Chapter1 Introduction
1.1MembraneProtein
Biologicalmembranesseparatecellsfromthesurroundingenvironmentaswellas enclosingcompartmentstomaintainspecifi cchemicalorbiochemicalconditions. Thesemembranesoftenconsistofphospholipidbilayersandhavecertaindistinctive tasksthatareessentialforfundamentalbiologicalfunctions.Membraneproteinsare acategoryofproteinfoundtointeractorembedontothebiomembrane,whichcarry speci ficfunctionsthatgovernthebehaviourofthebiomembrane[1].Membrane proteinscanhaveavarietyoffunctions.Someplaysimilarrolestosolubleproteins, suchasenzymes,carriersorpartsofensembleunits.Othersmightbemoreuniqueto thepropertiesofthemembrane,forexample,transportersandchannels,whichallow ionsorsmallmoleculestopermeatethemembranebarriersunderselectiveconditions.Membraneproteinsfunctioningasreceptorsareimportantduringsignal transductionpathways,triggeringthebiochemicalchainofeventsandallowingthe celltointeractwiththesurroundingenvironmentsandpromptthecorrectresponses. Genomicsequenceanalysisestimatesthatasmuchas30%oftheproteinexpressed in Homosapiens, Escherichiacoli and Saccharomycescerevisae isintegralmembraneprotein[2–6].Withtheiressentialphysiologicalrolesandthefactmembrane proteinislocatedonthesurfaceofthemembrane,theyarethetargetofalmost60% ofmedicaldrugs[7–9].Withsuchinterestinthefunctionalunderstandingand pharmaceuticalapplication,experimentallysolvingthedetailedthree-dimensional structuresofmembraneproteinshasbecomeafocusinmanyresearchprograms.
The firststructureofmembraneproteinwasrevealedin1975[10].Atwo dimensionalcrystalofpurplemembrane(PM)from Halobacteriumsalinarum containingbacteriorhodopsin(bR)wascharacterizedbyHendersonandUnwin usingelectronmicroscopy,andprovidedathreedimensionalmapat7 Å resolution. Theirresults,showninFig. 1.1,showedsevencylindricalrodsofdensityandthey concludedthattherodsweretransmembrane a helices.Hendersonwaslater awardedNobelPrizeinChemistry2017forhiscontributiontowardsdevelopment
© SpringerNatureSingaporePteLtd.2019
V.Yeh, StudyofBacteriorhodopsininaControlledLipidEnvironment, SpringerTheses,https://doi.org/10.1007/978-981-13-1238-0_1
ofcryo-electronmicroscopyforthehigh-resolutionstructuredeterminationof biomoleculesinsolution.In1985,DeisenhoferandMichelreportedtheatomic resolutionstructureofthephotosyntheticreactioncenterfrom Rhodopseudomonas viridis [11],afterbeingabletoobtainbettercrystalsbyadjustingthedetergentsand micellemodulatingadditives[12].Theirresearchbroadenedtheunderstandingof themechanismofphotosynthesisinbacteria,butmoreimportantlytheirresults suggestedthepossibilitiesofcrystalizingmembraneproteintoallowatomicresolutionX-raycrystallography.TogetherwithHuber,thethreesharedtheNobelPrize inChemistry1988fortheirachievements.
Withfurthersuccessesandbreakthroughs,theprogressofstructuraldeterminationofmembraneproteinacceleratedandthetotalnumberofuniquestructuresof membraneproteinsolvedincreasedexponentiallyeveryyear,asshowninFig. 1.2. Theprogressionresemblesthetrendofsolubleproteins,thoughwithasmallshift towardsthelowernumbers[13].However,despitetheprogressinscienceand advancesintechnology,characterizationofmembraneproteinremaineda tremendouschallengemostlyduetodifficultiesinsamplepreparation.Moreover, duetothenatureofmembraneproteinfunctionsinrelationtoitsnaturalenvironments,characterizationundernativeornearnativeenvironmentssuchaslipid bilayersormembranemimicsareessentialforabetterunderstandingofthe membraneprotein’sstructureorfunction.SuchmembranemimicswillbeintroducedandfurtherdiscussedinSect. 1.2
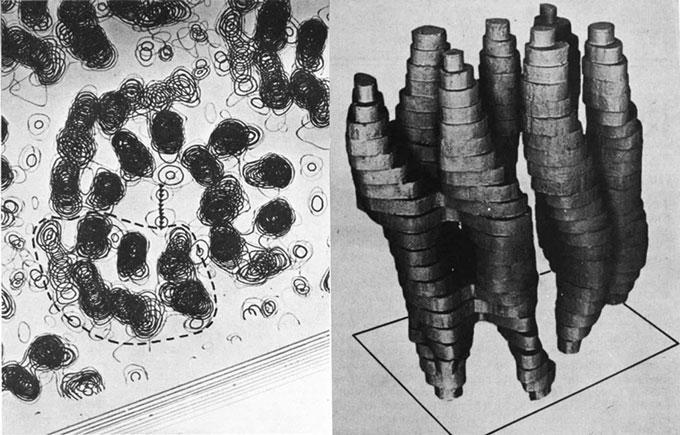
Fig.1.1 Partofthe3Dpotentialmap(left)and3Dreconstructionmodelofasingle bacteriorhodopsininpurplemembrane(right),derivedbyHendersonandUnwinin1975using electronmicroscopystudyingthe2Dcrystals.ReproducedandadaptedwithpermissionfromRef. [10],copyright1975NaturePublishingGroup
Fig.1.2 Progressinmembraneproteinstructuredetermination,whereonlyuniquestructuresare includedinthestatistic.Datapublishedat http://blanco.biomol.uci.edu/mpstruc/ (Accessed14 June2017)
1.1.1MembraneProteinStructures
Membraneproteincanbecategorizedintointegralmembraneproteinandperipheralmembraneprotein.Integralmembraneproteinsareproteinsthatarepermanentlyembeddedonthemembrane,andcanbesub-categorizedeitheras transmembraneproteinsorthosewhichresideononlyonesideofthemembrane throughananchor.
