Human Error at Work
Building a Delegation Profile for the Qualified Person Responsible for Pharmacovigilance (QPPV)
Results of PIPA’s 2023
Stability Survey
No more, no less: A guide to the appropriate prescribing of medicines and the role of industry


£12.00 or Free for Members µPIPELINE
Issue 71 The Journal of the Pharmaceutical Information & Pharmacovigilance Association April 2024
CONTENTS
04 07 15
Human Error at Work
By Greg Fradd
Building a delegation profile for the Qualified Person Responsible for Pharmacovigilance (QPPV)
By Susanne Becker and Dora Amene
Medical Information: Results of PIPA’s 2023 Stability Survey
By Sinem Castro
No
no
By Folake Hafeesat Odeshina
03
more,
less:
to
appropriate
of medicines
of industry
A guide
the
prescribing
and the role
Copyright for any article accepted for publication in PIPELINE is transferred to PIPA once the article is submitted. Copyright covers the exclusive rights to reproduce & distribute the article in any form (such as photocopies or electronic copies) and applies to the complete article and any part within. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form without written permission from PIPA. PIPA will, wherever possible, grant permission to authors to subsequently use their articles, or to others to take limited numbers of copies, provided permission is obtained from the editors in advance. PIPA members are permitted to print a copy of PIPELINE and / or save a single copy of the electronic file for their personal use.While all reasonable efforts are made to ensure the accuracy of the information presented in this journal, the editors do not accept any liability for loss arising from reliance on the information presented.The opinions expressed in the journal are not necessarily those of PIPA, its Committee or companies to which members belong - unless otherwise stated. PIPELINE Editor: Dora Amene
HUMAN ERROR AT WORK
Why don’t we get everything ‘Right First Time, Every Time’?
What has life taught you about making mistakes? Not the errors in judgement that are the stuff of agony columns and Hollywood blockbusters, but the ordinary mistypes, misreads, lapses of concentration that we all make day-to-day?
One of the biggest myths we hear at The Accuracy People is that accuracy skills – or the lack of them - are innate, a part of who we are. At school, we were told to “check our work”. If we were lucky, we had a teacher who not only got us into good habits with checking work but showed us how. Most of us, though, did not.
People in your organisations handle vast amounts of data daily, and the repercussions of errors can be both costly and time-consuming to rectify.
Working in the world of medical information and pharmacovigilance demands precision and even minor mistakes can have a big impact.

You’ll be relieved to know that making mistakes isn’t down to laziness, stupidity or a lack of caring. The good news is that accuracy – getting things right first time, data checking and proofreading - is a learnable skill. It is possible to significantly reduce the frequency and impact of errors once we understand why we make them.

In our upcoming presentation, we are going to explore four of the main reasons why we do make mistakes: The first is to do with how our eyes move. The physical structure of the human eye means that it cannot move smoothly across a string of digits or characters, so we don’t always perceive information accurately.
The second is to do with how we process visual information. From an early age we learn to recognise word shapes rather than each letter or character in its exact location – wchih is wyh you cna rdea this prfctly. This inbuilt ability to ‘autocorrect’ what we are reading makes checking a document for completeness and accuracy more challenging.
Thirdly: - often the one we are most aware of - is our tendency to be distracted too easily. Developing and nurturing habits to concentrate more effectively can result in fewer mistakes and greater productivity.

The fourth reason is that we use our memory ineffectively. The mind is a wonderful tool for thinking, but a dreadful place for storage. Relying on our memory, particularly under stress, is a recipe for making mistakes.

It’s encouraging to know that central to attention-to-detail and accuracy are a set of skills that can be learned, practised and even measured. We don’t have to accept expensive, dangerous and stress-inducing errors as an inevitable part of work.
In our free PIPA webinar on 2nd July, we will explore all of this in more detail. We’ll cover:
• What we mean by mistakes
• What they cost you
• Why we make mistakes, and what you can do about it.
To book: https://pipaonline.org/civicrm/event/info/?reset=1&id=67
 Greg Fradd Director of ‘The Accuracy
Greg Fradd Director of ‘The Accuracy
People’ https://www.accuracyprogramme.co.uk/ 0330 133 5456
3
BUILDING A DELEGATION
PROFILE FOR THE QUALIFIED PERSON RESPONSIBLE FOR PHARMACOVIGILANCE (QPPV)
EU pharmacovigilance (PV) guidance (1) states that:
“The QPPV may delegate specific tasks, under supervision, to appropriately qualified and trained individuals, for example, acting as safety experts for certain products, provided that the QPPV maintains system oversight and overview of the safety profiles of all products. Such delegation should be documented”.
Based on the QPPV experience of the authors over many years and with numerous clients, the practicalities of the QPPV’s role makes delegation of tasks by the QPPV inevitable. The responsibilities of the QPPV as described in the PV guidance (1) are very broad, and it may become unrealistic for a single individual to perform all the tasks/responsibilities without support of additional experts. Prior to delegating any task, the QPPV should first set goals for the delegation, i.e., what is the QPPV’s objective in delegating a task. Two key aspects may require delegation of tasks by the QPPV:
1. Delegating operational activities (e.g., individual case safety report (ICSR) processing, periodic benefit risk evaluation report (PBRER) writing) will free capacity and allow the QPPV to focus on the oversight role.
2. Delegation to subject matter experts will provide access to expertise which the QPPV may not have, e.g., developing a post-authorisation safety study (PASS) protocol for an identified important risk, medical input into a benefit risk assessment, pharmaceutical expertise in a quality defect assessment etc.

