





“As the CRN comes to an end, the whole management team and executive group would like to thank everybody again for their amazing work over the past few years. What a few years it has been."
Professor Saul Faust Clinical Director, NIHR Clinical
Research Network Wessex



“We should be
Clinical Directors of NIHR Wessex Clinical Research Network (CRN).
incredibly proud of what we have collectively achieved,
and we
hope that the Research Hubs, now part of Wessex Health Partners, will be a legacy of this joint working for many years to come.”

We’d like to warmly welcome you to the final issue of VISION magazine for the Wessex Clinical Research Network.
As the CRN comes to an end, the whole executive team would like to thank everybody again for their amazing work over the past few years. What a few years it has been.
The 2020 pandemic gave Wessex CRN partners the opportunity to work together without boundaries, in completely new ways. And the way that we all committed to sharing staff and resources to undertake pandemic research has led to collective innovation that is the envy of many people elsewhere in the country and, indeed, the world.
We should be incredibly proud of what we have collectively achieved, and we hope that the Research Hubs, now part of Wessex Health Partners, will be a legacy of this joint working for many years to come.
Our success hasn’t been a happy accident. We’ve achieved this because everybody decided that whatever the way of working had been in the past, we were going to trust each other to develop a new system that would be better for our participants and patients.
This trust has led to an amazing feeling of togetherness and confidence that we can support each other across boundaries and make best use of all the Wessex-based NIHR infrastructure, from the Wessex Applied Research Collaboration and Health Innovation Wessex to the NIHR Southampton Clinical Research Facility and Biomedical Research Centre that support the Wessex Experimental Medicine Network.
Although Wessex will encompass two future Regional Research Delivery Networks, instead of one, from a groundlevel research infrastructure and on-theground delivery perspective we know that we will continue our collaborative way of working into the future.
Clinical and social care research is clearly a fundamental part of Government
strategy, so whilst the network might be changing, the task of bringing research to the public and NHS hasn’t changed. When change happens, there are always threats but there are also new opportunities. The opportunities we now have to collaborate, particularly for primary and community care, will benefit our participants. But it’s also important to remember that we now have an opportunity to improve on the things that other regions do well, and where we are still learning. These opportunities are significant and ones that our population and participants will benefit from.
“This trust has led to an amazing feeling of togetherness and confidence that we can support each other across boundaries and make best use of all the Wessex-based NIHR infrastructure."
It is up to all of us to ensure that the legacy of what we’ve all achieved endures, and that our relationships remain strong to the benefit of our participants.
With all of this in mind, we hope you will enjoy this last issue of VISION and be able to reflect on the immense amount of collaborative research that has been happening across our region. None of this would have been possible without your commitment and dedication, and the stories you will read are a testament to the fantastic people we have working within our network. Research within Wessex has gone from strength to strength – thank you for all of your efforts and commitment to being part of this transformation.
Saul and
Patrick

Over 1000 open Wessex led studies in the last 10 years
Over 590,000 participants recruited to studies from the Wessex region in the last 10 years
Over 3638 studies opened in Wessex over the last 10 years
During the Covid-19 pandemic 208,711 participants were recruited to trials
Over 850 recruiting sites in the Wessex region in the last 10 years
As the NIHR’s Clinical Research Network (CRN) Wessex reaches the end of its ten-year contract, we spoke to Kelly Adams about what the journey has been like for research within Wessex during the last decade and her expectations for the future.
As part of the work to transition from the NIHR Clinical Research Network to the NIHR Research Delivery Network, CRN Wessex will undergo a change to its geographical boundaries. Partner organisations in Hampshire and the Isle of Wight will become part of the new South Central Research Delivery Network (RDN) and Dorset and South Wiltshire partners will move across to become part of the South West Central RDN.
The most important part of working within a research network, is investing in, and developing, your
working relationships with different people. Collaborative working can be hard, you all come to the table equally, but everyone comes with their own priorities, challenges and red lines. So, to network and collaborate effectively, all partners must have a genuine understanding and empathy of each other to move forward together. But we know from experience that we can achieve much more together than in isolation. During the last transition in 2014, we moved from Hampshire and the Isle of Wight CLRN to CRN Wessex, welcoming trusts and partners from across Dorset and South Wiltshire into the new patch. For a good couple of years, we got to

“Since the pandemic, virtual meeting platforms have made it much easier to check in and build the frequency of contact with people that is essential for transformative collaboration and system-wide working.”
know each other and the organisations across the new region, to understand who we were, the differences in every partner organisation, the opportunities, challenges, strengths of research portfolios, capacity, and expertise because this varied hugely across the Wessex region. A lot of time was spent listening and understanding how we could support teams most effectively. What’s enabled us and helped to make this kind of collaborative working work, was the pandemic. We all had a shared priority – to deliver Covid-19 vaccine trials at scale and pace and ultimately save lives. That goal helped focus our minds on how we needed to work together and helped to see us through any obstacles we might face.
It’s been heartening that since the pandemic ended, everyone is keen to maintain a shared focus, collaboration, and partnership working. It’s clear we’re still benefiting from those relationships that we built and

“One of the biggest enablers to increasing collaboration has been the move away from a national funding model that in no way incentivised organisations to work together.”
strengthened during the pandemic. Ten years ago, I’d work in the office five days a week with perhaps one or two face-to-face meetings in that time. Travel across counties took large chunks of time which limited how often we could meet with colleagues from other organisations. Since the pandemic, virtual meeting platforms have made it much easier to check in and build the frequency of contact with people that is essential for transformative collaboration and system-wide working. It’s helped
us to build better, more meaningful relationships.
We’re working together to ensure our organisations are better able to deliver research and give our local communities and patient populations the opportunity to take part in research that is of importance to them.
One of the biggest enablers to increasing collaboration has been the move away from a national funding model that in no way incentivised organisations to work together. There is now every reason to work collectively to offer greater opportunities for patients and service users to participate in research that spans organisational boundaries. This means that in many respects it’s irrelevant where participants are recruited, the important thing is that they are offered the opportunity to take part.
We have a collaborative, single contracting model in our regional Research Hubs too. The four hubs are contracted from University Hospital Southampton, but we recruit participants from across the Wessex region at four different sites in Weymouth, Bournemouth, Southampton, and Portsmouth. It doesn’t matter which trust recruits them – more communities from across the geography have the chance to take part. For me, it doesn’t get much better than that for collaborative working and increasing access to research opportunities.
This approach to contracting was unique to Wessex, and we’ve shared this model with colleagues across the country, so now other regions are looking to establish similar models. It has been looked at as an exemplar nationally, and we’re extremely proud of that.
As we come to the end of this contract, we’ve delivered what we were asked to deliver which was to support NHS organisations and GP practices to expand their ability to deliver research. While it’s sad its coming to an end, we should be proud of what we’ve all achieved.

"It's great to have Wessex Health Partners (WHP) on board. I think our work with WHP will only increase as we continue to move forward."
I’ve really enjoyed working much more closely with regional partners over the last few years and opening up those opportunities for collaborative research. We would never have had our two new research buses without that collaboration.
The Wessex research buses are a regional resource that can be hired and used by any of the Wessex regional partners that want to use them. They can be used to fully deliver specific studies. There’s a mini-laboratory and pharmacy on board with a pharmacy fridge, lab freezer, centrifuge and a separate clinic room with an examination couch and a small toilet. The buses can also be used for community outreach and engagement events too – we’re off to Chris Evans’ CarFest next year to engage a different group of people in research, what we do and
how to get involved. We’ve benefited so much from everyone’s willingness to work together.
That’s why it’s great to have Wessex Health Partners (WHP) on board as a new partner too. I think our work with WHP will only increase, as we continue to develop and move forward.
The Wessex Research Hubs and buses were all funded with Wessex regional income, so they will remain a resource for Wessex partners which should further develop our opportunities to continue working together.
Another huge success is that we’re seeing an increase in decentralised trials, taking advantage of opportunities to deliver research that doesn’t require face-to-face contact. This approach was successfully trailblazed on a national scale through the Panoramic trial during the pandemic. Researchers didn’t need to see participants in person which meant it didn’t matter where you were as a clinician you could still support people safely through the trial.
Increasing the number of studies like this with nationwide recruitment, will increase our ability to collaborate across studies and give access to even more research opportunities to patients.
2024 is our transition year where we become part of South Central and South West Central RDNs.
We will have a big geographical boundary change under the new contract, which means we’ll be working with, and getting to know, a whole new range of organisations.
"It also provides another aspect to people’s jobs, if they work in the NHS or in care, that they might find interesting and rewarding – helping with staff recruitment and retention."
We still have so much shared work and ambitions with Dorset and South Wiltshire colleagues. There remains absolute commitment on both sides to continue working with each other and, hopefully, the learnings we’ve had together, particularly the last five years, will stand us in good stead to move forward with our new partners

and work collaboratively with them too. As well as working with different partner organisations, NHS trusts and GP practices, we’re now looking at extending our support into schools, care homes, local authorities, and universities; public health and social care settings.
This is something the whole team is excited about and are grateful for the opportunity to continue supporting organisations to increase and develop their research portfolios.
A personal focus for me for the future will be truly embedding and integrating research delivery within front-line clinical services. Understanding how we can work together to embed research in everybody’s roles, so we’re increasing opportunities for our patients to participate in research, is a vital part of our future working together.
It also provides another aspect to people’s jobs, if they work in the NHS or in care, that they might find interesting and rewarding – helping with staff recruitment and retention.
The RDN contract will run for six years, and I’d like to see collaboration across the country become the norm in this time because of the expertise and
opportunities that it might bring to the people across our region.
In Wessex, we have a high prevalence of dementia and Alzheimer’s because we have an ageing population. But when you look at the centres of excellence for research in dementia, it’s in places like Cambridge, which can be a barrier to our local populations being able to participate in that critical research.
My hope for the future is that those geographical distances and organisational boundaries are no longer a barrier to our local populations being able to participate in research that is of relevance and importance to them.

