TITLE:
MORPHOLOGICALANDAGRONOMICALCHARACTERIZATIONAND ESTIMATESOFGENETICPARAMETERSOFSESBANIASCOP(LEGUMINOSAE) ACCESSIONS
AUTHOR:
E.A.Veasey
E.A.Schammass
R.Vencovsky
P.S.Martins
GBandel

TITLE:
MORPHOLOGICALANDAGRONOMICALCHARACTERIZATIONAND ESTIMATESOFGENETICPARAMETERSOFSESBANIASCOP(LEGUMINOSAE) ACCESSIONS
AUTHOR:
E.A.Veasey
E.A.Schammass
R.Vencovsky
P.S.Martins
GBandel
Twenty-two accessions of seven Sesbania (Leguminosae) species: S. emerus, S. rostrata, S. tetraptera, S. exasperata (annu- als). S. grandifiora, S. sesban and S. virgata (perennials), used for ruminant fodder, firewood. wood products, soil improvement, and human food, were investigated, with the aim of characterizing both inter- and intraspecific genetic vanability, esti- mating genetic parameters for the characters evaluated and appraising the forage potential of the accessions. These were planted at the Instituto de Zootecnia, Nova Odessa, SP, Brazil, in a randomized complete block design with 22 treatments and four replications. Seventeen morphological and 17 agronomic characters were evaluated. Genetic parameters coefficient of intraspecific genetic diversity (b) and coefiicient of intraspecific genetic variation (CV,,) were obtained for the species repre- sented by more than one accession. Highly significant differences were observed among as well as within species for most characters, showing considerable genetic variability. S. exasperata showed intraspecific genetic variability for the largest number of morphological characters. The same was observed for S. sesban for the agronomic characters. Most of the char- acters gave high b, values, above 0.80, indicating the possibility of selecting superior genotypes. The CV,, values, on the other hand, which indicate the magnitude of the existing genetic variability relative to the character mean, varied according to the species and character evaluated. Differences between annual and perennial species were observed, with higher biomass yields presented by the annuals at the first cut and by the perennials after the second cut, reaching the highest yield at the third cut. The annual species had higher seed production. Accession NO 934 of S. sesban gave the highest biomass yields and regrowth vigor, showing promise as a forage legume plant.
The genus Sesbania Scopoli belongs to the subfamily Papilionoideae of the Leguminosae and the botanical tribe Robinieae. The approximately 60 tropical and subtropical species include annual and perennial herbs, shrubs or small trees and are distributed in four subgen- era: Agati, Daubentonia, Pterosesbania and Sesbania (Monteiro, 1984). Their nitrogen fixing ability enables these plants to grow rapidly on nitrogen deficient soils and allows their utilization for green manuring in paddy fields, for intercropping and ground cover, and for agroforestry and wood production (Ndoye er al., 1990).
Subgenus Sesbania, originating from the Old World and later dispersed throughout the New World, has the greatest number of species, although among the perennial species of this subgenus, only S. sesban has been widely utihzed in agriculture for livestock forage and green ma- nure (Brewbaker ¢t al., 1990). This is a highly variable diploid species with two subspecies: sesban, with four vaneties, and punctata (Monteiro, 1984; Bray, 1994). The varicties sesban and bicolor are very similar except for flower color. These 1wo varieties às well às var. nubica give vigorous growth and high yields. The zambesiaca variety and ssp. punciata are less well known (Brewbaker
Cenro de Farragiculiura e Pastagens, Instawio de Lootecnia, Kua Hettor Penteado. 36, Cuts Postul 60, 13460-000 Nova Odessa, SP Brasil Depariamento de Genetica. ESALQ/USP. Cuixu Postal 85, 1400-970 Piraciaba. SP Brasil Send correspondence to G 8
etal., 1990). Among the annual species. subgenus Sesbania is represented throughout the Americas by S. bispinosa. S. emerus, S. oligosperma and S. exasperata (Monteiro, 1984). The tetraploid S. grandiflora of subgenus Agari, originating in the Old World (Australia and Asia). together with S. sesban have been utilized extensively in traditional agroforestry systems (Bray, 1994). Their leaves and young twigs are used as high protein fodder for ruminants, while the thick branches and stem provide fuelwood and construction material. These species are also used to improve soil fertility and to reduce soil erosion (Heering er al.. 1996a). The species with winged-pods were divided into two subgenera: subgenus Daubentonia, a native of the New World with five species, including the species S. virgara and S. punicea, and subgenus Prerosesbania. represented by only one species, S. tetraptera (Monteiro, 1984).

Agronomical and phenological evaluations of Sesbania sp. germplasm collections have been conducted by the University of Hawaii and International Livestock Centre tor Africa (ILCA) in Ethiopia and Tanzania (Mengistu, 1990; Bray, 1994), the Commonwealth Scien- tific and Industrial Research Organisauon (CSIRO) in Australia (Wood and Larkens, 1987), and the Intemational Centre for Research in Agrotorestry (ICRAF) in Kenya (Ndungu er al.. 1994). In Bruzil, Rocha er al (1979) col- lected uccessions corresponding to four species of Sesbania located mainly in damip areas near nver banks in the States of São Paulo, Minas Gerais and Rio de Juneiro, from 1976 to 1978,
Evaluation of the genetic variability tound in these collections represents an important step for breeding pro-
Veasey et al.
grams. Wood and Larkens (1987) presented plant growth data and general characteristics of 192 accessions of the genus Seshania. The collection of 161 accessions of Sesbania spp. was reported by Mengistu (1990), who observed considerable polymorphism in S. seshan, occurring in a wide range of environments. The classification of a collection of 108 accessions of S. sesban based on morphological and agronomical attributes was done by Heering et al. (1996a,b), who observed distinct groups within the collection.
The objective of this study was the characteriza- tion of genetic variability based on phenological, morphological and agronomical data of 22 accessions belonging to seven Sesbania species, representing the four subgenera Sesbania, Pterosesbania, Agati and Daubentonia, as well as the estimation of genetic parameters for the characters evaluated, and an appraisal of their forage potential.

