
CLINICAL INITIATIVES, RESEARCH AND CURRENT UPDATES IN TREATMENT Article continued next page...
Want Circuit straight to your inbox? Sign up for our e-newsletter here!
World Antimicrobial Resistance Awareness Week (WAAW):
18 – 24 November 2025


World Antimicrobial Resistance (AMR) Awareness Week is an annual global campaign to improve awareness and understanding of AMR and encourage antimicrobial stewardship (AMS) best practices to help stop further emergence and spread of antimicrobial resistance.
This year’s theme is: Act Now: Protect Our Present, Secure Our Future AMR is no longer a distant threat - it’s a present and growing crisis affecting patient outcomes, healthcare systems, and global health. This year’s theme calls on all healthcare professionals – including prescribers, nurses, pharmacists, allied health professionals and hospital administrators – to engage in coordinated, sustained efforts to preserve the effectiveness of life-saving antimicrobials. Whether it’s through antimicrobial stewardship strategies, infection prevention measures, or patient education, every action counts.
Please enjoy this WAAW special edition of the CIRCUIT newsletter.
Rethinking β -lactam antibiotic allergy cross-reactivity: it’s a (side) chain reaction
Bettina Kirk, Quality Pharmacist – Antimicrobial
Stewardship,
Quality and Medication Safety Unit
Antibiotic allergy, most commonly to penicillins, is frequently reported by patients and documented in patient records.1-3 These reports often originate from childhood exposures or poorly defined clinical events, with limited patient recollection and diagnostic confirmation.1-3 However, most childhood reactions do not recur upon re-exposure in adulthood and are often attributable to non-allergic mechanisms, such as viral infections, drug-virus interactions, or pharmacological side effects.3,4 Antibiotic allergy testing, including skin testing and oral challenge, has been shown to exclude over 90% of reported penicillin allergies.1,3,4
Not all reactions are the same
It is important to distinguish between the various types and timing of adverse drug reactions (ADRs), including:
• ‘Type A’ pharmacological adverse reactions: non-allergic, predictable side effects (e.g. diarrhoea from many antibiotics, nephrotoxicity from aminoglycosides)5,6
- These make up 85-90% of ADRs and can potentially affect any individual, based on dose and exposure.5,6
• ‘Type B’ hypersensitivity reactions: predominantly mediated by immunologic and/or inflammatory mechanisms, unpredictable and unrelated to the pharmacological actions of the drug.5,6 Refer to Table 1 for key features of Type B reactions.
- These make up 10-15% of ADRs, affecting only susceptible patients.5,6
- Immediate: within 1 hour of administration (may be slower after oral absorption).4-6
- Delayed: after 1 hour, although commonly appear after 6 hours or after several days of treatment, or even after cessation.4-6
Table 1: Mechanisms and clinical manifestations of Type B hypersensitivity reactions1,4-6
Type Mechanism
Type I IgE-mediated
Timing
Examples of clinical manifestations
Immediate Mild: Urticaria, mild immediate rash
Severe: angioedema, bronchospasm, anaphylaxis
Type II (rare) Antibody-dependant cytotoxicity (IgG/IgM) Delayed Haemolytic anaemia, thrombocytopaenia
Type III (rare) Immune-complex reaction (IgG/IgM) Delayed Small-vessel vasculitis, serum sickness
Type IV Cell-mediated Delayed Mild: Contact dermatitis, delayed rash
Severe: Severe cutaneous adverse reactions (SCAR), e.g. SJS/TEN, AGEP, DRESS
IgE: Immunoglobulin E, IgG: Immunoglobulin G, IgM: Immunoglobulin M.
SJS/TEN: Stevens-Johnson syndrome/toxic epidermal necrolysis, AGEP: acute generalised exanthematous pustulosis, DRESS: drug rash with eosinophilia and systemic symptoms.
