FDA-approved for the treatment of primary immunodeficiency (PI) in adults & children 2 years of age & older


FDA-approved for the treatment of primary immunodeficiency (PI) in adults & children 2 years of age & older

Indications and Usage
Cutaquig® (Immune Globulin Subcutaneous (Human) - hipp) is a 16.5% immune globulin solution for subcutaneous infusion indicated for treatment of PI in adults and pediatric patients 2 years of age and older.
WARNING: THROMBOSIS
See full prescribing information for complete boxed warning
Thrombosis may occur with immune globulin products, including cutaquig. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling vascular catheters, hyperviscosity, and cardiovascular risk factors.
For patients at risk of thrombosis, administer cutaquig at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk of hyperviscosity.
Please see accompanying full Prescribing Information.
This brochure contains important information to help you get the most from your treatment with cutaquig. You’ll learn how cutaquig is made and infused, what to expect during treatment, how cutaquig works to prevent infection, what type of side effects may occur, and where you can go to for additional education and treatment resources.
Cutaquig is a ready-to-use liquid medicine made from human plasma donated by healthy people and contains human immunoglobulin G (IgG), also called antibodies. The main function of these antibodies is to help the body fight infection by attaching to and identifying bacteria, viruses, or other pathogens.1,2
Cutaquig is designed to be given subcutaneously (SC, under the skin), not intravenously (IV, in a vein). Subcutaneous immunoglobulin products are commonly referred to as SCIg. After proper training, cutaquig can be infused at home—by yourself or a parent or caregiver. Cutaquig can also be administered by a specialized healthcare provider. Cutaquig is a 16.5% SCIg solution for adults and children 2 years of age and older with primary immunodeficiency (PI).1,2
Cutaquig is used to replace missing or poorly functioning antibodies in people with PI.1,2
• Normally, our immune system protects us against infections by recognizing potentially harmful bacteria and viruses that enter our body every day
• In response, the immune system produces special proteins called antibodies (immunoglobulins) that target these infective agents
• When our immune system is not working properly, antibody production can be affected
• In people with PI, cutaquig temporarily replaces missing antibodies that their immune system does not produce, and helps to prevent infection
Without antibodies, you’re at greater risk for infections caused by bacteria & viruses
Cutaquig contains the antibodies you’re missing
With regular administration, cutaquig helps your body fight bacteria and viruses that cause infections
Cutaquig® is contraindicated in patients who have had an anaphylactic or severe systemic reaction to the subcutaneous administration of human immune globulin or to any of the components of cutaquig such as Polysorbate 80. Cutaquig is contraindicated in IgA-deficient patients with antibodies against IgA and a history of hypersensitivity to human globulin treatment.
Information.
Cutaquig is made from large pools of donated human plasma that is donated from healthy people. Each preparation is frozen within 8 hours of collection, also known as fresh frozen plasma.1,2
Cutaquig is manufactured following strict FDA regulations and guidelines that include:
Getting human plasma from FDA-approved donation centers
• Octapharma plasma donation centers are located throughout the US
Testing the collected plasma to help ensure it is virus- and disease-free
• All donated plasma is tested for evidence of a range of viruses, including human immunodeficiency virus, hepatitis B virus, and hepatitis C virus
Screening plasma donors for certain viruses and other diseases

