C H E M I S T R


C H E M I S T R

This collection highlights breakthroughs from the UNC Chemistry Department that are transforming our understanding of human health. From developing new tools for drug discovery to advancing molecular approaches in diagnostics and treatment, Tar Heels are continuing to make an impact at the intersection of chemistry and medicine. Whether you are currently walking the halls of Kenan Labs or continuing to follow UNC Chemistry research from afar, we invite you to explore how today’s Carolina chemists are building on UNC’s legacy of innovation.
Contributing



03 LIGHT-POWERED SYNTHESIS OF MEDICINAL PLANT COMPOUNDS
TO PERFECT A LIGHTEMITTING PROTEIN 05
INNOVATIVE NITRIC OXIDE THERAPY FOR SKIN CANCER 07

BEYOND CAROLINA: FOUNDATIONS FOR SUCCESS
FROM UNC TO EASTMAN’S R&D LEADER: INTERVIEW WITH CHRIS KILLIAN 09
CREATIVE CHEMISTRY SHAPING DNADECODING RESEARCH: KATHERINE ALBANESE 13

11
TAYLIZ RODRIGUEZ: REFLECTING ON LEADERSHIP AND LASTING IMPACT
LOWRY CAUDILL: ON CAROLINA CHEMISTRY’S POWER TO CHANGE LIVES 15
THE HIDDEN AFTERLIFE OF ANTIDEPRESSANTS 19
STUDENT AIMS TO TRANSFORM DIALYSIS TREATMENT 21
PROTEIN STABILITY IN CELLS IS A BALANCE 23 FROM CRUDE TO CURE: RESHAPING CHEMICALS 25
by Dave DeFusco
A new light-driven method developed by two UNC chemists dramatically simplifies and accelerates the synthesis of stemoamide alkaloids a class of bioactive compounds found in plants demonstrating how photochemistry can streamline the production of complex natural medicines, opening new possibilities for drug discovery and development.
Dr. Nicholas Akkawi, who holds a Ph.D. in organic chemistry, and Dr. David Nicewicz, William R. Kenan, Jr. Distinguished Professor of Chemistry, discuss the findings of their research in the study, “Photochemically Enabled Total Syntheses of Stemoamide Alkaloids,” in the Journal of the American Chemical Society.
“We wanted to show that photochemical reactions could do more than just one step here and there,” said Akkawi, lead author of the study “We designed an entire strategy that relies on light to build these intricate molecules from the ground up, in fewer steps and under much milder conditions than ever before.”
Stemoamide alkaloids are naturally occurring compounds found in the Stemona family of plants, which have been used in traditional medicine for centuries Known for their cough-suppressing antitussive and insecticidal properties, these compounds are challenging to produce in the lab due to their unusual and compact ring structures three of them, to be exact, tightly fused together like interlocked puzzle pieces.

A photoreactor used in the Nicewicz Lab to catalyze photochemical reactions in visible light Nicewicz has been on the forefront of developing novel photoreactors to advance his work, even creating a company to design and manufacture a 16-well reactor that reached market in 2018

Dr David Nicewicz, William R. Kenan, Jr. Distinguished Professor of Chemistry

Dr Nicholas Akkawi, who holds a Ph D in organic chemistry, is the lead author of the recently published study.
Previously, chemists needed anywhere from 12 to 22 separate steps to assemble these compounds. Akkawi and Nicewicz’s method trimmed that process to just a handful of well-orchestrated photochemical steps, the shortest known syntheses of these compounds to date.
“This is not just about making one molecule faster,” said Nicewicz, senior author of the study “It’s about opening a door to how we think about building complex molecules with precision and efficiency Light is the key ”
At the heart of their approach is a type of reaction called photoredox catalysis. This process uses light to excite a special catalyst in this case, an acridinium dye into an energetic state where it can either donate or accept electrons. That power is used to generate radicals, highly reactive molecules that can build chemical bonds in ways traditional methods cannot.
Akkawi and Nicewicz use these radicals in an elegant reaction called a polar radical crossover cycloaddition (PRCC), which helped them form the tetrahydrofuran ring, a key building block of the stemoamide core. They further manipulated the resulting structures using more light-driven reactions to form a unique oxaspirocyclic butanolide, a rare and complex feature found in these natural products.
“These radical reactions let us jump over roadblocks that usually slow down synthesis,” said Akkawi “Instead of carefully adding piece after piece, we can form multiple bonds in one go, often under room temperature and with simple reagents ”
One challenge the team faced was that pyrroles, a nitrogen-containing structure common in stemoamides, didn’t behave well in early stages of the reaction. As a result, they used a clever workaround building a pyridine version of the molecule that was more reactive under light conditions. Later, they chemically reshaped it back into a pyrrole, much like putting on a disguise for part of the journey and then taking it off.
“This was one of our more creative moves,” said Akkawi “We realized we couldn’t get the molecule we wanted if we started with the real thing So we made a ‘fake’ version that played nice in the reaction and only later transformed it into the real thing.”
The beauty of this work lies not only in the chemistry but in what it suggests for the future. By showing that multiple light-driven reactions can be strung together as the main strategy, not just as side tricks, Nicewicz’s lab is paving the way for faster, cheaper and greener ways to produce complex molecules, including drugs
This approach could be especially useful for creating rare natural products in the lab, particularly those with medicinal value but hard-to-source plant origins. It could also inspire new designs in drug discovery, agriculture and materials science.

Previously, chemists needed anywhere from 12 to 22 separate steps to assemble Stemoamide alkaloid compounds. A method developed by Nicholas Akkawi and David Nicewicz trimmed that process to just a handful of well-orchestrated photochemical steps, the shortest known syntheses of these compounds to date
“This is a paradigm shift,” said Nicewicz “Chemists often use photochemistry for a cool transformation here or there But what Nick has shown is that we can think in a fully photochemical mindset That means less waste, fewer toxic reagents and more sustainable processes.”
by Dave DeFusco

