








Driving sustainability and carbon neutrality, MECS specializes in sulfuric acid and environmental technology processes for non-ferrous metal, fertilizer and chemical industries. As a world leader in optimized solutions for carbonless energy generation, smelter air pollution control, oleum & sulfur trioxide production for e-grade acid in support of semiconductor manufacturing, high-efficiency mist elimination for green hydrogen processes, and much more, we are on the leading edge of technology advances for a cleaner tomorrow.
Contact our experts today.
MECS.ElessentCT.com








Welcome to the Spring/Summer 2023 issue of Sulfuric Acid Today magazine. We have dedicated ourselves to covering the latest products and technology for those in the industry, and hope you find this issue both helpful and informative.

As we are preparing this issue for press, we are also wrapping up the plans for our 2023 Australasia Sulfuric Acid Workshop, April 2-5, in Brisbane, Queensland. This will be our first international conference since the coronavirus pandemic shut down most of the world in early 2020. This year’s Australasian sulfuric acid workshop is packed with 2.5 days of informative technical presentations and panel discussions that will delve into topics such as: sulfuric acid operations, troubleshooting, acid towers, vertical acid/sulfur pumps, mist elimination, converters, catalyst handling, main blowers, analyzers, acid piping and ducting, sulfur melting, precipitators, heat exchangers, and hydrogen formation and risk mitigation. Fiona Boyd of Acuity Commodities will present the keynote address on the changing sulfuric acid market dynamics. Since Acuity Commodities’ last article in the Fall/Winter 2022 issue of Sulfuric Acid Today, sulfuric acid pricing was under downward pressure as market sentiment was eroding and freight rates for certain trade routes remained at elevated levels. The downward pressure is continuing to mount over the sulfuric acid market mainly due to weak spot demand. Please see Acuity Commodities’ informative article “Uncertainty Remains in Early 2023,” on page 10 for further details.


Also in this issue, we have several illuminating articles regarding state-of-the-art technology and projects. Our cover story, on page 7, focuses on how Industrias Básicas de Caldas (IBC) of Colombia brought wastewater into compliance and recycled SO2 into increased sulfuric acid production, which netted a host of other benefits as well; VIP International ex-

plains the importance of protecting workers from high risk exposures (page 12); INTEREP shares knowledge on how to gain efficiency from the main blower (page 14); Mercad delves into the history of the anodically protected acid cooler (page 16); Elessent MECS Technologies explains how to minimize halide and its effects in strong acid systems (page 18); Central Maintenance & Welding shares how communication is integral for a successful project (page 20); Ecorobotics explains how their robotic tank entry equipment can provide safety and save money (page 22); NORAM Engineering & Constructors shares their CFD-aided design for sulfur furnace optimization (page 24); Topsoe announces its newest catalyst that increases acid production and lowers carbon footprint (page 28); Mosaic shares its recent capital improvements in Florida (page 32); KCM 2000 Holding of Bulgaria overhauls smelting operation with flexible, cleaner solution (page 34); and Savino Barbera of Brandizzo, Italy, has replaced metal with plastic in its line of industrial pumps and mixers (page 36).
I would like to welcome our new and returning Sulfuric Acid Today advertisers and contributors, including: Acid Piping Technology Inc., Acuity Commodities, BASF, Beltran Technologies, Central Maintenance & Welding, Clark Solutions, Ecorobotics, Elessent MECS Technologies, Mercad, GORE, INTEREP, Knight Material Technologies, NORAM Engineering & Constructors, Optimus, Southwest Refractory of Texas, Savino Barbera, Spraying Systems Co., STEULER-KCH GmbH, Topsoe, VIP International, and Weir Minerals Lewis Pumps.


We are currently compiling information for our Fall/Winter 2023 issue. If you have any suggestions for articles or other information you would like included, please feel free to contact me via email at kathy@h2so4today.com. I look forward to hearing from you.
Sincerely,
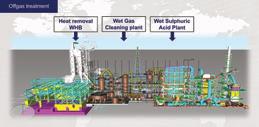
Kathy HaywardHELSINKI, Finland—Almalyk Mining and Metallurgical Company (AMMC) has awarded Metso Outotec an order for the delivery of two sulfuric acid plants to be built for AMMC’s zinc roasting facility in Almalyk, Uzbekistan. The value of the order is approximately EUR 70 million.
Metso Outotec’s scope of delivery includes the design and delivery of Planet Positive equipment for two gas cleaning and sulfuric acid plants, which will process all off-gases from the zinc roasters into industrial-grade sulfuric acid. In addition, Metso Outotec will deliver utility facilities, such as a common cooling tower system and a common air compressor system.
The two plants to be delivered by Metso Outotec will be identical and will replace AMMC’s existing facilities for gas cleaning and sulfuric acid production. The plants will improve operational efficiency and reliability and significantly reduce the facilities’ environmental impact. The sulfuric acid plants are expected to be operational by the end of the second half of 2025.
“We are extremely pleased that Almalyk has again selected us as the partner for providing gas cleaning and sulfuric acid technology. Metso Outotec’s advanced
Planet Positive gas cleaning and sulfuric acid plant solution will improve the environmental performance of AMMC’s metallurgical operations,” says Hannes Storch, Vice President, Metal and Chemical Processing at Metso Outotec.
For more information about Metso Outotec sulfuric acid plants, please visit www.mogroup.com.

RAVENSTHORPE, Western Australia—In an effort to lower carbon emissions, the Ravensthorpe nickel mine on Western Australia’s south coast has switched to wind power. The proposed installation will take advantage of the strong winds coming in from the Southern Ocean and will likely generate 18–20 megawatts, reducing the mine’s reliance on diesel generators.
First Quantum Minerals’ Perth-based regional manager of projects and operations Gavin Ashley described Ravensthorpe as a prime location for wind energy. “We’re not connected to the grid,” he said. “The way we produce power is from waste heat in steam turbines as we burn sulfur to generate the sulfuric acid, which we use in the nickel process. We also have stand-by diesel generators and recently we’ve identified that we’re short of power and we’re using our diesel generators a
lot more, so that’s triggered this motivation to look at how we can offset that.”
First Quantum Minerals, a Canadianbased company, owns 70 percent of the Ravensthorpe mine, with South Korean steelmaker POSCO holding 30 percent.
Ashley said the company was part of a wider movement in the mining industry to embrace renewable energy. “Five (turbines) is probably the most likely scenario…we’re just completing the study,” he said. “We’re hoping to have that wrapped up in the next month, and then we’ll be looking at presenting to the board for approvals and funding. The ultimate timeline will depend on two real factors—the time it will take to do the various environmental and government approvals and the supply chain availability of turbines in the market.” Ashley said the wind turbines should be operational by late 2024 or early 2025.
For more information, please visit gorno.com.au.
NERATOVICE, Czech Republic—Nuberg
EPC, a leading Indian Global EPC and turnkey project management company, has announced that it has been awarded a prestigious 550 tpd sulfuric acid plant project on an EPC-based contract in Neratovice. The project is owned by Spolana s.r.o, a leading chemical manufacturer located on the Elbe riverbank in Neratovice. Spolana a.s. is one of the largest chemical manufacturing companies in the Czech Republic, the only manufacturer of PVC and caprolactam, and a producer of ammonium sulfate and sulfuric acid. The plant is expected to be delivered in three years, including project stages 1, 2, and 3. The company is taking up the project on EPC based contract, including the technology.
sulfuric acid plant project for the International Company for Fertilizers and Chemicals in Egypt, and a hydrogen peroxide plant for Uzbekistan Hydrogen JU LLC in Uzbekistan.
For more information, please visit www. nubergepc.com.
RIBAS DO RIO PARDO, Brazil—Andritz has received an order from Brazilian pulp producer Suzano to deliver a SulfoLoop concentrated sulfuric acid plant for the new pulp mill that Suzano is building in the municipality of Ribas do Rio Pardo, state of Mato Grosso do Sul. The pulp mill, delivered by Andritz, is in the construction phase with start-up scheduled for the second half of 2024.
The sulfuric acid plant will have the capacity to produce 153 tons of commercial grade (>97%) sulfuric acid per day from the pulp mill’s concentrated odorous gases and elemental sulfur, thus making the mill completely self-sufficient in sulfuric acid and recycling sulfur from the waste streams. The Andritz SulfoLoop solution is based on the wet-gas sulfuric acid (WSA) technology for converting wet, sulfur-rich gases and elemental sulfur into sulfuric acid.
For the past 30 years VIP International has led the industry in innovative equipment and procedures in maintaining sulfuric acid plants. As the industry demands longer run times between catalyst screening, VIP’s patented catalyst handling system ensures the longest run time with lowest pressure drops to ensure maximum performance of your converter. Contact VIP International to learn how to reduce your downtime and increase your production and on stream factor.

Sulfuric acid produced by the 550 tpd sulfuric acid plant project in Czech Republic will be used for petrochemical- and fertilizer-based applications. It will also be used in the mining and processing of some ores and minerals, manufacturing batteries, and etching surfaces. Sulfuric acid is also used in the manufacturing of caprolactam, which is used in the production of nylon and perlon, and subsequently in the textile and clothing industry.
Upon receiving the project, Mr. A. K. Tyagi, MD, Nuberg EPC, said: “This undertaking illustrates Nuberg’s competency of combining intelligence and knowledge in terms of engineering aspects. It is a pleasure that Spolana has chosen our EPC services for this project.”
Other recent Nuberg EPC projects include the caustic soda plant project for Lineer Kostik Soda Sanayi Anonim Sirketi in Turkey, a sulfuric acid project for Sprea Misr in Egypt, a sulfuric acid plant project for Awash Melkassa Chemical Factory in Ethiopia, a
The sulfuric acid mill will help the new Suzano mill to control the sodium and sulfur balance and the sulfidity of the pulp mill. Also, the resource-efficiency of the mill will be improved because less sulfate needs to be discharged due to the optimized Na/S balance. An added advantage is that there is no need for hazardous sulfuric acid transport to the mill, which results in a major reduction in the truck transportation volume. The sulfuric acid plant also meets very strict air emission limits and therefore brings a significant improvement to the overall footprint of the new Suzano plant in Ribas do Rio Pardo.
For more information, please visit www. andritz.com.

PICKERING, Ontaria, Canada—Arafura
Rare Earths Ltd has awarded Chemetics Inc., a wholly owned subsidiary of Worley and leading provider of technology for sulfuric acid and other specialty chemical facilities, the contract to install Chemetics CORE-SO2 sulfuric acid technology at its Nolans Project in the Northern Territory of Australia.
The scope of the contract is to deliver the detailed engineering and supply of the CORE-SO2™ sulfuric acid plant plus the associated oxygen plant on a lump sum basis. The sulfuric acid plant at Arafura’s Nolans Project will be designed to meet to -
morrow’s emission performance and clean energy transition goals.
Leveraging CORE-SO2’s high turndown capability and the potential to idle the plant while keeping the catalyst warm for extended periods of time, Arafura’s new acid plant will operate with 95% reduced SO2 emissions when compared to traditional Double Contact Double Absorption (DCDA) plants. High pressure steam production within the CORE-SO2™ process will allow CO2-free electrical power to be generated. By removing the use of a diesel or natural gas start-up burner, further greenhouse gas emissions will be prevented.
“With this project, we will assist in Arafura’s goal to be a trusted global leader and supplier of choice for sustainably mined and processed rare earth products. With each year of plant operation, more than 700 mt of SO2 will be converted to usable sulfuric acid rather than emitted into the atmosphere,” Chemetics Inc. President Andrew Barr said.
Additionally, being 60% smaller in size than traditional sulfuric acid plants, CORE-SO2™ will grant Arafura significant construction advantages. Low internal gas flows and fewer pieces of equipment enable modularization. This will minimize construction and assembly work for the remote mine site at the Nolans Project, leading to increased workforce safety and improved cost-efficiency.
For more information, please visit www. worley.com/what-we-do/our-technology/chemetics.
ST. LOUIS—MECS, Inc. (MECS), a subsidiary of Elessent Clean Technologies (Elessent), recently announced several upcoming projects around the globe.
The first project includes providing a sulfuric acid plant to Lithium Americas Corp. (LAC). The new 3,000 MTPD sulfuric acid plant will feature MECS® HRS™ technology to harness waste heat for steam generation which will be converted to carbon free energy. The new plant will be located at Lithium Americas’ Thacker Pass operation, the largest lithium carbonate processing plant in North America. The State of Nevada has become a critical supplier of lithium within the U.S. as the nation’s leaders make plans to reduce reliance on imported lithium.
The MECS® sulfuric acid technology provides sulfuric acid for the processing of battery raw materials while ensuring the most stringent control of site emissions. Another measure to reduce environmental impact includes the use of MECS® HRS™ technology in the sulfuric acid plant which will play a key role in decarbonization of the region. When combined with more traditional means of energy recovery within sulfuric acid plants, HRS™ enables a plant to utilize up to 95% of the process heat it generates internally as steam, which can be converted to electricity that is either employed to power
the facility or sold to a neighboring industrial complex or the local power grid.
In Indonesia, PT Amman Mineral Industri (AMIN), has partnered with Elessent for the provision of a new smelter off-gas MECS® sulfuric acid plant equipped with MECS® DynaWave® wet gas scrubbing technology. The new plant will be constructed in Sumbawa, Nusa Tenggara Barat, Indonesia with anticipated startup in 2024.
The MECS® sulfuric acid process design for AMIN incorporates state of the art technology including both MECS® preconversion technology, which offers a novel approach for processing gas streams with elevated sulfur dioxide concentrations, and MECS® DynaWave® wet gas scrubbing technology.
The new sulfuric acid plant will enable AMIN to provide high quality sulfuric acid to the local Indonesian market, as well as benefiting the country and region by tightly controlling air emissions from the smelter.
Meanwhile in Brazil, Brazilian chemical maker Unigel RI (Unigel)–one of Latin America’s largest chemical companies and Brazil’s top manufacturer of nitrogen fertilizers–has contracted with MECS for the construction of a new sulfur burning sulfuric acid plant to displace the import of sulfuric acid for their downstream chemical processes. The new plant will be constructed in the coastal state of Bahia. With sustainability as a core value, Unigel’s new plant will also be used to generate utility steam that will provide reliable and carbon-free power throughout their industrial complex.
The MECS® sulfuric acid process design for Unigel incorporates state of the art products and technologies, such as MECS® GEAR® catalyst for ultra-low emissions and high conversion, Brink® AutoDrain™ technology for operational efficiency, as well as UniFlo® distributors, acid coolers and acid piping. The addition of the new plant will also facilitate increased capacity for Unigel to better serve their clients, as well as benefiting the region by contributing to a more competitive sulfuric acid supply chain.
MECS has also established itself as a leader in the technology and equipment needed for the production of high purity sulfur trioxide and oleum that is required for high purity e-grade acid production used in the rapidly growing semiconductor industry. Ninety percent of e-grade acid plants in the key production countries of Taiwan, Korea, United States, and Japan have selected MECS® technology and equipment to ensure the most reliable production of these critical e-grade acid feedstocks. Further solidifying the company’s foothold in the marketplace and deepening the experience gap among competitors, MECS was recently selected to supply propriety technology and equipment to clients in the U.S. and Taiwan to enable production of high-purity e-grade acid for the global market.
For more information, please visit www. ElessentCT.com. q



Op�mus delivered its rst sulfuric acid plant waste heat recovery system in 1996. Across the power and process industries, we’ve produced more heat recovery boilers, HRSGs, superheaters, and economizers than any ac�ve company in the USA.

