
(Continued)
COURSE DESCRIPTION
Join Us on Sept 14th and 15th, 2023 in Ann Arbor for Championship Updates in Sleep Medicine. Diverse & Multidisciplinary Speakers will provide evidence based and practical strategies to improve care of patients with sleep disorders and provide updates on innovations in sleep medicine. This 1.5-day conference will provide continuing education credits as applicable to attendees (Physicians, Advance Practice Providers and Sleep Technologists, etc.) commensurate with the extent of their participation in the activity. After this activity, sleep providers will be able to apply new knowledge regarding best practices and guidelines to the everyday care of the sleep medicine patient.
LEARNING OBJECTIVES
At the end of this activity, participant should be able to:
1.apply new knowledge regarding best practices and current society guidelines to the evaluation and management of patients with sleep disorders.
2.educate patients regarding treatment options for sleep disorders.
ACCREDITATIONS & CREDIT DESIGNATION
The University of Michigan Medical School is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
The University of Michigan Medical School designates this live activity for a maximum of 12.75 AMA PRA Category 1 Credit(s) ™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
PLANNING COMMITTEE
Educational Planners
Neeraj Kaplish, M.B., B.S.
Associate Professor, Neurology
University of Michigan Health
Cathy A Goldstein, MD
Clinical Professor, Neurology
University of Michigan Health
Activity Coordinator
Erika Laszlo
Outreach Manager
University of Michigan Health

Care of Pediatric Patients on Home Mechanical Ventilation.................................................................................311–353 Fauziya Hassan MD, MS Advanced PAP Modalities: Details Matter.............................................................................................................354–400 Megan Acho, MD, MS Establishing and Maintaining a Successful Sleep Disorders Center: Blue Print from an Academic Program.........401-445 Ronald D Chervin, MD, MS Dental Sleep Medicine: Collaborating to Enhance Treatment Options for OSA.....................................................446-510 Geoffrey Gerstner, DDS, MS, PhD, D.ABDSM
TABLE OF CONTENTS
Care of Pediatric Patients on Home Mechanical Ventilation
 Fauziya Hassan MD, MS Associate Professor, Service Chief
Fauziya Hassan MD, MS Associate Professor, Service Chief
Pediatric Home Ventilator and Pulmonology, Pediatric Sleep Medicine
311
Disclosures
None pertinent to this talk
Grant Funding – NHLBI, NIH
Consultant- Eli Lilly

•
•
•
312
Objectives • Guidelines in neuromuscular disorders


Supporting literature
Use of Polysomnography
•
•
313


Respiratory Management of Patients with Neuromuscular Weakness CHEST 2023; 164(2):394-413 314
Scoring Criteria for Hypoventilation
If electing to score hypoventilation during sleep - EITHER of the below occur (adults):
There is an increase in the arterial PCO2 (or surrogate) to a value
≥55 mmHg for ≥10 minutes

There is ≥10 mmHg increase in arterial PCO2 (or surrogate) during sleep (in comparison to awake supine value) to a value exceeding 50 mmHg for ≥10 minutes
Pediatrics – CO2 values ≥ 50 mm Hg for ≥2 % of sleep time or peripheral capillary oxygen saturation ≤ 90% for 2% of recording time
315
Assessments and Interventions for Respiratory Care of Patients with DMD by Stage of Disease
Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management

Considerations Working Group*
Contemporary care has been shaped by the availability of more sensitive diagnostic techniques and the earlier use of therapeutic interventions, which have the potential to improve patients’ duration and quality of life.
David J Birnkrant, Katharine Bushby, Carla M Bann, Benjamin A Alman, Susan D Apkon, Angela Blackwell, Laura E Case, Linda Cripe,Stasia Hadjiyannakis, Aaron K Olson, Daniel W Sheehan, Julie Bolen, David R Weber, Leanne M Ward, for the DMD Care
316
Assessments and Interventions for Respiratory Care of Patients with DMD by Stage of Disease






317
Diagnosis and Management of SMA



Finkel et al Neuromuscular Disorders 28 (2018) 197–207 318
Consensus Statement for Management of Congenital Myopathy



Wang et al. J Child Neurol. 2012 March ; 27(3): 363–38 319
Ambulatory Stage- Indications for PSG
Sleep studies with capnography can be used in ambulatory phase

When there is weight gain among patients on glucocorticoid therapy
There are symptoms of sleep disordered breathing even minimal snoring
Monitoring respiratory function when unable to monitor with pulmonary function testing (PFT)
•
320
Non-Ambulatory Stage
For patients with cognitive impairment and unable to perform PFT pre-operative PSG may be helpful
Late ambulatory stage indications for NIPPV include (needs one of the following)
Signs and symptoms of SDB or hypoventilation (regardless of PFT)
FVC < 50% predicted or MIP < 60 cm of water on lung function
Daytime ETCO2 or TCO2 value > 45 mm Hg, blood gas CO2 > 45 mm Hg (ABG- adults CBG allowed among children)
Pulse Oximetry value < 95%

321
Indications for Initiation of NIPPV
Abnormal sleep studies with ETCO2 or TCO2 values > 50 mm Hg for ≥ 2% of sleep time *
ETCO2 or TCO2 values > 10 mm Hg over awake CO2 values for ≥ 2% of sleep time
Adults- oxygen saturation of ≤ 88% for 5 continuous minutes (PSG) or ≤ 90 % for ≥ 2% of sleep time on overnight pulse oximetry**
Adults & children - AHI > 5?? ** or should it be OAHI > 1 among children
NIPPV can be used during and after procedures involving anesthesia/sedation or to extubate invasively ventilated patients- necessary if FVC < 30 %, recommended if FVC < 50%
Non-ambulatory patients should have baseline sleep studies as often as annually if possible
*Amaddeo et al Long Term CPAP and NIV in Children. Ped Pulmonology 2016 (51) 968-974

** Respiratory Management of Patients with Neuromuscular Weakness CHEST 2023; 164(2):394-413
*** ACCP Consensus Statement on Respiratory and Related Management of Patients with DMD Undergoing Anesthesia or Sedation CHEST 2007; 132:1977–1986)
322


323
Does NIPPV Help ??
Retrospective analysis of 120 non ambulatory patients (10.5 ± 6.1 yrs) with DMD were on NIPPV for symptomatic SDB, low SpO2 or high ETCO2
Patients used mechanically assisted cough and NIPPV, 26 patients on continuous NIPPV did not get hospitalized or have ARF and 7 lived to age > 40 *
Retrospective study of 197 patients seen from 1967 to 2002. Mean age of death in 1960s was 14.4 years **
Ventilation was started when patients were symptomatic or with abnormal pulse oximetry and decrease in FVC
* Bach JR et al. Duchenne muscular dystrophy: continuous noninvasive ventilatory support prolongs survival. Respir Care 2011(56) 744–50.
** Eagle M et al. Survival in Duchenne muscular dystrophy: Neuromuscul Disorder 2002 (12) 926–29.

324
Survival With and Without Ventilation
• Among patients who did not receive ventilation and died of respiratory failure, mean survival was 19.29 years (CI :18.61-19.97)
• Those receiving ventilation, mean survival was 25.3 years (CI: 23.11-26.58)
Eagle et al. Survival in DMD: improvements in life expectancy since 1967. Neuromuscular Disorders 2002; 12(10) 926-9


• DMD patients in Japan from 1964 to 2010 (N=187)
• Divided into 3 groups 1964-1984: oxygen only
• 1984-1991: oxygen or tracheostomy after CO2 narcosis or intubation for acute respiratory failure
• Since 1991 NIV for symptomatic hypercapnia, cough assist devices and cardio protective medications
Ishikawa et al Duchenne muscular dystrophy: Survival by cardio-respiratory interventions . Neuromuscular Disorders 2011 (21) 47-51

325
Duchenne Muscular Dystrophy and PSG
Retrospective study of 110 subjects 5-18 years of age with confirmed diagnosis of DMD

On steroid therapy, not on NIPPV
PFTs done for > 7 years of age with technically acceptable maneuvers in 41% and respiratory muscle strength acceptable testing in 67%
Some patients (18%) had SDB symptoms and 73% had abnormal screening PSGs (10-11 years) with ETCO2 and TCO2 monitoring
Apneas and hypopneas were scored as 2 respiratory cycles duration and hypopneas were 50% decrement from baseline
* Sawnani H et al. Sleep disordered breathing in Young Boys with Duchenne Muscular Dystrophy.
J Peds: 2015, 166 (3) 640-645. 326



Sawnani
J Peds
2015, 166 (3) 640-645. 327
H et al. SDB in Young Boys with DMD.
:
Spinal Muscular Atrophy- Shifting Paradigm

Proactive approach to introduction of therapies including ventilation
Non sitters
Pulse oximetry and ETCO2 or TCO2 monitoring during wakefulness
Sleep study with CO2 monitoring to assess for hypoventilation or sleep disordered breathing (SDB)
Sitters
No consensus on value of peak cough flow or timing of sleep study
Sleep study should be performed in symptomatic patients or even with minimal risk of hypoventilation or SDB
Walkers
Assessment of symptoms of sleep apnea or hypoventilation along with PFT
Pre-operative assessment
328
Early Respiratory Care and Survival in SMA type 1

Spinal muscular atrophy type 1: Are proactive respiratory interventions associated with longer survival?


