Expressed in a human cell line. B-domain deleted. No chemical modifications.
The Only Recombinant FVIII Produced From a Human Cell Line Without Chemical Modification or Protein Fusion
Powerful Bleeding Control
Prophylaxis, On-Demand, and
Surgery
Prophylaxis in adults and children1*
In adults & children
Median ABR for all bleeds was 0.9 in adults and 1.9 in children
2.5 mL diluent volume across ALL vials
Potential for 2X weekly infusions with personalized prophylaxis
Easy monitoring by chromogenic or one-stage assay
IN
ADULTS (N=32)
(N=59)
SPONTANEOUS BLEEDS Adults & Children
On-demand treatment in adults (N=986 bleeds)3,4
§ Most bleeds resolved with 1 infusion (90%) or 2 infusions (97%)
§ Flexibility for patients to redose at 12 to 24 hours
Perioperative control and prevention of bleeding (N=33 surgeries)1
§ 100% of minor surgeries, hemostasis rated as “excellent”
§ 92% of major surgeries, hemostasis rated as “excellent” or “good”
Provides bleeding protection with the potential for 2x weekly dosing
Indications and Use
NUWIQ® is a recombinant antihemophilic factor [coagulation factor VIII (Factor VIII)] indicated in adults and children with Hemophilia A for on-demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes. NUWIQ is not indicated for the treatment of von Willebrand Disease.
Please see accompanying full Prescribing Information.
Contraindications
NUWIQ is contraindicated in patients who have manifested life-threatening hypersensitivity reactions, including anaphylaxis, to the product or its components. Please see accompanying full Prescribing Information.
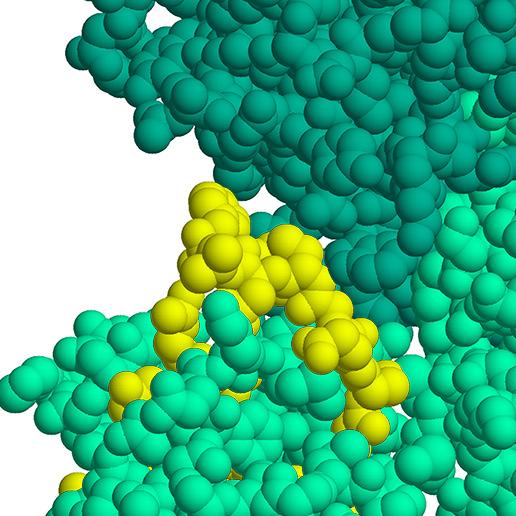
Not All Factor VIII Replacements Are the Same
FVIII Sulfation and Natural VWF Binding
§ Full tyrosine sulfation at Y1680 is essential for the interaction of FVIII with VWF and for full affinity for VWF11
§ Full sulfation increases binding affinity 5-fold naturally11
There are design differences in: 5,6
NUWIQ closely resembles natural FVIII 1,3,4,7-10
§ Produced in a human cell line, not hamster cell lines
§ B-domain deleted, no chemical modification or protein fusion
§ No animal proteins are added during production
NUWIQ has high binding affinity to endogenous VWF (99% bound) 6,7,9,10
§ VWF-binding prevents FVIII from being rapidly cleared from circulation
§ High binding affinity may contribute to prolonging FVIII half-life
NUWIQ is fully sulfated at Tyr1680—similar to plasma-derived FVIII10
Important Safety Information
Warnings and Precautions
Hypersensitivity reactions, including anaphylaxis, are possible with NUWIQ. Early signs of hypersensitivity reactions that can progress to anaphylaxis may include angioedema, chest tightness, dyspnea, wheezing, urticaria, or pruritus. Immediately discontinue administration and initiate appropriate treatment if hypersensitivity reactions occur. The formation of neutralizing antibodies (inhibitors) to Factor VIII can occur following the administration of NUWIQ.
Please see accompanying full Prescribing Information.
Other rFVIII concentrates show nonsulfated (minor or absent peak) Y1680-containing peptides10
Important Safety Information
Warnings and Precautions (continued )
Monitor all patients for the development of Factor VIII inhibitors by appropriate clinical observations and laboratory tests. If the plasma Factor VIII level fails to increase as expected, or if bleeding is not controlled after NUWIQ administration, suspect the presence of an inhibitor (neutralizing antibody).
Please see accompanying full Prescribing Information.
Type of FVIII Molecule Can Affect Inhibitor Potential
In the NuProtect study, cumulative incidence of high-titer inhibitors for NUWIQ was 17.6%; similar to pdFVIII in the separate SIPPET study1,2
Type of F8 Gene Mutation Can Impact Inhibitor Risk
Null F8 mutations are associated with a higher risk of inhibitors vs non-null F8 mutations 13
Cumulative Incidence of High-Titer Inhibitors By F8 Mutation in the SIPPET and NuProtect Studies2,12,14-16
Data from 105 PUPs treated with NUWIQ were analyzed for inhibitor development, with 95 patients reaching ≥100 EDs or inhibitor development.