Transmembraneproteinsspanacrossthemembraneandoftenfunctionas channels,transportersorreceptors.Theyareinsertedintothemembraneandare exposedtomostlynonpolarenvironments,sothatthesidechainsoftheaminoacids embeddedinthemembraneareusuallyhydrophobic.Thecarbonylandamide groupsonthebackbonesoftheproteinwillthereforeformhydrogenbonding networkswitheachotherwithinthehydrophobiccoreofthemembranetoachieve structuralstabilization.Tosatisfytherequirementstominimizeenergycosts,the formationof a helicalor b barrelsecondarystructures,showninFig. 1.3,isthus stronglyfavoredforintegralmembraneproteins.
Transmembraneproteinswith a helicalstructuresaremoreabundantduetothe factthat a helicesformlocalhydrogenbonds.Thispropertyincreasesthe flexibility ofthetransmembraneproteintoundergoconformationalchanges,suggestingthat a helicaltransmembraneproteinsaremoreversatileintermsofstructuresand functions.Indeed,itisestimatedthat27%ofthetotalhumanproteomeis a helical transmembraneproteins[14],withallplasma-membraneproteinsbeing a helical.
Fig.1.3 Schematicrepresentationdiagramofthedifferenttypesofintegraltransmembrane protein:bitopictransmembrane a helix(left), a helicesbundle(middle)and b barrels(right)
Theyfunctionasreceptors,channels,transporters,electrontransportersandredox facilitators[15].Mostnotably,G-proteincouplereceptor(GPCR),thelargest familyofmembraneproteininthehumangenome[16],sharesacommonstructure ofseventransmembranehelices[11, 16–21].Alphahelicesaremostlyperpendicularorclosetoperpendiculartothemembraneplane,wherethedifferenthelicesare connectedbetweentheends[22].Theyarealsoeasiertobeidenti fiedfrom sequenceanalysis,asthetransmembranesegmentsrequiremostlyhydrophobicside chains.Alphahelicalbundletransmembraneproteins,unlikethebitopicsingle-pass a helicalprotein,oftenhavetheactivesitewithinthetransmembranebundles. Alphahelixbundledmembraneproteinshaveremarkablediversitywithintheir topologyandquaternarystructures.ShowninFig. 1.4 arethreeverydifferent arrangements.MonomericbacteriorhodopsinshowninFig. 1.4aisaclassic seven-helixtransmembraneprotein(moredetailsdiscussedinSect. 1.4),where eachofthetransmembranehelicesliealmostperpendiculartothemembraneplane. TheClCCl channelsontheotherhand,showninFig. 1.4b,isahomodimerwith eachmonomerhavingaCl specificchannel[23].Thesubunitcontains18 a heliceswithmostofthehelicesfarfromperpendiculartothemembraneplaneas wellashelicesthatdonotspanacrossthemembranecompletely.Shownin Fig. 1.4cistheCa+-ATPase,whichisformedwithacentralpartandaperipheral part,whichtransfersCaionsafteramusclehascontractedandundergoessignificantconformationalchangesduringitsATP-drivenpumpingcycle[24, 25].
Alphahelicalproteinswithsingletransmembranedomainsarecharacterizedas single-passtransmembraneproteins,wheretype1membraneproteins(TM1)have theirN-terminusonthecytoplasmside.Single-passtransmembraneproteinsare involvedinsignalingpathways[26–28],andaresuggestedtocompromise6%of theproteins-codinggenesinhumans[29].Thetransmembranehelicesofsuch proteinswereinitiallyconsideredonlyasahydrophobicanchorconnectingtwo extra-membranouspartsoftheprotein[30],howevermodernstudiesshowed protein-proteininteractioninvolvingthesingle-passtransmembranehelix,where theproteinsdimerizeoroligomerizethroughtheirtransmembranedomains[31, 32].

