CHAPTER 6
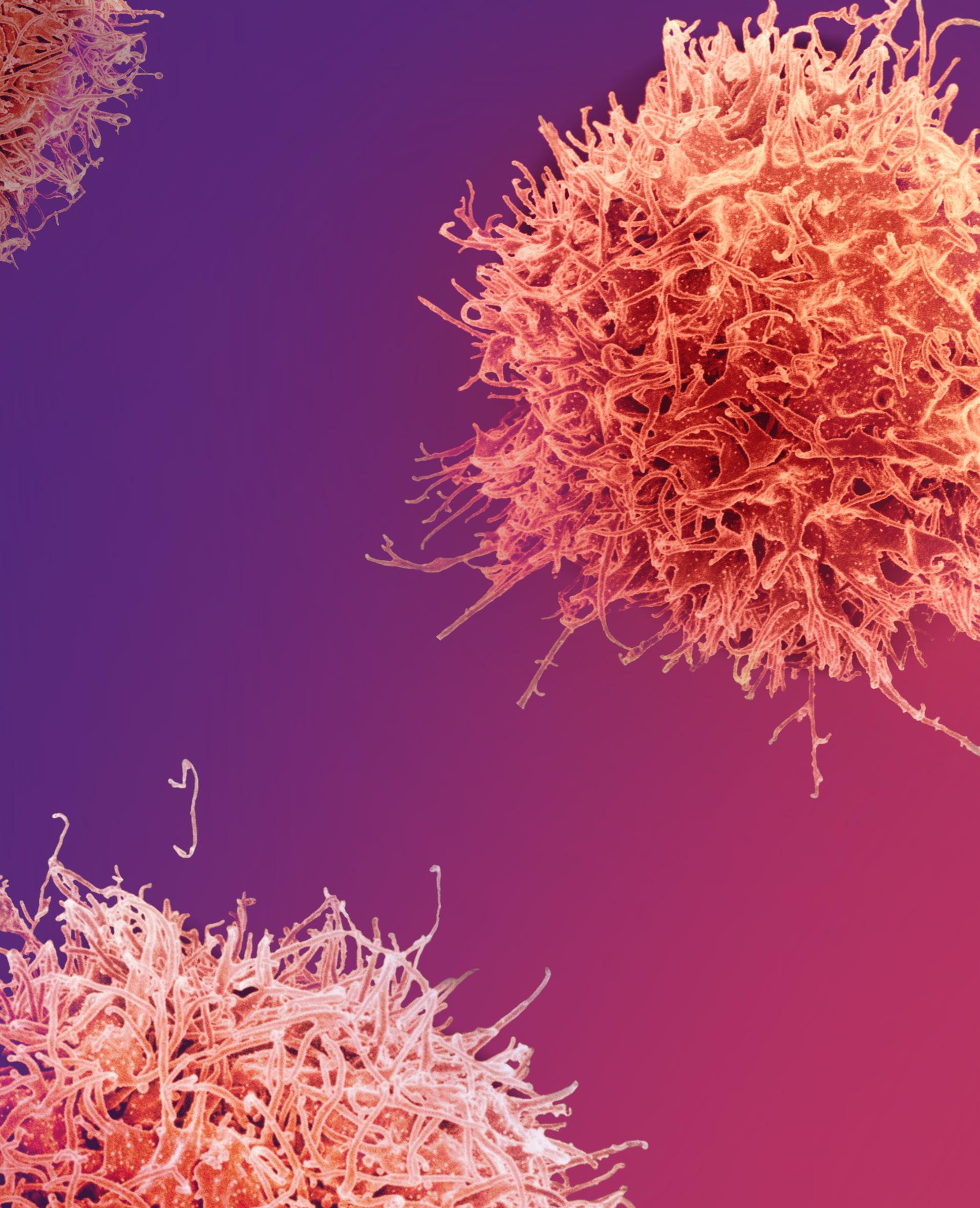
CHAPTER 6

Here are some essential terms used to understand how the immune system works. By the end of this chapter, you should be able to apply these concepts and understand how they relate to each other.
Adaptive Immune System
Allergies
Antibodies
Antigens
Antigen Presentation
Autoimmunity
B Cells
Cytokines
Humoral Immune Response
Immunological Memory
Innate Immune System
Phagocytes
Inflammation
T Cells
White Blood Cells (WBCs)
The immune system is your body’s way of protecting itself from outside threats and consists of many complementary lines of defense. Your innate immune system launches the first attack against any and all foreign threats, and the adaptive immune system follows with a specific attack tailored to the particular pathogen at hand. The immune system responds to pathogens at the moment of infection, and also keeps a record of every pathogen it has encountered. This helps the body quickly recognize and destroy a familiar pathogen if it enters the body again. This mechanism is known as immunological memory.
The innate immune system, your body’s first line of defense, provides nonspecific protection against various pathogens and foreign invaders. These mechanisms include mechanical barriers such as skin or mucous membranes, chemical barriers such as acidity, or destructive enzymes and biological barriers such as the microbiome. The innate immune system also has a cellular response component that broadly attacks foreign invaders; this includes phagocytes that engulf and digest invaders and natural killer cells that identify and destroy compromised cells.
The adaptive immune system is your body’s second line of defense. The adaptive immune system has two separate components that work together. The first is the humoral immune response, driven by B cells that produce specialized proteins called antibodies. Antibodies specifically recognize and bind a unique part of a pathogen, called an antigen, to neutralize or tag them. The second component is the cell-mediated immune response, driven by T cells that search for and eliminate your own compromised cells that have been infected with the pathogen.
Insufficient or overactive immune responses can affect individuals’ health and aggravate infectious diseases. On one hand, an insufficient immune response, such as occurs in immunodeficiencies, renders the host entirely vulnerable to pathogens, as we often see with late-stage AIDS patients. On the other hand, an overactive immune response can cause damage to ourselves. We see this in the form of allergies against benign substances like pollen and certain foods causing serious reactions, or in the form of autoimmune diseases, when the body attacks its own cells.


