21 minute read
Original study: The utility of a non-mydriatic fundus camera in a tertiary diabetes clinic
The utility of a non-mydriatic fundus camera in a tertiary diabetes clinic
S Ben Barka, MBChB, FCP (SA), MMed (Int), Cert Endo and Metab (SA), Fellow, Division of Endocrinology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
ORCID: https://orcid.org/0009-0000-6904-574X
C Laurence, MBChB MSc, Clinical Epidemiology Medical Officer, Division of Endocrinology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
ORCID: https://orcid.org/0000-0002-9250-6193
L Du Toit-De Wet, MBChB, FC Ophth, MMED (Opt), Dip Ophth (SA), Consultant, Division of Ophthalmology, Department of Surgery, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
ORCID: https://orcid.org/0000-0001-55 67-1109
M Conradie-Smit, MBChB, MMed (Int), FCP (SA), Cert Endo and Metab (SA), Consultant, Division of Endocrinology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
ORCID: https://orcid.org/0000-0002-4252-6647
Corresponding author: Samia Ben Barka, email: ssbenbarka.bbss@gmail.com
Abstract
Background: Diabetes mellitus (DM) is rapidly escalating in prevalence globally. This is affecting health systems everywhere, especially in developing countries where these are already overburdened. Diabetes is the leading cause of blindness in the world and early detection of retinopathy is paramount in preventing unwanted sequelae.
Aim: The study aimed to assess the utility of a non-mydriatic fundus camera to screen for eye disease in the Autoimmune Diabetes Mellitus (DM) clinic (Endocrinology Division) of Tygerberg Hospital (TBH), in Cape Town, South Africa.
Our primary objective was to quantify what proportion of patients screened had severe eye disease requiring urgent referral to the Ophthalmology service at the same institution.
The secondary objective was to quantify the proportion of patients with mild or no disease that were not referred to the eye clinic and who were suitable to be followed up in the DM clinic by a trained medical officer.
Method: This was a retrospective descriptive study. Ethical approval was obtained from the Health Research Ethics Committee (Ethics Reference Number S21/08/156) of Stellenbosch University. In addition, institutional approval was obtained (WC_202206_020).
The study population included patients with Type 1 DM, or other auto-immune DM, who underwent digital photographic retinal screening during a six-month period from Sept 2019 to March 2020.
Results: A total of 177 patients were included in the study. The majority had Type 1 DM (n = 153, 86.4%) with latent autoimmune diabetes mellitus (LADA) accounting for the rest (n = 24, 13.6%). Severe diabetic retinopathy (DR) was detected in 7.9% of patients (n = 14), resulting in 2.8% (n = 5) requiring urgent referral to Ophthalmology and the rest semi-urgent. Normal screening or only mild disease were detected in 78% (n = 138). This resulted in 78.5% (n = 139) of patients not requiring referral to the Ophthalmology service. The remaining 17.7% (n = 31) were referred routinely.
Conclusion: The use of a non-mydriatic fundus camera to detect eye disease is known to be highly effective as a classifying/ evaluating tool of the severity of Diabetes Retinopathy. In our study it assisted with appropriate urgent referral to Ophthalmology services in patients with severe diabetic retinopathy. It also allows for not to refer mild retinopathy disease to Ophthalmology clinic, thereby preventing unnecessary hospital visits for patients. Consequently, the tertiary level diabetes clinic’s screening approach demonstrated an excellent tool for prioritising high-risk patients so they could receive appropriate care without delay.
Keywords: Diabetes mellitus, retinopathy, non-mydriatic fundus camera, screening, ophthalmology referral.
Funding: Nil received.
Conflict of interest: None.
Ethics approval, including consent: HREC no (S21/08/156) WCG no (WC_202206_020).
The research was done as part of an MPhil degree, with successful submission of the thesis and the degree to be awarded end 2023.
List of abbreviations
DM - Diabetes mellitus
HIV - Human Immunodeficiency Virus
LADA - Latent Autoimmune Diabetes in Adults
WHO - World Health Organization
HbA1c - Haemoglobin A1c (Glycated haemoglobin)
GDM - Gestational Diabetes mellitus
TBH - Tygerberg Hospital
PLWD - People living with diabetes
NMFC - Non-mydriatic fundus camera
DED - Diabetes eye disease
Introduction
Diabetes mellitus (DM) is a metabolic disease/ malady characterised by hyperglycaemia, with rapidly escalating prevalence globally.1 This is affecting health systems worldwide, especially in developing countries.2,3 In Africa specifically where health systems are already overburdened by communicable diseases such as HIV, tuberculosis and malaria, DM prevalence will double from 12 million in 2010 to 24 million by 2030.4 In 2019, a total of 463 million people worldwide were estimated to be living with DM, representing 9.3% of the global adult population (20-79 years of age). This number is expected to increase to 700 million (10.9%) in 2045.1 As DM prevalence increases, prevalence of DM complications will increase.
