
de Redacció / Secretaría de Redacción / Editorial Office Museu de Ciències Naturals de Barcelona Passeig Picasso s/n.
Barcelona, Spain Tel.
Fax
E–mail
de Redacció / Secretaria de Redacción / Managing Editor Montserrat Ferrer Alba Jiménez
lingüístic / Asesoramiento lingüístico / Linguistic advisers Carolyn Newey Pilar Nuñez Animal Biodiversity and Conservation 45.2, 2022 © 2022 Museu de Ciències Naturals de Barcelona, Consorci format per l'Ajuntament de Barcelona i la Generalitat de Catalunya Autoedició: Montserrat Ferrer ISSN: 1578–665 X eISSN: 2014–928 X Dipòsit legal: B. 5357–2013 Animal Biodiversity and Conservation es publica amb el suport de / Animal Biodiversity and Conservation se publica con el apoyo de / Animal Biodiversity and Conservation is published with the support of: Asociación Española de Ecología Terrestre – AEET Sociedad
de Etología y Ecología Evolutiva – SEEEE
de
Evolutiva – SESBE
gratuitament a internet / Disponible gratuitamente en internet / Freely available online at: museucienciesjournals.cat/abc/
/ Dibujo
Drawing
the cover Jordi
Ben
J. Hatchwell, Univ. of Sheffield, UK Secretaria
08003
+34–93–3196912
+34–93–3104999
abc@bcn.cat Secretària
Assessorament
Española
Sociedad Española
Biología
Disponible
Dibuix de la coberta
de la portada /
of
Domènech Vespa velutina nigrithorax, vespa asiàtica, avispón asiático o avispa asiática, sian hornet o yellow–legged asian hornet
Animal Biodiversity and Conservation 45.2 (2022)
Editor en cap / Editor responsable / Editor in Chief
Joan Carles Senar Museu de Ciències Naturals de Barcelona, Barcelona, Spain
Editors temàtics / Editores temáticos / Thematic Editors
Ecologia / Ecología / Ecology: Mario Díaz (AEET)
Comportament / Comportamiento / Behaviour: Adolfo Cordero (SEEEE)
Biologia Evolutiva / Biología Evolutiva / Evolutionary Biology: Santiago Merino (SESBE)
Editors
/
Editores
/ Editors
Pere Abelló Institut de Ciències del Mar CMIMA–CSIC, Barcelona, Spain
Pelayo Acevedo Instituto de Investigación en Recursos Cinegéticos IREC–UCLM–CSIC–JCCM, Ciudad Real, Spain
Javier Alba–Tercedor Universidad de Granada, Granada, Spain
Russell Alpizar–Jara University of Évora, Évora, Portugal
Marco Apollonio Università degli Studi di Sassari, Sassari, Italy
Pedro Aragón Museo Nacional de Ciencias Naturales MNCN–CSIC, Madrid, Spain
Miquel Arnedo Universitat de Barcelona, Barcelona, Spain
Beatriz Arroyo Instituto de Investigación en Recursos Cinegéticos IREC–UCLM–CSIC–JCCM, Ciudad Real, Spain
Francisco Javier Aznar Institut Cavanilles de Biodiversidad y Biologia Evolutiva, Universitat de Valencia, Spain
Xavier Bellés Institut de Biología Evolutiva UPF–CSIC, Barcelona, Spain
Agustín Camacho Instituto de Biociências–USP, São Paulo, Brasil
David Canal MTA Centre for Ecological Research, Vácrátót, Hungary
Gonçalo C. Cardoso CIBIO–InBIO, Universidade do Porto, Portugal
Salvador Carranza Institut de Biologia Evolutiva UPF–CSIC, Barcelona, Spain
Luís Mª Carrascal Museo Nacional de Ciencias Naturales MNCN–CSIC, Madrid, Spain
Martina Carrete Universidad Pablo de Olavide, Sevilla, Spain
Pablo Castillo Institute for Sustainable Agriculture–CSIC, Córdoba, Spain
Darío Díaz Cosín Universidad Complutense de Madrid, Madrid, Spain
José A. Donazar Estación Biológica de Doñana EBD–CSIC, Sevilla, Spain
Arnaud Faille Museum National histoire naturelle, Paris, France
Jordi Figuerola Estación Biológica de Doñana EBD–CSIC, Sevilla, Spain
Gonzalo Giribet Museum of Comparative Zoology, Harvard University, Cambridge, USA
Susana González Universidad de la República–UdelaR, Montivideo, Uruguay
Jacob González–Solís Universitat de Barcelona, Barcelona, Spain
Iain Gordon Australian National University, Mysterton, Australia
Sidney F. Gouveia Universidad Federal de Sergipe, Sergipe, Brasil
Gary D. Grossman University of Georgia, Athens, USA
Ben J. Hatchwell University of Sheffield, Sheffield, UK
Joaquín Hortal Museo Nacional de Ciencias Naturales MNCN–CSIC, Madrid, Spain
Jacob Höglund Uppsala University, Uppsala, Sweden
Damià Jaume Institut Mediterrani d'Estudis Avançats IMEDEA–CSIC–UIB, Esporles, Spain
José Jiménez Instituto de Investigación en Recursos Cinegéticos IREC–UCLM–CSIC–JCCM, Ciudad Real, Spain
Miguel A. Jiménez–Clavero Centro de Investigación en Sanidad Animal–INIA, Madrid, Spain
Jennifer A. Leonard Estación Biológica de Doñana EBD–CSIC, Sevilla, Spain
Andras Liker University of Pannonia, Veszprém, Hungary
Jordi Lleonart Institut de Ciències del Mar CMIMA–CSIC, Barcelona, Spain
Josep Lloret Universitat de Girona, Girona, Spain
Jorge Mª Lobo Museo Nacional de Ciencias Naturales MNCN–CSIC, Madrid, Spain
Pablo J. López–González Universidad de Sevilla, Sevilla, Spain
Ian MacGregor–Fors University of Helsinki, Lahti, Finland
Jose Martin Museo Nacional de Ciencias Naturales MNCN–CSIC, Madrid, Spain
Juan F. Masello Justus Liebig University Giessen, Giessen, Germany
Manuel B. Morales CIBC–Universidad Autónoma de Madrid, Madrid Spain
Joan Navarro Institut de Ciències del Mar, CMIMA–CSIC, Barcelona, Spain
Juan J. Negro Estación Biológica de Doñana EBD–CSIC, Sevilla, Spain
Daniel Oro Centre d'Estudis Avançats de Blanes CEAB–CSIC, Girona, Spain
Vicente M. Ortuño Universidad de Alcalá de Henares, Alcalá de Henares, Spain
Miquel Palmer Institut Mediterrani d'Estudis Avançats IMEDEA–CSIC–UIB, Esporles, Spain
Per Jakob Palsbøll University of Groningen, Groningen, The Netherlands
Reyes Peña Universidad de Jaén, Jaén, Spain
Silvia Perea Universidad Nacional Autónoma de México UNAM, Ciudad de México, México
Javier Perez–Barberia Estación Biológica de Doñana EBD–CSIC, Sevilla, Spain
Silvia Pérez–Espona The University of Edinburgh, UK
Juan M. Pleguezuelos Universidad de Granada, Granada, Spain
Montserrat Ramón Institut de Ciències del Mar CMIMA–CSIC, Barcelona, Spain
Alex Richter–Boix CREAF, Universitat Autònoma de Barcelona, Bellaterra, Spain
Diego San Mauro Universidad Complutense de Madrid, Madrid, Spain
Ana Sanz–Aguilar Institut Mediterrani d'Estudis Avançats IMEDEA–CSIC–UIB, Esporles, Spain
Rafael Sardà Centre d'Estudis Avançats de Blanes CEAB–CSIC, Girona, Spain
Ramón C. Soriguer Estación Biológica de Doñana EBD–CSIC, Sevilla, Spain
Constantí Stefanescu Museu de Ciències Naturals de Granollers, Granollers, Spain
Diederik Strubbe University of Antwerp, Antwerp, Belgium
José L. Tellería Universidad Complutense de Madrid, Madrid, Spain
Simone Tenan Institute of Marine Sciences CNR–ISMAR, National Research Council, Venezia, Italy
Francesc Uribe Museu de Ciències Naturals de Barcelona, Barcelona, Spain
José Ramón Verdú CIBIO, Universidad de Alicante, Alicante, Spain
Carles Vilà Estación Biológica de Doñana EBD–CSIC, Sevilla, Spain
Rafael Villafuerte Instituto de Estudios Sociales Avanzados IESA–CSIC, Cordoba, Spain
Rafael Zardoya Museo Nacional de Ciencias Naturales MNCN–CSIC, Madrid, Spain
Spatial ecology of jaguar (Panthera onca) outside protected areas in the Yucatan Peninsula, Mexico
A. González–Gallina, M. Equihua, F. Pérez–Garduza, J. A. Iglesias–Hernández, A. Oliveras de Ita, A. Chacón–Hernández, O. Vázquez–Zúñiga, M. G. Hidalgo–Mihart
González–Gallina, A., Equihua, M., Pérez–Garduza, F., Iglesias–Hernández, J. A., Oliveras de Ita, A., Chacón–Hernández, A., Vázquez–Zúñiga, O., Hidalgo–Mihart, M. G., 2022. Spatial ecology of jaguar (Panthera onca) outside protected areas in the Yucatan Peninsula, Mexico. Animal Biodiversity and Conservation, 45.2: 131–144, Doi: https://doi.org/10.32800/abc.2022.45.0131
Abstract
Spatial ecology of jaguar (Panthera onca) outside protected areas in the Yucatan Peninsula, Mexico. Jaguars (Panthera onca) are endangered in several countries and a priority species for conservation action. Despite extensive research efforts in Mexico most studies have been associated with natural protected areas far from human habitation. Because protected areas are too few to conserve the jaguar population over the long–term, a landscape approach that includes both protected and unprotected lands is needed. This is the case in Quintana Roo State where an ecological corridor linking two protected areas (Yum Balam and Sian Ka'an) is at risk of disappearing due to tourism–driven activities. Between 2013 and 2015, four male jaguars were captured and monitored using satellite telemetry inside the corridor. The mean home range size (± SD) was 101.5 km2 (± 75.9 km2) for the dry season and 172 km2 (± 107.29 km2) for the rainy season. The mean core area size (± SD) was 17.54 km2 (± 16.21 km2) for the dry season and 29.07 km2 (± 16.19 km2) for the rainy season. No significant seasonal differences were found for home ranges or for core areas. As expected, we observed that jaguars preferred forest or young secondary growth over profusely disturbed areas, using whatever vegetation was available in their home ranges. Although it is not protected, a biological corridor linking Yum Balam and Sian Ka'an still holds its own jaguar population, a population that has learned to coexist with human presence. Conservation actions are recommended at landscape level to maintain what remains of tropical mature forest and to promote the development of long–term secondary growth into close tree canopy.
Key words: Conservation, Corridor, Home range, Habitat use, Non–protected
Resumen

Ecología espacial del jaguar (Panthera onca) fuera de las zonas protegidas de la península de Yucatán, México. El jaguar (Panthera onca) se encuentra en peligro de extinción en países como México y es una especie prioritaria para las medidas de conservación. La mayoría de los esfuerzos de investigación en el país, aunque extensos, se han asociado principalmente a zonas naturales protegidas, alejadas de los asentamientos de población humana. Las zonas protegidas existentes son insuficientes para conservar la población de jaguares a largo plazo, por lo que se debe adoptar un enfoque de paisaje que incluya tanto las tierras protegidas como las no protegidas. Esto es lo que sucede en el estado de Quintana Roo, donde existe un corredor ecológico que une dos zonas protegidas (Yum Balam y Sian Ka'an) que corre el riesgo de desaparecer debido a las actividades impulsadas por el turismo. Entre 2013 y 2015 se capturaron cuatro jaguares machos a los que se siguió mediante telemetría satelital dentro del corredor. La superficie media de la zona de actividad (± DE) fue de 101,5 km2 (± 75,9 km2) durante la temporada seca y de 172 km2 (± 107,29 km2) durante la temporada de lluvias. La superficie media de la zona central (± DE) fue de 17,54 km2 (± 16,21 km2) durante la estación seca y de 29,07 km2 (± 16,19 km2) durante la temporada de lluvias. No se encontraron diferencias estacionales significativas con respecto al área de distribución ni con la zona central. Según lo previsto, se encontró que los jaguares prefieren los bosques o las zonas de vegetación joven secundaria a las zonas muy perturbadas de la zona de estudio, mientras que utilizan todos los tipos de vegetación a su alcance dentro de su área de distribución. A pesar de no estar protegido, el corredor biológico que une Yum Balam y Sian Ka'an aún
ISSN: 1578–665 X eISSN: 2014–928 X
© [2022] Copyright belongs to the authors, who license the journal Animal Biodiversity and Conservation to publish the paper under a Creative Commons Attribution 4.0 License
131 Animal Biodiversity and
45.2
Conservation
(2022)
alberga una población de jaguares que ha aprendido a convivir con la presencia humana. Se recomienda adoptar medidas de conservación a escala de paisaje para mantener las zonas de bosque tropical maduro que queden y permitir la máxima recuperación posible de las zonas con vegetación secundaria para que se conviertan en bosques densos.
Palabras clave: Conservación, Corredor, Área de distribución, Uso del hábitat, Zonas no protegidas
Received: 11 I 21; Conditional acceptance: 10 V 21; Final acceptance: 19 IV 22
Alberto González–Gallina, Miguel Equihua, Red de Ambiente y Sustentabilidad. Instituto de Ecología A.C, Xalapa, Veracruz, 91070, México.– Freddy Pérez–Garduza, Jesús A. Iglesias–Hernández, Mircea G. Hidalgo–Mihart, División Académica de Ciencias Biológicas, Universidad Juárez Autónoma de Tabasco. Villahermosa, Tabasco, 86040, México.– Adán Oliveras de Ita, Andrés Chacón–Hernández, Octavio Vázquez–Zúñiga, Sistemas Estratégicos para la Gestión Ambiental SEGA, S. A. de C. V. Benito Juárez, Ciudad de México 03230, México.
Corresponding author: Mircea G. Hidalgo–Mihart E–mail: mhidalgo@yahoo.com
ORCID ID: A. González–Gallina: 0000-0002-9352-8554; Mircea G. Hidalgo–Mihart: 0000-0002-8779-6886
132 González–Gallina
et al.
Introduction
Understanding how large cats move and use a landscape is important in order to accurately identify priority habitat areas and connectivity corridors, both of which play a key role in the effective design and implementation of conservation strategies (Rodriguez–Soto et al., 2011). Because of their body size, social behavior, prey and habitat requirements, large felids such as jaguar (Panthera onca) require large home ranges for their survival and population health, which in turn means their population densities are low (Macdonald et al., 2010).
The home range size of jaguars varies from 10 to 690 km2, depending on sex, prey availability, and habitat fragmentation level (see González–Borrajo et al., 2016; Morato et al., 2018; de la Torre and Rivero, 2019 for a review). Due to these spatial requirements, protected areas of suitable size are too few to guarantee long–term viability of jaguar as their conservation is hard–pressed to interweave a landscape that includes both protected and unprotected lands (Sanderson et al., 2002; Rabinowitz and Zeller, 2010). Currently, jaguar populations across their distribution range are mostly concentrated in 'Jaguar Conservation Units' (JCUs), that is, in areas that have a stable prey community and are known or believed to contain a resident population of at least 50 breeding individuals (Sanderson et al., 2002). The most important area for jaguar conservation in the northern jaguar range is the Selva Maya, a large region that includes several JCUs in the Yucatan Peninsula in Mexico, northern Guatemala and western Belize. These JCUs are connected by a complex corridor network (Sanderson et al., 2002; Rabinowitz and Zeller, 2010; fig. 1). The region is composed of both protected and unprotected land tracts. Jaguars inhabit a landscape across gradients that include heavily perturbed areas, large tracts of almost intact forests, and areas with intense human disturbance (Mitchell et al., 2013; Ellis et al., 2017). Research on spatial ecology of jaguar in the Selva Maya has mainly been associated with natural protected areas (de la Torre et al., 2017) or areas where large human population settlements are rare (Figueroa, 2013; Cruz et al., 2021). In these preserved areas, such as Calakmul, the jaguar home range varies from 37–436 km2 for females to 49–633 km2 for males (Cruz et al., 2021). Further variation has been reported in relation to habitat in areas where the forest is well–preserved and human disturbance is minimal, such as between Selva Maya and the Maya Mountains (Figueroa, 2013), Selva Lacandona, and Selva Maya (de la Torre et al., 2017).
Despite this well–established understanding of the jaguar home range, the only published data on jaguar home ranges from the corridor between Yum Balam and Sian Ka'an is based on the report of a single male (Gonzalez–Gallina et al., 2017a). This individual showed a particularly small seasonal home range (16 km2) that was attributed to the availability of unintended human subsidies. This male is also included in the present study.
Human population density alone does not account for past extirpations of jaguar or for decreases in populations. Similarly, human population increases will not necessarily determine jaguar declines. Jaguar extirpations, however, are potentially avoidable through the design and implementation of sustainable management and conservation programs (Jędrzejewski et al., 2017). Considering that jaguars will enter some non–protected areas with high human disturbance, it is essential to know how they respond to these mixed territories in order to develop comprehensive and effective conservation plans that consider further urban expansion into jaguar populated areas. One relevant area for jaguar conservation in the Selva Maya lies in the northeastern part of Quintana Roo state in Mexico, in an area popularly referred to as 'The Mayan Riviera'. The urban and touristic development presently being undertaken here is affecting the tropical forest therein (Ellis et al., 2017). Furthermore, the area is characterized by a high frequency of hurricanes followed by forest fires that largely influence the process of forest cover loss and recovery in the region (Whigham et al., 2003; Ellis et al., 2017). Such unprotected regions are well–preserved natural ecosystems and valuable biological corridors that link the JCUs (Salom–Pérez et al., 2010; Foster et al., 2020), as is the case, for example, of the area between Yum Balam (to the north) and Sian Ka´an (to the south) (Rabinowitz and Zeller, 2010; Rodríguez–Soto et al., 2013). A jaguar population with resident males and females has been reported in this region (González–Gallina et al., 2018). However, the continuous and accelerated expansion of the urban areas, and the growing road network crisscrossing the corridor is increasing the number of jaguars killed on roads (González–Gallina and Hidalgo–Mihart, 2018) and intensifying conflict between humans and jaguars (Remolina–Suárez, 2014; Carral–García et al., 2021).
Individual jaguars have shown differential use of space in relation to the degree of human disturbances (Morato et al., 2016). One study showed that jaguars can survive in areas with a relatively high degree of human presence (Morato et al., 2016; Boron et al., 2016; Hidalgo–Mihart et al., 2019). It has also been observed that seasonality plays a role in the size of the home range as animals adapt to changes such as the availability of cover and of resources (Núñez and Miller, 2019). In the present study, our main objectives were to assess the seasonal home range and core area sizes and analyze habitat use in a non–protected area with high human densities between the JCUs of Yum Balam and Sian Ka'an in the state of Quintana Roo, Mexico. The aim of this study was based on our interest to preserve the high ecological value of the area as much as possible despite the lack of formal protection. As the area not only contains jaguars but also plays a role in connecting JCUs it is necessary to promote management practices that will allow jaguars to persist in this increasingly disturbed area.
