



Dr Man-hei Ma BSc(PV), DVM, GCertSAUA, MANZCVS (Small Animal Radiology) RVA President





Dr Man-hei Ma BSc(PV), DVM, GCertSAUA, MANZCVS (Small Animal Radiology) RVA President
Dear Alumni and Newsworthy readers,
Welcome to our third issue of Newsworthy! This issue encompasses clinical news in avian medicine, behaviour medicine, pathology, anaesthesia in small mammals, management of twins in mares, IVFT management in nephrotoxicity; and recent research on the management of feral cats and foxes in Australia, and the use of cnnabinoids for neuropathic pain. We also welcome Channy McGowan from The Vet Kaleidoscope to introduce us to Australia’s first organistion which advocates for Diversity, Equity and Inclusion within the profession.
We hope you enjoy reading each article!
As we near the end of 2024, my three-year term as President of the University of Adelaide Roseworthy Veterinary Alumni also draws to an end.��It has beena huge honour and privilege to have been elected into this position to work with all the committee members over the past 3 years into forming the key foundations, growing the resources and support that the Alumni Network provides for the veterinary profession, and forming connections in Australia and now here in the UK.
In reflection over the past 3 years as a committee we have had many big wins and achieved majority of the goals we set out to achieve each year. The success of each event and resource produced for the alumni and veterinary community comes from the great work of the committee and their individual hidden talents who diligently worked behind the scenes in putting together everything. Additionally, we could not have been achieved it without our sponsors (Royal Canin, Petgood, Horizon Palliative Vets, Ziwi Peak, Apiam Animal Health Limited, Zoetis, SASH), donations from VetPrac, Black Hawk and Dr. Woof Apparel, University of Adelaide Alumni Relations support.
I look forward to continuing as editor of the RVA Newsletter and passing the presidency baton on and seeing how the Alumni Network evolves and grows from the foundations we have built.


BSc DVM MANZCVS (ECC)
Hannah graduated in 2017 and started her career in a small animal practice before following her passion for emergency medicine. She first worked in a 24-hour GP practice and has spent the last four years at multidisciplinary emergency and referral centres. In 2021 she completed membership exams in emergency and critical care. Recently, she spent 12 months travelling and locuming around the UK and Europe, gaining a huge amount of knowledge and experiences along the way.
“Reassessing Intravenous Fluid Therapy for Nephrotoxic Exposure in Pets”

BSc(Vet Bioscience) DVM
Louise graduated from the University of Adelaide with a Doctor of Veterinary Medicine degree in 2017, and pursued small animal clinical practice until late 2023. After adopting a stray rabbit that was handed in to the clinic she worked at in 2018, she developed a passion for learning about exotic animals, and translated this into allowing the availability of veterinary care for exotic animals within these clinics. At the end of 2023 she began her employment with Unusual Vet Pet in Adelaide, and although she enjoy all facets of exotic veterinary care, she has a special interest in rabbit medicine and surgery.
“Improving Anaesthetic Safety in Small Mammals”

BSc (Vet Bioscience), DVM, MANZCVS (Veterinary Pathology)
Krysten graduated in 2019 and worked as a small animal GP in both Mildura and Adelaide for two and a half years, alongside continuing education courses in pathology and tutoring final year DVM students during their pathology rotation. In 2022 she moved to Sydney to begin residency training in Anatomic Pathology, consisting of a double Master’s program in both coursework and research. Her particular interests include wildlife pathology and small animal infectious disease.
“Melioidosis in Dogs and Cats”
BSc, BVMS, MANZCVS (Veterinary Behaviour)
Resident in Veterinary Behavioural Medicine ANZCVS & ECAWBM
Ash graduated from Adelaide University in 2015 and undertook a true mixed practice role in Warrnambool in South-West Victoria. In 2018 she completed the CVE Veterinary Behaviour Course through Sydney University, and in 2019 obtained Memberships with the Australian and New Zealand College of Veterinary Scientists (ANZCVS) in Veterinary Behaviour.
In 2021 Ash embarked on a residency program in Veterinary Behaviour under Dr Jacqui Ley at Melbourne Veterinary Specialist Centre (MVSC). Ash sees behaviour referral consults at MVSC and through her role at Apiam Animal Health she consults across rural Victoria and provides staff training and clinical support to veterinarians around Australia.
“When Fluffy bites the hand that feeds her””
BSc, DVM, MANZCVS (Avian Health), MVS
Kathryn Johnson is a 2014 graduate from the University of Adelaide. After spending 3 years in mixed animal practice in New Zealand and South Australia, she completed a residency program in Zoo Animal and Wildlife Health at Massey University and Wellington Zoo. She has obtained avian memberships and a Masters degree, and works as a veterinarian for ZoosSA, and as a casual lecturer and practical demonstrator for the veterinary and veterinary technology courses at the University of Adelaide.
“Advances in Avian Medicine”
BSc, DVM
Dr Olivia Miller graduated from The University of Adelaide in 2020. She then made the move back to Victoria to undertake a 18 month long rotating internship at Ballarat Equine Hospital. After completing the internship, Olivia took up an associate position at South Eastern Equine Hospital. It is here she has realised her passion for equine reproduction and neonatal medicine. Olivia plans to pursue further study in these areas in the coming years.
“Management of Twins in the Mare”




BSc, PhD
Department of Clinical Sciences, Auburn University College of Veterinary Medicine
Patrick is an Ecologist with Bush Heritage Australia working in the South Australian Arid Rangelands. He enjoys applied science and research and has spent most of his time working on the management of pest animals and diseases at large spatial scales.
“Optimising the Lethal Management of Feral Cats and Foxes”
BSc (PV), DVM, PhD
After graduating in 2014, Sherelle completed a PhD at the University of Sydney focussing on the potential of medicinal Cannabis to treat neuropathic pain. Upon graduating from her PhD, Sherelle began working with a company developing medical cannabis products for the veterinary industry. She is passionate about the potential of compounds in the Cannabis plant to treat diseases in animals and continues working towards registration of veterinary products containing these compounds in Australia and beyond.
“Cannabinoids for Neuropathic Pain - A PhD”



A number of our graduates have become entrepreneurs starting up or buying in to established veterinary companies. Each of the following have provided an insight into their journey and lessons as business owners.
We are seeking contributors
If any of the following resonate with you, please email us.
ARTWORK
Do you have a hobby in photography or design and would like to have your creative work feature as our next cover page?
Are there any particular clinical topics (common or rare, case study) which you feel passionate about?
Have you recently been involved in new research and would like to update the community? CONTACT THE RVA COMMITTEE

Share your VETERINARY JOURNEY through our ‘STORIES OF ALUMNI’
Are there any topics you would like to see the RVA committee cover in the newsletter?

Connecting with our alumni across Australia and the world.
Our Roseworthy Veterinary Alumni (RVA) Network aims to nurture the relationship between the University of Adelaide, our veterinary alumni and members of the public through engagement, mentoring, advocacy and support to students and alumni.
Some of the committee’s key objectives are:
• Support ongoing evidence based veterinary medicine and advocacy
• To promote mentoring opportunities and provide careers and professional advice for alumni and students
• Be a representative body for veterinary scientists in South Australia
• To support alumni in times of need through links to mental health and other resources
• Organise and host events promoting the RVA Network
• To promote the interests of The University of Adelaide’s Roseworthy campus and the School of Veterinary and Animal Sciences (SAVS)
• To encourage discussion in all facets of veterinary science including, but not limited to; policy, veterinary public health and associated research
The past 2.5 years have highlighted the importance of remaining in touch with each other. Update your details with us to ensure we can stay in touch and contact you in the ways that best suit you.
RECONNECT WITH THE ALUMNI NOW
Being a part of the RVA community has many benefits:
• Gain access to ongoing CPD
• Reunions and social events
• Mental Health Support
• Job opportunities
2024 Annual Cocktail Evening The Richmond Hotel




Destigmatising mental health within the veterinary profession has been a key feature in many publications regarding improved veterinarian health and wellness.
My aim for this wellbeing article is to contribute to destigmatising professional help for veterinarians but this time through story and experience. In our own lives, as the veterinary professionals, it’s tricky to know where to start with accepting parts of ourselves, and when to seek mental health management and support strategies. For many of us, it can be hard to know what we’re feeling and experiencing internally, all we know is that something feels uncomfortable, or we’re not sure what we want our life to look like, but we feel that this isn’t it. Sometimes it’s how we’re living, our behaviours towards and interactions with others, our performance, or our general quality of life is less than what we were hoping for after studying and working so hard, for so long. These moments and experiences are the ‘call to adventure’, and professional help can guide us through these feelings, so we’re not left to figure it out on our own. In telling this story, my hope is that you feel a little more comfortable and connected in seeking support for yourself, however that looks for you. If anything, hopefully it’s an entertaining read!
My call to adventure begun at a BBQ with friends, when one asked how I was going at work. At the time, I was five years out of vet school, working long work weeks with high on-call demands. I remember smiling and saying, “I am functioning at a solid 30% energy level, but I am doing ok”. She looked at me, my baggy eyes, determined but fake smile; she nodded gently and replied, “yeh but how long can that last for?”. As with all reality-slaps, the clarity of truth within this question, stopped me in my tracks and to be honest, stung a lot. Deep down something within me was high fiving that someone had said what I had needed to

Dr Meg Learey BSc (PV), DVM Mental Health Advocate
hear, but on the surface, mental level I wasn’t quite ready to acknowledge and accept this truth. So, I continued with grit, determination and persistence, doing what I was doing except for one thing; I decided to have a discussion with my employment about my expectations and hours. I needed to discuss what I wanted to learn and achieve. The next call to adventure came one night while I was standing in the kitchen trying to fuel up and head out on another afterhours call. I was exhausted beyond belief and numbly spooning yogurt and blueberries into my mouth while tears silently rolled, unacknowledged down my cheeks. On reflection, clearly crying into blueberry yogurt is a LOW POINT but sometimes you don’t know what you don’t know until you do. As my housemate entered the kitchen they asked “Hey, are you ok? You don’t look ok.” I replied “Oh, yep I actually am, I just know this case is beyond my ability and the client pressure is high but there is no one else to help, so I’m going out there”. With that, off I went, as do many of us on the frontline do.
Assisting with surgery the following week, I asked a colleague about work-life balance and was met with a statement that makes me smile these days, they had said, “What are you, a hippie?”. As these moments lingered in my mind the sustainability question was raised again ‘how long can that last?’ as it was slowly unravelling into my reality. I thought, how sustainable is the way I’m living my life? Is the benefit I perceive, truly outweighing the cost? Am I experiencing a fair compromise and sustainable veterinary practice? Crying into my yogurt, perhaps showed me I’m not. Being called a hippie for wanting work-life balance

