
As a national association, MTAA is the collective voice representing Australia’s dynamic MedTech industry, providing Australians with life-saving technologies. CEO Ian Burgess discusses MTAA’s mission, and paving the way forward for MedTech in Australia
Writer: Alex Tuck | Project Manager: Krisha Canlas
The world is awakening to the potential of technology streamlining operations across the healthcare sector – whether through making patient management systems more efficient or delivering innovative solutions for worldwide problems.
Australia is well-established as a global leader in this dynamic field, thanks to the country’s thriving research environment that has witnessed the
invention of the world’s first bionic ear, the electronic heart pacemaker, and the leading innovation of spray-on skin for burns victims.
Yet challenges prevail within the industry, and new ground is yet to be covered in order to fully exploit the benefits of MedTech for the advantage of Australia’s health care system.
Under the common goal of delivering better health outcomes to communities across the country,

2 | Healthcare Outlook Issue 2 INDUSTRY SPOTLIGHT
the Medical Technology Association of Australia (MTAA) is the collective voice unifying the industry in leveraging the power of technology to drive modern, innovative, and reliable health care.


Serving as the peak body representing the industry, MTAA’s initiatives and interests within this wider goal are diverse; from representing MedTech innovators, to improving public and private sector cohesion, and redressing the gender balance across the sector.
Now, MTAA’s role has risen to the fore, proving critical throughout the course of the COVID-19 pandemic, as Australia‘s health care system comes under an unprecedented level of pressure. Aside from navigating this significant challenge, MTAA is championing Australia’s status as a global leader in MedTech, seeking to increase more local manufacturing and R&D as the country transitions towards a value-based health system.
INTRODUCTION
Healthcare Outlook Issue 2 | 3 MEDICAL TECHNOLOGY ASSOCIATION OF AUSTRALIA (MTAA)
APAC Outlook (AO): Briefly introduce us to Device Technologies, including your location and portfolio of services, number of employees, etc.
Mick Trevaskis, CEO, Device Technologies (MT): Since 1992 Device Technologies has been dedicated to improving patients’ lives through leading-edge technology and services. Successfully supplying healthcare facilities and healthcare professionals with the finest medical solutions for their patients, we continue to grow, with over 200 trusted brands and over 950 highly skilled staff throughout Australia, New Zealand and Asia.
AO: How has the company navigated the COVID-19 pandemic and what impact has this had on your operations?
MT: The COVID-19 pandemic had major impacts on all aspects of our business, such as being called upon to urgently supply ventilators. Elsewhere:
• Our warehouses quickly changed operations, moving to a shift-based model. The two shifts each day were separated by a cleaning period, To ensure continuity of supply to our customers, our warehouses all retain strict COVID-Safe rules, with regular cleaning, mask mandates, and minimal contact with non-essential staff and visitors.
• Within a week of health measures being announced, all employees who could work remotely did so. Device Technologies ensured that all staff were equipped with IT equipment, and security and network upgrades were quickly made to serve a remote workforce.
• Device Technologies was called upon by

government and healthcare organisations to provide support in the form of supplying ventilators at the beginning of the pandemic. Along with importing and deploying over 1,200 ventilators, Device Technologies provides configuration, thorough in-servicing and training, which, along with technical service and routine maintenance, had to be adjusted in line with various hospital visitation rules.
• Some tactics deployed for remote support included:
• Livestream ventilator training for two main ventilator models, as well as neonatal settings.