UNC-Chapel Hill researchers are pushing the boundaries of bio-imaging by leveraging the power of computational chemistry. Utilizing advanced computer simulations, the Pieri Lab in the Chemistry Department is making significant strides in designing better biosensors and understanding new light-based reactions.
Bio-imaging allows scientists to see inside live tissues using special molecules that react to light. Despite having many such molecules available, finding the perfect one for each specific situation is challenging This is where computational chemistry, which uses computer simulations to predict and design these molecules, comes into play.
The Pieri Lab’s primary focus is on photoactive biliproteins, which are proteins that emit light. By studying how changes in their structure affect their properties, they aim to find new systems that could work well for bioimaging and optogenetics using light to control cells.
“Understanding and designing these lightsensitive molecules is complex. Photoreactions involve multiple electronic states and happen very quickly, using a lot of energy,” said Pieri, an assistant professor of chemistry at UNCChapel Hill. “This makes them challenging to simulate accurately. However, with new techniques and better computers, we can now explore these reactions more systematically.”
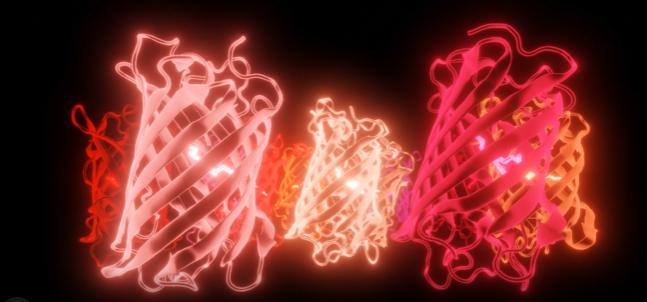
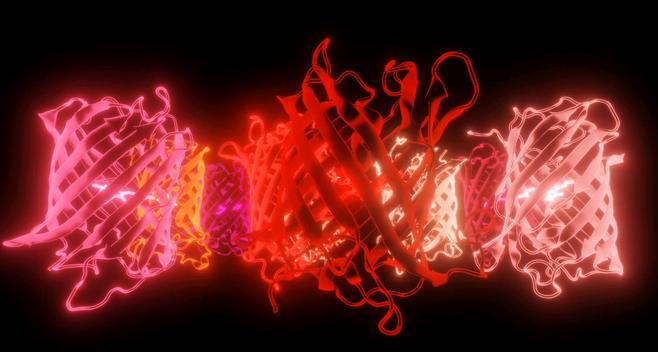
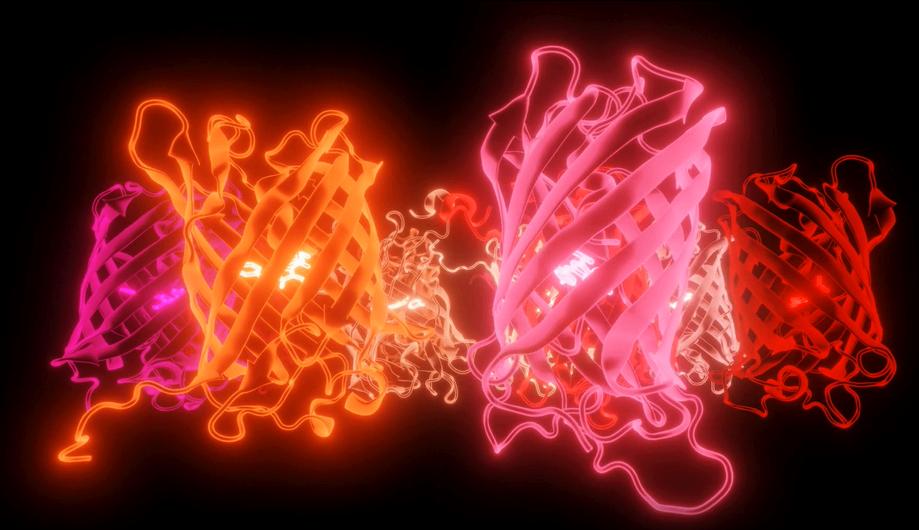
The Pieri lab believes that art and science work best together These images are stills from a video tribute to the red fluorescent proteins that span the whole red part of the spectrum Created by Dr Elisa Pieri
A study published in the Journal of the American Chemical Society highlights Pieri’s success in understanding the structural intricacies that dictate the brightness of red fluorescent proteins (RFPs), essential tools for bio-imaging. The study, “Conical Intersection Accessibility Dictates Brightness in Red Fluorescent Proteins,” conducted while Pieri was a postdoctoral researcher at Stanford University, focused on two RFPs, mScarlet and mRouge, which have similar sequences but different brightness levels
Using advanced computational simulations, the researchers identified key factors contributing to these differences. They found that mScarlet’s superior brightness is due to its more rigid structure, keeping the chromophore the part of the molecule responsible for light absorption and emission in a flat, strongly light-emitting configuration. In contrast, mRouge’s flexible structure allows the chromophore to twist, leading to energy dissipation and reduced fluorescence
The study traced differences in protein behavior to specific changes in the area around the chromophore, caused by mutations affecting the electric charge and spatial environment. In mScarlet, strong and numerous hydrogen bonds keep the chromophore flat and light-emitting. In mRouge, a mutation creates more space around the chromophore, allowing it to twist and preventing light emission.
“These insights will enable us to design better fluorescent proteins,” said Pieri “By understanding the structural factors that govern fluorescence, we can develop more efficient bioimaging tools.”
The potential applications of this research are vast. Improved fluorescent proteins could enhance the capabilities of bio-imaging technologies, allowing scientists to observe cellular and molecular processes in real-time with greater precision and brightness This can lead to advancements in various fields, from medical diagnostics to biological research.
“As we continue to explore new techniques and applications, our work promises to expand the possibilities for innovation in materials design and bio-imaging,” said Pieri, “offering a versatile tool for creating advanced materials suited to specific industrial and scientific needs ”
by Dave DeFusco
Melanoma, a highly aggressive form of skin cancer, has long posed significant challenges in both treatment and patient outcomes. Its increasing incidence and notorious resistance to conventional therapies highlight the need for innovative solutions. Traditional treatments like surgery, immunotherapy and chemotherapy have shown limited success, often accompanied by severe side effects and, in many cases, acquired resistance.
However, in a promising new study, “Nitric Oxide-Releasing Topical Treatments for Cutaneous Melanoma,” published in the journal, Molecular Pharmaceutics, a team of researchers led by Dr Mark Schoenfisch, Peter A Ornstein Distinguished Professor at UNC-Chapel Hill, explores the potential of nitric oxide as a topical treatment for melanoma, offering hope for a more effective, less toxic alternative
Nitric oxide, a naturally occurring gasotransmitter, has been gaining attention in cancer research for its dual role in regulating tumor biology. At low concentrations,
nitric oxide can promote tumor growth but at higher levels, it exhibits powerful anticancer effects. Nitric oxide triggers apoptosis, or programmed cell death, enhances oxidative stress, inhibits metastasis and even sensitizes tumors to chemotherapy and immunotherapy These tumor-suppressing effects make nitric oxide an attractive candidate for treating melanoma, which is notorious for its resistance to many conventional cancer therapies.

