for
pārongo
entities’ engagement with consumers and whānau Te tikanga mō te mahi tahi a ngā hinonga hauora ki ngā kiritaki me ngā whānau

for
pārongo
entities’ engagement with consumers and whānau Te tikanga mō te mahi tahi a ngā hinonga hauora ki ngā kiritaki me ngā whānau
This year has required careful navigation as we sought to balance much-needed infrastructure investment with prudent decisionmaking in a fiscally constrained environment.
We modified, deferred, or paused some significant projects to reduce expenditure while also identifying and giving effect to the small savings we could achieve as part of our wider efforts.
Given this challenging context, we are particularly proud of the projects and progress New Zealand Blood Service (NZBS)1 has realised over the year, including achieving our highest-ever number of plasma collections.
By June 30 we had collected nearly 235,000 units of blood, plasma, and platelets—up by nearly 15,000 units on last year, including a vital 13,500-unit increase in plasma. Our panel of plasma donors also increased by more than five percent. While there is much more still to do, this represents crucial progress as demand for our products continues to grow.
Increasing and retaining our donor numbers was also a driver behind the February introduction of our new Customer Service Standard and Promise. Donors who enjoy a positive experience are far more likely to return and to advocate for us, and while we traditionally achieve well over 90 percent in our Donor Experience Surveys, the new Standard formally articulates what’s required to consistently deliver exceptional service and care. The Standard and Promise resulted from comprehensive engagement with staff and donors, and customer service training for staff will be delivered next year.
Bolstering our infrastructure continued to be a priority over the year, and we identified and secured two new donor centre sites; one on Auckland’s North Shore, where fit-out work had begun before year-end, and another in Porirua. These sites will not only add fit-for-purpose capacity but reduce geographic barriers to access for potential and existing donors.
Our Henderson mobile venue has performed exceptionally well since its June 2024 opening, so work to convert it to a permanent donor centre also started this year, and we upgraded our Palmerston North site, ensuring it meets required standards.
October also saw the opening of Stage 1 of our largest infrastructure development, Highbrook, enabling logistics and plasma processing functions to relocate from our cramped Epsom site. Highbrook’s opening allowed us to move fully to using sea, rather than air, freight for shipping plasma to Australia for fractionation. This reduced both financial and environmental impacts, with our first containerised plasma shipment to leave Highbrook departing in December.
Rounding out a big month for the organisation, we started rolling out our long-awaited Self-Administered Health History (SAHH) system in October too. This introduced digital tablets and electronic forms for donors to complete their pre-donation ‘paperwork’ with us. Several years in the making, SAHH has removed pens and paper from the process, streamlining it and bringing us into line with blood services internationally. The system delivers a range of benefits, including improving the security of personal and health information, with further functionality still to come.
We were very pleased this year to renew our contract with the Malaghan Institute of Research in February, formalising our ongoing involvement in ENABLE, New Zealand’s first—and currently only— CAR T-cell therapy clinical trial. We are immensely proud of the contribution we can make to the field of cellular therapies and the difference this work is making to the lives of people fighting B-cell non-Hodgkin lymphoma.
In March, we also began delivering our new Dunedin Therapeutic Apheresis Service, providing close-to-home, ward-based care for people who would previously have been treated in Dunedin Hospital’s ICU or had to travel to Christchurch for therapeutic plasma exchange procedures. The result of a successful collaboration with Health New Zealand Te Whatu Ora, this service has freed up precious ICU space and will ultimately operate 24/7 to deliver a range of procedures, including stem cell collections.
This year also saw the continuation of our comprehensive and complex Process Migration Project for fractionated products, and more lifesaving work by our Organ Donation New Zealand (ODNZ) and New Zealand Bone Marrow Donor Registry teams. ODNZ facilitated 213 organ donations from 66 organ donors, as well as eye, heart, and skin donations, while NZBMDR’s work enabled 75 people to receive bone marrow transplants – a 30 per cent increase on last year.
So much more has been achieved throughout 2024/25, the details of which are traversed in the following pages. Delivering the breadth of activities outlined in this report, despite the constrained environment, has been no easy feat. As well as advancing strategic projects, our staff have worked exceptionally hard, day-to-day, to consistently deliver safe, high-quality products and services for our donors, health sector colleagues, patients, and recipients. For that, we extend our gratitude.
And of course, none of what we do would be possible without the generous contributions of our lifesaving donors. To them, a sincere and heartfelt thank you for empowering us to help improve and save thousands of lives this year.

Fiona Pimm Board Chair
Sam Cliffe Chief Executive
1 New Zealand Blood Service (NZBS) was established in 1998 under the New Zealand Public Health and Disability Act 2000 (replaced by the Pae Ora Healthy Futures Act from July 1, 2022). In 2019, the Organ Donors and Related Matters Act was passed, and Organ Donation New Zealand (ODNZ) was subsequently integrated into New Zealand Blood Service in 2020. As a result, New Zealand Blood Service’s legal name became New Zealand Blood and Organ Service. However, the organisation continues to operate as New Zealand Blood Service (NZBS). Where ‘New Zealand Blood Service’ and ‘NZBS’ appear in this report, they refer to the work of New Zealand Blood and Organ Service.
The Board of New Zealand Blood Service (NZBS)1 is responsible for:
• the preparation of New Zealand Blood Service’s financial statements and Statement of Performance, and for the judgements made in them,
• establishing and maintaining a system of internal control designed to provide reasonable assurance as to the integrity and reliability of financial reporting.
In our opinion, these financial statements and statement of performance fairly reflect the financial position and operations of NZBS for the year ended 30 June 2025.
Signed on behalf of the Board:

Fiona Pimm Board Chair
30 October 2025

Dr Bart Baker
Board member
30 October 2025
1 New Zealand Blood Service (NZBS) was established in 1998 under the New Zealand Public Health and Disability Act 2000 (replaced by the Pae Ora Healthy Futures Act from July 1, 2022). In 2019, the Organ Donors and Related Matters Act was passed, and Organ Donation New Zealand (ODNZ) was subsequently integrated into New Zealand Blood Service in 2020. As a result, New Zealand Blood Service’s legal name became New Zealand Blood and Organ Service. However, the organisation continues to operate as New Zealand Blood Service (NZBS). Where ‘New Zealand Blood Service’ and ‘NZBS’ appear in this report, they refer to the work of New Zealand Blood and Organ Service.
TŌ MĀTOU TŪMANAKO ME NGĀ WHANONGA PONO
To ensure the health needs of people in New Zealand are supported by the availability of safe and appropriate blood, blood products, tissues and related services.
To be recognised for excellence in meeting the needs of donors, patients, our staff, and the wider healthcare community.
We strive to achieve our vision and purpose in accordance with our shared values and driving principle of safety:

OUR SHARED VALUES: STRIVING FOR EXCELLENCE
KIA TAU KI TE TIHI

TE MAHI NGATAHI
TE PONO ME TE TIKA
INTEGRITY AND RESPECT
TE WHAKAWHITIWHITI WHAKAARO I
RUNGA I TE MAHARAHARA
OPEN COMMUNICATION
TE HAUMARU HOKI TE KATOA
HAUMARU
SAFETY FOR ALL
These values have been co-designed by staff, volunteers, blood donors and patients. Behind each value is a set of behaviours that guide the way we treat each other and conduct our business. They inspire us, motivate us and drive us to provide the very best service to the New Zealand public.
NGĀ TAU
141,261
THE ONLY BLOOD SERVICE IN NEW ZEALAND
OUR YOUNGEST DONOR 2009 OUR OLDEST DONOR 1942
583
MOBILE PLASMA DRIVES AT 9 LOCATIONS
BLOOD, PLASMA, AND PLATELET DONORS 1
10 FOUNDED IN 1998
DONOR CENTRES ACROSS THE MOTU
900 MORE THAN
TOTAL STAFF
4,435
AVERAGE NUMBER OF DONATIONS PER WEEK
3 MANUFACTURING SITES
6 BLOOD BANKS
3
SPECIALIST LABORATORIES
5
SITES OFFERING SUPPORT FOR CELLULAR THERAPIES (Auckland, Hamilton, Palmerston North, Wellington, Christchurch)
1,500
MOBILE BLOOD DRIVES AT 337 SEPARATE LOCATIONS
107,238 PLASMA UNITS
2,858 PLATELET UNITS
107 GRANULOCYTE UNITS
124,856
WHOLE BLOOD UNITS COLLECTED IN 2024/25
400
WONDERFUL VOLUNTEERS NATIONALLY AROUND
1. Check you are eligible to donate by viewing the eligibility criteria on our website: nzblood.co.nz
2. Eligible? Great! You can make an appointment at nzblood.co.nz, via the NZ Blood Donor app, or by calling 0800 GIVE BLOOD (0800 448 325).
• App for Android
• App for Apple
3. You can donate plasma at one of our donor centres or, if based in Auckland, at one of five mobile plasma drive locations available. A mobile plasma drive also operates in Wellington.
• In our laboratories, blood is spun in centrifuges to separate the red blood cells, platelets and plasma. Our scientists test every donation for infectious diseases and identify the blood type.
• Plasma can be made into two fresh blood components – Fresh Frozen Plasma (FFP) or Cryoprecipitate. We also send a large volume of the plasma we collect to CSL Behring in Australia, where it is processed and separated out (‘fractionated’) to make lifesaving treatments that are then returned to New Zealand for use in patients here (see page 10-11).
• Blood and blood products are stored until they are needed. The shelf life and temperature requirements are different for each blood component:
– Red blood cells are stored in refrigerators at 6°C for up to 35 days. They are used to treat people with chronic anaemia caused by problems such as kidney failure and cancer, and for acute blood loss resulting from trauma or surgery.
– Platelets are stored at room temperature for up to seven days. They are used to control bleeding following cardiac surgery and trauma, and to treat some blood diseases and cancer.
You can start donating blood any time after your 16th birthday and before your 71st birthday if you weigh more than 50kg, and meet the eligibility criteria, including:
1 / You must be in good health at the time you donate.
2 / If you have recently had a tattoo, you must wait three months from the date of the procedure before you can donate.
3 / If you have recently travelled overseas, a stand-down period may apply. Please check the online travel tool on our website (nzblood.co.nz) to check your eligibility.
There are no longer vCJD (‘mad cow’) donation restrictions for people who had lived in the United Kingdom, France, or the Republic of Ireland for six months or more between 1980 and 1996.
ELIGIBILITY CRITERIA ARE SUBJECT TO CHANGE.
FOR ADDITIONAL ELIGIBILITY CRITERIA, VISIT OUR WEBSITE: NZB LOOD.CO.NZ

KO WAI E HIAHIA TŌKU TOTO?
RED BLOOD CELLS ONLY LAST 35 DAYS AND HELP A VARIETY OF PATIENTS, WITH PARTICULAR CARE TAKEN TO ENSURE BLOOD TYPE COMPATIBILITY.

3%
Children, including those with cancer
Bone surgery patients 6%
7%
Pregnant women + babies
11% People with liver, kidney + heart disease
23% Accident and trauma patients
24% Cancer patients
26% Other medical conditions + surgical treatments
WAI ĀWHEO
PLASMA IS THE LIQUID PART OF OUR BLOOD. WE CALL IT ‘LIQUID GOLD’ BOTH BECAUSE OF ITS COLOUR AND BECAUSE OF ITS VALUABLE LIFESAVING PROPERTIES.
2
107,238
UNITS OF PLASMA COLLECTED IN 2024/25
24,319
PLASMA DONORS IN 2024/25
Plasma makes up more than half the volume of blood in our bodies and contains proteins and antibodies. Plasma’s proteins can be transformed into a number of specialised blood components and products.
This is plasma that has been frozen and carefully thawed before use. It is used to replace clotting factors when a patient is bleeding after major surgery or a serious accident.
Plasma that has been specially treated to produce a concentrated dose of clotting factors. It is mostly used for treatment of trauma patients and during cardiac and transplant surgery.
90 EVERY YEAR
TOTAL APPOINTMENT TIME TO GIVE PLASMA (APPROX.)
DEMAND FOR PLASMA IS GROWING BY MORE THAN 10%
If you feel fatigued by giving blood, then you may be a great candidate for giving plasma. This is because your blood cells are returned to your bloodstream as part of the donation process!
LEARN MORE ABOUT WHETHER YOU CAN DONATE PLASMA BY:
CALLING
0800 448 325
VISITING
NZBLOOD.CO.NZ/G IVE-PLASMA OR ASKING US NEXT TIME YOU’RE IN DONATING BLOOD!

Fresh frozen plasma is used to treat people who have lost a lot of blood due to accidents or other trauma, and patients who have severe bleeding during surgery. New Zealand Blood Service also sends plasma to Australia where it is fractionated, or separated, into its various components for therapeutic use. These fractionated products are then returned to New Zealand where they are used to treat up to 50 illnesses.
Boost the immune systems of people who have low levels of antibodies. Control some autoimmune disorders where the patient’s immune system is attacking their own tissues. Provide special clotting factor concentrates for some people with haemophilia and other bleeding disorders.
With plasma being used to treat an increasing number of life-threatening conditions, demand for this ‘liquid gold’ is growing by more than ten per cent every year.
To guarantee surety of supply for New Zealanders now and into the future, we need many more people to become plasma donors, and existing donors to donate more often if they can.
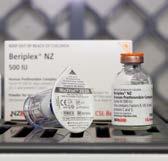
BERIPLEX ® NZ
Used in patients who require reversal of anticoagulant therapy, and for preventing and treating bleeding in patients deficient in one or more of the II, VII, IX, and X clotting factors. Inclusion of the VII clotting factor in this product eliminates the need to use fresh frozen plasma for anticoagulant reversal in patients.
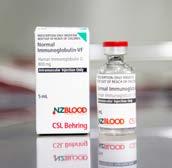
IMMUNOGLOBULIN
Provides antibodies to help protect people against hepatitis A, measles and other viruses - for example, when travelling overseas to high-risk areas areas or following exposure to infectious diseases.
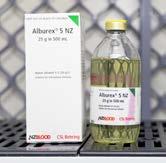
ALBUREX ® 5 NZ
Replaces albumin in people who lose this protein due to severe burns or who have very low albumin levels for other reasons. Also used to replace plasma during plasma exchange treatments and may be used to treat shock due to blood loss.
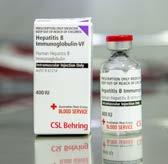
GLOBULINS
Provides temporary protection against a specific infection, such as chickenpox, tetanus or hepatitis B.
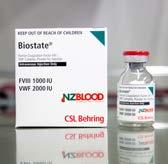
BIOSTATE ® (FACTOR VIII & VON WILLEBRAND FACTOR)
Contains blood clotting factor VIII and von Willebrand factor, two proteins that are essential for normal clotting. It is used in people with von Willebrand disease, a condition related to haemophilia. The treatment is used to prevent bleeding during surgery or after injury.
ALBUREX ® 20
Given to critically ill patients with a very low level of albumin in their blood due to factors such as extensive burns or acute blood or plasma loss.

PRIVIGEN NZ ® , HIZENTRA NZ ® , PRIVIGEN ® &HIZENTRA®
Used to treat some immune deficiencies by replacing antibodies that provide protection against many infections. Also used to treat patients with overactive immune systems that can lead to some autoimmune disorders. They are often used when other treatments are not effective.
RH(D) IMMUNOGLOBULIN-VF (ANTI-D)
Given to RhD negative women during pregnancy or after childbirth, this treatment prevents the mother from producing antibodies if her baby is RhD positive (i.e. incompatible with her own blood group). Without treatment, complications can arise for the baby (and for the mother’s future babies), ranging from jaundice to serious anaemia or even death.
The above treatments are a representative sampling of the 20 fractionated plasma products NZBS supplies to the New Zealand health sector. Where possible, they are manufactured using New Zealand source plasma, with commercial product acquired where appropriate and necessary.
For Auckland musician and guitar teacher Matt Hoyles, a normal life is possible only thanks to the plasma gifted by selfless donors.
In 2021, Matt was diagnosed with Common Variable Immunodeficiency (CVID), a rare condition that prevents his immune system from producing antibodies.
Without treatment, even a simple cold can become life-threatening but, thanks to immunoglobulin therapy derived from plasma, Matt’s been able to manage his condition and reclaim his life.
Before his diagnosis, Matt’s days consisted of chronic fatigue, constant and persistent infections such as sinusitis and bronchitis, and an underlying sense of fear and confusion, having previously been a ‘healthy’ person.
“My first infusion of immunoglobulin in September 2021 was a very long one, and I was unfortunately really wiped out for 10 days afterwards with headaches, nausea, severe joint pain, brain fog, and extreme fatigue, says Matt.
“But although it really knocked me, once I recovered, it was as if I could finally understand how my body was meant to feel. The boost I felt knowing that I was finally getting the help I needed was huge.”
Now, Matt manages his treatment at home with immunoglobulin infusions administered under the skin, giving him greater flexibility and control over his health.
“It’s been a total game-changer because it feels like I’ve taken back control of a life that my body sent off track without my consent.
“The more consistent energy levels and having fewer side effects have removed yet another barrier to getting out there and living fully.”
Matt’s wife Connie has also joined the New Zealand Blood Service donor community and is busy volunteering, donating blood, and organising workplace blood drives to encourage others to get involved.
The couple’s shared commitment reflects just how farreaching the impact of plasma donation can be – not just for patients, but for families, friends and communities too.
“Knowing that my continued health depends on the selfless act of strangers donating plasma is a truly mindblowing reality, and means more than it is possible to convey in words,” says Matt. “It’s a gift for which I will never truly be able to adequately express my gratitude.”
Since starting treatment, Matt has been able to resume many of the things he once thought were out of reach – from teaching music and travelling internationally to climbing mountains with his brothers.
For people like Matt, every plasma donation is life changing.

“KNOWING THAT MY CONTINUED HEALTH DEPENDS ON THE SELFLESS ACT OF STRANGERS DONATING PLASMA IS A TRULY MIND-BLOWING REALITY, AND MEANS MORE THAN IT IS POSSIBLE TO CONVEY IN WORDS,” SAYS MATT. “IT’S A GIFT FOR WHICH I WILL NEVER TRULY BE ABLE TO ADEQUATELY EXPRESS MY GRATITUDE.”

to
–

before getting sick.
Incorporating an assessment of NZBS’s Statement of Intent strategic goal delivery
The last 12 months focused on four key areas for our people:
• Supporting wellbeing
• Collective Employment Agreement bargaining
• Reward and remuneration, and
• Recognising and celebrating staff
Supporting wellbeing
We have delivered various sessions throughout the year to support staff wellbeing. The intent of these sessions is to foster a positive employee experience by supporting wellbeing both at work and at home (read more on page 84).
This year we also released new employee and manager guidance on how to manage acquired respiratory infections, including COVID-19. As part of this process, COVID-19 leave is now managed as part of our standard sick leave entitlements.
Bargaining
Seven months of bargaining throughout the 2023/24 year on the Public Services Association (PSA) and APEX Collective Employment Agreements concluded in July, when settlements were finally reached (read more on page 84).
Holidays Act Remediation
Following a comprehensive review of our payroll systems and processes in 2022/23, we rolled out a subsequent remediation phase in 2023/24. This year, following the July settlements reached with APEX and the PSA, we recalculated and made remediation payments to employees covered by the backdating arrangements within the two collective employment agreements for these unions (read more on page 84).
Celebrating staff
New Zealand Blood Service’s Vital Awards are presented to staff annually to acknowledge and showcase great work across three broad categories: Excellence in improvement and innovation, Service excellence, and Spirit of NZBS. Details of this year’s recipients can be found on page 86.
Gender and Ethnicity Pay Gap Action Plan
NZBS produced a report on progress against our Gender and Ethnicity Pay Gap Action Plan (read more on page 32).
Staff and Leadership Development Programmes
NZBS have promoted and supported training and development opportunities, continued to apply an impartial and merit based approach to employment and promoting staff as well as progressed workforce development activities (read more on page 32).
Mindful of the economic climate and pressures across the health sector during 2024/25, infrastructure development was constrained and limited to:
• essential works required to maintain a safe operating environment
• expanding our donor centre network to keep up with whole blood and plasma demand
• replacing and/or upgrading aging facilities and equipment to ensure they are fit-for-purpose and meet required standards.
Processing, laboratories, and head
The 71GSR redevelopment was largely paused during 2024/2025 due to budget constraints and the delay in Highbrook Stage 2 (see below). However, a small number of projects were completed, including seismic work at the rear of the site and removal of the old goods lift. Upgrades to the building’s HVAC and building management systems were also completed.
Highbrook satellite site, Auckland
Highbrook’s first stage was completed in 2024, and the site became operational in September (see page 52) following the relocation of Epsom-based Inwards Goods and Plasma Processing teams.
The facility is now handling refrigerated containerisation for the efficient shipping of source plasma to Australia for fractionation (see page 80)
Highbrook’s planned second stage, due to deliver cleanrooms and advanced cellular tissue therapies laboratories, was paused during 2024/2025 due to funding pressures. Further work on the funding requirements is planned for 2025/2026.
Work to expand the cryogenic storage area at our Christchurch site started this year to address the ongoing increase in demand for cellular therapies and stem cell storage. This complex work involves the creation of a plant area to accommodate increased HVAC equipment and provision for a second clean room in future years.
Work also started this year to ensure Auckland’s CTTB facilities can safely meet the service’s current requirements until a new facility is built (Highbrook, Stage 2).
• The new Waikato Collections Mobile storage facility on Kahikatea Drive, Hamilton, opened in January after a slight build programme delay. This new facility allows undercover storage of Collections vehicles, including the new Collections trucks.
• Our new mobile site at Henderson went live in June 2024 and has continued to grow the donor panels and collections for the Auckland area during the 24/25 year. The venue was temporarily closed in June 2025, enabling refurbishment works to occur that will bring the site up to the required standards for use as a permanent fixed centre. It will reopen in July 2025 with a new accessible bathroom, a new donor refreshment area, and the addition of two donor beds.
• The fitout of a new donor centre in Constellation Drive in the Rosedale/Albany area of Auckland’s North Shore started in June 2025 and has a projected opening date of November 2025.
• Our next donor centre in Porirua, Wellington, was approved in May 2025, and the leasing and design work is currently being finalised. Fit-out work is due to start in the first half of 2026.
Blood banks
A new location for the NZBS-operated Wellington Blood Bank was identified by Health New Zealand Te Whatu Ora and work is ongoing to prepare the site for handover. NZBS will then complete the building’s fit-out (the design phase is already well advanced). The new facility will ensure the Blood Bank is fit for purpose and provides a laboratory that meets the regulatory standards.
• Further work was completed on upgrades to the HVAC system at 71 GSR (see page 14), including the replacement of the main air handling unit (AHU), and upgrades to other hydraulic systems and controls. Work was also completed on the replacement of the HVAC system at the Manukau Donor Centre.
• The aging and unsupported security system at our Wellington site was upgraded to the new NZBS national system.
• A new safety line system was installed at our Wellington Donor Centre to improve contractor safety when servicing external plant.
• NZBS again delivered on its single enduring outcome and successfully met 100 percent of demand, 24-hours-a-day, sevendays-a-week.
• The organisation continued its work to maintain plasma self-reliance, consistent with its Plasma Strategy. This requires that enough plasma be collected to enable 85 per cent of New Zealand’s immunoglobulin product to be manufactured from locally sourced plasma. This target level is assessed as the baseline that safeguards New Zealand’s immunoglobulin product supply.
• In 2024/25, NZBS maintained its accreditation and compliance obligations with all pertinent regulatory bodies across its laboratories, manufacturing, and testing operations.
• One hundred percent testing was maintained for all blood donations.
• NZBS further developed its comprehensive, multi-disciplinary clinical governance framework. Formal meetings are conducted quarterly by each of the Patient Services, Blood, Plasma and Laboratories, and Organ and Tissue Donation subcommittees, who operate under the overarching Clinical Governance Committee. There has been a focus on developing a Clinical Risk Register and improving external stakeholder involvement in the subcommittees. Organ and Tissue Donation has introduced consumer representatives, and the other governance groups will follow.
• NZBS received Medsafe approval to remove the time-based donor deferral for all men who have had sex with another man (MSM) and move, instead, to an Individualised Donor Assessment (IDA) approach. The change is expected to enable more men who have sex with men to donate blood and plasma, when it is safe to do so. IDA is expected to be implemented in 2026.
• NZBS has begun establishing a new nucleic acid testing (NAT) pathway for Hepatitis A and Parvovirus B19, to align our testing of plasma donors with international best-practice. This work is required to enable the change to Individualised Donor Assessments (see above).
• It is anticipated that the final reviews of the Process Migration Project—entailing the roll out of Privigen NZ, Hizentra NZ, and other commercial products—will be completed in the 2025/26 year.
• The implementation of a national process and quality standards for therapeutic products to align them with other NZBS services is progressing as planned, with steady alignment of laboratory and clinical processes. Completion is expected in the 2025/26 financial year.
• Work to ensure the consistent delivery of therapeutic services throughout the country continues, providing a solid baseline for continuous improvement. A new Therapeutic Apheresis Service is being provided to Dunedin Hospital (see page 58), with further development of stem cell collection services anticipated in the next two years. Work is also under way to start providing therapeutic venesection services to Palmerston North Hospital. Increasing service provision to more hospitals reflects Health New Zealand Te Whatu Ora requirements and is progressing in parallel with the therapeutic products work.
New Zealand Blood Service remains committed to the delivery of its sustainability outcomes and goals, as detailed below (also see page 34):
Meet government-mandated decarbonisation targets.
GOAL 1
Reduce emissions without compromising operational effectiveness/capability.
Identify where emissions reductions can be made.
Begin where largest reduction can be made.
Support business units to identify initiatives that work for them.
Develop a common language.
GOAL 2
Develop a common language.
Clearly define sustainability and the importance of NZBS’s sustainability work programme .
Build knowledge and understanding of sustainability.
During the 2024/25 year the organisation has:
Embed sustainability into decision making.
GOAL 3
Embed sustainability into decision making.
Identify how and where we can improve and embed sustainable practices.
Work with our NZBS partners to leverage sustainable outcomes.
Leverage funding opportunities to transition faster where possible.
Circular economy thinking to become business as usual (BAU).
Ethically and sustainably source from our providers.
GOAL 4
Ethically and sustainability source from our providers.
Assess third party contracts to deliver value for money and support sustainability outcomes for NZBS and New Zealand.
• Continued to report to the Carbon Neutral Government Programme (CNGP) on its carbon emissions and any reduction ambitions. NZBS has captured and reported unaudited data for its sixth year, having established 2022/23 as the base year against which to monitor its reductions (with confirmation by external auditors).
• Reduced its carbon footprint by the ongoing conversion of its light vehicle fleet. Of the 40 vehicles NZBS operates nationally, 32 are classified as light vehicles. Currently, 41 percent of these light vehicles are either battery electric (BEV), plug-in hybrid electric (PHEV), or hybrid electric (HEV).
Based on CNGP guidance, NZBS has an ambition to reduce its gross emissions by 42 percent by 2030.
One of the most impactful sustainability milestones this year was the removal of dry ice from the international plasma freighting process. More than 196,000 units of plasma, equivalent to 31 shipping containers and 99 tonnes of products, have now been freighted using the new model—transport via refrigerated shipping container rather than by air.
Donor Relationship Management System
In January 2024, NZBS introduced a new Donor Relationship Management System (DRM), DRM365, to replace its DRM Touch predecessor, which was reaching end-of-life. After some initial setbacks following its introduction, this year we moved to an iterative improvement model, where regular updates and enhancements have been deployed (read more on page 70).
Self-Administered Health History system
This year our donor relationship systems were enhanced by the introduction of a new Donor Self-Administered Health History system that introduced tablets in place of paper forms (read more on pages 43, 61).
Organ Donation Referral System
This year we implemented the first phase of a new Donor Referral System (DRS) for Organ Donation New Zealand. This is a webbased management system that can be accessed and updated by those involved in the organ and tissue donation process (read more on page 70).
Vehicle rapid charging stations
NZBS endeavors to, where feasible, install vehicle rapid charging stations at new and existing donor sites. No new units were deemed feasible for installation in 2024/25.
Business Resilience: Emergency management, business continuity planning, disaster recovery
Throughout the year we undertook an audit of the systems and processes NZBS has in place to support business resilience and continued to carry out annual technical disaster recovery exercises for NZBS’s key business systems (read more on page 71).
Information management and security
Throughout the year, NZBS has continued its work to realise the Information Management Strategy introduced in 2023. This has involved ongoing collaboration with Archives New Zealand Te Rua Mahara o te Kāwanatanga to enhance our compliance with the Public Records Act, as well as educating staff and raising awareness of our information management policies and procedures.
We now have an updated, Chief Archivist-authorised Disposal Authority for the organisation that will remain in place for the next 10 years and have continued to roll out the Enterprise Content Management (ECM) system aligned to this authority. Our work in this area aims to ensure clear methods for accessing, maintaining, using, and sharing information to ensure we protect health information. Over the past year, in conjunction with industry partners, we’ve also maintained a strong focus on information security and cybersecurity across NZBS (read more in the Organisational Health and Capability section of this report, page 32).
Project Management Office and continuous improvement
Throughout the year the structure and activities of the Project Management Office (PMO) and continuous improvement functions were reviewed and enhanced to provide a platform for growth (see page 71).
Organisational KPIs and Strategic Transformation
NZBS continued to engage its Executive and Senior Leadership Teams in strategic workshops throughout the year, reaffirming the strategic direction set out in the 2023–2027 Statement of Intent. As part of our multi-year transformation programme to become a digitally enabled, insight-driven organisation, work is underway to develop an enterprise-wide set of organisational KPIs. These KPIs will underpin a simple dashboard designed to monitor organisational health and support timely, data-informed decision making. Delivery of this dashboard is planned for the 2026 calendar year, aligning with broader transformation milestones and strengthening our ability to respond to emerging trends and performance insights.
The 2024/25 financial year marked a significant improvement in NZBS’s financial performance, with a reported surplus of $16.9 million, a sharp reversal from the prior year’s deficit of $21.4 million. This turnaround was driven by a combination of stronger revenue, improved margins, and disciplined cost management. Revenue exceeded budget by $908k, with growth in fresh products and services (particularly platelet and plasma volumes), offsetting the planned exit from fractionated derived products such as Hizentra NZ to commercially purchased product. Gross margin improved by 2.9 percentage points compared with last year, reflecting a favourable shift in product mix towards higher-margin categories like the New Zealand Transplantation and Immunogenetics Laboratory’s (NZTIL) HLA antibody testing and fresh components. Production costs were significantly lower than budget, with $6.2 million in fractionation gains from improved IVIg and Biostate yields, and $2.7 million in higher recoveries from increased collection volumes. Consumables contributed a further $1.7 million in savings, largely due to deferred Nucleic Acid Testing (NAT-5) and lower AHF kit costs. Labour costs remained elevated, with $1.5 million in additional expense from backpay and leave accruals following union settlements but were partially offset by reduced contractor use and vacancy savings. Indirect costs were also favourable, with underspend in IT, consultancy, and call centre operations helping to absorb higher occupancy costs from new site openings.
Cashflow performance also rebounded significantly. NZBS generated $7.98 million in operating cash inflows for 2024/25, a marked improvement from the -$16.75 million outflow recorded in 2023/24, which was heavily impacted by pay equity and pay
parity settlements that contributed to elevated labour costs and a substantial drain on operating cash. The 2024/25 recovery was underpinned by the improved operating result, with favourable movements in trade and other payables helping to offset the cashflow impact of increased inventory holdings and receivables. Inventory build remained a pressure point, but was better managed than the prior year, which saw a $8.17 million increase. Unlike 2023/24, which relied heavily on $25.90 million in Crown equity injections and $10 million in debt repayments, FY25 was internally funded through tighter cost controls, deferred capital commitments, and modest financing activity.
• NZBS received an initial funding uplift from the Crown budget this year to support establishing the National Agency for Organ Donation. The focus has been on strengthening the delivery of clinical services by ODNZ, including work to ensure safe staffing levels and increased education provision for hospitals.
• ODNZ supported the first organ donation in the context of Assisted Death and Dying (ADD) this year. A comprehensive standard of care for ADD patients has been reviewed by the National Ethics Committee and is undergoing further rounds of stakeholder consultation.
• The ODNZ electronic Donor Referral System (Phase 1) is up and running, providing improved care, more consistent data collection and improved communication between ODNZ and Transplant Services.
• ODNZ is working closely with Health New Zealand Te Whatu Ora Quality teams to deliver a joined up biovigilance system across Organ and Tissue Donation. Further progress is anticipated in the next year.
• Organ Donation New Zealand also continues working closely with clinicians in the intensive care and transplant sectors, with a cross-sector operational working group. Organ donation education activities for hospitals continue to expand, as well as ongoing support for development pathways with hospital link teams.
• In a November 2024 survey, 95.56 percent of donors rated their experience at our donor centres as eight out of 10 or higher.
• Regional panels grew from 25 donors per 1,000 in FY24 to 28 per 1,000 in FY25, exceeding the 2023 - 2027 Statement of Intent (SOI) target of 26.3 per 1,000 by FY27.
• NZBS exceeded collection targets for whole blood, platelets and plasma in FY25, and built toward the target of 300,000 total donations by FY27. The 14 percent increase in plasma collections achieved compared to FY24 was especially pleasing. Strategies are in place to continue progress toward the FY27 SOI target of 300,000 total collections.
• NZBS continued to grow its overall donor panel (Active 24 Whole Blood and Platelets and Active 12 Plasma to 141,449, compared to 122,201 in FY24, an 11.7% increase)1
• Māori Active 12 donor panel decreased from 8,093 in FY24 to 7,485 in FY25. An action plan is being developed to lift performance in this area.
• The Youth Active 12 donor panel decreased from 11,910 in FY24 to 11,078 in FY25. Initiatives to improve performance in this area included making youth a particular focus of National Blood Donor week from June 9-15, 2025, and targeting events wellpatronised by those in the youth demographic.
1 Active 24 refers to the panel of donors who have made a donation in the previous two years (24 months). Active 12 refers to the panel of donors who have made a donation in the previous year (12 months).
• Work started between Health New Zealand Te Whatu Ora and NZBS to develop a Service Level Agreement (SLA) between the two organisations. This SLA will clarify and document how the two agencies will work together to further improve collaboration and enhance operational and financial efficiencies between the entities.
• NZBS has this year continued its efforts to strengthen the relationship with Health New Zealand Te Whatu Ora at: – the CEO and equivalent level – the procurement and finance levels, with NZBS providing monthly purchase data to Health New Zealand Te Whatu Ora – across clinical services, including at the well-established Hospital Transfusion Committee level.
• Close working relationships have been maintained with Asia Pacific Blood Network (APBN) members, with NZBS’s Chief Executive Officer the current Chair of APBN.
• NZBS has this year been part of, and contributed to, ABO’s Cost Modelling, Quality, and Sustainability working groups. ABO is an international grouping of not-for-profit blood services and provides NZBS with rich financial metric benchmarking data that is drawn from a broad international blood service grouping.
• As a member of the International Plasma Fractionators Association (IPFA), NZBS contributes to international development and research in plasma fractionation.
• As a member of the Biomedical Excellence for Safer Transfusion (BEST) Collaborative, NZBS contributes data and expertise to research programmes to support international improvements in transfusion and cellular therapy.
• NZBS contributes expertise to the European Directorate for the Quality of Medicines and Healthcare (EDQM) writing group for the ‘Blood Guide’, the most comprehensive expert-led guide to the collection, manufacture and distribution of blood and blood products.
• Also refer to the Training, research, and knowledge sharing section of this report, page 128-131.
• Provision of monthly clinical product utilisation data was delivered in a timely manner to hospitals every month throughout the 2024/25 financial year.
• The annual 2023 calendar year Haemovigilance Report was made available to all New Zealand hospitals in September 2024.
• The Clinical Oversight Programme for all blood banks not directly managed by NZBS was maintained throughout the year, with 31 clinical oversight visits to hospital blood banks.
• NZBS has worked closely with hospitals to ensure that ‘Process Migration’ of the CSL Behring plasma product portfolio, driven by manufacturing changes at CSL Behring in Melbourne, has been well communicated. The changes for patients are almost complete, and NZBS continues to actively manage and support the change, aiming for completion of the project in late 2025.
• NZBS has provided regular updates to the Transfusion Medicine Handbook, the national guideline on transfusion matters for clinical teams. It is delivered in a web-based, mobile-friendly format.
• Ongoing and productive relationships continue to be developed and maintained with other central Crown agencies and representative groups working in the broader health sector.
• Work to develop clinical governance pathways with the wider health sector has continued. This includes working closely with the Health Quality and Safety Commission towards complete delivery of the Code of Expectations (see page 76) and continued sharing of haemovigilance and quality improvement initiatives with healthcare leaders through the National Hospital Transfusion Committee meeting. We have also initiated a new Patient Blood Management National Meeting, with clinicians across the hospital sector, to drive improvements in hospital and community care. The inaugural meeting in November 2024, was well-received and attended.
New Zealand Blood Service made solid progress in the broad delivery of its strategic goals as outlined in its Statement of Intent - 1 July 2023 to 30 June 2027 (SOI).