• Any plasma with evidence of these viruses is rejected
Health screening, medical questionnaire, and
Removing & inactivating certain viruses
• Plasma goes through an extensive purification process for virus inactivation / removal of viruses
• Octapharma maintains stringent safety standards for the plasma used in all of its human protein therapies
• The process used to make cutaquig includes a multi step virus inactivation/removal process
• Octapharma pioneered a number of safety innovations that have set the standard for the protein products industry
• Octapharma was first to apply solvent/detergent (S/D) virus inactivation to a human plasma product, recognized as the gold standard for safety
• Octapharma was first to launch a liquid immunoglobulin preparation
Warnings and Precautions
Severe hypersensitivity reactions may occur with cutaquig, even in patients who tolerated previous treatment with human immune globulin. If a hypersensitivity reaction occurs, discontinue the cutaquig infusion immediately and initiate appropriate treatment. IgA-deficient patients with anti-IgA antibodies are at greater risk of severe reactions.
Cutaquig offers proven efficacy for children (2 years and older) and adults with PI1,2
ZERO serious bacterial infections (SBIs)
• In a 1-year pivotal study, 75 adults and children with PI treated with cutaquig experienced ZERO SBIs
• Similar results were seen in a follow-up extension study in 27 patients, with ZERO SBIs in children and 1 SBI in an adult
LOW hospitalizations due to infections
• In the pivotal study, there were ZERO hospitalizations due to infections in adults and 4 in pediatric patients
• In the extension study, there were ZERO hospitalizations due to infections in pediatric patients and 3 in adults
LOW rate of other infections
• In both the pivotal and extension studies, the rate of other infections was low (between 2.2 and 3.3 infections per person year)
LOW absences due to infection
• Days out of work, school, kindergarten, or daycare due to infections were low (between 2.4 and 3.6 days per person year)

As both a patient and a Nurse Educator for Octapharma, Laurel travels the country meeting with patients and healthcare professionals, educating them about cutaquig.
Similar to the results seen with cutaquig in the pivotal study, Laurel has had ZERO SBIs since starting cutaquig, and no hospitalizations due to an infection.
Laurel describes switching to subcutaneous infusions with cutaquig as a life-changer. “With cutaquig, I am finally able to plan my infusions around my life and schedule, instead of needing to plan my life around the scheduled infusions.”
Safety Information
Warnings and Precautions (continued)
Thrombosis may occur following treatment with immune globulin products, including cutaquig. For patients at risk of thrombosis, administer cutaquig at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity.
Infusion site reactions. As with all medications that are injected under the skin, infusion site (or local) reactions are most common with cutaquig, and may include1,2:
• Mild or moderate pain
• Redness
• Itching
• Swelling
• Tenderness
• Feeling of warmth
Infusion site reactions are often mild, and go away within 24 hours.
Other common side effects that may occur with cutaquig include1,2:
Headache Fever Diarrhea
Skin abrasion Dermatitis
Asthma
These are not all the possible side effects. Talk to your doctor or nurse about any side effect that bothers you or that does not go away.

Shannon tried several different IgG therapies, but didn’t tolerate them well. After 5 medication switches, she finally discovered cutaquig—and hasn’t looked back.
Since starting cutaquig, Shannon no longer needs premedication for side effects. She self-infuses once a week. Start to finish, her treatment takes about 2 hours.
Shannon reports that she is finally living the life that was waiting for her. “To be able to feel strong, to be in control, to do my job, and actually live my life to its fullest extent—because of this medication—there are no drawbacks for me. It’s such a gift, and I’m so grateful for that!”
Important Safety Information
Falsely elevated blood glucose readings may occur during and after the infusion of cutaquig with some glucometer and test strip systems. When administering cutaquig, measure blood glucose with a glucosespecific method.
As an SCIg, cutaquig is administered below the skin with an infusion pump and subcutaneous (SC) needles. To administer cutaquig, you’ll need supplies, including1,2:
• Cutaquig vials (per your healthcare provider’s prescription)
• An infusion pump and compatible syringes
• Subcutaneous needles
• Special tubing (provided by your specialty pharmacy)
• Sharps container (for disposing of used needles and syringes)
SC administration offers many key benefits compared to IV1-4
SCIg can be self–infused at home, school, work, or even while traveling. And unlike with IVIg, you can free yourself from clinic appointments for administration, and missing work or school to attend them. SCIg also offers each patient flexibility in their regimen design—where, how often, how much, and how long to infuse. SCIg generally takes less time per infusion than IVIg—the more often you choose to infuse, the lower the required dose and the shorter the duration of infusion. On average, treatment with cutaquig takes about 1.5 hours. Finally, SCIg provides a “steady state” IgG level in the body (no peaks and drops).
Cutaquig offers patients flexible dosing options1-2

