ClinicalToxicityOfAntibodyDrugConjugates
Antibody–drugconjugates(ADCs)havemadeconsiderableprogressin10 years.SeveralADCsarerankedamongthefirst-linetherapies,providing significantclinicalbenefitstooncologypatientsHowever,theirsideeffects shouldnotbeignored.Interstitiallungdisease,hematologictoxicity,hepatic andrenaltoxicity,andblackboxwarningscontinuetolimittheclinical performanceofADCstosomeextent.Therefore,theuseofADCrequires carefulmonitoringandresponsetoadversereactionsInaddition,weneedto optimizethestability,smallmoleculetoxicity,clearancerateandotherPK/PD parametersfromtheADCmoleculeitselfinordertofindanewgenerationof saferADCs.
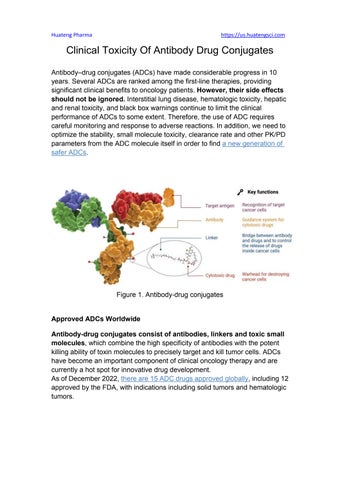
Figure1Antibody-drugconjugates
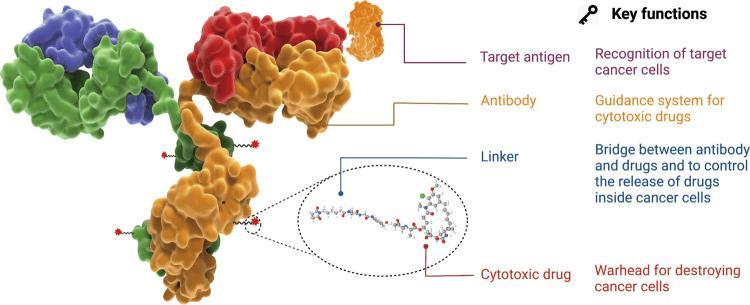
ApprovedADCsWorldwide
Antibody-drugconjugatesconsistofantibodies,linkersandtoxicsmall molecules,whichcombinethehighspecificityofantibodieswiththepotent killingabilityoftoxinmoleculestopreciselytargetandkilltumorcells.ADCs havebecomeanimportantcomponentofclinicaloncologytherapyandare currentlyahotspotforinnovativedrugdevelopment.
AsofDecember2022,thereare15ADCdrugsapprovedglobally,including12 approvedbytheFDA,withindicationsincludingsolidtumorsandhematologic tumors
HuatengPharma https://ushuatengscicom
ToxicSideEffectsofADCs
The"magicbullet"ADCcouldprovidesignificantclinicalbenefitstopatients andevenchangethewayoncologyistreated.
Adcetris,developedbySeagentotargetCD30,hasbecomeafirst-linetherapy forrefractoryHodgkin'slymphomaandperipheralT-celllymphoma,improving patientoutcomes
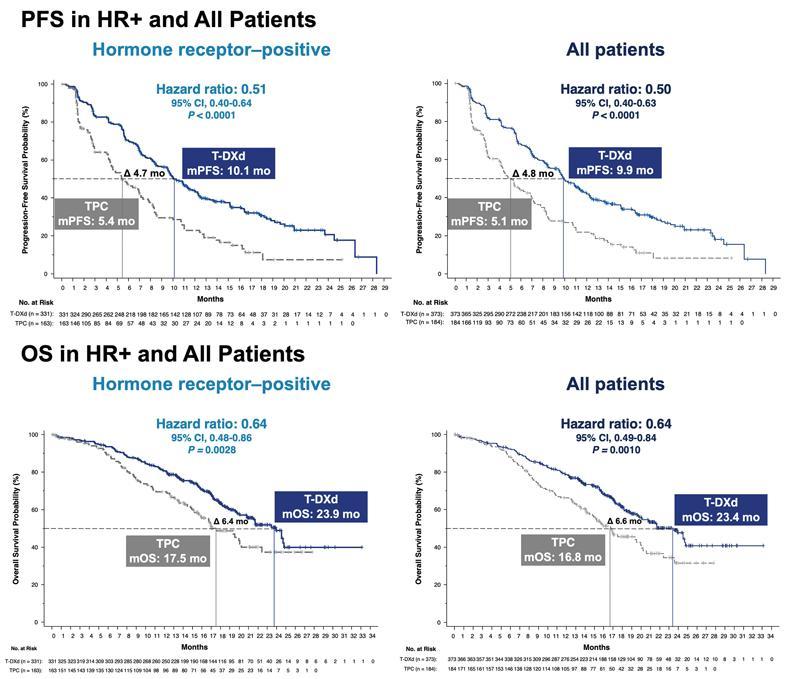
DaiichiSankyo/AstraZeneca'sDS-8201,ontheotherhand,hasrevolutionized theentireHER2+breastcancertreatmentlandscape,withequallyexcellent efficacyinpatientswithHER2-breastcancerIntheDESTINY-Breast04trial presentedatASCOthisyear,DS-8201extendedmedianprogression-free survivalfrom5.4monthsto10.1monthsandmedianoverallsurvival from17.5monthsto23.9monthsforpatientswithHER2low-expressing breastcancercomparedtochemotherapy,achievingabreakthroughinthe efficacyofHER2-targetedagentsinpatientswithHER2low-expressingbreast cancerOnAugust5,2022,theFDAapprovedDS-8201forunresectableor metastaticHER2low-expressingbreastcancer,rewritingtheclassification criteriaforbreastcancer