Animal Biodiversity and Conservation 45.2 (2022) 133
Material and methods
Study area
This study took place in the municipality of Solidaridad in the state of Quintana Roo, México (fig. 1), between the city of Playa del Carmen and the 180D Highway (Merida–Cancun; 87º 15' W, 20º 45' N´) in the vegetation corridor linking the protected areas of Yum–Balam (north) and Sian Ka´an (south) JCUs (Rabinowitz and Zeller, 2010). Average elevations in the area are between 5 and 10 m a.s.l. The climate is warm and sub–humid with average annual temperatures between 26 ºC and 33 ºC. Mean annual rainfall is 1,300 mm, mainly occurring from June to November. The dry season is well–defined (December to May) (INEGI, 2013). The original vegetation is tropical semi–deciduous forest with tree height around 12m and a well–defined understory (Rzedowski, 2006). Although this vegetation covers about 57 % of the state´s surface (The Nature Conservancy, 2006), due to the regular presence of hurricanes and agricultural–related fires, secondary growth in a patchwork of succession stages now occurs in most of the area. At the time of the study, the most extensive land cover was the old secondary–growth forests (regenerating for approximately 25 years after the fires that followed Hurricane Gilbert, 1988), characterized by trees of 8–10 m in height and a luxuriant understory. The second most extensive type is young secondary–growth forest (15 years or less of regeneration following recent fires, hurricanes, or human interventions), dominated by shrub and herbaceous strata. Amid these vegetation types there are small open areas used for slash–and–burn agriculture. To date, cattle farming is limited to only a few areas where people have cleared the forest and established pastures. Human presence in the study area is associated with the suburbs of the city of Playa del Carmen (population 149,923) (INEGI, 2010), with some 10 small towns of 100 to 500 inhabitants) and several outlying ranches. There are also a few illegal settlements scattered throughout, each with 5 to 10 inhabitants. The road network in the area is complex with numerous roads close to the Caribbean coast and the tourist areas. Inland, a few roads cut through the Sian Ka'an–Yum Balam corridor. These include the 305D Highway (Nuevo Xcan–Playa del Carmen), the 180D Highway, and some secondary paved and dirt roads.
Capture and telemetry
The jaguars were captured using Aldrich type foot–hold snare traps (Frank et al., 2003) set along trails with evidence of jaguar presence (e.g. scats, tracks, photos). Each trap was equipped with a VHF radio transmitter to monitor the triggering of traps (Halstead et al., 1995). Traps were set from January to April in 2013 and from April to May in 2014. They were checked using hand–held equipment with two antennae every four hours, except in the hottest months (March to April) when they were checked every two hours and turned off after the morning check (9:00) to prevent the animals' exposure to heat. They were reopened in the afternoon (17:00)
so as to avoid the hottest hours of the day. Once a jaguar was captured, it was immobilized with a mixture of ketamine/medetomidine for processing. Age, weight, and sex were estimated and each animal was fitted with a Vectronic GPS Plus Pro satellite collar with a Globalstar system and a drop–off device (Vectronic Aerospace GmbH, Berlin, Germany). Once the animal recovered from the anesthetic it was immediately released at the place it was captured The whole process complied with the guidelines of the American Society of Mammalogists (Sikes et al., 2011) under collection permit SGPA/DGVS/9611/12 (15 X 2012) and SGPA/ DGVS/975/14 (6 II 2014) granted to Mircea Gabriel Hidalgo Mihart by the Dirección General de Vida Silvestre–SEMARNAT–México.
The collars were programmed to record and send a GPS location every 6 hours, and the drop–off mechanism was set to release the collar approximately one year after activation. VHF telemetry was used to locate the collars after drop–off. Jaguar location data were classified for each animal seasonally, considering a dry season (January–May) and a rainy season (June–December). Jaguar 1 records were included even though some had been published previously (González–Gallina et al., 2017a). There were two reasons for this; first, to keep the variance of seasonality and habitat use of jaguars in the area in the current scenario and to increase the number of captured individuals, and second, because the previous analysis was focused on the home–range and core area size in relation to a distance to a landfill.
Home range and core areas
We calculated the home range so as to determine the space used by the jaguar over a given period. We calculated core areas because they indicate the most important areas. From this information we assessed landscapes where resources appeared to be clustered and hence important to the animal. These data were more likely to provide relevant clues on the specific life requirements of the animal rather than simply delimiting the peripheral areas (Harris et al., 1990; Powell, 2000). The home range of individual jaguars was calculated independently for each season as defined above, using only validated GPS locations (locations obtained with five or more satellites and with a dilution of precision of less than 10 meters). We estimated the seasonal home–range size and boundaries using the adaptive kernel method (Worton, 1989) at 90 % and seasonal core areas at 50 %. All home range and core areas were calculated using the Home Range Tools extension for ArcGis (Rodgers et al., 2007). The smoothing parameter (h) for each estimate was obtained using the least squares cross–validation method (Kernohan et al., 2001). To compare the jaguar home range with that of other studies (Gula and Theuerkauf, 2013), we also calculated the seasonal home range size of each jaguar using the Minimum Convex Polygon (MCP) estimator, applying the Animal Movement extension for 100 % of the locations (Hooge et al., 2001; table 1s in supplementary material).
134 González–Gallina et al.
Mexico
Campeche
BalamJCUKa'ax
Sian Ka'an JCU
Belize
Fig. 1. Location of the study area in the north–eastern portion of the Yucatan Peninsula in relation to the Jaguar Conservation Units (JCU) and jaguar corridors of the 'Selva Maya' region. (JCUs and jaguar corridors according to Rabinowitz and Zeller, 2010).
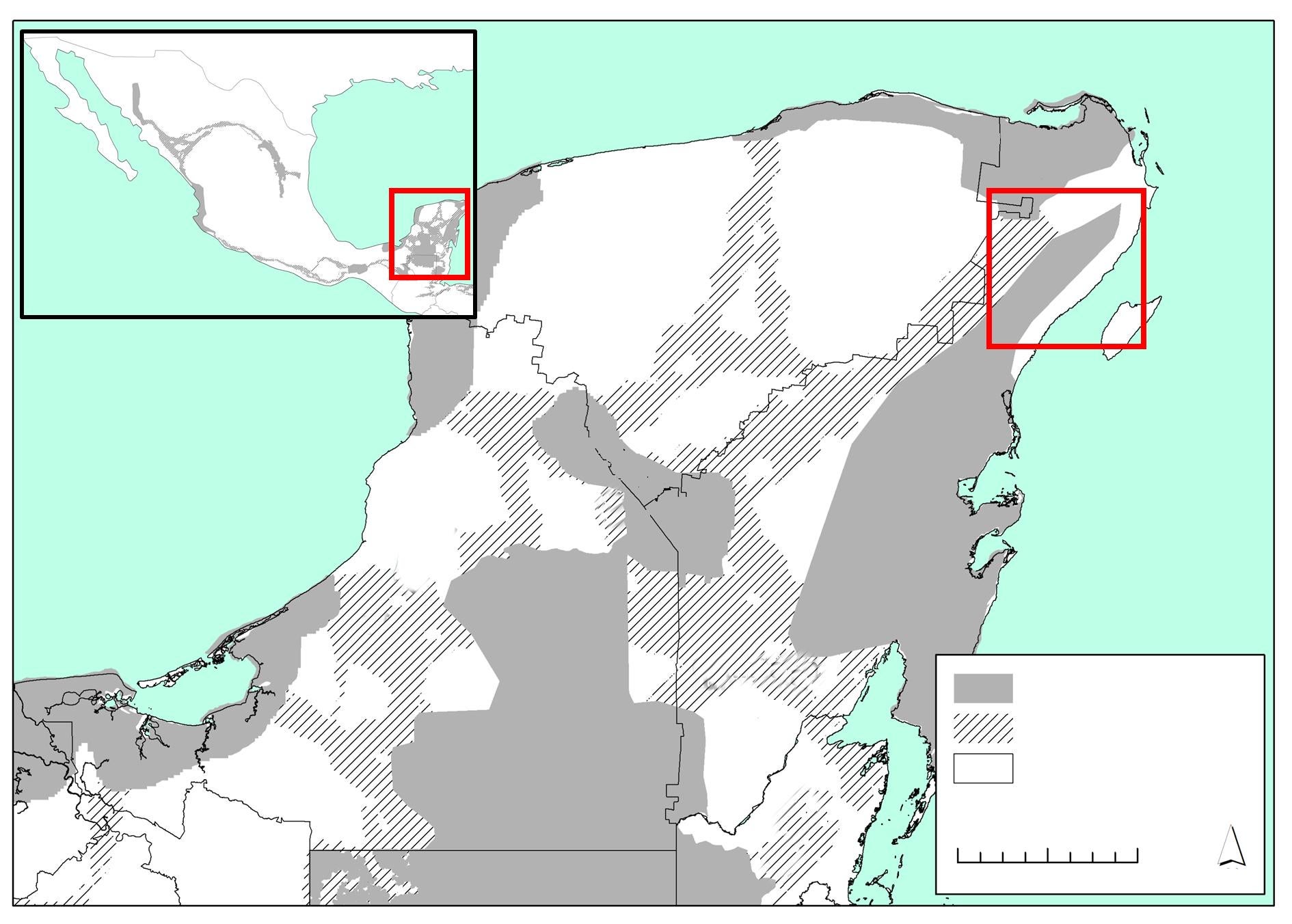
Fig. 1. Ubicación de la zona del estudio en el noreste de la península de Yucatán, en relación con las Unidades de Conservación de Jaguar (JCU, por sus siglas en inglés) y los corredores de jaguares de la región "Selva Maya". (Los corredores y las JCU para el jaguar se recabaron de Rabinowitz y Zeller, 2010).
To quantify the jaguars'degree of seasonal fidelity to their home range, we calculated the seasonal home range and core area overlap proposed by Kernohan et al. (2001). This method involves superimposing two–dimensional home range maps (HR1 and HR2). The measure of shared space use is the percent area overlap between the seasonal Kernel 90 % home ranges computed as:
HRi 3 HRj
SFIi,j = HRi
where SFIi,j is the Seasonal Fidelity index between animals i and j, resulting from the intersection of both animals in respect to seasonal home ranges (HR) relative to animal i seasonal home range.
A similar calculation was performed for seasonal core areas (CA); the resulting index was labeled CFI as Core Area Fidelity index:
CAi 3 CAj
CFIi,j = CAi
We analyzed the overlap of the seasonal home range for each individual because a simultaneous comparison between several individuals was marginal between seasons.
Jaguar habitat use
We characterized land use of the study regions from a mosaic of two ASTER (Advanced Spaceborne Thermal Emission and Reflection Radiometer) satellite scenes (pixel size 15 x 15 m) acquired in 2011. We performed a supervised classification of the mosaic image from 200 verification points distributed throughout the study area where the land cover was known and could be reliably assumed to be the same at the time the scenes were acquired. We classified the image based on the reflectance captured and stored by the ASTER sensor (visible and infrared) using the MaxLike algorithm in ENVI 4.5 (Exelis Visual Information Solutions, Boulder, USA). Four land cover categories were identified in the study area: tropical forest (including areas covered by tropical deciduous forest and old secondary growth forests with over 20 years' regeneration), young secondary growth forest
Animal Biodiversity and Conservation 45.2 (2022) 135
Yucatan
Guatemala
Quintana Roo
Yum Balam JCU
Calakmul JCU
JCU Jaguar corridor State and National limits 0 25 50 100 km
N
Table 1. Number of verified GPS locations (Loc), home range (HR) and core areas (CA) of four male jaguars that were radio–collared from 2013 to 2015 in a non–protected area in northeastern Quintana Roo, México. Home range and core area size (km2) were calculated using Kernel adaptive method at 90 % and 50 % respectively: J, jaguar number; W / A, weight (kg) and estimated age; T, total locations (Data from jaguar 1 was previously reported in González–Gallina et al., 2017a).
Tabla 1. Número de ubicaciones GPS verificadas (Loc), tamaño del ámbito hogareño (HR) y superficie de las zonas centrales (CA) de cuatro jaguares macho seguidos con collares satelitales entre 2013 y 2015 en una zona no protegida del noreste de Quintana Roo (México). El área de distribución y la superficie de la zona central (km2) se calcularon utilizando el método adaptativo Kernel al 90 % y 50 %, respectivamente: J, Jaguar; W / A, peso (kg) y edad estimada; T, ubicaciones totales. (Los datos del jaguar 1 se habían reportado previamente en González–Gallina et al., 2017a).
Dry season Rainy season Dry season Rainy season Dry season J W / A T
Loc HR CA Loc HR CA Loc HR CA Loc HR CA Loc HR CA
1 50 (adult)
466 16.22 2.5 561 97.46 2.55 508 82.38 2.51 1,535 2 48 (adult)
101 135.62 17.88 51 228.61 56.88 152 3 52 (adult) 239 129.98 18.22 854 318.04 38.29 304 218.1 43.61 95 170.27 35.22 1,492 4 48 (adult)
438 45.69 12.42 94 26.69 5.53 438
(under 10 years' regeneration), induced grasslands/ agriculture and urban areas. Despite its influence on jaguar behavior, we omitted the category 'water bodies'. It was not possible to identify this category because the karst landscapes in the Yucatan Peninsula of Mexico lacks flowing rivers or extensive water bodies on the surface (García–Gil et al., 2002). Surface water is either in 'aguadas' (topographic depressions in sparse tree cover that accumulate rainfall that usually dries out during the dry season) or in 'cenotes' (permanent water holes where the karst breaks, exposing underground water currents). Only a few are large enough and sufficiently exposed to be detected from above, and smaller ones are always hidden by the canopy (Delgado–Martínez et al., 2018) as is the case with 'sartenejas', that is, rock crevices that accumulate water (Delgado–Martínez et al., 2018). In our case, no reliable GIS layer showed either 'cenotes' or 'sartenejas'. We also included a null category (less than 3 % of the study area) for areas where we were unable to identify any land use due to cloud cover.
We investigated habitat selection following the framework developed by Johnson (1980) and Aebischer et al. (1993), under the assumption that animals make decisions about use at hierarchical stages, namely selection of home range within a study area (second–order selection) and selection of patches within the home range (third–order selection). To
determine second–order selection (hereafter called study–area selection), we defined habitat use as the percentage of total area occupied by each habitat type within the boundaries of the seasonal home range of each jaguar, obtained with the adaptive kernel method (ADK) at 90, and habitat availability as the total area occupied by each habitat type within the boundaries of the study area. For each season, we defined our study area as the boundaries of the home range obtained from all the GPS locations of all jaguars present during that season, estimated by the MCP method with 100 % utilization distribution. We used the MCP at 100 % because it is the smallest convex polygon that contains all locations (including those considered to be exploratory movements) and has been successfully used to determine the extension of a second order resource selection study (Horne et al., 2009). For the third–order selection (hereafter called home–range selection), we defined habitat use as the number of seasonal GPS locations of each jaguar in each land use type, and habitat availability as the percentage of the total area occupied by each habitat type within the boundaries of the seasonal ADK 90 % home range polygon of each jaguar (see table 2s in supplementary material for first, second and third order selection percentages).
We used compositional analysis (Aebisher et al., 1993) to examine seasonal habitat selection. We tested for differences of log–ratio habitat use and
136 González–Gallina et al.
Fig. 2. Seasonal home range configuration of the jaguars tracked by satellite in the north–eastern region of the Yucatan Peninsula, Mexico, obtained with the adaptive Kernel method (90 %): A, dry season of 2013; B, rainy season of 2013; C, dry season of 2014; D, rainy season of 2014; E, dry season of 2015; PR, pavement road; DR, dirt road; UA, urban area; W, water.
Fig. 2. Configuración del área de distribución estacional de los jaguares seguidos con collares satelitales en el noreste de la península de Yucatán (México) obtenida con el método adaptativo Kernel (90 %): A, temporada seca de 2013; B, temporada de lluvias de 2013; C, temporada seca de 2014; D, temporada de lluvias de 2014; E, temporada seca de 2015; PR, carretera pavimentada; DR, camino de tierra; UA, área urbana; W, agua.

availability across seasons at each habitat–selection order with a repeated measure multivariate analysis of variance (MANOVA). The small sample size precluded us from only using data of jaguars present in consecutive seasons. We thus combined data from jaguars that were present in different study years.
When habitat use was significantly non–random (P < 0.05), we calculated the mean and standard deviation for all log–ratio differences and constructed a matrix, ranking habitat types in their order of use (Aebischer et al., 1993) using 'adehabitat' HR version 0.3.15 in R (Calenge, 2006). If seasonal differences
Animal Biodiversity and Conservation 45.2 (2022) 137
PlayadelCarmenCaribbean Sea Leona Vicario J1 J2 J3 PR DR UA W J1 J2 J3 PR DR UA W 0 2 4 8 km N 0 2 4 8 km N 0 2 4 8 km N 0 2 4 8 km N 0 2 4 8 km N J1 J3 PR DR UA W J3 J4 PR DR UA W J4 PR DR UA W A B C D E 87º 30' 87º 15' 87º 30' 87º 15' PlayadelCarmenCaribbean Sea PlayadelCarmenCaribbean Sea PlayadelCarmenCaribbean Sea PlayadelCarmenCaribbean Sea Leona Vicario Leona Vicario Leona Vicario Leona Vicario 87º 30' 87º 15' 87º 30' 87º 15' 87º 30' 87º 15' 20º 45' 20º 45' 20º 45' 20º 45' 20º 45'
Table 2. Seasonal fidelity index (based on Kernohan et al.'s overlap index, 2001) of four male jaguars radio collared from year 2013 to 2015 in a non–protected area in northeastern Quintana Roo, México. Home range and core area size were calculated using Kernel adaptive method at 90 % and 50 % respectively: HR, home range overlap; CA, Core area overlap. (Data from jaguar 1 were previously reported in González–Gallina et al., 2017a).
Tabla 2. Índice de fidelidad estacional (basado en el índice de superposición de Kernohan et al., 2001) entre cuatro jaguares macho seguidos con collares satelitales entre 2013 y 2015 en un zona no protegida del noreste de Quintana Roo (México). La configuración del área de distribución y de la zona central se obtuvieron utilizando el método adaptativo Kernel al 90 % y 50 %, respectivamente: HR, superposición del área de distribución; CA, superposición de la zona central. (Los datos del jaguar 1 se habían reportado previamente en González–Gallina et al., 2017a).
Jaguar 1 Jaguar 2 Jaguar 3 Jaguar 4
Season 1 Season 2 HR CA HR CA HR CA HR CA
Dry 2013 Rain 2013 0.99 0.96 0.53 0.00 0.97 0.02
Rainy 2013 Dry 2013 0.13 0.94 0.31 0.00 0.40 0.01
Dry 2013 Dry 2014 0.89 0.87 0.79 0.42
Dry 2014 Dry 2013 0.18 0.88 0.47 0.18
Rainy 2013 Dry 2014 0.44 0.88 0.50 0.03
Dry 2014 Rainy 2013 0.66 0.90 0.73 0.03
Dry 2013 Rainy 2014 0.49 0.18
Rainy 2014 Dry 2013 0.37 0.10 Rainy 2013 Rainy 2014 0.34 0.38
Rainy 2014 Rainy 2013 0.49 0.34 Dry 2014 Rainy 2014 0.41 0.02 Rainy 2014 Dry 2014 0.52 0.03 Rainy 2014 Dry 2015 0.81 0.34 Dry 2015 Rainy 2014 0.47 0.77
in habitat selection were not detected by MANOVA, we pooled all GPS locations for each jaguar to determine a single–ranking matrix of habitat use and habitat availability.
Results
With a trapping effort of 1267 trap–nights, we captured four male jaguars (table 1) and later recaptured one of the four. Three of the animals were captured during the 2013 trapping season. One new capture and the recapture occurred during the 2014 trapping season. We retrieved the location data directly from two GPS collars that were recovered (jaguar 1 after drop–off and jaguar 3 after recapture). The data from the other three radio collars (jaguars 2 and 4, and the second year of data from jaguar 3) were retrieved from 'cloud data' stored at the Globalstar repository. We obtained 3617 GPS verified fixes from all the radio collared jaguars, with a maximum of 1,492 and a minimum of 152 per individual (table 1). A maximum of three individuals were monitored simultaneously per season.
Home range and core area size
The mean home range size (± SD) across the study period in the dry season for all the jaguars was 101.5 km2 (± 75.9 km2) and 172 km2 (± 107.29 km2) for the rainy season (table 1). We did not find statistically significant differences between seasonal home range sizes (F1,8 = 2.87, P = 0.12).
The mean core area size (± SD) for the four jaguars was 17.54 km2 (± 16.21 km2) for the dry season and 29.07 km2 (± 16.19 km2) for the rainy season (table 1). We found no statistically significant differences between seasonal core area sizes (F1,8 = 1.93, P = 0.20).
