HaloTag Introduction
HaloTag is a protein fusion tag system commonly used in molecular biology research for protein labeling, purification, and immobilization purposes. Developed by Promega Corporation, it offers several advantages over traditional protein tags like GFP (Green Fluorescent Protein) or His-tags.
The key feature of HaloTag is its covalent binding ability with specific ligands, such as HaloTag ligands or dyes, forming a highly stable and irreversible bond. This property enables precise and robust labeling of HaloTag-fused proteins both in vitro and in vivo, facilitating various applications including protein tracking, localization studies, and protein-protein interaction studies.
HaloTag fusion proteins can be purified using HaloTag ligands coupled to solid supports, allowing efficient isolation from complex biological samples with minimal background contamination. Additionally, HaloTag technology enables the immobilization of proteins onto surfaces for applications such as protein arrays or drug screening assays.
The versatility and reliability of HaloTag make it a valuable tool in cell biology, biochemistry, and drug discovery research, providing researchers with a powerful means to study protein function and dynamics in living cells and in vitro systems.
- HaloTag is a registered trademark of Promega Corporation.

Reviews:
(1) England CG, Luo H, Cai W. HaloTag technology: a versatile platform for biomedical applications. Bioconjug Chem. 2015 Jun 17;26(6):975-86. doi: 10.1021/acs.bioconjchem.5b00191. Epub 2015 May 22. PMID: 25974629; PMCID: PMC4482335.
(2) Cook A, Walterspiel F, Deo C. HaloTag-Based Reporters for Fluorescence Imaging and Biosensing. Chembiochem. 2023 Jun 15;24(12):e202300022. doi: 10.1002/cbic.202300022. Epub 2023 May 23. PMID: 36815462.
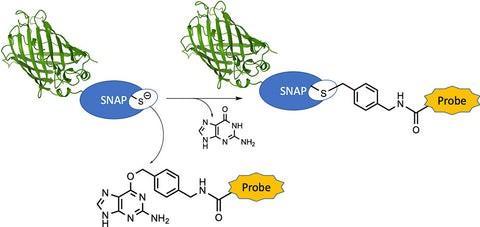
SNAP-Tag Introduction
The SNAP-tag is a protein fusion tag system used in molecular biology research, developed by New England Biolabs (NEB). It enables specific and covalent labeling of fusion proteins with various synthetic dyes or chemical probes.
Derived from O^6-alkylguanine-DNA alkyltransferase (AGT), a bacterial DNA repair enzyme, the SNAP-tag system involves mutating the active site of AGT to abolish its DNA repair function while retaining its ability to bind and covalently react with benzylguanine (BG) derivatives.
When a protein of interest is genetically fused to the SNAP-tag, it can be specifically labeled by incubating with a BG-conjugated dye or probe. This results in the covalent attachment of the dye or probe to the SNAP-tag fusion protein, enabling precise labeling and detection in various experimental settings, including live-cell imaging and flow cytometry.
The SNAP-tag system offers high specificity, minimal background labeling, and the ability to label proteins in living cells or in vitro. It has found wide applications in cell biology, neuroscience, and drug discovery research for tasks such as protein tracking, localization studies, and receptor-ligand binding assays.
- SNAP-tag and CLIP-tag are registered trademarks of New England Biolabs, Inc.
Reviews:
(1) Dreyer R, Pfukwa R, Barth S, Hunter R, Klumperman B. The Evolution of SNAP-Tag Labels. Biomacromolecules. 2023 Feb 13;24(2):517-530. doi: 10.1021/acs.biomac.2c01238. Epub 2023 Jan 6. PMID: 36607253.
(2) Hoelzel CA, Zhang X. Visualizing and Manipulating Biological Processes by Using HaloTag and SNAP-Tag Technologies. Chembiochem. 2020 Jul 16;21(14):1935-1946. doi: 10.1002/cbic.202000037. Epub 2020 Apr 2. PMID: 32180315; PMCID: PMC7367766.
