
Official journal of www.apscardio.org Volume 1 • 2022 www.JAPSCjournal.com










Radcliffe Lifelong Learning Vascular Lifelong Learning for Vascular Professionals Radcliffe Cardiology Lifelong Learning for Cardiovascular Professionals WEBINARS ROUNDTABLES EXPERT INTERVIEWS JOURNAL PUBLICATIONS ARTICLE PUBLICATIONS INDUSTRY NEWS CLINICAL TRIAL REVIEWS AND MORE... @radcliffeCARDIO @radcliffeVASCU Radcliffe Cardiology Radcliffe Vascular Radcliffe Cardiology radcliffe_cardiology FOLLOW US ON SOCIAL MEDIA FOR DAILY UPDATES Journal of Asian Paci c Society of Cardiology
Junya Ako
Kitasato University and Hospital, Tokyo, Japan
Alan Yean Yip Fong
Editor-in-Chief
Khung Keong Yeo
National Heart Centre, Singapore
Deputy Editors
Derek P Chew Flinders University, Adelaide, Australia
Associate Editors
Sarawak Heart Centre, Kota Samarahan, Malaysia
Teiji Akagi
Okayama University Hospital, Okayama, Japan
Juwairia Yousif Tahir Alali
Rashid Hospital, Dubai, United Arab Emirates
Wael Almahmeed
Cleveland Clinic Abu Dhabi, United Arab Emirates
Jeroen J Bax
Leiden University Medical Center, the Netherlands
Yee Ling Cham
Sarawak Heart Center, Kota Samarahan, Malaysia
Chern-En Chiang
Taipei Veterans General Hospital, Taipei, Taiwan
Eue-Keun Choi
Seoul National University Hospital, Seoul, South Korea
Jong-Il Choi
Korea University College of Medicine and Korea University Hospital, Seoul, South Korea
Jin Oh Choi
Sungkyunkwan University School of Medicine, Samsung Medical Center, Seoul, South Korea
László Göbölös
Cleveland Clinic Abu Dhabi, United Arab Emirates
Adrian Hernandez
Duke School of Medicine, Durham, NC, US
Abdul Ihdayhid
Fiona Stanley Hospital, Harry Perkins Institute of Medical Research, University of Western Australia, Perth, Australia
Hidehiro Kaneko
University of Tokyo, Tokyo, Japan
Sazzli Shahlan Kasim
University of Technology MARA, Shah Alam, Malaysia
Editorial Board
Dae-Hee Kim
Kyung Woo Park
Seoul National University Hospital Internal Medicine, Seoul, South Korea
Jonathan Yap
National Heart Centre Singapore; Duke-NUS Graduate Medical School, Singapore
Akihiro Nomura
Asan Medical Center, Seoul, South Korea
Hyung-Kwan Kim
Seoul National University College of Medicine, Seoul, South Korea
Michel Komajda
National Academy of Medicine, Paris, France
Takashi Kunihara
Jikei University School of Medicine, Tokyo, Japan
Kengo Kusano
National Cerebral and Cardiovascular Center, Suita, Japan
Koichiro Kuwahara
Shinshu University School of Medicine, Nagano, Japan
Alex Lee
The Chinese University of Hong Kong, Hong Kong, China
Jian-Jun Li
Fu Wai Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China
Yi-Heng Li
National Cheng King University Hospital, Tainan, Taiwan
Nitish Naik
All India Institute of Medical Sciences, New Delhi, India
Quang Ngoc Nguyen
Vietnam National Heart Institute, Hanoi Medical University, Hanoi, Vietnam
Stephen Nichols
South Australian Health and Medical Research Institute and University of Adelaide, Adelaide, Australia
Innovative Clinical Research Center, Kanazawa University (iCREK), Kanazawa, Japan
Arintaya Phrommintikul
Chiang Mai University, Chiang Mai, Thailand
Ding Zee Pin
National Heart Centre, Singapore
Sunu Budhi Rahardjo
Universitas Indonesia/National Cardiovascular Center Harapan Kita, Jakarta, Indonesia
Yasushi Sakata
Osaka University Graduate School of Medicine, Osaka, Japan
Zainab Samad
Aga Khan University, Karachi, Pakistan
Jack Wei Chieh Tan
National Heart Centre Singapore, Singapore
Shiro Uemura
Kawasaki Medical School, Kurashiki, Japan
Tzung-Dau Wang
National Taiwan University Hospital, Taipei, Taiwan
Rakesh Yadav
All India Institute of Medical Sciences, New Delhi, India
Osamu Yamaguchi
Ehime University Graduate School of Medicine, Matsuyama, Japan
Kazuhiro Yamamoto
Tottori University, Tottori, Japan
Yen-Wen Wu
Cardiovascular Medical Center, Far Eastern Memorial Hospital, New Taipei City, Taiwan
www.JAPSCjournal.comwww.apscardio.org Volume 1 • 2022 © THE AUTHORS. PRODUCED BY RADCLIFFE GROUP LTD. www.JAPSCjournal.com
Editorial
Publishing Director Leiah Norcott
Production Editor Garrett Ziolek | Senior Graphic Designer Lewis Allen
Peer Review Editor Nicola Parsons | Editorial Coordinator Jemima Hegerty-Ward
Contact leiah.norcott@radcliffe-group.com
Partnerships
Marketing Director Lizzy Comber
Digital Marketing Manager Kati Marandi | Marketing Coordinator Calum Barlow
Contact lizzy.comber@radcliffe-group.com
Radcliffe Medical Media

Managing Director Jonathan McKenna
Promotional Sales Director David Bradbury | Agency Sales Director Gary Swanston
Senior Account Managers William Cadden, Brad Wilson
Account Manager Steven Cavanagh
Contact david.bradbury@radcliffe-group.com
Radcliffe Medical Education

Managing Director Rob Barclay Education Sales Director Carrie Barclay Education Account Manager Meadbh Metrustry
Contact carrie.barclay@radcliffe-group.com
Leadership
Chief Executive Officer David Ramsey Chief Operations Officer Liam O’Neill
Official
of
Published by Radcliffe Cardiology. All information obtained by Radcliffe Cardiology, Asian Pacific Society of Cardiology and each of the contributors from various sources is as current and accurate as possible. However, due to human or mechanical errors, Radcliffe Cardiology, Asian Pacific Society of Cardiology and the contributors cannot guarantee the accuracy, adequacy or completeness of any information, and cannot be held responsible for any errors or omissions, or for the results obtained from the use thereof. Published content is for information purposes only and is not a substitute for professional medical advice. Where views and opinions are expressed, they are those of the author(s) and do not necessarily reflect or represent the views and opinions of Radcliffe Cardiology or Asian Pacific Society of Cardiology.
Radcliffe Cardiology, Unit F, First Floor, Bourne End Business Park, Cores End Road, Bourne End, Buckinghamshire SL8 5AS, UK © 2022 All rights reserved • eISSN: 2754-0650
www.JAPSCjournal.comwww.apscardio.org Volume 1 • 2022 © RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com
journal
Aims and Scope
• Journal of Asian Pacific Society of Cardiology is the official journal of the Asian Pacific Society of Cardiology. It is an English language, peer-reviewed journal that publishes articles continuously on www.JAPSCjournal.com
• Journal of Asian Pacific Society of Cardiology aims to provide a forum for cardiovascular research, promote understanding and allow the sharing of consensus across the Asia-Pacific region.
• Journal of Asian Pacific Society of Cardiology comprises of the latest original research, balanced and comprehensive review articles, expert opinion pieces and case reports, written by leading authorities from Asia-Pacific.
• Journal of Asian Pacific Society of Cardiology provides updates on a range of salient issues and will support physicians across the region to develop their knowledge and effectiveness in day-to-day clinical practice.
Structure and Format
• Journal of Asian Pacific Society of Cardiology publishes original research, review articles, expert opinions pieces and case reports.
• The structure and degree of coverage assigned to each category of the journal is the decision of the Editor-in-Chief, with the support of the Deputy Editors, Associate Editors and Editorial Board.
Editorial Expertise
Journal of Asian Pacific Society of Cardiology is supported by various levels of expertise:
• Overall direction from an Editor-in-Chief, supported by the Deputy Editors, Associate Editors and an Editorial Board comprising leading authorities from a variety of related disciplines.
• Peer review is conducted by experts appointed for their experience and knowledge of a specific topic.
• An experienced team of editors and technical editors.
Submissions and Instructions to Authors
• All submissions are subject to initial appraisal by the Editor-in-Chief, and, if found suitable for further consideration, sent forward for peer review by independent, anonymous expert referees. All peer review is double blind and submission is online via Editorial Manager.
• Instructions for authors and additional submission details are available at www.JAPSCjournal.com
• Leading authorities wishing to discuss potential submissions should contact Publishing & Editorial Director, Leiah Norcott, leiah.norcott@radcliffe-group.com
• Articles may be submitted directly at www.editorialmanager.com/jaspc
Ethics and Conflicts of Interest
The journal follows guidance from the International Committee of Medical Journal Editors and the Committee on Publication Ethics. We expect all parties involved in the journal’s publication to follow these guidelines. All authors must declare any conflicts of interest.
Open Access, Copyright and Permissions
Articles published in this journal are gold open access, which means the version of record is freely available, immediately upon publication, without charge. Articles may be published under a CC-BY-NC or CC-BY licence.
CC-BY-NC: Allows users to read, download, copy, redistribute and make derivative works for non-commercial purposes. The author retains all non-commercial rights for articles published herein under the CC-BY-NC 4.0 License (https://creativecommons.org/licenses/by-nc/4.0/legalcode). To support open access publication costs, Radcliffe charges an article publication charge upon acceptance of an unsolicited paper: £1,050 UK | €1,240 Eurozone | $1,380 all other countries. Waivers are available, as specified in the ‘For authors’ section on www.JAPSCjournal.com. Permission to reproduce an article published under CC-BY-NC for commercial purposes, either in full or part, should be sought from the Managing Editor.
CC-BY: Allows users to read, download, copy, redistribute and make derivative works for any purpose, including commercially. Radcliffe offers publication under the CC-BY 4.0 License (https://creativecommons.org/ licenses/by/4.0/legalcode) to authors funded by UK Research Councils (UKRI) or The Wellcome Trust. The article publication charge is £1,750 | €2,069 Eurozone | $2,299 all other countries. The author retains all rights under this option.
Peer Review
• On submission, all articles are assessed by the Editor-in-Chief, Deputy Editor or Associate Editor.
• Suitable manuscripts are sent for double-blind peer review.
• The Editors reserve the right to accept or reject any proposed amendments.
• Once a manuscript has been amended in accordance with the reviewers’ comments, it is assessed to ensure it meets expectations.
• The manuscript is sent to the Editors for final approval.
Distribution and Readership
Journal of Asian Pacific Society of Cardiology is an online publication. Articles are published continuously on www.JAPSCjournal.com. The journal is free to read online and PDF downloads are available for registered users.
Online
All published manuscripts are free to read at www.JAPSCjournal.com They are also available at www.radcliffecardiology.com, along with articles from the other journals in Radcliffe Cardiology’s cardiovascular portfolio – Arrhythmia & Electrophysiology Review, Cardiac Failure Review, European Cardiology Review, Interventional Cardiology and US Cardiology Review
Reprints
All articles included in Journal of Asian Pacific Society of Cardiology are available as reprints. Please contact the Promotional Sales Director, David Bradbury david.bradbury@radcliffe-group.com
www.JAPSCjournal.comwww.apscardio.org Volume 1 • 2022 © RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com
Shaping Our Destiny Together: A Shared Journey for the Asia-Pacific Region Begins Khung Keong Yeo https://doi.org/10.15420/japsc.2021.35
A Case of Osseocartilaginous Defects and Cardiovascular Anomalies: An Ambiguous Entity Aritra Paul, Aruni Ghose, Debasish Banerjee and Suvro Banerjee https://doi.org/10.15420/japsc.2021.06
Trio of Rheumatic Mitral Stenosis, Right Posterior Septal Accessory Pathway and Atrial Flutter: A Case Report
Jogendra Singh, Dibyasundar Mahanta, Rudra Pratap Mahapatra, Debasis Acharya and Ramachandra Barik https://doi.org/10.15420/japsc.2021.07
Characteristics and Outcomes of MI with Non-obstructive Coronary Arteries in a South-east Asian Cohort Cheney Jianlin Wong, Jonathan Yap, Fei Gao, Yee How Lau, Weiting Huang, Fazlur Jaufeerally, Ngiap Chuan Tan, Hee Hwa Ho, Mark Chan, Kelvin Bryan Tan and Khung Keong Yeo https://doi.org/10.15420/japsc.2021.04
Impact of COVID-19 on Acute MI and Percutaneous Coronary Intervention Rates and Outcomes in South East Asia and the Middle East
Paul Jie Wen Tern, Yilin Jiang, Yee How Lau, Wael Almahmeed, S Gunavathy Selvaraj, Jack Wei Chieh Tan, Wan Azman Wan Ahmad, Jonathan Yap and Khung Keong Yeo https://doi.org/10.15420/japsc.2021.12
Effects of the Current Japanese Guideline for Dedicated, Intensive Lipid-lowering Therapy on Lipid Profile and Coronary Events in Patients After Acute Coronary Syndrome
Ryotaro Yamada, Teruyoshi Kume, Hiroshi Okamoto, Masahiro Yamashita, Satoshi Koto, Kyo Kamisaka, Yoshitaka Sasahira, Yasuyuki Sudo, Ayano Enzan, Tomoko Tamada, Terumasa Koyama, Koichiro Imai, Takeshi Nishi, Yoji Neishi and Shiro Uemura https://doi.org/10.15420/japsc.2021.21
Intracoronary Imaging of Recanalised Coronary Thrombus: A Report of Two Cases Achmad Fauzi Yahya, Ibnu Adams and Aninka Saboe https://doi.org/10.15420/japsc.2021.08
Novel Mechanical Thrombosuction in an Ectatic Right Coronary Artery with Large Thrombus Burden: A Case Report Prabesh Neupane, Anish Hirachan and Kamaraj Selvaraj https://doi.org/10.15420/japsc.2021.15
Palliative Care in Advanced Heart Failure: A Description of Challenges in the Singapore Experience Samuel Ji Quan Koh, Gillian Li Gek Phua, David Kheng Leng Sim and Shirlyn Hui-Shan Neo https://doi.org/10.15420/japsc.2022.05
NT-proBNP Cut-off Values for Risk Stratification in Acute MI and Comparison with Other Risk Assessment Scores
Shirley Siang Ning Tan, Keng Tat Koh, Alan Yean Yip Fong, Mohammad Adam bin Bujang, Lee Len Tiong, Yee Ling Cham, Kian Hui Ho, Chen Ting Tan, Chee Sin Khaw, Nor Hanim Mohd Amin, Yen Yee Oon, Asri Said, Kent Ter Lau, Kar Ying Yong, Daniel Cheng Lee Pang, Chandan Deepak Bhavnani, Ing Tien Wong, Francis Eng Pbeng Shu and Tiong Kiam Ong https://doi.org/10.15420/japsc.2021.14
Role of Lipoprotein(a) in Cardiovascular Disease: A Review of Clinical Practice Yoshiyasu Minami, Daisuke Kinoshita, Yusuke Muramatsu, Takako Nagata and Junya Ako https://doi.org/10.15420/japsc.2021.31
Validation of the GRACE Risk Score for Acute Coronary Syndrome Patients in an Asian Medical Centre Wei Juan Lim, Ji Ken Ow, Xian Pei Cheong, Rusli bin Nordin and Chuey Yan Lee https://doi.org/10.15420/japsc.2022.01
The Use of Complementary Technologies in Calcified Left Main Disease: A Case Series
Dinakar Bootla, Pruthvi C Revaiah, Navjyot Kaur, Yash Paul Sharma and Himanshu Gupta https://doi.org/10.15420/japsc.2021.24
A Malaysian Expert Consensus on the Use of High-sensitivity Cardiac Troponin in the Emergency Department Raja Ezman Raja Shariff, Sazzli Shahlan Kasim, Subashini C Thambiah, Adi Osman, Asri Said, Farhi Ain Jamaluddin, Farina Mohd Salleh and Sarah Abd Karim https://doi.org/10.15420/japsc.2021.19
© RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com Contents
Impact of BMI and Type of Ablation Procedure on Atrial Fibrillation Recurrence in Japanese Patients
Aiko Takami, Junichiro Miake, Masaru Kato, Kazuyoshi Ogura, Akihiro Okamura, Takuya Tomomori, Daiki Tsujimoto, Syunsuke Kawatani, Masahiko Kato and Kazuhiro Yamamoto https://doi.org/10.15420/japsc.2021.27
Impact of 2016 American Society of Echocardiography/European Association of Cardiovascular Imaging Recommendations for the Evaluation of Left Ventricular Diastolic Function on Predicting Outcomes in Patients with Diabetes and Hypertension without a History of Cardiovascular Disease
Diana Hui Ping Foo, Kai Huat Lam, Macnicholson Igo, Mohammad Nor Azlan Bin Sulaiman, Mohamad Adam Bin Bujang, Ming Ying Ku, Teck Long King, Rose Hui Chin Jong, Sally Suriani Ahip, Mohammad Faiz Sahiran, Maila Mustapha, Jennett Michael, Azreen Abdullah and Alan Yean Yip Fong https://doi.org/10.15420/japsc.2021.25
Pericarditis and Myocarditis after mRNA-based COVID-19 Vaccination Rilong Hong, Jonathan Yap and Khung Keong Yeo https://doi.org/10.15420/japsc.2021.26
Dual Antiplatelet Therapy for 3 or 12 Months in Patients with Non-ST-elevation MI/ Unstable Angina or ST-elevation MI: Analysis of the REDUCE Trial
Wan Azman Wan Ahmad, Edouard Benit, Victor M Legrand, Achmad Fauzi Yahya, Huay Cheem Tan, Sodiqur Rifqi, Muhamad Ali SK Abdul Kader, Bruno Pironi, Robbert J de Winter, Chor-Cheung Frankie Tam, Eric Ligtenberg, Giuseppe DeLuca, Harry Suryapranata https://doi.org/10.15420/japsc.2021.29
Adverse Cardiac Effects of SARS-CoV-2 Infection
László Göbölös, Yosef Manla, István Rácz, Maurice Hogan, Ernő Remsey-Semmelweis, Bassam Atallah, Yazan AlJabery, Wael Almahmeed, Fahad AlSindi, Feras Bader, Gopal Bhatnagar, Tareq Aleinati, Emin Murat Tuzcu https://doi.org/10.15420/japsc.2022.04
Cross-sectional Study of Percutaneous Intervention in the Elderly in Malaysia: PIELD Study Ganapathi Palaniappan, Rhuban M Sundran, Afif Ashari, Mohd Saad Jalaluddin, Afrah Yousif Haroon, Rohith Stanislaus, Yee Sin Tey, Ahmad Farhan Abdul Hamid, Tjen Jhung Lee, Shaiful Azmi Yahaya, Balachandran Kandasamy, Zulaikha Zainal https://doi.org/10.15420/japsc.2021.34
Outcomes of Cardiac Arrest in Brunei Darussalam Sofian Johar, Nabilah Fadzilah Johani, Anne Catherine Cunningham https://doi.org/10.15420/japsc.2022.21-
Right Heart Catheterisation with Dobutamine Stress Test for Evaluation of Right Ventricular Outflow Tract Obstruction 30 Years After Surgical Repair of Tetralogy of Fallot Kengo Yasuda, Tomomi Watanabe, Aiko Takami, Toshihiko Akasaka, Yasushi Yoshikawa, Motonobu Nishimura, Kazuhiro Yamamoto https://doi.org/10.15420/japsc.2022.03
Rare Presentation of Cardiac Sarcoidosis With Recurrent Large Pericardial Effusion and Stress-induced Cardiomyopathy
Riyadh Qasim, Khalid Alkatout, Fatema Qaddoura, Ayman Nagib https://doi.org/10.15420/japsc.2022.10
European Examination in Core Cardiology (APSC Exit Examination)
Jack WC Tan, Jonathan Yap, Khung Keong Yeo, Derek P Chew, Alan Yean Yip Fong, Caitlyn Tan, Abdul Shehab, Quang Ngoc Nguyen, Terrence Chua, Clive Lawson, Danny Mathysen, Stephanie Thibault, Wael Almahmeed, Chris Plummer https://doi.org/10.15420/japsc.2022.15
Are There Differences in the Demographics and Clinical Outcomes Between Asian and European Patients Treated With the COMBO Dual Therapy Stent in the REDUCE Trial Populations?
Wan Azman Wan Ahmad, Edouard Benit, Cyril Camaro, Elvin Kedhi, Saman Rasoul, Lucia Barbieri, Jacques Lalmand, René J van der Schaaf, Tian H Koh, Arnoud W van‘t Hof, Stephen W Lee, Vincent Roolvink, Marc A Brouwer, Giuseppe DeLuca, Harry Suryapranata https://doi.org/10.15420/japsc.2022.02
High-sensitivity Troponins in the Emergency Department: Which Guideline to Recommend in Asia?
Laila Osama Abdel Wareth https://doi.org/10.15420/japsc.2022.09
© RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com www.JAPSCjournal.comContents Volume 1 • 2022
Shaping Our Destiny Together: A Shared Journey for the Asia-Pacific Region Begins
Khung Keong Yeo
Editor-in-Chief, Journal of Asian Pacific Society of Cardiology National Heart Centre Singapore, Singapore

Disclosure: The author has no conflicts of interest to declare.
Received: 21 December 2021 Accepted: 5 January 2021 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e01. DOI: https://doi.org/10.15420/japsc.2021.35
Correspondence: Khung Keong Yeo, National Heart Centre Singapore, 5 Hospital Drive, Singapore 169609, Singapore. E: yeo.khung.keong@singhealth.com.sg
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
The Journal of Asian Pacific Society of Cardiology (JAPSC) is a timely and important addition to the cardiovascular science sphere in Asia-Pacific. Until recently, there had been no dedicated general cardiovascular sciences journal for the region. In 2021, the Journal of the American College of Cardiology started JACC: Asia. This is important for the region, as the scientific community recognises the rising importance of Asia and, more importantly, of the science arising from Asia. At the same time, the combined leadership of the Asian Pacific Society of Cardiology (APSC), led then by Associate Prof Jack Tan from Singapore, decided that the time was ripe for a journal from the APSC. Thus, JAPSC was born.
JAPSC is envisioned to be a journal by the physicians of Asia-Pacific, for the people of Asia-Pacific. The editorial board is led by physicians from the countries of the region, with important members from Europe and the US. Scientific development in the cardiovascular sciences in the US and Europe is more mature and advanced than in Asia. Our friends, Prof Jeroen J Bax, Prof Michel Komadja and Prof Adrian Hernadez will provide expert insights to help guide the growth of the journal. Indeed, we hope and expect to invite more world-class experts to join our editorial board, including those from the US and Europe.
The APSC includes members from Australia to the Middle East. We are fortunate to have experts like Prof Stephen Nichols and Prof Wael Almahmeed support us on the journal’s editorial board. Within Asia, we
have editorial board members from across Asia, from China, Japan, South Korea and India to Singapore, Malaysia and Thailand. As we grow the journal, we will call upon more experts from our community to serve on the board.
JAPSC started its call for papers in July 2021. As of 7 December 2021, the journal had received 28 submissions. Six have been accepted and are near to publication, with others currently in peer review or being revised by the authors. The submissions represent a good spread across Asia and bode well for the future of the journal.
What kind of papers would have a home in JAPSC? Clearly, good quality original investigations from Asia-Pacific countries, regional or national statistics, consensus documents or guidelines, review papers with a focus on the Asia-Pacific region and interesting case reports with high learning value will be desirable for the journal.
The APSC is a professional cardiovascular medicine society, with a long history extending from 1956. Throughout its history, though the idea of a journal has been mooted several times, it is only now that we have finally coalesced our will as a region to come together. The journey will be long and there will be challenges along the way. Nonetheless, I am quietly confident that, together, we can build JAPSC into a world-class cardiovascular sciences journal.
© RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com Foreword
A Case of Osseocartilaginous Defects and Cardiovascular Anomalies: An Ambiguous Entity
Aritra Paul ,
Aruni Ghose ,
Debasish Banerjee 3 and Suvro Banerjee
1. Nil Ratan Sircar Medical College and Hospital, Kolkata, West Bengal, India; 2. Medway NHS Foundation Trust, Gillingham, Kent, UK; 3. Renal Transplantation Unit, St George’s University Hospitals NHS Foundation Trust, London, UK; 4. Apollo Gleneagles Hospitals, Kolkata, India
Abstract
A 24-year-old man presented with a history of palpitation and haemoptysis. He had a short stature, cardiac anomalies and physical deformities, including polydactyly, clubbing, cataracts and cyanosis. Echocardiography was performed, revealing both atrial and ventricular septal defects, along with severe pulmonary hypertension and Eisenmenger syndrome.
Keywords
Congenital heart disease, osseocartilaginous defect, case report, diagnosis

Disclosure: The authors have no conflicts of interest to declare.
Patient Consent: Written informed consent was obtained from the patient for publication of this case report.
Received: 4 August 2021 Accepted: 14 September 2021 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e02. DOI: https://doi.org/10.15420/japsc.2021.06
Correspondence: Suvro Banerjee, Apollo Hospitals, 58, Canal Circular Rd, Kankurgachi, Kolkata, West Bengal, 700054, India. E: drsuvrob@gmail.com
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Various congenital diseases affect the heart, with some involving multiple organ systems and encompassing multiple presentations. The broad literature on the subject of such syndromes describes various novel syndromes with presentations not seen previously. Our case is one such presentation, where the constellation of findings has not been reported before, nor do they satisfy the diagnostic criteria of any existing syndromes.
Case Report
A 24-year-old man, born of healthy non-consanguineous parents, presented with shortness of breath on walking half a mile and a 9-month history of palpitations, along with a cough with bouts of haemoptysis for 6 months. Developmental milestones were normal. His father died of a stroke at the age of 60, but his mother and six siblings (three men and three women) were well. He was treated with furosemide by his local doctor.
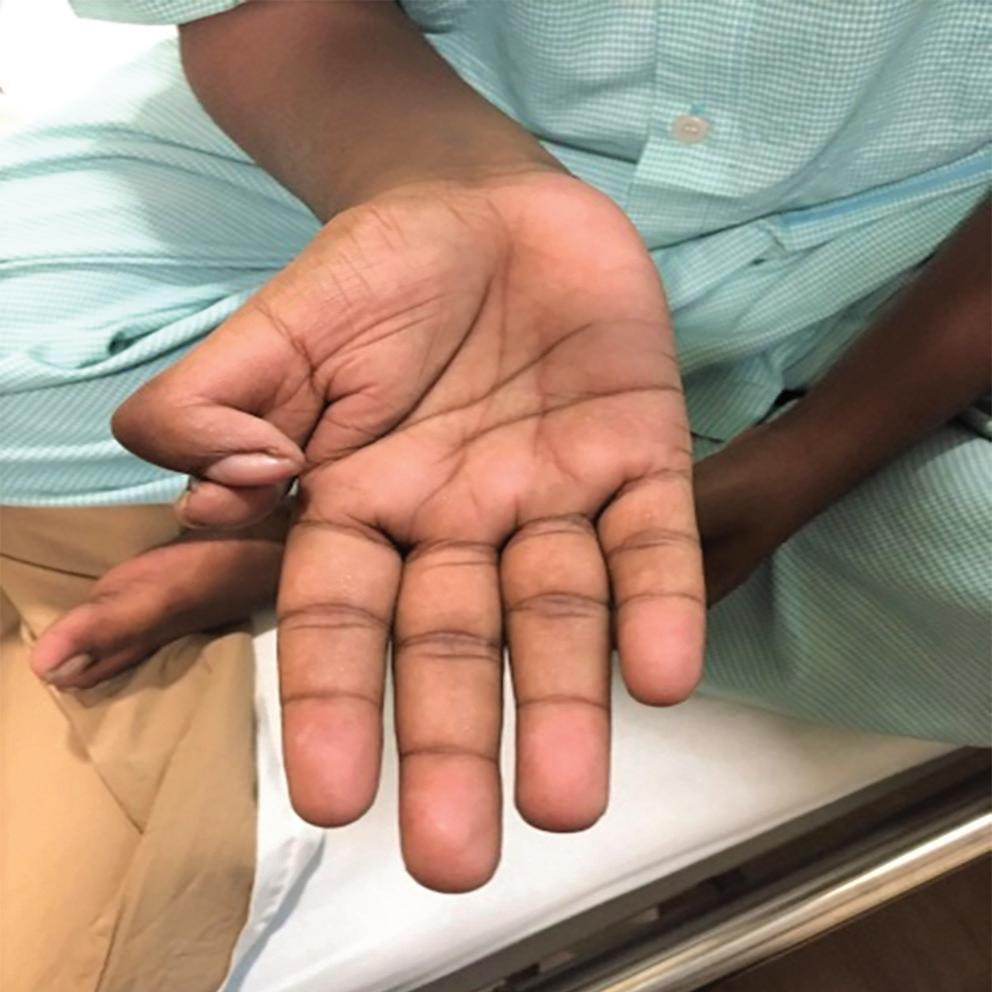
On examination, his weight was 40 kg and height 150 cm. He had a receding chin, a rudimentary right ear with normal auditory function (Figure 1), bilateral cataract, a drooping right upper eyelid and mild central cyanosis. Other significant findings included digital clubbing and polydactyly of the right thumb (Figure 2).
Vital signs were normal with a pulse of 84 BPM and blood pressure of 130/80 mmHg. Cardiovascular examination revealed raised jugular venous pressure, apex beat localisation to left fifth intercostal space, no pitting oedema, normal first and second heart sounds and a soft systolic murmur in the second left intercostal space. Respiratory auscultation was normal. Mini Mental State Exam and neurological examination were normal. Fundoscopy was difficult to perform because of bilateral cataracts,
but did not reveal any obvious abnormality. Abdominal and genital examinations were normal.
Chest X-ray revealed enlarged pulmonary arteries with peripheral pruning and an elevated cardiac apex due to right ventricular hypertrophy. ECG suggested features of right heart hypertrophy and frontal plane QRS right axis deviation. Ultrasonography of the abdomen was normal. Echocardiography revealed biatrial enlargement, biventricular hypertrophy, a large ostium secundum atrial septal defect and a perimembranous ventricular septal defect, each measuring approximately 18 mm with bidirectional flow shunting predominantly from right to left.
Severe pulmonary arterial hypertension was present with moderate tricuspid regurgitation. Pulmonary venous drainage was normal. Blood tests showed mild erythrocytosis with elevated haemoglobin. Urea and electrolytes, liver and thyroid function tests were normal. Karyotyping was performed, which was normal (46 XY).
The patient was offered cardiac catheterisation, which he declined. Conservative management was followed and his diuretic dosage was increased. After discharge, he was followed up over the next year. He remained adherent to medications and his symptoms improved.
Discussion
The patient had Eisenmenger syndrome with multiple upper limb and facial abnormalities along with cataracts, suggestive of a hereditary disorder. The diagnostic dilemma stemmed from the difficulty in unifying the clinical findings under one particular syndrome. Our findings bore resemblance to – but did not satisfy the diagnostic criteria for – existing syndromes. Hence, we narrowed down our differential diagnoses to the clinical entities described in the following paragraphs.
© RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com Clinical Cardiology CASE REPORT
1
2
4
Figure 1: Patient with Rudimentary Right Ear and Receding Chin
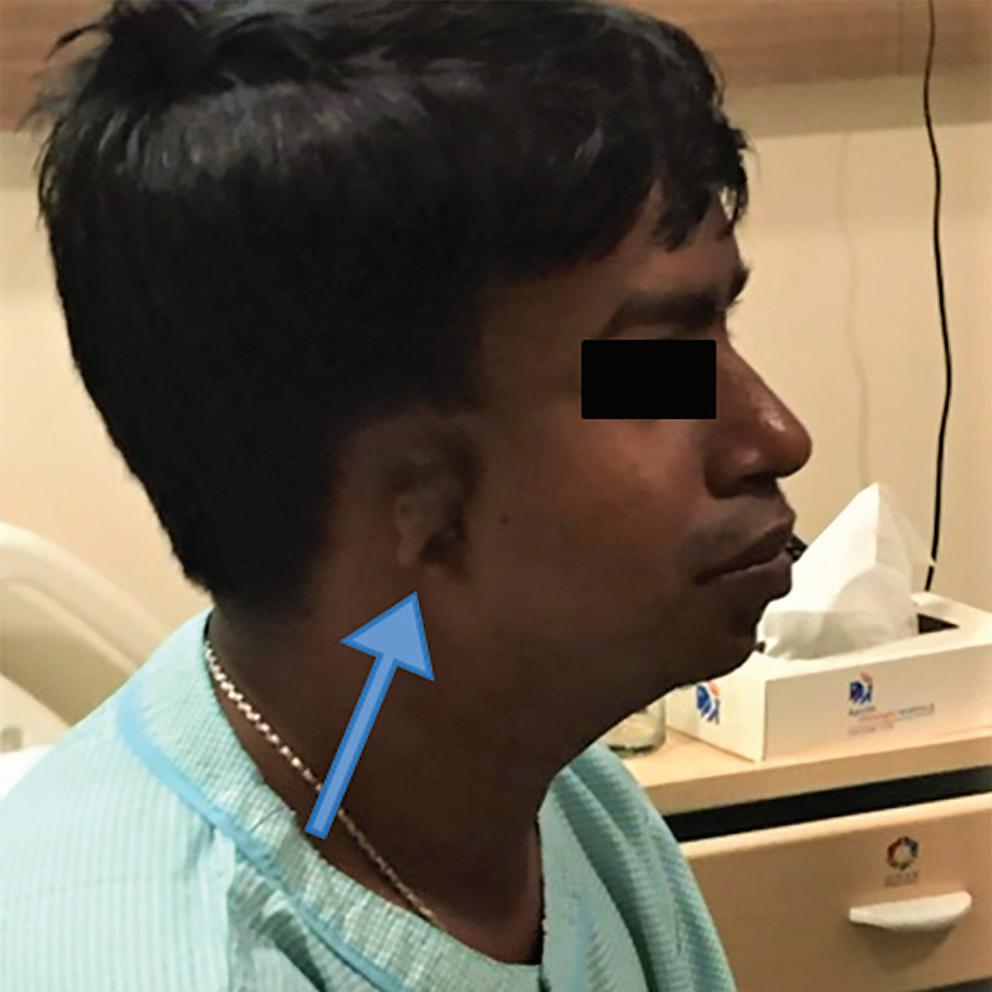
Congenital heart disease, ear deformity and polydactyly in our patient in line with the diagnosis of Patau syndrome, which involves trisomy of all or part of chromosome 13. However, our patient lacked other characteristic findings of Patau syndrome, such as mental retardation, umbilical hernia, cleft lip, coloboma and a congenital renal anomaly.1 In addition, survival until the third decade of life is extremely uncommon in Patau syndrome and when it occurs it is usually associated with mosaicism.2
Skeletal abnormalities of the upper extremities, along with cardiovascular anomalies raised the suspicion of Holt-Oram syndrome. However, the findings, such as facial deformities and cataracts and the absence of cardiac conduction defects or a family history of congenital heart disease, did not support this diagnosis.3
Smith-Lemli-Opitz syndrome is suspected in patients with distinctive facial features, microcephaly and intellectual disability along with cardiac, lung, renal or digital abnormalities.4 Apart from growth restriction and feeding difficulties, with features of autism, Smith-Lemli-Opitz syndrome has been associated with a characteristic atrioventricular canal defect.5
The term CHARGE syndrome encompasses coloboma, heart defects, choanal atresia, retarded growth and development, genital abnormalities
1. Williams GM, Brady R. Patau syndrome. In: StatPearls Treasure Island, FL: StatPearls Publishing, 2021. https:// www.ncbi.nlm.nih.gov/books/NBK538347
2. Peroos S, Forsythe E, Pugh JH, et al. Longevity and Patau syndrome: what determines survival? BMJ Case Rep 2012;2012:bcr0620114381. https://doi.org/10.1136/bcr-062011-4381; PMID: 23220825.
3. Krauser AF, Ponnarasu S, Schury MP. Holt Oram syndrome. In: StatPearls. Treasure Island, FL: StatPearls Publishing, 2021. https://www.ncbi.nlm.nih.gov/books/NBK513339
4. Nowaczyk MJM, Wassif CA. Smith-Lemli-Opitz syndrome. In:
Figure 2: Digital Clubbing with Post-axial Polydactyly of Right Thumb
and ear anomalies. However, our patient did not satisfy any of the three major criteria (Verloes updated criteria) for this diagnosis.6
Levin et al. described four patients with atrioventricular septal defects and postaxial polydactyly, without the presence of any other major deformity.7 These cases resembled Ellis-van Creveld syndrome, which includes short limb dwarfism, polydactyly, abnormal development of fingernails, normal IQ and motor development and congenital heart defects in over half of the cases.8 Our patient bears a strong resemblance to these cases, with the coexistence of cardiac abnormalities and upper limb deformities, albeit the facial deformity and early cataracts remain unexplained.
Hence, our patient may present a variant of Ellis-van Creveld syndrome, although the exact diagnosis is uncertain.
Apart from karyotyping, no detailed chromosomal analysis could be carried out on this patient. This lack of detailed chromosomal analysis makes it impossible to make a definite diagnosis, which is a limitation of this case report. The question of whether our case is the first reported case of its kind, or a phenotypic variant of an already reported syndrome, remains unanswered.
Adam MP, Ardinger HH, Pagon RA, Wallace SE, eds. GeneReviews. Seattle, WA: University of Washington, 2020. https://www.ncbi.nlm.nih.gov/books/NBK1143
5. Digilio MC, Marino B, Giannotti A, et al. Specific congenital heart defects in RSH/Smith-Lemli-Opitz syndrome: postulated involvement of the Sonic Hedgehog pathway in syndromes with postaxial polydactyly or heterotaxia. Birth Defects Res A Clin Mol Teratol 2003;67:149–53. https://doi. org/10.1002/bdra.10010; PMID: 12797454.
6. Verloes A. Updated diagnostic criteria for CHARGE syndrome: a proposal. Am J Med Genet A 2005;133A:306–8.
https://doi.org/10.1002/ajmg.a.30559; PMID: 15666308.
7. Levin SE, Dansky R, Milner S, et al. Atrioventricularseptal defect and type A postaxial polydactyly without other major associated anomalies: a specific association. Pediatr Cardiol 1995;16:242–6. https://doi.org/10.1007/BF00795716; PMID: 8524711.
8. Baujat G, Le Merrer M. Ellis-van Creveld syndrome. Orphanet J Rare Dis 2007;2:27. https://doi.org/10.1186/1750-1172-2-27; PMID: 17547743.
A Case of Osseocartilaginous Defects and CV Anomalies JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Trio of Rheumatic Mitral Stenosis, Right Posterior Septal Accessory Pathway and Atrial Flutter: A Case Report
Jogendra Singh , 1 Dibyasundar Mahanta , 1 Rudra Pratap Mahapatra , 2 Debasis Acharya 1 and Ramachandra Barik 1
1. Department of Cardiology, All India Institute of Medical Sciences, Bhubaneswar, India;
2. Department of Cardiothoracic Surgery, All India Institute of Medical Sciences, Bhubaneswar, India

Abstract
A 57-year-old man presented with recurrent palpitations. He was diagnosed with rheumatic mitral stenosis, right posterior septal accessory pathway and atrial flutter. An electrophysiological study after percutaneous balloon mitral valvotomy showed that the palpitations were due to atrial flutter with right bundle branch aberrancy. The right posterior septal pathway was a bystander because it had a higher refractory period than the atrioventricular node.
Keywords
Rheumatic mitral stenosis, right posterior septal accessory pathway, atrial flutter, right bundle branch, radiofrequency ablation, percutaneous balloon mitral valvotomy
Disclosure: The authors have no conflicts of interest to declare.
Patient Consent: Written informed consent was obtained from the patient for publication of this case report.
Received: 6 August 2021 Accepted: 16 September 2021 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e03. DOI: https://doi.org/10.15420/japsc.2021.07
Correspondence: Ramachandra Barik, Department of Cardiology, All India Institute of Medical Sciences, Bhubaneswar, Odisha, Pin-751019, India.
E: cardioramachandra@gmail.com
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
In India, the prevalence of rheumatic heart disease in the general population is 1.5–2.0 per 1,000.1,2 However, based on echocardiography, the prevalence of rheumatic heart disease is likely to be greater.3 The prevalence of Wolff–Parkinson–White (WPW) syndrome or the WPW pattern in the general population ranges from 0.1 to 0.3%, with a higher prevalence among men than women.4 The coexistence of rheumatic mitral stenosis and WPW syndrome is so rare that a search of the literature using Google Scholar and PubMed yielded 17 cases between 1960 and 2021. Among these, balloon mitral valvotomy and radiofrequency ablation were performed simultaneously in only two cases.5,6
Case Report
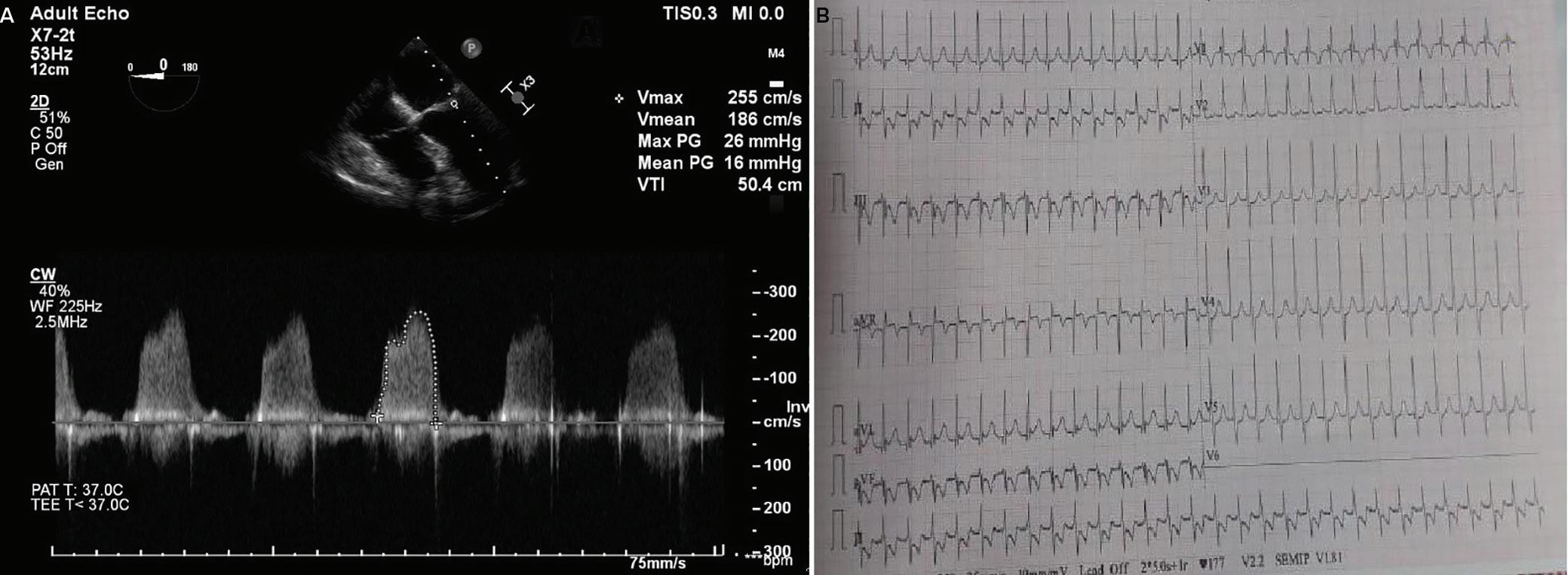
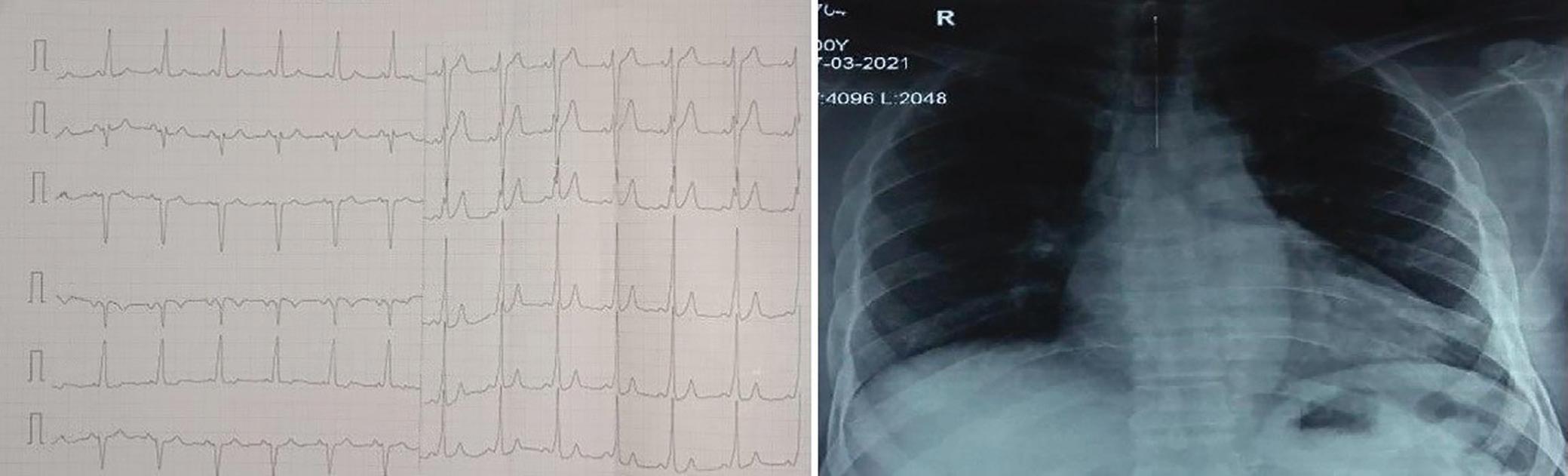
A 57-year-old man presented with a three-decade history of recurrent episodes of palpitation with alarming jugular venous pulsation, but without syncope. Each episode spontaneously reverted to the normal sinus rhythm 6–8 hours after the onset. Cardiac auscultation raised the suspicion of underlying rheumatic mitral stenosis. A 12-lead ECG was suggestive of a WPW pattern with a right posterior septal accessory pathway (Figure 1A). A chest X-ray in the posterior–anterior view was consistent with cardiac auscultation (Figure 1B). A transoesophageal echocardiogram confirmed rheumatic mitral stenosis (Figure 2A and Supplementary Material Video 1). The pliable mitral valve area was 0.8 cm2 and the mean gradient was 17 mmHg at a heart rate of 87 BPM. The coronary angiogram was normal.
An electrophysiologist, cardiothoracic surgeon, cardiac anaesthetist and cardiologist suggested mitral valvotomy followed by ablation of
the accessory pathway in a single procedure if possible to avoid repeated septal puncture. Informed consent was obtained for the procedure.
The day before the procedure, the patient developed an episode of palpitation during the clinical round. A 12-lead ECG revealed atrial flutter with right bundle branch aberrancy on metoprolol succinate (Figure 2B). The patient’s blood pressure was 124/80 mmHg. Oral verapamil was initiated and the atrial flutter reverted to normal sinus rhythm.
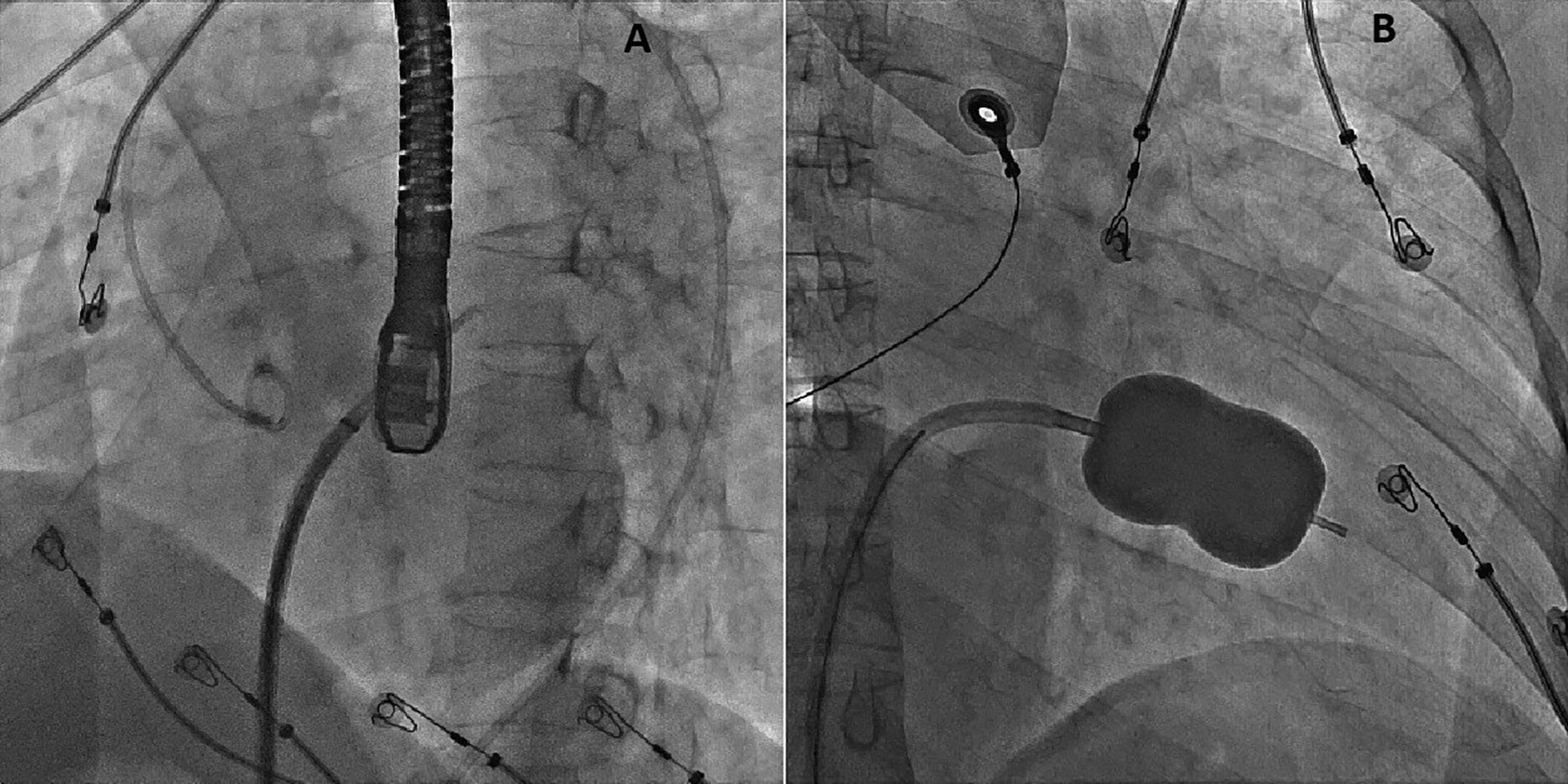
On the day of the procedure, the first balloon mitral valvotomy was performed from a right femoral approach using a 23–26 mm Accura balloon (Vascular Concepts) after transeptal access using an 8 Fr SL-1 sheath and a BRK-0 needle (St Jude Medical). A transeptal puncture was performed after proper needle tip position was confirmed by fluoroscopy (right anterior oblique, left anterior oblique and 90° lateral views) and transoesophageal echocardiography (bicaval and short axis views; Figure 3A). The mean left atrial pressure prior to the valvotomy was 31 mmHg. The balloon was inflated to 26 mm in the right anterior oblique 20° position under fluoroscopy (Figure 3B) because the patient was 160 cm tall. The mitral valve area increased to 2.2 cm2 without any additional mitral regurgitation, and the mean left atrial pressure decreased to 12 mmHg without any mitral valve gradient. Immediate transthoracic echocardiography showed that the mitral valve gradient had decreased to 7/2 mmHg with negligible mitral regurgitation.
The left atrial wire was reintroduced into the left atrium before the stretched balloon was removed from the left atrium for the
© RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com Intervention CASE REPORT
Figure 2:
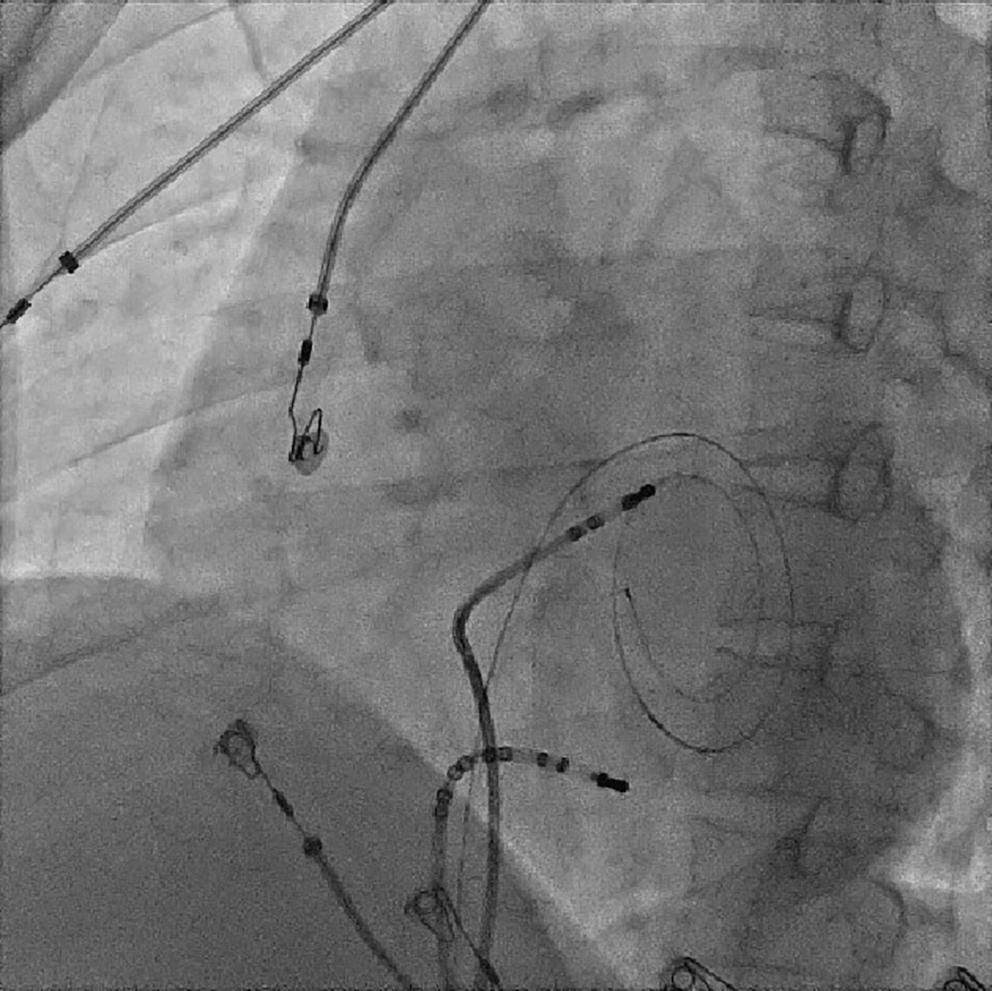
electrophysiological study and for possible radiofrequency ablation (Figure 3C). The electrophysiologist proceeded with the ablation plan.
Because the patient had baseline pre-excitation through the right posterior septal path and atrial flutter with orthodromic conduction with right bundle branch aberration, one decapolar catheter in the coronary sinus and a quadripolar catheter in the right ventricle were used to study the effective refractory period (ERP) of the accessory pathway rather than using the routine four electrophysiology catheters (three quadripolar [high right atrial, His bundle, right ventricle apex] and one decapolar catheter in the coronary sinus; Figure 4C). The supra-His conduction time was 65 ms and the infra-His conduction time was 9 ms. The baseline ECG was suggestive of a right posterior septal pathway. Right ventricular pacing showed eccentric conduction up to 450 ms, which suggested a retrograde pathway ERP of 450 ms. On pacing the integrated pathway, the ERP was found to be 450 ms. Due to the weak nature of the accessory pathway, ablation was not performed. On rapid atrial pacing, atrial flutter with right bundle branch aberrancy was induced, similar to the clinical tachycardia observed earlier. Ablation for the atrial flutter was not performed, and the patient was maintained on metoprolol succinate and an oral anticoagulant
During Palpitation
in the anticipation of spontaneous remission of atrial flutter after both remodelling of the atrium and haemodynamic improvement after balloon valvotomy.
This patient has remained asymptomatic over a follow-up period of 15 months.
Discussion
Balloon mitral valvotomy is preferred to surgery in the case of pliable rheumatic stenosis. Of the treatments available for valvular AF, less is known about the efficacy of radiofrequency ablation because of the lack of a significant number of randomised control trials.7 Arrhythmia-related death in asymptomatic pre-excitation is as low as 0.05 to 0.9 per 1,000.8 Therefore, the treatment of rheumatic mitral stenosis with bystander involvement of an accessory pathway and an ERP that is greater than that of the atrioventricular node is not a challenge. Ablation of the accessory pathway is not indicated if the bystander pathway has a high ERP.9 In the present case, the patient had recurrent episodes of palpitation, but the right posterior septal accessory pathway did not contribute to these, which is quite an unusual scenario and unlike the case reported by Jagadheesan et al.10
Pre-excitation Associated with Rheumatic Mitral Stenosis JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Figure 1: ECG and Chest X-Ray
Transesophagial Echocardiography and ECG
Report confirmed by I II IR aVR V1 V2 V3 V4 V5 V5 aVL aVP A B A: A 12-lead ECG showing the Wolff–Parkinson–White pattern with a possible right posterior septal accessory pathway because the R/S ratio is <0.5 in V1 and V2 and <1 in the inferior leads. B: Chest X-ray in the posterior–anterior view showing mitralisation of the left heart border, double atrial shadow on the right
side and a horizontal left bronchus.
A: There was significant mitral stenosis and the mean mitral valve gradient of 16 mmHg prior to the balloon mitral valvotomy. B: Atrial flutter with 2:1 atrioventricular block and right bundle branch aberrancy was evident during the electrophysiological study by pacing the atria with a decapolar catheter in the coronary sinus at cycle length of 200 ms.
Figure 4: Electrophysiology Study to Map the Accessory Pathway
Patients with rheumatic mitral stenosis who routinely seek help for fibrillation or flutter are in the 30- to 50-year age group, but the coexistence of a bystander right posterior septal accessory pathway, as in the present case, is unusual.11
The incidence of AF in a patient with an accessory pathway is 10–38%, but the association with common atrial flutter is not known.12 Our patient had orthodromic conduction of atrial flutter through the atrioventricular node
because the coexisting right posterior septal pathway had an ERP of ≥450 ms. Neither the atrial flutter nor right posterior septal pathway were ablated, with the expectation that, during follow-up, both would become non-functional over time because of left atrial remodelling and favourable haemodynamic changes.13 It is well established that mitral stenosis causes AF, and the incidence of AF is higher in older age groups. It is also known that the results of balloon mitral valvotomy are worse in older patients because of persistent AF.14 Therefore, it has been suggested that balloon mitral valvotomy is performed at an early age for favourable atrial remodelling to reduce the occurrence of AF or atrial flutter.15
Conclusion
Palpitations caused by atrial flutter with right bundle branch aberrancy in a patient with rheumatic mitral stenosis and a right posterior septal accessory pathway with an ERP higher than that of the atrioventricular node are rare. Whether left atrial remodelling after percutaneous balloon mitral valvotomy further reduces atrial flutter requires additional investigation in larger studies with a longer follow-up period.
Clinical Perspective
• Severe rheumatic mitral stenosis associated with a right posterior septal accessory pathway is rare.
• It is very unusual that recurrent palpitations are caused by atrial flutter with right bundle branch aberrancy; rather, palpitations are likely caused by right posterior septal accessory pathwaymediated Wolff–Parkinson–White syndrome because the right posterior septal accessory pathway has a lower effective refractory period than the atrioventricular node.
• Treating both conditions with a single intervention (i.e. by balloon mitral valvotomy and radiofrequency ablation) is rare.
• The almost complete resolution of atrial flutter 15 months after balloon mitral valvotomy in this patient is an interesting finding.
Pre-excitation Associated with Rheumatic Mitral Stenosis JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Figure 3: Transsepetal Puncture for Balloon Mitral Valvotomy and Electrophysiology Study in One Go
A: Septal puncture using fluoroscopy and transesophageal echocardiography guidance. B: A 23–26 mm Accura balloon was inflated to 26 mm in the right anterior oblique view.
Electrophysiological study using one quadripolar catheter for right ventricle pacing and one decapolar catheter in the coronary sinus.
1. Kumar RK, Tandon R. Rheumatic fever & rheumatic heart disease: the last 50 years. Indian J Med Res 2013;137:643–58. PMID: 23703332.
2. Watkins DA, Johnson CO, Colquhoun SM, et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med 2017;377:713–22. https://doi. org/10.1056/NEJMoa1603693; PMID: 28834488.
3. Weinberg J, Beaton A, Aliku T, et al. Prevalence of rheumatic heart disease in African school-aged population: extrapolation from echocardiography screening using the 2012 World Heart Federation Guidelines. Int J Cardiol 2016;202:238–9. https://doi.org/10.1016/j.ijcard.2015.08.128; PMID: 26402451.
4. Hiss RG, Lamb LE. Electrocardiographic findings in 122,043 individuals. Circulation 1962;25:947–61. https://doi. org/10.1161/01.cir.25.6.947; PMID: 13907778.
5. Nwe N, K. Shein KK, Latt T. Mitral stenosis with WPW syndrome. J Arrhythmia 2011;27(Suppl):PE4_093. https://doi. org/10.4020/jhrs.27.PE4_093
6. Alkindi F, Abed H, Thajudeen A, et al. Rheumatic mitral stenosis with incidental Wolff–Parkinson–White syndrome: a rare association. Treated by percutaneous transmitral
commissurotomy and radiofrequency ablation. Heart Views 2018;19:58–62. https://doi.org/10.4103/HEARTVIEWS. HEARTVIEWS_42_18; PMID: 30505396.
7. Iung B, Leenhardt A, Extramiana F. Management of atrial fibrillation in patients with rheumatic mitral stenosis. Heart 2018;104:1062–8. https://doi.org/10.1136/ heartjnl-2017-311425; PMID: 29453328.
8. Benson DW, Cohen MI. Wolff–Parkinson–White syndrome: lessons learnt and lessons remaining. Cardiol Young 2017;27(Suppl 1):S62–7. https://doi.org/10.1017/ S1047951116002250; PMID: 28084962.
9. Brugada J, Keegan R. Asymptomatic ventricular preexcitation: between sudden cardiac death and catheter ablation. Arrhythm Electrophysiol Rev 2018;7:32–8. https://doi. org/10.15420/aer.2017.51.2; PMID: 29636970.
10. Jagadheesan KS, Rangasamy S, Selvaraj RJ. A deadly mix –rheumatic mitral stenosis, preexcited atrial fibrillation, left atrial appendage thrombus and left atrial appendage accessory pathway. Indian Pacing Electrophysiol J 2017;17:183–5. https://doi.org/10.1016/j.ipej.2017.09.001; PMID: 29231823.
11. Strasser T, Dondog N, El Kholy A, et al. The community control of rheumatic fever and rheumatic heart disease:
report of a WHO international cooperative project. Bull World Health Orga. 1981;59:285–94. PMID: 6972819.
12. Kobza R, Toggweiler S, Dillier R, et al. Prevalence of preexcitation in a young population of male Swiss conscripts. Pacing Clin Electrophysiol 2011;34:949–53. https:// doi.org/10.1111/j.1540-8159.2011.03085.x; PMID: 21453334.
13. Iliceto N, Ginevrino P, Leone A. Mitral stenosis and the Wolff–Parkinson–White syndrome. (ECG findings in a case treated with commissurotomy with long-term disappearance of the pre-excitation syndrome). Acta Chir Ital 1968;24:695–712 [in Italian]. PMID: 5737767.
14. Aslanabadi N, Ghaffari S, Khezerlouy Aghdam N, et al. Poor outcome following percutaneous balloon mitral valvotomy in patients with atrial fibrillation. J Cardiovasc Thorac Res 2016;8:126–31. https://doi.org/10.15171/jcvtr.2016.26; PMID: 27777698.
15. Shaw TR, Sutaria N, Prendergast B. Clinical and haemodynamic profiles of young, middle aged, and elderly patients with mitral stenosis undergoing mitral balloon valvotomy. Heart 2003;89:1430–6. https://doi.org/10.1136/ heart.89.12.1430; PMID: 14617555.
Pre-excitation Associated with Rheumatic Mitral Stenosis JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Characteristics and Outcomes of MI with Non-obstructive Coronary Arteries in a South-east Asian Cohort
Cheney Jianlin Wong , 1 Jonathan Yap , 1,2 Fei Gao , 1 Yee How Lau,1 Weiting Huang,1 Fazlur Jaufeerally , 3 Ngiap Chuan Tan , 4 Hee Hwa Ho,5 Mark Chan,6 Kelvin Bryan Tan 7 and Khung Keong Yeo 1,2
1. National Heart Centre Singapore, Singapore; 2. Duke-NUS Medical School, Singapore; 3. Singapore General Hospital, Singapore; 4. SingHealth Polyclinics, Singapore; 5. Tan Tock Seng Hospital, Singapore; 6. National University Heart Centre, Singapore; 7. Ministry of Health, Singapore
Abstract
Background: MI with non-obstructive coronary arteries (MINOCA) is caused by a heterogenous group of conditions with clinically significant sequelae. Aim: This study aimed to compare the clinical characteristics and prognosis of MINOCA with MI with obstructive coronary artery disease (MICAD). Methods: Data on patients with a first presentation of MI between 2011 and 2014 were extracted from the Singapore Cardiac Longitudinal Outcomes Database and patients were classified as having either MINOCA or MICAD. The primary outcomes were all-cause mortality (ACM) and major adverse cardiac events (MACE), defined as a composite of ACM, recurrent MI, heart failure hospitalisation and stroke.
Results: Of the 4,124 patients who were included in this study, 159 (3.9%) were diagnosed with MINOCA. They were more likely to be women, present with a non-ST-elevation MI, have a higher left ventricular ejection fraction and less likely to have diabetes, previous stroke or smoking history. Over a mean follow-up duration of 4.5 years, MINOCA patients had a lower incidence of ACM (10.1% versus 16.5%) and MACE (20.8% versus 35.5%) compared with MICAD. On multivariable analysis, patients with MINOCA had a lower risk of ACM (HR 0.42; 95% CI [0.21–0.82]) and MACE (HR 0.42; 95% CI [0.26–0.69]). Within the MINOCA group, older age, higher creatinine, a ST-elevation MI presentation, and the absence of antiplatelet use predicted ACM and MACE. Conclusion: While patients with MINOCA had better clinical outcomes compared with MICAD patients, MINOCA is not a benign entity, with one in five patients experiencing an adverse cardiovascular event in the long term.
Keywords
MI with non-obstructive coronary arteries, non-obstructive coronary arteries, Singapore
Disclosure: KKY is editor-in-chief and JY is an associate editor of Journal of Asian Pacific Society of Cardiology; this did not influence peer review. All other authors have no conflicts of interest to declare.
Acknowledgements: The authors thank the SingCLOUD Publication Committee and chairperson Prof Mark Arthur Richards of National University Hospital (Singapore) for reviewing this article. This study was supported by the SingCLOUD Governance Committee, which includes representatives from the Ministry of Health, National Heart Centre Singapore, National University Hospital (Singapore), Tan Tock Seng Hospital, Khoo Teck Puat Hospital, Singapore General Hospital, Changi General Hospital, Sengkang General Hospital, Ng Teng Feng General Hospital, Alexandra Hospital, SingHealth Polyclinics, National Healthcare Group Polyclinics and National University Polyclinics.
Ethical Approval: This study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Data Availability: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Received: 31 July 2021 Accepted: 8 September 2021 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e04. DOI: https://doi.org/10.15420/japsc.2021.04
Correspondence: Khung Keong Yeo, National Heart Centre Singapore, 5 Hospital Drive, Singapore 169609. E: yeo.khung.keong@singhealth.com.sg
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
MI with non-obstructive coronary arteries (MINOCA) is an increasingly recognised entity that occurs in 6% of all patients diagnosed with acute MI.1 The Fourth Universal Definition of Myocardial Infarction Expert Consensus Document defines MINOCA as the absence of ≥50% diameter stenosis in a major epicardial coronary vessel identified on coronary angiography.2 Nevertheless, MINOCA should only be considered a working diagnosis and further evaluation should be undertaken to identify the underlying aetiology.3,4
Two meta-analyses on the use of cardiac MRI (CMR) in MINOCA identify myocarditis and true MI as the two most common causes, accounting for one-third and one-fifth of all cases, respectively.1,5 Other aetiologies include takotsubo cardiomyopathy and coronary vasospasm,

although no diagnosis is found in up to one-quarter of patients with MINOCA.
Existing literature on MINOCA is largely focused on Western populations. When compared with MI with obstructive coronary artery disease (MICAD), patients with MINOCA are more likely to be women, younger and have fewer traditional cardiovascular risk factors, such as diabetes and hypertension.3 The prognosis of MINOCA is generally more favourable than MICAD, although some studies have demonstrated similar if not poorer outcomes.1,6–15 Moreover, outcomes vary depending on the underlying cause of MINOCA. Dastidar et al. found that cardiomyopathies have the poorest prognosis, with a mortality rate of 15% at 3.5 years, followed by true MI (4%) and myocarditis (2%).16
© RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com General Cardiology ORIGINAL RESEARCH
Age (years), mean (SD)
Men, n (%)
Ethnicity
(13.5)
(11.3)
(11.4) 0.21
(57.2) 3,256 (82.1) 3347 (81.2) <0.0001
(65.4) 2,467 (62.2) 2,571 (62.3) Malay, n (%) 26 (16.4) 762 (19.2) 788 (19.1)
Chinese, n (%)
Indian, n (%)
(13.8) 533 (13.4) 555 (13.5) Other, n (%)
(4.4) 203 (5.1) 210 (5.1)
Medical History
Hypertension, n (%)
(59.1) 2,332 (59.0) 2,426 (59.0) 0.97 Dyslipidaemia, n (%) 90 (56.6) 2,171 (54.9) 2,261 (55.0) 0.68 Diabetes, n (%) 41 (25.8) 1,345 (34.0) 1,386 (33.7) 0.031 History of heart failure, n (%) 9 (5.7) 121 (3.1) 130 (3.2) 0.065
History of ischaemic stroke, n (%) 0 (0) 140 (3.5) 140 (3.4) 0.016
Peripheral artery disease, n (%) 1 (0.6) 50 (1.3) 51 (1.2) 0.48
Current/past smoking, n (%) 51 (32.1) 1,764 (44.5) 1,815 (44.0) 0.002 Creatinine, mmol/l (SD) 94 (132) 109 (117) 109 (118) 0.11
LVEF, % (SD) 62 (11.0) 54 (14.3) 54 (14.3) <0.0001
Presentation
STEMI, n (%) 28 (17.6) 2,140 (54.0) 2,168 (52.6) NSTEMI, n (%) 131 (82.4) 1,825 (46.0) 1,956 (47.4)
Angiography Findings
1-vessel disease, n (%) 1,265 (31.9) 2-vessel disease, n (%) 1,451 (36.6) 3-vessel disease, n (%) 1,249 (31.5)
Medications at Discharge
ACE-I/ARBs, n (%) 78 (49.1) 2,690 (67.8) 2,768 (67.1) <0.0001 β-blockers, n (%) 85 (53.5) 3,258 (82.2) 3,343 (81.1) <0.0001 Statins, n (%) 137 (86.2) 3,584 (90.4) 3,721 (90.2) 0.078 Aspirin, n (%) 122 (76.7) 3,501 (88.3) 3,623 (87.9) <0.0001 P2Y 12 inhibitor, n (%) 105 (66.0) 3,461 (87.3) 3,566 (86.5) <0.0001 Any antiplatelet use, n (%) 135 (84.9) 3,645 (91.9) 3,780 (91.7) 0.002
ACE-I = angiotensin
inhibitor; ARB = angiotensin
blocker; LVEF = left ventricular
MICAD = MI with obstructive coronary artery disease; MINOCA = MI with non-obstructive coronary arteries; NSTEMI = non-ST-elevation MI; PCI = percutaneous coronary intervention; STEMI = ST-elevation MI.
Previous studies evaluating the prognostic benefit of medical therapy support the use of renin–angiotensin system inhibitors (angiotensinconverting enzyme inhibitors/angiotensin receptor blockers), statins and β-blockers in MINOCA patients. In contrast, antiplatelet therapy has not been found to confer a protective effect against major adverse cardiovascular events (MACE).16–22
The aim of this study was to compare the clinical characteristics and outcomes of MINOCA and MICAD patients in Singapore.
Methods Study Protocol and Population
The Singapore Cardiovascular Longitudinal Outcomes Database (SingCLOUD) is an integrated national registry of adult patients with cardiovascular disease on follow up with public hospitals and outpatient clinics.23 Patients are identified using the Singapore Cardiac Data Bank, a
quality improvement database tracking cardiac interventions and surgery, and heart failure admissions in public hospitals; and discharge diagnoses associated with cardiovascular disease (e.g. MI, acute coronary syndrome) or heart failure based on ICD coding.
Clinical, laboratory, procedural, prescription, outcomes, administrative and financial data are obtained via the Singapore Cardiac Data Bank, participating public healthcare institutions and the Ministry of Health. Data from these sources flow through a Ministry of Health data grid comprising of six data warehouses prior to entering SingCLOUD.
Data collection for this study was performed as part of the approved SingCLOUD protocol (NCT03760705). Baseline data, such as patient characteristics, medical history, laboratory results, electrocardiogram at presentation and medications prescribed at discharge, were obtained from the database.
MINOCA in a South-east Asian Cohort JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Table 1: Baseline Characteristics and Medications
at
Discharge MINOCA
(n=159) MICAD (n=3,965) Overall Population (n=4,124) p-value
59
60
60
91
104
22
7
94
converting enzyme
receptor
ejection fraction;
All-cause mortality 10.1 (16) 16.5 (656) 0.58 [0.35–0.95] 0.030 0.42 [0.21–0.82] 0.011 MACE 20.8 (33) 35.5 (1,409) 0.51 [0.36–0.72] <0.0001 0.42 [0.26–0.69] 0.001
Recurrent MI 6.9 (11) 17.0 (673) 0.36 [0.20–0.65] 0.001 0.35 [0.15–0.85] 0.021
Hospitalisation for heart failure 3.8 (6) 10.1 (401) 0.34 [0.15–0.77] 0.010 0.51 [0.19–1.40] 0.19
Ischaemic stroke 3.1 (5) 3.0 (118) 1.0 [0.41–2.44] 0.91
Cumulative incidences over the study duration are presented. Multivariable adjustment made for age, sex, hypertension, dyslipidaemia, diabetes, smoking history, left ventricular ejection fraction, creatinine, renin-angiotensin inhibitors, β-blocker, statins and any antiplatelet use when these factors are statistically significant on univariate analysis. MACE = major adverse cardiovascular events; MICAD = MI with obstructive coronary artery disease; MINOCA = MI with non-obstructive coronary arteries.
This study was conducted in accordance with the standards set forth by the National Medical Research Council Institutional Review Boards and Declaration of Helsinki.
We identified 4,124 patients with a first presentation of acute MI who underwent coronary angiography between 1 January 2011 and 31 December 2014. Patients were classified into two groups: MICAD, defined as the presence of ≥50% stenosis in a major epicardial vessel; and MINOCA, defined as <50% stenosis. This was based on the Fourth Universal Definition of Myocardial Infarction Expert Consensus Document.2
Outcomes
Follow-up data were collected up until 31 December 2017 over a mean duration of 4.5 years. The primary endpoints for this study were all-cause mortality and MACE defined as a composite of all-cause mortality, recurrent MI, hospitalisation for heart failure and ischaemic stroke.
Statistical Analysis
Continuous variables are presented as the mean ± standard deviation and compared using the independent samples t-test, while categorical variables are expressed as absolute numbers and frequencies in percentages, and compared using the χ-squared test. The cumulative survival rate was analysed using the Kaplan–Meier estimator and the logrank test was used to compare outcomes in MINOCA and MICAD patients. The multivariate Cox proportional hazards model was used to assess the impact of various predictors on the primary endpoints and the corresponding HRs and 95% CI were reported. A p-value of <0.05 was significant. All statistical analyses were performed using Stata version 13.1 (StataCorp).
Results
The baseline characteristics of the 4,124 patients included in this study are shown in Table 1. The mean age was 60 years and 81.2% were men. The majority of patients were Chinese (62.3%) followed by Malay (19.1%) and Indian (13.5%), which is consistent with the demographics of Singapore. A total of 159 (3.9%) patients had a diagnosis of MINOCA. Compared with MICAD patients they were more likely be to be women, present with a non-ST-elevation MI, have a higher left ventricular ejection fraction (LVEF) and less likely to have diabetes, previous ischaemic stroke or smoking history. There were no significant differences in the ethnic composition of the two groups. In terms of medications, MICAD patients were more frequently prescribed angiotensin-converting enzyme inhibitors/ angiotensin receptor blockers (67.8% versus 49.1%, p<0.0001), β-blockers (82.2% versus 53.5%, p<0.0001) and antiplatelet therapy (91.9% versus 84.9%, p=0.002). The use of statins (90.4% versus 86.2%, p=0.078) was similar in both groups.
Figure 1: Cumulative Incidence of Primary Outcomes in MINOCA
A
Probability of survival
Number at risk MICAD 3,965 MINOCA
B
Probability of MACE free
Number at risk MICAD MINOCA
0
3,573
Peto test p=0.06
Year of follow-up
100% 75% 50% 25% 0% 2 4 6 8 2,474 861 0 0151159 111 43
0
All-cause mortality MICAD MINOCA 3,021
Peto test p=0.0001
Year of follow-up
100% 75% 50% 25% 0% 2 4 6 8 1,965 608 0
MACE MICAD MINOCA 3,965 141 99 39 0159
Kaplan–Meier curves with cumulative hazards of all-cause mortality (A) and MACE (B). MACE = major adverse cardiovascular events; MICAD = MI with obstructive coronary artery disease; MINOCA = MI with non-obstructive coronary arteries.
Event Rates and Outcomes
Patients diagnosed with MINOCA experienced a lower incidence of allcause mortality (10.1% versus 16.5%, p=0.030), overall MACE (20.8% versus 35.5%, p<0.0001), recurrent MI (6.9% versus 17.0%, p=0.001) and hospitalisation for heart failure (3.8% versus 10.1%, p=0.009) compared
MINOCA in a South-east Asian Cohort JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Table 2: Comparison of Outcomes Between MINOCA and MICAD
MINOCA (n=159), % (n) MICAD (n=3,965), % (n) Unadjusted Multivariable-adjusted HR [95% CI] p-value HR [95% CI] p-value
Table 3: Independent Predictors of All-Cause Mortality in Patients with MINOCA
Unadjusted HR [95% CI] p-value
Age 1.07 [1.02–1.11] 0.002
Male 0.43 [0.16–1.19] 0.10
Ethnicity
Chinese 1.0
Malay 0.32 [0.04–2.45] 0.27
Indian 0.84 [0.19–3.76] 0.82
Others 1.70 [0.22–13.2] 0.61
Hypertension 2.17 [0.70–6.74] 0.18
Dyslipidaemia 0.60 [0.22–1.60] 0.31
Diabetes 2.30 [0.86–6.18] 0.098
Current/past smoking 0.70 [0.22–2.16] 0.53
Creatinine 1.003 [1.001–1.005] <0.0001
LVEF 0.99 [0.94–1.05] 0.85
Presentation
STEMI 1.0
NSTEMI 0.17 [0.07–0.47] <0.0001
Medications at Discharge
ACE-I/ARBs 1.61 [0.58–4.43] 0.36
β-blockers 0.62 [0.23–1.66] 0.34 Statins 0.40 [0.13–1.24] 0.11
Any antiplatelet use 0.30 [0.10–0.87] 0.027
Multivariable-adjusted
Age 1.08 [1.03–1.13] 0.001
Creatinine 1.003 [1.001–1.005] 0.004
Presentation
STEMI 1.0
NSTEMI 0.16 [0.05–0.45] 0.001
Medications at Discharge
Any antiplatelet use 0.27 [0.08–0.90] 0.033
Cox proportional hazards analysis for all-cause mortality. Multivariable analysis only includes significant variables on univariate analysis. ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; LVEF = left ventricular ejection fraction; MINOCA = MI with non-obstructive coronary arteries; NSTEMI = non-ST-elevation MI; STEMI = ST-elevation MI.
with MICAD patients over the follow-up period. Using multivariable adjustment, MINOCA patients were at significantly lower risk of all-cause mortality (HR 0.42; 95% CI [0.21–0.82]; p=0.011), MACE (HR 0.42; 95% CI [0.26–0.69]; p=0.001) and recurrent MI (HR 0.35; 95% CI [0.15–0.85]; p=0.021; Table 2). Kaplan–Meier analysis also showed poorer outcomes in the MICAD group (Figure 1).
Independent Predictors of Outcomes in MINOCA
Older age, higher creatinine levels and ST-elevation MI at presentation were identified as independent predictors of all-cause death and MACE using multivariable Cox regression analysis. In addition, the use of antiplatelets (aspirin and/or P2Y12 inhibitors) was associated with a significantly lower risk of all-cause mortality (HR 0.27; 95% CI [0.08–0.90]; p=0.033).
Sex, ethnicity, hypertension, dyslipidaemia, diabetes, smoking status,
LVEF, renin–angiotensin inhibitors, β-blockers and statins did not have a significant impact on the primary endpoints. These results are displayed in Tables 3 and 4 Independent Predictors of Outcomes in MICAD Supplementary Material Tables 1 and 2 show the independent predictors in MICAD of all-cause mortality and MACE, respectively. On multivariate analysis, older age, hypertension, diabetes, higher creatinine levels, LVEF, statins and antiplatelet use were associated with an increased risk of allcause death and MACE. The use of renin–angiotensin inhibitors reduced the risk of all-cause mortality, while male sex and ethnicity were independent predictors of MACE.
Discussion
In this nationwide multicentre observational study conducted over a mean follow-up duration of 4.5 years, the prevalence of MINOCA among MI patients was about 4%. Patients with MINOCA had a lesser burden of cardiovascular risk factors and experienced better outcomes than MICAD patients.
The prevalence of MINOCA among patients diagnosed with MI has been estimated to be 6%, which is comparable to the prevalence of 3.9% observed in our cohort.1 Previous studies have shown that MINOCA is associated with female sex, a non-ST-elevation MI presentation, lower rates of smoking and lower prevalence of other traditional cardiovascular risk factors, such as hypertension, dyslipidaemia and diabetes.4 MINOCA patients are also more likely to have a higher post-MI LVEF.16,18 The baseline characteristics of patients with MINOCA in this study were largely consistent with existing literature. Compared with MICAD patients, they were more likely to be female, present with a non-ST-elevation MI, have a higher LVEF and lower rates of diabetes, prior ischaemic stroke and smoking. There were no intergroup differences in ethnic composition.
It is generally accepted that patients with MINOCA have a better prognosis than MICAD patients.1,6–10 A large Medicare study involving more than 276,522 patients found that the rates of all-cause mortality, MACE and rehospitalisation for MI and heart failure at 12 months were lower in MINOCA patients than in MICAD patients, while rehospitalisation for stroke was similar in both groups.11 MINOCA patients in the study also had a 43% lower risk of MACE over 1 year. This is consistent with the results of our study, with MINOCA patients having a lower incidence and risk of allcause mortality, MACE and recurrent MI. This contrasts with some studies that have reported that MINOCA patients experience similar if not poorer outcomes when compared with patients with MICAD.12–16 This discrepancy likely reflects the heterogeneity of MINOCA, the small (and therefore potentially non-representative) sample size of some previous series, and the influence of variation in underlying aetiologies ranging from true MI and myocarditis to takotsubo cardiomyopathy and coronary vasospasm.1,5 Regardless, the event rate in this group of patients is not insignificant and highlights the importance of secondary prevention.
Patients diagnosed with MINOCA represent an undertreated population, and there are currently no completed randomised controlled trials to date that have investigated secondary prevention medical therapy in MINOCA. Recruitment of adequate sample sizes for controlled trials obviously presents a major challenge. The recent 2020 European Society of Cardiology guidelines recommend performing a CMR in all patients with MINOCA to determine the underlying aetiology and to institute treatment according to the disease-specific guidelines.24 For patients without an established underlying cause, the guidelines recommend treating as per the secondary prevention guidelines for atherosclerotic disease.
MINOCA in a South-east Asian Cohort JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Unadjusted HR [95% CI] p-value
Age 1.04 [1.01–1.07] 0.005
Male 0.87 [0.44–1.73] 0.69
Ethnicity
Chinese 1.0
Malay 0.33 [0.08–1.38] 0.13
Indian 1.60 [0.68–3.75) 0.28
Others 1.62 [0.38–6.91] 0.51
Hypertension 1.94 [0.90–4.18] 0.089
Dyslipidaemia 0.89 [0.45–1.77] 0.74
Diabetes 1.26 [0.60–2.65] 0.54
Current/past smoking 1.24 [0.61–2.52] 0.55
Creatinine 1.003 [1.002–1.005] <0.0001
LVEF 0.98 [0.94–1.02] 0.39
Presentation
STEMI 1.0
NSTEMI 0.31 [0.15–0.63] 0.001
Medications at Discharge
ACE-I/ARBs 1.61 [0.80–3.23] 0.18 β-blockers 0.58 [0.29–1.15] 0.12 Statins 0.65 [0.27–1.57] 0.33
Any antiplatelet use 0.45 [0.20–1.0] 0.050 Multivariable-adjusted
Age 1.05 [1.02–1.08] 0.001
Ethnicity
Creatinine 1.003 [1.002–1.005] <0.0001
Presentation
STEMI 1.0
NSTEMI 0.28 [0.13–0.61] 0.001
Cox proportional hazards analysis for major adverse cardiovascular events. Multivariable analysis only includes significant variables on univariate analysis. ACE-I = angiotensin converting enzyme inhibitor; ARB = angiotensin-receptor blocker; LVEF = left ventricular ejection fraction; MINOCA = MI with non-obstructive coronary arteries; NSTEMI = non-ST-elevation MI; STEMI - ST-elevation MI.
However, the management of MINOCA varies widely in clinical practice for various reasons, including accessibility to CMR. It is not uncommon for some physicians to prescribe the same combination of drugs used in atherosclerotic MI for all patients with MINOCA without prior evaluation with CMR. These medications are primarily aimed at plaque stabilisation, reducing atherosclerotic progression and improving endothelial function.25,26 However, MINOCA represents a diverse group of conditions
1. Pasupathy S, Air T, Dreyer R, et al. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation 2015;131:861–70. https://doi.org/10.1161/ CIRCULATIONAHA.114.011201; PMID: 25587100.
2. Thygesen K, Alpert J, Jaffe A, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 2018;72:2231–64. https://doi.org/10.1016/j.jacc.2018.08.1038; PMID: 30571511.
3. Agewall S, Beltrame J, Reynolds H, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J 2016;38:143–53. https://doi.
and involves varying pathophysiological processes besides atherosclerosis.1 This challenges the therapeutic basis of using standard MICAD treatment in all MINOCA patients.
Similar to our cohort, prior studies have shown that MINOCA patients were less frequently started on conventional secondary prevention treatments, such as renin–angiotensin system inhibitors, β-blockers and antiplatelets.8,16,18 Several observational studies have suggested a beneficial effect of renin–angiotensin system inhibitors, statins and β-blockers on both short- and long-term outcomes when used in MINOCA patients.16,18,19,22,23 However, these medications did not appear to have a significant prognostic benefit when used in MINOCA patients in our cohort. In contrast, and as expected, renin–angiotensin inhibitors and statins were associated with improved outcomes in our MICAD cohort. Interestingly, the use of antiplatelets in our MINOCA cohort was independently associated with a reduction in all-cause mortality. This stands in contrast with existing literature and may reflect the aetiological heterogeneity of MINOCA.
The ongoing MINOCA-BAT trial is a randomised multinational study investigating the use of β-blockers and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers in MINOCA patients, and represents a crucial first step towards establishing guidelines for secondary prevention in MINOCA (NCT03686696).27 Future trials should also aim to individually identify the underlying aetiology, so as to facilitate tailored medical therapy for each specific condition.
Study Limitations
The limitations of this study are its observational methodology and the small sample size of MINOCA patients, which may not be powered to study the impact of medications on outcomes. In addition, the various exact underlying aetiologies of MINOCA were not established due to a lack of further cardiac evaluation with CMR and other modalities.
Conclusion
While patients with MINOCA had better clinical outcomes compared with MICAD patients, MINOCA is not a benign entity, with about one in five patients experiencing a major adverse cardiovascular event in the long term.
Clinical Perspective
• Patients with MINOCA have fewer cardiovascular risk factors and experience better outcomes compared with MICAD patients.
• MINOCA is not a benign entity and is associated with significant adverse cardiovascular events.
• Future studies on MINOCA should incorporate investigations to determine the underlying aetiology and evaluate the impact of medical therapy on each specific condition.
org/10.1093/eurheartj/ehw149; PMID: 28158518.
4. Tamis-Holland J, Jneid H, Reynolds H, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation 2019;139:e891–908. https://doi. org/10.1161/cir.0000000000000670; PMID: 30913893.
5. Tornvall P, Gerbaud E, Behaghel A, et al. Myocarditis or “true” infarction by cardiac magnetic resonance in patients with a clinical diagnosis of myocardial infarction without obstructive coronary disease: a meta-analysis of individual patient data. Atherosclerosis 2015;241:87–91. https://doi.
org/10.1016/j.atherosclerosis.2015.04.816; PMID: 25967935.
6. Smilowitz N, Mahajan A, Roe M, et al. Mortality of myocardial infarction by sex, age, and obstructive coronary artery disease status in the ACTION Registry-GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With the Guidelines). Circ Cardiovasc Qual Outcomes 2017;10:e003443. https://doi.org/10.1161/ circoutcomes.116.003443; PMID: 29246884.
7. Larsen A, Nilsen D, Yu J, et al. Long-term prognosis of patients presenting with ST-segment elevation myocardial infarction with no significant coronary artery disease (from the HORIZONS-AMI trial). Am J Cardiol 2013;111:643–8.
MINOCA in a South-east Asian Cohort JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Table 4: Independent Predictors of Major Adverse Cardiovascular Events in Patients with MICAD
https://doi.org/10.1016/j.amjcard.2012.11.011; PMID: 23261001.
8. Pizzi C, Xhyheri B, Costa G, et al. Nonobstructive versus obstructive coronary artery disease in acute coronary syndrome: a meta-analysis. J Am Heart Assoc 2016;5:e004185. https://doi.org/10.1161/jaha.116.004185; PMID: 27986756.
9. Barr P, Harrison W, Smyth D, et al. Myocardial infarction without obstructive coronary artery disease is not a benign condition (ANZACS-QI 10). Heart Lung Circ 2018;27:165–74. https://doi.org/10.1016/j.hlc.2017.02.023; PMID: 28408093.
10. Patel M, Chen A, Peterson E, et al. Prevalence, predictors, and outcomes of patients with non-ST-segment elevation myocardial infarction and insignificant coronary artery disease: Results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines (CRUSADE) initiative. Am Heart J 2006;152:641–7. https://doi.org/10.1016/j. ahj.2006.02.035; PMID: 16996828.
11. Dreyer R, Tavella R, Curtis J, et al. Myocardial infarction with non-obstructive coronary arteries as compared with myocardial infarction and obstructive coronary disease: outcomes in a Medicare population. Eur Heart J 2020;41:870–8. https://doi.org/10.1093/eurheartj/ehz403; PMID: 31222249.
12. Safdar B, Spatz E, Dreyer R, et al. Presentation, clinical profile, and prognosis of young patients with myocardial infarction with nonobstructive coronary arteries (MINOCA): results from the VIRGO study. J Am Heart Assoc 2018;7:e009174. https://doi.org/10.1161/jaha.118.009174; PMID: 29954744.
13. Kang W, Jeong M, Ahn Y, et al. Are patients with angiographically near-normal coronary arteries who present as acute myocardial infarction actually safe? Int J Cardiol 2011;146:207–12. https://doi.org/10.1016/j.ijcard.2009.07.001; PMID: 19664828.
14. Planer D, Mehran R, Ohman E, et al. Prognosis of patients
with non-ST-segment-elevation myocardial infarction and nonobstructive coronary artery disease. Circ Cardiovasc Interv 2014;7:285–93. https://doi.org/10.1161/ circinterventions.113.000606; PMID: 24847016.
15. Choo E, Chang K, Lee K, et al. Prognosis and predictors of mortality in patients suffering myocardial infarction with non-obstructive coronary arteries. J Am Heart Assoc 2019;8:e011990. https://doi.org/10.1161/jaha.119.011990; PMID: 31284804.
16. Dastidar A, Baritussio A, De Garate E, et al. Prognostic role of cardiac MRI and conventional risk factors in myocardial infarction with nonobstructed coronary arteries. JACC Cardiovasc Imaging 2017;10:1199–210. https://doi.org/10.1016/j. jcmg.2018.12.023; PMID: 30772224.
17. Paolisso P, Bergamaschi L, Saturi G, et al. Secondary prevention medical therapy and outcomes in patients with myocardial infarction with non-obstructive coronary artery disease. Front Pharmacol 2019;10:1606. https://doi. org/10.3389/fphar.2019.01606; PMID: 32082147.
18. Manfrini O, Morrell C, Das R, et al. Effects of angiotensinconverting enzyme inhibitors and beta blockers on clinical outcomes in patients with and without coronary artery obstructions at angiography (from a register-based cohort study on acute coronary syndromes). Am J Cardiol 2014;113:1628–33. https://doi.org/10.1016/j. amjcard.2014.02.015; PMID: 24698468.
19. Kovach C, Hebbe A, O’Donnell C, et al. Comparison of patients with nonobstructive coronary artery disease with versus without myocardial infarction (from the VA Clinical Assessment Reporting and Tracking [CART] program). Am J Cardiol 2021;146:1–7. https://doi.org/10.1016/j. amjcard.2021.01.015; PMID: 33539858.
20. Abdu F, Liu L, Mohammed A, et al. Effect of secondary prevention medication on the prognosis in patients with myocardial infarction with nonobstructive coronary artery disease. J Cardiovasc Pharmacol 2020;76:678–83. https://doi.
org/10.1097/fjc.0000000000000918; PMID: 33284169.
21. Lindahl B, Baron T, Erlinge D, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation 2017;135:1481–9. https://doi. org/10.1161/circulationaha.116.026336; PMID: 28179398.
22. Ciliberti G, Verdoia M, Merlo M, et al. Pharmacological therapy for the prevention of cardiovascular events in patients with myocardial infarction with non-obstructed coronary arteries (MINOCA): insights from a multicentre national registry. Int J Cardiol 2021;327:9–14. https://doi. org/10.1016/j.ijcard.2020.11.040; PMID: 33242505.
23. Yeo KK, Ong H, Chua T, et al. Building a longitudinal national integrated cardiovascular database - lessons learnt from SingCLOUD. Circ Rep 2020;2:33–43. https://doi. org/10.1253/circrep.cr-19-0106; PMID: 33693172.
24. Collet J, Thiele H, Barbato E, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289–367. https://doi.org/10.1093/eurheartj/ ehaa575; PMID: 32860058.
25. Calabrò P, Yeh E. The pleiotropic effects of statins. Curr Opin Cardiol 2005;20:541–6. https://doi.org/10.1097/01. hco.0000181482.99067.bf; PMID: 16234628.
26. Werner C, Baumhäkel M, Teo K, et al. RAS blockade with ARB and ACE inhibitors: current perspective on rationale and patient selection. Clin Res Cardiol 2008;97:418–31. https://doi.org/10.1007/s00392-008-0668-3; PMID: 18454336.
27. Nordenskjöld A, Agewall S, Atar D, et al. Randomized evaluation of beta blocker and ACE-inhibitor/angiotensin receptor blocker treatment in patients with myocardial infarction with non-obstructive coronary arteries (MINOCABAT): rationale and design. Am Heart J 2021;231:96–104. https://doi.org/10.1016/j.ahj.2020.10.059; PMID: 33203618.
MINOCA in a South-east Asian Cohort JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Impact of COVID-19 on Acute MI and Percutaneous Coronary Intervention Rates and Outcomes in South East Asia and the Middle East
Paul Jie Wen Tern , 1,2 Yilin Jiang,2 Yee How Lau,2,3 Wael Almahmeed , 4 S Gunavathy Selvaraj , 5 Jack Wei Chieh Tan , 2 Wan Azman Wan-Ahmad , 6 Jonathan Jiunn Liang Yap 2 and Khung Keong Yeo 2
1. Department of Medicine, Singapore General Hospital, Singapore; 2. Department of Cardiology, National Heart Centre Singapore, Singapore; 3. Singapore Cardiac Data Bank, National Heart Centre Singapore, Singapore; 4. Heart and Vascular Institute, Cleveland Clinic Abu Dhabi, Abu Dhabi, United Arab Emirates; 5. National Heart Association of Malaysia, Kuala Lumpur, Malaysia; 6. Department of Medicine, University Malaya Medical Center, Kuala Lumpur, Malaysia
Abstract
Background: Previous studies have reported mixed findings regarding the effects of COVID-19 on percutaneous coronary intervention (PCI) and acute MI (AMI) procedural volumes and case fatalities, as well as on ST-elevation MI (STEMI) door-to-balloon time (DTB). This study characterised the effects of COVID-19 on AMI and PCI volumes and mortality outcomes in countries from Asia and the Middle East, which faced repeated waves of COVID-19. Methods: Pooled data on AMI and PCI case volumes were collected in 4-weekly blocks from Malaysia, Singapore and Abu Dhabi from 2019 (pre-COVID-19) and 2020 (during COVID-19). These were compared against reported COVID-19 case numbers. Case fatality rates, STEMI patient demographics and STEMI DTB times were compared between the pre- and during COVID-19 periods. Results: During the COVID-19 pandemic, there was a comparative reduction in non-STEMI (NSTEMI) cases in Singapore (from 814 to 722; p=0.025) and Malaysia (from 925 to 604; p<0.001), but not in Abu Dhabi (from 144 to 188; p=0.010). PCI volumes fell significantly in Singapore (from 13,089 to 11,449; p=0.020), but not in Malaysia or Abu Dhabi. STEMI volume remained similar before and during COVID-19. There were no significant differences in in-hospital mortality for NSTEMI, STEMI or PCI between the two periods. Conclusion: COVID-19 resulted in a fall in NSTEMI and PCI cases, potentially as a result of patients deferring contact with healthcare institutions. With appropriate protocols and systems, it is possible to provide coronary intervention services in the middle of a pandemic without compromising on mortality or DTB outcomes.
Keywords
COVID-19, acute MI, percutaneous coronary intervention, case volume, mortality, door-to-balloon time

