








President: Christopher Stevenson - McCormick & Co. Inc.
Vice President:
Susan Bergman - Griffith Foods Inc.
Treasurer: Paul Hargarten - Hawkins Inc.
Secretary: Kerri Goad Berrios – Kalustyan Corp.
Past President: Tim Sonntag – Wixon Inc.
DIRECTORS
Associate Member Directors
Danelle Boehme - Solina
Theresa Dubin - R. L. Schreiber Inc.
Wendy Epstein - First Spice Mixing
Denise Johnson – Newly Weds Foods, LLC
Alina Lastra - Badia Spices Inc.
Liz Morris - Elite Spice, Inc.
Susan Perez – Pacific Spice Co.
Peter Losee – Bluegrass Ingredients
Aret Meyer – Sensient Natural Ingredients
Executive Director
Robert C. Post, Ph.D., MEd., MSc. Marketing & Member Services
Geraldina A. Cristantiello


The National Seasoning Manufacturers Association, Inc. (NSMA), is the trade association representing the blended seasoning industry. Founded in 1973, NSMA members manufacture and market about 98 percent oftheseasonings consumed in theUnitedStates. Membership is available to all companies actively engaged in seasoning manufacturing and companies that support the industry with ingredients, packaging, food safety applications, testing, and other services.
As an educational and policy-informing organization, the purposes of the Association are to:
Anticipate, interpret and addressthetechnical andregulatoryneeds oftheseasoning industry;
Advocate on behalf of the members to shape, guide, and inform regulatory actions and public food policy dialogues toward improved policies;
Maintain singular focus on solutions to challenges the blended seasoning industry faces;
Encourage the exchange of technical and regulatory expertise among industry peers; and
Promote the safety, quality, and wholesomeness of seasonings and their components used to enhance the quality and appeal of food and beverage products.
Our role is to represent the interests of the seasoning manufacturer sector with food regulatory agencies by providing a practical, science-based voice to inform regulators about issues affecting the industry. We also keep our members updated on all priority issues. Through NSMA representation, face-to-face meetings, and ongoing interactions with HHS/FDA, USDA/FSIS, AMS officials, and other food and health agencies and organizations, as well as legislative officials, we build trust and credibility to advise and shape the rules and policies that govern the industry.
We are committed to promoting education within the seasoning industry and for related government officials about laws, regulations, policies, and guidance related to them. We support quality and safety standards and advocate for compliance with acceptable practices for handling and distributing products in the international spice, blended seasonings, and seasoning-containing products industries.
NSMA works to continually improve the quality and safety of incoming spices and other functional ingredients. For its members, it also addresses issues related to imported ground spices and the quality, safety, wholesomeness, and accurate labeling of seasoning blends. These blends include spices as just one component among many functional ingredients used in various products for sensory, functional, safety, andhealth benefits.
TheNSMAExecutive Director aims tokeepourOfficers, Board ofDirectors, and most importantly, all members, informed about emerging and real-time food regulation and policy issues that challenge member companies; create sector confusion regarding compliance, supply chains, procurement, and operations; and require communication to promote better or improved policies through meetings, gatherings, and engagement with policymakers, scientists, and other trade groups. Using insights and intelligence, the goal is for members to have the information their companies need to meet regulatory, customer, and consumerdemands inthemarketplace.

TheNSMA's keyfoodpolicyandscientificissues (not necessarilylisted bypriority) are:
Food and Ingredient Labeling
Criteria for Ingredient/Ingredient Technology Approval/Acceptance by Federal Agencies
Changes in Federal Processes for Food Ingredient Reviews, Notifications, and Approvals
FDA Food Ingredients and Contaminants Post-Market Safety Assessments
Contaminants, Such as Heavy Metals and PFAS
GMO/Bioengineered Ingredients – Safety, National Bioengineered Food Disclosure Standard, and Other Aspects of BE Product and Ingredient Labeling
Allergens – Updated Federal and International Policies and Trade Standards and Guidelines (e.g., Codex), New and Emerging Allergens (Including Sesame)
Bioterrorism and Biodefense Plans
Guiding Principles for Modernizing Food Standards of Identity – HHS/FDA and USDA/FSIS
Codex Alimentarius Standards and Guidelines
The Legislative and Regulatory Policy Landscapes and Liaisons for Informing Policies
FoodSafetyModernizationActof2011,e.g.,ProcessValidationGuidanceforProcessCategories, andPreventiveControls
EmergingIssuesinRisksPosedbyContaminants,IncludingHeavyMetals,Pesticides,Pathogens, andOtherPotentialEnvironmentalForeignMatter;andRelatedQuality,SafetySpecificationsand NecessaryFoodTestingMethodsandCapabilitiestoSupportSafeProductsintheMarket
Supply Chain Management: Traceability, Data-Sharing Platforms/ERP
The Status of Nutrition and Health Science Relative to Seasonings and the Foods to Which They Are Added, and in Support of Federal Dietary Guidance




