DevelopmentofNasalSprayVaccines
Nasalsprayvaccinesareapromisingrouteofvaccinationandofferuniqueadvantages, especiallyinthepreventionofinfectiousdiseasestransmittedviatherespiratorytract Comparedtoinjectablevaccines,nasalsprayvaccinesalsohavetheadvantageofbeing lessinvasiveandeasiertostoreanddistribute
Thenasalsprayvaccineforinfluenzahasbeenusedstablyformorethanadecade,and thefirstnasalsprayCOVID-19vaccinehasbeenapprovedforemergencyuseinChina andIndiaHere,wefocusontheinfluencingfactorsandcurrentstatusofthenasalspray vaccinedevelopment
PhysiologicalStructureandImmunogenicityof theNasalCavity

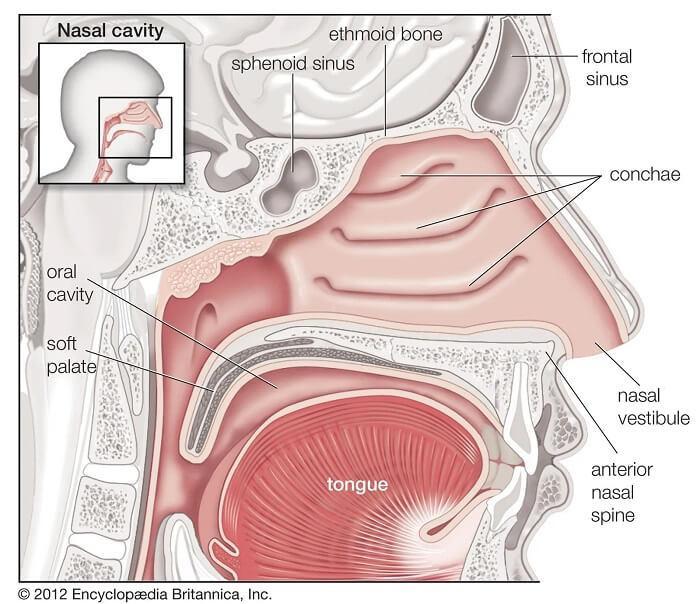
Thenasalcavityreferstotheinteriorofthenoseorthestructurethatopensexteriorlyat thenostrils.Itistheentrancetotheinhaledairandisthefirstofaseriesofstructuresthat makeuptherespiratorysystemThenasalcavityiscompletelylinedbythenasalmucosa, oneoftheanatomicalstructuresthatformthephysicalbarriertothebody'simmune systemThesebarriersprovidemechanicalprotectionagainsttheinvasionofinfectious andallergicpathogens.
Vaccinesarealsoforeignantigens,andthenasalcavityactivatesimmunemechanisms whenexposedtoforeignantigens(vaccines)Uponenteringthenasalcavity,theantigen istrappedonthemucosalepitheliumandthemicrofoldcells(Mcells)ofthelymphoid follicularepitheliumthenabsorbtheantigenparticlesSubsequently,theantigenismoved tothebasalendofthecellandpassedontoantigen-presentingcells(APCs),suchas
dendriticcells(DCs)andmacrophagesThereafter,DCcellsmigratethroughafferent lymphaticvesselstonearbydraininglymphnodeswhereantigenispresentedtonaiveT andBcellsviatheMHC-IIcomplex
Nasal-associatedlymphoidtissues(NALTs)areanimportantcomponentofmucosal immunityintherespiratorytractnALTshaveahighdistributionofMcellsandarethe mainsiteofuptakeofmacromoleculardrugsandparticlesinthenasalcavity,aswellas themainsiteofantigendeliveryinthenasalimmuneresponseNALTshave antigen-specificeffectorBandTcellsthatdifferentiateintoplasmacellsthatproduce aggregatedIgAandIgGandeventuallydifferentiateintosIgA,triggeringanimmune responsethatinterceptsantigensandpathogensMucosalimmunityalsohasaunique setofmechanismsforco-immunitythatinducesanimmuneresponseindistalmucosa, suchasthedigestiveandgenitaltractmucosa,butusuallytheintestinalimmunity activatedbynasalsprayvaccinesislesseffective.