DM is one of the major noncommunicable diseases (NCD) causing death.5,6 NCD include heart disease, stroke, cancer, chronic lung disease and DM, and these are collectively responsible for almost 70% of all deaths worldwide. Concerningly, 77% of all NCD deaths are in low- and middle-income countries, of which South Africa is one.7
DM causes macrovascular and microvascular (diabetic eye disease (DED), diabetic kidney disease and neuropathy) complications. DED is a group of ocular conditions that may occur in patients with DM. These conditions include, but are not limited to, cataracts at a younger age, diabetic retinopathy (DR), diabetic macular oedema (DME) and glaucoma.8 The complications of DM, including retinopathy, are correlated with the duration of DM, poor glycaemic control (high level of glycated haemoglobin [HbA1c]),9 and the presence of other vascular risk factors such as dyslipidemia10 and high blood pressure.11,12 Therefore, the importance of early detection of microvascular complications is paramount in mitigating risk, both by stricter glycaemic control and by optimising the management of other risk factors. The presence of microvascular complications is associated with macrovascular complications and cardiovascular risk.13,14,15 With advanced retinopathy the risk is increased for ischemic heart disease (IHD), as well as chronic kidney disease (CKD), stroke and dementia.16,17
There are several barriers impeding the early detection of retinopathy in resource limited settings.18 These include a lack of screening programmes in certain areas, lack of education of both patients and health care workers at the primary care level regarding the importance of DR, financial barriers and barriers in access to eye care such as rural settings.19 The use of dilating eye drops prior to fundoscopy is an added limitation due to the inconvenience of cycloplegia preventing accommodation and hence affecting the ability to read, drive and return to work for 4 to 10 hours.18,20 The process may also be timeconsuming and requires special skills.
As the prevalence of DM increases, the burden of screening for the complications, especially DED, becomes more challenging.21 Modern techniques for screening for diabetic eye disease have improved significantly in the last few years, becoming more practical and more accurate.22 This is achieved by using technology such as non-mydriatic fundus photography and artificial intelligence software to detect pathology.23,22 In addition, systems have been developed to grade DR as well as to possibly detect other pathology such as hypertensive retinopathy or cataract.24,25 Screening effectively detects the early development of DED and enables timeous treatment of eye disease,26 also in our setting.27
The study aimed to assess the utility of a NMFC (non-mydriatic fundus camera) to screen for DED in patients from the autoimmune DM clinic of TBH, a tertiary level facility in the Western Cape province of South Africa. Our primary objective was to quantify what proportion of screened patients had severe DED requiring urgent referral to the Ophthalmology service. The secondary objective was to quantify the proportion of patients with mild or no disease who were not referred to the eye clinic and who could therefore undergo further retinal screening at the DM clinic.
Materials and methods
This was a retrospective descriptive study where patients were selected using consecutive sampling. Ethical approval was obtained from the Health Research Ethics Committee of Stellenbosch University (Ethics Reference Number S21/08/156). In addition, institutional approval was obtained from Tygerberg hospital (WC_202206_020).
We included all patients who attended the auto-immune DM clinic (new and followup patients) and had retinal photographic screening during a six-month period from Sept 2019 to March 2020. This DM clinic was attended by adult and adolescent patients as it is dependent on the age of diagnosis in certain cases. We excluded patients with Type 2 DM, ketosis prone Type 2 DM and pregnant patients, should they have been screened. The reason was that we wanted to describe the impact of this intervention in the auto-immune DM population, prior to expansion to other cohorts.
We used a Canon Digital Retinal Camera CR-2 AF (CANON INC 30-2, SHIMOMARUKO 3-CHOME, OHTA-KU, TOKYO, JAPAN) to photograph the full fundus of each eye without dilating the pupil. Photos were repeated until a good quality photo was obtained. All photographic screening was performed by one endocrinology medical officer who was trained by staff from the ophthalmology service to do screening and worked in a darkened room adjacent to the DM clinic. The images were then reviewed by a doctor from the Ophthalmology clinic on the day. We used the Early Treatment Diabetic Retinopathy Study (ETDRS) classification to grade retinopathy.28
Data were collected from the database on the camera as well as medical records where applicable and recorded using a Microsoft Excel spreadsheet. Data included were age of the patient in years, sex of the patient, type of DM (type one or Latent Autoimmune Diabetes of Adulthood), duration of DM in years, weight (kilograms), HbA1c (%) and the presence of other cardiovascular risk factors (dyslipidaemia, smoking and blood pressure), other documented complications of diabetes (microvascular/ macrovascular) and applicable socioeconomic information such as employment. The privacy of personal information was ensured by using coded identities and the data were stored on a single computer with password protection.
