PMES 23 Interviews
With One Health, Sidra Medicine, Leader Life Sciences, and Biorus
PMES 23 Guide

Everything you need to know about this month’s PrecisionMed Exhibition & Summit 2023
COVER STORY
Dr. Ahmad Abou Tayoun

PMES 23 Interviews
With One Health, Sidra Medicine, Leader Life Sciences, and Biorus
PMES 23 Guide

Everything you need to know about this month’s PrecisionMed Exhibition & Summit 2023
Dr. Ahmad Abou Tayoun
The Al Jalila Genomics Center director who wants to ensure that no child in the region goes untreated
(to make precision medicine accessible for all)

At Sheikh Shakhbout Medical City, we don’t just treat conditions. We care for people. As one of the UAE’s largest and most advanced hospitals, we partner with the world-renowned Mayo Clinic to offer a global team of experts who focus on one thing: you.

DAVID STRADLING
Managing Director
It is with great pleasure that I welcome you to the PrecisionMed Exhibition & Summit 2023, the premier event dedicated to advancing this important field in the GCC and wider Middle East and North Africa (MENA). As Managing Director of PMES, I am honoured to be part of this transformative journey that unites a plethora of distinguished healthcare leaders and experts from around the world.
It’s hard to believe that our debut was only 12 months ago, and I am absolutely delighted to be welcoming back our partners and sponsors who believed in us and our mission in 2022. I’d also like to offer my gratitude to our brand-new partners and sponsors who have contributed to PMES 23 becoming twice as large as our first event.
Precision medicine represents the way forward in healthcare, offering us the opportunity to revolutionise disease treatment through a targeted and personalised approach. By understanding the intricate complexities of genetics, we can unlock a new era of healthcare where each patient receives tailored and effective treatments. The global market for precision medicine has already shown remarkable growth, with a value of $73.5 billion in 2022, projected to exceed $175 billion by 2030.
PMES 23 is not only a platform for knowledge sharing and collaboration but also a testament to the region’s commitment to advancing precision medicine. Generously supported by the UAE’s Ministry of Industry and Advanced Technology (MoIAT), Ministry of Health and Prevention (MoHAP), Department of Health (DoH) Abu Dhabi, and the Dubai Health Authority (DHA), I look forward to everyone fostering engaging discussions, insightful presentations, and interactive exhibits that showcase the latest breakthroughs and technologies in precision medicine. Also returning this year is EMERGE 2050. Co-located with PMES 23, this forum focuses
on innovation and investment in healthtech. connecting technology disruptors at startup and scale-up stage with the capital and partnering needed to take projects to fruition.
As we gather in Dubai, we have an unprecedented opportunity to shape the future of healthcare in the Middle East. The region’s precision medicine market is projected to witness a compound annual growth rate (CAGR) of approximately 7.37% and could exceed a value of $8.6 billion by 2032. This makes it one of the top four growth regions globally, just behind Eastern Europe, North America, and Asia Pacific. PMES 23 represents a crucial milestone in this journey. By reviewing the practical application of precision medicine and encouraging clinical adoption, we will contribute to the transformation of healthcare delivery in the region. Together, we will explore innovative solutions, discuss emerging trends, and forge collaborations that will enable us to harness the power of precision medicine for the benefit of patients across the Middle East.
Once again, I extend my deepest gratitude to you – the participants – partners, sponsors, exhibitors, speakers and more who have made PMES 23 possible. Your commitment to advancing precision medicine is inspiring, and I have no doubt that this event will serve as a catalyst for ground-breaking discoveries and advancements in the field.
The future of medicine is precise.
 David Stradling Managing Director
David Stradling Managing Director

PrecisionMed Exhibition & Summit (PMES) EMERGE 2050

PrecisionMed Exhibition & Summit (PMES) and EMERGE 2050
Managing Director David Stradling d.stradling@precisionmedexpo.com
Event Director Tea Vutmej t.vutmej@precisionmedexpo.com
Marketing Director Jasmir Bains j.bains@precisionmedexpo.com
International Sales Director Simon Willard s.willard@precisionmedexpo.com
Organised by Smart Planet Media Limited Exhibitions & Conferences
PrecisionMed International Magazine
Managing Editor Rachel McArthur rachel@digitalinkdubai.com
Creative Director Ben White ben@thewhitecreative.com
Cover Photography Paul Macleod
Editorial Contributors Jennifer Bell, Orla Browne, Lily Lawes, Karim Mansour
All images © iStock/Getty Images unless supplied by PMES 23 and EMERGE 2050 exhibitors, partners, interviewees and similar. All rights reserved. No part of this magazine may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or other electronic or mechanical methods, without the prior written permission of the publisher, except in the case of brief quotations embodied in critical reviews and certain other noncommercial uses permitted by copyright law.
precisionmedexpo.com emergeghi.com
Aiming for a future where healthcare is precise, personalised, and transformative…










































38.

47. PMES 23 EXHIBITOR LIST Who’s appearing?

77. EMERGE 2050


What not to miss Meet the speakers, innovators, and learn about the conference lineup



Minister of State for Public Education and Advanced Technology at the UAE Ministry of Industry & Advanced Technology, and Chairwoman of the Emirates Scientists Council
In her role as Minister of State for Public Education and Advanced Technology, Her Excellency Sarah Al Amiri spearheads the efforts of the UAE’s Ministry of Industry & Advanced Technology to empower the adoption of Fourth Industrial Revolution technologies and promote research and development in the advanced science and technology sector to shift towards a knowledge economy. Her Excellency is also the Chairwoman of the UAE Council of Scientists that aims to create an environment conducive to innovation and scientific research in the country and raise the impact of scientific research by representing the scientific community nationally and internationally, attracting, and building a generation of scientists in various scientific fields, and stimulating cooperation among research institutions in all sectors (government, private and higher education).



Dr. Asma Al Mannaei leads the implementation of quality measures and frameworks for the healthcare system, conducts inspections to ensure compliance with regulations, enforces standards, and promotes the adoption of world-class best practices and performance targets among healthcare service providers in Abu Dhabi.

The introduction of the JAWDA programme in 2014 marked a significant milestone in fostering a culture of healthcare quality across the sector, thereby enhancing the emirate’s position as a hub for medical tourism.
In 2018, Dr. Al Mannaei collaborated with stakeholders to launch the TIP in Healthcare Award, which fosters research-industry partnerships in conjunction with the Department of Economic Development (DED). She also spearheads a team dedicated to establishing the Healthcare Innovation Center in Abu Dhabi, in collaboration with partners such as the Abu Dhabi Global Market, Plug and Play, and Mubadala.


President at Vision Care Inc., Japan
Dr. Takahashi is a Japanese medical physician, ophthalmologist, and stem cell researcher, who is the president of Vision Care Inc., and also serves as a project research leader at the Riken Center for Developmental Biology focusing on the clinical application of iPS Cell (induced Pluripotent Stem Cell) technology on macular degeneration.
In 2014, Dr. Takahashi was named by the British science journal, “Nature” as one of “Five to Watch” global scientists for her groundbreaking work in regenerative medicine.
In March 2017, a team led by Dr. Takahashi completed the first successful transplant of iPS-derived retinal cells into the eye of a patient suffering from advanced wet age-related macular degeneration.

Chief Research Officer and Chair of the Precision Medicine Programme at Sidra Medicine, Qatar


Dr. Khalid Fakhro joined Sidra Medicine as a principal investigator in July 2014, and became director of th e facility’s Human Genetics department, where he built a robust genomic medicine research pipeline for the hospital.
In 2018, he became the first Director of Precision Medicine at Sidra, embedding research in genomics and personalised medicine in the heart of Sidra Medicine’s academic medical enterprise. He holds adjunct faculty appointments at Weill Cornell Medicine, Qatar and Hamad Bin Khalifa University, where he mentors the next generation of PhD students in human genetics and genomic medicine.

Vice Chancellor of Research and Director at Thumbay Institute for Precision Medicine, Gulf Medical University, UAE
In 1986, Dr. Salem Chouaib was appointed as a research asso ciate and joined the Tumour Biology department at the Institut Gustave Roussy. For over twenty years, he led a research unit at the French National Institute of Health and Biomedical Research (INSERM). His research was constantly directed at promoting the transfer of fundamental concepts to clinical application, especially in the fields of cancer vaccine and cancer immunotherapy.
Prof. Chouaib is a member of several notable societies including the American Association of Immunologists, the New York Academy of Sciences, the French Society of Immunologists and the American Association for Cancer Research.


Chair of Preventive Medicine, Medical Subspecialties Institute and Lead, Executive Health Program at Cleveland Clinic Abu Dhabi
Dr. Nicole Sirotin is among the first medical professionals globally to be certified as diplomates of the ABLM/American College of Lifestyle M edicine (ACLM) and the International Board of Lifestyle Medicine.
Prior to joining Cleveland Clinic Abu Dhabi, Dr. Sirotin was assistant professor of medicine at Weill Cornell Medical College and served as medical director of the Weill Cornell Center for Human Rights. Prior to Weill Cornell, Dr. Sirotin was associate program director of the primary care/social medicine residency program at Albert Einstein College of Medicine, Montefiore Medical Center.
DR. JUN
Director and Professor, Center for iPS Cell Research and Application (CiRA) at Kyoto University, Japan
Dr. Jun Takahashi is the director and a professor at the Center for iPS Cell Research and Application (CiRA), Kyoto University, Japan, and will be conducting a plenary speech on Parkinson’s disease cell therapy using iPS cells and a clinical trial.

After returning to Kyoto University Hospital, he conducted functional neurosurgery including deep brain stimulation and also research work on stem cell therapy for Parkinson’s disease. In 2012, he became a full professor at CiRA, pursuing stem cell therapies for Parkinson’s disease patients. He started the world’s first clinical trial for Parkinson’s disease using iPS cells in 2018.



Chief Science Officer and Director of Genomic Medicine, and Senior S&T Advisor, Healthcare at Sanimed International Lab and Management (UAE) – an International Holding Company
With over thirty years of professional experience in national laboratories, academia and private companies, Dr. Min has developed, managed, and executed more than $400 million in R&D projects in cancer, genomics, and renewable energy in territories including the United States, Korea, China, and the United Arab Emirates.
Amongst the companies he Dr. Min has served at include the Los Alamos National Laboratory in the UDA, the Korea Advanced Institute of Science and Technology, the Chinese Academy of Sciences, the China State Development and Investment Co, China’s GrandOmics Biosciences, and G42 Healthcare UAE as the founding director of the Emirati Genome Program. He has served on international scientific committees such as NIH-USA, DOE-USA, NSF-USA, NSERC-NSF-Canada, the World Health Organization, the Wellcome Trust Ltd UK, National Science Foundation (NSF) China, and National S&T Panel, Korea.


Chair of Genetics and Genomics Department at the College of Medicine & Health Sciences at UAE University


Also serving as metabolic consultant at Tawam Hospital, Prof. Fatma Al Jasmi pursued her postgraduate studies at University of Toronto, and Hospital for Sick Children, Canada.
In 2006, Prof. Al Jasmi received Canadian Board of Pediatrics after completing the paediatric residency program. Subsequently, she conducted her fellowship in biochemical genetics and in 2008 was certified via the Canadian College of Medical Genetics Board in biochemical genetics.
Prof. Al Jasmi is one of the founders of the UAE Rare Disease Society and has established the Biochemical Genetic Fellowship at UAEU in collaboration with Tawam Hospital. She has received several prestigious awards, including the Prime Minister Award for Excellence in a Specialised Job (2017), the Chancellors’ Innovation Award (2015), induction into the Women in Science (WiS) Hall of Fame as an outstanding woman in science across the Middle East and North Africa region (2015), the L’Oreal UNESCO For Women in Science Pan Arab Award (2013), and the Sheikh Rashid Bin Saeed Al-Maktoum Award for Excellent Achievements in Medicine (2000).
Professor in Molecular and Genetic Medicine for the Department of Genetics and Genomics at the College of Medicine and Health Sciences at UAE University
Prof. Bassam Ali obtained his PhD from Cambridge University and spent ten years working at Imperial College London before joining UAEU in 2006. With over 150 published original articles and reviews, his research focuses on the cellular and molecular mechanisms of human disease and pharmacogenomics.
Recognising his outstanding contributions, Prof. Ali has received prestigious accolades such as the 2018 Khalifa Education Award for Distinguished University Professor in Research and the 2019 Shoman Award for Arab Scientists in Health Sciences. He also serves on the editorial boards of several renowned international scientific journals, including his roles as an associate editor for “Human Genomics,” “Frontiers in Genetics,” and “Frontiers in Pediatrics.”


Principal Investigator at the Biotechnology Research Centre for the Technology Innovation Institute (TII), UAE Dr. Shaikha Almazrouei is an accomplished researcher and speaker in the field of biotechnology. Dr. Almazrouei received her PhD from King’s College London, where she studied liver transplant using hepatocyte microbes. She also holds an MSC from UAEU, where she researched the genetic basis of familial hypercholesterolemia, and a Bachelor’s degree in cellular and molecular biology from the same institution.
As an active member of the International Stem Cells Research Association (ISCCR) and the International Cryobiology Society, Dr. Almazrouei is committed to advancing research in the field of biotechnology. She also serves as the Head of the UAE Stem Cell Group and as a Lead Researcher with the Biotechnology Research Centre at the Abu Dhabi Technology Innovation Institute.
Dr. Almazrouei has contributed to several high-profile projects, including the establishment of the Emirate Genome with G42, the establishment of DNA fingerprinting for the UAE Armed Forces and AD police fingerprint unit, and as a research assistant at Khalifa University.
Dr. Almazrouei is dedicated to empowering biotechnology researchers and fostering innovation in the UAE. Her research interests include genomics and regenerative medicine.
DR. AHMAD ABOU TAYOUN


Director of Al Jalila Genomics Center at Al Jalila Children’s Hospital (AJCH), and Associate Professor of Genetics at the Mohammed Bin Rashid University of Medicine and Health Sciences


Receiving his PhD in genetics from Dartmouth Medical School, followed by a clinical molecular genetics fellowship at Harvard Medical School, Dr. Ahmad Abou Tayoun has co-authored over 100 peer-reviewed publications and his main interests include characterising the genomic landscape of rare diseases in the Middle East, developing and translating new diagnostic tools and assays for clinical use, and establishing guidelines for variant interpretation in collaboration with various expert groups, mainly ClinGen, ACMG, AMP, and CAP.
Prior to moving to Dubai, he was a director of genomic diagnostics and assistant professor at the Children’s Hospital of Philadelphia, University of Pennsylvania, USA.

MOHAMED NAGY
Pharmacy Director and Head of the Personalised Medication Management Unit at Children’s Cancer Hospital Egypt (57357)
Nagy is an experienced clinical pharmacist with over 15 years of specialisation in oncology. He is the founder and head of the Personalized Medication Management Unit at the Children’s Cancer Hospital 57357. With a master’s degree in biotechnology from the American University in Cairo (AUC), Mohamed is recognised as one of the leading experts in clinical pharmacogenetics implementation and lifestyle genetics in the Middle East and Africa. His passion lies in utilising genetic knowledge to benefit patients, enabling them to discover personalised medication, nutrition, and sports routines tailored to their unique genetic makeup, resulting in optimal health outcomes.
Furthermore, Mohamed is a founder of the Pharmacogenomics Access & Reimbursement Coalition (PARC) and the Standardizing Laboratory Practices in Pharmacogenomics (STRIPE). He currently leads the Pharmacogenomics Research Network Developing Countries Committee for the Middle East and Africa region. Additionally, he serves as the Leader of the IVPN Personalized Medicine Listserv, further showcasing his leadership and expertise in the field.
Professor and Head of Laboratory Department at the University of Patras, Greece
Prof. George Patrinos is a distinguished Professor of Pharmacogenomics and Pharmaceutical Biotechnology at the University of Patras’s Department of Pharmacy. He also holds adjunct Professorships at Erasmus MC, Faculty of Medicine in Rotterdam (the Netherlands) and the United Arab Emirates University, College of Medicine, Department of Pathology in Al Ain. For a period of 12 years (2010-2022), Prof. Patrinos served as a full member and Greece’s national representative in the CHMP Pharmacogenomics Working Party of the European Medicines Agency (EMA). Since 2018, he has held the position of co-chair of the Global Genomic Medicine Collaborative (G2MC), and in 2020, he took on the role of Editor-in-Chief for the esteemed “The Pharmacogenomics Journal”, published by
Dr. Decock is a group leader at the Translational Cancer and Immunity Center, Qatar Biomedical Research Institute (QBRI) and holds a joint assistant professor position at the College of Health & Life Sciences, Hamad Bin Khalifa University (HBKU). Her current research addresses two pivotal and emerging key areas in breast cancer research: tumour antigen discovery and tumour-immune ecosystem dynamics.
Her research efforts focus on exploring the potential of cancer testis antigens as prime therapeutic targets and modulators of anti-tumour immunity. A second line of her research aims to gain further insight into the molecular complexity and immune contexture of tumours that can dictate clinical outcome and treatment responses.

Dr. Decock is the recipient of several research grants and her track record includes more than 40 papers in high-tier scientific journals with over 2,100 citations and an h-index of 26.


VP, Molecular Biotech and Genomics for the Biotechnology Research Center at the Technology Innovation Institute (TII), UAE
Dr. Thomas Launey oversees a team of talented scientists committed to addressing key issues in the UAE pharma economy and healthcare, focusing on synthetic biology, immunology, and human genomics.

He obtained his PhD in brain science and biophysics before moving to Japan to work with M. Ito, one of the founders of modern neuroscience and the former Director of the Human Frontier Program, at the RIKEN Center for Brain Science in Tokyo.

Prior to joining TII, Dr. Launey led research projects ranging from live imaging and single-neuron biophysics to cell development and transcriptomics. He has contributed significantly to understanding the cellular and molecular mechanisms of long-term memory retention and to studying transcription in neurons, as well as translation regulation in sub-cellular neuronal compartments, all published in prestigious journals. Previously, he held professorships at Waseda University and Tsukuba University in Japan and is an active member of several Japanese, US and European Science Societies.
the Nature Publishing Group.
Prof. Patrinos boasts an extensive publication record with over 300 scientific papers in peer-reviewed journals, including renowned publications like “Nature Genetics”, “Nature Rev Genet”, “Nucleic Acids Res”, and “Genes Dev”. He has co-edited the acclaimed textbook “Molecular Diagnostics,” now in its third edition, published by Academic Press. Additionally, he has contributed to several international textbooks and serves as the editor of the “Translational and Applied Genomics” book series. Furthermore, Prof. Patrinos plays a pivotal role as the main co-organiser of the Golden Helix Conferences, a prestigious international meeting series focusing on Pharmacogenomics and Genomic Medicine.
ealthcare is moving swiftly and surely in the direction of precision medicine. Tailored treatments will provide better patient outcomes and help healthcare systems become much more efficient.
The potential of this field is not lost on the world’s largest health, science and technology corporations, who are making huge investments. The Middle East and Africa precision medicine market will expand at a CAGR of 9.96% to $2.51 billion by 2023. And the field is expected to grow by 11% annually to 2026 to be worth $142 billion globally.
The United Arab Emirates has been quick to sense the opportunity to enhance its healthcare systems, while also capitalising on a new high-growth industry. In line with our economic diversification strategy, we have built a progressive science and technology ecosystem to support key sectors such as precision medicine to evolve at scale.
This ecosystem has provided an effective foundation for innovation. The success stories emerging from the Emirates, supported by Government initiatives and policies, are a clear
indication that the country is becoming an emerging hub for precision medicine.
In March of this year, the UAE launched the National Genome Strategy to help guide advanced healthcare research, innovation and future technology use. The programme is one of the most comprehensive genomic regulatory initiatives in the world and will improve preventive and personalised healthcare within research and medical institutions across the country.
The Emirati Genome Programme is a foundational project within the National Genome Strategy. The UAE will use genomic data from the project to develop national healthcare strategies which address the population’s specific needs now and, in the future, and support the advancement of preventive medicine in the country.
Similarly, the Abu Dhabi Department of Health launched the first Personalised Precision Medicine Programme for oncology in the region. Innovate Life Sciences Lab has also inaugurated a cutting-edge genomics testing lab, while G42 Healthcare is leveraging its experience in data and AI to support its state-of-the-art healthcare centres and digital platforms.
The UAE is also drawing increasing interest from international investors. Our friendly business environment and unique value proposition has attracted some of the world’s top healthcare leaders. For example, Illumina recently opened a stateof-the-art solutions centre in Dubai and several international institutions are exploring biomedical and genomics research centres.
As a global hub for science, advanced technology, and R&D – the UAE has all the key ingredients needed for the development of new healthcare sectors. The country’s science, technology and R&D ecosystems are designed to foster innovation and
Her Excellency Sarah Al Amiri is Minister of State for Public Education and Advanced Technology in the United Arab Emirates and Secretary-General of the Emirates Genome Council.As a global hub for science, advanced technology, and R&D –the UAE has all the key ingredients needed for the development of new healthcare sectors.”
promote collaboration. Further, flagship initiatives such as the Technology Transformation Program (TTP) are leading to new technologies that enable rapid advances in fields such as precision medicine.
Not only is the UAE transforming into an industrial, technological and scientific powerhouse, but it also offers a unique set of competitive advantages. We clearly benefit from a strategic location at the centre of Asia, Europe and Africa, but also have a vibrant startup ecosystem, access to funding and support facilities and offer world-class infrastructure and logistics capabilities, as well as free trade agreements supporting businesses in accessing global markets.
As the UAE’s move towards a knowledge-based, digital economy gathers pace and our leadership continues to prioritise enhancing quality of life in the Emirates, the country will continue to invest heavily in precision medicine and life sciences as part of our broad commitment to the development of science, technology, and research.