Important Safety Information
Warnings and Precautions (continued)
Aseptic meningitis syndrome (AMS) can occur with cutaquig. AMS has been reported after the use of human immune globulin administered intravenously and subcutaneously and may occur within 2 days following treatment. Discontinuation of immunoglobulin treatment has resulted in remission within several days without sequelae.
Acute renal dysfunction/failure, acute tubular necrosis, proximal tubular nephropathy, osmotic nephrosis, and death may occur with use of human immune globulin, especially those containing sucrose. Cutaquig does not contain sucrose.
You can infuse cutaquig in the following areas1,2:
• Upper arm
• Abdomen
• Upper leg/hip
• Thigh
Infusion training
• Your infusion training will typically be performed by a Registered Nurse who is a specialist with experience in the administration of SCIg. Usually, infusion training is scheduled in your home, your doctor’s office, or in an infusion clinic

• Standard infusion training is 3 visits, scheduled on the dates of your first 3 infusions. Each visit is designed to add skills so that you are comfortable and confident in self-infusion. Ask as many questions as you want!
• You may want to have a family member or friend present for support, and to take notes for you so that you can focus on the training. Initially, it may be helpful to have them available when you are starting independent self-infusion


Use this journal to keep track of your infusions and record key information including:
• Date and time of infusion
• Dose
• Lot number information
• Side effects you may have experienced
• Questions and notes for your healthcare provider
• Any recent infections
The journal and other resources are available at IgCares, a comprehensive patient and caregiver support program from Octapharma, providing education and treatment resources. See page 9 for more information, or go to IgCares to enroll.
Monitor patients for signs and symptoms of renal dysfunction. Monitor blood urea nitrogen, serum creatinine, and urine output in patients at risk of acute renal failure.
Monitor cutaquig recipients for clinical signs and symptoms of hemolysis, particularly patients with pre-existing anemia and/or cardiovascular or pulmonary compromise. Consider appropriate confirmatory laboratory testing if signs and symptoms of hemolysis are present after cutaquig infusion. Non-cardiogenic pulmonary edema may occur in patients administered human immune globulin products. Monitor for pulmonary adverse reactions. If transfusion-related acute lung injury is suspected, perform appropriate tests for the presence of anti-neutrophil antibodies in both the product and patient’s serum.
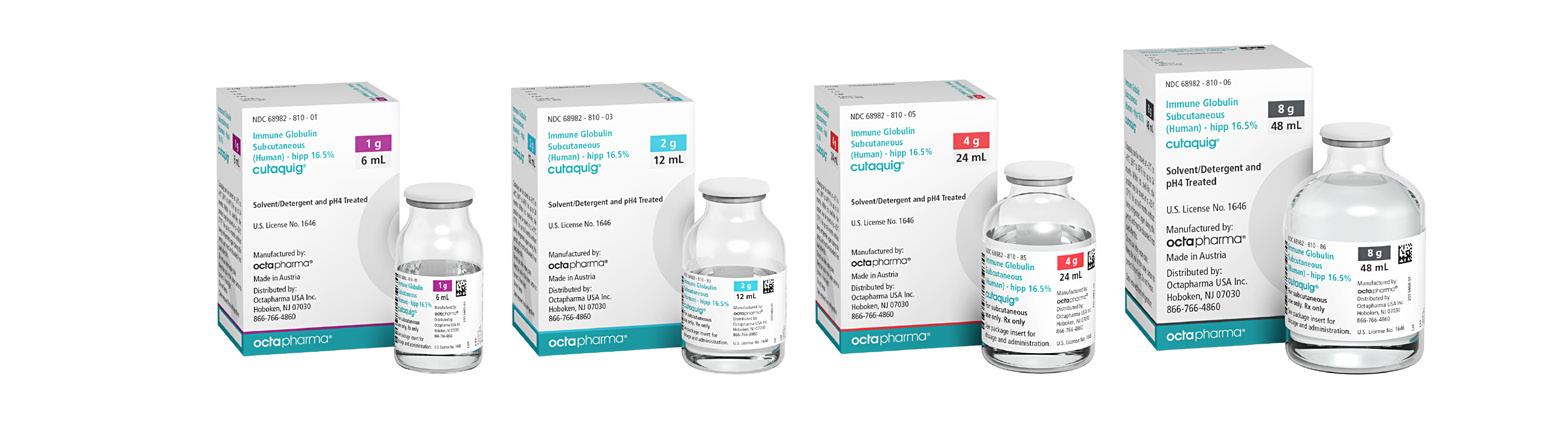
Cutaquig is available in a range of convenient, ready-to-use vial sizes for patients with varied needs
Store cutaquig in the refrigerator at 36°F to 46°F (+2°C to +8°C) for up to 36 months from date of manufacture.
Within its shelf-life, cutaquig may be stored at room temperature up to 77°F (+25°C) for up to 6 months without being refrigerated again during this period, and must be discarded if not used after this.
Keep the cutaquig vial in the outer carton to protect it from light.
When preparing and handling cutaquig1:
• Check the expiration date on the vial label. Do not use beyond expiration date
• Do not freeze cutaquig, and do not use frozen product
• Do not mix cutaquig with other products
• Do not shake the cutaquig vial
• Use aseptic technique (wash hands /clean work surface) when preparing/handling
• Prior to administration, look closely at each vial of cutaquig for any particles, whenever the solution and container permit. Do not use if the solution is cloudy or contains particulates
Each cutaquig vial is for single-use only. Discard any unused product after each infusion, in accordance with local requirements.
Cutaquig is made from human plasma and may carry a risk of transmitting infectious agents, e.g. viruses, the variant Creutzfeldt-Jakob disease agent, and, theoretically, the Creutzfeldt-Jakob disease agent.
www.cutaquigus.com
• Tailored to patients, families, and caregivers
• Provides educational content, product and patient support tools and resources, including co-pay assistance, reimbursement support, and clinical data