4

The QPPV should clearly define the task and the desired result, including what needs to be achieved, budget/resources available and time frame. The person taking over the task should understand the scope of the activity, the expectation of the QPPV, and a clear framework of what the person needs to deliver.
Clear setting of tasks and expectations may be explained by the following example: where the QPPV delegates the task of “PBRER Management”. This delegation may include different aspects of PBRER writing; e.g., delegation to a project manager for the organisation of the process including the management of different stakeholders as well as the timely submission to health authorities. The expected result may be improved efficiency of the writing process and reduced cycling times. On the other hand, delegation may also pertain to the generation of the content itself, i.e. the benefit-risk assessment of the product and the objective may be to gain specific medical or pharmaceutical input. In both scenarios, the QPPV is expected to create a mutual understanding of the desired “end product”, the time frame and the budget.
When setting goals, the person taking over a task should communicate back what was understood from the briefing and that the task and its objective are accepted. Clear agreement on objectives and scope is particularly important when the PV systems are spread across diverse cultures and misunderstandings are more likely to happen. In the PBRER example above, this feedback loop should include the project manager’s understanding of the QPPV expectations regarding effective PBRER generation. Feedback may already contain a more detailed breakdown of the project, i.e., “involved parties providing complete and accurate data in a timely fashion”, “medical writer generates a report within given timeframe” etc... Initial feedback by the project manager can then transfer into the communication plan with subsequent loops, which should continue throughout the execution (see below).
The QPPV should ensure that persons who take over tasks are qualified and knowledgeable to perform the task. This may include a formal process and qualification step (e.g., review of education, previous experience), or in long-standing collaborations, where the qualification is already established, a review and discussion of the delegated task. In the PBRER example, the person who takes on the task of managing the process should be experienced in the regulatory framework of PBRERs and have
project management skills to get the desired results in collaboration with the various parties. The subject matter expert who generates the content should be experienced in the PBRER structure/ templates and have both product and medical backgrounds to generate the reports.
The QPPV should encourage the person who will work on the task to assume ownership for the deliverables. They should be empowered to work independently and be self-sufficient in achieving the desired results. The empowerment should permit them to solve unanticipated problems/obstacles during the process and find appropriate strategies to manage the tasks. The QPPV should respect and appreciate the contribution of the person who takes over a task.
The QPPV should not micromanage the team. In the PBRER example, the project manager would conduct and improve the process together with the stakeholders and ensure that the collaboration is efficient. The project manager would organise the individual steps and ensure progress. In a best-case scenario, typical obstacles (e.g., non-responder to an information request, delayed or incomplete deliverables, unanticipated / late changes) would be resolved by the project manager within the framework of delegation and without intervention by the QPPV. It is important to strike the balance between challenging the team to get optimal results and growth whilst maintaining PV system compliance (i.e., PBRER is accurate, complete and submitted in time).
In case the QPPV delegates to subject matter experts (e.g., benefitrisk assessment of the product), the delegation is likely to be more collaborative and the QPPV will liaise with the expert to put the results into perspective for the safety of the product. In this type of delegation, the QPPV needs to understand the expert’s conclusions and their impact on product safety.
The QPPV should ensure that the person who takes over the task is in a position to fulfil it and has the necessary capacity. This includes an assessment of required resources (budget, infrastructure) and time. The person should also be installed in a position within the organisation to achieve the desired results. In case of the PBRER project management, where multiple stakeholders are providing input and feedback, senior management and the QPPV should foster an environment where the collaboration between PV and other departments is acknowledged as an inherent part of day-to-day activities.
The QPPV should establish a communication plan, including routine/ regular feedback and expectations when to communicate ad-hoc (unexpected, unplanned) issues. It is equally important that the person who takes over the task is able to decide when it is necessary to escalate a topic for QPPV review and support. Communication should be tailored to the task and the person. A more junior person is likely to need more routine communication and feedback than an experienced / senior team member. In the PBRER example, the cycling of the reports is determined by the regulatory framework defined in the EU PV guidance (2) which covers the scope, objectives, format, content and submission timelines of the PBRER report. The QPPV would set up early feedback loops to ensure that the project is running in the right direction. Defining milestones will help to keep an oversight of the progress.
For operational tasks (e.g. ICSR submission to authorities) the establishment of key performance indicators (KPIs) helps to maintain oversight and ensure supervision by the QPPV for delegated tasks. Complex tasks (e.g., a benefit-risk assessment
5
for a product) which cannot be measured in a one-dimensional KPI should have a more task-specific feedback structure, e.g., interim reviews at defined milestones.
At the end of the delegation, the person should hand over the results and feedback should be given as to whether the objectives were achieved, and what impact the achievement has had on the organisation.
“To err is human”. The QPPV needs to be able to accept that errors and failures during the delegation may occur. The QPPV should feel comfortable in working with fellow colleagues and be supportive of the team during the corrective and preventative action (CAPA) process in cases of errors or deviations.
Annex A of the pharmacovigilance system master file (PSMF) is where the list of all the activities delegated and maintained by the QPPV should be documented (3).