Christopher Stevenson – McCormick & Co. Inc.
It has been mycontinued privilegeto presideas President oftheNationalSeasoningManufacturers Association during this 52nd year of the organization. This past year has brought many new challenges to our industry. The influence of the new Presidential Administration’s policies has required all our members to be nimble and reactive to manage changing regulations, potential tariffs, and new or changing HHS initiatives. Considering the unprecedented levels of activity we have experienced; I want to acknowledge the value that Dr. Robert Post provides to our organization as its Executive Director. His engagement with regulators and his regular “News You Can Use” Newsletter provide our membership with insight into how the regulatory landscape is evolving. Unfortunately, the changes made at the USDA and FDA have impacted our ability to create a direct opportunity for members to interface directly with regulators. Please know it is our intent to pursue restoration of the valuable agency interaction that the Government Liaison Meeting provides.
This past year, like the last, active recruiting as part of our management helped us to retain and grow membership. We currently represent 59 member companies, an increase of 4 from last year. Continued membership growth shows there’s a value in serving as a voice and conduit for policy issues, which helps our members’ businesses meet the challenges that our sector faced in the last year.
We continue to be a registered non-profit educational organization and mindful of working within the budget from membership dues. We continue to look for ways to improve the value of the Association for our membership. Continuously evaluating and adapting our Association to a changing policy and member services landscape, using lessons we’ve learned through the year. Considering what we have accomplished, I am certain we will continue to change, grow and remain inclusive as we move into the next year.
Lastly, I would like to say thank you to all the member companies for your continued support and involvement with the National Seasoning Manufacturers Association. I am certain that your support will continue to sustain NSMA for many years to come.

Christopher Stevenson, McCormick & Co. Inc. NSMA President 2024-2025




Robert C. Post, PhD
This Annual Report celebrates another successful year for NSMA, now in its 52nd year of supporting and leading the food policy efforts for the seasonings industry! It has been a year of building strong relationships with an impressive membership and collaborating with a dedicated and insightful President and Board of Directors to address key food policy issues and gather input on matters affecting our members. It also marked another year of monitoring policy developments and regulatory actions, providing timely updates and guidance, and representing the Association at national and international conferences, events, convenings, and meetings. In-person and virtual representation of NSMA and its members increased this past year, marking the transition to the post-pandemic era. In-person events and hybrid gatherings have made it even easier than before to connect with key opinion leaders, trade groups, research and policy-setting organizations, and policymakers.
To keep members up to date, more time was dedicated to our content-rich “NSMA News You Can Use” policy updates, which are distributed exclusively to members through our online NSMA Technical Forum. These updates covered regulatory, policy, science/research, safety, and market news, including emerging policy priorities for a new Administration, HHS, and USDA; federal actions toimprovepost-market food chemical safetyassessment programs; supplychain issues; new and emerging allergens; increasing agency policy interest in regulating contaminants such as heavy metals; the evolving FSMA rules on validation of treatment processes and guidance on preventive controls and inspections; advice on RTE and hazard controls; FAQs related to the not-so-new Nutrition Facts label; EPA and foreign agency actions on ethylene oxide; FDA’s nutrition agenda, including defining “healthy” and establishing a front-of-pack nutrition labeling scheme; USDA’s and FDA’s unified regulatoryagenda; and links to webinars, conferences, and public meetings. This year, the update was expanded to include policy actions at the Office of Management and Budget and Congressional legislation, from bills introduced to laws enacted, along with updates and links to major scientific and international health organization reports and consumer-related studies. An excellent schematic appears on the following pages to illustrate the policy priorities that I address for NSMA as we move into the next year.
Thanks to regular communication with members, the Association continues to receive feedback that the “News You Can Use” update is valuable and essential for members of a modern trade organization. The challenge of staying current with regulatory agency policy priorities and their effects on seasoning manufacturers has grown over the past year. While food safety and food labeling remain top concerns, understanding the impact of the FDA’s restructured Human Foods Program has taken prominence, as members have had to simultaneously update their facilities and operations to meet public health and regulatory standards, as well as new market and customer expectations. Keeping members informed remains a priority, and updated guidance and resources from industry alliances and research groups have helped to better inform members.