Pediatric Critical Care Medicine13(3):e161-e165, May 2012. doi: 10.1097/PCC.0b013e3182388ad1
Survival with proactive care within 90 days of diagnosis was hazard ratio of 2.44 (0.847.1) compared to institution of proactive care later, HR 1.33 (0.38-4.59) when compared to children in supportive care group
Copyright ©
Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 19
2019 by the
Lemoine, Tara J.; Swoboda, Kathryn J.; Bratton, Susan L.; Holubkov, Richard; Mundorff, Michael; Srivastava, Rajendu
329
SMA Type 1: Management and Outcomes

All patients without ventilation died by 13 months of age
From birth to 3rd birthday those on NIPPV had higher rate of hospitalization than those with trach and IPPV (p value 0.039)
From third birthday onward there was no significant difference between those on NIPPV and IPPV (p value 0.598)

No support
Trach and vent NIPPV
et al Pediatric Pulmonology.2002 (34): 16-22 330
Bach
So if You Did Check CO2 During the Day…
52 children with neuromuscular disorder ( DMD=20, SMA= 10, NMD=22)

Lung function testing, CBG was done and overnight PSG with pulse oximetry and TCO2 monitoring
FVC was 36.5 % predicted (29-48%), daytime CO2 values mean 39 mm Hg (37-42), normal HCO3 of 24
*Bersanini C et al. Nocturnal hypoxemia and hypercapnia in children with neuromuscular disorders. Eur Resp J 2012. 39: 1026-1212
331
Correlation between Daytime and Nighttime
Using daytime PaCO2 measurement of 38 mm Hg had 80% sensitivity and 56.7 % specificity (AUC 0.74) to predict nocturnal elevation in CO2 values
PSG in 27 patients and 74% were obstructive, 21% central and 5% mixed with AHI > 5 in 19 patients

Daytime PaCO2 and HCO3 as well as lung function did not correlate with AI or AHI

332
HSAT in Vulnerable Populations
In-lab PSG versus level III HSAT done among 28 children ages 6-18 years with diagnosis of neuromuscular disorder, DMD being most common

HSAT with ETCO2 monitoring done at home
Patients had mild to borderline restrictive lung disease
Based on PSG findings 46% of patients had moderate to severe SDB and 1 patient with hypoventilation and with HSAT only 36% had moderate to severe SDB. No hypoventilation noted
Using an AHI >1 sensitivity and specificity of 68% and 67%
50% of patients had incomplete or falsely low ETCO2 values on HSAT due to signal loss or mouth breathing
Fishman et al J Clin Sleep Med 2018: 14 (12): 2013-2020
333
Need for Daytime Ventilation
Daytime ventilation should be considered when awake PCO2 > 50 mm Hg
And/or hemoglobin saturation <92% while awake
Modes of ventilation
Non-invasive - Sip and puff
AVAPS in PC mode via tracheostomy

Volume ventilation or pressure ventilation via tracheostomy

334
BiLevel PAP
BPAP use for OSA or hypoventilation

Starting PS and use of high intensity BPAP (high PS)
In hospital or sleep lab

CO2 monitoring and mask leak
What about sleep stages and position
Best Clinical Practices for the Sleep Center Adjustment of Noninvasive Positive Pressure Ventilation (NPPV) in Stable Chronic Alveolar Hypoventilation Syndromes – JCSM 6(5) 2010 335
INSURANCE




336
Updated Recommendations
• PSG may not be necessary among adult patients if PFT or ONO criteria support using NIV


• PSG may be helpful if PFT and clinical symptoms clinical evaluation may not capture hypoventilation
• PSG is indicated at least once among children and among adults with EDS, tiredness, fatigue, snoring, apneic episodes
• Adequacy of NIPPV support can be assessed by pulse oximetry > 90 % for 90% of the study
337
Indications – BPAP or AVAPS
Patients with hypoventilation-either central or peripheral:
congenital central hypoventilation syndrome (CCHS) or acquired hypoventilation
neuromuscular diseases - muscular dystrophy, spinal muscular atrophy (SMA), amyotrophic lateral sclerosis (ALS)

restrictive lung disease - neuromuscular scoliosis
AVAPS/iVAPS is also indicated in patients with COPD and obesity hypoventilation
338
Other features

Trilogy in AVAPS mode Trilogy EVO in AVAPS mode AVAPS Tidal volume (ml) 50 to 2000 35-2000 200 to 1500 IPAP minimum (cm of water) 4 Depends on circuit 4 IPAP maximum (cm of water) 50 60 30 Rise (ms) 1 to 6 0 to 6 1 to 6 EPAP (cm of water) 4 to 25 0 to 35 3 to 25 4 to 20 I time (seconds) 0.3 to 5.0 0.3-5.0 0.5 to 3.0 Rate (maximum) 1-60 (1-40 for AE) 0-80 30 AVAPS rate (pressure) 1-6 1-6 1 Sensitivity 1.0-9.0 l/min ATS, AT 0.5-9.0 l/min ATS, AT 3.0-9.0 AT
Dual prescription Back up battery (6 hr) NIPPV and IPPV 5kg 4 prescription Back up battery (15 hr) NIPPV and IPPV ETCO2, SpO2 monitor 2.5 kg none 339




Intubated 340


341

BiPAP 15/8, rate 14, I time 1.4 342


TV 400, IPAP, 30/12, EPAP 8, R10, I time 1.4 343


TV 450, IPAP, 30/12, EPAP 8, R10, I time 1.4 344


TV 450, IPAP, 30/12, EPAP 8, R10, I time 1.4 345
Bilevel PAP Lateral Position


346



level PAP Supine 347
Bi
AVAPS TV 390, IPAP max 20, IPAP min 10 EPAP 6, RR11, I time 1.5


348
AVAPS TV 440, IPAP max 20, IPAP min 12 ( for hypopneas) EPAP 6, RR12, I time 1.5


349



350
TV 620, EPAP 6 cm, different mask
Tidal volume 635, EPAP 7, different full-face mask


351
• Early ventilation is key
In Summary
• Think sleep study even with minimal symptoms especially if child is in a wheelchair

• SDB starts during REM sleep so consider PSG
• There is a role for titration studies in lab and for downloads
• Always do sleep studies with CO2 monitoring and look at tidal volumes
• Treat at minimum with Bilevel PAP with back up rate
352
Michigan Difference
Pediatric Cardiology

Pediatric Neurology
Pediatric Pulmonology
Physical Medicine and Rehabilitation
Palliative Care
Wheelchair Seating and Orthotics
Nutrition
Nursing
Respiratory Care
Social Work
Physical & Occupational Therapy
Sleep Disorders Center

353
Advanced PAP Modalities: Details Matter Megan Acho, MD, MS Championship Updates in Sleep Medicine September 15, 2023 354
Disclosures
• I have no conflicts of interest to disclose
• This talk is heavily influenced by work done by Lisa Wolfe, as well as Jennifer Newitt and Pat Strollo. I am indebted to their excellent teaching!
355
Goals and objectives
• Review key components of Positive Airway Pressure (PAP) delivery
• Discuss Volume Assured Pressure Support (VAPS) technology
• Describe the differences in VAPS technology based on manufacturer (Philips Respironics vs ResMed)
• Recognize common complications of VAPS therapy
356
Background: CPAP
• CPAP = Continuous Positive
Airway Pressure
• One set level of pressure delivered to the upper airway during sleep via face mask
• Standard CPAP: fixed level of pressure between 4-20cm H20
• No synchrony between the patient and the machine
• Utilized for OSA
Time
Pressure
357
Background: BPAP
• BPAP = Bilevel Positive
Airway Pressure
• Two set levels of pressure
• IPAP: Inspiratory Positive
Airway Pressure
• EPAP: Expiratory Positive
Airway Pressure
• PS = IPAP - EPAP
• Key: need to achieve synchrony between the patient and the device
Pressure
Time
358
Background: BPAP
Phase variables during the respiratory cycle:
• EPAP: Expiratory Positive Airway Pressure
• IPAP: Inspiratory Positive Airway Pressure
• PS: Pressure Support = IPAP – EPAP
• Ti: Inspiratory time
• Rise: Time to pressurize
• Trigger: Transition point from EPAP to IPAP (start of inspiration)
TV ���� (IPAP-EPAP) x R Ti
• Cycle: Transition point from IPAP to EPAP (end of inspiration)
Pressure Time
EPAP IPAP PS
Figure adapted from Selim et al. Chest 2018; 153(1):251-265. Wolfe. ATS Aspire Lecture. July 2020.
Ti
359
Rise Time
• Time to pressurize (transition from EPAP to IPAP)
• Slope of the line between EPAP and IPAP
• ResMed: measured in milliseconds (100-900ms)
• Philips-Respironics: qualitative measure (1-6)
• Adjust the rise time based on patient’s physiology
• Decreased rise time (fast pressurization):
• High respiratory drive
• COPD
• Muscle weakness
• Longer rise time (slow pressurization):
• Chest wall stiffness
• Bulbar disease
Pressure Time EPAP IPAP PS Selim
153(1):251-265. 360
et al. Chest 2018;
Inspiratory Trigger
• Transition from EPAP to IPAP (start of inspiration)
• May be:
• Time-triggered (mandatory breath)
• Device initiated breath based on back-up respiratory rate
Pressure Time EPAP IPAP PS Selim et al. Chest 2018; 153(1):251-265. 361
Inspiratory Trigger
• May be:
• Patient-triggered (spontaneous breath)
• Device senses a deflection in flow with patient effort
• Trigger sensitivity: level of deflection in flow needed for device to trigger the breath