According to EU PV guidance (1), the QPPV retains accountability for the entire PV system; therefore, they must maintain oversight over the functioning of the system including all the tasks they have delegated. This includes monitoring of PV processes, assessing performance/results and advising on areas for improvement. If updated as and when new information becomes available, PSMF annex F provides the QPPV with a good overview of performance metrics relating to delegated tasks (3). Although this is not a requirement in the EU PV guidance (3), adding CAPA metrics to annex F of the PSMF should be considered a useful way of maintaining oversight. There should be other ways to ensure oversight aside from the PSMF, as the PSMF does not provide oversight on other activities such as PV training compliance, withdrawn products, and information on mergers and acquisitions or commercial due diligence activities.
In conclusion, a QPPV should be able to decide how they can best apply delegation, oversight and authority in order to fulfil their responsibility for the establishment and maintenance of the marketing authorisation holder’s (MAH) PV system. The level of delegation and the oversight mechanism should be tailored to the PV system which the QPPV serves. Regardless of the model which best fits the needs of the specific PV systems, the MAH has to ensure that the QPPV has the necessary resources and support from cross-functional teams to implement and maintain their PV system (1).
References:
1. Guideline on good pharmacovigilance practices
(GVP) Module I – Pharmacovigilance systems and their quality systems; https://www.ema.europa.eu/ en/documents/scientific-guideline/guidelinegood-pharmacovigilance-practices-module-ipharmacovigilance-systems-and-their-qualitysystems_en.pdf
2. Guideline on good pharmacovigilance practices (GVP): Module VII – Periodic safety update report; https://www.ema.europa.eu/en/documents/ scientific-guideline/guideline-goodpharmacovigilance-practices-gvp-module-viiperiodic-safety-update-report_en.pdf
3. Guideline on good pharmacovigilance practices (GVP) Module II – Pharmacovigilance system master file (Rev 2); https://www.ema.europa.eu/en/ documents/scientific-guideline/guidelinegood-pharmacovigilance-practices-module-iipharmacovigilance-system-master-file-rev-2_ en.pdf
The contents of this article are solely the opinion of the authors and do not represent the opinions of PharmaLex GmbH or its parent Cencora Inc. PharmaLex and Cencora strongly encourage readers to review the references provided with this article and all available information related to the topics mentioned herein and to rely on their own experience and expertise in making decisions related thereto.
 Susanne Becker Senior Director, Pharmacovigilance and International Service Lead for Cencora PharmaLex QPPV Services
Susanne Becker Senior Director, Pharmacovigilance and International Service Lead for Cencora PharmaLex QPPV Services
 Dora Amene Associate Director, Pharmacovigilance at Cencora PharmaLex
Dora Amene Associate Director, Pharmacovigilance at Cencora PharmaLex
6
MEDICAL INFORMATION: PROVISION OF EXTENDED STABILITY DATA
RESULTS OF PIPA’S 2023 STABILITY
SURVEY
Healthcare Professionals (HCPs) often reach out to pharmaceutical companies for extended stability data in order to make a clinical decision about the use of a medicine which has or will be stored outside of its licensed storage conditions. Pharmaceutical medical information (MI) departments are often responsible for answering those questions, and this may involve liaising with their regulatory and quality department colleagues.
Pharmaceutical companies have been traditionally cautious about providing extended stability data for a variety of reasons. This, however, needs to be balanced with the importance of access to timely and accurate information regarding stability, to allow HCPs to make crucial decisions regarding the safe and cost-effective use of medicines.
WHAT IS A TEMPERATURE EXCURSION?
A temperature excursion is a deviation from a medicine’s licensed storage instructions.
WHY DO HCPS NEED EXTENDED STABILITY DATA FOR MEDICINES?
In clinical practice, incorrect storage or temperature excursion occurs frequently, and may happen for a variety of reasons e.g., refrigerator failure, human error, seasonal variation. Where temperature excursions occur, HCPs need stability information in a timely fashion to determine whether the medication can still be administered to a patient. Depending on the data provided, HCPs may need to follow a specified process to handle the impact on medicines.
WHAT RESOURCES ARE AVAILABLE FOR FINDING STABILITY INFORMATION FOR MEDICINES?
HCPs can obtain stability information from reputable resources such as:
• Manufacturer’s information sheet (summary of product characteristics [SmPC])
• Manufacturer’s medical information department
• British National Formulary (BNF)
Many HCPs, particularly hospital pharmacists, may also utilise the SPS Fridge Stability Tool ( https://www.sps.nhs.uk/home/ tools/refrigerated-medicines-stability-tool/ ). This tool does include some off-label information, although this is clearly explained on the website.
PIPA’S PARTNERSHIP WITH UKMI AND TOPRA
As many of you will be aware, PIPA works collaboratively with UK Medicines Information (UKMi) - an NHS pharmacy-based service which aims to support the safe, effective and efficient use of medicines by the provision of evidence-based medicines information and advice regarding therapeutic use. Our collaboration aims to help facilitate an effective working relationship between Pharma and UKMi Pharmacists, who are one of Pharma Medical Information’s largest customer groups.
The provision of stability data by the pharmaceutical industry continues to be topical. We are continuing to work with UKMi to help their members understand why stability data may not always be available in response to their enquiries, as well as educating Pharma MI teams around how they may improve the quality and timeliness of the information that they provide. To this end, in 2018 and 2020, PIPA undertook a survey of Pharma MI teams to identify how MI departments manage stability data requests, and to determine what limitations are put on the data they can provide (see Stability-Survey-2018-results.pdf (pipaonline.org) and Stability-survey-2020-summary-of-results-article.pdf (pipaonline.org) for the results of these two surveys).
With the NHS still struggling with huge monetary losses annually due to unused medicines that have been subject to temperature excursions, we have agreed with UKMi to run this survey again to determine whether there has been a change since our last survey around extended stability data provision.
This year, we’ve also partnered with TOPRA, The Organisation for Professionals in Regulatory Affairs. In recognition of the fact that regulatory teams often provide the extended stability data to their MI colleagues – and in some companies may handle such enquiries directly – PIPA have asked TOPRA to share this stability survey with their membership to achieve a wider insight into extended stability data provision and limitations.
7
Extended Stability Data Survey Results
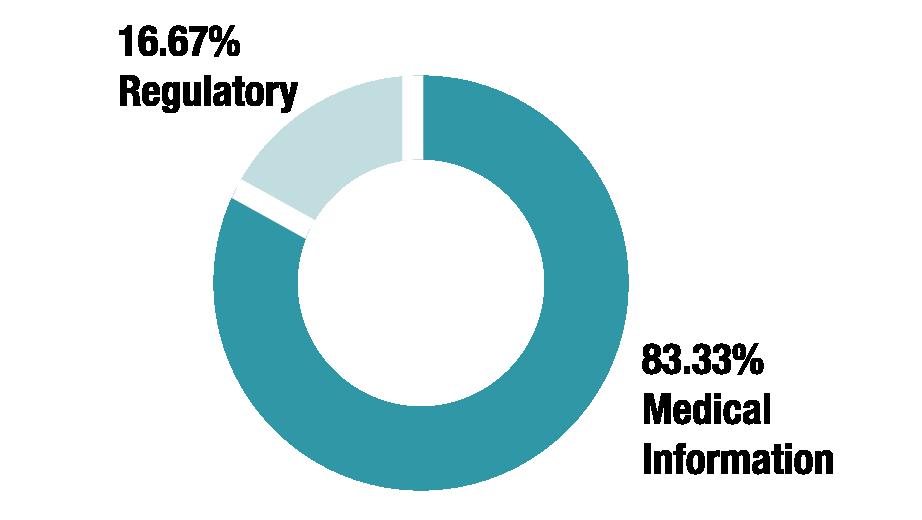
Do you work within the Medical Information or Regulatory team?
Do you provide extended stability data in response to temperature excursion enquiries?