Robert C. Post, Ph.D.
These updates contain valuable hyperlinks to policy documents and reports and are filed as resources readily available to all members on the Members-Only Technical Forum.
Along with the responsibility to update and keep members informed, on behalf of NSMA, I also attended and participated in food, health, and agriculture conferences and meetings. These events included serving on Codex Alimentarius committees focused on nutrition and food labeling, and working with the U.S. Codex Office to advise on trade standard positions. They also involved being an invited participant and advisor at White House stakeholder meetings and Congressional hearings on food, nutrition, and agriculture issues; attending HHS/FDA’s public meetings on the plan for regulatory science improvements, contaminant regulation, and enhancing nutrition and food labeling; andparticipatingininternationalmeetings hosted byWHOonimprovingfoodsystems and access to healthyfood. These opportunities allowed me to highlight NSMA’s mission and concerns. More importantly, in these roles, I’ve used these events to network and engage with advocates on NSMA issues.
This year, our members benefited from NSMA’s involvement in the Food and Beverage Issue Alliance (FBIA) (www.feedingus.org). The FBIA is a group of directors from food and beverage trade associations who work together to develop consensus views and share resources to inform federal food regulatory officials and policymakers about urgent and important issues impacting growers, manufacturers, retailers, and consumers. FBIA has become a vital industry partner collaborating with FDA, USDA, and other food and health regulatory agencies to gain practical policy insights that balance consumer protection, public health, and support innovation and market opportunities across the entire food production and distribution chain. Participation in FBIA has provided NSMA with a collective voice to give input to FDA and USDA on food chemical safety assessments, supply chain challenges; analytical methods for contaminants and setting limits; comments on the 2025 Dietary Guidelines Advisory Committee's charge; guidance on ERP and available technologyfor traceability, labeling, and e-commerce packaging trends; policies related to dietary guidelines, 'healthy” claims; and import safety.
Working with the President and Board of Directors to find new ways to support members is a top priority for the upcoming year, along with enhancing our webinar and educational platforms and creating more members-onlyresources for the Technical Forum on our website. One goal is to add a compilation of questions and answers to the NSMA Members-Only resources, covering topics where members have sought NSMA guidance beyond just including them in the News You Can Use.
I look forward to working with our members in the year ahead to support them with current policy news and help them advance regulations and policies that balance their needs and those of the customers and consumers they serve.


Kerri Goad Berrios, Kalustyan Corp.
Since our last Annual Meeting in July 2024, the Board of Directors met multiple times over Zoom to discuss various issues and plans for programming. The following summarizes the purpose of and decisions made at those meetings.
The Board met via Zoom on July 26, 2024 soon after our Annual Meeting to elect its officers. The current officers agreed to retain their positions and the remaining Board were all in agreement. The following are the Officers elected for the one year term:
President: Christopher Stevenson, McCormick & Co. Inc.
Vice President: Susan Bergman, Griffith Foods Inc.
Treasurer: Paul Hargarten, Hawkins Inc.
Secretary: Kerri Goad Berrios, Kalustyan Corp.
Past President: Tim Sonntag, Wixon Inc.
A second Zoom meeting was on December 9, 2024, the end-of-the-year Board meeting. Items discussed included NSMA’s engagement in regulatory and policy events, and comments on policy issues. An update and summarywas received from the events that occurred at IFT FIRST; namely, the NSMA Social Gathering on Sunday Night which provides a way for members to connect with others as well as an insider’s view for nonmembers. The Annual Report was created in digital form and placed on the website for members and nonmembers to learn about the workings of NSMA. It is also a reflection of our Annual Meeting. We also discussed that the Government Liaison Committee meeting should continue to be held at a venue where agency officials can present to our members. NSMA’s membership in FBIA (Food and Beverage Issue Alliance) has proven to be a great collaboration for insights and viewpoints on regulatory issues facing our industry and the Board supports this membership. During this meeting, it was agreed to continue to hold our Annual Meeting in conjunction with IFT FIRST with a desire to continue to hold the meeting in the morning. Other ideas and discussions related to marketing and recognition to be implemented in future plans.
The Board also convened virtually in its spring meeting on April 4, 2025. The meeting focused on updates regarding membership, upcoming board member term expirations, and considerations for boththe2025Government LiaisonCommittee(GLC)MeetinginWashington,DC,andtheAnnual Meeting and Social Gathering at IFT FIRST. It was discussed that the GLC meeting may not be possible if the key HHS and USDA officials are not in place and the new administration’s policy priorities are not aligned in a timely manner. Additional discussions included plans to survey members on topics of interest to help shape future programming, as well as the development of a streamlined platform to support website, email, and administrative functions affordable for a small organization.