Seven Sesbania species (22 accessions) from the germplasm bank of the Instituto de Zootecnia, in Nova Odessa. São Paulo, were evaluated (Table I), representing four different subgenera: subgenus Sesbania (S. emerus, S. exasperata, S. rostrata, S. sesban), originating from the Old World and later dispersed throughout the New World; subgenus Daubentonia (S. virgata), from the Americas; subgenus Agari (S. grandiflora), from Australia and Asia,
and subgenus Prerosesbania (S. tetraptera), originating from Africa (Monteiro, 1984)
The experiment started in October 1994 at the Instituto de Zootecnia at an elevation of 550 m, S. lat 22°42" and W. long. 47°18". The soil was a reddish yellow podzolic, with a pH of 5.3 (CaCl,), 3.33% organic matter. 0.19 meq/100 cm'K, 3.80 meq/100 cm Ca. 1.15 meg/100 cm Mg, 3.68 meg/100 cm' Al and 25.77 ug/dm' P. The mean annual maximum and minimum temperatures during the experimental period were approximately 29 and 17°C, respectively, with a mean annual rainfall of 1560 mm (mean of three years).
The experiment was set up as a randomized com- plete block design, with four replicates and 22 treatments (accessions). Scarified seeds were sown in 67 x 34 cmplastic trays, containing a mixture of sand and vermiculite as substrate, maintained in a greenhouse. Two months after sowing, well-developed seedlings were transplanted to the field. Plots consisted of single 16-m long rows of eight plants, spaced 2 m apart with 3 m between rows. Lime was applied at 1.92 t/ha. At planting. fertilizer was bandplaced at rates equivalent to 500 kg/ha single superphosphate, 150 kg/ha potassium chloride, 500 g/ha sodium molybdate, 8 kg/ha zinc sulfate, 4 kg/ha copper sulfate and 4 kg/ha borax. Two side dressings were applied dur- ing the experimental period, at the same rates described above, in September/95 and February/96. Seventeen morphological, including two pheno-
2562 Sesbania annual CENARGEN' - BRA 000914 / CPATU' 184
2 emerus 2563 s CENARGEN - BRA 000060 / CPATU 1212
3 S. exasperata 1017 Fazenda Santa Elisa - Campinas, SP
4 S 1545 Itumbiara, GO
5 2117 CNPMS' - Sete Lagoas, MG
6 - 2560 CENARGEN - BRA 000086 / CPATU 1214
7 S grandiflora 2559 Agati perennial Linhares, ES
8 S. rostrata 2566 Sesbania annual CENA/USP' - CNPAF/Goidnia, GO
9 S. sesban var. sesban 934 - perennial Cidade Univensitina - São Paulo, SP
10 var bicolor 1030 - Campinas, SP
" var bicolor 1375 Estrada Franca-ltuverava, SP
2 var. sesban 2419 Rongwa Research Centre - Tanzânia
3 var. bicolor 2529
Insttutode Pesquisas IRI - Matdo, SP
14 var. sesban 2567 Centrul São Jorge, Cape Verde (acc. 895)
15 S. tetraptera 1660 Prerosesbania annual Mozambique
16 S. virgata 1256 Daubentonia perennial Rodovia Pres. Dutra - Km 110, RJ - 1289 .
Miranda, MS
I 1634 Bicas, MG
19 2553
20 2554
2 2887
2 2565
Piracicaba, SP
Niterói, RJ
Amencana, SP
Corumbá, MS
'CENARGEN - Centro Nacional de Recursos Genéticos ¢ Biotecnolvgia; *CPATU - Centro de Pesquisa Agrondmica do Trópico Úmido; CNPMS - Centro Nacional de Pesquisa de Mato Grosso do Sul ENA/USP - Centro de Energia Nuclear na Agricultura / Universidade de São Paulo; CNPAF - Centro Nacional de Pesquisa de Arroz ¢ Feijão
logical characters were evaluated (Table 11 in the first four plants of each plot. A pachymeter was used to measure the characters stem diameter (SD), pod width (PW), seed length (SL) and seed width (SW). Seventeen agronomical characters (Table I11) were evaluated. With the exception of characters plant height at flowering (PHF), plant height (PH), seed production (SP) and plant survival of uncut plants (PS1), evaluated in the first four plants of each plot, all the others were evaluated in the four remaining plants. These plants were submitted to four cuts (April, August and December 1995, and April 1996). at a height of 1 m. At each cut, the plant
material was separated into two fractions: leaves (including small stems < 6 mm) and stems > 6 mm in diameter). representing edible and inedible components. respectively. Univariate analyses were undertaken, with the partitioning of the accessions within species and sources of variation, according to the mathematical model
+d, to Yu=p+p+a,+b+e o
where 4 = overall character mean; p = fixed effect of the ith species (i = 1,2,....7); a,,= fixed effect of the Ith acces-
Morphological characters evaluated in Sesbania spp.
Abbreviation Character Character description
A Floral initiation
FMP First mature pod
sSD Stem diameter
u Leaf length
NLP Number of leaflet pairs
LFL Leaflet length
LFW Leaflet width
L Inflorescence length
NF Number of flowers per inflorescence
PDL Pedicel length
AL Flower length
PL Pod length
PW Pod width
NSP Number of seeds per pod
DS Percentage of fully developed sceds per pod
sL Seed length
sw Seed width
Number of days from sowing to first open flower
Number of days from sowing to first mature pod
Measured six months after sowing. at m height
Measured at the 10th leaf from the apex (mean of three leaves)
Counted at the 10th leaf from the apex (mean of three leaves)
Measured on the middle leaflet at the 10th leaf from the apex (mean of three leaflets)
Measured at the widest point of the same leaflet. at the 10th leaf from the apex (mean of three leaflets)
Mean of five inflorescences
Number of flowers per inflorescence (mean of five inflorescences)
Mean of five flowers
Mean of five flowers
Mean of five pods. including the pedicel
Measured at the widest point (mean of five pods)
Mean of five pods
Mean of five pods
Mean of five seeds
Mean of five seeds
Table 11
Agronomical characters evaluated in Sesbania spp.
