NATURE-BASED SOLUTIONS
As one of the world’s leading planning, engineering and consulting firms, Michael Baker International believes in the power of naturebased solutions to reduce risk and improve infrastructure for a more resilient and sustainable future.
SOLUTIONS
For more information, contact Richard Beck, PWS, Michael Baker Practice Executive P: 949-855-3687 E: rbeck@mbakerintl.com
Constructed Wetlands
Dune Rehabilitation & Restoration
Ecosystem Restoration
Green Roofs & Rooftop Gardens
Habitat Preservation & Restoration
Hybrid Green-Gray Solutions
Living Shorelines
Mitigation Offsets & Banking
Phytoremediation
Recreational Resources
Regulatory Processing
Riparian Habitat
Creation & Restoration
Shoreline Restoration
Stream & Floodplain Restoration
Watershed Restoration
Wetland Delineation
PROUD SUPPORTER OF THE SOCIETY OF WETLAND
SCIENTISTS
Bow Creek Stormwater Park Flood Mitigation Improvements / City of Virginia Beach, Virginia
Boulder County Flood Recovery and Ecosystem Restoration / Boulder, Colorado
Westside Creeks Restoration / San Antonio, Texas
Thursday, November 20 | 12:00 PM - 1:00 PM (CST)
Topic: Monitoring of Man-Made Wetlands in the Semi-Arid Region of India Using Satellite Imagery (link)
Speaker: Raj Singh, M.Sc., PGD
Raj Singh, M.Sc., PGD, is Research Scholar in the Department of Environmental Science GITAM
Deemed to be University, Visakhapatnam, Andhra Pradesh, India. He served as Assistant Professor at Dr. K. N. Modi University, Newai, Rajasthan, and Tula’s Institute, Dehradun, India. He is Alumnus of the Indian Space Research Organization (ISRO), Department of Space, Gov. of India, and also worked on a France-funded IDDRI project at BITS Pilani, KK Birla Campus, Goa, India. He is Active Member of Wetland International, Wageningen, Netherlands, and the British Ecological Society London, UK. His expertise is in environmental science, wetland ecology, remote sensing, and GIS. He has published over 20 scientific articles in reputed national and international peer-reviewed journals, review, book chapters, presented 6 papers at international conferences, and edited 3 books with Jenny Stanford and Springer Nature publisher.
Description
This webinar is focused on the application of remote sensing techniques in wetland ecosystem monitoring. Wetlands are water-saturated ecosystems that support unique and diverse plant and animal communities.
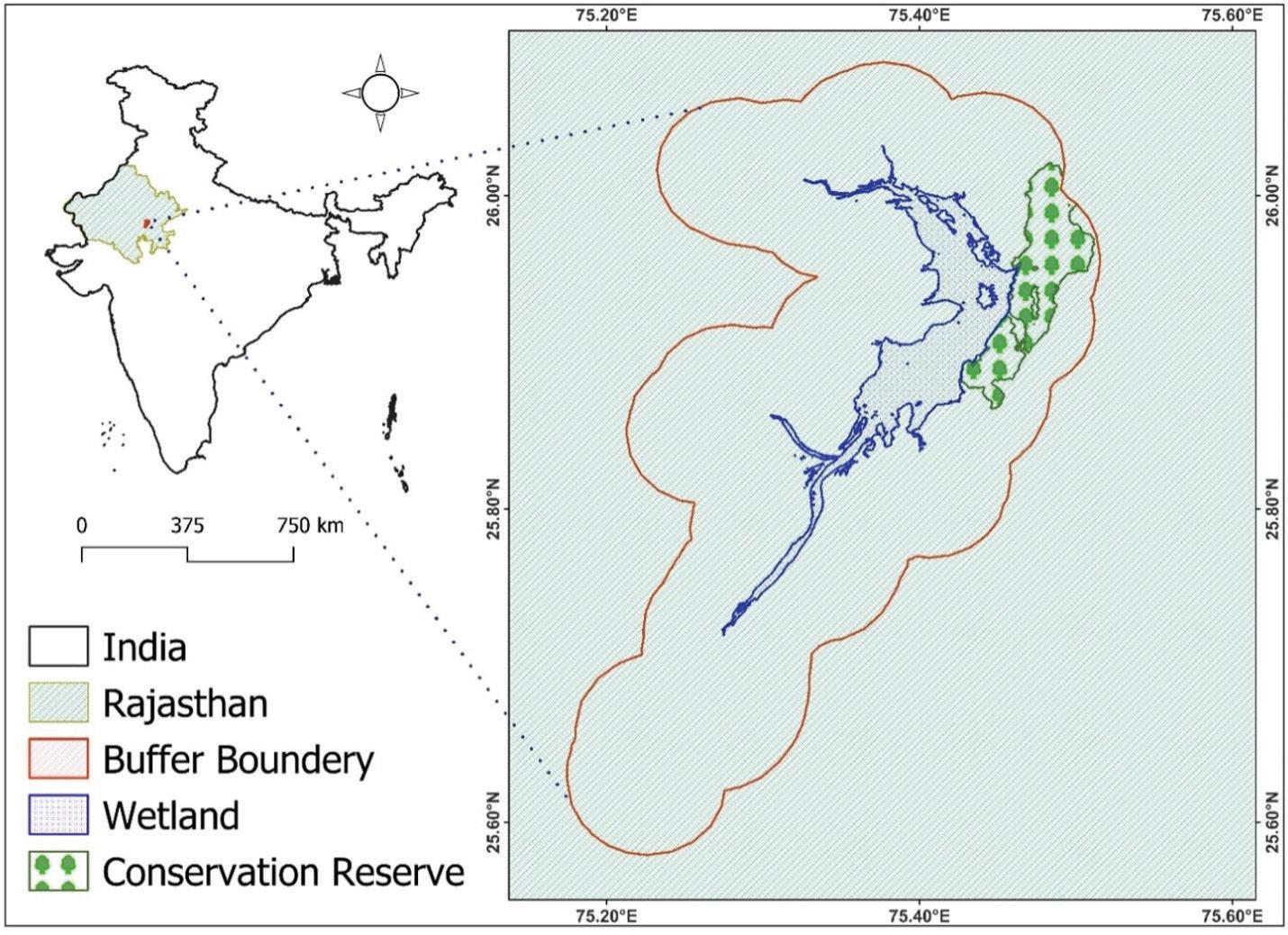
The current study focuses on Bisalpur Wetland, Rajasthan, India. Bisalpur wetland is the primary source of drinking water in major cities in Rajasthan. A wetland ecosystem in a place with high temperatures all year, notably in Rajasthan's arid and semi-arid areas, is critical for preventing water-related challenges and maintaining ecological balance. However, I utilize Landsat 8 (L8) Operational Land Imager (OLI) satellite imagery to map changes in Land Use and Land Cover (LULC), alongside analysis of Land Surface Temperature (LST) and vegetation health, with a focus on the Bisalpur wetland buffer zone in the post-monsoon seasons (October) of 2013 and 2022. Moreover, I employ remote sensing techniques to monitor the wetland turbidity of the Bisalpur wetland during both the pre and post-monsoon seasons in 2013 and 2022.



The study underscores that integrating remote sensing with climate data enhances wetland ecosystem understanding, guiding sustainable practices for resource management. Additionally, this research underscores the critical role of satellite imagery in assessing and managing water quality, particularly in situations where in-situ data is lacking.
The study area (Wetland site) includes two main components in the same landscape zone: the Bisalpur wetland buffer and the Bisalpur Conservation Reserve (BCR). Bisalpur Wetland is located on Bisalpur Dam in the Tonk district of Rajasthan, India.
More information here: https://www.sws.org/webinars/
SWS NEWS
Latest from the Journal Wetlands
To find the latest technical articles on wetlands from our companion journal Wetlands, go to https://link.springer.com/journal/13157.
The SWS Strategic Plan Update: A Common Vision for a Healthy and Effective Society
Eric D. Stein, SWS Immediate Past-President
The SWS Strategic Plan provides a road map for the Society’s activities and identifies priority objectives and initiatives to guide actions over the five-year period covered by the plan. The plan can influence membership and technical priorities as well as financial and administrative decisions. We are in the final year of our current five-year plan, and the SWS Board of Directors is scheduled to consider the 2026-2030 Strategic Plan for adoption at the mid-year meeting in February 2026.
The Strategic Plan update process began in 2024 with three guiding principles: 1) Focus on high-level goals and priorities for the Society. These high-level goals form the foundation for development of more specific implementation strategies/business plans by Chapters, Sections, and Committees. 2) The plan should be simple, easy to communicate, and readily tracked over time. 3) The plan should help establish a culture of purpose that should be referenced in our actions on an ongoing basis.
Broad member input was sought throughout the development process to help ensure that the plan reflects the vision and priorities of the membership as a whole. We began the process with a survey of the membership where we asked for feedback on the existing plan and priorities for the future. An initial brainstorming workshop was held during the SWS meeting in Taipei in fall 2024, followed by four facilitated brainstorming sessions with members from Chapters, Sections, and Committees. A half-day workshop held during the
annual meeting in Providence in July 2025 was used to discuss the initial draft plan and gain input on the structure, content, and implementation strategy and to identify potential measures of success.
Since Providence, an ad hoc working group has developed a final draft of the updated Strategic Plan. This plan contains four high-level goals for the Society over the next five years:
• Goal 1: Promote Protection and Restoration of Wetlands
• Goal 2: Improve Awareness About the Importance of Wetlands
• Goal 3: Ensure Relevance and Representation Among the Membership
• Goal 4: Provide for the Sustainability of the Society
A complete copy of the draft plan is below. We encourage all SWS members to review this plan and send any comments, questions, or suggestions to Immediate Past-President Eric Stein at erics@sccwrp. org. The full Board of Directors will vote on the Strategic Plan update in February 2026. We encourage you to provide input and help ensure that our Strategic Plan reflects the priorities of the Society membership.
Proposed SWS Strategic Plan Update - Draft, Sept. 15, 2025
Name Society of Wetland Scientists
Mission
To promote best practices and an inclusive and interactive community for wetland research, education, conservation, preservation, restoration, and management.
Vision
To ensure that wetlands are understood, their importance is recognized, and sound wetland science is used as a guide for wetland professionals and the public to collaborate on research, conservation, preservation, restoration, and management, and to inform current and future wetland policies.
Values
• Sound and credible science
• Effective communication
• Education and outreach
• Coordination and collaboration
• Integrity
• Diversity and inclusiveness
• Sustainability
• Partnership Overview
The Society’s strategic plan is a blueprint to guide and focus the Society’s actions over the next five years. It establishes long-term goals and measurable objectives to ensure that the Society is effective, impactful, and relevant to its members and to the global wetland community. The strategic plan reflects the challenges and opportunities anticipated over the forthcoming five-year period. Consequently, this plan emphasizes the importance of (1) sound science, (2) policy engagement, (3) societal relevance, (4) inclusiveness, (5) collaboration, and (6) sustainability.
Chapters, sections, and committees are encouraged to develop implementation plans that serve as mechanisms to measure, report, and achieve the society’s strategic goals. Implementation plans help to prioritize actions and provide accountability for the long-term success of the Society and will be used to track progress toward achieving the Society’s stated goals.
Strategic Goals
The Society will conduct/support activities and empower its members to achieve the following strategic goals:
Goal 1: Promote Protection and Restoration of Wetlands
Promote activities that contribute to the long-term protection and restoration of global wetland resources, including the production and dissemination of sound
science, development of strategic partnerships, engagement in wetland policy and management, and education and empowerment of the public. Specific implementation actions may include:
• Proactively engage partners to inform wetland policy and management decisions and inform broad constituencies on the importance of wetlands;
• Encourage conservation and wise use of wetlands by incorporation of sound science; and
• Partner with wetland regulators, practitioners, managers, and advocacy groups to provide them with science-based resources to support their efforts and help translate science into action.
Goal 2: Improve Awareness about the Importance of Wetlands
Develop science-driven, fact-based education and outreach materials on the importance of wetland protection, restoration, and management targeted at broad sectors, including students, the public, practitioners, researchers, and decision-makers. Specific implementation actions may include:
• Implement a diverse range of communication strategies that are accessible to people of all backgrounds and generations;
• Develop partnerships with academic programs and other organizations to support their educational mission and promote participation with the Society; and
• Develop and grow networks to allow exchange of scientific, practical, and policy information related to wetlands among researchers, practitioners, decision makers, and community groups.
Goal 3: Ensure Relevance and Representation Among the Membership
Implement programs that allow the Society to serve as an authoritative and meaningful forum for wetland professionals globally, including physical and social scientists, practitioners, decision makers, policy and legal experts, to collaborate on the exchange of ideas and research outcomes related to wetland assessment, conservation, restoration, and management. Specific implementation actions may include:
• Focus activities in ways that ensure representation and improve relevance for member interests and perspectives, globally;
• Improve accessibility to multiple sectors and career levels, and communicate the value of the Society in terms of professional advancement and opportunity;
• Create mechanisms by which the Society provides benefits throughout different career points (e.g., student, early career, experienced) and types of career paths (e.g., academic, practitioner, policy, etc.); and
• Foster an open, inclusive, and civil culture welcoming to people of all backgrounds, wetland interests, and views across Society activities and engagements.
Goal 4: Provide for the Sustainability of the Society
Ensure longevity and long-term sustainability of the Society through improved administrative efficiency and adaptive planning, increasing participation by students and early career professionals, and a diversified funding portfolio, and sound governance structure. Specific implementation actions may include:
• Develop mechanisms to ensure that the Society is agile and can focus on and respond to changing needs as the Society evolves and grows;
• Focus on advancing engagement by students and early career professionals to build the next generation of wetland professionals who are active in the Society; and
• Ensure fiscal and administrative strength and sustainability through a diversified funding portfolio, robust administration and management, and strategic partnerships.
Implementation
Chapters, Sections, and Committees are encouraged to develop implementation plans that support the strategic plan. The implementation plans should provide tangible actions to achieve the goals and include realistic measures, quantifiable objectives, and reporting measures to promote accountability. SWS leadership and management will provide support by reviewing and commenting on implementation plans and by reporting results to adaptively manage SWS in pursuit of the strategic plan objectives.
Progress toward meeting each of the strategic goals should be routinely reported to the membership
and disseminated through the SWS website, SWS newsletter, Wetland Science and Practice, SWS social media accounts, and other appropriate outreach mechanisms.
The following measures of success should be used to evaluate how effective the Society has been at achieving our strategic our goals and to connect to the specific actions that will be described in the implementation plan(s) developed by chapters, sections, and committees.
• SWS members know what the major goals of the Society are and how it guides our actions;
• SWS has a diverse funding portfolio to fund priority initiatives;
• SWS products or actions are cited for their role in guiding major policies at the local, regional, national, and international levels;
• SWS maintains globally affordable membership rates, while ensuring the Society’s long-term financial sustainability;
• SWS membership continues to grow and represents gender, race, age, and ethnic diversity;
• SWS maintains a relatively equal balance of members from the research, education, policy, and practitioner sectors;
• SWS membership includes representation from developed and developing countries and its members include students, early career, and experienced professionals;
• SWS strengthens its engagement and collaboration with associated professional societies, non-profit organizations, corporations, and groups (e.g., students, emerging economies)
• SWS has an effective Executive Director, a strong Board of Directors, and active committees, sections, and chapters;
• SWS continues to provide a pathway for launching careers and career continuity among wetland professionals (e.g., retaining and supporting wetland professionals throughout their careers); and
• SWS improves connection and engagement amongst members and the public via social media and other related communication mediums.
Foundations of Wetland Science Educational Modules
The Education Section of SWS has developed a program, Foundations of Wetland Science, which is designed to provide the general public, students, instructors, and professionals with freely accessible online educational modules about wetland science for general knowledge or as resources for the classroom and outreach activities. The first two modules were published in Wetland Science & Practice this past April, and are also freely available to all on the SWS website (https://www.sws.org/education-modules-wetlandscience/). Three new modules follow this introduction, and will also be posted on the SWS website.
We hope that the array of modules will grow over the years, and we encourage people who would like to develop a module on a topic of interest to them to contact the SWS Education Section Chair about their idea.
Module 3: Introduction to Wetland Plants
Contributor: Gary Ervin (Mississippi State University)
OVERVIEW
This module is meant to provide a coarse-scale overview of some of the more important aspects of wetland plant biology. Covered here is a bit about the biology and ecology of wetland plants, as well as an overview of wetland plant taxonomic diversity. The topic of invasive species in wetlands is included as an example of one of many applied topics where the biology of wetland plants and animals has important intersections with society. Although the examples used here come predominantly (but not exclusively) from freshwater wetlands, the processes discussed are also important in brackish and saline wetlands.
WETLAND PLANTS DEFINED
As mentioned in Module 1, wetlands are a type of ecosystem typically defined, at least in part, by the presence of shallow water, or temporarily flooded or saturated soils during some or all of the biologically active period(s) of the year. However, as is also covered in Module 1, there is more to the definition than simply
the presence of shallow water, otherwise, every puddle that forms after a rain shower could be called a wetland.
The depth of water is important, but so is the time of year during which standing water occurs and the length of time water is present. These factors influence biological and biogeochemical (biological + geological + chemical) conditions within a wetland, leading, under certain circumstances, to conditions that favor the development of wetland soils (a.k.a. hydric soils) and the establishment and persistence of wetlandadapted plant species. All three factors (water, soil, and plants) are intertwined in their influence on wetland characteristics, and this module will introduce some of the ways plants respond to the water and hydric soils in wetland ecosystems.
Wetland plants have been defined as those plants that are capable of establishing persistent populations in habitats where water is present at a sufficient depth and for a sufficient duration to have a biologically significant effect on the vegetation of the area. That effect is typically revealed in the characteristics of the plants and other organisms that live in the area from year to year. In the case of wetland plants, there are characteristic features of these species that indicate they would be expected to survive under the conditions created by the combination of standing water and hydric soils found in wetland ecosystems.
One such key feature in wetland plants is the presence of visible air passageways throughout their stems, roots, and leaves (Figure 1). These passageways often are associated with spongy tissues referred to as aerenchyma and provide many services to the plants, some of which will be discussed in more detail in later modules. However, one perhaps intuitive benefit of having air spaces throughout the leaves and stems of a plant living in an area with standing water is that the leaves and stems of the plant will be capable of floating at or near the surface of the water. Another benefit is that it shunts oxygen-rich air to the roots to support plant respiration. This floatation function of aerenchyma in wetland plants is a key feature that has led to a commonly used system for categorizing these plants.
It is estimated that there are more than 8,000 species of wetland plants in the United States alone (Lichvar
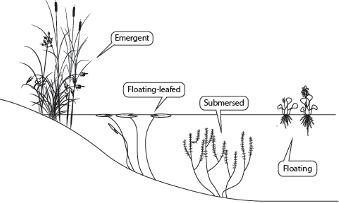
et al. 2014). In an effort to simplify this diversity of plants and relate species to their roles in the ecosystem, biologists have devised a few classification schemes based on plant traits. The simplest of these divides wetland plants among four groups referred to as growth forms. These growth forms are emergent plants, floating-leafed plants, submersed plants, and floating (or free-floating) plants (Sculthorpe 1967; Figure 2).
Emergent plants, which comprise the greatest diversity of species, are those that have their roots in the soil with their leaves and/or stems rising above the water surface. A few examples of emergent plants are shown in Figure 3A, but this group also includes species such as grasses, sedges, rushes, and cattails (Figure 3). Floating-leafed species typically have their roots and some form of belowground stem buried within the soil, with leaves floating atop the water surface at the end of long flexible
stems called petioles. Examples of these are waterlilies. Submersed species, like the floating-leafed plants, usually have their roots buried in the soil, but their leaves and stems float suspended within the water. Most species (> 90%) of submersed species will have stem tips or flowers at or above the water surface, but most of the plant still will remain below the water. Examples of submersed species are Hydrilla verticillata, milfoils (Myriophyllum species), and many pondweed species (Potamogeton species). The final growth form, floating or free-floating species, are just that; these species float at or on the water surface with roots, if present, hanging below them in the water. Pistia stratiotes, Pontederia crassipes, the duckweeds, and some of the aquatic ferns are some widespread examples of free-floating plants. All these groups are found not only in fresh water but also in saline or brackish waters.

Three of these growth forms, floating-leafed, submersed, and floating species, benefit directly from the aerenchyma tissues mentioned above through the buoyancy provided by the internal air spaces. These species benefit by having their photosynthetic tissues held at, above, or near the water surface, placing them in the best position for capturing solar energy. This placement reduces the intensity of competition from other species for light. At the same time, the buoyancy provided by aerenchyma in the non-emergent growth forms reduces the need to produce supportive tissues that typically are found in the leaves and stems of emergent plants. This distinction between emergent plants and the other three groups is also reflected in the
Figure 1. Examples of the air passageways (aerenchyma) that are common among wetland plants. The background shows a longitudinal section through the leaf stem (petiole) of Pontederia crassipes. Inset photos in the upper-left are sectioned petioles of Brasenia schreberi (left) and Nymphaea odorata (right). Bottom-right insets are sections of a leaf (upper) and root (lower) of Typha latifolia.
Figure 2. One commonly used scheme for categorizing wetland plants based on four growth form categories. Modified from Ervin (2023).
Figure 3. A few examples from the four growth forms mentioned. A: Several species of emergent wetland plants, including arrowheads (Sagittaria species), rushes, and pickerelweed (Pontederia cordata); B: Floating-leafed plants (Nymphaea odorata and Brasenia schreberi); C: Submersed plant (Hydrilla verticillata); D: Floating, or freefloating plant (Pistia stratiotes).
difference sometimes emphasized between wetland plants and the subset of those plants often referred to as aquatic plants. Aquatic plants typically are defined as those species who are supported by the water (via the buoyancy provided by aerenchyma) during all parts of their life cycles, and they make up a significant minority of all wetland plants, with only a few hundred species globally.
GROWTH FORMS AND WETLAND ZONATION
The great diversity of plant species present in wetlands makes organizing the floristics of these ecosystems somewhat complicated. There are thousands of species from among dozens of families, including everything from mosses and liverworts to cypress trees, mangroves, and sedges. The four-group scheme previously described (Figure 2) allows biologists to simplify this complexity to better study and understand the ecology of wetland plants.
This growth form classification system helps us think about the plants in light of some of their adaptations to the wetland environment while also reflecting their typical spatial organization within wetlands.
For example, emergent plant species, because their leaves and stems are physically supported above the water, can occupy a zone of wetland habitat along the water’s edge. At the same time, because these species also produce aerenchyma tissue that helps to aerate their root systems and other belowground organs, they can live in saturated soils where non-wetland species would experience severe physiological stress. Thus, these species can escape competition from upland species while maintaining optimal exposure of leaves to sunlight above the water. As a result of their situation along the wetland edge, where water, sunlight, and soil nutrients are often in abundant supply, emergent plant species tend to be the most productive of the wetland plant growth forms.
Commonly, in the shallow water just beyond the fringe of emergent species habitat, floating-leafed species can be found to dominate the wetland flora. This is, to some degree, the result of their ability to tolerate deeper water than the emergent plants while also maintaining their photosynthetic tissues atop the water for maximal sun
exposure. By having their roots in the soil at the bottom of the wetland, these species also have direct access to abundant soil nutrients.
Submersed plant species often have difficulty obtaining sufficient sunlight beneath the canopy of either emergent species or floating-leafed species. As a result, they are often relegated to deeper water, provided there is sufficient clarity to allow light to penetrate to the plant. Because most or all of their leaves are below water, their rates of photosynthesis will typically be lower than in emergent or floating-leafed species, despite having access to soil nutrients; seagrasses can be an exception to this rule. However, these species are very important in wetland and aquatic habitats because they provide dissolved oxygen and structural habitat for many aquatic invertebrates and foraging habitat for waterfowl, fish and other aquatic consumers.
Free-floating species are not confined to a particular zone within a wetland, by virtue of not being anchored within the wetland soil or sediments. However, these species do have an advantage in deeper waters by having direct access to sunlight, oxygen, and CO2, and these species sometimes will be found to shade out the submersed species if they develop dense populations.
As a result of these behaviors of the four wetland plant growth forms, wetland vegetation often tends to be organized into zones from the water’s edge out to the deeper portions of the wetland (Figure 2), although there often will be indistinct edges to the zones. This zonation plays into our understanding of wetland ecology, as well as our attempts to apply ecological understanding to solve problems such as water quality management with constructed wetland treatment systems. It has been found, for example, that different growth forms may complement one another in removing excess nutrients from contaminated waters (Vymazal 2007). Knowledge of the habitat requirements for the different growth forms, then, can facilitate could allow one to design a natural treatment system to efficiently improve water quality.
Some wetland scientists have developed much more complicated schemes for classifying wetland plants, in an effort to provide a more detailed summarization of the role vegetation plays in wetland function. Keddy, for
example, used more than two dozen different plant traits to develop a functional classification of North American wetland plants (Keddy 2023). The traits examined included plant growth rates, stress tolerance, biomass allocation (above- vs. belowground plant parts), plant morphology, sexual vs. asexual reproductive strategies, and plant longevity (e.g., annuals vs. perennials). This and similar approaches based on species traits are useful because they simplify the complexity of ecological communities while still retaining key information about species based on how they interact with each other and with the physical environment.
MAJOR TAXONOMIC GROUPS OF WETLAND PLANTS
Wetland plants are found across a broad range of plant groups. Taxonomically, wetland plants exist among the bryophytes (mosses, hornworts, and liverworts), the ferns and “fern allies” (including clubmosses, horsetails, and ferns proper; Figure 4), the gymnosperms (fir, spruce, larch, cypress), and the angiosperms (the flowering plants; Ervin 2023). The first three of these groups (bryophytes, ferns, and gymnosperms) include only about 32,000 species in total globally, only some of which are adapted to wetlands. In contrast, the flowering plants number somewhere between 250,000 to 400,000 species globally, the uncertainty arising from incomplete exploration of many parts of the world and ongoing research into the genetic relatedness of known species and those that are regularly being discovered and described. Again, only a small percentage of these are adapted to wetlands, indicative of the powerful ecological selection imposed by the wetland environment. In the United States, for example, there are approximately 8,000 plant species recognized as wetland plants (US Army Corps of Engineers 2022), out of approximately 38,000 total species (~ 20%).

org/licenses/by/4.0>, via Wikimedia Commons.
Among the mosses, Sphagnum species are probably best known, being important components of bog and fen communities, especially at the higher northern and southern latitudes. Ferns, horsetails, isoetids, and clubmosses are also commonly found in wetlands occupied by the mosses, but these species also are found in more temperate wetlands. Spruce (Picea species), fir (Abies species), and larch, or tamarack (Larix species), are often found in association with Sphagnum mosses in higher latitude peatland forests. In more temperate latitudes, river floodplain forests often serve as home to cypress (Taxodium species), many times in association with tupelos (Nyssa species, a genus of flowering plants).
Other trees within the angiosperms that often may be found in freshwater wetlands include the oaks (Quercus species), maples (Acer species), ash (Fraxinus species), and elms (Ulmus species). In saltwater habitats, another diverse group of trees is commonly encountered, that is the mangroves (Figure 5). Mangrove tree species come from more than fourteen somewhat distantly related families of plants, so they are defined by life form and habitat (i.e., trees that live in salt water) rather than by taxonomic relatedness. However, some of the more commonly recognized genera of mangrove tree species come from four families: red mangroves, Rhizophora species, from the Rhizophoraceae family, mangrove apple, Sonneratia species, from the Lythraceae, white mangrove, Laguncularia, from the Combretaceae, and the black mangroves, Avicennia, from the Acanthaceae.
Figure 4. Examples of sphagnum (Sphagnum squarrosum; A & B), clubmosses (C), horsetails (D), and aquatic ferns (E). Sphagnum photos courtesy of A: Tatiana Strus and B:
Виноградов (Georgy Vinogradov), CC BY 4.0 <https://creativecommons.

Also in the angiosperms are a number of families with many species of herbaceous plants that are quite important in wetlands. Perhaps most well-known among those are the grass family (Poaceae; ~11,300 species globally), the sedge family (Cyperaceae; ~5,600 species globally), and the rushes (Juncaceae; ~400 species globally). Although not all grass, sedge, and rush species occur in wetlands, a large percentage do, and they often are ecologically important components of the flora in those wetlands (Figure 6). Other very important families among wetland angiosperms are the Alismataceae (~90 species), Hydrocharitaceae (~140 species), and Potamogetonaceae (~100 species), which include the majority of truly aquatic flowering plant species. That is, most of the plant species in these three families are highly adapted to life in the water, with many of them even relying on the water itself to carry out pollination. This is in contrast to the majority of other flowering plant families, whose members use the wind or insects for pollination.

