











DIRECTOR: Mark A. Barker
INTERNATIONAL MEDIA DIRECTOR: Anthony Stewart anthony@senglobalcoms.com
EDITORIAL MANAGER: Beatriz Romao beatriz@senglobalcoms.com
DESIGN DIRECTOR: Jana Sukenikova www.fanahshapeless.com
FINANCE DEPARTMENT: Akash Sharma accounts@senglobal.co.uk
RESEARCH & CIRCULATION: Jessica Chapman jessica@senglobalcoms.com
COVER IMAGE: iStockphoto ©
PUBLISHED BY: Senglobal ltd. Unit 5.02, E1 Studios, 7 Whitechapel Road, E1 1DU, United Kingdom
Tel: +44 (0)20 4541 7569 Email: info@senglobalcoms.com www.international-biopharma.com
All rights reserved. No part of this publication may be reproduced, duplicated, stored in any retrieval system or transmitted in any form by any means without prior written permission of the Publishers.
The next issue of IBI will be published in Spring 2023.
ISSN No.International Biopharmaceutical Industry ISSN 1755-4578.
The opinions and views expressed by the authors in this journal are not necessarily those of the Editor or the Publisher. Please note that although care is taken in the preparation of this publication, the Editor and the Publisher are not responsible for opinions, views, and inaccuracies in the articles. Great care is taken concerning artwork supplied, but the Publisher cannot be held responsible for any loss or damage incurred. This publication is protected by copyright.
2022 Senglobal ltd.
Volume 5 Issue 4 – Winter 2022
www.international-biopharma.com
The lab at Riga East Clinical University Hospital provides clinical reporting of infectious diseases, pathology and oncology patient samples. Dr. Girts Skenders and Mr. Renis Zeltmatis shed light on how the COVID-19 pandemic brought significant changes to their hospital laboratory. Upgrading it in the midst of a global pandemic wasn’t ideal but a combination of shared insights and the right instruments helped open the door to success.
Success does not come about by coincidence. Success is an outcome of preparation. When it comes to successful U.S. Food and Drug Administration (FDA) inspection outcomes, preparation is at the foundation. Finding out that the Site is not in a position to meet the Agency’s requirements during the process of the Inspection is not a desirable position for any site. Unsuccessful FDA inspections can impose a lengthy time impact on a company’s strategic plan. Noelle Clifford at Pharmalex explains how to have a positive inspection.
Outsourcing all or part of the pharmacovigilance (PV) and patient safety services required during a project lifecycle is common practice in the current market. Ever evolving demands and increasing regulation presents additional challenges to determining the best solution for your PV and patient services needs. Karen Johnstone and Dr. Sabine Richter at ICON explore opportunities for outsourcing these services, potential models, and best practices for selecting a service provider and establishing a successful relationship.
RESEARCH / INNOVATION / DEVELOPMENT
The ideas behind integrated laboratory informatics are not new, but there are serious questions as to how many organisations are fully, or even partially, realising these benefits. Simon Wood at Autoscribe Informatics will re-emphasise the potential benefits and provide examples of successful digitalisation in the laboratory environment. It will also briefly explore factors that may prevent organisations realising the benefits of innovation.
The silkworm, Bombyx mori, has an interesting defense against bacterial and fungal invasion. This defense is found in the hemolymph and cuticle of the insect, as well as various other insect species as well. The defense mechanism involves a chemical cascade, the pro-phenol oxidase (proPO) cascade, that ultimately results in melanin formation around the foreign matter within the insect’s body. Timothy Francis at FUJIFILM Wako Chemicals outlines the use of SPL in the pharmaceutical industry
Promising broader patient access to critical medicines, biosimilars offer a more cost-effective alternative therapeutic option compared with originator products. With many patents for "blockbuster"
commercial biologics set to expire in the next few years, biopharma developers have become increasingly drawn to the biosimilars market. Understanding the benefits of having more readily available cutting-edge treatment options available for patients, a number of government and regulatory body-backed incentives are being introduced to streamline the biosimilar development process. In this article, Jim McNally and Lynn Kamen BioAgilytix Labs explore the incentives, regulations, and other trends pushing biopharma companies down the biosimilar development route.
The manufacturing and commercialisation of vaccines to tackle COVID-19 took place at an unprecedented speed and on a scale never before seen in the pharmaceutical industry. Billions of doses of effective mRNA vaccines have now been delivered to patients around the world, and Billions more continue to be manufactured. Jon Reed at Recipharm oulines how to the company addresses Product Finishing and Dispensing Challenges for Future Pandemics.
Gene therapy represents an expanding area of medicine with the potential to change the lives of people with life-limiting conditions. As the interest in gene therapies has increased over the years, so has the number of clinical trials, with 372 gene therapy trials active in the first half of 2022. Katie Roberts at OXGENE and Man-Shiow Jiang at WuXi Advanced Therapies explain how to build a AAV manufacturing platform.
The availability of a wide range of therapeutic biologics has revolutionised modern medicine. Whilst the majority of pharmaceutical drug products remain conventional, small molecule medicines; the emergence of biotherapeutics has allowed for the treatment of many disease areas which were previously out of
reach. Ross Blezard and Mara van Haandel at DFE Pharma analyse some strategies to reduce cold chain burden in biopharmaceuticals.
Plasmid-based methods have, for good reason, been a trusted method for the manufacture of adeno-associated viral (AAV) vectors for many years. As 2022 closes and we look towards 2023, plasmids will likely continue to be useful for gene therapies already in the pipeline. The refinement of robust plasmid-based manufacturing platforms, in particular, will further support drug developers as they prepare for the later stages of their therapeutic journey to commercialisation. Katie Roberts at OXGENE discusses the future for gene therapy manufacture.
The biopharma industry requires digital transformations to improve efficiencies and deliver critical therapeutics to patients at an accelerated rate. Although great efforts have gone into implementing data-driven business models and processes, the industry has seen mixed results in return, with the digital evolution remaining in the early phases of development. In this article, James Choi at Samsung Biologics delves into the shared vision of biopharma’s leaders to drive a digital transformation in their organisations. He also emphasizes the need for the industry to prepare for the road ahead.
Advancements in gene therapy and the approval of mRNA vaccines have dramatically increased the demand for well-characterised RNA and oligonucleotides in the biopharmaceutical industry. To support this emerging need, researchers have developed new analytical tools to analyse nucleic acid sequences by mass spectrometry. Christian Albers, Detlev Suckau, Guillaume Tremintin and Stuart Pengelley talk about the technological advancements support RNA and oligonucleotide sequence confirmation.











This issue features an interesting lineup of articles dealing with the different aspects from production to analysis of so-called Advanced Therapeutic Medicinal Products (ATMP), a very exciting class of therapies.
Fast and reliable DNA sequencing helped to identify the COVID-virus and find targets for vaccine development. The technology behind DNA sequencing has developed at a remarkable pace. Dr. Girts Skenders and Mr. Renis Zeltmatis at the Riga East Clinical University Hospital explain how they have implemented Next Generation Sequencing (NGS) technologies and how they are putting this technology to use. NGS is also one of the technologies key to the development and QC strategy of ATMP.
Plasmid DNA is the basic intermediate of most ATMPs developed to date. Ensuring and controlling sequence fidelity, relevant isoforms and host-cell contaminants is no easy task. Katie Roberts at OXGENE will talk us through complex steps of pharmaceutical grade plasmid DNA production and what we can expect to see in future.
Plasmid DNA by bacterial fermentation, requires the control of host cell contaminant such as endotoxins and pyrogens, which at even low concentrations can cause severe immune responses in patients. Having assays in place to test for theses complex compounds is mandatory. Timothy Francis at FUJIFILM Wako Chemicals explains how Silkworm Larvae Plasma may be real a alternative to the established LAL testing.
QC-Methods to determine the sequence fidelity and purity of mRNAs derived from plasmids and synthetic oligonucleotides are currently being implemented to support development projects. Mass spectrometry is a versatile tool and can be used to determine the sequence and purity of polynucleotides. Christian Albers, Detlev Suckau, Guillaume Tremintin and Stuart Pengelley report on how advances in mass spectrometry aid sequencing of mRNAs and Oligonucleotides and the current limitation of the technology.
• Ashok K. Ghone, PhD, VP, Global Services MakroCare, USA
• Bakhyt Sarymsakova – Head of Department of International Cooperation, National Research Center of MCH, Astana, Kazakhstan
• Catherine Lund, Vice Chairman, OnQ Consulting
• Cellia K. Habita, President & CEO, Arianne Corporation
• Chris Tait, Life Science Account Manager, CHUBB Insurance Company of Europe
• Deborah A. Komlos, Senior Medical & Regulatory Writer, Clarivate Analytics
• Elizabeth Moench, President and CEO of Bioclinica – Patient Recruitment & Retention
• Francis Crawley, Executive Director of the Good Clinical Practice Alliance – Europe (GCPA) and a World Health Organization (WHO) Expert in ethics
• Hermann Schulz, MD, Founder, PresseKontext
• Jim James DeSantihas, Chief Executive Officer, PharmaVigilant
Nucleic acids and their ability to add or even correct defective genetic information in cells hold a great promise for some of the severest illnesses. Unfortunately, these molecules do not readily enter cells where they are effective nor are they very stable outside of cells. Therefore, means to protect nucleic acids and to facilitate their transport into target cells are developed. Viruses are naturally very good at this, and Adeno-Associated Viruses (AAV) are one of the widely used viral vectors to date. Katie Roberts at OXGENE and Man-Shiow Jiang at WuXi Advanced Therapies will give an insight into how to setup a AVV production platform.

I want to end the foreword of the last issue for this year on an optimistic note. Now, that most of the countries are coming out of the COVID-19 pandemic, it is time to look back at how the pharmaceutical industry in collaboration with heath agencies and legislators managed to respond to the global health crises with both, more conventional and novel vaccines. Although, all stakeholders were operating under high speed and unprecedented public scrutiny to deliver efficacious and safe vaccines for the globes entire population. For vaccines, being a preventative therapy, the safety aspects are probably more important than for any other treatment. As vaccines are administered to principally healthy individuals and to whole populations, to be effective on an epidemiological scale, a thorough risk-benefit evaluation must be made. The past two years with lockdowns, homeschooling and in some cases, the tragic loss of life, has left it´s marks on all of us. Looking back at all of this and to see that we as individual, societies and humanity can achieve, I cannot help but to feel optimistic for the pressing challenges that lie ahead!
Dr. Steven A. Watt, Head of Business Development at A&M STABTEST GmbH• Jeffrey W. Sherman, Chief Medical Officer and Senior Vice President, IDM Pharma.
• Lorna. M. Graham, BSc Hons, MSc, Director, Project Management, Worldwide Clinical Trials
• Mark Goldberg, Chief Operating Officer, PAREXEL International Corporation
• Maha Al-Farhan, Chair of the GCC Chapter of the ACRP
• Rick Turner, Senior Scientific Director, Quintiles Cardiac Safety Services & Affiliate Clinical Associate Professor, University of Florida College of Pharmacy
• Robert Reekie, Snr. Executive Vice President Operations, Europe, Asia-Pacific at PharmaNet Development Group
• Stanley Tam, General Manager, Eurofins MEDINET (Singapore, Shanghai)
• Stefan Astrom, Founder and CEO of Astrom Research International HB
• Steve Heath, Head of EMEA – Medidata Solutions, Inc

SGS Health Science enables the medical and health innovators to deliver life-changing solutions in the quickest, safest and most efficient way, helping improve the lives of many Contact us healthscience@sgs com sgs com/healthscience sgs com/healthcommunity




Dr. Ģirts Šķenders and Mr. Reinis Zeltmatis shed light on how the COVID-19 pandemic brought significant changes to their hospital laboratory. Upgrading it in the midst of a global pandemic wasn’t ideal but a combination of shared insights and the right instruments helped open the door to success.
To develop more agile digital laboratories, QC leaders whose systems have become disconnected will need to reimagine an approach that advances a connected quality ecosystem. This requires thinking beyond specific tools and problems to consider a holistic approach to modernising QC and how it will fit within the organisation's overall technology modernisation efforts.
The lab at Riga East Clinical University Hospital provides clinical reporting of infectious diseases, pathology and oncology patient samples. However, for Dr Ģirts Šķenders, the laboratory’s Development Project Manager, and Laboratory Specialist Reinis Zeltmatis, things became a lot more complicated during the pandemic when the facility upgraded from Sanger sequencingbased approaches to next-generation sequencing (NGS). Fortunately, the duo is accustomed to challenges and problem-
solving and were therefore ideally equipped to lead their team through a successful transition to a new workflow.
The laboratory was originally part of the infectious disease department of the city’s University Hospital. This changed when five hospitals were brought together under the umbrella of the Riga East Clinical University Hospital, now the biggest hospital group in Latvia. Included in this consolidation were several independent laboratories focusing on pathology, oncology and infectious disease.
Although the University Hospital’s lab had been performing Sanger sequencing for around ten years, it only started integrating NGS workflows two years ago. “The plan to begin NGS of oncology and infectious disease samples started back in 2018,” explained Dr. Šķenders. However, some logistical and administrative challenges delayed the delivery of sequencing instruments and reagents.
Fortunately, the hospital received an additional sequencing grant from the Latvian Center for Biomedicine Studies and Research in 2020, which helped accelerate things. “The state decided it needed more sequencing and European governments were concerned about the public impact of COVID-19, so some additional money was given with the aim of increasing essential sequencing capabilities for critical laboratories,” said Dr. Šķenders.
Now, almost two years later, the lab’s workload is still focused on SARS-CoV-2: it analyses nearly 200 samples of the virus and around 12 oncology specimens each week.
Finding the Right Tools for the Job Building NGS workflows into a lab required benchmarking and optimisation. Sample quality control (QC) is a critical part of optimising NGS workflows, especially for labs that analyse formalin-fixed, paraffin-embedded (FFPE) tissue samples. However, a sample QC method was only selected after an investigation into which solutions other clinical researchers found to be the most successful for their genomics labs.
“We checked the other users’ experiences with kits or instruments and tried to choose the ones that had the greatest customer reviews,” said Mr Zeltmatis. The lab eventually settled on the Agilent 4200 TapeStation system.

“The TapeStation system is faster, much easier to use and the precision is much higher than other alternatives, so we can really determine what our average library size is. Using the original platform, we could only guess but the TapeStation system really shows the definitive number.”
Considering the large volume of samples processed weekly, including the additional labour of nucleic acid extraction and sample prep, speed was also a clear benefit. “We are able to
obtain results within 10 to 15 minutes, maximum,” said Zeltmatis. “It’s also simple to learn the workflow. Mix a little buffer with the sample, vortex, and you’re ready to go.”
Although NGS is a recent addition, the team plans to replace all the lab’s current genotyping methods with whole genome sequencing within the next 12 months. There will also be a specific focus on liquid biopsies. “We currently send those samples to external service labs for analysis, but liquid biopsies are a very promising direction, especially for oncology,” explained Dr. Šķenders.
RNA sequencing for oncology, as well as for rare genetic diseases, is another priority. They both agreed that Cell-free DNA and RNA ScreenTape devices will be essential for the more challenging sample types used in RNA sequencing and liquid biopsies.

SARS-CoV-2 may have generated the headlines but the lab has other infectious diseases in its sights. “We are going to do more bacterial work, such as sequencing tuberculosis, which is still an issue in Latvia because of multidrug-resistant cases,” said Dr. Šķenders. “We will also focus on other bacterial isolates and look into the possibility of finding bacterial DNA in the primary samples.”
Dr. Šķenders and Mr Zeltmatis have brought the Riga East Clinical University Hospital a long way in a very short period of time. By using NGS workflows, in conjunction with robust sample QC, they are already starting to unlock valuable insights around disease control.


Ģirts has 15 years of experience of diagnosing tuberculosis and drug-resistant tuberculosis at a National TB reference laboratory. He has participated in in two EU FP7 Collaborative Projects – TB PAN-NET and FAST-XDR-DETECT, has acted as temporary advisor for WHO and ECDC for assessment and optimisation of TB laboratory services in several countries. Research interests: Improvement of tuberculosis diagnostics, tuberculosis epidemiology, TB diagnostics and new TB drug clinical trials.
Reinis Zeltmatis began his career in IT working with medical insurance companies which led to a renewed interest in biology and medicine. Moving into the field of plant genetics, he followed his passion for genetics and medicine into molecular diagnostics. Recent advances in technology led Reinis and a colleague to formed the Sequencing Department as part of the National Microbiology Reference Laboratory at the Latvian Centere of Infectious Diseases in 2018. Since then Reinis has been a part of every field of sequencing performed in the department – from microbiology and SARS-CoV-2 to oncology, with latter becoming his main field of work.

