17 minute read
Original study: Microbial profile of vitreous and aqueous aspirates in patients with endophthalmitis at Inkosi Albert Luthuli Central Hospital: a retrospective study
Microbial profile of vitreous and aqueous aspirates in patients with endophthalmitis at Inkosi Albert Luthuli Central Hospital: a retrospective study
L Shelembe, MBChB (UKZN); Registrar, Department of Ophthalmology, University of KwaZulu-Natal
https://orcid.org/0009-0008-5497-4595
C Kruse, MBChB (UP), MMed (Ophth) UKZN, FCOphth (SA); Academic Head of Department, Department of Ophthalmology, University of KwaZulu-Natal
https://orcid.org/0000-0002-8805-8383
Corresponding author: Dr L Shelembe, e-mail: lindelani.shelembe@gmail.com
Abstract
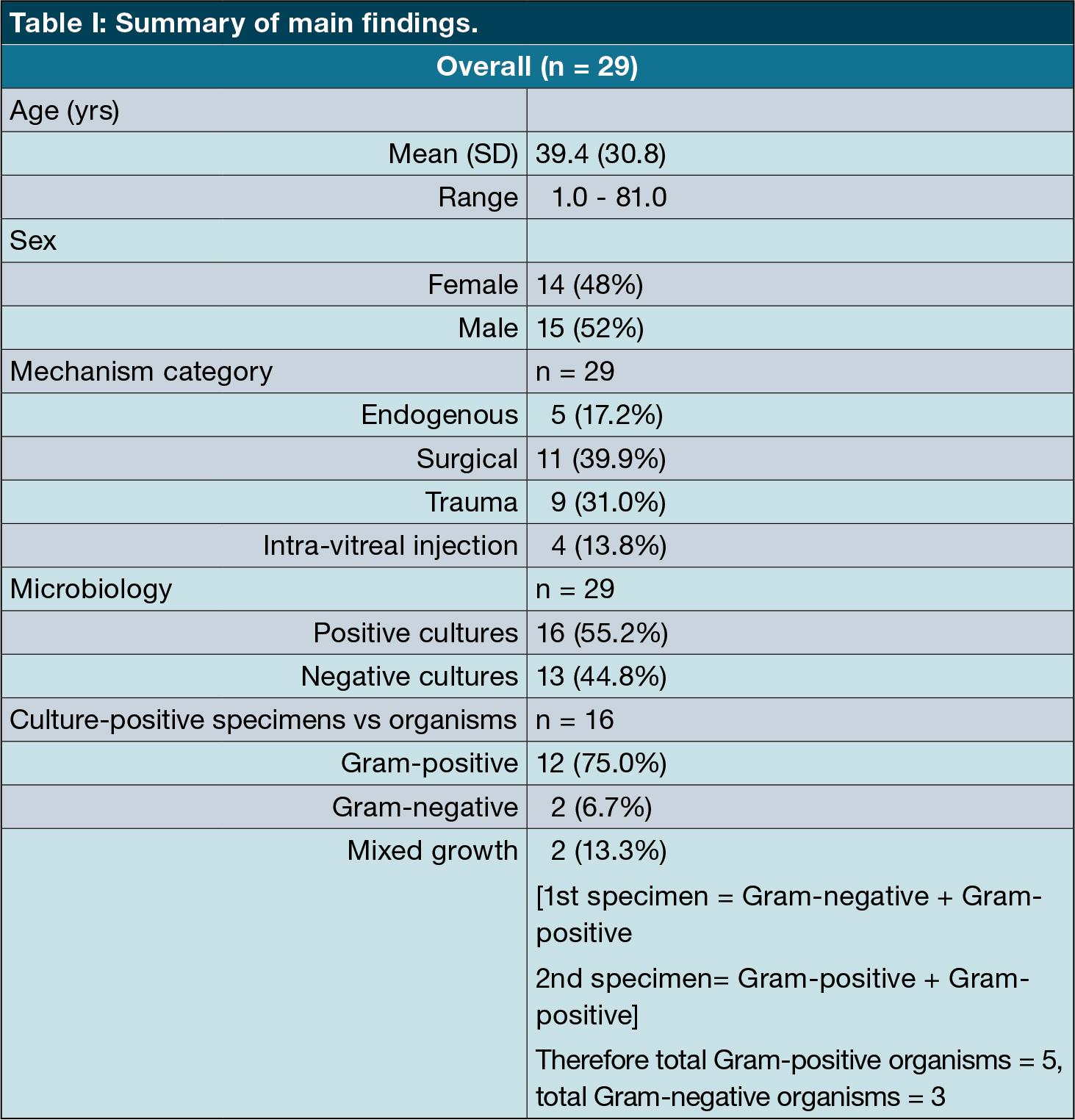
Background: Endophthalmitis is a rare but challenging condition to manage. It can result in significant and often permanent visual loss. Local microbial and sensitivity analyses are important to direct treatment guidelines.
Methods: A retrospective analysis of patients with clinically diagnosed endophthalmitis who had either a vitreous or aqueous specimen submitted between January 2016 and December 2022. Information on age, mechanism of inoculation, and microbial results were captured from the hospital electronic records.