1.1 Baseline savings
While NZBS was not assigned a specific baseline savings target by the Department of the Prime Minister and Cabinet (DPMC), the organisation delivered $8.80 million in savings against budget in the 2024/25 financial year. These savings were achieved through disciplined cost management and operational efficiencies, the establishment of a cost saving working group, and a focus on reducing the number and use of external contractors and consultants. Further detail is provided in Note 25 of the Financial Statements.
1.2 Major spending decisions
This section is not applicable to NZBS, as no major capital expenditure decisions were made during the reporting period that meet the materiality thresholds outlined in the Treasury guidance.
1.3 Government targets
In December 2023, the Government announced nine cross-agency targets to be achieved by 2030, focused on improving outcomes across health, education, law and order, employment, housing, and the environment:
1. Shorter stays in emergency departments
2. Shorter wait times for elective treatment
3. Reduced child and youth offending
4. Reduced violent crime
5. Fewer people on the Jobseeker Support Benefit
6. Increased student attendance
7. More students at expected curriculum levels
8. Fewer people in emergency housing
9. Reduced net greenhouse gas emissions
NZBS contributes meaningfully to Targets 1, 2, and 9:
1.3.1 Targets 1 and 2 — Health system efficiency
NZBS plays a critical enabling role in supporting timely emergency and elective care through the reliable provision of blood, plasma, and tissue products. Our national logistics network and clinical partnerships ensure that hospitals can deliver urgent and planned procedures without delay, directly supporting improved patient flow and reduced wait times.
1.3.2 Target 9 — Net emissions reductions
NZBS is committed to delivering on its sustainability outcomes and goals, aligned with the Carbon Neutral Government Programme (CNGP) (see page 34). Key achievements in 2024/25 include:
1.3.2.1 Reporting unaudited carbon emissions data for the sixth consecutive year, with 2022/23 established as the base year for tracking reductions.
1.3.2.2 Setting an ambition to reduce gross emissions by 42 percent by 2030, in line with CNGP guidance.
1.3.2.3 Converting 41 percent of the light vehicle fleet to electric or hybrid models.
1.3.2.4 Successfully removing dry ice from international plasma freight, enabling the shipment of over 196,000 units (99 tonnes) of plasma using a more sustainable model.
1.4 Other Government and Ministerial priorities of public interest
New Zealand Blood Service received several Letters of Expectation during the 2024/25 financial year from the Minister of Health. A consistent theme across these communications was the expectation that NZBS remain relentless in its pursuit of delivering more and better health services for New Zealanders, placing patient and consumer needs at the centre of all decisions, and prioritising services based on clinical need. New Zealand Blood Service has progressed several initiatives this year, including:
1.4.1 Establishing a Service Level Agreement with Health New Zealand Te Whatu Ora
Work was started to clarify clinical, financial, and operational arrangements between the two organisations. The
agreement aims to maximise return on investment, improve service delivery, and achieve better outcomes for all New Zealanders. The SLA is expected to be in place by late 2025.
1.4.2 Supporting a Ministry-led review of plasma self-reliance
NZBS contributed to a Ministry review of the commitment to meeting 85 percent of domestic demand for fractionated products from New Zealand-sourced plasma, including consideration of increased international sourcing. Work on this will continue into the next financial year.
1.4.3 Securing an extension of the Multi-option credit line Westpac financing facility
The facility was extended to 31 December 2028, enabling greater use of debt financing to support capital expenditure and working capital requirements.
New Zealand Blood Service’s Statement of Performance Expectations (SPE) 2024/25 sets out the organisation’s financial and nonfinancial performance expectations and a set of performance measures, with baseline figures and targets. The SPE is a statutory requirement that provides a base against which outputs can be assessed, ensuring we remain accountable to New Zealanders. The actual results of service performance (measured against the SPE’s set targets ) are included below in this Statement of Performance.
The SPE was prepared having regard to the Minister of Health’s letters of expectation for the 2024/25 financial year. In addition, Vote Health NZ, the Crown Entities Act (sections 136 and 151 (b)), and the Public Finance Act (19A and 19C), require the end of year performance information for the appropriation included in the annual report.
Who are we?
New Zealand Blood Service was established in 1998 under the New Zealand Public Health and Disability Act 2000 and is an appointed entity pursuant to section 63 of the Human Tissue Act 2008.
We are New Zealand’s sole provider of blood, blood products, and associated services, and coordinate deceased organ donation and tissue donation (eye tissue, heart valves, skin, and bone). We operate the National Heart Valve Bank and the New Zealand Bone Marrow Donor Registry and are home to Organ Donation New Zealand (ODNZ)
Why do we exist?
We play a crucial role in delivering the outputs of Health New Zealand Te Whatu Ora’s New Zealand Health Plan.
The core purpose of the New Zealand health system is to protect, promote, and improve the health and wellbeing of New Zealanders. The long-term vision for health and wellbeing is to achieve longer life expectancy and improved quality of life for all New Zealanders.
New Zealand Blood Service supports the health needs of people in New Zealand with the availability of safe and appropriate blood and tissue products and related services.
How did we perform?
New Zealand Blood Service has one reportable output class under section 149E(1)(a) of the Crown Entities Act 2004—the supply of safe and appropriate blood, blood products, and associated services. Performance is monitored through regular reporting to the Ministry of Health.
Statement of compliance
This Statement of Performance has been prepared in accordance with PBE FRS48 and is considered compliant with GAAP and Tier 1 Public Benefit Entity (PBE) standards. The Statement of Performance presented in this Annual Report covers the reporting period from 1 July 2024 to 30 June 2025.
Cost allocation policy
New Zealand Blood Service operates with a single reportable output class, which allows for straightforward allocation of both revenue and expenditure. All revenue generated by NZBS, along with its corresponding expenditures, are allocated entirely to this singular output. This approach ensures that all financial resources are directly associated with the primary operational activities of New Zealand Blood Service.
The Statement of Performance contains the performance indicators New Zealand Blood Service (NZBS) considers appropriate for monitoring its collective performance over the reporting period. Where performance indicators have either changed or are new, these will be clearly identified.
Most of the organisation’s performance indicators are objective in nature, being based on hard data outcomes provided by the organisation’s very comprehensive information systems. Subjective assessments have been applied in two indicators; 4.5 and 4.8
NZBS’s primary revenue-generating activities are the sale of blood and blood-related products and the delivery of services related to these products. The revenue associated with the core output/activities represent one overall Output Class, comprising three interrelated outputs related to:
• Donors (and patients)
• Products and services
• Demand management each of which collectively contributes to achieving the NZBS Outcome.
Provision of a safe and effective blood service for all New Zealanders through supply and delivery of:
• Fresh blood components;
• Fractionated blood products; and
• Other products and related services.
Health needs of people in New Zealand are supported by the availability of safe and appropriate blood and tissue products and related services.
Revenue of $236.72 with no price rebate declared to HNZ.
Expenses of $258.08m
Deficit of -$21.36m
Revenue of $287.38m with no price rebate declared to HNZ.
Expenses of $282.3m
Surplus of $5.06m
Revenue of $290.4m with no price rebate declared to HNZ.
Expenses of $273.5m
Surplus of $16.92m
Financial performance commentary
For 2024/25 we reported a surplus of $16.92m against a budgeted surplus of $5.06m.
Revenue from sales of goods was higher than budget by $908k, due to increased demand for fresh products and services. Fresh product revenue exceeded budget, driven by higher platelet and plasma volumes. Services activity was also above budget, reflecting elevated volumes in blood bank testing and New Zealand Transplantation and Immunogenetics Laboratory (NZTIL) Histotrac tissue type testing. These gains were partially offset by a shortfall in fractionated product sales, primarily due to the phasing out of Hizentra NZ and lower Biostate sales. While imported Hizentra and Gamunex contributed positively, this was outweighed by a decline in Privigen sales.
Expenses were less than budget by $8.80m or 3.12 percent, primarily due to a $9.0m reduction in inventory costs, driven by $6.2 million in fractionation gains from improved IVIg and Biostate yields, and $2.7 million in higher recoveries from increased collection volumes. Information technology costs were also lower by $2.9m, reflecting the deferral of operational IS projects and postponed pricing uplifts. These savings were partially offset by a $2.2m increase in personnel costs. Occupancy costs rose by $1.0m, largely due to new donor centres opened during the year, and an increase in premises reinstatement provisions for four sites in June.
The number of active donors increased from 122,201 to 141,261 reflecting an increase of 15.60 percent. From this donor pool, whole blood donations have increased by 12 percent to service a one percent increase in whole blood product demand. Plasmapheresis donations have also increased from 93,749 to 107,238, an increase of 14.4 percent— attributable to 12.45 percent more Cryoprecipitate Antihemophilic factor (AHF) plasma being shipped to our fractionator CSL Behring. Immunoglobulin (IVIg) derived product grams issued increased by eight percent, with this upward trend attributed to stock holdings and a product lifespan of three years.
1.1
Key products and services available at all times (24/7). Measure is instances when this is not achieved, and which had a negative consequence for a patient.
Note for clarification. For a failure to happen, it must have occurred within the control of the New Zealand Blood and Organ Services (NZBS) and be attributable to NZBS managed systems and processes. This measure is assessed via the NZBS Quality Management incident reporting system, where relevant issues are classified and investigated. Incidents indicating unavailability of key products or services are classified as a SAC1 incident, to note other incidents may be classified as a SAC 1 that are not linked to this measure. For this specific measure negative consequences to patient(s) are defined as potential outcomes which can cause death or harm due to the non or late supply of products or services by NZBS. This could include a delay in treatment or hospital procedures canceled. For this reporting period, no SAC1 incidents meeting this criterion were recorded.
2.1
2.2 Employee engagement Index.
Culture and Engagement Survey better than benchmark.
Not Achieved.
Our Voices engagement survey had a 76 per cent participation rate with an overall score of 7.3 out of a maximum 10.
2.3 Cultural Competency Programme
The organisation and its employees display good levels of cultural awareness in all things NZBS does.
2.4 Health and safety in the workplace
Ensuring the NZBS Health and Safety (H&S) programme is an important part of the day-to-day processes and culture of safety across the organisation.
2.5 Capital expenditure.
Capital programmes are focused on construction and refurbishment of facilities; procurement, upgrade, and implementation of information
Not Achieved
Appointment of new role deferred due to financial constraints.
Te Aka Whai Ora was disestablished.
Achieved.
The committee has held meetings twice per quarter. Health and Safety Representative to Employee ratio is 1:16.
Better than agreed benchmark.
Improvement on prior year and trending towards benchmark of 8 out of 10.
Not Achieved.
Stage 3C completed November 2023 as per key milestone for this year.
Maintain cultural competency and development across all staff.
National and Regional H&S committees all meet at least once per quarter.
The number of trained H&S representatives meets the minimum of one rep to 19 employees per Section 6 of the Health and Safety at Work Regulations 2016.
Project milestones achieved in line with project work programmes and business cases.
Not Achieved.
Employee engagement survey no longer undertaken due to cost. We have instead established Sessions with Sam (Cliffe, NZBS CEO). This is an opportunity for staff to hear directly from our CEO on NZBS’s strategic priorities and finances, and understand more about the business’s different functions in the business.
Not Achieved.
No cultural competency development training undertaken during 2024/25 due to budget restraints.
Achieved.
NZBS has five local committees and one national committee. One committee failed to meet in Q4, but had met six times over the previous three quarters NZBS has 64 Health and Safety Reps with a ratio of one rep to 14 staff. This exceeds the prescribed ration of 1:19.
Achieved.
Stage 3C was completed during the year which comprised further seismic upgrades to
2.5 solutions; and procurement and upgrade of equipment. Delivery of projects should meet planned targets to the standards agreed
Other work deferred by 12 months due to financial constraints
the 71 Great South Road site.
An amended consent was lodged and approved by Auckland City Council to reflect the work to date and the amended timeline.
Work was undertaken and completed to replace one of the building’s main Air Handling Units, and associated plant and controllers.
Further planning and budgeting are underway for the following stages, 3E, Enabling and 3E in FY26.
3.1 Raw material (Collections) inputs – based on Demand Forecasts
- Whole blood donations
- Plateletpheresis donations
- Plasmapheresis donations
3.2 Clinical Oversight Programme
All blood banks located in main hospitals (other than the six hospitals where NZBS is responsible for blood bank provision) receive NZBS Clinical Oversight visits and audit reports to enable them to meet the requirements of ISO15189 for IANZ Accreditation.
3.3 Donation testing
Donations are tested in accordance with NZBS Manufacturing Standards (as approved by Medsafe).
No product is released for issue to a patient until it completes mandatory testing or is released under the Exceptional Release protocol
3.4 Regulatory compliance - Medsafe
NZBS will maintain Medsafe licences for each of its six main sites to provide an assurance of Good Manufacturing Practices compliance. NZBS is required to maintain a licence to manufacture medicines.
Achieved
of blood banks
Achieved 100% of all donations tested
Achieved 100% GMP licensing compliance
All blood banks receive at least one NZBS Clinical Oversight visit and audit report Achieved 100% of blood banks
All blood bank sites maintain Medsafe licensing 100% of the time throughout the period
Achieved 100% GMP licensing compliance2 1 Plasmapheresis
3.5 Regulatory compliance – IANZ (International Accreditation New Zealand)
NZBS will maintain IANZ accreditation at all NZBS diagnostic laboratories and the six blood banks we operate.
IANZ is the national authority for accreditation of testing and calibration laboratories, inspection bodies, and radiology services.
3.6 Regulatory Compliance – ASHI (American Society of Histocompatibility and Immunogenetics)
NZBS will maintain ASHI accreditation 100% of the time at the NZ Transplantation and Immunogenetics Laboratory (NZTIL).
ASHI accreditation is a programme to evaluate laboratory personnel, procedures, and facilities to ensure compliance with published ASHI standards. Maintaining ASHI accreditation is a mandatory NZBS requirement.
3.7 Financial management
Assure cost efficiency and value for money management through maintenance of financial sustainability in a demand driven environment.
Achieved
100% IANZ accreditation
All diagnostic laboratories maintain IANZ accreditation 100% of the time throughout the period
Achieved
100% ASHI accreditation
New Zealand Transplantation and Immunogentics Laboratory maintains ASHI accreditation 100% of the time throughout the period
Achieved
100% IANZ accreditation
3.8 NZBS reports for Health New Zealand Te Whatu Ora
Demand reports outlining purchase volumes by key product line are provided to New Zealand Health | Te Whatu Ora hospitals to help them manage local usage and costs.
3.9 Organ Donation New Zealand (ODNZ) – Performance Monitoring
NZBS to provide an ODNZ performance monitoring report to the Ministry of Health on a quarterly basis.
Not Achieved
A worse than Budget reported deficit of $21.4m on revenues of $236.7m.
The variance is arising from the settlement of collective payments and parity. Refer Note 24 for further explanations.
No price rebate paid to Health New Zealand Te Whatu Ora.
Achieved Reports have been provided to Health New Zealand Te Whatu Ora hospitals by the tenth working day of the following month
Achieved
Performance monitoring reports were provided to the Ministry of Health on a quarterly basis.
The 2023 calendar year ODNZ Annual Report was completed in the June 24 Quarter and published in July 2024 on the ODNZ website.
Achievement of budget or better.
Budget set as a surplus of $5.064m on revenue of $287.38m.
Achieved
100% ASHI accreditation
Monthly demand reports are provided by the tenth working day of each month.
Achieved
A better than Budget reported surplus of $16.92m on revenues of $290.43m.
No price rebate paid to Health New Zealand Te Whatu Ora.
Performance monitoring reports, as agreed, to be provided to the Ministry of Health on a quarterly basis
Achieved Reports have been provided to Health New Zealand Te Whatu Ora hospitals by the tenth working day of the following month.
Achieved
Performance monitoring reports were provided to the Ministry of Health on a quarterly basis.
The 2024 calendar year ODNZ Annual Report was completed in the March 25 Quarter and published in May 2025 on the ODNZ website
3.10 New Zealand Bone Marrow Donor Registry (NZBMDR) WMDA Accreditation
The World Marrow Donor Association (WMDA) promotes product quality and global collaboration through accreditation and standardisation. This accreditation ensures that the NZ Bone Marrow Donor Registry complies with international WMDA standards and can collaborate with our international partners.
Achieved
NZBS has maintained WMDA accreditation 100% of the time at NZBMDR.
4.1 Blood donor population
NZBS maintains a donor population capable of meeting the ongoing demand for blood and blood products (active whole blood, plateletpheresis and plasmapheresis donor panels).
4.2 Blood donation - Donor satisfaction*
The measure of overall satisfaction with the quality of service is that 90 per cent of donors giving an 8 or higher score out of 10 for their experience of /satisfaction with the service.
Achieved
122,201 donors engaged to meet demand
NZBS will maintain WMDA accreditation 100% of the time at NZBMDR.
Achieved NZBMDR has maintained WMDA accreditation 100% of the time.
Achieved 95.6% satisfied
123,000 donors engaged to meet demand
To maintain greater than 90% satisfaction benchmark for donor experience.
Achieved
141,261 donors engaged to meet demand
Achieved 95.6% satisfied
*The donor satisfaction survey was conducted online using DRM365 Click Dimensions from November 7 to November 12, 2024. A total of 4430 emails were sent over the course of six days, resulting in 1,595 responses, which corresponds to a response rate of approximately 36 percent. The rationale for using this survey method was its historical effectiveness and cost efficiency. It was distributed via email to recent donors of whole blood, plasma, and platelets.
4.3 Recruit 2,900 new and reinstated Māori donors to the active donor panel (each year measure).
Achieved 3,338 donors
2,900 donors Achieved 2,938 donors
4.4 Recruit 11,000 new and reinstated donors between the ages of 16-25 (‘youth’) on the active donor panel (each year measure). Not Achieved 8,299 donors3 11,000 donors Not Achieved 7,990 donors4
4.5 Planning and communication with Health New Zealand Te Whatu Ora hospitals
NZBS will demonstrate a productive and supportive strategic relationship with Health New Zealand Te Whatu Ora at all levels - whether at hospital, regional, or national office levels, including proactively engaging with them to ensure they are fully informed on all relevant matters.
NOTE: Exact measure has changed over recent years.
We have built on established working relationships across Health New Zealand Te Whatu Ora, working at the:
• Clinical level, including with hospital transfusion committees
• Procurement level, with detailed monthly volumes and purchase information provided, and
NZBS to demonstrate a productive and supportive relationship with Health New Zealand Te Whatu Ora at all levels of the strategic partnership, underpinned by the timely and relevant provision of information over the course of the 2024/25 financial year. Measured at CEOto-CEO level.
NZBS’s CEO has had multiple communications with the CEOs of Health New Zealand Te Whatu Ora during the 2024/2025 year. These communications have been collegial and constructive leading to a successful agreement on the 2025/2026 price settings. During this financial year we have begun to develop
3 Performance against this target was significantly disrupted in previous years due to the closure of schools and universities, which have proven challenging to fully reengage. Compounding this, the youth performance target for FY2024 appears to have been set without reference to prior year performance. A more targeted process for setting output targets is needed for future years.
4 Performance against the youth donor target in FY2025 remained below expectations, continuing challenges from previous years in re-engaging schools and tertiary institutions post-disruption. While youth-focused activities were delivered at similar volumes, engagement levels remained lower than historical norms. The target also appears to have been set without reference to prior year actuals, contributing to the shortfall. NZBS is actively rebuilding youth engagement through refined outreach strategies and a more data-informed approach to target setting that better reflects current conditions.
• CEO level, including communications and interactions pertaining to annual price setting
• Excellent collaborative clinical relationships were built during the recent industrial action
• Extensive collaboration with New Zealand Health | Te Whatu Ora’s finance team on mutually important issues
a mutually beneficial Service Level Agreement with Health New Zealand Te Whatu Ora. NZBS continues to provide a full suite of purchase and volume information to HNZ as well as further fostering excellent clinical relationships.
For 4.5, subjective judgment is applied, as these factors are not easily captured by hard data but are better assessed through the insights of those involved. This approach is necessary because there is no rigid, quantifiable guidelines for managing these relationships. The ability to interpret various factors and make context-based decisions is key to maintaining a productive and supportive strategic relationship.
4.6 Haemovigilance reporting - patient safety (a calendar year-based report)
To promote risk awareness and best practice in transfusion, NZBS will publish, in any given financial year, an annual Haemovigilance Report for the nominated calendar year. It will share this information with all Health New Zealand Te Whatu Ora hospitals to help them reduce the incidence of transfusion-related adverse events.
4.7 Haemovigilance reporting –patient safety
Number of transfusion related errors made by NZBS that result in an adverse reaction in the recipient with a severity score greater than 1 and an imputability score classified as likely/ probable or certain 5
4.8 Sustainability – carbon reduction Initiative
NZBS is committed to reducing its greenhouse gas (GHG) emissions and has set a target of reducing its carbon footprint intensity by 15 percent within five years of the baseline measurement being established in accordance with rule 59b of the Government’s reduction programme.
Achieved
The 2022 calendar year Haemovigilence report was provided to Health New Zealand Te Whatu Ora hospitals in September 2023.
The 2022 report was also published on the NZBS website at that same time.
2023 Annual Haemovigilance Report to be published and provided to all Health New Zealand Te Whatu Ora hospitals within the 2024/25 financial year and made available on the NZBS website.
Achieved
The 2023 calendar year Haemovigilence report was provided to Health New Zealand Te Whatu Ora hospitals in September 2023.
The 2023 report was also published on the NZBS website at that same time.
Achieved
First year of reporting provided to the Carbon Neutral Government Programme. This reporting has supported a restated baseline against which to measure reductions toward the target reduction of 42 percent by 2030
In the 2024/25 financial year NZBS will continue to progress to a target of 21 percent reduction in GHG emissions from baseline by the 2025/26 financial year.
Achieved
The second year of reporting has provided to the Carbon Neutral Government Programme 6
Our emissions reporting has been prepared in accordance with the GHG Protocol: A Corporate Accounting and Reporting Standard, using the operational control consolidation method. Notwithstanding this, quantifying GHG emissions is subject to inherent uncertainty as scientific knowledge and methodologies are still evolving in this area, as are GHG reporting and assurance standards. We may identify further emissions sources in future and, where practicable, we will include these in future reporting.
5 As defined by the International Society of Blood Transfusion (ISBT), a severity score of 1 is defined as “The recipient may have required treatment, but lack of such would not have resulted in permanent damage or impairment of a body function.
6 NZBS initially targeted a 15% emissions reduction over five years. After aligning with the Carbon Neutral Government Programme, we adopted a 42% reduction target by 2030. As of FY25/26, NZBS has achieved a 31% reduction (pre-audit), exceeding interim goals. We continue progressing toward the 2030 target, supported by a sustainability strategy.
4.9 Sustainability – Eliminating CO2 dry ice from source plasma shipments to Australia
4.10 Sustainability – Replacing existing petrol/diesel fleet vehicles with electric/ hybrid vehicles where a conversion option exists.
Not Achieved As sea shipments commenced in August 2023 and paused in January 2024.
Not Achieved
Seven vehicles (or 16% of fleet) replaced.
Shortfall arose from a delay installing charging infrastructure at Wellington Donor Centre
To reduce CO2 dry ice used for shipments of source plasma to manufacturing facility in Australia by 80%
Replace 100% of existing petrol/diesel fleet vehicles with electric/ hybrid vehicles where a conversion option exists
Achieved NZBS no longer using CO2 dry ice in our standard shipments of plasma to CSL Behring, Melbourne.
Not Achieved
As at 30 June 2025, out of a total fleet of 40 lease vehicles, 32 were classified as “Light” (i.e. <3200kg). Thirteen, or 41 percent, were classed as either BEV/PHEV/HEV.
Under Vote Health NZ, an appropriation has been allocated to New Zealand Blood Service (NZBS) to address claims arising from historical non-compliance with the Holidays Act 2003. This appropriation, received on behalf of the Crown, is intended to provide working capital to fund NZBS in covering the costs of rectifying and remediating liabilities associated with this non-compliance.
According to Vote Health NZ, the total budget for the appropriation was $1,6237 million (2024: $572 million), which includes Health NZ. For details regarding the remaining portion of the expenditure, please refer to Health New Zealand | Te Whatu Ora’s annual report. Of this amount, NZBS received $8,516 million across the current and prior year, with $0.769 million remaining, still to be disbursed. The remaining funds are reserved as a provision for former employees. The remediation of former employees started before 31 December 2023, and NZBS is actively working on various measures to remediate former employees.
Payment for former employees whose information has been validated will continue in 2024/25
Remediate all current employees within agreed timelines Completed by 30 June 2024
in Previous Year
Establish a national portal to facilitate the identification and remediation of former employees and commence the remediation payments process by 31 December 2023 Achieved Not Achieved8 Discontinued Measure Discontinued Measure
7 Vote Health includes the full amount for HNZ and NZBS. NZBS has reported the NZBS portion only.
8 NZBS did not establish a national portal to facilitate the identification and remediation of former emloyees as the cost of developing such a system was deemed to outweigh the benefits. Records were maintained internally within the Human Resources department.
9 The remediation of former employees commenced prior to 31 December 2023 and continued through the 2024/25 financial year. NZBS continues to actively work on various measures to try and chase down remaining former employees.