The Division of Biostatistics and Epidemiology of the University of Stellenbosch assisted with the statistical analysis of the data. Stata version 16 was used to analyse the data. Descriptive statistics were utilised to estimate the proportions of interest in the study population such as the proportion of patients referred on an urgent basis due to severe retinopathy (primary outcome) and the proportion of patients who continued screening at the DM clinic (secondary outcome). The proportion of the latter group of patients who were employed and who did not have to attend a second time for screening in the ophthalmology department, was also calculated.
Results
A total of 180 patients were screened over the six months period at the DM clinic. Three patients were excluded as they had Type 2 DM. The majority of patients were female (n = 107, 60.5%). The mean age was 30.2 ± 9.7 years (range 15-62 years). Most patients were known with Type 1 DM (n = 153, 86.4%) and the rest had LADA (n = 24, 13.6%), Mean DM duration was 12.3 ± 8.3 years. A total of 175 patients were using insulin with a mean total daily insulin dose of 54.0 ± 23.7 units. Mean HbA1c was 10.5 ± 2.5% (range 4.9 to 18.4%).
Baseline characteristics are shown in (Table I).

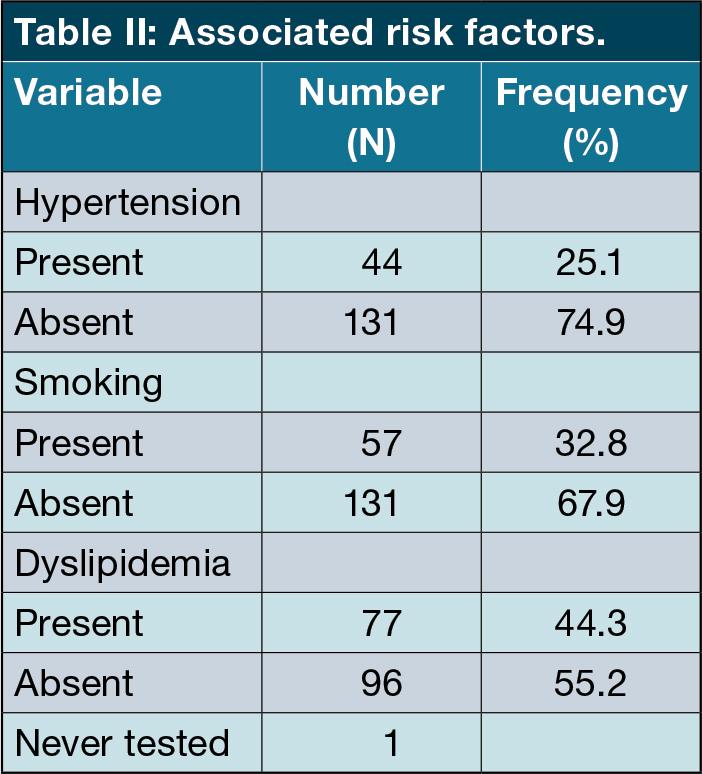
Other cardiovascular risk factors were documented. Dyslipidaemia was most common in 77 patients (44.3%), followed by smoking (57 patients, 32.85%) and hypertension (44 patients,25.1%). (Table II)
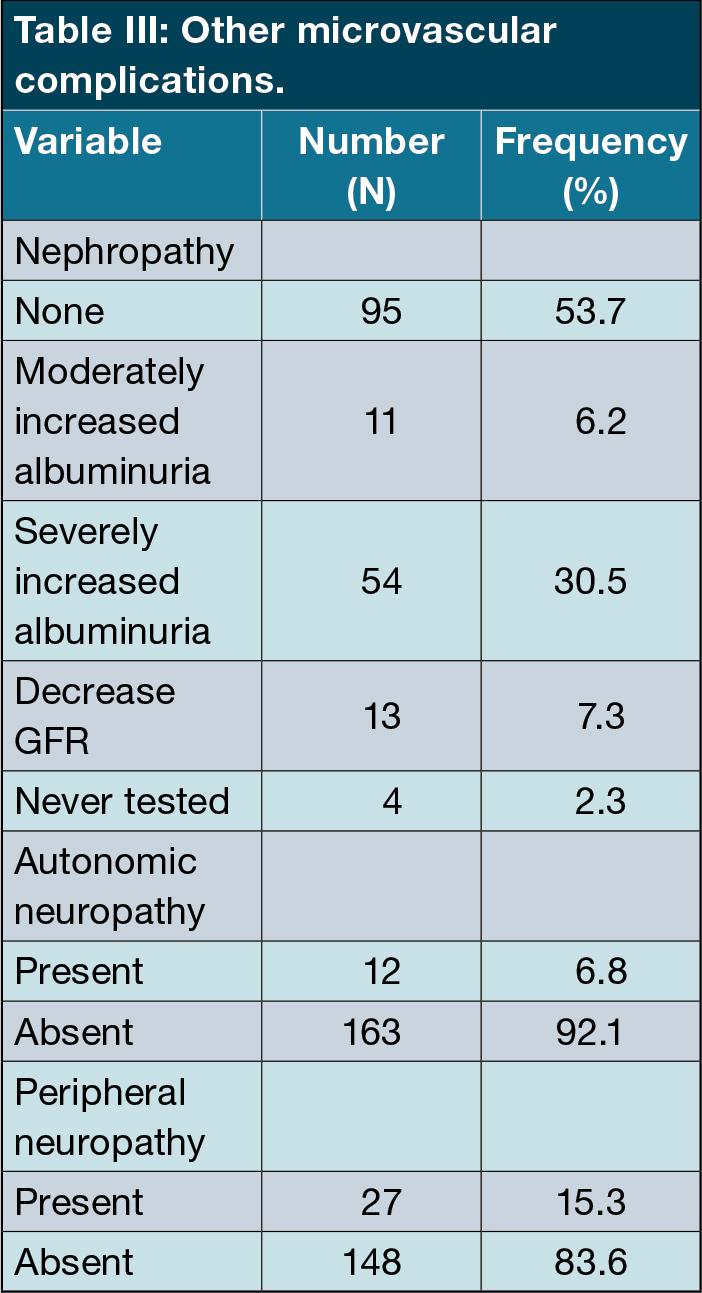
