Kadcyla-Top-SellingAntibodyDrugConjugate (ADC)
Kadcyla(adotrastuzumabemtansine,TDM1),asecondgenerationantibodydrug conjugate(ADC),isthemostcommerciallysuccessfulADC.In2021,theglobalADC marketexceeded$52billion,withKadcylasalesreaching$217billion
KadcylawasfirstapprovedbytheU.S.FoodandDrugAdministration(FDA)in2013,for patientswithHER2positive,latestage(metastatic)breastcancer.ItisthefirstADC approvedforsolidtumors
IntroductionofKadcyla
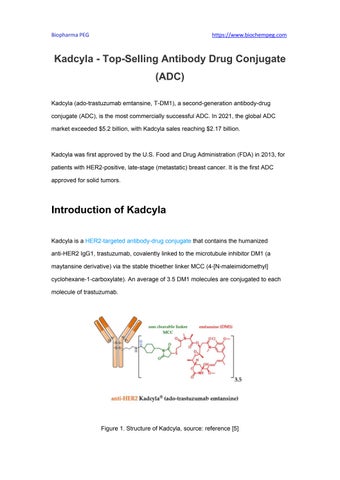
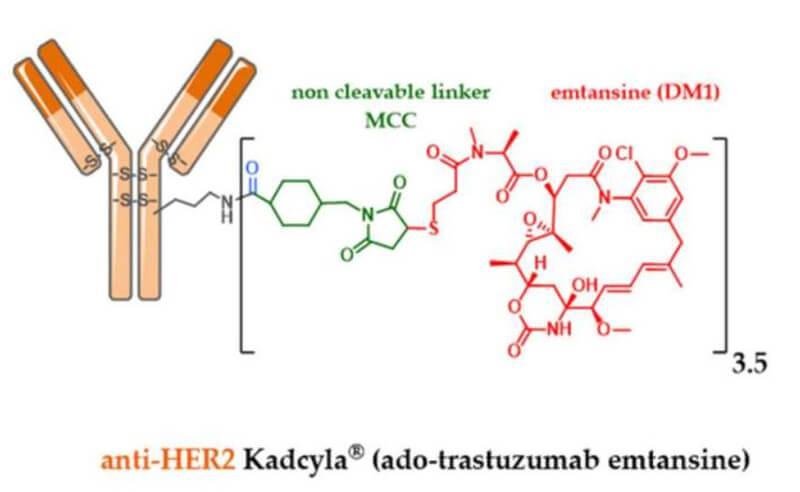
KadcylaisaHER2targetedantibodydrugconjugatethatcontainsthehumanized anti-HER2IgG1,trastuzumab,covalentlylinkedtothemicrotubuleinhibitorDM1(a maytansinederivative)viathestablethioetherlinkerMCC(4[Nmaleimidomethyl] cyclohexane1carboxylate).Anaverageof3.5DM1moleculesareconjugatedtoeach moleculeoftrastuzumab.
Figure1.StructureofKadcyla,source:reference[5]
BiopharmaPEG https://wwwbiochempegcom
DM1isamaytansinederivativeMaytansine,firstisolatedfromthebarkofAfricanshrub Maytenusovatusin1972,isapotentmicrotubuletargetedcompoundthatinducesmitotic arrestandkillstumorcellsatsubnanomolarconcentrationsTherefore,thisclassof cytotoxicdrugsalsobelongstotheclassofmicrotubuleproteininhibitors,andthetwo mostcommonlyusedinADCstodayareDM1andDM4
ClinicalEfficacyandMarketPerformanceof Kadcyla
ThelatestFDAapprovalin2019wasbasedonKATHERINE(NCT01772472),a randomized,multicenter,openlabeltrialof1486patientswithHER2positiveEBCwho hadresidualinvasivediseaseinthebreastand/oraxillarylymphnodesfollowing neoadjuvanttreatmentwithtaxane+trastuzumabbasedtherapy
TheprimaryobjectiveoftheKATHERINEstudyistoevaluate,theeffectivenessand safetyofadjuvanttreatmentwithKadcylaversustrastuzumabinpatientsaftersurgery
Theprimaryendpointofthestudyisinvasivediseasefreesurvival(IDFS),whichisthe timefromrandomizationgroupingtorecurrenceofinvasivebreastcancerordeathfrom anycauseSecondaryendpointsincludeddiseasefreesurvival(DFS)andoverallsurvival (OS). Patientsweregrouped1:1andreceivedKadcylaorHerceptin(trastuzumab),respectively, aswellasradiationand/orhormonaltherapyaccordingtolocaltreatmentguidelinesAta medianfollowuptimeof40months,patientsintheKadcylagrouphadastatistically significantimprovementinIDFScomparedtotheHerceptingroupanda50%reductionin theriskofdeathorrecurrenceofinvasivediseaseAt3years,88.3%ofpatientsinthe Kadcylagroupwererelapsefreecomparedto77.0%intheHerceptingroup.