Abbreviation Character Character description

PHF Plant height at flowering
PH Plant height
sP Secd production
LDMY! Leuf dry matter yield
LDMY2 Leaf dry matter yield
LDMY3 Lead dry matter yield
LDMY4 Leaf dry matter yield
SDMY1 Stem dry matter yield
SDMY2 Stem dry matter yield
SDMY3 Stem dry matter yield
SDMY4 Stem dry matter yield
cpl Crude protein
cP2 Crude protein
cpy
cpa
Crude protein
Crude protein
Psi Plant survival of uncut plants
Ps2 Plant survival of cut plants
Measured on the uncut plants at flowering, six months after sowing
(MAS) (April 1995).
Measured on the uncut plants, 18 MAS (April 1996)
Total seed from uncut plants.
First cut leal DM yield (April 1995)
Second cut leal DM yield (August 1995)
Third cut leal DM yield (December 1995)
Fourth cut leat DM yield (April 1996)
First cut stem DM yield (Apnl 1995)
Second cut siem DM yield (August 1995)
Third cut stem DM yield (December 1995)
Fourth cut stem DM yield (April 1996)
Crude protein percentage from leaf fraction f the first cut
Crude protein perventage trom leat fraction o( the second cut
Crude prote percentage trom leaf fraction of the thind cut
Crude protein percentage from leal fraction of the fourth cut
Perventage of surviving uncut plants, two years and four MAS
Percentage of surviving cut plants. two years and four MAS
sion, within the ith species (1 = ); b,= random effect of the jth block (j = 1,2 'm = random effect of plot error with the Ith accession of the ith species in the jth block. and d,,,, = random effect of kth individual, in the plot with the lth accession of the ith species in the jth block k=12,..4)
The analyses were conducted with plot means (Vencovsky and Barriga, 1992), obtaining the sums of squares and mean squares for blocks, species, accessions within species and error. The program SAS Version 6 (SAS Institute. 1993) was used for these analyses. Mean squares within plots were independently obtained through the means, weighted by the degrees of freedom, of the individual variance estimates within plots (Geraldi, 1977), such as:
OM,= X (GL)(QM,),/ X (GL), = X(5Q,), /% (GL), where OM, = mean squares within each plot; SQ, = sum of squares within each plot, and GL = degrees of freedom within each plot. The mean squares within plots (QM,,) for each of the four species with more than one accession was also obtained.
The analysis of variance and expected mean squares are summarized in Table [V, where o} = phenotypic variance among plants within plots; 62 = variance of the error among plots; V. = measure of genetic diversity among accessions of the ith species (i = 1, .., 4); V, = measureof genetic diversity among speci replicate number; k = mean number of plants per plot; L = mean number of accessions per species (arithmetic mean), and ¢ = coefficient obtained to correct for the different number of accessions per species.
There was a different number of plants per plot as a consequence of plant death; therefore, the mean number of plants per plot (k) was obtained through the harmonic mean, as follows:
k = N/ Zn(1/k), where N = total number of plots; n = number of plots with k plants, and k = number of plants
Table IV
Sources of variation, degrees of freedom (d.f.), and expected mean squares (MS) of the analysis of variance.
Sources of variantion a MS
Block ")
Expectation of mean squares
Species (1) Q8 o'k + ARV, Accession/species wn Q FAA
Access/S. emerus () Q6 A IV, Acces'S. exasperata (L) Q5 kel + IV,
Accesw'S. seshan ) Qs FAA
Access/S, virgata L) Q3 ke ol 4 IV, Emor n QQ ojk+ o, Within plots emror Q! o
per plot (k = 1, ..., 4). Similarly, the mean number of plants per plot for each species was obtained separately (k ). Due to the different number of accessions per species, a coefficient (¢) was calculated in order to correct for this prob- lem, according to the following equation described by Weir (1996): where p = number of species and ci = number of accessions of the ith species.
The phenotypic variance within species m ), error variance among plots (G ), genetic variation among species (V ) and among accessions within the species S. emerus (Vur). s exasperata (V.,), S. sesban (V,)andS. virgata (V , were estimated from the mean squares, according to the equations:
0:-0/k: V, = (Qr-QuV/6; & V) =(0s-QVI; V., = (Q-Q:i V., =(0-QYI: V., = (Q-Q:M.
These quantities permitted the estimation of intraspecific phenotypic variance (G7 ), intraspecific genotypic determination (b,), and intraspecific genetic varia- tion coefficient (CV, ), according to the following equations:

&=V+ (G/J) + (G2/Ik, ):
Considerable interspecific vari was observed for all the characters evaluated (P < 0.001). which is expected since the comparison is between annual and perennial species, belonging to different subgenera. In the measures of intraspecific variation, 76.5, 100, 82 and 71% of the morphological characters showed significant difterences (P < 0.05) between accessions of S. emerus, S. exasperata, S. sesban and S. virgata, respectively. S. exasperata had the highest intraspecific variation, followed in decreasing order by S. sesban, S. emerus and S. virgara (Tables V and VI). Intraspecific variation for S. grandiflora, S. rostrata and S. tetruptera could not be estimated as these species were represented by only one accession in this study.
In general, the genotypic determination coefticients (b) were high, above 0.80, indicating the possibility of selection for these characters. CV, values, however, var-
Table V
Estimates of variance among (V,) and within (V,) Sesbania spp.. coefficients of variation ( and coefficients of intraspecific genotypic determination (6)), for the morphological characters: floral initiation (FI), first mature pod stem diameter (SD), leaf length (LL), leaflet length (LFL) and width (LFW). number of leaflet pairs (NLP) and inflorescence length (IL)
Morphological and agronomical characterization of Seshania Scop (CV). coefficients of intraspecific genetic variation (CV,.). (FMP),
'Significant at P < 0.001 (a), P < 0.01 (b), P < 0.05 (c). *Values not estimated. Table V1
Estimates of variance among (V,) and within (V,) Sesbania spp.. coefficients of variation (CV), coefficients of intraspecific genetic variation (CV,), and coefficients of intraspecific genotypic determination (), for the morphological characters: number of flowers (NF), pedicel length (PDL). flower length (FL). pod length (PL) and width (PW), seed length (SL) and width (SW). number of seeds per pod (NSP) and percentage of fully developed sceds (FDS).