• 24-hour ventilator support line with direct access to specially trained staff.
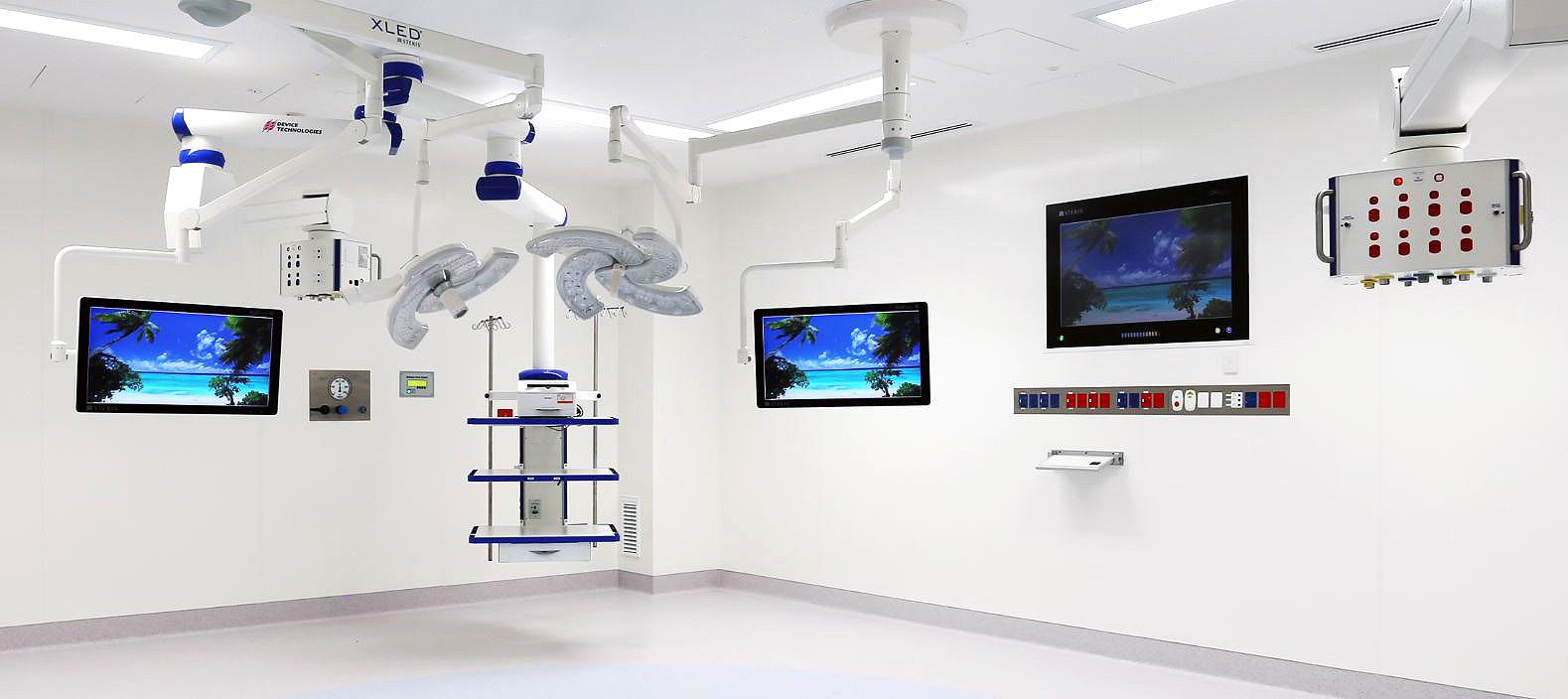
• On-demand virtual training for hospital groups and returning healthcare staff to ensure they could operate ventilators.
• Online Clinical Resource Hub, available to healthcare professionals via login, which housed operating manuals, COVID-19 manufacturer guidelines, operating checklists, educational material and videos.
• Working with state governments around border closures to ensure hospitals continued to receive vital clinical support and technical service.
AO: How did Device Technologies continue to support customers, people and the community during this time?
MT: The pivot to remote workforce. Virtual in-servicing and training for ventilators, followed by other critical care devices, meant that healthcare professionals could complete their training in their own time. The solutions will continue to be offered into the future, as part of our holistic product and service offering.
A large proportion of the workforce was, of course, home-schooling children and juggling all the issues that come with sudden remote working. There was a spirit of camaraderie and support at all levels of the company, with regular virtual team building events. The Executive group conducted quarterly business updates for the entire company, and there was a focus on both communication and inclusion, to ensure everyone felt their importance at Device.
While a significant area of our business, which relies on elective surgery, slowed down, the focus shifted again to education and remote training of surgeons on new products and technologies. Notably, the Robotic Surgery and Digital Education teams enabled a da Vinci surgery first; a surgical case in WA in which the proctoring surgeon was located overseas, and able to participate via livestream, in real time, with little-to-no delay.
AO: Are there any recent or ongoing projects that you would like to highlight?
MT: Device Online, our e-commerce and product catalogue solution, was launched in November 2020. Since then, over 9,000 products have been added to the catalogue, and over 1000 healthcare professionals and facilities have registered for an account, which
they utilise to view products, stock availability and some pricing. This platform continues to expand, with more product portfolios added, and more product information, to become a holistic destination for all Device Technologies product information and education.
Following cancellations of conferences throughout 2020 and again in 2021, our Ophthalmic Diagnostic business needed a solution to reach customers digitally. A campaign was created through LinkedIn and email marketing to ophthalmic customers, communicating a month-long promotion encompassing exclusive packages and discounts. This was the first time Device Technologies had promoted a sales event digitally, and the success and customer feedback ensured that it will be repeated and built upon in years to come, in conjunction with in-person conferences when they return.
AO: How integral is the concept of innovation to Device Technologies and how do you incorporate this within your service offering?
MT: Innovation is integral to the success of Device Technologies, on all levels, from employee satisfaction and engagement to clinical research and digital solutions. The entrepreneurial culture of the company stems from its grassroots beginnings, and all ideas are welcomed.
Device Technologies is renowned for its holistic offering, providing not only products, but ongoing clinical support and technical service solutions.