Success does not come about by coincidence. Success is an outcome of preparation. When it comes to successful U.S. Food and Drug Administration (FDA) inspection outcomes, preparation is at the foundation. Finding out that the Site is not in a position to meet the Agency’s requirements during the process of the Inspection is not a desirable position for any site. Unsuccessful FDA inspections can impose a lengthy time impact on a company’s strategic plan.
Those of us who have had the experience of FDA inspections are well aware that they can be challenging. However, the chances of having the inspection process run as smoothly as possible can be greatly improved by implementing the proper preparational tools. In the following article we will discuss a few simple but useful tools and offer guidance that you, and your team, can use to help you set up for success in your next FDA inspection.
First impressions are everything. You have worked hard as a team to get your Site to where it is, now take pride and show it off at its best. It is important that the areas the Inspectors are going to see are clean, tidy and in a state of control. This does not just include the inspection areas, all areas that the Inspectors are going to see need to be treated in the same manner. If your areas are well presented, clean, tidy, and organised this will build the Inspector’s confidence in the Site. Sites that are visually well maintained and well-presented speak to the Site attitude as a whole. Consider the implementation of a 5S program in your area. 5S helps to establish an orderly work environment in addition to improving efficiency. Some helpful tips on how to implement a 5S program can be found here.
This is a common pitfall during inspections. Many observations have been assigned to sites for lack of procedural accuracy. Always be clear and concise in your SOPs. Ensure that the overall requirements of your procedures are described in enough detail
to document the procedural activities. However, it is essential to have the appropriate balance in your level of detail. Do not be tempted to add too much granularity and get into minute detail as you will inevitably be caught out. Extensive detail in procedures opens you up to non-compliance when the Inspector reviews the process.
Be sure to keep on top of periodic review of your procedures. To keep procedures accurate, efficient and effective in terms of their intended use, it is important during this process to involve the people who use those procedures on a regular basis. They can provide valuable hands on experience and insight resulting in robust procedures.
It is important to understand the FDA’s inspection process and it is an expectation from the Inspectors that sites have an understanding of their inspection process. The FDA Website contains some useful guides which can be referred to such as What should I expect during an inspection?. The Site also contains a set of Inspection Guides which are useful in preparation for inspections as they contain reference material that the Inspectors will use during the inspection. These guides will provide information as to what the Inspectors will be looking for, specific to that inspection area.
A good tip is to put together a folder of documents in advance of the Inspection that you anticipate the Inspector may request. This way the documents will be quickly to hand should they need to be provided during the Inspection. For documents that may simply be too large to do this with, compile a categorised list including location, so you can easily find the document. Make sure that you, or the identified SME, are familiar with the documents and prepared for any potential queries that can be anticipated from the Inspectors.
An effective Self Inspection program is vital in preparing the Site for inspection success. Self-Inspections may seem an inconvenience at times, however they are a great opportunity to ensure your area is compliant in advance of inspections and remediate areas of concern. Discuss the Self Inspection in advance with the Self Inspection Team and request a review of any areas of concern within your department. Whilst this may be challenging, it is far more preferable to identify potential deficiencies and resolve them than to have them identified by an Inspector as an observation. An effective Self-Inspection process will help ensure a compliant state within your department and place it in a strong position of inspection readiness.
In recent times where COVID-19 has resulted in travel restrictions and therefore the requirement for virtual audits, inspection skills may not be as honed as they once were, and some personnel may have no direct inspection experience at

all. Self-Inspections are a great training opportunity where you can practice and develop your inspection approach, Use this time to role play, polish your inspection skill set and train in-experienced personnel.
The internal audit schedule needs to be at an appropriate frequency and needs to be effective. Senior management should empower the Self Inspection Team to be able to make effective changes. Rather than simply make recommendations, Internal Inspectors should have the required level of authority to hold functional areas accountable and ensure that they push actions through to completion in order to ensure compliance.
The internal audit program should also be assessed to ensure that the Self Inspections are not only performed on independent functional areas, but also laterally across departments, using a system based approach so that all the interconnected elements between processes are audited.
Each team should review their list of investigations/ discrepancies for their area identifying those they anticipate the Inspector may request to review, or the ones believed to be an area of concern. The person who is likely to discuss the issue with the Inspector should be well versed in all aspects of the investigation. Have all required supporting documentation readily available. Consider putting together a presentation to ‘tell the story’. This can provide clear context to the event should the Inspector take a deep dive into it. This strategy can be applied to not just investigations, but any area of concern. Try to identify other areas that review of an investigation, or similar document, may lead to. An FDA inspection can feel like threads being pulled, leading to larger and larger holes! To prevent this,
make sure that every question arising from the review of any issue, leads to a dead end, with documented information and if required, a documented position or strategy to prevent risk to the product.

Prepare your Team Area Subject Matter Experts (SMEs) should be identified in advance of the Audit. Ensure that they are confident in their abilities to engage with the Inspectors and are well prepared to discuss any areas of concern that may be identified.
Personnel should be appropriately equipped with the tools to interact with the Inspector. Inspection skills and behaviours should be part of an internal training programme and should be retrained upon appropriately . As part of this training try to include role play workshops where personnel present on their particular area of specialty, as they would in a real inspection situation. This can really help to build confidence and therefore result in stronger and more confident responses to the Inspectors queries.
Ensure that personnel are fully trained on the procedures required to complete their tasks and that training records are up to date, reviewed and readily available during the Inspection.
Promoting the correct mindset in this area is also very important. Remember why the FDA are inspecting your Site. They are there to ensure that the products being supplied are safe for the patient; you, your family and your friends. This is a necessary and positive process.
On the Front Line
When speaking with the Inspectors there are a few golden rules that need to be observed:
As the leading supplier of Recombinant Insulin for innovative biologics, we can help you keep development on track and production flowing.

Novo Nordisk Pharmatech A/S remains the best supplier of Insulin Human AF or cell culture processes by providing excellence at every step of the supply chain –beginning with the quality of our insulin sourced directly from Novo Nordisk, the world’s largest producer.

Manufacturing and quality control, precision delivery and a risk mitigation strategy that assures continuous availability are a few of the reasons why you should grow your cells with our insulin.
Learn more about our Insulin Human AF at novonordiskpharmatech.com

• Be prompt with a documentation request. Should the Inspector request a document, provide it as quickly as possible. If it is not available do not evade the Inspector’s request, admit this up front.
• Admit to, rather than hide known deficiencies, the plan for remediation should be prepared during inspection readiness and readily available. It is better to show you have identified issues and have a plan in place to remediate them, rather than hoping an Inspector doesn’t find an issue and give the impression the Site was unaware.
• Provide only the information directly related to answering the Inspector’s question, don’t offer any additional information.
• If you do not know the answer to a question, say so. Tell the Inspector that you will find the answer and come back to them as soon as possible.
• Do not use language like, ‘Sometimes’, ‘Occasionally’, ‘To be Honest’.
• You may find that there are pauses in conversation with the Inspector, do not be tempted to add more information in order to break an uncomfortable silence. Wait for the Inspector to re-initiate the discussion.
• Maintain a very high level of professionalism. Questions should be directed at the auditor in a polite and professional manner.
• Be courteous to the Inspector and do not get into an argument.
Inspectors can vary widely in terms of their inspection style or approach in order to obtain the required information and sometimes those approaches can be testing. If you are finding the interaction with the Inspector challenging, remember the tips above and try to keep in mind that they are just a person, like you, and you are simply interacting with them.
During the course of, and at the end of, the inspection the Inspectors may have a list of observations for the Site. Review these with the Inspector as they arise during the Inspection and attempt to remediate them immediately if this can be achieved. It will show the Inspector that the Site is committed to achieving and maintaining a compliant state. This helps to promote and build a positive relationship with the Agency. Fostering a good relationship with Inspection Agencies is important as it shows the Agency that the site is supportive of the process. This will stand to the site in future inspections.
Sites should strive to be in an audit ready state at all times. This approach not only promotes a constant state of control and continuous compliance but saves much energy and resources in the long run. It also saves your team a lot of pressure and stress by removing the possibility of ‘audit scramble’.
In a hectic inspection it is often easy to forget that not everyone is experienced in the inspection process, some team members may be new to the industry and unfamiliar with inspections. As discussed above, COVID-19 has resulted in the need for many inspections to be conducted virtually meaning that not everyone has had direct interaction with Inspectors. Be mindful of this and offer support/advice where necessary. Also, be
It is advisable, in advance of any upcoming inspections, that you perform some reconnaissance. Complete a review of publications and articles and see what the current trends are in terms of what areas or topics the Agency is frequently looking into during their inspections. Information on FDA observations given to sites, or 483s, can be found on the Agency’s website and can be reviewed according to the industry area and fiscal year. These findings should be communicated across the team so that all are aware of the current trends.
If you happen to have a network of sites within your company, it is in your mutual interest to maintain lines of communication and share your inspection experiences to aid in and support each other’s preparation. Often, when an Inspector discovers a discrepancy at one site they will look for the same at another site.

Noelle, Manager/Consultant in Pharmalex, is a qualified and experienced Microbiologist with a BSc (Hons) in Industrial Microbiology from UCD. She has worked within the pharmaceutical industry for over 15 years in Quality Control roles from ground level through to management. Noelle has worked for several multinational pharmaceutical companies gaining experience with Sterile Products, Dry Powder Inhalation Products, Oral Solid Dosage and Topical Products.

www.qualogy.co.uk


The primary focus of this one-day course is on the implementation of archive systems and procedures within an organisation and the operation of an archive in compliance with GLP. The principles of archiving covered in this course are also relevant for any research or scientific archive.
For further information on courses, dates and costs, please refer to our website: www.qualogy.co.uk/archive-training-courses/
Location Qualogy, in Rushden, Northamptonshire.
of the Archivist and the operation of a GLP archive
The primary focus of this one-day course is on the implementation of archive procedures for clinical trials conducted in accordance with GCP regulatory requirements.
The course provides guidance on the GCP regulations and the specific needs for archiving of Trial Master Files (TMF) and Investigator Site Files (ISF) in compliance with GCP.
For further information on courses, dates and costs, please refer to our website: www.qualogy.co.uk/archive-training-courses/
Location Qualogy, in Rushden, Northamptonshire.
Qualogy’s Regulatory Archive Service is a dedicated solely to the archiving of GxP regulated clients and their material.
Providing a high standard, secure archive facility. We offer a personal service for each client based upon knowledge of the requirements which are then geared to the needs of individual clients
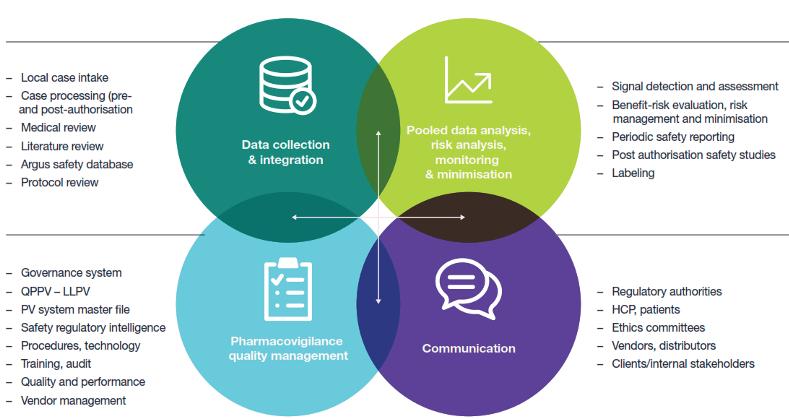
Outsourcing all or part of the pharmacovigilance (PV) and patient safety services required during a project lifecycle is common practice in the current market. Ever evolving demands and increasing regulation presents additional challenges to determining the best solution for your PV and patient services needs. We explore opportunities for outsourcing these services, potential models, and best practices for selecting a service provider and establishing a successful relationship.
Outsourcing pharmacovigilance and patient safety activities has become a standard business practice within a continuously expanding market segment. Innovations and rapid changes in technology and regulatory environments have created opportunities for sponsors to re-evaluate their requirements for successful outsourcing of their pharmacovigilance and patient safety obligations. While outsourcing provides access to unique expertise, specialised intellectual property, multidisciplinary knowledge and an unbiased view, there are certain challenges associated with the increasingly competitive market. These challenges include stricter regulatory environments with variations across the globe, a wider range of safety indicators and a higher demand for transparency that puts added pressure on biotech and pharmaceutical companies, especially within the pharmacovigilance and patient safety scopes.
Choosing a service provider that is a qualified to meet your needs is crucial to the success of your program. Outsourcing PV and patient safety can lower costs for staffing (based on reduced effort), recruitment, management and training. For organisations operating globally in the pre- and/or post-marketing setting, outsourcing PV can also provide improved access to important resources in non-traditional cost-effective regions. With the right service provider, the partnership can result in innovative solutions that maximise the value of the product portfolios while improving patients’ lives. In this article, we explore opportunities for outsourcing these services, potential models and best practices for selecting a service provider and establishing a successful relationship.
Small biotech, mid-size and large pharmaceutical organisations will have differing requirements for outsourcing their PV and patient safety services. At every phase of development, there are opportunities for outsourcing either as a full-service model from phase 1 through post-launch surveillance or as discrete, stand-alone services. Elements such as company size, pharmacovigilance and patient safety department size, in addition to existing license or development partnerships can impact these outsourcing decisions. Any of the services in the figure below can be outsourced individually, in a tailored combination of services or as part of a full-service contract:
Increasingly, pharma companies seek to establish a fit-topurpose, cost-effective operating model for the services they require. Traditional models for outsourcing are either resource-based or activity-based. Resource-based models operate on forecasted project workloads translated to full-time equivalent (FTE) per qualification/role requirements on programs and are cost-effective options for projects with predictable workloads. Activity-based models contain costs which are based on agreed unit pricing and allow for partially allocated resources to support the program. A blended model, leveraging aspects of both the resource- and activity-based models, can be tailored more specifically to the client’s needs. Blended models can provide a cost-effective FTE team to support an agreed baseline workload while more flexible, unitised services support the team during periods of higher volume.
A client’s needs may change over time and the established blended, resource or activity models may no longer fit the program as comfortably. In such situations, operating models require change or adjustment. ICON, for example, has implemented a transformational model with clients to convert them into the best-fit model while maintaining existing team knowledge and supporting business continuity. In any model,
the service provider must be flexible and agile enough to meet the dynamic needs of the client as scopes grow and evolve.

Outsourcing PV and patient safety can yield a mutually successful and positive outcome for the client if the right expectations for partnership are established. To achieve a beneficial partnership, this article provides guidance on:
• Selecting a provider that has the qualifications to perform the service
• Ensuring the contractual agreement and service models cover your needs
• Establishing collaborative partnerships with clear communication and well-defined responsibilities
In a highly competitive marketplace, there are various types of service providers available. The first step in a successful cooperative relationship is selecting a provider that is best suited to your current need as the required pharmacovigilance and patient safety activities can vary significantly. The typical range of service providers includes individual consultants,
specialty CROs, larger full-service CROs, or global service providers under the umbrella of business process outsourcing (BPO). To choose the best provider, we suggest first following these steps to inform your decision-making:
1. Set clear goals and objectives: establish a clear definition of the tasks to be contracted and assess the expertise and the resources required. Then evaluate the potential service providers against these objectives
2. Establish a realistic timeline: plan for enough time to conduct a thorough selection process with clearly defined scopes and appropriately determined outsourcing models

3. Conduct due diligence: we highly recommend checking references for past performance, confirming the provider’s regulatory compliance and comparing the tenure and technical expertise of the senior staff
4. Maintain flexibility: when timelines are tight or scopes are a moving target, communicate openly and stay flexible
Determining a service provider should also include an evaluation of potential business models for the services offered. Choosing a model will depend on factors including the services outsourced, complexity and variability, volume of work and duration of the project. Typical models can include a time and materials contract, fixed unit price or FTE. Time and materials contracts may include a budget cap and are often
good options for ad hoc services. Fixed price contracts serve well on projects that have well-defined scopes over a limited period. For projects with well-defined tasks but undefined or variable volumes of work, clients may opt for an FTE price. Large-scale projects with variability will benefit from a unit price model where the provider can ensure sufficient trained resources are available even when volumes fluctuate frequently and significantly.
Building a strong partnership with the selected service provider can benefit the project through improved communication, increased efficiency and opportunities for innovation. Establishing a collaborative relationship between sponsor and provider begins with clearly defined roles and responsibilities, definitive escalation procedures, a joint governance structure and contingency plans. With these key elements in place, both parties can enter the relationship with more trust and investment in the process.
From this foundation, we suggest investing time and resources into regular oversight committee meetings to deepen the collaboration, harmonise processes and value systems and leverage opportunities for innovation and process optimisation.
Delivering quality services and managing cost remain strong motivators for the common practice of outsourcing pharmacovigilance and patient safety tasks in today’s market. The increasing complexity of clinical studies, higher regulatory PV hurdles, wider range of safety indicators and a higher demand for transparency across the growing global footprint add challenges that push pharmaceutical and biotech companies to rely on outsourcing. With the right service provider, outsourcing provides smarter, leaner, more flexible solutions that contain costs without compromising quality. This article outlined some best practices for outsourcing PV and patient services, evaluating service models, selecting a service provider and establishing a successful relationship to support overall projects.
Karen Johnstone, Executive Director –Customised Solutions Safety and Risk Management, has been with ICON for 20 years with previous positions at GlaxoSmithKline (GSK) and Wyeth. She has held various pharmacovigilance leadership roles providing oversight for safety project management, operations, and project delivery.
 Dr. Sabine Richter
Dr. Sabine Richter
Dr. Sabine Richter, Vice President, Pharmacovigilance & Patient Safety, has over 30 years of research experience in the pharmaceutical and CRO industries, and in academia. As Vice President, she is responsible for all aspects of ICON's pharmacovigilance and patient safety services.




