






















President: Christopher Stevenson - McCormick & Co. Inc.
Vice President: Susan Bergman - Griffith Foods Inc.
Treasurer: Paul Hargarten - Hawkins Inc.
Secretary: Kerri Goad Berrios - Kalustyan
Past President: Tim Sonntag - Wixon
Eric Baylin - Chesapeake Spice Co.
Danelle Boehme - Asenzya, Inc.
Theresa Dubin - R. L. Schreiber Inc.
Wendy Epstein - First Spice Mixing
Alina Lastra - Badia Spices Inc.

Liz Morris - Elite Spice, Inc.
Susan Perez – Pacific Spice Co.
Associate Member Directors
Steve Markus - Sterigenics
Aret Meyer – Sensient Natural Ingredients
Executive Director
Robert C. Post, Ph.D., MEd., MSc.
Marketing & Member Services Geraldina A. Cristantiello National

The National Seasoning Manufacturers Association, Inc. (NSMA), is the trade association representing the blended seasoning industry. Founded in 1973, NSMA members manufacture and market about 98 percent of the seasonings consumed in the United States. Membership is available to all companies actively engaged in seasoning manufacturing and companies that support the industry with ingredients, packaging, food safety applications, testing, and other services.
As an educational and policy-informing organization, the purposes of the Association are to:
Anticipate, interpret and address the technical and regulatory needs of the seasoning industry;
Advocate on behalf of the members to shape, guide, and inform regulatory actions and public food policy dialogues toward improved policies;
Maintain singular focus on solutions to challenges the blended seasoning industry faces;
Encourage the exchange of technical and regulatory expertise among industry peers; and Promote safety, quality and wholesomeness of seasonings and their components.
Our role is to represent the seasoning manufacturer sector’s interests with the food regulatory agencies, providing a practical and science-based voice for informing regulatory officials on issues affecting the industry, as well keeping members up to date on all the priority issues. Through NSMA representation, face-to-face meetings and ongoing interactions with HHS/FDA and USDA/FSIS and AMS officials, and legislative officials, builds trust and credibility to inform and shape the policies that govern the industry.
We are dedicated to promoting the goal of education for the seasoning industry and the affiliated government liaisons, about laws, regulations, policies, and guidances pertaining to them. We endorse quality and safety standards and endorse compliance with acceptable handling and distribution approaches within the international spice, blended seasonings, and seasoningcontaining products industries.

NSMA works to promote the continual improvement in quality and wholesomeness of incoming spices and other functional ingredients, and to address issues of imported ground spices and the quality, safety, wholesomeness, and accurate labeling of seasoning blends. These blends include spices as a component in addition to many other functional ingredients in products that are added to many product categories for sensory, functional, food safety, and health benefits.
The NSMA Executive Director strives to keep our officers, Board of Directors, and most importantly, all members, apprised of emerging and real-time food regulatory and policy matters that challenge member companies; cause sector confusion about compliance, supply chain, procurement, and operations; and require informing to advance better or improved policies through convenings / meetings / engagement with policy makers, scientists, and other trade groups. Through

insights and intel, the goal is for members to have the information their company needs to meet regulatory, customer, and consumer requirements and demands in the marketplace.
The NSMA focal food policy and scientific issues (not necessarily in priority order) are:
Food and Ingredient Labeling
Criteria for Ingredient/Ingredient Technology Approval/Acceptance by Federal Agencies
GMO/Bioengineered Ingredients – Safety, National Bioengineered Food Disclosure Standard, and Other Aspects of BE Product and Ingredient Labeling
Allergens – Updated Federal and International Policies and Trade Standards and Guidelines (e.g., Codex), New and Emerging Allergens (Including Sesame)

Bioterrorism and Biodefense Plans
Guiding Principles for Modernizing Food Standards of Identity – HHS/FDA and USDA/FSIS
Codex Alimentarius Standards and Guidelines
The Legislative and Regulatory Policy Landscapes and Liaisons for Informing Policies
Food Safety Modernization Act of 2011, e.g., Process Validation Guidance for Process Categories, and Preventive Controls
Emerging Issues in Risks Posed by Contaminants, Including Heavy Metals, Pesticides, Pathogens, and Other Potential Environmental Foreign Matter; and Related Quality, Safety Specifications and Necessary Food Testing Methods and Capabilities to Support Safe Products in the Market
Best Practices for Food Manufacturers During a Pandemic
Supply Chain Management: Traceability, Data-Sharing Platforms/ERP


It has been my sincere privilege to preside as President of the National Seasoning Manufacturers Association during this 50th Anniversary year. Our longevity is a testament to the value our association has provided its membership since our inception in 1973. I am certain that the membership would agree this past year has seen a return to near normal after a couple of years of pandemic stresses – challenges from the supply chain, a flurry of catch-up policy and regulatory actions from the federal agencies, and a customer and consumer marketplace eager for recovery and the dependability of familiar products and enticing new products. The repercussions of the pandemic have heightened the importance of supply chain management, a theme that has taken the attention of manufacturers, customers, and policy makers. In this recovery, issues like contaminants, the availability of food safety treatments, the availability of essential ingredients for processing and functionality, and acceptable ingredient labeling have become intertwined with potential for supply chain repercussions and the need for issuances of new and updated guidances and regulations. Even with these challenges, as I reflect on the past year as President of the National Seasoning Manufacturers Association, I am pleased with the advancements our Association has made to keep current with and effectively engage in policy developments for members.

