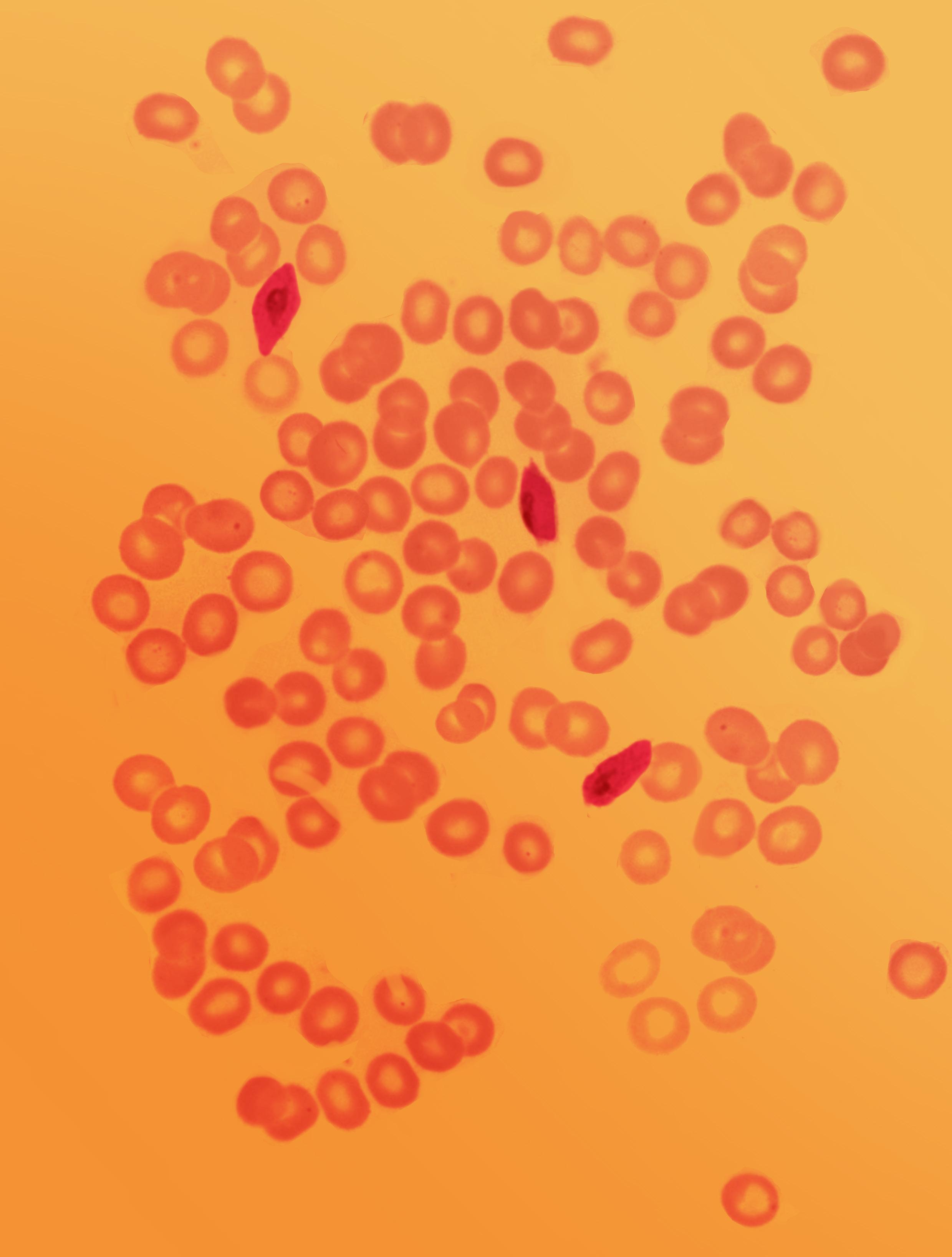
FIGURE 7.0 | Composite of microscopic images of blood smears showing red blood cells infected with Plasmodium vivax trophozoites.
CHAPTER 7

FIGURE 7.0 | Composite of microscopic images of blood smears showing red blood cells infected with Plasmodium vivax trophozoites.

CHAPTER 7
Here are some essential terms used in diagnostic tests. By the end of this chapter, you should be able to apply these concepts and understand how they relate to each other.
Antibody Tests
Antigen Tests
Bacterial Culture
Blood Smear
False Negative
False Positive
Invasive Tests
Non-Invasive Tests
Polymerase Chain Reaction (PCR)
Pooled Testing
Sensitivity
Specificity
Wastewater Testing
A diagnostic test is a procedure designed to determine the presence of a specific pathogen or illness in an organism. The first diagnostic tests used simple methods, such as examining the properties of urine, or directly visualizing microbes to help identify diseases. As newer technologies have been developed, diagnostic tests have become increasingly accurate and accessible.
Diagnostic tests for infectious diseases rely on biomarkers to either detect the pathogen itself or the footprint of the pathogen’s presence – current or past. There are five common diagnostic approaches for the identification of infectious disease. These methods detect: (1) the whole microbe, (2) its genome, (3) its proteins, (4) the host’s immune response to the microbe, or (5) a biological marker of the disease process.
Diagnostic tests can be deployed using different types of samples, in different settings, and using different underlying approaches. Diagnostic tests can also be classified by the way they are administered, the settings in which they are performed, the number of samples included in a single test, and the number of diseases they can target per test.
The utility of a diagnostic test relies on the ability to provide accurate results, giving a positive result when the pathogen or disease state is present, and negative when it is not. Scientists evaluate the performance and clinical utility of diagnostic tests by measuring sensitivity, specificity, and predictive value, which are parameters assessing how accurately a test can detect both the presence of a pathogen as well as its absence.
Healthcare professionals decide which diagnostic approaches best suit their patients’ and their communities’ needs. Factors that influence these decisions include the features of specific diseases, the economics of the region, and the available healthcare services.


By the end of this chapter you will have a foundational understanding of the different types of diagnostic tests used to detect either current or past presence of a pathogen, and the specific scientific mechanisms by which they work. You will also understand the different settings and circumstances in which we use these diagnostics, how we measure their performance, and the advantages and disadvantages of different tests within different contexts. Overall, you will understand the importance of diagnostic testing for public health and outbreak response.
It’s July 20, 2014, and you are a doctor at First Consultant Hospital in Lagos, Nigeria. As you continue to check on your patients late into the evening, a new patient is rushed into the hospital. You are told that the man is a Liberian-American lawyer named Patrick Sawyer, who has fallen ill at the Lagos airport. He was traveling to a work conference from Liberia, the center of a known, brewing Ebola outbreak. When questioned, he denies having contact with anyone infected with Ebola, and is subsequently given anti-malarials for his malaria-like symptoms. He is clearly in no shape to leave the hospital; nonetheless, he insists on being discharged.
You are not yet sure what exactly is causing Sawyer’s illness, so you take a blood sample for further diagnostic testing and begin to consider all possible causes. For the past few months, you’ve been observing the spread of Ebola – first in Guinea, and then in Liberia and Sierra Leone. The virus has propagated
“By recognizing that infectious disease is not some faraway exotic issue but a global problem, and by sharing the responsibility for its prevention, diagnosis, and control, the whole world will be a lot safer.”
—Seth Berkley, American epidemiologist
destruction, decimating communities and tearing families apart. Guinea’s healthcare systems weren’t able to identify the first emerging cases until it was too late, and the virus has now spread beyond control. Having watched this tragedy unfold abroad, you recognize how important early and accurate diagnostic testing is in mitigating a devastating public health crisis.
You send out Sawyer’s samples to be tested for Ebola. The city of Lagos is fortunate to have two labs near the city that can perform the essential diagnostic testing without delay. Both labs have facilities for PCR-based testing—a procedure that allows scientists to detect and highlight part of a pathogen’s genome if it is present.
When the samples arrive at the labs, the scientists immediately get to work processing them. One lab has a test that targets a part of the genome present in all filoviruses, the viral family that includes Ebola, and they confirm the presence of a filovirus. The second lab has access to a test that specifically detects the Ebola virus genome, and it confirms that your patient does, in fact, have an Ebola infection.
Sawyer remains adamant that he has malaria, a non-contagious and treatable infection, and is so insistent on leaving the hospital to attend a business conference that he is trying to fight his way out. Now certain of the dire threat his infection poses to others, you barricade Sawyer’s room and, with the help of staff members, wrestle him to the ground to keep him isolated from the general public. You soon discover that Sawyer had not been forthcoming regarding his contacts; back in Liberia, he had cared for an Ebola patient and was even hospitalized himself before boarding the plane to Lagos.
You know that time is now of the essence, and in order to make public health decisions, you must answer a number of questions very quickly including: who has Sawyer come into contact with since first encountering the disease? How many positive Ebola cases are there in Nigeria right now? The stakes are high; Lagos’ population is over 15 million people and home to one of the busiest international airports in
Africa, with frequent travel in and out of the city. If immediate, decisive action is not taken, Nigeria may soon tumble into a cataclysmic Ebola crisis with potentially enormous reach.
Thankfully, your diagnostic efforts allowed Nigerian health agencies to quickly enact impressive measures, with screens for elevated body temperature at every port of entry, strict isolation protocols, and rigorous sanitation measures. Health officials extensively track 351 individuals with whom Sawyer may have come into contact, as well as a total of 989 potential contacts of all of the original 351, and use diagnostic tests accordingly. The collective public health response saved Nigeria from a widespread Ebola outbreak. A very different outcome would have befallen the city of Lagos, and likely all of Nigeria, Africa, and perhaps even the world, if Sawyer’s true illness had not been identified, and if he had been permitted to leave the hospital and attend his conference.
Tragically, however, through your and your team’s heroic acts to keep Mr. Sawyer in the hospital, you are exposed to Ebola. You are Dr. Stella Ameyo Adadevoh, Nigerian physician, and you risked and lost your own life to protect your country (Figure 7.1) Since your passing on August 19th, 2014, you have become an emblem of the selfless courage and sacrifice of frontline healthcare workers. Heralded as a hero, you have inspired the world by the power of your commitment and dedication to public health. Furthermore, your actions demonstrate the importance of swift and effective diagnostic testing, allowing heroes like you to stop a transmissible disease in its tracks, and save lives. You are remembered as “the Ebola Heroine.”