made me realise I was going to have to step-outsidethe-norm. I made a deal with myself that I would have another three ‘I need something to change’ discussions with my employment in a bid to make my practice sustainable. I would commit and work hard but if things didn’t change, I would need to, and that meant quit no matter how loud my fears and insecurity about the future told me not to. Unbeknownst to me at the time, this deal was my form of self-protection and defence against intense burnout. My current practice certainly wasn’t suitable or sustainable for the life I wanted for myself. The life of seeing friends and family, taking up a hobby, preparing and enjoying nutritious meals for myself instead of last-minute grab and goes. The life in which I felt I had the energy and vitality to offer to those around me but more so feel for myself. The situation didn’t change, and I handed in my notice and entered the free fall through the great unknown. Fortunately, some locum work came up at a much slower paced clinic which allowed time to reflect on my experience. Most of my questions were just ‘why?’. Why did I think 30% was an ok energy capacity to run my life on, why did I allow myself to continue to be overworked and disrespected, why did I put my career above my own health and wellbeing? Then the question turned into, what didn’t I know about myself that let this all play out like it had? What was there to learn and who could help me figure it out?
So, my call to adventure turned into booking an appointment with a doctor to ask them where to start. I discussed the patterns I was living through at work; burn out, low energy levels, on-call stress, numbness, confusion, doubt. She wrote everything down and advised me to book in for a mental health consult and plan which hopefully leads to a Medicare rebate plan for affordable psychology sessions. As the doctor wrapped up the consult she said, “Let me know when you find the answer”. It didn’t exactly give me confidence about the pathway and healthsystem I was entering, but I pursued it none the less. The mental health consult gathered brief background history on why I required a mental health professional for this time in my life. I could either choose my own psychologist or be referred to one, so I went with their recommendations. Prior to the meeting, I could see my own thoughts and stigmas popping up about accessing support. Do I really need this? I’m sure I can figure it out myself. The bargaining to cut and run, and fear of the unknown, was very real. Luckily another colleague, who had sought professional help before, asked me, “Well what are you most scared of about going to therapy?”. The answer was so vulnerable it even surprised me, you know the sentences that come from the depths of yourself and spill out your

mouth before you can filter them? “What if I am actually, really messed up and completely fall apart?” What if it changes my life as I know it? What does going to therapy mean about me? After the “What if” conversation, I went to YouTube and searched ‘What is your first psychology appointment like?’ to try and get some baseline understanding and preparation. It was helpful but also set my expectations to be very different from reality. My first psychologist reminded me of an old schoolteacher, and although she was nice and I could tell her things, I found I didn’t want to. I tried a couple of sessions, but we just didn’t have the right fit. After a failed attempt, I wasn’t sure about professional help, but I figured this wasn’t how it was supposed to feel so I tried another person in a different practice that was available at short notice but much further away. She was open, comfortable, and casual and I watched as I just talked about my experience and life. She guided me through a few questions and helped formulate a few thoughts into words so they could be articulated and understood. After that first session, I realised going to therapy wasn’t anywhere near as big, scary and intense as I was making it out to be. It certainly didn’t unravel my life in an unmanageable way, but it did help to piece a few things together and understand myself more.
As I continued sessions with her, I developed my own interest in psychology and wellness. In addition to appointments with her, I ventured down different rabbit holes, learning about and experiencing Eastern and Western modalities for health and wellness. What I came to realise is that there are so many ways of understanding ourselves and the things that we struggle with. We are a container of thoughts, emotions, nervous system actions, physical matter, memories, spirit, and diverse experience. Some of us will resonate with different practices and at different times of our life. This is because we are all so unique in our life history, and unique in what we perceive as stressful or overwhelming, so our healing and maintenance of health is unique too.
Psychologists are incredible for helping establish a blueprint for our mind and helping us navigate and create shifts in our perspective to new and sustainable beliefs about the stressors we experience regularly. Psychologists help us navigate mental and emotional suffering. Sometimes we need them for ongoing management as we experience changes throughout life from new grad, to mid-, to late- career vet, or perhaps change into alternate pathways and life stages. Sometimes too, we establish our blueprint and outgrow them. Psychologists are not the only source of wisdom in the realm of inner understanding and mental wellbeing; they are not the only answer, but they can be a great start before we venture into healing and management strategies that work for us individually. What all wellbeing modalities agree on, is that supporting your own health, takes practice and is as unique to you, asyou are.
So in reflection, I think of mental health professionals as a guide. They can help discover and work through singular problems. They can be a safety net and provide reassurance from someone with an educated, non-biased, third-party opinion, someone you can soundboard anything with, as you move through life.
In my earlier years of veterinary practice, I experienced and witnessed a lot of stigmas around seeking professional help coming from others. With recent awareness campaigns and education, the stigma in our community has improved significantly. It has
always been up to me to see my self-stigmas. Now that I answered the call to adventure, am I worried when I face stigma internally or externally? Absolutely not. I have seen the quality of life that I am living now compared to back then. A quality of life bought about through learning how to navigate different aspects of life and the inherent challenges it brings. I know firsthand the impact changing our own perspective can have. This is not just a grateful tribute to psychology and the wellbeing modalities available to all of us, but more so, to the part of myself that was on the quest for answers that resonate with the why do I do what I do, and what gives my life meaning.
Therefore, I would like to leave you with a question; Why not?
Why not give yourself the grace to grant a permission slip, from yourself to yourself, to advocate for yourself and what you need; physically, emotionally, mentally. The sooner we seek support, understand and develop tools for ourselves to navigate the world we live in and the things that cause us distress, the greater our ability is to move through the hard times when adversity naturally surfaces.
We can’t hope to change the things that cause us discomfort until we can acknowledge them. That first step comes down to our willingness to try something new or, even and more so, again. Who knows, it might turn out to be one of the best decisions you have made.



13 11 13
24-hour counseling and crisis support chat trained crisis supporters available 24 hours a day, 7days a week.
1300 224 636
Telephone counseling service 24hours, 7 days a week.
1300 659 467
Free nationwide professional telephone and online counseling service for anyone affected by suicide.

1300 726 306
PANDA supports women, men and families affected by anxiety and depression during pregnancy and in the first year of parenthood. They also provide support relating to postnatal psychosis.

1300 687 327
Counseling Service available to AVA members, the vet professionals that work for them and family members – available 24 hours, 7 days a week.

Dr Mary McQuillan
Lecturer, DVM, DVSTud, MANZCVS (Medicine of Sheep)
SAVS Representative
The School of Animal and Veterinary Science has had an eventful year filled with exciting news, achievements, and events. The Hope Cup Cricket Match launch dinner in June was a fantastic success, bringing together staff and supporters for a night of great food, drinks, and inspiring cricket speakers. The auction saw spirited participation, especially from our very own Gustavo Fellini Agne and Mary McQuillan. We’re also thrilled to announce that Maddie Bing, a current DVM3 student, will represent Roseworthy in the Riverside All-Stars Team for the Hope Cup match on November 17th. This event is not only about cricket but also supports the Riverside Gawler Salvation Army, with funds directly benefiting local community projects for those at risk of homelessness. We encourage everyone to contribute to this worthy cause through Maddie’s fundraising page Madelaine Bing • HOPE CUP 2024 (salvationarmy.org.au).
A big thank you to everyone who contributed to the success of Open Day at North Terrace. Our displays, including the main tent, Ag and Vet Lawn exhibits, the horse, the cow, and the Green Feed trailer, were all huge hits with visitors. Special thanks go to Bec Forder for her incredible coordination.
We are pleased to introduce Dr. Darcie Kinnaird, who has recently joined as an Associate Lecturer
in Veterinary Anaesthesiology. A 2018 graduate of Murdoch University, Darcie completed an internship in anaesthesia and analgesia and gained her graduate diploma before moving back to Adelaide in early 2020. With experience in ECC practice, general practice, and teaching veterinary nursing at TAFESA, Darcie has always been passionate about anaesthesia and teaching. She has two rescue cats, Rizz and Fidget, and is also a competitive powerlifter! Darcie started with RVH earlier this year, working in the spay/neuter clinic and as a casual anaesthetist before accepting her academic role. We’re excited to have Darcie on board and know she will be a fantastic addition to the team.
We are excited to welcome Dr. Sita Withers as a Senior Lecturer in Companion Animal Medicine (Oncology). Sita graduated from the University of Melbourne in 2008 and later moved to California to complete a medical oncology residency at UC Davis, where she became a board-certified specialist in medical oncology. Sita also holds a PhD in Integrative Pathobiology and has spent five years as a tenure-track Assistant Professor at Louisiana State University. She is thrilled to return to Australia and join the University of Adelaide faculty. Outside of work, Sita enjoys jogging, swimming, practicing yoga, and spending time with her two dogs and two cats. We are thrilled to have Sita’s expertise and passion for oncology on our team.
We also welcome Dr. Chantell Jukic, who joins us as a Lecturer in Equine Surgery. Chantell graduated from the University of Queensland and completed a rotating internship in surgery and medicine at UQ VETS Equine
Specialist Hospital, followed by an orthopedic translational fellowship at the University of Pennsylvania. She returned to the University of Queensland to complete her surgical residency and doctorate, focusing on innovative techniques in equine surgery. Chantell has a particular interest in soft tissue surgery and joint arthrodesis techniques. We are excited to have Chantell’s expertise and enthusiasm for equine surgery as part of our team.
We are pleased to announce that Arin Collins officially joined us as an Associate Lecturer in Veterinary Technology on 29th July. Arin brings advanced qualifications in Veterinary Nursing and 15 years of clinical experience, including time at the Royal Veterinary College in London and our own Roseworthy Veterinary Hospital. With a strong background in veterinary nursing education, particularly in animal care, handling, anesthesia, and surgical nursing, Arin has been instrumental in the success of the Vet Tech rotations. She is currently completing a certificate in Clinical Education and is passionate about guiding students through their veterinary studies and into their careers. Outside of work, Arin enjoys time with her cat Tootie and her Welsh Cob, Dylan. We’re confident that Arin will be a valuable addition to our team.
We also welcome two new members to our Technical Services team: Adrian Hines and Sharna Coleman. Adrian, who has been with the Roseworthy Veterinary Hospital (RVH) since 2011 as a Necropsy Technical Officer, will focus on supporting lab-based
practical teaching activities. Sharna, who has worked at RVH since 2021 as a veterinary nurse, will support practical teaching related to clinical skills. We are excited to have Adrian and Sharna on board and look forward to their contributions.
Additionally, we are excited to welcome Nicky-Lee back to SAVS. Nicky-Lee completed her Animal Science degree in 2012 and her PhD at SAVS in 2017. After her PhD, Nicky-Lee worked at Central Queensland University in Yeppoon, where she completed an internship and gained extensive experience in microbiota trials and analyses, including a fascinating study on the intestinal microbiota of saltwater crocodiles. Returning to South Australia, Nicky-Lee has taken on various roles at the University of Adelaide, including a 1.5-year academic position and three years as a postdoctoral research fellow focused on poultry production and food safety. In January 2024, NickyLee started her new academic role as an Associate Lecturer in Ruminants teaching into the Veterinary Bioscience, Veterinary Technology and Animals Science degrees making friends with a few goats (or an entire mob) along the way. We’re delighted to have her back and look forward to her contributions to our team.
The SAVS community has seen several notable achievements. Chantelle Jukic won the best ACVS resident presentation at the American College of Veterinary Surgeons Conference and will present her talk at the European
meeting next year. Research at SAVS continues to thrive, highlighted by Kapil and Andrea McWhorter securing an ARC Linkage grant worth over $700,000.
As we approach the end of the year, we’re pleased to report that the Vet Science Program re-accreditation process has ended with the School being reaccredited. This success is a testament to the hard work and dedication of the SAVS team. Special thanks to Charles Caraguel, who played a crucial role in this effort as Associate HoS for Quality and Risk and now to Kandarp Patel for his continuing efforts as he has taken on this role. While we’re excited for Charles, who will be joining the University of Sydney for two years, his contributions to SAVS will be greatly missed. Peter Atkinson (a fellow RVA alumnus) has stepped in to coordinate the Veterinary Epidemiology and Evidence-based Medicine course during Charles’ absence.
We’re delighted to share that Hayley McGrice has received the UoA Stephen Cole the Elder Award for Excellence, recognizing her outstanding leadership in teaching practice.
As we wrap up the year, we look forward to continued success and growth in 2025.
The SAVS Student Awards Ceremony for the 2023 academic year was held on Sunday, 5th May. Congratulations to all the award winners who demonstrated outstanding academic and clinical proficiency.
Notable awards include the Hon Dr Bruce Eastick Prize and the Centre for Veterinary Education Clinical Competency Award, both awarded to Brianna Lambert.
The full list of award recipients is as follows:
• Principles of Animal Behaviour, Welfare & Ethics Prize (Animal Science) - Lok Ching Ho
• Principles of Animal Behaviour, Welfare & Ethics Prize (Animal Behaviour) - Riley Hayes Revitt
• Principles of Animal Behaviour, Welfare & Ethics Prize (Veterinary Technology) - Lily Cowan
• Principles of Animal Behaviour, Welfare & Ethics Prize (Veterinary Bioscience) - Melissa Chen
• Roseworthy Old Collegians Association Prize in Animal Science - Stephanie Warr
• Roseworthy Old Collegians Association Prize in Animal Behaviour - Bridget Cooper-Rogers
• Roseworthy Old Collegians Association Prize in Veterinary Bioscience - Xiao Chou Mok
• Roseworthy Old Collegians Association Prize in Veterinary Technology - Georgina King
• Hon Dr Bruce Eastick Prize in the Doctor of Veterinary Medicine - Brianna Lambert
• Centre for Veterinary Education Clinical Competency Award - Brianna Lambert
• Australian Society for Parasitology Prize - Sophia Seon Young Kim
• Parliamentary Medal for Merit in Veterinary Public Health - Isabella Kotasek
• Epidemiology Chapter of the Australian and New Zealand College of Veterinary Scientists Prize in Veterinary Epidemiology - Isabella Kotasek
• W V MacFarlane Prize - Bonnie Homer
• Thomas Gepp Prize in Veterinary Pathology - Lexie McKay
• Ern and Betty Wickes Prize in Animal Pathology (DVM 1) - Hiu Ming Cheung
• Ern and Betty Wickes Prize in Animal Pathology (DVM 3) - Sebastian Macaspac
• Dr Peter Irwin Prize for Equine Health - Sebastian Macaspac
• Mrs Audrey Abbie Veterinary Perpetual Prize - Marizaan Williams
• Wildlife and Exotic Special Interest Group (WESIG) Wildlife Prize - Nicholas Verco
• CRP Achievement Award - Nicole Staykov
• CRP Special Recognition Award - Sahara Craig and Si-En Ruth Khaw
• Heather Ridgway Prize - Emma Agardi
• The ASAV Award for Clinical Proficiency - Piya Coughlan
• W. J Colebatch Memorial Prize - Emily Kruger

