











About the Editor
Allan Knight is one of the science curriculum consultants with School
Curriculum and Standards He has taught science, including senior school chemistry and physics, at high school and been a university chemistry lecturer He has co-authored a number of senior secondary chemistry textbooks and written teacher resources for senior secondary physics for WA and other Australian states
Welcome to the first issue of SCIOS - Secondary
Regular readers will note this is an updated format for SCIOS
To share ideas and strategies specifically targeted at secondary teaching, we will have a ‘bite sized’ issue each term Each issue will have 1-3 articles on the same variety of topics as previously (teaching practices, teaching research, laboratory activities, assessment practices etc.) and continue to include promotional information about upcoming STAWA and other science events that may be of interest to teachers
The PDF format will enable easier access and the ability to download for repeated viewing without having to log into the SCIOS link via the STAWA website Please note all the previous online SCIOS issues are still available via the STAWA link
We are sure these shorter targeted issues of SCIOS will fit in readily with teachers’ busy schedules
We welcome feedback on the new look SCIOS - Secondary which can be sent to admin@stawa.net
In this issue, an update on the IUPAC naming of organic compounds relevant to the teaching of Years 11 and 12 ATAR Chemistry As well, information on research into new ways to synthesize ammonia, approximately 70% of which is used in the agricultural industry This is important as the current production process consumes large amounts of energy and emits large amounts of carbon dioxide
Allan Knight (SCIOS Editor)(Organic Edition)
Lyndon Smith
It has come to my attention that over the last 25 years, IUPAC has made various changes to their recommendations which have not been incorporated into West Australian syllabus nor our textbooks
In a previous time, this might not have been an issue, however, I find my students are seeking information on the Internet, and what we’re teaching and the information that Wikipedia, IUPAC and the Royal Chemical Society offers as answers, differs from our teaching.
I’ve collected the recent IUPAC recommendations (the most recent 25 years), and summarised them here I would encourage teachers and examiners to consider that there could be alternate answers to their questions
I’m eager to avoid the conflict that a teacher faces when a student says, “I looked this up on Wikipedia, and you’re wrong ”
Unit 1 – Chemical fundamentals: structure, properties, and reactions
Properties and structure of materials
Science Understanding
IUPAC nomenclature is used to name straight and simple branched alkanes and alkenes from C1–C8
Chemical reactions: reactants, products, and energy change
Science Understanding
the mole is a precisely defined quantity of matter equal to Avogadro’s number of particles
the mole concept relates mass, moles, and molar mass and, with the Law of
Conservation of Mass; can be used to calculate the masses of reactants and products in a chemical reaction
Unit 4 – Organic chemistry and chemical synthesis
Properties and structure of organic materials
Science Understanding
4 IUPAC nomenclature is used to name organic species, including those with a parent chain of up to 8 carbon atoms with simple branching and one of the following
functional groups: alkenes, alcohols, aldehydes, ketones, carboxylic acids, esters,
SCIOS
amines, and amides
9 empirical and molecular formulae can be determined by calculation and the structure of an organic compound established from the chemical reactions they undergo, and other analytical data
The International Union of Pure and Applied Chemistry (IUPAC) communicates its recommendations via the Blue Book, the Red Book, the Green Book, and the Gold Book with reference to their monthly journal. Most of the following changes are at least 25 years old.
IUPAC have issued updated versions of the Red Book (Nomenclature of Inorganic
Chemistry) and the Blue Book (Organic Nomenclature) along with a version of the Gold Book (Compendium of Chemical Terminology) and the Green Book (Quantities, Units and Symbols in Physical Chemistry) There are significant changes that will affect chemistry teaching in secondary schools
Blue Book: https://iupac org/what-we-do/books/bluebook/ Gold Book: https://goldbook.iupac.org/
Green Book: https://iupac org/what-we-do/books/greenbook/ Red Book: https://iupac.org/what-we-do/books/redbook/
PAC = “Pure and Applied Chemistry” is the official monthly journal of IUPAC
Preferred IUPAC names – PIN – alternates; some to accept and some current names to reject
“A major new principle is elaborated in these Recommendations The concept of ‘preferred IUPAC names’ is developed and systematically applied Up to now, the nomenclature developed and recommended by IUPAC has emphasized the generation of unambiguous names in accord with the historical development of the subject. In 1993, due to the explosion in the circulation of information and the globalization of human activities, it was deemed necessary to have a common language that will prove important in legal situations, with manifestations in patents, export-import regulations, environmental and health and safety information, etc However, rather than recommend only a single ‘unique name’ for each structure, we have developed rules for assigning ‘preferred IUPAC names’ , while continuing to allow alternatives in order to preserve the diversity and adaptability of the nomenclature to daily activities in chemistry and in science in general
Thus, the existence of preferred IUPAC names does not prevent the use of other