• Prefer paper (5 responses – 8%)
The ideas behind integrated laboratory informatics are not new, but there are serious questions as to how many organisations are fully, or even partially, realising these benefits. This article will both re-emphasise the potential benefits and provide examples of successful digitalisation in the laboratory environment. It will also briefly explore factors that may prevent organisations realising the benefits of innovation.
In the Winter 2021 edition of this magazine Autoscribe Informatics contributed an article titled ‘Digitisation versus Digitalisation – Understanding the Difference and the Role of LIMS in Achieving both’1. In this they described digitisation as the transformation of manual or paper-based operations to a digital format. While it was stated that digitalisation was more difficult to define it was described as the way that the digital environment, as enabled by the process of digitisation, can affect the way we work and do business.
Without doubt digitalisation is currently a hot topic with many articles, conference papers and webinars devoted to the subject. However, there is at least circumstantial evidence that it is a topic that may be more talked about than practiced. This article will outline why this statement has been made and reiterate the potential benefits of digitalisation. It will also identify some of the key barriers to implementing digitalisation in the laboratory that are often associated with an organisation’s resistance to change that can stifle innovation.
Autoscribe Informatics runs regular webinars on topics relevant to laboratory informatics. These are public events open to all. Two recent well attended events have been ‘Utilizing LIMS to Take Your Laboratory Paperless’ and ‘Incorporating ISO 17025 into your laboratory’. In these webinars polls are taken asking questions relevant to the topics, and the answers to these questions can be revealing.
To a question about what the attendees used to manage their lab data the following responses were received to the possible answers:
• We have a LIMS (12 responses – 13%)
• We have a LIMS but still need to use paper (40 responses – 43%)
• We use paper or spreadsheets (41 responses – 44%)
The question was then asked that if they were using paper why they were still using it:
• No funding to buy a LIMS at the moment (29 responses – 47%)
• Our LIMS does not meet our requirements (24 responses – 39%)
• We don’t believe a LIMS can meet our needs (4 responses – 6%)
Asked to select what the benefits of a LIMS might be the following responses were received:
• Automatic creation of Certificates of Analysis (6 responses– 7%)
• Compliance with regulatory requirements (20 responses – 23%)
• Free up laboratory staff time (21 responses – 24%)
• Keep all data in one place (40 responses – 46%)
In the second webinar the following questions were asked
How is your organisation currently managing controlled documents, employee training documentation and issue management or corrective action preventive actions (CAPA)
• Integrated in our LIMS (22 responses – 9%)
• Separate QMS electronic system (95 responses – 38%)
• Spreadsheets or paper based (121 responses – 49%)
• Not managing these (10 responses – 4%)
When asked to select what their organisation’s most significant challenge(s) for audits was, the following response were received (multiple options could be chosen)
• Too many places to look for records selected – chosen by 48% of 200 respondents
• Time required to compile records – chosen by 63% of 200 respondents
• Availability of staff to assist auditor in reviewing records –chosen by 41% of 200 respondents
• Lost/incomplete records – chosen by 34% of 200 respondents
As there were close to five hundred attendees at the first webinar and four hundred at the second it is clear that not everyone responded to the poll questions, however the answers still provide interesting observations on the state of digitalisation within the laboratory environment.
The use of paper records or spreadsheets, possibly in conjunction with an existing LIMS, is still highly prevalent. However, where a LIMS is in place it should be of concern to lab managers and executives that so many do not seem to meet their needs. It would also seem that even digitisation, the first step along the path to true digitalization, has some way to go as so many organisations are still using paper to a greater or lesser extent to manage their lab data and QMS. The need for better access to relevant data and information is clear, with attendees identifying time required to compile records for audit purposes as a significant challenge together with having too many places to look, and lack of resources to look in all these places.
However, the vast majority of respondents have records including controlled documents, training records and CAPA records either in a separate QMS or as a spreadsheet/paper-based system.
The state of true digitalisation would seem to be even worse with only 9% of respondents indicating that their LIMS and QMS were integrated into a single system. This is an area that has the potential to provide many benefits, for example linking training records to LIMS functions to check, in real time, that staff have the correct training to complete the task or linking CAPA directly to sample or batch records within LIMS.
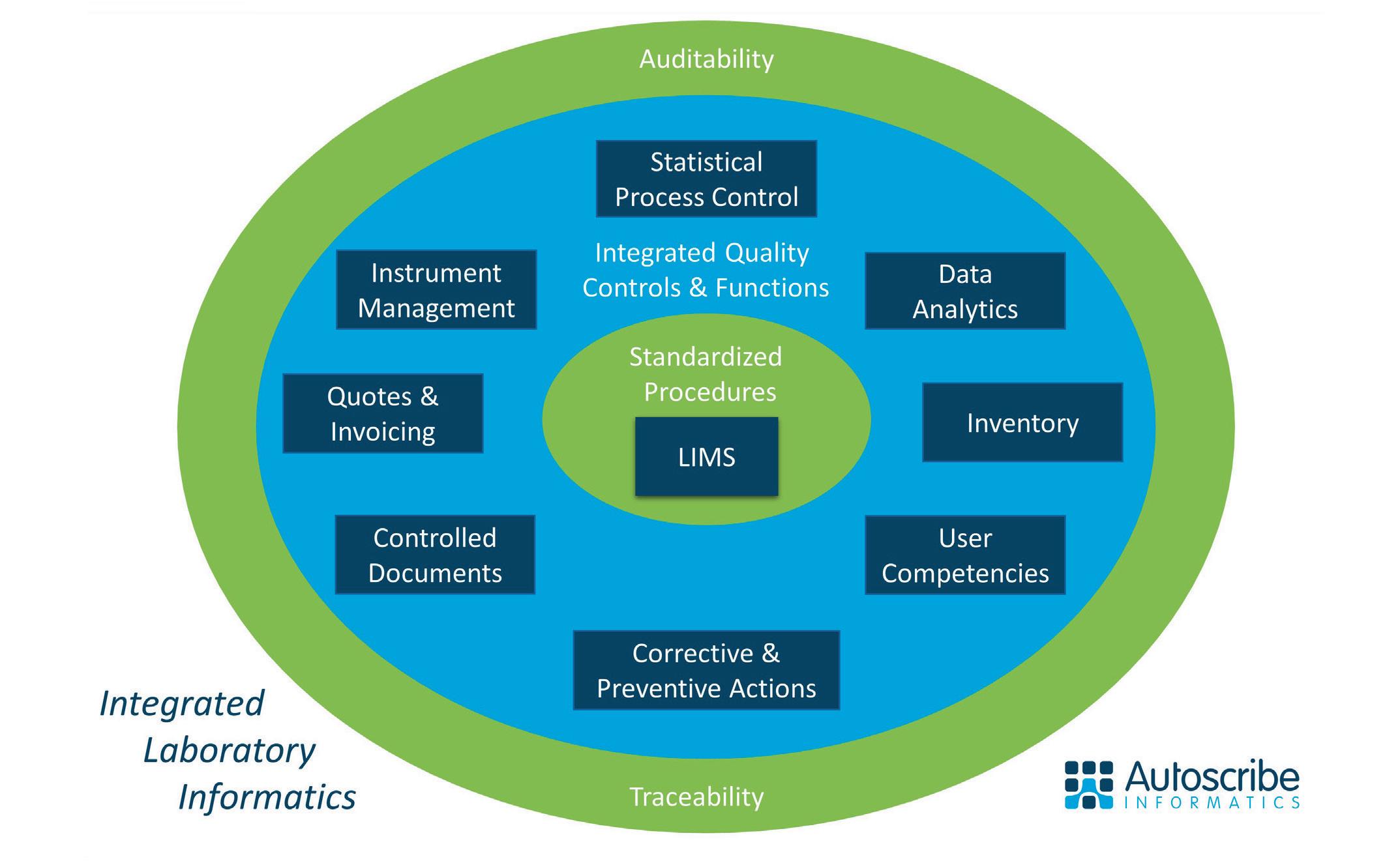
The benefits of digitalisation have been outlined many times before, but what may be missing is evidence of these benefits. With a large installed base Autoscribe Informatics have a number of case studies to draw on. These can provide insights into the benefits that organisations can gain from digitalization of the laboratory environment, and some examples are outlined below. They come from a broad cross section of industries, but the lessons to be learnt are applicable to all organisations with a laboratory, testing or QA/QC function.
A lubricants company that, among other sample types, tests field samples provided by customers replaced a hybrid paper and electronic system and, each day, reduced the time it took a technician to register samples from 5-6 hours to one hour. By integrating their instruments with LIMS and introducing other automation to replace paper-based steps, the number of manual touch points for each sample was reduced from around 11 to 3-4, with an associated reduction in transcription errors and improvement in turn-around time. Finally, having electronic records makes it simple to compare original batch results against results from field samples to see if there has been significant degradation of the product.
Similarly, a rapidly growing UK based specialist testing company needed to replace an existing hybrid paper and Microsoft Access system. As well as improving the sample management workflow and recording of results, the previously manual and time-consuming processes of creating certificates of analysis, customer quotes and invoices as well as the transfer of those invoice to a finance system were automated. Having all the information in a single system also means that required data is instantly available for reporting and audit purposes.
In a specialty chemical company, whose products include pharmaceutical APIs, a paper-based result approval process took 2–3 hours to complete with reactors idle while it was completed. The approval process was digitised, and reactors now rarely stand idle waiting for result approval.
Other examples include a veterinary testing lab that saved 2-3 hours per employee per day during their peak season through improved digitalisation of the laboratory, and a manufacturing organisation where the time to create lot traceability reports in response to customer complaints was reduced from up to two days using the previous paper-based system to a matter of seconds using an integrated LIMS solution. Case studies are available on the Autoscribe Informatics web site.2
It is clear that digitalisation and integration of laboratory operations can bring major benefits to organisations of many different types, but it is also clear that adoption is far from universal and paper-based systems persist. Lack of funding and either a belief that a LIMS cannot meet the organizations needs, or an existing LIMS that doesn’t meet these needs, are reasons cited in our polls for the continued use of paper. However, all too often we see potential customers thinking of buying a LIMS who have not created a compelling business case for the purchase.
Therefore, there is a lack of understanding of why digitisation is needed and little sense of the value of digitalisation. A business case should be the basis to obtain funding and ensure you get a LIMS that meets your needs. However, other barriers may be more difficult to overcome at both an organisational and individual level, and these may include:

• Lack of leadership and support for innovation
• Belief in superiority of existing processes and procedures
• Reluctancy to accept new technology
• Distrust of new technology
• Lack of vision
• Previous negative experience of new technology
• Lack of communication especially of potential benefits

• Unrealistic expectations especially of time required to achieve benefits
These objections can be difficult to overcome but it is worth doing if the true value of digital innovation is to be realised.

Simon Wood PhD, Product Manager at Autoscribe Informatics, has over 30 years' experience in the commercial LIMS environment. He is an acknowledged expert in the field of scientific and laboratory informatics. With a degree in Plant Biology from Newcastle University and a PhD in Mycology from the University of Sheffield, Simon successfully moved into the field of laboratory informatics.
Email: swood@autoscribeinformatics.com



The silkworm, Bombyx mori, has an interesting defense against bacterial and fungal invasion. This defense is found in the hemolymph and cuticle of the insect, as well as various other insect species as well. The defense mechanism involves a chemical cascade, the pro-phenol oxidase (proPO) cascade, that ultimately results in melanin formation around the foreign matter within the insect’s body. When one thinks of melanin, the first thing that that comes to mind is the tanning process in basal skin cells in response to ultraviolet light from the sun. This response is not simply aesthetic – the formation of melanin in human cells is a protective mechanism guarding the cells’ DNA against the ionization dangers of the radiation. In a similar manner, melanin is formed in the insects’ bodies to contain foreign matter.1
Thre proPO cascade can be activated by one of two unique receptor proteins. One is activated by peptidoglycan (PG), and the other is activated by (1, 3)- β-D-glucans (BG). These two molecules have three unique aspects that will be highlighted. First, these molecules are each signature components of different microorganisms. PG is a component of bacterial cell walls, and BG is a component of fungal cell walls. As a result, the proPO cascade is a defense against a wide variety of microorganisms. Second, this defense mechanism is not specific to living organisms. Any piece of bacterial or fungal matter containing PG or BG activates the proPO cascade. Third, these molecules themselves are pyrogens, fever producing substances. These three principles result in a means of detecting the presence of bacteria and fungi rapidly with no need of live cultures or the detection of an immune assay. Collection and formulation of the SLP has resulted in a rapid BG and PG detection method.1
By definition, a pyrogen is any substance that initiates a human immune febrile response. Exogenous pyrogens are therefore foreign substances that initiate a febrile response in the human body. By far, the most problematic pyrogen is bacterial endotoxin – the LPS molecule of the outer membrane of gram-negative bacteria. This pyrogen’s potency as well as resilience to sterilization techniques has prompted the pharmacopeial standard that all parenteral substances need be tested for bacterial endotoxin (USP 85). However, endotoxin is not the only form of pyrogen produced by microorganisms.2
PG and BG are both exogenous pyrogens. BG is easily detected long before the level of pyrogenicity is reached by the LAL assay – the same test method used to detect bacterial endotoxin. PG, however, is not detected by any pharmacopeial test. Problems with PG have arisen. Most notably, PG contamination of Icodextrin used in peritoneal dialysate led to negative patient effects. Since PG is typically not detectable apart from the detection of the living bacteria themselves, residual PG in a product after sterilization has eliminated the living producers of the PG can
remain both problematic and undetectable. The SLP assay can fill this need for both icodextrin production as well as any product that has the potential to lead to a human immune response due to the presence of undetected PG.3
The SLP assay’s unique features has led to the exploration of several novel applications of the assay. Once area that has seen much research in the past two decades has been the potential as a diagnostic tool to provide rapid detection and broad identification of bacterial infections. Meningitis can be bacterial or viral. Studies have indicated that the rate of gram-positive bacteria causing meningitis are on the rise, and the treatment of the disease is specific to the cause of the meningitis.1 The use of the SLP assay along with the LAL assay can be used to detect the presence of bacteria as well as provide the broad classification of gram-positive or gram-negative, all within an hour. Since PG is found in both gram-positive and gram-negative bacteria, the SLP test can be seen as a broad test for the presence of bacteria in general. The application of the LAL test can then be used to judge between the presence of gram-positive and gram-negative bacteria since the LPS molecules are only found in gram-negative bacteria. Studies have shown accuracy rates of around 90% of the SLP in detecting the PG of gram-positive bacteria in this situation.4
However, caution is raised since this test is an indirect means of detecting gram-positive bacteria. Specifically, the presence of peptidoglycan does not perfectly directly align to gram-positive bacteria counts of the sample. Because of this much research is still needed in the potential of the SLP assay in conjunction with the LAL assay to provide rapid, valuable data on the nature of a bacterial infection.5
SLP’s potential in peptidoglycan detection
SLP, as mentioned above, already has a proven track record in fulfilling the need of PG detection in the manufacture of icodextrin for peritoneal dialysis solutions. SLP shows the same potential for any parenteral product that is at risk of harmful effects from PG contamination. In similar manner to the adoption of LAL reagents to fill the need for the test of bacterial endotoxins, the need has already risen for one product, and may arise for other products, for the need to test for PG as an exogenous pyrogen. The risk of undetected PG, although less in magnitude than the risk of endotoxin contamination, is present as a member of the category of exogenous pyrogens.
The potential uses of SLP lead to many areas of manufacturing that may benefit from the use of the SLP assay to ensure that their product contains no peptidoglycan. Potential users could fall in one of three categories. First, manufacturers of products that need a test for bacteria may benefit from the use of the SLP reagent. As a product of bacteria, a test for peptidoglycan may provide a sensitive, rapid test for the presence of bacteria.

