







Sponsor Company: www.international-pharma.com Volume 14 Issue 3 Peer Reviewed Advances in Manufacturing and Processing Impacting Formulation Development Solid Form Services Bridging the Gap between Drug Substance and Drug Product Under Pressure Finding a More Sustainable Future for pMDIs What’s in Store for Aseptic Processing Technologies In 2022 and Beyond advanced liposomal technology

AUTOMATIC ENCAPSULATION MACHINE INTRODUCING THE NCF-45 A highly reliable encapsulator, producing up to 80% higher yields to typical hand-filling or semi-auto encapsulation machines. The smooth operation of the NCF-45 automates your encapsulation process to deliver up to 45,000 capsules per hour, making this Natoli machine ideal for medium-scale production. GMP & cGMP compliant I SCADA network compatible I 21 CFR part 11, CE Certified The solution to product loss. natoli .com
DIRECTOR: Mark A. Barker
BUSINESS DEVELOPMENT: Michael Hossain michael@senglobalcoms.com
EDITORIAL: Virginia Toteva virginia@senglobalcoms.com
DESIGN DIRECTOR: Jana Sukenikova www.fanahshapeless.com
FINANCE DEPARTMENT: Akash Shama accounts@senglobal.com
RESEARCH & CIRCULATION: Jessica Chapman jessica@senglobalcoms.com
COVER IMAGE: iStockphoto ©
PUBLISHED BY: Senglobal Ltd. Unit 5.02, E1 Studios, 7 Whitechapel Road, E1 1DU, United Kingdom
Tel: +44 (0) 2045417569 Email: info@senglobalcoms.com www.international-pharma.com
All rights reserved. No part of this publication may be reproduced, duplicated, stored in any retrieval system or transmitted in any form by any means without prior written permission of the Publishers.
The next issue of IPI will be published in Winter 2022. ISSN No.International Pharmaceutical Industry ISSN 1755-4578.
The opinions and views expressed by the authors in this journal are not necessarily those of the Editor or the Publisher. Please note that although care is taken in the preparation of this publication, the Editor and the Publisher are not responsible for opinions, views, and inaccuracies in the articles. Great care is taken concerning artwork supplied, but the Publisher cannot be held responsible for any loss or damage incurred. This publication is protected by copyright.
2022 Senglobal Ltd./Volume 14 Issue 3 – Autumn – 2022
REGULATORY
MARKETPLACE
08 A Prognosis of Sustainability in the Pharmaceutical Industry
The pharmaceutical sector is a key player in the race for a more sustainable future, and pharmaceutical companies are endeavouring to reduce their carbon footprint, eliminate pollution, conserve water, and use sustainable components. Suppliers and partners for drug delivery products are also working hard to ensure the entire supply chain improves its environmental, social and governance (ESG) standards. Pharmaceutical companies want to demonstrate action, and not just ambition, in moves towards greater sustainability. This Michael Earl at Owen Mumford Ltd will underline the achievements made to date as well as key areas for improvement.
12 Why We Recognise HIV Vaccine Awareness Day
May 18th is dedicated to HIV Vaccine Awareness Day. The significance of this recurrence is embedded in the years of research that have been dedicated to finding a reliable way for individuals to be protected against the virus. Research began shortly after the virus was discovered as being the cause of AIDS, with the first vaccine trial conducted in 1987. To this day, many clinical trials have been carried out, some with breakthrough results, yet no vaccines have been shown to be able to efficiently protect all individuals. Shameet Thakkar at Unimed Procurement Services, gives his expert opinion on the different factors that impact the effective delivery of vaccines and the power of prevention when it comes to global health crises such as HIV.
14 A Business Growth Tool: Patent Claiming Strategies
Developing a pharmaceutical product can take on the order of a decade from research to development, and finally to FDA approval. While Patent Term Extension can restore some time lost due to the regulatory approval process, the traditional strategy of relying on patents based on the drug itself or its general method of use will necessarily start – and stop – the protection clock much earlier. This limits the potential return on investment prior to generic copyists. Tom Irving, et al at Finnegan, discuss how additional strategies can be used to advance business growth with longer and stronger patents.
DRUG DISCOVERY, DEVELOPMENT & DELIVERY
18 Under Pressure: Finding a More Sustainable Future for pMDIs
The transition away from the use of ozone-damaging chlorofluorocarbon (CFC) gases has been one of the major environmental achievements of modern times. Today, hindsight affords us the ability to see how this transition enabled the Earth’s protective ozone layer to heal itself over several decades. An unintended consequence, however, was that the withdrawal of CFCs, ushered in greater use of hydrofluorocarbons, known as F-gases, which introduced their own environmental challenges. While this family of gases might not pose a risk to the atmospheric ozone layer, they were found to contribute to the greenhouse effect. Dr. John N. Pritchard, Dr. Jag Shur and Omar Usmani, explains why F-gases, compared to carbon dioxide (CO2) have a far higher global-warming potential, based on attributes including infrared radiation absorption and atmospheric lifetime.
22 Use of High-field NMR in Covid-19 Drug Development
The Covid-19 NMR Consortium, an international collaboration of NMR experts, is conducting research to determine the ribonucleic acid (RNA) structure of SARS-CoV-2 and its proteins using NMR spectroscopy. One of the consortium’s first discoveries, early in the pandemic, was that a protein within SARS-CoV-2 forms microdroplets with the RNA of the virus. Professor Markus Zweckstetter explains that in subsequent
wwww.international-pharma.com
INTERNATIONAL PHARMACEUTICAL INDUSTRY 1
Contents
06 Editor’s Letter
&
months, this discovery enabled scientists to set up experiments to study the interplay between the RNA and the protein, known as the nucleocapsid protein or N protein.
24 Virtual Screening in Modern Computational Chemistry
Virtual screening is a firmly established computational technique in computational drug design which has saved the pharmaceutical industry billions of dollars since its inception. The structure-based approach involves fitting potential small molecules to a protein target, to generate predicted ligand-protein interaction properties that can significantly reduce chemical search space. Scott Midgley and Mark Mackey at Cresset, discuss the technical and practical considerations for modern virtual screening to ensure projects are delivered efficiently, at optimal cost, and the time to get a drug to market is as short as possible.
30 The Pharma Drug Discovery Industry Should Look Towards Manufacturing as the Blueprint for Automation
Within the manufacturing sector – whether it’s transport, FMCGs, electronics or building materials – a modern approach to automation is already in place. Holistic, flexible, and automated processes are being used to carry out a wide range of tasks like formulation, blending, packaging, and cleaning – and enabling them to take place all at the same time. An industry that can also benefit from automation, is pharmaceutical drug discovery. Zoe Williams at Automata shows that by implementing a similar approach into laboratory spaces as is already happening in manufacturing, it will be possible to increase efficiencies, and ensure that wide scale, effective automation is in place, and for the long term.
CLINICAL & MEDICAL RESEARCH
32 The Use of Predictive Analytics to Improve Quality in Clinical Trials
The impact of poor quality in a clinical trial, often discovered late in the process, can not only add costs to addressing the re-work necessary, but in some cases can lead to rejection from a regulatory authority. Using predictive analytics and scorecards throughout the lifecycle of a study can, in many instances, prevent a quality issue occurring or mitigate the impact in other cases. Rose Kidd at ICON explains the need to apply this approach in Quality Assurance (QA) is becoming more important due to the increasing complexity of clinical research and the increased use of technology to capture patient data remotely, as is the case with decentralised and hybrid trials.
TECHNOLOGY
34 The Transformational Potential of Prioritising Data Today
Covid has spurred regulators towards a more agile and innovationsupporting future, paving the way for new opportunities for all. If life sciences companies embrace a data-driven approach to managing their product data now, they stand to reap the broadest benefits, including more robust and streamlined internal processes, supply continuity, and a level of transparency that really puts the patient first. Frits Stulp and Aida Demneri, partners at Deloitte, explore what’s possible.
36 New Technology and Automation in Labs: Data Risks and Privacy Compliance Issues
Within healthcare and life sciences, data science plays a major role in research and innovation. Improvements in computational speeds, storage capacity and connectivity across different platforms, systems and sampling equipment, mean that laboratories and research facilities are often the testing grounds for the use of advanced analytics including Big Data and Artificial Intelligence. Robert
Grosvenor, Managing Director with Alvarez & Marsal's Disputes and Investigations practice in London, points out that alongside the innovation and efficiency benefits of process automation, integrated workflow management, and collaborative research, comes increased exposure to data risks – some less obvious than others.
38 Why the Right Approach to Data Management Will Enable the Potential of Small-molecule RNA Modifiers as Drug Targets to be Fulfilled
Over the past 5 decades or so, the growing understanding that RNA can influence protein function through routes other than direct translation has opened the prospect of discovering small molecules for tackling diseases in novel ways. Using sources such as the UK Biobank, progress is now being made on using multi-omics data to derive mechanism of action (MoA) insights into the role of small molecules on RNA function, specifically the splice site selection process. However, such approaches are hindered by an approach to data management that is not designed to work with the large amount of interconnected, complex data in RNA splicing experiments. Zachary Pitluk, PhD., at Paradigm4, describes how a vector-based database approach can allow data to be managed, interrogated, processed, and shared much more effectively.
MANUFACTURING
42 Advances in Manufacturing and Processing Impacting Formulation Development
Achieving investigational new drug (IND) approval at an increasingly faster rate than competitor drugs is a widespread desire of biologics developers. This has been further amplified by the need for treatments and vaccines for the COVID-19 pandemic. As a result, the pressure to achieve speed in drug formulation development has escalated to new heights. Heonchang Lim of Samsung Biologics offers insight into the common hurdles that must be overcome in formulation development to meet these various requirements
46 Testing Excipients for 3D Printed Pharmaceuticals
3D printing or additive manufacturing (AM) technology has considerable potential to align tablet manufacture more closely with modern requirements and is a growing area of focus for the pharmaceutical industry. A key challenge for pharmaceutical manufacturers looking to exploit 3D printing technology is to determine requirements for excipients. Jamie Clayton at Freeman Technology, consider the role of powder rheometry within this context. Industries leading the way in powder-based AM have already established the importance of powder flowability and the relevance of dynamic powder properties.
54 Flexible Manufacturing Environments are Vital for Today's Potent Oral Dose Forms
Driven by advances in oncology treatments and personalised precision medicine, two trends are converging to increasingly challenge pharma developers and their external contract manufacturing suppliers. Highly potent active pharmaceutical ingredients (HPAPIs) and small-batch manufacturing. Leveraging the end-to-end capabilities of a specialist CDMO can be a route to accessing the small batch facility flexibility to manufacture HPAPI oral products reliably and cost efficiently. Stephane Guisado at Recipharm, explains that with better production and production data control, we can efficiently and safely deliver finished HPAPI drug products to patients and markets.
58 Solid Form Services – Bridging the Gap Between Drug Substance and Drug Product
The solid form of a drug substance has a huge bearing on its formulation as a drug product. In an ideal world, every API would
2 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Contents
nature innovation + our reputation
We are Scientific Protein Laboratories.




over
years of expertise in development and cGMPcompliant manufacturing,
trusted global source for innovation, customization, and the manufacturing of high quality

process
have become
naturally derived pharmaceutical
INTERNATIONAL PHARMACEUTICAL INDUSTRY 3wwww.international-pharma.com
With
40
we
a
API’s and
products. • Custom
development and formulations • Traceable supply chain of natural ingredients • Scale up and cGMP production • Worldwide regulatory support • Decades of experience manufacturing naturally derived materials including heparin and pancreatic enzymes Put our quality team to work on your product solution. Visit us at CPhl Worldwide 2022 in November in Frankfurt. Booth #121A102splpharma.com 700 E. Main Street Waunakee, WI 53597 USA +1 (608) 849-5944 Scientific Protein Laboratories LLC part of Shenzhen Hepalink Pharmaceutical Group Co.,Ltd.
reliably form beautiful, homogeneous crystals on isolation that would be perfect for creating a dosage form with excellent bioavailability. But that in the real world, that is unusual. Abhijeet Sinha at Lonza discusses that significant amount of work is commonly required to bridge the gap between the bulk API that comes out of synthesis and a solid form with all the attributes required by formulation scientists to make a good drug product.
62 What’s in Store for Aseptic Processing Technologies in 2022 and Beyond?
By 2028, the global market for injectable drugs is set to reach $69.13 billion, growing at a CAGR of 8.9%. There are many reasons for this boom in demand, from the drive to develop innovative treatments for rare diseases to heightened demand for faster vaccine rollouts. As a result of this growth, there have been several innovations and techniques adopted to enhance efficiencies across aseptic processes. As these drug products are injected directly into the body and therefore bypass the body’s natural defences, Ben Wylie at ChargePoint explains that parenterals such as injectables and topicals used in ophthalmics require aseptic processing to ensure total sterility.
64 Modular Cleanroom Technologies Have Always Been the Answer to Biopharma’s Global Growth and Flexibility Needs
For the last three decades or more, biopharma’s growth has been nothing short of amazing. From life changing biologics and monoclonal antibodies (MAbs) to mRNA-based and now cell-based immunotherapy medicines to fight viruses, cancers, and previously incurable diseases elevating biopharma’s position to even greater prominence. Mitch Gonzales at AES discusses that regulatory guidance and approvals of Advanced Therapeutic Medicinal Products (ATMPs) also highlight the assimilation of these advancements in the biopharmaceutical sector.
68 PCI Pharma Services – Acquire, Invest, Grow
The biologic market has grown expediently over the past few years, driven not only by the pandemic but also by the increasing demand for innovative therapies, the continued rise in chronic disease areas such as oncology and the loss of patent exclusivity of leading biologic drugs. That has led to rapid growth of aseptic processing, which is expected to reach $24.36 billion by 2031, growing from $10.63 billion in 2020, witnessing a CAGR of 7.9% (2021–2031). Tom McGrath at PCI Pharma Services notes that with the pharmaceutical industry’s growing pipeline of biologics, the need for technically advanced manufacturing and specialized packaging support has grown considerably.
PACKAGING
70 How Product Inspection Drives Quality in Liquid Pharmaceuticals
In liquid pharmaceuticals, the stakes could not be higher. The correct dosage and usage can literally be a matter of life and death. Marco Pelka, Market Manager PCE of Mettler-Toledo, explains how manufacturers and packaging companies in this field can harness product inspection to ensure they produce high-quality liquid pharmaceutical products.
74 SMIs and Soft Mist Nasal Sprays – a Key Defence in the Fight Against the Next Pandemic?
The COVID-19 outbreak has provided several lessons for the pharmaceutical industry to consider for future pandemics. argues that one key takeaway must be to change the way vaccines are administered. Wilbur de Kruijf at at Resyca discusses if soft mist inhalers (SMIs), and soft mist nasal sprays hold the key to improved vaccine delivery?
HEALTH OUTCOMES
76 Choosing and Developing User-friendly Osmotic Laxatives for a More Patient-centric Portfolio
At least 1 in 10 people worldwide suffer from constipation at some point in their lives. It affects people of all ages and has many causes. The symptoms of constipation include pain in the lower abdomen and irregular and painful bowel movements. Laxatives are often needed in addition to dietary changes to treat constipation. There are many laxatives to choose from, each with different mechanisms of action and, consequently, different advantages and disadvantages. Here Dr. Martin Koeberle and Dr. Verena Garsuch at HERMES PHARMA look at the important role of osmotic laxatives and how they overcome many of the side effects and drawbacks of other constipation treatments.
80 Patient-Centric Clinical Trials: Turning Opportunities into Standard Procedures
Over the past year, the industry has heard often, and at length about patient-focused decentralized trials (DCTs), as vendors and some early adopters report their early experiences in an area that is still evolving. It’s important to remember that the industry is still at a very early stage in digital clinical trial development. Truly, patient-focused trials are a goal that we have yet to attain. We still use ePRO and eCOA, as we have for 20 years, only now we talk about these tools as if they are brand new and operate under a decentralized banner. We may be using eConsent, but still have patients come to sites to initiate the process. Richard Young at Veeva Systems analyses the fundamental challenges facing the industry in developing standards for sharing clinical data that will enable digital decentralized trials.

LOGISTICS & SUPPLY CHAIN MANAGEMENT
86 Supply Chain Disruptions Bring Short Notice Changes to Ingredients – But Can Your Labeling and Artwork Management System Cope?
Supply chain and procurement industry figures have called this the ‘era of the shortage’, with widespread disruption to supply chains, raw materials, and ingredient availability. Ingredients substitution is a viable tactic to avoid product shortages or ceasing production, but it unlocks a whole host of operational challenges, argues Bob Tilling at Kallik. With product packaging, labeling and artwork also requiring updates every time an ingredient changes, business leaders must embrace technology to ensure ingredient substitutions do not become a disruptive ‘chop and change’ tactic that overshadows other operations.
4 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Contents
the potential of

INTERNATIONAL PHARMACEUTICAL INDUSTRY 5wwww.international-pharma.com Unlock
active ingredients Contract Manufacturing R&D White Labelling Create your own liposomal supplements Speak to us at stand number 41H53 www.plantacorp.com
Editor's Letter
Pharmaceuticals is one of the world's most researchintensive industries, generating a continuing steam of new products that save lives and raise the quality of life. The discovery of new drugs has evolved over time from a decidedly empirical process to one based to a considerable degree upon fundamental scientific knowledge. Rich linkages have emerged between profit-seeking manufacturers and basic research performers such as universities and national laboratories. The safety and efficacy of new pharmaceutical products are stringently regulated in most industrialized nations, adding to clinical testing costs. Because of high expenditures on research, development, and clinical testing and because new products, once proven, might be imitated easily, patent protection is unusually important. The extension of patent protection to third-world nations under Uruguay Round Treaty mandates has precipitated vigorous policy debates. Patents, first-mover advantages, and the lack of good substitutes for significant new drugs often give rise to substantial monopoly power, against which many national governments have counterpoised a diverse panoply of price control mechanisms. When patents expire, however, generic substitutes often introduce vigorous price competition. The extent to which generics capture market share from the branded original drugs depends upon government regulatory policies, the reimbursement strategies of health care insurers, and the organization of health care provider institutions.
Rising research and development (R&D) expenditures by pharmaceutical companies are, in part, a consequence of changing industry structure, particularly the rise of the biotechnology sector. The creation of a market for biomedical science and increased vertical competition within the industry are likely to spur innovation and raise productivity, but they also could induce socially wasteful spending and weaken academic science. With innovation increasingly dependent on financially vulnerable firms and complex
Editorial Advisory Board
Bakhyt Sarymsakova, Head of Department of International Cooperation, National Research, Center of MCH, Astana, Kazakhstan
Catherine Lund, Vice Chairman, OnQ Consulting
Deborah A. Komlos, Principal Content Writer, Clarivate

Diana L. Anderson, Ph.D president and CEO of D. Anderson & Company
Franz Buchholzer, Director Regulatory Operations worldwide, PharmaNet development Group
Francis Crawley. Executive Director of the Good Clinical Practice Alliance – Europe (GCPA) and a World Health Organization (WHO) Expert in ethics
Rick Turner, Senior Scientific Director, Quintiles Cardiac Safety Services & Affiliate Clinical Associate Professor, University of Florida College of Pharmacy
contractual arrangements, R&D investment might be becoming more sensitive to price controls or other cost containment measures.
The pharmaceutical industry has produced many drugs that have benefited man. Political frameworks designed to govern the industry must maintain these benefits. However, regulation needs to be sufficiently robust to protect public health from drugs that are unsafe, ineffective, or unnecessary. The extent of industry influence over drug regulation, at the expense of other interested parties, suggests that the current system could be more robust.
One of the key influences on government is environmental policies.
In this edition Michael Earl at Owen Mumford Ltd gives a “Prognosis of Sustainability in the Pharmaceutical Industry” Pharmaceutical companies want to demonstrate action, and not just ambition, in moves towards greater sustainability. This will underline the achievements made to date as well as key areas for improvement, and Dr. John N. Pritchard, Dr. Jag Shur and Omar Usmani, explains why F-gases, compared to carbon dioxide
(CO2) have a far higher global-warming potential, based on attributes including infrared radiation absorption and atmospheric lifetime.
Heonchang Lim of Samsung Biologics within his article “Advances in Manufacturing and Processing Impacting Formulation Development” offers insight into the common hurdles that must be overcome in formulation development to meet these various requirements.

Our very popular “Health Outcomes” section features articles by Dr. Martin Koeberle and Dr. Verena Garsuch at HERMES PHARMA who looks at the important role of osmotic laxatives and how they overcome many of the side effects and drawbacks of other constipation treatments, and Richard Young at Veeva Systems analyses the fundamental challenges facing the industry in developing standards for sharing clinical data that will enable digital decentralized trials.
I hope you all enjoy this edition of IPI, and I look forward to meeting most of you at CPHI in Frankfurt.
Georg Mathis Founder and Managing Director, Appletree AG
Jagdish Unni, Vice President – Beroe Risk and Industry Delivery Lead – Healthcare, Beroe Inc.
Jeffrey Litwin, M.D., F.A.C.C. Executive Vice President and Chief Medical Officer of ERT
Jeffrey W. Sherman, Chief Medical Officer and Senior Vice President, IDM Pharma
Jim James DeSantihas, Chief Executive Officer, PharmaVigilant
Mark Goldberg, Chief Operating Officer, PAREXEL International Corporation
Maha Al-Farhan, Chair of the GCC Chapter of the ACRP
Stanley Tam, General Manager, Eurofins MEDINET
(Singapore, Shanghai)
Steve Heath, Head of EMEA – Medidata Solutions, Inc
Patrice Hugo, Chief Scientific Officer, Clearstone Central Laboratories
Heinrich Klech, Professor of Medicine, CEO and Executive Vice President, Vienna School of Clinical Research
Robert Reekie, Snr. Executive Vice President Operations, Europe, Asia-Pacific at PharmaNet Development Group
Sanjiv Kanwar, Managing Director, Polaris BioPharma Consulting
Stefan Astrom, Founder and CEO of Astrom Research International HB
T S Jaishankar, Managing Director, QUEST Life Sciences
6 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
Virginia Toteva, Editorial Manager – IPI
wel-screen

A Prognosis of Sustainability in the Pharmaceutical Industry
The pharmaceutical sector is a key player in the race for a more sustainable future, and pharmaceutical companies are endeavouring to reduce their carbon footprint, eliminate pollution, conserve water, and use sustainable components. Suppliers and partners for drug delivery products are also working hard to ensure the entire supply chain improves its environmental, social and governance (ESG) standards. ESG credentials are becoming essential to pharmaceutical tenders at every step of the supply chain. Pharmaceutical companies want to demonstrate action, and not just ambition, in moves towards greater sustainability, to customers, policymakers and healthcare system stakeholders.
As a key delivery device partner for pharma companies, Owen Mumford Pharmaceutical Services has reviewed the current state of play on ESG compliance in the pharmaceutical industry among the top 25 companies reporting ESG scores. This article will underline the achievements made to date as well as key areas for improvement.
Outlining Targets
To really understand the progress of the pharmaceutical industry in its drive towards sustainability, it is valuable to begin by identifying what success would look like. Reports frequently focus on the same four main goals for achieving sustainability:
1. Reducing carbon emissions by improving energy use and setting net-zero targets.
2. Improving water sustainability by reducing manufacturing consumption and eliminating pharmaceutical waste from the water system.
3. Improving waste management by cutting down on excess packaging and more effectively recovering and disposing of used products.
4. Becoming more sustainable by design, through green chemistry initiatives,
chemical recovery and creating reuseable delivery devices.
The analysis focuses on ESG targets specific to the pharmaceutical sector and its suppliers. Additionally, the report not only focuses on where ESG policies have been put in place, but also where corporations have publicly set themselves concrete targets.
Areas of Progress
The pharmaceutical industry has begun to make significant strides towards a more sustainable future, with the October 2021 Climate Reporting Performance report from Ecoact featuring three biopharmaceutical giants in the global top twenty companies for sustainability.1 As an industry, biopharma performed considerably better than many other industries in each of the key categories, which included ambition and targets, governance and achievement. We can see important improvements in four areas in particular. With regards to energy, water, waste, and air emissions there have been valuable steps taken across the industry.
Air emissions are the area where pharma companies are most focused on pursuing targets. Close to 70% of pharma companies have specific targets for lowering air emissions, focusing both on reducing carbon emissions and gaseous pollutants. Typical pollutants to be filtered are acid gases, dust and aerosols, pharmaceutical ‘actives’ and volatile organic compounds which can all cause harmful damage to the environment.
Secondly, the energy intensive sector has made steps to reduce usage. Most energy policies focus on a combination of renewable energy sources, self-generation and increasing energy efficiency by reducing energy requirements in the manufacturing process.2 Reducing energy use in the manufacturing process can be either production line or industrial building focused – in both cases, savings of 25% are typical and are often much higher.3
Next, moves to improve water usage not only focus on reducing consumption but also on cleaning and reprocessing water
– either for re use or putting back into the water grid. One international giant aims to achieve 100% water neutrality by 2025, meaning all wastewater will be recycled, re-used and captured from rainwater. Our review shows that around 50% of pharma companies have already set hard targets in this category.
Lastly, progress on waste is trending in the right direction with goals being set by a large number of companies. Over a quarter of pharmaceutical companies have already set targets to reduce their waste emissions by at least 25%. Companies are trying to avoid reliance on landfills for waste disposal, while others pursue a zero-waste approach. Moreover, commercial incentives may become a factor as waste becomes more expensive to dispose of.4
To address the controversial subject of disposable plastic components in drug delivery devices, alternatives such as degradable plastics are being debated and scrutinised, but for now immediate progress is being made by reducing the number of disposable components. An example of sustainable design is Auto which will help partners reduce plastic waste within their supply chain.
Areas for Improvement
While there are certainly improvements being made in the industry, areas remain that need change; the statistics can hide underlying issues that need addressing. For example, although the industry as a whole achieves an ESG score of 61% in the Ecoact review considered earlier – well above the all-industries average of 53% –the performance of individual companies varies massively.
Our study uncovered a variance of 40% between top performers and those who have a way to go. The industry must focus on narrowing this band of variance before big pharma as a whole can be considered firmly on the path to sustainability. Furthermore, it appears that neither geography nor size are important to whether a corporation has begun to make improvements. Top performing small firms are not far behind the most committed multi-nationals – implying
8 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Regulatory & Marketplace
Versatile design intuitive delivery










INTERNATIONAL PHARMACEUTICAL INDUSTRY 9wwww.international-pharma.com Want to know more? Find out more about our new innovative Aidaptus® auto-injector by scanning the QR code or visiting ompharmaservices.com/ipi-sept2022 *In addition to an air bubble and overfill Aidaptus® is a registered trademark of Owen Mumford Ltd, ©️2022 OMPS/ipi/ad/ob/0922 Available now Now in collaboration with Your fill volume may change, with Aidaptus® auto-adjust plunger technology your auto-injector doesn’t need to 0.3mL - 1mL* 0.5mL - 2.0mL* Accommodates both 1mL and 2.25mL glass syringes in the same device
Auto-adjust plunger technology Auto-adjust plunger technology 1mL 2.25mL
&
that corporate will and commitment are as important as bigger budgets in the race to improve ESG scores.
As well as the large differential in ESG scores between the best and worst performing firms in the pharmaceutical industries, there are some areas where improvement is required industry wide. One such area of concern is with contamination. While 84% of companies have a policy on Pharmaceuticals in the Environment (PiE) and 36% have a policy on the related issue of Anti-Microbial Resistance (AMR) there are very few concrete targets in these areas.
The AMR Alliance, an industry initiative to address anti-microbial resistances, states “Manufacturing emissions from both the production of active pharmaceutical ingredients (APIs) and their formulation into drugs is another source of environmental emissions... In countries where discharges are not well controlled some studies have found very high levels of active residues in the discharge vicinity of antibiotic factories.”5 Various studies share these findings, which is just one of several in safeguarding the environment from pharmaceutical contamination.6 Clearly there is much work to be done in the area of contamination.
Another area where the lack of progress is concerning is packaging. This is a much
less complex area in which to take action. In many other industries, we have seen efforts to alter packaging in the distribution phase in particular. While 76% of pharma companies have policies on packaging, only 13% have actually set concrete targets. Packaging could be converted to sustainable alternatives – where clinically acceptable – and reductions in weight and packing efficiency could help to reduce the resources used in the shipping process.
A few of the leading companies have set themselves specific targets – focussing heavily on converting from plastic to sustainable paper packaging. They have begun to assess where replacements bring the biggest net environmental gain and where original packaging should be retained. This area should begin to gain traction within the rest of the industry within the next few years and large-scale changes should begin to take place.
Conclusions
While a variety of studies have confirmed that the pharmaceutical industry is on the right track with regard to sustainability –and performing better than other industries – there is certainly still work to be done. Standards need to be adopted throughout the supply chain of the pharmaceutical industry to meet scope 3 emissions if further progress is to be made. A collaborative approach

between pharma companies and their suppliers, or between suppliers themselves, could help to speed up the rate of change and facilitate action. Areas such as contamination and packaging need concrete targets for real progress to be made; those that being to formulate initiatives now will pave the way for other businesses to follow suit.

REFERENCES
1. Ecoact, The Climate Reporting Performance of the DOW 30, EURO STOXX 50 and FTSE 100: 11th edition, 2021 https://info.eco-act.com/en/ climate-reporting-performance-research-2021
2. Fierce Pharma, The energy switch: Big Pharma harnesses sun, wind and water in quest for a low-carbon future, 15 October 2021 https://www. fiercepharma.com/pharma/solar-wind-waterpharma-go-planet-astrazeneca-novo-nordisknovartis-and-amgen-talk-renewable
3. PwC, Towards a Net Zero future in pharma – the role of continuous manufacturing, 17 February 2021 https://pwc.blogs.com/health_ matters/2021/02/towards-a-net-zero-future-inpharma-the-role-of-continuous-manufacturing. html%20
4. Let’s Recycle.com, Waste bills to rise as costs jump, 28th January 2020, https://www. letsrecycle.com/news/waste-bills-to-rise-ascosts-jump/
5. AMR Industry Alliance, Making antibiotics responsibly: A common manufacturing framework to tackle antimicrobial resistance https://www.amrindustryalliance.org/wpcontent/uploads/2019/11/Making-antibioticsresponsibly_A-common-manufacturingframework-to-tackle-AMR.pdf
6. See, for instance, Pharmaceutical waste and antimicrobial resistance, Ahmad, Akram et al. The Lancet Infectious Diseases, Volume 17, Issue 6, 578–579 https://www.thelancet.com/journals/ laninf/article/PIIS1473-3099(17)30268-2/fulltext
Michael Earl
Michael Earl joined Owen Mumford as Director of Pharmaceutical Services in November 2020. He was previously the Commercial VP at Bespak, leading the commercial team there to drive growth in their substantial medical devices business. Prior to that, he worked for a number of pharma, biotech and device companies. In a career spanning 35 years, he has been responsible for all aspects and stages of drug and device development and commercialisation. Michael has also completed a substantial number of commercial, licensing and M&A transactions.
10 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
Regulatory
Marketplace
and Sterile



THE PCI WAY


www.pci.com talkfuture@pci.com YOUR BRIDGE BETWEEN LIFE-CHANGING THERAPIES AND PATIENTS OUR END-TO-END BIOLOGIC SOLUTIONS INCLUDE: • Sterile Formulation & Lyophilization Cycle Development • Lyophilization and Sterile Fill-Finish Manufacturing (including Complex Formulations) • Analytical Support • Clinical & Commercial Labeling & Packaging • Refrigerated/Frozen Storage & Distribution Lyophilization
Manufacturing
Specialist expertise and experience in driving development and connecting commercialization of sterile and lyophilized life-changing therapies, delivering peace of mind for our customers.
Why We Recognise HIV Vaccine Awareness Day
May 18th is dedicated to HIV Vaccine Awareness Day. The significance of this recurrence is embedded in the years of research that have been dedicated to finding a reliable way for individuals to be protected against the virus.
Research began shortly after the virus was discovered as being the cause of AIDS, with the first vaccine trial being conducted in 1987.1 To this day, many clinical trials have been carried out, some with breakthrough results, yet no vaccines have been shown to be able to efficiently protect all individuals.
While people worldwide are infected with HIV, those living in sub-Saharan Africa have the highest rates with Eswatini, Lesotho and Botswana being the three countries with the highest prevalence of the virus almost consistently between 2000 and 2020.2
Without a reliable vaccine being available, reducing the risk of transmission by providing assistance and resources to countries who struggle with accessing them should therefore be the main point of focus in the battle against HIV.
In this piece Shameet Thakkar, founder and managing director of leading healthcare procurement services organisation Unimed, gives his expert opinion on the different factors that impact the effective delivery of vaccines and the power of prevention when
it comes to global health crises such as HIV.

The Importance of Prevention
What the above stats tell us is that there are deep-rooted issues with individuals in certain countries being unable to limit their exposure to the risk factors that can lead to becoming infected with HIV.
In this regard, Shameet aims to highlights the importance of prevention. He says:
“What we really need to focus on is developing reliable preventative measures and services. By helping more individuals access vaccines and other forms of prevention such as contraception, we can solve the problem at the root and efficiently assist individuals affected by life-threatening illnesses.
What many don’t realise is that there is a lot that goes into supplying vaccines to populations or groups of individuals. There is an entire supply chain at the forefront of vaccine operations, which must provide the right equipment at the right time in order to be effective.” he continues.
Vaccines therefore play a fundamental role in prevention, but vaccine projects require a lot more than just syringes and the vaccines themselves. In order for vaccine delivery missions to be effective, the right shipment services and equipment must be provided.
These programmes require appropriate ultra-low freezers, vaccine carriers, alcoholic wipes, sharps containers and immunisation cards, and even one item missing or being damaged could compromise the whole operation. That is why using effective procurement services is key.
Using the right equipment can have a life-changing impact. A vital example of this is auto-disable syringes, which play a vital role in prevention, as they are designed to automatically lock after every use, meaning they cannot be reused.
Given the fact that sharing contaminated syringes or needles is one of the main ways in which individuals contract HIV, this type of equipment should be used as much as possible when administering any vaccine.
This is because it is a safe and effective way to save lives without accidentally contributing to the spread of HIV and other blood-borne viruses.
Reducing Waste by Using the Right Equipment
When it comes to providing medical services, reducing waste should be at the forefront of people’s minds. Considering the life-saving impact vaccines can have on preventing the spread of viruses such as HIV, wasting even a small amount of vaccine can be incredibly harmful.
12 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Regulatory & Marketplace
This is precisely where using the right equipment can make a difference. A relevant example is low dead-volume syringes, which can minimise waste by reducing the amount of liquid left in a syringe after an injection.