Individual site fidelity indexes for both SFI (for HR) and CFI (for CA) values varied from 0.99 to 0.01 (table 2). Variation between individuals was wide. We recorded near complete site fidelity for both HR and CA for jaguar 1 between the dry and rainy seasons of 2013, and for both dry seasons (2013–2014), and almost no site fidelity for jaguar 2 in 2013. HR fidelity did not imply CA fidelity as we can see with jaguar 3 in 2013, or the other way around jaguar 1 comparing the dry season in 2013 and the rainy season in 2014 (fig. 2).
138 González–Gallina et al.
Table 3. Mean habitat–selection ranks (Mean) and relation of use between four habitat types (Relation) at the study area level for jaguars in a non–protected area in northeastern Quintana Roo, México. Values for mean rank of habitat selection go from 0 (least–selected habitat type) to 3 (most–selected habitat type). The relation column indicates how jaguars use habitat type A compared to habitat type B. Each relation was replaced by its sign; a triple sign represents significant deviation from random at p < 0.05.
Tabla 3. Rango medio de la selección de hábitat (Mean) y la relación de uso de cuatro tipos de hábitat (Relation) presentes en la zona del estudio en una zona no protegida en el noreste de Quintana Roo (México). Los valores del rango medio de selección de hábitat van de 0 (tipo de hábitat menos seleccionado) a 3 (tipo de hábitat más seleccionado). En la columna de la relación (Relation) se indica cómo los jaguares usan el hábitat de tipo A en comparación con el hábitat de tipo B. Cada relación fue reemplazada por su signo; un signo triple representa una desviación significativa del azar a p < 0,05.
Mean Land use A Relation
Land use B
3 Tropical forest + Young secondary–growth forest +++ Induced grasslands/Agriculture +++ Urban areas
2 Young secondary–growth forest – Tropical forest +++ Induced grasslands/Agriculture +++ Urban areas
1 Induced grasslands/Agriculture
Tropical forest Young secondary–growth forest +++ Urban areas
0 Urban areas Tropical forest Young secondary–growth forest – Induced grasslands/Agriculture
Habitat use
We did not find any significant differences in the repeated measures MANOVA of the log–ratios between seasons, either at the study–area level (Wilks' λ = 0.69, F3,7 = 1.03, P = 0.44) or at the home–range level (Wilks' λ = 0.72, F3,7 = 0.92, P = 0.48). Because no seasonal differences were found, we pooled the seasonal data for all jaguars.
At the study area level (which considers all home ranges as a whole) we found that the proportional use of vegetation types was significantly different from availability (Λ = 0.003, d.f. = 3, P < 0.001). In order of relative selection at this level, jaguars selected forest land use, young secondary growth, induced grasslands/agriculture and urban areas (table 3). Because none of the GPS locations of the jaguars were in urban areas, so as to avoid the biases from missing habitat types (Aebisher et al., 1993) we eliminated this land use from the habitat use analysis at the home range level. In contrast, we found that jaguars within their home range level used all the habitats in proportion to their availability (Λ = 0.87, d.f. = 2, P = 0.76).
Discussion
Here we report on the home range of male jaguars and their habitat use in a non–protected area under increasing human influence from urban expansion and a growing road network. We recognize that our results could be biased by the low number of tracked individuals and the lack of information regarding female jaguars in the region.
Home range and core areas
The mean home range size of the jaguars in our study was smaller (128 km2 dry season and 190 km2 rainy season) than that of previously published home ranges in the 'Selva Maya' where mean male home ranges obtained with GPS tracking technology varied from 264 km2 in Belize (Figueroa, 2013), to 296 km2 in Calakmul (Cruz et al., 2021). The wide variation in the size of jaguar home ranges (González–Borrajo et al., 2016; Morato et al., 2018; de la Torre and Rivero, 2019) can be expected, however, as the geographic distribution of this species extends from southern
Animal Biodiversity and Conservation 45.2 (2022) 139
North America to the rest of the continent, and within this distribution, jaguars occur in various habitat types (Sanderson et al., 2002), leading to variation in their spatial ecology. When we look at individuals, male home ranges vary from a minimum of 109 km2 (Figueroa, 2013) to a maximum of 1,016 km2 (de la Torre et al., 2017). Our results seem unexpected as one could think that protected areas hold better habitat quality than non–protected areas, causing space use to increase as habitat quality decreases. Home range size and shape for a male jaguar is mostly influenced by prey availability (Sunquist and Sunquist, 1989; de Azevedo and Murray, 2007; McBride and Thompson, 2018), water availability (Delgado–Martínez et al., 2018) and the presence of females (Cavalcanti and Gese, 2009; Goodrich et al., 2010). Following this premise and our results, it seems that the Yum Balam–Sian Ka´an corridor holds enough resources to maintain a resident jaguar population with less than average home range sizes. Resource selection behavior varied considerably across individuals. Nevertheless, we noticed that jaguars in heavily forested areas (shift point = 58.4 %) showed a stronger tendency to avoid non–forest than individuals in more open landscapes. Also, a higher human population and livestock density does not appear to increase the strength of resource selection (Morato et al., 2018). Although we did not find differences between the rainy and dry seasons regarding home ranges and core areas, we observed that those in the rainy season were almost twice the size of those in the dry season (HR 101.5 and 172 km2; CA 17.54 and 29.07 km2). Núñez and Miller (2019) found differences in more contrasting seasonal areas in the coast of Jalisco where water appears to be the limiting factor. A different pattern is known for jaguars in Calakmul (Cruz et al., 2021) and Belize (Figueroa, 2013), although the sample size could also be limit detection of such an effect. This lack of change may indicate that the resources for male jaguars in the region do not change greatly across seasons, for jaguars will respond to shifts in the availability of prey by shifting the core areas of seasonal ranges to correspond with these shifts in prey availability (Figueroa, 2013).
In the Yum Balam JCU (a protected area), jaguars feed mostly on brocket deer (Mazama temama), collared pecari (Pecari tajacu) and armadillo (Dasypus novemecintus; Avila–Nájera et al., 2018), but in non–protected habitat there could be important changes in diet, as jaguars frequently prey on domestic dogs (Remolina–Suárez, 2014), and Jaguar 1 commonly visited the Playa del Carmen's landfill, probably searching for prey therein (González–Gallina et al., 2017a), such as black vultures (Coragyps atratus; González–Gallina et al., 2017b). We can thus assume that relative seasonal stability in the core area size in our study region could be attributed to the year–long availability not only of wild prey (Cavalcanti, 2008), which could be attracted by easy access to urban disposal zones, but also to prey items such as domestic dogs (Carral–García et al., 2021). Jaguar 1 home range size is the smallest for a male jaguar reported to date, and is considered largely attributable
to the availability of human–related prey at the Playa del Carmen landfill (Gallina et al., 2017a). Far from being a desirable situation, this reflects increasing jaguar–human conflict (Carral–García et al., 2021). This shift in prey items between forested areas and more urbanized areas is becoming a more common strategy among jaguars. Further research on the subject is needed. Prey availability and other resources, such as water, should be assessed. There is also the need to determine how widespread human presence is influencing resource availability.
We found the jaguars in our study showed a high degree of spatial overlap on the subsequent seasonal home range and core areas fidelity. The home range fidelity for at least two seasons in the case of jaguars 2 and 4, and for three seasons in jaguar 1 and four in jaguar 3, together with individual age, indicate they were all resident individuals. Long–term occupation of the same area has been used to distinguish resident jaguars from and transient jaguars (de Azevedo and Murray, 2007). As there is evidence of at least a few potentially reproductive females (González–Gallina et al., 2018) we can assume we were documenting a resident population (Karanth et al., 2006; Macdonald et al., 2010; Andersen et al., 2012).
We recommend jaguar conservation status of the area should be reconsidered. It could be incorporated into the region to the Sian Ka´an JCU or developed as a new JCU in the ‘Selva Maya’ region. This new JCU could consider encompassing the whole region between Cancun (to the north) between Leona Vicario and Puerto Morelos across the study area between Playa del Carmen and Tintal, connecting the natural protected area of Otoch Ma’ax Yetel Kooh, south to the Coba area. This would connect Yum Balam and Sian Ka'an JCUs. This whole area retains valuable jaguar habitat and resident populations, and it is under only minimal pressure of urban expansion.
Habitat use
According to GPS tracking, we found the jaguars were mainly located in tropical forests (defined as areas of tropical deciduous forest and old secondary growth forests with more than 20 years of regeneration), followed by young secondary forests (defined as areas with less than 10 years' regeneration). They were only occasionally located in induced grasslands/agriculture and never in urban areas. At the home range level, they appeared to use the habitat according to availability. At the core area level they preferred well developed tropical forests regardless of availability. Using photo trapping, Ávila–Nájera et al. (2019) observed that jaguars in Yum Balam JCU selected old secondary growth forests over other vegetation types. In general, we observed that jaguars in the study region preferred areas with low disturbance and dense vegetation, avoiding human modified areas as in other parts of the Selva Maya (Chávez, 2010; Conde et al., 2010; Figueroa, 2013; de la Torre et al., 2017)
Our results support previous observations that jaguars use forested areas in the northeastern portion of the Yucatán Peninsula, and highlight the importance
140 González–Gallina et al.
of conservation of these habitats throughout the region (Figueroa, 2013; de la Torre et al., 2017; Cruz et al., 2021). We emphasize the conservation value of young secondary growth forests resulting from forest fires. Though this vegetation type is structurally composed of seasonal bushes, thicker undergrowth and sparse tree cover, it represents the largest patches of natural vegetation in northern Quintana Roo. Not only is it the dominant vegetation type but it could become the jaguar’s preferred habitat if it is allowed 10 to 15 years to fully recover. Furthermore, this young secondary growth also harbors jaguar prey items such as white–tailed deer Odocoileus virginianus, collared peccary, and ocellated turkeys (Meleagris ocellate) (Urquiza–Haas et al., 2009).
Contrary to parts of the Selva Maya where the main threat to forest is the expansion of induced grasslands for cattle grazing (Chávez, 2010; Figueroa, 2013; de la Torre et al., 2017), in our study area the main threats to forest are fires and urban development (Ellis et al., 2017). Regarding the main conflict between jaguars and humans in the area, this is not livestock predation. Conflict is more likely the result of road kill (González–Gallina and Hidalgo–Mihart, 2018), retaliation after dog predation (Remolina–Suárez, 2014; Carral–García et al., 2021), or simply an increasing fear as the urban frontier expands further into the jaguar territory. This trend is likely to increase unless strong conservation actions are undertaken, such as establishing a new protected area in the regions to further protect the forest.
Researchers working with jaguars in cattle ranches (Hoogesteijn et al., 1993; Scognamillo et al., 2002; Polisar et al., 2003; Boron et al., 2016) have suggested that jaguars can live across unprotected human–use areas and co–exist with agricultural landscapes. The conditions conducive to this coexistence are the presence of sufficient natural areas and prohibition of hunting both jaguar and prey (Jedrzejewski et al., 2017). Conservation actions in the area are needed at both patch and corridor levels to maintain what remains of the tropical forest and to allow naturally disturbed patches to properly regenerate. Unless such action is taken, we risk further landscape alterations which, combined with other disturbance factors, will drive local extinctions not only of top predators such as jaguars but also second–growth tolerant mammal species (Urquiza–Haas et al., 2011; Ortiz–Lozada et al., 2017).
Acknowledgements
This study was made possible thanks to the support of SEGA S. A. de C. V. in charge of the biological survey for the highway project 'Ramales Cedral–Tintal, Tintal–Playa del Carmen con una longitud de 54 km en el estado de Quintana Roo, México'. We thank the DacBiol UJAT for logistical support. We also thank those who helped capture the jaguars: A. Rivera, F. Zavala, E. López, A. de la Torre, S. de Lara Carrillo, M. Tobler and D. Simpson and to the veterinary team: I. Cassaigne, S. Ortiz, S. Ilescas, O. Lofer and B. Portillo. To J. Equihua–Benítez for his help with the
GIS analysis. Special thanks to all the landowners of the ejidos of Agua Azul, Benito Juárez, Cedral, Laguna de Costa Rica, Guadalupe Victoria, Héroes de Nacozari and El Tintal for allowing us to do our research on their land.
References
Aebischer, N. J. Robertson, P. A., Kenward. R. E., 1993. Compositional analysis of habitat use from animal radio–tracking data. Ecology, 74(5): 1313–1325, http://www.jstor.org/stable/1940062 Andersen, L., Everatt, K. T., Somers, M. J., Purchase, G. K., 2012. Evidence for a resident population of cheetah in the Parque Nacional do Limpopo, Mozambique. African Journal of Wildlife Research, 42: 144–146
Ávila–Nájera, D. M., Palomares, F., Chávez C. Tigar, B., Mendoza, G. D., 2018. Jaguar (Panthera onca) and puma (Puma concolor) diets in Quintana Roo, Mexico. Animal Biodiversity and Conservation, 41(2): 257–266, Doi: 10.32800/abc.2018.41.0257
Ávila–Nájera, D. M., Lazcano–Barrero, M. A., Chávez, C. Pérez–Elizalde, S., Tigar, B., Mendoza, G. D., 2019. Habitat use of jaguar (Panthera onca) in a tropical forest in northern Quintana Roo, Mexico. Revista Mexicana de Biodiversidad, 90: e902186, Doi: 10.22201/ib.20078706e.2019.90.2186
Boron, V., Tzanopoulos, J., Gallo, J., Barragan, J., Jaimes–Rodriguez, L., Schaller, G., Payan, E., 2016. Jaguar densities across human–dominated landscapes in Colombia: the contribution of unprotected areas to long term conservation. Plos One, 11(5): e0153973, Doi: 10.1371/journal.pone.0153973
Calenge, C., 2006. The package 'adehabitat' for the R software: tool for the analysis of space and habitat use by animals. Ecological Modeling, 197: 516–519
Carral–García, M., Buenrostro, I., Weissenberger, H., Rosales, V., Pérez–Flores J., 2021. Dog predation by jaguars in a tourist town on the Mexican Caribbean. Neotropical Biology and Conservation, 16(4): 461–474, Doi: 10.3897/neotropical.16.e68973
Cavalcanti, S., 2008. Predator–prey relationships and spatial ecology of jaguars in the Southern Pantanal, Brazil: implications for conservation and management. PhD thesis, Utah State University.
Cavalcanti, S. M. C., Gese, E. M., 2009. Spatial ecology and social interactions of jaguars (Panthera onca) in the southern Pantanal, Brazil. Journal of Mammalogy, 90: 935–945, Doi: 10.1644/08-MAMM-A-188.1
Chávez, C., 2010. Ecología y conservación del jaguar (Panthera onca) y puma (Puma concolor) en la región de Calakmul y sus implicaciones para la conservación de la Peinínsula de Yucatán. PhD thesis, Universidad de Granada.
Conde, D. A., Colchero, F., Zarza, H., Christensen, N. L. Jr, Sexton, J. O., Manterola, C. ,Chávez, C., Rivera, A., Azuara, D., Ceballos, G., 2010. Sex matters: Modeling male and female habitat differences for jaguar conservation. Biological Conservation, 143(9): 1980–1988, Doi: 10.1016/j.
Animal Biodiversity and Conservation 45.2 (2022) 141
biocon.2010.04.049
Cruz, C., Zarza, H., Vidal–Mateo, J. ,Urios, V., Ceballos, G., 2021. Top predator ecology and conservation: Lessons from jaguars in souteastern Mexico. Conservation Science and Practice 3(2): e328, Doi: 10.1111/csp2.328 de Azevedo, F. C. C., Murray, D. L., 2007. Spatial organization and food habits of jaguars (Panthera onca) in a floodplain forest. Biological Conservation, 137(3): 391–402, Doi: 10.1016/j.biocon.2007.02.022 de la Torre, J. A. Núñez, J. M., Medellín, R. A., 2017. Spatial requirements of jaguars and pumas in southern Mexico. Mammalian Biology, 84: 52–60, Doi: 10.1016/j.mambio.2017.01.006
de la Torre, J. A., Rivero, M., 2019. Insights of the Movements of the Jaguar in the Tropical Forests of Southern Mexico. In: Movement Ecology of Neotropical Forest Mammals: 217–241 (C. Chapman, R. Reyna, Eds.). Springer, Cham.
Delgado–Martínez, C. M., Alvarado, F., Mendoza, E., Flores–Hernández, S., Navarrete, A., Navarrete, E., Botello, F., 2018. An ignored role of sartenejas to mitigate water shortage hazards for tropical forest vertebrates. Ecology, 99(3): 758–760, Doi: 10.1002/ecy.2078
Ellis, E. A., Romero–Montero, J. A., Hernández–Gómez, I. U., 2017. Deforestation Processes in the State of Quintana Roo, Mexico: The Role of Land Use and Community Forestry. Tropical Conservation Science, Doi: 10.1177/1940082917697259
Figueroa, O. A., 2013. The ecology and conservation of jaguars (Panthera onca) in central Belize: conservation status, diet, movement patterns and habitat use. PhD thesis, University of Florida.
Frank, L., Simpson, D., Woodroffe, R., 2003. Foot snares: an effective method for capturing African lions. Wildlife Society Bulletin, 31(1): 309–314, https://www.jstor.org/stable/3784391
Foster, R. J., Harmsen, B.,J., Urbina, Y.,L., Wooldridge, R. L., Doncaster, C. P., Quigley, H., Figueroa, O. A., 2020. Jaguar (Panthera onca) density and tenure in a critical biological corridor. Journal of Mammalogy, 101(6): 1622–1637, Doi: 10.1093/jmammal/gyaa134
García–Gil, G., Palacio-Prieto, J. L., Ortiz–Pérez, M. A., 2002. Reconocimiento geomorfológico e hidrográfico de la Reserva de la Biosfera Calakmul, México. Investigaciones Geográficas, 48: 7–23, http://www. redalyc.org/articulo.oa?id=56904802
González–Borrajo, N., López–Bao, J. V., Palomares, F., 2016. Spatial ecology of jaguars, pumas, and ocelots: a review of the state of knowledge. Mammalian Review, 47(1): 62–75, Doi: 10.1111/mam.12081
González–Gallina, A., Hidalgo–Mihart, M. G., Pérez–Garduza, F., Iglesias–Hernández, J. A., Oliveras de Ita, A., Chacón–Hernández, A., Vazquez–Zuniga, O., 2017a. Home range of a male jaguar spatially associated with the landfill of the city of Playa del Carmen, Mexico. Mammalia, 82(1): 1–8, Doi: 10.1515/mammalia-2016-0065
González–Gallina, A., Hidalgo–Mihart, M., 2018. A review of road–killed felids in Mexico. Therya, 9(2): 147–159, Doi: 10.12933/therya-18-584
González–Gallina, A., Hidalgo–Mihart, M. G., Caste-
lazo–Calva, V., 2018. Conservation implications for jaguars and other neotropical mammals using highway underpasses. Plos One, 13(11): e0206614, Doi: 10.1371/journal.pone.0206614
González–Gallina, A., Pérez–Garduza, F., Iglesias–Hernández, J. A., Oliveras de Ita, A., Chacón–Hernández, A., Vazquez–Zuniga, O., Hidalgo–Mihart, M. G., 2017b. A novel item, black vultures (Coragyps atratus) used as food by a jaguar (Panthera onca) in Quintana Roo, Mexico. American Midland Naturalist, 178(1): 158–164, Doi: 10.1674/0003-0031-178.1.158
Goodrich, J. M., Miquelle, D. G., Smirnov, E. N., Kerley, L. L., Quigley, H. B., Hornocker, M. G., 2010. Spatial structure of Amur (Siberian) tigers (Panthera tigris altaica) on Sikhote–Alin Biosphere Zapovednik, Russia. Journal of Mammalogy, 91(3): 737–748, Doi: 10.1644/09-MAMM-A-293.1
Gula, R., Theuerkauf, J., 2013. The need for standardization in wildlife science: home range estimators as an example. European Journal of Wildlife Research, 59: 713–718, Doi: 10.1007/s10344-013-0726-7
Halstead, T. D., Gruver, K. H., Phillips, R. L., Johnson, R. E., 1995. Using telemetry equipment for monitoring traps and snares. In: Twelfth Great Plains wildlife damage control workshop proceedings: 121–123 (R. E. Masters, J. G. Huggins, Eds.) Great Plains Agricultural Council, Tulsa. Harris, S., Cresswell, W. J., Forde, P. G., Trewhella, W. J., Woollard, T., Wray, S., 1990. Home–range analysis using radio–tracking data – a review of problems and techniques particularly as applied to the study of mammals. Mammalian Review, 20(2–3): 97–123, Doi: 10.1111/j.1365-2907.1990.tb00106.x Hidalgo–Mihart, M. G., Jesus–de la Cruz, A., Contreras–Moreno, F. M., Juárez–López. R., Bravata–de la Cruz, Y., Friedeberg, D., Bautista–Ramírez, P., 2019. Jaguar density in a mosaic of disturbed/preserved areas in southeastern Mexico. Mammalian Biology, 98: 173–178, Doi: 10.1016/j.mambio.2019.09.009
Hooge, P. N., Eichenlaub, W., Hooge, E. R., 2001. Animal movement 2.5 U.S. Geological Survey, Anchorage.