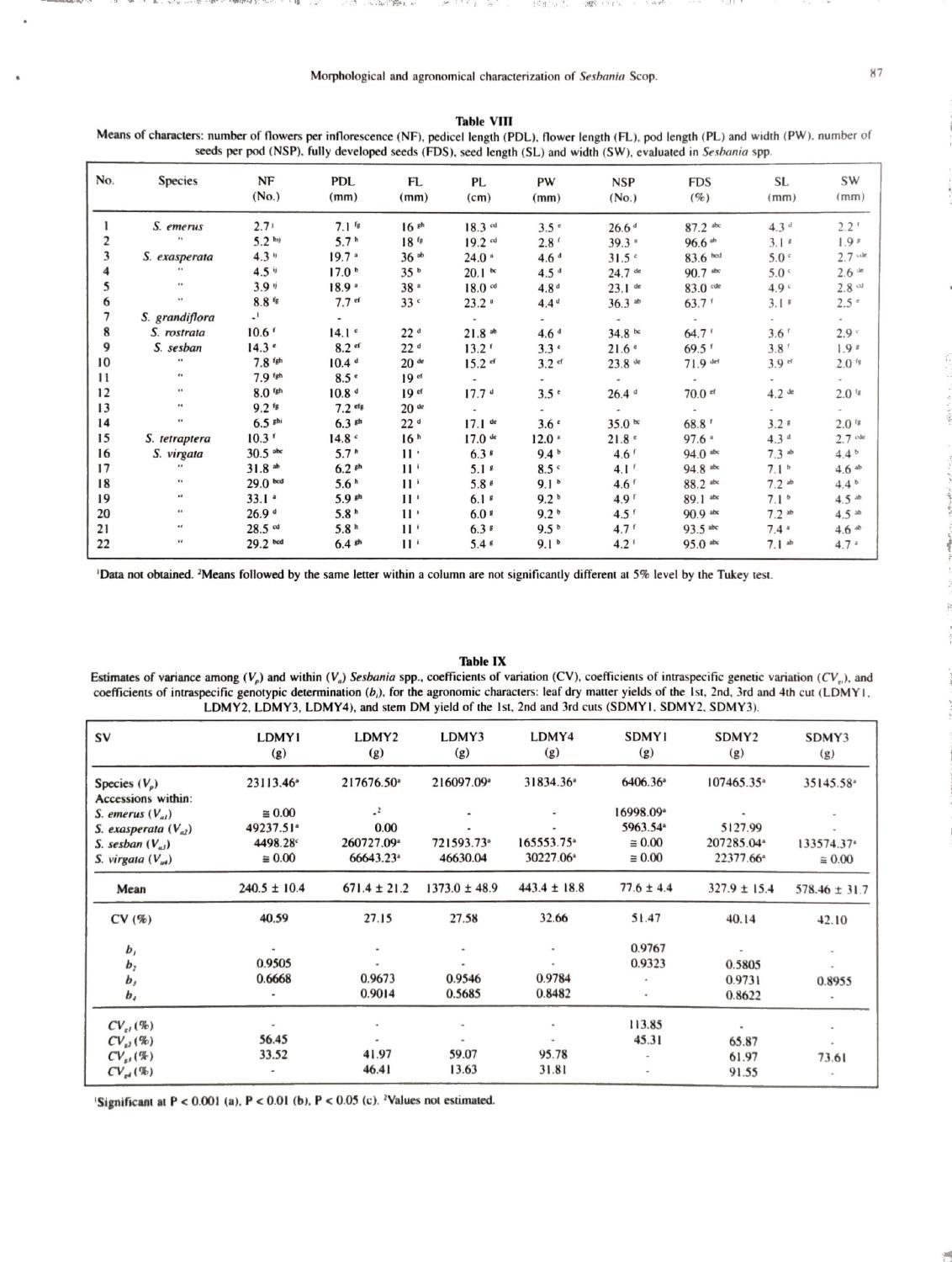
ied considerably according to the character and the species analyzed. For example. the character SD presented a high alue of 89.78% for S. emerus and also a high b, value of 0.9977. indicating the possibility of selection for this character. The averages presented by the two S. emerus accessions for SD were highly contrasting (Table VII). For S. seshan. the b, value for this same character was high, 0.9181. but the CV,, of 16.96% was low, with little genetic variability existing for selection to act upon this character. Another character that had a high b, as well as a high CV,, value was floral initiation (FI) for S. emerus (Table V), with means varying from 42.1 to 175 days (Table VII), indicating precocity for accession No. 1. The characters pedicel length (PDL). pod length (PL) and percentage of fully developed seeds per pod (FDS) presented low values for both b, and CV, for S. virgata, indicating low genetic variability for these traits. Variance was nil for the characters flower length (FL) and number of seeds per pod (NSP) for S. virgara. leaflet width (LFW) for S. emerus. and SW and FDS for S. sesban (Tables V. VI, VIl and VIII).