Op�mus and its Chanute Manufacturing plant have a long‐standing rep
uta�on for high‐quality workmanship and on‐�me performance. Cus‐tomers trust our unique manufacturing exper�se and have condence in our quality control and comprehensive project execu�on.
50 years of manufacturing experience
99% life�me on‐�me delivery performance
20 years experience in Sulfuric Acid waste heat recovery equipment
MONDI™ Pipe and fittings

- MONDI™
- Teflon Lined Piping

- Pipe Hangers
- Bolts - Gaskets

Tower & Converter Internals
- MONDI™ Distribution Systems



- MEEHANITE Converter Internals
- Ceramic Packing Saddles
- Ceramic Packing Support
- Quartz Rock
Flow Control
- Engineered Dampers & Valves for S02/S03 Applications
- PTFE/PFA Lined Valves
- 316SS, Alloy 20, 310SS Valves
- MONDI™ Thermowells
- PTFE Spargers
- Orifice Plates
- Pressure & Temperature Gauges
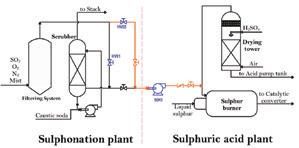
When the Colombian government tightened regulations on how much sulfate waste was permissible in wastewater, both Industrias Básicas de Caldas (IBC) and its customer next door were faced with a challenge. IBC supplies SO3 to an adjacent sulfonic acid facility that was releasing wastewater effluent with sulfates—and now the sulfate content must be reduced by at least 90%. After thoroughly testing several possible solutions, the final plan not only brought wastewater into compliance, but recycled SO2 into increased sulfuric acid production and netted a host of other benefits to boot.

Located in Manizales city, IBC produces sulfuric acid and provides diluted SO3 gas for a neighbor sulfonation facility. The company was established in 1988 by parent Química Básica Colombiana in association with investors from Caldas to serve the growing sulfuric acid market in central Colombia. Construction of the sulfuric acid plant began that year on the outskirts of Manizales city and by 1990 the first tons were produced and sold. In 1991, another investor built the sulfonic acid plant to use the SO3 supply from the sulfuric plant in the sulfonation process.
The sulfuric acid plant was designed and constructed by Conpancol-Ingenieros

Constructores (a subsidiary of Panamerican Consulting International Ltd.) with conventional technology—direct burning of sulfur followed by catalytic conversion via the contact method with double absorption in a (3+1) arrangement. Process gas flowed through the converter where most of the SO2 gas was converted to SO3. About 10% of the SO3 stream and remaining SO2 stream exited bed 3 and flowed to the customers’ sulfonation plant where the SO3 was used to make sulfonic acid and other surfactant products. Then the remaining stream was cleaned of SO2 and the clean gas released to the atmosphere.
This process worked well for many years while the sulfuric plant’s capacity remained at 100 MTPD. But when capacity increased to 125 MTPD in 2012, the sulfonic plant consumed much more caustic soda to clean the additional SO2 coming from the sulfuric plant’s converter, which in turn significantly increased the flow of sulfites and sulfates in the sulfonic plant’s wastewater. Then, in 2015 when the Colombian government passed stricter regulations for cleaner wastewater, both IBC and its SO3 customer began their quest for a resolution.
The new regulations allowed the plant five years to transition its process to compliance level. “The challenge,” explained IBC’s plant manager Héctor González, “was to research and implement an economical, functional, safe, and sustainable solution to remove 90% of sulfates from our customers’ wastewater or prevent their generation all together.”
An interdisciplinary engineering team of professionals from both sites was created to work on the project. After considering the risks, costs, and benefits of several options including replacing the converter and treating wastewater with reverse osmosis, the team determined the best long-term
By: April Smith, Editor, Sulfuric Acid Todaysolution was to return the SO2 gas stream back to the sulfuric plant, recover the SO2, and use it to make sulfuric acid. With the plan decided, both plants engaged in two years of feasibility testing to determine the best way to implement it.

Preliminary analysis considered the impacts to both plants given various gas entry points into the sulfuric plant. The team studied three possible entry locations: the drying tower, the converter, and the intermediate absorption tower (IAT). For each location, the team evaluated the pressure required to inject the return gas, the effect of the additional volume of gas, and the gas composition.
The pressure required depends on the distance between the main blower’s discharge and the gas injection point. The team had to select a blower that was suitably powered to accomplish the job, but not over-powered as to waste electricity.
The additional gas volume implies greater pressure loss of the equipment and an increase in flow rate through the beds of the converter, which in turn reduces conversion.
Finally, the composition of the gas is an important factor in the system. “The returning gas has a lower oxygen content than air and therefore will have a negative effect on conversion if fed to the first bed of the converter, but a positive effect if fed to the third or fourth bed of the converter,” said González. “Also, impurities in the return gas, particularly moisture and organic mist, can affect the catalyst, increase cor-
rosion, and reduce the quality of the sulfuric acid,” he said.
The team conducted a total of eight tests, analyzing results and modifying the system after each. They began with six tests that returned the gas stream to the IAT; then completed two additional tests directing the flow to the drying tower. Testing the return to the converter ultimately became unnecessary.
For the first six test runs, exit gases from the sulfonic plant were redirected to
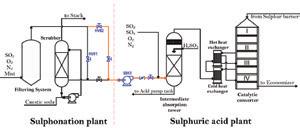
New converter pre-bed handles additional SO 2 -to-SO 3 conversion requirements. IBC’s new blower moves exhaust gas from customers’ sulfonic plant to IBC’s sulfuric plant and enables both plants to operate at capacity without significant pressure drop. IBC’s sulfuric acid plant in Manizales, Colombia with sulfur feedstock in foreground. ...To converterthe intermediate absorption tower of the sulfuric acid plant. A 10” diameter pipeline was installed to connect the plants and a centrifugal blower (50K1) was installed to move the gases. The direction of the gas flow was controlled by an HV01 control valve and pressure transmitters in the suction and discharge lines of the blower.
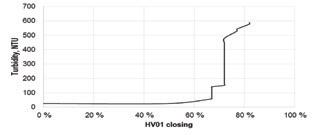
There was also very high turbidity in the sulfuric acid that increased as the return gas flow increased (see Fig. 2).
sulfur burner was the way to go and began implementation plans. The team worked with its catalyst supplier, Topsoe of Denmark, to further improve conversion.
“The new feed to the converter had a higher gas flow rate with a lower O2/SO2 ratio, which made the conditions more challenging for the catalyst,” explained Martin Alvarez, Solution Specialist at Topsoe. “Plus the existing converter had no free room available since the capacity had already been boosted 25% in the past,” he said.
The first test ran for 52 hours and results were mixed. Important successes included reducing sulfate content in the scrubber to below the new regulation level as well as significantly reducing consumption of caustic soda. Plus more than 80% of the SO2 present in the return gas was converted to SO3 in the fourth bed. However, the increased return gas flow (above 2.900 Nm3/h) resulted in considerable acid mist and a positive stick test in the IAT. At this point, the team stopped the test and determined the acid mist was caused by humidity in the gas stream from the sulfonation plant. So before the next test, the team identified the root cause and fixed the issue.
Because of turbidity and the high pressure drop, the team decided to try a variation of option two from the initial planning phase. The original plan directed the gas to the drying tower inlet, but the variation returned the gas directly to the sulfur burner (see Fig.3). The team ran two tests on the new flow design.
Topsoe ran simulations to determine the catalyst requirements needed to reach emission compliance while also minimizing costs. The team decided on an additional catalytic bed (a “pre-bed”) external to the converter positioned just before bed 1. Topsoe supplied 5.5m3 of vanadium catalyst for the new 3.5-m diameter, 304-stainless-steel vessel and replaced existing catalyst in beds 1 and 2.
Using the pressure loss data collected during testing, the team also defined specifications for a new blower capable of feeding all the return gas with no significant pressure loss in the equipment while both plants operate at capacity. For this, a two-stage, 3600 rpm, 450 mbar centrifugal blower was selected and purchased from FIMA, a supplier based in Germany.
To deal with strong and continuous changes of suction gas pressure, the team implemented a redundant and advanced control system. This was designed and installed by DCA Technology of Colombia.
Through the next three cycles of testing, analyzing results, and adjusting, the team moved the system closer to their goal. In addition to fixing the acid mist problem, they adjusted the inlet temperature to the converter’s third and fourth bed which increased conversion and reduced SO2 emissions from the sulfuric acid plant. They also achieved 100% gas return with the sulfuric plant operating below 100 MTPD. “This marked a significant milestone in reaching the required regulatory compliance and eliminating caustic soda consumption,” González said.
The next two test cycles focused on determining the maximum amount of SO2 that was possible to recover with the sulfuric acid plant operating at maximum capacity of 125 MTPD.
The results of these tests were a significant reduction in SO2 emissions and no acid mist in the IAT. But inlet pressure and pressure drop at this tower were very high with further pressure issues in downstream sulfonation plant equipment.
Feeding the gas return to the sulfur burner was very successful on several fronts. Pressure drop of the process equipment remained within normal ranges and the turbidity issue was resolved. The quality of the acid also met company specifications and there was no acid mist in the absorption towers. Sulfuric acid production increased by 10% to 114 MTPD for the same main blower discharge pressure, over 80% of sulfonic plant exhaust gas was returned, and SO2 emissions were within acceptable limits.
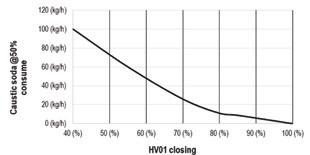
There was a clear reduction in caustic soda consumption by the scrubber as the HV01 valve was tightened to narrow the inlet and more gas was returned (see Fig. 4). Zero consumption was reached when the gas was returned completely by fully closing the HV01 valve.
Given the positive results of these last two tests, the team decided returning the gas to the sulfuric acid plant’s
The converter pre-bed was installed by Construcciones Montajes y Mantenimiento (CMM) of Colombia in July 2020 with the blower and control system installations completed later that year. By December 2020 the new system started operating at 120 MTPD capacity, returning 100% of the gas from the sulfonation plant. At maximum capacity of 125 MTPD however, the maximum recirculated gas was 90%. In both cases though, the new system complied with regulations on gas emissions and sulfate level in wastewater.
Some final tweaking to raise production capacity to 130 MTPD and eliminate bottlenecks was conducted during a general maintenance shutdown at the sulfuric plant in February 2022. The process gas-gas exchangers were replaced, catalyst in the pre-bed was increased, air fans were installed in the acid cooling tower distributors, and the final absorption tower internals were changed out. The changes enabled production greater than 130 MTPD, however returning 100% the gas was possible only when operating below 120 MTPD.
Along with increasing sulfuric acid production capacity to 130 MTPD, implementing the gas return system generated these benefits:

• SO2 emissions: reduced by more than 7.000 kg/year.
• Water consumption: reduced by over 3.500 tons/year.
• Sulfur consumption: reduced by more than 200 tons/ year.
• Sulfate discharge: reduced by more than 300 tons/year.
• Caustic soda consumption: reduced by more than 500 tons/year.
• Cost savings: more than $300,000 USD/year from reduced consumption of raw materials, supplies, and resources.
These achievements, from cost savings to the many environmental benefits gained by both sites, closely align with IBC’s corporate vision of providing value in a framework of conduct that is deeply responsible of people, society, and the planet. q
New gas-gas heat exchanger part of retrofit at IBC acid plant. Fig. 1: Testing flow of gas from sulfonation plant to IAT of sulfuric acid plant. Fig. 4: Caustic soda consumed vs. closing percentage of HV01 valve. Fig. 2: Turbidity in H2SO4 vs. HV01 control valve closing percentage.What is the real cost and danger of having inexperienced people on the job?
How much is 10-20% of your screening catalyst worth?
What does it cost for an extra day of down-time or worse yet, what does it cost to shut down to repair something that should have been caught during your last outage?
Is VIP expensive? Look at the value.
When you add it up… VIP Service pays for itself.