Similartonasaladministration,nasalsprayvaccinesneedtoovercomemultiple constraints,suchastheeffectofnasalmucosalciliaclearance,theproblemofshort residencetimeinthenasalcavity,andtheobstructionofantigensbythemucusand epitheliallayersofthenasalmucosalsiteThesolutionoftheseproblemsoftenalso requirestheassistanceofimmuneadjuvantsandpenetrationenhancers
KeyTechnicalFactorsintheDevelopmentof NasalSprayVaccines
Mostofthecurrentlymarketednasalvaccinesareliveattenuatedinfluenzavirusvaccines Wenowpresentseveralkeyfactorsthatshouldbeconsideredwhenresearchingand developingnasalsprayvaccines,mainlyfromtheperspectiveoftheimmuneprocess
SuccessfulnasaldeliveryfirstrequiresthecooperationofasuitabledeviceAlthoughthe nasalcavityhasalargemucosalsurfacearea,intranasaldeliveryofvaccinesislimitedby nasalanatomyandaerodynamics,wheredrugswithlargeparticlesizesareusually depositedintheanteriornoseandconsequentlyexpelledorwipedoff,whilesmallparticle sizes(ie,<10μm)maybypassthenoseandenterthelungsThus,asuitabledeviceis neededtodispersetheintranasalvaccineformulationintoappropriateparticlesizesfor deliverytotheposteriorregionofthenose,andachievingabalanceofparticlesizesis criticaltoincreasingantigenexposuretothenasalmucosaFluMistvaccinescurrentlyis deliveredusingtheAccuSpraynasaldeliverysystemInaddition,MADNasalfrom Teleflex,ViaNasefromKurve,PuffHalerfromAktiv-Dry,andSoloventfromBDalloffer nasalmucosalnebulizationsolutions
Todate,allapprovednasalsprayvaccineshavebeeninliquidformbecauseofthehigh solubilityandpotencyofliquidvaccines.However,liquidformulationsarefluidandany liquidthatexceedsthenasalvolumewillbedrainedfromthenasalcavity,andonlyhighly solubleorlowdoseantigenscanbedeliveredintranasallyvialiquidformulationsThe applicationofdrypowderformulations,ontheotherhand,requiresaprolongedresidence timeinconjunctionwithmucoadhesivepolymerssuchasstarchandchitosantoimprove antigenavailabilitytothenasallymphatictissue
2.ProlongingTheResidenceTimeOfVaccine–Mucoadhesives
MucoadhesivesarecommonlyusedinthedevelopmentofnasalsprayvaccinesOnce themucoadhesiveagentinthevaccinecomesintocontactwiththemucusofthenasal epithelium,thepolymerhydrates,swellsandbindstothemucus,prolongingtheresidence timeofthevaccineinthenasalmucosaInaddition,mucoadhesivesalsotemporarilyslow mucusciliaclearanceanddonotinhibitantigenreleasefromthevaccineCommonly usedmucoadhesivepolymersincludeegchitosan,starch,etc
3.PromotingTransmembranePenetrationofVaccinesPermeationEnhancers
Thenasalepitheliallayeriscoveredbyalayerofmucus,andnasalspray vaccines/antigensneedtocompletethepenetrationofthemucuslayerandepithelialcells. Firstly,itisnecessarytopenetratethemucuslayerPolyethyleneglycol(PEG)canbe usedasamucuslayerpenetrationenhancer,whilemannitolcandilutethemucus Therefore,theuseofPEG,mannitolorothermucuspenetrationenhancersinthe formulationcanhelpimprovethepenetrationofvaccines/antigensintothemucuslayer.
Thetransportofantigenacrosstheepithelialcellbarrierisacriticalstepintheinductionof immunityAntigenscanpenetratetheepitheliallayerthroughcellsoracrosscells,and permeationenhancerssuchaschitosanandbacterialtoxinsareoftenusedinthisprocess toincreasethepermeabilityofantigenstothemucosaBothchitosanandbacterialtoxins candisruptepithelialjunctionsandenhanceparacellularmotilitySpecifically,chitosan promotestranscellularuptakeofantigensbyMcellsinthenasalmucosaandintestinal mucosa.Incontrast,enterotoxinsdisruptthemucosalepitheliumandenhanceantigen uptakebyDCcells,leadingtoupregulationofCXCR4andCCR7,therebyallowingDC cellstomigratetolymphnodes
4.ReducingPotentialIntracranialTransport–Nose-to-Brain TransportInhibitors
Animportantsafetyconcernregardingnasalsprayvaccinesisthepotentialfortransport ofvaccineantigensoradjuvantsfromtheolfactoryepitheliumtothecentralnervous system(CNS),althoughresearchersanalyzing460adverseeventsassociatedwith FluMistfoundonlyonecaseofencephalitisandlaboratoryconfirmationthatitwascaused byanenterovirusHowever,thenasalmucosaprovidesadirectentrypointbetweenthe externalenvironmentandthecentralnervoussystem(nose-to-brainpathway),and compoundscanenterthecerebrospinalfluidthroughthenervesassociatedwiththe olfactoryarea.Therefore,neurophilicityneedstobeevaluatedinnasalsprayvaccine development
5.EnhancingtheImmunogenicityofAntigens-Vaccine Adjuvants
AdjuvantsthattargetAPCantigensintheprocessofimmunizationcanbeusefulas immunostimulants.Commonlyusedimmuneadjuvantsareinsolublealuminumsaltsand TLRagonists
InsolublealuminumsaltsareclassicalFDA-approvedimmuneadjuvantsSeveral aluminumsaltsarecurrentlyusedinvaccinesapprovedintheUnitedStates,suchas Alhydrogel(aluminumhydroxide),Adju-Phos(aluminumphosphate),andamorphous aluminumphosphatesulfate(AAPS)Theimmunostimulatoryeffectsofaluminumsalts arisefromavarietyofmechanisms,includingslowingthespreadofantigenfromthesite ofadministration,increasinginflammatorycellaccumulation,activatingcomplement,and inducingthedifferentiationofmonocytesintoDCsaswellasuptakeofantigen,antigen delivery,andactivationofCD4+TcellsbyDCs
TLRagonistscanactasadjuvantsformucosalimmunity;specifically,TLRagonists functionaspathogen-associatedmolecularpatterns(PAMPs)andactbybindingto pathogenrecognitionreceptors(PRRs)onDCcellsCommonlyusedareTLR2/6agonists, TLR3agonists,TLR3agonists