BiopharmaPEG https://wwwbiochempegcom
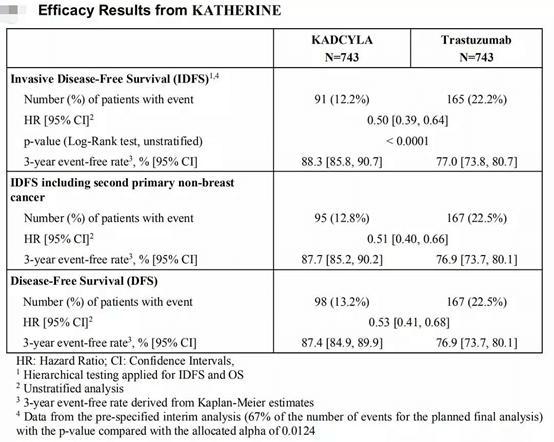

BiopharmaPEG https://wwwbiochempegcom Figure2.EfficacyResultsfromKATHERINE Figure33yeariDFSrateswere883%forKADCYLAvs770%forHerceptin,references [1]
Themostcommonadversereactions(ADRs)(≥25%)includednausea,fatigue,skeletal
peripheralneuropathy,withmostADRsbeingmildtomoderategrade1or2ADRsand theincidenceofsevereADRs>05%
Thereare2mainreasonsforitslimitedroomforfuturegrowth,oneisthehighpricingof
Ontheotherhand,biosimilarsarecomingtomarketoneafteranother,furthereroding
BiopharmaPEG https://wwwbiochempegcom
musclepain,bleeding,headache,elevatedtransaminases,thrombocytopenia,and
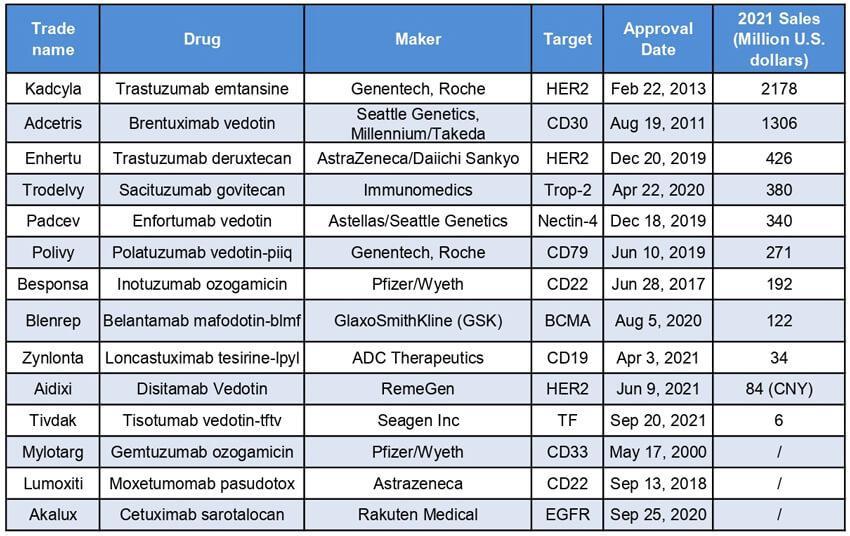
In2021,Kadcylahadglobalsalesof$218billion,rankingasthemostcommercially successfulADCAccordingtoanADCmarketsizereportbyNatureReviewsDrug Discovery,salesofKadcylaareexpectedtobe$23billionin2026,indicatinglimited roomforfuturegrowth Figure4SalesofADCdrugs
KadcylaThepricingofKadcylainUSAis$3,709/100mg,$5,929/160mg,whichlimitsthe useofthedrugforsomepatients
Kadcyla'sshareInIndia,ananalogue,Ujvira,hasbeenapprovedfor20%ofthepriceof
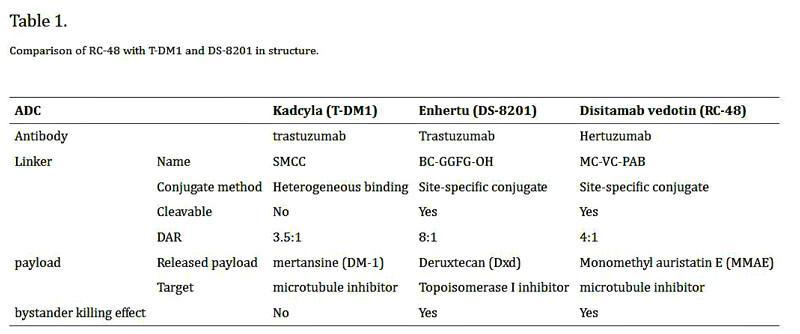
BiopharmaPEG https://wwwbiochempegcom KadcylaADCswiththesametarget,HER2,arealsobeingdevelopedand commercialized,undoubtedlyposingathreattoKadcyla. Kadcyla(T-DM1)ComparedtoDS-8201&RC48 Globally,twoADCproductstargetingHER2havebeenlaunched:Enhertu (DS8201)byDaiichiSankyoandDisitamabVedotin(RC48)byRemeGenCo Figure5Kadcyla(TDM1)ComparedtoDS8201&RC48,source:reference[3] 1.TrastuzumabDeruxtecan(Enhertu,T-DXd,DS-8201) ▶Company:DaiichiSankyo/AstraZeneca ▶Target:HER2 ▶Antibody:HumanizedIgG1monoclonalantibodyTrastuzumab ▶Drug/payload:DXd(topoisomerase) ▶Linker:GFLG(tetrapeptide)
hydrophobicpropertiesofthedrug,allowingeachantibodytobeloadedwith7or8DXd
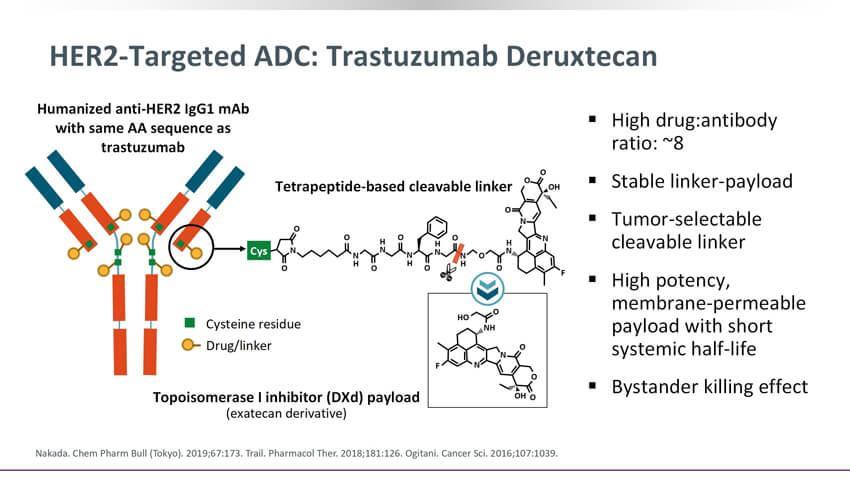
BiopharmaPEG https://wwwbiochempegcom ▶Indications:Breastcancer,gastriccancer,lungcancer,etc ▶Status:Availablein2019 EnhertuconsistsofanantiHER2antibody,Trastuzumab,coupledtothecytotoxicdrug DXdviaalinker,GFLGThelinkerisacleavabletetrapeptidyllinker(GlyPheLeuGly (GFLG)),whichisnotonlystablebutalsocanbespecificallycleavedandhasastrongcell membranepermeability,allowingittoexerta"bystandereffect" Figure6.StructureofEnhertu.Source:reference[2] ThecytotoxicdrugemploysDXd,anewDNAtopoisomeraseinhibitorthatimprovesthe
molecules.ThehighDARallowstheefficacyofEnhertutobeconsidered2to4times higherthanpreviouslyapprovedmarketedADCdrugs EnhertuwasfirstapprovedformarketingintheUnitedStatesin2019forthetreatmentof HER2positivemetastaticbreastcancer
antibodycoupledtoMMAEviaacleavablelinkerItisindicatedforthetreatmentof patientswithlocallyadvancedormetastaticHER2overexpressinggastriccancer, includingadenocarcinomaofthegastroesophagealjunction,whohavereceivedatleast2 systemicchemotherapies.
