President’s Message
Jessyka G. Lighthall, MD, FACS PAO-HNS President
Welcome to the Fall issue of Soundings!
I had the honor of assuming the role of president of the Pennsylvania Academy of Otolaryngology - Head and Neck Surgery in June 2023 and this is therefore my first Sounding's message as president of the PAO HNS. I want to thank Dr. David Cognetti, immediate past president, for his dedication, leadership and service to the Academy of PAO HNS. During his term, Dr. Cognetti spearheaded the reorganization of our PAO HNS committee structure encouraging more involvement from young faculty and trainees, helped us transition back to an in person annual meeting post COVID and engaged a new lobbyist, Philip Dunn, to continue the PAO HNS mission of providing strong advocacy for otolaryngologists in the state of Pennsylvania.

This years' annual PAO HNS scientific meeting was organized by program chairs Dr. Nick Purdy and Dr. Karen Choi and took place in June in Lancaster, Pennsylvania. The meeting was a great success! The chairs and program committee provided an excellent line up of speakers and panels from across the state as well as other disciplines discussing current management of otolaryngologic problems in pediatrics and adults, providing a patient safety section targeting optimization of postoperative pain control, and a session on how to incorporate advanced practice practitioners into our practices. We were honored to have the current president of the AAO-HNS, Kathleen Yaremchuk, MD, provide a keynote address during the general program titled " If We Knew Then, What We Know Now About Sleep" as well as being the key guest for the women in otolaryngology event. Additionally, the newly created Ellie Goldenberg Award in loving memory of the daughter of Drs. David and Renee Goldenberg. This was awarded to Kasra Ziai, MD and Nicole Molin, MD, residents who have a strong work ethic, diligence, initiation and kindness. Many thanks to PAMED
and meeting coordinators Jessica Winger and Audrey Dean for helping making this meeting a success. Please find more details of our annual meeting later in this issue of Soundings.

I look forward to the opportunity to serve the PAO HNS, working with PAMED and our President Elect Colin Huntley MD, SecretaryTreasurer Andy McCall MD, and the executive council.
Our goals are to continue to refine the committee structure, improve membership engagement, incorporate a more formal mentorship program within the PAOHNS, expand membership within the society, provide high quality educational and networking opportunities, and promote continued political advocacy.

President
Jessyka G. Lighthall, MD, FACS
Penn State Health Milton S. Hershey Medical Center Otolaryngology—Head & Neck Surgery


President-Elect Colin T. Huntley, MD Jefferson University—Otolaryngology Head & Neck Surgery
Secretary-Treasurer Andrew McCall, MD, FACS University of Pittsburgh Department of Otolaryngology
Administrative Office 400 Winding Creek Blvd. Mechanicsburg, PA 17050-1885
833-770-1544
855-918-3611 (fax)
Visit
Soundings accepts classified advertisements; however, there is no guarantee that they will be published. All submissions are subject to review. The advertisement should be of interest/ pertain to otolaryngologists, their practice, and health care in Pennsylvania. Submissions that are self-promotional or commercial in nature will not be accepted. Publication of advertising does not imply endorsement of the products advertised or the statements contained in such advertising by Soundings or the PAO-HNS. The opinions expressed in this newsletter do not necessarily reflect the opinion of PAO-HNS.
The Role of Cemiplimab in the Treatment of Head and Neck Cutaneous Squamous Cell Carcinoma
Pallavi Kulkarni, BS Neerav Goyal, MD MPH FACS The Pennsylvania State College of Medicine, Hershey, PA Penn State Health Department of Otolaryngology- Head and Neck Surgery, Hershey, PACutaneous squamous cell carcinoma (cSCC) is the second most common type of skin cancer in the world, and it constitutes 20% of non-melanotic skin cancers of the head and neck.1 The reported incidence in 2019 was 2.4 million, showing a 200% increase from the incidence seen 30 years prior.2 Exposure to solar ultraviolet light and Fitzpatrick skin types I-III increase a patient’s risk of developing cSCC; therefore, cSCC are commonly found on the head and neck.3,4 Factors leading to poor prognosis in head and neck cSCC include immunosuppression, lymph node involvement, and margin status, among others.5 In fact, cSCC now significantly contributes to cancer-related mortality in the elderly and immunocompromised populations.6
Primarily, cSCC is treated with surgical excision, which cures 95% of patients. However, 3-4% of patients will recur locally, and 2-4% will metastasize to regional or distant locations, with the parotid gland being the most common site.1,7,8 cSCC can also be treated with non-surgical methods such as chemotherapy (targeted or systemic) and/ or radiation. Interestingly, cSCC contains molecular and clinical characteristics that make it highly susceptible to systemic immune therapy. Until 2018, there were no FDA- approved therapies that were available for treatment of systemic cSCC, and cemiplimab was the first to be approved.9 Cemiplimab is a PD-1 antibody, binding to T-cells, allowing for immune system recognition of tumor cells.10 Typically, PD-1 binding to its
ligand (PD-1L) leads to a cessation of the immune response; however, tumor cells highly express PD-1L, leading to T-cell exhaustion.11
Currently, there are a few studies showing the efficacy of cemiplimab when treating advanced cSCC. An initial study, published in 2018 by Migden et al., with 69% of the sample consisting of head and neck patients, showed an impressive effect on disease modification, with response rates of about 50% for patients with advanced and metastatic cSCC. Additionally, most adverse events were labeled as grades I/ II, including diarrhea, constipation, fatigue, and nausea.12 In another study, patients specifically with metastatic cSCC of the head and neck were remarkably seen to have a better treatment response (64% vs. 38%) in comparison to other anatomical tumor sites.13
A subsequent study concluded that patients with resectable cSCC have an even better response to treatment. In a recent study published in 2022 by Gross et al., patients with stages II-IV (M0) disease received neoadjuvant cemiplimab prior to surgical resection. Administered dosage was 350 mg every 3 weeks for a total of 4 cycles. Ninety one percent of these patients had a primary tumor of the head and neck. Fifty one percent of all patients had complete response, and 13% had a major response (presence of viable tumor cells made up less than 10% of the surgical specimen) following surgical treatment of note, the rate of complete response was higher pathologically (51%) vs. when considering imaging only (6%), showing the importance of post-treatment surveillance modality. Sixty eight percent of patients with noted partial response on imaging had a complete pathologic response. Once again, most patients experienced mild side effects. Seventy two percent of side effects were treatment related, while 15% of patients experienced
ones that were immune-related.2 This study is a breakthrough for patients with head and neck cSCC. Similar results were seen in a small-scale study conducted by Ferrarotto and colleagues. Seventy five of patients receiving neoadjuvant cemiplimab for advanced stage (III, IV) head and neck cSCC, followed by surgical resection had a complete or major pathologic response. Patients received 2 cycles of cemiplimab every 3 weeks, with surgery planned for greater than 3 weeks following the last treatment.14
When considering treatment options for advanced head and neck cSCC, it is critical to consider cost for the patient. Although pembrolizumab, another PD-1 inhibitor, has been FDA-approved for the treatment of advanced cSCC, one study shows that cemiplimab may be the more cost-effective option, with greater life expectancy, more disease-free time, and lower cost in the United States compared to pembrolizumab.15 Overall, the medication still comes with significant cost, but cemiplimab has some payment assistance options available for patients.16
Cemiplimab can be an efficacious, moderate side-effect profile treatment for patients with advanced cSCC. Because it is a relatively newer treatment option, there are many considerations when evaluating patients to receive cemiplimab. More information is needed regarding dose amount and cycle frequency. There are many ongoing clinical trials investigating the role of cemiplimab in head and neck cSCC. Additionally, the studies that have been conducted looking at efficacy of cemiplimab had patients that were overwhelmingly white and male, with median ages of 73. Therefore, tumor response and adverse effects may not be representative of all patient types. Patients Continued on page
The Role of Cemiplimab in the Treatment of Head and Neck Cutaneous Squamous Cell Carcinoma
should be counseled on the “last resort,” nature of this therapy, the associated costs, and the side effects that accompany treatment. Modality of determining response rate of patient is important. Current recommendations by the American Head and Neck Society include reserving PD-1 antibody therapy for patients who cannot undergo surgery/radiation due to failure of previous treatment, comorbidities, disfigurement, and dysfunction, and/or wishes of the patient.17
References:
1. O'Hara J, Ferlito A, Takes RP, et al. Cutaneous squamous cell carcinoma of the head and neck metastasizing to the parotid gland--a review of current recommendations. Head Neck. 2011;33(12):1789-1795. doi:10.1002/ hed.21583
2. Gross ND, Miller DM, Khushalani NI, et al. Neoadjuvant Cemiplimab for Stage II to IV Cutaneous Squamous-Cell Carcinoma. N Engl J Med. 2022;387(17):1557-1568. doi:10.1056/NEJMoa2209813
3. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78(2):237-247. doi:10.1016/j. jaad.2017.08.059
4. Veness MJ. High-risk cutaneous squamous cell carcinoma of the head and neck. J Biomed Biotechnol. 2007;2007(3):80572. doi: 10.1155/2007/80572. Epub 2007 Apr 3. PMID: 17541471; PMCID: PMC1874675.
5. Lubov J, Labbé M, Sioufi K, et al. Prognostic factors of head and neck cutaneous squamous cell carcinoma: a systematic review. J Otolaryngol Head Neck Surg. 2021;50(1):54. Published 2021 Sep 7. doi:10.1186/s40463-02100529-7
6. Nehal KS, Bichakjian CK. Update on Keratinocyte Carcinomas. N Engl J Med. 2018 Jul 26;379(4):363-374. doi: 10.1056/NEJMra1708701. PMID: 30044931.
7. Karia PS, Jambusaria-Pahlajani A, Harrington DP, Murphy GF, Qureshi AA, Schmults CD. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol 2014; 32: 327–334.
8. Leibovitch I, Huilgol SC, Selva D, Hill D, Richards S, Paver R. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia I. Experience over 10 years. J Am Acad Dermatol 2005; 53: 253–260.
9. Lebas E, Marchal N, Rorive A, Nikkels AF. Cemiplimab for locally advanced cutaneous squamous cell carcinoma: safety, efficacy, and position in therapy panel. Expert Rev Anticancer Ther. 2021;21(4):355-363. doi:10.1080/147 37140.2021.1876567
10. Shiravand Y, Khodadadi F, Kashani SMA, Hosseini-Fard SR, Hosseini S, Sadeghirad H, Ladwa R, O'Byrne K, Kulasinghe A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr Oncol. 2022 Apr 24;29(5):3044-3060. doi: 10.3390/ curroncol29050247. PMID: 35621637; PMCID: PMC9139602.
11. Wang Q, Xie B, Liu S, et al. What Happens to the Immune Microenvironment After PD-1 Inhibitor Therapy?. Front Immunol. 2021;12:773168. Published 2021 Dec 23. doi:10.3389/fimmu.2021.773168
12. Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, Chung CH, Hernandez-Aya L, Lim AM, Chang ALS, Rabinowits G, Thai AA, Dunn LA, Hughes BGM, Khushalani
NI, Modi B, Schadendorf D, Gao B, Seebach F, Li S, Li J, Mathias M, Booth J, Mohan K, Stankevich E, Babiker HM, Brana I, Gil-Martin M, Homsi J, Johnson ML, Moreno V, Niu J, Owonikoko TK, Papadopoulos KP, Yancopoulos GD, Lowy I, Fury MG. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N Engl J Med. 2018 Jul 26;379(4):341-351. doi: 10.1056/NEJMoa1805131. Epub 2018 Jun 4. PMID: 29863979
13. Baggi A, Quaglino P, Rubatto M, et al. Real world data of cemiplimab in locally advanced and metastatic cutaneous squamous cell carcinoma [published correction appears in Eur J Cancer. 2022 May;166:309-310]. Eur J Cancer. 2021;157:250-258. doi:10.1016/j.ejca.2021.08.018
14. Ferrarotto R, Amit M, Nagarajan P, et al. Pilot Phase II Trial of Neoadjuvant Immunotherapy in Locoregionally Advanced, Resectable Cutaneous Squamous Cell Carcinoma of the Head and Neck [published correction appears in Clin Cancer Res. 2022 Apr 14;28(8):1735]. Clin Cancer Res. 2021;27(16):4557-4565. doi:10.1158/1078-0432.CCR-210585
15. Paul E, Konidaris G, Cope S, Chen CI, Keeping S, Xu Y, Atsou K, Ayers D, Guyot P, Sasane M, Mojebi A, Kuznik A. Cost-effectiveness analysis of cemiplimab vs pembrolizumab for treatment of advanced cutaneous squamous cell carcinoma. J Manag Care Spec Pharm. 2021 Nov;27(11):1513-1525. doi: 10.18553/ jmcp.2021.21164. Epub 2021 Aug 5. PMID: 34351214.
16. Official Patient Website. LIBTAYO. https://www.libtayo. Enjoy the following articles from the winners! Enjoy the following articles from the winners!
Kevin Kovatch, MD, Glen D Souza, MD, Christopher Tseng, MD, Terral Patel, MD, Kevin Stavrides, MD