Cutaquig is backed by the IgCares patient support program, designed to provide education and treatment resources for people living with PI (adults, children, parents, caregivers) at every step of the treatment journey—minimizing hassle, improving quality of life, and delivering on our company’s commitment to community and sustainability.
With four innovative and practical initiatives, IgCares is designed to help PI patients, parents, and caregivers while protecting our planet, championing advancement of knowledge, and supporting organizations dedicated to the PI community.
Care for your self
Exclusive access to educational and informational resources
Care for your causes
A special initiative that helps support the PI community
Care for your spirit
Personal connections to peers, the PI community, and patient advocates
Care for your world
A safety and sustainability program that transforms infusion supplies into energy
Membership in IgCares is free and open to anyone in the US who would like to join. IgCares offers a wide range of benefits and resources, including:


For eligible patients, the IgCares co-pay assistance program can provide significant savings on some of the costs associated with your treatment.
IgCares provides a wide range of exclusive educational resources—articles, self-infusion resources, personalized email streams, and more—all designed to help you become more knowledgeable and to make your life easier.

IgCares offers the opportunity to connect 1-on-1 with Patient Educators and Nurse Advisors. Our Patient Educators are actual patients with PI who can share their real-world experiences and provide valuable insight. Our Nurse Advisors are registered nurses who can answer your questions about treatment.

Mobile App
The IgCares mobile app provides anytime, anywhere support for adults with PI. The mobile app includes practical tools to help simplify treatment management and track the details of your infusions, whenever and wherever you need to.

The IgCares recycling service allows you to easily discard your sharps and other infusion supplies, as well as the packaging and shipping materials associated with your prescription. Also, 100% of what you return is repurposed into an industrial material used to generate electricity for homes and businesses.