By the end of this chapter, you will understand how our bodies protect themselves from foreign pathogens. You will learn about the nonspecific (innate) and the specific (adaptive) immune system responses, and you’ll be able to describe how these processes work together. You’ll become familiar with the key cells involved in each of these responses and the crucial role each cell plays in protecting against invading pathogens, as well as providing lasting immunological memory if a pathogen returns. Lastly, you will understand of what it means for the immune system to be compromised, as well as the wide-ranging impacts of immune-system errors, from everyday allergies to more serious immunodeficiency syndromes.
It’s early June 1915, and after a year of successfully evading conscription, you’ve finally been forced to take up arms in the Great War (known to later generations as World War I). You’re not a soldier by trade; in fact, you’re a painter, and a fairly prolific one. Despite your youth, you’ve already garnered a strong reputation in your home country of Austria as one of the most provocative and controversial artists of your generation. Before the war, you spent most of your time creating amatory works or self-portraits, often featuring yourself or female models such as your new wife, Edith. But just three days after your wedding, you’re ordered to journey to Prague as a soldier rather than an artisan.
Luckily for you, your commanding officers soon realize you’re more suited to hold a pen than a gun, and you’re given a job as a clerk
“I find it astonishing that the immune system embodies a degree of complexity which suggests some more or less superficial though striking analogies with
human language,
and that this cognitive system has evolved and functions without assistance of the brain.”
—Niels
K. Jerne, Danish Immunologist and Nobel Prize Laureate
to keep track of food storage. Your two years in the military pass fairly well, and as the war winds down, you’re able to shift your focus
back to your prospering career and building a family. Amidst the war turmoil, people are turning to your art in greater numbers, and both your popularity and income have skyrocketed. However, as you’ll all soon learn, there’s a new threat brewing in the background.
Spring of 1918 passes, and you hear news of a particularly nasty case of the flu spreading across the globe including amongst the art community. It quickly reaches your own doorstep; one of your mentors, Gustav Klimt, an Austrian painter, becomes infected with the mysterious illness while recovering from another sickness in the hospital, soon passing away. As the summer ends, the mysterious flu fully overtakes the Austrian city of Vienna. Your wife, six months pregnant with your first child, falls sick, and you become ill shortly after. Before long, your community is inundated with cases, and it seems as though nowhere is safe from the deadly influenza. As the cooling weather drives people indoors, and crowded wartime conditions persist, this new, dangerous influenza strain begins to spread like wildfire. Soon, it feels like everyone knows someone who’s had it—or worse, died. Although it soon gained the (erroneous, and later-discouraged) moniker “Spanish Flu” after the country’s intense media coverage of the disease, we now know this pandemic as the 1918 influenza pandemic.
Your wife is quickly deteriorating; she has a severe case of pneumonia, and you fear she and your unborn child will not survive. You write a brief letter to your mother stating, “I am preparing myself for the worst.” Sadly, your worst fears come true: on October 28, your pregnant wife passes away. Your health rapidly declines immediately afterward, and
you follow her in death just three days later at the young age of 28. Your story, while tragic, is not uncommon. Although influenza usually takes the very young and very old, this particular flu killed individuals in their mid-20s and 30s at alarming rates, as well. Why are individuals in the supposed primes of their lives, like you and your wife, dying at such disproportionate rates?
As later investigations would reveal, the particular flu strain responsible for the 1918 pandemic provoked unusually strong immune reactions in traditionally healthy patients. While immune cells exist to protect the body, too much of a good thing can become dangerous — as immune cells become overstimulated, they can begin to damage various parts of the human body. In the case of the 1918 influenza, damage to the lungs as a result of an overactive immune response caused a build-up of fluid, essentially drowning infected individuals.
The 1918 influenza pandemic was a horrible time, and for many, it was even more gruesome than the Great War itself. The disease was uniquely indiscriminate in whom and how many it affected, ultimately infecting a third of the global population and killing an estimated 50 million people. While influenza had the potential to affect everyone, regardless of occupation or nationality, its emphasis on the youth allowed it to erase countless futures — a fact acutely portrayed by the images of life and death created by you and other artists of your time. From Norwegian painter Edvard Munch’s harrowing series of self-portraits chronicling his recovery from the sickness, to the last few portraits of your wife you created before your own passing, the people within your era and those looking back a hundred years


FIGURE 6.1 | Last portraits of Gustav Klimt before passing of the 1918 flu (left) and Edith Schiele (right). “Gustav Klimt on his deathbed” provided a rare and intimate look into one of the pandemic’s early deaths. The sketch was made by Egon Schiele in 1918. Titled “Dying Edith,” features 25-year-old Edith Schiele during her final moments and is one of the last portraits her husband ever created.
later can see a glimpse into the reality of this unprecedented era. You are Egor Schiele, a famed Austrian expressionist artist, and your life and death, reflected in your art, provide a unique snapshot of the struggles of your time (Figure 6.1).
Your body’s immune system is an amazing security system that works around the clock to keep you healthy. You are in constant contact with potential infectious agents — in the air, on the surfaces you touch, and even on your own skin. Without a means of protecting itself from these outside threats, your body would be in danger.
We can imagine that your body is like an ancient city surrounded by a protective wall, and that pathogens are invading soldiers. Your immune system consists of all the elements the city uses to defend itself from invaders: walls, guards, soldiers, and more. The “walls” are physical barriers that you can easily spot, like your skin, while the specialized “soldiers” are unique immune cells that target and defeat specific pathogens, and so on. We’ll discuss each of these pieces in greater depth throughout this chapter.
Immunity is the ability to defend against a foreign pathogen after having been exposed to it. The process of gaining immunity consists of two key parts: the innate and adaptive immune responses. These parts work in tandem to neutralize – or render ineffective or harmless – threats, as well as remember them so that a quicker, more efficient response can be launched if the same pathogen tries to infect the body again.
When your body first encounters a pathogenic threat, the immune system quickly launches generalized defenses which do not depend on the particular pathogen at hand. This is called the innate immune response, and is governed by the innate immune system. The innate system doesn’t single out any particular pathogen, but instead recognizes the presence of any foreign threat. Think of it like traps surrounding the city. This first line of defense of the innate immune system includes physical, chemical, biological and behavioral barriers. If these prove ineffective, a cellular response is triggered, causing an inflammatory response in your body. Like the guards of our ancient city, the inflammatory response tells your immune cells to flock to the site of infection to respond, causing familiar symptoms like nasal congestion or headaches.
The inflammatory response kicks off the adaptive immune system, which is the other half of the immune system. The adaptive immune system is a specific and lasting, albeit slower, response to infection that seeks out and destroys a specific pathogen causing it. Adaptive immune cells are like trained assassins: when recruited to the site of infection by the innate immune system, they can learn key features of a pathogen in order to seek out that pathogen and destroy it.
The adaptive immune response is custom-made for the particular pathogen at hand, and is so named because it develops better responses to a given pathogen over time. To do so, the adaptive immune system recognizes and mounts a response against specific pathogen molecules known as antigens. Adaptive immune cells are fitted with specialized receptors, which you can think of as the “locks” into which the antigen “keys” fit. When a lock and key match, your adaptive immune cells get to work, producing more and more adaptive immune cells with receptors specific to the antigen.
The adaptive immune system also provides your body with a pathogen memory system, keeping a record of each pathogen which allows immune cells to rapidly recognize and neutralize the same pathogen in the case it strikes again. This is an impressive task; think of all the pathogens that your body has encountered over the years. Suppose any of these pathogens ever strike again in the future. In that case, your adaptive immune system will be able to identify it by running through all the pathogens it has “on file”, and then deploying a pathogen-specific attack. This process by which the immune system keeps a record of every pathogen it has encountered is called immunological memory











FIGURE 6.2 | The human immune system. The first response against pathogens involves anatomical and physiological barriers along with a group of specialized cells, together comprising the innate immune system. A second line of defense composed of a humoral immunity and a cellularmediated response makes up the adaptive immune system. All of these parts work together to protect the body from infections. Innate immunity is characterized by providing a nonspecific response whereas adaptive immunity consists of an acquired response, based on exposure to specific pathogens, leading to immunological memory.
The key players of the immune system include white blood cells (WBCs), also known as leukocytes. Blood, the body’s main circulating fluid, contains many different types of cells including red blood cells, which deliver oxygen
to the body’s tissues; platelets, which enable blood clotting; and WBCs, which are the protagonists of the immune system. There are many different types of WBCs, each with their own specialized jobs. WBCs are generated in the bone marrow and are found circulating in the blood. In Table 6.1 we present the different WBCs, their names, and their main roles in the fight against pathogens. We’ll look
into the specific functions of innate immune cells, such as dendritic cells and natural killer cells, and adaptive immune cells, such as B cells and T cells, throughout this chapter. We will also discuss another set of key players of our immune system known as cytokines, a special class of small molecules which act as a signal for different types of WBCs to communicate with each other and enhance the immune response.
Immune Response Immune System Cells
Innate Monocytes Dendritic Cells Alert other cells and link the innate with the adaptive immune system; antigenpresenting cells.
Macrophages A type of phagocyte; engulfs and destroys pathogens. Secrete cytokines.