names to take into account a specific context or to emphasize structural features common to a series of compounds Preferred IUPAC names belong to ‘preferred IUPAC nomenclature’ Any name other than a preferred IUPAC name, as long as it is
unambiguous and follows the principles of the IUPAC recommendations herein, is
acceptable as a ‘general’ IUPAC name, in the context of ‘general’ IUPAC
nomenclature ” -Blue Book Para P–10
The “Preferred IUPAC Name” (PIN) might not always be the systematic name Some examples you might have met are listed below
Retained names (can be used in publications)
acetic acid
benzoic acid
butyric acid
formic acid
oxalic acid
palmitic acid
stearic acid
terephthalic acid
Preferred IUPAC Name (PIN) Systematic Name
acetic acid
benzoic acid
butanoic acid
formic acid
oxalic acid
hexadecanoic acid (16 carbons)
octadecanoic acid (18 carbons)
ethanoic acid
benzenecarboxylic acid
methanoic acid
ethanedioic acid
P-34.3.2 Ketones and Aldehydes
Retained names (can be used in publications)
acetone
ethylene glycol
glycerol
phenol
(Blue Book – P-14.3.4.1)
benzene-1,4-dicarboxylic acid SCIOS
Preferred IUPAC Name (PIN) Systematic Name
propan-2-one
ethane-1,2-diol
propane-1,2,3-triol
phenol
benzenol
Omitting numbers in nomenclature – change in required locants
The locant is not omitted from propan-2-one, butan-2-one, prop-2-enoic acid and prop-2-ynoic acid although unambiguous without a locant. IUPAC specifically recommends propan–2–one and 2–methylpropane
There are some cases where the number (the locant) is omitted
1.
Certain functional groups can only occur at a particular position, so simply
stating the group defines the position unambiguously The most common examples are the aldehyde and carboxylic acid groups, which can only be at the terminal C
2
With (mono)-cyclic compounds carrying a single substituent, all positions are equivalent The position of the substituent is defined as the 1-position, and is omitted from the name
3
When all positions are full e g , octachloropropane There are only eight positions possible on propane, and "octachloro" means that all of them are Cℓ
Do not write 1,1,1,2,2,3,3,3-octachloropropane – the numbers are omitted
Functional groups that define the position
The aldehyde and carboxylic acid functional groups can occur only at the end of a chain When one of these is the main functional group, its position is defined as "1" Thus butanal (aldehyde) and butanoic acid (carboxylic acid) have the indicated functional group at the 1-position.
Similarly, butanedial and butanedioic acid have one functional group at each end; there is no other possibility, and IUPAC omitted the numbers
Cyclic compounds with one substituent
All positions on a ring are equivalent If there is one substituent, that substituent defines the "1" position Thus, bromocyclobutane and bromobenzene do not need numbers There is only one possible compound of each name, and the bromo position is defined as "1" This applies to single ring compounds, where all positions on the ring are equivalent
Special cases
There are several chemicals, generally small ones, where the number for a substituent is commonly omitted, even though they do not quite fit one of the specific exceptions discussed above However, they do fit the general criterion that the number is not needed, because there is no other choice A list of some of those special cases follows
Methanol
Alcohols usually need a number, but there is only one carbon here, so no number is needed
Ethanol
There are 2 carbons, but they are equivalent. There is only one alcohol possible based on ethane
Ethene and propene.
Double bonds usually need a number But these cases are unambiguous simply because the molecule is small, and there is only one possible structure However, note IUPAC requires propan–2–one and 2–methylpropane
Use “line formula” not “structural formula” – 1994
“Line Formula” has replaced the term “structural formula” as all representations are structural.
A two-dimensional representation of molecular entities in which atoms are shown joined by lines representing single or multiple bonds, without any indication or implication concerning the spatial direction of bonds For example, methanol is represented as:
H C O H H H
PAC, 1994, 66, 1077. (Glossary of terms used in physical organic chemistry (IUPAC Recommendations 1994)) on page 1136
Empirical formulae – Organics must be written in the form C₈H₈Br₂Cℓ₂F₂I₂ – CH first then alphabetic order while inorganics are in alpha order BrCℓH₃N₂NaO₂Pt
IR-4 2 1 – The Red Book
Empirical formulae
The empirical formula of a compound is formed by juxtaposition of the atomic symbols with appropriate (integer) subscripts to give the simplest possible formula expressing the composition For the order of citation of symbols in formulae, see Section IR-4 4, but, in the absence of any other ordering criterion (for example, if little structural information is available), the alphabetical order of atomic symbols should be used in an empirical formula, except that in carbon-containing compounds, C and H are usually cited first and second, respectively
Examples:
BrCℓH₃N₂NaO₂Pt 1
Empirical Formula
C₁₀H₁₀CℓFe 2
Note 1: In organic chemistry C and H are listed first, then the other elements in alphabetical order
Note 2: This differs from the molecular formula, in which the subscripts indicate how many of each element is included and which is an integer multiple of the empirical formula For example, the empirical formula of glucose is CH2O while its molecular formula is C₆H₁₂O₆
Note 3: The empirical formula is the information provided by combustion analysis, which has been largely superseded by mass spectrometry, which provides the molecular formula
Should we adopt the E-Z protocol?
cis-trans nomenclature applies only to those alkenes where the double bonded carbon atoms each only have one H atom attached to them
For example, trans-but–2–ene and cis-but–2–ene
Configurational
isomer (Molecular)
E/Z isomerism