2024 has been another very busy and successful year for the School of Animal and Veterinary Sciences! I thank all of the staff and students, other University of Adelaide supporters and collaborators and also all of our external stakeholders, including Alumni and other veterinarians and other professionals, who have helped contributed to the ongoing success of the School and our educational programs, veterinary enterprises and research.
From a University of Adelaide viewpoint, the key major focus and priority in 2024 has been progressing towards the merger of UoA and UniSA to form the new Adelaide University. Adelaide University was statutorily established by the Parliament of South Australia in March 2024 and registered by TEQSA, the Australian tertiary education accreditation body, as an Australian higher education provider on the 28th of May 2024. On the 15th of July 2024, the brand, logo and initial website for Adelaide University (adelaideuni. edu.au) were launched, and this included an initial suite of coursework programs, including animal and veterinary sciences, for potential students to consider for enrolment and commencement in 2026. Animal Science and Animal Behaviour programs will become majors of a Bachelor of Science qualification and the Bachelor of Veterinary Bioscience, the Doctor of Veterinary Medicine and the Bachelor of Veterinary Technology will transition to the new University in their existing states. Teaching for Adelaide University will commence from the 1st of January 2026.
All of SAVS’ programs have had program reviews in 2024 and Vet Science is also undergoing a detailed curriculum review into early 2025. This is all part of ongoing quality assurance to continue to deliver excellent contemporary educational programs and Dr Karen Kind has done an amazing job of coordinating the teams and all of the activities needed to achieve these major tasks.

Professor Rob Woodgate Head of School of Animal and Veterinary Sciences
It is also very pleasing to be able to report that the review and re-accreditation process for the Vet Science Programs is continuing well. The programs have retained accreditation, with only minor deficiencies needing attention during the coming months. The very detailed Self Evaluation Report and then full site visit, in October 2023, has been followed by a lot of further hard work from many staff. Special thanks, again, to Karen Kind as Associate Head of School for Learning and Teaching and also to Vet Science Program Directors, Natasha Speight and Gustavo Ferlini Agne. Charles Caraguel has also played a crucial role in this effort as Associate Head of School for Quality and Risk (this role has now transitioned to Kandarp Patel, while Charles is spending the next two years in a temporary role at the University of Sydney).
Facilities-wise, SAVS and Roseworthy have continued to receive excellent support from the University, with many projects completed or well underway this year. Many millions of dollars have been spent on new equipment and upgrades to existing facilities, including lecture theatres and labs. More than four million dollars alone will be spent on the new cattle and sheep yard complexes, with construction starting in November 2024 and completed by mid-2025. This will result in both areas completely undercover, including an approximately 60 metre by 50 metre roof over up to 18 new crushes and movement pens in the new cattle teaching area. We will share some photos in the 2025 annual update for the RVA!
Finally, I take this opportunity to wish everyone an enjoyable and relaxing summer break and I hope you all have a happy, healthy and prosperous 2025.
Its great to see our very special Roseworthy Veterinary Alumni Network go from strength to strength, year on year administered by an excellent committee and wonderful leadership that crafts CPD events, podcasts and social events for all its alumni.
As we stand now, there are 598 veterinary graduates who have qualified from Roseworthy since 2013. It seems from employer surveys, that our graduates do well - settling into their new jobs, achieving great things and making us very proud…..but there are things we can still do better and that’s what we are aiming for as we begin to review the entire veterinary science curriculum. We have started but to make real change we need to map programs, courses, learning objectives with AVBC Day One Competencies – then we can find any gaps, fill them as required and manage the timetable more effectively. We punch well above our weight for a small, new veterinary school but there is more we can do.

Associate Professor B Vet Med, MANZCVS, PHD, Dip ECZM Academic and RVA Founder
Our external advisory committee, Roseworthy External Veterinary Advisory Committee or REVAC meets twice yearly to provide commentary on our graduates and has been an invaluable source of feedback for the curriculum review.
As Associate Head of External Engagement, we are developing more formal relationships with (but not limited to the RSPCA, Zoos SA and Cleland Wildlife Park) as we seek more opportunities for all our students to get broader experiences. Slowly we are improving the school website, and we are also implementing opportunities for adjunct positions for distribution practices who teach our students on a regular basis.
On a personal note, 2024 has been busy with training trips to Sulawesi, electives in South Africa and Botswana and just recently a new elective in Tasmania with dedicated and enthusiastic students and hosted by one of our alumni, Alex Bullen (DVM 2017). We conducted health examinations on free ranging bandicoots, platypus (a huge highlight for me) devils and wombats but for me the most significant thing about the elective was the emergence of a new veterinary practice model. Both Alex and her boss James McGregor contribute hugely to the biology and biodiversity conservation of these free-ranging species and this is done as part of their normal day to day work activities and not in their spare time – and they really passionate about it, having written several peer-reviewed articles and one Phd so far. A fantastic new work model for those in practice who want to continue working with wildlife or in wildlife research while continuing general practice – perhaps the best of both worlds…..a new way of doing veterinary practice differently and definitely food for thought.
To our student alumni – a huge good luck message for the exams and for our DVM alumni, all the best for 2024
To paraphrase a wonderful student - Warmest wombat regards
At the start of 2024 we welcomed two alumni joining our committee - Dr Isabelle Goldsmith, Dr Meg Learey, and Dr Sofia Canala who have taken position as Secretary, Mental Health Advocate and Sponsorship Officer, respectively.





Thank you to our General sponsor this year for supporting the RVA program.
Thank you to our speakers for sharing their knowledge and advice to our veterinarians, alumni, nurses and students.
Thank you to all those who joined us in person or online! We hope you gained knowledge which you have been able to bring to your daily workplace.















This year our annual social event celebrated the 10 year reunion for the Class of 2014 and 5 year reunion for the class of 2019.








Guest: Steph, Annie, Britt, Emma
Join Steph, Annie, Britt, and Emma from the inaugural DVM class at The University of Adelaide as they share the joys and challenges of life as a rural GP vet.
Guest: Steph, Annie, Britt, Emma
Steph, Annie, Britt, and Emma from the inaugural DVM class are back to share how their careers have changed over time in response to their own mental health concerns and as their priorities shifted when they became mums.
Guest: Ellen McBryde
Join Dr Ellen McBryde as she shares how her career started out as a new graduate locum in tropical paradise and her advice to any graduates wanting to explore this career pathway.
Guest: Meg and Tobi Learey
This week Meg is joined by her sister Tobi, both Roseworthy veterinary graduates. In this vulnerable discussion they explore mental health, the importance of seeking support and developing mental health plans, as well as the challenges faced by both veterinary students and practicing vets alike.