One of the earliest known examples of a diagnostic test allowed for the detection of what we now know as diabetes. As far back as 1552 BCE, Egyptian physician Hesy-Ra documented frequent urination as a symptom of a mysterious disease that also caused emaciation, or thinness. Ancient healers then noted that ants seemed to be attracted to the urine of people afflicted by this illness. In 300 BCE, ancient Greek physician Hippocrates also noticed insects’ attraction to some individuals’ urine but not others’ (Figure 7.2). This observation took on an interesting dimension when, centuries later, some began to use taste tests as a diagnostic for diabetes. Yes, that’s right: physicians would actually taste the urine of people suspected to have diabetes to check for sweetness, which would then signal the presence of the disease. 17th century English physician Dr. Thomas Willis described diabetic urine as “wonderfully sweet as if it were imbued with honey and sugar,” and other doctors through the ages would attest to its saccharine taste and scent.
A diagnostic test, commonly termed diagnostic, is a procedure designed to confirm or rule out the presence of a specific illness or pathogen. While we currently use state-ofthe-art technologies for the development and deployment of diagnostics, diagnostic testing has a deep history, and the concepts they rely on have been used for millennia. The use of diagnostic testing has been traced back as far as Ancient Egypt, Babylonia, and Greece. As previously noted in Chapter 5: Clinical Symptomatology, the word diagnosis is Greek in origin, meaning to distinguish or discern. We use diagnostic tests to distinguish and discern the cause of medical ailments.
Similarly, in Ancient Egypt, women relied on natural signs for indications of pregnancy. These individuals would urinate on barley seeds,






















FIGURE 7.2 | An ancient diagnostics approach. Ants are attracted to the sweetness of the urine of people with diabetes. This observation was described by ancient Greeks back in 1552 BCE. Later physicians started to taste urine as a common practice to diagnose patients with diabetes. Today, there are many common diagnostic tests applied to urine.



realizing that if barley sprouted, she would be expecting a child. Modern science has shown this test is in fact a valid diagnostic, with over 70% accuracy for pregnancy identification.
Diagnostic capacity has improved by leaps and bounds since the days of urine tasting and barley sprouting. Advances include the invention and later development of the compound microscope in 1590 by the sonand-father team Hans and Zacharias Janssen; and the invention of the Polymerase Chain Reaction (PCR) by Kary Mullis, an American biochemist and Nobel Prize recipient. PCR is a method for DNA amplification, and has formed the basis of many medical techniques and exams still in use today. We will discuss PCR in more detail later in this chapter.
Although the techniques underlying diagnostic testing have advanced over the years, all diagnostic tests share the same fundamental goal: to determine which specific disease or condition an individual has. For most illnesses, diagnostic tests function by detecting — either directly or indirectly — the presence of an anomaly that may be responsible for the condition. For example, some diagnostics work by detecting physiological changes such as high hormone levels, the abnormal shape of red blood cells when viewed under a microscope, or a particularly high level of calcium in the blood.
When it comes to infectious diseases, we have an additional direct identifier—the pathogen itself, which acts as a “smoking gun,” or the undeniable evidence of something we are not expecting to see in our bodies. Some diagnostics identify the presence of the pathogen itself, while others look for indirect evidence — the metaphorical “footprint” the pathogen has left behind. An example of this pathogenic footprint is the presence of antibodies targeting the pathogen, or
a toxin known to be released by specific bacteria. Each of these approaches has unique benefits which we’ll discuss further over the course of this chapter.
One of the earliest microbial diagnostics was the microscopic visualization of the malaria-causing protozoa known as Plasmodium falciparum Soon after the parasite was first identified in the late 19th century, scientists developed the malaria smear. This is one of the first blood smears, a diagnostic method that involves smearing a drop of a patient’s blood on a microscope slide, staining the sample for easy visualization, and examining the slide for evidence of the pathogen (Figure 7.3). This elegantly simple diagnostic is so practical and effective that it is still in use today.
Since then, scientists have developed even more sophisticated approaches for diagnosing infectious diseases. Tests can be performed in many different locations, from a scientific laboratory, to a clinic, to a mobile pop-up lab — even in our own homes. Furthermore,

FIGURE 7.3 | Viewing blood samples of patients under a microscope allow for the detection of malaria parasites. A malaria smear is created by staining the patient’s blood sample with a chemical dye, known as “Giemsa stain,” then spreading it on a microscope slide. The dark purple rings are the malaria parasites that have been stained with the Giemsa dye. Image credit: With permission from Bobbi S. Pritt, MD, MSc, FCAP, DTMH.
diagnostic tests can vary in applications, modes of detection, routes of administration, and accuracy.
Regardless of the approach, diagnostic testing is integral to clinical care, as well as an effective surveillance and outbreak mitigation strategy. By confirming whether a patient has a specific
infectious disease through a diagnostic test, physicians can offer precise and therefore more effective treatments, better anticipate the course of the disease, and take appropriate protective measures if the disease poses a high transmission risk to others.
1. Describe one ancient diagnostic test covered in this section.
2. What unique advantage do we have in detecting infectious diseases in contrast to other diseases?
3. Why are diagnostics important for both individuals and public health at large?
In the world of infectious diseases, there are five different subsets of diagnostic tests, each used to detect current or past evidence of the pathogen’s presence. The types of evidence include:
1. The full pathogen.
2. A specific genetic sequence unique to the pathogen.
3. A pathogen’s protein,
4. A “footprint” the microbe left on its host’s immune system during or after infection.
5. Other biological markers linked to the impact of a pathogen.
Let’s dive into the details for each type of diagnostic test.
When scientists first discovered that microbes were the cause of infectious ailments, their only way to identify the specific microbes involved was to use a microscope to visualize the entire pathogen. The blood smear, a picture of which you saw in section 7.1 applied to malaria, is the quintessential example of this approach. Babesiosis, a disease caused by the tick-borne parasite Babesia , is also diagnosed by using a blood smear.
You’re actually already familiar with a diagnostic method that similarly involves making the pathogen easier to see: examining a microbe under a microscope. As you may recall from Chapter 5: Biology of Infectious Agents, a Gram stain is used for the easy identification of either grampositive or gram-negative bacteria based
on the composition of their cell wall. Gramnegative bacterial cell walls contain three layers with a thin peptidoglycan layer, and gram-positive bacterial cell walls contain two layers with a thick peptidoglycan layer. The differences in the peptidoglycan in the cell wall cause the different classes of bacteria to react differently to the Gram stain applied on the bacteria during the test. As a refresher, Gram stain itself has a crystal violet color; when applied to bacteria, the thicker layer of peptidoglycan in gram-positive cells retains the primary crystal violet stain, while the thinner peptidoglycan layer in gram-negative cells allows the crystal violet to wash out with ethanol. A subset of bacteria, referred to as acid-fast, cannot be captured by a Gram stain test. This is because the high mycolic acid (long fatty acids) content of their cell walls forms a waxy coat that makes them resistant to laboratory staining procedures.
Another common method of wholepathogen diagnosis is bacterial culture (Figure 7.4). You’ve likely had a bacterial culture performed if you’ve been tested for strep throat or a urinary tract infection. A bacterial culture involves taking a sample, such as a mouth or throat swab or a blood, urine, stool, or saliva sample, depending on the suspected location of infection. Then the sample is inoculated, which in this context means it is spread, across a Petri dish, a plastic circular dish that contains the nutrients that promote the growth of the specific bacteria the clinicians are suspecting. If the sample grows bacteria, the result is considered positive. If there is no growth, it is considered tentatively negative. Later we will discuss in detail how tests are interpreted and what happens when tests go wrong.
Genome-based tests usually target short genomic sequences that are shared between all members of the pathogen of interest’s species. For example, when searching for the SARS-CoV-2 virus in a nose swab, we look for a genetic sequence that is characteristic of the virus. As you may remember from Inoculate plate containing solid medium
FIGURE 7.4 | Bacterial culture is a common whole pathogen diagnosis test for the detection of bacterial infections. The test involves three steps: (1) Inoculating a sample containing a suspected bacteria on a Petri dish. The dish contains a medium, which is a substance that provides resources to promote bacterial growth. (2) Spreading the sample out across the dish. (3) Examining the surface for bacterial colonies to appear over time, indicating the presence of bacteria in the patient’s sample, and therefore body.
As we learned in Chapter 4: Fundamentals of Genetics, each species of microorganism has a unique set of nucleotides that make up its genome. Scientists can diagnose an infection by searching for a segment of the pathogen’s genome within the patient’s sample using genome-based methods, also known as nucleic acid testing methods , or NATs
Chapter 1: Emerging Pathogens, the complete genetic sequence for this virus was identified and released to the scientific community by virologist Zhang Yongzhen in 2019. By taking advantage of this shared information, and applying molecular biology techniques, we can detect viruses specifically and relatively quickly. But how do we isolate and detect such genetic sequences of pathogens? The most widely used form, PCR, employs nature’s own toolkit.
As you already know, in the human body, cells must make an entire copy of their genome through DNA replication in order to divide and generate two identical daughter cells. PCR is a laboratory approach that uses similar molecular machinery to replicate
genetic material outside of the cell, in a plastic tube. PCR allows scientists and laboratory technicians to generate large quantities of DNA quickly (typically less than two hours). PCR functions similarly to DNA replication by amplifying — making multiple copies of the sequence of interest. We call this sequence of interest an amplicon . You may wonder, “What about RNA viruses?” While the classic PCR technique creates copies of DNA, we still have ways to adapt this process and amplify RNA in the lab as well, as we will describe below.
In the context of infectious disease diagnostics, PCR is a powerful tool because it allows us to definitively identify a pathogen in a given sample by detecting DNA as it is amplified. Since it is one of the most fundamental tools in all biology and infectious disease diagnostics, let’s introduce you to the key steps and molecular mechanisms behind PCR.
Both in cellular DNA replication and PCR, one of the most crucial elements is a DNA polymerase , which you may remember is an enzyme responsible for copying and making DNA. Specifically, PCR uses a particularly heatresistant type of DNA polymerase that can withstand the high temperatures crucial to this process; in contrast, the DNA polymerase in the human body can only function within the normal body temperature range.
Whereas human DNA replication utilizes a number of enzymes which tell DNA polymerase where to begin, in PCR we use guides called primers to direct DNA polymerase to its starting point, i.e., the region that we want to amplify. Primers are single-stranded stretches of DNA (often
around 20-30 base pairs long) that are complementary to and flank the region of interest. These sequences can bind to the beginning and end of the sequence of interest on each DNA strand, thereby telling the DNA polymerase where to start and stop copying.
In order to create a new DNA chain in PCR, we also need free nucleotides , also known as “freely-available” or “raw” nucleotides, which are the building blocks of DNA. The researcher running the experiment adds these to the solution, where they float freely, ready to be incorporated into the new DNA strands being built. These components are mixed together in a special buffer , a liquid mixture of chemicals that provides a suitable environment for DNA polymerase activity. The buffer’s pH — a measurement of how acidic or basic a solution is — is usually between 8.0 and 9.5, and it has a special set of molecules that stabilizes the other components of the solution. The free nucleotides, buffer components, and heat-resistant DNA polymerase are often combined and sold as a singular solution known as a master mix . This is an all-in-one stable solution that simplifies the process, only requiring that you add the specific primers targeting the region you want to amplify as well as your sample. The sample itself is the final crucial component of the PCR reaction, because we suspect it is infected with the pathogen and therefore contains the sequence we want to amplify, which we call the DNA template
Once all the components are added, the solution is typically poured into a PCR tube , a thin plastic tube that holds small amounts of all the required materials. This tube is then placed in a thermocycler, which is a machine














