The Children’s Discovery Institute





















Founded in 2006, the Children’s Discovery Institute (CDI) is a partnership between St. Louis Children’s Hospital and Washington University School of Medicine (WUSM) to find innovative, more precise and personalized treatments for a spectrum of childhood diseases. Without this type of research, there is no progress.

The MD and PhD scientists and clinical staff at the CDI are relentlessly focused on speeding the path from laboratory research to lifechanging therapies. As a leading center for pediatric research, the CDI model is unique in its cross-disciplinary, collaborative approach to rigorous science intertwined with worldclass patient care. Washington University investigators are at the forefront of developing personalized medicine for children and families who currently have limited options for treatments or cures.
For nearly 15 years, the CDI primarily focused on four specific pediatric disease areas: congenital heart disease, cancer, lung and respiratory disorders and musculoskeletal diseases. Progress has been made and clinical trials have provided game-changing results. It’s now time to advance initial findings and propel St. Louis Children’s Hospital and WUSM forward into a new era of research and discovery.
Since 2006, St. Louis Children’s Hospital Foundation has granted more than $93.9 million to advance CDI pediatric research.
• This philanthropic investment of CDI investigator-led projects has had a remarkable return on investment, with the generation of more than 1,439 research papers.
• More than 240 grants have been awarded to researchers since the CDI’s inception.
• Awardees have leveraged their initial seed funding to gain more than $550 million in additional funding resources from the National Institutes of Health (NIH) and other organizations. In other words, for every $1 invested by the CDI to stimulate new research and treatment, the clinical investigators attract $5 in additional funding to advance discoveries.
The breakthroughs enabled through your ongoing support are providing answers for children and families living with pediatric illness. With the proven success of the CDI model, the team will expand the scope of research supported by the CDI to encompass many more areas of child health by establishing Centers of Excellence. These Centers will bring a laserlike focus on children’s diseases by combining clinical and laboratory-based approaches using diverse teams of investigators.
Donor gifts will continue fueling hope that earlier, more accurate diagnoses will lead to improved treatments and outcomes. They will also allow us to
invest in fostering and supporting young investigators in their early careers as we strive to build long-term collaborations with these talented scientists.
These changes in focus of disease and allocation of funds will spur faster scientific advancement and greater collaboration across disciplines creating a stronger, more expansive CDI. This vision has already enabled the establishment of multiple Centers thanks to donor support.
• The Hermann Center for Child and Family Development is designed to blend world-class research with a revolutionary model for whole-family behavioral healthcare.
• The Center for Pediatric Pulmonary Disease and Asthma was created with the goal of discovering more targeted, effective, rational and personalized treatments and interventions for children with respiratory issues.
• The McDonnell Pediatric Cancer Center will help advance some of the world’s most innovative research-based treatments and create pediatric clinical trials for children.
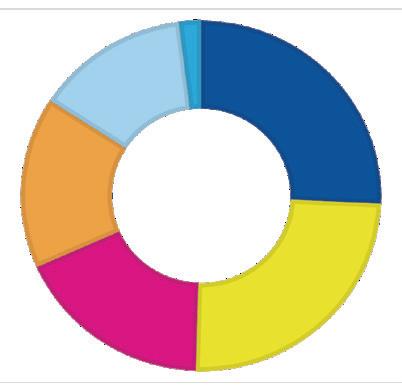
$93.9 million Awarded to Date
2006–2021: $85.6 million
Metabolism and Immunity — $22.1M
Cancer — $21.1M
Pulmonary Disease and Asthma — $15.6M
Unrestricted CDI Funding — $13.8M
Congenital Heart Disease — $11.5M
Personalized/Precision Medicine — $1.5M
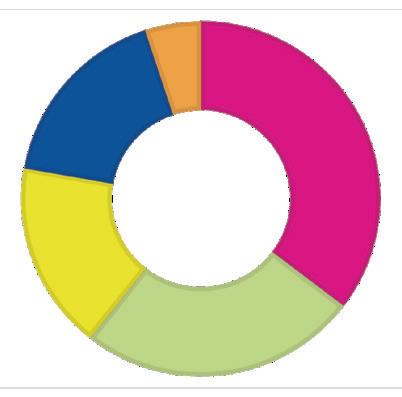
2022: $8.3 million
Center for Pediatric Pulmonary Disease and Asthma — $2.9M
Hermann Center for Child and Family
Development — $2.1M
McDonnell Pediatric Cancer Center — $1.4M
Metabolism and Immunity — $1.4M
Child Health Discovery Fund
(unrestricted) — $450K
We would like to introduce you to several patients and CDI investigators who have benefited from your extraordinary impact. These stories would not have been possible without donors like you, who have committed to funding groundbreaking scientific breakthroughs in hopes of helping more children — now and in the future.
Primary Investigators: Dr. Todd Fehniger and Dr. Jeffrey Bednarski
When a child is diagnosed with cancer, especially a cancer with few treatment options, families seek out physicians who are considered leaders in pediatric cancer research. They want a hospital that offers the latest and best therapies, which may only be available through groundbreaking clinical trials — often funded through generous donor support.
For Weston’s family, finding a trial for his acute myelogenous leukemia (AML) — a cancer of the blood and bone marrow — was a matter of life or death. AML accounts for about 18% of leukemia diagnoses in children but accounts for more than 50% of all leukemia-related deaths. Survival rates for kids diagnosed