We will continue working to attract and foster the best homegrown and international talent
and present ourselves as the ideal location for innovative healthcare companies. At the centre of these efforts is the “Make it in the Emirates” initiative, a campaign launched by the Ministry of Industry and Advanced Technology to attract industrialists, innovators and investors.
At global platforms such as PrecisionMed Exhibition & Summit (PMES), we are actively promoting this initiative and seeking to engage companies and investors from around the world and across industries, including healthcare.
By bringing the best and biggest to the UAE, we aim to drive healthcare transformation and boost the industry and technology sector’s contribution to GDP. In the process, we will continue driving our national development and economic goals to build a brighter future where people can enjoy longer, more fulfilling lives.

he UAE is leading the way in adopting advanced technologies to develop precision medicine and personalised healthcare, according to the CEO of one of the country’s leading medical and diagnostics devices distributors.
Across the globe, medical leaders are taking proactive steps to reduce the prevalence of certain diseases, enable early intervention and identify the most effective treatments. Increasingly, they are turning to precision medicine, which enables medical decisions, practices, and products to be customised to the individual patient according to their genetic footprint, lifestyle, and environment.
Kinjal Sureshchandra Zaveri, CEO of One Health, a subsidiary of PureHealth Group – the largest integrated healthcare platform in the United Arab Emirates (UAE), said that when it comes to precision medicine, the country is a frontrunner in the global race to adopt individual-specific diagnosis, prognosis, and treatment.
The UAE, he says, recognises the vast potential of customised medicine to meet patient needs, in turn increasing the average lifespan of people and improving their quality of life. This is PureHealth’s core vision about longevity which they believe revolves around a healthier, happier life, driven by personalised medicine, artificial intelligence (AI), and predictive diagnostics, that in turn will create a significant difference in people’s lives.
“Precision medicine will be playing a very important role [in healthcare] moving forward, and the Gulf Cooperation Council (GCC) states will definitely adapt to this,” Zaveri said. “The UAE, in particular, I think, is far ahead compared to any
other country in the world.”
This, the CEO elaborated, can be demonstrated by the UAE’s National Genome Strategy, one of the most ambitious programmes yet to analyse the genetic model of a country’s population. Under the strategy, the aim is to collect one million samples from citizens via cheek swabs or blood samples. From each sample, automated sequencing machines will be able to produce a profile of that individual’s genome, their complete set of genetic material.
This information may be able to accurately detect which diseases the person is more vulnerable to or indicate which medications would work most effectively on them.
“The UAE is the first country today already undergoing a whole genome sequencing program for its citizens,” Zaveri said. “The very essence of this project is to understand the genomic language of every citizen in this country, which will form the basis of personalised medicine going forward.
“So, I believe the UAE in particular will be one of the first countries to adopt and embrace this change of personalised medicine or precision medicine.”
For PureHealth, the group is establishing a datadriven technology platform that will enable them to engage with the entire population, baseline their health, provide them with their digital twin based on their genome sequencing, and monitor their health in real-time through wearables and smart homes.

But – as with any technology – comes certain challenges, said Zaveri. Especially when medicine –like many sectors across the world – is on the brink of an AI revolution.
Kinjal Sureshchandra Zaveri, CEO of One Health, delves into why precision medicine has the potential to alter the healthcare landscape in the region.
Precision medicine will be playing a very important role moving forward, and the GCC states will definitely adapt to this. The UAE, in particular, is far ahead compared to any other country in the world.”

He explained that successful precision medicine adoption involves a holistic approach; data analysis, including AI, which, in turn, involves algorithms and the history of a patient.
AI and precision medicine work together to refine medical management. However, this also brings about technical and ethical challenges while software trained on historical data sets can produce a raft of unintended consequences once they start interacting with unpredictable humans and fast-changing time, he pointed out.

As a result, Zaveri believes that AI can never be a substitute for a healthcare professional on the frontline.
“This area, I think, is a little challenging and debatable,” he said. “Especially with the talk of AI. It is useful, but it is debatable too. Because a lot of relevance is given to the historical data available. A lot of algorithms are based on historical data… this could be challenging moving forward because things are changing.
“What was true yesterday may not be true today. If you base your solutions on historical data, sometimes it could be debatable - you may deviate in terms of care.”
He continued: “So, does AI and machine learning provide a high degree of value proposition? Of course, yes. But this one-to-one intervention, this human-to-human intervention is always going to be the most important part of patient care.

“Yes, [AI] will support and will enhance and it will probably improve efficiencies, but it won’t be the final solution for sure. This is how I look at it today, unless things change again, in the next couple of years, or a decade.”
Regardless, precision medicine has the potential to alter the healthcare landscape, according to Zaveri.
“The way it is positioned today – it is basically an approach to tailor-make the disease treatment for an individual,” he said. “I mean, the way you have food or goods delivered at your home – this is exactly what you need if something would happen in the healthcare domain too.
“Every individual is different in terms of genomic sequence. By moving towards tailor-made personalised treatment where you will know exactly
what molecules will work for this treatment for this individual which will make a huge difference in terms of course treatment first, probably cost, and most important turnaround time.
“Getting the genomic data – incorporating other aspects like the molecular makeup, environment, lifestyle, etc – will make it more interesting and hopefully more, more better in treating those diseases and ailments.”

With an extensive network across the UAE – with an in-country customer base of more than 300 healthcare providers – One Health, part of the PureHealth portfolio – provides end-to-end medical solutions for healthcare service providers.
The company’s focus is on looking at ageing as an epidemic and using the latest treatments, technologies, and preventive and social strategies to help people lead optimal, healthier lives. It aims to introduce technologydriven, personalised, preventive healthcare solutions that focus on increasing the average lifespan and improving the health span in the UAE.
“PureHealth is the largest healthcare group in the region,” said Zaveri. “So, we manage and operate a significant amount of healthcare facilities in the UAE. And we are the largest
“The way it is advancing – the speed at which it is advancing – excites me the most and the number of solutions getting created worldwide research being done in this area.”
integrated platform focusing mainly on making healthcare convenient, affordable, and transparent. Being part of PureHealth, One Health is a vertical which focuses on bringing all new technologies of value proposition services to the country, in the healthcare domain. And as an entity, we are committed to delivering best in class solutions to all healthcare providers.”
At the helm of One Health, Zaveri said what excites him the most about his day-to-day role is the vast potential of precision medicine in the healthcare landscape today – and tomorrow.

“As CEO, a key focus is to keep a tab on all these changes happening [in] delivery systems, looking at various solutions and technologies penetrating the market now and, in the future, and making sure that One Health will be one of the relevant players in bringing all these things to all our healthcare providers,” he stated. “With AI coming into the picture, data analysis is becoming very, very relevant. Things are changing much more rapidly compared to what used to happen, say 10 or 15 years back. So, the whole definition of diagnostics will change moving forward.”
With diagnostics being primarily a preventive care tool, when it comes to curative care, the potential of this is genomic testing and follicular testing will become more relevant, said Zaveri.

“The way it is advancing – the speed at which it is advancing – excites me the most and the number of solutions getting created worldwide research being
done in this area.”
The PrecisionMed Exhibition & Summit 2023 (PMES), he added, will provide a perfect platform to engage with other stakeholders and experts in the field and join in the conversation about the wealth of opportunities precision medicine can offer.
“Precision medicine is going to play a big role moving forward in how we are delivering care to the patients,” he said. “In particular, [when it comes to] personalised diagnostics, pharmacogenomics, data analysis - this is where One Health is relevant today, and we can bring all these things which are very, very important to bring precision medicine to the country.”

Dr. Ahmad Abou Tayoun, Director of Al Jalila Genomics Center at Al Jalila Children’s Hospital (AJCH), and Associate Professor of Genetics at the Mohammed Bin Rashid University of Medicine and Health Sciences, believes that precision medicine can only become mainstream once it becomes truly inclusive across the world. Here is what he’s doing to make sure this happens.
 BY LILY LAWES
BY LILY LAWES

ith his impressive global experience and academic background, Ahmad Abou Tayoun, PhD is a key player in genomics research in the Middle East. As Director of Al Jalila Genomics Center at Al Jalila Children’s Hospital (AJCH) in Dubai, as well as Associate Professor of Genetics for the Center for Genomic Discovery at the Mohammed Bin Rashid University of Medicine and Health Sciences, the UAEbased clinical molecular geneticist is on multiple missions to ensure that no child goes untreated.
At present, Dr. Abou Tayoun heads up clinical and research programmes which are part of global efforts to characterise “normal” and “diseasecausing” variations in the human genome, establish genomic standards and guidelines, and create a more representative reference genome – or pangenome – as well as preparing to fight future pandemics. His clinical work at AJCH reinforces the hospital’s mission that every child, regardless of geographic or socioeconomic factors, has an equal opportunity for success in life.

“As a clinical molecular geneticist, my role is to provide genomic diagnostics services to support all paediatric specialties at AJCH. We mainly focus on rare diseases, and we offer comprehensive genomic testing, including rapid whole genome sequencing, whole exome sequencing, targeted disease gene panels, chromosomal microarrays, and targeted assays for methylation and repeat expansion disorders,” Dr. Abou Tayoun explained. “Besides genomic testing, our Genomics Center of Excellence offers genetic counselling services that educate

families on types of genomic tests and helps them understand how genetic conditions might affect them now and in the future.
“The team consists of American board-certified clinical molecular geneticists and genetic counsellors, genomic analysts, bioinformatics scientists, and molecular biologists.”

During PMES 23, Dr. Abou Tayoun is set to provide an update on the upcoming standards and guidelines for genetic sequence variant interpretation on behalf of the American College of Genetics and Genomics, the College of American Pathologists, the Clinical Genome Consortium (ClinGen), and the Association for Molecular Pathology (AMP) workgroup.
This work, which has been ongoing over the past few years to update the previous guideline published in 2015, aims to provide guidance to the genomics community on how to clinically interpret variants identified through genomic sequencing in clinical and research settings.
“In a nutshell, we want to be able to provide comprehensive genomic profiling for patients with suspected rare diseases as quickly, easily, and costeffectively as possible.”
Elaborating on his presentation, Dr. Abou Tayoun stated: “The guideline is moving toward a more objective quantitative approach, which will not only lead to more accurate interpretation, but also facilitate the automation of this cumbersome process.” Noting how important this update is to precision medicine, he added: “This work has a major impact in our field as can be seen from the previous guideline which was cited almost 20,000 times since 2015!” Dr. Abou Tayoun stressed that the update to the standards and guidelines will guide more accurate genomic interpretation and reduce misdiagnoses, while also facilitating the automation of this process and making sense of the vast amount of data downstream of genomic sequencing, which is currently a major bottleneck in the field.
Given its role in transforming paediatric care in the UAE, the AJCH was one of the entities brought under the recently-formed Dubai Academic Health Corporation (DAHC), which aims to advance health services in the emirate through a new academic system that integrates patient care, medical education, and scientific discovery. As part of its mandate, the Corporation seeks to “improve the efficiency, quality, and accessibility of Dubai’s healthcare services in accordance with the highest standards and best practices.” It will also seek to strengthen Dubai’s leadership in academic education and scientific research in medicine and health sciences, as part of the broader strategy to strengthen the knowledge economy. The establishment of the DAHC is set to strengthen the emirate’s position as a global hub for medical and life sciences.

“AJCH has always been committed to understanding the genomic landscape of rare diseases in the Middle East and bringing novel diagnostic tools to patients in this part of the world. The opportunity to work with DAHC, with the ultimate aim to enhance the quality of paediatric care in Dubai, has generated much excitement - and we look forward to progressing the journey,” Dr. Abou Tayoun continued.
Diving deeper into his work at AJCH, Dr. Abou Tayoun shared an update on the hospital’s agreement with Illumina to introduce rapid whole genome sequencing for critically ill children in the Middle East, which was signed in November of 2022.
“As part of this agreement, babies with varying medical conditions in the NICU [neonatal intensive care unit] will be offered a rWGS [rapid whole genome sequencing service] for free over the next two years,” he said, stating that this will benefit around 200 families. “The service will include the return of results to patients and caring physicians within 48-72 hours so they can use the genetic information to better care for patients in this setting.
“While we will be benefiting a lot of families throughout the duration of this study, the long term goal of this project is to generate necessary data demonstrating the clinical and economic utility of this service in the ICU setting,” he added, referring
specifically to diagnostic yield, feasibility, impact on treatment and management, and avoiding unnecessary diagnostic workup, among other advantages.
Further news from AJCH includes a pilot newborn genetic screening program for spinal muscular atrophy (SMA) over the next 2-3 years. Speaking of the initiative, Dr. Abou Tayoun said: “We will be offering genetic screening for 6,500 Emirati newborns for SMA, a life-threatening disease if untreated, and for which there is currently one of the first gene therapies. The goal of this project is to estimate the incidence, prevalence, and carrier frequency of this disease in the UAE population.
“This information will generate data to guide national newborn and/or premarital genetic screening programs for SMA as a prototype for many other rare diseases in the Middle East, which is expected to have a high prevalence of recessive diseases due to the higher proportion of marriages among relatives. In addition, early identification of affected babies will be key for more efficient gene therapy, which is extremely expensive.”
Other collaborations include projects to translate the latest technologies for clinical use for patients, such as with Oxford Nanopore to demonstrate the utility of long-read sequencing in rare disease diagnostics and joining forces with ThermoFisher Scientific and Gulf Scientific to assess the utility of
Part of Dubai Academic Health Corporation (DAHC), Al Jalila Children’s Hospital (AJCH) is the first dedicated children’s hospital in the UAE, established under the directives of His Highness Sheikh Mohammed Bin Rashid Al Maktoum, Vice President and Prime Minister of the UAE, and Ruler of Dubai. The pediatric medical facility was created to affirm His Highness’ belief that all children should have an equal opportunity for success in life, and that a child’s treatment of an illness should not be subject to geographical chance. AJCH’s 200-bed family-friendly medical facility is customised to eliminate any stress or anxiety that might affect children in a traditional hospital environment. Patients are able to enjoy glow-in-the-dark wallpapers
and screens with cartoon characters on the ceilings to provide children with a stress-free setting during scans and x-rays. The hospital design includes open spaces for children to participate in outdoor activities and several other child-friendly elements. Within each patient room, an interactive touch screen enables children to remain in contact with family and friends, play online games, access social media and educational portals when required. As a paediatric hospital, AJCH is equipped with numerous facilities that allow children to continue their education, such as a dedicated study space in every inpatient room and private teachers to assist in continuing their education directly from hospital grounds.
pharmacogenomics in paediatric settings.
Speaking more personally, Dr. Abou Tayoun revealed his dedication towards increasing access to precision medicine in the Middle East, North Africa, and Asia, as well as representation of diverse populations to fill the data gap in the NHGRI-EBI GWAS Catalog, a curated collection of all human genome-wide association studies, produced by a collaboration between the European Bioinformatics Institute (EMBL-EBI) and National Human Genome Research Institute (NHGRI). At present, 78% of genomic data in the catalogue comes from people of European descent.
Born in Kuwait and raised in Lebanon, Dr. Abou Tayoun was inspired to return to the region after his training in the United States to foster the field in the region and to help establish the Al Jalila Genomics Center.
“In a nutshell, we want to be able to provide comprehensive genomic profiling for patients with suspected rare diseases as quickly, easily, and cost-effectively as possible,” he said, noting the challenges of precision diagnostics in the Middle East. The goal, he elaborated, is to reduce the lengthy diagnostic odysseys – which can be longer
than seven years in this part of the world due to a higher prevalence of rare diseases – and to direct timely and effective personalised treatment and management plans.”
Looking to the future of precision medicine, Dr. Abou Tayoun sees an increase in the adoption of artificial intelligence (AI), which is already utilised in his research.

“We use a lot of algorithms and machine learning tools already to automate genomic analysis and interpretation,” he said. “So, we are adopters of AI and are keeping an eye on how to best utilise [it] safely and effectively.”
For the next 5-10 years, Dr. Abou Tayoun has a bold vision.
“From my perspective, I would love to see that every baby gets their whole genome sequenced and interpreted, cost-effectively and accurately, as part of a newborn screening program. This genomic data will be used longitudinally, through integration with electronic medical records, to guide the well-being of every child in their lifetime journey.
“Maybe this will take 5 to 10 years, but we are steadily headed there.”
“We want to be able to provide comprehensive genomic profiling for patients with suspected rare diseases as quickly, easily, and cost-effectively as possible.”

 BY KARIM MANSOUR
BY KARIM MANSOUR
sk Sukhdeep Sachdev’s team to describe him, and they’ll say he’s a “facts and figures wizard” whose “sharp intellect and business acumen is only matched by his humility.”
It is perhaps little surprise then that this modest approach to life, day-to-day drive in business, and overall passion for improving patients’ lives has led to the Global CEO of Leader Life Sciences becoming one of the region’s most sought-after professionals in precision medicine. In March of this year, Sachdev was even named one of the Middle East’s “Top 100 Healthcare Leaders for 2023” by Forbes Middle East. With four decades of experience in the industry – beginning his professional career as a medical representative in GSK to his current role at Leader Life Sciences – Sachdev has seen it all. But he’s as passionate as ever.
“Precision medicine is the need of the hour,” he tells us. “Unlocking human data gives the biggest potential to improve and impact patients’ health. The entire GCC is strategising on an institutional level to implement the same and enhance their population health, and as partners in discovery we help reach that goal.
“Omics-based diagnosis, personalised therapy in clinical practice, complete adoption [of] bioprinting in research, and mutation detection technology are some of the areas that the GCC is looking forward to developing in this regard.”

Part of the wider Leader Healthcare Group, Leader Life Sciences was established to introduce “innovative and disruptive solutions to solve “research and clinical problems faced by the scientific and medical community.” It covers scientific solutions that aim to be transformative for hospitals, research facilities and laboratories, and universities, covering human sciences, animal sciences, agriculture, pharma, environment, forensics, precision medicine and healthcare overall.
“Leader Healthcare Group began its journey of
expansion to the world of life sciences with the vision to equip the region’s scientists and researchers with knowledge along with the right solutions,” Sachdev states. “The idea was to strengthen them to nurture a self-reliant industry model for the current and future generations, and I can confidently say that we are on the right track to that end.”
At PMES 23, the company will obviously be focusing on precision medicine, showcasing several of its solutions including one “peculiar” concept, Sachdev reveals.
“This year we would like to introduce the audience to a very peculiar concept that helps you understand the true essence of partnering with Leader Life Sciences,” he says. “Our integration of solutions, combined with impeccable services, makes your process of building a lab or research centre as easy as going to a store and buying a ‘lab in a box’. Starting with pre-clinical research, to drug discovery [and] omics, we cater to the entire world of life sciences that can significantly impact the scientists of the region and help enhance their research workflow or diagnostic capabilities.”
Looking ahead, Sachdev is excited about the future of precision medicine.
“[Given] the exponential growth it is going… The future of precision medicine is, simply put, limitless! The nature of the health sector could be more predictive, preventive and precise with enhanced research quality and productivity.”
Nevertheless, he acknowledges there are challenges, the biggest of which is achieving the correct infrastructure.
“The biggest factor to consider is creating awareness on the importance of patient data and how it helps in developing [a] comprehensive [healthcare system],” Sachdev continues. “We need to build the roads before it becomes a city; similarly, the infrastructure to accommodate a workflow reliant on individual patient data needs to develop before the ecosystem can adopt it.
“This is the gap our scientific experts try to fill with each project they approach.”
And of course, artificial intelligence (AI) cannot be ignored in its role in managing data.
“Wherever data is involved, AI and machine learning becomes a crucial aspect to it. An integration of ‘human intelligence’ with ‘artificial intelligence’ is what will provide the best results and empower our health ecosystem.”
“Precision medicine is the need of the hour.”