Whilst it is crucial that an antibiotic is not administered to a truly allergic individual, it is also important to appropriately assess and confirm any reported hypersensitivity reaction, to ensure patients do not unnecessarily receive alternative antibiotics that can be inappropriate, less effective, more toxic or have an excessively broad spectrum, leading to inferior clinical outcomes.1-3
Allergy history documentation should be thorough and specific, and wherever possible should include the offending drug (i.e. not the drug class ‘penicillins’ or ‘cephalosporins’), the date, the type of reaction, the timing after administration, and any treatment received both for the reaction and since.3,4
To ensure patients with an antibiotic allergy receive the most suitable alternative treatment available to them, consideration must be given to severity of the allergy and any potential cross-reactivity of antibiotics, particularly amongst β-lactam antibiotics, including penicillins, cephalosporins, carbapenems and monobactams.1
There have been misconceptions historically that cephalosporin allergy would occur in approximately 10% of people allergic to penicillins, and that penicillin hypersensitivity was related solely to the central β-lactam ring structure, which is present in all β-lactam antibiotics.1,3
It is now estimated that less than 1.5% of patients with a confirmed penicillin hypersensitivity have a cephalosporin allergy.1,3
Additionally, whilst it is possible for any component of the molecule to cause a reaction (acyl side chain, β-lactam ring or thiazolidine/ dihydrothiazine ring – see Figure 1), true cross-reactivity has now been shown to predominantly relate to the R1 side-chain that distinguishes each β-lactam antibiotic from one another.1,3,4
Figure 1 demonstrates the rates of cross-reactivity between β-lactams, based on their structure.
Figure 1: Structure and cross-reactivity of β-lactam antibiotics, and shared R1 side chains clinically relevant to cross-reactivity1,3 Basic
Beta-lactam ring

Penicillin structure

Cephalosporin structure

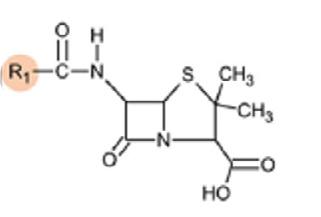



Similar side chains, Penicillins (R1)
• Penicillin VK (phenoxymethylpenicillin) & penicillin G (benzylpenicillin)
Shared side chans, Penicillins & cephalosporins (R1)
• Amoxicillin, ampicillin, cefaclor, cefalexin
Shared or similar side chains, Cephalosporins (R1)
• Cefaclor, cefalexin
• Cefepime, ceftriaxone, cefotaxime and cefuroxime
• Ceftazidime, aztreonam
No shared side chains, Penicillins & cephalosporins (R1)
• Cefazolin
Adapted from: Blumenthal K et al, Lancet 20191,3
The β-lactam antibiotics with similar or shared R1 side chains shown in Figure 1 have the highest likelihood of cross-reactivity, whereas those without shared side chains are much less likely to cross-react.1,3,4
Other points to note about β-lactam hypersensitivity cross-reactivity:
• Cefazolin does not share any side chains with any other cephalosporin or penicillin, so may be a good alternative option depending on the circumstance.1,3
• Cross-reactivity between carbapenems and penicillins or cephalosporins is approximately 1%.1,3
• There is no cross-reactivity with monobactams (aztreonam), except for ceftazidime which shares an R1 side chain.1,3
Figure 2 (over the page) can be used as a guide for the suitability of other β-lactam antibiotics in patients with confirmed penicillin allergy. This is based on the low prevalence of true penicillin allergy, known cross-reactivity rates and mechanisms, understanding of the role of R1 side chains, and the varying risks associated with different allergy types.1,3
Nonsevere - immediate (e.g. urticaria) OR - delayed (e.g. rash)
Safe to administer all cephalosporins, carbapenems and monobactams (ie. aztreonam).
Allergy delabelling
Confirmed allergy to a penicillin
Severe immediate (e.g. angioedema)
Considered safe to administer antibiotics which do not share a similar R1 side chain to hospitalised patients.
A thorough allergy assessment can also enable consideration of allergy delabelling (the removal of a patient-reported antibiotic allergy from their record).4 This may occur after allergy testing or oral rechallenge of the offending drug, or by direct delabelling (i.e. without testing or rechallenge), for example when the causative drug has since been tolerated, if there was a family history only (no hypersensitivity in patient) or with Type A ADRs (e.g. gastrointestinal side effects).1,4
There are considerable risks with inappropriate or unverified allergy labels (and subsequent use of inferior or unnecessarily broadspectrum alternative antibiotics), not only to individuals but also to public health.3,4,7 These include significant increase in side effects, Clostridioides difficile infection, surgical site infections, antimicrobial resistance (e.g. methicillin-resistant Staphylococcus aureus (MRSA)), patient mortality and hospital costs.3,4
Simple tools are now being developed to assess antibiotic allergy risk, which can be used by antimicrobial stewardship programs and
Severe delayed (Type IV reactions, e.g. SJS/TEN, DRESS)
Avoid all penicillins and cephalosporins (consider use of carbapenems). Safe to administer aztreonam and non-β-lactam antibiotics.