We documented the presence of other microvascular complications. For diabetic kidney disease, 95 patients (53.7%) were disease-free, while 76 patients (46.3%) were affected. The majority had severely increased albuminuria (more than (>300mg/day) (n = 54,30.5%), with moderately increased albuminuria in 11 patients (6.2%). A decreased glomerular filtration rate (GFR) was detected in 13 patients (7.3%). Four patients were not tested for kidney disease.
Peripheral and autonomic neuropathy was only detected in 27 (15.3%) and 12 (6%) patients respectively. (Table III)
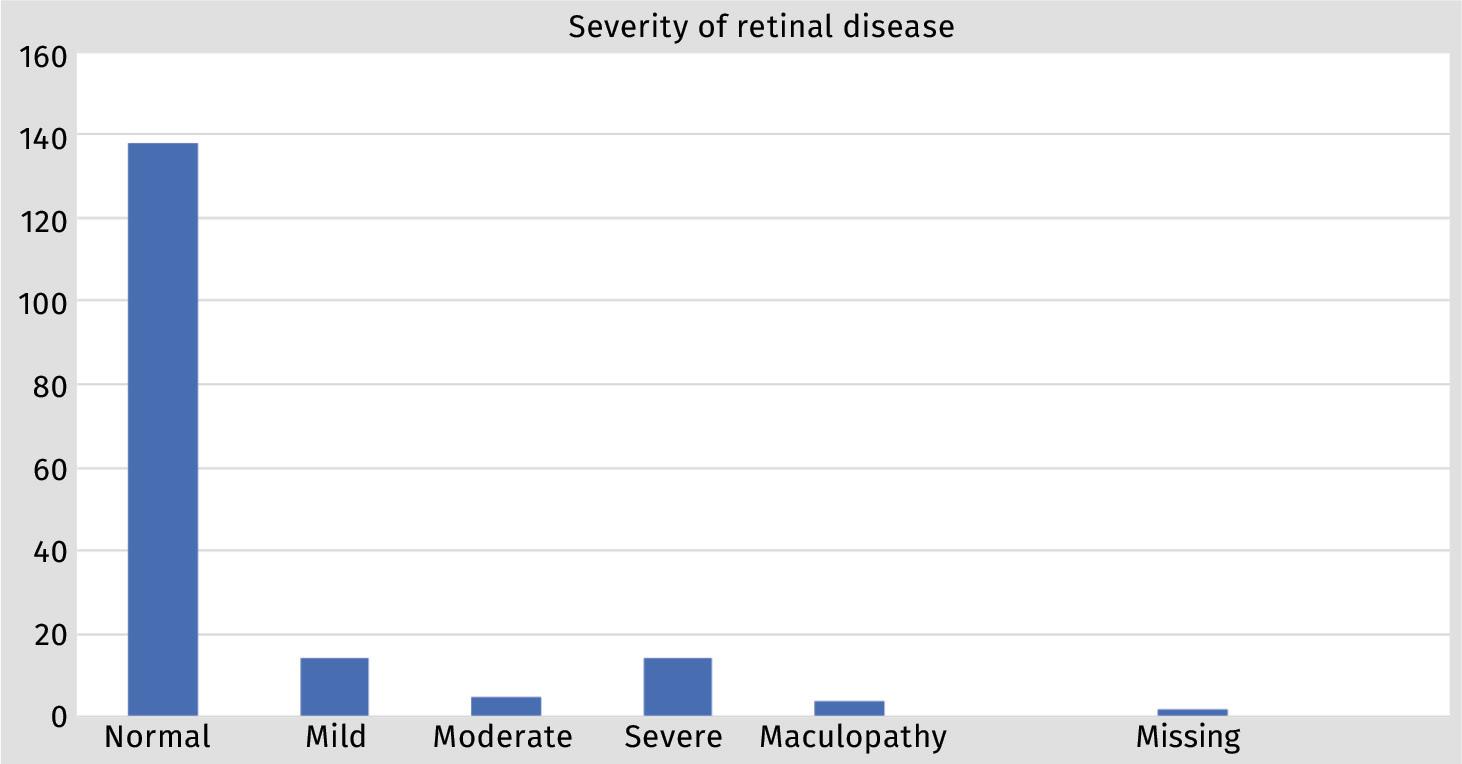
Diabetic retinopathy was not detected in 138 patients (78.0%), mild retinopathy occurred in 14 (8.0%), moderate in five (2.8%) and severe in 14 (8.0%) of patients. Maculopathy was detected in four patients (2.3%). In two patients (1.3%), evaluation was not possible due to the presence of cataracts. (Figure 1).
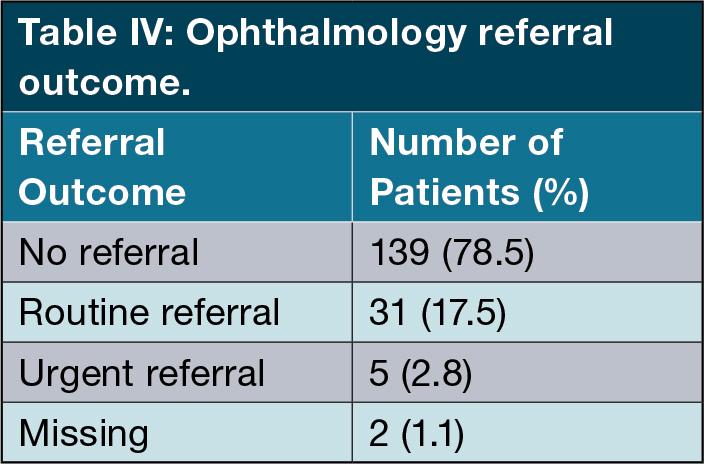
Therefore, a total of 139 patients (78.5%) were not referred for ophthalmology review. Furthermore, five patients (2.86%) were referred as priority cases for urgent ophthalmology review and 31 patients (17.71%) were referred for routine evaluation. (Table IV ).
Employment status was only recorded in 102 patients (57.6% of total). The majority of these patients were unemployed (60 patients). In the case of the employed patients, 34 (80.9%) were not referred to ophthalmology thereby avoiding attendance on another day and missing an additional day of work.
Discussion
This retrospective descriptive study evaluated the utility of NMFC in a tertiary auto-immune diabetes clinic in detecting diabetic retinopathy. It was noted in our clinic that patients who are followed in a tertiary setting do not present to these primary level screening programmes. Prior to this study these patients were all referred by our clinic to the Ophthalmology service on a regular basis for screening and follow-up.
In our study population, diabetic retinopathy was not detected in 138 patients (78.0%), mild retinopathy occurred in 14 (8.0%), moderate in five (2.8%) and severe in 14 (8.0%) of patients. This had two main advantages: firstly, patients with significant disease were detected earlier with urgent referral to the Ophthalmology service for timeous intervention and, secondly, patients with normal screening results or mild disease, which accounted for almost 80% of patients screened, could be followed up in the DM clinic, without unnecessarily overburdening the eye clinic. Early detection has major benefits in the outcome of individual patients as has been demonstrated in developed countries.10 However, preventing unnecessary referral to the eye clinic of patients with insignificant eye disease is also important in our setting, as healthcare services are overloaded. Patients were spared a second visit, which translates into cost-saving in transport and hospital visits and less time away from work for employed patients.