The Aria e-prescribing system has been in place across the Wessex Cancer Network for around 14 years. But with growing pressure on cancer services, particularly prescribing services, the pharmacy team at University Hospital Southampton (UHS) wanted to explore ways in which the Aria system could further help them to streamline processes, improve patient care and ease the pressure on a busy network of cancer services.
We spoke to James Allen and Nanda Basker, who explain how the Aria system is helping to benefit everyone in the network, patients, and staff, through the changes they’re making.
INTERVIEWEES:
JAMES ALLEN –Chief Pharmacist, University Hospital Southampton
NANDA BASKER –Lead Pharmacist for Cancer Services, University Hospital Southampton
NANDA: Aria is a collaborative e-prescribing system for our cancer network which includes Southampton, Portsmouth, Isle of Wight, Basingstoke, Chichester, Salisbury and Winchester. The system is around 14 years-old but we wanted to start using it to streamline processes and services further.
Chemotherapy protocols (or prescriptions) are often very complex and so the production of standardised protocols enable prescribing to be completed safely, quickly, and consistently.
Aria automates many elements of the chemotherapy prescribing process, e.g. users can enter a patient's height and weight, then Aria will automatically calculate their body surface area and subsequently what dose(s) of chemotherapy are required. Prescribers are then able to review this, and make any additional modifications required for their specific patient before sending this prescription to pharmacy.
Using Aria, we created standardised protocols and shared these across all Trusts within our network. This has had numerous benefits for a wide variety of staff within cancer care. Healthcare professionals e.g. nurses, doctors and pharmacists are
"Using Aria, we created standardised protocols and shared these across all Trusts within our network."
able move between Trusts all using the same protocols and processes, reducing the need for additional training and ensures staff are very familiar with the prescribing and administration system. Consultants regularly run clinics in multiple hospitals, and Aria enables them to prescribe remotely for any of the Trusts within our network.
Staff are able to view all historical prescriptions from all sites across the whole network, allowing us to maintain accurate treatment records for our patients. Additionally, staff are also able to interrogate this data, allowing us to quickly audit a wide variety of prescribing records and trends.
It has benefits for prescribers because they can move between trusts and know that the protocols are the same, there’s an efficiency in that, and they can prescribe for patients quickly and with confidence.
There are benefits for patients because it improves the safety of their prescriptions. If the treatments are standardised across the Trust,

we know that they are effective, and the amounts they need based on the patient’s information. It also has a practical benefit for patients too, if they needed to go for treatment at another centre then there are no gaps in their treatment records. Staff can see their treatment programme and records, including blood test results, so there are no delays. It’s a great continuation of care.
JAMES: This project was about using dedicated ring-fenced specialist pharmacist time to turbo-charge the development of Aria protocols across our already established network. It is expected that we could release the time of our pharmacists and maximise the care we’re giving patients by reducing duplication of effort and delivering a more co-ordinated centralised process. Essentially putting more resource behind what we do to build long term capacity. Additionally, if we can make the prescriptions in Aria and prepopulate them with standardised dose bands, then we can purchase those products ready to administer from
"If the treatments are standardised across the trust, we know that they are effective, and the amounts they need based on the patient’s information."
healthcare companies. This frees up time in our already stretched sterilized units to concentrate on medicines which must be prepared on-site due to their short lifespan or complexity of preparation.
The additional capacity we have using this approach, means this resource is shared across the entire network of sites and the capacity we release can improve access to highly specialist treatments such as clinical trials, where they need to be administered within a few hours of making, and patients who need a more bespoke prescription.
Our ultimate goal was to share information and reduce the duplication of effort.
"There’s potential for these mechanisms to become centralised to support clinical trials across the UK."
JAMES: We have an increasing number of clinical trials that need to be conducted and we need to maintain the quality of our prescribing across those trials and our patients. This involves using new medicines, old medicines and old medicines that are being used in new ways. On top of this, patients usually need supportive medicines that work alongside their treatment – either to alleviate their symptoms or side effects, or for another medical condition. So, this requires more resources. Aria is helping us to standardise what we can so that we can treat more people with the finite resources we have.
NANDA: Specialist Cancer Pharmacists are rare, so standardising across multiple sites allows patients to benefit from the knowledge of the few we have. By training pharmacists, doctors, nurses, and administration staff on how to fill out the information required in Aria and how to extract information from the data, it will suggest the supportive medications needed for that patient along with a schedule for their treatment. Pharmacists can then validate those prescriptions through the screening checks we have in place, like looking at blood test results that can indicate
suitability for that treatment and lessen the likelihood of side effects for them.
So, while it sounds more hands-off, it actually delivers more bespoke treatment and an improved quality of care for our patients.
We completed 19 protocols in the first five months of 2023 and 31 protocols in the same period in 2024. However, the funding was only partially implemented during that period, with the second pharmacist post starting in June. We expect the numbers to improve significantly for the second half of this year.
JAMES: We’re currently halfway through our first year of funding for the project, and I hope that we’ll continue to see improvements by increasing the knowledge and skills of people using Aria in the network. Many of the cancer treatment regimens will exist for years to come, so their value will remain for a long time. And with the advancement of knowledge around cancer and its treatment, it’s possible that we will be able to use the system to hold individual genetic marker data to personalise treatment even further. If we know a patient has a particular genetic marker, then we can provide
treatments where we know they’ll either be more effective, or they’ll have less side effects – which improves patient outcomes enormously.
NANDA: With Aria, we’re able to respond faster to expressions of interest from clinicians wanting to set up clinical trials. This means that the trial can be established quicker and patient recruitment can be accelerated, so that we ultimately help more people. This has the potential to impact people across the country.
JAMES: There’s potential for these mechanisms to become centralised to support clinical trials across the UK. But we need the workforce to be trained for succession and continuation if we are to achieve that. It could help many more people to access clinical trials. It also provides a career pathway for cancer pharmacists, who can help make the delivery of chemotherapy faster and more personalised for patients. The streamlining of the process alone has been a great result from the study, we’ve seen better capacity across all our sites. But it will be nice to look at more patient focused targets towards the end of the study, to see if we have higher numbers of people in clinical trials and what our patient outcomes have been.




Register online www.joindementiaresearch.nihr.ac.uk
• Click ‘Register by phone’ on the website
• Call one of the charity helplines
• Complete the paper registration form
If you need any help at all, please call:



INTERVIEWEE:
ANGUS PROSSER –Senior Research Fellow, University of Southampton
The current diagnosis process for dementia is slow and can yield unclear results – often delaying access to treatment or keeping patients stuck in the diagnosis pathway.
A new study hopes to improve the diagnostic process, providing more accurate evidence for clinicians and helping patients to get appropriate treatment quickly.
Angus Prosser told us more about the CONGA trial.
Alzheimer’s disease is the most common form of dementia. The disease progressively worsens over time, and can affect memory, problem solving, concentration, language and sometimes behaviour. It’s thought to be caused by abnormal protein changes in the brain, leading to the death of neurons, but there’s still research exploring the exact pathway for what causes the disease. It’s immensely complex.
Before someone is diagnosed with Alzheimer’s disease, they’re often
given a diagnosis of mild cognitive impairment (MCI). This is when someone has problems with their thinking, worse than what would be expected as part of normal ageing, but not so bad it affects their daily living. The difficulty is that not everyone with these symptoms progresses to a form of dementia. In the early stages, symptoms can also be subtle, and individuals can present differently. As you can imagine, this makes it really difficult to diagnose someone – people can present with the same symptoms for many different conditions.
Currently, it can take years for someone to get a clinical diagnosis
"The difficulty is that not everyone with these
symptoms progresses to a form of dementia. In the early stages, symptoms can also be subtle, and individuals can present differently."
WATCH THE VIDEO
Watch Angus talk about the CONGA trial and its goals.

