
Medications: discontinue glucagon-like peptide-1 agonists prior to pregnancy, and avoid prescribing metformin in pregnant individuals with pre-existing diabetes who are already on insulin.
Diabetes technology: recommend the use of hybrid closed-loop systems for pregnant individuals with type 1 diabetes.
Contraception: suggest that women with diabetes use contraception until they are ready to become pregnant.
The guideline has been published open access in both European Journal of Endocrinology1 and Journal of Clinical Endocrinology & Metabolism, and was presented at ENDO 2025.
It was developed by a multidisciplinary panel of topic-related experts in the field using a rigorous methodology. The panel relied on evidencebased reviews of the literature when developing the recommendations. They prioritised randomised controlled trials, and the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology was used to assess the certainty of evidence and guide recommendations.
The guideline working group was chaired by Jennifer Wyckoff (USA), who commented ‘Diabetes rates are rising among women of reproductive

age and very few women with diabetes receive proper preconception care. The guideline highlights the need for research and investment into preconception care, more randomised control trials to define glycaemic targets in pregnancy, and data on optimal nutrition and obesity management in pregnancy.’
Co-chair Annunziata Lapolla (Italy) added, ‘Given the increase in type 2 diabetes associated with obesity worldwide, and women with this pathology who become pregnant, these recommendations have also addressed the issues related to correct nutrition and therapeutic approach in such women.’
These other guidelines have either just been published or are in development.
Menopausal hormone therapy (see page 10)
Chronic hypoparathyroidism in adults (see page 11)
Guidance on transition (jointly with ESPE)
Hyponatraemia (jointly with the European Renal Association) AVP deficiency (jointly with the Endocrine Society)
Management of weight regain (jointly with EASO)
Male hypogonadism (jointly with the Endocrine Society)
This ESE Clinical Practice Guideline supports the evaluation and management of menopause by healthcare professionals.1
All women will go through the menopause if they live long enough. Since women make up 51% of the world’s population, there will be 2 billion women in postmenopause by 2030.
Many women experience a range of symptoms during the menopause and perimenopause that impair their quality of life and ability to lead full lives at work or at home. The most common include vasomotor symptoms (e.g. hot flushes and sweats), effects on mood (e.g. low mood) and urogenital symptoms (e.g. vaginal dryness).2 These symptoms are often short-lived and lessen or disappear over time. However, about 25% of women experience severe menopausal symptoms and, consequently, the numbers being seen within many specialties will increase.
Postmenopausal women are also at increased risk of a number of long term conditions, such as osteoporosis, cardiovascular disease and changes in the vagina and bladder. These occur because of natural ageing, although oestrogen depletion may have a role.3
Menopausal hormone therapy (MHT), also known as hormone replacement therapy, licensed in Europe to treat symptoms in postmenopausal women, will be an appropriate option to treat symptoms in most women, including those approaching menopause and in the menopause transition. Its role in the long term prevention of chronic disease is controversial.4−7
A holistic view is taken and the information that should be given to each woman is included, including alternatives to MHT. Thus women having hormone treatment of their vasomotor symptoms, for example, should know about other potential benefits, such as the impact on cardiovascular disease risk and bone health, as well as other available treatments. In addition, women should be made aware of potential risks and side effects, for example breast cancer, the rate of which changes with duration of hormone use, thus changing the risk/benefit equation as a woman ages.
Areas of controversy such as the impact of hormones on cancer risk and dementia are included, taking into account advice given by the major national and international menopause societies and licensing authorities.
The guideline’s conclusions
Diagnosis and hormone tests required at different ages
Diagnosis is clinical. Endocrine assessment, including follicle-stimulating hormone measurement, is not routinely required in women over 45, although it should be in younger women when there is a suspicion of POI.
Benefits of MHT that are discussed
• Symptom relief, where symptoms are due to hypo-oestrogenism (e.g. vasomotor symptoms, vulvo-vaginal atrophy)
• Improved quality of life
• Decreased emotional lability and improved mood
• Improved general health, including lipid profile and glucose metabolism, with some impact on fat distribution; a decreased chance of developing type 2 diabetes mellitus
• Maintenance of bone density and prevention of osteoporosis
• Decreased risk of developing cardiovascular disease.