FIGURE 7.5 | PCR components. PCR takes place in a PCR tube. The components of the reaction include: the DNA sample potentially harboring the DNA template (genomic target of the pathogen of interest), primers that will direct the enzyme to a specific genome region, free nucleotides that will build the new DNA strands, heatresistant DNA polymerase enzyme, and a buffer solution that allows the reaction to stably take place. The PCR tube with the final solution is then placed into a thermocycler machine.
that cycles between the temperatures required for each stage of the reaction to progress (Figure 7.5).
So how do all of these varied components required for PCR work together to make more and more copies of DNA? The process involves 3 key steps: denaturation, annealing, and extension (Figure 7.6).
Denaturation is the use of extremely high heat (~96°C) to break the bonds that hold the double-stranded DNA template together. This separates all the double-stranded DNA into unattached, single strands within the solution.
Annealing, which occurs at a lower temperature (~55°C), is when primers bind to the nowseparated free floating DNA strands. With the
primers attached to the template flanking the region of interest, everything is in place for replication to begin.
Extension, which takes place at an intermediate temperature (~72°C), is when heat-resistant DNA polymerase adds the free nucleotides to the end of the primer, following along the template strand to create a complementary strand of DNA alongside it.
The completion of these three steps constitutes one PCR cycle.
As each cycle of replication doubles the number of DNA strands of the target sequence, PCR produces exponential growth in DNA quantity. By the end of the first cycle, both strands of the template will have been copied, leaving you with 2 times the amount of DNA you started with. In cycle 2, all of those double, so now you have 2 times 2 the amount of original DNA, or 22 = 4
FIGURE 7.6 | DNA amplification through PCR involves three steps — denaturation, annealing, and extension. (1) Denaturation: the DNA template is first subjected to high temperatures, separating the strands. (2) Annealing: the primers bind to specific nucleotide sequences on the template strands at lower temperatures, allowing the DNA polymerase to attach at the correct locations. (3) Extension: the DNA polymerase adds the free nucleotides onto complementary strands (labeled in orange in this diagram), extending the sequence to generate a new copy of the original sequence.
times. In cycle 3, all of those double, so now you have 2 times 2 times 2 the amount of original DNA, or 23 = 8 times. So if you were to run n cycles, you expect to get 2n copies. By cycle 30, for example, you would have created 230, which is more than 1 billion times as many amplicons as you started with. You can see just how fast and effective this exponentially-increasing process can be in Figure 7.7.
We have a number of ways to interpret the results of a PCR reaction, all of which rely on tagging the DNA sequence of interest with a molecule that allows it to be visualized. The most basic information this provides is a binary “positive/negative” result, depending on whether the tagged molecule is observed in the sample. One common visual read-out is fluorescence, which has an added advantage of being quantifiable. A common quantitative fluorescent read-out is Quantitative PCR (qPCR), which
allows researchers to see how much DNA has accumulated after rounds of amplification. This is an indication of “how much” of a pathogen was present in the original sample.
qPCR works by measuring the rise in fluorescence as the reaction is occurring, and does it in real time. As the results of the PCR process are visualized at each cycle, scientists can then compute a cycle threshold (CT), which is essentially how many PCR cycles you have to complete to produce a predetermined number of DNA copies, which scientists have deem the threshold of evidence the pathogen is present. If you started with a lower initial amount of pathogenic DNA, you would need to run more PCR cycles to reach a particular threshold than if you had started with a higher amount. This means the higher the CT (i.e., the more cycles you need to detect a signal), the lower the amount of pathogen in the original sample. As
Chain reaction copies from copies produced
FIGURE 7.7 | DNA amplification during PCR demonstrates exponential growth. As the DNA template is amplified, each cycle doubles that amount of DNA present, thus exhibiting exponential growth. This exponential amplification of DNA allows for an easy readout during diagnostic testing. Scientists visualize the growth by incorporating a readout, usually fluorescent markers, into the PCR experiment. As the DNA increases, so does the fluorescence.
such CT allows us to work backward to estimate pathogen load, the amount of pathogen present in the patient’s body. This is an important metric as it can correlate to disease severity and infectious potential. The higher the CT, the lower the pathogen load, which likely indicates better outcomes for the patient.
As mentioned earlier, PCR can be performed on RNA as well. A PCR-based method commonly used to detect RNA viruses is

Scan this QR code or click on this link to join the PCR Virtual Lab to learn the basics of how PCR works.
Reverse Transcription-quantitative PCR (RT-qPCR) . This laboratory method can be performed thanks to a special enzyme that you’re already familiar with, called reverse transcriptase, which generates a complementary DNA or cDNA strand from an RNA template. Using this enzyme, scientists can run a traditional PCR protocol on an RNA template by first converting the RNA to a double-stranded DNA template, then using the cDNA to run the reaction as usual. This process can conveniently all take place in the same PCR tube.
You may remember that SARS-CoV-2 is an RNA virus, which means that RT-qPCR is highly effective in testing for SARS-CoV-2 infection. You might be familiar with the first steps of the RT-qPCR process if you’ve ever had a COVID test. The process starts when a sample is collected from an individual i.e. via
a nose swab. Then the sample is treated with special buffers to extract and purify its genetic information, RNA. Next, the reverse transcriptase enzyme generates the cDNA used for the main PCR process to amplify the desired material. The qPCR amplification and detection determine whether a patient is positive or negative for COVID, and the qPCR thermocycler generates a chart showing a growing slope when there is a positive result. There may still be a slope present in a negative result, as background material eventually becomes amplified much later in the reaction. The difference between a positive and negative result and the step by step of the method are shown in Figure 7.8.
There are a few key considerations required to develop PCR diagnostics that will (hopefully) allow them to work for some time. First, we
want to target a part of the genome we do not expect to change. Microbes often mutate quickly, so if the mutations change the sequence corresponding to a PCR or other amplification primer, the reaction may not amplify well or at all, rendering the test insensitive or altogether ineffective. Thus, for long-term efficacy, it is important to target a region of a pathogen’s genome that is resistant to mutation (due to, for example, encoding a crucial part of a gene). We call these regions conserved
Second, we want to be sure the region of the genome we target is unique to the pathogen we are aiming to detect. If the region is “too conserved,” it may have the same sequence in other closely or distantly related species, leading to cross-reactivity with these other species. Cross-reactivity occurs when a diagnostic test targeted against one specific pathogen can recognize a different pathogen, usually due to






















