The FIRST & ONLY Intravenous Immune Globulin (IVIg) Approved by the FDA for the Treatment of Dermatomyositis in
A TREATMENT GUIDE FOR PATIENTS

Octagam 10% is an immune globulin intravenous (human) liquid preparation indicated for the treatment of dermatomyositis (DM) in adults. Octagam 10% is also indicated for the treatment of chronic immune thrombocytopenic purpura (ITP) to rapidly raise platelet counts to control or prevent bleeding in adults.1
WARNING: THROMBOSIS, RENAL DYSFUNCTION AND ACUTE RENAL FAILURE
Please see accompanying full Prescribing Information for additional important information.
Thrombosis may occur with immune globulin intravenous (IGIV) products, including Octagam 10%. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors.
Renal dysfunction, acute renal failure, osmotic nephrosis, and death may occur in predisposed patients who receive IGIV products, including Octagam 10%. Patients predisposed to renal dysfunction include those with a degree of pre-existing renal insufficiency, diabetes mellitus, age greater than 65, volume depletion, sepsis, paraproteinemia, or patients receiving known nephrotoxic drugs. Renal dysfunction and acute renal failure occur more commonly in patients receiving IGIV products containing sucrose. Octagam 10% does not contain sucrose.
For patients at risk of thrombosis, renal dysfunction or acute renal failure, administer Octagam 10% at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity.
Please see accompanying full Prescribing Information.
Dermatomyositis may cause skin and joint problems, as well as permanent damage to muscles. It is important to speak with your healthcare professional about treatment.
What is dermatomyositis (DM)?
Dermatomyositis (dur-muh-toe-my-uh-SY-tis) or DM is a rare autoimmune disorder that causes rash, joint pain, and muscle weakness. You may hear dermatomyositis called by a more general name that refers to a group of disorders that cause muscle weakness: idiopathic inflammatory myopathy.
Symptoms can vary from person to person and can appear in different areas of the body. They may appear slowly, or all at once. Sometimes your symptoms can disappear for a while, then return.
Dermatomyositis most often occurs in adults in their late 40s to 60s. DM also occurs in young children.
Symptoms of DM

Indications and Usage
Octagam® 10% is an immune globulin intravenous (human) liquid preparation indicated for the treatment of dermatomyositis in adults. Octagam 10% is also indicated for the treatment of chronic immune thrombocytopenic purpura (ITP) to rapidly raise platelet counts to control or prevent bleeding in adults.
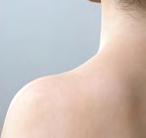

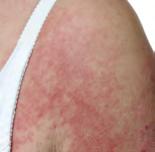
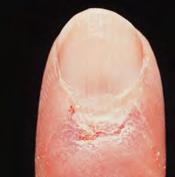
Red or violet bumps
Eyelids, chest, upper back
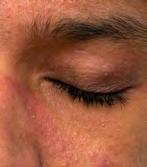
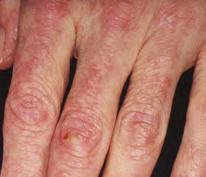
Discoloration
Fingers, cuticles, thighs
Important Safety Information
Contraindications
Fingers, elbows, knees, ankles
Octagam® 10% is contraindicated in patients who have a history of severe systemic hypersensitivity reactions, such as anaphylaxis, to human immunoglobulin. Octagam 10% contains trace amounts of IgA (average 106 µg/mL in a 10% solution). It is contraindicated in IgA-deficient patients with antibodies against IgA and history of hypersensitivity.
What causes dermatomyositis?
There are certain cells in the body called immune cells that fight off infection and disease. When a person has dermatomyositis, some of the body’s immune cells may release autoantibodies, which can attack the healthy blood vessels that supply blood to your muscles. This action may produce inflammation and cause dermatomyositis.
Autoantibodies
Immune Cells
Damaged Blood Vessel
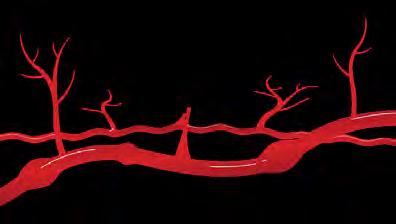
Muscle Inflammation
Important Safety Information
Adverse Reactions
Enlarged Blood Vessel
The most common adverse reactions observed in >5% of clinical study subjects with DM were headache, fever, nausea, vomiting, increased blood pressure, chills, musculoskeletal pain, increased heart rate, dyspnea, and infusion site reactions.
Please see accompanying full Prescribing Information.

Managing inflammation
There is no cure for dermatomyositis, but your healthcare professional can prescribe treatments to manage your symptoms. These may include:
• IVIg: Provides extra antibodies to the blood to help block the autoantibodies and decrease inflammation
• Steroids: Help reduce inflammation and relieve symptoms
• Steroid-sparing immunosuppressants: Used when steroids do not work or cause unwanted side effects
Because of the range of symptoms you might be experiencing, your care team may include specialists like neurologists, rheumatologists, dermatologists, pharmacists, and nurses, among others.