Fig.1.4 Schematicofthreedifferentstructuresof a helicalbundles,reproducedwithpermission fromRef.[182],copyright2006NaturePublishingGroup. a Monomericbacteriorhodopsinandco crystallizingmembranephospholipidshowninyellow. b HomodimericClCCl-/H+ antiporterfrom E.coli,withonesubunitshowninredandanotherinblue. c BovineCa2+-ATPase,whichhasa centralmembraneembeddingpartandaperipheralpart,experiencesdynamicchangesduring functionalcycles
Whileabundant a helicesbundlesarefoundinallbiologicalmembranes,the integraltransmembraneproteinswith b barrelstructuresareonlyfoundontheouter membranesofGram-negativebacteria,mitochondriaandchloroplasts[33].De fined by “long-range” hydrogenbondsbetweenthe b strands,unlikethelocalhydrogen bondsofan a helix,The b barrelstructureleavesonlyrestrictedmobilityallowing littleconformationchanges.Theyarehardertoberecognizedinthesequence comparedtothe a helicaltransmembraneproteins,sincenotallresiduesinthe b strandsarerequiredtobehydrophobic,makingthehydrophobicityof membrane-spanning b strandssimilartothoseofsolubleproteins.Transmembrane b barrelcanformwithanyevennumberof b strands,from8to22,andcanexistas monomers[34, 35],dimers[36]ortrimers[37].Betabarreltransmembraneproteins serveavarietyofdifferentfunctions,suchanon-speci fic[34]orspecificpores[38], activetransporters[35]andenzymessuchasproteasesorlipases[36].
Otherthanintegralmembraneprotein,anotherclassofmembraneproteinisthe peripheralmembraneprotein.Peripheralmembraneproteinsaredefinedasproteins thatcanassociateontothemembranenon-permanentlyaswellasexistinthe aqueousenvironmentsawayfromthemembrane[39, 40].Thiscoversawiderange
ofproteinfolds,structures,andfunctions.Peripheralmembraneproteinscanattach ontothemembraneviadifferentinteractions,includingnonspecificelectrostatic [41],hydrophobicpatch[42],covalentlyboundlipidanchor[43],speci fic lipid-bindingdomains[44]andprotein-proteininteractions[45].Peripheralmembraneproteinsarenotexclusivetoonlyonetypeofmembranebindingmethod. Whileperipheralmembraneproteinscanexistinaqueousenvironmentsunlike integraltransmembraneproteins,theinteractionswithbiologicalmembraneand mechanismremainagreatinterestinresearchduetotheirimportanceaswellas theircomplexity.
1.1.2MembraneProteinCharacterization
Membraneproteinsstructureshavebeensolvedwithvariousmethods,butthemain bottleneckofmembraneproteinstudiesremainstobesamplepreparation. Characterizationofsolubilizedmembraneproteincanbestudiedusingnumerous methodspriortodetailedstructuredetermination.Itisoftenthecaseinmembrane proteinresearchtohavethoroughpreliminarycharacterizationtocheckforsample homogeneity,structuralandfoldingintegrity,activityandstability.Sizeexclusion chromatography(SEC)[46, 47],analyticalultracentrifugation(AUC)[48]andlight scattering(LS)[49]methodshavebeenpopularinassessingthehomogeneityand theoligomerizationofthesolubilizedmembraneprotein.Circulardichroism spectroscopy(CD)[50],infraredspectroscopy(IR)[51]andRamanspectroscopy [52]hasbeendemonstratedtoprovideinformationofsecondarystructure,while isothermaltitrationcalorimetry(ITC)[53]and fluorescenceexperimentshavebeen usedforligandbindingmeasurementswhichcandeterminetheactivitiesofsome membraneproteins.
X-raycrystallographywasusedtodeterminethe firststructureofasoluble proteininthe1960sandthe fi rstatomicresolutionmembraneproteinin1985[11]. Althoughsomemembraneproteinsexistinlargequantitiesintheirnativemembrane,otherscanonlybeexpressedwithlowproductionyield,causingabig problemforcrystallographystudies[54].Proteinmustbeextractedfromthe membraneusingdetergentsbeforethemembraneproteincanbecrystalized. Howeverthetypeofdetergentappliedcansignifi cantlyinfluencethesuccessand thequalityofthecrystals.Withmostmembraneproteinstudies,proteinconstruct, bufferconditionsanddetergentusedtoextractorsolubilizemembraneproteinmust becarefullyscreenedandoptimizedpriortostructuredeterminationorfunctional observations[55].Membraneproteincrystalsarealsooftenfragiletohandleand suffereasilyfromradiationdamage[56].Butwithhighthroughputoptimization andincreasedknowledgeinhandlingmembraneprotein,x-raycrystallographycan provideastrongstructuralunderstandingofthemembraneproteins[57–59].