1st PLACE ORAL Does Hypoglossal Nerve Stimulation Surgery Improve Rhinologic Quality of Life?
Glen D Souza, MD; Alexander Duffy, MD; Andrew Corr, BS; Elina Toskala, MD, PhD, MBA; Mindy Rabinowitz, MD; Marc Rosen, MD; Gurston Nyquist, MD; Maurits Boon, MD; Colin Huntley MD
Thomas Jefferson University—Department of Otolaryngology Head and Neck Surgery, Philadelphia, PA, USA.
The prevalence of obstructive sleep apnea (OSA) has increased in the United States over the last few decades, affecting roughly 15-26% of the population [1-3]. This rise in OSAS has been linked to various health issues, including cardiovascular events, strokes, and metabolic syndrome while contributing to increased daytime sleepiness and risk of accidents [4-7] OSA is characterized by intermittent hypoxia and hypoxemia, which trigger a variety of responses in the body. While the cardiovascular and neurological effects of OSA are well-documented; experimental models have demonstrated that, intermittent hypoxia, a hallmark of OSAS, can reduce nasal ciliary beat frequency and increase inflammatory cytokines release into the
sinonasal mucosa [8,9]. Although the exact relationship between OSAS and chronic rhinosinusitis (CRS) is not fully understood, evidence suggests that patients with CRS are more likely to develop OSAS and possibly reduced sinonasal quality of life [10] Managing OSAS involves lifestyle modifications, continuous positive airway pressure (CPAP) therapy, and surgical procedures aimed at widening the airway. While CPAP therapy has been established as the gold standard for OSAS management and is known to enhance patients' quality of life, compliance has been an issue with continued CPAP usage.
Schwartz et al in 2001 discovered that stimulating the hypoglossal nerve could contract the genioglossus muscle which led to the discovery of Hypoglossal Nerve Stimulation (HGNS) devices. In 2014, the FDA approved the Inspire device, coinciding with the publication of results from the Stimulation Therapy for Apnea Reduction (STAR) trial [11], marking a significant milestone in OSAS treatment.
While multiple studies have demonstrated that Upper Airway Stimulation (UAS) surgery can significantly enhance the quality of life and sleep parameters in OSAS patients, the impact of HGNS on the sinonasal quality of life (QoL) has been largely unexplored [12,13] Hence the aim of the study was to assess if rhinologic quality of life changes following HGNS. Sinonasal quality of life (SNOT) -22, a questionnaire that covers both rhinologic/ nasal and sleep related (QoL) within a single instrument was used as an instrument to test the hypothesis. The SNOT-22 questionnaire comprises of 22 questions divided into four independently validated subdomains: rhinologic/nasal, sleep, otologic/facial, and behavioral. Each question is scored based on symptom severity, ranging from 0 (no problem) to 5 (severe problem) resulting in a total score between 0 and 110.
The methodology involved a retrospective chart review, approved by the ethics board of Thomas Jefferson University Hospital. Patients who had undergone UAS surgery with the Inspire device and had completed the SNOT22 questionnaire within a year before surgery
and were willing to retake it at least six months post-HGNS surgery were included.
Over 500 patients have undergone UAS surgery at our institute and 94 of them had completed the SNOT-22 questionnaire before surgery. Of these patients, 43 consented for the study and retook the SNOT-22 questionnaire. The majority of patients were men, with an average age of 62.18 years and an average BMI of 27.70. All patients had previously attempted CPAP, and a small subset of 4 patients also had a concurrent diagnosis of CRS.
Analysis of the SNOT-22 scores revealed a significant improvement in overall sinonasal quality of life after HGNS surgery. The mean SNOT-22 score reduced significantly from 32.27 to 26.54. The sleep subdomain, a significant portion of the questionnaire also exhibited substantial improvement following surgery.
The scores for the rhinologic subdomain also showed a notable reduction, emphasizing improved nasal function while the otologic/ facial subdomain displayed an insignificant change in scores.
Additional comparisons included Epworth Sleepiness Scores (ESS) and Apnea Hypopnea Index (AHI). ESS scores decreased post-surgery, albeit not significantly, while AHI showed a significant reduction.
OSAS significantly impacts various bodily systems, resulting in reduced quality of life and increased morbidity and mortality. The exact mechanisms behind these systemic effects are still under debate, but intermittent hypoxia (IH) and associated free radical injury, sympathetic excitation, and release of inflammatory mediators are among the leading theories. Intermittent hypoxia has been linked to craniofacial remodeling, turbinate hypertrophy, and increased nasal mucosal thickness. These changes bear similarities to the theories proposed for the development of chronic rhinosinusitis (CRS). Patients with OSAS have a notably higher risk of developing CRS, further
Congratulations to the 2023 PAO HNS Oral Abstract and Poster Abstract winners from the 2023 PAO HNS Annual Scientific Meeting!!
suggesting a potential connection. While proving causality between OSAS and CRS is beyond the score of the study, the findings are promising and indicate that HGNS enhances sinonasal quality of life along with sleep related quality of life. This improvement was observed even in patients without CRS, potentially attributable to better OSAS control following HGNS surgery, particularly in individuals who struggled with CPAP use.
Despite the findings, the study is limited by its small sample size and variations in SNOT-22 score administration intervals. Larger comparative studies are essential to gain deeper insights into the impact of OSAS and various treatment modalities on sinonasal outcomes.
In conclusion, untreated OSAS can have a significant adverse impact on nasal and rhinologic quality of life with a potential to result in craniofacial remodeling and CRS development over time. HGNS surgery has emerged as a valuable option, not only in improving sleep quality but also enhancing nasal and rhinologic quality of life. Future studies are necessary to explore the long-term effects of OSAS and UAS surgery on the nose and paranasal sinuses to provide an in-depth understanding of these interconnected health concerns.
References
1. Young, T., et al. (2009). Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. Wmj, 108(5), 246-249.
2. Peppard, P.E., et al. (2013). Increased Prevalence of Sleep-Disordered Breathing in Adults. American Journal of Epidemiology, 177(9), 1006-1014.
3. Benjafield, A.V., et al. (2019). Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. The Lancet Respiratory Medicine, 7(8), 687-698.
4. Yaggi, H.K., et al. (2005). Obstructive Sleep Apnea as a Risk Factor for Stroke and Death. New England Journal of Medicine, 353(19), 2034-2041.
5. Kareem, O., Tanvir, M., & Bader, G.N. (2022). Obstructive Sleep Apnea and Metabolic Syndrome. Sleep and Vigilance, 6(1), 85-99.
6. Jean-Louis, G., et al. (2008). Obstructive sleep apnea and cardiovascular disease: role of the metabolic syndrome and its components. J Clin Sleep Med, 4(3), 261-272.
7. Giampá, S.Q.C., Lorenzi-Filho, G., & Drager, L.F. (2023). Obstructive sleep apnea and metabolic syndrome. Obesity, 31(4), 900-911.
8. Tregear, S., et al. (2009). Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med, 5(6), 573-581.
9. Diamanti, C., et al. (2013). Depression, physical activity, energy consumption, and quality of life in OSA patients before and after CPAP treatment. Sleep and Breathing, 17(4), 1159-1168.
10. Weaver, T.E., & Grunstein, R.R. (2008). Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc, 5(2), 173-178.
11. Strollo, P.J., et al. (2014). Upper-Airway Stimulation for Obstructive Sleep Apnea. New England Journal of Medicine, 370(2), 139-149.
12. Huntley, C., et al. (2017). Upper Airway Stimulation for Treatment of Obstructive Sleep Apnea: An Evaluation and Comparison of Outcomes at Two Academic Centers. Journal of Clinical Sleep Medicine, 13(9), 1075-1079.
13. Huntley, C., et al. (2021). Comparison of Traditional Upper Airway Surgery and Upper Airway Stimulation for Obstructive Sleep Apnea. Ann Otol Rhinol Laryngol, 130(4), 370-376.
2ND PLACE ORAL Cost Effectiveness of Non-echo Planar Diffusion Weighted MRI in the Surveillance of Cholesteatoma
Terral Patel, MD, University of Pittsburgh Medical Center
Shaum Sridharan, MD Andrew McCall, MD
Cholesteatoma is a benign, locally destructive lesion comprised of keratin debris lined by differentiated squamous epithelium involving the middle ear and/or mastoid and is managed with surgical removal or exteriorization[1-3]. When cholesteatoma involves the mastoid, surgeons will commonly perform a tympanomastoidectomy surgery (in a canal wall up (CWU) or canal wall down fashion) to address the disease. However, residual disease rates for cholesteatoma can be high with rates
varying between 16-61% in the literature for CWU surgery[4]; such wide variance is multifactorial in nature and can be the result of factors such as specific surgical techniques utilized, including use of endoscopy, and patient factors such as surgical anatomy and disease burden. For many years, surgeons have advocated for planned second-look surgery after initial CWU surgery to reduce the risk of cholesteatoma regrowth from undetected residual disease[5]
The use of non-echo planar diffusion weighted MRI (non-EP DW MRI) has been recently described for the detection of residual cholesteatoma. There has been a growing body of evidence supporting its accuracy and precision allowing for the detection of occult cholesteatoma and has resulted in some advocating for its use as a replacement for planned second look tympanomastoidectomy[6]. Studies have shown that the size limit for recurrent cholesteatoma can be as small as 3-5mm [7]. However, this still allows for the presence of occult microscopic disease that remains undetected by the technique, resulting in a falsely negative study. This leads to a concern that utilization of imaging may delay the diagnosis of recurrent disease which could lead to increased morbidity or poorer outcomes. In contrast, non-EP DW MRI surveillance may reduce the cost and potential morbidity associated with planned second-look surgery, including that patients without residual cholesteatoma can avoid additional surgery altogether.
As healthcare reimbursement in the United States is trending toward delivery of valuebased care, there is a push to prioritize patient quality of life while minimizing costs[8-10] Although non-EP DW MRI may be an alternative option to second-look surgery, there is a paucity of data evaluating the costeffectiveness of this method. Cost-effectiveness analyses are critical tools that can inform the adoption of standardized healthcare guidelines. We recently performed a costeffectiveness analysis comparing planned second-look surgery versus non-EP DW MRI
for management of cholesteatoma following initial CWU tympanomastoidectomy surgery for cholesteatoma. At the 2023 PAO-HNS Annual Scientific Meeting we presented our data where we found both options to be costeffective solutions for addressing this clinical scenario. Based on our model, we concluded that treatment decisions in this clinical scenario should be individualized taking into account such factors as probability of residual disease, hearing status, and patient wishes. Further studies evaluating the cost-effectiveness of non-EP DW MRI in the detection of residual cholesteatoma are warranted, particularly if prospective series can be performed to enhance the underlying data these models rely on, to help otolaryngologists advocate for appropriate guidelines that enhance efficient resource utilization and maximize the value of medical interventions for the patients we serve.
References.
1. Rutkowska, J., N. Özgirgin, and E. Olszewska, Cholesteatoma definition and classification: a literature review. The journal of international advanced otology, 2017. 13(2): p. 266.
2. Kuo, C.-L., W.-H. Liao, and A.-S. Shiao, A review of current progress in acquired cholesteatoma management. European Archives of Oto-Rhino-Laryngology, 2015. 272(12): p. 3601-3609.
3. Stangerup, S.E., et al., Recurrence of attic cholesteatoma: different methods of estimating recurrence rates. Otolaryngol Head Neck Surg, 2000. 123(3): p. 283-7.
4. Kerckhoffs, K.G., et al., The disease recurrence rate after the canal wall up or canal wall down technique in adults. The Laryngoscope, 2016. 126(4): p. 980-987.
5. Tomlin, J., et al., Surgical technique and recurrence in cholesteatoma: a meta-analysis. Audiology and Neurotology, 2013. 18(3): p. 135-142.
6. Dhepnorrarat, R.C., B. Wood, and G.P. Rajan, Postoperative non-echo-planar diffusion-weighted magnetic resonance imaging changes after cholesteatoma surgery: implications for cholesteatoma screening. Otol Neurotol, 2009. 30(1): p. 54-8.
7. De Foer, B., et al., Detection of postoperative residual cholesteatoma with non-echo-planar diffusion-weighted magnetic resonance imaging. Otology & Neurotology, 2008. 29(4): p. 513-517.
8. VanLare, J.M., J.D. Blum, and P.H. Conway, Linking performance with payment: implementing the physician value-based payment modifier. Jama, 2012. 308(20): p. 2089-2090.
9. Johnston, K.J., J.M. Hockenberry, and K.E.J. Maddox, Building a better clinician value-based payment program in Medicare. Jama, 2021. 325(2): p. 129-130.
10 Lynn, J., A. McKethan, and A.K. Jha, Value-based payments require valuing what matters to patients. Jama, 2015. 314(14): p. 1445-1446.
1st PLACE POSTER
The Use of Actigraphy to Assess Sleep Improvement After Parathyroidectomy
Christopher C. Tseng, MD, Julio Fernandez-Mendoza, PhD and David Goldenberg, MD, FACS Pennsylvania State University College of Medicine