Significant differences (P < 0.05) between accessions of S. emerus. S. exasperata, S. sesban and S. virgata were presented by 80.0. 77.8, 86.7 and 33.3% of the agronomical characters, respectively (Tables IX and X). S. sesban had the greatest intraspecific variation in the agronomic characters. After the second cut, high values were observed both for b, and CV, in S. sesban for the characters leaf dry matter yield (LDMY) and stem dry matter yield (SDMY). Table

S. seshan. represented by six accessions. showed considerable genetic variation for several characters. such as first mature pod (FMP), number of leaflet pairs (NLP). leaflet length (LFL). inflorescence length (IL). number of flowers (NF). PDL, PL. SD. LFW. FL. NSP. SL. LDMY (2nd to 4th cut). CP (Ist to 4th cut). PHF and PH (Tables VIIL VIIL XI and XII). Significant differences (P < 0.05) were also observed between accessions of S. seshan 56 days after sowing (DAS) for the characters plant height and top growth dry weight (Veasey e al.. 1997). At this stage. the plants had already attained heights varying from 59 to 81 cm. reflecting the rapid growth of these species compared with the performance of Leucaena leucocephala. which varied from 18 to 22 cm
Mengistu (1990). evaluating 161 accessions of Sesbania spp. collected in various ecological zones of Tan- zania, observed considerable polymorphism for S. sesban. with variations in size, seed production and leafness. occurring over a wide range of altitudes (200-2200 m). rainfall (600-1400 mm, mean annual), vegetation (savannah. steppe, rain forest, semiarid) and a variety of soils. Varia- tion in leaf dry matter (DM) yield and seed production was also observed by Heering (1995) for S. sesban. Karachi er al. (1994) observed significant differences (P < 0.05) between accessions of S. sesban for height. basal collar diameter, primary branching, number of primary. secondary and tertiary branches, number of days to flowering and fodder and wood DM production. Heering er al. (1996a.b)
Means of characters: floral initiation (F1), first mature pod (FMP), siem diameter (SD), leaf length (LL), number of leaflet pairs (NLP), leaflet lengih (LFL) and width (LFW) and inflorescence length (IL), evaluated in Sesbania spp.
Means of characters: number of flowers per inflorescence (NF), pedicel length (PDL), flower length (FL). pod length (PL) and width (PW). number of seeds per pod (NSP), fully developed seeds (FDS), sced length (SL) and width (SW), evaluated in Sesbania spp.
'Data not obtained. *Means followed by the same letter within a column are not significantly different at 5% level by the Tukey test.
Estimates of variance among (V,) and within (V,) Sesbania spp., coefficients of variation (CV), coefficients of intraspecific genetic variation (CV, ), and coefficients of intraspecific genotypic determination (b), for the agronomic characters: leaf dry matter yields of the Ist, 2nd, 3rd and 4ih cut (LDMY 1. LDMY2, LDMY3, LDMY4), and stem DM yield of the 1st, 2nd and 3rd cuts (SDMY1. SDMY2. SDMY3).
Veasey el al. Table X n (CV), coefficients of intraspecific genetic variation (CV,). and coefficients of intraspecific genotypic determination (h). for the agronomic characters: stem DM yield of the 4th cut (SDMY4), crude protein percentage of the 15t, 2nd. 3rd and dth cuts (CP1. CP2, CP3, CP4), plant height at flowering (PHF). plant height (PH). and seed production (SP).

'Significant at P < 0.001 (a), P < 0.01 (b), P < 0.05 (c). *Values not estimated. Table XI
No. Species LDMY1 LDMY2 LDMY3 LDMY4 SDMYI SDMY2 SDMY3 SDMY4 ® ) &) @ [) & [) () S. emerus 160 < - - 22 - 2 - 193 s. - 208 * n: 3 S. exasperata 61 320 - 264+ 30 4 * 538 389 - 207 99¢ - 5 " 42 269 - 88 38º - - 6 " 160 186 384º 440 m» 252% 245 135 * S. grandiflora 17¢ 1047 491 me 84 n. 243 195 e 8 S. rostrata 674+ - - 285+ - - < 9 S. sesban 277 2123+ 3006 * 1146+ 42 1336+ 1076 n 10 - 240+ 1268 * 1455 210% u4o m. 586 2 s6* " 98« 7324 671+ 107 12+ - [ 250+ 115~ S8~ 285 . 3 90% 709% 660+ 690 7 12 a - 246 1353 1678+ 706 748~ 47 s S. teraprera 364 à - . 16 S. virgaia 147 481 2022 gy º 380 17 - 164 = 820 1180 = 832 S49 w501 18 126 453 1669 * a 688 336 19 145 3340 [A aa 698 2 239 20 95 asg 1688 * 459 808 & 36 2 160+ 302 1430~ 338% 687 9 2 28 1046 * 1339 m5 e 8
'Data not obtained. *Means followed by the same letter within u column are not significantly different at 5% level by the Tukey test
Means of characters: crude protein of the 1st, 2nd, 3rd and 4th cuts (CPI. CP2. CP3, CP4), plant height at flowering (PHF). plant height 18 months afer sowing (PH). seed production (SP). and plant survival of uncut (PS1) and cut plants (PS2). evaluated in Sesbania spp.
'Data not obtained. Means followed by the same letter within a column are not significantly different at 5% level by the Tukey test
classified a collection of 108 accessions of S. sesban into distinct groups based on morphological and agronomical data, separating accessions of the varieties sesban and bicolor from accessions of var. nubica. These two groups were separated principally because of the morphological characters leaflet size and number, seed size and color, and growth habit (Heering et al., 1996a). Considerable variation was also observed by Karachi and Matata (1997) in forage yield between accessions of S. sesban and S. macrantha.
S. emerus was represented by two accessions only, that contrast greatly in various characters. Indeed, because of the highly significant differences (P < 0.001) observed between them for characters FI, FMP, SD, leaf length (LL), NLP, PDL. PW, NSP, SL, SW, SDMYI, CPI and PHF (Tables VI, VIII, XI and XII), a hypothesis that these ac- cessions belong to different species was considered. However, the results obtained in the isozyme analyses, conducted in parallel to this trial (Veasey, 1998), confirm the identification of these two sions as belonging to the same species, as the band patterns for both accessions were very similar. Consequently, this confirms the existence of great genetic variability in S. emerus. which corroborates the extensive geographic distribution of this species. This last factor, associated with the morphological plasticity expressed in different areas of its occurrence. also generated a complex taxonomic history for this species (Monteiro, 1984).