FIGURE 7.8 | RT-qPCR is a molecular test used to diagnose SARS-CoV-2 and other RNA viruses. The procedure for COVID-19 diagnosis via RT-qPCR involves four main steps. (1) Sample collection through nose swab. (2) RNA extraction and purification. (3) Transcription of template RNA into cDNA, and (4) cDNA amplification and detection by quantitative PCR. The chart shows a positive result slope (green) and a negative result slope (gray) – there can still be a negative slope because, if given enough cycles, even negative samples can begin to show some amplification.
similarities in their targeted gene sequences. This can falsely report a positive result when in fact another species is present.
Third, it can be beneficial to target regions of the genome that the pathogen is expressing highly, meaning that the genes’ resulting proteins are frequently being produced within the cell. If the genes are highly expressed, the target nucleotide sequences or protein will more than likely be found in a patient sample. This is particularly important for RNA viruses that can vary widely in the number of copies produced for different parts of the genome.
Like all diagnostics, PCR has a number of pros and cons. One of PCR’s chief advantages is its accuracy; since it functions by recognizing highly specific DNA sequences and massively amplifies the target, it can detect whether or not a sample contains pathogenic material from a minuscule amount of the pathogen’s DNA. Designing a PCR test to identify a new pathogen is fairly straightforward as well: all a scientist needs is the target sequence of their pathogen of interest, and they can create primers to amplify the desired sequence with relative ease. However, the cost of equipment can be a major barrier to using this technology. For example, a thermocycler can cost many thousands of dollars and requires consistent access to electricity/ power, which can make it inaccessible in underresourced areas. PCR amplification also takes time – up to a couple of hours – which may limit the number of tests that one lab can process in a day, and potentially delay the reporting of critical information as a result.
A recent and promising type of genome-based diagnostic test that overcomes some of PCR’s
challenges is isothermal amplification, which also involves amplifying genomic material, but at a constant temperature, which means that it only requires a simple heating source rather than a thermocycler. As the COVID-19 pandemic surged around the world and exposed the need for widespread testing, isothermal amplification methods achieved renewed popularity due to their accessibility and feasibility with limited resources. These diagnostics have the potential to be deployed in many different healthcare settings, and can even be self-administered. One example of an isothermal amplification method, Loop Mediated Isothermal Amplification (LAMP), formed the basis of many popular commercial point-of-care tests, which are diagnostics that can be run wherever the patient first shows up to receive care, e.g., a doctor’s office. Moreover, many groups used LAMP to quickly roll out diagnostics into schools and communities because it could use very basic machinery, including common cooking devices, to maintain a temperature of 65°C.
Genome-based diagnostics is a very exciting field, and technological advancements are emerging from many fields — microbiology, synthetic biology, nanotechnology, and more — that hold immense promise for the future. One such approach is Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-based diagnostics. Originally found in nature, CRISPR is a family of DNA sequences that evolved to protect bacteria against viruses. CRISPR utilizes enzymes that recognize specific sequences, allowing bacteria to detect and then cut viral gene sequences at very specific points along the genome. CRISPR diagnostics pair this enzymatic cutting with a visual readout so that a signal will emerge when the specific cut occurs. The first CRISPR-based diagnostics to be approved were those targeting SARSCoV-2.
In what could be viewed as the ‘ultimate’ genome-based diagnostic method, scientists can also use genomic sequencing, which you were introduced to in Chapter 4: Fundamentals of Genetics. Genomic sequencing allows researchers to obtain a nucleotide-by-nucleotide readout of all the genetic material in a sample, including any from a pathogen. This is achieved by first collecting a sample from the patient, then extracting the genetic material present in the sample’s cells. The researchers then load the newly-extracted material into a sequencing machine and receive a report of the sequence of nucleotides contained within a sample, using it to identify a pathogen and even read out its entire genome. Sequencing was, until recently, an extremely difficult and expensive process. Now, novel sequencing methods are opening the door to a wide variety of diagnostics.
There are two main ways in which a pathogen’s genes can be sequenced. The first is metagenomic sequencing, which reads out all of the genetic material contained in a sample, not just the material of the pathogen of interest. This approach is described as “unbiased,” as it allows investigators to detect a wide array of pathogens without needing to know exactly what they are looking for in a sample. However, metagenomic sequencing is expensive, challenging, and time consuming, as researchers must recognize the gene sequence of a specific pathogen amongst countless other sequences in the sample, such as the patient’s own DNA or various nonpathogenic microbes found within the human host. The second is targeted sequencing, which will only produce specific pre-selected sequences from the pathogen of interest, or a defined genetic sequence. The most commonly-used form, called amplicon-based targeted sequencing, uses the same amplification processes as are used in PCR in order to make many copies of the targets of interest prior to sequencing. The targets of
interest could involve one amplicon, but often multiple sets of primes are used to amplify many target amplicons, or even the entirety of a pathogen’s genome.
Sequencing provides accurate and thorough reports of the genomes circulating within a tested sample, but it is still too costly to conduct across all individual patient samples. As the technology advances and costs continue to drop, sequencing may become a mainstay of everyday infectious disease diagnosis. For now, sequencing has been mainly used as a followup on samples that tested positive to identify novel viral genetic variants, i.e., information about minor changes in the genome that can’t be easily or systematically obtained through other methods. We will discuss the use of genome sequencing to further characterize pathogenic genomes in Chapter 14: Pathogen Evolution and Drug Resistance and Chapter 15: Genomic Epidemiology.
Another way to detect the presence of a pathogen is to detect one of its antigens by using antibodies; antigen tests are designed to do exactly that, informing us whether or not an individual is infected with a pathogen. The antigens used in diagnostic testing generally consist of proteins that exist on the surface of a pathogen, allowing for very specific detection of the pathogen itself.
As you may recall from Chapter 6: Immune System and Host Defenses, antibodies are the highly-specific result of the body’s immune response, and have the unique capacity to selectively bind to their antigen. We can take advantage of the selective binding in this natural
defense mechanism by raising antibodies in animal models, isolating the resulting antibodies, and using them for the detection of pathogens in the lab.
Antigen tests are often built on a molecular biology platform known as lateral flow. Lateral flow refers to the movement of molecules laterally (i.e., sideways) across a paper strip or other similar material. You might already be familiar with lateral-flow diagnostics if you’ve used an at-home test for COVID-19, or an athome pregnancy test. Antigen tests with lateral flow are the most user-friendly tests currently available for infectious disease diagnoses.
At-home antigen tests involve just 3 easy steps that altogether take only a few minutes to complete. First, you need to collect a sample. The aim of this step is to gather substances that you suspect contain the pathogen’s antigens. In the case of a respiratory virus, this could be mucus, along with some of the tissue cells gathered from the inner lining of your nose via a swab. Second, you’ll need to mix the swab in a buffer solution, which is usually included in the test kit; this process will break up infected cells and release any pathogen and other proteins into the solution. Third and finally, you’ll add the swab-mixed solution to the lateral flow device, where the solution will flow across the adsorbent paper strip to generate your positive or negative test result.
But what are the components within antigen capture tests, and how do they work together to visualize a pathogen antigen? It all starts with labeled antibodies. In molecular biology, labeling refers to the addition of a visible molecular marker to a protein, similar to the fluorescent tagging that you read about earlier in this chapter. In the case of antigen tests, the free floating antigen-specific antibodies are
labeled. The pathogen’s antigens in the sample will bind strongly to these labeled antibodies, which will allow them to be visualized as they concentrate down the flow of the strip. In the cases of many types of diagnostic tests, these labeled molecular markers can be visually observed — without any required equipment — in colorimetric readouts, where a certain color (e.g., red) demonstrates a positive test result.
As the solution flows along the strip, it will first encounter the test zone, which contains antigenspecific antibodies that are fixed in place, and to which the pathogen’s antigens will bind to at a different site from the antibody label. As the pathogen antigens bind to the fixed antibodies, they will begin to concentrate within the test zone. After some time, this buildup will cause a colored band visible to the naked eye to form at the test zone.
The labeled antigen-specific antibodies within the solution that are not bound to pathogen antigens, and therefore cannot attach to the immobilized antigen-specific antibodies on the test zone, will pass right through the test zone. These labeled but unbound antigenspecific antibodies will then continue on to what is called the control zone, located further down the lateral strip. There, they will bind to a different set of antibodies that are also fixed in place, the antibodyspecific antibodies, t designed to recognize, and therefore bind, the labeled antibodies themselves. A visible band will form within the control zone, regardless of whether the sample contains the pathogen or not. Because the control zone is positioned downstream of the test zone, the presence of a band here helps to confirm that the solution did fully progress and that the test worked; conversely, if the strip does not have a control line, the sample should be run again on a different
Antigen-specific antibody
Antibody-specific antibody
Immobilization of antibody
Pathogen antigen
Label