In the Spring/Summer 2022 issue of Sulfuric Acid Today, our article discussed sulfuric acid pricing being under downward pressure as market sentiment was eroding and freight rates for certain trade routes remaining at elevated levels.
At the time of this writing, downward pressure was continuing to mount over the sulfuric acid market mainly due to weak spot demand.
On the demand side, there was limited needs from two of the key global import markets: Chile and Morocco. In the case of Morocco, it imported around 2m t/yr in both 2021 and 2020. However, last year, its imports are likely not to have exceeded 1.4m t. Its imports dropped to as low as 25,000t in November 2022 after reaching as high as around 230,000t in March 2022 and compared to the 2021 monthly average of around 175,000t.
Morocco’s sulfuric acid import demand is solely to support phosphate fertilizer production. With market conditions in the phosphate market weakening in 2H22, it is understood the producer there, OCP, reduced its downstream production while maximizing its own sulfur-based production. Based on sulfur import data, it is estimated OCP produced a significant volume of around 20m t of sulfuric acid annually between 2019 and 2021. With its sulfur imports dropping last year based on its downstream market conditions, its sulfur-based production is likely to not have exceeded 18m t. The emphasis on maximizing its output rather than purchase sulfuric acid in 2H22 was likely impacted by the benefit of steam credit amid an energy crisis triggered by the RussiaUkraine crisis, and attractive sulfur pricing with its quarterly contract pricing reaching a low of $70-120/t CFR in 3Q22 following $400-500/t CFR in 2Q22.
Historically, Morocco has relied on sulfuric acid imports from northwest Europe. That trend began to change in 2019, however, as production from China increased and it became the top supplier to Morocco. Even last year, Morocco’s imports from China increased despite the notable drop in its overall import total. Therefore, with the combination of significantly reduced demand from Morocco and more competition from China, European sulfuric acid producers were becoming increasingly uncomfortable.
This was further pressured by reduced domestic demand in Europe for industrial applications such as caprolactam production due to the impacts of the energy crisis. Although the crisis was less of a threat
to the region than originally anticipated thanks to mild winter conditions in Europe, many industrial users’ operating rates, and subsequent sulfur/sulfuric acid consumption, were still curtailed at press time. At the same time, sulfur-based producers in the region were maximizing their own production for the benefits of creating energy through cogeneration.
There was some offset in Europe, however, by reduced throughput at some smelters due to the energy intensity of processes. This includes smelters still running at reduced rates at the time of writing in France, Germany, Italy, and the Netherlands. At the same time, however, the market is bracing for the restart of the Stolberg smelter in Germany which is now owned by Trafigura. It will be operated by Trafigura-owned Nyrstar with estimated annual sulfuric acid production of around 120,000t.
Still, with supply outstripping demand, European suppliers began relying more heavily on other markets, such as Latin America. As such, we began to see shipments from Europe to Chile become more prevalent in 2H22 due to length from the supply region on the depressed local and offshore demand. As an indication, cargoes were seen from Germany, Bulgaria, Poland, Spain, and Finland between July and December 2022 whereas in 1H22, the only European country to supply was Germany, according to customs data.
However, beginning in 4Q22, Chile began to have issues of its own that constrained its ability to receive sulfuric acid on a timely basis and kept it out of the spot market. This began with a fire at the key import point Port of Mejillones on October 1 that resulted in one of the two terminals there handling sulfuric acid being out of service until late December 2022. Demurrage time for vessels of several weeks was being reported and a bottleneck was emerging inland. Despite terminal
Freda Gordon, Acuity Commodities Fiona Boyd, Acuity Commoditiesoperations smoothing out going into 2023, a new threat emerged in the form of ocean swells that resulted in the Port of Mejillones being closed for several days on an on-andoff basis in January and February.
With swell threats easing at the time of writing, market participants are hopeful spot demand in Chile will resurface for 2Q23 with the country still expected to need a notable volume of at least 3m t this year. However, just as we saw with Morocco, China has become a more critical supplier to the Chilean market. Last year, Chile’s total imports were just under 3.7m t with China supplying an impressive 1.3m t of the annual total, almost double that seen in 2021 which was an historic high.
As we look forward, it is clear that demand in Morocco and Chile as well as supply availability out of Europe and China will be critical factors this year. A factor adding complexity to this is freight rates. As we discussed in our last article, freight was firming last year amid a lack of new stainless steel builds line up until 2025 amid firm demand for tankers. This was particularly the case in Asia with the firm need to move product such as palm oil in the region. While freight has since softened on slightly weaker demand, there remains a wide differential for spot freight between Europe and Chile versus China and Chile. As an indication, around mid-February 2023, while spot freight from Europe to Chile was pegged in the low $100s/t, freight from mid China to Chile was assessed as high as $150/t.
Turning to acid pricing, the above freight discussion highlights how suppliers can keep product moving at positive FOB pricing. Most sulfuric acid consumption in Chile is covered through annual contract pricing for both domestically produced and imported sulfuric acid. This year, Acuity assessed the annual contract price as $143148/t CFR Mejillones. On a spot basis, pricing at the time of writing was in the

$115-120/t CFR Mejillones range, which in both scenarios would equate to negative FOB pricing if considering the CFR range less spot freight. For this year, it is likely that those moving product from Asia were able to achieve more favorable rates under long-term commitment, but it suggests little wiggle room in terms of spot movements as CFR pricing weakens amid slow demand. While China’s export strategy for this year is unknown, many expect an improvement in copper concentrate availability and a continued ramp up in new smelter capacity that will result in more byproduct sulfuric acid production. Therefore, the health of domestic demand will be critical in determining need to export. It remains to be seen if the reopening of China, after close to three years of strict zero-Covid policies, will lend substantial support to domestic demand in 2H23. At the time of writing, all of the above meant the burden was on suppliers with FOB pricing being lowered to keep product moving. Annual offtake contracts for 2023 for Asian supply were mostly agreed in the mid/high $20s/t FOB and $25-45/t FOB for northwest European supply. At the time of writing, both spot assessments are in the single-digit range.
And while there has been steady spot demand from markets such as Brazil, Indonesia, and Saudi Arabia, for now it has not been enough to ease the emerging length with many fearing negative FOB pricing on the horizon. The last time we saw negative export pricing in the sulfuric acid market was in 1H20 amid the peak of the Covid-19 pandemic.
In addition to the needed return of spot demand, the market will also be influenced by sulfur pricing. Levels for the raw material dropped in the early weeks of 2023 as global production improved amid the rebound from the pandemic. Meanwhile, the phosphate market remains constrained and the outlook uncertain after production in that sector began to decline in July 2022, bringing sulfur pricing down along with it.
Acuity Commodities provides insight into the sulfur and sulfuric acid markets through price assessments, data, and supporting analysis. Offerings include weekly reports on the global sulfur and sulfuric acid markets. For North America, we offer a bi-weekly report on sulfur and sulfuric acid as well as a monthly report on industrial chemicals, including caustic soda and hydrochloric. In addition, Acuity does bespoke consulting work. Please visit www.acuitycommodities.com for detailed information. q



Arguably the most hazardous tasks encountered when performing maintenance in an acid plant involve the transfer or movement of highly acidic liquids. The major risk involved is the uncontrolled release of a substance. According to OSHA, a line break is defined as, the intentional opening of a pipe, line, or duct that is or has been carrying flammable, corrosive, or toxic material, an inert gas, or any fluid at a volume, pressure, or temperature capable of causing injury. Many facilities have a line break permitting system that is tailored to their specific chemical process. Does your company’s line break policy cover the special situations you may encounter from a maintenance perspective?
No matter the task you are preparing to perform, you should embrace the permit process and understand it is there to help you complete the work safely. Line break permits are typically designed to cover the specific process in the facility where you
are working, not necessarily some of the regularly required specialty maintenance work. That being said, permits are still a great resource to provide an extra layer of protection. Line break permits include critical items such as:
• Checklists to help identify things such as PPE and special safety concerns.
• Names of personnel involved with the work.
• Location of safety showers and eyewash stations.
• Emergency procedures.
• Information on the specific substance in line.
Permits are an important tool for any high-risk job. They give workers the opportunity to stop and evaluate the task, ask questions, and ensure everyone involved has the appropriate tools and understanding to complete the job safely.
When performing maintenance work in a sulfuric acid plant, there are numerous special situations that are not considered a





Now in it’s 39th year, the event continues to be an essential annual forum for the global sulphur and acid community to learn, connect and do business.
You can expect an expanded market outlooks agenda, including expert insights from CRU’s analysis teams on major supply and demand markets, including sulphur, sulphuric acid and phosphates, plus additional industry updates from key players from across the supply chain.
By: Alan Williamson, CSP, VIP Internationalline break, but should be treated with the same amount of caution. These situations can be encountered during all phases of maintenance work. They involve tasks that are often overlooked because they’re considered a separate entity from the chemical process itself.
An example is during the process of draining down liquid levels of acid towers and storage tanks. The facility’s process pumps can only pump down acid levels so far before they begin cavitating, which oftentimes results in a few inches of acid remaining in the vessels. Cavitation occurs when there is not enough pressure at the suction end of the pump causing the liquid in a pump to turn into a vapor at a low pressure. When performing maintenance on these vessels, the remaining acid must be manually drained using either a vacuum truck or some kind of pump, both requiring the use of chemical hose. The acid is typically either transferred back into another part of the process or into a tote or tank. Normally this is performed as a closed loop system. By closed loop system, we are referring to any system that has the potential to store pressure or hold liquid in any area. Many facilities have permits that cover the line breaks on their own process system. These permits typically do not cover the manual systems used, which are external to the facilities process, to get the rest of the liquid acid out of the vessel. We will share some steps to get the job done safely.
Before making any line break or hose disconnection, the first step is to ensure the line or hose is properly drained and deemed safe. When making line breaks or hose disconnects, there are four methods that could be used to prevent the accidental release of the substance: isolation, venting, draining, and purging. It is imperative that you understand the chemical you are handling so that you can select the safest method for releasing the pressure. For example, you do not want to add water to hoses full of acid, as this could create a reaction that could put workers at risk.
Setting your employees up for success is key when performing any type of highrisk work. The best way to do this is have a plan in place and ensure everyone involved has a complete understanding of the plan. Things to consider when developing the plan are:
• The hazards of the specific chemical being transferred.
• Size of an exclusion zone needed around the work area.
• Type of special PPE needed for the specific chemical transferred.
• Area the transfer is taking place.
• Secondary containment requirements in case of a potential spill.
• Prevention methods for a potential reaction in the planned process.
• Selection of equipment and methods that have the lowest risk of failure.
Creating policies and procedures for breaking any closed-circuit connections, training employees on how to properly block and bleed closed circuit lines, as well as having the proper PPE available for protection during the line break or hose disconnection could prevent a major incident and keep the worker(s) safe.
Breaking any closed-circuit line, whether it is process piping or hoses connected to pumps, should be treated as a line break. This is a best practice to ensure the utmost safety of your employees. Employees should be trained on the procedures and methods for isolating and bleeding lines prior to disconnecting them. In the unlikely event of a system failure, one way to eliminate the risk of employees coming in contact with the acidic material being transferred is to perform all line breaks in a “Class A Suit.” This provides your employees with the highest level of protection against unwanted chemical exposure. Every disconnect and line break should be treated as if it contains a substance that may be under pressure.
Have you reviewed your company’s line break policy lately? Hopefully this article will help raise awareness to areas that are often overlooked. The goal is for every employee to return home safely to their families every day. The key to achieving this goal is preparation. Setting your employees up for success is an integral part to completing any high-risk task safely. It is the employer’s responsibility to provide employees with the knowledge, equipment, and training needed to do their jobs safely and effectively.
For more information, contact Alan Williamson, Safety Coordinator, VIP International, Inc., at (225) 753-8575 or alan@vipinc.com. q
By leveraging oxygen from water electrolysis and ammonia production, CORE-SO2TM decreases acid plants’ environmental footprint and greenhouse gas emissions, recovers clean energy and enhances plants’ profitability.

SO2
Reduced stack emissions
It’s the same, but better.
• No fossil fuels required
• 98% lower emissions
• 60% less plot space
• 55% fewer construction materials
• 50% decrease in CAPEX
Lowest construction cost worley.com/chemetics
Decreased CAPEX
Produce CO2free power
Green ammonia/ hydrogen O2 use


Your main blower probably consumes about 10% of the electricity in your acid plant if you’re running at the industry average. That statistic begs the question: Are you running at the industry average? Perhaps you’re one of the lucky few who only spend 8% of your electrical budget on a blower, but you may be at or below average. This article outlines some simple steps you can take to perform an internal fan efficiency audit and determine if your blower is getting the attention it needs, so that you can demonstrate profitable production.
Before you head out into the plant to start taking pictures and measurements, grab a cup of coffee and get comfy in that office chair, because you’ve got some digging to do! First, open that perfectly organized “Blower Data & History” folder that you curated. The one that contains every drawing, inspection report, and operating snapshot since the plant was built. If you happen to have misplaced that folder, start re-creating it. Here’s what you want to start looking for:
1. Plant construction specs and drawings. Look for what was asked for when the bid package went out for these blowers and what was ultimately purchased.
2. Blower OEM construction or general arrangement drawings.
3. Blower OEM fan performance curves and aerodynamics data.
4. Blower OEM post-startup or plant commissioning report.
5. Blower O&M manual detailing procedures for care and daily operations of blower.
6. Routine inspection reports (should be annual at a minimum, preferably bi-annual or quarterly).
7. Engineering data or reports from any real or proposed changes that were explored or performed in the past.
Some of these are easier to ask for than they are to get, and it may take some armtwisting of your fan manufacturer. If you don’t have the bandwidth to get this put together, send this list to the fan consultant of your choice and see if you can get them to compile it for you. They’re probably quite familiar with your blower manufacturer and may know how to navigate their particular internal bureaucracy.
In addition to this “fan history folder,” you’ll want to start compiling specific technical data on your blower. We’ll call this one the “internal fan specification sheet.” This is the databank for all the technical nitty-gritty details on the various components that comprise your blower system. If you’ve got this in one place, you’ll be able to see if the components are all working together or whether one is an outlier that may be decreasing system performance.
1. Date the fan was manufactured and first started.
2. Who built the fan; who supplied it?
3. What are the current operating criteria for the fan: temperature, pressure, media, air density (altitude), relative humidity, flow rate?
4. Basic fan wheel/rotor/impeller information:
a. Weight
b. Diameter
c. Width of blades (measured at tip)
d. Width of blades (measured at base)
e. Diameter of inlet eye
f. Annular space between impeller OD (periphery) and casing ID
g. Housing area below-cutoff to housing scroll
h. Blade shape
i. Number of blades
5. Basic shaft data:
a. Weight
b. Major shaft diameter and estimated length
c. Minor shaft diameter and estimated length
6. Bearing details:
a. Manufacturer, O&M manual, etc.
b. Lubrication system details
7. Distance between fan bearings (center to center)
8. Impeller speed (RPMs)
9. Critical speed of shaft
10. Coupling details
11. I&C details: How is it driven? How is it modulated?
12. Damper details: OEM drawings, operating data
13. Motor details: OEM, supplier, type, dimensions, rated horsepower, I&C connection details (power load, voltage, amps, etc.), sole plate connection details, motor performance curve, O&M manual.
By: CJ Horecky, Executive Director, INTEREP Inc.
cess. In the case of a sulfuric acid plant, you’ll almost always see the blower downstream of the dry tower. This might seem obvious to you and the rest of the acid plant world, but if you need to call in a consultant or fan manufacturer, that diagram will make a world of difference when combined with the data above.
Congratulations! You have a solid data repository of all your relevant blower data. Now you want to start looking in your live data systems and interviewing operators so you can uncover signs that certain fans or blowers might be operating inefficiently.
First, let’s define what I mean by “operating inefficiently.” Simply put, this means that any part of your blower system is working harder than it needs to be, is operating at its maximum capacity, is shutting down when you don’t want it to, or generally isn’t providing your plant with the pressure or flow rate that your process calls for.
Some examples of this include historic electrical consumption trends showing that: the blower has run with lower motor-draw in the past and has steadily increased, the blower motor is running at 100% capacity, the blower will not run properly with the inlet/outlet louvers/valves fully opened, or that certain speeds (which you’d like to run the blower at) are not “safe” because of harmonic or vibration issues that operations has reported in the past. All of these are essentially anecdotal data, but they all point to the possibility that the blower equipment is working harder than it’s designed to, and thus consuming more electricity and/or generating less output for your process. When you’re interviewing your operators, listen for them to mention higher-than-desired oxygen levels in parts of the system, corrosion in the blower impeller or other pieces of process equipment, dilution of acids in-process, drips, leaks, or “buildups” at flanges, etc. These could all be signs that an expansion joint, gasket, or duct connection has failed or is leaking in a negative pressure environment, which not only introduces air and water to your system, but also consumes fan or blower capacity quickly, since the system is now working harder to suck in ambient air.
equipment, and if you’re seeing similar electrical consumption to your peers in the sulfuric acid world, then you’re probably fine, or at least good enough to go on and worry about other things! If that’s the case, kudos to you for compiling information that will be a boon to your plant and team for decades to come. You did not waste your time putting it together, time will prove that.
The art that I mentioned is not the comparison of datapoints and the uncovering of incongruities. That’s science. The art is the ability to recognize incongruities between your blower and all the other blowers like it out there in the world. Unfortunately, unless you have a large plant system and have seen hundreds of different blower installations, you’re just not going to have this kind of knowledge to draw from. If you’ve checked all the data, done the interviews, and you’re still dealing with a phantom loss of efficiency, you need to find somebody who has seen hundreds of these systems and can offer you some comparison to other sulfuric acid plants, other acid plants in general, and even other plants with similar processes that are completely outside the acid world. This is usually the golden ticket when it comes to solving problems that are hard to pin down. You might be able to find this kind of perspective in one of the old timers at the plant. If not, try a fan consultant or reach out to me and I’ll recommend one for you.
Your blower/fan system keeps your whole acid plant running, so it’s understandable that it’s the biggest consumer of electricity in the plant. Let’s keep pushing to proactively improve our systems and increase efficiency, rather than “putting out fires.” A 1-2% savings in electricity consumption will pay far more in dividends than just its cash value. If you do the hard work to collect data, conduct interviews with operations, and analyze patterns, you’re not just saving electricity. You’re saving on unplanned shutdowns, burned up motors, safety hazards, weekend overtime, and emergency service trips from vendors.
Once you collect the majority of this data (you may never get it all on your own), it’s a good idea to add process flow diagram information into this folder, so you can demonstrate exactly where the blower falls into your pro-
Once you have your list of issues and observations, this process involves a little more art than before. You’ve done the research, and the data in front of you will probably point you to any loss of efficiency in your system. Compare the litany of datapoints to the interview data you’ve collected from operations and see if any incongruity rises to the surface. If you’re not hearing stories or complaints from operations, if your data is all present and accounted for and you never encountered a big “we don’t know” during your research, if you are operating within the manufacturer’s recommended parameters for all of your blower
For more information, please visit www. interepinc.com or email CJ Horecky at cjh@ interepinc.com. q
Taking stock of your blower systems before there is a problem can save time and money.