One final group of plant families worth special mention are the families containing carnivorous plants (Figure 7). These species are often found in nutrient-poor highly acidic bog ecosystems, where they rely upon capture of insects and other small animals for part of their nutrition. Included among these are the Droseraceae (~200 species), which includes the sundews (Drosera species) and Venus flytraps (Dionaea species), the Sarraceniaceae (~30 species of pitcher-type plants; e.g., Sarracenia, Darlingtonia), and the Lentibulariaceae (~350 species), which includes the bladderworts (Utricularia species), among others.
INVASIVE SPECIES IN WETLANDS
Occasionally (although more commonly as the human population has expanded in size and interconnectedness), species may be introduced from wetlands in one part of the world to wetlands in another area, and some of those species might fit, ecologically speaking, differently into the community than does any one of the native species. This difference in ecological fit many times results in the newly introduced species having a competitive edge over many, or perhaps all, of the resident native species. There are many examples of such introduced species developing into highly invasive components of the ecosystem, overwhelming native species and causing important ecological and/or economic impacts to the invaded ecosystems.
A few noteworthy invasive plant species in wetlands are Pontederia crassipes, Hydrilla verticillata, Myriophyllum species, Alternanthera philoxeroides, Phragmites australis, Phalaris arundinacea, multiple
Figure 5. Rhizophora mangle (left) and Avicennia germinans (right) in southern Florida.
Figure 6. Lakeshore wetland in Rocky Mountain National Park, dominated by sedges (Carex species).
Figure 7. Examples of some carnivorous wetland plants; Sarracenia alata (left) and Drosera rotundifolia (right).
species of salt marsh grasses (Spartina species in areas where they are not native), and floating aquatic ferns in the genus Salvinia (Table 1; Figure 8). These species have diverse histories, in terms of how they came to be introduced to other parts of the world, how they have impacted native communities, and how humans have responded in attempts to manage the invasion.
8. A few examples of noteworthy invasive aquatic and wetland plants.
from top-left are: Pontederia crassipes, Myriophyllum spicatum, Alternanthera philoxeroides, Salvinia molesta, and Phragmites australis
Although the sources of every introduction are not known, some of these species have been introduced in the horticulture trade because of their attractive flowers (e.g., Pontederia crassipes and Lythrum salicaria). In the case of Lythrum salicaria, those large showy flowers have co-opted native pollinators, who preferentially visit flowers of loosestrife, rather than the smaller flowers of native plants (Brown et al. 2002). Other wetland invaders have been introduced in association with the aquatic plant trade. Submersed species like the milfoils are especially susceptible to be introduced in this way because, as mentioned above, they provide excellent habitat for fish and other aquatic animals and help to oxygenate waterbodies. These services make submersed aquatic plants valuable components of home aquaria, but this also results in the occasional dumping of non-native species into local waters or in accidental escapes of non-native species into the wild from aquatic plant nurseries.
Common Name Scientific Name Origin Region Invaded
Water hyacinth Pontederia crassipes
Hydrilla Hydrilla verticillata
Eurasian watermilfoil
Myriophyllum spicatum
Amazon basin in South America
India and southeast Asia
Europe, Asia, and northern Africa
Introduced into 146 countries worldwide; present on every continent but Antarctica
Introduced into 40 countries; present on every continent but Antarctica
Introduced into 19 countries; present on every continent but Antarctica; limited distribution in South America
Alligatorweed Alternanthera philoxeroides
Giant salvinia Salvinia molesta
Common salvinia
Paraná region of South America
Southeastern Brazil
Salvinia minima Central and South America, from southern
Mexico into Brazil and Argentina
Common reed Phragmites australis
Saltmarsh cordgrass
Spartina alterniflora
Native genotypes are known from many parts of this species’ range
Atlantic coast of North America
Introduced into 27 countries; present on every continent but Antarctica
Introduced into 61 countries; present on every continent but Antarctica
Introduced into 4 countries; widespread in the western hemisphere but with very limited distribution in Europe, Asia, and Africa
Recorded as introduced into 20 countries, but widespread occurrence of native genotypes complicates this species’ biogeography
Introduced into at least 10 countries; known to hybridize with native Spartina species
Table 1. Origins and regions invaded by some common species of invasive aquatic and wetland plants. Origin as reported by USGS NAS database (2024); Region invaded based on data in GBIF (2024).
Figure
Clockwise,
Free-floating species such as Pontederia crassipes and Salvinia molesta can have numerous negative impacts on aquatic and wetland habitats. For example, these species can shade the underlying water, resulting in decreased oxygenation of the water by submersed plants and algae, affecting populations of fish and other aquatic animals. They also can impede navigation of waterbodies causing economic impacts from reduced use of the water for transport and costs associated with managing the invasive populations.
In addition to the above well-known ecological or economic impacts, invasive plants can sometimes have less obvious impacts, such as hybridization with native species or introduction of non-native genotypes into different parts of a species’ global range. Invasion by salt marsh grasses in the genus Spartina, for example, have led to hybridization between native and non-native Spartina species, forming hybrids that eventually invaded local wetlands, outcompeting native species of Spartina and other native plants. In fact, until relatively recently, it was thought that Spartina alterniflora was native to both North and South America. Bortolus and colleagues (2015), however, have provided several pieces of evidence suggesting this species was introduced into South America, rather than originating there. In the case of Phragmites australis, there are genotypes that are considered native to the United States, but multiple genotypes have also been introduced into the US, sometimes complicating conservation efforts for this species and wetlands it occupies (Saltonstall 2002; Kettering and Mock 2012).
Management of invasive plant populations can involve physical or mechanical disruption of local ecosystems through efforts to harvest and remove plant material, application of herbicides to kills the invasive species, or introduction of herbivores meant to control the nuisance plant populations. This latter management approach, termed biological control (biocontrol for short), has made use of highly specialized herbivorous insects to target the introduced plant species or generalist herbivores, such as grass carp, for the wholesale removal of vegetation in invaded habitats. The use of specialist insects has been attempted for control of invasive aquatic plants (Figure 9), but long-term success of these efforts typically requires repeated
releases of the insects. In the case of the generalist fish herbivores, there are many examples of these species escaping from managed waterbodies and invading other habitats, leading to negative impacts on native aquatic communities and impeding use of the waterbodies by people. Physical collection of plants must be conducted carefully, especially with free-floating species, as small segments of plants left behind often regenerate into complete plants.

There also are many examples of introduced animals affecting wetland vegetation. Many of these are mammalian herbivores. For example, the nutria (Myocastor coypus) was introduced into North America as part of the fur trade, and it has had substantial impacts on coastal wetlands of the Gulf of Mexico and Atlantic coast of the US. Beaver (Castor species) similarly have been introduced outside their native range, where they have impacted local wetlands, as have water buffalo (Bubalus bubalis) and wild hogs (Sus scrofa). The Everglades, a large complex of wetlands in southern Florida (US) have experienced substantial numbers of invasions, not just by plants, but also by animal herbivores, and predators. Well known invaders in the Everglades include green iguanas (Iguana iguana), pythons, snails, and cane toads (Rhinella marina), but many more are present. In fact, it is estimated that there are more than 130 species of invasive animals in the Everglades.
Figure 9. Specialist insects introduced into the United States as potential biological control agents for invasive plants. Left: alligatorweed thrips (Amynothrips andersoni) on Alternanthera philoxeroides (at tips of black arrows); Right: water hyacinth planthopper (Megamelus scutellaris) on Pontederia crassipes.
ACKNOWLEDGEMENTS
Many thanks are owed to Darold Batzer, Steven Pennings, Lori Sutter, and Arnold van der Valk for their helpful comments and suggestions on earlier versions of this paper.
REFERENCES
Bortolus, Alejandro, James T. Carlton, and Evangelina Schwindt. 2015. Reimagining South American coasts: unveiling the hidden invasion history of an iconic ecological engineer. Diversity and Distributions 21: 1267-1283.
Brown, Beverly J., Randall J. Mitchell, and Shirley A. Graham. 2002. Competition for pollination between an invasive species (purple loosestrife) and a native congener. Ecology 83: 2328-2336.
Ervin, Gary. 2023. The Biology of Aquatic and Wetland Plants. Boca Raton, FL: CRC Press.
GBIF (Global Biodiversity Information Facility). 2024. https://www.gbif.org
Keddy, Paul. 2023. Wetland Ecology: Principles and Conservation, 3rd ed. Cambridge, UK: Cambridge University Press.
Kettering, Karin M. and Karen E. Mock. 2012. Genetic diversity, reproductive mode, and dispersal differ between the cryptic invader, Phragmites australis, and its native conspecific. Biological Invasions 14: 24892504.
Lichvar, R. W., M. Butterwick, N. C. Melvin, and W. N. Kirchner. 2014. The National Wetland Plant List: 2014 Update of Wetland Ratings. U.S. Army Corps of Engineers. Phytoneuron 41: 1-42.
Saltonstall, Kristin. 2002. Cryptic invasion by a nonnative genotype of the common reed, Phragmites australis, into North America. Proceedings of the National Academy of Sciences 99: 2445-2449.
Sculthorpe, C D. 1967. Biology of Aquatic Vascular Plants. New York: St. Martin’s Press.
US Army Corps of Engineers. 2022. National Wetland Plant List, version 3.6. https://wetland-plants.sec.usace. army.mil/
USGS NAS (US Geological Survey Nonindigenous Aquatic Species) Database. 2024. https://nas.er.usgs. gov.
Vymazal, Jan. 2007. Removal of nutrients in various types of constructed wetlands. Science of the Total Environment 380: 48-65.
OTHER SUGGESTED READINGS
Batzer, Darold and Rebecca Sharitz, eds. 2006. Ecology of Freshwater and Estuarine Wetlands. Los Angeles, CA: University of California Press.
Cronk, Julie and Siobhan Fennessy. 2001. Wetland Plants: Biology and Ecology. Boca Raton, FL: Lewis Publishers.
Lázaro-Lobo, Adrián and Gary Ervin. 2021. Wetland invasion: A multi-faceted challenge during a time of rapid global change. Wetlands 41:64
Mitsch, William, James Gosselink, Christopher Anderson, and Siobhan Fennessy. 2023. Wetlands, 6th ed. Hoboken, NJ, USA: John Wiley & Sons, Inc.
Rodgers, Leroy, Christen Mason, Ryan Brown, Ellen Allen, Philip Tipping, Mike Rochford, Frank Mazzotti, et al. 2018. 2018 South Florida Environmental Report, Volume I, Chapter 7 : Status of Nonindigenous Species Vol. I. West Palm Beach, Florida.
Module 4: Basic Biology of Wetland Animals
Contributors: Darold Batzer,1 Lora L. Smith,2 Joshua D. Stafford,3 Violet Harrison-Day,4 Samuel R. Kucia,5 William J. Severud,5 and Youzheng Zhang6
OVERVIEW
An assortment of animals inhabit wetlands, with many invertebrates, amphibians and reptiles, fishes, birds, and some mammals being wetland specialists. Wetlands provide water, food, and cover that benefits resident animals, but periodic high floods, frequent drying, and often harsh water quality (low-oxygen, acidic, saline) can stress wetland animals. Animals successful in wetlands have developed adaptations to cope with these stresses. Animal biodiversity in wetlands is particularly valued by people, from ecotourists and bird watchers who observe it, to hunters and fishers who harvest aspects of it. Animals such as alligators and beavers create or physically modify wetland environment. This activity is called ecosystem engineering and makes these animals among the most important organisms to wetland ecology. Wetlands have high biodiversity and support rich communities of wetland invertebrates, and are primary breeding habitats for most amphibians, and many migratory birds rely on wetlands during some part of their annual cycle. The interactions of animals with plants (herbivory, cutting) and each other (predation) strongly shape wetland environments and affect nutrient cycling. Human impacts on wetlands can negatively affect many animals through habitat loss, resulting in many wetland amphibians, reptiles, and birds being listed as threatened and endangered species. Thus, wetland conservation is vital to conserve many animal species.
INVERTEBRATES
Invertebrates are particularly important in wetland ecosystems. Most of the biodiversity in wetlands is found among the invertebrates, and invertebrates are the primary energetic link between plants and higher
1 Department of Entomology, University of Georgia, Athens, GA 30605, USA
2 The Jones Center at Ichauway, Newton, GA 39870, USA
animals (such as fish and birds). Invertebrates can also be useful bioindicators of wetland habitat quality.
Common invertebrate groups in wetlands include insects (dragonflies, beetles, aquatic flies), crustaceans (shrimps, crabs, microcrustaceans), mollusks (snails, clams, oysters, mussels), annelids (earthworms, leeches), and nematodes (roundworms). To succeed, wetland invertebrates must contend with periodic drying and low-oxygen conditions (anoxia), and these processes directly influence which groups do or do not thrive. For example, stoneflies are common in streams but are rare in wetlands because their nymphs are intolerant of both drying and anoxia.
Contending with drying involves tolerance or avoidance (Figure 1). Many wetland invertebrates have stages that persist even after surface water disappears. For example, mosquitoes and some crustaceans (such as brine shrimps) have desiccation resistant propagules that persist in dry substrates for long periods, sometimes years, and when wetlands reflood, propagules hatch and a new life cycle is initiated. Many beetles and shrimps simply bury themselves in moist substrates to survive. Some crabs and crayfish dig burrows that retain a connection to subsurface water; these burrows also provide temporary refuge for other organisms. Many winged insects are adept at avoiding drying; adult females fly to newly flooded wetlands to lay eggs, and the immature stages develop rapidly so new adults emerge before wetlands dry (to fly to other wetlands).
Surviving anoxia also involves either tolerance or avoidance. Some midge larvae tolerate low oxygen conditions using features of anatomy (soft, tubular bodies that maximize surface area), physiology (ability to respire anaerobically; presence of oxygenbinding hemoglobin in haemolymph), and behavior (build U-shaped tubes where water can be circulated, quiescence). Oysters and some worms also have hemoglobin to outlast low-oxygen conditions in wetlands. Many aquatic insects do not rely on dissolved
3 U.S. Geological Survey, South Dakota Cooperative Fish and Wildlife Research Unit, Department of Natural Resource Management, South Dakota State University, Brookings, SD 57007, USA
4 Geography, Planning, and Spatial Sciences, University of Tasmania, Sandy Bay, Tasmania 7005, Australia
5 Department of Natural Resource Management, South Dakota State University, Brookings, SD 57007, USA
6 Key Laboratory of Engineering Oceanography, Second Institute of Oceanography, Ministry of Natural Resources, Hangzhou, 310012, China
oxygen in the water but instead breathe air by accessing surface air with respiratory tubes (mosquito and some beetle larvae), capturing air bubbles under their wings or along their bodies (adult beetles, water bugs), or accessing oxygen sources in or surrounding plants roots. Most estuarine invertebrates can respire during both dry and wet periods.
Invertebrates in tidal wetlands must also contend with changing salinity and wave and tide action. In most estuaries, water salinities change constantly over daily tidal cycles. To survive, estuarine invertebrates must either tolerate or respond quickly to drastic changes in these environmental conditions.
Behaviors of invertebrates in wetlands can be divided into instinctive behaviors related to their physiological functions (feeding, molting, reproduction), and responses to environmental factors (light, water flow, substrate, dissolved oxygen, salinity, temperature). Nematodes are the most diverse and abundant invertebrates in many wetlands. This broad group of species can exploit variation in wetland habitats using a wide array of feeding strategies, including algalfeeding, herbivory, bacterivory, fungivory, carnivory, and omnivory, and can detect environmental changes using chemoreceptors (called amphids). To grow, arthropods periodically molt; molting has the added benefit of shedding external parasites and permits regeneration of damaged limbs.
Some aquatic beetles and bugs, called cyclic colonizers, migrate back and forth between temporary water wetlands and permanent water aquatic habitats. They take advantage of the temporary wetlands as foodrich and predator-free habitats for their progeny, while avoiding wetland dry periods by taking refuge in permanent water bodies. Many estuarine invertebrates reside in the substrate during the day (often in burrows), where they are protected from desiccation and predators and can conserve energy, and then emerge and become active at night.
Two major ecological transitions shape invertebrate communities in freshwater wetlands: 1) a permanence transition, and 2) a predator (fish) transition (Figure 1). If a wetland dries, resident invertebrates must tolerate or avoid desiccation and develop rapidly before surface
water disappears; organisms lacking these abilities do not survive. Typical residents of temporary waters include mosquitoes, fairy shrimps, water fleas, water beetles, and water bugs. Permanent water wetlands can support these same taxa, but also other taxa poorly adapted for drying, such as snails, mayflies, and dragonflies. However, permanent waters can support fish, some species of which visually target larger, active invertebrates as prey and may reduce their abundances. Invertebrates coexisting with fish tend to be small, cryptic (camouflaged), and sedentary, such as midges and worms. Thus, temporary wetlands are dominated by fast-developing invertebrates adapted for drying; permanent wetlands with fish are dominated by small, sedentary, slow-developing invertebrates that can avoid predation; and permanent wetlands without fish are dominated by large, active invertebrates (Figure 1; see Wellborn et al. 1996).
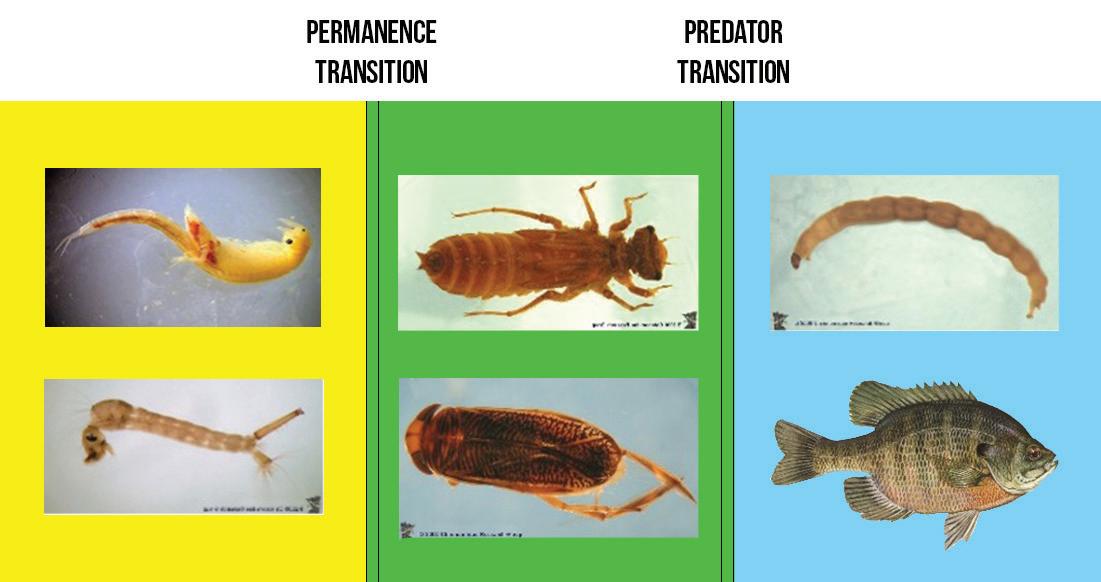
Invertebrates of freshwater wetlands are, however, notoriously difficult to predict with respect to abundances, distributions, and habitat associations (Batzer 2013). Most freshwater wetlands are highly variable in space and time and evolutionary and life histories of wetland invertebrates are adapted to this variability. Many wetland invertebrates are generalists adapted for environmental variation, coping with a range of hydrological and water quality conditions and consuming a range of foods (detritus, algae, other invertebrates). Conditions that develop in freshwater wetlands in any given year may be unknowable, and evolutionary strategies may develop that result in high
Figure 1. Key controls on wetland invertebrate assemblages: the permanence transition between temporary (yellow) and permanent (green, blue) waters and the predator transition between fish-bearing (blue) and fishless (green) permanent waters (adapted from Wellborn et al. 1996).
production in good years and high mortality in poor years.
Estuarine and tidal wetlands have been affected by tides for eons, leading to the gradual adaptation of invertebrates to environmental fluctuations (Jones and Candy 1981). The density and biomass of invertebrates are influenced by multiple factors (Levin and Talley 2002): organic detritus deposition controls nematode communities; temperature and silicate content controls polychaetes and echinoderms; salinity, dissolved oxygen and pH control mollusks; and inorganic nitrogen and phosphate control crustaceans. Polychaete worms grow best in muddy habitats with small grain sizes (Oyenekan 1986). Bivalves are burrowers and prefer habitats with sandy and clay substrates (Holland and Dean 1977). Salinity gradients act as physiological barriers to both saline marine species and freshwater species (Liu and He 2007). In general, estuarine invertebrate diversity increases with increased salinity. On the other hand, invertebrate densities tend to decrease with increased tidal levels, with burrowing species being most affected. Crabs of tidal flats follow zonal distributions along tidal gradients (Lee and Koh 1994).
More detail on invertebrate adaptations of invertebrates for wetland conditions can be found in Peterson (1991), Mendelssohn et al. (2014) and Batzer and Boix (2016). Lists of invertebrates common in freshwater and tidal wetlands can be found in Online Appendix 1 (https:// www.sws.org/education-modules-wetland-science/).
AMPHIBIANS AND REPTILES
The word amphibian is derived from the Greek amphibios, which means living both in water and on land. Most amphibians are closely tied to water for reproduction because their eggs are surrounded by a gelatinous capsule and are susceptible to desiccation. However, amphibian species range from being fully aquatic to truly amphibious (semiaquatic) and they occur in lotic (flowing water), lentic (still water), and ephemeral systems (relatively short-lived or quickdrying), and some even live an entirely terrestrial existence. Amphibians are grouped into three orders including the caecilians, Anura frogs, and the Urodela salamanders, which combined are estimated to include
>8400 species (Womack et al. 2022). They occur globally and inhabit freshwater, brackish, and even saltwater wetland systems from sea-level to mountain areas.
Amphibians display an array of reproductive adaptations for persisting across a gradient of wetland conditions. For example, species that breed in permanent water where large predators like fish are present may lay eggs singly or may have cryptically patterned larvae to avoid detection by predators. For species that breed in seasonally inundated wetlands, eggs may be laid on the moist substrate and hatch only after the wetland begins to fill, thus minimizing risk of predation. For other species using ephemeral wetlands, larval development to metamorphosis can be extremely rapid, allowing fully formed juveniles to exit before a wetland dries. Other species are not dependent on aquatic systems at all, with eggs developing to fully formed terrestrial juveniles on moist substrate, foam nests, or in small pools of water that collect in plants. A few species protect their eggs from desiccation by brooding eggs in special pouches in the oral cavity or on their backs, enveloped under a protective layer of skin (like the Pipa toads from Surinam).
With some exceptions, most amphibians have a biphasic life history with aquatic larvae that metamorphose to a juvenile stage (Figure 2a-b). Caecilians, which are earthworm-like in appearance with blunt heads for burrowing (Figure 2c), reproduce either through direct development to an adult form or have an aquatic larval stage. Larval caecilians and salamanders are morphologically similar to adults, whereas frogs produce free-swimming larvae (tadpoles) that undergo metamorphosis and transform into semi-aquatic or terrestrial frogs (see Online Appendix 2: https://www. sws.org/education-modules-wetland-science/). Many salamanders have a free-swimming larval stage, with external gills and a tail fin, and transform into terrestrial juveniles. However, some species (e.g., mole salamanders and striped newts) can attain sexual maturity and breed while in the aquatic larval life form with external gills and a tail fin, a phenomenon called paedomorphosis (see Online Appendix 2: https://www. sws.org/education-modules-wetland-science/). This allows salamanders to capitalize on opportunities to breed when water is available, rather than undergo
risky migrations through the terrestrial environment. Fully aquatic salamander taxa (e.g., Siren, Amphiuma) retain external gills and an aquatic larval morphology as adults.
Amphibians are an important component of wetland food webs, as both primary and secondary consumers, and as prey. Tadpoles filter particulate matter from the water column and may also use tooth-like keratinized mouth parts to scrape periphyton and algae from submerged macrophytes. Tadpoles undergo radical changes during metamorphosis, where larval mouth parts are replaced by jaws, teeth, and often a tongue, and the long-coiled intestine of herbivorous tadpoles becomes the shortened gut of a carnivorous frog. Salamanders are carnivorous as both larvae and adults and can be apex predators in some wetland systems (Urban 2013). Several salamander species develop a broad-headed morphotype that becomes cannibalistic under conditions of high density (Hoffman and Pfennig 1999). Both frogs and salamanders with aquatic larvae and terrestrial juvenile and adult stages transport nutrients across ecosystem boundaries (Atkinson et al. 2021, Smith et al. 2019, Bashinskiy et al. 2023).
Amphibians can occur in high abundance during breeding migrations or as juveniles emerging from wetlands. Although the movement of individuals can be considerable, studies documenting the magnitude of nutrient fluxes via amphibians are generally lacking. Amphibians are less common in saline wetlands (where they tend to suffer from dehydration) than in freshwater ones, but they are not entirely absent (Hopkins and Brodie 2015). More than 100 amphibian species are adapted to exploit coastal and inland saline habitats. The list includes representatives from 1 of the 10 caecilian families (10%), 5 of the 9 caudate families (56%), and 22 of the 56 anuran families (39%). Two anurans, the crab-eating frog and the European green toad, are among the most salt-tolerant species.





Reptiles (turtles and terrapins, lizards, snakes) are common in many saline and freshwater wetlands, and are particularly diverse in habitats occurring in warmer climates (tropics, sub-tropics). They can be the top predators in wetlands, with crocodilians (crocodiles, alligators, caiman; Figure 3), snapping turtles and some snakes feeding on an assortment of other vertebrates (amphibians, fish, birds, mammals, other reptiles). The thick skin of reptiles makes them more resistant than amphibians to saline conditions, and reptiles (most notably crocodilians) can be important components of saline wetland food webs. Given the predatory nature of so many reptilian species, it is important to exercise caution around them; however, most species will ignore you unless disturbed. American alligators (Figure 3) in wetlands of the southeastern United States (such as the Everglades, https://www.nps.gov/ever/learn/nature/ alligator.htm) can shape their environments by creating wallows, nests, and trails, which are exploited by many other plants and animals, an example of ecosystem engineering. Because alligators are both important engineers and predators, they and other large relatives are key species to the ecology of the wetlands where they reside (Somaweera et al. 2020).
Figure 2a. Adult and larva of the ornate chorus frog. (Photo: Gabe Miller)
Figure 2b. Adult and larva of the eastern tiger salamander. (Photo: Amanda Subalusky)
Figure 2c. Adult caecilian. (Photo: Kevin Stohlgren)
More details on amphibian and reptile diversity and their adaptations for wetland conditions can be found in Hopkins and Brodie (2015), Stebbins and Cohen (2021), and Zug (1993). A list of the taxonomic names of organisms listed in this subsection is available in Online Appendix 3 (https://www.sws.org/educationmodules-wetland-science/).
WETLAND FISHES
An assortment of fishes resides in wetlands (Batzer et al. 2014). Some migrate between deeper waterbodies, such as rivers, lakes or oceans, and adjacent wetlands. Others reside full-time in wetlands. Those species living in wetlands year-round tend to be small-bodied forms, such as killifishes, sticklebacks, mosquitofish, gobies or other diminutive species. Small immatures of larger fishes may use wetlands as nursery habitat, to hatch and grow, before migrating to deeper waters as adults. Often coastal wetlands form part of a mosaic of neighboring nursery habitats, which may also include seagrass, mudflats, and oyster reefs (Lefcheck et al. 2019). The ecology of many marine fisheries is highly dependent on coastal wetlands. Tidal coastal wetlands, such as mangroves and salt marshes, provide foraging and refuge opportunities for larvae and juveniles of species that are fished commercially or recreationally as adults, such as mullets (Figure 4), bream, and spot. Tidal coastal wetlands also provide foraging and refuge habitat for small fishes (and invertebrates) consumed as prey by the adult fisheries species.
Compared to lakes, rivers, and oceans, however, the number of fish species permanently residing in wetlands tends to be small, due to the harsh environmental conditions. Only a few fishes can tolerate habitat drying, and during drying events the majority of wetland fishes either migrate to deeper waters or perish from desiccation. The smallest species can persist
during dry periods in small residual pockets of water, such as crab/crayfish burrows or tree rot holes. Some of the killifishes can survive drying and hatch from eggs or revive when wetlands reflood (much as invertebrates do; see above). Interesting exceptions to this rule include the living fossil lungfishes; these air-breathing fish can not only move across grasslands in search of a new wet pond, but they can also endure regular periods of drought by digging into the mud and covering themselves with mucus that prevents dehydration. Due to periodic drying or isolation, many depressional wetlands are either completely fishless, or support only one or two species (such as mosquitofish, sticklebacks) (Zimmer et al. 2002).