for Alzheimer’s disease. And there’s a significant diagnostic gap, where 3040% of people with Alzheimer’s don’t have a diagnosis.
But a diagnosis is critical for care planning. And studies show that early diagnosis improves patients, and carers, quality of life and outcomes – reducing the likelihood of hospitalisation and delaying hospice care.
Identifying Alzheimer’s at an early stage is becoming even more critical with the emergence of new treatments for Alzheimer’s. Studies have shown that targeting a specific protein in the brain called amyloid can slow the progression of the disease, giving people more time to live well with Alzheimer’s.
These treatments have been shown to be most effective for those early in the disease, highlighting the importance of identifying and treating Alzheimer’s early. More accurate and earlier diagnosis would allow people

to be offered clinical trials earlier in the disease progression and ensure appropriate care plans are in place.
It could also help us to be more confident about identifying patients that don’t have dementia, relieving patient and family anxiety and stress that is often present before they are given the all clear.
Around four years ago we were approached by Oxford Brain Diagnostics (OBD), a company that has developed a new technique to analyse normal MRI brain scans, called Cortical Disarray Measurement (CDM). It’s a technique that can identify microscopic changes in the brain which are thought to be linked to Alzheimer’s disease pathology.
CDM uses the same brain scans that are already used as part of dementia
diagnosis, but a specialised sequence on the scanner which looks at the flow of water through the brain. This can provide information on tissue microstructure in the grey matter of the brain, and changes that are linked to potential underlying pathology.
Currently, an MRI scan is used clinically to look at basic structure of the brain to identify brain shrinkage. But gross volume loss in the brain is not seen until the later stages of the disease, so it often provides little information in the early Alzheimer’s and mild cognitive impairment stages. A more sensitive measure could help improve earlier detection and diagnosis.
Although OBD had promising preliminary data on the imaging technique, they needed to complete a larger scale analysis to see whether it could be effective in a real-world population. We developed the CONGA trial to see whether we can use this CDM technique to identify
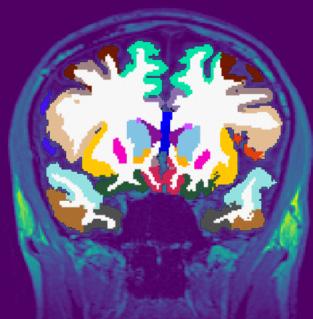
changes associated with Alzheimer’s earlier than what’s currently available.
We’re also working with the University of Oxford who are completing patient interviews and health economics in the CONGA trial to find out how the CDM technology could impact the NHS dementia diagnostic process and patients’ dementia journey. The CONGA study is recruiting until the end of October 2024 and currently we have 85 people enrolled – our target is to recruit 200 participants. Recruitment is completed at three sites: University Hospital Southampton, Dorset University Healthcare and Cardiff and Vale University Health Board. We’re working with the CRN to approach GP surgeries to screen participants at Primary Care level and we’ve added Patient Identification Centres to also help us with recruitment.
Ultimately, we want to know if we can use the CDM technique to improve the accuracy and the timeliness of dementia diagnosis and whether we can use it to identify individuals who are more likely to progress from MCI to Alzheimer’s, compared to current methods.
This study has had to be collaborative for us to succeed.
We worked closely with OBD, the University of Oxford, and Cardiff University, and were awarded an NIHR i4i Product Development Award to further develop the CDM technology and gather larger scale data to prove its effectiveness.
Professor Christopher Kipps, Clinical Director of Research & Development in Southampton & Professor of Clinical Neurology and Dementia, and I led on the development of the CONGA study to clinically validate this technology.
In addition to the main partners in the grant application, we’ve connected to Bournemouth University’s Institute of Medical Imaging and Visualisation, and The Healthshare Clinic Winchester, who joined the study to increase our scanning capacity.
Collaborating for recruitment has also been important. Identifying early-stage patients who are willing and able to take part can take time. To help with recruitment, we work closely with regional memory clinics and imaging services to make sure patients are aware of the study. We’ve also made new connections with Dorset Healthcare University to help recruitment, and worked closely with the CRN Wessex to provide additional staff for supporting the delivery of the project, to promote the study, and to link to primary care sites.
Although it has been challenging at times to make these connections, the network we have created has strengthened the project and provides a strong base for future collaboration. It has however been a steep learning curve.
It’s also important to note that we have strong Patient and Public Involvement in developing everything from the study protocol and patient information sheets, to how we structure the study, approach participants, and determine how much is appropriate to ask participants to do at the study visits. We have quarterly steering group meetings with patient representatives to move the trial on and help direct us.
Working collaboratively has been so important. Having lots of partners adds different perspectives and expertise, which opens up our eyes to different approaches. It’s not just about answering the questions we have, but paving the way for future trials, and learning to make the process as good an experience for participants as it can be.



INTERVIEWEE:
TOM SIMPSON –Research Delivery Manager NIHR, CRN Wessex
1 in 10,000 babies born are diagnosed with Spinal Muscular Atrophy (SMA) which can have a debilitating impact on their quality of life and for their families. A new multi-site study hopes to explore the impact that testing for the condition at birth can have on children and their outcomes for the future. We spoke to Tom Simpson to find out more about the study and the potential for it to change newborn screening in the UK.
BRINGING RESEARCH OPPORTUNITIES TO WESSEX
An important part of my role is to identify studies that could be delivered in Wessex to maximise research opportunities for patients. These studies may directly benefit participants but also add to our understanding of diseases and allow new treatments to be developed and tested.
In early 2023, the Newborn Screening for SMA Trial running in the Thames Valley and South Midlands region, but nowhere else in the country, was identified as a potential opportunity. It looked like a great study that might benefit the Wessex population,
so I contacted the study team to discuss the possibility of opening sites in Wessex.
The SMA trial uses the Guthrie Test, where the baby’s heel prick blood sample taken during the usual newborn screening undergoes an additional test in the laboratory to screen for the condition.
It’s a great study for patients and staff because it doesn’t require additional blood samples or interventions and the impact it can have is huge.
While SMA is quite rare, around 1 in 10,000 babies will be diagnosed with this potentially devastating disease.
This test allows the disease to be picked up before symptoms develop,
Spinal Muscular Atrophy (SMA) is a rare, but treatable, genetic disease that typically occurs in infancy and early childhood. SMA progressively, and irreversibly, destroys motor neurons in the brainstem and spinal cord, which control important voluntary and involuntary motions, in turn leading to deterioration or loss of muscle strength.
This can begin during the first three months of a child’s life, and in those with the most common and severe type of SMA, 95% of all motor neurons can be lost before the age of 6 months. The majority of children with this type of SMA, if untreated, will not survive beyond 2 years of age without permanent ventilatory support. Of those who do, many will not achieve independent sitting and few, if any, will ever walk independently.

which we know helps to improve outcomes for children with the disease. If babies are screened for SMA at birth and treated early it can be transformative for their quality of life. There’s obviously an immediate benefit for people who take part now, but the study’s wider aim is to evaluate the uptake and feasibility of screening in the UK. The findings could inform the case for a national screening programme for SMA which could potentially fit into the current testing system and be done as part of the newborn screening programme.
Following a few meetings with the University of Oxford based study team, they were keen to expand the study into Wessex. Locally, newborn screening samples are processed regionally by the laboratory team at Queen Alexandra Hospital in Portsmouth, so the study team
reached out to discuss logistics and feasibility of processing samples for the study. With agreement in place from a very supportive Portsmouth lab team, we offered the study opportunity out to sites across the Wessex region. Unsurprisingly, we received many expressions of interest from sites keen to be involved.
From here, the study team began conversations with interested sites to discuss feasibility and inform their site selection. After these discussions and a review of birth rate data we selected four sites in Wessex – Princess Anne Hospital (University Hospital Southampton NHS Foundation Trust), Queen Alexandra Hospital (Portsmouth Hospitals University NHS Trust), Poole Hospital (University Hospital Dorset NHS Foundation Trust), and Salisbury District Hospital (Salisbury NHS Foundation Trust). Because all the sites were setting up around the same time, we were able to regionally coordinate aspects of set
"We brought the teams together via regional calls which allowed us to share information, updates and progress in a way that reduced duplication."
up and communication to streamline the process. We brought the teams together via regional calls which allowed us to share information, updates and progress in a way that reduced duplication.
Adopting this collaborative approach also helped us to share best practice. Our regional calls included the Oxford study team, local research midwives, and R&D teams to ensure effective regional working. This collaboration helped to streamline the process and made set up more efficient, allowing
WATCH THE VIDEO
Watch Oscar and Sophia’s story to see the difference testing at birth can make for children with SMA.


us all to start recruiting sooner and screen even more babies.
The intention is to recruit as many babies as possible for this study –for every baby born at our sites to be offered this test. Our sites have enrolled 4,900 babies so far – which makes it the highest recruiting noncommercial study in Wessex this financial year.
Our regular regional calls have continued while the study has been recruiting as a forum to discuss what’s going well, raise any problems that need solving, and to share best practice. Everyone benefits from that. The teams can pre-empt problems and learn from each other’s experiences.
Every site has local nuances in how they deliver, but the benefit of regional collaboration has been evident. Now the study is established in Wessex, our sites and the Thames Valley and South Midlands sites now meet together to further share the benefits of collaborative working.
The study is due to end in March 2025, with new regions in discussion to open in 2024. So far, 23,000 babies have been recruited across Thames Valley, Oxford and Wessex. The hope is to recruit as many babies and gather as much data as possible to demonstrate the feasibility of newborn screening for SMA in the UK.
With all the conditions that could be screened for at birth, the Government and the UK National Screening Committee have lots of difficult decisions to make on which conditions should or shouldn’t be included in the newborn screening programme. Hopefully this study will provide the evidence to build a case for it to be included.
Some countries have already adopted this test as part of their newborn screening programmes. Economic modelling has also been undertaken to demonstrate the financial viability of newborn screening for SMA versus delayed diagnosis and the long-term treatment and care needs.
"This screening has already had a transformative impact on the lives of children with this condition, and their families, by being able to treat them before symptoms set in."
It’s been fantastic to get Wessex involved in this study, not just for the benefit of our local population but also potentially generating the evidence needed to inform its addition to our national newborn screening programme.
This screening has already had a transformative impact on the lives of children with this condition, and their families, by being able to treat them before symptoms set in. Early intervention can enable babies with SMA to lead relatively normal lives.