“Stewardship is a proactive duty of care for something that belongs to or exists for the benefit of others, now and into the future. Being a good steward requires active planning and management of resources so that the public’s medium and long-term interests can be protected.”
– Public Service Commission, Te Kawa Mataaho
For a comprehensive picture of our organisational health and capability, this section should be read in conjunction with the information provided elsewhere in this report.
New Zealand Blood Service has a range of policies and systems in place that support our commitment to being a good employer under the Public Service Act. A good employer is deemed to be one that operates an employment policy containing provisions generally accepted as necessary for the fair and proper treatment of employees in all aspects of their employment. We also work to anticipate, actively manage and plan for our future workforce so that we can meet the challenges ahead and continue delivering to our vision and purpose.
New Zealand Blood Service employs more than 900 staff nationally across a diverse range of roles, including Qualified Donor Technicians, Medical Laboratory Scientists, Clinical Coaches, Registered and Enrolled Nurses, Transfusion Medicine Specialists, Logistics personnel, Quality experts, plus many more. More than 85 per cent of our staff are classified as frontline, meaning they occupy a role directly related to collecting or providing blood, blood products, organs and tissues. Via legislative compliance (see below) and our organisational policies and frameworks, we endeavour to provide good, safe working conditions, opportunities for individual development and progression, gender and ethnicity pay equity, and equal employment opportunities. In keeping with these themes, this year we have:
• Published our Reward Philosophy and Remuneration Policy Read more on page 84.
• Progressed and concluded our Holidays Act Remediation Project
Read more on page 84.
• Prepared to launch our new Health and Safety Management System, Noggin
Read more page 36.
• Delivered a range of wellbeing sessions for staff
Read more on page 84.
• Promoted and supported training and development
This has included: offering scholarship opportunities; developing a one-stop-shop online induction process for new staff (for launch next year); providing learning and development opportunities in line with our Learning and Development Policy; encouraging and supporting staff to maintain their professional competencies, practising certificates, and/or professional registrations as required for their roles.
• Continued to apply an impartial and merit-based approach to employing and promoting staff
• Progressed workforce development activities Examples include hosting student nurses for placements and employing new nurse graduates— work informed by our Nursing and Donor Technician Strategy, in which two of the six strategic priorities are Creating a workforce ready for the future and Recruitment and retention of nurses and donor technicians.
• Produced our latest report on progress against our Gender and Ethnicity Pay Gap Action Plan. This report was the second NZBS has produced as part of its commitment to Kia Toipoto, the Government’s action plan to help close gender, Māori, Pacific, and other ethnic pay gaps in public service organisations. This year’s report showed an improvement in our overall gender pay gap, which was down from 8.9 percent to 8.7 percent compared with last year. This change was significant, given New Zealand Blood Service has a predominantly female workforce; 73.8 percent are female, 25.8 percent are male, and 0.3 percent are gender diverse. The report also detailed an improvement in the gender pay gap for staff working in Corporate Support Professional roles across functions like Supply Chain, IT, HR, Quality, and Marketing—down from 6.8 percent to 3.5 percent.
New Zealand Blood Service’s infrastructure is critical to the effective delivery of healthcare in New Zealand. Recognising this, we have robust systems and processes in place to protect and maintain our assets and systems for the long-term benefit of New Zealand and New Zealanders.
New Zealand Blood Service leases all its buildings from a combination of commercial and Health New Zealand Te Whatu Ora landlords. Leasing arrangements also extend to some of the equipment we use; in our laboratories, for example, this includes our large testing systems. Leasing this equipment reduces our capex burden and is a model we intend to adopt more widely into the future.
Because of the critical nature of our infrastructure for effective healthcare delivery, we have robust processes in place to protect those assets and manage our leasing arrangements.
At the operational level, our directorates have five-yearplus equipment replacement plans, where relevant, that form the basis of our capex and procurement plans.
We also have proactive maintenance agreements in place for all operational equipment, and our assets are managed within our Quality Management System (QMS).
Our Facilities and Property teams oversee preventative maintenance programmes for key building systems (electrical, hydraulic, mechanical), and a Building Management System that monitors and manages
our key plant and facilities equipment. Our Technical Equipment and Facilities Team has this year also trialled an equipment management software and a recommendation regarding the implementation of such a system will be presented to the Executive Team in the new financial year.
At the strategic level, we have a five-year Property Plan that guides how we manage, develop, and grow our infrastructure to meet our strategic objectives and health sector needs. We also maintain and operate our facilities in ways that meet the regulatory requirements we operate under, such as those prescribed by Medsafe and IANZ.
We apply four key principles to managing our physical assets:
1. The right condition: Our facilities and property meet the minimum standard to be safe, functional, and efficient.
2. The right size: Our workspaces are sized to meet the current and growing demands of the organisation
3. The right locations: Our workspaces are in the right locations to best meet the needs of donors, patients, and our teams
4. The right partners: We partner with suppliers that are committed to build long-term, effective relationships.
New Zealand Blood Service is committed to reducing its environmental impact and lowering its carbon emissions while continuing to deliver safe and highquality products and services.
Throughout the year, we have continued refining our strategic approach to sustainability, with a four-pillar ambition to:
• Become a carbon-zero organisation
• Become a sustainability leader in the blood service sector
• Embed sustainability into the core of our business
• Create a community of staff who think and act ‘sustainability’ both at work and at home.
To achieve this ambition, NZBS has committed to five priority areas:
Carbon Neutral Government Programme
Our commitment to the Carbon Neutral Government Programme is a key part of our broader sustainability strategy. By aligning with this initiative, we are contributing to New Zealand’s target of becoming net zero by 2050.
Sustainable resource use
We strive to use materials and resources efficiently with the aim of creating a sustainable business model for zero-carbon blood services by 2050.
Ethical supply chain practices
We promote and use ethical supply chain practices where possible. We engage with suppliers approved under New Zealand Government Procurement processes to ensure there are no modern slavery or other poor employment practices in our activities.
Collaborative partnerships
We are committed to partnering with Government agencies, other organisations, and iwi, to create a truly sustainable model through systemic change.
Responsible supplier practices
We work with our suppliers to understand their commitment to ethical, responsible, and sustainable business practices, as well as their adherence to the Government’s Supplier Code of Conduct.
This year’s highlights
Eliminating dry ice from plasma freight
One of the most impactful sustainability milestones we achieved this year was the successful removal of CO2 dry ice from our international plasma freighting process (see page 80). Led by our Logistics and Supply Chain teams, the change to temperature-controlled, containerised freight is an example of the systemslevel thinking required to decarbonise NZBS. The change integrated sustainability into our core business operations, while also improving safety, reliability, and financial performance, showing what’s possible as we work towards a 42 percent reduction in gross emissions by 2030 (from a 2022/23 base year).
Reducing emissions through our light vehicle fleet
Alongside our freight improvements, we continued improving our carbon footprint through the ongoing conversion of our light vehicle fleet. Of the 40 vehicles NZBS operates nationally, 32 are classified as light vehicles. Currently, 41 percent of these are either battery electric (BEV), plug-in hybrid electric (PHEV), or hybrid electric (HEV). As vehicle leases come up for renewal, we continue to prioritise low-emission options that meet operational needs without compromising service delivery.
sustainability actions
This year we have continued to utilise video conferencing in place of face-to-face meetings wherever possible, so our level of domestic air travel is no greater than required to maintain service.
We continue to explore options to reduce the waste we send to landfill, increase our recycling practices, and work with our suppliers to reduce non-recyclable packaging.
We continue to work with our landlords, and build/ redevelopment partners, to make our existing and new buildings as energy efficient as possible.
Beyond emissions
NZBS’s sustainability approach extends beyond simply capturing our carbon emissions and reducing our carbon footprint. It takes a long-term view across our interdependent ecosystems – our workforce, our business operations, the communities we serve, and our strategic partnerships – to achieve sustainable outcomes.
DIRECT EMISSIONS AND GREENHOUSE GAS REMOVAL:
INDIRECT EMISSIONS FROM IMPORTED ENERGY:
INDIRECT EMISSIONS FROM TRANSPORTATION:
INDIRECT EMISSIONS FROM PRODUCTS USED BY NZBS:
432
600
941
TOTAL CARBON EMISSIONS ( t CO 2 e ) FOR THE 2024/25 YEAR:
This total represents a 30% reduction compared to our 2022/23 base year.
NB: These numbers are subject to change after audit by Enviro-Mark Solutions Ltd (trading as Toitū Envirocare). Any significant changes will be stated in the NZBS 2025/26 Annual Report.
2023
In December, we submitted our first carbon emissions reporting to the programme.
2030
NZBS has the ambition of reducing its gross emissions by 42 per cent from the FY22/23 base year and offsetting its remaining emissions with carbon credits to achieve net-zero emissions.
2050
NZBS has an ambition of achieving zero emissions.
New Zealand Blood Service is committed to providing and maintaining a safe and healthy environment for all employees, donors, patients, volunteers, students, contractors, and visitors at all its locations, including blood banks, laboratories, donor centres, and mobile collection sites.
We maintain a comprehensive health and safety system, with processes and resources to manage hazards and risks; information, training, and supervision; accidents and near-misses; emergency management; employee participation; and contractor procurement, induction, and management. Led by our National Health and Safety Manager, NZBS has Health and Safety Advisors for our Central and Southern and Northern and Waikato regions, plus a network of more than 60 staff nationally who
voluntarily act as Health and Safety Representatives in their respective areas. These representatives attend committee meetings every second month to raise health and safety concerns and suggest areas for improvement.
Our National Health and Safety Steering Committee meets quarterly; regional committee reports are tabled, accidents and incidents reviewed, systems’ improvements discussed, and horizon-scanning is undertaken to identify emerging or potential operational issues.
This year, we have also progressed work to introduce a new, cloud-based, health and safety system which will automate and centralise our processes and resources to streamline how we report, track and respond to health and safety issues.
Preserving our institutional knowledge and information, including by meeting the requirements of the Public Records Act 2005, is an organisational priority for New Zealand Blood Service. This means we strive to maintain information practices in accordance with our legal obligations (see below) and ensure information is recorded and can be found. We also encourage continuous knowledge sharing and succession planning for critical roles in our agencies and teams.
This year we have strengthened stewardship of our digital and information assets through targeted improvements to governance, security, systems, and knowledge-sharing. Detailed below, this work has been carried out to support long-term organisational resilience, compliance, and service delivery.
Institutional knowledge and information
• Information Management Policy
We introduced a new Information Management Policy to replace our Records Management Policy and align with the Public Records Act 2005 and Archives NZ standards. The policy recognises information and records as strategic assets and embeds Te Tiriti o Waitangi principles to promote equity and responsible stewardship of Māori data.
• Information security governance
We implemented new Information Security, Operations Security, and Employee Security policies, strengthening governance around access control, encryption, backup, and incident response. Data activity monitoring tools were introduced to enhance resilience and detect anomalies.
• Artificial intelligence and knowledge systems
We refreshed our Artificial Intelligence Policy to align with ISO/IEC 42001 and OECD principles. Microsoft Copilot was authorised as the sole AI platform for use within NZBS, and a structured onboarding process was introduced for any new tools.
• Knowledge sharing and retention
We introduced a ‘secure-where-required, otherwiseavailable-to-everyone’ model to promote accessibility and retention. Our transition to the newly issued Disposal Authority DA732 ensures ongoing alignment with the Information and Records Management Standards and Public Records Act.
• Governance and oversight
The Information Management Strategy Group (IMSG) has continued to approve and govern information management and artificial intelligence initiatives. Progress is reported quarterly to the Executive Leadership Team.
Systems and processes
• Digital systems and process improvements
Our Enterprise Content Management (ECM) system supports compliant information lifecycle management. This year we’ve introduced enhanced data movement monitoring and an AI assessment framework that aligns with Government Chief Digital Office guidance.
• Automation and digitisation Initiatives
We have made key upgrades to the Donor Relationship Management platform, Donor Referral System, and Clinical Records ECM. These initiatives reduce reliance on paper, improve data accuracy, and enhance service delivery.
• Operational and cybersecurity enhancements
We have launched a cybersecurity awareness training programme and now have increased visibility of digital technology use through enhanced detection tools, regular audits and awareness initiatives.
• Governance and compliance
We have ensured our alignment with national and international standards and legislation, including the Public Records Act 2005, Privacy Act 2020, Health Information Privacy Code, Protective Security Requirements, Health and Information Standards Framework, and Artificial Intelligence Management ISO/IEC 42001:2023.
Training and knowledge sharing
• Training and upskilling initiatives
We have delivered internal campaigns on cybersecurity and information management, with staff also attending external sector events, including DigiGov, the CyberGov Leaders’ Summit, the Health Security Information Exchange, the Health Cybersecurity Forum, and the National Artificial Intelligence and Algorithm Expert Advisory Group.
• Educational content and publications
We have published regular guidance using staff platforms to promote digital literacy and secure practices.
• Sector collaboration and knowledge exchange
As well as attending the events detailed above, we have actively collaborated with Health New Zealand Te Whatu Ora, ACC, Ministry of Justice, Ministry of Health, Archives NZ, and the Government Chief Digital Office.
• Impact monitoring
While direct impact stories are still emerging, NZBS continues to monitor feedback and engagement to assess the effectiveness of its training and knowledge-sharing initiatives.
New Zealand Blood Service works within a highly regulated environment. Over the past year, we have worked in accordance with the following legislation to deliver on our obligations not only to the people of New Zealand, but to our staff, donors, patients, and health sector partners. The list below details the primary legislation, separated into eight categories, that we are statutorily required to operate under. In some instances, we will also have obligations under secondary legislation.
• Care of Children Act 2004
• Coroners Act 2006
• End of Life Choice Act 2019
• Epidemic Preparedness Act 2006
• Health Act 1956
• Health and Disability Commissioner Act 1994, and The Code of Health and Disability Services Consumers’ Rights
• Health and Disability Services (Safety) Act 2001
• Health Practitioners’ Competence Assurance Act 2003
• New Zealand Bill of Rights Act 1990
• Gene Technology Bill
• Hazardous Substances and New Organisms Act 1996
• Human Tissue Act 2008
• Land Transport Rule: Dangerous Goods 2005 (Rule 45001/1)
• Medicines Act 1981
• Organ Donors and Related Matters Act 2019
• Climate Change Response Act 2002
• Crown Entities Act 2004
• Radiation Safety Act 2016 and Radiation Safety Regulations 2016
• World Health Organization Guidance on Regulations for the Transport of Infectious Substances 2023-2024
• Commerce Act 1986
• Contract and Commercial Law Act 2017
• Copyright Act 1994
• Resource Management Act 1991
• Accident Compensation Act 2001
• Children’s Act 2014
• Crimes (Theft by Employer) Amendment Act 2025
• Domestic Violence—Victims’ Protection Act 2018
• Employment Relations Act 2000
• Employment Relations Act Amendment Bill 2025
• Employment Relations (Triangular Employment) Amendment Act 2019
• Pae Ora (Healthy Futures) Act 2022
• Public Service Act 2020
• Financial Reporting Act 2013
• Fair Trading Act 1986
• Goods and Services Tax Act 1985
• Public Finance Act 1989
• Equal Pay Act 1972
• Holidays Act 2003
• Human Rights Act 1993
• Immigration Act 2009
• KiwiSaver Act 2006
• Land Transport Act 1998
• Minimum Wage Act 1983
• Parental Leave and Employment Protection Act 1987
• Protected Disclosures (Protection of Whistleblowers) Act 2022
• Wages Protection Act 1983
• Fire and Emergency New Zealand Act 2017
• Health and Safety at Work Act 2015
• Smokefree Environments and Regulated Products Act 1990
• Volunteers Employment Protection Act 1973
New Zealand Blood Service does not have a formal Te Reo Māori Language Plan, but endeavours to utilise te reo and promote its use where possible. This includes:
• Having a dedicated staff intranet site, Ngā rauemi, with resources including:
– Te reo māori papakupu | Māori dictionaries
– Pae pāpāho pāpori | Suggestions for social media accounts to follow to support staff to learn te reo Māori
– Pūmanawa tautono | Apps, podcasts and other Māori language tools
– Pae tukutuku | Links to websites such as reomāori. co.nz and tpk.govt.nz
– Terewīhana | Links to language learning television shows
– Pukapuka | Links to books and Māori book shops
– Tuihono | Links to free, online te reo Māori and tikana Māori courses
– Waiata | Links to songs in te reo Māori
– Links to guidance on how to create your pepeha – Karakia for use during events and meetings
– Instructions for setting up keyboards to use macrons
– Quick reference cards with te reo Māori greetings, pronunciation, and karakia. NZBS has also previously printed these cards for staff to attach to their lanyards.
• Incorporating te reo Māori in strategy documents, such as NZBS’s Nursing and Donor Technician Strategy (2024-2027) and the organisation’s annual reports.
• Producing articles and staff engagement activities annually for Te Wiki o te Reo Māori and Matariki.
• Utilising Te Reo Māori names for facilities such as meeting rooms and having both a Māori and English organisational name.
Work to develop our comprehensive new Learning Management System for staff has also progressed this year. This work has included identifying options for a joint English and reo Māori name for the platform, and the intent is for kupu Māori to also be used within the platform, which is intended for launch next financial year.
New Zealand Blood Service has no responsibility for Treaty settlement commitments, so is not required to provide an update in accordance with the He Korowai Whakamana framework.
New Zealand Blood Service acknowledges its responsibilities to maintain the capability of the public service to engage with Māori and understand Māori perspectives. NZBS does not have a formal plan and recognises it is at the ‘Te Kākano | Seeding’ stage of developing its capabilities. Nevertheless, we have aimed to address our commitments in the following ways during the year.
WHĀINGA AMORANGI PHASE ONE: EMPOWERING PEOPLE
Te Reo Māori capability
Refer to the Māori Language Planning section above.
New Zealand history and Te Tiriti o Waitangi literacy
Since 2023, New Zealand Blood Service has supported more than 100 staff to undertake cultural capability training, developing and building their understanding of New Zealand history, Te Tiriti o Waitangi, and its significance to their work at NZBS. These training sessions also traversed topics such as racial equity, institutional racism, and unconscious bias.
Employee-led initiatives
New Zealand Blood Service has a staff-led Māori Staff Advisory Group, Kakapa Manawa (meaning heartbeat or pulse), which helps to promote te reo and tikanga in the workplace. The group also acts as an informal internal consultation service (for example, when clinicians wish to establish a study that may impact donors and/or patients and whānau), and provides guidance on NZBS’s standard operating procedures and training manuals. The rōpu provides support and feedback for internal and external communications.
Engagement with Māori
New Zealand Blood Service is conscious of the importance of engaging with Māori to better understand te ao Māori perspectives, and to reflect Māori cultural beliefs and values in the delivery of our services. While much more work is needed in this area, we have initiated the following over the past year:
• Research to help us better understand what motivates Māori donors and what factors act as barriers to donation for Māori non-donors. Māori blood types are uniquely valuable and rare, making them critical for both Māori and people from a wide
range of backgrounds. However, current donation rates among Māori remain disproportionately low, and there are important cultural concepts, values, and beliefs that need to be considered. The research findings will be available early in the next financial year and will help us better understand how we can provide respectful and culturally safe services and pathways for Māori participation in our work—whether that’s as a donor, consumer representative, or in some other capacity.
• Work to establish an Organ Donation New Zealand Māori Engagement Working Group. As part of our commitment to upholding Te Tiriti o Waitangi, this group will advance ODNZ’s understanding of, and engagement with, Te ao Māori, taking guidance from Dame Naida Glavish. The group would
New Zealand Blood Service’s workforce demographics are detailed on page 87 of this report. As noted earlier ( Long Term Capability and People ), NZBS applies a merit-based approach to making appointments and promotions. We also apply fair and equitable employment practices, including not discriminating against employees based on their race, sex, ethnic origin, disability, ethical believes, age, religion, politics, marital
comprise multiple senior ODNZ and NZBS staff, Kakapa Manawa (see above) representation, and a cohort of external Māori health and wellbeing experts from across the country, representing a diverse range of fields. It’s intended that this working group help realise our stated intention of ensuring active and meaningful partnership with Māori in the governance and processes of organ and tissue donation. It is hoped that this group will be operational in the first half of 2025/26.
NB: While NZBS planned to appoint a Cultural Capability Lead at Executive level in 2023/24— including scoping up the functions and responsibilities of this role and preparing for recruitment—funding constraints meant we could not proceed to market.
status, sexual orientation, employment status, family responsibilities or union affiliation. Our organisational values, including Teamwork, Integrity and Respect, and Open Communication also underpin our approach. Our staff composition is a reflection of these values, our employment practices, and the make-up of the available pool of potential employees in the market.
DIGITAL TECHNOLOGY + INFORMATION SYSTEMS
M ANU FACTU
M ANU FACTU R IN G COMPONEN T P R OCE SSI N G
M ANU FACTU R IN G
M ANU FA CTU R IN G COMPONEN T P R OCE SSI N G
DIGITAL TECHNOLOGY + INFORMATION TE S T I N G
COMPONEN T P R OCE SSI N G
Donor Services comprises four interdependent functions: Marketing and Communications – to build awareness and understanding, and attract new donors through campaigns and media; Donor Relations – to retain existing donors and maintain a sustainable donor population through relationship development and appointment-scheduling to achieve collection targets; Administration – to welcome our donors and patients and support the wider Donor Services team; Collections – to collect whole blood, plasma, and platelets while ensuring donor health is protected and maintained.
This year saw a massive 14 percent increase in plasma collections, with 107,238 units collected compared with a disappointing 93,749 units last year. This resulted from a combination of factors, including improved collections at all our existing sites, the collections from our new Henderson site (which opened at the end of last year, FY24), the unrelenting focus on plasma by our Donor Relations and Marketing and Communications teams, and the willingness and generosity of our donors.
The improvement this year was sorely needed, with New Zealand’s plasma self-reliance (the proportion of plasma products created using New Zealand-sourced plasma) having fallen to around 74 percent from a target of 85 percent.
Whole blood collections also met demand, with a one percent increase from 123,565 units last financial year to 124,856 units this year.
Our whole blood donor panels were boosted last year by the removal of the variant Creutzfeldt–Jakob disease (vCJD), ‘mad cow’, donation restriction. This affected individuals who had lived in the UK, Republic of Ireland, or France for more than six months between 1980 and 1996. The restriction’s removal resulted in around 6,000 previously affected people becoming blood or plasma donors. This boost in blood donors enabled us to encourage those interested to switch to plasma donation, while still meeting demand for whole blood. This, in turn, led to an almost 2,500 increase in plasma donors—up from 21,904 in July 2024 to 24,319 by June 2025—a major contributor to the outstanding plasma collections achieved this year.
Lincoln Rd, Henderson, Auckland
The Henderson mobile venue, our first new five-day-aweek Auckland collection for many years, opened on June 6, 2024, at 289 Lincoln Road. The six-bed centre was well received by the local community and has collected 3,768 units of plasma and 1,205 units of whole blood this year.
Work to expand the centre to eight beds (two dedicated to whole blood collection and six to plasma collection) saw it close in mid-June. It will reopen with the additional beds in place early in the new financial year (late July 2025).
Constellation Drive, Albany, Auckland
Work to identify, lease, plan, and refurbish an additional donor centre on Auckland’s North Shore progressed this year, with a new centre scheduled to open in November 2025. Having only four donor centres for a city of 1.7 million people has made travel a barrier to blood and plasma donation for many – something this new location will help reduce. The new Constellation Drive Donor Centre will have two whole blood and six plasma beds and is expected to be well supported by North Shore donors.
Heriot Drive, Porirua, Greater Wellington
Our Hospital Rd donor centre in Wellington’s Newtown is incredibly well supported by its community—so much so that it can be challenging for donors to find a suitable, and available, time to donate. There is also significant travel required of donors coming from Porirua and the Kapiti Coast. Consequently, a new site has this year been identified and approved for development in Porirua. It’s expected the centre will be operational by May 2026.
Waikato plasma mobile
While some of our larger cities haven’t been well served with options for donating plasma, there are also many people in regional centres who would like to donate. As populations are often insufficient to support a permanent donor centre, work began this year to extend the concept of our world-first, plasma mobile—which has been operating successfully in Auckland—to the regions. We began construction of a new mobile vehicle this year, which is intended to be operational across the Waikato region from the first half of the 2026 calendar year.
Blenheim mobile collection
The return of mobile whole blood collection to Blenheim in January, after an eight-year hiatus, made front page news in the Marlborough Express. Historically, we conducted regular mobile blood collections in Blenheim, with strong and reliable donor turnouts comparable to other major South Island centres. But, after the November 2016 Kaikoura earthquake, logistical challenges forced us to halt collections there, meaning we last visited in September
2016. The January collection was a great success, with 218 units of blood collected over three days, and we plan to be back in 2025/26.
Our Manukau Donor Centre marked its 20th anniversary in December. A big thank you to the staff who organised the celebratory event, with cake, to remind our donors how special they are.
Ensuring our donors have professional, clinically safe, and enjoyable donation experiences is fundamentally important. Our national Consumer Experience Lead sits within the organisation’s Clinical Team and works collaboratively across the organisation to support engagement with donors, patients, and health partners. Growing and developing our co-design capability by involving subject matter experts in direct consumer engagement is an ongoing commitment, and this year has seen the development of a co-designed Customer Service Promise, Customer Service Standard, and set of aligned survey metrics. The co-design process involved staff and donor focus groups and aligned with our stated strategic intent that ‘donors enjoy a tailored, positive experience and leave our donor centres well, feeling valued, and looking forward to donating again’.
Before they can donate, donors are required to complete questionnaires and participate in an interview with our Collections staff. The completed forms become the individual’s record of donation, capturing their responses
to our health and lifestyle questions, and their consent to the procedure and testing. After three years of planning and preparation, this year we introduced the Self-Administered Health History (SAHH) system in three phases for donors to complete their pre-donation forms with us electronically, using tablets. The staged roll-out of this critical process went very smoothly and was positively received by donors. SAHH’s introduction will ultimately see paper forms phased out, as we transform the way we interact with donors arriving to gift us their blood, plasma, or platelets. The system will also provide the foundation for a number of further enhancements to how we interact with donors in the years ahead.
One of the factors that held back plasma collections during 2023/24 was the implementation of our new donor relationship management system, DRM365, which underpins the NZBlood Service Donor app. Some early difficulties donors experienced with the new system led to complaints and loss of donations in FY24. But extensive work to bring the system in line with donor expectations saw complaints drop markedly this year. Despite the initial setbacks, the system has also provided powerful new features that were lacking in the old system. One such feature is the ability to display available appointments only to blood donors who have a blood type required at the time, an approach that minimises collections from donors where sufficient stock is already held. The system has also allowed for more streamlined communications with our donors and improved scheduling functionality.

Highest weekly bookings total in more than a year
An important annual event for raising awareness of the lifesaving importance of blood and plasma donation, National Blood Donor Week (NBDW) took place from June 9 to 15, with World Blood Donor Day falling on June 14.
Positioning blood and plasma donation as a ‘superpower’ we all possess, a range of marketing and communications activities were undertaken that resulted in one of our most successful ever National Blood Donor Weeks.
Our website traffic surged, with web bookings up by more than 70 percent compared with the previous week, alongside a 50 percent jump in the volume of inbound
calls, a 63 percent increase in traffic to our website homepage, and a 91 percent increase in the completion of our donor eligibility quiz.
We also engaged social media influencers for the first time, resulting in almost 65,000 views across six videos created for the week (154 hours or six days total viewing time). The average engagement rate also came in at 5.42 percent, well above the industry standard of 1.23 percent for sponsored content.
Most importantly, the outcome of this combined activity was our highest weekly bookings total in more than a year.