Disclosure: KKY is editor-in-chief, JJLY is an associate editor and WA is on the editorial board of the Journal of Asian Pacific Society of Cardiology; this did not influence peer review. All other authors have no conflicts of interest to disclose.
Acknowledgements: The authors thank the National Heart Association of Malaysia, all the NCVD investigators and all the sites that contributed towards the National Cardiovascular Disease Registry for permission to publish this manuscript.
Data Availability: The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethical Statement: This study was entirely based on registry data and approved by the relevant institutional review boards. Informed consent was not needed because anonymised registry data was used.
Received: 13 August 2021 Accepted: 4 November 2021 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e05. DOI: https://doi.org/10.15420/japsc.2021.12
Correspondence: Khung Keong Yeo, National Heart Centre Singapore, 5 Hospital Drive, Singapore 169609. E: yeo.khung.keong@singhealth.com.sg
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Since the outbreak of the severe acute respiratory syndrome coronavirus 2 pandemic in 2019, COVID-19 has spread across the globe, resulting in millions of deaths worldwide. Beyond an immediate impact on mortality and morbidity, this pandemic has also had secondary ramifications for the provision of healthcare as hospitals change existing workflows to accommodate the screening and treatment of COVID and governments impose lockdown measures that directly and indirectly affect patients’ presentation to hospitals.
Studies have been published about the effect of the COVID-19 pandemic on procedural volumes of elective percutaneous coronary intervention (PCI), as well as on the presentations and outcomes of acute MI (AMI).1–5 Many of these studies only capture the perspective of a single centre in a
particular country. Furthermore, study findings are mixed, with some studies reporting a decrease in non-ST segment elevation MI (NSTEMI) or ST-elevation MI (STEMI) presentations since the onset of the pandemic, and others finding no significant difference, particularly in the STEMI caseload.1–5 Similarly, reported effects on door-to-balloon (DTB) time and mortality outcomes are mixed.1
The aim of this study was to investigate the effects of the COVID-19 pandemic on AMI and PCI rates in countries from Asia and the Middle East to gain an insight into the burden COVID-19 was placing on cardiovascular care. This research is especially pertinent because many of these countries are facing repeated waves of COVID-19, and lessons drawn from earlier experiences will be instructive in preparing for future outbreaks.
© RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com Epidemiology ORIGINAL RESEARCH
Methods
Member countries from the Asian Pacific Society of Cardiology were invited to submit pooled data on cases with discharge diagnoses of AMI or PCI, as defined by prespecified ICD codes. Records for admissions for AMI were further subdivided into diagnoses of NSTEMI and STEMI. Inhospital case fatality data were collected for each of these admissions.
In addition, data on patient demographics, comorbidities and DTB time were collected for STEMI admissions. We chose STEMI cases for closer analysis because STEMI is a well-defined clinical entity, with established guidelines around the diagnosis and management of the condition, thus making it a good substrate for comparison of patient profiles before and during the COVID-19 pandemic.
Three centres responded to the call for data: the National Heart Association of Malaysia, which indexes all PCI and AMI data in Malaysia; the National Heart Centre Singapore, a tertiary hospital with the highest PCI case volume in Singapore; and the Cleveland Clinic Abu Dhabi, a private tertiary hospital in Abu Dhabi. AMI and PCI case volumes were reported in 4-weekly blocks and plotted against COVID-19 case numbers in each respective country for the corresponding time block (Figures 1 and 2).
Statistical Analysis
Statistical significance was set at two-tailed p<0.05. Continuous variables are presented as the median with interquartile range (IQR) and categorical
variables are presented as frequencies and percentages. Comparisons between the pre- and during COVID-19 periods were made using χ-squared or Fisher exact tests, as appropriate, for categorical variables and twosample t-tests or the Kruskal–Wallis test for continuous variables. The relative risk of mortality was calculated based on 2 × 2 contingency tables. Percentage changes were calculated by comparing the admissions and procedural volumes of the respective 4-week blocks for 2019 with the same period in 2020.
All statistical analyses were conducted using Stata version 16 (StataCorp).
Results
Acute MI
Overall, 3,661 patients were admitted for AMI during the pre-COVID-19 period, compared with 2,968 during the COVID-19 period (p<0.001). Although there was no difference in the number of STEMI admissions before and during the COVID-19 period (1,420 versus 1,233; p=0.081), there was a significant reduction in NSTEMI admissions (1,883 versus 1,514; p<0.001; Tables 1 and 2).
Looking at data from individual countries, both Singapore and Malaysia experienced a reduction in total AMI
driven primarily by a
in NSTEMI
the
to 722 during the pandemic (p=0.025). Similarly,
In Singapore, NSTEMI cases fell from 814
Malaysia, NSTEMI cases fell from 925 before the pandemic to
COVID-19 and AMI/PCI in SE Asia and the Middle East JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
admissions,
reduction
presentations.
before
pandemic
in
604 Figure 1: Acute MI Case Volumes Compared Against COVID-19 Case Numbers and National Lockdown Measures Number of admissions 50 100 150 200 Number of admissions 40 20 60 80 100 Number of admissions 20 40 60 80 Number of admissions 0 5 10 15 20 25 1–28 Jan 2019 21 May–17 June 2019 8 Oct–4 Nov 2019 25 Feb–23 Mar 2020 14 July–10 Aug 2020 1–28 Dec 2020 Number of COVID-19 cases 80,000 60,000 40,000 20,000 0 Number of COVID-19 cases 40,000 30,000 20,000 10,000 0 Number of COVID-19 cases 21,000 14,000 7,000 0 Number of COVID-19 cases 40,000 30,000 20,000 10,000 0 Aggregate data Malaysia lockdown Abu Dhabi curfew STEMI NSTEMI COVID-19 1–28 Jan 2019 21 May–17 June 2019 8 Oct–4 Nov 2019 25 Feb–23 Mar 2020 14 July–10 Aug 2020 1–28 Dec 2020 Singapore data STEMI NSTEMI COVID-19 1–28 Jan 2019 21 May–17 June 2019 8 Oct–4 Nov 2019 25 Feb–23 Mar 2020 14 July–10 Aug 2020 1–28 Dec 2020 Malaysia data STEMI NSTEMI COVID-19 1–28 Jan 2019 21 May–17 June 2019 8 Oct–4 Nov 2019 25 Feb–23 Mar 2020 14 July–10 Aug 2020 1–28 Dec 2020 Abu Dhabi data STEMI NSTEMI COVID-19 Singapore lockdown Malaysia lockdown Abu Dhabi curfew Singapore lockdown The vertical dotted lines on the plots represent the respective lockdown periods in Singapore and Malaysia, and the daytime partial curfew implemented in Abu Dhabi. NSTEMI = non-ST segment elevation MI; STEMI = ST-elevation MI.
Singapore
during the pandemic (p<0.001). However, in Abu Dhabi, there was a significant increase in NSTEMI cases (144 to 188; p=0.010). There was no significant difference in STEMI cases from before to during the pandemic in any of the three countries.
Patients admitted with STEMI during the COVID-19 period, compared with those admitted prior to the pandemic, were less likely to have diabetes (35.0% versus 38.7%; p=0.030), more likely to have dyslipidaemia (50.7% versus 41.2%; p<0.001), more likely to have had prior coronary artery bypass grafting (1.9% versus 0.8%; p=0.004) and more likely to have had prior coronary artery disease or PCI (16.6% versus 10.9%; p<0.001). Overall, there was a significant decrease in the proportion of cases achieving a DTB time of ≤90 minutes during the COVID-19 period (59.89% versus 76.81%; p<0.001 (Table 3).
In Malaysia, there was a significant reduction in proportion of cases with a DTB time ≤90 minutes during the pandemic (39.00% versus 71.21%; p<0.001). However, there was no difference in the proportion of cases with a DTB time ≤90 minutes in Singapore and Abu Dhabi with >95% of cases having a DTB ≤90 minutes in both countries. There was no significant difference before and during COVID-19 in in-hospital mortality for STEMI patients (5.49% versus 4.62%; p=0.309) or in in-hospital mortality for NSTEMI patients (3.03% versus 3.43%; p=0.503). Similarly, individual country analysis did not yield any significant differences in case fatality rates for STEMI or NSTEMI patients before and during COVID-19 (Table 4).
PCI Volume
Overall, there was a significant decrease in PCI cases from before to during the COVID-19 period (13,089 versus 11,449; p=0.020). This was driven mostly by data from Singapore, where the number of PCI cases decreased from 2,902 before the pandemic to 2,107 during the pandemic (p<0.001). In contrast, the PCI caseload in Malaysia and Abu Dhabi remained relatively constant (p>0.05). Overall, there was no significant difference in the case fatality rate from before to during the COVID-19 pandemic (1.55% versus 1.44%, respectively; p=0.480).
Discussion
Data pertaining to the impact of COVID-19 on cases of AMI are varied. Several centres have reported a decrease in admissions during the pandemic,1,2,6 whereas others have found no significant difference compared with pre-COVID-19 levels.5 With regard to the type of MI, most studies have shown a decrease in NSTEMI admissions during the COVID-19 pandemic, but mixed results pertaining to STEMI presentations.3,7
Similarly, in the present study
did not find any
symptoms
was a decrease in NSTEMI admissions,
difference in
It is conceivable that STEMI patients experience
will push them to seek medical attention.
will also have distinct ECG abnormalities that prompt
to tertiary centres by primary care or emergency medical
This stands in contrast to the symptoms in NSTEMI, which may
COVID-19 and AMI/PCI in SE Asia and the Middle East JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
there
but we
significant
STEMI presentations between the two periods.
more severe
that
Furthermore, they
urgent referral
services.
Figure 2: Percutaneous Coronary Intervention Case Volumes Compared Against COVID-19 Case Numbers and National Lockdown Measures Number of procedures 400 600 800 1,000 1,200 Number of procedures 200 400 600 800 1,000 100 150 200 250 15 10 20 25 30 21,000 14,000 7,000 0 80,000 60,000 40,000 20,000 0 40,000 30,000 20,000 10,000 0 Number of COVID-19 cases 40,000 30,000 20,000 10,000 0 Number of COVID-19 cases Number of COVID-19 cases Number of COVID-19 cases Number of procedures Number of procedures
lockdown Abu Dhabi curfewMalaysia lockdown 1–28 Jan 2019 21 May–17 June 2019 8 Oct–4 Nov 2019 25 Feb–23 Mar 2020 14 July–10 Aug 2020 1–28 Dec 2020 Aggregate data 1–28 Jan 2019 21 May–17 June 2019 8 Oct–4 Nov 2019 25 Feb–23 Mar 2020 14 July–10 Aug 2020 1–28 Dec 2020 Singapore data 1–28 Jan 2019 21 May–17 June 2019 8 Oct.–4 Nov 2019 25 Feb–23 Mar 2020 14 July–10 Aug 2020 1–28 Dec 2020 Malaysia data 1–28 Jan 2019 21 May–17 June 2019 8 Oct–4 Nov 2019 25 Feb–23 Mar 2020 14 July–10 Aug 2020 1–28 Dec 2020 Abu Dhabi data PCI COVID-19PCI COVID-19 PCI COVID-19 PCI COVID-19 Malaysia lockdown Abu Dhabi curfew Singapore lockdown The vertical dotted lines on the plots represent the respective lockdown periods in Singapore and Malaysia, and the daytime partial curfew implemented in Abu Dhabi. PCI = percutaneous coronary intervention.
Overall
PCI cases 24,538 13,089 1,035 (979–1,075) 11,449 976 (779–1,004) 0.020
Primary PCI 1,994 1,247
Admissions
(90–105) 747 53 (43–67) <0.001
AMI 6,629 3,661 285 (269–297) 2,968 241 (206–253) <0.001
STEMI 2,653 1,420 107 (101–116) 1,233 102 (80–111) 0.081
NSTEMI 3,397 1,883 145 (140–151) 1,514 124 (109–128) <0.001
Singapore
Procedural
PCI cases 5,009 2,902 221 (213–227) 2,107 159 (137–181) <0.001
Primary PCI 843 493 39 (34–42) 350 24 (23–28) 0.002
Admissions
AMI 2,846 1,574 123 (118–128) 1,272 97 (92–102) <0.001
STEMI 748 408 32 (29–34) 340 25 (21–30) 0.076
NSTEMI 1,536 814 63 (60–66) 722 59 (52–62) 0.025
Malaysia
Procedural
PCI cases 18,998 9,928 786 (750–816) 9,070 766 (589–829) 0.522
Primary PCI 1,060 719 58 (51–61) 341 23 (18–37) <0.001
Admissions
AMI 3,384 1,916 147 (134–160) 1,468 127 (93–131) 0.001
STEMI 1,838 985 78 (71–82) 853 72 (47–81) 0.317
NSTEMI 1,529 925 71 (66–78) 604 47 (44–53) <0.001
Abu Dhabi
Procedural
PCI cases 531 259 19 (18–21) 272 21 (16–26) 0.699
Primary PCI 91 35 2 (2–3) 56 4 (2–7) 0.052 Admissions
AMI 399
(9–16) 228 17 (15–19) 0.016 STEMI 67
(0–2) 40 2 (2–4) 0.104 NSTEMI
(8–13) 188 14 (13–16) 0.010
be less typical and less intense, such that they are tolerated by patients, who refrain from presenting to hospital for fear of being exposed to COVID-19.
There is high health avoidance behaviour during pandemics, especially when there are unconfirmed beliefs about modes of transmission, increased perceived severity of the outbreak and increased perception of susceptibility.8,9 Indeed, nadir levels of NSTEMI and STEMI admissions in Malaysia and Singapore were reached during the respective lockdown periods in each country. In particular, NSTEMI and STEMI admissions in Malaysia were lowest during the period 24 March–20 April 2020, which coincided with the start of the Movement Control Order (18 March–3 May 2020). This is despite the fact that the absolute number of COVID-19 cases
was higher in the latter part of 2020. This suggests that fear surrounding the initial outbreak of a hitherto unknown virus, along with the added psychological burden of a government-imposed curfew, may have been holding patients back from presenting to hospital, despite the fact that they were still allowed to seek medical attention during these lockdown periods.
NSTEMI and STEMI admissions in Abu Dhabi rose in tandem with the number of COVID-19 cases in the country, which is in contrast to data from Singapore and Malaysia. This reflects the impact of local policy, because the Cleveland Clinic Abu Dhabi was designated by the public health authorities as a centre responsible for care of non-COVID-19 conditions. This resulted in NSTEMI and STEMI cases being diverted to this centre.
COVID-19 and AMI/PCI in SE Asia and the Middle East JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Table 1: Comparison of Case Volumes in Singapore, Malaysia and Abu Dhabi Before and During the COVID-19 Pandemic Total No. Cases 2019–2020 Before COVID-19 During COVID-19 p-value (K-W test) Total No. Cases No. Cases per 4-week block, Median (IQR) Total No. Cases No. Cases per 4-week Block, Median (IQR)
Procedural
94
171 13
27 2
332 144 10
AMI = acute MI; IQR = interquartile range; K-W = Kruskal–Wallis; PCI = percutaneous coronary intervention; NSTEMI = non-ST segment elevation MI; STEMI = ST-elevation MI.
Overall
No. patients 3,220 1,854 1,366
Mean (±SD) age (years) 56.9 ± 12.2 56.9 ± 12.1 56.8 ± 12.8 0.819
Male sex 2,744 (85.2) 1,570 (84.7) 1,174 (85.9) 0.318
Diabetes 1,196 (37.1) 718 (38.7) 478 (35.0) 0.030
Hypertension 1,707 (53.0) 984 (53.1) 723 (52.9) 0.935
Dyslipidaemia 1,455 (45.2) 763 (41.2) 692 (50.7) <0.001
Smoking (current or past) 1,568 (48.7) 861 (46.4) 707 (51.8) 0.003 ESRD on dialysis 55 (1.7) 26 (1.4) 29 (2.1) 0.119
Any CKD 169 (5.2) 90 (4.9) 79 (5.8) 0.243
Prior CABG 40 (1.2) 14 (0.8) 26 (1.9) 0.004
Prior CVA 50 (1.6) 23 (1.2) 27 (2.0) 0.095
Prior known CAD or PCI 429 (13.3) 202 (10.9) 227 (16.6) <0.001
Singapore
No. patients 748 408 340
Mean (±SD) age (years) 62.5 ± 12.4 62.4 ± 12.5 62.6 ± 12.4 0.764
Male sex 601 (80.3) 332 (81.4) 269 (79.1) 0.440
Diabetes 320 (42.8) 181 (44.4) 139 (40.9) 0.338
Hypertension 470 (62.8) 263 (64.5) 207 (60.9) 0.313
Dyslipidaemia 514 (68.7) 270 (66.2) 244 (71.8) 0.101
Smoking (current or past) 382 (51.1) 205 (50.2) 177 (52.1) 0.621
ESRD on dialysis 23 (3.1) 8 (2.0) 15 (4.4) 0.053
Any CKD 109 (14.6) 54 (13.2) 55 (16.2) 0.256
Prior CABG 31 (4.1) 7 (1.7) 24 (7.1) <0.001
Prior CVA 15 (2.0) 7 (1.7) 8 (2.4) 0.536
Prior known CAD or PCI 237 (31.7) 97 (23.8) 140 (41.2) <0.001
Malaysia
No. patients 2,366 1,402 964
Mean (±SD) age (years) 55.2 (11.6) 55.4 (11.5) 54.9 (11.7)
Male sex 2,043 (86.3) 1,196 (85.3) 847 (87.9) 0.075
Diabetes 832 (35.2) 518 (36.9) 314 (32.6) 0.029
Hypertension 1,186 (50.1) 699 (49.9) 487 (50.5) 0.752 Dyslipidaemia 883 (37.3) 471 (33.6) 412 (42.7) <0.001 Smoking (current or past) 1,121 (47.4) 630 (44.9) 491 (50.9) 0.004 ESRD on dialysis 31 (1.3) 18 (1.3) 13 (1.3) 0.892
Any CKD 55 (2.3) 35 (2.5) 20 (2.1) 0.504
Prior CABG 7 (0.3) 6 (0.4)
(1.2)
(7.4)
Abu
(±SD)
sex
(years)
(11.7)
(94.3)
(41.5)
(48.1)
(54.7)
(current or past)
on dialysis
(61.3)
(0.9)
(1.0)
(7.1)
(10.6)
(95.5)
(43.2)
(50.0)
(50.0)
(59.1)
(0.1) 0.154
(1.6) 0.226
(7.8) 0.510
(12.5)
(93.5) 0.676
(40.3) 0.768
(46.8) 0.743
(58.1) 0.411
(62.9) 0.691
(1.6) 0.397
COVID-19 and AMI/PCI in SE Asia and the Middle East JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com Table 2: Demographics of Patients Admitted With ST-Elevation MI Before and During the COVID-19 Pandemic Overall 2019–2020 Before COVID-19 During COVID-19 p-value
1
Prior CVA 29
14
15
Prior known CAD or PCI 174
99
75
Dhabi No. patients 106 44 62 Mean
age
53.1
52.1
53.8
Male
100
42
58
Diabetes 44
19
25
Hypertension 51
22
29
Dyslipidaemia 58
22
36
Smoking
65
26
39
ESRD
1
0 (0.0) 1
Abu Dhabi
Any CKD 5 (4.7)
Prior CABG 2 (1.9)
Prior CVA 6 (5.7)
Prior known CAD or PCI 18 (17.0)
1 (2.3) 4 (6.5) 0.317
1 (2.3) 1 (1.6) 0.806
2 (4.5) 4 (6.5) 0.676
(13.6) 12 (19.4) 0.440
Unless indicated otherwise, data are given as n (%). CABG = coronary artery bypass grafting; CAD = coronary artery disease; CKD = chronic kidney disease; CVA = cerebrovascular accident; ESRD = end-stage renal disease; PCI = percutaneous coronary intervention.
Table 3: Number of Patients Meeting a Door-To-Balloon Time of ≤90 min Before and During the COVID-19 Pandemic
DTB Time
min
COVID-19 During COVID-19
Overall 709 (76.81) 318 (59.89) <0.001
NHCS 164 (96.47) 129 (96.27) 0.925
NHAM 512 (71.21) 133 (39.00) <0.001
Abu Dhabi 33 (97.06) 56 (100.00) 0.197
(χ2)
pandemic and reached a nadir during the government-imposed ‘circuit breaker’ (7 April–1 June 2020). The fall can be explained by guidance from the Ministry of Health in Singapore and individual institutions to postpone non-urgent cases to free up bed space in anticipation of a surge in COVID-19-related admissions. Recovery ensued as healthcare systems adapted.
Limitations
Unless indicated otherwise, data are given as n (%). DTB = door-to-balloon; NHAM = National Heart Association of Malaysia; NHCS = National Heart Centre Singapore.
In this study, the proportion of patients achieving a DTB of ≤90 minutes was reduced in Malaysia, but unchanged in Singapore and Abu Dhabi. Notably, of the three countries, Malaysia pursued a thrombolysis-first strategy, with primary PCI only if the patient was known to be COVID-19negative or as rescue therapy in the event of failed thrombolysis with medical staff in full personal protective equipment.10 This would likely explain the difference in DTB time among the three countries. Other studies with similar findings have attributed differences in DTB time to the time taken to perform preadmission screening chest X-rays and to ascertain travel and contact histories and symptomatology in patients whose COVID-19 status is unknown.1,11 Unfamiliarity of the emergency department (ED) with new workflows may also be a contributing factor, with subsequent recovery in the DTB time towards the later stages of the pandemic.12 Data from Singapore and Abu Dhabi suggest that with appropriate and well-rehearsed ED workflows, a strategy of primary PCI can still be pursued for STEMI care, with little effect on overall DTB time. This is especially important in a prolonged pandemic response, given the superior outcomes of a primary PCI strategy.13
Nevertheless, despite the increase in DTB time, we did not find a corresponding significant increase in STEMI in-hospital mortality. Similarly, case fatality rates for NSTEMI, PCI and primary PCI remained largely constant. This is in keeping with reports from other studies, and is testament to the robustness of these medical systems in maintaining a high level of cardiovascular care despite the pandemic.1,6,14
Data on PCI volumes provide some insights into how COVID-19 has affected elective procedures. The decrease in PCI in the present study was driven largely by data from Singapore, where there was an active push to reschedule non-urgent elective cardiac catheterisation. The decline in PCI in Singapore started with the onset of the COVID-19
Although this study sheds light on the effect of COVID-19 on cardiovascular care in Singapore, Malaysia and Abu Dhabi, the insights are largely limited to these countries and constrained by the particular circumstances of each country’s response to COVID-19. In addition, the data from Singapore and Abu Dhabi are from a single centre and may not be fully generalisable. As we highlighted earlier, reports on the effects of COVID-19 are context dependent and can differ greatly. Furthermore, our study concluded in December 2020, when case numbers in Malaysia and Abu Dhabi were still increasing. Further research into the effects of these second waves will be very instructive in ascertaining how healthcare systems deal with far higher COVID-19 case numbers despite being armed with protocols and experience in operating under pandemic conditions.
Conclusion
This study has shown that the COVID-19 pandemic has led to a fall in NSTEMI, but not STEMI, admissions. A reduction in the proportion of STEMI patients achieving a DTB time of ≤90 minutes may be related to the thrombolysis-first approach pursued by Malaysia. PCI volumes decreased, primarily driven by the deferral of non-urgent procedures in Singapore. There was no significant effect of the COVID-19 pandemic on in-hospital mortality.
Clinical Perspective
• The COVID-19 pandemic resulted in a fall in non-ST segment elevation MI but not ST-elevation MI (STEMI) admissions in Singapore and Malaysia. This decrease appears to be related more to the implementation of nationwide lockdown measures rather than absolute COVID-19 case numbers.
• A reduction in the proportion of STEMI patients achieving a door-to-balloon time of ≤90 min may be related to the thrombolysis-first approach pursued by Malaysia.
• PCI volumes decreased due to deferral of non-urgent procedures, especially in Singapore.
• There was no significant effect of the COVID-19 pandemic on case fatalities from acute MI admissions or percutaneous coronary intervention procedures.
COVID-19 and AMI/PCI in SE Asia and the Middle East JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Table 2: Cont. Overall 2019–2020 Before COVID-19 During COVID-19 p-value
6
≤90
p-value
Before
Overall
Procedural
PCI cases 13,089
PCI 1,247
Admissions
(1.55)
(7.30)
STEMI 1,420 78 (5.49)
1,883 57 (3.03)
Singapore
Procedural
165 (1.44) 0.9 (0.8–1.1) 0.480
60 (8.03) 1.1 (0.8–1.5) 0.548
57 (4.62) 0.8 (0.6–1.2) 0.309
52 (3.43) 1.1 (0.8–1.6) 0.503
PCI cases 2,902 69 (2.38) 2,107 54 (2.56) 1.1 (0.8–1.5) 0.676 Primary PCI 493 45 (9.13) 350 42 (12.00) 1.3 (0.9–2.0) 0.177 Admissions
STEMI 408 32 (7.84) 340 28 (8.24) 1.1 (0.6–1.7) 0.844 NSTEMI 814 34 (4.18) 722 35 (4.85) 1.2 (0.7–1.8) 0.527
Malaysia
Procedural
PCI cases 9,928 126 (1.27) 9,070 103 (1.14) 0.9 (0.7–1.2) 0.400 Primary PCI 719 45 (6.26) 341 16 (4.69) 0.7 (0.4–1.3) 0.306 Admissions
STEMI 985 43 (4.37) 853 28 (3.28) 0.8 (0.5–1.2) 0.230 NSTEMI 925 20 (2.16) 604 14 (2.32) 1.1 (0.5–2.1) 0.840
Abu Dhabi
Procedural
PCI cases 259 8 (3.09) 272 8 (2.94) 1.0 (0.4–2.5) 0.921 Primary PCI 35 1 (2.86) 56 2 (3.57) 1.3 (0.1–13.3) 0.853 Admissions
STEMI 27 3 (11.11) 40 1 (2.50) 0.2 (0.0–2.1) 0.145 NSTEMI 144 3 (2.08) 188 3 (1.60) 0.8 (0.2–3.7) 0.741 NSTEMI = non-ST segment elevation MI; STEMI = ST-elevation MI.
1. Rattka M, Dreyhaupt J, Winsauer C, et al. Effect of the COVID-19 pandemic on mortality of patients with STEMI: a systematic review and meta-analysis. Heart 2021;107:482–7. https://doi.org/10.1136/heartjnl-2020-318360; PMID: 33334863.
2. Meenakshisundaram R, Senthilkumaran S, Thirumalaikolundusubramanian P, et al. Status of acute myocardial infarction in southern India during COVID-19 lockdown: a multicentric study. Mayo Clin Proc Innov Qual Outcomes 2020;4:506–10. https://doi.org/10.1016/j. mayocpiqo.2020.06.010; PMID: 33043274.
3. Wu J, Mamas M, Rashid M, et al. Patient response, treatments, and mortality for acute myocardial infarction during the COVID-19 pandemic. Eur Heart J Qual Care Clin Outcomes 2021;7:238–46. https://doi.org/10.1093/ehjqcco/ qcaa062; PMID: 32730620.
4. Elliott JM, Crozier IG. Decreases in cardiac catheter laboratory workload during the COVID-19 level 4 lockdown in New Zealand. Intern Med J 2020;50:1000–3. https://doi. org/10.1111/imj.14922; PMID: 32881225.
5. Li YH, Huang WC, Hwang JJ. No reduction of ST-segment elevation myocardial infarction admission in Taiwan during coronavirus pandemic. Am J Cardiol 2020;131:133–4. https://
doi.org/10.1016/j.amjcard.2020.06.030; PMID: 32713656.
6. Showkathali R, Yalamanchi R, Sankeerthana MP, et al. Acute coronary syndrome admissions and outcome during COVID19 pandemic – report from large tertiary centre in India. Indian Heart J 2020;72:599–602. https://doi.org/10.1016/j. ihj.2020.09.005; PMID: 33357652.
7. Choudhary R, Singh K, Choudhary D, et al. Patterns of care and mortality outcomes in patients admitted with acute coronary syndrome during coronavirus disease 2019 pandemic in India. Coron Artery Dis 2021;32:590–2. https://doi.org/10.1097/MCA.0000000000001011; PMID: 33471481.
8. Lau JT, Griffiths S, Choi KC, Tsui HY. Avoidance behaviors and negative psychological responses in the general population in the initial stage of the H1N1 pandemic in Hong Kong. BMC Infect Dis 2010;10:139. https://doi.org/10.1186/14712334-10-139; PMID: 20509887.
9. Moroni F, Gramegna M, Ajello S, et al. Collateral damage: medical care avoidance behavior among patients with myocardial infarction during the COVID-19 pandemic. JACC Case Rep 2020;2:1620–4. https://doi.org/10.1016/j. jaccas.2020.04.010; PMID: 32835261.
10. National Heart Association of Malaysia. ACS during COVID-
19 pandemic. 2020. https://www.malaysianheart. org/?p=highlights&a=1430 (accessed 21 June 2021).
11. Tam CCF, Cheung KS, Lam S, et al. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-segment-elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes 2020;13:e006631. https://doi. org/10.1161/CIRCOUTCOMES.120.006631; PMID: 32182131.
12. Chew NW, Sia CH, Wee HL, et al. Impact of the COVID-19 pandemic on door-to-balloon time for primary percutaneous coronary intervention – results from the Singapore Western STEMI Network. Circ J 2021;85:139–49. https://doi. org/10.1253/circj.CJ-20-0800; PMID: 33162491.
13. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003;361:13–20. https://doi.org/10.1016/S01406736(03)12113-7; PMID: 12517460.
14. Mohamed MO, Banerjee A, Clarke S, et al. Impact of COVID19 on cardiac procedure activity in England and associated 30-day mortality. Eur Heart J Qual Care Clin Outcomes 2021;7:247–56. https://doi.org/10.1093/ehjqcco/qcaa079; PMID: 33079204.
COVID-19 and AMI/PCI in SE Asia and the Middle East JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com Table 4: Comparison of Case Fatality Rates for Percutaneous Coronary Intervention, Primary PCI, ST-Elevation MI and Non-ST-Elevation MI Admissions Before and During the COVID-19 Pandemic Before COVID-19 During COVID-19 RR (95% CI) p-value (χ2) Total No. Cases Mortality, n (%) Total No. Cases Mortality, n (%)
203
11,449
Primary
91
747
1,233
NSTEMI
1,514
Effects of the Current Japanese Guideline for Dedicated, Intensive Lipid-lowering Therapy on Lipid Profile and Coronary Events in Patients After Acute Coronary Syndrome
Ryotaro Yamada , Teruyoshi Kume , Hiroshi Okamoto , Masahiro Yamashita, Satoshi Koto, Kyo Kamisaka, Yoshitaka Sasahira, Yasuyuki Sudo, Ayano Enzan, Tomoko Tamada, Terumasa Koyama, Koichiro Imai, Takeshi Nishi, Yoji Neishi and Shiro Uemura
Department of Cardiology, Kawasaki Medical School, Kurashiki, Japan
Abstract
Background: Patients with acute coronary syndrome (ACS) still have high rates of recurrent adverse cardiovascular events. The purpose of this study was to evaluate the effects of changes in the Japan Atherosclerosis Society Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases for intensive lipid-lowering therapy on the prescription status, lipid profile and clinical outcomes among Japanese patients with ACS.
Methods: Data were retrospectively analysed for 283 consecutive patients with de novo ACS who underwent primary percutaneous coronary intervention and received follow-up coronary angiography between January 2012 and September 2019. Patients were divided into two groups according to the to onset of ACS relative to the guideline update – before (Group 1; n=182) and after (Group 2; n=101). Changes in prescription status, lipid profile and clinical outcomes (composite of cardiac death and non-fatal MI, coronary events from culprit and non-culprit lesions) were evaluated. Results: Post-treatment LDL concentrations were significantly lower in Group 2, but target LDL concentrations of <1.81 mmol/l were only achieved in 58.4% of patients. In Group 2, 85% of patients who received combination high-intensity statin/ezetimibe therapy achieved the target LDL. After propensity score matching, the incidence of coronary events from non-culprit lesions was significantly lower in Group 2 than Group 1 (3.0% versus 19.4%, respectively; p<0.01). Cox model analysis showed that the guideline update resulted in a lower rate of events from non-culprit lesions (HR 0.50; 95% CI [0.33–0.75]). Conclusion: The introduction of new guidelines improved the prescription status and lipid profile in ACS patients. Guideline-recommended more intensive lipid-lowering therapy may decrease events from non-culprit lesions in ACS patients.
Keywords
Acute coronary syndrome, major adverse cardiac event, lipid-lowering therapy, Japanese guidelines

Disclosure: SU is on the Journal of Asian Pacific Society of Cardiology editorial board; this did not influence peer review. All other authors have no conflicts of interest to declare.
Acknowledgement: The authors thank all the patients and staff who participated in this study.
Ethics This study was approved by the Ethics Committee of Kawasaki Medial School Hospital (Approval no. 3919) and was conducted in line with the requirements of the Declaration of Helsinki. Informed consent: Not required. This was a retrospective study in which all patient data were de-identified prior to use.
Data Availability: De-identified participant data will not be shared.
Received: 13 September 2021 Accepted: 16 November 2021 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e06.
DOI: https://doi.org/10.15420/japsc.2021.21
Correspondence: Ryotaro Yamada, Department of Cardiology, Kawasaki Medical School, 577 Matsushima Kurashiki 701-0192, Japan. E: ryotaro@med.kawasaki-m.ac.jp
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Several large studies have consistently demonstrated that lipid-lowering therapy with statins reduces cardiovascular risk, regardless of underlying coronary artery disease (CAD).1 Particularly in patients with stable CAD and acute coronary syndrome (ACS), statin therapy has been shown to achieve marked reductions in mortality and recurrent cardiac events.2–5 These data established very early clinical benefits that persisted on long-term followup. Furthermore, the PROVEIT-TIMI 22 and MIRACL trials have shown even better clinical outcomes with early, intensive statin therapy in ACS.3,5 Adherence to statin therapy in post-ACS patients is also well established as being directly related to statin initiation during the index admission.6
In Japan, the therapeutic target for LDL in patients after ACS was <2.59 mmol/l, but in 2017 the Japan Atherosclerosis Society (JAS) Guidelines
for Prevention of Atherosclerotic Cardiovascular Diseases changed the target to <1.81 mmol/l.7 Intensive lipid-lowering therapy, including the combination of a strong statin (atorvastatin, pitavastatin, and rosuvastatin), ezetimibe and a proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i), is now recommended for high-risk post-ACS patients.7,8
In addition to advances in lipid-lowering therapy, interventional cardiology in ACS is also progressing, with the introduction of intravascular imaging and newer drug-eluting stents. We thought it was clinically important to determine the effects of more intensive lipid-lowering therapy in accordance with current guidelines in the clinical revascularisation setting.
© RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com Intervention ORIGINAL RESEARCH
(71.7)
0.008 51 (76.1) 46 (68.7)
0.690 46 (68.7) 41 (61.2) 0.365
Diabetes 101 (35.7) 70 (38.5) 31 (30.7) 0.189 24 (35.8) 17 (25.4) 0.189
Smoking history 143 (50.5) 81 (44.5) 62 (61.4) 0.006 42 (58.3) 40 (55.6) 0.736
Current smoker 83 (29.3) 54 (19.1) 29 (10.3) 0.865 21 (31.3) 24 (35.8) 0.583
Dyslipidaemia 249 (88.0) 153 (84.1) 96 (95.1) 0.004 58 (86.6) 64 (95.5) 0.064 Family history of coronary artery disease 70 (24.7) 42 (23.1) 28 (27.7) 0.388 17 (25.4) 18 (31.3) 0.844
Lipid-lowering Therapies
Statins 253 (89.4) 156 (85.7) 97 (96.0) 0.004 62 (92.5) 63 (94.0) 0.730
Low intensity 181 (71.5) 151 (96.8) 30 (30.9) <0.001 64 (95.5) 29 (43.3) <0.001
High intensity 72 (28.5) 5 (3.2) 67 (69.1) <0.001 3 (4.5) 38 (56.7) <0.001
Ezetimibe 63 (22.2) 15 (8.2) 48 (47.5) <0.001 7 (10.5) 30 (44.8) <0.001
PCSK9i 7 (2.5) 1 (0.6) 6 (5.9) 0.006 0 (0.0) 4 (6.0) 0.017
Antiplatelet Therapies
Aspirin 283 (100.0) 182 (100.0) 101 (100.0) 1.000 67 (100.0) 67 (100.0) 1.000 P2Y 12 inhibitor 280 (98.9) 180 (98.9) 100 (99.0) 0.931 66 (98.5) 66 (98.5) 1.000
Anti-hypertensive Therapies β-blocker 169 (59.7) 91 (50.0) 78 (77.2) <0.001 47 (70.2) 48 (71.6) 0.849 ACEI/ARB 238 (84.1) 147 (80.8) 91 (90.1) 0.034 59 (88.1) 58 (86.6) 0.795
Aim
The primary purpose of the present study was to evaluate the effects of the introduction of new Japanese dyslipidaemia guidelines on the prescription status (not only the intensity of statin treatment but also changes in statin and other lipid-lowering prescriptions) and lipid profile of patients with ACS in the real-world clinical setting. As a secondary aim, we also investigated the effect of more intensive lipid-lowering therapy on clinical outcomes.
Methods Study Design and Patient Enrolment
The present retrospective observational cohort study was conducted at Kawasaki Medical School Hospital (Kurashiki, Japan). The KIBIDAN-Go (KawasakI BioImaging DAtabase for loNg term cardiovascular prognosis) Registry was reviewed and consecutive patients with de novo ACS admitted to Kawasaki Medical School Hospital between January 2012 and September 2019 were identified.
Using the June 2017 update of the Japan Atherosclerosis Society guidelines, patients were divided into two groups: those treated before (Group 1) and those treated after (Group 2) the guideline update. The exclusion criteria were a history of percutaneous coronary intervention (PCI) or coronary artery bypass surgery (CABG), loss to follow-up blood sampling and loss to follow-up 9–12 months after coronary angiography.
This study was approved by the Ethics Committee of Kawasaki Medial School Hospital (Approval no. 3919) and was conducted in line with the
requirements of the Declaration of Helsinki. Informed consent was not required because this was a retrospective study in which all patient data were de-identified prior to use.
Baseline Characteristics and Angiographic Assessment
Comprehensive clinical data were collected, including background characteristics, blood samples, medications and angiography procedural information, from reviews of charts and operation records. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg or the current use of antihypertensive medication. Dyslipidaemia was defined as serum LDL ≥3.62 mmol/l, HDL <1.03 mmol/l, triglyceride (TG) ≥1.69 mmol/ or the current use of cholesterol-lowering medication. Diabetes was defined as fasting plasma glucose ≥126 mg/dl, HbA1c ≥6.5% or the current use of antidiabetes medication. Smoking status was classified as either current smoker or ex-smoker.
The rates of intracoronary imaging-guided PCI and the use of second- or third-generation drug-eluting stents in the treatment of ACS were also evaluated. All patients underwent successful PCI on admission for the index ACS. Patients underwent follow-up examinations, blood sampling and coronary angiography after 9–12 months.
Qualitative and quantitative
with the
angiography (QCA) was evaluated in
expert consensus document from the
Association of Cardiovascular Intervention and Therapeutics
Effect of Japanese Guideline on Lipids and Coronary Events in ACS JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
coronary
accordance
clinical
Japanese
Table 1: Patient Characteristics Entire Cohort (n=283) No Matching After PSM Group 1 (n=182) Group 2 (n=101) p-value Group 1 (n=67) Group 2 (n=67) p-value Patient Characteristics Mean (± SD) age (years) 67.3 ± 10.9 66.7 ± 10.8 68.5 ± 11.1 0.196 66.9 ± 11.1 67.1 ± 11.5 0.921 Male sex 214 (75.6) 147 (80.8) 67 (66.3)
0.333 Hypertension 203
132 (72.5) 71 (70.3)
Unless indicated otherwise, data are given as n (%). ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; PCSK9i = proprotein convertase subtilisin/kexin type 9 inhibitor; PSM = propensity score matching.
0.299
0.760
Second- or third-generation DES 270 (95.4)
279 (98.6)
0.644 66 (98.5) 66 (98.5) 1.000
0.315 63 (94.0) 65 (97.0) 0.399 Intracoronary
type (n) <0.001 0.663
8
28 C 91
21 26 Culprit vessel 0.263 0.483 LAD 151
35 38 LCX 31
9 4 RCA 96
22 23 LMCA 6 3 3 1 2
QCA Analysis (Culprit Lesion)
Baseline Lesion length (mm) 26.0 ± 11.1 24.6 ± 9.2 29.1 ± 14.1 0.018 26.6 ± 11.6 26.0 ± 8.5 0.789 MLD (mm) 0.45 ± 0.51 0.48 ± 0.51 0.41 ± 0.52 0.270 0.42 ± 0.57 0.43 ± 0.48 0.893 RD (mm) 2.88 ± 0.69 2.91 ± 0.70 2.83 ± 0.67 0.394 2.95 ± 0.63 2.94 ± 0.72 0.944 DS (%) 0.83 ± 0.19 0.83 ± 0.18 0.84 ± 0.20 0.478 0.86 ± 0.19 0.85 ± 0.16 0.804
After stenting
MLD (mm) 2.60 ± 0.67 2.59 ± 0.72 2.61 ± 0.57 0.816 2.47 ± 0.75 2.64 ± 0.56 0.133 RD (mm) 2.94 ± 0.61 2.93 ± 0.63 2.95 ± 0.57 0.784 2.85 ± 0.66 3.01 ± 0.60 0.142 DS (%) 0.12 ± 0.14 0.12 ± 0.15 0.11 ± 0.13 0.521 0.14 ± 0.15 0.11 ± 0.13 0.259
Follow-up
MLD (mm) 2.38 ± 0.75 2.43 ± 0.71 2.29 ± 0.81 0.120 2.29 ± 0.79 2.38 ± 0.80 0.489 RD (mm) 2.88 ± 0.62 2.92 ± 0.61 2.81 ± 0.63 0.160 2.82 ± 0.59 2.79 ± 0.62 0.786 DS (%) 0.17
0.25
0.19 0.17
0.74 0.20
0.19
0.32
0.315 0.21
0.195 0.21
0.21 0.18
0.81 0.26
0.73 0.740
0.21 0.495 Late
using CAAS 7 (Pie Medical Imaging).9 Baseline, post-procedure and followup angiograms were assessed in all patients for whom angiograms were available for analysis. The target segment was defined as the entire segment involving the implanted stent and the edges 5 mm proximal and distal to the stent. A segment treated with multiple overlapping stents was regarded as a single target segment.
The following parameters were obtained through QCA: lesion length, minimum lumen diameter, reference diameter, diameter stenosis (DS) and late loss. In this cohort, angiographic restenosis was performed if angiography during follow-up showed DS ≥50%. Angiographic progression in a non-culprit lesion was defined as a progression in DS ≥50%.
Lipid Profile and Prescription
High-intensity statins were defined as atorvastatin 20 mg/day, rosuvastatin
mg/day and pitavastatin 4 mg/day. Low-intensity statins were defined as atorvastatin 5–10 mg/day, rosuvastatin 2.5–7.5 mg/day, pitavastatin 1–2 mg/day, pravastatin 2.5–15 mg/day or fluvastatin 20 mg/day. The primary endpoint was the change in prescription status and lipid profile.
Clinical Follow-up and Outcomes
Effect of Japanese Guideline on Lipids and Coronary Events in ACS JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
10
The first clinical outcome parameter was a composite of cardiac death and non-fatal MI; the second clinical outcome parameter was the incidence of coronary events, including events from culprit and nonculprit lesions. This study excluded staged PCI procedures, which were Table 2: Lesion Characteristics Entire Cohort (n=283) No Matching After PSM Group 1 (n=182) Group 2 (n=101) p-value Group 1 (n=67) Group 2 (n=67) p-value Lesion Characteristics Diagnosis (n)
0.417 AMI 205 129 77 53 49 UAP 78 54 24 14 18 Mean stent diameter (mm) 2.94 ± 0.50 2.93 ± 0.50 2.95 ± 0.50
2.88 ± 0.52 3.01 ± 0.53 0.148 Total stent length (mm) 27.1 ± 12.7 25.6 ± 10.4 30.0 ± 15.6 0.005 27.7 ± 13.0 26.1 ± 11.4 0.439
172 (94.5) 98 (97.0)
imaging
179 (98.4) 100 (99.0)
Lesion
A 18 12 6 4 6 B1 49 42 7
7 B2 126 86 40 34
43 48
99 52
24 7
57 39
±
± 0.18
± 0.21
±
±
loss (mm)
±
± 0.76
± 0.71
±
±
Unless indicated otherwise, data are given as the mean ± SD or n (%). AMI = acute MI; DES = drug-eluting stent; DS = diameter stenosis; LAD = left anterior descending artery; LCX = left circumflex artery; LMCA = left main coronary artery; MLD = minimum lumen diameter; PSM = propensity score matching; QCA = qualitative coronary angiography; RCA = right coronary artery; RD = reference diameter; UAP = unstable angina pectoris.
Baseline
TC (mmol/l)
LDL (mmol/l) 3.23
(mmol/l) 1.19
TG (mmol/l) 1.53
0.90
3.25
1.19
0.76 3.37
0.37 1.22
1.25 0.410
1.07 0.423
0.29 0.650
± 0.92 0.509 1.47 ± 0.83 1.61 ± 0.98 0.391
Non-HDL (mmol/l) 3.94 ± 1.01 3.93 ± 0.91 3.95 ± 1.19 0.855 3.96 ± 0.81 4.08 ± 1.26 0.493
LDL/HDL ratio 2.9 ± 1.1 2.9 ± 1.0 2.9 ± 1.1 0.919 3.0 ± 1.0 2.9 ± 1.1 0.845
9- to 12-month Follow-up
TC (mmol/l) 4.03 ± 0.84 4.26 ± 0.77 3.89 ± 2.94 0.114 4.30 ± 0.65 3.75 ± 0.83 <0.001
LDL (mmol/l) 2.13 ± 0.70 2.33 ± 0.66 1.77 ± 0.64 <0.001 2.33 ± 0.57 1.86 ± 0.70 <0.001
HDL (mmol/l) 1.24 ± 0.31 1.22 ± 0.32 1.27
Non-HDL (mmol/l) 2.79
LDL/HDL ratio 1.8
0.94 1.67
0.79 3.04
0.7
0.94 1.34
0.71 2.34
1.4
0.91 0.004 1.71 ± 1.08 1.45 ± 1.04 0.154
0.28 0.202 1.25 ± 0.34 1.29 ± 0.28 0.485 TG (mmol/l) 1.52
0.71 <0.001 3.05 ± 0.61 2.47 ± 0.78 <0.001
<0.001 2.0
defined as planned interventions after the first catheterisation. Information was gathered on clinical outcomes from medical charts or phone interviews. All patients were followed retrospectively up to October 2020 to assess survival status and clinical events.
Statistical Analysis
Values are presented as mean ± SD for quantitative variables and as numbers and percentages for qualitative variables. Continuous data were compared between subgroups using Student’s t-test for normally distributed data and the Wilcoxon rank-sum test for data that were not normally distributed. Qualitative data were compared between subgroups using Pearson’s χ-squared test.
Propensity score matching (PSM) was used to control for differences between Groups 1 and 2. Specifically, 1:1 nearest-neighbour matching was performed based on estimated propensity scores, with a calliper of 0.25 imposed to ensure that matching was within the zone of common support. After PSM, Pearson’s χ-squared test and, where appropriate, Fisher’s exact test were used to compare rates of the patient and angiographic lesion variables described above to ensure these factors were statistically equivalent between the two groups. After PSM, a Cox proportional hazard model was used to compare events.
All statistical analyses were performed using JMP version 15 (SAS Institute). Two-tailed p<0.05 was considered statistically significant.
Results Clinical Characteristics
Among the total patient population, 274 patients were excluded because they did not meet the inclusion criteria: 109 patients had history of previous PCI, 18 patients had history of CABG, 122 patients were lost to follow-up CAG and 20 patients were lost to follow-up blood tests. Thus, 283 consecutive patients with de novo ACS were enrolled in the study (Supplementary Material Figure 1). Patients were allocated to either Group 1 (treatment before the guideline update; n=182) or Group 2 (treatment after the guideline update; n=101) depending on the onset of the index ACS. During the observation period (up to October 2020), the overall
0.7 1.5
0.7 <0.001
median clinical follow-up period was 788 days (interquartile range [IQR] 385–1,293 days).
At baseline, mean age was 67 ± 11 years. Of the subjects, 214 (76%) were men. Significant differences were seen between Groups 1 and 2 in sex and dyslipidaemia. The percentage of patients using β-blockers and angiotensin-converting enzyme inhibitor (ACEI)/angiotensin II receptor blockers (ARBs) was significantly higher in Group 2 than in Group 1 (Table 1).
Lesion characteristics (lesion length, lesion type and total stent length) differed significantly between the two groups (Table 2). The rates of imaging-guided PCI and the use of second- and third-generation drugeluting stents in the treatment of ACS were >90% in both Groups 1 and 2, with no significant differences between (94.5% versus 97.0% [p=0.315] and 98.6% versus 99.0% [p=0.644], respectively). After PSM, all categorical variables (age, sex, smoking status, the use of β-blockers and ACEI/ARB, total stent length, lesion type and follow-up period) were acceptably matched between the two groups.
Lipid Profiles and Prescriptions
The percentage of patients receiving lipid-lowering therapies was significantly higher in Group 2 than in Group 1. The statin adherence rate was 98.4%. All cases of discontinuation occurred in Group 2. The reasons for statin discontinuation included liver dysfunction (n=1), digestive symptoms (n=1) and poor adherence (n=2). No significant differences in baseline lipid profiles were apparent between the two groups. Conversely, LDL and TG at follow-up were significantly lower and non-HDL was significantly higher in Group 2 (Table 3). The distribution of follow-up LDL concentrations before and after the guideline update is shown in Figure 1 Before the guideline update, only 22.0% of patients achieved an LDL target value of <1.81 mmol/l, with 63.7% of patients achieving an LDL target value of <2.59 mmol/l. However, after the guideline update, 58.4% of patients achieved an LDL target value of <1.81 mmol/l.
with low-intensity statins alone and half the patients treated with low- or high-intensity statin plus
one-quarter
Effect of Japanese Guideline on Lipids and Coronary Events in ACS JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
In Group 1, only
of patients treated
Table 3: Summary of Changes in Lipid Profiles Entire Cohort (n=283) No Matching After PSM Group 1 (n=182) Group 2 (n=101) p-value Group 1 (n=67) Group 2 (n=67) p-value
5.12 ± 1.04 5.11 ± 0.94 5.13 ± 1.21 0.854 5.15 ± 0.86 5.30 ±
± 0.89 3.18 ± 0.80 3.31 ± 1.03 0.262
±
±
HDL
± 0.32 1.18 ± 0.34 1.19 ± 0.29 0.689
±
±
±
1.50 ± 0.89 1.58
±
±
±
±
±
±
±
±
2.0 ± 0.7
± 0.6
±
±
Unless indicated otherwise, data are given as mean ± SD. PSM = propensity score matching; TC = total cholesterol; TG = triglycerides
7 (2.5)
Group
(n=182)
(3.3)
(1.0) 0.199 1 (1.5)
(n=101)
(0.0) 0.238
death 4 (1.4)
(1.7)
(1.0) 0.644 0 (0.0) 0 (0.0) 1.000 Non-fatal MI 3 (1.1) 3 (1.7) 0 (0) 0.103 1 (1.5) 0 (0.0) 0.238
event 55 (19.4) 37 (20.3) 18 (17.8) 0.608 15 (22.4) 7 (10.5) 0.060 Culprit 25 (8.8) 13 (7.1)
(11.9) 0.186 6 (9.0) 6 (9.0) 1.000 Non-culprit 36 (12.7)
(15.4)
(7.9) 0.062 13 (19.4) 2 (3.0) 0.002
ezetimibe achieved the new LDL target. In Group 2, 85% of patients who were treated with the combination of high-intensity statin plus ezetimibe therapy achieved the LDL target. The proportion of patients achieving the target LDL using the combination treatment tended to be higher in Group 2 than in Group 1 (84.9% versus 50.0%, respectively; p=0.268; Supplementary Material Figure 2).
Clinical Outcomes
During follow-up, seven patients (2.5%) developed clinical events and 55 patients (19.4%) developed coronary events. The median follow-up was 1,100 days (IQR 546–1,603 days) for Group 1 and 580 days (IQR 365–902 days) for Group 2. Before PSM, there were no significant differences in clinical and coronary events between the two groups (Table 4).
After PSM, the incidence of coronary events from non-culprit lesions was significantly lower in Group 2 than in Group 1 (3.0% versus 19.4%, respectively; p<0.01). There were no significant differences in the rates of clinical and culprit lesion events between the two groups. Using the Cox
model revealed that the guideline update resulted in a lower rate of events from non-culprit lesions (HR 0.50; 95% CI [0.33–0.75]).
Discussion
The major findings of this study are that: after the introduction of the new guidelines, although post-treatment LDL levels were significantly improved, target LDL levels of <1.81 mmol/l were achieved by 58.4% of patients; after the introduction of the new guidelines, 85% of patients who were treated with combination high-intensity statin/ezetimibe therapy achieved target LDL levels; and the change in the guideline for more intensive lipid-lowering therapy resulted in a decreased rate of nonculprit lesion progression in patients after ACS.
Intensive lipid-lowering
consisting of the combination of a strong statin, ezetimibe and PCSK9i, is now recommended for secondary prevention in high-risk patients after ACS. To the best of our knowledge, the present study is the first single-centre retrospective analysis to
the effect of guideline-compliant intensive lipid-lowering
Effect of Japanese Guideline on Lipids and Coronary Events in ACS JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
therapy,
investigate
Figure 1: Histogram of LDL Follow-up Before and After the Guideline Update
1
LDL (mmol/l) LDL (mmol/l) LDL <2.59 mmol/l n=116 (63.7%) LDL <1.81 mmol/l n=38 (22.0%) Number of patients 30 20 10 0 0 0.52 1.03 1.55 2.07 2.59 3.10 3.62 4.14 4.65 Group 2
LDL <2.59 mmol/l n=92 (91.1%) LDL <1.81 mmol/l n=59 (58.4%) Number of patients 30 20 10 0 Median 2.26 mmol/l (IQR 1.86–2.74 mmol/l) Mean (± SD) 2.33 ± 0.66 mmol/l Median 1.66 mmol/l (IQR 1.37–2.07 mmol/l) Mean (± SD) 1.77 ± 0.64 mmol/l 0 0.52 1.03 1.55 2.07 2.59 3.10 3.62 4.14 4.65 Table 4: Clinical Outcomes Entire cohort (n=283) No matching After PSM Group 1 (n=182) Group 2 (n=101) p-value Group 1 (n=67) Group 2 (n=67) p-value Follow-up period (days) 1,016.9 ± 592.7 1,223.3 ± 707.0 644.9 ± 287.0 <0.001 758.5 ± 461.0 715.3 ± 284.9 0.515 Clinical event
6
1
0
Cardiac
3
1
Coronary
12
28
8
Unless indicated otherwise, data are given as the mean ± SD or n (%). PSM = propensity score matching.
Effect
therapy on the lipid profile and secondary coronary events in patients after ACS.
As described previously, there is a close relationship between the LDL concentration achieved and coronary plaque regression, with the cut-off point at which coronary atherosclerotic development changes from plaque progression to regression being an LDL concentration of approximately 1.94 mmol/l.10 In patients with plaque regression, LDL concentrations achieved are significantly lower than in patients with plaque progression.10 Every 5 years, the JAS publishes guidelines for the treatment of dyslipidaemia and atherosclerosis. In accordance with the latest JAS (2017) guideline for secondary prevention in patients with familial hypercholesterolaemia, ACS and diabetes complicated by other high-risk conditions, such as non-cardiogenic cerebral infarction, peripheral artery disease, chronic kidney disease, metabolic syndrome, an overlap of major risk factors or smoking, stricter LDL control should be considered, with a concentration of <1.81 mmol/l set as the target.7
The REAL-CAD study, the first large-scale clinical trial in Japan, showed that high-dose pitavastatin (4 mg/day) significantly reduced major adverse cardiovascular events (MACE) compared with low-dose pitavastatin (1 mg/ day).11 Based on the results of the REAL-CAD study and a previous intravascular ultrasound trial, which showed coronary plaque regression using high-dose statins, the maximum tolerable dose of a high-intensity statin is recommended as first-line therapy for ACS patients, regardless of pre-intervention LDL concentrations.10 A systematic review demonstrated that combination therapy comprising of statin/ezetimibe and statin/ PCSK9i (particularly with high-intensity statins) could help attain the previously recommended strict LDL goals of <1.81 mmol/l.12 The results of the present study confirm that LDL target goals could be achieved following combination therapy with a high-intensity statin and ezetimibe. In addition, with LDL <1.81 mmol/l as the target, reductions in cardiac events were demonstrated in the IMPROVE-IT, FOURIER, ODYSSEY OUTCOMES trials.13–15 Using the same LDL target, the GLAGOV and PRECISE- IVUS trials showed plaque regression.16,17
In the present study, we confirmed that intensive lipid-lowering therapy decreased the rate of non-culprit lesion progression in patients after ACS. Conversely, we did not find any significant differences in the rate of instent restenosis between the two groups. This may represent the effect of advances in drug-eluting stents.
Current guidelines from the Japanese Circulation Society (JCS; issued in 2018), the American College of Cardiology/American Heart Association (ACC/AHA; issued in 2018) and European Atherosclerosis Society/ European Society of Cardiology (EAS/ESC; issued in 2019) regarding the treatment of hyperlipidaemia place particular emphasis on patients with very high cardiovascular risk and recommend stringent LDL lowering with statins.8,18,19 The JCS and ACC/AHA guidelines recommend fixed high-dose statins for high-risk patients with atherosclerotic cardiovascular disease (ASCVD), with the addition of ezetimibe to the maximum tolerated statin dose reasonable when the LDL concentration remains ≥1.81 mmol/l.8,18 Furthermore, EAS/ESC guidelines recommend a treatment goal of LDL <1.42 mmol/l or a >50% reduction in LDL.19 For patients with ASCVD who experience a second vascular event within 2 years (not necessarily of the same type as the first event) while taking the maximum tolerated dose of statin-based therapy, an LDL goal <1.03 mmol/l may be warranted.19
However, there is insufficient evidence to recommend a target value of LDL <1.42 mmol/l among Japanese ACS patients.
Recently, a subanalysis of the FOURIER trial suggested that patients who achieved the lowest LDL values had the lowest risk of future MACE.20 The clinical outcomes in the present study were comparable to those in that trial, but we recognise that our findings regarding clinical outcomes are not conclusive, but rather hypothesis generating, because of several study limitations (see below). However, we think it is important to elucidate the effects on ACS patients of intensive lipid therapy based on the newly revised guideline and in the current clinical revascularisation setting.
Limitations
Some potential limitations to our data need to be considered. First, although we adjusted for known confounders, the data are observational in nature and inherently subject to residual confounding. For example, detailed haemodynamic, echocardiographic and intracoronary imaging data could not be extracted from the dataset.
Second, patients were retrospectively and not randomly selected from a single centre, and the relatively small number of patients with selection biases may have affected the results. Although PSM can be used to adjust for measured independent variables, it sometimes struggles when there is a limited number of patients. Therefore, the results in the present study are for an exploratory analysis, with further investigations using data with greater accuracy required.
Third, the results of this study are applicable only to Japanese patients treated under the Japanese healthcare system. It may not be possible to generalise the results to other populations under different healthcare systems in other countries. For example, atorvastatin 20 mg/day, rosuvastatin 10 mg/day and pitavastatin 4 mg/day are classified as mild-intensity therapies in the US and Europe.18,19 However, the approved starting dose for aggressive lipid-lowering therapy in the present study was for Japanese patients, who generally have a lower body weight than non-Asian populations.
Finally, the follow-up period differed between the two groups because the guidelines were revised in 2017.
Conclusion
The introduction of new Japanese dyslipidaemia guidelines significantly improved the prescription status and lipid profile of patients with ACS, but almost 50% of ACS patients still did not achieve target LDL concentrations. Guideline-oriented, more intensive lipid-lowering therapy may decrease events from non-culprit lesions in patients after ACS.
Clinical Perspective
• This study evaluated the effect of changes in Japanese guidelines for dedicated, intensive lipid-lowering therapy on lipid profile and secondary clinical outcomes in patients with acute coronary syndrome (ACS).
• Most patients who received combination intensive statin/ ezetimibe therapy achieved a target LDL of <1.81 mmol/l.
• The guideline change for dedicated, intensive lipid-lowering therapy resulted in a decrease in the rate of non-culprit lesion progression in patients after ACS.
of Japanese Guideline on Lipids and Coronary Events in ACS JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Effect
1. Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective metaanalysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–78. https://doi. org/10.1016/S0140-6736(05)67394-1; PMID: 16214597.
2. Blazing MA, De Lemos JA, Dyke CK, et al. The A-to-Z Trial: methods and rationale for a single trial investigating combined use of low-molecular-weight heparin with the glycoprotein IIb/IIIa inhibitor tirofiban and defining the efficacy of early aggressive simvastatin therapy. Am Heart J 2001;142:211–7. https://doi.org/10.1067/mhj.2001.116959; PMID: 11479456.
3. Cannon CP, McCabe CH, Belder R, et al. Design of the Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE IT)-TIMI 22 trial. Am J Cardiol 2002;89:860–1. https:// doi.org/10.1016/S0002-9149(02)02201-4; PMID: 11909576.
4. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–504. https://doi.org/10.1056/NEJMoa040583; PMID: 15007110.
5. Kinlay S, Schwartz GG, Olsson AG, et al. High-dose atorvastatin enhances the decline in inflammatory markers in patients with acute coronary syndromes in the MIRACL study. Circulation 2003;108:1560–6. https://doi.org/10.1161/01. CIR.0000091404.09558.AF; PMID: 12975259.
6. Muhlestein JB, Horne BD, Bair TL, et al. Usefulness of in-hospital prescription of statin agents after angiographic diagnosis of coronary artery disease in improving continued compliance and reduced mortality. Am J Cardiol 2001;87:257–61. https://doi.org/10.1016/S00029149(00)01354-0; PMID: 11165956.
7. Kinoshita M, Yokote K, Arai H, et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb 2018;25:846–984. https://doi.org/10.5551/jat.GL2017;
PMID: 30135334.
8. Kimura K, Kimura T, Ishihara M, et al. JCS 2018 guideline on diagnosis and treatment of acute coronary syndrome. Circ J 2019;83:1085–196. https://doi.org/10.1253/circj.CJ-19-0133; PMID: 30930428.
9. Suzuki N, Asano T, Nakazawa G, et al. Clinical expert consensus document on quantitative coronary angiography from the Japanese Association of Cardiovascular Intervention and Therapeutics. Cardiovasc Interv Ther 2020;35:105–16. https://doi.org/10.1007/s12928-020-006537; PMID: 32125622.
10. Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very highintensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006;295:1556–65. https://doi.org/10.1001/jama.295.13.jpc60002; PMID: 16533939.
11. Taguchi I, Iimuro S, Iwata H, et al. High-dose versus lowdose pitavastatin in Japanese patients with stable coronary artery disease (REAL-CAD): a randomized superiority trial. Circulation 2018;137:1997–2009. https://doi.org/10.1161/ CIRCULATIONAHA.117.032615; PMID: 29735587.
12. Sharma M, Ansari MT, Abou-Setta AM, et al. Systematic review: comparative effectiveness and harms of combination therapy and monotherapy for dyslipidemia. Ann Intern Med 2009;151:622–30. https://doi.org/10.7326/00034819-151-9-200911030-00144; PMID: 19884623.
13. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–97. https://doi.org/10.1056/ NEJMoa1410489; PMID: 26039521.
14. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–22. https://doi. org/10.1056/NEJMoa1615664; PMID: 28304224.
15. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and
cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–107. https://doi.org/10.1056/ NEJMoa1801174; PMID: 30403574.
16. Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA 2016;316:2373–84. https://doi.org/10.1001/jama.2016.16951; PMID: 27846344.
17. Tsujita K, Sugiyama S, Sumida H, et al. Impact of dual lipidlowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention: the multicenter randomized controlled PRECISE-IVUS trial. J Am Coll Cardiol 2015;66:495–507. https://doi.org/10.1016/j.jacc.2015.05.065; PMID: 26227186.
18. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/ AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e1082–143. https://doi.org/10.1161/ CIR.0000000000000698; PMID: 30586774.
19. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–88. https://doi.org/10.1093/eurheartj/ehz455; PMID: 31504418.
20. Giugliano RP, Pedersen TR, Park JG, et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet 2017;390:1962–71. https://doi.org/10.1016/S01406736(17)32290-0; PMID: 28859947.
of Japanese Guideline on Lipids and Coronary Events in ACS JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Intracoronary Imaging of Recanalised Coronary Thrombus: A Report of Two Cases
Achmad Fauzi Yahya
Ibnu Adams
and Aninka Saboe
1. Department of Cardiology and Vascular Medicine, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia;

Division of Interventional Cardiology, Hasan Sadikin General Hospital, Bandung, Indonesia
Abstract
Advances in intracoronary imaging – particularly optical coherence tomography (OCT) – facilitate the visualisation of detailed vessel anatomy and plaque morphology, which is essential in determining the optimum percutaneous coronary intervention (PCI) strategy. The authors present two cases of OCT-guided PCI in spontaneous recanalisation of coronary thrombus lesions. They emphasise the role of OCT in detecting spontaneous recanalisation of coronary thrombus lesions, allowing optimisation of the PCI strategy and stent deployment. The cases described also illustrate the procedural challenges that can be encountered when managing lesions of this type.
Keywords
Coronary thrombus, honeycomb-like structure, intracoronary imaging, optimal coherence tomography, lesion preparation
Disclosure: The authors have no conflict of interests to declare.
Ethics: This study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Informed Consent: Written informed consent was obtained from the patients to participate in and to publish this case report and accompanying images.
Received: 6 August 2021 Accepted: 21 December 2021 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e07. DOI: https://doi.org/10.15420/japsc.2021.08
Correspondence: Achmad Fauzi Yahya, Department of Cardiology and Vascular Medicine, Universitas Padjadjaran Jalan Eyckman 38, Bandung 40161, Indonesia. E: a.fauzi.yahya@unpad.ac.id
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
The spontaneous recanalised coronary thrombus (SRCT) is defined as a coronary lesion with multiple intraluminal channels. The appearance of the lesions in imaging findings has been variously described as spiderweb like, or resembling Swiss cheese, honeycomb or lotus root. The complexity and structural variability of SRCTs poses challenges in coronary interventions. To date, no uniformity regarding the optimal intervention strategy in SRCT has been described.
We present two cases of optical coherence tomography (OCT)-guided percutaneous coronary intervention (PCI) in SRCT lesions. We emphasise the role of OCT in SRCT lesion recognition, providing insights into optimal lesion preparation and stent implantation. The cases also illustrate the procedural challenges in managing SRCT lesions.
Case 1
A 43-year-old man presenting with shortness of breath on moderate exertion was referred to our hospital for a coronary procedure. He had a history of an MI 3 months earlier, was a smoker and had diabetes. He was treated with furosemide 40 mg, dual antiplatelet therapy (acetylsalicylic acid 80 mg and clopidogrel 75 mg), atorvastatin 40 mg, bisoprolol 2.5 mg, captopril three × 12.5 mg and rapid insulin three × 4 units.
His vital signs were stable. A remarkable physical finding was cardiomegaly without any signs of congestion. ECG showed sinus rhythm with left axis deviation. Transthoracic echocardiography revealed left ventricle (LV) dilatation and marked reduction of LV systolic function with ejection
fraction 24%. Dobutamine stress echocardiography showed viable myocardium in all left ventricular segments.
Therapeutic Intervention
Coronary angiography revealed mild stenosis at the distal left main. Left anterior descending (LAD) evaluation showed moderate-severe stenosis at the mid–distal segment. The left circumflex was normal. Right coronary artery (RCA) evaluation revealed haziness at the proximal part with thrombolysis in myocardial infarction (TIMI) flow grade 3 (Figure 1A). We performed an OCT (Ilumien Optis System) evaluation of the RCA, which revealed channels of recanalised thrombus, divided by fibrous septa with variable thickness and high intensity and low signal attenuation at the proximal RCA (Figure 2A). Lesion preparation was performed using a 3.0/15mm Scoreflex balloon (OrbusNeich). OCT evaluation after scoring balloon dilatation showed adequate plaque disruption. A 3.5/18 mm XIENCE PRIME stent (Abbott Vascular) was implanted. Post-PCI OCT evaluation demonstrated mild stent malapposition (0.5 mm) at the middle part of the stent. Stent optimisation was performed using a 4.0/12 mm non-compliant (NC) Sprinter balloon (Medtronic). Final OCT evaluation showed good stent expansion (minimal stent area 11 mm2) and stent apposition with no stent edge dissection (Figure 2B). Contrast injection showed TIMI flow grade 3 (Figure 1B).
Follow-up and Outcomes
No symptoms or clinical complications were observed after the procedures. The patient was discharged from hospital the following day.
© RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com Intervention CASE REPORT
, 1,2
2
1,2
2.
Figure 1: Case 1 – Right Coronary Angiogram Before and After Percutaneous Coronary Intervention
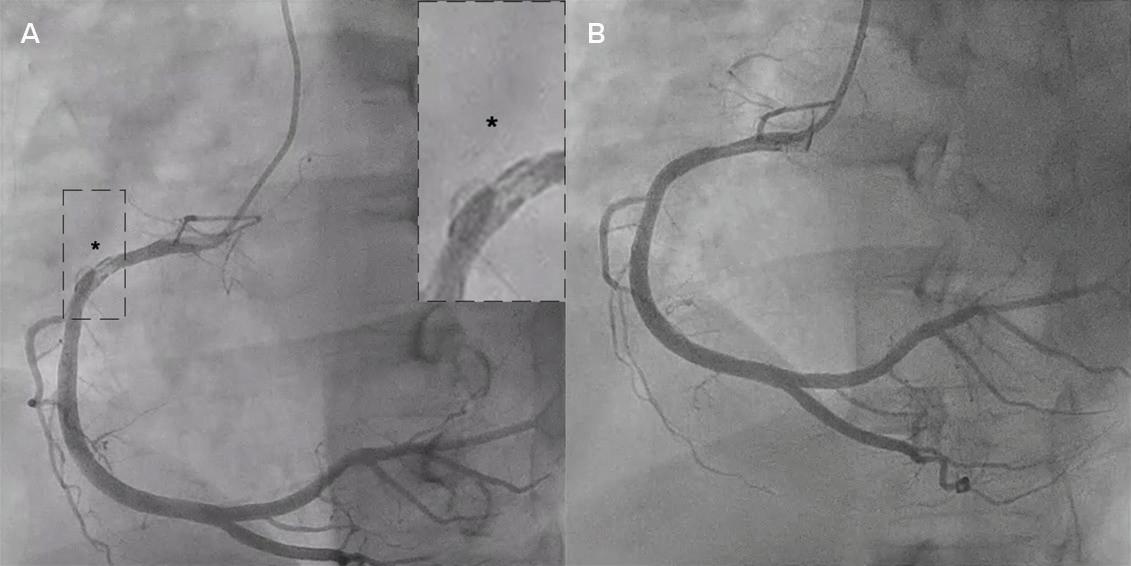
Figure 2: Case 1 – Optical Coherence Tomography Evaluation Before and After Percutaneous Coronary Intervention
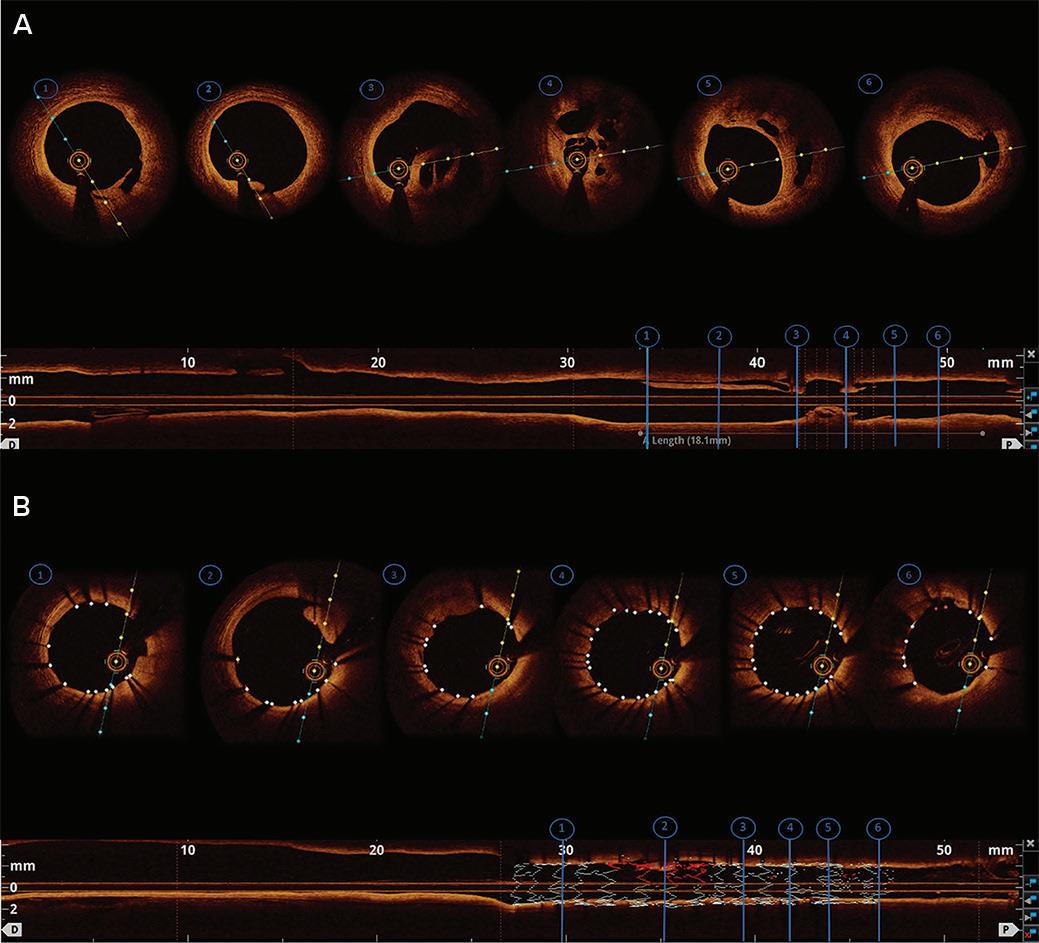
Case 2
A 46-year-old man presented with chronic coronary syndrome. He had a history of PCI of the LAD 1 year before. He smoked and had hypertension. The patient was receiving dual antiplatelet therapy (acetylsalicylic acid 80 mg and clopidogrel 75 mg), atorvastatin 40 mg, bisoprolol 10 mg and ramipril 10 mg.
His vital signs were stable, with no remarkable physical findings. ECG showed sinus rhythm with pathological Q waves in the inferior lead. An echocardiogram revealed mild LV dilatation and reduced LV systolic function (ejection fraction 44%), with hypokinesia on the basal-mid inferior, inferolateral and inferoseptal wall.
Therapeutic Intervention
Coronary angiography showed the left main was normal, with a right dominance system. The LAD stent was patent. A chronic total occlusion (CTO) lesion was visualised at the proximal part of the left circumflex. The RCA revealed moderate–severe stenosis at the mid part of the RCA with haziness (Figure 3A). We decided to perform fractional flow reserve (FFR) evaluation on the RCA before intervention.
The FFR wire was introduced to the RCA, but it failed to cross the lesion. We exchanged the wire for a Runthrough NS Floppy wire (Terumo). After several unsuccessful attempts crossing the lesion, we finally successfully crossed the lesion with the support of a Corsair 135 (Asahi) microcatheter. Considering prior wire crossing difficulties despite the angiography only showing moderate–severe lesions, we decided to perform intracoronary evaluation by OCT.
Pre-PCI OCT evaluation revealed a lesion with multiple channels of organised thrombus, divided by fibrous septa with variable thickness and high intensity/low signal attenuation, mimicking honeycomb features (Figure 4A). In light of this, we decided to intervene in the RCA.
Lesion preparation was performed using a 3.0/10 mm Scoreflex balloon to disrupt the fibrous septa. Post-scoring-balloon dilation OCT evaluation showed adequate plaque disruption. Therefore, it was followed by stent placement with a 3.0/38 mm XIENCE PRIME. Post-stenting OCT evaluation revealed stent malapposition at the proximal part, so we performed stent optimisation using a 3.25/12 mm NC Sprinter balloon. Final OCT evaluation showed good stent expansion and apposition (minimal stent area 6.9 mm2) and no stent edge dissection (Figure 4B). Contrast injection
Each image (1–6) corresponds with the longitudinal view (bottom image). A: Before percutaneous coronary intervention. A honeycomb-like lesion was visualised in images 3 and 4; B: After percutaneous coronary intervention. Final optical coherence tomography evaluation showed acceptable stent expansion, stent malapposition 0.5 mm, and minimal stent area 11 mm2
showed TIMI flow grade 3. FFR calculation was 0.89, without any complications (Figure 3B).
Follow-up and Outcomes
No symptoms or clinical complications were observed after the procedures. The patient was discharged from hospital the following day.
Discussion
SRCT is characterised by lumen irregularities with a braid-like appearance detected by intracoronary imaging. It was first observed by Terashima et al. in 2002 in patients with Kawasaki disease.1,2 The incidence and prevalence of SRCT are unknown. The pathological mechanisms are still unknown, but possibilities include spontaneous recanalisation of the thrombus because of plaque erosion, plaque rupture, coronary dissection or coronary thromboembolism.2,3 Suzuki et al. described the histopathologic assessment of septa as bilateral endothelial layers with collagen and elastin fibres between them.4
Intracoronary imaging allows detailed coronary lesion characterisation, guiding the PCI strategy and optimisation. OCT uses the scattering and absorption of near-infrared light, providing adequate tissue penetration with excellent axial and spatial resolution resulting in detailed identification of plaque characteristics and composition in real time. Intracoronary imaging of SRCT shows signal-rich, high-backscattering diaphragm-like protrusions, dividing the lumen into multiple small channels separated by fibrous septa, representing remnants of a late-stage recanalised thrombus.4
The fibrous septa were composed of high signal intensity and low signal attenuation, suggesting elastin and collagen fibres, as in our cases.4
The complexity and potential structural variability of SRCT pose challenges for coronary interventions. We encountered difficulties advancing the wire to cross the lesion in our second case. There are several possible causes of wiring difficulty in non-CTO lesions, such as severe tortuosity, extensive
Two Cases of Recanalised Coronary Thrombus JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
A: Before percutaneous coronary intervention. B: After percutaneous coronary intervention. Asterisk and box denotes haziness at the proximal right coronary artery. Lesion preparation using a 3.0/15 mm Scoreflex balloon, followed by stent implantation with 3.5/18 mm XIENCE PRIME and stent optimisation with a non-compliant Sprinter 4.0/12 mm balloon.
Figure 4: Case 2 – Optical Coherence Tomography Evaluation Before and After Percutaneous Coronary Intervention
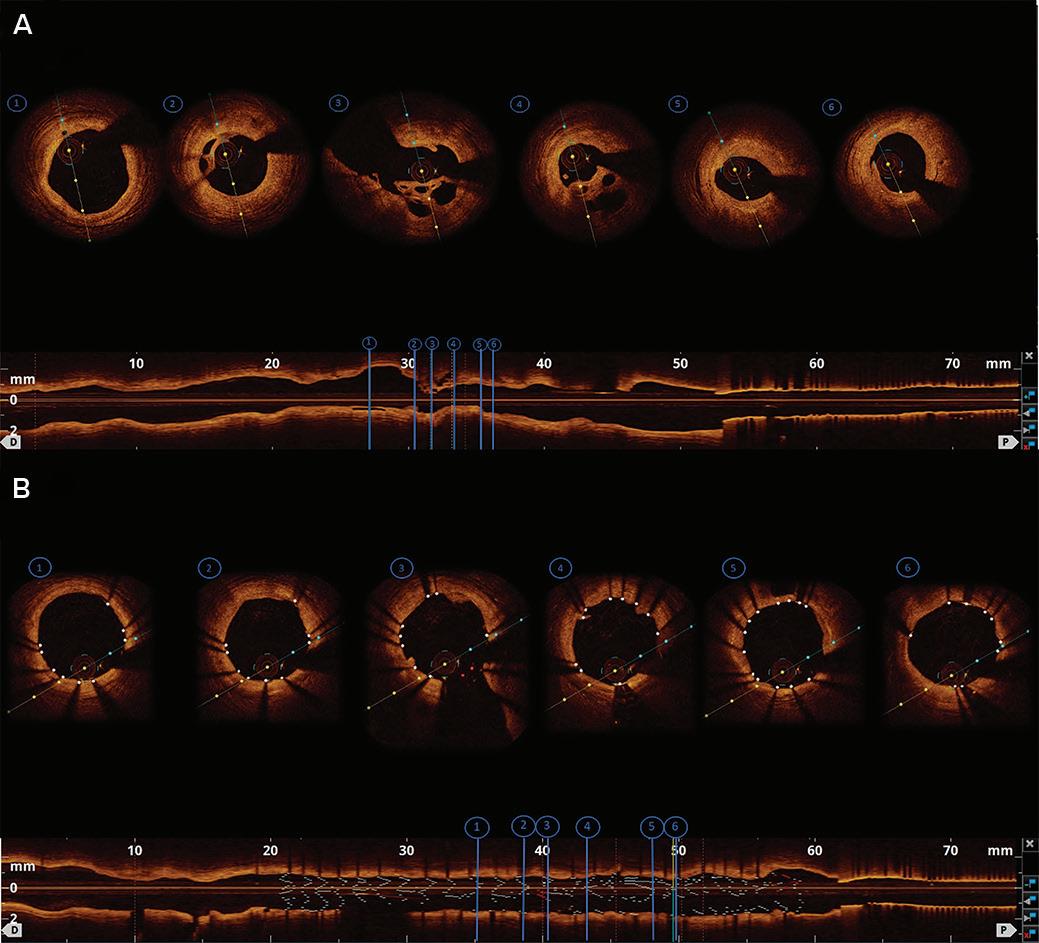
channel of recanalised thrombus, as opposed to tracking a microchannel in CTO lesion, which usually uses tapered wires. We also recommend considering using a microcatheter or balloon to increase wire support while crossing the lesion and to reduce the likelihood of wire tip deflection.
Unfavourable PCI outcomes have been described in SRCT lesions, such as in-stent thrombosis and in-stent restenosis.6 The primary factors contributing to stent failures are inadequate stent deployment and incomplete stent apposition, resulting from inadequate lesion preparation.7 Therefore, our strategy was to perform optimal lesion preparation in our cases.
There are several lesion preparation strategies for dealing with coronary lesions, such as using balloon dilatation (NC balloon, ultrahigh-pressure balloon, modified balloon), atherectomy devices or intravascular lithotripsy, the choice of which depends on specific scenarios. The NC balloon allows transmission of high pressures to resistant lesions while maintaining its shape and volume; therefore, the force is directed to the lesion rather than transmitting it to other artery parts, as with semicompliant balloons. The cutting balloon is an NC balloon with atherotomes along the surface that can cut into the plaque, creating controlled dissection and crack propagation. The scoring balloons consist of balloonmounted spiral scoring wires that anchor into fibrocalcific plaque.8,9
Despite these advances, to date there are no evidence-based data regarding the effectiveness of lesion preparation across various balloon dilation methods. We opted for the Scoreflex balloon as the method of lesion preparation because of the complex anatomy of SRCTs, particularly the presence of relatively thick fibrous septa. Compared with the NC balloon, whose force tends to be distributed to the segments with less resistance, Scoreflex, with its built-in integral wire and the coronary guidewire on the outside of the balloon, creates a focused force in a localised region of the plaque. The Scoreflex balloon can facilitate controlled plaque disruption.9 The Scoreflex balloon sizes were calculated using reference diameter from the OCT.
Adequate lesion preparation was shown by post-scoring balloon dilation OCT evaluation, characterised by complete disruption of the fibrous septa. In both cases, OCT-guided optimal stent implantations were successfully achieved after adequate lesion preparation.
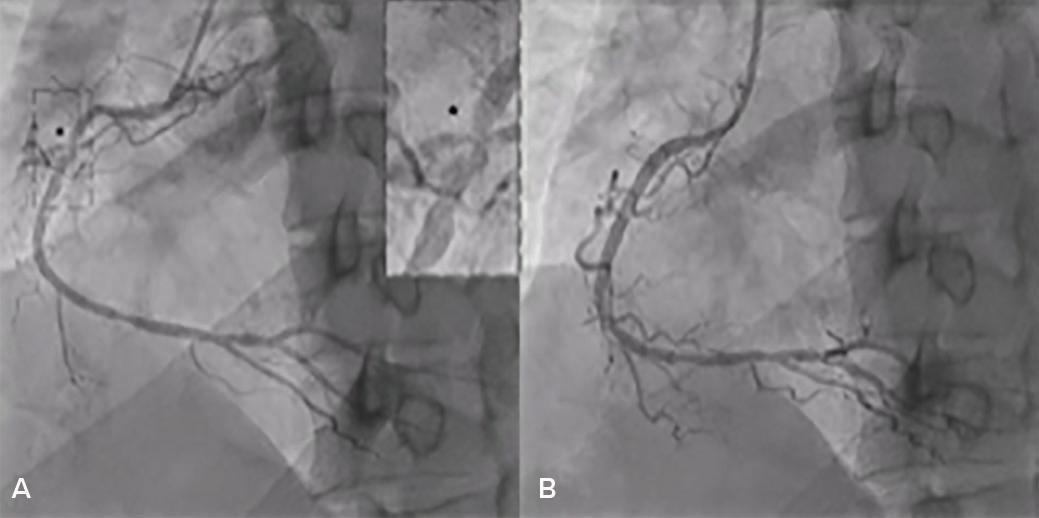
Conclusion
calcification, and severe stenosis or subtotal occlusion.5 However, in our cases, OCT revealed multiple channels of recanalised thrombus. Thus, the wiring difficulties may be caused by the complex architecture of the lesion, making the tangled yet narrow intraluminal channels.
Typically, step-wise approaches for difficulty wiring non-CTO lesions begin with first-line wire choices: a spring-coiled, hydrophobic, low gram workhorse wire. Escalating to an alternative wire is necessary after attempting and failing with a workhorse wire. However, the wire selection differs with specific conditions. Furthermore, using a wire support should also be considered.5
We suggest performing the sliding entry technique to facilitate tracking the channel of the SRCT lesion, as in our case. Regular floppy wire or hydrophilic wire is advanced with gentle tip rotation and probing to slide through the
The recanalised coronary thrombus can potentially become an obstacle in coronary interventions because of the complexity of the anatomy. To optimise PCI results, lesion preparation should be considered in managing recanalised coronary thrombus lesions. OCT may guide treatment decisions before, during and after PCI, giving optimal results.
Clinical Perspective
• Intracoronary imaging has provided insight into coronary lesions and vessel morphology, which plays a pivotal role in the optimisation of percutaneous coronary intervention.
• Recanalised coronary thrombus should be considered as a cause of hazy intracoronary angiographic images.
• Several procedural challenges may be encountered during percutaneous coronary intervention on spontaneous recanalised coronary thrombus lesions, such as wiring difficulties and opting for appropriate methods of lesion preparation, so physicians should have knowledge in dealing with lesions of this type.
Two Cases of Recanalised Coronary Thrombus JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Figure 3: Case 2 – Right Coronary Angiogram Before and After Percutaneous Coronary Intervention
A: Before percutaneous coronary intervention. B: After percutaneous coronary intervention.
Asterisk and
box denotes haziness
at
the proximal right coronary artery. Lesion preparation using a 3.0/10 mm Scoreflex balloon, followed by stent placement with 3.0/38 mm XIENCE PRIME and
stent
optimisation using
a non-compliant 3.25/12
mm Sprinter balloon.
Each image (1–6) corresponds with the longitudinal view (bottom image). A: Before percutaneous coronary intervention. A honeycomb-like lesion was visualised in image 4; B: After percutaneous coronary intervention. Final optical coherence tomography evaluation showed acceptable stent expansion and apposition with minimal stent area 6.9 mm2 and no stent edge dissection.
1. Lin M, Su Z, Li J, et al. Honeycomb-like structure in the right coronary artery treated with a drug-eluting stent: a case report and literature review. J Int Med Res 2018;46:2008–13. https://doi.org/10.1177/0300060518757605; PMID: 29529896.
2. Terashima M, Awano K, Honda Y, et al. “Arteries within the artery” after Kawasaki disease: a lotus root appearance by intravascular ultrasound. Circulation 2002;106:887. https:// doi.org/10.1161/01.CIR.0000030708.86783.92; PMID: 12176965.
3. Haraki T, Uemura R, Masuda SI, et al. A honeycomb-like structure in the left anterior descending coronary artery treated using a scoring device and drug-eluting stent implantation: a case report. J Med Case Rep 2016;10:80. https://doi.org/10.1186/s13256-016-0874-y; PMID: 27036624.
4. Suzuki S, Sotomi Y, Nakatani S, et al. Histopathologic
insights into the honeycomb-like structure in the coronary artery: in vivo multimodality imaging assessment with directional coronary atherectomy. JACC Cardiovasc Interv 2018;11:e157–9. https://doi.org/10.1016/j.jcin.2018.07.014; PMID: 30286864.
5. Groves E, Stinis C. Coronary stenting: practical considerations, equipment selection, tips and caveats. In: Topol E, Teirstein P, eds. Textbook of Interventional Cardiology 8th ed. Amsterdam: Elsevier, 2019.
6. Koyama K, Yoneyama K, Mitarai T, et al. In-stent protrusion after implantation of a drug-eluting stent in a honeycomblike coronary artery structure: complete resolution over 6 months and the role of optical coherence tomography imaging in the diagnosis and follow-up. JACC Cardiovasc Interv 2014;7:e39–40. https://doi.org/10.1016/j. jcin.2013.07.023; PMID: 24746653.
7. Lim M. Complications of percutaneous coronary interventions. In: Kern M, Sorajja P, Lim M, eds. The Interventional Cardiac Catheterization Handbook. Amsterdam: Elsevier, 2018. https://doi.org/10.1016/B978-0-323-476713.00010-7
8. McQuillan C, Jackson MWP, Brilakis ES, Egred M. Uncrossable and undilatable lesions – a practical approach to optimizing outcomes in PCI. Catheter Cardiovasc Interv 2021;97:121–6. https://doi.org/10.1002/ccd.29001; PMID: 32453918.
9. Bonaventura K, Schwefer M, Yusof AKM, et al. Systematic scoring balloon lesion preparation for drug-coated balloon angioplasty in clinical routine: results of the PASSWORD observational study. Adv Ther 2020;37:2210–23. https://doi. org/10.1007/s12325-020-01320-2; PMID: 32274746.
Two Cases of Recanalised Coronary Thrombus JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Novel Mechanical Thrombosuction in an Ectatic Right Coronary Artery with Large Thrombus Burden: A Case Report
Prabesh Neupane , 1 Anish Hirachan 1 and Kamaraj Selvaraj 2
1. Nepal Mediciti Hospital, Lalitpur, Nepal; 2. Hospital Serdang, Selangor, Malaysia
Abstract
Manual thrombosuction during primary angioplasty in an ectatic coronary artery with large thrombus burden carries the risk of incomplete thrombus extraction and thrombus migration. Here, the authors describe a case in which a novel mechanical thrombosuction device with a large lumen and sustained power aspiration enabled the safe and effective complete extraction of a large unfragmented clot. Further studies with such devices are required to shed light on the efficacy and safety of mechanical thrombosuction in coronary interventions.
Keywords
Ectatic coronary artery, primary angioplasty, mechanical thrombosuction, large thrombus
Disclosure: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient included in this case report.
Acknowledgments: This case study was initially presented as a poster at TCTAP and AP Valves Virtual 2020.
Received: 16 August 2021 Accepted: 16 November 2021 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e08. DOI: https://doi.org/10.15420/japsc.2021.15
Correspondence: Prabesh Neupane, Department of Cardiology, Nepal Mediciti Hospital, Sainbu, Lalitpur 44600, Nepal. E: nprabesh@gmail.com

Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Application of thrombosuction during percutaneous coronary intervention (PCI) is an adjunctive modality. It tends to be used in situations where ectatic vessels with a large thrombus burden are encountered. Conventional manual thrombosuction devices are limited by the possibility of incomplete extraction of the thrombus and the risk of thrombus migration because of the small lumen or insufficient suction force from a syringe. Newer dedicated mechanical thrombectomy devices with a wider lumen and continuous power aspiration might provide the potential for reduced time to perfusion, prevention of thrombus-related slow flow/ no flow and possibly reduce the risk of embolisation of the thrombus. We present a case in which a novel coronary mechanical thrombosuction device enabled the safe, rapid and effective complete extraction of a large thrombus from an ectatic coronary artery, resulting in complete reperfusion.
Case Presentation
A woman aged 67 years with underlying hypertension and dyslipidaemia presented at our centre with a sudden onset of left-sided chest pain associated with diaphoresis and dizziness. Her chest pain score was 8/10 and her pulse was 40 BPM and regular. Her blood pressure was 100/70 mmHg. Her chest and other cardiovascular examinations were within normal limits. Oxygen saturation was 96% in room air. The ECG conducted at the emergency department revealed inferior ST-elevation with reciprocal ST-depression in the septal and high lateral leads, complicated by second degree atrioventricular block (Mobitz type I; Figure 1). With a diagnosis of acute inferior wall ST-elevation MI, the patient received 300 mg aspirin and 40 mg rosuvastatin in the emergency department and was taken for an emergency coronary angiogram.
Diagnostic coronary angiography was conducted using a 5 Fr Optitorque (Terumo) catheter via the right radial artery. Left coronary artery angiogram revealed 80% stenosis in the mid left anterior descending artery, 80% stenosis in proximal ramus intermedius and minor disease in the nondominant left circumflex artery. Her right coronary angiogram revealed a large ectatic right coronary artery (RCA) with total thrombotic occlusion at the proximal segment (Figure 2A).
The patient received 180 mg of ticagrelor and a bolus of a total of 100 IU/kg unfractionated heparin. The RCA was engaged with a 6 Fr Short Amplatz Left (SAL) guide catheter (Launcher; Medtronic). The lesion was crossed with a Sion blue coronary wire (Asahi Intecc) and pre-dilatation of the lesion was done with a 3.0 × 15 mm semi-compliant (SC) balloon. Postballooning, a Thrombolysis in MI (TIMI) I flow with a large thrombus extending up to distal RCA was observed (Figure 2B). Given the large thrombus burden, a loading dose of intracoronary tirofiban (25 μg/kg) was given and a maintenance dose started at 0.15 μg/kg/min. The decision was made to use mechanical thrombosuction. Thrombus aspiration was done with a 5 Fr mechanical coronary power aspiration system (Indigo System CAT RX Aspiration Catheter, Penumbra). Aspiration successfully extracted a non-fragmented massive red thrombus (Figure 2C). Thrombosuction was uneventful with TIMI III flow seen post aspiration with grade 2 myocardial blush. The culprit lesion at the proximal RCA had significant stenosis with potential plaque rupture. The lesion was further predilated with a 3.0 × 15 mm SC balloon at 16 atm. The proximal RCA was stented with a self-apposing STENTYS XPosition (STENTYS) 3.0–3.5 mm × 27 mm at 12 atm. Post-dilatation was done with a 4.0 × 15 mm noncompliant balloon at 18 atm with good angiographic results (Figure 2D). The patient’s chest pain had subsided. Rhythm had reverted to sinus
© RADCLIFFE CARDIOLOGY 2022 Access at: www.JAPSCjournal.com Intervention CASE REPORT
Mechanical
in
Coronary Artery with Large Thrombus
aspiration was used in 7.1% of those cases when conventional PCI failed and a favourable outcome was noted with thrombectomy in the subgroup analysis of cases with high thrombus load.8 Furthermore, a randomised, prospective large study on the usage of abciximab infusion and aspiration thrombectomy showed that in a large anterior wall MI, thrombus aspiration resulted in a lower rate of new-onset heart failure and hospitalisation.10
Figure 2: Procedural Angiographic Views
Depicting Occluded Right Coronary Artery, Large Thrombus and Final Result
Manual aspiration thrombectomy carries a risk of thromboembolism either because of technical inadequacy or as a result of proximal clot migration and embolism because of inadequate suction pressure. Theoretically, devices with a larger lumen and sustained power aspiration throughout the procedure may offer an alternative approach to thrombosuction. These devices could limit distal embolisation as well as systemic embolisation by extracting unfragmented larger clots. Such power thrombectomy devices are an established technique in the neurovascular field for thrombus extraction and are a recommended modality. Recently, dedicated coronary mechanical thrombectomy devices have become available. In our case, we used a mechanical coronary power aspiration system (Indigo System CAT RX Aspiration Catheter), which is a 5 Fr, large-lumen continuous vacuum source power aspiration catheter system. Recently, the large prospective multicentre study CHEETAH examined this device for safety and performance, with results presented at Transcatheter Cardiovascular Therapeutics 2021. The study successfully met the primary endpoints, with low major adverse cardiac events rate and demonstrated high rates of thrombus removal, flow restoration and myocardial perfusion normalisation. TIMI flow grade 3 was achieved in 97.5% of patients, reduction of TIMI grade thrombus to zero was achieved post procedure and enhanced myocardial blush of 99.8% was reported in the study with no device related serious adverse events noted.11 In our experience, the device was technically simple to use and was able to efficiently extract a large thrombus in a single run. No adverse haemodynamic changes were noted during thrombosuction and procedure time was remarkably reduced.
Conclusion
In the setting of ectatic coronaries with high thrombus burden, mechanical thrombosuction with power aspiration might result in quicker perfusion, limited slow flow/no flow and improved immediate outcomes with, possibly, no device-related stroke. Further experience and studies with these devices are required to evaluate the efficacy and safety of mechanical thrombosuction in coronary interventions.
rhythm and significant resolution of the ST-segment was noted. The patient had no further events during her hospital stay and was discharged with aspirin 75 mg once daily and ticagrelor 90 mg twice daily as dual antiplatelet therapy.
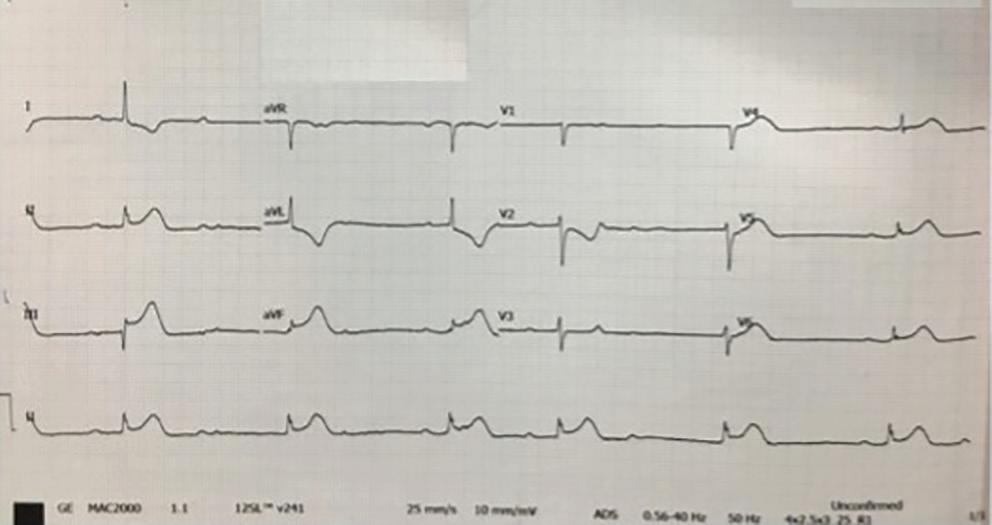
Discussion
Primary PCI in cases of high thrombus burden is a challenging task and is associated with an increased risk of acute and long-term complications, including stent thrombosis and death.1,2,3 A limited number of studies of aspiration thrombectomy in acute coronary syndrome have demonstrated improved myocardial perfusion demonstrated by better myocardial blush, enhanced resolution of ST-T changes and numerical reduction in in-stent thrombosis and target lesion revascularisation.4,5 Results from the singlecentre TAPAS trial revealed that routine thrombus aspiration resulted in improved myocardial reperfusion compared with conventional PCI, showing significant reduction in long-term mortality during the 1-year follow-up.6,7 However, TASTE and TOTAL, the major multicentre randomised controlled clinical trials on the efficiency of routine manual thrombosuction, revealed no mortality benefit and further concerns about the increased risk of stroke emerged.8,9 A bailout treatment strategy of thrombus
Clinical Perspective
• Thrombosuction during primary percutaneous coronary intervention is frequently practised to achieve good immediate reperfusion when thrombus-laden coronaries are encountered.
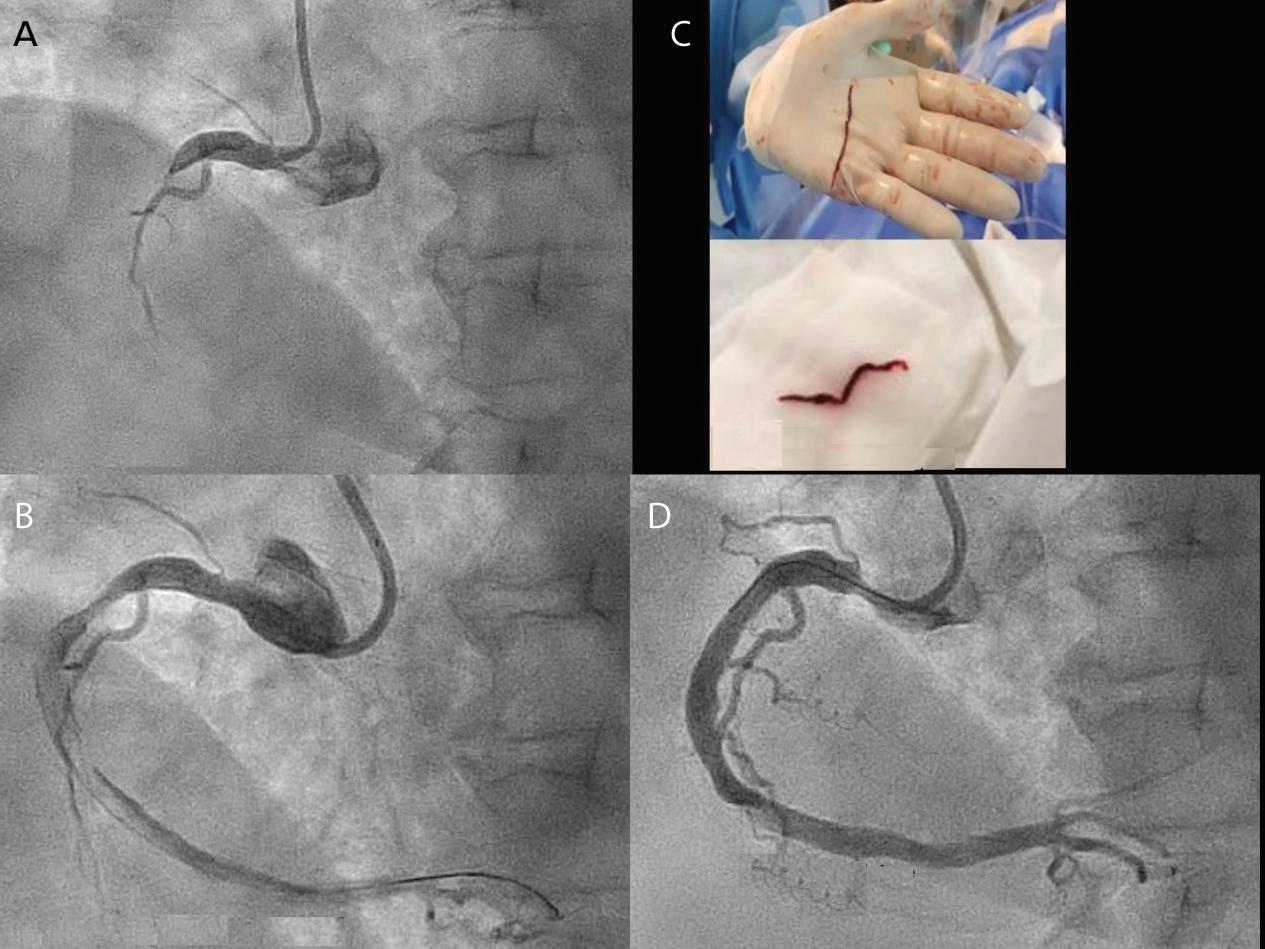
• Manual thrombosuction has a risk of possible thromboembolism owing to a smaller bore and insufficient suction force as the syringe fills with blood and special precautions with guidingcatheter handling are needed.
• The mechanical thrombosuction device described here overcame the limitations of manual thrombosuction because of a larger bore and sustained suction force throughout the procedure.
• This modality might assist in faster reperfusion by being able to efficiently extract larger unfragmented clots thereby avoiding risk the of no-flow and slow-flow phenomena attributed to distal thrombus migration post-ballooning.
Thrombosuction
Ectatic
JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY Access at: www.JAPSCjournal.com
Figure 1: Pre-procedure 12-lead ECG
The ECG shows ST elevation in the inferior leads and reciprocal ST-depression in the septal and high lateral leads with Mobitz type I atrioventricular block.
A: Occluded proximal RCA in left anterior oblique view; B: Large thrombus burden extending from proximal to distal RCA; C: Unfragmented thrombus aspirated with mechanical thrombosuction; D: Final angiogram showing patent RCA with Thrombolysis in Myocardial Infarction III flow. RCA = right coronary artery.
Mechanical Thrombosuction in Ectatic Coronary Artery with Large Thrombus
1. Kalyanasundaram A, Blankenship JC, Berger P, et al. Thrombus predicts ischemic complications during percutaneous coronary intervention in saphenous vein grafts: results from TARGET (do Tirofiban and ReoPro give similar efficacy trial?). Catheter Cardiovasc Interv 2007;69:623–9. https://doi.org/10.1002/ccd.20963; PMID: 17192960.
2. Sianos G, Papafaklis MI, Daemen J, et al. Angiographic stent thrombosis after routine use of drug-eluting stents in ST-segment elevation myocardial infarction: the importance of thrombus burden. J Am Coll Cardiol 2007;50:573–83. https://doi.org/10.1016/j.jacc.2007.04.059; PMID: 17692740.
3. Singh M, Reeder GS, Ohman EM, et al. Does the presence of thrombus seen on a coronary angiogram affect the outcome after percutaneous coronary angioplasty? An angiographic trials pool data experience. J Am Coll Cardiol 2001;38:624–30. https://doi.org/10.1016/s07351097(01)01445-0; PMID: 11527607.
4. Spitzer E, Heg D, Stefanini GG, et.al. Aspiration thrombectomy for treatment of ST-segment elevation
myocardial infarction: a meta-analysis of 26 randomized trials in 11,943 patients. Rev Esp Cardiol (Engl Ed) 2015;68:746–52. https://doi.org/10.1016/j.rec.2015.01.007; PMID: 25979551.
5. Burzotta F, De Vita M, Gu YL, et al. Clinical impact of thrombectomy in acute ST-elevation myocardial infarction: an individual patient-data pooled analysis of 11 trials. Eur Heart J 2009;30:2193–203. https://doi.org/10.1093/eurheartj/ ehp348; PMID: 19726437.
6. Svilaas T, Vlaar PJ, van der Horst IC, et. Al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med 2008;358:557–67. https://doi. org/10.1056/NEJMoa0706416; PMID: 18256391.
7. Vlaar PJ, Svilaas T, van der Horst IC, et.al. Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet 2008;371:1915–20. https://doi.org/10.1016/ S0140-6736(08)60833-8; PMID: 18539223.
8. Mahmood MM, Watt J, Ahmed JM. Thrombus aspiration during primary percutaneous coronary intervention for acute
myocardial infarction: a review of clinical evidence and guidelines. World J Cardiol 2015;7:889–94. https://doi. org/10.4330/wjc.v7.i12.889; PMID: 26730294.
9. Jolly SS, Cairns JA, Lavi S, et al. Thrombus aspiration in patients with high thrombus burden in the TOTAL trial. J Am Coll Cardiol 2018;72:1589–96. https://doi.org/10.1016/j. jacc.2018.07.047; PMID: 30261959.
10. Stone GW, Witzenbichler B, Godlewski J, et al. Intralesional abciximab and thrombus aspiration in patients with large anterior myocardial infarction: one-year results from the INFUSE-AMI trial. Circ Cardiovasc Interv 2013;6:527–34. https://doi.org/10.1161/CIRCINTERVENTIONS.113.000644; PMID: 24084626.
11. Mathews SJ, Parikh SA, Meizger C, et al. Results of CHEETAH study: a prospective, single-arm multicenter study of continuos mechanical aspiration thrombectomy prior to percutaneous coronary intervention. Presented at Transcatheter Cardiovascular Therapeutics (TCT) 2021, Orlando, FL, 4–6 November 2021.
JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY Access at: www.JAPSCjournal.com
Palliative Care in Advanced Heart Failure: A Description of Challenges in the Singapore Experience
Samuel Ji Quan Koh ,
Gillian Li Gek Phua ,
David Kheng Leng Sim
and Shirlyn Hui-Shan Neo
1. Department of Cardiology, National Heart Centre Singapore, Singapore; 2. Division of Supportive and Palliative Care, National Cancer Centre Singapore, Singapore; 3. Lien Centre for Palliative Care, Duke-NUS Medical School, Singapore
Keywords
Advanced heart failure, palliative care, quality of life, Singapore

Disclosure: The authors have no conflict of interests to declare.
Received: 25 February 2022 Accepted: 28 February 2022 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e09.
DOI: https://doi.org/10.15420/japsc.2022.05
Correspondence: Shirlyn Hui-Shan Neo, Division of Supportive and Palliative Care, National Cancer Centre Singapore, 11 Hospital Crescent, Singapore 169610, Singapore. E: shirlyn.neo.h.s@singhealth.com.sg
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Palliative care provides a holistic and comprehensive service that aims to improve the quality of life (QoL) of patients with life-limiting illnesses regardless of prognosis. Patients with advanced heart failure (HF) not only have significant mortality, but also experience debilitating morbidity because of physical, psychosocial, emotional and spiritual issues.
There are several US and European studies that demonstrate significant improvements in the QoL of advanced HF patients after the involvement of palliative care teams. This effect has been demonstrated in various settings, including inpatient, outpatient, primary healthcare and at home.1 5 Even though the evidence for palliative care in HF is robust, in many countries palliative care remains more likely to be delivered earlier in the illness for cancer patients than for non-cancer patients.6 This is reflected in Singapore, where palliative care has been primarily focused on cancer patients, despite the relatively higher prevalence of HF in the country. Up to 4.5% of Singaporeans live with HF as compared to 1–2% in the US and Europe.7 8
Given Singapore’s ageing population and the increasing prevalence of cardiovascular risk factors like hypertension and diabetes, the incidence of HF in Singapore is likely to grow. Recognising the value of palliative care in advanced HF, the 2020 guidelines from the Heart Failure Society (Singapore) advocate for palliative care involvement through early identification and subsequent support of patients with advanced HF.8,9
It is timely and important to understand the challenges of integrating palliative care into the usual care of a HF patient, so as to address these issues and ensure holistic care for all. Given the multiethnic and multicultural society of Singapore, the challenges and experiences identified here would also be applicable to the wider Asia-Pacific region.
Challenges of Integrating Palliative Care in Patients with Advanced Heart Failure
There are four constraints to the timely provision of palliative care to patients with advanced HF.
Cultural Avoidance of End-of-life Conversations
There remains significant cultural avoidance towards conversations pertaining to end-of-life (EOL) care.10 Because of this, patients and their families – and even cardiac specialists – often view the involvement of the palliative care team as defeatist, preferring to defer or even avoid palliative care referrals completely. With this unique cultural overlay, the negative connotation associated with EOL discussions itself presents a barrier to patients and their families receiving the support from the palliative care team.
Difficulty in Identifying Palliative Care Needs and Communicating with Seriously Ill Patients
The acceptability of palliative care in Singapore necessitates that healthcare professionals be equipped with good palliative care knowledge and be advocates for their patients to receive timely interventions to better address the misconceptions or cultural reluctance of palliative approaches. However, the role of palliative care in noncancer patients is still relatively new. Unlike cancer patients, those with advanced HF experience a non-linear deterioration, punctuated with periods of decompensation and exacerbation in their disease trajectory. Consequently, healthcare professionals have the added difficulty of identifying and prognosticating patients with advanced HF who require palliative care, which in turn limits the clarity that patients have regarding their treatment.11
Systemic Barriers to Palliative Care Use
While there have been recent acknowledgements at the national level of the increasing importance of palliative care in Singapore, current policies may need to be further refined to address systemic barriers to palliative care use.12
Current difficulties include the lack of community resources to support home inotrope infusions, as well as financial considerations for inpatient hospice stays because of limited coverage from private insurers.
Heart Failure LETTER © RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com
1
2,3
1
2
Limited Research Into Cultural Appropriateness of Palliative Care Assessment Tools
There is a paucity of research regarding the cultural appropriateness of palliative care tools and interventions in the Asia-Pacific region, and even more so in the Singaporean context. Among the published patientreported and healthcare-reported screening tools outlined in a systematic review by Ament et al., none have been studied in Asia-Pacific region.13 This limits the ability to identify and subsequently monitor the needs of patients with advanced HF in Singapore.
Addressing the Challenges
Some recommended solutions that could help to address these challenges include the following.
Equipping Healthcare Professionals to Communicate with Seriously Ill HF Patients
A recent pilot study has shown that cardiac specialists find communication training for serious illness conversations acceptable in the local setting.14 Further work can be done to explore implementing serious illness conversation training into the curriculum of specialist training.
Understanding the Needs of Local Patients and Caregivers
Recent studies have been published in identifying the care needs of patients with advanced HF unique to Singapore. One significant area facilitating the introduction of palliative care services to patients with advanced HF is the role of formalised advanced care planning (ACP).
A local study by Malhotra et al. demonstrated that ACP led to short-term improvements in decision making, whereby ACP lowered decisional conflict and facilitated discussion of the patients’ preferences with their caregivers.15 The results suggest that in our local cultural context, such processes could serve as an initial avenue for patients to communicate more effectively with their caregivers about their values and goals of care.
While patients with advanced HF with left ventricular assist devices (LVAD) are a minority, they represent a group who would have undergone a prolonged trial of optimal medical therapy and have had to cope with refractory disease for a much longer time. In addition, with the low rates of heart transplant in Singapore, many bridge-to-transplant recipients have had to live with their LVAD long term. Therefore, the experiences they have and those of their caregivers would be invaluable in identifying care needs in our local context.
Two local studies by Neo et al. investigated the long-term changes across the domains of physical, financial, social, psycho-emotional and spiritual for both patients and their caregivers and identified several significant
1. Hopp FP, Zalenski RJ, Waselewsky D, et al. Results of a hospital-based palliative care intervention for patients with an acute exacerbation of chronic heart failure. J Card Fail 2016;22:1033–6. https://doi.org/10.1016/j. cardfail.2016.04.004; PMID: 27079676.
2. Sidebottom AC, Jorgenson A, Richards H, et al. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J Palliat Med 2015;18:134–42. https://doi.org/10.1089/jpm.2014.0192; PMID: 25479182.
3. Rogers JG, Patel CB, Mentz RJ, et al. Palliative care in heart failure: the PAL-HF randomized, controlled clinical trial. J Am Coll Cardiol 2017;70:331–41. https://doi.org/10.1016/j. jacc.2017.05.030; PMID: 28705314.
4. Bekelman DB, Plomondon ME, Carey EP, et al. Primary results of the Patient-Centered Disease Management (PCDM) for heart failure study: a randomized clinical trial. JAMA Intern Med 2015;175:725–32. https://doi.org/10.1001/
findings.16 17 From the patient’s perspective, the authors identified the desire of patients to have an integrated and holistic healthcare support system. Through their illness, patients also yearned to have connection with others and society at large. From the caregiver’s perspective, practical stressors identified that could be addressed included the need for more emotional, social and financial support. It was also recognised that pre-existing personal notions and traditional expectations of the caregiving role have affected their own ways of coping with their circumstances. These insights would be invaluable in the development of culturally appropriate palliative care programmes in future.
Piloting Culturally Adapted Palliative Care Interventions and Assessment Tools
The authors have taken steps to culturally adapt palliative care tools that can help in identifying and tracking needs of local patients with HF, such as the Integrated Palliative Care Outcome Scale (IPOS), which was first developed in the UK. The IPOS is a brief tool that screens heart failure patients for symptoms and concerns. The IPOS was culturally adapted and validated at the National Heart Centre Singapore, for use for local heart failure patients.
A novel, nurse-led supportive care model in the US is the ENABLE CHF-PC programme, which demonstrated that advanced HF patients had improved pain intensity and reduced pain interference with daily life after 16 weeks.18 In line with the Singapore Ministry of Health’s 2020 healthcare strategy of ‘Beyond Hospital to Community’ and ‘Beyond Healthcare to Health’, a culturally adapted version of ENABLE CHF-PC is currently being planned in Singapore (ENABLE-HF-SG; NCT05211882) to investigate the cultural acceptability, feasibility and effectiveness of the programme locally, with the aim of enabling patients to continue living well in the community.
Conclusion
In our local context, more work can be done in identifying appropriate screening tools for directing timely referrals and monitoring needs, evaluating acceptability and effectiveness of palliative care interventions, and supporting cardiac specialists in providing basic palliative care for advanced HF patients. Cardiac specialists can, in turn, help shape patient, caregiver and societal perceptions regarding the acceptability and utility of palliative care interventions. The evaluation of the long-term effects of implementing palliative care interventions into routine care of HF patients and its cost effectiveness should also be examined in future.
Being a relatively new field, the landscape of palliative care in advanced HF in Singapore is a growing and promising area of development that would impact patient care locally, and potentially in the wider Asia-Pacific region as well.
jamainternmed.2015.0315; PMID: 25822284.
5. Ng AYM, Wong FKY. Effects of a home-based palliative heart failure program on quality of life, symptom burden, satisfaction and caregiver burden: a randomized controlled trial. J Pain Symptom Manage 2018;55:1–11. https://doi. org/10.1016/j.jpainsymman.2017.07.047; PMID: 28801001.
6. Boland J, Johnson MJ. End-of-life care for non-cancer patients. BMJ Support Palliat Care 2013;3:2–3. https://doi. org/10.1136/bmjspcare-2013-000446; PMID: 24644316.
7. Huang W, Lee SGS, How CH. Management of the heart failure patient in the primary care setting. Singapore Med J 2020;61:225–9. https://doi.org/10.11622/smedj.2020073; PMID: 32754766.
8. Lam CSP. Heart failure in Southeast Asia: facts and numbers. ESC Heart Fail 2015;2:46–9. https://doi.org/10.1002/ ehf2.12036; PMID: 28834655.
9. Kwok Wing Kui B, Ong YH, Lam Su Ping C, et al. 2020 Clinical
Practice Guidelines on the Diagnosis and Management of Heart Failure. Singapore Heart Failure Society, 2020. https://www. hfss.org.sg/wp-content/uploads/2018/07/Singapore-HeartFailure-Guidelines-2020.pdf (accessed 4 April 2022).
10. Goh SS. Singapore takes six steps forward in “the quality of death index” rankings. Asia Pac J Oncol Nurs 2018;5:21–5. https://doi.org/10.4103/apjon.apjon_66_17; PMID: 29379829.
11. Malhotra C, Wong GCS, Tan BC, et al. Living with heart failure: perspectives of patients from Singapore. Proc Singapore Healthc 2016;25:92–7. https://doi. org/10.1177/2010105815624121
12. Palliative care services to be boosted; topic of death needs open discussion: Ong Ye Kung. https://www.straitstimes. com/singapore/health/palliative-care-services-to-beboosted-topic-of-death-needs-open-discussion-ong-ye (accessed 4 April 2022).
13. Ament SM, Couwenberg IM, Boyne JJ, et al. Tools to help
Palliative Care in Advanced Heart Failure JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Failure
healthcare professionals recognize palliative care needs in patients with advanced heart failure: a systematic review. Palliat Med 2021;35:45–58. https://doi. org/10.1177/0269216320963941; PMID: 33054670.
14. Neo SHS, Zhou JX, Wong GC, et al. Teaching communication micro-skills to cardiologists managing seriously ill patients in Asia: challenges encountered amidst the COVID-19 pandemic and future perspectives. Cureus 2021;13:e19957. https://doi.org/10.7759/cureus.19957; PMID: 34976539.
15. Malhotra C, Sim D, Jaufeerally FR, et al. Impact of a formal
advance care planning program on end-of-life care for patients with heart failure: results from a randomized controlled trial. J Card Fail 2020;26:594–8. https://doi. org/10.1016/j.cardfail.2020.01.015; PMID: 31991216.
16. Neo SH, Ku JSM, Wong GCS, et al. Life beyond heart failure – what are the long-term challenges, supportive care needs, and views toward supportive care of multiethnic Asian patients with left ventricular assist device and their caregivers? J Pain Symptom Manage 2020;60:577–87.e1. https://doi.org/10.1016/j.jpainsymman.2020.03.022;
PMID: 32251690.
17. Neo SHS, Ku JSM, Tan JYT, Yoon S. Lived experiences and long-term challenges and needs of Asian left ventricular assist device caregivers. Palliat Med Rep 2021;2:84–92. https://doi.org/10.1089/pmr.2021.0001; PMID: 34223507.
18. Bakitas MA, Dionne-Odom JN, Ejem DB et al. Effect of an early palliative care telehealth intervention vs usual care on patients with heart failure: the ENABLE CHF-PC randomized clinical trial. JAMA Intern Med 2020;180:1203–13. https://doi. org/10.1001/jamainternmed.2020.2861; PMID: 32730613.
Palliative Care in Advanced Heart
JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Shirley Siang Ning Tan , 1,2 Keng Tat Koh , 3 Alan Yean Yip Fong , 1,3 Mohammad Adam bin Bujang , 1 Lee Len Tiong , 1 Yee Ling Cham , 3 Kian Hui Ho,3 Chen Ting Tan , 3 Chee Sin Khaw , 3 Nor Hanim Mohd Amin , 3 Yen Yee Oon , 3 Asri Said , 3,4 Kent Ter Lau,5 Kar Ying Yong,5 Daniel Cheng Lee Pang,6 Chandan Deepak Bhavnani , 3,6 Ing Tien Wong,7 Francis Eng Pbeng Shu3,8 and Tiong Kiam Ong 3
1. Clinical Research Centre, Sarawak General Hospital, Kuching, Sarawak, Malaysia; 2. Department of Pharmacy, Sarawak General Hospital, Kuching, Sarawak, Malaysia; 3. Department of Cardiology, Sarawak Heart Centre, Kuching, Sarawak, Malaysia; 4. Faculty of Medicine and Health Sciences, University of Malaysia, Kuching, Sarawak, Malaysia; 5. Department of Medicine, Hospital Miri, Miri, Sarawak, Malaysia;
6. Department of Medicine, Hospital Bintulu, Bintulu, Sarawak, Malaysia; 7. Department of Medicine, Hospital Sibu, Sibu, Sarawak, Malaysia;
8. Department of Medicine, Hospital Kapit, Kapit, Sarawak, Malaysia
Abstract
Background: N-terminal pro-brain natriuretic peptide (NT-proBNP) provides prognostic information regarding the risk of death, acute heart failure and the development of AF in patients with acute coronary syndrome. While there are established cut-off values for the association between clinical risk assessment scores and in-hospital mortality, there is no clear cut-off value for NT-proBNP to guide risk stratification in patients with acute MI (AMI). Our study sought to evaluate the cut-off values of NT-proBNP in all-cause mortality post AMI and to compare with other available risk assessment scores. Methods: We conducted a multicentre, prospective, observational study involving 411 patients admitted for AMI. Plasma NT-proBNP was assessed within 24 hours of admission. Results: One-year all-cause mortality occurred in 31 (7.6%) of 411 patients. NT-proBNP ≥404 pg/ml had an area under the curve of 0.66 (95% CI [0.54–0.77]; p=0.004) to predict all-cause mortality at 1 year (sensitivity 80.6%; specificity 36.9%; positive predictive value 9.47%; negative predictive value 95.89%). Using the Youden index, an NT-proBNP level ≥1,995 pg/ml was an independent predictor of all-cause mortality at 1 year (adjusted HR 2.6; 95% CI [1.3–5.5]; p=0.010), regardless of cardiovascular disease risk factors or revascularisation status. There were no significant differences among the predictive values of NT-proBNP, Thrombolysis in MI risk score, Global Registry of Acute Coronary Events risk score and left ventricular ejection fraction in predicting all-cause mortality at 1 year (p>0.05). Conclusion: NT-proBNP level ≥1,995 pg/ml measured within 24 hours of admission for AMI was associated with higher all-cause mortality at 1 year. Randomised controlled trials are needed to further validate the usefulness of NT-proBNP for risk stratification in patients with AMI.
Keywords
Acute MI, Global Registry of Acute Coronary Events risk score, N-terminal pro-brain natriuretic peptide, risk stratification, Thrombolysis in MI risk score
Disclosure: AYYF is an associate editor of Journal of Asian Pacific Society of Cardiology; this did not influence peer review. All other authors have no conflicts of interest to declare.

Acknowledgements: SSNT and KTK contributed equally to the conceptualisation of the manuscript. We acknowledge Alere Health (Malaysia) for supporting this study by providing the NT-proBNP test kits. We thank the patients, nurses, pharmacists, doctors and colleagues in all participating sites who were involved in this study. We express our gratitude to all reviewers for their invaluable comments on the manuscript. We thank the Director General of Health, Malaysia for his permission to publish this article.
Data Availability: The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Authors’ Contributions: Conceptualisation: SSNT, KTK, AYYF, MAB, LLT, YLC, KHH, CTT, CSK, NHMA, YYO, SA, KTL, KYY, DCLP, BCD, ITW, FPES, TKO; data curation: SSNT, KTK, AYYF, MAB, LLT, YLC, KHH, CTT, CSK, NHMA, YYO, SA, KTL, KYY, DCLP, BCD, ITW, FPES, TKO; formal analysis: SSNT, KTK, MAB; funding acquisition: KTK, AYYF; investigation: SSNT, KTK, AYYF, LLT, YLC, KHH, CTT, CSK, NHMA, YYO, SA, KTL, KYY, DCLP, BCD, ITW, FPES, TKO; methodology: SSNT, KTK, AYYF; project administration: SSNT, KTK, AYYF, MAbB, LLT, YLC, KHH, CTT, CSK, NHMA, YYO, SA, KTL, KYY, DCLP, CDB, ITW, FPES, TKO; resources: KTK, AYYF, TKO; software: SSNT, KTK, MAB; supervision: KTK, AYYF, TKO; validation: KTK, AYYF, TKO; visualisation: KTK, AYYF, TKO; writing – original draft preparation: SSNT, KTK, AYYF; writing – review and editing: KTK, AYYF, TKO.
Ethics: This study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Patient Consent: All patients have given written informed consent.
Received: 16 August 2021 Accepted: 17 January 2022 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e10. DOI: https://doi.org/10.15420/japsc.2021.14
Correspondence: Shirley Siang Ning Tan, Level 5, Clinical Research Centre, Sarawak General Hospital, Jalan Hospital, 93586 Kuching, Sarawak, Malaysia. E: tsnshirley@gmail.com
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
© RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com General Cardiology ORIGINAL RESEARCH
NT-proBNP Cut-off Values for Risk Stratification in Acute MI and Comparison with Other Risk Assessment Scores
Risk stratification and prognostication is an important step for the management of acute MI (AMI). The Thrombolysis in MI (TIMI) and Global Registry of Acute Coronary Events (GRACE) risk scores have been demonstrated to have reasonably good discriminatory value, including in Asian populations.1 Other predictors, including reduced left ventricular ejection fraction (LVEF) and poor recovery of LVEF after AMI, have also been long recognised in risk stratification for patients with AMI.2 3
N-terminal pro brain natriuretic peptide (NT-proBNP) is an established biomarker for the diagnosis of heart failure. However, its role for risk stratification in AMI remains debatable. The 2020 European Society of Cardiology guideline on the management of acute coronary syndrome (ACS) states that measurement of brain natriuretic peptide (BNP) or NTproBNP plasma concentrations should be considered to obtain prognostic information (class IIa, level of evidence B).4
While there are established cut-off values for the association between GRACE risk scores and in-hospital mortality, there is no clear cut-off value for NT-proBNP to guide risk stratification in patients with AMI. Moreover, most studies have been conducted in white populations and remain to be validated in Asian populations. In light of differences in genetic background and environmental factors, our study team decided to conduct this study in a local Asian, multi-ethnic population. The study sought to evaluate the NT proBNP cut-off value for predicting 1-year mortality in patients with AMI and to compare this with other available risk scores.
Study Objectives
The primary objective of the study was to explore the cut-off value of NTproBNP to predict 1-year all-cause mortality in AMI patients. The secondary objective was to compare the prognostic value of NT-proBNP with the TIMI and GRACE risk scores and with LVEF.
Methods
This multicentre, prospective observational study was conducted in one cardiology referral centre (Sarawak Heart Centre) and four non-cardiology general hospitals (Sibu Hospital, Miri Hospital, Bintulu Hospital and Kapit Hospital). Informed consent was obtained from each patient. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Malaysia Ministry of Health Medical Research and Ethics Committee (NMRR-16-1195-31525).
Patients and Eligibility Criteria
AMI was defined according to the fourth universal definition of MI.5 The exclusion criteria included age <18 years or >80 years, haemodynamic instability on presentation (cardiogenic shock, septicaemic shock, anaphylactic shock), high bleeding risk (unexplained anaemia, haematocrit <25%, history of blood transfusion within the 14 days prior to screening or active life-threatening bleeding), end-stage renal failure receiving dialysis, active cancer with life expectancy <1 year, pregnancy or being unable to provide informed consent.
Study Procedure
An NT-proBNP point-of-care (POC) test was performed within 24 hours from the diagnosis of AMI. Plasma NT-proBNP level was measured with the Alere Triage NT-proBNP POC test. The analytic range extends from 20 to 35,000 pg/ml, with a range of precision (% coefficient of variation) of 8.4–16.4%.
Approximately 3 ml of blood was drawn by direct venipuncture and collected in an ethylenediaminetetraacetic acid tube. Whole blood was
taken within 24 hours of diagnosis with an MI, irrespective of whether this was before or after percutaneous coronary intervention (PCI). The result was available in approximately 20 minutes.
TIMI Risk Score, GRACE Risk Score and Left Ventricular Ejection Fraction Measurement
For patients with non-ST-elevation MI (NSTEMI), TIMI risk score was assessed with the variables of age ≥65 years, three or more traditional risk factors for vascular disease, known coronary stenosis ≥50%, presence of ≥0.5 mm ST-segment deviation on admission ECG, two or more episodes of angina in the last 24 hours, positive biomarkers and the use of aspirin in the last 7 days.6 For patients with ST-elevation MI (STEMI), TIMI risk score was assessed with the variables of age, diabetes, hypertension or angina, systolic blood pressure <100 mmHg, heart rate >100 BPM, Killip class ≥2, weight >67 kg, anterior ST-elevation or left branch bundle block ECG and time to treatment of >4 hours.7 A TIMI risk score of ≥3 was used as the cut-off for comparison with NT-proBNP in predicting all-cause mortality.
The GRACE risk score was calculated based on the GRACE 2.0 ACS risk calculator using the online calculator or iPhone/Android app.8 Four continuous variables (age, systolic blood pressure, pulse rate, serum creatinine); three binary variables (cardiac arrest at admission, elevated cardiac biomarkers, ST-segment deviation); and one categorical variable (Killip class at presentation) were assessed. A GRACE risk score of ≥140 was used as the cut-off for comparison with NT-proBNP in predicting allcause mortality.
LVEF was measured by transthoracic echocardiogram using EPIQ7 ultrasound system (Philips) with X5-1 transducer. 2D echocardiogram images were obtained from apical four- and two-chamber views. Images were acquired using harmonic imaging at a frame rate of 40–80 frames per second. Manual tracing of left ventricle endocardial borders at end diastole and end systole was performed. Modified biplane Simpson’s rule was used to calculate left ventricular end-diastolic volume, left ventricular end-systolic volume and LVEF. An LVEF of <40% was used as the cut-off for comparison with NT-proBNP in predicting all-cause mortality.
Follow-up and Study Endpoints
All patients received the standard of care for AMI in hospital. Patients were followed up via telephone interviews at the end of 1, 6 and 12 months after discharge. Serial echocardiogram assessments were arranged at 6 and 12 months after discharge. The study endpoint was allcause mortality at 1 year. The Youden index was used to select the optimum cut-offs from the receiver operating characteristic (ROC) curve. The cut-off value was selected if the sensitivity was >80%. If the sensitivity of the cut-off value selected by the Youden index was <80%, then the nearest cut-off value with a sensitivity of ≥80% was selected.
Statistical Analysis
Numerical variables in this study were presented as mean ± SD and median (interquartile range [IQR]). Categorical variables were presented with counts (percentages). For univariate analyses of numerical variables, the independent sample t-test was used for parametric data and Mann–Whitney test in nonparametric variables. Survival analyses were generated using the Kaplan–Meier method, and the differences between the groups were assessed via the log-rank test. A Cox proportional hazard model was used to calculate the crude and adjusted HR with corresponding 95% CI. All analyses in this study were performed using the SPSS software package (Version 20, IBM). A value of p<0.05 was considered significant.
NT-proBNP in AMI JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
(86.1) 290 (87.6) 64 (80.0)
144 (35.0) 120(36.3) 24 (30.0) 0.293
Hypertension 218 (53.0) 175 (52.9) 43 (53.8) 0.887
Diabetes 104 (25. 3) 79 (23.9) 25 (31.3) 0.173
Family history of CVD 98 (23.8) 83 (25.1) 15 (18.8) 0.233
Smoker 290 (70.6) 243 (73.6) 47 (59.5) 0.013
History of MI 36 (8.8) 26 (7.9) 10 (12.5) 0.187
Documented CAD, stenosis ≥50% 46 (11.1) 35 (10.6) 11 (13.8) 0.419
New onset of angina (<2 weeks) 346 (84.2) 288 (87.0) 58 (72.5) 0.001
History of heart failure 9 (2.2) 4 (1.2) 5 (6.3) 0.016
History of stroke 16 (3.9) 8 (2.4) 8(10.0) 0.005
Peripheral vascular disease 1 (0.2) 0 (0) 1 (1.3) 0.195 AF 6 (1.5) 4 (1.2) 2 (2.5) 0.331
Type of AMI: STEMI NSTEMI 241 (58.6) 169 (41.1) 189 (57.3) 141 (42.7) 52 (65.0) 28 (35.0)
0.208
LVEF (%), mean ± SD 51.84 ± 14.42 53.71 ± 13.94 43.78 ± 13.58 <0.001
TIMI risk score, mean ± SD 3.0 ± 1.66 2.79 ± 1.56 3.89 ± 1.81 <0.001 GRACE risk score, mean ± SD 126.52 ± 31.14 122.21 ± 30.17 145.39 ± 29.10 <0.001
Coronary angiogram: LMS TVD 20 (4.9) 99 (24.0) 16 (4.8) 73 (22.1) 4 (5.0) 26 (32.5)
Revascularisation: None Partial Complete
0.556
0.02
155 (37.0) 99 (24.1) 156 (38.6)
116 (35.2) 78 (23.6) 136 (41.2)
39 (48.8) 21 (26.3) 20 (25.0)
Type of revascularisation: PCI CABG 235 (57.2) 20 (4.9) 195 (58.9) 19 (5.7) 40 (50.0) 1 (1.3)
0.214 Days from AMI to revascularisation, mean ± SD 21.64 ± 55.70 18.69 (48.67) 38.09 (83.90) 0.174
All-cause mortality: STEMI NSTEMI
Discharge medications: Aspirin Clopidogrel Statin β-blocker ACE inhibitor ARB Spironolactone
31 (7.6) 16 (3.9) 15 (3.6)
399 (97.1) 372 (90.5) 399 (97.0) 259 (63.0) 219 (53.3) 17 (4.1) 10 (2.4)
18 (5.4) 8 (2.4) 10 (3.0)
321 (98.5) 298 (91.4) 326 (98.5) 203 (62.3) 186 (57.1) 14 (4.3) 6 (1.8)
13 (16.3) 8 (10.0) 5 (6.3)
78 (97.5) 74 (92.5) 73 (91.3) 56 (70.0) 33 (41.3) 3 (3.8) 4 (5.0)
0.001
0.552 0.753 0.003 0.197 0.011 1.000 0.113
Results
A total of 411 patients who presented with AMI to the emergency department between 1 August 2016 and 6 October 2017 were recruited to the study. Mean age was 55.89 ± 10.62 years. In total, 70.6% of patients had a history of smoking, 84.2% had new onset of angina, and 25.3% had diabetes. A total of 58.6% of the patients presented with STEMI. Overall, 74.3% of STEMI patients received fibrinolytic therapy. Of the 411 patients, 255 (62.7%) underwent partial revascularisation (revascularisation to the infarct-related
arteries) or complete revascularisation within 1 year. Of the revascularisations, 235 (92.2%) used PCI while the remaining 20 (7.8%) used coronary artery
grafting (CABG). The mean duration from index event to revascularisation (PCI or CABG) was 21.6 ± 55.7 days (Table 1).
Primary Outcome
all-cause mortality occurred in 31 (7.6%) of 411 patients. Twenty-four patients (5.9%) had cardiac-related death and seven were
NT-proBNP in AMI JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
bypass
One-year
Table 1. Baseline Patient Characteristics All Patients (n=411), n (%) NT-proBNP <1,955pg/ml (n=331), n (%) NT-proBNP ≥1,955pg/ml (n=80), n (%) p-value* Age (years), mean ± SD 55.89 ± 10.624 54.65 ± 10.33 61.03 ± 10.33 <0.001 Male 354
0.077 Dyslipidaemia
*p-values were calculated using Student’s t-test, Pearson’s χ2 test or Fisher’s exact test, as appropriate. ACE = angiotensin-converting enzyme; AMI = acute MI; ARB = angiotensin II receptor blocker; CABG = coronary artery bypass grafting; CAD = coronary artery disease; CVD = cardiovascular disease; GRACE = Global Registry of Acute Coronary Events; IQR = interquartile range; LMS = left main stem involvement; LVEF = left ventricular ejection fraction (from transthoracic echocardiogram); NSTEMI = non-ST-elevation MI; PCI = percutaneous coronary intervention; STEMI = ST-elevation MI; TIMI = Thrombolysis in MI; TVD = triple-vessel coronary artery disease.
due to non-cardiac causes. The median (IQR) duration from the index AMI event to the primary outcome was 40 (9–211) days. The associations between all-cause mortality at 1 year and NT-proBNP level, TIMI risk score, GRACE risk score and LVEF are described in the subsequent sections (Figure 1).
NT-proBNP
Plasma NT-proBNP level was not normally distributed in the study population. The median (IQR) NT-proBNP level was 655 pg/ml (463–4,940 pg/ml; range 20–32,900 pg/ml). The NT-proBNP level was not statistically different between patients presenting with NSTEMI compared with STEMI (1,883.26 ± 4,723.41 pg/ml versus 1,536.75 ± 2,232.86 pg/ml; p=0.322). More patients in the group with STEMI had single- or double-vessel disease (75.9 versus 64.5%), while more patients in the NSTEMI group had triple vessel disease or left main stem involvement (35.5% versus 24.1%, p=0.012).
The ROC curve of the plasma NT-proBNP level for all-cause mortality had an area under the curve (AUC) of 0.66 (95% CI [0.54–0.77]; p=0.004). The first plasma NT-proBNP cut-off level with a sensitivity of 80.6% and
specificity of 36.9% was selected based on the Youden index. Plasma NTproBNP ≥404 pg/ml (frequency of 64.5%) was not associated with allcause mortality (HR 2.4; 95% CI [0.96–5.7]; p=0.062). The second plasma NT-proBNP level with a specificity of >80% (specificity 82.3%, sensitivity 41.9%) was selected using the Youden index. Plasma NT-proBNP level ≥1,995 ng/ml (frequency of 19.5%) was significantly associated with allcause mortality (HR 3.1; 95% CI [1.5–6.4]; p=0.002).
Multivariate analysis showed that NT-proBNP level ≥1,995 pg/ml was an independent predictor for all-cause mortality, regardless of the revascularisation status prior to the events (adjusted HR 2.6; 95% CI [1.3–5.5]; p=0.010) (Table 2). This demonstrated that AMI patients with NTproBNP level ≥1,995 pg/ml on presentation were 2.6 times more likely to have all-cause mortality within 1 year.
was higher all-cause
in the group of patients with NTproBNP <1,995 pg/ml who had medical therapy compared to those who had revascularisation (11.3 versus 2.3%; p=0.001). In the group of patients with NT-proBNP ≥1,995 pg/ml, there was no significant difference in all-
the two groups (20.5 versus 12.2%; p=0.313).
NT-proBNP in AMI JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
There
mortality
cause mortality between
Figure 1: Kaplan–Meier Curves for the Relationship Between 1-Year All-cause Mortality and NT-proBNP, LVEF, TIMI Risk Score and GRACE Risk Score Cumulative hazard Time (days) 0 0.00 0.02 0.04 0.06 0.08 0.10 0.12 A B C 100 200 300 400 NT-proBNP (pg/ml) <1,995 ≥1,995 LVEF (%) ≥40 <40 TIMI risk score <3 ≥3 Cumulative hazard Time (days) 0 0.00 0.02 0.04 0.06 0.08 0.10 100 200 300 400 Cumulative hazard Time (days) 0 0.000 0.025 0.125 0.150 0.100 0.075 0.050 100 200 300 400 D GRACE risk score <140 Cumulative hazard Time (days) 0 100 200 300 400 0.000 0.025 0.125 0.100 0.150 0.075 0.050 ≥140 A: NT-proBNP (cut-off ≥1,995 pg/ml); B: LVEF (cut-off <40%); C: TIMI risk score (cut-off ≥3); D: GRACE risk score (cut-off ≥140). GRACE = Global Registry of Acute Coronary Events; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro-brain natriuretic peptide; TIMI = Thrombolysis in MI.
GRACE risk score† 0.72 0.64–0.80 <0.001
[91.83–97.98]
[0.96–5.7];
2.3 [0.91–5.6]; p=0.08]
94.55 [75.02–83.09] 3.1 [1.5–6.4]; p=0.002 2.6 [1.3–5.5]; p=0.010
[9.19–13.03] 96.69 [93.38–98.37] 3.4 [1.4–8.4]; p=0.007 3.3 [1.3–8.0]; p=0.009 *
70.4 15.79 [12.32–20.01] 96.38 [94.08–97.80] 4.7 [2.2–9.9]; p<0.001 4.8 [2.2–10.2]; p<0.001†
LVEF (%) 0.68 0.55–0.80 0.001 <40 18.8 versus 4.4 53.3 81.6 18.82 [13.49–25.64] 95.62 [93.7–96.98] 4.6 [2.2–9.4]; p<0.001 4.1 [2.0–8.4]; p<0.001
All multivariate analysis were adjusted with fixed independent variables: Presence of dyslipidaemia, diabetes, hypertension, age ≥55 years, smoking history, revascularisation performed, diagnosis of STEMI. Multicollinearity checked. No interaction found. No outliers. *TIMI score was adjusted for revascularisation performed. †GRACE score was adjusted for presence of diabetes, dyslipidaemia, hypertension, smoking history, revascularisation performed. AUC = area under the curve; GRACE = Global Registry of Acute Coronary Events; LVEF = left ventricular ejection fraction; NPV = negative predictive value; NT-proBNP = N-terminal pro-brain natriuretic peptide; PPV = positive predictive value; STEMI = ST-elevation MI; TIMI = Thrombolysis in MI.
NT-proBNP (pg/ml) 0.0634 0.2804 0.00843 0.8893 0.00123 0.9859
GRACE risk score 0.0634 0.2804 0.0549 0.3329 0.0621 0.3506 TIMI risk score 0.00843 0.8893 0.0549 0.3329 0.00719 0.9238 LVEF (%) 0.00123 0.9859 0.0621 0.3506 0.00719 0.9238
GRACE = Global Registry of Acute Coronary Events; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro-brain natriuretic peptide; ROC = receiver operating characteristic; TIMI = Thrombolysis in MI.
Left Ventricular Ejection Fraction, NT proBNP and All-cause Mortality at 1 Year
LVEF was not normally distributed. The mean ± SD for LVEF was 51.84 ± 14.42% (range 12.0–88.0%). The ROC curve of the LVEF for all-cause mortality at 1-year had an AUC of 0.68 (95% CI [0.55–0.58]; p=0.001). LVEF of <40% was selected based on the Youden index with a sensitivity of 53.3% and specificity of 81.60%.
Patients with LVEF <40% had a four times increased risk of all-cause mortality at 1 year. (HR 4.6; 95% CI [2.2–9.4]; p<0.001), regardless of revascularisation status (adjusted HR 4.1; 95% CI [2.0–8.4]; p<0.001; Table 2). NT-proBNP level was inversely associated with lower LVEF (r −0.3, p<0.001). There were 86 patients (20.9%) with LVEF <40% upon admission and 55 (13.4%) with LVEF <40% at 1 year.
TIMI Risk Score, GRACE Risk Score, NT-proBNP and All-cause Mortality at 1 Year
The TIMI risk score ranged from 0 to 9 (mean ± SD 3.0 ± 1.7), and 224 (55.9%) patients had TIMI ≥3. The ROC curve of the TIMI risk score for allcause mortality at 1-year had an AUC of 0.67 (95% CI [0.57–0.76]; p=0.002). Patients with TIMI ≥3 were three times more likely to have all-cause mortality at 1 year (HR 3.4; 95% CI [1.4–8.4]; p=0.007) regardless of the revascularisation status (adjusted HR 3.3; 95% CI [1.3–8.0]; p=0.009).
The GRACE risk score ranged from 52 to 238 (mean ± SD 126.5 ± 31.1). A total of 133 (32.4%) of patients had a GRACE risk score ≥140. The ROC curve of the GRACE risk score for all-cause mortality at 1-year had an AUC
of 0.72 (95% CI [0.64–0.80]; p<0.001). A GRACE risk score ≥140 was significantly associated with all-cause mortality at 1 year (HR 4.7; 95% CI [2.2–9.9]; p<0.001), regardless of the revascularisation status (adjusted HR 4.8; 95% CI [2.2–10.2]; p<0.001). NT-proBNP level was significantly correlated with both TIMI risk score (r = 0.3, p<0.001) and GRACE risk score (r = 0.3, p<0.001).
The ROC curves for NT-proBNP, LVEF, TIMI risk score and GRACE risk score were compared using pairwise comparison. There was no significant difference among the four ROC curves in predicting all-cause mortality at 1 year (Table 3 and Figure 2).9
Discussion
Our study showed that higher NT-proBNP level measured within 24 hours of presentation of AMI was significantly associated with higher all-cause mortality at 1 year. NT-proBNP as a biomarker has the potential to serve as a reasonably good screening tool. A cut-off NT-proBNP level ≥1,995 pg/ml was independently associated with all-cause mortality at 1 year. Patients with acute MI and NT-proBNP level ≥1,995 pg/ml were 2.6 times more likely to have all-cause mortality at 1 year. Using the Youden index, NTproBNP level ≥404 pg/ml had good sensitivity (80.6%) and negative predictive value (95.89%) to predict all-cause mortality at 1 year. However, when adjusted for revascularisation status, it was not significantly associated with the primary outcome. NT-proBNP ≥1,995 pg/ml had a specificity of 82.3%
negative predictive value of 94.55% to predict all-cause
1 year. The association was significant regardless of the
status.
NT-proBNP in AMI JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
and
mortality at
revascularisation
Table 2: Characteristics of NT-proBNP, TIMI Risk Score , GRACE Risk Score and LVEF for Predicting 1-year All-cause Mortality Predictors AUC 95% CI p-value Cut-off Event Rate (%) Sensitivity Specificity PPV [95% CI] NPV [95% CI] Crude HR [95% CI] Adjusted HR [95% CI] NTproBNP (pg/ml) 0.66 0.54–0.77 0.004 ≥404 9.5 versus 4.1 80.6 36.9 9.47 [7.97–11.22] 95.89
2.4
p=0.062
≥1,995 16.3 versus 5.5 41.9 82.3 16.25 [10.84–23.65]
TIMI risk score* 0.67 0.57–0.76 0.002 ≥3 11.0 versus 3.3 80.6 46.3 10.96
≥140 67.7
Table 3. Pairwise Comparison of ROC Curves for NT-proBNP, GRACE Risk Score, TIMI Risk Score and LVEF to Predict All-cause Mortality at 1 Year Difference Between AUC NT-proBNP (pg/ml) p-value GRACE Risk Score p-value TIMI Risk Score p-value LVEF (%) p-value
Considering that higher NT-proBNP level was inversely associated with LVEF and given the fact that 92.2% of the revascularisation in our patients was PCI, in AMI patients with NT-proBNP level ≥1,995 pg/ml, revascularisation with PCI did not improve all-cause mortality. Similarly, medical therapy has comparable all-cause mortality at 1 year to revascularisation with PCI if the NT-proBNP level was ≥1,995 pg/ml.
The EXCEL trial demonstrated that elevated NT-proBNP levels were associated with higher mortality in patients with left main disease who underwent revascularisation, but were not associated with non-fatal major adverse cardiovascular events.10 In a cohort of 6,597 triple vessel coronary artery disease (TVD) patients (CABG, PCI or medical therapy), during a median follow up of 7 years, a higher baseline NT-proBNP level (quartile 4 as cut off; NT-proBNP ≥958.3 pmol/l) was strongly associated with an increased risk of all-cause mortality (adjusted HR 1.12), cardiac death (adjusted HR 1.13) and major adverse cardiac and cerebrovascular events (MACCE; adjusted HR 1.10), p<0.05.
Revascularisation was associated with lower risk of MACCE compared to medical therapy, except for patients in the lowest quartile. The outcomes after PCI versus CABG for revascularisation of left main coronary artery disease may be conditioned by baseline BNP level, with higher BNP levels favouring CABG and lower BNP levels favouring PCI. In our study, 20 (4.9%) and 99 (24%) patients had left main disease and TVD, respectively. There was no significant difference in the severity of coronary artery disease between patients with NT-proBNP level ≥1,995 or <1,995 pg/ml. Therefore, we were not able to find any association with the 1-year allcause mortality.
Nevertheless, early revascularisation guided by risk stratification with NTproBNP could be useful in preventing all-cause mortality at 1 year. In our cohort of patients, the median time from the index AMI event to primary outcome was 40 days. However, only 256 (63%) of the patients had partial or complete revascularisation (92% by PCI) with a mean duration from AMI to revascularisation of 21.6 (± 55.7) days.
All-cause mortality rates, particularly due to cardiac-related pathology, could be improved if an invasive strategy could be undertaken early, i.e. within 2 weeks. This can be a challenge in the clinical setting in a developing country like Malaysia. However, early revascularisation guided
by NT-proBNP level was not associated with improved clinical outcomes in patients with non-ST elevated ACS.
Further randomised controlled trials are needed to investigate the impact of NT-proBNP guided prognostication in patients with AMI. To date, there is no universal prognostic cut-off level for NT-proBNP. Therefore, the NTproBNP cut-off value of ≥1,995 pg/ml may guide future randomised controlled trials.
A study by Kontos et al., which measured serial NT-proBNP during admission and at 1-month post MI, demonstrated that patients who had high/high NT-proBNP (high was defined as >450 pg/ml for age ≤50 years, >900 pg/ml for 50–75 years and >1,800 pg/ml for >75 years) were four times more likely to develop all-cause mortality compared to patients who had low/low NT-proBNP at 2-year follow up.11 In a group of NSTEMI patients, Schellings et al. demonstrated that NT-proBNP and GRACE risk score had good predictive value on 1-month mortality (AUC >0.80) compared with TIMI risk score (AUC = 0.61). Adding NT-proBNP to the GRACE risk score did not have any augmented benefit.12 We did not measure serial NT-proBNP in our study and therefore are unable to comment on the prognostic benefit of serial measurement for NT-proBNP level.
The TIMI and GRACE risk scores are the widely used for risk stratification for patients with ACS. However, these risk scores are not well validated in Asian populations. The TIMI risk score has been shown to underestimate 30-day mortality in STEMI patients with diabetes and renal impairment.13 Souza et al. demonstrated that NT-proBNP had predictive value for cardiacrelated deaths (C-statistic = 0.78), albeit no evidence that NT-proBNP augmented the prognostic value when combined with GRACE risk score.14 In our study, the TIMI risk score, GRACE risk score and NT-proBNP were shown to have comparable predictive value for 1-year all-cause mortality.
Some researchers have established a cut-off of NT-proBNP for predicting mortality post-ACS.11 However, to date there is no universal cut-off and most studies were conducted in a largely white population. With the emergence of new biomarkers, for instance SGLT2, pentraxin-3 and D-dimer, more studies combining biomarkers as prognostic tools in predicting events post MI have been conducted. In this study, we examined the value of NT-proBNP as an independent prognostic tool.15–18 We found that NT-proBNP was an independent predictor for all-cause mortality in post-MI patients. Future studies could be conducted to combine other potential biomarkers to evaluate if there are added advantages when combined to further improve utility as a screening tool.
Our study has some limitations. Firstly, patients with cardiogenic shock on presentation were excluded from the study. These patients represent the highest risk group in AMI. However, these patients require urgent invasive procedures without further risk stratification or prognostication. Therefore, the authors felt that excluding these group of patients from the study represents the most relevant cohort of patients for prognostication.
Secondly, the baseline characteristics for patients divided by NT-proBNP level ≥1,995 pg/ml were heterogenous. Revascularisation was achieved in 62.3% (92% by PCI), with the mean duration to revascularisation of 22 days. There was a high rate of fibrinolytic therapy, which is explained by longer patient referral or transfer from rural healthcare facilities to the cardiac centre in an urban setting. Outcomes of the study might be different if more revascularisation was done with CABG and the duration to revascularisation shorter. The heterogeneity in revascularisation was
NT-proBNP in AMI JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Figure 2: Comparing ROC Curve ReferenceTrue-positive rate False-positive rate 0.0 0.0 0.2 0.4 0.6 0.8 1.0 0.2 0.4 0.6 0.8 1.0 NT-proBNP (pg/ml) TIMI risk score GRACE risk score LVEF (%) GRACE = Global Registry of Acute Coronary Events; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro-brain natriuretic peptide; ROC = receiver operating characteristic; TIMI = Thrombolysis in MI.
reflected in our local practice and may not be generalisable to other settings.
Thirdly, whole blood was taken within 24 hours of MI diagnosis, which could have been before or after revascularisation. Lastly, this study did not include the optimisation of medication therapy during the outpatient visits, which could be a factor determining outcomes. Randomised controlled trials are needed to further evaluate the prognostic value of this cut-off value. Nevertheless, our study provides a guide for future studies using an NT-proBNP cut-off value of ≥1,995 pg/ml.
This study was also conducted in a healthcare system environment where the single cardiology referral centre was located a significant distance from the other four general hospitals, the furthest of which was 800 km by road transfer. Therefore, in such settings – including in Asia – integrating NT-proBNP into the clinical workflow may provide rapid prognostic information to both the immediate healthcare provider and also the referral centre where the AMI patient might continue their care.
A universal cut-off was proposed in this population of patients post MI, STEMI or NSTEMI. NT-proBNP is a biomarker typically associated with the degree of myocardial stretch/stress. Therefore, in a patient presenting with chest pain diagnosed as an AMI early (with an ECG for STEMI or cardiac biomarker of myocardial damage), high NT-proBNP (e.g. >1,995 pg/ml) serves as a clear marker of worse prognosis, thereby improving the risk stratification process. In other words, NT-proBNP at this level independently identifies the patient as being at higher risk for allcause mortality. The rationale of choosing NT-proBNP over other biomarkers and the usual clinical features of myocardial stretch/strain in AMI is because other features characteristic of its clinical manifestations, such as acute pulmonary oedema, can be difficult to evaluate.
Firstly, in multi-ethnic populations – there are more than 30 distinct ethnic groups in Sarawak and Malaysia – language barriers can be an issue because patients may be most fluent in their own native language. It can be challenging to quickly obtain an accurate and reliable clinical history.
1. Chen YH, Huang SS, Lin SJ. TIMI and GRACE risk scores predict both short-term and long-term outcomes in Chinese patients with acute myocardial infarction. Acta Cardiol Sin 2018;34:4–12. https://doi.org/10.6515/ ACS.201801_34(1).20170730B; PMID: 29375219
2. Chew DS, Heikki H, Schmidt G, et al. Change in left ventricular ejection fraction following first myocardial infarction and outcome. JACC Clin Electrophysiol 2018;4:672–82. https://doi.org/10.1016/j.jacep.2017.12.015; PMID: 29798797
3. Multicenter Postinfarction Research Group. Risk stratification and survival after myocardial infarction. N Engl J Med 1983;309:331–6. https://doi.org/10.1056/ NEJM198308113090602; PMID: 6866068
4. Collet JP, Thiele H, Barbato E, et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation and coexistent atrial fibrillation – dual versus triple antithrombotic therapy. Eur Heart J 2021;42:2020–1. https:// doi.org/10.1093/eurheartj/ehaa909; PMID: 33186459
5. Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Circulation 2007;116:2634–53. https:// doi.org/10.1161/CIRCULATIONAHA.107.187397. PMID: 17951284.
6. Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000;284:835–42. https://doi.org/10.1001/jama.284.7.835; PMID: 10938172
7. Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk
Secondly, a chest radiograph, usually when performed in an A-P projection with a portable X-ray system in the emergency department setting, occasionally produces images that are difficult to interpret accurately. Thirdly, direct imaging of the heart such as portable echocardiography to determine left ventricular function and areas of new regional wall motion abnormality, is not universally available in the majority of hospitals in Malaysia, nor in hospitals in similar clinical settings across the Asia-Pacific region.
Therefore, measuring NT-proBNP using a POC system, especially when coupled with a biomarker for acute myocardial injury, provides the attending clinician a rapid tool for objective and quantifiable risk assessment in a patient with an AMI. By doing so, early identification of the patients at the highest risk can enable referral to tertiary centres with greater urgency, or transfer to higher-level-monitored beds at their own centres, both of which are typically in relatively short supply in most hospitals in Malaysia and the wider Asia-Pacific region.
Conclusion
NT-proBNP level ≥1,995 pg/ml measured within 24 hours upon admission for AMI was associated with higher all-cause mortality at 1 year. The predictive value for all-cause mortality was comparable to other risk assessment scores and LVEF. Randomised controlled trials will be needed to validate the usefulness of NT-proBNP for risk stratification in patients with AMI.
Clinical Perspective
• N-terminal pro-brain natriuretic peptide was an independent predictor of all-cause mortality in patients post MI in this study.
• N-terminal pro-brain natriuretic peptide measurement, using a point-of-care system, provides the attending clinician a rapid tool for risk assessment in a patient with an acute MI.
• This facilitates decision making regarding treatment strategy and enables referrals to tertiary centres with greater urgency.
score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation 2000;102:2031–7. https:// doi.org/10.1161/01.CIR.102.17.2031; PMID: 11044416
8. GRACE risk score calculator. https://www.mdcalc.com/graceacs-risk-mortality-calculator (accessed 13 March 2022).
9. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. https://doi.org/10.2307/2531595; PMID: 3203132
10. Redfors B, Chen S, Crowley A, et al. B-type natriuretic peptide assessment in patients undergoing revascularization for left main coronary artery disease: analysis from the EXCEL trial. Circulation 2018;138:469–78. https://doi.org/10.1161/CIRCULATIONAHA.118.033631; PMID: 29666071
11. Kontos MC, Lanfear DE, Gosch K, et al. Prognostic value of serial N-terminal pro-brain natriuretic peptide testing in patients with acute myocardial infarction. Am J Cardiol 2017;120:181–5. https://doi.org/10.1016/j. amjcard.2017.04.004; PMID: 28599802
12. Schellings DA, Adiyaman A, Dambrink JE, et al. Predictive value of NT-proBNP for 30-day mortality in patients with non-ST-elevation acute coronary syndromes: a comparison with the GRACE and TIMI risk scores. Vasc Health Risk Manag 2016;12:471–6. https://doi.org/10.2147/VHRM.S117204; PMID: 27920547
13. Selvarajah S, Fong AY, Selvaraj G, et al. An Asian validation of the TIMI risk score for ST-segment elevation myocardial infarction. PLOS One 2012;7:e40249. https://doi.org/10.1371/ journal.pone.0040249; PMID: 22815733
14. Souza TMB, Cerqueira Jr AMS, Suerdieck JG, et al. Prognostic value of NT-proBNP versus Killip classification in patients with acute coronary syndromes. Arq Bras Cardiol 2020;114:666–72. https://doi.org/10.36660/abc.20180345, PMID: 32074200
15. Choi S, Park D, Lee S, et al. Cut-off values of B-type natriuretic peptide for the diagnosis of congestive heart failure in patients with dyspnoea visiting emergency departments: a study on Korean patients visiting emergency departments. Emerg Med J 2007;24:343–7. https://doi. org/10.1136/emj.2006.041368; PMID: 17452702
16. Gupta DK, de Lemos JA, Ayers CR, et al. Racial differences in natriuretic peptide levels: the Dallas Heart Study. JACC Heart Fail 2015;3:513–9. https://doi.org/10.1016/j. jchf.2015.02.008; PMID: 26071618
17. Nakata K, Komukai K, Yoshii Y, et al. The optimal cut-off value of plasma BNP to differentiate heart failure in the emergency department in Japanese patients with dyspnea. Intern Med 2015;54:2975–80. https://doi.org/10.2169/ internalmedicine.54.4786; PMID: 26631879
18. Zagidullin N, Motloch LJ, Gareeva D, et al. Combining novel biomarkers for risk stratification of two-year cardiovascular mortality in patients with ST-elevation myocardial infarction. J Clin Med 2020;9:550. https://doi.org/10.3390/jcm9020550; PMID: 32085400
NT-proBNP in AMI JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Role of Lipoprotein(a) in Cardiovascular Disease: A Review of Clinical Practice
Department of Cardiovascular Medicine, Kitasato University School of Medicine, Sagamihara, Japan