trans-but–2–ene

cis–but–2–ene
For alkenes where the carbon atoms each have different non-H atoms (or groups) attached to them E/Z nomenclature is needed
The E/Z Naming Protocol – superior to cis-trans
For alkenes the terms cis and trans may be ambiguous and have therefore largely been replaced by the E, Z convention for the nomenclature of organic compounds
Source: PAC, 1996, 68, 2193 (Basic terminology of stereochemistry (IUPAC Recommendations 1996)) on page 2203 Blue Book (Guide), p 149
Configurational
isomer (Molecular)
E/Z isomerism
For each carbon of the double bond in question: Rank the two atoms directly attached to the carbon by atomic number Higher atomic number = higher seniority: e g I > Br > Cl > F > N > H (Note IUPAC has abandoned the term “priority” in favour of “seniority”

For both alkenes, compare Br vs F on the first carbon, and Cl vs H on the second carbon The atom with the higher atomic number is senior
The alkene where the highest seniority groups are on the same side is Z
The alkene where the highest seniority groups are on the opposite side is E

Geometric isomer – obsolete term – use strongly discouraged – 1996
geometric isomer [obsolete terminology] usage strongly discouraged – 1996 https://doi org/10 1351/goldbook G02620
Obsolete synonym for cis-trans isomerism (Usage strongly discouraged)
Geometric Isomer
Source: PAC, 1996, 68, 2193 (Basic terminology of stereochemistry (IUPAC Recommendations 1996)) on page 2209
Isomers : renamed – 1996
discouraged term for constitutional isomers
Structural Isomers
Note: The term “structural isomer” is discouraged, because all isomers differ in structure and because isomers may be constitutional or conformational
Conformation isomer
Constitutional isomers
Conformation refers to the spatial arrangement of atoms in a molecule that can be determined through single-bond rotation Examples include cis-trans or E/Z conformations
Constitutional isomers have the same molecular formula, but different connectivity For example, propan-2-ol and propan-1-ol, butane and 2-methylpropane are constitutional isomers
No longer do we name an alkene by finding the longest chain with the double bond–now simply the longest chain. Examples below-note new terms “ethenyl” , “methylidene” , “ethylidene” etc.
Nomenclature involving a double bond
Previously the molecule was named using the longest chain including the double bond
The nomenclature now gives seniority to the longest chain
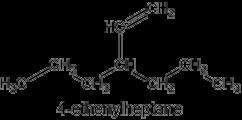