Studies conducted both internationally and locally at various levels of care demonstrated that screening people living with diabetes (PLWD) with a fundus camera improved the quality of care. In India, Wadhwani et al. screened PLWD aged 40 years and older using a portable handheld NMFC in screening camps in the slum communities of Delhi between January 2013 and June 201429 of the 9435 patients screened, 1273 (13.5%) had diabetic retinopathy; 351 (3.7%) of these had mild NPDR, 567 (6.0%) had moderate, and 92 (1.0%) had severe NPDR. PDR was noted in 77 cases (0.8%) and 49 (0.5%) had DME. All cases with DR were referred for tertiary care. In Pakistan, Fahadullah et al. also used a NMFC to screen type 2 DM patients for DR between January and May 2015. They screened 2970 eyes of 1485 patients and found DR in 646 (21.8%) of eyes.30
It has been recommended that effective screening programmes for diabetic eye disease should take place outside of the ophthalmology service.31 Numerous barriers to effective screening have been identified in various settings, which include access to the programme and resources.32
A study geographically comparable to ours is one by Mash et al. conducted in the Cape Town metropole. Of 400 individuals who underwent screening, 63% had retinopathy (22% severe nonproliferative retinopathy, 6% proliferative retinopathy) and 15% had maculopathy with 7% of all cases requiring immediate laser treatment. 27 However, only 2.8% of patients in our study required referral for urgent treatment while 17.5% needed routine referral and most of our patients had no retinopathy. The fact that most participants in the study by Mash et al. had Type 2 DM, were middle-aged and hypertensive may partially explain the differences between the two studies. Notwithstanding the differences, both these local studies have demonstrated that DR screening of patients using a NMFC is an effective way of detecting DR and preventing unnecessary referrals to eye clinics that are already overburdened. Another local study screened 14 541 patients between 2007 and 2010. They calculated a cost-effectiveness ratio of $1206 per blindness case averted which included screening and treatment costs. However, the cost to only screen a patient for DR was a mere $22.33
A recent study performed in a high-risk diabetic clinic in Soweto, South Africa, concluded that screening with digital nonmydriatic fundus photography was effective in detecting DR in their population, with 28% diagnosed with DR. Of these patients, only 27.02% had Type 1 DM.34
In the future, it may be possible to further improve the cost-effectiveness of this type of screening by introducing artificial intelligence (AI) into the grading of different types of retinal disease. For instance, a study conducted in China demonstrated that AI-based DR screening had a high sensitivity and specificity in detecting DR. DR was detected in 143 (16.1%) people by ophthalmologists and in 145 (16.3%) participants by AI.35 In a study from Zambia, retinal images were taken from 1574 PLWD which detected referable DR (moderate NPDR or worse, DME and ungradable images) in 22.5% of eyes and vision-threatening DR in 5.5% of eyes.28 The area-under-the-curve (AUC) of the AI programme for referable DR was 0.973 (95% CI 0.969 - 0.978) with sensitivity of 92.25% and specificity of 89.04%. The sensitivity for vision-threatening DR was 99.42%.23 This demonstrates the potential shown by AI technology to reduce preventable blindness in under-resourced countries. A systematic review of seven studies found that a telemedicine programme that involved taking a retinal image and sending it to an ocular imaging facility to assess the severity of DR could potentially result in significant cost savings by improving patients’ working capacity and lowering transport costs.36 A local study evaluated Google AutoML Vision AI trained using a DR dataset evaluated against realworld images classified by vitreoretinal trained ophthalmologists, resulting in a sensitivity of detecting referable DR of 85.7% (95% CI, 81.0% - 90.5%) and a positive predictive value of 96.3% (95% CI, 92.8%98.1%) making it an attractive option in South Africa.37
The other major benefit of adopting NMFC screening for DR is the number of unnecessary referrals to the Ophthalmology service that it prevented. The eye clinic at Tygerberg Hospital has been the busiest outpatient clinic at the facility for many years and is severely overburdened. By preventing the unnecessary referral of 139 patients over a six-month period the use of this type of screening allows for more capacity in the clinic.
This research has certain limitations. Firstly, the retrospective design was not ideal as some important data were not captured. Similarly, the challenge of evaluating the financial impact because of incomplete data. The patient numbers are also quite low, but this was a direct consequence of the Covid-19 pandemic and the periods of lockdown experienced in South Africa. Additionally, other types of diabetes mellitus were not evaluated during this study.
Studies have shown a correlation between nephropathy and retinal disease.38 Furthermore, it is indicated that there is a high association between HbA1c and retinal disease.39 Evaluating the correlation between factors like nephropathy, HbA1c and total daily insulin dose was not part of this study. This will be taken into consideration in future research.
