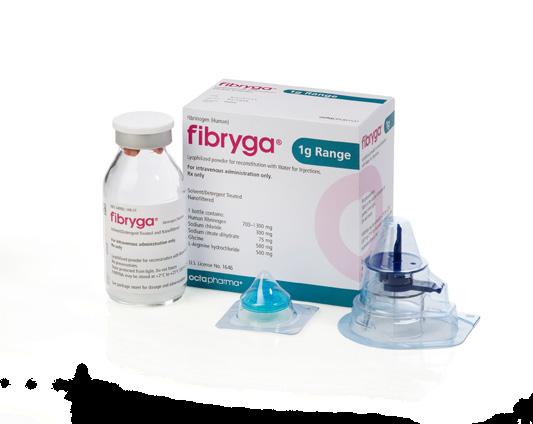
Fibryga is a highly purified, virus-inactivated, human fibrinogen concentrate indicated for the treatment of bleeding in adults and children with CFD, including afibrinogenemia and hypofibrinogenemia. Fibryga is not approved for dysfibrinogenemia.
Important Safety Information
Contraindications
Fibryga® is contraindicated in individuals who have manifested severe immediate hypersensitivity reactions, including anaphylaxis, to Fibryga or its components.
The most serious adverse reactions that may be observed with Fibryga are thromboembolic episodes and anaphylactic type reactions. The most common adverse reactions observed in more than one subject in clinical studies with Fibryga (> 5% of subjects) were nausea, vomiting, pyrexia (fever) and thrombocytosis.
? WHY Gaining Control in CFD
CONGENITAL FIBRINOGEN DEFICIENCY (CFD)
PURITY & SAFETY
Fibryga is a purified, virus-inactivated human plasma-derived fibrinogen concentrate3
BLEEDING CONTROL
Fibryga achieved 99% success, often with a single infusion4
EASE OF USE
Room temperature storage, no thawing; Octajet transfer device provides for rapid needleless reconstitution within 5 to 10 minutes, low infusion volume3
CO-PAY ASSISTANCE
Up to $10,000 per year on out-of-pocket costs for eligible patients
Contact Octapharma
Octapharma USA, Inc. Corporate Office 117 W. Century Road, Paramus, NJ 07652 Tel: 201-604-1130 www.octapharma.com
Customer Service General questions about fibrinogen, reconstitution, and product support. Tel: 866-766-4860 uscustomerservice@octapharma.com
Octapharma Medical Affairs usmedicalaffairs@octapharma.com
To contact your local Octapharma Representative Tel: 201-604-1130
For all inquiries relating to drug safety, or to report adverse events, please contact our Local Drug Safety Officer: Tel: 201-604-1137 Cell: 201-772-4546 Fax: 201-604-1141 or contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Indications and Usage
Fibryga® is a human fibrinogen concentrate indicated for the treatment of acute bleeding episodes in adults and children with congenital fibrinogen deficiency, including afibrinogenemia and hypofibrinogenemia. Fibryga is not indicated for dysfibrinogenemia.
References: 1. Amesse C, Lacroix S, Lupien G. et al. Canadian Hemophilia Society. Factor Deficiency, Fibrinogen: An inherited bleeding disorder. 2004. 2. Congenital Afibrinogenemia. National Organization for Rare Diseases (NORD). https://rarediseases.org/rare-diseases/afibrinogenemia-congenital/ Accessed September 29, 2020. 3. Fibryga full Prescribing Information. Paramus, NJ: Octapharma USA, Inc.; rev 2020. 4. Lissitchkov T, et al. J Thromb Haemost. 2020;18:815–824.
Treating CFD
GAIN CONTROL WHEN YOU NEED IT MOST OFFERS SIGNIFICANT COST SAVINGS
EQUALLY AFFECTS
• Body cannot form a stable blood clot
• CFD can result in mild to severe bleeding
Fibrinogen Concentrate Is the Preferred Choice for Treating CFD, Offering a Range of Benefits
men, women, and people of all races and ethnicities1
CFD OCCURS IN 1 out of 1,000,000 people WORLDWIDE 2
Bleeding Can Occur Anywhere in the Body2
• Bruising (skin)
• Umbilical cord (after birth)
• Nosebleeds
• Mucous membrane
Occurs spontaneously or after injury, trauma, or surgery1,2
• Gastrointestinal (digestive tract)
• Bladder (urinary) bleeding
• Menstrual bleeding
• Joints (knees, ankles)
Impact is greater for women due to menstruation, pregnancy, childbirth1
Multidisciplinary
Eligible patients with commercial insurance can receive up to a maximum amount of $2500 in copay assistance per enrollment.
VIRAL SAFETY
Less likely to be contaminated with viruses than older treatments 3,4 BLEEDING CONTROL
CONVENIENCE
Proven effectiveness for the treatment of acute bleeding episodes3,4
High Standards to Ensure Safety3
Enrollment can be renewed at Octapharma’s discretion up to 3 times within a calendar year—for a maximum of $10,000.
You must currently be using fibryga, or have a prescription to begin therapy, for the FDA-approved indication for fibryga.
• Co-pay assistance can be applied to co-payments, deductibles and co-insurance that may be associated with the cost of Octapharma products. The Octapharma Co-Pay Assistance Program does not cover costs associated with administration of therapy, such as office visits, infusion costs, or other professional services
Storage flexibility, no thawing, fast preparation, easy to administer without delay3 Important Safety Information
• Plasma is collected by FDA-licensed facilities; all donors undergo extensive screening and testing
• During manufacturing, fibryga undergoes a multistage process that includes solvent/detergent treatment and nanofiltration to inactivate/remove viruses Complete absence of fibrinogen in the blood.
Contraindications
Fibryga® is contraindicated in individuals who have manifested severe immediate hypersensitivity reactions, including anaphylaxis, to Fibryga or its components.
The most serious adverse reactions that may be observed with Fibryga are thromboembolic episodes and anaphylactic type reactions. The most common adverse reactions observed in more than one subject in clinical studies with Fibryga (> 5% of subjects) were nausea, vomiting, pyrexia (fever) and thrombocytosis.
• Those with Medicare, Medicaid, Medigap, VA, DOD, Tricare or other federal or state government insurance are not eligible
Reimbursement Assistance
Important Safety Information
Warnings and Precautions
Monitor patients for early signs of hypersensitivity or allergic reactions. If necessary, discontinue administration and institute appropriate treatment. Thrombotic events have been reported in patients receiving Fibryga®. Treatment with human fibrinogen concentrate has been associated with thrombosis at target plasma fibrinogen levels that were below 150 mg/dL. The thrombotic risks may be greater when the target fibrinogen plasma level is 150 mg/dL. Weigh the benefits of administration versus the risks of thrombosis.
Fibryga is made from pooled human plasma. Products made from human plasma may contain infectious agents, e.g., viruses and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent.