GLP-1RAgonistsforWeightLoss
Obesityisagrowingthreattopublichealth,increasingrisksofnumerousdiseasesand mortality,andimpairingqualityoflifeThereisastronginterestindevelopingobesity treatmentsbasedonglucagon-likepeptide-1(GLP-1)agonists,whichhaveprovedtolimit morbidityandmortalityintype2diabetes
Reference1 DataonweightlossofGLP-1Ragonistsarebeingcontinuouslyrefreshedalongwiththe emergenceofnewiterationsoftheproduct.NovoNordisk'ssemaglutidebeatsits counterpartliraglutidetobecomethenextgenerationofweightlossinnovation,andthen EliLilly'sGLP-1R/GIPRdual-targetagonisttirzepatideachievesa225%weightloss, surpassingsemaglutide'sweightlosshistory.Here,let'sfindoutmoreaboutGLP-1RAs forweightlossandtheirfutureprospects
ApprovedGLP-1AgonistsforWeightLoss
Currently,therearetwoGLP-1Ragonistsapprovedforthetreatmentofobesity, Saxenda(liraglutide)andWegovy(semaglutide),bothdevelopedbyNovoNordiskand approvedbytheFDAin2014and2021,respectively
TheemergenceofGLP-1Rasapopulartargetforweightlossismainlyattributedtoits mechanisticfeaturesandtheweightreductionefficacyshownbyliraglutideand semaglutide
StudieshaveshownthatGLP-1Ragonistsnotonlypromoteinsulinsecretionandexert glucose-loweringeffectsbutalsoeffectivelydelaygastricemptying,reduceintestinal motility,activateneuralpathwaysinthehypothalamusandappetiteregulationareas, resultingindecreasedappetiteandreducedfoodintake,thustreatingobesity
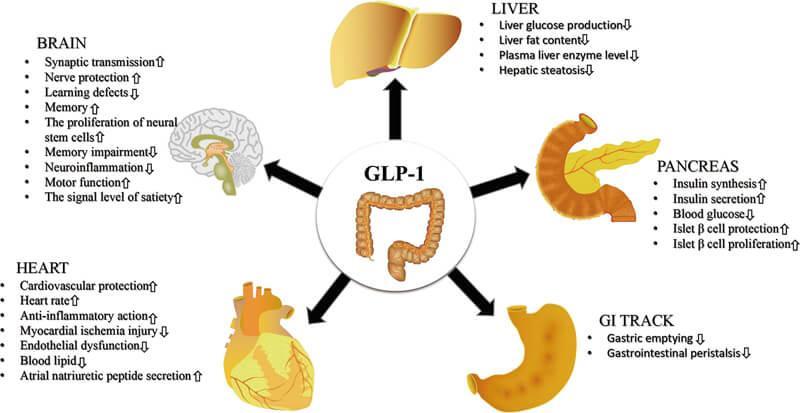
Figure2.TheeffectsofGLP-1RAsonmultiplehumanorganizations,source:reference[2]
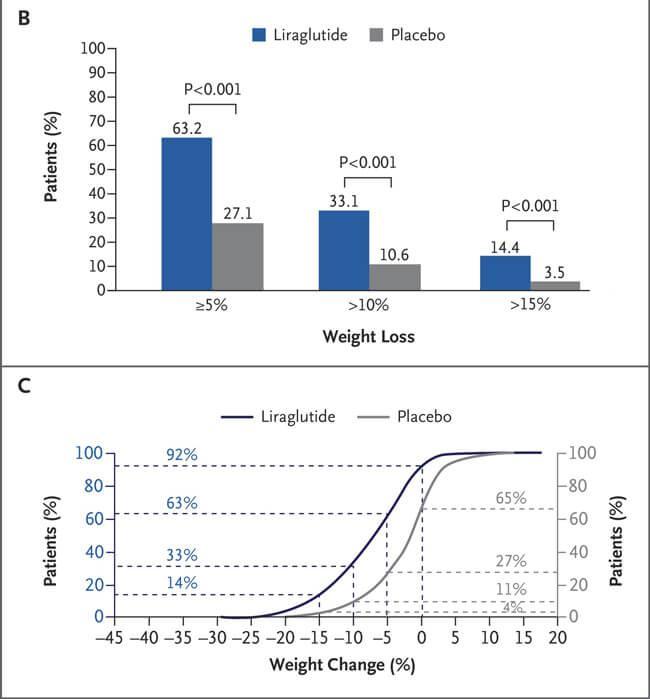
DatafromNovoNordisk'sPhaseIIIclinicaltrialshowedthatliraglutide30mgresultedin a5%weightlossinapproximately62%ofobesepatientsanda10%weightlossin34% ofpatientsaftera56-weektreatmentperiod.Thisdatasupportstheproduct'slaunchin 2014asthefirstapprovedGLP-1weightlossdrugInaddition,liraglutidewasexpanded toadolescentobesepatientsaged12yearsandolderin2020Afterthe56-week treatmentperiod,BMIstandarddeviationscores(BMISDS)decreasedbyameanof0.23 intheliraglutidegroup,withnochangeintheplacebogroup.