The risks of MHT
• Increased chance of a deep vein thrombosis with oral therapy, but not with transdermal therapy
• Increased incidence of breast cancer that is duration-dependent and depends on type and route of administration; long-term MHT use should be regularly reviewed, based on individual risk–benefit assessment
• The impact on cognition and dementia risk is uncertain.
In summary
The need for a guideline
ESE wanted practical advice on evaluation and management of menopause for healthcare professionals, particularly those working in endocrinology. The Society established a group of endocrinologists and gynaecologists, drawn from European and American menopause and endocrine societies, to compile an ESE Clinical Practice Guideline on menopause.1
The guideline not only covers women who go through the menopause in middle age, but also those with premature ovarian insufficiency (POI) and those for whom hormones are not appropriate, including women with, or at high risk of, breast cancer. The guideline does not cover trans women, as their needs from a hormone replacement standpoint are different.
Methodology and contents
Evidence is drawn from systematic reviews, randomised controlled trials and discussion of other national and international guidance to guide the recommendations. Three new systematic reviews, undertaken to answer specific questions, include the impact of dose of oestrogen on fracture risk, the impact of progestogen monotherapy and local oestrogen therapy.
Women are considered in three groups:
• those going through natural menopause over 45 years
• those between 40 and 45 (early menopause)
• those under 40 (POI).
The risks and benefits of administering hormones are discussed taking the woman’s age into account. Diagnosis of menopause transition is included, since this is important, particularly in younger women. History taking, examination and investigations required prior to starting hormones are included. This advice varies for women with and without medical co-morbidities.
• Menopausal women often experience symptoms that impair their quality of life and ability to lead full lives at work or at home.
• Many of the symptoms can be treated effectively.
• The new guideline on menopause informs clinicians who might see women with menopausal problems, and gives them advice which is easy to follow.
• Attention to lifestyle at this key time is a very important contributor to healthy ageing.
Mary Ann Lumsden and Leonie van Hulsteijn Chair and Post-doc Methodologist, Guideline Working Group
REFERENCES
1. Lumsden et al. 2025 European Journal of Endocrinology https://doi.org/10.1093/ejendo/lvaf206.
2. Nappi et al. 2021 Menopause https://doi.org/10.1097/gme.0000000000001793
3. Khoudary et al. 2019 Menopause https://doi.org/10.1097/gme.0000000000001424
4. Boardman et al. 2015 Cochrane Database of Systematic Reviews https:// doi.org/10.1002/14651858.cd002229.pub4
5. Marjoribanks et al. 2017 Cochrane Database of Systematic Reviews https://doi.org/10.1002/14651858.cd004143.pub5
6. Lobo & Gompel 2022 Lancet Diabetes & Endocrinology https://doi.org/10.1016/s2213-8587(21)00269-2
7. Gartlehner et al. 2022 JAMA https://doi.org/10.1001/jama.2022.18324
FURTHER READING
Collaborative Group on Hormonal Factors in Breast Cancer 2019 Lancet https://doi.org/10.1016/s0140-6736(19)31709-x
Greendale et al. 2021 Journal of Clinical Endocrinology & Metabolism https://doi.org/10.1210/clinem/dgab389
NICE 2024 Menopause: Identification and Management NG23 https://www.nice.org.uk/guidance/ng23
Rovinski et al. 2018 Thrombosis Research https://doi.org/10.1016/j.thromres.2018.06.014
Speksnijder et al. 2023 Diabetes Care https://doi.org/10.2337/dc23-0451
A revised ESE Clinical Practice Guideline on chronic hypoparathyroidism (HypoPT) in adults is now available.1
Ten years ago, ESE launched its first three clinical guidelines on the management of different endocrine diseases at ECE 2015 in Dublin, Ireland. Among these was a guideline on management of chronic hypoparathyroidism (HypoPT) in adults.2 The presentation was timely, as the first medical replacement therapy for chronic HypoPT was approved at that time by the US Food and Drug Administration and the European Medicines Agency.
Over the past decade, significant new evidence regarding the understanding of the disease, including the development of morbidities and patient burden, has evolved. This has led to changes in clinical management and surveillance of patients alongside new treatment modalities. In 2023, in line with ESE policy, the Clinical Committee decided to update the 2015 guideline based on up-to-date scientific evidence.
Management of HypoPT has, until recently, involved an indirect approach to treatment, based on vitamin D and calcium supplementation (primarily calcium by diet or supplements). This first-line treatment (termed conventional treatment) aims primarily to increase the intestinal absorption of calcium, and seems to work well for many patients. The algorithm is, however, non-physiological, as it does not restore the action of parathyroid hormone (PTH) on bone and renal tubules.