This equipment plays a vital role in increasing the amount of people vaccinated, which is particularly relevant in the context of worldwide healthcare crises such as Covid-19, as the use of low dead-volume syringes was proven to increase Covid-19 vaccine supply by providing an extra dose per vial.3
These syringes can also reduce the risk of spreading viruses like HIV due to the fact that less dead volume means there is less space for potentially contaminated blood to be left in the syringe.
Medical procurement services are therefore crucial in the battle against viruses such as HIV, as medical professionals – and those in need of a vaccine – have to rely on these providers to deliver efficient equipment and develop the right solutions to distribute it, once ready.
In the context of distribution services, tracking performance KPIs such as OTIF (On Time in Full) can guarantee more control and efficiency in the delivery of products, helping organisations meet their targets effectively.
Maximising OTIF scores is then a great way to optimise operations and identify issues within a supply chain, potentially avoiding detrimental and wasteful time delays.

Thinking About the Future
Unimed’s mission revolves around staying ahead of evolving needs within the healthcare industry in order to be prepared to provide imminent aid to individuals globally as and when required, no matter the circumstances.
Although there is a long road ahead for medical researchers when it comes to developing a vaccine, HIV vaccine awareness day should be dedicated to recognising the vaccine development journey so far and the clinical trials that have brought us a step closer to our goal.
Unimed’s sustainable procurement efforts will continue to be directed towards
supporting research and contributing to the fight against global health issues such as HIV.
REFERENCES
1. https://www.niaid.nih.gov/diseases-conditions/ hiv-vaccine-research-history
2. https://www.statista.com/statistics/270209/ countries-with-the-highest-global-hiv-prevalence/
3. https://www.ncbi.nlm.nih.gov/pmc/articles/ PMC8418643/
Shameet S. Thakkar
Shameet S. Thakkar is the Founder and Managing Director of Unimed Procurement Services, an MHRA licensed healthcare procurement company. With over 10 years of experience, Shameet has successfully delivered healthcare projects to global clients in over 25 countries.Shameet is an expert in harnessing procurement activity to engage supply markets in innovative ways, reduce costs and maximise commercial benefits.
HIGH PURITY. HIGH DEMANDS.
High
Safety valves













traps

INTERNATIONAL PHARMACEUTICAL INDUSTRY 13
Pressure regulators Control valves Pipeline ancillaries Special equipment Steam
purity equipment for clean steam adca@valsteam.pt www.valsteam.com +351 236 959 060 Zona Ind. da Guia, Pav. 14 - Brejo 3105-467, Guia PBL PORTUGAL PRODUCTS MANUFACTURED IN PORTUGAL AV 001 AP EN 01.20
Regulatory & Marketplace
A Business Growth Tool: Patent Claiming Strategies
Developing a pharmaceutical product can take on the order of a decade from research (first inventing the drug) to development, and finally to FDA approval. While Patent Term Extension can restore some time lost due to the regulatory approval process, the traditional strategy of relying on patents based on the drug itself or its general method of use will necessarily start – and stop – the protection clock much earlier. This limits the potential return on investment prior to generic copyists. But, if one is creative and coordinated, additional strategies can be used to advance business growth with longer and stronger patents.
While each and every product presents new and unique facts that must be separately evaluated, creative strategies such as coordinating patent claims with the product label, claiming unexpected discoveries from later product development, and using the oft overlooked “means plus function” claiming format have each been shown to provide opportunities for valuable patent protection.
I. Coordinating the Claims and the Label
With some exceptions, a generic or bio-similar manufacturer must copy the innovator’s FDA approved package insert (“label”). This makes innovations reflected in and recommended on the label – such as from pivotal Phase III clinical trials – a potentially valuable source for additional patent protection.
The successful use of clinical trial results in this way was affirmed in Sanofi v. Watson, 875 F.3d 636 (Fed. Cir. 2017), in relation to Sanofi’s antiarrhythmic dronedarone product, marketed as Multaq®. The net result for Sanofi was achieving 10 more years of patent exclusivity for Multaq® compared to the dronedarone composition of matter patent.
Prior to the approval of Multaq®, a Phase III clinical trial conducted by Sanofi showed decreased cardiovascular hospitalisation and death in a particular high-risk patient population, specifically patients at least age 75 or those having hypertension, diabetes,
or other cardiovascular risk factors. Sanofi’s patent in the case relied on the Phase III data to claim a method of administering dronedarone to decrease a risk of cardiovascular hospitalisation in these patients. Once approved, the Multaq® label included the same Phase III results with all the relevant details in its “Clinical Studies” section, which was also cross-referenced from the label’s “Indications and Usage” section.
The generic party (Watson) copied the Multaq® label, including the “Clinical Studies” section with the Phase III results and the “Indications and Usage” section. Watson argued, however, that this did not induce the claimed method in part because its “Indications and Usage” did not specify treating the claimed high-risk patients. The courts – district court and Federal Circuit – disagreed. They found that the generic label induced infringement by encouraging the claimed treatment of the claimed highrisk patients. Id. at 646. Relying in part on the fact Watson’s “Indications and Usage” section also cross referenced to the “Clinical Studies” section, the courts found that Watson’s label “directs medical providers to information identifying the desired benefit for only patients with the patent-claimed risk factors.” Id. at 645.
The strategy of protecting non-obvious innovations developed from late-phase clinical trials included on the product label has proved successful in other cases as well. For instance, Vanda Pharmaceuticals obtained stronger patent exclusivity by developing, claiming, and including on its product label a method of using iloperidone to treat patients suffering from schizophrenia, where the dose was adjusted based on determining whether the patient was a poor CYP2D6 metaboliser.1
There are several key considerations to successfully rely on late-developed clinical innovations included in the label. For example, a patent application based on later clinical trials is best filed before the product is approved and before such results are published. Otherwise, the product label or publication could become prior art, as set out under 35 U.S.C. § 102(a)(1), in the absence of any 35 U.S.C.
§ 102(b)(1) exceptions. Importantly, with timing being everything, IP teams should coordinate early and frequently with clinical and regulatory teams to implement this strategy.
Another important consideration is whether the specific information included in the label and covered by the claims can be carved out by the generic or biosimilar manufacturer. In general, label information related to safety, dosage adjustments, and references to the clinical studies section in the “Indication and Usage” section must be copied, such that they are particularly suitable when considering how to best protect the product. In addition, the relied-upon clinical trial results should be recommended or encouraged by the label, such as the population to be treated or the dose to be used to minimise the effect of drug-drug interactions.
II. Claiming Unexpected Discoveries
Unexpected discoveries from late-stage pharmaceutical development can also be relied upon for novel and non-obvious inventions that provide additional protection. A good example of this is addressed in Teva Pharms. USA, Inc. v. Corcept Therapeutics, Inc., 18 F.4th 1377 (Fed. Cir. 2021), where the Federal Circuit affirmed that Teva failed to show that Corcept’s patent was obvious in a Post Grant Review (“PGR”) before the Patent Trial and Appeal Board (“the Board”).
Corcept markets Korlym®, mifepristone— an old drug – in a 300 mg tablet for the treatment of Cushing's syndrome. In approving Corcept’s New Drug Application (“NDA”) for Korlym®, the U.S. Food and Drug Administration (“FDA”) required Corcept to conduct a post-marketing (Phase IV) drug-drug interaction clinical trial of mifepristone and ketoconazole (a strong CYP3A inhibitor). Id. at 1379. The FDA also provided a memorandum (the “Lee Memorandum”) explaining that the drug-drug interaction study was necessary to determine whether there was a safety risk in co-administration of CYP3A inhibitors and mifepristone. Id.
Corcept’s original Korlym® label recommended a starting dose of 300 mg once daily up to a maximum of 1200 mg
14 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Regulatory & Marketplace

INTERNATIONAL PHARMACEUTICAL INDUSTRY 15wwww.international-pharma.com www.lifescienceaustria.at Advancing Austrian life science at the heart of Europe www.lifesciencesdirectory.at Find your right partner in Austria Find out more about our excellence
once daily, but with a warning to limit the mifepristone dose to 300 mg once daily when used with strong CYP3A inhibitors. Id. Based on the post-marketing drug-drug interaction study, Corcept unexpectedly discovered that doses higher than 300 mg could be used with concomitant administration of a strong CYP3A inhibitor. Based on this, Corcept applied for and received U.S. Pat. No. 10,195,214 (“the ’214 patent”), claiming the use of up to 600 mg of mifepristone in combination with a strong inhibitor.
In the PGR, Teva argued that the claims of the ’214 patent were obvious based on the original Korlym® label, the Lee Memorandum, and other references. Id. at 1380. The Board disagreed, finding that Teva failed to show “a reasonable expectation of success for safe co-administration of more than 300 mg of mifepristone” – the limit set by the original Korlym® label – "with a strong CYP3A inhibitor.” Id. The Board relied on, among other evidence, an admission from Teva’s expert’s testimony that the skill artisan “would have no expectation as to whether the co-administration of 600 mg of mifepristone with ketoconazole would be safe.” Id. at 1381-82. (emphasis in original). The Federal Circuit affirmed based on precedent that that “reasonableexpectation-of-success requirement is not satisfied when the skilled artisan would have had no expectation of success.” Id.
Claiming late-developed unexpected discoveries has been successful for other companies as well. For example, Cumberland Pharmaceuticals obtained and successfully enforced a patent based on its unexpected discovery – also based on a Phase IV study –that its Acetadote® (acetylcysteine) Injection for the treatment of acetaminophen poisoning could be formulated as a stable product without a chelating agent (EDTA) that been used in all prior commercial acetylcysteine formulations.2
As these cases illustrate, claiming an unexpected discovery helps the patent owner bolster its defense against obviousness challenges since showing obviousness requires a reasonable expectation of success in arriving at the claimed invention. The claims are no longer simply dosage and formulations of specific amounts, but dosage and formulations with specific and important properties that could not have been expected.
III. Means-Plus-Function
Means-plus-function (“MPF”) claims define
an element, in a combination claim, by its function instead of its structure:
35 U.S.C. §112(f) ELEMENT IN CLAIM FOR A COMBINATION. – An element in a claim for a combination may be expressed as a means or step for performing a specified function without the recital of structure, material, or acts in support thereof, and such claim shall be construed to cover the corresponding structure, material, or acts described in the specification and equivalents thereof.
MPF claims, often thought of as very narrow and only for use in the mechanical or electrical arts, can in fact provide greater protection than can other types of claims, including for claims drawn to pharmaceutical subject matter.3 That is because the statutory term “equivalents” does not reference the doctrine of equivalents, but rather refers to literal equivalents of structure, material, or acts that perform the same function. By encompassing literal equivalents, an inventor’s claims can better protect an inventor’s rights. MPF claims can also better prevent competitors from drafting around the literal scope compared to claims that recite particular elements, as MPF claims can encompass alternatives that provide the same function as claimed. MPF claim language may also provide more accuracy and clarity than purely structural characterisation and may avoid written description and enablement issues.
Overall, MPF language can be a powerful tool in drafting pharmaceutical claims to encompass broader claim scope and thus more fully protect an inventor’s rights and provide greater protection against infringement. Practitioners are cautioned, however, that 35 U.S.C. § 112(b) also applies. If one of ordinary skill in the art would be unable to recognize the structure, material, or acts in the specification that is clearly linked to the means plus function element, the claim will be invalid as indefinite under §112(b)!
IV. Applying These Creative Claiming Strategies for Business Growth
Using creative patenting strategies to advance business growth means, by definition, not simply relying on routine patents and claim formats. Making the most of one’s innovations requires looking deeper, such as considering all the potential claimable subject matter:
• Actives
• Formulations
• Delivery systems
• Drug-device combos
• Methods of treatment
• Methods of making
• Diagnostic, personalised medicine
If you have access to pivotal clinical trial data, try a Sanofi v. Watson application with narrow claims that capture the clinical trial results recommended in the drug product label, e.g., specific indication, dosing, specified target patient population, contraindicated symptoms, warnings and risk factors. Consider, in particular, safety signals, dosing regimens, and other disclosures that are difficult or impossible to carve out of a label.
If you make unexpected discoveries that lead to novel methods or formulations, protect these too. As described above, they can be the basis for particularly strong patent protection.
In addition to capturing innovations with other claim styles, consider meansplus-function claiming such as:
A composition comprising:
• component A and means for [achieving some desirable outcome]
• possibly effective against an ANDA applicant; or
A composition comprising:
• means for [achieving some desirable outcome]; and a pharmaceutically acceptable carrier
• possibly effective against an 505(b) (2) applicant.
Draft the specification to cover all the embodiments that you want to cover with this language (include alternatives). Link the function recited by the “means for” in your claim to the specification’s description of the structure(s), materials or acts of that function. This can be done in a preliminary amendment if not explicit in the specification. In other words, be intentional and thorough in your decision to use MPF claims.
Successfully implementing any of these strategies also requires making sure the specification provides adequate written
16 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Regulatory & Marketplace
description support for the claim, such as the results of the clinical study or other relevant (unexpected) discoveries, and the MPF support. And coordination between the clinical, regulatory, and IP teams is critical.
There are necessarily multiple moving parts to track to implement advanced patenting strategies for pharmaceutical products. But, if properly considered, they can be leveraged to advance business growth and increase return on investment.4

REFERENCES
1. See Vanda Pharms. Inc. v. West-Ward Pharms. Int'l Ltd., 887 F.3d 1117 (Fed. Cir. 2018), US Patent No. 8,586,610 (FANAPT®); see also AstraZeneca AB v. Macleods Pharmaceuticals Ltd., No. 1-20cv-01180 (D.DEL.), US Patent No. 10,300,065 (BRILINTA®).
2. See U.S. Patent No. 8,399,445; Cumberland Pharm. Inc. v. Mylan Institutional LLC, 846 F.3d 1213, 1214 (Fed. Cir. 2017).
3. Examples of issued MPF bio/pharm claims can be found in U.S. 8,722,872; U.S. 10,413,611; U.S. 9,149,464; U.S. 7,579,380; U.S. 7,670,617; U.S. 9,446,076; U.S. 10,335,405; and U.S. 6,974,595 (note, successfully enforced in Nautilus Neurosciences, Inc. v. Wockhardt, United States LLC, 2:11-cv-01997 (D. NJ Feb. 27, 2013) (not for publication). See also Examples 5 and 6 in the USPTO Training Materials at https://www. uspto.gov/patents/laws/examination-policy/ examination-guidance-and-training-materials
4. These materials have been prepared solely for educational and entertainment purposes to contribute to the understanding of U.S. intellectual property law. These materials reflect only the personal views of the authors and are not individualised legal advice. It is understood that each case is fact specific, and that the appropriate solution in any case will vary. Therefore, these materials may or may not be relevant to any particular situation. Thus, the authors and FINNEGAN, HENDERSON,
Regulatory & Marketplace Tom Irving
Tom Irving is a partner at Finnegan and has some 45 years of experience in intellectual property law. His U.S. pharma practice includes America Invents Act (AIA) post-grant proceedings, due diligence, counselling, patent prosecution, reissue, and re-examination.
In addition to advising on procuring strong U.S. patents, Tom counsels clients on a wide range of mainly pharmaceutical matters, including pre-litigation, Orange Book listings of patents covering FDA-approved drugs, infringement issues, enforceability, supplemental examination, and validity analysis.
Email: tom.irving@finnegan.com
Stacy Lewis
Stacy Lewis is called to the bar in New York and has worked as a law clerk at Finnegan since 1995. She focuses on research and writing, particularly in the life sciences. She is the coordinating editor of the books Global Patent Litigation: How and Where to Win and Design Patent Law, as well as the coursebook Chemical Patent Practice. Stacy sits on the AIPLA Quarterly Journal and IPO Law Journal editorial boards.
Email:
FARABOW, GARRETT & DUNNER, LLP (including Finnegan Europe LLP, and Fei Han Foreign Legal Affairs Law Firm), cannot be bound either philosophically or as representatives of their various present and future clients to the comments expressed in these materials. The presentation of these materials does not establish any form of attorney-client relationship with these authors or FINNEGAN, HENDERSON, FARABOW, GARRETT & DUNNER, LLP (including Finnegan Europe LLP, and Fei Han Foreign Legal Affairs Law Firm). While every attempt was made to ensure that these materials are accurate, errors or omissions may be contained therein, for which any liability is disclaimed.
Mark Feldstein

Mark Feldstein, Ph.D. is a partner at Finnegan. His practice focuses on U.S. district court litigation, primarily concerning the enforcement of U.S. patent rights and trade secret issues, and postgrant trial proceedings at the USPTO.

His experience encompasses a range of technologies, including pharmaceuticals, chemicals, biochemistry, polymers, small molecule chemistry, nanotechnology, optics, and medical and analytic devices.
Mark has particular experience with litigations arising from Abbreviated New Drug Applications (ANDAs) under the Hatch-Waxman Act.
Email: mark.feldstein@finnegan.com
Adriana Burgy
Adriana Burgy is a partner at Finnegan. Her practice focuses on opinion work, client counselling, patent prosecution and management, and litigation in the chemical, pharmaceutical, and biotechnology arts. Recognised by The Legal 500 U.S. for patent prosecution, re-examinations, and post-grant proceedings, Adriana counsels her clients on a diverse range of patent issues. She has managed the prosecution of hundreds of patent applications domestically and internationally directed to consumer products, pharmaceuticals, and small molecules.
Email: adriana.burgy@finnegan.com

INTERNATIONAL PHARMACEUTICAL INDUSTRY 17wwww.international-pharma.com
stacy.lewis@finnegan.com
Drug
Development & Delivery
Under Pressure: Finding a More Sustainable Future for pMDIs
The transition away from the use of ozone-damaging chlorofluorocarbon (CFC) gases has been one of the major environmental achievements of modern times.
Today, hindsight affords us the ability to see how this transition enabled the Earth’s protective ozone layer to heal itself over several decades. An unintended consequence, however, was that the withdrawal of CFCs (Chlorofluorocarbons) ushered in greater use of hydrofluorocarbons, known as F-gases, which introduced their own environmental challenges.
While this family of gases might not pose a risk to the atmospheric ozone layer, they were found to contribute to the greenhouse effect. Compared with carbon dioxide (CO2), F-gases have a far higher global-warming potential (GWP), based on attributes including infrared radiation absorption and atmospheric lifetime. These characteristics mean F-gases are now also subject to a phasing down, impacting a variety of processes and applications where they currently play an essential role.1
In medicine, the use of F-gases is widespread across the globe, with HFC134a and HFC-227ea relied upon as safe and effective propellants within inhalation devices, helping millions of patients manage respiratory conditions such as asthma and chronic obstructive pulmonary disease (COPD). As the negative environmental impact of these gases becomes more understood, there are implications for every stakeholder in the chain, from device manufacturers and pharmaceutical companies to healthcare professionals (HCPs) and patients.
The phasing down of F-gases is already well underway, with global efforts falling in line with the Montreal Protocol of the Vienna Convention on Substances that Deplete the Ozone Layer. The Montreal Protocol has provided a framework for safeguarding the ozone layer since it went into effect in 1989 to define the transition away from CFCs.2 This framework was expanded on January 1,
2019, through the Kigali Amendment, which binds all 198 signatories to also take action to reduce the production and use of F-gases, including the HFCs used in medical devices.
Of all territories across the world, Europe has historically implemented the most stringent targets for HFC reduction. On April 5, 2022, The European Commission set out proposals for two new regulations that would strengthen F-gas legislation and pave the way for a series of changes to accelerate the phasing down process. In announcing the proposals, Frans Timmermans, Executive Vice-President for the European Green Deal, said that while the EU’s current ambitious policies have been successful, “science urges us to go further and faster now”.3
Currently, F-gases account for 2.5% of total greenhouse gas emissions across Europe. The European Commission (EC) said the proposals to accelerate their phasing down would contribute to reducing these emissions by at least 55% by 2030 and support the continent’s overarching plan to be climate-neutral by 2050.
In the United States, the world’s largest market for pressurised metered dose inhalers (pMDIs), the phase-down programme is scheduled to follow behind Europe through the American Innovation and Manufacturing Act, which came into effect in December 2020. While pMDIs are encompassed within ‘set-aside’ allocations to ease the transition in the region, these are only in place for a period of five years and the US is expected to achieve a phasedown level of 15% by 2035. Other territories across the rest of the world, including India
and countries across the Middle East, are expected to follow behind the US by a period of around 15 years.
Although these robust environmental targets are fixed in place, less certainty surrounds the knock-on effects of meeting them. Of primary concern here are patients, whose ongoing needs must be met in terms of consistent access to relief and prevention medication – something that can only be achieved through the transition either to alternative inhalation devices with lower GWP, such as dry powder inhalers (DPIs), or to pMDIs using lower GWP propellants.

While it would be hoped that such a transition would follow some of the precedent set by the move away from CFCs, it is clear that the phasing down of F-gases must overcome a different set of complex hurdles to ensure device and drug are optimised in a way that satisfies patient need.
In the most acute circumstances, patient need can manifest itself as the administration of life-saving medicine to a potentially incapacitated individual who might not have the respiratory force required to deliver the necessary dose via a DPI. Over decades, pMDIs have proved their worth in this scenario, with the force of a propellant driving successful drug deposition and, therefore, patient outcomes when used in tandem with a spacer or nebuliser. As an indication of their importance to patients, pMDIs account for 77% of inhaled device doses in the world’s top 15 markets.
Dry powder inhalers, while highly effective, do not represent a like-for-like ‘swap’

18 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
Discovery,
Drug Discovery, Development & Delivery
in this regard. For elderly populations, drug delivery can be impaired when respiratory muscle weakness limits a patient’s inhalation action, while younger children can struggle to comply with the technique required for optimal dose delivery. As such, the use of DPIs as a rescue medication remains limited, accounting for only 3% of prescriptions containing a short-acting beta-agonist (SABA).
Running in parallel with the requirement to answer patient need is the need to consider limiting the disruption to the current economic models that successfully underpin pMDI use. It cannot be ignored that delivering medication via HFC-based pMDIs is both highly efficient and highly cost-effective, with the average cost per dose of salbutamol estimated at $0.06.
However, analysis of the global economic impact of switching from pMDIs to DPIs, using prescribing data sourced from the IQVIA database, has revealed that in all the world’s top 15 markets for respiratory drugs by value, costs would rise if SABA pMDIs were replaced with DPIs. The only exception is Brazil, where there is no equivalent DPI registered.4
That is not to say that the economics for existing pMDIs are fixed. As regulations drive down volumes of industrial-grade F-gases, from which medical-grade propellants are manufactured, prices are likely to increase. Indeed, the market has already experienced this dynamic in recent years, with escalating propellant cost pressures only eased by the allowance of imports under the ‘exempted’ classification.
The answer to providing patients with device continuity therefore rests on the development of a new era of pMDIs using propellants with lower GWP than existing options. Of the candidate gases being evaluated across the sector, HFA152a and HFO-1234ze show promise in terms of balancing high performance and environmental credentials with low toxicity.

Simply switching to these new propellants might be an appealing notion, but, of course, their potential must first be fully evaluated via an assessment of their characteristics to ensure device performance, clinical efficacy, toxicology and patient safety are all satisfied. Nanopharm, an Aptar Pharma company, has conducted extensive testing in this area, revealing the influence of variable propellant attributes, such as solubility, and their interaction with the drug formulation. During their research, Nanopharm has uncovered, for example, that the electrostatic charge for HFA-152a is comparatively higher than HFO-1234ze, and that the two gases impact on particle dynamics differently, with higher oropharyngeal deposition observed for HFO1234ze.
As well as overcoming any formulation and aerosolisation challenges for devices using new propellants, regulatory hurdles must also be cleared to ensure patient safety is guaranteed in the final marketed product. As urgency builds around the accelerated phase down of F-gases, any transition will require stakeholders to engage closely with regulatory bodies to facilitate approval with the requisite clinical data. Pathways may be available to expedite this process. The
US Food & Drug Administration (FDA), for example, points to section 505(b)(2) of the Federal Food, Drug, and Cosmetic Act as an option for providing an abbreviated route for approval, potentially opening the door to applications being augmented with data from bioequivalence studies.
Particularly in light of the compressed development timeframe presented by the EU’s recent proposals, the emphasis on accelerating the transition to low-GWP propellants has intensified. It could also be said, however, that this has constricted the potential for wider reflection and innovation around inhalation devices. Tighter controls on imports and the removal of exemptions for medical use mean that by 2027, F-gas levels are projected to be at just 10% lower than the baseline and, without significant change, almost all that allowance would be consumed by MDIs.
Considering these figures, it is understandable that healthcare professionals (HCPs) are being encouraged to move patients to DPIs. However, there is also the risk that such an isolated switch does not take into account the realities of human behaviours and therefore does not fit into a holistic healthcare strategy for controlling the serious risks associated with respiratory conditions.
For example, if patients have difficulty in self-managing their condition, it is possible that they will require additional support within primary or secondary care settings. Aside from the health risk involved, this places an unnecessary burden on the health system while also carrying an additional carbon cost.
INTERNATIONAL PHARMACEUTICAL INDUSTRY 19wwww.international-pharma.com
& Delivery
Data from the SABINA CARBON UK study found that excess greenhouse gas emissions per capita were eight times higher for patients with uncontrolled asthma (as defined by one or more exacerbations in the past 12 months or being prescribed three or more SABA inhalers per year) compared with patients with controlled asthma (defined by no exacerbations and being prescribed up to two SABA inhalers per year).5 In addition, a single hospitalisation for a respiratory patient has been found to have a larger carbon footprint than 1.5 years of daily pMDI use.6


Findings like the above show that there is a wider picture to be considered when tackling the global challenge of climate
change. It is essential that the use of F-gases is controlled, but it is also critical that this process is managed within a holistic approach to the future of pMDIs, and that the contribution of associated factors such as dose efficiency, adherence and compliance are all equally considered. Within a managed transition to low-GWP alternatives, pMDI devices can not only continue to fulfil their vital purpose in delivering rescue and relief medication for patients with respiratory conditions, but they can also fulfil their potential in areas such as nasal drug delivery and as a platform for sensitive biologics, where low-GWP propellants show promise in supporting formulation stability and the delivery of high payloads.
Within the EU in particular, the drive to phase down F-gases and switch to DPIs means opportunities for more holistic reflection and innovation might be limited. In other territories, however, the elongated phase-down schedule could allow companies in these regions to leverage their position, using the introduction of low-GWP propellants to also usher in complementary device innovation.

One of the important lessons from the phasing out of CFCs via the Montreal Protocol was collaboration: all stakeholders were aligned on a singular vision, taking action to support a common goal. Today, however, we are faced with different circumstances in the challenge to deliver a sustainable strategy for the future of managing respiratory conditions. Converging on a collective vision must involve a comprehensive assessment of the changing role of different device formats within a multi-layered ecosystem over time.
Simpler strategies might hold more immediate appeal, but arguably come with risk attached.
Targets to reduce greenhouse gas emissions are of immediate interest to many across the globe but trying to make progress by exclusively focusing efforts on certain directions may carry consequences that are not immediately visible. From policymakers to healthcare providers, drug companies and device manufacturers, it is incumbent to find the right balance on the path to delivering better outcomes for patients and the planet long into the future.
REFERENCES
https://ec.europa.eu/clima/eu-action/ fluorinated-greenhouse-gases_en
https://ozone.unep.org/treaties/montrealprotocol
https://ec.europa.eu/commission/ presscorner/detail/e%20n/ip_22_2189
https://www.dovepress.com/the-climate-ischanging-for-metered-dose-inhalers-and-
20 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Drug Discovery, Development
1.
2.
3.
4.
Drug Discovery, Development & Delivery
action-is-needed-peer-reviewed-fulltextarticle-DDDT#T0002
https://www.astrazeneca.com/media-centre/ medical-releases/carbon-studies-showeduncontrolled-asthma-is-associated-with-an-
increased-carbon-footprint-of-asthma-care.html

https://www.astrazeneca.com/media-centre/ articles/2020/investing-in-a-sustainablefuture-for-patients-with-respiratory-disease.
Omar Usmani


Dr. Jag Shur
Dr. Jag Shur, Vice-President, Science & Technology, Nanopharm, an Aptar Pharma company, and Co-Founder of Nanopharm is an internationally recognised expert in the investigation of bioequivalence of OINDPs. Holding a BSc (Hons) in Chemistry, he completed his PhD entitled ‘Formulated Muco-Regulatory Agents in the Airways of Patients with Cystic Fibrosis’ from Portsmouth School of Pharmacy in the UK. Dr. Shur is also a post-doctoral fellow at the London School of Pharmacy, having investigated the fabrication of micro particles for vaccine delivery using supercritical fluid technology.
Dr. John N. Pritchard

Dr. John N. Pritchard, PhD is a private consultant specialising in strategic approaches for developing respiratory devices, drugs and digital health. He sits on several Scientific Advisory Boards as well as the UN Committee that makes recommendations on the medical uses of propellants covered by the Montreal Protocol and is Director for a number of SMEs. Having worked previously at GSK, 3M, AZ and Philips, he has worked on MDIs, DPIs and nebulized products, and was associated with the launch of 11 major products. At RDD 2018, John received the Charles Thiel award for outstanding research and discovery in respiratory drug delivery.
Professor Omar Usmani is Professor of Respiratory Medicine at the National Heart and Lung Institute (NHLI), Imperial College London and Consultant Physician at the Royal Brompton Hospital and St Mary’s Hospital London. He is currently Head of the ERS (European Respiratory Society) Assembly 5 Airway Diseases. His clinical research themes are Inhaled Drug Delivery, Airways Physiology, Lung Imaging and Biomarkers, and Digital E-health. He was co-investigator and lead Clinical PI, on an EU HORIZON 2020 grant, myaircoach, to investigate the role of E-health in asthma self-management. In 2015, he received, the Thomas T Mercer Award from the American Association of Aerosol Research (AAAR) and ISAM, recognizing his international research excellence in pharmaceutical aerosols and inhalable materials.
STEAMING SOLUTIONS FOR ALL INDUSTRIES

















INTERNATIONAL PHARMACEUTICAL INDUSTRY 21
Pressure regulators Control valves
Pipeline ancillaries
Steam
traps
Special equipment
adca@valsteam.pt www.valsteam.com +351 236 959 060 Zona Ind. da Guia, Pav. 14 - Brejo 3105-467, Guia PBL PORTUGAL PRODUCTS MANUFACTURED IN PORTUGAL AV 001 IN EN 01.20
5.
6.
html
Use of High-field NMR in Covid-19 Drug Development
The Covid-19 NMR Consortium, an international collaboration of NMR experts, is conducting research to determine the ribonucleic acid (RNA) structure of SARS-CoV-2 and its proteins using NMR spectroscopy. One of the consortium’s first discoveries, early on in the pandemic, was that a protein within SARS-CoV-2 forms microdroplets with the RNA of the virus. In subsequent months, this discovery enabled scientists to set up experiments to study the interplay between the RNA and the protein, known as the nucleocapsid protein or N protein.
At the invitation of Prof. Schwalbe, Prof. Markus Zweckstetter joined the consortium in late 2020 after the publication of his research on SARS-CoV-2. As part of this 50-strong international collaboration of NMR experts, Prof. Zweckstetter and his team at the Max Planck Institute for Multidisciplinary Sciences in Göttingen, Germany, have been uncovering insights into the hidden workings of Covid-19. Using one of only a few 1.2 GHz NMR instruments in the world, these researchers are helping to elucidate the structure and interactions of the nucleocapsid protein within the SARS-CoV-2 virus and, in doing so, identify promising options for drug targets.
Mechanisms of Covid-19
The etiologic agent of the Covid-19 pandemic is the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Many early studies of SARS-CoV-2 focused on the socalled ‘spike protein’ because this is involved in communication with the host cell. But as understanding of SARS-CoV-2 has evolved, it has become apparent that the nucleocapsid protein, or N protein, also plays a key role because it not only protects the RNA from degradation, but enables the transcription machinery to cluster, and so enhances its ability to replicate.
The viral membrane of SARS-CoV-2 surrounds a helical nucleocapsid in which the viral genome is encapsulated by the nucleocapsid protein. The nucleocapsid
protein of SARS-CoV-2 is produced at high levels within infected cells, enhances the efficiency of viral RNA transcription, and is essential for viral replication. Scientists at the German Center for Neurodegenerative Diseases (DZNE) and the Max Planck Institute have now shown that this protein and the RNA can jointly condense into tiny droplets when the virus releases its insides into a host cell.
Continued research determined that RNA induces cooperative liquid–liquid phase separation of the SARS-CoV-2 nucleocapsid protein, where the viral droplets float inside the fluid medium inside the cell. However, this droplet formation is not a specialty of the coronavirus. Such dynamic compartments composed of proteins and other molecules occur naturally inside cells, and they are used as storage sites and reaction chambers. Research by Prof. Zweckstetter and his team shows that the coronavirus also exploits these possibilities, and this is suspected to happen with other pathogens as well.
In agreement with its ability to phase separate in vitro, protein associates in cells with stress granules – cytoplasmic RNA/ protein granules that form through liquidliquid phase separation and are modulated by viruses to maximize replication efficiency. This process generates high-density protein/ RNA condensates that recruit the RNAdependent RNA polymerase complex of SARS-CoV-2, providing a mechanism for efficient transcription of viral RNA. Inhibition of RNA-induced phase separation of the nucleocapsid protein by small molecules or biologics thus can interfere with a key step in the SARS-CoV-2 replication cycle.1
Identifying Potential Drug Targets
Prof. Zweckstetter and his team believe these findings may offer starting points for drug development. Thanks to collaboration efforts, many groups around the world are now studying the N protein, with a view to assessing its potential as a target for treatment of Covid-19. For example, by interfering with the formation of N protein microdroplets, the viral RNA might become more vulnerable to external damage and less able to replicate reliably.
About the COVID-19 NMR Consortium
The Covid-19 NMR Consortium was initiated at the Goethe University in Frankfurt, Germany, in March 2020 by Prof. Dr. Harald Schwalbe and it has since grown rapidly into an international consortium. Today, scientists from all over the world are collaborating based on open science principles in a unique effort to investigate SARS-CoV-2 using NMR spectroscopy. The goals and the shared scientific targets of the project are coordinated by Prof. Schwalbe and his team at Goethe University. The core team includes five professors and junior group leaders from Darmstadt and Frankfurt and nine senior scientists from the Biological Magnetic Resonance Center (BMRZ) in Frankfurt. The overall goal of this consortium is to join forces to achieve meaningful scientific results in Covid-19 research as quickly as possible.
Another aspect of the work on N protein is to investigate the involvement of kinases in phosphorylating certain residues of N protein, as these enzymes could be promising targets for small-molecule inhibitors. Prof. Zweckstetter’s team also discovered that the enzyme SRPK1 kinase, which occurs naturally in the human body, chemically modifies the nucleocapsid protein and influences the formation of the viral droplets.
Insights from NMR Spectroscopy Consortium scientists investigated these events using NMR spectroscopy and other methods to examine the structure and dynamics of molecules. The Zweckstetter group’s research has involved a number of high-field NMR spectrometers from 600 MHz to 950 MHz, which have been central to carrying out the highly sensitive investigations into proteins. Now the group has access to the 1.2 GHz NMR instrument at Göttingen, which they believe will enhance the resolution of three- and higherdimensional NMR experiments by at least a factor of two compared to existing 950 MHz instrument. That will allow further studies of the structural dynamics of biomolecular
22 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Drug Discovery, Development & Delivery
Drug Discovery, Development & Delivery
Figure 1: Using NMR to investigate the properties of the A182–S197 region of the nucleocapsid (NCP) protein within SARS-CoV-2, which has a high proportion of serine and arginine resides (known to bind both RNA and proteins). (A) Chemical shift analysis (blue) agrees with molecular dynamics simulations (red) that the residues in this region are very flexible, with a small propensity for α-helical structure next to R189. (B) Re-running the simulations in the presence of polyuridylic acid (a simplified RNA) showed a large number of intermolecular contacts between the arginine residues and the RNA phosphate groups, with a maximum for R189. This agrees with the observation that R189 is the only residue in the region A182–S197 that is not mutated in most of the currently known strains of SARS-CoV-2 (gray bars). Reprinted from ref. [1] under a Creative Commons license (CC BY 4.0, http://creativecommons.org/licenses/by/4.0/). by enzymes. The capabilities of high-field NMR stand in contrast to techniques such as cryoelectron microscopy, which requires frozen samples, and so can only provide snapshots of the molecular action.

markers on huge range of time and length scales. The 1.2 GHz NMR instrument uses high-temperature superconductors for the inner coil and regular, low-temperature superconductors for the outer coil. That enables the uniform magnetic field of 28.2 Tesla.
With this boost to NMR science at Göttingen, Prof. Zweckstetter is integrating NMR with other structural biology techniques, thereby obtaining a more complete view of the inner workings of the SARS-CoV-2 virus. For example, further characterisation using high-resolution NMR, molecular dynamics simulations and phase separation experiments could help pinpoint how potential drugs interact with N protein, and whether RNA replication could be influenced.
NMR enables the study of how the biomolecule moves around in solution, and the different shapes it takes on to perform different activities. It also helps with the visualisation of molecules in realtime, gaining crucial insights into how they perform their function and are modified
For the SARS-CoV-2 research, the team combined molecular dynamics simulations with NMR spectroscopy of 1H, 13C and 15N nuclei, using NOESY, HSQC and total correlation spectroscopy (TOCSY). An example of the insights obtained is shown in Figure 1.
Future Steps
This work has the goal of improving treatment for those with Covid-19. Because the virus is constantly adapting and evolving, it brings a risk of re-infection. To counter the threat it poses, the consortium plans to continue to provide the data to understand the inner workings of the virus and work towards new and better drugs to treat Covid-19.