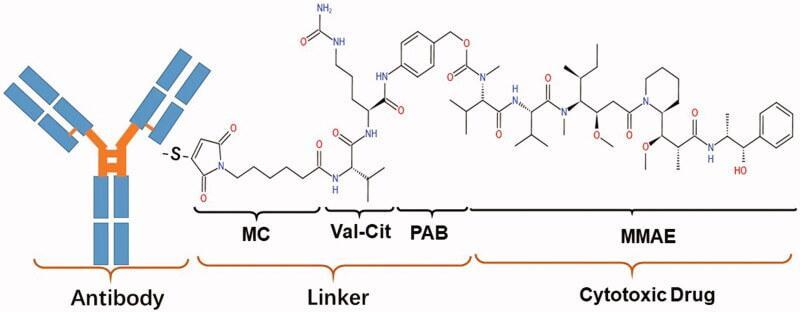
BiopharmaPEG https://wwwbiochempegcom 2.DisitamabVedotin(Aidixi,RC48) ▶Company:RemeGenCo ▶Target:HER2 ▶Antibody:recombinanthumanizedIgG1monoclonalantibodyHertuzumab ▶Drug/Payload:Duocarmycin/SecoDUBA ▶Linker:MCValCitPAB ▶Indication:Gastriccancer ▶Status:Availablein2021 DisitamabVedotin,consistsofarecombinanthumanizedHER2IgG1monoclonal
Figure7StructureofDisitamabVedotin,source:Reference[3]
InJune2021,theNationalMedicalProductsAdministration(NMPA)grantedconditional approvalforDisitamabVedotin.
Asanovelagent,RC48asmonotherapyoradjuvanttreatmentinclinicalpracticeforthe therapyofothercancerintheworld,includingUC,biliarytractcancer(BTC),nonsmall celllungcancer(NSCLC),andHER2+andHER2lowexpressingBC
Anti-Her2ADCsinClinicalTrials
ExceptforthethreeantiHer2ADCapprovedformarketing,therearemorethan20ADCs haveenteredclinicaltestingAmongthem,trastuzumabduocarmazine(SYD985)making thebestprogress.
BiopharmaPEG https://wwwbiochempegcom
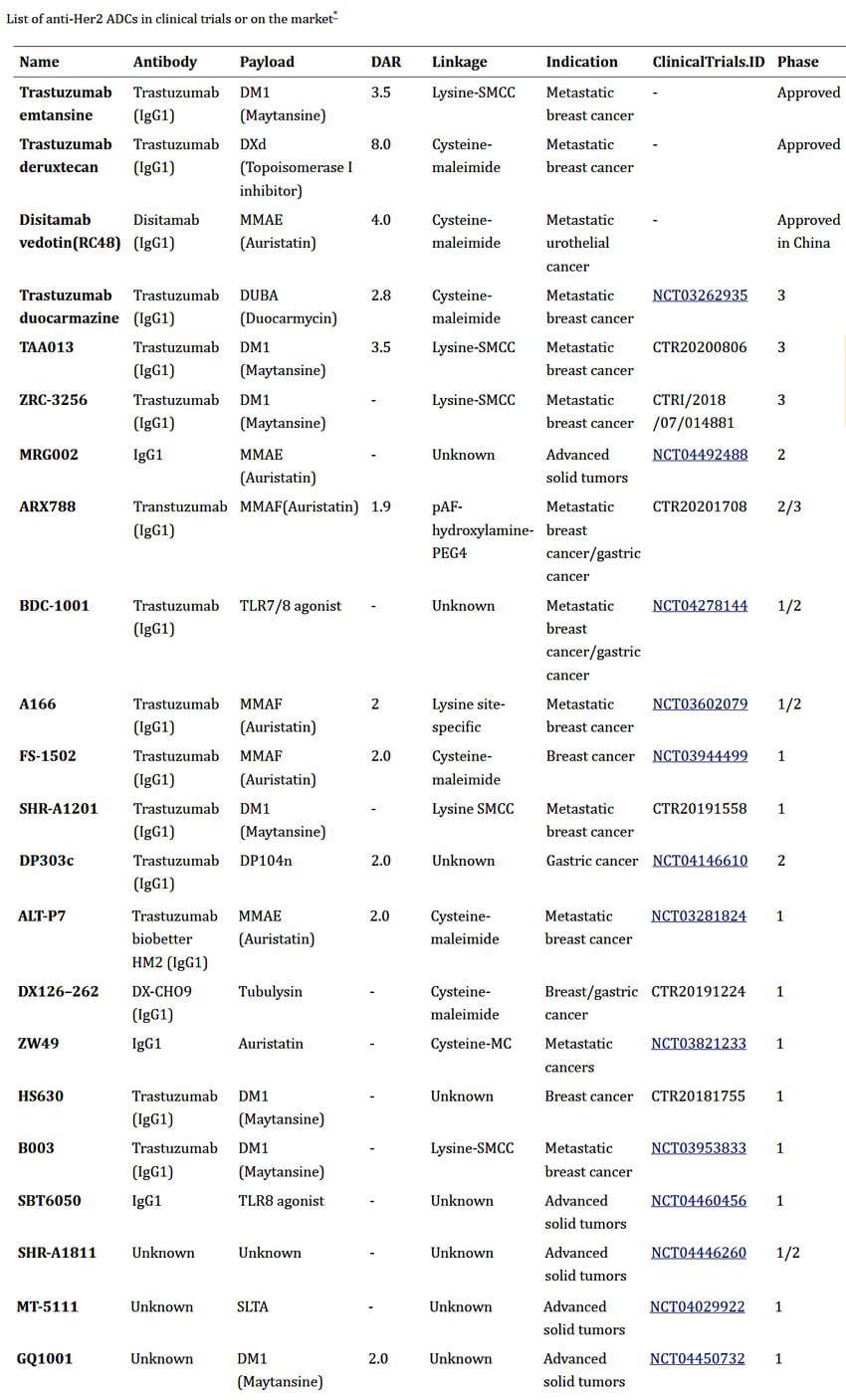
BiopharmaPEG https://wwwbiochempegcom Figure8Listofanti-Her2ADCsinclinicaltrialsoronthemarket,source:reference[4]
FastTrackDesignationbytheFDAinJanuary2018Inaddition,itsUSmarketing applicationwasacceptedbytheFDAonJuly12,2022,withaPDUFAdatesetforMay12, 2023
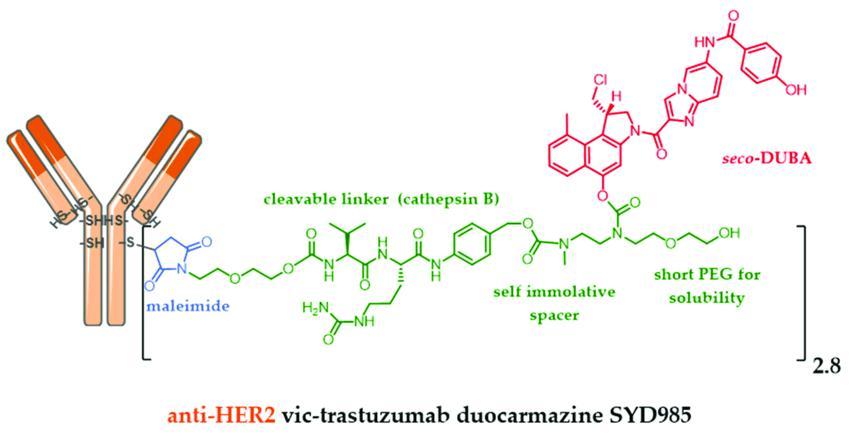
calledvalinecitrullinesecoDUocarmycinhydroxyBenzamideAzaindole(vcsecoDUBA). whichisdesignedusingByondis'proprietaryByonZinetechnologyplatform-duocarmazinelinkerdrugtechnology
