What Is TMS?
Transcranial Magnetic Stimulation (TMS) is an evidence-based, noninvasive procedure used to treat individuals experiencing treatmentresistant depression. TMS uses fluctuating magnetic fields to increase brain activity.
Does TMS Work?
Yes, TMS is highly effective. PrairieCare’s clinical results show that half of patients experience at least a 50% reduction in their depressive symptoms. On average, patients start TMS with severe or moderate depression, which drops into the mild range after treatment. For patients who have tried multiple antidepressant medications and therapeutic modalities without success, TMS can be life changing.
Who Qualifies for TMS?
• Patients who have a diagnosis of major depressive disorder (MDD)
• Patients who are 18 years of age or older
• Patients who have not seen an improvement in symptoms after trying multiple antidepressant medications from two different classes, medication augmentation trials, and a sufficient trial of psychotherapy
Who Does NOT Qualify for TMS?
• Patients who have any type of non-removable metal in their head or neck (braces and dental fillings are acceptable)
• Patients with a history of stroke or a neurodegenerative disorder
• Patients who have a history of seizures or a current seizure disorder
• Patients who have a history of mania or psychosis
• Patients who are pregnant or planning to become pregnant


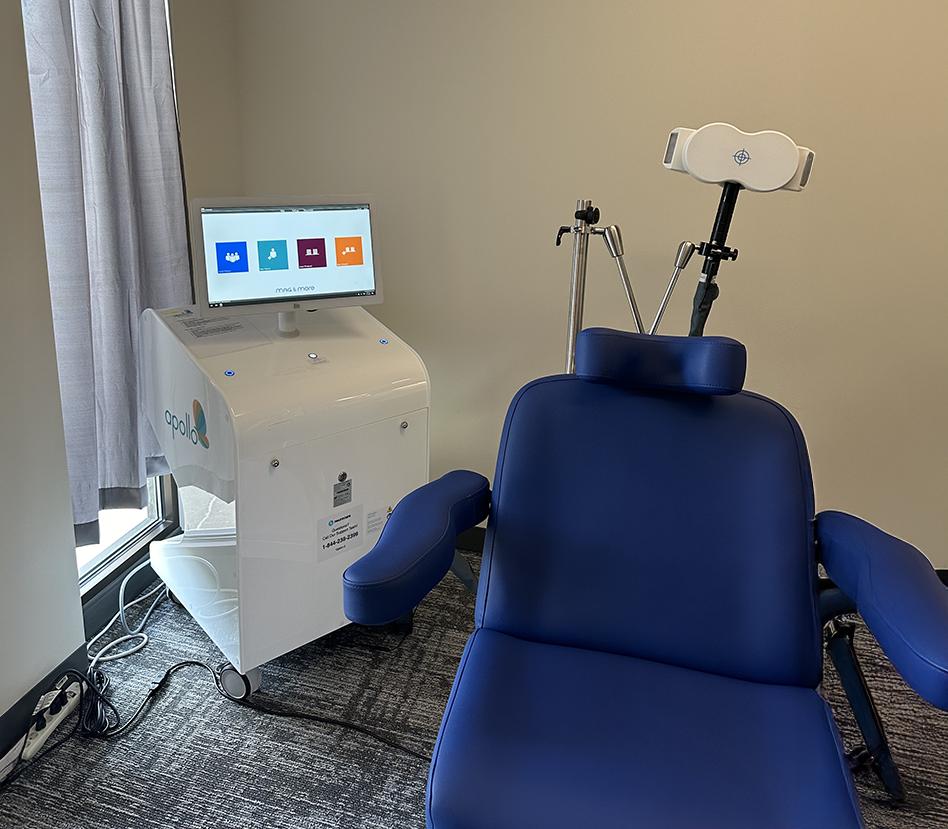















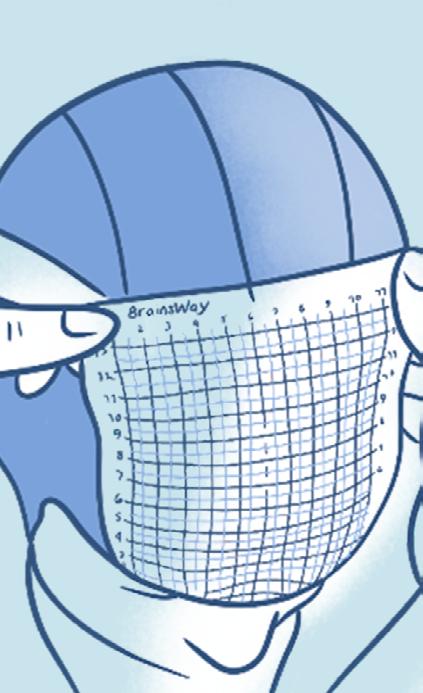


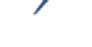











Placing the TMS coil



What to Expect During a TMS Treatment
During the first TMS session, several measurements are made to ensure the TMS coil is properly positioned over the patient’s head. The coil is then secured to the patient’s head. Once treatment begins, patients will hear a series of clicking sounds during the treatment and feel a tapping sensation under the treatment coil.
Each treatment session lasts about 20 minutes, and after the TMS technician removes the coil, the patient can safely resume their daily activities. A typical course of TMS treatment consists of 36–37 treatments over 8–10 weeks, as recommended by your TMS doctor or as covered by your insurance.
How Long Does Remission Last?
Remission lengths and the need for re-treatment vary by patient. According to a study published in the Journal of Clinical Psychiatry, 60% of people who responded to TMS initially had maintained their improvement after 12 months1 .
Potential Risks and Side Effects of TMS
• TMS is well-tolerated and has few side effects. The most common side effects are headache and fatigue. These are mild and generally diminish throughout the course of treatment.
• The most serious risk of TMS is seizures. However, the risk of a seizure is exceedingly low2, at less than 1%. At PrairieCare, we follow upto-date safety guidelines designed to minimize the risk of seizures.


• No long-term side effects associated with TMS have been observed.















Benefits of TMS
• Effective: Proven to reduce depression symptoms by 50% or more
• Safe: FDA approved and non-invasive
• Minimal side effects: Minimal site pain, without any ongoing effects
• Covered by insurance: Accepted by most major plans, including Medicaid/ Medical Assistance plans
• Convenient: 20-minute sessions—then continue with your day right away
How Do I Start TMS Treatment?
As part of PrairieCare’s full continuum of care, Transcranial Magnetic Stimulation (TMS) is a unique service offered at our Edina and Woodbury locations. Contact us today to get started.
952-737-4510