IF YOU DON’T PROVIDE DATA, WHY NOT? IF YOU ONLY PROVIDE DATA SOMETIMES, WHAT DETERMINES THIS?
We deal with each temperature excursion individually rather than giving extended stability data.
If we have information we can provide a statement. We do not send out actual data.
It is not always available - i.e. extended stability studies may have not been conducted and in many cases the information (at least in our company!) is not located in one place for each drug therefore can take time to obtain. In addition, we can assess individual temperature excursions (if we have access to the information) on a case by case basis if batch information is provided. However response provided would be based on transport risk history - meaning the data is applicable only whilst under the responsibility of the manufacturer, once the product has been provided at handover point this responsibility ceases. Therefore we can provide in good faith but the licenced product information should always be considered first and we cannot officially recommend use outside of this. It would always be under the responsibility and clinical judgement of the HCP to use the product.
Do not have it.
We will provide data where available and relevant to the question asked.
Only if we have robust data.
Provided we have the parameters of the excursion and the batch number and expiry date we refer the request to a quality colleague who assesses the available stability data and provides an impact assessment on product quality. I.e whether product is or is not impacted by the specific temperature excursion. This process can vary between countries and products.
Need to have all relevant information before providing information.
only if there has been stability testing completed for such enquires.
The dossiers for some products are highly confidential - we can provide a ‘yes it’s okay’/’no it’s not okay’ answer and information within a given temperature range but can’t for example provide the full data tables.
We provide an answer that confirms if we have stability to support the excursion, but not the documentation as often it contains more information than was requested.
Mostly yes - if the excursion falls outside the time we have stability data we state that the product should not be used.
Depends on the product.
8
We don’t provide the data, but we do provide a YES/NO response using an in-house stability tool calculator according to the excursion that has occurred.
Our Quality team will only provide us with additional stability data if the temperature excursion happened within the supply chain. If it’s outside the supply chain we are unable to provide additional information
Will provide if any extended stability data is available
We provide information on a case by case basis as long as we have all the information such as duration, max temp and batch number
If we have data available and depending on whether full parameters of the excursion are provided (i.e. not in response to prospective enquiries)
As per client instructions, we only provide what is stated in the product label. Only for some of the oncology products, we might have previously approved internal data that the client agreed to create a standard response. The info would be for clinical trial locations to support the preparation of the drugs during the clinical trials.
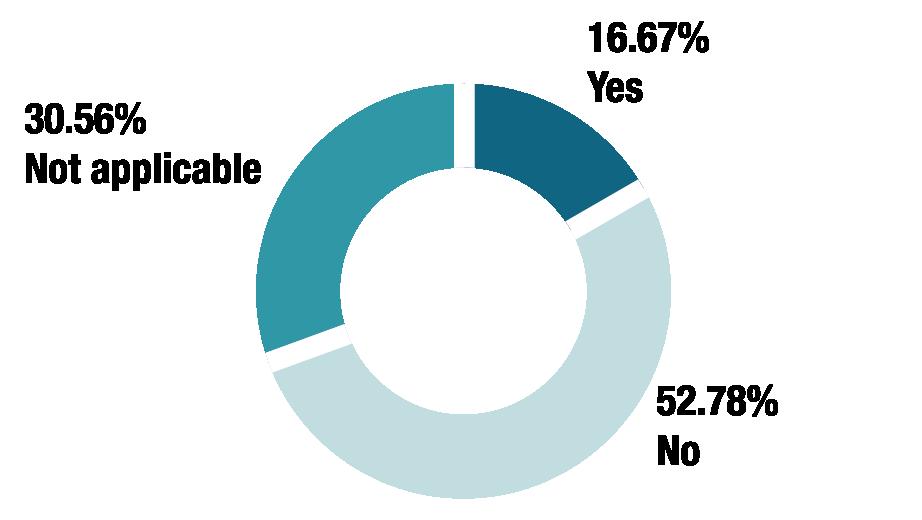
Are you permitted to share stability data for certain medicines but not others?
IF YES, WHAT IS THE RATIONALE FOR THIS?