In 2023, a rare complication of glandular fever left 22-year-old Dylan Butler-Ellis fighting for his life.
The usually fit and healthy gym-goer needed eight units each of red blood cells and plasma products just to stabilise him enough for a diagnostic scan.
Though Dylan himself has little-to-no memory of his time in the emergency department and ICU, his mum, Kim Butler, remembers every detail with startling clarity.
Dylan spent almost a week in hospital, including a night in ICU.
She credits the generosity of the 16 anonymous blood and plasma donors, along with the fast response of numerous medical professionals, for stopping her worst nightmare from becoming reality.
“Blood donors saved Dylan’s life,” says Kim. “They’re our real-life superheroes and not a day goes by that I’m not grateful.”
Twenty-four hours before winding up in the ICU, Dylan had been feeling unwell with what he thought was a second bout of strep throat; A&E staff recommended he be tested for glandular fever the next morning.
But when Dylan woke the next day, he collapsed while trying to stand and, moments later, briefly stopped breathing.
An ambulance rushed him to hospital and on the way, unbeknownst to Kim, he began vomiting blood.

Kim, who had packed an overnight bag and was 20 minutes behind the ambulance in her car, received a call from the hospital asking her to come straight to the emergency department.
“I thought it was strange at the time,” she recalls. “Then when I arrived, everyone kept saying, ‘I’m so sorry’, but I didn’t understand why until I walked into the room.”
Dylan, usually fit and strong from his work as an arborist, was lying unconscious under a heat blanket, surrounded by more than a dozen medical staff. A doctor stood at his bedside, urgently calling for more units of blood.
The cause of the bleeding was later traced to a ruptured spleen – a rare but known complication of glandular fever. Dylan would spend the night in ICU, nearly a week in hospital, and months recovering at home.
Today, you wouldn’t know that just two years ago, Dylan was fighting for his life in the ICU.
He’s back to full health and has set off on an OE with his girlfriend, Eve, but Kim will never forget how close they came to losing him – and who made the difference.
“No one wants to think that they, or someone they love, will be in the situation of needing blood to survive,” Kim says.
“When it happens, you realise just how vital that lifeline is. Dylan was in the right place at the right time – and the blood he needed was there. That’s only possible because someone else gave it.”




EACH YEAR, BLOOD AND PLASMA DONORS HELP SAVE OR IMPROVE THE LIVES OF AROUND 30,000 PEOPLE ACROSS AOTEAROA, WITHOUT EVER MEETING THEM.
New Zealand Blood Service is grateful to every single person who makes a life-changing or lifesaving blood or plasma donation. Among our lifesaving whānau are our ‘milestone donors’; those people who have donated up to 500 times or more.
Our highest donation count sits with stalwart donor Paul Tomlin, who had gifted his 712th donation with us by the end of this year!


New Zealand Blood Service is lucky to have a community of volunteers across the country who help us provide the best possible experience for donors – sometimes, just by making a mean cup of tea.
This year, we farewelled one of the best, Pauline O’Rourke, after a short battle with cancer.
Mum-of-two Pauline had supported New Zealand Blood service as both a donor and a volunteer, starting out as a donor in 2003 and going on to make more than 160 plasma donations.
It was also in 2003 that Pauline became a volunteer – an act that helped not only our donors, but Pauline too.
“I didn’t have a job at the time and thought it was a good place to start doing some meaningful work,” Pauline told us, shortly before her passing. “I’ve lived with depression which affected my ability to work and meant I’d always been on a small wage, but I treated my volunteer work at New Zealand Blood Service like a full-time job and be there at 7am in the morning.”
Pauline, who enjoyed knitting, reading, and watching TV (David Lomas Investigates was her favourite), would often work at Epsom Donor Centre in the morning, before heading out to help with mobile collections in the afternoons.
“I’d serve the teas and coffees — the most important job of the lot! — make sure donors had plenty to drink before and after their donations and, back in the day, would pre-package biscuits for the college students who were donating.”
Pauline’s contribution as a volunteer and donor continued a family legacy.
“My grandfather was a donor, my mother was a donor, and my daughter is a donor,” she says. While her diagnosis meant Pauline had to stop volunteering, she was clear that she would still have been donning her red volunteer’s apron if she could.
“It’s just the pleasure of meeting so many different people from all walks of life. Some people don’t get many people to talk to in a day, and that social connection is really important.”
The message Pauline left for those thinking of volunteering? “Go for it! It’s really interesting, you meet different people and make new friends.”
Thank you, Pauline. You are missed.


The Technical Services Directorate is responsible for manufacturing all of New Zealand’s blood, plasma, and platelet products and freezing plasma for clinical use and fractionation. It also delivers specialist services at the request of clinicians, including processing advanced cellular therapy products. Other specialist products and services supplied to Health New Zealand Te Whatu Ora include tissue banking (skin and bone banks and the National Heart Valve Bank), washed red cells, and serum eye drops. Technical Services operates New Zealand’s National Reference Laboratory, six of the country’s largest blood banks, and the New Zealand Transplantation and Immunogenetics Laboratory (NZTIL), where specialised testing and diagnostics are performed, including crossmatching organ donors and recipients. In NZBS’s Donation Accreditation laboratories, Technical Services tests every blood donation received for grouping and infectious diseases.
This year we have continued to experience growth in the demand for products and services from Health New Zealand Te Whatu Ora. This impacts our Collections and Logistics functions as well as our Technical Services’ operations across manufacturing, testing, and dispensing.
The increase in clinical demand has been particularly evident in the area of Advanced Cellular Therapies (ACT), managed by our Cellular Therapy and Tissue Banking (CTTB) team. Requests have become progressively more complex, including importing ‘unapproved products’ such as virus-specific T (VST) cells for specific patients and performing advanced procedures like cell selection for targeted therapies.


Work to expand our cryostorage (liquid nitrogen) infrastructure has also continued, in order to support the growing and diverse inventory of autologous and allogeneic products.
A major milestone this year was confirming our ongoing involvement in the Malaghan Institute’s groundbreaking cancer research. During Phase 1 of ENABLE, the Institute’s CAR T-cell therapy clinical trial, New Zealand Blood Service performed the stem cell apheresis required, collecting these cells from the patients and providing them to the trial team. A new agreement to continue delivering this service for phase two of this trial, which has now expanded from Wellington to include Auckland and Christchurch patients, was signed in February.

This year we continued our rolling replacement of essential laboratory equipment and successfully concluded three major procurement processes valued at around $3 million.
Of note, we appointed new suppliers for our liquid nitrogen freezers (used for storing cellular products) and the associated inventory system, national calibration service (for compliance testing of equipment such as our fridges and freezers), and Flow Cytometers, used by our New Zealand Transplantation and Immunogenetics Laboratory (read more, pg 81).
We also initiated Request for Proposal (RFP) processes to replace the presses used in blood component manufacturing; for phenotype testing reagents; and for the provision of quality control materials for the new Parvovirus B19 and Hepatitis A testing. We anticipate this testing will be in place in our Donation Accreditation laboratories in the first half of 2025/26.
These RFPs will conclude next financial year.
Within our New Zealand Transplant and Immunogenetics Laboratory (NZTIL) we have continued a high-paced expansion and upgrade programme. Following the development of a fiveyear ‘roadmap’, we have been investing in new equipment and improving how we manage data.
We’ve also been preparing to introduce ‘virtual crossmatching’—a significant advancement in the laboratory testing required before solid organ transplantation.
For more than 40 years, NZTIL and the New Zealand solid organ transplant programme have relied on lymphocyte crossmatching to optimise compatibility and improve transplant outcomes. This involves a laboratory assay where patient serum is incubated with donor lymphocytes (white blood cells) to identify any antibodies against proteins on the donor’s lymphocytes (donor specific antibodies). The method is time-consuming and dependent on aging equipment but thanks to technological advancements, NZTIL will be able to perform virtual crossmatching from the start of 2025/26, improving our speed of response, better supporting transplant decisions, and improving our service to Health New Zealand Te Whatu Ora and the organ transplant programmes.
In the last 12 months we have also continued transforming our two Donation Accreditation laboratories, where we test every donation for infectious diseases. We installed new Neo Iris blood grouping analysers, which increased our testing capacity in Auckland and Christchurch, and have begun preparations to replace one of our other key platforms in Donation Accreditation, the Abbott Architect analysers.
As our transformation programme continues into the next financial year, it will enable us to optimise our testing regimes and minimise our operating costs.



Following the successful completion of commissioning and qualification activities, our Highbrook facility (Stage 1) was operationalised this year. The manufacturing team now freezes and stores large volumes of plasma at this facility, in readiness for shipping to CSL Behring in Melbourne. Once with CSL, it is fractionated into a variety of life-changing and lifesaving products (see pages 11 and 80) which are then returned to New Zealand for use by patients. Highbrook is also the organisation’s national distribution hub and houses thousands of consumable products.
Design and construction of Highbrook’s second stage, providing new cleanroom facilities for Cellular Therapy and Tissue Banking (CTTB) operations, has been put on hold while funding is secured.
Learning underpins how we maintain competent and confident operational teams and supports us to meet the standards set by our regulators. Our Technical Services workforce of more than 270 staff includes registered scientists and technicians who are required to demonstrate competency in their operational tasks every year.
With New Zealand Blood Service preparing to introduce a Learning Management System (LMS) that centralises all learning on a single digital platform, we have this year mapped learning pathways for all Technical Services roles. As a result, we have more than 63 learning modules ready to move into the new system, revolutionising how staff fulfil their learning requirements.
NZBS continues to support the wider health sector in various ways. This includes providing training and support for regional blood banks (as agreed with IANZ) and accommodating Medical Laboratory Science (MLS) university students via student placements (10 this year). NZBS also contributes to sector learning and development in other ways, as detailed below.

NZBS staff hold important positions in several external organisations. We have scientists on the Asia Pacific Histocompatibility and Immunogenetics Association (APHIA) education committee and staff who, as organising committee members, have contributed to conferences that have taken place this year or will take place in 2025/26. These include the International Society for Cell and Gene Therapy (ISCT) Regional Meeting 2025, the NZIMLS Annual Scientific Meeting 2025, and APHIA 2026. This year we have also had representation on the New Zealand Institute of Medical Laboratory Scientists (NZIMLS) Council.
This year we participated in the ISCT Australia New Zealand regional meeting, enabling a number of our team to present papers and meet international experts.
NZBS also continues to be a core supporter of the National Immunohaematology Continuing Education (NICE) meeting, which brings together about 50 Scientists from across New Zealand. In addition to being on the organising committee, NZBS contributed 19 presentations to this year’s meeting and, on the eve of the gathering, also delivered a workshop to upskill blood bankers in Red Cell Serology.
For further information on our meeting presentations and journal publications this year, refer to the Training and Knowledge Sharing section of this report, page 128-131.



DIAGNOSTIC SAMPLES TESTED BY NZBS’S BLOOD BANKS & REFERENCE LABORATORY
37
HEART DONATIONS RESULTING IN 48 CARDIOVASCULAR HOMOGRAFTS BEING BANKED AND 61 IMPLANTED
5,536
REQUESTS TO NZTIL FOR DISEASE ASSOCIATION TESTING 17,493
53
DIFFERENT BLOOD COMPONENTS MANUFACTURED
768 STEM CELL UNITS ISSUED NATIONWIDE
140,000 cm 2 OF SKIN ALLOGRAPHS ISSUED
2,045,320
MANDATORY AND DISCRETIONARY TESTS ON DONATED PRODUCTS
185,480
COMPONENTS AND PRODUCT VIALS ISSUED BY NZBS BLOOD BANKS



224,488
UNITS OF PLASMA PROCESSED FOR FRACTIONATION
964
ALLOGENEIC BONE DONATIONS RECEIVED 658 ISSUED
1,418
TESTS COMPLETED BY OUR NEW ZEALAND TRANSPLANTATION AND IMMUNOGENETICS LABORATORY (NZTIL) AHEAD OF HAEMOPOIETIC STEM CELL (HPC) TRANSPLANTS
91
RED CELL GENOTYPE TESTS CARRIED OUT
646
STEM CELL DONATIONS PROCESSED INTO 932 UNITS READY FOR ISSUE
232
ANNUAL COMPETENCY REVIEWS COMPLETED BY REGISTERED MEDICAL LABORATORY SCIENTISTS AND TECHNICIANS
2,045
INDIVIDUALS COMPLETED BY NZTIL ORGAN TRANSPLANT COMPATIBILITY TESTS FOR
166,016
PRE-TRANSFUSION TEST SAMPLES RECEIVED BY NZBS’S BLOOD BANKS AND REFERENCE LABORATORY





Thirty-one-year-old Ashley Longstaff is a technology teacher and house dean at an all-girls’ school. She is also the recipient of a donated heart valve. Here, she shares her story…
When I was a toddler, I was diagnosed with a heart murmur that was soon revealed to be a severe aortic valve leak; I had surgery in 2006 to repair the valve but always knew a valve replacement would eventually be necessary.
The time came when, after 18 years of virtually no symptoms at all, I abruptly started experiencing fatigue, dizziness, and shortness of breath. It was so sudden and surreal that sometimes it feels like a dream in hindsight.
The heart valve surgery was difficult to plan. Being a teacher, I was adamant that my extended period of leave wouldn’t impact the students too much, but most of all I had to consider my partner and two-year-old son; knowing he would have a very different version of his mother for a while gave me a lot of anxiety.
After the surgery, I was very lucky to have family support to help out at home with my very active toddler, and the mundane tasks of day-to-day life.
Doing a load of washing, brushing my hair, changing my son’s nappy; they were all tasks that became near
impossible with minimal strength and a constant ache in my chest.

But six months post-surgery I felt as though I was emerging from the recovery phase. I was able to start exercising again and slowly regaining my strength.
I’ve always been active, and like to stay as fit as possible, so getting back to working out has felt like a breath of fresh air for my mental and physical health.
I’m in two minds about my donor valve. On the one hand I feel incredibly lucky to have had this opportunity. On the other, I’m mindful of the donor and their whānau, and so thankful to them.
I would love to know more about them, their occupation, their hobbies, if we had anything in common.
When talking with colleagues, students, and friends, it always blows my mind to learn how many people are affected by heart disease, or some form of transplant, or even just know someone who will eventually need a donor.
I also can’t help feeling a little unusual that my valve, that caused me years and years of appointments and testing, is all of a sudden gone, replaced with someone else’s!
Mostly though, I just feel grateful.


“I
ALSO CAN’T HELP FEELING A LITTLE UNUSUAL THAT MY VALVE, THAT CAUSED ME YEARS AND YEARS OF APPOINTMENTS AND TESTING, IS ALL OF A SUDDEN GONE, REPLACED WITH SOMEONE ELSE’S! MOSTLY THOUGH, I JUST FEEL GRATEFUL.”



The Clinical Team is a multi-disciplinary group that works to provide medical, nursing, and scientific advice and expertise within NZBS and across the New Zealand health sector. Involved in the support and delivery of all NZBS products and services, the team also comprises Organ Donation New Zealand (ODNZ) and the New Zealand Bone Marrow Donor Registry (NZBMDR). We aim to ensure clinical safety in all that we do and to improve our services through innovation and development. These are some of our highlights from the year.
The 2024/25 year has been another busy and productive one, with progress made across numerous workstreams to improve services and reduce inequities for donors and patients around the country.
In March, following close collaboration with Health New Zealand Te Whatu Ora, we launched a new Therapeutic Apheresis Service based at Dunedin Hospital. This service enables patients living in the country’s south to access a therapy (plasma exchange) offered by NZBS in only four other sites in New Zealand. It provides ward-based care for people who would previously have received treatment in the hospital’s ICU or had to travel to Christchurch. These people can now receive their treatment locally, close to their family, whānau, and support networks.

“I’M FEELING SO MUCH BETTER ALREADY. I’VE HAD RELIEF FROM ALL MY SYMPTOMS…MY SPEECH ARTICULATION IS IMPROVING, AND I DON’T HAVE BODY WEAKNESS. IT’S AMAZING, BECAUSE BEFORE THE TREATMENT I HAD
SYMPTOMS FROM THE MOMENT I WOKE UP UNTIL THE MOMENT I WENT TO SLEEP.”
— Xanthe following her first therapeutic plasma exchange treatments
In April, we also introduced a new National Therapeutic Apheresis Register. Developed by the Clinical Team, this tool enables apheresis teams around the county to capture information about the procedures they are performing. The resulting data pool facilitates better clinical governance and enables individual patient follow-up. Crucially, it also supports best practice and the ability to compare performance not only across our centres in New Zealand, but with those operating internationally.
Over the year, a range of activities were undertaken to improve monitoring, reduce the potential negative effects of blood donation, and to improve the donor experience. These included:
• A scientific literature review on fainting to inform further work to reduce the potential for this complication.
• An audit of how the practice of Applied Muscle Tension (AMT)—encouraged in donors to help reduce the likelihood of faints—is currently being used at NZBS.
• Using data visualisation software to provide timely and interactive donor adverse event information to Collections staff.
• Improving the robustness of donor adverse event data by systematically reducing clerical errors.
As well as our work to introduce Individualised Donor Assessments (see below), we have also begun an extensive update of policies and procedures to better achieve a safe, inclusive, and respectful environment for transgender and non-binary donors. This work will continue in 2025/26.
Our focus on reducing barriers to donation, enhancing safety, and making our eligibility criteria more inclusive has made strong progress over the year.
We achieved a significant milestone in early 2025 when Medsafe approved our submission to introduce Individualised Donor Assessments (IDAs) for screening potential blood and plasma donors. These evidence-based assessments will ask the same questions of every donor—irrespective of gender, sex, or sexual orientation—when assessing potential risk for sexually transmitted infections, while maintaining the highest level of blood safety.
We know that donor eligibility criteria relating to gender-based sexual activity, while necessary to ensure a safe blood supply in the past, have contributed to the stigma faced by men who have sex with men (MSM) in New Zealand. Introducing individualised risk assessments will ensure a more inclusive approach.
With Medsafe approval now in place, we began work this year to make the necessary modifications to our laboratory testing and, pending its successful completion, plan to introduce Individualised Donor Assessments in 2025/26.
These changes demonstrate our ongoing commitment to modifying our processes where it is clinically safe to do so, something that is vital to growing our lifesaving community of donors.
From the Greek language and meaning to ‘take away’, apheresis is what we do at our donor centres when we collect plasma and platelets. We draw whole blood from the donor and after separating and collecting (‘taking away’) the plasma and platelets we return the rest back to the donor.
In the case of therapeutic apheresis, we use the same method to treat patients by taking away the problematic component in their blood, or to collect lifesaving stem cells. NZBS performs seven different therapeutic apheresis procedures.
Our Clinical Governance Framework, established in 2023/24, has continued to go from strength to strength this year.
Consumer representatives have now joined each of our Clinical Governance subcommittees—Organ and Tissue; Blood Plasma and Laboratories; and Therapeutics and Patient Services which operate under the umbrella of our overarching Clinical Governance Committee, chaired by Chief Medical Officer Dr Sarah Morley.
Endorsed by NZBS’s Executive Leadership Team and Board, these committees exist to further enhance quality and safety at New Zealand Blood Service.
Throughout the year, the committees have met regularly to analyse clinical quality reports, including adverse event and consumer feedback data. These enable trends and emerging clinical risks to be identified and addressed. Clinical risks are recorded in a Clinical Risk Register for escalation and review across the organisation as required.
Our nurses and donor technicians make up around a third of our workforce and perform unique, highly specialised work across a range of settings. This year, under the leadership of NZBS’s Chief Nurse, progress has been made across two broad areas:
1. Nursing and Donor Technician Strategy implementation
Following last year’s launch of New Zealand Blood Service’s inaugural Nursing and Donor Technician Strategy (2024-2029), this year’s focus has been on bringing the strategy’s six priority areas to life.
• Embodiment of an inclusive culture
By year-end, a team had been identified to lead this workstream and activity was underway to appoint group members and engage cultural support and advice, particularly in relation to Te Tiriti o Waitangi.
• Promoting health and wellbeing of colleagues
In partnership with our Human Resources and Organisational Development colleagues, our focus this year has been on embedding wellbeing into daily practice. This has included making small changes that can deliver quick wins. For example, creating structured opportunities for staff checkins by devising a nationally consistent meeting agenda with wellbeing as a standard item. Taking advantage of this year’s International Nurses Day theme, ‘Caring for the carer’, nursing teams also explored what wellbeing means to them and what colleagues can do to support it.

• Maximising the professional contribution of nurses and donor technicians
A range of activities has taken place across the year, including running workshops to socialise the new standards of competence for nurses and scope of practice for enrolled nurses. A variety of nurse leadership opportunities have also been made available, including as members on NZBS’s Medicines and Therapeutic Management and Infection Prevention and Control Clinical Oversight Committees, and as Clinical Quality Improvement champions.
• Recruitment and retention and Creating a futureready workforce
This year we have hosted nurse students for placements in Auckland, Palmerston North, and Christchurch, and employed new graduates in Hamilton, Tauranga, and Wellington. We also received Health New Zealand Te Whatu Ora funding approval as a community health provider for new graduate registered and enrolled nurses. And, in a project that will extend into next year, we began revamping the nursing section of NZBS’s careers website.
In aligned work, we’ve turned our attention this year to activities that have the potential to help create a future-ready workforce, such as instructional and experiential design, and simulation-based training. While our nurses demonstrate skills that are uniquely human and can’t easily be replicated by machines, there is ample scope for technology and digital health tools like these to be harnessed to good effect; we plan to continue this work in 2025/26, and to deliver digital literacy workshops.
• Delivering safe, effective, and compassionate care
This Clinical Nurse Specialist-led workstream has been newly established this year. The group utilises data, such as Donor Adverse Event reporting, to inform improvements that will clinically enhance the donor experience. During the year, the
group performed a full review of haemolysis management, developed a process improvement pathway, and made Standard Operating Procedure updates.
Across the organisation, our nurses and donor technicians have this year worked on several clinical quality initiatives to reduce harm. These have included the:
• Vein Grading Programme
Following a successful pilot at our Dunedin Donor Centre in 2023/24, our Vein Grading Programme was extended to our Waikato Donor Centre for trialling in a larger setting. The initiative aims to improve the donor experience and reduce the rates of phlebotomy injury by undertaking a thorough vein assessment. The intention is to roll the programme out nationally by the end of the 2025 calendar year.
• Orange Dot Programme
By March, following its successful pilot, we had also rolled out our Orange Dot Programme nationally. Orange Dot is designed to reduce phlebotomy injury by minimising the number of times a needle is manipulated in the donor’s arm. A donor whose needle has already been repositioned once has an orange dot applied to their arm so that staff know not to manipulate the needle further.
• Infection Prevention Control Clinical Oversight Committee (IPCCOC)
Established this year, this committee supports health and safety at New Zealand Blood Service. Chaired by NZBS’s Chief Nurse, it has a multidisciplinary membership, including a microbiologist and transfusion medicine specialist. The group meets quarterly to review incidents and identify areas for improvements. It also oversees hand hygiene audits.
• Medicine and Therapeutic Management Advisory Committee (MTMAC)
Also established this year, this group supports staff who deliver donor and patient services. The committee provides guidance on safe practice and medicines management. Its three focus areas this year have included reviewing standing orders and dispensing processes and developing a method for sharing prescriptions with Health New Zealand Te Whatu Ora.
• National Hand Hygiene Programme
A new National Hand Hygiene Programme, aligned with World Health Organization (WHO) guidelines, was rolled out this year, including online education modules for staff. Initial findings already indicate improvements in compliance with hand hygiene best practice.
The Clinical Development Team has this year progressed or completed the following projects:
• The Self-Administered Health History system
As noted elsewhere in this report (see pages 2, 43, 73, 81), donors this year began completing their pre-donation health and lifestyle questionnaires using tablets rather than paper forms.
The introduction of the Self-Administered Health History (SAHH) system marked a significant advancement as it enables us to capture donors’ demographic information electronically, supporting more informed and data-driven service decisions.
SAHH also allows us to tailor questions to specific donor groups, such as plasma donors, reducing the number of unnecessary questions and streamlining the process. Looking ahead, this technology lays the foundation for a web-based version that donors can complete on their own devices, before even arriving at a donor centre.
• Fetal RHD genotyping
There has been significant progress this year towards implementing fetal RHD genotyping, a non-invasive prenatal test, with Phase 2 verification underway.
We anticipate completing the Phase 2 testing in the first half of next year. Once finalised, we will be ready to offer this test to all RhD-negative pregnant women.
This milestone represents a significant step forward in enhancing prenatal care and improving outcomes through more targeted and less invasive testing.
Fetal RHD genotyping is a non-invasive prenatal test (NIPT) that identifies the Rhesus D (RhD) blood type of a fetus by analysing cell-free fetal DNA in the mother’s blood. The test helps determine if the fetus is RhD positive or negative, which is crucial for managing any incompatibility that could result in the mother’s immune system attacking the baby’s red blood cells.
• The Donor Accreditation Testing Transformation Project
This year we launched a comprehensive programme to modernise and enhance the testing performed by our Donation Accreditation teams. The first phase involves introducing B19 (for Parvovirus B19) and HAV (for Hepatitis A virus) testing to our existing testing suite. Other key components of the programme include:
– Implementing pooling platforms to streamline sample handling and reduce costs
– Deploying new middleware to manage samples, tests, and results more efficiently
– Optimising testing protocols for plasma-forfractionation, reducing unnecessary testing
– Upgrading current serology and DNA testing platforms to improve accuracy and performance
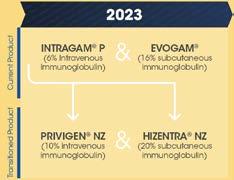
This transformation work has been designed to enhance patient safety, improve operational efficiency, and deliver cost savings.
Changes to New Zealand’s plasma products successfully managed
In 2022, our Australian plasma fractionator began work to expand its facilities and introduce new global manufacturing processes. This impacted five of our domestic plasma products, which needed to move across to the new manufacturing practices in what would be a complex, multi-year project.
The changes impacted thousands of patients and required careful collaboration with blood banks and clinicians across New Zealand.
That project is approaching completion this year, with all five products having safely transitioned to the new process.
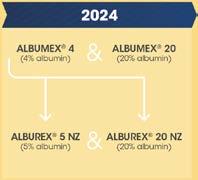
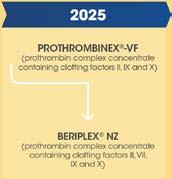

The New Zealand Bone Marrow Donor Registry (NZBMDR) team works with registries around the world to find unrelated volunteer donors for New Zealand patients requiring a stem cell transplant, and who do not have a suitable family donor. This international cooperation improves the chances of finding matches. The team also recruits volunteer donors from across New Zealand who would be willing to donate their bone marrow to a patient here or overseas.
A total of 147 searches for suitable donors were conducted during 2024/25, representing a 40 percent increase on the previous year.
While an unrelated, matched donor couldn’t be found for all patients, many were able to proceed with haploidentical transplants using family members.
Encouragingly, 75 patients received transplants from unrelated donors, a 30 percent increase compared with the previous year. This can partly be attributed to advances in therapies that allow for greater patient tolerance of mismatched donors, and that means the potential donor pool is greater, especially for patients from underrepresented ethnic backgrounds on the global registry.
In addition to donations from New Zealand donors, 70 cell products were also imported from 14 countries, including four cord blood units and four donor lymphocyte infusions for patients undergoing second transplants.
NZBMDR has continued its work over the year to recruit new donors, with a focus on engaging the 18–25-year-old age group. Since the COVID-19 pandemic, blood donation rates among this demographic have declined, which has also proven challenging for registry growth.
To address this, we’ve introduced promotional materials with QR codes that link directly to the website, allowing potential donors to request swab kits online.
There are also plans to expand the website to support full online swabbing registration, alongside existing blood donation-based recruitment.
Further work is planned to develop a marketing strategy that raises brand awareness and reaches target audiences via channels such as social media platforms.
In 2016, while attending a university blood drive, Kurt Mathes made a seemingly small choice that would later completely change someone’s life.
After filling in his blood donor form, one of the nurses noticed Kurt’s part-Samoan heritage—a crucial characteristic for the New Zealand Bone Marrow Registry (NZBMDR), which needs an ethnically diverse range of donors. They asked if he’d be happy to join the registry and he agreed, not thinking much of it at the time.
Years passed without knowing if that choice had amounted to anything.
Then, in 2022, Kurt missed a call from an unknown number that he’d considered ignoring. There was no ignoring the follow-up text that came from the New Zealand Bone Marrow Donor Registry though.
“They said I’d been identified as a potential match and asked if I’d be willing to donate,” Kurt says.
He knew what this could mean for a recipient, so saying yes was easy.
After some initial testing, however, things ground to a halt. The recipient had decided to pursue alternative treatments.
More time passed until, in 2024, Kurt again got the call, this time having saved NZBMDR’s number in his phone.
The recipient, based in the United States, had exhausted their options and was ready to proceed.
Kurt was in a busy stage of life—he’d just started a new job and had recently returned from an overseas trip—but he was eager to help. To his immense relief, the process was extremely smooth.