Sulfuric acid coolers have been around for so long that there must be a lot of readers who have never heard the story of their beginning. By 1976 their fabrication and marketing was well on the way. That was when I “discovered” this very interesting piece of equipment when I started working for the inventor/marketer/fabricator as their technical service representative. By that time, these fantastic inventions were such a big success that about 60 vessels were being made per year. (Each needed about 14 weeks to fabricate). These shell and tube heat exchangers immediately solved a big headache in the acid plant, i.e. shutdowns due to acid leaks in the acid cooling section. The coolers of the day were simply banks of iron pipe with cooling water falling over them to take away the process heat—very inefficient, dangerous, and problematic. Of course, safety was a big issue too.
The first anodically protected (AP) cooler was “made” circa 1969 as an experiment. I’m saying “made” because the AP components were added to an almost new existing acid cooler that had started failing due to acid corrosion at the hot end. The use of stainless steel and a shell and tube design made a much more efficient cooler design. Furthermore, the plant real estate that was needed to cool the circulating acid immediately shrank and the materials of choice were an improvement for strong acid circuits. However, hot acid was still something to be reckoned with and the designers thought that thicker tubes would give them better run time. No doubt it did; but 5 months instead of 3 months wasn’t quite what they’d hoped for.
Meanwhile, there had been some lab experiments going on at the plant that used an experimental acid cooler. Anodic protection already existed for over 10 years on carbon steel storage tanks and someone thought that it might work on this SS acid cooler. The test results in the lab were quite encouraging and when the new SS acid cooler started failing the management saw an opportunity to test it out on something new. The old cast iron coolers were again hooked up to keep the acid plant operating while the new but corroded SS cooler was isolated, neutralized, and converted to an anodically protected unit.
Conversion to AP required some basic items: a) a cathode inserted in such a way that DC current could be distributed inside the acid side, b) a reference electrode or two that could provide voltage readings from the acid side, c) a large enough DC
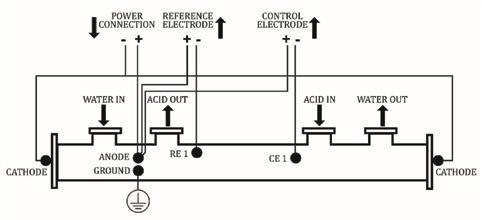 By: R. Barry Krentz, President, Mercad Equipment Inc.
By: R. Barry Krentz, President, Mercad Equipment Inc.


power supply to “passivate” the acid-wetted surfaces, and d) a controller that would regulate the amount of amperage and voltage inside the cooler.
a) Getting a cathode into an existing tube bundle was accomplished by removing a tube and a rosette of tubes around it, and then a rod inside a perforated PTFE sheath (cathode assembly) was inserted through the water channel in one end of the vessel. The sheath kept the cathode from shorting out against the anode (cooler baffles, etc.) but still allowed the acid to contact the cathode material. Due to the high conductivity of the acid, it became an integral part of the DC current being applied.
b) Holes were drilled through the shell and couplings welded on to allow two reference electrodes to be mounted, one at the acid inlet end and one at the acid outlet end. The Mv readings from the electrodes were sent to a controller.
c) Some power wires were fitted from a DC power supply to the cooler (anode) and cathode; and these were connected to the cooler in a similar manner as the storage tanks.
d) A very crude controller was installed having a current meter and a voltage meter c/w a set point switch to turn the AP amps “ON” and “OFF” to keep the reference electrode potential at or around set point.
SUCCESS! The anodically protected acid cooler was born! See Fig. 1.
The fact that this corroded cooler’s life was extended from 5 months to 10 more years definitely proved that AP worked and worked well. It was now very easy to convince the first customers to gamble on the new design and purchase several for their plants. Okay, they didn’t know yet that the damaged cooler would run another 10
years before actually being re-tubed, but they certainly could see it running day after day, cooling acid in a very economical and safe way. Cooler orders started coming in and the good news travelled very quickly around the sulfuric acid community worldwide. Sales people basically became order takers and a lot of money was made with very little competition for about 8-9 years. Hundreds of units were sold in countries all over the world by the time I came along in 1976, just 6 years after the first commercial unit was sold and started up.
The types of H2SO4 that were cooled ranged from 86-99.5% plus Oleum in various strengths. But 86% was a very cold acid circuit and I wouldn’t want some of you getting any ideas (corrosion rates were not negligible). The use of stainless steel in various grades is limited to “strong acid,” meaning greater than 90%, and is very dependent on the temperature and velocity.
The cooling medium varied from demineralized water, fresh water, brackish, and sea water. I seem to recall that glycol circuits were even used. An interesting attempt was made at “air coolers” that were finned tubes with acid inside, and a blower pushing a large volume of ambient air through the finned tube bundle. Let’s just
say that results were less than acceptable and not many were ever sold.
The successful way to build an AP shell and tube acid cooler is to have the acid located inside the shell and the water, etc., inside the tubes. The original designers chose correctly because it’s the waterside that usually needs cleaning and tube bores are good for doing that, whereas the shell is a lot more difficult to clean. You know when you have a good product when 10,000+ units have been made by multiple competitors over the past 50 years.
Other designs for acid coolers were sold with the cooling water on the shell side, but they didn’t end up being a great success because it’s difficult to throw anodic current down the tubes from each end. This fact was learned the hard way when the “air cooler” was first introduced.
It’s also interesting that non-anodically protected PTFE tubes were attempted in the early 70s and this included the “spaghetti coolers” which had the bundle lowered into the acid pump tank. The design also had a short life due to water-side debris plugging small i.d. thin-walled tubing and then overheating took place causing ruptures.
The only type of cooler that did have some success was in later years when new high silicon containing stainless steel came on the market. These materials did not require AP because they could withstand the hotter acid temperatures with minimal corrosion. However the down side was an acid cooler costing perhaps 50% more than standard AP coolers using 316L and 304L materials.
I hope I’ve given you something interesting to read. Cheers!
For more information, contact Barry Krentz at Mercad Equipment Inc. by email rkrentz@mercad.com, or visit www.mercad.com. q





Halides such as fluorides and chlorides can have significant negative effects on materials in the strong acid system and the acid plant as a whole. These effects include generalized corrosion, stress corrosion cracking and pitting, attack of mist eliminator fibers in the acid system, as well as catalyst damage in the converter. These effects can occur at very low concentrations and in relatively short periods of time.
From a business perspective, high halide product sulfuric acid is more difficult to sell or sells only at a discounted price.
Primary means, and maybe the only means, of managing halide content in the acid plant is to not allow it into the acid system in the first place. There are methods to remove halides upstream of the acid plant and maintaining effective removal methods prior to reaching harmful concentration levels deserves full attention.
Fig. 1 shows the classis periodic table, developed in the late 19th century, with elements sharing similar chemical properties organized into columns.
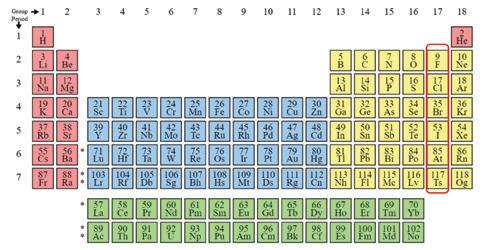
Halides are the ionic form of halogen compounds. Halogen is the name applied to group 17 circled in Fig. 1. They are highly reactive elements, seeking one additional electron to get to a stable outer electron shell. They can strip that electron from almost any other element. Hence, in nature it is highly unusual to find these in their pure state (fluorine and chlorine as diatomic gases). Rather, they are found in a bound form, as fluoride or chloride ions, which are present in nature as acids or salts. The name halogen itself comes from the Greek words for salt generator.
Referencing school chemistry textbooks, the chemical aggressiveness of compounds can be observed by the reduction potential table shown in Fig. 2. The scale is arbitrary, and the convention
is that hydrogen has a potential reduction of zero. Compounds that are more chemically aggressive than hydrogen to oxidize (take an electron from another compound) have a positive reduction potential value. Those compounds more chemically aggressive than hydrogen to be oxidized (give up an electron to another compound) are negative. The greater the absolute value of this reduction potential, the more reactive the element. Note that the halides are at or near the top of the list as the most oxidizing compounds. That includes fluorine and chlorine – circled in Fig. 2. These are among the most reactive elements on Earth.
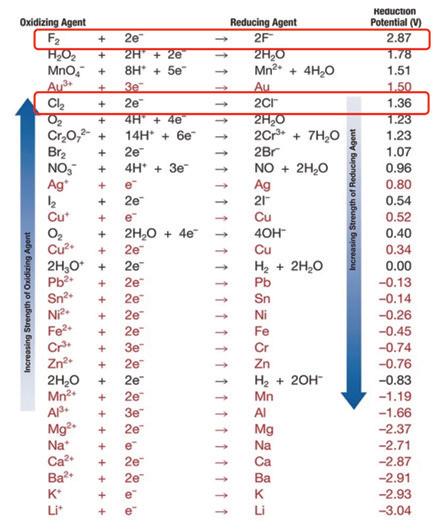
In a sulfuric acid plant, as noted earlier, it is not common to find halides in their elemental form. They will have already reacted, normally with hydrogen, to form acids—hydrofluoric (HF) and hydrochloric (HCl) acids. These acids are still quite aggressive to steel materials because the hydrogen bound to the halogen still has a higher reduction potential than the metals. Hence, corrosion is possible at any concentration. At higher concentrations, corrosion is more likely.
Halides often enter the acid system with the incoming gas from an upstream process, such as gas cleaning in a metallurgical or spent acid plant. But dilution water may contain unacceptable levels of halides—enough to increase acid concentrations to problematic levels. This has been observed when using “pond water” for dilution in a sulfur burning plant. In a few cases, sulfur burning plants in coastal areas with marginal air filtration equipment can see elevated levels of acid chloride content due to salt spray getting into the process.
High silica stainless steels used in sulfuric acid plant towers and piping, 316 SS
used in acid plant coolers and demisters, and 310 SS used in HRS™ applications are all austenitic stainless steels. These steels rely on an oxide surface layer to provide a corrosion resistant barrier, making the materials passive. The presence of higher chloride levels changes the passive film to active. As a result, these materials become subject to chloride-induced stress corrosion cracking. This is intergranular cracking which is often microscopic in nature and difficult to visually observe.
These materials are susceptible to chloride pitting as well. Pitting is normally started with under-deposit corrosion where chlorides can concentrate. Minimizing deposits becomes nearly as important as minimizing chloride content. Deposition and pitting also may be observed in wet/ dry zones where there may again be means for a chloride concentration cell to develop under deposition.
Factors that favor corrosion attack from chloride bearing acid include temperature and oxygen content. Temperatures in the literature suggest cracking begins at temperatures as low as 60° C (140° F). But this has been seen at lower temperatures as well, such as 50° C (122° F) or lower. Insofar as strong acid systems typically operate
from 40° C (104° F) to 120° C (248° F), this overlaps with the temperature range in which the chloride induced stress corrosion cracking is observed. The higher the temperature, the greater the rate of cracking.
Lab testing of allowable chloride content suggests that for this family of austenitic stainless steels, it is necessary to limit the chloride content of strong sulfuric acid to 2 ppmw or below. These lab tests are normally limited to common austenitic steels and in a natural state. It is still unclear how the addition of silica to specialized steels, such as ZeCor ®-Z, impacts this upper chloride concentration limit, and it is also unclear how the passivation system of cooler tubes impacts the limit. With future study, both may withstand higher chloride levels and current suggested maximums may be conservative. For the present, it is suggested that 2 ppmw be the upper limit.
The presence of molybdenum in the steel can provide enhanced chloride resistance, and the addition of nickel can substantially increase chloride resistance as well. Most of the susceptible austenitic stainless steels have nickel content from 8 to 10%. Higher nickel alloys are an option to minimize attack.
• 316 SS (16-10-2) has slightly better
chloride resistance characteristics than 304 SS (18-8). This difference is somewhat academic, though, as both materials experience significant attack, and neither material is advised for acid service with meaningful levels of chloride content.
• Hastelloy C-276 (15-57-15) is a high nickel high molybdenum alloy. Per the literature, this can exhibit corrosion levels of less than 5 mpy at chloride levels of 200 ppmw in strong sulfuric acid at temperatures up to 80° C (176° F).