We sometimes don’t have extended stability data for some medicines. If we have it though, we share it.
If the extended stability studies have been conducted and published then this can be shared if asked for. If not then it really depends on if we are able to obtain the information or not. For popular medicines it is sometimes easier to obtain an assessment from QA based on transport risk history via batch numbers but for others it is not as easy.
Only for the products that are manufactured by our company
Don’t supply for unlicensed medicines
No rationale provided by quality, it’s their decision.
IP threat
It is situation dependent rather than product dependent
Do you require the enquirer to provide specific information (e.g. the temperature reached and the length of time the medicine was stored at that temperature) before extended stability data is shared?
IF YES, WHAT INFORMATION DO YOU REQUIRE?

The max temperature reached and the length of time the medicine was stored at that temperature.
Temperature reached, length of time, amount of product affected, batch number of product, expiry date
Max/ Min temperature attained, length of excursion
Temperature reached, length of excursion, we try to confirm batch details and (for fridge products) if it has been returned to the fridge.
9
Lot number, temperature reached and length of time.
Sometimes, quality department would ask for excursion report to provide the most relevant information.
Maximum or minimum temperature reached, batch number (if available), expiry date (if available), number of previous excursions the product suffered, quantities of affected product, length of excursion.
For batch assessments we require batch numbers, date of excursion, max temp, min temp, has product been reconstituted or not if applicable and reason for temperature excursion. How it is currently being stored after the temp excursion can also be useful. Note we do not provide response if it has occurred with a patient only if in a pharmacy or hospital setting.
N/A, we don’t have the data
Temperature data Time duration of storage of the product at that temperature Location of the product
The temperature reached and the length of time the medicine was stored at that temperature
We require batch number, temperature reached, length of time and to confirm the product has been acquired commercially (as opposed to use in clinical trials)
Batch number and expiry date of product Temperature in degrees C of the excursion Length of time the product was subjected to the excursion Was the product unopened and unpunctured/unreconstituted.
Max/min temperature, duration of exposure
Country purchases, temperature and environment/ length of time the medicine was stored at
Min/max temp and duration
Duration, max or min temperature, batch details.
What is the presentation, batch number and expiry date of the product(s)? What was the maximum/minimum temperature reached and the duration of the excursion? Has the product been reconstituted/diluted? Did the product undergo a freeze/thaw cycle? If so, how many times? Is this product a clinical trial stock? If so, please specify if it is concerning a company sponsored trial and provide the trial number
Min/Max temps, length of time the medicine was stored at that temperature
Require temperature reached, length of time, batch # & expiry date and the customer type (physician/pharmacist/hospital, patient or wholesale/ distributor)
Length of time the medicine was stored at the specific temperature kept at
Temperature, length of time and when it happened
Batch no, exact temps and duration
Length of time, temperature reached. We also collect reason for excursion for PV reporting
Do you require the batch number and expiry date of the medicine?