“The team accommodated my schedule, arranged morning appointments and transport options, and covered my expenses,” Kurt says.
The pre-donation care was intense. The injections, which stimulate stem cell production, left Kurt feeling fatigued and a bit run down.
“I was lucky that my job affords me a lot of flexibility,” he says. “And NZBMDR supported me every step of the way.”
On donation day, the process was far simpler than he’d expected. He showed up with a laptop and watched movies until the donation session was complete, interrupted only by nurses checking on his status and bringing him food.
Reflecting on the experience, Kurt says the whole process was easy and seamless.
“It’s like a well-oiled machine, but a machine with great bedside manner!”
Kurt is among a special handful of people who have been able to make this type of life-changing donation; every year, about 14 Kiwis agree to donate stem cells to others in need.
While there are currently around 13,700 people, and growing, on the New Zealand registry, NZBMDR belongs to a global network of registries who have access to more than 42 million donors worldwide.
If you’d like to join the donor registry, ask about bone marrow donation at your next blood donation. Or, scan the code (right) or visit https://www.bonemarrow.org.nz/ to get in touch directly.
WHILE THERE ARE CURRENTLY AROUND 13,700 PEOPLE, AND GROWING, ON THE NEW ZEALAND REGISTRY, NZBMDR BELONGS TO A GLOBAL NETWORK OF REGISTRIES WHO HAVE ACCESS TO MORE THAN 42 MILLION DONORS WORLDWIDE.



IF YOU’D LIKE TO JOIN THE DONOR REGISTRY, ASK ABOUT BONE MARROW DONATION AT YOUR NEXT BLOOD DONATION. OR, SCAN THE CODE BELOW OR VISIT BONEMARROW.ORG.NZ/ TO GET IN TOUCH DIRECTLY.

JOIN THE REGISTRY:
Scan the QR code above for more information about how to join the New Zealand Bone Marrow Donor Registry. You may never be called on, but you may also save a life.
The year has been one of energising growth and progress for Organ Donation New Zealand (ODNZ). We have expanded our team and capabilities, forged stronger connections across the health sector and community, and laid the foundations for a new strategic plan that will guide our work over the next five years. Collaboration has been a driving force behind our work, and we would like to acknowledge the effort and expertise of our people, and the many other professionals and organisations we’ve had the privilege of working alongside.
ODNZ extends its heartfelt gratitude to the whānau who considered donation for their loved ones at a time of immense grief, and to the donors whose gift of life, out of personal tragedy, has changed the lives of others.
This financial year, 66 people in New Zealand donated organs following their death leading to lifesaving transplants. A further 67 tissue-only donations, facilitated by the ODNZ team, meant many more people also had their lives improved.
Of the deceased organ donors, 54 donated following brain death (DBD), which is when a person has died after a severe brain injury. Another 12 donated following circulatory death (DCD), which is when their heart has stopped beating.
One DCD donation was from an individual who participated in the Assisted Dying Programme. Our ability to honour the wishes of donors in these circumstances is the culmination of extensive consultation, research, and preparation to develop a national programme for Assisted Dying Donation.
The selfless act of organ and tissue donation always begins with a conversation. Encouraging everyone in New Zealand to have a donation conversation with their whānau remained at the core of our messaging and communications this year.
Staff working at hospitals where donations are performed are central to these conversations. Their time, skill, and compassion are vital to identifying potential donation opportunities, initiating conversations around donation, and providing support to donor whānau.
A highlight was our November 30 Thank You Day when, with the support of several major media outlets, recipients shared their personal transplant stories with the public.
In May, we held our annual Donor Thanksgiving Services in Auckland and Christchurch, providing a cherished time for donor whānau, recipients, and healthcare professionals to gather and connect.
Our ODNZ Link Symposium was held on October 17 and 18, bringing together our donation link teams from across the country for two days of inspiring online sessions covering a wide range of topics.
Donor Coordinators also held multiple education days with the donation link teams, as well as wider intensive care unit and operating theatre teams across the country. These sessions provide frontline staff with the tools and knowledge to identify and facilitate organ donation opportunities.
During the past year, we’ve worked hard to lay the foundations for a new ODNZ Strategy, which will be completed and rolled out in the coming year. This has included collaborating with colleagues from across the health sector, as well as in Australia; knowledgesharing with and learning from the incredible teams at DonateLife Queensland and the Australian Organ and Tissue Authority in Canberra has been invaluable.
The strategy will take a five-year view to make the most of every opportunity to give the gift of organs and tissues after death and builds on the existing national strategy. It will provide organisational direction and actionable steps towards increasing deceased organ and tissue donation rates in New Zealand.
In the coming year, we will continue to develop our team, improve our resources, expand our education programme, and collaborate with partner organisations across the country.
We look forward to another year of helping to change and save New Zealanders’ lives thanks to the generosity of donors and their whānau. We would like to reiterate our sincere gratitude to everyone who has joined us on this remarkable journey. Thank you.
ORGANS
TISSUE DONORS BY TYPE:
NB:
Thinking about whether you would want to be a donor, or not, and having a conversation with your family or whānau, will mean that in the event of your death, they will know what to do.
To learn more about Organ Donation New Zealand, and how to have that conversation, visit the ODNZ website www.donor.co.nz

Tamar (Tami) Neuman shares the story of her daughter, Yahel.
When our daughter was born, we decided to call her Yahel, which means the halo of the light in Hebrew. A fitting name, she brought light to every place she went. She was a beautiful baby, very stubborn, and always happy.
At five years old she was diagnosed with absence seizures, a type of epilepsy that typically causes freezing instead of muscle spasms.
We were determined for her to live as normal of a life as possible, like every other kid, and that her condition would not define or limit her. By the time she turned eight, her epilepsy had all but stopped presenting and we had decided to immigrate to New Zealand, around the end of 2002.
We bought a lifestyle block in a small South Island village; a dream come true for all of us, but Yahel most of all. She got a cat, dog, lambs, chickens, plenty of room to run, and an amazing view.
Yahel was so smart. When we first arrived, she had a vocabulary of about 50 English words. Within three months she was as fluent in English as the local kids.
She thrived at school, falling in love with drawing and photography, sports, dancing, horse-riding. She loved the outdoors.
She made lots of friends who would come to visit and fill our home with the sound of laughter.
When Yahel turned 11, her epilepsy started presenting again, this time as myoclonic seizures. She was very disappointed, but it never stopped her from living the way she wanted.

As high school came to an end, she decided to take a gap year, before coming home to start working and looking toward the next thing: university. We went to an open day at Victoria University and were impressed; Yahel decided to move to the big city.
Independence was her goal, and she did it very well. She made new friends and thrived again in her new environment.
Yahel was in her last semester, finishing a bachelor’s in business and marketing, when she died. Paramedics successfully managed to resuscitate her, but it was too late. She was on life support for three days, and we decided to take her off after we learned she was brain dead. We asked if it was possible to donate her organs and the doctor said yes.
Her friends gathered with us to say goodbye. During chats with them we were able to confirm her wishes; she had told them that if something happened to her, she wanted to be a donor. So that was that.
In Judaism we have a book known as the Mishnah, which was written after the Bible. In it there is a verse: “He who saved one soul, is the saviour of a whole world”. According to the Mishnah, each one of us is a whole world of its own, and we are all contributing to a community of worlds.
For us, achieving Yahel’s wishes best represents who she was: the most inspiring, caring person, always thinking about others first, doing her best to help those in need and the less fortunate.
We farewelled our daughter with great sadness in our hearts, but also great pride. Yahel gave a second chance to five transplant recipients. Five worlds. We are very happy to know they are living their lives to the fullest.

“WE FAREWELLED OUR DAUGHTER WITH GREAT SADNESS IN OUR HEARTS, BUT ALSO GREAT PRIDE. YAHEL GAVE A SECOND CHANCE TO FIVE TRANSPLANT RECIPIENTS. FIVE WORLDS. WE ARE VERY HAPPY TO KNOW THEY ARE LIVING THEIR LIVES TO THE FULLEST.”


NZBS’s Digital Technology and Information Management team ensures that fit-for-purpose technology and information systems are in place across the organisation. This includes providing and maintaining safe, secure, and robust digital solutions to support both the organisation’s operational activities, and the staff who perform them. The team is also responsible for ensuring business resilience by coordinating systems that support emergency management business continuity planning, and disaster recovery when needed. New Zealand Blood Service’s Project Management Office and continuous improvement capabilities also reside with this directorate, ensuring projects and initiatives are appropriately prioritised, governed and delivered.
Throughout the year we worked to improve our information systems’ delivery, with a particular focus on business engagement and technical design decision-making. We made ongoing cybersecurity improvements, consolidated and standardised our collaboration and hosting platforms, and moved to a ‘rolling refresh’ approach for our user and network device maintenance.
In January 2024, NZBS introduced a new Donor Relationship Management System, DRM365, to replace its DRM Touch predecessor, which was reaching end-of-life. The organisation uses DRM365, alongside several other systems and platforms, to enable donor bookings, appointment scheduling, donor communications and engagement, donor feedback and management, and as a gateway for donors to access the NZBS website and mobile app. After some initial setbacks following the system’s introduction, this year we moved to an iterative improvement model, where regular updates and enhancements
have been deployed. The system is now proving a very valuable tool, with even greater functionality still to be realised (also see page 43).
This year, our suite of donor relationship tools were enhanced by the introduction of a new Donor Self-Administered Health History system (also see page 43). This was a major step forward in improving the donor experience, maximising the benefits of digital technologies and streamlining the donor registration process by introducing tablets in place of paper forms.
Similarly, we implemented the first phase of a new Donor Referral System (DRS) for Organ Donation New Zealand. This is a web-based management system that can be accessed and updated by those involved in the organ and tissue donation process. The system has reduced workloads, doublehandling, and manual processes, and provided consistency, standardisation, and improved reporting.

Throughout the year, we undertook an audit of the systems and processes NZBS has in place to support business resilience. This identified areas of strength as well as areas for improvement, which we are now using to inform a range of continuous improvement activities.
We continued to carry out annual technical disaster recovery exercises for NZBS’s key business systems, as well as participating in business continuity tabletop exercises, often with a cybersecurity incident focus. And we used real-time scenarios to test our emergency management procedures, processes and documentation.
Throughout the year, NZBS has continued its work to realise the Information Management Strategy introduced in 2023. This has involved ongoing collaboration with Archives New Zealand Te Rua Mahara o te Kāwanatanga to enhance our compliance with the Public Records Act, as well as educating staff and raising awareness of our information management policies and procedures.
We now have an updated, Chief Archivist-authorised Disposal Authority for the organisation that will remain in place for the next 10 years and we have continued to roll out the Enterprise Content Management (ECM) system aligned to this authority. Our work in this area aims to ensure clear methods for accessing, maintaining, using, and sharing information to ensure we protect health information. Over the past year, in conjunction with industry partners, we’ve also maintained a strong focus on information security and cybersecurity across NZBS.
Throughout the year the structure and activities of the Project Management Office and continuous improvement functions were reviewed and enhanced to provide a platform for growth. This involved:
• Improving visibility of NZBS’s project portfolio
• Introducing governance frameworks for project initiation and delivery, and
• Developing greater capability, including establishing consistent delivery frameworks.
Our digital direction has continued to evolve around six themes:
1. Donor experience
2. Employee experience
3. Patient experience
4. Connected partnerships
5. Better insights
6. Safe, secure, and considerate.
These themes support our continuous efforts to deliver excellence in meeting the needs of donors, recipients, patients, our staff, the wider healthcare community and, more broadly, the New Zealand public. At the end of the year, we refreshed this Digital Direction, maintaining its themes while homing in on four key areas we will continue working on in 2025/26:
• Sales and Operational Planning (S&OP) digitisation
• Data and Analytics - enhanced data governance and quality
• Financial Management Information System (ERP) refresh
• Identity and Access Management.


New Zealand Blood Service must comply with national legislation and international accreditation standards to ensure the safe delivery of its products and services. These standards underpin our operations and protect donors, patients, and recipients across New Zealand. NZBS has a comprehensive internal Quality Management System (QMS) and an expert Quality and Regulatory Affairs Team; Quality business partners based at the organisation’s main sites support day-to-day operations, while a team of specialists provides expertise on development work and projects. Dedicated Regulatory Affairs and Audit staff support compliance. Each Quality function helps NZBS’s operational teams to work effectively within a complex compliance environment.
During the year, external regulatory and accreditation bodies (see Figure 2) carried out 10 audits. No critical findings were reported and only one major nonconformance was raised, marking a performance improvement for the second consecutive year (Figure 1). The non-conformance is being addressed through targeted corrective and preventive actions within the
required timeframes. Notably, all other deficiencies and observations have been resolved and closed. These results reflect NZBS’s ongoing commitment to strengthening compliance, improving audit outcomes, and working collaboratively with external bodies to enhance safety and quality.
During the year NZBS was also audited by CSL Behring (CSLB), the Melbourne-based manufacturer of our tollfractionated products. The CSLB team conducted a remote audit of the new plasma manufacturing floor
MEDSAFE licenses us to manufacture medicines. They ensure we are working to good practice guidelines (GxP).
WMDA is the World Marrow Donor Association. They promote global collaboration and best practices in Cellular Therapies.
ASHI is the American Society for Histocompatability and Immunogentics. It is an International society that sets standards for our Histocompatibility and Immunogenetics laboratory, NZTIL.
at our Highbrook facility, identifying three minor nonconformances and one recommendation. All findings were addressed and closed.
During 2024/25, the Quality and Regulatory Affairs (QRA) team conducted 30 internal audits, 24 hospital audits, and two supplier audits, providing valuable insights and identifying potential areas of risk across the organisation.
This year QRA colleagues supported operational teams to manage more than 2,000 internal quality incidents, helping to identify trends and implement robust corrective and preventive actions to reduce recurrence. Of these incidents, 57 were escalated to the senior leadership team for further oversight.
The Regulatory Affairs and Audit Team supported 15 regulatory submissions to Medsafe, enabling practice changes and driving safety and compliance improvements across NZBS. These submissions covered a range of areas, including updates to consumables used in blood product manufacturing and NZBS site development or refurbishment. In addition, the team reviewed 66 supplier change notifications and 97 internal change control records. These reviews helped identify potential impacts on product quality and operational processes, allowing NZBS to proactively manage risks, update documentation, and implement the necessary controls to maintain compliance and ensure continuity of supply.
IANZ certifies our laboratories such as our Blood Banks, NZTIL and Reference Laboratory to ensure they are working to international laboratory standards
FACT is an International accreditation body that sets standards for cellular therapy products such as stem cells. They ensure we collect, test, process and store Stem Cells to international best practice standards.
QRA team has continued its focus on increasing visibility, fostering collaboration, and supporting all staff to adopt a “Quality is my business” mindset. Over the past 12 months, the team has advanced the QRA Strategy, designed to build capability and deepen understanding across NZBS. Key initiatives included:
• Deploying online training: Moving key training modules such as Q-Pulse Essentials and Managing Equipment Activities to online platforms, improving accessibility and consistency across regions.
• Process and procedure reboot: Launching Phase 1 of the Incident Management Project to enhance how incidents are documented, investigated, and managed. Improvements were also made to quality risk management processes.
• Regulatory alignment: Working closely with regulators and accreditation bodies to align NZBS practices with updated standards and legislation, including ISO 15189:2022 and the proposed Medical Products Bill.
• Quality Management System planning: Started planning for a new electronic Quality Management System by developing a user requirement specification through stakeholder engagement and international collaboration.
INSPIRE A QUALITY CULTURE
COLLABORATE & SUPPORT FUTURE GROWTH
At NZBS, we take our role as kaitiaki of sensitive personal information—entrusted to us to deliver essential services to New Zealanders—very seriously. This information is treated as a taonga; all staff and contractors are expected to handle it with care and respect, in line with the principles of the Privacy Act 2020 and the Health Information Privacy Code 2020. Our policies and processes are designed to uphold these standards and foster a strong culture of privacy and accountability.
In early 2025, NZBS reviewed the Office of the Privacy Commissioner’s (OPC) draft guidance on the Biometric Processing Privacy Code to understand its potential impact on our operations. We also utilised the OPC’s tool Poupou Matatapu: Doing Privacy Well, which helped shape a targeted privacy work programme focused on continuous improvement. This confirmed that NZBS has
ASSURE SAFE PRODUCTS & SERVICES
well-established privacy practices, while also identifying areas for enhancement—work that will be progressed during 2025/26.
As part of our ongoing commitment to privacy compliance, NZBS completed 22 Privacy Impact Assessments (PIAs) in support of internal policy and system changes.
We also managed 20 internal privacy incidents. Each was reviewed by the NZBS Privacy Officer and assessed to determine whether reporting to the Office of the Privacy Commissioner was required. All incidents were handled in accordance with the Privacy Act 2020. Notably, there were no privacy breaches during this reporting period that required external notification.


The Code of Expectations1 for health entities’ engagement with consumers and whānau (‘the code’) was introduced in July 2022 and is required by the Pae Ora (Healthy Futures) Act 2022. The code sets the expectations for how health entities must work with consumers, whānau and communities in the planning, design, delivery, and evaluation of health services.
All health entities must act in accordance with the code and are required to report against a Quality and Safety Marker (QSM) on a six-monthly basis. The QSM is a framework to measure what successful consumer, whānau, and community engagement looks like; it has three domains and four self-ratings scores:
The environment created to support community engagement.
Responding to and acting on what consumers are saying about the service and having the right information at the right time for consumers accessing services.
The systems in place to capture consumer experience, and act upon the results.
1 = Minimal 2 = Consultation 3 = Involvement 4 = Partnership and shared leadership
NEW ZEALAND BLOOD SERVICE RATINGS as at March 2025
Established in 2023, New Zealand Blood Service’s Consumer Experience Lead role aligns with Section 7 of the Pae Ora (Healthy Futures) Act 2022 and delivers to The Code of Expectations for Whānau and Consumer Engagement.
This national role sits within NZBS’s Clinical Team, works collaboratively across the organisation to support engagement with donors, patients, and health partners, and involves subject matter experts in direct consumer engagement to grow and develop co-design capability internally.
The Consumer Experience Lead sits on all NZBS Clinical Governance committees (see page 78) and the National Consumer Voice Reference Group, led by the Health Safety and Quality Commission.
Under the leadership of the Consumer Experience Lead, work has this year progressed across three strategic priority areas:
1 The Code of Expectations does not replace the Code of Health and Disability Services Consumers’
1. Consumer interface
• Two consumer representatives have been identified and appointed to each of NZBS’s three Clinical Governance subcommittees (see page 60), representing a new era of customer focus for the organisation. An induction process to ensure the consumers’ safe introduction to the groups was developed before their appointments.
“NZBS HAS DONE SO MUCH TO HELP ME WITH MY CONDITION AND AS A RESULT I HAVE A QUALITY OF LIFE.
I’M VERY HAPPY AND HONOURED TO BE INVOLVED AND BE ABLE TO GIVE BACK TO SUCH AN IMPORTANT ORGANISATION.”
• A range of resources have been developed to support consumer engagement activities, including an intranet site with resources to support staff wishing to create and maximise consumer engagement opportunities.
• Consumer focus groups have been held to inform new projects and service development.
“THIS CONVERSATION WITH PATIENTS HAS BEEN A HIGHLIGHT OF MY CAREER, I’M SO DELIGHTED THAT WE HAVE THIS OPPORTUNITY TO DEVELOP A SERVICE FROM SCRATCH AND THAT WE ARE DOING THAT IN PARTNERSHIP WITH THE PATIENTS.”
— Senior nurse, NZBS
• New service evaluation surveys have been created for our laboratories and patient services, and we’ve been measuring users’ experience of our new electronic donor screening questionnaire, SAHH (see page 43, 61), to inform its next phases of development.
2. Feedback mechanisms
• We have improved and streamlined our feedback management processes.
• We are now reporting feedback data through the Clinical Governance committees to the Executive Team and the NZBS Board, and
• We are actively using feedback to inform quality improvement initiatives.
3. Professional development
We have begun work to develop a suite of Customer Service and Quality Conversations training resources for our public-facing teams (see over).
DONORS ENJOY A TAILORED, POSITIVE EXPERIENCE AND LEAVE OUR DONOR CENTRES WELL, FEELING VALUED, AND LOOKING FORWARD TO DONATING AGAIN.
This year we co-designed a Customer Service Promise, Standard, and set of aligned survey metrics, with the co-design process for this work involving both staff and donor focus groups.
As noted above, we also began developing the customer service training that will support our teams to consistently deliver to the Standard and Promise. We plan to deliver this towards the middle of the next financial year, and it will also then be embedded into the orientation process for new staff joining the organisation. It will be the first in a series of training modules we’ll be deploying to support quality conversations and great experiences for our donors. Further modules will traverse constructive conversations, sensitive conversations, and mastering donor deferral conversations in our unique environment. Our teams have been involved in the training’s development from the outset and are looking forward to the learning opportunity.
Bringing staff, clinicians, consumers, and the healthcare sector together
Last year, we introduced a new Clinical Governance Framework at NZBS, comprising an overarching Clinical Governance Committee, chaired by NZBS’s Chief Medical Officer, and three sub-committees (Organ and Tissue; Blood Plasma and Laboratories; Therapeutics and Patient Services). The primary objective of the framework is to improve clinical quality and patient and consumer safety.
This year, that framework has grown and strengthened. At regular meetings throughout the year, the subcommittees have analysed clinical quality reports, including adverse events and consumer feedback. This report data reveals trends and themes that can make it easier to identify and address emerging clinical risks early. These are logged in a risk register for escalation and review as appropriate; the subcommittees report into the overarching Clinical


Governance Committee, which in turn reports into the Executive Team and the NZBS Board.
As noted above, consumers are now represented on each of the three subcommittees, and we’ve also achieved a greater level of connection with the wider healthcare sector; our overarching Clinical Governance Committee distils the outputs of our subcommittees, taking that information to Health New Zealand Te Whatu Ora’s Clinical Governance and National Clinical Quality forums. By working together, we can reduce avoidable harm and side effects for patients receiving our products or services.
The Planning and Supply Chain Directorate provides a nationally integrated planning, supply chain, and logistics solution to enable the safe delivery of the right blood products and services, to the right places, at the right times. It also leads organisational procurement, and facilities and property functions.
Continuing the intent of last year’s work, FY25 has seen the ongoing upgrade of existing equipment and facilities, alongside the introduction of new infrastructure. As NZBS progresses through the most transformative period in its history, this year’s activity has helped advance the organisation’s three strategic goals1 to ensure New Zealanders receive safe, quality, products and services now and into the future.
Significant savings as move from air to sea freight fully realised
This year we completed our transition from using air freight to containerised sea freight for transporting plasma to Australia for fractionation.
The phased change began in Christchurch in August 2023, when we started shipping containerised plasma from the South Island’s largest port, Lyttleton.
Then, in December this year, we despatched our first, 20-foot, shipping container of plasma from our Highbrook facility in Auckland.
During the year, more than 230,200 units of plasma have been transported to Melbourne (departing from both Lyttleton and Auckland), via 31 flights and 22, temperature-controlled (-20 °C),

shipping containers. This equates to around 115 tonnes of plasma.
Our last air-freight delivery from Christchurch was despatched in January, and from Auckland in October 2
The move away from air freight has made the process both more economical and more environmentally sustainable. Freighting by air used to occur weekly and required dry ice and polystyrene to maintain the plasma at a temperature of -20°C during transit.
Using sea freight and refrigerated containers, shipping can occur every three to four weeks (plasma can be frozen for up to two years) without the need for dry ice or polystyrene. It also uses considerably less fuel per unit of cargo than air freight.
This year, around 70 tonnes of dry ice have been removed from the process and more than $825,000 saved by NZBS, from removing both the dry ice and the packaging required to freight by air.


Supporting operational effectiveness through prudent procurement
This year we have also gone to market for new or upgraded systems and equipment to futureproof and streamline our processes, and to support the safe and effective delivery of our products and services. This has involved:
• Completing the procurement phase for, and selecting, a new Supplier Management System (SMS) that will enable us to better oversee supplier lifecycles, supplier performance, and value for money. An implementation discovery phase has begun, and an implementation plan will be finalised and deployed in the new financial year.
• Ordering three new high-efficiency liquid nitrogen freezers to increase our stem cell storage capacity in Auckland (one freezer) and Christchurch (two freezers). These large freezers can each accommodate 300 stem cells and use less energy and, crucially, less liquid nitrogen than the existing freezers. A fourth new freezer is destined for Wellington to replace a smaller capacity freezer currently in place there. All four freezers will be installed in the new financial year.

• Taking delivery of 112 digital tablets, and ordering a further 20, to support the rollout of the new SelfAdministered Health History (SAHH) system for donors (see pg 43, 61).
• Acquiring 20 new Macopress Smarter machines, used for separating blood components. This new machine had begun validation by year-end, and the 20 new units will replace the existing Macopress Smart machines in our processing laboratories; these have now reached end-of-life.
• Purchasing two new Flow Cytometers, equipment that is critical to detecting antibodies and evaluating the compatibility of transplant donors and recipients.
PLASMA-FORFRACTIONATION FREIGHTED BY AIR AND SEA:
230,200 UNITS OR 115 TONNES
VOLUME OF DRY ICE REMOVED BY CHANGING TO SEA FREIGHT FROM AIR FREIGHT: 70 TONNES
NUMBER OF CONSUMABLE ITEMS DELIVERED BY OUR LOGISTICS TEAMS: ~ 930,000
NUMBER OF COMPONENTS DELIVERED TO BLOOD BANKS, LABORATORIES, AND HOSPITALS: ~ 600,000
NUMBER OF WAREHOUSES MANAGED: 5
COST SAVING FROM SHIFTING FROM AIR FREIGHT TO SEA FREIGHT:
> $ 825,000
• Sourcing a new supplier for the liquid phenotyping reagents used for testing by blood banks and our National Reference Laboratory. The change, a result of our standard retender process, resulted in around $60,000 in savings. We also changed to a new calibration service provider for our lab equipment this year—again, a result of our retendering process.
• Going to market for External Quality Control (EQC) materials that will be used to ‘test the testing’ when we introduce Nucleic Acid Tests (NAT) for Parvovirus and Hepatitis A (B19/ HAV) in FY26 (see pg 62).
• Beginning the process of reviewing our biscuit supplier for stocking post-donation donor refreshment areas. We will be sourcing direct from the manufacturer to secure bulkbuying pricing.
Ensuring fit-for-purpose facilities and infrastructure
Palmerston North
This year we completed a refurbishment of our Palmerston North site, ensuring it offers a clean, fit-for-purpose space that meets Good Manufacturing Practice (GMP) standards.
Refurbishment of the site, which had not been upgraded for around two decades, delivered a revamped donor refreshment area, an improved blood consignment room, new floor coverings and desk furniture in the reception area, and fresh paint. The new consignment area where donations are packed and processed for shipment now meets the latest regulatory requirements.
Epsom
Redevelopment of our Epsom site (71 Great South Road, Auckland) has been a multi-year project that started in 2018 3. The programme of work has been extensive and complex but, recognising budget limitations, was largely paused this year except for some essential work such as seismic strengthening and the replacement of an air handling unit that had reached end-of-life. Planning for the project’s next stages, in FY26, has nevertheless continued through the year, but further progress will be subject to budget constraints.
Our Wellington Blood Bank has long been a concern due to its size, condition and suitability, particularly from an accreditation perspective. This year, Health New Zealand Te Whatu Ora identified and approved a new site for an upgraded facility, and we subsequently finalised a design for the new location. Fit-out work is anticipated to start in the next financial year, following handover of the space by Health New Zealand Te Whatu Ora.
The success of our Henderson mobile venue since its June 2024 opening resulted in a decision this year to make that site a permanent donor centre (also see pg 42). We began work in May to support that change, which entails installing an accessible toilet, upgraded heating, ventilation, and air conditioning (HVAC), and ensuring the facility’s storeroom is GMP-compliant. We anticipate this work will be complete early in the first quarter of the new financial year.
We also secured two new donor centre sites in FY25, in Porirua and on Auckland’s North Shore, to support the increased collection volumes required to meet clinical demand, particularly for plasma (see pg 42). By the end of the year, we had begun the fit-out of the new Constellation Drive Donor Centre, with fit-out of the Porirua Donor Centre scheduled to start in the new financial year. The establishment of further new donor centres is subject to budget constraints and approval of NZBS’s Plasma Collection Strategy.
In the south, we began work in June to redevelop the cryogenic storage area at our main Christchurch site in Lester Lane. With current storage at capacity, this redevelopment is essential to meet the growing clinical demand for stem cell storage. Once complete, the facility will have two new extra-large, high-efficiency cryogenic freezers (see page 79), relocated and increased HVAC capability to support the plant’s operation, and a reconfigured laboratory space.
BEFORE AND AFTER: Refurbishment of the Palmerston North Donor Centre (below and right) site has created a brighter, fitfor-purpose space for donors and staff and, most importantly, ensured the facility meets GMP standards.







