Huateng Pharma
https://us.huatengsci.com
From ADC (Antibody-Drug Conjugate) to PDC (Peptide-Drug Conjugate) ADC drugs are favored by innovative pharmaceutical companies because of their combined therapeutic effects. PDC (Peptide-Drug Conjugate), as a new type of conjugated drug, has also begun to attract attention in the research and development field due to its unique advantages. There are currently 14 ADC drugs approved in the world, which have undergone three generations of technological changes. However, only two PDC products were approved, namely Lutathera and Pluvicto (both polypeptide-nuclide conjugates), which were approved by Novartis in 2018 and 2022. Compared with ADC, PDC has lower molecular weight, strong tumor penetration and low immunogenicity. In addition, compared with the complex process of antibody production, PDC is easier to synthesize and purify, with good uniformity and lower production cost. PDC is expected to become a new generation of targeted anticancer drugs following small molecule drugs, monoclonal antibodies and ADC drugs.
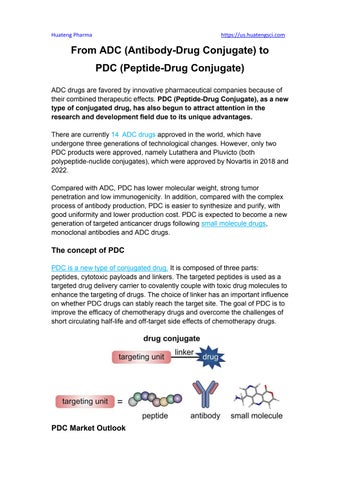
The concept of PDC PDC is a new type of conjugated drug. It is composed of three parts: peptides, cytotoxic payloads and linkers. The targeted peptides is used as a targeted drug delivery carrier to covalently couple with toxic drug molecules to enhance the targeting of drugs. The choice of linker has an important influence on whether PDC drugs can stably reach the target site. The goal of PDC is to improve the efficacy of chemotherapy drugs and overcome the challenges of short circulating half-life and off-target side effects of chemotherapy drugs.
PDC Market Outlook