BiopharmaPEG https://wwwbiochempegcom TrastuzumabDuocarmazine(SYD985) ▶Company:Byondis ▶Target:HER2 ▶Antibody:Monoclonalantibodytrastuzumab ▶Drug/Payload:MMAE(monomethylauristatinE) ▶Linker:vcsecoDUBA ▶Indication:BreastCancer,Endometrialcancer ▶Status:PhaseIII SYD985isaninvestigationalnextgenerationHER2ADCthatwaspreviouslygranted
SYD985consistsofthemonoclonalantibodytrastuzumabandacleavablelinkerdrug
Figure9SYD985formula,anti-HER2trastuzumabconjugatedtoseco-DUBAviaa cleavablelinkersensitivetocathepsinBSource:reference[5]
OnJuly18,2022,ByondisannouncedthattheEuropeanMedicinesAgency(EMA)has validatedtheMarketingAuthorizationApplication(MAA)forSYD985inpatientswith HER2positiveunresectablelocallyadvancedormetastaticbreastcancer(MBC)
TheMAAforSYD985isbasedondatafromthepivotalphaseIIITULIP.Thetrialwasa randomized,multicenter,open-labelclinicaltrial(n=436)comparingtheefficacyof SYD985withphysician'schoice(PC)forthetreatmentofpatientswithHER2positive unresectablelocallyadvancedormetastaticbreastcancer.Theprimaryendpointis progressionfreesurvival(PFS)
ByondispresentedpreliminaryresultsfromTULIP3attheESMO2021CongressThe resultsshowedthatSYD985significantlyimprovedPFSinpatientswithHER2positive unresectablelocallyadvancedormetastaticbreastcancer,withaPFSof7.0monthsin theSYD985groupand49monthsinthePCgroupInaddition,theoverallsurvival(OS) inthegroupreceivingSYD985showedatrendofimprovementwithanHRof083