The genetic variability observed for S. exasperatu
(Tables V, VI, IX and X) is mostly due to accession No. 6, which differs significantly (P < 0.05) from other accessions of this species for the characters FI, FMP. LL. NLP. IL, NF, PDL, FL, NSP, FDS, SL, CP1, CP2, CP3, PHF and SP (Tables VII, VIIL XI and XII). This accession was classified in isolated groups in cluster analyses with morphological, agronomical and isozyme data (Veasey, 1998).
S. virgata also presented significant differences (P < 0.05) between accessions for characters FI, FMP, LL, LFL, LFW, IL, NF, PW, LDMY2. LDMY4 and SDMY2 (Tables VII, VII and XI). This species belongs to subgenus Daubentonia and is native to the New World (Monteiro, 1984), commonly occurring along river banks and waterlogged areas in various regions of Brazil. The seven accessions evaluated in this study were collected in the States of São Paulo, Minas Gerais, Rio de Janeiro and Mato Grosso do Sul (Table 1), including the Mato Grosso swampland, which indicates the wide geographic distribution of this species in Brazil. Its use as a forage plant is doubtful as it is suspected that it has anti-nutritive and toxic factors. Tsuhako e al. (1989) reported toxic factors in seed of S, virgata. The use of this species to recuperate poor soils and for erosion control was evaluated by Franco e al. (1990) in soils whose horizons A and B had been removed, obtaining better results with the species Mimosa caesalpiniaefolia and Acacia auriculiformis than with Sesbania virgata and Gliricidia sepium
As for the crude protein percentage, with the exception of the annuals S. rostrata and S. retraptera, high
values were observed at the first cut, above 22%. for all accessions (Table XII). However, there was a sharp drop after the second cut, except for S. virgata, which maintained high values up to the third cut. Accession No. 9 of S. sesban. which stood out in terms of LDMY and SDMY, presented a higher value at the first cut but showed a strong reduction following the second cut, with values of 28, 13, 17 and 13% from the first to the fourth cut, respectively, and a mean value of 18% of CP.
A small number of accessions per species was evaluated in this study, especially S. emerus with only two accessions, which is far from an ideal number to represent a species. However, even with such small accession num- bers. high levels of genetic variability were observed.
Tables XIII and XIV show the amplitude of varia- tion for each character evaluated for morphological data compared with data observed by Monteiro (1984) for S. emerus, S. exasperata. S. sesban, S. virgata and S. grandi- flora and by Gutteridge ef al. (1995) for S. sesban. Some observations come close to data obtained by Monteiro (1984). such as characters FL and PL for S. emerus, LL, LFW. PDL. PW and SW for S. sesban and PW and SL for S. virgata, but the majority of the observations differ from data obtained by Monteiro (1984). Character LL in S. exasperata, for example, varied from 10.5 to 26.6 cm in this study and from 20 to 40 cm in Monteiro (1984). Therefore, S. exasperata has high variability for LL. Another example would be the character NSP in S. virgata, varying from one to eight seeds in this study and from five to nine in Monteiro (1984). Monteiro (1984) based his taxonomic review for Sesbania species from the New World
on herborized material and some morphometric restrictions always exist in dehydrated specimens. However, a wide geographical range was considered in this review.
Similarly, Gutteridge et al. (1995) observed a variation of 10 to 50 NSP for S. seshan, while in this study it varied from 11 to 46 NSP. Gutteridge er al. (1995) also observed a range in NLP from six to 27, while this study revealed a range of nine to 30 NLP.

As for phenological data, S. sesban presented floral initiation in the months of March and April. varying from 128 to 195 days after sowing (DAS) (Table XIII) The first mature pod for S. sesban accessions appeared after 162 to 378 days. Sedi and Humphreys (1992) established that flowering of S. sesban var. nubica is sensitive to temperature but not to daylength. Wood and Larkens (1987) observed greater variability for FI between accessions of S. sesban, from 75 to 218 days. Karachi es al. (1994) evaluated more precocious accessions flowering from 59 to 97 DAS, while Heering (1994) evaluated accessions flowering from 102 to 153 DAS when 50% of the plants had flowered.
The most precocious of all species was S. emerus, whose accession No. I flowered from 38 to 45 DAS. Flowering for this species occurred in the months of December to April and presented the FMP from February to June. S. virgata presented the latest flowering accessions, with FI varying from 121 to 384 DAS, from February to November, with the FMP occurring from April to December. S. exasperata flowered in the months of February to June, from 119 to 248 DAS. S. retraptera flowered from February to March, from 119 to 149 DAS. S. rostrata initiated
"Characters described in Table
Variation range of morphological characteres of Seshania grandiflora, S. rostrata and S. terraprera obiained in this study (Veasey er al.). compared with data obtained by Monteiro (1984) for S. grandiflora.
'Characters described in Table II. *Days after sowing. 'Data not obtained.
flowering in March, from 128 to 139 DAS, and seeding in April, from 174 to 184 DAS (Table XIV). Earlier flowering was observed by Joshua and Ramani (1993) for S. rostrata, 35 to 40 DAS for both control and M, plants in a mutation induction trial with gamma rays.
Wood and Larkens (1987) evaluated only one ac- cession of S. virgata, that initiated flowering at 115 DAS, of S. exasperata, which flowered at 106 DAS and started seeding at 141 DAS, and of S. tetraptera, which flowered at 111 DAS and initiated seeding at 158 DAS. The au- thors also observed FI at 112 DAS and FMP at 173 DAS for S. rostrata.
S. grandiflora did not flower throughout the ex- perimental period and presented low leaf and stem DM yields when compared with the other perennial species, indicating that it is not suitable for this region. However, before a more definitive conclusion can be drawn, more accessions of species should be tested.