Healthcare Outlook Issue 2 | 5

www.device.com.au
Another key offering is digital education. Our dedicated team specialises in filming surgical cases within operating theatres, and live-streaming these cases around the world, often in conjunction with industry conferences or training webinars.
Device Online, our e-commerce solution, has been built from scratch to enable healthcare professionals the ability to browse the Device product catalogue. Key account holders have the ability to request quotes as well as place orders. The convenient portal also houses product information in the form of downloadable flyers, educational videos and even operating instructions.
Device Technologies is committed to partnering with local key opinion leaders including leading surgeons and hospital executives. A move towards local manufacturing is also allowing us to collaborate with surgeons to develop innovative solutions to meet their needs.
Currently, Device Technologies is sponsoring an Australian-led randomised, controlled, comparative, multicentre study evaluating JointRep and Microfracture in Repair of Focal Articular Cartilage Lesions on the Femoral Condyle or Trochlea.

AO: Could you outline Device Technologies’ growth strategy for the years ahead?
MT: Our growth strategy for FY21-24 is currently in progress, centred around our promise to enable superior outcomes for patients by providing them with access to the best medical technology available worldwide. Over the next three years our key strategic pillars are: Organic Business Growth in Australia and New Zealand, Asia Expansion, Organisational Excellence and Strategic Acquisitions. These pillars are led by our values: Innovation; Ownership; Collaboration and Practising Good Business, and enabled by our continued focus on leveraging data, digital solutions, our people, providing education and delivering exceptional customer experience.
AO: Finally, do you have any further goals for diversification, or key priorities for the near future?
MT: Our continued focus on data and digital innovation has enabled collaboration with leading suppliers around the globe in the digital health space. Currently we’re focusing on technology that enables remote diagnosis
and monitoring. These devices and complementary technology are designed to enhance efficiency and accuracy for healthcare provide comfort and convenience for patients.
Wherever possible, we’re helping to initiate, sponsor and support Australian-led clinical studies, whether in research institutes or by practicing academic surgeons. Currently we’re supporting the above mentioned JointRep and Microfracture clinical trial, for which the Principal Investigator is Australian Surgeon and past President of the Australian Knee Society, Dr Bruce Caldwell. Participants in this trial are being enrolled by surgeons across Australia, New Zealand and Canada.
We are launching a portfolio of Australian manufactured products in 2022, which will encompass a range of specialties including ophthalmic, orthopaedic and infection prevention devices and medical products. This partnership with onshore manufacturers will enable us to deliver consistency, reliability and quality on particular product lines that measure the same products currently imported from overseas.
Medical Technology Association of Australia (MTAA)

that medical technology will be valued as a key driver of a healthier Australia.
HO: Since inception, how has the MTAA developed and progressed in terms of its key objectives and the messages it tries to get across?
IB: Since its inception, membership of the MTAA has grown steadily and our influence over health policy, funding and regulation in Australia has increased.
Healthcare Outlook (HO): Can you tell us about MTAA, and what role it plays in Australia’s MedTech industry?