BiopharmaPEG https://wwwbiochempegcom
BiopharmaPEG https://wwwbiochempegcom
BiopharmaPEGprovidesGMPstandardPEGderivativesandbulkordersviacustom synthesis,offeringtheopportunitytomatchcustomers'specialqualityrequirements.ADC linkerswithmolecularweights,branching,andfunctionalgroupsnotlistedinouronline catalogmaybeavailablebycustomsynthesis
References:
[1]https://wwwkadcylahcpcom/earlybreastcancer/efficacy/clinicaltrialresultshtml
[2]NakadaT,SugiharaK,JikohT,AbeY,AgatsumaTTheLatestResearchandDevelopmentintothe AntibodyDrugConjugate,[fam]TrastuzumabDeruxtecan(DS8201a),forHER2Cancer TherapyChemPharmBull(Tokyo)2019;67(3):173185doi:101248/cpbc1800744
[3]ShiF,LiuY,ZhouX,ShenP,XueR,ZhangMDisitamabvedotin:anovelantibodydrugconjugates forcancertherapy.DrugDeliv.2022Dec;29(1):13351344.doi:10.1080/10717544.2022.2069883.PMID: 35506447;PMCID:PMC9090390
[4]ZhangX,HuangAC,ChenF,ChenH,LiL,KongN,LuoW,FangJNoveldevelopmentstrategies andchallengesforantiHer2antibodydrugconjugatesAntibTher2022Jan27;5(1):1829doi: 101093/abt/tbac001PMID:35146330;PMCID:PMC8826051
[5]Joubert,N;Beck,A;Dumontet,C;DenevaultSabourin,CAntibodyDrugConjugates:TheLast DecadePharmaceuticals2020,13,245https://doiorg/103390/ph13090245
RelatedArticles: GlobalAntibodydrugConjugates(ADCs):Approvals&ClinicalTrailsReview HER2TargetedTherapiesInBreastCancer ADCDrugsGlobalSalesof2021andFutureProspects TheBystanderEffectofADCs