HypoPT is the last endocrine deficiency disease where the hormone which is lacking is not, itself, routinely replaced. Important new treatment modalities have been developed since the first guideline and introduced in preclinical trials and clinical management. However, so far, systematic PTH replacement therapy is not available for all patients in need.
What is chronic HypoPT?
HypoPT is a rare (orphan) disease, most often secondary to neck surgery (postoperative HypoPT) or related to various genetic syndromes or without known reasons (idiopathic); these latter are designated non-surgical HypoPT.
HypoPT is, in principle, a two-hormonal deficiency syndrome, where low circulating calcium levels are caused by a lack of PTH as the primary defect (low or inappropriately low PTH levels in relation to calcium concentrations). As PTH is the key regulator of vitamin D activation, PTH insufficiency is followed by low levels of activated vitamin D.3 As a result, calcium levels are low due to the lack of calcium mobilisation from bone (PTH action), lack of calcium reabsorption from the renal tubules (PTH action), and insufficient calcium absorption from the gut (activated vitamin D action) (see Figure). Importantly, the patient burden of HypoPT is high, leading to low quality of life (QoL) for patients and their relatives, with important economic liabilities for society.4,5
Procedure for guideline revision
A panel of international experts, comprising endocrinologists (including an Endocrine Society representative), methodologists, an ESE Nurse
Calcium absorption
representative, a representative of the ESE Young Endocrinologists and Scientists, a nephrologist and an endocrine surgeon (representing the European Society of Endocrine Surgeons), formed the guideline’s working group. They collaborated with representatives from several HypoPT patient organisations. The working group had two in-person meetings and several online conferences to develop the guideline over almost two years.
Literature was systematically searched during September−November 2024 for the individual three research (clinical) questions, whereas literature for discussion was updated until June 2025. As HypoPT is an orphan disease, solid evidence for most outcomes was scarce. Thus, recommendations were derived from a majority consensus of the guideline panel when scientific evidence was lacking or low.
We defined chronic postsurgical HypoPT as a condition persisting for more than 12 months following surgery. Importantly, recovery can be expected even after that time (clinical question I: QI), and should be checked for regularly in ongoing monitoring.
For QII, the optimal treatment of chronic HypoPT, relevant data regarding optimal conventional treatment regimens are lacking for clinically relevant endpoints, such as biochemical control and QoL, and long-term effects. PTH replacement therapy reduces the pill burden of conventional therapy, improves various biochemical parameters, and potentially improves QoL. Therefore, the guideline panel recommends initiating PTH replacement therapy in patients with chronic HypoPT who continue to have signs or symptoms of HypoPT despite optimised conventional treatment. Practical advice is given on how to do this.
QIII examined evidence for a potential role for parathyroid allotransplantation in management of chronic HypoPT. This was reviewed but proved very low, so the panel decided not to recommend this option.
The updated 2025 guideline presents recommendations for the diagnosis, management and monitoring of chronic HypoPT in adults, to give healthcare providers practical clinical guidance on the management of patients with this two-hormone deficiency syndrome. The guideline will serve as a source for preparation of educational materials to empower patients and clinicians.
Leonie van Hulsteijn and Jens Bollerslev
Post-doc Methodologist and Clinical Chair, Guideline Working Group
Urinary calcium excretion (before treatment)
Phosphate reabsorption
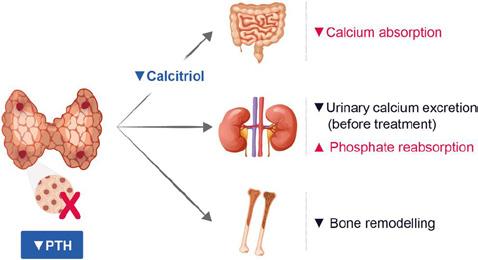
Bone remodelling
REFERENCES
1. Bollerslev et al. 2025 European Journal of Endocrinology https://doi.org/10.1093/ejendo/lvaf222
2. Bollerslev et al. 2015 European Journal of Endocrinology https://doi.org/10.1530/eje-15-0628
3. Bollerslev et al. 2019 European Journal of Endocrinology https://doi.org/10.1530/EJE-19-0316
4. Astor et al. 2016 Journal of Clinical Endocrinology & Metabolism https://doi.org/10.1210/jc.2016-1477
5. Siggelkow et al. 2020 Clinical Endocrinology https://doi.org/10.1111/cen.14128
6. Bollerslev et al. 2022 European Journal of Endocrinology https://doi.org/10.1530/EJE-21-1044
Some of the latest high-quality research published in ESE’s open access journal dedicated to all aspects of obesity, selected by Editor-in-Chief Melania Manco.