But higher alloy materials are expensive and rarely used for more than small components in the acid system. Options to reduce chloride content are often more cost effective than increasing metallurgy to handle it more effectively.
Fluoride is quite aggressive to almost all acid plant components. It seeks the presence of silica in the material to produce hexaflourosilicic acid (H2SiF6). By this means, fluorides can attack glass fiber in mist eliminator elements, as well as piping and vessel components in the acid circuit. In weak acid circuits of the gas cleaning system, extra resin is added to the FRP to protect against higher levels of fluorides. However, there remain limits placed on fluoride content of the weak acid (1000–2000 ppmw) without special treatment additives. Even in the gas phase, fluorides can attack acid plant catalyst by attacking the carrier, which consists of silica bearing diatomaceous earth, resulting in crumbling of the rings.
The austenitic stainless steels described earlier experience non-uniform attack. The surface corrosion barrier becomes compromised. Corrosion cells then develop.
Lab testing of fluoride corrosion rates in sulfuric acid is difficult due to the hazardous nature of handling the materials. There is some room for disagreement, but the typical recommendation is to limit fluoride content in the acid to 2 ppmw. By empirical observation in the field, concentrations below this level appear to be much less problematic.
As noted earlier, the way to minimize halide content of the strong acid system is to not allow halides into the process. For dilution water addition, higher quality dilution water is an option if this is a source of either fluorides or chlorides. Demineralized water is the best quality option, while using wastewater streams for dilution may introduce a source of problems. Wastewa-
ter can introduce other impurities into the acid in addition to the halides.
For chlorides carried into the process with the combustion air, such as salt spray, improved filtration is an option. This has additional benefits for reducing blower wheel erosion, boiler tube fouling and catalyst bed pressure drop.
However, the primary means of halides entering the strong acid system is through gas cleaning. For both chlorides and fluorides, there is a solubility in water or aqueous weak acid systems that is advantageous for their removal. This solubility is equilibrium limited such that the temperature plays a part as does the dissolved content of HF and HCl present in the weak acid. Henry’s law determines the vapor pressure of these gases above a liquid with a given halide content. For a given temperature, the higher the liquid concentrations, the greater the remaining vapor phase concentration that goes downstream to the acid plant. Suggested historical upper bounds of halides entering the drying tower have been suggested as follows:
HCl: 1.2 mg/Nm3 (0.0005 grains/scf)
HF: 0.25 mg/Nm3 (0.0001 grains/scf)
For a 12% gas strength metallurgical plant, this will provide product acid at 2 ppmw HCl and 0.5 ppmw HF. This is deemed acceptable. However, in a 6.5% gas strength metallurgical plant, these limits will provide product acid at over 4 ppmw HCl and 0.9 ppmw HF. Per the textbook,
this is less desirable. However, there are plants that do operate with this level of halides. Rules of thumb are valuable guides in general, but they need to be reviewed more closely on a case-by-case basis.
To achieve these removal limits, the normal control degrees of freedom are as follows:
• Evaluate and blend furnace ores based on fluoride content to not overload the gas cleaning section and acid plant.
• Adjust purge water flow to generate a more favorable Henry’s Constant and keep the halides in the liquid phase.
• Add an additional contacting stage called a Halide Tower.
• Add sodium silicate solution in doses as necessary to bind the fluorides as sodium hexafluorosilicate (Na2[SiF6]) and remove them from the Henry’s Law correlation.
As halide content of ores can vary from mine to mine, a particular focus is often put on fluoride analysis of the ores. It is fairly common practice to blend higher fluoride ore with some lower content ores. This requires some discipline within the smelter operation but the benefits to the acid plant operation often justify it in terms of reduced maintenance, as well as maintaining a saleable product quality.
A typical gas cleaning flow scheme for a metallurgical plant is shown below in Fig. 4. Purge water flow in gas cleaning is provided through wet electrostatic precipitator (WESP) fogging and wash water, pump seal flushes, safety seal purges, and an intended make up water line. This joins any condensed vapor in the incoming gas to push all impurities through the gas cleaning system and to effluent through the purge or blowdown line. These impurities include the halides. The greater the flush water flow, the lower the halide content in the weak acid system.
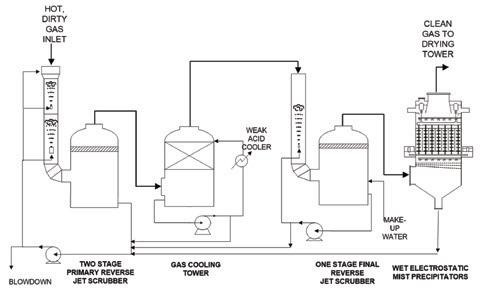
To a large degree, purge water flows counter current to the gas flow. Fluoride
content decreases along the flow path in the gas phase from equipment item to equipment item while fluoride content increases in the liquid phase along its flow path.
Required minimum purge water flow is often determined by other impurities such as arsenic, lead or selenium and is so noted on the design material balance. However, additional purge water flow beyond material balance figures may be allowed if the effluent system can manage it. Pump and piping systems will eventually provide a hydraulic limit.
A more commonly observed limit to the purge is the environment in which the plants are located. Often these are located at the mine sites high in the mountainous deserts where water availability is scarce and even meeting minimum purge requirements is difficult or sometimes impossible. This may not always be an option.
To maintain or increase fluoride removal, an added stage of contact has been used. Not shown in Fig. 4, this involves adding a halide tower in the flow scheme prior to the WESP. Fresh water is charged to halide tower, the liquid circulated to collect halides, and the outflow by level control goes to the final reverse jet scrubber taking absorbed fluorides and chlorides with it. This will add a bit of gas side pressure drop to the front end of the acid plant and blower limits potentially may rule this option out without adding additional gas compression capability.
These limits in existing plants suggest that the option of sodium silicate (aka water glass) addition may be the preferred or only remaining choice. This additive is specific to fluoride removal. Per the flow scheme in Fig. 4, this chemical additive may be injected into the primary reverse jet scrubber to knock down the fluoride gas content at its highest concentrations, improving the fluoride effectiveness of each downstream stage, or it may be added to the final reverse jet scrubber for polishing–or both. In the sites where it is used, both scrubbers are normally equipped with injection capability. This allows optimization of injection of the water glass.
Even with water glass injection, sometimes additional purge water is desired as the added chemicals can make the circulated solution become gelatinous and difficult to deal with. Many operators look for solutions other than water glass.
Any combination of removal options may be employed. These options are not exclusive. Added flush water, if available, is normally recommended as this is the lowest capital cost.
For more information, please visit www.MECS.ElessentCT.com or email Walter Weiss at Walter.Weiss@ElessentCT. com. q

In this article, I wanted to share some issues we have seen growing in our industry and hope that putting it out there helps someone. The idea came to me from attending a seminar that my company put on for our team to help us be the best we can be.
This is something all contractors, owners, operators, and probably anyone reading this have had an issue with at some point in their careers or life: I’m talking about communication. I have been in many meetings where poor communication was the root cause of many workplace, personal, or business issues. We all understand there are good and bad communications, but there should be a focus in the business world for clear and precise communications if we want successful outcomes.
Effective communication is the common denominator for any company, person, or project that has met or exceeded goals. Strong communication throughout the workplace allows your team members to know what they are working toward, contribute to overall goals, and help overcome challenges along the way.
It’s ironic that it doesn’t take much investment to improve communication, but poor communication can be one of the largest factors causing higher costs, poor timing of events, or schedule discrepancies. Although

we all could probably polish up our communication skills, all it takes is strategies by leaders to prioritize and make a commitment to improve them.
No matter how the industry displays it, there seems to be a decline in overall communication, which affects relationships. Some are hesitant to call it that, but working with a contractor, owner, or client is a relationship. In my opinion, longstanding relationships are the backbone of our company and this is even more true in the sulfuric acid industry. These relationships were founded on trust and loyalty. Good communication between both parties is what helps build that trust.
In business or any relationship, both parties benefit from effective communication. These benefits include reducing conflict, increasing performance and employee engagement, uncovering new talents, aiding in proper utilization of resources, and building trust and loyalty.
As a contractor in the heavy industrial sector, I will point out three of the most important benefits of improving communication at your company.

#1 SAFETY: How many safety meetings or incident follow-ups have you been involved in where poor communication was a factor? Good open lines of communication up front and on the job site have proven to
minimize safety risks and could save someone’s life.



#2 EFFICIENCY: When tasks are improperly communicated, they take longer to perform and quality could be affected. When instruction isn’t clear, it is easier to be confused or make mistakes. Good communication of what is expected will prove faster and help maintain high standards in the long run. This increases efficiency and reduces wasted time, effort, and resources.
#3 PROBLEM SOLVING: When an issue arrives (for example discoverable or unplanned work on an outage) if a piece of info is communicated improperly or passed on poorly, the message can quickly become misunderstood by either or both parties resulting
in escalation. By the time it reaches the highest level, the info is misconstrued causing mismanagement or even anger in employees. A good communication process can resolve issues quickly and minimize inaccuracies.
In conclusion, I want to leave you with a takeaway that could help anyone communicate, whether in a business or personal setting. The best communication tools are your EARS. Listening is probably the most important aspect of good communication and the one most often in disrepair. I bet we all are guilty of this one at times. I challenge everyone to be a better listener and see if your lines of communication improve.
To contractors, listen to your clients/ owners to understand their goals so you can provide a better service.

To owners/clients, listen to your contractors. They may have a wealth of knowledge and experience to help you reach successful outcomes.
One of our end goals here at CMW is to maintain our longstanding relationships with our valued clients that have trusted us since 1966. If you need a new relationship built on trust and integrity with an experienced contractor, start with good communication.
For more information, please contact Ian Legg at 813-365-2085 or iLegg@cmw.cc, or visit www.cmw.cc. q
Good communication is the key to all successful projects.
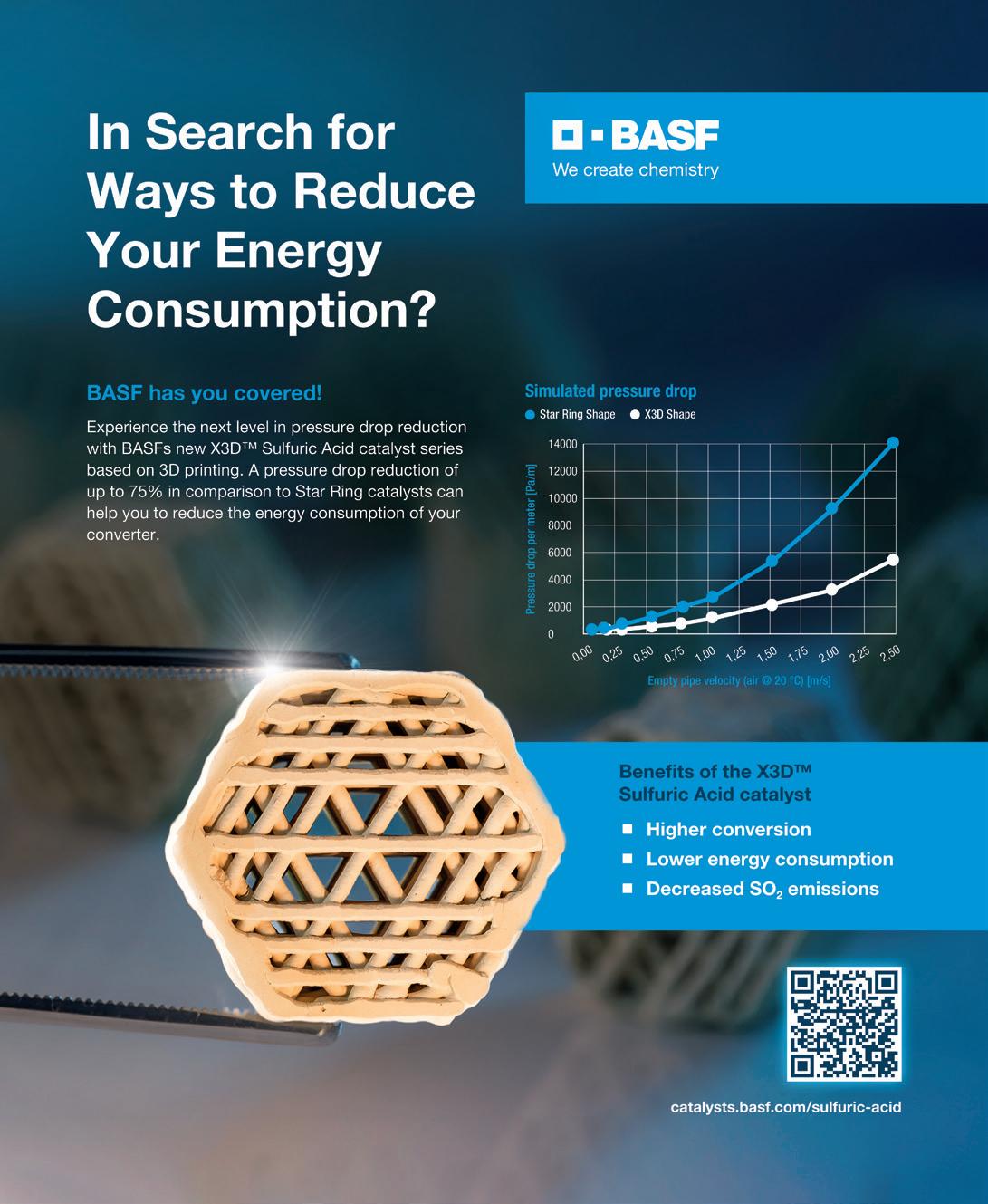

No matter how well-trained or careful plant personnel are, all confined-space entries come with safety risks. Ecorobotics has developed world class robotic tank cleaning technology that eliminates the need for confinedspace entry during the material removal and rinse phases of tank cleaning activities. This not only eliminates the risks associated with entering a storage tank, but also frees up personnel for other maintenance tasks at the plant, saving time and money.
Ecorobotics is a specialized technology company supplying Robotic Cleaning Systems (RCS) for vessel washing and bulk material removal. Employing groundbreaking technology designed to provide a more efficient and safe work process, these robotic cleaning systems are best used for removing hazardous and nonhazardous materials from industrial containment, such as aboveground storage tanks, sumps, ponds, and culvert/ ditches. Human entry into confined spaces is eliminated by providing engineered, efficient, and cost-effective robotic solutions for washing and material removal.
The RCS utilizes visual programming language (VPL) for controlling the robots performing the work. The robots can replace an entire crew of human workers. The electricover-hydraulic system is intrinsically safe and allows for remote monitoring. The H5 Robot with Vacuum Head is utilized for removal of hazardous and non-hazardous
materials, while the H5 Robot with Articulating Arm is utilized for motivating material or a final wash-down of containment.
In addition to the safety benefits, Ecorobitics’ unique technology for bulk material removal significantly reduces the overall project cost by: —greatly reducing asset off-line time.
—minimizing waste generated in the cleaning process. —allowing for a reduction in process-related manpower without worry of confined space injury or death.

