

Team Members…




Company Management
Dr. Anupama Patel, Chief Executive Officer







Worldwide Education & Research Work Experience
• Pre-PhD training at the Indian Institute of Chemical Technology (India)
• Ph.D. in Organic Synthesis from Hamburg & Kiel Universities (Germany)
• Post Doctoral Fellow at the University of British Columbia (Canada)
• Senior Research Scientist positions at Albany Molecular Research Inc.
• Published several research articles in Peer-reviewed International journals
• Held Teaching and leadership roles prior to incorporating Pharma Inventor Inc.




Prof. Thisbe Lindhorst
Company Management
Dr. Kashinath Sadalapure, Chief Scientific Officer






Recognitions & Awards
• Sharabayya Gada Gold Medal
• Council of Scientific & Industrial Research (CSIR) Fellowships
• Wiley book Prize (Kiel, Germany)
• Best Researcher (CytoMed Inc. USA)
• Employee of the Year, 2005 (NAEJA Pharmaceuticals Inc. ) Leadership
Industrial Work Experience
• IICT-Cytomed Inc. (USA & India)
• Xenon Pharmaceuticals Inc. (Canada)
• NAEJA Pharmaceuticals Inc. (Canada)
• Albany Molecular Research Inc. (USA)
• Phyton Biotech LLC. (Canada)
• Methylation Sciences Inc. (Canada)
• Multiple clinical development candidates






Pharma Inventor Advantages ...

• Vancouver based Business, Easy access & Quick Turn-around Times
Vancouver is one of the most livable cities in the World
• Family Owned Business, Founders Themselves are Skilled Scientists
Vancouver is a magnet for global and regional scientific talents
• Inventive, Cost-Effective, High Quality & Time Efficient Services

Positioned to deliver high quality services with increased productivity at reduced cost
• Absolute IP Protection
Our clients own Intellectual Property (IP) and we work under strict confidentiality.
• State-of-the-Art Scientific Infrastructure
Opportunities for scaled growth
• Strict Adherence to Health & Safety Standards
WorkSafe BC and WHMIS Health & Safety Standards in Practice

Comprehensive Services...


PHARMA INVENTOR INC,. is a Canadian Preclinical Chemistry R&D and Analytical services company established to serve the needs of Pharmaceutical, Biotech and Agrochemical industries as well as research institutes/academic research groups in North America to support their innovative Chemistry R&D/Analytical/ Biological testing activities.
Drug Discovery Capabilities ...


In-house Chemistry Capabilities …

• Biological Target Selection and Database Review
Literature review using Sci-Finder, Reaxys, POC data search in patents and research articles.
• Novel Structure Design and Synthesis of Compound Libraries
Compound design & synthesis based on biological activity, selectivity, pharmacokinetic (i.e. ADME) and toxico-kinetic properties, as well as novelty search for patentability
• Hit to Lead Generation
Lead hopping and screening using Biochemical assays.
• Lead Optimization
Skilled medicinal chemists with proven track record of innovative patents and publications.
• Back-up Candidate Identification
Synthesize diverse chemical scaffolds with optimized pharmacokinetic & toxicology properties.
• Process Development
Green and environmentally friendly synthetic processes, technologies for manufacturing under cGMP conditions.
In-house Chemistry Capabilities …


Custom Peptide Synthesis…

PHARMA INVENTOR INC. is very proud to offer high quality and cost-effective custom peptide synthesis service in a timely manner regardless of peptide complexity. Our extensive experience in peptide synthesis technologies has afforded us the capability to take on a wide range of sequences, purity levels, and modifications to meet our client's research/product needs.
PHARMA INVENTOR Advantages
Consistent Results: >99% success rate
Large number of modification
High purities: from 90% to >99%
Best Quality Control
No limitation
Rapid Turnaround
Competitive pricing

UPLC-CAD Analysis…

PHARMA INVENTOR INC. is very pleased to offer charged aerosol detector (CAD) analysis, which is highly sensitive, consistent, high precision and wide dynamic range system to provide accurate results for any non-volatile and many semi-volatile analytes.
Applications of CAD Analysis:
Lipids
Proteins
Peptides
Carbohydrates
Polymers
Steroids
Oligosaccharides
Surfactants
Polysorbate quantification and profiling

SFC Purification Capabilities…

PHARMA INVENTOR INC., offers chiral purification services using preparative Supercritical Fluid Chromatography (Prep-SFC) and Preparative High Performance Liquid Chromatography (PrepHPLC). Our expert scientists produce high quality compounds from milligrams to hundreds of grams in a fast turnaround time.
Analytical SFC
PHARMA INVENTOR Advantages
Purification of chiral compounds.
Isolation of impurities in starting materials, intermediates, APIs and drug products.
Purification of either single compounds or libraries.
High-throughput purification for synthetic compounds.
Chiral separation method development.
Rapid Turnaround.
Competitive pricing.