Everyone’s path in research is different, but it only takes minutes to start and can make a lifetime of difference. Clinical research is essential to our NHS. Whatever your role, you can be part of it.
For more information, please visit

Dr Patrick Moore, Associate Director of Clinical Research Network Wessex, explains why he’s passionate about Wessex Research Buses.
Have you seen our two new buses out and about? February saw the official launch in Southampton Guildhall Square with a fantastic ceremony. In the spring, the vehicles hit the road –fully operational and, with our excellent research teams across wessex, able to deliver a wide range of clinical trials.
The buses are a great example of collaboration. We worked with members of the public and students at Arts University Bournemouth to help design them. They will be an important resource for our Wessex research teams and partner organisations.
The buses are now busy travelling across the area to work with people in
their own communities, meaning many more people can take part in research. Now we can come to our patients and participants, rather than always asking them to come to us. Such an approach completely changes the ask and puts our volunteers at the centre of research, where they should be.
As a local GP in Poole, I am always trying to involve my patients in research, because I know how much this benefits them. I also see on a daily basis how hard it is for some people to engage in research. Coming into GP practices or visiting our hospitals can be stressful at the best of times. In addition to this, many GP surgeries also lack the space to be able to run research studies, and that stops them expanding into this area.
When it comes to taking part in studies which are based in hospitals, the barriers are even greater. Parking is a big issue, and as our studies often
involve repeat visits this can be really off-putting. Although we always reimburse travel costs, some people can’t afford to pay for public transport upfront. The reality is that the need to pay for a bus ticket might be make or break for someone agreeing to take part. And that’s not right.
Going mobile with these buses removes many of these barriers, and will significantly help improve the diversity and inclusion of our work. This is important to ensure we have good representation from all of our population so we know the results of research are attributable to the whole population.
Many of our participants tend to be from the same demographic –white, middle aged people, often of retirement age. We don’t want to stop this demographic from taking part and we appreciate the time and commitment they give to research.

"We
need to ensure our participants are representative of all our diverse communities across Hampshire, Dorset, the Isle of Wight and parts of Wiltshire. Historically some of these communities have been underserved by research opportunities."

However, we also need to ensure our participants are representative of all our diverse communities across Hampshire, Dorset, the Isle of Wight and parts of Wiltshire. Historically some of these communities have been under-served by research opportunities.
We must urgently address this, because research is for everyone. We know that our patients and participants who take part in research have better health outcomes. It is also important when trialling new and innovative medicines we include a diverse population so we can be sure that people from diverse ethnicities, with diverse genetic make ups, react in the same way, ensuring the medicines are equally effective in all of our population. We need our data to be robust. And I am absolutely convinced our buses will help with this. One of the first studies to run on the buses is likely to be the Prescription
Alerts for Reliever inhalers in Children (PARC) Project – an NIHR funded study researching childhood asthma run by researchers at the University of Southampton/University Hospital Southampton NHS Foundation Trust. This research focuses on children with asthma who are using a high number of reliever inhalers.
"We hope these families will find it easier to come along. They’ll be invited on board for a one-to-one check-up with a specially trained nurse, and given updated guidance on managing their condition."
Children with asthma may be using high numbers of reliever inhalers because they find it difficult to access health care. By taking the bus and parking near their homes,
in community settings, we hope these families will find it easier to come along. They’ll be invited on board for a one-to-one check-up with a specially trained nurse, and given updated guidance on managing their condition. Crucially, this study is helping us understand better how to prevent severe asthma attacks in a high risk group – and ultimately may help save lives.
Just one study which shows what great work the buses are going to deliver.


ARTICLE BY: CHRISSY STURT –
Communications Lead for CRN Wessex
Wessex Research Hubs began as an emergency response to the pandemic, focusing minds on the need to work together at a time of national crisis. Now they’re here to stay.
When the pandemic took hold in March 2020, CRN Wessex teamed up with local NHS Trusts to set up vaccination research centres. Our shared goal was to help test potential Covid-19 vaccines at scale and pace. Never before had we needed to recruit such large numbers into vaccine studies. Never before had these studies been so urgent. There was simply no time to lose.
Southampton’s hub was first on the scene. Initially set up in a university sports hall, the team later moved to the Royal South Hants. At the height of the pandemic, people queued around the block to take part.
Portsmouth came next, in the John Pounds Centre. This was followed by Bournemouth and Weymouth. All four hubs were well supported and easily met their recruitment targets - such was the demand for protection against Covid-19 and its variants. The vital work undertaken in these settings helped with the national roll-out of vaccines – testing doses, combinations and boosters. Ultimately this mapped a way out of lockdown and restored our freedoms. But was there any future for the model?

By the time the pandemic was over, our hubs had developed into a unique and special service. With the infrastructure, skills and staffing solidly in place, dismantling such centres was unthinkable. Not only was there continued demand for the testing of Covid-19 vaccines, but for many other vaccines and treatments. And we had hundreds of participants keen to stay involved and keep taking part. Today, the network has expanded beyond Covid-19 vaccines, but retains a special focus on researching infectious diseases. The hubs are able to run international studies ranging from RSV (respiratory syncytial virus) to whooping cough, Mpox, shingles and flu.
The future vision is to cater for an even greater depth and variety of trials, increasing access to research


for patients and the public across Wessex. Our big ambition is to align our research with the health needs of local communities – particularly the conditions and diseases we know really affect our populations in Wessex –asthma, diabetes and cancer. This way, we can offer local people innovative treatments which have the potential to really save and improve lives.
The network has expanded into four linked centres which share staff, expertise and resources. Each draws its participants from very different local populations, meaning recruitment reach is far greater than a single site could ever achieve.
Weymouth is the most recent facility to open, and showcases a desire to serve under-served areas – in this case a



"I've come to the conclusion that if people don't come forward to help with research, we're never going to move forward with vaccines or treatments."
coastal community with high levels of economic and social deprivation. The hub has been supported by a specialised programme of engagement to help build trust in clinical research.
With the hubs so embedded in their local communities, participants report very high satisfaction rates. They are happy to make return visits and often take part in multiple studies. They tell us they feel well informed about the study, understand what is involved, and find taking part a rewarding experience. Such high recruitment and retention rates are a major advantage of our unique model. Active social media accounts are helping boost engagement and interaction.
Our hubs are also supported by local primary care networks, with local GP surgeries contracted to send out text alerts to patients who meet specific study criteria and eligibility. This again supports recruitment in a highly targeted way. Many GPs and primary care
nurses also work within the hubs, and we have ideas about how these spaces might also be used to run primary care studies in the future.
The network is likely to expand, with further centres due to open in towns and cities in the Wessex area. This makes the hubs a shining example of collaborative working and how to take research out into the community, for the benefit of the Wessex population.
"By getting involved with your local hub, you could help future generations with illnesses which, at the moment, there are no treatments for."
TO FIND OUT MORE ABOUT WESSEX RESEARCH HUBS, PLEASE VISIT: wessexresearchhubs.nhs.uk


INTERVIEWEES:
HELEN SLOAN –Public Contributor
STEVE BOND –Public Contributor
ZOË SHEPPARD –Research Delivery Manager
All from Clinical Research Network Wessex
Patient and public involvement and engagement (PPIE) is a fundamental part of the National Institute for Health and Care’s (NIHR) strategy. Across Wessex, the Clinical Research Network (CRN) has been working hard to elevate the role of patients and the public within research. We spoke with public contributors, Helen and Steve, and Zoë (the lead for patient and public involvement and engagement) to find out how their contributions have been making an impact across Wessex.
SHAPING PATIENT AND PUBLIC INVOLVEMENT IN RESEARCH
STEVE: During COVID-19, I found an advert looking for public contributors in health and care research. It blended a number of things I was interested in at the time, one was health inequalities (e.g. in cancer) and the other was research which I had a background in at the Office for National Statistics.
HELEN: I got involved in PPIE about 10 years ago while having hospital treatment and then started working on PPIE at Southern Health including patient led research.
Myself and my family have various health conditions that I wanted to know more about and to contribute to improving. Through that, I found out about the national Coordinating Centre for CRN (CRNCC) and joined their Strategic Public Involvement Group. I found it difficult to understand the national situation and was glad to be able to join CRN Wessex as a public contributor.
My previous role as a curator of Art and Science collaborations brings a different perspective on how we could approach research to the role.
“Myself and my family have various health conditions that I wanted to know more about and to contribute to improving."