Export Opportunities:
ARC Regulatory, founded in Northern Ireland, is a leading provider of global regulatory and clinical research solutions to precision medicine, IVD and CDx industry. With in-region talent, ARC helps you meet regulatory requirements to export with ease.

Let ARC Regulatory expedite your regulatory approvals around the world.
Export Services:
Device CE-Marking project support and consultation for EU market entry.

Clinical Study Monitoring global IVD study sites and study compliance with local and global Good Clinical Practice (GCP) requirements.
FDA Pre-submissions, 510(k) and PMA submissions.
our talented team: www.arc-regulatory.com Explore ARC’s our Digital Regulatory Solution: www.arc360.app Email: info@arc-regulatory.co.uk

General manager Ahmad Shweiki shares insights on the company’s mission and innovative approaches to personalised treatment solutions in genomics, molecular diagnostics, and bioinformatics.
BY KARIM MANSOURaunching in the midst of a pandemic is no easy feat, but for Biorus, it was a determination to not neglect other areas in healthcare – such as precision medicine – that propelled its mission.

In its short time in the United Arab Emirates, Biorus has fast established itself as a pioneering company at the forefront of precision medicine, dedicated to revolutionising the field through innovative approaches and cutting-edge technologies. With a strong emphasis on research and development, Biorus mission is to deliver personalised and targeted treatment solutions in genomics, molecular diagnostics, and bioinformatics to enable healthcare professionals to tailor medical interventions to each individual patient’s unique genetic makeup and specific disease characteristics.
Ahead of exhibiting at PMES 23, we met Ahmad Shweiki, general manager for Biorus, to discuss what’s coming up for the company. With over 15 years of experience in the industry, Shweiki – who works out of Biorus’ headquarters in Dubai –plays a pivotal role in leading the company’s strategic direction and driving its growth and success in the precision medicine sector. Here’s what he had to say.
Can you give us an overview of Biorus as a company and its role in helping shape precision medicine?
There are two main aspects: diagnostics and life science research. In the field of diagnostics, precision medicine plays a crucial role in identifying and diagnosing diseases with accuracy. In the
realm of life science research, precision medicine contributes to drug discovery and development. At Biorus, we provide comprehensive solutions in both research and diagnostics, making us a leader in the field of precision medicine. It’s important to note that precision medicine is not just a concept for the future; it is already a reality today. While advancements and innovations will continue to shape the field in the future, precision medicine is actively happening and making a difference in healthcare today.
You are showcasing Biorus’ portfolio at PMES 23. Tell us a little more about what visitors can expect. The one I would particularly like to highlight is that of nextgeneration sequencing (NGS). In terms of diagnostics, we pride ourselves on having one of the widest selections available in the Middle East, and potentially even in Europe. Our precision medicine and diagnostic solutions set us apart from other companies, as we provide tests specifically designed for diagnostic use, not just for research purposes. For individuals with cancer, for example, our testing services allow for the identification of mutations, supporting precision medicine as a licensed diagnostic approach. This is an essential aspect of our capabilities. Additionally, in the field of life science, we offer innovative solutions and streamlined workflows for drug discovery in precision medicine. These advancements contribute to the overall development of precision medicine, enabling researchers and healthcare professionals to make significant strides in improving patient outcomes.
So, who are your ideal clients or partners?
Our primary client segments consist of professionals working in diagnostic labs within hospitals and reference labs, as well as researchers and universities. These two groups form the core of our targeted clientele.
What does precision medicine mean to you personally?
Both personally and as a company, Biorus is committed to making valuable contributions to the UAE community. Living and operating in the UAE, we believe there is a significant need for greater awareness and attention to precision medicine. This field has made remarkable advancements, and our aim is to further promote, develop, and implement precision medicine in the local market.

Our objective is to bring the necessary focus and resources to elevate precision medicine to a new level in the UAE.
We believe it is crucial to collaborate with key opinion leaders (KOLs), not only in the UAE but
also across the region. This allows us to engage with leading solution providers and experts from around the world, enabling us to stay at the forefront of precision medicine.
By fostering these collaborations and partnerships, we strive to advance precision medicine and its benefits to the healthcare ecosystem in the UAE and beyond. We are dedicated to playing an active role in shaping the future of precision medicine by bringing together top experts, innovative technologies, and strategic solutions for improved patient outcomes.
And what’s next for Biorus?
Our approach is centred around being a true partner to our clients, rather than simply being a product provider. We are committed to offering customised and tailored solutions that address their specific needs, whether it’s solving current challenges or supporting their future objectives. Our aim is to enhance patient care and drive advancements in research.
As part of our commitment, we actively engage in collaborations, including providing machines to universities for research purposes without any charges. Our focus is not solely on financial gains, as there are other lucrative business opportunities available in Dubai. Instead, our objective is to contribute to the success of our clients and institutions, aligning ourselves with their goals and ultimately improving patient care. We aspire to be a valuable contributor to the healthcare sector in both the country and the region.

We believe there is a significant need for greater awareness and attention to precision medicine.”
prominent figure in the field of precision medicine and healthcare innovation in the Gulf Cooperation Council (GCC) and beyond, Dr. Khalid Fakhro requires little introduction. As chief research officer at Qatar’s Sidra Medicine – and chair of the organisation’s precision medicine programme – Dr. Fakhro is pushing the boundaries of paediatric medicine and genomics, using cutting-edge technology to identify the root causes of disease and develop personalised treatments.
But his ambitions don’t stop there, for he and his wider team are determined to ensure that Arab, Middle Eastern and Asian populations are not left behind in the global race for medical breakthroughs, working to establish stronger collaborations and more inclusive research practices.
Appearing at PMES 23, guests will have the opportunity to find out more about Sidra Medicine’s
groundbreaking work, including delving into how the once “separate worlds” of population genetics and disease genetics are now finding common ground.
“The lines are becoming blurred between what used to be considered population genetics and what used to be considered rare disease programmes,” Dr. Fakhro told us. “Now, these two worlds are sort of intersecting where each one plays off the other as we sift through mountains of data, making discoveries that serve both patients and the general population on a daily basis.”
With extensive experience in education and research, particularly in clinical settings, Dr. Fakhro has been at the forefront of transforming the healthcare industry in Qatar and the region, contributing significantly to advancing our understanding of Arab populations and the genetic basis of human disease.
Under his leadership, Sidra Medicine has built a robust reputation in recent years as a leading site for Precision Medicine and clinical research in the region, and currently collaborates with around 80 different centres around the world, studying various areas of paediatric diseases and conditions, such as monogenic diabetes, autism, epilepsy, and rare forms of cancer.

Here, Dr. Fakhro shares more on Sidra Medicine’s journey as well as his vision for precision medicine in the future, including the formation of a human genomics consortium in the GCC.
For those not familiar with Sidra Medicine’s work, how would you describe it?
Firstly, I run the lab of human genetics and genomics at Sidra. As part of that, we work primarily on gene discovery in paediatric rare disease, as we are Qatar’s only paediatric tertiary care facility. We use genomic
Dr. Khalid Fakhro is on a mission to give children in Qatar and beyond access to groundbreaking treatments, as well as place Arab populations on the map of global research.
approaches to study why children [who are admitted] are sick, to discover new genes or biomarkers that explain disease.
The other half of the lab effort is on doing largescale human genomics in the Middle East. We published our first big paper on this in 2016, where we were the first to describe the population structure of the Middle East as being descendant from the earliest of Africa migrations. From that day, we have continued to publish numerous studies related to population genetics, the proclivity for disease amongst different sub-populations in the Arab world, and so forth. To drive that, we benefit immensely from the visionary Qatar Genome Program, and our strategic partnership together [Sidra Medicine is the sequencing and high-performance computing partner of QGP].
In the GCC, Qatar is one of the market leaders in precision medicine. What has been driving the space?


We’ve been fortunate, because [the country’s] push for genomics happened very early on. The first sequencer was bought in Qatar in 2011 – right at the beginning of sequencing technology. But the big push came with the announcement from our Highness [Sheikha Moza bint Nasser] who spoke about establishing a national genome program at the World Innovation Summit of Health in 2014, which really propelled the country to accelerate and facilitate this kind of work.
So, we’ve had a head start in that we worked early on to establish national regulations for genome research, and create a consent structure that allows future access and use for data, as well as responsible sharing of data for the purposes of research.
Making our data accessible to the world is important; if people are looking for Arab genotype phenotype correlations they can come knock on our door”
The reason I think the vision is so powerful is because, even within the limited numbers that we have sequenced so far – as a proportion of the GCC population, Qatar is going to be very small – we have a lot of families that represent pretty much every country or ethnicity across the MENA to South Asia. Thus, our projects and publications are an excellent proxy for our region of the world that is traditionally under-represented in public databases, and we can already see this data being useful and highly cited. So, imagine if we start doing this at scale!
And your data is accessible, isn’t it?
Making our data accessible to the world is important, so if people are looking for Arab genotype-phenotype correlations they know they can come knock on our door and they would have a very active partner helping them do that research and taking it forward.
What we need is just a few centres to follow a similar path around the GCC, because with 10% of centres, you can get 90% of the data you need, which would make this part of the world a hotbed for genomics research. But it all has to do with building resources from the ground-up the right way, and ensuring the participants are enrolled with informed consent that protects their rights and privacy while allowing sharing for scientific gain. That’s really what it boils down to at the end.
In this region, are there struggles with finding willing participants?
What has surprised me actually, is how quickly we were able to build a research mentality in the population. At the beginning, I thought that people would shy away from participating in research. But it turns out that our populations very willing to be involved in research. I think it has something to do as well with our Islamic traditions; many people consider it an act of charity to participate in genomic research that may eventually help others.
So, what challenges is the region facing in precision medicine at the moment?

There are always going to be challenges, and part of the challenge is managing expectations. Precision medicine comprises two different worlds – those working in the field and those looking from the outside, like looking at someone’s life on social media. The latter group only really get curated success stories, with no visibility of the blood, sweat and tears it took to produce them. This creates a somewhat skewed view of what this technology can and cannot do. And while I agree that there’s a lot of promise, what’s not commonly shown are the number of failures and challenges we all face.
That’s something that our part of the world is still not used to, because of the competitive nature of our people. When you go into an innovative field in R&D, you need to have a very high appetite for failure. And part of the challenge, to date, is teaching that, and making sure that stakeholder expectations are in line with our capabilities. You need to understand that there are going to be nine failures for every great thing you build. That’s just innovation in general.
It’s also important for those wanting to pursue
research to understand that this field is based on merit and peer review – something our part of the world still struggles with. I hate to say this, but it’s good to acknowledge our shortcomings, in that we have to move away from a mentality of privilege.
There’s also the issue of funding, isn’t there?
There’s the conversation around being able to continue having sustained funding for research, and to continue building the research enterprise. The way the government might look at it – since most research is government-funded in this part of the world – is that they keep spending all this money on R&D, but they don’t see immediate returns. And if economic pressures require fiscal tightening, research funding usually suffers disproportionately.
This effect is amplified in the Middle East partly because we don’t have any real “industry” or private sector funding. In all mature bioeconomies around the world, there’s a role that’s played by the government, there’s a role played by academia, and a big role played by industry. A theme that I think is going to continue growing over the next few years, is a bigger role for industry and industry partners – such as big pharma and big tech –coming to our region and establishing alliances and funding models for the future.
What would you like to see from pharmaceutical and tech companies?
With pharma, it’s launching clinical trials in our part of the world. There are very interesting combinations of Arab genotypes and phenotypes. So a specific drug might work very well on Asian populations, for example, but not on Arabs. You need to do the right
clinical trials to test these.
Apart from pharma, there are so many companies you can start in the precision medicine space... AI companies, disruptive research start-ups, regulatory companies, new insurance models, direct-toconsumer companies, etc. There’s so much that you can build around the data that we’re producing and the space between what research is producing, and the needs and wants of the healthcare enterprise at the other end of spectrum.
We’ve obviously got to touch upon AI in precision medicine. Thoughts?
I’m very excited by this. One of the most difficult things in this field – due to the explosion of precision medicine, genomic technologies, and data production – is keeping up with the literature. One thing that new language models like GPT have helped us with is to shorten the amount of time in which we search the literature. We’ve used it in many ways, for example, to find connections between genes and diseases that we did not previously know about… this frees up time for the scientists to do higher level jobs. I realise this is a very niche area, but the adoption is only going to continue growing.
I think we will see more of these forks of AI models specialised for biomedical research, because of the amount of data being generated, and the pace with which it is produced. This data is highly heterogeneous, containing genome sequence, multiomics at different time points, electronic health record, wearable data, questionnaires, etc. The possibilities for making ground-breaking discoveries are astounding!
Of course, I still think that every one of these
findings needs to be reviewed manually. A very strong and necessary human element is still required. But you can at least get through the first levels of big data organization and screening with AI very, very quickly.
And finally, what does precision medicine mean to you?
My vision of precision medicine has always been based around using the latest research technologies to improve patient outcomes. If you look at the term Precision Medicine, I see the ‘medicine’ coming from the hospital and ‘precision’ has to come from research. And unless you’re a hospital doing advanced research, it’s very hard for you to claim you’re doing precision medicine. That’s one of the things that is special about our setup here at Sidra Medicine, is that we’ve built an entire research enterprise inside a hospital. Ensuring every patient has access to cutting-edge research along their journey of care.. it’s quite unique for this part of the world.
Our precision medicine programme is pioneering a culture of academic medicine in the region, and thankfully, we’ve been quite successful to date with high impact research and improved patient outcomes. Being able to demonstrate [that Sidra successfully treats children through genomics research and advanced therapies] is very important, because while this commonly exists elsewhere, it does not yet exist in the Middle East at large. Imagine a world where no patient ever needs to leave the comfort of their home country to get the best treatment the world has to offer. That’s my vision for precision medicine in the future.

MUTASEM AL TITI Group Chief Commercial Officer AL BORG DIAGNOSTICS


DR. FADI YOUNIS Regional Sales Manager, EMEA ANALYTIK JENA GMBH


DR. IBRAHIM ABU-GHEIDA Staff Physician, Radiation Oncology, Oncology Institute CLEVELAND CLINIC ABU DHABI RIFAAT RAWASHDEH

Licensed Certified Genetic Counselor

CLEVELAND CLINIC ABU DHABI
SABA SAMIR FLAIHAN HAMASHA Patient Support Group UAE RARE DISEASE SOCIETY

PROF. PUTHEN VEETTIL JITHESH
Associate Professor & Program Coordinator, Genomics & Precision Medicine
HAMAD BIN KHALIFA UNIVERSITY
DR. STEVEN QUISTAD Senior Application Scientist DNA SCRIPT
DR. SUSANNA AKIKI
Consultant Clinical Scientist, Cancer Genomics Assistant Professor of Clinical Pathology HAMAD MEDICAL CORPORATION
PANKAJ KHANNA Manager & Application Scientist BIORUS


MOHAMMAD SABERI
MOHAMAD Director, Health Data Science Lab, Department of Genetics & Genomics COLLEGE OF MEDICINE & HEALTH SCIENCES, UAE UNIVERSITY
PROF. MINGGUANG (MING) HE

Principal Investigator CENTRE FOR EYE RESEARCH, AUSTRALIA
DR. KADHEM ALKHENAIZI Managing Director EXPRESSMED LABORATORIES

DR. FATMA AL HASHIMI Director HORTMAN STEM CELL CENTER

DR. KAROLINA KOBUS


Head of Molecular Genomics Precision Medicine Laboratory Technology & Innovation Advisor
EXPRESSMED LABORATORIES
DR.



President, Emirates

Society, Deputy International Commissioner, College of American Pathologists, Adj Professor, MBRU School of Medicine, HoD, King’s College Lab, Dubai, and Chief Medical Officer, Alliance Care Technologies













RESEARCH INSTITUTE (QBRI)

DR. PARHAM
MARDI
Researcher & Medical Doctor, Gynaecology, Obstetrics & Infertility Research Center
SAREM WOMEN’S HOSPITAL, IRAN UNIVERSITY OF MEDICAL SCIENCES
DR. AMAL AL TENAIJI

Metabolic Genetics Consultant
SHEIKH KHALIFA MEDICAL CITY
DR. WOUTER HENDRICKX


Principal Investigator, Pediatric Cancer Omics Lab

SIDRA MEDICINE
DARRAGH MCART
Founder
& CEO SONRAI ANALYTICS

Developed
DR. ABEER ALSAYEGH Head of Genomics



SULTAN QABOOS COMPREHENSIVE CANCER CARE & RESEARCH CENTRE
ZENAH CHABAN Scientific Advisor TESTATY
DZIHAN ABAZOVIC Co-founder & Supervisory Board Member VINCULA BIOTECH GROUP



JAMIE LI Product Manager BGI


SEUNG-YONG SEONG CEO SHAPERON

DR. SHOAIB AL ZADJALI Head of Research Laboratories
SULTAN QABOOS COMPREHENSIVE CANCER CARE & RESEARCH CENTRE
DR. YASER AMMAR ALSARRAJ Research Specialist & Sequencing Head QATAR GENOME PROGRAM

ANUPAM J DAS Chief Operating Officer BASESOLVE INFORMATICS

KERMEN BOLAEVA Area Sales Representative Middle East & CIS NEW ENGLAND BIOLABS

DR. GREGORY GUIRMAND

DR. SIRIN ADHAM Associate Professor SULTAN QABOOS UNIVERSITY
PHILIP JERMANN Director, Medical Affairs EMEA THERMO FISHER SCIENTIFIC
Lead Researcher – Molecular Biotech and Genomics TECHNOLOGY INNOVATION INSTITUTE
DR. NIALL HICKEY Business Support Scientist, EMEA BIONANO, USA

DR. THOMAS LAUNEY

VP Molecular Biotech & Genomics, Biotechnology Research Center TECHNOLOGY INNOVATION INSTITUTE, UAE
PROF. MILOS LJUBISAVIJEV Professor, Department of Physiology UAE UNIVERSITY
RAFFAELA CAMPANA Product Manager
MADX – MACRO ARRAY DIAGNOSTICS GMBH