Most patients with primary hyperparathyroidism present with incidental findings of mild hypercalcemia. Although these patients are generally classified as asymptomatic, many report neurocognitive difficulties, including chronic fatigue, malaise, memory impairment, emotional lability, lack of focus, and insomnia1. Sleep disturbances are
relatively common, occurring in up to 69% of these patients by subjective measurements2. Despite evidence of underdiagnosed and often subtle neuropsychological symptoms associated with sleep impairment in primary hyperparathyroidism patients, there are no universally accepted recommendations for specifically managing these issues3,4. Current NIH indications for parathyroidectomy for asymptomatic primary hyperparathyroidism include age younger than 50 and lab findings such as serum calcium >1.0 mg/ dL above the upper limit of normal and 24hour urinary calcium >400mg/dL3. While parathyroidectomy has been shown to improve neurocognitive deficits post-operatively, the mechanism for neurocognitive impairment, insomnia, and primary hyperparathyroidism has yet to be understood, indicating the need for further research4-6. As a result, demonstrating both objective and subjective deficits in sleep will help further delineate the need to manage this symptomatology6. Actigraphy has been validated as a cost-effective and noninvasive approach for circadian rhythm analysis in the patient's natural sleep environment7,8. Towards this end, our study applied actigraphy to objectively quantify changes in sleep following parathyroidectomy and improve our understanding of the clinical significance of sleep pathology in primary hyperparathyroidism patients.
We performed a longitudinal prospective study to investigate the sleep habits of primary hyperparathyroidism patients at three separate time points: pre-parathyroidectomy (pre-op), one-week post-parathyroidectomy (post-op), and three months post-parathyroidectomy (follow-up) (Figure 1).

Congratulations to the 2023 PAO HNS Oral Abstract and Poster Abstract winners from the 2023 PAO HNS Annual Scientific Meeting!!
Congratulations to the 2023 PAO HNS Oral Abstract and Poster Abstract winners from the 2023 PAO HNS Annual Scientific Meeting!!
Continued from page 7
Patients wore an Actigraph sleep monitor continuously for one week at each time point and recorded a sleep log. At the end of the week, patients completed three surveys: Pittsburgh Sleep Quality Index (PSQI), Depression Anxiety Stress Scales (DASS), and Insomnia Severity Index (ISI). Collected sleep pattern measures for each patient were then statistically evaluated using a repeated measures model as separate time points and trends over time. Throughout this study, adult primary hyperparathyroidism patients aged 18-89 years old, scheduled to undergo a parathyroidectomy at Penn State Hershey Medical Center were enrolled. Notable exclusion criteria included pregnant patients or patients who became pregnant during the study period, patients on chronic narcotic or sleep aid medication, and patients with known sleep disorders such as sleep apnea. Baseline characteristics of the 22 patients
in our current cohort with complete data showed 85% were female, with an average age of 60.7 years old, 91% were poor sleepers with PSQI score ≥ 5, and 56% had clinically significant insomnia with ISI score ≥ 10. These patients ' average actigraphy metrics and survey scores by time point were analyzed. Our results generally show improvement in actigraphy metrics (Figure 2) and survey scores (Figure 3) throughout the study when assessing change in post-op and follow-up measurements compared to baseline pre-op measurements. Statistical analysis reveals a significant decrease across the time points concerning average minutes in bed and total sleep time, evaluated as separate time points (p=0.017 and p=0.044, respectively). The test for trends also showed a significant negative trend over time (p=0.005 and p=0.012, respectively). Additionally, there was a significant decrease

among the time points concerning the average ISI score (p=0.004), which also resulted in a significant negative test for trend over time (p=0.015). In conclusion, average time in bed, total sleep time, and ISI score were significantly lower post-operatively compared to pre-operatively. These findings objectively point to more efficient sleep while in bed and clinically significant improvement of insomnia after parathyroidectomy. This study is the first of its kind to demonstrate objective sleep improvement using pre- and post-operative actigraphy for primary hyperparathyroidism patients following parathyroidectomy.
References
1. Silverberg SJ, Lewiecki EM, Mosekilde L, Peacock M, Rubin MR. Presentation of asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. J Clin Endocrinol Metab. 2009;94(2):351-365.
2. La J, Wang TS, Hammad AY, Burgardt L, Doffek K, Carr AA, Shaker JL, Carroll TB, Evans DB, Yen TW. Parathyroidectomy for primary hyperparathyroidism improves sleep quality: A prospective study. Surgery. 2017;161(1):25-34.
3. Eigelberger MS, Cheah WK, Ituarte PHG, Streja L, Duh Q-Y, Clark OH. The NIH criteria for parathyroidectomy in asymptomatic primary hyperparathyroidism: are they too limited? Ann Surg. 2004;239(4):528-535.
4. McDow AD, Sippel RS. Should symptoms be considered an indication for parathyroidectomy in primary hyperparathyroidism? Clin Med Insights Endocrinol Diabetes. 2018;11:1179551418785135.
5. Shah-Becker S, Derr J, Oberman BS, Baker A, Saunders B, Carr MM, Goldenberg D. Early neurocognitive improvements following parathyroidectomy for primary hyperparathyroidism. Laryngoscope. 2018;128(3):775780.