• Philips Respironics- AutoTrak
• ResMed- very high, high, medium, low, very low
Pressure Time EPAP IPAP PS
et al. Chest 2018; 153(1):251-265. ResMed 362
Selim
Inspiratory Cycle
• Transition point from IPAP to EPAP (end of inspiration)
• May be time- or flow-cycled
• Time cycle: based on the set inspiratory time (Ti)
• Philips Respironics: set Ti
• ResMed:
• Minimal Ti set (breath needs to be at least this long)
• Maximal Ti set (breath cannot exceed this amount of time)
Pressure Time EPAP IPAP PS Selim et al. Chest 2018; 153(1):251-265. 363
Inspiratory Cycle
• Transition point from IPAP to EPAP (end of inspiration)
• May be time or flow cycled
• Flow cycle: the completion of inspiration is determined by decrements in flow (relative to patient’s peak inspiratory flow)
• Cycle sensitivity: sets the level of peak flow below which the device changes from IPAP to EPAP
• Respironics: All spontaneous breaths are flow cycled
• ResMed: Any breath within the set Ti window will be flow cycled
Pressure Time EPAP IPAP PS
Selim et al. Chest 2018; 153(1):251-265. 364
Inspiratory Cycle


ResMed 50% 25% 8% 80 LPM 40 LPM 20 LPM 6.4 LPM 365
BPAP-S (Spontaneous)
• E0470 Devices
• ResMed: AirCurve
10S, AirCurve V-Auto
• Respironics:

DreamStation BiPAP

• Patient (flow) triggered breath
• RR, respiratory pattern, TV determined by the patient
Selim et al. Chest 2018; 153(1):251-265.
366
BPAP-ST (Spontaneous/Timed)
• E0471 Devices (Respiratory Assist Devices)
• ResMed: AirCurve 10ST
• Respironics: DreamStation BiPAP S/T


• S: Provides PS for any patient (flow) triggered breath
• Breath cycles to EPAP with decrement in inspiratory flow
• T: If patient fails to trigger, device will deliver breath
• Breath cycles to EPAP at the end of the prespecified Ti

Selim et al.
367
Chest 2018; 153(1):251-265.
BPAP-PC (Pressure Control)

• Set RR, patient also able to trigger spontaneously
• PS provided for S and T breaths
• No flow cycling; S and T breaths both last for the prespecified Ti
• Similar TVs between S and T breaths
Selim et al. Chest 2018; 153(1):251-265. 368
What is VAPS?
• VAPS = Volume Assured Pressure Support
• Device senses changes in patient’s respiratory flow over prior breaths
• Adjusts IPAP/PS to reach a respiratory target
• Used in S, ST, or PC mode (Respironics)
• In ResMed, VAPS is its own mode
Selim et al. Chest 2018; 153(1):251-265. 369
What is VAPS?

370
Selim et al. Chest 2018; 153(1):251-265.
AVAPS
• VAPS = Volume Assured Pressure Support
• Two types based on manufacturer
• AVAPS (Average Volume Assured Pressure Support)
• Philips Respironics
• RAD E0471- Respironics DreamStation BiPAP AVAPS
• Respiratory target: exhaled TV (VTe)
• Adjusts PS by varying IPAP level between the IPAPmin and IPAPmax to attempt to meet (or exceed) desired TV
• Gradually changes IPAP (0.5-1 cm H20/min) to maintain TV
• Backup rate can be fixed or autoset (2 beats/min less than average rate of the last 6 spontaneous breaths)
Selim et al. Chest 2018; 153(1):251-265
Philips Respironics
371
AVAPS
• AVAPS (Respironics)
• Set parameters:
• Mode (S/ST/PC)
• (Turn AVAPS on)
• IPAPmin, IPAPmax
• EPAP
• RR
• Ti
• Vt (desired)
• Typically start at 8cc/kg PBW
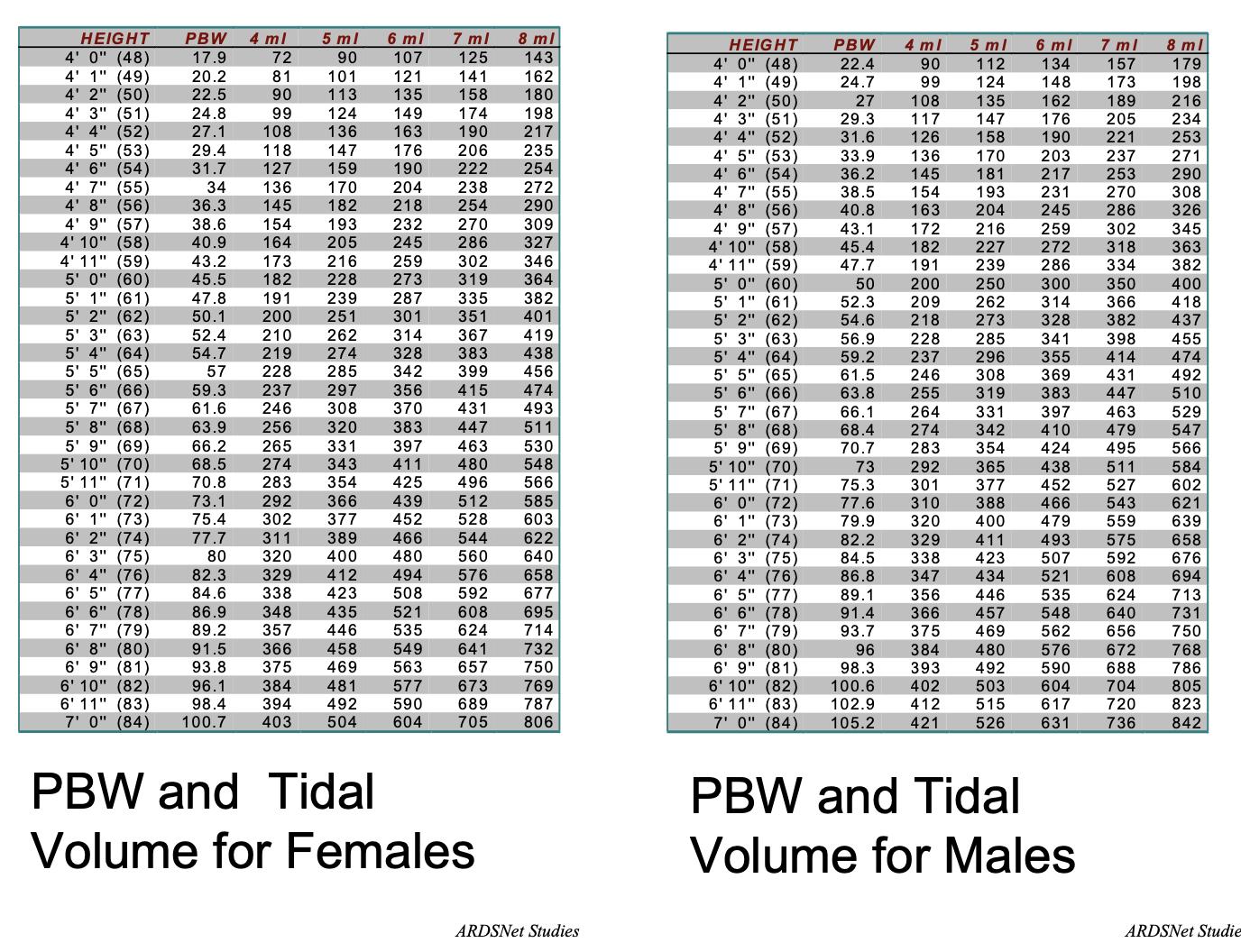
Selim et al. Chest 2018; 153(1):251-265
Philips Respironics ARDSNet
372
IVAPS
• VAPS = Volume Assured Pressure Support
• Two types based on manufacturer
• iVAPS (Intelligent Volume Assured Pressure Support)
• ResMed
• RAD E0471- ResMed AirCurve 10 STA, Astral
• Respiratory target: alveolar ventilation (VA)
Alveolar ventilation = VA = (TV – Vd) x f
• Targets alveolar ventilation using the patient’s height and backup rate
• “Intelligent Backup Rate” (iBR)
Selim et al. Chest 2018; 153(1):251-265 ResMed
373
IVAPS


ResMed 374
IVAPS

ResMed 375
IVAPS

ResMed 376
IVAPS
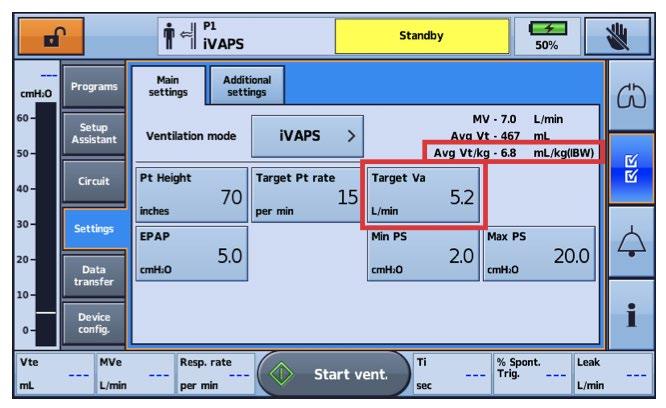
ResMed 377
IVAPS
• iVAPS (ResMed)
• Set parameters:
• You are not setting a mode! iVAPS is the mode!
• PSmin, PSmax
• EPAP
• Target breath rate
• Timin, Timax
• Target VA
• Patient height (inches)
• Trigger sensitivity, cycle sensitivity
• Rise time
ResMed 378
AutoEPAP
• Only available on ventilators (2nd generation device- E0466)
• Respironics: AVAPS-AE
• Trilogy 100/200, Trilogy Evo
• ResMed: iVAPS with autoEPAP
• Astral
• EPAP range allows for automatic adjustments based on device algorithm
• Overcome upper airway obstruction
• PS range allows for adjustments to maintain target TV
Selim et al. Chest 2018; 153(1):251-265. 379
Indications for VAPS
• Severe COPD
• Obesity Hypoventilation Syndrome
• Neuromuscular Disease
• ALS (and more)
380
Case 1
A 65-year-old man, former smoker, with a history of Chronic Obstructive Pulmonary Disease (COPD) presents to clinic for evaluation of nocturnal ventilation. He was admitted with an acute exacerbation of COPD (AECOPD) three months prior, requiring non-invasive ventilation with BPAP while in the intensive care unit. Since discharge, he has returned to his baseline functional status and is currently enrolled in pulmonary rehab. PSG in the interim demonstrated an oxygen saturation of <88% for 25 minutes with an Apnea Hypopnea Index of 3 events/hour.
Pulmonary function testing reveals an FEV1/FVC 35% and FEV1 18%. Labs from the day of this visit demonstrate a PaCO2 of 60mm Hg.
Is there a role for NIV and, more specifically, VAPS?
381
NIV in COPD
• Proposed rationale:
• Nocturnal NIV may facilitate CO2 clearance and potentially reset the respiratory center sensitivity for CO2
• Chronic nocturnal NIV could provide respiratory muscle rest enhanced recovery from chronic muscle fatigue
• Improve muscle strength, lung function, and gas exchange
• Potentially help to recruit poorly ventilated lung units in order to improve V/Q matching
Weir et al. Chronic Obstr Pulm Dis (Miami) 2015; 2(4):313-320. 382
VAPS in COPD
• So… is there a role for VAPS in stable severe COPD?
• Crisafulli et al (2009)
• Investigated nighttime efficacy of and compliance and physiologic responses to AVAPS versus PS ventilation.
• Looked at stable hypercapnic COPD patients over two 5-day periods during consecutive weeks.
• Patients received 8cc/kg of IBW as targeted TV
• IPAP ranged from EPAP up to 30cm H20 for AVAPS vs highest tolerated IPAP level for PS
• EPAP was set to a minimum level for both
• Primary outcome: PaCO2
Crisafulli et al. Lung 2009;187:299-305. 383
VAPS in COPD