Day 1
Tuesday 23 May
09:00
Welcome Note
Rachel McArthur
PMES 23 MC
Managing Editor & Healthcare Journalist, Digital Ink Media (UAE)
09:05
Opening Remarks
Dr. Min S Park
Chief Scientific Officer, Sanimed International (UAE)
09:10
Congratulatory Remarks
H.E. Sarah Al Amiri
Minister of State for Public Education and Advanced Technology (MOIAT) (pre-recorded session)
09:15
Congratulatory Remarks
Ministry of Health and Prevention (MoHAP) (UAE)
09:20
Congratulatory Remarks
Dr. Asma Al Mannaei
Executive Director, Research and Innovation Centre, Department of Health (DoH) Abu Dhabi (UAE)
09:25
My Journey
Saba Samir Flaihan Hamasha
Patient Support Group for UAE Rare Disease Society (UAE)
09:30
TBC
Dr. Yendry Ventura
CEO, Abu Dhabi Stem Cells Center (UAE)
09:35
Advancing Precision Medicine in the GCC
Dr. Khalid Fakhro
Chief Research Officer, Sidra Medicine (Qatar)
10:15
Developing an Integrated Comprehensive Multidisciplinary Genomics Programme in a Health System
Dr. Parth Shah
Director of Genome Informatics, Assistant Professor of Medicine, Dartmouth-Hitchcock (USA)
SESSION 1: PRECISION MEDICINE FOR RARE DISEASES
Chair: Prof. Fatma Al Jasmi
Chair of Genetics & Genomics Department, UAE University, College of Medicine & Health Science (UAE)
10:45
Translating Genomic Medicine in the Clinic
Prof. Fatma Al Jasmi Chair of Genetics & Genomics Department, UAE University, College of Medicine & Health Science (UAE)
11:15
Rare Diseases: New Treatments & Trials
Dr. Amal Al Tenaiji Metabolic Genetics Consultant Sheikh Khalifa Medical City (UAE)
11:35
Update on the ACMG Guideline
Dr. Ahmad Abou Tayoun Director, Al Jalila Children’s Genomics Center of Execllence, Associate Professor of Genetics, MBRU (UAE)
11:55
Emerging Technologies & Enhancing Precision MedicineIt’s the Future of Healthcare
Nirmal Kumar Managing Director, Leader Life Sciences (UAE)
12:15
Methods for Application of Personalised Medicine Approach in In-vitro Fertilization (IVF) in Middleincome Countries
Dr. Pharm Mardi Researcher & Medical Doctor Gynecology, Obstetrics, and Infertility Research Center, Sarem Women’s Hospital, Iran University of Medical Sciences (IUMS) (Iran)
12:35
SYNTAX System Enables Rapid Probe Synthesis & Testing to Accelerate Custom qPCR Assay Development
Dr. Steven D. Quistad Senior Application Scientist DNA Script (France)
12:50
Health Data Science for Precision Medicine
Mohammad Saberi Mohamad Director of the Health Data Science
Lab-Department of Genetics and Genomics, College of Medicine and Health Science, UAE University (UAE)
13:10
Networking Lunch Break
SESSION 2: ADVANCED IN PRECISION MEDICINE
Chair: Dr. Min S Park, Chief Scientific Officer, Sanimed International, (UAE)
14:00
Keynote Speech
Precision Medicine & AI: Trends & Challenges Relevant to Patient-Centred Care
M. Walid Qoronfleh
Executive Director, Q3CG Research Institute (QRI) (Qatar)
14:30
Machine Learning Paradigms for Variant Prioritisation: Going Beyond Mendelian Diseases
Dr. Imane Boudellioua
Assistant Professor-Information and Computer Science Department, King Fahd University of Petroleum and Minerals (KSA)
14:50
Addressing Unmet Needs in Predictive Biomarkers for Immunotherapy
Dr. Ofer Sharon CEO, OncoHost (Israel)
15:10
AI-powered Drug Classification & Modelling pipelines
Dr. Michel Bernanos Mesquita CEO & Founder, L&M Data Science Ltd (Spain)
15:30
AI in Ophthalmology: From Algorithm to Real-world Solutions & Products
Prof. Mingguang (Ming) He Department of Ophthalmic, Epidemiology, University of Melbourne and CERA (Australia)
15:45
Turning Analog Blood into Digital – A Health Data Revolution for Precision Medicine
Dr. Ali Tinazli
CEO, Lifespin (USA)
16:00
Data Integration & Analytics for Precision Medicine, Biomarker Discovery & Research Platforms
Darragh McArt
Founder & CEO, Sonrai Analytics (UK)
16:15
Networking Coffee Break
SESSION 3: STEM CELL THERAPY & PRECISION MEDICINE
Chair: Dr. Fatma Al Hashimi, Director, Hortman Stem Cell Center (UAE)
16:30
Unlocking the Power of Precision
Joanne M. Hackett
Medicine Vic e President, Genomic and Precision Medicine, EMEA IQVIA (USA)
16:45
Stem Cell Treatment for Vision
Dr. Masayo Takahashi
President, Vision Care Inc. (Japan)
17:05
Induced pluripotent Stem Cell Technology in Diabetes Precision Medicine
Dr. Essam Abdelalim
Scientist, Qatar Biomedical Research Institute (QBRI) (Qatar)
17:25
Hepatocyte Microbeads Cryopreservation for Transplantation
Dr. Shaikha AlMazrouei Lead Researcher, Technology Innovation Institute (UAE)
17:45
End of Conference Day 1
Day 2
Wednesday 24 May
SESSION 4: CURRENT STATUS OF PRECISION ONCOLOGY
Chair: Dr. Julie Decock Scientist, Qatar Biomedical Research Institute (Qatar)
Co-Chair: Dr. Sirin Adham
Associate Professor, Sultan Qaboos University (Oman)
09:00
Keynote Speech Cancer Immunotherapy: Where are we now?
Prof. Salem Chouaib
Vice Chancellor of Research and Director of Thumbay Institute for Precision Medicine, Gulf Medical University (UAE)
09:30
AC-ICAM Story on Colon Cancer Immune Infiltration & Intratumoral Microbiome
Dr. Wouter Hendrickx
Principal Investigator, Pediatric Cancer Omics Lab Sidra Medicine (Qatar)
09:50
Enabling Precision Oncology Through Rapid Molecular Profiling of Solid Tumours
Philip Jermann
Director Medical Affairs EMEA, Thermo Fisher Scientific (Switzerland)
10:05
Establishing a Genetic Cancer Risk Assessment Clinic –Lessons Learned So Far
Dr. Abeer Alsayegh
Head of Genomics, Sultan Qaboos Comprehensive Cancer care and Research Center (Sultanate of Oman)
10:25
Using Genetic Testing to Improve Health & Wellbeing: The Future Is Here For Lifestyle Genetics
Mohamed Nagy Ahmed Pharmacy Director, Head of Personalized Medication Management Unit, Children’s Cancer Hospital (Egypt)
10:45
Oncology Session on Brain
Tumours
Dr. Aaron Han
Vice President, Emirates Pathology Society, Deputy International Commissioner, College of American Pathologists, Adj Professor, MBRU School of Medicine, HoD, King’s College Lab, Dubai & CMO, Alliance Care Technologies (UAE / USA)
11:00
Present & Future Opportunities of Precision Medicine in Oncology & Rare Disease
Pankaj Khanna
Manager - Application Scientist, Biorus (India)
11:15
Panel Discussion
The Use of Oncomine Solutions in the Research of Personalised Medicine
Chair: Philip Jermann
Director Medical Affairs EMEA, Thermo Fisher Scientific (Switzerland)
Dr. Shoaib Al Zadjali
Head of research Laboratories, Sultan Qaboos Comprehensive Cancer Care and Research Centre (Sultanate of Oman)
Dr. Nada Abdullah AL Tassan Principal Specialist, King Faisal Specialist Hospital and Research Centre (KSA)
Dr. Susanna Akiki
Consultant Clinical Scientist, Qatar Rehabilitation Institute (Qatar)
12:00
Plenary Speech Parkinson’s Disease Cell Therapy Using iPS Cells & Performing Clinical Trials
Dr. Jun Takahashi
Director and Professor of the Center for iPS Cell Research (CiRA), Kyoto University (Japan)
12:45
Lunch Break
13:50
Cancer Genomics & Precision Oncology: A Middle Eastern Perspective
Dr. Karolina Kubos
Head of Molecular Genomics & Precision Medicine Laboratory Technology & Innovation Advisor, Express Medlabs (UAE)
14:05
From Omics to CDx: Advancing Biomarker Discovery
Megan Blair
Chair: Prof. George Patrinos
Professor and Head of Laboratory Department, University of Patras (Greece)
15:30
Keynote Speech Towards the Implementation of Pharmacogenomics in the UAE for Cardiovascular Diseases
5: ADVANCES IN SCIENCE & TECHNOLOGY FOR PRECISION MEDICINE
Chair: Dr. Thomas Launey
VP Molecular Biotech & Genomics Biotechnology Research Center, Technology Innovation Institute (TII) (UAE)
13:30
Precision Prevention: The Usage of Proteomics & Genomics to Better Understand Risk of Disease
Dr. Nicole Sirotin
Chair of Preventive Medicine in the Medical Subspecialties Institute & Executive Health Program Lead at Cleveland Clinic Abu Dhabi (UAE)
Pharma Collaborations Manager, Randox Laboratories Ltd (UK)
14:20
Breaking the $100 Genome Barrier: Our Journey From Bp to Pb
Toby Huang
Regional Director Middle East, MGI (UAE)

14:35
Introducing Illumina’s Complete Long Read Technology
Samih Al Qawlaq Senior Clinical Sales Specialist, Illumina (UAE)
14:50
Transforming The Future of Healthcare, One Nanopore at a Time
Tonya McSherry
Vice President, Genomic and Precision Medicine, EMEA, Oxford Nanopore Technologies (UK)
Bassam Ali Professor in Molecular and Genetic Medicine, Department of Genetics and Genomics College of Medicine and Health Sciences, UAE University (UAE)
16:00
Genome: Guided Treatment in Psychiatry
Prof. George Patrinos Professor and Head of Laboratory Department, University of Patras (Greece)
16:20
Pharmagenomics of Multiple Sclerosis & Neurodegenerative Disorders in the Middle East Prof .Milos Ljubisavijev Professor, Department of Physiology UAE University (UAE)
17:00
Actionable Pharmacogenomic Landscape of the Qatari Population
Prof. Puthen Veettil Jithesh Associate Professor and Program Coordinator, Genomics & Precision Medicine, Hamad Bin Khalifa University (HBKU) (Qatar)
17:15
Qatar Biobank: Immunomics Enhancing Phenotypic Profiling – A Diabetes Example
Dr. Eleni Fthenou Scientist, Qatar Biobank (Qatar)
17:30
MedicineMultiplexing
Immunofluorescent: A New Era in Cancer Companion Diagnostic & Precision Medicine
Dr. Kadhem Alkhenaizi
Managing Director, ExpressMed Diagnostics and Research (UAE)
17:45
End of Conference

“Nearly all diseases are linked to metabolism, which makes lifespin’s technology so powerful.”



Day 1
Tuesday 23 May
09:15
Combined Combined molecular Profiling of Tissue & Liquid Biopsy: A Holistic Approach to Address the Tumour Heterogeneity in Precision Oncology
Innovate life Sciences / Wipro
Hitesh Goswami
CEO, 4baseCare (India) (partnered with Innovate Life Sciences)
10:00
Sustainable & Secured Bio-production of Anti-cancer Drug Precursors by Yeast Cell Factories
Technology Innovation Institute
Dr. Gregory Guirmand
Lead Researcher – Molecular Biotech and Genomics, Technology Innovation Institute (TII) (UAE)
10:45
Next-Generation Cytogenomics with Optical Genome Mapping (OGM)
Genetrics
Dr Niall Hickey
Business Support Scientist EMEA, Bionano (USA)
11:15
Host-Microbiome Interactions: The Next Frontier in Precision Medicine
Eagle Genomics
Dr. Christian Roghi
Director of Microbiome Solutions, Eagle Genomics (UK)
12:00
A New Gateway to Personalised Genomics Using Twist NGS Solutions
Genetrics
Dr. Vishal Dixit
NGS Application Scientist - Europe & EMEA support, Twist Bioscience (USA)
12:00
Higher Library Yields & Accuracy with NEBNext NGS Workflows
New England Biolabs
Kermen Bolaeva
Area Sales Representative Middle East & CIS, New England Biolabs (UAE)
12:30 Break
13:00
Presentation by CareCloud CareCloud
14:00
Translating Research into Clinical Implementation / Reproductive Genetic Carrier Screening: A Prerequisite Thermo Fisher Scientific
Marco Pirotta
Genetic Science Business Manager EM, Thermo Fisher Scientific (Switzerland)
Dr Yaser Ammar AlSarraj Research Specialist and Sequencing Head, Qatar Genome Program (Qatar)
Dr. Lova Satyanarayana Matsa Scientific Director, Middle East & India, Igenomix (UAE)
15:00
Pharmacogenetics: Precision Medicine beyond Oncology Indus Health Plus
Dr. Nikhat Khan Manager - Genetics Department, Indus Health Plus (India)
15:45
Unlocking the Potential of Cancer Testis Antigens for Breast Cancer Precision Medicine
Qatar Biomedical Research Institute (QBRI)
Dr Julie V. Decock Scientist, Qatar Biomedical Research Institute (QBRI) (Qatar)
09:15
Proteomics of Human Aging & Longevity
Olink
Tarif Awad
Vice President, Scientific Affairs, Olink Proteomics (USA)
10:00
Proteomics Multiplex Technologies: An Ideal Clinical Tool for Biomarker Discovery Overview of the Genomics Core Facility at HBKU / The Use of Eye Tracking Technology as an Objective Tool in the Early Screening & Diagnosis of Autism Spectrum Disorder
Qatar Biomedical Research Institute (QBRI)
Dr Houari Abdesselem
Core Manager - Proteomics Core Lab, Qatar Biomedical Research Institute (QBRI), Hamad Bin Khalifa University, Qatar Foundation (Qatar)
Dr Khalid Ouararhni
Core Manager - Genomics Core Lab, Qatar Biomedical Research Institute (QBRI), Hamad Bin Khalifa University, Qatar Foundation (Qatar)
Dr. Fouad Al Shaban
Principle Investigator - Neurological Disorders Research Center, Qatar Biomedical Research Institute (QBRI), Hamad Bin Khalifa University, Qatar Foundation (Qatar)
11:15
Importance of Animal Research Facility Design in Precision Medicine
Leader Life Sciences
Mehdi Mobarek
Chief Strategy Officer (CSO)International, Allentown LLC (UK)
12:00
Break

12:15
The Next Wave in Precision Genomics: Presenting AUGMET’s Fully Automated Genomics Data Ananlysis, Reporting & Management Solution / Bye Bye Dye –Cell Viability Without Cytotoxic Effects Medscience

Anupam J Das Chief Operating Officer, BaseSolve Informatics Pvt. Ltd (India)
Dr. Fadi Younis Regional Sales Manager, Analytik Jena GmbH (Germany)
Dr Benjamin Usai Key Account Manager, anvajo GmbH (Germany)
13:00
Going “MAD” in Allergy Practice: The Use of Molecular Allergy Diagnosis in Clinical Practice
Babirus
Raffaela Campana
Product Manager, MADx – Macro Array Diagnostics GmbH (Austria)
14:00
Real-world Application of Precision Medicine for Whole Life Cycle Healthcare
BGI Genomics Co., Ltd
Jamie Li
Product Manager, BGI (USA)
14:20
Non-Communicable Diseases as a Megatrend & an Increasing Global Cost Burden – New Affordable & Scalable Precision Medicine Tools for Population Health Lifespin Health
Dr. Ali Tinzali CEO, Lifespin (Germany)
15:05
Testaty: Revolutionising Health Services
Testaty
Zenah Kabakibo
Scientific Advisor, Testaty (UAE)
15:30
Cellular & Subcellular Therapies –Latest Breakthroughs in Regenerative Medicine
Vincula Biotech Group
Dzihan Abazovic
Co-founder and Supervisory Board Member, Vincula Biotech Group (Serbia)
16:15
First-in-Class Inflammasome Inhibitors & Nanobody Therapeutics
Shaperon
Seung-Yong Seong CEO, Shaperon (Republic of Korea)
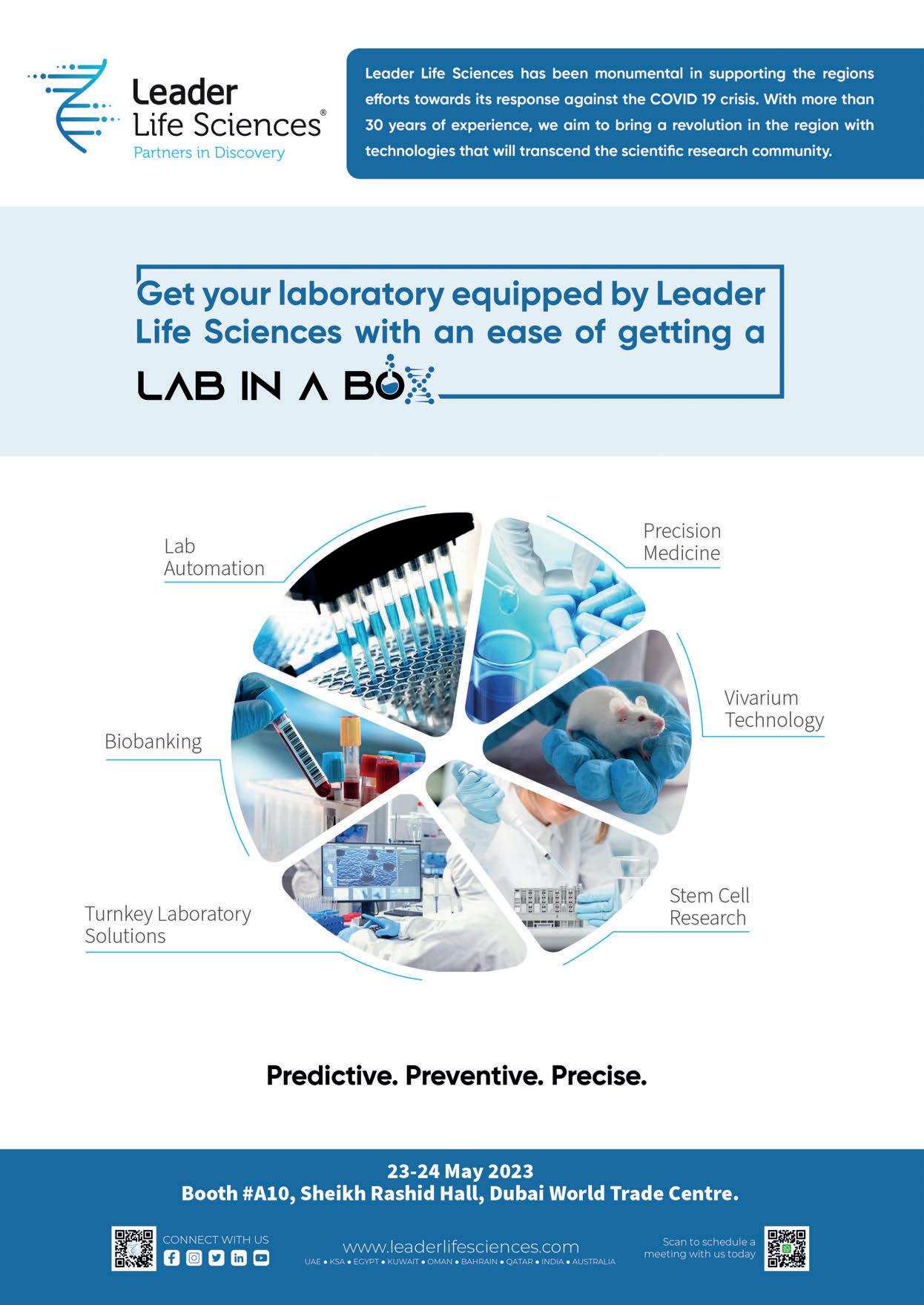
Precision Medicine is a personalised approach to health and wellness in which an individual’s genetic profile is used to customise their health management plan, and which combines genetic information with family history, lifestyle and environmental factors, to ensure that the most e ective treatment and/or preventative measures are delivered to the right person at the right time.
Our comprehensive range of genetic tests includes:
•Non-Invasive Prenatal Testing - basic and genome wide
•Solid Tumour Profiling
•Whole Exome Sequencing
•Carrier Genetic Screening
•Preimplantation Genetic Testing – PGT-A (chromosomal aneuploidies) and PGT-M (monogenic disorders)
•Targeted Disease-Specific Panels
•Comprehensive Hereditary Cancer Screening
care requirements unique to the residents and communities of the United Arab Emirates.
Stand Number: B07
United Arab Emirates
onehealth.ae


With an extensive network across the UAE, One Health is a PureHealth asset which provides end-to-end medical solutions for healthcare service providers. OneHealth has dedicated sales, service support, and engineering teams that work with healthcare facilities in supporting their operations.
With a customer base of over 300 healthcare providers, One Health’s Diagnostics team offers advanced technologies to support the delivery of patient care with the highest standard of diagnostic instruments and reagents. One Health partners with globallyacclaimed diagnostics equipment and product manufacturers to offer a complete spectrum of laboratory solutions in preanalytical and general lab services and other related products.
One Health’s Diagnostics unit supplies a comprehensive range of scalable diagnostic solutions that covers biochemistry, immunology, haematology, haemostasis, point of care testing, blood banking, molecular diagnostics and more.
One Health’s Diagnostics is also focused on consulting and advising on laboratory turnkey projects by bringing in the latest innovations in the field of in-vitro diagnostics.
PRODUCT CATEGORIES: Molecular Diagnostics, AI & Big Data, Digital Health, Targeted Therapeutics, Immunology, Haematology
United Arab Emirates
clevelandclinicabudhabi.ae