6. Bilezikian JP, Brandi ML, Eastell R, Silverberg SJ, Udelsman R, Marcocci C, Potts JT Jr. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the fourth international workshop. J Clin Endocrinol Metab. 2014;99(10):3561-3569.
7. Marino M, Li Y, Rueschman MN, Winkelman JW, Ellenbogen JM, Solet JM, Dulin H, Berkman LF, Buxton OM. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36(11):1747-1755.
8. Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389-396.
Congratulations to the 2023 PAO HNS Oral Abstract and Poster Abstract winners from the 2023 PAO HNS Annual Scientific Meeting!!
2nd PLACE POSTER
To Glue or Not to Glue: Do Fibrin Sealants Reduce Postoperative Complications in Facial Plastic Surgery?
Hanel W. Eberly BS; Bao Y. Sciscent BS; Jessyka G. Lighthall MD, FACSFibrin sealants have shown to promote hemostasis, aid in free flap or graft neovascularization, decrease seroma formation, and reduce dead space.1 This is achieved largely due to the unique ability of a fibrin sealant to thoroughly coat and adhere the entire surface of the graft to the wound.2 Fibrin sealants are made up of human fibrinogen, thrombin, and bovine aprotinin, which are mixed together to stimulate clot formation and coagulation.3 Recent studies have shown that fibrin sealants have added benefits such as reducing the need for dressing changes or drains, increasing patient satisfaction and convenience, and reducing pain.4–6
The first reports of fibrin sealant use in facial plastic surgery date back to 1988, when they were used for various otolaryngologic and facial plastic surgeries. 7 Reports of its use comment on its utility in minimizing venous bleeding, oozing, drain output, and even capillary arterial bleeding.3,7 However, it is relatively unknown whether fibrin sealants are effective in reducing postoperative complications such as hematoma, seroma, or wound infection. While some studies have found that fibrin sealant usage reduces these complications,8,9 others have not found significant differences between patients treated with fibrin sealants vs. those treated without.10,11 Furthermore, much of the existing research has focused on the use of fibrin sealants in rhytidectomy, with little data on other procedures such as rhinoplasty or regional flaps.
To investigate the effect of using a fibrin sealant during surgery on complication rates and morbidity, we utilized the TriNetX national database to identify patients that underwent facial plastics procedures, including rhinoplasty, rhytidectomy, regional flaps, and grafts/
implants. Two cohorts were created. One cohort contained patients with a fibrin sealant HCPCS code (C9250) recorded in their chart on the same day as the procedure, and the other cohort included patients without any documentation of fibrin sealant use. Demographic and comorbidities, including diabetes mellitus, anticoagulant use, bleeding and clotting disorders, as well as smoking and alcohol use, were collected for the cohorts. Postoperative complications were investigated including hemorrhage, wound infection, pain, seroma, paresthesia, and wound disruption, identified via ICD-10 codes. Statistical analyses included with Chi square tests for categorical measures, and t-tests for continuous measures.
A preliminary analysis showed that overall, there was no difference in the odds of postoperative complications between groups (OR 1.28 [95% CI 0.86-1.89], p=0.2254). Rates of comorbidities (0.1% vs 0.5%, p=0.0120), and anticoagulant usage (27.7% vs 48.7%, p<0.001) were higher in the fibrin sealant group. A sub analysis of postoperative hemorrhage and infection by procedure found no differences in complications between groups for free flaps (2.3% vs 5.9%; 4.0% vs. 5.9%, p=0.5912), rhinoplasty (0.9% vs. 0.5%; 0.6% vs. 0.5%, p=0.7558), and rhytidectomy (0.8% vs 0.0%; 0.6% vs 1.0%, p=0.5640). However, patients in the fibrin sealant group receiving regional flaps (1.3% vs. 2.7%; 0.8% vs 3.6%, p=0.002) and grafts (0.8% vs. 2.2%; 0.6% vs. 1.7%, p<0.001) had more complications.
Our results showed that there were no differences in the complication rates between groups using fibrin sealants compared to those not using fibrin sealants, but that rates of comorbidities and anticoagulant usage were significantly higher in groups using fibrin sealants. Furthermore, patients in the fibrin sealant group who also underwent regional flap and graft procedures had higher rates of complications and comorbidities. This leads us to believe that the higher rates of complications and comorbidities could
have been contributing to the increased complications seen in this group.
Anticoagulant and aspirin use have been shown to increase risk of adverse events in patients undergoing facial plastic surgery.12,13 Many clinicians choose to discontinue antiplatelet and/or anticoagulant therapy prior to surgery, especially if the procedure involves highly vascular areas.12 However, for some patients discontinuation of these drugs may not be feasible due to preexisting comorbidities. Our preliminary results showed that the fibrin sealant group had higher rates of comorbidities including anticoagulant and antiplatelet drug use, which may have contributed to the clinicians’ decision to use sealants instead of or as an adjunct to conventional methods used for hemostasis. Furthermore, we saw increased rates of complications for patients in the fibrin sealant group who underwent regional flap and graft procedures but also elevated rates of comorbidities, suggesting a similar conclusion.
Limitations of our study include its retrospective nature, as we were unable to assess the quality of the data collected, and analysis relied on information already available in patient records. However, our study encompassed multiple institutions, thereby improving the generalizability of our results as several of the procedures studied are not commonly performed. In addition, patient-level data on specific outcomes such as disease severity and adjunctive treatments were not available due to the nature of the database. However, the researchers posit that such data would have had minimal impact on the outcome of this study.
Although fibrin sealants have been in use for decades, there remain barriers to use including the increased cost, concern for disease transmission or allergic reaction in animal or human donor-derived sources, and relative unfamiliarity clinicians may have with the technology.10,14 However, several studies examining fibrin sealant use various procedures have demonstrated that the use of fibrin sealants may contribute to decreased length of stay14,15 (and operative time10,16 despite its increased cost compared to conventional sutures, dressings, and drains. Data on facial
Congratulations to the 2023 PAO HNS Oral Abstract and Poster Abstract winners from the 2023 PAO HNS Annual Scientific Meeting!!

Continued from page 9
plastic surgery is limited, although similar data has been published regarding rhytidectomy.10 Furthermore, no cases of viral transmission of disease such as HIV or hepatitis due to fibrin sealant use have been reported in the literature.10 Fibrin sealants hold much potential for surgeons to improve patient outcomes and decrease overall cost and further studies should be conducted to examine the utility of this treatment compared to traditional methods of promoting hemostasis or tissue adherence.
References
1. Toriumi DM, Chung VK, Cappelle QM. Surgical Adhesives in Facial Plastic Surgery. Otolaryngol Clin North Am. 2016;49(3):585-599. doi:10.1016/j. otc.2016.02.012
2. Paw E, Vangaveti V, Zonta M, Heal C, Gunnarsson R. Effectiveness of fibrin glue in skin graft survival: A systematic review and meta-analysis. Ann Med Surg (Lond). 2020;56:48-55. doi:10.1016/j. amsu.2020.06.006
3. Hamilton MM, Chan D. Adjunctive procedures to neck rejuvenation. Facial Plast Surg Clin North Am. 2014;22(2):231-242. doi:10.1016/j.fsc.2014.01.008
4. Marchac D, Sándor G. Face lifts and sprayed fibrin glue: an outcome analysis of 200 patients. Br J Plast Surg. 1994;47(5):306-309. doi:10.1016/00071226(94)90087-6
5. Saltz R, Sierra D, Feldman D, Saltz MB, Dimick A, Vasconez LO. Experimental and clinical applications of fibrin glue. Plast Reconstr Surg. 1991;88(6):1005-1015; discussion 1016-1017.
6. Janik S, Hirtler L, Traxler H, Weninger WJ, Seemann R, Erovic BM. The vascularized fascia lata free flap: an anatomical study and clinical considerations. Eur Arch Otorhinolaryngol. 2020;277(6):1733-1739. doi:10.1007/s00405-020-05861-8
7. Ellis DA, Shaikh A. The ideal tissue adhesive in facial plastic and reconstructive surgery. J Otolaryngol. 1990;19(1):68-72.
8. Kamer FM, Nguyen DB. Experience with fibrin glue in rhytidectomy. Plast Reconstr Surg. 2007;120(4):10451051. doi:10.1097/01.prs.0000278092.28351.c9
9. Zoumalan R, Rizk SS. Hematoma rates in drainless deep-plane face-lift surgery with and without the use of fibrin glue. Arch Facial Plast Surg. 2008;10(2):103-107. doi:10.1001/archfaci.10.2.103
10. Fezza JP, Cartwright M, Mack W, Flaharty P. The use of aerosolized fibrin glue in face-lift surgery. Plast Reconstr Surg. 2002;110(2):658-664; discussion 665-666. doi:10.1097/00006534-200208000-00044
11. Lee S, Pham AM, Pryor SG, Tollefson T, Sykes JM. Efficacy of Crosseal fibrin sealant (human) in rhytidectomy. Arch Facial Plast Surg. 2009;11(1):29-33. doi:10.1001/archfacial.2008.511
12. Kraft CT, Bellile E, Baker SR, Kim JC, Moyer JS. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015;17(2):103-107. doi:10.1001/jamafacial.2014.1147
13. Stewart CM, Bassiri-Tehrani B, Jones HE, Nahai F. Evidence of Hematoma Prevention After Facelift. Aesthet Surg J. Published online August 5, 2023:sjad247. doi:10.1093/asj/sjad247
14. Butts CC, Sahawneh J, Duffy A, et al. Cost-benefit analysis of outcomes from the use of fibrin sealant for fixation of skin grafts in small-size burns compared to staples as historical controls: a retrospective review. Ann Plast Surg. 2015;74(2):173-175. doi:10.1097/ SAP.0000000000000397
15. Cunniffe HA, Wong BLK, Hilger AW, Burgan OT. Drainfree parotidectomy: a pilot study using ARTISS fibrin sealant. Eur Arch Otorhinolaryngol. 2019;276(7):20252029. doi:10.1007/s00405-019-05449-x
16. Bouhout S, Kam J, Robert MC, Harissi-Dagher M. Cost-effectiveness analysis : fibrin glue versus sutures for conjonctival fixation during pterygion surgery. Can J Ophthalmol. 2022;57(1):41-46. doi:10.1016/j. jcjo.2021.02.016
Board of Governors update: The Board of Governors of the American Academy of Otolaryngology Head and Neck Surgery had its General Assembly meeting on September 30th, 2023, at the Nashville Convention Center prior to the opening of the American Academy of Otolaryngology Annual Meeting.
BOG leaders were elected for the chair elect and secretary positions. An update was given on the reorganization of the regional representative program and keynote speaker Mr. John Carr, Director of Administration for the Tennessee Health Facilities Commission, was enjoyed by all. Mr. Carr discussed the importance of otolaryngology in state advocacy and how specialty societies can help influence policy and regulation at the state level through interfacing with the legislature as well as state medical society.
The Board of Governor’s awards for the model society went to the Georgia Society of Otolaryngology Head and Neck Surgery. The BOG Practitioner Excellence Award was presented to Dr. David Edelstein from New York. A state panel was convened that explored ways the Board of Governors can provide opportunities to link otolaryngologists across Tennessee to the BOG with stressing the importance
of communication and sharing of information between the AAO and state specialty societies. The meeting ended with Dr. Stephen Kmucha MD JD, an otolaryngologist from California, assuming the new chair position with the passing of the gavel from Dr. Karen Rizzo to Dr. Kmucha.
Legislative advocacy highlights include forward movement on the prior authorization battle. United Healthcare announced it would eliminate the prior authorization requirement for a number of procedure codes utilized by our specialty. Analysis suggests that this will count for approximately 20% of the prior authorization volume. Regarding audiology and scope of practice: Senator Warren from Massachusetts has again proposed legislation that would inappropriately expand the scope of practice for audiology and reclassify them as Medicare providers. This legislation was introduced in prior sessions and again there is movement to undermine physicians as the hearing health experts. The BOG encourages physicians to contact their federal representatives asking them to oppose Senate Bill 2377. Concern over the ability to prescribe hearing aids is noted in recent Illinois legislation house bill 2443 which allows audiologists and other hearing aid dispensers to prescribe and fit
hearing aids without an exam or input from an otolaryngologist or any other physician. This is of significant concern. The importance of donating to the ENT PAC cannot be overstated. These monies are utilized to combat challenges and issues facing our specialty at the congressional level. Please consider donating towards these important causes. The response to a federal court ruling from lawsuits brought by the Texas Medical Association against the Centers for Medicare and Medicaid Services has suspended the independent abuse resolution process of the No Surprises Act effective August 24th. CMS has stated it will temporarily suspend all federal IDR process operations in order to make changes necessary to comply with the recent court’s opinion and order.
The BOG encourages grassroot members to stay informed and reach out to the BOG and their State specialty society when concerns occur regarding regulatory or legislative challenges as well as insurance issues.