FIGURE 7.9 | Antigen test mechanisms of action, results and interpretation. A) Antigen-specific antibody binds the antigen of the pathogen. B) Antibody-specific antibody binds antigen-specific antibody independently of whether the pathogen is present or not. C) Test preparation. An antigen test begins when a sample in buffer is placed on the lateral flow strip in the sample zone alongside labeled, free-floating antigenspecific antibodies. If the pathogen is present, it will bind to these antibodies. As the solution flows along the strip, it will encounter bound antigen-specific antibodies in the test zone (T) that will recognize and bind different parts of the pathogen antigen. The solution will then continue to flow to the control zone (C), where it will encounter bound antibodies (antibody-specific antibodies) that recognize the labeled antibodies themselves. Test results and interpretation. We call an antigen test positive when the antigen-bound, labeled antibodies build up at the sites of the fixed antibodies, causing a colored band to form at both the test zone and the control zone. We call it negative when labeled antibodies only build up in the control zone, causing a colored control band. The presence of the control band means that the solution made it all the way through the lateral flow strip. Any test that does not have a band in the control zone is invalid.
strip. Tests missing the band in the control zone are invalid as it indicates that either the test was manufactured incorrectly, or that for some reason the anti-antigen antibodies were not able to flow across the strip; these are both systematic errors that indicate a test is not functioning as intended. When both bands appear we have a positive test result. When the band over the test zone is missing, but the control band is present, we have a negative test result (Figure 7.9).
There are important considerations when choosing a target antigen, or more broadly, protein, for a diagnostics test. Not all surface proteins are alike; they each have unique features that inform our choices when designing diagnostics. Some surface proteins help to mediate host cell entry (like the SARS-CoV-2 spike) whereas others are present for structural reasons (like the SARS-CoV-2 nucleocapsid).
Each provides different diagnostic utility. As with genomic-based tests, we aim to use protein regions that are relatively conserved. The prime reason we select the nucleocapsid protein instead of the spike protein for COVID-19 antigen tests is because the spike is more prone to evolutionary pressures to mutate. If an antigen mutates too much, its shape may change, potentially rendering all of the mass-produced tests to be ineffective if they can no longer recognize the pathogen. At the same time, we want to ensure that the protein sequence is distinct enough from other species that we do not observe cross-reactivity.
While they are frequently compared to PCRbased tests, as they both can be used to detect the same pathogen, antigen capture tests have their own utility and drawbacks. Antigen tests are generally faster and less expensive than PCR tests, with the capacity of producing results in mere minutes. Without the need for expensive or complex equipment, they are also more accessible and can be carried out almost anywhere, frequently at the patient’s home, without any interaction with the healthcare system. However, it cannot detect small pathogen loads as effectively as PCR since antigen tests do not have a way to generate multiple copies of the sequence of interest; this makes them far less accurate. These tests therefore have a higher rate of mistakenly reporting that an individual is pathogen-free when they are actually infected: a phenomenon called a false negative, which we’ll explore in the next section. Another key challenge of a protein-based test design is that each protein is extremely specific in shape and size. The process of designing custom, individualized antibodies can be time-consuming, expending critical resources in the crucial early stages of
an outbreak, particularly in contrast to genomebased tests such as PCR that require less specificity.
Rather than detect a pathogen itself, antibody tests detect antibodies mounted in response to, and left in the aftermath of, pathogen infection. Antibody tests are the most well known form of serology test, which is any form of a diagnostic or research test based on blood serum – the component of blood that harbors immune cells.
If positive, an antibody test indicates that a person has developed antibodies in response to a current or prior infection of a particular pathogen. Since it may take several days, or even a few weeks, for antibodies to be generated in response to a given pathogen, antibody tests are not usually able to detect early infection. They however can uniquely signal both later-stage and past infection, as well as successful vaccination. Moreover the tests can detect different classes of antibodies that arise at different time points after an infection, which can allow clinical providers to distinguish the different stages of infection.
Antibody tests and antigen tests can be understood as two sides to the same coin –in both, researchers use the interaction between pathogen antigens and the antibodies that recognize and bind to them to detect the other. Antigen testing relies on labeled antibodies that bind to pathogen antigens in a patient sample, whereas antibody tests rely on labeled pathogen antigens that bind to the host’s antibodies in a patient sample.
Clinical providers can use lateral flow readouts for antibody tests, but they often also use a lab-based method called an enzyme-linked immunoassay (ELISA) , which involves a plate that is coated with antibodies that will capture the antigen of interest. While ELISA takes more time, speed is usually less important than accuracy and precision for antibody tests because, as we noted, antibody tests usually can only detect late or previous infection, where urgent reporting of active infection no longer has to be the priority.
Since patients who have recovered from an infectious disease often no longer have the pathogen’s genome or antigens in their cells, antibody tests can serve as a key tool in identifying these previously infected patients in a way that PCR-based and antigen-based diagnostics can not. This unique ability makes antibody tests particularly useful for detecting “hit-and-run viruses,” such as the West Nile virus, where the pathogen itself is cleared fairly quickly before the patient can come to clinical attention, while severe and even debilitating symptoms can last much longer.
Antibody tests provide two key insights for outbreak response and surveillance efforts. Firstly, they inform our understanding of a given pathogen’s spread through a particular population. Secondly, when paired with detection of antibodies due to vaccination (if vaccines are available), or other data on vaccination, it also helps inform how close the population is to herd immunity Herd immunity is the threshold of immune individuals within a population that greatly restricts the pathogen’s ability to spread
through the population, a concept we will discuss in Chapter 9: Vaccines and Immunizations.
Despite its utility, there are limitations to antibody tests that can cause them to yield inaccurate results. For example, 25% of samples that are truly positive for mononucleosis are not detected by an antibody test, giving a false negative result. False negatives can be due to low concentration levels of antibodies in the sample, when the infected individual does not produce enough of the specific antibody that the test is designed to detect, or if it’s too early on in the infection for the patient to have produced a detectable level of antibodies. Antibody tests can also yield false positives, typically when the antibodies bind cross-reacting antigens that have structural similarities to the antigen of interest, but are not the antigen that the test is actually designed to detect.
Biomarkers are any measurable, biological indication of a changed health state, which will differ in some discernible amount when a person is sick versus healthy. Genome and antigen-based tests detect biomarkers, but physicians can employ diagnostics that search for other biomarkers of illness such as hormones, proteins, waste products our bodies produce during metabolism, and cell count in the blood. For example, one of the earliest biomarkers of pregnancy is human chorionic gonadotropin (hCG), commonly known as the “pregnancy hormone.” By measuring the level of hCG in

Prostaglandin Metabolites





FIGURE 7.10 | Biomarkers can be used to determine the patient’s health state. While biomarkers aren’t necessarily direct markers of a specific pathogen, they provide information to the physicians about the state of the body. This information can help to make a prediction about what kind of infection the patient may have and decide for the pathogen test which should be performed next. Some common biomarkers include kidney toxicity, the presence of antibodies and growth factors, hormone levels, or prostaglandin metabolites.
a woman’s urine, these pregnancy tests can compare this biomarker to its expected levels in non-pregnant individuals to determine whether or not she is pregnant.
In the context of infectious disease, biomarker tests can measure the amounts of key – albeit non-specific – immune cells, such as neutrophils and helper T cells. Detecting abnormal amounts of these cells can be supporting evidence in diagnosing a bacterial or viral infection, or revealing a compromised immune system. Importantly, biomarkers are
less likely to confirm the presence of a specific pathogen, but rather indicate the process of infection within a host or whether the host is capable of adequate immune response in general. Biomarker tests can also detect inflammation in the body, or organ damage (pathogen-infected or otherwise) through a range of different proteins and waste products (Figure 7.10).
We provide examples of diagnostic tests by category, target, type of samples, and pathogen detection in Table 7.1
Diagnostic Test Category
Full pathogen detection
Genome-based testing
Diagnostic Test Target
The whole pathogen (e.g., virus, bacteria or others)
Pathogen gene or genome (DNA or RNA)
Antigen testing An antigen of the pathogen
Antibody testing (aka serology testing)
Biomarker detection
Antibodies produced by our immune system in response to pathogen infection
Biomarkers of disease or pathogen, such as readout of immune response or organ function
Type of Samples Diagnostic Test Examples
Blood, urine, saliva, cerebrospinal fluid
Blood, nose swab, mouth swab, cerebrospinal fluid, saliva
Pathogen Detection Examples
Microscopy, Gram staining and blood culture Malaria and babesia microscopy, bacterial culture
PCR, qRT-PCR, Isothermal amplification, (e.g., LAMP), CRISPR, genetic sequencing PCR tests for SARS-Cov-2, Influenza virus, and HIV, LAMP for SARS-CoV-2
Blood, nose swab, mouth swab Lateral flow, ELISA Rapid lateral flow tests for SARSCov-2, HIV and Influenza virus
Blood (serum)
ELISA, lateral flow SARS-Cov-2 rapid tests and serology surveys, West Nile virus detection
Urine, stool, blood, saliva WBC count, kidney function tests, liver function tests, urine pH
WBC count to determine if likely bacterial infection, liver function tests in Lassa fever
1. What are the five main categories of infectious disease diagnostics introduced in this section? Give a brief description and example of each.
2. How are antigen tests different from genome-based tests like PCR?
3. How are antigen tests different from antibody tests?
In addition to the scientific approach employed to target microbes, infectious disease diagnostics can be characterized by the way in which they are administered, the various settings in which they are performed, and other characteristics of the test.
Depending on the disease being investigated and the needed biomarker measurement, medical professionals administer diagnostics in one of three ways: non-invasively, minimally invasively, or invasively (Figure 7.11).
Non-invasive tests do not require physically breaking the skin or “invading” the body. These include simple tests we can administer ourselves like through collecting urine or saliva, or tests run by medical professionals like magnetic resonance imaging, MRI, or electrocardiogram, ECG.
Minimally invasive tests do enter the body, but are generally quite easy to administer. Examples of minimally invasive tests include blood spots,



which utilize just a few drops of blood, and anterior nasal or throat swabs, which may or may not require the assistance of a healthcare professional to collect.
In contrast, invasive tests enter the body using medical instruments and require a medical professional to administer, which can be a barrier in resource-limited settings. A common invasive technique frequently used in the diagnostics world is the blood draw. Although the initial prick of the needle might be uncomfortable or offputting, drawing blood for further analysis is a comparatively simple procedure that can provide lifesaving information. Other invasive tests commonly used in infectious disease diagnosis are taking a nasopharyngeal sample from way up in the nose or taking cerebrospinal fluid (CSF) from the spine.
Many diagnostic tests are lab-based, meaning that they are processed and analyzed in a laboratory or specialized facility outside the location of where the test is taken. Such diagnostics often require special equipment and training. Although it often takes a longer time to generate the results of lab-based diagnostics,



