
13 minute read
MAKING SENSE OF THE MACULA
from OT April/May 2023
by TheAOP
IN BRIEF This article reviews the anatomy and physiology of the normal macula and examines the presentation and management of four common macular conditions.
Introduction
Advertisement
The number of UK residents over the age of 75 is predicted to rise from 4.9 million in 2010 to 8.9 million in 2035.1 An inevitable consequence of this is a subsequent rise in the number of patients with ophthalmic disease. It is predicted that there will be a 64% rise in cases of neovascular age-related macular degeneration (nAMD) between 2015 and 2035.2 With an increasing number of practices using optical coherence tomography (OCT) as part of an eye examination, practitioners are now able to identify, and potentially diagnose, conditions at an earlier disease stage as well as make a more accurate assessment of disease severity. When considering macular disease, it is helpful to recall the anatomy and physiology of this region of the retina. The key players in the normal functioning of a healthy macula are the neurosensory retina itself, the retinal pigment epithelium (RPE), Bruch’s membrane and the choriocapillaris. The macula is the 5.5mm central region of the retina. The fovea is a depression of the central 1.5mm of the macula, composed purely of cone photoreceptor cells. Photoreceptor cells convert photons of light into electrical impulses; however, this comes at a cost. To function properly, these cells need constant regeneration of the outer segment and subsequently have an enormous metabolic demand. In fact, photoreceptor cells have the highest level of oxygen consumption per gram of any cell type in the body.3
The RPE is a single layer of hexagonal cells found between the neurosensory retina and the choroid. RPE cells are known as ‘phagocytic cells’; this means that they ingest waste material and dead or dying cells to support the continuous renewal of the outer segment of photoreceptors.4 The inner side of the RPE is made up of microvilli which interdigitate with the outer segment of photoreceptors, while the basal side of these cells form strong attachments to the underlying choriocapillaris, forming the innermost layer of Bruch’s membrane. The RPE is only weakly attached to the neurosensory retina and the potential space between the two structures is the subretinal space.5 The RPE and the surrounding tight junctional complexes form the blood-retinal barrier. This barrier prevents extracellular fluid leaking into the subretinal space from the choriocapillaris and also works to actively pump ions and water out of the subretinal space.4
Bruch’s membrane separates the RPE from the choriocapillaris. It is used to transport metabolic waste from the retina and is also thought to play an important role in suppressing choroidal neovascularisation.6
The choriocapillaris is the innermost structure of the choroid. Its role is to supply the outer retina with metabolites and remove waste products; this is particularly important at the macula, where there is no inner retina circulation. Subsequently, the density and width of the vessels of the choroid are greatest in the area beneath the macula. Figure 1 shows a schematic of the outer retinal layer, the RPE, Bruch’s membrane, and the choriocapillaris.
There are a number of conditions that can cause a change to the structure of the macula, the most common of which is age- related macular degeneration (AMD). Patients with acute macular disease will often present with sudden unilateral painless loss or change to their vision. This article will consider four common macular conditions that may present in primary care, outlining their presentation and management.

AGE-RELATED MACULAR DEGENERATION
As we age there are a number of ocular changes that occur, which make the eye more susceptible to AMD. There are decreases in choroidal thickness, circulation within the choroid and choriocapillaris density.3 Changes to collagen and proteins in Bruch’s membrane cause it to increase in density, and as a result, it becomes more resistant to the passage of waste materials.7 The RPE has reduced phagocytosis of waste materials and RPE density decreases with age.8 The consequence of these ageing changes is the formation of drusen between the RPE and Bruch’s membrane. The exact composition and mechanics behind the formation of drusen is unclear but it is known that they are deposits of biomaterials under the RPE.8 Although the presence of small drusen (<63μm in diameter) in the absence of pigmentary changes are considered a normal ageing change, they are a risk factor for the development of AMD.9 Drusen are rare prior to the age of 40 years but commonly found in patients over 70 years. Table 1 (see page 69) shows a classification system for AMD.9 Risk factors for the development of AMD include:
• Age (three-fold risk in over 70s)
• Family history of AMD
• Caucasian
• Light irides
• Genetics
• Smoking
• Obesity
• Hypertension
• Poor diet
• Lack of exercise.