The principal chain has the greater number of skeletal atoms In acyclic parent structures the order of seniority between unsaturation and length of chain given in earlier recommendations is reversed Thus, the first criterion to be considered in choosing a preferred parent acyclic chain is the length of the chain; unsaturation is now the second criterion.
Blue Book – P-44 3 2
Hyphens – When a name needs to break over two lines where does the break occur? 2020
It is recommended that when breaking a name over two lines keep the locant and its component together For example, in the pronunciation of ‘1,2-dicholorobutane’ it was concluded that the ‘1,2’ is closely associated with the name component ‘dichloro’
In general, locants are to be considered part of the name component that follows
Accordingly, the hyphen between the locant or locant set and the name component must be considered as a non-breaking hyphen
e g 1,2–dichloro–butane
not 1,2–
dichlorobutane
Source: Pure Appl Chem 2020
IUPAC Recommendations Albert J Dijkstra* , Karl-Heinz Hellwich, Richard M
Hartshorn, Jan Reedijk and Erik Szabó End-of-line hyphenation of chemical names
(IUPAC Provisional Recommendations)
Received October 16, 2019; accepted January 21, 2020
The synthesis of ammonia is important for many industries, in particular agricultural industries. Ammonia is used to produce nitrogen-based fertilisers including ammonium nitrate and urea These fertilisers are vital in our current agricultural practices however, the Haber-Bosch process used to produce ammonia uses large quantities of energy accounting for about 2% of the world’s energy usage As well, the steam reforming process which is used to get the hydrogen needed to synthesise the ammonia release large amounts the greenhouse gas carbon dioxide The total emissions associated with ammonia (direct and indirect emissions in its production and carbon dioxide released when urea is applies to soil) is estimated to be about 2% of global emissions
Increasing global population means there is a need to at least maintain and even
increase world-wide agricultural output Thus, ammonia production needs to continue and so there is a need to find ways to produce ammonia that eliminate its contribution to greenhouse gas emissions, and much research is going on in this area
We reprint here, with permission, an article originally published in Chemistry World in October 2023 describing some research to produce ammonia without the use of fossil fuels as a feedstock and so eliminate that component of the emissions arising from the current production process
Author: Fernando Gomollon-Bell
Lasers could revolutionise nitrogen fixation, offering a new way to synthesise ammonia under ambient conditions For the first time, researchers have used
commercial carbon dioxide lasers to break the nitrogen–nitrogen triple bond, offering a new green alternative to the Haber–Bosch process
The international team of researchers used lasers to convert lithium oxide into metallic lithium, which spontaneously reacts with nitrogen in air to form lithium nitride
This salt is easily hydrolysed into ammonia, breaking all current records in terms of yield

Source: © Huize Wang et al 2023
The new laser-based process is more efficient at producing ammonia than the conventional Haber–Bosch process
‘We have introduced a pioneering concept, [which] harnesses high-energy lasers to facilitate the conversion of various oxides into nitrides, ’ says first author Huize Wang, from the Helmholtz Institute for Renewable Energy, in Germany ‘We have achieved an unprecedented yield [ ] under room temperature and atmospheric pressure conditions, notable when compared to other methods, ’ he adds The yield is two orders of magnitude higher than other state-of-the-art solutions, including electrochemical and mechanochemical methods
‘It’s a new method for the production of green ammonia, ’ says Victor Mougel, an expert in the electrochemical transformation of small molecules based at ETH Zurich, Switzerland ‘[Alternative] methods are potentially more sustainable than the
Haber–Bosch process, which is very energy intensive as it operates at high temperature and pressure and [ ] contributes to carbon dioxide emissions ’ As the process works in ambient conditions it ‘offers operational flexibility, as well as the environmental benefits’ This process could also allow ammonia to be produced where it is needed, cutting the cost of transportation
The team generated metallic lithium from lithium oxide, thanks to an infrared laser that provides enough energy to dissociate the lithium–oxygen bonds When exposed to air, metallic lithium spontaneously binds nitrogen breaking the nitrogen–nitrogen triple covalent bond and generating lithium nitride ‘[We then] hydrolyse this lasergenerated lithium nitride to obtain ammonia gas and lithium hydroxide, ’ explains
Wang Moreover, this approach offers the opportunity for chemical cycling ‘A laser
[can] induce the conversion of lithium hydroxide back into lithium nitride, effectively closing the lithium cycle, ’ he adds ‘This [is] also another novel concept – the conversion from hydroxide to nitride ’
Ifan Stephens, an expert in electrochemistry and nitrogen fixation at Imperial College London, UK, is still sceptical ‘I’m not certain [these] high rates can be sustained for long periods of time, ’ he says. ‘Moreover, […] the fact that it is a batch process, as opposed to a continuous process, would pose significant limitations to its viability ’ In contrast, electrochemical technologies work continuously, which ‘offers a significant advantage over the new laser-induced method’ , according to Stephens Additionally, the energy needs of the lasers could pose problems for scaling-up ammonia synthesis ‘If you [ ] make ammonia on a small scale, as a fertiliser for remote locations, then the energy efficiency becomes less important, ’ he adds
‘Compared to electrochemistry, our method offers significant advantages [such as] desolvation and simplification, ’ argues Wang Plus, ‘the scale-up [ ] presents the most significant challenge for all emerging approaches for ammonia synthesis’ The researchers envision scaling up the process by distributing lithium oxide powder on a gridded surface, then irradiating the arrays of reaction cells with the laser, sequentially Additionally, researchers have observed similar behaviour with other oxides, such as magnesium, aluminium, zinc and calcium – although the yield is lower ‘[It] could be because these other oxides are more difficult to dissociate and hydrolyse, ’ explains Stephens However, the reactivity of alkaline and alkaline earth metals towards nitrogen seems promising ‘Our recent work shows that more abundant metals, such as magnesium and calcium can also dissociate nitrogen, ’ he says
References
H Wang et al, Nat Commun , 2023, 14, 5668, (DOI: 10 1038/s41467-023-41441-0)

Here are just some of the benefits and opportunities for STAWA Members Please check our website for details, and to find out what else STAWA does - PLUS what STAWA can offer you! (www stawa net)
SERVICES AND SUPPORT
Resources
A range of resources for both Primary and Secondary teachers is available on the website Look for the Resources Tab There are resources available to members and non-members Members can also share resources through the STAWA website You can download resources directly from the website or request to upload resources by contacting the Office (admin@stawa.net).
Catalist (Secondary teachers)
Catalist is an email communications list which reaches over 800 Science Educators and is used to share information, ask questions, and discuss current issues To subscribe to Catalist, look under the Teachers Tab on the website
Australian Science Teachers’ Association (ASTA) Affiliation
Full fee-paying members enjoy the benefits of affiliated membership to the national association This includes access to ASTA’s online journal, Teaching Science, and the
Chrysalis online learning community
PUBLICATIONS
STAWA Members receive:
SCIOS (STAWA online journal)
STAWA SPARKs! (Primary Science Committee online publication)
Teaching Science (ASTA journal),
Spotlight on STAWA (e-newsletter)
Information about Science activities for students and teachers
Professional Development & Conference programs
Members receive discounts for STAWA Professional Development Workshops, a range of services and attractions, STAWA texts and resources, plus attendance at STAWA
Conferences and events, including:
CONSTAWA (WA Science Educators Conference)
Future Science (WA Science Educators Conference)
Psychology Teachers Convention
Marine and Maritime Teachers Convention
CONASTA (Australian Science Educators Conference, ASTA)
PROFESSIONAL RECOGNITION
STAWA recognition of teacher achievement and service through annual awards –de Laeter Medal
Jeff Cahill Early Career Teacher Award
Support for primary Science teachers is given through the STAWA Early Career
Primary Science Scholarship
OPPORTUNITIES
Teaching employment opportunities, curriculum review and development, government policy input, science equipment advice and professional development.
An independent voice through STAWA’s representatives on many education bodies and committees.
WELCOME PACK
New members receive a Welcome Pack containing a Members USB, Pen, and Notepad
STAWA LIFE MEMBERSHIP
Each nomination for STAWA Life Membership is considered on its individual merits
Nominations, accompanied by relevant supportive evidence, must be submitted to the
President of STAWA Life Membership, if awarded, is bestowed at the AGM Please refer to the specific details on the STAWA website
STUDENT OPPORTUNITIES
Science Talent Search
Physics Day Synergy Schools Solar Challenge
MEMBERSHIP QUERIES
If you have any queries with regards to your membership, please email us at admin@stawa net