Results: Most of our cases were from post-surgical endophthalmitis (n = 11; 37.9%) and post-traumatic (n = 9; 31.0%) followed by endogenous (n = 5; 17.2%), and post-intravitreal injection (n = 4; 13.8%).
The most common organism group cultured was Grampositive cocci (n = 15; 83%). Within this Gram-positive group, staphylococci were predominant (n = 12; 80%) followed by streptococci (n = 2; 13.3%) and enterococcus (n = 1; 6.7%). Overall yield was positive in just over half of the samples (n = 15; 51.7%).
Our results showed no case of fungal endophthalmitis. None of the cases in our study showed any resistance to a combination of Vancomycin and Ceftazidime.
Conclusion: Our study demonstrated microbial profiles that are comparable with those of similar studies. We found no evidence of resistance to any of our first-line agents. Intravitreal vancomycin and ceftazidime, therefore, continue to be an appropriate first-line treatment for bacterial endophthalmitis across different ages, causes, and types of inoculation in our population.
Keywords: endophthalmitis, KwaZulu-Natal, microbiology, culture, exogenous, endogenous.
Funding and conflict of interest: none. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Introduction
Endophthalmitis is ocular inflammation involving the vitreous cavity along with the retinal and uveal components of the eye, mostly due to an infectious agent(s).1 It is a rare, but often challenging disease to manage and can result in significant and often permanent visual loss.
The incidence and mechanism of infectious endophthalmitis vary in different regions. Cataract surgery is by far the most common cause of exogenous endophthalmitis, with some estimating it to be responsible for 90% of cases.2 In their study, Tien Yin Wong et al. reported rates of 0.07%-0.32% of exogenous endophthalmitis following cataract surgery in 44 803 patients in the Singapore National Eye Centre.3 In their study, Chee et al. reported endogenous endophthalmitis as accounting for 2%-8% of as all cases of endophthalmitis also in Singapore.4 Similarly, the proportional spread between endogenous and exogenous endophthalmitis differs between regions, however, it is a generally accepted phenomenon that the occurrence of exogenous endophthalmitis tends to supersede endogenous endophthalmitis. One of the highest numbers of endogenous endophthalmitis comes from a report by Krause et al., where endogenous endophthalmitis accounted for 41% of cases in London.5 Ramakrishnan et al. suggested that as much as 92.6% were exogenous in origin in their study of the Indian population.6
A variation exists in microbial spectra between regions, mechanism of inoculation, and duration of exposure to possible inoculation. In the landmark study of the early to mid-1990s, the Early Vitrectomy Study looked at post-operative endophthalmitis, confirmed microbiologic growth showed a strong Gram-positive, coagulase-negative staphylococci predominance.7 This trend was echoed in the French Institutional Endophthalmitis Study group.8 In their study looking at visual outcomes post-surgery, Gupta et al. found a predominance of fungal isolates in cases of exogenous endophthalmitis in India, particularly post-cataract surgery.9 Connell et al. looked at endogenous endophthalmitis at a tertiary referral centre in Australia and also found higher rates of fungal isolates compared to bacterial cultures.10 These all point to varying microbiological trends between regions.
The increasing use of intravitreal injections is a risk factor for inoculation, albeit rare.11 In one study, Fintak et al. found the incidence of endophthalmitis post intravitreal bevacizumab and ranibizumab injection to be 1 in 4500.12 Interestingly, in this large study of more than 26 000 injections, they had only six cases of infectious endophthalmitis; four of them culturing Streptococci species, while the other two showed no growth.
The current empiric antibiotic regimen we use in our local eye centres is intravitreal vancomycin to cover Gram-positive organisms and intravitreal ceftazidime to target Gram-negative bacilli and Pseudomonas. These guidelines are largely influenced by North American practices.2
In South Africa, and particularly in KwaZulu-Natal, there is a paucity of comprehensive, and clear epidemiological and microbiological studies looking at the occurrence of endophthalmitis, its microbial profile, and susceptibilities.
From a South African perspective, some studies looked at the prevention of endophthalmitis. In particular, Du Toit et al. looked at the role of prophylactic antibiotics in open-globe injuries to prevent endophthalmitis while Van Der Merwe et al. looked at the role of intracameral cefuroxime particularly during cataract surgery for prevention of postoperative endophthalmitis.13,14 There still exists a need to document microbial patterns for our local population to determine prevailing susceptibility trends. Understanding the microbial profile of our region will guide us in determining whether we need to adopt different empiric antimicrobial protocols.
This study aimed to identify the microbial spectrum of endophthalmitis and to determine the antibacterial susceptibilities of microbial isolates of patients treated at Inkosi Albert Luthuli Central Hospital (IALCH), in the subtropical coastal city of Durban, South Africa. This would assist in identifying the most appropriate empirical therapy for infectious endophthalmitis within the province.
Methods
A retrospective analysis of patients with clinically diagnosed endophthalmitis who had either a vitreous or aqueous specimen submitted between January 2016 and December 2022. Information on age, mechanism of inoculation, and microbial results was captured from the hospital’s electronic records.
Samples not taken from clinically confirmed cases of endophthalmitis and samples not marked specifically as vitreous or aqueous specimens, as well as specimens submitted before or after the study period (January 2016 to December 2022), were excluded.