Cleveland Clinic Abu Dhabi, part of Mubadala Health’s network of world-class healthcare facilities, is a multispecialty hospital on Al Maryah Island in Abu Dhabi, UAE. It is a unique and unparalleled extension of US-based Cleveland Clinic’s model of care, specifically designed to address a range of complex and critical
Cleveland Clinic Abu Dhabi is composed of 15 institutes designed to put Patients First and align with the region’s specific healthcare needs. In all, more than 50 medical and surgical specialties are represented at Cleveland Clinic Abu Dhabi and are integrated to offer coordinated, multidisciplinary care for adult patients.
PRODUCT CATEGORIES:
Healthcare provider
Stand Number: A01
United Arab Emirates biorus.ae
As technology advances across the world, so do the capabilities of the healthcare sector. Medical and diagnostic solutions are becoming simpler, faster, and more accurate by the day, taking the entire healthcare system to new heights.
Stand Number: D06
United Arab Emirates
ssmc.ae

One of the UAE’s largest hospitals, Sheikh Shakhbout Medical City (SSMC) was established as part of the Abu Dhabi Economic Vision 2030 to elevate healthcare services in the emirate. In November 2019, SEHA partnered with the U.S. non-profit organisation, Mayo Clinic, to establish a joint venture to operate SSMC. The world-class partnership reinforces SEHA’s vision for positioning Abu Dhabi as a global healthcare hub.
SSMC provides patients with worldclass health care and medical services, and cutting-edge facilities, technologies and diagnostics. Its medical complex is a cornerstone of Abu Dhabi’s healthcare services which are aligned with global quality and safety standards to consolidate a new meaning for excellence.
PRODUCT CATEGORIES
Healthcare provider
Proudly located in Dubai Science Park, Biorus is a progressive company that aims to facilitate innovation in healthcare. Our team offers bespoke scientific solutions for the industry, leveraging the pillars of reliability, trust, and loyalty. It isn’t only about what we do, but why we do it. Our multinational team loves their work and shares a mutual vision of improving the population’s collective health. After all, a healthy society leads to a successful future.
PRODUCT CATEGORIES:
Chronic Disease, Molecular Diagnostics, Next-Generation Sequencing (NGS), Digital Health, Other

Stand Number: A08
United Arab Emirates leaderlifesciences.com
Leader Life Sciences has been monumental in supporting the region’s effort to strengthen the research and diagnostic community over the years. With more than 30 years of experience, the Leader Group aims to bring a revolution through innovative solutions and services with the ease of getting a LAB IN A BOX.
PRODUCT CATEGORIES:
Precision Oncology, Rare and Orphan Disease, Pharmacogenomics (PGX), Chronic Disease, Molecular Diagnostics, Next-Generation Sequencing (NGS), AI & Big Data, Drug Discovery, Digital Health, Targeted Therapeutics, Immunology, Epigenetics, Healthcare provider

Stand Number: A02
United Kingdom
thermofisher.com
Thermo Fisher Scientific is the world leader in serving science. Customers worldwide trust our tools, services and solutions to help them accelerate innovation and enhance productivity. Together, we are making advancements that make a real difference. We do that by providing an unmatched combination of innovative technologies, purchasing convenience, and comprehensive support through product and service brands that include Thermo Scientific, Applied Biosystems, Invitrogen, Fisher Scientific and Unity Lab Services.
Our Mission is to enable our customers to make the world healthier, cleaner and safer.
PRODUCT CATEGORIES:
Precision Oncology, Pharmacogenomics (PGX), Molecular Diagnostics, NextGeneration Sequencing (NGS), Targeted Therapeutics, Healthcare provider
academic, scientific partners by offering access to knowledge and research collected from a diverse, distinguished talent base. We serve the best clients by offering proprietary knowledge and data, a clear path from research to innovation, and a shared commitment to advancing the long-term public good.
PRODUCT CATEGORIES: AI & Big Data, Other
Stand Number: C12
Kingdom of Saudi Arabia bgi.com
Stand Number: D05 Kingdom of Saudi Arabia alborgdx.com
Since opening its first laboratory in Jeddah in 1998, Al Borg Diagnostics has expanded to become the largest chain of private laboratories in the Gulf Cooperation Council (GCC) countries, with a daily footfall of 15,000 visiting customers, and partnerships with over 5,000 hospitals, clinics and pharmaceutical companies in the field of precision medicine.

Stand Number: A14
United Arab Emirates
tii.ae
The Technology Innovation Institute (TII) is a leading global research centre dedicated to pushing the frontiers of knowledge. Our teams of scientists, researchers and engineers work in an open, flexible and agile environment to deliver discovery science and transformative technologies. Our work means we will not only prepare for the future;we will create it. Working together, we are committed to inspiring innovation for a better tomorrow.
We exist to help create a better world. We develop the most advanced, disruptive technological innovations designed to solve society’s greatest challenges.
We attract the best scientific talent by offering world-class facilities and resources. We partner with the best
As a leading company in the Arab Gulf and North African region, Al Borg Diagnostics prides itself on providing high-quality services, adopting best practices, and supplying the latest technologies to improve community health. With more than 20 years of experience, it has become the goto provider of integrated laboratory services and solutions for citizens, residents, healthcare providers, and international pharmaceutical companies and research centres in the countries it operates in.
With the continued support and trust of its customers, Al Borg Diagnostics looks forward to further contributing towards the Kingdom’s 2030 vision for the health sector.
PRODUCT CATEGORIES:
Precision Oncology, Rare and Orphan Disease, Chronic Disease, Pharmacogenomics (PGX), Molecular Diagnostics, Next-Generation Sequencing (NGS), Targeted Therapeutics, Immunology, Epigenetics
BGI Genomics is the world’s leading provider of genomic sequencing services and proteomic services, now serving customers in more than 100 countries. We provide academic institutions, pharmaceutical companies, health care providers and other organisations with integrated genomic sequencing and proteomic services and solutions across a broad range of applications spanning basic research covering human, plant, animal and microbial species; clinical research in human health; drug discovery and development, and agriculture and Biodiversity preservation and sustainability.
We have almost 20 years of genomics experience helping our customers achieve their research goals by delivering rapid, high quality results using a broad array of cost-effective, cutting-edge technologies, including our own innovative DNBSEQ sequencing technology.
BGI Almanahil Health for Medical Services is BGI Genomics’ subsidiary in the KSA.
PRODUCT CATEGORIES:
Precision Oncology, Molecular Diagnostics, Next-Generation Sequencing (NGS), Healthcare provider
Stand Number: B06
United Arab Emirates
firstgenomix.ae
First Genomix (formerly known as Viafet Genomics Laboratory) is a provider of genetic diagnostic services offering a wide range of specialised testing solutions to a wide network of local, regional, and international healthcare providers in the GCC and Middle East region.

The company was founded in 2012 by Dr. Micheal Fakih, a pioneering consultant in reproductive and fertility medicine, in partnership with Dr. Ali Hellani, a renowned reproductive geneticist, having recognised the importance and growing need of genetic testing in the field of reproductive medicine.
Since its inception, First Genomix has made great strides in providing unparalleled expertise using the most advanced molecular diagnostic technologies to help advance diagnostic and treatment outcomes.
Today, the company offers a comprehensive portfolio of highly specialised reproductive, prenatal, diagnostic, and screening solutions, all performed in-house and complemented by genetic counselling support by a highly qualified team of geneticists and professional counsellors.
PRODUCT CATEGORIES:
Next-Generation Sequencing (NGS), Molecular Diagnostics

Stand Number: A03
United Arab Emirates
gulf-scientific.com
Gulf Scientific Corporation (GSC) is a leading provider of complete laboratory solutions across the Middle East. At GSC, we provide our clients with a broad spectrum of technologies in the fields of analytical, life and material sciences serving a wide range of industries.
PRODUCT CATEGORIES:
Pharmacogenomics (PGX), Precision Oncology, Rare and Orphan Disease, Drug Discovery
Stand Number: A06
United States infusion51a.com
Infusion 51a is an impact-focused investment fund on a mission to invest in, develop, and advance world changing innovation initially seeking disruptive precision-based opportunities within healthcare.

PRODUCT CATEGORIES:
Precision Oncology, Rare and Orphan Disease, Molecular Diagnostics, AI & Big Data, Drug Discovery, Digital Health, Targeted Therapeutics, Immunology

Stand Number: B12
United Arab Emirates iqvia.com
IQVIA is a leading global provider of advanced analytics, technology solutions, and clinical research services to the life sciences industry. IQVIA creates intelligent connections to deliver powerful insights with speed and agility, enabling customers to accelerate the clinical development and commercialization of innovative medical treatments that improve healthcare outcomes for patients.
With approximately 86,000 employees, IQVIA conducts operations in more than 100 countries.
PRODUCT CATEGORIES:
Digital Health, AI & Big Data, Precision Oncology, Rare and Orphan Disease, Next-Generation Sequencing (NGS), Drug Discovery, Investor / VC, Other

Stand Number: B01
United Arab Emirates igbiosystems.com
With close to 25 years of excellence, Integrated Gulf Biosystems (IGB) has established itself in the Middle East region as one of the trusted partners of choice for life science solutions providers. Since its inception in 1999, IGB has differentiated itself through exemplary support (service and application), workflow solutions, and good sales support. We are the channel partner for some industry giants like Thermo Fisher, Hamilton (Robotics & Storage), Lexogen, LGC Seracare, Scale Bio, Menarini Silicon Biosystems, Angle and many more in the region. Being headquartered in Dubai, United Arab Emirates, our operations are spread across Saudi Arabia, Qatar, Bahrain, Libya, and India.
We are a complete solution provider in molecular and genomic solutions for human identification, human genetics, and the inherited and infectious disease diagnostics space. We have a great team who are committed to providing the latest technological advances in basic research, oncology, and reproductive health solutions.
PRODUCT CATEGORIES:
Precision Oncology, Pharmacogenomics (PGX), Next-Generation Sequencing (NGS), Molecular Diagnostics, Immunology, Epigenetics
Stand Number: D01
United Arab Emirates mediclinic.ae/en/corporate/mediclinicprecise
At Mediclinic Middle East, Mediclinic Precise uses state-of-the-art technology to transform healthcare delivery in the region, by interpreting genetic information to create personalised prevention and treatment plans for its patients. Mediclinic Precise’s goal is to deliver effective, innovative, and high-quality precision medicine services at an affordable price, with the purpose of enhancing the lives of the UAE community.
At Mediclinic Precise, we believe every patient is unique, and that every patient’s health management plan should be too. Through genomic testing we can target treatment to you and your DNA, by taking into account your risk for certain genetic conditions, understanding how well you respond to certain medications and dosage, and leading to a better quality of life.
PRODUCT CATEGORIES: Next-Generation Sequencing (NGS)
MEDSOL

Stand Number: E03
Egypt
medicalsolutionme.com
Headquartered in Cairo, Medsol was established in 2014 as a supplier of laboratory equipment, specialising in genetics and molecular biology. Our mission is to provide innovative solutions to the healthcare industry, helping to overcome diagnostic and research challenges.
Since our inception, we have expanded our presence in other markets including KSA, UAE, and our newest branch in Morocco. Our expert team, coupled with our unique portfolio, ensures technology is user-friendly, and supports diagnostic confidence for all.
At Medsol, we pride ourselves on our traditional business model, which prioritises quality and reliability to exceed customer expectations.
PRODUCT CATEGORIES: Molecular Diagnostics, Next-Generation Sequencing (NGS), Drug Discovery, Epigenetics
MGI
Stand Number: C11
United Arab Emirates en.mgi-tech.com
MGI Tech Co., Ltd. (referred to as MGI) is committed to building core tools and technology to lead life science through intelligent innovation. With a focus on R&D, production and sales of DNA sequencing instruments, reagents, and related products, MGI provides real-time, panoramic, and full-life-cycle equipment and systems for precision medicine, precision agriculture, precision healthcare and other relevant industries.
MGI is a leading producer of clinical high-throughput gene sequencers, and its multi-omics platforms include genetic sequencing, medical imaging, and laboratory automation.
Founded in 2016, MGI operates in more than 80 countries and regions, serving more than 1,300 customers. It has established scientific research and production bases, plus global training and service networks in many countries and regions around the world. MGI is one of the few companies in the world that can independently develop and mass-produce clinical high-throughput gene sequencers. Providing real-time, comprehensive, lifelong solutions, its vision is to lead life
science innovation.
PRODUCT CATEGORIES:
Molecular Diagnostics, Next-Generation Sequencing (NGS), Drug Discovery, Targeted Therapeutics, Immunology, Epigenetics, Precision Oncology, AI & Big Data, Rare and Orphan Disease, Pharmacogenomics (PGX)
Stand Number: C02
United Kingdom randox.com
Randox Biosciences is dedicated to advancing scientific discovery, drug development and diagnostics. We offer complete tailored solutions for clinical and research use, aiming to help pharma, biotechs, and CROs move away from a “one treatment fits all” approach, get a better understanding of the complexity of diseases and increase the efficacy of treatment.
Key elements to realising the potential for personalised medicine is companion diagnostics (CDx) development and omics services.
Randox has key expertise in all elements of CDx and diagnostics development, offering a fully flexible partnership. Randox is the UK’s first commercial partner for large scale proteomics.
PRODUCT CATEGORIES:
Pharmacogenomics (PGX), NextGeneration Sequencing (NGS), Molecular Diagnostics, Immunology

Stand Number: A05
United Arab Emirates illumina.com
At Illumina, our mission is to improve human health by unlocking the power of the genome. Our sequencing by synthesis chemistry is used to generate high-accuracy DNA and RNA sequence data in studies around the globe. Our microarrays also provide accurate, highthroughput genotyping for a range of applications. The innovative products that we provide are helping researchers make pharmacogenomic discoveries that improve treatment, reduce adverse drug reactions, and decrease the potential for side effects or dependency.
We’re empowering our partners to predict and prevent disease, make personalised medicine a reality, and help people
understand more about themselves. And this is only the beginning.
PRODUCT CATEGORIES:
Precision Oncology, Rare and Orphan Disease, Pharmacogenomics (PGX), AI & Big Data, Next-Generation Sequencing (NGS), Targeted Therapeutics, Epigenetics

Stand Number: A12
United Arab Emirates innovatelifesciences.org
Innovate Life Sciences is a Dubai-based genomics lab that offers a comprehensive range of next-generation sequencing and bioinformatics solutions to global life science and pharma customers. Our lab has partnered with WIPRO Limited to develop precision oncology solutions and genetic disorders, using advanced computational analysis powered by AI/MLbased technologies.
With a team of highly qualified professionals with multi-disciplinary expertise, Innovate Life Sciences provides end-to-end comprehensive services covering a range of NGS core business lines, including whole-genome sequencing, clinical exome sequencing, transcriptome sequencing, metagenomics, epigenomics, qPCR, and large-scale multi-omics studies. Our services accelerate biological interpretation using data and advanced analytics, combined with AI/ML and quantum platforms, while ensuring quality, security, and regulatory compliance. Innovate Life Sciences aims to aid customers in outgrowing their competitors by building stronger and more resilient businesses, while contributing to the UAE’s health systems and research, allowing for international comparisons and benchmarking.
PRODUCT CATEGORIES:
Precision Oncology, Next-Generation Sequencing (NGS), Rare and Orphan Disease, AI & Big Data, Molecular Diagnostic

Stand Number: A09
United Arab Emirates babirus.ae
Established in 2008, Babirus is a leading provider of innovative diagnostic solutions in the MENA region. Our commitment to improving people’s health by working with healthcare partners has helped us grow and thrive in the fast-evolving healthcare industry.
With our highly dedicated and qualified team, we provide tailored and efficient medical and diagnostic solutions to bridge the gap between healthcare providers, clinical laboratories, and research institutions.
Babirus partners with global healthcare companies to provide market-leading and technologically innovative products in molecular medicine, immunology, microbiology, immunohematology, special haematology, and quality control programs.
Our goal is to be the partner-of-choice for best-in-class healthcare innovative solutions providers in the region and consistently exceed the expectations of clients and partners. We ensure growth, profitability, continuity, and satisfaction for all our partners and clients.
PRODUCT CATEGORIES:
Precision Oncology, Chronic Disease, Molecular Diagnostics, Immunology, Other, Targeted Therapeutics
Stand Number: B09
United States carecloud.com
CareCloud, Inc. is a leading healthcare technology company with a comprehensive suite of proprietary, cloudbased solutions for growing healthcare organisations.
In today’s challenging healthcare landscape, healthcare organisations need an innovative partner who can help enhance clinical workflows, increase revenue, modernise the patient experience, and reduce operational expenses. CareCloud (formerly known as MTBC), delivers the most comprehensive technology-enabled solutions to healthcare organisations of all sizes, spanning a wide range of specialties, all across the country.
Our cloud-based platform for revenue cycle management (RCM), practice management (PM), electronic health record (EHR), business intelligence, telehealth, and patient experience management (PXM) empowers clients to optimise care
delivery, reduce operational expenses, increase collections, improve the patient experience, grow their practice, and expand patient access to care.
PRODUCT CATEGORIES:
Digital Health, AI & Big Data
Germany
lifespin.health

Lifespin is building a smart health diagnostic platform that combines biology, deep data, and artificial intelligence to generate metabolic insights for precision medicine. We are transforming blood samples into digital samples, making it accessible to data science methods. Our AI-enabled technology is highly scalable and built to provide moderate cost diagnostics to both traditional healthcare and new consumer verticals. Lifespin products are software algorithms and do not require advanced chemical reagents. We have proven performance against legacy methods in clinical chemistry.
Our first commercial products for healthcare providers will be launched mid2023.
PRODUCT CATEGORIES:
Chronic Disease, Precision Oncology, AI & Big Data, Digital Health
United States sengenics.com
Sengenics is a precision medicine company working to improve patient outcomes through physiologically relevant, data-guided decision making. Our solutions enable the discovery and validation of autoantibody biomarker signatures for patient stratification, therapeutic response prediction and development of companion diagnostics.

PRODUCT CATEGORIES:
Immunology, Precision Oncology, Drug Discovery, Targeted Therapeutics
United States eaglegenomics.com
Eagle Genomics is a techbio company pioneering the application of network science to biological discovery and innovation across the food and nutrition, agBio, beauty and personal care, and biopharma industries. Its AI-augmented knowledge discovery platform, the e[datascientist], enables enterprise customers to trace digital journeys in data, driving transformative innovations, exploiting leading-edge scientific discovery in silico and supporting differentiated product claims.
Eagle Genomics’ purpose is to accelerate the bioeconomy through the digital reinvention of life sciences R&D, and the company is increasingly focused on supporting customers’ aspirations as they address “One Health” challenges towards more sustainable and impact-oriented innovation and product outcomes.

PRODUCT CATEGORIES: Other, Start-up Innovator, AI & Big Data
Stand Number: E11
United States medicaspace.com
Medicaspace is a committed medical networking and marketing channel for improving the communication between all members in the medical pool.