The process of apoptosis, which can be blocked in cancerous cells While in the process of apoptosis, a normal cell shrinks, causing chromatin to condense The membrane of the cell then starts blebbing, or developing blister-like bulges of the membrane while the nucleus and organelles collapse Finally the cell breaks into apoptotic bodies Normal Cell Shrinking Cell Blebbing Apoptotic Bodies
release it in a controlled manner. However, the efficacy of these nitric oxide donors has remained underexplored, particularly in the context of melanoma. This is where the new research originates, aiming to optimize the nitric oxide release for targeted melanoma treatment.
The key challenge with nitric oxide as a therapeutic agent is its reactivity and short half-life. To harness its full potential, scientists have turned to chemical nitric oxide donors compounds that store nitric oxide and Dr. Schoenfisch and his collaborators investigate the use of macromolecular nitric oxide donors for the treatment of cutaneous melanoma. The study evaluated three different nitric oxide donor systems cyclodextrin, mesoporous silica nanoparticles and hyaluronic acid each with tunable nitric oxide-release kinetics.
By analyzing their cytotoxicity against melanoma and healthy skin cells, as well as their ability to permeate the skin, the team sought to identify the most effective donor for topical melanoma therapy.
“Our approach focused on optimizing the delivery of nitric oxide directly to the tumor site, bypassing the need for systemic administration,” said Dr. Schoenfisch. “This topical approach could potentially reduce the adverse side effects commonly associated with traditional therapies and increase the concentration of the drug at the site of the tumor.”

The researchers found that the cytotoxicity of nitric oxide-releasing systems was primarily dependent on the nitric oxide payload and not the donor identity or the release kinetics. For example, the three nitric oxide donor systems cyclodextrin, mesoporous silica nanoparticles and hyaluronic acid differed in their release profiles, but their anticancer activity was largely determined by the amount of nitric oxide they could deliver.
Representative images of tumors in each treatment group at days 3, 5, and 7 post-tumor cell inoculation
Among these systems, cyclodextrin-based nitric oxide donors exhibited the largest therapeutic indices, meaning they were more toxic to cancer cells versus the healthy cells. hyaluronic acid-based nitric oxide donors also showed favorable results, with attractive therapeutic indices, while mesoporous silica nanoparticles had lower therapeutic indices. This suggests that biopolymerbased nitric oxide donors, such as cyclodextrin and hyaluronic acid, are more effective for melanoma treatment as they combine high nitric oxide payloads with better biocompatibility.
A significant challenge in developing topical treatments is ensuring that the therapeutic agents can penetrate the skin’s protective barrier The study employed Franz diffusion cells to assess the skin permeation of the nitric oxide-releasing materials Cyclodextrin-based nitric oxide donors, due to their small size and favorable charge characteristics, demonstrated the highest skin permeation The researchers also found that smaller, more neutral donors were more successful at permeating the skin, a critical factor for the success of topical treatments.
The team used a Pluronic F127 organogel formulation to optimize the delivery of the cyclodextrin-based nitric oxide donor to tumors below the skin. This formulation not only improved the skin permeation of the nitric oxide donor but also showed promising results in reducing tumor growth in an in vivo model.
The final step in the study was to evaluate the in vivo efficacy of the most promising nitric oxide donor formulation. The results showed that the cyclodextrin-based nitric oxide donor, delivered through the optimized organogel formulation, significantly reduced tumor growth in a melanoma mouse model. This promising outcome suggests that the topical delivery of nitric oxide could be a viable therapeutic strategy for addressing cutaneous melanoma
“The controlled delivery of nitric oxide through macromolecular donors offers a novel approach to overcoming the limitations of traditional melanoma therapies,” said Dr. Schoenfisch. “By reducing the risk of systemic toxicity and potentially overcoming drug resistance, nitric oxide-releasing topical treatments could revolutionize melanoma care, providing patients with a safer, more effective option for managing this aggressive cancer.”
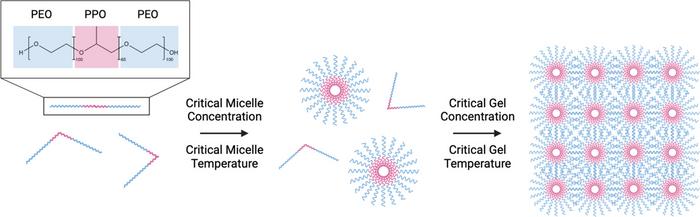
Pluronic F127 consists of poly(ethylene oxide)-poly(propylene oxide)poly(ethylene oxide) (PEO–PPO-PEO) units In aqueous solutions, micelles are formed Upon surpassing a critical gel concentration or temperature, gelation occurs due to strong packing of the micellar structures
A multisport athlete growing up, Chris Killian, who holds a Ph.D. in chemistry from UNC-Chapel Hill, credits football, baseball and track with instilling in him a deep respect for teamwork and interdependence. He’s carried that ethos into the executive suite at Eastman, where he champions collaboration over command, development over directives.

