GlobalSalesofADCsin2022–UPto7Billion
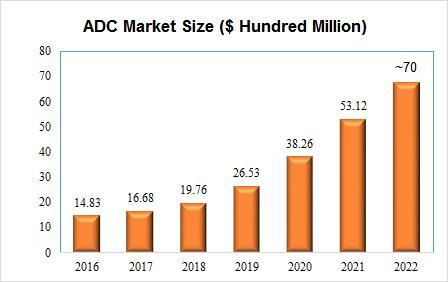
Currently,atotalof15antibody-drugconjugates(ADCs)arecurrentlyapprovedfor marketinggloballyTheglobalADCsmarketsizewasvaluedatUSD531billionin 2021Andin2022,thetotalsalesoftheseADCdrugsarenearly$7billion
FromthedisclosedFY2022financialstatements,ADCdrugssuchasSeagen/Takeda's Adcetris,Roche'sKadcylaandPolivy,Gilead'sTrodelvy,andDaiichi Sankyo/AstraZeneca'sEnhertu,havebeenapprovedfornewindicationsandaccelerated toseizethemarket.OfparticularinterestistheexplosivesalesgrowthofEnhertu fromDaiichiSankyo/AstraZeneca,whichsurgedfrom$426millionto$1234billion
Kadcyla:TopADCSeller
Kadcyla(Adptrastuzumabemtansine),co-developedbyRocheandImmunoGen,wasfirst approvedbytheFDAonFebruary22,2013forHER2-positivemetastaticbreastcancer
KadcylareportedrevenuesofCHF2.08billion,orapproximately$2.265billion,in thefullyear2022,withyear-over-year(CER)growthof7%,showingslowergrowth comparedtothe16%growthratein2021
In2023,Rocheannouncedapricecutofmorethan50%forKadcylainChina,hopingto capturethemarketthroughpricecuts.
AdcetrisSalesin2022
Adcetris®(brentuximabvedotin),originallydevelopedbySeagen(formerly SeattleGenetics)andlaterco-developedwithTakeda,isthefirstADCapprovedfor first-linetreatmenttargetingCD30/CD16,andhasgainedworldwidepopularitysinceits
launchFrom2011to2021,AdcetrishasbeenapprovedbytheUSFDA,ChinaNMPA andEUEMAforthetreatmentofHodgkinlymphoma(HL)andrelapsedsystemic anaplasticlargecelllymphoma(sALCL)
Adcetrishasglobalsalesofupto$659millionin2020;doublingto$1.306billionin 2021andfurthergrowingto$1.48billion(JPY194.3billion)in2022.
EnhertuSalesin2022
Enhertu,co-developedbyDaiichiSankyoandAstraZeneca,hasshownthefastestsales growth
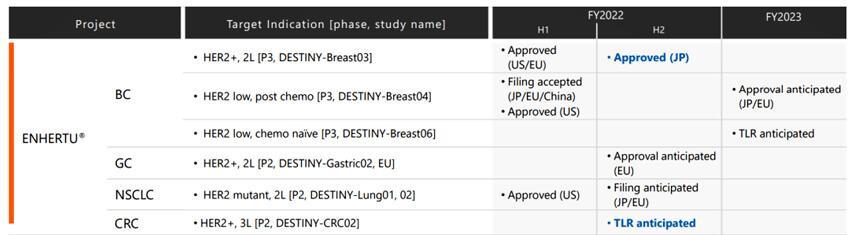
In2019,EnhertufirstwonFDAapprovalforsomepatientswithHER2-positive unresectableormetastaticbreastcancerTwoyearslater,thedrugwonanindicationfor previouslytreatedHER2-positiveadvancedgastriccancerandnon-smallcelllungcancer (NSCLC).Itisalsotheworld'sfirsttherapyforHER-2low-expressingbreastcancer.
FigureEnthertuIndications
Benefitingfromtheapprovalofnewindicationsin2022andthegradualpenetrationinto moremarkets,Enhertubrokethebillion-dollarbarrierthistimewithsalesofJPY 1616billion(approximately$1234billion),analmosttwofoldincreasecomparedto thepreviousyear'ssalesof$426million.
Inaddition,EnhertuisnotjustsatisfiedwithitscurrentresultsInadditiontobreast,lung andgastriccancers,Enhertuisalsoconductingclinicaltrialsforawiderangeofcancers, includingcolorectal,uterine,pancreaticandbiliarytractcancers
TrodelvySalesin2022
TRODELVY®(sacituzumabgovitecan-hziy)isaTrop-2-directedantibodyand topoisomeraseinhibitorconjugateCurrently,Trodelvyhasbeenapprovedinmorethan 40countriesworldwide,withthefirstapprovalin2020bytheFDAforthetreatmentof patientswithmetastatictriple-negativebreastcancer(TNBC)whohavefailedtwoormore systemictherapies,aswellasacceleratedapprovalforthesecond-linetreatmentof patientswithmetastaticuroepithelialcancer
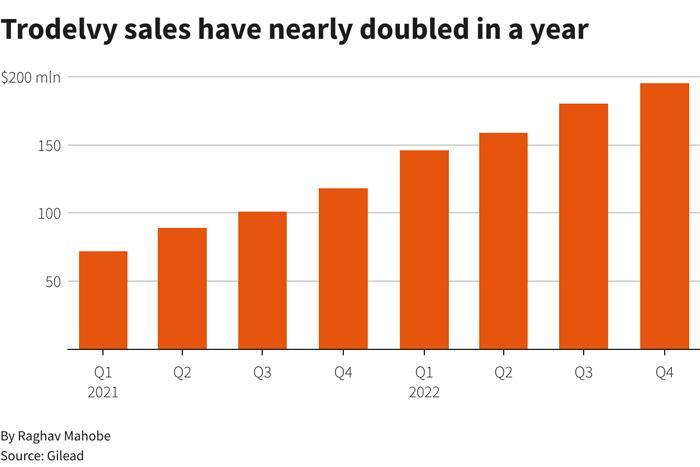
Trodelvysalesincreased78.9%to$680millionforthefullyear2022comparedto 2021.Meanwhile,thedrughasjustbeenapprovedforthetreatmentofHR+/HER2-breast cancerandisexpectedtocontinueitsexplosivegrowthin2023