Dry AMD is the most common type of the condition accounting for 85 to 90% of cases.10 It presents as a gradual loss of vision, often affecting both eyes, although one eye may be worse than the other. Vision may fluctuate but is often better in bright light. Fundus examination will show visible drusen. OCT can confirm the presence of drusen, increased hyper-reflectivity, and in advanced cases, reverse shadowing caused by RPE atrophy and photoreceptor cell death. Unfortunately, there is currently no treatment for dry AMD, but patients should be counselled on appropriate advice for reducing their risk of the progression of AMD, and if appropriate, a referral to low vision services or signposting to an eye clinic liaison officer (ECLO) may be helpful. nAMD, otherwise known as wet AMD, occurs as a result of choroidal neovascularisation (CNV). Vascular endothelial growth factor (VEGF) is one substance among many enzymes and growth factors that promotes the formation of new blood vessels within the macula. VEGF is upregulated in response to ischaemia, hypoxia, inflammation and trauma.11 With regards to nAMD, it is thought that the accumulation of oxidised lipids in Bruch’s membrane and ischaemia caused by the atrophy of the choriocapillaris, drive the upregulation of VEGF leading to the formation of CNV.11 These new vessels penetrate through Bruch’s membrane and proliferate between Bruch’s membrane and the RPE or in the subretinal space. They are fragile and can leak proteins and lipids or haemorrhage. Patients with nAMD will typically present with a sudden onset of reduced vision in the affected eye. They may be aware of metamorphopsia or a scotoma but might only offer this information when asked directly. A fundus examination will often show a visible subretinal or intraretinal haemorrhage (see Figure 2) and/or exudates. However, it is worth being aware that a CNV complex may exist and the patient could have symptoms without an obvious haemorrhage.

If assessing a patient presenting with a marked reduction in visual acuity and it is difficult to get an adequate view of the macula, then it is worth dilating them. With a dilated, stereoscopic view, CNV may be visible as a grey-green submacular lesion. OCT is an invaluable tool for assessing patients with macular disease. The presence of subretinal fluid (SRF), intraretinal fluid (IRF) or a pigment epithelial detachment (PED) in the presence of acute symptoms is often enough to form a likely diagnosis of nAMD.
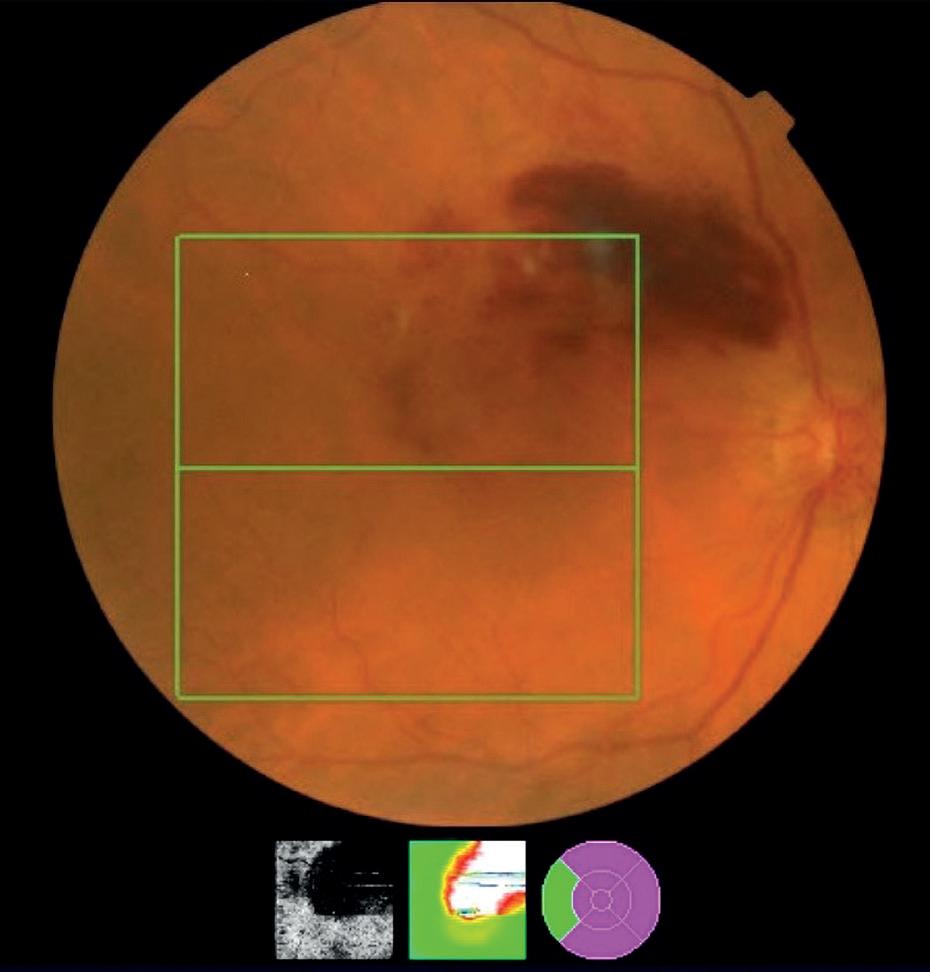

Although nAMD usually presents as an acute loss of vision, it is surprising how many patients can be unaware of unilateral vision loss that is only detected during a vision check at the start of a routine sight test. A marked drop in vision should always be treated with suspicion and a dilated fundus examination is recommended.
