














Center for Breakthrough Medicines is striving for a future where advanced therapies are available and affordable for every patient. We discuss the miraculous development of biotechnology and the company’s hunger for innovation with Chief Strategy Officer, Alan Moore
Writer: Ed Budds | Project Manager: Felix Revell

Center for Breakthrough Medicines (CBM) is on a mission to save lives. An advanced therapy focused Contract Development and Manufacturing Organization (CDMO) dedicated to delivering life-changing treatments to the patients that need them, CBM’s leaders have successfully led cell and gene therapy (CGT) programs at innovator companies and are bringing their expertise to solving the challenges associated with CGT manufacturing. CBM also boasts partnerships with industry leading pioneers who have taken advanced therapies to mass commercialization. The company leads with science, developing the platforms and processes that turn its clients’ ideas into treatments that go on to transform patients’ lives.

Spearheading identification of new technologies for CBM, as Chief Strategy Officer (CSO), Alan Moore oversees new client development and on-boarding, building strategic partnerships and collaborations. He is now able to utilize and call upon 40 years of experience in the life sciences contract services field.
 Alan Moore, Chief Strategy Officer
Alan Moore, Chief Strategy Officer
“My father was a physician, and my mother was a head nurse. I remember my dad used to take us to the hospital to visit the patients after Sunday school, so I was destined to be a physician too. However, this was shortly after the discovery of restriction enzymes, and I became fascinated with biotechnology, so my dad gave me permission to not seek the family trade,” he recalls.
Starting out as a laboratory technician at Microbiological Associates, just as the biotechnology (biotech) revolution was starting and the company was simultaneously experiencing rapid growth, Moore moved into a management position where he helped build a strong organization to test and ensure the

Alan Moore, Chief Strategy Officer: “Technology is absolutely crucial, from AI and machine learning (ML) to a number of different applications that range across the analysis of patient outcomes and relating those back to the manufacturing processes and the performance of the manufacture of products.
“Some of these applications are helping to design highquality viral vectors, making them more specific, and giving them the ability to target the exact tissue that is required to deliver the specific therapy needed for each individual patient.
“Technology can also be applied to help establish the knowledge that’s necessary to conduct gene editing, making sure that you know what the target is and are able to finely conduct the gene editing required to deliver the desired effect.
“Very early on, I saw the success of ML in the development of diagnostic tests or pediatric cancers. There were a series of cancers that were identified as small, round, blue cell tumors and some were very invasive, requiring aggressive surgery. Through ML, researchers were then able to identify a subset of genes that could uniquely identify these various types of cancers, and that was very important to pediatric oncologists.
“As a result of this, it’s clear that there are numerous key applications of ML and AI across diagnosis, design and development and manufacturing of these advanced therapies.”
safety of biotech products, gaining invaluable experience.
Later, in a role at Genzyme, Moore subsequently became enthralled with the concept of cell therapy. At this time, he was working with the National Cancer Institute and his team manufactured patient specific products for B cell lymphoma and multiple myeloma.

“I was then eventually attracted to CBM because of the specific mission presented to me, which was to build an end-to-end capability to advance cell therapies as rapidly as possible. Our founder was insistent that any of the roadblocks that face these products needed to be removed, which was a challenge that I was very excited to take up,” explains Moore, who remains fascinated by his profession.
“When you’re working on personalized cell therapies, you are as close to the patient as you’re ever going to get in support of the development of these products. The industry is moving rapidly as the technologies are advancing at light speed, so the progress that’s being made is exciting to witness.”
Seamless extension of your team managing the design and construction of your facility to be revenue ready as quickly and efficiently as possible

LET’S TALK ABOUT YOUR UNIQUE PROJECT VISION
thecarlincollaborative.com

Presently, due to the infancy of the technologies and processes that are being employed in the current incarnation, the manufacturing of these products can be inefficient and costly. However, CBM actively partners with state of the art companies to work towards an improvement.
“One of the other things that’s equally fascinating and encouraging is seeing our partner companies and tool providers jumping in with both feet now that the products have demonstrated success and that there’s been significant investment,” Moore adds.
CBM is partnering as broadly as possible with innovators so that the company is constantly aware of the latest developments and the everevolving future of processes and tools that will soon be utilized to efficiently produce key products.
The recent devaluation of many biotech companies due to shrinking stock prices has sparked a sectorwide shift towards focusing on the development of fewer, but later stage products. Previously, it was believed that in order to maximize value, a company had to establish its own manufacturing platform. In this capital-constrained environment, the concept of “build versus buy” is losing

