OngoingInvestigationofWeightLoss
Therapies:3PositiveTrends
Howtoloseweight?Wheneverthistopiccomesup,exerciseanddietingare thepreferredmethods,whiledrugsalwaysleavetheimpressionofbeing unsafeandineffective
Recently,someweightlosstherapieshavemadequitepositiveprogressA paperinaNaturesub-publicationhasidentifiedakeygenemutationthat reducesabdominalfatthatcouldeliminatelovehandlesinthefuturewithjust oneinjectionayear.IntheNewEnglandJournalofMedicine,EliLilly announcedtheresultsofthephase3clinicaltrialoftirzepatide,inwhich patientslostupto225%oftheirbodyweightafter72weeksoftreatment, settinganewbenchmarkforweightlosswiththedrug
Onthepremiseofsafetyandeffectiveness,drugtherapyisashortcut comparedtoexerciseanddietingwhichrequirepersistenteffortsBasedon thehugeunfinishedneedsinthefieldofweightloss,wehavecompiledthe latestclinicalpipelineofdrugsunderinvestigationforthisindication(asof August2022)topresentthecurrentstatusandfuturetrendsofweightloss drugdevelopment
Accordingtoincompletestatistics,atotalof10weightlosstherapies havebeenmarketed,and21areinclinicalphase2(17),phase3(2),and pre-registration(2)statusTherearethreetrendsofweightlossdrug development.
HuatengPharma https://ushuatengscicom
Targets:DominatedbyGLP-1receptors,followedby multi-mechanismincretin
ThelargestnumberoftherapiestargetingtheGLP-1receptorhavebeenused inallphasesofdevelopmentfromPhase2clinicaltomarketGLP1 (glucagonlikepeptide1)belongstoagroupofhormonescalledincretin,which isproducedbyendocrinecellslocatedinthegutandsecretedintothe bloodstreamwithinminutesaftereatingItsphysiologicaleffectsinclude regulatingthereleaseofinsulinaftereatingandactingonthebody's hypothalamustoregulateappetiteInadditiontoGLP1,GIP (glucose-dependentinsulinotropicpolypeptide)isalsoacommon incretinSemaglutideandliraglutide,bothapprovedbyFDAinrecentyears, areGLP-1receptoragonists,andthesafetyandefficacyofthistargethas beenverifiedinstudies
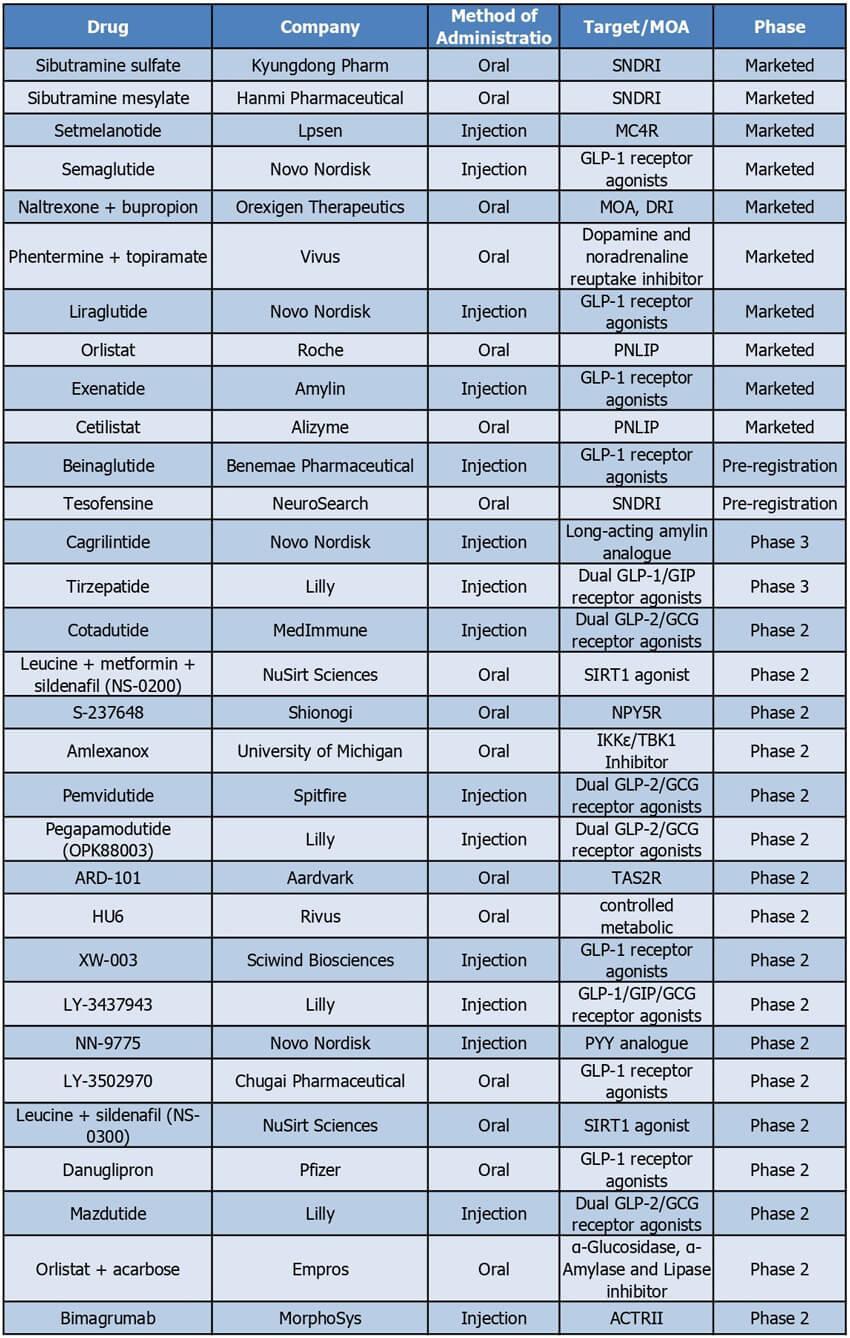
Figure2TargetsofWeightlossdrugsinclinicaltrials
Specifically,GLP1receptoragonistsaccountfor30%ofmarketeddrugs,50% ofpre-registereddrugs,and18%ofphase2drugs.Thisshowsthatthe proportionofsingleGLP1receptoragonistsinearlyclinicaldevelopment therapiesisgraduallydecreasing,whiledualandtriplereceptoragonistssuch asGLP1/GCG,GLP1/GIP,andGLP1/GCG/GIParemoredistributedin phase2andphase3clinicaltrials.ThesafetyandefficacyofGLP-1targeted therapiesonthemarkethasledtoresearchonincretintherapiestargeting multiplemechanisms,astheregulationofenergymetabolismitselfinvolves multiplehormones,anddrugtargetingmultiplesignalingpathwaysmay producesynergisticeffects,thusmakingtheefficacymoresignificant
Besidesincretin,thereisatrendofmorediversifiedandnovel targetsfrommarketedtophase2therapies.Fiveofthe10marketeddrugs