Second, as an exogenous pyrogen, manufacturers will want to ensure that their product remains peptidoglycan free as well as endotoxin free. A third potential user of SLP may be research institutes that may wish to explore the potential of the SLP reagent to detect peptidoglycan in a diagnostic setting. The manufacturers and distributors of the SLP reagent, FUJIFILM Wako Pure Chemicals in Japan and FUJIFILM Wako Chemicals U.S.A., are willing to assist and provide samples to any users that may potentially benefit from the adoption of the SLP reagent for the detection of peptidoglycan.6

1.
https://academic.oup.com/femspd/article/28/1/49/486267
2. https://www.usp.org/sites/default/files/usp/document/ harmonization/gen-method/q06_current_webpage_stage_6_ monograph_23_nov_2011.pdf 3. https://www.researchgate.net/publication/9021064_Exposure_ to_the_Peptidoglycan_Contaminant_in_Icodextrin_May_Cause_ Sensitization_of_the_Patient_Maintained_on_Peritoneal_Dialysis 4. https://onlinelibrary.wiley.com/doi/ epdf/10.1111/j.1348-0421.2003.tb03439.x
Timothy Francis is the Technical Specialist for the LAL Division of FUJIFILM Wako Chemicals U.S.A. Corporation. He comes into the Technical Specialist role with 5 years of experience teaching the natural sciences at a college level. He is proficient at taking the complex, technical aspects of a topic and breaking them down into clear, understandable pieces that all connect back to the big picture. He draws upon this experience to provide professional technical support and training for the PYROSTAR™ line and to help you with your technical needs. Outside of the work environment, he enjoys backyard astronomy, disc golf, kayaking, and enjoying time with his wife as they care for their pets.

• Endotoxin-specific reagent eliminates the risk of false positives from (1-->3)ß-D-Glucan

• 100% animal free • Quantitative range: 0.001 to 50EU/mL
• High sensitivity with less lot-to-lot variation
• Stable storage after dissolution (4 hrs. at 2-8°C and 2 weeks at -30°C)
Promising broader patient access to critical medicines, biosimilars offer a more cost-effective alternative therapeutic option compared with originator products. With many patents for "blockbuster" commercial biologics set to expire in the next few years, biopharma developers have become increasingly drawn to the biosimilars market.
Understanding the benefits of having more readily available cutting-edge treatment options available for patients, a number of government and regulatory body-backed incentives are being introduced to streamline the biosimilar development process. In this article, Jim McNally, Chief Scientific Officer and Lynn Kamen, the company's Scientific Officer, explore the incentives, regulations, and other trends pushing biopharma companies down the biosimilar development route.
At the onset of 2010, the biopharmaceutical industry witnessed an unprecedented wave of top-selling drugs reaching their patent expirations. Drug developers reacted quickly, recognising the potential for new biosimilar products when entering a market previously dominated by one player and acting to further establish the biosimilars market. With a total of 26 blockbuster molecule patents set to expire between 2022 and 2026, it can be expected that more developers in biotherapeutics will turn their hand to the biosimilars market.1
The potential to benefit financially from new exposure in the market is not the only driving force toward biosimilars. Lower development costs and more competitive product pricing quickly result in broader patient access to critical medicines. Innovator products that may have previously been too costly to distribute to patients in developing countries widely must make way for these often more affordable alternatives.
With the many advantages that can come from entering the biosimilars market as soon as possible after patent expiration, time is of the essence in what can be expected to be a competitive race to market. If timelines to critical milestones in biosimilar development cannot be accelerated, there is a risk that one of the many competitors will overtake, gaining the market share.
Moving towards an era with a continually steady stream of blockbuster therapeutic molecules coming off patent, biosimilar developers must carefully consider all areas where opportunities for increased speed lie - from process development to bioanalytical assessment. However, the biopharma industry is not alone in the push for more biosimilars to enter the market. Several noticeable trends have begun to emerge, driven by the desire of drug developers as well as government and regulatory
changes to provide a wider patient population access to vital therapeutics.
Policy Changes in the US
One notable trend in the market is the promotion of biosimilar use over innovators through policy changes. An example of this can be seen in the US, where policies have been introduced to provide favourable reimbursement for biosimilars.
In 1992, the US federal government introduced the 340B drug pricing program. The aim of 340B was to facilitate outpatient drug access to uninsured and/or low-income patients by allowing hospitals, clinics, and outpatient facilities to purchase these therapeutics from manufacturers at discounted prices. However, in 2018, the Centres for Medicare and Medicaid Services (CMS) significantly reduced drug reimbursement for therapeutics purchased through the 340B drug pricing program. These Medicare cuts severely impacted sales of both innovators and biosimilars, discouraging developers from entering the market.
In 2021, policy changes in the US awarded biosimilars a "pass-through status". This means that for 340B-designated hospitals (those recognised by Section 340B of the Public Health Service Act to care for many uninsured and low-income patients), biosimilars are now reimbursed at a higher rate than before. Now, biosimilars are reimbursed at a rate of average sales price (ASP) plus 6%, as opposed to the previous rate of ASP minus 22.5% which still applies to the innovator products.2
This policy revision has completely changed the face of the biosimilars market, incentivising 340B-designated hospitals to prescribe biosimilars over innovator products. However, there has been some backlash from developers of originator products claiming that biosimilars should not be awarded pass-through status as they are highly similar to existing products rather than being "innovative". Despite this, these policy changes are likely to be retained and continue to help patients access critical therapeutics.
Improved patient access to biological drugs is not only being facilitated in the US but is now predicted to be seen globally through more streamlined methods of biosimilar approval. The World Health Organization (WHO) is revising its 2009 guidelines for developing biosimilars following scientists highlighting the need for policies to effectively make biosimilars less costly to attain.
The 2009 guidelines were introduced as a "living" document, providing scientific principles and a stepwise approach to demonstrate the similarity between a biosimilar and the originator. The WHO Committee recommended a revision of the guidance in 2020 to evaluate new developments and identify areas where guidance could improve flexibility without compromising its basic principles.



The revisions propose that if biosimilarity can be inferred from other parts of the comparability exercise, an "adequately powered" comparative efficacy trial may not be necessary.3 This means that the large and often expensive Phase III trials previously required to demonstrate confirmatory efficacy could be forgone with suitable alternatives, effectively reducing development and manufacturing costs.
The WHO has highlighted that the decision to revise these guidelines is to reflect the anticipated increase in biosimilar availability, leading to increased competition and decreased pricing, which should improve access to biosimilar products.
With WHO guideline revisions remaining on the horizon, for now, some developers are aiming to outsource to Australia, where clinical trials can be conducted under a streamlined timeframe and at a lower cost.
Currently, Australia offers developers increasingly attractive tax incentives that aim to enable innovation and growth by offsetting some of the costs of certain R&D activities. This means that some biopharma companies are eligible to receive a tax rebate of up to 43.5% on clinical trial-related R&D expenses.4
Relief of the biosimilar clinical trial burden is further supported by the flexibility of the Australian clinical trial process, which offers increased speed while achieving comparable quality to data sourced from US clinical trials. In a market where accelerated timelines to market can make or break gaining a market share, it is not surprising that more than 50% of global clinical trials have treatment sites in the Asia-Pacific region.
With these incentives and benefits set to drive developers towards an increasingly cost-effective biosimilars market, those taking on the challenge will need to carefully consider options that can minimise delays and compress timelines. If not, there will be a risk of missing out due to growing competition.
Speed to market requires development programs that can operate under compressed timelines while minimising delay risks. A key element that must be considered to achieve this is the need for thorough and complete assay development and validation of bioanalytical assays that support biosimilar programs.
Initial assay development and validation of bioanalytical assays supporting a biosimilar program must also be thorough and complete. This will involve incorporating all necessary evaluations to assess critical program characteristics, ultimately allowing for meaningful interpretation of necessary comparability study data.
As process development and analytical method development typically go hand in hand, appropriately designed and robust assays will be required promptly to avoid bottlenecks and process hold-ups. However, analytical development and validation take time, especially if platforms or basic methods do not already exist. Relying on support from those with analytical
expertise and experience in development and validation could prevent a product from being delayed on its journey to market and potentially missing the opportunity for success.
As the patents for approved blockbuster drugs naturally come to an end, the desire for increased patient accessibility is being further facilitated by regulatory revisions, policy changes, and reduction in development costs. Offering attractive prospects for financial gain, the biosimilar market will likely continue to swell, with competition translating into a reduced cost burden for patients.
However, being first to the post in the race to market is no simple feat. Biosimilar developers will need to contemplate how processes can be accelerated without detrimentally impacting safety and efficacy. One important area that cannot be overlooked is bioanalytical development, where the results of the assays performed will influence the determination of whether the drug is "highly similar" enough to be considered a biosimilar.
1. https://www.statista.com/statistics/1247142/expiring-patentsblockbuster-small-molecule-drugs-worldwide/
2. https://www.ropesgray.com/en/newsroom/alerts/2022/06/ Supreme-Court-Overturns-Reduction-in-Reimbursement-forOutpatient-Drugs-Purchased-by-Hospitals
3. https://cdn.who.int/media/docs/default-source/biologicals/ who-guidelines-on-evaluation-of-biosimilars---4-nov-2021. pdf?sfvrsn=f17799ae_5
4. https://www.svb.com/industry-insights/healthcare-life-science/ the-advantage-of-oz-offshore-tax-rebates-for-lshc-rd-inaustralia#:~:text=Key%20Takeaways,and%20accelerate%20 time%20to%20market.
Jim McNally, Ph.D., Chief Scientific Officer has an extensive background in bioanalytical assay development and program leadership spanning nearly 20 years working in the pharmaceutical and biotechnology industry.

Lynn Kamen is a Scientific Officer at BioAgilytix. She earned her Ph.D. in Immunology at the University of Michigan and completed a postdoctoral fellowship in immunology at the University of California San Francisco. Lynn has over a decade of experience working in drug development, from early target discovery through clinical development for both large and small molecules at several companies including Portola Pharmaceuticals, and Alector. More recently, Lynn was a principal scientist at Genentech where she supported the in vitro biological characterization of large molecules and lead the development of immunogenicity assays including ADA, NAb and immunogenicity risk ranking assays. She is co-lead of the AAPS NAber working group and member of the AAPS NAb drug tolerance sub-team.



































The refinement of sterile manufacturing processes had a central part to play in enabling the pharmaceutical industry to successfully deliver billions of doses of COVID-19 vaccines to tackle the coronavirus pandemic. But, are there further efficiency savings that can be made to enable an even faster response in the future? Jon Reed, Head of Strategy, Recipharm argues “yes”.


The manufacturing and commercialisation of vaccines to tackle COVID-19 took place at an unprecedented speed and on a scale never before seen in the pharmaceutical industry. Billions of doses of effective mRNA vaccines have now been delivered to patients around the world, and Billions more continue to be manufactured.
Overall vaccine spending in 2021 has been higher than any other year on record,1 and total cumulative expenditure for the COVID-19 vaccine is projected to reach $157 billion by 2025.2
The last 24 months have demonstrated the value of mRNA vaccine technology. Harnessing messenger RNA (mRNA) technology and commercial manufacturing partnerships, pharma companies were able to accelerate development and deliver an effective mRNA-based response to the virus. They have shown how the pharmaceutical development and manufacturing process can be taken to new levels of speed and efficiency, all
while maintaining optimum levels of sterility required for vaccine production.
In addition, they highlighted the importance of partnerships with contract development and manufacturing organisations (CDMOs). These outsourcing partners were able to provide ready-made sterile processing infrastructure for large-volume aseptic fill and finish of the vaccines. For example, with the help of their commercial partners, Moderna and Pfizer/ BioNTech have been able to deliver more than 4 billion doses to patients to date. The support of experienced sterile processing specialists with aseptic manufacturing capabilities was essential, providing pharmaceutical companies with immediate access to the infrastructure needed to develop mRNA vaccines and commercialise them successfully.
Nevertheless, the success of the COVID-19 vaccine roll-out does pose a challenge for pharma companies and their outsourcing partners when developing new vaccines and biopharma treatments for the next influenza or coronavirus outbreak. The general public, investors and other key stakeholders now have heightened expectations about what the industry can achieve and how quickly they can deliver. As such, there is pressure on pharma companies to achieve even greater speed and effectiveness next time.
With this in mind, companies must reflect on what they got right and what went wrong during the vaccine drive over the past year to ensure they are well prepared for future wide-scale drug developments.
Having received both EU and FDA approvals for its COVID-19 vaccine, a developer was poised to commercialise its mRNA vaccine candidate and to distribute it to global healthcare providers (HCPs) as quickly as possible. In keeping with contemporary drug development and commercialisation business models, the decision was made to outsource to Recipharm and two other external manufacturing partners to deliver the capabilities and capacity to fill and finish billions of doses of its vaccine before the end of 2021.
As one of the two mRNA vaccine candidates approved to fight the current strain of COVID-19, this vaccine developer needed to commercialise its formula with minimal delay. Once engaged, Recipharm defined the capacity and capabilities it would need to fill, finish and package the vaccine, enabling it to meet the company’s ambitious commercialisation goals.
Recipharm also had to work flexibly to overcome the temperature requirements – a maximum of 96 hours was allowed from the thawing of the drug substance to the final freezing of the drug product in secondary packaging. Exposing the formulation to room temperature conditions for longer than this period would impact stability and in turn efficacy of the vaccine.

The many advances the pharmaceutical industry made in its efforts to tackle coronavirus resulted in many difficult lessons. Nevertheless, this education has been invaluable, delivering a new development paradigm with better practices, new efficiencies and technologies. For Recipharm, the features of this new paradigm include:
• Capability must be flexible and adaptable
A crucial asset for any future large-volume sterile processing project is flexible and dynamic capacity. During the COVID-19 pandemic, existing sterile filling infrastructure had to be upgraded and changed over rapidly to support the filling of large vaccine batches.
In addition, many new lines had to be constructed from the ground up to expand capacity. Recipharm worked with a supplier of prefabricated modular sterile production lines to deliver additional vaccine filling capacity at its site at Monts, France, within months instead of years, in order to support a COVID-19 vaccine developer.
Now that the intensity of COVID-19 vaccine production is diminishing, these lines – new and old – are being changed over to support new projects. Flexibility is crucial to enable the rapid and efficient transitioning of sterile filling lines to new projects during “normal” non-pandemic conditions. This can ensure the new infrastructure delivers value for the industry day-to-day, while also making sure required capacity is on hand in the event it is needed for a future outbreak.
• Sterile filling infrastructure needs to handle a diversity of packaging
To support with flexibility, sterile filling capacity needs to be capable of handling a diverse array of primary packaging ranging from vials and ampoules to pre-filled syringes and cartridges for auto-injector devices. This is vital to enable sterile fill and finish infrastructure to be easily upgraded and changed over either to ramp up vaccine production in an outbreak situation, or to allow production of new injectable products in non-pandemic conditions.
Digital automation and adaptable processing equipment can help achieve this diversity in the future, enabling CDMOs and other manufacturers to deliver sterile filling support to any project that needs it.
Knowledge sharing of process performance is key
Another key lesson from the COVID-19 is the importance of collating and sharing insight into process best practice to support
companies in delivering high-quality vaccine doses as quickly and efficiently as possible.
For each COVID vaccine, the product’s chemistry and formulation attributes had to be carefully evaluated, then optimised during manufacturing to minimise dead volume and to enhance the efficiency of process flows.
These process enhancements offer benefits for future products, potentially optimising manufacturing efficiency – as such, we should consider sharing knowledge of COVID efficiency successes and apply learnings to new projects.
Vaccine transport and storage considerations and other formulation challenges need to be factored in as early as possible
Many of the vaccines developed to tackle COVID-19 presented challenges for innovators with regard to their formulations, as well as the safe storage and transit of finished doses. Some early vaccines were difficult to formulate effectively and consistently at scale, impacting on manufacturing speed and efficiency.
Adding to the complexity, several mRNA vaccines in particular had to be stored and shipped at temperatures as low as -80°C to ensure they arrived at clinics in top condition. Developing the required supply chain and infrastructure to enable this can be difficult even in mature economies such as Europe and North America. Doing the same for some emerging markets is an even greater challenge.
As development of COVID-19 vaccines and boosters has continued, innovators have begun to address the cold-chain issue. Formulation innovation has taken place to allow vaccine doses to be stored at higher temperatures without degradation of performance. As such, they have not only streamlined the delivery of doses to mature markets, but have also made it easier to ship to emerging economies and remote geographic regions, helping increase access to vaccination for billions of people.
These formulation advances should be taken into consideration by vaccine innovators. They can help to inform future vaccine development, potentially further improving the speed and efficiency of a vaccine roll-out in the event of a future pandemic.