Besides habitat drying, another major constraint on fishes in wetlands is a lack of oxygen in the water. Respiration with gills requires sufficient dissolved oxygen levels to be effective, and only a few fish species can tolerate hypoxia. Some fishes (such as carp) will gulp air to survive temporary periods of oxygen stress. Salinity is another important constraint on some fishes, especially in salt marsh, mangrove, or estuarine habitats. Fishes in brackish (moderately saline) water tend to either have a connection to the adjacent saltwater habitat or alternatively to the adjacent freshwater habitat, but rarely both. Fish productivity in brackish waters can, however, be very high because of these connections, and because of the abundant invertebrate food resources found in estuaries. Some fishes (such as some hardyheads) have wide salinity tolerances and survive easily in wetlands subject to changing salinities. Other fishes (such as eels and salmon) occupy saline and brackish wetlands as transitory visitors when moving between marine and freshwater environments during different life stages. Finally, acidity is another water quality constraint on
Figure 3. American alligator. (Photo: Jonathan Freedman, U.S. Geological Survey)
Figure 4. A juvenile mullet, a common saltmarsh fish. (Photo: Violet Harrison-Day)
fishes, and highly acidic (low pH) peatlands tend to support only a few fish taxa. Acid conditions impair some physiological processes in fishes (Mendelssohn et al. 2014).
Historically, many coastal wetlands were converted from saline or brackish marshes to freshwater wetlands. Changes to salinity and other aquatic conditions in these wetlands led to a transition from salt tolerant to freshwater fish assemblages. Interest in returning tidal flows to impounded and levied coastal wetlands (for fisheries and other ecosystem service benefits) is growing, however, and many are being restored, allowing saline-tolerant wetland fishes to return (Raposa and Talley 2012). The restoration process requires careful planning, taking into consideration the site-specific habitat characteristics, conditions, and fish species currently and previously present. The presence or absence of fishes is considered one of the major controls on other wetland animals. Large fishes may be the top aquatic predators in wetlands, and as visual predators tend to target some of the larger, more-active prey animals. Many amphibians (tadpoles, salamanders) and large, active-swimming invertebrates (water bugs, beetles) do poorly in the presence of fish predators, and the overall structure of animal communities in many freshwater wetlands is determined by fishes (Wellborn et al. 1996; see Figure 1). Fish predation may indirectly promote algal growth by reducing the numbers of invertebrate grazers. In tidal wetlands, shallow vegetated habitat can provide important refuge for small fishes (juveniles and small bodied adults) that are avoiding predation by large fishes. Different fishes can occupy tidal habitats at different times and water depths, balancing the benefits of refuge (and foraging opportunities) with the risks of stranding, adverse water conditions, and the energetic costs of travelling with or against the tide (Rountree and Able 2007). Fish themselves are primary food items for many wetland birds and mammals. For example, wading birds such as herons, egrets, and storks target fish prey in shallow waters, and thus find wetlands to be critical foraging habitats (Batzer et al. 2014). Similarly, American mink, North American river otter, and brown bear rely on fish found in wetland ecosystems. In addition to predation, fishes can exert other ecological influences. Bioturbation of substrates by
carp can affect wetland water clarity and water quality (Batzer et al. 2014). Excretion by fishes can fertilize wetland waters, affecting algal growth.
Many freshwater wetlands are naturally fishless, and the introduction of fishes into these habitats can be ecologically damaging. Zimmer et al. (2002) found that fathead minnows can exert important ecological controls on invertebrates and amphibians in natural prairie potholes of north central North America, and introductions of these fish into otherwise fishless potholes (to commercially rear them as baitfish) is a concern. The mosquitofish is native to the southeastern United States, but the species has been actively introduced to many other places in an effort to control mosquitoes. Thus, the mosquitofish is now the most wide-spread freshwater fish in the world and can have unintended negative effects on native species (Pyke 2008).
Introduced plant species can also affect wetland habitat conditions for fishes. Rapidly spreading salt marsh grasses such as cordgrass (Spartina spp.) were introduced in many parts of the world to help stabilize shorelines and have become major invaders of native wetland habitats. Changes to habitat structure and conditions resulting from vegetation change may affect nursery habitat quality, prey type and availability, and accessibility of the habitat for fishes.
A list of the taxonomic names of organisms listed in this subsection is available in Online Appendix 3 (https:// www.sws.org/education-modules-wetland-science/).
WETLAND BIRDS
Many birds rely on wetlands for some portion of their annual cycle; thus, conservation and management of wetlands is critical to bird conservation. Approximately 138 species of birds in the United States are considered obligate wetland species, meaning that their survival and reproduction is entirely dependent on resources provided by wetlands; these include ducks, geese, swans, cranes, herons, rails, plovers, sandpipers, grebes, storks, and various passerines, such as some blackbirds and sparrows. An even larger group of birds are facultative wetland species and use wetlands for certain critical life-history events, such as foraging, roosting, nesting, and brood rearing (e.g., many passerines, owls, and hawks). Although many bird species are declining,
waterbirds in general, and ducks in particular, have increased 18 percent and 34 percent, respectively, in North America since 1970 (North American Bird Conservation Initiative, U.S. Committee 2017).
Birds that depend heavily on wetlands have many adaptations to exploit wetland resources (Table 1). Most species are migratory, primarily because of the transient nature of wetlands and the resources they provide. In many regions, some of the most productive wetlands for birds have temporary or seasonal water regimes, meaning they only contain water for a few weeks or months. These wetlands provide abundant invertebrates and seeds that offer forage for birds when inundated. Due to the variable hydrologic conditions, birds must not only be able to exploit wetland foods when there is water but also be able to relocate or otherwise adapt during dry periods. In a similar fashion, where winters are cold and wetlands freeze, birds have evolved a mostly north-south pattern of seasonal movements in the spring and autumn.
Anatomy and Morphology
Leg placement for easier swimming and diving
Bone and lung modifications for light weight and oxygen intake at altitude
Eye adaptations for underwater vision to aid foraging
Flight (wing) adaptations for diving or taking off from water
Webbed and lobed feet for swimming and diving
Long legs for wading to improve foraging efficiency
Bill and beak specializations for filtering, grasping, digging
Oil glands for water resistance
Countercurrent heat exchange to warm legs and feet in wet and cold conditions
Behavioral
Water-responsive behaviors, such as preening, drying
Specific foraging strategies, such as dabbling, tipping-up, diving
Seasonally variable social behaviors, gregariousness vs. spacing and aggression
Energetic efficiencies, use of thermal cover for roosting
In some cases, diet plasticity in response to wetland availability
Predation avoidance strategies, hiding, safety in numbers
Unique foraging behaviors, including kleptoparasitism
Table 1. Some avian adaptations to wetland habitats (adapted from Weller 1999). More information on adaptations of birds to wetlands may be found in Weller (1981) and Baldassarre (2014).
Once a waterbird arrives at a wetland and determines that conditions are adequate to settle on that site (e.g., Johnson 1980), it must be able to exploit available resources to meet various life-history needs. To this end, evolution has shaped many adaptations to exploit specific wetland resources while avoiding competition and depredation (Table 1). Most wetland birds eat plant material (e.g., seeds), invertebrates, or both and these foods exist in wetlands at varying water depths and sediment types. Duck bill morphology provides an excellent example of phenotypes that evolved to forage efficiently and promote niche partitioning (evolved strategies by which species can coexist when there is competition for resources), thereby minimizing competition. Specifically, bill lamellae, which are comb-like structures that line the upper and lower bills of ducks, vary considerably among duck species, and relate directly to the foraging niche they occupy. Ducks that mostly feed in shallow water are called dabbling ducks. For example, the northern shoveler has a scoop-like bill with fine, dense lamellae that allows them to efficiently strain invertebrates from shallow water (Figure 5). In contrast, some ducks such as canvasbacks can dive and have bills with thick, less dense lamellae that help them forage in deeper water and dense sediments for hard-shelled invertebrates (e.g., fingernail clams) or the roots and tubers of submersed aquatic plants. Intermediate examples include the wood duck, which eats plant seeds and invertebrates and has a shorter bill with less-dense lamellae and prominent bill nails to aid in grabbing submerged acorns or plant bulbs. Finally, mergansers eat almost exclusively animal matter; their bill resembles a set of needle-nose pliers with a sharp hook for grasping fish, crayfish, or other aquatic organisms. Their teeth-like lamellae aid in retaining prey even though ducks do not chew their food.
Body shapes, eye positions, and foot and leg morphologies also help birds exploit wetlands (Table 1). Bitterns, egrets, and herons have long legs that allow them to hunt for prey in water of various depths, and long necks that enable a top-down view of aquatic prey that reduces surface glare. They also possess sharp,
knife- or spear-like bills for impaling or grabbing fish, amphibians, and larger invertebrates. Their eyes are placed on the side of their head to promote peripheral vision for detecting threats yet are far enough forward to offer some binocular vision important to predators that stalk their prey.
Because birds are often prey themselves, their morphologies balance the needs of foraging and vigilance (i.e., predator avoidance). Northern shovelers and Eurasian green-winged teal typically filter feed with their eyes above the water surface; lateral eye placement allows peripheral vision of 220°, so they can see in front, above, and behind while feeding (Guillemain et al. 2002). In contrast, Eurasian wigeon have eye placement that puts the bill in front of the field of vision, restricting its field of view and causing a slight blind spot behind the head (Guillemain et al. 2002). A tradeoff for improved forward vision requires them to spend more time with their head up scanning for predators, reducing total foraging time.
Variation in leg length and bill size among shorebirds allows them to all forage in the same wetlands for the same prey species with minimal competition. The curlew, American oystercatcher, and sanderling may occur at the same sites, but each targets different sizeclasses of clam prey (Myers et al. 1980; Zwarts and Wanink 1984). Different shore birds have evolved different foraging strategies, such as visually pecking (e.g., willet, killdeer) versus feeding tactilely (e.g., Pectoral sandpiper), which allows for coexistence with less competitive overlap (Rojas et al. 1999; Thomas et al. 2006). Visual feeders run a short distance, quickly stop and watch for movement from prey which they grab from the surface when spotted. In contrast, longbilled shorebirds probe deep into mud substrates to feel and capture prey not visible from the surface. Despite being unable to see their prey, some shorebirds have finely tuned sensory mechanoreceptors in their bill tips that help them identify vibrations of buried invertebrates (Gerritsen and Meijboom 1986; Piersma et al. 1998).
Bird abundance and species composition in wetlands are driven primarily by: 1) hydrologic regime; 2) heterogeneity of avian habitat features (mixtures of water and plants); and 3) geographic location. In general, wetlands with fluctuating water levels are
more productive ecosystems due to increased nutrient cycling, vegetation regeneration, and abundant macroinvertebrates (Harris and Marshall 1963; Niemuth et al. 2014). More stable wetlands, however, serve as refugia when water levels are low (Mattsson et al. 2012).
Depressional wetlands experience periodic drawdown and reflooding, which strongly influence their avian communities. Northern pintails in North America, for example, vary the latitude at which they breed depending on how wet or dry wetlands are when they arrive in spring (Figure 5). During periods of drought, they may migrate further north while readily settling into southern areas in times of deluge (Mattsson et al. 2012). However, periodic drawdown and reflooding of wetlands is critical to maintaining productivity and the most diverse avian communities are typically found after wetlands reflood (Fredrickson and Taylor 1982; Taft et al. 2002; Farley et al. 2022).
The most important factor influencing abundance and diversity of wetland birds is the relative availability of multiple habitat types at local (i.e., within wetland and immediate watershed) and landscape extents. Due to niche partitioning, each bird species thrives in slightly different conditions (see above). Thus, avian diversity typically increases as heterogeneity of wetland resources (e.g., cover, forage) increases (Figure 5). Wetlands with interspersed submergent and emergent aquatic vegetation that approximate a 50:50 cover to water ratio (i.e., a hemi-marsh; Weller and Spatcher 1965; Masto et al. 2022) generally support the greatest abundance and diversity of wetland birds (Smith et al. 2004). Bird use is driven more by the structure and cover patterns within a wetland than the actual species composition of plants (Fairbairn and Dinsmore 2001). Copious emergent vegetation can support multiple vegetation zones and, therefore, greater habitat and bird diversity (Stewart and Kantrud 1971). At the landscape extent the greatest avian diversity is usually supported by a high density of wetlands (Webb et al. 2010; Fairbairn and Dinsmore 2001) that encompass all stages of the wetland cycle, offer greater wetland area, and an assortment of cover types and water depths, which each fill the requirements for foraging, loafing, or roosting habitats for individual species. To sustain wetland birds, conserving wetland complexes at local and landscape scales is an important consideration.
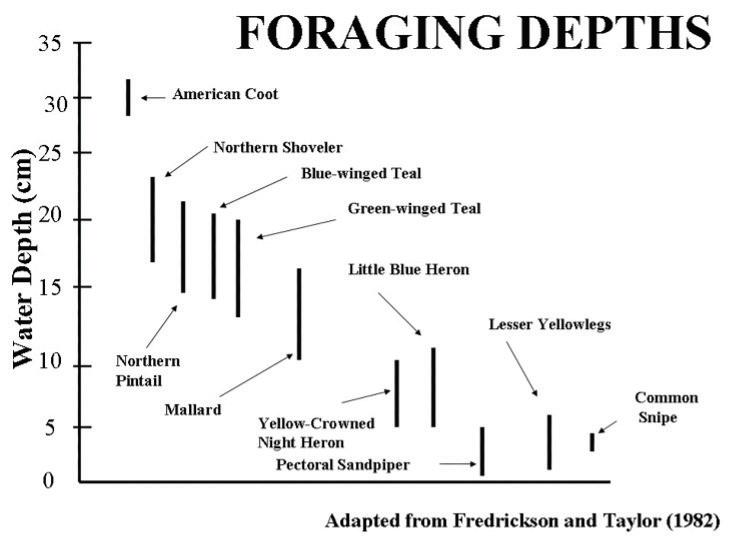
Wetlands in many regions are considered endangered ecosystems due to climate and land-use pressures driving habitat fragmentation, degradation, and loss. Conversion of wetlands to agriculture has been a primary driver of wetland loss. In North America, king rail, sora, Virginia rail, and least bittern are notable wetland-dependent species that forage in areas of wetlands with emergent vegetation and highstem densities that have been negatively impacted by human development (Rahlin et al. 2022); these species populations are estimated to have declined by ~25% since 1970 (Bolenbaugh et al. 2012). For most wetlanddependent birds, habitat loss in breeding areas may result in reduced population sizes or in the forcing of birds to less-suitable habitats where reproduction and survival may be lower (Stewart 1996). Because so many bird species use wetlands for food, shelter, and/ or breeding (Kroodsma 1979), these ecosystems need protection to prevent species loss and ensure healthy populations into the future.
A list of the taxonomic names of organisms listed in this subsection is available in Online Appendix 3 (https:// www.sws.org/education-modules-wetland-science/).
Wetland Mammals
Of the 6,399 recognized extant mammal species (Burgin et al. 2018), approximately 4,000 have evolved to exploit wetland habitats and depend on wetland resources to meet at least some life-history needs (Vaughan et al. 2013). As with other terrestrial taxa, there is a gradient in the dependence of individual
species on wetland resources, with some species being entirely dependent on wetlands (e.g., muskrats, capybaras, and beavers). Many other species of mammals are facultative wetland species and may thrive in wetland habitats but are not totally dependent on them for survival and reproduction and may persist where wetlands are scarce (e.g., raccoon). Finally, many species of mammals do not explicitly associate with wetlands but benefit from the resources they provide when available (e.g., white-tailed deer, American black bears). This gradient in wetland dependence is reflected in mammalian species morphology and behaviors.
Mammals play important roles in wetland ecosystems. As consumers, mammals may eat invertebrates, plants, birds, fish, and even other mammals, all of which may be abundant in and near wetlands (Dertien et al. 2020). These feeding activities add nutrients to wetlands, may cultivate the soil, and can influence vegetation patterns and abundance (May 2001). Mammals that transfer nutrients between aquatic and terrestrial systems are deemed ecotone specialists (Johnston 2017; Tischler et al. 2022). For example, brown bears feed extensively on salmon and move nutrients from the river to adjacent floodplain and upland areas. Similarly, bats feed on aerial insects with aquatic life stages, connecting the aquatic and terrestrial realms. Conversely, beavers fell trees and those terrestrially sourced resources increase the nutrient content of wetlands as well as provide underwater structure for aquatic species.
Some mammals hold the special distinction of being able to alter or create wetland habitats. The bestknown example is the North American beaver, which creates extensive networks of aquatic areas through their works, such as building dams and developing canals. These activities can create new wetlands that not only benefit the beavers themselves, but a host of other species and ecosystem services (Johnston 2017). The muskrat, although a much smaller rodent than beavers, can also alter wetlands through their foraging patterns (Bomske and Ahlers 2021). Muskrats create heterogeneity in vegetation structure in marshy wetlands and can create open water areas that are used by a variety of taxa. An even smaller rodent, the North American water vole, acts as an ecosystem engineer via its burrowing activities which enhance diversity of riparian vegetation (Bryce et al. 2013).
Figure 5. Relationship of some wetland-dependent bird species to variation in water depth.
The activities of large mammals may actually alter waterways themselves. Hippopotamuses can wear down deep channels and expand wetlands to surrounding areas. Elephants can similarly build water channels as they move between isolated ponds and rivers, and their tracks can eventually link distinct wetlands. Asian elephants divert monsoon rains directly into rivers (Sidle and Ziegler 2010). The wallows of American bison and peccaries in the Neotropics create ephemeral wetlands that are used by frogs (Gerlanc and Kaufman 2003; Beck et al. 2010).
Because wetland ecosystems are threatened throughout the world, some mammals have also experienced significant population declines due to losses of critical wetland habitats. One of the best-known and charismatic examples is the Florida panther, which was on the brink of extinction for many years. Results of GPS tracking research have shown these animals tend to spend their days in and around wetlands, particularly cypress swamps, and move into grasslands at night (Onorato et al. 2011). Their diet reflects their tendency to use wetlands, including items such as waterfowl and alligators (McBride and McBride 2010). In recent decades, the population of Florida panthers has increased due to significant conservation actions and genetic management, but human development in southern Florida continues to put pressure on this iconic species. In South America, the aquatic rat is an endangered rodent endemic to Ecuador and Colombia. Although little is known about this species, it is semiaquatic and relies on arthropods and other insects that are abundant in wetlands. It appears the species has a small native range, which makes it especially vulnerable to disturbances to aquatic habitats, such as pollution or wetland loss. Therefore, conservation efforts for this species may consider protection of critical habitats within its narrow areas of distribution. As with the other aforementioned taxa, many wetland habitats that mammals rely on are threatened by loss or degradation. These species evolved to exploit the seasonally abundant resources that wetlands provide. In addition to efforts to restore and protect aquatic ecosystems everywhere they are under threat, management is beginning to re-embrace wetland reliant mammals as important parts of aquatic ecosystems. For example, ranchers in some areas of the Western
U.S. now support increased North American beaver abundances and the natural infrastructure they provide, such as ponded water that cattle may use (e.g., https:// www.beefmagazine.com/policy/beaver-power-providesyear-long-water-to-idaho-ranch). Such attitudes towards aquatic species may help preserve wetlands systems while taking advantage of the ecosystem services they can provide.
A list of the taxonomic names of organisms listed in this subsection is available in Online Appendix 3 (https:// www.sws.org/education-modules-wetland-science/).
Disclaimer
Any use of trade names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Link to Supplemental Online Appendices: https://www. sws.org/education-modules-wetland-science/
REFERENCES
Atkinson, C.L., D.D. Knapp, and L.L. Smith. 2021. Long-term patterns of amphibian diversity, abundance and nutrient export from small, isolated wetlands. Diversity 13(11): 598.
Baldassarre, G.A. 2014. Ducks, Geese, and Swans of North America. Johns Hopkins University Press.
Figure 6. A North American beaver. (Photo: Courtesy of Kansas State University Research and Extension Service)
Bashinskiy, I.W., Y.Y. Dgebuadze, N.N. Sushchik, V.V. Osipov, and M.L. Gladyshev. 2023. Spadefoot Pelobates vespertinus (Amphibia, Pelobatidae) as a transmitter of fatty acids from water to land in a foreststeppe floodplain. Science of The Total Environment p.162819.
Batzer, D.P. 2013. The seemingly intractable ecological responses of invertebrates in North American wetlands: a review. Wetlands 33: 1-15.
Batzer, D.P. and D. Boix (eds.). 2016. Invertebrates in Freshwater Wetlands. Springer International, Switzerland.
Batzer, D. P., R. Cooper, and S. A. Wissinger. 2014. Wetland animal ecology. In Ecology of Freshwater and Estuarine Wetlands. pp. 151-183. University of California Press.
Beck, H., P. Thebpanya, and M. Filiaggi. 2010. Do Neotropical peccary species (Tayassuidae) function as ecosystem engineers for anurans? Journal of Tropical Ecology 26: 407–414.
Bolenbaugh, J.R., T. Cooper, R.S. Brady, K.L. Willard, and D.G. Krementz. 2012. Population status and habitat associations of the King Rail in the Midwestern United States. Waterbirds 35: 535-45.
Bomske, C. M. and A. A. Ahlers. 2021. How do muskrats Ondatra zibethicus affect ecosystems? A review of the evidence. Mammal Review 51: 40–50.
Bryce, R., R. Van Der Wal, R. Mitchell, and X. Lambin. 2013. Metapopulation dynamics of a burrowing herbivore drive spatio-temporal dynamics of riparian plant communities. Ecosystems 16: 1165–1177.
Burgin, C.J., J.P. Colella, P.L. Kahn, and N.S. Upham. 2018. How many species of mammals are there? Journal of Mammalogy 99: 1–14.
Dertien, J.S., S. Self, B.E. Ross, K. Barrett, and R.F. Baldwin. 2020. The relationship between biodiversity and wetland cover varies across regions of the conterminous United States. PLOS ONE 15: e0232052.
Fairbairn, S.E. and J.J. Dinsmore. 2001. Local and landscape-level influences on wetland bird communities
of the prairie pothole region of Iowa, USA. Wetlands 21:41-47.
Farley, E.B., M.L. Schummer, D.J. Leopold, J.M. Coluccy, and D.C. Tozer. 2022. Influence of water level management on vegetation and bird use of restored wetlands in the Montezuma Wetlands Complex. Wildlife Biology 2022: e01016. https://doi.org/10.1002/ wlb3.01016
Fredrickson, L.H. and T.S. Taylor. 1982. Management of seasonally flooded impoundments for wildlife. U.S. Dep. of Interior Resource Publication 148. 29 pp.
Gerlanc, N.M. and G.A. Kaufman. 2003. Use of bison wallows by anurans on Konza Prairie. American Midland Naturalist 150: 158–168.
Gerritsen, A.F.C. and A. Meijboom. 1986. The role of touch in prey density estimation by Calidris alba. Netherlands Journal of Zoology 36: 530–562.
Guillemain, M., G.R. Martin, and H. Fritz. 2002. Feeding methods, visual fields, and vigilance in dabbling ducks (Anatidae). Functional Ecology 16: 522–529.
Harris, S.W. and W.H. Marshall. 1963. Ecology of water-level manipulations on a northern marsh. Ecology 44: 331–343. https://doi.org/10.2307/1932180
Hoffman, E.A. and D.W. Pfennig. 1999. Proximate causes of cannibalistic polyphenism in larval tiger salamanders. Ecology 80: 1076-1080.
Holland, A. F. and J. M. Dean. 1977. The biology of the stout razor clam Tagelus plebeius: II. Some aspects of the population dynamics. Chesapeake Science 18: 188196.
Hopkins, G.R. and E.D. Brodie, Jr. 2015. Occurrence of amphibians in saline habitats: a review and evolutionary perspective. Herpetological Monographs 29: 1-27.
Johnson, D.H. 1980. The comparison of usage and availability measurements for evaluating resource preference. Ecology 61: 65–71.
Johnston, C.A. 2017. Beavers: Boreal Ecosystem Engineers. Springer International Publishing, Cham. http://link.springer.com/10.1007/978-3-319-61533-2.
Jones, G., and S. Candy. 1981. Effects of dredging on the macrobenthic infauna of Botany Bay. Marine & Freshwater Research 32: 379-398.
Kroodsma, D.E. 1979. Habitat values for nongame wetlands birds. In Greeson, P. E., J. R. Clark, and J. E. Clark (eds.). Wetland Functions and Values – The State of our Understanding. American Water Resources Association, Minneapolis MN. p. 320-343.
Lee, Y. H. and C.H. Koh.1994. Biogenic sedimentary structures on a Korean mud flat: spring-neap variations. Netherlands Journal of Sea Research 32: 81-90.
Lefcheck, J.S., B.B. Hughes, A.J. Johnson, B.W. Pfirrmann, D.B. Rasher, A. R. Smyth, B. L. Williams, M. W. Beck, and R. J. Orth. 2019. Are coastal habitats important nurseries? A meta-analysis. Conservation Letters 12: e12645.
Levin, L.A. and T.S. Talley. 2002. Natural and manipulated sources of heterogeneity controlling early faunal development of a salt marsh. Ecological Applications 12: 1785-1802.
Liu, W. and W. He. 2007. The Benthic Macroinvertebrates in the Yangtze Estuary. Shanghai Scientific & Technical Publishers.
Masto, N. M., R. M. Kaminski, and H. H. Prince. 2022. Hemi-marsh concept prevails? Kaminski and Prince (1981) revisited. Journal of Wildlife Management 86: e22301. DOI: 10.1002/jwmg.22301.
Mattsson, B. J., M. C. Runge, J. H Devries, G. S. Boomer, J. M. Eadie, D. A. Haukos, J. P. Fleskes, D. N. Koons, W. E. Thogmartin, and R. G. Clark. 2012. A modeling framework for integrated harvest and habitat management of North American waterfowl: Casestudy of northern pintail metapopulation dynamics. Ecological Modelling 225: 146–158.
May, L. H. 2001. Wetland Mammals. U.S. Department of Agriculture, Natural Resource Conservation Service, Fish and Wildlife Habitat Management Leaflet 21.
McBride, R. and C. McBride. 2010. Predation of a large alligator by a Florida panther. Southeastern Naturalist 9: 854–856.
Mendelssohn, I. A., D. P. Batzer, C. R. Holt, and S. A. Graham. 2014. 3. Abiotic Constraints for Wetland Plants and Animals. In Batzer, D. P and R. R. Sharitz (eds.) Ecology of Freshwater and Estuarine Wetlands pp. 61-86. University of California Press.
Myers, J. P., S. L. Williams, and F. A. Pitelka. 1980. An experimental analysis of prey availability for Sanderlings. (Aves: Scolopacidae) feeding on sandy beach crustaceans. Canadian Journal of Zoology 58: 1564-1574.
Niemuth, N.D., K.K. Fleming, and R.E. Reynolds. 2014. Waterfowl conservation in the US Prairie Pothole Region: confronting the complexities of climate change. PLoS One 9(6). DOI: 10.1371/journal.pone.0100034
North American Bird Conservation Initiative, U.S. Committee. 2017. The State of the Birds 2017: A Farm Bill Special Report. Cornell Lab of Ornithology, Ithaca, NY.
Onorato, D. P., M. Criffield, M. Lotz, M. Cunningham, R. McBride, E. H. Leone, O. L. Bass Jr, and E. C. Hellgren. 2011. Habitat selection by critically endangered Florida panthers across the diel period: implications for land management and conservation. Animal Conservation 14: 196–205.
Oyenekan, J. A. 1986. Population dynamics and secondary production in an estuarine population of Nephtys hombergii (Polychaeta: Nephtyidae). Marine Biology 93: 217-223.
Peterson, H. C. 1991. Intertidal zonation of marine invertebrates in sand and mud. American Scientist 79: 236-249.
Piersma, T., R. van Aelst, K. Kurk, H. Berkhoudt, and L. R. M. Maas. 1998. A new pressure sensory mechanism for prey detection in birds: the use of principles of seabed dynamics? Proceedings of the Royal Society of London B 265: 1377–1383.
Pyke, G. H. 2008. Plague minnow or mosquito fish? A review of the biology and impacts of introduced Gambusia species. Annual Review of Ecology, Evolution, and Systematics 39: 171-191.
Rahlin, A. A., S. P. Saunders, and S. Beilke. 2022. Spatial drivers of wetland bird occupancy within an urbanized matrix in the Upper Midwestern United States. Ecosphere 13(9). https://doi.org/10.1002/ ecs2.4232
Raposa, K.B. and D.M. Talley. 2012. A meta-analysis of nekton responses to restoration of tide-restricted New England salt marshes. In Roman, C. T. and D. M. Burdick (eds) Tidal Marsh Restoration. The Science and Practice of Ecological Restoration. pp. 97-118. Island Press, Washington, DC.
Rojas, L. M., R. McNeil, T. Cabana, and P. Lachapelle. 1999. Diurnal and nocturnal visual capabilities in shorebirds as a function of their feeding strategies. Brain, Behaviour and Evolution 53: 29–43.
Rountree, R.A. and K.W. Able. 2007. Spatial and temporal habitat use patterns for salt marsh nekton: implications for ecological functions. Aquatic Ecology 41: 25-45.
Sidle, R.C., and A.D. Ziegler. 2010. Elephant trail runoff and sediment dynamics in northern Thailand. Journal of Environmental Quality 39:871–881.
Smith, L. L., A. L. Subalusky, C. L. Atkinson, J. E. Earl, D. M. Mushet, D. E. Scott, S. L. Lance, and S. A. Johnson. 2019. Biological connectivity of seasonally ponded wetlands across spatial and temporal scales. Journal of the American Water Resources Association 55: 334-353.
Smith, L. M., D. A. Haukos, and R. M. Prather. 2004. Avian response to vegetative pattern in playa wetlands during winter. Wildlife Society Bulletin 32: 474–480.
Somaweera, R., J. Nifong, A. Rosenblatt, et al. 2020. The ecological importance of crocodylians: towards evidence-based justification for their conservation. Biological Reviews 95: 936-959.
Stebbins, R. C. and N. W. Cohen. 2021. A Natural History of Amphibians. Princeton University Press: Princeton, NJ.
Stewart, R. E., Jr. 1996. Technical aspects of wetlands: wetlands as bird habitat. Washington, D.C.: U.S.
Government Printing Office National water summary on wetland resources Report No. 2425.
Stewart, R.E., and H.A. Kantrud. 1971. Classification of natural ponds and lakes in the glaciated prairie region. U.S. Fish and Wildlife Service. Resource Publication 92.
Taft, O. W., M. A. Colwell, C. R. Isola, and R. J. Safran. 2002. Waterbird responses to experimental drawdown: implications for the multispecies management of wetland mosaics. Journal of Applied Ecology 39: 987–1001.
Thomas, R. J., T. Szekely, R. F. Powell, and I. C. Cuthill. 2006. Eye size, foraging methods and the timing of foraging in shorebirds. Functional Ecology 20: 157–165.
Tischler, K. B., W. J. Severud, R. O. Peterson, J. A. Vucetich, and J. K. Bump. 2022. Aquatic areas provide high nitrogen forage for moose (Alces alces) in Isle Royale National Park, Michigan, USA. Alces 58: 75–90.
Urban, M.C. 2013. Evolution mediates the effects of apex predation on aquatic food webs. Proceedings of the Royal Society B: Biological Sciences 280(1763):20130859.
Vaughan, T. A., J. M. Ryan, and N. J. Czaplewski. 2013. Mammalogy. 6th edition. Jones & Bartlett Learning, Burlington, Mass.
Webb, E.B., L.M. Smith, M.P. Vrtiska, and T.G. LaGrange. 2010. Effects of local and landscape variables on wetland bird habitat use during migration through the Rainwater Basin. Journal of Wildlife Management 74:109–119. DOI: 10.2193/2008-577.
Wellborn, G.A., D.K. Skelly, and E.E. Werner. 1996. Mechanisms creating community structure across a freshwater habitat gradient. Annual Review of Ecology and Systematics 27: 337-363.
Weller, M.W. 1981. Freshwater Marshes, Ecology and Wildlife Management. University of Minnesota Press, Minneapolis.
Weller, M. 1999. Wetland Birds: Habitat Resources and Conservation Implications. Cambridge: Cambridge University Press. doi:10.1017/CBO9780511541919
Weller, M.W. and C.E. Spatcher. 1965. Role of habitat in the distribution and abundance of marsh birds. Special Report 43. Iowa Agriculture and Home Economics Experiment Station, Iowa State University of Science and Technology.
Womack, M.C., E. Steigerwald, D.C. Blackburn, D.C. Cannatella, A. Catenazzi, J. Che, M. S. Koo, J. A. McGuire, S.R. Ron, C.L. Spencer, and V.T. Vredenburg. 2022. State of the Amphibia 2020: A review of five years of amphibian research and existing resources. Ichthyology & Herpetology 110: 638-661.
Zimmer, K.D., M.A. Hanson, and M.G. Butler. 2002. Effects of fathead minnows and restoration on prairie wetland ecosystems. Freshwater Biology 47: 20712086.
Zug, G.R. 1993. Herpetology: An Introductory Biology of Amphibians and Reptiles. Academic Press: San Diego, CA.
Zwarts, L. and J. Wanink. 1984. How oystercatchers and curlews successively deplete clams. In Evans, P. R., J. D. Gross-Custard, and W. G. Hale (eds). Coastal Waders and Wildfowl in Winter. Chapter 4. Cambridge University Press.
Module 5: Wetland Community Assembly
Contributors: Steven C. Pennings, Department of Biology and Biochemistry, University of Houston, Houston TX 77204, USA
Alejandro Bortolus, Instituto Patagónico para el Estudio de Ecosistemas Continentales (IPEEC), CONICET, Boulevard Brown 2915, Puerto Madryn (U9120ACD), Chubut, Argentina
OVERVIEW
Wetland habitats are occupied by species that can tolerate flooded and—in the case of coastal wetlands— salty soils (Figure 1). Relatively few species can tolerate these conditions, but species with the right adaptations can be very abundant in wetland habitats. Wetlands vary in how stressful the conditions are, and plant and animal biomass varies correspondingly, from almost
zero to extremely high. Within a wetland, plants and animals are often arranged in “zones” along a gradient of physical stress, with the best competitors occupying the least stressful habitats and excluding all others. In turn, the species that are best able to tolerate stress, but are poor competitors, occupy the most stressful habitats. In contrast to the negative effects of competition, the presence of stress-tolerant plants and animals may reduce the level of physical stress in the habitat and benefit other species, a process called “facilitation.” In addition, wetland species are negatively affected by consumers. Animals that eat plants may strongly reduce plant biomass, the abundance of these “herbivores” may be reduced by predators, and organisms on all trophic levels may be affected by parasites. Most wetlands vary over time in how “wet” they are, and this variation creates constantly changing abiotic conditions that affect all these organisms and their interactions. Wetland organisms are also affected by a variety of disturbances such as floating mats of dead vegetation, sediment deposited by floating ice, and fire. These and other disturbances reduce competition and create opportunities for species that would otherwise be excluded from the habitat. Due to global change, individual wetlands will transform over time as climate and water inputs vary. At the same time, humans directly affect wetlands by increasing nutrient supplies, removing predators, and filling or draining wetlands. All these factors—abiotic stress, species interactions, disturbance, global change, and human impacts—vary geographically, meaning that an understanding of a particular wetland requires an understanding of its geographic context.