The Covid-19 NMR project holds further implications that extend beyond the virus. While collaboration is valued in
science, the inherent competition between research groups has limited the scope of that collaboration. Whereas normally scientists might work with one or two other teams, the Covid-19 consortium shows the potential when dozens of research teams can collaborate globally. The ability to pool expertise, equipment, and reagents enables much faster progress. Setting up large scientific collaborations to tackle major challenges could be a complementary and maybe even more powerful way of making rapid progress.
REFERENCES
1. Savastano A, Ibanez de Opakua A, Rankovic M and Zweckstetter M, Nucleocapsid protein of SARS-CoV-2 phase separates into RNA-rich polymerase-containing condensates, Nature Communications, 2020, 11: 6041, https://doi. org/10.1038/s41467-020-19843-1
Dr. Markus Zweckstetter
Prof. Dr. Markus Zweckstetter is head of the research group on Structure determination of proteins using NMR at the Max Planck Institute for Multidisciplinary Sciences in Göttingen, Germany. Since 2012, he has also led the Translational Structural Biology group at the German Center for Neurodegenerative Diseases (DZNE), and is a professor at the University Medical Center in Göttingen. Prof. Zweckstetter received three European Research Council (ERC) grants, which have helped his team uncover protein structure and function using the power of NMR spectroscopy.

INTERNATIONAL PHARMACEUTICAL INDUSTRY 23wwww.international-pharma.com
Virtual Screening in Modern Computational Chemistry
Virtual screening is a firmly established technique in computational drug design which has saved the pharmaceutical industry billions of dollars since its inception. The structure-based approach involves fitting potential small molecules to a protein target, to generate predicted ligand-protein interaction properties that can significantly reduce chemical search space. Ligand-based virtual screening does not involve direct use of a 3D representation of the biological target, but instead uses known active compounds as a reference structure for screening in chemical space. With a multitude of approaches available to the end-user, we discuss the technical and practical considerations for modern virtual screening to ensure projects are delivered efficiently, at optimal cost, and the time to get a drug to market is as short as possible.

Hit identification in drug discovery presents an immense task to researchers, with current estimates putting total chemical space at over 1060 plausible compounds. The odds of finding active molecules against a particular biological target are, therefore, stacked against researchers, prompting the need for high-throughput screening stages in most projects. The monetary, material, and time waste in lab-based screening is enormous, which has driven widespread adoption of in silico techniques for earlystage hit identification.
Virtual Screening Increases Efficiency in Early-stage Drug Design
Virtual screening of compounds has several benefits when compared to labbased approaches. Firstly, there are enormous gains in speed. Unlike labbased approaches, virtual techniques do not require the purchase of chemical compounds, and do not need to be physically loaded into laboratory apparatus prior to screening. A computational chemist only needs access to chemical structure databases, which are readily available in the modern era. There is also far less waste generated by computational approaches.
Figure 1. Virtual screening server platforms can search millions of potential actives in a matter of hours.
Most compounds purchased for lab-based screening do not yield a hit, thereby wasting the chemical material and time investment in running the experiment, as well as incurring unnecessary environmental impact. In virtual screening however, waste is minimised, because the process can be heavily automated, and the only cost is computing time. In addition to speed and efficiency gains, in silico screening makes it possible to consider millions of compounds in a matter of hours, which is orders of magnitude higher than can be achieved in a lab-based screen. Virtual screening can, therefore, explore chemical space much more widely than lab-based wet screening setups, meaning that more hits can be identified in a given timeframe.
Outsourcing Virtual Screening Enables Access to Specialised Knowledge without Incurring Overheads

Virtual screening can have significant implications for the success of drug discovery projects, which is why expert knowledge is needed to maximise hit rates. The demand for this specialised expertise has led to the formation of Contract Research Organisations (CROs), focussed on providing outsourced computational modelling for early-stage drug design. The benefit of this kind of working model is that drug discovery organisations can very quickly access specialised knowledge centres at different stages of the project, without incurring the initial overheads of hiring experts internally. This kind of model has become popular and has driven development of computational tools for collaboration between project stakeholders, specifically tailored to aid the collaborative
Design-Make-Test-Analyse (DMTA) chemical design workflow.
In silico Virtual Screening Increasingly Performed by Non-computational Chemists
Computational chemistry is often perceived as being extremely challenging because it involves mastery of concepts from both physical and computational sciences, which are often less familiar to lab scientists, introducing a barrier to their widespread use. Though collaboration with CROs makes this specialist knowledge immediately accessible, there is benefit in medicinal chemists adopting in silico techniques as part of their standard workflows. Recent years have, therefore, seen a focus on the development of scientific software which emphasises user friendliness and the ability to be used ‘out-of-the-box’, meaning that virtual screening is becoming increasingly performed by non-computational chemists. Despite advances in the usability of computational modelling software packages, however, a good theoretical understanding of in silico methods remains essential in maximising the practical benefits of computational chemistry. As we discuss some of the key concepts from structure and ligand-based drug design in the following paragraphs, it should be emphasised that neither structure nor ligand-based screening is superior; they are often regarded as orthogonal/complementary techniques, providing value in different scenarios.
3D Structure Fundamental to Virtual Screening
Structure-based virtual screening involves computer simulation of the interactions between a known biological target and
24 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Drug Discovery, Development & Delivery
Health Science
Inspired, Quality Driven
Nitrosamine Testing Solutions

The presence of the nitrosamine, N-nitrosodimethylamine (NDMA), in certain sartan API’s has resulted in several regulatory warnings and recall of contaminated products. Concerns over the presence of this class of genotoxins has now widened to include other medicines such as the well-known diabetes drug, Metformin. The US Food & Drug Administration (USFDA) and European Medicines Agency (EMA) have responded by publishing documents for the pharmaceutical industry that address requirements and limits related to nitrosamine contaminants. Pharmaceutical Manufacturers are now taking a pro active approach to risk assessment and mitigation of genotoxic contaminants within global pharmaceutical supply chains. Central to these activities is a coordinated analytical capability to identify and quantify contaminants across global geographies and regulatory zones.



Why use SGS
SGS Health Science has considerable expertise in the method development of nitrosamine determination in pharmaceutical products. SGS has established a specific method for NDMA which can be applied to various different matrices. Alternatively, a platform method, based on trace-level detection by LC-MSMS, is also available and provides rapid and simultaneous determination of up to ten different, targeted nitrosamines. Although with more limited application, the SGS network is also able to support specialist methodologies such as GC-MSMS. Our experience in optimizing extraction allows application of these methods to drug products, API’s, and raw materials.
By establishing these nitrosamine methods within centers of expertise across a global laboratory network, SGS can provide an unrivaled service offering that incorporates a harmonized methodological approach together with flexible management of capacity and capability requirements.SGS offers a variety of partnership models and can collaborate in such testing programs using fee-forservice to outsourced staffing models all exploiting resources of the SGS network.
About SGS
SGS is the world’s leading inspection, verification, testing and certification company. We are recognized as the global benchmark for quality and integrity. With more than 94,000 employees, we operate a network of more than 2,600 offices and laboratories around the world.Our conveniently located network of laboratories and clinical trial facilities offer an array of integrated services and expertise, providing you with the knowledge, flexibility and ability to scale.
Wide-range of laboratories and clinical research sites and qualified partners. Size and diverse testing capabilities matching biologics and small molecules needs International network across America, Europe and Asia-Pacific
INTERNATIONAL PHARMACEUTICAL INDUSTRY 25wwww.international-pharma.com
Health
Contact us healthscience@sgs.com sgs.com/healthscience sgs.com/healthcommunity
Drug Discovery, Development & Delivery
one-or-more ligand(s). To begin, structural representations of ligands and 3D biological targets must be obtained from chemical or biological database(s), such as the protein data bank (PDB). Structures from the PDB have usually been obtained from experimental techniques such as protein X-ray crystallography or cryogenic electron microscopy. In cases where a protein structure is not available, homology modelling techniques can be used to improvise a protein structure if sequence information for the unknown target is available.
Though protein structure determination represents cutting edge science, there are several important limitations of these methods when using an experimental structure for computational modelling. One of these considerations is that proteins are active in solution, where their structural and behavioural characteristics may be very different from the solid state which is typically used for protein structure determination. The structure of the protein, therefore, requires intensive manipulation before it can be used reliably. There are tools that can automate protein preparation and are incorporated into premium structurebased screening software packages. However, due to the enormous complexity of proteins and the extreme sensitivity of structure-based virtual screening, some manual structural manipulation is usually required.
3D structure is fundamental to virtual screening because biological activity is almost always attributable to specific 3D interactions, such as in a protein active site. Ligand-protein binding occurs when

an arrangement of chemical groups in 3D space (pharmacophore) matches the 3D arrangement of complementary chemistries in the biological target. Accordingly, the success of structure-based virtual screening can be critically dependant on the quality of 3D representation for the protein.
Optimising Ligand Pose with docking in Structure-based Virtual Screening Docking is a widely adopted method for structure-based virtual screening, where each ligand is placed into the biological target, e.g., a protein active site, and its structure adjusted to optimise binding contacts. A ‘docking score’ is then calculated, typically using an atomistic potential energy method to predict binding properties between the ligand with the protein. A new orientation of the ligand is then tried, and the score is recalculated at the new

orientation, and so on. Each orientation is referred to as a pose, and the ligand pose is optimised with respect to the pseudo energy function using a global optimisation method, such as a genetic algorithm.
Following the experiment, ligands that have high scoring poses can be regarded as having better theoretical potential for binding to the protein than ligands that have lower scoring poses. High scoring ligands are, therefore, hit candidates in structure-based virtual screening.
Though structure-based screening is extremely powerful, there are some limitations on its use. Firstly, a reliable protein structure is required. Proteins can be extremely difficult to isolate and characterise, therefore, it is quite common for drug discovery projects to start without a protein structure, meaning that structurebased virtual screening cannot be employed.
Another limitation of structure-based methods is that due to the extreme complexity of ligand-protein binding, many approximations must be made in docking algorithms to enable reasonably fast compute times. This adversely affects accuracy, meaning that state-of-the-art docking scores can give an estimation of the ligand-protein binding physics, but cannot be strongly correlated with biological activity. Lastly, given that structure-based methods require full atomistic representation of the biological target, calculations can be very intensive. This means that structure-based screening of many millions of chemical compounds is only plausible with access to significant high-powered computing,
26 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
Figure 2. Computational chemistry tools can represent the binding contacts between a protein active site and an inhibitor.
Figure 3. Docking ligands into a protein active site can easily be performed using computational chemistry software packages. The protein is shown here with a hydrophobic surface.
Drug Discovery, Development & Delivery
which reduces the accessibility of the virtual screening method to those with sufficient resources.
Ligand-based Virtual Screening Using Molecular Similarity
Ligand-based virtual screening offers an alternative approach for computational hit identification. In ligand-based screening, knowledge of the biological target is not required. Here, only a known active ligand is required, which is referred to as the ‘query’ compound. Ligands from a chemical database are then compared to the reference structure, to try and find hits which are similar (according to a certain metric or score) to the query ligand, and accordingly have a higher likelihood of being active at the target of interest. The assumption here is that molecules that are ‘similar’ should have similar biological activity.
Different methods use different definitions of similarity, from computing similarity in terms of the molecular graph (using fingerprints or the like), to using 3D properties such as shape and electrostatics. As protein-ligand binding is a 3D interaction, the expectation is that 3D metrics can capture more interesting details of molecular similarity and hence are attractive despite their increased computational cost.
Descriptors based on molecular interaction points, which are a simplified model of the molecular interaction potential, have been shown as extremely effective for quantifying molecular similarity. The 3D arrangement of certain modes of interaction, e.g., regions of positive and negative electrostatics, and regions of hydrophobicity,
are used to quantify molecular similarity, rather than molecular structure. This approach focusses on the important physical properties that contribute to ligand binding with the biological target and can generate high scoring molecules even if the molecular structure is very different. This facilitates identification of analogues with very different chemistries to the reference.
Ligand-based virtual screening with an effective similarity descriptor allows for very rapid exploration of many millions of chemical compounds, at a much lower computational cost than equivalent structure-based approaches. The technique may be further divided into two categories. The first is screening whole molecules, which aims to identify novel active compound analogues, with similar electrostatics


to the reference. The other approach involves screening for partial bioisosteric replacement, where only part of the refence molecule is searched against a database of fragments.
Often, bioisosteric replacement of just a small portion of a molecule can enhance the binding properties of the ligand with respect to the biological target. This approach often allows for significant gains in ligand chemistry during the screening experiment, while incurring a lower computational cost than full molecule screening, making it an attractive option for high-throughput workflows.
Establishing Virtual Screening as a Widespread Solution
Despite significant efforts to improve the accessibility of virtual screening in recent years, computational chemistry can never be recommended for use as a ‘black box’, meaning that the user will always need some theoretical knowledge to make informed decisions about the computational experiment. Though this may be daunting for some life scientists, this is paralleled by prior knowledge required to run an NMR experiment or a Suzuki cross-coupling reaction. There is, therefore, a lower limit on how far computational methods can be simplified to aid accessibility before the integrity of the experiment is compromised.
However, it is the job of scientific software developers and product managers to increase the usability of computational chemistry tools, such that barriers for new users are minimised. Computational chemistry has long been the domain of
INTERNATIONAL PHARMACEUTICAL INDUSTRY 27wwww.international-pharma.com
Figure 4. The electrostatics between a ligand and a protein can be calculated following a docking calculation and visualised using computational chemistry software packages, to help the prioritisation of the hits.
Figure 5. Partial bioisosteric replacement experiment imidazopyridine group in 1OIT cyclin-dependant kinase inhibitor compound. Results of a partial bioisosteric replacement experiment are shown overlayed on the reference, with electrostatic and hydrophobic interaction positions shown as points of molecular interaction.
Drug Discovery, Development & Delivery
expert computer users, who interact with complex codes using nothing more than a black-and-white computer terminal. Development of easy-to-use graphical user interfaces along with extensive user training and support is slowly changing this perception, with computational chemistry tools now resembling everyday computer applications.
Despite the availability of open-source software, premium software products encourage user confidence, offering an intuitive experience so that a wide range of scientific staff can perform in silico virtual screening experiments, without needing an extensive computational chemistry background. With premium software products, the user feels that they are making informed decisions on the computational experiment, and, therefore, confident that they are gaining excellent scientific insight. This maximises return on investment for any organisation investing in premium in silico computational chemistry tools, and
ultimately improves efficiency of chemical discovery workflows globally.
Conclusion
Virtual screening offers significant improvements for hit identification by increasing time efficiency and cost effectiveness during the initial stages of a drug development project, compared to lab-based highthroughput screening. Virtual screening can be categorised as either structure-based or ligand-based.
In the structure-based approach, knowledge of the biological target is required such that prospective drug candidates can be screened against the receptor for their binding physics. In the ligand-based approach, knowledge of the biological target is not required and instead, compounds are assessed in terms of their similarity to a reference compound that is known to be active. Similarity can be effectively assessed using a molecular descriptor that is not based on 2D molecular structure, but


is instead a simplified interaction model of the ligand electrostatics and hydrophobics.
Virtual screening techniques are already widely adopted by computational chemists in modern drug discovery, but there are several benefits to increasing accessibility of virtual screening software such that drug discovery scientists with a wider range of backgrounds feel confident employing the techniques. Premium software solutions on the market today offer easy-to-use interfaces, such that the barrier to using virtual screening techniques for noncomputational chemists is significantly reduced.
In cases where expert use is still required, outsourcing to specialist computational chemistry CROs remains a popular choice.
Mark Mackey was one of the founders of Cresset when the company was formed in 2002. Appointed as CSO in 2013, he leads the scientific team and has made fundamental contributions to Cresset's underlying scientific approach and to the development of the software. Mark's previous experience includes roles at Napp Pharmaceuticals and Merck, Sharpe and Dohme. He obtained a PhD in Chemistry from the University of Cambridge and is a Fellow of the Royal Society of Chemistry.

Scott Midgley
Scott Midgley joined Cresset as a Postdoctoral Scientist in 2021 after completing his PhD in Theoretical Materials Chemistry at the University of Reading. His experience lies in classical and quantum mechanical modeling methods in photochemistry, thermodynamics of geochemical processes, and electronic properties of conductive materials. Since joining Cresset, Scott has applied his computational skills towards studying the behavior of ligands and proteins in drug design, including in software development and in consulting projects with Cresset Discovery.

28 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
Mark Mackey
SLP-HS Single Reagent Set II

Endotoxin detection methods that make lab technician’s lives easier. www.wakopyrostar.com ~ wkuspyrostarinfo@fujifilm.com FUJIFILM Wako Chemicals U.S.A. Corp. © FUJIFILM Wako Chemicals U.S.A. Corp. - 2022 © 2022 FUJIFILM Wako Chemicals USA Accurate and sensitive quantification of peptidoglycan(PG) and β-glucan with tube readers.
• Testing for microbial contamination in dialysate and its raw materials. • Detection of fungal compounds in pharmaceuticals, medical devices, biologics, and genetically engineered products. • Investigation of bacterial pollutants in commercial water. • Study of the structure-activity relationship, biosynthesis, metabolism, and etiological significance of PG.
The Pharma Drug Discovery Industry Should Look Towards Manufacturing as the Blueprint for Automation
Within the manufacturing sector – whether it’s transport, FMCGs, electronics or building materials – a modern approach to automation is already in place. Holistic, flexible and automated processes are being used to carry out a wide range of tasks like formulation, blending, packaging, and cleaning – and enabling them to take place all at the same time.
An industry that can also benefit from automation is pharmaceutical drug discovery. By implementing a similar approach into laboratory spaces as is already happening in manufacturing, it will be possible to increase efficiencies, and ensure that wide scale, effective automation is in place, and for the long term.
Automation is not a new concept for the pharma and drug discovery industries. In fact, it has been used to carry out tasks like library preparation or liquid handing for some time. The real opportunity comes from being able to apply it more flexibly and across entire workflows, as well as for more accurate data collection and analysis. However, change and advancements to date have often been a challenge and the industry has taken a fairly rigid approach to the use of automated technologies.
To free scientists’ time away from repetitive tasks and admin and give them more opportunity to work on projects that make the best use of their valuable skills, this modern approach to automation is key. With the continued advancement of technology, more complex processes and even full workflows can now benefit from being automated. This is now allowing scientists to advance work on plate reading and interpretation, for example, and ultimately bring much needed discoveries to market at a pace that has not been possible until now.
Putting the Focus on Flexibility
It is standard practice in manufacturing for a single tool to be optimised to perform multiple processes at the same time, in what is called a capability-first approach.
In contrast, the pharma and drug discovery industries often require one instrument to carry out many tasks without being truly optimised for the specific process.
By embracing this capability-first model, single automation tools in the lab will be able to perform multiple processes – such as liquid handing or thermocycling – more efficiently and enable instruments in the lab to work far more flexibly. The same piece of automation technology can be optimised to carry out new tasks when needed, enabling labs to meet the rising demand for new drugs and medicines. What’s more, as batch sizes become smaller and both product lifecycles and time to market both become shorter, increasing efficiencies and optimising any automation tools will be critical.
For example, when working with us, one biotech organisation realised that while an automated liquid handler alone worked in an accurate and repeatable way, it couldn’t support the lab to scale and increase throughput. This was partly because the physical lab space limited how many liquid handlers could be installed in the space, and because they were using a workflow design that meant processes must happen sequentially rather than in parallel.
The result was an expensive piece of equipment, like a liquid handler, becoming a hindrance in many lab setups. If, for example, the liquid handler is waiting on a plate reader or thermocycler to be able to carry out its role, looking at optimising when and how the handler is used can be a valuable way to keep efficiency up.
Connecting the Entire Workflow
The manufacturing industry has also stopped relying solely on workers to move parts between various automated systems and is now making use of technology to connect different parts of the production line. In comparison, lab spaces often have effective automated systems in place, but they stand alone in a ‘partial automation’ model. It’s rare to see a fully connected and automated workflow.
While partial automation does remove the repetitive tasks involved in one process,
many of the in-between steps such as barcode scanning still have to be done by lab workers – which can slow down the process. The benefits that automation brings are limited when only applied to one small part of the assay in this way, as it can result in reproducibility and analysis being more difficult.
Automating end to end laboratory workflows, on the other hand, integrates multiple processes and workflows, across both hardware and software. By using robotics to link each process, lab technicians can transform previously clunky, step-bystep systems into one continuous flow that increases both scale and precision. This type of continuous flow is also able to prevent the hold-up that can happen when only a small part of a system is automated and maximises the capabilities of equipment to produce high quality results without manual intervention.
Collaboration Between Science and Engineering
In order to bring this next generation of lab automation to reality, more than just the right technology is required. Collaboration between science and engineering will also be incredibly important, as scientists will only feel empowered to work with new technologies – and ultimately deliver better outcomes – if new ideas and approaches to automation are embraced by everyone.
Understandably, change can be challenging to implement as it requires a great deal of trust in the new tools, something especially true when it comes to technology that can accurately collect and analyse results of important trials without human intervention. This is partly because of scientists’ desire to move away from ‘black box’ approaches, and instead be able to see and understand every part of a process.
To overcome this, scientists and the engineers working on automation tools must work more closely together. For example, designing tools that take a human-first approach, and taking the time to understand exactly how scientists work and the type of support they need can both be effective ways of ensuring that products are effective,
30 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Drug Discovery, Development & Delivery
Drug Discovery, Development & Delivery
and drive earlier adoption. It's also about finding the right scientists that are open to new ways of working and look at lab processes in a holistic, end-to-end way.
For example, this was evident when the University Hospital Southampton NHS Trust began to implement automation tools as part of their COVID-19 saliva testing programme. The Trust found that having access to expert engineers was critical to be able to implement the technology effectively and at pace, and it has helped to build trust with scientists. As a result of strong buy-in from scientists, the Trust was able carry out up to tens of thousands of COVID-19 tests per day, and laboratory staff ran up to 40 robots at once to process tests, all from one iPad.
Optimising Data Collection and Analysis
As well as applying automation more flexibly and fostering stronger working relationships between scientists and engineers, the pharma and drug discovery
industry can learn a lesson about optimising data analysis from the manufacturing sector.
In both industries, collecting data is vital. In manufacturing, it helps to monitor and control costs and ensures the smooth operation of the plant. For the pharma industry, it is critical for better understanding the results of research and bringing new drugs to market successfully. In both cases, mistakes cannot be made.
With the application of automated tools, much of the manufacturing industry now benefits from more streamlined and accurate data collection. However, the pharma industry still often relies on manual data entry. This can become fragmented across teams and lead to human error or inefficiencies as time is taken up recording data. It is not uncommon for organisations to track progress and results of multiple trials on paper – and paper tracking is incredibly susceptible to manual error.
This is especially true during clinical trials, where up to ten trials can be running at once, or when hundreds of samples need to be managed per hour. In these circumstances, a single mistake can be costly and slow down the development of much needed drugs and treatments.
If the pharma industry embraced automated data collection, scientists would have access to richer and more accurate data and cut down the lengthy data analysis process that elongates the time it takes for the drug to get to market.
As well as cutting down these timelines, automation also ensures a level of repeatability and traceability, which gives lab technicians increased visibility to every step of the process. This enables them to eliminate potential mistakes and work more efficiently – much like the manufacturing industry when monitoring its operations to keep costs down.

Closing Thoughts
By looking at the great success that the manufacturing industry has had from applying automation flexibly, laboratories will be empowered to increase productivity, free up scientists to work on more complex tasks, and bring innovative drugs and treatments to market faster. The pharma and drug discovery industry are already embracing automation and robotics, but with the capability-first mindset used in manufacturing and a focus on optimising data collection, labs will be set to take automation to the next level.
Zoe Williams
Zoe is Head of Drug Discovery at Automata, with over 30 years' experience working in the life sciences industry. She brings strong and agile teams together to help provide labs with the technology they need to empower scientists in the pursuit of progress. Before joining Automata, Zoe worked for Pfizer in roles including account management, service re-design and programme management and for IQVIA as a Business Development Director delivering a breadth of solutions for clients, from optimising NHS patient care to developing strong business strategies in the biopharma space.

INTERNATIONAL PHARMACEUTICAL INDUSTRY 31wwww.international-pharma.com
Clinical and Medical Research
The Use of Predictive Analytics to Improve Quality in Clinical Trials Quality Assurance:
Independent or an Enabler – or Can it Be Both?
The impact of poor quality in a clinical trial, often discovered late in the process, can not only add costs to addressing the re-work necessary, but in some cases can lead to rejection from a regulatory authority. Using predictive analytics and scorecards throughout the lifecycle of a study can, in many instances, prevent a quality issue occurring or mitigate the impact in other cases. The need to apply this approach in Quality Assurance (QA) is becoming more important due to the increasing complexity of clinical research and the increased use of technology to capture patient data remotely, as is the case with decentralised and hybrid trials.
Quality Assurance – An Evolving Discipline
The traditional role of the QA department is to provide oversight of process and patient safety by way of audits. This approach involves an auditor looking at a sample of data in a process and making a judgement on if the process is robust and fit-forpurpose, or has gaps. The challenge with such an approach is that it is reactive and involves a deep assessment as to why the process isn’t working or why there was noncompliance to the process, otherwise known as Corrective Action Preventative Action, (CAPA). However, too often we see repeat CAPAs with the same root cause and the same preventative action, i.e. retraining.
However, similar to how technology and the insights we can gain from innovations is changing and evolving many areas across the clinical trial process, there is a real opportunity to develop new and innovative processes in the area of QA.
The role of QA departments is moving beyond audits at a given point in time, and building processes and procedures that will enhance the value it delivers on an ongoing basis. Predictive analytics is one of the key approaches that is enabling this expansion of how QA departments support the clinical trial process. This, coupled with the use of scorecards in the appropriate settings, can prevent serious data quality issues occurring in a study. In this article we’ll outline how using data analytics can deliver
greater value and driving an expansion of the QA department’s role.
What’s Enabling this Evolution?
Predictive analytics is an approach that examines data, trends and content to answer the question, “What is likely to happen?” based on the data trends we are seeing. It involves looking for patterns and trends in real time throughout the lifecycle of a clinical trial.
In the field of clinical trials, we already have a vast amount of data available to us from external audits, regulatory inspections and internal audits. Putting the findings into agreed consistent categories before data mining is a critical step in being able to refresh the data in real time. Following this step, we can examine the root cause of each category with an experienced expert and data analyst. This allows the QA department to direct the business in the correct areas that lead to repeat findings. For example, it may be a sub-optimal process that is the issue, or a repeat human error in what can be an over-complicated process.
The development of scorecards can also bring considerable insight to the process. They are the product of a snap assessment of a process (or multiple processes) embedded in a study, providing a “score” or colour code indicating if a process is under control or out of compliance, even before a study has begun.
The Benefits of Predictive Analytics and Scorecards
Our experience has shown a number of clear benefits from the use of predictive analytics, not least the ability to assess risks both before and during a study, thereby allowing us to combine initial, inherent and operational risks.
It has allowed for timely conversations with clinical trial team members in advance of a process being executed. The approach steers the team in the right direction, thereby avoiding the need to perform root cause analysis explaining why something went wrong.
With more and more hybrid studies being implemented, there is a great need
for early intervention of the QA department to evaluate the flow of data from the onset. Technology is and will continue to play a greater role in collecting patient data. The traditional role of the project manager has evolved, and now requires more technologyminded individuals who can identify the need for audit trails, identify the original source data and ensure that the data has not changed as it travelled throughout the various tools. Partnering with the QA department earlier will enable the project manager to implement controls, plans and enhanced communication plans across the study to ensure the data flow map is understood by all key stakeholders, with the critical end point data always as a priority. Ultimately, predictive analytics and early intervention provide a greater opportunity for clinical trial teams to implement a change to process at the right time, therefore preventing repeat errors occurring in the trial and potentially risking fatal errors being made that could impact the outcome of the entire study.
Embedding a QA Process Into the Clinical Trial Process
By way of example, let’s look at how this expanded QA process can be embedded into the overall clinical trial framework.
It is critical that the QA department and clinical trial teams understand respective responsibilities and collaborate from the outset to ensure reduction of quality errors on a study. The QA department then assesses and determines the categories that require more attention as the study is being set up. The study can be evaluated against key risk indicators such as phase of study, patient populations, and this can also include data sources to be collected and mode of collection. If a study is considered a high risk study or is planning accelerated approval, the approach of a scorecard can be applied. The auditors in the QA department will evaluate, via a snap assessment approach, the planned processes put in place against regulations and compliances to approved internal processes. Each process will be assigned a rating of red, amber or green, with details behind the score indicating where the process is at risk.
32 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
The clinical trial is then empowered with the data at the right time and is required to address the gaps/risks identified in real time. The QA department can and should, at a later stage, conduct the standard independent audits or inspection readiness as would be routine. The reduction of average critical or major findings per audit is a measure of how successful the team embraced the data provided.
Key Considerations When Using Predictive Analytics
There are a number of considerations that should be borne in mind when implementing QA processes using predictive analytics.
When building the QA process, after identifying the correct data points and root cause information, consideration needs to be given to whether there is enough data to accurately represent the relationship between root cause and the potential outcome, should no intervention be taken. Data should be refreshed regularly to ensure the risk profile is kept up to date. Internal data sources are best for internal root causes such as employee behaviour and inadequate processes, for example. External data sources should be used as
Clinical and Medical Research
Figure 1: Example of a QA scorecard

best-fit for risks driven by the external environment such as regulatory changes and new data sources e.g. remote patient monitoring or new technologies that enable decentralised trials.
In addition, a commitment to change management by the clinical trial teams is critical to them embracing the concept of “potential risk” and willing to invest time up front in the preventative activity. This speaks to the culture of quality within an organisation and whether it is willing to prevent rather than fix the issues, thereby embedding quality into the study design up front.
Conclusion
Predictive analytics and early intervention is becoming a necessity for QA departments. As the pressure continues to get much-needed drugs to the market to meet the needs of patients, there is a need for real time quality assurance support throughout the study to address/prevent any delays due to quality concerns. At this time of transformation in clinical development there is a significant opportunity to be innovative in the qualitative assurance approach to increase the quality and integrity of data throughout
the trial. This allows for more enhanced insights and more timely corrective actions and reduces waste in re-work at the end of a clinical trial. Becoming a partner to clinical trial teams by providing predictive data enables the QA department to both be an enabler of high quality while still maintaining diligent independent oversight.
Rose Kidd
In her role as Executive Vice President, Quality and Compliance at ICON, Rose leads an international team of experts who are specialised in Quality and Compliance. Rose provides leadership and strategic vision for the implementation and management of a quality and compliance infrastructure and a global Quality Management System at ICON. This involves proactive engagement with ICON operational service line leaders to advise and support on regulatory compliance and operational excellence.