Multilayered Flap Closure of Persistent Tracheocutaneous Fistulae
Persistent tracheocutaneous fistula (TCF) can occur in up to 3% of patients after tracheostomy. Common reasons patients may develop this complication include retained sutures, chronic infection, history of radiation to the head and neck, or underlying systemic illnesses that prevents healing. The risk also positively correlates with the duration of the tracheostomy. Although not immediately life threatening, a persistent TCF delays decannulation, worsens respiratory function, and puts the patient at greater risk of respiratory infection.
Surgical closure is the primary treatment for persistent TCFs. Many techniques have been described which include suture ligation, fistulectomy, use of a regional flap, cartilage grafts,

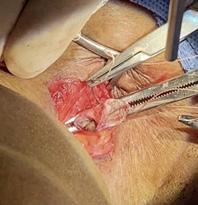

mucosal grafts, and even alloplasts. At Temple University Hospital, we have vast experience with repair of tracheoesophageal fistulae over many years and have developed an effective, reliable technique for TCF closure even in challenged wound healing scenarios. Our technique starts by making a horizontal elliptical incision around the TCF. The fistula tract is dissected down to the trachea with care not to perforate the tract. Once this is completed, the inner surface is gently de epithelialized, clamped with a curved hemostat or tonsil forceps just a few millimeters from the trachea to allow room for suturing. The tract is then closed using a 2-layer, running horizontal mattress 3-0 Vicryl suture.
The strap muscles are then freed bilaterally, medially advanced over the closure, and sutured together in the midline using