Information relating to patients’ age, sex, mechanism of inoculation, and microbial culture outcome of the samples were received from the IALCH MEDITECH® health record system. This information was captured on an Excel® data capture tool using a de-identified ‘flat’ database design to facilitate statistical analysis: Each row contained one complete case (anonymized) and each column a specified data field. Missing data was ‘entered’ as empty fields.
Data was analysed was done in SPSS® version 27. Frequencies and percentages were calculated to summarise categorical variables. Central tendency and dispersion of numerical data were measured using means and standard deviations, if these variables are normally distributed, and medians and interquartile ranges if the variables are skewed.
Ethical approval was obtained from the University of KwaZulu-Natal Biomedical Research Committee (BREC/00005284/2023).
Results
Our overall number of cases with endophthalmitis was 29. These cases were seen either by a registrar/resident or medical officer and discussed with a consultant ophthalmologist at the referring base hospital before referral to IALCH.
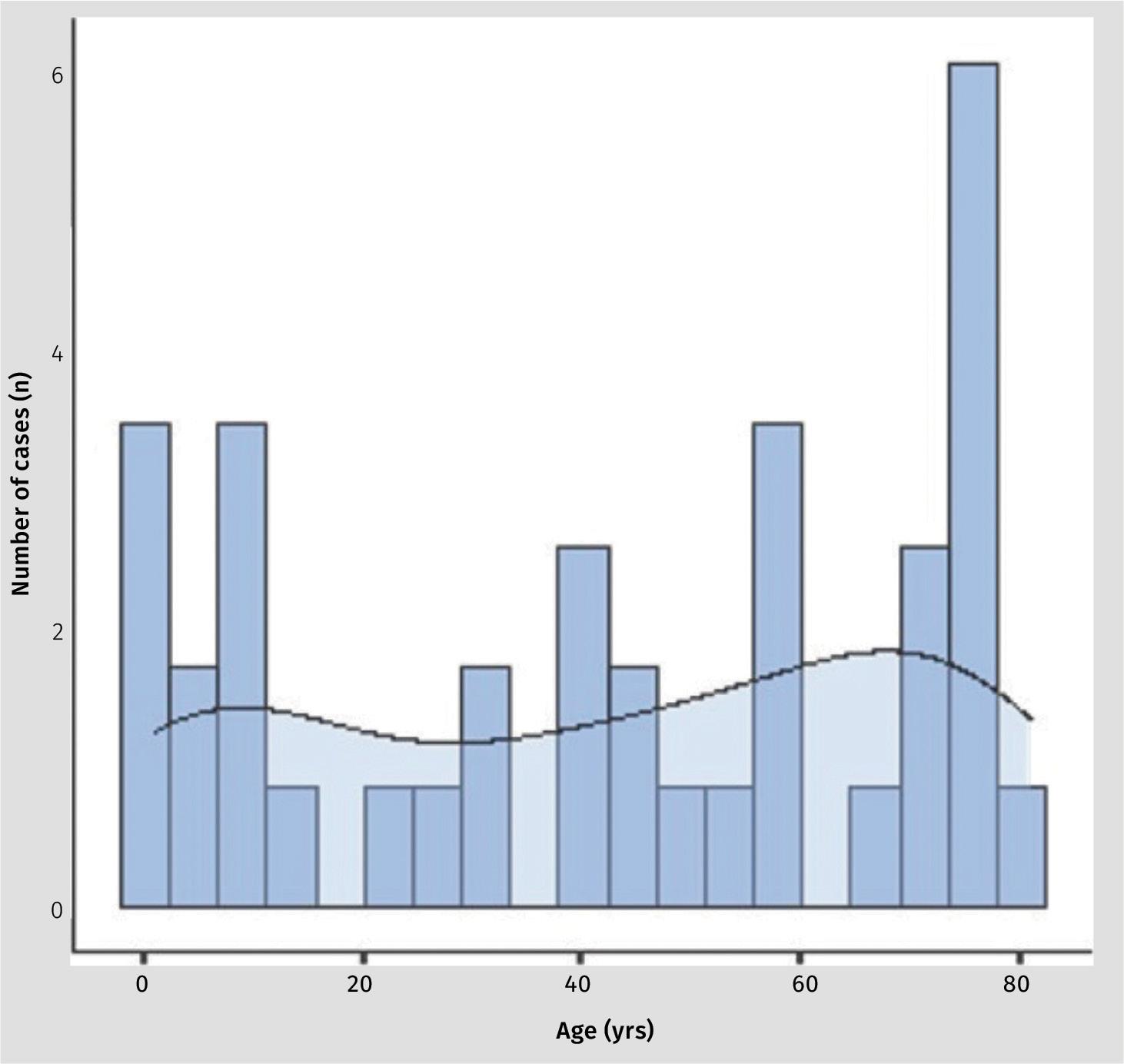
The mean age of patients was 39.4 years (SD 30.8; range 1.0 - 81.0). The spread between sexes was essentially equal with 14 (48%) being female and 15 (52%) being male. Figure 1 shows an even spread of cases over the age groups with a slightly denser preponderance of cases in the first and 8th decade of life.