Granulocytes
Adaptive Lymphocytes
Neutrophils Arrive at the scene of infection before any other granulocytes. Contain toxic granules to destroy pathogens.
Eosinophils Contain acidic toxic granules to destroy pathogens, produce histamine.
Basophils Play a role in the inflammatory response and produce histamine.
NK Cells Detect infected cells, and trigger the release of cytokines.
Adaptive (humoral)
Adaptive (cell-mediated)
B cells Produce antibodies against a specific antigen; differentiate into plasma B cells and memory B cells.
T Cells Search for infected host cells that need to be destroyed; differentiate into killer T cells and helper T cells.













For most people, the immune system works like a well-coordinated security team, keeping your body up to date and well protected against common pathogens. However, sometimes things can go wrong. Some people may be immunocompromised, meaning that they have a diminished ability to properly fend off pathogens. As you can imagine, infections in an immunocompromised person can have devastating consequences. On the other end of the spectrum, some people can develop an overactive immune response to stimuli leading to what is known as an autoimmune response. Autoimmunity is the result of the
immune system mistakenly attacking the body’s own tissues or organs; autoimmune destruction can occur while actively fighting off a pathogen, or at a steady state. For example, a type of psoriasis known as psoriasis guttate is thought to be triggered by streptococcal infection, and typically resolves completely (eventually). On the other hand, type I diabetes appears to have both a genetic basis and possible links to viral infection, but its effects are typically lifelong, erroneously destroying the insulin-producing cells in the pancreas. We will discuss scenarios of autoimmunity in more detail in section 6.4: Malfunctioning Immune Responses and Their Impacts.
1. What is the main job of the immune system?
2. What are the two main divisions of the immune system? Briefly describe their main roles.
3. What are the key players of the immune system?
Let’s take a closer look at your body’s initial defense: the innate immune system, which encompasses your body’s nonspecific protections against foreign threats. “Nonspecific” means that these mechanisms have one general method of blocking out a wide range of potential invaders; they are not designed to go after specific pathogens.
Your innate immune system has a few different ways of providing this nonspecific protection, frequently separated into the following subtypes: mechanical barriers, such as the skin, which prevent some pathogens from entering your body in the first place; chemical deterrents, like sweat and stomach acids, which neutralize pathogens before they harm your body; and biological barriers such as the typically-benign microorganisms in your body, which work together with the immune system cells to fight pathogens. If pathogens escape these barriers and enter the body, your innate immune cells can launch their nonspecific defenses.
Mechanical barriers in the immune system block unwanted microbes from entering the body. Imagine these barriers as the large walls surrounding our ancient city, physically blocking the invading soldiers from entering. This is how the epidermis , the outermost layer of the skin keeps pathogens out of the body (Figure 6.3). Unless there is an opening in this protective layer, such as a cut or a burn, many pathogens are blocked from entering your body and causing harm.
What about the areas of the body that are not covered by skin, such as the eyes, nose, and



F IGURE 6.3 | Our skin is a primary barrier to pathogens. A diagram of the different layers of the skin — a prime example of our bodies’ mechanical barriers preventing pathogens from invading our systems. The top layer, the epidermis, acts as armor against pathogens.
ears? These parts of the body have their own unique barriers. For example, your eyelashes and nose hairs prevent dust and airborne microorganisms from getting inside your body. Your ears contain earwax, which has antibacterial and antifungal properties and blocks the entrance of microbes into the ear canal. Lastly, some parts of your body, such as the lips, genitals, nose, and anus, have mucous membranes that produce mucus (also known as snot), a gooey substance that can trap microbes.
The mucous membranes in your nose and mouth release mucus as you inhale and exhale to trap any pathogens that could irritate your airways. This mucus is stored in the sinuses , a system of hollow spaces around your nose. An infection of the sinuses is medically referred to as sinusitis . Some mucous membranes have an additional layer of protection called cilia , which are thin, hair-like projections responsible for pushing pathogens out of the airways and away from the membrane (Figure 6.4). Cilia are tiny, only about 5-10 micrometers long
Cilia
Ciliated cell
Goblet cell
FIGURE 6.4 | The sinuses with their lining of cilia work together to provide another mechanical barrier to prevent pathogens from entering the body. The sinuses produce mucus that traps pathogens while the cilia protect the body by sweeping foreign particles out of the nose and airways. Note: while the sinuses are a system of hollow spaces, in this diagram only one is depicted.
(1 mm = 1000 μm), which is hundreds of times smaller than a grain of sand.
There are other barriers like coughing and sneezing which are common ways unwanted material is dislodged from our airways (Figure 6.5). In addition, the olfactory system, which deals with your sense of smell, is one of these
subtle yet important physiological protective responses. Think about how we react when we are near something that smells bad: we want to move away. This is because our bodies have learned to recognize certain odors as warnings for things that could make us sick. That’s why we make a point to avoid smelly dog poop, and know not to take a sip of spoiled milk after a single whiff. Receptors in the nose take in the unpleasant odor, and send it up to the brain for guidance, which triggers our instincts to stay away.
FIGURE 6.5 | Coughing is another example of a mechanical barrier provided by the innate immune system. Coughing is a mechanical barrier that can force debris and foreign material out of the body’s respiratory system.
Physical barriers aren’t your body’s only nonspecific defense mechanism – there is an additional layer of chemical barriers. Chemical barriers are antimicrobial substances that protect against infection. In our ancient city, chemical barriers are like traps — think rolling boulders and spear-covered corridors — set up in and around the city to prevent invasion. In the body, these “traps” take the form of destructive enzymes, acids, or other chemical compounds that can stop an invading pathogen.
Sweat, in addition to an oily substance called sebum, is produced in skin glands and contains lysozymes, which protect the body from pathogens. Lysozymes are enzymes capable of inducing lysis, or breakdown, of bacterial cell walls. These helpful enzymes are also found in tears and saliva, allowing us to combat bacteria entering through the eyes or mouth acting as chemical barriers.
In addition to specialized enzymes like lysozymes, there are a few highly-acidic regions in your body that are especially toxic for many pathogens. For example, the stomach contains acidic juices which can destroy bacteria and toxins that may otherwise harm the host.
As you might remember from Chapter 5: Biology of Infectious Agents, the microbiome refers to the collection of microorganisms
(bacteria, fungi, protozoa and/or viruses) that live together in any given habitat. We find commensal microorganisms, or “good microorganisms,” in pretty much every tissue of the body– the skin, the gut, the lungs and more. These microorganisms make up the human microbiome which, among other things, functions as a biological barrier against pathogens (Figure 6.6).
Along with our own 30 trillion human cells, scientists have quantified about 40 trillion microbial cells making up the human body. That means that there are about the same number of microbial cells as human cells in our bodies. You may be wondering why our bodies host so many microorganisms and what the role of the microbiome is in the immune system. This is an active area of research, and there seem to be many ways that the human microbiome works together with the immune system to keep pathogens away. For example, some