* Information from the NuProtect study is presented in parallel to the SIPPET study for context, but please note that these trials were performed under different conditions and with different populations. The observed incidence of inhibitor formation may be influenced by a number of factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease.
In the SIPPET study, rFVIII products from hamster cell lines (CHO or BHK) had a higher incidence † of inhibitor development than pdFVIII products12
SIPPET | Cumulative Incidence of High-Titer Inhibitors (N=251)12
†Differences in high-titer inhibitor rates between pdFVIII and rFVIII were not found to be statistically significant. SIPPET authors suggested this may have been due to the small sample size of the study.
The SIPPET Study compared the incidence of inhibitors in PUPs with severe Hemophilia A treated with either pdFVIII or rFVIII derived from hamster cell lines (Chinese hamster ovary [CHO] cells or baby hamster kidney [BHK] cells). Patients were followed for 50 consecutive EDs or 3 years or until inhibitor development was confirmed.
ED, exposure days; pdFVIII; plasma-derived factor VIII; SIPPET, Survey of Inhibitors in Plasma-Product Exposed Toddlers.
There were ZERO high-titer inhibitors in PUPs with non-null F8 mutations treated with NUWIQ
SIPPET
In PUPs with non-null F8 mutations treated with pdFVIII, there were ZERO high-titer inhibitors
In PTPs | ZERO Inhibitors With NUWIQ 1,14
In clinical trials of PTPs (N=135) who switched to NUWIQ, there were ZERO FVIII inhibitors after ≥50 EDs and ≥6 months of treatment1,14‡
Important Safety Information
Adverse Reactions
The most frequently occurring adverse reactions (>5%) in clinical trials were upper respiratory tract infection, headache, fever, cough, lower respiratory tract infection, rhinitis, chills, abdominal pain, arthralgia, anemia, and pharyngitis.
Please see accompanying full Prescribing Information. 7
Convenient Usage, Comprehensive Support
A broad range of dosage strength vials
§ Available in 250 IU, 500 IU, 1000 IU, 1500 IU, 2000 IU, 2500 IU, 3000 IU, and 4000 IU vials
§ Low 2.5 mL diluent volume across the entire range of vial strengths
Factor My Way Assistance
Includes free trial of NUWIQ (up to 6 doses) and co-pay assistance for eligible patients (up to $12,000 per year) on out-of-pocket treatment costs.
Factor My Way Events
Join scheduled live and on-demand digital information programs and events.
Factor My Way Connection
Personal support with Patient Experience Managers, educators, and nurses.
Factor My Way Learning
Comprehensive bleeding disorders library (articles, videos, and downloads).
Octapharma USA, Inc.
117 W. Century Road
Paramus, NJ 07652
Free Trial, Co-Pay Assistance, and Reimbursement
Tel: 201-604-1130 Medical
usmedicalaffairs@octapharma.com
For all inquiries relating to drug safety, or to report adverse events, please contact our local Drug Safety Officer: Tel: 201-604-1137 | Cell: 201-772-4546 | Fax: 201-604-1141 or contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
References: 1. NUWIQ full Prescribing Information. Paramus, NJ: Octapharma USA, Inc.; rev 2021. 2. Liesner RJ, et al. Thromb Haemost. 2021;121:1400–1408. 3. Valentino LA, et al. Haemophilia. 2014;20(Suppl. 1):1-9. 4. Kessler C, et al. Haemophilia. 2015;21(Suppl. 1):1-12. 5. Lissitchkov T, et al. Haemophilia. 2017;23:697-704. 6. Cafuir LA, et al. Ther Adv Hematol. 2017;8(10):303-313. 7. Casademunt E, et al. Eur J Haematol. 2012;89:165-176. 8. Winge S, et al. Protein Expr Purif. 2015;115:165-175. 9. Sandberg H, et al. Thromb Res. 2012;130:808-817. 10. Kannicht C, et al. Thromb Res. 2013;131:78-88. 11. Leyte A. et al. J Biol Chem. 1991;266:740-746. 12. Peyvandi F, et al. N Engl J Med. 2016;374:2054-2064. 13. Oldenburg J, Pavlova A. Haemophilia. 2006;12:15–22. 14. Data on file. Paramus, NJ: Octapharma USA, Inc. 15. Rosendaal F, et al. Blood. 2017;130:1757–1759. 16. Astermark J, et al. Haemophilia. 2010;16:747-766.
©2023. Octapharma USA, Inc. All rights reserved. Date of preparation: 5/2023. NUW-0713-PSA
usreimbursement@octapharma.com Tel: 800-554-4440 www.NUWIQUSA.com