This past year, like the last, active recruiting as part of our management helped us to retain and grow membership. Compared to pre-pandemic numbers, it has enabled us to grow and sustain 50 member companies. That shows there’s a value in serving as a voice and conduit for policy issues, which helps our members’ businesses meet the challenges of supply and demand that our sector faced in the last years.
We continue to be a registered non-profit organization and mindful of working within the budget from membership dues. The small extra cost for our membership in the Food and Beverage Issue Alliance (FBIA, feedingus.org) has resulted in great value for our members, enabling our Association to be active in a platform for coalition building among directors of other food trade groups on hot policy issues and public comments, adding to opportunities in addition to his regular interactions with senior policy officials. The regular and ongoing interactions of our Executive Director with policy officials also produces new information sources and resources, which enable us to be responsive with immediate and regular communications on emerging and timely policy with updates and breaking news. We continue to look for ways to improve the value of the Association for our membership.
As a hold-over from the ways of working these past couple of years, we have emerged with a hybrid approach to networking – offering virtual channels and in-person channels. But we believe that we’ve worked well to support members last year through virtual access to events, such as our Annual Government Liaison Meeting with FDA in June 2023. We’ve supported members with









 Christopher Stevenson
Christopher Stevenson
speakers on topics of importance at the 2022 in-person NSMA Annual Meeting, and through opportunities to seek out advice and insights among members through the members-only Technical Forum. The Association received twice as many inquiries on policy positions and requests for advice on policies and regulatory requirements, than the previous year, showing that we continue to be connected effectively to member companies and their needs through our Technical Forum and email.
We understand the need to adapt our Association to a changing policy and member services landscape using lessons we’ve learned through the year. Considering what we have accomplished, I am certain we will continue to change, grow and remain inclusive as we move into the next year.

Thank you to all the member companies for your continued support and involvement with the National Seasoning Manufacturers Association. I am certain that your support will continue to sustain NSMA for the next 50 years!



 Christopher Stevenson, McCormick & Co. Inc. NSMA President 2022-2023
Christopher Stevenson, McCormick & Co. Inc. NSMA President 2022-2023














 Robert C. Post, Ph.D.
Robert C. Post, Ph.D.
This Annual Report marks a tremendous milestone for NSMA: 50 years of support and leadership for the seasonings industry! And, another year of building great relationships with an impressive membership and working with a dedicated and insightful President and Board of Directors to manage issues and gather Association input on important food policy matters affecting members. It also marked another year of monitoring policy issues, providing timely updates and advice, and representing the Association at national and international conferences, events, and meetings. The emergence of hybrid gatherings, made it even more convenient than in the past to connect with policy makers, key opinion leaders, and research organizations.

To keep members current, more time was devoted to content-filled “NSMA News You Can Use” policy updates through our online NSMA Technical Forum. These updates covered regulatory, policy, science/research, safety, and market news, with topics such as supply chain issues like a nitrite shortage and FSIA and FDA advice; recalls on allergens and growing policy interest in contaminants like heavy metals; the evolving FSMA rules on validation of treatment processes and guidance on preventive controls and inspections; guidance on RTE and hazard controls; FAQs on the not-so-new Nutrition Facts label; FDA’s Era of Smarter Food Safety and e-commerce; EPA registrations of products; FDA’s Nutrition Innovation Strategy, including defining “healthy” and modernizing food standards; USDA’s and FDA’s unified regulatory agenda; and links to webinars, conferences, and public meetings This year, the Update was expanded to cover policy actions at the Office of Management and Budget and Congressional actions, and updates and links to major scientific and international health organization reports.
Thanks to a regular exchange with members, the Association received feedback that the “News You Can Use” update is of value and a necessity for a modern trade association. The challenge to keep current with regulatory agency policy priorities and their impacts on seasoning manufacturers increased in the past year. While food safety and food labeling remain top priorities, supply chain issues have taken center stage as members have had to simultaneously fit or retrofit new ways of working into their facilities and operations to meet public health and regulatory guidelines and new market and customer demands. Keeping membership informed is a priority, so updated guidance and resources from industry alliances and research organizations helped to better inform members.
These updates contain valuable hyperlinks to policy documents and reports and are filed as resources readily available to all members on the Members-Only Technical Forum.
Along with the role to update and keep members apprised, on behalf of NSMA, I also attended and participated at food, health, and agriculture conferences and meetings. These events included serving as a US Delegate on Codex committees; as an invited participant and advisor at White House stakeholder meetings and at congressional hearings on food, nutrition, and agriculture matters; as an attendee at FDA’s public meetings on the culture of food safety and the era of smarter food safety, and for planning for a White House Conference on Hunger, Nutrition and Health; and at international meetings held by WHO on improving food systems and access to healthy food. These offered opportunities to highlight NSMA’s mission and issues of concern. More importantly, in these roles, I’ve been able to use these events to network and engage on NSMA issues.
This year, our members benefited from NSMA membership in the Food and Beverage Issue Alliance (FBIA) (www.feedingus.org). The FBIA is an organization comprised of directors of food and beverage trade associations who work together to form consensus views and collective resources to inform federal food regulatory officials and policymaking on issues of urgency and importance to growers, manufacturers, retailers, and consumers. FBIA has emerged as a key industry group that FDA, USDA, and other food and health regulatory agencies are conferring with to gain insights and about practical and practicable policies that balance the needs of consumer protection, assuring public health, and enabling innovation and market opportunities for all segments of the food production and delivery chain. Engagement in FBIA for NSMA has enabled a collective voice to inform FDA and USDA with inputs on supply chain issues; analytical methods for contaminants in considering limits; comments on the charge of the 2025 Dietary Guidelines Advisory Committee; advice on ERP and available technology for traceability, labeling and packaging trends in ecommerce; advice on policies for dietary guidelines/healthy/”natural”; and advice on import safety.
Working with the President and Board of Directors on new ways to support members is on the top of the list of goals for the year ahead, along with building our webinar and educational platforms and capabilities; and creating more members-only resources for the Technical Forum on our website.
I look forward to working with our members in the year ahead to support them with current policy news and help them advance regulations and policies that balance their needs and those of the customers and consumers they serve. Happy 50th members!