Preparative SFC

Radiopharmaceuticals…

PHARMA INVENTOR INC., is proud to announce radiolabeling capabilities for synthesis, method development and manufacturing of radiolabeled (C-14 and Tritium) small molecules of almost any complexity for use in pre-clinical and clinical studies.
PHARMA INVENTOR Advantages








Radiosynthesis and synthetic routes development.
Synthesis of radiolabeled (C-14 and Tritium) APIs to GMP.
Full Certificate of Analysis and analytical profile.
Chiral separation and salt formation.
Storage and management of radiolabeled compounds.
Safe and fast shipment to all destinations in North America.
Rapid re-purification service.
Competitive pricing.

Supporting AI in Drug Discovery…

PHARMA INVENTOR INC., has a great reputation for its exceptional track record and commitment to excellence. With our expanding scientific team of highly skilled Medicinal/Synthetic Chemists, we consistently deliver outstanding results that meet the highest professional standards. We have an exquisite reputation to provide medicinal chemistry support to synthesize novel lead drug candidates identified by the Deep Docking AI platform, for several Pharma Industries across North America. Our dedication to accuracy, clear communication and timely execution ensures that your customized synthetic chemistry project is handled with the utmost care and precision.



https://www.pharmaceutical-technology.com/news/ rakovina-expands-collaborations-to-leverage-ai-
Benefits of AI in Drug Discovery
Co-crystal & Salt Screening

Selecting the optimal solid form of an API during early drug development can mitigate the risk of failure later in development. This can be achieved through polymorph, salt and cocrystal screening.
PHARMA INVENTOR Co-crystal, Salt and Polymorph Screening Services
Co-crystal Screening : Choose suitable co-formers to design experiments; Co-crystal form identification and characterization; Thermodynamic phase relationship study; Stability and solubility evaluation; Co-crystal form recommendation.
Salt Screening: Design various salt-forming experiments according to the physicochemical

Focused Form Screening
o Physiochemical Property
o Developability assessment
PHARMA INVENTOR Capabilities - Solid Form Screening

Polymorph/Salt/Co-crystal Screening
o Thermodynamic phase relationship
o Stability and solubility evaluation
o Optimal form recommendation
Extensive Form Screening
o Form evaluation
o Patenting of polymorph/salt
Diverse Synthesis Capabilities …
Thio-CMI-977
5-LO Inhibitor
Anti-asthmatic agent

Setmelanotide
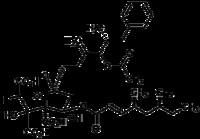

Metal Catalyst (R,R)-Salen Co (III) OAc
Stable, Brick Red Solid
Key Intermediates

Analytical Services…
PHARMA INVENTOR INC.

provide broad range of analytical services including Method
Development, Validation, Subject sample analysis, Quality Control Testing and Stability Studies.

Pharmaceutical Compounds
Natural Health Products
Herbal and Botanical Products/ Pesticides Analysis
o Ultra Higher Performance Liquid Chromatography - Mass Spectrometer (Waters UPLC-MS/MS)
o UPLC-MS/MS integrated with Charged Aerosol Detector (CAD)
o Tripple Quadrupole Liquid Chromatography - Mass Spectrometer (TQD LC-MS/MS)
o High Performance Liquid Chromatography (Agilent 1100 HPLC)
o Preparative High Performance Liquid Chromatography (Agilent Prep 1200 HPLC)
o Karl Fischer Titration (KF Titration)
o Nuclear Magnetic Resonance (NMR) Spectrometer (1D, 2D, 13C, 31P, 15N, 19F on 400 MHz & 600 MHz)
o Trapped Ion Mobility Spectrometry coupled to High-Resolution Time-of-Fight Mass Spectrometry (Bruker TIMS TOF)
o Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
o Multi Angle Light Scattering (MALS)
o Dynamic Light Scattering (DLS)
o Viscometer
o Fourier Transfer-Infrared Spectroscopy (FT-IR) with attenuated total reflectance (ATR)
o Gas Chromatograph (GC) with Flame Ionization Detector (FID) and Thermal Conductivity Detector (TCD), Gas Chromatograph Mass Spectrometry (GC-MS)
o Fluorescence Spectrophotometer
o Polarimeter, Stopped Flow Instrument
o Lambda 25 UV/Vis Spectrophotometer
Cosmetics Products
Cannabinoid/Terpenoid Compounds (Under development)