Dr Ashleigh Hargreaves BSc, BVMS, MANZCVS (Veterinary Behaviour)
An approach to the case of a dog starting to show aggression towards family members in the home.
It can be a daunting task in GP practice when your next 15-minute consultation is for the ‘dog showing aggression to people in the home’. There are many factors that make treating such a case in primary care practice challenging, from the lack of time in a standard consultation, perceived lack of training in Veterinary Behavioural Medicine by most veterinarians and the human factor of heightened client emotions involved in such a case.
Despite these challenges, many of these cases can be managed well in primary practice when they are approached logically like any other problem a patient presents with to the clinic.
Just like any patient presented to you, a thorough history should first be collected. Within the short time allocated, at least 5-10 minutes should be spent listening to the client before approaching the patient. Take notes and focus your history focusing on the specifics of when the problem started and how it has progressed. Concentrate on the details and context of when the aggressive behaviour occurs. Ask for examples and ask for details of what happened before, during and after the event. Ask the clients to describe it to you as if you were watching it unfold, including the body language the dog was showing. Steer clients towards objective descriptions (What was the tail position? Were the hackles up? Could you see the whites of the eyes?) rather than perceived motivations for the behaviour.
The final part of history taking should focus on physical health history. Ensure you have read the patient history prior to the consultation if available. For patients showing aggression to familiar people, especially when it is a new behaviour, or if it occurs when the animal is
approached when resting or being handled, there is a high likelihood that pain and physical discomfort may be a contributing factor to the behaviour. It is important to keep in mind breed predispositions here, especially for young dogs when pain is generally not considered (hip or elbow dysplasia, spinal conformation, dermatological or gastrointestinal disease). A thorough physical examination is important, however should not be considered effective to rule out pain. Short videos of mobility at home such as movements up and down stairs or on and off furniture can be helpful to request. A comprehensive blood test for haematology and biochemistry, imaging, and pain trials with appropriate pain relief medications for a minimum of 3 weeks can all be helpful diagnostic tools in some of these cases.
Dogs use aggression when they are feeling uncomfortable and threatened for their safety, usually as a way to move the target of the aggression away. Common list of diagnosis for aggression towards familiar people listed in Veterinary Behavioural Medicine textbooks include:
• Pain related aggression
• Resource related (protective) aggression of food, toys, resting places, and favourite people.
• Conflict related aggression (often when previous aversive handling has been used)
• Impulse control aggression
However, many specialists in the field globally are using descriptive diagnoses rather than a pathophysiological diagnostic label. A descriptive diagnosis provides information about when the behaviour is occurring and how to target a treatment plan. This is helpful as it is understood that often the underlying cause of the behavioural problem is one of a mental health or developmental disorder caused by a malfunction of the emotional systems of the individual. For many behavioural problems in our patients, it may be an underlying anxiety disorder that causes the dog to have reactions that appear disproportionately large (or irrational) for the situation. It is important to remember, there is always the effect of genetics, temperament and previous learning at play in any behaviour that
our patient’s present with. Many factors may be contributing to a presenting behaviour, and each may need to be addressed separately to improve the behaviour and the welfare of the animal.
Treatment depends on the underlying motivations of the dog and much of this will be learnt through thorough history taking, especially the context of the aggression, and investigation and analysis of the complete health profile of the animal.
Treatment plans should consider:
1. Treating any underlying causes of physical discomfort and pain (from any body system).
2. Simple management strategies that are specific to the context in which the aggression is occurring and practical for the client to implement.
• Preventing the situations where aggression occurs e.g. For a food guarding dog-feed the dog away from others. For a dog showing aggression on the couch-prevent their access to a couch or bed or allocate one area of the couch for them to rest.
3. Psychopharmacology to treat a diagnosed anxiety disorder may be helpful including:
• Starting a daily anxiety medication such as Fluoxetine or Clomipramine
• Using shorter acting situational medication such as, Clonidine, Trazodone, Gabapentin or Benzodiazepines, may be appropriate.

Figure 1: Asking a dog for a simple task they know how to do, such as asking for a ‘touch’ (nose to palm), can act as a way for a dog to give ‘consent’ and opt-in for an interaction if they chose to perform the task. The dog should be left alone if they choose not to engage.
behaviour modification recommendations to help the patient learn to strategies to interact without aggression such as:
• Consent based handling- calling the dog to you for a pat, and leaving them be if they do not movew towards someone for the pat. “Pat and pause,”patting for a second or two then pausing to check in if the dog wants more (leans in vs moves away or shows other signs of discomfort) and responding to the dog’s signals. Figure 1 shows teaching a ‘touch’ cue to use as a way of consent based handling.
• Teaching appropriate handling to family, pat chin and chest, not over the head.
Prognosis will vary depending on underlying motivations and the severity of aggression. Aggression is context specific and therefore a dog showing aggression around handling on the couch, is not necessarily at a risk of showing aggression to a stranger on a walk.
As with treating any patient in veterinary medicine, in a case of aggression it is the veterinarian’s role to provide information about all treatment options, and how to reduce risk factors for safety, so then clients can make informed decisions about their next steps.
Referral of patients showing aggression to familiar people is appropriate at any time from first presentation and should always be offered. However, many cases of aggression towards familiar people can be managed effectively in primary practice when a logical process is followed to ascertain motivations, and develop a treatment plan, and ongoing follow-up care can be provided. Treating behaviour patients requires time to work through these steps thoroughly and continue to assess response to treatment. A 15-minute consult can be a great starting point to assessing these cases in-house, however the expectation should be that more time is needed, so book a follow up consult as you do have the skills to provide care for these patients and their caregivers.
There are 4 registered specialists across Australia and a growing number of residents in veterinary behavioural medicine to provide help with cases and take referrals when required.
References
Affenzeller, Nadja, et al. “Human directed aggressive behaviour as the main presenting sign in dogs subsequently diagnosed with diskospondylitis.” Veterinary Record Case Reports 5.4 (2017): e000501.
Denenberg, S 2021, “Small Animal Veterinary Psychiatry”, CABI Publishing. Camps, Tomàs, Marta Amat, and Xavier Manteca. “A review of medical conditions and behavioral problems in dogs and cats.” Animals 9.12 (2019): 1133.
Landsberg, G 2023, “Behavior Problems of the Dog and Cat”, 4th Edition, Elsevier. Mills, Daniel S. “Perspectives on assessing the emotional behavior of animals with behavior problems.” Current opinion in behavioral sciences 16 (2017): 66-72.
Mills, Daniel S., et al. “Pain and problem behavior in cats and dogs.” Animals 10.2 (2020): 318.
Overall, K 2013, “Manual of Clinical Behavioral Medicine for Dogs and Cats”, Elsevier.

I developed a fascination for birds after I built several aviaries and started keeping birds as a child, deciding very quickly that they are a seriously underrated group in the Animal Kingdom. I shortly realised there were very few vets in Adelaide who held a genuine interest for birds or who had done further study at the time, which was one of the driving factors for me to pursue more study in this area. I was also drawn to avian medicine because of the huge number of different species of birds in the world. It is believed there are approximately 9700 species of birds (Callaghan, Nakagawa & Cornwell, 2020), with some avian species being as closely related taxonomically as a cow is to a horse. This therefore presents some unique challenges to the veterinary practitioner, as inevitably some degree of extrapolation is required to make clinical decisions and treat patients. However, the body of research that informs avian medicine is growing with new insights being constantly discovered.
One growing area of research is avian pharmacology. In general, avian drug doses required to deliver aw therapeutic effect are quite high compared to doses used in other domestic animals. In some instances, doses are as much as 10-fold higher than commonly used doses used in dogs and cats. For example,

amoxicillin clavulanic acid is often used at 125mg/ kg in birds (instead of 12.5mg/kg), meloxicam at 1mg/ kg (instead of 0.1mg/kg) and doxycycline at 50mg/kg (instead of 5mg/kg) (Carpenter & Harms, 2022).
Most of the pharmacological information available in birds is based on research with parrots, pigeons, birds of prey, penguins and chickens (Carpenter & Harms, 2022). When considering species to study, researchers are likely limited by the physical size of some species (to ensure they can safely collect sufficient blood volumes for testing), as well as available species in universities or research institutions where these studies are performed. There are few studies that pair both the pharmacokinetics and pharmacodynamics of a drug in the same bird species, therefore assumptions are often made such as the plasma levels required for a therapeutic effect in a non-avian species (e.g. human or dog) is the same in the bird species studied. The pharmacodynamic studies available usually involve an assessment of latency of foot removal from a hot plate or electrical stimulus, or improved weight bearing of a leg after inducing arthritis (Aliansyah, Chng & Xie, 2022). Due to these studies inflicting painful stimuli on patients, pharmacodynamic studies aren’t as common as pharmacokinetic studies. There is also a dearth of multi-dose pharmacological studies available in birds.
With a growing number of studies investigating the pharmacology of drugs in various bird species, it is becoming quite evident that there is considerable pharmacological variation between bird Orders. Single-dose meloxicam trials in brown pelicans show that oral meloxicam in this species has the longest half-life recorded in any bird species of 36 hours (Horgan et al. 2020). Compare this to a half-life of 4 hours in red-tailed hawks (Lacasse, Gamble, & Boothe, 2012) and 16 hours in ringneck parrots (Wilson, Divers & Budsberg, 2005). This suggests dosing frequency should be varied accordingly. Pelicans in the above study were given a single dose of meloxicam of 0.2mg/ kg orally, which is regarded as a low dose for most bird species. Additionally, there are reports of pelicans with proventricular ulceration or perforation, visceral gout and coagulopathies from meloxicam at doses that would be considered safe in other bird species (Horgan


Dr. Kaori Takeoka BVSc, DVM, CPEV
Dr. Ana Carolina Springford BVM, BVS, CPEV

et al. 2020). At this stage it is not recommended to use this drug in pelicans until further multi-dose studies become available (Horgan et al. 2020). Similarly, recent studies about meloxicam use in African penguins has concluded that this species should be dosed with meloxicam 1mg/kg PO q48 hours or 0.5mg/kg IM q24h (Morrison et al. 2018), and I currently apply the same dosages to Australasian penguin species.
When considering multimodal analgesia, there’s scientific evidence to suggest a variety options are effective in birds. Tramadol, gabapentin and butorphanol are recognised for providing effective analgesia across a range of bird Orders (Carpenter & Harms, 2022). Some new research suggests that high doses of fentanyl given subcutaneously in birds may provide effective analgesia, although more research is required to determine the efficacy of fentanyl patches in providing analgesia to birds (Aliansyah, Chng & Xie, 2022). A recent study even investigated the use of high dose cannabidiol (120mg/kg) in birds, although this showed that it had a short half-life in parrots (76 mins) and thus concluded it may not be an effective form of analgesia (Carpenter et al. 2022).