INTERNATIONAL PHARMACEUTICAL INDUSTRY 33wwww.international-pharma.com
The Transformational Potential of Prioritising Data Today
Covid has spurred regulators towards a more agile and innovation-supporting future, paving the way for new opportunities for all. If life sciences companies embrace a data-driven approach to managing their product data now, they stand to reap the broadest benefits, including more robust and streamlined internal processes, supply continuity, and a level of transparency that really puts the patient first. Frits Stulp and Aida Demneri, partners at Deloitte, explore what’s possible.
In the light of the pandemic, there is a new sense of urgency within the EC and EMA around transforming approval processes so that expedited approvals don’t continue to rely on reviewers working overtime.
The need for lasting transformation isn’t just about approving new products more swiftly. The pandemic also highlighted entire populations’ vulnerability to breaks in supply chains and shortages of critical medicines, and indeed medical devices, and the need to be able to track and manage
inventories and stock movement across multiple geographies in ever more agile ways.
The answer to all of these real-world challenges lies in greater data centricity supported by the adoption of new technologies, and life sciences companies must play their own part in driving change. But this requires the right mind-set, and that conversations and plans are happening and being enacted at the right levels.
Looking Beyond the Guidance
Tracking the granular details of what EMA is or isn’t mandating at any given time can be a distraction, as companies develop and execute their own data-first strategies.
After all, the whole point of prioritising data over static documents is to transform what can be done with that information, over time and in all kinds of use cases. It also paves the way for information to be shared in different formats, while maintaining its integrity. (In the case of electronic patient information (ePI), the data might drive the population of HCP-/patient-friendly information through channels other than a paper insert, for instance.)
This has organisational implications, which companies could be tackling now. Should Regulatory Affairs or the company’s RIM lead automatically continue as the owner of the master data source, given that regulatory applications are just one aspect of the product lifecycle?
In the bigger picture – the vision WHO and its supporters have set out – standardised data sets, which can be understood by any stakeholder and any system across the international ecosystem, transform product traceability and transparency. They inspire confidence in all users that the information in front of them is the latest approved truth about a product and its status at any given time. This suggests a move away from running multiple, discrete systems each drawing from their own, slightly different data repositories.
Data Harmonisation is a Global Goal
There is another consideration for teams that have become too deeply preoccupied with the specifics of EMA’s IDMP implementation roadmap. Development of internationallyagreed standards to underpin data-driven processes elsewhere in the product lifecycle is on the cards, too. At the recent DIA 2022

34 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Technology
event, discussions included the evolution of shared plans by EMA, FDA and Japan’s PMDA to promote standardised data for CMC content, something akin to a global take on PQ/CMC. This would pave the way for one part of regulatory dossiers to be created and managed more dynamically, and for more than one region (potentially making lighter work of variations management, as just one potential use case).
It’s this kind of expanded vision for data use the major pharma companies are working towards today, appreciating that this is the scenario that everything is pointing towards (so to prepare for anything less would be unwise). And actually, adoption of data standardisation is already filtering down to a national authority level in some cases.
Devising an Enterprise Data Layer
Harnessing the fuller future potential involves creating a product data capability that transcends individual operational functions, teams and use cases. Ideally, it means creating a non-proprietary, crossorganisation data layer which is more likely to attract enterprise-level funding because this master data core will underpin the entire company, and a whole range of connected processes.
Another example of the difference this will make will be as the various authorities strive for greater harmony in their treatment of medicines and medical devices, and as companies are required to provide consistent data across both portfolios.
To maximise the value of today’s data investments over the long term, it makes sense that life sciences companies should adhere to the core standards being set out with ISO IDMP. But waiting for the day that individual variations of this have been finalised is futile, as this is a continuously evolving environment. Rather, futureproofing is about adhering to the agreed core, and tweaking as needed, following an agile approach to adding functions or amending features. And of course one of the advantages of creating a master data layer is that each set of adaptations, when they are needed, will only need to be made once, at the source.
Keeping the End Goal in Focus
The work that needs to happen now continues to be around data surveying, assessment, standardisation and enrichment. In time, the growing reliance on data – internally to the business, and externally in exchanges with regulators, supplychain partners, healthcare providers and
ultimately patients – will pave the way for process transformation. This will include growing degrees of automation (eg through structured authoring of routine documents, with minimal manual intervention).

It helps to approach all of this with the end goal in mind, which is about patient benefits, enabled by faster approvals, more accurate and timely medicines monitoring. In time, the ability to harness data to its fullest potential will allow life sciences companies to transform not only their own operations, but also their role within the Future of Health – perhaps via a keener focus on unmet medical needs, or by reducing the negative impact of ingredients and manufacturing processes on the planet.
Frits Stulp
Frits Stulp is Managing Director of Iperion, a Deloitte company, where he leads a team of regulatory/IDMP experts active in various projects to deliver value to both pharmaceutical companies as well as regulators. Iperion, now part of Deloitte, is a globally-operating life sciences consultancy firm which is paving the way to digital healthcare. Frits Stulp is also involved with CTADHL, as part of his efforts to support transatlantic data harmonisation based on IDMP.

Email:
Aida Demneri

Aida Demneri is a Partner in Deloitte’s Risk Advisory practice based in the Netherlands. She leads the European and the Netherlands’ Life Sciences and Health (including MedTech) Risk Advisory and Regulatory practice. With her team Aida works to help clients overcome challenges in their journey towards a responsible Future of Health.
Email:
INTERNATIONAL PHARMACEUTICAL INDUSTRY 35wwww.international-pharma.com Technology
frits.stulp@iperion.com Web: www.iperion.com
ademneri@deloitte.nl Web: www.deloitte.nl
Technology
New Technology and Automation in Labs: Data Risks and Privacy Compliance Issues
Within healthcare and life sciences, data science plays a major role in research and innovation. Improvements in computational speeds, storage capacity and connectivity across different platforms, systems and sampling equipment, mean that laboratories and research facilities are often the testing grounds for the use of advanced analytics including Big Data and Artificial Intelligence (AI).
Alongside the innovation and efficiency benefits of process automation, integrated workflow management, and collaborative research, comes increased exposure to data risks – some less obvious than others.
Often this is also compounded by competitive pressures and agile working methods, resulting in demands for upgrades to data solutions and IT systems that bring new functionality, quicker results or more efficient processes.
Such pressures can then put greater responsibility on those testing and approving these systems when it comes to identifying potential new data risks or privacy compliance issues.
Data Protection and Privacy Regulation
The strengthening of data protection and privacy laws such as the EU General Data Protection Regulation (EU GDPR), as well as sectorial obligations and targeted rules relating to the use of medical records, diversity indicators, genetic data, and human tissue samples etc. provide a complicated framework for compliance.
Often, they also provide the legal grounds for challenges, complaints, compensation and regulatory enforcement when things go wrong.
Under GDPR, there have been a number of high-profile investigations and enforcement actions involving data breaches or mishandling of data by pharmaceuticals and their respective technology and solution providers.
In the instance of a medical software solution provider, records of nearly half a million individuals were exposed during the migration from one software solution to another. The data protection authority raised a number of concerns, including the lack of data encryption and that there was no automatic deletion of data after the migration process had been completed.
The Cost of Non-compliance
Failure to properly manage data within the laboratory may have significant commercial implications when it comes to loss of intellectual property and delays to product development due to data quality issues. There are also indirect costs relating to reputational harm with investors, partners and professionals.
According to IBM’s 2022 Cost of a Data Breach Report,1 the pharmaceutical industry ranked third – behind healthcare and financial services – when it came to the highest average total cost of a data related breach.
The average cost for dealing with a data breach in pharmaceuticals currently stands at over USD $5 million, and over USD $10 million in healthcare. For the same period, the industrial industry including chemical, engineering and manufacturing, saw an increase to an average of nearly USD $4.5 million per breach.
Factors for these costs include the regulated nature of the sectors, the volume of confidential and sensitive data being processed, and the scale and nature of the technology and data services being used to support these industries.
Five Key Considerations When Undertaking Data Protection Impact Assessments
Data protection impact assessments are
essential to avoid falling foul of the regulation and paying the price for non-compliance.
Companies should pay particular attention when updating laboratory technology and automating laboratory processes, as there are many factors involved in identifying and addressing the associated data risks:
1) Extent of Data Collected
Firstly, it should be determined whether all of the data collected or held by the system is necessary to support the intended purposes.
Where possible, datasets should be anonymised or pseudonymised beforehand, or in some cases it might be preferable to use synthetic data (namely data that reflects the computational value of the dataset but does not refer to real individuals).
Note that even where data has been deidentified, it may be possible to re-identify the individual through other data sources held by the organisation or a third-party, therefore care should be taken to assess the risk prior to publishing or sharing even anonymised data sets.
2) Managing Data Access
Even where equipment is housed in a secure environment, systems and devices may still be accessible through a corporate network or via remote internet access.
Consider the minimum level of connectivity required to perform the tasks and ensure that physical and technical access is limited to authorised staff or representatives. Comprised user credentials remain a common cause of data breaches.
Maintaining logs of system access can also help to identify where access rights have not been updated or where access is attempted from unauthorised locations or devices.
In the case of a COVID-19 testing laboratory, the UK ICO noted that failures in data protection planning and poor cybersecurity controls led to test results being displayed on a website without

36 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
Technology
adequate user authentication. There have also been instances where clinical data has been discovered or accessed on unsecured cloud storage.
3) Using Third-party Solutions
It is also often the case that the delivery and maintenance of these systems are dependent on different technology vendors, service providers and their own technology partners.
Particular consideration should be given to situations where third parties may be hosting data or require physical or virtual access to systems, as vulnerabilities in third-party solutions and misconfigured cloud services are common causes for data breaches.
Consider how best to monitor ongoing vendor compliance and steps to mitigate risks involving access to sensitive and confidential data. From a contractual standpoint, it is important to address privacy and security obligations including responsibilities to report data breaches or issues impacting on the security, integrity and confidentiality of the data. The contract should also clarify in what circumstances the vendor may have access to confidential data and ownership of system generated data and logs which may be used to monitor and improve system performance, but which may contain identifiable personal data or information which is commercially sensitive.
4) Data Sharing
Opportunities to leverage Big Data and AI to radically improve research and development are often dependent on obtaining very large data sets. As a result, there are many commercial and social benefits to collaborating with other research grounds and potentially combining data sets from different sources.
Where data sharing is required between systems or between different organisations, consider not only the nature of the security controls, but also if applicable laws or commercial data sharing arrangements permit sharing and secondary use of the data, including whether appropriate disclosure notices, consent approvals and contractual measures are in place.
From a legal and regulatory standpoint, there may be additional requirements and considerations where data is stored or accessed from a third country, particularly
where it may not offer the same data protection laws and rights.
5) Data Retention and Deletion
Even where there is a commercial benefit from retaining data for future research, due consideration must be given to data retention and deletion practices.
Data protection rules generally require that data is only held for as long as is necessary to fulfill the original purposes for processing, and any secondary usage of data may require consent of the data subject or additional legal grounds.
There may also be regulatory or contractual requirements requiring data to be retained linked to the nature of the processing and type of data involved.
Alongside any system or process related retention schedule or policy, there needs to be clear operational responsibility, whether it is an automated or manual deletion. Responsibility around retention and deletion also extends to data that is held by third-party vendors and also to copies of data that may exist for business continuity purposes, audit and quality control review.
Mitigating Risk
Practical ways to address or mitigate data risks include undertaking a data protection impact assessment to confirm the nature and volume of the data sets which will be collected and processed and to identify any significant requirements or gaps in compliance with corporate policies and standards, as well as any specific considerations relevant to the nature of the system and process.
It is important that changes to these systems and processes are also reviewed to identify if they impact on risk or trigger additional regulatory or contractual requirements. This also applies to the handling of data and records during migration, when additional measures may be required to manage interim duplicate data sets or prevent data quality issues during the migration.
It is also important that staff are provided with appropriate training and guidance on the use of new systems and that any concerns or issues can be escalated during and after testing and deployment.
In some cases, new technology may also be the catalyst for significant changes to
ways of working, for example shifting from recording experiments through paper-based notation to using a laboratory notebook or structured workflow management tool. Staff may need to take time for adjustment in the same way in which a software solution is tested and refined.
When selecting a vendor or service provider, consider your priorities and the risks involved. Implementing next generation technology and functionality may be the priority, but there are also considerations around stability, out of the box readiness and interoperability with other systems and processes. If you require a reliable, secure and stable solution for critical data processing, then you also need a vendor and technology that is tried and tested for these purposes.
In conclusion, managing data risk around laboratory information management systems and other laboratory process automation services, needs to start at the inception phase with appropriate input across responsibilities for technology, cybersecurity, procurement, privacy and data governance.
Take the time to understand your technology and business needs, and with it the priorities, risk and challenges associated with such tasks.
Finally, the job does not stop with a successful deployment. Ongoing assurance monitoring of compliance and risk management, along with process improvement, are vital post-integration activities.
REFERENCES
1. IBM’s 2022 Cost of a Data Breach Report: https://www.ibm.com/uk-en/security/databreach
Robert Grosvenor
Robert Grosvenor is a Managing Director with Alvarez & Marsal's Disputes and Investigations practice in London. He brings more than 20 years of experience in advising on global and cross-border privacy, secrecy, records management and related data regulation requirements.

INTERNATIONAL PHARMACEUTICAL INDUSTRY 37wwww.international-pharma.com
Why the Right Approach to Data Management Will Enable the Potential of Small-molecule RNA Sodifiers as Drug Targets to be Fulfilled
Over the past 5 decades or so, the growing understanding that RNA can influence protein function through routes other than direct translation has opened the prospect of discovering small molecules for tackling diseases in novel ways. Using sources such as the UK Biobank, progress is now being made on using multi-omics data to derive mechanism of action (MoA) insights into the role of small molecules on RNA function, specifically the splice site selection process. However, such approaches are hindered by an approach to data management that is not designed to work with the large amount of interconnected, complex data in RNA splicing experiments which relies on analysing changes in the distribution of ranges of transcripts along the genomic coordinate axis, and thereby requires a flexible, scalable and shareable analysis platform. In this article, we describe how a vector-based database approach can allow data to be managed, interrogated, processed and shared much more effectively, offering a faster, less costly route to small-molecule insights, and hence future success in developing drugs for a range of conditions.
The Evolution in Drug Development
The approaches used for drug discovery have changed markedly over the last 50 years, as new enabling technologies have emerged and evolved. Prominent amongst these is the revolution in genomics and protein sequencing tools that enabled highthroughput screening of small-molecule libraries to become viable – and indeed this remains a widely used route today. However, powerful as this route is, it is time-consuming and resource-intensive, prompting scientists to develop new tools to speed up the identification of candidate drugs.
For example, advances in molecular modelling and computing power now enable in silico screening to play a major role in drug development, while improving capabilities in the analysis of ligand–protein

complexes by NMR and XRD have aided the development of fragment-based lead discovery. Above and beyond this, and drawing on an ever-growing portfolio of analytical and biotechnological methods, biologics are now playing a major role as therapeutics, with peptides, antibodies, nucleic acids, blood components and cell therapies all seeing success in recent years.
A Promising Paradigm –
RNA as a Drug Target
Joining this evolving portfolio of approaches to drug discovery is a paradigm – first suggested thirty years ago1 – that suggests that RNA structures could be targeted using small molecules, in a way that is analogous to the targeting of protein structures. Termed the ‘RNA revolution’,2 this approach has for a number of years been developed using antisense oligonucleotides and small interfering RNAs, which work by complementary base-pairing with the target and have helped researchers to identify therapeutically tractable target RNAs. However, small molecules are now receiving increased interest, thanks to their generally better pharmacological properties and easily tunable structures, but particularly because of their ability to ‘reprogram’ RNA processing.
This reprogramming capability opens the possibility of treating diseases in entirely new ways that overcome challenges associated with protein-based targets. Perhaps most importantly, by targeting RNAs directly, the activities of proteins that are difficult to drug, or prone to give rise to undesirable side-effects, could be modulated. This potential is already beginning to be realized, with the small-molecule drug risdiplam (Evrysdi™), developed by Roche/Genentech in collaboration with PTC Therapeutics, having received FDA approval in 2020 for the treatment of spinal muscular atrophy.3
Addressing the Challenge of Data Processing
There are two interconnected data analysis challenges in RNA targeted therapy development: 1. The identification of disease modifying intervention points in the RNA splicing process and, 2. Detecting alterations in splicing at genome scale both broadly
(selectivity) and specifically at the disease modifying intervention points. Developing RNA-targeting small molecules as drug targets first requires identification of areas on the mRNA molecule that are relevant to the disease or trait in question. This can be done using genome- or phenomewide association screening (GWAS and PheWAS) in conjunction with quantitative trait loci (QTL), to identify mutations in specific regions of mRNA that occur more frequently in people with that disease or trait, compared to those without it.
Mutations that occur in regions of precursor mRNA involved in the splicing process are of particular interest, because cellular differentiation is understood to result from restriction of transcript expression stemming from the coordinated retention of introns. For this reason, RNA mis-splicing is believed to be the root cause of an array of human diseases.4 It is known that mis-splicing occurs in specific cell types within tissues, which is essential information of MoA determination of interventions.
As a result, QTLs are seen as valuable for identifying splicing steps that may be useful to modulate. However, because of the amount of data involved, the standard approach up to now has been to select a small number of relevant genes for study, but this is inflexible because if it is decided to change them at a later date, then a new database must be created.
A more complete approach would be to use the full genome in the database, and
38 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Technology
this indeed has been done in a recent study used GTEx consortium data to look at eQTLs for 89 large GWAS studies.5 Such work has considerable potential, and the authors demonstrate that tissue specific information can be gleaned from the analyses. Moreover, a recent bioRxiv paper from Aviv Regev’s laboratory at the Broad Institute, shows that the methodology can further be refined to offer cell type specific eQTLs which provide detailed mechanistic insights into associations that are masked by bulk tissue analysis like the GTEx consortia’s.6
In Practice – The Challenge of Data Processing
The work by the GTEx consortium is an excellent starting point for understanding how QTLs could be used in the identification of new drugs. However, it does illustrate that the value of mechanism of action information in a national biobank data depends crucially on the database tools that are used to store and process it.
For example, current approaches in many genomic studies rely on file based systems with repetitive operations to extract, curate, transform and load data, that increases the time taken (and hence the computational costs) for every question asked. In addition, scientifically accepted tools are often extremely inefficient in analysing population scale datasets, making analysis of population scale datasets prohibitively expensive or simply impossible. Alongside these factors is a need to improve data management, using tools tailored for the purpose and designed to enhance the ability to collaborate across teams.
We think that a fundamentally different data storage model is needed to tackle these multiple challenges: one that dispenses with row wise indexing used by so-called ‘relational databases.’ We believe that complex, heterogeneous datasets should be arranged in multidimensional arrays, allowing data to be stored and accessed horizontally and vertically. This approach allows much greater flexibility in curating and accessing multi-attribute data across multiple datasets, including metadata such as ontologies and reference data. It also allows multiple queries to be run on multiple datasets at the same time, all in a secure environment.
To add functionality to this underlying database, rather than assembling (and needing to maintain) an ad-hoc group of open-source algorithms or analysis
packages, a suite of application programming interfaces (APIs) can be called into play, tailored to the requirements of the application. These provide purposebuilt data schema, interfaces, and taskrelevant functionality, using vocabulary appropriate to the application. To ensure ease of use by those without programming expertise, interfaces can be set up focused on achieving easy data visualisation and exploration by non-specialists, allowing multiple researchers to cross-analyse and share hypotheses that they’re working on.
Scaling-up analyses without experiencing a slow-down in responsiveness is now needed more than ever for genomics studies, and using the approach described here, we can readily apply it to the study of small-molecule RNA modifiers. To illustrate potential time and cost savings, we recently enabled a protein QTL to run 40 times faster than previously, while at the same time reducing computing costs 30-fold. In another case, a large GWAS computation involving 109 linear regressions was completed in just one hour, with a computing cost of less than $300.
The Future with an Abundance of Multi-omic Data
The abundance of multi-omics data now available means a new era in the development of small-molecule drugs that target RNA, rather than proteins directly. This approach has great potential for circumventing the limitations of proteinbased targets, but we believe it requires a new approach to data management.
Specifically, it is time to dispense with relational databases and the restrictions they impose, and instead use a vectorbased approach that allows disparate datasets to be stored in multidimensional arrays. This allows databases to be made ‘future-proof’, enabling a wide variety of queries to be run without the need for laborious reconfiguration, and the whole infrastructure to be readily scalable without incurring heavy computing costs. Enabled by and enhancing this novel database approach are ‘application programming interfaces’, allowing users to access and query data in an environment that doesn’t require familiarity with programming languages, while facilitating easy collaboration between geographically separated teams.
With such database systems in place, we think that future work in the world of smallmolecule targets for RNA should become
far easier than at present, enabling insights into new drugs based on this paradigm to emerge far more readily.
REFERENCES
1. L.S. Ratmeyer, R. Vinayak, G. Zon and W.D. Wilson, An ethidium analogue that binds with high specificity to a base-bulged duplex from the TAR RNA region of the HIV-I genome, Journal of Medicinal Chemistry, 1992, 35: 966–968, http://doi.org/10.1021/jm00083a024.
2. T.R. Cech and J.A. Steitz, The noncoding RNA revolution – Trashing old rules to forge new ones, Cell, 2014, 157: 77–94, http://doi. org/10.1016/j.cell.2014.03.008.

3. FDA approves oral treatment for spinal muscular atrophy [News release], US FDA, 2020, https://www.fda.gov/news-events/ press-announcements/fda-approves-oraltreatment-spinal-muscular-atrophy.
4. C.R. Neil, M.W. Seiler, D.J. Reynolds, F.H. Vaillancourt, P.G. Smith and A.A. Agrawal, Reprogramming RNA processing: An emerging therapeutic landscape, Trends in Pharmacological Sciences, 2022, 43: 437–454, https://doi.org/10.1016/j.tips.2022.02.011.
5. Barbeira, A.N., Bonazzola, R., Gamazon, E.R. et al. Exploiting the GTEx resources to decipher the mechanisms at GWAS loci. Genome Biol 22, 49 (2021). https://doi.org/10.1186/s13059020-02252-4
6. Identifying disease-critical cell types and cellular processes across the human body by integration of single-cell profiles and human genetics Karthik A. Jagadeesh, Kushal K. Dey, Daniel T. Montoro, Rahul Mohan, Steven Gazal, Jesse M. Engreitz, Ramnik J. Xavier, Alkes L. Price, Aviv RegevbioRxiv 2021.03.19.436212; doi: https://doi.org/10.1101/2021.03.19.436212
Zachary Pitluk
Zachary Pitluk, PhD., VP of Life Sciences at Paradigm4, has worked in sales and marketing for 23 years, from being a pharmaceutical representative for BMS to management roles in Life Science technology companies. Since 2003, his positions have included VP of Business Development at Gene Network Sciences and Chief Commercial Officer at Proveris Scientific. Zach has held academic positions at Yale University Department of Molecular Biophysics and Biochemistry (Assistant Research Scientist, NIH Postdoctoral Fellow and Graduate Student), and has been named as co-inventor on numerous patents.
INTERNATIONAL PHARMACEUTICAL INDUSTRY 39wwww.international-pharma.com Technology
Email: zpitluk@paradigm4.com
Handling with Care: Aseptic Dip Coating for Osteoporosis Treatment
Kindeva Drug Delivery is a contract development and manufacturing organisation (CDMO) specialising in complex drug formulation and delivery. Seeking to scale its production of coated transdermal devices for a biologic API, the company enlisted systems integrator Keller Technology to design, build and integrate an aseptic dip coating system. Because the application demands extreme precision, the solution would depend on a Stäubli robot.
In the effort to bring life-changing medicines to a highly regulated market, pharmaceutical manufacturers must maintain a delicate balance between volume, repeatability and sterility. Drug delivery systems like those developed by Kindeva are the culmination of a series of carefully coordinated steps, where the API meets the patient. Preserving the sterility and efficacy of the product, and ultimately the safety of the patient, is paramount.
With a nearly 175-year history, Kindeva has played a role in bringing hundreds of drug products to fruition. Among the Minnesotabased company’s innovations is a proprietary solid microstructured transdermal system (sMTS) platform. The microneedle-based device is highly patient-friendly, making it ideal for self-administering abaloparatide, a biologic that stimulates bone formation for postmenopausal women at high risk for bone fractures due to osteoporosis.
Since abaloparatide is a biologic API, it cannot be terminally sterilised. Therefore the processes involved in coating and packaging the abaloparatide-sMTS combination must take place entirely within an ISO Class 5 environment. In the lead-up to an NDA filing, Kindeva sought to reduce the product’s manufacturing cycle time. Only an automated system, capable of operating optimally within an aseptic isolator, could accomplish all of this.
The company turned to Keller Technology, a trusted partner with extensive experience in robotic applications for controlled environments in biotech and



pharmaceuticals, including combination products like Kindeva’s sMTS. It was up to Keller, as the systems integrator, to identify a key component: a robot that meets all of the customer’s specifications.
The Right Robot for a Sensitive Environment
Stäubli Robotics was the clear choice to complete the triumvirate of expertise required for the sterile coating and packaging system. The company has a strong record of innovation in the automation of life science applications in hygienic and aseptic environments. Its full Stericlean range of robots is designed specifically for sterile use.
A variety of features enable Stäubli’s Stericlean robots to operate in a Grade A environment and maintain high performance under strict aseptic conditions: A fully enclosed structure with special seals keeps airborne particles to a minimum. The robot’s completely smooth surface, protected by a high-resistance coating, eliminates retention
At the dipping station, the API is meticulously loaded onto the microneedles. A uniform coating on each unit results in high repeatability.
areas where antigens can proliferate. This design also enables the robot to withstand harsh VHP decontamination processes. All connections run through the base of the robot, safely outside the isolator.
Past experience was also a factor in choosing a robotics supplier. Keller had been integrating Stäubli robots successfully for years in various applications – including a Stericlean for a nearly identical sMTS application for Kindeva. They knew exactly what to expect: exceptional cleanliness, repeatability and accuracy. A Stericlean sixaxis robot, exceeding Kindeva’s requirements with an ISO Class 4 rating, was selected for integration into the system.
High Precision at Commercial Scale
The system Keller devised performs precision dip coating as well as primary packaging, all within an aseptic isolator. It begins when the sMTS devices are transferred into the isolator on trays, while the sterile liquid API is fed into a coating system, designed previously by Keller.

40 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Manufacturing Application Note
Kindeva’s proprietary solid microstructured transdermal system (sMTS) serves as an ideal delivery system for abaloparatide, a biologic API for the treatment of osteoporosis.
A Stäubli 6-axis Stericlean robot picks up one of the diminutive sMTS devices within the ISO 5 aseptic dip coating system built by Keller to scale production.
The robot uses a custom gripper to pick the sMTS from its plastic housing before taking it to the dip station, then returns it with pinpoint accuracy.
The Stericlean’s pinpoint accuracy is critical in the dip coating operation that follows. The dexterous robotic arm picks up the individual sMTS devices, each smaller than a postage stamp, and immerses them in the liquid API bath, loading the microneedles with the biologic API. The process is carefully calibrated to achieve a uniform coating on each unit, resulting in high repeatability.
The robot then lifts the coated sMTS out vertically, carefully places it back on the tray, and repeats the process at a constant speed. Once the tray is full, it is transferred to a tray sealer, also custom-designed and built by
Keller. The sealed trays are transferred out of the isolator, completing the sterile primary packaging operation.
The isolator itself is equipped with a monitoring system to ensure that no septic antigens are present. It also provides laminar airflow, so the entire body of air within the isolator is uniform in velocity and direction. Likewise all system components are designed to minimise airflow disruption – as exemplified by the sleek, fully enclosed structure of the Stericlean robot. This prevents disturbances such as eddies, voids and shadows that could retain antigens.
Dip Coating, Reinvented
What has traditionally been a slow and difficult manufacturing process – made all the more complex in pharmaceutical applications – is transformed by the inventiveness of Keller’s automated system, along with the precision and repeatability of the Stäubli robot.


Crucially, the risks that are inherent to exposing a biologic API such as abaloparatide during dip coating are eliminated. Keller engineered and integrated its customised system into an aseptic isolator to maintain sterile conditions and shield the product from contamination. The specialised design of the Stericlean robot brings added assurance. This protects the operators, the product, and ultimately the patient.
While careful precautions are taken at every step of the production process, the automated system also delivers the high speed and efficiency Kindeva needed to achieve its goal of reducing its abaloparatide-sMTS combination product’s manufacturing cycle time. Speed and efficiency gains, through automation, have also enabled the company to scale up the product for commercial manufacture. Further, the enhanced traceability provided by the robot’s control software optimises process control, which has the potential to bring long-term benefits for years to come.
Stäubli Robotics
Stäubli Robotics is a leading global player in robotics, consistently delivering engineering as effective and reliable as our service and support. A complete solutions provider for digitally networked production, Stäubli offers a broad range of 4- and 6-axis robots including robotic arms designed specifically for sensitive environments, autonomous mobile robots, driverless transport systems (AGVs) and cobots for human-robot collaboration.
INTERNATIONAL PHARMACEUTICAL INDUSTRY 41wwww.international-pharma.com Application Note Manufacturing
For more information please contact: Stäubli Robotics Cynthia Jamison-Brashier Marketing, Events Coordinator Robotics Division North America Phone: +1 864 764 4729 Email: c.jamison-brashier@staubli.com
The Staubli TX-40 Stericlean is rated for ISO 4 and GMP Grade A operations, and designed to withstand harsh VHP decontamination processes.
Keller’s smart design gives operators easy access using glove ports during production as well as for maintenance operations without compromising hygiene and safety.
Advances in Manufacturing and Processing Impacting Formulation Development
Achieving investigational new drug (IND) approval at an increasingly faster rate than competitor drugs is a widespread desire of biologics developers. This has been further amplified by the need for treatments and vaccines for the COVID-19 pandemic. As a result, the pressure to achieve speed in drug formulation development has escalated to new heights.
With research and development into new drug modalities and technological advances in manufacturing processes over the past decade, biologic drug formulation development requirements have evolved. These range from finding new formulation conditions for multiple new drug modalities to minimising the burden of treatment, easing patient administration, and ensuring device development for drug compatibility.
In this article, Heonchang Lim of Samsung Biologics, offers insight into the common hurdles that must be overcome in formulation development to meet these various requirements. Lim also explores the need for manufacturers to adopt strategies considering these challenges that will enable timelines to be reduced and essential drugs to be delivered to patients faster.
Tools to Assess Drug Formulation Feasibility

The formulation of biologic drugs, such as protein-based products like monoclonal antibodies (mAbs) is a difficult and timeconsuming process, in part due to the often-complex protein structure. The success of most biologics is dependent on the active form being delivered to its site of action. To achieve this goal, there are many characteristics of the drug that must be considered, including pharmacokinetics, toxicity, clinical indication, and physicochemical stability.
Prior to formulation development, verifying drug formulation feasibility will help developers to select an optimal
candidate in terms of developability among several molecular candidates. Formulation feasibility studies can be conducted using various in silico and in vitro assessment tools.
In silico assessments rely on data on the molecule’s amino acid sequence and structure and aim to minimise the risks in terms of the molecule’s stability. For mAbs, this could include evaluating the liability of the molecule’s complementaritydetermining region (CDR) using its protein sequence.
If utilising deep learning technologies for the prediction of a protein’s tertiary structure, in silico assessments can be used exclusively for feasibility studies. However, deep learning systems are at an early stage and may not be suitably effective for all analytical functions.
Therefore, in silico assessments will more commonly utilize SWISS-modelling-based structural predictions. These can be used to evaluate mAb formulation feasibility. However, the structure of proteins like bispecific antibodies (BsAbs) or Fc fusion proteins, including the folding and other aspects of their tertiary structure are generally too complex for SWISS-modelling methods. Therefore, most developability evaluations are confirmed using in vitro methods.
In vitro tools can be used to evaluate the molecule’s thermal and chemical stability using various techniques. For example, the stability of the drug can be determined using size-exclusion chromatography (SEC) analysis after high-temperature treatment in low pH conditions. At present, these in vitro analytical tools are generally more accurate in predicting the molecule’s stability and developability than in silico assessments.
Challenges in Formulation Development
During formulation development, feasibility studies can help select optimal candidates but there are still a number of challenges that can arise throughout the process. Expertise and experience are needed to identify these challenges early and to solve them with minimal disruption to the project.
• Visible Particle Issues
One of the biggest challenges that formulation developers will encounter is the need to overcome visible particle issues. This includes formulations having visible particulate, sub-visible particulate, turbidity, and opalescence. These phenomena can occur with various root causes such as aggregation or precipitation. Having particulate matter in drug formulations is a significant problem, as these particles can lead to severe side effects including inflammation issues following injection.
Manufacturers must actively monitor the formula to prevent particle issues to reduce the risk to patients as well as ensure compliance with relevant pharmacopeia, which will often state that drug formulations should essentially be free of particulate and outline the acceptance criteria of subvisible particles in terms of size.
In some instances when particle issues are detected during a screening step, there may be a need for the molecule to be reengineered in the discovery stage. In most cases, however, particle issues in drug products can be prevented by identifying optimal conditions (such as pH, buffer composition, and excipient condition) via screening studies.
• Missing Molecular Information
It is important to obtain as much information about the drug molecule as possible during a feasibility study, because understanding the molecular characteristics, such as the pI value, is critical in determining the optimal conditions such as pH or buffer constitutions for formulation.
Having the molecular characteristics readily accessible will prevent the need to conduct further screening activities and allow for more focus on finding the optimal solutions which will ultimately save time. It also means that developers are able to reduce the use of valuable drug substance material in these screening activities. If the information is available, communication between developers and those supporting
42 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Manufacturing
the formulation of the project will be essential.
• Determining the Optimal Conditions
It is a common misconception made by many developers that conducting a wider array of various excipient, pH, and buffer type screening studies will help achieve better results in formulation development. In fact, as pH and buffer conditions are typically within a certain range for biopharmaceutical drug products, these discursive studies aren’t always necessary. Studies that focus on narrowed-down, specific conditions are enough to find the optimal solutions.
Selection from a set of excipients, pH ranges, and buffers that are already widely used in similar biologic drug product manufacturing processes will often allow a company to find the optimal conditions and combinations more effectively. As the drug molecule’s characteristics will heavily determine the optimal conditions, this approach will also rely on understanding these properties, such as their glycosylation and molecular structure.
• The Need for In-process Controls
One of the most common missteps
that can occur during the formulation development process is making an error when making reagent or buffer stocks. As the stocks will be used to make many all subsequent reagents or buffers at that step of the process, these errors can lead to a large amount of time being lost and work having to be repeated.
Human errors can be easily made when working with chemicals with very similar names, for example, switching the amount of sodium phosphate monobasic and phosphate dibasic when making a buffer stock. If such errors are made and undetected and go through the manufacturing process for drug substance and drug product, in a worst-case scenario, all formulation materials will become unusable.
To prevent this from happening, inprocess controls (IPCs) must be conducted once the buffer or reagent is made. This could include measuring and recording the pH and conductivity of the stock upon completion to ensure it is within a set range. As errors are more likely to occur with under-trained employees, it is also important to rigorously train the employees to strictly adhere to all the necessary steps throughout the process.
Considerations When Scaling
With many challenges to overcome, it can be easy to forget to consider how scaling may impact the formulation processes, especially when moving from small scale to large scale. Mixing, filtration sizing, and compatibility in manufacturing scale-up processes are common stages in which issues can arise. For example, as the scale of the project increases, micro-filter size and pressure must be adjusted and optimised.
As a result, the conditions when scaling up should be considered from the start and throughout formulation development. It is therefore important to consider seeking support from manufacturers with experience and expertise working across a wide range of scales, that will be aware of these potential difficulties.
Achieving Faster Timelines
With an understanding of the challenges throughout formulation development and the methods that can be used to solve them, strategies can be designed aiming to achieve faster timelines to meet increasing demand.
One strategy that can be used to shorten the time taken for drug formulation is to unify processes from start to finish. This

INTERNATIONAL PHARMACEUTICAL INDUSTRY 43wwww.international-pharma.com Manufacturing
Manufacturing
will involve producing a standardised screening matrix and process for each type of molecule, including standardisation of the analytical tools used throughout screening steps.
Having a unified process can allow for more consistent formulation conditions to be determined and minimise risks from occurring when scaling up, as there will be confidence and experience with the standardised approach.
Ultimately, it is crucial to unify the process in a way that allows for tailoring to the molecule based on its characteristics. Utilising tools such as high throughput screening (HTS) systems that incorporate sample preparation and sample analysis to characterise the stability and activity of protein formulations will help to reduce timelines.
Adapting to New Drug Modalities
Advances and innovations in drug design and development have meant that formulation considerations must be made to a new wave of drug modalities, including Fc-fusion proteins, multi-specific antibodies, antibody-drug conjugates (ADCs), and gene therapy products such as mRNA vaccines. For example, formulation development for mRNA products must aim to ensure stability
is maintained when in deep freezing conditions, requiring carefully selected “anti-freeze” excipients.
Similarly, ADC and recombinant proteins formulation will benefit from the production of appropriate formulation matrix sets with HTS systems to reduce the development timeline.