10
Do
IF YES, WHAT FORM DO THEY TAKE?
Internal process documents.
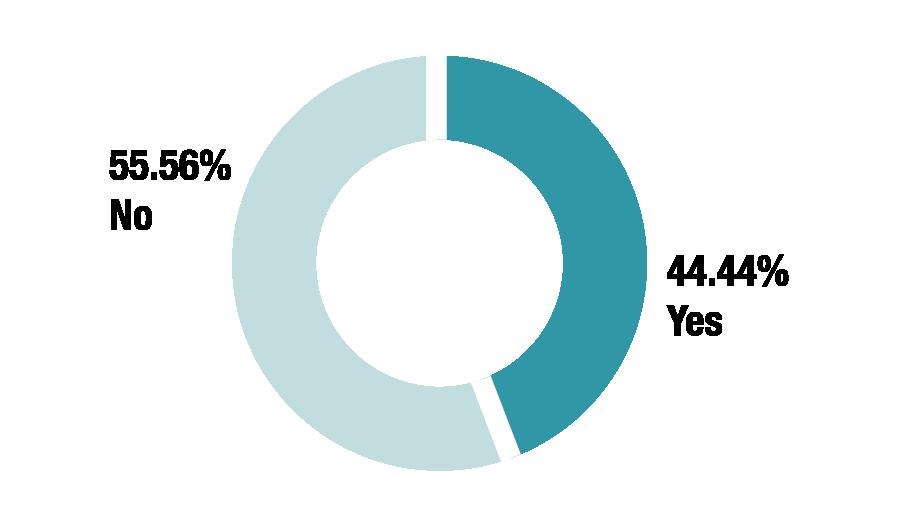
Quality own these SOPs.
Global Quality SOP on dealing with temperature excursions.
Standard response documents, global and local SOPs.
I think QA may have, although we do not have anything as prescriptive in MI.
standard operating principles and working instructions.
SOP and work instruction (rolling doc). We also have a sign off process for new starters. AllTE responses get checked until the team member is comfortable doing them independently.
A single work instruction with decision making aid.
SOP to outline use of Stability Tool, data not shared externally.
Working practice / standard responses.
Has your policy around sharing extended stability data changed over the past few years?
IF YES, IN WHAT WAY?

We have become stricter about not sharing extended stability data as we want to deal with each temperature excursion individually.
Stricter control.
Generally we should only provide info in alignment with SPC. However in good faith we try to provide info via case-by-case assessments if we can based on batch transport risk history as mentioned above if possible.
We didn’t use to share the data, we only used to say if the product has been compromised or not.
It changes regularly depending on quality teams process and preference.
It evolves constantly as we try to be as customer focused as possible while keeping it clear and straight forward.
Company merger: one legacy company shared extended stability data for HCP to make own decision; other legacy company did not share data, but would make assessment & provide response (as outlined above).
We no longer provide general extended data - only on a case by case basis.
Requesting parameters and not providing prospective data.
11
you have documented policies around sharing extended stability data?
What type of data do you provide?

IF OTHER, PLEASE PROVIDE FURTHER INFORMATION:
We do not share data, we look at each individual temperature excision and advise whether product is OK to be used or needs to be quarantined and destroyed.
We will provide a statement based on the information available from the manufacturer.
Only the conclusion of stability, not the original study data.
We cannot provide full studies if they are not published externally. We can only assess on a case-by-case basis.
We only provide what’s in the SmPC.
Product stored at various temperature and relative humidity eg, in fridge, outside fridge, frozen, etc.
Studies regarding storage at elevated temperatures Studies with or without light protection.
Who provides the extended stability data?
IF OTHER, PLEASE SPECIFY
We don’t provide stability data, we just inform the enquirer if the product can still be used after a temperature excursion.
Data from original product trials for determining shelf life.
We don’t send the data out we provide an impact assessment based on the stability data available which can include shipping/freeze/thaw studies.
An assessment of whether the product can be considered for continued use, based on available data. No specific data is provided.
Text answer in relation to specific queries - we would not provide the studies
We do not provide studies.
We are not sharing externally data, but either mention that we have data to support the temperature excursion.

12
Product
Manufacturing sites. Quality. QA.
manufacturer.
Sometimes we have to ask the licensor, as our Reg Affairs might only have limited studies based on which the dossier was approved.
Clients who contracted our services.
QA, suppliers.
N/A.
Local Medical Information Department.
Quality.
QA teams to Med Info to enquirer.
Quality Department.
Global organisation delivered through MI.
Quality Assurance provide the data internally for Med Info to make assessment & response.
Quality team.
Quality Assurance.
Quality Assurance.
Quality assurance department.
PLEASE PROVIDE ANY FURTHER RELEVANT INFORMATION
All temperature excursions are checked with Global Quality.
We have a local process that has been made between quality and MIOther affiliates follow different processes.
Sometimes there are different formulations that require local approval rather than global.
Are you aware of extended stability data that is available in other countries that you are not permitted to use in the UK?

IF YES, DO YOU KNOW WHY YOU ARE NOT ABLE TO SHARE IT?
However - different countries SPCs could differ so we can only provide info relevant to UK batches.
Global process that the company doesn’t provide the data.
13
Is there variation in the data you obtain from different sources (for example, local vs global?)
If you are a generics company, do you undertake your own stability studies?