Figure3Progression-FreeSurvivalandOverallSurvivalResultsin HR-PositiveandAllPatientPopulationsfromtheDESTINY-Breast04Trial OnAugust11,2022,theFDAalsoacceleratedtheapprovalofDS-8201for non-smallcelllungcancerwithHER2mutations,makingitthefirst HER2-targetedtherapyforlungcancer.
HuatengPharma https://ushuatengscicom
However,themultiplecombinationsofantibody-linker-toxinmoleculesarelike adouble-edgedsword,whichbringspowerfulefficacytoADCdrugs,butalso bringsthedisadvantageofhigherincidenceandseverityoftoxicsideeffects thangeneralmonoclonalantibodiesandsmallmolecules.
Amongthe12ADCsapprovedbytheFDA,onlyLoncastuximabtesirine targetingCD19approvedin2021andPolatuzumabvedotintargeting CD79approvedin2019werenotaddedwithblackboxwarnings,while theremaining10ADCswereaddedwithblackboxwarningsTheprobabilityof blackboxwarningsismuchhigherthanthatofcommonsmallmoleculesand monoclonalantibodies,andthetoxicitiesinvolvedincludesevere hepatotoxicity,oculartoxicity,severeskinreactions,neutropenia,progressive multifocalleukoencephalopathyandinterstitiallungdisease(ILD)
(1)Accordingtoameta-analysisencompassing169clinicaltrialswithatotalof 22,492patients[1],theoverallincidenceofallgradesofadversereactions whentreatedwithADCdrugswas912%,andtheincidenceofgrade≥3 adversereactionswas46.1%,withcommonadversereactionssimilartothose reportedwithchemotherapydrugs,suchaslymphocytopenia(530%),nausea (441%),neutropenia(437%),blurredvision(405%),andperipheral neuropathy(39.6%),whichweremainlydose-limitingoff-targettoxicities. PatientdeathduetoADCtreatmentwasreportedin153ofthese169studies, withanoverallincidenceof1.3%,andtheimmediatecausesofdeathwere primarilypneumonia,sepsis,andrespiratoryfailure
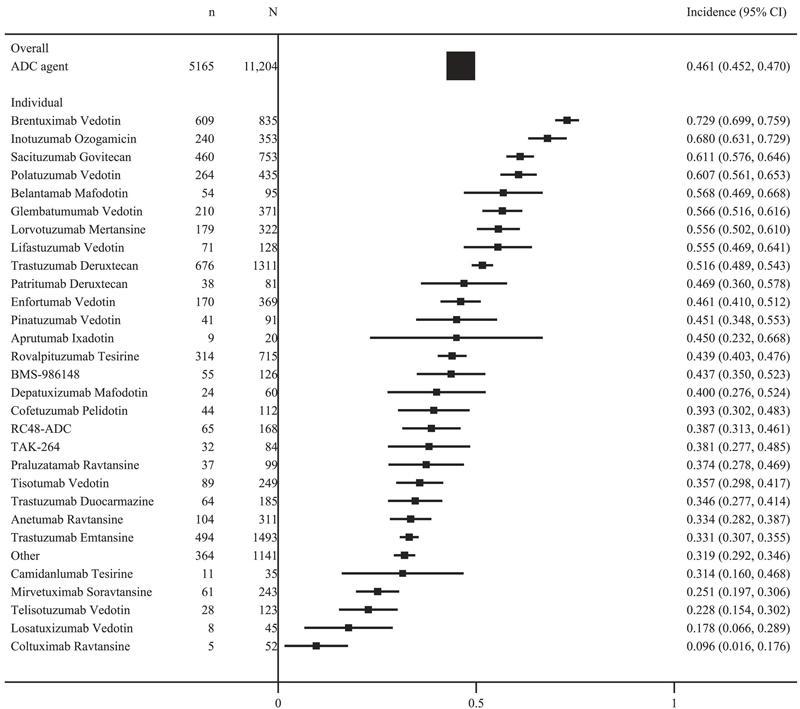
HuatengPharma https://ushuatengscicom Figure4Theoverallincidenceofgrade≥3treatment-relatedadverseevents inADCregimens.ADCindicatesantibody–drugconjugate.Source:rference [1]