A leading US-based clinical-stage mRNA medicines company approached Recipharm in mid-2020 to support the manufacture of clinical trial supplies of a promising COVID-19 mRNA vaccine candidate for a Phase III trial.
Recipharm was asked to fill and finish 160,000 units of mRNA vaccine and prepared the finished drug product for distribution to clinical Phase III trial patients.
When the vaccine innovator presented the candidate for technology transfer, it was originally proposed as an end-to-end fill, finish, and packaging project to deliver a ready-to-administer sterile, liquid injectable for clinical trial.
However, as an mRNA product, the vaccine candidate had cryogenic logistics and storage requirements that posed challenges when it came to its intended market –under-served populations with limited access to developed healthcare infrastructures. As such, a different formulation approach was needed. Lyophilisation was chosen to ease the complexities of distribution and extend, without freezer storage, the shelf-life of vaccines at the point of care.
Developed in collaboration with the intellectual property (IP) owner, this innovation was a unique first in the history of mRNA vaccines. Being a vaccine intended to fight a current strain of COVID-19, keeping to strict timelines was of paramount importance. As such, there was little room for error, let alone a major change to the vaccine’s Target Product Profile (TPP), during technology transfer.
Complex formulation requirements also added to the programme’s technology transfer challenges. The original formulation called for manufactured lipid nanoparticles (LNP) to be combined in-train with mRNA from a third-party.
Engaging internal technical transfer development experts as well as the resources of the entire biochemistry department Recipharm identified potential bottlenecks and arrived at a viable solution. Recipharm de-risked formulation processes and quickly integrated and optimised new LNP and mRNA combining processes. Chemistry, Manufacturing and Controls (CMC) filings quickly followed to validate the change to the original formulation during technology transfer.
Manufacturing and product handling must be superefficient. Regardless of the batch size a manufacturer doesn’t have long from receiving the sterile product, filling, lyophilising, and finishing each dose before performing secondary packaging. For any external contractor that means maintaining strict storage temperature requirements throughout the course of the project.
To ensure the success of the vaccine programme, Recipharm fully engaged its broad range of technologies including advanced digital manufacturing management platforms, a diverse array of product and data analytics, and aseptic high-speed fill/finish and packing systems. On-site LnP mixing capabilities also proved effective. Ultimately, tight collaboration and experienced capabilities combined to deliver the finished drug product on time and in full.
From the rise of biologic treatments requiring injectable delivery, to an increase in the popularity of ophthalmic administration, a number of megatrends in the pharmaceutical space have resulted in an increase in investment in sterile fill/finish capabilities. Nevertheless, sterile capacity can be demanding, complex and costly for pharmaceutical companies to install and operate themselves.
As the COVID-19 pandemic demonstrated, sterile fill and finish capacity provided by outsourcing partners is vital to the flexibility and success of the pharmaceutical industry, providing essential infrastructure when and where it is needed.

Recognising that human beings represent the most significant risk to contamination in an aseptic environment, there has been a growing focus on applying technology to remove people from the sterile manufacturing process almost entirely. Industry focus is also turning towards rapid microbiological methods due to their ability to streamline manufacturing and enhance environmental monitoring performance.
Modern sterile manufacturing facilities are designed to maintain a high degree of segregation between process steps and to minimise product exposure to the external environment. In support of this change, high levels of automation are being adopted to limit operator intervention and, as a result, reduce the potential for contamination. In addition, new regulations, such as the European Union’s (EU) GMP annex 1, are having a major effect on how the industry must approach processes on aseptic production lines.
The demand for aseptic manufacturing capacity is likely to remain high for the foreseeable future. New COVID-19 strains mean that boosters are going to be required to continue to protect vulnerable demographics. mRNA vaccines will be developed for influenza and other treatments. New non-vaccine biopharma treatments will also likely require sterile fill and finish.
Whatever the nature of the sterile filling project, outsourcing these capabilities to expert partners still represents the most cost-effective and time-efficient option for even the most prominent pharmaceutical companies.
1. https://www.statista.com/statistics/263102/pharmaceutical-marketworldwide-revenue-since-2001/
2. https://www.iqvia.com/insights/the-iqvia-institute/reports/globalmedicine-spending-and-usage-trends-outlook-to-2025
Jon is the Head of Strategy and works within the sterile fill finish business unit at Recipharm, one of the biggest CDMOs with in-depth knowledge of sterile filling, oral solid dose, and drug delivery systems.
Gene therapy represents an expanding area of medicine with the potential to change the lives of people with life-limiting conditions. As the interest in gene therapies has increased over the years, so has the number of clinical trials, with 372 gene therapy trials active in the first half of 2022. Adeno-associated Viral (AAV) vectors are the most common gene delivery agents for most of these trials, creating a substantial industry need for AAV vector manufacture.1
When bringing a new AAV-based gene therapy through the pipeline and towards clinical trials, it is important for a drug developer to onboard a robust, efficient manufacturing process. Although it is important for a Contract Development and Manufacturing Organization (CDMO) to build custom manufacturing processes when needed, many projects can make use of manufacturing platforms pre-built and pre-tested by the CDMO.
The advantages of platform processes are numerous compared with building a new process every time. The standardisation of processes improves consistency between runs. Using pre-validated, pre-optimised technology helps guarantee good performance while minimising delays. Staff are already trained on the specific processes, with no need for comprehensive onboarding. Keeping materials in stock helps to guarantee supply chain security. These factors combine to create a shorter end-to-end process and an accelerated time to market, as well as reduced manufacturing costs compared with a custom platform.
OXGENE and WuXi Advanced Therapies have combined our technologies and expertise to create a platform approach for transient AAV manufacture. As a Contract Testing Development and Manufacturing Organization (CTDMO), WuXi Advanced Therapies provides end-to-end services to cell and gene therapy companies seeking to discover, develop, manufacture and test

innovative drug candidates at scale for global commercialisation. We incorporated OXGENE’s AAVEX™ plasmids into our transient production system, creating a tried-and-tested workflow suitable for a wide range of projects. This AAVEX™ platform combines plasmid and cell line engineering, upstream and downstream process development, GMP manufacture and testing to deliver consistent improvements in AAV yields and quality.

HEK293 cells act as mini factories in the production of AAV, using genetic instructions encoded by plasmids. The quality of the cell line can affect the yield and quality of the resultant AAV particles, so optimisation can have substantial benefits.
As such, we screened several thousands of single cell clones in suspension for high AAV production, high cell density, and low aggregation. This helped us to find the best-performing clones.
In a head-to-head comparison between the top performing HEK293 clone and the original cell line, AAV productivity was 2–3 times higher in the top performing clone across multiple AAV serotypes (Figure 2). This clone was further demonstrated to result in robust cell growth, viability, and stability. Master cell banks (MCB) and working cell banks (WCB) were fully banked and released ready for internal and external client projects within our AAV manufacturing platform.
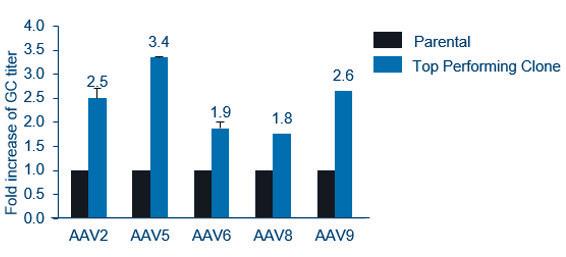
Our AAVEX™ plasmids, used within our AAV transient production platform, have been carefully engineered for optimal performance. The RepCap plasmid has been engineered to place Rep and Cap under different promoters, so that cap is expressed at a higher level than rep. This reconfiguration results in up to an eightfold increase in AAV expression compared with the industry standard (Figure 3). In addition to yield, this reconfiguration improved infectivity and packaging efficiency of AAV vectors.
Figure 3: Reconfiguration of the Rep-Cap system improves viral vector yield, here shown across three AAV subtypes
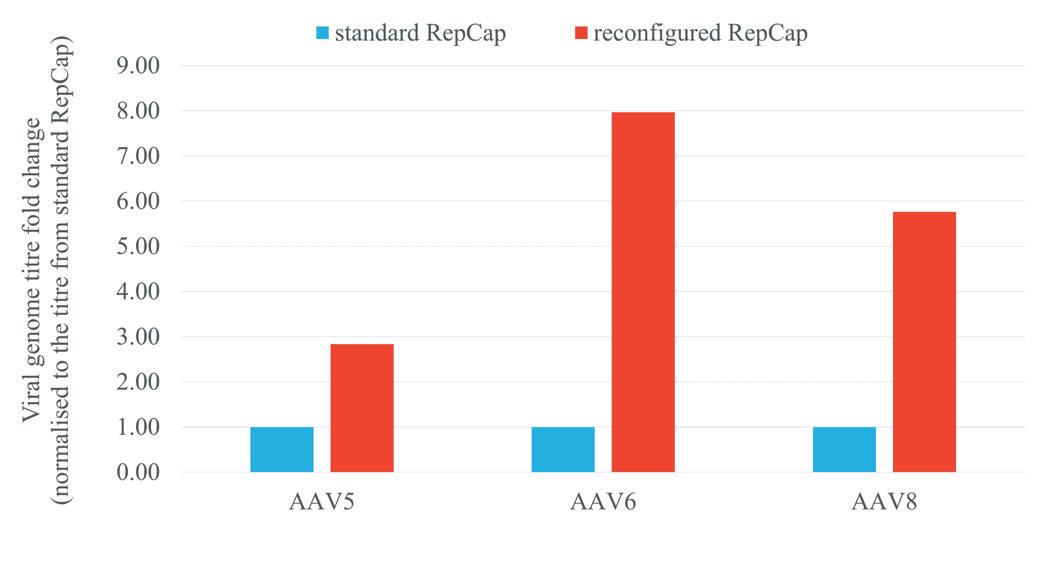
In addition, we have simplified the Ad5 helper plasmid to remove unnecessary adenoviral genes. This further improves the plasmid yield while also providing a regulatory advantage.
Careful selection of the HEK293 cell line and plasmid engineering have substantial benefits for AAV yield and quality, but found that the system could be further improved by optimising other aspects of the upstream process.
These features can be divided into three main groups: transfection conditions, bioreactor parameters, and medium development (Figure 4). By carefully optimizing each parameter within the upstream development process, we have found further improvements to AAV yield across multiple serotypes.
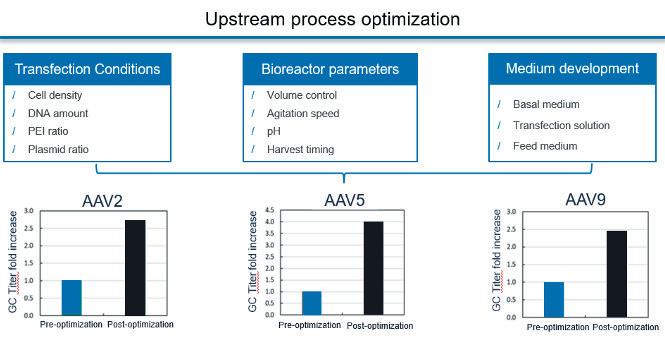
processes to ensure purity. Ultracentrifugation, by contrast, takes less time to develop but is less scalable. A combination of these two methods combines some benefits from both techniques, requiring moderate development work but yielding a high percentage of full capsids (88–94%).
The AAVEX™ platform is a proprietary, optimized AAV manufacturing platform that provides excellent yields, scalability and process robustness using a high performing single clonal cell line and carefully engineered plasmids. We have further developed the upstream and downstream processes, such as by tweaking the transfection conditions, bioreactor parameters, medium development, harvest time, affinity capture, and polishing steps to create a system that doesn’t need to be reinvented for each project. In this way, customers working with WuXi Advanced Therapies for AAV manufacture benefit from our experience operating in the AAV-based gene therapy space.
1. Alliance for Regenerative Medicine H2 2022 report, available here: Regenerative Medicine: The Pipeline Momentum Builds - Alliance for Regenerative Medicine (alliancerm.org

Dr. Katie Roberts is Content Manager at OXGENE, a WuXi Advanced Therapies company. She has been writing about science and communicating science, first as a Medical Writer and then within biotech marketing, for most of the last five years. Before this, she completed her PhD in cellular signalling at the University of Manchester, helping to decode oscillatory cell signalling patterns using molecular biology, live-cell confocal microscopy, and mathematical modelling.

We have also optimised the downstream purification process to deliver the best results. Careful material selection and optimised buffers, for example, have led to greater enrichment of full capsids and improved AAV recovery.
Our platform offers three options for the AAV full capsid purification and polishing step, depending on specific project needs. Ion exchange chromatography provides an option, which is easy to scale up and fewer steps, but takes longer to develop
Dr. Man-Shiow Jiang, Executive Director of Viral Vector Process Technology Development at WuXi Advanced Therapies, has over 22 years of experience with bioprocesses for cell and gene therapies. Man-Shiow is responsible for developing viral vector manufacturing platforms to reduce therapeutic timelines from research to commercialization while improving processes and cost effectiveness. She has extensive experience working in CDMO environments, and with private companies and government contractors. Man-Shiow earned a master’s in chemistry from National Taiwan University and holds a PhD. in biochemistry, biophysics, and molecular biology from Johns Hopkins University.

The availability of a wide range of therapeutic biologics has revolutionised modern medicine. Whilst the majority of pharmaceutical drug products remain conventional, small molecule medicines; the emergence of biotherapeutics has allowed for the treatment of many disease areas which were previously out of reach.1 Despite the positive growth in biotherapeutics (Figure 1), significant challenges will need to be overcome in order to fulfil the promise of biotherapeutics across society. With a growing number of protein therapeutics and vaccines in development2,3 one of the biggest challenges facing the product developers is the stability of these molecules.4 Biologics including vaccines are inherently unstable and prone to degradation. Therefore these medicines typically require low temperature (cold chain) transport and storage. The result is a much higher economic burden and in some cases limited access.
Biopharmaceuticals comprise of medium to large, complex, macromolecules that can be divided into 4 major classes; proteins, nucleic acids, lipids and carbohydrates. For the purposes of this article the focus will be proteins.