Octagam 10% is the first and only FDA-approved IVIg treatment for dermatomyositis in adults1,2
In a clinical study, 95 adults with dermatomyositis were given octagam 10% or placebo.
Patients in both treatment groups could continue taking their other medications while they were part of the study.
This study looked at patient improvements in:
• Muscle strength
• Amount of visible rash
• Activity level
• Quality of life
• Protein levels in muscle
How is octagam 10% made?1,2
Octagam 10% is made from healthy human plasma, which comes from blood. IVIg therapies like octagam 10% are made under strict Food and Drug Administration (FDA) guidelines to ensure they’re safe to use. These include:
• Getting human plasma from FDA-approved donation centers
• Checking plasma donors for prior exposure to certain viruses and other diseases
• Testing the collected plasma to help ensure that it is virus- and disease-free
• Virus inactivation/removal steps in the manufacturing process
Important Safety Information
Octagam® 10% contains maltose, a disaccharide which is derived from corn. Patients known to have corn allergies should avoid using Octagam 10%.
Hemolysis that is either intravascular or due to enhanced red blood cell sequestration can develop subsequent to Octagam 10% treatments. Risk factors for hemolysis include high doses and non-O-blood group. Closely monitor patients for hemolysis and hemolytic anemia.
Aseptic meningitis syndrome may occur in patients receiving Octagam 10%, especially with high doses or rapid infusion.

Indications and Usage
Octagam® 10% is an immune globulin intravenous (human) liquid preparation indicated for the treatment of dermatomyositis in adults. Octagam 10% is also indicated for the treatment of chronic immune thrombocytopenic purpura (ITP) to rapidly raise platelet counts to control or prevent bleeding in adults.

Octagam 10% was shown to help improve symptoms of dermatomyositis1-3
How was improvement measured?
There were 3 different categories that defined how much symptoms improved: minimal, moderate, and major
All three improvement categories were measured after 16 weeks.
PATIENTS TAKING octagam 10% PATIENTS IN THE Placebo Group
How will I receive octagam 10%?
Octagam 10% is given intravenously (through a needle inserted in a vein)1
Patients in the clinical study received their infusion over 2 to 5 days. Your infusion may be given either at an infusion clinic or in your own home and will usually be given every four weeks.
Getting ready for your infusion
Make sure to drink a lot of water the day before and the day of your IVIg therapy, and avoid caffeine and alcohol
Have an activity available to help pass the time (eg, reading a book)
During and after your infusion
Your IVIg therapy will be given as an infusion through a needle inserted into your vein
Your blood pressure and temperature will be checked during treatment
TIS=Total Improvement Score.
Important Safety Information
Contraindications
Octagam® 10% is contraindicated in patients who have a history of severe systemic hypersensitivity reactions, such as anaphylaxis, to human immunoglobulin. Octagam 10% contains trace amounts of IgA (average 106 µg/mL in a 10% solution). It is contraindicated in IgA-deficient patients with antibodies against IgA and history of hypersensitivity.
Your infusion time will vary and could take several hours
You can continue with the regular activities of your day, as tolerated
Call your doctor, nurse, or pharmacist with any questions, or if you have side effects

Most common drug-related side effects
In a clinical study, more than 5% of patients had the following side effects1:
• Headache
• Fever
• Nausea
• Vomiting
• Chills
• Muscle pain
• Increased blood pressure
Important Safety Information
The following serious adverse reactions were observed in the DM study: muscle spasms and dyspnea in one subject, loss of consciousness in one subject, and thromboembolic events (TEEs) in five subjects, including deep vein thrombosis and pulmonary embolism in one subject, cerebrovascular accident in one subject, cerebral infarction in one subject, hypoesthesia in one subject and pulmonary embolism in one subject.
Co-pay assistance and reimbursement

The Octapharma Support Center offers comprehensive advice and support on:
Octagam® 10% contains maltose, a disaccharide which is derived from corn.
Patients known to have corn allergies should avoid using Octagam 10%.
Hemolysis that is either intravascular or due to enhanced red blood cell sequestration can develop subsequent to Octagam 10% treatments. Risk factors for hemolysis include high doses and non-O-blood group. Closely monitor patients for hemolysis and hemolytic anemia.
Benefits investigation & prior authorization support
Co-pay assistance support
Ongoing support for access & reimbursement
The Octapharma Co-Pay Assistance Program offers significant cost savings
RECEIVE UP TO PER CALENDAR YEAR $ 2,500 on the out-of-pocket costs associated with octagam 10% for adult DM
Eligible patients may receive up to $2,500 in co-pay assistance for octagam 10% usage in the current calendar year. Patients must currently be using octagam 10%, or have a prescription to begin therapy, for the FDA-approved indication of adult DM.
To contact the Octapharma Support Center, call 800-554-4440
The FIRST & ONLY Intravenous Immune Globulin (IVIg)
Approved by the FDA for the Treatment of Dermatomyositis in Adults

Why octagam 10% for the treatment of DM in adults?
Octagam 10% has been shown to improve dermatomyositis symptoms in a clinical study1-3
• Almost twice as many patients taking octagam 10% saw improvement in their symptoms (79%) when compared with patients taking placebo (44%)

Important Safety Information
Aseptic meningitis syndrome may occur in patients receiving Octagam 10%, especially with high doses or rapid infusion.
Octapharma USA, Inc.
117 W. Century Road Paramus, NJ 07652
Tel: 201-604-1130
IgCares/Reimbursement Assistance
usreimbursement@octapharma.com
Tel: 800-554-4440
Medical Affairs
usmedicalaffairs@octapharma.com
For all inquiries relating to drug safety, or to report adverse events, please contact our local Drug Safety Officer:
Tel: 201-604-1137 | Cell: 201-772-4546 | Fax: 201-604-1141 or contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
References: 1. Octagam 10% Full Prescribing Information. Paramus, NJ: Octapharma USA Inc; rev June 2021. 2. Octapharma. Data on file. 3. Aggarwal R, et al. (ProDERM study). Submitted for publication.
Please see accompanying full Prescribing Information.
©2022. Octapharma USA, Inc. All rights reserved. Date of preparation: 2/2022. GAM10-0318-COT