Most of our cases were from postsurgical endophthalmitis (n = 11; 39.9%), followed by post-traumatic endophthalmitis (n = 9; 31.0%), endogenous (n = 5; 17.2%) and post-intravitreal injection (n = 4; 13.8%) (Table III).
Overall microbiology yield was positive in just over half of the samples (n = 16; 55.2%). No growth of any organism on culture was found in 13 (44.8%). The vast majority of the microbiological samples were from vitreous aspirates (n = 28; 97%) versus one aqueous aspirate (3%).
Table II displays, in greater detail, the microbiology results on the positive culture yields.
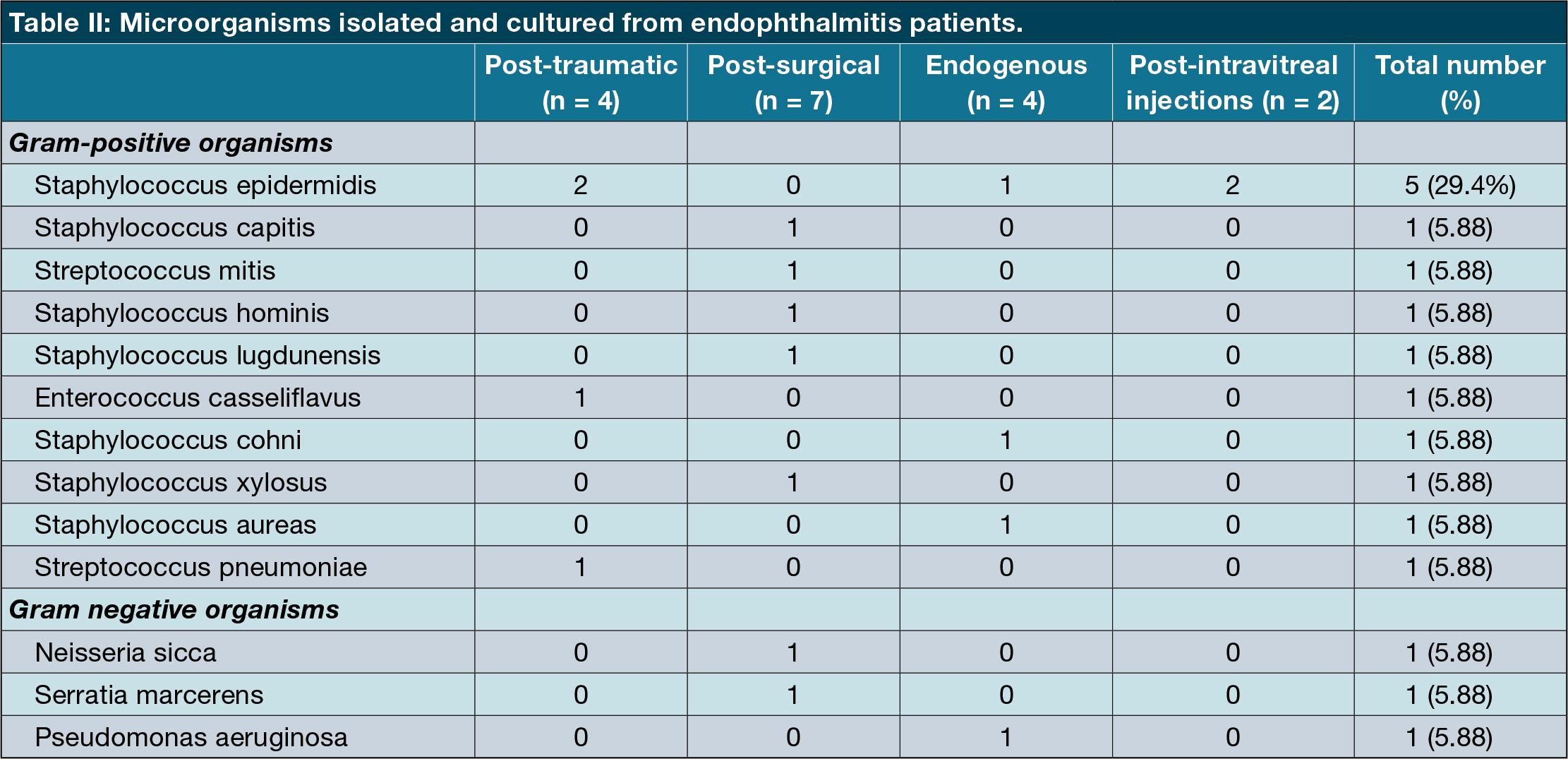
The most common organism group cultured was Gram-positive cocci (n = 15; 83%). Within this Gram-positive group, staphylococci were predominant (n = 12; 80%) followed by streptococci (n = 2; 13.3%) and Enterococcus (n = 1; 6.7%). The most common staphylococcus organism was staphylococcus epidermidis (five cases) which was common across all mechanism categories. Gram-negative were far fewer with only three organisms cultured (17%). It is worth mentioning that two of the samples (from different patients) cultured two organisms each. One of these multiple-growth samples showed a mixture of Gram-positive (Streptococcus miti) and Gram-negative (Neisseria sicca).