The IgCares program includes a charitable giving program created to donate funds to organizations that provide support to the PI community, including the Immune Deficiency Foundation, the Jeffrey Modell Foundation, the Foundation for Primary Immunodeficiency, and the International Patient Organization for Primary Immunodeficiencies
After infusion of cutaquig, the transitory rise of the various passively transferred antibodies in the patient’s blood may yield false positive serological test results, with the potential for misleading interpretation.
Adverse Reactions
The most common adverse reactions (≥ 5% of study subjects) were local infusion site reactions (such as redness, swelling, itching), headache, fever, dermatitis, asthma, diarrhea, and cough.
Download the Welcome to Cutaquig Handbook
Available at the IgCares website, the handbook provides helpful tips, insights, and advice for living with PI, FAQs on cutaquig, and how to save with our reimbursement and co-pay information, and much more! Go to IgCares to enroll.


The IgCares program supports well-known foundations in the PI community, including:
• The Immune Deficiency Foundation (IDF), dedicated to improving the diagnosis, treatment, and quality of life of people with PI. Visit primaryimmune.org
• The Jeffrey Modell Foundation, a global resource for the most up-to-date information and support for people with PI. Visit info4pi.org
• The Foundation for Primary Immunodeficiency Diseases (FPID), supporting PI education, early diagnosis, counseling, therapy, and research. Visit fpid.org
• International Patient Organisation for Primary Immunodeficiencies, inspired by its mission to improve awareness, access to early diagnosis, and optimal treatments for PI patients worldwide. Visit ipopi.org
Malea has been living with PI for over a decade. As a Patient Educator for Octapharma, she works in the trenches, staying connected to patients well beyond the initial diagnosis.

Malea recommends that every person who is taking cutaquig enroll in IgCares. Whether you’re switching to cutaquig or a newly diagnosed patient, the program will guide you through the cutaquig process.
As a full-time schoolteacher, Malea’s big motivator is education, and to engage new patients. “Patients feel most alone when they are first diagnosed. That’s when 1-to-1 support is really important. Just being around your fellow patients helps so much.”