Crisafulli et al. Lung 2009;187:299-305. 384

Crisafulli et al. Lung 2009;187:299-305. 385
VAPS in COPD
Case 2
A 33-year-old female with a past medical history of pre-diabetes presents to clinic after her primary care doctor noted an elevated serum bicarbonate of 32 mEq/L on routine labs. Her vitals are notable for a body mass index (BMI) of 40.
Pulmonary function testing reveals an FEV1/FVC 80% and FVC 56%. Labs from the day of this visit demonstrate a PCO2 of 50 mm Hg.
Is there a role for NIV and, more specifically, VAPS?
386
NIV in Chronic OHS
• Obesity Hypoventilation Syndrome (OHS)
• Definition: Obesity (BMI > 30 kg/m2), daytime hypercapnia (PaCO2 > 45 mm Hg) not attributable to other causes of hypoventilation.
• 90% have concurrent OSA (nearly 70% have severe)
• American Thoracic Society (ATS) Clinical Practice Guidelines for patients with confirmed OHS
• + severe OSA: CPAP titration and treatment
• If inadequate treatment, change to NIV
• + no OSA or mild/moderate OSA: NIV initiation and treatment with consideration of bariatric surgery
• Per the ATS, if admitted with acute on chronic hypercarbic respiratory failure in the setting of OHS, patients should be discharged with NIV
Mokhlesi et al. Am J Respir Crit Care Med 2019. 200(3):e6-e24. 387
VAPS in Chronic OHS
• Potential utility of VAPS
• In obese patients, movement from sitting to supine increases respiratory muscle exertion with an associated fall in TV
• Fixed PS may not maintain adequate ventilation due to variable pulmonary mechanics throughout sleep; autotitrating device may be more effective
Murphy et al. Thorax 2012;67:727-734. 388
VAPS in Chronic OHS
• Murphy et al (2011)
• Investigated whether the addition of VAPS to standard fixed bilevel PS improves physiologic and clinical outcomes in the treatment of OHS
• Looked at stable OHS patients as well as those admitted for acute on chronic decompensated respiratory failure.
• Patients received AVAPS (goal VT 8-10cc/kg) vs BPAP fixed PS (IPAP 18-22, EPAP 8-10), titrated via protocol
• Primary outcome: PaCO2
389
Murphy et al. Thorax 2012;67:727-734.
VAPS in Chronic OHS

Murphy et al. Thorax 2012;67:727-734. 390
Case 3
A 60-year-old man with a recent diagnosis of ALS presents to clinic. He complains of some recent upper extremity weakness and denies any bulbar symptoms. He notes frequent morning headaches and frequent arousals at night. He also reports some mild exertional dyspnea, though denies any shortness of breath at rest.
A nocturnal oximetry study is obtained, which demonstrates desaturations below 88% for 45 minutes.
Is there a role for NIV and, more specifically, VAPS?
391
VAPS in Neuromuscular Disease
• Nicholson et al (2017)
• Retrospective chart review of patients receiving PS or VAPS for ALS
• Looked at tidal volumes, rapid shallow breathing indices
• Rapid shallow breathing index = respiratory rate (f) / tidal volume (VT)
• In ALS, more severe disease is associated with decreased VT and increased rapid shallow breathing pattern
Nicholson et al. Ann Am Thoracic Soc 2017;14(7):1139-1146. 392

Nicholson et al. Ann Am Thoracic Soc 2017;14(7):1139-1146. 393
Common Complications of VAPS
• Dyssynchrony
• Trigger
• Ineffective trigger
• Autotrigger
• Cycle
• Premature cycling
• Delayed cycling
• Mask leak
394


395
Leak Decreased tidal volume Increased pressure
396
Image courtesy of Jennifer Newitt (UPMC)
Thank you!
397
Works Consulted
ARDSNet.
Bourke, S. C., et al. (2006). "Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial." Lancet Neurol 5(2): 140-147.
Crisafulli, E., et al. (2009). "Subjective sleep quality during average volume assured pressure support (AVAPS) ventilation in patients with hypercapnic COPD: a physiological pilot study." Lung 187(5): 299305.
Donovan, L. M., et al. (2015). "New developments in the use of positive airway pressure for obstructive sleep apnea." J Thorac Dis 7(8): 1323-1342.
Dreher, M., et al. (2010). "High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial." Thorax 65(4): 303-308.
Howard, M. E., et al. (2017). "A randomised controlled trial of CPAP versus non-invasive ventilation for initial treatment of obesity hypoventilation syndrome." Thorax 72(5): 437-444.
Kohnlein, T., et al. (2014). "Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial." Lancet Respir Med 2(9): 698-705.
Mokhlesi, B., et al. (2019). "Evaluation and Management of Obesity Hypoventilation Syndrome. An Official American Thoracic Society Clinical Practice Guideline." Am J Respir Crit Care Med 200(3): e6e24.
Murphy, P. B., et al. (2012). "Volume targeted versus pressure support non-invasive ventilation in patients with super obesity and chronic respiratory failure: a randomised controlled trial." Thorax 67(8): 727-734.
Nicholson, T. T., et al. (2017). "Respiratory Pattern and Tidal Volumes Differ for Pressure Support and Volume-assured Pressure Support in Amyotrophic Lateral Sclerosis." Ann Am Thorac Soc 14(7): 11391146.
Philips Respironics. https://www.usa.philips.com/healthcare/solutions/sleep.
Piper, A. J., et al. (2008). "Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation." Thorax 63(5): 395-401. ResMed. https://www.resmed.com/en-us/.
Selim, B. J., et al. (2018). "Initiation of Noninvasive Ventilation for Sleep Related Hypoventilation Disorders: Advanced Modes and Devices." Chest 153(1): 251-265.
Storre, J. H., et al. (2006). "Average volume-assured pressure support in obesity hypoventilation: A randomized crossover trial." Chest 130(3): 815-821.
Weir, M., et al. (2015). "High Intensity Non-Invasive Positive Pressure Ventilation (HINPPV) for Stable Hypercapnic Chronic Obstructive Pulmonary Disease (COPD) Patients." Chronic Obstr Pulm Dis 2(4): 313-320.
Wolfe, L. “VAPS Therapy For Sleep Disordered Breathing.” (Presentation, ATS Aspire Webinar, June 25, 2020).
398
399
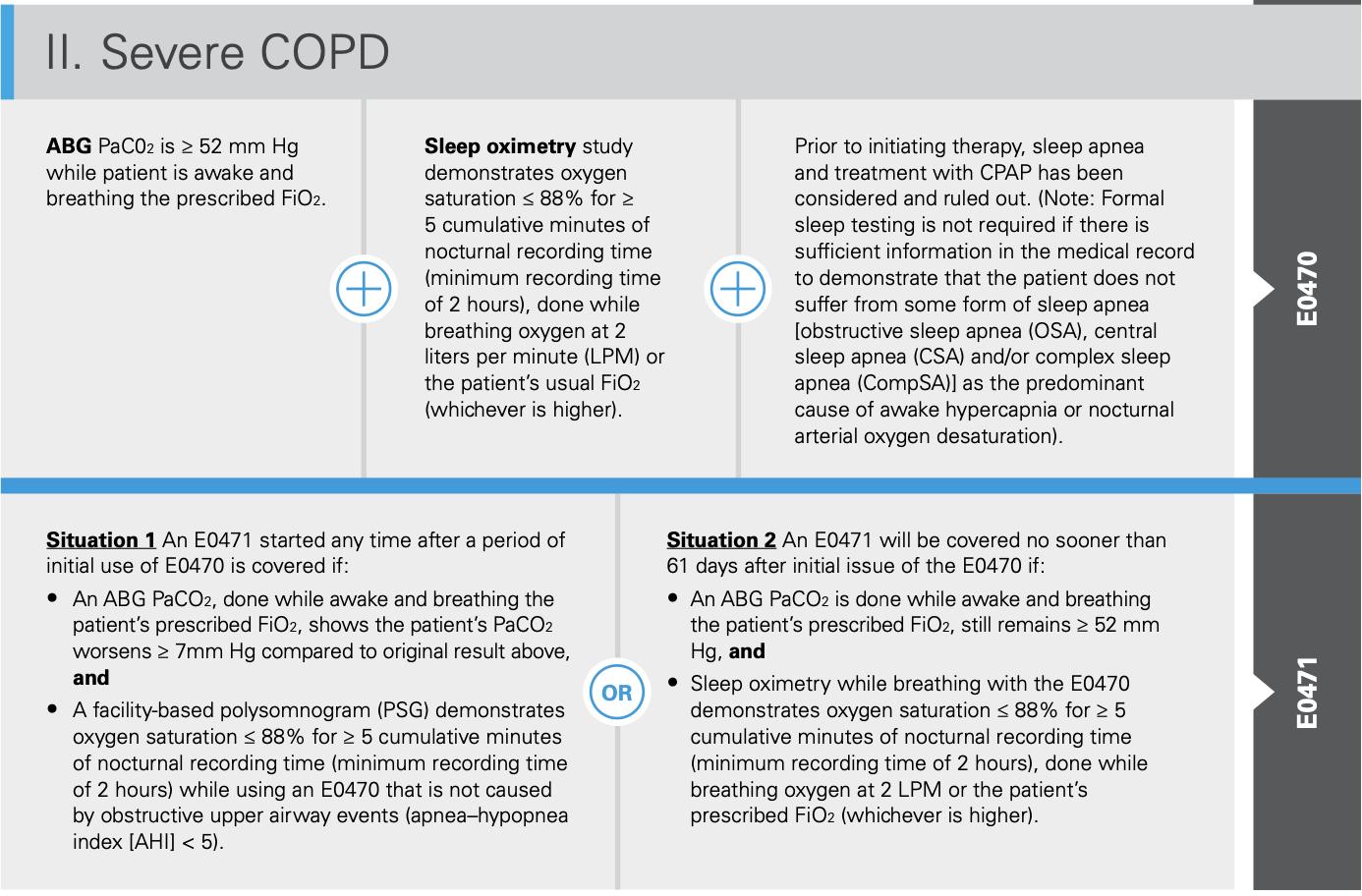
IV. Hypoventilation