Electronmicroscopy(EM)canreconstructthree-dimensionalstructuresofbiologicalsamplesbyobtainingasetof2Dprojections.Duetotheneedtoreduce radiationdamagetobiologicalspecimens,highresolutioninformationcanonlybe
determinedbyaveragingmanydifferentimagesofidenticalmoleculesunderthe sameorientation.Cryo-electronmicroscopy(cryo-EM)isaparticularlypowerful methodinthestudyofproteinormembraneprotein.Thebiologicalsampleis frozenrapidlywherethewaterisfrozenintoathinlayerofamorphousice,which notonlyincreasesthecontrastoftheimagescomparedtotheuseofstaining,but alsocanpreservethebiologicalsamplesintheirnativeconformation[60].For cryo-EM,thesampleisnotrequiredtobecrystallineandonlyasmallamountof materialisrequired.However,thoughthosequalitiesarewellsuitedformembrane proteinstudies,itisnotwithoutitsownchallenges.Forexample,thepresenceof detergentsisoftennecessarytosolubilizemembraneproteins,butdetergentcan reducetheresolutionduetoitschemicalcompositionaswellascausingproblems duringvitri fication.Moreover,studiesofmembraneproteinusingsingle-particle electronmicroscopyrequiresextensivedataanalysis,includingimagesortingand classi ficationofprojectionsanddeterminationoforientationwithrespecttoeach averagedview.Extensivestructuralcalculationsandmolecularmodelingwould thenbeperformedbeforecomparisontoexperimentaldatatodeterminethe necessityoffurtherrefinement.Anotherapproachtoelectronmicroscopy,other thanthesingleparticleapproach,iselectroncrystallography.Althoughacrystalline sampleisrequiredasinthecaseinx-raycrystallography,insteadof3Dcrystals electroncrystallographyusescrystalsofasinglelayerthickness,referredtoas2D crystals[10, 61].Thetwodimensionalcrystalappearssimilartoamembrane environmentandisoftenachievedbyreconstitutingpuri fiedmembraneproteininto alipidbilayer.Ithasbeendemonstratedthatsomemembraneproteincanundergo distortionorconformationalchangeswhenpackedinathreedimensionallattice, causingsomedisadvantagesforx-raycrystallography.
Solid-statenuclearmagneticresonance(NMR)hasbeenusedtostudymembraneproteinsinceitcanstudyinsolublemacromoleculesthatcannotbecrystallized[62, 63].NMRprobesthenuclearmagneticmomentswithastrongstatic magnetic fi eldandasecondary fieldgeneratedbyanexternalradiofrequency(rf). InthecaseofNMRstudies,thesamplecanbeisotopicallylabelled,forexample with 13C, 15Nor 2H,inordertoenhancethesignal-to-noiseratiooftheNMR signals.Structuredeterminationbysolid-stateNMRisnotinfluencedbythesizeof theproteinbutbysensitivityandspectralresolution.Toimproveresolution,magic anglespinning(MAS)[64]isoftenemployed,wherethesamplecontaineris rapidlyspunathighspeeds(upto80kHz)aroundaspeci ficangleinrelationtothe staticmagnetic field.Atthisangle,anisotropicinteractionssuchasdipolarcoupling andchemicalshiftanisotropy(CSA)canbereducedorremoved.
Aswithanymethodofcharacterizingmembraneprotein,samplepreparation remainsthebiggestfactorinobtaininghighqualityresultsassamplehomogeneity hassignificanteffectonthespectralresolution.Lyophilizationhasbeenused extensively,butithasbeendemonstratedtocauseside-chainconformational inhomogeneity.Microcrystallineproteinsampleisalsoapopularmethod,asthe qualitiesofthecrystalarenotrequiredtobeatthelevelwhichx-raycrystallographydemands.
Solution-stateNMRisadrasticallydifferentmethodologytosolid-stateNMR.In solution,themoleculestumblerapidlywhichaveragesoutanisotropicinteractions. Thissuggestsasizedependenceofthemoleculeofinterestsinceastumblingrateof biggermoleculedecreases,linebroadeningoccursduetoincreasesofanisotropic interactions,thusleadingtoasignifi cantdecreaseofresolution.Moreover,structuralstudiesofmembraneproteinusingNMRrequiresthesampletobe monodisperseandstableformoderateamountsoftimeduringdataacquisition,as wellasisotopiclabelstoenhancesensitivity.However,recentdevelopmentshave enabledsuccessfulstructuredeterminationofintegralmembraneproteininsmall membranemimeticsuchasdetergentmicelles,lipidbicellesandnanodisc.NMRis apowerfultoolinstructuraldeterminationofmembraneproteinduetoitsunique abilitytoprobelocalstructureswhileabletoresolvedetailedthreedimension structuresthroughdifferentcorrelationassignments,providingNMRgreatpotential inthestudyofmembraneprotein.
1.2MembraneMimics
Asmentionedintheprevioussection,themainchallengeinmembraneprotein studyisthesamplepreparation.Membraneproteinsarenaturallyfoundembedded innativelipidbilayers.Oftenmembraneproteinsarenotsolubleinaqueous solutionssuggestingamembraneenvironmentmustbeprovidedinordertosatisfy thehighhydrophobicityofthemembraneproteinsbeforecarryingoutbiophysical characterization.However,biologicalmembranesarecomplex,heterogeneous, dynamicalsystemswhichprovetobedifficulttoworkwith.Itisthereforedesirable totransferthemembraneproteinofinteresttoacontrolledandsimplerenvironment thatcansatisfythehydrophobicnatureofthemembraneproteinaswellasprovidinganear-nativeenvironmenttoallowtherelevantstructuresorfunctionsofthe targetmembraneproteinstobecharacterized.Inthissectionafewcommonlyused membrane-mimickingsystemsareintroduced,whilelipidnanodisc,shownin Fig. 1.5f,willbediscussedfurtherinSect. 1.3.