The combination of RCS generated benefits results in a safe and efficient work environment, while eliminating confined space entry.
Ecorobotics’ industry leading robotic technology, combined with the vast experience of the company’s team, has resulted in the completion of many successful projects in the refining and chemical manufacturing industries. One such client was an acid plant on the U.S. Gulf Coast. Late last year, Ecorobotics was asked to provide tank cleaning services on a steel, closed-top tank, 55 feet in diameter and 40 feet high. It presented a perfect use case for the company’s tank cleaning robots.
After a site visit, it was determined that the scope of work on the spent acid tank would include degassing, bulk removal of one foot of acid sludge, final rinse, and neutralizing. The H5 robot with vacuum head was successfully used for bulk removal, eliminating the need for tank entry by personnel. Then the H5 robot with viper arm was used to rinse the tank and spray neutralizing solution. In total, 422 bbl. of acid sludge was removed from the spent acid tank. The project was safely completed over the course of 7 days (including degassing).
Ecorobotics’ combined strength of technology and depth of expertise provides clients like this Gulf Coast facility with a strategic partner they can count on for their bulk material removal needs.
For more information, please visit www.ecorobotics.com, or email David LaCoste at dlacoste@ecorobotics.com. q







The combustion of liquid sulfur to produce sulfur dioxide represents a key step in several important industrial applications. These include the production of commercial sulfuric acid, cyanide removal in mining applications, the bleaching stage of pulp and paper plants, and other uses in chemical industries. Liquid sulfur is typically introduced into a sulfur furnace where it combusts in air at high temperature to produce sulfur dioxide gas. The sulfur furnace diameter and length, volumetric gas flow rates, sulfur atomization effectiveness (i.e. droplet size, initial velocity), internal baffle arrangement, and heat losses are important design considerations for an efficient furnace.
Sizing of sulfur furnaces has typically been based on residence time, which may work well for traditional large capacity furnaces but results in inefficient designs for furnaces with very high turn-downs and either very high or low SO2 concentrations. Computational Fluid Dynamics (CFD) analysis is a useful design tool to facilitate the design and to evaluate the performance of the furnace / reduce the risk of incomplete combustion across the entire operating envelope.
Furnace design requirements are dictated by the plant process requirements which are application dependent. For example, sulfuric acid plants use the sulfur furnace as a heat sink during the pre-heating of the converter catalyst beds and use the energy generated to produce steam. These requirements for thermal inertia and heat conservation directly impact the furnace length, diameter, and refractory. Conversely, smaller furnaces producing sulfur dioxide for the mining and pulp-and-paper industries have requirements to minimize start-up time without the requirements for heat conservation or thermal inertia resulting in smaller (diameter and length) furnaces with thinner refractory lining. In addition to the application requirements, the internal processes, as illustrated in Fig. 1, need to be accounted for.
Upon introduction of liquid sulfur into the furnace, the sulfur vaporizes in the preheated furnace and combusts with the ambient oxygen to form SO2 and traces of SO3 The furnace “flame” is produced by the emission of visible radiation from the combustion gases and liquid sulfur droplets. The
sulfur flame produced in a furnace with a poor versus an excellent atomization process is shown in Fig. 2, which illustrates the relationships between atomization performance, gas temperatures, and overall furnace performance.
The designer has a wide selection of furnace configurations and options that can be employed to meet the requirements. However, these options, considered in isolation, provide limited insight into the operation of the system without performing a computational assessment. Table 1 summarizes some of the design options that may be available.
Sulfur atomization
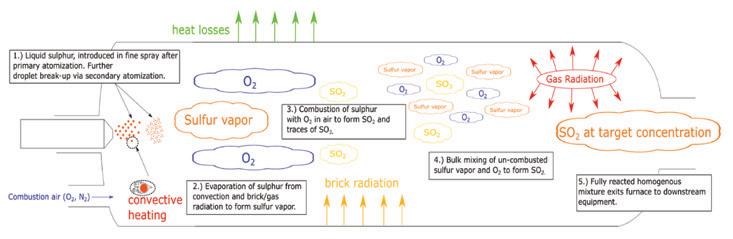
Available Options Advantages / Disadvantages
• Air atomized
• Maintains good atomization at 1050% sulfur turn-down.
• Finer atomization - improved combustion at the cost of increased compressed air usage.
Number of sulfur guns
Mixing element design
• Pressure atomized
• Simplified gun design.
• Does not require instrument air, works well provided manufacturer recommended nozzle operating envelope is strictly adhered to.
Combustion air introduction
• Varies, typically 2 – 6 for pressure atomized guns.
• Baffle wall(s)
• Checker wall
• Orifice baffle
• Primary air only (air introduced in furnace wind-box).
• Primary and secondary air inlets (air introduction split between wind-box and 2 or more inlets on shell).
• Increasing quantity of guns improves atomization performance at turn-down for pressure-atomized guns.
• Enhanced mixing resulting in improved droplet vaporization and faster/complete combustion. (Selection of mixing elements needs to be assessed in computational model to obtain optimal furnace configuration.)

• Robust, simple design.
• Enhanced mixing and droplet vaporization.
Internal refractory design and external insulation
• Hot shell design (i.e. thinner refractory lining and a thin layer of insulation on the outside of the shell)
• Advantage: Temperature at the brick/shell interface is maintained above the dew point of the gas which minimizes corrosion risk.
• Disadvantage: larger shell expansion and large differentials between summer and winter operation.
• Shell temperature range: 200-250°C
• Cold shell design (maximizes the refractory lining thickness with no insulation on the outside of the shell)
• Advantage: Minimizes the shell expansion.
• Disadvantage: potential for corrosion at the brick/shell interface due to condensation of the acid on the cold shell.
• Some form of protective barrier (other than insulation) should be provided to prevent burns from accidental contact with shell.
• Shell temperature range: 100-150°C
• Cooling Jacket
• Potential for refractory thickness reduction and reduction in furnace preheat time.
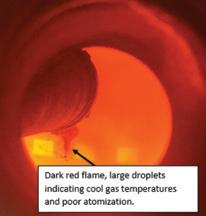 Fig. 1: Schematic of sulfur combustion process.
Fig. 2: Poor atomization and cool flame (top), efficient atomization and hot flame (bottom).
Table 1: Furnace design options
Fig. 1: Schematic of sulfur combustion process.
Fig. 2: Poor atomization and cool flame (top), efficient atomization and hot flame (bottom).
Table 1: Furnace design options
NORAM’s approach to furnace design, regardless of application, is to conduct a thorough CFD simulation to evaluate design configurations. NORAM uses OpenFOAM® CFD software with the quasi-steady state Lagrangian particle field solver SprayFOAM. Turbulence modeling is performed using the standard k-Epsilon closure model. Combustion modeling is performed using a multistep reaction mechanism developed by Sebar et al. at the Karlsruh Institute of Technology (Energy&Fuels 2018 Vol 32). Typical CFD quality metrics (e.g. convergence tolerances and mesh independence for the main quantities of interest) are applied in the simulation. The models are run using AWS (Amazon Web Services) cloud computing services for approximately 400 hours of compute time on a 128 vCPU (virtual CPU) instance with 32 Gb of RAM which was sufficient to obtain a steady state particle and gas field in the two case studies described here.
Although NORAM has implemented this design approach on a number of furnace designs over the last few years, the following two examples are recent case studies illustrating NORAM’s analysis procedure.
Case study 1: Sulfuric acid producer using pressure atomized sulfur guns


NORAM was approached by a sulfuric acid producer in North America to replace their existing sulfur furnace. The existing furnace, shown in Fig. 3, was suffering with gas leaks, incomplete sulfur combustion, sulfur permeating through the brick lining, sulfur pooling on the bottom of the furnace,
and significant shell deformation and bulging. The replacement furnace was to be installed in the same location as the existing in an already congested plant area leaving little latitude to increase the furnace dimensions. The physical phenomena occurring inside the furnace, including the sulfur spray dispersion, secondary atomization, vaporization, mixing, and combustion, was investigated in a detailed CFD model.
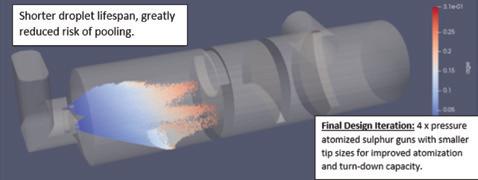
Fig. 4 shows one of the initial CFD design iterations suggesting possible sulfur pooling at the bottom surface of the furnace. Key aspects of the system such as baffle design placement, quantity of sulfur guns, and sulfur gun tip size were changed until, multiple iterations later, it was determined that increasing the number of sulfur guns, with smaller atomization nozzles, resulted in the best overall performance as shown in Fig. 5. Comparison of the initial and final designs shows a significantly reduced risk of incomplete combustion and sulfur pooling. The design in Fig. 5 was ultimately selected, constructed, and commissioned in 2021. Quali-
tative sight-glass measurement and temperature measurements of the furnace interior validated the CFD model results (see RHS of Fig. 2 for sight-glass observations). Field photos taken of the furnace during final stages of site installation are shown in Fig. 6.
Case Study 2: Sulfur dioxide system using air atomized sulfur guns (NORAM Cellchem™ Furnace)
NORAM was awarded the contract to design and supply a new sulfur dioxide plant, located in Africa, to produce 612 TPD SO2 at 10.5% strength (69 TPD sulfur combusted in air). The plant utilizes a NORAM Cellchem™ furnace with some novel design changes including air-atomized sulfur guns equipped with a pre-mix chamber and design for pressurized operation up to 650 kPa(g). A CFD analysis was performed to optimize the design and reduce the risk of any liquid sulfur droplets being carried into the downstream equipment.
Fig. 7 shows the first CFD design iteration using a pressure atomization sulfur gun
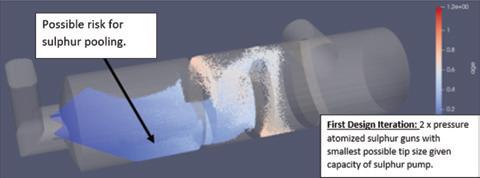
indicating high risk of sulfur droplets being carried into downstream equipment and potential for accumulation of liquid sulfur on the interior surfaces of the furnace.


Multiple additional design iterations were performed to reduce the risk of sulfur carry-over. Furnace length, inner diameter, baffle location, and primary vs. secondary air split were modified to improve furnace performance. The most sensitive parameter to improving sulfur combustion efficiency was determined to be the atomization effectiveness. The final CFD design iteration with a NORAM SB-60 air atomized sulfur gun with pre-mix chamber is shown in Fig. 8. Compared to Fig. 7, Fig. 8 shows a dramatic improvement in sulfur vaporization and reduced sulfur carry-over risk. The furnace length and diameter were maintained, despite the dramatically improved sulfur vaporization, to adequately mix the combustion gases before exiting the furnace.
Atomization effectiveness represents a critical parameter in minimizing droplet
Fig. 3: Previous furnace showing sulfur pooling (top) and deteriorating shell (bottom). Fig. 4: CFD design iteration 1. Fig. 5: Final CFD design iteration. Fig. 6: New sulfur furnace in final stages of site installation. Contnued on page 26Atomization effectiveness represents a critical parameter in minimizing droplet impingement and sulfur carry-over to downstream equipment.
impingement and sulfur carry-over to downstream equipment. This should not be considered in isolation from other design parameters such as number of guns in operation, placement of baffles, furnace size, etc. CFD is a useful tool to evaluate the entire system performance, but care needs to be taken during the model set-up and results assessment by applying typical CFD quality metrics and assessing transient versus steady state features in the model.
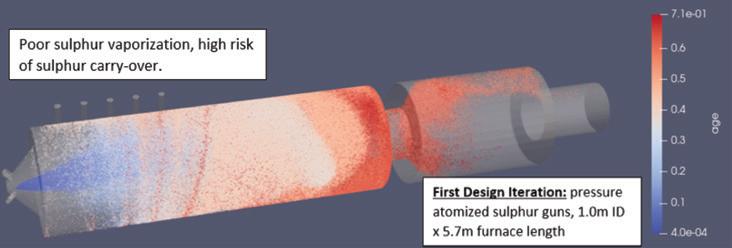
In Case 1, the atomization effectiveness was improved by increasing the number of sulfur guns and using smaller spray nozzles, whereas in Case 2 using an air atomized sulfur gun was the optimal solution. Each case needs to be considered individually as there may be factors in play. There is no golden solution that will serve all users equally well.
NORAM can evaluate sulfur combustion systems for both new and existing systems and develop a tailor-made solution to improve your plant’s operation.
The NORAM Group is a vertically integrated portfolio of businesses offering industrial processes and scale-up through engineering, R&D, pilot plants, demonstration plants, modular plants, custom fabrication, and site assistance. NORAM offers sulfuric acid technologies and equipment for clients worldwide. For more information, visit www. noram-eng.com, email sulfuric@noram-eng. com, or call (604) 681-2030. q
The robotic cleaning system employs groundbreaking technology designed to provide a more environmental and worker friendly process of acid and caustic tank cleaning. The revolutionary patented technology is making significant changes in the chemical, petrochemical and petroleum industry by eliminating confined space entry, while increasing efficiency and productivity of the industrial cleaning process.

• Above Ground Acid/Caustic Storage Tanks
• Run-Off Ditches
• Sumps (Online/Offline)
• Pits
• Below Ground Acid/ Caustic Storage Tanks
• Large Diameter Pipe
• Cooling Towers
• Reactors
• Eliminate confined space entries and other safety hazards
• Bulk Removal Rates up to 150 bbl./hr
• Adaptable 4” and 6” Vacuum Heads
• Electric over Hydraulic System

• Caple of High-Pressure Water Jetting
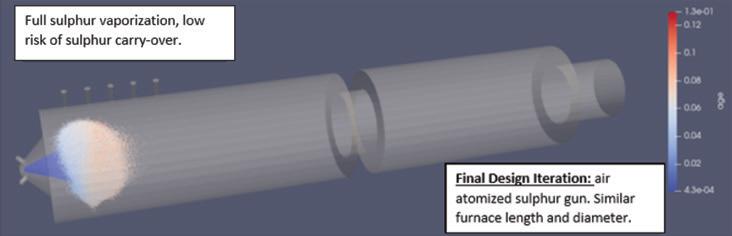
• Clean large projects faster and more efficiently than conventional crews


• Reduce water consumption
• Reduce waste management and disposal costs
• Video capability with DVR system
• Class 1 Division 1 Camera and Lighting System
• Rig Up/Rig Down Time approximately 2 hours
• Equipment outside the tank is Class 1 Division 2, which includes 4 gas detection
From our strategic location in Houston, TX, we are able to supply a variety of brick shapes (straights, arches, wedges, keys) and sizes from stock for immediate purchase.

Please reach out to us for technical data and pricing. Samples available upon request.
Meeting ASTM C279 standards for use in new construction and refurbishment of existing structures in sulphuric acid plants including towers, process vessels, floors, sumps, pits, etc.

