Biopharma PEG
https://www.biochempeg.com
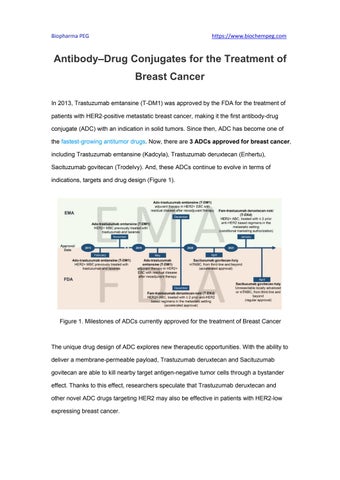
Antibody–Drug Conjugates for the Treatment of Breast Cancer In 2013, Trastuzumab emtansine (T-DM1) was approved by the FDA for the treatment of patients with HER2-positive metastatic breast cancer, making it the first antibody-drug conjugate (ADC) with an indication in solid tumors. Since then, ADC has become one of the fastest-growing antitumor drugs. Now, there are 3 ADCs approved for breast cancer, including Trastuzumab emtansine (Kadcyla), Trastuzumab deruxtecan (Enhertu), Sacituzumab govitecan (Trodelvy). And, these ADCs continue to evolve in terms of indications, targets and drug design (Figure 1).
Figure 1. Milestones of ADCs currently approved for the treatment of Breast Cancer
The unique drug design of ADC explores new therapeutic opportunities. With the ability to deliver a membrane-permeable payload, Trastuzumab deruxtecan and Sacituzumab govitecan are able to kill nearby target antigen-negative tumor cells through a bystander effect. Thanks to this effect, researchers speculate that Trastuzumab deruxtecan and other novel ADC drugs targeting HER2 may also be effective in patients with HER2-low expressing breast cancer.