Octapharma USA, Inc. 117 W. Century Road Paramus, NJ 07652 Tel: 201-604-1130
IgCares/Reimbursement Assistance usreimbursement@octapharma.com Tel: 800-554-4440
usmedicalaffairs@octapharma.com
For all inquiries relating to drug safety, or to report adverse events, please contact our local Drug Safety Officer:
Tel: 201-604-1137 | Cell: 201-772-4546 | Fax: 201-604-1141 or contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
References: 1. Cutaquig® (Immune Globulin Subcutaneous (Human) - hipp), 16.5% solution. Full Prescribing Information. Paramus, NJ: Octapharma USA Inc.; October 2021. 2. Octapharma USA Inc. Data on File. 3. Berman K. Safety, Efficacy, Tolerability, Advantages and Disadvantages of Intravenous and Subcutaneous Immune Globulin Therapy. Highlights from IG Living Teleconference December 10, 2015. Accessed January 11, 2022. http://www.igliving.com/life-with-ig/ teleconference/advantages-and-disadvantages-of-intravenous-and-subcutaneous-immune-globulin-therapy.html 4. Jolles S, et al. Biol Ther. 2011;1(1):1:003.
Please see Important Safety Information throughout inside pages and full Prescribing Information, including complete BOXED WARNING, in pocket.
©2022. Octapharma USA, Inc. All rights reserved. Date of preparation: 2/2022. CUTA-0576-COT
Patient Information
CUTAQUIG
(kew’ ta kwig)
Immune Globulin Subcutaneous (Human)hipp, 16.5% solution
Information for Patients
The following summarizes important information about CUTAQUIG. Please read it carefully before using CUTAQUIG and each time you get a refill, as there may be new information. This Patient Information does not take the place of talking with your healthcare provider about your medical condition or your treatment. If you have any questions after reading this, ask your healthcare provider.
What is CUTAQUIG?
CUTAQUIG is a ready-to-use liquid solution of Immunoglobulin G (IgG), also called antibodies, which protect the body against infection. CUTAQUIG is used to treat patients with primary humoral immunodeficiency (PI).
There are many forms of PI. The most common types of PI result in an inability to make a very important type of protein called antibodies, which help the body fight off infections from bacteria or viruses. Regular administration of CUTAQUIG will help your body to fight bacteria and viruses that cause infections. CUTAQUIG is made from human plasma that is donated by healthy people.
CUTAQUIG contains antibodies collected from these healthy people; these antibodies replace the missing antibodies in PI patients.
Who should NOT use CUTAQUIG?
Do not use CUTAQUIG if you have ever had a severe allergic reaction to immune globulin or other blood products.
Tell your healthcare provider if you:
• ever had any severe reaction to other immune globulin medicines.
• were told that you have a condition called IgA deficiency.
• have a history of heart or blood vessel disease.
• have had blood clots or “thick blood”.
• have been immobile for some time.
What should I tell my healthcare provider before using CUTAQUIG?
Talk to your healthcare provider about any medical conditions that you have or have had.
Tell your healthcare provider that you are taking CUTAQUIG before you get vaccination as vaccines may not work while you are taking CUTAQUIG.
Tell your healthcare provider about all of the prescription and non-prescription medicines you take, including over-the-counter medicines, dietary supplements, or herbal medicines.
Tell your healthcare provider if you are pregnant or plan to get pregnant, or if you are nursing because CUTAQUIG might not be right for you.
Tell your healthcare provider if you have diabetes. If you need to do glucose testing, your healthcare provider may tell you to use a different way to monitor your blood sugar levels on the day that you receive a CUTAQUIG infusion. Some types of blood glucose testing systems (so called glucometers) falsely interpret the maltose contained in CUTAQUIG as glucose. If you are uncertain ask your healthcare provider which glucose testing system you can use while using CUTAQUIG.
How should I use CUTAQUIG?
CUTAQUIG is given under the skin (subcutaneously). This type of infusion can be given at home by yourself or by your caretaker after appropriate training. Administration of CUTAQUIG should be done at regular intervals. Only use CUTAQUIG by yourself after you have been instructed by your healthcare provider.
What are the possible or reasonably likely side effects of CUTAQUIG?
The most common side effects of CUTAQUIG are:
• Infusion site reactions (including but not limited to redness, swelling, itching, fluid in tissue, pain, mass, bruising)
• Headache
• Elevated body temperature
Call your healthcare provider or emergency department immediately if you have any of the following symptoms: difficulty breathing, chest tightness, itching, swelling of the face, rash, hives or dizziness. These could be signs of a serious allergic reaction.
Tell your healthcare provider immediately if you have any of the following symptoms. They could be signs of a serious drug reaction:
• Decreased urination, sudden weight gain, or swelling in the legs. These could be signs of a kidney problem.
• Bad headache with nausea, vomiting, stiff neck, fever, and sensitivity to light. These could be signs of meningitis, which is irritation of the lining around your brain.
• Pain, swelling, warmth, discoloration of an arm or leg, unexplained shortness of breath, chest pain, discomfort that worsens on deep breathing, unexplained rapid pulse, a lump in your legs or arms, or numbness or weakness on one side of the body. These could be signs of a blood clot.
• Chest pain or trouble breathing, or blue lips or extremities. These could be signs of a serious heart or blood problem.
• Brown or red urine, fast heart rate, yellow skin or eyes. These could be signs of a liver or heart problem.
• Fever over 100°F (38°C). This could be sign of an infection.
These are not all of the possible side effects from CUTAQUIG. Ask your healthcare provider for more information. You are encouraged to report side effects to Octapharma USA Inc. at 1-866-766-4860 or FDA at 1-800-FDA-1088.
Talk to your healthcare provider about any side effect that bothers you or that does not go away. You can ask your healthcare provider for more information on possible side effects.
Whenever giving yourself treatments at home, make sure another responsible person
is present to help treat side effects or help if a serious adverse reaction occurs. Ask your healthcare provider whether you should have rescue medications, such as antihistamines or epinephrine. If prescribed epinephrine for severe allergic reactions, make sure you receive adequate training from a healthcare provider for its proper use.
How should I store CUTAQUIG?
Keep CUTAQUIG in the outer carton to protect it from exposure to light.
Do not freeze CUTAQUIG.
Store CUTAQUIG at 36°F-46°F (+2°C to +8°C) for up to 36 months from the date of manufacture. Within its shelf-life, the product can be stored at room temperature up to 77°F (up to +25°C) for up to 6 months without being refrigerated again during this period, and must be discarded if not used after this.
Do not use CUTAQUIG after the expiration date printed on the vial.
Dispose all materials, including any unused CUTAQUIG, in an appropriate container.
What else should I know about CUTAQUIG?
Do not use CUTAQUIG for a medical condition for which it was not prescribed. Do not share CUTAQUIG with other people, even if they have the same diagnosis and symptoms that you have.
Resources at Octapharma available to patients
For more product information on CUTAQUIG, please visit www.cutaquig.com.
For more information on patient assistance programs that are available to you, please contact the Octapharma Patient Support Center at 1-800-554-4440.
Manufactured by Octapharma Pharmazeutika Produktionsges.m.b.H.
Oberlaaer Strasse 235
1100 Vienna, Austria U.S. License No. 1646
Distributed by Octapharma USA, Inc.
117 W Century Road
Paramus, NJ 07652
CUTAQUIG® is a registered trademark of Octapharma.
CUTAQUIG
Immune Globulin Subcutaneous (Human)hipp, 16.5% solution
Detailed patient handling instructions for administration of CUTAQUIG