An initialABG PaCO2, done while awake and breathing the patient's prescribed Fl02, is :.:: 45 mm Hg
Spirometry shows an FEV1/FVC :.::70%
• An ABGs PaCO2, done during sleep or immediately upon awakening, and while breathing the patient's prescribed FiO2, shows the patient's PaCO2 worsened :.:: 7mm Hg compared to the original result, or
•A facility-based PSG or home sleep testing (HST)* demonstrates oxygen saturation::;; 88% for:.:: 5 minutes of nocturnal recording (minimumrecording time of 2 hours) thatis notcaused by obstructive upper airway events (AHi < 5).
Covered E0470 is Spirometry being used shows an FEV1/FVC :.::70%

• An ABGs PaCO2, done while awake and breathing the patient's prescribed FiO2, shows the patient's PaCO2 worsens :.::7 mm Hg compared to the ABG result performed to qualify the patient for the E0470 device, or
•A facility-based PSG or HST* demonstrates oxygen saturation ::;; 88% for :.:: 5 minutes of nocturnal recording time (minimum recording time of 2 hours) that is notcaused by obstructive upperairway events (AHi < 5 while using an E0470).

(±)
(±) (±)
400
Establishing and Maintaining a Successful Sleep Disorders Center: Blue Print from an Academic Program
 Ronald D Chervin, MD, MS University of Michigan
Ronald D Chervin, MD, MS University of Michigan
401
Disclosures
Potential conflicts of interest could conceivably arise from:
• NIH – grant funding
• UpToDate – royalties for serving as Co-Editor-in-Chief (Sleep Medicine); Section Editor; Author
• Eli Lilly & Company – to be compensated as consultant through contract with Michigan Medicine
• International Pediatric Sleep Association (IPSA) – unpaid BOD member, Treasurer
• Pajama Program – unpaid advisory board member for this non-profit
Today’s presentation content directly related to these outside interests -- none 402
Learning Objectives
Objective 1
Discuss the tripartiteplus mission of an academic SDC
Objective 2
Examine what fosters success in each mission
Objective 3
Identify what are metrics for success
Source of icons for this presentation: the Noun Project



403
First Sleep Disorders Center

 Dr. Christian Guilleminault
1938 - 2019
Dr. Christian Guilleminault
1938 - 2019
404
Dr. William C. Dement, 1928 - 2020
Third Sleep Disorders Center
Montefiore, 1981

Charles Pollak
John Adler
Elliot Weitzman
Arthur Spielman
Mark Pressman
Charles Czeisler
Janet Zimmerman
Richard Coleman
Source: MJ Thorpy, Sleep Med
405
Why me?

Sleep Res 1981;10:222 406
ABMS Board-Certified Sleep Medicine Physicians
AASM 2016 13 155 245 275 1,502 3,845 6,035 0 2000 4000 6000 Anesthesiology Family Medicine Otolaryngology Pediatrics Psychiatry and Neurology Internal Medicine TOTAL 407
Aldrich Sleep Disorders Center Proposal, April 1985: Preamble by
Michael S Aldrich, MD
“The Sleep Disorders Center (SDC) will be a multidisciplinary center within the University of Michigan administered through the Department of Neurology. Initially, the SDC will be composed of the Sleep Disorders Laboratory and the Department of Neurology Sleep Disorders Outpatient Clinic…
the roles of the Departments of Neurology and Psychiatry are defined in the Sleep Disorders Center Working Agreement. … it is anticipated that other departments will participate in the SDC as it develops.
The purposes of the SDC will be to:
1) diagnose and treat patients with sleep disorders;
2) educate medical students, house officers, University faculty, and referring physicians concerning sleep disorders; and
3) contribute to increased understanding of sleep disorders medicine through research into the causes and treatments of sleep disorders.”
408
Mission Statement – UM SDCs
The mission of the Sleep Disorders Centers is to provide excellence and leadership in sleep medicine patient care, research, education, and service

409
Patient Care
• Unique role of patient care in the tripartite-plus mission -- the “Sid Gilman” postulate
• Ensure access to adequate patient referral base, in context of regional care
• 3 foundational keys to success: 1) people, 2) people, and 3) people
• Faculty
• Team members
• Technical lead and staff
410
• Beginning rather than end
Accreditation


• Team effort
• Resources from AASM




411
• “ROI”
Justifying Establishment and Growth
• Administration of sleep lab services
• Self-managed vs outsourced
• Service line
• Targeted growth: to what size?
412
Referral Base and Education of Colleagues
• Little sleep medicine covered in medical school curricula, rotations, textbooks

• Success of SDC depends on education of referral sources
• Institution, practice group, in-network clinicians
• PCPs, FP, Neurology, ENT, Pediatrics, Psych …
• Dentistry, Procedure Units, PACU
• Community, media
• Relationships with DME companies and vendors
413
What sets
apart from the competition in patient care?
an academic program
• Poor access, high costs, lack of continuity, factory-like setting, loss of focus on patient-centered care, trainees without good supervision? basis for success?
• Faculty
• Well-educated, fellowship-trained, BC/BE
• Evidence-based care / outcomes
• Subspecialized and multidisciplinary clinics
• Research opportunities, field leadership, and national visibility
• Technologist staff, lab services offered, equipment, call center staff, local vs. central oversight, effective integration with other service lines
414
Education
• Originally ABSM, now ACGME-accredited clinical sleep medicine fellowship
• Key mission, highly rewarding
• Magnifies SDC impact
• Improves public health
• Augments national visibility
• Attracts top faculty

415
Clinical Fellow Education – Nuts and Bolts
• Funding models vary, but can be cost-effective
• Depends critically on program director, effective staff, and department support
• Challenges include continual evolution of targets, recruitment of best talent, attracting a diverse applicant pool, funding sources, and optimal balance and alignment between patient care and education
416
Education Across the Board: Faculty, Residents, and Students
• Faculty mentorship, wellness, and satisfaction
• Why stay in academics? The cost-benefit calculation
• Resident experiences with sleep
• Two-week rotations – goals and expectations?
• Student shadowing
• Didactic offerings to medical, dental, graduate, and undergrad students
417
Public Education
• Public Relations or “Institutional Positioning” – another distinguishing feature of academic medical centers • Always try to accommodate – quickly -requests from reputable media sources
• Try to distribute opportunities among faculty: balance expertise, ability, equity, and availability
You can get better sleep with wearables. Just focus on the right data. The Washington Post, April 20, 2023, quoting Dr. Cathy Goldstein 418