The Human Resources and Organisational Development Directorate provides strategic people leadership to enable a high-performing culture among staff in support of NZBS’s single enduring outcome1
Throughout the year, we have focused on four key areas amid our business-as-usual activity:
• Supporting wellbeing
• Collective Employment Agreement bargaining
• Reward and remuneration, and
• Recognising and celebrating staff
We have delivered various sessions throughout the year to support staff wellbeing. These have included: mental health awareness, including how to support colleagues and learn techniques that enable safe conversations; employee-led health conversations on topics such as menopause; understanding and celebrating neurodiversity; and financial planning, including sessions on improving financial capability and preparing for retirement. The intent of these sessions is to foster a positive employee experience by supporting wellbeing both at work and at home.
This year we also released new employee and manager guidance on how to manage acquired respiratory infections, including COVID-19. As part of this process, COVID-19 leave is now managed as part of our standard sick leave entitlements.
Seven months of bargaining throughout the 2023/24 year on the Public Services Association (PSA) and APEX Collective Employment Agreements concluded in July, when settlements were finally reached. This year we have also continued to monitor the progress of Health New Zealand Te Whatu Ora bargaining with the Association of Salaried Medical Specialists (ASMS) and the New Zealand Nurses Organisation (NZNO), noting that our collective agreements with these unions expired on 31 August 2024 and 11 March 2025 respectively.
New Zealand’s Holidays Act 2003 sets out how payment for leave and final pay is to be calculated and paid to staff. However, the Act has historically proven complex for some employers to interpret and, as a result, some entitlements for annual leave, bereavement and sick leave, public holidays, and alternative holidays (including lieu days) were
incorrectly calculated. Following a comprehensive review of our payroll systems and processes in 2022/23, we rolled out a subsequent remediation phase in 2023/24. This year, following the July settlements reached with APEX and the PSA, we recalculated and made remediation payments to employees covered by the backdating arrangements within the two collective employment agreements for these unions.
Of the $3,287,385 owed to former employees, $559,565 remains to be paid. Work to locate former employees was carried out as part of our Holidays Act Remediation Project. With that project having now concluded, any payments are now actioned, as they are identified, via our business-as-usual processes.
New Zealand Blood Service’s Reward Philosophy and Remuneration Policy were both published for the first time this year.
The Reward Philosophy has seven key principles that guide how we attract, retain, and reward all staff at NZBS. It emphasises fair, competitive, and equitable remuneration that supports our people’s career development and progression.
The Remuneration Policy, on the other hand, applies to the approximately 25 percent of NZBS staff who are on Individual Employment Agreements (IEAs). For these staff, the Policy clearly explains the factors that influence our reward and remuneration decisions. These include career bands, pay ranges, how we evaluate or size jobs, how we position ourselves against the market, and how we manage promotions and annual salary reviews; it’s detailed and designed to make things as transparent as possible for staff.
The organisation’s Executive Directors held meetings with their teams and with individual employees on IEAs to share the policy. Supporting resources were also distributed and shared on the staff intranet, upholding our commitment to transparency about how IEA staff are remunerated.
The development of the Philosophy and Policy was also a response to the findings of our recent NZBS staff surveys, which showed some staff were unclear about how their pay is decided.
This work is ongoing and, next year, NZBS will publish all pay bands and information about job grades.
1New Zealanders health needs are supported by the availability of safe and appropriate blood and tissue products and related services.








New Zealand Blood Service’s Vital Awards are presented annually to acknowledge and showcase great work at across three broad categories: Excellence in improvement and innovation, Service excellence, and Spirit of NZBS.
EXCELLENCE IN IMPROVEMENT AND INNOVATION
This award recognises individuals or teams who show leadership, enthusiasm and commitment to creating a culture of positive change, innovation, and/or sustainable improvement at NZBS.
WINNER
Sue Haines, Medical Laboratory Technician, Christchurch
Sue was recognised for being an outstanding role model and leader in adopting change. She is the lead user for many of the Donation Accreditation analysers used in our laboratories and is the subject matter expert for many processes and procedures. She has played an essential role in numerous lab-based change projects in her more than 46 years with New Zealand Blood Service, including being central to the selection, installation, validation, and implementation of the Neo Iris blood group analysers last financial year. She consistently upholds NZBS’s ‘safety is our cornerstone’ philosophy.
COMMENDED
• Jaen Cabias, Qualified Donor Technician, Christchurch
• Morwenna Glenie, Learning and Development Manager, Technical Services, Auckland SERVICE EXCELLENCE
For individuals or teams who promote a philosophy of service excellence by demonstrating an unwavering commitment to the communities we serve both in and outside our organisation.
The Quality Team was recognised for their significant contribution to the ISBT128 project, which saw the rollout of the global standard for labelling and identifying medical products of human origin at NZBS. The team had to update hundreds of organisational documents, including almost 600 Standard Operating Procedures, within challenging timeframes, and did so in a consistently helpful and friendly manner.
The HR and Payroll Team:
• Pat Lim, HR and Payroll Administrator, Auckland;
• Colin Skelton, HR Centralised Services Manager, Auckland;
• Chiung-Yi Huang, HR and Payroll Administrator, Auckland;
• Helen Sarabojee, HR and Payroll Administrator, Auckland;
• Georgina Blackmore, HR and Payroll Administrator, Auckland
• Jamie Nicholson, Senior Blood Management System Support Analyst (IS), Wellington
• Craig Smith, Technical Architect (IS), Auckland
• Kamelot Venus, Clinical Nurse Leader, Donor and Product Safety, Auckland
For individuals or teams who cultivate a community spirit, consistently make a social difference and help shape our work environment for the good of our organisation and our people.
WINNER



WINNER
The Quality Team:





• Tanvi Karnik, Quality Systems Manager, Christchurch;
• Meagen Thompson, Quality Systems Officer, Christchurch;
• Hannah Capon, Quality Systems Officer, Christchurch;
• Alexandra Dean, Document Control Officer, Hamilton;
• Teresa Awan, Quality Systems Officer, Christchurch;
• Kate Dellow, Quality Systems Officer, Christchurch;
• Sandra Jacobs, Quality Systems Officer, Christchurch
Mabel Nyashanu, Medical Laboratory Scientist, Hamilton
Mabel embodies the Spirit of NZBS and was recognised for consistently supporting her colleagues to better themselves and aim high and prioritising the welfare of others. She has become the ‘go to’ person for any issues at the Tissue Bank and regularly puts forward ideas for improving the service. She has trained many students at Waikato Blood Bank and is a patient and thorough teacher who goes the extra mile to ensure students leave well-prepared to progress their learning.
• Sue Nix, Donor Relations Coordinator, Hamilton
• Ashleigh Parata, Administration Assistant, Dunedin
Fiona is an independent consultant and director with more than 40 years’ experience in the health sector and extensive governance experience. She has previously worked in senior executive roles in primary care and District Health Board (DHB) management, as well as in Māori Health with Ngāi Tahu. Fiona holds a diverse range of governance roles and currently represents Arowhenua on the Board of Te Runanga o Ngāi Tahu. She is also a Director of Ngāi Tahu Holdings Corporation and has a Postgraduate Diploma in Public Health and an MBA from Massey University.
Bart is a consultant haematologist at Palmerston North Hospital’s Regional Cancer Treatment Service. He is an active and experienced committee member, including for Leukaemia & Blood Cancer New Zealand. He is a member of the Haematology Society of Australia and New Zealand and the American Society of Haematology and is registered with the New Zealand Medical Council as a specialist in Internal Medicine and in Pathology (Haematology).
Roger Jarrold is a Chartered Accountant with more than four decades’ experience. He has worked across the engineering, construction, and health sectors, including in Chief Financial Officer roles in construction and health. Alongside several other positions, Roger is currently Chair of the Health Research Council’s Audit and Risk Committee and a Board member at Health New Zealand Te Whatu Ora, where he is also a member of the Infrastructure Committee. Roger is a trustee of the Auckland Hospitals Research and Endowment Fund and joined New Zealand Blood Service’s Board in July 2023.
Nicole Anderson is a chartered professional director (CMInstD) with a background in financial management, economic development, and public health. She is a member of various public and private sector governance bodies, including being a Director of Top Energy Ltd, Far North Holdings Ltd, and Te Ohu Kaimoana Boards, and Chair of the International Accreditation Council (IANZ). Ko Ngāpuhi tōna iwi, nō Hokianga ia.
Anthony is an independent director and business strategy and expansion capital consultant. He has more than 30 years of investment management, banking and finance, and mergers and acquisitions experience, and has held CEO and senior executive leader positions in financial services’ entities. Anthony’s governance roles include Chair of the Medical Radiation Technologists Board and Chair of Whai Rawa Fund Limited. He has a Bachelor of Commerce from the University of Auckland and holds Chartered Accountant designation from the professional accounting bodies of New Zealand and Singapore.
Edward is a consultant ophthalmologist at Wellington and Kenepuru hospitals, and at Wairau Hospital, Blenheim. He holds a Bachelor of Medicine and a Bachelor of Surgery from the University of Auckland, and a postgraduate diploma in Ophthalmology from the University of Otago. He is a fellow of the Royal Australia and New Zealand College of Ophthalmologists, and has undertaken subspecialty fellowship training in corneal surgery, retinal surgery and ocular oncology.
Ko Ngāi Tahu/Waitaha toku iwi. Huirapa toku hapu, Puketeraki toku marae.
(DBS, BBS, PGDBA, MBS)
Edie is Independent Chair of New Zealand Blood Service’s Finance and Audit Subcommittee.
She is a Chartered Fellow of the Institute of Directors New Zealand and an independent director with more than 25 years’ governance experience across organisations related to health, broadcasting, audio-visual archiving, regional development, philanthropy, and kaupapa Māori community services.
Edie is also Independent Chair of the New Zealand Human Rights Commission’s Finance and Audit Subcommittee, an independent member of the Ministry of Health’s Risk Assurance Committee, and a member of the International Accreditation NZ Council (IANZ) Board. She is also IANZ’s Audit and Risk Committee Chair. Edie is not a member of the NZBS Board.
TE RĀNGAI MATUA

SAM CLIFFE
Chief Executive Officer
As CEO Sam leads the Executive Leadership Team (ELT), has overall responsibility for organisational performance, and is accountable to New Zealand Blood Service’s Board. The Executive Leadership Team members act as partners to the CEO, providing critical advice and support to enable the organisation to perform effectively and deliver on its objectives.
DR SARAH MORLEY
Chief Medical Officer
The Chief Medical Officer leads the Clinical Services Team, which plays a key role in ensuring that clinically appropriate blood products and services are provided to patients. The multi-professional expert team provides transfusion and blood donation advice, and clinical expertise in cellular therapies and apheresis, and organ and tissue donation. Sarah’s team works closely with clinicians across the health sector and includes Organ Donation New Zealand and the New Zealand Bone Marrow Donor Registry.
JOSHUA BANKERS
Director, Digital Technology and Information Management
Josh provides vision and leadership for strategically developing and managing information, information resources, digital platforms, and technology. He is responsible for enabling New Zealand Blood Service’s business strategy through the smart application and management of technology and information. He is also accountable for information services’ systems and infrastructure across all locations.
KYLE BEUTH
Chief Financial Officer
As Chief Financial Officer (CFO) Kyle plays a key role in ensuring New Zealand Blood Service meets its financial and legal obligations. The CFO also contributes to the strategic direction and overall performance of the organisation. Kyle leads a team responsible for delivering effective financial management, financial strategy, policy development, and operational analysis. A key function of the CFO is also to drive business improvement and enhanced performance using analytics and business intelligence practices.
FIDELMA MURPHY
Director, Quality and Regulatory Affairs and NZBS Privacy Officer
Fidelma is accountable for ensuring NZBS works within required legislation and international best practice standards for blood, tissue, and cells’ services. NZBS works within a complex regulatory framework for the collection, testing, manufacturing, and distribution of substances of human origin in New Zealand. Fidelma’s role ensures NZBS is fully compliant with all the required standards, ensuring the organisation provides safe products and services. She is also the organisation’s Privacy Officer and ensures compliance with New Zealand’s Privacy Act 2020.
KAREN DIDOVICH
Director, Human Resources and Organisational Development
The Director, Human Resources and Organisational Development is responsible for providing strategic leadership across these areas. The role ensures policies, programmes and HR services support a high-performing culture that is underpinned by NZBS’s values and contributes to NZBS’s ‘single enduring outcome’1. Working in partnership with other Executive Leadership Team members and senior management teams, HR&OD work to attract, select, motivate, and retain a highly qualified and diverse workforce; promote effective leadership and management practices; manage salary and benefits; develop employee recognition programmes; promote fair and equitable treatment of employees through employee relations services; lead health and safety, inclusive of wellbeing initiatives; and provide training and development.
DR MANDY SUDDES
Director, Technical Services
Mandy leads and is accountable for the Technical Services function, from strategy development to operational service delivery, including managing major change programmes. This work is central to ensuring we can meet the clinical demand for products and services and deliver them in accordance with statutory and regulatory requirements. Technical Services’ functions include manufacturing and donation accreditation, and operating six national blood banks, the National Reference Laboratory, and the New Zealand Transplantation and Immunogenetics Laboratory. Technical Services also carries out cellular therapy and tissue banking activities with support from NZBS’s Learning and Development and Technical Facilities and Equipment teams.
BRETT PARADINE
Director, Donor Services
Brett leads and is accountable for the Donor Services’ function, from strategy development through to operational service delivery. This work is central to ensuring collection targets are achieved so that clinical demand for products and services can be met. Donor Services’ functions include donor strategy, planning, marketing, donor administration, donor recruitment and collections, training, the Donor Product and Safety (DaPS) Team, therapeutic services, and contact centre oversight.
JUSTIN SCOTT
Director, Planning & Supply Chain
Justin is accountable for providing strategic direction and oversight of NZBS’s Planning and Supply Chain function. He leads the operational activity that sees the timely distribution of blood and blood products to New Zealand hospitals and, with his team, heads up NZBS’s integrated planning process to ensure product demand can be met. Justin’s role also manages the organisation’s facility, property, and procurement functions, and he is the lead executive for NZBS’s emergency planning management.
1New Zealanders health needs are supported by the availability of safe and appropriate blood and tissue products and related services.
TE ANGA ME TE KĀWANATANGA
NZBS IS A CROWN ENTITY ESTABLISHED IN 1998 UNDER THE NEW ZEALAND PUBLIC HEALTH AND DISABILITY ACT 2000 1 .

Its legislated primary purpose and core activity is the safe, timely, high quality and efficient provision of blood, blood products, and services to clinicians for the people of New Zealand. In addition, NZBS provides services for matching patients and donors prior to organ/tissue transplantation, tissue banking (skin and bone), and stem cell services.
These activities contribute to the organisation achieving its single Enduring Outcome:
The health needs of people in New Zealand are supported by the availability of safe and appropriate blood, blood products, tissues and related services.
NZBS is required under the Crown Entities Act 2004 (the Act) to give effect to Government policy as directed by the responsible Minister, the Minister of Health. The NZBS Board is appointed by and responsible to the Minister of Health and performs strategic and governance functions for the organisation in accordance with the Act.
The collective duties of the Board under the Act include ensuring that NZBS:
• Acts consistently with its objectives, functions, Statement of Intent, and Annual Statement of Performance Expectations
• Performs its functions efficiently, effectively, consistently, and with the spirit of service to the public, and
• Operates in a financially responsible manner.
Board members who have a range of appropriate and complementary skills and experience to govern this complex collections’, manufacturing, and distribution organisation also have individual duties to: comply with the Act (including with respect to disclosure of information), act with honesty and integrity, act in good faith and not at the expense of NZBS’s interests, and act with reasonable care, diligence and skill.
The NZBS Board appoints the Chief Executive Officer (CEO) who reports directly to them. An Executive Leadership Team (see page 89) supports the CEO.
The NZBS Board ensured the organisation’s activities contributed to achieving the following three strategic objectives for the 2024/25 year:
• Building foundations for growth
• Delivering operational effectiveness
• Providing exceptional service
1 Replaced by the Pae Ora Healthy Futures Act from 1 July 2022
Collections & production volumes
performance ($000)