Networking space: The main purpose of our website is to create a strong network connecting all medical members (Medical companies, biomedical engineers, doctors, professors, nurses, medical students) for all medical specialties. This aims to facilitate the exchange of experiences among them by providing a free registration to build the medical community.
Marketing space: The main objective of any medical company is to fulfil their goals and reach their targets easily, quickly, smartly and effectively with an affordable cost. Our website provides this privilege by offering a space for marketing to all medical companies.”
PRODUCT CATEGORIES: Digital Health
Stand Number: A04
United States
10xgenomics.com

10x Genomics was founded on the vision that this century will bring advances in biomedicine and transform the way we understand and treat disease. We build products to interrogate biological systems at a resolution and scale that matches the complexity of biology. Our end-to-end single cell and spatial solutions include instruments, consumables, and intuitive software, letting you resolve highly intricate biological systems, while bringing into focus the details that matter most. Explore biology at true resolution.
PRODUCT CATEGORIES:
Precision Oncology, Next-Generation Sequencing (NGS), Pharmacogenomics (PGX), Drug Discovery, Targeted Therapeutics, Immunology, Epigenetics, Other, Chronic Disease, Rare and Orphan Disease, Molecular Diagnostics, Digital Health

Stand Number: C03
United Arab Emirates
W alnawrasmls.com
Founded in 1988 and located in the UAE, Al Nawras is one of the very first wellestablished companies in the sector of healthcare and life science in the GCC region.
PRODUCT CATEGORIES:
Molecular Diagnostics, Next-Generation Sequencing (NGS), Epigenetics, Precision Oncology, Rare and Orphan Disease, Other, Chronic Disease

Stand Number: C18
United Arab Emirates
biggulf-zte.com
Al Zubair Trading LLC is a subsidiary of the Oman-based business conglomerate Zubair Corporation, with a presence in both Oman and the UAE. The company is a leading supplier of laboratory diagnostics, life science, analytical, and general laboratory products. In the UAE, Al Zubair Trading LLC represents companies such as Thermo Fisher Scientific.
With a strong commitment to quality and customer satisfaction, the company has established itself as a reliable supplier of laboratory products in the region.
Product Categories: Precision Oncology, Rare and Orphan Disease, Pharmacogenomics (PGX), Molecular Diagnostics, Next-Generation Sequencing (NGS), Drug Discovery, Targeted Therapeutics, Epigenetics

Stand Number: C15 Switzerland alitheagenomics.com
Alithea Genomics is a global leader in sample and library prep solutions for large-scale RNA sequencing. Thanks to DRUG-seq and our proprietary Bulk RNA Barcoding and sequencing technology (BRB-seq), we drastically simplify the preparation of large numbers of bulk RNAseq libraries.
Our flagship products are the RNAextraction-free DRUG-seq and Organoid DRUG-seq systems, which are specifically tailored to early drug discovery efforts, as well as BRB-seq, which provides the same data quality as Illumina’s Truseq, but 100x more scalable and for 1/10th of the price.
PRODUCT CATEGORIES:
Next-Generation Sequencing (NGS), Drug Discovery, AI & Big Data

Stand Number: B09
United Arab Emirates alliancecaretech.com
ACT develops value-based healthcare solutions leveraging advanced technology and artificial intelligence to solve today’s most pressing healthcare challenges. Our solutions are designed to enhance the patient engagement, automate administrative tasks, diagnostic decisions, manage costs, and ultimately, improve healthcare outcomes.
PRODUCT CATEGORIES:
Precision Oncology, Chronic Disease, Molecular Diagnostics, Next-Generation Sequencing (NGS), AI & Big Data, Digital Health, Targeted Therapeutics, Pharmacogenomics (PGX)
Stand Number: A04
United Arab Emirates agbl.net
As the largest biomedical distribution group in the Middle East, Africa, and Asia region, Alliance Global (AGBL) is dedicated to bringing innovative technologies and products to researchers, clinicians, and diagnostic centres in emerging healthcare markets around the world. AGBL employs a team of product and commercial specialists and has more than 14 offices across the region, offering distribution, consulting, and product development services to manufacturers of biomedical technologies.
PRODUCT CATEGORIES:
Next-Generation Sequencing (NGS), Molecular Diagnostics, Precision Oncology

Stand Number: B11 China
amoydiagnostics.com
Amoy Diagnostics Co., Ltd. is a pioneer and globally leading company in the field of molecular diagnostics for precision oncology, focusing on companion diagnostics product development and commercialisation.
Product Categories: Precision Oncology, Molecular Diagnostics, Next-Generation Sequencing (NGS), Targeted Therapeutics

Stand Number: E04 United Kingdom arc-regulatory.co.uk
ARC Regulatory is a niche provider of global regulatory and clinical research solutions to the precision medicine and in vitro diagnostic medical device industry. Our mission is to support companies, from the world’s top pharmaceutical and biotech leaders to medical device start-ups, in their search to find kinder treatments and guide them through complex, myriad regulatory and research requirements as they bring new medical discoveries to market. We are a trusted partner in the global ambition to improve patient care, leveraging our expertise in delivering high quality, accelerated clinical research for biomarker diagnostics.
PRODUCT CATEGORIES:
Precision Oncology, Molecular Diagnostics, Rare and Orphan Disease, Targeted Therapeutics
Stand Number: D10
United Arab Emirates astragene.com
AstraGene is the UAE’s first molecular diagnostic manufacturing company located at Dubai Science Park. Established in 2020, AstraGene’s vision is to emerge as a leading manufacturer and marketer of in-vitro diagnostic reagents, kits and instruments both nationally and internationally. We make lab tests easy, with at-home testing kits for a wide

variety of tests. We have established collaborations across the globe for collaboration and contract manufacturing.
PRODUCT CATEGORIES:
Molecular Diagnostics, Next-Generation Sequencing (NGS), AI & Big Data, Digital Health, Other
Stand Number: A08 India basesolve.com
BaseSolve is a genomics technology company providing customisable largescale data analysis and management solutions in facilitating accurate insights for clinical diagnostics as well as scientific research.

We are on the mission of transforming the way data is being managed and analysed in the field of genomics; by making it seamless, fast, easy and accurate. Our vision is to make genomic research and diagnoses incomparably insightful and accurate by providing the most powerful technologies.
PRODUCT CATEGORIES:
Precision Oncology, Rare and Orphan Disease, Chronic Disease, Pharmacogenomics (PGX), Molecular Diagnostics, Next-Generation Sequencing (NGS), AI & Big Data, Drug Discovery, Digital Health, Targeted Therapeutics, Immunology, Epigenetics
Stand Number: C06 Germany cegat.com
CeGaT is a global provider of genetic analyses for a wide range of medical, research, and pharmaceutical applications. The owner-managed company stands for independence, comprehensive personal customer service, and outstanding quality. Founded in 2009, the company combines state-of-the-art sequencing technology with medical expertise, with the aim of identifying the genetic causes of diseases and supporting patient care. For researchers and pharmaceutical companies, CeGaT offers a broad portfolio of sequencing services and tumour analyses.
PRODUCT CATEGORIES:
Precision Oncology, Rare and Orphan
Disease, Pharmacogenomics (PGX), Molecular Diagnostics, Next-Generation Sequencing (NGS), Targeted Therapeutics, Immunology, Epigenetics
Stand Number: C13
Korea, Republic of celemics.com

We are a Next Generation Sequencing (NGS) full solution provider for human and non-human applications that focuses on bringing the right solution to customers by catering to individual customers’ specific needs and goals. We confidently offer customisation services and high-quality preset panels, covering fields such as inherited diseases, oncology, and pathogen diagnostics.
Products and services include diagnostics and inherited diseases, cancer research, microbiology and virus research, molecular breeding, antibody discovery, synthetic biology, and BTSeq (Barcode Tagged Sequencing) services.
PRODUCT CATEGORIES:
Precision Oncology, Rare and Orphan Disease, Pharmacogenomics (PGX), Next-Generation Sequencing (NGS), Drug Discovery, Targeted Therapeutics, Epigenetics
Stand Number: B10
United States coatconnect.com
CoatConnect is the first online hub for healthcare professional, educational and business opportunities. A multi-sided marketplace connecting healthcare professionals to opportunities from verified accredited healthcare institutions by profession, specialty and location. Our technology helps healthcare institutions showcase their events to an ever-growing network of healthcare professionals and let them easily find, book and pay for the opportunity anytime, anywhere.
PRODUCT CATEGORIES: Other

Stand Number: C16
United Kingdom congenica.com
Congenica is a digital health company providing software and solutions for the analysis and interpretation of genomic data at scale. We drive precision medicine and help reduce the burden on healthcare systems by delivering automated analysis, diagnosis and treatment solutions to healthcare providers and patients worldwide.

Our products and services are consolidated on one single scalable, automated analysis and diagnostic platform that encompasses inherited disease, somatic cancer, pharma insights and pathogen surveillance.
PRODUCT CATEGORIES:
Precision Oncology, Rare and Orphan Disease, Pharmacogenomics (PGX), Digital Health, Next-Generation Sequencing (NGS)

Stand Number: D07
Italy
diatechpharmacogenetics.com
Diatech Pharmacogenetics is a leading Italian company in the development, production and commercialisation of molecular diagnostic tools for precision oncology medicine. Founded in 1996, the company aims to simplify complex molecular diagnostic procedures into easy end-to-end solutions, from nucleic acid extraction and molecular analysis to final reporting. Diatech has shown strong growth in recent years and today is among the first players in the EU market for the oncology diagnostics market.
PRODUCT CATEGORIES:
Pharmacogenomics (PGX), Molecular Diagnostics, Next-Generation Sequencing (NGS), Precision Oncology
Stand Number: D02
United Arab Emirates digi7.ae
Digi7 is a UAE-based digital healthcare company with specific expertise in healthtech, insure-tech, digital medicine, and artificial intelligence. Focusing on attracting global technology firms to the UAE and MENA region, and establishing, nurturing innovative locally-based technology startups.
Digi7 is a disruptor in the digital healthcare market, bringing a broad portfolio of innovative products that can improve healthcare outcomes, reduce costs, and increase efficiencies in healthcare systems.

PRODUCT CATEGORIES:
Precision Oncology, Rare and Orphan Disease, Pharmacogenomics (PGX), Next-Generation Sequencing (NGS), AI & Big Data, Digital Health, Epigenetics, Healthtech Unicorn, Startup Innovator

Stand Number: D08
United Kingdom enbiosis.com
Enbiosis provides the world’s first clinicallyproven AI-assisted gut health solutions. We have developed an AI algorithm that holistically evaluates the human gut microbiome to drive each individual’s microbiome profile to a healthier state.
PRODUCT CATEGORIES:
Digital Health, AI & Big Data, NextGeneration Sequencing (NGS), Chronic Disease

Stand Number: D04
Poland evispine.com
Evispine is a startup that has been developing unique personalised spinal implants using methods of robotics and up-to-date biomaterials. Contrary to the existing solutions, the implants allow a surgeon a better adjustment of a surgical procedure to various spinal diseases and the level of pain in individual patients. Another area of Evispine activity is implementation of AI in the dedicated spinal software.
PRODUCT CATEGORIES: Digital Health, Other, AI & Big Data

Stand Number: A16 Bahrain
expressmedlabs.com
ExpressMed Laboratories is a CAP accredited multi omics facility based in the Kingdom of Bahrain that provides highly advanced diagnostic and research services. Established in 2015, ExpressMed Laboratories is specialised in nano imaging, electron microscopy, genomics and precision medicine, clinical and anatomical pathology. Each department is wellequipped with cutting edge technologies, operated and managed by highly-skilled specialised personnel.
PRODUCT CATEGORIES:
Molecular Diagnostics, Next-Generation Sequencing (NGS)

Stand Number: Find at PMES 23 hub Egypt
falakstartups.com
Powered by the Egyptian Ministry of International Cooperation, General Authority for Investment and their venture capital arm, Egypt Ventures, Falak Startups’ mission is to find and empower talented and ambitious tech startup founders and help them thrive in the region’s rapidly changing entrepreneurship
landscape.
The company’s sector-agnostic offering focuses on improving startups’ productmarket fit; fine-tuning their business, operating and revenue models; growing their customer base; and raising follow-on investments.
PRODUCT CATEGORIES: Startup Innovator

Stand Number: A20 Kingdom of Saudi Arabia havensci.com
We are a Saudi company that specialises in the development and production of scientific products in the genomics field. Our mission is to provide affordable, highquality genomics products for the medical and research sectors.
PRODUCT CATEGORIES:
Stand Number: E06
United Arab Emirates focp.ae
Friends of Cancer Patients is a non-profit organisation, founded in 1999 under the directives and patronage of Her Highness

Molecular Diagnostics, Next-Generation Sequencing (NGS), Epigenetics
more than 80 different sensors such as temperature, humidity, carbon dioxide etc.
PRODUCT CATEGORIES:
Precision Oncology, Pharmacogenomics (PGX), Molecular Diagnostics, Targeted Therapeutics, Next-Generation Sequencing (NGS), Chronic Disease, Rare and Orphan Disease, Epigenetics, Immunology

Stand Number: D12
United Kingdom lmdatascience.com
SheikhaJawaher bint Mohammed Al Qasimi, wife of the Ruler of Sharjah in the UAE, and is licenced by the Ministry of Community development.
PRODUCT CATEGORIES: Non-profit Organisation

Stand Number: A07 India indushealthplus.ae

Stand Number: B08
United Arab Emirates
genetricsinc.com
Genetrics is a specialised solution provider for genetics instruments, reagents, consumables and softwares for molecular diagnostics and research for various labs and hospitals. It represents leaders in molecular and NGS solutions such as Certest, Bioneer, Twist Biosciences, Bionano, MRC Holland, and Sophia Genetics.

PRODUCT CATEGORIES:
Precision Oncology, Pharmacogenomics (PGX), Molecular Diagnostics, NextGeneration Sequencing (NGS), AI & Big Data, Epigenetics
Indus Health Plus has been trailblazing the preventive healthcare and wellness industry since its inception in 2000. With the aim of making quality healthcare available, accessible, and affordable, Indus Health Plus has taken its services across the globe. Indus has a range of genetic products under the brand- DNAWise; to give a complete analysis of your body type, health risks and a recommended lifestyle.
PRODUCT CATEGORIES:
Pharmacogenomics (PGX), Digital Health, Healthcare provider
Stand Number: C10 United Arab Emirates vivision.com.co
Invention Vision is committed to distributing a wide range of unique technologies. Our medical wing is mainly focused on providing diagnostic solutions based on real-time PCR for oncology, genetics, infertility and infectious assays, point-of-care solutions, rapid test assays, analysers for biochemistry, immunological assays, haematology, fully automated semen, urine, and faecal analysis. We also represent products from European and US-based companies, which offer centralised monitoring systems for
L&M Data Science is a provider of preclinical neuroimaging services for the pharmaceutical industry. Our cuttingedge technology and highly-trained team of experts enable us to provide precise and accurate imaging data that is critical to the success of preclinical drug development programs. Our services are tailored to meet the unique needs of each client, and we work closely with our customers to ensure that they receive the highest quality data in a timely and cost-effective manner. With a focus on innovation and customer satisfaction, L&M Data Science is the ideal partner for pharmaceutical companies seeking to accelerate their drug discovery and development programs.
PRODUCT CATEGORIES: AI & Big Data, Drug Discovery
LABOSHOP
Stand Number: E01
United Arab Emirates laboshop.ae
LaboShop is your go-to platform in MENA for everything related to molecular biology Including high-tech lab products, premium services and news.

PRODUCT CATEGORIES:
Precision Oncology, Molecular Diagnostics, Next-Generation Sequencing (NGS)


Stand Number: C07a India diagnostics.medgenome.com
MedGenome Labs is the market leader in genetic diagnostic testing and genomic wellness in India and South Asia. We deliver AI-driven and validated genetic testing solutions through new-age proprietary tools and technologies to empower clinicians for better management of disease cohorts in rare inherited genetics, cancer and reproductive genetics, ophthalmology, neurology, nephrology, cardiovascular genetics disorders and more.
PRODUCT CATEGORIES:
Precision Oncology, Rare and Orphan Disease, Chronic Disease, Pharmacogenomics (PGX), Molecular Diagnostics, Next-Generation Sequencing (NGS), AI & Big Data, Drug Discovery, Digital Health, Immunology, Epigenetics, Healthtech Unicorn
among healthcare providers, patients and their families, and public can be achieved through scientific conferences, family meetings, social activities, workshops, training programs, and online tools such as electronic newsletters and educational webpages. Connecting people to exchange knowledge about rare disease is achieved by establishing patient support groups for families with rare diseases and healthcare provider networks. Supporting individuals with rare diseases is accomplished through connecting patients to healthcare providers and facilitating support from healthcare institutions and community services.
PRODUCT CATEGORIES: Non-profit Organisation

Stand Number: C08 United States myengene.com
MyEngene started with a vision to truly personalise medicine. Our mission is to create a positive healthcare experience by elevating the standard of care that patients deserve.
PRODUCT CATEGORIES: Pharmacogenomics (PGX)
Stand Number: A08
United Arab Emirates medscience.ae

Established in 2018, Medscience aims to deliver best-in-class services and solutions in the field of life science research (human, veterinary and plant), pharmaceuticals, clinical diagnostics, natural sciences and food sciences.
PRODUCT CATEGORIES: Digital Health, Molecular Diagnostics, AI & Big Data, Other

Stand Number: Find at PMES 23 hub United Arab Emirates menararediseases.org
The MENA Organization for Rare Diseases was established to serve and support people with rare diseases in the MENA region. The organisation’s goals are to educate, connect, and support. Education, spreading knowledge, and increasing awareness about rare diseases
Stand Number: B02
United States neb.com
Founded in the mid-1970s as a collective of scientists committed to developing innovative products for the life sciences industry, NEB is now a recognised world leader in the discovery and production of enzymes for molecular biology applications.
A supplier-of-choice for scientists across the globe, NEB offers the largest selection of recombinant and native enzymes for genomic research. Our ever-expanding catalogue also includes products related to restriction enzymes, PCR, gene expression, sample preparation for next generation sequencing, synthetic biology, glycobiology, epigenetics and RNA analysis.
PRODUCT CATEGORIES:
Molecular Diagnostics, Next-Generation Sequencing (NGS), Immunology, Epigenetics
S tand Number: B04
Sweden
olink.com
Olink Proteomics is dedicated to innovation, quality, and transparency, providing advanced products and services for protein biomarker discovery. Our ground-breaking, scalable proteomics solutions help scientists conduct highquality proteomics research over a wide range of multiplexing levels and sample throughput to meet their individual needs. Olink’s mission is to accelerate proteomics together with the scientific community to understand real-time biology and gain actionable insights into human health and disease. We believe this will drive the development of better, more targeted therapies and help revolutionise future healthcare through the implementation of precision medicine.
PRODUCT CATEGORIES:
Precision Oncology, Rare and Orphan Disease, Chronic Disease, Drug Discovery, Immunology, Targeted Therapeutics
Stand Number: B04A
United Kingdom
nanoporetech.com
Oxford Nanopore Technologies goal is to bring the widest benefits to society through enabling the analysis of anything, by anyone, anywhere. The company has developed a new generation of nanoporebased sensing technology that is currently used for real-time, high-performance, accessible, and scalable analysis of DNA and RNA. The technology is used in more than 120 countries, to understand the biology of humans, plants, animals, bacteria, viruses and environments as well as to understand diseases such as cancer. Oxford Nanopore’s technology also has the potential to provide broad, high impact, rapid insights in a number of areas including healthcare, food and agriculture.
PRODUCT CATEGORIES:
Rare and Orphan Disease, Next-Generation Sequencing (NGS), Epigenetics, Startup innovator
Stand Number: B04
Qatar hbku.edu.qa
QBRI is a leading research institute under Hamad Bin Khalifa University (HBKU). As a national centre of excellence and a global hub for biomedical and translational research, the institute works to improve and transform healthcare in Qatar through discoveries in the prevention, diagnosis, and treatment of diseases, affecting the people of Qatar and populations across the region. The institute addresses the Qatar National Health priorities outlined in the Qatar National Research Strategy through its dedicated research centres.
PRODUCT CATEGORIES:
Chronic Disease, Molecular Diagnostics, Next-Generation Sequencing (NGS), AI & Big Data, Drug Discovery, Targeted Therapeutics, Immunology, Epigenetics

Stand Number: E12
Korea, Republic of shaperon.com
Shaperon is a clinical stage biotech company developing novel inflammasome inhibitors. Its unique mechanism of action of GPCR19-P2X7 modulation suppresses a broad spectrum of inflammatory cytokines by controlling both priming and activation phase of inflammasome. With this novel modality which is best suited to address complex immune-mediated inflammatory disorders, we are currently developing multiple clinical programs in atopic dermatitis, cytokine release syndrome, Alzheimer’s disease, NASH, etc. In addition to inflammasome R&D programs, Shaperon has anti-cancer nanobody bispecifics and anti-viral nanobody therapeutics.
PRODUCT CATEGORIES:
Chronic Disease, Molecular Diagnostics, Drug Discovery, Targeted Therapeutics, Immunology
Stand Number: B02
United Arab Emirates scientechnic.com
Scientechnic Lifesciences is a business unit of Scientechnic, a leading technology solutions provider in the Middle East. Scientechnic Lifesciences is dedicated to advancing the field of life sciences by providing innovative genomics and proteomics solutions and services for research, clinical, and industrial applications. Our portfolio includes a broad range of products and services, such as NGS systems and associated reagents, real-time PCR machines, diagnostic kits, molecular biology reagents, automated liquid handling systems, antibodies, ELISA assays, microbiology solutions, sequencing services etc .
PRODUCT CATEGORIES:
Molecular Diagnostics, Next-Generation Sequencing (NGS), Epigenetics

Stand Number: C14
United States somalogic.com
SomaLogic’s SomaScan Assay enables researchers to measure an industry leading 7,000 proteins with only 55 uL of body fluid. The proprietary SomaScan Assay measures proteins with high specificity, high throughput, and high reproducibility, which enables the possibility of faster, more precise drug discovery. Further, through machine learning-powered bioinformatics algorithms operated in tandem with a growing database of more than 550,000 protein samples, SomaLogic has created a suite of SomaSignal tests. These tests are clinical proteomic diagnostics that provide additional insights into the current health status of patients and the future risk of conditions and diseases.
PRODUCT CATEGORIES:
Chronic Disease, Molecular Diagnostics, Targeted Therapeutics

Stand Number: C05
India strandls.com
Strand Life Sciences is a pioneer in advancing precision medicine through cutting-edge genomics and bioinformatics software and services. With over two decades of legacy, we’re proud to be India’s first genomics lab certified by CAP and NABL. Our end-to-end clinical genomics testing services cover various diseases, including oncology, rare diseases, and reproductive health. We work closely with leading hospitals and healthcare organisations worldwide for their clinical research.
PRODUCT CATEGORIES:
Precision Oncology, Rare and Orphan Disease, Molecular Diagnostics, NextGeneration Sequencing (NGS), Targeted Therapeutics, Pharmacogenomics (PGX), Healthcare provider, AI & Big Data, Drug Discovery, Digital Health, Epigenetics
SYNAGENE
Stand Number: C01
Malaysia synagene.com
Synagene is a prominent healthcare group that strives to advance healthcare by integrating cutting-edge technologies and pushing beyond the confines of traditional healthcare.
Through innovative solutions and a commitment to excellence, Synagene seeks to enhance the quality of life for individuals and communities alike. With a strong focus on integrating digital technologies and personalised medicine, Synagene is at the forefront of healthcare innovation. Whether it’s through clinical care, research, and development, or partnerships with other healthcare organisations, Synagene is dedicated to improving healthcare outcomes and making a meaningful difference in lives.
PRODUCT CATEGORIES:
Precision Oncology, Rare and Orphan Disease, Chronic Disease, Pharmacogenomics (PGX), Molecular Diagnostics, Next-Generation Sequencing (NGS), AI & Big Data, Drug Discovery, Digital Health, Targeted Therapeutics, Immunology, Healthtech Unicorn, Healthcare Provider
Stand Number: E02
United Arab Emirates testaty.com
A medical tests advisor and locator, Testaty was developed as the only well-connected portal in the country linking together the healthcare providers (clinicians) on one side with the authorised medical laboratories on the other for the end benefit of the patient, facilitating processes for all.