The difference between annual and perennial species can be observed in Figure |, where at the first cut the annual species S. exasperata (accessions Nos. 3 and 4), S. rostrata (No. 8) and S. retraptera (No. 15) were the most productive. The annual S. emerus, however, presented lower forage yields. After the second cut, the superiority of the perennial S. sesban, followed by S. virgata, was observed. as well as the reduction of LDMY of the annual species. The peak of production for the perennial species. was reached at the third cut, made in December 1995, one year after the seedlings were transplanted to the field. The superiority of accessions No. 9 and 14 of §. sesban, both
from subspecies sesban var. sesban, was noted. pri ncipally accession No. 9 (NO 934). At the fourth cut, in April 1996, a sharp fall was observed when compared with the yields at the third cut, clearly showing the weak perenniality of the Sesbania species.
Heering (1995) established that the majority of the S. sesban accessions evaluated attained their maximum production before or at the second cut, with DM yields decreasing rapidly soon afterwards. However, some ac- ions of S. sesban evaluated by Heering er al. (1996b), ied in a separate group in the cluster analysis, main- tained high productivity even after the second cut. Mois- ture is one of the factors limiting production in S. sesban and S. macrantha, according to Karachi and Matata (1997), who concluded that the usefulness of sesbania as a poten- tial source of fodder is limited to wet season growth. Bray et al. (1997) also observed less perenniality for S. seshan and S. grandiflora, as well as for Cajanus cajan, Codario- calyx gyroides and Desmodium discolor. These authors commented on the important role that these species can play when cultivated with more perennial species, as early sources of animal feed in the form of protein banks, dur- ing the period in which the perennial species are develop- ing before they attain their peak of production.
Another comparison between annual and perennial species can be made for seed production which was much greater for the annual species 8. emerus (No. 2), 8. exasperata, S. rostrata and 8. tetraptera than for the perennials (Table XI1). Reis (1984) also observed greater production of seeds, bructs and Howers by the annual Stylosanthes humilis when

compared with the perennial S. guianensis, S. hamata and S. scabra, in agreement with the r and k selection theory (MacArthur and Wilson, 1967), which attributes greater reproductive effort for shorter life-cycle populations of precocious sexual maturity than perennial, late flowering populations subject to more stable environments.
A plant survival evaluation was carried out at the end of the experiment, two years and four months after sowing (Table XII). The annual species did not survive up to that time. In general, plants that did not undergo cutting presented greater persistence, with high variation being observed for S. sesban, from 6.2 to 93.7%.

The authors are grateful to Professor Reinaldo Monteiro from the University of the State of São Paulo, Rio Claro, for identification of Sesbania accessions and reviewing the manu- script; to Dr. Joaguim Carlos Werner and Dr. Darcy A. Beisman, from the Instituto de Zootecnia, Nova Odessa, for technical as- 10 Centro Nacional de Recursos Genéticos e Biotec- nologia (CENARGEN/Embrapa), Brasilia, The Henry Doubleday Research Association, Coventry/UK, Companhia do Vale do Rio Doce, Linhares, and Instituto de Zootecnia, Nova Odessa, for providing the seed; and to Conselho Nacional de Desenvolvimento Cientifico ¢ Tecnológico (CNPq) for finan- cial support. Publication supported by FAPESP.
Sete espécies de Sesbania (Leguminosse): S. emerus, S, exasperata, $ rostrata, S. tetraptera (anuais), 8. grandiflora, 8. sesban ¢ $. virgata (perenes), utilizadas como forrageira para Tuminantes, madeira para lenha e construção, melhoramento do solo ¢ alimentação humana, foram avaliadas neste trabalho, num total de 22 acessos, com o vbjetivo de caracterizar a variabilidade
genética tanto inter como intraespecífica, estimar parimetros genéticos para os caracteres avaliados e avaliar o potencial forrageiro dos acessos. O experimento foi conduzido no Instituto de Zootecnia, em Nova Odessa, SP, em blocos ao acaso com 22 tratamentos e quatro repetições. Foram avaliados 17 caracteres morfológicos, incluindo dois de fenologia, e 17 caracteres agronômicos. Foram estimados os parâmetros coeficiente de diversidade genotípica intraespecífica (b,) e coeficiente de variação genética intraespecifica (CV,,) para as espécies repre- sentadas por mais de um acesso. Diferenças altamente signiti- cativas foram observadas tanto entre como dentro de espécies para a maioria dos caracteres avaliados, mostrando a grande variabilidade genética do material em estudo. S. exasperata apresentou variação intraespecífica para o maior número de caracteres morfológicos, o mesmo sendo observado para S. sesban para caracteres agronômicos. Valores elevados de b, acima de 0,80, foram obtidos para a maioria dos caracteres, indicando possibilidade de seleção de genótipos superiores. Já os valores de CV,, que indicam à magnitude da variabilidade genética existente com relação à média do caráter, variaram de acordo com a espécie e o caráter avaliado. Observaram-se diferenças entre espécies anuais e perenes, com as anuais apresentando maior produção de biomassa no primeiro corte ¢ às perenes à partir do segundo corte, atingindo o pico de produção no terceiro corte. As espécies anuais apresentaram maior produção de sementes, O acesso NO 934 de S. sesban se destacou quanto à produção de biomassa e vigor de rebrota, sendo indicado como promissor para uso como leguminosa forrageira.
Bray, RA. (19%4). Diversity within tropical tree and shrub legumes. tn Foruge Tree Legames in Tropical Agricultare (Gutendge, R.C and Shelton, H M . eds) CAB Intermational, Wallagtord, pp 111-119
RA Paltmer, B. and Ibrahim, TM. (1997), Pertormance of shrub leguinies at four sites 1n Indonesia and Australia, Trop. Grussl. 31 s
Brewbaker, . s Mucklin, B. and Evans, D.0. (1990). The perennial
Morphological and agronomical characterization of Seshania Scop
Seshanias. In: Perennial Sesbania Production and Use (Evans. D.O. and Macklin, B.. eds ) Nitrogen Fixing Tree Association. Waimanalo. p 62
Franco, A.A.. Monteiro, R. and Faria, S.M. (1990) Regional field expe- mence with Seshania species in Latin America. In: Perennial Seshania Species in Agrmforestiy Svystems (Macklin, B. and Evans, D.0. eds.). Nitrogen Fixing Trec Association, Waimanalo, pp. 151-158

Geraldi. 1.0. (1977), Extimagho de parámeiros genéticos de caracteres do pendão em milho (Zea mavs L) e perspectivas de melhoramento Masters thesis. ESALQ/USP. Piracicaba, SP.