Abstract
The association between elevated lipoprotein(a) (Lp(a)) and an increased risk of cardiovascular disease has been demonstrated. Although the impact of Lp(a) plasma level on the onset of disease depends on the type of disease and the patient’s comorbidities, an Lp(a) plasma level >1.29 mmol/l (50 mg/dl) has been proposed as a practical cut-off. Approximately 10% of the general Asian population may have Lp(a) >1.29 mmol/l, as do 15–30% of the global population. An alternative cut-off for Asian populations may be 0.78 mmol/l (30 mg/dl). Measurements may have to be considered at least once in each adult’s lifetime, particularly for people with a family or personal history of premature atherosclerotic cardiovascular disease. Although plasma level is mostly consistent throughout life, some therapies, such as proprotein convertase subtilisin/ kexin type 9 inhibitors and antisense oligonucleotides, may reduce the Lp(a) plasma level.
Keywords
Atherosclerosis, cholesterol, coronary artery disease, ischaemic stroke, statin
Disclosure: JA is a deputy editor of Journal of Asian Pacific Society of Cardiology; this did not influence peer review. All other authors have no conflicts of interest to declare.
Received: 22 November 2021 Accepted: 17 January 2022 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e11.
DOI: https://doi.org/10.15420/japsc.2021.31
Correspondence: Yoshiyasu Minami, Department of Cardiovascular Medicine, Kitasato University School of Medicine, 1-15-1 Kitasato, Minami-ku, Sagamihara, Kanagawa 252-0373, Japan. E: nrg12391@yahoo.co.jp
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
The lipoprotein(a) (Lp(a)) molecule consists of an apolipoprotein (Apo) B-containing LDL-like segment and a plasminogen-like glycoprotein Apo(a) segment. An increased risk of cardiovascular disease, including acute coronary syndrome, ischaemic stroke and aortic stenosis, in patients with high plasma Lp(a) has been demonstrated in clinical studies, genome-wide association studies and Mendelian randomisation studies.1–4 The increased risk is often explained by the enhanced progression of atherosclerosis in the arterial system through the proatherogenic and anti-fibrinolytic nature of Lp(a) in combination with other factors, including age, sex, ethnicity, comorbidities and LDL cholesterol level. The independent association between elevated Lp(a) and an increased risk of cardiovascular events has been demonstrated, therefore the role of residual risk in patients with statin-based conventional therapy has been emphasised.2 The level of Lp(a) is established during childhood and is mostly consistent throughout the lifetime because it is regulated primarily by the LPA gene locus. However, several therapies, including proprotein convertase subtilisin/ kexin type 9 (PCSK9) inhibitors, inclisiran and antisense oligonucleotides, have been reported to reduce the plasma level.5–8 Approximately 20–30% of people have elevated Lp(a), so more clinical attention is required.9
In this review, we summarise the clinical impact of elevated Lp(a), the evaluation of Lp(a) in daily practice, and the potential therapeutic approaches for high Lp(a) (Figure 1).
Epidemiology of High Plasma Lp(a)
The distribution of plasma Lp(a) level is skewed and ranges widely between individuals, with no sex differences.3 Plasma concentration is
determined primarily by the LPA gene locus, without significant acquired or environmental influences.10 Patients with established cardiovascular disease had higher levels of Lp(a) (Figure 2). There are ethnic differences in median Lp(a) level between countries caused by the difference in the prevalence of LPA single nucleotide polymorphisms and Apo(a) isoforms.11,12 The median Lp(a) level in the general Asian population is 0.21–0.36 mmol/l, which is lower than that in black (1.01 mmol/l), Hispanic (0.49 mmol/l) and Arab (0.39 mmol/l) populations.11,13–15
Cut-off of Lp(a) for Risk Stratification
Several practical cut-offs for Lp(a), including 0.78 mmol/l (30 mg/dl) and 1.29 mmol/l (50 mg/dl) for the risk stratification of coronary artery disease (CAD) and ischaemic stroke, have been proposed. The Copenhagen City Heart Study (n=7,524) found that Lp(a) >0.78 mmol/l is associated with an increased risk of MI in a dose-dependent manner, with an adjusted HR of 1.2 (95% CI [0.9–1.6]) for Lp(a) 0.13–0.75 mmol/l (5–29 mg/dl), 1.6 (95% CI [1.1–2.2]) for Lp(a) 0.78–1.97 mmol/l (30–76 mg/dl), 1.9 (95% CI [1.2–3.0]) for Lp(a) 1.99–3.03 mmol/l (77–117 mg/dl), and 2.6 (95% CI [1.6–4.1]) for Lp(a) >3.03 mmol/l (117 mg/dl) versus for Lp(a) <0.13 mmol/l (5 mg/dl).3
A meta-analysis of 126,634 participants showed that the risk of MI started to increase steeply at Lp(a) >0.62 mmol/l, and curvilinearly increased according to the increase in Lp(a) and became significant at around 1.24 mmol/l.2 A genome-wide association study also showed a steep increase in risk at >0.78 mmol/l and an added risk at >1.22 mmol/l.16 Current guidelines in the US, Canada and Europe define 1.29 mmol/l (50 mg/dl) as a risk-enhancing factor.17–19
© RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com Clinical Cardiology REVIEW
Yoshiyasu Minami , Daisuke Kinoshita, Yusuke Muramatsu, Takako Nagata and Junya Ako
Globally, the estimated number of people with elevated Lp(a) is >1 billion. Approximately 10% of the general Asian population may have Lp(a) >1.29 mmol/l, compared to 15–30% of the global population.20 A recent report investigated ethnic differences in the HR of high Lp(a) (>1.29 mmol/l) for the incidence of MI.15 The HR in Chinese, Southeast Asian and South Asian populations is reported as 1.62, 1.83, and 2.14, respectively, while it is 1.36 in European and 1.67 in Latin American populations. Although few reports have focused on the practical cut-off point of Lp(a) for Asian populations, Lp(a) ≥0.78 mmol/l (30 mg/dl) may be more suitable for the Chinese population.20,21 Several studies have suggested that elevated Lp(a) remains a risk factor for cardiovascular disease, even in patients with LDL cholesterol <1.81 mmol/l.22–24 Meanwhile, a recent study reported that the association of elevated Lp(a) and the risk of cardiovascular disease is attenuated in a primary prevention setting at LDL cholesterol <2.5 mmol/l.25
Impact of Elevated Lp(a) on the Incidence of Cardiovascular Disease
Elevated Lp(a) is associated with an increased risk of premature onset of CAD, ischaemic stroke, peripheral artery disease (PAD) and aortic valve degradation.2,26–28 The association of elevated Lp(a) and an increased incidence of recurrent cardiovascular disease in patients treated with statin and percutaneous coronary intervention (PCI) has been also reported.29–32
Elevated Lp(a) and Coronary Artery Disease
In the general population, a significant increase in the risk of coronary death and non-fatal MI has been observed in people with Lp(a) >1.29 mmol/l (50 mg/dl).2 In patients with established CAD, elevated Lp(a) increases the risk of recurrent clinical events, particularly in those with LDL cholesterol ≥3.37 mmol/l.33,34 The increased risk of recurrent cardiovascular events, according to the increase in Lp(a), has been shown even in patients treated with statins.29 The increased incidence of CAD in people with elevated Lp(a) is often explained by the higher prevalence of coronary plaque through the effect of the proatherogenic properties of Lp(a) particles.35,36 The association of elevated Lp(a) with a greater number and degree of coronary stenosis on angiography, a larger number of
stenoses on CT, and a greater change in the plaque-plus-media area on serial intravascular ultrasound has also been reported.35–37 Chieng et al. investigated the impact of Lp(a) and LDL cholesterol levels on the angiographic disease severity assessed using the SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) score in patients with premature CAD admitted to hospital in Australia.38 They showed that patients with both elevated Lp(a) and elevated LDL cholesterol comprised the majority of patients in the highest SYNTAX tertile, while patients with non-elevated Lp(a) and non-elevated LDL cholesterol were predominant in the lowest SYNTAX tertile.38
Muramatsu et al. investigated the impact of Lp(a) and LDL cholesterol level on the prevalence of vulnerable coronary plaque using optical coherence tomography in a Japanese population.39 They noted an increased prevalence of thin-cap fibroatheroma in the culprit coronary plaque with an increase in Lp(a) level, particularly in patients with LDL cholesterol ≥2.59 mmol/l.39 The increased incidence of recurrent cardiovascular events after PCI in patients with elevated Lp(a) has been shown in a Japanese cohort with chronic kidney disease and diabetes.30,31
A recent report from Korea has also shown the impact of elevated Lp(a) on the increased incidence of recurrent ischaemic cardiovascular events including repeated revascularisation and ischaemic stroke after PCI.32 The authors showed that the HR of recurrent events in patients with Lp(a) ≥0.78 mmol/l (30 mg/dl) during a median follow-up of 7.4 years was 1.17 (95% CI [1.05–1.30]; p=0.004). They noted that the enhanced proliferation of smooth muscle cells in addition to accelerated atherosclerosis via elevated Lp(a) might be a contributing factor.
Elevated Lp(a) and Peripheral Artery Disease
A modest but significant association between Lp(a) and PAD was noted in a study of Mendelian randomisation.26 The authors showed that increases in genetically predicted Lp(a) were associated with an increased risk of PAD (OR 1.04 per 0.26 mmol/l increase in Lp(a)). They also found that the association was not attenuated in multivariable analyses, accounting for the association of these genetic variants with ApoB. Further studies are needed to confirm the impact of elevated Lp(a) on the increased incidence of PAD.
Elevated Lp(a) and Stroke
A recent meta-analysis showed a significant association between elevated Lp(a) and an increased risk of ischaemic stroke compared with control subjects.27 The association between elevated Lp(a) and the increased risk of ischaemic stroke was also demonstrated in Asian patients.40–42 The meta-analysis further showed that elevated Lp(a) was significantly associated with an increased risk of the large artery atherosclerosis subtype as well as an increased risk of intracerebral haemorrhage. Most of the studies included in the meta-analysis used 0.52–1.04 mmol/l (20–40 mg/dl) as the cut-off for elevated Lp(a).
A recent prospective and observational study investigated the relationship between acute ischaemic stroke and serum Lp(a) level in the Chinese population.41 The authors reported a significant difference in median serum Lp(a) between patients with acute ischaemic stroke and control cases (0.85 versus 0.39 mmol/l [33 versus 15 mg/dl]; p=0.000). The authors also reported that elevated Lp(a) was an independent factor for stroke, and serum Lp(a) ≥0.78 mmol/l (30 mg/dl) was associated with a 2.23-fold increase in acute ischaemic stroke when adjusting for other possible risk factors. The pathogenesis of an increased incidence of ischaemic stroke in people with elevated Lp(a) could be explained by
Lipoprotein(a) and Cardiovascular Disease JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Figure 1: Lipoprotein (a) and Cardiovascular Disease ApoB LDL N - SC Apo(a) Lp(a) >1.293 mmol/l (50 mg/dl): practical cut-o for risk stratification 0 7758 mmol/l (30 mg/dl) may be an alternative cut-o for Asian populations Lipoprotein (a): Lp(a) Venous thromboembolism? Peripheral artery disease? Heart failure? Reduced incidence of cardiovascular disease? …has not been demonstrated in dedicated randomised trials Indications for measurement: • Established CVD • Family history of premature CVD • Screening (at least once in the lifetime of each adult person) Risk: • Coronary artery disease • MI • Aortic valve degradation • Ischaemic stroke Potential Lp(a)-lowering therapy: • PCSK9 inhibitors • Antisense oligonucl otides • Apheresis • Inclisiran? The Lp(a) molecule consists of an apolipoprotein (Apo) B-containing LDL-like segment and a plasminogen-like glycoprotein Apo(a) segment connected to each other by a disulphide bridge (-S-). CVD = cardiovascular disease; Lp(a) = lipoprotein (a); PCSK9 = proprotein convertase subtilisin/ kexin type 9.
advanced carotid atherosclerosis.43,44 The association between increased Lp(a) and a higher prevalence of vulnerable plaque characteristics in the carotid artery in patients with symptomatic carotid artery stenosis has been demonstrated in a previous study.45
Elevated Lp(a) and Aortic Valve Disease
The association between elevated Lp(a) and calcification and stenosis of the aortic valve has been reported in several studies.46,47 A study combining two prospective studies of the general population showed that elevated Lp(a) and the corresponding genotypes were associated with an increased risk of aortic valve stenosis in the general population, with Lp(a) >2.33 mmol/l (90 mg/dl) predicting a threefold higher risk.28 A recent study with serial observations using multiple modalities showed that patients in the highest Lp(a) tertile had a higher progression of valvular calcification, faster haemodynamic progression, and a higher risk of aortic valve replacement and death compared with patients in the lower tertiles.48 Increased alkaline phosphatase activity, hydroxyapatite, cell apoptosis and phosphorylation of signal transduction proteins have been proposed as a conceivable pathway of aortic valve degradation by elevated Lp(a).49
Although the ethnic difference in the effect of elevated Lp(a) on degradation of the aortic valve remains unclear, a recent study reported that there were no associations between Lp(a) level and the extent of aortic valve calcification in South Asian people, although the association was seen in white people and black people.50
Indications for Lp(a) Measurement
The potential of Lp(a) measurement as a tool for the stratification of risk of future cardiovascular events was shown in a study of 826 people in the general population.51 Elevated Lp(a) was associated with an increased risk of cardiovascular disease over a 15-year follow-up period: the adjusted HR for cardiovascular events was 1.37 per 1 SD higher level of Lp(a) (SD=0.83 mmol/l [32 mg/dl]) and 2.37 compared with the top fifth quintile. The evaluation of Lp(a) has been shown to improve the quality of risk stratification in the general population with borderline risk, defined using the Framingham risk score.25,52
Measurements of Lp(a) enable the reclassification of the risk of cardiovascular disease and facilitate shared decision-making for the
initiation of treatment, especially in younger patients, particularly those with a family history of premature cardiovascular disease. Current guidelines from Europe and North America recommend measuring Lp(a) in patients with a family history of premature cardiovascular disease, with Lp(a) >1.29 mmol/l (50 mg/dl) considered a risk-enhancing factor.17–19 A Mendelian randomisation study showed that extremely elevated Lp(a) (>5.172 mmol/l [200 mg/dl]) is associated with a three- to fourfold increased risk of cardiovascular disease, representing a similar lifetime risk of cardiovascular disease in heterozygous familial hypercholesterolaemia.53
Although it remains unclear whether universal testing of Lp(a) is recommended for healthy people regardless of the presence of a family history, the potential causal association between Lp(a) and future cardiovascular events has been shown in several Mendelian randomisation studies, which could justify universal screening.1–4 Some of the current guidelines recommend Lp(a) measurement at least once in the lifetime of each adult person to identify people who may have inherited extremely high Lp(a) equivalent to the risk associated with familial hyperlipidaemia.18,19 In patients with established cardiovascular disease, elevated Lp(a) is associated with an increased incidence of recurrent cardiovascular events irrespective of LDL cholesterol.6,54 However, no randomised controlled trial has demonstrated a significant reduction in recurrent clinical events through lowering of Lp(a). Therefore, plasma Lp(a) cannot be a treatment goal in current clinical practice, and the serial measurement of Lp(a) is not recommended in guidelines.17–19 The possible indications for Lp(a) measurement in daily clinical practice are listed in Table 1
Issues of Lp(a) Measurement
Lp(a) level may sometimes need to be interpreted with caution. Because of the wide range in the size of the Apo(a) segment and lipid content, the molecular mass of the Lp(a) particle also varies widely. The size of the Apo(a) segment depends on the number of kringle (KIV2) repeats, which is genetically determined.55 This may cause an ethnic difference in the size distribution of the Lp(a) molecule and raise difficulties in the application of results to patients of a different ethnicity.56 Although most currently available assays provide acceptable accuracy in the measurement of Lp(a) level to differentiate high-risk patients, the system of different assays
requires standardisation
calibration.1,56
Lipoprotein(a) and Cardiovascular Disease JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
still
and appropriate
Figure 2: Lipoprotein(a) Levels in Patients with Acute Coronary Syndrome Number of patients 0 20 40 60 80 Lp(a) (mmol/l) 0 0.26 0.52 0.78 1.04 1.30 1.56 1.82 2.08 2.34 2.60 2.86 3.12 3.38 3.64 3.90 4.16 4.42 4.66 Lp(a) was measured in 342 consecutive patients with acute coronary syndrome requiring percutaneous coronary intervention between January 2016 and December 2020 at Kitasato University Hospital, Japan (Minami Y, 2021, unpublished data). The median Lp(a) level is 0.41 mmol/l (IQR, 0.21–0.83 mmol/l; 16.0 mg/dl; IQR, 8.0–32.0 mg/dl). A total of 26.3% of patients had Lp(a) >0.78 mmol/l (30 mg/dl) and 10.5% had Lp(a) >1.29 mmol/l (50 mg/dl). Lp(a) = lipoprotein (a).
Table 1: Lp(a) Measurement in Daily Clinical Practice
Cohort Indications for Lp(a) Measurement
Universal screening at least once in the lifetime of each adult person
Should be considered18,19
Elevated Lp(a) is associated with an increased risk of future cardiovascular events. The Lp(a) level is mostly consistent throughout an individual’s lifetime
starting statin therapy.34,60–64 A meta-analysis showed that statin increased Lp(a) by 8.5–19.5% from baseline, although it remains to be elucidated whether the statin-mediated increase in Lp(a) contributes to the increased risk of clinical events.65 Another meta-analysis found that statins may modify the association between Lp(a) and the risk of cardiovascular disease.29 In the analysis, Lp(a) level was shown to be more strongly associated with an increased risk of cardiovascular disease in patients with statins than in those with placebo.29
People with a family history of premature CVD
Recommended17–19
Elevated Lp(a) might facilitate shared decision-making for initiation of treatment
Patients with established CVD Should be considered19
Elevated Lp(a) is associated with recurrent cardiovascular events
Serial measurement of Lp(a) Not recommended17–19
Lp(a) level is mostly consistent through an individual’s lifetime. Its role as a treatment target has not been established
CVD = cardiovascular disease; Lp(a) = lipoprotein(a).
Table 2: Potential Therapies for Lp(a) Lowering
Potential Therapy Lp(a) Level Risk Reduction by Lp(a) Lowering
Statin 9–20% increase Niacin 20–30% decrease Unknown
PCSK9 inhibitors 23–30% decrease Further risk reduction independently from LDL cholesterol reduction was reported in sub-analyses6
Inclisiran 14–26% decrease (NS) Unknown
Mipomersen (ASOs) 24–32% decrease Unknown Apo(a)-LRx (ASOs) 35–91% decrease Ongoing trial (NCT04023552) in patients with a history of CVD and Lp(a) >1.81 mmol/l (70 mg/dl)
Lipoprotein apheresis 70% decrease 94% reduction in an observational study79
ASO = antisense oligonucleotide; CVD = cardiovascular disease; Lp(a) = lipoprotein(a); NS = not significant; PCSK9 = proprotein convertase subtilisin/kexin type 9.
Potential Approaches for Lowering Lp(a)
There have been no dedicated randomised clinical trials on the impact of Lp(a) lowering on the reduction of cardiovascular disease. Although the pharmacological mechanisms are unclear, a potentially significant decrease in plasma Lp(a) by several drugs has been reported.7,8,57 For primary prevention in people with intermediate risk, defined as a cardiovascular disease risk of 7.5–20% calculated using the Framingham risk score, elevated Lp(a) may be considered a risk-enhancing factor to start statin therapy.17,58 In high-risk or very-high-risk patients with LDL cholesterol ≥6.98 mmol/l on maximally tolerated statin treatment, it might be reasonable to consider more intensive treatment based on elevated Lp(a).59 In the setting of secondary prevention, the presence of a high Lp(a) level is strongly predictive of recurrent cardiovascular events and suggests the need for more intensive therapies, including PCSK9 inhibitors. Table 2 summarises the current status of potential therapies for Lp(a) lowering and subsequent reduction of cardiovascular events.
Statins
The role of statins in the primary and secondary prevention of cardiovascular events has been established. However, several prospective statin studies have shown a significant increase in plasma Lp(a) after
Niacin
Niacin, also known as vitamin B3, is an essential vitamin in the human body. Niacin can decrease total cholesterol, LDL cholesterol and triglyceride levels, and increase HDL cholesterol in large doses. Its lipidlowering function is associated with the inhibition of hormone-sensitive lipase activity in adipose tissue, reduction of free fatty acid entry into the liver, and decrease in very-low-density lipoprotein secretion.66 Niacin also reduces Lp(a) levels by 20–30% by inhibiting the LPA promoter.23 However, HPS2-4THRIVE, a double-blind randomised trial, demonstrated that niacin failed to reduce cardiovascular events in patients with CAD. The addition of niacin to statin treatment resulted in significant increases in adverse effects, including disturbances in diabetes control, dysplasia, diarrhoea, myopathy, serious infection and skin ulcerations.67 The combination of niacin and statins in lipid-lowering therapy is no longer recommended in most countries.18
PCSK9 Inhibitors
PCSK9 inhibitors have been shown to reduce Lp(a) by 7–36%.54,57,68 This effect is consistent and reproducible for both alirocumab and evolocumab, although the pharmacological mechanism of reduction of Lp(a) by PCSK9 inhibitors remains unclear.69,70 In the FOURIER trial that included patients with cardiovascular disease and maximum tolerated dose of statin, evolocumab significantly reduced Lp(a) by a median of 26.9% at 48 weeks.54 Evolocumab has been shown to reduce the risk of cardiovascular events by 23% in patients with high Lp(a) and by 7% in those with low Lp(a). This indicates that the higher the baseline Lp(a), the greater the potential benefit of PCSK9 inhibitors in reducing cardiovascular events.54 A subanalysis of the Odyssey Outcomes trial showed that alirocumabinduced reductions of Lp(a) independently predicted lower risk of recurrent cardiovascular events, after adjustment for baseline concentrations of both LDL cholesterol and Lp(a) and demographic and clinical characteristics (i.e. a 0.03 mmol/l [1 mg/dl] reduction in Lp(a) with alirocumab was associated with a HR of 0.994 for recurrent cardiovascular events).6
Inclisiran
A potential for Lp(a) lowering by inclisiran has been reported although the pharmacological mechanism remains unclear. Inclisiran is a small interfering RNA (siRNA) molecule that reduces LDL cholesterol through the inhibition of PCSK9 synthesis. The safety and efficacy of inclisiran given twice a year by subcutaneous injection have been demonstrated.7,71,72 The current ongoing randomised trial ORION-4 (NCT03705234) will provide information on the effect of inclisiran on the incidence of cardiovascular events. In addition to the reduction of LDL cholesterol levels by 52.3% without serious adverse effects, inclisiran also reduced Lp(a) by 14–26%, although this did not reach statistical significance.7,73
Antisense Oligonucleotides
Mipomersen is an antisense oligonucleotide (ASO) that is designed to inhibit the synthesis of the ApoB100 protein and which produces a modest reduction in Lp(a). The percentage change in LDL cholesterol and Lp(a) with mipomersen therapy was −24.0% and −31.6%, respectively.74 Apo(a)-
Lipoprotein(a) and Cardiovascular Disease JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
L Rx is also an ASO, and it is rapidly and specifically taken up by hepatocytes and inhibits the synthesis of Apo(a).75,76 Apo(a)-LRx has been shown to reduce Lp(a) by 66–91% in a dose-dependent manner in healthy participants with elevated Lp(a), as well as in patients with established cardiovascular disease and elevated Lp(a).8,77
In a randomised trial of patients with established cardiovascular disease who had Lp(a) ≥1.55 mmol/l (60 mg/dl) on screening, Apo(a)-LRx at a dose of 20 mg weekly for 6 months resulted in an 80% reduction in Lp(a) level, and 98% of the patients on this dose attained an Lp(a) level <1.29 mmol/l (50 mg/dl).8 Another randomised trial compared the Lp(a)-lowering and anti-inflammatory effects of Apo(a)-LRx with PCSK9 inhibitors.78 In that study, the percent change in Lp(a) in patients with Apo(a)-LRx and PCSK9 inhibitors was −46.6% and −16.1%, respectively. Furthermore, potent and highly selective Lp(a) lowering by Apo(a)-LRx resulted in the reduction of inflammatory gene expression in circulating monocytes in patients with cardiovascular disease. In contrast, PCSK9 inhibitors did not alter Lp(a)induced proinflammatory profiles.78 These findings support the potential benefit of improving clinical outcomes through a greater reduction of Lp(a) in patients with established cardiovascular disease. The current Phase III study (HORIZON; NCT04023552) will clarify the treatment effect of Apo(a)-LRx on clinical outcomes in patients with cardiovascular disease.
Lipoprotein Apheresis
Lipoprotein apheresis (LA) is the final escalating option to reduce blood LDL cholesterol levels in patients with severe hypercholesterolaemia, such as familial hypercholesterolaemia or other forms of hypercholesterolaemia resistant to or intolerant to lipid-lowering medication. LA should be considered for patients with elevated Lp(a) and progressive cardiovascular disease and is the only FDA-approved therapy to lower Lp(a) in the US. LA reduces Lp(a) by 70% in patients with elevated Lp(a) and cardiovascular disease.79 Although no randomised controlled studies have been conducted to demonstrate the effectiveness of LA in reducing cardiovascular events, LA has been shown to be associated with a 94% reduction in cardiovascular events over a mean treatment period of 48 months.79 In the Pro(a)LiFe study, patients with high Lp(a) were prospectively followed, and the incidence rates of cardiovascular events
1. Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol 2017;69:692–711. https://doi.org/10.1016/j. jacc.2016.11.042; PMID: 28183512.
2. Emerging Risk Factors Collaboration, Erqou S, Kaptoge S, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 2009;302:412–23. https://doi.org/10.1001/jama.2009.1063; PMID: 19622820.
3. Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA 2009;301:2331–9. https://doi.org/10.1001/jama.2009.801; PMID: 19509380.
4. Clarke R, Peden JF, Hopewell JC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med 2009;361:2518–28. https://doi. org/10.1056/NEJMoa0902604; PMID: 20032323.
5. Schmidt K, Noureen A, Kronenberg F, Utermann G. Structure, function, and genetics of lipoprotein (a). J Lipid Res 2016;57:1339–59. https://doi.org/10.1194/jlr.R067314; PMID: 27074913.
6. Bittner VA, Szarek M, Aylward PE, et al. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol 2020;75:133–44. https://doi.org/10.1016/j.jacc.2019.10.057; PMID: 31948641.
7. Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med 2020;382:1507–19. https://doi.org/10.1056/ NEJMoa1912387; PMID: 32187462.
8. Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, et al. Lipoprotein(a) reduction in persons with cardiovascular
between 2 years before and 2 years after LA treatment were compared. The mean rate of cardiovascular events declined from 0.41 for 2 years before LA to 0.09 for 2 years during LA.80 LA effectively lowered the incidence of cardiovascular events with a significant reduction in Lp(a). Both the German and UK apheresis guidelines use Lp(a) >1.55 mmol/l (60 mg/dl) to specifically allow reimbursement for patients with isolated Lp(a) elevation and recurrent cardiovascular events or in conjunction with uncontrolled elevated LDL cholesterol.81,82
Conclusion
Several scientific approaches, including observational studies, genetic studies, and Mendelian randomisation studies, have clarified the clinical impact of elevated Lp(a). A level of 1.29 mmol/l (50 mg/dl) has been suggested as a practical cut-off point for risk stratification for future cardiovascular events in daily practice, while a level of 0.78 mmol/l (30 mg/dl) may be an alternative cut-off for Asian populations. In addition to people with a high risk of atherosclerotic cardiovascular disease, the healthy general population may also be a potential candidate for Lp(a) measurement. Although the optimal pharmacological intervention for elevated Lp(a) has not been established, some ongoing trials may open the door to novel treatment strategies for people with elevated Lp(a). Healthcare providers must pay more attention to the risk stratification of cardiovascular disease according to Lp(a) level.
Clinical Perspective
• Elevated lipoprotein (a) (Lp(a)) is associated with an increased risk of cardiovascular disease.
• Plasma Lp(a) >1.29 mmol/l (50 mg/dl) has been proposed as a practical cut-off.
• An alternative cut-off of Lp(a) for Asian populations may be 0.78 mmol/l (30 mg/dl).
• Measurements may have to be considered at least once in the lifetime of each adult.
• Proprotein convertase subtilisin kexin type 9 inhibitors and antisense oligonucleotides may reduce the Lp(a) plasma level.
disease. N Engl J Med 2020;382:244–55. https://doi. org/10.1056/NEJMoa1905239; PMID: 31893580.
9. Tsimikas S, Stroes ESG. The dedicated “Lp(a) clinic”: a concept whose time has arrived? Atherosclerosis 2020;300:1–9. https://doi.org/10.1016/j. atherosclerosis.2020.03.003; PMID: 32234580.
10. Kronenberg F. Human genetics and the causal role of lipoprotein(a) for various diseases. Cardiovasc Drugs Ther 2016;30:87–100. https://doi.org/10.1007/s10557-016-6648-3; PMID: 26896185.
11. Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J 2010;31:2844–53. https://doi.org/10.1093/eurheartj/ehq386; PMID: 20965889.
12. Lee SR, Prasad A, Choi YS, et al. LPA gene, ethnicity, and cardiovascular events. Circulation 2017;135:251–63. https:// doi.org/10.1161/CIRCULATIONAHA.116.024611; PMID: 27831500.
13. Matthews KA, Sowers MF, Derby CA, et al. Ethnic differences in cardiovascular risk factor burden among middle-aged women: Study of Women’s Health Across the Nation (SWAN). Am Heart J 2005;149:1066–73. https://doi. org/10.1016/j.ahj.2004.08.027; PMID: 15976790.
14. Sandholzer C, Boerwinkle E, Saha N, et al. Apolipoprotein(a) phenotypes, Lp(a) concentration and plasma lipid levels in relation to coronary heart disease in a Chinese population: evidence for the role of the apo(a) gene in coronary heart disease. J Clin Invest 1992;89:1040–6. https://doi.org/10.1172/ JCI115645; PMID: 1541665.
15. Paré G, Çaku A, McQueen M, et al. Lipoprotein(a) levels and the risk of myocardial infarction among 7 ethnic groups. Circulation 2019;139:1472–82. https://doi.org/10.1161/
CIRCULATIONAHA.118.034311; PMID: 30667276.
16. Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and improved cardiovascular risk prediction. J Am Coll Cardiol 2013;61:1146–56. https://doi. org/10.1016/j.jacc.2012.12.023; PMID: 23375930.
17. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/ AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e1082–143. https://doi/org/10.1161/ CIR.0000000000000625; PMID: 30586774.
18. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–88. https://doi.org/10.1093/eurheartj/ehz455; PMID: 31504418.
19. Pearson GJ, Thanassoulis G, Anderson TJ, et al. 2021 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol 2021;37:1129–50. https://doi.org/10.1016/j.cjca.2021.03.016; PMID: 33781847.
20. Tsimikas S, Fazio S, Ferdinand KC, et al. NHLBI working group recommendations to reduce lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol 2018;71:177–92. https://doi.org/10.1016/j. jacc.2017.11.014; PMID: 29325642.
21. Guan W, Cao J, Steffen BT, et al. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 2015;35:996–
Lipoprotein(a) and Cardiovascular Disease JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
1001. https://doi.org/10.1161/ATVBAHA.114.304785; PMID: 25810300.
22. Khera AV, Everett BM, Caulfield MP, et al. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the Jupiter Trial (Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). Circulation 2014;129:635–42. https://doi. org/10.1161/CIRCULATIONAHA.113.004406; PMID: 24243886.
23. Albers JJ, Slee A, O’Brien KD, et al. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes). J Am Coll Cardiol 2013;62:1575–9. https://doi. org/10.1016/j.jacc.2013.06.051; PMID: 23973688.
24. Nestel PJ, Barnes EH, Tonkin AM, et al. Plasma lipoprotein(a) concentration predicts future coronary and cardiovascular events in patients with stable coronary heart disease. Arterioscler Thromb Vasc Biol 2013;33:2902–8. https://doi. org/10.1161/ATVBAHA.113.302479; PMID: 24092750.
25. Verbeek R, Hoogeveen RM, Langsted A, et al. Cardiovascular disease risk associated with elevated lipoprotein(a) attenuates at low low-density lipoprotein cholesterol levels in a primary prevention setting. Eur Heart J 2018;39:2589–96. https://doi.org/10.1093/eurheartj/ehy334; PMID: 29931232.
26. Levin MG, Zuber V, Walker VM, et al. Prioritizing the role of major lipoproteins and subfractions as risk factors for peripheral artery disease. Circulation 2021;144:353–64. https://doi.org/10.1161/CIRCULATIONAHA.121.053797; PMID: 34139859.
27. Kumar P, Swarnkar P, Misra S, Nath M. Lipoprotein (a) level as a risk factor for stroke and its subtype: a systematic review and meta-analysis. Sci Rep 2021;11:15660. https://doi. org/10.1038/s41598-021-95141-0; PMID: 34341405.
28. Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol 2014;63:470–7. https://doi.org/10.1016/j.jacc.2013.09.038; PMID: 24161338.
29. Willeit P, Ridker PM, Nestel PJ, et al. Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet 2018;392:1311–20. https://doi. org/10.1016/S0140-6736(18)31652-0; PMID: 30293769
30. Konishi H, Miyauchi K, Tsuboi S, et al. Plasma lipoprotein(a) predicts major cardiovascular events in patients with chronic kidney disease who undergo percutaneous coronary intervention. Int J Cardiol 2016;205:50–3. https:// doi.org/10.1016/j.ijcard.2015.12.007; PMID: 26710333.
31. Konishi H, Miyauchi K, Shitara J, et al. Impact of lipoprotein(a) on long-term outcomes in patients with diabetes mellitus who underwent percutaneous coronary intervention. Am J Cardiol 2016;118:1781–5. https://doi. org/10.1016/j.amjcard.2016.08.067; PMID: 27712648.
32. Yoon YH, Ahn JM, Kang DY, et al. Association of lipoprotein(a) with recurrent ischemic events following percutaneous coronary intervention. JACC Cardiovasc Interv 2021;14:2059–68. https://doi.org/10.1016/j.jcin.2021.07.042; PMID: 34556280.
33. Bucci M, Tana C, Giamberardino MA, Cipollone F. Lp(a) and cardiovascular risk: investigating the hidden side of the moon. Nutr Metab Cardiovasc Dis 2016;26:980–6. https://doi. org/10.1016/j.numecd.2016.07.004; PMID: 27514608.
34. O’Donoghue ML, Morrow DA, Tsimikas S, et al. Lipoprotein(a) for risk assessment in patients with established coronary artery disease. J Am Coll Cardiol 2014;63:520–7. https://doi.org/10.1016/j.jacc.2013.09.042; PMID: 24161323.
35. Dahlen GH, Guyton JR, Attar M, et al. Association of levels of lipoprotein Lp(a), plasma lipids, and other lipoproteins with coronary artery disease documented by angiography. Circulation 1986;74:758–65. https://doi.org/10.1161/01. CIR.74.4.758; PMID: 2944670.
36. Kral BG, Kalyani RR, Yanek LR, et al. Relation of plasma lipoprotein(a) to subclinical coronary plaque volumes, threevessel and left main coronary disease, and severe coronary stenoses in apparently healthy African-Americans with a family history of early-onset coronary artery disease. Am J Cardiol 2016;118:656–61. https://doi.org/10.1016/j. amjcard.2016.06.020; PMID: 27530333.
37. Hartmann M, von Birgelen C, Mintz GS, et al. Relation between lipoprotein(a) and fibrinogen and serial intravascular ultrasound plaque progression in left main coronary arteries. J Am Coll Cardiol 2006;48:446–52. https:// doi.org/10.1016/j.jacc.2006.03.047; PMID: 16875967.
38. Chieng D, Pang J, Ellis KL, et al. Elevated lipoprotein(a) and low-density lipoprotein cholesterol as predictors of the severity and complexity of angiographic lesions in patients with premature coronary artery disease. J Clin Lipidol 2018;12:1019–26. https://doi.org/10.1016/j.jacl.2018.03.090;
PMID: 29703625.
39. Muramatsu Y, Minami Y, Kato A, et al. Lipoprotein (a) level is associated with plaque vulnerability in patients with coronary artery disease: an optical coherence tomography study. Int J Cardiol Heart Vasc 2019;24:100382. https://doi. org/10.1016/j.ijcha.2019.100382; PMID: 31245530.
40. Fu H, Zhang D, Zhu R, et al. Association between lipoprotein(a) concentration and the risk of stroke in the Chinese Han population: a retrospective case-control study. Ann Transl Med 2020;8:212. https://doi.org/10.21037/ atm.2020.01.38; PMID: 32309359.
41. Li SY, Gao Y, Ma WN, et al. The relationship between serum lipoprotein (a) levels and ischemic stroke risk: a cohort study in the Chinese population. Inflammation 2014;37:686–93. https://doi.org/10.1007/s10753-013-9785-x; PMID: 24292801.
42. Sun L, Li Z, Zhang H, et al. Pentanucleotide TTTTA repeat polymorphism of apolipoprotein(a) gene and plasma lipoprotein(a) are associated with ischemic and hemorrhagic stroke in Chinese: a multicenter case-control study in China. Stroke 2003;34:1617–22. https://doi.org/10.1161/01. STR.0000078370.12085.02; PMID: 12791946.
43. Gencer B, Kronenberg F, Stroes ES, Mach F. Lipoprotein(a): the revenant. Eur Heart J 2017;38:1553–60. https://doi. org/10.1093/eurheartj/ehx033; PMID: 28329241.
44. Kronenberg F, Kronenberg MF, Kiechl S, et al. Role of lipoprotein(a) and apolipoprotein(a) phenotype in atherogenesis: prospective results from the Bruneck study. Circulation 1999;100:1154–60. https://doi.org/10.1161/01. CIR.100.11.1154; PMID: 10484534.
45. van Dam-Nolen DHK, van Dijk AC, Crombag GAJC, et al. Lipoprotein(a) levels and atherosclerotic plaque characteristics in the carotid artery: the Plaque at RISK (PARISK) study. Atherosclerosis 2021;329:22–9. https://doi. org/10.1016/j.atherosclerosis.2021.06.004; PMID: 34216874.
46. Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997;29:630–4. https://doi. org/10.1016/S0735-1097(96)00563-3; PMID: 9060903.
47. Bozbas H, Yildirir A, Atar I, et al. Effects of serum levels of novel atherosclerotic risk factors on aortic valve calcification. J Heart Valve Dis 2007;16:387–93. PMID: 17702363.
48. Zheng KH, Tsimikas S, Pawade T, et al. Lipoprotein(a) and oxidized phospholipids promote valve calcification in patients with aortic stenosis. J Am Coll Cardiol 2019;73:2150–62. https://doi.org/10.1016/j.jacc.2019.01.070; PMID: 31047003.
49. Yu B, Hafiane A, Thanassoulis G, et al. Lipoprotein(a) induces human aortic valve interstitial cell calcification. JACC Basic Transl Sci 2017;2:358–71. https://doi.org/10.1016/j. jacbts.2017.03.015; PMID: 30062157.
50. Makshood M, Joshi PH, Kanaya AM, et al. Lipoprotein (a) and aortic valve calcium in South Asians compared to other race/ethnic groups. Atherosclerosis 2020;313:14–9. https:// doi.org/10.1016/j.atherosclerosis.2020.09.010; PMID: 33002750.
51. Willeit P, Kiechl S, Kronenberg F, et al. Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15-year outcomes in the Bruneck Study. J Am Coll Cardiol 2014;64:851–60. https://doi.org/10.1016/j. jacc.2014.03.061; PMID: 25169167.
52. Zhang W, Speiser JL, Ye F, et al. High-sensitivity C-reactive protein modifies the cardiovascular risk of lipoprotein(a): Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol 2021;78:1083–94. https://doi.org/10.1016/j.jacc.2021.07.016; PMID: 34503676.
53. Burgess S, Ference BA, Staley JR, et al. Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: a Mendelian randomization analysis. JAMA Cardiol 2018;3:619–27. https:// doi.org/10.1001/jamacardio.2018.1470; PMID: 29926099.
54. O’Donoghue ML, Fazio S, Giugliano RP, et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation 2019;139:1483–92. https://doi.org/10.1161/ CIRCULATIONAHA.118.037184; PMID: 30586750.
55. Lackner C, Cohen JC, Hobbs HH. Molecular definition of the extreme size polymorphism in apolipoprotein(a). Hum Mol Genet 1993;2:933–40. https://doi.org/10.1093/hmg/2.7.933; PMID: 8395942.
56. Rhainds D, Brodeur MR, Tardif JC. Lipoprotein (a): when to measure and how to treat? Curr Atheroscler Rep 2021;23:51. https://doi.org/10.1007/s11883-021-00951-2; PMID: 34235598.
57. Desai NR, Kohli P, Giugliano RP, et al. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: an analysis from the LDL-C assessment with proprotein convertase subtilisin kexin type 9 monoclonal antibody
inhibition combined with statin therapy (LAPLACE)Thrombolysis in Myocardial Infarction (TIMI) 57 trial. Circulation 2013;128:962–9. https://doi.org/10.1161/ CIRCULATIONAHA.113.001969; PMID: 23884353.
58. Wilson DP, Jacobson TA, Jones PH, et al. Use of lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol 2019;13:374–92. https://doi. org/10.1016/j.jacl.2019.04.010; PMID: 31147269.
59. Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–81. https://doi.org/10.1016/S01406736(10)61350-5; PMID: 21067804.
60. Tsimikas S, Witztum JL, Miller ER, et al. High-dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B-100 in patients with acute coronary syndromes in the MIRACL trial. Circulation 2004;110:1406–12. https://doi.org/10.1161/01.CIR.0000141728.23033.B5; PMID: 15353498.
61. Rodenburg J, Vissers MN, Wiegman A, et al. Oxidized lowdensity lipoprotein in children with familial hypercholesterolemia and unaffected siblings: effect of pravastatin. J Am Coll Cardiol 2006;47:1803–10. https://doi. org/10.1016/j.jacc.2005.12.047; PMID: 16682304.
62. Capoulade R, Chan KL, Yeang C, et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol 2015;66:1236–46. https://doi.org/10.1016/j.jacc.2015.07.020; PMID: 26361154.
63. Choi SH, Chae A, Miller E, et al. Relationship between biomarkers of oxidized low-density lipoprotein, statin therapy, quantitative coronary angiography, and atheroma: volume observations from the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) study. J Am Coll Cardiol 2008;52:24–32. https://doi.org/10.1016/j. jacc.2008.02.066; PMID: 18582631.
64. Yoshida H, Shoda T, Yanai H, et al. Effects of pitavastatin and atorvastatin on lipoprotein oxidation biomarkers in patients with dyslipidemia. Atherosclerosis 2013;226:161–4. https://doi.org/10.1016/j.atherosclerosis.2012.10.069; PMID: 23174369.
65. Tsimikas S, Gordts PLSM, Nora C, et al. Statin therapy increases lipoprotein(a) levels. Eur Heart J 2020;41:2275–84. https://doi.org/10.1093/eurheartj/ehz310; PMID: 31111151.
66. Kamanna VS, Kashyap ML. Mechanism of action of niacin on lipoprotein metabolism. Curr Atheroscler Rep 2000;2:36–46. https://doi.org/10.1007/s11883-000-0093-1; PMID: 11122723.
67. HPS2-THRIVE Collaborative Group, Landray MJ, Haynes R, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203–12. https://doi. org/10.1056/NEJMoa1300955; PMID: 25014686.
68. Raal FJ, Hovingh GK, Blom D, et al. Long-term treatment with evolocumab added to conventional drug therapy, with or without apheresis, in patients with homozygous familial hypercholesterolaemia: an interim subset analysis of the open-label TAUSSIG study. Lancet Diabetes Endocrinol 2017;5:280–90. https://doi.org/10.1016/S22138587(17)30044-X; PMID: 28215937.
69. Raal FJ, Giugliano RP, Sabatine MS, et al. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol 2014;63:1278–88. https:// doi.org/10.1016/j.jacc.2014.01.006; PMID: 24509273.
70. Gaudet D, Kereiakes DJ, McKenney JM, et al. Effect of alirocumab, a monoclonal proprotein convertase subtilisin/ kexin 9 antibody, on lipoprotein(a) concentrations (a pooled analysis of 150 mg every two weeks dosing from phase 2 trials). Am J Cardiol 2014;114:711–5. https://doi.org/10.1016/j. amjcard.2014.05.060; PMID: 25060413.
71. Fitzgerald K, White S, Borodovsky A, et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med 2017;376:41–51. https://doi.org/10.1056/NEJMoa1609243; PMID: 27959715.
72. Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med 2017;376:1430–40. https://doi.org/10.1056/ NEJMoa1615758; PMID: 28306389.
73. Ray KK, Stoekenbroek RM, Kallend D, et al. Effect of an siRNA therapeutic targeting PCSK9 on atherogenic lipoproteins: prespecified secondary end points in ORION 1. Circulation 2018;138:1304–16. https://doi.org/10.1161/ CIRCULATIONAHA.118.034710; PMID: 29735484.
74. Raal FJ, Santos RD, Blom DJ, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet 2010;375:998–1006. https://doi.org/10.1016/S0140-6736(10)60284-X;
Lipoprotein(a) and Cardiovascular Disease JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
PMID: 20227758.
75. Merki E, Graham M, Taleb A, et al. Antisense oligonucleotide lowers plasma levels of apolipoprotein (a) and lipoprotein (a) in transgenic mice. J Am Coll Cardiol 2011;57:1611–21. https://doi.org/10.1016/j.jacc.2010.10.052; PMID: 21474042.
76. Graham MJ, Viney N, Crooke RM, Tsimikas S. Antisense inhibition of apolipoprotein (a) to lower plasma lipoprotein (a) levels in humans. J Lipid Res 2016;57:340–51. https://doi. org/10.1194/jlr.R052258; PMID: 26538546.
77. Viney NJ, van Capelleveen JC, Geary RS, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 2016;388:2239–53. https://doi.org/10.1016/S01406736(16)31009-1; PMID: 27665230.
and
Disease
78. Stiekema LCA, Prange KHM, Hoogeveen RM, et al. Potent lipoprotein(a) lowering following apolipoprotein(a) antisense treatment reduces the pro-inflammatory activation of circulating monocytes in patients with elevated lipoprotein(a). Eur Heart J 2020;41:2262–71. https://doi. org/10.1093/eurheartj/ehaa171; PMID: 32268367.
79. Moriarty PM, Gray JV, Gorby LK. Lipoprotein apheresis for lipoprotein(a) and cardiovascular disease. J Clin Lipidol 2019;13:894–900. https://doi.org/10.1016/j.jacl.2019.09.010; PMID: 31753721.
80. Leebmann J, Roeseler E, Julius U, et al. Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: prospective observational multicenter study. Circulation 2013;128:2567–
76. https://doi.org/10.1161/CIRCULATIONAHA.113.002432; PMID: 24056686.
81. Klingel R, Heibges A, Fassbender C, Pro(a)LiFe-Study Group. Prevention of cardiovascular complications in patients with Lp(a)-hyperlipoproteinemia and progressive cardiovascular disease by long-term lipoprotein apheresis according to German national guidelines. Clin Res Cardiol Suppl 2017;12(Suppl 1):38–43. https://doi.org/10.1007/s11789-0170082-3; PMID: 28185214.
82. Howell C, Douglas K, Cho G, et al. Guideline on the clinical use of apheresis procedures for the treatment of patients and collection of cellular therapy products. British Committee for Standards in Haematology. Transfus Med 2015;25:57–78. https://doi.org/10.1111/tme.12205; PMID: 26013470.
Lipoprotein(a)
Cardiovascular
JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Validation of the GRACE Risk Score for Acute Coronary Syndrome Patients in an Asian Medical Centre
Wei Juan Lim , 1 Ji Ken Ow , 2 Xian Pei Cheong , 2 Rusli bin Nordin 3 and Chuey Yan Lee 4
1. Cardiology Department, National Heart Institute, Kuala Lumpur, Malaysia; 2. Medical Department, Hospital Sultanah Aminah, Johor Bahru, Malaysia; 3. School of Medicine, MAHSA University, Jenjarom, Selangor, Malaysia; 4. Cardiology Department, Hospital Sultanah Aminah Johor Bahru, Malaysia

Abstract
Background: The Global Registry of Acute Coronary Syndrome (GRACE) risk score is used to provide an estimate of 6-month mortality among patients admitted for acute coronary syndrome (ACS). Methods: This study validated the GRACE score in a contemporary cohort of 428 patients aged ≥18 years admitted to Hospital Sultanah Aminah Johor Bahru between January and April 2018 for ACS. The survival status of patients 6 months after hospital discharge was calculated using the GRACE risk score, and the validity of the GRACE risk score was evaluated by assessing its calibration (Hosmer–Lemeshow test) and discriminatory capacity. Results: Of the 428 patients in this study, 92 (21.5%) were admitted for STelevation MI (STEMI), 128 (29.9%) were admitted for non-STEMI and 208 (48.6) were admitted for unstable angina. By 6 months after discharge, 66 (15%) patients had died. The GRACE risk score was calibrated and validated, showing an adequate capacity for discrimination with a receiver operating characteristic area under the curve of 0.831 (95% CI [0.778–0.884]; p<0.001). Conclusion: This study validated the GRACE score for predicting 6-month mortality among patients admitted to an Asian medical centre for ACS and recommended that it is used routinely.
Keywords
GRACE risk score, acute coronary syndrome, validation, Asian medical centre
Disclosure: The authors have no conflicts of interest to declare.
Funding: This research received no specific grant from any funding agency.
Ethics Approval: This study was conducted in accordance with the amended Declaration of Helsinki and approved by the local ethics committee, National Medical Research Register (NMRR-19-3476-51813; IIR).
Author Contributions: Conceptualisation: WJL, XPC; data curation: WJL, JKO, XPC; formal analysis: WJL, JKO, XPC; investigation: WJL, JKO, XPC; methodology: WJL, JKO, XPC; project administration: WJL, JKO, XPC; resources: WJL, JKO, XPC; software: WJL, JKO, XPC; supervision: RbN, CYL; validation: RbN, CYL; visualization: WJL; writing –original draft preparation: WJL, JKO, XPC; writing – review & editing: RbN, CYL.
Data Availability: The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Informed Consent: This is a retrospective study with ethics approval and consent to publish. Informed consent was not required for this retrospective work. Trial Registration: https://nmrr.gov.my/ (NMRR-19-3476-51813 [IIR])
Received: 2 January 2022 Accepted: 15 February 2022 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e12. DOI: https://doi.org/10.15420/japsc.2022.01
Correspondence: Wei Juan Lim, Cardiology Department, National Heart Institute, Kuala Lumpur 50480, Malaysia. E: omegakimia@yahoo.com
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
There are various risk scores to predict mortality risk among patients admitted for acute coronary syndrome (ACS), such as the Thrombolysis in MI (TIMI) score, Global Registry of Acute Coronary Events (GRACE) risk score and the Platelet glycoprotein IIb/IIIa in Unstable angina: Receptor Suppression Using Integrilin (eptifibatide) Therapy (PURSUIT) score.1
The GRACE registry, a global registry of ACS patients from 94 hospitals in 14 countries, developed two models to estimate the risk of in-hospital and 6-month mortality among all patients with ACS.2
The GRACE risk score predicts 6-month mortality after a patient has been discharged following hospital admission for ACS. It uses a predictive logistic model with eight prognostic variables (Supplementary Material Figure 1) to determine a patient’s probability of death due to any cause during the first 6 months after discharge.3
We must be mindful of the geographical differences and patient characteristics in the original GRACE study when applying this risk score to other populations, with validation required to avoid erroneous results in risk calculations.4 Thus, the aim of this study was to validate the GRACE risk score in an Asian medical centre to determine whether the probabilistic model can be used outside of the geographical and patient environment in which it was created.
Methods Patients
This study included 428 patients aged ≥18 years who were admitted to Hospital Sultanah Aminah, Johor Bahru, Malaysia, between January and April 2018 for acute coronary syndrome (ACS). The initial cohort contained 440 patients, but 12 (2.7%) patients were excluded because they did not have valid data for calculating the GRACE score or vital status 6 months
© RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com General Cardiology ORIGINAL RESEARCH
after discharge. The study included all patients presenting with chest pain suggestive of ACS and admitted for further treatment. A diagnosis of ACS was made based on presenting symptoms, ECG and cardiac biomarkers. All patients were treated with dual anti-platelet therapy, anticoagulants, statins, angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers and β-blockers where possible.
Risk Score
The GRACE score was calculated using the online calculator (Version 2.0; https://www.mdcalc.com/grace-acs-risk-mortality-calculator), as described previously.5,6 The eight variables that constitute the GRACE score are age, history of heart failure, history of acute MI, heart rate and systolic blood pressure (SBP) at admission, ST-segment depression, serum creatinine at admission and elevated myocardial necrosis markers or enzymes.
Data Collection
For this retrospective study, a database was created to collect relevant data, which were then stored in spreadsheets, and in accordance with the
GRACE score variables. Most of the information was obtained between January and April 2018 from an in-house database, which included 10 patient-related factors, five cardiac-related factors and three operationrelated factors. Out-of-hospital data, including death or late complications, were obtained via reviewing patient outpatient clinic notes.
The GRACE score was calculated for each patient by adding the points for each of the eight prognostic variables used to calculate the score, namely age, history of heart failure, history of acute MI, heart rate and SBP at admission, ST-segment depression, serum creatinine at admission and elevated myocardial necrosis markers or enzymes (Supplementary Material Figure 1).
Statistical Analysis
Universal sampling was used. Data were entered into a Microsoft Excel database and analysed using SPSS version 22.0. Continuous variables are reported as the mean ± SD. Univariate analysis of dichotomous variables was performed using the χ2 or Fisher’s exact test. Pearson and Spearman tests were used for correlational analyses of continuous variables.
Receiver operating characteristic (ROC) curve analysis was performed to estimate the discriminant ability of the risk scoring method to predict immediate postoperative adverse events. The calibration of the riskscoring method was estimated as the area under the ROC curve (AUC) with 95% CIs. Survival analysis was performed by the Kaplan–Meier method. ROC curve analysis was used to estimate the performance of the risk score in predicting mortality at the 6-month follow-up. P-value of <0.05 was considered statistically significant.
Calibration of Analysis
Model calibration was evaluated using the Hosmer–Lemeshow goodnessof-fit test because this test is useful for validating an existing logistic model with an external database.7 Values of p>0.05 indicate that the model is well adjusted to the data and is therefore a good predictor, in this case, of patients’ probability of death.
Results
Baseline Characteristics
The baseline characteristics of the study population are provided in Supplementary Material Table 1. Of the 428 patients in this study, 307 (72%) were male and 50% of the study population was Malay. Most patients had hypertension and nearly half had diabetes with dyslipidaemia.
Of the 428 patients in this study, 208 (48.6%) were admitted for unstable angina and 92 (21.5%) were admitted for ST-elevation MI (STEMI). Thirteen (3%) patients had a cardiac arrest upon arrival at hospital. The frequencies of GRACE risk score variables in our cohort are presented in Supplementary Material Table 2
Mortality
By 6 months after discharge, 66 (15%) patients had died. Figure 1 shows the number of mortalities at the end of the 6-month follow-up period, whereas Figure 2 shows the distribution of mortality at 6 months in each of the low-, intermediate- and high-risk categories. The mortality rate increased significantly as the GRACE risk category increased. In addition, the GRACE risk score increased with advancing age (Supplementary Material Figure 2).
Factors Affecting Prognosis
Univariate
the risk
GRACE Risk Score Validation JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
and multivariate analyses were conducted to assess
Figure 1: GRACE Score: Actual Mortality at 6 Months 66 (15%) Alive Dead 362 (85%) Figure 2: GRACE Score: Mortality at 6 Months 13 10 11 45 0 1 2 3 24 8 87 0 Total STEMI NSTEMI Unstable angina Low risk Intermediate risk High risk 5 10 15 20 25 30 35 40 45 50 Percentage NSTEMI = non-ST-elevation MI; STEMI = ST-elevation MI.
Table 1: Univariate Analysis for Association of Risk Factors with Event (Death)
Risk Factor SE Wald χ2 p-value OR [95% CI]
Age >65 years 0.277 22.27 <0.001 3.686 [2.143–6.340]
Sex: male 0.289 0.483 0.487 1.223 [0.694–2.155]
Diabetes 0.272 4.531 0.033 0.561 [0.329–0.955]
Hypertension 0.298 0.657 0.148 0.785 [0.438–1.409]
Dyslipidaemia 0.268 1.702 0.192 0.705 [0.417–1.192]
Smoking 0.269 0.818 0.366 0.784 [0.462–1.329]
CKD/ESRF 0.333 8.095 0.004 2.576 [1.342–4.943]
Previous history of IHD 0.269 0.137 0.711 1.105 [0.652–1.874]
Previous aspirin use 0.268 0.549 0.459 1.220 [0.721–2.063]
Family history of IHD 0.380 3.065 0.080 1.943 [0.924–4.089]
Cardiac biomarkers 0.279 5.769 0.016 0.512 [0.296–0.884]
Systolic blood pressure 0.537 13.142 <0.001 0.143 [0.050–0.409]
Heart rate 0.252 1.779 0.182 1.400 [0.854–2.295]
ST-segment deviation 0.273 7.376 0.007 0.476 [0.278–0.813]
Serum creatinine level 0.296 8.544 0.003 2.373 [1.329–4.236]
NHYA class:
I 2.791 0.425
II 0.822 0.490 0.484 0.563 [0.112–2.815]
III 0.835 0.221 0.638 0.675 [0.131–3.470]
IV 0.932 0.051 0.821 1.235 [0.199–7.673]
Killip class:
I 30.342 <0.001
II 0.391 18.803 <0.001 0.183 [0.085–0.395]
III 0.485 8.358 0.004 0.246 [0.095–0.636]
IV 0.575 0.273 0.601 1.350 [0.438–4.163]
CABG = coronary artery bypass graft; CAG = coronary angiogram; CKD = chronic kidney disease; ESRF = end-stage renal failure; IHD = ischaemic heart disease; NYHA = New York Heart Association; PCI = percutaneous coronary intervention.
associated with the baseline characteristics and to evaluate the predictive accuracy of the composite GRACE risk score (Tables 1 and 2).
Age
The mortality rate increased with increasing age, with multivariate analysis revealing a good association between age and death (OR 9.642, 95% CI [4.077–22.803], p<0.001). In this study, we used a cut-off value of 65 years and compared GRACE risk scores between those aged ≥65 and <65 years. The mean GRACE risk score was significantly (p<0.001) higher for patients aged ≥65 than <65 years (116.17 ± 30.16 versus 84.85 ± 34.32, respectively).
Dyslipidaemia
Dyslipidaemia is strongly associated with cardiovascular mortality. In the present study, 189 (44%) patients had high cholesterol, and this showed a trend towards statistical significance (OR 2.812, 95% CI [1.141–6.928], p=0.025).
Chronic Kidney Disease
The median serum creatinine level in our study population was 88 mmol/l. Multivariate analysis indicated that patients with chronic kidney
Table 2: Multivariate Logistic Regression Analysis for Predicting the Event (Death/MI)
Risk factor SE Wald χ2 p-value Adjusted OR
Age >65 years 0.439 26.630 <0.001 9.642
Sex: male 0.487 0.629 0.411 0.679 Diabetes 0.419 1.936 0.160 1.79
Hypertension 0.482 2.377 0.120 0.476
Dyslipidaemia 0.460 5.047 0.025 2.812 Smoking 0.469 1.409 0.235 1.744 CKD/ESRF 0.604 4.858 0.028 3.786
Previous history of IHD, CAG, CABG or PCI 0.614 0.722 0.396 0.522
Previous aspirin use 0.650 3.316 0.069 3.265 Family history of IHD 0.516 4.445 0.035 0.337 Cardiac biomarkers 0.469 0.373 0.541 0.751 Systolic blood pressure 0.794 8.894 0.003 0.094 Heart rate 0.342 1.636 0.201 1.550 ST-segment deviation 0.428 1.491 0.222 1.686 Serum creatinine level 0.529 0.396 0.529 0.717
NHYA class: I 7.806 0.050 II 1.381 4.301 0.038 17.535 III 1.320 1.859 0.173 6.050 IV 1.313 2.982 0.084 9.656
Killip class: I 8.323 0.040 II 0.749 1.070 0.301 0.461 III 0.856 0.043 0.836 0.837 IV 0.922 1.958 0.162 3.631
CABG = coronary artery bypass graft; CAG = coronary angiogram; CKD = chronic kidney disease; ESRF = end-stage renal failure; IHD = ischaemic heart disease; NYHA = New York Heart Association; PCI = percutaneous coronary intervention.
disease (CKD) had a poorer prognosis (OR 3.786, 95% CI [1.159–12.367], p=0.028).
Family History of Ischaemic Heart Disease
Despite only 94 (21.9%) patients having a family history of ischaemic heart disease, it proved to be strongly associated with mortality (OR 0.337, 95% CI [0.123–0.926], p=0.035).
Systolic Blood Pressure
The mean SBP in the study population was 149 ± 32 mmHg. There was a significant difference in mean SBP between patients alive at 6 months and those who died (153 ± 31 versus 131 ± 27 mmHg, respectively; p<0.001). Multivariate analysis revealed that decreasing SBP was associated with mortality (OR 0.094, 95% CI [0.020–0.444], p=0.003).
New York Heart Association Class
New York Heart Association (NYHA) class is not one of the parameters used to calculate the GRACE risk score but, in our study, there was a statistically significant association between NYHA class II and mortality (OR 17.535, 95% CI [0.112–2.815], p=0.038). This is likely to be due to most patients being classified as NYHA class I or II.
GRACE Risk Score Validation JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Killip Class
A higher Killip class was associated with higher mortality and worse prognosis. In the present study, Killip class I was statistically significant compared with mortality (p=0.040), but this was most likely due to most patients (70%) being in Killip class I, which skewed the statistical calculations.
Calibration and Discrimination
Model calibration was excellent for our cohort population, with the validated model showing an adequate capacity for discrimination after calibration (Hosmer–Lemeshow goodness-of-fit test). The AUC was 0.831 (95% CI [0.778–0.884], p<0.001; Figure 3).
Discussion
Accurate prediction of risk in ACS is important to ensure appropriate and optimised interventions. The GRACE risk score stands out as being simple to use in the clinical setting, with evidence showing that it performs better than similar ACS risk models.8 Moreover, the GRACE risk score can be applied across all cases of ACS, be they non-STEMI (NSTEMI) or STEMI. Furthermore, the validity of the GRACE risk score has already been tested in populations from several countries.8
The use of the GRACE score has been recommended by clinical practice guidelines.9,10 However, proper use of the scoring system requires a properly contextualised validation to ensure that it will provide correct information and probabilities. This study is the first validation study on the GRACE risk score in Malaysia. In this study, the GRACE risk score was calibrated using the Hosmer–Lemeshow goodness-of-fit test with an AUC of 0.831 (95% CI [0.778–0.884]). This indicates that, in our cohort, the model offers an excellent calibration of probability of mortality at 6 months after discharge from hospital following admission for ACS.
In this study, increasing age showed a strong association with mortality,
with patients aged >65 years being at particularly high risk. Elderly patients aged >65 years with ACS tend to be under-represented in clinical trials and undertreated, with both invasive and pharmacological therapy.11 Hence, we chose a cut-off age of 65 years for analysis in the present study. The mean GRACE risk score for patients aged ≥65 years was 116.17 ± 30.16 years, significantly (p<0.001) higher than the mean GRACE risk score of 84.85 ± 34.32 among those aged <65 years.
As observed in the GRACE registry (OR 1.4 for every 10 mmHg decrease in SBP), in the present study a lower SBP on admission was associated with higher mortality at 6 months after discharge.3 In addition, a number of studies have shown that patients with CKD, with or without long-term dialysis, are at a prognostically significantly higher risk of death.12,13 Patients with ACS and CKD are associated with poorer short- and longterm outcomes; these cases are more complex and there is a lack of welldesigned randomised clinical trials assessing therapeutic strategies in this group of patients.12,13
Dyslipidaemia with high total cholesterol, LDL, triglycerides and low HDL is one of the major risk factors in patients admitted for ACS.14 Current guidelines advocate that the lower the LDL, the better the prognosis of patients, with a target LDL of <1.4 mmol/l in the very high-risk group.15 Our analysis was consistent with these findings, showing that LDL level was a significant predictor of mortality (p=0.025).
A family history of ischaemic heart disease in patients with ACS is important, especially in younger patients or those with no initial elevations in high-sensitivity troponin T concentrations.16,17 A positive family history of ischaemic heart disease is a major risk factor for coronary artery disease, which predisposes to atherosclerosis at a younger age.17 A study in South Korea showed that patients with a family history of ischaemic heart disease were younger and more likely to be male.18 In the present study, having a family history of ischaemic heart disease was associated with more cardiovascular events (p=0.035).
In this study, mortality was higher than in the GRACE registry (15% versus 4.8%, respectively).3 Of the 92 patients admitted for STEMI, only 43.5% had a coronary angiogram including primary percutaneous coronary intervention (PCI) or rescue PCI, whereas 47.8% had thrombolysis. The remaining cases were late presentations MI or spontaneous resolutions of MI. The higher number of patients receiving thrombolysis is due, in part, to the unavailability of cath labs. As can be seen in Figure 2, a relatively high number of patients with low GRACE risk scores died compared with intermediate- and high-risk patients. Most of the deaths were due to recurrent admission for heart failure, hospital-acquired infections or non-cardiac causes.
In the present study, there was a significant association between NYHA class and mortality (p<0.050), but NYHA class is not included in the GRACE risk score. NYHA class is strongly associated with mortality, with higher NYHA class therefore warranting close attention during admission.19 Higher Killip class is associated with severe angiographic coronary artery disease and worse ventricular function.20 In the present study, only Killip class I was a significant predictor of mortality, and this could be because most of the patients were in Killip class I and experienced recurrent admissions for complications of MI, such as heart failure, or other noncardiac problems.
The GRACE risk score calculator is easily available via a smartphone application and fulfils the fundamental criteria for a prognostic score, namely accuracy, ease of use and generalisability.
GRACE Risk Score Validation JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Figure 3: Receiver Operating Characteristic Curve Analysis of the Capacity of Discrimination of the GRACE Risk Score 1 0 0.8 0 6 0.4 0.2 0 0 0 0 0.2 0.4 0 6 0.8 1 0 Sensitivity 1 – Specificity ROC Curve The area under the receiver operating characteristic curve was 0.831. Diagonal segments are produced by ties.
Study Limitations
Because of this was a single-centre study with a relatively smaller database, some prognostic variables may not be statistically significant despite showing good association with mortality. In real-word practice, recalculation of risk scores during a patient’s hospital stay is needed for a more dynamic assessment because risk stratification is a continuous process.
1. De Araújo Gonçalves P, Ferreira J, Aguiar C, Seabra-Gomes R. TIMI, PURSUIT, and GRACE risk scores: sustained prognostic value and interaction with revascularization in NSTE-ACS. Eur Heart J 2005;26:865–72. https://doi. org/10.1093/eurheartj/ehi187; PMID: 15764619.
2. Simons M, Alpert JS, Wilson PWF, et al. Risk stratification after non-ST elevation acute coronary syndrome. UpToDate 2019. https://www.uptodate.com/contents/risk-stratificationafter-non-st-elevation-acute-coronary-syndrome (accessed 4 April 2020).
3. Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA 2004;291:2727–33. https://doi.org/10.1001/ jama.291.22.2727; PMID: 15187054.
4. Cooney MT, Dudina AL, Graham IM. Value and limitations of existing scores for the assessment of cardiovascular risk: a review for clinicians. J Am Coll Cardiol 2009;54:1209–27. https://doi.org/10.1016/j.jacc.2009.07.020; PMID: 19778661.
5. Hung J, Roos A, Kadesjö E, et al. Performance of the GRACE 2.0 score in patients with type 1 and type 2 myocardial infarction. Eur Heart J 2021;42:2552–61. https://doi. org/10.1093/eurheartj/ehaa375; PMID: 32516805.
6. GRACE ACS Risk and Mortality Calculator. https://www. mdcalc.com/grace-acs-risk-mortality-calculator (accessed 12 April 2022).
7. Lemeshow S, Hosmer DW. A review of goodness of fit statistic for use in the development of logistic regression models. Am J Epidemiol 1982;115:92–106. https://doi. org/10.1093/oxfordjournals.aje.a113284; PMID: 7055134.
8. D’Ascenzo F, Biondi-Zoccai G, Moretti C, et al. TIMI, GRACE and alternative risk scores in acute coronary syndromes: a
Conclusion
The GRACE risk score for predicting mortality was validated in our population and could be used to estimate the risk of death at 6 months for our ACS patients. More studies, preferably multicentre studies, are needed to confirm our findings and validate all the GRACE risk score parameters.
meta-analysis of 40 derivation studies on 216,552 patients and of 42 validation studies on 31,625 patients. Contemp Clin Trials 2012;33:507–14. https://doi.org/10.1016/j. cct.2012.01.001; PMID: 22265976.
9. Collet JP, Thiele H, Barbato E, et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2021;42:1289–367. https://doi.org/10.1093/ eurheartj/ehaa575; PMID: 32860058.
10. Amsterdam EA, Wenger NK, Brindis RG, et al. AHA/ACC guideline for the management of patients with non-STelevation acute coronary syndromes. Circulation 2014;130:e344–426. https://doi.org/10.1161/ cir.0000000000000134; PMID: 25249585.
11. Zuhdi AS, Ahmad WA, Zaki RA, et al. Acute coronary syndrome in the elderly: the Malaysian National Cardiovascular Disease Database–Acute Coronary Syndrome registry. Singapore Med J 2016;57:191–7. https:// doi.org/10.11622/smedj.2015145; PMID: 26768171.
12. Marenzi G, Cabiati A, Assanelli E. Chronic kidney disease in acute coronary syndromes. World J Nephrol 2012;1:134–45. https://doi.org/10.5527/wjn.v1.i5.134; PMID: 24175251.
13. Moisi MI, Rus M, Bungau S, et al. Acute coronary syndromes in chronic kidney disease: clinical and therapeutic characteristics. Medicina (Kaunas) 2020;56:118. https://doi. org/10.3390/medicina56030118; PMID: 32182690.
14. Lee JS, Chang PY, Zhang Y, et al. Triglyceride and HDL-C dyslipidemia and risks of coronary heart disease and ischemic stroke by glycemic dysregulation status: the strong
heart study. Diabetes Care 2017;40:529–37. https://doi. org/10.2337/dc16-1958; PMID: 28122840.
15. Mach F, Baigent C, Catapano AL, et al. ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–88. https://doi.org/10.1093/eurheartj/ehz455; PMID: 31504418.
16. Wahrenberg A, Magnusson PK, Discacciati A, et al. Family history of coronary artery disease is associated with acute coronary syndrome in 28,188 chest pain patients. Eur Heart J Acute Cardiovasc Care 2020;9:741–7. https://doi. org/10.1177/2048872619853521; PMID: 31124704.
17. Hoseini K, Sadeghian S, Mahmoudian M, et al. Family history of cardiovascular disease as a risk factor for coronary artery disease in adult offspring. Monaldi Arch Chest Dis 2008;70:84–7. https://doi.org/10.4081/monaldi.2008.427; PMID: 18754276.
18. Kim C, Chang HJ, Cho I, et al. Impact of family history on the presentation and clinical outcomes of coronary heart disease: data from the Korea Acute Myocardial Infarction Registry. Korean J Intern Med 2013;28:547–56. https://doi. org/10.3904/kjim.2013.28.5.547; PMID: 24009450.
19. Siegersma KR, Groepenhoff F, Onland-Moret NC, et al. New York Heart Association class is strongly associated with mortality beyond heart failure in symptomatic women. Eur Heart J Qual Care Clin Outcomes 2021;7:214–5. https://doi. org/10.1093/ehjqcco/qcaa091; PMID: 33346810.
20. El-Menyar A, Zubaid M, AlMahmeed W, et al. Killip classification in patients with acute coronary syndrome: insight from a multicenter registry. Am J Emerg Med 2012;30:97–103. https://doi.org/10.1016/j.ajem.2010.10.011; PMID: 21159479.
GRACE Risk Score Validation JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Postgraduate Institute of Medical Education and Research, Chandigarh, India

Abstract
Calcified coronary artery lesions are challenging to treat and are generally associated with suboptimal results. With improvements in technology, these lesions can now be treated with better short- and long-term outcomes. The authors present three cases of heavily calcified left main and diffuse coronary artery disease that were managed with a combination of rotablation and intravascular lithotripsy.
Keywords
Rotatripsy, heavy calcification, intravascular lithotripsy, rotablation, medial calcification, left main calcification
Disclosure: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from all patients.
Ethics Approval: Institutional ethics approval was granted for publication.
Acknowledgements: The authors thank Mr Prashanth Sareen who helped retrieve and interpret intravascular ultrasound images.
Received: 15 October 2021 Accepted: 10 January 2022 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e13. DOI: https://doi.org/10.15420/japsc.2021.24
Correspondence: Himanshu Gupta, Department of Cardiology, Advanced Cardiac Centre, Postgraduate Institute of Medical Education & Research, Sector 12, Chandigarh – 160 012, India. E: himanshu2883@gmail.com
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Calcified coronary artery lesions are one of the most challenging lesions to treat and are associated with worse clinical outcomes.1 Initially, rotablation or orbital atherectomy were the only treatment options available, with both being associated with a steep learning curve and difficult-to-treat complications. With time, balloon-based devices have evolved and intravascular lithotripsy (IVL) has come into the fore.2 However, any single technology may not be enough, and, in some cases, the complementary use of balloon-based calcium ablators, such as IVL, and rotational or orbital atherectomy may be needed for optimal plaque preparation and stent deployment.3
Here, we describe three cases in which the complementary use of two technologies helped successfully manage calcified coronary artery lesions.
Case Presentation
Case 1
A 77-year-old man, with known diabetes, hypertension and chronic kidney disease, presented with accelerating angina over the previous 9 days. The patient had severe left ventricular dysfunction, with an ejection fraction of 35% and severe global hypokinesia. Coronary angiography revealed severely calcified left main (LM) and diffuse left anterior descending artery (LAD) disease (Figure 1A) and mid-right coronary artery (RCA) chronic total occlusion. He had a SYNTAX score of 40 and a EuroSCORE II of 18.45%.
The patient underwent intra-aortic balloon pump (IABP)-supported percutaneous intervention (PCI) with a plan for rotational atherectomy to
mid-LAD lesion followed by IVL, if required. Initial rotablation was successfully performed in the proximal and mid-LAD with a 1.5 mm burr (see Figure 1B). After rotablation, intravascular ultrasonography (IVUS) revealed an arc of thick circumferential calcium in the LM and proximal LAD, along with deep medial calcification in the LM and LAD (Figures 2A–2C). After IVUS, a 3 mm NC balloon could not be expanded, even at 20 atm (Figure 1B). Considering the large reference minimum lumen diameter (MLD) and thick circumferential calcium, the next best option was to use an IVL or a very-high-pressure OPN balloon. We decided to use a 3.5 mm IVL balloon because the calcium appeared very thick and we considered IVL safer than using a very-high-pressure OPN balloon.
IVL was successfully performed with 3.5 mm × 12 mm balloon with 40 pulses delivered in LM and 40 pulses in the proximal LAD. The patient tolerated prolonged balloon inflation in the LM despite poor left ventricular function because he also had IABP support. We deployed a cross-over 3.5 mm × 38 mm zotarolimus-eluting stent from the LM to proximal LAD, followed by proximal optimisation (POT) of LM stent with 4.5 mm and 5 mm non-compliant (NC) balloons. We deployed a 2.5 mm × 28 mm everolimus-eluting stent from the mid- to distal LAD with final post-dilation with a 3.0 mm NC balloon.
Post-stent IVUS revealed multiple cracks in the calcium arc (Figure 2D) with an LM minimum stent area (MSA) of 16.5 mm2 (Figure 2F), and proximal and mid-LAD MSAs of 10 mm2 and 8 mm2, respectively (Figure 2E), with good stent apposition (Figure 1D). The patient’s IABP was removed the next day and was discharged after 2 days. The patient is currently asymptomatic at 1-year follow-up.
© RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com Intervention CASE REPORT
The Use of Complementary Technologies in Calcified Left Main Disease: A Case Series
Dinakar Bootla , Pruthvi C Revaiah, Navjyot Kaur, Yash Paul Sharma and Himanshu Gupta
and
Case 2
A 75-year-old man with diabetes, hypertension, severe Parkinson’s disease and coronary artery disease presented to the emergency department with severe angina. Coronary angiography revealed severely calcified LM and LAD disease, with 70% stenosis of the distal LM and a diffusely diseased, very tortuous proximal LAD with diffuse 80% stenosis (Figure 3A). The distal left circumflex artery (LCx) had 70% stenosis, and the RCA had mild disease. His SYNTAX score was 33 and his EuroSCORE II was 13.07.
The patient was taken up for PCI with rotablation and IVL, if required. An initial rotablation was performed with a small, 1.25 mm burr in the distal LM and proximal LAD due to severe tortuosity. IVUS after rotablation revealed circumferential calcium on the luminal surface along with deep medial calcification (Figures 4A and 4B). Although luminal calcium was modified by rotablation, the MLD and minimum lumen area (MLA) remained low in the proximal LAD and distal LM (Figures 4C and 4D) and the lesion could not be dilated with 3 mm NC balloon, even at high pressures (Figure 3B), due to heavy medial calcification.
Hence, we proceeded with IVL. IVL was performed with a 3 mm balloon, with 40 pulses given in the proximal LAD and another 40 pulses in the distal LM. After IVL, the lesions were easily modified with a 3 mm × 15 mm NC balloon followed by stenting with a zotarolimus-eluting stent (3.5 mm × 22 mm) from the LM to LAD and an everolimus-eluting stent (2.25 mm × 24 mm) in the proximal to mid-LAD. The LAD stent was postdilated with a 3.25-mm NC balloon and LM POT was done with 4 mm and 4.5 mm NC balloons. Post-stenting IVUS revealed an MSA of 12.74 mm2 with 9.7 mm2 and 9.7 mm2 in the LM and proximal LAD, respectively, with good apposition and expansion (Figures 3C,4E and 4F). The patient was discharged on day 2 and continues to do well at the 10-month follow-up.
Intravascular Ultrasound During Angioplasty
Case 3
An 82-year-old man with diabetes, hypertension and chronic kidney disease who had been medically managed for chronic stable angina for the past 6 years presented with rest angina for 4 days. Coronary angiography revealed heavily calcified critical LM disease, diffusely diseased and calcified proximal LAD disease, tight proximal LCx disease (Figure 5A–C), dominant RCA with mid-diffuse disease and chronic total occlusion of the right posterior descending artery, with collaterals from the LAD, and an ejection fraction of 40% with inferior wall akinesia. His SYNTAX score was 36 and his EuroSCORE II was 14.85.
The patient was taken up for high-risk ‘protected’ PCI; an IABP was inserted and initial rotablation was performed using a 1.5 mm burr in the LM, proximal and mid-LAD. After rotablation, IVUS revealed circumferential diffuse calcification in the proximal to mid-LAD and a calcified nodule in the distal LM (Figures 6A–6C). A 2.5 mm NC balloon failed to dilate fully even at 24 atm, in the mid- and proximal LAD lesions (Figure 5D). Due to very heavy calcification and a large MLD after initial rotablation and the high risk of slow/no reflow with a larger-sized rotablator device, we decided to perform IVL.
Successful IVL was performed with 3 mm and 3.5 mm balloons in the proximal to mid-distal LAD and distal LM, respectively (Figure 5E). While performing IVL with a 3 mm balloon in the ostial LCx lesion, the balloon ruptured after 20 pulses, causing flow-limiting dissection in the LM. The reason for IVL balloon rupture was not clear, but it was probably due to nodular calcium at this location.
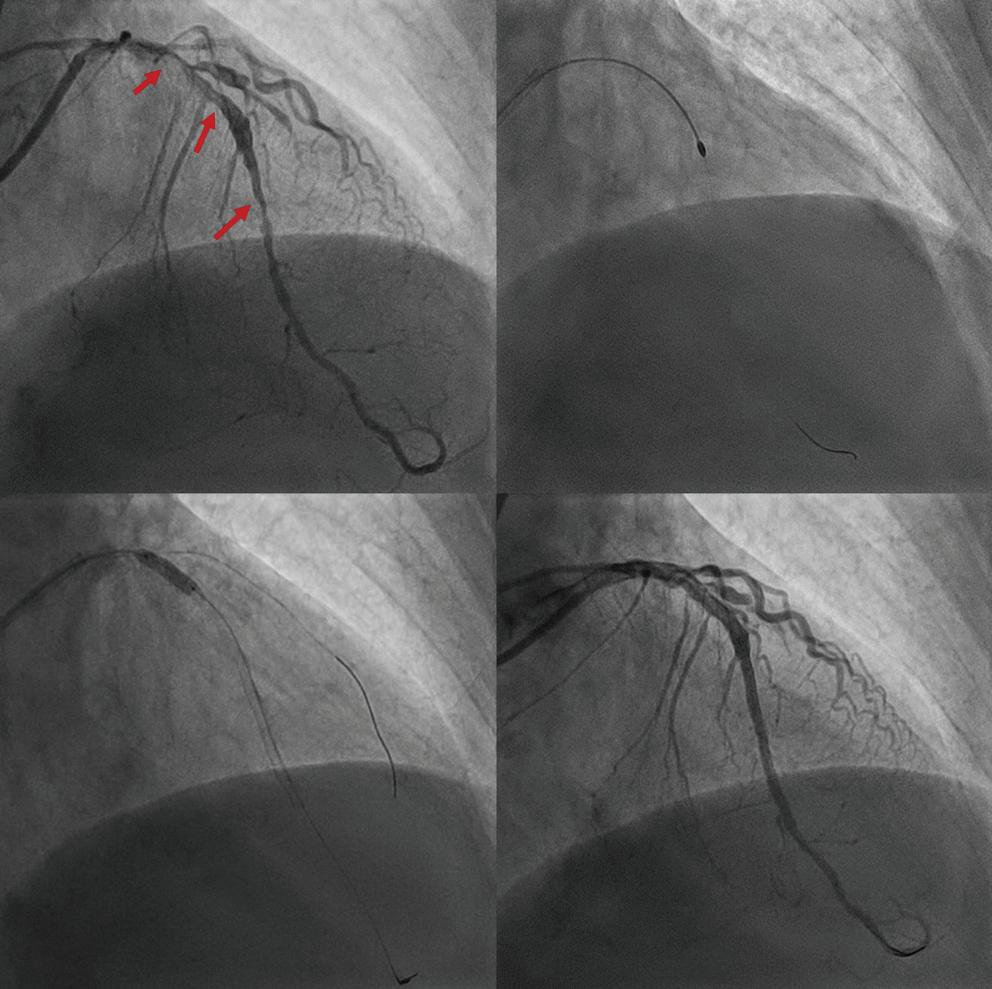
Emergency LM bifurcation stenting was done with a minicrush technique and the LM ostium to LAD was stented with a zotarolimus-eluting stent (3.5 mm × 26 mm), with a 3 mm × 26 mm zotarolimus-eluting stent deployed from the ostium to mid-LCx. LM POT was performed using a 5 mm NC balloon followed by final kissing balloon inflation with 3.5 mm and 3 mm NC balloons in the LAD and LCx. The mid- to distal LAD was successfully stented with two overlapping stents.
Post stenting IVUS images of LM and proximal LAD are shown in Figures
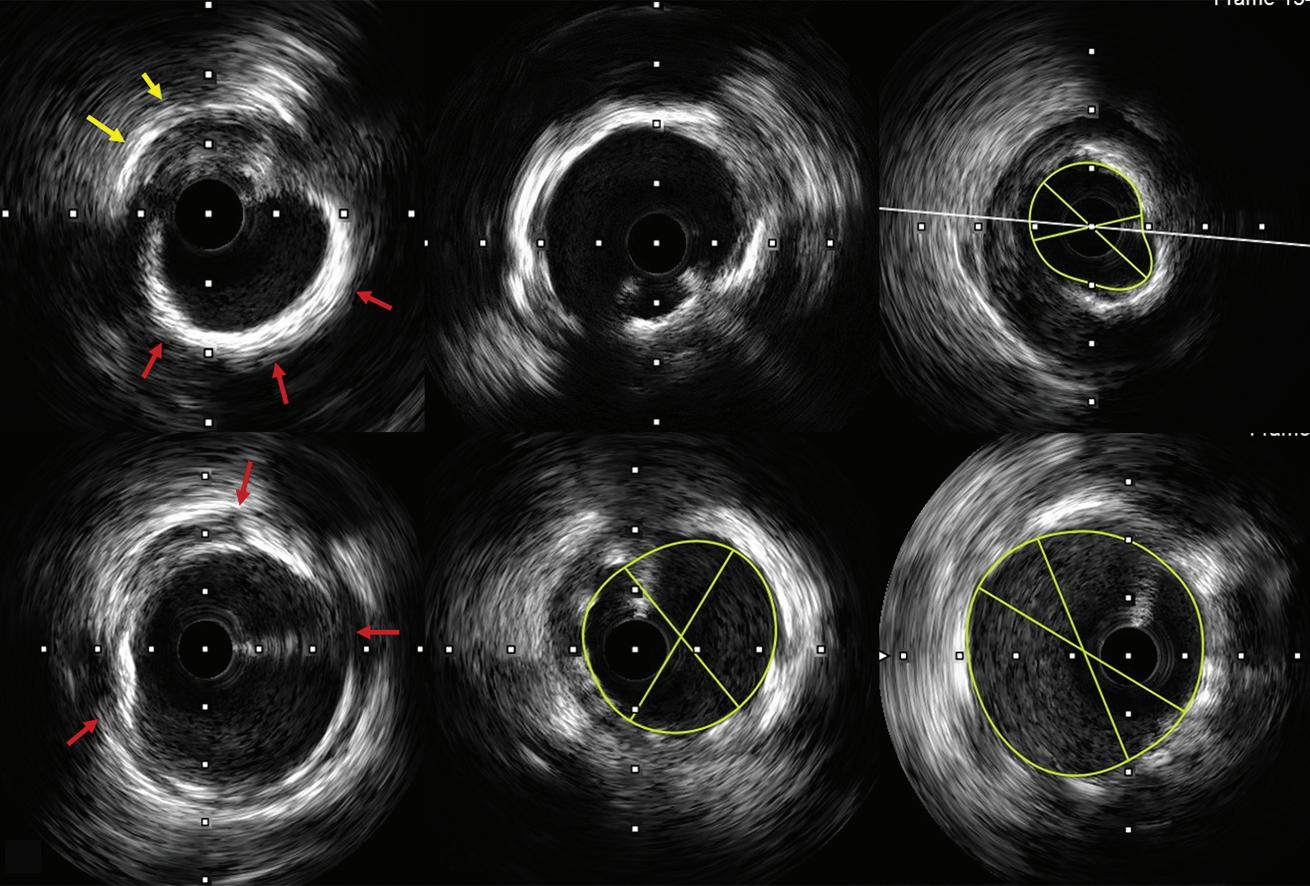
Rotatripsy in Left Main Disease JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Figure
1: Case 1, Coronary Angiography
Angioplasty
Figure
2: Case 1,
A B C D A
B C
D E F
A: Angiogram showing severe, long segment, calcified left main and left anterior descending artery disease (arrows). B: Initial rotablation with a 1.5-mm burr. C: Complete expansion of the non-compliant balloon after intravascular lithotripsy. D: Final angiogram showing a good angiographic result.
A–C: Intravascular ultrasound (IVUS) showing luminal (red arrows) and deep (yellow) calcification (A), circumferential calcification (B) and a minimum lumen diameter of 2.71 mm in the mid-left anterior descending artery (LAD; C). D–F: IVUS showing cracks (red arrows) in the luminal calcification after intravascular lithotripsy (IVL; D), a minimum stent area (MSA) of 8 and 10 mm2 in the mid- and proximal LAD, respectively (E), and an MSA of 16.5 mm2 (F) in the LM after stenting following lesion modification with rotablation and IVL.
Figure 4: Case 2, Intravascular Ultrasound During Angioplasty
A:
showed adequately expanded and apposed stents with a minimum stent area of 9.7 and 12.74 mm2 in the proximal LAD (E) and LM (F), respectively.
6D–6F and final angiographic result is in Figure 5F. The IABP was removed 24 hours after the procedure and the patient was discharged after 72 hours. The patient is currently asymptomatic during daily activities and moderate exertion.
Discussion
Calcified coronary stenoses are one of the most difficult lesions to treat and are associated with worse clinical outcomes.1 Currently, there are several devices available for dealing with calcified lesions.3 Balloonbased devices include scoring balloons, cutting balloons, super-highpressure NC balloons and IVL. Ablation-based devices include rotablation and orbital atherectomy.
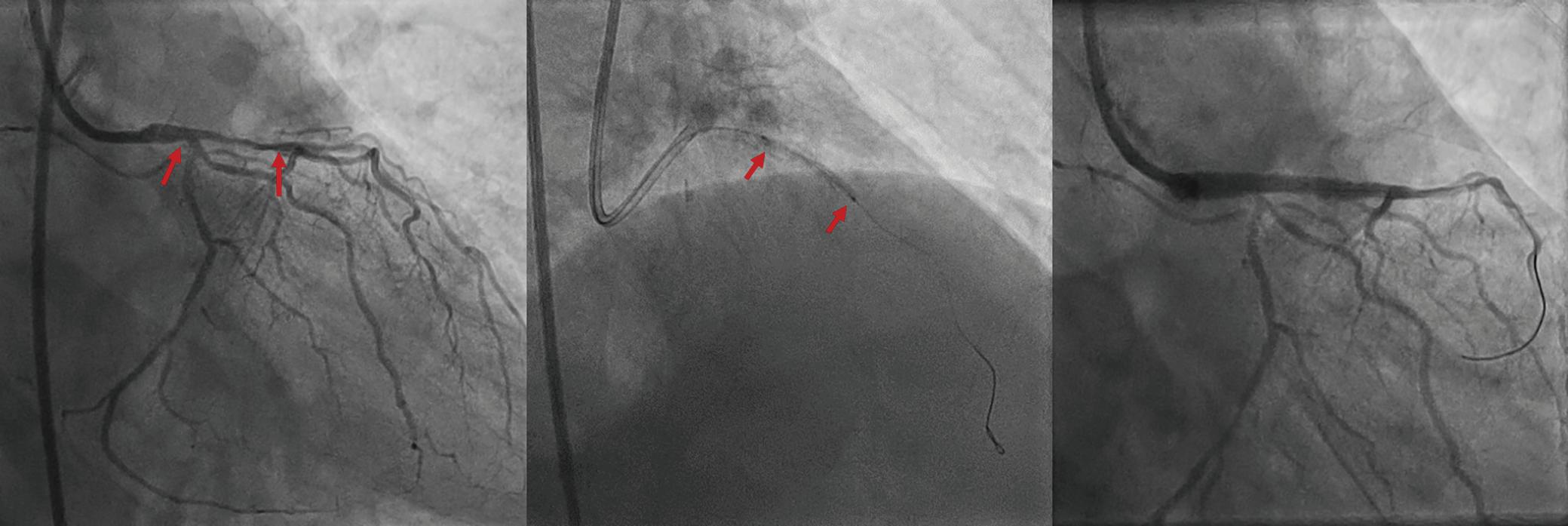
Although rotablation has been available for the treatment of coronary lesions for the past three decades, it is not commonly used because of the steep learning curve, its ability to modify only luminal calcium and difficulty in managing complications related to its use.4 These considerations have
Figure 6: Case 3, Intravascular Ultrasound During Angioplasty
A,B: Intravascular ultrasound showing long-segment circumferential calcification (red arrows; A) and a calcium nodule (orange arrow) in the distal left main, luminal calcium (red arrows), deep calcium (yellow arrows) (LM; B). C: Mid-left anterior descending artery (LAD) circumferential calcium with a minimum lumen area and minimum lumen diameter (MLD) of 1.9 mm2 and 1.06 mm, respectively, at the tightest point. D–F: After stenting, the minimum stent area and MLD increased to 12.7 mm2 and 3.66 mm, respectively, in the ostial LAD (D; note the calcium nodule [arrow]), to 16.08 mm2 and 4.32 mm, respectively, in the distal LM (E) and to 17.6 mm2 and 4.52 mm, respectively, in the proximal LM (F).
prompted research for alternative modalities, such as IVL. The main advantages of IVL are that it is easy to use, it can modify deep calcium and its use is associated with less chance of slow/no reflow and fewer coronary perforations.2 Rotablation and IVL can be used together in cases where IVL cannot cross a very tight stenosis, in cases of eccentric calcified nodules or long diffuse disease, where multiple balloons may be required.5–7
In all three cases described above, we performed upfront rotablation to ablate luminal calcium because the lesions were long, diffuse or nodular. In all three cases, IVUS was performed only after initial rotablation because IVUS could not cross the lesion before initial plaque modification. In this scenario, upfront use of the rotablator with a small burr is the best choice and IVL can be performed later depending on IVUS findings and/or results of initial balloon dilatation, as in all three cases described in this report. Rotablation with a small burr is safe but sometimes insufficient in terms of significantly modifying plaque,
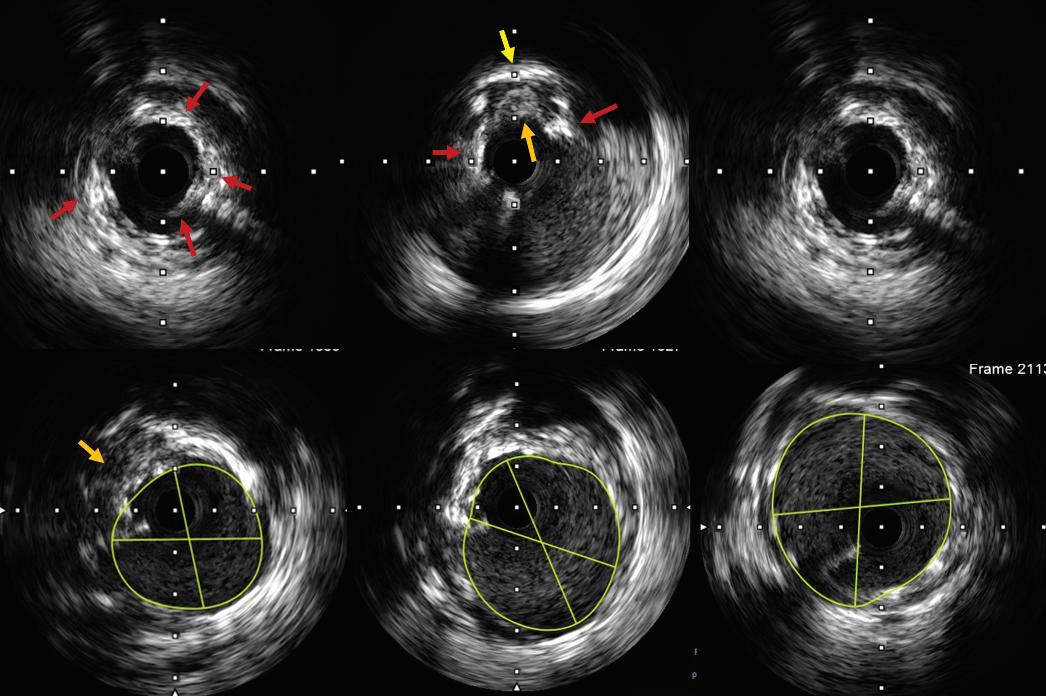
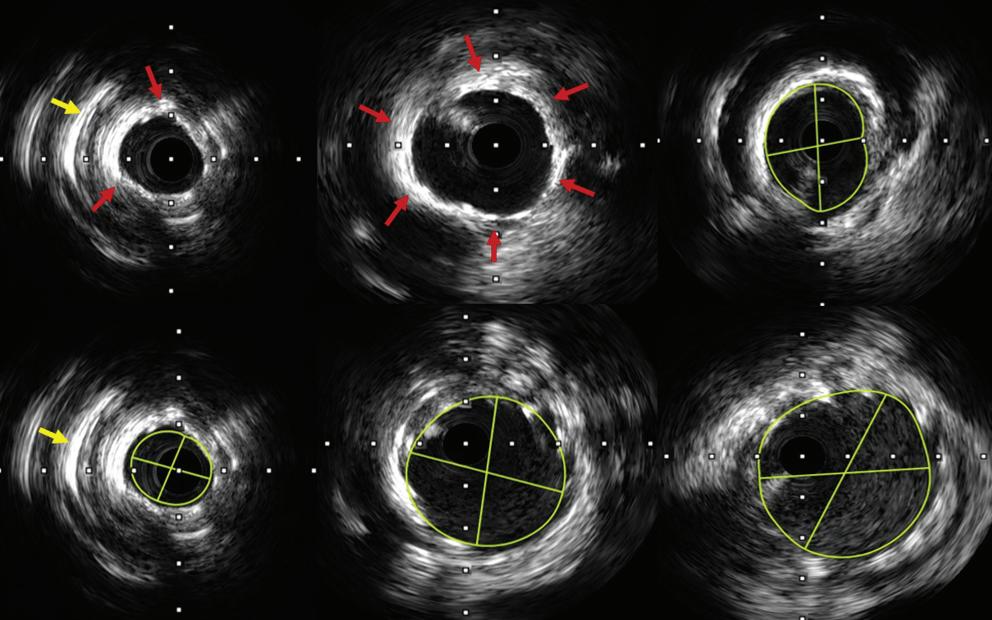
Rotatripsy in Left Main Disease JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Figure 3: Case 2, Coronary Angiography and Angioplasty A B C
A: Angiography showing diffuse calcified left main (LM) and left anterior descending artery (LAD) disease (arrows). B: An undilatable LAD lesion (arrows), despite high balloon pressures, after initial rotablation. C: Final angiogram showing acceptable results after intravascular lithotripsy and stenting of the LM and proximal LAD.
A B C
D
E F
Intravascular ultrasound (IVUS) images of luminal (red arrows) and deep (yellow arrow) medial calcification. B: Circumferential calcification (arrows). C,D: After initial rotablation, the minimum lumen diameter and minimum lumen area were 3.05 mm and 5.85 mm2, respectively, in the distal left main (LM; C) and 1.79 mm and 2.20 mm2, respectively, in the proximal left anterior descending artery (LAD; D). E,F: After IVL and stenting, IVUS
A B
C
D E F
especially in large vessels with significant deep calcium and if the calcium thickness is >500 µm.8
Using a larger-sized burr may be effective in decreasing the calcium volume and helping to make calcium cracks with further balloon dilatation, but can be associated with risks such as slow/no reflow or coronary perforation if there is unfavourable wire bias or significant vessel tortuosity. This is where its combination of rotablation with IVL is very effective, because IVL is very efficient in treating large vessels with deep and circumferential calcification (Table 1). Furthermore, the upfront use of IVL in diffuse calcified lesions can be challenging because the IVL balloon
has a large crossing profile. In addition, in lesions with long diffuse calcified disease, more than one IVL balloon may be required, which may be cost prohibitive.
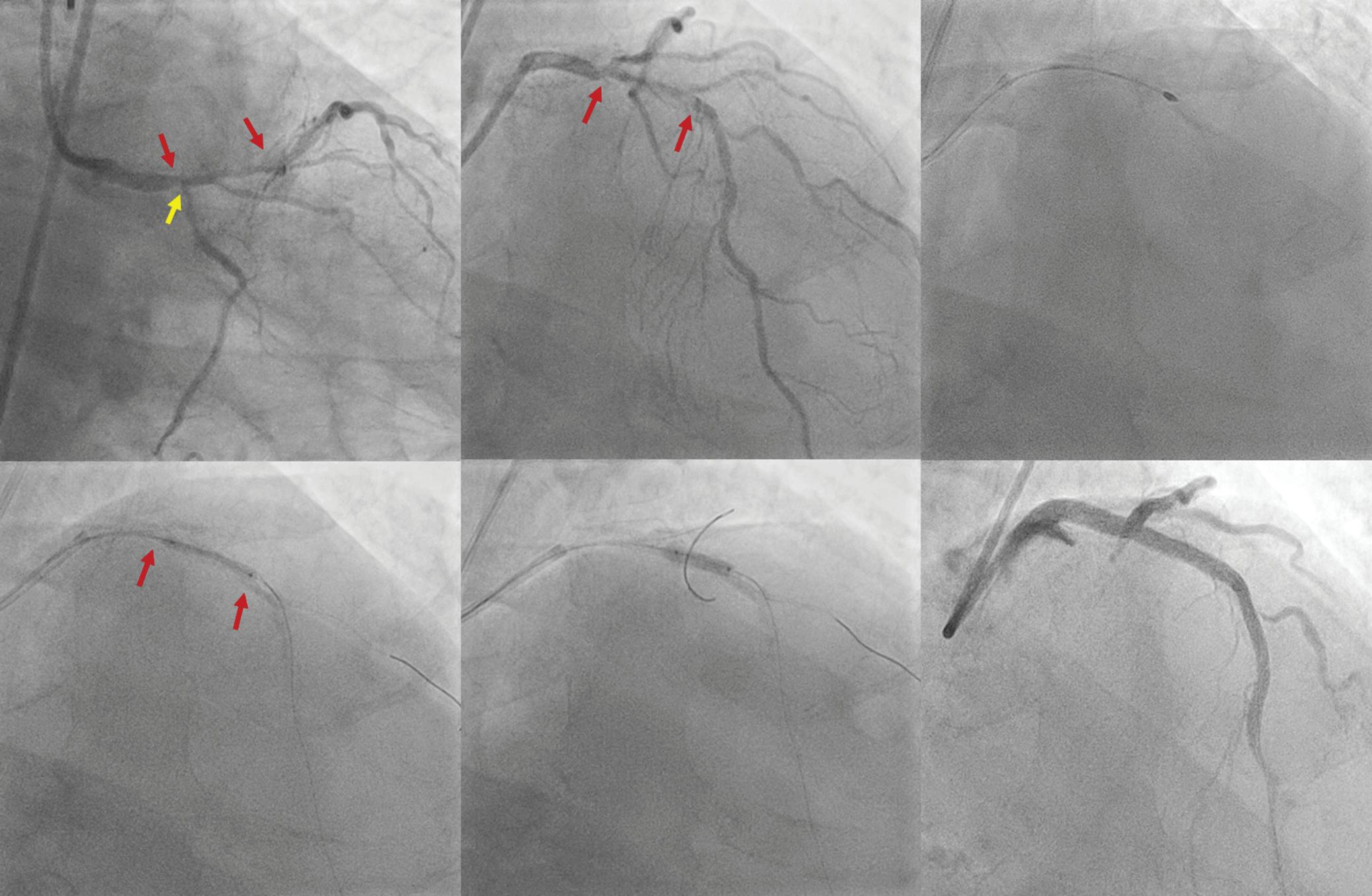
It has also been observed that the upfront use of IVL in eccentric calcified nodules may lead to IVL balloon rupture, as occurred in our Case 3 and previously reported.9 A large rotablator burr or orbital atherectomy remain the best modalities to modify eccentric nodular calcium if there is favourable wire bias.
Combining upfront rotablation and IVL has several advantages. Initial rotablation can treat the diffuse intimal calcium and/or calcified nodule and IVL can be selectively used later in lesions with circumferential and/or deep calcium, which are not modified with balloon dilatation after initial rotablation. Initial rotablation may also prevent IVL balloon rupture, as has been reported previously.9 In addition, prior rotablation favourably modifies the vessel for the easy delivery of the lithotripsy balloon and other devices.5
Ideally, diffuse calcified lesions should be treated with imaging guidance. Calculation of the calcium score can provide guidance as to the need for advanced plaque preparation techniques, such as atherectomy or IVL.10,11 Imaging also helps define calcium morphology, such as eccentricity, nodularity, thickness, length and location, especially at bifurcations, to enable further planning of plaque preparation strategies.12
Rotatripsy in Left Main Disease JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Figure 5: Case 3, Coronary Angiography and Angioplasty A � C D E F A: Coronary angiogram showing severe and diffusely calcified left main (LM) and left anterior descending artery (LAD) disease (red arrows) and tight proximal left circumflex artery (LCx) disease (yellow arrow). B: Long tram-track calcium was seen on fluoroscopy (arrows). C: Rotablation in the LM and proximal LAD. D: Undilatable proximal LAD lesion (arrows), even at high pressures, using a non-compliant balloon after rotablation. E: Intravascular lithotripsy being performed in the proximal LAD and LM. F: Final angiogram showing good angiographic results after LM and LAD stenting. Table 1: Use of Rotablation Versus Intravascular Lithotripsy in Calcified Coronary Lesions Type of calcium Rotablation IVL Balloon-uncrossable lesion ++ Eccentric nodular calcium ++ Diffuse calcified lesions ++ + Thick focal circumferential calcium (calcium thickness >500 µm) +* +++ Deep calcium ++ *Efficacy depends on feasibility and wire bias; large burr sizes may be needed. + = mildly effective; ++ = moderately effective; +++ = highly effective; IVL = intravascular lithotripsy.
Optical coherence tomography has the advantage of providing calcium thickness, but its use may be limited in patients with compromised renal function or LM disease due to poor visualisation of the ostium of the LM.13
For these reasons, IVUS was chosen as the imaging modality in all three cases described here.
Conclusion
IVL and rotablation are complementary technologies for the treatment of diffuse calcified lesions. Upfront rotablation with a small-sized burr is safe and favourably modifies the superficial calcium, also helping in the efficient delivery of the IVL balloon, if required later.
Upfront use of IVL in the case of diffuse calcified lesions may be unsuccessful or associated with complications.
1. Madhavan MV, Tarigopula M, Mintz GS, et al. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol 2014;63:1703–14. https://doi. org/10.1016/j.jacc.2014.01.017; PMID: 24530667.
2. Hill JM, Kereiakes DJ, Shlofmitz RA, et al. Intravascular lithotripsy for treatment of severely calcified coronary artery disease. J Am Coll Cardiol 2020;76:2635–46. https://doi. org/10.1016/j.jacc.2020.09.603; PMID: 33069849.
3. De Maria GL, Scarsini R, Banning AP. Management of calcific coronary artery lesions: is it time to change our interventional therapeutic approach? JACC Cardiovasc Interv 2019;12:1465–78. https://doi.org/10.1016/j.jcin.2019.03.038; PMID: 31395217.
4. Barbato E, Carrié D, Dardas P, et al. European expert consensus on rotational atherectomy. EuroIntervention 2015;11:30–6. https://doi.org/10.4244/EIJV11I1A6; PMID: 25982648.
5. Gonzálvez-García A, Jiménez-Valero S, Galeote G, et al. ‘RotaTripsy’: combination of rotational atherectomy and intravascular lithotripsy in heavily calcified coronary lesions:
Clinical Perspective
• Intravascular lithotripsy (IVL) works best in concentric calcified lesions and is very effective in the case of thick and focal circumferential calcified lesions.
• Rotablation is most effective for nodular calcified lesions and lesions that a balloon cannot cross.
• The combination of rotablation and IVL is very effective in diffuse calcified lesions, with the initial rotablation modifying superficial calcium and helping in the efficient delivery of the IVL balloon, which can further modify deep and thick calcium, if required.
• Upfront use of IVL in the case of diffuse calcified lesions should probably be avoided without imaging so that it is used more effectively and prevents complications such as balloon rupture.
a case series. Cardiovasc Revasc Med 2022;35:179–84. https://doi.org/10.1016/j.carrev.2021.04.011; PMID: 33903037.
6. Aznaouridis K, Bonou M, Masoura C, et al. Rotatripsy: a hybrid “drill and disrupt” approach for treating heavily calcified coronary lesions. J Invasive Cardiol 2020;32:e175. PMID: 32479423.
7. Giacchi G, Contarini M, Ruscica G, Brugaletta S. The ‘RotaTripsy Plus’ approach in a heavily calcified coronary stenosis. Cardiovasc Revasc Med 2021;28(Suppl):203–5. https://doi.org/10.1016/j.carrev.2021.04.022; PMID: 33958306.
8. Maejima N, Hibi K, Saka K, et al. Relationship between thickness of calcium on optical coherence tomography and crack formation after balloon dilatation in calcified plaque requiring rotational atherectomy. Circ J 2016;80:1413–9. https://doi.org/10.1253/circj.CJ-15-1059; PMID: 27087360.
9. Kaur N, Pruthvi CR, Sharma Y, Gupta H. Rotatripsy: synergistic effects of complementary technologies: a case report. Eur Heart J Case Rep 2021;5:ytab083. https://doi. org/10.1093/ehjcr/ytab083; PMID: 34124544.
10. Fujino A, Mintz GS, Matsumura M, et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention 2018;13:e2182–9. https://doi.org/10.4244/EIJ-D-17-00962; PMID: 29400655.
11. Liu S, Neleman T, Hartman EMJ, et al. Automated quantitative assessment of coronary calcification using intravascular ultrasound. Ultrasound Med Biol 2020;46:2801–9. https://doi.org/10.1016/j.ultrasmedbio.2020.04.032; PMID: 32636052.
12. Ueki Y, Otsuka T, Hibi K, Räber L. The value of intracoronary imaging and coronary physiology when treating calcified lesions. Interv Cardiol 2019;14:164–8. https://doi.org/10.15420/ icr.2019.16.R1; PMID: 31867063.
13. Räber L, Mintz GS, Koskinas KC, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J 2018;39:3281–300. https://doi. org/10.1093/eurheartj/ehy285; PMID: 29790954.
Rotatripsy in Left Main Disease JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
A Malaysian Expert Consensus on the Use of High-sensitivity Cardiac Troponin in the Emergency Department
Raja Ezman Raja Shariff , 1 Sazzli Shahlan Kasim , 1 Subashini C Thambiah , 2 Adi Osman , 3 Asri Said , 4 Farhi Ain Jamaluddin , 5 Farina Mohd Salleh6 and Sarah Abd Karim7
1. Cardiology Department, Universiti Teknologi MARA (UITM) Sungai Buloh, Sungai Buloh, Selangor, Malaysia; 2. Pathology Department, Universiti Putra Malaysia, Seri Kembangan, Selangor, Malaysia; 3. Emergency Department, Hospital Raja Permaisuri Bainun, Ipoh, Malaysia;
4. Cardiology Department, Pusat Jantung Negara Sarawak, Kota Samarahan, Sarawak, Malaysia; 5. Pathology Department, Universiti Malaya Medical Centre, Kuala Lumpur, Malaysia; 6. Emergency Department, Institut Jantung Negara, Kuala Lumpur, Malaysia;