Figure 1. Diverse plant community in a Netherlands salt marsh. (Photo: Steve Pennings)
THE BASICS OF WETLAND COMMUNITY ASSEMBLY
We know a great deal about how wetland communities are assembled. Wetlands have been attractive study systems for research on community ecology, because they combine simplicity in plant biodiversity with strong abiotic gradients that produce striking community patterns. In addition, the soft sediments of wetlands make them suitable for some kinds of experiments, such as transplant experiments (moving plants and animals around and among different ecological conditions), because organisms are easy to excavate and relocate. At the same time, the importance of wetlands in regulating water chemistry, mediating the global carbon cycle, supporting fisheries and other wildlife, and protecting coastal communities from storms has generated a high level of interest in wetland processes and conservation. One lesson we have learned is that not all wetlands are the same: different types of wetlands in different parts of the world can function in different ways. What follows are broad generalizations that may not apply everywhere.
Plant species richness is low in most wetlands, compared with neighboring terrestrial communities, because most plant species cannot tolerate the abiotic stresses present in flooded soil (Module 3, Introduction to wetland plants; https://www.sws.org/educationmodules-wetland-science/). For example, the anoxic soils caused by waterlogging prevent the majority of plant species that might occur in a terrestrial grassland from colonizing a freshwater wetland. The high porewater salinity encountered in brackish and saltwater wetlands further reduces the number of plant species that can persist in coastal and other saline wetland ecosystems. The flooded soils and high salinity can be conceptualized as two major “filters” that prevent species from colonizing wetlands (Figure 2). The low water potential and sulfidic conditions caused by flooding with seawater further prevent the majority of flood-tolerant plants from colonizing saline wetlands. Despite low species richness in wetlands, the plant species that do occur in wetlands are usually different from those found in terrestrial habitats, so wetlands contribute to plant diversity across the broader landscape.
Figure 2. Species richness in wetlands is limited by two strong abiotic “filters.” The lack of oxygen in flooded, waterlogged soils prevents many species that occur in a region from establishing in freshwater wetlands. The high salt and sulfide concentrations in saline soils prevent many species that could live in freshwater wetlands from establishing in saline wetlands.
Animal species richness is low in wetlands for similar reasons (Module 4, Basic biology of wetland animals; https://www.sws.org/education-modules-wetlandscience/). Animals must not only be able to tolerate the abiotic conditions presented by flooded, and perhaps saline, soils, but often must also be able to tolerate alternating periods of flooding and exposure due to variable wetland hydroperiods (temporal variation in water depth due to tides, seasonal rainfall, etc.). Moreover, because many herbivores and pollinators only interact with a limited number of plant species, the low plant species richness of wetlands favors low species richness in the herbivore and pollinator fauna. Nevertheless, wetlands can be important in the conservation of declining species by providing safe habitat within otherwise developed regions (Fantinato and Bufa 2019). Moreover, as with plants, wetlands support groups of animal species such as aquatic insects and amphibians that are rare in terrestrial habitats, and so contribute to animal diversity across the broader landscape. In particular, wetlands provide essential habitat for many birds, some of which use wetlands occasionally and others of which are
wetland specialists. As parts of a landscape, wetlands also provide access to drinking water for a variety of terrestrial organisms.
Although species richness is usually low, some wetlands are highly productive. Species that have evolved the necessary physiological mechanisms may flourish in wetlands where they have access to abundant water and nutrients, and experience limited competition from other species. Standing above-ground plant biomass in freshwater wetlands dominated by cattails (Typha), Phragmites, or wild rice (Zizaniopsis) is on the order of 1,000-2,000 g m-2, and in forested wetlands aboveground biomass can exceed 10,000 g m-2 (Mitsch and Gosselink 1993). Similarly, the marine invertebrates found in salt marshes, such as snails and burrowing crabs, can reach densities well over 50 m-2 (Silliman and Bortolus 2003). Productive wetlands often support high densities of birds adapted to wetlands. Not all wetlands are highly productive, however. Those with extreme levels of abiotic stress, such as highly saline salt marshes in regions with little rainfall, or low nutrients such as bogs, may not be able to support higher plants at all (Osland et al. 2014), and have correspondingly low animal biomass.
In many wetlands, plants and animals occur in distinct zonation patterns, in which one species replaces another over one or more spatial gradients of abiotic stress. These patterns are usually understood as resulting from a tradeoff between stress tolerance at one end of a gradient and competitive ability at the other end (Figure 3). According to this understanding, species that are competitively dominant occupy the least stressful habitats, but are excluded from the more stressful habitats by an inability to tolerate abiotic stress. The more stressful habitats are occupied by species that are more tolerant to the locally prevailing stress(es), but are poorer competitors—a consequence of tradeoffs in resource allocation to traits favoring stress tolerance versus competition. For example, in freshwater wetlands in North America, Typha latifolia occupies less-flooded habitats and limits Typha angustifolia to more stressful, more flooded habitats (Grace and Wetzel 1981). Similarly, in northeastern USA salt marshes, the competitively dominant species Juncus gerardii occurs in less-flooded high marsh habitats, and limits the more
stress-tolerant but poorer competitor, Spartina patens, to lower, more-flooded elevations (Bertness 1991a). S. patens in turn limits S. alterniflora to the mostflooded marsh habitats (Bertness 1991b). Finally, along estuaries, it is common for tidal fresh marshes (the less stressful habitat) to be dominated by highly competitive plant species, and tidal salt marshes (the more stressful habitat) to be dominated by stress-tolerant but competitively inferior species (Crain et al. 2004; Engels and Jensen 2010).
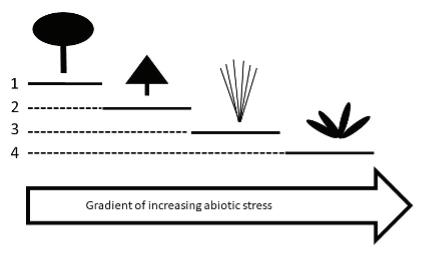
Figure 3. Competition-stress tolerance tradeoff. Four hypothetical species occur (solid lines) in zones across a gradient of abiotic stress, with species 1 in the least-stressful habitat and species 4 in the most-stressful habitat. Species 2 could survive where species 1 occurs, but is excluded from this habitat (dotted line) by competition. Similarly, species 3 and 4 could survive in less-stressful parts of the gradient (dotted lines) but are excluded from these areas by competition.
In cases where there are multiple types of stressful habitats, one can conceptualize this as a “centrifugal” model (Figure 4), in which a single, low-stress “core habitat” is occupied by the competitive dominant, and the various peripheral habitats, each with its own type of abiotic stress, are occupied by species tolerant of those particular stresses (Keddy 1990). Although the ecological processes creating these zonation patterns have been studied most extensively in plants, similar zonation patterns do occur in wetland animals. For example, in salt marshes in North and South America, crab species segregate both across elevation, from high to low marsh, and also along estuarine gradients from marine to freshwater (Teal 1958; Costa and Davy 1992). Herbivorous insects also segregate across elevation in response to variation in plant quality and abiotic factors (Denno et al. 1996; Denno et al. 2000).
Figure 4. Centrifugal model. The competition-stress tolerance tradeoff can be generalized to scenarios in which there are multiple gradients of abiotic stress. The core habitat is the least-stressful habitat, and is occupied by the competitively dominant species. Different species, all of which are excluded by competition from the core habitat, occupy peripheral habitats representing different types of abiotic stress. These might include, for example, gradients in flooding, low nutrient availability, high wave action, and so on. Although for clarity the figure shows only one species at the extreme of each gradient, there might be intermediate species along each gradient as shown in Figure 3.
FACILITATION
The severe abiotic stresses present in wetlands create the opportunity for species to benefit each other by ameliorating harsh physical conditions in a process called facilitation (Figure 5). Plants that are capable of transporting oxygen from shoots to roots, for example, may oxidize the rhizosphere, improving conditions for other plant species (Callaway and King 1996). Plants that shade the substrate and block the wind may reduce heat stress on the soil surface while also limiting evaporation that concentrates salts in the rhizosphere of salt marshes, benefiting neighboring plant species (Bertness and Shumway 1993) and associated invertebrates (Bortolus et al. 2002; Sueiro et al. 2013). Burrowing crabs may improve soil conditions for plants by improving oxygenation of the soil and nitrogen availability (Montague 1982). Marsh bivalves may benefit both plants and other invertebrates by fertilizing the soil, enhancing water storage and reducing salinity
stress (Angelini et al. 2016) and increasing the biotic complexity of the intertidal communities. Finally, the physical structure of wetland plants may benefit other organisms, for example by trapping mangrove propagules and enhancing establishment (Peterson and Bell 2012) or by altering the effectiveness of predators (Denno et al. 2002).
TOP-DOWN CONTROL, ABIOTIC VARIABILITY AND DISTURBANCE
Although wetland organisms may benefit from positive interactions with other species, they may also be subject to top-down pressure from consumers such as herbivores, predators, and parasites. When conditions favor either high herbivore density or reduced plant resistance, herbivores can strongly suppress plant biomass (Silliman and Bortolus 2003). Herbivores in turn may be strongly suppressed by their predators. For example, in southeast USA salt marshes, predators largely exclude marsh snails from creekbank habitats that are easily accessed by fish and swimming crabs (Silliman and Bertness 2002). Human activity has largely removed the largest predators, such as tigers, alligators, seals and sea otters, from modern marshes, but where these species are protected they are increasing in abundance and resuming important ecological roles as apex predators (Gaskins et al. 2020). Finally, many wetland species support multiple parasite species that can strongly suppress the abundance and performance of their hosts (Lafferty and Morris 1996; Pennings and Callaway 1996).
While abiotic stress, competition, facilitation, and consumer pressure can combine to create strong patterns in community composition in wetlands, a variety of other factors may combine to create changing conditions that obscure these patterns. The
Figure 5. Burrowing crabs and bivalves are examples of wetland invertebrates that may benefit plants by decreasing abiotic stress or increasing soil nutrients.
most obvious of these is changing water levels. Water levels in many freshwater wetlands vary seasonally and among years as a function of rainfall. As a result, abiotic conditions are constantly changing, and this increases local species diversity because changing conditions limit the ability of any single species to competitively exclude others (van der Valk 1981; Seabloom et al. 2001).
Another common ecological process that creates spatial and temporal variation in wetlands is disturbance. A wide variety of disturbances can remove plant biomass at particular locations in wetlands, creating opportunities for species that are not strong competitors and initiating secondary succession. In some wetlands, periodic drought can kill vegetation, which allows a new set of plants to establish from seeds. Another wellstudied example is wrack (floating plant debris), which is deposited into the marsh at high tide, smothering underlying vegetation (Bertness and Ellison 1987). When wrack decomposes or is later rafted away by the tides, a series of competitively inferior plants can temporarily occupy the disturbed patches until such time as the competitively dominant plants recolonize. In high latitude marshes of North America, ice may raft chunks of sediment onto the marsh platform, with similar effects (Hardwick-Witman 1985). In other areas, fire may burn aboveground vegetation and leaf litter, again creating opportunities for suppressed plant species (Baldwin and Mendelssohn 1998). When common, herbivores may also suppress dominant plants and favor species that are weaker competitors but less palatable (Bazely and Jefferies 1986; He et al. 2015). Finally, a number of wetlands experience periodic largescale dieback of vegetation for reasons that are not yet fully understood (McKee et al. 2004). These factors tend to obscure the simple zonation patterns that might otherwise have been present across abiotic gradients in a wetland (Brewer et al. 1998).
Over long time periods, wetland community structure may change due to changing abiotic conditions, such as those caused by climate change or long-term internal dynamics. Freshwater wetlands may disappear if groundwater supplies dry up or climate change reduces precipitation. Coastal marshes may experience more flooding if relative sea level rise outpaces sediment
accretion, or less flooding if the land is rising out of the ocean. Depressional wetlands in terrestrial habitats may become more terrestrial as they gradually fill in with soil and organic matter. In a warming climate, marsh plants and animals may migrate to higher latitudes, such that community composition changes. Because global climate change is altering patterns of precipitation and sea level, it is likely that many wetlands will experience changes in species composition and structure in coming decades.
HUMAN ACTIVITIES
The species composition of today’s wetlands is also strongly affected by human activities on the landscape. Humans have deliberately or accidentally introduced many species into geographic locations where they historically did not exist. For example, non-native genotypes of Phragmites australis have been introduced into North America where they have spread widely, invading brackish marshes where Phragmites previously did not occur (Kettenring and Mock 2012). Similarly, the salt marsh grass Spartina alterniflora has been introduced around the world, where it transforms estuaries from mudflats into salt marshes, often hybridizes with native species of Spartina, and dramatically changes the composition of the local biota (Strong and Ayres 2013). One of the most dramatic examples of the invasive potential of Spartina alterniflora comes from China, where it was deliberately introduced. In 40 years, Spartina alterniflora has spread to cover most of the Chinese coastline, replacing native intertidal ecosystems and obliterating the human industries, such as aquaculture, that depended on them (Nie et al. 2022). Another example comes from Central and South America, where the introduction of S. alterniflora was overlooked for centuries, leading to a radical misunderstanding of “native” coastal landscapes (Bortolus et al. 2015). Although ecologically and economically devastating, introductions like these offer ecologists novel opportunities to understand how communities assemble and are structured, and in this sense can provide valuable insights into wetland ecology.
Humans have had other important effects on wetlands. We have increased nitrogen availability in many wetlands, favoring large, competitively dominant
plants at the expense of weaker competitors (Levine et al. 1998). Humans have also removed many predator species, likely contributing to increased densities of herbivores (Coverdale et al. 2013). Finally, humans have filled or drained many wetlands, removing the habitat entirely.
All of the factors affecting the community structure of wetlands are likely to vary geographically as a function of climate and human population density, even within a particular wetland type (Osland et al. 2014). For example, the standing biomass of coastal salt marshes varies with latitude on the east coasts of both China and the United States due to variation in temperature and tidal range (Liu et al. 2020). Ice rafting is a known disturbance in high-latitude salt marshes, whereas hypersaline, low-productivity salt marshes are most common in warm, low-latitude climates (Osland et al. 2014).
This chapter has provided a brief introduction to the assembly of wetland communities, but has only touched on what is a vast literature. There are a wide variety of types of wetlands, and the generalities in this short chapter don’t always apply to every specific situation. Readers wanting more information can turn to many review chapters and book length treatments of wetland ecology for more examples, greater detail, and treatment of specific wetland types. Although we know a great deal about community assembly of wetlands, a disproportionate share of this knowledge comes from temperate latitudes of North America and Europe. Studies from other parts of the world still have much to teach us about how processes that mediate the assembly of wetland communities vary geographically. Studying wetlands around the world will increase the generality of our concepts of wetland community assembly and improve local management of wetlands in all the places where they occur.
OTHER ELEMENTS
The figures from this module and supplemental photographs are provided in a PowerPoint file online at https://www.sws.org/education-modules-wetlandscience/
REFERENCES CITED
Angelini, C., J.N. Griffin, J. Van de Koppel, L.P.M. Lamers, A.J.P. Smolders, M. Derksen-Hooijberg, T. Van der Heide, and B.R. Silliman. 2016. A keystone mutualism underpins resilience of a coastal ecosystem to drought. Nature Communications 7: 12473.
Baldwin, A.H., and I.A. Mendelssohn. 1998. Response of two oligohaline marsh communities to lethal and nonlethal disturbance. Oecologia 116: 543-555.
Bazely, D.R., and R.L. Jefferies. 1986. Changes in the composition and standing crop of salt-marsh communities in response to the removal of a grazer.
Journal of Ecology 74: 693-706.
Bertness, M.D. 1991a. Interspecific interactions among high marsh perennials in a New England salt marsh. Ecology 72: 125-137.
Bertness, M.D. 1991b. Zonation of Spartina patens and Spartina alterniflora in a New England salt marsh. Ecology 72: 138-148.
Bertness, M.D., and A.M. Ellison. 1987. Determinants of pattern in a New England salt marsh plant community. Ecological Monographs 57: 129-147.
Bertness, M. D., and S. W. Shumway. 1993. Competition and facilitation in marsh plants. American Naturalist 142: 718-724.
Bortolus, A., J.T. Carlton, and E. Schwindt. 2015. Reimagining South American coasts: unveiling the hidden invasion history of an iconic ecological engineer. Diversity and Distributions 21: 1267-1283.
Bortolus, A., E. Schwindt, and O. Iribarne. 2002. Positive plant-animal interactions in the high marsh of an Argentinean coastal lagoon. Ecology 83: 733-742.
Brewer, J. S., J.M. Levine, and M.D. Bertness. 1998. Interactive effects of elevation and burial with wrack on plant community structure in some Rhode Island salt marshes. Journal of Ecology 86: 125-136.
Callaway, R. M., and L. King. 1996. Temperaturedriven variation in substrate oxygenation and the balance of competition and facilitation. Ecology 77: 1189-1195.
Costa, C.S.B., and A.J. Davy. 1992. Coastal saltmarsh communities of Latin America. Pages 179-199 in U. Seeliger, editor. Coastal Plant Communities of Latin America. Academic Press, San Diego.
Coverdale, T.C., N.C. Herrmann, A.H. Altieri, and M.D. Bertness. 2013. Latent impacts: the role of historical human activity in coastal habitat loss. Frontiers in Ecology and the Environment 11: 69-74.
Crain, C. M., B.R. Silliman, S.L. Bertness, and M.D. Bertness. 2004. Physical and biotic drivers of plant distribution across estuarine salinity gradients. Ecology 85: 2539-2549.
Denno, R.F., C. Gratton, M.A. Peterson, G.A. Langellotto, D.L. Finke, and A.F. Huberty. 2002. Bottom-up forces mediate natural-enemy impact in a phytophagous insect community. Ecology 83: 14431458.
Denno, R.F., M.A. Peterson, C. Gratton, J. Cheng, G. A. Langellotto, A.F. Huberty, and D.L. Finke. 2000. Feeding-induced changes in plant quality mediate interspecific competition between sap-feeding herbivores. Ecology 81: 1814-1827.
Denno, R.F., G.K. Roderick, M.A. Peterson, A.F. Huberty, H.G. Dobel, M D. Eubanks, J.E. Losey, and G.A. Langellotto. 1996. Habitat persistence underlies intraspecific variation in the dispersal strategies of planthoppers. Ecological Monographs 66: 389-408.
Engels, J.G., and K. Jensen. 2010. Role of biotic interactions and physical factors in determining the distribution of marsh species along an estuarine salinity gradient. Oikos 119: 679-685.
Fantinato, E., and G. Bufa. 2019. Animal-mediated interactions for pollination in saltmarsh communities. Plant Sociology 56: 35-42.
Gaskins, L.C., A.B. Paxton, and B.R. Silliman. 2020. Megafauna in salt marshes. Frontiers in Marine Science 7: 561476.
Grace, J.B., and R.G. Wetzel. 1981. Habitat partitioning and competitive displacement in cattails (Typha): experimental field studies. American Naturalist 118: 463-474.
Hardwick-Witman, M.N. 1985. Biological consequences of ice rafting in a New England salt marsh community. Journal of Experimental Marine Biology and Ecology 87: 283-298.
He, Q., A.H. Altieri, and B. Cui. 2015. Herbivory drives zonation of stress-tolerant marsh plants. Ecology 96: 1318-1328.
Keddy, P. A. 1990. Competitive hierarchies and centrifugal organization in plant communities. Pages 265-290 in J. B. Grace and D. Tilman, editors. Perspectives on plant competition. Academic Press, Inc., San Diego.
Kettenring, KM., and K.E. Mock. 2012. Genetic diversity, reproductive mode, and dispersal differ between the cryptic invader, Phragmites australis, and its native conspecific. Biological Invasions 14: 24892504.
Lafferty, K.D., and K. Morris. 1996. Altered behavior of parasitized killifish increases susceptibility to predation by bird final hosts. Ecology 77: 1390-1397.
Levine, J M., J.S. Brewer, and M.D. Bertness. 1998. Nutrients, competition and plant zonation in a New England salt marsh. Journal of Ecology 86: 285-292.
Liu, W., X. Chen, D.R. Strong, S.C. Pennings, M.L. Kirwan, X. Chen, and Y. Zhang. 2020. Climate and geographic adaptation drive latitudinal clines in biomass of a widespread saltmarsh plant in its native and introduced ranges. Limnology and Oceanography 65: 1399-1409.
McKee, K.L., IA. Mendelssohn, and M. D. Materne. 2004. Acute salt marsh dieback in the Mississippi River deltaic plain: a drought-induced phenomenon? Global Ecology and Biogeography 13: 65-73.
Mitsch, W.J., and J.G. Gosselink. 1993. Wetlands. 2 edition. Wiley.
Montague, C.L. 1982. The influence of fiddler crab burrows and burrowing on metabolic processes in salt marsh sediments. Pages 283-301 in V. S. Kennedy, editor. Estuarine Comparisons. Academic Press, New York.
Nie, M., W. Liu, S. C. Pennings, and B. Li. 2022. Lessons from the invasion of Spartina alterniflora in coastal China. Ecology 104: e3874.
Osland, M J., N. Enwright, and C.L. Stagg. 2014. Freshwater availability and coastal wetland foundation species: ecological transitions along a rainfall gradient. Ecology 95: 2789-2802.
Pennings, S.C., and R.M. Callaway. 1996. Impact of a parasitic plant on the structure and dynamics of salt marsh vegetation. Ecology 77: 1410-1419.
Peterson, J.M., and S.S. Bell. 2012. Tidal events and salt-marsh structure influence black mangrove (Avicennia germinans) recruitment across an ecotone. Ecology 98: 1648-1658.
Seabloom, E. W., K. A. Moloney, and A. G. van der Valk. 2001. Constraints on the establishment of plants along a fluctuating water-depth gradient. Ecology 82: 2216-2232.
Silliman, B.R., and M.D. Bertness. 2002. A trophic cascade regulates salt marsh primary production. Proceedings of the National Academy of Science, USA 99: 10500-10505.
Silliman, B.R., and A. Bortolus. 2003. Underestimation of Spartina productivity in western Atlantic marshes: marsh invertebrates eat more than just detritus. Oikos 101: 549-554.
Strong, D.R., and D.R. Ayres. 2013. Ecological and evolutionary misadventures of Spartina Annual Review of Ecology, Evolution and Systematics 44: 389-411.
Sueiro, M., E. Schwindt, M.M. Mendez, and A. Bortolus. 2013. Interactions between ecosystem engineers: A native species indirectly facilitates a nonnative one. Acta Oecologica 51:11-16.
Teal, J.M. 1958. Distribution of fiddler crabs in Georgia salt marshes. Ecology 39:185-193.
van der Valk, A.G. 1981. Succession in wetlands: a gleasonian approach. Ecology 62:688-696.
TheGhostsofIraq’sMarshes:AHistoryof Conflict,Tragedy,andRestoration
by
Steve Lonergan and Jassim al-Asadi, published by the American University of Cairo Press.
ISBN 978 1 649 03325 3
By Dr. Michelle Stevens, Emeritus Professor, California State University Sacramento. 2024.
The book The Ghosts of Iraq’s Marshes: A History of Conflict, Tragedy, and Restoration tells the story of the history of the Iraqi marshes, their people, and the efforts to protect both the natural and cultural resources. At the confluence of the Tigris and Euphrates Rivers, the Mesopotamian Marshes of southern Iraq and Iran are iconic wetlands and were at one time the largest wetland ecosystem in the world. From antiquity, the marshes are the original Eden to the Abrahamic world’s religions. Mesopotamia—“the land between two rivers,” the Tigris and Euphrates—is where civilization began. The al Ahwar marshes, derived from Aramaic and means “whiteness” or “the illumination of sun on water,” are the homeland of a distinct cultural group—the Marsh Arabs or Maʻdan. The Marsh Arabs are integral to the marsh ecosystem through their management of the ecosystem over millennia. These marshes have been the battleground involving ecocide and genocide since the second world war. This story is unknown to most Americans.
The Ghosts of Iraqi’s Marshes tells the story of the marshes and Marsh Arabs from the perspective of co-author Jassim al-Asadi, champion of the marshes. Jassim’s story is the most compelling part of the book. He is a contemporary wetland advocate and my hero, telling the story of his life in Iraq, including being jailed, tortured, kidnapped, and restored to his loving family and friends. How many of us have spent our lives enduring torture and kidnapping to advocate for wetlands?
I met Jassim when I was the project manager for the Eden Again Project in 2001, hired by Azzam Alwash and Suzanne Reynolds Alwash to work with a blue ribbon panel of wetland scientists to study restoration of the Mesopotamian Marshes. When the Iraqi regime was toppled in 2003, Azzam founded Nature Iraq, the first nonprofit Iraqi organization. Jassim collaborated
with Azzam as an irrigation engineer, working with Nature Iraq, the Iraq Ministry of Water Resources, the Center for Restoration of the Iraqi Marshes, and Ministry of Environment. Jassim acted as a “crucial link between the engineering solutions and the people of the marshes.”
Jassim was born in the marshes, and tells the story that he loved the marshes so much he was born in a traditional “mashoof” boat in the marshes themselves. He belongs to the Bani Asad tribe from Chibayish, known by their family name: Al-Asadi. Above all, Jassim loved the birds, and as a young man built his own binoculars to study them. He worked with Birdlife International and Nature Iraq to publish a book on birds of Iraq, as well as a children’s bird book. The avifauna of Iraq include a total of 421 species, and 20 species are globally threatened.
The marshes play a key role in an intercontinental flyway of migratory birds; they support globally endangered species and sustain the productivity of the Gulf fishery. The ecoregion is a “river of grass,” with Phragmites dominated marshes, swamps, shallow freshwater lakes, and seasonally inundated plains fed by the Tigris and Euphrates Rivers. An entire flyway of migratory waterfowl and shorebirds made their way from Siberian nesting grounds to the Mesopotamian Marshes and northern Africa in the winter. Marsh biodiversity includes 28 species that are deemed to be of Conservation Concern, six species of which are Globally Threatened. Five of these species of Conservation Concern are “endemic species” found only in the Mesopotamian Marshes in Iraq.
This book documents (from Jassim al-Asadi’s personal experience) the terrible history of the marshes and the people that inhabit them. As the authors state, “To say that Iraq has had a tumultuous history since Independence would be a considerable understatement.” At the end of World War I, the Ottoman Empire was divided, and the British were awarded Iraq (due to the known oil resources). The marshes have been the site of three military conflicts: the Iran-Iraq War (1980-1988), the Gulf War (1990-1991), and the 2003 invasion of Iraq led by the United States and Great Britain. For thirty-five years, the Iraqi people and marshes have been in the middle of a war zone. The Maʻdan
homeland has been a frontline combat zone, and the people have been environmental refugees faced with cultural genocide and the deliberate destruction and drainage of their marsh home and way of life.
The biodiversity and cultural integrity of the TigrisEuphrates River Basin is jeopardized by water scarcity, inequitable allocation of water rights, and desertification. Dams and upstream water diversions in Turkey, Syria, and Iran have reduced mean annual flows, resulting in water scarcity and impaired water quality throughout the watershed. Salinity has increased and fish populations, reed availability, and grazing quality for water buffalo forage has declined. The Maʻdan are now becoming environmental refugees.
The authors do point to the success of conservation and rehabilitation of the marshes. These include designation of three Ramsar Wetlands of International Importance: creation of the Mesopotamian Marshlands National Park, a UNESCO World Heritage Site, the Ahwar of Southern Iraq: Refuge of Biodiversity, and the Relict Landscape of the Mesopotamian Cities in 2016. Given the war, ecocide, genocide, and conflicts in the marshes, this conservation success is astonishing. Jassim played a pivotal role organizing and facilitating community workshops to initiate public buy-in and participation in the national park initiative.
The fate of the marshes is uncertain. Upstream withdrawals from upstream dams on the Tigris and Euphrates, pollutants from agricultural runoff and wastewater, and saltwater intrusion from reduced flows severely reduces water availability and quality. Climate change exacerbates severe drought conditions, resulting in dust storms and impaired air quality. Widespread poverty along with limited educational opportunities, human health, and veterinary care are additional challenges to protecting and restoring these wetlands. On a positive note, the Eden in Iraq Wastewater Project treats wastewater to supplement clean water supplies.
There is always hope; the marshes and people are very resilient. As Jassim says, “But as man can be broken but never defeated, nature can revive and marshes could be resurrected to embrace her lovers once again, as is happening in some areas of the Mesopotamian Marshlands.”
The authors are to be complimented for the complex and in-depth information provided in The Ghosts of Iraq’s Marshes. However, the information is clouded by the book’s convoluted organization and the lack of clear maps to orient readers to the region. The history of Iraq and multiple conflicts over time is so complex that a clear, chronological order is essential for readers unfamiliar with the region to help clarify and comprehend the context. Information is not given in chronological order in the book as a whole, or even in individual chapters. For readers unfamiliar with the complex ecological and social history of the marshes, the book jumps back and forth in time in a confusing manner. While the authors provide a comprehensive and complex history of the marshes, a consistent chronological timeline throughout the book would make it easier to follow. Regardless, the book is essential reading for members of the Society of Wetland Scientists.