Since our last Annual Meeting in July 2022, the Board of Directors met multiple times over zoom. The following summarizes the purpose of and decisions made at those meetings.
The Board met via Zoom on January 4th . The Board received updates on Members who were delinquent in dues and agreed to drop those that had not paid their 2022 membership dues.
Dr. Robert Post reported that Government Liaison Meeting could not be held in person as government officials were not all in their offices. The Board agreed, upon Dr. Post’s recommendation, that our Government Liaison meeting should be held virtually again this year. The Board suggested that this would allow more flexibility in scheduling the meetings with FDA and USDA.
Plans for the annual meeting were discussed and the Board agreed to hold the 2023 Annual Meeting in conjunction with IFT FIRST Meeting & Expo on July 18, 2023 in Chicago, IL. Gerri would work on the location and logistics. It was also noted that 2023 marks NSMA’s 25th Anniversary and a plan would be put into place for a celebration.
Under New Business a director advised that he was contacted by Laura Shumow of the American Spice Trade Association (ASTA) advising that the Association wants to collaborate with NSMA and that he was invited to participate in a meeting. It was agreed that Rob Post and Gerri Cristantiello would also participate on this call as well as other members of NSMA’s Board.
Other issues addressed include:

Review of Treasurer’s Report Nominations Committee
NSMA’s Website and Email Platform
On February 14, some members of NSMA’s Board of Directors, Rob Post and Gerri Cristantiello met with Laura Shumow of the American Spice Trade Association via Zoom to discuss synergies expressed by ASTA between the two associations. The purpose was to see how the organizations can collaborate in 2023 regarding meetings, advocacy, and committee work.
On May 26, 2023 the NSMA Board of Directors met via Zoom to discuss the NSMA 50th Anniversary Celebration. Topics included venue, budget and ideas. Gerri and Rob worked very hard on this celebration, and we appreciate them very much.
On June 9th the Annual Government Liaison Committee meeting with FDA was held via Zoom, since the majority of government staff are still working remotely. It was a successful meeting.
Over the last 12 months, NSMA has been signatories on multiple industry letters, including but not limited to:
America COMPETES Act
White House Conference on Hunger, Nutrition and Health
FBIA Letter to FDA on Supply Chain
The Reagan Udall Report
The Research Questions and Protocols for the 2025 Dietary Guidelines Advisory Committee
Titanium Dioxide Color Additive Revocation

FDA Front-of-Pack Labeling Notice
The website is being reconsidered, as it needs to be updated for better navigation and to include more information. It is limited in space right now.
Domestically Produced High-Quality Ingredients:


➤ Acetates
➤ Benzoates
➤ Calcium Chloride
➤ Carbonates
➤ Cheese-Phos®
➤ Citric Acid (Liquid)
➤ Citrates
➤ Diacetates
➤ Dipotassium Phosphate
➤ Disodium Phosphate
➤ Lactic Acid
➤ Lactates
➤ Magnesium Chloride
➤ Malic Acid
➤ Phosphoric Acid
➤ Potassium Citrate
➤ Potassium Hydroxide
➤ Propionates
➤ Sodium Citrate
➤ Sorbates
➤ Liquid & Dry Vinegar
Also available: Customized Product Solutions Tailored to Your Needs
1.877.751.2787
food.ingredients@hawkinsinc.com www.hawkinsinc.com

When SECURITY OF SUPPLY is essential, turn to Hawkins – your reliable & trusted source since 1938.

Now in its 50th year, NSMA has progressively grown as an organization since its inception in 1973, with 50 active member companies today and more than 130 individuals as contacts in the NSMA Technical Forum. Company representation is about 98 percent of the seasoning blenders in the U.S., with a combined marketplace footprint around the world and in all forms of commerce –food service, retail, B2B and e-commerce (D2C, etc.). Income has been increasing as membership has grown. Even so, the dues NSMA charges ($1475/year for Regular voting members and $975/year for Associate members) are minimal in contrast to the value of access to timely and regular insights on immediate and emerging policy and trade issues; a library of resources; and the ability to network and glean advice from others in the business. Other trade groups charge 10 times what NSMA charges for dues with similar or less services, or only for policy issue monitoring. Thus, value for dues, NSMA is a hands-down success.

As a registered non-profit organization, NSMA is limited in ways it can achieve income. Thus, membership dues are and have always been a primary source of income. In the last year, NSMA increased its membership slightly – up four members which resulted in a slightly larger expected income from dues, compared to 2021. In 2022 NSMA member dues income was up some $4,300 from 2021.
From a finance accounting standpoint, NSMA bookkeeping is not comprehensive. It is similar to a checkbook register involving a cash -basis accounting method. As a non-profit, NSMA has no investments or losses to report, nor does it rent space, or need to project budget needs to maintain contingency funds for facility or equipment maintenance or services. NSMA receives dues income, which are simply added to income, recorded as “income.” It has administrative and operating expenses for management, communications, and reporting, which are simply deducted from the NSMA checking account and recorded as “expenses.”
Regarding expenses in 2022, from January 01 to December 31, 2022, with the impact of the pandemic still limiting some travel and promoting virtual meetings, the management staff travel and meeting venue and related costs was less than in 2019, the last pre-pandemic year of activities. Compared to 2021, about half are related to administrative costs. In the past, costs associated with staff travel and meeting/event venues were the predominant expenses. This past year, the Association did not expend for management travel or meeting space for the annual GLC meeting with FDA officials, in Washington, DC, as it was virtual as in 2021. However, the organization had costs associated with the NSMA Annual Board of Directors meeting (held on July 12, 2022) and the NSMA Annual Meeting program held on July 14, 2022, concurrent with the IFT Annual Meeting in Chicago. Expenses for management staff travel, and meeting venue room, AV, food,