ZOË: It is vital to embed PPIE in all stages of the research cycle of health and care research and I have seen first-hand the value of this. Indeed, it improves the quality of research as well as leading to greater impact. PPIE has the potential to change every aspect of health and care research – from the research question that is being asked, to how the data are collected, and how the findings are shared.
People like Helen and Steve are fundamental to the work of the NIHR as they importantly remind us why we are all working in research – for the patients and public.
It takes time to develop good quality PPIE, and since being in post from September 2022, we have made great strides towards changing the culture around PPIE within the network. For example, whilst initially Helen and Steve were brought in to
represent the patient perspective on the Executive Group, their roles have expanded across all workstreams within the network.
ZOË: Helen and Steve have coproduced a strategy with us to embed patient and public involvement and engagement throughout the work of the network to ensure research is patient-centred. For example, it is now an agenda item at relevant meetings, our public contributors represent the patient/public voice at many meetings, have been involved in the recruitment of staff, and I hope feel integrated as important members of the team.
STEVE: After I got into post, my role quickly evolved from attending meetings to working on projects and PPIE initiatives, including reviewing documents, communications, and videos.

HELEN: Within our public contributor roles, we sit on various committees, we attend meetings, read papers and comment on them and review research funding applications. We have an involvement in shaping how CRN Wessex delivers research with the public. We have contributed to and developed information sheets about the Wessex Research Hubs and buses, the Join Dementia Research initiative and Be Part of Research offer for CRN Wessex.
We’ve also been involved in showcase events, where we’ve raised awareness of the CRN’s innovative research projects involving patients and the public. A project I’ve been really happy to be involved with, and lead on, is the Patient Advocacy in Patient and Public Involvement and Engagement in
"We've been involved in showcase events, where we've raised awareness of the CRN's innovative projects involving patients and the public."
An artist's response to presentations at a showcase event
Research (PAPPIER) project – a scoping project about the level of delivery of PPIE across the Wessex region in order to share and develop practice.
I feel very involved, Wessex CRN has really engaged us in a meaningful way.
STEVE: When I first sat on board meetings as part of this role, I wanted to address inequality in research participation. There were large groups of people who simply weren’t contributing to research and felt excluded from taking part. So, I wanted to make sure at every opportunity, researchers were thinking about: “Is what I’m doing open and accessible to all, and bringing in all parts of the community in terms of designing, participating and evaluating research?”
Sometimes it just takes an outside person who has good connections with different communities to bring that awareness. I don’t say that I represent communities, because I can’t represent Black and Asian communities, for example, but I can make sure that their struggles and problems accessing research are heard and make introductions to people in those communities.
The other thing I’ve done is try to make communications more accessible. The language within the CRN was difficult to understand in meetings, so I needed researchers and the network to know that it needed to change to get people to understand it.
I spend a lot of time advocating and heading out and about, introducing research to people. When I was at the Wickham Festival, I was talking to the public about Be Part of Research, listening to people’s stories about their health successes and failures and helping them to understand how important research is as a part of that.
What we say is valued and we’re welcomed and supported by the CRN, which allows us to make a difference.
HELEN: PPIE is all about involving people and doing research with and by people, rather than it being done to them or for them. Our approach enables us to have those voices heard and involve people from an increased number of communities across Wessex.
STEVE: Communication is key to gaining PPIE – getting the CRN to consider how it talks to people and how it communicates information, and being more open and transparent about what it does and who it helps. Our role very much sits in there and helps to make that happen locally.
We want to encourage researchers to collaborate with voluntary and community sector organisations, to design and deliver research that makes the community the champions of that research.
HELEN: I also feel that there’s a public out there that doesn't engage with community organisations. There are lots of isolated people who would benefit from research, perhaps in care homes or at home, and different approaches would benefit them. We’ve had good engagement from working with GP surgeries, for example, but we need to collaborate further to reach isolated people too. By working with communities who are under-served by research, we are starting to see these different types of research punch through and we’re beginning to see the public defining the themes of the research and what that research is, and sometimes even doing the research themselves. That’s been an amazing breakthrough. They’re experts by experience and able to have a say in their conditions or public health situation.
STEVE: We are also able to spot areas where competition is creeping in, and remind the researchers that collaboration is key – and advocate for the need to overcome that to benefit patients and the public. We add value by simply being people.
HELEN: Everyone has the potential to be a patient. At some point most of us go to the doctor and experience being on the other side of treatment – a situation that is mirrored in health and care research. I hope that we bring empathy and representation for the patient and public, their lives and experiences.

Tackling stigma associated with mental health is an ongoing challenge. It can act as a barrier for people reaching out for help and treatment but also as a barrier within the treatment itself. When people open up and share an unusual experience, hearing voices, for example, how the therapist responds in that moment can have an impact on the patient and their outcomes.
INTERVIEWEES:
LIZI GRAVES –Research and Innovation Manager, Southern Health
PROFESSOR KATHERINE
NEWMAN-TAYLOR –Chief Investigator
A collaborative study, ARMS (At Risk Mental State) in IAPT (Improving Access to Psychological Therapies), across the Isle of Wight, Solent and Southampton is looking to explore how common these unusual and unshared experiences are and whether a change in how their experience is acknowledged can lead to better patient outcomes.
The study was born out of a collaboration between researchers at the University of Southampton and clinical staff within the NHS. They came together to identify areas of service need, locally within Wessex. Particularly
prominent was this gap between people presenting at NHS Talking Therapy Services (formerly IAPT) and our secondary care mental health service. There were people who had psychotic type experiences, such as hearing voices, but were not unwell enough for secondary care, where these problems can be addressed.
Alongside this, there was a paper published by Clare Knight that showed high levels of people having unusual experiences within NHS Talking Therapies, so seeing things, hearing things that other people don’t hear – unshared experiences as we call them. From this, the ARMS in IAPT
"It’s important for us to find out how many people presenting with anxiety and depression are also experiencing low-level unusual, or unshared, experiences."



study was formed to see if we could address this within the NHS Talking Therapies model.
For people who are suitable for the service (not people who are classified as having psychosis, who would go into secondary care services), we wanted to take into account low-level unusual experiences to see whether it’s feasible, and also helpful for those people, to augment the CBT that’s provided. By allowing for that experience to be shared in talking therapies sessions, and acknowledged as part of normal human distress, would it help to reduce the isolation and stigma experienced by people with these symptoms?
What we’ve seen through our control arm of this study is that people with unshared experiences tend to come into the services being more unwell, reporting higher levels of distress and they leave still reporting high levels of distress.
So, the goal is to account for their experiences in their sessions and explore whether this approach helps with their depression and anxiety symptoms and to find out if this prevents them from becoming more unwell in the future.
It was also important for us to look at how prevalent these experiences are for people who are eligible for NHS Talking Therapies. We know that unshared experiences are common, but we don’t know how common.
So, it’s important for us to find out how many people presenting with anxiety and depression are also experiencing low-level unusual, or unshared, experiences.
In early 2021 we began the process of bringing the study together and by the summer we were bringing
the protocol together and applying for ethics. Initially we were given an Impact Acceleration Award through the University of Southampton which allowed us to recruit a Research Fellow to the study. This was invaluable because it was a study across three sites – Solent NHS Trust, Isle of Wight Health Trust, and Dorset Healthcare – Southampton Service. This meant the study could be coordinated well. It was then portfolio adopted through the NIHR underserved communities funding.
The team based in Psychology at the University of Southampton held a training course with the current CBT high-intensity therapists to provide education around unshared experiences and how to discuss these within NHS Talking Therapies CBT. They also provided supervision to follow that up and talk about cases as they came through.
"We want to be able to ensure that the people accessing our services have a better experience and, hopefully, improved outcomes."