FIGURE 6.6 | The human microbiome. Our immune system collaborates with commensal bacteria and fungi to protect us from pathogens. Interestingly, it is estimated that at least 50% of the total cells found in the human body belong to microorganisms.
bacteria produce vitamins and anti-inflammatory compounds that play a role in the inflammatory response to regulate, for example, swelling. The human microbiome also plays an important role in the training of the host immune system from the moment we are born; your immune system is trained not to kill the “good bacteria” from the outset. We let them live in our body, and in exchange for some of our nutrition and resources, they help us fight harmful pathogens – a pretty good deal for both sides.
Now that we’ve covered all of the immune system’s noncellular defense systems, let’s think back to our ancient city analogy to see how they all fit in. When invading soldiers approach, the city has more than just its own walls and traps (e.g., skin and saliva) to rely on. A group of citizens in the city, such as the gut microbiome, might alert our city that the invaders (particularly viruses) are on the move or even try to slow their progress.
In our ancient city, if invading soldiers breach the walls and dodge the traps, how can the city defend itself? This is where our city’s specialized responders come in: its guards. When a pathogen Virus


gets past your body’s physical, chemical, and behavioral barriers, your innate immune cells are alerted to attack. We can imagine innate immune cells as our city’s general guards, patrolling just inside the city walls, ready to quickly respond to any sign of a threat.
Found in blood vessels and various tissues, innate immune cells aim to catch these invading pathogens before they multiply out of control and impact your health. Since innate immune cells are part of the nonspecific immune response, they deal with any substances that appear foreign to your body. They do so by carrying cell surface receptors which can recognize many broad molecular features of pathogens.
Phagocytes, a class of WBCs, are the main cellular players of the innate immune system. They are monocytes, macrophages, dendritic cells, and granulocytes. Phagocytes engulf and digest a diverse range of pathogens, destroying them. This process is known as phagocytosis, stemming from the Greek prefix phago which means “to eat” (Figure 6.7). As the name suggests, phagocytes patrol the bloodstream, “eating” whatever potential threats they find.


FIGURE 6.7 | Phagocytosis is a first line of cellular defense inside the body. Once phagocytes first detect a target particle, they induce a signal that activates the cell to engulf the pathogen, then a membranous cell structure called phagosome forms to encapsulate the pathogen. Finally, the pathogen is destroyed inside the phagosome and its components are ultimately absorbed by the cell.
Phagocytes recognize foreign substances such as certain lipids and common nucleic acid sequences using different receptors on their cell membranes. These receptors recognize common pathogenic features. For example, some phagocytic receptors detect peptidoglycan, a key component of bacterial cell walls that you might remember from Chapter 5: Biology of Infectious Agents. When a phagocyte detects this molecule on an invading bacterium, its peptidoglycan receptor binds to the cell wall, and the phagocyte is prompted to engulf the bacterium. Since this receptor can bind any bacterium in the body with peptidoglycan in its cell wall, it is a nonspecific response.
Phagocytes can be divided into a few different classes based on the different actions they perform. The most important classes are macrophages, dendritic cells, and granulocytes. Macrophages are classic phagocytic cells which reside in tissues, ready to engulf invading pathogens. When macrophages “eat” pathogens, they release inflammatory signals via cytokines, launching the larger immune response.

Scan this QR code or click on this link to watch a video of a macrophage in action under a microscope.
Dendritic cells are named for their characteristic “dendritic” or web-like projections. Like macrophages, dendritic cells engulf pathogens they come across. Often, phagocytosis is enough to neutralize the threat, but if not, macrophages and dendritic cells can recruit additional partners from the adaptive immune system to help in the fight. Once this happens, dendritic cells can use their dendritic projections to display pieces of the pathogen and alert adaptive immune cells to the infection. This process is known as antigen presentation, and as we will look into it in more detail, we’ll learn just how vital this immune process is for the activation of the adaptive system T cells. This linkage of the innate and adaptive immune systems is key to the progression of the immune response.
Granulocytes contain toxic granules which are used to destroy pathogens (Figure 6.8). There are three main types of granulocytes: neutrophils, eosinophils, and basophils. Neutrophils are the first to arrive at the scene of infection. To kill pathogens, they first “eat” them and then they destroy them by releasing harmful, neutrophilic granules to eliminate the pathogen at hand. Neutrophils are plentiful, speedy, and short-lived, frequently dying as they complete their immune functions. Consequently, dead neutrophils

FIGURE 6.8 | Granulocytes are a type of phagocyte containing toxic granules used to destroy pathogens. Neutrophils, eosinophils and basophils are types of granulocytes, named for the presence of granules within the cells.
quickly build up at the site of infection, and their remains are cleaned up by resident macrophages. This buildup of dead neutrophils is what causes the familiar yellow-white pus that you might notice around a cut.
Basophils and eosinophils cause inflammation during the immune response by releasing histamines – small molecules that often cause symptoms like the hyperpigmented, itchy splotches that accompany allergic reactions.
Now that we’ve discussed the phagocytes, let’s talk about the other key cell type in the innate immune system: natural killer (NK) cells. These cells do exactly what their name suggests: identify and destroy your own compromised cells. While phagocytes target pathogens, NK cells target infected cells. To identify which cells need to be destroyed, NK cells inspect their surfaces and can determine whether a particular cell has been compromised; some infected cells will “look” different, missing certain easily-




recognizable proteins on their surfaces. But killing cells identified for destruction isn’t always simple: exploding a virus-infected cell, for example, could lead to the release of more copies of the virus in the body, ultimately worsening an infection. Imagine trying to get rid of a smelly garbage bag by ripping it open — bad idea. To avoid this, once NK cells have identified their target, they poke holes in the target’s membrane and inject the target cell with toxic molecules. These toxic molecules break down the infected cell, leading to a type of controlled cell death called apoptosis Then, other phagocytes such as macrophages will ingest these dying or apoptotic cells.
NK cells are traditionally classified as part of the innate system. However, more recent studies have suggested they have memory-like functions, providing a compelling argument for their classification as adaptive immune cells as well (Figure 6.9). We will introduce the adaptive immune system and how immunological memory is formed in section: 4.3 Adaptive Immune System.







| Innate and adaptive immune cells. The cellular defenses of the innate immune system include macrophages, dendritic cells, neutrophils, eosinophils and basophils. The adaptive immune cells include B and T cells. NK cells are classified as part of the innate immune system but appear to have some properties of both innate and adaptive immunity.
By now, you’ve learned that our city has a lot of defense mechanisms that need to coordinate with each other to organize a counterattack against invading soldiers. But how do these parts work together?
Immune cells, such as macrophages and helper T cells, use cellular signaling molecules, known as cytokines, to communicate with each other. Cytokines can act on the cells that secrete them, on the surrounding cells, or sometimes on more distant cells. There are many different types of cytokines, including chemokines, interferons, interleukins, lymphokines, and tumor necrosis factors, each with specific signaling functions. Some cytokines tell immune cells where to go, alerting the immune system to bring a higher level response. For example, when macrophages encounter a pathogen, they release specific cytokines which attract other immune cells to the site of infection (Figure 6.10). Other types of cytokines induce the production of more immune cells. Cytokines can also be thought of as distress signals that recruit additional resources in the fight against a pathogen. Cytokine functions and mechanisms are extremely broad, and researchers continue to study their various categories and how they regulate cell function.