and other costs at the Annual meetings and for one other meeting for Association business were $8,911.57, plus $1,470.00 for meeting registrations, equaling $10,381.57. Even so, we had well attended and successful virtual meetings that incurred no significant costs for web meeting platform access.
Looking at 2021, NSMA’s income of $62,890.62 came from member dues. In 2021, NSMA lost 3 Regular voting members and gained 1 Voting and 3 Associate members. Expenses in 2021 totaled $46,375.28, which includes management services fees for the NSMA Executive Director and the Marketing & Member Services Manager.
Expenses in 2021 included dues for membership in the Food and Beverage Issue Alliance (FBIA), as well as other administrative fees, for example, business registrations and filings, website and email account hosting and cloud storage. Different than past years, there were no expenses for inperson events, e.g., travel for the Executive Director or Member Services lead, for meeting room space, meals, or IT rental.
From January 01 to December 31, 2022, the Association received $67,279.11 in Member Dues income, which is roughly $4,300 more than in 2021. Funds expended were mostly for NSMA management fees to the firms contracted to support the Association, i.e., FoodTrition Solutions, LLC, for our Executive Director, Dr. Post; and GC Business Services, LLC, for the service of our Membership and Marketing Services manager, Gerri Cristantiello. Our expenses for these management services total $38,400for the year. Other management expenses were in the categories of: (1) “Dues to Other Organizations” ($3,500, for the Food and Beverage Issue Alliance); (2) “Business Costs” (i.e., annual business reporting, website/domains/email services, dues collection services, and mail services) ($257.95); (3) “Other Than Ongoing Business Costs” (i.e., business filings and registrations, finalizing digitizing of NSMA files) ($1,213.25); and (4) “Travel, Meeting, and Registrations Costs”, which were reported above ($ 10,381.57). The total expenses for 2022 were $53,752.77. This is $7,377.49 more than what was expended in 2021, essentially reflecting the increased costs of resuming with in-person annual meetings and increased costs for some services.
Looking to 2023, the financial picture for NSMA looks satisfactory. Member Dues income should be in excess of expenses. However, advancing the services of the organization further by updating the NSMA website with outreach, resources, references, educational tools, and webinars for members; and enhancing NSMA communications platforms, will use the bulk of NSMA’s funding outside of administrative costs. Further, as administrative costs are increasing at present, and many costs are being absorbed by the Executive Director’s firm and the Marketing and Member Services manager’s firm, the projected finances for 2023 will be a point of discussion for the NSMA Board of Directors.
The Treasurer’s Report of finances is shown on the next page.
National Seasoning Manufacturers Association, Inc.

Treasurer’s Report
January 01-December 31, 2022
January 01 – December 31, 2022, Income and Expenses
Expenses – January 01 – December 31, 2022 – Includes:
Fees for management services: $38,400.00
Dues to Other Organizations: $3,500
Business Costs (i.e., annual business reporting, website/domains/email services, dues collection services, and mail services): $257.95
Other Than Ongoing Business Costs (i.e., business filings and registrations, finalizing digitizing of NSMA files): $1,213.25
Travel, Meeting, and Registrations Costs; $10,381.57
*Income minus expenses for 2022 appears to be $13,526.34. However, as of December 31, 2021, some checks and payments for 2022 services, such as management fees and email storage space, issued at the end of December 2021, had not been cashed by the recipients until January 2022 or later in 2022, totaling $1973.89. Taking this into account, the actual income for 2022 was $11,552.45.
For Reference: 2021 Income vs Expenses
– December 31
Expenses (fees for management services, annual business reporting, website/domains/email services, dues collection services, web conferencing services)
Respectively Submitted to the Treasurer, Robert C. Post, Executive Director, NSMA
$ 46,375.28 (includes $3000 (FBIA) membership for 2022)











NSMA members did not miss out on the Annual Government Liaison Committee (GLC) Meeting with FDA officials in 2023. Like last year, government officials are still working remotely, so a virtual meeting was the only way to go. We’ve always enjoyed the one -on-one and face-to-face interactions with federal officials at agencies and on the Hill, which in-person meetings afford. However, a major benefit of a virtual format this year was that more members could attend, save on their travel budgets, and get to ask questions outside of the agenda.
As our policy maker colleagues have developed different ways of working in a remote format, we were able to convene our GLC meeting with FDA officials on June 09, 2023. The Official FDA agenda for this meeting is below. It reflects the hot topics and challenging policy issues of importance to the NSMA membership on which we asked for updates.