We offer in-home palliative care and euthanasia services for pets We are a mobile service that provides a dignified end of life journey at home, where the pets are most comfortable. In palliative care, we focus on improving the wellbeing of our patients while supporting our clients in some of the most difficult times
Quality of Life Assessments
Palliative Care and Pain Management
Compassionate In-home Euthanasia
Deceased Pet Transportation
Aftercare Organisation
Personalised Plan suited to our client’s and the pet’s needs
Support and Guidance


When treating birds in clinical practice, it’s worth searching for species-specific information particularly regarding pharmacology, which is widely variable. In reality, it is rare to find pharmacological information on all the exact species you’re working with, but quite frequently there’ll be information available from another species within the same Order, and this information can be extrapolated. The most comprehensive and userfriendly resource for this is Carpenter’s Exotic Animal Formulary, which references up-to-date information based on both pharmacology studies and anecdotal evidence for a range of exotic species. There are also useful recent review papers detailing pharmacological information about analgesia and antibiotics in birds, including Soh et al. (2022) and Aliansyah, Chng & Xie (2022). For any aspiring avian veterinarians, consider joining the Association of Avian Vets (AAV) and the Association of Avian Veterinarians Australasian Committee (AAVAC), which provide networks of other avian vets and high-quality journal publications detailing the latest avian research.
References
Aliansyah, E.; Chng, H.T., Xie, S. (2022) ‘A critical review of the pharmacokinetics and pharmacodynamics of opioid medications used in avian patients’, Birds. 3, pp. 1-28. Callaghan, C.T., Nakagawa, S., Cornwell, W.K. (2020) ‘Global abundance estimates for 9,700 bird species’, Biological Sciences. 118 (21), e2023170118.
Carpenter, J.W. & Harms, C. (2022) Carpenter’s Exotic Animal Formulary. 6th edn. Elsevier, US.
Carpenter, J. W., Tully Jr, T.N., Rockwell, K., KuKanich, B. (2022) ‘Pharmacokinetics of Cannabidiol in the Hispaniolan Amazon Parrot (Amazona ventralis)’, Journal of Avian Medicine and Surgery. 36(2), pp. 121-127.
Horgan, M. D., Knych, H.K., Siksay, S.E., and Duerr, R.S. (2020) ‘Pharmacokinetics of a Single Dose of Oral Meloxicam in Rehabilitated Wild Brown Pelicans (Pelecanus occidentalis)’, Journal of Avian Medicine and Surgery, 34(4), pp. 329-337.
Lacasse, C., Gamble, K.C. & Boothe, D.M. (2012) ‘Pharmacokinetics of a Single Dose of Intravenous and Oral Meloxicam in Red-tailed Hawks (Buteo jamaicensis) and Great Horned Owls (Bubo virginianus)’, Journal of Avian Medicine and Surgery, 27(3), pp. 204210.
Morrison, J. Greenacre, C.B., George, R., Cox, S. & Martín-Jiménez, T. (2018) ‘Pharmacokinetics of a single dose of oral and intramuscular meloxicam in African penguins (Spheniscus demersus)’, Journal of Avian Medicine and Surgery, 32(2), pp.102–108. Soh, H.Y., Tan, P.X.Y., Ng, T.T.M., Chng, H.T., Xie, S. (2022) ‘A critical review of the pharmacokinetics, pharmacodynamics, and safety data of antibiotics in avian species’, Antibiotics, 11(6), pp. 741-781.
Wilson, G.H. & Divers, S. & Budsberg, S. (2005) ‘Pharmacokinetics and use of meloxicam in psittacine birds’, Proceedings of the Association of Avian Veterinarians Conference. pp.7-9.
Through my residency program I have been involved in collating a large collection of melioidosis cases in companion animals dating back to 1997, with the goal of publishing an in-depth case series that could help to improve the rate of successful diagnosis and treatment of this often serious and frequently fatal disease. To date, published information on melioidosis in companion animals is sparse, with only few reports of intraocular infection or disseminated disease in cats (O’Brien et al., 2003; Parkes et al., 2006).
Melioidosis is the disease caused by Burkholderia pseudomallei, a Gram-negative bacterium which is endemic to the tropical and subtropical regions of Asia and northern Australia (Quinn et al., 2015). The bacteria are present in soil and water, and infection occurs through ingestion, inhalation, or skin contamination after penetrating injury. Contamination of multidose vials can give rise to case clusters in veterinary practices. Rates of infection tend to increase during periods of high rainfall and flooding, as the bacteria is brought to the soil surface where it can readily infect both humans and animals. Although humans and animals can both be infected from the same environmental source, zoonotic transmission from infected animals appears to be extremely rare. Any animal species can be infected, including companion animals, livestock, and wildlife (Choy et al., 2000).


The majority of the collated cases were diagnosed in the known endemic regions of the Northern Territory and far north Queensland, with the exception of one cat in Brisbane (Figure 1). This cat was diagnosed shortly after flooding events in Queensland earlier that year. However, in both humans and animals, sporadic cases have been diagnosed in desert and temperate regions including southwestern Western Australia and Ipswich, Queensland. The bacterium can also have a long latent subclinical period with subsequent reactivation up to decades later.
As previously mentioned, melioidosis is often associated with periods of high rainfall. In the collated cases, the vast majority were diagnosed during the months of highest rainfall in northern Australia (wet season, January and February), although six were diagnosed during dry season (June and July; Figure 2). The reason for this is unclear, although in many cases diagnosis may be delayed either due to a chronic disease course (latent subclinical infection reactivating due to stress or immunosuppression months later), or difficulty in achieving a definitive diagnosis.

There is a wide variety in clinical presentation of cases of melioidosis, which can often lead to difficulty in early recognition and diagnosis. Pyrexia, anorexia, and lethargy are common presenting signs in all disease syndromes.
• Non-healing soft tissue wounds and abscesses (Figure 3) are a common presentation in both dogs and cats, often accompanied by pyrexia and lethargy. These infections are usually not disseminated and carry a better prognosis with appropriate treatment.
• Intraocular infection appears to be unique to cats and may be associated with soil contamination of penetrating corneal injuries due to cat fighting.
• The neurologic presentation is seen in both dogs and cats, often as an acute onset of head tilt, circling, ataxia, and hindlimb weakness. This is associated with encephalitis of the brainstem on postmortem examination. This is a difficult presentation to diagnose, and prompt and aggressive treatment is needed to avoid mortality.
• Pneumonia is not common as a primary presentation (in comparison to humans where it is the most common disease manifestation) but can occur with dissemination and in some cats presumably after exposure to aerosols.
• Less common presentations include urinary tract infection (diagnosed on urine culture), and reproductive problems (lateterm abortion, swollen testicles).

Haematology and biochemistry findings are often non-specific inflammatory changes, including non-regenerative anaemia, neutrophilia with left shift, monocytosis, and hyperglycaemia (or hypoglycaemia with sepsis).
Cytology and histopathology reveal suppurative, necrosuppurative, or pyogranulomatous inflammation in the affected tissues, often with Gramnegative bacilli. Culture `is required for definitive diagnosis.
There are currently no published treatment guidelines for melioidosis in small animals. B. pseudomallei is inherently resistant to numerous antibiotics, including penicillin, ampicillin, first and second generation cephalosporins, macrolides, and aminoglycosides, along with intermediate resistance to fluoroquinolones. In humans, treatment is initiated with an intensive intravenous protocol with meropenem or ceftazidime, often with the addition of TMS. This is then followed by prolonged oral therapy with TMS for 3 months. Doxycycline and amoxicillin-clavulanic acid are alternative choices if TMS is not well-tolerated. Establishing drainage of large or deep abscesses is also an important component of therapy. Importantly, in vitro susceptibility testing often over-reports resistance to TMS, which is not reflective of a true resistance in vivo and rather difficulty in reading diffuse disc edges.
As most companion animal vets do not stock meropenem or third generation cephalosporins, usually combinations are used of TMS (ideally by injection at first), pradofloxacin, amoxicillin-clavulanic acid and doxycycline (usually using 2-3 of these agents).
• Melioidosis is more common in dogs and cats than is currently reflected in the literature, endemic to northern Australia, and strongly associated with high rainfall periods. It is an important cause of sepsis, severe disease and non-healing wounds in the NT and far north QLD.
• Recognition of the clinical syndromes in endemic areas should prompt early culture and appropriate antimicrobial treatment – IV in acute disseminated cases, followed by long courses of multiple oral antimicrobials (TMS, doxycycline, pradofloxacin and amoxicillin-clavulanic acid are all good choices).
• Although zoonotic transmission is possible, it appears to be rare, and standard PPE practices (wearing gloves when dealing with wounds and hand hygiene) should be sufficient for immunocompetent veterinary staff and owners.
References
Choy JL, Mayo M, Janmaat A, Currie BJ. Animal melioidosis in Australia. Acta Trop. 2000 Feb 5;74(2-3):153-8.
O’Brien CR, Krockenberger MB, Martin P, Parkes H, Kidd M, Malik R. Disseminated melioidosis in two cats. J Feline Med Surg. 2003 Apr;5(2):83-9.
Parkes HM, Shilton CM, Jerrett IV, Benedict S, Spratt BG, Godoy D, O’Brien CR, Krockenberger MB, Mayo M, Currie BJ, Malik R. Primary ocular melioidosis due to a single genotype of Burkholderia pseudomallei in two cats from Arnhem Land in the Northern Territory of Australia. J Feline Med Surg. 2009 Oct;11(10):856-63.
Quinn PJ, Markey BK, Leonard FC. Concise Review of Veterinary Microbiology. Newark, UNITED STATES: Wiley; 2015.

Dr Louise Folwell BSc(VB) DVM
Anaesthesia in small mammals has continued to cause anxiety in the veterinary community for many years. This may be multifactorial including the ability of these animals to mask disease and the perceived increased likelihood of perianaesthetic mortality compared to other domestic animals.
It is generally accepted in the veterinary industry that more risk factors are present and associated with anaesthetic-related death in small mammals compared to dogs and cats; however, literature surrounding peri-anaesthetic mortality rates in small mammals is limited, with variability in sample sizes and results. Broadbelt et al. 2008 had a considerably larger sample size compared to other available research and demonstrated that mortality rates in small mammals to be quite low to that that may be expected by veterinarians. This study reported a 1.39% mortality rate in 8209 rabbits (114 of 8209), 3.8% in guinea pigs (49/1288) and 2% in rats (8/398). The aim of this article is to identify risk factors to small mammal anaesthesia, and outline mitigation measures that may be taken to reduce the risk to anaesthetic-related complications.
Risk factor: Unidentified illness prior to anaesthetic
• Always perform a consult and health check with the client, preferably on a separate day to the planned anaesthetic. Clients may perceive their animals as “healthy”; however, it’s extremely important to question them thoroughly in regards to any subtle signs of disease. Time should also be taken to ensure appropriate diet and husbandry is being provided.
• A thorough clinical exam may reveal signs of dental disease, cardiac or respiratory disease, ear disease, or poor body condition, which may not have been picked up by the client.
• As with most species, anaesthetic mortality is significantly higher for unwell rabbits (Brodbelt et al. 2008; Ishida et al. 2014), with underlying cardiopulmonary diseases accounting for the vast majority of peri-anaesthetic deaths (Lee et al. 2018). Therefore, all unexpected signs of illness need to be identified and addressed before any procedure.
Risk factor: Stress
• Stress should be minimised as much as possible prior to anaesthesia to reduce the possibility of gut stasis and myocardial sensitivity to anaesthetic agents. To assist with this prey animals should be housed in an area with minimal foot traffic, away from the smells and sounds of predators and where an adequate hiding area is available.
• Small mammals are social, and bond strongly with their conspecifics. Having the client bring their bonded friends to the hospital with them can help to make them feel more relaxed in an unfamiliar environment.
Risk factor: Hypothermia
• As small mammals have a large surface area to volume ratio, they are susceptible to heat loss and hypothermia during anaesthesia and surgery. Ensuring appropriate warming is imperative to maintain normothermia and can be assisted using forced air warmers during sedation, anaesthesia, and during recovery.
Risk factor: Unestablished intravenous access
• Establishing intravenous access is essential to allow fluid therapy, drug administration, and IV access for emergency medications if required. Applying local anaesthetic cream to the intended venipuncture site at least five minutes before can improve
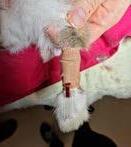
patient tolerance. Rabbits are generally quite tolerant of lateral ear vein cannula placement even when unsedated.
• Smaller mammals such as rats and guinea pigs will often require sedation prior to cannula placement.
The lateral tail vein in rats is easily accessible. Due to their thick skin, intravenous access in guinea pigs can often be challenging; however, piercing the skin with a larger gauge needle prior to introducing the cannula can help facilitate placement – typically, the saphenous or cephalic vein is the most easily accessible.
Risk factor: Lack of appropriate oxygenation and airway access
• Pre-oxygenation before anaesthesia, and establishing airway access is critical. It should be noted that in some small mammals (rats, mice, guinea pigs) intubation is generally not possible in which case a tight-fitting nasal mask should be used.
• In rabbits, it is extremely important to establish airway access, particularly when the procedure is expected to be long or involve significant discomfort. Always apply local anaesthetic to the larynx prior to intubation to reduce risk of laryngospasm. Either uncuffed endotracheal tubes or V-gels can be used. Both techniques have their risks and benefits, and there is no clear evidence in the literature as to which method is superior, so it is the clinician’s preference as to which is used.