When comparing culture-positive yields according to the mechanism of inoculation, endogenous cases had more culture positivity (80%) followed by postsurgical cases at 64%; post-intravitreal injection had a 50% positivity rate, while post-traumatic cases had the least positivity rate at 44% (Figure 2).
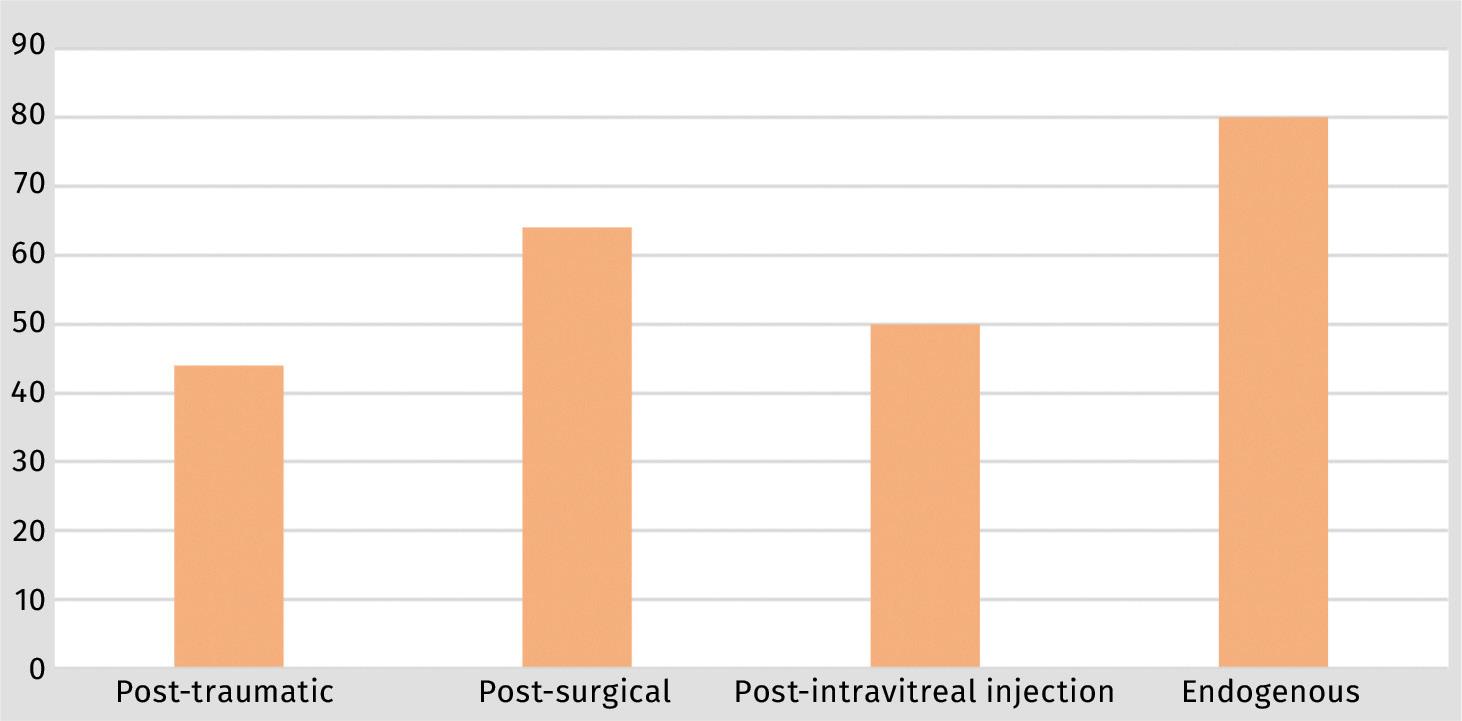
The most commonly cultured organisms by far across all mechanisms were Grampositive cocci (80%) and all Gram-positive organisms were either staphylococci or streptococci.
All 17 different microorganisms cultured were sensitive to the commonly tested antibiotics in our local NHLS laboratory, with only one Gram-positive isolate showing resistance to cloxacillin, erythromycin, and azithromycin. All Gramnegatives were sensitive to ceftazidime and all Gram-positives were sensitive to vancomycin. None of the cases in our study isolated fungal organisms despite being a subtropical coastal facility.
We had nine paediatric cases (28% of overall cases). The age range for this group was 1-9 years (median 5). The majority of these cases were post-surgical 4 (44%), three cases were post-traumatic (43%) and two were endogenous (22%). Of the total nine paediatric cases, only four were culture-positive (44%). Three cases showed Gram-positive cocci (two staphylococci and one streptococci). One case cultured a Gram-negative organism (Pseudomonas aeruginosa).
Discussion
The age spread of our cases showed a particular preponderance of paediatric and geriatric patients (see Figure 1). Most of these cases were post-surgery. This is similar to findings in other similar studies.15,16 The cultured pathogenic organisms proved similar to those found in studies from other regions with predominant Gram-positive cocci with similar sensitivity profiles.1,6,17-23 The higher number of Staphylococcus epidermidis positive cultures could also be attributed to contamination with skin commensals, however, that is hard to determine as a matter of certainty by looking at the results as they are.