Moving into an era focusing on these previously unfamiliar drug modalities, it will become of increasing importance to be supported by those with formulation expertise and experience identifying formulation challenges.
A Look to the Future
In the future, it is likely that the high demand for speed in formulation development will continue. As improvements in formulation techniques advance, there will also be higher expectations of the final product.
Viscosity control throughout the process will make way for high concentration (>100 mg/mL) and ultra-high concentration (>200 mg/mL) formulation development. In protein formulations that are highly concentrated, viscosity increases due to enhanced interaction between the molecules. This increase in viscosity could result in injectability and product quality
issues. Therefore, control of viscosity throughout formulation will become even more crucial.
In-depth studies on the delivery system will also become increasingly important with highly viscous drugs. Challenges surrounding the safety and convenience of the patient can arise, as injection of highly viscous drugs has been associated with cannulas being pushed out and syringes breaking if not designed carefully.
Key Lessons
Formulation development is associated with a wide array of challenges and hurdles that biologic drug developers must overcome to meet the demand for speed to market. Having a strong understanding of the characteristics and properties of the molecule is essential during formulation and will rely on strong communication with those manufacturing the product and carefully designed feasibility studies.
With new drug modalities on the horizon, support from dedicated contract development and manufacturing organisations (CDMOs) with extensive formulation experience will become increasingly important. With well-applied formulation strategies, these organisations can ease the process from start to finish, from small to large scale, while delivering speed to market without compromising quality.
Heonchang
Lim
Heonchang Lim has over 15 years of R&D experience in the biopharmaceutical industries, including the fields in protein drugs and vaccines. He leads Drug Product group in the CDO division at Samsung Biologics, a leading contract development and manufacturing organisation (CDMO). He successfully performed more than 30 formulation development, non-GMP DP manufacturing projects. Prior to joining Samsung Biologics in 2017, he was a scientist at LG Chem conducting R&D for purification and formulation development in several vaccine and protein drug projects. He acquired a master's degree of medicine from Seoul National University and Bachelor of molecular biology from Catholic University.

44 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
a


































INTERNATIONAL PHARMACEUTICAL INDUSTRY 45wwww.international-pharma.com KahleAutomation.com We’ve merged with BBS Automation Our ideas just got
whole lot brighter Announcing a partnership in automation equipment that brings a whole new light to medical and pharmaceutical device manufacturing. Visit www.KahleAutomation.com or contact Kahle@KahleAutomation.com U.S.A. | ITALY | CHINA Kahle® is dedicated to providing custom automation machinery solutions for the Medical Device, Pharmaceutical, and Healthcare Industries around the world.
Testing Excipients for 3D Printed Pharamceuticals
3D printing or additive manufacturing (AM) technology has considerable potential to more closely align tablet manufacture with modern requirements and is a growing area of focus for the pharmaceutical industry. A unique benefit is the ability to deliver customisation at marginal cost with printers easily switched from product to product simply by changing feedstock and loading the appropriate file. This flexibility makes 3D printing especially interesting for the manufacture of tablets with sophisticated geometries and delivery profiles, for personalised medicines, and for decentralised, ‘on-demand’ manufacture in inaccessible locations. The potential to use 3D printing to accelerate drug development is also considerable as evidenced by a Merck initiative to develop AM technology in the first instance for clinical trial tablet production.1 Savings in time and API requirements, along with the flexibility to easily modifiy parameters such as a dosage and release profile are all notable gains.
A key challenge for pharmaceutical manufacturers looking to exploit 3D printing technology is to determine requirements for excipients. In this article, we consider the role of powder rheometry within this context. Industries leading the way in powder-based AM have already established the importance of powder flowability and the relevance of dynamic powder properties. Here we consider the properties that can be usefully measured to identify excipients that will print well referencing a study carried out by researchers at Deakin University (Victoria, Australia).2
3D Printing Technologies for Drug Product Manufacture
Looking across the current 3D printing landscape for pharmaceuticals it is clear that multiple technologies may emerge as valuable. For example, the M3DIMaker platform (FabRx, UK) is a fused filament fabrication (FFF) desktop system designed for drug product manufacture at the point
of dispensing, in hospitals and pharmacies.3 This commercialised design enables both fused deposition modelling (FDM) and direct powder extrusion a novel technique that utilises a print head based on a single screw hot melt extruder. Direct powder extrusion eliminates the need to form a filament prior to printing thereby simplifying manufacture and addressing an important limitation of FDM for certain actives and excipients.4
Proof-of-concept and comparative studies from Merck, on the other hand, highlight the benefits of powder bed fusion, specifically selective laser sintering. These include the ability to cost-effectively produce tablets in substantial quantities that meet shape and surface roughness specifications, have satisfactory levels of mechanical variability and deliver uniform distribution of the active ingredient.1 Spritam, the only 3D printed drug to have achieved FDA approval is also manufactured using a powder-based processing technique, binder jetting. This technology is particularly useful for the production of highly porous and orodispersible products but is more generally suitable for high dose loadings and for more complex formulations.5
This brief survey points to the centrality of powder-based processes and by extension powder handling in the application of 3D printing in pharmaceutical manufacture. Learning how to formulate powders for optimal performance in the print environment is therefore vital if the industry

is to take full advantage of this innovative manufacturing technique.
Looking Inside the Printer: A Unique Environment for Powders. In AM processes powders are stored in and discharged from hoppers and other feeders, as in conventional unit operations but printing itself applies a specific and less frequently encountered environment.
Figure 1 shows the key features of a generic printer for binder jetting. The roller spreads powder dispensed onto the build plate to sequentially form uniformly packed, fine layers. The printer head then releases binder droplets to selectively bind areas of successive layers to form the desired product. Printing success, particularly speed and by extension throughput, is heavily reliant on the ability of the powder to flow and spread easily across the build platform and pack consistently within a layer that is typically just tens of microns in depth. Porosity or voidage in the deposited bed inhibits binding of the layers, increasing the likelihood of poor mechanical integity and non-uniformity in the finished product. Other powder bed fusion processes, notably selective laser sintering, are equally dependent on this core step of powder layer formation.
The preceding analysis highlights the conditions to which powders are subject within the printer and by extension the behaviours of interest when it comes

46 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Manufacturing
Figure 1: Schematic of a binder jetting printer
Manufacturing
to characterising powders for printing. Identifying appropriate powders for 3D printing calls for information on:
• The impact of consolidation – how does storage in a feeder or hopper influence flow properties? How easily will the powder discharge when subject to moderate compression under its own weight?

• Spreadability – how will the powder flow and spread under the low stress, unconfined thin layer flow conditions that prevail? And when subject to more forcing flow conditions at the roller face?
• Particle packing – will the powder particles pack closely to form a uniform bed?
In all powder bed fusion processes only a relatively small proportion of powder is bound into the emerging product making powder recycling critical for economic application. Powder stability is therefore an additional concern. Furthermore, if processes such as direct powder extrusion are established as part of the long-term mix of technologies for pharmaceutical 3D printing then powder behaviour under a broader set of conditions are of interest. In this process, formulations are subjected to relatively high compressive force within the confined environment of an extruder.
To maximise the potential of 3D printing formulators need access to information relevant to all these outlined processing environments. Particle characterisation has a role to play with both particle size and shape, for example, known to impact flowability and packing behaviour. However,
these behaviours are also influenced by many other parameters such as the porosity, stiffness, surface texture and electrostatic charge of the particles, and equally importantly, system variables such as the degree of aeration of the powder. Because of this complexity, reliably predicting bulk powder properties is not feasible. Indeed, even extrapolating powder properties measured under one set of conditions to predict behaviour under another is unreliable. The solution is to measure bulk powder properties under stress and flow regimes that are representative of those applied within the specific process.
The Benefits of Multi-faceted Powder Characterisation
Early adopters of AM, in sectors such as aerospace and automotive, have already amassed considerable learning about how best to characterise powders for powder bed fusion.6 Those leading the way incorporate both particle and bulk powder properties in feedstock specifications, an approach that pharmaceutical manufacturers can readily capitalise on. Multi-faceted powder characterisation based on the measurement of dynamic, shear and bulk properties such as bulk density, compressibility and permeability already has an established track record in AM.
Figure 2 shows a powder rheometer (FT4 Powder Rheometer®, Freeman Technology, Tewkesbury, UK) for the measurement of dynamic powder properties. Dynamic properties are quantified by measuring the axial and rotational forces acting on the blade, or impeller, of the instrument as it rotates along a prescribed path through the powder sample. A downward traverse of the blade pushes the powder against the confining based of the test vessel generating
values of Basic Flowability Energy (BFE). Here, the powder is under low stress but subject to forcing conditions. An upward traverse, in contrast, applies a lifting action producing Specific Energy (SE) values that reflect how the powder flows in a low stress state when subject to gravitational forces. Dynamic properties have been successfully correlated with print performance, but a defining attraction of the associated instrumentation is the complementary ability to measure:
• Shear properties – to investigate powder flow behaviour under moderate stress and assess hopper discharge performance.
• Bulk powder properties – to investigate packing behaviour via measurements of bulk density, susceptibility to compressive force via compressibility measurements, and the ease with which the powder release air via permeability measurements.
These capabilities allow formulators to implement repeatable, reproducible, and reliable multi-faceted powder testing across the entire stress and flow range associated with 3D printing. Repeat measurements of BFE enable an assessment of stability and flow energy can be measured as the powder is aerated to directly quantify correlations between degree of aeration and flow behaviour. Spreadability can be characterised at different speeds and shear rates by measuring flow energy at different blade speeds, and the effect of environmental conditions such as humidity can be quantified directly.
The following case study illustrates the application of this approach in the
INTERNATIONAL PHARMACEUTICAL INDUSTRY 47wwww.international-pharma.com
Figure 2: Instrumentation for dynamic testing with the versatility to also measure shear and bulk powder properties has proven value for 3D printing applications.
Table 1: Dynamic properties and compressibility data for a broad range of commercially available pharmaceutical excipients and blends.
selection of suitable excipients for 3D printing.
Case Study: Using Powder Testing to Screen Pharmaceutical Excipients for 3D Printing
Table 1 shows dynamic properties and compressibility data measured for a wide range of commercially available excipients and blends by researchers at Deakin University, using an FT4 Powder Rheometer. Ref 3 provides further details of excipient

product names and manufacturers. The measured properties were selected on the basis of their relevance to 3D printing applications, based on previous studies. Wetting characteristics were determined for powders with acceptable bulk powder properties via drop penetration, depth and spread experiments to form a refined selection for print trials with a binder jetting printer (Projet CJP 360 Plus 3D printer, 3D systems Inc, Rock Hill, USA). The overall aim of the project was to determine whether
powders with acceptable binder jetting printability could be identified on the basis of powder testing and wettability assessment.
The excipients can been grouped into three sets on the basis of BFE measurements and an important initial point to note is that these groups cannot be robustly differentiated on the basis of particle size alone. For example, the Dv50 values of powders with a BFE of between 100 and 250 mJ lie in the range 31 to 86µm but so
48 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Manufacturing





















INTERNATIONAL PHARMACEUTICAL INDUSTRY 49wwww.international-pharma.com // Flexibility is the key to successful pharma production – our CombiSys is perfectly in line with your needs. ///
Flexibility is your key to success
Do you want the flexibility to process a variety of packaging materials with just one platform?













The CombiSys transport system with grippers also gives you the ability to process a wide range of container sizes in a single production run without the need for changing size parts. With up to three dosing systems in one unit, CombiSys gives you maximum dosing flexibility. Get ready for the future today.

benefits

50 INTERNATIONAL PHARMACEUTICAL INDUSTRYBAUSCH+ STRÖBEL SE + Co. KG | 74532 Ilshofen | Germany
Your
+ Zero reject principle + 100 % IPC at full output + Re-capping ensures 100 % sealed containers + All-in-one platform for various packaging materials + RABS/isolator compatible + Resource-e icient parts management
Figure 3: Excipients can be screened for suitability for 3D printing on the basis of BFE and compressibility data, via comparison with commercial powders specified for the chosen printer. too do those of powders with a BFE in excess of 250 mJ.

Figure 3 shows BFE and compressibility data for the excipients along with analogous data generated for five commercially available binder jetting powders. This analysis identifies seven powder samples as clustering with the commercial powders with respect to flowability, as quantified by BFE, and compressibility. Low compressibility tends to indicate efficient particle packing since there is little scope for densely packed powders to reduce in volume upon application of a compressive force.
BFE values are also influenced by particle packing. Denser packing increases the efficiency with which force transmits through the bed as the rheometer blade advances
through the powder. In powders with low compressibility the flow zone associated with blade movement therefore tends to be large giving rise to relatively high BFE values. Powders with low compressibility in combination with low to moderate BFE values are therefore exhibiting relatively free-flowing behaviour and high packing efficiency, a beneficial combination for 3D printing.
A primary aim was to identify pharmaceutical powders similar to commercial powders for this printer with respect to BFE
and compressibility, to identify excipients with acceptable printability. Table 2 presents values for additional variables that have proven value for differentiating print performance – SE and Flow Rate Index (FRI), a parameter that quantifies how flow energy varies as a function of blade speed. FRI values were measured exclusively for excipients with a BFE in the range 100–250 mJ and it was found that all these powders met both the FRI and SE specification. On that basis, Lactohale, Pharmacel 101 and Pharmacel 102 were selected as core excipients for the development of blends for further screening and printing.
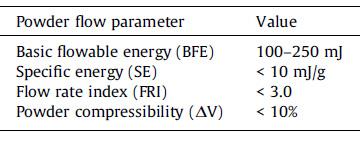

Figure 4 shows images of tablets printed using a subset of five excipients/excipient blends refined on the basis of wetting characteristics. The key point to note is that all five powders were successful with respect to acceptable flow through the printer and the production of printed constructs. This is a significant result. In the absence of relevant testing suitable 3D printing feedstocks can only be identified via print trials, a costly, time and material consuming process, particularly when the printer becomes blocked.
The dimensional accuracy and mechanical properties of the resulting tablets varied considerably with all the lactose-based formulations producing tablets with some type of defect. These results can be rationalised with reference to the compressibility of the formulations in combination with binder penetration characteristics. The PH101 and PH102 formulations produced highly friable tablets of low hardness but dimensional accuracy
INTERNATIONAL PHARMACEUTICAL INDUSTRY 51wwww.international-pharma.com Manufacturing
Figure
4: Images of printed constructs. The lactose formulated powders all have some type of defect either due to printing, inadequate formulation and/or powder processing
was much improved. Poor mechanical characteristics can be linked with the low porosity of the tablets.
This trial therefore suggests that though work remains to be done with respect to the improving the mechanical properties of the printed tablets the core principle of using bulk powder testing and wettability assessments to screen powders for 3D printing by binder jetting is sound. 3D printing is inherently different with respect to direct compaction in terms of the mechanical properties of the finished tablet; there remains much work to do to both rationalise and exploit this difference.
In Conclusion
3D printing has considerable potential for
the manufacture of pharmaceutical tablets and is closely aligned with efforts towards accelerating time to market, personalized medicine, decentralised production. Powder based AM technologies are a particularly fertile area for the industry given its powder handling expertise and the dominance of tablets as a drug delivery vehicle. Going forward powder bed fusion processes such as binder jetting and selective laser sintering look set to become important tools for the industry with the first approved 3D printed drug – a product made by binder jetting –already in use.

To use powder-based 3D printing technology effectively formulators need to be able to reliably measure powder properties that are relevant to the process
environment within the printer. Multi-faceted powder testing based on dynamic, shear and bulk powder property measurement has proven merit within this context that pharmaceutical manufacturers can draw on. The work presented here suggests that this process is already underway as the application of 3D printing becomes increasingly routine.
REFERENCES
1. C. Huls ‘Digitalizing the production of pills’ Presentation given at 3D Medical Printing Conference, Feb 2021. Available to view at: https://www.3dpharmaprintingconference. com/wp-content/uploads/2021/02/Merck_3DMedical-Printing-Conference-2021_C-Huels.pdf

2. A. Antic et al “Sreening pharmaceutical excipient powders for use in commercial 3D binder jetting printers” Advanced Powder Technology https://doi.org/10.1016/j. apt.2021.05.014
3. K. Sertoglu ‘FabRx launch the M3DIMAKER for personalized medicine 3D printing’ News Item, 3Dprintingindustry.com 14th April 2020: https://3dprintingindustry.com/news/fabrxlaunches-the-m3dimaker-for-personalizedmedicine-3d-printing-170695/
4. A. Goyanes et al. ‘Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process’ Int J of Pharmaceutics 567 (2019) 118471
5. Aprecia Zipdose Technology web page. Available to view at: https://www.aprecia.com/ technology/zipdose
6. Powder Flow Testing for Additive Manufacturing. Ebook available for download from: https:// www.freemantech.co.uk/learn/ebooks
Jamie Clayton
Jamie Clayton is Operations Director at powder characterisation company Freeman Technology, based at the company’s headquarters in Tewkesbury, UK. He graduated from University of Sheffield with a degree in Control Engineering and is responsible for overall management of company activities, including the R&D, production, sales and customer support teams. During his time with the company, Jamie has worked as a mentor with several academic groups and is an active member of ASTM F42. Jamie is also a regular contributor to conferences and workshops on the topic of powder rheology and works closely with clients on the application of the company’s technology.
52 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
Manufacturing
DropControl minimizes the spontaneous dropping of ophthalmic preparations


—
The newest developments of eye drop solutions are focused on non-water based solutions, which require a different design of the eye dropper to prevent unintended dropping.
Our solution is an insert into our existing eye dropper, which controls the flow of water-free eye drop solutions.

The DropControl fits our existing dropper bottle system A, E and F.

INTERNATIONAL PHARMACEUTICAL INDUSTRY 53wwww.international-pharma.com gerresheimer.com CPHI Frankfurt | November 1 – 3, 2022 | Frankfurt, Germany | Hall 3, booth 30B21
Flexible Manufacturing Environments are Vital for Today's Potent Oral Dose Forms
Driven by advances in oncology treatments and personalised precision medicine, two trends are converging to increasingly challenge pharma's developers and their external contract manufacturing suppliers: highly potent active pharmaceutical ingredients (HPAPIs) and small batch manufacturing.
Currently, approximately 25% of drugs on the market today contain HPAPIs.1 As of 2022, the global market for these drugs was worth $21 billion.2 Finished HPAPI drug products range from anti-cancer therapies containing small molecule cytotoxic compounds, to potent largemolecule bio logic compounds, to antibody drug conjugates (ADCs) and hormonal therapies.
Spending on cancer medicine, which includes small molecules, biologics, and emerging modalities, such as cell and gene therapies, rose to $164 billion globally in 2020 increasing at an average 14.3% over the past five years (2015 to 2020), according IQVIA.3
Even though IQVIA projects that the overall oncology market will slow slightly, with a compound annual growth rate projected at 8.7%, Market Data Forecast analysts predict the market for HPAPIs will climb to more than $31 billion by 2027.2 With this demand on the horizon, drug sponsors will increasingly be challenged to source effective specialist HPAPI manufacturing capabilities, as well as expertise to formulate and finish them into patient-ready drug products.3
Development of Biologic Molecules for Oral Drug Delivery
Oral biologics are another emerging market segment of highly potent API drugs requiring highly specialised development and manufacturing capabilities. Oral biologics already on the market mostly target chronic disease treatments to eliminate the need or provide a substitute for repeated, frequent injections. Targeted indications in this segment include diabetes (e.g., similitude), irritable bowel syndrome (e.g., linaclotide)
and diseases requiring immunosuppressants (e.g., cyclosporin A).
Even if at present, there is only a very limited number of drugs in the market, an estimated 4% of ongoing oral clinical trials are biologics, some of them potential blockbusters (e.g., oral insulin). There is still a way to go in these clinical trials and there is a risk that they will never come to market, as development trends and oral biologics begin to attract substantial investment. This said, should the efficacy of oral biologics in the medium term be proven in existing ongoing clinical trials these developers will have to secure the specialised capacity to manufacture these drug products safely and compliantly.

Tackling the Challenges of Small Batch Manufacturing Flexibly Manufacturing large and small molecule HPAPIs compliantly demands manufacturing under high-containment conditions and processing operational controls. Specialised operator training with the level of containment based on occupational exposure limits is another prerequisite. While HPAPIs have the potential to treat many medical conditions and therefore represent a lucrative business opportunity, sponsors with promising formulations can face significant challenges developing these innovative medicines and bringing them to market safely and cost effectively.
From ensuring the environmental health and safety (EHS) of operators to demonstrating to regulators the presence of adequate controls to mitigate crosscontamination risk, pharma manufacturers will always be challenged to deliver compliant HPAPI capacity cost effectively. Market trends for HPAPIs, notes IQVIA, suggest that CDMOs will be tasked with handling even more demand for HPAPI
manufacturing services in the near future. However emerging drug development trends are raising the bar on all manufacturers to deliver such high-demand capacity cost efficiently because HPAPI batch volumes are trending lower not higher.4
Trends in personalised medicine and patient centred drug design have turned pharmaceutical manufacturing economics and business models on their head. Drugs in the blockbuster era of development used to justify enormous capital investment into large-scale dedicated facilities. However, the rise in the number of orphan-drug approvals and an increase in the development of more targeted therapies with smaller patient populations is resulting in lower-volume drugs entering the market.4
The development of these and similar modalities will require strong adaptations by drug manufacturers, as it both affects economies of scale and containment controls at a given site. Small batch manufacturing implies exponential complexity at an operational level, to deal with multiple substances and products as well as the consequent need to shift production between batches. The emphasis on processing smaller batches more efficiently represents significant institutional change. In response the industry's contract manufacturers are investing heavily in engineering flexible small volume HPAPI operations with flexible facilities configured to meet the high demand for low volume batch manufacturing services.
Solubility Adding Formulation and Manufacturing Complexity
In formulation, highly potent compounds and similar drug substances need to address two pharmacokinetic problems: the ability to transit the gastro-intestinal tract and bioavailability. Due to advances in
54 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Manufacturing
Single Use Transfer Solutions

&
for
55
Providing purity
performance
your products www.thechargepoint.com Transfer powder ingredients economically, with a single use solution that delivers outstanding containment and sterility assurance performance.
combinatorial chemistry and high-throughput screening most of the HPAPIs in today's oncology drugs are poorly soluble. The implication is that these substances require either chemical modifications to increase stability, improved coating solutions, or other processing/manufacturing methods to optimise therapeutic effect and patient outcomes.
However, it is the compound that ultimately dictates the individualised solution. When it comes to contemporary OSD manufacturing capabilities, most leading providers are building on decades of experience overcoming the challenges relative to HPAPIs and controlling solubility. Equipment manufacturers are also contributing a great deal to the body of knowledge and experience to optimise the delivery of highly potent, poorly soluble compounds. The latest challenge for pharma manufacturers (internal and external), however, is how to reconcile the extra processing steps (cost and complexity) with economies of scale associated with small batch, low volume production scales.4
Optimising Poorly Soluble Compounds for Bioavailability and Dose Control
Controlling and optimising the release of poorly soluble HPAPIs pose a particular challenge to pharma manufacturers, often requiring specialised technologies, excipients and processing to overcome solubility and associated bioavailability challenges. Further complicating the issue is that no two APIs are alike; each compound or molecule has its own unique features that mean there is no one-size-fits-all technology or formulation approach to successfully accomplish solubility enhancement.
Nevertheless, in most cases, holding to the principles of Quality by Design (QbD) and Design of Experiments (DoE) still provides the most effective approach. Well-established contemporary Good Manufacturing Practice (cGMP) principles help to lay a systematic foundation for identifying the best most effective solubilisation and drug product delivery solution to ensure the commercial viability of the finished formulation.
Modifying the release of actives and controlling dose delivery to improve therapeutic performance and patient outcomes (e.g., dose compliance and side effects) has become a popular strategy to mitigate solubility issues. Controlling drug release during a defined period at a specific rate at targeted locations makes it
possible for the active substance to be more bioavailable once it arrives at the target site.
This is particularly relevant for targeted HPAPI oncology therapies. Controlledrelease formulations are typically achieved by utilising high-molecular-weight, watersoluble polymers to form hydrophilic matrix tablets or by film coating using predominantly water-insoluble polymers. The most important property when designing a controlled-release formulation is to ensure robust and accurate API release, particularly for those with a narrow window of absorption and/or therapeutic range.
Lower molecular weight polymers, on the other hand, are typically used to generate a more erosion-based drug-release profile for low-solubility drugs, whereas higher molecular weight polymers that swell more and afford a diffusion-based drug-release profile are paired with high-solubility drugs. The rate of API release from a sustainedrelease coating is modified by changing the ratio of the water-insoluble and watersoluble polymers in the formulation and the film coating thickness.
Bigger Efficiencies from Smaller Batches
Need to be Built-in
To achieve economies at small scale, flexibility must be "built" in, and integrated into a cohesive risk-based operational frame that drives efficient safe production regardless of batch size or neighbouring product programs. However, managing the
increased complexity of smaller batches in controlled environments will continue to require scrupulous attention to detail and continued technical innovation and investment. Contract manufacturers across the industry are investing heavily in process innovation to advance the quality and safety of HPAPI drug substance and drug product manufacturing at low-volume scale.4
Pharma manufacturers will need to integrate similar innovations to keep up with the demands HPAPI compounds place on processing, quality, and compliance. Information and analytical technologies, as well as digital controls and automation are prerequisites, but machine learning, artificial intelligence, and robotics all present opportunities to optimise small batch HPAPI drug processing and finished dose form manufacture.

With so much at stake, leveraging the endto-end capabilities of a specialist CDMO can be a route to accessing the small batch facility flexibility to manufacture HPAPI oral products reliably and cost efficiently. With better production and production data control, the better a facility and its operators can deal with product change-outs and in-between batches, manage product and process data and more efficiently and safely deliver finished HPAPI drug products to patients and markets.
REFERENCES
1. https://www.europeanpharmaceuticalreview. com/news/129187/research-finds-25-percentof-drugs-contain-highly-potent-compounds/
2. https://www.marketdataforecast.com/marketreports/high-potency-active-pharmaceuticalingredients-market
3. https://www.dcatvci.org/features/hpapimfg-the-market-drivers-and-cdmo-cmoexpansions/
4. https://www.dcatvci.org/features/industryperspectives-low-volume-drugs-andmanufacturing/
Stephane Guisado
Stephane is the site manager at Recipharm Leganés site and prior to this he was the site head at the Fontaine site. Stephane has worked at Recipharm since 2009 with a background in pharmaceutical manufacturing dating back to 1995.

56 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
Manufacturing
With our solution you will have IFU and warranty documents in all languages printed in one thin paper booklet – leading to less warehousing space, simplified packaging process and significant cost savings in logistics.
UNIQUE ALL-IN -ONE BOOKLET
This picture tells it all: One stitched booklet vs. four folded leafletsboth with a space of 1,2 m2.
• Thin paper really means thin paper! With our specialized printing technology, we can use paper grades from 29 g/m2 up to 40 g/m2

• Lower paper substance and caliper also mean less space and weighat. With the Booklet your package is lighter and smaller in size.
• All-in-one solution. All language versions of your IFU documents, warranty information and legal texts are printed in one Booklet that can be easily slipped into the product package.

• Streamlined packaging process: our Booklet works excellently on automated high-speed packaging lines.
• Only one stock item number for your Booklet – meaning less warehousing space, faster inventory cycle and no unnecessary reserves. The tied-up capital will be released for your other needs.
• Lighter and smaller package means significant savings in shipping costs and increased sustainability of your business.
Heikki Suomala

Sales & Marketing
Olli Kivijärvi Sales Manager

440 350269
YOUR THINPRINTING SPECIALIST
With almost 40 years of experience,
are one of Europe’s leading printing houses specialized in printing on thin and ultrathin paper.
use only high-quality, totally wood free thin print paper from reliable manufacturers in Finland,
solid logistics network, we guarantee fast and timely deliveries all over the world.
together with
we
We
and
our
Visit www.thinprinting.com
BENEFITS CONTACT
Director,
Tel. +358 440 350268 info@thinprinting.fi
Tel. +358
info@thinprinting.fi
Solid Form Services – Bridging the Gap Between Drug Substance and Drug Product
The solid form of a drug substance has a huge bearing on its formulation as a drug product. In an ideal world, every API would reliably form beautiful, homogeneous crystals on isolation that would be perfect for creating a dosage form with excellent bioavailability. But in the real world, that is unusual. Rather, a significant amount of work is commonly required to bridge the gap between the bulk API that comes out of synthesis and a solid form with all the attributes required by formulation scientists to make a good drug product.
That bridge is provided by experts in solid form services (SFS), who will look for a solid form that meets all the needs of the formulation team, but, crucially, one that can also be scaled up reliably to commercial scale. This will include its full characterisation, including an assessment of whether it has the solubility and bioavailability properties that are required.
The first step is likely to be a search for the most stable crystal polymorph of the API. Molecules can exist in multiple different crystal forms (see Figure 1). One of these is likely to be more thermodynamically stable than the others, and therefore this is by far the most advisable choice. Going with a metastable polymorph may cause
significant problems down the line if it interconverts into another form, leading to batch failure.
If this crystalline form meets all the solubility and bioavailability criteria, this would be ideal. But if it is insufficiently soluble, the next step would likely be the same as it would be were there no stable polymorph – to look for a salt or a cocrystal.
A salt would be the simplest option, and more than half of all marketed medicines are sold in salt form, ranging from NSAID Voltaren (diclofenac sodium) to erectile dysfunction med Viagra (sildenafil citrate). If the API has an ionisable group, then it can be paired with a suitable acidic or basic salt former, and the solubility, bioavailability and stability of the resulting salt crystals assessed to see if they are suitable for formulation.
For APIs with no ionisable groups, cocrystals are a viable option. These tend to be more stable than salts, and therefore might also be a good alternative if the salt form’s stability is poor. The difference between a salt and a cocrystal is charge transfer: salts are ionic (proton transfer), whereas cocrystals are a combination of two or more neutral species in a crystalline lattice. A small number of cocrystals are already on the market. Exemestane, a drug

used in the treatment of early breast cancer in women, is poorly water soluble. It has been formulated as a maleic acid cocrystal, with a polymer added to the dissolution medium to vastly improve dissolution compared to the parent drug.
Once the search for a lead crystalline form, either polymorph, salt and cocrystal, has been exhausted, whether because one cannot be found or because they do not have the right performance attributes, it may still be possible to formulate for oral delivery. While a crystalline form of a drug is preferable, if one is not available then alternate technologies such as amorphous spray dried dispersions can be a viable way to make oral formulations.
Working Through the Workflow
The standard workflow for a solid form screen of a drug would start with an extensive characterisation of the API, including assessing its solubility in various solvents, particularly those that are processrelevant and might be used in later stages of development (see Figure 2). With these data in hand, an extensive screen would be run to look for polymorphs, using different methods such as slurry crystallisation, cooling crystallisation and solvent/ antisolvent techniques. It is also common to look for polymorphs at both ambient and higher temperatures. The latter is useful if a higher temperature downstream processing
58 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Manufacturing
Figure 1: Possible solid forms of a drug
step is likely to be used, as it will assess whether a new polymorph might form on heating. The idea here is to work out whether another polymorph could appear at some point within the process-relevant space to avoid the potential for batch failure.
Extensive characterisation would be carried out on each of these polymorphs, followed by competitive slurry experiments to define the relationships between the different forms, assessing stability and which other polymorphs they have a tendency to convert into, if any. Ideally, one would want to progress the most thermodynamically stable polymorph rather than one that is metastable, as there is a risk that the latter might revert to the former down the line. This is even the case if the metastable form has the right properties for formulation, and the thermodynamically stable one does not. In this instance, it might be wise to look to an amorphous form instead, or even look to make a salt or cocrystal that might not have similar polymorphism issues.
If a stable polymorph cannot be found, the next step is usually to look for a salt or a cocrystal, depending on whether the molecule is acidic or basic. In silico calculations can be used to determine the pKa values, which will highlight whether a salt form would be feasible. A standard salt screen involves two to three different methods of crystal forming techniques coupled with eight to 10 counterions. All newly identified salt forms are fully characterised to see if any of them tick all the developability boxes.
There are many potential counterions to choose from. Typically, they are split into three categories: (a) Class 1 = unrestricted use; (b) Class 2 = low toxicity and good tolerability; (c) Class 3 = of potential interest,
but may have limited precedence or their own pharmacological activity. The list of counterions in Class 1, all of which the FDA deems approvable, is extensive, and it is unlikely that a suitable one will not be found in that list. Class 2 counterions are also likely to be approvable although FDA will require better definition. Those in Class 3 are unlikely to be used for a marketed drug, but it can be useful to include them in patent coverage to give more extensive IP protection.
If salts do not work, either because the properties are unsuitable or because the API has no ionizable functional groups, a cocrystal is another option in the drive for a crystalline form. However, despite their potential advantages, they remain unusual among marketed products. The screening process for a cocrystal is analogous to that for a salt.