Hoogesteijn, R., Hoogesteijn, A., Mondolfi, X., 1993. Jaguar predation and conservation: cattle mortality caused by felines on three ranches in the Venezuelan Llanos. In: Mammals as Predators: 391–407 (N. Dunstone, R. L. Gorman, Eds.). Zoological Society, London.
Horne, J. S., Tewes, M. E., Haines, A. M., Laack, L. L., 2009. Habitat partitioning of sympatric ocelots and bobcats: implications for ocelot recovery in southern Texas. Southwestern Naturalist, 54(2): 119–126, https://www.jstor.org/stable/40263680
INEGI (Instituto Nacional de Estadística y Geografía), 2013. Anuario estadístico y geográfico de Quintana Roo 2013. Instituto Nacional de Estadística y Geografía, Aguascalientes.
– 2010. Censo de Población y Vivienda 2010. Principales resultados por localidad. Instituto Nacional de Estadística y Geografía, Aguascalientes.
Jędrzejewski, W., Boede, E. O., Abarca, M., Sánchez–Mercado, A., Ferrer–Paris, J. R., Lampo, M., Velásquez, G., Carreño, R., Viloria, A. L., Hooges-
142 González–Gallina et al.
teijn, R., Robinson, H. S., Stachowicz, I., Cerda, H., Weisz, M. M., Barros, T. R., Rivas, G. A., Borges, G., Molinari, J., Lew, D., Takiff, H., Schmidt, K., 2017. Predicting carnivore distribution and extirpation rate based on human impacts and productivity factors; assessment of the state of jaguar (Panthera onca) in Venezuela. Biological Conservation, 206: 132–142, Doi: 10.1016/j.biocon.2016.09.027
Johnson, D. H., 1980. The comparison of usage and availability measurements for evaluation of resource preference. Ecology, 61: 65–71.
Karanth, U. K., Nichols, J. D., Kumar, N. S.,Hines, J. E., 2006. Assessing tiger population dynamics using photographic capture–recapture sampling. Ecology, 87: 2925–2937, Doi: 10.1890/0012-9658(2006)87[2925:atpdup]2.0.co;2
Kernohan, B. J., Gitzen, R. A., Millspaugh, J. J., 2001. Analysis of animal space use and movements. In: Radio tracking animal populations: 125–166 (J. J. Millspaugh, J. M. Marzluff, Eds.). Academic Press, San Diego.
Macdonald, D. W., Mosser, A., Gittleman, J. L., 2010. Felid society. In: Biology and Conservation of Wild Felids: 125–160 (D. W. Macdonald, A. J. Loveridge, Eds.). Oxford University Press, Oxford.
McBride, R. T., Thompson, J. J., 2018. Space use and movement of jaguar (Panthera onca) in western Paraguay. Mammalia, 82(6): 540–549, Doi: 10.1515/mammalia-2017-0040
Mitchell, T. A,. Clark, M. L., Grau, H. R., López–Carr, D., Levy, M. A., Redo, D., Bonilla–Moheno, M., Riner, G., Andrade–Núñez, M. J., Muñiz, M., 2013. Deforestation and reforestation of Latin America and the Caribbean (2001–2010). Biotropica, 45(2): 262–271, Doi: 10.1111/j.1744-7429.2012.00908.x
Morato, R. G., Connette, G. M., Stabach, J. A., De Paula R. C., Ferraz, K. M. P. M., Kantek, D. L. Z., Miyazaki, S. S., Pereira, T. D. C., Silva, L.C., Paviolo, A., De Angelo, A., Di Bitetti, M. S., Cruz P., Lima, F., Cullen, L., Sana, D. A., Ramalho, E. E., Carvalho, M. M., da Silva, M. X., Moraes, M.,D. F., Vogliotti, A., May Jr, J. A., Haberfeld, M., Rampim, L., Sartorello, L., Araujo, G. R., Wittemyer, G., Riberio, M. C., Leimgruber P., 2018. Resource selection in an apex predator and variation in response to local landscape characteristics. Biological Conservation, 228: 233–240, Doi: 10.1016/j.biocon.2018.10.022
Morato, R. G., Stabach, J. A., Fleming, C. H., Calabrese, J. M., De Paula, R. C., Ferraz, K. M. P. M., Kantek, D. L. Z., Miyazaki, S. S., Pereira, T. D. C., Araujo, G. R., De Angelo, A. P. C., Di Bitetti, M. S., Cruz, P., Lima, F., Cullen, L., Sana, D. A., Ramalho, E. E., Carvalho, M. M., Soares, F. H. S., Zimbres, B., Marina, M. X., Silva, X., Moraes, M. D. F., Vogliotti, A., May Jr., J. A., Haberfeld, M., Rampim, L., Sartorello, L., Ribeiro, M. C., Leimgruber, P., 2016. Space use and movement of a Neotropical top predator: The endangered jaguar. Plos One, 11(2): e0168176, Doi: 10.1371/journal.pone.0168176
Núñez, R., Miller, B., 2019. Movements and Home Range of Jaguars (Panthera onca) and Mountain Lions (Puma concolor) in a Tropical Dry Forest of Western Mexico. In: Movement Ecology of Neo-
tropical Forest Mammals: 243–262 (C. Chapman, R. Reyna, Eds.). Springer, Cham. Ortiz–Lozada, L., Pelayo–Martínez, J., Mota–Vargas, C., Demeneghi–Calatayud, A. P., Sosa, V. J., 2017. Absence of large and presence of medium–sized mammal species of conservation concern in a privately protected area of rain forest in southeastern Mexico. Tropical Conservation Science, Doi: 10.1177/1940082917738093
Polisar, J., Maxit, I., Scognamillo, D., Farrell, L., Sunquist, M. E., Eisenberg J. F., 2003. Jaguars, pumas, their prey base, and cattle ranching: ecological interpretations of a management problem. Biological Conservation, 109(2): 297–310, Doi: 10.1016/S0006-3207(02)00157-X
Powell, R. A., 2000. Animal home ranges and territories and home range estimators. In: Research techniques in animal ecology: controversies and consequences: 65–110 (L. Boitani, T. Fuller, T, Eds.). Columbia University Press, New York.
Rabinowitz, A., Zeller, K., 2010. A range–wide model of landscape connectivity and conservation for the jaguar, Panthera onca Biological Conservation, 143(4): 939–945, Doi: 10.1016/j. biocon.2010.01.002
Remolina–Suárez, J. F., 2014. Reporte de Reubicación de un jaguar que estuvo atacando perros en un asentamiento irregular que invade el territorio de su distribución al Oeste de Playa del Carmen, Quintana Roo Technical report for the Comisión Nacional de Áreas Naturales Protegidas, SEMARNAT, Cancún, México.
Rodgers, A. R., Carr, A. P., Beyer, H. L., Smith, L., Kie, J. G., 2007. HRT: Home Range Tools for ArcGIS–Version 1.1. Ontario Ministry of Natural Resources, Thunder Bay, Ontario, Canada.
Rodríguez–Soto, C., Monroy–Vilchis, O., Maiorano, L., Boitani, L., Faller, J. C., Briones, M. A, Núñez, R., Rosas–Rosas, O., Ceballos, G., Falcucci, A., 2011. Predicting potential distribution of the jaguar in Mexico: identification of priority areas for conservation. Diversity and Distributions, 17: 350–361, Doi: 10.1111/j.1472-4642.2010.00740.x
Rodríguez–Soto, C., Monroy–Vilchis, O., Zarco–González, M. M., 2013. Corridors for jaguar (Panthera onca) in Mexico: Conservation strategies. Journal of Natural Conservation, 21: 438–443, Doi: 10.1016/j.jnc.2013.07.002
Rzedowski, J., 2006. Vegetación de México Primera Edición digital. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Distrito Federal, Mexico D.F.
Salom–Pérez, R., Polisar, J., Quigley, H., Zeller K., 2010. Jaguar Corridor Initiative: A biological corridor and a long–term commitment to conservation. Mesoamericana, 14(3): 25–34.
Sanderson, E. W., Redford, K. H., Chetkiewicz, C. B., Medellin, R. A., Rabinowitz, A. R., Robinson, J. G., Taber, A. B., 2002. Planning to Save a Species: The Jaguar as a Model. Conservation Biology, 16: 58–72, Doi: 10.1046/j.1523-1739.2002.00352.x
Scognamillo, D. Maxit, I. Sunquist, M., Farrell, L., 2002. Ecología del jaguar y el problema de la depredación
Animal Biodiversity and Conservation 45.2 (2022) 143
de ganado en un hato de Los Llanos Venezolanos. In: El Jaguar en el Nuevo Milenio: 139–150 (R. A. Medellín, C. Equihua, C. Chetkiewicz, P. G. Crawshaw Jr., A. Rabinowitz, K. H. Redford, J. G. Robinson, E. W. Sanderson, A. B. Taber, Eds.). Fondo de Cultura Económica, Universidad Autónoma de México y Wildlife Conservation Society. México.
Sikes, R. S. Gannon, W. L., Animal Care and Use Committee of the American Society of Mammalogists, 2011. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. Journal of Mammalogy, 92(1): 235–253, Doi: https://doi.org/10.1644/10-MAMM-F-355.1
Sunquist, M. E., Sunquist, F. C., 1989. Ecological constraints on predation by large felids. In: Carnivore Behavior, Ecology, and Evolution: 238–301 (J. L. Gittleman, Ed.) Cornell University Press, Ithaca.
The Nature Conservancy, 2006. Una visión para el futuro, una agenda para hoy. Plan ecorregional de las selvas mayas, zoque y olmeca. The Nature Conservancy, San Joé, Costa Rica.
Urquiza–Haas, T., Peres, C. A., Dolman, P. M., 2009. Regional scale effects of human density and forest disturbance on large–bodied vertebrates throughout the Yucatan Peninsula, Mexico. Biological Conservation, 42(1): 134–148, Doi: 10.1016/j. biocon.2008.10.007
– 2011. Large vertebrate responses to forest cover and hunting pressure in communal landholdings and protected areas of the Yucatan Peninsula, Mexico. Animal Conservation, 14: 271–282, Doi: 10.1111/j.1469-1795.2010.00426.x
Whigham, D. F., Olmsted, I., Cano, E. C., Curtis, A. B., 2003. Impacts of hurricanes on the forests of Quintana Roo, Yucatán Peninsula, Mexico. In: The lowland Maya: three millennia at the human–wildland interface: 193–216 (S. Fedick, M. Allen, J. Jiménez–Osorno, A. Gómez–Pompa, Eds.) Haworth Press, Binghamton. Worton, B. J., 1989 Kernel methods for estimating the utilization distribution in home–range studies. Ecology, 70.1: 164–168.
144 González–Gallina et al.
Population density and daily activity patterns of bobcat in its southernmost continental distribution
M. C. Lavariega, M. Briones–Salas, A. G. Monroy–Gamboa, D. Ramos–Méndez
Lavariega, M. C., Briones–Salas, M., Monroy–Gamboa, A. G., Ramos–Méndez, D., 2022. Population density and daily activity patterns of bobcat in its southernmost continental distribution. Animal Biodiversity and Conservation, 45.2: 145–160, Doi: https://doi.org/10.32800/abc.2022.45.0145
Abstract
Population density and daily activity patterns of bobcat in its southernmost continental distribution. Estimating density and activity patterns is useful for management and conservation of species. Data for Mexican bobcat (Lynx rufus) populations are scarce. Here we estimated the density of a bobcat population in Oaxaca, southern Mexico, and evaluated its daily activity patterns. We also evaluated macroecological patterns of bobcat density across its distribution range to determine any geographical (latitudinal, longitudinal, elevation, or range centroid) or climatic effects on the population density. Camera–trap data were divided into four 60–day periods (two in the dry season and two in the rainy season). Density was calculated using the random encounter model and daily activity patterns were analyzed fitting a kernel density function. The mean estimated density for the four periods was 17.3 bobcats/100 km2, with the highest densities occurring during the dry periods. Bobcat daily activity pattern presented two peaks, one after midnight and the other after dawn, with very slight changes between seasons. In the study area, density and activity patterns were associated with anthropogenic perturbation and prey availability. Bobcats increased their population density in the dry season, and showed a preference for activity at night and early morning hours when it is cooler and there are likely fewer competitors but more prey. Across its range, bobcat density was mainly related to annual precipitation and mean temperature of the driest quarter at 100 km radius buffers, and between annual precipitation and longitude on a smaller scale (50 km radius buffers). These findings support their preference for the arid or mesic environments that enabled them to reach southern areas of the Neartic region.
Key words: Camera–trap, Lynx rufus, Mesocarnivore, Oaxaca, Random Encounter Model
Resumen

Densidad demográfica y patrones de actividad diaria del gato montés en su área de distribución más meridional del continente. La estimación de la densidad demográfica y de los patrones de actividad diaria es útil para el manejo y la conservación de las especies. Los datos disponibles en relación con las poblaciones mexicanas de gato montés (Lynx rufus) son escasos. Los objetivos de este trabajo fueron estimar la densidad de una población de gato montés y evaluar sus patrones de actividad diaria en Oaxaca, al sur de México. Asimismo, evaluamos patrones macroecológicos de la densidad del gato montés a lo largo de su área de distribución para comprobar si existe algún efecto geográfico (latitud, longitud, elevación y centroide de su área de distribución) o climático en la densidad de la población. Los datos recabados mediante fototrampeo se agruparon en cuatro períodos de 60 días (dos en la estación seca y dos en la estación lluviosa). La densidad se calculó mediante un modelo de encuentros aleatorios y los patrones de actividad diaria se analizaron mediante ajustes de modelos Kernel. La densidad media estimada de los cuatro períodos fue de 17,3 gatos montés/100 km2, con las densidades más altas en las estaciones secas. El patrón de actividad diaria presentó dos picos: uno después de la medianoche y otro después del amanecer, con ligeros cambios entre temporadas. En la zona del estudio, la densidad y los patrones de actividad podrían estar relacionados con la perturbación antropogénica y la disponibilidad de presas. En la estación seca, la población aumentó y los gatos montés prefirieron estar activos durante la noche y la madrugada, cuando la temperatura es más fresca y tienen menos competidores y más presas. En su área de distribución, la densidad del gato montés
ISSN: 1578–665 X eISSN: 2014–928 X © [2022] Copyright belongs to the authors, who license the journal Animal Biodiversity and Conservation to publish the paper under a Creative Commons Attribution 4.0 License
145 Animal
Biodiversity and Conservation 45.2 (2022)
Lavariega et al.
está ligada principalmente a la precipitación anual y a la temperatura media del cuarto trimestre más seco en un radio de 100 km, y entre la precipitación anual y la longitud a una escala menor (radio de 50 km), lo que respalda el hecho de que el gato montés prefiere ambientes áridos o mésicos, que le permitan llegar a las zonas más meridionales de la región neártica.
Palabras clave: Cámara trampa, Lynx rufus, Mesocarnívoro, Oaxaca, Modelo de conteo aleatorio
Received: 9 III 21; Conditional acceptance: 9 VIII 21; Final acceptance: 27 IV 22
Mario C. Lavariega, Miguel Briones–Salas, Dagoberto Ramos–Méndez, Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional, Unidad Oaxaca, Instituto Politécnico Nacional. Hornos 1003, Santa Cruz Xoxocotlán, Oaxaca, México, 71230.– Alina Gabriela Monroy–Gamboa, Centro de Investigaciones Biológicas del Noroeste, S. C. Av. Instituto Politécnico Nacional 195, La Paz, Baja California Sur, México, 23096.
Corresponding Author: Miguel Briones–Salas. E–mail: mbriones@ipn.mx
ORCID ID: M. C. Lavariega: 0000-0003-2513-8244; M. Briones–Salas: 0000-0003-1413-9946; A. G. Monroy–Gamboa: 0000-0002-3277-855X
146
Introduction
Estimating the number of individuals in an area (population density) is useful to calculate the size of a population, and to promote management and conservation actions (Lawton, 1993; Mills, 2012). Patterns of mammal densities have shown that intrinsic factors, such as body mass and trophic level, can explain the population size of species. For example, large–bodied species, species with strictly carnivorous diets, and species at higher trophic levels occur at lower densities, whereas species with generalist diets and small body size have higher densities (Robinson and Redford, 1986; Fa and Purvis, 1997). In addition, density appears to be correlated with the size of the distribution range (density–range relation; Blackburn et al., 1997; Komonen et al., 2013) as it has been observed that population densities decline gradually toward the range boundaries (centroid distance–abundance relation; Brown, 1984). Among the extrinsic factors explaining density, body size and over–exploitation such as hunting are probably the causes of some small populations, making species more susceptible to local extinction (Lawton, 1994; Cardillo et al., 2005). In association with density estimates, the study of activity patterns sheds light on the circadian rhythms of animals, and the factors influencing them (Ridout and Linkie, 2009; Nouvellet et al., 2012; Rowcliffe et al., 2014; Frey et al., 2017; Sollmann, 2018).
At the intraspecific level, depending on sex and age, individuals use their diurnal and nocturnal time differently (Di Bitetti et al., 2006; Biggerstaff et al., 2017; Stone et al., 2018). The most widely studied temporal segregation has likely been investigated at an interspecific level, that is, for two or more species such as in predator–prey systems (Harmsen et al., 2011; Linkie and Ridout, 2011; Monterroso et al., 2013; Ross et al., 2013; Porfirio et al., 2016) or to evaluate competition between species of the same guild (e.g Harmsen et al., 2009; Lucherini et al., 2009; Dröge et al., 2017). For management purposes, it is useful to determine species' peaks of activity when planning to monitor a species (Jarnemo et al., 2017; Lavariega et al., 2019).
Camera–traps are a convenient means to record species with minimal interference (O'Connell et al., 2011). Early population studies using this approach focused on naturally marked animals, which in association with capture–recapture models provided the possibility to estimate abundances and densities (Karanth, 1995; Silver et al., 2004; Trolle and Kéry, 2003; Jackson et al., 2006). Nowadays, advances in the technology of camera–traps (e.g. delay time, resolution, and storage capability) have allowed advances in analytical approaches, improving population estimates for a wide range of species (Sollmann, 2018). For example, most recent mathematical models to estimate density have been developed for animals with few or no natural marks (Nakashima et al., 2018; Jimenez et al., 2019; Murphy et al., 2019). The Random Encounter Model (REM) is in the group of models for unmarked individuals calculates population density based on the encounter rate between camera–traps and animals, the velocity of movement of focal species, the sampling
effort, and the detection angle of the devices (Rowcliffe et al., 2008). REM has been applied to estimate population density of Harvey's duiker (Cephalophus harveyi; Rovero and Marshall, 2009), European pine marten (Martes martes; Manzo et al., 2012), and Baird's tapir (Tapirella bairdii; Carbajal–Borjes et al., 2014; Lavariega et al., 2016), providing adequate estimates such as capture–recapture methods.