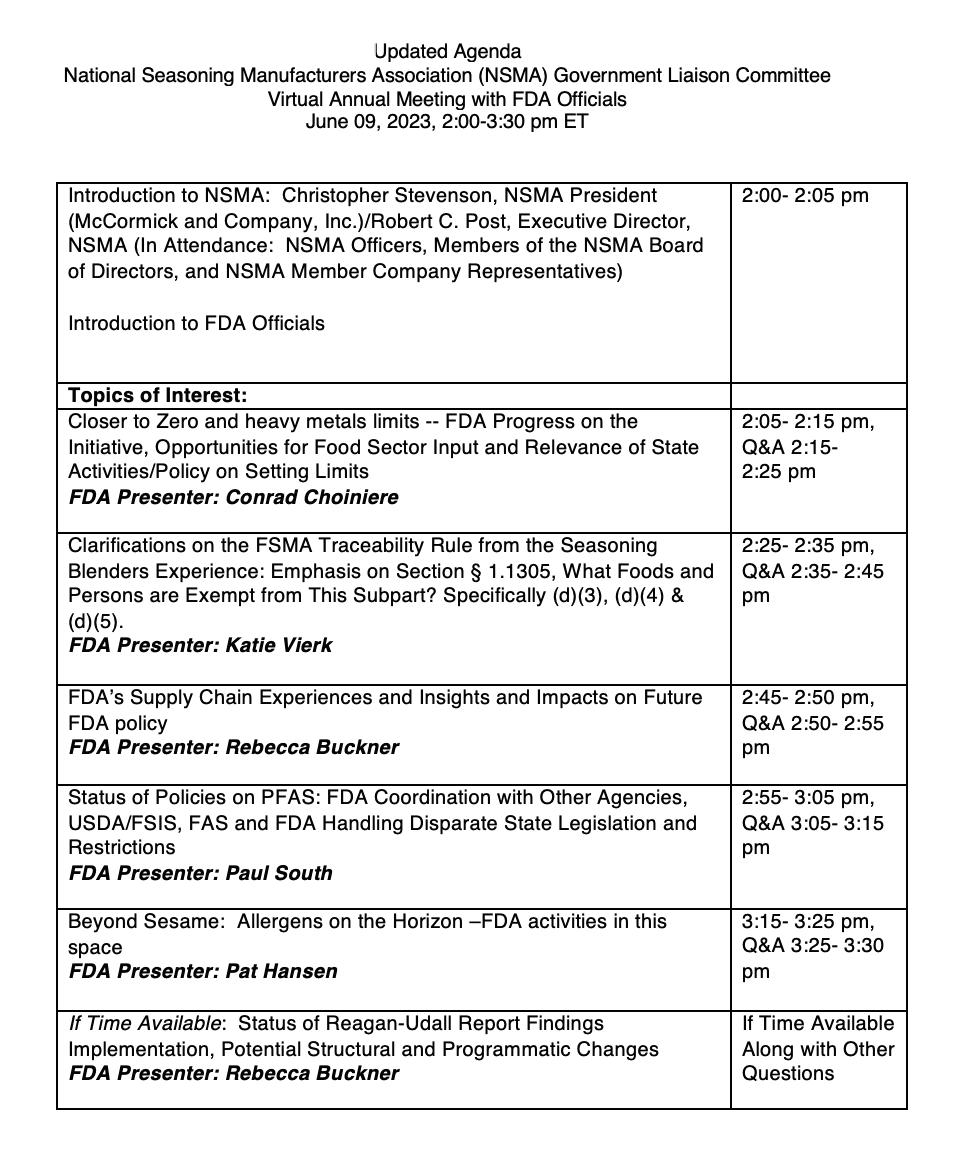

The Government Liaison Committee met via Zoom with staff from various areas of FDA, as the agenda reflects. The discussion followed the agenda above, with additional questions and topics raised by members in attendance. The Zoom recording for this meeting, which includes slides, can be found on the Members-Only section of the NSMA website.
On the top of the agenda, Dr. Conrad Choiniere, Director, Office of Analytics and Outreach, CFSAN, FDA, described the concerns that led FDA to undertake, starting in 2017, the Closer to Zero initiative. The work is focused on reducing relevant toxicity and exposure of infants and children to arsenic, cadmium, lead, and mercury. There are several prongs to the program; one is to analyze environmental source of contamination; work being conducted with EPA. In April 2021, FDA published an action plan that includes reference levels for lead in infant blood, with the work continuing to develop reference levels and draft action levels for the other toxic elements. The focus is on risk to the most vulnerable population, infants. Dr, Chioniere described the data sets FDA is using for determining exposure through different food categories and key sources of exposure for infants. Thus far, action levels for lead (Pb) juices are 10 ppm for apple, and 20 ppm for other juices. He explained that the initiative must tie closely to the Dietary Guidelines for Americans (DGA) recommendations for children, although the infant age segment is a target for future action. The goal is to mitigate likely impacts of exposure in food groups that are promoted in healthy eating patterns. They can do this through analysis conducted in the FDA Total Diet Study. Their work will focus on determining reference levels of arsenic, cadmium, and mercury, in the diet within this year; work will include determining dose responses. He reported that FDA issued a report in 2022 on sources of Pb, Cd, and Hg in the food supply, including baby foods. Work forward includes examining foods commonly consumed by young children and ways to mitigate and reduce exposure, citing that establishing action levels is very difficult without disrupting the supply chain with the unintended effect of people avoiding food groups that should be consumed. He noted that it is difficult to get exposure data on certain ingredients like spices and the proxy may be the foods in which they are used, which has limitations. In response to members’ questions, Dr, Choiniere indicated getting spice that they plan to advise states like New York that have stepped into the limit-setting space, and to harmonize with global trade standards in Codex and in Canada and Europe. He noted, like many issues these days, the heightened concern for creating supply chain problems presses for sound science, practical action levels, and seamless trade.
Katie Vierk, Director, Division of Public Health Informatics & Analytics, CFSAN, FDA, spoke on the FSMA Traceability Rule from the perspective of aspects that challenge seasoning manufacturers. She focused on processing exemptions and receiving records. Her presentation gave an overview of the Food Traceability List and the foods covered by the rule, noting certain foods, like dried herbs are not covered. Dr. Vierk explained the basis for processing exemptions, e.g., foods subjected to a kill step are not covered by the rule, and when traceability records are not needed. Her presentation gave pertinent cites, including the online ExemptionsTool, and the Critical Tracking Event and Key Data Elements. With the January 20, 2026, compliance date,