A new study published in Obesity and Endocrinology by Andrew Agbaje challenges long-standing reliance on body mass index (BMI) for diagnosing overweight and obesity in children. Drawing on data from over 7600 participants in the UK-based Avon Longitudinal Study of Parents and Children (ALSPAC), the research reveals that BMI significantly overestimates the prevalence of overweight compared to waist-to-height ratio (WHtR) − a simpler, more sensitive, anthropometric measure of central adiposity.
The study followed participants from age 9 through adolescence to age 24, demonstrating that BMI classified 2.3 to 2.8 times more children and adolescents as overweight than WHtR did. Nearly two-thirds of children who were identified as overweight by BMI actually had normal fat levels when assessed by WHtR. Conversely, WHtR was more accurate in capturing those with clinically relevant excess fat: individuals at higher risk for future metabolic diseases.
To strengthen these findings, Agbaje externally validated the WHtR cut-offs using a cohort of over 3300 adults from the USA (the
2021–2023 NHANES dataset). WHtR-defined excess fat was associated with markedly increased odds of prediabetes and type 2 diabetes, independent of traditional risk factors. Participants with high or excess WHtR had up to sixfold increased odds of diabetes, underscoring the metabolic relevance of this anthropometric marker.
Importantly, the study proposes a reframing of obesity terminology. Agbaje recommends replacing the stigmatising labels ‘overweight’ and ‘obese’ with ‘adiposopathy grade 1’ and ‘adiposopathy grade 2’ respectively. This shift aligns with recent calls from the European Association for the Study of Obesity to conceptualise obesity not as excess weight, but as a chronic, adiposity-based disease.
In light of these findings, WHtR emerges as a practical, low-cost and non-invasive tool for clinical and public health settings, especially in paediatrics where misclassification can lead to both over- and undertreatment.
Read the full article in Obesity and Endocrinology https://doi.org/10.1093/obendo/wjaf002
From genes to care: the expanding therapeutic horizon in rare genetic obesities
Rare genetic obesities − such as those involving POMC, LEPR, PCSK1, MC4R, and syndromes such as Bardet–Biedl and Prader–Willi − are increasingly recognised as neuroendocrine disorders rooted in hypothalamic dysfunction. These conditions often present with early-onset obesity, hyperphagia and complex behavioural and endocrine profiles, making conventional interventions insufficient.
Recent advances have led to the development of targeted therapies that modulate appetite regulation pathways. Chalopin et al. offer an expert’s opinion, exploring the use of different drugs in genetic and syndromic obesities.
• Setmelanotide, an melanocortin 4 receptor agonist, is approved for use in biallelic mutations in key melanocortin pathway genes and Bardet–Biedl syndrome.
• Glucagon-like peptide-1 (GLP-1) receptor agonists have shown variable benefits in syndromic forms like Prader–Willi syndrome.
• Tirzepatide, a dual GLP-1/glucose-dependent insulinotropic polypeptide agonist, and retatrutide, a triple agonist, are emerging as potent agents with promising results even in syndromic obesities.
• In Prader–Willi syndrome, novel agents like DCCR (recently approved by the US Food and Drug Administration for hyperphagia), carbetocin and neuromodulation techniques (e.g. vagus nerve stimulation) are under active investigation.
These therapies offer new hope. They require careful integration into multidisciplinary care frameworks, with attention to long-term safety, adherence and equitable access. Early genetic diagnosis is essential to guide treatment and improve outcomes.
Read the full article in Obesity and Endocrinology https://doi.org/10.1093/obendo/wjaf007



Obesity and Endocrinology is the official ESE journal dedicated to high-quality clinical and translational research on all aspects of obesity, including its complexity as an endocrine disease, its biology, diagnostics and treatment, and its connections to other endocrine and non-endocrine conditions.
ESE members are eligible for a 50% APC discount when publishing in Obesity and Endocrinology