Camera–trapping studies aiming to obtain information on abundance, density, and activity patterns have been mainly focused on large–sized mammals, whereas medium–sized species have been largely overlooked throughout their range (O’Connell et al., 2011). This is the case of the bobcat (Lynx rufus). Bobcat populations have been widely studied in the USA for many years, with estimates ranging from 3 to 48 indiv./100 km2 (Thornton and Pekins, 2015). The total population size has been estimated at 1,419,333 to 2,638,738 individuals, indicating that the bobcat population is stable in this country (Roberts and Crimmins, 2010). However, data on population size and densities in Mexico are scarce, precluding comparisons of regional variations and trends for the species (CITES, 2009). To date, Medellín and Bárcenas (2010) have evaluated the density of bobcat in six localities across Mexico, with estimates varying from 5 to 53 indiv./100 km2 in four localities. López–González et al. (2015) estimated a minimum density of 17 indiv./100 km2 for El Cimatario National Park in central Mexico and suggested that the lower densities found are due to the higher rates of habitat loss and urbanization. More recently, Greenspan et al. (2020) estimated a bobcat density of 16/100 km2 in a private ranching area in Sonora at north of Mexico, and Vega–Flores and Farías–González (2021) estimated a population density of 15 indiv./100 km2 in the Tehuacán–Cuicatlán Biosphere Reserve in the state of Puebla in the border with Oaxaca. However, density estimates for the southernmost limit of the bobcat's range in Mexico are lacking.
Regarding the activity patterns of bobcat, many authors report that the species is cathemeral, but activity peaks occur at crepuscule in response to environmental factors (Witmer and Decalesta, 1986; Bradley and Fagre, 1988; Neale and Sacks, 2001; Harrison, 2010; Rockhill et al., 2013; Symmank et al., 2014; Farías et al., 2015; Serna–Lagunes et al., 2019a). Most studies on bobcats in Mexico consist of reports concerning their presence (Bárcenas and Medellín, 2007; Valenzuela–Galván et al., 2013; Elizalde–Arellano et al., 2014; Ramírez–Albores et al., 2014; Sosa–Guerrero et al., 2017; Monterrubio–Rico et al., 2019), whereas studies on their ecology, such as feeding habits (Delibes et al., 1997; Aranda et al., 2002; Islas and Ceballos, 2018), competition (Sánchez–Cordero et al., 2008; Flores–Morales et al., 2019; Serna–Lagunes et al., 2019a), and population estimates (Medellín and Bárcenas, 2010; López–González et al., 2015; Vega–Flores and Farías–González, 2021) are scarce.
This scarcity of general information further limits macroecology analyses. For instance, Thornton and Pekins (2015) found a longitudinal and climatic relation
Animal Biodiversity and Conservation 45.2 (2022) 147
with the densities of bobcat in USA, but they were concerned about the need to include data regarding southern estimates. Using ecological niche modeling with presence data only, Loveless et al. (2016) found that seasonal climatic variables are relevant to predict habitat suitability of bobcat, and Pérez–Irineo et al. (2019) found a positive relation between bobcat densities and distance to the climatic niche centroid rather than a relationship with habitat suitability.
Here we aimed to expand the analyses of Thornton and Perkins (2015) by testing for a latitudinal effect, plus geographic and climatic relations of densities including density estimates for Mexico. It has been observed that bobcat densities are lower in central and southern Mexico than in the north of the country (Kelly et al., 2016). We estimated the population density of bobcat and evaluated its daily activity patterns in the southernmost limit of their current distribution in North America in order to test the latitudinal effect and geographic and climatic relations. We hypothesized that the density and the daily activity patterns could be affected by stochastic climatic variables, with differences between seasons as a response to the changing conditions of the environment (e.g. availability of resources, hours of light, and temperature). Thus, we predicted that bobcat population densities in the southern portion of their distribution would be lower than in the north due to its larger longitudinal space (cone shape effect; Sánchez–Cordero et al., 2008) and because bobcat populations in Mexico are far from the centroid of their range (Brown, 1984). Bobcat subspecies in Mexico are smaller than those in the USA (Loveless et al., 2016), and they consequently have greater densities (Robinson and Redford, 1986; Fa and Purvis, 1997; Vega–Flores and Farías–González, 2021). We considered that their daily activity patterns would be higher in the dry season than in the rainy season because they would spend more time hunting and searching for water.
Material and methods
Study site
The study was performed in the Municipality of Cosoltepec, Oaxaca, Mexico, on the borders with the Puebla State (18º 8' 13.08'' N and 97º 47' 26.16'' W; fig. 1). The area is characterized by mountains with an elevational range from 1,600 to 1,800 m a.s.l., a semi–warm and semi–humid climate, a rainy season in the summer (May–September), and a dry season in the winter (October–April). The average annual precipitation is 800 mm and the annual average temperature is 18–22 ºC (maximum 40 ºC and minimum 4 ºC). The landscape is composed of a matrix of grazing areas, temporary crops, patches of crasicaule scrubland, deciduous forest, and secondary vegetation (INEGI, 2008; Guizar, 2011).
Camera–trap survey
From December 2013 to September 2014 we deployed 14 camera–traps (Cuddeback ® models Capture
and Expert) in a grid, covering 19.7 km2 (fig. 1). The distance between camera–traps was approximately 1.5 km, considering the average home range of a bobcat (11.54 km2) in Oaxaca, Mexico (Monroy and Briones–Salas, 2012), and consistent with a travel distance of 1.76 ± 0.41 km recorded in Puebla, close to north Oaxaca (Vega–Flores and Farías–González, 2021). Camera–traps were set 30 cm above the ground, programmed to take photographs only, and to function 24 hrs with a minimum delay time (30 sec). The traps were checked monthly to download information and change batteries. We also recorded the presence of other terrestrial vertebrates.
Data analyses
The camera–trapping survey was divided into four 60–day periods, two in the dry seasons (period one: 31 January to 31 March; period two: 1 April to 30 May) and two in the rainy seasons (period three: 31 May to 29 July; period four: 30 July to 27 September). Periods of 60 days allowed us to comply with the assumption of population closure. We calculated the camera–trap capture rate (CR) for bobcat and for their potential prey for the whole study and for each camera–trap period using the quotient of independent event (IE) and the effort survey (ES) per 1,000 (CR = IE/ES x 1,000; Lira–Torres and Briones–Salas, 2012). An event was considered independent: a) when consecutive photographs of different identifiable individuals appeared; b) when a period of 24 hours passed between photographs, even if it was the same species or individual; and c) when photographs of the same species at the same place were not consecutive (Monroy–Vilchis et al., 2011; Lira–Torres and Briones–Salas, 2012).
To test the variations between dry and rainy seasons in the capture rate, we applied a two–sample test for equality of proportions with continuity correction in R program v. 2.15.0 (R Development Core Team, 2019). To estimate the bobcat density, we used the equation of Rowcliffe et al. (2008):
D = (y/t) (π/vr (2 + θ)
where D is the density, y/t is the number of photographs per unit time, v is the animal movement, r is the radial distance and θ the angle of detection of cameras. Values of r and θ were taken directly from camera–traps with trials of passing in front of them. We then used the means of 9 m for r and 19° for θ For v, we used the value of 0.3 km/hr, which was the mean velocity of bobcats recorded throughout GPS radio–tracking in a desert of Chihuahua, northern Mexico (Elizalde–Arellano et al., 2012). To calculate density we used the RandEM package (Caravaggi, 2017) in the R program. According to the photographic records, bobcats were active day and night, so bobcat activity parameters over 24 hrs were used.
Bobcat daily activity was analyzed fitting a kernel density function to quantify overall levels of activity. Using the package Overlap (Meredith and Ridout, 2018) in R program, we used a circular kernel to calculate data. A smoothing parameter of h = 1 was
148 Lavariega
et al.
Fig. 1. Study area for the estimate of density of bobcat (Lynx rufus) in the Mixteca region, Oaxaca, southern Mexico: the location of camera–trap stations is indicated in white dots.

Fig. 1. Área de estudio para la estimación de la densidad del gato montés (Lynx rufus) en la región Mixteca, en Oaxaca, en el sur de México: las estaciones de cámaras–trampa se indican con círculos blancos.
selected following the Ridout and Linkie (2009) recommendations for small samples. To compare activity level estimates of bobcat between the dry and rainy seasons, we applied a Wald test on a x2 distribution with 1 degree of freedom, in the package Activity (Rowcliffe, 2015) in R program.
Wide range density patterns
Using regression linear models we followed procedures of Thornton and Pekins (2015) to test the relationship of bobcat population density estimates with latitude, longitude, elevation, and bioclimatic variables. To test for a density–range centroid relationship, we used the distance between density studies and range centroid as covariables.
First, we compiled information on methods, locations, and bobcat density estimates from studies cited by Thornton and Pekins (2015). To add estimates from recent studies (> 2015) and to include studies in Mexico, we performed a search in Google Scholar (https://scholar.google.com/), using as keywords: density, bobcat, and Lynx rufus, in both Spanish and English. In all cases, for the study areas, we estimated a centroid according to geographic coordinates or maps provided by authors using Google Earth. From every centroid, we obtained the latitude, longitude, and elevation, and applied two buffers to characterize the surroundings, with a radius of 50 km and 100 km, similarly to Thornton and Pekins (2015). Climatic data was retrieved from the WorldClim project (www.worldclim.org; Hijmans et al., 2005), including 19 bioclimatic variables with 30 arcseconds of resolution (~1 km) generated from precipitation and
temperature covering a time span of 1950 to 2000. In QGIS (2012), we cut the bioclimatic variables with both buffer polygons 50 km and 100 km. We then calculated the average values of the clipped variables.
To test the relationship between density and range centroid, we downloaded a map of geographic distribution of the bobcat from the International Union for Conservation of Nature (IUCN; Kelly et al., 2016). In QGIS (2012), we calculated the geographic centroid, and estimated the distance between density studies centroids and the bobcat geographic centroid (fig. 2).
A Pearson correlation test revealed some variables were correlated (r > 0.7). In these cases, we maintained the variable most ecologically significant for bobcats (Loveless et al., 2016).
Linear regression models were tested with log–transformed density estimates as response variable. Latitude, longitude, elevation, annual mean temperature (Bio1), mean diurnal range (Bio2), mean temperature of wettest quarter (Bio8), mean temperature of the dries quarter (Bio9; only for 100 km buffers), annual precipitation (Bio12), precipitation of wettest month (Bio13), precipitation seasonality (Bio15), distance to bobcat geographic centroid, and method used to obtain data (invasive for radio–tracked individuals, and noninvasive when fecal DNA sampling or camera–trapping) were used as explanatory variables. We tested the models with up to two variables in order to avoid overfitting. The best model was selected using the Akaike Information Criteria (AICc) adjusted to small samples (Burnham and Anderson, 2002). All analyses were performed in the R program (R Development Core Team, 2019).
Animal Biodiversity and Conservation 45.2 (2022) 149
– 97º 52' – 97º 50' – 97º 49' – 97º 48' – 97º 47' – 97º 52' – 97º 50' – 97º 49' – 97º 48' – 97º 47' 18º 6' 18º 7' 18º 8' 18º 10' 18º 6' 18º 7' 18º 8' 18º 10'
Mexico Puebla State Oaxaca State
50º 0'
40º 0'
40º 0'
30º 0'
30º 0'
Mexico
20º 0'
50º 0' 20º 0'
– 120º 0' – 110º 0' – 100º 0' – 90º 0' – 80º 0'
Fig. 2. Locations of bobcat (Lynx rufus) density estimates in USA and Mexico. The dark grey area depicts the distribution range of the bobcat according to International Union for Conservation of Nature (IUCN; Kelly et al., 2016). The white star represents the total range–centroid for North America, and the black dots, are the local centroids of the study areas. Numbers correspond to the studies in table 1s in supplementary material

Fig. 2. Localidades de estimación de densidad poblacional de gato montés (Lynx rufus) en Estados Unidos de América y México. El área gris oscuro demuestra la distribución de acuerdo con la Unión Internacional para la Conservación de la Naturaleza (IUCN por sus siglas en inglés; Kelly et al., 2016). La estrella blanca representa el centroide obtenido de la distribución total para América del Norte y los puntos negros son los centroides de cada una de las áreas de estudio referidas y documentadas. Los números son los mismos que se muestran en la tabla 1s en material suplementario.
Results
The overall sampling effort (eight months) was 3,360 camera–traps/days. We recorded a total of 559 independent events (photographs of the same species separated by 24 hrs) of wild animals, 199 of which corresponded to carnivore mammals (tables 2s and 3s in supplementary material). Bobcats' potential prey were also recorded: Order Lagomorpha, Lepus spp. (n = 185), Order Didelphimorphia, Virginia opussum (Didelphis virginiana; n = 8), and Order Cingulata, nine–banded armadillo (Dasypus novemcinctus; n = 9). We also recorded 127 independent records of bird species, mainly lesser roadrunner (Geococcyx velox), plain chachalaca (Ortalis vetula), and white–tipped dove (Leptotila verreauxi).
We obtained 32 photographic records of bobcats (fig. 3), 28 of which were independent events, giving a capture rate of 8.33 captures per 1,000 days camera–trap. The number of independent events and the capture rate were higher in the two dry periods than in the rainy periods (table 1). However, we did not find differences between seasons regarding the
capture rate (x2 = 3.2749; df = 1; p–value = 0.0703). The mean estimated population density for the entire sampling period was 17.3 indiv./100 km. Considering a mean speed of bobcat movement of 0.30 km/H, the estimated density was higher during the dry seasons than during the rainy seasons (table 1).
Daily activity patterns
The bobcat had two peaks of activity, the first around 2:00 hrs and the second around 09:00 hrs (fig. 4). The pattern of activity was similar between the dry and rainy seasons (kernel density in dry season = 0.228, confidence interval = 0.18–0.28; kernel density in rainy season = 0.412, confidence interval = 0.21–0.44). There was no statistically significant difference between seasons, possibly due to the small sample size (Wald test = 3.88; p–value = 0.050).
Wide range analyses
We compiled 47 bobcat density estimates, including nine for Mexico (fig. 2; table 1s in supplementary ma-
150 Lavariega et al.
– 120º 0' – 110º 0' – 100º 0' – 90º 0' – 80º 0'
United States of America
Fig. 3. Un gato montés (Lynx rufus) fotografiado en este estudio con cámaras–trampa en la región Mixteca, en Oaxaca, en el sur de México.

terial). Overall, from a total of 56 models, the model that best explained the variation in density estimates was the one containing annual precipitation and the mean temperature of driest quarter in buffers of 100 km radius (AICc = 11.88; table 2). In buffers of 50 km radius, the best model included annual mean temperature and longitude (AICc = 12.77; table 3). The best model with 100 km buffers explained 53 % of the variation on log–transformed bobcat densities (model weight 0.35, AICc = 11.88), similarly to 52 % with 50 km buffer (model weight 0.94, AICc = 12.77). At 100 km radius buffers, we found that annual precipitation (Bio12) had a negative relation with densities, whereas the relation was positive for the mean temperature in the driest quarter (Bio 9). At 50 km radius buffers, we found a positive relation of densities with the annual mean temperature, and a negative relation with longitude.
Discussion
Our findings contribute to current knowledge of bobcats in the southernmost limit of their distribution range, adding data concerning capture rates, population density, and daily activity patterns. The bobcat capture rate in all periods of the dry and rainy seasons in the Mixteca region of Oaxaca was similar to or higher than rates reported previously for camera–trapping surveys in Mexico (table 4). Higher capture rates (> 12.0) were observed in coniferous forests and meadows in southern Mexico City, and in
pasturelands and scrubs in northwestern Chihuahua (Medellín and Bárcenas, 2010) In contrast, capture rates were low (< 2.0) in areas of submontane scrub and pine–oak forests in northwestern Guanajuato (Charre–Medellín et al., 2016), and also in areas with scrublands in western Zacatecas (Sánchez–González et al., 2018). Low capture rates could be explained by the highly disturbed habitats, likely associated with consequent low prey abundance. The lower capture rate in the rainy season in our study contrasts with findings from other studies with similar capture rates along seasons (Cruz–Jácome et al., 2015; Serna–Lagunes et al., 2019b). However, no studies have reported statistical differences between seasons. This suggests that the bobcat has a Nearctic affinity, which could explain the low capture rates in Neotropical latitudes and ecosystems, in addition to the great anthropogenic influence.
The mean bobcat density estimated in this study (17.32 indiv./100 km2) corresponds to one–third of the range recorded for populations in the USA and Mexico (0.05 to 53 indiv./100 km2; Medellín and Bárcenas, 2010; Thornton and Pekins, 2015). Particularly for Mexico, our estimates are lower than those reported by Medellín and Bárcenas (2010) in the Sierra Seri, Sonora (17.4–31.9 indiv./100 km2), San Ignacio, Sinaloa (31.8–47.8 indiv./100 km2) and Janos, Chihuahua (30.9–53.6 indiv./100 km2), but slightly higher than those recorded in Acatlán de Osorio, Puebla (6.5–12.2 indiv./100 km2), and San Miguel Topilejo, Mexico City (5.3–12.4 indiv./100 km2). They
Animal Biodiversity and Conservation 45.2 (2022) 151
Fig. 3. A bobcat (Lynx rufus) photographed in this study using camera–traps in the Mixteca region, Oaxaca, southern Mexico.
03/06/2014 3:50 a.m.
Lavariega et al.
Table 1. Photographic sightings and density of bobcat (Lynx rufus) in four sampling periods over two seasons in their southern–most continental distribution range, in the Mixteca region, Oaxaca, southern Mexico: N, number of independent detections.
Tabla 1. Detecciones fotográficas y densidad del gato montés (Lynx rufus) en cuatro periodos de muestreo en dos estacionalidades en la región Mixteca, en Oaxaca, en el sur de México, su distribución continental más sureña: N, número de detecciones independientes.
Period Season N
Capture rate
Density
Capture rate prey (indiv./100 km2)
Period 1 Dry 9 10.7 53.6 22.2
Period 2 Dry 10 11.9 84.5 24.7
Period 3 Rainy 5 5.9 58.3 12.4
Period 4 Rainy 4 4.8 30.9 9.9
are similar to the estimates of López–González et al. (2015) in the National Park El Cimatario, Querétaro (17 indiv./100 km2), and also to those of Greenspan et al. (2020) in Sonora (15.88 indiv./100 km2) and Vega–Flores and Farías–González (2021) in the Puebla portion of the Cuicatlán–Tehuacán Biosphere Reserve (15.4 ± 3.5 indiv./100 km2; table 1s in material supplementary). Medellín and Bárcenas (2010) found low bobcat densities in the more disturbed habitats, possibly as a consequence of habitat and refuge loss, and low availability of prey such as leporids. In contrast, intermediate and high densities have been reported in sites with moderate or low perturbation, such as in Sierra Seri, Sonora, San Ignacio, Sinaloa, and Janos, Chihuahua.
Considering climate only, for populations in the USA, Thornton and Pekins (2015) found that bobcat density increased linearly with the mean temperature in the study area (i.e., bobcats were more abundant at higher temperatures) and the more western the localities. However, they did not exclude the possibility that other variables, such as human disturbance, suitable habitat, or the presence of competitors and predators, have a negative effect on population size. Although the bobcat is a species with the ability to use landscapes with some level of anthropic disturbance (Zanin et al., 2015), forested habitat seems to be play a role in maintaining healthy populations.
Another factor that possibly plays an important role in the abundance of bobcats is prey availability.
Fig. 4. Daily activity pattern of bobcat (Lynx rufus) in the Mixteca region, Oaxaca, southern Mexico.
Fig. 4. Patrón diario de actividad del gato montés (Lynx rufus) en la región Mixteca, en Oaxaca, en el sur de México.

152
0.05 0.00
12:00 18:00 0:00
0.15 0.10
Density
6:00 12:00 Time
Table 2. Linear regression analysis of range–wide density estimates for bobcats (Lynx rufus) with 100 km radius buffers. Only the best 10 models are presented, and in order. The first corresponds to the best model. Covariables are: Bio1, annual mean temperature; Bio2, mean diurnal range, Bio8: mean temperature of wettest quarter; Bio9, mean temperature of the dries quarter; Bio12, annual precipitation; Bio13, precipitation of wettest month; Centroid, distance to the range centroid; and Method, type of method used to record bobcats (invasive versus no–invasive methods); Mw, model weight.