Protein structure can be divided into four levels as shown in Figure 2. The 3D structure of the protein is highly complex, and inherently linked to therapeutic effectiveness. If the 3D structure is not maintained, there is a risk the therapeutic effect of the drug is reduced or lost entirely. There is also the possibility the molecule can present and immunogenicity risk to the patient. It is therefore vital to maintain the structure of the protein.
The difficulty arises due to the structure of a protein being relatively weak, compared to small molecules. Numerous chemical, physical and colloidal degradation pathways can occur in biologics, a full review of these pathways is beyond the scope of this article. Henceforth, a simplified term of
“stability” will be taken to mean that all degradation pathways that negatively impact activity or safety are reduced.
As a result of these numerous degradation pathways, it is typically the case that biopharmaceutics require a cold-chain to be maintained throughout the supply chain, and ultimately until the medicine is administered. If the material cannot be maintained under the required conditions then the consequence may be; reduced availability of medicines, product waste, loss of therapeutic effect or even risk of immunogenicity.
Even if a robust cold chain is in place, the financial impact of maintaining it is a significant contributor to the high price of biological medicines. Additionally, when a cold chain is available and the medicine affordable, the shelf life of biological drug product is typically short relative to conventional small molecule medicines. Below we briefly discuss the multiple strategies employed to enhance the stability of biologics and thus potentially reduce the cold chain burden.
There are different approaches to reducing the burden posed by
the need for cold chain storage, a non-exhaustive summary is discussed in the following sections. It is reasonable to consider that multiple strategies will ultimately be required.
Through the many steps of discovery and development of biotherapeutics there are opportunities to enhance the stability profile. Powerful in silico modelling tools enable screening of molecules with higher chances of developability.5,6 During upstream processing, post translational modifications may be included to enhance therapeutic effect as well as stability. Further downstream the efficient isolation, purification and the use of an optimised buffer system offers further gains.
One of the clearest examples of a stability enhancement opportunity occurs during downstream isolation and purification. Where it is crucial to employ highly selective process steps to remove products that could lead to degradation; for example protein fragments. An often less considered area is selection of fully optimised buffer. This is a crucial step and should be made with the end use in mind. Typically the main response that is maximised is the yield. Whilst stability is also a consideration analytical methods to predict longer term stability may not be representative. As an example even trace levels of impurities introduced by lower purity ingredients, can lead to stability issues later in the products life, however rapid screening technique often cannot discern these long terms effects. Impurities may ultimately lead to aggregation and can be very challenging to debug when the aggregation is only detected in the final formulation, but actually activated during downstream processes. It is therefore prudent to take a conservative risk based approach when selecting buffer media.
Many stability optimisation approaches are highly specific to a single molecule. They often involve expensive and time consuming studies for which the conclusions cannot be extrapolated. It would be advantageous to instead take a more generalised approach to protein stability enhancement. Degradation pathways all have a common pre-requisite, they require molecular mobility on some scale.5 Thus if one can reduce the molecular mobility, a reduction in the rate of degradation could be expected. Removal of the continuous phase of a biological system, i.e. water, offers a great potential in the preparation of robust and stable drug product.
Several technologies are capable of removal of water from an aqueous protein solution, lyophilisation, also known also freeze drying in particular shows great promise in biopharmaceutical applications. During lyophilisation the product is initially frozen, followed by removal of water ice via sublimation. This is followed by secondary drying step resulting in a powder where a residual water content of < 1 % w/w can be achieved.7
There is however a complicating factor, during the freezing and drying process, the biomolecules are exposed to stresses that can lead to physical or chemical degradation of the protein. Stresses such as; pH shift, increased ionic strength, mechanical shear stress and dehydration stress may all occur7 In order to protect the protein, a suitable combination of process parameters and excipients is required. A well-executed process
The understanding of active-excipient interactions is critical in the rational design of formulations to stabilise proteinbased therapeutic drugs and vaccines. Selection and use of the appropriate excipients enables the development of novel therapies and robust pharmaceutical products.
BioHale® key promises:
• Uncompromised Quality
Unlike taking medications orally or via inhalation, introducing a drug into the body by parenteral administration poses a greater risk since the body's natural defenses are bypassed. As such, they must be exceptionally pure and free from physical, chemical, and biological contaminants. Therefore, similar requirements are applicable for the excipients for which DFE Pharma has a specific BioHale® portfolio of high purity, low endotoxin excipients.
By making use of the best available active purification technologies in state-of-the-art cGMP, FDA inspected manufacturing facility in the Netherlands, Europe, the level of purity and endotoxin are fully controlled, resulting in market leading specifications. Our excipients, produced according to ICHQ7 guidelines, have multi-compendial specification and comply with the global regulatory requirements of the pharmaceutical industry (Ph. Eur., USP-NF, JP, ChP). BioHale® Sucrose and BioHale® Trehalose are used to stabilise the fragile biomolecules in during processing and in their final applications.
•
The established stability in the supply chain has gained customers trust due to our long history of providing reliable security of supply – even during the pandemic. DFE Pharma has local stocks in Europe, US and Japan and capacity available to ensure flawless supply of BioHale® excipients today and in future. The active purification process makes our product less dependent on raw material variability, raw material supply and raw material production campaigns.
•
Our BioHale® team of experts, no matter if is in formulation support, analytical support, excipient expertise, regulatory or quality related, we are there to support you.
optimisation and rational formulation design may result in a drug product that has good stability, in some cases a cold-chain can be entirely avoided.
Carbohydrate Systems to Maximise Stability. The selection of excipients is critical; as the vast majority of biologics are administered parenterally3,4 the need for high
purity excipients with an excellent safety profile is paramount, options are fairly limited. One class of molecules that show great promise are carbohydrates. Sucrose, Trehalose and Mannitol have long been known to offer cryo and lyo protection.7 There is no general rule for best excipient selection, however trehalose has shown great promise across a wide range of difficult to stabilised molecules such as monoclonal antibodies (mAbs).
Durvalumab
Ocrelizumab
Adalimumab
Blinatumomab
Obinutuzumab

Ranibizumab
Bevacizumab
Trastuzumab
Brentuximab
Pertuzumab
Liquid injection
Liquid injection
Liquid injection
Lyophilised
Liquid injection
Liquid injection
Liquid injection
Lyophilised
Lyophilised
Liquid injection
Table 1. Marketed monoclonal antibody formulations containing trehalose dihydrate (non-exhaustive)11
The general mechanisms by which sugars stabilise proteins during lyophilisation is described by two predominant theories; water replacement theory and vitrification theory. A critical requirement in both these theories is the formation
of an amorphous glass matrix, composed of the biologic, sugars and other excipients. In order to remain a glass, the product must remain below the glass transition temperature Tg of the matrix. Therefore if a glass with a very high Tg can be prepared, it is feasible that the protein may remain stable even at temperatures above ambient. Moisture also needs to be considered and it acts as a plasticiser, effectively reducing the Tg and allowing re-crystallisation at lower temperatures.
It common to utilise sugar glasses to stabilise proteins. For successful protection of the protein the sugar should have a high Tg, low hygroscopicity and a slow crystallisation rate. Additionally a high glass transition at the maximally freeze concentrated fraction (Tg’) is preferable.8 The disaccharide trehalose dihydrate exhibits all these properties and is therefore widely used. However there is compelling research indicating that incorporation of higher molecular weight carbohydrates with trehalose could enhance the stabilisation further.
In a recent study, binary mixtures of trehalose (a disaccharide) and varying levels of pullulan (a polysaccharide) were used during the preparation of lyophilised formulations using the model protein β-galactosidase.9 It was observed that approximately 80% activity of β-galactosidase was retained for binary mixtures of specific ratios stored at 30°C / 56% RH after for 4 weeks. In environment with very low relative humidy the benefit of the binary mixture over trehalose alone was limited. However in the more realistic environment with exposure to RH > 50% the benefits were clear. The researchers noted that pullulan itself may not be the optimal polysaccharide. Despite the high molecular weight and high Tg of the material used (261ºC), pullulan is a bulky molecule with a linear, rigid structure. The researchers hypothesized this could prevent tight molecular packing and limit the stabilising potential.9
Oligo and polysaccharides are a highly complex class of molecules and further research is ongoing.10 However to date this formulation strategy shows great promise of a universal approach in the development of stable lyophilised protein drug product, without the necessity of an uninterrupted cold chain.
In order for biopharmaceuticals to reach their maximum potential across society, the industry needs to reduce the burden of cold-chain. A crucial step in achieving this ambitious goal is the availability of technologies able to stabilise proteins under more demanding environmental conditions. Lyophilisation is discussed as a critical enabling technology. Next generation sugar glass’ are proposed as another critical technology. Specifically, by combining the established benefits of trehalose with higher molecular weight sugars such as inulin, pullulan or other oligo and polysaccharides, biotherapeutics that do not require cold chain storage may become reality.
1. Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730.
2. Kitney, R. I., Bell, J., & Philp, J. (2021). Build a sustainable vaccines industry with synthetic biology. Trends in Biotechnology, 39(9), 866-874. doi:10.1016/j.tibtech.2020.12.006
3. Ngo HX, Garneau-Tsodikova S. What are the drugs of the future?. Medchemcomm. 2018;9(5):757-758. Published 2018 Apr 23. doi:10.1039/c8md90019a
4. Batta A, Kalra BS, Khirasaria R. Trends in FDA drug approvals over last 2 decades: An observational study. J Family Med Prim Care. 2020;9(1):105-114. Published 2020 Jan 28. doi:10.4103/jfmpc. jfmpc_578_19
5. Chennamsetty N, Voynov V, Kayser V, Helk B, Trout BL. Design of therapeutic proteins with enhanced stability. Proc Natl Acad Sci U S A. 2009;106(29):11937-11942. doi:10.1073/pnas.0904191106
6. Voynov V, Chennamsetty N, Kayser V, Helk B, Trout BL. Predictive tools for stabilization of therapeutic proteins. MAbs. 2009;1(6):580582. doi:10.4161/mabs.1.6.9773
7. Bjelošević M, Zvonar Pobirk A, Planinšek O, Ahlin Grabnar P. Excipients in freeze-dried biopharmaceuticals: Contributions toward formulation stability and lyophilisation cycle optimisation. Int J
Pharm. 2020;576:119029. doi:10.1016/j.ijpharm.2020.119029.
8. de Jonge J, Amorij JP, Hinrichs WL, Wilschut J, Huckriede A, Frijlink HW. Inulin sugar glasses preserve the structural integrity and biological activity of influenza virosomes during freeze-drying and storage. Eur J Pharm Sci. 2007;32(1):33-44. doi:10.1016/j.ejps.2007.05.112.
9. Teekamp N, Tian Y, Visser JC, et al. Addition of Pullulan to Trehalose Glasses Improves the Stability of β-Galactosidase at High Moisture Conditions. Carbohydr Polym. 2017;176:374-380. doi:10.1016/j. carbpol.2017.08.084
10. Singh RS, Kaur N, Hassan M, Kennedy JF. Pullulan in biomedical research and development – A review. Int J Biol Macromol. 2021;166:694-706. doi:10.1016/j.ijbiomac.2020.10.227

11. Dickhoff, B et al, The importance of disaccharide excipients in biologics, accessed on 6 Oct 2022, https://www.european pharmaceuticalreview.com/article/143124/the-importance-ofdisaccharide-excipients-in-biologics/2/
Ross Blezard is Product and Application specialist Biopharma at DFE Pharma. A physicist by education Ross began his career in inhalation and transdermal drug delivery. After leaving his position as head of formulation development at a UK based CDMO, Ross spent several years working as a senior scientist in material sciences. In 2019 Ross joined DFE Pharma where he has supported our inhalation and biopharmaceutical customers with their technical challenges.

Mara van Haandel is Marketing Director Biopharma at DFE Pharma. Prior to becoming the marketing lead Biopharma at DFE Pharma she has been working for almost 20 years in R&D. Uncovering and realising excipient solutions that can support overcoming the challenges of customers in drug development and manufacturing is what excites her.

Plasmid-based methods have, for good reason, been a trusted method for the manufacture of adeno-associated viral (AAV) vectors for many years. As 2022 closes and we look towards 2023, plasmids will likely continue to be useful for gene therapies already in the pipeline. The refinement of robust plasmid-based manufacturing platforms, in particular, will further support drug developers as they prepare for the later stages of their therapeutic journey to commercialisation. Improvements in viral vector yield, as well as reduced costs and timelines, are of particular importance and are possible to achieve using platform approaches.
Key priorities for 2023 and beyond will be to reduce the costs of gene therapies and expand their accessibility to patients who need them, once novel therapies obtain regulatory approval. The ability of plasmid-based processes to meet increasingly ambitious challenges is likely to be limited and novel, disruptive technologies are keenly needed. One such disruptive technology, TESSA™ technology, has the potential to substantially increase yields compared with plasmid-based systems; could it be the future of gene therapy manufacture?
Plasmids: An Old Standard but a Gold Standard AAV vectors are commonly manufactured in HEK293 cells transiently transfected with a set of plasmids, containing all the genetic instructions needed to produce AAV. Advancements in plasmid engineering have reinforced the successes of plasmid-based manufacturing platforms. Reconfiguration of the RepCap genes, as well as removal of additional hexon genes in the adenoviral helper plasmid, have been found to enhance viral vector yield and performance. In addition to plasmid optimisation, fine-tuning of other aspects of platform approaches, which hold benefits in terms of pre-validated methods, equipment, and in-stock raw materials, can bring down timelines and costs.
There are, however, inherent limitations of working with plasmids. Transient transfection of plasmids into HEK293 cell lines is variable, limiting the maximum yield of AAV and introducing batch-to-batch variability. Plasmids may be genetically unstable, limiting quality and consistency when scaled up to the substantial quantities needed for treatment. The plasmids supply chain is beset with bottlenecks, with relatively few facilities able to produce plasmids to GMP grade.
One option for a high yielding, plasmid-free approach is to use wildtype adenovirus to manufacture AAV. However, this method introduces the real possibility of adenovirus contaminating the final product, with expensive decontamination steps required to remove this contamination. Although high yielding, the decontamination costs may be a barrier to success.
Could it be possible to use an adenoviral system to produce high AAV yields while avoiding contamination? To do this, scientists closely examined the adenoviral lifecycle, which contains two distinct temporal phases; early and late, with the early phase providing adenoviral help for AAV production but the late phase contributing to adenoviral contamination. Using a doxycycline-inducible system, they found a way to switch on the useful early genes while tightly repressing late-phase genes. This resulted in high yields of AAV vector but reduced adenoviral contamination by 99.99999–100%. The adenoviral vectors they built were termed TESSA™ vectors, short for tetracycline enabled self silencing adenovirus.
To produce AAV, the Rep and Cap genes, as well as the gene or capsid of interest, are stably encoded into two TESSA™ vectors which are then used to infect HEK293 cells (Figure 1). Using the TESSA™ system, the cells’ resources are entirely directed to making AAV rather than adenovirus, resulting in even greater yields than manufacture using wildtype adenovirus.

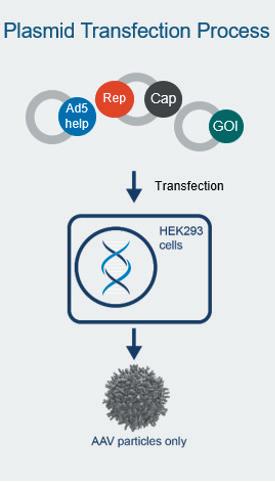
TESSA™ technology has been scaled up through 50 L and to 200 L so far, with the potential to be scaled up further to over 2000 L due to the lack of limitation by cell or volume density. At a 200 L scale, TESSA™ has demonstrated a 30 to 40-fold increase in AAV titre compared with plasmid-based manufacture at the same scale. The ability of TESSA™ to generate substantially more AAV, compared with plasmid-based manufacture, could mean that more patients are treated from a from a single manufacturing run. This in turn could open the door to treating more patients in a cost-effective way (Figure 2).
In addition to producing higher yields, TESSA™ has been shown to produce more infectious AAV particles than plasmidbased manufacture at a 200 L scale, and with ~95% full capsids.