The service will be extended to additional cohorts, for example pregnant patients with DM.
Conclusion
NMFC imaging demonstrated value in a low-middle income setting to prioritise cases by detecting significant diabetic retinopathy and avoiding unnecessary referrals to the ophthalmology clinic in patients with no or mild eye disease.
References
Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019;157:107843. doi:10.1016/j.diabres.2019.107843.
Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87(1):4-14. doi:10.1016/j. diabres.2009.10.007.
Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014;103(2):137-149. doi:10.1016/j. diabres.2013.11.002.
Gouda HN, Charlson F, Sorsdahl K, et al. Burden of non-communicable diseases in subSaharan Africa, 1990–2017: results from the Global Burden of Disease Study 2017. Lancet Glob Health. 2019;7(10):e1375-e1387. doi:10.1016/ S2214-109X(19)30374-2.
Saloni Dattani, Fiona Spooner HR and MR (2023) “Causes of DP online at OurWorldInData org. R from: “https://ourworldindata. org/ causes of death” [Online R. No Title.
Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018;138:271-281. doi:10.1016/j.diabres.2018.02.023.
Bigna JJ, Noubiap JJ. The rising burden of noncommunicable diseases in sub-Saharan Africa. Lancet Glob Health. 2019;7(10): e1295-e1296.
Klein R, Klein BEK. Diabetic eye disease. The Lancet. 1997;350(9072):197-204.
Jampol LM, Glassman AR, Sun J. Evaluation and Care of Patients with Diabetic Retinopathy. N Engl J Med 2020;382(17):1629-1637. doi:10.1056/ nejmra1909637.
Chang YC, Wu WC. Dyslipidaemia and diabetic retinopathy. Rev Diabet Stud. 2013;10(2-3):121132. doi:10.1900/RDS.2013.10.121.
Li YT, Wang Y, Hu XJ, et al. Association between systolic blood pressure and diabetic retinopathy in both hypertensive and normotensive patients with type 2 diabetes: Risk factors and healthcare implications. Healthc (Switz). 2021;9(5):1-13. doi:10.3390/ healthcare9050580.
Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556-564. doi:10.2337/dc11-1909
Avogaro A, Fadini GP. Microvascular complications in diabetes: A growing concern for cardiologists Int. J. Cardiol 2019;291:29-35. doi:10.1016/j.ijcard.2019.02.030.
Modjtahedi BS, Wu J, Luong TQ, Gandhi NK, Fong DS, Chen W. Severity of Diabetic Retinopathy and the Risk of Future Cerebrovascular Disease, Cardiovascular Disease, and All-Cause Mortality. Ophthalmology. Published online 2021. doi:10.1016/j.ophtha.2020.12.019.
Brownrigg JRW, Hughes CO, Burleigh D, et al. Microvascular disease and risk of cardiovascular events among individuals with type 2 diabetes: A populationlevel cohort study Lancet Diabetes Endocrinol 2016;4(7):588-597. doi:10.1016/ S2213-8587(16)30057-2.
Xie J, Ikram MK, Cotch MF, et al. Association of diabetic macular edema and proliferative diabetic retinopathy with cardiovascular disease a systematic review and metaanalysis. JAMA Ophthalmol. 2017;135(6):586593. doi:10.1001/jamaophthalmol.2017.0988.
Modjtahedi BS, Wu J, Luong TQ, Gandhi NK, Fong DS, Chen W. Severity of Diabetic Retinopathy and the Risk of Future Cerebrovascular Disease, Cardiovascular Disease, and All-Cause Mortality. Ophthalmology (Rochester, Minn). Published online 2020. doi:10.1016/j.ophtha.2020.12.019.
Liu Y, Swearingen R. Diabetic Eye Screening: Knowledge and Perspectives from Providers and Patients. Curr Diab Rep 2017;17(10). doi:10.1007/s11892-017-0911-2.
Sheppler CR, Lambert WE, Gardiner SK, Becker TM, Mansberger SL. Predicting Adherence to Diabetic Eye Examinations: Development of the Compliance with Annual Diabetic Eye Exams Survey. Ophthalmology. 2014;121(6):12121219. doi:https://doi.org/10.1016/j. ophtha.2013.12.016.
Hipwell AE, Sturt J, Lindenmeyer A, et al. Attitudes, access and anguish: a qualitative interview study of staff and patients’ experiences of diabetic retinopathy screening. BMJ Open. 2014;4(12):e005498. doi:10.1136/ bmjopen-2014-005498.