There was a lot of engagement with clinical services to find out how we could run this study in each service. We also engaged with Patient and Public Involvement (PPI) representatives involved in the study to ask how we should talk about these sorts of experiences and why people may not wish to discuss them. It was important for us to really understand why people might keep their experiences secret when being assessed. We wanted to look at how we could overcome the stigma –because it’s perceived to be much more acceptable for people to say they feel low or anxious, than to say they hear voices, for example. We used information from the PPI group to shape the questions we asked in initial assessments and to train the therapists.
Then in October 2021, there was a service change implemented across IOW, Solent and Southampton Talking Therapies, where they all started asking the questions about unshared experiences in their assessment process.
Of those people answering yes to the assessment questions around unusual/unshared experiences, and who were then offered high intensity
CBT through NHS Talking Therapies, more than 90% of people approached agreed to taking part.
The study finishes at the end of March 2024 and there will be three papers published. These will look at the prevalence of these experiences, the psychosocial and clinical presentations associated with these experiences, and the outcomes in therapy.
This research wouldn’t have happened without the collaboration we established, because the clinicians simply wouldn’t have had the time to address the issues they were seeing. And without the clinician input alongside the expertise of the University in how to address these issues and their research experience, it wouldn’t have come together.
Each of the clinical teams involved provided research assistant time, therapist time, and principal investigator time from their services, alongside helping us to establish PPI groups. One of the big successes of this study so far is that it’s a home-grown Wessex study in mental health. We have so much local expertise involved in this study, I would
like to see more of these collaborations in mental health with service driven research that meets local needs and collaborates across organisations.
If we see a positive indication from the results, then our larger goal would be to apply for more funding in Summer 2024. So far the study has only tested 43 participants, so we would like to test further and include more people.
If we’re able to further reduce the distress of people coming into services with these unshared experiences then we’ll be able to know if it’s feasible, accessible and possible to roll out across NHS Talking Therapies services.
We want to be able to ensure that the people accessing our services have a better experience and, hopefully, improved outcomes.



JEMMA AUSTIN –Clinical Research Occupational Therapist, Southern Health NHS Foundation Trust
Making research accessible and inclusive to the wider community is an important part of building equality within healthcare access. Southern Health NHS Foundation Trust (SHFT) and Hampshire Hospitals Foundation Trust (HFFT) came together to initiate a study to explore the under-served populations of Mid-North Hampshire and find out how they could be accessed to improve research outcomes and opportunities for all.
We spoke to Jemma Austin, to see how her work is impacting research projects within the region.
It’s important that we reach out to more diverse communities within the Mid-North Hampshire area, because the under-representation of ethnic minorities in clinical trials affects the generalisability of study findings. Ultimately, this contributes to inequities in access to healthcare. There are also noticeable ethnic disparities in reported mental health outcomes which impacts
improving clinical practice. We needed to find out who our underserved communities were and find out why we were underserving them. It’s important we understand everyone’s health needs to make a difference to their wellbeing. We’d also noticed barriers to participation in trials around mental health and/ or psychiatric interventions. Stigma attached to discussing mental illness, anxieties related to understanding the implications of participation, worries
"It's important we understand everyone's health needs to make a difference to their wellbeing."

about the effects of trial medications, as well as cost and time concerns, all create anxiety around taking part.
SHFT and HFFT submitted an application together for a researcher to support engagement of underserved communities in our Mid-North Hampshire patch. I was recruited to undertake the research over a fourmonth period in November 2022. The study began as exploring racially minoritised groups, but then expanded to under-served communities as I discovered more and more about who our population was and what problems they were encountering.
There was no existing model for how this work should be done so I started by looking at population demographics in Mid-North Hampshire via the census. I looked at specific data and demographics I could extract from that such as ethnicity, gender, and
economics. Anything that could impact peoples’ involvement with healthcare. But the census is only done in 10 year periods, so it was hard to gather all the information accurately. I began comparing that data with data we collect around clinical trials on EDGE, our research database. It’s used to log participants approached or accepted into trials and, while it’s not all complete, I was able to gather some more information on ages, races and gender to help build a picture of our population.
By looking at the data on the census and on EDGE, it became clear that we really do need to start engaging young people with health and wellbeing as we don’t have a lot of data on young people in our area. I want to try and make sure we’re reaching young people too.
Because there was a limit to the data and how much I could learn from it,
"We need to start engaging young people with with health and wellbeing as we don't have a lot of data on young people in our area."
I began networking with the 44 local GP surgeries in our patch and seven areas of health visitors. I went along to the Health Visitors’ Working Action Group meetings to find out about their under-served communities – using their local knowledge to help build a better picture.
They understand their community needs well and building relationships is important if we’re to reach people and help with early intervention. All these areas have safeguarding leads as well, so it was helpful to connect with them as they understand a lot about their
"If we can work with children and family services, we can make a difference by embedding research early."

vulnerable population and what their needs are. If we can work with children and family services, we can make a difference by embedding research early and maybe helping them to not need health services as much in the future.
I found out through these conversations that we have a lot of refugees locally, as well as homeless and military communities that we’re underserving in terms of their health. These are communities which would be beneficial to engage with more.
What was surprising for me, was that we are recruiting quite well from people with different ethnic backgrounds, but we still have big gaps in some ethnicities and communities that we’re not reaching.
A focus on engaging people from Asian/Asian British, any Mixed and Black/African/Black British ethnic backgrounds is needed.
It was so important to this research to learn that people from military backgrounds and teenage mums were vulnerable groups that we’re not reaching currently.
There are also lots of rural areas in our patch, so gaining access to services can be a challenge for those people.
As part of this project, I used my newly made connections and understanding of our population to begin embedding research into the community. This study collaborated with HHFT on their Harmonie study – a study that looks at the effectiveness of a vaccination for babies against Respiratory Syncytial Virus (RSV). At the time I came into this position to help with the research, the challenge was recruiting out into the local community. They were mainly recruiting on hospital wards which limited the amount of people they were reaching.
So, it was a good chance to get out into the community and gain access to our under-served communities within the area. When I initially met with HHFT Clinical Researchers at end of December 2022, recruitment for Harmonie was 65 in total with a target 100. At the end of my research period (March 2023) the
recruitment was 128 patients overall. Another fantastic example of how we’re using this study to reach new populations and adapt our research approaches is CaFI (Culturally-adapted Family Intervention). CaFI therapy is being evaluated in a Randomly Controlled Trial (RCT) with African and Caribbean heritage service users diagnosed with Schizophrenia and related psychoses compared with the usual treatment they would experience.
Our Project Co-ordinator has worked collaboratively engaging in local church community groups for carers and service users, embedding research for this trial. He also organised a promotion day at the church, involving service users, carers and clinicians, promoting CaFI, where the sponsor joined online and we had an opportunity to eat food across different cultures. Around 80 people attended which was incredible.
We’ve also had discussion around producing leaflets in different languages and using visual imagery for people with learning disabilities. This research into our under-
"Collecting data on our population will help us understand our underserved communities further."

Find out more about the Culturally adapted family intervention (CaFI): WATCH THE VIDEO

served communities wouldn’t have happened without collaboration. I’ve collaborated across trusts and GP surgeries, but it would now be nice to start collaborating further with the communities we serve and develop those relationships. An important next step for us it to become more embedded within the community
"We're beginning to engage with Practice Managers so that we can put posters and leaflets out in GP surgeries to promote our studies."
and to create strong links with health visitors and safeguarding leads, so that we can reach more people and engage them with research.
Accessing more funding, to develop these networks and to continue to scope our population and understand their problems, means that we will be able to embed research into day-today practice so that it becomes familiar and reduces any stigma that people associate with it.
It would also be great to start collaborating via the EDGE database so that we can use it to its full potential, collecting data on our population that will help us understand our underserved communities further.
In the future, we're hoping to use one of the Wessex Research Buses to access our rural and underserved communities. To travel around and engage people with their health and wellbeing, would highlight how important it is that everyone has access to a safe space to take part in research if they wish.
We’re beginning to engage with Practice Managers so that we can put posters and leaflets up for our studies in GP surgeries across the area and it would be wonderful to link up with schools to further talk about health which would then lead into the wider community and research.
Ultimately, what we want is for our communities to access health services and research earlier, so that we can help them earlier and alleviate their problems.

End-of-life care is an underdeveloped area of research for many illnesses. But new approaches can have a big impact on a patients’ quality of life and their experience towards the end of their illness.
INTERVIEWEES:
SOFIA PIRES –
Research Sister at University Hospital Southampton
FAITH PANILAGAO-BUSHI –
Research Nurse at University Hospital Southampton
Sofia Pires and Faith Panilagao-Bushi spoke to us about the work they’re doing on the REDUCe2 study at the University Hospital Southampton, that hopes to improve palliative care for patients with advanced liver disease.
Ascites is an end stage complication of liver cirrhosis. It is a build-up of fluid in a patient’s abdominal cavity that can cause symptoms like pain, nausea, and breathlessness. In its early stages it can be treated with medication, but as the disease progresses treatment options diminish, and palliative care are involved. NICE guidelines advise the ascites can be safely drained up
to every one to two weeks in hospital, via large volume paracentesis (LVP) and this is the standard of care we offer currently. LVP is often performed as a palliative care procedure especially if the patient can’t be treated with medication or have any other treatment options such as a shunt or liver transplant.
A typical day for LVP involves the patient to be admitted as a day case in hospital, where the patient would have bloods taken beforehand to check renal function and clotting. The drain is inserted under local anaesthetic by a doctor or advanced nurse practitioner by the bedside and the drain can stay in for up to six hours. The patient would also have