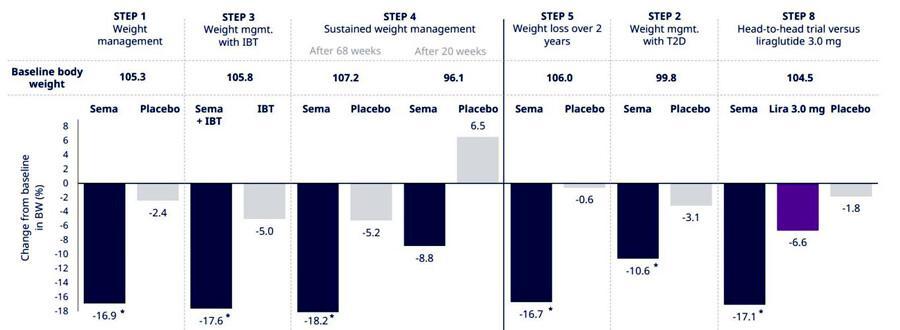
SemaglutideisanotherGLP-1productdevelopedbyNovoNordiskwithabetterweight losseffectthanliraglutideInalargePhase3clinicaltrial(STEPseries),semaglutide demonstratedexcellentweightlossandweightmaintenanceafterweightloss18.2% averageweightlossatweek68wasobservedinpatientsintheSTEP4trial,alongwith reducedbloodglucoselevels,improvedlipidprofileandsignificantimprovementsin overallqualityoflifeandhealthstatusscoresInaddition,ahead-to-headtrialof semaglutideandliraglutidewasconductedintheSTEP8trial,withameanweightlossof 171%inthesemaglutidegroup,whichwassignificantlymoreeffectivethanliraglutide (meanweightlossof66%)
Novo'stwoobesityproducts,WegovyandSaxenda,havegeneratedDKK16.86bn(USD 25bn)insalesthroughout2022,doublingtheprecedingyear'sresultofDKK84bn(USD 12bn)–anincreaseof101%NovoNordiskplanstoachievemorethan25billionDKK ($372billion)inobesitysalesby2025
DualAgonistsUnderInvestigation
Tirzepatide(Mounjaro®)wasapprovedbytheFDAonMay13,2022forthetreatmentof type2diabetesinadults,makingitthefirstandonlyGIP/GLP-1receptoragonist Althoughtirzepatideisnotcurrentlymarketedasaweightlossdrug,itappearsthatitis onlyamatteroftimefromwherewestandThePhaseIIIclinicalSURMOUNT-1 (NCT04184622)fortirzepatide,isexpectedtobecompletedinthefirsthalfof2024
AccordingtotheresultspublishedinTheNewEnglandJournalofMedicine(NEJM), participantstakingtirzepatideachievedaverageweightreductionsof160%(35lbor16 kgon5mg),214%(49lbor22kgon10mg)and225%(52lbor24kgon15mg), comparedtoplacebo(24%,5lbor2kg)
AMG133isanovelbispecificglucose-dependentinsulinotropicpolypeptidereceptor (GIPR)antagonistandglucagon-likepeptide-1(GLP-1)receptoragonistmolecule
AmgenupdateddatafromitsPhaseIstudyoftheweightlossdrugAMG133atthe2022 AmericanHeartAssociationAnnualScientificSessions(AHA)Atday85(approximately 12weeks),patientsinthelow-dosegrouplostanaverageof7.19percentoftheirbody weightandthehigh-dosegrouplostanaverageof1452percentoftheirbodyweight, whiletheplacebogroupgainedanaverageof149percentoftheirbodyweightAmgen planstoadvanceAMG133toPhaseIIclinicalinearly2023

AreGLP-1WeightLossDrugstheNext Blockbusters?