Patients presenting with nAMD require urgent referral to secondary care. Most regions have a rapid access referral pathway in place, so practitioners should be familiar with arrangements in their local area. Patients are typically treated with anti-VEGF intravitreal injections. Traditionally this was with Lucentis (ranibizumab) or Eylea (aflibercept); however, in 2020 the UK Court of Appeal ruled that Avastin (bevacizumab) could be used off label to treat nAMD and in circumstances where patients would not otherwise meet the treatment criteria (if vision is better than 6/12 or worse than 6/96).12 The National Institute for Health and Care Excellence (NICE) guideline 82 (NG82) provides a comprehensive overview of the treatment of nAMD.13 Vabysmo (faricimab) is another anti-VEGF injection that was approved by NICE for the treatment of nAMD in June 2022. In addition to targeting anti-VEGF, it also targets the angiopoietin-2 pathway.14
It is worth discussing modifiable risk factors with patients, including smoking cessation and the benefits of a healthy diet and lifestyle. The Royal College of Ophthalmologists advise a healthy diet rich in fresh fruit, vegetables, eggs and oily fish.15 Dietary supplements following the age-related eye disease study (AREDS) 2 formulation may reduce the five-year risk of developing late AMD by up to 25%; however, NICE guidelines acknowledge the limitations of the AREDS 2 study design, and highlight that further research is required to assess the effect of AREDS 2 on disease progression.15
It is also important to explain self-monitoring to those with AMD. Practitioners should advise patients with AMD to report any new symptoms or changes of a blurred or grey patch in their vision, distortion, or objects appearing a different size to normal. Providing patients with an Amsler chart is a cheap, simple and effective way to allow them to monitor their vision.
Adult Vitelliform Dystrophy
Adult vitelliform dystrophy is a type of pattern dystrophy, a genetic disease, that may be discovered by chance during a routine sight test. The patient may present with a gradual reduction in their central vision or can be asymptomatic. It typically occurs in patients over 40 years of age. Clinical signs include a symmetrical, round, yellow deposit visible on ophthalmoscopic examination in one or both eyes. The appearance is similar to Best disease, but the lesion is typically smaller and presents later in life. An OCT examination may show subretinal material and atrophy in the area of the lesion (see Figure 3).
There is currently no treatment for adult vitelliform dystrophy. Although its appearance on an OCT can look alarming, adult vitelliform lesions without CNV do not necessarily require referral to secondary care. NG82 classifies a vitelliform lesion without significant visual loss (best corrected visual acuity better than 6/18) as early AMD at high risk of progression and it should be managed as such.15 If the visual acuity is 6/18 or worse, NG82 classifies the condition as late AMD (dry). Visual acuity is often preserved or only mildly affected despite the presence of vitelliform material in the subretinal space.16 However, as there is an increased risk that the patient will develop CNV in their lifetime, these patients should be monitored in primary care on an annual basis and given an Amsler chart to monitor for any change to vision or onset of distortion. If the patient does go on to develop CNV they should be referred to secondary care for the commencement of anti-VEGF treatment.