PRODUCT CATEGORIES:
Healthcare Provider
Stand Number: B03
France
tsp-diffusion.com/actigraph
Founded in 1995, TSP Diffusion provides online tools and databases dedicated to scientific research and engineering. ActiGraph is recognised as a pioneer in the field of motion-sensing wearable

technology. Our usual partners are clinics and hospitals, and medicine and sports universities. Pharmaceutical research is more and more interested in our actigraphy objective data.
PRODUCT CATEGORIES:
Digital Health, AI & Big Data, Other, Targeted Therapeutics, Chronic Disease, Drug Discovery
Stand Number: C07
United States veracyte.com
Veracyte is a global diagnostics company that empowers clinicians with the highvalue insights they need to guide and assure patients at pivotal moments in the race to diagnose and treat cancer. Our growing menu of diagnostic tests answers important clinical questions to help patients avoid risky, costly procedures and interventions, and accelerate time to appropriate treatment.

PRODUCT CATEGORIES:
Precision Oncology, Molecular Diagnostics, Digital Health
Stand Number: B13
Spain
veritasint.com

At Veritas, we apply science and our global resources to bring genetic and genomic diagnostics to people to extend and significantly improve their lives. Our global portfolio includes preventive medicine, perinatal medicine, and genomic diagnostic services.
In keeping with our responsibility as a leading biotechnology company, we collaborate with healthcare providers, governments, and local communities to support and expand access to genetic and genomic medicine reliably and affordably around the world.
PRODUCT CATEGORIES: Rare and Orphan Disease, Pharmacogenomics (PGX), Immunology, Next-Generation Sequencing (NGS)

Stand Number: B07
United Arab Emirates
adscc.ae
The Abu Dhabi Stem Cell Center (ADSCC), a PureHealth asset, is a renowned healthcare institution in Abu Dhabi, UAE, specializing in advanced stem cell therapy, research, and regenerative medicine. ADSCC is the incubator of the Abu Dhabi Bone Marrow Transplant (AD-BMT) programme, the first comprehensive program to provide autologous and allogeneic hematopoietic stem cells transplant (HSCT) for adult and pediatric patients in the UAE since 2020.
As a Center of Excellence in Hematopoietic Stem Cell Transplantation accredited by the Department of Health Abu Dhabi, ADSCC's holistic service model includes advanced research, clinical trials, translational care, and manufacturing capabilities. ADSCC has one of the region's most advanced and sophisticated research labs and a robust multidisciplinary hospital. It is the only center in the UAE to encompass a cell processing laboratory, a state-of-theart apheresis unit, a stem cell collection unit, a Good Manufacturing Practice (GMP) laboratory, and dedicated multi-specialty outpatient clinics and inpatient wards.
As the UAE’s first and most experienced stem cell transplant center, ADSCC has received multiple prestigious recognitions and conducted strategic collaborations, solidifying its position as a center of excellence. For inquiries, please email communications@adscc.ae

Liaison MDX

An innovative and powerful thermocycler with two consumable disc options: the 8-well Direct Amplification Disc for sample-to-answer testing and the 96-well Universal Disc for higher volume testing.
Supported by an expanding menu of molecular assays, the LIAISON MDX is flexible allowing you to run real-time PCR for qualitative, quantitative and multi-analyte detection. The instrument can perform both IVD as well as Laboratory Developed Tests.
The LIAISON MDX software provides easy to understand results with the ability to check amplification curves after a run. The software also plots QC Charts and can be bi-directionally interfaced with LIS for easy integration into any lab workflow. You can also access and navigate the software during a run.
The instrument is compact with an extremely small footprint measuring only 12” by 8” by 12” so that it can fit into laboratories of any size.
The Spark Multimode Microplate Reader by TECAN

Easy selection of one of the four preset configurations for a specific application segment provides a fast solution to solve your research problems. The fully configurable platform also gives you the freedom to personalise your own Spark reader. The Spark is also upgradeable so that you can add modules at any time in the future.
Mercurius BRB-SEQ Library Preparation Kits
The MERCURIUS BRB-seq library preparation kits for Illumina contain all the oligos and enzymes needed to go from purified RNA to sequencing-ready DNA libraries.
CancerPrecision enables targeted treatment for your patients. It provides optimal molecular genetic tumour profiling using NGS and forms the basis for personalised, biomarker-based cancer therapy.


NEW ENGLAND BIOLABS
NEBNext Ultra II FS DNA Library Prep Kit for Illumina


The NEBNext Ultra II FS DNA Library Prep Kit for Illumina provides a fragmentation system: a fast and reliable solution for DNA fragmentation and library construction. A new DNA fragmentation reagent is combined with end repair and dA-tailing reagents, allowing these steps to be performed in the same tube, with no clean-ups or sample loss. The same fragmentation protocol is used for any input amount or GC content.
The kit also includes the NEBNext Ultra II Ligation Master Mix for adaptor ligation, the NEBNext Ultra II Q5 Master Mix for uniform, high-fidelity library amplification, and the gold standard SPRI select size selection and clean-up beads.
Adaptors and primers are not included in the kit and are available separately.
As one of MGI’s single-cell series products, the DNBelab C series highthroughput single cell RNA library preparation set V2.0 contains the C4 device used for droplet generation, the flow cell for loading, library preparation reagents and data analysis package- C4Tools to support the whole process of single cell 3’RNA sequencing data analysis. Currently, based on MGI’s DNBelab C-series high-throughput single-cell RNA library preparation
with high-resolution scanning and automation to dramatically improve efficiency in gene expression and genetic analysis applications.



When used with the GeneChip AutoLoader, the GeneChip Scanner 3000 7G provides complete walk-away freedom for scanning your arrays. As with all GeneChip Scanner 3000 instruments, the GeneChip Scanner 3000 7G fits easily into a benchtop environment.







Olink Target
Olink Target 96 & 48 panels for targeted protein biomarker discovery enable faster, better-informed decisions in human protein biomarker research by providing high-multiplex immunoassays that do not compromise on either data quality or performance. Identifying multiple proteins that form a signature is more powerful and reliable than looking at a single protein.
But how does it work? The unique technology behind the Olink platforms, Proximity Extension Assay (PEA) technology, is an innovative dual recognition, DNA-coupled methodology providing exceptional readout specificity. PEA enables high multiplex, rapid throughput biomarker analysis without compromising on data quality.
Now in its 8 edition, Precision Medicine & Functional Genomics, will be taking place from 11 – 14 November in Doha, Qatar.








PMFG 2023 will explore the latest developments and innovations in functional genomics and how cutting-edge discoveries translate into precision medicine solutions. www.sidra.org/pmfg2023

The United Arab Emirates (UAE) is a small healthcare market with a population of 10 million. It makes up for its small population with an abundance of governmental vision and support to become the global leader in precision medicine and digital healthcare transformation.
The UAE has empowered leadership through a comprehensive digital infrastructure, responsive and engaged regulatory bodies, as well as the most diverse global population providing a single site for population health research. An openness to biotech and healthtech ensures the future of Sustainable Development Goal Number 3 (SDG #3) focused on “good health and wellbeing”, and a commitment to supporting emerging healthcare nations ensures access and support to achieve this goal by the year 2030.
Study monitoring assures the rights, safety and welfare of study subjects as well as ensuring the highest quality data for regulatory submissions.
The UAE sets itself apart in three areas that demonstrate leadership, attract investment and innovation. They are genome sequencing, health data infrastructure, and regulatory structure.
Firstly, the UAE has launched the most aggressive national genome sequencing program anywhere in the world, with a goal to sequence 100% of its citizens. Currently, more than half of the nation’s citizens have been sequenced.
According to the country’s Ministry of Health and Prevention (MoHaP): “The programme will equip healthcare practitioners with quality information that will enable them to provide advanced diagnosis, treatment options, and personalised and preventive programmes tailored to an individual’s unique genetic makeup.
“It will also help to predict and prevent present and future genetic diseases better and implement new therapies for rare and chronic diseases.”
This model will likely be expanded to the expatriate population, which makes up 90% of the nation’s population creating what could be the largest global repository of population health genome sequencing.
Secondly, the country has built a modern infrastructure that includes 100% of patient electronic medical records. The UAE’s national Health Information Exchange (HIE) digital policy currently includes three regional HIEs. Future plans for a regional Gulf Cooperation Council (GCC) HIE will provide a tremendous repository of data for research and clinical trials.
And thirdly, while the government has traditionally adopted regulatory and procedural approaches of the United States and United Kingdom, the UAE recognises the need to consider new approaches as digital technology changes the way healthcare and research are conducted. The creation of ‘regulatory sandboxes’ allows researchers, pharma, biotech and medical device organisations to work with authorities to adapt and expand research and care. The UAE is uncompromising in its
dedication to privacy and data protection, but open to new approaches that remove archaic barriers of regulation.
Alliance Care Technologies International (ACT) set up operations in the UAE in 2020 to partner with the governmental vision in precision medicine and digital transformation. We believe the next paradigm shift in healthcare will emerge through leapfrogging technology in Africa. The UAE – with its commitment to SDG #3 – plus modern infrastructure, value of personalised and precision medicine, and an open-minded regulatory policy make this a great environment for designing and creating the future of healthcare.
ACT leverages technology to create value-based healthcare solutions and represents strategic partners who desire to expand their presence in the Middle East and Africa. ACT provides full service regional representation including market research, business development, medical product registration, and sales, service and support staffing.
For more information, contact value@alliancecaretech.com

Driven by a vision for equitable, universal access to value-based healthcare, Alliance Care Technologies develops and deploys advanced technology and artificial intelligence solutions that solve today’s most pressing healthcare challenges. We work with physicians, patients, researchers, industry leaders, and entrepreneurs to design valuebased, individualized solutions that:

• Enhance patient - physician collaboration



• Expand workforce capacity and streamline administrative processes


• Support diagnostic decisions
• Manage costs
• Enable global research for population health and pandemic response
And ultimately, improve outcomes, equity, and access across the healthcare continuum.
Visit


www.alliancecaretech.com
value@alliancecaretech.com

In the rapidly evolving field of healthcare, artificial intelligence (AI) is carving a transformative path, paving the way for personalised and optimised patient care. Among the organisations leading this revolution, we stand at the forefront, harnessing the power of AI to deliver bespoke healthcare solutions. Our state-ofthe-art healthcare organisation is on a mission to revolutionise patient care by merging technology, data, and scientific breakthroughs.
Aiomix explores revolutionising healthcare with AI, linking genetic variants to diseases and developing novel molecules for precision medicine.
The core of our approach lies in the utilisation of Knowledge Graphs. These complex information networks amalgamate data from a multitude of sources, including public databases, research articles, and clinical trial databases. By fostering a holistic view of vast volumes of data, Knowledge Graphs empower us to discern patterns and associations that might otherwise remain hidden.
A crucial application of this tool is the correlation of genetic variants with specific diseases. By making these connections, we can develop personalised treatment plans that cater to the unique genetic makeup of each patient. This approach goes a long way in enhancing health outcomes and the overall quality of life for patients. Moreover, our organisation is making significant strides in the realm of AI Drug Discovery. Our advanced AIdriven methodologies enable us to generate novel molecules, offering groundbreaking potential in the development of precision medicine. The ability to create such unique molecular structures signifies a paradigm shift in medical treatment strategies. This innovative approach will yield more targeted and efficacious treatment options for patients, thereby addressing their unique
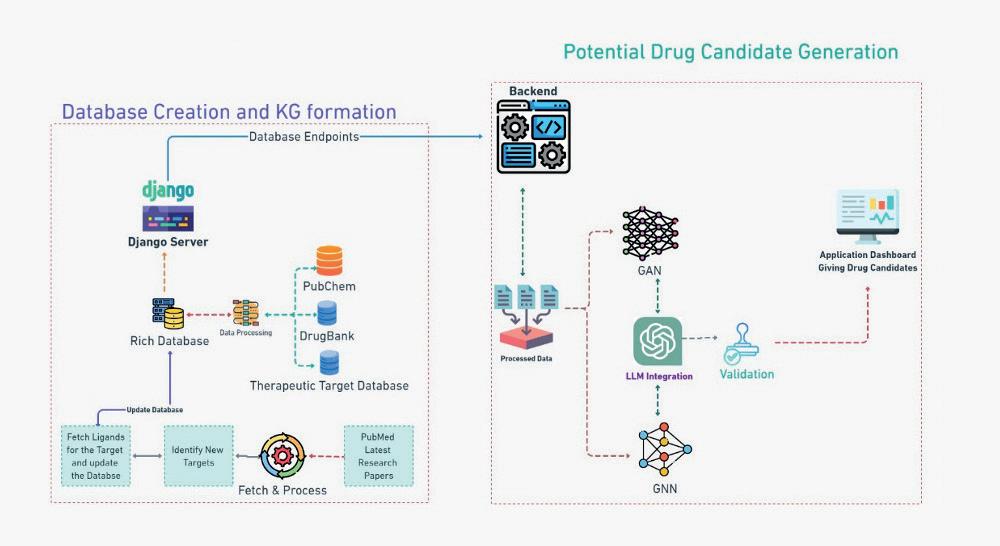
needs and genetic constitution.
Our drive to innovate and adapt ensures that we remain at the cutting edge of technology and healthcare. By exploiting the power of AI, we aim to unlock new opportunities and possibilities in patient care, creating a future where healthcare solutions are as unique as the individuals they are designed for.
In conclusion, as a pioneering healthcare organisation, our commitment lies in harnessing the potential of AI to deliver customised healthcare solutions. By leveraging technology and scientific breakthroughs, we are optimising patient care and propelling the healthcare industry into a new era. Our utilisation of Knowledge Graphs and AI in drug discovery exemplifies our innovative approach, enabling us to cater to the unique needs of patients and improve their health outcomes. Our journey is a testament to the transformative power of AI in healthcare, and we remain committed to pushing the boundaries of what is possible in this exciting field.
For more information, visit aiomixuae.com


Artificial intelligence has been shown to better digitise patient experiences, thus improving outcomes and lowering cost burdens.
In recent years, the importance of artificial intelligence (AI) in precision medicine has grown exponentially, with its potential to digitise patient experiences – and optimise diagnostic processes – leading to better outcomes for all.
One such groundbreaking method, developed by Lifespin, leverages a single droplet of blood to perform a comprehensive health assessment and diagnostics at minimal cost, making it accessible and affordable for the masses.
This innovative approach utilises proprietary electromagnetic scanning technology to capture and digitise metabolic information from the blood, allowing algorithms to analyse and assess health markers with unprecedented accuracy. When performed on a monthly or quarterly basis, this method could dramatically improve individual health and prevent
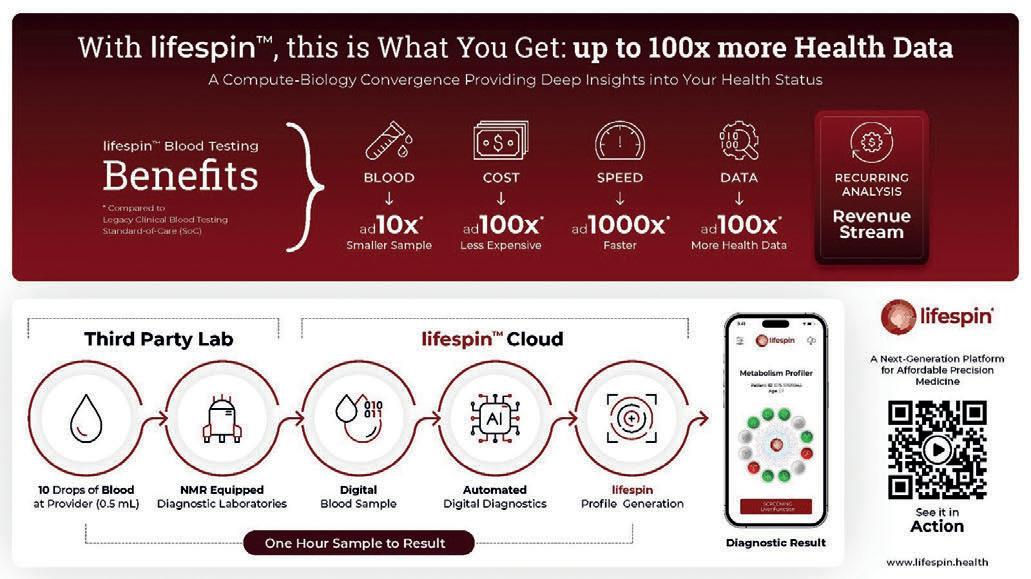
non-communicable diseases such as obesity, diabetes, and cardiovascular conditions.
The implications of this technology are far-reaching, particularly for nations struggling with the burden of healthcare costs. By keeping track of blood markers and implementing preventative measures, countries can elevate their overall health status and reduce the financial strain on their healthcare systems. Physicians stand to benefit as well, as the AI takes care of the technical aspects, allowing them to focus on providing more personalised and human-centric care to their patients.
Lifespin’s system also has the potential to revolutionise healthcare delivery on a population level through the use of cloud-based digital and in-silico tools. This is especially relevant in the Middle East, where non-communicable diseases such as obesity and diabetes are rampant. By utilising AI-powered technology, healthcare becomes more accessible and affordable for millions of individuals.
The Lifespin system operates on a proprietary and highly scalable platform that scans blood samples within minutes using nuclear magnetic resonance (like MRT). This process digitises metabolic health data, providing real-time insight
into an individual’s health status. The system then employs proprietary AI-based algorithms to add multiple layers of health information. Once digitised, the obtained metabolic profiles can be used for numerous testing events without the need for new sample collection, such as simultaneous testing for the impact of drug treatment, organ function, or the presence of neurological diseases.
Compared to traditional methods, the Lifespin system generates up to 100 times more information at a fraction of the cost. Its scalability based on an enabling model for third party laboratories, cost structure, and decentralised computing access make it particularly suitable for population-wide health monitoring. Lifespin and its partners are working tirelessly to fulfil this vision at both national and health system levels.
In conclusion, the integration of AI in precision medicine, as exemplified by Lifespin’s innovative blood scanning technology, promises a new era of affordable and accessible healthcare. By digitising patient experiences and harnessing the power of AI, we can better prevent, diagnose, and manage non-communicable diseases on a global scale.