7. Emergency Department, Hospital Sungai Buloh, Sungai Buloh, Selangor, Malaysia
Abstract
Recent guidelines have recommended using high sensitivity cardiac troponin (hs-cTn) assays to triage patients with suspected acute coronary syndrome. Despite this, less sensitive point-of-care testing is often the preferred choice in majority of hospitals. The aim of this evidence-based, expert consensus is to provide guidance for healthcare professionals in understanding the role of hs-cTn, specifically its level of sensitivity and specificity as well as its practical application in the emergency department setting, particularly in resource-limited centres in Malaysia. An expert panel with clinical and research expertise in the diagnosis and treatment of acute coronary syndrome was convened. Recommendations were based on a comprehensive review of the existing literature using MEDLINE and Embase databases, alongside individual clinical experience within the regional and international context. This expert consensus provides a structured approach to using hs-cTn in the emergency department and remains the only one to date produced by a group of Malaysian experts to help guide Malaysian clinicians dealing with acute chest pain on a daily basis.
Keywords
High-sensitivity troponin, troponin, chest pain, emergency department, expert consensus
Disclosure: SSK is on the editorial board of Journal of Asian Pacific Society of Cardiology; this did not influence peer review. All other authors have no conflicts of interest to declare.
Acknowledgements: The authors thank Universiti Teknologi MARA (UITM) Sungai Buloh, Universiti Putra Malaysia, Hospital Raja Permaisuri Bainun, Pusat Jantung Negara Sarawak, Universiti Malaya Medical Centre, Institut Jantung Negara and Hospital Sungai Buloh for supporting the submission of this work. The authors acknowledge the following individuals: Benjamin Samraj Prakash Earnest (General Medicine), Farnaza Ariffin (Family Medicine), Johan Rizwal Ismail (Cardiology), Rahal Mohd Yusoff (Cardiology), Yong Chee Keong (Emergency Medicine), Hanita Othman (Chemical Pathology) and Hidayah Shafie (Emergency Medicine), for attending the three meetings that were held between August 2019 and February 2020, with an agenda to formulate recommendations on hs-cTn testing.
Received: 1 September 2021 Accepted: 27 December 2021 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e14.
DOI: https://doi.org/10.15420/japsc.2021.19
Correspondence: Raja Ezman Raja Shariff, Universiti Teknologi Mara Sungai Buloh, Jalan Hospital, 47000, Sungai Buloh, Selangor, Malaysia. E: rajaezman@gmail.com
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Globally, ischaemic heart disease (IHD) remains a main cause of mortality, amounting to more than 9 million deaths in 2016 alone.1 The burden of IHD is particularly prevalent in low- to middle-income countries, where the rates of mortality and risk for IHD among those aged <60 years are reported to be high.1,2 In Malaysia, IHD is the major cause of national mortality, contributing to 15.6% of all deaths in 2018.3 The 2019 National Cardiovascular Disease Database for acute coronary syndrome (ACS) revealed a total of 21,327 patients being admitted for ACS between 2016 and 2017; 44.6% of which were because of ST-elevation MI (STEMI), 28.0% for non-STEMI (NSTEMI) and 27.4% for unstable angina.4
Data describing the common characteristics of patients presenting with chest pain to emergency departments (EDs) remain sparse in Malaysia and regionally. A study from an Ecuadorian rural hospital revealed 2.8% of
patients aged <60 years and 5.4% of those aged >60 years presented to the ED with chest pain, whereas a study from Pakistan (involving seven major EDs) revealed a rate of approximately 7%.5,6
It is important for ED clinicians to triage patients presenting with chest pain as either cardiac, likely cardiac or non-cardiac in nature.7 Furthermore, decisions are needed to identify patients who would benefit from hospitalisation versus those who can be safely discharged.8 Failure to do so effectively may lead to not only financial and institutional consequences (i.e. exit blocks), but also to compromised care for those requiring immediate clinical management. Patients with MI who are prematurely discharged have a doubled risk of mortality compared with those who are hospitalised.8,9 Therefore, rapid and accurate diagnosis, followed by thoughtful risk stratification of ACS in the ED is paramount.10
© RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com Clinical Cardiology REVIEW
Cardiac troponins (cTn) I and T remain the conventional biomarkers used to detect myocardial injury, risk-stratify patients suspected of ACS, and subsequently diagnose MI.11 Recent guidelines have recommended using high-sensitivity cardiac troponin (hs-cTn) assays to triage patients with suspected ACS.11–14 According to the International Federation of Clinical Chemistry (IFCC) and Laboratory Medicine Task Force on Clinical Applications of Bio-Markers, for an assay to be defined as high-sensitivity, two analytical criteria need to be fulfilled: analytical imprecision (% coefficient of variation) at the 99th percentile upper reference limit (URL) should be ≤10%; and highly-sensitive assays should measure cTn at or above the assay’s limit of detection in ≥50% of healthy subjects.15,16 Despite strong recommendations in adopting routine use of hs-cTn, less sensitive point-of-care testing (POCT) is often a preferred choice in majority of hospitals due to rapid access of results without on the need for central laboratory involvement.
The aim of this evidence-based expert consensus is to provide guidance for healthcare professionals in understanding the role of hs-cTn, specifically its level of sensitivity and specificity, as well as its practical application in the ED setting, particularly in resource-limited centres. Discussions will be based on case scenarios throughout this guide.
Methods
An expert panel with clinical and research expertise in the diagnosis and treatment of ACS was convened. This consisted of cardiologists, family medicine physicians, emergency medicine physicians and chemical pathologists from public, university and private healthcare settings in Malaysia. Three meetings were held between August 2019 and February 2020, with an agenda to formulate recommendations on hs-cTn testing. These recommendations were based on a comprehensive review of the existing literature using MEDLINE and Embase databases, alongside individual clinical experience. Level of evidence was based on an adaptation of the Levels of Evidence by the Oxford Centre for Evidence-Based Medicine. During these meetings, participating members identified problems, created frameworks and suggested input statements, which were used in subsequent meetings to develop this paper. After incorporating all feedback, a unanimous agreement was reached for final evidenced-based decisions on an algorithm (Figure 1) and consensus recommendations for the use of hs-cTn to diagnose ACS in the ED.
Results
Assessing for Acute Coronary Syndrome in the Emergency Department: The Five Steps
To address the gaps in current ACS diagnosis in the ED, particularly in resource-limited centres, the panel agreed on a five-step approach using hs-cTn. These steps are:
1. Obtaining patient history and determining symptom onset.
2. Performing a clinical examination, ECG and echocardiography.
3. Performing initial hs-cTn testing.
4. Performing risk stratification.
5. Determining diagnosis.
These recommendations are based on the need to fully use hs-cTn in ensuring prompt diagnosis of patients with possible ACS and prompt discharge of patients at lower risk of ACS or other serious conditions. The panel’s five-step approach is incorporated in the proposed algorithm for ruling in or ruling out ACS in the ED (Figure 1) and these steps are elaborated in the subsequent paragraphs.
Step 1: Patient History and Symptom Onset
By obtaining a comprehensive patient history, physicians can identify possible serious and life-threatening causes for chest pain and exclude less critical differentials. Essential components include information on the nature of the pain itself (i.e. site, onset, characteristics, radiation, duration, alleviating and/or exacerbating factors and severity). The presence of associated symptoms such as shortness of breath, palpitations, ankle oedema, orthopnoea and paroxysmal nocturnal dyspnoea is also pertinent. Detailed descriptions of pain and discomfort can also help exclude other non-cardiac causes of pain.
It is recommended that physicians establish the exact onset, duration and pattern of symptoms by allowing patients to describe the symptoms themselves (i.e. avoiding coaching). This will not only improve patient recall, but also allow for more accurate diagnosis, which subsequently leads to better patient care.17,18 It is also important to establish cardiovascular risk factors, such as diabetes, hypertension, hyperlipidaemia, a family history of premature heart disease, smoking and a sedentary lifestyle. However, it should be noted that women can present with atypical symptoms and hence under-diagnosis is common in this cohort who are further under-represented in clinical trials. This is also similar in patients aged >75 years. Thus, additional attention should be paid to these two cohorts.7
Acute chest ‘pain’ or ‘discomfort’ may encompass a tightness, heaviness, pressure, fullness, or squeezing sensation that starts gradually and worsens, or does not improve with resting, lying down, drinking water or other measures. Patients may present a few hours – or even up to several days – after the onset of chest pain. As the timing of the onset is important in determining the role of hs-cTn testing, the panel recommends a cut-off time of 2 hours. There is evidence that physicians can safely identify patients who only need a single troponin level based on symptom onset, which justifies the recommendations on incorporating this element of history taking. However, the authors acknowledge that setting a cut-off time point is often challenging as in practice patients are sometimes unable to determine the exact onset of their symptoms, although efforts should be made by clinicians to estimate the timing.19–21
Step 2: Physical Examination, ECG and Bedside Echocardiography
Physical examination remains key in identifying the patient’s general condition and determining the level of illness severity. Physicians should look for signs of flushing, discomfort or sweating and review the patient’s vital signs upon arrival to the ED. Discrepancies in pulse or blood pressure should alert to the possibility of aortic dissection. An elevated jugular venous pressure, presence of gallop rhythm, leg oedema and basal crackles may indicate presence of congestive cardiac failure. One key diagnostic tool for MI is the ECG.22 However, we would remind readers that the ECG should be performed concurrently with the initial triage of the patient in ED, and possibly even before a thorough history taking is performed. This is to help identify a STEMI prior to other steps in this pathway, which would alter the management pathway significantly. Furthermore, additional ECG leads such as leads V3R, V4R and V7 to V9, are recommended if on-going ischaemia is suspected when standard leads are inconclusive to help exclude right-sided and posterior MI.
Bedside transthoracic echocardiography has also proven to be a useful tool in the initial management of acute chest pain. It allows clinicians to identify left ventricular regional wall motion abnormalities suggestive of underlying ischaemic pathology, and helps to identify important
High-sensitivity Cardiac Troponin in the Emergency Department JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
of acute myocardial ischaemia or infarction (NSTEMI, unstable angina, or STEMI).
hs-cTn
0
2 hours
the centre
produce results within a good turnaround time. The 0/2 hour algorithm is preferable because a shorter length of stay would prevent overcrowding in the
thus being ideal for resource-limited centres. If retesting within 2 hours is challenging, the 0/3 hour algorithm is recommended. ‡A baseline result of >99th percentile of the URL can assist in the decision for admission, whereas a result of ≤99th percentile of the URL may benefit from further risk stratification and/or serial hs-cTn measurements. §The HEART score is recommended because it is better at distinguishing patients at low risk for MACE and risk stratification than the GRACE and TIMI scoring systems; it also has a lower rate of missed MACE.31–33,36–39 Low risk indicates a HEART score of 0–3; intermediate to high risk indicates a HEART score >3.19 ||Decision thresholds include a RCV of >20% from a baseline value of >99th percentile URL; and an RCV>50% from a baseline value of ≤99th percentile URL.11 (Refer to Supplementary Material Table 1 for values reported in whole numbers for sex-specific 99th percentile URL). ¶Risk scores are not needed for patients who present with chest pain onset <2 hours. **Patients with negative troponin results and a low HEART risk score have a 6–18% risk of MACE.19 Patients with chest pain onset ≥2 hours and hs-cTn result with undetectable or very low levels of cardiac troponin (<40%) at the time of arrival, can be discharged without depending on the risk score.19,40–42,45–51 ED = emergency department; GRACE = Global Registry of Acute Coronary Events; HEART score = history, ECG, age, risk factors, troponin; hs-cTn = high-sensitivity cardiac troponin; MACE = major adverse cardiovascular events; NSTEMI = non-ST-elevation MI; RCV = reference change value; STEMI = ST-elevation MI; TIMI = Thrombolysis in MI; URL = upper reference limit.
differentials such as an acute pulmonary embolism and aortic dissection. It is also important to highlight the need for adequate training in performing and interpreting results from echocardiography before including it into routine clinical practice.7,23
Step 3: Initial High-sensitivity Cardiac Troponin Testing
Initial hs-cTn testing should be conducted as soon as possible if ACS is suspected.12 The hs-cTn assay’s diagnostic troponin concentration cut-off for MI is the 99th percentile value of the reference population. Levels of cTn above the 99th percentile upper reference limit (URL) indicate myocardial injury.11 A multicentre study to examine the diagnostic accuracy of cTn assays found hs-cTn assays to have a negative predictive value (NPV) of 99%.24 As the various cTn assays have distinct biological and analytical characteristics, the clinical decision limits to suggest possible MI for each assay would be different.25 Different populations may also have different URLs, and the use of cohort-specific URLs would further improve the diagnosis of ACS.26–28 Supplementary Material Table 1 shows variable 99th percentile URLs for assays based on studies in Malaysia and two other developing countries.
Major organisations, such as the European Society of Cardiology (ESC), American College of Cardiology (ACC), American Heart Association, World Heart Federation and IFCC, have recognised lower values of hs-cTn assay URLs for women than for men in their recommendations .11,29 With sexspecific thresholds, women are five times more likely to be identified with myocardial injury than men.30 Supplementary Material Table 1 includes sex-specific 99th percentile URLs for the assays showing a lower URL for women. However, an Asia-Pacific consensus has otherwise underscored the routine need for sex-based cut-off values, as little was to be gained in clinical outcome when patients were reclassified.13 It should be noted that criteria on hs-cTn assays differed between studies versus that recommended by the IFCC. Laboratories should, therefore, establish their own hs-cTn reference values based on the assays available.
Readers should also note several other causes for raised hs-cTn levels beyond myocardial injury, such as chronic kidney disease (CKD), that should be taken into consideration during interpretation of the test. Given the lack of data in the Malaysian population, it should be acknowledged
High-sensitivity Cardiac Troponin in the Emergency Department JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Figure 1: Proposed Algorithm to Rule-in and Rule-out Acute Coronary Syndrome in the Emergency Department using High-sensitivity Cardiac Troponin Patient presenting to the ED with chest pain ECG Suspected ACS* Time of onset: <2 hours or uncertain hs-cTn test at baseline (0 hour)† Negative test result‡ Negative test result|| DISCHARGE¶ ADMIT Positive test result|| Repeat hs-cTn test† + ECG Positive test result‡ Negative test result‡ Low risk DISCHARGE** ADMIT Negative test result|| Positive test result|| High risk Risk score§ Positive test result‡ Time of onset: ≥2 hours hs-cTn test at baseline (0 hour)† Repeat hs-cTn test† + ECG *Suspicion
†Serial
measurements at
and
are recommended if
has the capacity to
ED,
that both the positive predictive value and NPV of tests, including hs-cTn, is dependent upon the population of interest. A handful of studies have since be published describing the accuracy and also limitations of hs-cTn, which is a topic beyond the scope of this paper.12,13,27
Step 4: Risk Stratification
Risk assessment tools such as the HEART score should be incorporated to distinguish between patients who are at low, intermediate, or high risk for ACS-related outcomes (Table 1).31 The HEART score was first developed in 2008 as a rapid risk-stratification tool to determine the short-term risk of major adverse cardiovascular events (MACE) in patients with chest pain and has been extensively validated for use in the ED. Although the original study included patients with an identified acute MI (AMI), the HEART score is generally not used to assist with the management of patients with ACS, but to risk stratify patients who have a negative work-up in the ED who may need further testing or observation.31,32 By excluding the patient identified with an AMI from the ED cohort, this substantially changes the estimated risk for MACE in an ED population.33 Clinicians should note that scores between 0–3 indicate low risk, while scores ≥3 indicate higher risk, but this may differ depending on the patient population.20,34
The HEART score has been shown to be a better risk tool for patients with low risk for MACE and for risk stratification versus Global Registry of Acute Coronary Events and Thrombolysis in Myocardial Infarction scoring, with its lower rate of missed MACE (Supplementary Material Table 2).31,32,35–39 When performed together, patients with negative troponin results and low HEART scores have a 6–18% risk of MACE.19
It should be noted that there is also evidence that the combination of both troponin and risk stratification can be more reliable than a troponin level alone, although the evidence is not as substantial.40,41 Clinicians should be encouraged to use clinical judgement to identify such patients based on history, the patient’s HEART score and ECG findings. Clinicians should bear in mind that risk scores are not recommended in patients presenting within 2 hours of chest pain onset. Such patients, if accompanied by a negative hs-cTn result with undetectable or at very low levels of hs-cTn at the time of arrival, can be discharged without depending on risk scoring.19,42–51
Step 5: Diagnosis
During this stage, serial measurements of hs-cTn may be necessary. Myocardial injury may be acute, evident by a newly detected dynamic rise and/or fall of cTn values above the 99th percentile URL, or chronic in the setting of persistently elevated levels.11 Serial hs-cTn measurements at 0 and 2 hours from the first blood test are recommended if the laboratory is able to produce results within the expected turnaround time. The 0/2 hour algorithm is ideal as shorter length of stay within ED would prevent overcrowding especially in resource-limited centres. Studies have shown that with hs-cTn, MI can be excluded within 2 hours of presentation in the
ED.52 If retesting within 2 hours is challenging, the 0/3 hour algorithm is recommended.
The reference change value (RCV) is defined as the critical difference that must be exceeded between two sequential results for a significant (or true) change to occur. It is used to determine whether a significant change between two serial troponin values from the same cTn assay is demonstrated. This is calculated using the following formula:
Reference change value = 2 h value − 0 h value
The RCV considers both the biological and analytical variation of hs-cTn.53 For a baseline value of ≤99th percentile URL, an RCV of >50% is recommended as the decision threshold.54,55 For a baseline value of >99th percentile URL, an RCV >20% is suggested as the decision threshold.10 When the RCV is high, ACS is likely (high specificity, lower sensitivity for MI), whereas when the RCV is low, ACS becomes unlikely (higher sensitivity, lower specificity for MI).56 The use of an absolute RCV (in ng/l), which is assay dependent is preferable to relative RCV (in %) because it gives a varying set of criteria depending on the baseline value, hence maintaining sensitivity.11,57,58 For the sake of brevity, this consensus paper has not included the cut-off values for various high-sensitivity troponin assays, which are readily available online.58–61
Discussion
The proposed algorithm remains to be validated in larger populations, but nevertheless provides a useful foundation to improve ED triaging of patients presenting with acute chest pain. Patients presenting to the ED with chest pain who are deemed likely to have ACS should be admitted to an intensive care unit (ICU) or a non-ICU monitored setting, depending on their level of risk as evaluated in the ED. Patients presenting to the ED with chest pain who are unlikely to have ACS may be discharged and evaluated as outpatients.
Subsequent investigations, such as an echocardiogram, stress test, nuclear perfusion study and MRI, should be considered.62 Low-risk patients discharged with follow-up evaluation through a primary care physician and/or cardiologist have a significantly reduced risk of death or MI at 1 year.63 Patients unlikely to suffer from ACS may also benefit from referrals to other specialties to identify alternative causes for chest pain.59
It should be noted that data to suggest a significant reduction in mortality remain scarce at present from such practice. However, there have been several publications highlighting the benefit of incorporating highsensitivity troponin testing in helping to identify low-risk patients, allowing for deferral of early non-invasive cardiac testing safely while simultaneously decongesting the ED. This may lead to better patient care in the acute setting as it allows for redirection of resources and services to those who truly require them.64–67
Our expert consensus document was developed following difficulty in incorporating recommendations from international guidelines, such as those of the ESC, ACC and Asian Pacific Society of Cardiology. We provide several hypothetical scenarios below to better demonstrate the role of the proposed algorithm.
Rule-out Protocol for Acute Coronary Syndrome
Recommendation 1: Chest pain onset of <2 hours, with an initial hs-cTn ≤99th percentile URL followed by second hs-cTn with RCV of <50% may rule out ACS.
High-sensitivity Cardiac Troponin in the Emergency Department JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
with High-sensitivity Cardiac Troponin
Table 1: Items in the HEART Score Item Score History 0–2 ECG 0–2 Age 0–2 Risk factors 0–2 Troponin 0–2 Total score 0–10 Total score: 0–3 points = low risk; 4–6 points = intermediate risk; 7–10 points = high risk.
Case scenario 1: A 58-year-old man presented to the ED with central chest pain, which occurred <2 hours earlier. He described the pain as pricking and lasting 10 minutes with no associated symptoms. He had a history of diabetes, hypertension and hypercholesterolaemia, and smoked regularly. On physical assessment, he was pain free. His vital signs and clinical examination were unremarkable. His ECG revealed sinus rhythm with no ischaemic changes.
This man presented with a short duration chest pain, which occurred <2 hours earlier. He has several cardiovascular risk factors. Although his ECG was normal, an initial hs-cTn test, followed by a second test 2 hours after the initial blood test, is recommended. If both the hs-cTn test results are normal, MI can be safely ruled out. The hs-cTn algorithms have high sensitivity and high NPV, and further investigations to rule out MI are unnecessary. The patient can be discharged from the ED and safely evaluated as an outpatient. An alternative approach, outside the proposed algorithm, would be to perform a second hs-cTn test and to observe if the results are similarly ≤99th percentile URL (as opposed to observing RCV), which can appear much safer and clinicians should be encouraged to use the clinical gestalt to identify such patients based on history, the patient’s HEART score and ECG findings. Furthermore, early follow-up outpatient assessment by a cardiologist or a general physician should also be considered.
Recommendation 2: Chest pain onset of ≥2 hours before presentation, low HEART score, and hs-cTn ≤99th percentile URL may rule out ACS.
Case scenario 2: A 50-year-old man with underlying hypertension complained of central chest pain, which occurred >2 hours prior to hospital arrival. The pain lasted for 10 minutes and was associated with mild breathlessness. He denied any palpitations, sweating or loss of consciousness. On physical assessment he was alert and not in distress. His pain score was 2/10. His vital signs and clinical examination were unremarkable. His ECG revealed sinus rhythm with no ischaemic changes.
This 50-year-old man presented to the ED with chest pain >2 hours prior. His ECG was normal. The first hs-cTn test was negative (≤99th percentile URL). Despite a history of hypertension, his cardiovascular risk remains low based on his HEART score of 3. A second hs-cTn test is not deemed necessary and the patient can be discharged with subsequent outpatient follow-up, as described by Wassie et al. where safe discharge based on history and a single troponin testing was considered safe.21
Recommendation 3: Chest pain onset of ≥2 hours, and hs-cTn ≤99th percentile URL followed by second hs-cTn with RCV of <50% even with a high HEART score may rule out ACS.
Case scenario 3: A 65-year-old woman with underlying hypertension, diabetes and dyslipidaemia presented with chest heaviness associated with breathlessness, which occurred 4 hours earlier. The pain occurred when she was climbing up a staircase and resolved with rest. At the time of physical assessment, she was pain free. Her vital signs and clinical examination were unremarkable. Her ECG revealed sinus rhythm changes consistent with left ventricular hypertrophy.
This woman complained of chest pain on assessment. The ECG revealed sinus rhythm with left ventricular hypertrophy changes. The first hs-cTn was negative (≤99th percentile URL). As the patient had multiple cardiovascular risk factors (hypertension, diabetes and dyslipidaemia), her HEART score was 6 (moderate risk). For this patient, hs-cTn test should be repeated 2 hours from initial testing. Following a second test that shows no significant change from baseline (RCV ≤50%) the patient can be discharged with subsequent follow-up within an outpatient setting.
However, similar to case scenario 1, an alternative approach could be undertaken, which can appear much safer. Again, clinicians should be encouraged to use clinical gestalt to identify such patients based on history, HEART score and ECG findings, alongside early outpatient followup. Even in the absence of positive results through repeated hs-cTn testing, it would be reasonable for clinicians to observe the patient within a medical assessment unit or observation bay which has since become a common adjunct in most EDs in Malaysia. This may allow for medical optimisation of cardiovascular risk factors including blood pressure and glycaemic control acutely, prior to discharging the patient for outpatient follow-up.
Rule-in Protocol for Acute Coronary Syndrome with High-sensitivity Cardiac Troponin
Recommendation 4: Chest pain onset of <2 hours before presentation and initial hs-cTn >99th percentile URL may rule in ACS.
Case scenario 4: A 46-year-old man presented to the ED with central chest pain, which occurred an hour earlier. He had no history of diabetes, hypertension, or hypercholesterolaemia but smoked regularly. His ECG showed poor R wave progression. His renal profile was normal on laboratory testing.
The patient had short-duration chest pain with cardiovascular risk factors. Based on the ECG finding, coronary artery disease was suspected. An initial hs-cTn test followed by a second test (2 hours from presentation) was recommended. If the first hs-cTn test was elevated, the patient should be referred to a cardiologist or responsible physician. A second hs-cTn test is not necessary. The patient was admitted and treated for NSTEMI with dual antiplatelet therapy and low-molecular-weight heparin (LMWH).
Case scenario 5: A 65-year-old man with hypertension, dyslipidaemia, stage 3 CKD and diabetes complained of left-sided chest discomfort, which occurred 30 minutes before arrival to the hospital. The pain lasted for 20 minutes and was associated with mild sweating. On physical assessment, he was alert and had minimal pain. A coronary angiogram conducted 8 years ago was normal. His pain score was 3/10. His vital signs and clinical examination were unremarkable. His ECG revealed sinus rhythm with T-wave inversions in lead III and aVF. His renal profile was deranged, with an estimated glomerular filtration rate of 45 ml/min/1.73 m2
This patient presented to the ED, with complaints of chest pain that occurred <2 hours earlier. His clinical history indicated high suspicion for acute coronary syndrome. Serial ECGs taken 15 minutes apart, revealed non-specific repolarisation disturbance. The first hs-cTn test result was positive (>99th percentile URL). The patient was admitted and treated for NSTEMI with dual antiplatelets and LMWH.
High-sensitivity Cardiac Troponin in the Emergency Department JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
A caveat to consider would be that the patient has concomitant CKD, as this may lead to elevation in hs-cTn levels, both in presence or absence of myocardial injury. However, the expert panel believes that patient suffering from CKD, especially those in the later stages (i.e. stage 4 and 5) are of a particularly higher risk and would benefit from closer evaluation as an inpatient whenever feasible. Inpatient review should incorporate serial hscTn testing to better assess risk and allow closer monitoring for acute cardiopulmonary decompensation in this otherwise fragile group of patients. This is also largely supported by evidence that even among patients with renal dysfunction without ACS, raised troponin levels are observed in about 40%, often not because of reduced renal clearance, but instead via myocardial injury through uraemia, increased left ventricular pressure, hypotension, microvascular dysfunction and anaemia.13
We would recommend further reading on differences in definition of MI, differentiating both Type 1 and 2 MI as well, which is beyond the scope of this consensus paper.11
Recommendation 5: Chest pain onset of <2 hours before presentation and initial hs-cTn ≤99th percentile URL followed by second hs-cTn with RCV of >50% may rule in ACS.
Case scenario 6: A 35-year-old woman with hypertension and diabetes complained of left-sided chest discomfort, which occurred an hour prior to arrival to the hospital. The pain lasted for 15 minutes and was associated with mild dyspnoea. This was the patient’s first episode, and it was brought on by moderate exertion. On physical assessment, she was alert and pain free. Her vital signs and clinical examination were unremarkable. Her ECG revealed sinus rhythm with no ischaemic changes.
This patient presented to the ED complaining of chest pain that occurred within 2 hours. Her clinical history indicates moderate suspicion for coronary artery disease. Initial ECG showed non-specific repolarisation disturbance. The initial hs-cTn test was negative (≤99th percentile URL) and her HEART score was 3, indicating low risk. The patient was observed for further chest pain, while remaining pain free.
Serial ECGs, taken 15 minutes apart, revealed no progressive changes. However, the second hs-cTn test result (conducted 2 hours after the first test) showed an RCV of >50% from baseline value. The patient was admitted and treated for NSTEMI with dual antiplatelets and LMWH. There was also emphasis on better blood pressure and glycaemic control, with regular close monitoring of her capillary blood glucose levels, as diabetic ketoacidosis remains a possible life-threatening differential.
Recommendation 6: Chest pain onset of ≥2 hours before presentation and initial hs-cTn >99th percentile URL may rule in ACS.
Case scenario 7: A 64-year-old man presented to the ED with central chest pain, which occurred 4 hours earlier. He had history of diabetes, hypertension and hypercholesterolaemia and smoked regularly. His ECG was normal.
This patient presented with chest pain that occurred beyond 2 hours from presentation. He had multiple cardiovascular risk factors. His ECG was
normal. An initial hs-cTn test, followed by a second test (2 hours from initial testing) was performed and returned positive (>99th percentile URL). The patient should be referred to a cardiologist or responsible physician. In this case, a second hs-cTn is unnecessary.
Case scenario 8: A 36-year-old male smoker complained of retrosternal chest pain, which occurred 2 hours prior to hospital arrival. The pain lasted for an hour and was associated with sweating, nausea and vomiting. He required parenteral opioid and isosorbide dinitrate infusion to control the pain. On physical assessment he appeared anxious and his pain score was 6/10. His vital signs and clinical examination were unremarkable. His ECG revealed sinus rhythm with ST depression at leads l and V2–V6, and T-wave inversions at II, III and aVF.
This patient presented to the ED complaining of chest pain that occurred more than 2 hours earlier. Based on ECG results and his prolonged chest pain (lasted for 1 hour) and accompanying symptoms, ACS was suspected. As the first hs-cTn test result was positive (>99th percentile URL), he was admitted for NSTEMI and treated with dual antiplatelets and LMWH. As the patient is in a high-risk category, risk scoring and a second hs-cTn were not necessary.
Recommendation 7: Chest pain of unknown onset and initial hs-cTn ≤99th percentile URL followed by second hs-cTn with RCV of ≤50% may rule in ACS.
Case scenario 9: A 68-year-old male smoker with hypertension and dyslipidaemia developed central chest pain, which radiated to both shoulders 3 hours prior. The pain lasted for 20 minutes before it gradually subsided. He vomited once. On physical assessment, his pain score was 3/10. His vital signs were unremarkable. Examination revealed a third heart sound with bibasal crepitations. His ECG revealed sinus rhythm with ST-segment depression at leads II, III and aVF, and T-wave inversions at V4–V6, I and aVL.
This patient presented to the ED complaining of chest pain that occurred prior to his arrival. The time of onset of chest pain was unknown. He had multiple cardiovascular risk factors. His ECG revealed sinus rhythm with significant ST deviation. Based on ECG and clinical history, ACS was highly suspected. An initial hs-cTn test was conducted upon arrival, but results were negative (≤99th percentile URL). The HEART score was 8, indicating high risk for cardiovascular disease. Although a second hs-cTn test repeated 2 hours after the initial test demonstrated an RCV ≤50%, the patient was still admitted for observation and inpatient work-up for ischaemic heart disease.
The reason for introducing this case scenario is to remind clinicians that guidelines and recommendations should never replace clinical gestalt. Despite two negative hs-cTn tests, the patient’s clinical history, HEART score and ECG were worrying enough to support a decision for observation and further investigation, ideally as an inpatient. This may include a transthoracic echocardiogram to look for new regional wall motion abnormalities, serial hs-cTn testing, coronary artery assessment via CT imaging or imaging for evidence of ischaemia via cardiac MRI or nuclear myocardial perfusion imaging.
High-sensitivity Cardiac Troponin in the Emergency Department JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
The Use of Other Cardiac Troponin Assays in the Emergency Department
Although conventional cTn tests have high diagnostic accuracy, hs-cTn testing has been shown to be more sensitive.60,68,69 A meta-analysis revealed that hs-cTn had significantly greater sensitivity (0.884 versus 0.749; p<0.001) and NPV (0.964 versus 0.935; p<0.001) compared to the conventional assay in patients with chest pain.60 However, when hs-cTn testing is unavailable or inaccessible, POCT may be a reasonable alternative to diagnose ACS in the ED.12 These tests have a short turnaround time, making them suitable in EDs where there is overcrowding or poor access to laboratories.70,71 However, it should be noted that many POCT cTn assays have lower sensitivity, diagnostic accuracy and NPV than central laboratory tests.63,64,72–74 One study showed that a POCT cTn assay had 68% sensitivity compared to a hs-cTn assay, which had a 98% sensitivity for excluding MI.70 The NPV was 95% for POCT troponin compared to 98% for hs-cTn.70 Additionally, POCT has high rates of underdiagnosis. In one study, the POCT for troponin missed 29% of patients with acute MI or unstable angina.75 Therefore, extreme caution is required when using POCT troponin to rule out ACS in the ED.
Non-ischaemic Myocardial Injuries
In cases with low pre-test probability for myocardial ischaemia, efforts should be made to identify alternative causes for symptoms. Physicians should be familiar with various conditions where non-ischaemic myocardial injury could occur and cause raised troponin levels (Table 2) to avoid premature diagnosis of ACS, which then leads to inappropriate cascading of investigations and interventions.76–78 However, raised troponins are often useful for prognosis even in such cases, and can guide management. Information from other diagnostic modalities such as transoesophageal echocardiography, late gadolinium enhancement in cardiac MRI, CT coronary angiogram and – when necessary – the conventional coronary angiogram, should be used in conjunction with serial cTn measurements to establish a diagnosis.
Late Presentation in Patients with NSTEMI
It should be noted that in patients presenting beyond 12 hours with an initial elevated hs-cTn, care should be taken in the interpretation of differences in values between subsequent results. Patients with adjudicated NSTEMI with less acute presentations, and those with longer ischaemic times, are more likely to present closer to their peak hs-cTn value. Subsequent values may experience reduction or plateau, which can at times provide false reassurance to clinicians away from the diagnosis of NSTEMI. This is important because small changes in cardiac enzyme levels are common in patients with NSTEMI who remain at substantial risk for mortality.79 A way to circumvent this is by identifying high-risk features linked to ACS. Although this remains beyond the scope of this manuscript, we provide the necessary references for the perusal of readers.80
Conclusion
Our expert consensus provides a structured approach to using hs-cTn in the ED. It remains the only one to date produced by a group of Malaysian
1. Nowbar AN, Gitto M, Howard JP, et al. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes 2019;12:e005375. https://doi.org/10.1161/ CIRCOUTCOMES.118.005375; PMID: 31163980.
2. Seligman B, Vedanthan R, Fuster V. Acute coronary syndromes in low- and middle-income countries: Moving forward. Int J Cardiol 2016;217(Suppl):S10–2. https://doi. org/10.1016/j.ijcard.2016.06.213; PMID: 27381860.
Table 2: Conditions that Can Lead to Non-ischaemic Myocardial Injury and Raised High-sensitivity Cardiac Troponin75–78
• Chronic kidney disease
• Chemotherapy-associated cardiotoxicity
• Amyloidosis
• Heart failure
• Pulmonary embolism
• Sepsis
• Takotsubo syndrome
• Critical illness
• Supraventricular tachycardia and AF
• Myocarditis and myopericarditis
• Infections
• Hypertension and left ventricular hypertrophy
• Aortic dissection
• Post-transplantation monitoring
• Blunt chest trauma
• Post-operative monitoring in non-cardiac surgery
• Acute kidney injury
• Systemic lupus erythematosus
• Subarachnoid haemorrhage
• Stroke
• Endocarditis
• Cardiopulmonary resuscitation
• Diabetes
• Pregnancy
experts to help guide local healthcare providers in dealing with patients presenting with acute chest pain. A local consensus statement was felt necessary as it would incorporate essential information based on local availability of diagnostic and therapeutic modalities. Having a scenarioguided consensus statement would also supplement existing recommendations on the use of high-sensitive troponin testing in the ED setting, while enforcing the need for practicing clinicians to use their clinical acumen when in doubt, to avoid test-related issues.
Clinical Perspective
• High-sensitivity cardiac troponin assays are currently recommended to help triage patients with suspected acute coronary syndrome.
• However, despite strong recommendations in adopting high-sensitivity cardiac troponin, less sensitive rapid assays are often a preferred choice in majority of hospitals because of rapid results without the need for central laboratory involvement.
• Our expert consensus provides guidance for healthcare professionals in understanding the role of high-sensitivity cardiac troponin, specifically its level of sensitivity and specificity as well as its practical application in the emergency department setting, particularly in resource-limited centres.
3. Department of Statistics Malaysia. Statistics on causes of death, Malaysia, 2019. 2019. https://dosm.gov.my/v1/index. php?r=column/pdfPrev&id=RUxlSDNkcnRVazJnakNCNVN2VG grdz09 (accessed 21 April 2022).
4. National Heart Association of Malaysia. Annual report of the NCVD-ACS registry 2014–15. https://www.malaysianheart. org/?p=ncvd&a=1250 (accessed 28 April 2022).
5. Johnson T, Gaus D, Herrera D. Emergency department of a
rural hospital in Ecuador. West J Emerg Med 2016;17:66–72. https://doi.org/10.5811/westjem.2015.11.27936; PMID: 26823934.
6. Paichadze N, Afzal B, Zia N, et al. Characteristics of chest pain and its acute management in a low-middle income country: analysis of emergency department surveillance data from Pakistan. BMC Emerg Med 2015;15(Suppl 2):S13. https://doi.org/10.1186/1471-227X-15-S2-S13; PMID: 26691439.
High-sensitivity Cardiac Troponin in the Emergency Department JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
7. Gulati M, Levy PD, Mukherjee D, et al. AHA/ACC/ASE/CHEST/ SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;144:e368–454. https://doi.org/10.1161/CIR.0000000000001029; PMID: 34709879.
8. Januzzi JL Jr, McCarthy CP. Evaluating chest pain in the emergency department: searching for the optimal gatekeeper. J Am Coll Cardiol 2018;71:617–9. https://doi. org/10.1016/j.jacc.2017.11.065; PMID: 29420957.
9. Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med 2000;342:1163–70. https://doi. org/10.1056/NEJM200004203421603; PMID: 10770981.
10. Garg P, Morris P, Fazlanie AL, et al. Cardiac biomarkers of acute coronary syndrome: from history to high-sensitivity cardiac troponin. Intern Emerg Med 2017;12:147–55. https:// doi.org/10.1007/s11739-017-1612-1; PMID: 28188579.
11. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019;40:237–69. https://doi.org/10.1093/eurheartj/ehy462
12. Tan WCJ, Inoue K, AbdelWareth L, et al. The Asia-Pacific Society of Cardiology (APSC) expert committee consensus recommendations for assessment of suspected acute coronary syndrome using high-sensitivity cardiac troponin T in the emergency department. Circ J 2020;84:136–43. https://doi.org/10.1253/circj.CJ-19-0874; PMID: 31852863.
13. Chieh JWC, Lam CSP, Kasim SS, et al. Asia-Pacific consensus statement on the optimal use of high-sensitivity troponin assays in acute coronary syndromes diagnosis: focus on hs-TnI. Heart Asia 2017;9:81–7. https://doi.org/10.1136/ heartasia-2016-010818; PMID: 28466882.
14. Roffi M, Patrono C, Collet JP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. https://doi.org/10.1093/eurheartj/ehv320; PMID: 26320110.
15. Apple FS, Jaffe AS, Collinson P, et al. IFCC educational materials on selected analytical and clinical applications of high sensitivity cardiac troponin assays. Clin Biochem 2015;48:201–3. https://doi.org/10.1016/j. clinbiochem.2014.08.021; PMID: 25204966.
16. Aloe R, Lippi G, Di Pietro M, et al. Improved efficiency and cost reduction in the emergency department by replacing contemporary sensitive with high-sensitivity cardiac troponin immunoassay. Acta Biomed 2019;90:614–20. https://doi. org/10.23750/abm.v90i4.8769; PMID: 31910198.
17. Kalitzkus V, Matthiessen PF. Narrative-based medicine: potential, pitfalls and practice. Perm J 2009;13:80–6. https:// doi.org/10.7812/TPP/08-043; PMID: 21373252.
18. Rosti G. Role of narrative-based medicine in proper patient assessment. Support Care Cancer 2017;25(Suppl 1):3–6. https://doi.org/10.1007/s00520-017-3637-4; PMID: 28220317.
19. Chenevier-Gobeaux C, Sebanne M, Meune C, et al. Is highsensitivity troponin, alone or in combination with copeptin, sensitive enough for ruling out NSTEMI in very early presenters at admission? A post hoc analysis performed in emergency departments. BMJ Open 2019;9:e023994. https:// doi.org/10.1136/bmjopen-2018-023994; PMID: 31209082.
20. Wildi K, Nelles B, Twerenbold R, et al. Safety and efficacy of the 0 h/3 h protocol for rapid rule out of myocardial infarction. Am Heart J 2016;181:16–25. https://doi. org/10.1016/j.ahj.2016.07.013; PMID: 27823689.
21. Wassie M, Lee MS, Sun BC, et al. Single vs serial measurements of cardiac troponin level in the evaluation of patients in the emergency department with suspected acute myocardial infarction. JAMA Netw Open 2021;4:e2037930. https://doi.org/10.1001/jamanetworkopen.2020.37930; PMID: 33620444.
22. Hollander JE, Than M, Mueller C. State-of-the-art evaluation of emergency department patients presenting with potential acute coronary syndromes. Circulation 2016;134:547–64. https://doi.org/10.1161/CIRCULATIONAHA.116.021886; PMID: 27528647.
23. Flachskampf FA, Daniel WG. Cardiac imaging in the patient with chest pain: echocardiography. Heart 2010;96:1063–72. https://doi.org/10.1136/hrt.2009.172114; PMID: 20584859.
24. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of acute myocardial infarction using more sensitive cardiac troponin assays. N Engl J Med 2009;361:858–67. https://doi. org/10.1056/NEJMoa0900428; PMID: 19710484.
25. Aw TC, Huang WT, Le TT, et al. High-sensitivity cardiac troponins in cardio-healthy subjects: a cardiovascular magnetic resonance imaging study. Sci Rep 2018;8:15409. https://doi.org/10.1038/s41598-018-33850-9
PMID: 30337631.
26. Gaggin HK, Dhang PV, Do LD, et al. Reference interval evaluation of high-sensitivity troponin T and N-terminal B-type natriuretic peptide in Vietnam and the US: the North South East West trial. Clin Chem 2014;60:758–64. https://doi. org/10.1373/clinchem.2013.216275; PMID: 24568795.
27. Li S, Zuo Y, Huong W. Establishment of a reference interval for high-sensitivity cardiac troponin I in healthy adults from the Sichuan area. Med (Baltim) 2017;96:e6252. https://doi. org/10.1097/MD.0000000000006252; PMID: 28383401.
28. Lim SM, Thambiah SC, Zahari Sham SY, et al. Determination of the 99th percentile upper reference limit for highsensitivity cardiac troponin I in Malaysian population. Malays J Pathol 2017;39:135–40; PMID: 28866694.
29. Wu AHB, Christenson RH, Greene DN, et al. Clinical Laboratory Practice Recommendations for the use of cardiac troponin in acute coronary syndrome: expert opinion from the Academy of the American Association for Clinical Chemistry and the Task Force on Clinical Applications of Cardiac Bio-Markers of the International Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem 2018;64:645–55. https://doi.org/10.1373/ clinchem.2017.277186; PMID: 29343532.
30. Lee KK, Ferry AV, Anand A, et al. Sex-specific thresholds of high-sensitivity troponin in patients with suspected acute coronary syndrome. J Am Coll Cardiol 2019;74:2032–43. https://doi.org/10.1016/j.jacc.2019.07.082; PMID: 31623760.
31. Brady W, de Souza K. The HEART score: a guide to its application in the emergency department. Turk J Emerg Med 2018;18:47–51. https://doi.org/10.1016/j.tjem.2018.04.004; PMID: 29922729.
32. Aarts GWA, Camaro C, van Geuns RJ, et al. Acute rule-out of non-ST-segment elevation acute coronary syndrome in the (pre)hospital setting by HEART score assessment and a single point-of-care troponin: rationale and design of the ARTICA randomised trial. BMJ Open 2020;10:e034403. https://doi.org/10.1136/bmjopen-2019-034403; PMID: 32071186.
33. Sharp AL, Wu YL, Shen E, et al. The HEART score for suspected acute coronary syndrome in U.S. Emergency departments. J Am Coll Cardiol 2018;72:1875–7. https://doi. org/10.1016/j.jacc.2018.07.059; PMID: 30286933.
34. Than M, Herbert M, Flaws D, et al. What is an acceptable risk of major adverse cardiac event in chest pain patients soon after discharge from the emergency department?: a clinical survey. Int J Cardiol 2013;166:752–4. https://doi. org/10.1016/j.ijcard.2012.09.171; PMID: 23084108.
35. Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART score. Crit Pathw Cardiol 2010;9:164–9. https://doi. org/10.1097/HPC.0b013e3181ec36d8; PMID: 20802272.
36. Six AJ, Cullen L, Backus BE, et al. The HEART score for the assessment of patients with chest pain in the emergency department: a multinational validation study. Crit Pathw Cardiol 2013;12:121–6. https://doi.org/10.1097/ HPC.0b013e31828b327e; PMID: 23892941.
37. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol 2013;168:2153–8. https://doi.org/10.1016/j.ijcard.2013.01.255; PMID: 23465250.
38. Mahler SA, Miller CD, Hollander JE, et al. Identifying patients for early discharge: performance of decision rules among patients with acute chest pain. Int J Cardiol 2013;168:795–802. https://doi.org/10.1016/j.ijcard.2012.10.010; PMID: 23117012.
39. Poldervaart JM, Langedijk M, Backus BE, et al. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol 2017;227:656–61. https://doi.org/10.1016/j.ijcard.2016.10.080; PMID: 27810290.
40. Chapman AR, Hesse K, Andrews J, et al. High-sensitivity cardiac troponin I and clinical risk scores in patients with suspected acute coronary syndrome. Circulation 2018;138:1654–65. https://doi.org/10.1161/ CIRCULATIONAHA.118.036426; PMID: 30354460.
41. Bularga A, Lee KK, Stewart S, et al. High-sensitivity troponin and the application of risk stratification thresholds in patients with suspected acute coronary syndrome. Circulation 2019;140:1557–68. https://doi.org/10.1161/ CIRCULATIONAHA.119.042866; PMID: 31475856.
42. Haaf P, Drexler B, Reichlin T, et al. High-sensitivity cardiac troponin in the distinction of acute myocardial infarction from acute cardiac non-coronary artery disease. Circulation 2012;126:31–40. https://doi.org/10.1161/ CIRCULATIONAHA.112.100867; PMID: 22623715.
43. Reiter M, Trewenbold R, Reichlin T, et al. Early diagnosis of acute myocardial infarction in patients with pre-existing coronary artery disease using more sensitive cardiac troponin assays. Eur Heart J 2012;33:988–97. https://doi. org/10.1093/eurheartj/ehr376; PMID: 22044927.
44. Apple FS. A new season for cardiac troponin assays: it’s
time to keep a scorecard. Clin Chem 2009;55:1303–6. https://doi.org/10.1373/clinchem.2009.128363; PMID: 19478023.
45. Rubini Gimenez M, Trewenbold R, Reichlin T, et al. Direct comparison of high-sensitivity-cardiac troponin I vs. T for the early diagnosis of acute myocardial infarction. Eur Heart J 2014;35:2303–11. https://doi.org/10.1093/eurheartj/ehu188; PMID: 24842285.
46. Reichlin T, Trewenbold R, Reichlin T, et al. Introduction of high-sensitivity troponin assays: impact on myocardial infarction incidence and prognosis. Am J Med 2012;125:1205–13.e1. https://doi.org/10.1016/j. amjmed.2012.07.015; PMID: 23164485.
47. Body R, Carley S, McDowall G, et al. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol 2011;58:1332–9. https://doi.org/10.1016/j.jacc.2011.06.026; PMID: 21920261.
48. Rubini Giménez M, Hoeller R, Reichlin T, et al. Rapid rule out of acute myocardial infarction using undetectable levels of high-sensitivity cardiac troponin. Int J Cardiol 2013;168:3896–901. https://doi.org/10.1016/j.ijcard.2013.06.049; PMID: 23876467.
49. Zhelev Z, Hyde C, Youngman E, et al. Diagnostic accuracy of single baseline measurement of Elecsys troponin T highsensitive assay for diagnosis of acute myocardial infarction in emergency department: systematic review and metaanalysis. BMJ 2015;350:h15. https://doi.org/10.1136/bmj.h15; PMID: 25646632.
50. Body R, Burrows G, Carley S, et al. High-sensitivity cardiac troponin T concentrations below the limit of detection to exclude acute myocardial infarction: a prospective evaluation. Clin Chem 2015;61:983–9. https://doi.org/10.1373/ clinchem.2014.231530; PMID: 25979953.
51. Shah AS, Anand A, Sandoval Y, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet 2015;386:2481–8. https://doi.org/10.1016/S0140-6736(15)00391-8; PMID: 26454362.
52. Cullen L, Aldous S, Than M, et al. Comparison of high sensitivity troponin T and I assay in the diagnosis of non-ST elevation acute myocardial infarction in emergency patients with chest pain. Clin Biochem 2014;47:321–6. https://doi. org/10.1016/j.clinbiochem.2013.11.019; PMID: 24316100.
53. Kozinski M, Krintus M, Kubica J, Sypniewska G. Highsensitivity cardiac troponin assays: from improved analytical performance to enhanced risk stratification. Crit Rev Clin Lab Sci 2017;54:143–72. https://doi.org/10.1080/10408363.2017.12 85268; PMID: 28457177.
54. Thygesen K, Mair J, Giannitsis E, et al. How to use highsensitivity cardiac troponins in acute cardiac care. Eur Heart J 2012;33:2252–7. https://doi.org/10.1093/eurheartj/ehs154; PMID: 22723599.
55. Chenevier-Gobeaux C, Bonnefoy-Cudraz É, Charpentier S, et al. High-sensitivity cardiac troponin assays: answers to frequently asked questions. Arch Cardiovasc Dis 2015;108:132–49. https://doi.org/10.1016/j.acvd.2014.11.001; PMID: 25669958.
56. Korley FK, Jaffe AS. Preparing the United States for highsensitivity cardiac troponin assays. J Am Coll Cardiol 2013;61:1753–8. https://doi.org/10.1016/j.jacc.2012.09.069; PMID: 23395074.
57. Vasile VC, Jaffe AS. High-sensitivity cardiac troponin for the diagnosis of patients with acute coronary syndromes. Curr Cardiol Rep 2017;19:92. https://doi.org/10.1007/s11886-0170904-4; PMID: 28840515.
58. Vasile VC, Jaffe SA. High-sensitivity cardiac troponin in the evaluation of possible AMI. American College of Cardiology, 2018. https://www.acc.org/latest-in-cardiology/ articles/2018/07/16/09/17/high-sensitivity-cardiac-troponin-inthe-evaluation-of-possible-ami (accessed 21 April 2022).
59. Apple FS, Sandoval Y, Jaffe AS, et al. Cardiac troponin assays: guide to understanding analytical characteristics and their impact on clinical care. Clin Chem 2017;63:73–81. https://doi.org/10.1373/clinchem.2016.255109; PMID: 28062612.
60. Tjora HL, Steiro OT, Langørgen J, et al. Cardiac troponin assays with improved analytical quality: A trade-off between enhanced diagnostic performance and reduced long-term prognostic value. J Am Heart Assoc 2020;9:e017465. https:// doi.org/10.1161/JAHA.120.017465; PMID: 33238783.
61. Melanson SE, Tanasijevic MJ, Jarolim P. Cardiac troponin assays: a view from the clinical chemistry laboratory. Circulation 2007;116:e501–4. https://doi.org/10.1161/ CIRCULATIONAHA.107.722975; PMID: 17967982.
62. Ministry of Health Malaysia. Clinical practice guidelines on stable coronary artery disease 2018, 2nd ed. Kuala Lumpur: National Heart Association of Malaysia, 2018. https://www. malaysianheart.org/?p=cpg&a=1296 (accessed 28 April 2022).
High-sensitivity Cardiac Troponin in the Emergency Department JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
63. Czarnecki A, Wang JT, Tu JV, et al. The role of primary care physician and cardiologist follow-up for low-risk patients with chest pain after emergency department assessment. Am Heart J 2014;168:289–95. https://doi.org/10.1016/j. ahj.2014.05.016; PMID: 25173539.
64. Natsui S, Sun BC, Shen E, et al. Higher emergency physician chest pain hospitalization rates do not lead to improved patient outcomes. Circ Cardiovasc Qual Outcomes 2021;14:e006297. https://doi.org/10.1161/ CIRCOUTCOMES.119.006297; PMID: 33430609.
65. Kawatkar AA, Sharp AL, Baecker AS, et al. Early noninvasive cardiac testing after emergency department evaluation for suspected acute coronary syndrome. JAMA Intern Med 2020;180:1621–9. https://doi.org/10.1001/ jamainternmed.2020.4325; PMID: 33031502.
66. Foy AJ, Liu G, Davidson WR Jr, et al. Comparative effectiveness of diagnostic testing strategies in emergency department patients with chest pain: an analysis of downstream testing, interventions, and outcomes. JAMA Intern Med 2015;175:428–36. https://doi.org/10.1001/ jamainternmed.2014.7657; PMID: 25622287.
67. Natsui S, Sun BC, Shen E, et al. Evaluation of outpatient cardiac stress testing after emergency department encounters for suspected acute coronary syndrome. Ann Emerg Med 2019;74:216–23. https://doi.org/10.1016/j. annemergmed.2019.01.027; PMID: 30955986.
68. Lipinski MJ, Baker NC, Escarcega RO, et al. Comparison of conventional and high-sensitivity troponin in patients with chest pain: a collaborative meta-analysis. Am Heart J 2015;169:6–16.e6. https://doi.org/10.1016/j.ahj.2014.10.007; PMID: 25497242.
69. Fox WR, Diercks DB. Troponin assay use in the emergency department for management of patients with potential acute coronary syndrome: current use and future directions. Clin Exp Emerg Med 2016;3:1–8. https://doi.org/10.15441/ ceem.16.120; PMID: 27752608.
70. Ammirati E, Dobrev D. Conventional troponin-I versus highsensitivity troponin-T: performance and incremental prognostic value in non-ST-elevation acute myocardial infarction patients with negative CK-MB based on a realworld multicenter cohort. Int J Cardiol Heart Vasc 2018;20:38–9. https://doi.org/10.1016/j.ijcha.2018.07.002; PMID: 30094334.
71. Bingisser R, Cairns C, Christ M, et al. Cardiac troponin: a critical review of the case for point-of-care testing in the ED. Am J Emerg Med 2012;30:1639–49. https://doi.org/10.1016/j. ajem.2012.03.004; PMID: 22633720.
72. Aldous S, Richards AM, George PM, et al. Comparison of new point-of-care troponin assay with high sensitivity troponin in diagnosing myocardial infarction. Int J Cardiol 2014;177:182–6. https://doi.org/10.1016/j.ijcard.2014.09.026; PMID: 25499373.
73. Collinson PO, Saenger AK, Apple FS. High sensitivity, contemporary and point-of-care cardiac troponin assays: educational aids developed by the IFCC Committee on Clinical Application of Cardiac Bio-Markers. Clin Chem Lab Med 2019;57:623–32. https://doi.org/10.1515/cclm-2018-1211; PMID: 30530880.
74. Alan HB. Recent advances in point-of-care diagnostics for cardiac markers. EJIFCC 2014;25:170–7. PMID: 27683464.
75. Ter Avest E, Visser A, Reitsma B, et al. Point-of-care troponin T is inferior to high-sensitivity troponin T for ruling out acute
myocardial infarction in the emergency department. Eur J Emerg Med 2016;23:95–101. https://doi.org/10.1097/ MEJ.0000000000000225; PMID: 25536392.
76. Nilsson S, Andersson PO, Borgquist L, et al. Point-of-care troponin T testing in the management of patients with chest pain in the Swedish primary care. Int J Fam Med 2013;2013:532093. https://doi.org/10.1155/2013/532093; PMID: 23365746.
77. Newby LK, Jesse RL, Babb JD, et al. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: a report of the American College of Cardiology Foundation task force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2012;60:2427–63. https://doi.org/10.1016/j.jacc.2012.08.969; PMID: 23154053.
78. Song D, de Zoysa JR, Ng A, Chiu W. Troponins in acute kidney injury. Ren Fail 2012;34:35–9. https://doi.org/10.3109/ 0886022X.2011.623440; PMID: 22010639.
79. Bjurman C, Larsson M, Johanson P, et al. Small changes in troponin T levels are common in patients with non-STsegment elevation myocardial infarction and are linked to higher mortality. J Am Coll Cardiol 2013;62:1231–8. https://doi. org/10.1016/j.jacc.2013.06.050; PMID: 23933541.
80. Stepinska J, Lettino M, Ahrens I, et al. Diagnosis and risk stratification of chest pain patients in the emergency department: focus on acute coronary syndromes. A position paper of the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care 2020;9:76–89. https://doi. org/10.1177/2048872619885346; PMID: 31958018.
High-sensitivity Cardiac Troponin in the Emergency Department JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Impact of BMI and Type of Ablation Procedure on Atrial Fibrillation Recurrence in Japanese Patients
Aiko Takami , 1 Junichiro Miake , 2 Masaru Kato , 1 Kazuyoshi Ogura , 1 Akihiro Okamura , 1 Takuya Tomomori , 1 Daiki Tsujimoto , 1 Syunsuke Kawatani , 1 Masahiko Kato 1 and Kazuhiro Yamamoto 1
1. Department of Cardiovascular Medicine and Endocrinology and Metabolism, Faculty of Medicine, Tottori University, Yonago, Japan; 2. Division of Pharmacology, Department of Pathophysiological and Therapeutic Science, Faculty of Medicine, Tottori University, Yonago, Japan
Abstract
Background: AF recurs more frequently after catheter ablation in individuals with a high BMI than it does in those with a normal BMI. However, the association between AF recurrence and BMI may be influenced by race. The authors investigated the relationship between BMI and AF recurrence after catheter ablation in Japanese patients. Methods: They enrolled 726 consecutive patients with paroxysmal or persistent AF (241 patients classified as overweight/obese [BMI ≥25 kg/m2] and 485 classified as non-overweight/obese [BMI <25 kg/m2]) who underwent cryoablation or radiofrequency ablation. The relationship between BMI and AF recurrence was assessed. Results: AF recurred in 183 patients (25.2%; 105 with paroxysmal AF and 78 with persistent AF). Median BMI differed significantly between patients with and without AF recurrence (23.9 kg/m2 [interquartile range: 21.6–26.9] versus 23.2 kg/m2 [interquartile range: 21.3–25.4]; p=0.011). Fisher’s exact test showed that the AF recurrence rate increased significantly with BMI in the radiofrequency ablation group (non-overweight/obese: 23.9% versus overweight/ obese: 44.6%; p<0.001) but not in the cryoablation group (non-overweight/obese: 19.3% versus overweight/obese: 18.0%; p=0.883). Patients classified as overweight/obese had a significantly larger left atrial dimension, and left atrial dimension increased synergistically with persistent AF and increasing BMI. Conclusion: The AF recurrence rate in Japanese patients who underwent radiofrequency ablation, but not in those who underwent cryoablation, increased with BMI (especially BMI ≥25 kg/m2). Cryoablation may be superior to radiofrequency ablation in Japanese patients classified as overweight/obese.
Keywords
AF, BMI, cryoablation, left atrial enlargement, overweight, radiofrequency ablation, recurrence

Disclosure: KY has received lecture fees from Otsuka Pharmaceutical, Daiichi-Sankyo and Novartis, and research grants from Abbott, Otsuka Pharmaceutical, Medtronic Japan, Daiichi-Sankyo, Boston Scientific, Biotronik Japan, Japan Lifeline, Mitsubishi Tanabe Pharma, Fukuda Denshi, Takeda Pharmaceutical, Ono Pharmaceutical and Novartis; and is on the editorial board of Journal of Asian Pacific Society of Cardiology; this did not influence peer review. All other authors have no conflicts of interest to declare.
Informed Consent: Informed consent was obtained from all patients to participate and for this paper to be published.
Data Availability: Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval: This study was conducted in accordance with the Declaration of Helsinki.
Author Contributions: Conceptualisation: AT, JM, MK, KO, AO, TT, DT, SK, MK, KY; data curation: AT, JM, MK, KO, AO, TT, DT, SK, MK, KY; formal analysis: AT, JM, MK; funding acquisition: AT, JM, MK; investigation: AT, JM, MK, KO, AO, TT, DT, SK, MK, KY; methodology: AT, JM, MK, KO, AO, TT, DT, SK, MK, KY; project administration: AT, JM, MK; resources: AT, JM, MK; software: AT, JM, MK; supervision: JM,MK, KY; validation: JM,MK, KY; visualisation: JM,MK, KY; writing – original draft preparation: AT, JM, MK; writing – review & editing: AT, JM, MK, KY.
Received: 4 November 2021 Accepted: 6 March 2022 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e15. DOI: https://doi.org/10.15420/japsc.2021.27
Correspondence: Junichiro Miake, Division of Pharmacology, Department of Pathophysiological and Therapeutic Science, Faculty of Medicine, Tottori University 36-1 Nishi-cho, Yonago, 683-8504, Japan. E: jmiake@tottori-u.ac.jp
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
AF is the most common arrhythmia worldwide.1 It decreases quality of life and increases mortality in affected individuals.2,3 Recent studies have demonstrated that ablation therapy for rhythm control achieves a better clinical outcome than rate control therapy.4 However, the high recurrence rate of AF has halted the clinical impact of ablation therapy.
The risk factors for AF recurrence after ablation remain to be determined. Obesity has been reported as an independent risk factor for incident AF.5 A meta-analysis of western and Asian patients revealed that obesity is a
significant risk factor for AF recurrence after radiofrequency ablation but that being overweight is not.6 However, the impact of increased BMI on cardiovascular disease may differ among races. Epidemiological studies have revealed that the distribution of BMI differs significantly among countries. For example, people in Asian countries have lower BMI values, and it has been suggested that a BMI of ≥25 kg/m2 in Asian people is similar to a BMI of ≥30 kg/m2 in white people.7 Although Asian people were included in the previous meta-analysis, the number was small, and the effects of ethnic differences were not assessed.6 Thus, the impact of
© RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com Electrophysiology ORIGINAL RESEARCH
Efficacy
BMI on AF recurrence after ablation in Japanese patients remains to be clarified.
There is a variety of ablation methods available, including radiofrequency ablation, cryoablation, laser balloons, hot balloons and pulse-field ablation. It has been reported that cryoablation is non-inferior to radiofrequency ablation.8 Specifically, cryoablation is more effective in reducing AF recurrence than radiofrequency ablation.9 Nevertheless, it is unclear whether BMI influences the likelihood of AF recurrence after cryoablation or radiofrequency ablation.
This study aimed to determine the impact of BMI on AF recurrence in Japanese patients and to assess the relationship between the impact of BMI and the ablation method. We prospectively examined AF recurrence in Japanese patients who underwent radiofrequency ablation or cryoablation.
Methods Patients
We enrolled consecutive Japanese patients with non-valvular paroxysmal or persistent AF who underwent first-time single-catheter ablation from March 2013 to December 2019 at Tottori University Hospital, Japan. They were followed prospectively to detect AF recurrence after the procedure. We defined patients with a BMI of ≥25 kg/m2 as the overweight/obese group.
Paroxysmal AF was defined as self-terminating AF lasting for up to 7 days or AF episodes that exhibited cardioversion within 7 days. Persistent AF was defined as AF lasting between 7 days and 1 year or AF episodes that terminated either by cardioversion with drugs or by direct current cardioversion. The AF type and burden were determined using clinical reviews at least once a year, 12-lead ECG, device interrogation and 24hour or 7-day Holter monitoring. Although there is no consensus on the duration of the blanking period, we defined the blanking period in accordance with current guidelines.10 The CHA 2 DS2-VASc score comprised congestive heart failure, hypertension, age ≥75 years, diabetes, stroke/ transient ischaemic attack, vascular disease, age 65–74 years and female sex.11 We evaluated the body surface area using the formula of Du Bois [body weight (kg)0.425 × height (cm)0.725 × 0.007184].12 We calculated alcohol consumption of 20 units weekly as ethanol according to the HAS-BLED score.13 All laboratory measurements were assessed on admission (from 1 to 3 days before ablation procedures).
Recurrences of AF during the first 90 days after the ablation were not counted in the determination of the primary endpoints, and still included in the study.8
Study Protocol
The study protocol was approved by the institutional review board of our institution and written informed consent was obtained from each patient before the procedure. This investigation conformed to the principles outlined in the Declaration of Helsinki.
Intracardiac echocardiography-guided transseptal puncture was performed using a Brockenbrough needle. Immediately after transseptal puncture and pre-ablation blood sample collection from the left atrium (LA), an IV heparin bolus (150 IU/kg body weight) was administered, followed by continuous infusion to maintain a minimum target activated clotting time of >300 ms. LA sheaths were flushed continuously with heparinised saline at a steady rate of approximately 30 ml/h, and additional fluid replacement was initiated in cases of hypotonia throughout the procedure.
in
In the radiofrequency ablation procedure, pulmonary vein isolation (PVI) was achieved using a focal ‘point-by-point’ catheter approach, delivering radiofrequency energy to the cardiac tissue. A 3.5 mm irrigated-tip ablation catheter (Thermocool, Biosense Webster) was advanced into the LA to achieve bilateral circumferential pulmonary vein isolation with the endpoints of bidirectional conduction block between the LA and pulmonary vein using electroanatomical mapping (CARTO3, Biosense Webster) and fluoroscopy guidance. Details of the radiofrequency ablation procedure are described in our previous study, and cryoablation was performed as previously reported.14,15 PVI was performed with a singleballoon technique using a second-generation cryoballoon (CB; Arctic Front Advance, Medtronic). A 28 mm CB catheter was used in all patients. A spiral mapping catheter (Achieve; Medtronic) was used to advance the CB and to map the pulmonary vein (PV) potentials. Complete sealing at the antral aspect of the PV was confirmed via injection of contrast medium. To avoid phrenic nerve injury, diaphragmatic compound motor action potentials were monitored with phrenic nerve pacing during each CB application.
The procedural endpoints were defined as the establishment of bidirectional PV–LA block, which was verified via a circular mapping catheter. If electrical isolation was not achieved after CB applications (180 seconds for each application) on each vein, additional touch-up ablation was performed using conventional radiofrequency. Both an entrance block (i.e. the elimination of all PV potentials) and exit block (i.e. noncapture of the LA during placement of a circular catheter) were confirmed as endpoints following a 30 mg bolus injection of adenosine triphosphate, which unmasked any dormant PV conduction. After PVI, a bidirectional conduction block line was created at the cavotricuspid isthmus (CTI) in all patients, but the CTI was incomplete in five patients. The ablation procedure was performed under conscious sedation with continuous blood pressure and oxygen saturation monitoring. The decision of anti-arrhythmic drugs handled post-ablation was made by each attending physician.
The transthoracic echocardiography was performed from 1 to 30 days before catheter ablation. Echocardiographic data were measured according to the recommendation of the American Society of Echocardiography, which our previous studies followed.16
Statistical Analysis
Categorical data are presented as frequency (%) and were compared with Fisher’s exact test. Continuous data are presented as mean (SD) or as median (interquartile range) for skewed distributions. Normality was tested with the Kolmogorov-Smirnov test. Normally distributed continuous variables were compared using the independent Student’s t-test, and skewed data were compared using the non-parametric Mann-Whitney U-test. The Student’s t-test, analysis of variance and ANCOVA were used to compare the means between groups.
Analyses were performed after natural logarithmic transformation (ln) of skewed biomarkers. Freedom from AF was reported as crude event rates and by means of a time-to-event analysis using the Kaplan–Meier method. Variables with p<0.10 in the group comparison were further evaluated by the Cox proportional hazards regression analyses to determine their associations with the recurrence of AF. The variables with p<0.10 in the univariate Cox proportional hazards regression analysis were further evaluated by the multivariate Cox proportional hazards regression analysis. A trend analysis was performed using the Cochran-Armitage test and the Jonckheere-Terpstra test. All statistical analyses were performed
of Cryoablation
Patients with Obesity JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
(years), median [interquartile range] 66.0 [60.0–72.0] 67.0 [61.0–73.0] 65.0 [57.0–72.0] 0.115 67.0 [61.0–72.0] 65.0 [58.0–71.0] 0.360
Women, n (%) 224 (30.9) 122 (33.2) 41 (39.0) 0.295 39 (22.3) 26 (33.3) 0.086 BMI (kg/m2), median [interquartile range] 23.7 [21.5–25.7] 23.0 [21.2–25.3] 23.7 [21.3–25.8] 0.241 23.7 [21.9–25.5] 25.1 [22.0–27.8] 0.023
Overweight/obesity, n (%) 241 (33.2) 105 (28.5) 38 (36.2) 0.148 58 (33.1) 40 (51.3) 0.008 BSA (m2 ), median [interquartile range] 1.7 [1.6–1.8] 1.7 [1.6–1.8] 1.7 [1.6–1.8] 0.191 1.7 [1.6–1.8] 1.8 [1.6–1.9] 0.168 CHA 2 DS2-VASc, median [interquartile range] 2.0 [1.0–3.0] 2.0 [1.0–3.0] 2.0 [1.0–3.0] 0.402 2.0 [1.0–3.0] 2.0 [1.0–3.0] 0.232 Congestive heart failure, n (%) 110 (15.7) 32 (8.7) 9 (8.6) 1.000 45 (25.7) 24 (30.8) 0.617 Hypertension, n (%) 389 (53.6) 196 (53.3) 59 (56.2) 0.657 87 (49.7) 46 (59.0) 0.220 Alcohol, n (%) 38 (5.2) 18 (4.9) 3 (2.9) 0.590 11 (6.3) 6 (7.7) 0.786 Diabetes, n (%) 109 (15.0) 59 (16.0) 14 (13.3) 0.544 24 (13.7) 11 (14.1) 1.000 Vascular disease, n (%) 44 (6.1) 21 (5.7) 6 (5.7) 1.000 13 (7.4) 4 (5.1) 0.596
Stroke or transient ischaemic attack, n (%) 49 (6.8) 27 (7.3) 6 (5.7) 0.668 19 (10.9) 4 (5.1) 0.163
Obstructive sleep apnoea, n (%) 12 (1.7) 7 (1.9) 1 (1.0) 0.691 2 (1.1) 2 (2.6) 0.589 BNP (pg/ml; n=698), median [interquartile range] 89.6 [40.2–175.2] 54.3 [23.1–117.7] 84.4 [37.1–172.2] 0.005 155.5 [100.1–246.3] 146.6 [99.7–230.4] 0.611
Creatinine (mg/dl), median [interquartile range] 0.8 [0.7–0.9] 0.8 [0.6–0.9] 0.8 [0.6–0.9] 0.163 0.8 [0.7–1.0] 0.8 [0.7–1.0] 0.625 ACEI/ARB, n (%) 290 (39.9) 140 (38.0) 45 (42.9) 0.428 75 (42.9) 31 (39.7) 0.680
β-blockers, n (%) 280 (38.6) 116 (31.5) 44 (41.9) 0.061 82 (46.9) 38 (48.7) 0.787 Loop diuretics, n (%) 97 (13.4) 28 (7.6) 12 (11.4) 0.233 36 (20.6) 22 (28.2) 0.197
Aldosterone blockers, n (%) 42 (5.8) 15 (4.1) 4 (3.8) 1 15 (8.6) 6 (7.7) 1 Anti-arrhythmic drugs, n (%) 276 (38.0) 138 (37.5) 50 (47.6) 0.071 51 (29.1) 30 (38.5) 0.148
Left atrial dimension (mm), mean ± SD 39.9 ± 6.8 37.7 ± 5.9 39.4 ± 6.5 0.011 42.7 ± 6.2 44.5 ± 8.1 0.060 LVEF (%), median [interquartile range] 62.8 [67.8–57.0] 65.0 [60.1–69.0] 65.0 [61.0–69.2] 0.719 57.9 [52.1–63.0] 58.9 [53.0–63.0] 0.696
Radiofrequency ablation/cryoablation, n 377/349 162/206 60/45 0.02 98/77 57/21 0.012
ACEI = angiotensin-converting enzyme inhibitors; ARB = angiotensin II receptor blockers; BNP = B-type natriuretic peptide; BSA = body surface area; LVEF = left ventricular ejection fraction.
using R (version 3.4.0; R Foundation for Statistical Computing), and p<0.05 was considered statistically significant.
Results
In total, 726 patients with AF (65.2% with paroxysmal AF and 34.8% with persistent AF) were included in this study (Supplementary Material Figure 1). The baseline characteristics of the patients are summarised in Supplementary Material Table 1. Most patients (69.1%) were men, and the median age of patients was 66.0 years [range 60.0–72.0]. The median follow-up duration for censored cases was 506.4 days [range 362.0–730.0]. Of all patients, 241 were classified as overweight/obese (BMI ≥25 kg/m2), and they had a significantly larger LA dimension (p<0.001). The median LA dimension was 39.8 mm and the median B-type natriuretic peptide was 89.6 pg/ml.
AF recurred in 183 patients (25.2%; 105 with paroxysmal AF and 78 with persistent AF). At baseline, no significant differences were noted in sex, age or left ventricular ejection fraction between patients with and without AF recurrence (Table 1). Table 1 shows the medication at the first day of hospitalisation. There were significant differences in the type of AF (paroxysmal versus persistent) and LA dimension between patients with and without AF recurrence. Median BMI was significantly higher in those with AF recurrence than in those without AF recurrence (23.9 kg/m2 [range 21.6–26.9] versus 23.2 kg/m2 [range 21.3–25.4], respectively; p=0.011). Moreover, the prevalence of patients treated with radiofrequency ablation
Table 2: Subgroup Analysis Between Overweight/ Obese and Non-overweight/obese Patients
Variables Univariate Analysis
HR 95% CI p-value
BMI ≥25 kg/m2
Ablation procedure (radiofrequency ablation = 1) 2.07 1.23–3.47 0.006
Left atrial dimension 1.07 1.03–1.11 0.001 lnBNP 1.37 1.08–1.73 0.011
Loop diuretics (use = 1) 2.02 1.15–3.55 0.015 BMI <25 kg/m2
Ablation procedure (radiofrequency ablation = 1) 1.02 0.69–1.50 0.930
Left atrial dimension 1.01 0.99–1.04 0.312 lnBNP 1.21 1.02–1.44 0.029
Loop diuretics (use = 1) 1.29 0.78–2.12 0.317 lnBNP = log-transformed B-type natriuretic peptide.
higher in patients with AF recurrence than in those without AF recurrence (p<0.001). The multivariate Cox proportional hazards regression analysis identified overweight/obesity (BMI ≥25 kg/m2) as an independent predictor of AF recurrence (adjusted HR 1.40, 95% CI [1.00–1.94]; p=0.049) (Supplementary Material Tables 2 and 3).
Efficacy of Cryoablation in Patients with Obesity JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
was
Table 1: Predictors of Post-blanking Atrial Arrhythmia Relapse after an Ablation Procedure
Variables Overall Paroxysmal AF Persistent AF No Recurrence Recurrence p-value No Recurrence Recurrence p-value n 726 368 105 175 78 Age
Subgroup Analysis of Risks of the Recurrence of AF for BMI
Based on the results above, we performed a subgroup analysis between the overweight/obese and non-overweight/obese groups. Fisher’s exact test showed that the AF recurrence rate increased significantly with BMI (non-overweight/obese: 21.6% versus overweight/obese: 32.4%; p<0.001). The AF recurrence rate in the overweight/obese group was significantly lower in the cryoablation group than in the radiofrequency ablation group (18.0% versus 44.6%, respectively; p<0.001). The multivariate Cox proportional hazards regression analysis identified larger LA dimension and radiofrequency ablation as independent predictors of AF recurrence in the overweight/obese group (radiofrequency ablation: adjusted HR 1.86; 95% CI [1.07–3.23]; p=0.03). The ablation procedure had no significant impact on AF recurrence in the non-overweight/obese group (Table 2). We stratified patients into four groups based on BMI and ablation procedure: BMI <25 kg/m2 and cryoablation; BMI <25 kg/m2 and radiofrequency ablation; BMI ≥25 kg/m2 and cryoablation; and BMI ≥25 kg/m2 and radiofrequency ablation. The AF recurrence rate significantly differed among the four groups according to the Kaplan–Meier analysis (log-rank test, p<0.001) (Figure 1).
We further analysed the impact of AF type on AF recurrence. Fisher’s exact test showed that the recurrence rate increased significantly with BMI in the radiofrequency ablation group (normal BMI: 22.4% versus overweight/obese BMI: 37.9%; p=0.018) but not in the cryoablation group (normal BMI: 18.4% versus overweight/obese BMI: 16.9%; p=0.859) in patients with paroxysmal AF (Figure 2). Similarly, for patients with persistent AF, the recurrence rate increased significantly with BMI in the radiofrequency ablation group (normal BMI: 26.8% versus overweight/ obese BMI: 51.6%; p<0.001) but not in the cryoablation group (normal BMI: 21.9% versus overweight/obese BMI: 20.6%; p=0.883). The CochranArmitage trend test showed similar results.
Association Between Left Atrial Dimension and BMI
Because LA dimension was an independent predictor of AF recurrence in the overweight/obese group, we examined the association between LA dimension and BMI in patients with paroxysmal versus persistent AF. The Jonckheere-Terpstra test showed that LA dimension increased in association with the increase in BMI and persistent AF (p<0.001; Figure 3A). Following the inclusion of AF type as an indicator variable and
adjustment for baseline BMI, ANCOVA showed that LA dimension was higher in the persistent AF group than in the paroxysmal AF group (p<0.001; Figure 3B).
Safety of Ablation Procedures
Major complications occurred in 22 patients in the cryoablation group and in 13 patients in the radiofrequency ablation group. There was no significant difference in the occurrence of complications with ablation between normal weight and overweight/obese patients. As in previous studies, cryoablation was more frequently associated with diaphragmatic neuropathy, but all patients improved at 1-month post-ablation.8 A full list of postprocedural adverse events is provided in Supplementary Material Table 4
Discussion
The aim of this study was to investigate the effect of BMI and type of ablation procedure on AF recurrence after ablation. Our results demonstrate that BMI was significantly higher in patients who presented with AF recurrence. We specifically identified that the AF recurrence rate of patients who underwent radiofrequency ablation, but not those who underwent cryoablation, increased with BMI. Patients in the overweight/ obese group had a significantly larger LA dimension, and LA dimension enlarged synergistically with persistent AF and increasing BMI.
Although some meta-analyses have suggested that AF recurs more frequently in patients with a higher BMI after ablation, many subjects in these meta-analyses had a BMI of ≥25 kg/m2, and both western and Asian patients were included in the analysis.6 17 The studies in western patients reported obesity (BMI ≥30 kg/m2), but not overweight (BMI 25–30 kg/m2), as a risk factor for AF recurrence after ablation.18 19 In our study, the risk of post-ablation AF recurrence increased in Japanese patients with a BMI ≥25 kg/m2. BMI is a typical criterion to assess obesity, but it is influenced by race. It has been reported that Asian people have relatively lower BMI values compared with white people.7 Previous studies have indicated that the cut-off value for BMI as a risk factor for AF recurrence after ablation should depend on race.6 7
In the FIRE AND ICE trial, cryoablation was non-inferior to radiofrequency ablation with respect to their efficacy in treating paroxysmal AF.8 However, in recent years, cryoablation has been reported to reduce the incidence of paroxysmal AF recurrence compared with radiofrequency ablation.9 Another study has also shown that cryoablation is less operatordependent and more reproducible than radiofrequency ablation in patients with paroxysmal AF.20 The pathological difference caused by ablation procedures may affect the AF recurrence rate after ablation. Reportedly, the LA–PV lesion caused by cryoablation is transmural, and the width of the lesion is 6 mm, which is greater than that of the lesion caused by radiofrequency ablation.21 Wider lesions caused by ablation may reduce re-conduction after ablation. Lesions caused by cryoablation exhibit less endocardial bleeding, are more clearly delineated and are more uniformly replaced with fibrous tissue. All these characteristics are expected to result in a low arrhythmogenic risk.21
The effect of different ablation procedures on AF recurrence in patients with obesity has not been well studied. Because LA dimension is an independent risk factor for recurrence in patients classified as overweight/ obese, we analysed the relationship between BMI and LA dimension (Figure 3). LA size has been reported to be enlarged with persistence of AF and increasing body weight.22 23 In our study, LA dimension and BMI were correlated and LA dimension enlarged synergistically with persistent
Efficacy of Cryoablation in Patients with Obesity JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Figure 1: Kaplan–Meier Analysis of AF Recurrence Rate 0 100 200 300 500 BMI ≥25kg/m2 Cryoablation BMI ≥25kg/m2 Radiofrequency ablation BMI <25kg/m2 Cryoablation BMI <25 kg/m2 Radiofrequency ablation Days 600 700 0.0 0.2 0.4 0.6 0.8 1.0 AF recurrence-free survival p<0.001 The AF recurrence rate significantly differed among the four groups classified by BMI and the type of ablation procedure.
AF
increasing
With an increase in
to 30 kg/m2,
increased by approximately 5 mm. With LA
radiofrequency ablation can increase the possibility of re-conduction because of an increase in the number of ablation points. This may at least partly explain the higher recurrence rate after
in the overweight/obese group in this study and in previous studies.
Obesity has been reported to increase the percentage of epicardial
tissue (EAT), and excessive pericardial fat is associated not only with a large LA volume and increased AF severity, but also with poor
for
insights may be
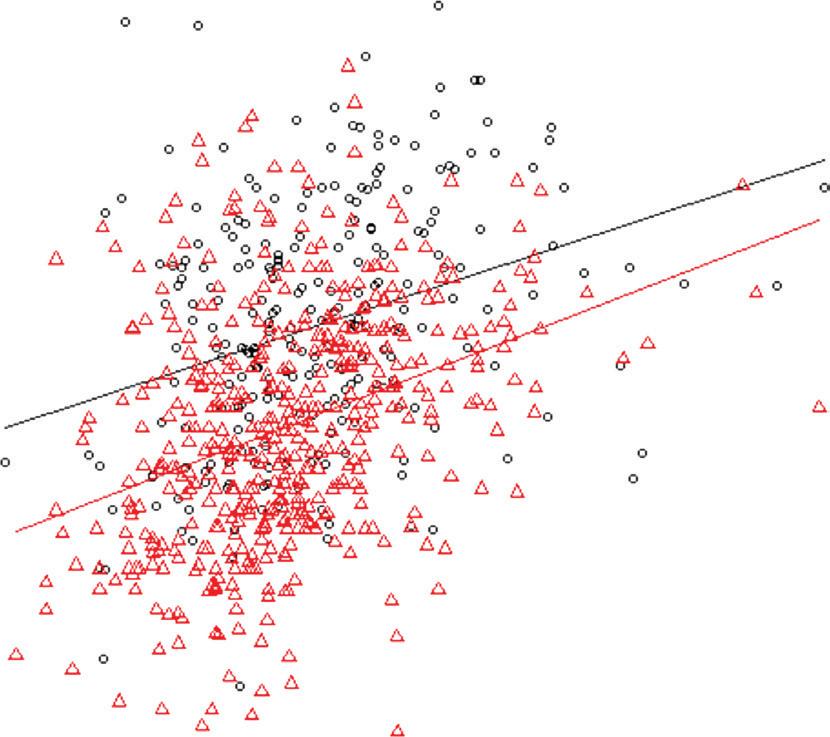
examining the impact of EAT from the perspective of the ablation procedure.
Clinical
post-ablation
of
Okumura
of
al.,
ablation, suggesting that
taken into account in evaluating the
the other hand, the
Efficacy of Cryoablation in Patients with Obesity JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
and
BMI.
BMI from 25
LA dimension
enlargement,
radiofrequency ablation
adipose
outcomes after radiofrequency ablation
AF.24–27 New
gained by
Implications The current study, in line with the findings reported by
et
showed that overweight/obesity was associated with a higher rate
AF recurrence in Japanese patients after catheter
racial differences in BMI should be
risk of
recurrence
AF.28 On
subgroup Figure 2: Fisher’s Exact Test Analysis of Paroxysmal and Persistent AF Recurrence Rate Figure 3: Association between Left Atrial Dimension and BMI by AF Type 1.0 Paroxysmal AF Persistent AF 0.8 0.6 0.4 0.2 0.0 1.0 0.8 0.6 0.4 0.2 0.0 BMI, kg/m2 Recurrence/total Cryoablation Radiofrequency ablation <25 32/174 ≥25 13/77 <25 35/156 ≥25 25/66 p=0.859 p=0.018 Cryoablation Radiofrequency ablation <25 14/64 ≥25 7/34 <25 24/91 ≥25 33/64 p=0.883 p<0.001 Frequency Frequency Recurrence Non-recurrence 60 50 40 30 LA dimension, mm 60 50 40 30 15 20 25 30 35 40 LA dimension, mm BMI, kg/m2 BMI, kg/m2 Paroxysmal AF <25 ≥25 <25 ≥25 Persistent AF p<0.001 p<0.001 A B The recurrence rate increased significantly with BMI in the radiofrequency ablation group. A: There was a significant trend in LA dimension in the four groups classified by obesity and AF type (p<0.001; Jonckheere-Terpstra test); B: BMI-adjusted LA dimension and AF type were correlated (p<0.001; ANCOVA). LA = left atrial.
Efficacy of Cryoablation in Patients with Obesity
analysis of our study showed that, for cryoablation, overweight/obesity was not a significant risk for AF recurrence. Our results showed a positive correlation between LA dimension and BMI, which may explain the reason why differences in ablation procedures modified the relationship between BMI and the rate of AF recurrence. To further test the modifying effects of ablation procedures on the relationship between BMI and the postablation AF recurrence, it would be useful to study the electrical and histological changes of the LA after ablation procedures using catheterbased potential mapping and cardiac MRI, respectively.
Study Limitations
Our study has some limitations that should be noted. First, this is a singlecentre, non-randomised study; thus, large-scale randomised studies are needed to confirm our findings. Second, intermittent rhythm monitoring might underestimate the actual AF recurrence rate. As in previous studies, the rate of AF detection by device interrogation was also significantly higher in this study.29 Thus, reassessment with other modalities may be necessary. Third, LA diameter rather than LA volume was used in our analysis. Fourth, this study cannot make a clear conclusive remark about whether obesity is a confounder or an effect modifier of success rates of ablation from the perspective of persistent versus paroxysmal AF versus LA dimension. Finally, this study performed a race-specific BMI assessment. Providência et al. have shown comparable efficacy between cryoablation and radiofrequency ablation in patients with a BMI >30 kg/m2 in Europe.18 Although a study stratified by the same BMI would be needed, this was difficult in this study because most patients had a BMI <30 kg/m2
1. Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 study. Circulation 2014;129:837–47. https://doi. org/10.1161/CIRCULATIONAHA.113.005119; PMID: 24345399
2. Dorian P, Jung W, Newman D, et al. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol 2000;36:1303–9. https://doi.org/10.1016/s0735-1097(00)00886-x; PMID: 11028487
3. Miyasaka Y, Barnes ME, Bailey KR, et al. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol 2007;49:986–92. https://doi.org/10.1016/j.jacc.2006.10.062; PMID: 17336723
4. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. https://doi.org/10.1056/NEJMoa2019422; PMID: 32865375
5. Tedrow UB, Conen D, Ridker PM, et al. The long- and shortterm impact of elevated body mass index on the risk of new atrial fibrillation the WHS (Women’s Health Study). J Am Coll Cardiol 2010;55:2319–27. https://doi.org/10.1016/j. jacc.2010.02.029; PMID: 20488302
6. Guijian L, Jinchuan Y, Rongzeng D, et al. Impact of body mass index on atrial fibrillation recurrence: a meta-analysis of observational studies. Pacing Clin Electrophysiol 2013;36:748–56. https://doi.org/10.1111/pace.12106; PMID: 23437987
7. Chang CJ, Wu CH, Chang CS, et al. Low body mass index but high percent body fat in Taiwanese subjects: implications of obesity cutoffs. Int J Obes Relat Metab Disord 2003;27:253–9. https://doi.org/10.1038/sj.ijo.802197; PMID: 12587007
8. Kuck KH, Brugada J, Fürnkranz A, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374:2235–45. https://doi.org/10.1056/ NEJMoa1602014; PMID: 27042964
9. Hoffmann E, Straube F, Wegscheider K, et al. Outcomes of cryoballoon or radiofrequency ablation in symptomatic paroxysmal or persistent atrial fibrillation. Europace 2019;21:1313–24. https://doi.org/10.1093/europace/euz155; PMID: 31199860
10. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design.
(Figure 3B). However, the severity of obesity is different between Asian and western people, and information about the effects of ablation on AF recurrence, specifically in Asian patients with a BMI >25 kg/m2, may be important. Research using a more accurate method to assess obesity is desirable.
Conclusion
We found that AF recurrence in Japanese patients undergoing radiofrequency ablation, but not in those undergoing cryoablation, increased with BMI, especially in patients with a BMI of ≥25 kg/m2. Thus, cryoablation may be superior to radiofrequency ablation in Japanese patients who are classified as overweight/obese.
Clinical Perspective
• Multivariate logistic regression analysis identified larger left atrial dimension and radiofrequency ablation as independent predictors of AF recurrence in patients classified as overweight/ obese.
• Higher BMI was significantly associated with AF recurrence in the radiofrequency ablation group, but not in the cryoablation group.
• Left atrial dimension was positively correlated with BMI and was larger in the persistent AF group than in the paroxysmal AF group.
Heart Rhythm 2012;9:632–96.e21. https://doi.org/10.1016/j. hrthm.2011.12.016; PMID: 22386883
11. Kornej J, Hindricks G, Kosiuk J, et al. Renal dysfunction, stroke risk scores (CHADS2, CHA2DS2-VASc, and R2CHADS2), and the risk of thromboembolic events after catheter ablation of atrial fibrillation: the Leipzig Heart Center AF Ablation Registry. Circ Arrhythm Electrophysiol 2013;6:868–74. https://doi.org/10.1161/CIRCEP.113.000869; PMID: 24047706
12. Du Bois D, Du Bois EF. Tenth paper. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med (Chic) 1916;17:863–71. https://doi. org/10.1001/archinte.1916.00080130010002
13. Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation. Eur Heart J 2010;31:2369–429. https://doi.org/10.1093/eurheartj/ehq278; PMID: 20802247
14. Kondo T, Miake J, Kato M, et al. Impact of postprocedural antiarrhythmic drug therapy with bepridil on maintaining sinus rhythm after catheter ablation for persistent atrial fibrillation. J Cardiol 2016;68:229–35. https://doi. org/10.1016/j.jjcc.2015.09.012; PMID: 26654806
15. Tscholl V, Lsharaf AK, Lin T, et al. Two years outcome in patients with persistent atrial fibrillation after pulmonary vein isolation using the second-generation 28-mm cryoballoon. Heart Rhythm 2016;13:1817–22. https://doi. org/10.1016/j.hrthm.2016.05.022; PMID: 27241353
16. Yamada K, Kinugasa Y, Sota T, et al. Inspiratory muscle weakness is associated with exercise intolerance in patients with heart failure with preserved ejection fraction: a preliminary study. J Card Fail 2016;22:38–47. https://doi. org/10.1016/j.cardfail.2015.10.010; PMID: 26505812
17. Zhuang J, Lu Y, Tang K, et al. Influence of body mass index on recurrence and quality of life in atrial fibrillation patients after catheter ablation: a meta-analysis and systematic review. Clin Cardiol 2013;36:269–75. https://doi.org/10.1002/ clc.22108; PMID: 23494488
18. Providência R, Adragão P, de Asmundis C, et al. Impact of body mass index on the outcomes of catheter ablation of atrial fibrillation: a European observational multicenter study. J Am Heart Assoc 2019;8:e012253. https://doi. org/10.1161/JAHA.119.012253; PMID: 31581876
19. Glover BM, Hong KL, Dagres N, et al. Impact of body mass index on the outcome of catheter ablation of atrial fibrillation. Heart 2019;105:244–50. https://doi.org/10.1136/ heartjnl-2018-313490; PMID: 30279268
20. Providencia R, Defaye P, Lambiase PD, et al. Results from a
multicentre comparison of cryoballoon vs. radiofrequency ablation for paroxysmal atrial fibrillation: is cryoablation more reproducible? Europace 2017;19:48–57. https://doi. org/10.1093/europace/euw080; PMID: 27267554
21. Hirao T, Nitta J, Adachi A, et al. First confirmation of histologic changes in the human heart after cryoballoon ablation. HeartRhythm Case Rep 2019;5:93–6. https://doi. org/10.1016/j.hrcr.2018.10.012; PMID: 30820405
22. Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol 2008;51:802–9. https://doi.org/10.1016/j.jacc.2007.09.064; PMID: 18294563
23. Aiad NN, Hearon C, Jr, Hieda M, et al. Mechanisms of left atrial enlargement in obesity. Am J Cardiol 2019;124:442–7. https://doi.org/10.1016/j.amjcard.2019.04.043; PMID: 31133275
24. Pandit SV, Anumonwo J, Jalife J. Atrial fibrillation susceptibility in obesity: an excess adiposity and fibrosis complicity? Circ Res 2016;118:1468–71. https://doi.org/10.1161/ CIRCRESAHA.116.308686; PMID: 27174946
25. Abed HS, Samuel CS, Lau DH, et al. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm 2013;10:90–100. https://doi.org/10.1016/j.hrthm.2012.08.043; PMID: 23063864
26. Wong CX, Abed HS, Molaee P, et al. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol 2011;57:1745–51. https://doi. org/10.1016/j.jacc.2010.11.045; PMID: 21511110
27. Kim TH, Park J, Park JK, et al. Pericardial fat volume is associated with clinical recurrence after catheter ablation for persistent atrial fibrillation, but not paroxysmal atrial fibrillation: an analysis of over 600 patients. Int J Cardiol 2014;176:841–6. https://doi.org/10.1016/j.ijcard.2014.08.008; PMID: 25176630
28. Okumura Y, Watanabe I, Nakai T, et al. Impact of biomarkers of inflammation and extracellular matrix turnover on the outcome of atrial fibrillation ablation: importance of matrix metalloproteinase-2 as a predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol 2011;22:987–93. https://doi.org/10.1111/j.1540-8167.2011.02059.x; PMID: 21489023
29. Blomström-Lundqvist C, Gizurarson S, Schwieler J, et al. Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation: the CAPTAF randomized clinical trial. JAMA 2019;321:1059–68. https:// doi.org/10.1001/jama.2019.0335; PMID: 30874754
JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Diana Hui Ping Foo , 1 Kai Huat Lam,2 Macnicholson Igo , 1 Mohammad Nor Azlan Bin Sulaiman,1 Mohamad Adam Bin Bujang , 3 Ming Ying Ku,1 Teck Long King , 1 Rose Hui Chin Jong , 1 Sally Suriani Ahip , 3 Mohammad Faiz Sahiran,4 Maila Mustapha , 5 Jennett Michael,6 Azreen Abdullah7 and Alan Yean Yip Fong 1
1. Clinical Research Centre, Sarawak General Hospital, Ministry of Health Malaysia, Kuching, Malaysia; 2. Assunta Heart Centre, Petaling Jaya, Malaysia; 3. Klinik Kesihatan Kota Sentosa, Ministry of Health Malaysia, Kuching, Malaysia; 4. Klinik Kesihatan Petra Jaya, Ministry of Health Malaysia, Kuching, Malaysia; 5. Klinik Kesihatan Jalan Masjid, Ministry of Health Malaysia, Kuching, Malaysia; 6. Klinik Kesihatan Tanah Puteh, Ministry of Health Malaysia, Kuching, Malaysia; 7. Klinik Kesihatan Batu Kawa, Ministry of Health Malaysia, Kuching, Malaysia.
Abstract
Background: The prognostic value of diastolic dysfunction estimates in clinical settings is not well established. We aimed to evaluate the impact of the 2016 American Society of Echocardiography and the European Association of Cardiovascular Imaging recommendations on estimates of diastolic dysfunction and predicting cardiovascular outcomes in patients with diabetes and hypertension. Methods: In total, 111 patients with diabetes and hypertension without a known history of cardiovascular diseases were enrolled. All patients had preserved left ventricular ejection fraction on echocardiography at screening. Echocardiography was performed at baseline. The 2009 and 2016 algorithms were applied in diastolic function assessment. All patients were followed up for 1 year to assess clinical outcomes. Results: There were 65 (58.6%) female patients. The mean age was 59.86 ± 7.45 years, and the mean duration of diabetes was 10.5 ± 5.41 years. Fifty-five (50.5%) patients had left ventricular hypertrophy on echocardiography. The prevalence of diastolic dysfunction was lower and that of indeterminate diastolic function was higher with 2016 recommendations. Concordance between 2016 and 2009 recommendations was poor with a reclassification rate of 41.4%. No patients diagnosed with indeterminate and normal diastolic function using the 2016 algorithm and diastolic dysfunction using the 2009 algorithm developed major adverse cardiac events at 1 year. Two of 11 patients diagnosed with diastolic dysfunction using both recommendations and two of five patients diagnosed with diastolic dysfunction and indeterminate diastolic function using 2016 and 2009 recommendations, respectively, developed major adverse cardiac events at 1 year. The 2016 algorithm showed better accuracy in predicting major adverse cardiac events at 1 year. Conclusion: The updated 2016 criteria detect more advanced diastolic dysfunction cases and predict 1-year cardiovascular outcomes. Further studies are warranted to investigate the prognostic impact of these criteria. Trial registration number: NMRR-16-436-29619.
Keywords
Diastolic dysfunction, diabetes, hypertension, heart failure, echocardiography
Disclosure: AYYF is an associate editor of Journal of Asian Pacific Society of Cardiology; this did not influence peer review. All other authors have no conflicts of interest to declare.