CENTRAL SEROUS CHORIORETINOPATHY
Central serous chorioretinopathy (CSCR) is a condition predominantly affecting men of working age and is characterised by a localised serous detachment of the sensory retina and the presence of subretinal fluid. It can be acute – typically resolving within three to six months – or chronic. The pathophysiology of this condition is poorly understood. It is thought that in CSCR, hyperpermeability of the choroid causes ischaemia and increased hydrostatic pressure. This in turn leads to damage of the RPE and the breakdown of the normal function of the blood-retinal barrier, leading to the accumulation of fluid in the sub-retinal space.17
Patients with CSCR may present with unilateral blurred vision, a reduction in contrast sensitivity and colour vision, metamorphopsia and a hyperopic refractive shift. Often, they will complain of an awareness of a scotoma and may compare it to a feeling of having looked at a light for too long. Vision may only be slightly reduced in spite of the presence of SRF, with studies finding an average best corrected logMAR visual acuity of approximately 0.20 (Snellen 6/10).18,19 Dilated fundus examination may show a round elevated clear lesion; however, often there may be no visible sign of CSCR on a fundus image. OCT will show an elevated dome where the sensory retina has detached from the RPE. Risk factors for the development of the condition include:
• Male
• Between fourth and sixth decades of life
• ‘Type A’ personality and/or psychological stress –possibly causing elevated corticosteroids
• Pregnancy
• Sleep apnoea
• Systemic corticosteroids
• Exogenous testosterone exposure.
There is a known relationship between the use of glucocorticoid steroids (for example, prednisolone, betamethasone, dexamethasone) and the presence of CSCR. Cortisol, a naturally occurring steroid hormone, can be elevated in response to high stress and during pregnancy. It is thought that the elevated level of cortisol may be the trigger for the development of CSCR in pregnancy.20 A detailed history is essential. It can be helpful to ask specifically about steroid
Ischaemic Non-ischaemic
Visual acuity (VA) worse than 6/60
Relative afferent pupillary defect (RAPD)
Multiple dark deep intraretinal haemorrhages
Multiple cotton wool spots
Macular oedema
Severe venous tortuosity
Marked optic disc swelling
New vessels elsewhere
New vessels at disc
New vessels at iris (usually after two to four months) inhalers, nasal sprays or topical creams as many patients might not think of these as medicine and may forget to mention them when asked about medication. Consider the patient’s overall demeanour and their occupation. Enquiring about their general stress levels can often lead to patients reporting that they have had an abnormal amount of recent stress and/or anxiety.
In the vast majority of cases, CSCR is self-limiting and will resolve without treatment within four months. In a small percentage of cases, SRF and serous retinal detachment persists. CSCR is chronic if it has not resolved after four to six months. Chronic CSCR may be treated with photodynamic therapy (PDT).
The College of Optometrists’ guidelines recommend referring central serous retinopathy urgently;21 however, some regions may have local schemes in place. For example, Suffolk LOC recommend urgent referral if the optometrist is unsure about the diagnosis, particularly if they do not have access to an OCT, or cannot rule out other causes, for example nAMD. However, if the optometrist is confident in their diagnosis, Suffolk LOC recommend routine referral alongside advice to the patient should their condition fail to improve, or worsen, and the patient should be given an Amsler chart for self-monitoring.22 Practitioners can check if there are guidelines within their local area. Differential diagnosis of CSCR includes: CNV, AMD, polypoidal choroidal vasculopathy, hypertensive choroidopathy and optic pit maculopathy.
Retinal Vein Occlusion
Retinal vein occlusion (RVO) is the second most common retinal vascular disease after diabetic retinopathy23 and occurs as a result of obstruction of
VA 6/30-6/60
No RAPD or mild RAPD
Mild to moderate dot, blot and flame haemorrhages
Mild cotton wool spots
Mild venous dilation and tortuosity
Mild macular oedema the venous system due to thrombus formation. The thrombus formation associated with the development of a retinal vein occlusion is often as a result of atherosclerosis (where arteries develop plaque). The most common associations of RVO can, therefore, be defined as risk factors for atherosclerosis.24 Other systemic considerations include conditions that cause hyperviscosity or affect blood flow through retinal veins. Risk factors for RVO include:25
• Age
• Hypertension
• Hyperlipidaemia
• Hyperviscosity syndromes
• Blood dyscrasias
• Inflammatory vasculitis, for example, systemic lupus erythematosus
• Open-angle glaucoma
• Ischaemic optic neuropathy
• Optic nerve head changes including disc drusen and tilted optic nerve heads
• Some medications including oral contraceptives and diuretics.