Paul Hargarten, Hawkins, Inc. (Treasurer) & Robert C. Post, Ph.D., Executive Director (Bookkeeper)
Now in its 52nd year, NSMA has continued to grow since its founding in 1973, with 59 active member companies todayand more than 150 individuals listed as contacts in the NSMA Technical Forum. Company representation accounts for about 98 percent of the seasoning blenders in the U.S., with a combined marketplace presence worldwide and across all channels food service, retail, B2B, and e-commerce (D2C, etc.). Income has risen as membership has expanded. Even so, the annual dues NSMA charges are modest compared to other trade groups and, more importantly, given the value of access to timely insights on current and emerging food policy and trade issues, a resource library, and opportunities to network and seek advice from industry peers. Other trade groups charge ten times what NSMA charges for dues, often offering similar or fewer services or only policy issue monitoring. Therefore, in terms of value for dues paid, NSMA stands out as a highly cost-effective and successful organization.
As a registered non-profit educational (501(c)(3)) organization, NSMA faces limitations on how it can generate income. Therefore, membership dues have always been a primarysource of revenue. In 2024, NSMA member dues income was about $11,000 more than the previous year. In addition, with the inductionofsponsorshipsin2024attheNSMAAnnualmeeting,additionalincomewasgenerated,some of whichoffset thecosts oftheNSMAannual meeting.
From a finance accounting standpoint, NSMA bookkeeping is not at all comprehensive. It is similar to a checkbook register involving a cash-basis accountingmethod. As a non-profit, NSMA has no investments or losses to report, nor does it rent space, pay for equipment, or need to project budget needs to maintain contingency funds for facility or equipment maintenance or services. NSMA receives member dues income, which are added to sponsorship income, and recorded as “income.” It has administrative expenses for meetings and operating expenses for management, communications, and reporting, which are simply deducted from the NSMA checking account register and recorded as “expenses.”
Regarding expenses in 2024, from January 1 to December 31, 2024, the majority of funds expended were for costs associated with the in-person NSMA networking event during the NSMA Annual Meeting in Chicago in July 2024, as well as the cost of holding the Annual Meeting in conjunction with the Annual Board meeting In 2024, the NSMA Annual Government Liaison Committee meeting returned to an in-person event at a new location in the Washington, DC area, which had associated expenses for the venue and meals.

NSMA Treasurer’s Report for Fiscal Year 2024 – (cont’d.)
Paul Hargarten, Hawkins, Inc. (Treasurer) & Robert C. Post, Ph.D., Executive Director (Bookkeeper)
Expenses for the NSMA networking event, the Annual Meeting Program, and the Board meeting in July 2024 included meeting venue rooms and amenities, audiovisual equipment, food, printing and signage, and branded giveaways totaling $23,169.35.
In addition, travel and registration costs for NSMA management staff attending the Annual Meeting and other events totaled $ 5,563.49. The next largest expense was for fees paid to NSMA management by the Executive Director, supported by the Marketing and Member Services Manager. Finally, NSMA pays $3,500 in dues for membership in the Food and Beverage Issue Alliance. Overall, expenses amounted to $83,267.60.
In 2024, total expenses were slightly below income. Still, the organization maintained a healthy bank balance that is about equal to its income without having to resort to using any of its savings for any purposes.
Looking to 2025, the financial picture for NSMA looks satisfactory. Member dues income should exceed expenses. Investments in enhancing the website and other administrative functions, should the Board of Directors agree to them, will be covered byincome.
The Treasurer’s Report of NSMA finances for FY 2024 is shown on the next page.


NSMA Treasurer’s Report for Fiscal Year 2024 – (cont’d.)
Paul Hargarten, Hawkins, Inc. (Treasurer) & Robert C. Post, Ph.D., Executive Director (Bookkeeper)
National Seasoning Manufacturers Association, Inc. (NSMA) Treasurer’s Report for Board of Directors Meeting July 13, 2025 January - December 2024
January 01 – December 31, 2024: Income and Expenses
Income
Includes:
Member Dues, January 01 – December 31, 2024
Other Income (Sponsorships for NSMA Event at IFT, July 16, 2024 (2 Gold, 3 Silver, 2 Bronze, 3 Chrome) $ 77,267.60** $ 6,000.00
Total Income for 2024 $ 83,267.60
Expenses
Includes (for January 01 – December 31, 2024):
Fees for management services from FoodTrition Solutions, LLC and GC Business Services, LLC
Annual business reporting, website/domains/email services, dues collection services
Travel and conference registrations for Executive Director and Mktg/Member Services Manager
Travel-related administrative costs such as shipping
NSMA GLC Meeting Venue, Food, A/V, Group Dinner
NSMA annual meeting, BOD meeting (venues, food, swag, printing, annual report)
2024 Member dues for FBIA $ 80,811.47
Total Expenses for 2024 $ 80,811.47 Bank Account Balance as of December 31, 2024 $ 82,783.73** * Member dues total: 55 ** Some checks that have been issued or pending receipt have not cleared the bank as of December 31, 2024
Respectively Submitted to the Treasurer, Paul Hargarten, Hawkins
Robert C. Post, Executive Director, NSMA