While improving solubility is often the focus of solid form services, optimising the solid form can have other advantages. Studies have also been done on the drug pirfenidone which is too soluble, and by forming a cocrystal the dissolution can be slowed down.1
Solubility isn’t the only issue that can be addressed with a cocrystal, however. Perhaps the most notable is a combination heart drug, where the cocrystals comprise two different APIs in a single crystal: sacubitril and valsartan.2 In another example, ertugliflozin is formulated as a cocrystal with L-pyroglutamic acid to prevent the active from disintegrating, which it tends to do when formulated with standard excipients.3
Overall, the goal of a comprehensive solid form screening can be a combination of any of the following targets:
1. Finding a viable solid form.
2. Robustness of process, stability and testing.
3. Ensuring regulatory compliance.
4. Offering intellectual property protection.
Crystallisation Process Development
Whether a polymorph, a salt or a cocrystal is found to be the viable solid form, the process to make it must be scaled up reliably. If the first batch is polymorph A, the next batch is polymorph B and the one after that a mixture of the two, the resulting batch failure will not only be expensive, but a hindrance to what are often tight timelines in a drug development process. The crystallisation space must be tied down so that the preferred form is made every time.
Another important aspect of crystallisation process development concerns morphology and particle size. It is often possible to control particle morphology and size by changing the solvent system (key considerations include dielectric constant and hydrogen bonding capability of solvent), the crystallisation process (seeded vs unseeded, solvent/anti-solvent), or the process parameters for the crystallisation (rate of addition of solvent/anti-solvent or rate of heating/cooling). A more uniform particle size distribution is preferable to a very wide range of different sizes. Getting these process parameters correct in the beginning so that the target product profile is met can assist subsequent processes to run more smoothly, including filtration and drying.
It is not unusual for tweaks to be required to the crystallisation conditions as the scale increases. What starts out at a milligram scale in the lab will gradually increase in
INTERNATIONAL PHARMACEUTICAL INDUSTRY 59wwww.international-pharma.com Manufacturing
Figure
2:
Standard
workflow for
solid form
screening of
a
drug
Manufacturing
size, such as to 10g, then 100g, and so on into the kilo lab. Even when it is transferred to the manufacturing plant, further tweaks are likely to be required. However, by putting in all this work at a smaller scale it narrows down the process for the large scale crystallisation. Operators in the plant might have to tweak the rate of addition of an antisolvent, or the concentration, or even the quantity of seed crystals that is added, but these should be small changes, not a wholesale re-engineering of it. It should limit the number of experiments that have to be done on a plant scale to optimise the final process.
The Missing Link
There are occasions where, despite a comprehensive screening programme, a suitable solid form that meets all solubility and bioavailability requirements proves elusive. Typically, the next step in these cases is to investigate the possibility of other bioavailability enhancing techniques such as spray dried dispersions. In cases where an appropriate lead form is found, sustainment in bio-relevant media can be a problem that requires more formulation work in the presence of sustaining polymers.
Consequently, it is recommended that solid form screening is carried out in conjunction with these other bioavailability enhancing technologies, and these are evaluated in parallel under the same programme. This allows a seamless transition from drug substance synthesis to drug product formulation, rather than being a stand-alone operation, or tacked onto either the end of synthesis or before formulation. This is likely to be faster, not least because the process chemists will be thinking about optimising the solid form rather than simply delivering the correct molecule in any form. The drug formulation team will have a fairly good idea of how they are likely to be able to formulate the product, and what they will be looking for from the drug substance team. Solid form services is the link between the two, and also into the bioavailability enhancement team should further technologies be required. With all the disparate teams working together, a robust, scalable route from synthesis to drug product should be found more quickly.

Abhijeet Sinha
Abhijeet Sinha is a Senior Scientist in Solid Form Services at Lonza. He works on product development and internal R&D in preformulation, polymorph, salt and co-crystal screens. Abhijeet joined Lonza in 2020 from Kansas State University's Chemistry Department, where he served as a Senior Scientist and Director of the university's x-ray crystallography facility. His expertise includes molecular modeling, in silico screening of active pharmaceutical ingredients (APIs) and polymorphs, particle engineering, industrial crystallization, organic, inorganic chemistry, and x-ray crystallography. He earned his B.Sc. and M.Sc. degrees in Chemistry at Delhi University and received a Ph.D. in Inorganic Chemistry from Kansas State University. After graduation, Abhijeet completed 2 years of postdoctoral work at University College Cork mapping the solid-form landscape of APIs for the pharmaceutical industry.

60 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
REFERENCES 1. N. Kumari et al. Cryst. Growth Des. 2019, 19, 6482 2. M.A. Yousef et al. Cryst. Growth Des. 2019, 19, 7420 3. N.K. Duggirala et al. Cryst. Growth Des. 2020, 20, 617

INTERNATIONAL PHARMACEUTICAL INDUSTRY 61wwww.international-pharma.com ROBOTICS Discovering new pharma automation solutions Robots for Life Sterile or standard environments, high-end or routine tasks, Stäubli robots deliver clean, consistent performance ensuring the highest levels of product hygiene, safety, flexibility and productivity. Benefit from our know-how and discover the new automation possibilities of intelligent and safe robot technology. CPHI Frankfurt 1 – 3 November 2022 Hall 6, Booth 60C70 Stäubli – Experts in Man and Machine www.staubli.com Stäubli Faverges SCA, Tél. +33 (0)4 50 65 62 87, robot.sales@staubli.com
What’s in Store for Aseptic Processing Technologies in 2022 and Beyond?
By 2028, the global market for injectable drugs is set to reach $69.13 billion, growing at a CAGR of 8.9%.
There are many reasons for this boom in demand, from the drive to develop innovative treatments for rare diseases to heightened demand for faster vaccine rollouts.
However, as these drug products are injected directly into the body and therefore bypass the body’s natural defences, parenterals such as injectables and topicals used in ophthalmics require aseptic processing to ensure total sterility.
Aseptic processing is a fast-growing market, expected to reach 12.5 billion USD by 2027. Recent spikes in growth for this market are driven by the latest innovations in vaccine development for the COVID-19 pandemic, as well as the rise of biopharma treatments to manage serious chronic conditions.
As a result of this growth, there have been a number of innovations and techniques adopted to enhance efficiencies across aseptic processes.
The Aseptic Processing Technology Landscape in 2022
The legal requirements for aseptic product manufacturing have undergone many changes in recent years. As a result, containment technology experts have had to explore further innovations to ensure these regulations are met, resulting in rising growth of the aseptic technology market.
Aseptic procedures must follow longestablished strict guidelines to ensure the safety of all drug products. Most prominently, all cleanroom environments must adhere to Good Manufacturing Practice (GMP) guidelines for the manufacture of sterile products – meaning that all cleanroom spaces must be classified according to the required characteristics of the environment.
These environments must meet rigorous classifications to remain compliant with ISO14644, which stipulates that cleanroom spaces must be categorised as Grade A, B, C
or D. As a result, pharmaceutical technologies have been developed alongside these regulations to ensure safe and compliant aseptic processing techniques are followed across cleanrooms.
Alongside these well-established guidelines however, manufacturers must also ensure that their aseptic processes are aligned with any new updates that come into force.
In 2018, amendments to Annex 1 of the GMP were drafted, and are set to come into effect imminently. These revisions offer significant changes to the aseptic requirements across cleanroom spaces.
These amendments place a stronger emphasis on pharmaceutical companies to minimise the number of manual interventions across aseptic cleanroom processes as much as possible, while still maintaining optimal sterility.
As a result, this has further motivated technology manufacturers to ramp up their development of innovative new technologies to not only help boost efficiencies and productivity, but to also help their customers remain compliant.
These technologies have been designed to minimise manual interventions by allowing manufacturers to sidestep lengthy cleaning and validation procedures, resulting in less production downtime.
In previous versions of Annex 1, all connections for aseptic processes were required to be performed under highlyclassified Grade A environments.
However, some of the latest technological amendments take into account recent technological advancements being implemented across cleanrooms, making it possible in some cases to declassify cleanroom environments while still performing compliant aseptic manufacturing processes.
Limitations of Existing Aseptic Processing Technologies
Across sterile manufacturing environments, there are many potential sources of
contamination that can pose serious risks when it comes to drug manufacturing.
From the microbes and potential pathogens carried by human operators to the particles of other APIs, there are many potential contaminants that must be contained. If any were to make their way into the drug manufacturing areas and processes, they can pose a serious risk to the health and safety of patients taking these drug products.
To maintain high levels of sterility across cleanrooms and production lines, drug manufacturers are required to implement highly specialised equipment and infrastructures, as well as following stringent operating processes.
Achieving sterility across critical cleanroom areas and operations (e.g. fill/finish processes) has traditionally been done through the use of equipment such as restricted access barrier systems (RABS).
Manufacturers using RABS must ensure they have the right airflow measures in place, provide a physical barrier for operator interventions across critical zones, and have automated processes and procedures in place to minimise manual interventions as much as possible.
Isolator systems are also used across pharmaceutical cleanrooms to separate operators from the drug product during manual handling. These isolators help prevent any ingress of contaminants, as enclosures are accessed by operators via attached gloves to perform manual interventions, while remaining totally separate from the drug product material.
Alongside these systems, drug manufacturers must implement and follow regulatory guidelines for their cleanroom processes, stringent cleaning techniques and rigorous air classification management procedures.
While both of these systems are proven to be highly effective, they can be hugely expensive to acquire and install into cleanroom spaces. They also call for strict cleaning regimes to be carried out between each use, which can lead to significant
62 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Manufacturing
Manufacturing
production downtime and impact on overall productivity.
For isolators, operators are often faced with difficulties when transferring materials into and out of their chambers. When this happens, it may be necessary for operators to implement a docking isolator to aid the transfer process.
As well as incurring an additional step in the process, the sterilisation of the interior of the isolator may be carried out before any drug product materials can be transferred through. This, however, may not always be necessary as isolators can be decontaminated via automated processes.
When it comes to RABS equipment, there are further complexities that manufacturers must adhere to that cannot be achieved via automated processes. As all components that come into contact with the drug product material be steamed-in-place (SIP) prior to use, they must be cleaned and re-validated as soon as the door to the RABS is opened.
Manufacturers using RABS equipment must also ensure that their cleanrooms and gowning procedures are ISO 7 compliant, as their ability to open in the event that operator intervention is needed means they do not offer complete isolation from drug product materials.
The Latest Innovations in Aseptic Processing Technologies
Aseptic processing tech is evolving to keep up with Annex 1 and other changes in the aseptic landscape, helping to not only ensure compliance but also enhancing efficiencies while further minimising the potential for contamination.
One example of these advancements is the single use variant of the Split Butterfly Valve (SBV) – traditionally stainless steel devices consisting of two components –containing an ‘active’ and a ‘passive’ part which form a single ‘butterfly’ disk.
SBVs can help to reduce the need for pharma manufacturers to carry out additional processes within RABS equipment, such as manual cleaning and validation. This is because SBVs allow for decontamination within a closed environment, as they create an opening between the discs when the valve is sealed which enables a decontaminating gas to be flushed through. As a result, only the SBV component requires cleaning and validation, as opposed to the entire RAB system.
Now available as disposable components, SBVs connect to the container, process vessel, isolator or to a machine within a RABS aseptic filling line to allow drug product material to flow while preventing contact with the outside environment.
Single use aseptic components have become more prevalent across sterile processing environments, offering the same level of contained sterility before their disposal after use.
Single use charge bags are also increasingly used for the storage and transportation of drug product materials. These feature a valve which connects to the passive half of an SBV which transfers drug product material into the contained bag, preventing exposure to the outside environment.
These charge bags are designed for disposal after use, and much like the SBVs, they promise the uncompromised sterility and integrity of the drug product material.
Single use technologies such as these offer manufacturers greatly enhanced efficiencies and ease of use across their processes. Gamma sterilised before use, these components are pre-sterilised to ensure no contamination can occur before use.
Additionally, they can help companies remain compliant with Annex 1 by reducing the need for manual intervention required for cleaning and validation after each use. Provided that all other Annex 1 requirements are followed, drug manufacturers using single use components such as the SBV may be able to declassify their cleanroom environment while still performing compliant aseptic transfers.
Looking Ahead
In an industry as complex and dynamic as the pharmaceutical industry, advancements in aseptic processing technologies are everchanging.
Pharmaceutical companies must continually strive to explore the latest technologies that not only help them stay competitive, enhance efficiencies and streamline processes, but they must also search for equipment that helps them stay compliant with the latest regulatory updates.
Technologies such as RABs and Isolators have been used across cleanrooms for decades, and they are expected to remain
in place for years to come. However, these technologies have significant limitations, which is where newer innovations such as single use technologies come into play.
SUTs are increasingly popular across manufacturing spaces for the efficiency and productivity benefits they provide, and the latest updates to Annex 1 which recognise their benefits indicates that we’ll see them become a staple of standard cleanroom technologies.
As we look ahead to the future of aseptic processing technologies, we can also expect to see greater digitalisation across production lines. Smart factory technologies are increasingly being implemented, helping to automate manual procedures.
These are devices and components that can be implemented within existing manufacturing lines to help automate and monitor production lines. They can help alert operators to any potential breaches in containment or flag any maintenance needs before they become an issue, further optimising aseptic processes.
As these technologies can automate key processes, they help to reduce the amount of manual operator interventions required to a minimum, helping pharma companies ensure further compliance with Annex 1 sterility requirements.
Overall, it’s likely that the cleanrooms of the future will look drastically different from those that existed just a few years ago, helping to enhance the safety of drug products and boost compliance with existing and future regulations.
Ben Wylie
Ben Wylie is the Senior Product Manager at ChargePoint. He joined ChargePoint in 2005 and is responsible for the dayto-day and strategic management of the product portfolio, including the development of the ChargePoint single use solutions. Ben has 14 years of experience in Pharma with a focus on marketing and product management of powder processing and containment handling.

INTERNATIONAL PHARMACEUTICAL INDUSTRY 63wwww.international-pharma.com
Modular Cleanroom Technologies Have Always Been the Answer to Biopharma’s Global Growth and Flexibility Needs
Modular building technologies have well proven to meet the pharmaceutical industry’s demanding capacity and speed imperatives.
For the last three decades or more, biopharma’s growth has been nothing short of amazing. From life changing biologics and monoclonal antibodies (MAbs) to mRNA-based and now cell based immunotherapy medicines to fight viruses, cancers, and previously incurable diseases elevating biopharma’s position to even greater prominence. Regulatory guidance and approvals of Advanced Therapeutic Medicinal Products (ATMPs) also highlight the assimilation of these advancements in the biopharmaceutical sector.
From recent growth projections it is evident that the patient reward, demand, and investment in cell therapies will continue to fuel the continuing rapid global expansion of the biopharmaceutical industry. This will require the industry to quickly expand its manufacturing capacity in alignment with the advanced scientific achievements on the verge of a new wave of discovery.
Expanding ATMP capacity is a complex and expensive proposition, requiring enormous outlays of capital, resources and niche experience that can realise the requirements of the inherent novel manufacturing smallbatch process design methodologies. Although traditional biopharmaceutical manufacturing facilities have always been capital intensive to construct owing to the aseptic cleanroom sterile environments were intuitively rationalised by more efficient (and profitable) large-scale biologic manufacturing processes and their respective facility investments. There was a time when the business/financial case for a particular biologics facility and drug product would prioritise and rationalise a “speed to patient over expense” project strategy for new greenfield capital investments. Smaller-scale production requiring more nimble facility designs have shifted this economic paradigm requiring greater diligence to ensure long term value of significant capital outlays. New facilities
for highly specialised processes need to be designed and constructed in a manner that accommodates future novel and improved manufacturing processes resulting from a coming wave of therapeutic advances and expected capacity increases.
In 2020, the global biopharmaceuticals market was valued at some $291 billion, according to market research company NextMSC. Dramatic growth is expected note analysts who project the market to increase to nearly $1 trillion by 2030.1 Fuelling overall growth in the sector Visiongain projects the global ATMP market to reach $59.91 billion by 2030.2
Rapidly Deployed Flexible Manufacturing Capacity Needed Fast
Because of the global success of biologic products, industry data clearly shows that the demand for these aseptically processed therapeutics will continue to rise along with the corresponding increase in manufacturing capacity to serve growing patient populations, payer access, as well as the multi-decade appetite for venture capital investment. Biopharma’s response to this demand is drawing an astonishing amount of capital to the sector and with it, a call from the industry for millions of square feet of new, efficient cleanroom manufacturing space to produce the next generation of novel biologics, sophisticated cell based manufactured therapies.
With worldwide sales approaching $300 million per year and increasing at ~12% annually, BioPlan Associates’ technical research director Ronald A. Rader explains that growth in bioprocessing supplies and services will also likely keep pace noting that new capital investments in bioprocessing equipment is growing at 8.7% on average.2 He also noted the industry’s bioprocessing sector is extremely dynamic and will continue to expand in revenue, importance and diversity.
Industry analysts confirm that almost all biopharmaceutical and biotech firms outsource at least some of their cGMP manufacturing operational needs. For example, outsourcing final product container vial filling and finishing labelling
is a common strategic operational practice enabling capital investment risk mitigation. Following the growth of the biopharmaceutical sector, according to a report by Grand View Research, Inc. the global fillfinish pharmaceutical contract manufacturing market size is projected to grow at a compound annual growth rate (CAGR) of 6.1% and expand to reach $14.0 billion by 2030.3
In the early years, traditional biologic (aseptic) manufacturing processes (e.g. cell culture, fermentation) addressed large markets with the applied therapies. Product areas were dedicated, batches were large, equipment automation was applied, and yields were quick to improve.
In perspective, cell and gene cell therapies manufacturing process generally treat small patient populations, have manual aseptic production techniques that often induce proportionally greater facility design space and flexibility requirements including multi-product manufacturing. Also, these complex processes inherently have yield and scale challenges which impede efficiency and supply initiatives.
According to BioPlan’s survey respondents whose facilities perform cell and gene therapy bioprocessing, fear a “capacity crunch” similar to what mainstream bioprocessing feared at the turn of the century. Notable from BioPlan Associate’s market analysis was the fact that respondents were projecting the distribution of advanced-therapy capacity to be insufficient to meet coming demand for these specialised therapeutics.
When considering the noted operational methodology differences and their consequential space demands, the current capacity shortfall for cell and gene therapies is estimated at 5× or 500%. Rader explains that means five times current capacity would be in use if such capacity were available. Most developers, including leading contract development and manufacturing organisations, are working to catch up with early and mid-phase clinical capacity needs, and a few developers he notes have scaled up capacity for commercial and GMP manufacturing.2
64 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
Manufacturing
Your Drug Delivery Device Solutions Partner



Manufacturing
All injectable drugs have one thing in common; they must be sterile and they require the highest levels of operational and compliance excellence ensuring the same level of patient safety of established biologics products. Cell and gene small batch processing necessitate manual “unit” aseptic processing steps, therefore making it even more challenging for the demand of space applied toward people and materials flows as well as to induce meaningful physical separation to qualify sterile technique correlated to product safety.
Regulatory approved life-saving therapies, rapid advances in ATMP science, relatively small aseptic operations, multiproduct flexibility, and eager capital investors elucidate the current need for speedto-patient and the immediate need for manufacturing capacity.
Speed-to-patient Cleanroom Solutions Delivered to Your Door
Traditionally cleanroom solutions were defined, designed and constructed onsite over long end-to-end serial project timelines. These solutions not only negate speed-to-patient accelerated FDA approval structures (e.g., Breakthrough, Orphan Drug, Fastrack, PRIME), they also employ the use of outdated materials of construction and methods using stick-built wall-board construction never intended for an aseptic manufacturing environment. This conventional cleanroom methodology emanated from the biotech industry genesis era more than three decades ago.
Current cleanroom solutions have evolved rapidly in step with the advancing biopharmaceutical processing sciences. Modular cleanrooms solutions use fitfor-purpose robust materials to construct their wall & ceiling component systems that are manufactured off site under strict quality controls. The pre-manufactured modular approach enables an installation (or assembly) of these components in a parallel sequenced schedule activity format resulting in project completion reduction by months while reducing overall risk (e.g., cost, schedule, safety). And as with any technology, this kind of capital project’s value proposition has taken time to establish itself but, modular cleanroom solutions are now well aligned with industry and regulators’ quality and safety requirements.
Often, to deliver a defined, engineered commercial biopharma cleanroom space to facility owners, the project would
traditionally assemble a list of related contractors and technology integrators to deliver the cleanroom space. Depending on the project manager’s preferred approach and/or experience, such a list might include a myriad of different contractors possessing a wide range of experience and qualifications for a project. This is often considered the most highly regulated form of construction; cleanroom manufacturing facilities. This may include:
1. Flooring
2. Walls and ceiling
3. Glass curtains
4. Lighting, fixtures and controls
5. Heating ventilation and Air Conditioning (HVAC) ducting, hardware, controls
6. Fire suppression and safety systems
7. EHS and operator system
8. Shell and mechanical structural support design
9. Personnel egress/access systems (life safety)
10. Containment integrity and environmental monitoring systems
From this incomplete list, it becomes evident the exponential complexity in experience, resources, and risk in coordinating a fast-track multi-million-dollar project in managing contractors, costs, and users. The probability of success is often low in achieving the project goals of budget, schedule, and quality.
The Future is Now for Pre-engineered Modular Cleanrooms
Modular cleanroom solutions offer significant proven advantages over the conventional methodology by consolidating accountability, leverage expertise, increase lower cost risk, vastly improving quality, and expediting completion delivery. If need is the mother of invention, then the modular cleanroom approach has been specifically developed to address the specific challenges and drivers necessitated by complex biopharma projects. By current compliance standards, the component materials, fabrication, and installation methods are superior. (Relatively speaking an apples to oranges comparison.)
This engineering ethos continues to grow in popularity because of modularity’s inherent efficiencies. Further, many plant operators understand one key benefit of having a single-source system supplier comes well after construction and commissioning.
A common complaint new plant operators often have is that once the A/E leaves the
building there is no one company on the hook to resolve “issues” with all the critical cleanroom subsystems. So, instead of a gang of minimally invested contractors and equipment suppliers integrating a cleanroom system on-site, the industry has access to a single source supplier capable of delivering a completely defined, modular turn-key system to the site. An experienced modular cleanroom provider can deliver the full breadth of services to complement their product offerings. These include full cleanroom engineering design A&E services, component design/fabrication and expert installation services. This is backed by the ability to assume the full design and installation of the ISO classified HVAC mechanical cleanroom systems. A reputable modular provider likely has experience with thousands of cleanroom projects, and guarantees their product and its performance, not to mention being contractually accountable to the client’s project schedule.
Pre-integrated Enabling Technologies
Designed to Define the Space
These drivers have led to significant innovation, primarily in the form of integrating a systems approach to modular cleanroom design and engineering. Biopharma understands the best way to implement high-value cleanrooms faster and with significantly less risk is to leverage the technology’s advanced modularity, and prefabricated components. Developed with standard modular factorymanufactured components, cleanroom systems are purpose-built to simplify implementation and integration of the sterile, aseptic manufacturing space biopharma needs to meet future SI capacity demand.
Currently, several key enabling technologies leveraging modular concepts and factory fabrication are available from modular cleanroom providers offering the flexibility and configurability to meet specific facility and product cleanroom requirements including:
• Prefabricated, sealed microbial resistant wall panels
• Walkable ceilings with fully integrated HVAC systems
• Specialty advanced integrated LED lighting and controls
• Multiple full panel and window product viewing options
• Preinstalled integrated electrical raceways and standardized utility connections
• A single-source system supplier and service provider responsible for the
66 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
Manufacturing
delivery and quality of the entire integrated cleanroom system
How Modularity Can Accelerate Biologics Facility Delivery Timelines
Modularity cleanrooms accelerate project schedules and lend itself to fundamental and superior capital investment benefits that traditional methods can’t accommodate. This includes a “showroom” interior fit and finish that has become the gold standard for CDMOs competing for very demanding biopharma clients. The industry’s modular cleanroom integrators understand critical coordination, from process systems to infrastructure tie-ins, is required to ensure there are no gaps in responsibility that can disrupt project timelines or operations later.
The modular cleanroom systems available today are engineered to accommodate virtually any commercially viable floor plan both horizontally and vertically. This approach is more flexible and cost-effective and offers higher quality at a competitive price. The concept also offers expedited validation timelines than traditional stickbuilt facilities through the early availability of commissioning documentation packages.
Modular configurable cleanroom systems can relieve project managers from the many “unpredictabilities” of project execution by directly sharing the risk with an experienced
provider. When executed properly, it’s a well proven fact that modular technology can shave months off construction timetables because it streamlines installation and minimises coordination with all other trades and contractors on site.
Although there are many factors to consider, the cost of a modular cleanroom capital projects is often comparable to traditional solutions. But if you consider the value proposition of quality, speed, and flexibility over a facility’s lifecycle the capital project benefits become self-evident.
All evidence suggests modular innovation is winning in the market. According to fact. MR analysts, the global modular cleanroom solutions market is set to experience rapid growth at a compound annual growth rate of more than 10% from 2020 to 2030. Further, they report advancements in cleanroom construction and the adoption of integrated IoT-based HVAC systems are also expanding the demand for modular cleanroom systems.5
Modularity’s Ongoing Value and Utility Ideal for Biopharma’s Expansion
It is clear that over the past 15 years or more, modular cleanroom facilities have become a key enabler in the safe and effective manufacture of biologic large-molecule drugs, and with each breakthrough, the

technology is proving how efficient the solution can be helping biopharma build out processing and manufacturing capacity in an economically sustainable way. With unprecedented pandemic response, COVID vaccine supply was vastly enabled within modular cleanroom manufacturing environments via the public-private partnership national project WarpSpeed (OWS). In a global crisis mode, modular cleanroom technologies have proven they can accelerate project timelines and exceed industry compliance standards.
Modular cleanroom deployment also delivers significant capital expense control and ongoing operational benefits at a competitive cost– the kind biopharma’s most innovative developers need to meet aggressive market objectives faster.
REFERENCES
1. https://www.statista.com/statistics/1293077/ global-biopharmaceuticals-market-size/
2. https://bioprocessintl.com/business/ economics/top-trends-in-biopharmaceuticalmanufacturing-2020/
3. https://www.bloomberg.com/press-releases/ 2022-07-05/fill-finish-pharmaceutical-contractmanufacturing-market-worth-14-0-billion-by2030-grand-view-research-inc
4. https://resources.workstationindustries.com/ blog/how-much-does-a-modular-clean-roomcost-per-square-foot#:~:text=The%20cost%20 of%20a%20modular,the%20controls%20 and%20specifications%20needed.
5. https://www.factmr.com/media-release/1823/ global-modular-cleanroom-solutions-market
Mitch Gonzales
As VP of Process Technology, Mitch is a newer member of the AES leadership team and brings three decades of experience working directly for large and small biopharmaceutical companies including Amgen, Merck, Novartis, and Baxter. He was VP of Manufacturing at Pfenex, a biosimilar company who recently launched a generic (Forteo®) osteoporosis multi use injection pen. Most recently, he comes to AES from Tocagen a gene therapy company in San Diego. He earned degrees in Mechanical Engineering Technology and Mathematics and holds certifications in Six Sigma Green Belt and Project Management.

INTERNATIONAL PHARMACEUTICAL INDUSTRY 67wwww.international-pharma.com
Note
PCI Pharma Services –Robotic Aseptic Processing
The biologic market has grown expediently over the past few years, driven not only by the pandemic but also by the increasing demand for innovative therapies, the continued rise in chronic disease areas such as oncology and the loss of patent exclusivity of leading biologic drugs. This has led to a rapid growth of aseptic processing which is expected to reach $24.36 billion by 2031, growing from $10.63 billion in 2020, witnessing a CAGR of 7.9% (2021–2031)1
With the pharmaceutical industry’s growing pipeline of biologics, the need for technically advanced manufacturing and specialised packaging support has grown considerably. Global contract development and manufacturing organisations (CDMOs) such as PCI Pharma Services are playing a vital role, stepping in to fulfil industry needs, providing the expert capabilities and scalable capacities for the development, manufacture, packaging and supply of these life-changing drug products for patients.
PCI Pharma Services – Acquire, Invest, Grow
In response to the evolving industry landscape and as part of PCI Pharma Services’ global strategy to increase our sterile fill-finish capabilities and help alleviate the worldwide capacity shortage for sterile drug manufacturing and packaging, we continue to invest and grow our service offering through acquisition and investment in state-of-the-art technology and our people.
In December 2021, PCI Pharma Services acquired Lyophilization Services of New England (LSNE), a premier CDMO headquartered in Bedford, New Hampshire. The acquisition was a key step for PCI, as LSNE expanded the breadth of our services as a global CDMO, building on our expertise in specialty manufacturing, clinical trial supply and pharmaceutical packaging. PCI now offers integrated large and small molecule solutions for our clinical and commercial clients, including global manufacturing capabilities in complex
formulations, high potency, sterile fill-finish, and lyophilization.
Lyophilization is a common commercially validated process used to address formulation stability challenges and reduce the complexity associated with cold chain logistics for biologics. The use of lyophilization for pharmaceutical drug products has grown over recent years with demand for this process due to increase as more biosimiliars and novel biologics are developed.
Meeting this growing demand, PCI Pharma Services has doubled our large scale lyophilization capacity at our Madison, Wisconsin facility providing a seamless solution to help our clients progress their drug products through the clinical cycle to commercialisation and product launch.
Further enhancing our integrated development to commercialisation sterile fill-finish offering, we have committed to over $100 million investment at our Bedford, New Hampshire campus. This investment will see new facilities, capabilities and increased capacity all using state-of-the-art isolator technology including high speed, large volume sterile fill-finish lines.
Aseptic Filling
Administered parenterally to patients, products such as vaccines and other biologics, require specific and unique production processes to optimise both particulate and bioburden control whilst ensuring uncompromised sterility throughout the manufacturing process to maximise patient safety. This has led to the development of aseptic transfer and containment methods to protect drug products from contamination for clinical and commercial scale sterile injectable products.
As a result, the industry is looking for advanced ways of improving efficiency, reducing cost and increasing sterility assurance, with robotic processing gaining huge popularity for the primary filling of Ready to Use (RTU) containers such as vials, syringes and cartridges. Robotic aseptic fillfinish technology and the use of isolatorbarrier systems are emerging as key to
keeping pace with these requirements, and PCI Pharma Services is leading the way by investing in this cutting-edge technology to further enhance our global sterile fill-finish operations.
Robotic Aseptic Platforms at PCI Pharma Services
Complementing our global sterile fillfinish and lyophilization manufacturing capabilities across North America and Europe, PCI Pharma Services has invested significantly in state-of-the-art robotic technology platforms at both our San Diego, US and Melbourne, Australia facilities. These technologies are positioned at locations where a high volume of early phase clinical trials take place and also allow our clients to benefit from the 43% tax rebate incentive for clinical trials conducted in Australia.
Utilising the latest advancements of the Microcell and SA25 technologies, we deliver flexible aseptic fill-finish solutions for both small and larger-scale production runs across a variety of dosage forms including vials, prefilled syringes and cartridges for use in auto injectors, helping to meet our clients’ needs delivering products to patients safely and efficiently. Our investment in multiple robotic technologies provides a scalable solution for our clients, meeting requirements from smaller Phase I trials through to commercialisation and beyond.
The Microcell platforms in San Diego and Australia offer fully automated, gloveless filling, performed through closed robotic isolator technology that provides both small batch flexibility and standardised manufacturing. The fast changeover and agility of the technologies make it suitable for the production of both personalised medicine batches and clinical trial supplies delivering true speed to patients. Superior drug product quality is assured through advanced automation, removing the need for operator intervention during the filling process and limiting product contact. Importantly, the Microcell technology can fill up to 1,200 units per batch with fill volumes ranging from 1.0–50mL.
Providing a scalable aseptic solution in support of clients progressing through the
68 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Manufacturing Application
clinical lifecycle towards commercialisation, the SA25 Aseptic Filling Workstation is a larger-scale, gloveless, isolator-based filling technology offering small and large scale batch production of up to 20,000 units, supporting fill volumes from 0.2–50mL. It provides flexible and standardised manufacturing, and the ability to fill multiple delivery device formats including vials, syringes and cartridges through an aseptic process.
For both the Microcell and SA25 technologies, precise, programmable robotic functions cover all aspects of the fill process, including isolator leakage tests, VHP sterilisation of the container closures, filling into the CCS of choice, capping and batch delivery. They are also compatible with ready-to-use (RTU) containers and closures, removing the container and closure preparation stage, aiding speed of delivery of a quality and sterility assured drug product.
Robotic Advantages for Our Clients Speed and Accuracy
Expediting the filling process with automation while also increasing accuracy, the robotic platforms with high-precision Dynamic Peristaltic Pumps ensure accurate fill volumes with minimal product losses. This is a significant benefit to our clients, who are often developing life-changing, high-value drug products with the need of progressing through the clinical pipeline with efficiency and speed. Minimising product losses not only conserves valuable drug product and therefore reduces cost, but ensures our clients clinical supply needs are met and key milestones are achieved as the product progresses towards commercialisation.
Quality Assurance and Sterility
With a robot performing the processes in a recipe driven, validated system and utilising single-use parts, pre-sterilised flow paths and ready-to-use (RTU) containers, multiple sources of risk are eliminated including; cross-contamination, human error, electromechanical filling and closure activity failures, environmental control failures, cleaning and set-up errors; and product loss.
Using press-fit vial closures with integrated rubber stoppers not only reduces the risk of particle contamination but also simplifies the manufacturing process with the press-fit closures being a one-step application that presses closures on top of the vials, versus the traditional two-
step process of stoppering and aluminium crimp capping. All this combined means our clients are able to move more rapidly through the clinical stages and provide safe, life-changing therapies to patients.
Flexibility
Robotic technologies are designed for maximum flexibility while maintaining high aseptic processing rates. Meeting the needs to fill many different products and process multiple projects with minimal changeover time between batches, provides ultimate savings in time allowing us to better support clients who have urgent drug product supply needs.
Seamless Sterile Solutions
Our clients have the aim of accelerating their sterile drug development lifecycle and seek readily available capacity, reducing time to clinic and ultimately commercial launch. They seek experienced partners able to meet their fill demands with state of the art technology to minimise the risk of failure particularly in the early-stage development of complex, high value products that by nature can be difficult to handle. Any failure during the aseptic fill-finish process can lead to product degradation affecting a drug’s efficacy and safety. The importance of mitigating risk cannot be overstated: failure in a batch can mean significant time to replace it at substantial cost, and may put the entire timeline in jeopardy, delaying clinical trials, launch plans, commercialisation and ultimately impacting patient lives.
The addition of these innovative robotic aseptic fill-finish platforms in our San Diego and Australian facilities enhances our renowned sterile and lyophilization capabilities across our global network. These advanced technologies not only expedite the filling process with automation but being able to pivot between filling different sterile medications into multiple dosage formats, brings even broader sterile fill-finish solutions to PCI clients across the entire drug product lifecycle bringing therapies to market with increased speed and safety.
Providing integrated end-to-end solutions and helping to reduce supply chain complexity and risk, our clients can also access our expert secondary packaging, labelling and distribution solutions available across our extensive global network, leveraging the benefits of with working with a single supplier. Combining our expertise in sterile fill-finish manufacturing with
specialist biologic packaging, labelling and cold chain distribution provides a valuable end-to-end solution, simplifying the supply chain while delivering time and cost efficiencies.
Future Outlook
As more new biologically derived therapies such as antibody, mRNA and SRNA treatments are discovered, the need for specialised sterile fill-finish capabilities and capacities will continue to increase. And, as the biopharmaceutical industry seeks to reduce the time to market for new therapies and lower the risk associated with high-cost product loss whilst increasing sterility and patient safety, robotic platforms will be a true differentiator, offering an additional level of assurance and delivering products to clinic as rapidly as possible without compromising drug product quality or patient safety.
REFERENCES
1. Aseptic Pharma Processing Market – Industry Analysis, Trends & Forecast 2031 | BIS Research Tom McGrath

Tom McGrath is currently the VP, Global Quality, Manufacturing at PCI Pharma Services, a pharmaceutical full service CDMO. Mr. McGrath has over 25 years of in drug development, quality, and regulatory roles, with the last decade+ focused on aseptically processed pharmaceuticals and medical devices. Prior to assuming his current role in 2014, he was Sr. Director of Quality for Aseptic Services at AMRI–Burlington(now Curia), where he led successful remediation efforts. Before that he held development and quality positions of increasing responsibility at AMAG Pharmaceuticals, CombinatoRx and Praecis Pharmaceuticals. Tom received his B.A. degree in Chemistry from The College of the Holy Cross.