Ian Burgess, CEO (IB): The Medical Technology Association of Australia (MTAA) was created as the national association representing manufacturers and suppliers of medical technology used in the diagnosis, prevention, treatment and management of disease and disability.

Our members manufacture and supply a diverse range of medical technology, ranging from syringes and wound dressings, through to high technology implanted devices such as pacemakers, defibrillators, hip and other orthopaedic implants. MTAA members also manufacture hospital and diagnostic imaging equipment including ultrasounds and magnetic resonance imaging machines.
As well as producing and distributing medical devices and technologies, MTAA member companies also play a vital role in providing healthcare professionals with essential education and training to ensure safe and effective use of medical technology.
Our members include large, established global companies as well as local start-ups and SMEs and currently total 123 companies across Australia.
MTAA’s mission is to champion medical technology for a healthier Australia. Our vision is
We have developed close relationships with governments and worked closely with Ministers and parliamentarians in relevant portfolios, including health and industry. We have represented the interests of the MedTech industry to government in consultations, reviews, and inquiries, including developing an agreement in 2017 on the reform of funding for medical devices in the private hospital sector.
Over this time, MTAA has developed productive relationships with other health peak bodies, such as the Australian Medical Association, the Australian Private Hospitals Association, and the Consumers Health Forum of Australia. On many issues of mutual concern, MTAA has worked collaboratively with these organisations to develop joint positions on key health issues and present a united health sector voice to government.

INTERVIEW
8 | Healthcare Outlook Issue 2 INDUSTRY SPOTLIGHT
Ian Burgess Chief Executive Officer, MTAA
MTAA has also been working to address broader MedTech industry issues such as the underrepresentation of women.
In 2015, the MTAA Board launched the Women in MedTech initiative, to lead the debate and promote the benefits of gender diversity in the workplace and within the MedTech Industry. The Board established a Women in MedTech Committee to support the mission of the organisation, industry and wider community.
The Women in MedTech (WiMT) mission aims to proactively support gender diversity within the MedTech industry, effectively supporting women to unlock their full potential.
HO: What do you find most exciting about working in Australia’s healthcare sector?
IB: MedTech is one of the most advanced and dynamic manufacturing sectors in Australia and has the potential to provide substantial health gains and highly skilled employment and export opportunities for Australia.

Despite our small population, Australia is a global force in MedTech. With a total market for medical devices in Australia over US$4.9 billion[1], Australia is ranked at 13th in terms of total market value worldwide, according to the Worldwide Medical Device Factbook.[2]
The MedTech industry in Australia is also a substantial employer. In 2014, it was estimated the industry employs approximately 19,000 people, excluding those working in digital health. Overall, 78 percent of all MedTech employees have graduated with a university degree, demonstrating the highly educated nature of the workforce. Of these employees, 52 percent earned an undergraduate degree, and a further 25 percent completed a postgraduate degree. [3]
Australia has a proud history of innovation and
commercial success in this industry, with companies such as Prism Surgical, Cochlear Australia and ResMed, exporting Australian innovation in medical devices to the world. It is exciting to be part of this fast-moving industry sector which harnesses the talents of Australia’s world class scientists, clinicians, and medical researchers to solve the health problems of today and tomorrow.
Over the past two years of the COVID-19 pandemic, the MedTech sector has played a leadership role in coordinating supply of medical devices and equipment and working with government to meet challenges caused by global supply chain disruption. The sector has played a key role in quantifying existing stock of crucial medical devices, ensuring additional supply in the context of global supply constraints, and ramping up domestic manufacturing to address gaps in the market.
Two standout examples of industry innovation during the COVID-19 pandemic, are the consortium formed by Grey Innovation (with the support of the Federal and Victorian state governments) to