Our study revealed a preponderance of post-surgical and post-traumatic causes of endophthalmitis of which post-procedural surgical cases (either cataract surgery or intravitreal injections) were more common. This is similar to studies in high-income countries like the United Kingdom and the United States of America 24 as well as in low to middle-income countries like China,1 Thailand, 25 and India.6,17
Interestingly, these results were different from research from a study in northern China where Liu et al. found a significant proportional predominance of post-traumatic endophthalmitis (49.6%) compared to post-surgical causes (26.7%).20
Our positivity rates were comparable to other similar studies. Duan et al. also found a greater positivity yield in endogenous cases.1 However, what was slightly different in our study was that post-surgical cases tended to have a slightly better positivity rate than posttraumatic cases (Figure 2).
None of our cases were secondary to infective keratitis, unlike studies from other regions where they were often the majority.6,17,20,22,26 This is most likely attributable to our departmental protocol of not doing intraocular procedures (including diagnostic taps and antibiotic injections) in cases of infective keratitis, especially in the first three days after initiating topical antibiotic therapy.
Two out of our four culture-positive paediatric cases grew staphylococci. This result is dissimilar to that from a paediatric population study in China by Yang et al.22 where they found more streptococci, especially in the lower-age paediatric groups.
The current empiric antibiotic regimen we use in our local eye centres is intravitreal vancomycin to cover Grampositive organisms, and intravitreal ceftazidime to target Gram-negative bacilli and Pseudomonas. These guidelines are largely influenced by North American research.7 These two antibiotics have been established as the standard empiric treatment in many eye centres around the world for many years, particularly since the Early Vitrectomy Study.27
Some authors in other regions like India28,29 and Australia30 have demonstrated growing, albeit mild, resistance of pathogenic organisms to these two antibiotics. However, none of the cases in our study showed any resistance to either of these antibiotics in vitro.
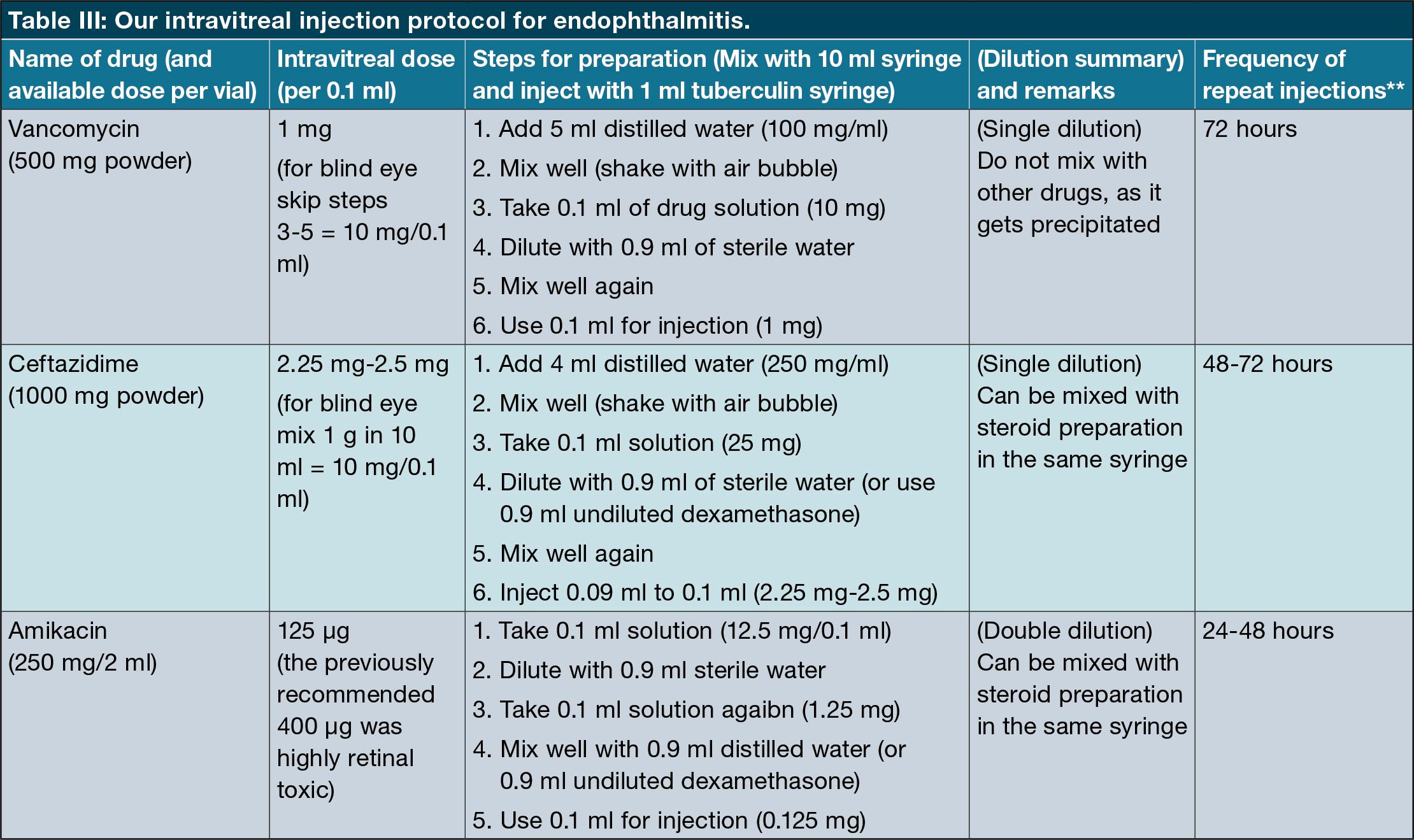
One of our objectives was to determine whether this current empiric antimicrobial regimen is an appropriate first-line choice for all cases of bacterial endophthalmitis in our region. None of the cases in our study showed any resistance to a combination of Vancomycin and Ceftazidime. Furthermore, we have demonstrated no evidence of emerging resistance to these agents. Intravitreal vancomycin, with ceftazidime, therefore, continues to be an appropriate first-line treatment for bacterial endophthalmitis across all mechanisms of inoculation in our population. Table III shows our protocol specifics and preparation instructions.