Fishermen in the Hawizeh Marsh wetlands year 2012. This area of the Mesopotamian Marshes is now dry. Photo by Jassim al-Asadi.
Co-author Jassim al-Asadi with Marsh Arab children and iconic water buffalo in the desiccated Abu-Zaraq Marsh in Dhi Qar area of the Mesopotamian Marshes. Photo: Jassim al-Asadi.
Wetland Research Expedition in Torres del Paine Biosphere Reserve (Chile): Field Observations
Rafaela Retamal,1,2 Isha Goyal,2,3 Samantha Stuhlmiller,2,4
Kiara Bonilla,2,5 Owen Jakel,2,6 Cole Maclin Sullivan,2,7
Michael Reed Keegan,2,7 and Mariana Woeber Gardner2,8
Abstract
From April 11-20, 2024, we conducted an expedition to the wetlands located in the Torres del Paine Biosphere Reserve. During this exploration, we examined wetlands situated in the Core zone (dedicated to conservation), those in the Buffer zone (where livestock activities take place), and those in the Transition zone (where there are multiple uses). In situ water samples were collected, and observations were made to understand the linkages between wetland management, water quality and climatic threats.
Resumen
Del 11 al 20 Entre los días 11 y 20 de abril de 2024, realizamos una expedición a los humedales que se ubican ubicados en la Reserva de la Biosfera Torres del Paine. Durante esta exploración, examinamos humedales situados en la zona Núcleo (dedicada a la conservación), en la zona de Amortiguamiento (donde se desarrolla la actividad ganadera) y de aquellos en la zona de Transición (zona de múltiples usos). En dichos humedales se tomaron muestras de agua in situ y se realizaron observaciones con el propósito de entender las relaciones entre la gestión de los humedales, la calidad de agua y las amenazas climáticas.
Introduction
The Torres del Paine National Park, with its majestic massif featuring Almirante Nieto, the Cuernos (Horns), and its iconic towers, serves as the central area of
the Torres del Paine Biosphere Reserve (TDP BR). Biosphere reserves are recognized as “sites of learning for sustainable development” (UNESCO 2024), where a socioecological approach is applied. This approach aims to prevent conflicts between human uses and biodiversity conservation. Additionally, these reserves promote cultural conservation, preserving both natural and cultural heritage. Within these spaces, three distinct zones are identified: the Core, Buffer, and Transition zones.
The TDP BR received its designation in 1978. The Core zone is the protected area—the Torres del Paine National Park. The Buffer zone primarily supports livestock activities, while the Transition zone lies near the city of Puerto Natales (see Figure 1). Across these three zones, a diverse range of continental wetlands can be found, including rivers, lakes, meadows, and peatlands. These wetlands have been identified by the Chilean Ministry of the Environment through the development of a National Wetland Inventory (MMA 2020; see Figure 1).
Two elements prompted our socioecological investigation: (1) there are wetlands that remain unidentified in the National Inventory, and (2) the community’s assessment of wetlands and the quality of their water is unknown. Given that water is a critical component of wetlands, its quality can be affected by surrounding activities, potentially leading to ecological shifts and degradation. Therefore, the objective of this research was to characterize the wetlands within the TDP BR using a socioecological approach and water quality sampling over a two-year period (2023–2024).
1 Fundación Planeta Agua, Puerto Natales, Chile, corresponding author: rafaelaretamal@gmail.com
2 Center for Climate Studies, School for Field Studies, Puerto Natales, Chile
3 Olin College of Engineering, Needham, Massachusetts
4 Hollins University, Roanoke, Virginia
5 University of Colorado at Boulder, Boulder, Colorado
6 Whitman College, Walla Walla, Washington
7 Williams College, Williamstown, Massachusetts
8 Denison University, Granville, Ohio
Field Expedition
Our expedition takes place within the context of the School for Field Studies, Chile (SFS-Chile), located in Puerto Natales. At SFS-Chile, undergraduate students from various universities in the United States participate each semester in a study abroad program. Most of these students are studying sciences and environmental studies. In the guided research course focused on “conservation: political and social aspects,” students assist the academic staff in a long-term research project. The project focuses on wetlands within the TDP BR from a socioecological perspective. It involves in situ water quality measurements and interviews with local stakeholders.
From April 11-20, 2024, the field expedition was conducted in the TDP BR and vicinity. Seven students collected water quality samples at 24 monitoring points, each representing a distinct wetland. These
wetlands span across the Core, Buffer, and Transition zones. Parameters measured included flow, depth, pH, temperature, dissolved oxygen, total sediments, turbidity, nitrogen (nitrates), and phosphorus (phosphates). These measurements were complemented by wetland observations related to threats, conservation evidence, management, monitoring, and ecosystem services provided by these ecosystems (Figures 2, 3, 4, 5, 6, and 7).
Simultaneously, interviews were conducted with stakeholders, covering topics such as wetland value, water quality perception, wetland history, and the impact of climate change on wetlands.
Figure 1. Water Quality Monitoring Sites and Locations for Interviews on Wetland Perception, History, Water Quality, and Climate Threats (Source: Goyal and Stuhlmiller 2024)
Figure 2. Riparian wetland, Natales River, in a peri-urban area in the Transition zone of TDP BR.
Figure 3. Riparian wetland, Natales River, in a rural area in the Transition zone of TDP BR.
Figure 4. Riparian wetland, Las Chinas River, in a rural area in the Buffer zone of TDP BR.
Figure 5. Lacustrine wetland in Jara Lagoon, a rural area in the Buffer zone of TDP BR.
Figure 6. Lacustrine-Palustrine wetland, in a rural area in the Core zone of TDP BR.
Figure 7. Field equipment used for nitrate measurements.
Findings of the Expedition
During the nine-day field expedition, we visited a total of 24 wetlands. Notably, the TDP BR contains a significant number of wetlands, which are often underrepresented and little-known in online community portals dedicated for tourism or education. Most of those websites are dedicated to Paine massif and there is little or no information about wetlands (e.g., Biosphere Reserve official webpage).
We could observe a gradient of use in each of the BR zones. In the core zone, TDP National Park, we observed two dominant types of wetlands: lacustrine and riverine.
The lakes are characterized by endorreic watershed, located in the pre-Andean scrubland ecoregion, and some wetlands used to be part of previous ranch (estancias in Spanish) that were donated to become part of the National Park, so the level of anthropocentric disturbance can be attributed to historical land use and the Chilean mega-drought. Among all the lacustrine wetlands visited (Figure 8), we observed that Laguna Los Juncos is a shallow lake, where we can find neneo macho (Anarthrophylum desideratum), guanacos (Lama guanicaoe) and puma (Puma concolor), as well as a very recent expansion of Juncacea sp. into areas that had been open water (Figure 9). Key informants attributed this change in Juncacea distribution to a slow-drying of the laguna, due to the upgrading of the gravel road that cuts across the laguna’s narrow valley into a raised tarmac, which has meant less local runoff entering this laguna. Similar to these wetlands were two other small ones with no official name. We visited a wetland named Laguna Los Cisnes, which is very important since it is possible to observe many different birds like flamingos (Phoenicopterus chilensis), black neck swan (Cygnus melancoryphus), and coscoroba swan (Cosocoroba coscoroba). It has been dry due to the mega-drought that affected Chile from 2012–2022, but the last two winters have been wet and some birds have returned, according to the interviewees. Additionally, we could observe the behavior of birds due to puma presence as all the birds were united in the center of the wetland.
We also visited the two main rivers, Serrano and Grey Rivers, that receive water from glaciers. The Serrano River receives melting water from Dickson Glacier and flows around the national park from east to west until confluence with fjord Ultima Esperanza (Figures 10 and 11). The Grey River receives melting water from Grey Glacier and becomes a tributary of Serrano River (Figure 12). Both rivers are located in the pre-Andean scrubland, where riverine areas are cover by low and high deciduous beech (Nothofagus antarctica and N. pumilio). The epiphyte, Old man´s beard (Protousnea sp), cover these trees all year around. In the Serrano River, we observed Dydimo (Didymosphenia geminata), an invasive microalgae, and in the Grey River area where there is a large high deciduous beech forest, it is possible to observe huemul (Hippocamelus bisiculus), which is a charismatic species, because it is one of Chile’s national animals and is prominently displayed on the national coat of arms.


Figure 8. Laguna Los Juncos, lacustrine wetlands in the core area of TDP BR.
Figure 9. Type of Juncacea sp. observed in core area wetlands of TDP BR.
In the Buffer zone, we observed three different wetland types: riverine, palustrine, and lacustrine. They are located either in Patagonian steppe or pre-Andean scrubland and in the ecotone among them. A common feature to all of them is they have been currently or very recently (less than 3 years) impacted by ranching. So, the species presented are very similar to the ones in the Core zone, but its abundance depends on how well-conserved each property is. There are just a few estancias dedicated to tourism in the Buffer zone (Figures 13 to 16).
Figure 10. View of Serrano River and Torres del Paine Massif in the core area of TDP BR.
Figure 11. Serrano River with presence of Dydimo algae.
Figure 12. Serrano River with presence of high deciduous beech forest in its shore, after the confluence with Grey River.
Figure 13. Riverine wetlands (Jara River) in the Buffer area of TDP BR, characterized by steppe or pre-Andean scrubland.
Figure 14. Palustrine wetland in TDP BR Buffer area. The water has been channeled to a cattle watering.
Finally, in the Transition zone, we could observe mainly riverine wetlands, surrounded by rural and urban land uses. Although there are palustrine wetlands, we did not have access because it was difficult to contact the properties’ owners. We visited different segments of Natales River, from the headwater to the confluence with the Ultima Esperanza fjord. In the upper part of the watershed, we found grass for ranching along with low and high deciduous beech (Nothofagus antarctica and N. pumilio); in the middle part we observed much more grass and trash; and in the lower part we found chocho (Lupinus polyphyllus), which is an invasive species that was introduced for ornamental reasons and now is widespread along the TDP BR (Figures 2, 3, 17 and 18).
Figure 15. Palustrine wetland whose banks are covered with reeds (La Fina 2 lake) in the Buffer area of TDP BR.
Figure 16. Lacustrine wetland in TDP BR Buffer zone with presence of reeds and flamingos inserted in a property dedicated to livestock.
Figure 17. Riverine wetland in the transition area of TBP BR (Natales River), the bank river has been widened for cattle watering.
We observed a relationship between the reserve’s zones and the ecosystem services (ES) they provide. For instance:
• Core zone wetlands contribute to recreational ES, based on the amount of tourists that navigate on the cruise to observe Glacier Grey.
• Buffer zone wetlands support agricultural and livestock provisioning ES—for instance, estancias (ranchers) use wetlands for water diversion to provide water to sheep and cows.
• Transition zone wetlands provide ES for human consumption, agriculture, and water purification regulation. For example, the agricultural sector in Puerto Natales bases its water source from a riverine wetland and the city was founded in its surroundings because of the availability of water access for living and ranching.
Field data indicate that the water quality generally falls within established Chilean water quality standards, except for phosphate levels. Wetlands with elevated values of phosphate are found in both the Core and Buffer zones. By observing the hydrographic watershed, we identified that these wetlands are endorheic, located near gravel roads, which are subject to ongoing maintenance. Additionally, they exhibit a high presence of aquatic plants, such as Juncaceae, potentially transforming lacustrine wetlands into palustrine ecosystems. Table 1 presents a list of dominant flora and fauna for each wetland type characteristic in this expedition.
Figure 18. Riverine wetland in transition area of TBP BR (Natales River) with presence of Nothofagus antarctica and grass for livestock purpose.
Wetland type Dominant species observed in the field
Low deciduous beech is located in the shoreline (Nothofagus antartica)
Riverine
High deciduous beech is located in the shoreline (Nothofagus pumilio)
Didymo located in the Serrano River is an invasive species (Didymosphenia geminate)
Neneo macho (Anarthrophylum desiderátum)
Lacustrine
Palustrine
Lacustrinepalustrine
Puma (Puma concolor) can be found in the pre-Andean scrubland
Guanaco was observed in this location (Lama guanicoe) Gato Jeofrey
Guanaco was observed in this wetland type (Lama guanicoe)
Low deciduous beech is in the palustrine wetland (Nothofagus antartica)
Junco de Magallanes can be found in lakes moving to become palustrine wetlands (Marsippospermum grandiflorum or Juncus spp.)
Table 1. Dominant flora and fauna per wetland type characteristc
Our survey of wetlands in the region indicates the need to understand the factors responsible for the changing plant communities. It is essential to develop water quality monitoring for these wetlands, including additional types of measurements. For example, wetlands transitioning to reed beds could alter habitat and life cycles for certain bird species, while also influencing birdwatching tourism opportunities in public protected areas and private properties dedicated to ecotourism.
Acknowledgments
The authors gratefully acknowledge the key field and logistical support provided by The School for Field Studies Center for Climate Studies, Chile. Similarly, we deeply appreciate the National Forest Corporation for granting the research permit through Resolution No. 211 in 2023. This permit, valid for two years, will enable the continued monitoring of these wetlands. Our gratitude extends to the landowners who allowed us to visit and sample the wetlands on their properties, and who generously shared their knowledge with us. We also thank the Municipality of Cerro Castillo for providing accommodation, allowing us to be closer to the expedition sites. Special thanks go to the
individuals interviewed during this research. Lastly, we acknowledge all the tourism services for their efforts in ensuring a successful expedition.
Declaration of Originality
This is an original work that has not been published before. Images, figures, and quotations included in the article have been properly cited and permission has been granted for any that are not those of the author.
Literature
Goyal, I. and S. Stuhlmiller. 2024. Wetlands in Torres del Paine Biosphere Reserve: Perceptions and Interactions of Park Rangers, Estancieros, and Other Residents. SFS - Chile, Puerto Natales.
MMA (Ministerio del Medio Ambiente). 2020. Tipología de humedales según el Inventario Nacional de Humedales https://humedaleschile.mma.gob.cl/wpcontent/uploads/2022/08/Clasificacion-de-humedales. pdf
UNESCO. 2024. What are biosphere reserves? https:// www.unesco.org/en/mab/wnbr/about?hub=66369
Discovering Bioremediation Microorganisms in a Temporary Wetland of Central Mexico Highlands using Environmental DNA Metabarcoding
María José Hernández-de Santos1 and Tatiana Lobato-de Magalhães1
Abstract
This study employed environmental DNA (eDNA) analysis through metabarcoding, alongside brightfield microscopy, to assess biodiversity and identify microorganisms with bioremediation potential in the temporary wetlands of Querétaro, Mexico. Environmental DNA provides a non-invasive and innovative method for monitoring various organisms, from microbes to higher taxa. Samples were collected from a temporary wetland in Pedro Escobedo. WilderLab® processed environmental DNA samples, and microscopy (using a UNICO G504 trinocular microscope) was conducted at UAQ’s Laboratory of Molecular Genetics and Evolutionary Ecology (100 hours of observation, 90 wet mounts). Our findings revealed 136 Operational Taxonomic Units (OTUs) across 25 phyla, including bacteria, green algae, ciliates, crustaceans, and rotifers. In addition, 32 species and morphospecies of green algae, diatoms, and cyanobacteria were identified microscopically. The literature review highlighted that several identified bacterial and green algae species could degrade organic and inorganic pollutants, e.g., Exiguobacterium sp. Gemmatimonas sp., Methylobacterium sp., Psychroglaciecola sp. for arsenic, lead, mercury; Chryseobacterium sp. and Acholeplasma sp. for radioactive elements such as uranium. The findings contribute to understanding microbial diversity in Querétaro’s wetlands and support future research on employing native microorganisms for aquatic ecosystem remediation.
Keywords: eDNA, biodiversity, bioremediating bacteria, Operational Taxonomic Unit, Querétaro state.
1 Introduction
Mexico’s rich topography, soil, climate, and vegetation have contributed to a high diversity of wetland types, covering approximately 114,000 km² of this territory (56 % inland wetlands and 44% coastal wetlands) (Olmsted 1993; Instituto Nacional de Estadística y Geografia [INEGI] 2025). These ecosystems provide critical ecosystem services, including food, water, flood control, groundwater recharge, biogeochemical regulation, and cultural value (Davidson et al. 2019; Wood et al. 2024). Wetlands offer various ecosystem services, including regulating water quality through bioremediation. Therefore, identifying microorganisms with bioremediation potential is key, particularly in the temporary/ephemeral wetlands of Querétaro state, a unique ecosystem (like Californian vernal pools) that is poorly studied (Lobato-de Magalhães et al. 2022).
Microorganisms in wetland sediments can break down various pollutants, such as organic waste, industrial chemicals, hydrocarbons, pesticides, heavy metals, and nanoparticles, through specialized metabolic pathways. These processes are essential for the ecosystem’s self-purification and resilience. Environmental DNA (eDNA) analysis through metabarcoding allows large-scale biodiversity assessment to identify and monitor organisms, ranging from microorganisms to higher organisms such as plants and animals. This novel and non-invasive technique has been applied for studying biodiversity (Bird et al. 2024; Blackman et al. 2024; Foulquier et al. 2024). This powerful, non-invasive tool for studying microbial diversity in aquatic systems allows culturable and unculturable species to be detected. This research aimed to identify microbial assemblages of a temporary wetland in Pedro Escobedo, Querétaro, focusing on its bioremediation potential. Understanding microbial diversity and its remediation potential in highland temporary wetlands is crucial for identifying new taxa and underscoring the importance of this unique ecosystem. It will support efforts to protect these threatened ecosystems. To gain deeper insights into these ecosystems and their potential for bioremediation, the following research questions were proposed: (1) What is the diversity (at
1 Faculty of Natural Sciences, Universidad Autónoma de Querétaro, México; corresponding author email address: tatiana.lobato@uaq.mx
a lower taxonomic level) of microorganisms in the wetland? (2) Are there any microorganisms identified as remediators? and (3) Which microorganisms would be particularly relevant for future remediation studies?
2 Material and Methods
2.1 Study Area and Sampling
The study area was located in a temporary wetland, municipality of Pedro Escobedo, Querétaro (Lat. 20°24’38.2’’, Long. -100°15’58.6’’, altitude 2,305 m a.s.l.), covering approximately one hectare of land (Lobato-de Magalhães et al. 2022). The climate in this region is semi-arid and semi-warm, classified as Cwb in the Köppen climate classification; this implies that the region experiences warm, dry summers with maximum temperatures ranging between 25°C and 32°C. Winters are temperate, with temperatures fluctuating between 18°C and 22°C. The rainy season extends from May to September, with July and August being the wettest months, while the dry season lasts from October to April, with December and January being the driest. The average annual precipitation in the region ranges from 500 to 600 mm (INEGI 2025).
The wetland exhibits fluctuations in hydroperiods, being intermittently flooded, with the water level varying seasonally according to rainfall. The site is flooded chiefly from June to September, while from October to December, the water level decreases, resulting in shorter hydroperiods. These fluctuations are the main drivers of community dynamics, and by supporting biota adapted to temporary waters, these wetlands contribute to the diversity of aquatic and facultative plants and animals (Calhoun et al. 2017). The aquatic flora of the temporary wetland consists of species such as Eleocharis acicularis, E. densa, Luziolla fluitans, Marsilea mollis, Najas guadalupensis, Nymphoides fallax, Sagittaria demersal (Lobato-de Magalhães and Martínez 2018; Lobato-de Magalhães et al. 2022) (Figure 1).