1.2.1DetergentMicelle
Detergentshavebeenwidelyusedinthestudyofmembraneproteinduetotheir abilitytoextractmembraneproteinfromthemembranewhilesolubilizingthe hydrophobicmembraneproteins[65–68].Thispropertyallowsforpuri ficationof themembraneproteinaswellasimprovingqualityofcrystallization[69].Typical detergentmoleculescontainapolarhydrophilic “headgroup” andahydrophobic hydrocarbonchain “tail”.Atlowconcentrations,thedetergentappearstobe monomericmoleculesinsolution.However,whentheconcentrationissufficiently high,detergentmoleculesinaqueoussolutionwouldspontaneouslyself-assemble

Fig.1.5 Schematicdiagramshowingdifferenttypesofcommonmembranemimicssystems: a smalldetergentmicellesurroundingthetargetmembraneprotein; b amphipolsmadeupof amphiphaticpolymerscarryinghydrophobicchains,whichwraparoundthehydrophobicportion ofthemembraneprotein; c smalllipidbicellecomposedofamixtureofdetergentmoleculesand lipidmoleculesatspecificratios; d liposomeconsistingofphospholipidvesiclemimicking membranebilayerstructure; e SMALPsofsmallcentralcoreoflipidbilayerstabilizedby amphipathicpolymercalledstyrenemalicacidcoploymerand f lipidnanodiscofsmallcentral disc-shapecoreoflipidbilayerwrappedbytwocopiesofamphipathichelicalproteinscalled membranescaffoldproteins
intosphericalmicelles,wherethepolarhydrophilicheadwouldbesolventexposed whilethehydrocarbontailspackintoahydrophobiccore.Withthisproperty, detergentmoleculescanimitatethemembraneenvironmentandprotectthe hydrophobicportionofthemembraneprotein,generatingasoluble protein-detergentcomplex(PDC)showninFig. 1.5a.Thelowestconcentrationfor detergentmicelleformationiscalledcriticalmicelleconcentration(CMC).Above theCMC,themonomericdetergentmoleculeconcentrationisindependentofthe overalldetergentconcentration,andtheadditionaldetergentmoleculeswouldbe incorporatedintothedetergentmicelle[67].CommondetergentandtheirCMC valuecanbefoundinTable 1.1
Detergentscanbeclassifiedaccordingtotheirstructures:ionic,non-ionic,bile acidsaltsandzwitterionic[70].Ionicdetergentscontainaheadgroupthatcarriesan overallcharge,eitheranionicorcationic.Ionicdetergentssuchastheanionic sodiumdodecylesulfate(SDS),showninFig. 1.6a,canbeharshbuteffectivein solubilizationofmembraneproteins,oftencausingdenaturingofthemembrane proteinduetothefactitcandisruptthehydrophobicinteractionwithintheprotein. Insomecasesthemembraneproteincanberenaturedorrefoldedafterbeing
Table1.1 Commondetergentsusedinthestudyofmembraneproteinforstructuralanalysis
DetergentMolecularweightCMC(mM)Aggregationnumber
Ionic
SDS2887–1062
Sodiumcholate4319–152–3
CTAB3640.961–169
Non-ionic
TX-1006250.2–0.9100–155
DDM5110.1598
DM483 *1.8 *69
OG29220–2584
Digitonin1229<0.560
Zwitterionic
CHAPS6156–1010
CHAPSO631811
DPC3521.1–1.554 C7-DHPC4821.425 LDAO2291–274
transferredtoanotherenvironmentandtheremovalofSDS.Bileacidsaltsarealso ionic,butunlikeionicdetergentsbileacidsaltscontainrigidsteroidalgroups.Bile acidsaltssuchassodiumcholate,showninFig. 1.6b,haveapolarandapolarface andbehaveaslipidemulsifiers.Theyareconsideredasrelativelymilddetergents anddonotformtraditionalsphericalshapedmicelles.Inbothcasesofionic detergents,theiraggregationisstronglyaffectedbytheionicstrengthoftheir chargedheadgroups.
Non-ionicdetergents,ontheotherhand,aregenerallyconsideredtobemildand relativelynon-denaturingdetergents,astheybreakonlythelipid-proteinand lipid-lipidinteractionsbutnottheprotein-proteininteractionsasinthecasethe ionicdetergents.Thisallowsfunctionalandstructuralstudiesofthemembrane proteinuponsolubilization.ShowninFig. 1.6cisTritonX-100,acompound containinganeutralheadgroupfollowedbyaromaticringsformingitshydrophobic parts.Duetoitsaromaticfeature,TritonX-100absorbsUVlightandinterfereswith manyspectroscopicmethodsmonitoringproteinconcentrationwith280nm wavelengthlights.AnotherexampleofanonionicdetergentisshowninFig. 1.6d. n-Dodecyl b-D-maltoside(DDM)whichisanalkylglucoside,hasbeendemonstratedtosuccessfullyextractandisolatefunctionalmembraneproteinfromthe membraneenvironments[71].