Limitations
This was a retrospective study which inherently limits the strength of evidence.
We had a relatively small sample number. This is due to multiple factors, including the fact that endophthalmitis is a rare complication – most academic studies on this subject have similar or fewer numbers.23,25,26,31 Also, our study was conducted at a quaternary level site that deals predominantly with the most difficult referrals, most of whom require pars plana vitrectomy, and therefore see fewer cases. We presume that cases in peripheral centres that were managed conservatively, with intravitreal antibiotics only, often did not make it to our study site.
Differences between in-vivo tests and clinical response to antibiotic treatment do exist. However, as outcomes were not assessed in this study, this could not be determined. Future studies could look at clinical outcomes with each variable measured (e.g. microbial spectrum measured against response to empiric treatment). To find out if these outcomes are universal in our setting, it would be interesting to see future endophthalmitis studies from other eye units within the broader South African context.
Acknowledgements
Competing interests:
The authors have declared that no competing interest exist.
Authors’ contributions:
LGS prepared the protocol, collected the data and wrote the manuscript.
C-HK supervised the writing of the protocol, analysed the data and assisted with the preparation of the final manuscript.
Data availability statement:
The anonymised data underpinning this study will be shared upon reasonable request to the corresponding author.
Disclaimer:
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
No artificial intelligence-assisted technologies such as Large Language Models, chatbots, or image creators were used in the production of submitted work.
References
Duan F, Wu K, Liao J, Zheng Y, Yuan Z, Tan J, et al. Causative Microorganisms of Infectious Endophthalmitis: A 5-Year Retrospective Study. J Ophthalmol. 2016;2016:6764192.
Kernt M, Kampik A. Endophthalmitis: Pathogenesis, clinical presentation, management, and perspectives. Clin Ophthalmol. 2010;4:121-35.
Wong TY, Chee S-P. The epidemiology of acute endophthalmitis after cataract surgery in an Asian population. Ophthalmology. 2004;111(4):699-705.
Chee S-P, Jap A. Endogenous endophthalmitis. Curr. Opin. Ophthalmol. 2001;12(6):464-70.
Krause L, Bechrakis NE, Heimann H, Kildal D, Foerster MH. Incidence and outcome of endophthalmitis over a 13-year period. Can J Ophthalmol. 2009;44(1):88-94.
Ramakrishnan R, Bharathi MJ, Shivkumar C, Mittal S, Meenakshi R, Khadeer MA, et al. Microbiological profile of culture-proven cases of exogenous and endogenous endophthalmitis: a 10-year retrospective study. Eye (Lond). 2009;23(4):945-56.
Han DP, Wisniewski SR, Wilson LA, Barza M, Vine AK, Doft BH, et al. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol. 1996;122(1):1-17.
Brillat-Zaratzian E, Bron A, Aptel F, Romanet JP, Cornut PL, Vandenesch F, et al. FRIENDS Group: clinical and microbiological characteristics of post-filtering surgery endophthalmitis. Graefes Arch Clin Exp Ophthalmol. 2014;252(1):101-7.
Gupta A, Gupta V, Gupta A, Dogra MR, Pandav SS, Ray P, et al. Spectrum and Clinical Profile of Post Cataract Surgery Endophthalmitis in North India. Indian J. Ophthalmol. 2003;51(2):139-45.
Connell PP, O’Neill EC, Fabinyi D, Islam FMA, Buttery R, McCombe M, et al. Endogenous endophthalmitis: 10-year experience at a tertiary referral centre. Eye. 2011;25(1):66-72.
Mccannel CA. Meta-analysis of endophthalmitis after intravitreal injection of anti–vascular endothelial growth factor agents: Causative Organisms and Possible Prevention Strategies. Retina 2011;31(4):654-61.
Fintak DR, Shah GK, Blinder KJ, Regillo CD, Pollack J, Heier JS, et al. Incidence of endophthalmitis related to intravitreal injection of bevacizumab and ranibizumab. Retina. 2008;28(10):1395-9.