CUTAQUIG is for subcutaneous use only.
Use CUTAQUIG only after you have been properly instructed and trained by your healthcare provider.
Follow the administration guidance below step by step and use aseptic/sterile technique when administrating CUTAQUIG. Use gloves if you have been told to do so when preparing the infusion.
1. Prepare the necessary number of CUTAQUIG vials
– If stored in the refrigerator, put the vials at room temperature at least 90 minutes prior to infusion.
– Do not heat the vials or put them into the microwave.
– Do not shake the vials to avoid foaming.
2. Getting ready for infusion
– Choose and prepare a clean work area using antiseptic wipes or disinfecting solution.
– Gather your infusion equipment:
– Infusion pump and compatible syringe(s) Needle or needleless transfer device (for drawing up product from the vial) Infusion set (varies according to manufacturer’s instructions) Infusion tubing and Y-connector (if required)
Ancillary supplies: disinfectant wipes, gauze or transparent dressing, tape and sharps container
Treatment diary and pen
– Wash and clean your hands thoroughly and let them dry as has been shown to you during the training (Figure 1). You can wear gloves during the preparation of the infusion if you have been told so during the training.
– If necessary, program the pump according to the user manual and as you have been shown during the training by your healthcare provider.
3. Checking and opening the vials
– Allow products to reach room temperature (77°F / ≤ 25°C).
– Inspect each vial carefully: Check that the labelled dose is correct and based on your prescription. Check that the expiration date has not been passed.
Check the appearance of the solution (it should be clear and colorless to pale yellow or slightly opalescent).
Do not use the solution if it is cloudy or contains particles.
Make sure the protective cap is not broken or missing.
– Remove the protective cap.
– Disinfect the rubber stopper by using a sterile wipe and allow it to dry (Figure 2).

4. Preparing and filling the syringe
– Open sterile syringe and needle or needleless transfer device.
– If a needleless transfer device is used, follow the instructions of the device manufacturer.
– If the transfer is done using needle and syringe, follow the instructions below: Attach the needle to the syringe with a screw action.
Draw back on the plunger to fill the syringe with air. The amount of air should be roughly equal to the amount of solution needed from the vial. Insert the needle into the center of the
vial stopper and slowly turn the vial upside down. To avoid foaming, ensure that the tip of the needle is not in the solution; then inject air by pushing the plunger of the syringe. Next, move the needle so that the tip is in the solution; then slowly draw up the desired volume of CUTAQUIG solution, making sure that the needle tip is always in the solution (Figure 3).