clinicians at the point-of-care.4,7,8 PEN-FAST is an externally validated clinical decision rule which accurately identifies low-risk penicillin allergies, enabling oral rechallenge, safe β-lactam use and allergy delabelling without formal allergy testing.4,7 Similarly, CEPH-FAST has recently been validated in the assessment of low-risk cephalosporin allergies.8
Antibiotic allergies should be clarified in all patients, and removed where appropriate.4 Importantly, when an allergy label can be removed, ensure the patient’s medical record is updated accordingly, adequate communication is provided to all relevant healthcare providers, and most crucially, the patient is involved and educated about their renewed allergy status.3
Scan QR code for references
What’s new in the Therapeutic Guidelines?
Peter Parashou, Reliever Hospital Pharmacist, based at Epic Pharmacy Newcastle
The Australian Therapeutic Guidelines (TGs) provide independent, evidence-based guidelines and therapeutic information on the management of a range of conditions. Significant updates to the Antibiotic TGs chapter were released in March 2025, revising management recommendations of many infections, several driven by rising antimicrobial resistance (AMR).
Antimicrobial Resistance
AMR occurs when bacteria, viruses, or fungi develop the ability to resist medicines making infections harder to treat.1 It has been shown that inappropriate use of antimicrobials can drive the growth of AMR.2 The 2022 Australian Antimicrobial Prescribing Practice in Australian Hospital Survey showed 21.7% of hospital antimicrobial prescriptions were inappropriate, such as prescribing for conditions which did not require antimicrobial therapy, incorrect duration, inappropriate spectrum of activity, or prescribing inconsistent with guidelines or microbiological culture results.2 The changes to the guidelines highlight the importance of appropriate use of antimicrobials in minimising resistance.
Several key updates relevant to common clinical practice are outlined below and over the page.
Multidrug-resistant bacteria management
Due to an increase in bacteria developing resistance to multiple drugs, a new section was added to the guidelines on the “Management of suspected infection with a multidrug-resistant (MDR) gram-negative bacterium” covering risk factors for MDR, information on managing infections and antimicrobials with and without activity against MDR gram-negative bacterium.3
Changes to Urinary Tract Infection (UTI) treatment
Empirical antibiotic treatment has changed for acute cystitis in adults – refer to Table 1.
These changes are due to rising resistance to trimethoprim in Escherichia coli (E. coli) where adult urine isolates showed resistance rates above 20%, compared to 5% of urine isolates being resistant to nitrofurantoin and less than 5% resistant to fosfomycin.4
However, whilst fosfomycin is now a recommended empirical treatment option, it is also often classified as a ‘red’ (restricted) antimicrobial in many hospitals’ “traffic light” antimicrobial restriction policies, reserving its use for MDR infections and requiring approval from an Infectious Diseases service before use.5 Fosfomycin is not PBS (Pharmaceutical Benefits Scheme) listed and costs more than nitrofurantoin or trimethoprim, therefore subsequent cost implications to the hospital and/or patient should also be considered. Cefalexin is reserved for males and non-pregnant females as a last line agent if other options are not suitable.4
Urine testing Medicare Benefits changes
The Medicare Benefits Schedule (MBS) requirements for urine testing (item 69333) and examination have also been updated to reduce unnecessary testing of asymptomatic patients.6
Asymptomatic bacteriuria (ABU), the presence of bacteria in the urine without signs or symptoms of a UTI, does not require antibiotic treatment or screening. However, it is often detected if urine testing is conducted, leading to the unnecessary prescription of antibiotics and increasing the risk of antimicrobial resistance without patient benefit.7,8
The MBS change limits testing to only when symptoms of UTI or kidney disease are present.6 Exceptions are made for specific patient groups who may be asymptomatic including:6
• Pregnant
• Less than 16 years of age
• Recipients of renal transplants
• Suffering from recurrent urinary tract infections
• Being investigated or monitored for kidney disease
• Undergoing urinary tract instrumentation, urological procedures, or transurethral resection of the prostate MRSA treatment changes
