
7 minute read
BFP: Hydrogen as fuel for domestic boilers
from openME 52.2
by Simon Stevin
Bachelor Final Project
Hydrogen as fuel for domestic boilers
Advertisement
Hello, my name is Derk de Jong. I am a fourth-year Mechanical Engineering student. Last semester I did my final bachelor project at the Power & Flow research group under the supervision of Yuriy Shoshyn. In this project I did research on the combustion behaviour of premixed hydrogen-air flames in a perforated plate burner. This project was done in collaboration with Bosch TT Deventer, who work on an application of hydrogen as fuel in domestic boilers.
Written by Derk de Jong
Introduction
With a growing world population resulting in an increasing energy demand, we must look for alternative energy sources and carriers in order to make the world less dependent on fossil fuels. One of those alternative energy carriers could be hydrogen. Hydrogen can be produced using clean energy sources like solar or wind energy. It could then be used in domestic boilers instead of natural gas. Unfortunately, it is not possible to safely run a normal domestic boiler, designed for natural gas, on hydrogen. To use hydrogen in domestic boilers, more research needs to be done about its combustion behaviour to be able to design a boiler that can safely and efficiently run on hydrogen. In this project, the combustion behaviour of premixed hydrogen-air flames is investigated on a perforated plate burner. For a burner to work in a boiler application there are multiple important requirements; it needs to be fuel-efficient and silent. The flame length can not be too high or it will damage the heat exchanger. Furthermore, they need to have a high turndown ratio. That is the ratio between the minimal power and maximum power at which the flames in the burner are stable. The goal of this project is to find the effects of flow rate, equivalence ratio (ratio between the air-fuel ratio and the stochiometric air-fuel ratio), and burner plate layout on flame stability, flame length, radiative power, burner plate temperature and NOx emissions. This is done using a set of experiments.
Setup
An existing setup was modified for usage in this project.The setup consists of a burner that is fed a mixture of hydrogen and air which is controlled by a set of mass flow controllers. A ball valve is added before the burner to quickly shut off the gas supply in case of flashback (when the mixture underneath the burner plate is ignited). Next to this, there is an extra air hose connected to the burner to cool the burner housing. To measure the temperature of the burner plate, a pyrometer is used. Furthermore, a camera equipped with a UV-lens and two optical filters is used to take UV-images to determine the flame length and radiative power. To measure NOx emissions, a gas analyser is used in combination with a water-cooled probe. A thermostat is used to keep the cooling water around 40°C to protect the probe from overheating, while preventing the forming of condensation on the probe. The burner can be fitted with different burner plates. In this project 5 different burner plates with different slit width and pitch where installed to investigate the effects of the burner plate layout.
Experiments and analysis

During the experiments the flow rate and equivalence ratio were varied, the burner plate temperature and NOx emissions were measured and pictures were taken to determine the length and radiative power of the flames.
Furthermore, the stability limits where determined. This is very important, because if a burner gets unstable, this can lead to very dangerous situations, when not acted upon immediately. There are two ways a burner can get unstable. The first one is called ‘blow off’ and this happens when the flame is blown off the burner deck. This typically happens at very high flow rates and low equivalence ratios. The second cause of instability is ‘flashback’, which is when the mixture

below the burner plate is ignited. Flashback can occur by either the flame shooting through one of the holes in the burner plate or the plate getting so hot that it ignites the mixture below. Flashback is the main problem with using hydrogen instead of natural gas. This is caused by the higher burning velocity of hydrogen, which results in shorter flames and thus more heat production close to the burner plate. This all results in the following formula:


Shown in the figures below, experimental and theoretical data show approximately the same shape, but the absolute value differs significantly. This indicates a significant impact of flame stretch and preferential diffusion on the flame length. Furthermore, a higher flow rate will result in a higher flame length and a higher equivalence ratio will result in a shorter flame length.
Next to the experimentally determined flame length, a theoretical flame length is calculated. This is done in order to compare it with the experimental results and see if preferential diffusion and flame stretch have a significant impact on the flame length. In this simple calculation diffusion and flame stretch effects have been neglected and a few assumptions have been made: there is a uniform cold mixture velocity profile across the slit, which does not change with the height above the burner plate. The flame front is infinitely thin and all mixture that flows through the slit burns at the flame front. The burning velocity is equal to the laminar burning velocity (which is known form literature and dependent on the equivalence ratio) for each front location. In this assumption the cross section of the flame shape will be an isosceles triangle with a base equal to the slit width.
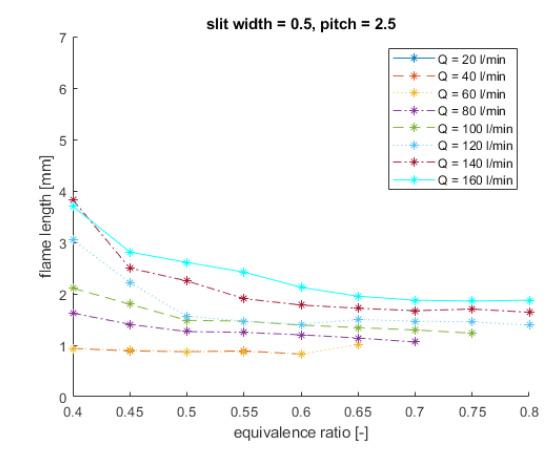


The radiative flame power is calculated in arbitrary unit by integrating the image intensity over the projected flame area. These relative values can be converted to absolute values to, for instance, compare these experimental results with simulations. This can be done by determining a few calibration factors by taking pictures (with the same camera and filters) of a known radiation source, like a tungsten ribbon lamp. While finishing this project, someone at Bosch TT was still working on these calibration factors. While analysing the measured burner plate temperature, an interesting trend was found: at low equivalence ratios, a higher flow rate results in a higher temperature, while at higher ratios, a higher flow rate results in a lower temperature. An explanation for this phenomenon has not been found in this project, but would be interesting to investigate in further research. After analysing the NOx emission data, it could be concluded that, within the measurement range, the NOx emission increases exponentially with the equivalence ratio and does not depend on the flow rate very much. The colored figures below show the stability limits of the burner with the different burner plates. On the horizontal axis the flow rate is shown and on the vertical axis the equivalence ratio. In the green area the burner behaviour is stable, in the red area flashback occurs. The yellow area is the area where it transitions from stable to instable. The width of this area is determined by the step size used in the experiments. As can be seen, in the measured range only flashback occurs and no blow off.


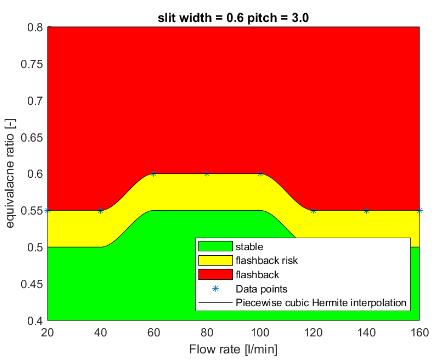
The top two figures have the same slit width and an increasing slit pitch. As can be seen, a bigger pitch results in a more stable burner behaviour. Looking at the bottom two figures with both different slit width and pitch and by taking the previous in mind, it can be concluded that wider slits will result in less stable burner behaviour.