Environmental DNA was measured in water samples and were collected from the temporary wetland at the beginning and end of the rainy season 2024. The recommendations provided by the manufacturer of the Comprehensive Freshwater eDNA Mini Kit WilderLab® for analysis were strictly followed for sample collection, handling, and storage. Genetic material was collected from the equivalent of 1 L of water from 20 random points within the wetland, using a filter while taking necessary precautions to minimize exposure to air, sunlight, and contamination from the collector’s DNA. Factors can affect the integrity of the eDNA in the samples and compromise the quality of the results. For shipment to the laboratory, the samples were preserved in collecting filters with the addition of a preservative provided by the manufacturer, allowing for storage for up to six months, ensuring optimal sample preservation until the metabarcoding analysis.
For water samples to be observed under microscopy, three 100 ml samples were collected at three random points distributed throughout the wetland, using sterile bottles and discarding two sample volumes before collecting the final definitive sample. The samples were kept under cold chain conditions, using a cooler with refrigerant bags, and then stored at 4°C. Additionally, there was an in situ analysis of the water’s physical and chemical parameters at three points within the wetland using a Hanna HI 91130 probe as follow (range) pH 6.23-6.80, dissolved oxygen 3.47-4.83 mg/L, conductivity 75,00-127.0 μSTm, total dissolved solids 20.50-63.50 ppm, salinity 0.02-0.06 PSU.
Figure 1. Highland temporary wetland (2305 m a.s.l.), Pedro Escobedo, Querétaro, Mexico. (Photo: T. Lobato-de Magalhães)
2.2 Environmental DNA Metabarcoding
The Comprehensive Freshwater eDNA analysis (WilderLab®, https://www.wilderlab.co.nz) was used to identify crustaceans, algae, and other microorganisms. The DNA extraction, sample quality control, barcode library preparation/indexing, sequencing, and bioinformatics analysis were performed by WilderLab®, including noise removal and taxonomic identification. This mass DNA sequencing method uses a specific region of the genome, referred to as a ‘barcode’ or ‘marker’, which enables the identification and classification of species present in a sample, since it is unique to each species. We employed a variant of environmental DNA barcoding called metabarcoding, which amplifies and sequences multiple barcodes simultaneously (Shea et al. 2023).
2.3 Optical Microscopy
For the preparation of permanent slides, the process involved fixing the samples onto the slides using 70% ethanol, as it is less aggressive to cells and minimizes the contraction or distortion that may occur with higher ethanol concentrations. Once the ethanol evaporated, a coverslip was placed, and the sample was sealed with clear varnish. Each slide was labeled with the date, the sampling site, and the name of the student responsible for its preparation. Given the ephemeral nature of the wetland, these permanent slides serve as invaluable tools for documenting the microbial communities of the ecosystem. They also facilitate comparison between microscopic observations and the eDNA analysis obtained.
Water samples were analyzed using bright-field optical microscopy on a UNICO G504T microscope, with the primary goal of describing the taxa present in the wetland by identifying microorganisms such as diatoms, green algae, dinoflagellates, cyanobacteria, amoebae, ciliates, rotifers, cladocerans, copepods, pollen grains, and fungi. Ten observations of each freshwater sample were conducted at 10x and 40x magnification (100 hours of observation, 90 wet mounts). As an integral part of the process, the observations were documented through photographic and video recordings of the identified microorganisms, using a digital AmScope camera mounted on a C-mount and coupled to a
trinocular UNICO G504T optical microscope. This photographic archive was supplemented with written descriptions and the application of taxonomic keys for microorganism identification. Optical microscopy was conducted at the Laboratory of Molecular Genetics and Evolutionary Ecology at UMBA, in the Applied Microbiology Unit of UAQ. Taxonomic guides were used as references for identifying the diversity of the samples, and digital tools such as Algae Database (https://www.algaebase.org), iNaturalist (https://www. inaturalist.org), and Encyclopedia of Life (https://eol. org/) were consulted.
3 Results and Discussion
3.1 Environmental DNA Metabarcoding
We identified 136 Operational Taxonomic Units (OTUs), distributed across 25 phyla, 37 classes, and 53 orders, including the following: bacteria (73 OTUs), green algae (19 OTUs), ciliates (10 OTUs), crustaceans (9 OTUs), rotifers, cryptomonads, flagellates (5 OTUs apiece), heterokont algae (4 OTUs), diatoms (2 OTUs), fungi, oomycetes, protists (1 OTU apiece) (Figure 2 and 3; Table 1).
Figure 2. Operational Taxonomic Units richness and percentage by taxonomic group by environmental DNA samples of a highland temporary wetland, Pedro Escobedo, Querétaro, Mexico.
Figure 3. Operational Taxonomic Units richness by the three major taxonomic groups (highlighting major families) by environmental DNA samples of a highland temporary wetland, Pedro Escobedo, Querétaro, Mexico.
Taxonomic group Families/taxa
Bacteria (73 OTUs, 38 families)
The class Alphaproteobacteria is the richest (14 OTUs), followed by Betaproteibacteria (11), Cytophagia (eight), Actnomycetes (seven), Flavobacteriia, Gammaproteobacteria (five apiece), Bacilli (four), Chitinophagia, Clostridia, Sphingobacteriia (three apiece), other encompassed one OTU.
The richest families are Chitinophagaceae, Comamonadaceae, Hymenobacteraceae, Sphingobaceriaceae (three apiece). Taxa that have not been classified into a family (15 OTUs or 21%).
Green algae (19 OTUs, seven families) The richest family is Chlamydomonadaceae (three species), followed by Desmidiaceae, Oedogoniaceae, and Volvocales (two species apiece). Taxa that have not been classified into a family (seven OTUs or 37%).
Ciliates (ten OTUs, five families)
The richest families are Oxytrichidae and Trachelophyllidae (two OTUs apiece). Taxa that have not been classified into a family (three OTUs or 30%).
Crustaceans (nine OTUs) The richest families are Daphniidae and Macrotrichidae (two species apiece). Taxa that have not been classified into a family (five OTUs or 44%).
Rotifers (five OTUs) The families Brachionidae and Flinidae have one OTU apiece. Taxa that have not been classified into a family (three OTUs or 60%).
Cryptomonads (five OTUs) The families Chroomonadaceae, Cryptomonadaceae, Pyrenomonadaceae have one OTU apiece. Taxa that have not been classified into a family (two OTUs or 40%).
Flagellates (five OTUs) The family Euglenaceae has three species. Taxa that have not been classified into a family (two OTUs or 40%).
Heterokont algae (four OTUs) The family Chromulinaceae has one OTU. Taxa that have not been classified into a family (three OTUs or 75%).
Diatoms (two OTUs) This community is 100% dominated by the family Bacillariaceae.
Fungi (one OTU) Sporobolomyces sp.
Protist (one OTU) Order Bigyra
Oomycetes (one OTU) Family Pythiaceae
Microscopic animal Family Chaetonotidae
Table 1. Observed taxa in environmental DNA samples of a highland temporary wetland, Pedro Escobedo, Querétaro, Mexico.
3.2 Optical Microscopy
Sixteen taxa of green algae (Actinastrum sp., Gonium sociale, Scenedesmus quadricaudata, Tetrabaena sociales, Tetraedhron sp., Volvox sp. and ten morphospecies), eight diatoms (Cymbella sp., Navicularis sp, Nitzschia acicularis, Nitzschia sp., Synedra sp., and three morphospecies), five flagellates (Phacus sp., Trachelomonas bacilari, T. hispida, T. volvocinensis, and Strombomonas sp.), two
cyanobacteria (Anabaena sp. and a morphospecies) and one crustacean (Daphnia sp.) were observed under microscopic examination, in total 32 species and morphospecies (Fig. 4). From the 32 taxa, six morphospecies of green algae, four diatoms, and two cyanobacteria were only observed by optical microscopy and not detected in the eDNA analysis.
4. Examples of taxa from a highland temporary wetland (Pedro Escobedo, Querétaro, Mexico) observed via optical microscopy: Green algae (a) Actinastrum sp., (b) Scenedesmus quadricaudata, (c) Volvox sp.; Diatoms (d) Nitzschia sp., (e) Synedra sp.; Cyanobacteria (f) Anabaena sp.; Crustacean (g) Daphnia sp. Copyright: M. J. Hernández-de Santos.
3.2 Remediation Application
Wetlands are critical players in regulating and improving water quality, acting as natural filters for the retention, transformation, and recirculation of nutrients, sediments, and pollutants. These ecosystems actively prevent eutrophication and enhance water quality in downstream water bodies (Davidson et al. 2018) due to the diversity of microorganisms inhabiting wetlands, especially bacteria, which colonize a wide range of environments or microhabitats generated by the combination of water, nutrients, soil, sediments, and vegetation (Liu et al. 2020; Kumar et al. 2022). The main phylum of functional bacteria in treatment wetlands to remove pollutants are Proteobacteria, Bacteroidetes, Actinobacteria and Firmicutes (Wang et al. 2022).
We conducted a literature review to identify the potential of target taxa to degrade various organic compounds, metals, and radioactive elements. Some of the key families of bacteria identified through the eDNA analysis are the following: Acholeplasmataceae, Bacillaceae, Burkholderiaceae, Caulobacteraceae, Chitinophagaceae, Comamonadaceae, Cytophagaceae, Flavobacteriaceae, Gemmatimonadaceae, Geodermatophilaceae, Hymenobacteraceae, Methylobacteriaceae, Microbacteriaceae, Moraxellaceae, Nocardioidaceae, Paenibacillaceae, Pseudomonadaceae, Sphingobacteriaceae, and Sphingomonadaceae (Table 2 and Appendix 1). Li et al. (2022) found that nitrogen enrichment enhances bacterial communities in hydrocarbon-contaminated soils, particularly boosting the PAH-scavenging capacity of Chitinophagaceae, possibly through cometabolism with fungi. Similarly, Blanco-Enríquez et al. (2018) reported that Chitinophagaceae bacteria can degrade pyrene, a key water pollutant. Révész et al. (2020) identified Malikia (Comamonadaceae) as dominant in benzene- and toluene-degrading microbial communities in activated sludge, with their activity linked to oxygenase-encoding genes. Some Comamonadaceae species also possess regulatory systems for low-oxygen environments, and genetic diversity within the family may influence degradation abilities. Wang et al. (2023) showed that Ramlibacter can degrade sulfadiazine in ryegrass-planted soil, highlighting root–microbe synergy in rhizosphere bioremediation. Ajibade et al. (2023) also reported Ramlibacter’s ability to degrade sulfamethoxazole in wetlands. Lastly, Guo et al. (2020) demonstrated that Hymenobacter can break down the pesticide imidacloprid in lake surface waters via hydroxylation.
Figure
Family
Acholeplasmataceae
Bacillaceae
Burkholderiaceae
Caulobacteraceae
Chitinophagaceae
Comamonadaceae
Cytophagaceae
Flavobacteriaceae
Gemmatimonadaceae
Geodermatophilaceae
Hymenobacteraceae
Methylobacteriaceae
Microbacteriaceae
Moraxellaceae
Nocardioidaceae
Remediation use/Species observed in this study with prior research on bioremediation
Bacteria
Species: Acholeplasma sp. Uranium reduction.
Species: Exiguobacterium sp. Degradation of polistireno, PAHs, PGPR, remediation of arsenic, cadmium, copper, mercury, tellurium, chrome.
Species: Polynucleobacter sp. Degradation of sulfonamides, DeSOx.
Species: Brevundimonas sp. Degradation of DeSOx, alquibencenos sulfonados (TritonX-100, detergents)
Species: Segetibacter sp., Sediminibacterium sp. Fenantreno, hydrocarbon-contaminated soils, polycyclic aromatic hydrocarbons (PAHs), pyrene.
Species: Malikia sp., Macromonas sp., Ramlibacter, sp. Benzene and toluene in activated sludge, cDCE and DeSOx in subterranean waters, sulfamethoxazole, pharmaceutical contaminants such as sulfadiazine.
Species: Spirosoma sp.
Degradation of phosphates, resistant to gamma rays.
Species: Flavobacterium sp.
Degradation of PCBs, diesel, organophosphates, nylon
Species: Gemmatimonas sp.
Degradation of phenols, sulfonamides, remediation of copper, zinc, arsenic, lead.
Species: Blastococcus sp. Remediation of copper, zinc, arsenic, lead.
Species: Siccationidurans sp., Hymenobacter sp., Rufibacter sp. Degradation of imidacloprid, an agricultural pesticide that can persist in soils for over a year and eventually move into surface waters or leach into groundwater. Resistant to UV rays.
Species: Methylobacterium sp., Psychroglaciecola sp.
Remediation of cadmium, chrome, mercury, arsenic, PGPR, organophosphates, PAHs.
Species: Rhodoluna sp, Curtobacterium sp. Chrome reduction
Species: Acinetobacter sp.
Remediation of diesel, PAHs, acrylamides, cypermethrin
Species: Nocardiodes sp.
Degradation of DEHP, nitrophenols, pesticides, dyes, explosives, PAHs, dibenzofurans, ibuprofen.
References*
Leavitt et al. 2012
Kumar and Chandra 2020
Parthasarathy et al. 2022
Maroof et al. 2022
Xiao et al.2024
Gu et al, 2022
Zhang et al. 2024
Saravanan et al.2022
Cortés-Lorenzo et al. 2013
Song et al. 2023
An et al. 2007
Blanco-Enríquez et al. 2018
Kariyawasam et al. 2022
Benedek et al. 2018
Révész et al. 2020
Ajibade et al. 2023
Song et al. 2023
Wang et al. 2023
Lee et al. 2014
Li et al. 2017
Negoro et al. 1994
Ning et al. 2012
Khalid et al. 2021
Liang et al. 2020
Kong et al, 2024
Liu et al. 2022
Zhang et al. 2022
Sbissi et al. 2025
Srinivasan et al. 2015
Guo et al. 2020
Lee et al. 2021
De Marco et al. 2004
Onder Erguven et al 2021
Gutiérrez et al. 2010
Dahal et al. 2023
Gur Ozdal and Algur 2022
Ebert et al. 2001
Kubota et al. 2005
Zhu et al. 2020
Ma et al. 2023
Family
Paenibacillaceae
Pseudomonadaceae
Sphingobacteriaceae
Sphingomonadaceae
Spirosomaceae
Weeksellaceae
Chlamydomonadaceae
Chlorellaceae
Desmidiaceae
Oedogoniaceae
Remediation use/Species observed in this study with prior research on bioremediation
Species: Paenibacillus sp. Remediation of copper and zinc.
Species: Pseudomonas sp.
Remediation of lead, copper, oxyaniones, pesticides, PHAs, phenolic composts, dye.
Species: Mucilaginibacter sp., Pedobacter sp. Degradation of PCBs, DeSOx, PGPR, PAHs.
Species: Sphingomonas sp., Novospingobium sp. Degradation of PGP, PAHs, diesel, remediation of copper, zinc, cadmium.
Species: Arcicella sp.
Degradation of polyphenols, nitrogen and phosphates.
Species: Chryseobacterium sp.
Degradation of pesticides organochlorides (OCPs), DDT, glyphosate, PAHs, remediation of uranium, cadmium, lead, zinc.
Green algae
Species: Chlamydomonas spp.
Degradation of various organic compounds, including the biotransformation of trichlorfon, PAHs such as benz(a)anthracene, polymers like polystyrene, bisphenol A, and pharmaceuticals such as enrofloxacin, carbamazepine, ciprofloxacin, erythromycin, estrone, norfloxacin, ofloxacin, paracetamol, progesterone, roxithromycin, salicylic acid, sulfadiazine, sulfadimethoxine, sulfamethoxydiazine, fametazine, triclocarban, triclosan, trimethoprim, ibuprofen, azithromycin, sulfapyridine, estradiol, and diclofenac.
Species: Chlorella sp.
Biotransformation or absorption of pesticides (lindane, naphthalene, mirex, DDT, organophosphates, pyrethroids, and oxadiazoles), and removal of nickel, zinc, and lead, degradation of cephalosporins, ceftazidime, cefradine, cefalexin, amoxicillin, azithromycin, enrofloxacin, florfenicol, and levofloxacin.
Species: Cosmarium sp., Staraustram sp.
Removal of synthetic dyes from wastewater.
Species: Oedogonium sp.
Remediation of inorganic nitrogen and phosphates, bioaccumulation of Ca and Mg.
References*
Govarthanan et al. 2016
Gilani et al. 2015
Li et al. 2017
Palanivel et al. 2020
Fakhar et al. 2020
Ivanova et al. 2022
Shah et al. 2025
Vélez et al. 2021
You et al 2022
Mastny et al. 2021
Vasconcelos et al. 2022
Tabata et al., 2000
Li et al 2019
Chen et al. 2014
Lyu et al. 2014
Rodríguez-Conde et al. 2016
Xu et al. 2018
Asaf et al. 2020
Hu et al. 2023
Chen et al. 2013
Chai et al. 2017
Szoboszlay et al. 2008
Gurav and Jadhav 2013
Qu et al. 2015
Jadhav 2016
Majewska et al. 2022
Zhang et al. 2022
Khare and Acharya 2024
Zhou et al. 2014
Hom-Díaz et al. 2015, 2022
Xiong et al. 2016
Li et al. 2022
Carbó et al. 2023
Liakh et al. 2023
Seoane et al. 2023
Avila et al. 2021
Ferreira et al. 2011
Xiong et al. 2021
Aranguren Díaz et al. 2022
Daneshvar et al. 2007
Sudha et al. 2020
Oberholster et al. 2018
Lawton et al. 2021
Table 2. Practical remediation applications identified through the literature on the microorganism list discovered in the environmental DNA samples of a highland temporary wetland, Pedro Escobedo, Querétaro, Mexico.* See Appendix 1.
Understanding bacterial diversity in wetlands and its potential for bioremediation has become an increasingly relevant area of research. The analysis of environmental DNA extracted from wetland water samples enables the identification and characterization of microorganisms present in the ecosystem, even those difficult to identify using optical microscopy. This technique has revealed a previously unexplored wealth of bacteria and green algae in the wetland and has provided invaluable information on the diversity and remediation capabilities of its microbial communities. Overall, bacterial species are studied for the degradation of organic and inorganic pollutants, as well as radiation, while green algae are known for degrading various organic compounds, including the biotransformation of trichlorfon, PAHs such as benz(a) anthracene, polymers like polystyrene, bisphenol A, and pharmaceuticals like enrofloxacin, carbamazepine, ciprofloxacin, erythromycin, estrone, norfloxacin, ofloxacin, paracetamol, progesterone, roxithromycin, salicylic acid, sulfadiazine, sulfadimethoxine, sulfamethoxydiazine, fametazine, triclocarban, triclosan, trimethoprim, ibuprofen, azithromycin, sulfapyridine, estradiol, and diclofenac (Table 2 and Appendix 1).
We also highlight for remediation the green algae families: Chlamydomonadaceae, Chlorellaceae, Desmidiaceae, and Oedogoniaceae (Table 2 and Appendix 1). Microalgae from the genus Chlamydomonas have demonstrated the ability to degrade a wide range of organic pollutants, including trichlorfon, PAHs like benz(a)anthracene, polystyrene, bisphenol A, and numerous pharmaceuticals such as antibiotics, hormones, and painkillers. Similarly, Cosmarium (Desmidiaceae) has shown potential for removing synthetic dyes from wastewater. Within the Oedogoniaceae family, Oedogonium species have been reported to remove inorganic nitrogen and phosphates while bioaccumulating minerals such as calcium and magnesium. In the Chlorellaceae family, Chlorella species identified through eDNA metabarcoding have been associated with the uptake or degradation of various pesticides (e.g., lindane, DDT) and metals (nickel, zinc, lead), as well as a wide range of antibiotics including cephalosporins and fluoroquinolones.
Therefore, microorganisms present in wetlands are key players in regulating water quality, particularly bacteria (Kumar et al. 2023) and microscopical green algae (Fang et al. 2023).
4 Conclusion
Overall, the study recorded 148 microorganism taxa. Environmental DNA has proven to be a powerful technique for assessing the microbial diversity of wetlands. We observed more taxa through environmental analysis (136 OTUS) than optical microscopy (32 species and morphospecies). This was because most of the taxa identified via eDNA included bacteria (73 OTUS), which are not easily visible with optical microscopy techniques. In contrast, 12 taxa identified through optical microscopy were not detected by eDNA analysis.
We found potential remediation uses in the literature for several taxa on our list (e.g., Exiguobacterium sp., Malikia sp., Macromonas sp., Ramlibacter sp., Sphingomonas sp., Novospingobium sp., Mucilaginibacter sp., Pedobacter sp.) (Table 2 and Appendix 1). Microorganisms from the temporary wetlands of Querétaro state should be included in future research on remediation issues, particularly given the current growth in the industrial sector in this region and its associated environmental challenges.
Our findings would be relevant for identifying potentially new records of microorganisms in Querétaro’s freshwater wetlands and compiling a list of selected species for future studies on remediation, using microorganisms associated with aquatic ecosystems. Continued exploration using eDNA techniques is likely to uncover further functional diversity. We are optimistic that using the eDNA method could improve our knowledge and understanding of the environment, benefiting science and society by conserving inland wetlands in the Central Mexico Highlands.
Author Contributions
María José Hernández-de Santos wrote the first draft manuscript; conducted fieldwork, a literature review on remediation, and bright-field microscopy analysis; and prepared graphics. Tatiana Lobato-de Magalhães conceptualized the study, secured funding, translated
the draft manuscript into English, and reviewed the final version.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Declaration of Originality
This is an original work that has not been published before. Images, figures, and quotations included in the article have been properly cited and permission has been granted for any that are not those of the authors.
Acknowledgements
We thank Débora O. Lobato for her support with the manuscript revision. This study is part of an Undergraduate Thesis in Microbiology led by the first author. It was funded by the Council of Science and Technology of the Querétaro State, project FNB202011, agreement CACTI-096-2024.
References
Ajibade, F. O., W. Yin, A. Guadie, et al. 2023. Impact of biochar amended on antibiotic removal and resistant genes accumulation in constructed wetlands for low C/N wastewater treatment. Chemical Engineering Journal 459. https://doi.org/10.1016/j.cej.2023.141541
Bird, S., P. Dutton, S. Wilkinson, J. Smith, I. Duggan and A. McGaughran. 2024. Developing an eDNA approach for wetland biomonitoring: Insights on technical and conventional approaches. Environmental DNA 6(3). https://doi.org/10.1002/edn3.574
Blackman, R., M. Couton, F. Keck, D. Kirschner, L. Carraro, E. Cereghetti, K. Perrelet, R. Bossart, J. Brantschen, Y. Zhang and F. Altermatt. 2024. Environmental DNA: The next chapter. Molecular Ecology 33(11). https://doi.org/10.1111/mec.17355
Blanco-Enríquez, E.G., F.J. Zavala-Díaz de la Serna, M.D.R. Peralta-Pérez, L. Ballinas-Casarrubias, I. Salmerón, H. Rubio-Arias, and B.A. Rocha-Gutiérrez. 2018. Characterization of a microbial consortium for the bioremoval of polycyclic aromatic hydrocarbons (PAHs) in water. International journal of environmental
research and public health 15(5): 975. https://doi. org/10.3390/ijerph15050975
Calhoun, A.J., D.M. Mushet, K.P. Bell, D. Boix, J.A. Fitzsimons and F. Isselin-Nondedeu. 2017. Temporary wetlands: challenges and solutions to conserving a ‘disappearing’ecosystem. Biological conservation 211: 3–11. https://doi.org/10.1016/j.biocon.2016.11.024
Davidson, N.C., E. Fluet-Chouinard, and C.M. Finlayson. 2018. Global extent and distribution of wetlands: trends and issues. Marine and Freshwater Research 69(4): 620–627. https://doi.org/10.1071/ MF17019
Davidson, N.C., A.A. van Dam, C.M. Finlayson and R.J. McInnes. 2019. Worth of wetlands: revised global monetary values of coastal and inland wetland ecosystem services. Marine & Freshwater Research. https://doi.org/10.1071/mf18391
Fang, W.T., C.H. Hsu and B. LePage. 2023. Bioremediation and Biofuel Production Using Microalgae. In: Lobato de Magalhães, T. and M.L. Otte (eds) Wetlands for Remediation in the Tropics. Springer, Cham. https://doi.org/10.1007/978-3-03123665-5_9
Foulquier, A., T. Datry, R. Corti, et al. 2024. Unravelling large-scale patterns and drivers of biodiversity in dry rivers. Nature Communications 15: 7233. https://doi.org/10.1038/s41467-024-50873-1
Guo, L., Z. Daí, J. Guo, et al. 2020.Oligotrophic bacterium Hymenobacter latericoloratus CGMCC 16346 degrades the neonicotinoid imidacloprid in surface water. AMB Express 10(7). https://doi. org/10.1186/s13568-019-0942-y
INEGI (Instituto Nacional de Estadística y Geografia). 2025. Atlas Nacional Interactivo De México. http:// www.inegi.org.mx/
Kumar, S., N. Thakur, A. Singh, B. Gudade, D. Ghimire, and S. Das. 2022. Microbes-assisted phytoremediation of contaminated environment: Global status, progress, challenges, and future prospects. Phytoremediation Technology for the Removal of Heavy Metals and Other Contaminants from Soil and Water
555–566. https://doi.org/10.1016/B978-0-323-857635.00007-6
Kumar, V., T. Bera, S. Roy, et al. 2023. Investigating bio-remediation capabilities of a constructed wetland through spatial successional study of the sediment microbiome. NPJ Clean Water 6(1): 8. https://doi. org/10.1038/s41545-023-00225-1
Li, C., Q. Tian, Y. Zhang, et al. 2022. Sequential combination of photocatalysis and microalgae technology for promoting the degradation and detoxification of typical antibiotics. Water research 210. https://doi.org/10.1016/j.watres.2021.117985
Liu, Q., Y. Zhang, H. Wu, et al. 2020. A Review and Perspective of eDNA Application to Eutrophication and HAB Control in Freshwater and Marine Ecosystems. Microorganisms 8(3). https://doi.org/10.3390/ microorganisms8030417
Lobato-de Magalhães, T. and M. Martínez. 2018. Temporary freshwater wetlands floristics in central Mexico highlands. Botanical Sciences 96: 138–156. https://doi.org/10.17129/botsci.1532
Lobato-de Magalhães, T., L.E. Olguín-Chávez, M. Martínez and N.P. Tippery. 2022. Insights into sexual reproduction and morph compatibility of the distylous Nymphoides fallax Ornduff (Menyanthaceae). Aquatic Botany 183, 103573. https://doi.org/10.1016/j. aquabot.2022.103573
Olmsted, I. 1993. Wetlands of Mexico. In: Wetlands of the world: Inventory, ecology and management. Volume I: Africa, Australia, Canada and Greenland, Mediterranean, Mexico, Papua New Guinea, South Asia, Tropical South America, United States (pp. 637–677). Dordrecht: Springer Netherlands.
Révész, F., M. Farkas, B. Kriszt, et al. 2020. Effect of oxygen limitation on the enrichment of bacteria degrading either benzene or toluene and the identification of Malikia spinosa (Comamonadaceae) as prominent aerobic benzene-, toluene-, and ethylbenzene-degrading bacterium: enrichment, isolation and whole-genome analysis. Environmental Science and Pollution Research 27: 31130–31142. https://doi.org/10.1007/s11356-020-09277-z
Shea, M.M., J. Kuppermann, M.P. Rogers, D.S. Smith, P. Edwards and A.B. Boehm. 2023. Systematic review of marine environmental DNA metabarcoding studies: toward best practices for data usability and accessibility. PeerJ 11: e14993. https://doi.org/10.7717/peerj.14993
Wang, J., Y. Long, G. Yu, et al. 2022. A review on microorganisms in constructed wetlands for typical pollutant removal: species, function, and diversity. Frontiers in microbiology 13: 845725. https:// doi.org/10.3389/fmicb.2022.845725
Wang, H. R., X. R. Du, Z. Y. Zhang, F. J. Feng and J. M. Zhang. 2023. Rhizosphere Interface Microbiome Reassembly by Arbuscular Mycorrhizal Fungi Weakens Cadmium Migration Dynamics. iMeta 2(4). https://doi. org/10.1002/imt2.133
Wood, K.A., L.L. Jupe, F.C. Aguiar, A.M. Collins, S.J. Davidson, W. Freeman, L. Kirkpatrick, T. Lobatode Magalhães, E. McKinley, A. Nuno, J.F. Pagès, A. Petruzzella, D. Pritchard, J.P. Reeves, S.M. Thomaz, S.A. Thornton, H. Yamashita, J.L. Newth. 2024. A global systematic review of the cultural ecosystem services provided by wetlands. Ecosystem Services. https://doi.org/10.1016/j.ecoser.2024.101673
Appendix 1. References cited in Table 2.
Ajibade, F.O., W.Yin, A. Guadie, T.F. Ajibade, Y. Liu, M.N. Kumwimba, ... and A.J. Wang. 2023. Impact of biochar amended on antibiotic removal and resistant genes accumulation in constructed wetlands for low C/N wastewater treatment. Chemical Engineering Journal 459. https://doi.org/10.1016/j.cej.2023.141541
An, D., H. Lee, W. Im, Q. Liu and S. Lee. 2007. Segetibacter koreensis gen. nov., sp. nov., a novel member of the phylum Bacteroidetes, isolated from the soil of a ginseng field in South Korea. International journal of systematic and evolutionary microbiology, 57: 1828-33. https://doi.org/10.1099/ijs.0.64803-0
Aranguren Díaz, Y., E. Monterroza Martínez, L. Carillo García, M.C. Serrano and E. Machado Sierra. 2022. Phycoremediation as a strategy for the recovery of marsh and wetland with potential in Colombia. Resources, 11(2): 15. https://doi. org/10.3390/resources11020015
Asaf, S., M. Numan, A.L. Khan and A. Al-Harrasi. 2020. Sphingomonas: from diversity and genomics to functional role in environmental remediation and plant growth. Critical Reviews in Biotechnology 40(2): 138152. https://doi.org/10.1080/07388551.2019.1709793
Avila, R., A. Peris, E. Eljarrat, T. Vicent and P. Blánquez. 2021. Biodegradation of hydrophobic pesticides by microalgae: Transformation products and impact on algae biochemical methane potential. Science of the Total Environment, 754: 142114. https://doi. org/10.1016/j.scitotenv.2020.142114
Benedek, T., F. Szentgyörgyi, I. Szabó et al. 2018. Aerobic and oxygen-limited enrichment of BTEXdegrading biofilm bacteria: dominance of Malikia versus Acidovorax species. Environmental Science and Pollution Research 25: 32178–32195. https://doi. org/10.1007/s11356-018-3096-6
Blanco-Enríquez, E.G., F.J. Zavala-Díaz de la Serna, M.D.R. Peralta-Pérez, L. Ballinas-Casarrubias, I. Salmerón, H. Rubio-Arias, and B.A. Rocha-Gutiérrez. 2018. Characterization of a microbial consortium for the bioremoval of polycyclic aromatic hydrocarbons (PAHs) in water. International journal of environmental research and public health, 15(5): 975. https://doi. org/10.3390/ijerph15050975
Carbó, M., P. Chaturvedi, A. Álvarez, D. PinedaCevallos, A. Ghatak, P.R. González, ... and L. Valledor. 2023. Ferroptosis is the key cellular process mediating Bisphenol A responses in Chlamydomonas and a promising target for enhancing microalgae-based bioremediation. Journal of Hazardous Materials, 448: 130997. https://doi.org/10.1016/j.jhazmat.2023.130997
Chai, X., B. Wu, Z. Xu, N.Yang, L. Song, J. Mai, et al. 2017. Ecosystem activation system (EAS) technology for remediation of eutrophic freshwater. Scientific reports, 7(1): 4818. https://doi.org/10.1038/s41598-01704306-3
Chen C.Y., X.Q. Zhao, H.W. Yen, S.H. Ho, C.L. Cheng, D. Lee, F.W Ba, J.S.Chang. 2013. Microalgae-based carbohydrates for biofuel production. Biochemical Engineering Journal 78: 1–10. https://doi.org/10.1016/j. bej.2013.03.006
Chen Q., J. Zhang, C.H. Wang, J. Jiang, S.W. Kwon, L.N. Sun, W.B. Shen, J. He. 2014. Novosphingobium chloroacetimidivorans sp. nov., a chloroacetamide herbicide-degrading bacterium isolated from activated sludge. International Journal of Systematic and Evolutionary Microbiology, 64(8): 2573-2578. https:// doi.org/10.1099/ijs.0.062950-0
Cortés-Lorenzo, C., M. del Mar Sánchez-Peinado, B. Oliver-Rodríguez, J.L. Vílchez, J.J. GonzálezLópez and M.Rodríguez-Díaz. 2013. Two novel strains within the family Caulobacteraceae capable of degradation of linear alkylbenzene sulfonates as pure cultures. International Biodeterioration & Biodegradation, 85: 62-65. https://doi.org/10.1016/j. ibiod.2013.06.001
Dahal, U., K. Paul and S. Gupta. 2023. The multifaceted genus Acinetobacter: from infection to bioremediation. Journal of Applied Microbiology, 134(8). https://doi.org/10.1093/jambio/ lxad145
Daneshvar, N., M. Ayazloo, A.R. Khataee and M.J.B.T. Pourhassan. 2007. Biological decolorization of dye solution containing Malachite Green by microalgae Cosmarium sp. Bioresource technology, 98(6): 11761182. https://doi.org/10.1016/j.biortech.2006.05.025
De Marco P, C.C. Pacheco, A.R. Figueiredo, P. Moradas-Ferreira. 2004. Novel pollutant-resistant methylotrophic bacteria for use in bioremediation. FEMS Microbiology Letters, 234: 75–80. https://doi. org/10.1111/j.1574-6968.2004.tb09515.x
Ebert, S., P. Fischer and H.J. Knackmuss. 2001. Converging catabolism of 2, 4, 6-trinitrophenol (picric acid) and 2, 4-dinitrophenol by Nocardioides simplex FJ2-1A. Biodegradation, 12(5): 367-376. https://doi. org/10.1023/A:1014447700775
Fakhar, A., B. Gul, A.R. Gurmani, S.M. Khan, S. Ali, T. Sultan, et al. 2020. Heavy metal remediation and resistance mechanism of Aeromonas, Bacillus, and Pseudomonas: A review. Critical Reviews in Environmental Science and Technology, 52(11): 18681914 https://doi.org/10.1080/10643389.2020.1863112
Ferreira, L. S., M.S. Rodrigues, J.C.M. de Carvalho, A. Lodi, E. Finocchio, P. Perego and A. Converti. 2011. Adsorption of Ni2+, Zn2+ and Pb2+ onto dry biomass of Arthrospira (Spirulina) platensis and Chlorella vulgaris. I. Single metal systems. Chemical Engineering Journal, 173(2): 326-333. https://doi.org/10.1016/j. cej.2011.07.039
Gilani, R. A., M. Rafique, A. Rehman, M.F.H. Munis, S.U. Rehman and H.J. Chaudhary. 2015. Biodegradation of chlorpyrifos by bacterial genus Pseudomonas. Journal of basic microbiology, 56(2): 105-119. https://doi.org/10.1002/jobm.201500336
Govarthanan, M., R. Mythil, T. Selvankumar, et al. 2016. Bioremediation of heavy metals using an endophytic bacterium Paenibacillus sp. RM isolated from the roots of Tridax procumbens. 3 Biotech 6(242). https://doi.org/10.1007/s13205-016-0560-1
Gu, Q., M. Sun, T. Lin, Y. Zhang, X. Wei, S. Wu, et al. 2022. Characteristics of antibiotic resistance genes and antibiotic-resistant bacteria in full-scale drinking water treatment system using metagenomics and culturing. Frontiers in Microbiology, 12. https://doi. org/10.3389/fmicb.2021.798442
Guo, L., Z. Daí, J. Guo et al. 2020.Oligotrophic bacterium Hymenobacter latericoloratus CGMCC 16346 degrades the neonicotinoid imidacloprid in surface water. AMB Express 10(7). https://doi. org/10.1186/s13568-019-0942-y
Gur Ozdal, O. and O.F. Algur. 2022. Biodegradation α-endosulfan and α-cypermethrin by Acinetobacter schindleri B7 isolated from the microflora of grasshopper (Poecilimon tauricola). Archives of Microbiology, 204(159). https://doi.org/10.1007/ s00203-022-02765-5
Gurav, R. G. and J.P. Jadhav. 2013. Biodegradation of keratinous waste by Chryseobacterium sp. RBT isolated from soil contaminated with poultry waste. Journal of basic microbiology, 53(2): 128-135. https://doi. org/10.1002/jobm.201100371
Gutiérrez, A. M., J.J.P. Cabriales and M.M. Vega. 2010. Isolation and characterization of hexavalent chromium-reducing rhizospheric
bacteria from a wetland. International journal of phytoremediation, 12(4): 317-334. http://dx.doi. org/10.1080/15226510902968118
Hom-Diaz, A., M. Llorca, S. Rodríguez-Mozaz, T. Vicent, D. Barceló and P. Blánquez. 2015. Microalgae cultivation on wastewater digestate: β-estradiol and 17α-ethynylestradiol degradation and transformation products identification Journal of environmental management, 155: 106-113. https://doi.org/10.1016/j. jenvman.2015.03.003
Hom-Diaz, A., A. Jaén-Gi, S. Rodríguez-Mozaz, D. Barceló, T. Vicent and P. Blánquez. 2022. Insights into removal of antibiotics by selected microalgae (Chlamydomonas reinhardtii, Chlorella sorokiniana, Dunaliella tertiolecta and Pseudokirchneriella subcapitata). Algal Research, 61. https://doi. org/10.1016/j.algal.2021.102560
Hu, W., Z. Li, H. Ou, X. Wang, Q. Wang, Z. Tao, et al. 2023. Novosphingobium album sp. nov., Novosphingobium organovorum sp. nov. and Novosphingobium mangrovi sp. nov. with the organophosphorus pesticides degrading ability isolated from mangrove sediments. International Journal of Systematic and Evolutionary Microbiology, 73(4), 005843. https://doi.org/10.1099/ijsem.0.005843
Ivanova, A.A., S.A. Mullaeva, O.I. Sazonova, K.V. Petrikov and A.A. Vetrova. 2022. Current research on simultaneous oxidation of aliphatic and aromatic hydrocarbons by bacteria of genus Pseudomonas. Folia Microbiologica, 67(4): 591-604. https://doi. org/10.1007/s12223-022-00966-5
Jadhav, S. S. and M. David. 2016. Biodegradation of flubendiamide by a newly isolated Chryseobacterium sp. strain SSJ1. 3 Biotech, 6(1): 31. https://doi. org/10.1007/s13205-015-0347-9
Kariyawasam, T., G.S. Doran, J.A. Howitt and P.D. Prenzler 2022. Polycyclic aromatic hydrocarbon contamination in soils and sediments: Sustainable approaches for extraction and remediation. Chemosphere, 291. https://doi.org/10.1016/j. chemosphere.2021.132981
Khalid, N., M. Aqeel, A. Noman, S.M. Khan and N. Akhter. 2021. Interactions and effects of microplastics with heavy metals in aquatic and terrestrial environments. Environmental Pollution, 290. https:// doi.org/10.1016/j.envpol.2021.118104
Khare, D. and C. Acharya. 2024. Uranium biomineralization by immobilized Chryseobacterium sp. strain PMSZPI cells for efficient uranium removal. Journal of Hazardous Materials, 465. https:// doi.org/10.1016/j.jhazmat.2024.133503
Kong, D., L. Xu, M. Dai, Z. Ye., B. Ma and X. Tan. 2024. Deciphering the functional assembly of microbial communities driven by heavy metals in the tidal soils of Hangzhou Bay. Environmental Pollution, 360. https:// doi.org/10.1016/j.envpol.2024.124671
Kubota M, K. Kawahara, K. Sekiya, T. Uchida, Y. Hattori, H. Futamata, A. Hiraishi. 2005. Nocardioides aromaticivorans sp. nov., a dibenzofuran-degrading bacterium isolated from dioxin-polluted environments Systematic and Applied Microbiology, 28(2):165-74. https://doi.org/10.1016/j.syapm.2004.10.002
Kumar, V., and R. Chandra. (2020). Metagenomics analysis of rhizospheric bacterial communities of Saccharum arundinaceum growing on organometallic sludge of sugarcane molasses-based distillery. 3 Biotech, 10(7): 316. https://doi.org/10.1007/s13205020-02310-5
Lawton, R. J., C.R. Glasson, P.M. Novis, J.E. Sutherland and M.E. Magnusson. 2021. Productivity and municipal wastewater nutrient bioremediation performance of new filamentous green macroalgal cultivars. Journal of Applied Phycology, 33: 4137-4148. https://doi.org/10.1007/s10811-021-02595-w
Leavitt, J. 2012. Biosorption of uranium and its effect on uranium transport in groundwater. (Doctoral dissertation, The University of New Mexico). https:// digitalrepository.unm.edu/ce_etds/3
Lee, J.J., S. Srinivasan, S. Lim, M. Joe, S. Im,S.I. Bae., K.R. Park, J.H. Han, S.H. Park, B.M. Joo, S.J. Park and M.K. Kim. 2014.. Spirosoma radiotolerans sp. nov., a gamma-radiation-resistant bacterium isolated from
gamma ray-irradiated soil. Current microbiology, 69(3): 286–291. https://doi.org/10.1007/s00284-014-0584-x
Lee J.H., J.H. Jung, M.K. Kim, H.N. Choe and S. Lim. 2021. Hymenobacter taeanensis sp.nov., radiation resistant bacterium isolated from coastal sand dune. Antonie Van Leeuwenhoek 114(10): 1585–1593. https:// doi.org/10.1007/s10482-021-01624-5
Li, Y., M.J. Ai, Y Sun, Y.Q. Zhang and J.Q. Zhang. 2017. Spirosoma lacussanchae sp. nov., a phosphatesolubilizing bacterium isolated from a freshwater reservoir. International journal of systematic and evolutionary microbiology, 67(9): 3144-3149. https:// doi.org/10.1099/ijsem.0.001778
Li, J., C. Luo, D. Zhang, X. Cai, L. Jiang, X. Zhao and G. Zhang. 2019. Diversity of the active phenanthrene degraders in PAH-polluted soil is shaped by ryegrass rhizosphere and root exudates. Soil Biology and Biochemistry, 128: 100-110. https://doi.org/10.1016/j. soilbio.2018.10.008
Li, C., Q. Tian, Y. Zhang, Y. Li, X. Yang, H. Zheng, ... and F. Li. 2022. Sequential combination of photocatalysis and microalgae technology for promoting the degradation and detoxification of typical antibiotics. Water research, 210. https://doi. org/10.1016/j.watres.2021.117985
Liakh, I., D. Harshkova, P. Hrouzek, K. Bišová, A. Aksmann and B. Wielgomas. 2023. Green alga Chlamydomonas reinhardtii can effectively remove diclofenac from the water environment - A new perspective on biotransformation. Journal of Hazardous Materials, 455. https://doi.org/10.1016/j. jhazmat.2023.131570
Liang, J., S. Tang, J. Gong, G. Zeng, W. Tang, B. Song, et al. 2020. Responses of enzymatic activity and microbial communities to biochar/compost amendment in sulfamethoxazole polluted wetland soil. Journal of Hazardous Materials, 385. https://doi.org/10.1016/j. jhazmat.2019.121533
Liu, C., J. Zhuang, J. Wang, G. Fan, M. Feng and S. Zhang. 2022. Soil bacterial communities of three types of plants from ecological restoration areas and plant-growth promotional benefits of Microbacterium
invictum (strain X-18). Frontiers in Microbiology, 13. https://doi.org/10.3389/fmicb.2022.926037
Lyu,Y., W. Zheng, T. Zheng and Y. Tian. 2014. Biodegradation of polycyclic aromatic hydrocarbons by Novosphingobium pentaromativorans US6-1. PloS one, 9(7), e101438. https://doi.org/10.1371/journal. pone.0101438
Ma, Y., J. Wang, Y. Liu,X. Wang, B. Zhang,W. Zhang, T. Chen, G. Liu, L. Xue and X. Cui. 2023. Nocardioides: “Specialists” for hard-to-degrade pollutants in the environment. Molecules, 28(21). https://doi.org/10.3390/molecules28217433
Majewska, M., S. Wdowiak-Wróbel, M. MarekKozaczuk, A. Nowak,and R. Tyśkiewicz. 2022. Cadmium-resistant Chryseobacterium sp. DEMBc1 strain: characteristics and potential to assist phytoremediation and promote plant growth Environmental science and pollution research international, 29(55): 83567–83579. https://doi. org/10.1007/s11356-022-21574-3
Maroof, L., I. Khan, H. Hassan, S. Azam and .W. Khan. 2022. Microbial degradation of low density polyethylene by Exiguobacterium sp. strain LM-IK2 isolated from plastic dumped soil. World Journal of microbiology and biotechnology, 38(11): 197. https:// doi.org/10.1007/s11274-022-03389-z
Mastny, J., J. Barta., E. Kastovska and T. Picek. 2021. Decomposition of peatland DOC affected by root exudates is driven by specific R and K strategic bacterial taxa. Scientific Reports, 11. https://doi. org/10.1038/s41598-021-97698-2
Negoro, S., K. Kato, K. Fujiyama et al. 1994. The nylon oligomer biodegradation system of Flavobacterium and Pseudomonas. Biodegradation 5: 185–194. https://doi. org/10.1007/BF00696459
Ning, J., G. Gang, Z. Bai, Q. Hu, H. Qi, A.Ma, X. Zhuan and G. Zhuang. 2012. In situ enhanced bioremediation of dichlorvos by a phyllosphere Flavobacterium strain. Frontiers of Environmental Science & Engineering, 6: 231-237. https://doi. org/10.1007/s11783-011-0316-4
Oberholster, P.J., P.H. Cheng, A.M. Botha et al. 2018. Assessment of selected macroalgae for use in a biological hybrid system for treating sulphur in acid mine drainage (AMD). Journal of Applied Phycology, 30: 1361–1370. https://doi.org/10.1007/ s10811-017-1314-0
Onder Erguven, G., Ş. Tatar, O. Serdar and N.C. Yildirim. 2021. Evaluation of the efficiency of chlorpyrifos-ethyl remediation by Methylobacterium radiotolerans and Microbacterium arthrosphaerae using response of some biochemical biomarkers. Environmental Science and Pollution Research, 28: 2871-2879.
Palanivel, T. M., N. Sivakumar, A. Al-Ansari and R. Victor. 2020. Bioremediation of copper by active cells of Pseudomonas stutzeri LA3 isolated from an abandoned copper mine soil. Journal of environmental management, 253. https://doi.org/10.1016/j. jenvman.2019.109706
Parthasarathy, A., R.R. Miranda, N.C. Eddingsaas, J. Chu, I.M. Freezman, A.C. Tyler and A.O. Hudson. 2022. Polystyrene degradation by Exiguobacterium sp. RIT 594: preliminary evidence for a pathway containing an atypical oxygenase. Microorganisms, 10(8). https:// doi.org/10.3390/microorganisms10081619
Qu, J., Y. Xu, G.M. Ai, Y. Liu and Z.P. Liu. 2015. Novel Chryseobacterium sp. PYR2 degrades various organochlorine pesticides (OCPs) and achieves enhancing removal and complete degradation of DDT in highly contaminated soil. Journal of Environmental Management, 161: 350-357. https://doi.org/10.1016/j. jenvman.2015.07.025
Révész, F., M. Farkas, B. Kriszt et al. 2020. Effect of oxygen limitation on the enrichment of bacteria degrading either benzene or toluene and the identification of Malikia spinosa (Comamonadaceae) as prominent aerobic benzene-, toluene-, and ethylbenzene-degrading bacterium: enrichment, isolation and whole-genome analysis. Environmental Science and Pollution Research, 27: 31130–31142. https://doi.org/10.1007/s11356-020-09277-z
Rodriguez-Conde S, L. Molina, P. González, A. García-Puente and A. Segura. 2016. Degradation of
phenanthrene by Novosphingobium sp. HS2a improved plant growth in PAHs-contaminated environments. Applied Microbiology Biotechnology, 100(24): 1062710636. https://doi.org/10.1007/s00253-016-7892-y
Saravanan, S., P.S. Kumar, B. Chitra and G. Rangasamy. 2022. Biodegradation of textile dye Rhodamine-B by Brevundimonas diminuta and screening of their breakdown metabolites. Chemosphere, 308. https://doi. org/10.1016/j.chemosphere.2022.136266
Sbissi, I., F. Chouikhi, F. Ghodhbane-Gtari et al. 2025. Ecogenomic insights into the resilience of keystone Blastococcus Species in extreme environments: a comprehensive analysis. BMC Genomics 26 (51). https://doi.org/10.1186/s12864-02511228-2
Seoane, M., K. Conde-Pérez, M. Esperanza, Á. Cid and C. Rioboo 2023. Unravelling joint cytotoxicity of ibuprofen and oxytetracycline on Chlamydomonas reinhardtii using a programmed cell death-related biomarkers panel. Aquatic Toxicology, 257. https://doi. org/10.1016/j.aquatox.2023.106455
Shah, S.M., S. Khan, N. Bibi. et al. 2025. Indigenous bacteria as potential agents for trace metal remediation in industrial wastewater. Scientific Reports 15 (13141). https://doi.org/10.1038/s41598-025-97711-y
Song, C., Y. Shi, H. Gao, P. Liu and X. Liu. 2023. Bio-Augmentation of S2− Oxidation for a Heavily Polluted River by a Mixed Culture Microbial Consortium. Fermentation, 9(7). https://doi. org/10.3390/fermentation9070592
Srinivasan, S., E.S. Joo, J.J. Lee et al. 2015. Hymenobacter humi sp. nov., a bacterium isolated from soil. Antonie van Leeuwenhoek 107: 1411–1419. https://doi.org/10.1007/s10482-015-0436-0
Sudha, P. N., K. Vijayalakshmi, D. Hemapriya, M. Saranya and S.K. Kim. 2020. Microalgal efficiency for wastewater treatment, In: Se-Kwon, K. (Ed.) Encyclopedia of Marine Biotechnology, 1: 459-495. https://doi.org/10.1002/9781119143802.ch15
Szoboszlay, S., B. Atzel, J. Kukolya, E.M. Toth., K.Marialigeti, P. Schumann and B. Kriszt. 2008. Chryseobacterium hungaricum sp. nov., isolated from
hydrocarbon-contaminated soil. International journal of systematic and evolutionary microbiology, 58(12): 2748-2754. https://doi.org/10.1099/ijs.0.65847-0
Tabata, K., H. Abe,and Y. Doi. 2000. Microbial degradation of poly(aspartic acid) by two isolated strains of Pedobacter sp. and Sphingomonas sp. Biomacromolecules, 1(2): 157–161. https://doi. org/10.1021/bm9900038
Vasconcelos, A. L., F.D. Andreote, T. Defalco, E. Delbaje, L Barrientos, A.C. Dias, et al. 2022. Mucilaginibacter sp. strain metal (loid) and antibiotic resistance isolated from estuarine soil contaminated mine tailing from the Fundão dam. Genes, 13(2): 174. https://doi.org/10.3390/genes13020174
Vélez, J. M. B., J.G. Martínez, J.T. Ospina and S.O. Agudelo. 2021. Bioremediation potential of Pseudomonas genus isolates from residual water, capable of tolerating lead through mechanisms of exopolysaccharide production and biosorption. Biotechnology Reports, 32. https://doi. org/10.1016/j.btre.2021.e00685
Wang, H. R., X.R. Du, Z.Y. Zhang, F.J. Feng and J.M. Zhang. 2023. Rhizosphere Interface Microbiome Reassembly by Arbuscular Mycorrhizal Fungi Weakens Cadmium Migration Dynamics. iMeta 2(4). https://doi. org/10.1002/imt2.133
Xiao, M., Z.E. Long, X. Fu and L. Zou. 2024. Versatile applications and mechanisms of genus Exiguobacterium in bioremediating heavy metals and organic pollutants: A review. International Biodeterioration & Biodegradation, 194. https://doi.org/10.1016/j. ibiod.2024.105884
Xiong J.Q., M.B. Kurade, R.A. Abou-Shanab, M.K. Ji, J. Choi, J.O. Kim, B.H. Jeon. 2016. Biodegradation of carbamazepine using freshwater microalgae Chlamydomonas mexicana and Scenedesmus obliquus and the determination of its metabolic fate. Bioresource Technology, 205:183-190. https://doi.org/10.1016/j. biortech.2016.01.038
Xiong, Q., L.X. Hu, Y.S. Liu, J.L. Zhao, L.Y. He and G.G. Ying. 2021. Microalgae-based technology for antibiotics removal: From mechanisms to application
of innovational hybrid systems. Environment international, 155, 106594. https://doi.org/10.1016/j. envint.2021.106594
Xu, X., W. Liu, S. Tian, W. Wang, Q. Qi, P. Jiang, X. Gao, F. Li, H. Li and H.Yu. 2018. Petroleum Hydrocarbon-Degrading bacteria for the remediation of oil pollution under aerobic conditions: a perspective analysis. Frontiers in microbiology, 9. https://doi. org/10.3389/fmicb.2018.02885
You, X.Y., J.H. Liu, H. Tian, Y. Ding, QY. Bu.,K.X. Zhang, et al. 2022. Mucilaginibacter phenanthrenivorans sp. nov., a novel phenanthrene degradation bacterium isolated from wetland Soil. Current Microbiology, 79(382). https://doi. org/10.1007/s00284-022-03085-z
Zhang, W., J. Li, Y.Zhang, X. Wu, Z. Zhou, et al. 2022. Characterization of a novel glyphosatedegrading bacterial species, Chryseobacterium sp. Y16C, and evaluation of its effects on microbial communities in glyphosate-contaminated soil. Journal of Hazardous Materials 432. https://doi.org/10.1016/j. jhazmat.2022.128689
Zhang, Y., Ai, D., K. Liu, S. Sun, Y. Li, D. Huang and J. Zhang. 2024. Microbial succession and enrichment of antibiotic resistance genes during algal-bacterial biofilm purification of aquaculture wastewater. Journal of Water Process Engineering, 64. https://doi.org/10.1016/j. jwpe.2024.105642
Zhou, G. J., G.G. Ying, S. Liu, L.J. Zhou, Z.F. Chen and F.Q. Peng. 2014. Simultaneous removal of inorganic and organic compounds in wastewater by freshwater green microalgae. Environmental Science: Processes & Impacts, 16(8), 2018-2027. https://doi.org/10.1039/ c4em00094c
Zhu F., E. Doyle, C. Zhu, D. Zhou, C. Gu, J. Gao. 2020. Metagenomic analysis exploring microbial assemblages and functional genes potentially involved in di (2-ethylhexyl) phthalate degradation in soil. Science of the Total Environment,715. https://doi.org/10.1016/j. scitotenv.2020.137037
Remote Sensing in Iberá Wetlands, Argentina:
Uncrewed Aircraft Systems and Service Learning
Supporting Environmental Resilience
Nickolas D. Macchiarella, James T. Deal, and Kevin A. Adkins1
Abstract
Mapping wetland regions is challenging. Uncrewed aircraft systems (UAS) have inherent abilities to rapidly and efficiently remotely sense difficult to access locations. Wetland regions around the world are under pressure due to increasing human presence and climate change. The Iberá Wetlands in Argentina is an example of a region that greatly rebounded from detritus effects from human activity. Although the Iberá Wetlands suffered great environmental degradation with numerous large animals being driven from the region, visitors today see a biologically diverse region bearing the fruit of restoration. It serves as a prime location for demonstrating the ability of UAS to efficiently collect data to monitor the environment. A team traveled to the Iberá Wetlands to work with local stakeholders with a common interest in the health of the regional environment. The team flew UAS equipped with RGB and multispectral cameras with local stakeholders. The UAS could generate vegetative indices in real time for viewing as well as record video and still images for post hoc analysis. The team worked with concerned parties from the local community and agencies from the nongovernmental sector to gather and show how UAS could readily collect data facilitating the understanding of the Iberá Wetlands and its current state of being.
Keywords: UAS; remote sensing; wetlands; service learning; NDVI; Iberá
Introduction
Wetland communities across the world are grappling with a need to better understand, mitigate, and adapt to the interlinked environmental stressors caused by increasing human presence and climate change driven effects. The Iberá Wetlands in Argentina are an example of wetlands under stress, and an example
of implemented responses designed to restore the natural environment while enhancing its resilience. Environmental resilience addresses understanding and managing the impacts of change while preventing those impacts from growing worse. The Iberá Wetlands have some unique stressors and ensuing tailored responses that included restoration of natural waterflow and reintroduction of animals. Uncrewed aircraft systems (UAS) (i.e., drones) have applications for gathering data through remote sensing that enables real-time data gathering for interpretation and presentation to concerned stakeholders. University students, while studying abroad, participated in operational phases of UAS missions for remote sensing to include planning, preparing aircraft and sensors, collecting data, and processing data. Data were prepared into photogrammetric products and imagery for local stakeholders and the international community. The data helped educate about readily available remote sensing technologies and the positive effects that restoring the Iberá Wetlands has on livelihoods in rural communities.
UAS are ideal platforms for remotely sensing critical wetlands due to the inherent advantage of aerial perspective, flexible application for hard to access locations, and precise flight path management (Davis et al. 2022). Innovative applications of UAS are made possible by the ever-increasing power of microprocessors and increases in data bandwidth; these technological advantages are occurring while relative costs decrease for levels of computing power and increasing bandwidth (Macchiarella et al. 2019a). Intelligent automation makes ultra-precise flight path control and navigation possible for UAS. A UAS provides users with a versatile remote sensing platform that can attain vantage points that were impossible in the past with manned aircraft or terrestrial means. Imagery obtained by remote sensing during UAS flight is being used in varying instances to create orthomosaic maps with levels of detail previously unavailable. Multispectral imaging enables rapid assessment of the health and wellbeing of plant life. Furthermore, UAS obtained imagery can be processed using photogrammetric techniques to create three dimensions of data for developing digital surface models (DSM),
and terrain visualization through 3D virtualization. Hence, UAS obtained data and derived products can aid in understanding the environment.
Mapping wetlands is challenging (Jeziorska 2019). The physicality of the surface (i.e., diversity and concentration of vegetation combined with water saturation and surface instability) makes access by ground vehicle and boat difficult. Aerial platform mobility is unaffected by wetland surface conditions. Traditional piloted aviation and satellites can serve as solutions; however, both are associated with higher cost and lower temporal and spatial resolutions. Higher spatial resolution pictures, higher temporal frequency, relative ease of use, and flexibility of deployment are hallmarks of UAS when used for remote sensing imagery. Specifically, the National Oceanic and Atmospheric Administration (NOAA) determined that, “UAS are a valuable addition to wetland monitoring efforts and will dramatically increase the ability to detect change at the temporal and spatial scales that are important to wetland scientists and managers” (Davis et al. 2022).
A team composed of faculty, students, and local nationals participated in a study abroad servicelearning project in the Iberá Wetlands. The team flew UAS equipped with multispectral and RGB cameras over the wetlands during June 2024 to help gauge the impact of conservation efforts. The data were processed to determine vegetation reflectance values in visible light and infrared. Images were converted into orthomosaics and normalized difference vegetative index (NDVI) maps to demonstrate the ability to comprehensively assess vegetative health in a targeted portion of the Iberá National Park. This effort aimed to assess the impact that conservation efforts have had on the region and demonstrate UAS as a valid tool for performing environmental monitoring in these remote regions. These efforts highlight the contribution that innovative uses of UAS have for guiding conservation strategies and managing sustainable land practices in an ecologically significant wetland.
University students from three different aerospacerelated undergraduate degree programs participated as members of multidisciplinary teams. The teams accomplished group projects centered on learning
outcomes associated with applying UAS for remote sensing in a wetland environment. Teams analyzed given problems for data collection that could be solved using UAS platforms and sensors. Students determined and implemented the steps that were necessary for collecting data, analyzing data, drawing conclusions, generating usable information, and making presentations to stakeholders in the region. The nature of assigned teamwork required capturing multispectral and visible light imagery that documented the current environmental health of select areas.
The Iberá Wetlands an Environmentally Important Region
Background
Situated in northeastern Argentina, the Iberá Wetlands stand as the world’s second-largest wetland with a size of over 13,000 square kilometers, hosting a remarkable array of over 4,000 plant and animal species, and representing 30% of the nation’s biodiversity (Allen 2023). Despite its ecological significance, this remote region faces challenges arising from climate change and human activities. Commercial foresting, poaching, and cattle ranching significantly degraded the region to the point that several species—including jaguars, giant otters, tapirs, and giant anteaters—had completely vanished, and several more species faced the same fate
Figure 1. Iberá Wetlands located in Argentina (Pettersson and de Carvalho 2021)
(Allen 2023). To address these concerns and ensure the preservation of this critical ecosystem, the Iberá Provincial Park was established in 1983, signifying a strategic commitment to conservation and sustainable management practices, including the reintroduction of key species and the promotion of ecotourism.
The Iberá Wetlands have historically faced threats from climate change and human activities. Extensive conservation efforts, led by organizations like the Rewilding Argentina Foundation and the Argentinian government, have successfully revitalized the region (López 2022). Noteworthy achievements include the reintroduction of key species and enhancements in local plant life through sustainable farming practices and restoring water flow.
Between December 2022 and February 2023, the Iberá Wetlands suffered a large wildfire burning over 5200 square kilometers or over a third of its total area (Cassidy 2023). The contributing factors to the fire’s generation were a prolonged drought and high temperatures that were attributed to climate change and the La Niña weather pattern. The fire greatly impacted conservation efforts, but despite this setback, the region continues to be revived. Continuing assessment of the progress of conservation efforts, amid temporary setbacks and climate change is important. These continuing assessments provide crucial information that is necessary for making informed decisions that help to increase the resilience of the wetlands.
Importance
In 2015, member nations of the United Nations (UN) adopted Sustainable Development Goals. The goals aim to protect the world’s environment from humaninduced effects and climate change. Biodiversity loss is an environmental problem for nations to mitigate. Terrestrial ecosystems are essential elements to overall health and viability of the land for supporting humanity. UN Sustainable Development Goal 15 addresses Life on Land. It describes healthy land environments, challenges, and solutions (United Nations Environment Programme 2015). Maintaining soil quality, enabling biodiversity, preserving water quality, regulating proper water flow, and implementing erosion control all help reduce possibilities of natural
disaster (e.g., floods, landslides). These efforts help the climate while maintaining agricultural productivity. Preserving healthy ecosystems greatly supports mitigation of climate change and adaptation efforts to help local communities thrive in a world of increasing environmental pressures.
The Iberá Wetlands represents an extremely ecologically significant region for Argentina and the entire world. The wetland contains over 30% of the nation’s total biodiversity while only making up a small fraction of the of the country’s landmass (Allen 2023). The wetlands are densely packed with vegetation and wildlife that are faced with threats from climate change and human activities. The protection of this region is imperative for preserving native animals. Over the years, many restoration and conservation efforts have been undertaken to bring the wetlands back to health and save several species from extinction in the region (López 2022). Locally extinct species included the giant anteater, tapir, collared peccary, pampas deer, ocelot, giant river otter, and jaguar. Restoration efforts have successfully reintroduced these animals to the region.
Restoration
Through the conservation efforts of Doug and Kristine Tompkins, Conservation Land Trust, and later Rewilding Argentina Foundation, the area covered by the Iberá Provincial Park was expanded significantly into the largest protected wetland in the country. It includes 7,580 square kilometers. The Rewilding Argentina Foundation allowed the region to return from biodiversity loss that was caused mainly by agriculture practice and overhunting. The ongoing restoration effort has reintroduced animal species, restored water flow, and enhanced the health of local vegetation. The Iberá Wetlands now consists of replenished wetlands that teem with traditional wildlife (López 2022).
Locales for UAS Operations
Two sites were chosen as representative of the land use in the wetlands. These locations supported a robust sample for data collection while serving as an exemplar of the overall conditions in the Iberá Wetlands. The first site is a mixed-use location consisting of a cattle ranch and wetlands environment. This site represents
the cohabitation of human activities and conservation areas, including sustainable farming and grazing practices along with ecotourism. The pasturing fields are regularly cycled to prevent overgrazing and allowing fields to regenerate over time. The second site is purely a conservation region with a focus on ecotourism. The area presents a minimalization of human activity and is well preserved in its natural state. It generates revenue from ecotourism to help continue conservation efforts. The site consisted of a small building facility that is surrounded by nature trails and the wetlands environment. This location provided the most encounters with native wildlife and often serves as an area to introduce visitors to the local ecosystem.
Camera Technologies
The DJI Mavic 3M (DJI 2024) served as the primary UAS for remotely sensing the selected areas (DJI 2024). Its compact form factor and weight of 951 grams makes it easy to transport and take to remote locations by various means to include kayak and horseback (Figure 2). The DJI Mavic 3M integrates both an RGB camera and four multispectral cameras that are sensitive in green, red, red edge, and near infrared (Tables 1 and 2). The combination of these cameras allows for highly detailed RGB and multispectral imagery to be captured simultaneously. The UAS supports viewing the RGB camera and processed vegetative indices (e.g., NDVI) in real time.
Attribute Specification
Image Sensor
Lens
ISO Range
Shutter Speed
Image Size
4/3 CMOS
Effective Pixels: 20 MP
FOV: 84°
Equivalent focal length: 24 mm
Aperture: f/2.8 to f/11
Focus: 1 m to ∞
100-6400
Electronic shutter: 8-1/8000 s
Mechanical shutter: 8-1/2000 s
5280×3956
Table 1. Mavic 3M RGB camera specifications (DJI 2024)
Attribute Specification
Image Sensor
Lens
1/2.8-inch CMOS, effective pixels: 5 MP
FOV: 73.91° (61.2° x 48.10°)
Equivalent focal length: 25 mm
Aperture: f/2.0
Focus: Fixed Focus
Multispectral Camera Band Green (G): 560 ± 16 nm;
Red (R): 650 ± 16 nm;
Red Edge (RE): 730 ± 16 nm;
Near infrared (NIR): 860 ± 26 nm;
Shutter Speed
Electronic Shutter: 1/30~1/12800 s
Max Image Size 2592×1944
Video Format MP4 (MPEG-4 AVC/H.264)
Video Resolution
H.264
FHD: 1920 x 1080@30fpsse
Video content: NDVI/GNDVI/NDRE
2024)
Flight Methodology
Automated flight transects and free-flight missions were used to collect aerial imagery. Images captured by the UAS were processed using commercially available Structure-from-Motion (SfM) software, Pix4Dmapper Pro (v. 4.9.0) (Pix4D 2024a). The resulting highresolution orthomosaic image was 18.7 times more detailed than commonly available satellite imagery.
The orthomosaic was produced using photogrammetry, a process that creates spatially accurate, map-like images by stitching together multiple overlapping
Figure 2. DJI Mavic 3M, left, and hand controller, right.
Table 2. Mavic 3M Multispectral camera specifications (DJI
photos (Figure 3). Photogrammetry is defined as “the art and science of obtaining useful information from the environment by processing imagery and then applying exacting measures that can provide 3D characteristics” (Macchiarella et al. 2019a).
Figure 3. Representative transects for automated positioning of drone to create image overlap enabling SfM parallactic perspectives (Jensen 2007)
Indices and Visualization
The Mavic 3M’s multispectral camera supports the creation of several indices for determining plant health. The most common vegetative index for assessing plant health is the NDVI. Plants strongly absorb visible light (from 400 nm to 700 nm) for use in photosynthesis. Conversely, the plant leaf cell structure strongly reflects near-infrared (NIR) light (above700 nm to 1100 nm) because of photosynthesis (Weier and Herring 2000). The combination of NIR with red bands is often adopted for biomass estimation, canopy structure, and assessment of specific plant health. The NDVI formula is (NIR-RED)/(NIR+RED). These indices and ratios between bands are normalized, obtaining values that range between –1 and +1, and allow the use of thresholding values to differentiate between distinct land covers (e.g., a value of NDVI greater than 0.4 usually indicates the presence of live vegetation) (Bevington et al. 2018). Also, Mavic 3M also allows a real-time view of vegetation indices (e.g., NDVI).
Service Learning
Overview
Service learning is a dynamic and impactful form of experiential education in which students acquire knowledge and skills through active engagement in real-world tasks that address specific, identified community or societal needs. Unlike traditional classroom instruction, service learning integrates meaningful service activities with structured opportunities for reflection, critical analysis, and the application of academic content. In this pedagogical model, learning objectives are central—they shape the design of the learning experience and define clear, measurable outcomes for students. According to Astin and Sax (1998) and Caspersz and Olaru (2017), this approach fosters not only the achievement of academic goals but also a deeper and more authentic understanding of the subject matter, as students recognize the relevance and broader implications of their work.
Rather than being an add-on to existing curriculum, service learning reframes education as an interactive and transformative event. It challenges students to think beyond theoretical knowledge, encouraging them to apply their learning in ways that generate tangible benefits for individuals, communities, or partner organizations. As Aceves and Aceves (2008) argue, service learning represents a paradigm shift where education and community needs intersect, thereby cultivating a sense of social responsibility and mutual benefit.
Within aerospace and aviation disciplines, service learning is particularly well-suited to fields that emphasize applied technologies, such as the use of UAS for environmental remote sensing. These tools offer students the opportunity to execute real-world missions in authentic environments, reinforcing technical competencies while simultaneously contributing to meaningful data collection and analysis. For example, in the service-learning project conducted in the Iberá Wetlands of Argentina, student missions were directly tied to ecological monitoring goals. These missions provided valuable environmental data to local stakeholders while offering students hands-on
experience in mission planning, data acquisition, and sensor integration.
Assessment plays a critical role in maximizing the educational value of service learning. Both formative and summative assessments are used to evaluate student progress and ensure alignment with instructional goals. Formative assessments, conducted during the execution of UAS missions, provide timely feedback that helps refine skills and correct misunderstandings in real-time. Summative assessments, on the other hand, are conducted after the completion of a project or mission set and measure student achievement against established performance standards. These assessments verify that students have met intended learning outcomes, including technical proficiency, problemsolving ability, teamwork, and effective communication.
The Iberá Wetlands project illustrates how experiential missions tied to service-learning principles can result in positive educational gains. Under faculty supervision, students achieved mission readiness and ultimately demonstrated competence in conducting remote sensing operations in ecologically sensitive areas. They were also able to engage with local communities, enhancing both their technical and interpersonal skills. The dual benefit of meeting academic objectives while providing actionable information to local stakeholders exemplifies the multifaceted value of this instructional strategy.
A growing body of research supports the broad and lasting benefits of service learning. Bettencourt (2015), building on earlier work by Astin and Sax (1998), found that participation in service-learning projects significantly enhances students’ domain-specific knowledge, cultivates vital life skills, and promotes civic engagement. Further studies have highlighted additional gains, including personal development, improved interpersonal communication, stronger leadership abilities, and enhanced career readiness (Macchiarella et al. 2019b). Moreover, service learning can be particularly effective in immersive, crosscultural contexts where students also gain language proficiency, cultural awareness, and the ability to integrate interdisciplinary knowledge across diverse subject areas (Gebert et al. 2019).
In summary, service learning is a powerful instructional strategy that blends academic rigor with practical application. In higher education, it offers a pathway for students to become not only competent professionals but also socially engaged and globally conscious citizens. When thoughtfully implemented, service learning deepens academic learning, strengthens community ties, and equips students with the competencies needed to address real-world challenges in meaningful and lasting ways.
Local Stakeholders
Three principal local stakeholders in the Iberá Wetlands directly benefited from the service-learning project: a working cattle ranch, a local elementary school, and an ecotourism operation. The broader community also derives significant economic benefit from ecotourism, which serves as a key driver of sustainable development in the region.
The profitability of ecotourism plays a critical role in justifying governmental and non-governmental investments aimed at establishing an “ecologically complete” national park (Pettersson and de Carvalho 2021). By involving area residents in the development and operation of sustainable businesses, ecotourism can help strengthen communities. This engagement not only supports job creation but also contributes to the preservation of local culture and traditions. Moreover, ecotourism has the potential to raise environmental awareness among both visitors and locals. It fosters a deeper understanding of ecological issues, promotes respect for nature, and encourages the protection of natural surroundings, all while delivering enriching experiences to tourists. Through this multifaceted impact, ecotourism serves as a powerful tool for conservation, education, and community development.
The Buena Vista Estancia is a working cattle ranch in the Iberá Wetlands. The ranch also hosts ecotourism activities as a supplemental economic endeavor. These activities help to build environmental and cultural awareness and provide financial benefits for conservation. The ranch staff were oriented to UAS for remote sensing (Figure 4).
International Engagement
Escuela Albergue Nº 364 is a small rural public elementary school that is located adjacent to Bueno Vista Estancia. Rural education in Argentina largely occurs in a multigrade teaching setting. The students from Escuela Albergue Nº 364 were included during UAS operations and provided with orientation activities that allowed hands-on UAS flight experience, application of remote sensing, and exposure to the possibilities made available by drones (Figure 4).
Puerto Galarza is a commercial ecotourism activity that provides tours through the estuaries and lagoons of the Iberá Wetlands. Tour activities include bird watching, boat rides, flora and fauna lectures, and walks through estuaries, grasslands, and native forests (Figure 5). The tour guides provide educational experiences that elucidate to visitors how organisms in the wetlands interact in a semi-aquatic zone with both water and dryland characteristics. The present state of wetland health and biodiversity are directly observable.