Healthcare Outlook Issue 2 | 9 MEDICAL TECHNOLOGY ASSOCIATION OF AUSTRALIA (MTAA)
produce ventilators domestically and the move by 3D Meditech to pivot their manufacturing facilities to 3D print the swabs needed for COVID-19 testing.
It has been extremely rewarding for the MTAA to be able to support companies on the front line of our health system to ensure the supply of equipment and devices needed to diagnose, treat, and support patients during the course of the pandemic.

Ultimately, the fundamental purpose of the MedTech industry is all about patients – savings lives, extending lives and providing people a better quality of life.

HO: On the flip side, what are its biggest challenges?
IB: The fragmentation of Australia’s health system creates ongoing challenges for the MedTech industry. Responsibility for the delivery of health care in Australia is split between the Commonwealth and State/Territory governments which means that there is often duplication of processes and systems. There are also inconsistencies between the public and private health sectors which adds an additional level of complexity for companies operating across both domains.
This year, a major challenge for the MedTech industry has been the ongoing negotiations with government over changes to the Prostheses List, the funding mechanism for devices in the private hospital system. This has involved MTAA and our members working hard to combat the efforts of private health insurers to exert more power over device funding and undermine clinical freedom and patient choice.
The negotiation process over the future of the Prostheses List has been an important focus of our industry over the past 12 months, with member companies engaging on multiple fronts with policymakers to ensure positive reforms using direct advocacy, collaboration, and strategic communications.
The COVID-19 pandemic has also presented a number of challenges, with far-reaching social, environmental and economic impacts. Our health system has been put under pressure with increased demands on hospitals and additional pressures on an already stressed health workforce. Cancellations of elective surgery in many states have had negative impacts on patients waiting for procedures to treat their pain and disability. They have also impacted MTAA member companies which have faced a substantial reduction in demand for products and services over this period, leading to cuts in revenue up to 90 percent for some companies.
Freight costs have dramatically increased over the course of the pandemic, in some cases by up to 800 percent, adding to the enormous pressure on our industry. Companies have had to adjust their operations to enable employees to work from home and implement split shifts for manufacturing or warehousing workforces. Limitations on company representatives entering hospitals has also restricted the capacity of companies to support clinicians and nurses before, during and after operations.
While all areas of the health system have been
INTERVIEW
10 | Healthcare Outlook Issue 2 INDUSTRY SPOTLIGHT
Celebrating 30 Years of
Since 1992 Device Technologies has been dedicated to improving patients’ lives through leading-edge medical technology and services. Successfully supplying hospitals and healthcare professionals with the finest medical solutions for their patients, Device Technologies represents leading global suppliers and manufacturers across all areas of healthcare.





Device Technologies continues to grow and innovate, with over 200 trusted brands and over 950 highly skilled staff throughout Australia, New Zealand and Asia.

To find out more, head to device.com.au
These products are not available for purchase by the general public.
adversely impacted by COVID-19, the pandemic has had a particularly serious impact on companies involved in clinical trials, as many of these have been delayed or put on hold, and on start-up companies which do not have the capacity to absorb market downturns.

Despite these multiple challenges, MTAA member companies have continued to support Australia’s COVID-19 response and the ongoing functioning of other areas of the health system during the course of the pandemic.
HO: What trends are currently transforming the healthcare sector across Australia, and how is the MTAA responding to them?