FIGURE 7.11 | Diagnostic tests can be classified as non-invasive, minimally invasive and invasive according to the procedure involved. Different tests require different ways for sample collection; some are less invasive than others. Non-invasive include urine or saliva collection, minimally invasive include throat or nose swabs, and invasive testing involves the breaking of the skin, for example collecting blood or CSF.
these types of tests are very common, and the machinery and expertise behind the test may yield more accurate results than others.
As technology progresses, more and more tests can be both performed and processed at the same location. These tests, referred to as pointof-care diagnostic tests, provide results relatively quickly at the healthcare site in which the test is administered. These tests do not require sending patient samples to a laboratory for analysis — a key advantage when we require faster results, and facilities lack the equipment needed to run a more advanced diagnostic. However, due to the fact that point of care tests are administered across a wide variety of locations and sites, these tests can be more susceptible to random error caused by their unstable environments in comparison to lab-based procedures. Regardless, during outbreaks, point-of-care tests can be especially useful, as they are relatively portable and quick, allowing responders to distribute them throughout disease hotspots, provide swift diagnoses, and deploy quick and effective containment strategies.
A subset of point-of-care tests are point-of-need diagnostic tests, also referred to as direct-toconsumer (D2C) diagnostic tests or at-home


FIGURE 7.12 | Point-of-need diagnostic tests permit consumers to self-administer at home. These tests are generally simple to perform and analyze, for example, the self-test antigen rapid test for COVID-19 gives results within 15 minutes using a nasal swab kit, and does not require a health professional to perform.
tests, which permit consumers to self-administer the diagnostic without the assistance of a healthcare provider (Figure 7.12). These tests are generally simple to perform and analyze, and can be critical tools during situations in which individuals lack easy access to health professionals. A common example of pointof-need diagnostic tests include pregnancy tests, as well as glucose monitoring systems, which allow individuals with diabetes to check their blood sugar in a matter of seconds. One of the first point-of-need diagnostic tests for infectious disease is the at-home tests for HIV infection, which has become highly reliable and can provide results within minutes. During the COVID-19 pandemic, numerous antigen capture tests for SARS-CoV-2 have been widely used for at-home testing, and other tests have been moving closer and closer to point-of-need accessibility.
In the context of infectious disease, point-ofneed diagnostics that can be self-administered can play a pivotal role in slowing disease spread. When an infected person goes to and from a clinical testing site or interacts with a medical professional, they can infect others. The ability to test without interacting with healthcare workers is a huge benefit of pointof-need diagnostics, no matter whether the point-of-need diagnostic generates results instantly, or is just collected at home and then sent off for analysis. In this way, point-of-need diagnostics are a powerful tool in determining when a person can safely test out of isolation.
Most diagnostic tests, including those we’ve already discussed, are singleplex diagnostics, in which each diagnostic tests a single person for a single candidate pathogen. However, COVID-19 has highlighted that there is a clear

Scan this QR code or click on this link to learn more about how the Broad Institute ran daily qRT-PCR tests during the COVID-19 pandemic.
need for a much higher volume of testing and a much broader set of pathogens for which we must test.
Technological advances like robotics and high-capacity testing machines now enable the evaluation of thousands of tests at once. This high-throughput testing method allows scientists to test the population more effectively. During the COVID-19 pandemic, for example, the Broad Institute of MIT and Harvard was able to run up to 200,000 qRT-PCR tests daily using a high-throughput approach.
Pooled testing is one approach to increase testing throughput by combining or “pooling” samples from several individuals and looking for the presence of an active pathogen anywhere in the pool (Figure 7.13). If the pathogen is detected, the researchers will follow up with all the individuals whose samples were part of the positive result pool to see which individuals specifically were positive for the disease. While on its surface this may seem like creating more work, it’s actually very efficient when performed properly, as a pool with no pathogen detected would clear all the individuals in that pool with only one test.
The pooled testing strategy is particularly useful in mass surveillance efforts, providing a general idea of the frequency and location of infection without necessarily requiring a test for each individual. During outbreaks, pooled testing can be incredibly effective, as a limited supply and the sometimes prohibitive cost of tests often makes frequent testing of individuals difficult. Furthermore, reducing the number of
FIGURE 7.13 | A pooled testing approach for 100 individuals, tested in 20 pools of 5. Only 5 of the pools included infected individuals, thereby eliminating the need to test everyone in the other 15 pools individually. This approach was used in Boston, Massachusetts, US in 2021-22 to offer weekly testing in the Boston Public Schools, running classroom-pooled testing for the detection of COVID-19 and continuing to monitor the students during the pandemic.
tests saves time and allows health professionals to generate results rapidly enough to provide targeted recommendations for follow-up testing quickly.
It should be noted that pooled testing isn’t always a perfectly advantageous approach. This method saves the most resources when the expected number of cases in a population are low. As case numbers get higher and higher, it is more likely that many pools will test positive, increasing the number of pools that have to be retested individually. Pooled testing can be made more efficient when the individuals who interact with each other (e.g., co-workers on the same shift, or students in the same home room) are tested together, such that positive cases will be most likely found within the same pool. Pooled testing also does not work well for pathogens that are present at low levels in a patient sample, for which
combining many samples would make it too hard to detect a small amount of pathogenic material amid the volume of testing material that is combined together.
Wastewater testing is another way to conduct effective pooled sample testing when virus is shed in urine and feces, and is an example of infectious disease surveillance through environmental sampling. Wastewater detection methods involve the collection of sewage water samples from toilets, showers, sinks, water runoff, and other sources. By grouping the sewage samples into community pools, scientists can test for the presence of infectious microbes within the wastewater, thus providing an efficient mechanism for early detection and mitigation. Most recently, the CDC and many health departments across the US have undertaken efforts to test wastewater for the