Du Toit N, Mustak S, Cook C. Randomised controlled trial of prophylactic antibiotic treatment for the prevention of endophthalmitis after open globe injury at Groote Schuur Hospital. Br. J. Ophthalmol. 2017;101(7):862.
van der Merwe J, Mustak H, Cook C. Endophthalmitis prophylaxis with intracameral cefuroxime in South Africa. J Cataract Refract Surg. 2012;38(11):2054.
Relhan N, Forster RK, Flynn HW, Jr. Endophthalmitis: Then and Now. Am J Ophthalmol. 2018;187:xx-xxvii.
Peck TJ, Patel SN, Ho AC. Endophthalmitis after cataract surgery: an update on recent advances . Curr Opin Ophthalmol. 2021;32(1):62-8.
Bhattacharjee H, Bhattacharjee K, Gogoi K, Singh M, Singla BG, Yadav A. Microbial profile of the vitreous aspirates in culture proven exogenous endophthalmitis: A 10-year retrospective study. Indian J Med Microbiol. 2016;34(2):153-8.
Jindal A, Pathengay A, Mithal K, Jalali S, Mathai A, Pappuru RR, et al. Microbiologic spectrum and susceptibility of isolates in acute postcataract surgery endophthalmitis: are they same as they were more than a decade ago? Br J Ophthalmol 2014;98(3):414-6.
Kannan NB, Sen S, Mishra C, Lalitha P, Rameshkumar G, Rajan RP, et al. Comparative Study of Microbiological Profile and Management Outcomes of Acute Endophthalmitis after Microincision Vitrectomy Surgery versus Intravitreal Injections. Ocul Immunol Inflamm. 2021;29(5):838-44.
Liu Q, Wan L, Zhou J, Huang Y. Ten-Year Analysis of Pathogenic Factors and Etiological Characteristics of Endophthalmitis from a Tertiary Eye Center in North China. Infect Drug Resist. 2022;15:3005-12.
Lu LJ, Chen X, Adelman RA. Clinical Etiologies, Microbial Spectrum, Antibiotic Susceptibilities, and Visual Acuity Outcomes of Acute Endophthalmitis. J Ocul Pharmacol Ther. 2020;36(7):534-9.
Yang Y, Lin L, Li Y, Jiang Z, Li C, Liu M, et al. Etiology, microbiological isolates, and antibiotic susceptibilities in cultureproven pediatric endophthalmitis: a 9-year review. Graefes Arch Clin Exp Ophthalmol 2021;259(1):197-204.
Gupta A, Orlans HO, Hornby SJ, Bowler IC. Microbiology and visual outcomes of culture-positive bacterial endophthalmitis in Oxford, UK. Graefes Arch Clin Exp Ophthalmol. 2014;252(11):1825-30.
Miller JJ, Scott IU, Flynn HW, Jr., Smiddy WE, Newton J, Miller D. Acute-onset endophthalmitis after cataract surgery (2000-2004): incidence, clinical settings, and visual acuity outcomes after treatment. Am J Ophthalmol. 2005;139(6):983-7.
Bhurayanontachai P, Klongthanakit P. A 14-Year Retrospective Analysis of Endogenous Endophthalmitis in a Tertiary Referral Center of Southern Thailand. J Ophthalmol. 2020;2020:6689081.
Sridhar J, Yonekawa Y, Kuriyan AE, Joseph A, Thomas BJ, Liang MC, et al. Microbiologic Spectrum and Visual Outcomes of AcuteOnset Endophthalmitis Undergoing Therapeutic Pars Plana Vitrectomy. Retina. 2017;37(7):1246-51.
Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Endophthalmitis Vitrectomy Study Group. Arch Ophthalmol 1995;113(12):1479-96.
Shivaramaiah HS, Relhan N, Pathengay A, Mohan N, Flynn HW, Jr. Endophthalmitis caused by gram-positive bacteria resistant to vancomycin: Clinical settings, causative organisms, antimicrobial susceptibilities, and treatment outcomes. Am J Ophthalmol Case Rep. 2018;10:211-4.
Dave VP, Pathengay A, Nishant K, PappuruRR, Sharma S, Sharma P, et al. Clinical presentations, risk factors and outcomes of ceftazidime-resistant Gram-negative endophthalmitis. Clin Exp Ophthalmol. 2017;45(3):254-60.
Stevenson LJ, Dawkins RCH, Sheorey H, McGuinness MB, Hurley AH, Allen PJ. Gramnegative endophthalmitis: A prospective study examining the microbiology, clinical associations and visual outcomes following infection. Clin Exp Ophthalmol. 2020;48(6):813-20.
Malhotra S, Mandal P, Patanker G, Agrawal D. Clinical profile and visual outcome in cluster endophthalmitis following cataract surgery in Central India. Indian J Ophthalmol. 2008;56(2):157-8.
Copyright © 2023 Dr Lindelani G Shelembe. All rights reserved. Copyright subsists in the Author of this work. No part of this article or included photographs may be reproduced, published, performed, broadcast, transmitted or adapted in any form or by any electronic, mechanical or other means without the written permission of the copyright holder. This article is published by New Media, a division of Media24 (Pty) Ltd with consent of the Author. Any unauthorised reproduction, publishing, or adaption of this work will constitute copyright infringement and render the doer liable under both civil and criminal law.