Funding: This work was supported by a research grant from the Ministry of Health of Malaysia (grant number NMRR-16-436-29619). The sponsor had no role in study design; in the collection, analysis and interpretation of data; and in the writing of the report. The sponsor approved the article to be submitted for publication.
Acknowledgements: The authors thank the Director General of Health Malaysia for his permission to publish this article. We extend our deepest gratitude to all medical staff involved in conducting the study from the participating sites (Klinik Kesihatan Kota Sentosa, Klinik Kesihatan Petra Jaya, Klinik Kesihatan Jalan Masjid, Klinik Kesihatan Tanah Puteh, Klinik Kesihatan Batu Kawa and Clinical Research Centre Sarawak General Hospital). Abstracts of the preliminary results were presented at the National Heart Association of Malaysia Congress, 12−14 April 2019, and European Society of Cardiology Congress, 29 August–2 September 2020.
Data Availability: All data are incorporated into the article.
Ethics: This study was performed in accordance with the principles of the Declaration of Helsinki. Ethical approval was obtained from the Medical Research and Ethics Committee, Ministry of Health Malaysia (Research ID: NMRR-16-436-29619).
Informed Consent: Written informed consent was obtained from the patients to participate in this study.
Authors’ Contributions: Conceptualisation: DHPF, KHL, AYYF; data curation: DHPF; formal analysis: MABB; funding acquisition: DHPF; investigation: DHPF, KHL, MI, MNABS, MYK, TLK, RH, CJ; methodology: DHPF, KHL, MI, MNABS, MYK, TLK, RH, CJ, MABB; project administration: DHPF; resources: SSA, MFS, MM, JM, AA, AYYF; supervision: AYYF, KHL; validation: KHL, MABB; writing – original draft preparation: DHPF; writing – review & editing: KHL, AYYF. All authors approved the final version of the manuscript.
Received: 23 October 2021 Accepted: 15 February 2022 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e16.
DOI: https://doi.org/10.15420/japsc.2021.25
Correspondence: Diana Hui Ping Foo, Clinical Research Centre, Sarawak General Hospital, Jalan Tun Ahmad Zaidi Adruce, Kuching, Sarawak 93586, Malaysia. E: dianafoo.crc@gmail.com
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
© RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com Echocardiography ORIGINAL RESEARCH
Impact of 2016 American Society of Echocardiography/European Association of Cardiovascular Imaging Recommendations for the Evaluation of Left Ventricular Diastolic Function on Predicting Outcomes in Patients with Diabetes and Hypertension without a History of Cardiovascular Disease
Historically, type 2 diabetes (T2D) has been associated with higher cardiovascular morbidity and mortality.1 Diastolic dysfunction (DD) is an early manifestation of diabetic cardiomyopathy and is linked to an increased risk of heart failure and mortality independent of systolic function decline.2 DD occurs early in diabetes, even when patients with diabetes are asymptomatic with normal blood pressure, normal contractility and no vascular complications.3–5
It is challenging to diagnose DD because evaluation of diastolic function (DF) using several parameters and different criteria is complex and the heterogeneity and ambiguity of different definitions from previous diagnostic algorithms results in significant variability in the reported prevalence and grading of DD.6–10 In 2016, a joint task force of the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI) proposed a new and simplified algorithm for diagnosing and classifying DF.11
Several studies evaluated the impact of the 2016 recommendations compared to that of the 2009 recommendations and showed poor concordance and a significantly lower prevalence of DD when applying the 2016 recommendations.12–14 Nevertheless, outcome-based studies are lacking to demonstrate the prognostic implications of such comparisons in predicting outcomes in the T2D population.
In the present study, we prospectively evaluated the impact of the 2016 ASE/EACVI recommendations on estimates of DD, concordance between 2009 and 2016 recommendations in the diagnosis of DD and the prognostic accuracy of the 2016 algorithm in predicting cardiovascular outcomes in patients with hypertension and T2D but without a history of cardiovascular disease.
Methods Study Population
This prospective cohort study included adults aged 18–65 years with T2D who were treated for at least 3 years. Individuals with known cardiovascular disease – defined as a history of MI, percutaneous coronary revascularisation, coronary artery bypass graft surgery, at least moderate valvular heart disease, bundle branch block, AF or atrial flutter, permanent pacemaker, arrhythmia, heart failure hospitalisation, or reduced left ventricular (LV) ejection fraction (<55%) – were excluded.
In addition, individuals with renal impairment (defined as an estimated glomerular filtration rate of <30 ml/min/1.73 m2), known history of bronchial asthma, pancreatitis, underlying endocrine disorders (such as acromegaly, Cushing’s syndrome, hyperthyroidism, pheochromocytoma and aldosteronism), uncontrolled hypertension (defined as systolic blood pressure of ≥200 mmHg, diastolic blood pressure of ≥120 mmHg or use of more than three antihypertensive drugs), life-threatening conditions with <1 year of life expectancy and poor echocardiographic windows were excluded.
All included participants underwent physical examinations and evaluation of cardiovascular risk factors and detailed anthropometric parameters. Blood samples were collected for evaluation of fasting lipid profiles, HbA1c levels, and renal profiles. All participants underwent comprehensive transthoracic echocardiography examinations at baseline and were followed up for 1 year to assess clinical outcomes. Data on clinical outcomes measures, a composite of death, MI, heart failure, stroke, AF or atrial flutter and hospital admissions for adverse cardiovascular events, were collected.
This study was performed in accordance with the principles of the Declaration of Helsinki. Ethical approval for this study was obtained from the Medical Research and Ethics Committee, Ministry of Health Malaysia (Research ID: NMRR-16-436-29619). All participants provided written informed consent before enrolment in the study.
Echocardiography
Transthoracic echocardiography examinations were performed using the Philips CX50 System (Philips Medical Systems) equipped with S3 probes (2–4 MHz). Stored images were analysed offline by an echocardiographic technician blinded to the clinical data. Three readings were obtained for each echocardiographic variable and averaged over three consecutive cardiac cycles. Chamber quantification and function were assessed based on the most recent 2015 ASE/EACVI recommendations.15 Mitral inflow velocities (E-wave and A-wave), E/A ratio, deceleration time (DT), tissue Doppler imaging septal and lateral e’ and a’ diastolic peak velocities, E/e’ ratio and tricuspid regurgitation (TR) peak velocity were obtained for diastolic function assessment.
Classification of DF
Both 2009 ASE/European Association of Echocardiography (EAE; now the EACVI) and 2016 ASE/EACVI recommendations were applied to determine LV DF and grades.6 11 According to the 2009 ASE/EAE recommendations, the determination of DF consisted of two steps. The first step started with the septal and lateral e’ velocities and the indexed left atrial (LA) volume.6 Two of three measures exceeding the cut-off value suggested were required to proceed to the subsequent step in grading of DD. The second step involved the assessment of E/A ratio, DT, and average E/e’ ratio. At least two positive measures were required to attribute the DD grade. DF was considered indeterminate it was not possible to grade DD due to discrepancies in measurements between these parameters.
In the 2016 ASE/EACVI recommendations, DF was evaluated using four variables: septal or lateral e’ velocity, indexed LA volume, average E/e’ ratio, and TR peak velocity.11 Based on the measurements obtained from all four variables suggested in 2016 recommendations, participants were classified as having normal DF (less than two of four positive criteria), DD (more than two of four positive criteria), or indeterminate DF (two of four positive criteria).
DF classification was performed by two independent investigators blinded to participant characteristics (MI and MNABS). All findings were then validated by a board-certified cardiologist with expertise in echocardiography (KHL), who reviewed all echocardiographic data and images acquired and was blinded to the clinical data of each participant.
Statistical Methods
Clinical characteristics and echocardiographic variables are presented as mean ± SD, and DF categories are presented as absolute numbers and percentages. Concordance between the 2009 and 2016 classifications was determined using the κ coefficient and the proportion of agreement. Concordance was defined as poor (0–0.20), fair (0.21–0.40), moderate (0.41–0.60), good (0.61–0.80), and optimal (0.81–1).16 The reclassification rate was calculated using the formula: 100% − proportion of agreement (%). Statistical significance was set at p<0.05. All data analyses were performed using SPSS software (IBM SPSS Statistics for Windows, Version 21.0).
Results
A total of 324 individuals with T2D and hypertension who attended the diabetes outpatient clinic follow-up in primary healthcare settings were
2016 Diastolic Function Criteria and Outcomes Prediction in Patients with Diabetes JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
screened for eligibility. Among the screened individuals, 111 who met the inclusion and exclusion criteria were enrolled. Table 1 shows the clinical and echocardiographic characteristics of the study cohort. There were 65 (58.6%) women. The mean age was 59.86 ± 7.45 years, and the mean duration of diabetes was 10.5 ± 5.41 years. Most patients were obese (BMI >25 kg/m2 and waist circumference >90 cm). The mean systolic blood pressure was elevated at 144.29 ± 18.18 mmHg. Echocardiography revealed LV hypertrophy in 50.5% patients.
The prevalence of DD was much lower with the 2016 algorithm than with the 2009 algorithm (14.4% [n=16] versus 43.2% [n=48]; Figure 1). However, the prevalence of indeterminate (18.0% [n=20] versus 12.6% [n=14]) and normal DF (67.6% [n=75] versus 44.1% [n=49]) were higher with 2016 recommendations than with 2009 recommendations. Concordance between 2016 and 2009 recommendations was poor (k=0.02, p<0.05), with a reclassification rate of 41.4% (Figure 2). Among the 48 patients classified as having DD according to 2009 recommendations, 22 (46%) were reclassified as having normal DF and 15 (31%) were reclassified as having indeterminate DF according to 2016 recommendations. Nevertheless, there was concordance in 11 (23%) participants classified as having DD according to the 2016 and 2009 guidelines. Among the 20 patients classified as having indeterminate DF according to 2016 recommendations, 15 (75%) were reclassified as having DD according to 2009 recommendations. There was agreement only in five (25%) patients classified as having indeterminate DF according to both guidelines.
Based on the prevalence of DD determined according to previous and current recommendations, the impact of the application of the current 2016 algorithm was evaluated by assessing the incidence of major adverse cardiac events (MACE) at 1 year. As demonstrated in Table 2, none of the patients classified as having indeterminate and normal DF using the 2016 algorithm and DD and indeterminate DF using the 2009 algorithm developed MACE at 1 year. Among the 16 patients classified as having DD according to the 2016 algorithm, four (25%) developed MACE at 1 year; among them, two were also classified as having DD according to the 2009 algorithm, and the other two were classified as having indeterminate DF according to the 2009 algorithm.
Supplementary Material Table 1 shows components of MACE developed at 1 year in our study cohort. Among the 48 patients classified as having DD based on the 2009 algorithm, only 2 (4.2%) developed MACE at 1 year. A comparison of the diagnostic accuracy of the 2016 ASE/EACVI and 2009 ASE/EAE algorithms is summarised in Table 3. The 2016 algorithm showed better diagnostic accuracy (88.29%, sensitivity 80.00%, specificity 88.68%, positive predictive value 25.00%, negative predictive value 98.95%) than the 2009 algorithm (46.85%, sensitivity 80.00%, specificity 45.28%, positive predictive value 6.45%, negative predictive value 97.96%) for predicting MACE at 1 year.
Discussion
DD is an important precursor of heart failure with preserved ejection fraction (HFpEF). A study from the ASIAN-HF registry found that many patients in southeast Asia who had HFpEF had associated diabetes.17 For example, in Malaysia, one-third of patients admitted for HFpEF had underlying T2D.18 These patients were found to have the worst, if not, the second worst outcomes, with a high rate of heart failure hospitalisation and mortality events within 1 year.17
The 2021 European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic heart failure have also included
Table 1: Clinical and Echocardiographic Characteristics of the Study Cohort
All patients (n=111)
Age (years) 59.86 ± 7.45
Sex (female) 65 (58.6%)
BMI (kg/m2) 28.95 ± 4.78
Waist circumference (cm) 97.15 ± 9.04
Duration of diabetes (years) 10.5 ± 5.41
Systolic blood pressure (mmHg) 144.29 ± 18.18
Diastolic blood pressure (mmHg) 78.98 ± 9.45
Heart rate (BPM) 75.94 ± 12.93
Ejection fraction (%) 67.62 ± 6.03
Left ventricular mass index (kg/m2) 106.81 ± 23.22
Left ventricular hypertrophy 55 (50.5%)
Left atrial volume index (ml/m2) 27.53 ± 8.42
E/A ratio 0.81 ± 0.24
Deceleration time (s) 0.232 ± 0.055
Septal e’ peak velocity (cm/s) 6.65 ± 1.70
Lateral e’ peak velocity (cm/s) 8.19 ± 2.17
Average septal-lateral E/e’ ratio 9.84 ± 3.01
Tricuspid regurgitation peak velocity (cm/s) 191.84 ± 86.09
Data are presented as mean ± SD for continuous variables and as n (%) for categorical variables.
Figure 1: Prevalence of Diastolic Dysfunction According to 2009 ASE/EAE and 2016 ASE/EACVI Algorithms
echocardiographic LV DD/raised LV filling pressure as an objective evaluation of cardiac functional abnormalities to diagnose HFpEF.19
Furthermore, the results of the EMPEROR-PRESERVED study have demonstrated benefits of sodium-glucose cotransporter 2 inhibitors (SGLT2I) − as a treatment option − in improving outcomes and lowering the risk of composite cardiovascular death and heart failure hospitalisation in patients with HFpEF.20 Hence, in practice, DD characteristics on echocardiography similar to those of HFpEF in patients with T2D should prompt clinicians to intervene early while patients are still asymptomatic for primary prevention to reduce the burden of the disease.
Early screening of patients who are at risk of developing adverse cardiac events and aggressive management of risk factors have been shown to
2016 Diastolic Function Criteria and Outcomes Prediction in Patients with Diabetes JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
2016 guidelines 2009 guidelines 0.0 40.0 30.0 20.0 10.0 50.0 60.0 70.0 80.0 Percentage Normal Indeterminate diastolic function Left ventricular diastolic dysfunction Elevated filling pressure 67.6% 44 1% 18.0% 12.6% 14.4% 43.2% 9.0% 26 1% ASE = American
Society of
Echocardiography; EACVI =
European Association
of Cardiovascular Imaging; EAE = European Association of Echocardiography (now the EACVI).
2009 recommendations 2016 recommendations
Normal (n=49)
Left ventricular diastolic dysfunction (n=48)
Indeterminate diastolic function (n=14)
Normal (n=49)
Left ventricular diastolic dysfunction (n=0)
Indeterminate diastolic function (n=0)
Normal (n=22)
Left ventricular diastolic dysfunction (n=11)
Indeterminate diastolic function (n=15)
Normal (n=4)
Left ventricular diastolic dysfunction (n=5)
Indeterminate diastolic function (n=5)
Table 3: Diagnostic Accuracy of the 2016 and 2009 Algorithms for Predicting MACE within 1 Year
Statistic 2016 Algorithm 2009 Algorithm
Value 95% CI Value 95% CI
Sensitivity 80.00% 28.36–99.49% 80.00% 28.36–99.49% Specificity 88.68% 81.06–94.01% 45.28% 35.59–55.25%
Positive likelihood ratio 7.07 3.54–14.09 1.46 0.91–2.34
Negative likelihood ratio 0.23 0.04–1.30 0.44 0.08–2.58 Disease prevalence 4.50% 1.48–10.20% 4.50% 1.48–10.20%
Positive predictive value 25.00% 14.33–39.92% 6.45% 4.13–9.95%
Negative predictive value 98.95% 94.21–99.82% 97.96% 89.15–99.64% Accuracy 88.29% 80.81–93.61% 46.85% 37.31–56.55%
MACE = major adverse cardiac events.
Concordance, κ=0.02, p<0.05
Table 2: Incidence of Major Adverse Cardiac Events at 1 Year in Different Groups According to 2016 and 2009 Algorithms
1-year MACE
No
DD (2016); DD (2009) 9
(n)
(n)
11
5 Indeterminate DF (2016); DD (2009) 15
DD (2016); Indeterminate DF (2009) 3
15 Indeterminate DF (2016); Indeterminate DF (2009) 5 0 5
Normal (2016); DD (2009) 22 0
Normal (2016); Indeterminate DF (2009)
Normal (2016); Normal (2009)
ASE/EAE
criterion in this study) because of pressure overload. Consistent with the findings of previous studies, our study showed that application of the 2016 ASE/EACVI DF criteria reduced the prevalence of DD significantly because of the use of a strict definition of DD (more than two of four positive criteria).12–14 According to our observation, the 2016 DF algorithm detected more advanced cases and performed better in terms of diagnostic accuracy than the previous 2009 algorithm. Nevertheless, although the current 2016 DF recommendations simplify the diagnosis of DD, they resulted in a higher number of indeterminate DF cases in our study cohort. It was observed that 75% of the indeterminate DF cases classified using the 2016 algorithm were reclassified as DD cases using the 2009 algorithm. Although none of the patients developed MACE, the 1-year follow-up period was very short to deduce whether patients who met two of four positive criteria could be considered as having normal DF. These patients did not fulfil sufficient criteria to be diagnosed with DD. Further investigations and closer monitoring are warranted to exclude DD, especially among patients with T2D and a dilated LA. An increased LA volume index has been associated with worse outcomes in patients with T2D and heart failure.23–27
ventricular diastolic
ASE/EACVI
American Society of Echocardiography;
diastolic
diastolic
EACVI = European Association of Cardiovascular Imaging;
European Association of Echocardiography (now the EACVI); MACE = major adverse cardiac events.
reduce the rate of LV dysfunction and heart failure progression and improve clinical outcomes.21 22 In the context of local settings, this could prevent at least one-third of HFpEF hospitalisations.
This study is the first to assess the implications of the 2016 DF recommendations compared to those the 2009 DF recommendations in predicting cardiovascular outcomes in patients with T2D without a history of cardiac disease. The study cohort involved, i.e. patients with both T2D and hypertension, represented majority of our T2D population. Nevertheless, we believe that the inflammatory process of diabetes was the driver for the diastolic function in our study population as DD is usually associated with uncontrolled hypertension (which was an exclusion
The implication of this study is important as it is only the tip of the iceberg of cardiovascular risk prediction in diabetes. Indeed, a longer follow-up period should be considered in future studies. However, titration of treatments, which can change the natural course of T2D and hypertension, usually occur while managing patients with T2D at diabetes outpatient clinics in primary care settings. Therefore, the LV should be assessed by echocardiography every time HbA1c is measured as routine assessment of diabetes control as per recommendations for patients with diabetes.28 Then, from trajectory analysis of serial data obtained at different time points over a longer follow-up period, rather than distinguishing between normal and abnormal diastolic function, a structured outcome-driven predictive model with age- and sex-specific reference frames could be generated to better risk stratify asymptomatic patients with T2D. The resultant model will be more clinically relevant to the Asian population, which has a higher prevalence of T2D; younger age at heart failure presentation; higher association with HFpEF than with heart failure with reduced ejection fraction; and unique female heart failure characteristics associated with lean diabetic phenotype, higher prevalence of concentric LV hypertrophy , and worse outcomes.29–33,17 This will be parallel with recent changes introduced in clinical practice when therapeutic options – such as SGLT2Is that reduce risk of cardiovascular deaths or hospitalisations for heart failure − become available for patients with HFpEF, with or without diabetes.19 20
2016 Diastolic Function Criteria and Outcomes Prediction in Patients with Diabetes JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Figure 2: Reclassification of Diastolic Function and Concordance in the Prevalence of Diastolic Dysfunction According to 2009 ASE/ EAE and 2016 ASE/EACVI Algorithms
ASE = American Society of Echocardiography; EACVI = European Association of Cardiovascular Imaging; EAE = European Association of Echocardiography (now the EACVI).
MACE
MACE
Total (n)
2
2
0
22
4 0 4
48 1 49 2009 denotes the 2009
recommendations for the evaluation of left ventricular diastolic function; 2016 denotes the 2016
recommendations for the evaluation of left
function. ASE =
DD =
dysfunction; DF =
function;
EAE =
Study Limitations
This study has some limitations. First, this is a single-centre study involving a local Southeast Asian population. Thus, there is limitation with regard to generalisability to other populations particularly in the context of a heterogenous Asian population accompanied with geographical variances. Second, it included only asymptomatic patients with diabetes and LV ejection fraction of >55%. Therefore, the observations cannot be extrapolated to patients with diabetes and reduced LV ejection fraction on echocardiography. Third, pulmonary vein flow parameters, which are included in the 2009 ASE/EAE algorithm for the evaluation of LV DF,6 were not included in LV DF assessment in this study. However, this is not expected to significantly change the reported results. Finally, the duration of follow-up was very short to demonstrate the role of the current 2016 ASE/EACVI recommendations in distinguishing patients with T2D and hypertension who are at risk of developing MACE. A longer-term follow-up of a larger sample of the population with T2D involving serial investigations will be considered in future studies.
Conclusion
The application of the new 2016 recommendations resulted in a much lower prevalence of DD in this cohort. The updated criteria detect more
1. Emerging Risk Factors Collaboration, Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–22. https://doi. org/10.1016/S0140-6736(10)60484-9; PMID: 20609967.
2. From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction: a population-based study. J Am Coll Cardiol 2010;55:300–5. https://doi.org/10.1016/j. jacc.2009.12.003; PMID: 20117433.
3. Patil VC, Shah KB, Vasani JD, et al. Diastolic dysfunction in asymptomatic type 2 diabetes mellitus with normal systolic function. J Cardiovasc Dis Res 2001;2:213–22. https://doi. org/10.4103/0975-3583.89805; PMID: 22135479.
4. Nadeau KJ, Regensteiner JG, Bauer TA, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab 2010;95:513–21. https://doi.org/10.1210/jc.2009-1756; PMID: 19915016.
5. Bradley TJ, Slorach C, Mahmud FH, et al. Early changes in cardiovascular structure and function in adolescents with type 1 diabetes. Cardiovasc Diabetol 2016;15:31. https://doi. org/10.1186/s12933-016-0351-3; PMID: 26879273.
6. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009;10:165–93. https://doi.org/10.1016/j.echo.2016.01.011; PMID: 27037982.
7. Paulus WJ, Tschöpe C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007;28:2539–50. https://doi.org/10.1093/eurheartj/ ehm037; PMID: 17428822.
8. Appleton CP. Doppler assessment of left ventricular diastolic function: the refinements continue. J Am Coll Cardiol 1993;21:1697–700. https://doi.org/10.1016/07351097(93)90389-i; PMID: 8496539.
9. Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 2000;102:1788–94. https://doi.org/10.1161/01. cir.102.15.1788; PMID: 11023933.
10. Selmeryd J, Henriksen E, Leppert J, Hedberg P. Interstudy heterogeneity of definitions of diastolic dysfunction severely affects reported prevalence. Eur Heart J Cardiovasc Imaging 2016;17:892–9. https://doi.org/10.1093/ehjci/jev211; PMID: 26374880.
11. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European
advanced cases and predict short-term 1-year cardiovascular outcomes. Further studies are warranted to investigate the prognostic impact of these criteria.
Clinical Perspective
• This study is the first to compare 2016 versus 2009 diastolic function recommendations in cardiovascular risk prediction in patients with type 2 diabetes.
• The 2016 algorithm showed better accuracy than the 2009 algorithm in predicting major adverse cardiac events at 1 year.
• Left ventricular assessment by echocardiography may be recommended when the HbA 1c level is measured if titration of treatments is involved.
• The implication of this study is important to enhance future study designs to adopt a more comprehensive approach involving the collection of clinical and imaging data and a longer follow-up period to better stratify cardiovascular risk in patients with type 2 diabetes, particularly when treatment options that improve outcomes in patients with diabetes and diastolic heart failure become available.
Association of Cardiovascular Imaging. Eur J Echocardiogr 2016;17:1321–60. https://doi.org/10.1016/j.echo.2016.01.011; PMID: 27037982.
12. Almeida JG, Fontes-Carvalho R, Sampaio F, et al. Impact of the 2016 ASE/EACVI recommendations on the prevalence of diastolic dysfunction in the general population. Eur Heart J Cardiovasc Imaging 2018;19:380–6. https://doi.org/10.1093/ ehjci/jex252; PMID: 29236978.
13. Huttin O, Fraser AG, Coiro S, et al. Impact of changes in consensus diagnostic recommendations on the echocardiographic prevalence of diastolic dysfunction. J Am Coll Cardiol 2017;69:3119–21. https://doi.org/10.1016/j. jacc.2017.04.039; PMID: 28641802.
14. Wan SH, Pumerantz AS, Dong F, et al. Comparing the influence of 2009 versus 2016 ASE/EACVI diastolic function guidelines on the prevalence and echocardiographic characteristics of preclinical diastolic dysfunction (stage B heart failure) in a Hispanic population with type 2 diabetes mellitus. J Diabetes Complications 2019;33:579–84. https:// doi.org/10.1016/j.jdiacomp.2019.04.015; PMID: 31155469.
15. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39. https://doi.org/10.1016/j.echo.2014.10.003; PMID: 25559473.
16. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. https://doi.org/10.2307/2529310; PMID: 843571.
17. Tromp J, Tay WT, Ouwerkerk W, et al. Multimorbidity in patients with heart failure from 11 Asian regions: a prospective cohort study using the ASIAN-HF registry. PLoS Med 2018;15:e1002541. https://doi.org/10.1371/journal. pmed.1002541; PMID: 29584721.
18. Ling HS, Chung BK, Chua PF, et al. Acute decompensated heart failure in a non cardiology tertiary referral centre, Sarawak General Hospital (SGH-HF). BMC Cardiovasc Disord 2020;20:511. https://doi.org/10.1186/s12872-020-01793-7; PMID: 33287705.
19. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. https:// doi.org/10.1093/eurheartj/ehab368; PMID: 34447992.
20. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451−61. https://doi.org/10.1056/NEJMoa2107038; PMID: 34449189.
21. Ledwidge MT, O’Connell E, Gallagher J, et al. Costeffectiveness of natriuretic peptide-based screening and collaborative care: a report from the STOP-HF (St Vincent’s Screening TO Prevent Heart Failure) study. Eur J Heart Fail 2015;17:672–9. https://doi.org/10.1002/ejhf.286; PMID: 26139583.
22. Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic
peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA 2013;310:66–74. https://doi.org/10.1001/jama.2013.7588; PMID: 23821090.
23. Tsang TS, Barnes ME, Gersh BJ, et al. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol 2002;90:1284–9. https://doi.org/10.1016/s00029149(02)02864-3; PMID: 12480035.
24. Gottdiener JS, Kitzman DW, Aurigemma GP, et al. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons ≥65 years of age (the Cardiovascular Health study). Am J Cardiol 2006;97:83–9. https://doi. org/10.1016/j.amjcard.2005.07.126; PMID: 16377289.
25. Takemoto Y, Barnes ME, Seward JB, et al. Usefulness of left atrial volume in predicting first congestive heart failure in patients ≥65 years of age with well-preserved left ventricular systolic function. Am J Cardiol 2005;96:832–6. https://doi. org/10.1016/j.amjcard.2005.05.031; PMID: 16169372.
26. Poulsen MK, Dahl JS, Henriksen JE, et al. Left atrial volume index: relation to long-term clinical outcome in type 2 diabetes. J Am Coll Cardiol 2013;62:2416–21. https://doi. org/10.1016/j.jacc.2013.08.1622; PMID: 24076532.
27. Tsang TS, Abhayaratna WP, Barnes ME, et al. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol 2006;47:1018–23. https://doi.org/10.1016/j.jacc.2005.08.077; PMID: 16516087.
28. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41:255–323. https://doi.org/10.1093/eurheartj/ehz486; PMID: 31497854.
29. Volgman AS, Palaniappan LS, Aggarwal NT, et al. Atherosclerotic cardiovascular disease in South Asians in the United States: epidemiology, risk factors, and treatments. Circulation 2018;138:e1–34. https://doi.org/10.1161/ CIR.0000000000000580; PMID: 29794080.
30. Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 2017;70:1–25. https://doi. org/10.1016/j.jacc.2017.04.052; PMID: 28527533.
31. Cainzos-Achirica M, Vela E, Cleries M, et al. Cardiovascular risk factors and disease among non-European immigrants living in Catalonia. Heart 2019;105:1168–74. https://doi. org/10.1136/heartjnl-2018-314436; PMID: 30819763.
32. Nanditha A, Ma RC, Ramachandran A, et al. Diabetes in Asia and the Pacific: implications for the global epidemic. Diabetes Care 2016;39:472–85. https://doi.org/10.2337/dc151536; PMID: 26908931.
33. Lam CS, Teng TK, Tay WT, et al. Regional and ethnic differences among patients with heart failure in Asia: the Asian Sudden Cardiac Death in Heart Failure registry. Eur Heart J 2016;37:3141–53. https://doi.org/10.1093/eurheartj/ ehw331; PMID: 27502121.
2016 Diastolic Function Criteria and Outcomes Prediction in Patients with Diabetes JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Pericarditis and Myocarditis after mRNA-based COVID-19 Vaccination
Rilong Hong ,
1. Department of Cardiology, National Heart Centre

Yap
and Khung Keong Yeo
Singapore,
Duke-NUS Medical School, Singapore
Keywords: Pericarditis, myocarditis, COVID-19, vaccine
Disclosure: KKY is Editor-in-Chief and JY is an Associate Editor of Journal of Asian Pacific Society of Cardiology; this did not influence acceptance. RH has no conflicts of interest to declare.
Acknowledgements: RH and JY contributed equally.
Received: 28 October 2021 Accepted: 22 April 2022 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e17.
DOI: https://doi.org/10.15420/japsc.2021.26
Correspondence: Khung Keong Yeo, National Heart Centre Singapore, 5 Hospital Drive, Singapore 169609, E: yeo.khung.keong@singhealth.com.sg
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has resulted in much morbidity and mortality. The development of vaccines has provided an important tool in the battle against this pandemic. The recent randomised clinical trials published on the mRNAbased COVID-19 vaccines have demonstrated their efficacy and safety.1 2 However, with large-scale global uptake, rarer side-effects not seen in the earlier clinical trials have been reported.
Israel, one of the early leaders of vaccination uptake (using predominantly the Pfizer–BioNTech vaccine), first raised concerns about the risk of myocarditis, with reports of 275 cases of myocarditis between December 2020 and May 2021 among more than 5 million vaccinated people.3 Since then, several other reports have surfaced worldwide. In this article, we aim to summarise some of the major international data available on the incidence of myocarditis and/or pericarditis after mRNA COVID-19 vaccination, and provide an overview of the topic. Please refer to Supplementary Material Table 1 for a more detailed breakdown of the data.
Incidence of Myocarditis and Pericarditis After mRNA COVID-19 Vaccination
Overall, the combined rates of myocarditis and pericarditis post-SARSCoV-2 vaccination ranged between 0.45 and 24.7 per million doses across the different countries, as summarised in Supplementary Material Table 1 Separately, the rates of myocarditis and pericarditis varied from 0.37 to 26.8 per million and 0.08 to 12.9 per million, respectively. These numbers were collated from government reports and health warnings from the individual countries. In Singapore, the combined rates of myocarditis and pericarditis reported by Health Sciences Authority as of 31 August 2021 were approximately 10.6 for every million doses of vaccine administered, which was comparable to the numbers reported overseas.4
Comparison with background rates of myocarditis and/or pericarditis were performed to determine if these reported rates reflect a true increase in incidence. The Communicable Disease Center compared US Vaccine Adverse Event Reporting System (VAERS) data with populationbased background incidence rates in the US; this demonstrated an increase in the incidence of observed over expected rates of myocarditis
or pericarditis after the SARS-CoV-2 vaccination.5 6 This was further supported by a nationwide observational study in Israel, which compared incident rates of myocarditis in vaccinated versus unvaccinated groups –the risk of myocarditis increased by a factor of three after vaccination (21 versus 6 in the control group).7
Of note, not all countries published rates of myocarditis and pericarditis separately. However, in countries that did, the numbers between groups were comparable. In the US, VAERS rates of myocarditis and pericarditis were between 2.8 and 4.5 per million vaccinations, and 2.1 and 2.6 per million vaccines, respectively.5 In Europe, the rates were between 0.8 and 0.9 per million vaccines for both myocarditis and pericarditis individually.8 Finally, in the UK, rates of myocarditis and pericarditis post-mRNA vaccination were between 7.4 and 28.5 per million vaccines, and 5.5 and 17.3 per million vaccines, respectively.9
The cumulative data suggest a trend towards an increase in incidence over background rates of myocarditis and pericarditis associated with SARS-CoV-2 vaccination.
First Versus Second Dose
The occurrence of myocarditis and/or pericarditis is seen more commonly with the administration of the second dose of the vaccine. According to the data published by US VAERS, the risk of developing myocarditis and/ or pericarditis postvaccination was up to fivefold more likely after the second dose, as opposed to the first dose (1.5 versus 5.6 per million for first versus second dose, respectively).5
This trend is also consistent with data from Israel, which demonstrated myocarditis rates of five versus 24 per million doses of vaccine after the first dose versus second dose, respectively.10 Canadian Ministry of Health reports rates of myocarditis and pericarditis of 9.1 cases per million after the first dose versus 15.6 per million doses after the second dose.11
Though no specific numbers were provided by the UK’s Medicines & Healthcare products Regulatory Agency’s Yellow Card reporting and the European Medicines Agency, both mentioned that occurrence of myocarditis and pericarditis were more common after the second dose.8 9
COVID-19 EDITORIAL © RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com
1 Jonathan
1,2
1,2
Singapore,
2.
Table 1: Recommendations for Management of Myocarditis and Pericarditis
Evaluation for the following symptoms
Myocarditis:
• Chest pain, tightness or discomfort
• Rapid or irregular heartbeat shortness of breath
• Fluid build-up – lower limb oedema, pulmonary congestion
• May have non-specific signs, such as fever, fatigue, body aches
Pericarditis:
• Chest pain that is usually sharp, worse when lying down, and relieved by sitting up and leaning forward
• Pain on deep inspiration
Symptom onset Within a few days after vaccination (median 2 days)
Initial evaluation Clinical history and examination ECG, cardiac troponin level, as well as inflammatory markers, such as erythrocyte sedimentation rate and C-reactive protein
Review all the results of initial investigations promptly to decide further management
For suspected cases Evaluation:
• Cardiology consult
• Echocardiography ± cardiac MRI
• Coronary evaluation for presentations suggestive of acute coronary syndrome to rule out obstructive coronary artery disease
• Endomyocardial biopsy (this is rarely indicated)
Monitoring:
• Patients with evidence of congestive cardiac failure, myocardial injury, ECG changes, cardiac imaging abnormalities, arrhythmia or haemodynamic instability will require hospitalisation and close follow-up
Further treatment:
• Patients with heart failure, life-threatening arrhythmia or hemodynamic instability can be considered for intravenous steroids and/or intravenous immunoglobulin together with other circulatory support measures
pericarditis, they both mentioned that majority of the affected were men aged <30 years.
These statistics are further supported by a recent local nationwide publication on the incidence of pericarditis and myocarditis in Singapore post-COVID-19 vaccination.12 It found a total of nine cases of pericarditis; all of which were male, except one. This fell predominantly in the 12–19 years age range, with an incidence of 1.11 cases per 100,000 doses. A further 25 cases of myocarditis (11 with concomitant pericarditis) were diagnosed, 80% (20 cases) were male and the median age was 23 years (range 12–55 years), with 16 cases after the second dose. Similarly, a higher-than-expected number of cases was seen in males aged 12–19 and 20–29 years.12
Differences Between Vaccine Brands
There appear to be some differences in myocarditis or pericarditis rates between the two types of mRNA vaccines (Pfizer–BioNTech and Moderna). A signal of this possible discrepancy was noted in both UK and Canadian data. In the UK, the rates of myocarditis were 7.4 per million Pfizer–BioNTech vaccines compared with 28.5 per million Moderna vaccines.9 Pericarditis rates followed this similar trend, with 5.5 per million doses after Pfizer–BioNTech and 17.3 per million vaccines after Moderna. In Canada, the combined rate of myocarditis and pericarditis was 10.3 per million vaccines and 21.1 per million vaccines after Pfizer–BioNTech and Moderna vaccines, respectively.11
It is not clear if this is a significant observation, and what the possible reasons for this could be. Postulations include the higher concentration in the Moderna vaccine.
Of note, in Canada, Europe and the UK, where data on AstraZeneca, an adenovirus-based vaccine, were collected, the rates of myocarditis and pericarditis ranged from 2.2 to 5.8 per million doses.
Patients with minor symptoms
Patients with normal ECG, troponin and inflammatory markers may be monitored in the outpatient setting
Advice to avoid strenuous activities
Source: Bozkurt et al. 202113 and Australian Government, Department of Health 2021.16
Influence of Age and Sex
The current data suggest a preponderance for young men to develop myocarditis and/or pericarditis postvaccination. Data from VAERS demonstrate that myocarditis and pericarditis were up to 10-fold more likely to occur postvaccination in men aged <30 years when compared with the US population-based background incidence rates. This difference was considerably less for adults aged >40 years.5
Canadian Ministry of Health data corroborated these findings; the median age of incidence was 30 years after Pfizer–BioNTech vaccines, and 28 years after Moderna vaccines. The majority of those affected were men; accounting for 62% and 70% of myocarditis after the Pfizer–BioNTech and Moderna vaccine, respectively.11
In an Israeli nationwide cohort study, among those with myocarditis, the median age was 25 years (interquartile range 20–34 years), and 90.9% were men. Once again, this highlights the increased risk in this particular population.7
Although the Medicines & Healthcare products Regulatory Agency and European Medicines Agency did not provide individual breakdowns of age and sex of the patients who developed possible myocarditis and/or
Discussion
From the data, there is a signal of increased risk of pericarditis and myocarditis in men aged <30 years, although the absolute risk is very low.
Several pathophysiological mechanisms for the risk of pericarditis and myocarditis after mRNA vaccine have been proposed. Molecular mimicry between the spike protein of SARS-CoV-2 and self-antigens, triggering of pre-existing dysregulated immune pathways in predisposed individuals,nd direct immune response to mRNA have been put forth.13 Some of these would also account for the increased rates of myocarditis seen in SARSCoV-2 infections.7
The reasons for young male predominance in pericarditis and myocarditis cases are being worked up, but possible explanations relate to sex hormone differences in immune response and myocarditis, and underdiagnosis of cardiac disease in women.13
These potential risks need to be balanced against the risk of SARS-CoV-2 infection. Of hospitalised SARS-CoV-2 patients in a similar age group (18–34 years); 21% required ventilation and 2.7% died (almost double the mortality of acute MI).14 Vaccination can significantly reduce these risks.1 2 Our local real-world data support this, with 6.6% of unvaccinated patients becoming severely ill or dying compared with just 0.9% in the fully vaccinated population.4 Additionally, COVID-19 infection can itself raise the risk of myocarditis, the risk ratio of myocarditis from SARS-CoV-2 infection is 18.28 compared with 3.24 from vaccination.7
Pericarditis and Myocarditis after mRNA-based COVID-19 Vaccination JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Pericarditis
There are some limitations to these findings. First, as the data were largely collated from self-reported national databases, the diagnosis may not necessarily fulfil strict diagnostic criteria for peri- or myocarditis. Second, inherent in such data is the inability to match for other confounding variables. Patients also possibly possessed increased clinical awareness of the risk of myocarditis/pericarditis and were therefore more likely to seek medical attention for a potentially otherwise self-limiting condition. Finally, heterogenicity of the type of data collected makes direct comparison challenging. For example, not all data sources collated data according to the brand of vaccine or first versus second dose, and often there was no clear distinction between myocarditis and pericarditis, which can have different disease processes.
Recommendations
While there is an increased risk of pericarditis and myocarditis in younger
1. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–15. https://doi.org/10.1056/ NEJMoa2034577; PMID: 33301246.
2. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403–16. https://doi.org/10.1056/NEJMoa2035389; PMID: 33378609.
3. Heller J. Israel sees probable link between Pfizer vaccine and myocarditis cases. Reuters 2 June 2021. https://www. reuters.com/world/middle-east/israel-sees-probable-linkbetween-pfizer-vaccine-small-number-myocarditiscases-2021-06-01 (accessed 7 September 2021).
4. Singapore Ministry of Health. Update on local COVID-19 situation and vaccination progress. 7 September 2021. https://www.moh.gov.sg/news-highlights/details/update-onlocal-covid-19-situation-and-vaccination-progress-(7-sep) (accessed 8 September 2021).
5. Shimabukuro T. COVID-19 vaccine safety updates. Centers for Disease Control and Prevention, 23 June 2021. https:// www.cdc.gov/vaccines/acip/meetings/downloads/slides2021-06/03-COVID-Shimabukuro-508.pdf (accessed 7 September 2021).
6. Gubernot D, Jazwa A, Niu M, et al. U.S. population-based background incidence rates of medical conditions for use in
males, the absolute risk is low, and the US Centers for Disease Control and Prevention have recommended vaccination in those aged ≥12 years to mitigate the risk of serious SARS-CoV-2 infection.15 Similarly, all the countries discussed in Supplementary Material Table 1 have adopted similar recommendations. This is because of the efficacy of mRNA-based vaccines in preventing serious illness and transmission of disease, and the relatively small numbers of adverse side-effects of myocarditis/ pericarditis.1 2
Additional precautions may be put in place to mitigate the risks, such as raising awareness of these conditions, advice on seeking early medical attention and reducing physical activity in the immediate phase postvaccination. Although uncommon, patients, particular younger males, who begin feeling unwell shortly after COVID-19 vaccinations, should be further evaluated. See Table 1 for more details.
safety assessment of COVID-19 vaccines. Vaccine 2021;39:3666–77. https://doi.org/10.1016/j. vaccine.2021.05.016; PMID: 34088506.
7. Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med 2021;385:1078–90. https://doi.org/10.1056/ NEJMoa2110475; PMID: 34432976.
8. European Medicines Agency. COVID-19 vaccines: update on ongoing evaluation of myocarditis and pericarditis. European Medicines Agency, 11 June 2021. https://www. ema.europa.eu/en/news/covid-19-vaccines-update-ongoingevaluation-myocarditis-pericarditis (accessed 6 October 2021).
9. Medicines & Healthcare products Regulatory Agency. Coronavirus vaccine – weekly summary of Yellow Card reporting. 2021. https://www.gov.uk/government/ publications/coronavirus-covid-19-vaccine-adversereactions/coronavirus-vaccine-summary-of-yellow-cardreporting#annex-2-glossary (accessed 6 October 2021).
10. Ministry of Health Israel. Surveillance of myocarditis (inflammation of the heart muscle) cases between December 2020 and May 2021. Israel: Ministry of Health, 2 June 2021. https://www.gov.il/en/Departments/ news/01062021-03 (accessed 7 September 2021).
11. Public Health Agency of Canada. Canadian COVID-19
vaccination safety report. Ottawa: Public Health Agency of Canada, 2021. https://health-infobase.canada.ca/covid-19/ vaccine-safety/ (accessed 7 September 2021).
12. Yap J, Tham MY, Poh J, et al. Pericarditis and myocarditis after COVID-19 mRNA vaccination in a nationwide setting. Ann Acad Med Singap 2022;51:96–100. https://doi. org/10.47102/annals-acadmedsg.2021425; PMID: 35224605.
13. Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021;144:471–84. https://doi. org/10.1161/CIRCULATIONAHA.121.056135; PMID: 34281357.
14. Cunningham JW, Vaduganathan M, Claggett BL, et al. Clinical outcomes in young US adults hospitalized with COVID-19. JAMA Intern Med 2020;181:379–81. https://doi. org/10.1001/jamainternmed.2020.5313; PMID: 32902580.
15. Centers for Disease Control and Prevention. COVID-19 vaccines for children and teens. 2021. https://www.cdc.gov/ coronavirus/2019-ncov/vaccines/recommendations/ adolescents.html (accessed 26 September 2021).
16. Australian Government, Department of Health. COVID-19 vaccination – guidance on myocarditis and pericarditis after mRNA COVID-19 vaccines. 2021. https://www.health.gov.au/ sites/default/files/documents/2021/12/covid-19-vaccinationguidance-on-myocarditis-and-pericarditis-after-mrna-covid19-vaccines_0.pdf (accessed 2 April 2022).
and Myocarditis after mRNA-based COVID-19 Vaccination JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Dual Antiplatelet Therapy for 3 or 12 Months in Patients with Non-ST-elevation MI/ Unstable Angina or ST-elevation MI: Analysis of the REDUCE Trial
Wan Azman Wan Ahmad , 1 Edouard Benit , 2 Victor M Legrand , 3 Achmad Fauzi Yahya,4 Huay Cheem Tan , 5 Sodiqur Rifqi,6 Muhamad Ali SK Abdul Kader , 7 Bruno Pironi,8 Robert J de Winter,9 Chor-Cheung Frankie Tam,10 Eric Ligtenberg,11 Giuseppe De Luca12 and Harry Suryapranata13
1. Cardiology Unit, Department of Medicine, University Malaya Medical Centre, Kuala Lumpur, Malaysia; 2. Department of Invasive and Interventional Cardiology, Jessa Ziekenhuis, Hasselt, Belgium; 3. Service de Cardiologie, University Hospital of Liège, Liège, Belgium; 4. Department of Cardiology and Vascular Medicine, Hasan Sadikin Hospital, Jawa Barat, Indonesia; 5. Department of Cardiology, National University Heart Centre, Singapore; 6. Department of Cardiology and Vascular Medicine, Dr Kariadi Central General Hospital- Semarang, Jawa Tengah, Indonesia; 7. Cardiology Department, Hospital Pulau Pinang, George Town, Malaysia; 8. Department of Cardiology, Ospedale Madre Giuseppina Vannini, Rome, Italy; 9. Department of Cardiology, Amsterdam University Medical Centre, Amsterdam, the Netherlands; 10. Cardiology Division, Department of Medicine, The University of Hong Kong, Hong Kong, China; 11. OrbusNeich Medical, Hoevelaken, the Netherlands; 12. Department of Cardiology, AOU Maggiore della Carità, Eastern Piedmont University, Novara, Italy; 13. Department of Cardiology, Radboud University Medical Centre, Nijmegen, the Netherlands
Abstract
Background: The aim of this subanalysis of the REDUCE (Short-term Dual Antiplatelet Therapy in Patients with ACS Treated with the COMBO Dual-therapy Stent) trial was to evaluate differences between non-ST-elevation MI/unstable angina (NSTEMI/UA) patients and ST-elevation MI (STEMI) patients in terms of patient characteristics, procedures and outcomes. Methods: In the REDUCE trial, 1,496 acute coronary syndrome patients undergoing successful COMBO stent implantation were randomised to 3 or 12 months of dual antiplatelet therapy (DAPT) treatment. In total, 789 (52.8%) patients had NSTEMI/UA and 706 (47.2%) had STEMI. For this analysis, NSTEMI/UA patients (n=789) were compared with STEMI patients (n=706). Results: In the analysis of the NSTEMI/UA and STEMI cohorts comparing 3-month DAPT (751 patients) and 12-month DAPT treatment (745 patients) there were no statistically significant differences in the primary endpoints of all-cause mortality, MI, stent thrombosis, stroke, target vessel revascularisation or bleeding (Bleeding Academic Research Consortium [BARC] II, III, V) at 360 and 720 days between the two cohorts. Comparing the overall primary endpoints in the NSTEMI/UA cohort, 3-month DAPT was better than 12-month DAPT (9.6% versus 10.0% at 360 days and 12.1% versus 15.0% at 720 days). In the STEMI cohort, 12-month DAPT was better than 3-month DAPT (8.4% versus 11.0% at 720 days). Conclusion: For NSTEMI/UA patients, a numerically lower occurrence of outcomes was observed with 3-month DAPT at 360 and 720 days, whereas for STEMI patients, 12-month DAPT appeared to be better at 720 days. NSTEMI patients seemed to have less favourable long-term outcomes than STEMI patients in this subanalysis. Further adequately powered randomised trials are needed to confirm the findings.
Keywords
COMBO stent, non-ST-elevation MI, outcomes, ST-elevation MI, unstable angina
Disclosure: All authors were included in the original trial. EL is an employee of OrbusNeich Medical. All other authors have no conflicts of interest to declare Trial Registration: The REDUCE trial is registered (NCT02118870).
Funding: No funding was involved in the analysis or the writing of the manuscript.
Author contributions: Conceptualisation: WAWA, HCT, MASKAK, RJdW, CCFT, GDL, HS; data curation: EB, MASKAK, RJdW, CCFT, GDL, HS; formal analysis: GDL; funding acquisition: EL, GDL, HS; investigation: EB, VML, AFY, SR, MASKAK, BP, RJdW, CCFT, GDL, HS; methodology: WAWA, AFY, HCT, CCFT, HS; project administration: EB, HCT, MASKAK, RJdW, EL, HS; resources: VML, CCFT, HS; software: HS; supervision: HCT, MASKAK, GDL, HS; validation: WAWA, VML, AFY, SR, CCFT, GDL, HS; visualisation: GDL; writing – original draft preparation: WAWA; writing – review & editing: WAWA, EB, VML, HCT, MASKAK, RJdW, CCFT, EL, GDL.
Informed Consent: Acute coronary syndrome patients who were successfully treated with a COMBO stent were subsequently randomised, after obtaining written informed consent, during index hospitalisation (before discharge), in a 1:1 ratio, to either 3-month or 12-month DAPT. The Investigator or designee, who has been trained on the protocol, explained the nature and scope of the trial, potential risks and benefits of participation to the patients and answered their questions. All patients signed and personally dated the approved informed consent prior to any study-specific procedures.
Ethics: The ethics committees of each participating centre and the competent authorities approved the study. This study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).

Data Availability: The data that support the findings of this study are available from Diagram BV, Dokter Stolteweg 96, 8025 AZ Zwolle, the Netherlands. Restrictions apply to the availability of these data, which were used under licence for this study. Data are available from the authors with the permission of Diagram BV.
Received: 15 November 2021 Accepted: 20 December 2021 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e18.
DOI: https://doi.org/10.15420/japsc.2021.29
Correspondence: Wan Azman Wan Ahmad, Department of Medicine, University Malaya Medical Centre, Kuala Lumpur 59100, Malaysia. E: wanazman@ummc.edu.my
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
© RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com Intervention
TRIAL ANALYSIS T1
DAPT in NSTEMI/UA or STEMI: Analysis of REDUCE
Acute coronary syndrome (ACS) occurs when an atherosclerotic plaque disrupts the flow of blood, resulting in activation of platelets and coagulation factors and leading to the formation of a thrombus. Depending upon the degree and acuteness of thrombus formation and coronary occlusion, the patient can present with non-ST-elevation ACS or STelevation MI (STEMI). Non-ST-elevation ACS is further classified as non-STelevation MI (NSTEMI) or unstable angina (UA). In NSTEMI there is myocardial injury and the cardiac biomarkers are elevated, whereas in UA the cardiac biomarkers are normal.
Dual antiplatelet therapy (DAPT) with aspirin plus a P2Y12 inhibitor is the cornerstone of treatment after percutaneous coronary intervention (PCI) for ACS. DAPT has a twofold benefit in these patients. First, DAPT is crucial to reduce the risk of stent thrombosis, MI and target lesion revascularisation at the PCI coronary site. Second, patients with ACS are at high risk of recurrent ischaemic events at other sites, which can be prevented by DAPT. European and American guidelines recommend the use of DAPT for 12 months after ACS and PCI, with the highest level of evidence.1,2
While 1-year DAPT has been recommended with first-generation drugeluting stents (DES) to prevent late stent thrombosis, with newergeneration DES the duration of DAPT can be significantly reduced.3,4 Bleeding risk increases in line with DAPT duration regardless of which P2Y 12 inhibitor is used.5 Major bleeding in patients receiving DAPT has an impact on mortality after ACS that can be greater than that of recurrent MI.6
There is emerging evidence that shorter-duration DAPT with the newergeneration DES has similar efficacy and safety to the gold standard DAPT (1 year) in the setting of ACS and PCI.7–10
The COMBO stent (OrbusNeich Medical) was used in the REDUCE (Shortterm Dual Antiplatelet Therapy in Patients with ACS Treated with the COMBO Dual-therapy Stent) trial.11 COMBO is a dual-therapy stent with abluminal sirolimus elution from a biodegradable polymer and a luminal pro-healing anti-CD34+ antibody layer, which attracts circulating endothelial progenitor cells to provide rapid endothelialisation. This novel technology may allow for a shorter duration of DAPT after PCI in the setting of ACS. The objective of this analysis was to evaluate differences between NSTEMI/UA and STEMI patients from the REDUCE trial population in terms of their characteristics, procedures and outcomes.
Methods
REDUCE (NCT02118870) was an investigator-initiated prospective, multicentre trial that randomised ACS patients after treatment with the COMBO stent to either 3 or 12 months of DAPT.12 The study was conducted at 36 investigational sites in Europe and Asia and was designed to enrol 1,500 ACS patients. The study design and randomisation, ethics approval, treatment and follow-up procedures, study endpoints, statistical consideration and results were published in 2019.11 The primary endpoints were all-cause mortality, MI, stent thrombosis, stroke, target vessel revascularisation (TVR) and bleeding (Bleeding Academic Research Consortium [BARC] II, III and V). Of the 1,500 eligible ACS patients undergoing successful COMBO stent implantation, four patients revoked their informed consent soon after randomisation, leaving 1,496 patients for analysis. Of these 1,496 patients, 789 (52.8%) had NSTEMI/UA and 706 (47.2%) had STEMI. In the NSTEMI/UA cohort when 3- and 12-month DAPT were compared, the only significant differences were for body weight (mean 80.97 ± 15.81 kg versus 78.88 ± 14.85 kg; p=0.0487) and spironolactone use (3.2% versus 1.0%; p=0.037), both of which were
higher in the 3-month group. In the STEMI cohort, the 3-month group had a higher rate of previous PCI than the 12-month group (7.0% versus 3.6%; p=0.0422). The patient profiles were otherwise similar. Thus, in this current analysis, 789 NSTEMI/UA patients were compared with 706 STEMI patients in terms of patient characteristics, PCI procedures and outcomes.
Statistical Analysis
Cumulative event rates were estimated using the Kaplan–Meier method and compared using the log-rank test. The incidence of the events was also compared using the χ2 test or Fisher’s exact test. Normally distributed variables were compared using the Student’s t-test. Otherwise, the Mann–Whitney U-test was used. Categorical variables are summarised in terms of number and percentages and were compared using the twosided χ2 test or Fisher’s exact test when applicable; p≤0.05 was considered statistically significant.
Results
The overall number of patients in the NSTEMI/UA cohort (789; 52.8%) was compared with the overall number of STEMI cohort patients (706; 47.2%). In this subgroup analysis of the REDUCE trial for the NSTEMI/UA and STEMI cohorts, comparing 3-month versus 12-month DAPT, there were no statistically significant differences in the primary endpoints at 360 days and at 720 days between the two cohorts. However, in the NSTEMI/UA cohort, 3-month DAPT was better than 12-month DAPT regarding the primary endpoint outcomes (9.6% versus 10.0% at 360 days and 12.1% versus 15.0% at 720 days). In contrast, for the STEMI cohort, primary endpoint outcomes for 12-month DAPT were better than for 3-month DAPT (3.3% versus 4.1% at 180 days, 6.6% versus 6.6% at 360 days, 8.4% versus 11.0% at 720 days).
Patients from the NSTEMI/UA cohort were older (mean age, 63.39 ± 11.48 years versus 57.99 ± 11.59 years) and more of them were women (24.2% versus 15.4%) than in the STEMI cohort. In the NSTEMI/UA cohort, patient recruitment was higher at the European sites than Asian sites (78.2% versus 60.8%). They had higher rates of conventional cardiovascular risk factors than the STEMI cohort (diabetes, 22.5% versus 18.2%; hypercholesterolaemia, 49.0% versus 41.7%; and hypertension, 56.9% versus 41.5%). Tobacco use was higher in the STEMI cohort than in the NSTEMI/UA cohort (52.5% versus 33.2%). NSTEMI/UA patients had a higher rate of family history of coronary artery disease than STEMI patients (37.4% versus 33.4%) and higher rates of previous ACS (17.0% versus 6.8%) and known angina (18.0% versus 6.9%) prior to the current event. They also had higher rates of previous PCI (15.7% versus 5.4%), previous coronary artery bypass graft surgery (3.9% versus 1.6%) and previous stroke (2.2% versus 1.3%) than the STEMI cohort. NSTEMI/UA patients had higher body weight than STEMI patients (mean ± SD: 79.89 ± 15.34 kg versus 78.70 ± 16.17 kg) and had a higher mean BMI. Their mean creatinine level was higher (85.71 ± 27.00 µmol/l versus 84.94 ± 23.71 µmol/l). However, mean glucose, mean HbA1c, total cholesterol and LDL cholesterol were lower in the NSTEMI/UA cohort than the STEMI cohort (Table 1).
In the NSTEMI/UA cohort, more procedures were performed using radial site access (81.8% versus 70.8%) and this cohort had a higher rate of initial thrombolysis in MI (TIMI) III flow (66.5% versus 27.2%) than the STEMI cohort. They also had higher balloon pre-dilatation (73.9% versus 64.5%). However, post-balloon dilation was similar (55.4% versus 60.0%). PCI success rates were also similar between the two cohorts (almost 100%), along with the number and length of stents used. Thrombosuction devices
JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
were more frequently used in the STEMI cohort than in the NSTEMI/UA cohort (27.8% versus 5.2%; Table 2).
Length of hospital stay was longer in the STEMI cohort than in the NSTEMI/ UA cohort (mean ± SD: 3.45 ± 2.47 days versus 1.97 ± 3.01 days). Discharge medications were similar except for the calcium antagonists, which were prescribed at higher rates in the NSTEMI/UA cohort than in the STEMI cohort (17.3% versus 6.8%; Table 3).
Outcomes
The number of patients from the overall NSTEMI/UA and STEMI cohorts available for outcome analysis at various time intervals is as follows: 782 versus 703 at 90 days; 779 versus 700 at 180 days; 777 versus 700 at 360 days; and 765 versus 694 at 720 days.
When comparing the overall NSTEMI/UA cohort with the overall STEMI cohort for the primary endpoint indices of all-cause mortality, MI, stent thrombosis, stroke, TVR and bleeding (BARC II, III, V), rates in the NSTEMI/ UA cohort were consistently higher than in the STEMI cohort: 2.3% versus 2.1% at 90 days (p=0.8262); 4.9% versus 3.7% at 180 days (p=0.2721); 9.8% versus 6.6% at 360 days (p=0.0252); and 13.6% versus 9.8% at 720 days (p=0.0247). This was attributed to higher all-cause mortality at 180 days (0.8% versus 0.4%), 360 days (1.7% versus 1.0%) and 720 days (3.3% versus 2.0%); higher TVR at 180 days (1.8% versus 1.1%), 360 days (4.5% versus 2.1%) and 720 days (5.8% versus 3.7%); and higher MI at 180 days (1.4% versus 0.7%), 360 days (2.8% versus 1.3%) and 720 days (4.1% versus 2.4%). Revascularisation within 360 and 720 days was also higher in the NSTEMI/UA cohort than in the STEMI cohort (7.6% versus 3.7% and 10.8% versus 6.1%, respectively).
There were no differences in cardiac mortality and bleeding (BARC II, III and V) between the two cohorts. Despite these being ACS patients undergoing PCI, the incidence of stent thrombosis was similarly very low in the two cohorts (Table 3).
Discussion
The REDUCE trial shows that in ACS patients treated with the COMBO stent, 3-month DAPT is non-inferior to 12-month DAPT regarding the primary endpoint indices of all-cause mortality, MI, stent thrombosis, stroke, TVR and bleeding (BARC II, III and V). Similar outcomes between the two groups were observed at 2-year follow-up (11.6% versus 12.1%).11 In the REDUCE trial population, of the 1,496 patients eligible for analysis, 789 (52.8%) were in the NSTEMI/UA cohort and 706 (47.2%) were in the STEMI cohort. This subgroup analysis of NSTEMI/UA and STEMI patients therefore provides a unique opportunity to compare the differences in terms of clinical characteristics, PCI procedures and outcomes between the two cohorts in the era of the new-generation DES in the setting of ACS and contemporary practice.
Although NSTEMI/UA and STEMI share many similarities in terms of risk factors, demographics and pathogenesis of plaque rupture, they are two distinct pathophysiological entities. STEMI is usually characterised by severe and/or total coronary flow obstruction with transmural ischaemia, which predisposes to myocardial necrosis and pump dysfunction. The pathogenesis of NSTEMI differs from that of STEMI in that it usually results from a flow-limiting coronary stenosis with resultant downstream myocardial ischaemia. However, total coronary artery occlusion does exist in a significant number of patients presenting with NSTEMI/UA, thus this analysis will provide insights into the DAPT strategy between NSTEMI/ UA and STEMI in the era of the new-generation DES.
Table 1: History and Investigation in the NSTEMI/ Unstable Angina or STEMI Cohorts
NSTEMI/UA (n=789) STEMI (n=706)
Age (years), mean ± SD 63.39 ± 11.48 57.99 ± 11.59
Sex (male/female), % 75.8/24.2 84.6/15.4
European study sites, n (%) 617/789 (78.2) 429/706 (60.8)
Asian study sites, n (%) 172/789 (21.8) 277/706 (39.2)
Diabetes, n (%) 177/788 (22.5) 128/705 (18.2)
Tobacco use, n (%) 258/776 (33.2) 369/703 (52.5)
Hypercholesterolaemia, n (%) 385/786 (49.0) 293/703 (41.7) Hypertension, n (%) 447/785 (56.9) 306/702 (41.7)
Family history of CAD, n (%) 291/779 (37.4) 234/700 (33.4)
Previous ACS (before current ACS), n (%) 138/788 (17.0) 48/706 (6.8)
Previous PCI, n (%) 124/789 (15.7) 38/706 (5.4)
Previous CABG, n (%) 31/789 (3.9) 11/706 (1.6)
Previous CVA, n (%) 17/789 (2.2) 9/706 (1.3)
Known angina before current ACS, n (%) 142/788 (18.0) 49/706 (6.9) Killip class on arrival at PCI centre, n (%): Class I 683/756 (90.3) 619/705 (87.8)
Class II 59/756 (7.8) 71/705 (10.1)
Class III 10/756 (1.3) 4/705 (0.6) Class IV 4/756 (0.5) 11/705 (1.6)
Weight (kg), mean ± SD 79.89 ± 15.34 78.70 ± 16.17
BMI (kg/m²), mean ± SD 27.12 ± 4.22 26.84 ± 4.52
Creatinine (µmol/l), mean ± SD 85.71 ± 27.00 84.94 ± 23.71 Glucose (mmol/l), mean ± SD 7.22 ± 2.90 8.30 ± 3.78 HbA1c, mean ± SD 8.93 ± 1.00 9.06 ± 1.05
Total cholesterol (mmol/l), mean ± SD 5.00 ± 1.29 5.18 ± 1.14 HDL cholesterol (mmol/l), mean ± SD 1.13 ± 0.35 1.07 ± 0.31 LDL cholesterol (mmol/l), mean ± SD 2.98 ± 1.15 3.39 ± 1.07
ACS = acute coronary syndrome; CABG = coronary artery bypass grafting; CAD = coronary artery disease; CVA = cerebrovascular accident; NSTEMI = non-ST-elevation MI; PCI = percutaneous coronary intervention; STEMI = ST-elevation MI; UA = unstable angina.
In this subgroup analysis of the REDUCE trial in the NSTEMI/UA cohort and STEMI cohort comparing 3-month DAPT versus 12-month DAPT, the primary endpoint indices at 360 and 720 days were not statistically significantly different.11 However, in the NSTEMI/UA cohort, 3-month DAPT appears to be safe in terms of 360- and 720-day outcomes. In contrast, for the STEMI cohort, 12-month DAPT appears to be better for the 720-day outcomes.
With the introduction of newer generation DES, the minimum duration of DAPT recommended by the guidelines has been reduced to 6 months in patients with stable coronary artery disease.1 2 Whether a shorter duration of DAPT is safe in patients presenting with ACS in the contemporary era remains controversial. Furthermore, limited data exist comparing the duration of DAPT between NSTEMI/UA and STEMI patients.
In a network meta-analysis by Yin et al. that included 17 studies with 46,864 patients, eight trials reported endpoints in ACS subgroups (12,376 patients).10 In the subgroup analysis of patients with ACS, short-duration DAPT (3–6 months) showed similar efficacy and safety to standard 12-month DAPT.
DAPT in NSTEMI/UA or STEMI: Analysis of REDUCE JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Vessel disease, n (%):
One 503/789 (63.8) 468/706 (66.3)
Two 213/789 (27.0) 181/706 (25.6)
Three 73/789 (9.3) 57/706 (8.1)
Infarct-related vessel, n (%):
Right coronary artery 219/789 (27.8) 262/706 (37.1)
Left anterior descending 349/789 (44.2) 339/706 (48.0)
Circumflex 210/789 (26.6) 100/706 (14.1)
Left main 1/789 (0.1) 1/706 (0.1)
Graft 10/789 (1.3) 4/706 (0.6)
Initial TIMI flow III, n (%) 510/767 (66.5) 191/701 (27.2)
Radial access site, n (%) 632/773 (81.8) 499/705 (70.8)
Balloon pre-dilatation, n (%) 583/789 (73.9) 454/704 (64.5)
Number of stents used, n (%):
1 645/789 (81.7) 584/706 (82.7)
2 123/789 (15.6) 102/706 (14.4)
3 17/789 (2.2) 19/706 (2.7)
4 4/789 (0.5) 1/706 (0.1)
Total stent length (mm), mean ± SD 24.75 ± 12.33 26.03 ± 13.18
Post-dilatation, n (%) 437/789 (55.4) 423/705 (60.0)
PCI successful (culprit lesion), n (%) 785/789 (99.5) 703/706 (99.6)
Additional devices used, n (%) 41/789 (5.2) 196/705 (27.8)
Additional segments dilated during hospitalisation, n (%) 178/789 (22.6) 137/706 (19.4)
NSTEMI = non-ST-elevation MI; PCI = percutaneous coronary intervention; STEMI = ST-elevation MI; TIMI = Thrombolysis in Myocardial Infarction; UA = unstable angina.
Verdoia et al. conducted a meta-analysis of 17,941 ACS patients undergoing PCI, which also showed that a shorter duration of DAPT (3–6 months) may be safely considered.13 The authors reported similar rates of recurrent thrombotic complications and mortality compared with the standard 12-month DAPT and a significant reduction in major bleeding complications.
In a recent network meta-analysis of randomised trials by Kuno et al., 14 eligible trials enrolling a total of 31,837 patients comparing different DAPT durations (≤3, 6, 12 and >12 months) in ACS patients undergoing PCI were examined. The main findings were that short-term DAPT (≤3 or 6 months) did not increase ischaemic outcomes compared with long-term DAPT (12 and >12 months).14 For bleeding outcomes, ≤3-month DAPT was associated with significant reduction in bleeding compared with 6-, 12- or >12-month DAPT.
Data from these meta-analyses of randomised trials support short-term (≤3- and 6-month) DAPT, even in patients with ACS undergoing PCI using the newer generation DES. However, there is a paucity of data comparing the different durations of DAPT in NSTEMI/UA and STEMI patients. Our analysis suggests that 3 months of DAPT for NSTEMI/UA patients results in numerically lower outcomes of the primary endpoints.
However, for STEMI patients, the 12-month DAPT outcomes may be numerically better. This may be explained by the higher thrombotic risk in
Table 3: Discharge and Primary Endpoint Outcomes in the NSTEMI/Unstable Angina and STEMI Cohorts
NSTEMI/ UA (n=789) STEMI (n=706) p-value
Hospital stay (days), mean ± SD 1.97 ± 3.01 3.45 ± 2.47 <0.01
All-cause mortality, MI, stent thrombosis, stroke, TVR or bleeding (BARC II, III, V), n (%)
90 days: 18/782 (2.3) 15/703 (2.1) 0.8263
180 days: 38/779 (4.9) 26/700 (3.7) 0.2721
360 days: 76/777 (9.8) 46/700 (6.6) 0.0252
720 days: 104/765 (13.6) 68/694 (9.8) 0.0247
90 days: 3/782 (0.4) 2/703 (0.3) 1.0000
All-cause mortality, n (%)
Cardiac mortality, n (%)
Definite/probable stent thrombosis, n (%)
180 days: 6/779 (0.8) 3/700 (0.4) 0.5121
360 days: 13/777 (1.7) 7/700 (1.0) 0.3677
720 days: 25/765 (3.3) 14/694 (2.0) 0.1471
90 days: 2/782 (0.3) 1/703 (0.1) 1.0000
180 days: 2/779 (0.3) 2/700 (0.3) 1.0000
360 days: 7/777 (0.9) 4/700 (0.6) 0.5534
720 days: 13/765 (1.7) 8/695 (1.2) 0.5102
90 days: 3/782 (0.4) 2/703 (0.3) 1.0000
180 days: 5/779 (0.6) 4/700 (0.6) 1.0000
360 days: 7/777 (0.9) 5/700 (0.7) 0.7772
720 days: 10/765 (1.3) 8/694 (1.2) 0.8174
90 days: 4/782 (0.5) 4/703 (0.6) 1.0000
180 days: 14/779 (1.8) 8/700 (1.1) 0.3906
TVR, n (%)
360 days: 35/777 (4.5) 15/700 (2.1) 0.0139
720 days: 44/765 (5.8) 26/694 (3.7) 0.0857
90 days: 8/782 (1.0) 6/703 (0.9) 0.7357
Bleeding BARC II, III or V, n (%)
Stroke, n (%)
180 days: 14/779 (1.8) 12/700 (1.7) 0.9036
360 days: 20/777 (2.6) 19/700 (2.7) 0.8667
720 days: 29/765 (3.8) 23/694 (3.3) 0.6238
90 days: 0/782 (0.0) 3/703 (0.4) 0.1059
180 days: 0/779 (0.0) 3/700 (0.4) 0.1058
360 days: 0/777 (0.0) 5/700 (0.7) 0.0237
720 days: 0/765 (0.0) 6/694 (0.9) 0.0115
90 days: 5/782 (0.6) 2/703 (0.3) 0.4567
180 days: 11/779 (1.4) 5/700 (0.7) 0.2181
MI, n (%)
360 days: 22/777 (2.8) 9/700 (1.3) 0.0452
720 days: 31/765 (4.1) 17/694 (2.4) 0.0105
BARC = Bleeding Academic Research Consortium; NSTEMI = non-ST-elevation MI; STEMI = ST-elevation MI; TVR = target vessel revascularisation; UA = unstable angina.
STEMI, which requires a longer DAPT duration than in NSTEMI/UA. Moreover, in patients with STEMI, the daily risk of ischaemia significantly exceeded the daily risk of bleeding beyond 30 days, supporting the use of a longer duration of DAPT after STEMI.15
Patients from the NSTEMI/UA cohort were older, were more often women and had higher rates of cardiovascular risk factors (diabetes, hypercholesterolaemia and hypertension). Tobacco use was higher in the STEMI cohort. NSTEMI/UA patients had higher rates of previous ACS, known angina, previous stroke and a higher mean creatinine level. Length of hospital stay was longer in the STEMI cohort than in the NSTEMI/UA cohort. Discharged medications were similar except for
DAPT in NSTEMI/UA or STEMI: Analysis of REDUCE JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Table 2: Procedures in the NSTEMI/ Unstable Angina and STEMI Cohorts
NSTEMI/UA (n=789) STEMI (n=706)
calcium antagonists, which were prescribed more frequently in the NSTEMI/UA cohort than in the STEMI cohort. These findings are similar to the Malaysian Annual Report of the NCVD-ACS Registry, 2014–15.16 Similar findings were also reported in the Second Euro Heart Survey on ACS.17
Patients presenting with STEMI are known to have a higher risk of early mortality, but patients with NSTEMI/UA have been shown to have a higher risk of long-term mortality, attributed to a higher burden of comorbidities and multivessel disease.18–20
In our analysis, the NSTEMI/UA cohort appeared to have higher rates of primary endpoint indices than the STEMI cohort at 90, 180, 360 and 720 days. This was attributed to higher all-cause mortality, TVR and MI. Data from the ACTION Registry–Get With The Guidelines indicated that STEMI patients had significantly lower comorbidities than NSTEMI patients.21 They were more likely to present with a larger infarct and had higher predicted in-hospital mortality. However, the unadjusted cumulative incidence of all-cause mortality and a composite outcome including mortality and non-fatal cardiovascular and cerebrovascular outcomes, was lower for STEMI patients from hospital discharge through 2 years.21 Similar findings were noted from the Malaysian NCVD-ACS egistry.16 Outcomes for STEMI compared with NSTEMI for in-hospital mortality were 10.6% versus 8.0%, whereas at 1 year they were 17.9% versus 23.0%.16
Study Limitations
This study was a subgroup analysis with all the inherent limitations in the interpretation of data. The REDUCE trial was adequately powered. However, in this subgroup analysis, comparing NSTEMI/UA versus STEMI cohorts, the sample size was not adequately powered to determine the
1. Levine GN, Bates ER, Bittl JA, et al. ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. J Am Coll Cardiol 2016;68:1082–115. https://doi. org/10.1016/j.jtcversus.2016.07.044; PMID: 27751237.
2. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213–60. https://doi.org/10.1093/ eurheartj/ehx419. PMID: 28886622.
3. Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Clinical outcomes with bioabsorbable polymer- versus durable polymer-based drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol 2014;63:299–307. https://doi.org/10.1016/j. jacc.2013.09.061; PMID: 24211507.
4. Moon JY, Franchi F, Rollini F, Angiolillo DJ. Evolution of coronary stent technology and implications for duration of dual antiplatelet therapy. Prog Cardiovasc Dis 2018;60:478–90. https://doi.org/10.1016/j.pcad.2017.12.004; PMID: 29291426.
5. Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet 2017;389:1025–34. https://doi.org/10.1016/S01406736(17)30397-5; PMID: 28290994.
6. Mehran R, Pocock S, Nikolsky E, et al. Impact of bleeding on mortality after percutaneous coronary intervention: results from a patient-level pooled analysis of the REPLACE-2 (Randomized Evaluation of PCI linking Angiomax to Reduced Clinical Events), ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy), and HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) trials. JACC Cardiovasc Interv
difference in outcomes between the two cohorts for 3- and 12-month DAPT. Furthermore, for simplicity of comparison, the overall patients in the NSTEMI/UA and STEMI cohorts were compared to determine the difference between patient profile and outcomes, although there were some minor differences between the two cohorts.
Conclusion
In the REDUCE trial population, a numerically lower occurrence of outcomes was observed with the shorter duration of DAPT for NSTEMI patients, whereas for STEMI patients, 12-month DAPT appeared to be better. There is no statistical interaction across the subgroups and this observation is exploratory. These findings will need to be tested in a randomised controlled trial with adequate sample size. In this analysis, the overall NSTEMI/UA cohort tended to have significantly higher primary endpoint indices at 360 and 720 days. This highlights the importance of risk assessment tools to identify high-risk patients, early PCI and good guideline-directed medical therapy to further improve long-term outcomes in the NSTEMI/UA population.
Clinical Perspective
• There is a paucity of data on the duration of dual antiplatelet therapy (DAPT) comparing non-ST-elevation MI (NSTEMI) and ST-elevation MI (STEMI) in contemporary percutaneous coronary intervention in the era of new-generation drug-eluting stents.
• Our analysis suggests that shorter DAPT may be suitable for NSTEMI patients and that longer DAPT may be suitable for STEMI patients.
• These findings need to be further tested in a randomised controlled study.
2011;4:654–64. https://doi.org/10.1016/j.jcin.2011.02.011; PMID: 21700252.
7. Naber CK, Urban P, Ong PJ, et al. Biolimus-A9 polymer-free coated stent in high bleeding risk patients with acute coronary syndrome: a Leaders Free ACS sub-study. Eur Heart J 2017;38:961–9. https://doi.org/10.1093/eurheartj/ehw203; PMID: 27190095.
8. Lohaus R, Michel J, Mayer K, et al. Six versus twelve months clopidogrel therapy after drug-eluting stenting in patients with acute coronary syndrome: an ISAR-SAFE study subgroup analysis. Sci Rep 2016;6:33054. https://doi. org/10.1038/srep33054; PMID: 27624287.
9. Kedhi E, Fabris E, van der Ent M, et al. Six months versus 12 months dual antiplatelet therapy after drug-eluting stent implantation in ST-elevation myocardial infarction (DAPTSTEMI): randomised, multicentre, non-inferiority trial. BMJ 2018;363:k3793. https://doi.org/10.1136/bmj.k3793; PMID: 30279197.
10. Yin S-HL, Xu P, Wang B, et al. Duration of dual antiplatelet therapy after percutaneous coronary intervention with drugeluting stent: systematic review and network meta-analysis. BMJ 2019;365:l2222. https://doi.org/10.1136/bmj.l2222; PMID: 31253632.
11. De Luca G, Damen SA, Camaro C, et al. Final results of the randomised evaluation of short-term dual antiplatelet therapy in patients with acute coronary syndrome treated with a new-generation stent (REDUCE trial). EuroIntervention 2019;15:e990–8. https://doi.org/10.4244/EIJ-D-19-00539; PMID: 31422929.
12. Camaro C, Damen SA, Brouwer MA, et al. Randomized evaluation of short-term dual antiplatelet therapy in patients with acute coronary syndrome treated with the COMBO dual therapy stent: rationale and design of the REDUCE trial. Am Heart J 2016;178:37–44. https://doi.org/10.1016/j. ahj.2016.04.016; PMID: 27502850.
13. Verdoia M, Kedhi E, Ceccon C, et al. Duration of dual antiplatelet therapy and outcome in patients with acute coronary syndrome undergoing percutaneous revascularization: a meta-analysis of 11 randomized trials. Int J Cardiol 2018;264:30–8. https://doi.org/10.1016/j. ijcard.2018.02.095; PMID: 29776573.
14. Kuno T, Ueyama H, Takagi H, et al. Optimal duration of dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome: insights from a network meta-analysis of randomized trials. Cardiovasc Revasc Med 2021;28:50–6. https://doi. org/10.1016/j.carrev.2020.07.039; PMID: 32893157.
15. Giustino G, Mehran R, Dangas GD, et al. Characterization of the average daily ischemic and bleeding risk after primary PCI for STEMI. J Am Coll Cardiol 2017;70:1846–57. https://doi. org/10.1016/j.jacc.2017.08.018; PMID: 28982497.
16. Wan Ahmad WA (ed). Annual report of the NCVD-ACS Registry 2014 & 2015. http://www.acrm.org.my/ncvd/ pciReport_14.php (accessed 19 May 2022).
17. Mandelzweig L, Battler A, Boyko V, et al. The second Euro Heart Survey on acute coronary syndromes: characteristics, treatment, and outcome of patients with ACS in Europe and the Mediterranean Basin in 2004. Eur Heart J 2006;27:2285–93. https://doi.org/10.1093/eurheartj/ehl196; PMID: 16908490.
18. Chan MY, Sun JL, Newby LK, et al. Long-term mortality of patients undergoing cardiac catheterization for ST-elevation and non-ST-elevation myocardial infarction. Circulation 2009;119: 3110–7. https://doi.org/10.1161/ CIRCULATIONAHA.108.799981; PMID: 19506116.
19. Chin CT, Wang TY, Chen AY, et al. Trends in outcomes among older patients with non-ST-segment elevation myocardial infarction. Am Heart J 2014;167:36–42. https://doi. org/10.1016/j.ahj.2013.10.008; PMID: 24332140.
20. Roe MT, Parsons LS, Pollack CV Jr, et al. Quality of care by classification of myocardial infarction: treatment patterns for ST-segment elevation versus non-ST-segment elevation myocardial infarction. Arch Intern Med 2005;165:1630–6. https://doi.org/10.1001/archinte.165.14.1630; PMID: 16043682.
21. Vora AN, Wang TY, Hellkamp AS, et al. Differences in shortand long-term outcomes among older patients with ST-elevation versus non-ST-elevation myocardial infarction with angiographically proven coronary artery disease. Circ Cardiovasc Qual Outcomes 2016;9:513–22. https://doi. org/10.1161/CIRCOUTCOMES.115.002312; PMID: 27601458.
DAPT
in NSTEMI/UA or STEMI: Analysis of REDUCE
JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Adverse Cardiac Effects of SARS-CoV-2 Infection