INTERNATIONAL PHARMACEUTICAL INDUSTRY 69wwww.international-pharma.com Application Note Manufacturing
How Product Inspection Drives Quality in Liquid Pharmaceuticals
In liquid pharmaceuticals, the stakes could not be higher: the correct dosage and usage can literally be a matter of life and death. Marco Pelka, Market Manager PCE of Mettler-Toledo explains how manufacturers and packaging companies in this field can harness product inspection to ensure they produce high quality liquid pharmaceutical products.
As at the end of May 2022, more than 1.28 billion COVID-19 doses had been administered in Europe1 since this unprecedented immunisation campaign began in 2020. Both here and worldwide, there is no doubt that the pandemic vaccination programme has done much to raise the profile of research into, and deployment of liquid pharmaceutical drugs. The liquid pharmaceutical market has been growing due to the importance of biologics and cancer drugs that are produced more effectively in liquid form. Historically drugs were produced in solid form, which takes years of development to ensure shelf-life stable solid doses. As demand has pushed for faster-to-market drugs, manufacturers must keep pace with production methods to make that happen. The market has changed, and the production of the COVID-19 vaccine has been a catalyst for change in producing more liquid pharmaceuticals directly into bottles, vials and ampules as standard packaging formats.
Manufacturers in liquid pharma understand that they will face the most serious consequences if they get things wrong. If, for example, they provide a product with an incorrect dosage size, it could be fatal for a patient. The other main issue is counterfeit medicines. The US has addressed this in the Drug Supply Chain and Security Act (DSCSA) in order to provide measures to minimise counterfeiting, and most other territories follow this lead.
With these issues in mind, liquid pharma manufacturers must operate at the forefront of product quality standards, ensuring that their dosages are correct and safe to protect consumers and patients. To comply with DSCSA, the packaging, labelling and in-
pack product information must be accurate and up-to-date as well as be able to trace their products through manufacturing processes and the supply chain. Delivering transparency and proof of their legitimacy against counterfeits will help in securing safer pharmaceuticals.
Of course, these issues must be addressed while still operating efficiently, to get safe, costeffective liquid pharma products to market quickly. There can be no compromise on quality standards, and this means that product inspection solutions must be deployed in both manufacturing and packaging processes in order to follow Current Good Manufacturing Practice (CGMP) guidelines set by the US Food and Drug Administration (FDA). For manufacturers, there are also some very specific needs to address. As discussed below, these steps support quality assurance in liquid pharma packs:
1. Fill Level Control
The nature of liquid pharma products is that they must contain an exact amount of dosage, usually held in small, fragile packs such as glass vials and bottles. There is a lot that can go wrong here: the dosage amount might be under- or over-filled; the bottles themselves may not be completely uniform, with slight, but very important, fluctuations in tare weight; the size and shapes of these vessels makes them inherently prone to toppling over and breaking during processing.
Both the unfilled and filled weight of each bottle or vial might be extremely light, and therefore weighing systems must be capable of very sensitive and accurate weighing. Checkweighers using high-performance Electro-Magnetic Force Restoration (EMFR) load cells can meet the precision and accuracy required. Careful handling of this kind of fragile primary packaging, including sorting and rejection mechanisms, is a must to reduce the risk of downtime caused by broken or damaged vials. All of this is required to happen at standard production speeds.
The use of a checkweigher that is capable of these levels of precision and product handling delivers further benefits to liquid pharma manufacturers. It provides weighing
data that demonstrates the attainment of certified weighing standards required to support compliance with industry and government regulations. It also brings with it an economic argument, as well as a product quality argument. Accurate weighing that identifies over- and under-filling errors helps meet weights and measures compliance, reduces the costs involved in over-filling and product give-away, and lessens the potential for reputational damage – and the costs that extend from that – to the manufacturer.
2. Completeness Control
In liquid pharma, a product is only complete when its packaging includes instructions for use, most commonly in the form of a folded leaflet. The difference in weight between a pack with a leaflet and that of one without can be very subtle and weighing technology of great precision and sensitivity is required. Checkweighers are able to detect these slight variations in weight and can do so without disrupting production speeds. They can also feature a countercheck function, which checks rejected products to ensure that they have been correctly identified as off-weight.
As with fill levels, while the primary benefit is ensuring the safety of users, having the product inspection capability to identify and correct these kinds of issues before a product is distributed can save manufacturers a great deal in terms of cost and reputation through avoiding product recalls. It also supports them in meeting compliance requirements, such as those set by FDA 21 CFR Part 11, which requires an audit trail of changes to be created and stored electronically.
3. Serialisation
Serialisation is not just sensible but is a requirement. With each product assigned its own unique serial code there is now full track and trace of individual packs, from origin to point of sale. If a problem arises and a product recall is deemed necessary, serialization potentially enables more targeted recalling of affected products, rather than larger batches.
Serialisation also supports regulatory compliance, and in liquid pharma it can
70 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Packaging








INTERNATIONAL PHARMACEUTICAL INDUSTRY 71wwww.international-pharma.com CE-MARKED PRIVATE LABEL PRODUCTS FOR YOUR BRAND •EMOLLIENT SPRAY •ADHESIVE REMOVER SPRAY •BURN GEL www.aurenalabs.com • contact@aurenalabs.com LET’S COLLABORATE TO CREATE YOUR NEXT PRODUCT. CONTACT AURENA TODAY. •SPARKLING SALINE NASAL SPRAY •SALINE/SEAWATER NASAL SPRAY •EAR CLEANSING SPRAY •WOUND WASH SPRAY •EYE WASH SPRAY CONTRACT MANUFACTURING OF LIQUID DRUGS AND MEDICAL DEVICES •BAG-ON-VALVE •NASAL SPRAY PUMPS •DERMAL SPRAY PUMPS •AIRLESS SPRAY PACKAGING •PureHale® CONTRACT MANUFACTURING AND PRIVATE LABEL PRODUCTS
also play a specific role in combatting counterfeit medicines, giving assurance that a legitimate medication is being distributed. Packs of medicines bearing a unique and verifiable serial code bear a hallmark of quality, in that they immediately suggest legitimacy and responsibility on behalf of the pharma manufacturer. This impression of quality can be enhanced or degraded by the quality of the coding itself. Whether it is presented on a pre-printed label or printed directly onto the package, the code needs to be clear and legible, with no smearing or skewing.
With vision inspection systems, these issues come sharply into focus. They can read serial codes and verify them and identify those products with incorrect or poor-quality print. Liquid pharma products typically come in a variety of bottles or vials of differing shapes and sizes. Unfortunately, with these types of round containers, they may rotate on the conveyor line, and potentially obscure the visible printed codes. Serialisation equipment must be flexible in its ability to print and inspect these containers, and it may be desirable to choose a vision system that provides a complete 360-degree view of the product surface, so that the codes can be verified.
4. Aggregation
Aggregating products into larger batches makes supply chain logistics run more efficiently, with easier tracking of products. Aggregation is growing as a mandatory requirement in many countries (legislation specifically in the US is coming into effect in 2023) and is therefore necessary for compliance. Along with serialisation, it also combines to help in the fight against counterfeit medicines.
There are some technical challenges involved in aggregation, in particular, where unoriented products such as bottles, or vials are packed into a case and the codes are therefore difficult to read. Printed helper codes on the tops or bottoms of bottles can help to mitigate this situation. However, it is a challenge requiring co-ordination as this increases the number of steps on the production line requiring additional equipment.
It is important for liquid pharma manufacturers to make sure that their aggregation systems offer support for a variety of round containers, can inspect top or helper codes, and can verify small detail datamatrix codes. Advanced camera
technologies will offer either a fixed focal lens or liquid lens to accommodate inspection of various detailed datamatrix codes. In addition, because the serial and aggregation data need to be shared with the supply chain, it is critical that these systems can interface with Level 4 and Level 5 systems effectively.
5. Software Connectivity
It cannot be emphasised enough: software is crucial in accurate and effective serialisation and aggregation. The tracking of products, real-time process monitoring, and inventory management cannot happen without it. Software connectivity is a huge challenge, but addressing it is a must-have. By equipping product inspection systems with the right software connectivity, manufacturers can gain that real-time picture which will help to identify problems, and possibly prevent them from occurring in the first place.
Typically, liquid pharma manufacturers will have checkweighing (for fill-level and completeness checks), vision inspection and track and trace software within their product inspection regime. While these systems perform very different functions and record different data, they must provide robust user security and process monitoring, as well as the ability to connect and communicate with standard systems and processes already in place in the production line. With FDA CFR 21 Part 11 requirements in mind, they must also produce a local audit trail that records activity such as user logins and adjustments to machine set-up.
Conclusion
Quality in liquid pharma is a multifaceted aspiration, incorporating the manufacture of the medication itself, and
the processing and packaging stages that bring it into the supply chain. A robust quality assurance program has strong product inspection capabilities at its centre. Product inspection technology such as checkweighing, vision inspection and track and trace are the manufacturer’s friend in helping to check that products are safe, complete, compliant, and traceable through the increasingly digitalised and connected supply chain.
That they must do so without compromising the manufacturer’s speed to market, and cost-efficiency performance makes the challenge of quality management still greater. However, with the application of good planning and the right technology, it is a challenge that liquid pharma manufacturers can meet.


Marco Pelka
Marco Pelka is Head of Market Management for Mettler-Toledo PCE Track & Trace and Mark & Verify solutions and has been with the business since 2010 in various positions as a Trainee and Sales Support. Marco is a passionate expert in his industry, providing guidance for customers to be compliant with global Serialization & Aggregation regulations plus supporting them in their traceability journey.
72 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
REFERENCES 1. https://www.statista.com/topics/1764/globalpharmaceutical-industry/#dossierKeyfigures Packaging
The packaging makes a difference!
Hypodermic Needles in Hard Plastic Unit Packaging

Optimized packaging process
Smooth administrations
Secure connection to syringes

INTERNATIONAL PHARMACEUTICAL INDUSTRY 73wwww.international-pharma.com NIPRO PHARMAPACKAGING INTERNATIONAL Blokhuisstraat 42, 2800 Mechelen, Belgium | pharmapackaging@nipro-group.com | www.nipro-group.com
Hard plastic unit pack | Takes littles space | Supports compatibility with feeders/automated pick-and-place systems | Enables in-line product identification
Easy opening | Sharp lancet point | Excellent gliding performance | Tamper-proof label
Hub complies to ISO 80369-7
SMIs and Soft Mist Nasal Sprays – A Key Defence in the Fight Against the Next Pandemic?
The COVID-19 outbreak has provided a number of lessons for the pharmaceutical industry to consider for future pandemics. Wilbur de Kruijf at Recipharm argues that one key takeaway must be to change the way vaccines are administered. Could soft mist inhalers (SMIs), and soft mist nasal sprays in particular, hold the key to improved vaccine delivery?
Spotlighting the potential of messenger RNA (mRNA) vaccines to speed up vaccine development and the importance of inter-company partnerships, the COVID-19 pandemic has reinforced key learnings for the pharmaceutical industry.
Perhaps the most crucial lesson has been the value of collaboration with contract development and manufacturing organisations (CDMOs). With the formulation and upscaling support of these specialist partners, pharmaceutical companies had access to the infrastructure they needed to develop their vaccines at record speeds. They also benefited from the capacity to commercialise their products and deliver billions of doses, as well as expertise in solving cold-chain transport challenges. In addition, they had an insight into overcoming administration issues to minimise discomfort for patients.
With so many advances made in our efforts to tackle coronavirus, it is now time for us to look ahead, exploring what we can take away from the present to further improve our work developing vaccines to fight future pandemics. We need to consider how we can further streamline development, and how to improve commercialisation processes. Just as importantly, we need to think about how to enhance the administration of vaccines, perhaps without the need for patients to attend crowded vaccination centres.
Thanks to technological advances in recent years, one solution to this last challenge could be to use innovative soft mist inhalers (SMIs), and soft mist nasal spray device technology in particular, to
harness the useability benefits of nasal vaccine delivery.
The Advantages of Nasal Administration Parenteral administration has long been the standard approach when developing vaccines for two key reasons. First, formulation development is streamlined due to the enhanced bioavailability inherent to injected drugs. Second, efficacy is optimised as injection bypasses the defence mechanisms in the gastrointestinal tract that can damage the active ingredients within the vaccine.
Nevertheless, non-invasive administration routes are beginning to be explored for their ability to enhance the patient experience and potentially improve performance. A number of such routes have been investigated for delivery of biologics, including oral,1 buccal,2 transdermal,3 and inhalation,4 including nasal. 5
The nasal route in particular is rapidly establishing itself as a viable alternative. As recent studies suggest, mucosal delivery of vaccines provides a better, longer-lasting effect than the traditional injection route,6,7 and can trigger robust protective immune responses at the predominant sites of pathogen infection. Inducing adaptive immunity at mucosal sites, involving secretory antibody responses and tissue-resident T cells, can prevent an infection, particularly from coronavirus and influenza viruses, from establishing itself in the first place. Other approaches may simply reduce the severity of the infection and protect against the development of disease symptoms.
Nasal administration may also allow the volume of the formulation needed per patient to be considerably reduced, helping to make more doses available sooner in the event of a pandemic without the need to increase manufacturing capacity.
Developing a nasal vaccine offers advantages in the regulatory arena over injectables. As nasal products are noninvasive and allow for more targeted delivery compared with parenteral, they potentially offer reduced side effects and a faster response.
For all of these reasons, we are seeing significant growth in the nasal vaccine segment, which is forecast to grow in value from $122.287 million in 2021 to more than $250.91 million by the end of the decade.8
Unique Development Challenges
The benefits of nasal vaccine administration are clear. Nevertheless, there are challenges facing pharmaceutical companies seeking to develop them. Failure to deal with these issues could undermine future growth in the segment.
One problem that needs to be addressed is that biologics, including vaccines, are fragile and vulnerable to damage when dispensed from a nasal spray or from a nebuliser. A study from the University of Amsterdam demonstrated that lipid nanoparticles, such as those used in mRNA vaccine formulations, can be harmed when administered by an oral inhalation nebuliser.9
Another challenge unique to biologic formulations for nasal administration is that they are incompatible with benzalkonium chloride – a preservative commonly used in single and multi-dose nasal sprays –complicating the development and filling process.
Finally, dose volume and the area of the nasal cavity you are able to target is critical for the efficacy of a vaccine and other biologics. This can pose issues when selecting a delivery device for a nasal vaccine. Standard nasal sprays feature swirl nozzles, which have a typical spray cone angle of between 60 and 90 degrees. This is wider than desirable for a biologic, leading to the bulk of the formulation hitting the walls of the nose, rather than reaching deeper into the nasal cavity, with implications for dose uniformity and therapeutic effect.
A new generation of soft mist nasal sprays hold the key to overcoming these development obstacles to drive growth in the nasal vaccine segment.
The Rise of Nasal Sprays
The global inhalation market boasts a wide
74 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Packaging
Packaging
range of nasal spray devices, each with benefits that make them ideal for specific applications. However, many of these were not developed with the needs of biologic and vaccine formulations in mind, often requiring considerable customisation to the design to optimise their effectiveness.
A relative newcomer to the market, SMIs and soft mist nasal sprays offer considerable potential for addressing the delivery needs of nasal vaccines. SMIs are liquid inhalers capable of producing a slow-moving cloud of aerosolised drug formulation. As such, they can provide convenient, optimal drug delivery to the lungs or nasal cavity, while reducing the need for patient inspiratory effort and coordination – potentially optimising the therapeutic effect of a vaccine while being easy to self-administer.
These aren’t the only benefits of SMIs and nasal sprays that make them ideal for use in delivering vaccines and biologic formulations. Many of these devices feature a new generation of spray nozzles with the ability to produce a narrower spray cone that can allow the formulation to penetrate the nasal cavity to improve dose uniformity.
One example of this new generation allows the fine-tuning of the spray cone angle from 0 to 30 degrees. This delivers a higher dose in the upper turbinates and olfactory centres of the nasal cavity.10 Research shows that a soft mist nasal spray featuring such a nozzle can evenly distribute a soft mist into the nasal cavity, ensuring an effective and even dose11 capable of optimising the efficacy of a nasal vaccine.
Another benefit of these devices is their ability to dispense a sensitive vaccine or biologic product while minimising the degradation of lipid nanoparticles within the formulation. As a result, they can support the effectiveness of the product.
Many SMIs and nasal sprays are designed from a standardised device platform, which has the potential to streamline the development journey, the regulatory approval process and enhance the economic viability of nasal vaccine products. The device platform significantly reduces the need for customisation to the specific needs of a formulation, which eases the burden of regulatory approval for any new product.
For instance, there are soft mist nasal sprays being developed that combine a
multi-use device with a disposable syringe containing the drug formulation – this eliminates the need to design a complex new device, as well as the requirement for high-volume device production. The simplified design of SMI and nasal spray device platforms, combined with the ability to customise the spray cone, simplifies development, allowing projects to be completed more rapidly.
Innovations in unit dose nasal spray platforms mean that advanced devices feature a user-independent delivery mechanism and modular nozzle design. These can allow consistent and directed dose delivery tailorable to the specific needs of the formulation. Such devices meet the needs of both the emergency and non-emergency markets, overcoming device design issues that in the past have proven expensive for vaccine developers to overcome.
These are just some of the innovations in SMI device design that are helping to make nasal administration a potential route for future vaccine development. As a result, soft mist nasal sprays can help streamline development timeframes and enhance the efficiency of the manufacturing process. By making nasal vaccine delivery possible, SMIs and nasal sprays can go a long way towards making future vaccination programmes more convenient and efficient for healthcare providers, and more pleasant for patients too.
Device Innovation Make Nasal Vaccines the Solution to Fighting the Epidemics of the Future
It is impossible to know which pathogen will trigger the next pandemic, nor when and where it will happen. Nevertheless, we can be certain that we will face a new global outbreak sooner or later. By heeding the lessons of COVID-19 now, we can be confident we will be better prepared for whatever the future holds. One of the lessons from the present that we must act on is that we must change the way we develop, manufacture and deliver vaccines to help us launch global vaccination programmes more efficiently.
Harnessing alternative vaccination approaches, such as nasal administration, can help streamline these processes, but only if we use suitable device technology. SMIs and soft mist nasal sprays offer benefits that mean we can truly deliver on the potential of nasal vaccines, improving dosing precision, ensuring the
quality of even the most sensitive biologic formulations, while optimising device costefficiency. As a result, they can help support growth in the nasal vaccine market, and further speed up our response to the next global public health emergency.
REFERENCES
1. Vllasaliu D., Thanou M., Stolnik S., Fowler R. Recent advances in oral delivery of biologics: Nanomedicine and physical modes of delivery. Expert Opin. Drug Deliv. 2018
2. Montenegro-Nicolini M., Morales J.O. Overview and future potential of buccal mucoadhesive films as drug delivery systems for biologics. 2017
3. Morales J.O., Fathe K.R., Brunaugh A., Ferrati S., Li S., Montenegro-Nicolini M., Mousavikhamene Z., McConville J.T., Prausnitz M.R., Smyth H.D.C. Challenges and Future Prospects for the Delivery of Biologics: Oral Mucosal, Pulmonary, and Transdermal Routes. 2017
4. Ferrati S., Wu T., Kanapuram S.R., Smyth H.D. Dosing considerations for inhaled biologics. Int. J. Pharm. 2018
5. Rohrer J., Lupo N., Bernkop-Schnürch A. Advanced formulations for intranasal delivery of biologics. Int. J. Pharm. 2018
6. Mucosal vaccines — fortifying the frontiers, Ed C. Lavelle & Ross W. Ward, 2021
7. https://www.tandfonline.com/doi/abs/10.108 0/17425247.2020.1714589
8. https://www.databridgemarketresearch.com/ reports/global-nasal-spray-vaccine-market
9. Klein D, Poortinga A, Verhoeven F, Bonnet S, van Rijn C, ‘Degradation of lipid based drug delivery formulations during nebulization’, medRxiv, March 24 2021
10. https://www.sciencedirect.com/science/ article/pii/S2090123222001072
11. Mucosal vaccines — fortifying the frontiers, Ed C. Lavelle & Ross W. Ward, 2021
Wilbur de Kruijf
Wilbur is General Manager and Chief Technology Officer at Resyca, based in Enschede, The Netherlands. Wilbur has a background in medical product design and human factors engineering. Prior to joining Resyca, Wilbur held a role at Medspray for 15 years, developing the micro spray nozzles and aerosol devices based on that technology. Wilbur has experience working with design consultancy firms, developing a range of medical devices such as wheelchairs, hospital beds, x-ray scanners and drug delivery devices.

INTERNATIONAL PHARMACEUTICAL INDUSTRY 75wwww.international-pharma.com
Choosing and Developing User-friendly Osmotic Laxatives for a More Patient-centric Portfolio


At least 1 in 10 people worldwide suffer from constipation at some point in their lives.1,2 It affects people of all ages and has many causes. The symptoms of constipation include pain in the lower abdomen and irregular and painful bowel movements. Laxatives are often needed in addition to dietary changes to treat constipation. There are many laxatives to choose from, each with different mechanisms of action and, consequently, different advantages and disadvantages. Here we look at the important role of osmotic laxatives, how they overcome many of the side effects and drawbacks of other constipation treatments, and the manufacturing expertise needed to make user-friendly laxatives part of a patient-centric product portfolio.
Constipation: Prevalence, Causes and Treatment
Constipation affects many of us, but it is more common in older people, women and children.2 The prevalence of constipation

increases with age because of factors such as medicine use, underlying disease, change in drinking and diet habits, weakened pelvic floor muscles and long-term hospitalisation or institutionalisation. Women are twice as likely to be affected by constipation as men, especially during pregnancy where hormones influence bowel muscle movement.2,3
For most people, constipation is caused by not drinking enough water or not eating enough fiber, but it is also a common side effect of taking certain medicines (e.g., opioids and diuretics), and can be associated with conditions such as anxiety or depression and gastrointestinal disease.
Some people experience acute constipation, which lasts for only a few hours and often results from lifestyle changes, such as travel or stress. For others, constipation becomes a chronic condition (obstipation) where symptoms persist for more than three months and have a significant impact on quality of life. They may not be able to pass stools at all, or they may pass stools that are hard, cause pain and lead to secondary
issues such as anal tears (fissures) or even fainting or heart attacks in the elderly.2
Treatment for adults usually involves increasing water and fiber intake as well as upping exercise levels, and laxatives may be offered if symptoms don’t improve. In children, laxatives are usually offered as first-line standard care because constipation can worsen quickly.
Types of Laxatives
There are many different laxatives available, each varying in their mechanisms of action, ease of administration and the side effects they can cause:
76 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
Health Outcomes
Illustration
1: The
prevalence of constipation
Type of laxative Mechanism of action Examples
Contact stimulant laxatives
Increase fluid secretion into the bowel and prevent resorption of water
Stimulate nerves in the bowel muscles, which increases bowel movement (peristalsis)
Bisacodyl (available as oral tablets and as suppositories)
Sodium picosulfate (given as oral tablets often before a colonoscopy or bowel surgery)
Anthraquinones found in plants such as Senna
Health Outcomes
Softeners and emollients
Lubricate the intestine wall and soften stools to make them easier to pass
Paraffin
Glycerol
Sorbitol
Considerations/restrictions on use
Side effects include irritation of the bowel lining and gastrointestinal spasms
Can cause heart dysfunction
May not be suitable for diabetics as they reduce the effectiveness of insulin
Bulk-forming laxatives
Contain non-digestible substances that add bulk to stool
Absorb water to increase the volume of stools
Larger, softer stools stretch the internal bowel wall and trigger bowel movements
Psyllium husk (taken as a drink)
Flaxseed
Can lead to steatorrhea (fatty stools) and diarrhea
Can reduce absorption of fat-soluble vitamins, such as vitamin K
Some (e.g., glycerol) need to be administered as suppository or clysta (enema) so are fast-acting but only work within the rectum and not the large bowel
In some countries, paraffin is not recommended for use in children under 18 or during pregnancy
Additional stool bulk can cause bloating and abdominal pain
Essential to drink plenty of water to avoid risk of bowel obstruction
Osmotic laxatives
Facilitate the drawing and retention of water into the gut, increasing stool volume and softness and triggering bowel movement
Different types of osmotic laxatives stimulate water release into the gut in different ways
Lactulose
Sugar alcohols
Saline laxatives
Macrogols
Some osmotic laxatives are metabolised, and others are not, leading to differences in side effects
Most are given orally, but some are also available as enemas
In the rest of this article, we will look more closely at osmotic laxatives and discuss why these are considered one of the most patient-friendly and effective treatments for constipation in all age groups.
Osmotic Laxatives –
A Patient-friendly Solution
Osmotic laxatives can be further divided into four groups: lactulose, sugar alcohols, saline laxatives and macrogols (polyethylene glycol).
• Lactulose is a non-absorbable disaccharide that is metabolised in the
Table 1. Types of laxatives and considerations for use
gut. The metabolites bind water and draw it into the colon, softening stools.
• Sugar alcohols work in a similar way to lactulose: they are metabolised by intestinal bacteria to form acids which stimulate peristalsis, and the metabolites draw water from the gut mucosa, softening stools.
• Saline laxatives contain magnesium or potassium and work primarily by drawing water into the bowel and making stools softer and easier to pass.
• Macrogols (polyethylene glycol) work by binding the water they are consumed with to make stools slightly larger (which stimulates bowel movements) and softer (which makes stools easier to pass). There are different types of macrogols available, characterised by their mean chain-length. Macrogol 3350 and Macrogol 4000, which are mainly used, have approximately 3350 and 4000 ethylene units respectively, and they have virtually the same properties.
INTERNATIONAL PHARMACEUTICAL INDUSTRY 77wwww.international-pharma.com
From Table 2, macrogols stand out as the osmotic laxative that can be most widely used and that causes the fewest side-effects. They are easy to take as an oral solution and are also available as a dosage strength that is suitable for children from 6 months to 14 years. Further, they do not cause fermentation in the gut, so they avoid bloating and abdominal pain. Unlike softeners and emollients, macrogols do not affect the uptake of nutrients and, because they don’t cause any changes to the gut microbiome, they can be taken long-term.
Macrogols can be classed as either a medicine or a medical device in the EU because it only has a physical mode of action and is non-resorbable. This makes it faster and easier for such products to be registered in Europe as the process
is centralised rather than being country specific. For the patient, the benefit comes through a simple physical mechanism of action that also ensures there is no loss of effectiveness over time. It is easy to add other substances to macrogol-containing products such as electrolytes to maintain salt balance, or prebiotics such as inulin, citrus fibers, or partially hydrolysed guar gum (PHGG) to improve intestinal flora, all of which contributes to improving bowel function long-term.

Avoiding Electrolyte Loss
A key general drawback with laxatives is the vicious cycle that occurs when they are used over a long time. Many laxatives cause electrolyte loss from the body into the stool, and this can cause stools to thicken. In turn, this leads to increased constipation and a
need to take more laxatives. In addition, when stools are hard, the body sends fewer reflexes to go to the toilet, which further worsens constipation. Thus, the patient needs greater laxative levels to achieve a bowel movement and the vicious cycle continues.
Some laxatives include salts to mitigate for this issue but taking these can be problematic for patients who have cardiovascular or renal disease and need to be on a low potassium or sodium diet. The impact of losing salts is also much worse for these patients. For example, in someone with cardiovascular disease, the heart already has a high workload, but an electrolyte imbalance requires it to work even harder.
Because macrogols have only a physical mode of action they avoid this issue with salt loss. Some macrogol products do contain electrolytes, but these are added in such a way as to balance the electrolyte
levels in the body and in the stool. This reduces the risk that electrolytes move from the body into the stool and keeps the patient in control of their condition.
The fact that these products are also classed as medical devices rather than medicines indicates that the notified bodies accept that the electrolytes are not absorbed by the body but simply balance electrolyte levels and have no pharmacological effect.
And that’s Not All
Beyond constipation, a physical laxative has several other applications. They can be used to empty the intestine before surgery, to induce labor when babies are overdue, and to flush the system ready for colonoscopy. The doses used will vary depending on the objective of treatment. For constipation and
78 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Health Outcomes These four different classes of osmotic laxatives have different advantages and disadvantages, as summarised in Table 2. Illustration 2: Mechanism of action of macrogols in the large intestine Table 2. Advantages and disadvantages of osmotic laxatives. * For short term use only ** Although lactulose is often used long-term with patients in retirement/care homes. Ease of administration Causes bloating Causes abdominal pain Affects microbiome or nutrient uptake Safe for children? Safe for those with renal impairment or diabetes? Safe and effective for longterm use? Lactulose Oral Y Y Y Y* N N** Sugar alcohols Oral or rectal Y Y Y Y* N N Saline laxatives Oral or rectal Y Y Y N N N Macrogols Oral (as a drink) N N N Y Y Y
Health Outcomes
stool softening, a daily dosage of around 10g for adults is sufficient. To clear the bowel before a colonoscopy, a dosage of around 40g is used.

Overcoming Formulation and Manufacturing Hurdles
Despite their many advantages for the patient, user-friendly macrogol products can be challenging to create, requiring a thorough understanding of the product and its components, as well as deep technical expertise in manufacturing and formulation. Product development should focus on end-product homogeneity and content uniformity. Specifically, the particle size distributions of macrogol and other ingredients must match to ensure good blend homogeneity. Even minor variability in particle size distribution can lead to dust formation, which compromises proper sachet sealing and risks ingress of moisture. Resultant product agglomeration can then create poor dissolution characteristics when mixed with water, or a change in the product’s appearance when still in the sachet. Products suffering from such issues risk not meeting patient expectations, compromising brand reputation.
Flavoring macrogol products is not straightforward either, primarily because the large volume of macrogol dominates product composition. Creating a palatable and enjoyable product that patients are happy to buy repeatedly thus requires deep expertise in flavoring. For example, in macrogol products containing inulin or PHGG, which are generally included in the composition at doses above 500 mg, knowledge of which flavors will work optimally can avoid unnecessary trial and error, and accelerate product development and time to market.
Macrogol is also incompatible with several excipients, as they can cause the formation of formaldehyde upon storage, impacting product quality, reducing shelf life, and necessitating inconvenient storage restrictions (storing below 25°C, for example). Knowledge of these incompatibilities not only reduces product development time but also eliminates the need for additional storage restrictions, offering better convenience for the patient and additional selling points to differentiate from competing products.
To obtain high product quality, managing excellent supplier relations can’t be overstated. Access to the right, highquality raw materials and excipients, as well as reliable and consistent delivery performance, must be secured in order to deliver On Time and In Full (OTIF) – reducing the chances of being impacted by stock shortage.
Conclusion
Of the many types of laxatives available to treat constipation, osmotic laxatives are some of the easiest to use by virtue of their easy route of administration. Within this class, there are options that avoid the side effects that are common with other types of constipation treatment – namely, bloating, abdominal pain, and flatulence.
We know that patients are more likely to use laxatives if they are user-friendly: easy to take and avoid the discomfort and embarrassment of these common side effects, especially when they are needed for long term use.
As an example, macrogol is an easyto-use alternative available in a range of formulations and doses that are safe for children as young as six months, and for older adults, including those with long-term health complications such as heart and renal conditions, and diabetes. It’s wide applicability to all patients with constipation and the ability to register as a medical device rather than a medicinal product in the EU makes it a straightforward and patient-friendly addition to any company’s portfolio of constipation treatments.
Although easy to use, macrogols are challenging to formulate, and so technical expertise in their formulation and manufacturing is key to ensuring they are effective, user-friendly and purchased again and again.
REFERENCES
1. Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and metaanalysis. Am J Gastroenterol. 2011;106(9):15821592. doi:10.1038/ajg.2011.164
2. Camilleri M, Ford AC, Mawe GM, et al. Chronic constipation. Nat Rev Dis Primers. 2017;3:17095. Published 2017 Dec 14. doi:10.1038/nrdp.2017.95
3. https://www.nhsinform.scot/illnesses-andconditions/stomach-liver-and-gastrointestinaltract/constipation
4. Gandell D, Straus SE, Bundookwala M, Tsui V, Alibhai SM. Treatment of constipation in older people. CMAJ. 2013;185(8):663-670. doi:10.1503/ cmaj.120819
Dr. Martin Koeberle

Dr. Martin Koeberle is Head of Analytical Development & Stability Testing at HERMES PHARMA, where he is responsible for the analytical and stability aspects of all products developed and manufactured by the company. Koeberle has deep know-how in working with a broad range of APIs, vitamins, and minerals, and is an expert in developing user-friendly dosage forms. He is experienced in defining and justifying product specifications against international regulatory scrutiny, and has authored numerous technical articles in leading pharmaceutical publications.
Dr. Verena Garsuch
Dr. Verena Garsuch is Manager Analytical and Clinical Development & Stability Testing at HERMES PHARMA, where she organizes and evaluates stability studies of food supplements and manages GCP activities. Garsuch has profound knowledge of the formulation and production of user-friendly dosage forms. She is also proficient in assessing stability data, setting up and managing bioequivalence studies, and has authored numerous technical articles in leading pharmaceutical publications. Prior to HERMES PHARMA, Garsuch held formulation development roles at Hexal/ Novartis and Acino Pharma.