HuatengPharma https://ushuatengscicom targetnonincretintargets,sibutraminesulfateandsibutraminemesylateare serotoninnorepinephrinedopaminereuptakeinhibitors(SNDRIs),orlistatand cetilistattargetgastrointestinallipase,andnaltrexoneandbupropiontarget opiatereceptorsandadrenergicreceptors,respectively
Ofthe2preregisteredtherapies,tesofensineremainsanSNDRI,whileofthe phase3therapies,tirzepatideisadualGLP-1/GIPreceptoragonistand cagrilintideisalongactingamylinanalogueAmylin,apeptidethatis co-secretedwithinsulin,reducesfoodintakethroughasignalingpathwaythat regulatessatiety
Finally,amongthe17therapiesundergoingphase2clinicaltrials,atotalof8 therapiesadoptednon-incretinmechanismsofaction,namelycontrolled metabolicaccelerators(CMAs),neuropeptideYY5receptors, appetite-regulatinghormones,IKKε/TBK1,animportantregulatorofmetabolic disease,SIRT1protein,activintypeIIreceptor,bittertastereceptorTAS2R, amylase/glucosidases,whicharerelativelynovelmechanisms.Bittertaste receptorsmediatetheperceptionofbittertastenotonlyintheoralcavitybut arealsoexpressedinothertissuesofthebody.
Aardvark'sleadproduct,ARD101isafirstinclassoralcompositiontargeting extraoralbittertastereceptors(TAS2R).ARD101waslargelyrestrictedtothe gutwhilestillinducingsystemiceffects,includingincreasedexpressionof endogenousgutpeptidehormones.HU6isacontrolledmetabolicaccelerator (CMA)thatcanactivateprotonleakandmitochondrialuncoupling,anatural processinthebodythatregulatesanddissipatesenergy.Byferryingprotons outofthemitochondrialintermembranespace,CMAscuetheincreased oxidationofsugarsandfats,whilemaintainingthesamebaselineproductionof adenosinetriphosphate(ATP)Activatingthisprocessresultsinthereduction ofaccumulatedfatthroughoutthebody.
Dosageform:Slightlymoreoralformulationsthaninjectable formulations,betterdevelopmenttrendoforalformulationsin thefuture
Ofthe31drugs,16areoraldrugsand15areinjectabledrugs.Currently,the oralformaccountsfor60%ofthemarketedweightlossdrugs,whilethe proportionoforaldrugsdecreasesto50%and53%fordrugsinthe preregistrationandphase2,andthetwodrugsundergoingphase3clinical trialsareadministeredbyinjection.Amongthedrugsinthemarketand preregistrationphase,theincretintherapiesrepresentedbyGLP1receptor agonistswerealladministeredbyinjection.Ofthesevenincretintherapiesin Phase2,onlytwowereadministeredorallyThisshowsthatincretintherapies haveashorterhalf-lifeandarebetterabsorbedbyinjectionthanbyoral administrationThisexplainsthefluctuationsintheproportionoforaltherapies
HuatengPharma https://ushuatengscicom atdifferentstagesofclinicaltrialsAmongthedrugsinthemarket, preregistrationandphase3,theproportionoforaldrugsdecreasesasthe proportionofincretintherapyrisesFordrugsinPhase2,thepercentageof oraldrugsreboundedwiththediversificationoftargetdistributionandthe developmentoforalincretintherapy.