INDUCING STIMULUS
Cytokine-producing Cell
Cytokine Gene
Cytokines

Target Cell
Gene Activation Signal
BIOLOGICAL EFFECTS
FIGURE 6.10 | Cytokines enhance the immune response. This image shows how cytokines released by macrophages are received by another cell, activating cell signaling processes that induce the inflammatory response. This response can result in swelling, increased temperature, and increased blood vessel permeability.
Cytokine signaling directly causes inflammation, a response including heat, redness, swelling, and pain that we typically associate with being sick. These symptoms are caused by the production of lots of new innate immune cells and cytokines fighting the ongoing infection.
1. What are the different types of barriers your innate immune system uses to protect your body? List an example of each type.
2. How does a natural killer cell kill its target cell?
3. What are cytokines?
If a pathogen gets past your body’s physical, chemical, and behavioral barriers, as well as its initial nonspecific cellular defenses, your adaptive immune system – the body’s second line of defense – is prepared to respond specifically to the threat at hand. The adaptive immune system is like an elite, specialized team of assassins for our city, trained to seek out specific, wanted criminals. The walls, traps, and general guards of the innate immune system mount the initial attack, but these special agents of the adaptive immune system
collect evidence of the invader’s identity, like fingerprints, and use these to track down and eliminate specific invaders.
Recall that during the initial inflammatory response to a threat, the innate immune system calls the adaptive immune system into action using specialized signals, such as cytokines. The two components of the adaptive system, called the humoral immune response and the cell-mediated immune response, then work together to search for and destroy the specific pathogenic threat that they identify (Figure 6.11). The B cells in the humoral immune response create special proteins called
antibodies that patrol the blood looking for antigens, and the T cells of the cell-mediated immune response search for infected cells that need to be destroyed.
In our ancient city, the B cells are special agents who are patrolling the streets to identify and take out the enemy. The T cells, on the other hand, are the immune system’s spies, watching out for and eliminating any suspicious citizens, i.e., compromised human cells infected by the pathogen. The humoral and cell-mediated responses of the adaptive immune system, though separate, rely on similar patrolling mechanisms and machinery.
All adaptive immune cells use antigens, the “fingerprints” or evidence of a given pathogen, to specifically identify and/or destroy pathogens. Antigens are molecules including proteins and lipids, all of which are unique to each invading pathogen. When your highlyspecific B and T cells recognize a particular antigen, they can launch a specialized, potent attack which improves over time, as the adaptive immune cells hone the response to the specific pathogen profile.
The adaptive immune system also keeps a record of the pathogens it has encountered. The special agents of our city are on constant watch for foes they have defeated before, looking specifically for the wanted enemy using the fingerprints collected in the previous invasion. If and when these agents ever come across the same fingerprint, they sound a strong and powerful siren that signals the body to enter attack mode. Your immune cells remember each antigen they encounter to ensure a specific and faster response if the same pathogen tries to infect you again. Now
let’s dive into how exactly the adaptive immune system does its job by looking into the humoral and the cell-mediated immune responses.
Humoral immunity deals specifically with the immune system’s role in monitoring your blood. The term humoral comes from the Latin word humor, which means fluids or bodily fluids. The humoral immune response relies on antibodies, also known as immunoglobulins, which are produced by B cells. As you will see, the structure of antibodies helps them recognize and respond to their target antigens, and the way your body creates antibodies ensures they can take on a boundless number of diverse forms (Figure 6.12).

Antibody
Antigen A

Antigen B
Pathogen
FIGURE 6.12 | B cells produce antibodies specific to a pathogen’s antigens. Each antibody is uniquely shaped to match a specific antigen, such as a protein on the surface of the pathogen.
Because of their unique structure, antibodies are able to bind specific antigens, allowing them to systematically target and eliminate
specific threats. Antibodies are Y-shaped: the base of the Y is known as the constant region , and the top of the Y is known as the variable region (Figure 6.13). The constant region is found in every antibody of a particular type. Different antibody types are found at different times during the course of infection, and as we’ll talk about in greater detail in Chapter 7: Diagnostic Tests, we can measure these to determine the progression of infection in patients.
The variable region is what recognizes and binds to a specific antigen. As the name implies, these vary from antibody to antibody, and our immune system has an infinite range of different potential variable regions. You can think of a variable region as a custom-made lock that matches exactly one type of key: the antigen from a specific pathogen.
It’s critical to remember that the humoral immune response relies upon the antibody’s ability to correctly recognize the invading antigen. This is truly an amazing biological feat; despite containing only twenty thousand genes in total, your body can make hundreds of billions of different antibodies. To get an idea of how your body does this, let’s talk about how antibodies are made.
VARIABLE REGION
CONSTANT REGION
F IGURE 6.13 | Structure of a typical antibody. Antibodies are produced to specifically bind a pathogen antigen. They have two main regions: a constant region which does not change across antibodies and a variable region which matches a specific antigen.
Antibodies are produced and released into the bloodstream by specialized B cells, a type of WBC that originates in the bone marrow. B cells have B cell receptors (BCRs), which are very similar to antibodies in structure, and bind to antigens just like antibodies, but are embedded in the B cell membrane. Each B cell expresses a single type of BCR with a unique binding capability that is produced via genetic reshuffling. Shuffling entails that BCR-specific pieces of DNA are shuffled around to combine in random, nearly-infinite permutations that allow for binding to far more antigens than any predetermined combinations could provide. These BCRs are unique from other proteins of our genome, in that they are not explicitly encoded by your genome, but rather a product of this shuffling process. When a naive B cell encounters an antigen in the bloodstream that matches its BCR, more B cells are produced with the same BCR in a process called clonal selection and proliferation. These B cells produce corresponding antibodies which can fight off the pathogen (Figure 6.14).
B cells also differentiate into another celltype that is crucial to the immune response: plasma cells. Plasma cells both synthesize and release the antibodies that are key to this whole process. After the plasma cell produces

Antigen
Naive cells
Antibodies

If antigen appears later, the memory cells are stimulated, divide and produce many plasma cells very quickly.