with AML are 65-75%, and for those with relapsed AML, the survival rate drops drastically to under 10%. Children diagnosed with AML currently have only two approved treatment options: high-dose chemotherapy and stem cell transplant.
At just 3 years old, Weston came to St. Louis Children’s after both options failed to put his cancer into remission and enrolled in a first-ofits-kind treatment designed to fight the exact kind of AML he was diagnosed with at just 7 months old.

While creating new immunotherapies for diseases such as AML is complex, the NK1 Trial was built on basic scientific research at Washington University School of Medicine.
Megan Cooper, MD, PhD, Director of the Clinical Immunology program at St. Louis Children’s, discovered in her lab that taking Natural Killer (NK) cells and pre-exposing them to immune activators to mimic an infection allows them to perform their natural killing function better when they are called to duty a second time. Essentially — these cells have memory.
That work captured the attention of Dr. Cooper’s husband, Todd Fehniger, MD, PhD, Professor of Medicine and Bone Marrow Transplant Specialist at Siteman Cancer Center, who knew that NK cells were particularly good at fighting AML. His lab went even further in their research, and after showing that NK cells have a “supercharged” attack against leukemia, performed the first adult trial for patients with relapsed AML. Thanks to this new treatment, 67% responded positively and 47% achieved a complete remission. These are the same patients who were told they were no longer candidates for any other therapies — the exact same news Weston and his parents received.
When Jeff Bednarski, MD, PhD, a Washington University pediatric hematologist and oncologist at St. Louis Children’s Hospital, learned of the
trial’s success in adults with relapsed AML, he wanted to bring the same trial to pediatric patients who had unsuccessfully undergone a bone marrow transplant. Using NK cells from the same donor ensures the cells won’t be destroyed, since the donor and patient now share the same immune system.
The trial itself, which was supported by CDI funding, involves a round of chemotherapy to reduce the leukemia to give the NK cells the best possible advantage. Within two weeks after starting chemotherapy, the patient receives an infusion of T cells and NK cells from the donor, which only takes a matter of minutes. After discharge, the patient and family must reside locally for three months for monitoring.
After a lot of ups and downs, Weston and his family received the news they so desperately had been searching for: Weston was officially in remission! No one knows exactly what the future holds for Weston, but Dr. Bednarski and his NK1 trial have given the family hope not offered anywhere else.
Primary Investigators:
Dr. Phillip Tarr and Dr. Barbara Warner
A body’s gut microbiome — made of up to 1,000 species of bacteria — is crucial to a human’s health. And the body is affected by its microbiome the moment a baby is born. Healthy gut microbiomes have been linked to reduced risk of a variety of immune and metabolic disorders.
Nearly all premature babies receive antibiotics in their first weeks of life to ward off or treat potentially deadly bacterial infections. However, the concern remains that while such drugs are lifesavers, they also cause long-lasting collateral damage to the developing microbial communities in the babies’ intestinal tracts.
Phillip I. Tarr, MD, who specializes in pediatric gastroenterology, and Barbara Warner, MD, MSc, who focuses on newborn medicine, wanted to gather