Withdraw the needle from the vial. This procedure might be repeated if you need multiple vials for the calculated dose.
When finished, remove the needle and dispose of it into the sharps bin. Once finished, proceed to the next step.
5. Preparing the infusion pump and tubing
– Follow the manufacturer’s instructions for preparing the infusion pump.
– To prime the administration tubing, attach the filled syringe to the infusion tubing and gently push the plunger to fill the tubing with CUTAQUIG, as has been shown to you during training (Figure 4).
– Stop priming before fluid reaches the needle tip.


– The number and location of injection sites depends on the volume of the total dose.
– The infusion sites should be at least 2 inches apart. Do not use more than 6 infusion sites at the same time.
– Rotate sites between infusions.
– Avoid inserting the needle into scars, tattoos, stretch marks or any skin that has signs of infection (such as injured/inflamed/ red skin area).
– Clean your skin at your selected infusion site(s) with an antiseptic skin wipe, starting at the center and working outward in a circular motion. Allow each site to dry before proceeding.
– Pinch the skin between your thumb and forefinger around the injection site (Figure 6). Carefully remove the needle cover and insert the needle into the skin (Figure 7). The angle of the needle will depend on the type of infusion set being used.
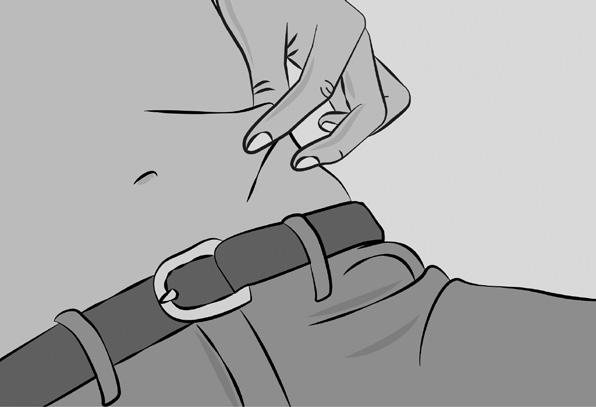

6. Preparing infusion site(s) and inserting the infusion needle(s)
– CUTAQUIG can be infused into the following areas: abdomen, thigh, upper arm, and/or upper leg/hip area (Figure 5).
7. Checking the infusion
– Check needle placement by pulling back on the syringe plunger. There should not be any blood return in the tubing (Figure 8).

– If blood return is seen, remove the needle and restart from step 6 with new tubing at a different location.
8. Starting the infusion
– Start the infusion. Follow infusion pump manufacturer’s instructions.
9. Recording the infusion
– On each vial of CUTAQUIG, you will find a peel-off portion of the label with the batch number details. Stick this label in your patient’s treatment diary or infusion log book. Record details of the dose, date, time, infusion site location and any infections, side effects or other comments in connection with this infusion.
10. After infusion is complete
– Gently remove the dressing and the needle(s) and immediately place into the sharps bin.
– Press a small piece of gauze on the needle site and apply a dressing.
– Discard all used disposable supplies as well as any unused product and the empty vial(s) as recommended by your healthcare provider and according to local requirements.
– Tidy up and securely store all the reusable equipment (e.g., pump) until the next infusion.
If you encounter any problems or experience side effects during or after the infusion, contact your healthcare provider. When doing so, keep your treatment diary or log book with you to be able to give all necessary information.
You can also report side effects to the FDA at 1-800-FDA-1088 or online under www.fda. gov/medwatch.
For more product information on CUTAQUIG, please visit www.cutaquig.com
Manufactured by Octapharma Pharmazeutika Produktionsges.m.b.H. Oberlaaer Strasse 235 1100 Vienna, Austria U.S. License No. 1646
Distributed by Octapharma USA, Inc. 117 W Century Road Paramus, NJ 07652
CUTAQUIG® is a registered trademark of Octapharma.
Issued 10 2021.
– Put transparent dressing or sterile tape and gauze over the administration site to keep the needle in place during the infusion.