Vancomycin is recommended instead of clindamycin for intravenous therapy of methicillin-resistant Staphylococcus aureus (MRSA) due to rising rates of resistance to clindamycin.3 For oral treatment, treatment with trimethoprim + sulfamethoxazole is preferred to clindamycin, however clindamycin may be a suitable alternative depending on local susceptibility data.3
Pneumonia
The pneumonia section of the TGs has also undergone several changes; alongside updated treatment duration and dosages, there have been major updates to how pneumonia severity is assessed and defined. For community-acquired pneumonia (CAP), severity is now assessed using features indicating hospital admission and red flags for intensive care support instead of severity scoring tools.3 As the scoring tools had limited sensitivity and specificity and relevance only to the population groups for which they were developed, assessment with these new features allows for a more objective measure.9
The new categories are (refer to Figures 1 and 2):9
• Low severity – no features indicating hospital admission
• Moderate – at least one feature indicating hospital admission, but no intensive care support red flags
• Severe – at least one intensive care support red flag present, including adult with signs of sepsis or septic shock
The definition of Hospital Acquired Pneumonia (HAP) was expanded to include patients who have discharged within 7 days of a hospital stay over 48 hours, and those in acute care for more than 48 hours.3
Other changes
• Influenza treatment guideline updates introduced two new drugs Baloxavir and Peramivir. Baloxavir is for non-severe influenza and post-exposure prophylaxis.3 Peramivir can treat severe influenza in patients who cannot tolerate or absorb oseltamivir.3 These treatments are not PBS listed and they are more expensive than oseltamivir so should be used judiciously.
• When an aminoglycoside is recommended, Tobramycin is now included and ranked equally with gentamicin, except for Pseudomonas aeruginosa treatment where tobramycin is preferred.3
• Aminoglycoside dosages were updated and new initial dose calculators for amikacin, gentamicin, and tobramycin were added to the guidelines.3
• Directed therapy for Staphylococcus aureus infection now includes cefazolin as an equal first-line alternative to flucloxacillin as evidence has shown equal effectiveness and lower risk of acute kidney injury.1
• For Clostridioides difficile (C. difficile) treatment, oral vancomycin is now first-line for initial treatment of mild to moderate infection due to greater efficacy than metronidazole.3
Alongside many changes to the structure, algorithms, and recommended treatments in the TGs, a key causative feature of several changes was the rise of antimicrobial resistance. Ensuring the use of up-to-date guidelines in clinical practice will help prevent the growth of AMR and optimise treatment for patients. There are more updates expected for the TGs later in 2025 and 2026.

Adults with CAP and any of the following features need close clinical observation and may need hospital admission:
• tachypnoea (respiratory rate 22 breaths per minute or more)
• tachycardia (heart rate more than 100 beats per minute)
• hypotension (systolic blood pressure less than 90 mmHg)
• acute-onset confusion
• oxygen saturation less than 92% on room air (or less than baseline in patients with comorbid lung disease)
• multilobar involvement on chest X-ray (if available)
• blood lactate concentration more than 2mmol (if available).
Patients with any of the following parameters may require intensive care support; assess the patient's preferences, goals of care and suitability for intensive care management:
• signs of severe acute respiratory insufficiency
- respiratory rate 30 breaths per minute or more
- oxygen saturation less than 90% on room air, Pa02 less than 60 mmHg, or Pa02/Fi02 less than 250
- multilobar or rapid progression of chest X-ray infiltrates
• signs of acute extrapulmonary organ dysfunction
- hypotension (systolic blood pressure less than 90 mmHg)
- acute-onset confusion
- poor peripheral perfusion or mottled skin
- acute oliguria, elevated serum creatinine (above baseline) or uraemia (serum urea more than 7 mmol/L or BUN more than 19 mg/dL)
- blood lactate concentration more than 2 mmol/L.
BUN = blood urea nitrogen; Fi02 = fraction of oxygen in inspired air; Pa02 = partial pressure of oxygen
Scan QR code for references