more information regarding the role of antibiotics in the development of the microbiome of premature infants. While they already understood that antibiotics cause harmful disruption, they were seeking to understand how long the disruption would last. The team analyzed 437 fecal samples collected from 58 infants, ages birth to 21 months. Forty-one of the infants were born around 2.5 months premature, and the remainder were born at full term.
All of the premature infants had been treated with antibiotics in the newborn intensive care unit (NICU), with nine receiving just one course, and the other 32 given an average of eight courses — spending about half their time in the NICU on antibiotics. None of the full-term babies had received antibiotics.
Drs. Tarr and Warner discovered that preemies who had been heavily treated with antibiotics carried significantly more drug-resistant bacteria in their gut microbiomes at 21 months of age than preemies who had received just one course of antibiotics and full-term infants who had not received antibiotics. The presence of drug-resistant bacteria did not necessarily cause any immediate problems for the babies because most gut bacteria are harmless — as long as they stay in the gut. But gut microbes sometimes escape the intestine and travel to the bloodstream, urinary tract or other parts of the body. When they do, drug resistance can make the resulting infections very difficult to treat.
They then took their study further to reveal the babies developed diverse microbiomes by 21 months of age — a good sign since lack of microbial diversity is associated with immune and metabolic disorders in children and adults. But heavily treated preemies developed diverse microbiomes more slowly and the
makeup of the gut microbial communities differed, with heavily treated premature infants having fewer healthy groups of bacteria and more unhealthy kinds.
The findings already have led Dr. Warner, who is the current chief of newborn medicine at St. Louis Children’s Hospital, and her fellow neonatologists to scale down their use of antibiotics in the NICU.
Researchers also looked at the gut microbiome in early life from another angle, comparing differences in infant formula and breast milk on microbe populations that develop in the digestive tract of healthy infants.
Infant formula is designed to mimic human breast milk, not only in nutrients but also by nurturing a similar set of microbes in the digestive tract. Such microbes are indispensable in keeping humans healthy. They crowd out disease-causing bacteria, influence our metabolism and produce many vitamins and amino acids, the building blocks of proteins.
Gautam Dantas, PhD, who specializes in laboratory and genomic medicine, and Aimee Baumann-Dudenhoeffer, MD, a neonatologist, studied samples collected monthly from 30 sets of twins born in the St. Louis area as part of Drs. Warner and Tarr’s original neonatal gut microbiome project a decade ago. They identified the components of the gut microbiome in each child as well as nutrient molecules that these bacteria were capable of producing or breaking down. The investigators also examined information on how the babies were fed, including the specific brands of formula.
Older studies have looked at how formula makers have created the right mix of bacteria, but not how it’s performing. This new study has determined that while formula and breast milk encourage the growth of similar kinds of bacteria in babies’ digestive tracts, the bacteria work differently. The health implications of these differences are as yet unclear and point to a need to better understand this given the importance of early infancy for brain and overall development.
This research has led Drs. Dantas and BaumannDudenhoeffer to consider ways to determine which babies could benefit from changes to their gut microbiome and how to do that.
Primary Investigators:
Dr. Ahmed Said and Dr. Adam Eggebrecht
When identical twins Ryder and Wyatt were born, they received a clean bill of health and were sent home with their parents. But after just 12 days, it was clear something wasn’t right with not just one, but both twin boys. Their increasing sleepiness, lack of appetite and missing two feedings in a row was concerning, and their mom called their pediatrician. He advised their parents