Zwitterionicdetergentscombinethepropertiesofbothionicandnonionic detergents,thustheirdenaturingstrengthliesomewherebetweenthetwotypes. Someexamplesofzwitterionicdetergentsinclude3-([3-Cholamidopropyl] dimethylammonio)-2-hydroxy–1-propanesulfonate(CHAPSO)whichisshownin Fig. 1.6e,Lauryldimethyl –amine-N-oxide(LDAO),whichisconsideredaharsh
Fig.1.6 Molecularstructuresofafewcommonlyuseddetergentsinmembraneproteins: a ionic detergentSDS, b bileacidsaltsodiumcholate, c, d non-ionicdetergentTritonX-100andDDM, and e, f zwitterionicdetergentCHAPSOandC7-DHPC
detergentandn-dodecylphosphocholine(DPC),azwitterionicdetergentclosely resemblingaphospholipidwithaphosphocholineheadgroupdirectlybondedtoa hydrocarbonchain.Anotherzwitterionicdetergentis 1,2-diheptanoyl-sn-glycero-3-phosphocholine(C7-DHPC),showninFig. 1.6f,
whichisaphosphocholinewithshorthydrophobicchains.Forsomemembrane proteinszwitterionicdetergentsremaintooharshforfunctionalstudies,however zwitterionicdetergentshavebeencommonlyusedforcrystallography.
Theoptimalchoiceofdetergentforsolubilizingaparticularmembraneprotein canonlybefoundthroughscreeningandtrial-and-error[72–74].Thestabilityof thePDC,activityandstructuralintegrityofthemembraneproteininPDCremains complexandvariesdrasticallybetweendifferentmembraneproteins.Moreover, membraneproteinsarenotguaranteedtobecorrectlyfoldedevenwithinmild detergents,andnotalldetergentsaresuitableforeverycharacterizationmethod. Sizeandstructureofthedetergentmicellesandtemperaturestabilitymustallbe takenintoaccountaswellasitseffectsonthemembraneprotein.Insomecases systemswithmixeddetergentmicelleoramixtureoflipidanddetergentcan improvethequalityofthedesign.
Anexcessofdetergentmightbeusedtoensurecompletesolubilizationof membraneproteins,howeversuchexcessdetergentcanoftencomplicatethesystem ordisruptfurtherexperimentalstudies.Detergentscanberemovedorexchanged fromthesystemthroughvariousmethods.Dilutionofdetergenttoaconcentration belowitsCMCwouldleadtothedissolutionofdetergentmicelles,andthe monomericdetergentmoleculecanthenberemovedviamethodssuchasdialysis. However,fordetergentswithlowCMCvalues,suchasTritonX-100andDDM wheretheirCMCisabout0.2mM,suchprocedurecanbeineffective.Another methodisusinghydrophobicresins,orbeadssuchasBioBeads(Bio-Rad),which interactwiththehydrophobicdetergentandabsorbthedetergent,thusremovingthe detergentfromthesystem.ThisparticularmethodiseffectiveinremovingdetergentswithlowCMC,andthedetergentcoatedbeadscanbeseparatedfromthe sampleusingcentrifugationor fi ltration.Chromatographymethodssuchas ionic-exchangeandgel filtrationhasbeenemployedtoremoveorexchange detergentwithinthesystem.Becausemembraneproteincanaggregateorbecome insolubleintheabsenceofdetergents,bufferusedforchromatographymethods oftencontainsafurtherdetergentbasedonthechromatographymethods.For example,ifthemembraneproteinofinterestcontainspolyhistidinetags,nickel columncanprovideanefficientwayoftransferringproteinfromonedetergent environmenttoadifferentone.Theproteinboundtothecolumncanbewashed withthenewdetergentbufferpriortoelution.
Detergentcanbeusefulduringextractionandpuri ficationofmembraneproteins, buttheconformationandstructureofdetergentmicellecandeviatefromthe structureofthebiomembrane,suggestingtheuseofdetergentmicelleforthestudy ofmembraneisnotalwaysideal.
1.2.2Amphipols
Amphipolsareafamilyofamphipathicpolymersthatcarryhydrophobicside chainsandhydrophilicbackbones,whichcanwraparoundthehydrophobicportion
Another random document with no related content on Scribd:
ERICA
margaritacea.
CHARACTER SPECIFICUS
E����, antheris cristatis, inclusis; stylo exserto; corollis globoso campanulatis; floribus terminalibus, sub-umbellatis; foliis quaternis, linearitrigonis, glabris, erectis.
DESCRIPTIO
C����� erectus, ramosus; ramuli frequentissimi, erecti, glabri.
F���� quaterna, lineari-trigona, erecta, subtus sulcata, glabra; petiolis brevissimis, adpressis.
F����� terminales, nutantes, sub-umbellati; pedunculi filiformes, bracteis tribus, linearibus instructi.
C����. Perianthium tetraphyllum, foliolis subulatis, carinatis, sulcatis, lævibus, adpressis.
C������ urceolata, alba, calyce paulo longior; laciniæ limbi obtusæ, erecto-patulæ.
S������. Filamenta octo capillaria, apice inflexa. Antheræ ovatæ, brunneæ, cristatæ, inclusæ.
P��������. Germen globosum, sulcatum. Stylus filiformis, exsertus. Stigma tetragonum.
Habitat ad Caput Bonæ Spei.
Floret a mensi Junii, in Septembrem.
REFERENTIA.
1. Calyx, et Corolla.
2. Calyx, lente auctus.
3. Stamina, et Pistillum.
4. Stamen unum, lente auctum.
5. Pistillum, lente auctum.
SPECIFIC CHARACTER.