Robert C. Post, Ph.D., Executive Director
NSMA has been dedicated to building and sustaining reliable and collegial relationships with policymakers in the food and health regulatory, research, and agricultural marketing agencies, as well as congressional offices and international trade standard organizations.
The Government Liaison Committee (GLC) meetings with these officials have been a highlight of each year since NSMA's inception.
In May 2024, NSMA members convened the GLC at the Marriott Hotel & Conference Center in College Park, Maryland, with a comprehensive agenda that spanned a day and a half. We heard from HHS/FDA and USDA/FSIS officials on the regulatory priorities and actions impacting our industry. However, in 2025, with the change in Administrations, there was a delay in establishing the food and health regulatory policy priorities and the policy leaders with whom to engage. A GLC meeting in the spring of 2025, with sufficient insights into the policy landscape for the next four years for HHS and USDA, was not feasible.
We are pleased, however, to have the content on page 17 of this Report (NSMA Works to Inform Policy Makers…) and page 28 (Chronology of Matters of NSMA Engagement) to reflect on the everyday, ongoing interactions of NSMA's Executive Director in representing policy matters of importance to NSMA members. These policy issues are also reflected in the routine and comprehensive NSMA News You Can Use Policy Updates that are provided to members through our NSMA Technical Forum.
NSMA plans to hold its next GLC meeting in 2026 with full representation of policy officials on matters of importance to members. In the meantime, we are pleased that Mark Hartman, Director of the FDA's Office of Food Chemical Safety, Dietary Supplements, and Innovation within the Human Foods Program, will be able to speak at the 2025 NSMA Annual Meeting.

Government Liaison Meeting Attendees - Washington, DC | February 24-25, 2020


Geraldina A. Cristantiello, Marketing & Member Services

Aswe reflect on thepast year, NSMA’scommitmentto communication, engagement, andindustry connection has remained strong. Our goal is to ensure that every member both Voting and Associate feels informed, connected, and valued.
This year, I had the opportunityto attend numerous industrytrade shows, where I engaged directly with both members and non-members. These conversations are essential to understanding what current and prospective members are seeking from NSMA. The insights gained continue to shape our outreach, retention efforts, and the development of meaningful member benefits.

For the first time, we introduced Products & Services Spotlight Tables during our Social Gathering reception. These highboy-style tables introduced a fresh, informal way for Associate Members to highlight their offerings during the event. Designed to spark genuine conversation and build relationships, the format offered strong visibilitywithout theconstraints ofaformal exhibit setup. Interest is growing, and we anticipate increased participation moving forward.
This initiative reflects our ongoing commitment to enhancing the value of Associate Membership and encouraging broader participation. We will continue developing creative ways for Associate Members to connect meaningfully with the full NSMA community.
We continue to strengthen NSMA’s communications strategy through multiple channels:
NSMA Technical Forum: Our members-only Google Group remains a key space for collaboration, questions, and the sharing of regulatory and industry insights. If you're new to the group or need help accessing it, we’re happy to assist.

Geraldina A. Cristantiello, Marketing & Member Services
News You Can Use: Now in its seventh year, this biweekly newsletter continues to be one of the most valued member benefits, offering timely updates on regulatory policy, food safety, science, and market trends.
Online Presence: Our website (seasoningmanufacturers.org) remains the hub for member resources. We also continue to grow our visibility across LinkedIn, Facebook, and Instagram (@seasoningmanufacturersassoc), with plans to expand our digital presence further in the coming year.
As we continue to build on this momentum, NSMA remains focused on listening to members, evolving our offerings, and creating more touchpoints for connection. These efforts across various venues such as tradeshowsandin-person gatherings, andthrough multiplecommunication channels including digital outreach and social media have contributed to the addition of several new members this year. Just as importantly, they have played a key role in strengthening member retention by consistently reinforcing the value of being part of NSMA.
Thank you for being an essential part of this thriving, well-seasoned community.


Robert C. Post, PhD., Executive Director
Food policy issues and regulatory requirements can be and often are challenging for our sector. Compliance expectations, testing needs, analytical support, supply chain impacts, and labeling are just some factors that can make regulatory and policy actions arduous. One of the benefits for NSMA members is the availabilityof expert advice from our Executive Director and our network, provided through the Members-Only Technical Forum. On behalf of our members, I’ve been able to give a voice to inform senior policy officials at HHS/FDA, USDA/FSIS and AMS, and Codex/FAO on a variety of issues, providing guidance and promoting practical solutions to challenges. This past year has been especiallydemanding, with changes to the FDA Human Foods Program structure and pending changes to Administration leadership and priorities.