• Embrace customers
• Be committed
• Respect all, always
• Ignite innovation
• Empower all teams
• Give your all, at all times
favor, as companies opt to allocate their limited capital towards therapy development while outsourcing the manufacturing process.
This trend continues to underline the importance of a CDMO, as well as the role it plays in helping biotech products move forward proactively.
“Another emerging trend is a greater capability on the part of CDMOs. For example, here at CBM, we established at the outset that we were going to have commercial scale and commercial ready facilities because our products can quickly move through the development cycle, from clinical, early clinical to late clinical and then finally, commercial manufacturing,” he declares.
Elsewhere, regulatory agencies have recognized the importance of many of these products and have subsequently provided mechanisms
to accelerate its approval process, which is gradually benefiting and enhancing the industry.
“We exist in a high growth environment where things are rapidly changing, and being able to keep your head above water and keep focused on the end goal is important,” expresses Moore.
“Often in science, we are very skittish around anything that we don’t have all of the answers to, and one of the things that I find constantly rewarding is being able to call on your core understanding and concepts and then apply those to new challenges.”
CBM was established with the goal of providing a complete end-to-end solution for developers of advanced
“WE HAVE ONE OF THE MOST EXPERIENCED TEAMS IN THE INDUSTRY AT PRESENT, INCLUDING PIONEERS IN CELL THERAPY AND VIRAL VECTOR MANUFACTURING”
– ALAN MOORE, CHIEF STRATEGY OFFICER, CENTER FOR BREAKTHROUGH MEDICINES





















A unique isolator workstation integrated with Bioquell bio-decontamination, distinct features, and more





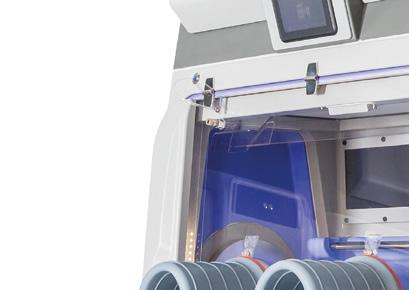

◥ 2, 4 or 6 glove options with up to 2 transfer chambers or RTPs














◥ Innovative build allows for installation and validation in as fast as 16 weeks from order date










◥ No construction, additional electrical work, or HVAC required for installation




Room bio-decontamination systems for automated, mobile and scalable bio-decontamination.
















Bioquell
L-4


Help eliminate microbial contamination from exposed surfaces with systems providing validated cycles for enclosures and equipment.









A scalable service response option with validated results to help quell emergency contamination events to scheduled shutdown periods.

therapies, including support that provides the efficient transfer of a process all the way from development into manufacturing.
“One of the ways that CBM accomplishes this is by establishing the same scale of manufacturing platform in the process development area that is involved in manufacturing. This is pretty unique as companies usually don’t have large scale process development capabilities,” explains Moore.
“We’ve also recognized that there are unique challenges for cell therapies. We are dealing with patient specific cell therapies as well as the shelf cell therapies, and they differ vastly in complexity and scale.”
One of the challenges commonly encountered with patient-specific cell and gene therapies (CGT) is being able to efficiently receive patient material to engineer, move it rapidly, and then through the manufacturing process get it back to the patient quickly.

CBM has innovatively established

121 North Center Drive
North Brunswick, NJ 08902
+1 732 422 7585
bione@distekinc.com
www.distekinc.com
At Distek, quality and innovation are at the forefront of our bioreactor solutions. Our BIOne 1250 control station has revolutionized bioprocessing for both mammalian and microbial models. With a sleek appearance and intuitive touchscreen interface, the BIOne 1250 is designed to be the easiest-to-use and most capable standalone controller on the market, making it ideal for both novice and experienced users.

Our BIOne interface puts full control of all bioreactor parameters in your hands, providing real-time data on all bioreactor parameters. This empowers you to easily optimize bioprocess performance for optimal control and efficiency.
The standard controller options, including OPC and Additional Input & Outputs, significantly expand the capabilities of the controller, providing end-users with almost infinite process expansion potential. This feature allows you to engineer and develop future bioprocesses with ease.
In addition, the BIOne 1250 can seamlessly transition between Distek’s autoclavable and single-use bioreactors in seconds, offering versatility for all bioprocessing needs.
Distek’s bioprocessing solutions offer a range of options to fit various process needs, including the BIOne single-use bioreactor available in 2L, 5L, and 10L working volumes, which can be customized above and below the headplate for specific applications.