H����, with crested tips, within the blossom; shaft without; blossoms globularly bell-shaped; flowers terminate the branches in small bunches; leaves grow by fours, linearly three-sided, smooth and upright.
DESCRIPTION
S��� upright, and branching; the small branches are numerous, upright, and smooth.
L����� grow by fours, linearly three-sided, upright, furrowed on the under part and smooth; with very short foot-stalks, pressed to the branches.
F������ terminate the branches, are a little bent downwards, and grow in small bunches; the foot-stalks are thread-shaped, with three linear floral leaves on them.
E���������. Cup four-leaved, the little leaves are awl-shaped, keeled, furrowed, smooth, and pressed to the blossom.
B������ pitcher-shaped, white, and a little longer than the cup; the segments of the border are blunt, upright and spreading.
C�����. Eight hair-like threads, bent inwards at the ends. Tips eggshaped, brown, crested and within the blossom.
P������. Seed-bud globular, and furrowed. Shaft thread-shaped and without the blossom. Summit four-cornered.
Native of the Cape of Good Hope.
Flowers from June, till September.
REFERENCE.
1. The Empalement, and Blossom.
2. The Empalement, magnified.
3. The Chives, and Pointal.
4. A Chive, magnified.
5. The Pointal, magnified.
ERICA
Massonia.
CHARACTER SPECIFICUS
E����, antheris muticis, inclusis; corollis sub-cylindricis, viscosis, speciosissimis, tricoloratis; foliis subquaternis, pilis longis vestitis.
DESCRIPTIO.
C����� fruticosus, erectus, bipedalis, basi simplicissimus, dein ramosus, rami suberecti, foliis toti tecti.
F���� subquaterna, oblonga, serrata, pilis longis vestita, supra plana, subtus sulcata, petiolis brevissimis adpressis.
F����� subterminales, plures, simplice verticillati, cernuo-patenti, pedunculi pilosi, foliolis tribus subtus instructi.
C����. Perianthium tetraphyllum, foliolis lanciolatis, hirsutis, adpressis, apicibus revolutis.
C������ subcylindracea, micans, viscosa, rubra, lutea et viridia speciosissime colorata, ore arctato, quadrisido.
S������. Filamenta octo capillaria, longitudine tubi, receptaculo inserta. Antheræ muticæ.
P��������. Germen clavatum, sulcatum. Stylus filiformis, subexsertus. Stigma tetragonum.
Habitat ad Caput Bonæ Spei.
Floret a mensi Augusti ad Decembrem.
REFERENTIA.
1. Calyx et Corolla.
2. Calyx lente auctus.
3. Stamina et Pistillum.
4. Stamina a Pistillo diducta, anthera una lente aucta.
5. Stylus et Stigma lente aucta.
SPECIFIC CHARACTER.
H����, with beardless tips, within the blossom, which are nearly cylindrical, clammy, most beautiful, and three-coloured; the leaves grow mostly by fours, and are entirely covered with long hairs.
DESCRIPTION
S��� shrubby, upright, grows two feet high, simple at the base, then branching, the branches nearly upright, and quite covered by the leaves.
L����� grow mostly by fours, oblong, sawed, clothed with long hairs, smooth on the upper, and furrowed on the under part, having very short leafstems pressed to the branches.
F������ are numerous, in single whorls, near the summit of the branches, bending downward, and spreading, the foot-stalks hairy, with three small leaves fixed on their under part.
E���������. Cup four-leaved, which are lance-shaped, hairy, pressed to the blossom, and rolled back at the point.
B������ nearly cylindrical, shining, clammy, and most beautifully coloured with red, yellow, and green, narrow at the mouth, which is divided into four segments.
C�����. Eight hair-like threads, the length of the blossom, fixed into the receptacle. Tips beardless.
P������. Seed-vessel club-shaped, and furrowed. Shaft thread-shaped, rather without the blossom. Summit four-cornered.
Native of the Cape of Good Hope.
Flowers from August till December.
REFERENCE.
1. The Empalement and Blossom.
2. The Empalement magnified.
3. The Chives and Pointal.
4. The Chives detached from the Pointal; one tip magnified.
5. The Shaft and its Summit magnified.
ERICA melastoma.
CHARACTER SPECIFICUS
E����, antheræ muticæ, exsertæ, attenuata in filamenta plana; corollis sessilibus, solitariis, luteis, terminalibus; oris laciniis nigris; calyx duplicatus, heptaphyllus, imbricatus; foliis subulatis, quaternis.
DESCRIPTIO
C����� laxus, erectus, bipedalis; rami pauci, simplices; ramuli sparsi, brevissimi, frequentissimi, foliosi.
F���� quaterna, subulata, apice, recurvata, sub-scabrida, rigida; petiolis brevissimis, adpressis.
F����� sessiles in apicibus ramulorum, solitarii, dependenti; pedunculi brevissimi.
C����. Perianthium duplex; interius tetraphyllum, foliolis concavis, ovatis, glabris, imbricatis, integris, luteis; exterius triphyllum, priori brevioribus, consimilibus.
C������ conica, lutea, parum curvata, basi quadrifariam sulcata, apice attenuata; laciniis erectis, obtusis, longissimis, adpressis, nigris.