The state of Massachusetts implemented wastewater testing for Covid-19 tracking and in this site you can see what a chart and data look like. Scan the QR code or click on this link.
genetic code of SARS-CoV-2 and monkeypox, which can inform surveillance efforts and elucidate the changes in total infection within certain areas and communities.
New technologies have also made it possible to carry out multiplex diagnostics, which can detect many different pathogens in a single patient’s sample. These diagnostics are especially useful when a patient presents symptoms that can accompany a number of diseases; rather than administering individual tests to rule out each potential pathogen one at a time, a multiplex diagnostic allows clinical providers to administer a single test and search the patient sample for numerous candidate pathogens simultaneously. Metagenomic sequencing, which we discussed previously, is one form of multiplex diagnostic testing that can detect all sequences in a sample. However, there are many emerging approaches based on sequencing, PCR, CRISPR, and other technologies to select a subset of pathogens to search for in a patient sample.
1. What is the difference between non-invasive and invasive methods to conduct a diagnostic test?
2. What is the difference between point-of-need and lab-based diagnostics?
3. What is the difference between singleplex and multiplex diagnostics?
In order for a diagnostic to be considered reliable, we must be certain that it is able to identify the pathogen or disease process that we’re after, and do so with a high level of accuracy. Reliable tests have the ability to detect when a pathogen or disease process is present, as well as an ability to indicate the absence of a pathogen or disease process when it is not present. Sometimes a diagnostic test can give a result that is not correct. In this section, we’ll discuss these possibilities, explain why and how false results may occur, shedding light on implications for diagnostic development.
Despite scientists’ best efforts, tests aren’t perfect. Diagnostics can sometimes falsely generate a positive result when the individual is not infected. Conversely, they can sometimes falsely generate a negative result when the individual is infected. These possibilities for error are critical to consider when developing and using a diagnostic test (Figure 7.14).
In infectious disease diagnostics, a true positive result occurs when the test correctly identifies the presence of the disease and a true negative occurs when the test correctly indicates the absence of the disease. True positives and true negatives are accurate results; however, diagnostic tests can also give incorrect readings, known as false positives and false negatives.
A false positive result occurs when a test incorrectly indicates the presence of the disease when there is no infection. If you were tested for strep throat and the test came back positive, but in reality, you hadn’t been infected at all, that result would be a false positive. False positives usually occur due to cross-reactivity with a related pathogen or contamination . Contamination can occur, for example, when the target pathogen gets into the sample or lab equipment, which can happen when many samples are being processed in the same lab without proper controls, or when samples are mislabeled or accidently mixed up.
FIGURE 7.14 | Accurate and inaccurate diagnostic test outcomes in diagnostic testing. There are four possible outcomes of a diagnostic test, with accurate results given in green (true positive and true negative) and inaccurate results given in red (false negative and false positive).
On the other hand, a false negative occurs when the test incorrectly indicates the absence of the disease when in fact there is an infection. This would be the case if an individual’s COVID-19 test came back negative when, in reality, they were infected with SARS-CoV-2. False negatives usually occur when the test is unable to detect the pathogen in the sample. Oftentimes you’ll get a false negative when the concentration of the sample is too low for the test to pick up. This can happen when the pathogen load is still minimal at the early stages of infection, or if the pathogen is generally at low concentration, like Zika virus, or hard to find in the body i.e., residing in tissues that are difficult to access, like in the spinal
or joint fluid in the case of some Borrelia burgdorferi infections, the causative agent for Lyme disease. False negatives can also occur if a sample is handled poorly such that the pathogen material degrades in the sample.
False positives and false negatives can have serious ramifications, both for individuals and overall outbreak containment and response. False positives can cause individuals to be placed on unnecessary treatment plans or perhaps exaggerate our data on the number of infections within a given community. This inaccuracy becomes even more significant when false positive cases are sent into quarantine alongside true positive cases because you risk infecting the false positive individuals by patients who are truly ill. False negatives could prevent an individual from receiving their needed treatment, or, at the very least, delay treatment until the disease advances in severity. This individual could also return to their daily activities, operating under the assumption that they are not infected, and continue passing on their infection to others.
Rates of false positive and false negative results are carefully documented as diagnostic tests are developed, and optimal tests are able to minimize inaccuracies.
In order to measure the accuracy of new tests, we would ideally know the ground truth – whether the person is or is not truly infected with the pathogen – so that we can accurately measure how frequent true positives and negatives are. Since the ground truth can be hard to know, we instead compare it to a gold standard – the best available test that can be used to benchmark
other tests. The accepted gold standard varies depending on which disease a new diagnostic is meant to detect; of course it itself is sometimes imperfect. However, the gold standard still serves as a high-fidelity benchmark against which scientists can compare new diagnostics.
Researchers perform a few basic calculations to measure the likelihood of false positives and false negatives. They also use these calculations to determine important values like sensitivity and specificity that measure a test’s accuracy, as well as the positive and negative predictive values that inform a test’s clinical utility. Let’s briefly dive into these concepts and how to determine them.
Scientists use false positives and false negatives to calculate two values that are critical to understanding the accuracy of a given diagnostic test: sensitivity and specificity.
Sensitivity measures how reliably a test can correctly identify disease in an infected person. It is defined as the proportion of the total group of subjects that are truly infected by the pathogen, i.e., total infected, that received positive test results.
Specificity measures how effective the test is at correctly identifying that a person is truly not harboring the pathogen. It is defined as the proportion of the total group of subjects not infected by the pathogen, i.e., total negatives, that received negative test results.
has received a positive result is to actually be infected. Specifically, it is the ratio of patients truly diagnosed as positive to all those who had positive test results (including those incorrectly diagnosed as positive for the disease).
The value of sensitivity is fairly intuitive – of course we want a test that picks up 100% of cases. However, specificity is equally important! Suppose a diagnostic test for COVID-19 returns a positive result from every single nasal swab. This would definitely give a positive result to every single case, but it wouldn’t be very actionable information –it would be full of errors, and unhelpful in deciding who should be isolated and treated. Specificity helps us feel confident in the accuracy of a positive (or negative) result.
There are two other ways to calculate measures that help us determine whether to trust the result of a diagnostic test: positive and negative predictive value. Predictive values are quantitative measures to predict how likely it is that a test will give a correct result. While sensitivity and specificity measure a test’s accuracy, predictive values inform a test’s clinical significance.
Positive Predictive Value (PPV) indicates the proportion of the cases who received positive test results who are truly positive for the disease — in essence, how likely a person who
Negative Predictive Value (NPV) indicates the proportion of the cases who received a negative test result who are healthy — in essence how likely is a negative result to correctly predict a healthy person. Specifically, it is the ratio of subjects truly diagnosed as negative to all those who had negative test results (including those who were incorrectly diagnosed as healthy).
Unlike sensitivity and specificity, PPV and NPV can change with a disease’s prevalence, and speaks to a test’s clinical utility. For example, when the number of positive cases are far greater than the number of false positives produced due to error, then PPV will be high. But when prevalence is so low that a false positive becomes as likely as real positive, PPV is low.
Ultimately, no test in our current arsenal is perfect: each has its own set of strengths and weaknesses that must be taken into account when deciding what tests to deploy in different settings. Oftentimes, leaders need to consider a wide range of factors beyond the accuracy of the test itself, depending on the context of its use.
The most common trade-off with diagnostics lies between accuracy and accessibility, which is often reflected in a test’s cost, turnaround time, and equipment needs. For example, during the COVID-19 pandemic, certain public health experts advocated for officials to prioritize widely distributing rapid, at-home antigen tests because of their potential for
widespread, easy deployment, despite these tests having a lower sensitivity value relative to other more comprehensive methods such as PCR testing.
You’ll learn more about these challenges and considerations regarding diagnostic deployment in the next section.
1. How would a high false negative rate impact the outbreak response procedures against a disease that often spreads asymptomatically?
2. Practice Problem: Your team is trying to evaluate the efficacy of a new diagnostic test for Ebola. In a clinical trial, 250 participants are afflicted with the disease and 250 participants are healthy. However, upon testing all participants, 325 tests came back positive. All the individuals who tested negative were determined to be healthy.
a) What is the specificity of your test?
b) What is the sensitivity of your test?
In an ideal world, healthcare providers around the world would have the resources and infrastructure to provide diagnostic testing to all of the individuals in a community. This is not always the case, though, and specifically during an outbreak, the high demand for diagnostic testing can cause even more challenges.
Diagnostic challenges range from accessibility of the required equipment and technologies, to the space and conditions needed for storing test supplies, biosafety regulations, and the availability of healthcare personnel for the processing and interpretation of diagnostic tests, among many other considerations. These needs can become obstacles when there aren’t sufficient resources or infrastructure in place or when a novel pathogen emerges. Let’s explore some factors that affect the use of diagnostics in different settings.
Economic obstacles often impede our efforts to effectively distribute and utilize diagnostic tests. For example, the cost of reagents to conduct a PCR test, on top of the alreadyexpensive PCR machine required, could create a significant accessibility barrier for underresourced communities and underfunded health systems around the world. This may prompt health officials to restrict testing and prioritize only patients showing very specific or severe symptoms, and it leaves a gaping opportunity for disease transmission to continue under the radar. Increased
infection rate in low income communities — a phenomenon observed in nearly every outbreak — creates a negative feedback cycle: the large gap between the number of individuals infected and the number of tests available prevents these communities from implementing fully-informed mitigation strategies, allowing transmission to continue unchecked.
Local economic factors and capitalist incentives can also divert limited resources away from communities in greatest need. During the COVID-19 pandemic, professional sports teams, businesses, colleges, and other institutions with great resources and an eagerness to remain open began testing many thousands of asymptomatic people — sometimes daily — often while their surrounding communities struggled to meet basic clinical testing needs. By shifting resources away from hypothesis-driven testing (e.g., symptomatic persons, contacts, and high-risk groups), they used up most of their community’s limited testing capacity on low-risk cases. Since resources were finite and mostly used on low-risk cases, this strategy likely increased the burden of disease for the community, leading to overall containment failure.
Beyond physical and financial resources, utilization of the majority of diagnostics requires that communities have enough trained healthcare professionals to perform the tests. Though some diagnostics are easier to administer than others, many still require stringent protocols that need thoroughly trained individuals to execute. Lack of training infrastructure, limited resources for the