Industrial Linings for Sulphuric Acid Plants. Absorption Towers, Pump Tanks, Sulphur Pits, Secondary Containment, Acid Resistant Linings. Acid Brick, Acid Resistant Mortar, Membranes, Carbon Brick, Polymer Concrete, Refractories, Teflon, Ceramic Paper and Blanket, Ceramic Rope, Borosilicate Block

As the world moves to decarbonize, the demand for electric vehicles and renewable fuels continues to grow. Metals, like nickel and copper, are critical for a transition to an electrified world and as a consequence, the world’s miners are racing to keep up with the booming demand. Haldor Topsoe’s new high-activity catalyst, VK38+, can help acid plants maximize acid production to make the most out of this metal boom while simultaneously securing important economic benefits and reducing their carbon footprint.
Electric vehicle demand has skyrocketed in the last couple of years influenced by government subsidies, ambitious climate targets, bans on internal combustion engine vehicles, new technologies, and rising fuel prices. And since the batteries that power the EVs need minerals like copper and nickel, it is expected that as the EV uptake gains speed, metal demand will also increase. Now, most of these metals originate from sulfide ores, or require sulfuric acid in their production, which implies that increased production of metals will translate into more demand for the already squeezed sulfuric acid plants. Being able to produce more sulfuric acid by processing higher gas rates or using stronger SO2 gases while keeping the SO2 emissions at low levels, will require more or a higher activity catalyst installed in the converter. The extra activity needed can be gained by upgrading to new potassium-promoted VK38+ catalyst,
which was introduced in 2020, to tip the scales in the operators’ favor.
Let’s take the example of a sulfurburning plant that needs to produce sulfuric acid for the leaching of nickel ores. One of the ways to increase the acid production is by increasing the amount of sulfur that is burned in the furnace, maintaining the air flow rate constant. This result would be very attractive for most acid producers because it would enable, at the same time, an increase in steam production without increasing the gas volume handled by the plant. However, keeping the airflow unchanged while burning more sulfur will increase the SO2 concentration at the inlet of the SO2 converter. And if the catalyst solution isn’t upgraded, then a stronger gas will inevitably generate higher SO2 emissions. There are two alternatives for utilizing stronger feed gas without experiencing such an emission increase. The first is to increase catalyst volume, but free converter space is usually very limited or unavailable altogether. The second alternative is to use a more active catalyst.
By: Martin Alvarez, Haldor TopsoeOutputs Extra heat, MW (compared to VK38/VK48 case)
Extra Steam produced, ton/day (compared to VK38/VK48 case)
Extra acid produced, ton/day (compared to VK38/VK48 case)
+3.10 +110 +91
Inputs Additional liquid sulfur burnt, ton/h (compared to VK38/VK48 case) +1.20
Benefits Extra steam earnings, MMEUR/year
Extra acid production earnings, MMEUR/year
Extra sulfur costs, MMEUR/year Total earnings, MMEUR/year
+1.90 +3.60 - 2.38 +3.12
thermal capacity available to recover this additional heat, these 3.10 MW are equivalent to an extra HP steam production of approximately +110 tons/ day. The extra steam production is achieved without increasing the pressure drop of the unit and therefore improving the energy efficiency of the plant.
Using the natural-gas cost of the steam generation, 110 tons of extra steam, delivered daily, would represent around 1.90 MM EUR/year, according to the current gas prices at the Dutch TTF (65 EUR/MWh). On the other hand, assuming a liquid sulfur price of 250 EUR/ton, the additional 1.2 tons/hour of liquid sulfur that needs to be added to the furnace in order to increase the SO2 strength to 11.5% would have an extra cost of 2.38 MM EUR/year.
Fig. 1 displays the catalyst volume needed for an S-burner unit to reach a certain level of emissions at different acid production rates. Both curves reflect a constant airflow of 91,000 Nm³/h, and the different production capacities have been achieved by increasing the strength of the feed gas. The VK38+ curve remains consistently at the right of the VK38/VK48 curve, showing that a VK38+ solution can, in general, accept stronger gases and higher capacities from the same catalyst volume.
Fig. 2 below explains how this concept can be applied to a 1000 MTPD plant operating initially with 10.5% SO2 gas. By switching the catalyst from VK38/VK48 to the same volume of VK38+, the catalytic converter would be able to admit the same feed gas flow at a higher SO2 concentration, keeping the emission level unchanged. The increase in the gas strength from 10.5% to 11.5% will lead to a production increase from the original 1000 MTPD to 1091 MTPD. Using a sulfuric acid price of 120 EUR/ ton, these 91 extra daily tons of acid will bring an annual benefit of around 3.60 MM EUR.
As this increase in the gas strength is achieved by burning more sulfur in the furnace, the sulfur combustion heat increases by around 3.10 MW. Provided that the steam system has
Overall, after deducting the liquid sulfur costs, the economic benefit of the VK38+ solution would be around 3.12 MM EUR/year. This is summarized in Table 1.
The extra steam generated will result in reduced carbon emmisions of approximately 4,500 metric tons of CO2 annually.
VK38+ is already in operation in 10+ units. The list includes copper smelters, sulfur burning units, and sulfonation plants.
The following industrial example illustrates how VK38+ can be used to increase acid production while generating more steam and decreasing the plant pressure drop. The plant in question is a 1,000 MTPD sulfur burning
 Table 1: Benefits of using VK38+ in an S-burner unit.
Fig. 1: Acid production for different loading sizes for VK38 and VK38+.
Table 1: Benefits of using VK38+ in an S-burner unit.
Fig. 1: Acid production for different loading sizes for VK38 and VK38+.
Record-breaking metal demand and the path toward sustainability push sulfuric acid plants, many of which already operate with fully loaded converters
“ ”
unit located in Western Europe that had been operating for decades with mostly competitor catalyst. The plant had the following specifications:





• SO2 source: sulfur burning


• Configuration: 3+1 DA
• Design capacity: 1,000 t/d



• Loading size: 220 L/TPD

The client decided to replace the catalyst in all the beds with VK38+ to increase production by burning more sulfur without jeopardizing the emission level, and to be able to run at this new condition for several years. Fig. 3 shows the loading diagram before and after the replacement.
Based on Topsoe simulations,
it was assessed that even in the new more challenging conditions, with the considerably higher VK38+ activity, it will not be necessary to use a full bed of expensive cesium-promoted catalyst, as was previously the case. In the new design, as shown in Fig. 3, only half of bed 4 was loaded with Cs-promoted catalyst (VK69), while the other half was loaded with VK38+.
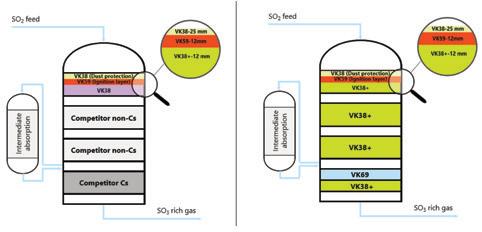
Two TOPGUN™s were performed before and after the replacement of the converter. See Table 2.
The performance with the VK38+ has allowed the customer to increase production by +6.7% with practically an

unchanged emission level. As an added bonus, the pressure drop of the unit was decreased by 20% and the steam generated in the plant was increased by around 2.1 MW (73 ton/h of HP steam @ 60 barg). The increase in steam production and the blower energy savings due to the lower pressure drop in the unit will result in a reduction of 3,200 tons CO2 annually.
Record-breaking metal demand and the path toward sustainability push sulfuric acid plants, many of which





already operate with fully loaded converters. With VK38+, operators can tip the scales in their favor and increase the capacity of their units without the cost increase associated with a Cs-catalyst solution. VK38+ benefits are not limited to increasing capacity, but include reducing the environmental footprints of sulfuric acid units. Also, operators can use VK38+ to reduce blower energy consumption by reducing the pressure drop when using lower volumes of more active catalyst or maximizing the steam generated by processing stronger gases. q

A HIGHLY BREATHABLE PPE OPTION FOR WORKERS POTENTIALLY EXPOSED TO LIQUIFIED ANHYDROUS AMMONIA, HARSH ACIDS, OR CAUSTIC CHEMICALS.
GORE-TEX Liquid Chemical Splash Garments are made with a combination of three unique technologies:
1 A lightweight and breathable GORE-TEX single ply fabric using our unique ePTFE membrane, which offers liqud chemical penetration resistance and moisture vapor permeability (allows sweat to escape).
BASF recently introduced the novel X3D™ technology, a new additive manufacturing technology for catalysts based on 3D printing. Catalysts produced with this technology feature an open structure, resulting in a reduction of pressure drop across the reactor and a high surface area, significantly improving the catalysts’ performance. BASF has capabilities to supply commercial quantities.

2 Sewn and seam sealed using GORE® TENARA® sewing thread and GORE® Seam Tape for additional performance.
3 Seams are vulnerable areas prone to chemical penetration. Garments made with GORE-TEX Liquid Chemical Splash fabric are finished with GORE-SEAM® Tape and sewn with GORE® TENARA® sewing thread, a specifically designed thread that is highly thermally stable and chemically inert, which prevents seams from failing during chemical splash incidents.

• Resists penetration of a broad list of chemicals (upwards of 100) (Refer to the Gore-Tex Liquid Chemical Splash Chemical List).
• Breathability reduces heat stress and improves productivity.
• Tough, durable 3 layer laminate provides long-lasting garment life.

Seams sewn and sealed with GORE® technology provide overall penetration resistance.
• NFPA 1990/1992 compliance assures quality and performance.

• Durable washability and reuse decrease costs over time.
• Light weight, comfort and breathability enable proper use of PPE.
The technology offers a greater freedom of catalyst design compared to conventional production technologies. It brings catalysts’ performance to the next level and helps to customize catalysts to customers’ specific conditions and needs by designing infill pattern, fiber diameter, and orientation. Customers can benefit from increased reactor output, higher product quality, and lower energy consumption. The novel catalysts are mechanically
robust and proven in commercial plant operation externally and for several years in BASF.
BASF can apply the technology to a wide variety of existing catalytic materials, including base or precious metal catalysts as well as carrier materials.
BASF’s sulfuric acid catalysts O4-111 X3D and O4115 X3D are the first catalysts produced with the new technology and are used in industrial plants.
“With this technology, we are able to provide catalysts that are tailored to our
customers’ needs to help significantly boost their plant performance while reducing energy consumption and increasing sustainability at the customer level,” said Detlef Ruff, Senior Vice President, Process Catalysts at BASF. “BASF’s technical service team will work with customers to identify the best catalytic technology for their individual projects,” said Chris Wai, Vice President, Global Chemical Market Catalysts at BASF.
For more information, please visit www.basf.com. q
TWINSBURG, Ohio— In response to increased demand for system design, CG Thermal recently promoted Product Manager Ethan Schrader to oversee AirBTU VPRR. After joining CG Thermal as a Project Manager in 2022, Schrader quickly became an integral part of their systems group. With his experience in SOLIDWORKS modeling, and formal training in both CFD and FEA, his contributions have become invaluable.

“Based on Ethan’s track record, he is ideally suited for this newly formed management position. AirBTU VPRR is a high profile product for CG Thermal, and we are very confident that Ethan is the one to lead the group to early successes,” said CG Thermal
Senior Vice President Greg Becherer.
Schrader will devote more time to developing and implementing the AirBTU VPRR as the premier solution for high-temperature process streams. The AirBTU VPRR features unique design considerations, including a proprietary baffle design and variable radial pitch tube arrangements.
Ultimately, the design’s reliability and efficiency proves to outlast and outperform current standards.
As the team at CG Thermal continues to grow, they will continue to forge a path of new technologies and offering customers industry-best solutions and services.
For more information, visit www.cgthermal.com. q
BASF introduces X3D™, catalyst shaping technology for optimized catalyst performance
Our geometrically arranged corrugated sheets provide greater resistance to fouling than random packing, while our design provides higher capacity, higher efficiency and lower pressure drop than any random packing that is available today.

Up to 30% more gas flow for 30% more acid production
50% lower pressure drop with the same gas flow
Contact us for delivery timelines.
ACID-RESISANT CONSTRUCTION
TOWER INTERNALS
HEAT TRANSFER MEDIA
MASS TRANSFER PACKING
ENGINEERING SERVICES
Knight has acquired Electro Chemical Engineering and Manufacturing Co., a leading supplier in high-performance fluoropolymer-lined vessels. Together, we will work to expand services and technology in the corrosion-resistant materials industry.
INSTALLATION SYSTEMS
Over the last five years, global fertilizer producer Mosaic has been steadily improving the asset reliability of sulfuric acid plants across multiple facilities in Florida and Louisiana. The long-range capital improvement plan, with projects continuing into the next few years, is replacing converters and acid towers that have been in service for four decades or more.
Mosaic is a leading integrated producer of concentrated phosphate and potash fertilizers. With nine locations in North America and South America, the company produces 25m tonnes finished product annually and serves customers in 40 countries.
Several of Mosaic’s Florida-based sulfuric acid plants have been replacing their converters that have reached the end of their useful life. The New Wales location installed two new converters, one in 2020 and the other in 2021. Also in 2021, the South Pierce plant replaced its

converter. In the Bartow location, three new converters will be replacing 50-yearold equipment over the next three years.
The new installations are part of the MECS ® sulfuric acid plant technology and are based on MECS ® patented Staid stainless steel converter design. This time-tested design has been in use since 1980 with the longest running units in service for over 40 years.

“The all-welded design eliminates the instability problems commonly



associated with carbon steel converters and their support castings,” said John Horne, Sales Manager of MECS, Inc. “Internal columns distribute the static load to the foundation evenly and reduce the load on the shell. MECS specifies the minimum carbon content of the stainless steel to ensure structural integrity at elevated operating temperatures. The Staid converter is capable of handling variations in gas strengths to over 14% SO2 gas,” Horne said.
 MECS-designed converters installed at Mosaic’s acid plant facilities in South Pierce, Fla., left, and New Wales, Fla., right.
MECS-designed converters installed at Mosaic’s acid plant facilities in South Pierce, Fla., left, and New Wales, Fla., right.
In all the Florida replacements, converters were installed on existing foundations, stainless gas duct work was redesigned to minimize multi-bellow expansion joints, and a new heatup duct into pass 3 was added to improve start up time.