CAN YOU CONTRIBUTE TO SCIOS?
YES, of course you can Contributions from teachers, laboratory technicians, students, academics and industry are all welcome
We are keen to increase the number and variety of types of articles published in the
SCIOS So, if the answer is YES to any of the following questions, please consider submitting an article to the editor
Have you recently conducted an experiment (investigation or hands-on activity)
that worked well?
Is there a great demonstration that always gets your students’ attention?
Have you tried a new teaching technique that really engaged your students?
Do you have some helpful hints for new (and not-so-new) teachers?
Are there some safety hints and tips that you would like to pass on?
Are you using some new technology that has improved the effectiveness of your students’ learning?
Are your students involved in a science project outside of school?
Have you recently attended a useful/interesting professional development activity?
Email your contributions to admin@stawa.net.
GUIDELINES FOR AUTHORS
These notes are a brief guide to contributors who should also refer to recent issues of the journal for guidance with style
Longer articles - should not normally exceed 3000 words plus figures, tables and any references Please use headings and sub-headings to give your article structure
Shorter articles - We also welcome shorter articles of approximately 500-1000 words plus figures, tables and any references Again, use of headings and subheadings may assist to give your article structure
Send the following to the editor:
1
Please send your document as a word file with photographs and other images
embedded where you need them to be
2
Photographs and other images (e g diagrams) should be sent as separate files
3.
Photographs often increase the clarity and interest level of your work Send your
photographs as tiff or highest quality jpeg files with a resolution of at least 300
dot per inch (dpi) Note to teachers: a signed parent permission slip must be
obtained for any photographs of students to be included in SCIOS.
4. COPYRIGHT
Copyright for any part of your contribution that is copyright of a third party needs to be obtained in writing (email acceptable)
No other publisher should have published your manuscript, nor should you submit for publication elsewhere If SCIOS publishes your manuscript then your text and graphics will become copyright of STAWA STAWA will, however, agree to your use of the contents of your paper for most reasonable non-commercial purposes
Contact admin@stawa net to submit your articles

Website: https://scienceiq net
ScienceIQ is an online science quiz for school teams of four students
Teams have one hour to complete up to 12 science questions and problems in two separate rounds of competition Each round is a week apart and teachers choose the days and time to do each quiz To win, students will need to finish in the shortest time with the most questions correct
Participation certificates, first, second and third place prizes, and other great stuff
Years 6 and 9 Year 8 and a joint Years 5 & 6 competition
Rd 1 week of 12 Aug
Rd 2 week of 19 Aug
Rd 1 week of 4 Nov Rd 2 week of 11 Nov
Registration fee:
$25 (+ GST) per year group per school
ScienceIQ will test student skills and understandings of the Natural Sciences: astronomy, physics, chemistry, Earth science, and biology
Register via the STAWA website.
Log-in details and instructions to access the practice quiz will be sent to the teachers of the registered schools prior to the competition date



School Registrations for the 2024 Science Talent Search are NOW OPEN!
Register here
WHATISTHESCIENCETALENTSEARCH?
An amazing opportunity for your students to do their own real science.
WHO IS IT FOR?
Students from Kindergarten to Year 12 can enter, though different categories have some specific age requirements. Make sure you check the rules for each category
CAN GROUPS OF STUDENTS ENTER?
Yes - be sure to check the requirements
WHATCATEGORIESARETHERE?
Science Investigations
Science Communication - including posters, videos and photography
Engineering
HOWMANYCATEGORIESCANMYSCHOOLSUBMIT?
There are requirements for schools to provide teachers to judge entries over a certain number of student entrants, so be sure to check this out very early
WHERECANIFINDMOREINFORMATION?
Look under the Student Activities tab on the STAWA website where all your questions will be answered
NEVERBEENINVOLVEDWITHSTS?
Why not have a go this year? Perhaps start with just a few student entries and learn how it all happens.