Risk factor: Cardiorespiratory depression
• There are numerous anaesthetic protocols described, all of which have their pros and cons. As a general rule, avoiding agents that cause significant cardiorespiratory depression or hypotension are ideal, especially in older patients.
• Due to the small thorax in comparison to large abdomen, positioning the patient by raising the head and thorax up slightly will
help reduce pressure on the diaphragm from the large abdominal viscera and minimise respiratory complications.
Risk factor: Dehydration
• Providing fluid therapy is essential as many small mammals won’t eat or drink in hospital (especially post-anaesthesia) which can result in rapid dehydration. Intravenous fluid therapy is ideal however in cases where intravenous access can’t be established, subcutaneous will often suffice. As small mammals have a large surface area to volume ratio, they are susceptible to heat loss and hypothermia during anaesthesia and surgery. Ensuring appropriate warming is imperative to maintain normothermia and can be assisted using forced air warmers during sedation, anaesthesia, and during recovery.
• Post-anaesthetic recovery is the most critical time for small mammals, with greater than 50% of deaths occurring post-anaesthesia (Brodbelt et al. 2008; Lee et al. 2018;), with the largest study showing most deaths in rabbits occurred within three hours of the procedure (Brodbelt et al. 2008). This is due to a combination of factors such as gastrointestinal complications, hypothermia, and underlying cardiorespiratory disease (Lee et al. 2018) so stringent monitoring of patients post procedure is critical for a good recovery.
Risk factor: Gastrointestinal motility reduction
1. Pain, stress, and anaesthesia can all contribute to reduced gastrointestinal motility, with one study showing that peri-anaesthetic gastrointestinal complications occurred in in 38% of rabbits undergoing routine surgery (Lee et al. 2018). Ensuring sufficient (and often multi-modal) pain relief, as well as supplemental feeding upon recovery, can significantly reduce complication rates. Guinea pigs and rabbits can be syringe-fed hospital feeds such as Critical Care or Emeraid Herbivore until voluntarily eating. Rats will often readily eat pureed feeds such as baby food.
2. Clients should be instructed on recognising signs of pain in their pets, as most clients are not aware that they present differently to dogs and cats. Clients should also be educated on how to syringe feed their pets should this be necessary. Providing a handout on post-operative care in small mammals can be very useful.
Although anaesthetic in small mammals can be sometimes appear daunting, awareness of the main risk factors to anaesthetic safety and implementing appropriate measures and precautions can reduce the risk of complications and hopefully increase general confidence in approaching anaesthesia in exotic patients within the veterinary clinic.
References
Brodbelt, DC, Blissit, KJ, Hammond, RA, Neath, Young, LE, Pfeiffer, DU, Wood, JL 2008, ‘The risk of death: the confidential enquiry into perioperative small animal fatalities’, Veterinary Anaesthesia and Analgesia, vol 35, pp. 365-373, DOI: 10.1111/j.1467-2995.2008.00397.
Ishida, T, Onuma, M, Ono, S, Murakami, A, Sano, T 2014, ‘Anesthesia-associated death in 160 rabbits’, Japanese Journal of Veterinary Anesthesia & Surgery, vol. 45, no. 1, pp 7-12.
Lee, HW, Machin, H, Adami, C 2018 ‘Peri-anaesthetic mortality and non-fatal gastrointestinal complications in pet rabbits: a retrospective study on 210 cases’, Veterinary Anaesthesia and Analgesia, vol. 45, no. 4, pp. 520-528, DOI: 10.1016/j.vaa.2018.01.010.
There has been an increasing focus on the use of fluid therapy in veterinary practice. Just this year, AAHA have released their updated fluid therapy guidelines, with a strong focus on fluid balance and appropriate prescribing of fluids. No two patients or plans are the same, and the patient and fluid plan should frequently be reassessed during hospitalisation.
As an emergency veterinarian, I frequently encounter animals exposed to nephrotoxic substances, such as grapes, NSAIDs and lillies. The conventional treatment approach includes gastrointestinal decontamination, charcoal administration, and intravenous fluid therapy, at a variable rate usually above maintenance, for 48 to 72 hours. The goal has been to induce diuresis, theoretically accelerating toxin elimination and preventing acute kidney injury. Interestingly, this common practice is lacking in evidence, and more recently there is concern it could potentially be harmful.
While increased fluid administration does boost urine production, this is not only due to increased glomerular filtration rate, but also the secretion of atrial natriuretic peptide. Due to the intricate nature of renal physiology and the varying mechanisms of nephrotoxins, the actual benefits of forced diuresis and elevated ANP remain unclear and require research for each toxin.

BSc DVM MANZCVS (ECC)
In human medicine, similar practices have been reconsidered due to lack of evidence for benefits and potential risks. Excessive fluid therapy, particularly in a patient vulnerable to acute kidney injury, can lead to hypervolemia and interstitial edema. This can lead to worsened kidney injury rather than prevent it.
Fluid therapy should be tailored to each animal’s specific needs. Animals showing signs of hypovolemia, dehydration, or significant fluid losses should receive appropriate fluid treatment. Conversely, animals without these signs who can maintain euvolaemia should be considered to be monitored at home may not need prolonged IVFT.
With the current level of research, my approach to these cases includes rapid decontamination, then a discussion with the owners about what we know on the particular toxin (and what we don’t), management options and their potential outcomes, and the expected clinical signs and course of disease. More and more, this leads to less patients being hospitalised, but rather having outpatient care, baseline bloods, and serial creatinine measurements each day for 72 hours. Owners are counselled on the possibility that at any stage their pet could develop an AKI and hospitalisation indicated. This may be based off onset of clinical signs, or serial creatinine raising ≥ 26.4umol/L above baseline.
In conclusion, while IVFT is an important tool in managing nephrotoxic exposure, its use should be carefully considered. Current practices should be guided by evidence and tailored to individual patient needs rather than relying on a one size fits all approach.
References
1. Cole, L., 2017. *Management of acute kidney injury in the dog and cat* [Online]. Veterinary Ireland Journal. Available at: <https://www.veterinaryirelandjournal.com/images/pdf/small/ sa_dec_2017.pdf> [Accessed 1 August 2024].
2. Cowgill, L., 2016. *Grading of acute kidney injury* [Online]. IRIS. Available at: <http://www.iris-kidney.com/pdf/4_ldc-revised-grading-of-acute-kidney-injury.pdf> [Accessed 1 August 2024].
3. Ghannoum, M. and Gosselin, S., 2013. Enhanced poison elimination in critical care. *Advances in Chronic Kidney Disease*, 20(1), pp.94–101.
4. Malbrain, M.L., Marik, P.E., Witters, I., et al., 2014. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. *Anaesthesiology and Intensive Therapy*, 46(5), pp.361–380.`
5. Zhang, L., Chen, Z., Diao, Y., Yang, Y. and Fu, P., 2015. Associations of fluid overload with mortality and kidney recovery in patients with acute kidney injury: a systematic review and metaanalysis. *Journal of Critical Care*, 30(4), pp.860.

Dr Channy McGowan
BVSc MANZCVS (Behaviour) Elite FFCP-V

Did you know that 80% of disability is acquired, and approximately 1 in 6 people are disabled (Welfare, 2018)? The disabled community make up the largest marginalised population, with people discovering or acquiring their disability every minute, however tend to be the quietest in advocating for their Equity and Inclusion. And with the veterinary profession ranking highly among industries for rates of injury, disability and conversations around accommodation are very important. So, The Veterinary Kaleidoscope was born.
The Veterinary Kaleidoscope is the first Diversity, Equity and Inclusion organisation in Australasia supporting all veterinarians, vet nurses, vet techs, support staff, industry workers and vet students. Further to supporting vet professionals with disability, we advocate for Neurodivergents, Culturally and Linguistically Marginalised (CALM), Religious diversity, Socioeconomic marginalised, Aboriginal and Torres Strait Islander identities, Queer folk and other intersectional identities.
The Veterinary Kaleidoscope advocates for inclusion, diversity & belonging in our intersectional veterinary community by celebrating authenticity, visibility, curiosity & kindness.
Originating as a podcast between Veterinarians Kate Toyer and Cam Raw, The Veterinary Kaleidoscope (TVK) has exploded with action across the country thanks to a hard-working committee of diverse folk of lived experience. From advocating to support students affected by placement poverty to organising a world-first DEI Summit for the veterinary profession in 2023 and again in 2024, TVK Committee aims to serve with integrity, accountability and compassion, promoting understanding, connection & representation with Safety, Visibility and Empowerment, not only of our members, but all intersectionalities.
Dr Chantelle (Channy) McGowan is owned by two mischievous kitties, Mittens & Smudge. After nearly a decade in SA clinical practice, a side step to industry for a few years and then launching their own behaviour consulting business, Channy realised their passion lay with promotion of Diversity, Equity & Inclusion (DEI). Channy holds various volunteer roles, helping launch the first DEI conference for the veterinary profession (TVK Summit 2023 & 2024) as Treasurer for the Australian Rainbow Vets and Allies, and Vice President of The Veterinary Kaleidoscope. Dr Channy is an openly proud disabled, non-binary, neurodivergent, pansexual, intersex veterinarian.
We have had TVK committee members formally take on mentoring roles in the Pinnacle Foundation (Who provide educational scholarships, mentoring and opportunities for young LGBTQIA+ Australians to realise their full potential), and also represented our organisation at a ‘veterinary profession crisis’ round-table event run by Cherished Pets, hosted by the RSPCA, attended by organisations & charities across the veterinary profession and social work space. In receiving an award for their contributions to DEI in the veterinary profession, Drs Kate and Tara have also attended the 100 year anniversary of the Veterinary Surgeons Act of 1923 reception, by invitation from the Governor of New South Wales.
“We want the [veterinary] profession to see that diversity, equity and inclusion drives innovation, reduces cognitive load and burnoutas people no longer have to hide their needs or their identities. It also reduces workforce attrition, widens the pool of prospective talent, and increases profitability. Productivity gains are substantial. It gives a competitive edge and is a positive disruptor. However, we also don’t want to be reductionist by viewing diversity, equity and inclusion only through an economic lens as ultimately, The Veterinary Kaleidoscope is about visibility, protection and empowerment (Harrison, 2024)” – Dr Alex Harrison, Treasurer

To find out more and how you can be a member, contact us!
References Harrison, A., 2024. Veterinary scene Down Under: DEI advancements, plus title protection and more. [Online]
Available at: https://www.dvm360.com/view/veterinary-scene-down-under-dei-advancements-plus-titleprotection-and-more [Accessed 2024].
Welfare, A. I. o. H. a., 2018. [Online]
Available at: https://www.aihw.gov.au/reports/disability/people-with-disability-in-australia/contents/ people-with-disability/prevalence-of-disability [Accessed 2024].