(2)ThespectrumandincidenceofadversereactionsvaryfordifferentADC drugstheresultsoftheDESTINY-Breast01trialshowedthattheadverse reactionsofEnhertuweremainlyderuxtecan-relatedsideeffects,suchas nausea(79%),decreasedwhitebloodcellcount(70%),decreased hemoglobin(70%),decreasedneutrophilcount(62%),fatigue(59%),vomiting (47%),alopecia(46%),increasedaspartateaminotransferase(41%), increasedalanineaminotransferase(38%),decreasedplateletcount(37%), constipation(35%),decreasedappetite(32%),anemia(31%),diarrhea(29%), hypokalemia(26%),andcough(20%),witha9%incidenceofinterstitiallung diseasewarnedbyblackboxesanda26%incidenceofdeathdueto interstitiallungdisease[5].
(3)TherecentlyapprovedMirvetuximabSoravtansine,ontheotherhand,has ablackboxwarningaddedforoculartoxicity,whichmaycauseseriousocular sideeffectsincludingvisualdisturbances,keratopathy,dryeye,anduveitis. Accordingtosafetydatapresentedatthe2022ASCOCongress[7],themost commonadverseeffectsofMirvetuximabSoravtansineincludedblurredvision (63%),fatigue(58%),keratopathy(43%),anddryeye(35%).Thisissimilarto BelantamabmafodotindevelopedbyGSK,whosemainadverseeffectwas alsooculartoxicity,probablyduetothetendencyofthepayloadofbothto accumulateinthecornealepithelium
Off-TargetToxicityasaMajorSourceofAdverseReactions
BothcytotoxicdrugsandantibodiesofADCcanaffectnormalcells,whichcan leadtoadversereactionsItsadversereactionscanbedividedinto on-targetandoff-targettoxicity,whereon-targettoxicityreferstothekilling ofnormaltissuesexpressingtargetantigensbyADCdrugsandoff-target toxicityreferstothekillingofADCsintissuesnotexpressingtargetantigens Accordingtoclinicalobservations,off-targettoxicitycausedbycytotoxicdrugs isthemainsourceofADCadversereactions
On-targettoxicity:Antibody-mediatedADCCandCDCeffectscanalloccur onnormalcellsexpressingthetargetantigenandleadtoadverseeffects,such assecondaryrenalinjuryInaddition,antibodiescanalsoblockthesignaling ofnormalcellulartargetantigens,forexample,HER2-targetedADCcanacton HER2-expressinglungepithelialcells,cardiomyocytes,andcauseadverse effectssuchaslunginjury,hepatotoxicity,andreducedLVEFbyeither blockingtheHER2pathwaybyantibodiesordirectkillingbycytotoxicdrugs
HuatengPharma https://ushuatengscicom
Off-targettoxicity:Thesheddingofcytotoxicdrugsinthecirculation,the bystanderkillingeffectonnormalcells,andtheendocytosis/uptakeofADCs bynormalcellscanleadtooff-targettoxicity,exposingnormalcellstodamage bycytotoxicdrugsLymphocytes,granulocytes,andplateletsinthecirculation arethefirsttosufferdamage,followedbytheliver,whichtendstoaccumulate lipophilicsmallmolecules,andthekidneys,wherethedrugisexcreted,aswell asthelungs,nerves,skin,andothertissues,causingadversereactionssimilar tothoseobservedclinicallywithchemotherapydrugs