The Lifespin system is poised to transform healthcare delivery, ushering in a healthier and more equitable future for all.
Visit lifespin.health for more.




Study monitoring assures the rights, safety and welfare of study subjects as well as ensuring the highest quality data for regulatory submissions.
By Claire Jones, Clinical Project Manager at ARC RegulatoryStudy monitoring is a requirement under FDA and IVDR regulation of any IVD irrespective of the stage of clinical development. It assures the rights, safety, and welfare of study subjects, as well as ensures the highest quality data for regulatory submissions.
A clinical monitor or CRA (Clinical Research Associate) is a professional who is employed by a sponsor or by a CRO (contract research organisation) acting on a sponsor’s behalf, who monitors the progress of investigator sites participating in a study.
The monitor confirms that procedures are performed at the site in accordance with the approved protocol. Monitoring will also identify any problems and recommend solutions.
Clinical monitoring can be conducted in several ways:

Onsite, the CRA reviews source documentation and other essential documentation as outlined in the sponsors monitoring plan. In depth reviews take place to help ensure the correct protocols, instructions, samples and test kits are being used, stored and destroyed.
This type of site includes institutions such as hospitals, CROs, or central clinical testing facilities. Clinical, drug, device, and hospital testing also often takes place here. The CRA reviews source documentation and other essential documentation as outlined in the sponsor’s device monitoring plan. Often, centralised testing sites are selected for device studies being used in the context of a companion diagnostic development.
A full or hybrid approach to onsite monitoring. CRAs are given access to source data via electronic platforms such as eTMF (Trial Master file) or eDC (Data Capture) which can facilitate remote review as the source documentation is electronic rather than paper.
When monitoring, the CRA may identify common pain points, including:
• Typographical errors
• Missing documentation
• Incomplete documents
• Incorrect storage of investigational product
• Deviation from protocol
• QMS non-Compliance
Undoubtedly, successful monitoring can be achieved by taking a collaborative approach and the benefits are numerous. Here are the top reasons why monitoring matters:
• Understanding relevant regulatory requirements (USA, Europe and MENA)
• Compliance to regulations
• Data integrity
• Establishing good relationships with test sites and supporting staff
• Consistent review of essential documentation
• Effective team communication
• Study progress
• Real time tracking
• Harmonisation across sites
• Operational efficiencies
The above has given you a snapshot of what matters when looking to successfully monitor a clinical study, the common pain points, and the kind of monitoring activities that will ensure smooth regulatory submissions and audits, readying your company for an optimistic future.
By actively engaging with a clinical research organisation like ARC Regulatory to conduct your study monitoring, you can feel confident knowing you have experts at your side when tackling the nuances of international regulations. Learn more at arc-regulatory.com
The Eyetelligence Assure platform works in conjunction with any fundus camera and PC, and does not require image upload via the internet.
Eyetelligence, an Australian-based, international medtech company, has developed Eyetelligence Assure, an advanced AI software solution that analyses retinal images for features of the three major blinding eye diseases: glaucoma, referable diabetic retinopathy and referable neovascular age-related macular degeneration.

Backed by peer-reviewed publications and journals, Eyetelligence’s deep learning technology is designed to empower clinicians to make more informed clinical decisions with speed, accuracy, and confidence to ensure the best health outcomes for their patients.
Professor Mingguang He, Professor of Ophthalmic Epidemiology at the University of Melbourne and Eyetelligence Chief Medical Officer, says: “AI has evolved from computer software in the laboratory to clinical adaptation in many imagedriven practices, including ophthalmology.
“Enabled by a combination of the availability of large datasets and substantially improved computing power, deep learning algorithms (DLAs) have created unprecedented opportunities for substantially improved accuracy in automated diagnosis and classification of medical images. This automated diagnosis, which makes eye care less dependent on human input, is improving accessibility, efficiency, and cost-effectiveness, making ocular disease diagnosis and management quicker, cheaper, and more consistent.”
The Eyetelligence Assure platform works in conjunction with any fundus camera and PC, and does not require image upload via the internet, which means every patient’s identity, information and clinical data are protected.
“In the optometry setting, our aim is to provide practitioners and patients alike with peace of mind – because they know that Eyetelligence detects cataract, age related macular degeneration, glaucoma and diabetic retinopathy with 95% accuracy regardless of the practitioner’s level of experience,” Prof. Ming explained.
As the eye is the body’s only organ that provides a microvascular profile Eyetelligence has also developed Eyetelligence Microvascular, a non-invasive platform to detect early signs of cardiovascular disease.
Eyetelligence Microvascular makes it easy to understand the patient’s level of risk so that management with counselling and medicines can begin to minimise the risk. Eyetelligence Microvascular report shows:
• 5-year risk score as a percentage of the potential for a cardiovascular event
• Comparison of health status against other people with a similar age and gender
Eyetelligence harnesses the power of AI To provide affordable clinical support tools that make disease screening easy, anytime, anywhere. Visit eyetelligence.ai




















tumour-model-based platform accurately predicts drug efficiency and toxicity by mimicking the native environment of human tissues and organs. Our platforms are userfriendly, easily preserved for long periods, and costeffective to maintain.
The second
that for some could reach millions of storage tubes. Our solution utilises paper-based platforms to help organisations store five hundred times more cell line product units using the same space, which translates into substantial cost savings.
We are at advanced stages of technology development, as our Technology Readiness Level (TRL) is six. We have a working prototype of our paper platforms, tested in
scientific journals. Our unique process has been filed for an International Patent (PCT/ US2018/037347).
In the first two years of operation, we plan to have our product at a TRL of nine, and test it with early adopters. We have received AED 1.5 million in research funds, and we are looking forward to attracting investments to move out of New York University and establish our independent research/ production lab. Visit wp.nyu.edu

Alithea Genomics, a spin-off of the Swiss Federal Institute of Technology (EPFL), is a pioneering life science company focused on commercialising innovative sample preparation solutions for RNA sequencing. Founded by two postdocs – Riccardo Dainese and Daniel Alpern – along with EPFL’s full professor Bart Deplancke, the company has developed a technology that offers 100x higher scalability and 10x higher cost efficiency in RNA sequencing
With Alithea Genomics’ revolutionary sample preparation solutions, researchers can now conduct large-scale transcriptomics studies, ranging from hundreds to tens of thousands of samples. This breakthrough opens new possibilities for biomarker and drug discovery studies, empowering the next generation of healthcare with personalised medicine and big data analysis.
By leveraging their advanced RNA sequencing technology, Alithea Genomics aims to transform the field of genomics, accelerating the development of novel therapies and treatments. Their innovative solutions hold the potential to revolutionise healthcare, enhance patient outcomes, and contribute to a more personalised future of


As a leading player in the life science industry, Alithea Genomics is poised to drive significant advancements in transcriptomics research, ushering in a new era of precision medicine and contributing to a better understanding of complex diseases.

Visit alitheagenomics.com
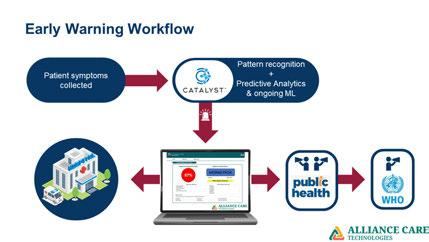


The economic and global health devastation caused by the COVID-19 pandemic created a societal imperative to develop effective syndromic surveillance systems capable of detecting and preventing future pandemics. The existing diagnosis-based surveillance systems proved inadequate due to their inability to detect pathogens quickly, during the critical, pre-epidemic
The only way to identify pernicious diseases earlier in their natural history timelines is to analyse patients’ symptoms to predict diagnosis rather than having to wait for a confirmed diagnosis. Currently patients waste precious time deciding if and when to seek medical care and, when they do, must wait for the clinician's ordered culture to identify the organism. That wasted time - from identification of initial symptoms to confirmed diagnosis – increases the time it takes to take action to contain and quarantine infectious and deadly pathogens.
Alliance Care Technologies’ (ACT) Catalyst S4 solution overcomes these challenges by using early symptom identification to identify probable pathogens rather than waiting for a confirmed diagnosis. When utilised in conjunction with other surveillance methods, Catalyst S4 can eliminate epidemics and pandemic outbreaks, saving countless lives and reducing economic hardships.
Contact value@alliancecaretech.com or
Saving one life means saving all. This is the mantra of HiRO Health founder Jad Akkary, whose mission is to change the way electronic medical data is utilised.
“Someday, one of us – our beloved ones – will become a patient, therefore patients are not just numbers mentioned in the news; one patient’s life means a lot,” Akkary says. “Since my journey as a patient has begun, many questions have come to mind: Why are my doctors not connected together wherever they are, and why don’t I have immediate access to my personal medical info? How many patients have passed away because of all of that?”
Thus, in a mission to save more lives and change the reality of healthcare, Akkary –along with co-founder Dr. Rezkalla Akkary, and a great team of advisors, doctors and engineers – launched HiRO Health.
“The idea of the startup is to build a decentralised electronic health record
region, more than 145,000 patients and 750 doctors are using HiRO Health.
“We have introduced a new concept of sustainable medical journey where patients are in full control over their medical data wherever they want. Healthcare infrastructure is digitilised with zero cost, which allowed us to build the first real time medical data set in the MENA region.”
Visit hiro-health.com
$200-400/month. They want to pay as little as possible, they are very demanding and expect premium software that caters to all their daily needs, and so they continue to change applications, or use multiple applications at once.

On the other hand, no software is able to provide clinics with everything they need to go paperless, centralise their operations, and be on the cloud, for such a low fee and be able to expand, resulting in a highly
solution out there for dental clinics, for the low price point they are willing to part with.
We decided to focus first on one city, and one specialty (dentistry), we have received and completed over 2,500 customisation requests from our clients, and we did that for FREE, with a main goal in mind to give them everything they are asking for, while improving user interface and workflows so it continues to become easier to use with time; as for our survival through the process,
Dhabi, over 200 clinics have been onboarded, and utilising the software in close to full capabilities, with increase of 50% in production, lowering operations cost by 30%, being paperless, and on the cloud.
We are now catering to other medical specialties and other geographical markets, replicating the same model we applied in dentistry, allowing us to be years ahead of any competition.
Visit dok32.com
The Middle East, North Africa and Türkiye (MENA) is home to over 600 million people, a rapidly evolving healthcare infrastructure and demand, and some of the fastest-growing tech ecosystems in the world. At a time when the transformative power of healthtech is just starting to hit its stride, this makes MENAT well-placed to lead in, and capitalise on, the future of precision medicine, digital therapeutics, clinical intelligence, and much more within healthtech innovation.
The tech ecosystem in the MENAT has undergone one of the most dramatic transformations of anywhere in the world in recent years.
Following steady growth in entrepreneurial activity in the last decade, a major step change happened in 2021 and 2022. An explosion of venture capital (VC) investment, from domestic and international investors, has launched the MENAT into the higher echelons of global venture ecosystems. An estimated $10 billion was raised by startups based in the region last year – a 20-fold
increase compared to a decade earlier.
While VC activity globally has cooled in 2022 and 2023, early-stage investment in the MENAT is still very robust. This is an important leading signal of future innovation, and a strong sign for continued scaleup success. Early-stage startups that raised big rounds in Q1 2023 include Remp People, Almentor, Tané, and Meshini.
Meanwhile, the region has so far produced 16 startups valued or exited at over $1 billion, which collectively form the MENAT unicorn club.
The MENAT is now also home to some of the fastest-growing tech ecosystems anywhere in the world, by amount of VC raised in 2022 compared to the previous year. The UAE, Türkiye and Saudi Arabia raised the most investment in 2022 of any MENAT countries, while Tunisia, Bahrain, and Jordan grew fastest.
Globally, healthtech is the fifth biggest VC investment category after fintech, energy, transportation, biotech, and pharma – which are separate from healthtech. In 2022, $42 billion in VC was invested in healthtech companies globally. Healthtech encompasses software-driven technology for patients (direct-to-consumer); technology for healthcare providers (professionals and hospitals); and technology for biotech and pharma. These are distinct categories from biotech and pharma which deal with atoms (leveraging biology and chemistry, respectively) rather than bits. That said, collectively, biotech, pharma and healthtech are increasingly overlapping. Combined, these three make up the biggest VC investment category – ahead of fintech – with $85.8 billion invested in 2022. This leading position can be explained by the massive size of the healthcare and biopharma industries.
Total VC in health, biotech, and pharma peaked at $116 billion in 2021, up from $30 billion in 2015. This was in part driven by pandemic innovation, coinciding with a surge in overall VC deployment.
As healthtech gains momentum, the Middle East, North Africa and Türkiye have the potential to lead in precision medicine, digital therapeutics, and clinical intelligence.
While healthtech investment fell in 2022 compared to 2021, early and mid-stage investment remained largely unchanged. Late-stage megarounds of over $100 million were most affected by a global pullback in VC.
MENAT healthtech may be nascent, but it is fierce. Healthtech companies in the MENAT region have increased in value eight-fold since 2017, reaching an estimated $1.7 billion.
The region’s large and growing population, increasing chronic disease burden, and shortage of healthcare professionals, makes healthtech an attractive solution to address these challenges. Pair this with institutional and governmental support for innovation, and an entrepreneurial scene that’s already taking off, and it has the potential for a potent combination.
Healthtech investment in MENAT has reached $106 million in 2022, still a relatively small proportion of all MENAT VC and global healthtech funding, but nonetheless an all-time-high in an anticyclical year, and a stark increase from $6 million in just 2016.
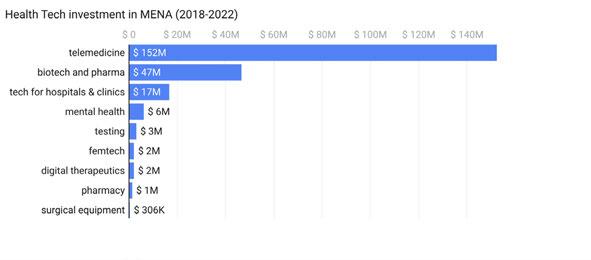
The UAE is the undisputed leader in healthtech in MENAT so far, having raised more than half of all the region’s healthtech funding in the last five years.
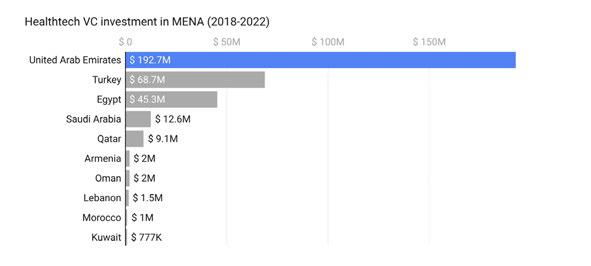
Telemedicine is the most funded healthtech category in MENAT. Out of pocket expenditure makes up more than 35% of all healthcare spending in MENAT, compared to less than 15% in Europe and the US. This makes a compelling case for cheap, scalable care finding consumer demand.
Research intensive biotechnology startups come second by funding raised in MENAT, leveraging the region’s rich life science talent pool.
Overall, the healthtech landscape in the MENAT region is still relatively early stage, but there remains a large opportunity in the region, concerted political will, and an ever growing pool of promising companies looking to capitalise.
Discover the world’s most promising companies and tech ecosystems at dealroom.co
















Discover more about the vital questions we’re asking at tii.ae
















 PILAR FERNANDEZ HERMIDA MD & Founder, I-EXPAND
ABHISHEK SHAH Founder & CEO, WELLTHY THERAPEUTICS
MARK R.L. KRUL AGLAIA ONCOLOGY FUNDS
DR. SADYK GUSNIEV Health Tech Expert & Researcher, VENTURE CAPITAL
FAISAL AL-HUSRY Principal, HIKMA VENTURES
OBEDIAH AYTON Director, DHABI HOLD CO.
CEO & Founder, Postdoctoral Research Associate,
PILAR FERNANDEZ HERMIDA MD & Founder, I-EXPAND
ABHISHEK SHAH Founder & CEO, WELLTHY THERAPEUTICS
MARK R.L. KRUL AGLAIA ONCOLOGY FUNDS
DR. SADYK GUSNIEV Health Tech Expert & Researcher, VENTURE CAPITAL
FAISAL AL-HUSRY Principal, HIKMA VENTURES
OBEDIAH AYTON Director, DHABI HOLD CO.
CEO & Founder, Postdoctoral Research Associate,


 MOHAMED GAMAL CTO & Director of Information Technology, ACCUMED UAE - KSA
MOHAMED GAMAL CTO & Director of Information Technology, ACCUMED UAE - KSA







 DR. DIEGO PALACIO Co-founder & Chief Commercial Officer, COAT
SCOTT VAN DER MEER
DR. MUSSAAD M. ALChief Business Development KUWAIT LIFE
DR. DIEGO PALACIO Co-founder & Chief Commercial Officer, COAT
SCOTT VAN DER MEER
DR. MUSSAAD M. ALChief Business Development KUWAIT LIFE
Marina Solovyeva
CEO & Founder, diagnio
Kawthar Bukhari
Partner Success Manager - Health, Plug and Play
Farah Dehmouni
Founder, iameno
Founder & CEO, Wellthy Therapeutics
14:00
Shaping the Future of Funding: Exploring Emerging Trends & their Impact on Investment Structures
Mark R.L. Krul
Director, Aglaia Oncology Funds
Director, Aglaia Oncology Funds
Zehan Teoh
Senior Vice President, Head of Venture
Builder / Platform, Qatar Insurance Group

Obediah Ayton
Director, Dhabi Hold Co.
Jad Akkary
CEO & Founder, HiRO Health
Ayoub Glia
Postdoctoral Research Associate, CellTiró
Keswin Suresh
Co-founder and COO, DarDoc
Maha Sallam
President, Vuessence
Viviana Mucci, CEO, Bequalise
Samia Rias
Health is Youth
Kyungme Boo
Managing Director, Arang Corporation
Wednesday 24 May
Value-based Innovation in Healthcare (powered by
Cofounder and Managing Director meta(bolic)/GluCare Health
Director – Health, Plug and Play
obal Ventures
Co-pilots in Accelerating Transformation (powered by

Head of Marketing & Corporate Communications, Dubai National Ventures Associate, Plug and Play
Unlocking the Potential of Big Data & IoT in Healthcare: Overcoming Fragmentation, Cost & Ownership Challenges
Viktoriya Vasilenko
Founder and CEO, Knowledge Gate Group
13:40
Empowering Physicians: How AI Can Reshape Healthcare Services
Alex Aliper Founder, Insilico Medicine
Where Are We On Localisation? Key Imperatives for Life Sciences
Eleonora Brero
Principal Life Sciences, IQVIA
Case Study
The Strategic Adoption of Machine Learning & AI in the Healthcare Sector
Shiraz Bajwa
Senior Director of Transformation, Sheikh Shakhbout Medical City (SSMC)
Engineered T Cells Targeting Cancer: Accelerating Discovery, Eradicating Recurrence, Saving Lives
Dr. Tony Bais
CSO, Adoptive Therapeutics LLC
AI in Healthcare will Save Lives & Reduce Costs
Dr. Diego Palacio
Co-founder & Chief Commercial Officer, Coat Connect
Dragon’s Den
Innovations to Include AI & ML-driven Solutions
Pilar Fernandez Hermida
MD & Founder, i-expand
CEO and Founder, Shawerna
Dr. Mussaad M. Al-Razouki
Chief Business Development Officer, Kuwait Life Sciences
Jules Chasles
Principal, Global Ventures

Zehan Teoh
Senior Vice President, Head of Venture Builder / Platform, Qatar Insurance


Riccardo Dainese
Co-founder, Alithea Genomics
Michelle Tarnow
Founder, CEO, Alliance CareTech
Mohamad Hijazi
CEO/Founder, IT Flakes
Ömer Özkan
CEO, ENBIOSIS Biotechnology
Piotr Szydlik
CEO, Evispine
Bilal AlKhizzi
General Manager, Haven Scientific