Some of the policy issues and actions on which members sought advice from NSMA are:
Supply chain disruptions for ingredient sourcing and availability, transportation, packaging materials and the likelihood of FDA guidance on regulatory compliance, included insights on specific ingredient substitutions.
The FDA’s priorities on issuing guidance and timelines for implementation.
Guidance on the FDA food chemical safety post-market assessment program enhancements.
Guidance on potential changes at the FDA to the programs and processes for food additive, color additive, and food contact substance approvals, and no-objection reviews.
FDA GRAS Notifications and the process for inquiries and the timeliness of such
The FDA’s traceability proposed rule.
Amenability of products to USDA-FSIS jurisdiction and inspection versus FDA jurisdiction/inspection.
Import requirements for food safety and labeling.

FDA sources of irradiation regulations and provisions for foods, ingredients, and components of seasonings.
FDA regulatory allowances for ETO use and controls, and international trade standards and challenges
Heavy metals, the FDA’s Closer to Zero initiative, and the potential impact of recommended action levels on seasoning products.
E-commerce and potential guidance on labeling meal kits with seasonings.
The likelihood of additional allergens on the FDA’s “big” allergen list.
The USDA AMS BE Disclosure Standard and the commodities and ingredients on the “required disclosure” list.
Classifying Dried Vegetables as RTE and Determining the Appropriate Preventive Control Strategy
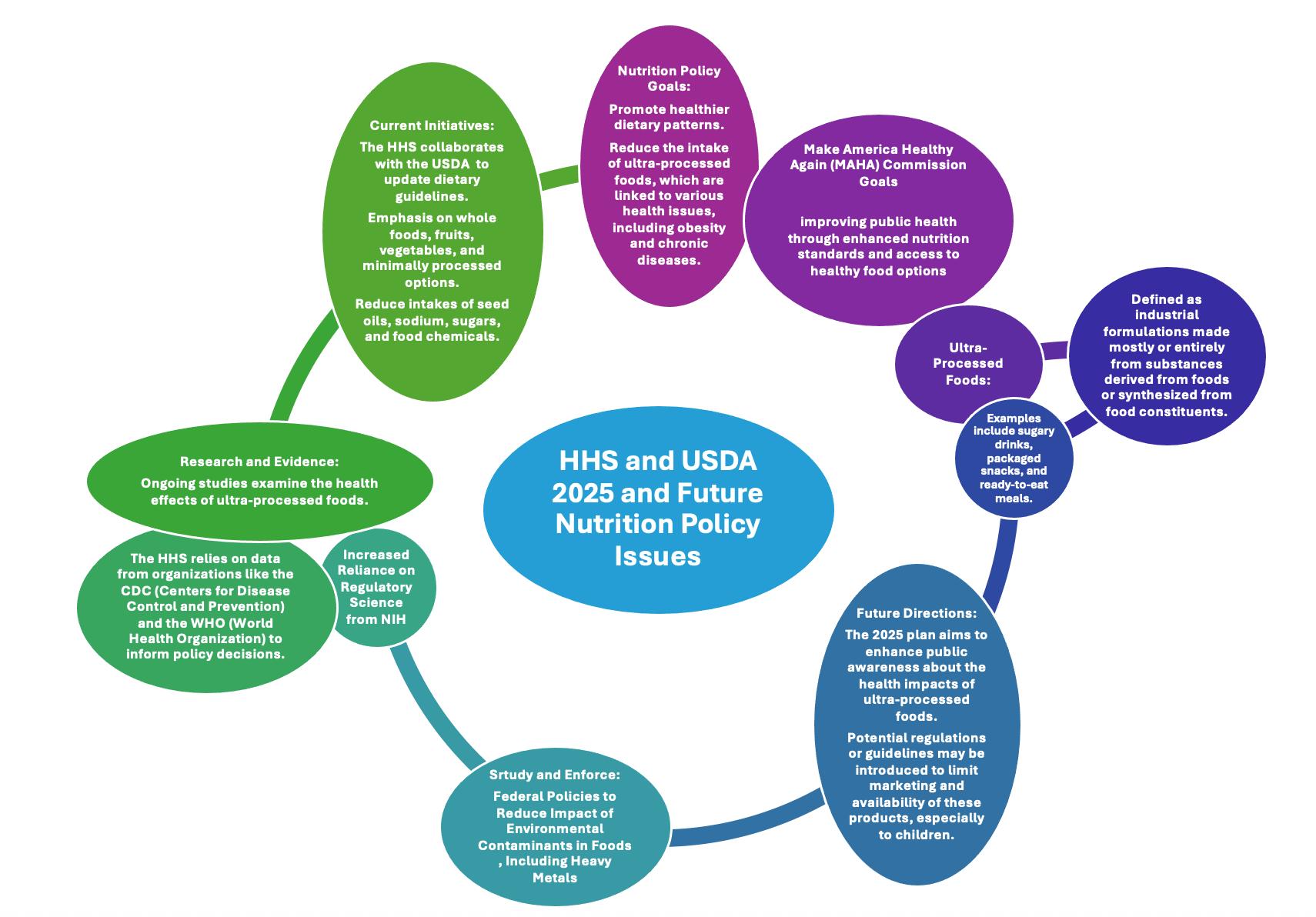