Peer Reviewed, IPI looks into the best practice in outsourcing management for the Pharmaceutical and BioPharmaceutical industry.

www.international-pharma.com
Peer Reviewed, JCS provides you with the best practice guidelines for conducting global Clinical Trials. JCS is the specialist journal providing you with relevant articles which will help you to navigate emerging markets.

www.journalforclinicalstudies.com
Peer Reviewed, IAHJ looks into the entire outsourcing management of the Veterinary Drug, Veterinary Devices & Animal Food Development Industry.

www.international-animalhealth.com
Peer reviewed, IBI provides the biopharmaceutical industry with practical advice on managing bioprocessing and technology, upstream and downstream processing, manufacturing, regulations, formulation, scale-up/technology transfer, drug delivery, analytical testing and more.

www.international-biopharma.com

PNP
Pharma Nature Positive, is a platform for all stakeholders in this industry to influence decision making by regulators, governments, investors and other service providers to achieve Nature Net Positive Results. This journal will enable pharma the ability to choose the right services to attain this goal.

www.pharmanaturepositive.com
Listen to industry experts on the latest in drug discovery, development, research, industry regulations and much more at Pharma,s DNA, the podcast channel by Senglobal Ltd., available on Sound Cloud, Spotify, iTunes and YouTube.
An increase in infectivity or improvement in the ratio of full capsids could have particular relevance to the question of how we can reduce the therapeutic dose of gene therapy needed to treat a patient. Currently, the high doses of AAV often needed for therapeutic effect represent a possible safety issue.1 Improving particle infectivity or percentage of full capsids, therefore reducing the total volume of AAV needed for treatment, could provide a solution.
Positive results for TESSA™ vectors were published in Nature Communications in March 2022, helping to cement the promising future of the technology.2,3 Based on these findings, WuXi Advanced Therapies launched TESSA™ for small-scale evaluation ahead of GMP manufacture. The company offers TESSA™
evaluation kits to academic labs and therapeutics companies, allowing them to assess TESSA™ in their own lab far ahead of GMP-grade manufacture.
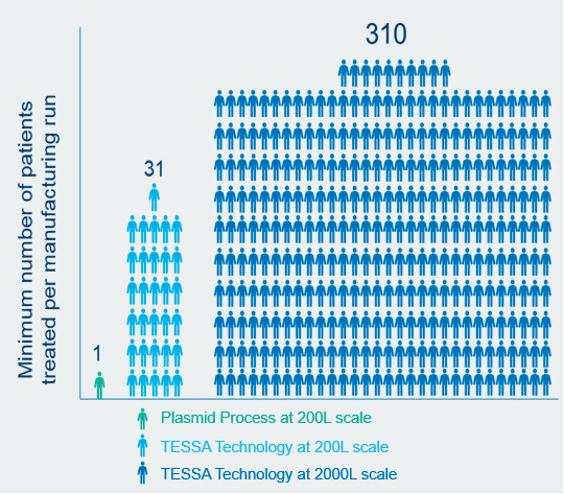
Plasmids have been, and will continue to be, important tools for the manufacture of AAV-based gene therapies, especially those currently in the therapeutic pipeline. Optimisation of plasmid-based platform technologies will continue to be relevant and will help to shape the success of gene therapies yet to reach the market. However, over the next few years, as more gene therapies enter the therapeutic pipeline, TESSA™ technology could help make even more treatments scalable and accessible to patients in need.
1. Kishimoto TK and Samulski RJ (2022). Addressing High Dose AAV Toxicity –‘One and Done’ or ‘Slower and Lower’? Expert Opinion on Biological Therapy.
2. Su W, Patrício MI et al (2022). Self-Attenuating Adenovirus Enables Production of Recombinant Adeno-Associated Virus for High Manufacturing Yield Without Contamination. Nature Communications.
3. Launch of TESSA™ Technology – https://oxgene.com/news_and_ views/news/81/launch_of_tessa_technology
Dr. Katie Roberts is Content Manager at OXGENE, a WuXi Advanced Therapies company. She has been writing about science and communicating science, first as a Medical Writer and then within biotech marketing, for most of the last five years. Before this, she completed her PhD in cellular signalling at the University of Manchester, helping to decode oscillatory cell signalling patterns using molecular biology, live-cell confocal microscopy, and mathematical modelling.

Research to commercial supply of high-quality plasmid DNA, RNA, and Protein

• Over 24 years of manufacturing experience
• ~ 3,000 lots produced for cGMP manufacture of cell and gene therapies
• 140,000+ lots produced for research, clinical trials, and commercial products
• Extensive experience in consistently achieving lots released at the 25g+scale (supercoiled and linear pDNA)
• Standardized, in-stock products at research and clinical grades for viral vector manufacturing, gene editing, and more
Contact us today to get started.
4055 41st Avenue South, Fargo, ND 58104 USA +1 (701) 297-9256 Toll-free (U.S. and Canada): +1 (877) 787-3362 a ldevron.com
The biopharma industry requires digital transformations to improve efficiencies and deliver critical therapeutics to patients at an accelerated rate. Although great efforts have gone into implementing data-driven business models and processes, the industry has seen mixed results in return, with the digital evolution remaining in the early phases of development.
Lack of standards, vision, and appropriate strategies keep digitalisation programs from success, possibly preventing biopharma from achieving its goals for a data-driven future. Clearly, biopharma needs a shared vision and strategic roadmap to facilitate this transformation.
In this article, Samsung Biologics’ Chief Information and Marketing Officer, Process Improvement, James Choi delves into the shared vision of biopharma’s leaders to drive a digital transformation in their organisations. He also emphasizes the need for the industry to prepare for the road ahead.
Need to Accelerate Biopharma’s Digital Transformation
In the biopharma industry, mergers and acquisitions (M&As) have helped elevate companies, translating into a wider variety of offerings, greater levels of expertise, and expanded geographical reach. However, when organisations join together, siloed digital infrastructures can arise and lead to difficulties. The challenges and costs involved in adopting holistic data management systems to rectify these issues mean that many of these companies are running disparate legacy technologies that are decades old.
Consequently, the biopharma industry is constrained from developing efficient data-driven operations and is in danger of a future filled with data integrity (DI) challenges. This is highlighted by the fact that 75% of FDA manufacturing warning letters in 2018 cited DI as an issue, more than doubling the 28% seen in 2014.1
Despite this need for the industry to accelerate its digital transformation, recent polling has identified some hesitancy in adapting data-driven digital business models.2 In fact, in a survey of equipment vendors involved in designing and upgrading their biopharma clients’ facilities, 27% stated digitization is rarely part of the discussion.2
This lack of consistency in digital innovation is not sustainable. However, with industry-developed standards and digital plant maturity models, the biopharma industry can start to address these issues and accelerate progress.
Embracing digital transformations can be daunting for biologics manufacturers, especially considering the growing complexity
of these drugs, which can require complex, multi-step manufacturing processes. However, ensuring product quality not only relies on expertly designed and operated processes, but also an appreciation of the intricate supply chains, cold-chain logistics, and handling required. Manual processes in biologic production can further compound the complexity, as with manual handling and operation comes the increased probability of human error.
Being able to secure DI (making sure data is complete, consistent, and accurate throughout its lifecycle) is vital to drug quality and patient safety. The consequences of complexity, error, and uncertainty surrounding DI in biologic development and manufacturing are multifaceted. In worst-case scenarios, failure to ensure DI can lead to safety risks, delays, recalls, remediation costs, and denied approvals. Ultimately, all have the potential to generate huge financial losses and operating consequences for the biopharma companies involved.
Avoiding these issues will rely on the biopharma industry embracing digital tools and operational platforms to mitigate risk and assure compliance. Pharma also needs to embrace data-driven business and operational models to ensure DI is sustained throughout biologic development and manufacturing.
The importance of DI in ensuring the safety and quality of biologics has long been understood by regulatory authorities. All major regulatory bodies, including the FDA and EMA, have developed similar guidelines surrounding DI, and these have been regularly updated to abide by current good practice (cGxP) practices.
The first guidance issued by the FDA in the early 1960s has been regularly updated with the understanding that managing the wealth of data that arises from biologics manufacturing relies on an array of digital systems. Regulators understood these technologies were needed to help implement controls and safeguard DI.
The FDA highlights that complete, consistent, and accurate data need to be ALCOA (attributable, legible, contemporaneous, original, and accurate). Regulators then introduced ALCOA+ principles to further emphasize that data should be complete, consistent, enduring, and available.
Despite available guidance, understanding and implementing DI compliantly remains a challenge for biopharma developers and manufacturers. These difficulties largely stem from a lack of strategic goals, poor risk assessment, and effective approaches for digital transformations that focus on establishing and maintaining a robust DI response.
Setting Goals Strategically From the Outset
True digital transformations focus on minimising complexity
and simplifying the experience employees have when managing and interacting with the systems and operations being implemented. Ideally, the first step will centre on optimising business and operational processes by applying well-understood process improvement principles. Intrinsic to operational excellence, these principles should be applied with the client and program in mind every step of the way.
However, before embarking on such a transformational journey, the organisation must be clear on the goals it aims to achieve. If objectives are not clear and well-prioritised, these initiatives may have trouble achieving the desired results. Companies that rely on a technology-first approach to digital transformation may not have their priorities straight or the effective strategy they need to digitally transform operations and achieve DI across the enterprise.
There are many digital technologies available that can help those in the biologics manufacturing industry record, manage, and store data. When correctly implemented with robust DI controls, users have the tools to be proactively compliant and minimise human error. This can be seen in the application of validated laboratory information management systems (LIMS). In support of lab operations efficiency, LIMS can help standardise and harmonise workflows, enabling enhanced data flow management.
However, the success of these systems in the improvement and enhancement of DI is often attributed solely to the fact that a digitised solution has been incorporated into the process. This is a false assumption, leading to a common issue when approaching digitalisation: focusing on the technology and not the process it is trying to improve.
Converting paper entries into electronic logs via a digital logbook solution can make assets easier to track and, in theory, could sharpen transformation strategies. However, from an end-user perspective, switching from manual entry to a keyboard and screen is only marginally more convenient.
Instead, analysing the information needed from the source and eliminating the need to manually collect it altogether may be the better approach. By connecting the source (e.g., a lab instrument or equipment) to a centralised system, information that would have been entered into the logbook can be collected automatically. This obviates the need for a logbook – paper or electronic – to begin with.
Although a paperless environment can be achieved as a consequence of digitalisation, it should not be the sole key performance indicator of success, nor the initial objective of the effort. Identifying and converting paper-based forms and procedures to digital processes without broader, strategic reasoning merely shifts manual processes to automated ones without actually optimising the process of generating any true efficiency or productivity gains.
The SOAO method – Simplify, Optimise, Automate, Outsource – is an approach to digitalisation that ensures processes
are digitised in a way that ensures DI while guiding optimal digitally-enabled process development with four key steps:
The first step of the SOAO method involves concept-guided simplification, the goal of which is to streamline all the steps and procedures involved in the process and digitising them to maximise efficiencies and deliver customer value. Simplification will involve the evaluation and assessment of each step in the process to determine whether it is critical to quality and whether it adds value.
Once the process has been streamlined with potentially redundant steps removed, developers and manufacturers can move on to optimising the remaining steps. Various methodologies and tools can be adopted by the organisation to achieve this, including:
• Suppliers, Inputs, Process, Outputs, and Customers (SIPOC) analysis
• Define, measure, analyse, improve, and control (DMAIC) strategies
• Lean Six Sigma philosophies
By optimising the process before introducing digital technologies, businesses can avoid generating problematic processes and subsequently prevent introducing new inefficiencies into established operational systems.
With a simplified and optimised process, organisations can begin to incorporate digital tools and automation into the business environment. By carefully selecting the right technologies, using critical thinking, and evaluating how the solution could meet its intended use, unnecessary existing procedures could be eliminated, as seen in the previous logbook example.
Finally, businesses will need to decide whether outsourcing digital processes could offer a longer-term strategic solution that could ultimately be in the best interest of both clients and shareholders. Making this decision will rely on the evaluation of all processes to determine whether each adds strategic value or presents a critical competitive differentiator. By outsourcing processes that do not offer this value, agility and focus can be improved elsewhere while leveraging internal cost structures and scalability options.
Implementing the SOAO method as opposed to a technologyfirst approach to solving process efficiency problems can offer operators several benefits. Enhancements to DI will ensure the reliability of data and support data-driven decision-making. Artificial intelligence has a role here as well, delivering more actionable information from enterprise data. Improvements in productivity may also arise due to a better employee experience and more value from the data, ultimately helping to drive business growth and innovation. For contract development and manufacturing organisations (CDMOs) implementing the SOAO method, there are several additional advantages to improving digital efficiencies including:
The most obvious benefit that can come from applying the SOAO method is it could minimise human error and subsequently improve quality. With digital tools correctly implemented to harmonise processes, the potential for errors can be reduced by a significant level. As a result, the quality and safety of the biologics produced can be better safeguarded.
When producing biologics, there are many parts, partners, and processes involved in delivering a secure supply chain. These are often data-driven and can present several challenges:
• Raw material supplies require monitoring to avoid potential disruptions
• Track and trace and serialisation can be needed to meet supply chain security compliance
• More stringent demands in supply chain security surround cold chain custody and personalised medicines
By implementing SOAO, developers and manufacturers can introduce digitised solutions with the industrial strength to overcome these challenges. Block chain technologies, for example, offer the connectivity and interoperability biopharma needs to create the more secure, transparent supply chains it needs to reach patients.
To effectively support their clients, CDMOs must ensure clients have easy and rapid access to their projects’ data (e.g., equality information) as well as receive updates on the project status. CDMOs must also offer transparent communications with regulators, helping to build trust and prevent project delays.
Using the SOAO method to improve communication processes and security, CDMOs are able to access and leverage the public cloud. This can provide an infrastructure that is scalable and accessible by contract partners and biopharma, regardless of where they are. A variety of other tools are also available that enable optimised, more strategic communications:
• Secure portals for sensitive program data and information with 24/7 access to documentation
• Virtual plant tours
• Notes on regulatory inspections and virtual regulatory systems
Securing Biopharma’s Digital Future
Digital technologies that have the potential to improve efficiencies and enhance DI in biologic production are becoming increasingly powerful and the biopharma industry must redouble its efforts to accelerate digital transformation.

However, to gain the benefits that these technologies offer, biopharma's leadership may need to integrate digital technologies into processes thoughtfully with process improvement in mind. By incorporating methods like SOAO, biopharma and their manufacturing partners have the tools they need to truly make processes and operations better through the application of digital technologies and systems data.
By partnering with CDMOs embracing digital transformation and DI, ones that are implementing advanced information technologies methodically and strategically, the more likely they will be able to produce complex biologics efficiently and safely and deliver better patient outcomes.
1. Redica Systems, “Data Integrity Trends in 483s and Warning Letters: Part 1,” redica.com, May 16, 2019.
2. https://www.pharmamanufacturing.com/information-technology/ data-management-analytics/article/11291070/smart-pharmasurvey-results-pharmas-digital-prowess-put-to-the-test
James Choi, Chief Information and Marketing Officer, Process Improvement at Samsung Biologics. Prior to joining Samsung Biologics in 2014, James Choi held various technology and operations leadership positions at major global healthcare and informatics corporations.

Cellbox- an active portable CO2 incubator for the transport of living cells and complex biological materials under optimal laboratory conditions. It provides precise CO2 concentration, highly accurate temperature control, data logging and export. In addition, Cellbox makes worldwide shipment of living cells possible due to its international flight allowance and the self-sufficient runtime of up to 48hours! More Information: cellbox-solution.com




Advancements in gene therapy and the approval of mRNA vaccines have dramatically increased the demand for well-characterised RNA and oligonucleotides in the biopharmaceutical industry. To support this emerging need, researchers have developed new analytical tools to analyse nucleic acid sequences by mass spectrometry (MS). Software advancements can now take data from high isotopic fidelity Quadrupole time-of-flight (QTOF) instruments and enable the fully automatic confirmation of sequences based on intact mass and MS/MS data, as well as the quantitation and identification of synthesis by-products. Corresponding innovations in analytical instrumentation also have provided the robustness, sensitivity, and high dynamic range necessary for RNA and oligonucleotide sequence confirmation.
The growing importance of oligonucleotides in research, diagnostics, and gene therapy – including tRNA, siRNA, and other modified oligos up to 120 bases – has increased interest in developing tools to verify their sequence, as well as identify and quantify related impurities. Liquid chromatography coupled with ultraviolet detection (LC-UV) and LC-MS/MS are the preferred methods to characterise highly modified oligonucleotide sequences. QTOF mass spectrometers are widely used for this purpose, as they can determine monoisotopic masses of intact oligonucleotides and their fragment ions with high mass accuracy.
Improved data quality from high isotopic fidelity QTOF instruments have generated a growing need for enhanced capabilities from analytical software to automate confirmation of sequences based on intact mass and MS/MS data, as well as the quantitation and identification of synthesis byproduct. This technology holds the potential to simplify the data interpretation, e.g. by accommodating user-defined sequence definitions to easily include the wide range of modified nucleotides typically used by the biopharmaceutical industry.
RNA and oligonucleotide sequence accuracy is a critical attribute impacting molecular safety profiles to avoid off target effects or toxicity, as well as compound efficacy to ensure correct activity and expression. While useful for oligonucleotides and RNA molecules made of canonical nucleotides, next-generation sequencing methods do not offer comprehensive characterisation of highly modified RNA-based therapeutics.
Additionally, oligonucleotide by-products may include failed nucleotide additions, nucleotide variants, and cytidine-touridine conversions with either no or +1 Da molecular weight differences to the target product sequence, respectively. As these can be difficult to characterise solely based on their intact
mass, information at the nucleotide level is required to improve oligonucleotide sequence confirmation.
Well established tools for the oligonucleotide characterisation process include LC-MS/MS based methods. However, a challenge for adopting LC-MS/MS methods is the complexity of oligonucleotide MS/MS spectra. The manual interpretation of spectra is time-consuming and requires in-depth analysis skills, which renders the full analysis of several samples per day in a routine lab both difficult and expensive.
Several advancements in both analytical software and instrumentation have shown to be promising to enable the fully automatic confirmation of sequences based on intact mass and MS/MS data, as well as the quantitation and identification of synthesis by-products.
Trapped Ion Mobility Spectrometry (TIMS)
Trapped ion mobility spectrometry (TIMS) is first and foremost a separation technique in gas phase, which resolves sample complexity with an added dimension of separation in addition to high-performance LC (HPLC) and MS, increasing both peak capacity and confidence in compound characterisation. Equally as important, the TIMS device also serves to accumulate and concentrate ions of a given mass and mobility, enabling a unique increase in sensitivity and speed along with the additional dimension of separation. By measuring the collisional cross sections (CCS) of the two isobaric oligonucleotide clipping products with an LC-TIMS-MS approach, it’s possible to differentiate the molecules using an intrinsic biophysical property. Such measurements simplify method transfer as such parameters are independent from the analytical setup.
As an example, two isobaric 9mer oligonucleotides were analysed. These two forms are clipping products originating from a 10mer molecule with guanine as base at both termini. The two 9mer oligonucleotides are obtained by a loss of a guanosine nucleotide either at the 5'- or 3'-end, providing two isobaric molecules. The samples were measured with HPLC, utilising a short gradient for desalting, connected to a MS instrument with TIMS functionality for differentiation based on the characteristics of the molecules' gas-phase behaviour. Extracted Ion mobilogram (EIM) traces were created for the measured different charge states of each oligonucleotide. CCS values of detected charge states were then calculated to validate if a differentiation of these isobaric molecules is possible (Figures 1 and 2).
Compared to standard electrospray, the development of the vacuum insulated probe (VIP) HESI ion source offers a solid gain in sensitivity for many compounds of interest. For a wide range of compounds, the VIP-HESI achieves sensitivities that rival those of industry-leading triple quad technology, while still providing the known benefits of accurate mass values with true isotopic
Figure 1. Extracted ion mobilogram traces of charge state 6- for original 10ner oligonucleotide and isobaric clipping variants. The measured collisional cross section of clipping variants allows clear differentiation of the two molecules.