she emphasized that the resources on her slides 17 and 18 (shown on NSMA’s recording of the GLC meeting) are essential to know and use now.
Dr. Rebecca Buckner, Acting Deputy Director for Regulatory Policy, Nutrition and Engagement, CFSAN, FDA covered supply chain issues, a major topic of interest to NSMA members. This issue comes to FDA, she explained, when the agency is not missioned to handle supply chain matters. She cited some provisions in the December 2022 Omnibus Appropriations bill (which funds, sets priorities, and creates new authorities for agencies) that created a new section of the FDCA with provisions for FDA to oversee the response to significant disruptions of critical foods, like infant formula, by manufacturers who have to have resiliency plans. In this case, manufacturers will have to notify FDA – like they have to with shortages of drugs and devices
of the actions they need to take. Again, she emphasized that generally, FDA has no part of their mission to step into supply chain matters.
Dr. Paul South, Director, Division of Plant Products and Beverages, Office of Food Safety, CFSAN, FDA, was asked to cover PFAS and the status of actions on these substances at FDA. He reminded us that there are literally 1000’s of PFAS, and short and long chain with diff erent toxicities. One reason why they are on top of the list of contaminants needing limits is their prevalence and exposure capability because they travel in water and the detection level is in the quadrillions. That’s why EPA has regulations on drinking water for 6 PFAS. Like heavy metals, he explained that FDA is relying on the Total Diet Study to for taking samples to determine where there’s most exposure in foods. Right now, they are focused on commercial seafood and on method of analysis development. And they are working with states who need help setting action levels, using EPA’s Tox Reference Levels. Dr. South’s slides (which are part of NSMA’s recording at the members-only website) described the FDA involvement with interagency and state level of coordination, as well as international efforts to set voluntary action levels.
Dr. Pat Hansen, Deputy Director, Office of Nutrition and Food Labeling, CFSAN, FDA, was asked to speak about allergens and what might be on the horizon for FDA policy following the addition of sesame to the big-9. Dr. Hansen explained that there are many petitions submitted to FDA for recognizing potential allergens in barley, rye, garlic, and many other foods. They are using systematic reviews of research coupled with factors like how these foods are used, how broad is exposure to determine the public health importance. They expect to issue guidance by the end of the year on their findings of what may be potential allergens in common foods and ingredients. In addition, FDA plans to issue guidance stemming from the introduction of new foods that are alternates of foods which are known allergens, i.e., non-bovine and non-avian “milk” and “eggs”, which may be sources of components which cause sensitivities. And, it was added, that FDA intends to finally address the mis-categorization of “coconut” as a tree nut (it’s a fibrous oneseeded drupe). Stay tuned.
All in all, it was a great collegial session with FDA, confirming once again that NSMA has established helpful relationships with policy officials.

Last year we set out to develop a communication and marketing plan that would outline all the benefits NSMA offers and their value to our members and nonmembers. Although it is still being worked on, we found that NSMA has many benefits for our members as well as nonmembers. As we began to create this document, we realized that 2023 marks our 50 th anniversary and appropriately we began to organize an anniversary celebration to commemorate 50 years.

It is not every day that associations celebrate 50 years. To reach that milestone it takes a group of volunteer industry leaders who recognize the importance of coming together for the betterment of their industry. It also takes a dedicated staff to execute those initiatives. NSMA continues to be the only trade organization whose purpose is to serve the needs of comp anies involved in the seasoning industry. Congratulations on achieving this milestone and we look forward to a seasoned future!
I would like to point out our accomplishments this year in the area of communication:
News You Can Use – A biweekly report that provides our members with updates on Food Regulatory, Policy, Science, Safety and Market News. This continues to be the most valuable publication to our members. It is especially useful and timely with resources and links that you and your colleagues can use on a daily basis.
Technical Forum – A private members-only online tool in which you can send an email to the membership. This is a great way to communicate among NSMA members. You can use it to ask for opinions or guidance from members or share something of interest. If you are not familiar with this resource, please reach out and we can provide you with instructions.
Website – Our website has a public and private members -only section that provides valuable resources. Members have access to our Regulatory Manual, a document that contains specific policies, relevant regulations, and NSMA positions on current topics dating as far back as 1973 when our organization was formed. This document is only available to our members. Members also have access to past presentations, Government Liaison Meetings, and Annual Meetings
Social Media – We have a presence on LinkedIn and on Facebook as National Seasoning Manufacturers Association and on Instagram

Annual Report – The creation of this document came about in 2020 during COVID to make it easier to follow our Zoom Annual Meeting. We have continued to provide it as a way to communicate our accomplishments and future goals. It also serves as a way to tell our story to future members.
Sponsorship Program – As you can see in this Annual Report, we have initiated our Sponsorship Program. Members who sponsor provide us with an advertisement.
In this next year, we plan to complete our Marketing and Communication Plan and present it to the Board of Directors for review and approval. With that we plan to improve on our Sponsorship Program, our Annual Report, and Social Media postings. We will revamp our website to provide more resources on our members-only section as well as our public section. Additionally, we will add educational workshops for both members and nonmembers.
We also plan to increase our services to our Associate Members that are suppliers of ingredients, packaging or provide other services to our Voting Members.
As I continue to meet with prospects and industry professionals in the seasonings industry, I continue to receive positive comments on how our association has taken major steps forward. The value that NSMA gives its members far exceeds the cost of membership.