Analytical Services…
Chemistry Testing Services
Method development & validation
Analysis of Lipids and Carbohydrates
using Charged Aerosol Detector (CAD)
Residual solvent analysis
Physicochemical techniques such as:
UPLC-CAD, LC-MS/MS
UV, XRPD, TGA, DSC, CE-DAD, FT-IR, GC-MS-FID…
Elemental impurities
Solubility & Comparative
Drug product dissolution & release testing
Pharmacopeial / raw
Investigative analysis of drug products
Karl Fischer Moisture Analysis
Validation of cleaning
Biopharmaceutical Testing Services
Amino acid sequence analysis
Structural characterization and confirmation
Physio-chemical properties

Analytical support for clinical trial supplies
Toxic heavy metals analysis (As, Hg, Cd, etc.)
Comparative dissolution testing

Bioanalytical Services…

PHARMA INVENTOR INC. provides method development, method validation and sample analysis for all relevant in-vitro and in-vivo matrices. Our expert bioanalytical team offers our clients a comprehensive range of state-of-the-art bioanalytical services including:
Analysis in Chemistry
• Isotope purity determination.
• Residual solvent determination in new chemical entities.
Analysis in DMPK
• Bioanalysis of in-vivo pharmacokinetic (PK) samples from different matrices, such as plasma and tissues.
• Bioanalysis with well-established procedures for all routine in-vitro ADME studies, such as plasma binding, permeability assays, metabolic stability and solubility.
• Bioanalysis of small, volatile or hydrophilic compounds using UPLC-MS/MS and GC-MS.
Analysis in Protein Science
• Targeted protein quantification including therapeutic biologics and biomarkers.
• Protein characterization including protein MW determination.
Discovery Biology Services…

As a Chemistry R&D company mainly focusing on APIs development, PHARMA INVENTOR INC., has continuous investment into bench-side Discovery Biology capabilities and expertise tailored to small molecule therapeutic projects…
In-Vitro Biology
Drug Metabolism and Pharmacokinetics (DMPK)
Non-GLP Bioanalysis
In-Vivo Pharmacology Studies
Pharmaceutical Microbiology Testing
Toxicology


We offer a comprehensive service that brings together all
End to End Patent Lifecycle Management

Proven Discovery Track Record...



A Novel Synthesis of a Triazolesulfonyl Chloride as a Precursor for GlyT-1 Inhibitors
Kashinath Sadalapure etal., , Discovery R&D, AMRI, 26 Corporate Circle, Albany, NY.
UK-Singapore Symposium on Contemporary Strategies and Practices in Medicinal Chemistry, 8 September 2011, Biopolis, Singapore

DD-Hydantoin recreational water treatment
Case Studies

Case study #1: Seed-stage start-up company
• Private Investor
• No science background
• Short timelines
Developed novel dug compounds
• Fast results & IP position
• Going to clinical





FFS and FTE Services

Each R&D project has different challenges and PHARMA INVENTOR INC. designs the project depending on the level of complexity utilizing either a fee for service (FFS) or a research-based full time equivalent (FTE) approach.

Fee For Service (FFS)



FFS chemistry R&D services approach is chosen depending on the specific needs of each individual project. FFS approach is usually suitable when a project has strong precedent or literature background without the need for extensive development.
Full Time Equivalent (FTE)
For technically more challenging and research-intensive chemistry R&D projects, FTE approach is highly recommended. FTE works very well for dedicated R&D projects and/or for the resolution of complexity/challenging chemistry problems. We can allocate specific team(s) of either one or more scientists with excellent expertise in your field to work on your project.
Pre-Clinical Clinical Phases 1, 2 and 3 Market
SR&ED Benefits: FTE vs FFS

Recognition by Life Sciences Communities