S������. Filamenta octo, plana, linearia. Antheræ muticæ, lineares, exsertæ, longitudine corollæ, attenuata in filamenta.
P��������. Germen ovatum, glabrum, integrum. Stylus exsertus, filiformis, staminibus paulo longior. Stigma marginatum, virescens.
Habitat ad Caput Bonæ Spei.
Floret a Mensi Februarii, in Julium.
REFERENTIA.
1. Calyx.
2. Corolla, et Stamina.
3. Stamina, et Pistillum.
4. Stamina a Pistillo diducta.
5. Stamen unum, lente auctum.
6. Pistillum, lente auctum.
SPECIFIC CHARACTER.
H����, with beardless tips, without the blossom, tapering into the threads which are flat; blossoms sitting close to the branches singly, are yellow, and terminate them; the segments of the mouth are black; the cup is doubled, of seven leaves and tiled; leaves awl-shaped, growing by fours.
DESCRIPTION.
S��� weak, upright, grows two feet high; the branches are few, and simple; the small branches are scattered, very short, numerous, and covered with leaves.
L����� grow by fours, awl-shaped, bent back at the point, roughish and stiff; foot-stalks very short, and pressed to the stem.
F������ sitting on the ends of the smaller branches are solitary, and hang down; the foot-stalks are very short.
E���������. Cup double; the inner one has four leaves, which are concave, egg-shaped, smooth, tiled, entire, and yellow; the outer threeleaved, shorter than the former, and like them.
B������ conical, yellow, slightly curved, having four furrows at the base, and tapering at the point; the segments of the border grow upright, are blunt, very long, pressed to the chives, and black.
C�����. Eight flat, linear, threads. Tips beardless, linear, without the blossom, and of its length, tapering into the threads.
P������. Seed-bud egg-shaped, smooth and entire. Shaft without the blossom, thread-shaped, a little longer than the chives. Summit bordered, and greenish.
Native of the Cape of Good Hope. Flowers from February, till July.
REFERENCE.
1. The Empalement.
2. The Blossom, and Chives.
3. The Chives, and Pointal.
4. The Chives detached from the Pointal.
5. A Chive magnified.
6. The Pointal magnified.
ERICA
monadelphia.
CHARACTER SPECIFICUS
E����, antheris muticis, exsertis, attenuatis in filamenta plana. Corolla conica, sanguinea, oris laciniis erectis, adpressis. Folia terna.
DESCRIPTIO.
C����� erectus, sesquipedalis, scaber, ad basin parum ramosus. Ramuli sparsi.
F���� terna, obtusa, sub-tomentosa, subtus sulcata, petiolis brevissimis adpressis.
F����� in ramulis, bini vel terni, terminales, cernui, in medio ramorum racemum formantes speciosissimum; pedunculis brevibus, bracteis nullis.
C����. Perianthium duplex, coloratum; exterius triphyllum, foliolis latoovatis, apicibus virescentibus; interius tetraphyllum, foliolis latioribus majoribus.
C������ conica, sub-pollicaris, glabra, sanguinea; oris laciniis erectis.
S������. Filamenta octo plana, corolla multoties longiora, receptaculo inserta; antheris muticis, exsertis, attenuatis in filamenta.
P��������. Germen ovatum, tenuissime sulcatum. Stylus filiformis. Stigma tetragonum.
Habitat ad Caput Bonæ Spei.
Floret a mense Augusti in Decembrem.
REFERENTIA.
1. Calyx et Corolla.
2. Calyx lente auctus.
3. Stamina et Pistillum.
4. Stamina a Pistillo diducta; antherâ unâ lente auctâ.
5. Stylus et Stigma lente aucta.
SPECIFIC CHARACTER.
H����, with beardless tips without the blossoms, and tapering into filaments, which are flat. The blossom is conical, of a blood colour, having the segments of the mouth upright, and pressed to the threads. The leaves grow by threes.
DESCRIPTION.
S��� upright, a foot and a half high, rough, branching but little at the base. The smaller branches are scattered.
L����� grow by threes, blunt-ended, rather downy, channelled underneath, having very short foot-stalks pressed to the branches.
F������ grow by twos and threes at the end of the smaller branches, hanging down, forming a beautiful spike near the middle of the larger branches. The foot-stalks are short, without floral leaves.
E���������. Cup double, and coloured; the outer three-leaved, the leaves of a broad oval-shape, the ends greenish: the inner is four-leaved, broader and larger than the former.
B������ cone-shaped, near an inch long, smooth, deep red; the segments of the mouth upright.
C�����. Eight flat threads, much longer than the blossom, fixed into the receptacle; the tips beardless, without the blossom, and tapering into the filaments.
P������. Seed-vessel egg-shaped, and slightly furrowed. Shaft threadshaped. Summit four-cornered.
Native of the Cape of Good Hope.
Flowers from August till December.
REFERENCE.
1. The Empalement and Blossom.
2. The Empalement magnified.
3. The Chives and Pointal.
4. The Chives detached from the Pointal; one tip magnified.
5. The Shaft and Summit magnified.


