One of the benefits to NSMA members is the availability of advice on challenging policy issues and regulatory requirements from our Executive Director and our network via the Members-Only Technical Forum. On behalf of members, the Association has been able to provide a voice for informing senior policy officials at HHS/FDA, USDA/FSIS, and at Codex/FAO and WHO, on a variety of issues to inform guidance and promote practicable solutions to challenges.
Just some of the policy issues and actions on which members sought advice from NSMA are:
Supply chain disruptions for ingredient sourcing and availability, transportation, packaging materials and the likelihood of FDA guidance on regulatory compliance, included insights on specific ingredients such as lecithin.
FDA guidance priorities and timelines.
FDA’s traceability proposed rule.
FDA GRAS Notifications, inquiries, and timeliness.
Amenability of products to USDA-FSIS jurisdiction and inspection versus FDA jurisdiction/inspection.
Product labeling flexibilities post-pandemic.
The Strategic Plan from the White House Conference on Hunger, Nutrition, and Health, and potential issues and actions to come from it.
FDA sources of irradiation regulations and provisions for foods, ingredients, and components of seasonings.

FDA regulatory allowances for ETO use and controls, and international trade standards. Heavy metals, FDA’s Closer to Zero initiative and potential impact of recommended action levels on seasoning products.
E-commerce and potential guidance on labeling meals kits with seasonings. Sesame and the likelihood of additional allergens on FDA’s “big” list.
AMS’ BE Disclosure Standard and commodities and ingredients on the “required disclosure” list.
These topics reflect the swirl of regulatory actions and policy issues FDA and USDA are addressing, as well as issues related to international food trade standards. As an example to show just how current these member inquiries are, below is the July 06, 2023, update from FDA’s Center for Food Safety and Applied Nutrition (CFSAN) and Office of Food Policy and Response (OFPR) on their list of planned guidance priorities. This is an updated list of draft and final guidance topics that are a priority for the FDA Foods Program to complete during 2023.

























FDA: Possible New Topics for Guidance In 2023
Questions and Answers Regarding Food Allergens, Including the Food Allergen Labeling and Consumer Protection Act of 2004 (Edition 5); Guidance for Industry
Evaluating the Public Health Importance of Food Allergens Other Than the Major Food Allergens Listed in the Federal Food, Drug, and Cosmetic Act; Guidance for FDA Staff and Stakeholders
Preparation of Premarket Submission for Food Contact Substances (Chemistry Recommendations): Draft Guidance for Industry
Premarket Consultation on Cultured Animal Cell Foods: Draft Guidance for Industry
Foods Derived from Plants Produced Using Genome Editing; Draft Guidance for Industry
Compliance Policy Guide Sec. 555.320 Listeria monocytogenes in Human Food; Draft Guidance for FDA Staff
Evaluation and Establishment of Safety of Low-Moisture Ready-to-Eat Foods Following Equipment Microbiological Contamination Event: Guidance for Industry (Planned for Release at End of 2023)
Action Levels for Arsenic in Food Intended for Babies and Young Children: Draft Guidance for Industry
Action Levels for Cadmium in Food Intended for Babies and Young Children: Draft Guidance for Industry
Hazard Analysis and Risk-Based Preventive Controls for Human Food; Appendix 1: Potential Hazards for Foods and Processes; Draft Guidance for Industry
Hazard Analysis and Risk-Based Preventive Controls for Human Food; Chapter 11: Food Allergen Controls; Draft Guidance for Industry
Hazard Analysis and Risk-Based Preventive Controls for Human Food; Chapter 9: Validation of Process Controls; Draft Guidance for Industry
Hazard Analysis and Risk-Based Preventive Controls for Human Food; Chapter 17: Classifying Food as Ready-To-Eat or Not Ready- to-Eat; Draft Guidance for Industry
Hazard Analysis and Risk-Based Preventive Controls for Human Food; Chapter 16: Acidified Foods; Draft Guidance for Industry
Labeling of Plant-Based Alternatives to Animal-Derived Foods; Draft Guidance for Industry

NSMA continues to be actively engaged with FDA and USDA policy makers in their efforts to provide continued transparency for stakeholders regarding foods program priorities. This example of Guidance Documents represents the FDA’s current thinking on a specific topic and the information can help stakeholders plan for potential changes that may impact their businesses and organizations. Guidance documents do not impose legally enforceable requirements but are helpful insights as to how policy makers view issues.

Although the FDA's intent is to publish all draft and final guidance topics on the list, modifications in plans may be needed to support emerging issues and Administration priorities. FDA also may issue additional guidances that are not on the list. Therefore, NSMA and the Executive Director will remain constant in ways and efforts to interact with FDA and USDA, and Codex, officials to inform them on policies that affect our sector and will bring back the learnings and updates to the NSMA membership.


NSMA Membership is based on a calendar year and as such this report will provide you with a recap of where we finished our year in 2022 and a status report on this year’s membership as of June 30, 2023. New members that apply for membership remit pro-rated dues to the closest quarter.
The chart below shows our status as of December 2022. We currently have some members who have not yet paid their dues for 2023. The next page shows our members to date. Although we have grown to sustain 50 members, we’ve lost three in the past year. As evident in the chart, we will need to continue to work on recruiting new companies to grow our membership..
Since our last Annual Meeting in 2022, two members were dropped for nonpayment of dues and as such we closed the year with 48 members. It is worth noting that the loss of membership was in the Associate category.
Since January 2023, we have gained three new members. If we receive 100 percent of our dues from our current members, we will end our 2023 year with 50 members plus any additional new members that join after the Annual Meeting.
Our new members since our last Annual Meeting in July 2022 are:
PAX Spice & Labs (Associate)
iSpice Foods (Voting)