AccordingtoCiteline'sPharmaprojectsanalysis,theglobalmarketforweightlossdrugsis expectedtogrowdramaticallyfrom$282billionin2022toover$13billionby2029

However,compliantweightlossdrugsareextremelyscarceinthefaceofstrongdemand forweightlossTheFDAhasonlyapprovedsixweightlossdrugs,including:
●Bupropion-naltrexone(Contrave)
●Liraglutide(Saxenda)
●Orlistat(Xenical,Alli)
●Phentermine-topiramate(Qsymia)
●Semaglutide(Wegovy)
BiopharmaPEG
●Setmelanotide(Imcivree)
Currently,thechoiceofweightlossdrugsremainsverylimitedandisdividedintotwo maincategories:pancreaticlipaseinhibitorsandappetitesuppressantsthatactonthe centralnervoussystem
Appetitesuppressantsarerestrictedduetotheadverseneurologicaleffectstheycan causeThepancreaticlipaseinhibitororlistatlosesweightbyinhibitingpancreaticlipase activity,whichinturninhibitsthebreakdownandabsorptionoffatfromfoodHowever,it cancausesteatorrhea,resultinginfat-solublevitamindeficiency,andcanevencause liverdamage.
GLP-1canactonthecentralGLP-1receptors(especiallythehypothalamus)andthe centralsatietycenter,causingafeelingofsatietyandreducingfoodintake,thusachieving weightlossthroughappetitesuppressionIncontrast,theweightlosseffectofGLP-1 drugsismorepronounced
ItisforeseeablethatGLP-1weightlossdrugswithsuperioreffectsandsafetywillbecome thenextblockbuster
BiopharmaPEGsuppliespolyethyleneglycol(PEG)derivatives,monodispersePEGs, customPEGderivativesynthesisandPEGylationservicesworldwide,fromR&Dthrough GMPcommercialquantities,forpreclinical,clinicaltrials,andcommercialproductsfor pharma,biotech,medicaldevices,anddiagnosticsAnd,BiopharmaPEGnow providessemaglutidesidechainwiththebestpriceandhighquality
CASNo.166108-71-0,Fmoc-NH-PEG2-CH2COOH
CASNo134978-97-5,NH2-PEG2-CH2COOH
CASNo108466-89-3,Boc-NH-PEG2-CH2COOH
CASNo134979-01-4,CH2COOH-PEG2-NH2HCl
BiopharmaPEG
References:
[1]GribbleFM,ReimannF.MetabolicMessengers:glucagon-likepeptide1.NatMetab. 2021;3(2):142-148doi:101038/s42255-020-00327-x
[2]ZhaoX,WangM,WenZ,LuZ,CuiL,FuC,XueH,LiuY,ZhangYGLP-1Receptor Agonists:BeyondTheirPancreaticEffectsFrontEndocrinol(Lausanne)2021Aug 23;12:721135.doi:10.3389/fendo.2021.721135.PMID:34497589;PMCID:PMC8419463.
[3]AStudyofTirzepatide(LY3298176)inParticipantsWithObesityorOverweight (SURMOUNT-1)ClinicalTrialsgovhttps://clinicaltrialsgov/ct2/show/NCT04184622
RelatedArticles
FDAApprovesSemaglutideAsAnPowerfulWeight-lossDrug
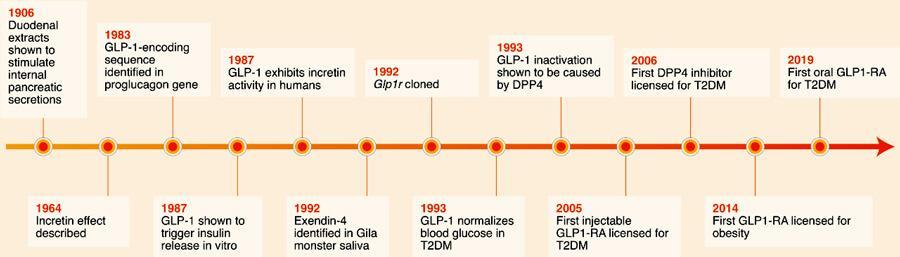
EvolutionofGLP‐1ReceptorAgonistsforDiabetesTreatment
FDAApprovesTirzepatide,APotentialBlockbusterDrugforType2Diabetes
Anti-obesityMedications(AOMs):HistoryAndProgress