(cont’d.)
These topics reflect the swirl of regulatory actions and policy issues FDA and USDA are addressing, as well as issues related to international food trade standards As an example to show just how current these member inquiries are, the link to current FDA Human Foods Program Guidances under development is here, and a link to the Guidances that have been published for expected adherence by industry is here (https://www.fda.gov/food/guidance-documentsregulatory-information-topic-food-and-dietary-supplements/foods-program-guidance-underdevelopment; and https://www.fda.gov/food/guidance-regulation-food-and-dietarysupplements/guidance-documents-regulatory-information-topic-food-and-dietary-supplements), respectfully).
The FDA has issued the following guidances that were on the list (accessed here):

NSMA remains actively engaged, through its Executive Director, with FDA and USDA policymakers to promote transparency for stakeholders regarding foods program priorities. The list of Guidance Documents reflects the FDA’s current perspective on specific topics and can help stakeholders anticipate potential changes that could affect their businesses and organizations. While guidance documents do not carry legally enforceable requirements, they offer useful insights into how policymakers view certain issues.

(cont’d.)
Although the FDA's intent is to publish all guidance topics as final on the list, modifications in plans may be needed to support emerging issues and Administration priorities. FDA also may issue additional guidances that are not on the list. Therefore, NSMA and the Executive Director will remain constant in ways to engage FDA and USDA, and Codex, officials to inform them on policies that affect our sector and will bring back the learnings and updates to the NSMA Membership.
As for regulatory actions, NSMA monitors and reports on activities related to rule development and publications as they occur. NSMA’s Executive Director routinely interacts with the agencies on current regulatory priorities at the FDA, for example, seeking clarity of intent and timing to gauge impact on members’ products and operations and attending in-person expert panels, roundtables and convenings. NSMA has been involved in convenings on plans for the FDA and NIH collaboration on modern approaches to developing regulatory science, and enhancing the program for post-market assessments of food chemical safety. Updates are routinely provided, along with pertinent links, in the NSMA News You Can Use policy updates.







Geraldina A. Cristantiello, Marketing & Member Services
NSMA Membership is based on a calendar year and as such this report will provide you with a recap of where we finished our year in 2024. Also included is a status report on this year’s membership as of June 30, 2025. New members that apply for membership remit pro-rated dues to the closest quarter.
The chart below shows our status as of December 2024. We currently have some members who have not yet paid their dues for 2025
Below is a chart comparing resignations to total number of members over the last ten years:

Geraldina A. Cristantiello, Marketing & Member Services
We are pleased to report that our Membership has been steadily growing. Since our last annual meeting in July 2024, we have welcomed four (4) new members, listed below:
Herbospice SL
ASSOCIATE MEMBERS
Artiste Flavor / Essence Inc. DBA Artiste
Jascole/ Haospice
Omya Inc.
As of June 30, 2025, we have 59 members. Although we gained 4 new members, we did have one resignation (Olds Products Company). At the time of this writing, we are awaiting payment from some members.
July 1 - June 30 Yearly except where noted


Geraldina A. Cristantiello, Marketing & Member Services
The chart below illustrates our increasing membership, notably with a significant rise in our Associate Members. We attribute this growth to newly introduced benefits tailored specifically for Associate members, reinforcing the value of remaining members of NSMA.
July 1 - June 30 yearly except where noted
Membership in the National SeasoningManufacturersAssociation (NSMA) is companybased and its benefits extend to all employees of your company. I strongly encourage you to involve others and highlight how NSMA’s resources can be of benefit them daily.
In lookingto the future, we will continue to recruit members and appreciate your referrals and leads. Please send them to me by email (gerri@seasoningmanufacturers.org), text/phone: (201) 657-1989 and I will be pleased to let them know the importance and value of membership.