Burgess PI, Msukwa G, Beare NAV. Diabetic retinopathy in sub-Saharan Africa:| c. BMC Medicine. 2013;11(1). doi:10.1186/1741-7015-11-157.
Panwar N, Huang P, Lee J, et al. Fundus photography in the 21st century - a review of recent technological advances and their implications for worldwide healthcare. Telemed J E Health. 2016;22(3):198-208. doi:10.1089/tmj.2015.0068.
Bellemo V, Lim ZW, Lim G, et al. Artificial Intelligence Using Deep Learning to Screen for Referable and Vision-Threatening Diabetic Retinopathy in Africa. SSRN Journal. Published online 2020. doi:10.2139/ssrn.3324738.
Ting DSW, Cheung CYL, Lim G, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMAJ. Am. Med. Assoc. JAMA. 2017;318(22):2211-2223. doi:10.1001/jama.2017.18152.
Lu L, Ren P, Lu Q, et al. Analyzing fundus images to detect diabetic retinopathy (DR) using deep learning system in the Yangtze River delta region of China. Ann. Transl. Med. 2021;9(3):226-226. doi:10.21037/atm-20-3275.
DeBuc DC. The Role of Retinal Imaging and Portable Screening Devices in Teleophthalmology Applications for Diabetic Retinopathy Management. Curr. Diab. Rep. 2016;16(12). doi:10.1007/s11892-016-0827-2.
Mash B, Powell D, Du Plessis F, Van Vuuren U, Michalowska M, Levitt N. Screening for diabetic retinopathy in primary care with a mobile fundal camera -evaluation of a South African pilot project. S. Afr. Med. J. 2007;97(12):1284-1288. doi:10.7196/SAMJ.203.
Solomon SD, Goldberg MF. ETDRS Grading of Diabetic Retinopathy: Still the Gold Standard? Ophthalmic Research. 2019;62(4):190-195. doi:10.1159/000501372
Wadhwani M, Vashist P, Singh SS, et al. Diabetic retinopathy screening programme utilising non-mydriatic fundus imaging in slum populations of New Delhi, India. Trop. Med. Int. Health 2018;23(4):405-414.
Fahadullah M, Memon NA, Salim S, et al. Diagnostic accuracy of non-mydriatic fundus camera for screening of diabetic retinopathy: A hospital based observational study in Pakistan. J Pak Med Assoc. 2019;69(3):378-382.
Lanzetta P, Sarao V, Scanlon PH, et al. Fundamental principles of an effective diabetic retinopathy screening program. Acta Diabetol. 2020;57(7):785-798. doi:10.1007/ s00592-020-01506-8.
Nishantha Piyasena MMP, Murthy GVS, Yip JLY, et al. Systematic review on barriers and enablers for access to diabetic retinopathy screening services in different income settings. PLoS One. 2019;14(4). doi:10.1371/ journal.pone.0198979.
Khan T, Bertram MY, Jina R, Mash B, Levitt N, Hofman K. Preventing diabetes blindness: Cost effectiveness of a screening programme using digital non-mydriatic fundus photography for diabetic retinopathy in a primary health care setting in South Africa. Diabetes Res. Clin. Pract. 2013;101(2):170-176. doi:10.1016/j. diabres.2013.05.006.
De Jager W MI. Determining the prevalence of diabetic retinopathy in a high-risk diabetic clinic in Soweto, South Africa, using digital colour fundus photographs for screening. SA Ophthalmology Journal. 2023;18(1):10-14.
He J, Cao T, Xu F, et al. Artificial intelligencebased screening for diabetic retinopathy at community hospital. Eye (Basingstoke) 2020;34(3):572-576. doi:10.1038/ s41433-019-0562-4.
Avidor D, Loewenstein A, Waisbourd M, Nutman A. Cost-effectiveness of diabetic retinopathy screening programs using telemedicine: A systematic review. Cost Effect Resour Alloc. 2020;18(1):1-10. doi:10.1186/ s12962-020-00211-1.
Abdoola F KC. Evaluation of Google AutoML Vision artificial intelligence in detecting referable diabetic retinopathy in South Africa. SA Ophthalmology Journal. 2023;18(1):17-21.
Antwi-Boasiako C, Obeng KA, Amissah-Arthur KN, et al. Association between albuminuria and retinal microvascular dysfunction in type 2 diabetes with and without hypertension. Diabetes Epidemiology and Management. 2023;11:100139. doi:10.1016/j.deman.2023.100139.
Thomas RL, Distiller L, Luzio SD, et al. Incidence and progression of diabetic retinopathy within a private diabetes mellitus clinic in South Africa. J Clin Endocrinol Metab. 2015;20(3):28-34. doi:10.1080/16089677.2015.1090159.