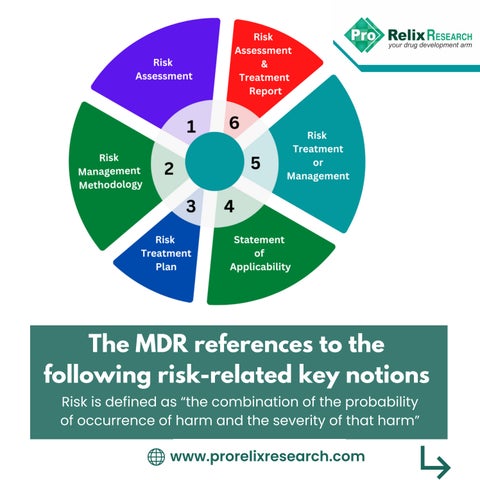
The MDR references to the following risk-related key notions www.prorelixresearch.com Riskisdefinedas“thecombinationoftheprobability ofoccurrenceofharmandtheseverityofthatharm”
www.prorelixresearch.com Benefit-RiskDeterminationisdefinedas“the analysisofallassessmentsofbenefitandrisk ofpossiblerelevancefortheuseofthedevice fortheintendedpurpose,whenusedin accordancewiththeintendedpurposegiven bythemanufacturer”
Generalobligationsaredefinedas “Manufacturersshallestablish, document,implementandmaintain asystemforriskmanagement” www.prorelixresearch.com
www.prorelixresearch.com QMS for Risk Management: TheQualityManagementSystemsshall addressthefollowingmatter–“risk management”
Establishanddocumentarisk managementplanforeachdevice; Identifyandanalyzetheknownand foreseeablehazardsassociatedwith eachdevice; Estimateandevaluatetherisks associatedwith,andoccurringduring, theintendeduseandduring reasonablyforeseeablemisuse; Eliminateorcontroltherisks; The following requirements by the MDR should be addressed in order to ensure compliance and correct benefit/risk management: www.prorelixresearch.com
Evaluatetheimpactofinformation fromtheproductionphaseand,in particular,fromthepost-market; Surveillancesystem,onhazardsand thefrequencyofoccurrencethereof, onestimatesoftheirassociated risks,aswellasontheoverallrisk, benefit-riskratioandrisk acceptability; Amendcontrolmeasuresif necessary. www.prorelixresearch.com
ProRelix Research is here for the timely execution of your projects!
www.prorelixresearch.com