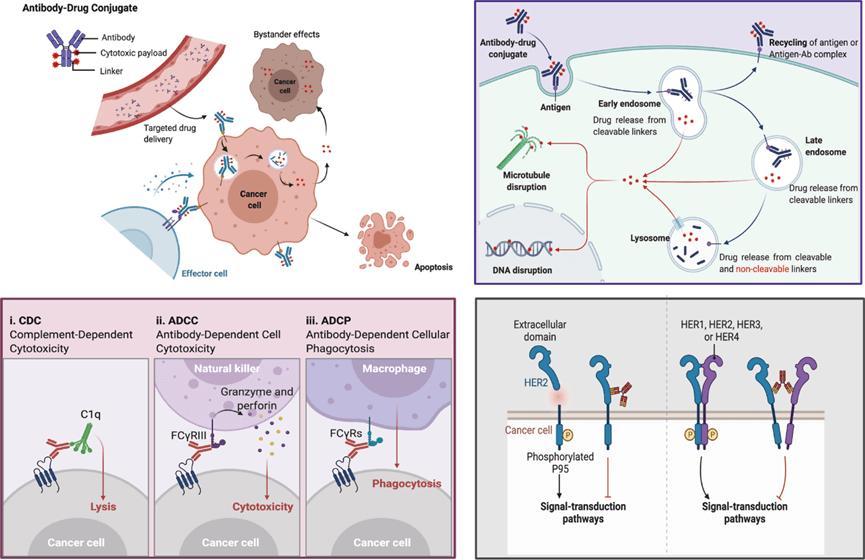
Figure5TheoverviewofthemechanismsofADCforkillingcancer cellsviadifferentapproaches.(Fu,2022)
Conclusion
ADCdrugshavebenefitedmanyoncologypatientsbyimprovingthetargeting ofsmallmoleculesbylinkingcytotoxicdrugstoantibodiesHowever,current ADCdrugshavenotyetdemonstratedbettersafetythanchemotherapeutic smallmoleculesormonoclonalantibodiesduetosignificantlyhigherpayload toxicityandunstablelinkercirculation.
Therefore,ontheonehand,weneedtomonitorandmanagetheriskofside effectsintheclinicalapplicationofADCsandrespondtotheadverseeffectsin atimelymanner;ontheotherhand,weneedtocontinuouslyoptimizetheir antibodies,linkersandcytotoxicdrugstoimprovePK/PDpropertiesand enhancedeliveryefficiency,soastoachievestrongdrugefficacyunderweak toxicity
HuatengPharma https://ushuatengscicom
References:
[1]ZhuY,LiuK,WangK,ZhuH.Treatment-relatedadverseeventsof antibody-drugconjugatesinclinicaltrials:Asystematicreviewand meta-analysisCancer2022Nov21
[2]CarrieWynn,RiteshPatel,Shou-ChingTangetal.Increasedsystemic toxicitiesfromantibody-drugconjugates(ADCs)withcleavableversus non-cleavablelinkers:Ameta-analysisofcommerciallyavailableADCs. JournalofClinicalOncology202240:16suppl,3032-3032
[3]Ma,F,Xu,B.Expertconsensusontheclinicalapplicationofantibody-drug conjugatesinthetreatmentofmalignanttumors(2021edition)Cancer Innovation.2022;1:3–24.
[4]Lievano,FA,Scarazzini,LJ,Tyczynski,JEetalRiskMinimizationof Antibody–DrugConjugatesinOncology:AReview.DrugSaf44,733–742 (2021)
[5]https://www.enhertuhcp.com/en/breast/efficacy/destiny-breast-01
[6]EatonJS,MillerPE,MannisMJetalOcularAdverseEventsAssociated withAntibody-DrugConjugatesinHumanClinicalTrials.JOculPharmacol Ther2015Dec;31(10):589-604
[7]https://www.immunogen.com
RelatedArticles:
DirectionsforNextGenerationAntibody-DrugConjugates
ApprovedAntibody–DrugConjugates(ADCs)andInClinicalTrials
WhatArePEGLinkersandTheirApplications?
Trodelvy:AnyChanceAgainstDato-DXdandEnhertu?
FromADC(Antibody-DrugConjugate)toPDC(Peptide-DrugConjugate) BispecificAntibodies–FastGrowingTherapies