The BIOne single-use bioreactor exemplifies the industry’s growing preference for single-use technologies, eliminating the need for assembly, cleaning, or sterilization, resulting in an efficient and hassle-free solution for bioprocessing applications. Assembled in Distek’s advanced ISO-7 cleanroom, the BIOne SUB is then gamma-sterilized, significantly reducing the risk of contamination, ensuring that the bioreactor is ready to use immediately upon delivery.
The BIOne’s versatility and compatibility with a range of bioprocess controllers make it an ideal choice for researchers, scientists, and bioprocess engineers seeking a reliable and efficient system for their experiments or production processes. Whether working on complex cell culture models or developing new gene therapies, the BIOne single-use bioreactor provides an easy-to-use and versatile platform for bioprocessing applications, while its scalable design allows for seamless integration into existing workflows.
Contact Distek today at bione@distekinc.com to learn more or schedule your free evaluation.
digital manufacturing capabilities, which take the complexity of paper records and look at ways of reviewing data and streamline it, creating a more efficient process.
CBM’s process development team begins its operations by transferring knowledge from their clients to ensure a robust and scalable process. The company’s platform enables clients to reduce the development timeline and deliver the potentially life-saving therapies to patients sooner.
“We can map the entire journey of a product from early development to commercialization, building in the requisite milestones to track progress and ensure quality all the way down
the path,” Moore sets out.
In this way, CBM is fully transparent in its partnerships, with an experienced program management team providing clear oversight of a project through regular updates, on-site access, and the potential for dedicated office space with the Partner-in-Plant program.
CBM, is conveniently located in near Philadelphia- the birthplace of CGT and operates the largest fully integrated single-site development and manufacturing facility for advanced therapies.
CBM serves as a CDMO dedicated
to overcoming manufacturing challenges and providing timely access to life-saving cell therapies. Their 700,000 sq. ft facility has plans to build out 20+ suites each- for viral vector production and cell therapy, with the site offering customization and expansion options to meet client needs.
Their experienced team supports clients from early development to commercialization, leveraging a large facility and client-centric program management. CBM offers end-toend capabilities, including process and analytical development, CGMP manufacturing, and rigorous quality control measures.

The Universal Machine and Engineering Corporation specializes in the automating industry. Our fully degreed engineering team is a key part of being a single source solution provider to our customers. Our engineers design and ensure that each control panel, electrical panel, automated system or robotic device works as specified. Quality, reliability, accuracy and safety are at the core of every design that Universal Machine produces. Our customers return again and again because they understand that when they invest in Universal Machine technology, they get the support of a team of Universal engineers to keep their technology running reliably and profitably.
Our symbol, Oscar the Octopus, represents our many arms. These arms symbolize our experience, knowledge and dedication to serve our customers. They are hands which perform in all areas of the house –which means complete control of all aspects of your project.
The Universal Machine and Engineering Corporation team wraps its arms around all areas of system integration, including: design, fabrication, assembly, testing, controls, installation, specification, validation, training and maintenance.




The CBM team is committed to ensuring program integrity through a well-structured chain of identity including streamlined manufacturing processes, execution, and quality systems to minimize hiccups and enhance efficiency.
“Our single-source integrated services simplify the management of programs and support rapid advancement to clinical trials and commercial production. CBM has the expertise and production agility to keep projects moving, as well as the crucial knowledge of the regulatory environment,” Moore expands.

Forming strategic partnerships within the CGT space requires high levels of collaboration from all parties involved. At present, the cost is high, the production is inefficient, and newer, better methods must be found through these collaborations.
Already in 2023, CBM has kickstarted this process and
has invested in a series of new agreements. In January, the company signed a licensing deal with UK-based Autolomous, a developer of critical manufacturing management systems for CGT.
This will prove to be vital as the future is all digital, and companies require digital batch records. Therefore, artificial intelligence (AI) is needed to help manufacture more efficiently, understand the surrounding manufacturing environment, analyze what makes the best optimal batch requirements, and have data to help support that decision making.
Also, earlier this year, CBM
signed a deal with Boston-based Asimov, to license Asimov’s HEK293 viral vector production platform for use in preclinical and clinical manufacturing. This was followed by the announcement of a new partnerships with Virion Therapeutics, a clinical-stage biotech company focused on the development of novel, adaptable, and accessible CD8+ T cell-based technologies focused on cancers and infectious diseases, as well as a separate partnership with Stoic Bio and Nucleus Biologics, making the company the preferred supplier of cell culture media and other critical biological solutions for CBM.
“WE BRING ALL OF OUR FOCUS AND ENERGY TO WORK, HAVE AUTHENTIC CONVERSATIONS AND HAVE FUN WORKING WITH OUR COLLEAGUES”
– ALAN MOORE, CHIEF STRATEGY OFFICER, CENTER FOR BREAKTHROUGH MEDICINES
Gemini Bio is proud to support Center for Breakthrough Medicines with custom manufactured cGMP buffers.
GeminiBio has the capability, agility, and speed C> CDMOs require:





Industry Leading Scalability
– cGMP batch sizes from 10 liters to 10,000 liters
Industry Leading Customer Experience
– 48 hour feasibility and 10-week lead times
Industry Leading Solution Capabilities
– water for injection, acids, bases, media hydration, salt solutions, solvents, etc.

Optima is your strategic turnkey partner. Based on renowned platforms using the most advanced technologies, we help you to make fill-and-finish processes more secure, flexible and productive.
The new OPTIMA FillCell is one of our innovative highlights in the ATMP sector. A flexible and modular system for filling valuable cell and gene products with product saving features. Every drop is precious.
- Flexible and modular design
- Maximization of product yield with comprehensive product-saving features
- Flexible, gentle, and safe handling with a format-less transport system
Our partner Center for Breakthrough Medicine, established as a world class advanced therapies CDMO, is equipped with a fully automated and isolated FillCell to enhance their drug product capabilities. The top-of-the-line filling solution reduces product loss while significantly increasing filling speeds.





“The difficulty with early-stage CGT companies is manufacturing and development, which was exacerbated during the COVID-19 pandemic. It was very difficult to procure some of these media and cell culture solutions, so the partnership with Stoic and Nucleus Biologics allows us to be a

preferred partner of choice,” Moore expands.
Part of an exciting time for the company, creating an alignment of interests is crucial to CBM’s plans moving forward as it aims to continue adding new partnerships to its collaborative roster.
From the outset, CBM recognized that it must empower its employees, and because of the breadth of services and specialization that the company offers, this cannot be driven from the top down. Specific staff achievements are regularly recognized at town hall meetings where the heads of the business units recognize the teams that have had success and accomplished
great things.
Looking to increase its already excellent strand of community engagement, CBM works with the Orphan Disease Center at the University of Pennsylvania. This is alongside its ongoing work with the Emily Whitehead Foundation and the Friedreich’s Ataxia Research Alliance.

Individuals or spokespersons for these companies are often invited to come and visit on site to motivate CBM’s team and interact with them.
Similarly central to all its success is CBM’s remarkable team of people that includes some of the brightest minds in the industry, who continue to build and innovate the extraordinary.
Everyone involved at CBM shares the same passion for saving and improving patients’ lives. This unifying culture drives its staff every day and
as the operation continues to expand, CBM is always seeking to add and enhance like-minded talent to its team.
“We have one of the most experienced teams in the industry at present, including pioneers in cell therapy and viral vector manufacturing – and the largest single-site facility in the world that is truly state of the art and located in an

ecosystem of heavily invested, worldclass biotechnology companies,” Moore outlines.
Upon joining CBM, new team members find themselves immersed in a culture dedicated to progressing advanced therapies to the clinic as fast as possible. Through this successful environment, CBM empowers its people to share ideas, innovate and collaborate, as every
voice is valued in the mission of bringing life-changing therapies to patients in need.
Ensconced among a team of peers and mentors with strong scientific and technical prowess, and proven experience in bringing advanced therapies to market, CBM inspires its staff to play their part in unearthing the next big breakthrough.
With the support of cutting-edge technology, an elite level of industry pioneers and a patient-centric company culture, there is no limit to what CBM can achieve.
Tel: 866-274-4009
breakthroughmedicines.com

“WE CAN MAP THE ENTIRE JOURNEY OF A PRODUCT FROM EARLY DEVELOPMENT TO COMMERCIALIZATION, BUILDING IN THE REQUISITE MILESTONES TO TRACK PROGRESS AND ENSURE QUALITY”
– ALAN MOORE, CHIEF STRATEGY OFFICER, CENTER FOR BREAKTHROUGH MEDICINES