RVO is classified according to where the occlusion occurred. In a central retinal vein occlusion (CRVO), the occlusion typically occurs posterior to the lamina cribrosa. In a hemiretinal vein occlusion, the occlusion occurs in a hemicentral vein affecting the entire superior or inferior retinal hemisphere. In a branch retinal vein occlusion (BRVO), the occlusion typically occurs at an artero-venous crossing.
Retinal vein occlusions have a distinctive appearance (see Figure 4). In a central retinal vein occlusion there will be extensive, widespread dot, blot and flame haemorrhages in all four quadrants of the retina (‘blood and thunder appearance’), there will also be cotton wool spots, dilation and tortuosity of retinal vessels, along with possible disc and macular oedema. Patients may go on to form collateral vessels at the disc. In a hemiretinal vein occlusion, this will affect either the superior or inferior retina, respecting the horizontal demarcation. In a BRVO, signs will occur in the affected venous segment, often in a wedgeshaped pattern.
The most common cause of visual loss as a result of a vein occlusion is macular oedema.25 When an occlusion occurs, increased vascular pressure can cause failure of the capillary endothelium blood-retinal barrier, causing fluid and small molecules to leak across the vascular wall resulting in oedema. This oedema can then lead to the presence of inflammatory mediators including VEGF. Macular oedema can occur in ischaemic and non-ischaemic CRVO (see Table 2). In ischaemic RVO, increased venous pressure causes decreased capillary perfusion, meaning the retina does not receive enough oxygen; the retina becomes hypoxic and permanent cell damage or death can occur.26 Ischaemia can lead to neovascularisation at the retina or iris (rubeosis iridis), increasing the risk of neovascular glaucoma, tractional retinal detachment or vitreous haemorrhage. In an ischaemic vein occlusion, cotton wool spots will be more prominent, and haemorrhages may appear deeper and more extensive. Fundus fluorescein angiography (FFA) will show capillary non-perfusion. The prognosis for an ischaemic retinal vein occlusion is generally poor.
The College of Optometrists’ guidelines recommend that retinal vein occlusions should be referred urgently to ophthalmology (to be seen within at least two to four weeks) andthey should be referred urgently to their GP for medical management and investigation for underlying systemic conditions, specifically for cardiovascular risk factors.27 In secondary care, patients may be treated with anti-VEGF or an intravitreal steroid such as Ozurdex (dexamethasone) therapy to treat macular oedema and/or neovascularisation. Patients with neovascularisation of the retina, disc or iris may also require pan-retinal photocoagulation (PRP) laser treatment.
Referring Macular Conditions
When confronted with an unfamiliar macular condition, it can be difficult to know whether to refer it and how urgently it needs to be seen. Practitioners should check their local pathways to see what guidance is in place. The College of Optometrists’ clinical management guidelines are useful tools when making decisions. If in doubt, refer, particularly if fluid is present at the macula.

In the referral letter it is helpful to describe what the patient’s visual symptoms are, or whether they are asymptomatic and the duration of the symptoms, particularly whether it is an acute onset. Include a description of what can be seen on the fundus image or OCT; this is often more helpful than a tentative diagnosis and helps to triage the patient. Ideally, an image of the OCT and fundus photo should be provided alongside the letter. Including previous visual acuities in the referral letter is also useful where available. It is also helpful to advise the patient that they will likely have dilating drops at their hospital appointment and should not drive to it.
Conclusion
Patients presenting with a sudden, unilateral, painless loss of vision will often have macular disease. A careful history including the description, onset and duration of visual symptoms will aid in making a differential diagnosis. Remember to consider the patient’s age, medical and family history, and the presence of risk factors. Although OCT is a valuable tool, its absence should not be a barrier to assessment; an Amsler chart and a dilated fundus examination can provide the information needed to guide appropriate management. Any sudden loss of vision, distortion or scotoma should be regarded with a high degree of suspicion even in the absence of an obvious haemorrhage and should warrant further investigation.
There are a number of materials available for practitioners looking to further their knowledge, but in particular, NG82, the Royal College of Ophthalmologists’ guidance, and the College of Optometrists’ clinical management guidelines are all free, easily accessible, valuable resources.
0