Top Chance Foods Inc. (Associate)
These members joined after January 1, 2023, our new calendar and fiscal year.
The chart below shows our Voting Members has remained on a steady pace, while our Associate Members have been declining. We are working on developing some incentives and benefits specifically aimed to Associate members so that they can see the value in remaining members of NSMA.
I would also like to note that we have had a name change: BDS Natural Products is now Sabater USA.
Membership in the National Seasoning Manufacturers Association (NSMA) is on a company basis and the benefits are open to all employees of your company. I highly encourage you to get others involved and let them know how NSMA’s resources can be of benefit to them on a daily basis.
In looking to the future, we will continue to recruit members and appreciate your referrals and leads. Please send them to me by email (gerri@seasoningmanufacturers.org), text/phone: (201) 657-1989 and I will be pleased to let them know the importance and value of membership.
As of June 30, 2023
*Indicates Associate Member
A.C. Legg, Inc.
Adams Flavors, Foods & Ingredients
Advanced Spice & Trading, Inc./Valdez Spice
Asenzya, Inc.
Badia Spices, Inc.

Bag Supply Company *
Bluegrass Ingredients *
Blue Line
Carolina Ingredients LLC *
Certified Laboratories, Inc.*
Chesapeake Spice Co. LLC
Deep South Blenders
Elite Spice, Inc.
Everson Spice Company, Inc.
First Spice Mixing Co. Inc.
Fuji Foods
Givaudan
Griffith Foods, Inc.
Hawkins Inc.
Illes Seasonings & Flavors
International Spices
iSpice Foods
Kalsec Inc. *
Kalustyan Corporation
Kutas North America *
Louisiana Fish Fry Products, Ltd. *
Macco Organiques, Inc.
Magic Seasoning Blends, LLC
MANE
McCarthy Spice & Blends
McClancy Seasoning Co.
McCormick & Co. Inc.
MG Spices
Newly Weds Foods Inc.
Nu Products Seasoning Co.
Old World Spices and Seasonings
Olds Products Company
Pacific Farms *
Pacific Spice Co. Inc.
PAX Spice & Labs *
Rio Valley Chili
R.L. Schreiber, Inc.
Rocky Mountain Spice Company
Sabater USA
Saratoga Food Specialties
Sensient Natural Ingredients *
Sterigenics Intl. Inc. *
Topchance Foods Inc. *
Wixon Inc.
World Flavors Inc. *
The Nominating Committee Tim Sonntag, Kerri Goad Berrios, and Alina Lastra is pleased to present the following slate of nominees for the Board of Directors for approval by the membership at the 2023 Annual Meeting.
These members will serve a 3-year term, ending in 2026:
Danelle Boehme, Asenzya
Denise Johnson, Newly Weds Foods, Inc.
Chris Stevenson, McCormick & Company, Inc.

Additionally, Aret Meyer of Sensient Natural Ingredients agreed to serve as Associate Member Director for a two-year term ending in 2025.
On behalf of the Board of Directors, Rob Post and myself, we extend our gratitude and thanks to Mr. Eric Baylin who has served on the NSMA’s Board of Directors for the past five years. He has been an invaluable member of our Board.
We trust that this 50th Anniversary 2022-2023Annual Report has provided members with a comprehensive account of the Association’s activities for this past year. As we move toward 2024, we will continue with the valued resources for members as well as undertake some new initiatives. Here’s what our plan will include:
Continue to build our membership and find new ways to promote networking.
Enhance our website to give a more thorough view of NSMA -- both the public and members-only sections.
Continue to provide members with the “News You Can Use: NSMA’s Food Regulatory, Policy, Science, Safety and Market News” policy updates, and archiving them and the helpful links to references and resources in them on the members-only section of the website.
Enhance our participation in the Food and Beverage Issue Alliance (FBIA). (feedingus.org), sharing NSMA viewpoints and insights that contribute to improved and informed federal and state food safety and nutrition policies.
Add additional resources to the members-only portion of the website based on members’ needs.
Conduct webinars on timely topics that will be open to members and non-members. Partnering on educational training opportunities for our members.
Sponsor sessions on topics that challenge and promote seasoning manufacturers and the seasoning industry.

Develop communication vehicles for our website and for members, consumers, and professionals about seasonings, i.e., quality, nutritional benefits, contributions to make healthy foods more enjoyable.
As we continue to build this organization, we look to you, our members, to guide us in our role as your voice of the blended seasoning industry in promoting balanced and effective regulations and policies. We value your membership and thank you for your trust in us.

Allergens Labeling
Sulfites/Sulfiting
Flavor/Natural Flavor Labeling
FSMA: Preventive Controls for RTE and Not RTE Ingredients
Heavy Metals and Other Contaminants: Limits and Controls
Supply Chain Challenges for Ingredients




Dear National Seasoning Manufacturers Association, Congratulations on reaching the significant milestone of your 50th anniversary! As a founding member and proud annual supporter, First Spice Mixing Company is thrilled to celebrate this remarkable achievement with you.

Throughout the years, NSMA has played a crucial role in fostering unity and collaboration within the seasoning industry. NSMA’s dedication to keeping the industry informed on important news and updates is invaluable in navigating the ever changing regulatory landscape.
On this momentous occasion, we extend our heartfelt gratitude to the entire NSMA team, past and present, for their dedication, hard work, and vision. Your tireless efforts have made the association a beacon of knowledge, advocacy, and support for the seasoning industry.
As First Spice Mixing Company looks ahead to the next 50 years and beyond, we are excited to continue our partnership with NSMA. Together, we will shape the future of the industry, embrace emerging trends, and address the challenges that lie ahead. We are confident that with NSMA’s leadership and the collective strength of our fellow members, the association will continue to thrive and make a lasting impact.
Cheers to Another 50 Years!
Warmest regards,