Figure3.Routofadministrationofweightlossdrug

Lookingahead,oralweightlosstherapiesmaybepreferredoverinjections,as compliancewithoraldosingissignificantlyhigherthanthatofinjectionsItis assumedthattheindustrywillinvestmoreindevelopingoralformulationsof weightlossdrugs,asincretin,adrugthatisdifficulttomakeintooraldosage forms,isbeingexploredfororaluse.
Figure4Bodyweightlossachievedthroughlifestylechanges,currently
HuatengPharma https://ushuatengscicom approvedantiobesitymedications(AOMs)andbariatricsurgery(parta)and correlationofdruginducedbodyweightlossinrodentsandhumans(partb).
Source:Reference[1]
Theeffectivenessofweightlosstherapiesisatopicofgreatinterest,asshown hereinareviewofweightlosstherapiesfromNatureReviewsDrugDiscovery Oftheavailableweightlossmethods,bariatricsurgeryisthemosteffective, withanaverageweightlossofabout30%,buttheconditionsforperforming bariatricsurgeryaredemanding,andtherisksofsurgeryandpostoperative recoveryareuncertaintiesthatcannotbeignoredIncontrast,weightloss drugsgreatlysurpasssurgeryintermsofconvenienceandsafety,butareless effectivethantheformer,withmostweightlossdrugsonlyreducingweightby 5%-10%andveryfewby10%-15%.Historically,thedevelopmentofweight lossdrugshasbeenfraughtwithupsanddownsAccordingtotheliterature, drugswereapprovedforweightlossasearlyasthe1930s,yetmostwere withdrawnfromthemarketbecauseofserioustoxicsideeffects
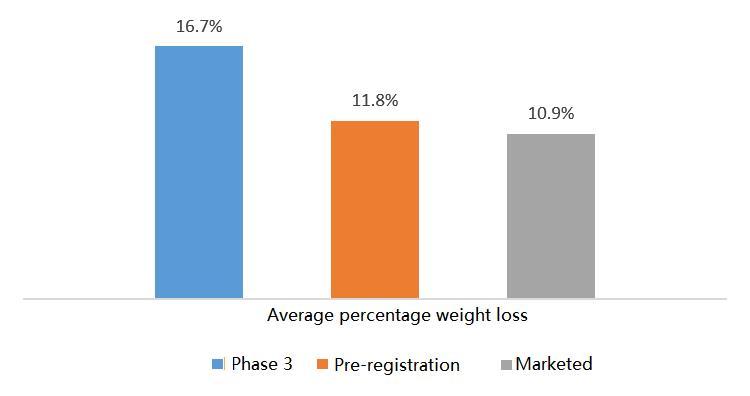
Figure5Averagepercentageweightloss
Theaveragepercentageofweightlossformarketed,preregistered,and Phase3weightlossdrugsisincreasingsequentially,andtheupperlimitofthe drug'sweightlossefficacyisincreasingForexample,resultsfromtheSTEP Phase3aclinicaltrialofsemaglutideshowedthatobesepatientstreatedwith thedruglostanaverageof1718%oftheirbodyweightandthattheeffectwas maintainedformorethan68weeksOnJuly26,EliLillypublishedintheNew EnglandJournalofMedicinetheresultsoftheclinicalPhase3trialof tirzepatide,SURMOUNT1,inwhichpatientslostupto225%oftheirbody weightafter72weeksoftreatment,aneffectcomparabletobariatricsurgery.
HuatengPharma https://ushuatengscicom
Althoughdrugsarenotcurrentlythepreferredmethodofweightlossinthe publicperception,andmostweightlossdrugsarestillintheearlytomidstage ofdevelopment,thethreemajortrendsoftargetdiversification,increased convenienceofdosageforms,andimprovedweightlosseffectsreflectthe industry'sconcernandcommitmenttoweightlosstherapies.Weexpectmore effective,safeandconvenientweightlossdrugstoemergeassoonas possibletoalleviatethepublichealthcrisisoftheexpandingglobalobesity population
HuatengPharma,foundedin2013,isaone-stopcontractdevelopmentand manufacturingorganization(CDMO)tosupplyresearchersandcompanies withPEGderivativesandproductsusedacrossthepharmaceuticalvalue chainincludingintermediates,excipients,APIs,andreagents.
Reference: 1Mülleretal,(2021)Antiobesitydrugdiscovery:advancesandchallengesNature ReviewsDrugDiscovery,https://doi.org/10.1038/s415730210033782. 2Lilly'stirzepatidedeliveredupto225%weightlossinadultswithobesityoroverweight inSURMOUNT1RetrievedAugust25,2022,from https://investorlillycom/news-releases/news-release-details/lillys-tirzepatide-delivered-22 5weightlossadultsobesityor
RelatedArticles:
AntiObesityDrugs:HistoryAndDevelopment OverviewofDiabetesTreatmentStrategies