"Our community research nurses are essential for conducting this trial. Their support is particularly valuable when patients are assigned to the long-term abdominal drain arm or face challenges attending the hospital."
an IV infusion of protein (albumin) for every few litres of ascitic fluid removed, which can be up to 10 litres at a time. The patients are usually also very dependent on their caregiver to travel to and from hospital. This is a huge time commitment for both the patient and the carer. Sometimes, a patient may phone in themselves to arrange a time to come and have LVP but when they arrive at hospital the level of fluid they have isn’t considered enough to require draining, so they get sent home, which can be hugely disruptive for them.
The aim of the REDUCe 2 study is to understand if a long-term abdominal drain (LTAD), that can stay in for a longer period of time and be drained at home, by a community nurse, is effective and can improve the patient’s quality of life without any increased risk of infection or complications. If we can prove that the long-term drain is suitable, then it
“It’s so exciting to be involved in this important trial for people with advanced liver disease. The promise of the study is that it will, hopefully, meaningfully impact people’s symptoms and quality of life. It’s a different trial from those we normally do, as the endpoints focus on what is really, good quality palliative care – an area where evidence is often sadly lacking. The multicentre nature of the trial brings together teams from the whole of the UK and will form the basis for future collaboration in this area.”
DR MARK WRIGHT, Principal Investigator REDUCe 2 Study
could have a transformational effect on patients’ lives.
Recruitment for REDUCe 2 in Southampton began in March 2023, and our target is to recruit four patients per year, with four years of recruitment. Each patient is in the trial for three months, but sometimes, due to the high mortality rate of the patients we treat, it can be less. Recruitment for this trial is scheduled to end in 2027.
Patients are referred to us via the clinicians in the hospital, and via a cirrhosis multidisciplinary meeting every week where patients with cirrhosis who require palliative care are discussed. We work collaboratively with the advanced nurse practitioners in the hospital who have a designated area for patients with ascites, Same Day Emergency Care (SDEC). We also have
an ascites clinic that refers patients into the study.
Due to the palliative care nature of the study, it can be difficult to get patients that fully meet the criteria to be eligible for the trial. With end stage liver disease, patients can often be confused and quite vulnerable, so even if they are eligible clinically, we can’t consent them into the trial because we need their full understanding to take part.
Our community and research nurses are vital for helping us conduct this trial in the community because some patients can’t attend the hospital. Therefore, the nurses will have to conduct a home visit and take their bloods, do the daily draining if the patient is randomised into the intervention arm of the trial, along with assisting with patient questionnaires and checking for infections. If the patient is randomised into the control
"Ultimately, we want this collaboration to positively impact our patients’ quality of life, and the lives of their care givers."

arm of the trial, then they are treated in-hospital via LVP as per normal.
An important part of this trial will be the financial and economical modelling of this treatment by the team at the University of Sussex, to check it is feasible and worthwhile for the NHS for the long term.
Before REDUCe 2, we ran the pilot study, REDUCe, here at Southampton, so being able to run REDUCe 2 is a nice continuation and helps us to build on our current provisions for people with ascites, like the SDEC clinic.
Collaboration is crucial for REDUCe 2 to happen. There are currently 24 sites involved in the study (with a target of 35 sites and 310 patients), with the central study team based in Sussex. We have contact with the central
team regularly when we have to ask questions about individual patients.
The nurse practitioner team regularly refer patients to us, having had initial conversations with them about the study. And the CRN research nurses are the ones going to patients’ homes and performing all the required activities for the study – we couldn’t do it without them.
We also coordinate with the interventional radiology team at UHS when the drain needs to be inserted through ultrasound guidance. In addition to this, there is a multidisciplinary weekly cirrhosis meeting so we can talk about patients and their treatment, which helps us hugely with the study because that channel of communication is already there. We need to be aware of all our patients because of the chance the patient will deteriorate quickly.
If the study proves successful, then this new approach would grow our collaborative network, with community nurses working much more closely with us and training them for the drain management. We would rely on our community nurses for spotting infections and leading on treatment, so our collaboration would increase hugely.
Ultimately, we want this collaboration to positively impact our patients’ quality of life, and the lives of their care givers.


INTERVIEWEES:
ANDY CLAXTON – Research
Paramedic, South Central Ambulance Service
CRAIG JACKSON – Research
Paramedic, South Central Ambulance Service
ANNA FOSTER – Research
Nurse, University Hospital
Southampton
KATE SHEPPARD – Research
Nurse, University Hospital
Southampton
CRASH-4 is a multi-site randomised placebo-controlled trial, co-ordinated by the London School of Hygiene and Tropical Medicine, that aims to provide evidence about the effects of early administration of intramuscular Tranexamic Acid (TXA) on intracranial haemorrhage, disability, death, and dementia in older adults with a symptomatic head injury.
CRASH-4 follows on from the CRASH-3 study, which showed the reduction in head injury deaths was largest in patients with mild to moderate head injuries if they were treated with TXA soon after injury. South Central Ambulance Service (SCAS) has been instrumental to the establishment of the trial in Wessex, and we caught up with the team to find out how their work is changing the lives of patients.
CRAIG & ANDY: The CRASH-2 and CRASH-3 trials provided significant evidence for paramedic practice relating to the administration of TXA, so having the opportunity to be involved in CRASH-4, was something we did not want to miss. The outcomes of CRASH-3 identified the benefits of administering TXA to patients with a Glasgow Coma Scale of less than 13
after a severe head injury. The study found that mortality was reduced by a third, and the sooner TXA is administered the greater the positive effect. In fact, the last iteration of head injury guidelines from NICE suggests TXA within two hours for head injuries.
Our patients are pre-hospital, so no CT scan has taken place, and they often have a more mild or moderate head injury, which is not always obviously serious.
“The study found that mortality was reduced by a third, and the sooner TXA is administered the greater the positive effect.”


When assessing older people for head injuries, it’s surprising how few symptoms they can present with but still have an underlying significant brain injury. This trial is designed to follow on from the impact of CRASH-2 and 3, to see how we can deliver the best care using TXA in a quick timeframe for patients with mild to moderate traumatic brain injury.
In the sporting world we’ve seen what blows to the head can do with regards to onset of dementia and Alzheimer’s disease and the study has considered these diseases as a secondary outcome. It asks whether this medicine can also stop or reduce the likelihood of developing these diseases post-head injury.
The study started back in 2021, and is recruiting until Nov 2025 across multiple ambulance service and hospital sites. Initially, the patient is randomised via administration of
Investigational Medicinal Product (IMP) within three hours of head injury. The discharge follow up, considers the patient’s condition and treatment whilst in hospital such as whether they had a CT scan, or a bleed, for example. This is followed by a day-28 post randomisation followup to monitor for adverse events requiring re-admission into hospital. After 12 months, the London School of Tropical Medicine and Hygiene clinical trials team review patients’ medical records for adverse events and dementia diagnosis.
CRAIG & ANDY: We’ve currently recruited 309 patients to the trial, making us the leading site for patient randomisation. Our model of working has been a major contributing factor in this success and collaborative working with hospital sites has supported

our recruitment. Many hospital sites initially didn’t have the capacity to complete patient follow-up due to post COVID-19 workloads, however, University Hospital Southampton (UHS) and Queen Alexandra Hospital (QA) in Portsmouth provided us with remote access to patient data, through letters of access, to enable us to follow up the patients we randomised.
This was only possible, with the help and support of the central study team at the London School of Hygiene and Tropical Medicine, working to change the protocol from one of divided functions (ambulance service enrols and hospital team follows up), to one of integrated functions which allow us to do both. Without the help of the hospital teams, we wouldn’t have been able to make such an impact on the trial.
Alongside this, we are the only ambulance service to have dedicated research rapid response vehicles.
This fleet of research-dedicated vehicles attend these incidents specifically, so that we can seek consent from patients regarding trial participation thereafter randomising them without delay. If full patient consent is not appropriate at the time of treatment we can record verbal assent or defer consent and written consent can be sought later in hospital.
With reductions in pressures, the UHS Research Team can now complete follow up data collection for CRASH4 randomised participants transported to their Emergency Department (ED). This then increases our availability to respond to potential eligible patients.
ANNA & KATE: We receive an email alert from the paramedic team about the patient when they’ve been given CRASH-4 IMP, so that we can identify them when they come into the ED.
If we are in hours, we find out what sort of consent the paramedic team has received (patient or relative) and we can seek consent if they were unable to get it from a patient or relative at the time of the injection.