Laszlo Göbölös , 1 Yosef Manla , 2 István Rácz , 3 Maurice Hogan , 4 Ernő Remsey-Semmelweis,5 Bassam Atallah , 6 Yazan AlJabery,1 Wael AlMahmeed , 2 Fahad AlSindi,2 Feras Bader,2 Gopal Bhatnagar , 1 Tareq Aleinati 1 and Emin Murat Tuzcu 2
1. Department of Cardiac Surgery, Heart and Vascular Institute, Cleveland Clinic, Abu Dhabi, UAE; 2. Department of Cardiology, Heart and Vascular Institute, Cleveland Clinic, Abu Dhabi, UAE; 3. Winramed Health Care Services Limited Company, Siófok, Hungary; 4. Department of Cardiothoracic and Vascular Intensive Care, Auckland City Hospital, Auckland, New Zealand; 5. Royal Brompton and Harefield NHS Foundation Trust, London, UK; 6. Department of Clinical Pharmacotherapy, Cleveland Clinic, Abu Dhabi, UAE
Abstract
The coronavirus pandemic has spread globally and resulted in the registered deaths of over 5.5 million people, with nearly 380 million infected, straining health systems focused on transmission suppression and supportive care because specific treatment options are limited. COVID-19 is a microvascular disease with dominant respiratory representation, but a significant number of patients experience multisystem or extrarespiratory organ involvement. Although severe acute respiratory syndrome coronavirus-2 has some degree of a direct cytopathic effect on cardiomyocytes, the oxidative burst on a microvascular level seems to be the key for both short- and long-term adverse health effects. Targeted diagnostics and treatment without substantial delay may reduce the amplified immune response; otherwise, considerable tissue damage may occur with unfavourable consequences, including acute and chronic cardiac syndromes. This paper reviews the pathomechanisms relevant to the shortand long-term cardiac effects of COVID-19. Data were identified by searching the PubMed database and reviewing references from relevant articles published in English; abstracts and meeting reports were excluded.
Keywords
SARS-CoV-2, activated phagocytes, microvascular disease, heart failure, cardiac biomarkers, extracorporeal membrane oxygenation
Disclosure: LG and WAM are on the Journal of Asian Pacific Society of Cardiology editorial board; this did not influence peer review. All other authors have no conflicts of interest to disclose.
Received: 4 February 2022 Accepted: 7 April 2022 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e19. DOI: https://doi.org/10.15420/japsc.2022.04
Correspondence: Laszlo Göbölös, Department of Cardiac Surgery, Heart and Vascular Institute, Cleveland Clinic Abu Dhabi, Sowwah Square, Al Maryah Island, Abu Dhabi, United Arab Emirates. E: gobolol@clevelandclinicabudhabi.ae
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), arising in Wuhan in autumn 2019, has severely affected both economies and health worldwide over the past 2 years. Although most infected individuals are asymptomatic, 10–15% of cases foster critical conditions featured by pneumonitis or multiple organ failure.1 The published mortality rate for critically ill patients requiring mechanical ventilation is persistently high, and the recuperation period for these patients is prolonged.2 The scale of long-term restoration of integrity is not known in every respect yet. However, clinical data reveal that unimpaired recovery is often not obtainable, particularly in individuals with associated viral myocarditis or multiorgan involvement.3 Numerous diverse therapeutic interventions have been proposed for viral pneumonitis with some degree of success, but a specific treatment has not yet been established. Ongoing vaccination programs limit disease spread and the extent of disease severity in affected patients.4 This review analyses the role of COVID-19 and the pathomechanisms underlying its short- and long-term cardiac effects.
Search Strategy and Selection Criteria
Appropriate data were identified by searching the PubMed database, as well as reference lists in relevant articles using the search terms ‘SARS’,
‘COVID-19’, ‘renin–angiotensin system’, ‘phagocyte’, ‘reactive free radical’, ‘antioxidant’, ‘ARDS’, ‘thrombosis’, ‘acute myocardial’, ‘ischaemia’, ‘reperfusion’, ‘microvascular’, ‘ACE2’, ‘heart failure’, ‘haemodynamics’, ‘cardiac markers’, ‘myocarditis’, ‘cardiomyopathy’, ‘transplantation’, ‘ECMO’, ‘ventricular assist device’, ‘vaccine’ and ‘medical therapy’. Abstracts and meeting reports were not included in the present study. Only articles published in English between 1976 and 2021 were reviewed.
This paper is a scoping review; therefore, a detailed risk of bias assessment was not conducted. The data were abstracted by investigator one (LG) and verified by the second investigator (YM); discrepancies were resolved by discussion. Final inclusion was determined by the senior investigator (EMT).
COVID-19 Development and Angiotensin-converting Enzyme 2
Angiotensin-converting enzyme (ACE) 2 is a transmembrane peptidase. It is the receptor for the Severe Acute Respiratory Syndrome (SARS) coronavirus that caused epidemics in Guangdong, China, and Canada in 2003.5 ACE2 is expressed in numerous organs, including the intestinal
© RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com General Cardiology REVIEW
epithelium, heart, central nervous system and kidneys, with high density in the microvascular endothelium. SARS-CoV-2 targets the ACE2 isoform located on the surface of Type I and II alveolar pneumocytes during initial respiratory infection.6 In addition, ACE2 expression is observed on alveolar phagocytes and lymphocytes, having a pivotal role in the leucocyte activation process.7
ACE2 splits angiotensin I (ATI), into angiotensin (1–9), thereby inhibiting the renin–angiotensin system (RAS). In addition, ACE2 transforms angiotensin II (ATII) into angiotensin (1–7), with angiotensin (1–7) activating the MAS-related G-protein-coupled receptor (MAS R), resulting in an antiinflammatory effect and vasodilation.8–11 The coronavirus connects via its spike protein (S-protein) to ACE2 outside the enzyme’s catalytic domain, triggering virus internalisation into the cell expressing the receptor and viral replication.8,9,12
In SARS and Middle East Respiratory Syndrome, mortality ensued not from a direct cytopathic effect of the coronavirus, but rather acute respiratory distress syndrome (ARDS), a culmination of maladaptive and excess immune responses of the host, generated by RAS dysfunction leading to reduced ACE2 activity.13,14 As a result of the RAS imbalance (ATI-angiotensin 1–7), the elevated ATII level triggers a pathologic inflammatory response by the angiotensin II type 1 receptor (AT1R). Activation of AT1R initiates the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) signalling pathway, resulting in a surge in tumour necrosis factor-α (TNF-α) and interleukin IL-1 and IL-6, activating the circulating neutrophil (PMN) cells and monocytes/macrophages (MPH). This activation causes further adhesion and migration of phagocytes by facilitating the expression of Vascular cell adhesion molecule-1, monocyte chemoattractant protein-1 and intercellular adhesion molecule-1.15–19 The vascular marginalisation, endothelial rolling, diapedesis and tissue migration of phagocytes leads to an oxidative burst (i.e. exaggerated production of free radicals/reactive oxygen species; Figures 1, 2).20,21 The expression of novel viral proteins (e.g. ORF3b, ORF6, nucleoporin Nsp1, papain-like protease) intensely influences the innate immunity on numerous targets. Hindered production of critical antiviral interferons has been demonstrated in human cell cultures and murine models, and amplified synthesis of cytokines (IL-6, IL-8) regulated by NFκB signalling has been reported.16 The latter may play a critical role in the pathogenesis of ARDS.22–24
Phagocyte Actions in Host Tissue Damage
Circulating PMNs use reactive oxygen species to defend the body against invading germs and foreign particles.25 However, these defensive pathways can also pose a risk for the host; in the case of an imbalanced enzymatic or oxidative response to a harmful substance, there may be damage caused to host tissues.26 After leaving the bone marrow, PMNs travel to the capillaries and then reach post-capillary high endothelial venules (HEV); diapedesis starts through the intercellular junctions. Over a 5- to 6-day life cycle, the PMNs migrate through the tissues and eradicate all external threats via degranulation.27 In the case of PMN activation by any trigger, including the AT1R pathway, NADPH oxidase initiates the oxidative burst by creating highly reactive superoxide radicals from molecular oxygen, which are then converted by superoxide dismutase into hydrogen peroxide, followed by the creation of hydroxyl ions, potent free radicals, via the Fenton reaction. Another by-product of the cascade is the hypochlorite radical, formed by the conversion of hydrogen peroxide into hypochlorite ion by myeloperoxidase (MPO).28 The free radicals destroy cell membranes and DNA, resulting in activation of poly(ADP) ribose and the exhaustion of intracellular ATP stores. In addition, the free radical burst results in an increase in intracellular ionised iron and calcium concentrations, causing the destruction of many biomolecules (Figure 1).20
Free radicals also affect cellular phospholipid metabolism, initially in the endothelium, the first point of contact of PMNs with tissues, resulting in the enhanced synthesis of lipid mediators, specifically leukotriene B4 (LTB4) and the platelet-activating factor (PAF), both of which are adept at promoting the further chemotaxis of phagocytes. In addition, intracellular xanthine oxidase generates additional superoxide radicals from xanthine, and the superoxide anions activate phospholipase A2, resulting in a notable change in arachidonic acid metabolism that leads to considerable changes in leukotriene and prostaglandin (PG) synthesis. The hypochlorite anion forms a protein–amino–hypochlorite after reacting with the free amino terminals of proteins. The protein–amino–hypochlorite inactivates nitric oxide (NO), a potent PMN inhibitor. In addition, the hypochlorite activates the proteases released from the azurophilic granules of PMNs.20,28 The decreases in NO lead to vasoconstriction and microvascular blood stasis, initiating microthrombosis.20
PMNs and MPHs share a common dependent pathway, and the IL-8 produced facilitates the evolution of immature neutrophils into functionally active granulocytes. MPHs cells behave in a similar manner to PMNs during their maturation, diapedesis and migration to the tissues; they also phagocytose foreign particles and release soluble factors.27,28 Autocrine, paracrine and endocrine mechanisms coordinate the regulation of the phagocyte system. The primary regulators are the cytokines, including IL-1, IL-6 and interferon-γ. The expression of histamine and catecholamine receptors on the phagocytes reveals a further neuroendocrine regulatory pathway of this defensive system. In contrast, glucocorticoids effectively suppress both the destructive biosynthetic processes and phagocytosis.20,28 Disproportionally high phagocyte activation in patients with severe COVID-19 results in ‘hitand-run’-type damage primarily in the heart and lungs, making therapeutic outcomes less favourable.20
The typical sites of PMN/MPH extravasation are in the post-capillary HEV. The local vascular smooth muscle cells secrete NO, IL-1 and IL-6, thereby activating the HEV. In addition, the expression of PAF and P-selectin on the endothelial cellular surface intensifies within minutes of HEV activation.20 After the PMNs adhere to the endothelial cellular surface, the endothelium secretes IL-8 and expresses E-selectin after some hours to days; meanwhile, phosphorylation of lymphocyte function-associated antigen (LFA)-1 leads to G-actin polymerisation and the production of F-actin required for phagocyte diapedesis.20,29,30 Experimental polysaccharide blockade of selectins limited myocardial reperfusion injury, revealing the destructive nature of phagocyte overstimulation in the involved tissues.20,31
P-Selectin molecules are excessively expressed in diseased organs, but not in ‘resting’ endothelium, and play a role in the homing and migration of phagocytes.29 Furthermore, activated granulocytes may damage the endothelial cellular surface, triggering the coagulation cascade.32–35 In addition, the free radicals have a prothrombotic effect, promoting platelet aggregation by modifying prostacyclin synthase function and inhibiting antithrombin III.36,37 Thrombin is an effective P-selectin activator, so a vicious cycle of phagocyte attraction, endothelial damage, local coagulation and PAF-triggered coagulation on the HEV endothelial cellular surface may explain the predisposition to microthrombosis described in diseaseaffected organs in severe COVID-19 infections (Figure 2).38,39
Coronavirus Infection and Established Ischaemic Heart Disease
The effects of phagocytes and free radicals on ischaemic heart disease, especially reperfusion injury and arrhythmias, is well-established. However,
Cardiac Effects of the SARS-CoV-2 Infection JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
the impact of SARS-CoV-2 infection on the heart is not yet entirely known, although there are reports that SARS-CoV-2 infection can lead to myocarditis and may exacerbate pre-existing ischaemic heart disease.40,41 As discussed in the previous section, virus-activated leucocytes can cause direct tissue damage or lead to end-organ microthrombosis, a critical phenomenon in the acute manifestation of chronic, stable ischaemic myocardial disease.38,39
Both experimental and clinical investigations have revealed significant effects of free radicals and phagocyte activation in unstable angina. P-Selectin expression is increased in atherectomy specimens from patients with unstable angina, predominantly in the endothelium. In addition, the expression of cellular adhesion molecules on circulating phagocytes and soluble P-selectin levels are markedly elevated in unstable angina, and increased expression of the adhesion molecule MAC-1 was detected on phagocytes in blood samples collected from the coronary sinus.30 In a canine model, histological samples of reperfused ischaemic heart muscle in the initial hour of reperfusion contained CD64positive monocytes in the HEV and perivascular connective tissue.42 In addition, the phagocyte count in the extracellular fluid of the canine heart was elevated from 1 to 4 hours after successful reperfusion.42 Initially, the PMN proportion was dominant, but this shifted to a predominance of MPH by 4 hours.42 The initial chemotactic activity results from C5a then shifts to TGF-β1, which is coordinated by the monocyte chemoattractant protein (MCP-1).43 These pathways are crucial in repairing ischaemic damage; however, if they become dysregulated, they can lead to further myocardial injury, which could be the case in fulminant SARS-CoV-2 infections. The hypertensive excess resulting from viral ACE2 blockade may also trigger acute angina or acute heart failure (HF) in some individuals with established coronary disease (Figure 2).
Emerging data from the pandemic indicates that an over-reactive immune response may be seen in severe or fatal outcomes.23,24 The phagocytes first attack the microvasculature binding to the HEV and then cause destruction in the affected tissue, not only at a cellular level but also in the intercellular space. The initial 4- to 6-day ‘honeymoon period’ in mildly symptomatic SARS-CoV-2 infection and the sudden escalation in respiratory compromise parallels the tissue lifecycle of phagocytes. If the activation of these cells is limited because of a lower viral load, for example, or the host immune reaction/RAS response remains within the physiological range, a smooth recovery may be more likely. In progressive infections, microvascular thrombosis is the first event of the process (Figure 2).20 Procoagulant activity has been described in SARSCoV-2-infected lung tissue samples containing diffuse microthrombi.44 Therefore, early anticoagulation is essential in patients with elevated coagulation markers; a relatively increased cumulative incidence of venous thromboembolism was reported in the absence of typical acute disseminated intravascular coagulopathy in COVID-19 pneumonia, even when patients were administered pharmacological thromboprophylaxis.45,46 Furthermore, heparin binds to the viral S-proteins and downregulates IL6, supporting its early administration during the course of infection. Based on these data, numerous proactive approaches using early therapeutic anticoagulation or intensified pharmacological prophylaxis have been suggested.20,47 However, more data and prospective trials are needed to establish the role of anticoagulation in mitigating the coagulopathy associated with the COVID-19 pandemic.
There are reports that even minor plaques can result in adverse coronary events, mainly in combination with the prothrombotic status caused by the coronavirus disease.41 Aggressive antithrombotic therapy and/or
2
azurophilic granules; H2O
·OH
molecule; H2O2 =
peroxide;
= myeloperoxidase;
hypochlorite
polymorphonuclear
prompt coronary revascularisation may provide a satisfactory solution; in case of failed therapeutic attempts, mechanical circulatory support is the bailout.48 It has been suggested that the timing of surgical interventions, including cardiac surgery, be postponed by 7 weeks after the initial SARSCoV-2 infection due to the increased postoperative morbidity and mortality of patients with active COVID-19 disease at the time of surgery, although, of course, this decision must be balanced by the urgency of the condition requiring surgical intervention.49 Several risk factors associated with adverse outcomes with COVID-19 are potentially modifiable. Primary and secondary prevention strategies that target cardiovascular risk factors may improve outcomes for people with COVID-19.50 Therefore, maintaining follow-up for chronic heart disease is essential in protecting the population.
Despite the virus-derived leucocyte activation and risk of exacerbation of pre-existing ischaemic heart disease, the number of cardiac emergencies decreased in numerous countries during COVID-19 lockdown periods. As a consequence of curfews, many patients with chronic coronary disease stayed home and experienced lower stress levels than usual, or did not exercise at all, resulting in reduced provocative effects on their existing coronary burden. This observation is reinforced by a large-scale database analysis from France, in which a progressive reduction in ambulatory blood pressure values was registered compared with the pre-lockdown era.51 In addition, there may have been a fear component involved in the reduction in cardiac emergencies, whereby people did not seek medical attention for complaints relating to cardiac symptoms.52 However, patients experiencing ST-elevation MI who were admitted during the first wave of the COVID-19 pandemic experienced longer total ischaemic times, resulting in a more severe disease status upon hospital admission and a higher rate of in-hospital adverse events compared with a comparable pre-COVID-19 period.40,41,52 These adverse outcomes resulted from multiple confounding factors, including viral infection triggering additional ischaemia, healthcare systems that were overwhelmed by the pandemic and a delay in procedures following admission because of the need for precautionary infection control measures.
Myocarditis
of patients hospitalised with COVID-19 experience additional
involvement, with a smaller proportion being caused by direct
Cardiac Effects of the SARS-CoV-2 Infection JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
One-quarter
myocardial
Figure 1: Neutrophil Granulocyte Activation and Free Radical Cascade Lactoferrin–Fe3+ Fe3+ Fe2+ Fe3+ •OH NADPH oxidase O2 O2 SOD H2O2 Cl H2O HOCl Proteases Neutrophil granulocyte SG AG Tissue damage PMN MPOFenton reaction AG =
= water
hydrogen
HOCl =
ion; O
= oxygen free radical;
= hydroxyl radical; MPO
PMN =
leucocyte; SG = specific granules; SOD = superoxide dismutase.
Angiotensinogen
Blue arrows indicate a facilitating effect, whereas red arrows indicate inhibition.
= angiotensin-converting enzyme; AT = angiotensin; AT1R = angiotensin II type 1 receptor; AT2R = angiotensin II type 2 receptor; COX = cyclo-oxygenase; MAS R = MAS-related G-protein-coupled
NFκB = nuclear factor-κB; PAF = platelet-activating
= reactive oxygen species; SARS-CoV-2 = severe acute respiratory syndrome coronavirus-2.
cytopathic, myocarditic changes. The possibility of autoimmune reactions in this process has been raised.53 There are insufficient data as to whether successful acute treatment of the infection affects long-term myocardial recovery and cardiac pump function. Some patients experience subclinical cardiovascular abnormalities, and even those with recovered heart function may remain at risk of delayed cardiomyopathy and cardiac arrhythmias (Figure 2). The most appropriate long-term follow-up cardiac testing, also taking into account cost-effectiveness, for post-COVID-19 myocardial dysfunction and arrhythmias must be established. Standard ECG and echocardiogram 1 and 6 months after recovery, similar to cardiosurgical postoperative follow-up, may be reasonable. However, even these tests may not reveal subtle clinical abnormalities, and advanced imaging, including magnetic resonance imaging with gadolinium enhancement or an echocardiographic strain test, may be required in selected cases when initial testing reveals abnormalities, or as clinically indicated.54,55
Takotsubo syndrome is caused by a catecholamine surge, which may occur in COVID-19 disease due to the cytokine storm. Despite a lower prevalence of cardiac comorbidities in COVID-19-infected patients with takotsubo syndrome, direct myocardial injury, stress and inflammation may result in takotsubo syndrome with a high complication rate, although most patients recover successfully; in addition, this patient population is predominantly composed of elderly females.56
Cardiac Arrhythmias
Cardiovascular involvement in COVID-19 may result in arrhythmias. Offerhaus et al. analysed 5,782 hospitalised COVID-19 patients, finding that 11.0% experienced AF during hospitalisation and 1.6% experienced
atrial flutter (AFL).57 Ventricular arrhythmias were observed in <0.8% of patients, and a conduction disorder was found in 6.3%. New-onset AF/AFL was associated with increased in-hospital mortality, mainly in males aged 60–72 years.57 In another analysis, the left atrial volume index and left ventricular mass index were significantly increased in patients with cardiac injury who had COVID-19 pneumonia, with an AF occurrence of 40.0%. These patients required invasive or non-invasive mechanical ventilation and had a higher mortality rate. Arrhythmias are multifactorial and complex in this patient population and result from hypoxia, acidosis, metabolic derangements and neurohormonal and catecholaminergic stress. Pathophysiological mechanisms of arrhythmogenesis include spontaneous release of calcium from the sarcoplasmic reticulum, autonomic nervous system-induced calcium entry into cardiac myocytes and possible direct atrial injury resulting from coronary artery disease accompanied by small vessel thrombosis.58
Ventricular arrhythmias affect a modest proportion of SARS-CoV-2infected patients, although occasionally represent the only initial symptom. QT prolongation inconsistently predicts ventricular arrhythmias; in addition, hydroxychloroquine is seldom associated with malignant arrhythmias (<1%). ST-T wave changes are not clearly related to ventricular arrhythmias; nevertheless, elevated systemic inflammatory and cardiac injury markers are significantly higher in individuals presenting with lifethreatening arrhythmias.59
There is a correlation between COVID-19 and new-onset atrioventricular (AV) blocks, but direct causality cannot be established due to various confounding factors. New-onset AV block is often benign and likely does not affect immediate and long-term clinical outcomes; the decision to
Cardiac Effects of the SARS-CoV-2 Infection JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Figure 2: Cardiovascular Effects of SARS-CoV-2 Infection
Direct cytopathic
e ect
(myocarditis,
pneumonitis) Heart failure ACE2 ACE2 Proinflammatory response Platelet activation Phagocyte activation Acute coronary syndrome SARS-CoV-2 NADPH oxidase NKĸB pathway Degranulation AT (1–7)AT (1–9) AT1R AT2R MAS R Proteases ROS Primary tissue damage Secondary tissue damage (thrombosis)
Angiotensin I Angiotensin II Endothelium Cathepsin kinases PAF COX ACE
ACE
receptor;
factor; ROS
undertake appropriate rhythm follow-up should be considered on a caseby-case basis.60 ECG features of SARS-CoV-2 are not characteristic, especially during the initial disease phase. ECG changes are usually late onset and do not occur in parallel with pulmonary abnormalities. Echocardiography may be a useful tool to distinguish between COVID-19related myocardial damage and primary cardiac disease, and to monitor and assess COVID-19-related cardiovascular complications.61
Heart Failure
HF is a common condition that may develop at different stages during the course of a SARS-CoV-2 infection; severe inflammatory reaction exaggerates pre-existing myocardial disease.62 In combination with the higher metabolic demand due to sepsis, this can cause myocardial depression and either new-onset HF or acute decompensation of chronic HF. Another contributor to myocardial depression could be the coagulation dysfunction induced by sepsis.62 Elevated natriuretic peptides suggest HF with a worse prognosis in COVID-19 and warrant at least an echocardiogram to further assess cardiac function.63 Cardiac markers, such as troponin and creatine kinase-MB, increase significantly more in patients with cardiovascular risk factors and are associated with notably higher morbidity and mortality.40,50 Table 1 presents the prevalence of myocardial injury, and its prognostic value, in several studies in hospitalised COVID-19 patients.64–73
In the initial months following the emergence of the COVID-19 pandemic, several repurposed medications, such as hydroxychloroquine, antimicrobials and antivirals, were touted as potential treatments for the SARS-CoV-2 infection. This raised concerns regarding pharmacodynamic and pharmacokinetic drug-drug interactions, mainly involving concomitant QTc-prolonging agents and cytochrome P450 inhibitors.51,74 This is particularly relevant in patients with existing HF or after transplantation, given that these patients are likely to be on medication with the potential for significant interactions. As more data emerged that showed such treatments to be ineffective, the use of these repurposed agents and therefore the aforementioned concerns have subsided. However, QTc prolongation continues to be an essential aspect of drug monitoring for all hospitalised patients and should not be ignored despite the decline in the use of ‘red flag’ medications such as hydroxychloroquine, lopinavir/ritonavir and azithromycin in this particular setting. Evaluating the baseline risk of QTc prolongation for all hospitalised SARS-CoV-2 patients using the Tisdale risk score could offer insights into an individual patient’s risk of this complication and the subsequent risk of torsade de pointes.75
The emergence of the SARS-CoV-2 also led to early speculation regarding the chronic implications of certain cardiovascular medications, including ACE inhibitors, in relation to the infection. These medications are the backbone of guideline-directed medical therapy for HF. Patients with chronically upregulated ACE2 receptors, such as those on ACE inhibitors, have been shown not to have an increased risk of viral uptake. Cardiovascular societies issued statements early on during the pandemic to correct this perception of increased risk and avoid having patients stop these medications when they would otherwise benefit from evidencebased therapies. High-quality evidence later emerged from clinical trials, including REPLACE COVID and BRACE CORONA, providing assurance that these medications are safe and should not be suspended in patients hospitalised with mild or moderate COVID-19, which is consistent with the recommendations of international societies.76,77 Nonetheless, the practice of withholding RAS inhibitors in patients who develop systemic hyperinflammation and progress to vasoplegic shock should continue to
Table 1: Prevalence of Myocardial Injury and Its Prognostic Value Among Hospitalised COVID-19 Patients
Study n Pre-existing
Cardiac
ICU
Mortality
Stefanini et al.64 397 8.4 32.7 23.2
Raad et al.65 1,020 12.1 38.2 50.2 17.6
Barman et al.66 607 19.1 24.7 32.1 17
Majure et al.67 6,247 13.3 29.1 30.2 22.4
Zhou et al.68 191 8 17 26 28
Yang et al.69 52 10 23 100 38
Arentz et al.70 21 42.9 33.3 100 52.4
Wang et al.71 138 14.5 7.2 26 Huang et al.72 41 15 12 31 15 Buckner et al.73 105 38 19 (13/67) 32 33
CVD = cardiovascular disease; ICU = intensive care unit.
be implemented in the setting of severe COVID-19, similar to other scenarios in critically ill patients.
If the developing cardiogenic shock necessitates advanced therapy, this means venoarterial (VA) extracorporeal membrane oxygenation (ECMO) rather than venovenous (VV) extracorporeal ECMO in COVID-19 respiratory distress. It was recently reported that in case of an escalation of therapy from VV- to VA-ECMO, a relatively low-flow VA perfusion was required, highlighting the efficacy of such mild circulatory support without inducing left ventricular distension in patients with COVID-19-related cardiopulmonary shock.78 The timing of care escalation is crucial to achieving improved outcomes. COVID-19 patients on mechanical ventilation with increased positive end-expiratory pressure (PEEP) may have compromised right ventricular filling, resulting in extra fluid resuscitation or pressor support. Therefore, lung-protective ventilation is recommended with an early escalation strategy. In the literature, high preECMO PEEP (>15 cmH20) on ventilator and low respiratory system compliance (<30 ml/cmH20) are independent predictors of mortality, and delaying tracheostomy is an additional risk factor for death. The inflammatory burst and concomitant procoagulative status may significantly influence outcome, an elevated pre-ECMO C-reactive protein level is an additional risk factor for mortality and the required ECMO flow is higher in non-survivors than survivors.79 In severe SARS-CoV-2 respiratory failure, a long period of invasive ventilation and extracorporeal support is often required. There is a non-negligible burden of thromboembolic disease and renal injury associated with ECMO treatment. A large proportion of patients need tracheostomy and steroids to facilitate the challenging weaning process.80 Therefore, a thorough risk–benefit analysis of escalated therapy for each candidate is suggested so that patients with an increased probability of survival can benefit from scarce regional resources during the pandemic.81
One report compared the 6-month mortality of patients receiving VVECMO support for COVID-19 with a historical viral ARDS cohort.82 Patient characteristics, ECMO parameters, complications and mortality were compared between the two cohorts. At 6 months, survival was significantly higher in the COVID-19 than in non-COVID-19 viral pneumonia group. Patients with COVID-19 who survived to decannulation had a higher Murray score, decreased burden of organ dysfunction, higher incidence
Cardiac Effects of the SARS-CoV-2 Infection JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
CVD (%)
injury (%)
admission (%)
(%)
of pulmonary embolism and longer ECMO runs than those who did not. However, survival in patients on ECMO for COVID-19 was similar to those treated for non-COVID-19 viral ARDS.82
Cellular immunity is altered in long-term ventricular assist device (VAD) recipients.83 However, there is no evidence that these patients have a higher risk of acquiring SARS-CoV-2 infection. Although cardiac output is maintained at a constant level by the VAD, optimal preload and afterload are essential to keep actual output steady, even in the context of a systemic infection. If haemodynamics are compromised, various VADassociated complications may occur, including low-flow and suction events, concomitant pump thrombosis or right ventricular failure. Studies have reported similar complications and risk profiles for COVID-19infected VAD recipients and patients receiving ECMO.84
In heart transplant patients, emerging infections are always a concern given life-long immunosuppression to prevent rejection and preserve graft function. Although immunosuppression elevates susceptibility to viral infections such as herpes or cytomegalovirus, acute respiratory viral infections are uncommon. SARS-CoV-2-infected heart transplanted patients share similar demographic and clinical features with the general population regarding male predominance, comorbidities and clinical presentation. Whether heart transplanted patients are more prone to acquiring COVID-19 remains unclear. Furthermore, most of these patients presented with absolute lymphopenia, a common finding in SARS-CoV-2 infection, and this is independently associated with higher morbidity and mortality. However, myelotoxicity related to the immunosuppressive treatment in transplant recipients may also result in a similar picture. Modification of the baseline immunosuppressive regimen is diverse in the literature. The American Society of Transplantation suggests reducing immunosuppressive therapy with the maintenance of corticosteroids in SARS-CoV-2-infected patients who have not had recent rejection episodes, especially in severe COVID-19 cases.85,86 The International Society for Heart and Lung Transplantation (ISHLT) has similar recommendations for moderate to severe COVID-19.87 Regarding patients recovering from COVID-19 on the waiting list, it is essential to obtain two negative polymerase chain reaction tests 2 weeks after the initial SARSCoV-2 diagnosis in order to proceed with transplantation depending on the acuity of the disease and organ availability. The ISHLT recommendations support testing for the SARS-CoV-2 in donors, if available, and donors should be rejected in case of positive testing and imaging signs of viral pneumonitis.87 Moreover, due to the possibility of nosocomial crossinfections, a second test for donors who previously tested negative should be considered before organ harvesting.62
A similar trend was observed among HIV-positive patients; the absolute cumulative COVID-19 mortality was low, at <0.1%, reflecting the young age profile of this population. Conversely, patients with comorbidities show an increased death rate compared with the general population. The association between HIV and COVID-19 mortality is more pronounced among people of African origin, with a 4.3-fold higher risk of COVID-19 death in this group. Nevertheless, this group is also at increased risk of adverse HIV outcomes, including virological rebound.88
Vaccination and the Heart
Vaccines against SARS-CoV-2 infection are effective within clinical trials of immunocompetent people, significantly reducing severe disease probability, hospitalisation and mortality. Most vaccines use viral mRNA to replicate the S-protein subunits of SARS-CoV-2 to hinder viral entry and stimulate both cellular and humoral immune responses. Nevertheless,
some studies have demonstrated low or nearly no response to vaccination in heart transplant recipients, although immune paresis, a weaker cellular and humoral response than expected to an antigenic stimulus in heart transplant hosts is not unique to the COVID-19 vaccine in single-organtransplanted recipients; hence, lower rates of immune response have been reported with other vaccines in this vulnerable cohort.89
Myocarditis is a rare complication of COVID-19 mRNA vaccinations, commonly occurring in young adult men. The incidence of myocarditis measures 12.6 cases per million second-dose mRNA vaccinations in those aged 12–39 years. Patients with post-vaccination myocarditis invariably present with chest pain accompanied by elevated cardiac troponin levels, usually 2–3 days after the second vaccination dose. ECG abnormalities with ST elevation are common; cardiac MRI is suggestive of myocarditis in all affected patients. In some cases, autoantibody titres against certain self-antigens and levels of natural killer cells are increased. The mechanisms underlying the development of myocarditis are unclear; triggered pre-existing dysregulated immune pathways in certain individuals, a hyperactive immune response to mRNA and dysregulated cytokine flair-up have been assumed. The reason for male predominance is also unknown; a possible explanation may rely on sex hormoneinfluenced differences in immune response, and an underdiagnosis of cardiac disease in women may also contribute. Almost all patients show resolution of signs and symptoms, as well as improvements in diagnostic markers and imaging studies, with or without treatment.90
Cerebral microthrombotic activity has been reported at a very low incidence in freshly vaccinated people, mainly on the venous side. The thrombotic tendency results predominantly from the phagocyte activation cascade and free radical discharge. In strong responders on the RAS–phagocyte activation pathway, the S-protein trigger itself may ignite the microthrombotic tendency. Therefore, additional low-dose postvaccination acetylsalicylic acid therapy for 1 week could be beneficial in avoiding this complication, and the risk of this brief therapy is negligeable.20
Innate immunity triggers responsible for infection limitation or a hyperinflammatory response in SARS-CoV-2 infection are primarily uncharted territories. The SARS-CoV-2 S-protein primes inflammasome formation and the release of mature IL-1β from macrophages in COVID-19 patients, but not from macrophages in healthy SARS-CoV-2-naïve individuals. S-protein-driven inflammasome activation in macrophages from convalescent COVID-19 patients with distinct epigenetic and gene expression signatures is suggestive of innate immune memory after recovery from the SARS-CoV-2 infection. S-protein-driven IL-1β secretion from macrophages demands non-specific monocyte preactivation in vivo to trigger NOD-, LRR-, and pyrin domain-containing protein (NLRP3)inflammasome signalling. The NLRP3 inflammasome is a multiprotein complex required for the secretion of the proinflammatory cytokine IL-1β that is crucial in COVID-19 hyperinflammatory syndromes. The SARSCoV-2 S-protein, even as a vaccine antigen, triggers NLRP3 inflammasome activation and cytokine secretion in COVID-19 patient-derived macrophages. SARS-CoV-2 infection results in the reprogramming of human macrophages that enables rapid inflammasome assembly. Profound changes in macrophage gene activation and expression for several weeks to months after infection in COVID-19 patients may facilitate a better understanding of post-COVID-19 inflammatory syndromes.91
Supportive Therapy
Early introduction of an angiotensin receptor blocker to therapy, as long the patient’s blood pressure asymptomatically allows (≤110 mmHg), may
Cardiac Effects of the SARS-CoV-2 Infection JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
result in favourable outcomes by weakening the cytokine burst.92–94 Microthrombotic activity on a capillary level requires low-molecularweight heparin prophylaxis even in the early phase of the disease.95 Additional antiplatelet therapy must be considered because the thrombotic activity is caused by the phagocyte activation cascade and free radical burst.32,33
Remdesivir was developed for Ebola virus infections and is a monophosphoramidate nucleoside prodrug that can easily penetrate the cell membrane. Upon entering target cells, remdesivir monophosphate is rapidly converted into its active triphosphate, and, in RNA viruses, it acts as a substrate for the viral replicase, whereby it competes with endogenous ATP for incorporation in elongating RNA strands. After its incorporation, remdesivir triphosphate causes synthesis arrest by inducing delayed chain termination.96 Thus, remdesivir improves recovery from SAR-CoV-2 infection in hospitalised patients and reduces morbidity, mortality and time to clinical improvement. Furthermore, in patients who do not require mechanical ventilation or ECMO, a 5-day course of remdesivir may provide similar outcomes and fewer side effects than double-length treatment.97
Molnupiravir is another antiviral agent that has received emergency authorisation from the US Food and Drug Administration (FDA) by a marginal vote in its favour. The initial Phase III trial showed that molnupiravir reduced hospital admissions by 50%, but the data presented to the FDA revealed only a 30% admission rate reduction. Molnupiravir triggers an accumulation of errors in the viral genome during the replication phase, and this raised a concern regarding the possibility of human genome alteration. However, animal studies revealed a low risk for adults at therapeutic doses.98
Another antiviral agent, PF-07321332, a 3-chymotrypsin like protease (3CLpro) inhibitor targeting the main SARS-CoV-2 protease essential in viral replication, is currently in phase III trials in combination therapy with ritonavir. The scheduled interim analysis in the trial demonstrated an 89% reduction in the risk of COVID-19-related hospitalisation or death from any cause compared with placebo-treated study subjects within 3 days of symptom onset.99
A combination of the monoclonal antibodies imdevimab and casirivimab is also a promising treatment strategy for SARS-CoV-2. This combination drug has effectively reduced both the number of medical visits and the viral load in COVID-19 patients, and shows in vitro activity against current concerning variants of the virus. The main limitation of this therapy at the moment is primarily financial.100
A further target in the fight against COVID-19 is in microvascular phase, by limiting the tissue damage caused by activated leucocytes. However, this therapy is not microbe specific and effective in any vasculitis accelerated on the phagocyte pathway. Vioprolide A is a natural product isolated from the myxobacterium Cystobacter violaceus. It is a highly potent substance, showing promising effects on the vascular endothelium in inflammatory processes by reducing phagocyte–endothelial cell interactions and targeting the importin family of carrier proteins without exerting cytotoxic effects. The inhibition of importindependent NFκB p65 nuclear translocation is crucial in the action of vioprolide A, resulting in reduced NFκB promoter activity and inflammatory gene expression.101
Limitations
This paper is a scoping review, providing an overview of the existing evidence in respect of adverse cardiac effects and relevant pathomechanisms involved in SARS-CoV-2 infection, regardless of the methodological quality of the included articles. In addition, we did not conduct a risk of bias analysis on the articles included. With ever-growing evidence regarding the COVID-19 pandemic, it remains challenging to provide a comprehensive and final overview relevant to daily cardiovascular clinical practice.
Conclusion
The SARS-CoV-2 pandemic represents a critical challenge to society worldwide over an extended period. Emerging experience reveals the microvascular target and multiorgan involvement of the infection, in contrast with the initial assumption that the respiratory system was the only target. Excessive activation of the RAS leading to an uncontrolled surge of the phagocyte system is the main driver of adverse outcomes, including short- and long-term cardiovascular complications. Furthermore, it has been established that complete restitution of integrity does not always commence after going through a SARS-CoV-2 infection, and that post-COVID-19 syndromes emerge in several organs, resulting in new or aggravating existing disease burdens.
Clinical Perspective
• Uncontrolled overstimulation of the phagocyte system results in microvascular disease in SARS-CoV-2 infection that may lead to unfavourable clinical consequences, including acute and chronic cardiac syndromes.
• Patients with STEMI hospitalised during the COVID-19 pandemic experienced longer total ischaemic time, resulting in a more severe disease status upon hospital admission and a higher rate of in-hospital adverse events compared with a parallel period. These adverse outcomes result from various confounding factors, including additional ischaemia triggered by the viral infection, overwhelmed healthcare systems during the pandemic and a delay in procedures because of precautionary infection control measurements on admission.
• HF may develop at different stages during the course of a SARS-CoV-2 infection; the severe inflammatory reaction can exaggerate pre-existing myocardial disease. Therefore, regular follow-up on established HF patients, even in the form of remote videographic consultations, is essential during the pandemic.
• If developing cardiogenic shock necessitates advanced therapy, this means VA-ECMO in contrast to VV-ECMO in COVID-19 respiratory distress. In case of treatment escalation from VV- to VA-ECMO, a relatively low-flow VA perfusion is required, highlighting the efficacy of such mild circulatory support without inducing left ventricular distension in patients with COVID-19related cardiopulmonary shock. Therefore, the timing of care escalation is crucial to achieving improved outcomes.
• Myocarditis is a rare complication of COVID-19 mRNA vaccinations, commonly occurring among young adult men, with an incidence of 12.6 cases per million second-dose mRNA vaccinations in those aged 12–39 years. Therefore, it is crucial to thoroughly investigate post-vaccination atypical chest discomfort in this at-risk population.
Cardiac Effects of the SARS-CoV-2 Infection JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
1. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417–8. https://doi.org/10.1016/S01406736(20)30937-5; PMID: 32325026.
2. Zareifopoulos N, Lagadinou M, Karela A, et al. Intubation and mechanical ventilation of patients with COVID-19: what should we tell them? Monaldi Arch Chest Dis 2020;90. https:// doi.org/10.4081/monaldi.2020.1296; PMID: 32268719.
3. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:811–8. https://doi. org/10.1001/jamacardio.2020.1017; PMID: 32219356.
4. Ayoub HH, Chemaitelly H, Makhoul M, et al. Epidemiological impact of prioritising SARS-CoV-2 vaccination by antibody status: mathematical modelling analyses. BMJ Innov 2021;7:327–36. https://doi.org/10.1136/ bmjinnov-2021-000677; PMID: 34192020.
5. Chan-Yeung M, Xu RH. SARS: epidemiology. Respirology 2003;8(Suppl):S9–14. https://doi.org/10.1046/j.14401843.2003.00518.x; PMID: 15018127.
6. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005;11:875–9. https://doi.org/10.1038/ nm1267; PMID: 16007097.
7. Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–7. https://doi. org/10.1002/path.1570; PMID: 15141377.
8. Kuba K, Imai Y, Ohto-Nakanishi T, Penninger JM. Trilogy of ACE2: a peptidase in the renin–angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther 2010;128:119–28. https://doi.org/10.1016/j. pharmthera.2010.06.003; PMID: 20599443.
9. Kuba K, Imai Y, Rao S, et al. Lessons from SARS: control of acute lung failure by the SARS receptor ACE2. J Mol Med (Berl) 2006;84:814–20. https://doi.org/10.1007/s00109-0060094-9; PMID: 16988814.
10. Iwai M, Horiuchi M. Devil and angel in the renin–angiotensin system: ACE–angiotensin II–AT1 receptor axis vs. ACE2–angiotensin-(1–7)–MAS receptor axis. Hypertens Res 2009;32:533–6. https://doi.org/10.1038/hr.2009.74; PMID: 19461648.
11. Bader M, Alenina N, Young D, et al. The meaning of MAS. Hypertension 2018;72:1072–5. https://doi.org/10.1161/ HYPERTENSIONAHA.118.10918; PMID: 30354821.
12. Li F. Receptor recognition and cross-species infections of SARS coronavirus. Antiviral Res 2013;100:246–54. https://doi. org/10.1016/j.antiviral.2013.08.014; PMID: 23994189.
13. Perlman S, Dandekar AA. Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol 2005;5:917–27. https://doi.org/10.1038/nri1732; PMID: 16322745.
14. Ng DL, Al Hosani F, Keating MK, et al. Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. Am J Pathol 2016;186:652–8. https://doi.org/10.1016/j.ajpath.2015.10.024; PMID: 26857507.
15. Dandona P, Dhindsa S, Ghanim H, Chaudhuri A. Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J Hum Hypertens 2007;21:20–7. https://doi.org/10.1038/sj. jhh.1002101; PMID: 17096009.
16. Stegbauer J, Coffman TM. New insights into angiotensin receptor actions: from blood pressure to ageing. Curr Opin Nephrol Hypertens 2011;20:84–8. https://doi.org/10.1097/ MNH.0b013e3283414d40; PMID: 21076298.
17. Ruiz-Ortega M, Ruperez M, Lorenzo O, et al. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl 2002;82:S12–22. https://doi.org/10.1046/j.1523-1755.62.s82.4.x; PMID: 12410849.
18. Sano M, Fukuda K, Kodama H, et al. Interleukin-6 family of cytokines mediate angiotensin II-induced cardiac hypertrophy in rodent cardiomyocytes. J Biol Chem 2000;275:29717–23. https://doi.org/10.1074/jbc. M003128200; PMID: 10843995.
19. Ju X, Ijaz T, Sun H, et al. IL-6 regulates extracellular matrix remodeling associated with aortic dilation in a fibrillin-1 hypomorphic mgR/mgR mouse model of severe Marfan syndrome. J Am Heart Assoc 2014;3:e000476. https://doi. org/10.1161/JAHA.113.000476; PMID: 24449804.
20. Göbölös L, Rácz I, Hogan M, et al. The role of renin–angiotensin system activated phagocytes in the SARS-CoV-2 coronavirus infection. J Vasc Surg 2021;73:1889–97. https:// doi.org/10.1016/j.jvs.2020.12.056; PMID: 33348007.
21. Lantos J, Temes G, Göbölös L, et al. Is peripheral blood a reliable indicator of acute oxidative stress following heart ischemia and reperfusion? Med Sci Monit 2001;7:1166–70; PMID: 11687725.
22. Totura AL, Baric RS. SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon. Curr Opin Virol 2012;2:264–75. https://doi. org/10.1016/j.coviro.2012.04.004; PMID: 22572391.
23. Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol 2020;92:424–32. https://doi. org/10.1002/jmv.25685; PMID: 31981224.
24. Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–4. https://doi.org/10.1016/S01406736(20)30628-0; PMID: 32192578.
25. Weissmann G, Smolen JE, Korchak HM. Release of inflammatory mediators from stimulated neutrophils. N Engl J Med 1980;303:27–34. https://doi.org/10.1056/ NEJM198007033030109; PMID: 6246431.
26. Weiss SJ. Tissue destruction by neutrophils. N Engl J Med 1989;320:365–76. https://doi.org/10.1056/ NEJM198902093200606; PMID: 2536474.
27. Klein J. Immunology. Oxford: Blackwell Science Ltd, 1991.
28. Das DK. Cellular, biochemical, and molecular aspects of reperfusion injury. Introduction. Ann N Y Acad Sci 1994;723:xiii–xvi. https://doi.org/10.1111/j.1749-6632.1994. tb36711.x; PMID: 8030856.
29. Actin LE. Alpha-actinin, and tropomyosin interaction in the structural organisation of actin filaments in nonmuscle cells. J Cell Biol 1976;68:202–19. https://doi.org/10.1083/ jcb.68.2.202; PMID: 1107334.
30. Dustin P. Microtubules. Sci Am 1980;243:66–76. https://doi. org/10.1038/scientificamerican0880-66; PMID: 7423178.
31. Tenaglia AN, Buda AJ, Wilkins RG, et al. Levels of expression of P-selectin, E-selectin, and intercellular adhesion molecule-1 in coronary atherectomy specimens from patients with stable and unstable angina pectoris. Am J Cardiol 1997;79:742–7. https://doi.org/10.1016/S00029149(96)00861-2; PMID: 9070552.
32. De Servi S, Mazzone A, Ricevuti G, et al. Granulocyte activation after coronary angioplasty in humans. Circulation 1990;82:140–6. https://doi.org/10.1161/01.CIR.82.1.140; PMID: 2163778.
33. Harlan JM. Consequences of leukocyte–vessel wall interactions in inflammatory and immune reactions. Semin Thromb Hemost 1987;13:434–44. https://doi. org/10.1055/s-2007-1003520; PMID: 3321436.
34. Hessler JR, Robertson AL Jr, Chisolm GM 3rd. LDL-induced cytotoxicity and its inhibition by HDL in human vascular smooth muscle and endothelial cells in culture. Atherosclerosis 1979;32:213–29. https://doi.org/10.1016/00219150(79)90166-7; PMID: 223585.
35. Henriksen T, Evensen SA, Carlander B. Injury to human endothelial cells in culture induced by low density lipoproteins. Scand J Clin Lab Investig 1979;39:361–8. https:// doi.org/10.3109/00365517909106120; PMID: 230571.
36. Moncada S, Vane JR. Prostacyclin and blood coagulation. Drugs 1981;21:430–7. https://doi.org/10.2165/00003495198121060-00002; PMID: 7018874.
37. Gray E, Barrowcliffe TW. Inhibition of antithrombin III by lipid peroxides. Thromb Res 1985;37:241–50. https://doi. org/10.1016/0049-3848(85)90012-X; PMID: 3975871.
38. Mori N, Horie Y, Gerritsen ME, Granger DN. Ischemia–reperfusion induced microvascular responses in LDLreceptor –/– mice. Am J Physiol 1999;276:H1647–54. https://doi.org/10.1152/ajpheart.1999.276.5.H1647; PMID: 10330250.
39. Uhl E, Pickelmann S, Baethmann A, Schürer L. Influence of platelet-activating factor on cerebral microcirculation in rats: part 1. Systemic application. Stroke 1999;30:873–9. https:// doi.org/10.1161/01.STR.30.4.873; PMID: 10187894.
40. Fardman A, Zahger D, Orvin K, et al. Acute myocardial infarction in the Covid-19 era: incidence, clinical characteristics and in-hospital outcomes – a multicenter registry. PLoS One 2021;16:e0253524. https://doi.org/10.1371/ journal.pone.0253524; PMID: 34143840, PMCID: PMC8213163.
41. Bangalore S, Sharma A, Slotwiner A, et al. ST-segment elevation in patients with Covid-19 – a case series. N Engl J Med 2020;382:2478–80. https://doi.org/10.1056/ NEJMc2009020; PMID: 32302081.
42. Dreyer WJ, Smith CW, Michael LH, et al. Canine neutrophil activation by cardiac lymph obtained during reperfusion of ischemic myocardium. Circ Res 1989;65:1751–62. https://doi. org/10.1161/01.RES.65.6.1751; PMID: 2573438.
43. Birdsall HH, Green DM, Trial J, et al. Complement C5a, TGFbeta 1, and MCP-1, in sequence, induce migration of monocytes into ischemic canine myocardium within the first one to five hours after reperfusion. Circulation 1997;95:684–92. https://doi.org/10.1161/01.CIR.95.3.684; PMID: 9024158.
44. Yao XH, He ZC, Li TY, et al. Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-fordischarge patient. Cell Res 2020;30:541–3. https://doi. org/10.1038/s41422-020-0318-5; PMID: 32346074.
45. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–7. https://doi.org/10.1016/j.thromres.2020.04.013; PMID: 32291094.
46. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020;191:9–14. https://doi.org/10.1016/j. thromres.2020.04.024; PMID: 32353746.
47. Mummery RS, Rider CC. Characterisation of the heparinbinding properties of IL-6. J Immunol 2000;165:5671–9. https://doi.org/10.4049/jimmunol.165.10.5671; PMID: 11067924.
48. Valchanov K, Krishnan U, Hoole SP, et al. COVID-19 patient with coronary thrombosis supported with ECMO and Impella 5.0 ventricular assist device: a case report. Eur Heart J J Case Rep 2020;4:1–6. https://doi.org/10.1093/ehjcr/ytaa342; PMID: 33442588.
49. COVIDSurg Collaborative, GlobalSurg Collaborative. Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia 2021;76:748–58. https://doi.org/10.1111/anae.15458; PMID: 33690889.
50. Harrison SL, Buckley BJR, Rivera-Caravaca JM, et al. Cardiovascular risk factors, cardiovascular disease, and COVID-19: an umbrella review of systematic reviews. Eur Heart J Qual Care Clin Outcomes 2021;7:330–9. https://doi. org/10.1093/ehjqcco/qcab029; PMID: 34107535.
51. Girerd N, Meune C, Duarte K, et al. Evidence of a blood pressure reduction during the COVID-19 pandemic and associated lockdown period: insights from e-health data. Telemed J E Health 2022;28:266–70. https://doi.org/10.1089/ tmj.2021.0006; PMID: 34101507.
52. Tern PJW, Jiang Y, Lau YH, et al. Impact of COVID-19 on acute MI and percutaneous coronary intervention rates and outcomes in South East Asia and the Middle East. Journal of Asian Pacific Society of Cardiology 2022;1:e05. https://doi. org/10.15420/japsc.2021.12.
53. Yeleti R, Guglin M, Saleem K, et al. Fulminant myocarditis: COVID or not COVID? Reinfection or co-infection? Future Cardiol 2021;17:1307–11. https://doi.org/10.2217/fca-20200237; PMID: 33615872.
54. Mitrani RD, Dabas N, Goldberger JJ. COVID-19 cardiac injury: implications for long-term surveillance and outcomes in survivors. Heart Rhythm 2020;17:1984–90. https://doi. org/10.1016/j.hrthm.2020.06.026; PMID: 32599178.
55. Siripanthong B, Nazarian S, Muser D, et al. Recognising COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020;17:1463–71. https://doi.org/10.1016/j. hrthm.2020.05.001; PMID: 32387246.
56. Singh S, Desai R, Gandhi Z, et al. Takotsubo syndrome in patients with COVID-19: a systematic review of published cases. SN Compr Clin Med 2020;2:2102–8. https://doi. org/10.1007/s42399-020-00557-w; PMID: 33043251.
57. Offerhaus JA, Joosten LPT, van Smeden M, et al. Sex- and age specific association of new-onset atrial fibrillation with in-hospital mortality in hospitalised COVID-19 patients. Int J Cardiol Heart Vasc 2022;39:100970. https://doi.org/10.1016/j. ijcha.2022.100970; PMID: 35136831.
58. D’Andrea A, Russo V, Manzo G, et al. Association of atrial fibrillation and left atrial volume index with mortality in patients with COVID-19 pneumonia. Eur J Prev Cardiol 2022;29:e44–6. https://doi.org/10.1093/eurjpc/zwaa138; PMID: 33624089.
59. Tarantino N, Della Rocca DG, Zou F, et al. Prevalence, outcomes, and management of ventricular arrhythmias in COVID-19 patients. Card Electrophysiol Clin 2022;14:11–20. https://doi.org/10.1016/j.ccep.2021.10.002; PMID: 35221078.
60. Lao N, Lim J, Bashir H, et al. Incidence of atrioventricular blocks and its association with in-hospital mortality and morbidity in patients with coronavirus disease 2019. J Cardiol 2022;79:482–8. https://doi.org/10.1016/j. jjcc.2021.10.025; PMID: 34848117.
61. Varney JA, Dong VS, Tsao T, et al. COVID-19 and arrhythmia: an overview. J Cardiol 2022;79:468–75. https://doi. org/10.1016/j.jjcc.2021.11.019; PMID: 35074257.
62. Bader F, Manla Y, Atallah B, Starling RC. Heart failure and COVID-19. Heart Fail Rev 2021;26:1–10. https://doi. org/10.1007/s10741-020-10008-2; PMID: 32720082.
63. Gao L, Jiang D, Wen XS, et al. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir Res 2020;21:83. https://doi.org/10.1186/s12931-020-01352-w; PMID: 32293449.
64. Stefanini GG, Chiarito M, Ferrante G, et al. Early detection of elevated cardiac biomarkers to optimise risk stratification in patients with COVID-19. Heart 2020;106:1512–8. https://doi. org/10.1136/heartjnl-2020-317322; PMID: 32817312.
65. Raad M, Dabbagh M, Gorgis S, et al. Cardiac injury patterns and inpatient outcomes among patients admitted with
Cardiac Effects of the SARS-CoV-2 Infection JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
COVID-19. Am J Cardiol 2020;133:154–61. https://doi. org/10.1016/j.amjcard.2020.07.040; PMID: 32829913.
66. Barman HA, Atici A, Sahin I, et al. Prognostic significance of cardiac injury in COVID-19 patients with and without coronary artery disease. Coron Artery Dis 2021;32:359–66. https://doi.org/10.1097/MCA.0000000000000914; PMID: 32568741.
67. Majure DT, Gruberg L, Saba SG, et al. Usefulness of elevated troponin to predict death in patients with COVID-19 and myocardial injury. Am J Cardiol 2021;138:100–6. https:// doi.org/10.1016/j.amjcard.2020.09.060; PMID: 33058800.
68. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. https://doi.org/10.1016/S0140-6736(20)30566-3; PMID: 32171076.
69. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475–81. https://doi.org/10.1016/ S2213-2600(20)30079-5; PMID: 32105632.
70. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020;323:1612–4. https://doi.org/10.1001/ jama.2020.4326; PMID: 32191259.
71. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalised patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. https://doi.org/10.1001/jama.2020.1585; PMID: 32031570.
72. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. https://doi.org/10.1016/S01406736(20)30183-5.
73. Buckner FS, McCulloch DJ, Atluri V, et al. Clinical features and outcomes of 105 hospitalised patients with COVID-19 in Seattle, Washington. Clin Infect Dis 2020;71:2167–73. https:// doi.org/10.1093/cid/ciaa632; PMID: 32444880.
74. Mallah SI, Ghorab OK, Al-Salmi S, et al. COVID-19: breaking down a global health crisis. Ann Clin Microbiol Antimicrob 2021;20:35. https://doi.org/10.1186/s12941-021-00438-7; PMID: 34006330.
75. Tisdale JE, Jaynes HA, Kingery JR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalised patients. Circ Cardiovasc Qual Outcomes 2013;6:479–87. https://doi.org/10.1161/ CIRCOUTCOMES.113.000152; PMID: 23716032.
76. Cohen JB, Hanff TC, William P, et al. Continuation versus discontinuation of renin–angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med 2021;9:275–84. https://doi.org/10.1016/S2213-2600(20)30558-0; PMID: 33422263.
77. Lopes RD, Macedo AVS, de Barros E Silva PGM, et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with
COVID-19: a randomised clinical trial. JAMA 2021;325:254–64. https://doi.org/10.1001/jama.2020.25864; PMID: 33464336.
78. Fried JA, Ramasubbu K, Bhatt R, et al. The variety of cardiovascular presentations of COVID-19. Circulation 2020;141:1930–6. https://doi.org/10.1161/ CIRCULATIONAHA.120.047164; PMID: 32243205.
79. Loforte A, Di Mauro M, Pellegrini C, et al. Extracorporeal membrane oxygenation for COVID-19 respiratory distress syndrome: an Italian Society for Cardiac Surgery report. ASAIO J 2021;67:385–91. https://doi.org/10.1097/ MAT.0000000000001399; PMID: 33470643.
80. Akhtar W, Olusanya O, Baladia MM, et al. SARS-CoV-2 and ECMO: early results and experience. Indian J Thorac Cardiovasc Surg 2020;37:1–8. https://doi.org/10.1007/s12055020-01084-y; PMID: 33250591.
81. Haiduc AA, Alom S, Melamed N, Harky A. Role of extracorporeal membrane oxygenation in COVID-19: a systematic review. J Card Surg 2020;35:2679–87. https://doi. org/10.1111/jocs.14879; PMID: 32717771.
82. Garfield B, Bianchi P, Arachchillage D, et al. Six-month mortality in patients with COVID-19 and non-COVID-19 viral pneumonitis managed with veno-venous extracorporeal membrane oxygenation. ASAIO J 2021;67:982–8. https://doi. org/10.1097/MAT.0000000000001527; PMID: 34144551.
83. Kimball PM, Flattery M, McDougan F, Kasirajan V. Cellular immunity impaired among patients on left ventricular assist device for 6 months. Ann Thorac Surg 2008;85:1656–61. https://doi.org/10.1016/j.athoracsur.2008.01.050; PMID: 18442560.
84. Birati EY, Najjar SS, Tedford RJ, et al. Characteristics and outcomes of COVID-19 in patients on left ventricular assist device support. Circ Heart Fail 2021;14:e007957. https://doi. org/10.1161/CIRCHEARTFAILURE.120.007957; PMID: 33813838.
85. Diaz-Arocutipa C, Carvallo-Castañeda D, Luis-Ybañez O, et al. COVID-19 in heart transplant recipients during February–August 2020: a systematic review. Clin Transplant 2021;35:e14390. https://doi.org/10.1111/ctr.14390; PMID: 34159650.
86. American Society of Transplantation. COVID-19 information. 2020. https://www.myast.org/covid-19-information (accessed 4 May 2021).
87. International Society of Heart and Lung Transplantation. Guidance from the International Society of Heart and Lung Transplantation regarding the SARS CoV-2 pandemic. 2020. https://ishlt.org/ishlt/media/documents/SARS-CoV-2_Guidance-for-Cardiothoracic-Transplant-and-VAD-centers.pdf (accessed 5 May 2021).
88. Bhaskaran K, Rentsch CT, MacKenna B, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV 2021;8:e24–32. https://doi.org/10.1016/S2352-3018(20)30305-2; PMID: 33316211.
89. Aslam S, Danziger-Isakov L, Mehra MR. COVID-19 vaccination immune paresis in heart and lung
transplantation. J Heart Lung Transplant 2021;40:763–6. https://doi.org/10.1016/j.healun.2021.04.018; PMID: 34144891.
90. Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021;144:471–84. https://doi.org/10.1161/CIRCULATIONAHA.121.056135; PMID: 34281357.
91. Theobald SJ, Simonis A, Georgomanolis T, et al. Long-lived macrophage reprogramming drives spike protein-mediated inflammasome activation in COVID-19. EMBO Mol Med 2021;13:e14150. https://doi.org/10.15252/emmm.202114150; PMID: 34133077.
92. Mehra MR, Desai SS, Kuy S, et al. Cardiovascular disease, drug therapy, and mortality in COVID-19. N Engl J Med 2020;382:e102. https://doi.org/10.1056/NEJMoa2007621; PMID: 32356626.
93. Rea F, Corrao G, Ludergnani M, et al. A new populationbased risk stratification tool was developed and validated for predicting mortality, hospital admissions, and health care costs. J Clin Epidemiol 2019;116:62–71. https://doi. org/10.1016/j.jclinepi.2019.08.009; PMID: 31472207.
94. Reynolds HR, Adhikari S, Pulgarin C, et al. Renin–angiotensin–aldosterone system inhibitors and risk of COVID-19. N Engl J Med 2020;382:2441–8. https://doi. org/10.1056/NEJMoa2008975; PMID: 32356628.
95. Atallah B, Mallah SI, AlMahmeed W. Anticoagulation in COVID-19. Eur Heart J Cardiovasc Pharmacother 2020;6:260–1. https://doi.org/10.1093/ehjcvp/pvaa036; PMID: 32352517.
96. Simonis A, Theobald SJ, Fätkenheuer G, et al. A comparative analysis of remdesivir and other repurposed antivirals against SARS-CoV-2. EMBO Mol Med 2021;13:e13105. https://doi.org/10.15252/emmm.202013105; PMID: 33015938.
97. Wilt TJ, Kaka AS, MacDonald R, et al. Remdesivir for adults with COVID-19: a living systematic review for American College of Physicians practice points. Ann Intern Med 2021;174:209–20. https://doi.org/10.7326/M20-5752; PMID: 33017170.
98. Dyer O. Covid-19: FDA expert panel recommends authorising molnupiravir but also voices concerns. BMJ 2021;375:n2984. https://doi.org/10.1136/bmj.n2984; PMID: 34857644.
99. Macchiagodena M, Pagliai M, Procacci P. Characterization of the non-covalent interaction between the PF-07321332 inhibitor and the SARS-CoV-2 main protease. J Mol Graph Model 2022;110:108042. https://doi.org/10.1016/j. jmgm.2021.108042; PMID: 34653812.
100. Weinreich DM, Sivapalasingam S, Norton T, et al. REGENCOV antibody combination and outcomes in outpatients with COVID-19. N Engl J Med 2021;385:e81. https://doi. org/10.1056/NEJMoa2108163; PMID: 34587383.
101. Burgers LD, Luong B, Li Y, et al. The natural product vioprolide A exerts anti-inflammatory actions through inhibition of its cellular target NOP14 and downregulation of importin-dependent NF-ĸB p65 nuclear translocation. Biomed Pharmacother 2021;144:112255. https://doi. org/10.1016/j.biopha.2021.112255; PMID: 34607110.
Cardiac Effects of the SARS-CoV-2 Infection JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Cross-sectional Study of Percutaneous Intervention in the Elderly in Malaysia: PIELD Study
Ganapathi Palaniappan , Rhuban M Sundran, Afif Ashari, Mohd Saad Jalaluddin, Afrah Yousif Haroon, Rohith Stanislaus, Yee Sin Tey, Ahmad Farhan Abdul Hamid, Tjen Jhung Lee, Shaiful Azmi Yahaya, Balachandran Kandasamy and Zulaikha Zainal
Department of Cardiology, Institut Jantung Negara, Kuala Lumpur, Malaysia
Abstract
Background: Worldwide, people are living longer. Most trials do not include elderly patients. Studies in the elderly are primarily subgroup analyses of major trials. Methods: This study investigated the outcomes of percutaneous coronary intervention (PCI) in the elderly (age ≥70 years). The primary outcomes were all-cause mortality and major adverse cardiovascular events (MACE) during the periprocedural period (up to 30 days) and 1 year after PCI. We also investigated the relationship between baseline characteristics and these cardiovascular outcomes. All elderly patients enrolled in the study underwent either urgent or elective PCI between 2007 and 2016. Data were obtained from electronic medical records. Patients (n=3,659) were divided into three groups: early-elderly (age 70–74 years, n=2,316), mid-elderly (age 75–79 years, n=1,037) and late-elderly (age ≥80 years, n=306). Results: All-cause mortality and MACE 30 days after PCI were significantly higher in the lateelderly group. One year after PCI, all-cause mortality remained highest in the late-elderly group, but MACE was highest in the mid-elderly group. Kaplan–Meier survival analysis showed that survival probability 1 year after PCI was highest in early-elderly (92.0%), followed by mid-elderly (88.9%), and lowest in late-elderly group (84.9%). Conclusion: Immediate outcomes (30 days) after PCI, in terms of both MACE and all-cause mortality, favour patients without chronic renal failure undergoing PCI in an elective setting. Mid-term outcomes (1 year) after PCI, in terms of all-cause mortality, favour patients without chronic heart failure or renal failure. In conclusion, revascularisation via PCI is safe, with acceptable short- (30 days) and mid-term (1 year) outcomes, in the elderly population.
Keywords
Percutaneous intervention, elderly, geriatric, acute coronary syndrome, primary PCI, elective PCI, Asia

Disclosure: The authors have no conflicts of interest to declare.
Informed consent/consent to publish: This is a single-centre, retrospective cross-sectional study, no patient-identifying data were used. Informed consent was not required.
Data availability statement: The data supporting the findings of this study are available in the supplementary material of this article.
Ethics approval: The study was approved by our institutional research ethics committee (IJNREC).
Authors’ contributions: Conceptualisation: GP, RMS, BK, SAY; data curation: GP, RMS, AA, MSJ, AYH, RS, YST, AFAH, TJL; formal analysis: ZZ; funding acquisition: N/A; investigation: GP, RMS, BK, SAY; methodology: GP, RMS, BK, SAY; project administration: GP, ZZ; resources: GP, RMS; software: ZZ; supervision: BK, SAY; validation: BK, SAY; visualisation: GP, ZZ, AA; writing – original draft preparation: GP, RMS, AA; writing – review & editing: GP, RMS, AA, MSJ, AYH, RS, YST, AFAH, TJL, BK, SAY.
Received: 11 December 2021 Accepted: 21 April 2022 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e20.
DOI: https://doi.org/10.15420/japsc.2021.34
Correspondence: Ganapathi Palaniappan, Department of Cardiology, Institut Jantung Negara, 145, Jalan Tun Razak, 50450 WP Kuala Lumpur, Malaysia. E: dr.gana.palani@gmail.com
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
The World Health Organization’s Global Health Observatory 2019 reports that people worldwide are living longer. By 2050, the world’s population aged ≥60 years is expected to reach 2 billion, up from 900 million in 2015.1 The shift in the distribution of the population towards older age started in high-income countries, with the proportion of the population aged ≥60 years in Japan reaching >30% in 2017.1 The most significant changes in population age structure are currently being experienced in lower- and middle-income countries.1 The Global Health Observatory’s 2019 report lists ischaemic heart disease and stroke as the top two causes of death globally, accounting for 16% and 11% of total deaths worldwide, respectively.1 These two conditions have been the top two causes of death globally since 2000. Even in low-income countries, ischaemic heart
disease is the third leading cause of death after neonatal and infectious causes. However, there is little evidence suggesting that older people generally are in better health. Globally, the main contributor to disabilityadjusted life years (DALY), after neonatal conditions, is the ischaemic heart disease burden.1
The MSD Manual (https://www.msdmanuals.com/professional; accessed 23 October 2021) defines elderly or geriatric as age >65 years, but most people do not require expert geriatric care at 70, 75, or even 80 years of age. Most cardiac intervention trials do not include elderly patients, with studies in the elderly primarily from subgroup analyses of major trials. In the setting of acute coronary syndrome (ACS), subgroup analysis from
© RADCLIFFE CARDIOLOGY 2022 www.JAPSCjournal.com Intervention ORIGINAL RESEARCH
many randomised control trials, such as PAMI-1, GUSTO-IIb, and DANAMI-2, has shown significant reductions in the combined endpoint of death, stroke and reinfarction in the percutaneous coronary intervention (PCI) arm compared with fibrinolysis in elderly patients with ST-elevation MI (STEMI). A meta-analysis of the SENIOR PAMI, TACTICS TIMI-RCT and PCAT-2 trials also found favourable outcomes in the elderly group undergoing cardiac intervention compared with medical therapy.2 In all these trials, patients in the very elderly group (age >80 years) are underrepresented. As such, clinicians are less assertive when it comes to providing cardiac intervention in elderly patients.
Long-term mortality is similar in elderly patients (age >80 years) treated with coronary artery bypass graft (CABG) surgery or PCI, but reintervention is higher in the coronary intervention group.3,4 Conrotto et al. reported that the risk for the primary composite endpoint of all-cause death, cerebrovascular accident and MI in very elderly patients was similar between those undergoing PCI versus CABG, despite a greater prevalence of baseline comorbidities in the PCI group.4 Another meta-analysis of 32 studies of PCI performed in patients aged ≥80 years found a pooled 30day estimate of mortality of 5.4% and a 1-year survival estimate of 87%.5
The aim of this study was to investigate the safety outcomes by analysing total all-cause mortality and major adverse cardiovascular events (MACE) during the periprocedural period (up to 30 days) and 1 year after PCI in a cohort of the elderly patients stratified according to age (70–74, 75–79 and ≥80 years old). In addition, we analysed the relationships between these cardiovascular outcomes and both clinical characteristics and elective/urgent PCI status. Overall survival curves across all age groups were also analysed.
Methods
This study was a single-centre retrospective cross-sectional study that included every elderly patient (age ≥70 years) who underwent PCI between 2007 and 2016. Data were collected through an electronic medical record (EMR) database at Institute Jantung Negara (National Heart Institute), Kuala Lumpur, Malaysia (the national cardiovascular referral centre). The study was approved by the Institutional Research Ethics Committee of the Institute Jantung Negara.
Patients admitted to the Institute Jantung Negara between 2007 and 2016 who underwent PCI as either an urgent or elective procedure were screened for inclusion in this study. Within this population, all patients who were aged ≥70 years at the time of admission were in the study. Patients <70 years of age were excluded from the study. Urgent PCI was defined as an admission for ACS with the patient requiring PCI immediately upon admission; STEMI, both primary and rescue, ongoing chest pain with unstable angina (UA) or non-STEMI (NSTEMI). Elective PCI was defined as a planned admission for PCI because of symptoms, positive results on functional studies, staged PCI/post ACS; facilitated/pharmaco-invasive/ delayed PCI among stable ACS patients or referral from other centres. Most of the patients included in the study were Malaysian (Malay, Chinese, Indians and other indigenous people of Malaysia, such as Orang Asli, Iban and Kadazan, among others).
Baseline patient characteristics, including demographics (sex, age, PCI status [elective or urgent]) and comorbidities (smoking, dyslipidaemia, hypertension, diabetes, history of ischaemic heart disease, family history of ischaemic heart disease, heart failure, defined as heart failure with a significantly reduced left ventricular ejection fraction [LVEF] ≤35%, cerebrovascular accident, chronic renal failure), were compared between
the three predetermined age groups: early-elderly (age 70–74 years), mid-elderly (age 75–79 years) and late-elderly (age ≥80 years).
All hospital admissions, readmissions and redo PCI data were collected from electronic medical records. MACE was defined as a composite of total cardiovascular events, including fatal and non-fatal MI/stroke, hospitalisation for revascularisation, stent thrombosis and angiographic target vessel revascularisation (TVR)/target lesion revascularisation (TLR). TVR was defined as any repeat intervention to any segment of the previously intervened vessel, including upstream and downstream branches. TLR was defined as an intervention to a segment of a previously intervened target vessel. In all, 42 angiograms were reviewed to determine TVR/TLR. Information regarding clinical status was collected based on follow-up obtained 1 year after the procedure during a clinic visits, via telephone interviews or from the Malaysia National Cardiovascular Disease Registry database and cross-checked against the Malaysia National Registration Department if the patient could not be contacted or had died.
The revascularisation strategy was determined by the treating interventional cardiologist in the institution and included plain old balloon angioplasty (POBA), implantation of a drug-eluting balloon or drug-eluting stent, atherectomy on any native vessels and grafts; all strategies were accepted and included in the study. In addition, therapy with any single antiplatelet agent (due to bleeding risk) or dual antiplatelet therapy (e.g. aspirin or triflusal with clopidogrel, prasugrel, ticlopidine or cilostazol) was also accepted.
All statistics are descriptive. Data are presented as percentages or the mean ± SD. The significance of differences in proportions was tested with the Chi-squared test. Logistic regression was used to describe relationships. Cumulative events curves were generated using the Kaplan–Meier method.
Results
Over the 10 years from 2007 to 2016, 28,407 patients were admitted to the Cardiology Department at Institute Jantung Negara and underwent PCI (either urgent or elective). Of these patients, 3,659 (12.9%) were aged ≥70 years at the time of admission. After further evaluation, all these patients were found to be eligible for inclusion in this study (Figure 1). Of the 3,659 patients included in the study, 3,175 (86.8%) completed at least 1 year of follow-up (via clinic visits, telephone interviews or inclusion in the National Cardiovascular Disease Registry database); data for 463 (12.7%) patients were obtained through cross-checks with the National Registration Department. The 21 (0.6%) patients who were lost to follow-up were also included in the study. Most of the patients lost to follow-up were from other states in Malaysia, with logistical or financial constraints being the leading causes of loss to follow-up.
In each age group, the number of elderly patients admitted for PCI increased over time. Overall, the total number of elderly patients admitted for PCI increased from 244 in 2007 to 438 in 2016. Most admissions were from the early-elderly group (n=2,316 [63.3%]; Table 1). The oldest patient enrolled in the study was a 98-year-old man admitted for NSTEMI who underwent successful elective PCI to the mid-left anterior descending (LAD) and left circumflex (LCX) arteries in 2012. Baseline characteristics for each age group are summarised in Table 2. Most patients were male (70%).
The most common comorbidity among the elderly patients who underwent PCI was hypertension (84.5%), followed by dyslipidaemia (70.7%) and
PIELD Study JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
diabetes (52.9%); the least common comorbidity was a previous history of stroke (3.1%). Among the entire cohort, 39.9% of patients had a previous history of MI, 6% had heart failure (LVEF <35%) and 9.7% had chronic renal failure, defined as an estimated glomerular filtration rate <60 ml/min/1.73 m2
Most of the patients in this study (94.3%) underwent PCI as an elective procedure; only 209 (5.7%) patients underwent urgent PCI.
The clinical characteristics that differed significantly across the three age groups were a family history of ischaemic heart disease (p=0.014) and a previous history of CABG (p=0.03). Over the 10 years of the study, only 16 of 306 (5.5%) patients aged ≥80 years (late-elderly group) had a positive family history of ischaemic heart disease. This rate was significantly lower than in the early- and mid-elderly age groups (10.7% and 9.1%, respectively).
The percentage of patients with a history of CABG was significantly higher in the late-elderly than mid- and early-elderly groups (17.3% versus 13.6% and 11.1%, respectively).
As indicated in Supplementary Table 1, the percentage of patients presenting with unstable angina was significantly higher among the lateelderly group compared with the early- and mid-elderly groups (15% versus 10.7% and 12.9%, respectively; p=0.027). However, there were no significant differences among the three groups for all other types of coronary heart disease. Most admissions (51%) were for chronic stable angina. However, it should be noted that coronary heart disease data were not available in the electronic medical records for 482 (13.2%) patients. There was no significant difference in LVEF among the three age groups.
Supplementary Table 2 details the angiographic findings and procedures undertaken. Of all PCI procedures performed among elderly patients, 92.6% were for de novo coronary lesions; this is statistically significant (p<0.001). Regarding the number of vessels involved, although overall most elderly patients required interventions for single-vessel disease (51.7%), the percentage of patients in the late-elderly group with multivessel disease requiring intervention (55.6%) was significantly higher than in the mid-elderly and early-elderly (49.7% and 46.8%, respectively; p=0.009). However, there was no significant difference among age groups with regard to the coronary artery (i.e. LAD, LCX and right coronary artery) being treated. There was also no significant difference in the type of lesion being treated, although most lesions were Type C lesions (52.7%).
Generally, although fewer left main stem (LMS) vessels (i.e. only LMS and LMS-LAD/LCX bifurcation stenting) were treated in the early-elderly group, the difference did not reach statistical significance. However, rates of provisional LMS-LAD (3.0%) and LMS-LCX (0.7%) stenting were significantly lower in the early-elderly age group than in the mid-elderly (LMS-LAD 4.2% and LMS-LCX 1.5%) and late-elderly (LMS-LAD 4.6% and LMS-LCX 3.0%) groups. One possible explanation for the lower rates of PCI for patients with LMS disease in the early-elderly age group may be that complete revascularisation via CABG is still considered safe in most of these patients compared with patients in the older age groups.
Most lesions treated in this study were ostial lesions (11.0% of all lesions across all age groups), with a significant higher rate of ostial lesions among late-elderly patients compared to early-elderly and mid-elderly (15.2% versus 9.5% and 12.9%, respectively; p<0.001). The next most common lesions were bifurcation lesions (9.5% of all lesions across all age groups). With regard to intracoronary devices, the use of rotablation was significantly higher among the late-elderly group compared with the early- and mid-
Recruitment Flow Diagram
Transfer
Run
elderly groups (5.2% versus 3.0% and 4.4%, respectively; p=0.007). This finding is not surprising because vessel calcification is a hallmark of ageing. There were no differences in the types of stents or drug-coated balloons used in elderly patients across the three age groups.
Primary endpoint analysis, summarised in Tables 3 and 4, showed that allcause mortality at 30 days and 1 year after PCI differed significantly between the three age groups. At 30 days, all-cause mortality was higher in the lateelderly age group than in the early- and mid-elderly groups (6.5% versus 2.8% and 4.1%, respectively; p=0.002). Mortality within 30 days after PCI
PIELD Study JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Figure 1:
Start e-NCVD PCI database from 2007 to 2016 (n=28,407) Check with EMR database Error Cannot be contacted for follow-up
data from Excel to IBM SPSS
appropriate statistical analysis Interpret data and reporting End Cross-check with NRD Exclude: age <70 years (n=24,748) Eligible patients (n=3,659) Download data from databases to Excel Data cleaning EMR = electronic medical records; e-NCVD = electronic National Cardiovascular Disease registry; NRD = National Registration Department; PCI = percutaneous coronary intervention. Table 1: Number of Patients in Each Age Group Per Year of Admission Year Age Group Total Patients (n)Early-elderly (Age 70–74 Years) Mid-elderly (Age 75–79 Years) Late-elderly (Age ≥80 Years) 2007 160 69 15 244 2008 188 69 15 272 2009 186 84 14 284 2010 250 92 35 377 2011 208 79 20 307 2012 282 125 45 452 2013 272 126 35 433 2014 259 113 44 416 2015 244 149 43 436 2016 267 131 40 438 Total 2,316 1,037 306 3,659
Male 1,639
677 (29.2)
700
215 (70.3) 2,578 (70.5) 0.853
(30.2) 91 (29.7) 1,081 (29.5)
(33.4) 81 (32.9) 1,067 (35.6) 0.118
Dyslipidaemia 1,651 (71.3) 724 (69.8) 211 (69) 2,586 (70.7) 0.542
Hypertension 1960 (84.6) 881 (85) 252 (82.4) 3,093 (84.5) 0.530
Diabetes 1,226 (52.9) 550 (53) 159 (52) 1,935 (52.9) 0.943
History of MI 942 (40.7) 402 (38.8) 115 (37.6) 1,459 (39.9) 0.403
Family history 228 (10.7) 88 (9.1) 16 (5.5) 332 (9.8) 0.014
History of heart failure 123 (5.3) 73 (7) 23 (7.5) 219 (6.0) 0.074
Cerebrovascular disease 73 (3.2) 30 (2.9) 11 (3.6) 114 (3.1) 0.813
Chronic renal failure 209 (9) 107 (10.3) 39 (12.7) 355 (9.7) 0.086 PCI status†
Urgent 121 (5.2) 69 (6.7) 19 (6.2) 209 (5.7) 0.238 Non-urgent 2,195 (94.8) 968 (93.3) 287 (93.8) 3,450 (94.3)
Previous history of CABG 258 (11.1) 141 (13.6) 53 (17.3) 452 (12.4) 0.003 Single antiplatelet upon discharge 63 (2.8) 33 (3.3) 10 (3.4) 106 (3.0) 0.846 Unless indicated otherwise, data are given as n (%). †Urgent PCI was defined as PCI for STEMI (primary and rescue PCI), and NSTEMI with ongoing chest pain (who underwent PCI directly upon admission). Non-urgent PCI was defined as elective ad hoc PCI, elective PCI and PCI performed for stable NSTEMI (non-urgent) and STEMI (facilitated, pharmaco-invasive and delayed PCI). CABG = coronary artery bypass graft; PCI = percutaneous coronary intervention; STEMI = ST-elevation MI; NSTEMI = non-ST-elevation MI.
was mostly related to cardiac deaths. Rates of MI and pure cardiac death 30 days after PCI were significantly higher in the late-elderly population than in early-elderly and mid-elderly (MI: 2.0% versus 0.6% and 0.6%, respectively; p=0.032 and pure cardiac death: 4.9% versus 1.9% and 2.9%, respectively; p=0.002). All-cause mortality at 1 year was also significantly higher in the late-elderly group compared with the mid- and early-elderly groups (9.1% versus 7.4% and 5.3%, respectively; p=0.008). The primary contributor to all-cause death at 1 year was cardiac death in all age groups (Tables 3, 4).
Overall, the risk of MACE within 30 days after PCI was significantly higher in the late-elderly group than in the early- and mid-elderly groups (5.9% versus 2.3% and 3.2%, respectively; p=0.002), with the main contributors being cardiac death (66.7%) and cardiac death associated with MI (16.7%).
There were no significant differences in rates of TLR/TVR among the age groups within 30 days after PCI. However, at 1 year after PCI, rates of MACE were significantly higher in the mid-elderly group than in the earlyand late-elderly groups (4.9 versus 2.1% and 2.8%, respectively; p<0.001). This was contributed to, in part, by the higher, albeit not significant rate of TVR in the mid-elderly population.
Correlation Analyses Between Clinical Characteristics and Primary Endpoints After Percutaneous Coronary Intervention
Further analyses were conducted to assess correlations between clinical characteristics and both all-cause mortality and MACE at 30 days and 1 year after PCI, as detailed below.
Univariate and Multivariate Analyses at 30 Days After Percutaneous Coronary Intervention All-cause Mortality
The results of univariate analysis assessing correlations between clinical characteristics and all-cause mortality at 30 days after PCI are summarised in Supplementary Table 3. Significant linear correlations were found for chronic renal failure and urgent PCI status with all-cause mortality in each of the three age groups. Conversely, univariate analysis revealed that only urgent PCI status was linearly associated with MACE 30 days after PCI (Supplementary Table 4).
As indicated in Supplementary Table 5, multivariate analysis revealed that dyslipidaemia (OR 2.4; 95% CI [1.146–4.947]; p=0.02) was associated with higher all-cause mortality at 30 days after PCI in the early-elderly group (age 70–74 years). In the mid-elderly (age 75–79 years) and late-elderly group (≥ 80 years old), although statistically not significant, dyslipidaemia was still associated with higher all-cause mortality at 30 days after PCI (mid-elderly: OR 1.9; CI [0.857–4.411], p=0.111 and late-elderly: OR 2.5; CI [0.783–8.133], p=0.121).
Major Adverse Cardiovascular Events
Among patients in the early-
status (OR
at
was significantly associated only with
95% CI [4.181–14.638]; p<0.001). Among mid-
PIELD Study JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
The results of multivariate analysis of MACE at 30 days after PCI are summarised in Supplementary Table 6. Across all age groups, Urgent PCI status is the significant predictor of MACE.
elderly group, MACE
30 days
urgent PCI
7.8;
Table 2: Baseline Characteristics Comorbidities/Risk Factors Age Group All Patients p-value (Chi-squared)Early-elderly (Age 70–74 years) Mid-elderly (Age 75–79 years) Late-elderly (Age ≥80 years) Total no. patients 2,316 (63.3) 1,037 (28.3) 306 (8.4) 3,659 (100) Age (years) Mean ± SD 72.2 ± 1.43 77.0 ± 1.36 82.6 ± 2.39 74.4 ± 3.58 Median [IQR] 72.1 [70–74] 76.8 [75–79] 81.9 [80–98] 73.6 [72–76] Sex
(70.8) 724 (69.8)
Female
313
Smoking (current/former)
(37) 286
(2.8)
(0.9)
Old age 2 (0.1)
15 (0.6)
Stroke 3 (0.1)
54 (2.3)
Cardiac death
(64.8)
Cardiac death + stroke 2 (3.7)
Cardiac death + MI 5 (9.3)
(4.1)
(2.9)
(8.4)
(100)
(6.5) 128 (3.5)
(4.9) 88 (2.4) 0.002
(0.8) 5 (1.6) 34 (0.9) 0.378
(0.4) 6 (0.2) 0.107
(0.6) 6 (2.0) 27 (0.7) 0.032
(0.2) 5 (0.1) 0.716
(3.2) 18 (5.9) 105 (2.9) 0.002
(75.8) 12 (66.7) 72 (68.6) 0.008
(3.0) 3 (2.9) 0.868
(3.8) 3 (16.7) 12 (11.4) 0.083
Cardiac death + TLR 1 (1.9) 1 (1.0) 0.748
Stroke
(1.9)
MI 8 (14.8)
MI + TLR
(3.0) 2 (1.9) 0.758
(6.1) 3 (16.7) 13 (12.4) 0.125
(1.9) 1 (1.0) 0.748
MI + TVR 1 (1.9) 1 (1.0) 0.748
indicated otherwise, data are given as n (%). †MACE includes MI, stroke, cardiac mortality, TLR and TVR. MACE = major adverse cardiovascular events; TLR = target lesion revascularisation; TVR = target vessel revascularisation.
elderly patients, MACE was significantly associated with chronic renal failure (OR 3.3; 95% CI [1.404–7.935]; p=0.006) and urgent PCI status (OR 14.8; 95% CI [6.934–31.374]; p<0.001). In late-elderly patients, MACE was significantly associated with urgent PCI status only (OR 6.6; 95% CI [1.909–22.896]; p=0.003).
Univariate and Multivariate Analyses 1 Year
After Percutaneous Coronary Intervention
Results of univariate and multivariate analyses assessing correlations between clinical characteristics and all-cause mortality and MACE 1 year after PCI are summarised in Supplementary Tables 7–10. Neither univariate nor multivariate analysis for all-cause mortality at 1 year after PCI revealed any specific correlations. Univariate analysis for MACE at 1 year after PCI did not reveal any specific correlations either. However, multivariate analysis for MACE at 1 year post PCI among the early-elderly age group showed significant correlation to dyslipidaemia (OR 2.4; CI [1.052–5.298]; p=0.037) and history of heart failure (OR 2.5; CI [1.030–6.034]; p=0.043). Mid-elderly patients showed MACE correlation in males (OR 3.2; CI [1.361–7.671]; p=0.008). Late-elderly patients showed MACE correlation to chronic renal failure (OR 7.5; CI [1.798–31.402]; p=0.006).
Kaplan–Meier Survival Analysis
Kaplan–Meier survival analysis revealed that the probability of survival at 30 days after PCI was highest for the early-elderly group (97.1%), followed by the mid-elderly group (95.8%) and then the late-elderly group (93.4%; Figure 2; Supplementary Table 11). A similar trend was seen for survival at 1 year after PCI, with the highest survival rate in the early-elderly group, followed by the mid-elderly and then late-elderly groups (92%, 88.9% and 84.9%, respectively).
Discussion
The findings of this study suggest that all-cause mortality and MACE at 30
days and 1 year after revascularisation via PCI increases with age. Overall, revascularisation via PCI in elderly patients (age ≥70 years old) is associated with a mean rate of all-cause mortality at 30 days and 1 year of 3.5% and 6.2%, respectively. The rate of all-cause mortality at 30 days and 1 year after PCI is highest among the late-elderly (6.5% and 9.1%, respectively), followed by the mid-elderly (4.1% and 7.4%, respectively). The early-elderly have the lowest rate of all-cause mortality at 30 days and 1 year after PCI (2.8% and 5.3%, respectively). Overall, the risk of MACE 30 days and 1 year after PCI in elderly patients (age ≥70 years) is 2.9% and 3.0%, respectively. The risk of MACE 30 days after the procedure follows the same trend as seen for allcause mortality, with the highest risk among late-elderly patients. However, the risk of MACE 1 year after the procedure is highest in the mid-elderly group, with a significant contribution from high rates of TLR/TVR.
The number of patients undergoing urgent PCI was highest in the midelderly group, and univariate analysis shows a statistically significant correlation between MACE and urgent PCI. In this study, risk of MACE at 30 days after PCI were significantly higher among patients with urgent compared with elective PCI in all age groups. This finding provides similar prognostic insights as the GUSTO-IIb and PAMI-1 trials and highlights the importance of both early intervention in patients with chronic stable angina and screening for coronary heart disease before a cardiac event happens. Identifying high-risk baseline clinical characteristics, such as those highlighted in the present study, and further investigating or considering early angiograms, as recommended by the TACTICS-TIMI trial, among the geriatric groups, regardless of age group, is crucial.
PAMI-1 trial demonstrated that the significant reduction in the incidence of recurrent ischaemia and reduced mortality after angioplasty at 6 months (from the PAMI trial) extended to 2 years. Similar observations were made in the present study, but only up to 1 year after PCI. Similarly, there was a reduction in MACE in the group undergoing urgent PCI
PIELD Study JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
The
Table 3: Primary Endpoints at 30 Days After Percutaneous Coronary Intervention Primary Endpoint at 30 days Age Group All Patients p-value (Chi-squared)Early-elderly (Age 70–74 Years) Mid-elderly (Age 75–79 Years) Late-elderly (Age ≥80 Years) Total no. patients 2,316 (63.3) 1,037 (28.3) 306
3,659
All-cause mortality 66
42
20
0.002 Cardiac death 43 (1.9) 30
15
Non-cardiac death 21
8
4
MI
6
2
MACE†
33
35
25
1
4
1
1
2
1
Unless
known 43 (1.9)
11 (0.5)
Stroke 3 (0.1)
MACE† 48 (2.1)
Cardiac death 28 (58.3)
Cardiac death + stroke 3 (6.3)
Cardiac death + MI 3 (6.3)
Cardiac death + TLR
MI 8 (16.7)
TLR 2 (4.2)
TVR 4 (8.3)
TLR + TVR
(1.7)
(0.8)
(0.2)
(0.5) 0.853
(3.1) 69 (2.0) 0.293
(1.0) 22 (0.6) 0.365
(0.7) 7 (0.2) 0.128
(4.9) 8 (2.8) 105 (3.0) <0.000
(53.1) 2 (25.0) 56 (53.3) 0.007
(4.1) 2 (25.0) 7 (6.7) 0.128
(10.2) 2 (25.0) 10 (9.5) 0.073
(4.1) 2 (1.9) 0.078
(6.1) 1 (12.5) 12 (11.4) 0.970
(8.2) 1 (12.5) 7 (6.7) 0.151
(12.2) 10 (9.5) 0.071
(2.0) 1 (1.0) 0.280
Unless indicated otherwise, data are given as n (%). †MACE includes MI, stroke, cardiac mortality, TLR and TVR. MACE = major adverse cardiovascular events; TLR = target lesion revascularisation; TVR = target vessel revascularisation.
age groups. However, 30 days after PCI, all-cause mortality was highest among patients who had undergone urgent PCI and those with chronic renal failure. At 1 year after PCI, all-cause mortality was correlated with chronic heart failure (among early-elderly group) and renal failure (among late-elderly group).
This principal finding of this study is that intervention in the elderly is tolerated reasonably well, which is comparable to reports from other centres.5–9 Unfortunately, the quality of the available evidence is low, being limited to patient registries. Inevitably, the elderly population is more likely to be vulnerable, hence limiting their participation in trials.10 This restricts the possibility of a randomised controlled trial in this group, and so the present study was designed as a retrospective cross-sectional study.
Elderly patients have more cardiovascular risk factors and are at higher risk of subsequent cardiovascular events with a more severe disease burden (less ischaemic reserve). A less compliant heart (less tolerant to myocardial oxygen supply–demand mismatch) in elderly patients compared with that in younger patients may derive a more significant benefit from revascularisation. In this regard, trials of coronary revascularisation have generally shown that patients at high risk derive more significant benefits from revascularisation than patients at low risk.8 11
(involving primary PCI), as well as substantial reductions in readmissions for revascularisation. The observations in the present study were not extended beyond 1 year because the follow-up of most patients was changed to their respective referring hospitals (as per local government policy to reduce overcrowding and to allow for new referrals to tertiary treatment centres).
This study shows that 30 days after PCI, MACE outcomes depend primarily only on urgent PCI status. There was no significant association between MACE 1 year after PCI and any clinical characteristics in any of the three
The combination of improved patient selection, the evolution of revascularisation techniques, newer stents and the broader use of evidence-based periprocedural medications that balance the risks of thrombosis
among the
bleeding will significantly reduce PCI-associated
the world’s elderly population grows
too
outcomes following PCI in the elderly are
among the elderly, and the outcome seems promising. Overall,
status and in the absence of chronic renal failure
by an elective
chronic heart failure.
PIELD Study JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
and
mortality
elderly.3 8 11 As
rapidly, so
will revascularisation
good
favoured
PCI
and
Figure 2: Kaplan–Meier Survival Analysis 1.0 0.8 Age group (years) 70–74 75–79 ≥80 70–74 censored 75–79 censored ≥80 censored p<0.05 Probability of survival Duration (months) 1 2 3 4 5 6 7 8 9 10 11 12 0.6 0.4 0.2 0.0 0 Table 4: Primary Endpoints 1 Year After Percutaneous Coronary Intervention Primary Endpoints at 1 year Age Group All Patients p-value (Chi-squared)Early-elderly (Age 70–74 Years) Mid-elderly (Age 75–79 Years) Late-elderly (Age ≥80 Years) Total no. patients 2,250 (63.7) 995 (28.2) 286 (8.1) 3,531 (100) All-cause mortality 120 (5.3) 74 (7.4) 26 (9.1) 220 (6.2) 0.008 Cardiac death 34 (1.5) 35 (3.5) 6 (2.1) 75 (2.1) 0.001 Non-cardiac death 32 (1.4) 16 (1.6) 9 (3.1) 57 (1.6) 0.093 Old age 11 (0.5) 6 (0.6) 2 (0.7) 19
Not
17
9
MI
8
3
2
2
49
26
2
5
2
3
4
6
1
A potential confounding factor in this study is bias in patient selection for intervention; this is particularly relevant among the elderly population, for whom decisions regarding interventions are based on factors such as frailty, family support and the presence of other debilitating conditions, such as malignancy with poor prognosis. Further studies examining correlations between a frailty index (e.g. based on a clinical frailty scale) with PCI outcomes may further rationalise the basis for the selection of patients for revascularisation intervention.
The main limitation of this study is that we used observational data obtained from institutional electronic medical records and administrative databases. We may have missed post-procedural cardiovascular events among patients living in other Malaysian states, especially if these patients sought treatment at their respective local state hospitals and failed to update their clinicians during subsequent clinic visits. In addition, the data used in this study were for the period 2007–16. Various newer techniques, devices and medicines may have changed the current setting and therefore limit the extrapolation of our findings. Furthermore, we did not consider the effects of the treating physician’s preferences, the patient’s clinical status and the operator’s skill on PCI outcomes. All patients who underwent PCI performed by senior and junior interventional cardiologists, regardless of experience and lesion complexity, were enrolled in the study. More importantly, we enrolled patients on any antiplatelet medication (aspirin, ticlopidine, clopidogrel, prasugrel), those on dual, single or no (high bleeding risk patients) antiplatelet agents and those receiving antiplatelet therapy for any duration (none to maximum dual antiplatelet therapy up to 1 year), although it should be noted that there were no specific data on the use of antiplatelet agents in the electronic medical records. Nevertheless, these last two limitations are advantages for the generalisability of the study findings to the realworld setting.
1. World Health Organization. World health statistics 2019: monitoring health for the SDGs, sustainable development goals. 2019. https://apps.who.int/iris/handle/10665/324835 (accessed 5 September 2021).
2. Shanmugasundaram M. Percutaneous coronary intervention in elderly patients: is it beneficial? Tex Heart Inst J 2011;38:398–403. PMID: 21841868.
3. Šerpytis R, Puodžiukaitė L, Petrauskas S, et al. Outcomes of a percutaneous coronary intervention versus coronary artery bypass grafting in octogenarians. Acta Med Litu 2018;25:132–9. https://doi.org/10.6001/actamedica. v25i3.3860; PMID: 30842702.
4. Conrotto F, Scacciatella P, D’Ascenzo F, et al. Long-term outcomes of percutaneous coronary interventions or coronary artery bypass grafting for left main coronary artery disease in octogenarians (from a drug-eluting stent for left main artery registry substudy). Am J Cardiol 2014;113:2007–12. https://doi.org/10.1016/j.amjcard.2014.03.044; PMID: 24793677.
Conclusion
In conclusion, revascularisation via PCI is safe with acceptable short(30 days) and mid-term (1 year) outcomes in the elderly population. Immediate outcomes (30 days after PCI) in terms of both MACE and allcause mortality are better in patients undergoing elective PCI and those without chronic renal failure or dyslipidaemia (only MACE). Mid-term outcomes (1 year after PCI) in terms of all-cause mortality are better in patients without chronic heart failure or chronic renal failure. With the changing demographics of the heart disease patient population leaning towards the elderly, we should focus on appropriate patient selection for revascularisation. Clinicians and patients need to weigh these intervention risks against the significant potential improvement in cardiovascular wellbeing instead of deciding PCI risks based on age alone.
Clinical Perspective
• This study investigated the real-world clinical outcomes following PCI (both emergency and elective) in elderly patients.
• The findings provide clinicians with actual risks of PCI in specific age groups to use during angiogram and angioplasty counselling, rather than using an approximate generalised value for all; the actual risk at advanced age is much higher than for the general population.
• Safety from all-cause mortality and MACE at the periprocedural time and up to 1 year after PCI among the elderly population favours those with elective PCI and those without chronic renal failure, dyslipidaemia, or chronic heart failure.
5. McKellar SH, Brown ML, Frye RL, et al. Comparison of coronary revascularization procedures in octogenarians: a systematic review and meta-analysis. Nat Clin Pract Cardiovasc Med 2008;5:738–46. https://doi.org/10.1038/ ncpcardio1348; PMID: 18825133.
6. Singh M, Peterson ED, Roe MT, et al. Trends in the association between age and in-hospital mortality after percutaneous coronary intervention national cardiovascular data registry experience. Circ Cardiovasc Interv 2009;2:20–6. https://doi.org/10.1161/circinterventions.108.826172; PMID: 20031689.
7. Batchelor WB, Anstrom KJ, Muhlbaier LH, et al. Contemporary outcome trends in the elderly undergoing percutaneous coronary interventions: results in 7,472 octogenarians. National Cardiovascular Network Collaboration. J Am Coll Cardiol 2000;36:723–30. https://doi.org/10.1016/s0735-1097(00)00777-4; PMID: 10987591.
8. Wang TY, Gutierrez A, Peterson ED. Percutaneous coronary intervention in the elderly. Nat Rev Cardiol 2011;8:79–90. https://doi.org/10.1038/nrcardio.2010.184; PMID: 21139558.
9. Johnman C, Oldroyd KG, Mackay DF, et al. Percutaneous coronary intervention in the elderly changes in case-mix and peri-procedural outcomes in 31 758 patients treated between 2000 and 2007. Circ Cardiovasc Interv 2010;3:341–5. https://doi.org/10.1161/circinterventions.109.928705; PMID: 20606133.
10. Arrant K. Ethical considerations when conducting research with older adults. Online Journal of Interprofessional Health Promotion 2020;2:Article 5. https://repository.ulm.edu/ojihp/ vol2/iss1/5
11. Arisha MJ, Ibrahim DA, Abouarab AA, et al. Percutaneous coronary intervention in the elderly: current updates and trends. Vessel Plus 2018;2:14. https://doi.org/10.20517/25741209.2018.29
PIELD Study JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Outcomes of Cardiac Arrest in Brunei Darussalam
1. Cardiac Centre, Raja Isteri Pengiran Anak Saleha (RIPAS) Hospital, Bandar Seri Begawan, Brunei; 2. Pengiran Anak Puteri Rashidah Sa’adatul Bolkiah (PAPRSB), Institute of Health Sciences, Universiti Brunei Darussalam, Bandar Seri Begawan, Brunei

Abstract
Cardiac arrest outcomes in Brunei Darussalam have not been previously reported. The mean (SD) time of arrest to cardiopulmonary resuscitation in hospital was 1.6 min (2.31 min; range 0–6 min) compared to the mean (SD) time of arrest to first cardiopulmonary resuscitation out of hospital of 19 min (23.91 min; range 0–87 min). The majority (71.2%) were out-of-hospital cardiac arrests (OHCA) (versus in-hospital cardiac arrests (IHCA)). One-year median survival for cardiac arrests was 1.4% which is below survival rates reported by other groups previously. Median 1-month survival rates were 25% for IHCA versus 5% for OHCA. Survival rates at 1 month for shockable rhythms were better (40%; p<0.001) for IHCA versus 16.7% for OHCA. Survival from cardiac arrests is low in Brunei Darussalam. This is associated with low rates of bystander cardiopulmonary resuscitation and may need to be addressed to improve outcomes.
Keywords
Cardiac arrest, resuscitation, VF, ventricular tachycardia
Acknowledgements: The authors thank the Ministry of Health, Brunei Darussalam, for giving permission for data collection.
Disclosure: The authors have no conflicts of interest to declare.
Received: 12 May 2022 Accepted: 20 May 2022 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e21. DOI: https://doi.org/10.15420/japsc.2022.21
Correspondence: Sofian Johar, Cardiac Centre, RIPAS Hospital, Jalan Putera Al-Muhtadee Billah, Bandar Seri Begawan BA 1712, Brunei Darussalam, Brunei. E: Sofianjohar@hotmail.com
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Almost one-third of the global population die of cardiovascular diseases annually, and cardiovascular disease is the second leading cause of mortality in Brunei after cancer.1 Survival rates can range from 0% to 42% for in-hospital cardiac arrests (IHCA) and are generally lower for out-ofhospital cardiac arrests (OHCA).2 This is the first report to determine the outcomes of cardiac arrests in Brunei.
A 3-year retrospective record review of patients who experienced cardiac arrest in the Brunei Muara district of Brunei Darussalam (January 2017 to December 2019) identified 145 patients who met the inclusion criteria (age >12 years). A further 34 patients were excluded (26 do-not-resuscitate patients, six trauma patients, two patients with missing outcomes).
The majority were Malay (82.1%), had hypertension (97%) and were male (66.2%). The mean age of this population was 50.3 years (SD 16.1). A ‘Utstein style’ template was used to collect data on the arrest, resuscitation, first cardiac rhythm and return of spontaneous circulation.2 R Studio V3.5.2 (The R Foundation) was used to analyse data, and p<0.050 was considered statistically significant.
Most arrests were OHCA (71.2%), and vital signs not recorded within the 3 h prior to the arrest. Although they were often witnessed by a lay person (73.1%), bystander cardiopulmonary resuscitation (CPR) was not performed in all cases. Emergency medical services were called in 81.4% of cases and 44% were resuscitated.
Time to treatment was faster for IHCA patients. The mean (SD) time of arrest to first CPR in hospital was 1.6 min (2.31 min; range 0–6 min), whereas the mean (SD) time of arrest to first CPR out-of-hospital was 19 min (23.91 min; range 0–87 min).
There was a higher median 1-month (27.3%) and 1-year (18.2%) survival in patients with shockable compared with non-shockable rhythms (p>0.005). Survival of IHCA patients with shockable rhythms (40% 1 month; 20% 1 year) was significantly higher than OHCA patients (16.7% 1 month/year; p<0.001). Overall survival rates were higher in IHCA patients (25% 1-month median survival) compared with OHCA patients (5% median 1-month survival; p<0.001).
There was a very low 1-year survival rate in this patient cohort (1.4%, n=2), which was below the ranges for OHCA observed in other countries in the Asia-Pacific region (2.7–8.0%), Europe (6.4–12.0%) and North America (2.8–5.3%), and IHCA (13%).2,3 This may be explained by a lack of bystander CPR and delays to the first defibrillation attempt, especially for OHCA. Survival rates were higher in the hospital setting, 40 out of 41 IHCA patients were witnessed and immediately resuscitated, in common with most published studies.2 Shockable rhythms (VF, ventricular tachycardia) had significantly higher survival rates to discharge than non-shockable rhythms, as this is more easily treated by defibrillation.
This is the first report of cardiac outcomes from Brunei following cardiac
General Cardiology SHORT COMMUNICATION © 2022 The Author(s). Published by Radcliffe Group Ltd. www.JAPSCjournal.com
Sofian Johar , 1 Nabilah Fadzilah Johani 2 and Anne Catherine Cunningham 2
arrest. A cardiac arrest registry to monitor resuscitation practices and outcomes, including implementation of a simple and standardised ‘Utstein’ template data collection form, is recommended to help improve outcomes.4 Further training and community engagement are needed to improve the observed very low rates of bystander resuscitation in Brunei Darussalam.
Clinical Perspective
• Survival rates for cardiac arrests in countries with low bystander rates of cardiopulmonary resuscitation can be low.
• As expected, outcomes for IHCA are better than OHCA with a shorter time of arrest to first cardiopulmonary resuscitation.
• The observed survival rates appear to be lower compared to data from the region and internationally, and concerted efforts may need to be undertaken involving multiple stakeholders in order to improve outcomes.
1. Ministry of Health, Brunei Darussalam. Health Information Booklet 2017 (Revised as of 11 December 2018). http://moh. gov.bn/Downloadables/Health%20Information%20 Bookler%202017%20(revised%20as%20of%20January%20 2019).pdf (accessed 13 May 2020).
2. Andersen LW, Holmberg MJ, Berg KM, et al. In-hospital cardiac arrest: a review. JAMA 2019;321:1200–10. https://doi. org/10.1001/jama.2019.1696; PMID: 30912843.
3. Yan S, Gan Y, Jiang N, et al. The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: a systematic review and meta-analysis. Crit Care 2020;24:61. https://doi.org/10.1186/ s13054-020-2773-2; PMID: 32087741.
4. Nolan JP, Berg RA, Andersen LW, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein resuscitation registry template for in-hospital cardiac
arrest: a consensus report from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia). Resuscitation 2019;144:166–77. https://doi. org/10.1016/j.resuscitation.2019.08.021; PMID: 31536777.
Cardiac Arrest in Brunei Darussalam JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Kengo Yasuda,1 Tomomi Watanabe , 2 Aiko Takami , 2 Toshihiko Akasaka,2 Yasushi Yoshikawa,3 Motonobu Nishimura 3 and Kazuhiro Yamamoto 2
1. Center for Clinical Residency Program, Tottori University Hospital, Yonago, Japan; 2. Division of Cardiovascular Medicine, Tottori University Hospital, Yonago, Japan; 3. Division of Cardiovascular Surgery, Tottori University Hospital, Yonago, Japan