STATEMENT OF TOTAL COMPREHENSIVE INCOME
For the year ended 30 June 2025
Explanations of major variances against budget are provided in Note 25. The accompanying notes form part of these financial statements.
As at 30 June 2025
For the year ended 30 June 2025
Explanations of major variances against budget are provided in Note 25. The accompanying notes form part of these financial statements.
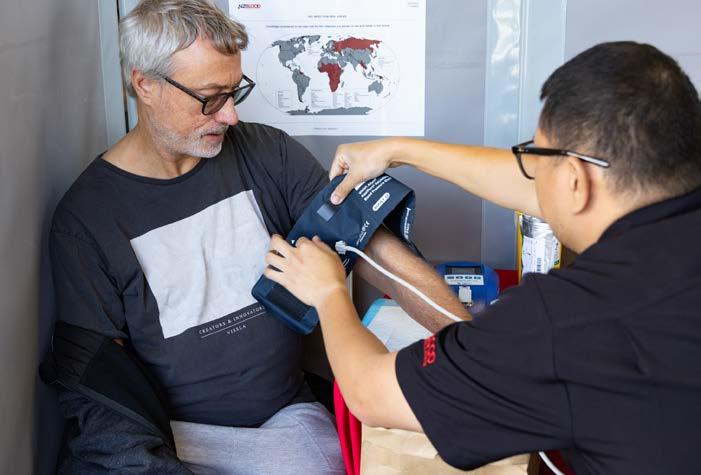
For the year ended 30 June 2025
The accompanying notes form part of these financial statements.
For the year ended 30 June 2025
The accompanying notes form part of these financial statements
For the year ended 30 June 2025
New Zealand Blood Service is a Crown entity as defined by the Crown Entities Act 2004 and is domiciled and operates in New Zealand. The relevant legislation governing New Zealand Blood Service’s operations includes the Crown Entities Act 2004, the Pae Ora (Healthy Futures) Act 2022 and the Human Tissue Act 2008. New Zealand Blood Service’s ultimate parent is the New Zealand Crown.
New Zealand Blood Service’s primary objective is to provide services to the New Zealand public. It is New Zealand’s sole provider of blood, blood products, and associated services. It co-ordinates deceased organ donation (NZBS is home to Organ Donation New Zealand), tissue donation (eye tissue, heart valves, skin, and hip bone donations), and operates the national Heart Valve Bank and the New Zealand Bone Marrow Donor Registry. New Zealand Blood Service does not operate to make a financial return.
New Zealand Blood Service has designated itself as a public benefit entity (PBE) for financial reporting purposes.
The financial statements for New Zealand Blood Service are for the year ended 30 June 2025, and the Board approved them for issue on 30 October 2025.
The financial statements have been prepared on a going concern basis, and the accounting policies have been applied consistently throughout the year.
Statement of compliance
New Zealand Blood Service’s financial statements have been prepared in accordance with the requirements of the Crown Entities Act 2004, which includes the requirement to comply with generally accepted accounting practice in New Zealand (NZ GAAP).
New Zealand Blood Service is a Tier 1 entity and the financial statements have been prepared in accordance with and comply with PBE standards.
Presentation currency and rounding
The financial statements are presented in New Zealand dollars, and all values are rounded to the nearest thousand dollars ($000).
New or amended standards adopted
2024 Omnibus Amendments to PBE Standards issued October 2024
The 2024 Omnibus Amendments issued by the External Reporting Board include several general updates and amendments to several Tier 1 PBE accounting standards, effective for reporting periods starting 1 January 2024. New Zealand Blood Service has adopted the revised PBE standards, and the adoption did not result in any significant impact on New Zealand Blood Service’s financial statements.
Disclosure of Fees for Audit Firms’ Services (Amendments to PBE IPSAS 1)
Amendments to PBE IPSAS 1 Presentation of Financial Reports changed the required disclosures for fees relating to services provided by the audit or review provider, including a requirement to disaggregate the fees into specified categories. The amendments to PBE IPSAS 1 aim to address concerns about the quality and consistency of disclosures an entity provides about fees paid to its audit or review firm for different types of services. The enhanced disclosures are expected to improve the transparency and consistency of disclosures about fees paid to an entity’s audit or review firm. This is effective for the year ended 30 June 2025 and New Zealand Blood Service has adopted the revised PBE standards in Notes 7, expenses.
Other changes in accounting policies
There have been no changes in New Zealand Blood Service’s accounting policies since the date of the last audited financial statements.
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Significant accounting policies are included in the notes to which they relate.
Significant accounting policies that do not relate to a specific note are outlined below.
Foreign currency transactions
Foreign currency transactions (including those that forward foreign exchange contracts are held for) are translated into NZ dollars (the functional currency) using the spot exchange rates at the dates of the transactions. Foreign exchange gains and losses resulting from the settlement of such transactions and from the translation at year-end exchange rates of monetary assets and liabilities denominated in foreign currencies are recognised in the surplus or deficit.
Goods and services tax
Items in the financial statements are presented exclusive of GST, except for receivables and payables, which are presented on a GST-inclusive basis. Where GST is not recoverable as input tax, it is recognised as part of the related asset or expense.
The net amount of GST recoverable from, or payable to, the IRD is included as part of receivables or payables in the statement of financial position.
The net GST paid to, or received from, the IRD, including the GST relating to investing and financing activities, is classified as a net operating cash flow in the statement of cash flows.
Commitments and contingencies are disclosed exclusive of GST.
Income tax
New Zealand Blood Service is a public authority and consequently is exempt from paying income tax.
Accordingly, no provision has been made for income tax.
For the year ended 30 June 2025
Budget figures
The budget figures are derived from the Statement of Performance Expectations as approved by the Board at the beginning of the financial year. The budget figures have been prepared in accordance with NZ GAAP, using accounting policies that are consistent with those adopted by the Board in preparing these financial statements.
Critical accounting estimates and assumptions
In preparing these financial statements, New Zealand Blood Service has made estimates and assumptions about the future. These estimates and assumptions might differ from the subsequent actual results. New Zealand Blood Service continually evaluates its estimates and assumptions, which are based on historical experience and other factors, including expectations of future events that are believed to be reasonable under the circumstances.
The estimates and assumptions that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities within the next financial year are:
• assessing the useful lives and residual values of property, plant, and equipment – refer to Note 13
• assessing the useful lives of software assets – refer to Note 14
• estimating the current costs, inflation rates, discount rates, and other assumptions used in measuring the provision for lease make-good provisionrefer Note 18
• year-end valuation of inventory and the provision raised for obsolescent inventory items. Further detail to the estimates and assumptions mentioned is in Note 12
• estimating the rates of future salary increases, discount rates, and other assumptions used in measuring retirement and long service leave – refer to Note 17.
Critical judgements in applying accounting policies
Management has exercised the following critical judgements in applying accounting policies:
• leases classification – refer to Note 16.
Accounting policy
The specific accounting policies for significant revenue items are explained below:
Sale of goods
Revenue from the sale of goods is recognised when the goods are sold to the customer.
Provision of services
Services provided to third parties on commercial terms are recognised as revenue once the services have been fully provided.
Price rebates
New Zealand Blood Service’s Board may from time to time approve price rebates to Health New Zealand Te Whatu Ora. Price rebates are recognised as a contra to revenue.
Funding from the Crown
Funding from the Crown is restricted in its use for the purpose of New Zealand Blood Service meeting the objectives specified in Output Agreements with the Crown. Where conditions are attached to funding from the Crown, any annual surpluses arising from the efficient delivery of services may be retained by New Zealand Blood Service for use in subsequent years. Funding from the Crown is recognised as revenue only to the extent that economic benefits of the funding have been consumed. The fair value of revenue from the Crown has been determined to be equivalent to the amounts due in the funding arrangements.
Interest revenue
Interest revenue is recognised by accruing on a time proportion basis the interest due for the investment.
Breakdown of interest revenue
Interest earned from financial assets measured at amortised cost
For the year ended 30 June 2025
New Zealand Blood Service received funding from a non-exchange Output Agreement with the Ministry of Health to provide deceased organ and tissue donation services. The Output Agreement contains conditions attached to funding, and surpluses arising from the efficient delivery of services during the year are to be retained by New Zealand Blood Service for use in subsequent years.
Accounting policy
Salaries and wages
Salaries and wages are recognised as an expense as employees provide services. Superannuation schemes
Defined contribution schemes
Employer contributions to KiwiSaver, the Medical Assurance Society Retirement Savings Plan, the Mercer Individual Retirement Plan, the Lump Sum National Scheme, and the AMP NZ Retirement Trust are accounted for as defined contribution superannuation schemes and are expensed in the surplus or deficit as incurred.
Expired and written down product
New Zealand Blood Service is the sole supplier of blood, blood products, organs and tissues needed within New Zealand’s heath system and must maintain sufficient inventory of products so that key products and services are always available. New Zealand Blood Service continually monitors inventory to minimise the inventory of products held to product expiry.
Changes in inventory represent an aggregated reporting amount, which includes cost of goods sold, production recoveries, consumables, and inventory valuation adjustments. This is consistent with the application of manufacturing standard costing methodologies and generally accepted inventory valuation principle.
Accounting policy
The capital charge is expensed in the financial year that the charge relates to.
Further information on the capital charge
The capital charge paid to the Crown is calculated based on New Zealand Blood Service’s equity as at 30 June and 31 December each year. The capital charge rate for the year ended 30 June 2025 was five percent (2024: 5%).
Accounting policy
Borrowing costs are expensed in the financial year that they are incurred in.
No borrowing costs were capitalised during the period (2024: nil).
Accounting policy
An
or
as a reduction of rental expense over the
and
For the year ended 30 June 2025
Disclosure of Fees for Audit Firms’ Services (Amendments to PBE IPSAS 1)
Disclosure of Fees for Audit Firms’ Services
Operating leases as lessee
The future aggregate minimum lease payments to be paid under non-cancellable operating leases are as follows:
New Zealand Blood Service leases 23 premises (2024: 20 premises) across New Zealand from which it operates donor collection services, warehousing and component processing, testing services and national support services.
The prior year operating lease component for premises has been restated to reflect the minimum future lease payment obligations.
Significant leasing arrangements
Lester Lane, Christchurch
This facility was purpose built for New Zealand Blood Service and is used for donor collection services and component processing for the South Island. The lease arrangement was entered into in November 2014 for an initial term of 30 years with two rights of renewal for an additional 12 years each. Rent reviews occur every three years with rent escalating at the minimum of CPI or 2.5 percent per annum compounded over the preceding three years. A market rental review is undertaken in the 15th year and each renewal period thereafter.
71 Great South Road, Auckland
This site has been significantly redeveloped by New Zealand Blood Service and is used for donor collection services encompassing the greater Auckland region and Northland, component processing for the upper North Island, and national support services. The lease arrangement was entered into in October 2018 for an initial term of 20 years with three rights of renewal for an additional three years each. Rent reviews occur annually with rent escalating at two percent per annum. A market rental review is undertaken every 10 years.
Highbrook Drive, Auckland
Highbrook has been purpose built for New Zealand Blood Service and is used for national warehousing and specialised component processing. The lease arrangement was entered into in February 2023 for an initial term of 20 years with two rights of renewal for an additional seven years each. Rent reviews occur annually with rent escalating at 2.6 percent per annum. A market rental review is undertaken every 10 years.
London Street, Hamilton
This facility is used for donor collection services encompassing the Waikato, Bay of Plenty, and Central North Island regions. The lease arrangement was entered into in June 2023 for an initial term of 20 years with three rights of renewal for an additional five years each. Rent reviews occur annually with rent escalating two percent per annum. A market rent review is undertaken every five years.
Crawford Street, Dunedin
This site is used for donor collection services in Dunedin. The lease arrangement was entered into in May 2017 for an initial term of 12 years with two rights of renewal for an additional nine years each. Rent reviews occur every three years with rent escalating at CPI or prevailing market rates. The landlord made a cash contribution of $311,465 towards the fitout of the premises which may be clawed back by the landlord in the event the lease agreement is terminated during the initial 12-year term of the agreement.
There are no restrictions placed on New Zealand Blood Service by any of its leasing arrangements.
Total future minimum sublease payments to be received under non-cancellable subleases for office space at balance date are $nil (2024: $nil).
For the year ended 30 June 2025
Accounting policy
Cash and cash equivalents include cash on hand, deposits held on call with banks, and other short-term, highly liquid investments with original maturities of three months or less.
of cash and cash equivalents and further information
A loss allowance for expected credit losses has not been recognised because the estimated loss allowance for credit losses is trivial.
Cash at bank is deposited with counter parties with Standards & Poor’s credit ratings of AA- or better. The carrying value of cash at bank, cash on hand and short-term deposits with maturities less than three months from the date of acquisition approximates their fair value.
Accounting policy
Short-term receivables are recorded at the amount due, less an allowance for expected credit losses (ECL). New Zealand Blood Service applies the simplified ECL model of recognising lifetime ECLs for short-term receivables.
In measuring ECLs, short-term receivables have been assessed on a collective basis because they possess shared credit risk characteristics. They have been grouped based on the days past due. A provision matrix is then established based on historical credit loss experience, adjusted for forward-looking factors specific to the debtors and the economic environment.
Short-term receivables are written off when there is no reasonable expectation of recovery. Indicators that there is no reasonable expectation of recovery include the debtor being in liquidation or the receivable being more than one year overdue. There were no trade and other receivables from non-exchange transactions in 2025 and 2024.
Receivables from interest earned on term deposits - 208
The expected credit loss rates for receivables at 30 June 2025 and 30 June 2024 are based on the payment profile of revenue on credit over the prior two years at the measurement date and the corresponding historical credit losses experienced for that period. The historical loss rates are adjusted for current and forward-looking macroeconomic factors that might affect the recoverability of receivables. Given the short period of credit risk exposure, the impact of macroeconomic factors is not considered significant. The expected credit loss is zero percent (2024: 0%).
There have been no changes in the estimation techniques or significant assumptions used in measuring the loss allowance during the reporting.
For the year ended 30 June 2025
Accounting policy
Bank term deposits
Bank term deposits are initially measured at the amount invested. A loss allowance for expected credit losses is recognised if the estimated loss allowance is not trivial.
Breakdown of investments and further information
Current portion
Non-current portion
non-current portion
Term deposits
New Zealand Blood Service considers there has not been a significant increase in credit risk for investments in term deposits because the issuer of the investment continues to have low credit risk at balance date. Term deposits are held with banks that have a long-term AA- investment grade credit rating, which indicates that the bank has a very strong capacity to meet its financial commitments. No loss allowance for expected credit losses has been recognised because the estimated 12-month expected loss allowance for credit losses is trivial.
The carrying amounts of term deposits with maturities of 12 months or less approximate their fair value.
Accounting policy
Derivative financial instruments are used to manage exposure to foreign exchange risk arising from New Zealand Blood Service’s operational activities. New Zealand Blood Service does not hold or issue derivative financial instruments for trading purposes. New Zealand Blood Service has not adopted hedge accounting.
Derivatives are initially recognised at fair value on the date a derivative contract is entered into and are subsequently remeasured to their fair value at each balance date, with the resulting gain or loss recognised in the surplus or deficit.
A forward foreign exchange derivative is classified as current if the contract is due for settlement within 12 months of balance date. Otherwise, the full fair value of a forward foreign exchange derivative is classified as non-current.
Further information on derivative financial instruments
The notional principal amount of outstanding forward foreign exchange contracts in NZ$ was $92.11m (2024: $79.54m). The foreign currency principal amount was AUD$84.61m (2024: AUD$73.00m).
The notional principal amount of outstanding forward foreign exchange contracts in NZ$ was $1.27m (2024: $0). The foreign currency principal amount was USD$0.75m (2024: USD$0).
The fair values of forward foreign exchange contracts have been determined using a discounted cash flows valuation technique based on quoted market prices. The inputs into the valuation model are from independently sourced market parameters such as currency rates. Most market parameters are implied from forward foreign exchange contract prices.
Accounting policy
To the extent that inventory was received through non-exchange transactions (i.e. donated goods) for no or a nominal cost, the cost of the inventory is its fair value at the date of acquisition.
However, as NZBS is not legally permitted to purchase blood from the public, the fair value for accounting purposes of blood from donors is considered to be nil. Therefore, the cost of inventories comprises all costs of collection, costs of conversion, and any other costs incurred in bringing the inventories to their present location and condition.
After initial recognition, inventory is measured at the lower of cost and net realisable value. The cost of inventory is determined using the FIFO or weighted average methods. The valuation includes allowance for slow moving items. Obsolete inventories are written off.
Inventories are recognised as an expense when deployed for utilisation or consumption in the ordinary course of NZBS’s operation. Any write-down from cost to net realisable value is recognised in surplus or deficit in the year of the write-down.
For the year ended 30 June 2025
Fractionated derived products manufactured from a principal plasma pool
Fractionated derived products are manufactured into finished blood products by a third-party manufacturer on a “toll” manufacturing basis using NZBSprovided source plasma collected from New Zealanders.
The work in progress (WIP) is included at full standard cost, as the final output that the manufacturer must produce is locked in via the agreed production plan and contract yields per the toll manufacturing agreement. This high level of certainty enables the WIP to be viewed in the same light as finished fractionation product for the purposes of inventory valuation.
Valuation of fractionated derived (both finished goods and WIP), is based on allocating the actual input cost of manufacturing a plasma pool (NZBS source plasma input plus third-party toll fractionation manufacturing fee) to prorated finished/WIP product output using actual product plasma yield, as reported by the manufacturer.
Fractionated inventory items are now carried at actual cost without any reallocation to other immunoglobulin products where the cost exceeds the net realisable value. The excess of net realisable value is charged in full to the current financial year profit and loss account and carried to the next
year under a provision to be released over future months in line with the sales profile of the affected products. The shift in accounting policy and treatment enables NZBS to have increased visibility of the true cost of fractionated derived products and further aids in future price setting considerations and decisions around production of derived fractionated products versus commercial purchases.
Breakdown of inventories and further information
Inventories were written down by $1.88m (2024: $5.72m). There were reversals of write-downs of $0.18m (2024: $0.18m).
No inventories are pledged as security for liabilities (2024: $nil).
Accounting policy
Property, plant, and equipment consists of four asset classes, which are measured as follows:
• leasehold improvements, at cost less accumulated depreciation and impairment losses;
• plant and equipment, at cost less accumulated depreciation and impairment losses;
• furniture and office equipment, at cost less accumulated depreciation and impairment losses; and
• motor vehicles, at cost less accumulated depreciation and impairment losses.
Additions
The cost of an item of property, plant, and equipment is recognised as an asset only when it is probable that future economic benefits or service potential associated with the item will flow to New Zealand Blood Service and the cost of the item can be measured reliably.
Work in progress is recognised at cost less impairment and is not depreciated.
In most instances, an item of property, plant, and equipment is initially recognised at its cost. Where an asset is acquired through a non-exchange transaction, it is recognised at its fair value as at the date of acquisition.
Costs incurred after initial acquisition are capitalised only when it is probable that future economic benefits or service potential associated with the item will flow to New Zealand Blood Service and the cost of the item can be measured reliably.
The costs of day-to-day servicing of property, plant, and equipment are expensed in the surplus or deficit as they are incurred.
Disposals
Gains and losses on disposals are determined by comparing the proceeds with the carrying amount of the asset. Gains and losses on disposals are reported net in the surplus or deficit.
Depreciation
Depreciation is provided on a straight-line basis on all property, plant, and equipment at rates that will write off the cost of the assets to their estimated residual values over their useful lives. The useful lives and associated depreciation rates of major classes of property, plant, and equipment have been estimated as follows:
• Lease hold improvements 2 - 30 years 3%-50%
For the year ended 30 June 2025
• Plant and equipment 2 - 20 years 5%-50%
• Furniture and office equipment 3 - 15 years 6%-33%
• Motor vehicles 8 - 12 years 8%-13%
Leasehold improvements are depreciated over the unexpired period of the lease or the estimated remaining useful lives of the improvements, whichever is the shorter.
Impairment of property, plant, and equipment
New Zealand Blood Service does not hold any cash-generating assets. Assets are considered cash generating where their primary objective is to generate a commercial return.
Property, plant, and equipment are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount might not be recoverable. An impairment loss is recognised for the amount that the asset’s carrying amount exceeds its recoverable service amount. The recoverable service amount is the higher of an asset’s fair value, less costs to sell, and value-in-use.
Value-in-use is the present value of an asset’s remaining service potential. It is determined using an approach based on a depreciated replacement cost approach, a restoration cost approach, or a service units approach. The most appropriate approach for measuring value-in-use depends on the nature of the impairment and availability of information.
If an asset’s carrying amount exceeds its recoverable service amount, the asset is regarded as impaired and the carrying amount is written down to the recoverable service amount.
The total impairment loss is recognised in the surplus or deficit.
Critical accounting estimates and assumptions
Estimating useful lives and residual values of property, plant, and equipment
At each balance date, the useful lives and residual values of property, plant, and equipment are reviewed. Assessing the appropriateness of useful life and residual value estimates of property, plant, and equipment requires several factors to be considered, such as the physical condition of the asset, how long New Zealand Blood Service expects to use the asset, and expected disposal proceeds from the future sale of the asset.
An incorrect estimate of the useful life or residual value will affect the depreciation expense recognised in the surplus or deficit and carrying amount of the asset in the statement of financial position. New Zealand Blood Service minimises the risk of this estimation uncertainty by:
• physical inspection of assets, and
• asset replacement programmes.
There have been no material changes to useful lives, depreciation methods, and residual values.
Breakdown of property, plant, and equipment and further information
Cost or valuation
For the year ended 30 June 2025
Carrying amounts
Property, plant, and equipment totalling $0.46m (2024: $6.44m) was acquired by means of finance leases during the year.
Restrictions
There are no restrictions and pledges on New Zealand Blood Service’s property, plant, and equipment.
Finance leases and term financing
The net carrying amount of assets under term financing is leasehold improvements of $12.14m (2024: $13.21m) and equipment of $0.57m (2024: $0.61m).
The net carrying amount of assets under finance leases is leasehold improvements of $1.83m (2024: $1.38m) and equipment of $10.87m (2024: $7.48m).
Capital commitments
The value of contractual commitments for the acquisition of property, plant, and equipment at the reporting date is:
Accounting
Software acquisition and development
Computer software licenses are capitalised based on the costs incurred to acquire the specific software and bring it to use.
Costs that are directly associated with developing software for internal use are recognised as an intangible asset where this results in an asset controlled by New Zealand Blood Service. Direct costs include software development employee costs and an appropriate portion of relevant overheads.
Where software is provided under a Software-as-a-service (SaaS) arrangement, costs of configuration and customisation are recognised as an intangible asset only if the activities create an intangible asset that New Zealand Blood Service controls and asset recognition criteria are met. Costs, including ongoing fees for software use, that do not result in an intangible asset or a software finance lease are expensed as a service contract as incurred. However, where fees represent payment for future services to be received, New Zealand Blood Service recognises these as a prepayment and expenses these as subsequent services are received.
Staff training costs are recognised as an expense when incurred.
Costs associated with maintaining computer software are expensed when incurred.
Costs associated with developing and maintaining New Zealand Blood Service’s website are expensed when incurred.
For the year ended 30 June 2025
Amortisation
The carrying value of an intangible asset with a finite life is amortised on a straight-line basis over its useful life. Amortisation begins when the asset is available for use and ceases at the date when the asset is derecognised. The amortisation charge for each financial year is expensed in the surplus or deficit.
The useful lives and associated amortisation rates of major classes of intangible assets have been estimated as follows:
• Acquired
Impairment of intangible assets
Refer to the policy for impairment of property, plant, and equipment in Note 13. The same approach applies to the impairment of intangible assets.
Breakdown of intangible assets and further information
Accumulated amortisation and impairment losses
Classes of intangible asset
New Zealand Blood Service has not generated software internally. All software has been acquired. Restrictions
There are no restrictions over the title of New Zealand Blood Service’s intangible assets, nor are any intangible assets pledged as security for liabilities. Capital commitments
The value of contractual commitments for the acquisition of intangible assets is $nil (2024: $0).
Accounting policy
Short-term payables are recorded at the amount payable.
Breakdown of payables and deferred revenue, and other information
For the year ended 30 June 2025
Accounting policy
Borrowings on normal commercial terms are initially recognised at the amount borrowed plus transaction costs. Borrowings are carried at amortised cost using the effective interest method.
Borrowings are classified as current liabilities unless New Zealand Blood Service has an unconditional right to defer settlement of the liability for at least 12 months after balance date.
Finance leases
A finance lease transfers substantially all the risks and rewards incidental to ownership of an asset to the lessee, whether or not title is eventually transferred.
At the start of the lease term, finance leases are recognised as assets and liabilities in the statement of financial position at the lower of the fair value of the leased asset or the present value of the minimum lease payments.
The finance charge is charged to the surplus or deficit over the lease period so as to produce a constant periodic rate of interest on the remaining balance of the liability.
The amount recognised as an asset is depreciated over its useful life. If there is no reasonable certainty whether New Zealand Blood Service will obtain ownership at the end of the lease term, the asset is fully depreciated over the shorter of the lease term and its useful life.
Critical judgements in applying accounting policies
Determining lease classification
Determining whether a lease is a finance lease or an operating lease requires judgement as to whether the lease transfers substantially all the risks and rewards of ownership to New Zealand Blood Service.
Judgement is required on various aspects that include, but are not limited to, the fair value of the leased asset, the economic life of the leased asset, whether to include renewal options in the lease term, and determining an appropriate discount rate to calculate the present value of the minimum lease payments. Classification as a finance lease means that the asset is recognised in the statement of financial position as property, plant, and equipment, but no such asset is recognised for an operating lease.
Breakdown of borrowings and further information
For the year ended 30 June 2025
New Zealand Blood Service has a finance lease limit of $8m with Westpac New Zealand Limited and other parties. Term financing
New Zealand Blood Service has a term debt facility with Westpac New Zealand Limited. The maximum amount available under this committed funding line is $15m (2024: $15m) and any borrowing against it is secured against assets. Unsecured loans
New Zealand Blood Service has a Multi Option Credit Line facility (MOCL) with Westpac New Zealand Limited. The maximum amount available under this committed funding line is $25m (2024: $10m) and any borrowing against it is unsecured and subject to a negative pledge undertaking.
Security
Finance lease liabilities and Term financing are effectively secured, because the rights to the leased asset revert to the lessor in the event of default.
Fair value
Because interest rates on floating rate debt reset to a market rate every 29 days, the carrying amounts of secured loans approximate their fair value.
The fair value of finance leases is $9.1m (2024: $8.8m). Fair value has been determined using contractual cash flows discounted using a rate based on market borrowing rates at balance date of 4.6 percent (2024: 6.6%).
The fair value of term financing is $13.1m (2024: $13.2m). Fair value has been determined using contractual cash flows discounted using a rate based on market borrowing rates at balance date of 4.6 percent (2024: 6.6%).
Analysis of finance leases:
Finance leases as lessee
The net carrying amount of assets under finance leases is leasehold improvements of $1.83m (2024: $1.38m) and equipment of $10.87m (2024: $7.48m).
New Zealand Blood Service is not permitted to pledge the leased assets as security, nor can it sublease the leased equipment without the permission of the lessor. There are no other restrictions placed on New Zealand Blood Service by any of the leasing arrangements.
Analysis of term financing:
For the year ended 30 June 2025
Term financing:
The net carrying amount of assets under term financing is leasehold improvements of $12.14m (2024: $13.21m) and equipment of $0.57m (2024: $0.61m).
The Westpac New Zealand Limited borrowing is unsecured and operates via a negative pledge undertaking. The maximum amount available under the Multi Option Credit Line facility (MOCL) is $25m, all of which is a committed funding line on a term facility (2024: $10m).
The specific requirements of the negative pledge are stated below:
(a) NZBS must not grant a security interest over more than five percent of its adjusted tangible assets (defined as total assets less intangible assets) to any third party without the prior consent of Westpac New Zealand Limited.
The Westpac New Zealand Limited unsecured loan becomes repayable on demand in the event New Zealand Blood Service breaches any of the obligations under the negative pledge and ratio’s undertaking. New Zealand Blood Service has complied with all negative pledge undertakings and borrowing obligations during the financial year. Current facility arrangements operate to 31 December 2028.
The MOCL borrowing facility provides construction phase funding to the multi-year redevelopment of 71 Great South Road, Auckland. On completion of each phase, the cost will transfer from the MOCL to the long-term Westpac Master Lease Agreement (MLA) that provides a 10-year funding and repayment facility with interest rates struck off the 10-year swap rate ruling at each phase’s MLA execution.
Accounting policy
Short-term employee entitlements
Employee benefits that are expected to be settled wholly before 12 months after the end of the reporting period during which the employees provided the related service are measured based on accrued entitlements at current rates of pay. These include salaries and wages accrued up to balance date, annual leave earned but not yet taken at balance date, and sick leave.
A liability and an expense are recognised for bonuses where there is a contractual obligation or where there is past practice that has created a constructive obligation, and a reliable estimate of the obligation can be made.
Long-term employee entitlements
Employee benefits that are not expected to be settled before 12 months after the end of the reporting period during which the employees provide the related service, such as long service leave and retirement gratuities, have been calculated on an actuarial basis. The calculations are based on:
• likely future entitlements accruing to employees, based on years of service, years to entitlement, the likelihood that employees will reach the point of entitlement, and contractual entitlement information; and
• the present value of the estimated future cash flows.
Presentation of employee entitlements
Sick leave, annual leave and vested long service leave are classified as a current liability. Non-vested retirement and long service leave expected to be settled within 12 months of balance date are classified as a current liability. All other employee entitlements are classified as a non-current liability.
Critical accounting estimates and assumptions
Measuring retirement and long service leave obligations
Measuring the long service leave and retirement gratuities obligations depends on several factors that are determined on an actuarial basis using several assumptions. Two key assumptions used in calculating this liability include the discount rate and the salary inflation factor. Any changes in these assumptions will affect the carrying amount of the liability.
Expected future payments are discounted using discount rates derived from the yield curve of New Zealand Government bonds. The discount rates used have maturities that match, as closely as possible, the estimated future cash outflows. The salary inflation factor has been determined after considering historical salary inflation patterns and obtaining advice from an independent actuary. A weighted average discount rate of 4.77 percent (2024: 4.77%) for retirement leave and 4.30 percent (2024: 4.61%) for long service leave, and an inflation factor of 1.75 percent (2024: 1.75%) was used.
If the discount rate were to decrease by one percent, or the salary inflation rate were to increase by one percent from the rate used, with all other factors held constant, the carrying amount of the retirement and long service leave liability would be approximately $0.23 million higher.
If the discount rate were to increase by one percent, or the salary inflation rate were to decrease by one percent from the rate used, with all other factors held constant, the carrying amount of the retirement and long service leave liability would be approximately $0.20 million lower.
For the year ended 30 June 2025
Breakdown of employee entitlements
Accounting policy
General
A provision is recognised for future expenditure of uncertain amount or timing when:
• there is a present obligation (either legal or constructive) resulting from a past event;
• it is probable that an outflow of future economic benefits or service potential will be required to settle the obligation; and
• a reliable estimate of the amount of the obligation can be made.
Provisions are measured at the present value of the expenditure expected to be required to settle the obligation using a pre-tax discount rate that reflects current market assessments of the time, value of money, and the risks specific to the obligation. An increase in the provision because of the passage of time is recognised as a finance cost (refer Note 6).
At the expiry of the lease terms for 17 leased premises (2024: 12) New Zealand Blood Service is required to make good any damage caused to the premises and to remove any fixtures or fittings that it installed. New Zealand Blood Service has the option to renew several of these leases, which affects the timing of expected cash outflows to make good the premises.
In measuring the provision, New Zealand Blood Service has assumed that it will not exercise options to renew any premises. The cash flows associated with the non-current portion of the lease make-good provision are expected to occur between 2025 and 2043. Significant cash flows are expected for 71 Great South Road, Auckland, in 2038 of $0.73m and Highbrook Drive, Auckland, in 2043 of $0.48m.
Further information about New Zealand Blood Service’s leasing arrangements is disclosed in Note 7.
For the year ended 30 June 2025
New Zealand Blood Service has accrued lease payments related to the lease liability for the remaining lease payments. Refer to Note 7 for details on significant lease arrangements.
Contingent liabilities
New Zealand Blood Service has no contingent liabilities (2024: $nil).
Contingent assets
New Zealand Blood Service has no contingent assets (2024: $nil) Accounting
Equity is measured as the difference between total assets and total liabilities. Equity is disaggregated and classified into the following components:
• contributed capital;
• accumulated surplus/(deficit); and
• adverse event reserves.
Adverse event reserves
This reserve comprises the transfer of surpluses from accumulated comprehensive revenue and expense. The reserve was established during the year ended 30 June 2015 to mitigate financial risk associated with the manufacturing and production of fractionated derived products.
For the year ended 30 June 2025
Breakdown of equity and further information
Capital management
New Zealand Blood Service’s capital is its equity, which comprises accumulated funds and adverse event reserves. Equity is represented by net assets.
During the year ended 30 June 2023, NZBS’s Board approved reducing the adverse event reserves to $nil and making the remaining $2.0m of reserves available for operational requirements.
New Zealand Blood Service is subject to the financial management and accountability provisions of the Crown Entities Act 2004, which impose restrictions on borrowings, acquisition of securities, issuing of guarantees and indemnities, and the use of derivatives.
New Zealand Blood Service has complied with the financial management requirements of the Crown Entities Act 2004 during the year.
New Zealand Blood Service manages its equity as a byproduct of prudently managing revenues, expenses, assets, liabilities, investments, and general financial dealings to ensure it effectively achieves its objectives and purpose while remaining a going concern.
New Zealand Blood Service is controlled by the Crown.
Related party disclosures have not been made for transactions with related parties that are:
• within a normal supplier or client/recipient relationship, and
• on terms and conditions no more or less favourable than those that it is reasonable to expect New Zealand Blood Service would have adopted in dealing with the party at arm’s length in the same circumstances.
During the period, New Zealand Blood Service:
• paid the Ministry of Health a capital charge of $5,486,840 (2024: $5,511,669). The amount outstanding at the end of the period was nil (2024: nil).
• received $1,366,411 as an equity injection from the Ministry of Health (2024: $24,563,522)
• received $5,067,500 (2024: $4,067,500) from the Ministry of Health related to Organ Donation New Zealand.
Further, transactions with other government agencies (for example, government departments and Crown Entities) are not disclosed as related party transactions when they are on normal terms and conditions consistent with the normal operating arrangements between government agencies.
For the year ended 30 June 2025
The full-time equivalent for Board members has been determined based on the frequency and length of Board meetings and the estimated time for Board members to prepare for meetings.
An analysis of Board member remuneration is provided in Appendix 1.
23 Financial instruments
23A Financial instruments categories NOTES TO THE FINANCIAL STATEMENTS
The carrying amounts of financial assets and liabilities in each of the financial instrument categories are as follows:
instruments categories
23B Fair value hierarchy
For those instruments recognised at fair value in the statement of financial position, fair values are determined according to the following hierarchy:
• Quoted market prices (Level 1) – Financial instruments with
• Valuation techniques using observable inputs (Level 2) – Financial instruments with
• Valuation techniques with significant non-observable inputs (Level 3) – Financial instruments valued using models where one or more significant inputs are not observable.
For the year ended 30 June 2025
The following table analyses the basis of the valuation of classes of financial instruments measured at fair value in the statement of financial position.
30 June 2025
30 June 2024
23C Financial instrument risks
New Zealand Blood Service’s activities expose it to a variety of financial instrument risks, including market risk, credit risk, and liquidity risk. New Zealand Blood Service has policies to manage these risks and seeks to minimise exposure from financial instruments. These policies do not allow it to enter transactions that are speculative in nature.
Market risk
Price risk
Price risk is the risk that the value of a financial instrument will fluctuate because of changes in market prices.
Fair value interest rate risk
Fair value interest rate risk is the risk that the fair value of a financial instrument will fluctuate due to changes in market interest rates. New Zealand Blood Service’s exposure to fair value interest rate risk is limited to its bank deposits, which are held at fixed rates of interest. New Zealand Blood Service does not actively manage its exposure to fair value interest rate risk.
Cash flow interest rate risk
Cash flow interest rate risk is the risk that the cash flows from a financial instrument will fluctuate because of changes in market interest rates. Investments and borrowings issued at variable interest rates expose New Zealand Blood Service to cash flow interest rate risk.
New Zealand Blood Service manages interest rate risks arising from contractual commitments and liabilities by entering into interest rate swaps, interest rate caps, and fixed rate loans to manage the interest rate risk exposure.
2025
to conditions)
For the year ended 30 June 2025
2024
(excluding income in advance, taxes payable and grants received subject to conditions)
New Zealand Blood Service currently has no variable interest rate investments.
Sensitivity analysis
At 30 June 2025, if the 90-day bank bill rate had been 50 basis points higher or lower, with all other variables held constant, the surplus/deficit for the year would have been $0.10m (2024: $0.11m) lower/higher. This movement is attributable to increased or decreased interest expense on borrowings. The sensitivity is lower in 2025 than in 2024 because of a slight reduction in outstanding borrowings.
Currency risk
Currency risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in foreign exchange rates.
New Zealand Blood Service makes purchases of goods and services overseas that require it to enter transactions denominated in foreign currencies. As a result of these activities, exposure to currency risk arises.
New Zealand Blood Service manages foreign currency risks arising from contractual commitments and liabilities by entering forward foreign exchange contracts to manage the foreign currency risk exposure.
Sensitivity analysis
At 30 June 2025, if the NZ dollar had weakened/strengthened by five percent against the Australian dollar, with all other variables held constant, the surplus for the year would have been:
• $1.32m (2024: $1.32m) lower if the NZ dollar had weakened.
• $1.20m (2024: $1.20m) higher if the NZ dollar had strengthened.
This movement is attributable to foreign exchange gains/losses on translation of Australian dollar denominated creditors and bank balances.
At 30 June 2025, if the NZ dollar had weakened/strengthened by fiver percent against the United States dollar, with all other variables held constant, the surplus for the year would have been:
• $0.65m (2024: $0) lower if the NZ dollar had weakened.
• $0.59m (2024: $0) higher if the NZ dollar had strengthened.
This movement is attributable to foreign exchange gains/losses on translation of United States dollar denominated creditors and bank balances.
Credit risk
Credit risk is the risk that a third party will default on its obligation to New Zealand Blood Service, causing it to incur a loss.
New Zealand Blood Service is exposed to credit risk from cash and term deposits with banks, receivables, and derivative financial instrument assets. For each of these, the maximum credit exposure is best represented by the carrying amount in the statement of financial position.
• Risk management
For receivables, New Zealand Blood Service reviews the credit quality of customers before granting credit. It continues to monitor and manage receivables based on their ageing and adjusts the expected credit loss allowance accordingly. There are no significant concentrations of credit risk.
Because of the timing of its cash inflows and outflows, New Zealand Blood Service invests surplus cash with registered banks with a Standard & Poor’s credit rating of at least A+. New Zealand Blood Service limits the amount of net credit exposure to any one financial institution to $15 million. New Zealand Blood Service invests funds and enters into derivative financial instruments only with registered banks that have a Standard & Poor’s credit rating of at least A+. New Zealand Blood Service’s investments in term deposits are considered low-risk investments. The credit ratings of banks are monitored for credit deterioration.
• Security
No collateral or other credit enhancements are held for financial assets that give rise to credit risk.
• Impairment
Cash and cash equivalents (Note 8), receivables (Note 9), and term deposit investments (Note 10) are subject to the expected credit loss model. The notes for these items provide relevant information on impairment.
For the year ended 30 June 2025
Credit risk exposure by credit risk rating grades, excluding receivables
The gross carrying amount of financial assets, excluding receivables, by credit rating is provided below by reference to Standard & Poor’s credit ratings.
Liquidity risk
Management of liquidity risk
Liquidity risk is the risk that New Zealand Blood Service will encounter difficulty raising liquid funds to meet commitments as they fall due. Prudent liquidity risk management implies maintaining sufficient cash and the ability to close out market positions. New Zealand Blood Service manages liquidity risk by continuously monitoring forecast and actual cash flow requirements.
Contractual maturity analysis of financial liabilities, excluding derivatives
The table below analyses financial liabilities (excluding derivatives) into their relevant maturity groupings based on the remaining period at balance date to the contractual maturity date. Future interest payments on floating rate debt are based on the floating rate of the instrument at balance date. The amounts disclosed are the undiscounted contractual cash flows.
Contractual maturity analysis of derivative financial liabilities
The table below analyses derivative financial instrument liabilities that are settled net and all gross settled derivatives according to their relevant maturity groupings based on the remaining period at balance date to the contractual maturity date. The amounts disclosed are the undiscounted contractual cash flows.
For the year ended 30 June 2025
23D Reconciliation of movements in liabilities arising from financing activities
The table below provides a reconciliation between the opening and closing balances of finance lease liabilities and unsecured borrowings at balance date.
borrowings
24 Events after the balance date
There were no significant events after the balance date.
For the year ended 30 June 2025
Explanations of major variances from New Zealand Blood Service’s budgeted figures in the Statement of Performance Expectations are as follows:
Statement of comprehensive revenue and expense
Revenue
Revenue from sales of goods were higher than budget by $908k, due to increased demand for fresh products and services. Fresh product revenue exceeded budget, driven by higher platelet and plasma volumes. Services activity was also above budget, reflecting elevated volumes in blood bank testing and New Zealand Transplantation and Immunogenetics Laboratory (NZTIL) Histotrac Tissue Type testing. These gains were partially offset by a shortfall in fractionated product sales, primarily due to the phasing out of Hizentra and lower Biostate sales. While imported Hizentra and Gamunex contributed positively, this was outweighed by a decline in Privigen sales.
Expenses
Expenses were less than budget by $8.80m or 3.12 percent, primarily due to a $9.0m reduction in inventory costs, driven by $6.2 million in fractionation gains from improved IVIg and Biostate yields, and $2.7 million in higher recoveries from increased collection volumes. Information technology costs were also lower by $2.9m, reflecting the deferral of operational IS projects and postponed pricing uplifts.
These savings were partially offset by a $2.2m increase in personnel costs. Occupancy costs rose by $1.0m, largely due to new donor centres opened during the year, and an increase in premises reinstatement provisions for four sites in June.
Statement of financial position
Cash is $7.3m higher than budget, reflecting stronger operating performance. Inventories of plasma-derived products are $4.0m above budget, due to higher stock levels. Property, plant, and equipment and intangible assets are $6.4m below budget, as several additions and project completions have been deferred to future years. Employee entitlements are $3.5m higher than budget, driven by anticipated backpay from union settlements, movements in long service and sick leave provisions, and Holidays Act 2003 remediation costs. Borrowings are $10.6m below budget, reflecting lower financing requirements. Payables and deferred revenue is $12.7m higher than budget mainly due to an un-budgeted plasma pool being processed by CSL in late May.
Cash flows
Net cash flows from operating activities were $11.9m higher than budget, underpinned by the improved operating result, with favourable movements in trade and other payables helping to offset the cashflow impact of increased inventory holdings and receivables. This was partially offset by the deferral of property redevelopment works and other capital investment programmes totalling $0.73m, and an additional capital contribution of $1.36m from the Crown for Holidays Act 2003 remediation costs. Net cash outflows of $0.2m from financing activities were due to the repayment of finance leases and term financing.

Employee remuneration
remuneration paid or payable that is or exceeds $100,000:
$390,000 - $399,999
Employee remuneration
During the year ended 30 June 2025 no (2024: 1) employee received compensation or other benefits in relation to cessation (2024: $20,000).
Board member remuneration
The total value of remuneration paid or payable to each Board member during the year was:
There have been payments made to Edie Moke, a committee member appointed by the Board who was not a Board member during the financial year, totalling $16,000 (2024: $15,911).
New Zealand Blood Service has provided a deed of indemnity to Board members for certain activities carried out in the performance of New Zealand Blood and Organ Service’s functions.
New Zealand Blood Service has taken out Directors’ and Officers’ Liability and Professional Indemnity insurance cover during the financial year for the liability or costs of Board members and employees.
No Board members received compensation or other benefits in relation to cessation (2024: $nil).