Education in Sleep Research

• Within clinical fellowship
• Didactics
• Project / poster
• NIH T32 (2 or 3-year fellowship)
• Neurology; Sleep and Genetics; Behavioral Pediatrics; Pulmonary; Anesthesiology
• Some clinical sleep fellowships started historically as NIH-funded T32 programs
• NIH R25 award for resident or undergraduate research experiences
• Foreign institution, government, or self-funded fellowship for international fellows
419
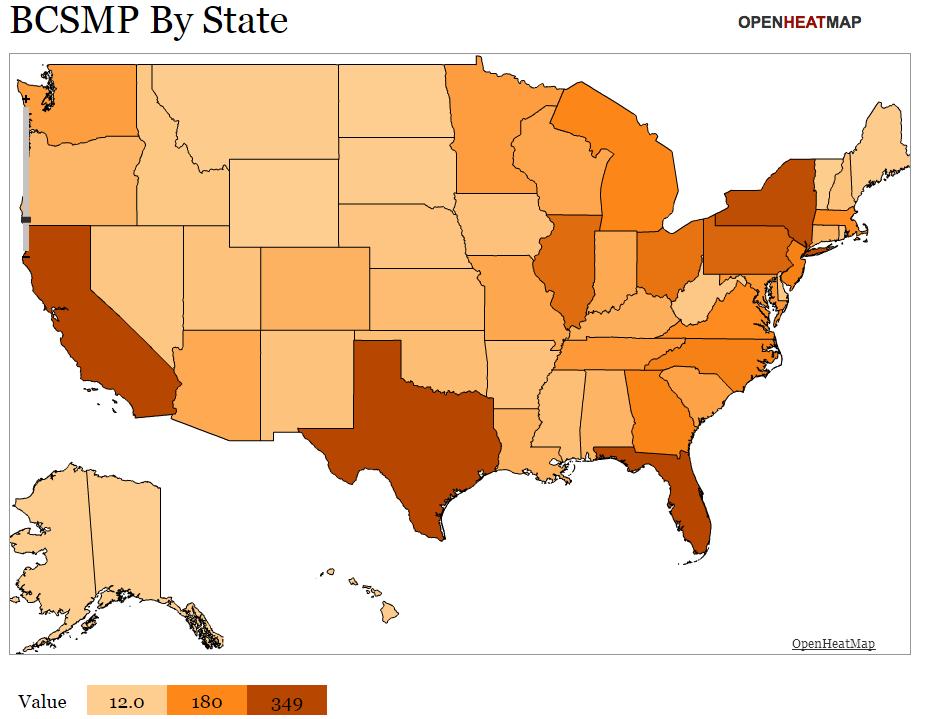
Is the BCSMP Pipeline in Peril?
420

421
Data from NRMP, courtesy of Dr. Michelle Zeidler, SMFDC Chair

422
Data from NRMP, courtesy of Dr. Michelle Zeidler, SMFDC Chair
Is the Investigator Pipeline in Peril?

423
New Individual Fellowship and Career Development Awards
Across NIH (2018 – 2022)

0 2 4 6 8 10 12 14 16 18 20 F31 F32 K01 K08 K23 K24 K99 Number of New Awards Training Mechanism 2018 2019 2020 2021 2022 Total: 44 52 53 52 42 https://report.nih.gov/categorical_spending.aspx ; courtesy of Marishka K. Brown, Ph.D., Director, National Center on Sleep Disorders Research 424
NIH funding

Research


•
425


NIH Funding for Sleep Research
426
https://report.nih.gov/funding/categorical-spending#/
• Central importance of R01 or R33/R61 awards
• K awards – Career Development Awards – key building blocks for research careers
• T32, R25, and K12 awards to institution; candidates apply for slots locally

• R21 and R03 awards for smaller projects
• SBIR and STTR awards for academic / industry partnerships
• Program Project Grants (P01, P30, P50)
• U01 awards – Research Project Cooperative Agreement -- typically for large multicenter projects
427
•
Research – Beyond NIH





Department of Defense (DoD)

•
Foundations

• PCORI
428
Industry-Sponsored Sleep Research
• Create opportunities for your patients, add variety to your work week, keep sleep lab busy, build revenue, secure academic opportunity … but none is guaranteed
• Negotiate for your aims up-front
• Take care not to plan, or later achieve, a negative budget!
• Consider applying for investigator-initiated trials, or industry assistance with NIH or foundation-supported research
429
Logo 1 Logo 2 Logo 3 Logo 4 Logo 5
Research – Five keys to Success
• Multidisciplinary collaborations

• Lure investigators in other fields into yours, with opportunities for them, and you
• Full range of preclinical, translational, clinical, and human research – if possible
• Mentorship and research training
• Identify, recruit, train, and retain an effective core of sleep research coordinators
• Communicate important findings to public, institution, and colleagues

430
Service
• Institutional, local, regional, national, international


• Apply early and often

• Public vs professional vs governmental
• Align with clinical, education, or research interests




• Seek opportunities for field leadership

431
DEI and Welcoming Environment

https://medicine.umich.edu/dept/neurology/about-us/ourcommitment-diversity-equity-inclusion




432
In
case
Michael S Aldrich, MD, Collegiate Professorship in Sleep Medicine
Gene and Tubie Gilmore Fund for Sleep Research and Education
Malhotra Academic Sleep Medicine Endowment

Johnathan A Covault Memorial Foundation for Sleep Disorders Research
Bequests: Yvonne Greull, John N. Casella, and Gene Gilmore
Philanthropy
Generous contributions of alumni -- Go Blue! 433
you feel inspired by Championship Updates –Allison Clark Associate Director for Development ajmayer@umich.edu 734-763-1638
Administrative Structure for a Successful SDC
• System should incorporate traditional department support, and transcend temptations and limitations of department silos
• Administrative structure must support central “city planning” for sleep medicine: department/division vs center structures

• Technical fees and grant indirects
• Constant need to “make the case for sleep” – share vision of what can be attained
SLEEP 2013;36(6):795-801 SLEEP 2013;36(6):803-811 434
Administration of SDCs: Highly Variable


435
Lessons Learned #1
• Inherent multidisciplinary nature of sleep means collaborations are key
• Within Sleep Disorders Center
• Around SDC -- many departments
• Several overlapping, highly relevant administrative structures

436
• Engagement
Lessons Learned #2
• Distinguish experience from practice outside academia
• “The team, the team, the team”
• Find and help develop each person’s unique strengths
• Combined contributions key to success of program
• Hail Sleep Tailgate

437
Lessons Learned #3
• Asking for support to grow – Department, Faculty Group Practice, or Grant Agency
• Always be sure to supply vision of what support will secure or enable
• Strategy for positive margin – at least neutral – is critical; show feasibility
• Highlight improved care, safety, grant competitiveness, or other compelling advantage
• Consider in-house vs. outsource, e.g. for technical operations, data entry, etc.
438
Lessons Learned #4
• Work/life balance vs burnout
• Constant effort to ensure faculty, staff, learner satisfaction and wellness
• Academics must prove different – in unique, rewarding ways – to remain attractive
• Good communication is important, with balance between virtual and F2F

439
What are Metrics for “Success” at Your Academic Sleep Center?
• Clinical outcomes? Measured how?
• Reputation? Measured how?
• Grants and publications?
• Productivity, compensation, and margin?
• People – recruitment and retention of top talent in all categories
• Exciting place to work at, day in and day out

440
Future Changes and Opportunities for Academic Sleep Disorders Centers
• Rapid transformation of the field (Academic and Non-Academic)
• Population health issues in sleep remain to be addressed
• AI
• Wearables
• Race to less complicated, expensive, precise, and comprehensive assessment
• Less reimbursed cost per unit of service
• Improved value proposition for patients, insurance, and purchasers of health care
• Demonstrable outcomes
441
Future Changes and Opportunities for Academic Sleep Disorders Centers
• Opportunities for Academic Sleep Disorders Centers
• Telemedicine
• Partnerships with sleep-savvy PCPs and other non-sleep specialists
• Effective training, expansion, and integration of sleep caregiving teams
• Lead clinical advances in:
• Patient-centered care
• PAP adherence
• Alternatives to CPAP

442
Future Changes and Opportunities for Academic Sleep Disorders Centers
• Address plethora of central, unanswered research questions in sleep medicine
• Partner more effectively with industry, at early stages of innovation
• Improve impact on the public health burden of sleep disorders
• Demonstrate impact and value
443
Future Changes and Opportunities for Academic Sleep Disorders Centers
• Lead efforts to educate public
• Make compelling argument for education about sleep health, starting at pre-school
• Ensure strong pipeline of clinicians, educators, and investigators into sleep medicine
• Secure a seat at the table for sleep during all phases of public health planning
https://pajamaprogram.org/our-programs/

444
One Final Opportunity
Make sure everyone remembers who are the leaders and the best!

445

 Geoffrey Gerstner, DDS, MS, PhD, D.ABDSM Associate Professor
Geoffrey Gerstner, DDS, MS, PhD, D.ABDSM Associate Professor
446
University of Michigan School of Dentistry
Conflict of interest disclosure for speakers
• Akervall Technologies Inc (consultant/advisory role)
• Relationship is not relevant to content discussed today

Mini-Residency in Dental Sleep Medicine
447
Topics
• Oral appliance therapy (OAT)
– Alternative to PAP
– Improving PAP compliance
– Other OAT-combination therapies
• Thoughts on standards for practice
• Collaboration and consensus
–
What constitutes treatment success
–
What are the treatment goals and outcomes
– When and how is treatment modified
• Brief OAT Overview
– Indications and contraindications
– Expectations and limitations to treatments
–
The role of collaboration
448
Oral
An alterative to PAP • Improving PAP compliance • Other combination therapies 449
appliance therapy •
Comparison of PAP & OAT

• Compliance vs efficacy

Sutherland, et al. Efficacy versus effectiveness in treatment of obstructive sleep apnea: CPAP and oral appliances. JDSM 2015;2(4),175-181 450
Comparison of PAP & OAT: SARAH Index
AHITreatment = 4.7 HoursTreatment = 7.6 hr (95%TST)



451
Strategies to improve compliance and efficacy

J, Oral Appliance Therapy; the definition of effectiveness. CE Published 3.18.21, accessed 8.21.23.
(used with permission) 452
Viviano
https://dentalsleeppractice.com/ce-articles/oral-appliance-therapy-the-definition-of-effectiveness/
Combo vs CPAP or MAD
• N = 10 subjects
• PAP intolerant, using MAD
• 1 wk washout off MAD
• APAP + MAD for 3 nights
• Optimal PAP pressure reduced; 9.4(2.3) to 7.3(1.4), P<0.001
• Residual AHI on MAD decreased; 11.2(3.9) to 3.4(1.5), P<0.001
• N SpO2 desats <90% reduced MAD v Combo (P<0.001), PAP v Combo (NS)
• ESS score reduced from 12.7(2.1) to 7.5(4.1) P=0.007
El-Solh, Ali A. et al. Combined oral appliance and positive airway pressure therapy for obstructive sleep apnea: a pilot study. Sleep Breath (2011) 15:203-208
453
Combo vs CPAP or MAD
Liu H-W, et al. (2017) Combining MAD and CPAP as an effective strategy for treating patients with severe sleep apnea intolerant to high-pressure PAP and unresponsive to MAD. PLoS ONE