Chris Killian’s story isn’t about a linear climb to the top of a Fortune 500 company It’s about staying grounded while rising through the ranks from research chemist to Eastman’s C-Suite It’s about a scientist and R&D Group Leader who bet his early career on a small team of scientists and a nascent idea to develop a new to the world polymer and stayed with it long past when conventional wisdom might’ve urged a career pivot. It’s about choosing authenticity over authority, vulnerability over bravado. And it’s about the quiet and confident clarity that comes from overcoming a serious illness and getting a second chance at living a full life.
“I am who I am,” said Killian, who holds a Ph D in chemistry from UNC-Chapel Hill and is senior vice president and chief technology and sustainability officer at Eastman chemical company “Whether I’m talking to the CEO or someone on the shop floor.”
At 48, after decades of managing a slow, silent illness, Killian received a life-saving kidney transplant from a statistically improbable match: his wife, whose kidney turned out to be as compatible as a sibling’s The gift didn’t just save his life it reshaped his understanding of what it means to lead
by Dave DeFusco
Killian’s pivotal moment came early, between 2001 and 2007, when he embraced a quiet rebellion disguised as a research project. A brilliant chemist in another group was pursuing a novel high-temperature polymer that didn’t fit his group’s mission. That chemist was reassigned to Killian’s team and with him came the bold idea of what would become Eastman’s Tritan™ copolyester, a sustainable plastic and now a flagship product.
“It wasn’t branded yet. It was an early-stage exploratory research project,” said Killian. “But we nurtured it in my lab and eventually brought it into the business ”
That long-odds project eventually became the fastestgrowing polymer launch in 50 years, generating hundreds of millions of dollars annually But it didn’t just make money it shaped Killian as an innovation leader He stayed with the project from lab to successful commercial launch, transitioning with it from technical incubation to commercial integration.
“Being a part of bringing something new to the world
in partnership with hundreds of Eastman men and women is one of my proudest moments,” he said. “There were times I wondered if I was moving fast enough in my career, but I was doing something I loved. I stuck with it. And it taught me everything business, marketing, leadership and the conditions required for innovation success ”
This wasn’t just a project. It was his crucible. Today, Killian leads a division of 1,500, but he’s quick to note that leadership isn’t about hierarchy or spotlight
“If you focus too much on self-promotion, you lose the ability to harness the power of the organization,” he said “No matter how talented you are, you’re not more talented than the hundred people I was leading in 2007 or the 1,500 I’m leading now.”
Killian said his career has been greatly influenced by two former UNC chemistry professors, Maurice Brookhart and Joseph DeSimone. In Brookhart’s lab, Killian researched nickel and palladium catalysts for olefin polymerization, which attracted industrial sponsorship and collaboration and taught him the importance of combining fundamental science and practical application
He said Professor Brookhart had an “indelible impact” on him by demonstrating the importance of collaboration and collegiality, and Professor DeSimone taught him to believe in himself, while sparking his interest in business and entrepreneurship.
“I would not have accomplished the things I have without UNC Chemistry and these two men,” said Killian “These critical influences have shaped my career and the way I lead Eastman R&D.”


A multisport athlete growing up, Killian credits football, baseball and track with instilling in him a deep respect for teamwork and interdependence He’s carried that ethos into the executive suite, where he champions collaboration over command, development over directives.
“The job is to motivate, to unlock what others can do,” he said “You do that by focusing on them their goals, their
growth, how they can have the greatest impact on the company not your own résumé or recognition.”
In a corporate world that often rewards polished facades and confident certainty, Killian leads with something more disarming: honesty. “Vulnerability is being real. It’s saying, ‘I made a mistake.’ It’s asking for help. It’s being human,” he said. “When I mess up with my kids, I own it. That carries over into work ”
Killian doesn’t just model vulnerability, he expects it. And he believes it’s more necessary than ever in a complex, highpressure corporate environment “When you’re vulnerable, people trust you more,” he said “And when they trust you, they follow you not out of obligation, but because they believe in the journey.”
Killian’s journey took an irreversible turn in 2018, when he received his kidney transplant He had known for years it would be necessary. A bout of strep throat at 16 had triggered an autoimmune condition IgA nephropathy that slowly destroyed his kidneys. But the timing was unknowable. “Some people need a transplant five years in Others last 45 I fell somewhere in between ”
For years, he compartmentalized the disease, but when the moment finally came, and his wife turned out to be a one-in100,000 match, the experience recalibrated his entire outlook
“You wouldn’t be talking to me today without God’s grace and her love,” he said “It made me grateful in ways I can’t describe It made me see what really matters ”
The experience amplified his vulnerability and deepened his authenticity “I used to resist acknowledging my weaknesses I didn’t want to ask for help,” he said “But going through something like that, you realize how much strength there is in being human.”
With nearly three decades at Eastman, Killian’s still learning what it takes to build resilient, high-performing teams across both science and business. But one principle guides him: hire for talent, not just expertise. Skills can be learned. But raw capability, drive, curiosity that’s what you can build a company on He emphasizes intentional investment in people
“If people feel like they’re growing, like someone cares enough to invest in them they’ll stay,” he said. “People most often leave leaders not companies ”
And what of the scientists and engineers working under him today? “I want them to be better than me,” he said. “I want them to surpass me That’s the legacy ”

by Emily Sherman

When Tayliz Rodriguez arrived at UNC Chemistry, she wanted it all: not just a doctorate, but a supportive community to grow with her, the freedom to pursue her passions, and the skillset to tackle anything that came her way. From investigating untreatable diseases and sustainable energy storage to creating programs for a more connected Carolina, Rodriguez crafted a dynamic Ph.D. experience that enabled her to thrive in the halls of Kenan and in BASF’s prestigious Leadership Development Program.
Rodriguez, a UNC Chemistry Ph D alumna, always loved a challenge Drawn to the complexity of life at the atomic level, the Puerto Rico native arrived in Chapel Hill for a coveted NSF Research Experience for Undergraduates. As a member of the Waters Lab, Rodriguez and graduate mentor Lauren St Louis developed a method to control the three-dimensional shape of peptide therapeutics proteins that could cure currently untreatable diseases like cancer and Alzheimer’s.
“My favorite memory from that summer was synthesizing a peptide for the first time not on an automated synthesizer, but by hand,” said Rodriguez. “The process of linking together each
amino acid in the peptide chain piece by piece took an entire week and the help of all the Waters Lab graduate students By the end, I had a much deeper understanding of peptide chemistry and had made something that could cure diseases. The Waters Lab community valued and encouraged my learning and gave me a picture of what research at UNC Chemistry could and would be.”

From incurable disease treatments and sustainable energy storage devices to novel materials design, Rodriguez’s impact extends beyond her UNC Chemistry and BASF communities.
After earning her B.S. in Chemistry from Florida International University, that vision catalyzed Rodriguez’s decision to return to Chapel Hill for graduate school, where she lost no time in jumping into the Chemistry community in and out of lab While co-leading Allies for Minorities and Women in Science (AM-WISE) and representing fellow graduate students on the Chemistry Diversity and Inclusion Committee, Rodriguez saw an opportunity to connect incoming graduate students with their peers. The Graduate Achievement through Mentoring (GrAM) program, now in its 3 year, partners current and new graduate students to answer questions, provide support, and ease the transition into graduate school. rd
“I think it’s critical for students to have support on all fronts from peers, mentors, and our department,” said Rodriguez. “That enables us to implement initiatives that advocate for the Chemistry community as a whole.”
As co-chair of the Graduate Committee for Professional Development, Rodriguez also helped establish Industry InSight, a two-day long celebration of science that connects hundreds of
UNC students with industry representatives to share ideas and build professional networks.
“Initiative and connectivity are must-haves for strong leadership,” said Rodriguez. “Leaders don’t just have a vision: they take initiative to bring it to life and work with a diverse team to find out how. By choosing to get involved with these committees, I was able to build confidence in taking charge and learn effective interpersonal skills which have been crucial to my professional impact at BASF.”
After spending her graduate career investigating charge transfer excited state chemistry for nextgeneration energy storage in the Dempsey Lab, the NSF Graduate Research Fellow earned a spot in the competitive BASF Leadership Development Program (LDP), which took her far from her field of expertise and her comfort zone From scouting sustainable energy projects at the University of California at Berkley, to synthesizing new materials to transport biocatalysts in New York, Rodriguez took on some of today’s biggest scientific challenges. Now, she has returned to North Carolina and entered yet another new field at BASF: designing chemical additives that go into everyday materials like paints, adhesives, construction materials.
“At its core, a PhD is bigger than just one area of expertise,” said Rodriguez “It enables you to quickly grasp new ideas and contribute to new projects Throughout BASF’s LDP, I encountered problems in fields I had never studied, but the skills I earned during my PhD gave me – and my employer – confidence that I was up for the challenge.”
Reflecting back on her experience, Rodriguez characterized her Ph.D. experience at UNC as “dynamic.”
“I found numerous opportunities to grow as a scientist and leader all while being supported by my peers and mentors like Marcey Waters and Jillian Dempsey. I found that taking advantage of opportunities like these is crucial to developing as a leader, no matter what career you pursue ”
By Dave Defusco