PLEASE PROVIDE ANY OTHER RELEVANT INFORMATION
Our manufacturers undertake stability studies for us.
I am not sure, maybe sometimes. Usually would go to the licensor.
If you are happy to do so, please share any standard wording you use in response to temperature excursion enquiries.
RP or Pharmacist on customer side must agree to using the product.
There is no standard wording it depends on the excursion.
Any decision to use a product that has been stored outside of the recommendations stated in SmPC will be considered off license and will be a clinical decision to be made at the discretion of the treating healthcare professional.
Always quote that the company can only recommend use of our medicines that are stored within the terms of the SPC and that we are providing them in answer to their specific query and that the information is off label
Based on the excursion information provided in the report there is no perceived impact to product quality. We would also like to emphasize that stability data is not meant to be repeatedly relied upon to address distribution challenges.
Assuming that PRODUCT has been stored as mentioned; at a maximum of XX degrees C for a single time period of maximum XX hours, the product quality is considered intact, and the product can still be used.
BUSINESS CASE
Should you deem the above data to have not impacted the product integrity, no adjustment to the product expiry dates are required if the excursion falls within the limits above. The products can then be returned to the licensed SPC storage conditions. In addition, the use of the products will not be in accordance with the licence. Please be advised that the data only applies to products stored in their original container that have not yet been reconstituted, diluted or altered in any way. This stability data is intended for use as a guide by the healthcare professional (HCP) to assess the likely impact of the temperature excursion on the quality and suitability for use of the products. Ultimately, the HCP is responsible for the decision whether or not the products are safe, efficacious and suitable for use. In addition, the use of the products will not be in accordance with the licence. The quality of the products have not been checked by scientific analysis by the company. The stability data provided is based on the history of the batch, including when the product is transported to the warehouse, storage in the warehouse and transport to the customer. It has been prepared from information provided by yourself who is responsible for its accuracy and completeness. The data has been prepared with the assumption that the product has been stored correctly at all other times by the customer once received from the company. This information must not be used for any future temperature excursions. Please contact the company Medical Information for any future temperature excursions.
We provide one of 2 responses: “According to the information provided the use of the product is supported/is not supported.”
After provision of data: Please note, any use of [product] outside of the recommendations in our SmPC would be the clinical decision of the treating practitioner.
If you are planning to challenge your current process with internal stakeholders –consider preparing a business case which could include the following:
• Rationale behind the requests for extended stability data
• HCP concerns regarding patient safety and financial risk
• PIPA Survey results which demonstrate that other pharma companies do provide extended stability data
• Prepare in advance template disclaimers that would be provided in the MI response alongside the extended stability data
By educating internal stakeholders about the challenges faced by HCPs and MI departments relating to the handling of stability enquiries, we can help to ensure the best decisions are made regarding patient care.
 By Sinem Castro
By Sinem Castro
14
Medical information Workstream; Training Workstream, PIPA
NO MORE, NO LESS: A GUIDE TO THE APPROPRIATE PRESCRIBING OF MEDICINES AND THE ROLE OF INDUSTRY
A SUMMARY OF GUIDANCE FOR THE PHARMACEUTICAL INDUSTRY
The Association of the British Pharmaceutical Industry (ABPI) released a new guidance in February 2024 directed at healthcare professionals (HCPs) and the UK pharmaceutical industry. The guide provides recommendations to both the pharmaceutical industry and prescribers with the aim to shed light on the complexities surrounding the prescribing process and the role that pharmaceutical companies can play in ensuring appropriate prescribing practices. It acknowledges the increasing options and existing challenges faced by HCPs in making treatment decisions in specific populations such as prescribing in pregnancy, paediatrics, and the elderly, as well as the importance of online prescribing, and drug interactions.
Prescribers are essentially the gatekeepers in ensuring the safe and effective use of medicines. The guide touches upon prescribers considering the risk and benefits of opioids, selective serotonin uptake inhibitors (SSRIs), cannabis-based medicinal products, and promoting antimicrobial stewardship for optimal patient outcomes. This article will focus on the recommendations for the pharmaceutical industry and the role that pharmaceutical companies can – or already are – playing in appropriate prescribing and improving patient outcomes through supporting HCPs in providing evidence-based patient-centred care.
THE ROLE OF THE PHARMACEUTICAL INDUSTRY IN HELPING WITH APPROPRIATE PRESCRIBING
Appropriate prescribing involves prescribing the right medicine, at the right dose, for the right duration and for the right patient through shared decision-making between HCPs and patients. Pharmaceutical companies have a crucial responsibility to work with regulators and experts with regards to the quality, safety, and efficacy of pharmaceutical medications. This includes assisting with appropriate prescribing when engaging with HCPs regarding