2017 12(10):e0187032

* * * * * 454

Options available with combo therapy Viviano, Oral Appliance Therapy; the definition of effectiveness. CE Published 3.18.21, accessed 8.21.23. https://dentalsleeppractice.com/ce-articles/oral-appliance-therapy-the-definition-of-effectiveness/ (used with permission) 455
Hybrid
CPAP + OAT




456
Inspire - MAD combo, case report
•
PSG data Pre-tx HNS only OAT only HNS + OAT AHI 43.7 11.6 29.2 2.1 Lee,
OSA
HNS
457
et al. Severe
treated with combination
and OAT. JDSM 2015;2:185-186
Positional therapy



•
Djeltjens, M, et al. Sleep Breath 2015; 19: 637-644 458
SPT: sleep position trainer
Take home message
• OAT is not merely a stand-alone alternative to PAP
• It improves PAP compliance
• Its effectiveness (compliance x efficacy) and impact on co-morbid conditions are equal to PAP
• Its efficacy is maximized in combination with other therapies
• It maximizes the efficacy of other therapies
• How does the above knowledge impact standards for practice?
459
Standards for Practice
• Dentists in screening
–
As part of routine dental examinations, dentists can recognize a small upper airway and other anatomic risk factors for OSA, and use the opportunity to identify potential patients through use of simple screening questions and/or questionnaires.
• Dentists in treatment
– Choosing the proper OAT appliance, adjusting the OAT appliance and assessing the patient for adverse effects
Quan SF, Schmidt-Nowara W. The Role of Dentists in the Diagnosis and Treatment of Obstructive Sleep Apnea: Consensus and Controversy. J Clin Sleep Med. 2017;13(10):1117–1119.
460
Access to Care
• 18,000,000 - 24,000,000 undiagnosed OSA sufferers
• Number of dentists in USA: ~200,000 (90-120/DDS; many of these would already be dental patients)
• Sleep clinics in USA: ~2500 (7,200 - 9,600 / clinic; most if not all of these would be new patients to the sleep clinics)
• Sleep physicians in USA: ~7500 and decreasing (2,400 - 3,200 / physician; most if not all of these would be new sleep physician patients)
461
• Effective communication among physicians and dentists is essential to comprehensive, coordinated care.
• In 2010, the Joint Commission identified poor communication as the most commonly cited root cause of sentinel events.1
• AASM: “Effective communication between the sleep physician, the patient, the PCMH PCP, and other physicians and health care entities is essential for optimal care delivery.”2
1. Joint Commission on Accreditation of Healthcare Organizations. The Sentinel Event Data Root Causes by Event Type, 2004-Fourth Quarter 2010.
2. Strollo PJ; Badr MS; Coppola MP; Fleishman SA; Jacobowitz O; Kushida CA. The future of sleep medicine. SLEEP 2011;34(12):1613-1619
Communication
462
Levine, et al. JDSM 2022
• Collaborative care between dentists and physicians is key in treating patients with SRBDs
• Patients should be screened for OSA using validated methods
• A Qualified Dentist should complete a comprehensive DSM exam
• OSA diagnosis is completed by a sleep medicine physician
• OAT should be prescribed as first-line therapy for primary snoring and may be prescribed as first-line therapy for OSA
• Patients should be educated on medical conditions associated with OSA
• After OAT, the patient should be referred to the medical provider to verify treatment efficacy
463

Levine, et al. JDSM 2022 464
Screening patients in dental operatories


Levine et al. 2022 465
Education in screening


• UM Dental students

–
~13 hr Didactics—sleep physiology and pathophysiology, PSG, OSA
–
~ 3 - 6 hr Clinical—screenings on each other, but not on patients
–

~ 6 hr SP that vary in medical hx and clinical findings. Assessed based on decision to refer or not refer and rational for decision

466
Education in referring
• Students have little exposure to clinical sleep medicine
• UMSOD does not use Epic
• Graduating students learn to screen, but not how to refer – If students did refer patients suspected of having OSA
– Where are ~120 - 240 pts / yr going to be referred?
–
Assume a prevalence rate of 16%, 1,760 OSA patients in the pre-doc clinics
467
Screening patients, reality


Levine et al. 2022 * 468
HSATS and screening


Levine et al. 2022 * 469
ADA Statement on Screening, 2021
“Order and administrating” HSAT within dentist’s scope of practice law or regulation
What would make this acceptable in the medical profession?
Assigned reading: ADA House of Delegates. Policy Statement on the Role of Dentistry in the Treatment of Sleep Related Breathing Disorders (Trans.2017:269; 2019:270; 2021;92)
•
•
470
Communicating regarding HSATs
• Clarify that oximetry/HSAT you use is a tool to measure one objective element of treatment efficacy.
• Clarify that you are not making a diagnosis.
• Note that any objective measurement you make is not in place of any confirmatory testing that physicians may order later.
• Indicate that you follow AADSM and AASM Standards, including being skilled in recognizing medical contraindications for HSAT use.
• Reassure that all patients are sent back to referring physician for any further diagnostic testing (usually HSAT or PSG).
• And, if a dentist orders an HSAT and the test comes back negative, the result should ALWAYS be forwarded to the physician.
471
Education
• Dentists: – Currently, little additional training required to practice DSM
– Define necessary and sufficient training for scope of practice
–
Precedents: dental specialties; OFP specialty and the wild west of TMD
–
UM—105 hr CE Mini-residency
– Accredited by the AADSM
–
Team taught by medical and dental faculty
– Achieve qualified dentist status, opportunity to take ABDSM board
–
OFP residents will take this course while in residency (OFP accreditation requires DSM exposure)
–
–
Is this sufficient?
Future: AOFP, AADSM, AASM, ADA, AMA
472
OAT titration needs objectivity for efficiency







Levine et al. 2022 * * * 473
Referrals & Letters
Lost in system [Dr Staff/Epic/Axium Dr]
Lost to FU [Dr Pt Dr]
Appointment Scheduling [Pt Staff]



Insurance [DDS Staff PCP Insurance]
Insurance denials [Insurance Staff ?] *
Levine et al. 2022
474
* * * * * * *
Communicating With Referring Physicians
Receipt of LoMN/Rx & start of tx
Letter to Sleep Physician
Exam/records
OA Delivery
Calibration
OA at presumed MMI
MD approves
Long-Term Management
Letter to Sleep Physician
Letter to Sleep Physician
Letter to Sleep Physician
(repeat q6mo/yearly)
475
Improving collaboration
• Residents:
–
OFP-Progressive clinical training in OAT, rotations to SM
SM-Rotations to OFP – Addresses some collaboration challenges
–
• Dental faculty
– Time in sleep medicine
– Retreats
–
Face-to-face time
476
Oral Appliance Therapy


*At what point should combo tx be considered

Levine et al. 2022
? * * * ? ? 477
Goal of OAT
• Improvement of signs, symptoms & objective indices
•Determination of improvement is agreed upon by patient, dentist and medical provider using
•Clinical experience
•When available, evidence based approaches
• Appliance can be used comfortably on a nightly basis
Sheats R, Essick G, Grosdidier J, Katz S, Kim C, Levine M, Patel I. Identifying the appropriate therapeutic position of an oral appliance. J Dent Sleep Med. 2020;7(4).
478
Realities of OAT
• Neither the patient nor the sleep physician should be expecting a complete relief from OSA with OAT
• An AHI < 5, snoring resolution, and 8 hours of perfect sleep are not necessarily feasible goals
• Therefore, it is important to set reasonable goals of care with patient and their physician from the start.
479
MRI Sagittal View: Patterns of Motion

33%-Movement en bloc, lower AHI
27%-Anterior movement at base of tongue
40%-Elongation, limited anterior movement
Brown EC, et al. Sleep. 2013 Mar 1;36(3):397-404.480
P: Pcrit –
CollapsibilityHIGH
M
A: Arousal
Threshold - LOW
L: Loop GainHIGH
M: Muscle Response –Ineffective - POOR
Lai V, Carberry JC, Eckert DJ. Sleep Apnea Phenotyping: Implications for Dental Sleep Medicine. J Dent Sleep Med. 2019;6(2)

P
A L
OSA 481
Literature-based definitions of successful management
• An AHI/RDI/REI reduction of more than 50% from baseline
• A final AHI/RDI/REI of 5 (or 10) events/hr
• ESS score decrease of at least 2 points (implies clinically significant reduction in sleepiness)
Ramar K, Dort LC, Katz SG, Lettieri CJ, Harrod CG, Thomas SM, Chervin RD. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. Journal of Dental Sleep Medicine 2015;2(3):71– 125.
482
Patient-Centered Definitions
• Patient (and bed partner) goals of care should be met
• OAT comfortable enough to use nightly
• QoL , chief complaints and symptoms controlled
• This information should be shared with the sleep physician
483
Consider non-custom appliances
• Temporary device to predict patient compliance, efficacy, position

• Interim device if custom is lost, damaged




484
Non-custom appliance



Levine et al. 2022 * 485
Caveat




486
~ 17 mm average
Oropharyngeal Geometry




487
Implications
• TAP PAP without mandibular component increases VD without protrusion


• Non-custom appliances are thicker than custom appliances
• Bite splints for sleep bruxism increase VD without protrusion