In her lab at Wake Forest University, Assistant Professor Katherine Albanese is reimagining proteins as tools for probing life at the molecular level decoding how subtle chemical tags on DNA-packaging proteins affect gene expression and, ultimately, health.
It’s a mission shaped as much by her academic journey as by her scientific imagination Albanese’s lab at Wake Forest is a reflection of the interdisciplinary rigor, collaborative spirit and experimental freedom she experienced during her Ph.D. at the University of North Carolina at Chapel Hill, where she trained in the lab of Dr. Marcey Waters, Glen H. Elder, Jr. Distinguished Professor of Chemistry.
When Dr Katherine Albanese was a member of the Waters Lab at UNC, one of her main projects was engineering proteins that can detect a specific chemical tag on other proteins called methylated lysine This helped set the stage for how her lab now designs new proteins to study how genes are turned on or off
“I’m extremely grateful for the latitude Marcey gave us to explore our own ideas,” said Albanese “We planned our projects from start to end, and when things didn’t work, we came up with new hypotheses for why That ability to shape and reshape a problem has stuck with me.”
Albanese’s research today focuses on epigenetics how gene activity is controlled by reversible chemical tags on histone proteins. These posttranslational modifications, or PTMs, can turn genes on or off without changing the underlying DNA The proteins that “read” these modifications are critical for maintaining healthy cell function. Misreading them, as occurs in many cancers, can lead to disease.
Understanding these readers, however, is no small feat. Histones are notoriously disordered proteins, meaning they don’t hold a rigid structure a feature that challenges conventional protein design tools, which rely on welldefined shapes like helices and sheets
“The hardest part is designing something to bind to a protein that doesn’t have structure,” she said “We’re tweaking current computational pipelines to accept sequence inputs instead of structure It’s a very different problem ”
This kind of challenge engineering “reader proteins” that can selectively recognize these shapeshifting signals grew out of her work in the Waters Lab, where she investigated how proteins interact with methylated lysine, a common histone modification.
When Albanese joined Waters’ group at UNC in 2016, the lab was shifting from studying small molecule receptors to investigating full proteins with biological relevance It was a pivotal time, and Albanese, along with a small cohort of graduate students, helped shape the lab’s new research focus
“Katherine was fearless in blazing new directions in my lab,” said Waters “My research program wouldn’t be where it is today without her leading us all in a new direction. I’m excited to see where she takes her own research program. I definitely feel like a proud parent!”
Albanese said they spent a lot of time bouncing ideas around and exploring possibilities together. “Marcey was always encouraging. Even if I had a wild idea, she never said, ‘No, that’s bad ’ It was always, ‘Let’s talk through it ’”
One of her main projects was engineering proteins that can detect a specific chemical tag on other proteins called methylated lysine This helped set the stage for how her lab now designs new proteins to study how genes are turned on or off. The experience also introduced her to high-level interdisciplinary collaborations with leaders like Ken Houk and Brian Kuhlman that continue to shape her philosophy today
“Science is better when you bring more people to the table,” she said “That’s something I carry into how I run my lab now I’m always looking for new collaborators at Wake or in the surrounding area ”
During her postdoctoral fellowship at the University of Bristol in the UK with Professor Dek Woolfson, Albanese immersed herself in computational protein design, helping develop α-helical barrels, which are cylindrical protein
structures built with precision While her current work doesn’t use these barrels directly, the philosophy of “rational seeding” lives on.
“Rather than letting AI spit out a protein for us, we use our intuition what we know about molecular recognition and build from that,” she said
In her own lab, this looks like identifying a key feature, such as an aromatic cage known to recognize methylated lysine, and using it as a starting point to design new proteins from scratch. These proteins become experimental tools for uncovering how histone modifications work, and how mutations may disrupt their function.

Albanese said she is grateful for the latitude that Dr Marcey Waters, Glen H Elder, Jr Distinguished Professor of Chemistry, gave her to explore her own ideas.
Now, as a faculty member at her alma mater, Albanese teaches courses in biochemistry and protein design that mirror the creativity and structure of her own scientific path. In one graduate seminar on protein design, students analyze scientific literature, build proteins computationally, and propose their own experimental projects.
“The goal is for students to understand how protein sequence relates to structure and function,” she said. “That way, when they build something from scratch, they really appreciate the nuances ”
In her undergraduate biochemistry lab, students study enzymes like beta-galactosidase the same enzyme found in lactose pills “I love when students realize, ‘Oh, this thing I bought at the pharmacy? I now understand how it works.’ That kind of moment makes biochemistry come alive.”
Her teaching style open-door and supportive, but handsoff when needed draws directly from her time at UNC. “I try to be hands-off for students who want to explore, and very present for students who want direct input That balance was something I really valued in Marcey’s lab ”
Though she now leads her own lab just an hour away from Chapel Hill, she still stops by when she can “I’m grateful to still have Marcey nearby,” she said “It feels like coming full circle by bringing that spirit of exploration forward.”
by Dave DeFusco
Walk through UNC’s modern chemistry complex and you’ll see Lowry Caudill’s influence everywhere on the facilities, the fundraising campaigns, the student experiences and, perhaps most enduringly, the values that guide the department’s mission
A 1979 graduate with a bachelor’s degree in chemistry, Caudill has returned to the university again and again, not just in spirit but in substance: as Chair of the Board of Trustees, Adjunct Professor of Chemistry, Chair of the Chancellor’s Innovation Circle, and one of the most influential champions of Carolina’s scientific infrastructure and entrepreneurial vision.
Over a lifetime that has bridged science, business, education and philanthropy, Caudill has built a legacy defined by his belief in chemistry’s power to change lives, and his relentless work to ensure Carolina remains at the forefront of that mission
Caudill’s path began in the classrooms and labs of UNC, where mentors inspired his love for science and encouraged him to pursue graduate study at Indiana University There, under the guidance of faculty who traced their own academic lineage to Carolina his advisor Mark Wightman earned his Ph.D. under UNC legend Royce Murray Caudill sharpened the analytical skills that would define his early career