The Brevard Zoo, located in Melbourne, Florida, USA, is engaged in conservation, community outreach, and education. University students remotely sensing the Iberá Wetlands for service learning connected in real time with zoo summer camp students. The educational event included live-casting UAS video conferencing of the wetlands, wildlife, and on location participants. Through the live cast, a guided discussion with online videoconferencing software occurred. The zoo summer camp students participated in question-and-answer sessions. Specifically of interest were animals like the capybara (Figure 6) that live both at the zoo and in the Iberá Wetlands. Zoo summer camp students were familiarized with UAS operations in wetlands and how drones are uniquely suited for remote sensing in difficult to reach locations. This learning experience was enriching for the zoo summer campers, and it reinforced the need to protect and preserve the Iberá Wetlands and conversely wetlands globally.

Remote Monitoring Iberá Wetlands for Environmental Resilience
Two NDVIs, One Camera
The Mavic 3M drone can generate live, real-time NDVI visualizations using video from its multispectral camera, while also capturing high-resolution still images for later post-processing. Post-processing generated an indexed NDVI map, using red and near
Figure 4. Left: Rancher examining drone with university student during a servicelearning event. Right: Local elementary school students participating in a remote sensing flight.
Figure 5. Ecotourism boat transitioning through the Iberá Wetlands.
Figure 6. Wild capybara located in the Iberá Wetlands.
infrared images, and an orthomosaic based on RGB images with SfM software (Figure 7). Figure 8 shows drone positions, where still images were captured during programmed flight above the working area of the Buena Vista Estancia. Captured still images were processed using SfM methods with Pix4Dmapper Pro.
Two Types of Sites: Mixed Use Wetland with Cattle Ranch and Natural Wetlands
Two sites were analyzed during the project. One site was a cattle ranch embedded in the wetlands and the other location comprised purely preserved wetlands. Both sites offered unique perspectives of how regenerative efforts were fruitful in restoring and subsequently maintaining the health of the wetlands. The cattle ranch demonstrated the effects of sustainable farming practices while the natural wetlands demonstrated the effects of conservation and preservation with human presence in the form of ecotourists.
Buena Vista Estancia Mixed Use Wetland
The cattle ranch included a central area of buildings with a residence and equipment facilities surrounded by various fields for mixed uses (e.g., temporary holding of animals and hosting ecotourist activities). The associated fields and surrounding wetlands were imaged with programmed flight to ensure parameters were obtained for virtual models, orthomosaic development, and production of an indexed map of vegetative health that are all products of post-processing (Figures 7 and 8). The flight patterns were lawnmower-like (i.e., back and forth rows). The programmed flights occurred at a height of 29 meters altitude above takeoff (ATO). The post-processing project yielded an average ground sampling distance (GSD) of 0.83 cm per pixel with root mean square positional error in meters with values of x = 0.497, y = 0.574, and z = 0.664. Due to this project’s nature, positional accuracy was adequate using the inherent precision of global navigation satellite systems. No further positional enhancements were required (i.e., real-time kinematic or post-processed kinematic). Live RGB imagery and multispectral imagery of the NDVI were also obtained to allow examination of these same areas in real time with local stakeholders (Figures 10, 11, and 12).
Both post-processed and live NDVI deliver useful information that is derived from the same camera multispectral sensor. Examining the post-processed NDVI index map of the cattle ranch (Figure 7) shows that highly grazed areas are evident and clearly identifiable indicating lower IR reflectance using the applied traditional indexing scale. Green indicates lower IR reflectance and lower health vegetation when using a traditional indexing scale. The red, or healthy, areas are typically fields that are either unused or only lightly grazed by livestock, and are covered with trees, wetland shrubs, and grasses. The live NDVI paints a similar picture. The healthy regions are indicated in red, while the less healthy regions are colored green. Water and other surfaces are indicated in black or blue. The live images corroborate the post-processed NDVI index map, for the same locations, by indicating heavily grazed areas as less healthy regions. However, the live NDVI does show more context of the surrounding environment than the NDVI index map or RGB orthomosaic due to its oblique perspectives (Figures 10 and 11). However, planar and nadir examination angles provide the viewer with a comprehensible image of a location that is 2D and map like. Oblique perspectives provide foreground and surrounding scenes placing the area of interest in context. The live NDVI captures the relatively dense vegetation surrounding the cattle ranch and identifies it as a healthy environment. The live NDVI also provides a more detailed view of the surrounding trees, including those that have lost their leaves due to seasonal change (i.e., it is fall in the southern hemisphere). The trees lacking leaves are clearly identified as green, standing out from the surrounding healthy trees, which are colored in red. The trees unaffected by seasonal changes, confirmed with the RGB orthomosaic, were evergreen trees and shrubs. The imaging occurred during June in the southern hemisphere. Due to the latitude and local geography, fall and winter conditions are very mild in temperate northern Argentina. The adjacent wetlands, visible in the background of Figure 9, display varying levels of healthy vegetation, with no visibly dead or barren areas.
The central area of buildings (Figure 9) contains Locations A, B, and C each with a vector arrow indicating camera positions and viewing directions. Location A (Figure 10) shows the main ranch building as seen from the northwest while facing southeast. The wetlands visible at the top of Figure 10 correspond to the southwest section of the orthomosaic shown in Figure 9. Additionally, in both figures, healthy and unhealthy trees are discernable in the area surrounding the main building. One deciduous tree is noteworthy. Location B, facing southeast (Figure 11), shows a communal field adjacent to a corral. The ground of the communal field is worn and indicates lower health due to use from recreational activities. This is shown by the RGB and NDVI imagery (Figure 11). Location C (Figure 12) provides a nadir view of the main ranch building, small shed, and two vehicles while facing southwest. The three locations display different land uses and their appearance in RGB and multispectral imagery from both a post processed orthomosaic nadir perspective and live viewing perspective.