Tabla 2. Análisis de regresión linear en una estimación de amplio rango de densidad para gato montés (Lynx rufus) en buffers de 100 km de radio. Solo los 10 mejores modelos se presentan en orden, el primero corresponde al mejor. Las covariables son: Bio1, temperatura promedio anual; Bio2, promedio del rango diurno; Bio8, temperatura promedio en el cuarto trimestre más húmedo; Bio9, temperatura promedio del cuarto trimestre más seco; Bio12, precipitación anual; Bio13, precipitación del mes más húmedo; Centroid, distancia al centroide de la distribución del gato montés; y Method, método usado para registrar a los gatos montés (invasivo y no–invasivo.); Mw, peso modelo.
Model AICc dAICc Mw
Bio12 + Bio9 11.886 0 0.350 Centroid + Bio8 14.144 2.258 0.113 Bio9 14.511 2.625 0.094
Bio1 + Longitude 15.113 3.227 0.070 Method + Bio13 15.490 3.605 0.058 Longitude + Elevation 15.509 3.623 0.057 Method + Bio2 15.628 3.742 0.054 Bio1 + Bio9 15.638 3.752 0.054
Method + Bio8 15.980 4.094 0.045 Latitude + Bio9 16.551 4.665 0.034
Medellín and Bárcenas (2010) and López–González et al. (2015) did not discuss prey availability but it is feasibly lower in crowded central Mexico than in the northern wilderness areas such as Sonora and Chihuahua. In the Mixteca region, we found that the main prey recorded, the cottontail (Sylvilagus sp.), presented a higher capture rate (55.06 independent events per 1,000 day/camera) than in many sites in Mexico (Cortés–Marcial and Briones–Salas, 2014; Martínez–Hernández et al., 2017; Hernández et al., 2018; Serna–Lagunes et al., 2019a), and it was only surpassed by a site in the Tehuacán–Cuicatlán Biosphere Reserve in southern Mexico (Cruz–Jácome et
Table 3. Linear regression analysis of range–wide density estimates for bobcat (Lynx rufus) 50 km radius buffers. Only the best 10 models are presented, in order. The first corresponds to the best model. Covariables are: Bio1, annual mean temperature; Bio2, mean diurnal range; Bio8, mean temperature of wettest quarter; Bio13, precipitation of wettest month; Bio15, precipitation seasonality; and Centroid, distance to the range centroid; Ww, Model weight.
Tabla 3. Análisis de regresión linear en una estimación de amplio rango de densidad para gato montés (Lynx rufus) en buffers de 50 km de radio. Solo los 10 mejores modelos se presentan en orden, el primer corresponde al mejor. Las covariables son: Bio1, temperatura promedio anual; Bio2, promedio del rango diurno; Bio8, temperatura promedio en el cuarto trimestre más húmedo; Bio13, precipitación del mes más húmedo; Bio15, estacionalidad de la precipitación; y Centroid, distancia al centroide de la distribución del gato motés; Mw, peso modelo.
Model AICc dAICc Mw
Bio1 + Longitude 12.777 0 0.939
Bio1 + Bio2 20.442 7.665 0.020
Bio1 + Bio8 21.706 8.929 0.011 Bio1 + Bio13 22.571 9.794 0.007 Bio1 + Bio15 22.964 10.187 0.006
Latitude + Longitude 23.389 10.612 0.005 Bio1 23.537 10.760 0.004 Bio1 + Elevation 23.942 11.166 0.004 Bio1 + Centroid 25.594 12.817 0.002
Bio1 + Latitude 25.619 12.842 0.002
al., 2015), but further north of our study area. Other potential prey were not as frequent as Sylvilagus sp. (table 2s in material supplementary).
In our study area the weather is predominantly warm throughout the year. Although it is being heavily deforested, it is composed of fragments of crasicaule scrub and deciduous forest mixed with patches of grassland for cattle, and maize crops, providing bobcat with habitat cover to forage. In addition, prey availability is high. The area supports mesocarnivores such as coyote, gray fox, and white–nosed coati, although others, such as ocelot (Leopardus pardalis; Cervantes and Riveros, 2012) and large carnivores such as mountain lions (Puma concolor) and jaguars (Panthera onca; Briones–Salas et al., 2015; Padilla–Gómez et al., 2018) are absent. These characteristics could help explaining the high capture rate and density of bobcats in our study site.
Animal Biodiversity and Conservation 45.2 (2022) 153
Comparing densities between seasons in the Mixteca region, in accordance with our prediction, we found densities were higher in the dry season than in the rainy season. Similar results were observed by Medellín and Bárcenas (2010) in the Sierra Seri, Sonora, whereas for Janos, Chihuahua, the authors found that densities between seasons were similar (Medellín and Bárcenas, 2010).
We found that bobcats had a daily activity pattern with two peaks. The first peak began at night, reached the highest point around midnight, and then decreased until the sunrise. The second peak began mid–morning and decreased abruptly at noon. Our results are similar to those from other studies in Mexico, with two peaks of activity and the notoriously low activity after noon (Elizalde–Arellano et al., 2012). The temperature at our study area does not present such a wide variation as in some other areas. The maximum temperature range is 26 to 32 ºC, and the minimum temperature is 8 to 15 ºC). Daytime temperatures are predominately warm, contrasting with the extreme temperatures that occur in the deserts in the north of the country. Nevertheless, our results show that activity decreases at noon –when temperatures reach their maximum– and increases around midnight, when temperatures are lowest. Our results are similar to those concerning bobcats in the Chihuahuan desert where they show a significant negative correlation between temperature and daily activity patterns (Elizalde–Arellano et al., 2012). The highest temperatures occur during daylight, resulting in an increase in their nocturnal activity (George and Crooks, 2006; Wang et al., 2015; Lendrum et al., 2017; Flores–Morales et al., 2019). Harrison (2010) showed that bobcats avoid extremely high temperatures. Elizalde–Arellano et al. (2012) mention that distance traveled and the daily activity patterns of bobcats are positively related to energy requirements, prey availability and behavior.
Other potential explanations for concentrating activity at night could be competition with other mesocarnivores in the region, such as gray fox, coyote, and white–nosed coati (Cervantes and Riveros, 2012). These competitors are an important factor for the bobcat in its southernmost limit of the distribution range because of the cone–shape of this Mexican region; as the territory and/or extension of suitable habitat is smaller than that in the north, the species are closer and are more prone to compete. The bobcat share preys with other felids, such as ocelot ( Leopardus pardalis ) and margay ( Leopardus wiedii ; Sánchez–Cordero et al., 2008), and with other carnivores such as coyote and gray fox. It has been observed that the coyote and the bobcat also share activity patterns (Monroy–Gamboa, 2007; Serna–Lagunes et al., 2019). In the Mixteca region they share activity at dawn, but the coyote is more active in the evening and early night hours. In contrast, gray fox and bobcat share activity peaks after midnight, but the gray fox is active in the evening and early night hours. It is therefore possible that the competition could explain the difference between activity patterns in Oaxaca and other places in northern Mexico.
In its southernmost limit, the bobcat distribution range overlaps with the some of its competitors, but it is absent in the south of the Tehuantepec Isthmus despite the suitable habitat (Sánchez–Cordero et al., 2008). The explanation for this is that the Tehuantepec Isthmus is a continental strait with a width of 200 km. On both sides, the elevation is between 200 and 2,000 m a.s.l., resulting in a geographic and ecological barrier for montane species that are unable to move through the lowlands because of the weather and the multiple changes in vegetation (Barrier et al., 1998).
Thornton and Pekins (2015) found temperature and geographic longitude within 50 km radius buffers were the best variables to explain bobcat densities in the USA. This pattern was consistent when they included density estimates from Mexico at 50 km radius buffers. For the 100 km radius buffers, the best model obtained from a total of 56 models tested included annual precipitation and the mean temperature of the driest quarter In macroecological studies, variations in the relationship between species and environment variables, as observed here, are a common phenomenon, suggesting that species respond to environment in hierarchical ways throughout the buffers (Thornton and Pekins, 2015). However, both buffers offer support for selectivity of bobcat for arid or mesic environments. The northernmost distribution range of the bobcat (Lynx rufus) in Mexico occurs in the southernmost limit of the Neartic region. Individuals in this southern area are smaller than those in the north Neartic region, so their prey are also smaller. They are more tolerant and adapted to the tempered weather (coniferous forests with scarce snow precipitations) and also to the warmer conditions (deserts and scrubs). It is in this type of environment where the species mainly occur in Mexico (Bárcenas and Romero, 2014).
Contrary to our expectations, distance to geographic centroid or latitude (fig. 2) did not emerge in the best models to explain bobcat densities, i.e., bobcat densities did not fit the theory of abundance–centroid or a latitudinal pattern (which is also related to a body–size pattern). Through ecological niche modeling, Pérez–Irineo et al. (2019) found a positive relationship between bobcat densities and distance to the climatic niche centroid, which is opposed to the theory of abundance–centroid. Instead, they found that the sites with high bobcat densities were those with high climatic suitability. Climatic variables therefore seem to be related to bobcat densities on a wider scale. However, we do not rule out the possibility that population size at local scales is driven by the interrelation of factors such as habitat availability, productivity, and anthropogenic disturbance. Further studies on bobcat densities in Mexico are needed in order to elucidate the macroecological pattern of the species.
In summary, bobcats of the Mixteca region in Oaxaca, Mexico, increase their population density during the dry season and when there are higher temperatures. They prefer to be active in nocturnal and early morning hours when it is cooler, and when they may have fewer competitors. From a macroecological perspective, bobcat density seems to be
154
Lavariega et al.
Table 4. Comparison of capture rate of the bobcat (Lynx rufus) in camera–trapping studies in the Mixteca region, Oaxaca, southern Mexico.
Tabla 4. Comparación de la tasa de captura de gato montés (Lynx rufus) en estudios usando cámaras–trampa en la región Mixteca, en Oaxaca, en el sur de México.
Capture
Locality
Vegetation type rate Bibliographic reference
Northwestern Guanajuato Pine–oak forests 0.9 Charre–Medellín et al. (2016)
Northwestern Guanajuato Submontane scrub 1.5 Charre–Medellín et al. (2016)
Western Zacatecas Scrubland 1.9 Sánchez–González et al. (2018)
Northwestern Sonora Semi–desert grassland, 2.0 Coronel–Arellano et al. (2018) evergreen woodland, and plain, and great basin grassland
Northwestern Sonora Montane conifer forest 3.6 Coronel–Arellano et al. (2018)
Northwestern Oaxaca Tropical deciduous forests 4.2 Pérez–Solano et al. (2018) and evergreen woodland
Northwestern Sonora Semi–desert grassland, evergreen 4.3 Coronel–Arellano et al. (2018) evergreen woodland, and plain, and great basin grassland
Northeastern Coahuila Deciduous thorn forest 5.5 Gómez–Naranjo et al. (2017)
Northwestern Sonora Evergreen woodland 7.3 Coronel–Arellano et al. (2018) and semi–desert grassland
Northwestern Sonora Evergreen woodland 7.4 Coronel–Arellano et al. (2018) and thorn scrub
Northwestern Sonora Evergreen woodland, 7.5 Coronel–Arellano et al. (2018) montane conifer forest, and semi–desert grassland
Western Sonora Sarcocaule scrub 8.3 Medellín and Bárcenas (2010) and microphilic desert scrub
Northwestern Oaxaca Tropical deciduous forests, 8.3 This study crasicaule scrub, pasturelands, and crops
Central Veracruz Pine forest, subalpine vegetation, 9.1 Serna–Lagunes et al. (2019b) and paramo
Northwestern Sonora Semi–desert grassland 10.3 Coronel–Arellano et al. (2018) and evergreen woodland
Northwestern Oaxaca Tropical deciduous forests, 10.9 Cruz–Jácome et al. (2015) crasicaule scrub, pasturelands, and crops
Southern Mexico City Coniferous forests and meadows 12.5 Medellín and Bárcenas (2010)
Northwestern Chihuahua Pasturelands and scrub 12.5 Medellín and Bárcenas (2010)
Animal Biodiversity and Conservation 45.2 (2022) 155
Lavariega et al.
related to the driest periods and warmer climate, rather than reflect abundance–centroid or latitudinal pattern. However, more population studies throughout Mexico are needed to achieve a balanced sample size. Our findings add further data to our knowledge of the species and may contribute to more efficient management and conservation planning for bobcats in their southernmost distribution range.
Acknowledgements
Thanks to the Municipal and ejidal authorities of Cosoltepec for the facilities to perform this study, and to Y. M. Martínez–Ayón and B. Riveros–Lara for their valuable field support. M. Briones–Salas thanks the Comisión de Operación y Fomento a las Actividades Académicas (COFAA) and the Programa de Estímulos al Desempeño a la Investigación (EDI) at the Instituto Politécnico Nacional for their support, and also the Sistema Nacional de Investigadores (SNI) for its recognition and support. M. C. Lavariega thanks the Programa de Estímulos al Desempeño a la Investigación (EDI) at the Instituto Politécnico Nacional. A. G. Monroy–Gamboa thanks the Consejo Nacional de Ciencia y Tecnología (CONACYT) for the postdoctoral fellowship (CVU 206047).
References
Aranda, M., Rosas, O., Ríos, J. D. J, García, N., 2002. Análisis comparativo de la alimentación del gato montés (Lynx rufus) en dos diferentes ambientes de México. Acta Zoológica Mexicana. Nueva serie, 87: 99–109.
Bárcenas, H. V., Medellín, R. A., 2007. Registros notables de mamíferos en el sur del Distrito Federal, México. Revista Mexicana de Mastozoología (Nueva Época), 11: 73–79, Doi: 10.22201/ ie.20074484e.2007.11.1.131
Barcenas, R. H. V., Romero, F. R., 2014. Bobcat (Lynx rufus). In: Mammals of Mexico: 503–504 (G. Ceballos, Ed.). Comisión Nacional para el Conocimiento y el Uso de la Biodiversidad, Johns Hopkins University Press, Baltimore, USA.
Barrier, E., Velasquillo, L., Chavez, M., Gaulon, R., 1998. Neotectonic evolution of the Isthmus of Tehuantepec (southeastern Mexico). Tectonophysics, 287 (1–4): 77–96, Doi: 10.1016/S00401951(98)80062-0
Biggerstaff, M. T., Lashley, M. A., Chitwood, M. C., Moorman, C. E., DePerno, S. C., 2017. Sexual segregation of forage patch use: support for the social–factors and predation hypotheses. Behavioural Processes, 136: 36–42, Doi: 10.1016/j. beproc.2017.01.003
Blackburn, T. M., Gaston, K. J., Quinn, R. M., Arnold, H., Gregory, R. D., 1997. Of mice and wrens: the relation between abundance and geographic range size in British mammals and birds. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 352(1352): 419–427, Doi:
10.1098/rstb.1997.0030
Bradley, L. C., Fagre, D. B., 1988. Coyote and bobcat responses to integrated ranch management practices in south Texas. Journal of Range Management, 41: 322–327.
Briones–Salas, M., Cortés–Marcial, M., Lavariega, M. C., 2015. Diversidad y distribución geográfica de los mamíferos terrestres del estado de Oaxaca, México. Revista Mexicana de Biodiversidad, 86(3): 685–710, Doi: 10.1016/j.rmb.2015.07.008
Brown, J. H., 1984. On the relationship between abundance and distribution of species. American Naturalist, 12: 255–279.
Burnham, K. P., Anderson, R. P., 2002. Model Selection and Multimodel Inference: A Practical Information–Theoretic Approach. 2nd ed. Springer Verlag, New York.
Caravaggi, A., 2017. remBoot: An R package for Random Encounter Modelling. Journal of Open Source Software, 2: 176, Doi: 10.21105/joss.00176
Carbajal–Borjes, J. P., Godínez–Gómez, O., Mendoza, E., 2014. Density, abundance and activity patterns of the endangered Tapirus bairdii in one of its last strongholds in southern Mexico. Tropical Conservation Science, 7(1): 100–114, Doi: 10.1177/194008291400700102
Cardillo, M., Mace, G. M., Jones, K. E., Bielby, J., Bininda–Emonds, O. R., Sechrest, W., Orme, C. D., Purvis, A., 2005. Multiple causes of high extinction risk in large mammal species. Science, 309(5738): 1239–1241, Doi: 10.1126/science.1116030
Cervantes, F. A., Riveros, B., 2012. Mamíferos del Municipio de Cosoltepec, Oaxaca, México. Therya, 3(3): 311–325, Doi: 10.12933/therya-12-87
Chamberlain, M. J., Leopold, B. D., Conner, L. M., 2003. Space use, movements and habitat selection of adult bobcats (Lynx rufus) in Central Mississippi. American Midland Naturalist, 149(2): 395–405, https://www.jstor.org/stable/3566775
Charre–Medellín, J. F., Magaña–Cota, G., Monterrubio–Rico, T. C., Tafolla–Muñoz, R., Charre–Luna, J. L., Botello, F., 2016. Mamíferos medianos y grandes del municipio de Victoria, Reserva de la Biosfera Sierra Gorda Guanajuato, México. Acta Universitaria, 26: 62–70, Doi: 10.15174/au.2016.1438
CITES, Convention on International Trade in Endangered Species of Wild Fauna and Flora, 2009. Evaluación de la situación de las poblaciones de lince rojo (Lynx rufus) en México. CITES, Ginebra.
Coronel–Arellano, H., Lara–Díaz, N. E., Moreno, C. E., Gutiérrez–González, C. E., López–González, C. A., 2018. Biodiversity conservation in the Madrean sky islands: community homogeneity of medium and large mammals in northwestern Mexico. Journal of Mammalogy, 99: 465–477, Doi: 10.1093/ jmammal/gyx151
Cortés–Marcial, M., Briones–Salas, M., 2014. Diversidad, abundancia relativa y patrones de actividad de mamíferos medianos y grandes en una selva seca del Istmo de Tehuantepec, Oaxaca, México. Revista de Biología Tropical, 62(4):1433–1448, Doi: 10.15517/RBT.V62I4.13285
Cruz–Jácome, O., López–Tello, E., Delfín–Alfonso, C.
156
A., Mandujano, S., 2015. Riqueza y abundancia relativa de mamíferos medianos y grandes en una localidad en la Reserva de la Biosfera Tehuacán–Cuicatlán, Oaxaca, México. Therya, 6: 435–448.
Delibes, M., Zapata, S. C., Blázquez, M. C., Rodríguez–Estrella, R., 1997. Seasonal food habits of bobcats (Lynx rufus) in subtropical Baja California Sur, Mexico. Canadian Journal of Zoology, 75(3): 478–483, Doi: 10.1139/z97-058
Di Bitetti, M. S., Paviolo, A., De Angelo, C., 2006. Density, habitat use and activity patterns of ocelots (Leopardus pardalis) in the Atlantic Forest of Misiones, Argentina. Journal of Zoology, 270: 153–163, Doi: 10.1590/1678-476620151053367371
Dröge, E., Creel, S., Becker, M. S., M'soka, J., 2017. Spatial and temporal avoidance of risk within a large carnivore guild. Ecology and Evolution, 7: 189–199, Doi: 10.1002/ece3.2616
Elizalde–Arellano, C., López–Vidal, J. C., Hernández, L., Laundré, J. W., Cervantes, F. A., Alonso–Spilsbury, M., 2012. Home range size and activity patterns of bobcats (Lynx rufus) in the southern part of their range in the Chihuahuan Desert, Mexico. American Midland Naturalist, 168: 247–264.
Elizalde–Arellano, C., López–Vidal, J. C., Hernández–García, L., Laundré, J. W., Cervantes–Reza, F. A., Morales–Mejía, F. M., Ramírez–Vargas, M., Dávila–Galaviz, L. F., González–Romero, A., Alonso–Spilsbury, M., 2014. Registro de presencia y actividades de algunos mamíferos en el Desierto Chihuahuense, México. Therya, 5(3): 793–816, Doi: 10.12933/therya-14-252
Fa, J. E., Purvis, A., 1997. Body size, diet and population density in Afrotropical forest mammals: a comparison with neotropical species. Journal of Animal Ecology, 66: 98–112.