Abstract
Right ventricular outflow tract obstruction was suspected in a 32-year-old man with repaired tetralogy of Fallot and borderline indications for surgical. Right heart catheterisation with a dobutamine stress test was useful in assessing the dynamics and severity of right ventricular outflow tract obstruction and in determining the treatment strategy.
Keywords
Tetralogy of Fallot, right ventricular outflow tract obstruction, right heart catheterisation, dobutamine stress test
Conflict of interest: KYam is on the Editorial Board of the Journal of Asian Pacific Society of Cardiology; this did not influence peer review. All other authors have no conflicts of interest to disclose.
Informed consent/consent to publish: The Ethics Committee of Tottori University Hospital has approved the publication of case reports on an opt-out basis. Thus, the need for written informed consent for this report was waived.
Acknowledgements: The authors thank Angela Morben from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Received: 26 January 2022 Accepted: 5 April 2022 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e22.
DOI: https://doi.org/10.15420/japsc.2022.03
Correspondence: Tomomi Watanabe, Division of Cardiovascular Medicine, Tottori University Faculty of Medicine, 36-1 Nishi-cho, Yonago 683-8504, Japan. E: t-wata@tottori-u.ac.jp
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
The survival rate of patients with repaired tetralogy of Fallot (TOF) has improved markedly, and most patients reach adulthood. Therefore, residual complications in adulthood have emerged as new problems among these patients. Right ventricular outflow tract obstruction (RVOTO) is one such residual complication, and the number of reoperations for TOF in adulthood has been increasing.1 However, the optimal timing of and indications for reoperation remain controversial. In this report we describe a patient with repaired TOF in whom right heart catheterisation (RHC) with a dobutamine stress test was useful in assessing the dynamics and severity of RVOTO, and determining the treatment strategy.
Case Report
A 32-year-old man presented to Tottori University Hospital with shortness of breath and syncope on exertion. He had been diagnosed with TOF at 1 month of age. At 1 year of age, he had undergone interventricular septal closure and right ventricular outflow tract (RVOT) plasty with a monocusp valved outflow patch (MVOP) at another hospital. Although he had regularly visited the Department of Paediatrics at the same hospital, he had stopped going to the hospital and taking medication at 24 years of age. Eight years later, he developed shortness of breath on exertion. One day, he developed syncope on his way home. He quickly regained consciousness and presented at Tottori University Hospital the next day.
Chest auscultation revealed a systolic murmur (Levine 3/6) from Erb’s point to the second left sternal border. Laboratory tests revealed no obvious abnormalities. The B-Type natriuretic peptide concentration was 7.3 pg/ml. An ECG revealed complete right bundle branch block, right axis deviation and QRS prolongation to 187 ms (Figure 1A). On transthoracic echocardiography, left ventricular (LV) systolic function was normal, and no residual shunt was detected. The estimated systolic right ventricular (RV) pressure at rest was approximately 50 mmHg, and narrowing of the RVOT at the MVOP was suspected although poorly visualised. The LV was compressed by the RV (Figure 2A). RV enlargement and slight impairment of contractility were observed. Pulmonary regurgitation was mild. A 24-h Holter ECG showed no serious arrhythmia. Contrast-enhanced CT showed a narrowing in the RVOT near the patch and calcification of the nearby endocardium on the epicardial side (Figure 1B,C). No significant narrowing was detected in the RV other than at the site of the MVOP. There were no atherosclerotic changes and no aortic enlargement. Cardiac MRI showed that the RV end-systolic volume index was 75.9 ml/m2 and the RV ejection fraction was 30.9%. The regurgitant fraction at the pulmonary valve was 15.7%.
Although we considered that the patient’s symptoms may have been caused by RVOTO, it was difficult to obtain clear echocardiographic images of the RVOT and to calculate the pressure gradient at the RVOT in this patient. RHC was planned to evaluate the pressure gradient between
© 2022 The Author(s). Published by Radcliffe Group Ltd. www.JAPSCjournal.com Clinical Cardiology CASE REPORT
Right Heart Catheterisation with Dobutamine Stress Test for Evaluation of Right Ventricular Outflow Tract Obstruction 30 Years After Surgical Repair of Tetralogy of Fallot
the RV and pulmonary artery (PA) to determine the indication for surgical treatment of the RVOTO.
The RV–PA systolic pressure gradient was recorded as a peak-to-peak pressure gradient of 25 mmHg. The RV/LV systolic pressure ratio was 0.32 (Table 1). Because these data at rest did not meet the criteria for reoperation,2 we added a dobutamine stress test during RHC to evaluate the severity of RVOTO, following a protocol for the stress test established by the Japanese Society of Echocardiography.3 Dobutamine infusion was
initiated at a rate of 5 μg/kg/min and increased at 5-min intervals to 10, 20, 30 and 40 μg/kg/min until one the following termination criteria is met: a target heart rate of (220 − age) × 0.85 (BPM); a significant decrease or increase in systolic blood pressure (≤80 or ≥220 mmHg); manifestation of significant tachyarrhythmia; or manifestation of intolerable symptoms. The dobutamine stress test was performed in the cath lab to facilitate an emergency response because the patient’s symptoms, including syncope, placed him at high risk.
Infusion of dobutamine at 10 μg/kg/min increased systolic RV pressure without changing PA pressure, and the RV–PA pressure gradient increased to 84 mmHg. In association with these haemodynamic changes, the patient felt the same symptoms as those on exertion, and the dobutamine stress test was terminated. Following termination of the dobutamine infusion, the patient’s symptoms improved and his systolic RV pressure decreased (Table 2). The stress test was not associated with serious arrhythmia.
Based on these findings, we considered that the indication for reoperation of RVOTO after TOF repair was met. The patient underwent pulmonary valve replacement and RVOT patch reconstruction. He was discharged 11 days postoperatively with no complications. His subjective symptoms during exertion improved significantly, and syncope did not recur. An exercise stress test was performed before discharge, and no symptoms were observed, even when the systolic blood pressure was around 200 mmHg. Echocardiography 3 months postoperatively showed that the RV overload was improved (Figure 2B) and that the tricuspid regurgitation pressure gradient had dropped to 22 mmHg. The RV diameter decreased from 43.7 to 31.9 cm.
Discussion
The most common cause of death in patients with repaired TOF is reportedly sudden cardiac death caused by ventricular tachycardia and fibrillation.4 Thus, the cause of syncope should be identified to avoid sudden cardiac death. In the present case, a 24-h Holter ECG revealed no serious arrhythmia, and resting RHC data did not meet the criteria for reoperation of RVOTO.
Because we suspected that the RV–PA pressure gradient may increase during exertion with reference to the patient’s medical history, we added a dobutamine stress test during RHC. This unmasked the changes in the severity of the RVOTO and reproduced the same symptoms as experienced by the patient during exertion without serious arrhythmia; therefore, it seemed reasonable to assume that the cause of the syncope and symptoms during exertion was RVOTO. Our strategy was supported by the relief of the patient’s symptoms after the reoperation. Medical treatment, such as a beta-blockers, may be suitable for mild symptoms due to dynamic obstruction. However, our patient’s symptoms were severe with syncope, and the effect of drug treatment was likely limited considering the mechanism of stenosis inferred by the CT imaging.
It was important to consider the mechanism underlying the changes in the severity of RVOTS to decide whether RVOT myectomy was required in addition to pulmonary valve replacement and RVOT patch reconstruction. In this case, CT showed calcification in the MVOP but no muscular obstruction in the RVOT. These findings were also confirmed intraoperatively.
LV outflow tract obstruction provoked by the dobutamine stress test has been reported as an independent predictor of future episodes of syncope.5 However, few reports have evaluated the usefulness of the
 RHC With Dobutamine Stress Test for RVOTO in an Adult TOF
RHC With Dobutamine Stress Test for RVOTO in an Adult TOF
JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Figure 1: ECG and Contrast-enhanced CT A I II III aVR aVL aVF V1 V2 V3 V4 V5 V6 B C A: The ECG revealed complete right bundle branch block, right axis deviation and QRS prolongation to 187 ms. B: Contrast-enhanced CT showed a narrowing in the outflow near the patch on the front of the right ventricle (arrow). C: The narrowest part (dashed line with white arrow in B) was 12.3 mm × 23.2 mm, with a cross-sectional area of 273 mm2 Figure 2: Echocardiography Before and After Reoperation A B A: Echocardiography on admission showed that the left ventricle was compressed by right ventricular overload. B: The right ventricular overload was improved after the reoperation. Table 1: Right Heart Catheterisation at Rest Measurement Item Numerical Value SBP/DBP (mmHg) 143/73 Mean PAWP (mmHg) 9 PAP (mmHg) Systolic 22 Diastolic 10 RVP (mmHg) Systolic 47 End-diastolic ~12 Mean RAP (mmHg) 9 Cardiac output (l/min) 6.3 Cardiac Index (l/min/m2) 3.43 DBP = diastolic blood pressure; PAP = pulmonary artery pressure; PAWP = pulmonary artery wedge pressure; RAP = right atrial pressure; RVP = right ventricular pressure; SBP = systolic blood pressure.
stress test to assess RVOTO, and guidelines provide no recommendations for the stress test in adults with congenital heart diseases. Hasan et al. reported that exercise stress echocardiography was useful in evaluating the effects of labile RVOTO on RV function in patients with repaired congenital heart diseases, most of which were TOF.6 Although stress echocardiography is less invasive than catheter-based examinations, this method is only applicable to patients in whom echocardiographic assessment of RVOT is feasible. An exercise stress test may be more physiological than a dobutamine stress test; however, life-threatening arrhythmia is a contraindication to an exercise stress test.3 Thus, a dobutamine stress test was used in our patient with syncope suspected to be due to potentially fatal arrhythmia.
Although a consensus has not been achieved regarding the risk factors for sudden cardiac death in patients with repaired TOF, several studies have suggested that RV dysfunction, RV enlargement and elevated RV systolic pressure are risk factors.7 Labile RVOTO is likely to elevate RV systolic pressure on a daily basis, resulting in RV enlargement and dysfunction. Postoperative echocardiography in our case showed a decrease in RV volume and systolic pressure. Thus, in addition to the relief of dyspnoea and syncope on exertion, the reoperation for RVOTO may have helped prevent sudden death in our patient.
When considering the dobutamine stress test for adults with TOF, the risk of a sudden rise in blood pressure should be kept in mind, because such patients may have advanced aortic disease for their age.8 Even if there is no history of hypertension or obvious aortic enlargement, close attention should be paid to the dose of dobutamine, considering the potential aortopathy associated with TOF.
Mizuno A, Niwa
PMID: 24042321.
Matsuo
of
2. JCS Joint Working Group. Guidelines for management of congenital heart diseases in adults (JCS 2017). 2018 [In Japanese]. https://www.j-circ.or.jp/cms/wp-content/ uploads/2020/02/JCS2017_ichida_h.pdf (accessed 27 October 2021).
3. Suzuki K, Hirano Y, Yamada H, et al. Practical guidance for the implementation of stress echocardiography. J Echocardiogr 2018;16:105–29. https://doi.org/10.1007/s12574018-0382-8; PMID: 29876799.
4. Nollert GDA, Däbritz SH, Schmoeckel M, et al. Risk factors for sudden death after repair of tetralogy of Fallot. Ann Thorac Surg 2003;76:1901–5. https://doi.org/10.1016/s00034975(03)01065-8; PMID: 14667608.
Recent studies have suggested the usefulness of novel methods to evaluate the severity of repaired TOF.9,10 Future studies are necessary to establish the indications for reoperation in patients with and without RVOTO.
Conclusion
with a dobutamine stress test revealed the severity of labile RVOTO not found at rest in a patient with repaired TOF, and the stress test reproduced his symptoms on exertion. The results of the stress
were helpful in determining the need for reoperation, and the reoperation resulted in an improvement in the patient’s symptoms, with a decrease in RV systolic pressure and volume. When RVOTO is suspected to cause symptoms and/or progression of RV dysfunction but the indications for reoperation are not met, a stress test may be useful in evaluating the effects of labile RVOTO.
Clinical Perspective
of reoperations in adulthood increasing.
reoperation
cover all patients who are
terms of prevention of sudden death
be
when
met
5. Dawn B, Paliwal VS, Raza ST, et al. Left ventricular outflow tract obstruction provoked during dobutamine stress echocardiography predicts future chest pain, syncope, and near syncope. Am Heart J 2005;149:908–16. https://doi.org/10.1016/j.ahj.2004.07.029; PMID: 15894976.
6. Hasan BS, Lunze FI, McElhinney DB, et al. Exercise stress echocardiographic assessment of outflow tract and ventricular function in patients with an obstructed right ventricular-to-pulmonary artery conduit after repair of conotruncal heart defects. Am J Cardiol 2012;110:1527–33. https://doi.org/10.1016/j.amjcard.2012.07.013; PMID: 22858182.
7. Silka MJ, Bar-Cohen Y. A contemporary assessment of the risk for sudden cardiac death in patients with congenital heart disease. Pediatr Cardiol 2012;33:452–60. https://doi. org/10.1007/s00246-012-0165-3; PMID: 22311569.
8. Mongeon FP, Gurvitz MZ, Broberg CS, et al. Aortic root dilatation in adults with surgically repaired tetralogy of Fallot. A multicenter cross-sectional study. Circulation 2013;127:172–79. https://doi.org/10.1161/ CIRCULATIONAHA.112.129585; PMID: 23224208.
9. Gusseva M, Hussain T, Friesen CH, et al. Biomechanical modeling to inform pulmonary valve replacement in tetralogy of Fallot patients after complete repair. Can J Cardiol 2021;37:1798–807. https://doi.org/10.1016/j. cjca.2021.06.018; PMID: 34216743.
10. Gusseva M, Hussain T, Friesen CH, et al. Prediction of ventricular mechanics after pulmonary valve replacement in tetralogy of Fallot by biomechanical modeling: a step towards precision healthcare. Ann Biomed Eng 2021;49:3339–48. https://doi.org/10.1007/s10439-02102895-9; PMID: 34853921.
RHC With Dobutamine Stress Test for RVOTO in an Adult TOF JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
In this study, RHC
RHC
Table 2: Right Heart Catheterisation With Dobutamine Stress Test Stress Heart Rate (BPM) SBP/DBP (mmHg) PAP (mmHg) RVP (mmHg) Symptoms Systolic Diastolic Systolic End-diastolic Before infusion 71 143/73 17 10 51 ~10 Dobutamine infusion (μg/kg/min) 5 70 149/77 17 9 55 ~9 10 88 216/103 19 11 103 ~11 + After dobutamine discontinuation 2 min 84 182/83 17 11 94 ~12 + 5 min 88 170/67 18 5 73 ~21 + 7 min 86 143/64 17 10 54 ~9 DBP = diastolic blood pressure; PAP = pulmonary artery pressure; RVP = right ventricular pressure; SBP = systolic blood pressure.
• RVOTO is a residual complication in patients with repaired TOF, with the number
• Although current guidelines provide indications for
of RVOTO, they do not necessarily
eligible for reoperation in
and preservation of RV function. • Dobutamine stress RHC may
useful
RVOTO is suspected to be the cause of serious symptoms but the indications for reoperation are not
during the examination at rest. 1.
K,
K, et al. Survey of reoperation indications in tetralogy
Fallot in Japan. Circ J 2013;77:2942–7. https://doi.org/10.1253/circj.CJ-13-0673;
Riyadh Qasim,1 Khalid Alkatout,2 Fatema Qaddoura1 and Ayman Nagib 1
1. Cardiology Department, Cardiac Center, King Fahd Military Medical Complex, Dhahran, Saudi Arabia; 2. Faculty of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia

Abstract
A 65-year-old female known to have type 2 diabetes, with no significant past medical history, had presented to another hospital with a 2-week history of progressive dyspnoea, orthopnoea and, lower limb oedema. Echocardiography revealed a large pericardial effusion with echocardiographic signs of increased intrapericardial pressure. An emergency pericardiocentesis was performed. After 1 week, the patient was admitted to King Fahd Military Medical Complex (Dhahran, Saudi Arabia) because of re-accumulation of the large pericardial effusion, which mandated another pericardiocentesis. CT of the chest revealed enlarged mediastinal lymph nodes. Video-assisted thoracic surgery was performed to obtain a pericardial window and a biopsy from the mediastinal lymph nodes, which revealed non-caseating granuloma, highly suggestive of sarcoidosis. A few days after surgery the patient experienced a neurogenic bladder, acute renal shutdown, and metabolic acidosis, during which she developed stress-induced cardiomyopathy that improved a few days later. The patient’s symptoms improved within 2 weeks after receiving corticosteroids.
Keywords
Cardiac sarcoidosis, pericardial effusion, cardiac tamponade, echocardiography, pericardiocentesis, stress-induced cardiomyopathy
Disclosure: The authors have no conflicts of interest to declare.
Informed consent/consent to publish: Written informed consent was obtained from the patient.
Received: 7 March 2022 Accepted: 4 May 2022 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e23. DOI: https://doi.org/10.15420/japsc.2022.10
Correspondence: Ayman Nagib, King Fahd Military Medical Complex, PO Box 31932, Dhahran, Saudi Arabia. E: draymannaguib@gmail.com
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Sarcoidosis is a granulomatous disease of unknown aetiology, the pathological hallmark of which is non-caseating granulomas. Sarcoidosis can affect various organs, including the heart.1 Cardiac involvement by sarcoidosis (CS) can affect any portion of the heart, including the pericardium, atria, ventricles, papillary muscles and valves. Clinical presentation of CS varies widely, from asymptomatic to decompensated heart failure, heart block, and malignant arrhythmias.2 CS has various phenotypes in cardiac imaging with either ventricular septum thinning, especially in the basal portion, ventricular wall thickening, dilatation, regional wall motion abnormalities, valvular dysfunction or, rarely, pericardial effusion. This makes the diagnosis of CS challenging in many clinical settings.3
Case Report
A 65-year-old female known to have type 2 diabetes, on oral hypoglycaemic medications with no other significant past medical history, presented with a 2-week history of the gradual onset and progressive course of dyspnoea, orthopnoea, dry cough and lower limb oedema, as well as reduced appetite and lethargy. She had no other cardiac complaints and was admitted to another hospital. Clinically, the patient had signs of cardiac tamponade and echocardiography revealed a large pericardial effusion with echocardiographic signs of increased
intrapericardial pressure. An emergency pericardiocentesis was performed and the patient was discharged after 3 days, following improvement of her symptoms and follow-up echocardiography that revealed minimal pericardial effusion. One week later, the patient presented to King Fahd Military Medical Complex (Dhahran, Saudi Arabia) with recurrence of her initial symptoms. The physical examination revealed a low-bodyweight female lying in a semiseated position with respiratory distress, tachypnoea, tachycardia (heart rate 120 BPM), hypotension (blood pressure 80/40 mmHg) and engorged jugular veins. Local cardiac examination revealed distant heart sounds and decreased breath sounds at both lung bases. Urgent echocardiography revealed a large circumferential pericardial effusion with echocardiographic signs of cardiac tamponade. Urgent pericardiocentesis, with a drain of approximately 1.5 l, was performed.
Investigations
ECG showed sinus tachycardia, left axis deviation, low voltage and poor R wave progression on precordial leads. Initial echocardiography showed normal left ventricle systolic function, large pericardial effusion with signs of increased intrapericardial pressure (Supplementary Material Video 1 and 2; Figures 1 and 2). Pericardial fluid analysis showed clear serous fluid, where cells were mainly lymphocytes, remarkable exudative
© 2022 The Author(s). Published by Radcliffe Group Ltd. www.JAPSCjournal.com General Cardiology CASE REPORT
Rare Presentation of Cardiac Sarcoidosis With Recurrent Large Pericardial Effusion and Stress-induced Cardiomyopathy
effusion, with no malignant cells, and negative for acid-fast bacillus and tuberculosis polymerase chain reaction. The CT coronary angiogram showed non-significant coronary artery disease. A CT of the chest showed bilateral symmetrical hilar mediastinal lymphadenopathy and bilateral pleural effusion. Lymph node (LN) biopsy showed a picture of nonnecrotising granulomatous lymphadenitis. Cardiac MRI (CMR) was performed 3 months after the acute illness because of its unavailability at time of hospitalisation as a result of the COVID-19 pandemic. CMR revealed diffuse patchy epicardial late gadolinium enhancement (LGE) at the basal to mid-inferior, inferolateral and lateral myocardial walls with patchy mesocardium at the basal septum.
Differential Diagnosis
The differential diagnosis of this condition includes myopericarditis, cardiac sarcoidosis, tuberculosis and lymphoma.
Management
After the second pericardiocentesis and a review of the chest CT findings of with the thoracic surgeon in a multidisciplinary team discussion, the decision was made to undertake video-assisted thoracic
surgery. Biopsies were was taken from the mediastinal LN and the pericardium; a pericardial window was also performed. The patient was then started on oral corticosteroid therapy 25 mg once daily, ibuprofen and colchicine, and was discharged home to be followed up as an outpatient.
However, 5 days after discharge, the patient presented with anuria and shortness of breath. Her initial laboratory work-up revealed severe metabolic acidosis and acute renal injury. She was readmitted to the intensive care unit. A urogenital ultrasound did not reveal any obstruction. The urology team’s assessment was that the underlying cause was acute urine retention, secondary to urogenic autonomic bladder. A urinary catheter was placed and the patient’s condition improved.
During the intensive care unit admission because of acute kidney injury, echocardiography revealed mild left ventricular systolic dysfunction and new regional wall motion abnormalities in the form of hypokinesia and ballooning of the apical segments; other segments were hyperkinetic (Figure 3; Supplementary Material Video 3, 4 and 5).
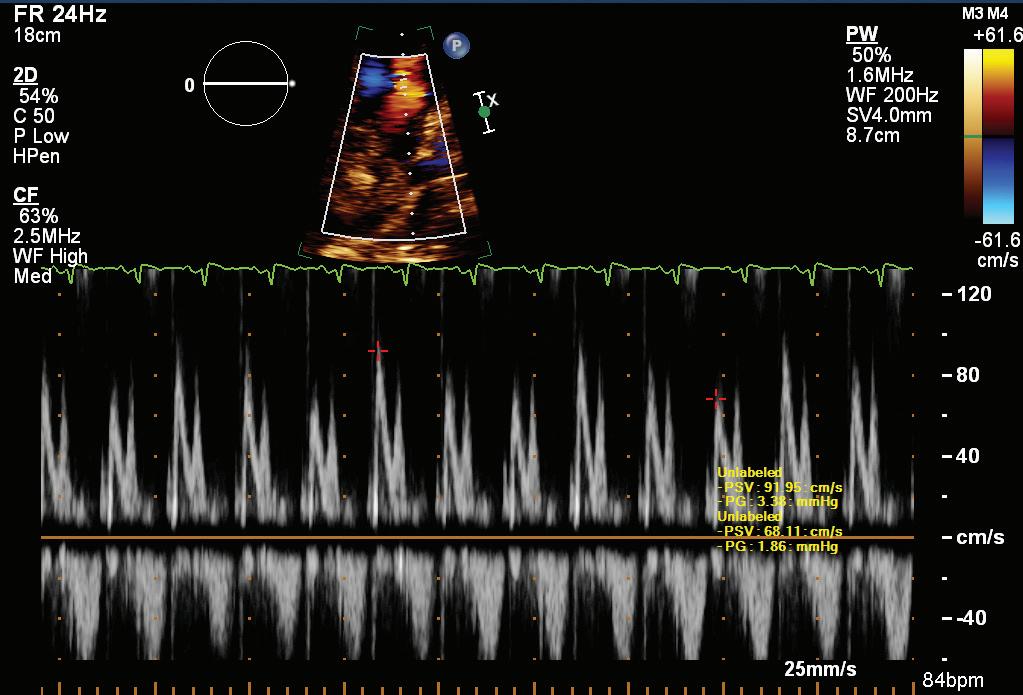
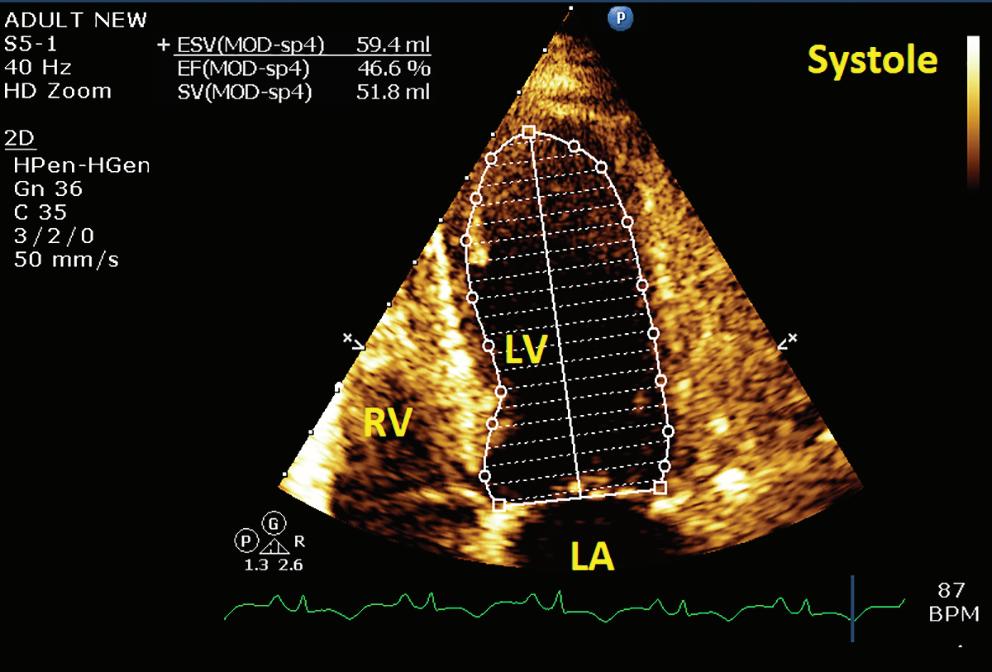
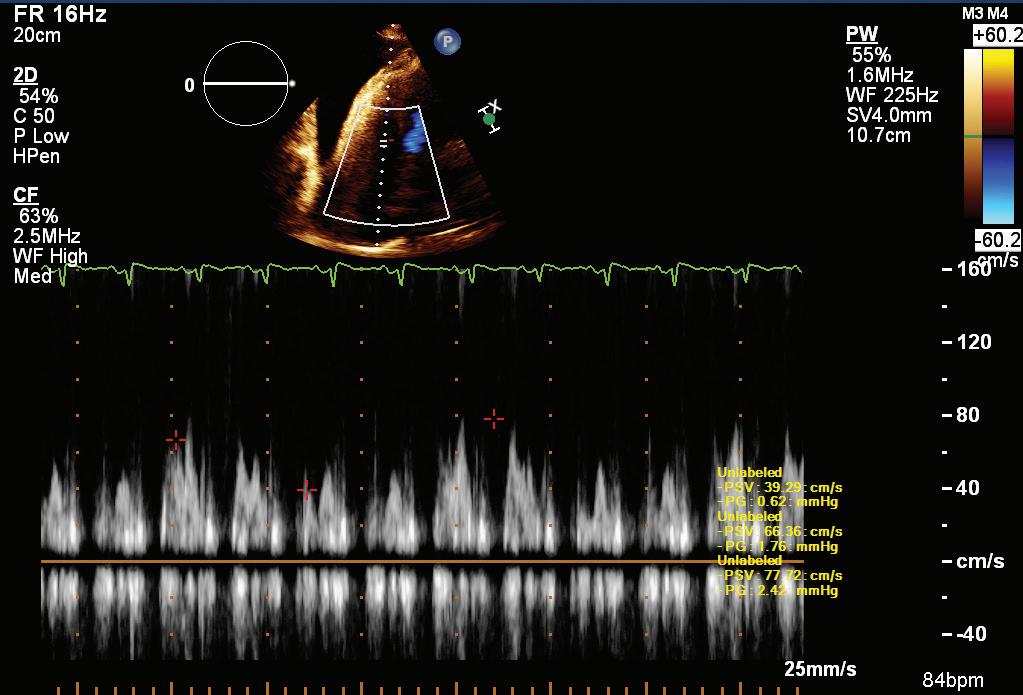
A few days after resolution of metabolic acidosis and normalisation of kidney function tests, echocardiography was repeated and revealed
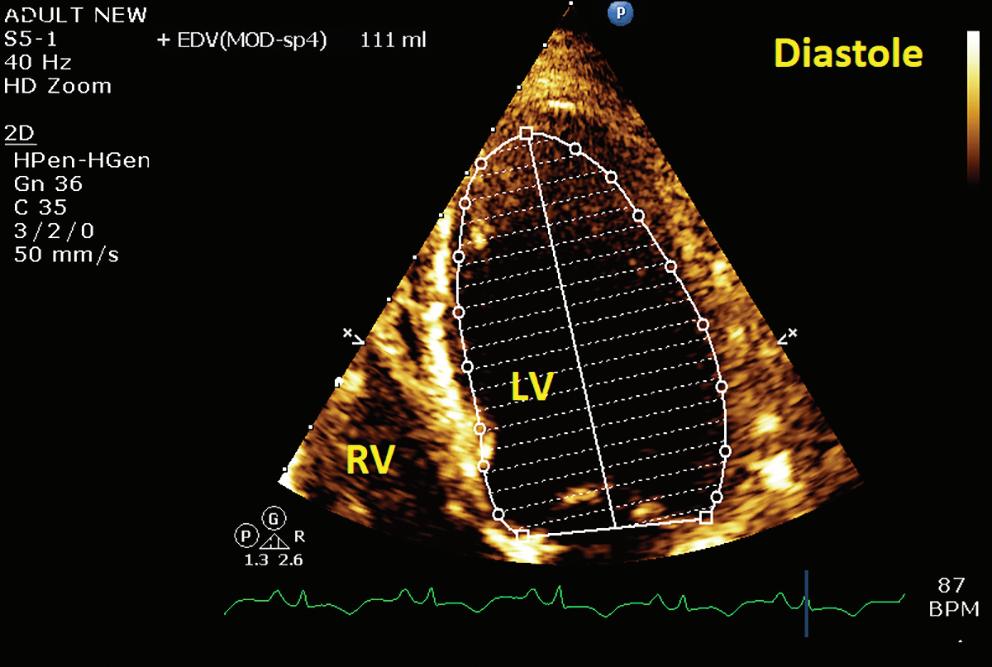
Cardiac Sarcoidosis, Pericardial Effusion and Stress-induced Cardiomyopathy JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Figure 1: Pulsed Wave Doppler Over the Tricuspid Valve Showing Significant Respiratory Variations
Figure
3: Apical Four-chamber View Showing Mild Left
Ventricular
Systolic Dysfunction
by the Modified Simpson’s Method
Figure 2: Pulsed Wave
Doppler
Over the Mitral Valve Showing Significant Respiratory Variations A B PG = pressure gradient; PSV = peak systolic velocity; PW = pulsed wave. A: End-diastolic frame; B: end-systolic frame. EDV = end diastolic volume; EF = ejection fraction; ESV = end systolic volume; LA = left atrium; LV = left ventricle; RV = right ventricle; SV = stroke volume. PG = pressure gradient; PSV = peak systolic velocity; PW = pulsed wave.
normal left ventricular systolic function and normal regional wall motion (Supplementary Material Video 6 and 7).
Discussion
This report presents a rare case of cardiac sarcoidosis with recurrent large pericardial effusion causing tamponade. Echocardiography played an important role in establishing this diagnosis; the significant respiratory variations in wave Doppler over tricuspid and mitral valves provided an indication of increased intrapericardial pressure and, in the clinical context of distress and haemodynamic instability, mandated the rapid performance of pericardiocentesis. The recurrence of the large pericardial effusion after 1 week is also a rare finding, reported in very few cases.4,5 Believing in the importance of investigating unexplained pericardial effusions, especially if large, symptomatic and recurrent, we undertook many investigations. Laboratory results did not help in our search for the specific aetiology of pericardial effusion in our patient. However, the presence of mediastinal lymphadenopathy and histopathology of the LN biopsy were major factors in establishing the diagnosis. The absence of significant coronary artery disease by cardiac CT helped rule out underlying coronary artery disease. The CMR findings were non-specific; this could be attributed to the lateness (after 3 months) of the imaging or
1. Birnie DH, Nery PB, Ha AC, Beanlands RS. Cardiac sarcoidosis. J Am Coll Cardiol 2016;68:411–21. https://doi.org/10.1016/j.jacc.2016.03.605; PMID: 27443438.
2. Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014;11:1305–23. https://doi.org/10.1016/j.hrthm.2014.03.043; PMID: 24819193.
3. Terasaki F, Azuma A, Anzai T, et al. JCS 2016 guideline on diagnosis and treatment of cardiac sarcoidosis – digest version. Circ J 2019;83:2329–88. https://doi.org/10.1253/circj.CJ-19-0508; PMID: 31597819.
due to the nature of the disease and heterogeneity of the LGE affection pattern.6,7 During the clinical course, the patient developed acute renal shut down and severe metabolic acidosis; echocardiography showed a picture of stress-induced cardiomyopathy with transient ballooning of the apical segments, which improved to normal after 2 days. This is rare and, to the best of our knowledge, has not been reported before in such a clinical context.
Follow-up
During clinical follow-up 2 months later, the patient showed good improvement of her symptoms and complete recovery of acute renal failure. The patient was on steroids for 2 months, which were gradually tapered over 4 weeks, and she has remained symptom-free. The patient also had elevated blood glucose levels, for which she was started on insulin.
Conclusion
Pericardial effusion is a rare presentation of cardiac sarcoidosis and can be not only large enough to cause cardiac tamponade, but also recurrent. Stress-induced cardiomyopathy occurring in such a clinical context has not been reported previously.
4. Whiteside H, Jyothidasan A, Sorrentino R, et al. Cardiac sarcoidosis: an unusual cause of recurrent large pericardial effusion. J Am Coll Cardiol 2016;67:1073. https://doi.org/10.1016/S0735-1097(16)31074-9.
5. Navaneethan SD, Venkatesh S, Shrivastava R, et al. Recurrent pleural and pericardial effusions due to sarcoidosis. PLoS Med 2005;2:e63. https://doi.org/10.1371/journal.pmed.0020063; PMID: 15783254.
6. Okada DR, Bravo PE, Vita T, et al. Isolated cardiac sarcoidosis: a focused review of an under-recognized entity. J Nucl Cardiol 2018;25:1136–46.
https://doi.org/10.1007/s12350-016-0658-1; PMID: 27613395.
7. Smedema JP, Van Kroonenburgh MJ, Snoep G, et al. Cardiac sarcoidosis in a patient with hypertrophic cardiomyopathy demonstrated by magnetic resonance imaging and single photon emission computed tomography dual-isotope scintigraphy. Circulation 2004;110:e529–31.
https://doi.org/10.1161/01.CIR.0000149749.95902.A4; PMID: 15596554.
Cardiac Sarcoidosis, Pericardial Effusion and Stress-induced Cardiomyopathy JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
European Examination in Core Cardiology (APSC Exit Examination)
Jack WC Tan,1,2,3 Jonathan Yap,1,2 Khung Keong Yeo,1,2 Derek P Chew,4 Alan Yean Yip Fong,5,6 Caitlyn Tan,7 Abdul Shehab,8 Nguyen Ngoc Quang,9 Terrence Chua,1 Clive Lawson,10 Danny Mathysen,11,12 Stephanie Thibault,13 Wael Al Mahmeed14 and Chris Plummer15
1. Department of Cardiology, National Heart Centre Singapore; 2. Duke-NUS Medical School, Singapore; 3. Department of Cardiology, Sengkang General Hospital, Singapore; 4. Department of Cardiology, Flinders University College of Medicine and Public Health, Adelaide, Australia; 5. Department of Cardiology, Sarawak Heart Centre, Kota Samarahan, Sarawak, Malaysia; 6. Faculty of Medicine and Health Sciences, University Malaysia Sarawak, Kota Samarahan, Sarawak, Malaysia; 7. National University of Singapore Yong Loo Lin School of Medicine, Singapore; 8. College of Medicine and Health Sciences, UAE University, Al Ain, United Arab Emirates; 9. Department of Cardiology, Hanoi Medical University, Hanoi, Vietnam; 10. Department of Cardiology, Maidstone and Tunbridge Wells NHS Trust, Maidstone, UK; 11. Department of Ophthalmology, Antwerp University Hospital, Edegem, Belgium; 12. Faculty of Medicine and Health Sciences, University of Antwerp, Wilrijk (Antwerp), Belgium; 13. European Society of Cardiology (ESC), Biot, France; 14. Heart and Vascular Institute, Cleveland Clinic, Abu Dhabi, United Arab Emirates; 15. Department of Cardiology, Freeman Hospital, The Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK
Keywords
European Examination in Core Cardiology (EECC), Asian Pacific Society of Cardiology (APSC), Asia–Pacific, exit examination, core cardiology
Disclosure: JWCT is on the Editorial Board of the Journal of Asian Pacific Society of Cardiology; this did not influence peer review. JWCT has received honoraria from AstraZeneca, Bayer, Amgen, Medtronic, Abbott Vascular, Biosensors, Alvimedica, Boehringer Ingelheim and Pfizer; research and educational grants from Medtronic, Biosensors, Biotronik, Philips, Amgen, AstraZeneca, Roche, Otsuka, Terumo and Abbott Vascular; and consulting fees from Elixir, CSL Behring and Radcliffe Publishing. JY is an Associate Editor of the Journal of Asian Pacific Society of Cardiology; this did not influence peer review. KKY is Editor-in-Chief of the Journal of Asian Pacific Society of Cardiology; this did not influence peer review. KKY has received institutional research funding from Medtronic, Boston Scientific, Amgen, AstraZeneca and Shockwave Medical; consulting or honoraria fees from Medtronic, Boston Scientific, Abbott Vascular, Amgen, Bayer and Novartis; and speaker or proctor fees from Abbott Vascular, Boston Scientific, Medtronic, Philips, Shockwave Medical, Alvimedica, Menarini, AstraZeneca, Amgen and Bayer. DPC is a Deputy Editor of the Journal of Asian Pacific Society of Cardiology; this did not influence peer review. DPC has received consulting fees from the Asian Pacific Society of Cardiology (APSC); support for travel to meetings for the study or otherwise from the APSC; grants/grants pending from Roche Diagnostics and AstraZeneca; and payment for the development of educational presentations, including service on speakers’ bureaus, from AstraZeneca AYYF is an Associate Editor and NNQ and WAM are on the Editorial Board of the Journal of Asian Pacific Society of Cardiology; this did not influence peer review. All other authors have no conflicts of interest to declare.
Acknowledgements: The authors thank Ivan Olegario for medical writing support.
Received: 5 April 2022 Accepted: 7 June 2022 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e24. DOI: https://doi.org/10.15420/japsc.2022.15
Correspondence: Jack Wei Chieh Tan, Department of Cardiology, National Heart Centre, 5 Hospital Drive, Singapore 169609. E: jack.tan.w.c@singhealth.com.sg
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
The European Examination in Core Cardiology (EECC), known as the European Examination in General Cardiology prior to 2020, is a joint venture between the European Society of Cardiology (ESC), the Union of European Medical Specialists Cardiology Section and participating national cardiac societies. The EECC is also supported by independent academic oversight.1
In 2012, the ESC launched the first pilot examination with 80 volunteers from Ireland, the Netherlands, Portugal, Spain and UK; since 2013, there have been annual sittings of the European Examination in General Cardiology/ EECC with the participation of the ESC National Cardiac Societies network.
The purpose of the examination is to provide a broad, balanced and upto-date test of the core knowledge required by cardiology specialty trainees for independent practice.1
The examination assesses knowledge from current evidence-based guidelines and published research, and has recently been aligned with
the ESC Core Curriculum for the Cardiologist, published in 2020.2 The examination is intended to complement workplace-based assessments as part of a comprehensive cardiology training programme, as well as to facilitate progressive improvement and harmonisation of cardiology training and clinical practice.1 Participating cardiac national societies use the EECC in different ways to support the medical education of cardiology trainees in their country. Trainees are advised to take the EECC once they have completed core training, so that unsuccessful candidates may have the opportunity to re-sit the examination before the end of their overall training.1
The Asian Pacific Society of Cardiology (APSC) is an umbrella organisation representing 22 cardiology societies in the Asia–Pacific region.3 Due to the heterogeneity of the region, the completion of cardiology training and entry into continuous professional development varies across the region. There is no uniform cardiology subspecialty training, and no uniform exit examination to assess the core cardiology knowledge of trainees.

General Cardiology EDITORIAL © 2022 The Author(s). Published by Radcliffe Group Ltd. www.JAPSCjournal.com
In 2020, the APSC approached the ESC to pilot the EECC, and to determine how the APSC could participate and support the deployment of the examination and its administration in the region for cardiac societies that are part of the APSC but may not be able to join the EECC on their own. Examinees from member countries of the APSC are nominated to take the same EECC examination as their Western counterparts. The examinees are advised to take the examination anytime within 6 months before to 6 months after their own country’s general cardiology exit examination.
In this article we describe the development and administration of the pilot EECC (APSC Exit Examination). The examination results of the APSC examinees over the first 3 years of implementation will be published in a future paper. This analysis is intended to benchmark the knowledge of cardiology trainees as part of the evaluation of the quality of training in the region and to guide future plans for the improvement and harmonisation of cardiology training.
Methodology
Examination Development
Every year, the writing of the EECC (APSC Exit Examination) starts with question writing meetings in August of the preceding year (August 2019 for the first pilot examination) and the following January (Figure 1). The examination questions are written and edited by groups of cardiologists representing their national cardiology societies and the APSC, all of whom come from all subspecialties within cardiology. The examination questions test knowledge that is mapped to the ESC core curriculum, in line with current guidelines and published clinical studies. The questions cover the following four sections:
• imaging and valvular heart disease;
• rhythm disorders;
• coronary artery disease, acute cardiovascular care, prevention, rehabilitation and sports; and
• heart failure and cardiac patients in other settings.
Each question comprises a short clinical scenario and a single multiple-choice question with five possible answers shown in alphabetical order (Table 1).4
A board meeting is held in January of the examination year to review the policies and procedures for the examination and, in February, cardiologists select 120 questions across all 62 topics of the core curriculum (30 questions from each examination section).4 Seventy per cent of the selected questions are text only and 30% contain a still image or short video clip.
After all 120 questions have been chosen, a standard-setting group reviews each question using a modified Angoff method to estimate the probability that a candidate who would just pass the examination will select the correct answer, and these assessments are used to inform the final pass mark.5 In May, all questions for the final examination are reviewed by the examination board chair and the chair of the standardsetting group to ensure that there are no errors before the examination is held in June.4
Examination Delivery and Pass Mark Determination
Countries participating in the EECC register in September in the year preceding the examination and need to start registering their candidates in November. The ESC manages all confirmations and instructions to the candidates up to the delivery, in June, of the 3-h online examination with remote proctoring.
After the examination, the performance of each question is reviewed. Questions where <30% or >90% of candidates answered correctly, or those where there was a negative correlation with candidates’ performance in the overall examination are reviewed by representatives of participating national societies. This is to ensure that the answer key was correct, that the question was not misleading and that the question tested an important point of cardiology knowledge.4 Any question that does not pass this assessment is excluded from the examination (historically, the number of questions excluded has been very low due to the robust quality assurance methods used in question development).
The marks are then given to the independent psychometrician and the EECC board to determine the pass mark. Using the Hofstee method, the
APSC Exit Examination JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Figure 1: Gantt Chart of the Pilot European Examination in Core Cardiology (Asian Pacific Society of Cardiology Exit Examinations) Year 1 Year of examination Aug. Sep. Oct. Nov. Dec. Jan. Feb. Mar. Apr. May June July Examination development Question writing Board meeting Question selection Standard setting Final review Examination delivery Country registration Candidate registration Examination sat Board meeting to set pass mark
Table 1: Example Question
A 42-year-old man was admitted to the Emergency Department with a 60-min history of central chest pain. He had no significant past medical history and no abnormal physical signs. His ECG showed 3 mm ST-elevation in V2–V6, I and aVL. He was given analgesia, 300 mg of aspirin and 180 mg of ticagrelor. There were no on-site facilities for PCI, so emergency transfer was arranged to the regional heart attack centre with an expected transfer time of 130 min.
What is the most appropriate additional treatment prior to transfer?
A: Bivalirudin
B: Full-dose fibrinolysis
C: Glycoprotein IIb/IIIa receptor antagonist
D: Glycoprotein IIb/IIIa receptor antagonist plus half-dose fibrinolysis
E: Half-dose fibrinolysis
B: Full-dose
pass mark is expected to fall in the rectangle formed on a graph of pass rate (percentage of candidates passing the examination) against pass mark (the percentage of questions answered correctly for a candidate to pass), bounded by the expected pass rates of 75–95%, and 2SDs around the mean expected pass mark determined by the standard-setting group.6
The final pass mark is the x-coordinate at the intersection between the diagonal across the rectangle and the plot of the candidates’ performance (Figure 2).
European Society of Cardiology. European exam in core cardiology (EECC). 2020.
European-Exam-in-Core-Cardiology-(EECC) (accessed 22 February 2022).
2. European Society of Cardiology. ESC curricula. 2020.
core-curricula#:~:text=The%202020%20Core%20
and%20patients (accessed 22 February 2022).
3. Asian Pacific Society of Cardiology. About APSC. 2022.
Schematic Diagram of the Hofstee Method of Determining the Exit Examination Pass Mark
Conclusion
The APSC pilot with the EECC is an opportunity to evaluate the core cardiology knowledge of trainees as part of the evaluation of the quality of training in the Asia–Pacific region, and is a starting point in harmonising the practice of evidence-based clinical cardiology among APSC member countries. The EECC examination is conducted to international standards and established standard-setting methodologies to ensure the validity of results in testing core cardiology knowledge. The methodology described in this article may be used for reference by future examinees, as well as cardiology trainers in the Asia–Pacific region.
(accessed 22 February 2022).
4. Plummer C, Bowater S, Hall J, et al. Behind the scenes of the European examination in general cardiology. Heart 2019;105:889–90.
https://doi.org/10.1136/heartjnl-2018-314495; PMID: 30712001.
5. Angoff WH. Scales, norms and equivalent scores. In: Thorndike RL, Angoff WH, Lindquist EF; American Council on Education, eds. Educational Measurement. 2nd ed. Washington, DC: American Council on Education; 1971;508–600.
6. Hofstee WKB. The case for compromise in educational selection and grading. In: Anderson SB, Helmick JS, eds. On Educational Testing. Washington, DC: Jossey-Bass; 1983;109–2.
7. Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J 2018;39:119–177. https://doi.org/10.1093/eurheartj/ehx393; PMID: 28886621.
APSC Exit Examination JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Correct answer
fibrinolysis7 aVL = augmented vector left; PCI = percutaneous coronary intervention. Pass mark (%) Pass rate (%) 95% 75% 2SD around expected pass mark 100 90 80 70 60 50 40 30 20 0 800 10 20 30 40 50 60 70 90 100 Adjusted pass mark Figure 2:
1.
https://www.escardio.org/Education/Career-Development/
https://www.escardio.org/Education/esc-and-subspecialty-
Curriculum%20for,Community%2C%20trainees%2C%20
https://www.apscardio.org/about/index.html
Wan Azman Wan Ahmad , 1 Edouard Benit , 2 Cyril Camaro , 3 Elvin Kedhi,4 Saman Rasoul,5,6 Lucia Barbieri , 7 Jacques Lalmand,8 René J van der Schaaf,9 Tian H Koh , 10 Arnoud W van‘t Hof,5,6 Stephen W Lee , 11 Vincent Roolvink,12 Marc A Brouwer,3 Giuseppe De Luca7 and Harry Suryapranata3
1. Department of Medicine, University Malaya Medical Centre, Kuala Lumpur, Malaysia; 2. Department of Cardiology, Jessa Ziekenhuis, Hasselt, Belgium; 3. Department of Cardiology, Radboud University Medical Centre, Nijmegen, the Netherlands; 4. Department of Cardiology, AZ Sint-Jan, Brugge, Belgium; 5. Department of Cardiology, Maastricht University Medical Center, Maastricht, the Netherlands; 6. Department of Cardiology, Zuyderland Atrium Medical Center, Heerlen, the Netherlands; 7. Department of Cardiology, AOU Maggiore della Carità, Eastern Piedmont University, Novara, Italy; 8. Department of Cardiology, Centre Hospitalier Universitaire, Charleroi, Belgium; 9. Department of Cardiology, Onze Lieve Vrouwe Gasthuis, Amsterdam, the Netherlands; 10. Department of Cardiology, National Heart Center, Singapore, Singapore; 11. Department of Cardiology, Queen Mary Hospital, University of Hong Kong, Hong Kong, China; 12. Department of Cardiology, Isala Hospital, Zwolle, the Netherlands

Abstract
Background: This study aimed to assess differences in clinical characteristics and outcomes between Asian and European patients treated with the COMBO dual therapy stent in the REDUCE trial. Methods: In the REDUCE trial, 1,496 patients who underwent successful COMBO stent implantation were randomised to 3- or 12-month dual antiplatelet therapy groups. Of these, 449 (30%) were Asian and 1,047 (70%) were European. Given that no significant differences were observed between the 3- and 12-month dual antiplatelet therapy cohorts for both sites, the overall data of Asian patients were compared with those of European patients in terms of clinical characteristics and outcomes. Results: The Asian cohort was younger and comprised fewer women than the European cohort. They also had more cases of type 2 diabetes (33.0% versus 15.1%), hypercholesterolaemia (54.6% versus 41.7%) and hypertension (55.7% versus 48.5%) but had fewer previous interventions compared with European patients. Regarding procedures, Asian patients had more cases of infarct-related vessel in the left anterior descending artery. The primary endpoint indices among Asian patients at 90, 180, 360 and 720 days were lower than those among European patients, which can be attributed to the lower number of cases of target-vessel revascularisation, bleeding (Bleeding Academic Research Consortium classification II, III or V) and MI among the Asian cohort. Conclusion: Despite having a higher incidence of cardiovascular risk factors, more ST-elevation MI cases and more cases of culprit lesion located at the proximal left anterior descending artery, Asian patients in the REDUCE trial showed lower trends of target-vessel revascularisation, bleeding and MI than European patients in the REDUCE trial.
Keywords
COMBO stent, Asian, European, demographic, outcome
Conflict of interest: The authors have no conflicts of interest to declare.
Informed consent/consent to publish: All patients have given written informed consent for the main REDUCE trial from which the data were collected.
Data Availability Statement: The data were provided by Diagram B.V, Zwolle, the Netherlands. The unpublished data are from the subgroup analysis report Asian Sites 12 February 2020 by Diagram B.V. Restrictions apply to the availability of these data, which were used under licence for this study. Data are available from the authors with the permission of Diagram BV.
Ethics approval: As this is a post-hoc subgroup analysis of existing trial data, research ethics approval is not needed. The main REDUCE trial from which the data were collected has been approved by the respective ethics committee of each site.
Trial registration number: NCT02118870
Authors’ contributions: Conceptualisation: WAWA, EK, GDL, HS; data curation: WAWA, EK, GDL, HS; formal analysis: GDL; funding acquisition: GDL, HS; investigation: EB, SR, RJdS, GDL, HS; methodology: WAWA, HS; project administration: EB, HS; resources: HS; software: HS; supervision: GDL, HS; validation WAWA, SR; visualisation: GDL; writing – original draft preparation: WAWA; writing – review & editing: EB, CC, SR, LB, JL, RJdS, THK, AWH, SWL, VR, MAB.
Received: 24 January 2022 Accepted: 11 May 2022 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e25. DOI: https://doi.org/10.15420/japsc.2022.02
Correspondence: Wan Azman Wan Ahmad, Department of Medicine, University Malaya Medical Centre, Kuala Lumpur, Malaysia, 59100. E: wanazman@ummc.edu.my
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
The COMBO stent (OrbusNeich Medical) is a novel stent comprising an abluminal coating of a biodegradable polymer for eluting sirolimus and a luminal prohealing anti-CD34+ antibody layer for capturing circulating
endothelial progenitor cells and providing rapid endothelialisation. This novel technology may shorten the duration of dual antiplatelet therapy (DAPT) after stenting. Evaluation of clinical outcomes after COMBO stent
© 2022 The Author(s). Published by Radcliffe Group Ltd. www.JAPSCjournal.com Intervention TRIAL ANALYSIS
Are There Differences in the Demographics and Clinical Outcomes Between Asian and European Patients Treated With the COMBO Dual Therapy Stent in the REDUCE Trial Populations?
implantation in randomised trials and large, prospective, multicentre registries showed low clinical event rates and the noninferiority of the COMBO stent versus ‘first in class’, second-generation and newergeneration drug-eluting stents (DES).1–5
The demographics and patterns of diseases differ between patients from the Asia-Pacific region and Europe. The results of coronary angiography reveal that Asia-Pacific patients have relatively smaller coronary arteries but longer lesion length than European patients.6 In addition, more recent antithrombotic treatment trials have shown that Asia-Pacific patients have a higher risk of bleeding (particularly gastrointestinal bleeding and haemorrhagic stroke) than European patients.7–9
With the advent of newer-generation DES, real-world clinical data comparing patient profile, procedure details and clinical outcomes between Asian and European patients remain scarce. Thus, this study aimed to assess these regional differences in the era of contemporary percutaneous coronary intervention (PCI) practice.
Methods
The REDUCE (Short-term Dual Anti Platelet Therapy in Patients With ACS Treated With the COMBO Dual-therapy Stent) trial (NCT02118870) is an investigator-initiated prospective, multicentre, randomised trial involving patients with acute coronary syndrome (ACS) after treatment with COMBO stent randomised to either 3 or 12 months of DAPT. The primary study endpoint was a composite of all-cause mortality, MI, stent thrombosis, stroke, target-vessel revascularisation (TVR) and bleeding at 12 months. The data management team of Diagram B.V. (Zwolle, the Netherlands), a contract research organisation specialising in tailored cardiovascular research, provided the data for this post-hoc analysis. These unpublished data (not included in the main paper of the REDUCE trial and not published elsewhere) were obtained from the subgroup analysis report of Asian sites dated 12 February 2020 using Diagram B.V. (Supplementary Material). The main study was designed to enrol 1,500 patients with ACS from 36 investigational sites in Europe and Asia. The Asian sites were Malaysia (252), Singapore (81), Indonesia (60) and Hong Kong (57), with a total of 450 patients, and the European sites included the Netherlands (589), Belgium (254), Italy (150), Germany (31), Poland (16) and Hungary (10), with a total of 1,050 patients.
The REDUCE protocol, which describes the study design and randomisation, ethics approval, treatment and follow-up procedures, study endpoints and statistical consideration, was published in the American Heart Journal, with the trial results published in EuroIntervention in 2019.5,10 The main inclusion criteria were patients with a diagnosis of ACS (ST-elevation MI [STEMI], non-STEMI [NSTEMI], or unstable angina), successful COMBO stent implantation (thrombolysis in MI [TIMI] III flow with residual stenosis of <20% based on visual estimation) and no clinical adverse events during hospitalisation (death, stent thrombosis, stroke, TVR, bleeding with Bleeding Academic Research Consortium [BARC] classification II, III and V). The main exclusion criteria were patients with cardiogenic shock, recent major bleeding complications or contraindication to DAPT, oral anticoagulation requirement, bleeding diathesis history, stroke or transient ischaemic attack history within the last 6 months, gastrointestinal or genitourinary bleeding history within the last 2 months and/or major surgery within 6 weeks.
Of the 1,500 eligible patients with ACS who underwent successful COMBO stent implantation, four revoked their informed consent soon after randomisation, leaving 1,496 patients for analysis. Of these, 449 (30%)
Trial
were Asian patients and 1,047 (70%) were European patients. The overall patient data were compared between the two sites.
Patient and Public Involvement Statement
The subgroup analysis of this study using patient data from the main REDUCE trial did not involve the patients and the public.
Statistical Methods
Cumulative event rates were estimated using the Kaplan–Meier method and compared using the log-rank test. The incidence of events was compared using chi-square or Fisher’s exact test. Student’s t-test was used for normally distributed variables and Mann–Whitney U-test for nonnormally distributed variables. Categorical variables were summarised using number and percentages and compared using two-sided chi-square tests or Fisher’s exact test, as applicable.
Results
At Asian sites, the 3-month DAPT cohort showed a higher proportion of hypercholesterolaemia cases than the 12-month DAPT cohort (59.6% versus 49.6%; p=0.0319). The number of cases of infarct-related vessel (IRV) was also significantly higher in the 3-month cohort than in the 12-month cohort (p=0.0078), with IRV in the left anterior descending (LAD) artery contributing to the largest difference (67.7% versus 56.6%). At European sites, the 12-month DAPT cohort comprised more women compared with the 3-month cohort (27.0% versus 19.1%; p=0.0026). Other patient characteristics, procedure details and discharge data showed no significant differences between these cohorts for both Asian and European sites.
Moreover, the Asian cohort was younger and showed a higher prevalence of conventional cardiovascular risk factors (type 2 diabetes, hypercholesterolaemia and hypertension) and STEMI, but a lower prevalence of known family history of coronary artery disease (CAD), ACS, PCI and coronary artery bypass graft (CABG) surgery than the European cohort. Furthermore, Asian patients had higher Killip classification levels upon arrival at the PCI centre as well as lower weight and BMI, higher serum creatinine and glucose levels and worse lipid profiles than European patients (Table 1).
In terms of the procedure, Asian patients had a notably higher number of IRV cases in the LAD artery and at the proximal segment of LAD than European patients. Furthermore, these Asian patients had lower rates of initial TIMI III flow and radial site access and a higher incidence of balloon pre-dilatation and post-dilatation before and after stent deployment. Nonetheless, the PCI success rate (approximately 100%) and the number and length of stents used were similar between the two geographical groups. The incidence of using additional devices, such as those for embolic protection and thrombosuction, was more frequent among Asian patients than among European patients (Table 2).
The length of hospital stay was similar between the two geographical groups. On discharge, fewer Asian than European patients were administered with calcium antagonists, β-blockers, oral anticoagulants and proton pump inhibitors. Asian patients were twice as likely to be receiving diabetic medications (insulin or oral antidiabetic medications) than the European patients (Table 3).
Outcomes
Figure 1 shows data regarding the number of patients available for the outcome analysis at various time intervals.
Asian Versus European Patients Treated With COMBO Stent in the REDUCE
JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Age (years),
Sex (male:female),
77.0%:23.0% <0.0001
Diagnosis for hospitalisation, n (%): <0.0001
• STEMI 277/449 (61.7%) 429/1,046 (41.0%)
• Non-STEMI 126/449 (28.1%) 446/1,046 (42.6%)
• Unstable angina 46/449 (10.2%) 171/1,046 (16.3%)
Type 2 diabetes, n (%) 148/449 (33.0%) 158/1,045 (15.1%) <0.0001
Hypercholesterolaemia, n (%) 245/449 (54.6%) 434/1,041 (41.7%) <0.0001
Hypertension, n (%) 250/449 (55.7%) 504/1,039 (48.5%) <0.0001
Family history of CAD, n (%) 101/449 (22.5%) 424/1,031 (41.1%) 0.0111
Previous ACS (before current ACS), n (%) 40/449 (8.9%) 142/1,046 (13.6%) <0.0001
Previous PCI, n (%) 34/449 (7.6%) 128/1,047 (12.2%) 0.0114
Previous CABG, n (%) 7/449 (1.6%) 35/1,047 (3.3%) 0.0079
Previous CVA, n (%) 10/449 (2.2%) 16/1,047 (1.5%) 0.0556
Killip class upon arrival at PCI centre, n (%): 0.3430
• Class I 331/422 (78.4%) 972/1,040 (93.5%) <0.0001
• Class II 75/422 (17.8%) 54/1,040 (5.3%)
• Class III 5/422 (1.2%) 9/1,040 (0.9%)
• Class IV 11/422 (2.6%) 4/1,040 (0.4%)
Weight (kg), mean ± SD 71.40 ± 13.59 82.83 ± 15.37 BMI (kg/m²), mean ± SD 26.18 ± 4.64 27.35 ± 4.19 <0.0001
Creatinine (µmol/l), mean ± SD 88.84 ± 26.27 83.88 ± 25.00 <0.0001
Glucose (mmol/l), mean ± SD 8.33 ± 3.95 7.54 ± 3.16 <0.0001
HbA 1c (mmol/l), mean ± SD 9.03 ± 1.19 8.98 ± 0.94 0.0028
Total cholesterol (mmol/l), mean ± SD 5.22 ± 1.33 5.04 ± 1.17 0.0860
HDL cholesterol (mmol/l), mean ± SD 1.03 ± 0.25 1.13 ± 0.36 0.0003
LDL cholesterol (mmol/l), mean ± SD 3.48 ± 1.28 3.05 ± 1.02 <0.0001
ACS
The composite primary endpoint indices for all-cause mortality, MI, stent thrombosis, stroke, TVR and bleeding (BARC II, III, or V) were lower among Asian patients than among European patients at 90, 180, 360 and 720 days (1.3% versus 2.6%, 2.7% versus 5.1%, 6.9% versus 8.9% and 9.2% versus 13.0%, respectively). This decrement among Asian patients is attributed to the lower number of cases of TVR (0.2% versus 0.7%, 0.7% versus 1.8%, 2.2% versus 3.9% and 2.9% versus 5.6%), bleeding (BARC II, III, or V) (0.7% versus 1.2%, 1.1% versus 2.1%, 2.5% versus 2.8% and 3.4% versus 3.7%) and MI (0.2% versus 0.6%, 0.7% versus 1.3%, 2.0% versus 2.1% and 2.7% versus 3.5%) at 90, 180, 360 and 720 days, respectively. However, no difference was found in allcause mortality and cardiac mortality between Asian and European patients. Although PCI was performed in patients with ACS, the incidence of stent thrombosis was extremely low in both groups (Table 4).
Discussion
Using the data of the main REDUCE trial, this study showed that among patients with ACS treated with the COMBO stent, the primary outcomes (all-cause mortality, MI, stent thrombosis, stroke, TVR and bleeding [BARC II, III, or V]) were not different between the 3- and 12-month DAPT cohorts
during follow-up at 1 and 2 years.5 This analysis provides a unique opportunity to compare the differences in the clinical characteristics, procedures and outcomes of patients with ACS in the era of newgeneration DES. Moreover, it provides an opportunity for comparing contemporary practices between the Asian sites (Malaysia, Singapore, Hong Kong and Indonesia), which share various similarities regarding ethnicity, patient profile, cultural and psychosocial factors and PCI procedures, and European sites (the Netherlands, Belgium, Italy, Germany, Poland and Hungary), which also share these similarities.
Our data revealed that the Asian cohort was younger, comprised a higher number of women, had a higher prevalence of cardiovascular risk factors, a lower prevalence of family history of CAD and a higher incidence of STEMI at presentation than the European cohort. Furthermore, Asian patients showed lower prevalence of history of ACS and revascularisation (PCI and CABG) than European patients. In addition, Asian patients showed a higher Killip classification level at presentation, with higher serum creatinine and blood glucose levels and worse lipid profiles than European patients. The incidence of the culprit segment being located at the proximal LAD was also higher among Asian patients than among European patients. Asian patients also had a lower rate of initial TIMI III
Asian Versus European Patients Treated With COMBO Stent in the REDUCE Trial JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Table 1: Patient Characteristics and Investigation Asian Sites Overall (n=449) European Sites Overall (n=1,047) p-value
mean ± SD 56.14 ± 11.76 62.85 ± 11.29 <0.0001
% 86.9%:13.1%
= acute coronary syndrome; CABG = coronary artery bypass graft; CAD = coronary artery disease; CVA = cerebrovascular accident; PCI = percutaneous coronary intervention; STEMI = ST-elevation MI. Source: Reproduced with permission from Diagram B.V.
Table 2: Procedure Characteristics at Asian and European Sites
Asian Sites Overall (n=449) European Sites Overall (n=1,047) p-value
Vessel disease, n (%) 0.985
• One 291/449 (64.8%) 661/1,047 (65.0%)
• Two 119/449 (26.5%) 275/1,047 (26.3%)
• Three 39/449 (8.7%) 91/1,047 (8.7%)
Infarct-related vessel, n (%)
• RCA 118/449 (26.3%) 361/1,047 (34.7%) 0.002
• LAD 279/449 (62.1%) 410/1,047 (39.2%) <0.001
• LAD proximal segment 195/449 (43.4%) 187/1,047 (17.9%) <0.001
• CX 48/449 (10.7%) 262/1,047 (25.0%) <0.001
• Left main 2/449 (0.4%) 0/1,047 (0.0%) 0.090
• Graft 2/449 (0.4%) 12/1,047 (1.1%) 0.252
Initial TIMI Flow 3, n (%) 156/448 (34.8%) 546/1,021 (53.5%) <0.001
Access site radialis, n (%) 317/449 (70.6%) 814/1,029 (79.1%) <0.001
Balloon pre-dilatation, n (%) 329/449 (73.3%) 709/1,045 (67.8%) 0.037
Number of stents used, n (%) 0.156
• One 359/449 (80.0%) 871/1,047 (83.2%)
• Two 80/449 (17.8%) 145/1,047 (13.8%)
• Three 8/449 (1.8%) 28/1,047 (2.7%)
• Four 2/449 (0.4%) 3/1,047 (0.3%)
Total stent length (mm) mean ± SD 28.47 ± 14.49 24.00 ± 11.67 <0.001
Post-dilatation, n (%) 349/449 (77.7%) 511/1,046 (48.9%) <0.001
PCI successful (culprit lesion), n (%) 447/449 (99.6%) 1,042/1,047 (99.5%) >0.950
Additional devices used, n (%) 92/449 (20.5%) 145/1,046 (13.9%) 0.001
Additional segments dilated during hospitalisation, n (%) 109/449 (24.3%) 206/1,047 (19.7%) 0.045
Statin,
Calcium
n
430/449 (95.8%) 984/1,045 (94.3%)
42/449 (9.4%) 143/1,045 (13.7%)
β-blocker, n (%) 347/449 (77.3%) 898/1,045 (85.9%)
inhibitor, n (%) 293/449 (65.3%) 649/1,045 (62.1%) 0.25 Angiotensin II blocker, n (%) 34/449 (7.6%) 108/1,045 (10.3%) 0.10
6/449 (1.3%) 21/1,045 (2.0%)
flow before PCI. In terms of procedure, Asian patients had a lower rate of radial site access but had higher rates of balloon pre-dilatation and postdilatation and the use of additional devices. This finding reflects the differences in practice between Asian and European PCI operators. Nevertheless, the procedural success rates and length of hospital stay were similar between the two geographical groups. At discharge, Asian patients were administered with fewer prescriptions of β-blockers, oral anticoagulants and proton pump inhibitors and were twice as likely to be receiving diabetic medication. The primary endpoint indices at 90, 180, 360 and 720 days were also lower among Asian patients than among European patients, which can be attributed to lower TVR, bleeding (BARC II, III or V) and MI cases among Asian patients. However, all-cause mortality, cardiac mortality and stent thrombosis were similar between the two geographical cohorts.
oral
Insulin, n (%) 44/449 (9.8%) 57/1,045 (5.5%) <0.1 Oral antidiabetic, n (%) 113/449 (25.2%) 117/1,045 (11.2%) <0.1 Proton pump inhibitor, n (%) 190/449 (42.3%) 668/1,045 (63.9%) <0.1
ACE
from Diagram B.V.
In the e-HEALING worldwide registry substudy by Klomp M et al., differences in cardiovascular risk factors and clinical outcomes were evaluated between Western European and Southeast Asian patients treated with the Genous Bio-engineered R stent (OrbusNeich Medical Technologies).11 The primary study outcome was target-vessel failure (TVF), which is the composite of cardiac death or MI and TVR, at 12-month follow-up. Of 3,604 eligible patients, 2,873 (80%) were European and 731 (20%) were Southeast Asian. The findings are similar to those of our study, which used patient data from the REDUCE trial. For instance, Southeast Asian patients were younger compared with European patients (mean
Asian Versus European Patients Treated With COMBO Stent in the REDUCE Trial JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
CX = circumflex; LAD = left anterior descending artery; PCI = percutaneous coronary intervention; RCA = right coronary artery; TIMI = thrombolysis in
myocardial
infarction. Source: Data reproduced
with
permission from Diagram B.V. Table 3: Hospital Stay and Medications at Discharge at Asian and European Sites Asian Sites Overall (n=449) European Sites Overall (n=1,047) p-value Hospital stay (days), mean ± SD 2.71 ± 3.53 2.65 ± 2.52 0.28 Medications at discharge
n (%)
0.23
antagonist,
(%)
0.02
<0.1 ACE
Oral anticoagulant or non-vitamin K
anticoagulant, n (%)
0.37
= angiotensin-converting enzyme inhibitor. Source: Data reproduced with permission
age 57.4 ± 9.9 versus 65.9 ± 11.2 years, p<0.01), with fewer women (15% versus 25%, p<0.01). Southeast Asian patients also had a higher incidence of type 2 diabetes (36.0% versus 24%, p<0.01) and a lower prevalence of MI or revascularisation history. In comparison with European patients, their average lesion was longer but their vessel diameter was smaller. At 12-month follow-up, the primary endpoint of TVF was found in 11.4% of European patients and only 5.6% of Southeast Asian patients (p<0.01). This previous study concluded that differences exist in patient clinical profiles and outcomes between the European and Southeast Asian patients who have undergone PCI.11 This suggests that reports from studies worldwide should include both overall and regional subgroup outcomes.
A more recent study by Krackhardt et al.12 assessed the regional and ethnic differences in an unselected patient population treated with newer-generation DES (polymer-free sirolimus-eluting stents) in Asia and Europe. Of the 7,190 eligible patients, 3,186 (44.0%), 2,317 (32.0%), 1,274 (17.6%) and 413 (5.7%) were from the Mediterranean region, Central Europe, South Korea and Malaysia, respectively.
Their findings also showed differences in all cardiovascular risk factors across the regions. Although Central European patients were the oldest (68.0 ± 10.9 years) and Malaysian patients were the youngest (59.7 ± 10.9 years), the incidence of type 2 diabetes was markedly different, with rates of 48.7% in Malaysia and 40.1% in Central Europe. The Malaysian subgroup also showed longer lengths of lesions and stents than the European subgroup (22.8 ± 8.6 versus 17.4 ± 9.8 mm and 23.7 ± 7.1 versus 20.7 ± 10.3 mm, respectively). The pre-dilatation rate at the Asian sites (South Korea, 94.5%; Malaysia, 86.2%) was higher than that in Central Europe (58.9%). This difference is attributed to smaller vessel diameter and longer lesion length among Asian patients. In the combined ACS/elective CAD cohorts, the primary endpoint target-lesion revascularisation (TLR) was borderline significant (p=0.046) across regions, with 2.4%, 2.5%, 1.5% and 0.8% in the Mediterranean region, Central Europe, South Korea and Malaysia, respectively. The accumulated TLR rates for both ACS and stable CAD were significantly lower in Malaysia than in Central Europe (ACS, 1.2% versus 3.1%; stable CAD, 0.8% versus 2.1%).
Moreover, the ASPECT collaboration examined the variation in clinical characteristics and outcomes between patients undergoing PCI across the Asia-Pacific region.13 Of a total of 52,608 cases from 27 hospitals, 48.4% were from Malaysia, 7.7% from Singapore, 4.8% from Hong Kong and 39.1% from Australia. Asian patients were younger and comprised fewer women than Australian patients. They also had higher incidence rates of diabetes, dyslipidaemia and renal failure but showed a lower prevalence of family history of ACS. However, the procedural success rates were similar across the region (>95%).
In summary, available data from the registries and randomised controlled trials from the eras of bare-metal stents and contemporary DES show that patient clinical profiles, procedure findings and outcomes – particularly the TVR rate – are significantly different between Asian and European patients, which is consistent with the findings of this study. These differences are important factors that should be considered for developing risk-adjustment models to enable meaningful outcome comparisons and for establishing benchmarks for the site to aid in providing quality care to patients.
These differences may have been caused by several factors, including sociocultural differences between the two populations, different risk factors in different populations and potential genetic influences. The
Table 4: Patient Outcomes at Asian and European Sites
Asian Sites Overall (n=449), n (%)
All-cause mortality, MI, stent thrombosis, stroke, TVR or bleeding (BARC II, III, V):
European Sites Overall (n=1,047), n (%)
p-value
• 90 days 7/449 (1.6%) 27/1,037 (2.6%) 0.216
• 180 days 12/448 (2.7%) 53/1,032 (5.1%) 0.034
• 360 days 31/448 (6.9%) 92/1,030 (8.9%) 0.198
• 720 days 41/445 (9.2%) 132/1,015 (13.0%) 0.039
All-cause mortality:
• 90 days 2/449 (0.4%) 3/1,037 (0.3%) 0.641
• 180 days 3/448 (0.7%) 6/1,032 (0.6%) 1.000
• 360 days 8/448 (1.8%) 12/1,030 (1.2%) 0.336
• 720 days 12/445 (2.7%) 27/1,015 (2.7%) 1.000
Cardiac mortality:
• 90 days 1/449 (0.2%) 2/1,037 (0.2%) 1.000
• 180 days 1/448 (0.2%) 3/1,032 (0.3%) 1.000
• 360 days 5/448 (1.1%) 6/1,030 (0.6%) 0.325
• 720 days 8/445 (1.8%) 13/1,015 (1.3%) 0.476
Definite/probable stent thrombosis:
• 90 days 1/449 (0.2%) 4/1,037 (0.4%) 1.000
• 180 days 2/448 (0.4%) 7/1,032 (0.7%) 0.731
• 360 days 4/448 (0.9%) 8/1,030 (0.8%) 0.762
• 720 days 5/445 (1.1%) 13/1,015 (1.3%) 1.000
TVR:
• 90 days 1/449 (0.2%) 7/1,037 (0.7%) 0.448
• 180 days 3/448 (0.7%) 19/1,032 (1.8%) 0.103
• 360 days 10/448 (2.2%) 40/1,030 (3.9%) 0.119
• 720 days 13/445 (2.9%) 57/1,015 (5.6%) 0.032
Bleeding BARC II, III or V:
• 90 days 3/449 (0.7%) 12/1,037 (1.2%) 0.387
• 180 days 5/448 (1.1%) 22/1,032 (2.1%) 0.180
• 360 days 11/448 (2.5%) 29/1,030 (2.8%) 0.695
• 720 days 15/445 (3.4%) 38/1,015 (3.7%) 0.726
Stroke:
• 90 days 2/449 (0.4%) 1/1,037 (0.1%) 0.219
• 180 days 2/448 (0.4%) 1/1,032 (0.1%) 0.219
• 360 days 2/448 (0.4%) 3/1,030 (0.3%) 0.643
• 720 days 3/445 (0.7%) 3/1,015 (0.3%) 0.377
MI:
• 90 days 10/449 (0.2%) 6/1,037 (0.6%) 0.682
• 180 days 3/448 (0.7%) 13/1,032 (1.3%) 0.418
• 360 days 9/448 (2.0%) 22/1,030 (2.1%) 1.000
• 720 days 12/445 (2.7%) 36/1,015 (3.5%) 0.524
BARC = Bleeding Academic Research Consortium; TVR = target-vessel revascularisation. Source: Data reproduced with permission from Diagram B.V.
Asian Versus European Patients Treated With COMBO Stent in the REDUCE Trial JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Figure 1: Outcome Analysis at Different Time Intervals after Randomisation
Outcome analysis at time intervals
Asian at randomisation n=449
90 days n=449 patients
180 days n=448 patients
360 days n=448 patients
720 days n=445 patients
European at randomisation n=1,047
90 days n=1,037 patients
180 days n=1,032 patients
360 days n=1,030 patients
720 days n=1,015 patients
difference in the PCI practices between the two sites may also contribute to the different outcomes between the two populations. The Asian patients seem to have higher rates of using pre- and post-dilatational balloons and additional devices during the PCI. However, these potential contributors to such differences still require further in-depth studies to solve this issue.
Study Limitations
This study has several limitations. It is a subgroup analysis with its inherent limitations in data interpretation. The sample size was not
1. de Winter RJ, Chandrasekhar J, Kalkman DN, et al. 1-year clinical outcomes of all-comer patients treated with the dual-therapy COMBO stent: primary results of the COMBO collaboration. JACC Cardiovasc Interv 2018;11:1969–78. https://doi.org/10.1016/j.jcin.2018.04.038; PMID: 30286855.
2. Kalkman DN, Kerkmeijer LS, Woudstra P, et al. Three-year clinical outcomes after dual-therapy COMBO stent placement: insights from the REMEDEE registry. Catheter Cardiovasc Interv 2019;94:342–7. https://doi.org/10.1002/ ccd.28047; PMID: 30565371.
3. Kalkman DN, Chandrasekhar J, de Winter RJ, Mehran R. Current evidence for the safety and efficacy of the bioengineered dual therapy COMBO stent. Minerva Cardioangiol 2018;66:262–72. https://doi.org/10.23736/S00264725.18.04612-1; PMID: 29381028.
4. Saito S, Krucoff MW, Nakamura S, et al. Japan–United States of America Harmonized Assessment by Randomized Multicentre Study of OrbusNEich’s Combo StEnt (Japan–USA HARMONEE) study: primary results of the pivotal registration study of combined endothelial progenitor cell capture and drug-eluting stent in patients with ischaemic coronary disease and non-ST-elevation acute coronary syndrome. Eur Heart J 2018;39:2460–8. https://doi.org/10.1093/eurheartj/ ehy275; PMID: 29931092.
5. De Luca G, Damen SA, Camaro C, et al. Final results of the randomized evaluation of short-term dual antiplatelet
Trial
powered to determine the difference in the outcomes between Asian and European patients. Furthermore, the overall patient data in the 3and 12-month DAPT cohorts for the two geographical sites were consolidated to simplify the comparison; even though most of the mentioned parameters were similar, minor differences were found between the two cohorts. Finally, this study only included patients randomised to the REDUCE trial; thus, the result may not be applicable to the general population.
Conclusion
In the REDUCE trial, patient clinical characteristics, procedures and outcomes were not significantly different between Asian and European patients, given that all p-values were not significant on univariate analysis. However, Asian patients tended to have lower primary endpoint indices for all-cause mortality, MI, stent thrombosis, stroke, TVR and bleeding (BARC II, III or V) for up to 2 years, despite having higher incidence of cardiovascular risk factors, STEMI and the culprit lesion located at the proximal LAD artery.
Clinical Perspective
• Differences between Asian and European patients with acute coronary syndrome in the era of new-generation stents are not well understood.
• Previous studies noted differences in patient demographics, percutaneous coronary intervention practices and outcomes between Asian and European patients, which require further exploration.
• Our outcome question is whether these differences translate into poorer outcomes in Asian patients than in European patients.
• Clinical trial data of European patients should be interpreted with caution because of their possible differences from those of Asian patients.
therapy in patients with acute coronary syndrome treated with a new-generation stent (REDUCE trial). EuroIntervention 2019;15:e990–8. https://doi.org/10.4244/EIJ-D-19-00539; PMID: 31422929.
6. Ong PJ, Zeymer U, Waliszewski M, et al. Differences in clinical and angiographic profiles between Asian and Western patients with coronary artery disease: insights from the prospective “real world” paclitaxel-coated balloon registry. Int J Cardiol 2014;175:199–200. https://doi. org/10.1016/j.ijcard.2014.04.239; PMID: 24820752.
7. Hori M, Connolly SJ, Ezekowitz MD, et al. Efficacy and safety of dabigatran vs. warfarin in patients with atrial fibrillation-sub-analysis in Japanese population in re-LY trial. Circ J 2011;75:800–5. https://doi.org/10.1253/circj.cj-11-0191; PMID: 21436594.
8. Kohsaka S, Kimura T, Goto M, et al. Difference in patient profiles and outcomes in Japanese versus American patients undergoing coronary revascularization (collaborative study by CREDO-Kyoto and the Texas Heart Institute Research Database). Am J Cardiol 2010;105:1698–704. https://doi.org/10.1016/j.amjcard.2010.01.349; PMID: 20538117.
9. Misumida N, Ogunbayo GO, Kim SM, et al. Higher risk of bleeding in Asians presenting with ST-segment elevation myocardial infarction: analysis of the national inpatient sample database. Angiology 2018;69:548–54. https://doi. org/10.1177/0003319717730168; PMID: 28905638.
10. Camaro C, Damen SA, Brouwer MA, et al. Randomized evaluation of short-term dual antiplatelet therapy in patients with acute coronary syndrome treated with the COMBO dual therapy stent: rationale and design of the REDUCE trial. Am Heart J 2016;178:37–44. https://doi.org/10.1016/j. ahj.2016.04.016; PMID: 27502850.
11. Klomp M, Damman P, Beijk MAM, et al. Differences in cardiovascular risk factors and clinical outcomes between western European and southeast Asian patients treated with the Genous Bio-engineered R stent: an e-HEALING worldwide registry substudy. Coron Artery Dis 2012;23:271–7. https://doi.org/10.1097/MCA.0b013e328351aaed; PMID: 22473083.
12. Krackhardt F, Waliszewski M, Wan Ahmad WA, et al. Polymer-free sirolimus-eluting stent use in Europe and Asia: ethnic differences in demographics and clinical outcomes. PLOS ONE 2020;15:e0226606. https://doi.org/10.1371/journal. pone.0226606; PMID: 31929543.
13. Reid CM, Yan B, Wan Ahmad WA, et al. The Asia-Pacific Evaluation of Cardiovascular Therapies (ASPECT) collaboration - improving the quality of cardiovascular care in the Asia Pacific region. Int J Cardiol 2014;172:72–5. https:// doi.org/10.1016/j.ijcard.2013.12.030; PMID: 24480180.
Asian Versus European Patients Treated With COMBO Stent in the REDUCE
JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Laila Osama AbdelWareth
Pathology & Laboratory Medicine Department, The National Reference Laboratory, Abu Dhabi, United Arab Emirates

Keywords
High-sensitivity cardiac troponin, guidelines, non-ST-elevation MI
Disclosure: The author has no conflicts of interest to declare.
Acknowledgements: The author thanks Professor Kunji Inoue and Professor Jack Tan for sharing the data of their studies.
Received: 20 March 2022 Accepted: 11 August 2022 Citation: Journal of Asian Pacific Society of Cardiology 2022;1:e26. DOI: https://doi.org/10.15420/japsc.2022.09
Correspondence: Laila Osama AbdelWareth, Pathology & Laboratory Medicine Department, The National Reference Laboratory, Abu Dhabi, PO Box 92323, United Arab Emirates. E: WarethL@nrl.ae
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
The use of high-sensitivity cardiac troponin (hs-cTn) in the emergency department is adopted as the standard of care in many parts of the world. The higher sensitivity and analytical precision at the lower concentrations improved the ability of hs-cTn to accurately detect minor changes in troponin concentrations associated with myocardial injury, including ischaemic events.1 This has led to changes in the universal definition of MI to include evidence of elevation of cTn values with at least one value above the 99th percentile upper reference limit, as well as the detection of kinetic changes in cTn concentrations as a rise and/or fall of cTn values.2
Elevated hs-cTn values suggest injury to the myocardial cells, although this does not indicate the underlying cause of injury; thus, the differential diagnosis of elevated hs-cTn includes a huge list of ischaemic and nonischaemic causes of cardiac injury.1
Several multicentre clinical trials examined the usage of hs-cTn for the accelerated triaging of patients presenting to the emergency department (ED) with chest pain and particularly in the absence of conclusive ECG changes, mainly non-ST-elevation MI (NSTEMI). This has led to the incorporation of hs-cTn in various guidelines and consensus group recommendations, including the European Society of Cardiology (ESC) and the Asian Pacific Society of Cardiology (APSC).3,4 Both guidelines proposed algorithms for the rapid rule-in and rule-out of acute MI patients presenting to the ED with NSTEMI within 2–6 hours of symptoms.3,4
Shariff et al. reported that the Malaysian Expert Consensus Group are proposing a national guideline on the use of high-sensitivity troponins in the ED in Malaysia.5 The group recommended the use of a sex-specific 99th percentile upper reference limit (URL) as the basis to classify patients as ‘negative’ or ‘positive’ for hs-cTn, applying the History, Electrocardiogram, Age, Risk factors and Troponin risk score for the negative patients to decide on the final disposition of discharge or admission with repeat testing in the high-risk group. The absolute reference change value (RCV) in ng/l for the assay is recommended by the group to be used to determine
whether the difference between the 0- and 2-hour values is significant. The use of the 0-hour/3-hour algorithm is recommended if retesting within 2 hours is a challenge.
The ESC and APSC guidelines recommend 0-hour/1-hour, 0-hour/2-hour and 0-hour/3-hour algorithms based on the limit of detection of the assay, the 99th percentile URL, the magnitude of increase and the delta changes in serial measurements to categorise patients with suspected NSTEMI into rule-in, rule-out or observe categories with suggested final dispositions to either discharge, admit to the ward or admit to the cardiac observation unit.3,4
Shiozaki et al. examined the APSC and ESC algorithms performances in a prospective study of the ESC 0-hour/1-hour algorithm in Japan and Taiwan.6,7 Using the cohort data, 903 patients with a prevalence rate of acute MI of 13.1%, the utility of the ESC 0-hour/1-hour algorithm and the APSC algorithm were compared. Major adverse cardiovascular events consisted of all-cause mortality, subsequent acute MI and unexpected coronary revascularisation in 30 days. The study excluded ST-elevation MI and chronic kidney disease, defined as >3.0 mg/dl of serum creatinine level. The distribution pattern of the stratification was similar (ESC versus APSC; rule-out 23% versus 22%, observation 34% versus 33%, rule-in 43% versus 45%); however, with lower sensitivity compared with the ESC algorithm in this population. The sensitivity was 99.9% (95% CI [94.0–99.9]) versus 95.7% (95% CI [89.2–98.8]) and specificity 76.6% (95% CI [72.8–80.2]) versus 78.3% (95% CI [74.5–81.7]), respectively (unpublished data). Table 1 summarises the main differences between the APSC Expert Committee consensus recommendations, ESC guidelines and Malaysian Expert Consensus Group in the use of high-sensitivity cardiac troponin T (hs-cTnT).
As the global adoption of hs-cTn continues, the paper by the Malaysian Expert Consensus Group provides yet another different perspective for the application of the rapid rule-in and rule-out algorithms using hs-cTn in
Clinical Cardiology EDITORIAL © 2022 The Author(s). Published by Radcliffe Group Ltd. www.JAPSCjournal.com
High-sensitivity Troponins in the Emergency Department: Which Guideline to Recommend in Asia?
Rule-in AMI
Rule-out AMI
Observe
Types or rapid
Use of RCV
Use of sex-specific 99th
Algorithm
Algorithm
Use of risk score
on Asian
APSC ESC Malaysian
Hs-cTnT >70 ng/l or >5–70 ng/l with 1–3 h delta ≥5 ng/l
Hs-cTnT <5 ng/l or <14 ng/l with 1–3 h delta of <3 ng/l
Hs-cTnT 5–70 ng/l and delta 3 to <5 ng/l at 1–3 h
Hs-cTnT >52 or >12 ng/l with 1–3 h delta ≥5 ng/l
Hs-cTnT <limit of detection (5 ng/l) or <12 ng/l with 1–3 h delta of <3 ng/l
Hs-cTnT 12–52 ng/l and delta 3 to <5 ng/l at 1–3 h
Hs-cTnT >sex-specific 99th percentile or high risk score and 2–3 h delta >RCV
Hs-cTnT <sex-specific 99th percentile and low risk score
Not applicable
0/1 and 0/3 h 0/1, 0/2 and 0/3 h 0/2 and 0/3 h
Recommends the use of HEART risk score
Recommends the use of GRACE risk score models for estimating prognosis
Requires the use of HEART score for final disposition
Hs-cTn used Hs-cTnT Hs-cTnT and TnI Hs-cTnT and TnI
AMI = acute MI; APSC = Asian Pacific Society of Cardiology; ED = emergency department; ESC = European Society of Cardiology; GRACE = Global Registry of Acute Coronary Events; HEART = History, Electrocardiogram, Age, Risk factors and Troponin; Hs-cTn = high-sensitivity cardiac troponin; Hs-cTnT = high-sensitivity cardiac troponin T; RCV = reference change value; Malaysian = Malaysian Expert Consensus Group; TnI = Troponin I.
the ED setting to triage patients with acute coronary syndrome.5
National guidelines unify the practice recommendation for the country and decrease variations between hospitals considering the countries’ own disease prevalence and practice limitations. This also facilitates and streamlines the transfer of patients between various medical institutions within the country. The Malaysian Expert Census Group is to be congratulated on their effort to drive for standardisation of practice in ED triaging patients with suspected acute coronary syndrome across Malaysia.
One caveat in the proposed guideline is the use of the 99th percentile URL as ‘positive’ or ‘negative’, as this may lead to increased uptake due to chronic elevations of hs-cTn in some conditions, such as chronic renal failure. This dichotomous classification does not apply only to hs-cTn assays, and detection of kinetic changes is required to enhance test performance. Another point to highlight is the use of the RCV as an indicator of a significant change in hs-cTn concentrations. Decision thresholds include RCV of >20% from a baseline value >99th percentile URL; and a RCV >50% from a baseline value ≤99th percentile URL. If we would apply the proposed RCV for hs-cTnT assay with an overall 99th percentile URL of 19 ng/l, this would translate into >9.5 ng/l from a baseline value ≤99th percentile URL, which is different to the 3–5 ng/l delta proposed by the APSC consensus recommendation and the ESC.3,4
RCV incorporates both biological variation as well as analytical variations in determining whether the change in the serial concentrations is significant or not. Although this is a very valid analytical argument, this approach has not been validated in clinical studies, and the values are different to the delta changes recommended by the ESC and APSC guidelines, which were tested and validated in several prospective clinical studies. The RCV for hs-cTnT is calculated to be between 20% and 44% by various authors using various platforms to measure hs-cTnT.8 In addition, biological variations will vary in individuals with chronic kidney diseases compared with the healthy individuals from which most of the data on biological variation are derived.
The use of age-specific 99th percentile thresholds is another difference between the Malaysian group recommendations and the APSC and ESC guidelines. The use of age-specific cut-offs is not required in the APSC and ESC guidelines, as they both use the limit of detection of the assay as the basis for rule-out, which is much lower than the 99th percentile reference limit, and this was validated prospectively on mixed cohorts.
Regarding the incorporation of the History, Electrocardiogram, Age, Risk factors and Troponin score for risk stratification, although it is a good recommendation and especially for those with ‘negative’ work-up to better identify patients at increased risk of cardiovascular events, it may not add much to the classification of patients based on hs-cTn and clinical judgement. In a comprehensive prospective study of the performance of chest pain scores in the ED in Singapore, although the History, Electrocardiogram, Age, Risk factors and Troponin score had the highest sensitivity of 88.1% (95% CI [81.5–92.6]) among the chest pain scores, it was extremely comparable with clinical judgement, at 85.5% sensitivity (95% CI [78.3–90.6]), with an overall area under curve value of 0.794.9
Similar to the APSC, the use of point-of-care cardiac troponin assays in the ED was not encouraged due to the lower sensitivity and negative predictive values of those point-of-care testing assays compared with hscTn assays, although there are newer devices in the pipeline that may have promising analytical sensitivity and performance.10
In summary, the paper from the Malaysian Expert Census Group provides a modified approach to the APSC and ESC for triaging patients with acute coronary syndrome for the ED in Malaysia.5 The proposed algorithm remains to be validated in a large population and its clinical performance compared with the algorithm proposed by other guidelines. The APSC guidelines, thus, remain the most recommended guidelines to follow in the Asia-Pacific region when using hs-cTnT, followed by the ESC when using high-sensitivity cardiac troponin I.
High-sensitivity Troponins in the ED: Which Guideline to Recommend in Asia? JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com
Table 1: Main Differences Between the Asian Pacific Society of Cardiology Expert Committee Consensus Recommendations, European Society of Cardiology Guidelines and Malaysian Expert Consensus Group
protocols
No No Yes
percentile cut-offs No No Yes
validated on ED cohorts Yes Yes No
validated
cohorts Yes No No
1. Januzzi JL, Mahler SA, Christenson RH, et al. Recommendations for institutions transitioning to highsensitivity troponin testing: JACC Scientific Expert Panel. J Am Coll Cardiol 2019;73:1059–77. https://doi. org/10.1016/j.jacc.2018.12.046; PMID: 30798981
2. Chapman AR, Adamson PD, Shah ASV, et al. High-sensitivity cardiac troponin and the universal definition of myocardial infarction. Circulation 2020;141:161–71. https://doi.org/10.1161/ CIRCULATIONAHA.119.042960; PMID: 31587565
3. Collet J-P, Thiele H, Barbato E, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289–367. https://doi. org/10.1093/eurheartj/ehaa575; PMID: 32860058
4. Tan WCJ, Inoue K, AbdelWareth L, et al. The Asia-Pacific Society of Cardiology (APSC) expert committee consensus recommendations for assessment of suspected acute coronary syndrome using high-sensitivity cardiac
troponin T in the emergency department. Circ J 2020;84:136–43. https://doi.org/10.1253/circj.CJ-19-0874; PMID: 31852863
5. Raja Shariff RE, Kasim S, Thambiah SC, et al. A Malaysian expert consensus on the use of high-sensitivity cardiac troponin in the emergency department. Journal of Asian Pacific Society of Cardiology 2022;1:e14. https://doi. org/10.15420/japsc.2021.19
6. Shiozaki M, Inoue K, Suwa S, et al. Utility of the 0-hour/1hour high-sensitivity cardiac troponin T algorithm in Asian patients with suspected non-ST elevation myocardial infarction. Int J Cardiol 2017;249:32–5. https://doi. org/10.1016/j.ijcard.2017.09.009; PMID: 28986063
7. Shiozaki M, Inoue K, Suwa S, et al. Implementing the European Society of Cardiology 0-h/1-h algorithm in patients presenting very early after chest pain. Int J Cardiol 2020;320:1–6. https://doi.org/10.1016/j.ijcard.2020.07.037; PMID: 32730826
8. Lan NSR, Bell DA. Revisiting the biological variability of cardiac troponin: implications for clinical practice. Clin Biochem Rev 2019;40:201–16. https://doi.org/10.33176/AACB19-00032; PMID: 31857741
9. Ng M, Tan HJG, Gao F, et al. Comparative prospective study of the performance of chest pain scores and clinical assessment in an emergency department cohort in Singapore. J Am Coll Emerg Physicians Open 2020;1:723–9. https://doi.org/10.1002/emp2.12242; PMID: 33145512
10. Apple FS, Fantz CR, Collinson PO, IFCC Committee on Clinical Application of Cardiac Bio-Markers. Implementation of high-sensitivity and point-of-care cardiac troponin assays into practice: some different thoughts. Clin Chem 2021;67:70–8. https://doi.org/10.1093/clinchem/hvaa264; PMID: 33279984
High-sensitivity Troponins in the ED: Which Guideline to Recommend
in
Asia? JOURNAL OF ASIAN PACIFIC SOCIETY OF CARDIOLOGY www.JAPSCjournal.com





































 RHC With Dobutamine Stress Test for RVOTO in an Adult TOF
RHC With Dobutamine Stress Test for RVOTO in an Adult TOF