As of June 30, 2025 *Indicates Associate Member
A.C. Legg, Inc.
Adams Flavors, Foods & Ingredients
Advanced Spice & Trading, Inc./Valdez Spice
Artiste Flavor / Essence Inc. *
Badia Spices, Inc.
Bag Supply Company *
BCFoods/Culinary Farms
Beaconpoint Labs *
Blue Line Foods
Bluegrass Ingredients *
Carolina Ingredients LLC *
Certified Laboratories, Inc.*
Chesapeake Spice Co. LLC
Deep South Blenders
Deibel Laboratories*
Elite Spice, Inc.
Everson Spice Company, Inc.
First Spice Mixing Co. Inc.
Fuji Foods
Givaudan
Griffith Foods, Inc.
Harris Spice
Hawkins Inc.
Herbospice SL
Illes Seasonings & Flavors
International Spices
iSpice Foods
Jascole/Haospice *
Kalsec Inc. *
Kalustyan Corp.
Kerry Ingredients
Kutas North America *
Louisiana Fish Fry Products, Ltd. *
Macco Organiques, Inc.
Magic Seasoning Blends, LLC
MANE
McCarthy Spice & Blends
McClancy Seasoning Co.
McCormick & Co. Inc.
MG Spices
Newly Weds Foods, LLC
Nu Products Seasoning Co.
Old World Spices and Seasonings
Omya Inc. *
Pacific Farms *
Pacific Spice Co. Inc.
Pristine Finds LLC
R.L. Schreiber, Inc.
Rio Valley Chili
Rocky Mountain Spice Company
Sabater USA
Savor Seasonings Inc.
Sensient Natural Ingredients *
Silgan Specialty *
Solina
Sterigenics Intl. Inc. *
Topchance Foods Inc. *
Wixon Inc.
World Flavors Inc. *

Geraldina A. Cristantiello, Marketing & Member Services
This past year NSMA had four (4) Director positions and one (1) Associate Member Director with terms expiring. With this in mind, the NSMA administrative office was asked to send out an email to the membership asking for nominations. We received some interest from both Voting and Associate members.
The Nominating Committee (comprised of Tim Sonntag, Kerri Goad Berrios, and Alina Lastra) reviewed the nominations and selected its candidates.
The Nominating Committee is pleased to present the following Slate of Nominees for the Board of Directors for approval by the membership at the 2025 Annual Meeting.
These members will serve a three-year term, ending in 2028:
Susan Bergman, Griffith Foods, Inc.
Danielle Dutton, Rocky Mountain Spice
Liz Morris, Elite Spice
Susan Perez, Pacific Spice Co
Additionally, the following representative from the Associate category agreed to serve a two-year term ending in 2027:
Aret Meyer, Sensient Natural Ingredients
On behalf of the Board of Directors, Rob Post and myself, we extend our gratitude and thanks to Ms. Kerri Goad Berrios who has served on the NSMA’s Board of Directors for the past six years. She has been a valuable contributor to our Board.

Robert C. Post, PhD., Executive Director
Geraldina A. Cristantiello, Marketing & Member Services
We trust that this Annual Report, covering the period from 2024 to 2025, has provided members with a comprehensive account of the Association’s activities for the past year, our 52nd. As we move into 2025, we will continue our efforts to deliver more valuable resources for members, while also undertaking some new initiatives. Here’s what our plan will include:
Continue to expand our membership and explore new ways to foster networking with complementary trade and professional organizations.
Within budgetary means, enhance our website to provide a more comprehensive view of NSMA, including both public and members-only sections.
Continue to provide members with the “News You Can Use: NSMA’s Food Regulatory, Policy, Science, Safety and Market News” policy updates, and archive them along with the helpful links to references and resources in the members-only section of the website.
Increase our involvement in the Food and Beverage Issue Alliance (feedingus.org), sharing NSMA viewpoints and insights that help shape better and more informed federal and state food safety and nutrition policies.
Add more resources to the members-only section of the website based on members’ needs.
Host webinars on relevant topics accessible to both members and non-members.
Collaborating on educational training opportunities for our members.
Sponsor sessions at conferences that challenge and advance seasoning manufacturers and the seasoning industry
Develop communication tools for our website and for members, consumers, and professionals about seasonings, including quality, nutritional benefits, and how they contribute to making healthy foods more enjoyable.
Aswecontinue to growthis organization,werelyon you, ourmembers, to guideus in representing your interests in the blended seasoning industry by advocating for balanced and effective regulations and policies. We appreciate your membership and thank you for your trust in us.


Disclosing
Ingredients in Proprietary Seasoning
Formulas and NSMA Certifications
Ethylene Oxide Residuals
Nitrates/Nitrites in Seasoning Blends
Color Additives in Seasonings Treating Ingredients with Sources of Ionizing Radiation
Sulfites/Sulfiting Agents and Labeling
Flavor/Natural Flavor Labeling
Allergens Labeling
Heavy Metals and Other Contaminants: Limits and Controls
Traceability:
FSMA: Preventive Controls for RTE and Not RTE Ingredients
Supply Chain Challenges for Ingredients
Federal Truck Inspection Policy