interrupted 3-0 Vicryl sutures. A sterile rubber band drain is placed deep to this layer to prevent the rare but potential complication of pneumothorax or pneumomediastinum in instances when air leaks from an incompletely ligated TCF. The rubber band drain is brought out of the wound in the midline and is removed by the patient the following day. Finally, the skin is closed in two layers starting with a deep layer of interrupted 3-0 Vicryl and a running subcuticular 4-0 Monocryl. Occlusive dressings are generally used and a post-op chest X-Ray is crucial to rule out any pneumothorax or pneumomediastinum.
Our technique is safe and effective over many years with no serious complication even in immunocompromised patients. It has held up to positive airway pressure ventilation even on the first postoperative day.
References:
1. Drezner DA, Cantrell H. Surgical management of tracheocutaneous fistula. Ear Nose Throat J. (1998) 77:534–7.
2. Bryant, Justin R et al. “Tracheocutaneous Fistula Closure with Turnover Flap and Polydioxanone Plate.” Plastic and reconstructive surgery. Global open vol. 5,10 e1515. 10 Oct. 2017, doi:10.1097/GOX.0000000000001515
3. Hauff SJ, Brisebois S, Moss W, Merati AL, Weissbrod PA. Suture-ligature technique for the closure of tracheocutaneous fistula in adults. Laryngoscope. 2019 Mar;129(3):574-577. doi: 10.1002/lary.27448. Epub 2018 Nov 9.
4. Wheller WB, Kurachek SC, Lobas JG, Lipscomb TS. (1991). Respiratory complications of tracheocutaneous fistula closure. Critical Care Medicine, 19 (4), 580-582.
Garrett Ni, MD and Ahmed M.S. Soliman, MDInnovation in OSA Treatment and the Role of the Otolaryngologist
W. Jack Palmer, MD Maurits Boon, MD Colin Huntley, MD“’Sleep!’ said the old gentleman, ‘he’s always asleep. Goes on errands fast asleep, and snores as he waits at table.’”1,2 Reading Charles Dickens’ description of Joe, “the Fat Boy,” a supporting character in his 1836 novel The Posthumous Papers of the Pickwick Club, conjures images of friends, family, and patients who we now understand suffer from obstructive sleep apnea (OSA).2,3 Despite Dickens’ striking description, formal treatments for Joe’s condition weren’t documented in the medical literature until the mid-20th century. Soft palate interventions for snoring were suggested in the 1950s, while the concept of OSA began to emerge in the 1970s, with tracheostomy the definitive treatment.3-6 Although offering patients expeditious and significant symptomatic relief, tracheostomy also brought with it medical and psychosocial complications.3,7 Physicians were challenged to innovate to improve their patients’ outcomes: in 1981, Fujita et al. described uvulopalatophatyngoplasty (UPPP) for OSA, and Sullivan et al. introduced continuous positive airway pressure (CPAP).8,9
Innovation has continued from the foundation laid by these landmark articles. Physicians treating OSA now have a host of surgical and non-surgical options for their patients. Improvements in PAP hardware, including better-fitting masks, heating, and humidification, and PAP delivery technology, including bilevel pressure and related derivatives, allow individualization of treatment and have been shown to improve patient compliance with therapy.10,11 Variations of Fujita’s classical UPPP, including lateral pharyngoplasty, expansion pharyngoplasty, and barbed suture pharyngoplasty, have added to the surgeon’s armamentarium with noted benefit for patients.12-14 Surgical options have expanded to include interventions at other levels of the upper airway; these
techniques can be used in isolation or as part of a multi-level treatment approach. Tongue base obstruction, a frequent driver of OSA particularly in cases refractory to palatal surgery, for instance, has been addressed with robotic or coblation tongue base resection, hyoid suspension, and maxillomandibular advancement.3,15,16 Despite these exciting advances, CPAP efficacy is still limited by imperfect patient adherence, and short of tracheostomy, no single surgical intervention has demonstrated uniform success in patients with OSA.3,17 The need for innovation persists.
Over the past decade, upper-airway stimulation (UAS) has emerged as a valuable treatment option. The pioneering device in this field, produced by Inspire Medical Systems, was introduced through the landmark stimulation therapy for apnea reduction (STAR) trial, published in 2014.17 By promoting tongue protrusion during inspiratory effort, this device effectively improved subjective and objective measures of OSA severity in a population with moderate-to-severe OSA.17 Encouragingly, improvements were noted to be durable at least to the five year mark during a follow-up study published in 2018 by the STAR group.18 While initially receiving FDA approval for use in moderate-to-severe OSA patients with apnea-hypopnea index up to 65, BMI up to 32, and no concentric velopharyngeal collapse on drug-induced sleep endoscopy, inclusion criteria were recently broadened to include AHIs up to 100 and BMIs up to 40.19,20
As our understanding of the Inspire device and the population that it can benefit grows, two additional UAS devices are awaiting FDA approval. The Nyxoah Genio differs from the Inspire by requiring only a single submental incision, limiting implanted material by forgoing sensor leads and operating through an external battery, and stimulating bilateral hypoglossal nerves.21 This device’s initial study, abbreviated the BLAST OSA study, demonstrated significant improvement in OSA severity, daytime sleepiness, and quality of life, similar to data for other
UAS devices, without any device-related complications.21 The aura6000 from LivaNova shares similarities with both the Inspire and Genio devices but focuses on targeting the proximal aspect of the hypoglossal nerve which may offer a less time-consuming surgical experience.22 The THN3 trial utilizing the aura6000 device was recently published and demonstrated significant improvement in objective and patient-reported measures of OSA severity between treated patients and controls.22 When considering the upper airway as it relates to OSA, it is important to not overlook the nasal passages. Although isolated nasal surgery, including septoplasty, nasal valve repair, and turbinate reduction, has not been shown to significantly reduce AHI in patients with OSA, it has been shown to significantly improve CPAP compliance in OSA patients with nasal obstruction.23,24 Nasal surgery remains an important tool in the multimodal treatment of OSA.
The field of sleep medicine has progressed significantly since the days when tracheostomy was the most viable solution to burdensome OSA. Innovations in PAP delivery, surgical techniques, and implantable devices have improved patient outcomes with reduced morbidity. Physicians now have multiple treatment options, allowing personalization of treatment plans. Despite these developments, no treatment algorithm exists that encapsulates all patients suffering from OSA; PAP compliance remains imperfect, disease can be refractory to surgical intervention, and certain patient populations may not qualify for UAS implantation. Innovation must continue in order to help these individuals.
Otolaryngologists are uniquely poised to answer this call. With an extensive
Continued on page 14
Innovation in OSA Treatment and the Role of the Otolaryngologist
Continued from page 13
medical and surgical background, Otolaryngologists will be able to optimize multimodal approaches with existing treatment options. And with a deep understanding of all levels of the upper airway, Otolaryngologists can lead the charge to develop novel treatments.
References:
1. Dickens C, Aldin CCW. The Posthumous Papers of the Pickwick Club, Volume 1 (of 2) (Illustrations). E. P. DUTTON & COMPANY, 2015.
2. Groopman J. The Secrets of Sleep The New Yorker, 2017.
3. Yaremchuk K, Garcia-Rodriguez L. The history of sleep surgery SleepRelated Breathing Disorders: Karger Publishers, 2017:17-21.
4. Simmons FB, Hill MW. Hypersomnia Caused by Upper Airway Obstructions; A New Syndrome in Otolaryngology. Annals of Otology, Rhinology & Laryngology 1974; 83:670-673.
5. Fee Jr WE, Ward PH. Permanent tracheostomy: a new surgical technique. Annals of Otology, Rhinology & Laryngology 1977; 86:635-638.
6. Weitzman ED, Pollack CP, Borowiecki B. Hypersomnia-sleep apnea due to micrognathia: reversal by tracheoplasty. Archives of Neurology 1978; 35:392-395.
7. Conway WA, Victor LD, Magilligan DJ, Fujita S, Zorick FJ, Roth T. Adverse effects of tracheostomy for sleep apnea. Jama 1981; 246:347-350.
8. Fujita S, Conway W, Zorick F, Roth T. Surgical correction of anatomic abnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngology—head and neck surgery 1981; 89:923-934.
9. Sullivan C, Berthon-Jones M, Issa F, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. The Lancet 1981; 317:862-865.
10. Ballard RD, Gay PC, Strollo PJ. Interventions to improve compliance in sleep apnea patients previously non-compliant with continuous positive airway pressure. J Clin Sleep Med 2007; 3:706-712.
11. Blau A, Minx M, Peter JG, Glos M, Penzel T, Baumann G, Fietze I. Auto bilevel pressure relief–PAP is as effective as CPAP in OSA patients—a pilot study. Sleep and Breathing 2012; 16:773-779.
12. Vicini C, Hendawy E, Campanini Aet al. Barbed reposition pharyngoplasty (BRP) for OSAHS: a feasibility, safety, efficacy and teachability pilot study.“We are on the giant’s shoulders”. European Archives of Oto-Rhino-Laryngology 2015; 272:3065-3070.
13. Pang EB, Pang KP, Cheong RCet al. Expansion sphincter pharyngoplasty in OSA: a 15 year review. European Archives of Oto-Rhino-Laryngology 2023:1-8.
14. Cahali MB. Lateral pharyngoplasty: a new treatment for obstructive sleep apnea hypopnea syndrome. The Laryngoscope 2003; 113:1961-1968.
15. Riley RW, Powell NB, Guilleminault C. Obstructive sleep apnea syndrome: a surgical protocol for dynamic upper airway reconstruction. Journal of oral and maxillofacial surgery 1993; 51:742-747.
16. Babademez MA, Gul F, Sancak M, Kale H. Prospective randomized comparison of tongue base resection techniques: robotic vs coblation. Clinical Otolaryngology 2019; 44:989-996.
17. Strollo Jr PJ, Soose RJ, Maurer JTet al. Upper-airway stimulation for obstructive sleep apnea. New England Journal of Medicine 2014; 370:139-149.
18. Woodson BT, Strohl KP, Soose RJet al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngology–Head and Neck Surgery 2018; 159:194-202.
19. Strohl MP, Yamauchi M, Peng Z, Strohl KP. Insights since FDA approval of hypoglossal nerve stimulation for the treatment of obstructive sleep apnea. Current sleep medicine reports 2017; 3:133-141.
20. Inspire Medical Systems, Inc. Announces FDA Approval for Apnea Hypopnea Index Indication Expansion and Increased Body Mass Index Labeling. June 9, 2023. Available at: https://investors.inspiresleep. com/investors/press-releases/ press-release-details/2023/ Inspire-Medical-Systems-Inc.Announces-FDA-Approval-forApnea-Hypopnea-Index-IndicationExpansion-and-Increased-Body-MassIndex-Labeling/default.aspx. Accessed August 28 2023.
21. Eastwood PR, Barnes M, MacKay SGet al. Bilateral hypoglossal nerve stimulation for treatment of adult obstructive sleep apnoea. European Respiratory Journal 2020; 55.
22. Schwartz AR, Jacobowitz O, Eisele DWet al. Targeted hypoglossal nerve stimulation for patients with obstructive sleep apnea: a randomized clinical trial. JAMA Otolaryngology–Head & Neck Surgery 2023.
23. Ishii L, Roxbury C, Godoy A, Ishman S, Ishii M. Does nasal surgery improve OSA in patients with nasal obstruction and OSA? A meta-analysis. Otolaryngology–Head and Neck Surgery 2015; 153:326-333.
24. Poirier J, George C, Rotenberg B. The effect of nasal surgery on nasal continuous positive airway pressure compliance. The Laryngoscope 2014; 124:317-319.
Unmasking Potential: Harnessing AI for Analysis of Facial Paralysis Outcomes
Hanel W. Eberly, BS, Bao Y. Sciscent, BS, Jessyka G. Lighthall, MD, FACSSocial interactions such as recognition and processing of emotion, effective nonverbal communication, and execution of actions such as eating, drinking, and speaking are all controlled by facial movements. The inability to control the facial muscles on one or both sides of the face is known as facial paralysis and a reduction in strength of facial nerve musculature is known as facial palsy. Both conditions can be caused by infections, neoplasms, Bell’s palsy, congenital defects, traumas, or other injuries.1,2 In addition to impaired functional outcomes, facial palsy can contribute to or worsen depression, anxiety, and body dysmorphia.1,3,4 Treatments include direct repair of the injured nerve via nerve grafts or transfers, muscle or free tissue transfers, static techniques such as fascia lata sling placement or upper eyelid weight placement, botulinum toxin injections, and facial physical therapy.5 Management is difficult and continued follow up is warranted due to the high complexity and variety of deficits.
Multiple grading systems for assessing severity and treatment progression of facial palsy are available. However, these scales rely on subjective assessment of patient outcomes that may vary among clinicians. While some scales have been extensively validated and studied, many have not been thoroughly validated.6 Commonly used grading scales such as the HouseBrackmann scale7 or Sunnybrook Facial Grading System8 ask clinicians to grade parameters such as the level of impairment, symmetry of voluntary movement, and the degree of involuntary muscle contraction associated with various facial expressions. While subjective analysis may have benefits such as ease of use and accessibility, an accurate universal and objective grading system to standardize assessment is important to advance care and clinical outcomes in these patients.
Several attempts have been made to apply machine learning algorithms to grading facial palsy. Dusseldorp et al. used Emotrics, a free available facial tracking application, to record facial landmarks in video clips and generate a plot of healthy and affected side values to be analyzed over time. Their group then used Affdex, another computer vision artificial intelligence software, to generate percentage probabilities of perceived emotions during smiling.9 Guarin et al. described the use of a previously proposed algorithm for providing accurate facial landmark localization to train the program on a smaller number of images using the Massachusetts Eye and Ear Facial Nerve Data Repository, a digital collection of patient photographs and video clips used to evaluate unilateral facial palsy.10 Studies have shown that using machine learning algorithms can be useful and accurate tools for evaluating outcomes following treatment, and have highlighted the reliability, time- and cost-effectiveness, and ease of use when implementing these programs.11–16 One study reported a 100% accuracy rate using an automated grading system for facial palsy, and demonstrated its compatibility with mobile-based applications.11 Such examples illustrate how these programs may provide a reliable and accurate method of grading facial palsy while improving accessibility for patients and cost-effectiveness for clinicians.
The primary constraints for assessing facial paralysis using objective measures include the need for specialized equipment such as cameras or software, and the time demands they impose. Some clinics are able to provide a dedicated photography/videography room, a staff photographer, standardized backgrounds, and specialized equipment to ensure a high degree of uniformity in their patient photographs. However, as this is not always feasible in busy clinics with limited resources, efforts have been made to develop more accessible methods of using artificial intelligence to grade facial paralysis. Mobile applications have been developed where patients can record their own facial expressions from home, allowing clinicians to quantify postoperative outcomes and objectively assess the success of various facial reanimation techniques.15
Another group designed a real-time facial asymmetry analysis algorithm designed for use with any standard laptop computer and built-in webcam that could be used anywhere.17 These examples show that with advancements in technology, objective and accurate assessment of outcomes in facial paralysis can be possible and even practical in busy clinical settings.
Substantial improvements in patient care and in outcomes research may be made with continued efforts to develop and use machine learning algorithms. In an ideal world, these tools would allow clinicians to be more consistent in their evaluations of patients and decrease positive confirmation bias that may be inherent in patient- or clinician-reported measures.18 Another advantage would be ease of use and greater accessibility for both clinicians and patients. While the use of specialized equipment and importance of software knowhow may be a current limitation, improvements in technology will continue to further this goal. Given the disruption in healthcare delivery caused by the COVID-19 pandemic and the continued rise of telemedicine, use of machine learning algorithms may enhance both accessibility and deliverability of care in these patients. For example, a better platform for assessment could contribute to shorter wait times for patients to be seen by specialists, and an enhanced quality of care that could be delivered remotely. Furthermore, a centralized database and analytical software will allow improved communication and collaboration between clinicians for increased collaboration.
The journey towards harnessing artificial intelligence for facial paralysis assessment is marked by its potential to revolutionize patient care and outcomes research. As technology continues to advance, the integration of such tools could improve accessibility and deliver effective care, thereby contributing to the overall wellbeing of patients with facial paralysis and palsy.
Continued on page 16
Unmasking Potential: Harnessing AI for Analysis of Facial Paralysis Outcomes
References
1. Vargo M, Ding P, Sacco M, et al. The psychological and psychosocial effects of facial paralysis: A review. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2023;83:423-430. doi:10.1016/j.bjps.2023.05.027
2. Homer N, Fay A. Facial Paralysis. Advanced Ophthalmology Optometry. 2018;3(1):357-373.
3. Moroco AE, Daher GS, O’Connell Ferster AP, Lighthall JG. Prevalence of Body Dysmorphic Disorder in an Otolaryngology-Head and Neck Surgery Clinic. Ann Otol Rhinol Laryngol. 2023;132(7):783-789. doi:10.1177/00034894221118772
4. Saadi R, Shokri T, Schaefer E, Hollenbeak C, Lighthall JG. Depression Rates After Facial Paralysis: Annals of Plastic Surgery. 2019;83(2):190-194. doi:10.1097/ SAP.0000000000001908
5. Snyder V, Frost AS, Ciolek PJ. Advances in Facial Reanimation. Otolaryngologic Clinics of North America. 2023;56(3):599-609. doi:10.1016/j.otc.2023.02.020
6. Hadlock T. Standard Outcome Measures in Facial Paralysis: Getting on the Same Page. JAMA Facial Plast Surg. 2016;18(2):85-86. doi:10.1001/ jamafacial.2015.2095
7. House J, Brackmann D. Facial Nerve Grading System. Otolaryngology Head and Neck Surgery. 1985;93(2).
8. Sunnybrook Facial Grading System. https://sunnybrook.ca/uploads/ FacialGradingSystem.pdf
9. Dusseldorp JR, Guarin DL, Van Veen MM, Miller M, Jowett N, Hadlock TA. Automated Spontaneity Assessment after Smile Reanimation: A Machine Learning Approach. Plastic & Reconstructive Surgery. 2022;149(6):1393-1402. doi:10.1097/PRS.0000000000009167
10. Guarin DL, Yunusova Y, Taati B, et al. Toward an Automatic System for Computer-Aided Assessment in Facial Palsy. Facial Plastic Surgery & Aesthetic Medicine. 2020;22(1):42-49. doi:10.1089/fpsam.2019.29000.gua
11. Knoedler L, Baecher H, Kauke-Navarro M, et al. Towards a Reliable and Rapid Automated Grading System in Facial Palsy Patients: Facial Palsy Surgery Meets Computer Science. JCM. 2022;11(17):4998. doi:10.3390/ jcm11174998
12. Gaber A, Taher MF, Wahed MA, Shalaby NM, Gaber S. Classification of facial paralysis based on machine learning techniques. BioMed Eng OnLine. 2022;21(1):65. doi:10.1186/ s12938-022-01036-0
13. Kollar B, Schneider L, Horner VK, et al. Artificial Intelligence-Driven Video Analysis for Novel Outcome Measures After Smile Reanimation Surgery. Facial Plastic Surgery & Aesthetic Medicine. 2022;24(2):117-123. doi:10.1089/ fpsam.2020.0556
14. Boonipat T, Asaad M, Lin J, Glass GE, Mardini S, Stotland M. Using Artificial Intelligence to Measure Facial Expression following Facial Reanimation Surgery. Plastic & Reconstructive Surgery. 2020;146(5):1147-1150. doi:10.1097/ PRS.0000000000007251
15. Fuzi J, Meller C, Ch’ng S, Hadlock TM, Dusseldorp J. Voluntary and Spontaneous Smile Quantification in Facial Palsy Patients: Validation of a Novel Mobile Application. Facial Plastic Surgery & Aesthetic Medicine. 2023;25(4):312-317. doi:10.1089/ fpsam.2022.0104