effective. We will learn more about social drivers of disease, such as geographic region and resourcing, in Chapter 10: Social Determinants of Health.
The specific pathogen circulating within a community also impacts diagnostic testing needs. Depending on the pathogen, a number of factors vary, such as the timeframe that governs the course of infection, ease of transmissibility, potential for asymptomatic spread, and severity of infection. Each of these variables must be considered when allocating limited resources to a testing strategy.
rapid dissemination of training information, and a shortage of medical professionals in underserved areas can pose significant obstacles to widespread testing (Figure 7.15).
Being unable to access healthcare providers and services is a function of system inequality. Health system disparities become increasingly apparent in rural areas, which are associated with fewer healthcare providers and higher incidences of chronic conditions. Because rural areas often have a low population density, it is difficult to establish large and well-resourced medical centers in locations that are accessible to all. It is oftentimes more burdensome for rural residents to commute long distances to access medical care, and many forgo such care. In the context of infectious disease, insufficient healthcare infrastructure across rural communities makes the deployment and utilization of diagnostic testing in these communities much more difficult and less
Different diseases demand different time frames within their respective response protocols. For example, a week-long delay in receiving a diagnosis for a swift and deadly disease, such as Ebola, can place the entire community at high risk in the interim and can even result in the patient’s death. Conversely, quick diagnostic results may not have as much of an impact when combating the spread of a disease like tuberculosis, since the duration of this disease averages three years, even with treatment.
For a virus that can spread asymptomatically, surveillance testing is indeed a useful addition to robust testing strategies in communities where clinical needs are already met. It can help to track the latest hotspots that can turn into epicenters for viral spread and eventually stop the pathogen in its tracks. On the other hand, for pathogens that are less infectious but cause diseases that are more severe, a government’s healthcare approach may emphasize treatments and broad messaging alongside a robust, but localized, testing strategy.
1. What are some challenges in diagnostic testing with regard to infrastructure and resources?
2. Why is it important to consider technical resources of a community (e.g., healthcare workers) when choosing which diagnostic tools to use?
3. How might a population’s response to a pathogen that is highly infectious and fast-acting differ from their response to a pathogen that is less infectious and remains in the host’s system for long periods of time?
1. Multiple possible answers, including ancient pregnancy detection using urine and barley, diabetes diagnosis via the taste, scent, or insect-attracting properties of urine.
2. We can detect a ‘smoking gun’ –the presence of the pathogen itself, or evidence of its presence– that noninfectious diseases do not possess.
3. By confirming whether a patient has a specific infectious disease through a diagnostic test, physicians can offer more effective, precise treatments, better anticipate the course of illness, and take appropriate protective measures if the disease is found to pose a risk to others. In this way, diagnostic testing helps public health experts contain a disease’s spread, stopping the pathogen in its tracks.
1. More or less details can be provided but answers should include the five groups:
1.1 Full microbe detection (blood smear staining and microscopy for whole pathogen detection)
1.2 Gene and genome analyses (detection of a pathogen’s gene, genome or protein by PCR, qRT-PCR or CRISPR/ Cas and gene or genome sequencing)
1.3 Antigen testing (lateral testing, ELISA for pathogen antigen detection)
1.4 Antibody testing (blood serum exploration for antibodies)
1.5 Biomarkers (state of the body markers)
2. Antigen tests detect the proteins of a microbe, while genome-based tests detect specific nucleotide sequences within the microbe’s genome.
3. Antigen tests indicate if an individual is currently infected with the pathogen whereas antibody tests determine whether someone has ever been infected with the pathogen of interest. While Antigen tests look for proteins of the microbe, antibody tests instead detect antibodies during or remaining after an infection.
1. Non-invasive methods rely on the use of biomarkers whereas invasive methods require tests that break skin to enter the body
2. Lab-based diagnostic tests are not analyzed in the same location that the test is administered, while point-of-need diagnostic tests are analyzed in the same location as administration. Therefore, point-of-need diagnostics typically yield quicker results.
3. Singleplex diagnostics test for one specific disease, whereas multiplex diagnostics test for multiple diseases at once.
1. A false negative could result in a patient not receiving the necessary treatment for a disease. They could also continue to pass along the infection -- especially if they are asymptomatic.
2.
a. In this chapter, we learned that :
Specificity=True NegativeTrue Negative + False Positive
In this example, the number of true negatives is the number of participants who tested negative who are truly healthy. The problem states that everyone who tested negative was healthy.
The number of people who tested negative can be determined by subtracting the positive tests from the total number of participants:
Total Participants = 500
Positive Tests = 325
Negative Tests = 500 - 325 = 175 = True Negatives
To find the number of false positives, we subtract the true positives from the total number of positive tests:
True Positives = 250
Number of Positive Tests = 325
False Positives = 325 - 250 = 75
Specificity=True NegativeTrue Negative + False Positive=175175 + 75=175250=70%
b. In this chapter, we learned that
The number of True Positives is equivalent to the number of sick participants who tested positive for the disease. If 250 participants are known to have the disease, and 325 tested positive, and all those who tested negative are healthy, that means that all of the 250 sick patients tested positive. Therefore:
True Positives = 250
The number of False Negatives is equivalent to the number of patients who tested negative who actually have the disease. The question tells us that all those who tested negative are healthy. That means no one who tested negative was actually sick. Therefore:
False Negatives = 0
Sensitivity=True PositiveTrue Positive + False Negative=250250 + 0= 100%
This example shows the importance of both Sensitivity and Specificity in evaluating diagnostic tests. If researchers had only calculated the Sensitivity for the diagnostic test depicted above, the test would have yielded a perfect result, and a key weakness in the Specificity would have been overlooked.
1. Because several considerations are needed to ensure that the infrastructure and budget allow for the right choice, answers may vary. Still, they should include some of these:
a. Accessibility to the technology required to run certain tests
b. Accessibility to space and conditions needed for the storage of test supplies (e.g., temperature, sterile condition, space)
c. Biosafety regulations
d. Availability of health personnel for the performance, processing and interpretation of diagnostic tests.
e. Pathogen specific transmission that may require a specific testing campaign
2. Because some types of diagnostics take special training and expertise to run fully, a community’s technical resources will determine whether they are able to correctly employ diagnostic techniques and obtain an accurate result/reading.
3. Answers may vary. Highly-infectious, fastacting pathogens may be better contained with rapid tests (along with aggressive control measures). Less infectious pathogens that remain in the host for a while could likely still be well-controlled with diagnostic methods that take longer to process, including PCR and antibody testing.
Amplicon : The genomic region of interest we are targeting to make many copies of, amplifying, in genomic amplification-based tests like PCR or LAMP.
Antibody Tests : A form of serology test, this type of test detects antibodies to a pathogen, which indicate whether an individual has already been infected by a pathogen.
Antigen Tests : A type of test designed to detect the presence of a specific antigen, informing us whether or not an individual is infected with a pathogen.
Asymptomatic : Showing no symptoms. In the case of pathogens, it is showing no signs or symptoms of infection with a pathogen.
Bacterial Culture : Is a type of diagnostic test that involves taking a patient sample that may have a bacteria and exposing it to conditions to promote growth of the suspected bacteria on a petri dish. The test is considered to be positive if colonies of bacteria grow.
Biomarker : A measurable biological property that can be used to determine a person’s specific health state, including whether or not they have been infected by a particular pathogen.
Blood Smear : A diagnostic test that involves smearing a drop of a patient’s blood on a microscope slide, staining the sample for easy visualization, and examining the slide for evidence of abnormalities, such as the presence of a pathogen.
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) : A component of the bacterial immune system that degrades pathogens attempting to reinfect the host. Recently, it has become a popular biomedical tool with several uses, including gene-editing and diagnostic technologies.
Conserved : Refers to a part of a genome that has been unchanged over time such that it is the same between all members of the pathogen species, or even in the larger evolutionary tree.
Contamination : That action or state of something being made impure by polluting. In the case of diagnostic tests, this refers to the unintentional presence of another biological material in the sample being tested, leading to a false diagnostic result.
Cross-Reactivity : When a test targeted against one particular pathogen can recognize a different pathogen, usually due to similarities in their genome or protein sequence.
Cycle Threshold (CT) : How many times you have to cycle a PCR reaction to reach a result (threshold) for detection. It is a useful proxy for pathogen load.
Diagnostic Test / Diagnostic : A procedure designed to confirm or rule out the presence of a specific pathogen or illness.
DNA Template : The region of DNA sequence that is targeted by primers and thus becomes copied during replication.
Enzyme-Linked Immunosorbent Assay (ELISA): A laboratory test that identifies and measures antibodies and other peptides and proteins. It yields quantitative results and can be performed at high-throughput.
False Negative: A test result that incorrectly identifies the absence of a pathogen when the pathogen is present.
False Positive: A test result that incorrectly identifies the presence of a pathogen when the pathogen is not present.
Gold-Standard: The best available existing test that can be used to benchmark other tests.
High-Throughput Testing: A broad term used to describe testing methods and technologies that can process many samples at once.
Invasive Tests: Clinical tests that require entering or “invading” the body with medical instruments. These include a blood draw or a nasopharyngeal swab.
Isothermal Amplification: A method that can be used as a diagnostic test, that involves amplifying genetic material at a single temperature.
Lab-Based Diagnostic Tests: Diagnostic tests that are processed and analyzed in a laboratory or specialized facility outside the location in which the test was administered.
Labeling: In molecular biology, this process involves adding a visible molecular marker to a protein.
Lateral Flow: Refers to the movement of molecules laterally, or sideways, across what is often a paper strip (e.g., most rapid COVID tests).
Loop-Mediated Amplification (LAMP): An isothermal amplification-based method that can be used as a point-of-care diagnostic test, amplifying pathogenic genetic material at a constant temperature in order to detect its presence.
Metagenomic Sequencing: A form of sequencing that reads out the entirety of genetic material of everything in a sample, including a microbe’s genome if it is present in a sample.
Minimally Invasive tests: Clinical tests that do require entering or “invading” the body, but are frequently easy to administer safely. These include blood spots and anterior nasal or salivary swabs.
Molecular Diagnostics: A family of medical techniques used to assess human health at a molecular level, analyzing biological markers in an individual’s genetic code (DNA or RNA).
Multiplex Diagnostics: Diagnostics that test a sample for many different things, such as potential pathogens present.
Non-Invasive Tests: Clinical tests that do not require physically breaking the skin or “invading” the body. These include simple tests we can administer ourselves like through collecting urine or saliva, or tests run by medical professionals like an MRI or EKG.
Pathogen Load: The amount of pathogen material present in the patient’s body.
Point-of-Care Diagnostic Tests: Diagnostic tests that are quickly processed and analyzed at the site they were administered.
Point-of-Need Diagnostic Tests: Diagnostic tests that individuals can take and analyze at home, or wherever a patient is at, and that often can be done without the help of a health professional.
Pooled Testing : A testing approach that groups or “pools” samples from multiple people, looking for the presence of an active pathogen from anyone in the pool.
Polymerase Chain Reaction (PCR) : An amplification method that uses several heating and cooling cycles to make many copies of a particular genetic sequence in order to detect its presence. This method is commonly implemented in diagnostic tests, which seek to amplify pathogenic sequences; if the sequence is present in the patient sample, it will be amplified by the PCR and can be subsequently visualized as a positive result in the diagnostic test.
Primers : Guides to direct DNA polymerase where to begin during the replication process. They are single-stranded stretches of DNA (often around 20-30 base pairs long) that are complementary to and flank – are on either side of – the region of interest.
Quantitative PCR (qPCR) : A form of PCR that harnesses the power of fluorescent labeling to measure the rise in fluorescence as the reaction is occurring, in real time, to help quantify how much of a particular genome sequence is in the sample
Reverse Transcription-qPCR (RT-qPCR) : A form of PCR, where scientists can use RNA as a template by first converting it to a double-stranded DNA template using a special enzyme called reverse transcriptase.
Sensitivity: A measure of how well a test can correctly identify the disease in an infected person. It is defined as the proportion of the total group of subjects infected by the pathogen (total positives = true positives + false negatives) that received positive test results (true positives).
Serology: A diagnostic or research test based on the use of blood serum, often used to detect the immune response to pathogens.
Singleplex diagnostics: Diagnostics that test one sample for just one thing, such as potential pathogens present.
Specificity: A measure of how effective the test is at correctly identifying that a person is truly not harboring the pathogen. It is defined as the proportion of the total group of subjects not infected by the pathogen (total negatives = true negatives + false positives) that received negative test results (true negatives).
Targeted Sequencing: A form of sequencing which will only produce sequences from the pathogen of interest. The most commonly-used form, amplicon-based targeted sequencing, uses the same amplification process using polymerases as PCR, to make many copies of the target(s) of interest prior to sequencing.
PCR Tube: A thin plastic tube closed at one end that holds small amounts (microliters) of material for lab experiments, typically used for PCR therefore the common name.
Thermocylcer: A lab instrument that regulates temperature during cyclical programs, used in PCR.
True Negative: A test result that correctly identifies the absence of a pathogen when the pathogen is not present.
True Positive: A test result that correctly identifies the presence of a pathogen when the pathogen is present.