Figure 7. Left: Post-processed NDVI map. Right: Orthomosaic of mixed-use wetland at the Buena Vista Estancia (Pix4D 2024).
Figure 8. Left: UAS camera locations depicted above point cloud. Right: A triangle mesh of the mixed-use wetland at the Buena Vista Estancia (Pix4D 2024).
Figure 9. Left: NDVI indexed map of reflectance values. Right: RGB orthomosaic at the main ranch site with location markers of the three live images with view orientation at Locations A, B, and C (Pix4D 2024).
Figure 10. Live image Location A in RGB (left) and NDVI (right) of wetlands abutting main ranch site with noteworthy tree – oblique angle.
Figure 11. Live image Location B in RGB (left) and NDVI (right) of the adjacent recreational field at the main ranch site – oblique angle.
Figure 12. Live image Location C in RGB (left) and NDVI (right) of the central building at the main ranch site with rooftops and vehicles – nadir angle.
Puerto Galarza Natural Wetlands
The natural wetland site is characterized by a wide variety of vegetation, open tracts of water, and canals (Figure 13). The live RGB imagery clearly shows a difference between water, bare land, and vegetation. The live NDVI easily distinguishes between the denser soil-based vegetation, shown in red, and the thinner suspended algae, shown in green (Figure 13). The cause of this distinction is that the soil raises the plants out of the water, allowing for the full reflectance values to reach the sensor. Algae can also be monitored using RGB and multispectral sensors. The purpose of the UAS operation over the Iberá Wetlands was not to fully assess the vegetative health of the region, but to demonstrate the technology to local stakeholders and assess possibilities using vegetative indices like NDVI. The inherent ability of the Mavic 3M’s multispectral camera in NIR, 860nm ± 26 nm, allows some data collection ascertaining the presence of algae and its proliferation. Studies by Pokrzywinski et al. (2022) and Cannizzaro et al. (2019) provide greater detailing on the remote sensing possibilities afforded by hyper and multispectral sensors mounted on drones and flown at wetlands of interest. UAS significantly reduces the time cost of collecting data for algae.
An orthomosaic and NDVI index map was not produced at this location. The decision was made to focus upon the live NDVI to examine the area based upon the interest and needs of the local collaborators. The homogeneous nature of the vegetation and abundance of water makes image mosaicking challenging in this type of wetland, but possible. Live RGB and NDVI imaging occurred at an altitude of 29 meters. If mosaic images are desired for locations with highly homogeneous coverings, increasing flight altitude opens the camera’s field of view (FOV) (Pix4D 2024). This increased FOV affords greater possibilities of capturing features that support the stitching of individual images into a mosaic. Also having large degrees of image overlap (e.g., 75% frontlap and sidelap, or greater) greatly assists with stitching. Altitude always represents a functional tradeoff between resolution (i.e., GSD) and mission flight time. One photogrammetric advantage for imaging wetlands is that the flatness lends itself to rectification.
Comparison of Post-Processed NDVI Map and Live NDVI Image
Multispectral data is crucial for assessing and monitoring vegetation (Pappalardo and Andrade 2022). Live NDVI simplifies this by providing immediate insights into plant health without the need for post-processing. Traditionally, a major drawback of multispectral sensors was the time-consuming post-processing needed to extract useful information. Creating an NDVI map requires images with sufficient overlap and resolution (GSD) to ensure accuracy (Davis et al. 2022). This is especially important in areas like wetlands, where the landscape often has uniform textures and complex shapes, making image analysis more challenging (Pix4D 2024).
The NDVI video is depicted in real time to the drone operator and user of information, in this case a stakeholder (e.g., cattle rancher, biologist, ecotourist guide). This real-time delivery of information can enhance comprehension of the environmental conditions and increase user engagement (Lv et al. 2022). When an area of interest is found, it is quick and simple to switch between live NDVI and RGB video using the hand controller. Cycling between live sensor modes presents two sets of images for the same area. The drone operator and stakeholder can analyze each image scene while qualitatively applying elements of aerial image interpretation, to include size, shape, shadow, tone, color, texture, pattern, height, and depth (Aber et al. 2019). Stakeholders can play a vital role by directing the UAS to designated areas of interest. The UAS operator can record video and still images affording opportunities for post hoc analysis and sharing.
Figure 13. Live RGB (left) and live NDVI (right) images of natural wetland at Puerto Galarza with examples of denser soil-based vegetation outlined, and suspended algae, outlined in purple.
Utility of Data Collected
Using UAS (drones) for remote sensing in environmentally important areas offers multiple benefits to stakeholders. It creates opportunities for collaboration among diverse groups, including residents, academic institutions, NGOs, government agencies, and others. These multidisciplinary teams—often with international representation—bring a wide range of perspectives and expertise, leading to outcomes greater than what any one group could achieve alone.
In the Iberá Wetlands service-learning project, local stakeholders gained access to processed environmental data for future planning and research. This data supports the use of advanced UAS technologies and provides timely spatial information. Additionally, by sharing data internationally—through live streams (e.g., with the Brevard Zoo) or academic outputs—the project helps raise global awareness of the Iberá Wetlands and showcases Argentina’s success in wetland and biodiversity restoration.
The data collected, processed, and shared from the Iberá Wetlands helps to further validate UAS as a cost-efficient, high-resolution means for monitoring while simultaneously increasing the extent of spatial coverage, resolution, and timeliness of monitoring. UAS have the potential to effectively monitor wetlands on short temporal scales and as fast as real time. RGB images, and the derived orthomosaic image, truly provide a remarkable level of detail as evidenced by the GSD of .83 cm. This level of detail allows experts to review images and identify specific plants, by type, using highly accurate colorization details. The resolution of UAS imagery allows for accurate classification of plant life in wetlands and associated habitats. The flight altitude of 29 meters ATO was selected with the aim of gathering ultra-high-resolution images. This level of detail is sufficient for experts to examine imagery and conduct classification of plants if desired. Previous work conducted by NOAA (Davis et al. 2022) corroborates this assessment.
Photogrammetric techniques do have limitations in geometrically complex and vegetatively dense locations like wetlands. The challenge remains to choose a GSD that provides a level of detail that is necessary to
meet the needs as identified for the specific project by the stakeholders. Over extremely uniform areas (i.e., covered with geometrically similar vegetation and water surfaces) imagery with a GSD greater than 10 cm will be necessary for stitching mosaics. This is confirmed by guidelines published for SfM processing (Pix4D 2024).
Live RGB video and real-time NDVI video are not constrained by the challenges of stitching during post processing. The live techniques can provide extremely detailed imagery in real time and be recorded. However, as stated, the live imagery with its oblique viewing angles provides the viewer with a picture that is set in context with the surrounding environment due to slanting perspectives (Figures 10 and 11).
Photogrammetrically derived orthomosaic images, with planar perspectives and nadir examination angles, create a comprehensible 2D map perspective. Examining wetland areas using a combination of oblique live viewing areas and planar orthomosaic maps provide a high level of detail. This combination of approaches to remote sensing was very helpful while examining the cattle ranch. The technique also worked in the natural wetland areas of Puerto Galarza. However, levels of detail were lower here for the planar orthomosaic image.
The Iberá Wetlands serves as one use case for UAS remote sensing. Each wetland has unique characteristics that need to be addressed. The decision to use real-time imagery, postprocessed imagery, or a combination of the two is a function of user needs, time, and resources.
Figure 14. Ecotourism in Iberá Wetlands kayaking (left) and horseback tours (right).
Figure 15. Wildlife in the Iberá Wetlands caiman (left) and fly catcher (right).
Conclusions
The collection of UAS remotely sensed data, obtained during service learning, demonstrated an effective means for assessing vegetative health in the Iberá Wetlands. This occurred from both post-processed and real-time perspectives. The post-processed NDVI index maps, orthomosaic maps, and recorded live video afforded opportunities to examine images, discuss findings in detail, and share data. The live RGB and NDVI imagery provided a real-time means of interacting with stakeholders, affording a high degree of cognitive connection between what is seen
in the real world with human eyes and what is seen through the aerial perspective from the drone’s camera. Insights gained here help validate the ability of UAS to remotely sense wetland environments to help concerned stakeholders understand the true state of vegetation. Integrating UAS-based remote sensing operations over wetlands into service-learning initiatives enriches academic curricula by exposing students and stakeholders to advanced methods for assessing vegetative health and ecosystem dynamics through realtime, data-driven analysis.
Although the Iberá Wetlands once experienced severe environmental degradation—marked by the loss of large wildlife, reduced water flow, and declining biodiversity—visitors today witness a thriving, biologically rich ecosystem. This transformation reflects a large-scale restoration initiative aligned with the UN Sustainable Development Goal for Life on Land. Intensive conservation efforts by both private and government organizations have successfully restored the region, promoted sustainable use, and increased biodiversity. At the study sites, the wetlands were vibrant with native vegetation and abundant wildlife.
Upon close inspection, from the aerial perspective afforded by UAS, the environment appeared healthy. The mixed-use site showed clear signs of humanrelated activities that included cultivation, grazing cattle, and horses. The mixed-use site is embedded into the wetlands. The immediate areas in the surrounding terrain were packed with dense wetland vegetation and associated animal life. The natural wetlands site at Puerto Galarza demonstrated the effectiveness of preservation in a purely natural state environment. There was no evidence of human presence other than ecotourist. The site was home to a large population of caimans and capybaras in their natural habitat and offered visitors the opportunity to explore the area via nature trails, kayaking, and guided boat tours. Overall, the chosen sites were representative of the two main use cases of land and water in the Iberá Wetlands and provided a sample to assess the effects of conservation strategies in the region. Despite numerous setbacks from natural and human activities, the Iberá Wetlands have rebounded and are thriving once more. The data collected, in the form of visible light and multispectral
Figure 16. Ecotourism journey on horseback through the Iberá Wetlands.
imaging, with subsequent inspection and consultation with local stakeholders, demonstrated a significant increase in the region’s health and the positive effects of conservation programs. The return of the region to its original state, where the wetlands are a functioning component of the ecosystem within Argentina, aligns with the UN Sustainable Development Goal 15 Life on Land.
AUTHOR DECLARATION
This is an original work that has not been previously published. Citations are given for quotations from published sources. Sources for all images are also given in figure legends.
References
Aber, J.S., I. Marzolff, J.B. Ries, and S.E.W. Aber. 2019. Small-Format Aerial Photography and UAS Imagery. Second Edition. Elsevier. doi: 10.1016/C20160-03506-4.
Aceves, R.I. and P.A. Aceves. 2008. Preparing Aviators for the 21st Century: A 3-Year Case Study of Service Learning in the Aviation Classroom. Collegiate Aviation Review International 26(1). doi: 10.22488/ okstate.18.100362.
Allen, D. 2023. The Iberá Wetlands: Argentina’s Answer to Yellowstone https://www.bbc.com/travel/ article/20230614-the-iber-wetlands-argentinas-answerto-yellowstone
Astin, A. and L. Sax. 1998. How Undergraduates Are Affected by Service Participation. Journal of College Student Development 39.
Bettencourt, M. 2015. Supporting Student Learning Outcomes Through Service Learning. Foreign Language Annals 48(3), pp. 473–490. doi: 10.1111/ flan.12147.
Bevington, A., H. Gleason, X. Giroux-Bougard, and T. de Jong. 2018. A Review of Free Optical Satellite Imagery for Watershed-Scale Landscape Analysis. Confluence: Journal of Watershed Science and Management 2. doi: 10.22230/jwsm.2018v2n2a18.
Cannizzaro, J.P. et al. 2019. Remote detection of cyanobacteria blooms in an optically shallow subtropical lagoonal estuary using MODIS data. Remote Sensing of Environment 231, p. 111227. doi: 10.1016/j. rse.2019.111227.
Caspersz, D. and D. Olaru. 2017. The value of servicelearning: the student perspective. Studies in Higher Education 42(4), pp. 685–700. https://doi.org/10.1080/0 3075079.2015.1070818.
Cassidy, E. 2023. Fires Burn in Argentina’s Iberá National Park https://earthobservatory.nasa.gov/ images/151011/fires-burn-in-argentinas-ibera-nationalpark.
Davis, J., R. Giannelli, C. Falvo, B. Puckett, J. Ridge, and E. Smith, E. 2022. Best practices for incorporating UAS image collection into wetland monitoring efforts: a guide for entry level users.
DJI. 2024. DJI Mavic 3M User Manual. 1.0. DJI. https://ag.dji.com/mavic-3-m/specs.
Esri. 2023. ArcGIS Pro 3.1.0.
Esri. 2024. Data Classification Methods. https://pro. arcgis.com/en/pro-app/latest/help/mapping/layerproperties/data-classification-methods.htm.
Gebert, G., L. Griffin, J. Lawlor, L. Davis, K. Vander Velde, and S. Ali. 2019. Advanced Photogrammetric Modeling of Dranoc Kullas Using Small Unmanned Aircraft Systems. https://commons.erau.edu/studentworks/144
Jensen, J.R. 2007. Remote Sensing of the Environment. Upper Saddle River, NJ: Prentice Hall.
Jeziorska, J. 2019. UAS for Wetland Mapping and Hydrological Modeling. Remote Sensing 11(17), p. 1997. doi: 10.3390/rs11171997.
López, M. 2022. Iberá Project https://www. rewildingargentina.org/Iberá-project/.
Lv, J., C. Cao, Q. Xu, L., Ni, X. Shao, and Y. Shi. 2022. How Live Streaming Interactions and Their Visual Stimuli Affect Users’ Sustained Engagement Behaviour—A Comparative Experiment Using Live
and Virtual Live Streaming. Sustainability 14(14), p. 8907. doi: 10.3390/su14148907.
Macchiarella, N.D., J. Robbins, and D. Cashdollar. 2019a. Rapid Virtual Object Development using Photogrammetric Imagery Obtained with Small Unmanned Aircraft Systems -Applications for Disaster Assessment and Cultural Heritage Preservation. AIAA SciTech 2019 Forum. Reston, Virginia: American Institute of Aeronautics and Astronautics. doi: 10.2514/6.2019-1974.
Macchiarella, S., R. McGinnis, K. Thaci, and N. Macchiarella,. 2019b. Cultural Heritage Preservation Through Service Learning: Applying Unmanned Technologies in the Balkans Bridging Technical and Social Science. CIEE Annual Conference. New York, New York.
Pappalardo, S. E. and D. Andrade. 2022. Drones for Good: UAS Applications in Agroecology and Organic Farming. 1st ed. CRC Press, pp. 122–148. doi: 10.1201/9780429052842-8.
Pettersson, H.L. and S.H.C. de Carvalho. 2021. Rewilding and gazetting the Iberá National Park: Using an asset approach to evaluate project success. Conservation Science and Practice 3(5). doi: 10.1111/ csp2.258.
Pix4D. 2024a. Pix4Dmapper Pro.
Pix4D. 2024. How to Improve the Outputs of Dense Vegetation Areas Using PIX4Dmapper?
Pokrzywinski, K., R. Johansen, M. Reif, S. Bourne, S., Hammond, and B. Fernando. 2022. Remote sensing of the cyanobacteria life cycle: A mesocosm temporal assessment of a Microcystis sp. bloom using coincident unmanned aircraft system (UAS) hyperspectral imagery and ground sampling efforts. Harmful Algae 117, p. 102268. doi: 10.1016/j.hal.2022.102268.no
United Nations Environment Programme. 2015. UNEP and the Sustainable Development Goals https://www. unep.org/explore-topics/sustainable-development-goals
Weier, J. and D. Herring. 2000. Measuring Vegetation (NDVI & EVI). https://earthobservatory.nasa.gov/ features/MeasuringVegetation
OBSERVATIONS FROM WETLANDS THIS SUMMER AND FALL
By Ralph Tiner
During this year, I’ve visited a number of wetlands— most during wetland training courses I’ve taught. Here’s some examples of what I’ve seen on these trips.
EARLY SUMMER
From the wetlands of eastern Massachusetts in June.
MID SUMMER
I noticed these wetlands while cycling along the Cape Cod rail trail in Massachusetts.
While looking like a disaster zone, this is the beginning of a wetland restoration project by the Harwich Conservation Trust (HCT) that involves converting a Cape Cod cranberry bog (cultivated) to a more naturally functioning wetland. This disturbance is breaking up the cranberry bog turf, creating microtopography for colonization by a variety of species. This is one of a number of HCT restoration projects; check out their work at: https://harwichconservationtrust.org/ecorestoration-projects/
Swamp Milkweed (Asclepias incarnata) from a wet meadow.
Smooth Winterberry (Ilex laevigata).
These bogs are a type of farmed wetland with their origin as some type of wooded swamp, often an Atlantic white cedar swamp.
FALL
In Illinois, the prairies were painted yellow at this time of year, yet a few other colored flowers were also found when you looked closely near the ground.
Typical commercial cranberry bog on Cape Cod.
View of a prairie in Lake County, Illinois during field exercise; plenty of Common Sneezeweed (Helenium autumnale) was present in the wettest areas.
Cup Plant (Silphium perfoliatum) in a Lake County (IL) prairie.
Prairie Dock or Prairie Rosinweed (Silphium terebinthinaceum).
Common Sneezeweed (Helenium autumnale).
Sawtooth Sunflower (Helianthus grosseserratus).
Praying Mantis (likely Tenodera sinensis) on a goldenrod in an Illinois prairie.
Closed Bottle Gentian (Gentiana andrewsii).
Wild Mint (Mentha arvensis).
Swamp Asters (Symphyotrichum puniceum) showing variable colors.
Button Eryngium (Eryngium yuccifolium).
Obedient Plant (Physostegia virginiana).
Purpleleaf Willowherb (Epilobium coloratum).
Salt marshes are most colorful in the fall... views from a Connecticut salt marsh at Barn Island Wildlife Management Area.
View across the marsh toward an area where I have a few permanent plots along a transect to document vegetation change with rising sea levels.
Flooded pannes in the high marsh, with island in the background. (Note: Purplish leaves of Black Gum Nyssa sylvatica on the island.)
Perennial Saltmarsh Aster (Symphyotrichum tenuifolium).
Common Glasswort (Salicornia depressa) turns from green to red in the fall.
Saltmarsh False Foxglove or Seaside Gerardia (Agalinis maritima).
The swamps of New Jersey offered a different collection of wild life.
Seaside Goldenrod (Solidago sempervirens).
Groundsel Tree or Eastern Baccharis (Baccharis halimifolia).
Blue Crab (Callinectes sapidus) in the high marsh at high tide.
Buttressed trunk of Pin Oak (Quercus palustris).
A crayfish (possibly the White River Crayfish, Procambarus acutus) that a student found buried in the soil (no crayfish “chimney” observed) when digging a soil pit in the Great Swamp...quite a surprise!
Local fisherman enjoying beautiful September day on the Passaic River (NJ). We all need to spend more time on the water—fishing, kayaking, canoeing, or on the banks—observing wildlife. No bugs and mild temperatures make for a wonderful outdoor experience.
Common Winterberry (Ilex verticillata).
Wood Frog (Lithobates sylvaticus or Rana sylvatica) in New Jersey’s Great Swamp. I saw many at this site in late September.
Wildlife from urban wetland in the park of the North Carolina Museum of Art in Raleigh, North Carolina.
They were moving along a stream in the park but stopped to gaze at the goats the park had fenced in as an experiment to control invasive and undesirable species (e.g., Japanese Stiltgrass or Nepalese Browntop -Microstegium vimineum and Poison IvyToxicodendron radicans). Two were entranced by the goats, but the youngest stared right at me. I was there to see the site that the museum has planned for wetland restoration along the stream.
Incredible pose of White-tail Deer (Odocoileus virginianus).
Listed below are some links to news articles that may be of interest. Links from past issues can be accessed on the SWS website news page. This section includes links to mostly newspaper, magazine, and news articles. Members are encouraged to send links to articles about wetlands in their local area. Please send the links to the WSP Editor at chrstphrcrft@gmail.com and reference “Wetlands in the News” in the subject box. Thanks for your support.
• From 14 birds to 557: Whooping Cranes reach record numbers in 2025
• Officials issue warning after invading carnivorous plants take over local waterways: ‘It can be blown around or moved around by waves’
• Hundreds Of Mountain Yellow-Legged Frogs Leap Back Into Wild In SoCal
• The call of a native frog is heard again in Southern California thanks to help from Mexico and AI
• Program to reduce conflicts with beavers keeping nature’s ‘engineers’ on the job
• Volunteers turn Ribble Valley grassland into network of wetlands
• Invasive — And Cannibalistic — Bullfrogs Are Taking Over The West
• Developers destroyed a Tampa wetland. Then they tried an unusual move.
• Time is running out to save this Texas bird refuge from beach homes
• Colorado’s subalpine wetlands may be producing a toxic form of mercury – that’s a concern for downstream water supplies
• No more flattened frogs: Oregon’s newest wildlife crossing latest in growing movement
• Strategically bringing back beavers could support healthy and climate-resilient watersheds
• ‘It’s destruction disguised as progress’: how the oil industry is sucking Iraq’s ancient wetlands dry
• Efforts to curb flooding at battleship memorial yield results
• Experts thrilled after successfully reintroducing endangered creature into wild after 30-year absence: ‘We’re hearing them again’
• ‘Livers of the river’ found in Grand Rapids area polluted creek offer hope for recovery
• Sri Lanka plans restoring revoked protection for an important mangrove patch in the island’s North
• Scientists issue urgent warnings about future of vital US land: ‘We don’t have a lot of time’
• Louisiana Pulls the Plug on the Nation’s Largest Ecosystem Restoration Project | Audubon
• Scientists issue urgent warnings about future of vital US land: ‘We don’t have a lot of time’
• Man hit with severe penalties for repeated crimes at protected wetlands: ‘Today, we are holding him accountable’
• Friends of the Everglades News
• NC Environmental Management Commission votes to update ‘wetlands’ definition
• Experts celebrate as long-lost species returns to wetlands for first time in nearly a century: ‘We’re delighted’
• Beavers helping restore native habitat in Portland metro area
• Scientist: Lake water treatment system working | The Daily Standard Stories
• Graduate students make astounding discovery while studying problem plant: ‘It just gets stronger and stronger’
• Recycled glass could help fend off coastal erosion
• Army Corps devises plan to address chronic Lake St. Clair muck
• Virginia Middle Peninsula marshes bring $90M annual economic benefits
• To Defend Against Russian Tanks, Finland and Poland Consider Restoring Wetlands
• Wealthy benefactor’s big donation could transform crucial wetlands: ‘Critically important to the future’
• Farmers flip fields to wetland for Central Valley shorebirds | Sacramento Bee
• Spokane River in crisis as wildlife habitats dry up | Local News
Please help us add new books and government wetland reports to this listing. If your agency, organization, or institution has published new publications on wetlands, please send the information to the Editor of Wetland Science & Practice. Your cooperation is appreciated.
BOOKS
• The Atchafalaya River Basin: History and Ecology of an American Wetland
• Bayou D’Arbonne Swamp: A Naturalist’s Memoir of Place
• Bayou-Diversity: Nature and People in the Louisiana Bayou Country
• Black Swan Lake – Life of a Wetland
• Coastal Wetlands of the World: Geology, Ecology, Distribution and Applications
• Constructed Wetlands and Sustainable Development
• Creating and Restoring Wetlands: From Theory to Practice
• Eager: The Surprising Secret Life of Beavers and Why They Matter
• Florida’s Wetlands
• Ghosts of Iraqi Marshes, A Conflict of History, Tragedy and Restoration
• History of Wetland Science: A Perspective from Wetland Leaders
• An Introduction to the Aquatic Insects of North America (5th Edition)
• Mid-Atlantic Freshwater Wetlands: Science, Management, Policy, and Practice
• Remote Sensing of Wetlands: Applications and Advances
• Salt Marsh Secrets. Who uncovered them and how?
• Sedges of Maine
• Sedges and Rushes of Minnesota
• Tidal Wetlands Primer: An Introduction to their Ecology, Natural History, Status and Conservation
• Tussock Sedge: A Wetland Superplant
• Wading Right In: Discovering the Nature of Wetlands
• Waubesa Wetlands: New Look at an Old Gem
• Wetland Ecosystems
• Wetland Indicators – A Guide to Wetland Formation, Identification, Delineation, Classification, and Mapping
• Wetland Landscape Characterization: Practical Tools, Methods, and Approaches for Landscape Ecology
• Wetlands (5th Edition)
• Wetland Restoration: A Handbook for New Zealand Freshwater Systems
• Wetland Soils: Genesis, Hydrology, Landscapes, and Classification
• Wetland & Stream Rapid Assessments: Development, Validation, and Application
• Wetland Techniques (3 volumes)
• Wildflowers and Other Plants of Iowa Wetlands
About WETLAND SCIENCE & PRACTICE (WSP)
Wetland Science & Practice (WSP) is the SWS quarterly publication aimed at providing information on select SWS activities (technical committee summaries, chapter workshop overview/abstracts, and SWS-funded student activities), articles on ongoing or recently completed wetland research, restoration, or management projects, freelance articles on the general ecology and natural history of wetlands, and highlights of current events. The July issue is typically dedicated to publishing the proceedings of our annual conference. WSP also serves as an outlet for commentaries, perspectives, and opinions on important developments in wetland science, theory, management and policy. Both invited and unsolicited manuscripts are reviewed by the WSP editor for suitability for publication. When deemed necessary or upon request, some articles are subject to scientific peer review. Student papers are welcomed. Please see publication guidelines herein. Electronic access to WSP is included in your SWS membership. All issues published, except the current issue, are available via the internet to the general public. The current issue is only available to SWS members; it will be available to the public four months after its publication when the next issue is released (e.g., the January 2025 issue will be an open access issue in April 2025). WSP is an excellent choice to convey the results of your projects or interest in wetlands to others. Also note that WSP will publish advertisements; contact info@sws.org for details.
HOW YOU CAN HELP
If you read something you like in WSP, or that you think someone else would find interesting, be sure to share. Share links to your Facebook, X, Instagram, and LinkedIn accounts. Make sure that all your SWS colleagues are checking out our recent issues, and help spread the word about SWS to non-members! Questions? Contact editor Christopher Craft (chrstphrcrft@gmail.com).
WSP MANUSCRIPT – GENERAL GUIDELINES FOR AUTHOR AND ARTICLES
AUTHOR ETHICS AND DECLARATION:
The work is original and has not been published elsewhere. Data reported in submission must be author’s own and/or data that the author has permission to use. Inclusion of results from previously published studies must be appropriately credited. It is vital that all contributing authors review the initial submission and subsequent versions. Upon submission of the final manuscript, the lead author must submit a declaration stating that all contributing authors have reviewed and approve the final manuscript. Failure to do this will lead to rejection of the manuscript. Also please include a statement of originality in the article after the Acknowledgements and before the References section. Such statement should be something like this:
Declaration of Originality
This is an original work that has not been published before. Images, figures, and quotations included in the article have been properly cited and permission has been granted for any that are not those of the author.
LENGTH:
Approximately 5,000 words; can be longer if necessary.
STYLE:
See existing articles from 2014 to more recent years available online at: https://members.sws.org/wetland-science-and-practice. Standard format/outline for articles: Title, authors (include affiliations and correspondence author email in footnotes), followed by Abstract, then Text (e.g., Introduction, Methods, Results, Discussion, and Conclusion), and ending with References. All articles must have an abstract. Keywords are optional.
TEXT:
Word document, 12 font, Times New Roman, single-spaced; keep tables and figures separate, although captions can be included in text. For reference citations in text use this format: (Smith 2016; Jones and Whithead 2014; Peterson et al. 2010). Do not perform formatting (e.g., capitalization of headings and subheadings). For example, do not indent paragraphs… just separate paragraphs by lines.
FIGURES:
Please include color images and photos of subject wetland(s) as WSP is a full-color e-publication. Image size should be less than 1MB; 500KB may work best for this e-publication. Figures should be original (not published elsewhere) or in the public domain. If the figure was published elsewhere (copyrighted), it is the responsibility of the author to secure permission for use. Be sure to provide proper credit in the caption.
Reference Citation Examples:
• Clements, F.E. 1916. Plant Succession: An Analysis of the Development of Vegetation. Carnegie Institution of Washington. Washington D.C. Publication 242.
• Colburn, E.A. 2004. Vernal Pools: Natural History and Conservation. McDonald & Woodward Publishing Company, Blacksburg, VA.
• Cole, C.A. and R.P. Brooks. 2000. Patterns of wetland hydrology in the Ridge and Valley Province, Pennsylvania, USA. Wetlands 20: 438-447. https://doi.org/10.1672/02775212(2000)020<0438:POWHIT>2.0.CO;2
• Cook, E.R., R. Seager, M.A. Cane, and D.W. Stahle. 2007. North American drought: reconstructions, causes, and consequences. Earth-Science Reviews 81: 93-134.
• Cooper, D.J. and D.M. Merritt. 2012. Assessing the water needs of riparian and wetland vegetation in the western United States. U.S.D.A., Forest Service, Rocky Mountain Research Station, Ft. Collins, CO. Gen. Tech. Rep. RMRSGTR-282.
• van der Valk, A. 2023. The beginnings of wetland science in Britain: Agnes Arber and William H. Pearsall. Wetland Science & Practice 41(1): 10-18. https://doi.org/10.1672/ ucrt083-01
Please be sure to add the DOI link to citations where possible. If you have questions, please contact the editor, Christopher Craft, at chrstphrcrft@gmail.com.