Caudill’s deep commitment to the UNC Department of Chemistry has taken many forms from mentoring faculty and students to helping raise millions of dollars in private and public support for research facilities
After earning his Ph.D., Caudill entered the pharmaceutical industry, drawn by the potential to apply science to real-world human needs. “In the life sciences,” he said, “you’re developing drugs that can eradicate or manage diseases helping people you’ll never meet, but whose lives are improved by the work you do.”
That work soon evolved into something more ambitious. In 1991, alongside Alfred Childers, Caudill co-founded Magellan Laboratories, a contract pharmaceutical development company built around a novel idea: that scientists deserved an environment where they could thrive By the time they sold the company, it employed hundreds many with advanced degrees and had contributed to numerous life-saving drugs now found in pharmacies across the country.

“I still smile when I walk into a drugstore,” said Caudill. “There are drugs on those shelves we helped develop. Even if our piece was small, it meant something.”
When asked what today’s chemistry Ph.D. students and postdocs should focus on, Caudill’s answer is clear: “Learn your science Get really good at what you do Become an expert ”
But he also urges students not to stop there In an age when AI and data science are transforming every industry, including pharmaceuticals, Caudill believes that chemists must be alert to broader trends and opportunities. He advises students to “pay attention,” and to seek out training in entrepreneurship, communication and business management, even if their ultimate path stays rooted in research
“The reality is,” he said, “many professors already are entrepreneurs They run labs, write grant proposals, manage teams and produce results. We just needed to reframe that mindset.”
At UNC, he helped do exactly that by co-creating and teaching courses in the university’s Entrepreneurship Minor, a program he now supports and funds. He also urges students to connect with industry while still in academia through the alumni network, departmental initiatives or simply by reaching out.
“You’d be surprised how many people are willing to talk to students. They remember what it was like to be starting out.”
Caudill’s deep commitment to the UNC Department of Chemistry has taken many forms from mentoring faculty and students to helping raise millions of dollars in private and public support for research facilities He was a pivotal figure in the successful campaign to build UNC’s Physical Sciences Complex, a state-of-the-art research facility, including a building that bears his name, made possible through a $3 billion statewide bond referendum, university funds and private philanthropy The Physical Science Complex received the largest funding $90 million from the bond referendum. He and Holden Thorp, former chancellor of UNC-Chapel Hill, raised approximately $25 million.
“You hear that people don’t give to bricks and mortar,” said Caudill “But we helped people understand that if you want world-class research, you need top professors, students, funding and facilities. That made it a compelling case.”
He’s proud of what’s been accomplished: “We now have buildings that rival any in the country. There’s no reason why UNC Chemistry can’t continue to be the top department in the nation ”
Looking ahead, Caudill sees opportunities for growth in emerging research areas such as artificial intelligence, clean energy and translational science.
“There’s so much happening in battery chemistry, solar energy and drug development,” he said. “When I started out, no one wanted to work on batteries. Now it’s one of the most exciting places to be, and chemistry is at the center of all of it.”
In an era when public skepticism toward science is growing, Caudill believes that institutions like UNC play an essential role in reinforcing the value and integrity of research
“Chemistry is foundational to so much medicine, materials, energy, the environment. We have to do a better job communicating that, especially to people outside the scientific community”
He sees this as a shared responsibility: not just for faculty and administrators, but also for students, alumni and supporters who understand science’s real-world impact. Philanthropy, he said, is one way of demonstrating that belief tangibly by investing in the tools and talent that make discovery possible
When reflecting on his greatest accomplishments, Caudill doesn’t dwell on awards, though they’re many from UNC’s Distinguished Alumnus Award to national entrepreneurial honors. Instead, he focuses on the impact of innovation.
“I’m proud of helping create a company where scientists could do meaningful work I’m proud of contributing to medicines that help people And I’m proud of helping Carolina grow.”
That growth continues, thanks in no small part to Caudill’s vision, energy and belief in what chemistry and Carolina can do. For the students walking today through the classrooms and labs that bear his imprint, his message is both a challenge and encouragement
“Pay attention. Learn deeply. Connect widely. And never forget that science, at its best, serves the public good.”