Gillett, J.B. (1963) Seshania in Africa (excluding Madagascar) and south- em Arabia Kew Bull 17:
R.C.. Shelton, H.M. and Oram. R.N. (1995). Register 0( Austra- lian herbage plant cultivars. B. Legumes. 24. Sesban (a) Sesbania sesban (L) Merril! (sesban)cv. Mount Cotton. Aust. J. Exp. Agric. 35: 561.
Heering. J.H. (1994). The reproductive biology of three perennial Sesbania species (Leguminosae). Euphytica 74: 143-148.
Heering. J.H. (1995). The effect of cutting height and frequency on the forage. wood and seed production of six Sesbania sesban accessions productivity of Sesbania sesban accessions under irrigated condi- tions. Agroforestry Syst. 30: 341-350.
Heering. J.H.. Nokoe. S. and Mohammed, J. (1996a). The classi of a Sesbania sesban (ssp. sesban) collection. 1. Morphological at- tributes and their taxonomic significance. Trop. Grassl. 30: 206-214,
Heering, J.H., Nokoe, S. and Mohammed, J. (1996b). The classification ofa Sesbania sesban (ssp. sesban) collection. Il. Agronomic atiributes and their relation to biomass estimation. Trop. Grassl. 30: 215-222.
Joshua, D.C. and Ramani, S. (1993). An induced mutant with extended vegetative phase in stem nodulating Sesbania rostrata. J. Agric. Sci. 120, 71-73.
Karachi,M. and Matata, Z. (1997). Effect of age of cutting on the produc- tvity and forage quality of fourteen Sesbania accessions in westem Tanzania. Trop. Grassl 31: 543-548.
Karachi,M., Lema, N., Sabas, E. and Shirima, D. (1994). Growth, bio- mass production and plant mortality of seven Sesbania sesban var. nubica and three Sesbania macrantha accessions at Tumbi, Tanza- nia. For. Ecol. Manage. 64: 153-159.
MacArthur. R.H. and Wilson, E.O. (1967). The Theory of Island Bioge- ography. University Press, Princeton.
Mengistu, S. (1990). Repon of a survey and collection mission for Sesbania in Tanzania. PGRC/E-ILCA Germplasm Newsl. 16: 3-14.
Monteiro, R. (1984). Taxonomic studies on Brazilian legumes with forage potential: Seshania. Lupinus. Doctoral thesis, University of St Andrews,St. Andrews, UK
Ndoye, 1. Tomekpe. K. Dreyfus, B. and Dommergues, Y.R. 117% Seshania and Rhizobium symbiosis nodulation and mitrogen fixa tion. In: Perennial Seshania Species im Agroforesiry Sitems (Macklin. B. and Evans. D.O.. eds.). Nitrogen Fixing Tree Avvxia tion. Wamanalo, pp. 31-38.
Ndungu, J.N., Llewellyn-Smith, R. and Boland, D.J. (1994 Collectine Seshania spp. germplasm in the vicinity of Nairobi. Kenya For Gener. Resour 22 43-48
Reis, M.S. (1984). Autoccologia de diferentes espécies de Stvlosanthes Sw Andlise da alocação de energia e estudos da hiologia da semente Doctoral thesis. ESALQ/USP. Piracicaba. SP
Rocha, G.L.R., Leitão Filho, H. de F.. Andrade. J.B.. Shepherd. G.J.. Semir, J.. Gouvéa, L.S.K., Taroda, N., Gibbs, P.E., Tamashiro. J., Monteiro, R., Alcantara, P.B.. Bufarah. G Alcintara, V.B.G., Almeida,J.E... Salgado,P.R... Pulz, ... Sigrist. J.M.M., Fonseca, T.C. and Paulino, V.T. (1979) Coleta identificagdo ¢ distribuigdo de leguminosas forrageiras tropicais brasileiras - Brasil Central - Fase |. Bol. Ind. Amim. 16 255.324
SAS Institute Inc. (1993). SAS/STAT User's Guide for Personal Compul ers. Version6. 3rd edn. Cary. NC.
Sedi, Y. and Humphreys, L.R. (1992). Temperature. daylength and the Nlow. ering and seed production of Sesbania sesban and S. cannabina Trop. Grassl. 26: 100-110.
os nas
Tsuhako, M.H., Santos, M., Haraguchi, M., Nobre, D., Leme, M. and Macruz, R. (1989). Presenca de principios ativos tóx sementes da leguminosa Sesbania virgata. Arq. Inst. Biol. 56 27-29.
Veasey, E.A. (1998). Variabilidade genética em acessos de espécies de Sesbania Scop. (Leguminosae). caracterização morfologica. agrond- mica e isoenzimtica. Doctoral thesis. ESALQ/USP.
Veasey, E-A., Guisi, O.M.A.A., Valarini, M.J., Otsuk, L. Cardelli, M.A... Sanchez, M.J.F. and Beisman, D.A. (1997). Early growth and nutive nodulation of leguminous shrub and tree species in Brazil. Trop. Grassl. 31: 40-48.
Vencovsky, R. and Barriga, P. (1992). Genética Biométrica no FitomeIhoramento. Sociedade Brasileira de Genética, Ribeirdo Preto
Weir, B.S. (1996). Genetic Data Analysis. Methods for Discrete Populu- tion Genetic Data. 2nd edn. Sinaver Associates Inc. Publishers. Sunderland
Wood, LM. and Larkens, A.G. (1987). Agronomic and phenological data for a collection of Sesbania species grown in Southeast Queensland, Australia. CSIRO. Brisbane, pp. 13 (Genetic Resources Communication 11).
(Received January 22, 1998)