Non-custom MAD
Bite splint
TAP PAP
488
Maxillomandibular relationships can impact MAD selection

“Excessive” curve of Spee
Extension distal to the red line requires increase in vertical dimension (VD)
•
•
489
• Indicate clearly to sleep physician that OAT results have fallen short of goal (e.g. AHI/REI remains elevated)
• Delineate what has been done in attempt to attain that goal
–
Patient counseling
–
Device adjustment
–
Device change
• Specify that the patient requires medical follow-up for consideration of other treatment options (which could be in addition to OAT or instead of OAT)
–
Is this the time to consider combo/hybrid?
When a “goal” is not attained, the dentist should…
490
Take home message
• What constitutes tx success on a case-by-case basis?
• What are the treatment goals?
• When and how should treatment change when goals aren’t met?
• SOP flowchart; consider how combo and hybrid therapies could impact flow
• The cautious use of HSAT to decrease burden on sleep clinics without increasing harm of patients
491
Oral Appliance Therapy



Levine et al. 2022 492
Oral Appliance Therapy
Unattached: Bilateral
Interlocking
Bilateral

Midline Traction
Compression
Attached:
Attached: Bilateral
Attached:
Traction
Essick et al. Part III Oral Appliance Therapy for Sleep-Disordered Breathing, Inside Dental Technology, September 2016 493
Attached midline traction






VD 494
Hybrid OAT-PAP designed into the attached midline traction; otherwise must be customized




495
Attached bilateral compression



496
Unattached bilateral interlocking “Dorsals”








VD 497
Bilateral traction




498
Decision making process
• Insurance restrictions
• Exclude most problematic components or design elements for the patient
– Allergies, buccal corridor space, tongue space or habits, missing teeth, malocclusions, arch 3-D shape
• Include most beneficial features for the patient
– Ease of use, force vectors, patient input
• Add custom design features
499
Custom design features
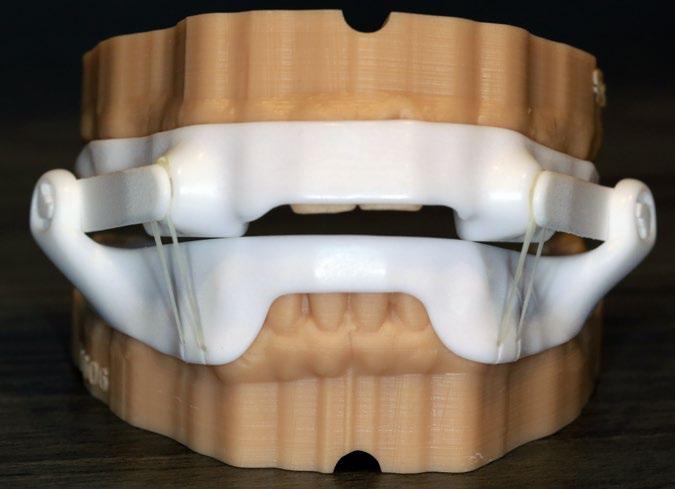



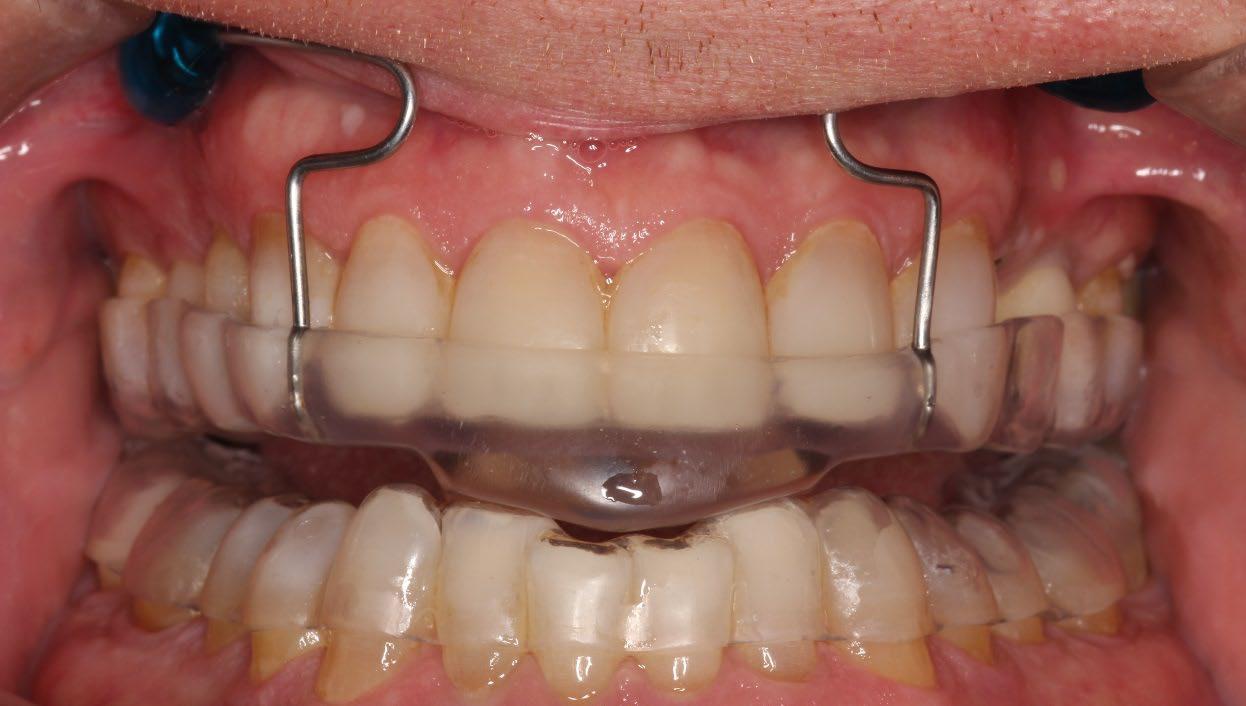

500
Reasons for Discontinuing OA

* * * * * *
501
*
Morning Occlusal Guide (MOG), AM




Positioner, AM Reprogrammer
502
State of the field
• New manufacturing methods
• Very few studies have compared devices for efficacy, compliance, side effects
• Objective compliance with embedded sensors
• Efficacy
503
What Makes It Work?
• Efficacy in eliminating sleep-disordered breathing in the individual patient lies in the amount of jaw advancement produced.
Aarab G, Lobbezoo F, Hamburger HL, Naeije M. Effects of an oral appliance with different mandibular protrusion positions at a constant vertical dimension on obstructive sleep apnea. Clin Oral Investig. 2010 Jun;14(3):339-45..

504
The Pterygomandibular Raphe
Sagittal diagram demonstrates the pterygomandibular raphe (arrows) extending from the hamulus of the medial pterygoid plate to the mandibular mylohyoid ridge ---Shimada, Anat Rec.

1989
• Aponeurotic white fibrous fascia that connects muscles with other muscles or bone
• Absent in 36% of adults
• Present in fetuses
Brown EC, et al. Tongue and lateral upper airway movement with mandibular advancement. Sleep. 2013 Mar 1;36(3):397-404.
505
of enlargement in lateral
planes” Kyung SH, Park YC, Pae EK. Obstructive sleep apnea patients with the oral appliance experience pharyngeal size and shape changes in three dimensions. Angle Orthod. 2005 Jan;75(1):15-22 506
“Greater degree
than sagittal

507
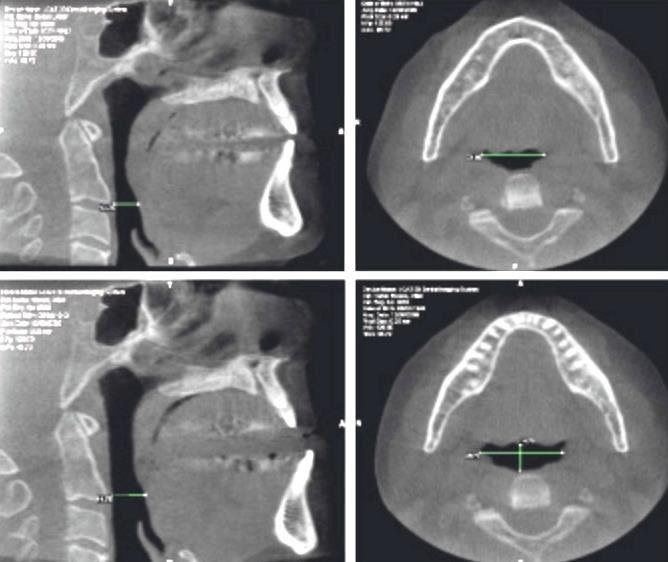
Does Airway Geometry of OSA Patients Affect Efficacy?
• No features are predictive of OAT success
• A multi-factorial disease
Cunha,etal, Braz. OralRes.2017 508
Take home message
• Various device designs and options increase the chances a referral will be a candidate for OAT
• The device may work in a number of different ways, i.e., opening the pharynx laterally, moving the tongue forward, opening the nasal airway, allowing / disallowing mouth breathing.
• Side effects, including tooth movement, development of myogenous or arthogenous pain, etc.. Most can be effectively treated if provider is vigilant and has regular communication with the patient.
509
Thank You


510



 Fauziya Hassan MD, MS Associate Professor, Service Chief
Fauziya Hassan MD, MS Associate Professor, Service Chief









































































 Ronald D Chervin, MD, MS University of Michigan
Ronald D Chervin, MD, MS University of Michigan



 Dr. Christian Guilleminault
1938 - 2019
Dr. Christian Guilleminault
1938 - 2019

























































 Geoffrey Gerstner, DDS, MS, PhD, D.ABDSM Associate Professor
Geoffrey Gerstner, DDS, MS, PhD, D.ABDSM Associate Professor

























































































