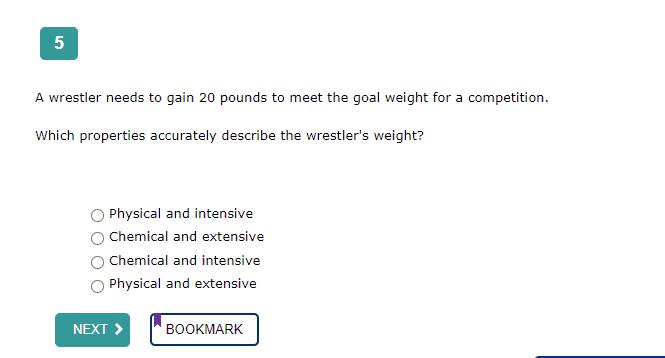
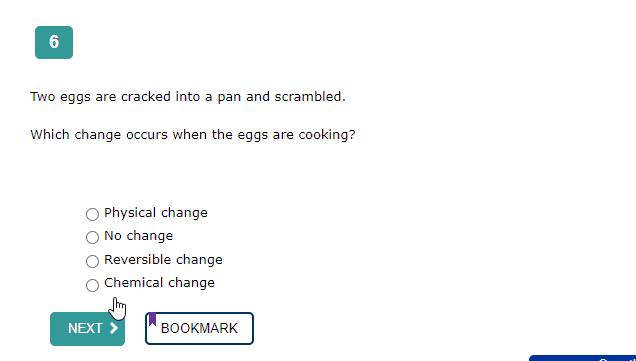
The correct answer is:
Homogeneous mixture
When table salt (sodium chloride) is dissolved in water, it forms a uniform solution where the salt is evenly distributed throughout. This is classied as a homogeneous mixture.

The substance has a freezing point of -114°C and a boiling point of 98°C. At 25°C, the temperature is between the freezing and boiling points.
Therefore, the physical state of the substance at 25°C is: Liquid.

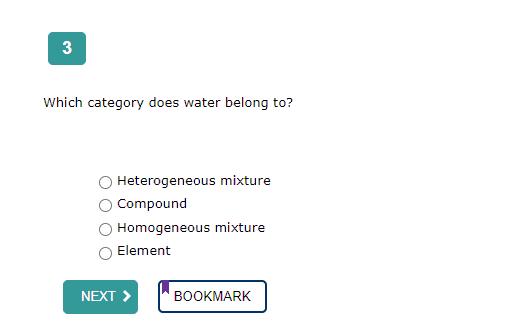
The correct answer is:
Compound
Water (H₂O) is a chemical compound composed of two hydrogen atoms covalently bonded to one oxygen atom.