of interest are shielded against heat by a vacuum barrier. The combination of heating and eluent stream into the probe makes it a simple and easy to maintain design. The active exhaust prevents re-circulation by utilising the Venturi-effect, showing smart implementation of long known physical principles into modern instrumentation.
Figure 3: Improved signal intensities with use of VIP-HESI technology
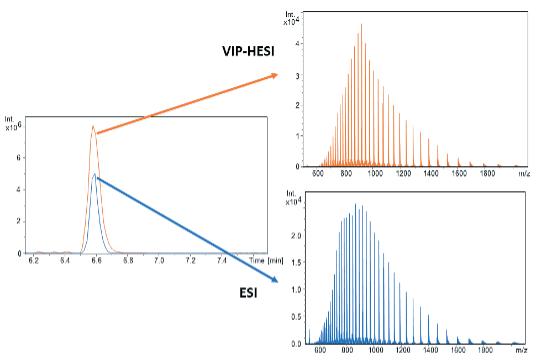
New software advances are bridging the MS/MS data gap by adding powerful tools for the interpretation of RNA and oligonucleotide data.i The development of a more flexible sequence editor supports a wide range of nucleic acid building blocks, both natural and synthetic. User-defined sequence nomenclature avoids a translation of lab-specific sequence definitions to a proprietary software format. Proven algorithms for peak detection and deconvolution of MS and MS/MS data obtained with high isotopic fidelity ensure correct mass assignments even in high dynamic range samples (Figure 4).
Figure 2. Extracted ion mobilogram traces of three different injections of the 3' clipped molecule. Shown is charge state 6-. Highly consistent CCS values without need of data file recalibration.

pattern (TIP). The VIP-HESI has simple operating parameters combined with efficient active exhaust to allow easiest upgrade from conventional sources.
The VIP-HESI ion source enhances sensitivity and lowers detection limits by delivering more efficient ionisation across a wide range of applications. Inside the VIP-HESI, an electrospray is maintained at a controlled vaporising temperature to enable enhanced desolvation of the analyte ions, even at high eluent flow rates in ultra-HPLC separations with sharp chromatographic peaks. Within the VIP the eluent stream including the compounds
Figure 4: Deconvoluted spectrum for a sgRNA and associated low level impurities.
Specifically, one propriety algorithm has been successfully used with top-down MS for the interpretation of spectra with large numbers of overlapping multiply charged ions. The MS/ MS annotation engine can automatically compute and identify terminal and internal fragments, allowing rapid analysis of complex MS/MS data in a straightforward, easy to interpret fashion. The MS/MS data is deisotoped with a proprietary algorithm and annotated by the software. The advanced peak picking helps with the confident interpretation of MS/MS data with complex and overlapping peaks. Matched peaks are annotated directly on the spectrum (Figure 5). Fragments with multiple explanations are denoted on the spectrum with the associated number of matches and not used analytically.
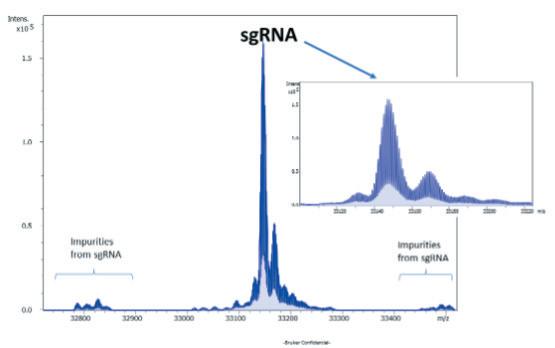
Figure 7: Sample analytical workflow.

A common challenge with longer molecules is the increased presence of internal fragments in the MS/MS data. Their interpretation is made difficult by the mass degeneracy due to the limited number of possible permutations between the basic building blocks. The software can annotate internal fragments with a unique explanation in a map view to complement the information that can be derived from terminal fragments (Figure 6). The delta mass between internal fragments allows for the confirmation of nucleotides not otherwise confirmed by terminal fragments.
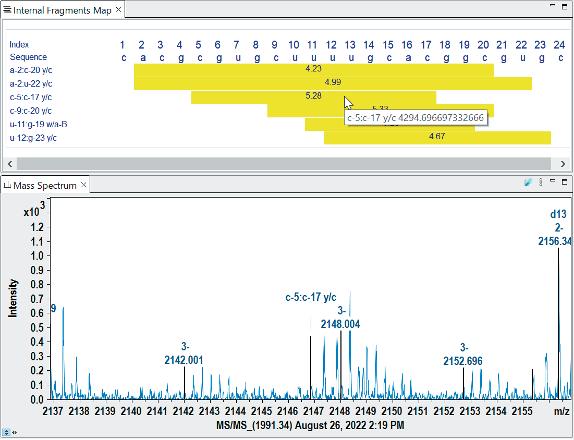
Combining all three of the advancements together has shown to be a robust and stable platform that maintains analytical flow rates, which benefits from increased sensitivity due to optimal ionisation in the VIP-HESI ion source (Figure 7). The combination of isotopic fidelity with high resolution enables measurement of biopolymers in the 30–50 kDa range with sub-ppm mass accuracy. Robustness, sensitivity and, most importantly, high dynamic
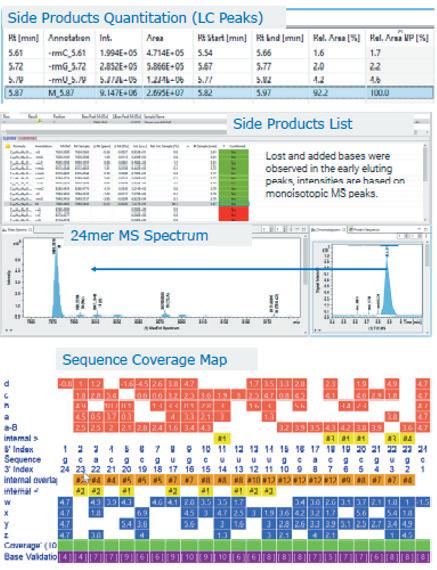
range in negative mode allow the detection and identification of impurities based on intact mass. These characteristics have shown benefits for the characterisation of analytes ranging from siRNA APIs, single guide RNA for gene editing (sgRNA) and mRNA specific assays (5’ capping, polyA tail).
New technological advances can automate the validation of oligonucleotide sequences using MS/MS datasets and quantitates by-products using LC-UV or LC-MS from the same dataset. They have been shown to enable the analysis of complex oligonucleotide sequences that were previously extremely time-consuming and limited to experts. In addition, a lab-specific sequence syntax can be used, thus facilitating the insertion of the software into existing processes. Isotopic fidelity combined with ultra-high-resolution MS has shown tremendous promise for the analysis of highly modified RNA. Simplifying this analysis with these advancements in commercial software and analytical instrumentation can dramatically accelerate research and development in the RNA space.

1. Rydzik, A. M., et al. Access to 1'-Amino Carbocyclic Phosphoramidite to Enable Postsynthetic Functionalization of Oligonucleotides. Organic letters, 23(17), 6735–6739.
I studied Food Chemistry at the University of Muenster and graduated with a state examination in 1998. After a practical year at the CVUA Muenster, I started my PhD thesis on the topic “Development of an Immunoassay for the hallucinogenic compound Psilocin” at the Institute of Pharmacy and Medical Chemistry and the Institute of Forensic Medicine, University of Muenster, and finished the thesis in 2004. Afterwards I worked as Postdoc at the National Institutes of Health (NIH), Bethesda, Maryland in the field of Plasma Proteomics. In 2006, I joined Bruker Daltonics in Bremen as application chemist for small molecules, proteomics, and biopharmaceutical applications. Starting in 2021 I am working as Business Development Manager Pharmaceutical Markets Focus for Bruker with the main responsibility for Germany, Switzerland, Austria, and Denmark.
Dr. Detlev Suckau is Global Biopharmaceutical Solutions R&D Manager at Bruker Daltonics. He is responsible for the development of new analytical workflows and software utilizing Bruker TOF and timsTOF mass spectrometers. Current development focus comprises methods for synthetic oligonucleotides characterization and design of the software support in BioPharma Compass. He graduated in Chemistry from the University of Constance in 1991 developing new methods to characterize the surface topology of proteins by MS. He spent a PostDoc year at Cornell Univ., Ithaca, NY, USA with Fred McLafferty, analyzing protein conformations in the gas phase of an FTMS, and a year in the Boehringer Mannheim Analytics Dept. for pharmaceutical drug development. He joined Bruker Daltonik in 1994 and leads the analytical methods developments group for more than 20 years. He published or co-authored more than 60 peer reviewed papers and is (co-)inventor of 17 patents.
Over 20 years international experience in the field of HPLC and MS method development, with expertise in multi-dimensional chromatography, proteomics and protein characterization. Now US business manager for Bruker Pharma business unit and supporting the development and deployment of Bruker innovative solutions for the pharmaceutical industry.



BSc Infectious Diseases, University of East London, UK, PhD Virology, Reading University, UK, Postdoc Mass Spectrometry, Proteome Center Tübingen, Germany, Bruker since 2009, Biopharma Application Development.

Over 1500 life science professionals attended the Drug Discovery 2022 conference this year. Organised by ELRIG – leaders in the provision of scientific meetings and conferences – the conference provided the perfect platform for the community to come together to restart the life science revolution; with over 50 world-class speakers and plentiful networking opportunities.
Through engaging presentations and innovative technologies presented by exhibitors, delegates had an immersive experience that provided them with an in-depth guide to the latest and most exciting trends in drug discovery. Here, we bring you some of the event’s many highlights.
Sharing Knowledge, Inspiring Action
ELRIG events are renowned for high-quality science. But this year, ELRIG partnered with the British Pharmacological Society (BPS), the Society for Laboratory Automation and Screening (SLAS), and the Royal Society of Chemistry (RSC), to bring delegates an even broader mix of topics on all aspects of drug discovery research.
For example, in her keynote presentation, Molly Stevens, Professor of Biomedical Materials and Regenerative Medicine at Imperial College London, explained how SPARTA® technology is enabling scientists to develop a portfolio of nanomedicines that interact with the biological environment to deliver a targeted biocargo. Meanwhile, Rab Prinjha, VP Head of Immunology Research Unit at GlaxoSmithKline shared how advances in genomic sciences are improving target identification, providing deeper insight, faster. As target identification is a stumbling block for many therapeutic areas, the hope is that more researchers now share a vision for how these advances can be incorporated into other drug discovery pipelines.
Our newly crowned winner of the Early Career Professional Impact Award, Dr. Shaun Pennington, also gave a presentation on his research. The Award recognises and celebrates early career contributions that have made a clear impact on the wider scientific community. Dr Pennington is an immunologist/ microbiologist working in drug discovery at the Liverpool School of Tropical Medicine. and received the award for his efforts in developing various screening platforms to better understand and target SARS-CoV-2.
The two-day event had a full programme, featuring eight-insight-packed tracks on subjects from High Content Imaging (HCI) to developments in preclinical models, and cell and gene therapies. Over 52 scientific speakers and 40 technology speakers shared expert insights, presented case studies and answered questions to share learnings that could help other scientists improve their drug discovery and development activities.
The exhibition hall was bursting with energy, occupying over 120 exhibiting companies from around the world. Delegates enjoyed numerous opportunities to discover the latest technologies, level-up current methods, and alleviate challenges to get better results, save time and drive their research forward. What’s more, the Innovation Zone – a platform for start-up companies to demonstrate their products and services – featured a diverse range of up-and-coming technologies that could move the field of drug discovery forward.
Also in the exhibition hall were 180 scientific posters from both industry and academic institutions alike. Having undergone rigorous scrutiny by the ELRIG scientific committee, each poster on display was of the highest quality, offering interesting information that elucidated methods and results that could be applicable to other areas of research. Congratulations go to
The intuitive pipetting robot, flowbot® ONE, is ideal for labs that need high flexibility, low-running costs, and maximum walk-away time

– enabling you to smoothly empower your lab. BOOK A DEMO TODAY FLOW-ROBOTICS.COM



ELRIG events provide a strong platform for career advancement, especially for early-career professionals (ECPs). The speed networking session titled, ‘Network like a Boss’ gave ECPs a chance to step outside inner circles and meet with experts in different industries such as pharma, biotech, CROs, and not-forprofit. Picking up great tips on how to successfully network in a supportive environment, many attendees said they’d recommend ‘Network like a Boss’ to their peers and would definitely join again.
In another career-related session, a discussion panel shared key insights on how to found a biotech start-up company,
encouraging the up-and-coming generation of scientific entrepreneurs to take their first steps into such a voyage.
With rich scientific content and high-quality networking, delegates fed back that it was one of the most insightful events in Europe this year. In 2023, Drug Discovery will return for its 17th year and provide another great opportunity to network, learn and explore.


There’s nothing quite like meeting face-to-face. The world implemented new levels of digital connectivity during the covid pandemic, of which there are many benefits. However, one thing has become clear since the return of in-person events – nothing fosters collaboration in the same way as physically meeting peers. The ELRIG 2023 calendar is live on the website, and registration for many events is already open. Visit elrig.org to learn more and join the mailing list so you don’t miss out.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organisation is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery. Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organisations, on an open access basis, as all our events are free-of-charge to attend!

























































































Page 43
Page 55
Aldevron
Anglo Nordic Life Science Conference
Page 21 Biotech i Kungsbacka AB
Page 55 BioTrinity 2023

Page 47
CELLBOX
Page 36–39 DFE Pharma
Page 25 FUJIFILM Wako Chemicals USA Page 53 Flow Robotics
IBC GenXPro GmbH
Page 17 Nemera
Page 11 Novo Nordisk Pharmatech A/S Page 3 Owen Mumford Ltd.
Page 34–35 OXGENE
Page 27 PCI Pharma Services Page 29 Peli Biothermal Page 23 Phage Consultants
Page 9 Precision for Medicine
Page 13 Qualogy Ltd
OBC Richter-Helm Biologics GmbH & Co. Kg IFC RGCC Group / Welmedis Group
Page 41 Senglobal Ltd
Richter-Helm is a Germany-based GMP manufacturer specialized in products derived from bacteria and yeasts, with a proven 30-year track record.


Count on us to flexibly provide a comprehensive range of services and customized solutions. Clients worldwide have already benefited from our commitment to good manufacturing practice and total transparency. Our work focuses on recombinant proteins, plasmid DNA, antibody fragments, and vaccines.

Richter-Helm consistently works to the highest standards of pharmaceutical quality. Contact us +49 40 55290-801 www.richter-helm.eu