by Dave DeFusco

In the UNC Chemistry Department, Ph.D. student Emily Crawford is staring down a mystery that most people never think about when they swallow a pill Every day, millions of Americans rely on antidepressant medications to stabilize their moods and reclaim their lives. But once those drugs have done their job inside the human body, up to 90 percent of their chemical remnants still biologically active don’t just disappear Instead, they pass quietly through wastewater treatment systems and into the rivers, lakes and streams that form the veins of our environment.
For Crawford, this intersection between mental health treatment and environmental chemistry is more than just an academic interest it’s a passion project with global consequences. Recognized with an honorable mention from the National Science Foundation’s Graduate Research Fellowship Program, Crawford is pioneering a multidimensional approach to quantify antidepressants in North Carolina’s waterways and evaluate their molecular impacts on aquatic life
Her findings could not only help clean up the water but also reframe how we think about the downstream effects literally of human health choices on the ecosystems we depend on Crawford’s project was born out of a simple yet provocative question: what happens to antidepressants after they leave the human body?
“We know these drugs are essential for mental health,” she said “But they’re also active in trace amounts, and our
wastewater systems weren’t designed to catch them. So they persist in the environment and fish and other aquatic species are exposed constantly ”
Antidepressants like sertraline and escitalopram among the most widely prescribed SSRIs have already been linked to behavioral and reproductive disruptions in aquatic organisms. Fish that become unusually bold or sluggish, or fail to reproduce on schedule may be responding to the same neurotransmitter-altering chemistry that helps people feel better But until now, little has been known about how these exposures might be affecting organisms at a molecular level
That’s where Crawford’s research breaks new ground After graduating from Georgia Tech with a 4 0 GPA and a string of scholarships including the Zell Miller and Women in Sciences Grant Crawford headed to Chapel Hill to join the lab of analytical chemist Dr. Erin Baker, who said she was “ecstatic” to gain Crawford as a lab member
“Emily has been an amazing addition to our lab,” said Dr Baker “She not only thinks deeply about scientific problems, but she has also driven this project by knowing the history of this area and finding unique strategies to address challenges that she has encountered in the lab. Emily is also an amazing mentor and teacher for our other students.”
Known for pioneering work in exposomics, Baker’s lab has built a strong reputation for studying so-called “forever chemicals” like PFAS. Crawford saw an opportunity to apply the lab’s sophisticated tools particularly their liquid chromatography–ion mobility–mass spectrometry platform (LC-IMS-MS) to something new: pharmaceuticals in the environment
“I was really interested in how human behaviors interact with our environment and vice versa,” she said. “We realized that no one had ever used our specific platform to analyze antidepressants That opened the door ”
The unique capabilities of the lab’s drift tube ion mobility spectrometer gave Crawford an edge Unlike conventional mass spectrometry, which can struggle to distinguish molecules with identical masses, the drift tube adds another layer of separation measuring molecules by their shape and gas-phase surface area, known as collision cross section (CCS). This allows for a level of structural resolution that’s vital when studying complex biological matrices like wastewater or fish tissue
Before Crawford could analyze anything in the wild, she had to build a reference library from the ground up a meticulous and time-consuming process that catalogued the key properties of 55 different antidepressant compounds and their metabolites, including SSRIs, SNRIs, MAOIs and tricyclics.
“It had never been done before on this platform,” she said. “These drugs are designed to break down in the body, and sometimes they were fragmenting in the instrument even when we didn’t want them to. It took a lot of optimization to keep them intact ”
She had another problem too: uncertainty. Would the pharmaceutical-grade standards she purchased from chemical suppliers be chemically identical to the forms showing up in real-world water samples? What about unknown degradation products?
“If those aren’t in the library,” she said, “we cannot identify them in the water samples with the same level of confidence That’s one of the biggest challenges in environmental chemistry what you don’t know can still hurt the ecosystem.”
With her library in place and methods validated, Crawford launched a two-part investigation
In Aim 1, she is sampling water from five sites across central North Carolina four near wastewater treatment plant outflows and one from a recreational lake that doesn’t receive wastewater effluent. She spikes each sample with “heavy” deuterated standards to enable precise quantification using her platform Early data suggest a direct correlation between antidepressant concentrations and population size served by the plant, but the story is complicated by variables like water body size and flow rate.
In Aim 2, Crawford will travel to the University of Luxembourg to collaborate with environmental toxicologist Dr. Emma Schymanski There, she’ll expose zebrafish embryos model organisms often used in toxicology to varying concentrations of antidepressants in a tightly controlled aquatic facility.
By analyzing lipid profiles from these embryos using her LC-IMS-MS platform, she hopes to determine whether antidepressants induce dyslipidemia a disruption in fat metabolism known to be associated with cardiovascular issues in humans.
“There’s this longstanding controversy,” said Crawford. “Are side effects like dyslipidemia caused by the depression itself through things like poor diet and sleep or are they a direct result of the medication? With fish, we can control for those behaviors and isolate the effects of the drugs themselves ” Her hypothesis: fish embryos exposed to high levels of antidepressants will show molecular changes particularly in polyunsaturated fatty acids (PUFAs) that correlate with previously observed behavioral disruptions. If confirmed, it would provide the first strong molecular evidence linking pharmaceutical pollution to ecosystem-wide health risks


“With North Carolina’s growing population and diverse geography, it’s an ideal setting for this research,” she says “But the issue is global By partnering with labs in Europe and mentoring students here, I’m trying to make sure the science reaches beyond just one lab or one town ”

Maria Furukawa’s academic journey is driven by a personal mission to improve the lives of those undergoing dialysis, including a close family member As a Ph D candidate in the department of chemistry at the UNC-Chapel Hill, Furukawa is developing liquid-crystal polymers that could transform the membranes used in hemodialysis, a life-saving treatment for patients with late-stage renal failure.
After earning her B S in chemistry from the Georgia Institute of Technology, Furukawa synthesized high-performance polymers in industry before she had a realization.
“I wanted a role where I could initiate ideas for projects,” said Furukawa, “and to do that, you need a Ph.D. I applied to UNC-Chapel Hill at a colleague’s recommendation and was really excited about the research. There were lots of good polymer
options, and I thought project-wise, PI-wise, I’d be happy to work in any of those groups ”
As a member of the Dingemans Group in the UNC department of applied physical sciences, Furukawa synthesizes liquid-crystal polymers. These unique materials have properties of solids and liquids which make them attractive options for a variety of applications, but one in particular drew Furukawa’s interest:



hemodialysis. A common treatment for late-stage renal failure, hemodialysis mimics the kidneys’ function by utilizing an external membrane to filter waste from the blood
To create these membranes, Furukawa links together chemical building blocks, called monomers, to create chemical chains called polymers. By mixing different types of monomers into the polymer chain, Furukawa can control how much water and waste can pass through the polymer two key considerations when designing dialysis membranes.
“It’s an area that needs improvement,” said Furukawa. “I have close family that are on dialysis which gives me more motivation to learn and hopefully make some contribution to the field to make an impact ”
She had the opportunity to do just that when she joined the Uhlmann Group in Dresden, Germany. Over the 10-week research experience,
Furukawa investigated modifications to her polymer that could improve the membrane’s performance by preventing protein fouling. This phenomenon occurs when proteins adsorb onto the surface of the membrane, compromising its ability to effectively filter waste over time
Creating the membrane required carefully layering three polymers onto a silicon wafer, each layer around 1,000 times thinner than a sheet of paper. A base layer anchored the membrane to the solid surface; the liquid-crystal polymer layer provided filtration, and the final polymer studied by the Uhlmann Group provided antiprotein fouling protection. The result? A new membrane that enabled high water permeability and decreased protein adsorption. By successfully demonstrating her liquid-crystal polymer could be modified with the anti-protein fouling polymer, Furukawa took a crucial step towards creating a
longer-lasting hemodialysis membrane that could effectively remove waste from the blood.
“The fact that the project worked as simply as it did over less than 10 weeks was pretty exciting,” Furukawa said “I thought it was a good combination of my interests and for a good purpose ”
Back in Chapel Hill, Furukawa focuses on replicating her results and synthesizing membranes of varying thicknesses. After optimizing the syntheses, she hopes to return to Dresden and perform more protein adsorption experiments.
“I learned something new that I could bring back to my group,” said Furukawa. “I feel like I am more confident in what I want to do and how to move forward. There are a lot of possibilities to explore.”
In the microscopic world inside our cells, space is at a premium. Proteins tiny molecular machines that perform essential tasks like digesting food, fighting infections and sending signals exist in extremely crowded environments In fact, inside a cell, the concentration of proteins can reach as high as 300 grams per liter. That’s like dissolving 60 teaspoons of sugar in a single cup of water.
Yet, scientists often study proteins in labs under much more diluted conditions less than 1 gram per liter mainly to simplify experiments. But how accurate are those results, given the vast
difference between test tubes and living cells?
That question drove a team of chemists at UNC-Chapel Hill to explore what really happens to a protein when it’s packed in tightly with its neighbors In a Protein Science study, “Crowding-induced Stabilization and Destabilization in a Single Protein,” Chemistry Ph.D. candidate Jordyn Markle, with Tarynn Neal, a Morehead-Cain scholar, and Hania Kantzer, Chancellor’s Carolina Scholar all working under the guidance of Dr. Gary Pielak, senior author and Kenan Distinguished Professor set out to better understand the
by Dave DeFusco
surprising effects of protein crowding.
Their research reveals a complex truth: when proteins crowd together, they can both stabilize and destabilize themselves sometimes in unexpected ways.
“For decades, scientists have believed that when proteins are crowded, they’re generally more stable,” said Dr. Pielak. “This idea comes from the concept of ‘excluded volume’ essentially, proteins take up space and can’t overlap, so they tend to stay in their compact, folded forms to avoid bumping into each other.

