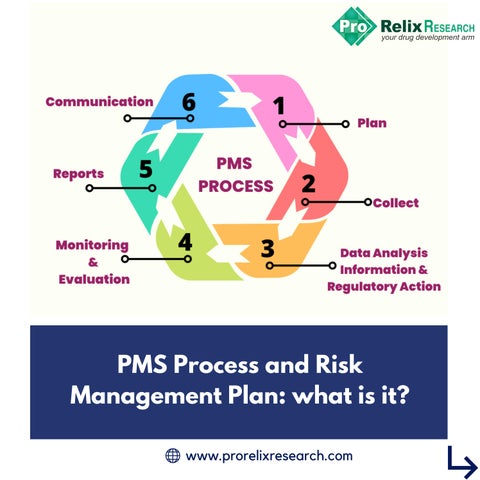
www.prorelixresearch.com PMS Process and Risk Management Plan: what is it?
The PMS process is focused on the collection and analysis of the data that comes from the various sources and is carried out according to a PMS plan to be established for each MD in order to: www.prorelixresearch.com
PMS Plan For Each MD
Update the design and manufacturing information, the instructions for use and the labelling;
Update the benefit-risk determination and improve the risk management;
Update the clinical evaluation;
Update the summary of safety and clinical performance;
www.prorelixresearch.com
Identify the needs for preventive, corrective or field safety corrective action;
www.prorelixresearch.com
Identify options to improve the usability, performance and safety of the device; Contribute to the post-market surveillance of other devices; Detection and reporting of trends. Risk management requirements for postmarket surveillance for medical devices.
www.prorelixresearch.com
With PMS becoming a duty for medical device manufacturers, the effective risk management system becomes a priority as well as one of the three basic elements that ensure compliance and safety, alongside with PMS and clinical evaluation
Plan
www.prorelixresearch.com
Risk Management
www.prorelixresearch.com
According to the MDR, manufacturers are expected to provide evidence of a risk management plan created for the whole lifecycle of products. Such plans should be used for tracking and reducing any potential hazards and ensuring the safety of the devices.
ProRelix Research is here for the timely execution
of your projects! www.prorelixresearch.com