Farías, V., Téllez, O., Botello, F., Hernández, O., Berruecos, J., Olivares, S. J., Hernández, J. C., 2015. Primeros registros de 4 especies de felinos en el sur de Puebla, México. Revista Mexicana de Biodiversidad, 86(4): 1065–1071, Doi: 10.1016/j.rmb.2015.06.014
Flores–Morales, M., Vázquez, J., Bautista, A., Rodríguez–Martínez, L., Monroy–Vilchis, O., 2019. Response of two sympatric carnivores to human disturbances of their habitat: the bobcat and coyote. Mammal Research, 64(1): 53–62.
Frey, S., Fisher, J. T., Burton, A. C., Volpe, J. P., 2017. Investigating animal activity patterns and temporal niche partitioning using camera–trap data: challenges and opportunities. Remote Sensing in Ecology and Conservation, 3: 123–132, Doi: 10.1002/rse2.60
George, S. L., Crooks, K. R., 2006. Recreation and large mammal activity in an urban nature reserve. Biological Conservation, 133: 107–117, Doi: 10.1016/j.biocon.2006.05.024
Greenspan, E., Anile, S., Nielsen, C. K., 2020. Density of wild felids in Sonora, Mexico: a comparison of spatially explicit capture–recapture methods. European Journal of Wildlife Research, 66(4): 168–179, DoI: 10.1007/s10344-020-01401-1
Gómez–Naranjo, M. V., León–Tapia, M. Á., Hortelano–Moncada, Y., 2017. Mammals of the Tamaulipeco
Thorny Scrubland, Northwestern Coahuila, Mexico. Therya, 8(1): 35–44, Doi: 10.12933/therya-17-445
Guizar, N. E., 2011. La vegetación de la Mixteca PhD thesis, Universidad Autónoma Metropolitana–Iztapalapa.
Harmsen, B. J., Foster, R. J., Silver, S. C., Ostro, L. E., Doncaster, C. P., 2009. Spatial and temporal interactions of sympatric jaguars (Panthera onca) and pumas (Puma concolor) in a neotropical forest. Journal of Mammalogy, 90(3): 612–620, Doi: 10.1644/08-MAMM-A-140R.1
– 2011. Jaguar and puma activity patterns in relation to their main prey. Mammalian Biology, 76: 320–324, Doi: 10.1016/j.mambio.2010.08.007
Harrison, R. L., 2010. Ecological relationships of bobcats (Lynx rufus) in the Chihuahuan desert of New Mexico. Southwestern Naturalist, 55: 374–382.
Hernández, J. C., Chávez, C., List, R., 2018. Diversidad y patrones de actividad de mamíferos medianos y grandes en la Reserva de la Biosfera La Encrucijada, Chiapas, México. Revista de Biología Tropical, 66(2): 634–646, Doi: 10.15517/rbt.v66i2.33395
Hijmans, R. J., Cameron, S.E., Parra, J. L., Jones, P. G., Jarvis, A., 2005. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology, 25: 1965–1978.
INEGI (Instituto Nacional de Estadística y Geografía), 2008. Prontuario de información geográfica municipal de los Estados Unidos Mexicanos. Cosoltepec, Oaxaca. INEGI, México.
Islas, F. L., Ceballos, G., 2018. Depredación de un cincuate (Pituophis lineaticollis) y una ardilla arborícola (Sciurus aerogaster) por un gato montés (Lynx rufus escuinapae) en el Parque Estatal Hermenegildo Galeana, Estado de México. Revista Mexicana de Mastozoología (Nueva Época), 8: 1–7, Doi: 10.22201/ie.20074484e.2018.1.1.252
Jackson, R. M., Roe, J. D., Wangchuk, R., Hunter, D. O., 2006. Estimating snow leopard population abundance using photography and capture–recapture techniques. Wildlife Society Bulletin, 34(3): 772–781, https://www.jstor.org/stable/3784707
Jarnemo, A., Jansson, G., Månsson, J., 2017. Temporal variations in activity patterns during rut–implications for survey techniques of red deer, Cervus elaphus Wildlife Research, 44(2): 106–113, Doi: 10.1071/WR16156
Jimenez, J., Chandler, R., Tobajas, J., Descalzo, E., Mateo, R., Ferreras, P., 2019. Generalized spatial mark–resight models with incomplete identification: An application to red fox density estimates. Ecology and Evolution, 9(8): 4739–4748, Doi: 10.1002/ ece3.5077
Karanth, K. U., 1995. Estimating tiger Panthera tigris populations from camera–trap data using capture–recapture models. Biological Conservation, 71: 333–338.
Kelly, M., Morin, D., López–González, C. A., 2016. Lynx rufus The IUCN Red List of Threatened Species, 2016: e.T12521A50655874. Available online at: http://dx.doi.org/10.2305/IUCN.UK.2016–1. RLTS.T12521A50655874.en [Accessed on 25 February 2019].
Animal Biodiversity and Conservation 45.2 (2022) 157
Lavariega et al.
Komonen, A., Henttonen, H., Huitu, O., Nissinen, K., 2013. Curvilinear interspecific density–range size relationship in small mammals in Finland. Journal of Biogeography, 40: 1194–1201, Doi: 10.1111/jbi.12078
Lavariega, M. C., Briones–Salas, M., Mazas–Teodocio, A., Durán–Medina, E., 2016. Ecology and local knowledge of the Baird´s tapir (Tapirella bairdii) in the Sierra Madre de Oaxaca. Integrative Zoolology, 11(5): 361–374, Doi: 10.1111/1749-4877.12183
Lavariega, M. C., Monroy–Gamboa, A. G., Padilla–Gómez, E., Olivera–Martínez, U., 2019. Patrones de actividad de la chachalaca pálida (Ortalis poliocephala). Huitzil, 20(2): e–536, Doi: 10.28947/ hrmo.2019.20.2.455
Lawton, J. H., 1993. Range, population abundance and conservation. Trends in Ecology, Evolution, 8: 409–413. – 1994. Population dynamic principles. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 344: 61–68.
Lendrum, P. E., Crooks, K. R., Wittemyer, G., 2017. Changes in circadian activity patterns of a wildlife community post high–intensity energy development. Journal of Mammalogy, 98(5): 1265–1271, Doi: 10.1093/jmammal/gyx097
Linkie, M., Ridout, M. S., 2011. Assessing tiger–prey interactions in Sumatran rainforests. Journal of Zoology, 284(3): 224–229, Doi: 10.1111/j.14697998.2011.00801.x
Lira–Torres, I., Briones–Salas, M., 2012. Abundancia relativa y patrones de actividad de los mamíferos de los Chimalapas, Oaxaca, México. Acta Zoológica Mexicana Nueva Serie, 28(3): 566–585.
López–González, C. A., Ávila–Aguilar, D., Cruz–Torres, M. F., 2015. Abundancia del gato montés (Lynx rufus escuinapae J. A. Allen, 1903) en el Parque Nacional el Cimatario, Querétaro, México. Acta Zoológica Mexicana, Nueva Serie, 31(1): 138–140, Doi: 10.21829/azm.2015.311531
Loveless, A. M., Reding, D. M., Kapfer, P. M. M., Papes, M., 2016. Combining ecological niche modelling and morphology to assess the range–wide population genetic structure of bobcats (Lynx rufus). Biological Journal of the Linnean Society, 117(4): 842–857, Doi: 10.1111/bij.12718
Lucherini, M., Reppucci, J. I., Walker, R. S., Villalba, M. L., Wurstten, A., Gallardo, G., Iriarte, A., Villalobos, R., Perovic, P., 2009. Activity pattern segregation of carnivores in the high Andes. Journal of Mammalogy, 90(6): 1404–1409, Doi: 10.2307/27755147
Manzo, E., Bartolommei, P., Rowcliffe, J. M., Cozzolino, R., 2012. Estimation of population density of European pine marten in central Italy using camera trapping. Acta Theriologica, 57: 165–172, Doi: 10.1007/s13364-011-0055-8
Martínez–Hernández, A., Rosas–Rosas, O. C., Tarango–Arámbula, L. A., Benitez–Alemán, H. E., 2017. Abundancia de algunas presas de mesodepredadores en la reserva de la Biosfera Sierra del Abra Tanchipa y áreas adyacentes, San Luis Potosí, México. Revista Chapingo Serie Zonas Áridas, 16(2), 37–49, Doi: https://doi.org/10.5154/r. rchsza.2017.10.005
Medellín, R. A., Bárcenas, H. V., 2010. Estimación de
la densidad y dieta del lince (Lynx rufus) en seis localidades de México. SNIB–CONABIO, Informe final No. ES003 y ES009, México.
Meredith, M., Ridout, M., 2018. Overlap: Estimates of a coefficient of overlapping for animal activity patterns. R package version 0.3.2. Available online at: https://CRAN.R–project.org/package=overlap [Accessed on 17 March 2020].
Mills, L. S., 2012. Conservation of wildlife populations. Demography, genetics, and management. 2nd ed. John Wiley, Sons, New Jersey.
Monroy–Gamboa, A. G., 2007. Uso de hábitat y ámbito hogareño del coyote Canis latrans cagottis en un área comunal protegida de la Sierra Madre de Oaxaca, México. Master thesis. Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional–unidad Oaxaca, Instituto Politécnico Nacional.
Monroy, G., Briones–Salas, M., 2012. Primeros datos sobre área de actividad de gato montés (Lynx rufus) en Oaxaca, México. Acta Zoológica Mexicana Nueva Serie, 28(2): 471–474.
Monroy–Vilchis, O., Zarco–González, M., Rodriguez–Soto, C., Soria–Díaz, L., Urios, V., 2011. Fototrampeo de mamíferos en la Sierra Nanchititla, México. Revista de Biología Tropical (International Journal of Tropical Biology), 59(1): 373–383, Doi: 10.15517/RBT.V59I1.3206
Monterroso, P., Alves, P. C., Ferreras, P., 2013. Catch me if you can: diel activity patterns of mammalian prey and predators. Ethology, 119(12): 1044–1056, Doi: 10.1111/eth.12156
Monterrubio–Rico, T. C., Charre–Medellín, J. F., López–Ortíz, E. I., 2019. Wild felids in the temperate forest remnants in an avocado plantation landscape in Michoacán, México. The Southwestern Naturalist, 63(2): 137–142, Doi: 10.1894/0038-4909-63-2-137
Murphy, S. M., Wilckens, D. T., Augustine, B. C., Peyton, M. A., Harper, G. C., 2019. Improving estimation of puma (Puma concolor) population density: clustered camera–trapping, telemetry data, and generalized spatial mark–resight models. Scientific Reports, 9: 4590, Doi: 10.1038/s41598019-40926-7
Nakashima, Y., Fukasawa, K., Sarmejima, H., 2018. Estimating animal density without individual recognition using information derivable exclusively from camera traps. Journal of Applied Ecology, 55(2): 735–744, Doi: 10.1111/1365-2664.13059
Neale, J. C., Sacks, B. N., 2001. Resource utilization and interspecific relations of sympatric bobcats and coyotes. Oikos, 94: 236–249.
Nouvellet, P., Rasmussen, G. S. A., Macdonald, D. W., Courchamp, F., 2012. Noisy clocks and silent sunrises: measurement methods of daily activity pattern. Journal of Zoology, 286(3): 179–184, Doi: 10.1111/j.1469-7998.2011.00864.x
O'Connell, A. F., Nichols, J. D., Karanth, K. U. (Eds.), 2011. Camera traps in animal ecology. Methods and analyses. Springer Science, Business Media, Japan.
Padilla–Gómez, E., Lavariega, M. C., García–Santiago, P. A., Santiago–Velasco, J., Méndez–Méndez, R. O., 2018. An open–access platform for camera–
158
trapping data. Biodiversity Informatics, 13: 1–10.
Pérez–Irineo, G., Ballesteros–Barrera, C., Santos–Moreno, A., 2019. Densidad, idoneidad ambiental y nicho ecológico de cuatro especies de felinos americanos (Carnivora: Felidae). Revista de Biología Tropical, 67(3): 667–678, Doi: 10.15517/ RBT.V67I3.34819
Pérez–Solano, L. A., González, M., López–Tello, E., Mandujano, S., 2018. Mamíferos medianos y grandes asociados al bosque tropical seco del centro de México. Revista de Biología Tropical, 66(3): 1232–1243, Doi: 10.15517/rbt.v66i3.30810
Porfirio, G., Foster, V. C., Fonseca, C., Sarmento, P., 2016. Activity patterns of ocelots and their potential prey in the Brazilian Pantanal. Mammalian Biology, 81: 511–517, Doi: 10.1016/j.mambio.2016.06.006
QGIS (Quantum GIS Development Team), 2012. Quantum Geographic Information System. Open Source Geospatial Foundation Project. Available online at: https://qgis.org/ [Accessed on 19 August 2019].
R Development Core Team, 2019. R: A Language and Environment for Statistical Computing. 2.15.0 ed. R Foundation for Statistical Computing, Vienna. Available online at: https://www.r–project.org/ [Accessed on 29 April 2019].
Ramírez–Albores, J. E., León–Paniagua, L., Navarro–Sigüenza, A. G., 2014. Mamíferos silvestres del Parque Ecoturístico Piedra Canteada y alrededores, Tlaxcala, México; con notas sobre algunos registros notables para el área. Revista Mexicana de Biodiversidad, 85(1): 48–61, Doi: 10.7550/rmb.30485
Ridout, M. S., Linkie, M., 2009. Estimating overlap of daily activity patterns from camera trap data. Journal of Agricultural, Botanical, and Environmental Statistics, 14(3): 322–337, Doi: 10.1198/ jabes.2009.08038
Roberts, N. M., Crimmins, S. M., 2010. Bobcat population status and management in North America: evidence of large–scale population increase. Journal of Fish and Wildlife Management, 1: 169–174.
Robinson, J. G., Redford, K. H., 1986. Body size, diet, and population density of Neotropical forest mammals. American Naturalist, 128: 665–680.
Rockhill, A. P., DePerno, C. S., Powell, R. A., 2013. The Effect of Illumination and Time of Day on Movements of Bobcats (Lynx rufus). Plos One, 8: e69213, Doi: https://doi.org/10.1371/journal. pone.0069213
Ross, J., Hearn, A. J., Johnson, P. J., Macdonald, D. W., 2013. Activity patterns and temporal avoidance by prey in response to Sunda clouded leopard predation risk. Journal of Zoology, 290: 96–106, Doi: 10.1111/jzo.12018
Rovero, F., Marshall, A. R., 2009. Camera trapping photographic rate as an index of density in forest ungulates. Journal of Applied Ecology, 46(5): 1011–1017, Doi: 10.1111/j.1365-2664.2009.01705.x
Rowcliffe, J. M., 2015. Package "activity"– Animal Activity Statistics Version 1.3. Available online at: https://cran.r–project.org/web/packages/activity/ index.html [Accessed on 12 September 2019].
Rowcliffe, J. M., Field, J., Turvey, S. T., Carbone, C., 2008. Estimating animal density using camera traps without the need for individual recognition. Journal of Applied Ecology, 45(4): 1228–1236, Doi: 10.1111/j.1365-2664.2008.01473.x
Rowcliffe, J. M., Kays, R., Kranstauber, B., Carbone, C., Jansen, P. A., 2014. Quantifying levels of animal activity using camera trap data. Methods in Ecology and Evolution, 5(11): 1170–1179, Doi: 10.1111/2041-210X.12278
Sánchez–Cordero, V., Stockwell, D., Sarkar, S., Liu, H., Stephens, C. R., Giménez, J., 2008. Competitive interactions between felid species may limit the southern distribution of bobcats Lynx rufus Ecography, 31: 757–764.
Sánchez–González, R., Martin, H. S., David, A., Rosas–Rosas, O. C., García–Chávez, J., 2018. Diet and abundance of bobcat (Lynx rufus) in the Potosino–Zacatecano Plateau, Mexico. Therya, 9: 107–112, Doi: 10.12933/therya-18-498
Serna–Lagunes, R., Álvarez–Oseguera, L. R., Ávila–Nájera, D. M., Leyva–Ovalle, O. R., Andrés–Meza, P., Tigar, B., 2019a. Temporal overlap in the activity of Lynx rufus and Canis latrans and their potential prey in the Pico de Orizaba National Park, Mexico. Animal Biodiversity and Conservation, 42: 153–161, Doi: 10.32800/abc.2019.42.0153
Serna–Lagunes, R., Hernández–García, N., Álvarez–Oseguera, L. R., Llarena–Hernández, C., Alavéz–Martínez, N., Vivas–Lindo, R., Núñez–Pastrana, R., 2019b. Diversidad de mamíferos medianos en el Parque Nacional Pico de Orizaba. Ecosistemas y Recursos Agropecuarios, 6: 423–434.
Silver, S. C., Ostro, L. E., Marsh, L. K., Maffei, L., Noss, A. J., Kelly, M. J., Wallace, R. B., Gómez, H., Ayala, G., 2004. The use of camera traps for estimating jaguar Panthera onca abundance and density using capture/recapture analysis. Oryx, 38(2): 148–154, Doi: 10.1017/S0030605304000286
Sollmann, R., 2018. A gentle introduction to camera–trap data analysis. African Journal of Ecology, 56(4): 740–749, Doi: 10.1111/aje.12557
Sosa–Guerrero, O., Campos–Rodríguez, J. I., Flores–Leyva, X., Yáñez–López, P., Mora–Villa, L. A., 2017. Registros notables de mamíferos para la Reserva de la Biósfera Sierra Gorda de Guanajuato, México. Revista Mexicana de Mastozoología (Nueva Época), 7: 52–60, Doi: 10.22201/ ie.20074484e.2017.1.1.241
Stone, D. B., Martin, J. A., Cohen, B. S., Prebyl, T. J., Killmaster, C., Miller, K. V., 2018. Intraspecific temporal resource partitioning at white–tailed deer feeding sites. Current Zoology, 65(2): 139–146, Doi: 10.1093/cz/zoy051
Symmank, M. E., Comer, C. E., Kroll, J. C., 2014. Using infrared–triggered cameras to monitor activity of forest carnivores. Southeastern Naturalist, 13: 172–183, http://www.bioone.org/doi/ full/10.1656/058.013.s501
Thornton, D. H., Pekins, C. E., 2015. Spatially explicit capture–recapture analysis of bobcat (Lynx rufus) density: implications for mesocarnivore monitoring. Wildlife Research, 42(5): 394–404, Doi: 10.1071/
Animal Biodiversity
159
and Conservation 45.2 (2022)
WR15092
Trolle, M., Kéry, M., 2003. Estimation of ocelot density in the Pantanal using capture–recapture analysis of camera–trapping data. Journal of Mammalogy, 84: 607–614, Doi: 10.1644/1545-1542(2003)084<0607:EOODIT>2.0.CO;2
Valenzuela–Galván, D., de León–Ibarra, A., Lavalle–Sánchez, A., Orozco–Lugo, L., Chávez, C., 2013. The margay Leopardus wiedii and bobcat Lynx rufus from the dry forests of southern Morelos, Mexico. Southwestern Naturalist, 58: 118–120, Doi: 10.1894/0038-4909-58.1.118
Vega–Flores, C. N., Farías–González, V., 2021. Densidad de puma ( Puma concolor ) y gato montés (Lynx rufus) en la Reserva de la Biosfera Tehuacán–Cuicatlán, México. Revista Mexicana
de Biodiversidad, 92: e923639, Doi: 10.22201/ ib.20078706e.2021.92.3639
Wang, Y., Allen, M. L., Wilmers, C. C., 2015. Mesopredator spatial and temporal responses to large predators and human development in the Santa Cruz Mountains of California. Biological Conservation, 190: 23–33, Doi: 10.1016/j.biocon.2015.05.007
Witmer, G. W., Decalesta, D. S., 1986. Resource use by unexploited sympatric bobcats and coyotes in Oregon. Canadian Journal of Zoology, 64: 2333–2338.
Zanin, M., Palomares, F., Brito, D., 2015. What we (don’t) know about the effects of habitat loss and fragmentation on felids. Oryx, 49: 96–106, Doi: 10.1017/S0030605313001609
160
Lavariega et al.