“Sometimes being surrounded by others keeps them calm,...

This entropic effect, a type of stabilizing force in classic crowding theory, was expected to dominate in crowded environments ”
But as more researchers have studied proteins under crowded conditions, strange things have emerged. Proteins don’t always get more stable. Sometimes they become less stable. That contradiction suggested that other forces, especially chemical interactions between proteins, might be at play.
To investigate, the UNC team used a simpler approach: they focused on just one kind of protein called GB1, a small, well-studied globular protein often used as a model. Rather than crowding it with synthetic materials or complex mixtures, they crowded GB1 with more GB1, allowing them to isolate the effects of protein-protein interactions what they call “self-crowding.”
Using a sophisticated technique called nuclear magnetic resonance (NMR) spectroscopy, they were able to monitor how individual parts, or residues, of the protein behaved under crowded conditions, specifically by measuring hydrogen exchange rates a marker of how stable or unstable a protein region is.
Their experiments showed that crowding can stabilize some parts of GB1 while destabilizing others,
while other times, the crowd makes them lose composure, or unfold.”

even within the same protein The key insight is that these opposing effects arise from two different types of interactions between proteins:
1.Repulsive interactions, like charges pushing away from each other, help stabilize parts of the protein that only become exposed when the whole protein unfolds. These regions were harder to unfold in crowded environments, likely because surrounding proteins pushed back against the unfolding
2 Attractive interactions, like weak chemical bonds or sticky patches, can destabilize parts of the protein that unfold locally, just a few segments at a time. These segments became easier to unfold as protein concentration increased, probably because nearby proteins were pulling on or subtly interfering with them.
“In other words, proteins are like introverts at a party,” said Markle, one of the lead authors. “Sometimes being surrounded by others keeps them calm and collected, or folded, while other times, the crowd makes them lose composure, or unfold ”
Understanding how proteins behave in crowded environments is vital for both basic biology and applied research. Inside our cells, protein crowding affects everything from how enzymes work to how diseases like Alzheimer’s and Parkinson’s begin, which are linked to misfolded or aggregated proteins
The new findings also highlight the importance of moving beyond overly simplified lab experiments. By showing that proteins can be both stabilized and destabilized by crowding, and identifying the underlying forces at play, the UNC team has provided a more realistic view of what happens inside living cells.
“Our study supports the idea that protein stability in cells isn’t just about space, it’s also about the chemistry between neighbors,” said Markle “It’s a complex balance of repulsion and attraction.”

by Emily Sherman
More eco-friendly chemical tools are needed to access novel compounds for drug discovery and mitigate the tons of chemical waste produced every year Chemists in the Johnson Lab at UNC-Chapel Hill tackled this problem in their Journal of Organic Chemistry study, “Formal Dearomative Hydroamination of 2 Arylphenols,” by converting chemicals from petroleum waste into compounds for study as anesthetics and antidepressants.
When lead author Dr. Bob Wiley began investigating a path to these compounds, he discovered two key roadblocks. First, many approaches used toxic, lead-containing chemicals. When disposed of, these can contaminate the soil, water and air, decreasing plant growth while
potentially harming neurological development in children and decreasing memory and concentration in adults.
Second, these methods only produce a few iterations of the core molecular structure preventing scientists from easily testing drug candidates Like trying on multiple pairs of jeans to find the right fit, quickly producing many variants is key to finding the bestperforming molecule.
“This investigation would allow us to put our own spin on a classic problem in chemistry,” said Wiley. “Other methods produced a select few variants of these biologically relevant structures and used harsh chemicals, so we hoped to synthesize a broader scope of these molecules with more benign conditions ”

Wiley and co-authors tackled these two issues with a chemical method called dearomatization which transformed phenols a type of molecule found in plant or petroleum waste into the target molecules.


While they sing, they tightly hold each other’s hands until a stimulus is introduced and at least one child will take a treat, disrupting their grip on their friend’s hand.
To understand how dearomatization works, think of a group of children playing “Ring Around the Rosie.” While they sing, they tightly hold each other’s hands until a stimulus is introduced like a tray of cupcakes Naturally, at least one child will take a treat, disrupting their grip on their friend’s hand.
Much like the children’s ring, the bonds in phenols are strong and unlikely to break unless a stimulus is introduced. When the chemists added a stimulus a weak nitrogen-oxygen bond a variety of inexpensive phenols reacted and rapidly transformed into new molecules. By using dearomatization, the chemists avoided hazardous lead-containing chemicals and developed a method with less toxic waste to quickly access molecules for drug discovery
“Without dearomatization, producing these molecules would require multiple chemical reactions,” said Michael Eng, the paper’s second author. “These additional synthetic steps would generate more waste and potentially higher production costs ”

“Synthetic methods like this dearomatization are challenging because of the stability of the phenols,” said Dr Jeffrey Johnson, senior author and A. Ronald Gallant Distinguished Professor. “By disrupting this stability without lead-based chemicals, a diverse array of inexpensive molecules can be transformed to advance available drug technologies.”