Dr Olivia Miller BSc, DVM
The phenomenon of twins is common in many large animal species, however when it comes to horses it can lead to disaster more often than joy.
The rate of twins in thoroughbred mares is 10-15% (1). If a mare is allowed to carry a twin pregnancy, the mare is likely to suffer complications. Most commonly, these include abortion, dystocia, retained foetal membranes and still birth of one or both of the foals(1,2). If a foal is born alive, it is often smaller, weaker and more susceptible to infection.
Due to the potential for negative outcomes with twin pregnancies, veterinarians use to use various management practices to help minimise the risk of a mare carrying a twin pregnancy. These included not breeding mares with two dominant follicles and short cycling mares that ovulate two follicles. While these methods are effective, they take up valuable time in the breeding season where the mare is not pregnant or getting breed.
There are now several methods implemented after insemination to ensure the mare only carries a singleton pregnancy through gestation.
After a mare is inseminated, an ultrasound per rectum is performed to confirm ovulation 12-24 hours post insemination. At this time, it is important to note how many follicles were ovulated. This is generally done by counting the number of corpus luteums (CLs) present on the ovaries. If the mare has ovulated more than one follicle, it is critical to do a thorough scan in 12-14 days to check for twin embryos (3). At this stage of gestation, the embryos are still free moving within the uterus, so it is important to have a thorough look through the entire uterus (3).
Some mares can develop uterine cysts, which can often resemble the size and shape of early embryos. A good way to differentiate a cyst from a 14 day embryo
is if lightly touched and it does not move away, it is likely a cyst. However a further scan in 2-3 days time is recommended to check the size again. Embryos should grow at approximately 2-4mm per day (4)
If twins are noted on an ultrasound, there are now several methods to reduce the pregnancy to a single embryo. These methods are split into two categories, prior to fixation and after fixation. There is a higher rate of success when reduction is done prior to fixation.
Methods of reduction up until day 40 of gestation are outlined below.

Equine embryos are free moving in the uterus up until approximately day 16-18 of gestation, allowing a simple manual reduction technique to be performed. You need to ensure the embryos are separated from one another, this can easily be done by lightly touching one with the ultrasound transducer. Once separated, one of the embryos should be manoeuvred to the distal tip of a uterine horn. To reduce the embryo, you need to exert pressure using either the thumb and forefinger or the ultrasound transducer against the pelvis (1,5). Manual reduction prior to fixation has the highest rate of success in the literature, and therefore is the preferred method of twin reduction (1).
The method of reduction once the embryos have been fixed, depends on the age of the pregnancy.
Day 16 - Day 30: Manual Reduction. This technique can be performed as described above in twin embryos that have fixed in different horns. Bilateral twins have a much lower chance of natural reduction, and therefore reduction should be attempted immediately once detected. Twin embryos that fix in the same horn have a higher incidence of natural reduction (85% compared to 4% in bilaterally fixed embryos) (6). Therefore, it is reasonable to wait for a few days to see if natural reduction occurs prior to attempting manual reduction.
Day 30 - Day 45 (1,5): Pinching technique. Over 5-10 days, gentle pressure with the ultrasound probe is applied to one embryo. This causes disruption of the chorioallantois without rupturing to induce gradual stress and eventful loss of heartbeat of the targeted embryo. This in turn causes the foetal fluid compartments to become hyperechoic and reduce in size. This generally takes up to 48 hours to occur after the disruption of the chorioallantois.
Deciding which embryo to reduce will rely on several factors being taken in to consideration. The size of each embryo, the position of each embryo and the ease of which the embryo can be manoeuvred (7). Typically, the smaller of the two embryos will be the one to be reduced. It is thought that this embryo is likely to be less viable and therefore the larger embryo has a higher rate of being maintained after reduction (1,6).
NSAID and progestin therapies should also be considered when reducing a twin pregnancy. Some studies have shown the use of these therapies to result in lower chance of early pregnancy loss (8). The mare’s age and the duration of the procedure are the two key factors to consider when determining if these additional therapies are needed (8, 9, 10).
For more comprehensive information on twin reduction, please see the articles listed in the reference list below.
References
1. Peere S, van Den Branden E, Papas M, Gerits I, Smits K, Govaere J. Twin management in the mare: A review. Equine Veterinary Journal. 2024 Apr 9.
2. Jeffcott LB, Whitwell KE. Twinning as a cause of foetal and neonatal loss in the Thoroughbred mare. J Comp Pathol. 1973;83:91–106.
3. Chevalier F, Palmer E. Ultrasonic echography in the mare. J Reprod Fertil Suppl. 1982;32:423–30.
4. Carnevale EM, Metcalf ES. Morphology, developmental stages and quality parameters of in vitro-produced equine embryos. Reproduction, Fertility and Development. 2019 Dec 12;31(12):1758-70.
5. McKinnon AO. Twin reduction techniques. In: Samper J, Pycock J, McKinnon A, editors. Current therapy in equine reproduction. 1st ed. St. Louis, Missouri: Elsevier; 2007. p. 357–73.
6. Ginther OJ. The nature of embryo reduction in mares with twin conceptuses: deprivation hypothesis. Am J Vet Res. 1989;50:45–53.
7. Wolfsdorf K. Management of twins in the mare. Equine Vet Educ. 2011;24:60–1
8. de Mestre AM, Rose BV, Chang YM, Wathes DC, Verheyen KLP. Multivariable analysis to determine risk factors associated with early pregnancy loss in Thoroughbred broodmares. Theriogenology. 2019; 124:18–23.
9. Sheerin P, Howard CE, LeBlanc MM, Stromberg AJ. Effects of operator, treatment and mare age on the live foal rate of mares after manual twin reduction. Anim Reprod Sci. 2010;121:312–3.
10. Schnobrich MR, Riddle WT, Stromberg AJ, Leblanc MM. Factors affecting live foal rates of Thoroughbred mares that undergo manual twin elimination. Equine Vet J. 2013;45:676–80







Dr
BSc, PhD
Feral cats cost the Australian economy >$6 billion annually through impacts on human health and livestock production [1]. They also consume millions of native birds, mammals and reptiles annually [2]. Similarly, foxes can have significant impacts on livestock production and wildlife [3]. To protect threatened species and domestic stock, land managers attempt to mitigate these impacts through the use of toxic baiting, most commonly 1080/ sodium monofluoroacetate impregnated baits.
Baits are cost-effective to distribute and so are uniformly distributed across vast areas of Australia ([4]). This is typically achieved by throwing baits out the door of a plane or helicopter while flying back and forth across the landscape (Figure 1), or alternatively, distributing them by car along farm tracks and dirt roads.


We now know that cats and foxes do not use the landscape uniformly. Hence, when toxic baits are distributed in a uniform manner many are likely never encountered or consumed. Instead, they selectively use the landscape based on the distribution and abundance of food and shelter resources, or landscape features that facilitate accessing these (Figure 2). To increase the number of baits that are encountered and potentially consumed by cats and foxes, it is therefore logical to target habitats that are preferentially used and avoid those that are infrequently visited. Through this, we should be able to achieve an equivalent or better population reduction at reduced labour and financial cost, with the added benefit that fewer baits remain in the landscape that may then be consumed by other non-target wildlife.

On three large Bush Heritage reserves here in South Australia, my colleagues and I are currently working towards optimising how large-scale toxic baiting is conducted. Our research is driven by the exact challenge I describe above. Specifically, we are catching and GPS collaring feral cats and foxes to identify what habitats they preferentially use or avoid. This data will then be used to inform large-scale trials comparing the effectiveness of targeted Vs uniform baiting strategies, with the hope that targeted baiting will achieve an equivalent reduction in cat and fox populations at reduced cost and non-target impacts. For vet practitioners, fewer toxic baits distributed in more targeted locations should also mean that our pets are at reduced risk of consuming them accidently.
References
1.Legge, S., et al., Cat-dependent diseases cost Australia AU $6 billion per year through impacts on human health and livestock production. Wildlife Research, 2020. 47(8): p. 731-746.
2.Woinarski, J., et al., How many birds are killed by cats in Australia? Biological Conservation, 2017. 214: p. 76-87.
3.Stobo-Wilson, A.M., et al., Sharing meals: Predation on Australian mammals by the introduced European red fox compounds and complements predation by feral cats. Biological Conservation, 2021. 261: p. 109284.
4.Campbell, K., et al., Review of feral cat eradications on islands. Island Invasives: Eradication and Management: Proceedings of the International Conference on Island Invasives. 2011, Gland, Switzerland: International Union for Conservation of Nature. 37-46.










Dr Sherelle Casey BSc (PV), DVM, PhD
Background: Chronic pain is prevalent in both humans and animals and current treatments are hampered by limited efficacy and side effects. Multi-modal treatments are generally most effective, with single treatments often not providing sufficient analgesia. New treatment options, particularly those acting at novel receptors, are needed.
The Cannabis sativa plant has been used in traditional medicine for millenia. While it contains more than 400 compounds, the majority of research has focussed on 9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Using a mouse model, I investigated the potential of THC and CBD alone and in combination for the treatment of neuropathic pain.
Key Findings
• When administered as a one-off dose in mice with established neuropathic pain, both THC and CBD alone administered via the subcutaneous, oral, intrathecal and intraplantar routes produced dose-dependent attenuation of mechanical allodynia and thermal hyperalgesia (1, 2, 3).
» THC was more effective and more potent than CBD across all administration routes (1, 2, 3).
» One-off oral doses of THC and CBD alone were less potent and less effective than subcutaneous doses (2).

• Systemically administered THC acted at the CB1 receptor and produced typical cannabinoid side effects (motor impairment, catalepsy and sedation). CBD did not act at the CB1 receptor and therefore did not produce typical cannabinoid side effects. CBD was a partial agonist at the CB2 receptor (1,2).
» THC did not produce typical cannabinoid side effects when administered via the intrathecal and intraplantar routes (3). This is likely because it is acting locally at spinal and peripheral receptors and not reaching CB1 receptors in the brain, where these side effects are mediated.
• There was a profound synergistic interaction between THC and CBD when co-administered as a one-off subcutaneous dose in a 1:1 ratio for pain relief, but not for side effects. Isobolographic analysis determined that this synergistic interaction was biphasic. At low doses, there was a high potency THC:CBD mode of action, with a 200-fold increase in anti-allodynic activity. At higher doses, there was a low potency THC:CBD mode of action.
» 1:1 was the optimal ratio for low dose THC:CBD combinations (superior to both 1:3 and 3:1).
• Based on the above findings, the most promising candidates for treatment of neuropathic pain are CBD alone and low dose THC:CBD combinations, likely as adjuncts to other therapies. Topical creams may also have potential given the findings for the intraplantar administration route and the highly lipophilic nature of cannabinoids.
At present, there is no regulatory body-approved Cannabis product in any country worldwide. There are, however, multiple start-ups aiming at getting such products registered, which should happen in Australia in the next couple of years.