Prescribers are essentially the gatekeepers in ensuring the safe and effective use of medicines.
15
development, promotion, and supply of medication. They also support in making informed decisions through provision of educational resources, clear Summary of Product Characteristics (SmPCs) as the reference document or ‘instruction manual’ of medicines information for prescribers and services such as medical information and medical affairs teams. This is discussed in more detail below.
MEDICAL INFORMATION SERVICE (MIS)
The MIS, in many instances, is the primary point of contact between pharmaceutical companies, HCPs and the public. The MIS provides information on clinical aspects of medicine by sharing non-promotional, evaluated, balanced information to support the use of medicines. The service can assist with areas such as addressing misuse of medicines and highlighting contraindications for specific patient populations. An example of this was provided in a case study in the guidance, where the MIS was able to use the SmPC to highlight the suitability of a medication in the elderly for a senior advanced pharmacist. The MIS took into consideration that elderly patients may have renal impairment, and since the medicine is excreted in the urine, it is contraindicated in cases of renal insufficiency. The MIS, in this case, provided evaluated and balanced information to help guide the pharmacist in their prescribing decision.
PHARMACOVIGILANCE
MEDICAL SALES REPRESENTATIVES
Pharmaceutical sales representatives are recognised for promoting pharmaceutical products to healthcare providers, such as GPs, hospital doctors, pharmacists, and nurses, ensuring to educate them of the effectiveness, safety, and appropriate usage of the company’s medications. The recommendation suggests that they should consider the wider prescribing context confronting a patient. This includes having a more holistic role during promotional calls and exploring scenarios which HCPs often encounter. For example, considering patients whom the medication is suitable for as well as those that it may not be suitable for and considering how the medication aligns with a complex co-morbid patients’ polypharmacy situation.

The internal drug safety or company pharmacovigilance department plays a vital role in answering complex safety-related HCP queries and continuously monitoring the safety profile of medicines after they receive regulatory approval. They collate adverse event reports on their medicines and report these events to regulatory agencies –in the UK, this is the Medicines and Healthcare products Regulatory Agency (MHRA). The majority of the reports are from HCPs or members of the public which include adverse events, serious adverse events, off-label/off-licence use of the medication, use in pregnancy or use of unapproved doses or routes of administration. The collected information is analysed to ensure the continual evaluation and update of the safety profile or benefit-risk profile of the medication. The pharmacovigilance team can therefore review safety reports and relevant data to provide prescribers with information regarding safety-related queries.
MEDICAL AFFAIRS AND MEDICAL SCIENCE LIAISONS (MSLS)
Medical advisers in medical affairs and MSLs are non-promotional subject matter experts who can support HCPs in making informed treatment decisions through disseminating scientific information and addressing clinical queries. They also ensure that promotional materials used in the marketing of a licensed medicine are medically accurate and in compliance with the product licence, ethical and legal requirements, and industry codes of practice. These teams have the knowledge and resources available to support prescribing decisions through providing clinical interpretation of scientific and clinical trial data related to the development of medicines which will play a part in improving patient safety.
To conclude, the ABPI’s guide provides valuable insights into the complexities of appropriate prescribing and the resources that the pharmaceutical industry has available to support HCPs with good and appropriate prescribing of medicines. Enhancing prescribing practices requires collaborative efforts and utilisation of the available resources. By embracing these recommendations, the goal of ensuring that patients receive the right medicines, in the right way, at the right time, ultimately enhancing patient care and safety will be achieved.
To access the full document, which includes case studies of how these teams can support HCPs in practice, please click here

16
Folake Hafeesat Odeshina Medical Information Specialist Johnson and Johnson

PIPELINE PIPA, PO Box 254, Haslemere, Surrrey, GU27 9AF pipa@pipaonline.org | https://pipaonline.org







 Greg Fradd Director of ‘The Accuracy
Greg Fradd Director of ‘The Accuracy


 Susanne Becker Senior Director, Pharmacovigilance and International Service Lead for Cencora PharmaLex QPPV Services
Susanne Becker Senior Director, Pharmacovigilance and International Service Lead for Cencora PharmaLex QPPV Services
 Dora Amene Associate Director, Pharmacovigilance at Cencora PharmaLex
Dora Amene Associate Director, Pharmacovigilance at Cencora PharmaLex





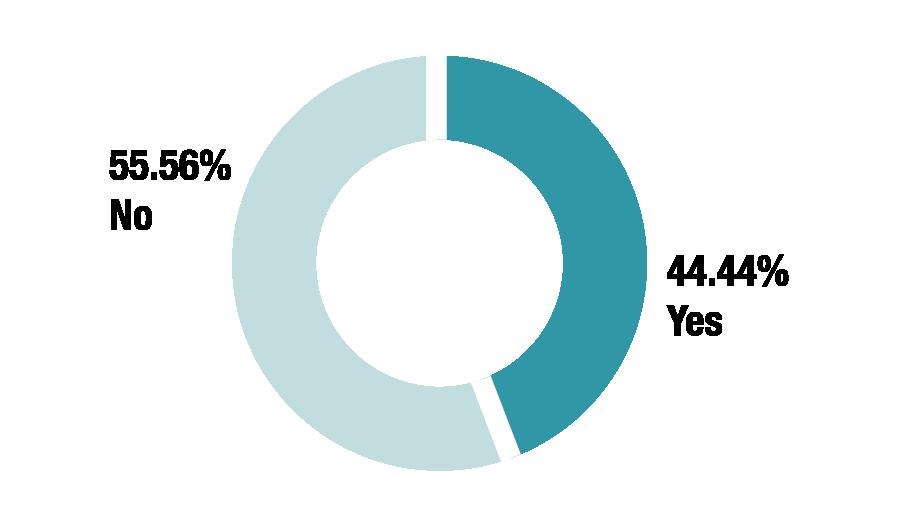






 By Sinem Castro
By Sinem Castro