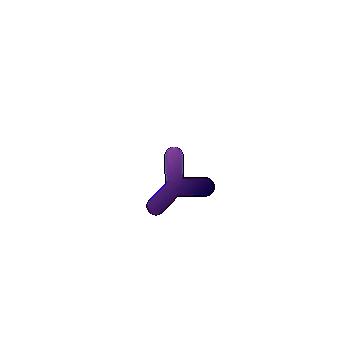

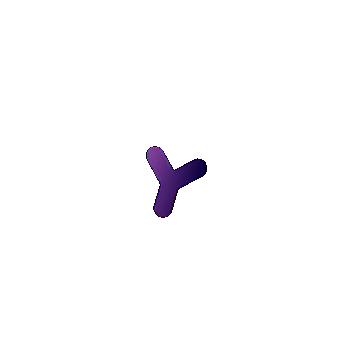




PLASMA CELLS

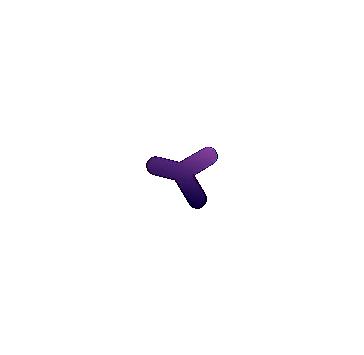











FIGURE 6.14 | Antibodies are produced and released into the bloodstream by B cells. B cells have BCRs embedded in their membrane. BCRs are specific and when a B cell encounters a BCR matching antigen in the bloodstream, more cells with that BCR are produced (clonal selection and proliferation). These plasma cells make antibodies and release them in the bloodstream to fight the target pathogen. During this process, memory B cells are also made. If that antigen appears again, the memory cells can rapidly produce more plasma cells to fight it.
an antibody, the antibody’s variable region can be “unlocked” by its corresponding antigen key, which amplifies your humoral response.
Antibodies have many ways of combating pathogens. Some antibodies are able to neutralize pathogens by binding to a part of the pathogen necessary for its invasion of host cells; if the pathogen cannot get in, it cannot cause damage. Other antibodies cause pathogens to clump together, physically preventing them from separating and invading your cells, a process called agglutination. In both cases, the antibodies are considered neutralizing antibodies, because they are capable of making their targets ineffective or harmless (Figure 6.15).
There are lots of other ways in which antibodies fight off pathogens that do not involve directly blocking an infection. Some antibodies can also induce cell rupture or cell death. Other antibodies may bind to antigens and recruit phagocytes to the scene to gobble up the antibody-bound pathogen. Destruction by
phagocytes can in turn activate the cellmediated immune system for an even more robust response. This is just one of the many instances in which the innate and adaptive immune systems work together to fight infection.
Immunological memory is built as our immune systems get exposed to pathogens. In the introduction of this chapter, we compared this to keeping pathogens “on file,” but how exactly does your immune system save this information?
After your body is exposed to an antigen, your cells develop antibodies specific to it in a process known as active immunity. As we’ll describe below, this type of immunity can be triggered by an infection or a vaccine. In either case, your body has the ability to “remember” the look of the attacker by saving antibodies specifically tailored for it, preparing your system for any future potential exposures.
FIGURE 6.15 | Antibodies can fight and destroy pathogens through several means, including neutralization and agglutination. Here are two examples of mechanisms by which antibodies take action: neutralization and agglutination.
1) Antibodies neutralize a pathogen by binding to a part of the pathogen necessary for its invasion of host tissue. 2) Antibodies neutralize a pathogen through agglutination. They make the pathogens clump together, preventing them from separating and invading the host cells.
The key players of this critical process are the memory B cells. They are the longlasting immune cells which retain the specific antibodies needed to fight off the invading pathogen, allowing them to quickly restart antibody production if the same antigen is ever detected again. It’s like facing an old foe for the first time after many years; since you already know how they operate, you can rapidly reconstruct the defenses that worked the first time to fight them off once again.
Memory B cells are produced when circulating B cells first encounter an antigen in the bloodstream. They are also the reason why vaccines work; when these B cells first encounter an antigen (e.g., through a vaccine), they store the DNA instructions for making
the perfect antibody that will recognize the pathogen’s antigens, preparing your body for future battles. We will learn about vaccines and how they induce this long-lasting immunity in more detail in Chapter 9: Vaccines and Immunizations.
Cell-mediated immunity refers to your body’s mechanisms of recognizing signs of infection within its own tissues. What sets this response apart is its role in monitoring your own cells to identify pathogen-compromised cells, as opposed to the humoral immune response, which monitors your blood for circulating pathogens to detect antigens. Since many pathogens attack by invading your cells, the ability to monitor your body’s cells and ensure their health is crucial. The main players doing this in the cell-mediated response are the T cells.
T cells patrol your cells, and must figure out which of them are infected in order to appropriately target them. How do they accomplish this? With the help of the adaptive immune system, specifically dendritic cells and macrophages. These cells consume pathogens,
chop the pathogens into pieces, and present specific pieces - antigens - on their cell surface. Cells capable of presenting antigens on their cell surfaces are called antigen-presenting cells (APCs). Once an APC presents an antigen, it can be detected by T-cell receptors (TCRs). TCRs are found on the surface of T cells. Once a TCR binds an antigen, it can relay a message into the T cell, telling the cell to launch a defense against the pathogen.
Just as B cells express antigen-specific BCRs on their cell surface, T cells express antigenspecific TCRs. Like BCRs, TCRs are able to bind antigens. When a T cell encounters an antigen on the surface of an APC that matches its TCR, it produces more T cells with the same TCRs capable of recognizing the same antigen through clonal selection and proliferation. Later, two types of T cells are produced: killer T cells and helper T cells. Killer T cells, also known as cytotoxic T cells, kill any cells already infected with a particular pathogen. Just like B cells, some killer T cells have the ability to “remember” antigens they have seen before through immunological memory, facilitating a faster immune response the second time around (Figure 6.16). Helper T cells “help” coordinate the army of cells fighting the infection and assist killer T cells (and B cells) in recognizing their targets by secreting cytokines.
Antigen

SPECIFIC BINDING
Naive T cells APC (Macrophage)
PROLIFERATION
T-KILLER LYMPHOCYTES
T-HELPER LYMPHOCYTES
T-killer cells bind to cells presenting the complimentary antigen and kill them

Killed
FIGURE 6.16 | Antigen presentation and T cell activation. The innate and the adaptive systems work together to amplify the immune response through the cell-mediated response. Dendritic cells phagocytose pathogens and present antigens on their cell surfaces (purple cells). T cells help other cells coordinate an adaptive immune response by recognizing antigens presented to them and secreting cytokines. Cytokines then activate B cells and killer T cells. T cells detect these antigens on APCs, bind them using their TCRs and then amplify the response by producing more T cells with the same TCRs (clonal cell selection and proliferation). This activates the production of the two specialized types of T cells: Killer T cells and helper T cells. Both cell types have the ability to recognize the antigen targets and create memory cells. The killer T cells in addition are cytotoxic and can bind the infected cells presenting the antigens and kill them.
1. Dendritic cells play a crucial role in helping your body develop a tailored adaptive immune response. Explain how they do this.
2. How does immunological memory work?
3. What two types of cells are best at providing long-term immunity against a pathogen?
a. Glial cells and natural killer cells
b. T cells and B cells
c. B cells and dendritic cells
As we’ve demonstrated throughout this chapter, a properly functioning immune system is necessary for your health. While this delicatelyorchestrated response works well in the majority of individuals, things can sometimes go wrong. Both deficient and overactive immune responses can be harmful; the former can leave you defenseless to pathogens, and the latter can cause your own immune cells to attack your organs. As we explore how the immune system can malfunction, we’ll discuss both immunodeficiency and overactive immune responses.
The term immunodeficiency covers a wide variety of disorders in which the immune response is either dampened in some way or eliminated completely. As you may recall from Chapter 5: Biology of Infectious Agents, people with immunodeficiencies are often referred to as immunocompromised. Immunodeficiency implies a weakened or deficient immune system, highlighting that people who are immunocompromised are more likely than healthy individuals to get infected by certain pathogens.
Immunocompromised individuals can suffer longer and more serious infections or become very ill from pathogens that typically do not cause disease. There are many causes of immunodeficiency, including exposure to pathogens, genetic disorders, chronic diseases, and environmental conditions. Immunodeficiency caused by genetic disorders is referred to as a primary immunodeficiency,
while any immunodeficiency caused by an external factor, such as an environmental factor, is called a secondary immunodeficiency One example of a primary immunodeficiency is Common Variable Immune Deficiency (CVID), a disease of the humoral immune system that results in low antibody levels. Although the cause of CVID remains unknown, genetic studies have suggested that CVID is polygenic, meaning there are multiple genes that contribute to the trait. The treatment for CVID is injections of antibodies to replace the ones that affected people do not make.
The best known example of a secondary immunodeficiency is AIDS, which is caused by HIV. HIV hijacks and kills T cells, weakening both the cellular and humoral immune responses. As a result, individuals with HIV are far more susceptible to many opportunistic infections like candidiasis, toxoplasmosis, and tuberculosis. Another example of a secondary immunodeficiency syndrome is neutropenia, which is characterized by decreased neutrophil levels – recall that neutrophils arrive first at the site of an infection to kill an invading pathogen using toxic granules, meaning that a lack of neutrophils entirely wipes out this early line of defense. Neutropenia can be caused by chemotherapy, a common therapy to treat cancer, as the chemotherapeutic agents are designed to stop the rapid proliferation of cancerous cells. A side effect of this mechanism is that other non-cancerous cells that rapidly divide are also affected, including hair follicles, cells in the gastrointestinal lining, and neutrophils. The main treatment for neutropenia is hospitalization during acute events, and a protective antibiotic regimen that the patient maintains until their neutrophil count returns to its original level.
Sometimes, your immune system mistakes your own healthy tissues as foreign and mounts an attack in response. This is called autoimmunity – when your body attacks itself, stemming from the Greek prefix “auto,” which means “self.” Autoimmunity represents a critical failure of the immune system: its primary job is to distinguish between self and non-self and to mount a response accordingly.
Autoimmunity can occur through broad overactivity of your innate and adaptive immune systems or by your adaptive immune cells wrongly targeting host cells that haven’t been compromised. On the broad end of the spectrum, in a process called cytokine storm, a very high level of cytokine activity can be triggered by the presence of pathogens or other sources, which can cause so much inflammation that it severely – and possibly fatally – harms the patient.
On the more specific side, the construction of a virtually infinite range of antibodies and antibody receptors (BCRs and TCRs) means that some of these receptors are inevitably built to recognize your body’s own proteins, lipids, and other molecules (called self-antigens). Luckily, your immune system knows how to detect and get rid of these autoreactive receptors. On the rare occasions when its detection mechanisms fail, some autoreactive receptors remain. When an autoreactive immune cell recognizes its corresponding self-antigen, an immune response is launched against that self-antigen. Other immune cell types are recruited to the scene and your immune system is prompted to attack your body’s normal tissues. With this, all of the actions you’d expect from a normal immune response occur: inflammation, production of antibodies against yourself
(called autoantibodies), destruction of “infected” cells, immunological memory of self-antigens, and more.
Autoimmunity can make the effects of a pathogenic infection worse. For example, in some COVID-19 patients, a hyperactive immune response is launched in response to the viral infection, and the resulting increased inflammatory response can cause lifethreatening symptoms.
As you may remember from our discussion of the 1918 influenza pandemic at the beginning of the chapter, millions of young adults like Egon and Edith Schiele perished in great numbers. This is because the virus ignited an autoimmune response within these young adults: their immune systems, called into action when the virus attacked, responded too ferociously, attacking their own lungs. This explains the unusual pattern in mortality observed across age groups during the 1918 pandemic. Typical influenza death tolls tend to follow a U-shaped graph, with the very young and the very old having the highest death rates and the young and middle aged adults having the lowest death rates. This makes sense, as infants lack the exposure to pathogens necessary to develop defenses against them, and the general health and immune systems of elderly individuals have deteriorated due to old age. Young adults are in a sweet spot: by now, their immune cells have seen many bad actors, and they are both welltrained to protect the body and strong enough to fend off whatever comes their way. The 1918 flu, however, followed a W-shaped graph, killing individuals in their mid-20s and 40s at alarming rates as a result of overactive immune responses in these individuals (Figure 6.17).
The concept of autoimmunity extends past infectious disease alone. Autoimmune disorders affect ~5% of the population, and they can result