INTERNATIONAL PHARMACEUTICAL INDUSTRY 79wwww.international-pharma.com
Patient-Centric Clinical Trials: Turning Opportunities into Standard Procedures
Over the past year, the industry has heard often and at length about patientfocused decentralised trials (DCTs), as vendors and some early adopters report their early experiences in an area that is still evolving. It’s important to remember that the industry is still at a very early stage in digital clinical trial development.
Truly patient-focused trials are a goal that we have yet to attain. We still use ePRO and eCOA, as we have for 20 years, only now we talk about these tools as if they are brand new and operate under a decentralised banner. They don’t — at least, not yet. We may be using eConsent, but still have patients come to sites to initiate the process. That is not decentralised practice.
At the same time, most trial protocols have not reached a point where patients can participate flexibly based on their own preferences, which change from day to day. If we use multiple technologies to capture data — in essence, the same data from different patients — we must have simple methods to integrate and review that data in one step. That does not happen today. The industry is just beginning to think about common standards for sharing clinical data.
Now that more clinical leaders have experienced, firsthand, the limitations of new tools designed to make trials more patient-centric, many have adjusted their expectations from new technology and are experimenting with different approaches. They’ve also seen that simply deploying more technology won’t make the patient journey any easier if it burdens research sites. In fact, we heard repeatedly at the recent DIA meeting (June 2022, Chicago) how technology overload was slowing down sites and their ability to work with patients. Today, some sites are reporting capacity as low as 30% of pre-COVID levels.
The Changing Role of Standards
One of the fundamental challenges facing the industry is developing standards for
sharing clinical data that will enable digital decentralised trials. In the 30 years that Clinical Data Interchange Standards Consortium (CDISC) standards have been in place, industry thinking has changed considerably. Today, standards are no longer seen as intellectual property to be protected, but approaches that must be open and shared, so that the industry can move to one set of clinical data standards. Results will improve efficiency, not only for trial stakeholders, but for regulators, who will be able to inspect data more quickly.
Standards for data transfer will ensure that data is exchanged in the right format. Work will need to focus on two very different areas, designed for two very different purposes: clinical data (under the CDISC)1 and healthcare data (under Health Level Seven HL 7).2 Connecting these different data sources, types, and aggregations will require ongoing effort. At this point, as the industry moves into patient-driven data collection, neither, on its own, is a fit. Patients are going to own more and more of their data, and this will likely afford a whole new category, that will lead to yet more complication if new approaches aren’t developed. The opportunity therefore is to find a way to generate, collate, and distribute data between point solutions that results in seamless connections between researchers and patients. Only once those connections exist can the industry apply scientific rigor to clinical data, and only then can AI, ML, and NLP be applied to the data in an efficient and scalable manner.
Modern standards development for clinical trial data sharing will require tremendous effort. Even though this work has only begun, interesting ethical questions are already coming up that the industry must address. For example, some healthcare providers have proposed giving every patient a unique record ID. This tokenisation approach would facilitate faster data exchange but could also lead to concerns about data security and privacy and could potentially be vulnerable to misuse. This idea requires open and frank discussion, but it does highlight the raw need to be able to share data quickly and effectively.
An Explosion of Data from Different Sources
Clearly, clinical trial stakeholders today must deal with far more data, from diverse sources, than they did in the past. Some clinical studies today have 10, 20, or even 30 distinct data sources, and, ideally, data should be ingested from these sources in real time. Currently, it must be aggregated and cleaned separately and then brought into the clinical database, a process that can take weeks. Data workbench platforms will be crucial to aggregating and cleaning that data and connecting it to other data in the CDMS. After all, every data point we collect has the potential to create a pattern of interest with every other data point.
These solutions will also fit in well with other approaches such as time-in-motion analyses3 and advanced analytics, and we can expect to see more collaboration between vendors that specialise in data management and those that apply analytics to that data. The clinical workbench would ingest and aggregate the data and store it in one place, accessible to CDMS, while separate efforts would, for example, consider the cost vs benefit of running more frequent biomarker analysis, or use artificial intelligence to test the soundness of a trial model or individual process. CluePoints,4 for example, uses AI algorithms, not only to analyse and challenge trial results, but the approaches being used. Analysing all the trial data, not just the clinical but operational, metadata, and audit trail data, offers a detailed picture of the patient’s journey. Over time, it will allow the industry to question why certain processes and jobs are even needed and replace them with better approaches. If we can compartmentalise the work that needs to be done, and work together, clinical trials will be able to advance much faster.
Connecting with Patients
The industry is clearly increasing use of eConsent and eCOA tools in today’s hybrid trials, and eCOA is already used in roughly half of today’s studies. But the question today is whether it will grow because more people are adopting a digital/decentralised trial strategy or just because they simply want more real time, everyday data. We shouldn’t confuse the use of those
80 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Health Outcomes
KLINGE TEMPERATURE CONTROL
The Biopharma Cold Chain Sourcebook (a leading resource to the pharmaceutical industry) recently reported:

“Recent Good Distribution Practice (GDP) guidelines, to which the industry is gradually adopting, require control of even room-temperature product, which is essentially everything that is not refrigerated or frozen.
With each passing year, the oversight of pharmaceutical and biologics shipping is getting tighter.”
Klinge Corporation has been safely transporting and storing pharmaceuticals for over 30 years. Protect your product with our qualified refrigerated and deep freezer containers.

REDUNDANT REFRIGERATED & -70°C DEEP FREEZER CONTAINERS FOR PHARMA PRODUCTS
Avoid Temperature Excursions with Redundant Reefer/Freezer Containers

Keep pharma products at required temperature down to -70°C
Meet international transport regulations
Safeguard pharma products with temperature recorder & online temperature tracking
INTERNATIONAL PHARMACEUTICAL INDUSTRY 81wwww.international-pharma.com inquiry@klingegroup.com | www.klingegroup.com USA: +1 717 840 4500
technologies with the delivery of a true digital strategy.
I think it is important to remind ourselves that decentralising means we push out more of the daily activities from a centralised clinical site to the patient’s home. This is not just about collecting more data; it is about changing the core thinking about data collection.
Industry practice will become more interesting, and much closer to the patientcentric ideal, when patients can be given more choice in how they participate in a trial. In one scenario, a patient would provide his or her consent remotely and would download an app to automatically become part of a trial. Day by day, she’d be able to choose whether to use home or remote monitoring or come to the clinic. This may not be possible with MRI or other procedures that require the patient to physically visit a location, but, in most cases, the patient would determine the degree of decentralisation. However, different clinical study teams would have to accept these options in order for this model to be delivered to patients.
At this point, sponsors are actively exploring the use of digital technology in clinical trials, but for each problem any new application solves, it may create several new challenges, especially for research sites,
which will see it as a burden if it doesn’t offer immediate benefits. In discussions with sponsors and partners, I advise them to consider both patient and site needs when they start writing the protocol; not afterwards. Applying new technology to a final trial design is simply too little too late. It is the same advice I consider for all technology companies - think about the user experiences we are seeking, across multiple different therapeutic needs, before you try to design a new product. Tackling the issues of the day, from concept to conclusion, is paramount and the only way to drive real change.
In the end, patient convenience is key to patient compliance and future trial enrollments. Today, most digital patientfacing technologies require too much work from patients. The following guidelines can help ensure that application development results in a win-win for patients, sites, sponsors and CROs.
• Consider closely the interests of each stakeholder in the trial. What are the key needs and interests for each group, and how are you delivering them? Are you currently using technology that solved one problem but created new problems that you couldn’t anticipate? The fewer changes to the patient’s normal daily activity we introduce, the better.
Success boils down to making the right data available at the right time in a highly consumable way. From a sponsor’s perspective, it’s about making processes repeatable, attainable, and defendable.

• Determine the impact that each application will have on each stakeholder in the trial and consider overall cost. How likely will auditors accept the data, how well will patients respond to the strategy, and how easily will sites be able to execute the approach? On top of that, of course, costs must also be considered. Remember that, if patients like the approach, accept it readily, and comply with requirements, you’ll also be able to recruit them more easily in the future.
• Don’t make research sites the help desk for each trial. Ensure strong sitepatient engagement and don’t burden sites with new technology that does not add value to the work they do. We’re hearing anecdotally that some patient-focused apps are adding to clinical staff workloads and slowing trials down significantly. Before the COVID-19 pandemic, some sponsors say, research sites used to see six to seven patients per day. Now, they are seeing one to two patients a day, as site staff adjust to the need to educate and support patients on the use of new apps. At the earliest planning stages, sponsors must consider the potential burden that any new technology will place on sites and ensure that any tools that they develop will benefit sites and strengthen engagement between sites and patients. Better site-patient engagement needn’t result in patients visiting sites more frequently. It means ensuring that the level of contact is right for the patient on that particular day. If a patient is doing well, there's no reason to have him or her come in. But if she isn’t, she will need to visit the site at the right time to ensure that the trial is right for her or determine whether changes are needed.
• Integrate applications into the devices that patients already use in their daily lives, whether phones, smart watches, or Fitbits, and reduce the number of steps required to register and use each tool.
Be sure that the tools selected for any trial can communicate with each other, to make it easier for clinical site staff to check patient health and treatment records.
82 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Health Outcomes
Health Outcomes
Think about how patients behave in the real world. Saddling them with apps on different devices plus an iPad will not be convenient. Integrating apps into tools that patients already use and accept, whether smartphones, smart watches, or Fitbits, will make it easier for them to comply with trial procedures. You may not be able to avoid having patients download an app, but why not give them a QR code to complete registration and other steps for them, carrying over user details and automatically creating data flows? This way, everything is connected in one step, rather than having patients go through a 20-step checklist just to get up and running.
• Use a holistic healthcare framework for developing trial technology. For example, ensure that trial apps also give patients data that will help them better manage their overall health and interact more effectively with their HCPs.
A scorecard approach will be extremely helpful for most patients. For example, diabetic patients who are overweight could receive a personalised daily diagnostic
message from their physician, as in: "This is the amount of insulin you've taken today, this is your HbA1c result from the lab, and this is your blood glucose level. You’re taken 10,000 steps today. Great work. Keep going.” Or "I need to see you. You haven’t taken any steps today, and your insulin, blood glucose and HbA1c levels are up. We need to talk." This type of approach would motivate patients and make the patient-physician interaction part of the trial, moving trials in the direction of holistic medical care. In the end, that is exactly what digital clinical trial technology should deliver.


Now that the initial buzz over DCTs has begun to fade, the real work is beginning as the industry explores how technology can best be used to bring patient centricity and efficiency to each trial. One test for digital will be adaptive trials, which would change continuously in response to trial results, requiring a central control point and seamless data transfer. At this point, we’re a long way from being able to run adaptive trials digitally, just as we can’t yet give patients real choice in how they participate in clinical trials every day. The
key to advancing clinical trials is realising that digital strategies are in their very formative days. Accepting this fact, instead of acting as if decentralised patient-focused trials had already arrived, will allow us to work together and advance to truly flexible, patient-centered digital trials in the future.

 Richard Young
Richard Young
Richard Young is Vice President, Vault CDMS, at Veeva Systems. With over 25 years of experience in life sciences, Richard is known for his executive vision and proven operational experience in data management, eClinical solutions, and advanced clinical strategies. His role at Veeva is helping to transform the way we approach clinical data, unifying every contributor and consumer in a single platform.
& Supply Chain Management
Paving the Way to a more Sustainable Future More Economy and Ecology in Logistics by Small and Thin Multilingual IFU Booklets
Sustainability is considered a priority across the entire pharmaceutical packaging value chain, and more and more companies are making efforts to be eco-friendly in everything they do.
The printing of Instructions for Use (IFU) or Patient Information Leaflets (PIL) represents a highly specialist print job. IFU packaging plays a critical role in delivering vital information to patients and consumers and involves printing booklets and inserts required for products, medical devices, and pharmaceuticals, under rigorous regulatory requirements. Every single prescription sold to consumers needs strictly regulated IFUs to be included as part of the pharmaceutical packaging. It is paramount, as the information included in an IFU helps to provide guidance on the proper use of a medication or device.
These days, device labelling has also become a critical business process for manufacturing companies and the manufacturer/marketing authorizing holder is required to continuously monitor product safety from PMS and update the label information as applicable. And the new European Medical Devices Regulation (MDR) has brought with it added complexity, urging companies to review their labelling structure and design.
Crucially, sustainable packaging implies using those materials with the most limited environmental impact possible, but also accounting for what goes into their production and what happens after they’re used. According to The European Organisation for Packaging and the Environment, “packaging needs to be able to fulfil its enabling role in a circular economy by optimising resource use, minimising waste and extending the value in a product and the economy.” However, in delivering ecofriendly packaging options, the pharmaceutical industry must carefully navigate multiple challenges, from the maintaining of the most cost-efficient processes to the preserving of ever-more complex global supply chains.
Thinprinting concept by St Michel Print has revolutionised the way IFU’s and similar documents are designed and printed. With their unique all-in-one Thin Paper Booklet solution, the company is contributing to a simplified warehousing, packaging and logistics process, thus enabling businesses to increase their economic efficiency and sustainability.

Have you ever been frustrated with having to print a multitude of different language versions of IFU’s, manuals and warranty documents for your product? Do you feel unease at the thought of wasting warehousing space for separate printed items included in packages destined for different market areas? Is your packaging process complicated to handle?
St Michel Print Has Got You Covered Located in South-eastern Finland, in the Land of Forests and Paper Making, St Michel Print is part of privately owned Länsi-Savo Group which boasts a rich heritage of 133 years in printing. With almost 40 years of experience in printing on thin and ultrathin paper, St Michel Print is currently one of Europe’s leading printing houses in the field.
Over the years, St Michel Print has refined their operations, focusing on minimising waste and maximising process automation and efficiency; they’re using a renewable resource, exclusively high-quality, totally wood free thin print paper sourced from reliable manufacturers in Finland and a streamlined process management.
Environmental sustainability is not easy to achieve, but St Michel Print is committed to making a difference. Close
collaboration with Europe’s leading thin paper manufacturers and logistics partners enables the company to ensure fast and reliable deliveries to customers all around the world.
The Concept of the Wood-free Paper
By using coated wood-free papers, St Michel Print has combined requirements of high opacity and brightness with the increasing sustainability expectations and environmentally friendly printing solutions.

In papermaking, the term “wood-free” means that the yellowing lignin in the pulp, wood’s natural binding agent, has been bleached off in chemical process. Cellulose fibres remain long and undamaged that means strength and durability in paper.
A paper is as strong as its weakest point, they say. High opacity, readability and durability are the key benefits of this type of ultra-thin paper, that is also completely recyclable and biodegradable. Manufacturing process of wood-free does not require the energy consuming mechanical grinding. Quite the contrary, the chemical pulp process is converted in a source of energy instead.
St Michel Booklet Solution: Simplify your Logistics, Why Don’t You!
Handling the stock item numbers can become easy just by combining all languages into one multilingual thin paper booklet. The extremely thin paper enables the use of a bigger font size and all the pages can still be bound in one single thin IFU or warranty booklet.
Thin paper really means thin paper! By virtue of a paper thickness measuring as low

84 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Logistics
Logistics & Supply Chain Management
as 29 g/m², there is practically an unlimited printing area that can accommodate lots of information and larger font size when needed. The high brightness ensures extremely high print contrast and easy readability, whilst the high opacity created by special fillers diminishes the risk of print through. All the written information can be printed in one colour and the hazard symbols in multiple colours on the flap cover.
Ultimately, the high opacity thin paper means less truckloads and less containers; higher quantity in one truckload or container, less CO2 emissions by transportation. And this is how you can reduce your carbon footprint and save money significantly.
Both paper thickness and weight play their important role. Lower paper substance

and caliper (thickness) means less space and weight, and this is how The Thin Paper Booklet makes sure your package is lighter and smaller.
Do You Want to Know Your Savings?
To help companies plan their tailored Booklet solution and know exactly the savings that can be achieved on a yearby-year basis, St Michel Print has devised The Thinprinting Simulator. Available on the company`s website, the innovative tool provides the customer the opportunity to choose the booklet size and number of languages in one booklet.
It`s all about the quintessential Allin-one solution. By combining Timson’s tailored web thinprinting technology with high-quality paper, St Michel print has
achieved the easy-to-handle, sustainable and consumer-friendly Booklet.

All language versions of IFU’s, warranty information and legal texts are printed in only one Booklet that can be easily slipped into the product package. Due to their characteristics, the booklets can be used with ease on automated high-speed packaging lines.

There will be only one stock item number for your thin paper booklet. This means less warehousing space, a faster inventory cycle and no unnecessary stock. The tied-up capital of your warehoused products can be released for your other needs.

With St Michel booklet solution what you get is a streamlined packaging process and significant savings in shipping costs. And it’s through simplified warehousing, packaging and logistics processes that increasing the economic efficiency and sustainability of any business can be achieved.
Heikki Suomala


Heikki Suomala is the Director of Sales & Marketing at the St Michel Print. Finnish printing house that specializes in printing on thin and ultra-thin papers. Heikki has worked at the company for 18 years. As M.Sc. in Business Economics of Forestry his professional experience includes management, sales and marketing positions in forestry, forest industry and heavy machinery organizations. Both in Finland and North America.
Olli
Kivijärvi
Olli Kivijärvi is the Sales Manager at the St Michel Print. Finnish printing house that specializes in printing on thin and ultra-thin papers. Olli has worked at the company for 10 years. As M.Sc. in Forestry Planning he has a strong experience in management, sales, controlling and product development in wood industry mainly in Europe.
INTERNATIONAL PHARMACEUTICAL INDUSTRY 85wwww.international-pharma.com Application Note
Supply Chain Disruptions Bring Short Notice Changes to Ingredients – But Can Your labelling and Artwork Management System Cope?
Supply chain and procurement industry figures have called this the ‘era of the shortage’, with widespread disruption to supply chains, raw materials and ingredient availability. Ingredients substitution is a viable tactic to avoid product shortages or ceasing production, but it unlocks a whole host of operational challenges, argues Bob Tilling, VP of Sales at Kallik. With product packaging, labelling and artwork also requiring updates every time an ingredient changes, business leaders must embrace technology to ensure ingredient substitutions do not become a disruptive ‘chop and change’ tactic that overshadows other operations.
Earlier in the year we saw the disruption caused by strikes at paper mills in Finland, which resulted in a major shortfall in packaging and labelling materials. This has been a drop in the ocean compared to the widespread business challenges posed today by raw material shortages, supply chain crunches, cost pressures such as inflation and even severe weather delaying or limiting harvests.
Popular Ingredients Are Few and Far Between
This isn’t strictly limited to the food and beverage market – affected industries cover healthcare products such as pharmaceuticals, and cosmetic lines, which have far more ingredients in each product than many consumers anticipate.
Although many ingredients have become scarce or expensive, manufacturers have had no issues sourcing alternatives. For example, a likely shortfall in sunflower oil caused by the conflict in Ukraine –the world’s largest supplier of the oil – has caused disruption for many food manufacturers, yet is already being actively mitigated by switching to rapeseed oil as a close alternative.
The problem lies not with this product reformulation, but the ripple effect on operations such as labelling, artwork and
packaging – all of which must be updated in line with any ingredient switches.
Reformulate, Reformulate and Then Reformulate Again – A Pattern is Emerging The ingredients issue is forcing change at a far greater pace than usual. U.S. consumer foods giant General Mills cited some products as being reformulated over 20 times between January and April. For comparison, many ingredient tweaks are typically a very rare occurrence, with adjustments to tried and tested formulations taking place perhaps every 2–3 years.
As manufacturers across multiple industries jostle to secure limited stocks of existing ingredients in what is a very fast-moving situation, some products may end up being repeatedly reformulated with alternatives on a regular basis during this ongoing ‘perfect storm’ of disruption.
But product formulation is far more than a simple ‘drag and drop’ activity during the manufacturing process – especially in highly-regulated industries such as pharmaceuticals, cosmetics, and food & beverage.
Don’t Let Product Labelling be the Forgotten Piece of the Puzzle – It’s an Essential Part!
Packaging and labelling can often be an oversight in this current laser focus on ingredients supply and reformulation to ensure production continuity – but it plays an equally vital role in the overall process, and manually adjusting these assets can be a long, laborious process.
Indeed, a UK Food Standards Agency report on food substitution and labelling places label change lead times at 6–12 weeks – assuming disruption such as supply chain issues is not extending this even further.

If labelling and artwork cannot be adjusted at the same pace as product ingredients, production could be severely delayed or even halted. It is clear advanced technology is needed to cope with the impact of short-notice changes and bring agility to the artwork and label management process.
Ensure your Digital Eye is on the Entire Production Process
Any business with multinational operations
86 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2 Logistics & Supply Chain Management



INTERNATIONAL PHARMACEUTICAL INDUSTRY 87wwww.international-pharma.com Development and manufacturing Thermal Blankets for the temperature protection of pharmaceuticals and healthcare products in airfreight (+15°C +25°C) and (+2°C +30°C) Qualifications & Validations Multilayer thermal blanket for PMC ULD Euro and Block pallets Temax 4000 blanket with multiple reflection technique Stress tested in summer (+46°C) and winter ( 15°C) profiles Tarmac tested on solar power and greenhouse effects Ecological notes Recyclable + Low ecological footprint NON laminated composition = easy to dismantle Re manufacturing of recycled compounds Available worldwide at freight forwarders KRAUTZ TEMAX Group Development and manufacturing Belgium Europe Phone +32 11.26.24.20 Website www.krautz.org Email info@krautz.org Temax Americas LTD Hanover park Illinois USA ISO 9001:2015 certified
Logistics & Supply Chain Management
or large product ranges will likely see a major impact to operations if they cannot identify and address ingredient changes on packaging in an agile, accurate manner –ideally from a ‘single source of truth’.
With many firms still outsourcing their product artwork and label management to local third-party agencies, dealing with ingredient shifts is a slow, costly process with no complete visibility of operations and an ever-present threat of inconsistencies introduced by human error.
This outdated process can today be phased out with the arrival of advanced, end-to-end label and artwork management (LAM) systems, which will offer features to identify every instance of a specific ingredient used on every product label – in every language – and update accordingly.
Disruption and Regulation are Now Par for the Future
Global disruption has reached unprecedented levels over the last few years – but further uncertainty for businesses and their supply chains could be on the horizon. Constantly shifting regulations on both a regional and national level are likely to cause business leaders to rethink their
product formulations – think ‘sugar taxes’ requiring lower sugar content in the food & beverage industry.
We’re also currently seeing businesses across multiple industries – from cosmetics to consumer goods – adopt a ‘shrinkflation’ tactic, where products reduce in size while remaining the same price. Again, this will likely demand a change in product composition, with a knock-on labelling effect.
Ultimately, any change – whether imposed by supply chain disruption or even seasonal crop availability – will require ingredients to be updated or listed in a differing order on product packaging.
Act Now to Avoid Further Supply Chain Headaches

As these various supply chain woes continue to pose problems to business worldwide, it has become apparent that a solid digital framework is required to provide the necessary agility to deal with problems as they arise.
LAM solutions will not replace creative tasks such as artwork design, but they can significantly increase the speed at which
businesses can react to shifting ingredients availability and in turn avoid operational disruption.
With strict regulations in place for many industries worldwide and constant concern over introducing unlabelled allergens into products, establishing a consistent, digitised approach to label and artwork management is a business priority that underpins many other critical operations.
Bob Tilling is the VP of Global Sales at Kallik, an enterprise labelling and artwork management company. He has a wealth of knowledge when it comes to the life sciences industry, particularly regarding medical devices. Bob helps businesses in highly regulated industries begin their journey of transforming their labelling and artwork management.

88 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
Bob Tilling
Email: bob.tilling@kallik.com
Real-time supply chain visibility is driving the industry forward
Cold Chain as a Service®
Integrated technology and services for your end-to-end supply chain and vaccine distribution.

Real-time temperature monitoring
location
monitoring and response services
Mission-critical analytics & insights
Validated for all lanes—air, road, sea. Pay as you go.
Controlant is pioneering the development of next-generation visibility solutions for digitally connected global supply chains that keep products safe. Our Cold Chain as a Service® Digital Visibility Platform solution consists of reusable Internet of Things (IoT) data loggers that send mission-critical quality data and insights in real-time to a proprietary, cloud-enabled software platform, and costreducing operational services..
Businesses are leveraging the improved visibility to collaborate with stakeholders, support their corporate sustainability objectives, and achieve supply chain improvements leading to a substantial return on investment.
For more information, visit controlant.com contact@controlant.com
Follow us on Twitter
Product
traceability
24/7
@controlant
Events Preview
CPHI Frankfurt 2022: At the Heart of Pharma
Event Overview
CPHI Frankfurt (previously called CPHI Worldwide) – the world’s largest pharma event – returns to Germany in 2022.
It remains the go-to event for global pharma attracting industry wide participation, making this event the best place to source, network and collaborate.
The 2022 edition brings a raft of exciting changes – including a full schedule of onsite content and in person sessions for the first time since 2019 – with event numbers expected to meet or exceed pre-pandemic levels as the pharma community turns out again en masse. The three day exhibition and in-person conference will be hosted at Messe Frankfurt, Germany, 1–3 November 2022 and will run in hybrid form – fusing the best elements of the traditional show with interactive online features to help attendees maximise their CPHI experience.
Extending the value delivered, CPHI opened its networking and learning platform on 28 Sep 2022 with the 'Connect to Frankfurt' digital platform that began the countdown to CPHI Frankfurt. Connect to Frankfurt will feature over 40 on demand session covering topics from all aspect of the pharma supply chain. The online platform is accessible to anyone registered for CPHI Frankfurt (November 1–3, 2022) and will remain open post event until 18th November.
What’s New at CPHI Frankfurt
One significant change, that will be immediately clear, is that the event has been rebranded as CPHI Frankfurt and the onsite experience enhanced for attendees’ ease of navigation. For example, the collocated events have been replaced by descriptively named zones for packaging, outsourcing, machinery and more.
“One of the reasons behind the new identity is that we wanted to deliver the
smoothest possible onsite experience so that attendees and exhibitors can concentrate on maximising opportunities. Everything we have done is to help the industry to match with exactly the right partners and to be able to do this more quickly,” Orhan Caglayan, Group Director CPHI Frankfurt.
Highlights at CPHI Frankfurt 2022
Who Attends: It’s truly the heart of pharma with participants from over 145 countries; with 78% having purchasing responsibility and 47% from the C-suite.
CPHI Frankfurt has been designed to empower attendees to more connections and make them count, with a full platform of enhancing digital tools to use pre-event.
Who Exhibits: CPHI Frankfurt is the beating heart of the entire global pharma supply chain – with everything from ingredients and finished dosage drugs to machinery, outsourcing providers, packaging and even bioprocessing at BioProduction.
CPHI Awards 2022: Held on 1st Nov 2022, the CPHI Awards will feature a welcome reception and ceremony to celebrate the industry’s achievements and brightest stars – with categories spanning a total of 10 awards.
Attending CPHI Frankfurt
Event Opening Hours: 10:30am to 6:30pm
Early Access: 9:30am to 6:30pm
VIP Access: 9:30am to 6:30pm
To register kindly visit: https://registration-smartevents.iiris.com/ CPHI%2F2022
Digital Features
Connect to Frankfurt: Take the first digital step on your journey to CPHI Frankfurt
Connect to Frankfurt marks the launch of the digital platform – a companion to CPHI Frankfurt – the online meeting place to connect with the entire supply chain. The platform empowers visitors to source suppliers, browse the exhibitors list and discover the full content agenda. Attendees can make contact online, pre-vet partners and then schedule in person meetings at the event.
The CPHI Frankfurt app provides both attendees and exhibitors with a timetable of each day’s activities, a full list of exhibitors and onsite navigation (including hall locations).

Content Overview
Connect to Frankfurt Agenda CPHI Frankfurt content experience begins
90 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
online on 28 September 2022, with Connect to Frankfurt. Highlights include a ‘trends outlook’ session hosted by leading marketing intelligence companies (including IQVIA and Accenture), the CPHI Learning Labs – with insights from leading pharma companies on new products & solutions – and an overview of the German pharma market and the CPHI Award finalists.
The on-demand webinars will span more than 40 sessions and cover all topics from ingredients to clinical trials.
A session on ‘Sustainability Strategies in the Life Sciences Industry’ will be hosted by Accenture AG, as well as analysis from GlobalData exploring ‘Predictions for Future Drugs’.
CPHI Frankfurt (1–3 Nov 2022) Content Agenda – The First Full Schedule of In-person Content Since 2019 The conference agenda, the largest ever put together by CPHI, will span some five tracks across three days in the main Conference theatre, with an additional
Events Preview
theatre dedicated to ‘Product Innovation and Sustainability’.
Reflecting the hottest trends in the industry and attendees’ interests tracks cover: ‘Ingredients and Formulation (Track 1), Future Therapies (Track 2), Digital (Track 3), Manufacturing Excellence (Track 4), and Patient Centricity (Track 5).
The opening day keynote explores the glut of recent research into Psychedelic therapies to treat an array of mental health issues from depression to anxiety and posttraumatic stress. David Erritzoe, Clinical Director and Deputy Head at the Centre for Psychedelic Research presents an ‘Introduction of Psychedelic Therapies into Mental Health – Current Status and Future Perspective’.
Each of the five main tracks will feature a keynote address, panel discussions and presentations, with the second hall focused on exhibitor product innovations – including biologicals – and industry wide efforts to improve sustainability with best practice case studies.


BioProduction Theatre
Running alongside CPHI Frankfurt content will be 3-days of biologics sessions curated by the team at BioProduction. In total BioProduction will feature some 30+ talks covering all aspects of the biologics supply chain from automation and digital to downstream and upstream processing.
91 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
92 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
global life science
BIO-Europe is back in person!
The 28th BIO-Europe will be held in Leipzig, Germany from October 24–26, 2002, to fulfil its pivotal role of bringing together the global biopharma community to accelerate dealmaking.
Over the years, BIO-Europe has become Europe’s flagship partnering event bringing together over 4,000 executives from biotech, pharma and finance companies from around the world. Your access to the entire life science ecosystem all under one roof. Be part of the 20,000+ one-to-one meetings that will take place at the event that will ultimately shape the future of our industry—one partnership at a time.

93 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
Your gateway to the
community Take advantage of the best registration rates available now! LEARN MORE: BIOEUROPE.COM OCT. 2426, LEIPZIG, GERMANY NOV. 24 DIGITAL PARTNERING 2022 Produced by: In collaboration with:
see the return of the Clinical Trial Supply Europe conference to Milan


pharma, large and small,
have the
discuss, debate and consider
never been a better time to exchange case studies, meet

distanced thought leaders,

latest solutions
clinical supply chain.
selection
two-day in person event for industry leading updates on a series of topics around temperature-controlled
how to avoid excursions, using data
monitor temperatures,
products at deep frozen
how your peers
with new Brexit regulations
supply chain
and out
Healthcare Events CLINICAL TRIAL SUPPLY EUROPE 2023 TEMPERATURE CONTROLLED LOGISTICS IN BIOPHARMACEUTICALS EUROPE 2023 Milan, Italy 15th-16th March, 2023 The ultimate events for the pharmaceutical and biotechnology community to discover how to excel in clinical supply strategy as well as form key connections for long-term success 2023 will
where
alongside biotechs will
opportunity to
new technologies and processes to streamline supply chain operations. There’s
socially-
and discover the
from our
of experienced exhibitors to create a robust
The
logistics. This includes
to
shipping
temperatures and
are coping
affecting the
in
of the UK. Register now Register now Use reference code: IPI Use reference code: IPI www.arena-international.com/ctseurope Milan, Italy 15th-16th March, 2023 www.arena-international.com/pharmatemp
IPI
Peer Reviewed, IPI looks into the best practice in outsourcing management for the Pharmaceutical and BioPharmaceutical industry.



INSIGHT / KNOWLEDGE /
SUPER PUBLICATIONS FOR SUPER PHARMACEUTICALS

JCS
Peer Reviewed, JCS provides you with the best practice guidelines for conducting global Clinical Trials. JCS is the specialist journal providing you with relevant articles which will help you to navigate emerging markets.



PHARMA’S DNA
Listen to industry experts on the latest in drug discovery, development, research, industry regulations and much more at Pharma,s DNA, the podcast channel by Senglobal Ltd., available on Sound Cloud, Spotify, iTunes and YouTube.
IAHJ
Peer Reviewed, IAHJ looks into the entire outsourcing management of the Veterinary Drug, Veterinary Devices & Animal Food Development Industry.


IBI
Peer reviewed, IBI provides the biopharmaceutical industry with practical advice on managing bioprocessing and technology, upstream and downstream processing, manufacturing, regulations, formulation, scale-up/technology transfer, drug delivery, analytical testing and more.


95 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
FORESIGHT
www.journalforclinicalstudies.com
www.international-animalhealth.com
www.international-biopharma.com
www.international-pharma.com
Page 71
Aurena Laboratories
Page 15 Austria Wirtschaftsservice Gesellschaft mbH
Page 49 & 50
Bausch+Ströbel SE + Co KG
Page 93 Bio Europe 2022
BC BSP Pharmaceuticals S.p.A
Page 55 ChargePoint Technology
Page 94 Clinical Trial Supply Europe 2023
Page 89 Controlant
Page 90 & 91
CPHI Frankfurt 2022
Page 92 DDL 2022
Page 29
FUJIFILM Wako Chemicals U.S.A. Corporation
Page 53 Gerresheimer AG
Page 45 Kahle Automation
Page 81 Klinge Corporation
Page 87 Krautz Temax
IFC Natoli Engineering Company
Page 65 Nemera
Page 73 Nipro
Page 9 Owen Mumford
Page 11 PCI Pharma Services
Page 5 PlantaCorp
Page 7
RGCC International GmbH
Page 95 Senglobal Ltd
Page 25 SGS
Page 3 SPL Scientific Protein Laboratories
Page 61 Stäubli International AG
Page 94 Temperature Controlled Logistics in Biopharmaceuticals Europe 2023

Page 57 ThinPrinting
IBC Tive
Page 13 & 21 Valsteam ADCA
Page 83 Woolcool
96 INTERNATIONAL PHARMACEUTICAL INDUSTRY Summer 2022 Volume 14 Issue 2
Subscribe today at www.international-pharma.com or email info@senglobalcoms.com I hope this journal guides you progressively, through the maze of activities and changes taking place in the pharmaceutical industry IPI is also now active on social media. Follow us on: www.twitter.com/ipimediaworld www.facebook.com/ipimediaworld www.plus.google.com/+ipimediaworldmagazine www.ipimediaworld.tumblr.com Advertisers Index











Real-time visibility Mitigate temperature excursions Assure product integrity
CYTOTOXIC

STERILE

NON-CYTOTOXIC
DS MANUFACTURING
ORAL
QC ANALYTICAL
DEVELOPMENT
Summer 2022 Volume 14 Issue 2 | | | 41Million units 2022 INSPECTED BY: EUROPE | USA | JAPAN | BRAZIL | KOREA | TAIWAN | TURKEY | SAUDI ARABIA | RUSSIA | IRAQ | KENYA | BIELORUSSIA
CAPABILITIES Preformulation and formulation development Analytical methods Development Process Development: oral solids, conjugation, liquid and lyo formulations, complex formulations
CAPABILITIES Method validation and transfer Full testing of small and large molecules Stability and Photostability studies
CAPABILITIES icated manufacturing area for tabs, minitabs, ule, LFHC velopment: 100g to 1000g P Clinical and Commercial: 4Kg to 100Kg Annual capacity: 50 Million units
CAPABILITIES Conjugation of ADC’s from development (10mg 5g) to clinical and commercial (20g 5 Kg) Liposomal Bulk Solutions Annual capacity: 410 Kg dditional 900 Kg by end of 2022
CAPABILITIES 7 Filling lines working in full containment, to produce liquid and lyo vials Annual capacity: liq/lyo vials 31 Mill. units end 2022
STERILE CAPABILITIES ling lines working in full containment roduce liquid and lyo vials ual capacity liquid/lyo vials: 9 2023 17 2024 26 2025 A CDMO TECHNOLOGICALLY DEVELOPED TO MEET THE NEEDS OF INNOVATORS, WITH ITS HIGH CONTAINMENT FACILITY DESIGNED TO OFFER SERVICES FOR PRE-CLINICAL GLP, CLINICAL AND COMMERCIAL SUPPLY BROCHURE HEADQUARTER and MANUFACTURING PLANT BSP PHARMACEUTICALS S.p.A. Via Appia km 65,561 04013 Latina Scalo (LT) – Italy Phone: +39 0773 8221 Web: bsppharmaceuticals.com Mail: business.development@bsppharmaceuticals.com
























































































































































































































































 Richard Young
Richard Young
















