IB: In Australia, as in many developed countries, the health system is under pressure due to the ageing of our population and the resulting increase in chronic disease and disability. This is placing increased demand for health services and highlights the importance of focusing on prevention and management to increase quality of life.
working with members to develop strategies to promote the value of evidence-based technologies to reduce pain and disability and save costs elsewhere in the system.
Innovations in technology will provide exciting opportunities for improving treatments as well as challenges to Australia’s regulatory and funding systems. For example, the increased use of robotic technologies throughout the health system, including in surgery, aged care, and pharmacy, will require the development of appropriate regulations and practices to ensure quality, safety and accountability standards are maintained.
Along with the move towards a value-based health system, there is an increased focus on consumer input in health policies, programmes, and funding decisions. This is occurring both at the services delivery level, for example through increased consumer representation on hospital boards and committees, as well as at the government level via consultation processes and the inclusion of consumer organisations on relevant committees and reviews.
Australia is also exploring ways to move from paying for services to a more value-based approach to health care to divert resources into the services or strategies which deliver greatest value.
MTAA is closely monitoring this debate and
This highlights the importance for MTAA of increased engagement between the MedTech industry and consumer organisations to support dialogue about key MedTech issues and ensure mutual understanding of each industry’s role in promoting a high quality and good value health system for Australia’s future.


INTERVIEW
12 | Healthcare Outlook Issue 2 INDUSTRY SPOTLIGHT
HO: Have you got any projects in the pipeline you wish to highlight?
IB: One important current focus for MTAA is to increase Australia’s ability to attract clinical trials. Australia has a number of benefits as a site for clinical trials: a high-quality health system; world class clinicians and medical researchers; and a record of ‘early adoption’ of medical technology by the Australian community. However, there are some regulatory and administrative barriers which prevent Australia from becoming a preferred destination for international companies looking to run clinical trials. As a result, Australia is missing out on the health and economic advantages that come from supporting this important sector of the health system.
MTAA is currently collaborating with Medicines Australia and AusBioTech on the Research and Development Task Force which aims to offer a unique industry perspective to stakeholders across Federal and State Governments as well as the broader health and research and development sector.

Key goals for this taskforce are to promote the federal government’s ‘One-Stop-Shop’ and ‘Front Door’ initiatives and to support a national, interconnected, rapid and streamlined approvals platform for clinical trials.
Improving Australia’s system of Health Technology Assessment (HTA) is another MTAA priority, in order to streamline current processes across sectors and jurisdictions to ensure Australians can access evidence-based innovative health technologies and treatments.


Currently, HTA in Australia is fragmented across jurisdictions and sectors. For example, drugs and vaccines are reviewed by one agency (Pharmaceutical Benefits Advisory Committee), whilst procedures, tests and devices are reviewed by a different agency (Medical Services Advisory Committee). This creates inconsistencies between these two areas and a lack of clarity for companies, particularly those involved in the development of co-dependent and hybrid technologies.
A key focus for the MTAA is to promote changes to HTA which streamline current processes and take into account the unique characteristics of medical technology. Another priority will be ensuring we implement the main learnings from the COVID-19 pandemic, including the need to protect Australia’s sovereign capability through more local manufacturing and R&D.

HO: How do you see the MTAA developing over the next five years?
IB: Over the next five years the MTAA will continue to advocate for regulatory and reimbursement reform to allow for better innovation and commercialisation and to make Australia a world leader in MedTech innovation. MTAA will also continue our work to promote greater harmonisation of regulatory and reimbursement with other jurisdictions to reduce the administrative burden on companies and support access to new technologies for Australian consumers.
MTAA believes that this requires a structure around government-industry engagement to ensure early collaboration on emerging health challenges and to avoid preventable problems. By working closely with government and other stakeholders, the MedTech sector will strengthen Australia’s capacity to respond to further health threats and to maximise our potential as a global MedTech leader.
INTERVIEW
Healthcare Outlook Issue 2 | 13 MEDICAL TECHNOLOGY ASSOCIATION OF AUSTRALIA (MTAA) Tel: (02) 9900 0600 www.mtaa.org.au/contact-us


PRODUCED BY HEALTHCARE OUTLOOK MAGAZINE
TECHNOLOGY ASSOCIATION OF AUSTRALIA (MTAA)
4, 97 Waterloo Road, Macquarie Park, NSW 2113 Tel: (02) 9900 0600
MEDICAL
Level
www.mtaa.org.au/contact-us












