FIGURE 6.17 | The 1918 influenza pandemic is an example in which the virus ignited an autoimmune response in a specific group of the population. This plot combines influenza- and pneumonia-specific death rates by age for the population in the US and compares these between the years 1911-1917 (dotted, U-shaped curve) and the year of the pandemic, 1918 (continuous line, W-shaped graph). The W-shaped curve shows an unusual pattern in mortality due to the virus igniting an autoimmune response within young adults causing their death.
in a variety of different symptoms depending on which immune cells are autoreactive in which part of the body. For example, in Type 1 diabetes, as your T cells try to fight off a foreign pathogen, they mistakenly destroy the cells in the pancreas responsible for producing insulin. Additionally, some autoimmune disorders, like lupus,are systemic, which means that they cause inflammation in many different organ systems.
The question of how autoimmunity arises is still under investigation, but a variety of factors are likely at play. Some individuals may be genetically more likely to develop autoimmune disorders, and environmental triggers including pathogen infections are also thought to play a role.
Another important form of overactive immune response is allergies. Allergies are the result of your body incorrectly identifying
harmless substances like cat and dog fur, flower pollen, and peanuts as dangerous invaders and signaling an immune response. These so-called allergens bind to and induce the production of a specific class of antibodies called IgE antibodies. These can cause granulocytes to release cytokines and histamine, inducing an inflammatory reaction that is considered hyperactive because it is out of proportion to the actual threat caused by the particles (Figure 6.18). Interestingly, this IgE response is believed to have been initially involved in protecting the body against helminths.
This inflammatory reaction can affect either a local area, resulting in swelling, or the whole body, which can lead to a life-threatening allergic reaction known as anaphylaxis Antihistamines are typically taken to treat allergic reactions because they block the action of histamine, halting the immune response.
Allergic Reactions