To the readers of New Zealand Blood Service’s annual financial statements and performance information for the year ended 30 June 2025
The Auditor-General is the auditor of New Zealand Blood Service. The Auditor-General has appointed me, René van Zyl, using the staff and resources of Audit New Zealand, to carry out, on his behalf, the audit of:
• the annual financial statements that comprise the statement of financial position as at 30 June 2025, the statement of comprehensive revenue and expenses, statement of changes in equity, and statement of cash flows for the year ended on that date and the notes to the financial statements that include accounting policies and other explanatory information on pages 94 to 122;
• the performance information that consists of:
the statement of performance for the year ended 30 June 2025 on pages 22 to 30; and
the end-of-year performance information for appropriations for the year ended 30 June 2025 on page 30.
In our opinion:
• The annual financial statements of the New Zealand Blood Service:
– fairly present, in all material respects:
- its financial position as at 30 June 2025; and - its financial performance and cash flows for the year then ended; and
comply with generally accepted accounting practice in New Zealand in accordance with Public Benefit Entity Reporting Standards.
• The statement of performance fairly presents, in all material respects, the New Zealand Blood Service’s service performance for the year ended 30 June 2025. In particular, the statement of performance:
– provides an appropriate and meaningful basis to enable readers to assess the actual performance of the New Zealand Blood Service for each class of reportable outputs; determined in accordance with generally accepted accounting practice in New Zealand;
– fairly presents, in all material respects, for each class of reportable outputs:
- the actual performance of the New Zealand Blood Service
- the actual revenue earned; and
- the output expenses incurred, as compared with the forecast standards of performance, the expected revenues, and the proposed output expenses included in the New Zealand Blood Service’s statement of performance expectations for the financial year; and
– complies with generally accepted accounting practice in New Zealand in accordance with Public Benefit Entity Reporting Standards.
• The end-of-year performance information for appropriations:
fairly presents, in all material respects:
- what has been achieved with the appropriation; and
- the actual expenses or capital expenditure incurred in relation to the appropriation as compared with the expenses or capital expenditure that were appropriated or forecast to be
incurred; and – complies with generally accepted accounting practice in New Zealand in accordance with Public Benefit Entity Reporting Standards.
Our audit was completed on 30 October 2025. This is the date at which our opinion is expressed.
We carried out our audit in accordance with the Auditor-General’s Auditing Standards, which incorporate the Professional and Ethical Standards, the International Standards on Auditing (New Zealand), and New Zealand Auditing Standard 1 (Revised): The Audit of Service Performance Information issued by the New Zealand Auditing and Assurance Standards Board. Our responsibilities under those standards are further described in the Responsibilities of the auditor section of our report.
We have fulfilled our responsibilities in accordance with the AuditorGeneral’s Auditing Standards.
We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.
Responsibilities of the Board for the annual financial statements and the performance information
The preparation of the financial statements and performance information of the New Zealand Blood Service is the responsibility of the Board.
The Board is responsible on behalf of the New Zealand Blood Service for preparing financial statements and performance information that are fairly presented and comply with generally accepted accounting practice in New Zealand. This includes preparing performance information that provides an appropriate and meaningful basis to enable readers to assess what has been achieved for the year.
The Board is responsible for such internal control as it determines is necessary to enable it to prepare annual financial statements, a statement of performance, and the end-of-year performance information for appropriations that are free from material misstatement, whether due to fraud or error.
In preparing the annual financial statements, the statement of performance, and the end-of-year performance information for appropriations, the Board is responsible on behalf of the New Zealand Blood Service for assessing the New Zealand Blood Service’s ability to continue as a going concern.
The Board’s responsibilities arise from the Crown Entities Act 2004 and the Public Finance Act 1989.
Responsibilities of the auditor for the audit of the annual financial statements and the performance information
Our objectives are to obtain reasonable assurance about whether the annual financial statements, the statement of performance, and the endof-year performance information for appropriations, as a whole, are free from material misstatement, whether due to fraud or error, and to issue an auditor’s report that includes our opinion.
Reasonable assurance is a high level of assurance, but is not a guarantee that an audit carried out in accordance with the Auditor-
General’s Auditing Standards will always detect a material misstatement when it exists. Misstatements are differences or omissions of amounts or disclosures, and can arise from fraud or error. Misstatements are considered material if, individually or in the aggregate, they could reasonably be expected to influence the decisions of readers, taken on the basis of the annual financial statements, the statement of performance, and the end-of-year performance information for appropriations.
For the budget information reported in the annual financial statements, the statement of performance, and the end-of-year performance information for appropriations, our procedures were limited to checking that the information agreed to the New Zealand Blood Service’s statement of performance expectations or to the Estimates of Appropriations for the Government of New Zealand for the year ended 30 June 2025.
We did not evaluate the security and controls over the electronic publication of the annual financial statements, the statement of performance, and the end-of-year performance information for appropriations.
As part of an audit in accordance with the Auditor-General’s Auditing Standards, we exercise professional judgement and maintain professional scepticism throughout the audit. Also:
• We identify and assess the risks of material misstatement of the annual financial statements, the statement of performance, and the end-of-year performance information for appropriations, whether due to fraud or error, design and perform audit procedures responsive to those risks, and obtain audit evidence that is sufficient and appropriate to provide a basis for our opinion. The risk of not detecting a material misstatement resulting from fraud is higher than for one resulting from error, as fraud may involve collusion, forgery, intentional omissions, misrepresentations, or the override of internal control.
• We obtain an understanding of internal control relevant to the audit in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the New Zealand Blood Service’s internal control.
• We evaluate the appropriateness of accounting policies used and the reasonableness of accounting estimates and related disclosures made by the Board.
• We evaluate whether the statement of performance and the endof-year performance information for appropriations:
– provide an appropriate and meaningful basis to enable readers to assess the actual performance of the New Zealand Blood Service in relation to the forecast performance of the New Zealand Blood Service (for the statement of performance) and what has been achieved with the appropriation by the New Zealand Blood Service (for the end-of-year performance information for appropriations). We make our evaluation by reference to generally accepted accounting practice in New Zealand; and – fairly present the actual performance of the New Zealand Blood Service and what has been achieved with the appropriation by the New Zealand Blood Service for the financial year.
• We conclude on the appropriateness of the use of the going concern basis of accounting by the Board.
• We evaluate the overall presentation, structure and content of the annual financial statements, the statement of performance, and the end-of-year performance information for appropriations, including the disclosures, and whether the annual financial statements, the statement of performance, and the end-of-
year performance information for appropriations represent the underlying transactions and events in a manner that achieves fair presentation.
We communicate with the Board regarding, among other matters, the planned scope and timing of the audit and significant audit findings, including any significant deficiencies in internal control that we identify during our audit.
Our responsibilities arise from the Public Audit Act 2001.
The Board is responsible for the other information. The other information comprises all of the information included in the annual report, but does not include the annual financial statements, the statement of performance, and the end-of-year performance information for appropriations, and our auditor’s report thereon.
Our opinion on the annual financial statements, the statement of performance, and the end-of-year performance information for appropriations does not cover the other information and we do not express any form of audit opinion or assurance conclusion thereon.
In connection with our audit of the annual financial statements, the statement of performance, and the end-of-year performance information for appropriations, our responsibility is to read the other information. In doing so, we consider whether the other information is materially inconsistent with the annual financial statements, the statement of performance, and the end-of-year performance information for appropriations or our knowledge obtained in the audit, or otherwise appears to be materially misstated. If, based on our work, we conclude that there is a material misstatement of this other information, we are required to report that fact. We have nothing to report in this regard.
We are independent of the New Zealand Blood Service in accordance with the independence requirements of the Auditor-General’s Auditing Standards, which incorporate the independence requirements of Professional and Ethical Standard 1: International Code of Ethics for Assurance Practitioners (including International Independence Standards) (New Zealand) issued by the New Zealand Auditing and Assurance Standards Board.
Other than in our capacity as auditor, we have no relationship with, or interests in, the New Zealand Blood Service.

René van Zyl Audit New Zealand
On behalf of the Auditor-General Auckland, New Zealand
New Zealand Blood Service contributes knowledge, research, and training to support local and international health sector colleagues and services for the benefit of the people they serve. Staff also deliver training internally and undertake professional development to ensure skills and knowledge remain current. Below is a representative summary of some of the outputs and activities from the year.
Technical Services
Publications
• Chin Zhong Ng, Leigh Mosen, Christopher Corkery, Meredith Smith, Gustavo C. Duarte. Transfusion-associated graft-versus-host disease and purine analogues: What are we missing? Vox Sanguinis May 2025. http://doi.org/10.1111/vox.70047
Meeting presentations
• Nalder, A. Poster: How we got here and where we are going - A look at Cellular Therapy Products collected at Wellington and Palmerston North Hospitals over the past 20 years. International Society for Cell and Gene Therapy (ISCT) Australia/New Zealand region, New Zealand, 7-9 August 2024.
• Atkinson, G.; de Carvalho Duarte, G.; Patel, H. Non-cryopreserved autologous stem cell transplant (ASCT) for multiple myeloma in New Zealand. International Society for Cell and Gene Therapy (ISCT) Australia/New Zealand region, New Zealand, 7-9 August 2024.
• Atkinson, G. Non-cryopreserved Autologous Stem Cell Transplant; a Pilot Study. New Zealand Institute of Medical Laboratory Science (NZIMLS) Annual Scientific Meeting, Christchurch, 28-30 August 2024.
• Prado, D. The Essence of Teamwork. New Zealand Institute of Medical Laboratory Science (NZIMLS) Annual Scientific Meeting, Christchurch, 28-30 August 2024.
• Tremblay, J (representing the Biotherapeutics Association of Australasia). Training and Education of Eye and Tissue Banking staff – an Australasian perspective. 32nd Annual Congress of the European Association of Tissue and Cell Banks (EATCB), Barcelona, 28-29 November 2024.
• Ruddell, J. Cardiovascular tissue banking – the New Zealand experience. 2nd Annual Eye & Tissue Banking Conference of Australasia, Brisbane, 4-5 March 2025.
• Tuinukuafe, A. Assembling the gift of donated skin. 2nd Annual Eye & Tissue Banking Conference of Australasia, Brisbane, 4-5 March 2025.
• Tremblay, J. NZBS Tissue Bank update. 2nd Annual Eye & Tissue Banking Conference of Australasia, Brisbane, 4-5 March 2025.

• Vanhecke, C. Poster: Effects of changes in transfusion management, population mix and fertility on red blood cells alloantibody prevalence in Christchurch, New Zealand. Presented at the 35th International Society of Blood Transfusion (ISBT) Regional Congress, Milan, 31 May - 4 June 2025.
• Takimoana, T. Rare Cause of HDFN: Have a MMMMM. Presented at the National Immunohaematology Continuing Education (NICE), Auckland, 23-25 May 2025.
• Murphy-Devlin, D.; Copedo, S. Mind your P’s and k’s. Presented at the National Immunohaematology Continuing Education (NICE), Auckland, 23-25 May 2025.
• Costas, M. Obstetrics, oxygen, overload - Obstetric MHP case study. Presented at the National Immunohaematology Continuing Education (NICE), Auckland, 23-25 May 2025.
• Seoraj, V. Ref Lab hunting down the rarest of the rare: Rare, high incidence, and autoimmune. Presented at the National Immunohaematology Continuing Education (NICE), Auckland, 23-25 May 2025.
• Du Preez, T. Blood, sweat and airways. Presented at the National Immunohaematology Continuing Education (NICE), Auckland, 23-25 May 2025.
• Grant, S. How low can you go? Presented at the National Immunohaematology Continuing Education (NICE), Auckland, 23-25 May 2025.
• Murphy, T. Looking beyond the blood bank for clues. Presented at the National Immunohaematology Continuing Education (NICE), Auckland, 23-25 May 2025.
• Hadley, J. When stabbing my friends was a good thing. Presented at the National Immunohaematology Continuing Education (NICE), Auckland, 23-25 May 2025.
• Lin, P: KHE. Presented at the National Immunohaematology Continuing Education (NICE), Auckland, 23-25 May 2025.
• Chaplin,K. CLL-imbing the challenge: a case study of CLL and AIHA. Presented at the National Immunohaematology Continuing Education (NICE), Auckland, 23-25 May 2025.
• Ponesca, C. Current practices in granulocyte transfusion. Presented at the National Immunohaematology Continuing Education (NICE), Auckland, 23-25 May 2025.
• Coker, J. It’s a surprise!!! Presented at the National Immunohaematology Continuing Education (NICE), Auckland, 23-25 May 2025.
• Prado, D. Mixed feelings on mixed field. Presented at the National Immunohaematology Continuing Education (NICE), Auckland, 23-25 May 2025.
• Ritchie, D. Mixed field RhD group and myelofibrosis. Presented at the National Immunohaematology Continuing Education (NICE), Auckland, 23-25 May 2025.
• Buckley, L. Where have all the Biostate users gone? Presented at the National Immunohaematology Continuing Education (NICE), Auckland, 23-25 May 2025.
• Behr, C. Post-transplant monitoring for kidney patients using donor-derived cell-free DNA (dd-cfDNA). Presented at the National Immunohaematology Continuing Education (NICE), Auckland, 23-25 May 2025.
• Mosen, L. “A Special Gift” - now comes in a box. Presented at the National Immunohaematology Continuing Education (NICE), Auckland, 23-25 May 2025.
• Griffiths, D. Role NZTIL plays in haematopoetic transplants; Donor options. Presented at the National Immunohaematology Continuing Education (NICE), Auckland, 23-25 May 2025.
• Timms, K. A tale of two screening cells. Presented at the National Immunohaematology Continuing Education (NICE), Auckland, 23-25 May 2025.
Training delivered across the New Zealand or international healthcare sector
• Radgoodarzi, S.; Dongoran, S. Education Day for Operating Theatre Charge Nurses: Femoral Head (bone) collections + Tissue Banking, Lifehealthcare, Auckland, September 2024.
• Dongoran, S.; Shah, S. Femoral Head (bone) collections – tamper mechanism. Manukau Superclinic, Auckland September 2024.
• Radgoodarzi, S. Femoral Head (bone) collections – tamper mechanism. Ascot Hospital Greenlane, Auckland, October 2024.
• Nalder, A. Femoral Head (bone) collections – tamper mechanism. Masterton Hospital, January 2025.
• Blood Banking Training: Timaru Hospital, Nelson Hospital. Christchurch Blood Bank and regional blood banks, November 2024 - January 2025.
Professional development
• Within Technical Services, NZBS is currently supporting a laboratory staff member working in the Cellular Therapies and Tissue Banking area to complete their PhD project in platelets and megakaryocytes.
Clinical Services
Meetings hosted
• Inaugural National Patient Blood Management Symposium, Wellington, November 2024.
Publications
• Badami KG, Faed JM. Warfarin reversal in patients with antiphospholipid syndrome: caution required, but clear guidance not available. Br J Haematol 2024 Oct 3.
• Badami KG, Hull S, Vanhecke C. Effects of change in transfusion practice, population mix and fertility on red blood cell alloantibody prevalence. Vox Sang 2025 Mar 27.
• Badami KG, McKellar M. Reactions to serum eye drops—New Zealand experience and review of the literature. Transfus Med 2024 Feb;34(1):61–5.
• Birrell JM, Webster AC, Cross NB, Driscoll T, Dunckley H, Beaglehole B, Dittmer I, Walker C, Jones M, Irvine J, Wyld ML, Wyburn KR, De La Mata NL. Geographic variation in kidney failure and transplantation in Aotearoa New Zealand: a population-based data linkage study. Nephrology (Carlton) 2024;29:941–54.
• Cain L, Lafrance C, Morton S, Latour C, Girard M, Tiberghien P, de la Taille V, Datta SS, Virk MS, Andrews J, Yahalom V, Pugliese AM, Alba R, Charlewood R, Kirwan S, Yokoyama APH, Kutner JM, Nogues EA, Martinez IL, Llonch N, Daly J, Irving DO, Schulze TJ, Huisman E, Le Poole K, Vrielink H, Saifee NH, Pagano MB, Stanworth S; Biomedical Excellence for Safer Transfusion Collaborative. Differences in the product characteristics and clinical use of granulocytes for transfusion: the BEST Collaborative study. Transfusion 2025 Jun;65(6):1111–23.
• Clausen FB, Hellberg Å, Toly-Ndour C, Nielsen ET, de Haas M, The Noninvasive Fetal Antigen Genotyping Survey Group. Noninvasive fetal antigen genotyping: results from a survey on the status of clinical implementation. Vox Sang 2025;Epub ahead of print. doi:10.1111/vox.70062
• De Simone B, Chouillard E, Podda M, Pararas N, De Carvalho Duarte G, et al. The 2023 WSES guidelines on the management of trauma in elderly and frail patients. World J Emerg Surg 2024 May 31;19(1):18.
• Duarte CDG, Ladvanszky L, Atkinson G, Burns M, Madola D, Sadani D, Yan-Fischer M, Patel H, Tremblay J, Butler A, Waikato X, Wei WH. Postthaw CD34+ cell recovery likely degraded under extreme graft platelet concentrations. Bone Marrow Transplant 2024;59(12):1704–9.
• Duarte CDG, Wei WH. Personalised autologous stem cell harvesting improves patient collection outcomes. Transfus Clin Biol 2024;31:157–61.
• Dunbar NM, Kaufman RM, Bary KS, Bellairs GRM, Cohn CS, Delettre F, Ditcham S, Duarte GC, et al. ABO-mismatched platelet and plasma transfusion practices and the potential for transfusion-related alpha-gal syndrome (TRAGS): the Biomedical Excellence for Safer Transfusion (BEST) Collaborative Study. Transfusion 2025 Jul 11.
• Gauld N, Hinks A, Gao R, Teu T, Gounder D. Implementation and mixed method evaluation of a unique midwife-prescribed, pharmacistadministered routine antenatal Anti-D prophylaxis model in pregnant people. Res Social Adm Pharm 2025;21:704–13.
• Han MH, Badami KG. ABO non-identical platelet transfusions, immune platelet refractoriness and platelet support. Br J Haematol 2024 May;204(5):2097–102.
• Hess AS. What have all the models done? Transfusion 2025 Jun;65(6):1212–3.
• Hess AS. The thromboelastogram is paradoxically confounded by haematocrit in clinical samples tested on both mechanical and acoustic platforms. Front Med (Lausanne) 2024 Nov 13;11:Pathology.
• Hill JF, Hess AS, Pretty CG, Chase JG. Non-invasive, continuous venous oxygen saturation and oxygen extraction estimation from the internal jugular vein. In: 47th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2025 Jul 14–17; Copenhagen
• Jacobs JW, Booth GS, Lewis-Newby M, Saifee NH, Ferguson E, Cohn CS, Delaney M, Morley S, Thomas S, Thorpe R, Raza S, Weaver MS, Woo JS, Sharma D, So-Osman C, Yurtsever N, Tormey CA, Waters A, Goldman M, Yan MTS, Fasano RM, Stephens LD, Allen ES, Erikstrup C, Infanti L, Schlafer TD, Warner MA, Winters JL, Tobian AAR, Bloch EM. Medical, societal, and ethical considerations for directed blood donation in 2025. Ann Intern Med 2025 Jul;178(7):1021–6.
• Kaminsky CJ, Mill J, Patel V, Pierce D, Haj A, Hess AS, Li L, Raife TR. The longevity factor spermidine is part of a highly heritable complex erythrocyte phenotype associated with longevity. Aging Cell 2024 Sep 7:e14311.
• Kwan PSL, Kirwan S, Tuinukuafe A, Morley S. Temporal dynamics of in vitro hemostatic function in platelets cryopreserved using a novel approach for rapid issuance. Transfusion 2024 Jul;64(7):1287–95.
• Lieshout-Krikke R, Hoad V, Chua SS, Kam G, Satake M, Hino I, Stramer SL, Groves JA, de la Taille V, Laperche S, Cheng A, Goodison K, Tsoi WC, Lee CK, Prati D, Pati I, Drews SJ, Bigham M, Gratz G, Jungbauer C, Charlewood R, Smith M, O’Flaherty N, Raftery A, Oyonarte S, Gubbe K, Luhm J, Ngcobo S, Slot E, Davison K, Brailsford S, Dunbar N. International Forum on donor- and recipient-triggered lookback for traditional transfusion-transmitted infections: summary. Vox Sang 2025 Feb;120(2):197–206.
• Lieshout-Krikke R, Hoad V, Chua SS, Kam G, Satake M, Hino I, Stramer SL, Groves JA, de la Taille V, Laperche S, Cheng A, Goodison K, Tsoi WC, Lee CK, Prati D, Pati I, Drews SJ, Bigham M, Gratz G, Jungbauer C, Charlewood R, Smith M, O’Flaherty N, Raftery A, Oyonarte S, Gubbe K, Luhm J, Ngcobo S, Slot E, Davison K, Brailsford S, Dunbar N. International Forum on donor- and recipient-triggered lookback for traditional transfusion-transmitted infections: responses. Vox Sang 2025 Feb;120(2):207–38.
• Lorenz N, James A, Van Rooyen T, Paterson A, Ramiah C, Carlton LH, Sharma P, Baker MG, Charlewood R, McGregor R, Moreland NJ. Decline of antibodies to major viral and bacterial respiratory pathogens during the COVID-19 pandemic. J Infect Dis 2025 Feb 4;231(1):e77–81.
• Maddock A, Warrington S, Lyver A, Badami KG. Early alloimmunization in an infant to red cell antigens—rare but possible in the ‘right’ circumstances. Transfus Med 2025 Feb 10.
• Metcalf RA, Nahirniak S, Guyatt G, Bathla A, White SK, Al-Riyami AZ, Jug RC, La Rocca U, Callum JL, Cohn CS, DeAnda A, DeSimone RA, Dubon A, Estcourt LJ, Filipescu DC, Fung MK, Goel R, Hess AS, Hume HA, Kaufman RM, Kranke P, Louw VJ, Møller MH, Murphy MF, Muszynski JA, O’Kelly CJ, Pagano MB, Patidar GK, Pavenski K, Poston JN, Saifee NH, Stolla M, Szczepiorkowski ZM, Tobian AAR, Uberoi R, Waters J, Williams B, Wood EM, Zantek ND, Zeller MP, Grossman BJ, Stanworth SJ. Platelet transfusion: 2025 AABB and ICTMG international clinical practice guidelines. JAMA 2025 May 29.
• Ng CZ, Mosen L, Corkery C, Smith M, Duarte GC. Transfusion-associated graft-versus-host disease and purine analogues: what are we missing? Vox Sang 2025.
• Poston JN, Andrews J, Arya S, Chou S, Cohn CS, Covington M, Crowe EP, Goel R, Gupta G, Haspel R, Hess AS, Ipe T, Jacobson J, Khan J, Murphy M, O’Brien K, Pagano MB, Panigrahi A, Salazar E, Saifee NH, Stolla M, Zantek ND, Ziman A, Metcalf RA. Current advances in 2024: a critical review of selected topics by the Association for the Advancement of Blood and Biotherapies (AABB) Clinical Transfusion Medicine Committee. Transfusion2024 Oct;64(10):2019–28.
• Ruxton K, Madola D, Duarte GC, et al. Development and implementation of a citrate-related toxicity identification tool for stem cell collection. J Clin Apher 2025;40:E70014,72-3.
• Shim L, Wensley C, Casement J, Parke R. What determinants impact deceased organ donation consent in the adult intensive care unit? An integrative review exploring the perspectives of staff and families. Aust Crit Care 2024 Jul;37(4):638–50.
• Wall L, Gounder D. International Forum: Genotyping of blood antigens in donors. Vox Sang 2025;1–16.
• Webber C, Sriamporn KT, Morley SL, Ritchie S, Hollingshead BM, McAllister S, Priest P, Fisher M, Saxton P. Why men who have sex with men in New Zealand intend to donate or not donate blood. Vox Sang 2025 Jul;120(7):664–70.
• Young SC, Paterson BG, Gounder DS. A curious case of haemolytic disease of the newborn caused by cold-reacting anti-M: a New Zealand case report. N Z J Med Lab Sci 2025;79(2).
Meetings presentations
• Kazemi, A.; Grayson, K. Poster: Safeguarding Last Wishes: A Collaborative Process for Developing an Ethical Framework for Donation after Assisted Dying. The Organ and Tissue Authority, Organ and Transplantation Conference, Melbourne, May 2025.
• Ruxton, K.; Aboy, K. Poster: Development and Implementation of a Citrate-Related Toxicity Identification Tool for Stem Cell Collection. 46th American Society for Apheresis (ASFA) Annual Meeting, Canada, 9-11 April 2025.
• Kwan, P.; Agustin G.; Kirwan, S.; Morley, S. Poster: National Supply and Utilization of Red Cells and Platelets Components: a Retrospective Analysis of Pre- and Post- Covid Trends in New Zealand, 2019 – 2024. Presented at the 35th Regional International Society of Blood Transfusion (ISBT) Congress, Milan, Italy, June 2025.
• Hill, JF.; Hess, AS.; Pretty, CG.; Chase, JG. Non-invasive, continuous venous oxygen saturation and oxygen extraction estimation from the
internal jugular vein. 47th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Copenhagen, 14-17 July.
• Hurley, NC.; Hess, AS. Performance Degradations of Cloud-based Large Language Models over Time. Midwest Anaesthesiology Residents’ Conference. Omaha, Nebraska, April 6, 2025.
Training delivered across the New Zealand or international healthcare sector
The training delivered by the Clinical Team this year has been extensive and is not listed here in its entirety.
• Organ Donation New Zealand: Link Symposium, 17-18 October 2024
• Organ Donation New Zealand: Education Days (multiple) for Link Teams, ICU, and Operating Theatre staff. These sessions provide frontline staff with the tools and knowledge to identify and facilitate organ donation opportunities.
Training delivered across the New Zealand or international healthcare sector
Murphy, F. Good Manufacturing Practice (GMP) Compliance within a Blood Establishment. Malaysian National Blood Centre, Kuala Lumpur, Malaysia, October 2024.
This initiative was undertaken at the request of the Clinical team leading quality efforts at the Malaysian National Blood Centre (Pusat Darah Negara, or PDN). Over the course of four days, 120 scientists and clinical staff from across Malaysia participated in a series of workshops and masterclasses designed to strengthen GMP compliance. The programme focused on core GMP principles and aimed to enhance participants’ understanding and practical application of these standards. Attendees were encouraged to take their learnings back to their respective regional blood banks to drive local improvements in quality and compliance. This engagement marked the beginning of a broader national initiative led by PDN, with the goal of embedding a culture of quality and regulatory excellence across Malaysia’s blood services.
Professional development
Privacy Capability Development
As part of strengthening organisational privacy capability, the Quality Business Partner within NZBS who also holds Privacy responsibilities completed two key training programmes:
• Privacy Officer Workshop: A two-day course covering the Privacy Act 2020, privacy programme development, data mapping, Privacy Impact Assessments, and breach management.
• Privacy in the Public Sector: A six-week course focused on applying privacy principles in the public sector, building a privacy culture, and managing breaches and complaints. The course included an assessment.
Quality Management System Training
• In 2024, a member of the Quality Systems team successfully completed the virtual, 10-week, National Association of Testing Authorities Australia (NATA) General Quality Management Systems Programme.
Internal Auditor Training
• Twenty Quality and Regulatory Affairs and Patient Services staff completed tailored Internal Auditor Training delivered virtually by NSF International. The course, run in two cohorts over three half-day sessions, covered audit principles, risk-based planning, and post-audit follow-up. The training reinforced the role of audits in driving continuous improvement and patient safety.
Professional development
Located within the Donor Services Directorate, NZBS’s Nurse Education Team has delivered a range of internal staff training over the past year. This has supported staff to effectively and safely adopt and deploy new and updated processes. Examples included:
• Training Donor and Technical Services’ staff in readiness for the go-live phase of the Self-Administered Health History (SAAH) system (see page 43).
• Rolling out Infection Prevention and Control (IPC) and hand hygiene training.
• Delivering a Qualified Medical Laboratory Technician (QMLT) programme to prepare Donor Technician trainees to sit the QMLT Pre-Analytical Technical exam.
• Launching a paediatric Therapeutic Plasma Exchange education module for NZBS’s Auckland and Christchurch Therapeutic Apheresis teams.
• Introducing, in Auckland, Principles of Blood Administration training. Developed in partnership with NZBS’s Transfusion Nurse Specialist team, this will be rolled out nationally next year and be tailored to each region’s hospital policies.
• Launching training for hemovigilance reporting and K2 (web-based system) donor adverse event reporting for therapeutic apheresis.
• Delivering regular training programmes, such as Workforce Coach training and Induction Days for Collections staff.
The Nurse Education Team also led the assessment of Professional Development Recognition Programme (PDRP) Expert level portfolios.
NZBS BOARD MEMBERS
Fiona Pimm (Board Chair)
DipAppSci, DPH, MBA
Dr Bart Baker
MBChB, FRACP, FRCPA
Nicole Anderson DBS, DipBus, DipMgt, PDGPH
Roger Jarrold B.Com FCA
Dr Edward Tanetoa Hutchins
BHB, MBChB, PGDipOphthBS, FRANCO
Anthony Bow BCom, CA, CA (Singapore)
Edie Moke, Independent Chair DBS, BBS, DBA, MBS, CMInstD
AUDITOR
Audit New Zealand (on behalf of the Auditor-General)
Level 14, Shortland & Fort, 88 Shortland Street, CBD, Auckland 1010
PRINCIPAL BANKERS
Westpac New Zealand Limited Level 6, 16 Takutai Square Auckland
SOLICITORS
Buddle Findlay
Auckland and Wellington
SOCIAL MEDIA
Facebook: New Zealand Blood Service
Instagram: nzbloodservice
LinkedIn: New Zealand Blood Service
Chief Executive Officer
Sam Cliffe
BSc (Hons)
Director Human Resources and Organisational Development
Karen Didovich
BA; MA (Political Studies); MA (Information Management and Finance)
Director Donor Services
Brett Paradine
PG Dip HSM, Master of Management (Dist)
Director Finance and Corporate Services
Kyle Beuth
NCB, NDA, Chartered Accountant
Chief Medical Officer
Dr Sarah Morley
MBBS, FRCPCH, FFICM, PhD
Director Planning and Supply Chain
Justin Scott
B.Com, MInstD, Ngāi Tahu
Director, Quality and Regulatory Affairs
Fidelma Murphy
MSc Distinction – Medical Ethics; BSc (Hons) –Health Studies and Health Care Management; Diploma in Professional Studies in Nursing
Director Technical Services
Dr Mandy Suddes PhD, PMP
Director, Digital Technology and Information Management
Joshua Bankers
Bachelor of Technology, Manufacturing and Industrial Technology
71 Great South Road, Epsom, Auckland
Tel: +64 (0) 9 523 5744
Fax: +64 (0) 9 523 5754