16. Greene JJ, Tavares J, Guarin DL, Hadlock T. Clinician and Automated Assessments of Facial Function Following Eyelid Weight Placement. JAMA Facial Plast Surg. 2019;21(5):387-392. doi:10.1001/ jamafacial.2019.0086
17. Hidaka T, Kurita M, Ogawa K, Tomioka Y, Okazaki M. Application of Artificial Intelligence for RealTime Facial Asymmetry Analysis. Plastic & Reconstructive Surgery. 2020;146(2):243e-245e. doi:10.1097/ PRS.0000000000007035
18. Dusseldorp JR, Van Veen MM, Guarin DL, Quatela O, Jowett N, Hadlock TA. Spontaneity Assessment in Dually Innervated Gracilis Smile Reanimation Surgery. JAMA Facial Plast Surg. 2019;21(6):551-557. doi:10.1001/ jamafacial.2019.1090
Greetings,
At the end of June, the Pennsylvania General Assembly and Governor Shapiro inked a spending plan for Fiscal Year 2023-2024. Although, at the time of this writing, the accompanying Code Bills (how to pay for the budget) have yet to be finalized.
The two departments that closely affect the physician community are the Department of Health and Department of Human Services.
The Department of Health saw its budget increase by 3.3% or a gain of 7.5 million over last year’s budget. The line items that saw the biggest increases were Local Health Departments, State Health Care Centers and Health Promotion and Disease Prevention.
The Department of Human Service’s total budget increase by 5.5% - It should be noted that Medical Assistance (Capitation) – increased by 8% or 355 million. A link to the entire state budget can be found on this page https://www.otopa.org/oto-pac.html
Legislation
As reported in the spring edition of Soundings, Senate Bill 25 (Independent Practice for Certified Registered Nurse Practitioners) was introduced in March and remains in the Senate Consumer Protection and Professional Licensure Committee. This legislation will need to be closely watched for possible movement in the fall.
Recently, the PA House of Representatives held a public hearing on House Bill 1000. This legislation amends the Professional Psychologist Practice Act to allow prescribing privileges for licensed doctoral degrees psychologists who have a master’s degree in clinical psychopharmacology. A further stipulation is that the psychologist would be required to be in a collaborative agreement with a primary care physician who would have final signoff.
The Pennsylvania Medical Society (PAMED) has indicated their opposition to this bill to the Committee Chairman. Additionally, PAMED will be sending letters to all members of the House of Representatives indicated its position.

Another issue that continues to loom on the horizon is the matter of noncompliant clauses/restrictive covenants. These type of arrangements in employee contracts limit where physicians can practice after leaving their current employer. This becomes more problematic as health systems continue to grow throughout the Commonwealth, and these covenants cover a larger radius of where physicians can practice.
Earlier this year, the Federal Trade Commission issued a draft proposal that bans employers from imposing non-compete restrictions. As read, the proposal appears to ban non-profits, including hospitals, from engaging in this practice.
As the legislature returns, in September, I will be monitoring the above issues as well as others that may be introduced. In the meantime, should you have any questions on legislation or become aware of proposals that should be brought to my attention, please email me at pfdunn444@gmail.com
Acclarent
Ambo
Anchor Medical Products, LLC
Atos Medical
Cochlear Americas
Cuesta Medical Solutions, LLC
DePuy Synthes
Ethicon US, LLC
Fuel Medical Group
Inspire Medical Systems, Inc.
Integra Lifesciences
KARL STORZ Endoscopy—America, Inc.
MD Surgical Products Inc.
Medtronic
Naveris
Neurent Medical
Regeneron
Smith + Nephew, Inc.
Stryker
Tactile Medical
Tungsten Medical Network
Industry Symposium Sponsor:
Regeneron
Women in Otolaryngology Sponsor:
Stryker
The Effects on Voice of Steroid Inhalers for Asthma
Dylan Vance, MD Resident Physician Department of Otorhinolaryngology –Head and Neck Surgery University of Texas Houston Health Science Center Robert T Sataloff, MD, DMA, FACS Professor and Chair Department of Otolaryngology –Head and Neck Surgery Senior Associate Dean for Clinical Academic SpecialtiesDrexel
University College of Medicine Director, Otolaryngology and Communication Sciences Research
Impact of Corticosteroid Inhalers on the voice
The most common and effective method of reducing asthma symptoms and exacerbations is the daily use of inhaled corticosteroids (ICS). Voice effects of ICS were reviewed by Vance et al1. Dysphonia is seen in up to 50 percent of asthmatics using steroid inhalers in some studies.
Use of high-dosage ICSs can cause systematic and local laryngeal side effects. Systemic effects included easy bruising, skin thinning, issues with calcium and phosphate metabolism, adrenocortical suppression, increased risk of osteoporosis, posterior subcapsular cataracts, dysphonia, pharyngeal discomfort and glaucoma.
Lankenau
Institute for Medical ResearchIntroduction.
Asthma affects roughly 25 million Americans. Many singers and other voice professionals are impacted by their struggle with asthma. In addition to making it difficult to breath and feeling increased air resistance, asthma can cause changes in air resistance and impair support impacting phonation and quality of the voice. Singers suffering from asthma may have lower lung volumes directly impacting strength of voice. Asthmatics tend to have restricted expiration, increase in abdominal wall muscle contraction to compensate for restricted breathing, lung hyperinflation with lowering of the diaphragm, and fluctuations in intra-thoracic and intraabdominal pressures. These effects help explain the significantly higher prevalence of dysphonia, phonatory effort and voice fatigue in asthmatics. Asthmatics also typically have shorter maximum phonation time, increased shimmer (perturbation of amplitude), increased jitter values (perturbation of frequency) and elevated Voice-handicap Indexes4
Dysphonia has been explained by the presence of thyroarytenoid muscle myopathy, laryngeal candidiasis and/ or steroid-induced laryngitis. Common laryngeal stroboscopic findings include bowing of the vocal folds, presence of glottic insufficiency, vocal fold edge irregularities, and presence of vascular lesions, mucosal thickening and leukoplakia. In patients with dysphonia on ICS, the vocal folds often have areas of hyperemia with plaque-like changes on the surface mucosa, as well as vascular lesions, dilated blood vessels, varices, hemorrhages, and thickness around the vocal folds following inhaler use. Reduced vibration and reduced mucosal wave propagation are common. Patients in professions with high voice demands are most likely to report dysphonia on ICS; and women and elderly patients also have a higher prevalence than men and younger patients.
Fungal and chemical irritation are other causes of dysphonia in asthmatics using ICS. Switching inhalers may improve dysphonia. Ciclesonide metered-dose inhalers may have less oropharyngeal deposition of steroid than standard inhalers and therefore might be associated with reduced oropharyngeal candidiasis and dysphonia compared with other inhaled corticosteroid delivery systems. Symptoms and signs generally improve after ICS is discontinued.
Type of ICS may influence impact on the voice. The aerosol triamcinolone acetonide reduces the vocal function compared with baseline, but aerosol beclomethasone dipropionate does not affect voice performance measurably. Patients using fluticasone as an ICS, abnormal mucosal wave symmetry/ periodicity, phase closure, glottic closure, mucosal wave amplitude/ magnitude, supraglottic hyperactivity, and negative mucosal quality are common, suggesting chemical irritation. Short-
Continued on page 20
Continued from page 19
term administration of corticosteroids as inhaled discs often results in increased incidence of cough, mouth and throat dryness, sensation of polydipsia and skin inflammation around the mouth. Longterm administration tends to produce dysphonia, voice fatigue, and laryngeal structural changes. Cellular markers of inflammation do not necessarily correspond with clinical appearances; however, there is an increase in intra-epithelial inflammatory cell markers after long-term use of ICS.
ICS also can improve voice quality. Since asthma can impact the voice negatively by impairing support (the power source of the voice), treatment with ICS can improve voice quality in the short term. However, inhaler use, especially ICS, is the preferred approach for most pulmonologists for asthmatics in general; but use of oral medications, reflux control, and avoiding or minimizing inhaler (especially ICS) use is the preferred strategy for singers and other voice professionals.
A meta-analysis looking at ICS oropharyngeal adverse events noted a difference in adverse events by type of device used to deliver the ICS. The