FIGURE 6.18 | Immunologic basis of allergies. Allergic reactions are the consequence of an overreactive immune response. 1) B cell receptors make the initial contact with the allergen. 2) The IgE is released. 3) The IgE released from the subsequent plasma cell then attach to the surface of a granulocyte. 4) When the allergen is re-encountered, the sensitized granulocyte prepares to release histamines and other inflammatory products. 5) Histamines, which are the chemicals responsible for allergy symptoms such as sneezing, are released.
1. What are some of the challenges that people with immunodeficiencies might face when exposed to pathogens?
2. Describe the two different types of immunodeficiency.
3. What is autoimmunity?
4. What happens in your body during an allergic reaction?
1. Protect the body from pathogens.
2. The innate immune system and the adaptive immune system. The innate immune system provides you with nonspecific protection against invading pathogens, including barriers to enter the body. The adaptive immune system, on the other hand, is the body’s second line of defense against threats, providing specialized responses that are unique to specific pathogens.
3. Answers may vary:
a. WBCs and cytokines
b. Dendritic cells, macrophages, neutrophils, eosinophils, basophils, NK cells, B cells, T cells and cytokines.
1. The three types of barriers the innate system uses to protect your body are: physical barriers (e.g. mucous membranes, epidermis), chemical barriers (e.g. lysozymes), and biological barriers (e.g. microbiome).
2. Once NK cells have identified their target by inspecting proteins on the surface of your body’s cells for invaders, they poke holes in the target’s membrane and inject the target cell with toxic molecules, leading to programmed cell death.
3. Cytokines are a broad category of small proteins, signaling molecules, that engage in cell-to-cell communication. These are released primarily by macrophages and T helper cells as distress signals to recruit additional resources to the site of infection. Cytokines jumpstart the rest of your immune response, setting off a chain reaction of
inflammatory responses, such as swelling, warmth, and increased blood vessel permeability, that make it easier for immune cells to jump in and out of tissues. There are many kinds including chemokines, interferons, interleukins, lymphokines, and tumor necrosis factors
1. Dendritic cells play a role in the adaptive immune system by showing T cells possible antigens to target. After eating the pathogen, they break it up and present the small pieces on their surface as antigens to T cells, allowing them to bind to TCRs. Subsequently, dendritic cells and other phagocytes capable of performing this job are referred to as APCs.
2. When you get invaded by a pathogen, your B cells will actively make specific antibodies against it, and memory B cells will keep a record of these antibodies in case you get infected again with the same pathogen. Memory B cells are critical to immunological memory because they “remember” which antibodies are needed to fight off specific pathogens, triggering antibody production once they come across a familiar pathogen.
3. b. T cells and B cells.
1. People with immunodeficiencies are particularly susceptible to opportunistic pathogens. Due to their weakened immune systems, they are also likely to remain sick longer than others and have complications in receiving vaccines.
2. A weakened or deficient immune system is called a primary immunodeficiency if it is caused by a genetic disorder. In contrast, a secondary immunodeficiency is caused by factors external to an individual, such as environmental factors, infection by a pathogen, or powerful medical treatment.
3. Autoimmunity can be the result of your body’s inability to distinguish itself from invading pathogens, leading it to mount an immune response against
itself. Other autoimmune responses can simply be the result of an overly strong immune response, in which the amounts of inflammation are so high that they damage your own organs.
4. Allergic reactions occur when your body mistakenly identifies a harmless substance as a threat, inducing an inflammatory reaction. This happens as your body releases specialized immune cells that then release cytokines and histamine.
Active Immunity: Process whereby antibodies develop in a person’s own immune system after the body is exposed to an antigen. This type of immunity can last for a long time.
Adaptive Immune System: Specific immune system response targeting specific antigens to destroy pathogens; composed of a humoral response and a cell-mediated response.
Allergies: Condition in which the body’s immune system recognizes a harmless substance, known as an allergen, as a threat.
Antibodies: Proteins that bind to specific antigens; the central component of the humoral immune response; also known as immunoglobulins.
Antigens: Unique proteins native to the invading pathogen or microbe; every pathogen species has a different set. Your body’s adaptive immune system records these antigens in its memory, thereby remembering the specific pathogen in case of reinfection.
Antigen Presentation: Immune system process that triggers the activation of T cells as phagocytes present antigens on their cell surface.
Antigen-Presenting Cells (APCs): Cells of the innate immune system that the antigen pieces to an adaptive immune cell, bridging the gap between the innate and adaptive immune systems; refers to dendritic cells and other phagocytes such as B cells and macrophages capable of antigen presentation.
Apoptosis: Type of programmed cell death.
Autoimmunity: Condition in which the immune system mistakenly attacks the body’s own tissues or organs; can occur while fighting off a pathogen.
Basophil: Type of white blood cell (WBC) that plays an important role in inflammatory reactions; releases histamine. It also contains specific enzymes that prevent blood clotting.
B Cell: Type of WBC that derives from and matures in bone marrow, contains a B cell receptor (BCR) critical to function, and creates antibodies; central players in the humoral immune response.
B Cell Receptor (BCR) : Immunoglobulin embedded in the cell membrane of a B cell that binds to antigens in the same way as the antibodies produced by that B cell. As B cells mature and develop, they express a single type of BCR with unique binding capabilities integral to developing immunological memory in your adaptive immune system. Your immune system forms a diverse population of B cells that constantly circulate throughout the body. BCRs are critical to the functionality of B cells.
Cell-Mediated Immune Response : Your body’s mechanisms of recognizing signs of infection within its own cells; monitoring your own cells to ensure they haven’t been infected by invading pathogens.
Cilia : Thin, hair-like projections found in ‘ciliated’ cells on mucous membranes; responsible for pushing pathogens out of the body and away from the membrane; also found on protozoa, giving them mobility.
Clonal Selection: Scientific theory of the immune system which suggests how some B and T cells are selected to help destroy specific antigens; these selected immune cells multiply (proliferate) to produce identical clones and make antibodies.
Cytokines: Broad category of small proteins that engage in inflammation and cell signaling to aid and/or enhance the immune response.
Dendritic Cell: A type of phagocyte that, when immature, patrols the blood and tissue and gobbles up foreign invaders. Immature dendritic cells uniquely become activated after “eating” a threat – in this process, they take pieces of the threat or pathogen to your lymph nodes and present them to the cells of your adaptive immune system. Dendritic cells connect your innate immune system to your adaptive immune system and are named for their physical appearance resembling dendrites, the spiderlike projections on neurons.
Eosinophil: A type of WBC called a granulocyte which contains granules with an acidic nature; responsible for combating multicellular parasites and certain infections in vertebrates.
Granulocyte: A cell of the innate immune system that has toxic granules used to destroy pathogens. There are three main types of granulocytes: neutrophils, eosinophils, and basophils.
Helper T Cells: One of two main types of T cells that activate other immune cells by secreting cytokines and expressing regulatory proteins; orchestrate the adaptive immune response.
Histamine: Small molecules that often cause symptoms like the red, itchy splotches that accompany allergic reactions and asthma; produced by basophils and eosinophils of the innate immune system.
Humoral Immune Response: Deals specifically with the immune system’s role in monitoring your blood, the main fluid of your body.
Immune System: A complex network of cells and proteins that defends the body against infection.
Immunity: The body’s ability to defend against a pathogen after it has been exposed.
Immunological Memory: The process by which the immune system keeps a record of every pathogen it has encountered so that it can quickly recognize and mount an immune response if the pathogen enters the body again.
Inflammation: A localized reaction including heat, redness, swelling, pain, and sometimes loss of function, indicative of an infection.
Innate Immune Response: Nonspecific response of the immune system including anatomical and physiological barriers and a cellular response to fight pathogens.
Innate Immune System : Refers to the mechanisms of your body that provide nonspecific protection against pathogens.
Killer T Cell: Specialized cell that kills any cells infected with a particular pathogen, stimulated by antigen; also known as a cytotoxic T cell.
Lysozyme: Enzyme that can kill invading bacteria by breaking down bacterial cell walls.
Macrophage: A type of phagocyte that typically resides in tissues and monitors the premises for any toxins or foreign agents, which they can then phagocytose accordingly; also plays a role in the adaptive immune system as an APC.
Memory B Cell: Long-lasting B cell that has the potential to induce specific antibody production if the antigen is ever detected in the future; results from clonal selection.
Mucus: Aqueous substance with defensive properties to trap microbes so that they cannot enter and spread to the rest of the body.
Natural Killer (NK) Cell: Cell of the innate immune system whose function, unlike a cytotoxic T cell, does not need to be stimulated by antigen; identifies and destroys compromised cells of the body.
Neutralize: To render something ineffective or harmless — in the context of outbreak science, a pathogen.
Neutralizing Antibodies: Antibodies that are able to neutralize pathogens by binding to one of their components necessary for attachment to or invasion of the host tissue, such as a cell surface protein.
Neutrophil : Main type of granulocyte that makes up 60% of the WBCs in circulation; the most plentiful type of immune cell in your body. They are often the first to arrive at the scene of an infection when macrophages send out distress signals, recruiting more neutrophils and other immune cells. They are plentiful, speedy, and short-lived, frequently dying in the process of completing their immunological functions; their buildup leads to things like the yellow-white pus on cuts, or snot when you’re sick.
Phagocyte: The main class of WBC at the epicenter of your innate immune system, whose function is to engulf and digest foreign invaders.
Phagocytosis: Process of ingesting and eliminating pathogens and apoptotic cells, performed by cells of the immune system called phagocytes.
Plasma Cell: B cell, central to the function of the adaptive immune system, that makes antibodies and releases them into the bloodstream.
Sinuses: A connected system of hollow spaces around your nose and other places in your head. During infection, inflammation around the sinuses can lead to excess mucus, nasal congestion, and discomfort.
T Cell: A type of WBC that derives from bone marrow and matures in the thymus; contains a T cell receptor (TCR) critical to their function and creates; central players in the cell-mediated immune response.
T Cell Receptor (TCR): A group of proteins embedded in the cell membrane of T cells that recognizes a specific antigen and activates T cells to mature, similar to BCRs in B cells. This specificity is what makes cell-mediated immunity a component of the greater adaptive immune system. TCRs are critical to the functionality of T cells.
White Blood Cells (WBCs): A broad category of immune cells that circulate in the blood in order to help fight pathogens; also known as leukocytes.