Mosaic also replaced acid towers that have reached end of life. To date
Mosaic has replaced 18 brick lined acid towers with MECS ZeCor®-Z alloy acid towers in Florida and Louisiana.
MECS ® ZeCor® and ZeCor®-Z alloy materials replace traditional brick lining in sulfuric acid plant towers with advantages such as reduced weight, easier installation, and lower foundation costs. The alloys are corrosion resistant

in a wide range of concentrations, temperatures, and applications. MECS’ line of ZeCor® materials provides the ideal balance of corrosion resistance and weldability. Combined with their design, fabrication expertise and process knowledge, MECS ® has provided more
than 350 ZeCor® towers around the world in a wide variety of applications. This makes MECS ® ideally suited to design the optimal solution.
The MECS® sulfuric acid technologies has been in use for nearly 100 years in the phosphate fertilizer, nonferrous metals (leaching & smelting), oil refining and general chemical industries. MECS® technologies feature breakthrough solutions, many of which have revolutionized the performance, quality and cost effectiveness of customer operations. They include MECS® heat recovery systems (HRS™), MECS® SolvR® regenerative SO2 scrubbing and MECS® MAX3™ sulfuric acid production technology. Integrated into these MECS® technologies are proven specialty products such as catalysts, Brink® mist eliminators, DynaWave® scrubbers, ZeCor® corrosion resistant alloy products, and acid coolers all of which are specifically designed for the most demanding operating environments. Licensed and marketed by Elessent Clean Technologies, the MECS® technology is the world-leading sulfuric acid production technology with more than 400 licensed acid plants worldwide since the 1960’s. Elessent Technologies is committed to long-term customer satisfaction and support for the life of customer assets. For more information, visit ElessentCT.com. q
Over the last five years, global fertilizer producer Mosaic has been steadily improving the asset reliability of sulfuric acid plants across multiple facilities in Florida and Louisiana.
KCM 2000 Holding of Bulgaria has a completely new process for separating lead from rock. The lead producer upgraded its Plovdiv smelting operation in the form of a new TSL furnace, wet gas cleaning unit, and sulfuric acid plant. The new facility answered the call for stronger emissions capture as well as steady demand for lead. In a dynamic system, the furnace cycles through two tasks— smelting and slag reduction—each generating different SO2 concentrations in the off-gas. The system allows for variation in feed materials and handles varying SO2 levels by making commensurate quantities of sulfuric acid.
KCM runs the largest lead and zinc production operation in South Eastern Europe and exports worldwide. The company began in 1961 with a zinc production plant followed by a lead production line two years later. In 2000, KCM 2000 Group was formed and a few years later a new zinc plant came online with the first production of sulfuric acid. Over the next 20 years, KCM expanded with acquisitions, a battery recycling unit, and the new lead production plant. In 2022 the company became KCM 2000 Holding—a group of some 12 companies operating in metallurgy and ore mining; processing and marketing of concentrates, nonferrous metals, and alloys; engineering; and industrial and other services.
In the early 2000s, stricter environmental regulations made clear to KCM that it could no longer run its lead plant using the original sintering and blast furnace technology.
“The original smelter plant was built in the middle of the last century,” explained Georgi Doganov, Smelting and Sulfuric Acid Manager at KCM. “It didn’t comply with the strict emission limits and required modernizing with environmentally friendly processes.”
So, beginning in 2008, the company started planning a new brownfield lead smelting plant that was commissioned in 2014. The facility was designed to produce 75,000 t/y crude lead and 45,000 t/y sulfuric acid in a manner that was both economically feasible and environmentally friendly.
The facility consists of three sections: a top-submerged lance (TSL) Ausmelt furnace for smelting and slag reduction, a wet gas cleaning plant to capture dust particles from the furnace’s SO2-containing off-gas, and a sulfuric acid plant to process the purified SO2 gas exiting the gas cleaning plant. See Fig. 1.
The TSL Ausmelt furnace both smelts raw materials and reduces slag inside the same vessel. It runs in batches where the first stage (smelting) lasts about 7 hours and the
By: April Smith, Editorsecond stage (slag reduction) runs for about 90 minutes. Then the slag is tapped, granulated, and stockpiled for zinc recovery. The air, oxygen, and natural gas needed for these processes are injected through the lance submerged in the bath.
Supplied by Metso-Outotec, the furnace provides environmental and economic benefits. “The Ausmelt furnace operates under negative pressure and is very tightly sealed, which reduces fugitive gas and dust/fume emissions,” said Jacob Wood, TSL Smelting Account Manager at Metso-Outotec.
The furnace also nets economic benefits. For one, it can treat lead sulfide concentrate directly, without the need for sintering, which saves energy. Second, the process can treat a wide range of lead-bearing feeds, including sulfide concentrates, battery paste and grids, slags, residues, and dusts. This operational flexibility is particularly important when the supply of feed materials is unsteady. Third, with the single-vessel approach, there’s no moving material from smelter to a separate slag reducer; and no duplication of plant equipment to deliver air and oxygen, distribute cooling water, process off-gas, or handle dust and fumes.
But the dual process furnace also had its challenges— notably varying SO2 concentrations in the exit gas. “The furnace generates SO2 concentrations from effectively zero (during slag reduction stage) up to about 10% (during smelting stage),” said Doganov. “The alternating oxidation and reduction atmosphere in the furnace requires the expert skills of the operators.” So training was planned with both theoretical and practical segments, and equipment suppliers participated actively with plant commissioning.
Fluctuating SO2 also spelled particular requirements for downstream gas cleaning and sulfuric acid manufacturing.
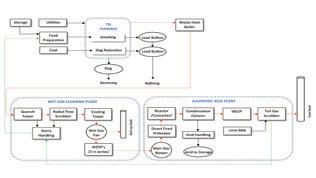
To accommodate fluctuating SO2, KCM built a wet gas cleaning plant because producing sulfuric acid by wet method doesn’t require the burning of sulfur to compensate for the low SO2 coming from the furnace. During the stage when no SO2 is produced, no acid is produced, and operational temperature in the converter is maintained by burning natural gas.
GEA Bischoff of Essen, Germany, provided the wet gas unit, which was specially designed to handle large dust loads in the inlet gas stream. “The quench tower in conjunction with the high efficiency radial flow scrubber are key to the design. They remove about 99.5% PM of dust from the process gas,” said Miguel González, Senior Director, Emission Control Non-Ferrous/Chemical at GEA. “Then, the special bottom design of the vessels combined with specific clarifier and thickener efficiently remove the total suspended solids (TSS) from the washing liquid,” he said.
An advantage to this design? No upstream hot electrostatic precipitator is needed. “This saves a process step and considerably lowers capex,” said González. But it also requires super efficiency in the quench tower and scrubber.
“Typical gas cleaning units use an ESP to handle dust. Without one, dust entering the system increases by 1,000 times a normal concentration. The design of the wet gas cleaning plant had to be adapted to this exceptionally high
dust concentration,” said González.
The next part of the total gas treatment package, the sulfuric acid plant, was installed downstream. Supplied by MECS Inc., the three-bed converter loaded with vanadiumcesium catalyst processes 4.5% SO2 concentration, diluted down from as much as 10%. The gas is then cooled via heat exchangers using molten salt as heat transfer media. Next, SO3/water vapor/acid mist mixture is condensed and further cooled by ambient air flow. Acid at 97-98% concentration is produced at rates as low as 0.5 t per 1 t lead bullion. Final SO2 capture amounts to 99.8% putting less than 300 mg/Nm³ into the atmosphere.
There are other planet-friendly aspects: no process water is used and no wastewater is produced.
“No chemicals are used either, only hydrated lime solution in the tail gas scrubber,” said Doganov. “The 20% gypsum solution generated from the scrubber is then mixed with the slurry from the wet gas cleaning plant and becomes part of the raw materials feed. By utilizing the calcium from the gypsum, the total demand of fresh lime ash required as a flux for the furnace is decreased by almost 30%.”
In terms of outcome, the statistics speak for themselves. Per ton of lead bullion produced, energy consumption of the new facility is down about 40% from the old sintering/blast furnace process. Other benefits include:
• Low emissions in compliance with European standards.
• Flexible technology gives opportunities for smelting a variety of feed materials.
• Several times lower coal consumption.
• Direct extraction of 73-74% lead from materials and higher metal production.
• Better recovery of zinc from slag.
• Carbon footprint decreased by almost 50%.
Over the next year, KCM is planning to expand metals production and upgrade the acid plant to accommodate the increase in SO2 concentration from 4.5% to 6.5%.
“We are planning to replace the heat exchangers between the reactor layers with larger ones and change the catalytic mass. Then we will increase the capacity of the temperature transfer system and the equipment treating the concentrated sulfuric acid,” said Doganov.
These changes will enable even more flexibility processing primary raw materials with high sulfur content while maintaining compliance with environmental regulations. q
Fig 1: Flow of KCM’s lead plant with TSL furnace, wet gas cleaning, and sulfuric acid unit.

When handling hazardous fluids or industrial liquids, the main hazard is usually corrosion. This is why Savino Barbera of Brandizzo, Italy, has replaced metal with plastic in its line of industrial pumps and mixers. In fact, all the wetted parts (those that touch corrosive liquid) are made entirely of thermoplastics, or coated with technical polymers, making them insensitive to chemical aggression.
The plastic impellers on pumps have no metal inserts rendering them resistant to attack from acids even on the inside. Their conical configuration protects the shaft and bushings from radial stress, conveying structural stability to the pump.

The few components that must be made of metal for mechanical support reasons, such as






nuts, bolts, and pump shafts, are protected with chemically inert material. With no metal parts in direct contact with aggressive liquids, there’s less corrosion and fewer problems.
The main corrosion-resistant plastics Savino uses to manufacture its equipment are PP, PVC, and PVDF. The particular plastic used is meticulously selected based on the characteristics of the corrosive liquid being handled and how the equipment will be
used in the field. Every plastic material has a different chemical compatibility with the various corrosive liquids. The fundamental data for selecting the material of the wetted parts of the pump are: chemical nature, concentration, temperature, specific weight, and the possible presence of solids. For example, different concentrations of the same acid may require different plastic materials. Over the years, Savino has honed its skills
in helping clients navigate the various options depending on the application.




For sulfuric acid applications, as a rule of thumb, PVDF pumps are rated for the following sulfuric acid temperatures and concentrations:









• 90°C at 90% concentration
• 60°C at 96% concentration
• 40°C at 98% concentration
• 20°C at 98% concentration
Savino Barbera makes horizontal and vertical chemical
pumps, magnetic drive pumps, double diaphragm pumps, topentry mixers, and other industrial equipment. Savino Barbera’s pumps and mixers are compatible with a variety of corrosive chemicals, including sulfuric acid, ferric chloride, hydrochloric acid, sodium hydroxide (caustic soda), chromic acid, sodium hypochlorite, seawater, phosphoric acid, and many others. For more information, visit www.savinobarbera. com. q

The
Prevent Pollution
Protect Equipment
Achive Quality
Mitigate Losses
Fiberbed has a highest removal efficiency of sub micron mists, reducing visible stack gas plumes.


Widely used in the sulfuric acid industry, to capture smaller particles without affecting capacity and increasing the service life of the equipment in extremely corrosive media.
Reduce Energy Use
Boost Capacity
Enhance Purity
Reduce Costs
Optimize Design
Handle Fluctuations
Promote high irrigation densities, reaching hundreds of points per square meter and allowing design for wide ranges of liquid flow.
Clark Solutions offers a wide range of innovative products and complete solutions for Sulfuric Acid processes.
Always aiming to help our customers around the world to keep their operations running with high level of efficiency and a reliability.
Follow
www.clarksolutions.com.br
Designed to allow more points of gas flow, increasing the efficiency, and reducing the pressure drop. Their area is highly effective and the low resistance to gas flow.
Widely used on the chemistry industry, to allow the mass transfer between highly corrosive substances, when it’s not possible to use random packing of plastic or metal.
Materials Of Construction
Brick Lined Systems
310S
High grade stainless steel Or CSX

To protect your plant, process vessels and storage tanks against chemical attack and corrosion.




LIGHTWEIGHT BREATHABLE LIQUID CHEMICAL SPLASH PROTECTION

Each year for the last 45 years, members of the AIChE Central Florida Section and colleagues from all over the world have gathered at Clearwater Beach to share their ideas concerning chemical process technology, specifically the production of phosphoric acid, phosphate fertilizers, and sulfuric acid. This year’s event is scheduled for June 9-10 at The Sheraton Sand Key Resort in Clearwater, Fla.
In addition to Saturday’s schedule, this year’s convention will include several Friday workshops on sulfuric acid and many other topics. Attendees can also earn up to 8 PDH for attending. To obtain PDH certification for conference sessions, members must attend the full session of the section for which they need credit, supply their P.E. number on or before Friday, June 7, 2023, and have attendance verified by proctors.
The convention also provides ample excuse to enjoy a relaxing getaway with friends and family, good food, and a lot of fun! Hospitality suites and dinners are always a highlight.
For more information, please contact convention chair Robert Andrew at vicechair@aiche-cf.org or visit www.aiche-cf.org.
For over 35 years, CRU’s Sulphur + Sulphuric Acid has been one of the premier networking and professional development event for those involved in the production, consumption, and trade of sulfur and sulfuric acid.

furic acid, plus additional industry updates from key players across the supply chain.
Covering technical updates on the production and processing of sulfur and sulfuric acid, the conference will feature industryleading presentations covering new innovations in process, technology, materials, and equipment developments, as well as practical case studies highlighting operational experience and improvement.
For more information, please visit www. crugroup.com.

The 2024 Sulfuric Acid Roundtable (SAR) will take place April 22-25, 2024 at the Omni Resort in ChampionsGate, near Orlando, Fla. The 2.5-day event will bring sulfuric acid producers and leading technology companies together to discuss the best practices for maintenance, operations, production, and safety. The event will include a keynote address on the global sulfuric acid market, producing plant panel discussions and presentations, new technology developments, safety and incident reviews, and exhibits.
Networking is an important part of the AIChE Clearwater Conference, giving plant personnel and industry leaders a chance to meet and share ideas informally.


CRU Group’s Sulphur + Sulphuric Acid conference continues to be an essential annual forum for the global sulfur and sulfuric acid community to learn, connect, and do business. Knowledge sharing is at the event’s core via both the respected dual-streamed technical program and the connections made with industry experts and solution providers attending.
This year’s event takes place November 6-8, 2023 at The Sheraton in New Orleans, La. Topics include an expanded market outlooks agenda with expert insights from CRU’s analysis teams on major supply and demand markets, including sulfur and sul-

Social activities include golf and fishing tournaments, plus additional networking opportunities during hospitality receptions and group dinners each evening. The event will also include a bus tour of a nearby acid plant.
For plant personnel interested in registering for the event, please visit www. acidroundtable.com. For vendors and technology providers interested in participating, please contact Kathy Hayward at kathy@ h2so4today.com or (985) 807-3868. q
Custom tower designs to match your plant needs
• Achieves performance requirements in all plant operating modes
• Customized SARAMET® metallurgy is selected for each application (Dry, Inter, Final, ALPHA™ or Oleum Towers)
• Proprietary gas inlet nozzle designs engineered to eliminate localized corrosion
• Designed for retrofit or new tower installations
• Allows use of existing or new tower foundations minimizing installation time
• Scope of supply ranges from detailed engineering and manufacturing drawings with material supply, to complete EPC
• Full tower life cycle support is available from Chemetics, including technical and inspection services
DETAILS MATTER
Get in touch with us to learn more!
chemetics.equipment@worley.com
Tel: +1 905 619 5200
worley.com/chemetics
Innovative solutions for your sulphuric acid plant needs