to bring the boys to his office right away. It became clear later that had they not made that phone call, both boys would not have survived the night.
The family arrived at the pediatrician’s office, and it was evident something was very wrong. The twins were so cold the office staff couldn’t even detect a temperature. An ambulance arrived, ready to take Ryder and Wyatt to St. Louis Children’s Hospital. The time between stepping through the doors of the emergency department and Ryder and Wyatt being admitted to the pediatric intensive care unit (PICU) was less than 30 minutes.
Wyatt had to be placed on a ventilator while the team of medical professionals tried to figure out what was causing these 12-day-old babies to go into cardiac failure. Unfortunately, both Ryder and Wyatt continued to rapidly decline, and both boys required extracorporeal membrane oxygenation (ECMO) within 24 hours of arriving at St. Louis Children’s. Their parents met with Ahmed Said, MD, PhD, a pediatric cardiac intensivist at Washington University School of Medicine, to have a difficult conversation about everything entailed with going on ECMO — a temporary heart and lung machine that circulates blood and oxygen throughout the body, allowing a child’s organs the chance to rest and recover.
Once the team of doctors realized that each baby was following the same path, a lot of questions needed answers. Was it something genetic or metabolic they were born with? Within a few days, Ryder and Wyatt were both diagnosed with enteroviral myocarditis caused by a specific virus — Coxsackie B5.
Both boys were ineligible for a heart transplant or a ventricular assist device, which is used to support heart failure patients who are too sick to wait for a donor heart to become available.
A pediatric infectious disease consultant was called to assist with a treatment plan that included the investigational antiviral drug Pocapavir. The team contacted the Food and Drug Administration (FDA) for emergency usage permission, and it was granted.
After 11 days, Ryder went off ECMO, and Wyatt joined him the next day. It was a very exciting day for everyone involved. After several more weeks in the hospital, the twins were released to go home to join their parents and big sister.
Today, Ryder and Wyatt visit St. Louis Children’s Hospital once a year for follow-up but they’ve returned to normal heart function and are no longer taking any medication. They also have no long-term neurological effects of ECMO that some patients retain long after their hospital stays conclude.
With support from CDI funding, Dr. Said has partnered with Adam Eggebrecht, PhD, who specializes in radiology, to conduct further ways to monitor brain health during ECMO. Dr. Said’s ECMO expertise is also currently being leveraged as he leads a research team to develop a model to predict the use of ECMO in COVID-19 patients, thanks to CDI support. The pediatric ECMO program at St. Louis Children’s Hospital is one of the highest-volume centers internationally, which positions this research team to be well-versed in the ECMO process. With highdensity diffuse optical tomography (HD-DOT) developed by Dr. Eggebrecht, they aim to provide direct access to realtime brain health measures that will inform clinicians of potential changes to a patient’s physiological status.
Zoe
Primary Investigators: Dr. Jennifer Silva and Dr. Jonathan Silva
For patients with cardiac rhythm abnormalities, procedures that intentionally scar heart tissue to block irregular electrical signals are the mainstay of modern therapy. These procedures have been enhanced by the development of technology that constructs 2D images of the interior heart surface. It’s extremely difficult to translate this flat image to 3D cardiac anatomy. The goal of this CDIfunded project is to develop a new display system to allow physicians to visualize patient-specific cardiac 3D holograms.
Jennifer Silva, MD, PhD, who specializes in pediatric cardiology, and Jonathan Silva, PhD, whose focus is biomedical engineering, have developed a mixed reality system to provide enhanced visualization of cardiac images in patients with heart rhythm abnormalities during minimally invasive procedures to treat these patients. The system uses holographic images to allow the physician to visualize catheters within a patientspecific heart 3D hologram. The team has
completed a first-in-human study utilizing this technology. They also created an ultrasound-mixed reality prototype that will allow physicians to see a patient’s vascular system.


This problem of translating a flat image into a 3D procedure also exists in the cardiac catheterization laboratory, where patients routinely undergo procedures for congenital heart defects with very complicated anatomies. With the catheter, physicians must map out where the heart’s electrophysiological abnormalities are by creating two 2D images that they must then interpret to decide whether to surgically remove tissue. It can lead to a lot of mental fatigue. But computer modeling helps to put this all together in a quantitative way.
Thanks to funding from the CDI, Drs. Silva and Silva were able to apply for and receive a coveted grant from the NIH, and their technology received approval from the FDA to be used as a medical device.
New research center will serve as a platform to transform behavioral health, starting with the family unit

Even before the COVID-19 pandemic, the Centers for Disease Control and Prevention (CDC) estimated that one in five kids would experience a mental health problem during their school years. In St. Louis, that translates to an estimated 125,000 kids, making behavioral health one of the greatest healthcare needs in our community.
In 2022, the Hermann Center for Child and Family Development was established as a new research center within the CDI to develop a new model of care to improve the behavioral health of children. The Hermann Center will upend the usual approach to mental health and aim to begin caring for kids as early as possible, even from birth, rather than waiting to treat problems long after they emerge. The Hermann Center will serve as the medical and psychiatric “home” for children. The first of its kind: a program focused on a holistic approach to behavioral health that engages the whole family, and that will track the results of this care for a decade or more.
The Hermann Center will identify children at increased genetic or environmental risk of experiencing a behavioral health impairment — and then provide education and support for parents, applying evidence-based preventive intervention and instituting behavioral health treatment, if and when it is necessary, at the earliest possible juncture in each child’s life. What the Hermann Center learns has the potential to improve key aspects of how behavioral healthcare needs for children are identified and managed — from how parents interact with their new babies to how medical professionals identify and address risk factors for behavioral health problems to the way kids learn coping skills.

“Signa and I want to ensure the future of children with a variety of conditions is limitless and families receive transformational care that leads to long and healthy lives.”
Bob Hermann
We are profoundly grateful for your support of our patients and CDI investigators who have benefited from your extraordinary impact. You allow our care teams to continue providing world-class care for kids in St. Louis, across the United States and around the globe.