"It has also generated more collaborative opportunities for us, and the close working has made it really easy."
Once they are in hospital, we give the patient more information about the trial, and then we follow up either by looking at the CRASH4 database for details around the treatment they received in hospital and their symptoms or via phone with the patient themselves.
CRAIG & ANDY: This approach has enabled us to seek an appropriate level of consent in all applicable emergency situations - giving patients the opportunity to be involved in research even if they might not have full capacity at that time. It helps us to administer the IMP quickly, as evidence shows it’s time-critical. Since TXA is a relatively safe drug that doesn’t have many interactions this feels like a safe and effective solution.
It has also generated more collaborative opportunities for us, and the close working has made it really easy.
ANNA & KATE: The level of communication between us and the paramedics has made this study so much easier. Their help recruiting is essential. Patients are normally already aware of the study when they come to us in hospital, because the paramedics have done so much already.
We’re able to give the best opportunities and care to patients because of this collaboration, the paramedics are so good when they bring patients into the ED. This means we can give high quality care, despite the challenges we’re facing.
CRAIG & ANDY: We are full time research paramedics, and we work across all of our trials. We can respond to any incident that may be appropriate and randomise to a trial if the patient is eligible. We then support the patient journey, complete appropriate data collection
"It allows us the ability to remain clinical and have patient contact whilst having research involvement and inclusion."

and administrative tasks in the background. It allows us the ability to remain clinical and have patient contact whilst having research involvement and inclusion.
This model also means that we can work from anywhere, so we work across lots of different ambulance stations, interacting with staff about research and answering questions. People now know us and know they can approach us to to discuss research.
In September 2023 our paramedic registering body, Health and Care Professions Council, amended the wording in their professional standards to require registered clinicians to be actively engaging in research. Our approach makes this happen, ensuring that paramedics are actively engaging patients appropriately in research activities.
Our research rapid response vehicles can respond to paramedic requests quickly, enabling them and patients to be involved in research easily. For the CRASH-4 study, we created pocket cards that we distributed to paramedics so that if they are on a call
with a head injured patient that may be suitable, they know how to quickly contact the rapid response vehicle so that we can attend and offer the opportunity to the patient.
"The relationships we have developed across the ambulance service and beyond makes us brilliant advocates for research and has grown our ability to bring on more studies."
We also provide opportunities for paramedics to complete on-station training for trials so that they can be involved and seek consent for trial participation from patients. This approach has provided the opportunity to develop research in the ambulance service in terms of broadening our research offering.
The relationships we have developed across the ambulance service and beyond makes us brilliant advocates for research and has grown our ability to
bring on more studies. We have hosted senior research staff from UHS and Wessex CRN on rapid response cars so that they can get a good understanding of what we can do and deliver. There is continuity and unity of all of the teams involved across services.
There are lots of challenges around data sharing and taking part in research, but we’re proving it can work. A lot of the innovations we’re bringing on involve bravery in stepping outside our comfort zone. The (calculated) risks you take to build these relationships have been backed by our forward thinking and open minded leaders. Without them, we wouldn’t be where we are now.
We need more ambulance-based research to ensure that we’re giving the patient the right treatment delivered by the right person at the right time.
ANNA & KATE: Patients should always be offered research if it’s appropriate – it’s how we can give better patient outcomes for them. We’re incredibly thankful for CRASH-4, the paramedics and for giving patients this opportunity.

INTERVIEWEES:
CHRISTINE MCGRATH –Managing Director
WILLIAM ROSENBERG
–Chair and Interim Academic Director
Wessex Health Partners (WHP) is the region’s strategic alliance of fifteen organisations across Dorset, Hampshire and the Isle of Wight (IOW), including four Higher Education Institutions (HEIs), ten NHS organisations (two Integrated Care Boards and eight NHS Provider Trusts), and Health Innovation Wessex. This strategic alliance of partners is dedicated to improving research, innovation and training in health and care for the benefit of local populations, with national and global reach. We caught up with Christine and William to talk about the goals for WHP and why collaboration in health and care research is fundamental to its future.
THE NEED FOR COLLABORATION FOR WESSEX HEALTH AND CARE RESEARCH AND INNOVATION
CHRISTINE: There’s a growing recognition that we can achieve more by working together and a good evidence base that shows collaborating outside of your own group or organisation is highly beneficial.
Over the last few years, there have also been legislative changes that promote collaboration. The new Health and Care Act absolutely requires organisations to collaborate,
whereas previous legislation for the NHS required competition. Universities now have civic responsibility too, so the momentum is there for collaboration which has been enabled by these changes.
WILLIAM: We have local challenges that are relevant to our local populations, but they resonate throughout the country and around the world. Particularly around air quality, coastal towns, and ageing. In some ways we are unusual. The average age of people in Dorset is
"There’s a growing recognition that we can achieve more by working together and a good evidence base that shows collaborating outside of your own group or organisation is highly beneficial."

a decade older than the rest of the country. In a decade, the vast majority of the Isle of Wight’s population will be older people, and we have the highest concentration of active and veteran service personnel too.
So, the purpose of promoting more research and innovation across Wessex is to address the health inequalities that exist in our region, that impact multi-morbidity, and ensure that people across Wessex are able to live better, healthier lives. It’s driving up the quality of health and care and levelling the benefits across Wessex.
CHRISTINE: Getting the pan-Wessex interdisciplinary expertise working together with our populations and partners, means that we can design research and innovation that meets those challenges. If we have an ecosystem that works together, we’ll be able to translate our research and innovation into practice more quickly.
WILLIAM: Around a decade ago, the National Institute for Health and Care Research (NIHR) and the NHS initiated their programme of establishing Academic Health Science Centres (AHSCs) – bringing together HEIs and NHS organisations to improve the discovery, development and deployment of health and care research and innovation.
About five years ago, partners across Wessex came together to submit an application for national accreditation that was highly commended. With this came the freedom to create something specific to Wessex and play to our talents and capabilities.
Most of the established AHSCs are dominated by one NHS trust and a single HEI. But what’s unique about Wessex is that it brings together so many complimentary structures that

can work synergistically rather than in service to one or two dominant actors. Ours is an equal partnership.
CHRISTINE: It’s also the largest alliance of it's kind in the UK, and an example of what could be done in other areas.
WILLIAM: Other AHSCs often apply their focus according to the contribution of their partners. What we’re recognising is that many of our wider stakeholders, like local authorities (LAs) for example, may not be particularly well funded, and have challenges, but that doesn’t mean they’re not important.
Traditionally, universities and NHS organisations compete for resources. We’re taking a region-wide approach to reduce the competition and increase the collaboration for the benefit of the whole ecosystem.
“Relationships with trust mean that partners can work together more quickly and more effectively.”

We introduce people to each other, spot good relationships that we can foster and nurture. And in a historically competitive landscape, we’re creating safe spaces for previous competitors to come together and work in collaboration.
CHRISTINE: Our work with wider stakeholders, like LAs, industry at all levels (startup and blue-chip companies), public and patients is very important. We’re bringing parties together to form a research and innovation ecosystem. In order for it to work well, it has to be introduced to itself. We’re making sure that our partners, individuals, and organisations become more aware of each other and what’s happening in the partnership, to work together and accelerate what they’re doing.
Relationships with trust mean that partners can work together more quickly and more effectively. And
this isn’t new to us. We were building these links before the pandemic, which meant our collaboration across Wessex during the pandemic showed us what was possible. It gave us confidence and accelerated how we work together.
WILLIAM: Exactly, through the pandemic Wessex was at the forefront of research and innovation, which opened people’s eyes.
WILLIAM: We are establishing a coherent and visible identity that external bodies can understand, to show them why they should work with us and what we can deliver. Big global corporations may not know much about our region. If we can promote awareness and showcase our strengths and expertise, we have the potential to be much more globally competitive together.
As a strategic alliance, we’ve already had success with some national funding initiatives including the Health Determinants Research Collaborations in Southampton and Portsmouth, the NIHR, and the Wessex Secure Data Environment.
CHRISTINE: It’s difficult for funders of all types to navigate their way through an ecosystem. We want to be able to say to them; “This is what we need and are interested in.” And when they approach us, we can direct funders to relevant groups and organisations within the partnership.
There’s expertise across Wessex and particular health challenges in Dorset populations that are also shared in Hampshire and IOW populations, so getting those individuals together to shape what the research questions might be, shape what the innovation needs are and put in collaborative grant applications will drive that success.

WILLIAM: WHP is a partnership alliance. We, as the core team, “serve at the pleasure” of our partners. We’re employed to help them achieve their goals. We can help identify opportunities they might not know about and help increase the level of safety of the relationships, but success is driven by the partnership itself.
CHRISTINE: The core team can act as the secretariat. The secretariat enables collaboration to happen more, but if the partner organisations make their connections and start working together, and they don’t need the core team, that’s fantastic. That is what it’s all about. We bring time and the opportunity for our partners to be organised and this can speed up the process of applying for grants, making the process better for them, with a better chance of the grants being awarded.
WILLIAM: Our ability to work with, nurture and support the Partners offers value for these reasons. We provide a bird’s eye view of our local challenges and commonalities, so that we can discover, develop, innovate and design services appropriately.
CHRISTINE: The re-organisation of NIHR Clinical Research Networks to NIHR Regional Research Delivery Networks, provides an opportunity for us to work supra-regionally too. We already have good relationships with Bristol Health Partners and Oxford Academic Health Partners, so the restructure will help us to work at that level and grow opportunities.
For anyone looking for opportunities, we do have small amounts of funding available to stimulate new collaboration. We’d advise people to go and speak to their directors of R&D and Associate Deans for Research in their organisations, to learn more about those opportunities.
wessexhp.org.uk