Figure 2: Dose-response curves for the effect of 1:1 THC:CBD on (A) mechanical PWT and (B) acetone responses. Also shown are the predicted additive dose response curve for combination THC:CBD, and the sigmoidal fits for THC and CBD monotherapy (reproduced from Figure 1). ***, and **** denote p < 0.001, and 0.0001 for THC:CBD experimental combination versus predicted additive at equivalent doses.
• When administered as a one-off oral dose, the synergistic effect observed for low dose THC:CBD was significantly less (2-fold rather than 200-fold) than that seen for the subcutaneous administration route (2). This, as well as the lower potency and efficacy of oral THC and CBD alone, are likely due to low bioavailability and the effects of first-pass metabolism.
References
CBD is currently a Schedule 4 veterinary medicine and THC (if comprising more than 2% of cannabinoid content) Schedule 9, with an application to the TGA to reduce this classification to S8 in 2022 being rejected. In Australia veterinarians are able to legally prescribe CBD products (>98% cannabinoid content must be CBD) through CBD Vets Australia and ECS Veterinary. Most veterinary studies have focussed on dogs, with fewer studies in cats and horses. There is some evidence that CBD has benefits for the treatment of canine osteoarthritis, post-surgical pain, epilepsy, anxiety/ stress and atopic dermatitis (4, 5, 6, 7, 8, 9). In both clinical trials and safety studies in dogs, CBD is generally regarded as safe. The most common side effect of CBD is gastrointestinal disturbance (diarrhoea, vomiting), generally mild and self-resolving (4, 5, 7, 10, 11). It is worth noting that elevated ALP (without concurrent increases in other liver enzymes or bilirubin) has been observed in some dogs on long term oral CBD (5, 12, 13). While the reasons for this remain unclear, it is possible that it is associated with hepatic microsomal enzyme induction without actual liver damage (5, 10, 12). Nonetheless, it may be prudent to monitor ALP concentrations in dogs taking CBD, particularly those with existing liver disease.
1. Casey SL, Atwal N, Vaughan CW. Cannabis constituent synergy in a mouse neuropathic pain model. PAIN. 2017 Dec; 158(12):2452-60
2. Mitchell VA, Harley J, Casey SL, Vaughan AC, Winters BL, Vaughan CW. Oral efficacy of Δ(9)-tetrahydrocannabinol and cannabidiol in a mouse neuropathic pain model. Neuropharmacology. 2021 May;189:108529. 3. Casey SL, Mitchell VA, Sokolaj EE, Winters BL, Vaughan CW. Intrathecal actions of the cannabis constituents Δ(9)-tetrahydrocannabinol and cannabidiol in a mouse neuropathic pain model. International Journal of Molecular Science. 2022 August; 23:8649
4. Verrico CD, Wesson S, Konduri V, Hofferek CJ, Vazquez-Perez J, Blair E, et al. A randomized, double-blind, placebo-controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain. Pain. 2020 Apr 24;161(9):2191–202.
5. Gamble LJ, Boesch JM, Frye CW, Schwark WS, Mann S, Wolfe L, et al. Pharmacokinetics, Safety, and Clinical Efficacy of Cannabidiol Treatment in Osteoarthritic Dogs. Frontiers in Veterinary Science. 2018 Jul 23;5. 6. Casas-Alvarado A, Martinez-Burnes J, Hernandez-Avalos I, Mora-Medina P, Miranda-Cortes A, Dominguez-Oliva A et al. Assessment of the nociceptive response to the use of cannabidiol alone and in combination with meloxicam through infrared pupillometry in female dogs undergoing elective ovariohysterectomy. Frontiers in Veterinary Science. 2024 July 11.
7. Rozental AJ, Weisbeck BG, Corsato Alvarenga I, Gustafson DL, Kusick B, Rao S, et al. The efficacy and safety of cannabidiol as adjunct treatment for drug‐resistant idiopathic epilepsy in 51 dogs: A double‐blinded crossover study. Journal of Veterinary Internal Medicine. 2023 Oct 27;
8. Flint HE, Hunt ABG, Logan DW, King T. Daily dosing of cannabidiol (CBD) demonstrates a positive effect on measures of stress in dogs during repeated exposure to car travel. Journal of Animal Science. 2024 Jan 1;102. 9. Loewinger M, Wakshlag JJ, Bowden D, Peters‐Kennedy J, Rosenberg A. The effect of a mixed cannabidiol and cannabidiolic acid based oil on client‐owned dogs with atopic dermatitis. Veterinary Dermatology. 2022 May 29;33(4):329.
10. Vaughn DM, Paulionis LJ, Kulpa JE. Randomized, placebo-controlled, 28-day safety and pharmacokinetics evaluation of repeated oral cannabidiol administration in healthy dogs. American Journal of Veterinary Research. 2021 May;82(5):405–16.
11. Vaughn D, Kulpa J, Paulionis L. Preliminary Investigation of the Safety of Escalating Cannabinoid Doses in Healthy Dogs. Frontiers in Veterinary Science. 2020 Feb 11;7.
12. Deabold KA, Schwark WS, Wolf L, Wakshlag JJ. Single-Dose Pharmacokinetics and Preliminary Safety Assessment with Use of CBD-Rich Hemp Nutraceutical in Healthy Dogs and Cats. Animals : an Open Access Journal from MDPI [Internet]. 2019 Oct 19
13. McGrath S, Bartner R, Rao S, Kogan LR, Hellyer PD. A report of adverse effects associated with the administration of cannabidiol in healthy dogs. Journal of the American Holistic Veterinary Medical Association. 2018 Fall; 52:34–38

29 Adamson St, Hayborough SA 5211
T: 08 8552 1188
W: www.victorvet.com.au
Describe why you wanted to start your own business.

2015 alumnus


Instagram: @_victor_vet_
Facebook: @victorvetclinic
It was always the ultimate goal, however it came about quicker than I was anticipating. I was working for another local vet clinic and I had been promised a partnership, however they decided to sell to a corporate structure behind the scenes. The work environment changed and I was constantly getting admonished for not meeting KPIs and sales targets. I quickly realised that I’m not a good salesperson and I don’t work well within rigid parameters. My mental health was terrible, the pay was worse, I had no work-life balance. I was very depressed and was constantly dreading going to work.
I was hesitant to become a business owner because I was still only recently graduated. The corporate owners were pushing a contract that would prevent me from working for any other clinic within a 25km radius - so this gave me the “now or never” push. I contacted a small clinic close to where I live and asked if they would be interested in selling. As luck would have it, they were.
What is your greatest win since starting your own business?
The greatest win is that I genuinely enjoy my work. The clinic already had a big client base but it has now quadrupled. I have a much better work-life balance and I am spending significantly more time with my family and surfing everyday when the waves are breaking.
What’s something you didn’t expect to learn/do/encounter while starting a business?
Business ownership is so much easier than you are led to believe. As vets we are problem-solvers and starting a business is just a series of problems that you work through and solve. The biggest challenge has been the “vet shortage”. We have had strong and consistent growth, but finding vets has been really challenging!
What advice would you give other graduates interested in business ownership or practice management?
If you have the desire, just do it. Get a good team of professionals to help you with the ‘business stuff’. Get a good accountant as they will help you keep more of your hard-earned dollars that you will otherwise lose to unnecessary taxation. You need a reliable and pragmatic IT guy. Lastly it is really useful to have a mentor - a vet or someone in a similar field, like a dentist - that you can chat through ideas and frustrations. Also... don’t spend anything on advertising or marketing. Provide good, honest service and ‘word of mouth’ is all you need.

Dr Benjamin Weir BSc (Veteirnary Bioscience) DVM 2017 alumnus

ProductionAnimalVets

Dr Ben is the director and owner of Production Animal Veterinary Services (PAVS). He offers a mobile large animal (mainly cattle) veterinary service to clients in the Fleurieu region and Adelaide Hills. Dr Ben is a 2017 alumnus of the University of Adelaide and started his veterinary career down in Mount Gambier where he learned how to be a successful cattle veterinarian from his mentor Dr Andrew Hoare. He then, as most people do after a few years, moved back to Adelaide and started his own business in 2023. He is kept plenty busy with over 400 large animal clients to look after, despite only being open for 15 months.
Describe why you wanted to start your own business.
I started my own business because when I moved back to Adelaide, I saw a large gap in the large animal veterinary market which wasn’t being filled. Since starting my own practice, my mental health has dramatically improved due to having control over a better work life balance. After working my butt off for someone else for a wage, it is an awesome feeling to work for yourself and be rewarded for all the effort you put in.
What is your greatest win since starting your own business?
I hired an incredible practice manager right at the start who had a wealth of experience in the veterinary industry. Initially, I thought about this business venture solo, due to the uncertainty of initial cash flow and the extra costs of hiring someone else. However, having someone who you can rely on for the administration part of the job has been so valuable, meaning I can focus more on the veterinary aspect of the job while knowing that the background activities (ordering/tax reconciliation/phones/scheduling)- are all under control while I’m out and about. I also have exceptionally good clients - I’d consider that the biggest win of them all!
What’s something you didn’t expect to learn/do/encounter while starting a business?
Cash flow in any veterinary business is always a constant source of anxiety, especially as most large animal clients are used to being on monthly accounts. Bur due to a combination of being selective with my client list and utilising the skills of my practice manager, I have a good client base that always pay on time. This makes that aspect of running a business less stressful, but in the early stages I had to fire a few of the bad paying clients. I can tell you, that’s not a super fun job to do. But my business is better off now because of it.
What advice would you give other graduates interested in business ownership or practice management?
Make sure you have a good business plan to follow. I took advice from several mentors, and this was very important to develop and progress as the business has. I would also recommend being extremely comfortable with all the areas of veterinary care that you will be providing. For me, that entailed working as a production vet for 5 years before jumping into ownership and management of a large animal business. But absolutely no regrets here. I’m loving it.





























Kaurna acknowledgement
We acknowledge and pay our respects to the Kaurna people, the original custodians of the Adelaide Plains and the land on which the University of Adelaide’s campuses at North Terrace, Waite, and Roseworthy are built. We acknowledge the deep feelings of attachment and relationship of the Kaurna people to country and we respect and value their past, present and ongoing connection to the land and cultural beliefs. The University continues to develop respectful and reciprocal relationships with all Indigenous peoples in Australia, and with other Indigenous peoples throughout the world.
Further enquiries
The University of Adelaide SA 5005 Australia enquiries alumni@adelaide.edu.au
phone +61 8 8313 5800 web adelaide.edu.au/alumni
Disclaimer The information in this publication is current as at the date of printing and is subject to change.