meta-analysis identified 23 studies that showed that incidence of oral candidiasis, dysphonia and pharyngitis were all increased with use of ICS. The study also found that the metered-dose inhaler carried a five-fold greater risk of candidiasis and dysphonia when compared to placebo, while the dry-powdered inhaler carried a threefold greater risk of candidiasis and dysphonia2. Increased research into the types of ICS and methods of delivery is needed to evaluate further the impact of ICS on voice, especially as new formulations including Ciclesonide are created.
A 2021 study looked at the impact of size of the formulation on the voice. The retrospective study assessed inhaled corticosteroids of small or standard particle size. The prevailing theory was that small particle size ICS distribute more effectively to the lungs, while standard size particles tend to deposit more heavily in the larynx. Small corticosteroid particle size was considered less than or equal to 2.0 micrometers (Ciclesonide, and beclomethasone-dipropionate HFA), and standard corticosteroid particle size was considered greater than 2.0 micrometers (Budesonide and Fluticasone). That study of 40 patients found that the small particle ICS caused less vocal fold atrophy than
the standard particle ICS group1. Further research also is needed with old and new formulations of ICS to determine the lasting impacts ICS can have on the voice.
Conclusion
Inhaled corticosteroids are used mostly for long-term treatment of asthma; and over the course of many months or years, they can have negative impact on voice including vocal fold atrophy and other structural changes and deterioration of voice quality. Most of the literature has examined standard ICS; but the new smaller particle-size inhalers may lead to fewer adverse effects on the voice, and further research is encouraged.
While further research is pursued, it is important to understand not only the potential problems that may occur with ICS, but also other treatment options. Pulmonologists who understand singers and other voice professionals try to control asthma with oral medicines, minimizing the use of inhalers, especially steroid inhalers. When ICS is necessary, Otolaryngologists should work closely with the pulmonologist to be sure that the pulmonologist understands voice issues and has prescribed the optimal particle size and delivery method, and the lowest dose of ICS required.
References
1. Vance D, Alnouri G, Valentino W, Eichorn D, Acharya P, Sataloff RT. Effects of Particle Size of Inhaled Corticosteroid on the Voice. J Voice. 2021 May;35(3): 455-457.
2. Rachelefsky GS, Liao Y, Faruqi R. Impact of inhaled corticosteroidinduced oropharyngeal adverse events: results from a meta-analysis. Ann Allergy Asthma Immunol. 2007 Mar;98(3):225-38.
Unique Demographics and Healthcare Resource Utilization for Trauma in the Amish Population
Bao Y. Sciscent, BS Hanel W. Eberly, BS, Richard Bavier, MD1, Jessyka G. Lighthall, MD, FACS Corresponding Author: Jessyka G. Lighthall, MD, FACSEmail:
jlighthall@pennstatehealth.psu.eduOverview of Amish in Pennsylvania
Pennsylvania, specifically Lancaster, York, Lititz, and New Wilmington counties, is home to the largest Amish population in the U.S. Because of religious beliefs, they generally eschew most modern technological advancements and work in traditional industries (e.g. farming, woodworking, and manufacturing) and travel by nonmotorized means. This makes them a unique patient population from a facial trauma standpoint. While studies have compared differences in facial trauma among urban and rural populations, less is known regarding facial trauma in the Amish population specifically.
Mechanisms of Trauma
Like urban and rural populations, falls are the most common cause of trauma in the Amish - making up 30-40% of cases.1,2 Similar to other populations, the highest proportion of trauma occurs in the summer months, and males are significantly more likely than females to sustain trauma.3
Hay hole falls are a mechanism relatively unique to the Amish. Hay holes are holes within the floor of the second story of a barn used to drop feed to animals. These are often left uncovered and are one of the leading causes of falls in the Amish.
A study of hay hole falls in Amish children found that 73% had skull fractures and 27% of patients presented with facial fractures. In this patient cohort, 26% were intubated, 41% required an intensive-care unit admission, and the median age of presentation was 4 years of age, indicating a high proportion
of trauma in young Amish patients. 4 Following falls, the most common causes of trauma in the Amish population are animal and buggy injuries. In a study of injuries sustained by buggy vs. motorized vehicle crashes, 16% of patients sustained facial injuries. 5 Buggies are a leading cause of trauma because they lack general safety features including seatbelts and airbags, are harder to visualize due to their black exterior, and move significantly slower than other road vehicles.
In an overview of Amish mortalities from trauma, pediatric and geriatric patients had the highest mortality rates. Mechanisms of injury associated with the highest injury severity scores (ISS) are hay hole falls and animal-related injuries. Animal injuries, namely horses, are associated with an increased need for surgery, increased length of stay, and craniomaxillofacial trauma.2,6 The high frequency of these injuries stems from a farming lifestyle and the use of horsedrawn buggies. Among pediatric Amish patients, farming machine-related injuries make up 10-40% of trauma cases.3,6 The farm is more often a place of work rather than play for the Amish as 49% of pediatric farm injuries occur while the child is working. 7 Children assist in farm work at a young age but do not have an adequate understanding of safety practices and injury prevention.8 Children often jump off moving vehicles or mow barefoot.9
Another group within the Anabaptist community with the Amish is the Mennonites. They share similar religious beliefs with the Amish but are more likely to use electricity and other modern technology including steel-wheeled tractors and motorized vehicles.10 A study of Amish and Mennonite traumas reported that Mennonites have significantly fewer buggy injuries, but still presented with a similar percentage of facial injuries likely due to a similar agrarian lifestyle.11
Current and Prior Interventions and Strategies
Assessments of trauma locations revealed injuries were most likely to occur at home and the farm, making these important targets for intervention.1 To address these issues, the Amish safety committees and farm safety days have been developed to partner Amish residents with medical and legal personnel to identify common hazards and determine best practices for change. In Ohio, counties with a high proportion of Amish residents, the U.S. Department of Transportation has provided grants to widen roads, create buggy lanes, post signs, and install buggy detectors. The Anabaptist Youth Trauma Prevention Consortium distributed 231 hay hole covers in south central Pennsylvania and analyzed trauma rates from hay hole falls before and after hay hole cover distribution. After hay hole cover distribution, there was a 50% decrease in hay hole falls and facial fractures from hay hole falls.12 While this study consisted of a small cohort, hay hole covers represent a simple but effective solution. Furthermore, with farm work playing such a prevalent role in Amish children’s lives, education-based interventions aimed at farm health and safety knowledge have been attempted with mixed results. A systematic review of interventions to prevent childhood farm injuries found that education on pediatric development or a farm visit by a dedicated specialist yielded improved retention and knowledge acquisition among patients.13
Continued on page 22
Unique
Utilization of Health Care Resources
and Healthcare Resource Utilization for Trauma in the Amish Population
To tackle these issues, it is important to understand how the Amish population interacts with the modern healthcare system. Cultural dissimilarity may be a barrier to seeking treatment. Amish often use local doctors and dentists and go to specialists and hospitals only if necessary. Furthermore, Amish patients’ primary language may not be English, and common languages include German, Swiss, and Pennsylvania Dutch. Addressing facial trauma is important because most Amish are self-pay where families and/or communities pool money to help cover costs. The Amish are exempt from the Affordable Care Act and many do not participate and receive social security benefits or Medicare.14,15 This emphasizes the importance of simple but effective interventions such as hay hole covers that cost approximately $100 compared to a hospital stay for facial trauma which can cost thousands of dollars. One study found that the mean costs of treating Le Fort I, II, and III fractures were $25,836, $28,415, and $47,333, respectively, which can be an immense burden on any patient’s family. 16
Conclusion
The Amish population is at increased risk for injury due to the prevalence of farming and other occupations involving old-fashioned technology. Other unique features of Amish life, such as the use of horse-drawn buggies and other teams of animals, hay holes, and the expectation that Amish children contribute actively to farm work, contribute to this increased risk. Given the distinct cultural and social aspects of Amish life, it is important to understand the unique mechanisms of facial trauma and incorporate culturefocused care when caring for this patient population. The most common causes of trauma in Amish patients include falls and

animal-related injuries. In particular, the use of hay holes and horse-drawn buggies contributes significantly to the trauma burden in these patients. Furthermore, craniofacial injuries were among the most common in these groups, emphasizing the importance of recognizing and treating this population. Interventions to reduce trauma incidence have included connecting these patients to dedicated personnel, adapting infrastructure, distributing hay hole covers, and implementing education-based interventions. Interventions should focus on being feasible and culturally appropriate, given that Amish societies provide little to no modern conveniences and many Amish patients may be reluctant to adopt practices readily accepted in other parts of the country.
Many of the traumatic injuries mentioned are preventable in this setting, and addressing the issue of facial trauma is
especially important as it can significantly impact the emotional, physical, and even financial well-being of these patients. Further research and intervention on this topic are imperative to improve the health of this patient population.
References
1. Whitney L, Bonneville K, Morgan M, Perea LL. Mechanisms of Injury Among the Amish Population in Central Pennsylvania. Am Surg. 2022;88(4):608-612. doi:10.1177/00031348211050592
2. Morgan ME, Brown CT, Whitney L, Bonneville K, Perea LL. An Overview of Amish Mortalities at a Level I Trauma Center. Am Surg. 2022;88(3):394-398. doi:10.1177/00031348211047218
3. Vitale MA, Rzucidlo S, Shaffer ML, Ceneviva GD, Thomas NJ. The impact of pediatric trauma in the Amish community. J Pediatr. 2006;148(3):359-365. doi:10.1016/j.jpeds.2005.10.036
Unique Demographics and Healthcare Resource Utilization for Trauma in the Amish Population

12. Batra EK, Gross BW, Jammula S, et al. Preliminary results of a novel hay-hole fall prevention initiative. J Trauma Acute Care Surg. 2018;84(2):295-300. doi:10.1097/ TA.0000000000001754
13. Hartling L, Brison RJ, Crumley ET, Klassen TP, Pickett W. A systematic review of interventions to prevent childhood farm injuries. Pediatrics. 2004;114(4):e483-e496. doi:10.1542/peds.2003-1038-L
14. Rohrer K, Dundes L. Sharing the Load: Amish Healthcare Financing. Healthcare (Basel). 2016;4(4):92. Published 2016 Dec 14. doi:10.3390/ healthcare4040092
4. Engbrecht BW, Kulaylat AN, Dias M, Kendig JW, Cilley RE. Childhood Injuries Due to Hay-Hole Falls: A 19Year Experience at a Rural Pediatric Trauma Center. Pediatr Emerg Care. 2016;32(7):455-458. doi:10.1097/ PEC.0000000000000450
5. Aaland MO, Hlaing T. Amish buggy injuries in the 21st century: A retrospective review from a rural level II trauma center. Am Surg. 2004;70(3):228-234.
6. Smith GA, Scherzer DJ, Buckley JW, Haley KJ, Shields BJ. Pediatric farm-related injuries: a series of 96 hospitalized patients. Clin Pediatr (Phila). 2004;43(4):335-342.
7. Gilliam JM, Jones PJ, Field WE, Kraybill DB, Scott SE. Farm-related injuries among Old Order Anabaptist children: developing a baseline from which to formulate and assess future prevention strategies. J Agromedicine. 2007;12(3):11-23. doi:10.1080/10599240701885855
8. Brewer JA, Bonalumi NM. Health care beliefs and practices among the Pennsylvania Amish. J Emerg Nurs. 1995;21(6):494-497. doi:10.1016/ s0099-1767(05)80258-2
9. Burgus S, Rademaker A. Testing a novel child farm safety intervention for Anabaptist audiences. J Agromedicine. 2007;12(4):63-70. doi:10.1080/10599240801986165
10. Kraybill DB, Gilliam JM. Culturally competent safety interventions for children in Old Order Anabaptist communities. J Agromedicine. 2012;17(2):247-250. doi:10.1080/10 59924X.2012.658303
11. Grandizio LC, Wagner BR, Graham J, Klena JC, Suk M. Orthopaedic trauma in the Anabaptist community: epidemiology and hospital charges. J Agromedicine. 2015;20(2):140-148. do i:10.1080/1059924X.2015.1010066
15. Weller GER. Caring for the Amish: What Every Anesthesiologist Should Know. Anesth Analg. 2017;124(5):1520-1528. doi:10.1213/ ANE.0000000000001808
16. Lee KC, Chuang SK, Eisig SB. The Characteristics and Cost of Le Fort Fractures: A Review of 519 Cases From a Nationwide Sample. J Oral Maxillofac Surg. 2019;77(6):12181226. doi:10.1016/j.joms.2019.01.060