Current genetic structure of European vendace Coregonus albula (L.) populations in Latvia after multiple past translocations J. Oreha, N. Škute
Oreha, J., Škute, N., 2022. Current genetic structure of European vendace Coregonus albula (L.) populations in Latvia after multiple past translocations. Animal Biodiversity and Conservation, 45.2: 161–173, Doi: https:// doi.org/10.32800/abc.2022.45.0161
Abstract
Current genetic structure of European vendace Coregonus albula (L.) populations in Latvia after multiple past translocations. The European vendace Coregonus albula (L.), also known as the European cisco, is a widespread fish species in northern Europe, often regarded as an example of a glacial relict. It is an economically valuable fish and has been artificially propagated in Latvia since 1900. Despite past translocations of larvae and fry and its current protection status, it can be found in only 15 Latvian lakes. We used nine microsatellite markers to study vendace populations from nine Latvian lakes. A higher mean allelic richness and private allelic richness in Lake Riču suggest that this population may be indigenous. Three complementary clustering methods revealed similar grouping into three distinct genetic groups. According to the results, European vendace populations in the Latvian lakes studied may currently be a mixture of several other populations after multiple translocations.
Key words: Population genetics, Fish transfer, Indigenous population, Divergence, Translocation
Resumen
Estructura genética de las poblaciones actuales de Coregonus albula (L.) como posible respuesta a múltiples translocaciones en el pasado. El corégono blanco Coregonus albula (L.) es una especie abundante en Europa septentrional que se suele considerar un ejemplo de vestigio de la era glaciar. El corégono blanco es una especie de alto valor económico y, en consecuencia, ha sido propagada artificialmente en Letonia desde 1900. A pesar de las translocaciones de larvas y alevines realizadas en el pasado y el actual estado de protección del corégono blanco, esta especie solo se puede encontrar en 15 lagos de Letonia. En el presente estudio utilizamos nueve marcadores de microsatélites para analizar las poblaciones de corégono blanco de nueve de estos lagos. La mayor riqueza alélica media y riqueza de alelos privados del lago Riču sugiere que esta población de corégono blanco puede ser autóctona. Se utilizaron tres métodos complementarios de agrupación que dieron resultados similares y revelaron la existencia de tres grupos genéticos diferenciados. De acuerdo con los resultados, es posible que las poblaciones europeas de corégono blanco de los lagos estudiados en Letonia sean en la actualidad una mezcla de distintas poblaciones tras múltiples translocaciones.
Palabras clave: Genética de poblaciones, Transferencia de peces, Población autóctona, Divergencia, Translocación
Received: 15 VI 21; Conditional acceptance: 8 XI 21; Final acceptance: 2 V 22
Jelena Oreha, Natalja Škute, Department of Ecology, Institute of Life Sciences and Technologies, Daugavpils University, Parādes street 1A–202, Daugavpils, Latvia.
Corresponding author: Jelena Oreha. E–mail: jelena.oreha@du.lv
ORCID ID: J. Oreha: 0000-0003-2389-0723

ISSN: 1578–665 X eISSN: 2014–928 X
© [2022] Copyright belongs to the authors, who license the journal Animal Biodiversity and Conservation to publish the paper under a Creative Commons Attribution 4.0 License
161 Animal Biodiversity and
Conservation 45.2 (2022)
Introduction
European vendace Coregonus albula (L.) is a widespread species in northern Europe. It appeared in North–eastern Europe after the last glacial period and is often regarded as a textbook example of a glacial relict (Mansfelds, 1936; Skrzypczak and Mamcarz, 2006; Borovikova and Makhrov, 2012). European vendace shows a high degree of morphological and genetic variability (Heikinheimo and Mikkola, 2004; Czerniejewski and Rybczyk, 2008; Umbrasaite et al., 2012; Borovikova et al., 2013; Dierking et al., 2014; Delling and Palm, 2019); and together with other whitefish and salmonids, it is considered an economically valuable fish.
The occurrence of European vendace in Latvia was mentioned in reports from the 19th century (Lake Puzes and Lake Usmas) (Kawall, 1858), and a survey of Latvian fish conducted in the 1930s showed that vendace could be found in about 30 lakes, mainly in Eastern Latvia (Mansfelds, 1936). Vendace populations later declined rapidly; they were found in only 11 lakes in the 1950–60s and in five lakes (Ežezers, Lejas, Nirzas, Rāznas and Usmas) in the 1990s (Plikšs and Aleksejevs, 1998). Consequently, European vendace was included in the list of specially protected species in Latvia in 2000 (Regulation no. 396 of the Cabinet of Ministers of the Republic of Latvia, November 14, 2000).
Due to its economic importance, European vendace has been artificially propagated in Latvia since 1900, being stopped only between 1916 and 1922 and between 1941 and 1946 because of the world wars. For artificial propagation, European vendace eggs were collected from Lake Peipus and Lake Ladoga up to 1959 and from Lake Rāznas and Lake Drīdzis ever since 1955 (Mosevich and Kumsare, 1955; Nikanorov and Nikanorova, 1956). Altogether, Peipus and Ladoga vendace larvae, fry and fingerlings, Ladoga ripus larvae (Coregonus albula infraspecies Ladogae (Prawdin, 1938 in Kottelat et al., 2005), whitefish (Coregonus lavaretus maraenoides (Poljakow, 1874)) and whitefish–ripus hybrids were released successively to many Latvian lakes in different years (1922–1941, 1946–1948, 1955–1959) (Kotov et al., 1958; Andrušaitis, 1960; Burmakin, 1963). As a result of these actions, the industrial catch of European vendace reached up to 13 tons in the 1930s, which was quite substantial for Latvian lakes. Yet despite past translocations of larvae and fry and current European vendace protection status, the species can be found in no more than 15 Latvian lakes. Furthermore, its share in the Latvian fishery is small, with rather insignificant and unstable catches (Aleksejevs, 2015, 2019).
Nowadays, the European vendace populations of West and East Europe have been relatively well investigated using various morphological features and molecular genetic markers (Vuorinen, 1984; Sendek, 2002; Huuskonen et al., 2004; Schulz et al., 2006; Oreha and Škute, 2009; Mehner et al., 2010; Borovikova et al., 2013; Præbel et al., 2013a, 2013b; Sendek et al., 2013; Delling and Palm, 2019). Microsatellite
genotyping is particularly useful for detecting genetic structure in closely related populations, regardless of whether they are in evolutionary equilibrium or not, and have been applied, for instance, in the study of the population genetic structure of whitefish all over the world. There are few similar studies on European vendace (Huuskonen et al., 2004; Schulz et al., 2006; Præbel et al., 2013a, 2013b), and little is known about the genetic structure of the European vendace populations in Baltic countries (Kaupinis et al., 2004; Škute and Oreha, 2016). In the present research, nine microsatellite markers were used to study European vendace populations from nine Latvian lakes. We hypothesized that their genetic structure in Latvian lakes might reveal the traces of translocated gene pools. We aimed to decipher whether vendace translocations in the middle of the last century, the consequent population flourishing, and the following decline had an impact on the genetic variability and genetic structure of vendace in Latvian lakes.
Material and methods
Sampling
Between 2007 and 2016 European vendace samples were collected in Latvia with the help of specialists from the Latvian Fish Resources Agency as part of the sampling efforts of its monitoring plan. Specimens were captured with 20 mm mesh size bottom–set gill nets, each measuring 70 m in length and 6 m in height. The material was collected from nine Latvian lakes (Alūksnes, Nirzas, Ežezers, Rāznas, Lejas, Drīdzis, Stirnu, Sventes and Riču; see locations, surface areas and depths of lakes in fig. 1, table 1). All studied lakes are eutrophic or meso–eutrophic and support commercial and recreational fishing, although commercial activities are currently insignificant and non–profitable. It is notable that fishing with a seine net is restricted in all lakes of Latvia (Regulation No. 159 of the Cabinet of Ministers of the Republic of Latvia, 2001). As the contribution of European vendace to the fishery is not large and the catch is insignificant and unstable, sample sizes taken for research purposes differed in each lake (table 1). Samples of fish tissue (skeletal muscles) were taken and stored at –80 ºC.
Microsatellite analysis
DNA was purified from skeletal muscle tissue according to the salt–extraction method of Aljanabi and Martinez (1997). The quality and quantity of DNA samples were determined using spectrophotometer BioSpec–Nano (Shimadzu). The extracted DNA was stored at –20 ºC. For subsequent molecular analysis, DNA was diluted to a final concentration of 20 ng/μL. Microsatellite amplification was performed using the ABI 9700 thermocycler. PCR (polymerase chain reaction) with fluorescently marked primers (the forward primer in each pair being labelled with the fluorescent label NED, HEX or FAM). PCR mixture components in a final volume of 12 μL were: 100 ng
162 Oreha
Škute
and
Lake
Fig.
of DNA sample, 10mM Tris–HCl buffer with 50mM KCl, 1.5 mM MgCl2, 2mM dNTPs mix, 0.06 U/μL Taq DNA polymerase, 0.4 μmol/μL of each primer. The individuals were genotyped at nine microsatellite loci: six of the nine were were dinucleotide repeat loci (Cisco106, Cisco90, Cisco126, Cisco157, Cisco200, BWF1; Patton et al., 1997; Turgeon et al., 1999) and three were tetranucleotide repeat loci (Clatet6, Clatet9, Clatet13; Winkler and Weiss, 2008). The PCR thermal cycling program had an initial denaturation at 94 ºC for 5 min, followed by 25 cycles with denaturation at 94 ºC for 30 s, annealing at locus specific temperature (see table 2) for 30 s, and extension at 72 ºC for 60 s, followed by a 7–min final extension at 72 ºC, and cooling at 4 ºC. Both positive and negative controls were used during PCR amplification.
PCR products were separated on an ABI 310 automated analyzer (Applied Biosystem) using Genescan ROX 500 size standard (Applied Biosystem), and alleles were scored with GeneMapper 3.7 software (Applied Biosystem). The Micro–Checker 2.2.3 program was used to check the data for typographic errors and to identify the null allele and genotyping errors: short allele dominance (large allele dropout) and scoring of stutter peaks (Van Oosterhout et al., 2004).
The following standard indices of genetic variation were measured: number and frequency of alleles per locus, occurrence of private alleles in each population, and observed (Ho) and expected (He) heterozygosity levels at each locus. The differences and statistical (x2) significance between observed and expected heterozygosity values were calculated using POPGENE 1.32 (Yeh et al., 1999) and GeneAlex 6.41 software
(Peakall and Smouse, 2006). Richness of alleles and private alleles in each population were determined, accounting for differences in the size of samples. The rarefaction procedure was used for the smallest sample size as implemented in the software HP–RARE 1.0 (Kalinowski, 2005).
In order to estimate and visualize the genetic structure and differentiation of the studied European vendace populations we used the computer programs STRUCTURE 2.3 (Hubisz et al., 2009) and STRUCTURE HARVESTER (Earl and von Holdt, 2012). A model assuming admixture and correlated allele frequencies between K populations (Burn–ins of 100,000 replications and 300,000 Markov chain Monte Carlo (MCMC) replicates) was used. Sampling locations were used as prior information to assist the structuring (the LOCPRIOR model) as recommended for weak signals of structuring (Hubisz et al., 2009). Values of K between one and nine were tested, running STRUCTURE ten times for each K and using Evanno's ∆K method to determine the most suitable number of clusters (Evanno et al., 2005). The most likely (highest ln Pr(Χ|Κ)) grouping was visualized using STRUCTURE HARVESTER (Earl and von Holdt, 2012).
The genetic relatedness of the populations was estimated with Nei's index of genetic distance (D; Nei et al., 1983) using the computer program Populations 1.2.32 (Langella, 2005). The corresponding dendrogram was created according to the neighbor–joining (NJ) method using the computer program TreeView (Page, 1996). Genetic divergence was estimated using pairwise FST values (Weir and Cockerham, 1984) with GeneAlex 6.41 software (Peakall and Smouse,
Animal Biodiversity and Conservation 45.2 (2022) 163
Fig. 1. Map showing sampling locations of nine European vendace populations.
1. Mapa de los puntos de muestreo de nueve poblaciones de corégono blanco.
Lithuania
Latvia
Estonia Russia
Latvia 22º 0' 0'' E 23º 0' 0'' E 24º 0' 0'' E 25º 0' 0'' E 26º 0' 0'' E 27º 0' 0'' E 28º 0' 0'' E 10º0'0''W 10º0'0''E 20º0'0''E 30º0'0''E 58º 0' 0'' N 57º 0' 0'' N 56º 0' 0'' N Coordinate system: LKS–92. Projection: Transverse Mercator (TM–1993). Axis meridian: 24 00 00 E. Reference points of coordinate system: from the meridian of the axis: 500 000 m, from the equator: 0 m. Scale factor: 0.999600. Reference ellipsoid: GRS–1980. Thematic layers from the GIS database were used as the basis for the map 'ĢIS Latvija' (SIA Envirotech ©2008). 60º 0' 0'' N 55º 0' 0'' N 50º 0' 0'' N 45º 0' 0'' N 40º 0' 0'' N 35º 0' 0'' N 58º 0' 0'' N 57º 0' 0'' N 56º 0' 0'' N 24º 0' 0'' E 25º 0' 0'' E 26º 0' 0'' E 27º 0' 0'' E 28º 0' 0'' E 0 15 30 60 90 km N O E S
Europe
Nirzas Lake Ežezers Lake Lejas
Lake Rāznas Lake Sventes
Lake Drīdzis
Lake Stirnu Belarus Lake Riču Lake Alūksnes Gulf of Riga
Oreha and Škute
Table 1. Characteristics of the Latvian lakes where European vendace were collected, acclimatization actions, and sampling dates: A, area in km2; Depht, average depth (max depth in m); Date, sampling date; 1 Andrušaitis (1960); 2 Kotov et al. (1958); 3 Burmakin (1963); 4 mention of vendace release but no specific data found.
Tabla 1. Los datos relativos al muestreo y las actuaciones de aclimatación, las principales características de los lagos de Letonia en los que se encontró el corégono blanco: A, área en km2; Depht, profundidad media (profundidad máxima en m) ; Date, fecha de muestreo; 1 Andrušaitis (1960); 2 Kotov et al. (1958); 3 Burmakin (1963); 4 mención de la liberación de corégono blanco pero no se encontraron datos específicos.
Action of acclimatization,
Lake Location Water drainage A Depht release dates and material Date N
Lejas 56º 10' N Daugava River 1.77 8.2 19482 (vendace larvae) IX 2012 21 27º 12' E (34)
Riču 55º 41' N Daugava River 12.84 9.7 No data4 VIII 2016 30 26º 43' E (39.7)
Rāznas 56º 19' N Daugava River 57.56 7 1930–19411 (vendace larvae) VIII 2010 7 27º 26' E (17) 19462 (vendace caviar) 19563 (Ladoga ripus, ripus and whitefish hybrid larvae) 19571 (Ladoga ripus) 19591 (Ladoga ripus and Peipus whitefish hybrid)
Sventes 55º 51' N Daugava River 7.35 7.8 1930–19411 IX 2012 30 26º 21' E (38) (vendace larvae, caviar)
Drīdzis 55º 58' N Daugava River 7.53 12.8 1930–19411 (vendace larvae) IX 2012 32 27º 17' E (65.1) 19462 (vendace larvae) 1947–19482 (vendace larvae) 1957–19591 (vendace larvae)
Stirnu 55º 55' N Daugava River 1.48 7.7 1930–19411 (vendace larvae) VI 2012 32 27º 23' E (25.8) 19462 (vendace larvae) 19482 (vendace larvae) 19591(vendace larvae)
Alūksnes 57º 27' N Daugava River 15.44 7.1 19553 (Ladoga ripus IX 2007 32 27º 50' E (20) and Peipus whitefish hybrid) 19581 (Ladoga ripus) 19591 (Peipus vendace larvae)
Ežezers 56º 10' N Daugava River 9.88 6.4 No precise data4 IX 2008 32 27º 36' E (21)
Nirzas 56º 23' N Velikaya River 5.52 8.2 No precise data4 XI 2007 13 27º 54' E (32)
2006). The P–values for the pairwise FST values were corrected for multiple comparisons using the Bonferroni correction (BFC) following Rice (1989). In addition, we estimated structuring of the studied European vendace populations with the principal component analysis (PCA) using GeneAlex 6.41 software (Peakall and Smouse, 2006). The computer program Bottleneck 1.2.02 (Cornuet and Luikart, 1997) was used to detect bottleneck effects on studied populations.
Results
Quality control of genotypic data
Microsatellite genotypic data had no typographic errors or large allele dropout. However, heterozygosity deficits due to the presence of null alleles were detected at 6 loci. Heterozygosity deficits were indicated at Cisco106 for samples from Lakes Lejas, Riču, Rāznas, Sventes,
164
Table 2. Main characteristics of the eight microsatellite loci used for the analysis of European vendace Coregonus albula populations: TA, annealing temperature; NA, observed number of alleles in each locus; FST, the global differentiation per locus; N, mean number of migrants; Ho, observed heterozygosity; He, expected heterozygosity.
Tabla 2. Principales características de los ocho loci de microsatélites utilizados para analizar las poblaciones de Coregonus albula: TA, temperatura de hibridación; NA, número observado de alelos en cada locus; FST, diferenciación general por locus; N, promedio de migrantes; Ho, heterocigosidad observada; He, heterocigosidad esperada.
Locus Size range (bp) TA (ºC) NA FST N Ho He
Cisco200 174–340 58 44 0.051 4.609 0.764 0.900
Cisco126 201–209 58 5 0.197 1.020 0.443 0.529
Cisco157 101–165 58 15 0.116 1.906 0.581 0.488
BWF1 203–283 58 23 0.122 1.791 0.530 0.662
Cisco90 108–140 58 15 0.192 1.049 0.763 0.723
Clatet6 184–200 61 9 0.363 0.438 0.249 0.509
Clatet9 165–289 61 24 0.069 3.384 0.820 0.876
Clatet13 214–294 57 19 0.159 1.321 0.772 0.738
Drīdzis, Alūksnes, Ežezers and Nirzas; at Clatet6 for samples from Lakes Lejas, Riču, Drīdzis, Stirnu, and Alūksnes; at Cisco200 for samples from Lakes Riču, Drīdzis, Stirnu and Ežezers; at BWF1 for samples from Lakes Rāznas and Alūksnes; at Clatet9 for samples from Lake Lejas; and at Cisco126 for samples from Lake Stirnu. STRUCTURE analyses were performed with and without those loci showing the most heterozygote deficits across populations (Cisco106, Clatet6, Cisco200). The number of inferred clusters and the population structure were found to differ when comparing results without Cisco106 locus but not without Clatet6 and Cisco200 (fig. 1s and 2s in supplementary material). Therefore, the locus Cisco106 was excluded from further analysis.
Genetic variation
Table 2 and table 1s in supplementary material show details of analysed microsatellite loci. The number of alleles at each microsatellite locus was variable. The greatest number of alleles (44) was found at locus Cisco200. The minimum numbers of alleles, namely five and nine, were found at loci Cisco126 and Clatet6, respectively. Accordingly, the differentiation values (FST) at these loci were the smallest. The number of migrants (Nm value) was < 1 only in locus Clatet6 and > 3 in two loci (Cisco200 and Clatet9). The estimated gene flow was > 1 in five loci (Cisco126, Cisco157, BWF1, Cisco90, Clatet13) (table 2). Ho and He differed at each locus. The largest values were found at loci Cisco200 and Clatet9. The smallest values were detected at loci Clatet6 and Cisco126. A heterozygote deficit was revealed at five out of eight
estimated loci (Cisco200, Cisco126, BWF1, Clatet6, Clatet9) and heterozygote excess at three loci (Cisco157, Cisco90, Clatet13). However, the differences in Ho and He were not significant (p > 0.05).
The standard parameters of genetic variation in studied Latvian populations of European vendace are shown in table 3 and 1s in supplementary material. A total of 154 alleles from among eight microsatellite loci were determined in nine European vendace populations. Allele number in different populations varied from 46 to 84. The mean number of alleles per locus or allelic richness (NRA) varied from 4.24 (Lake Stirnu) to 6.22 (Lake Riču). The mean number of private alleles (NRPA) varied from 0.29 (Lake Stirnu) to 0.87 (Lake Riču). Ho and He varied from 0.507 (Lake Nirzas) to 0.758 (Lake Riču) and from 0.526 (Lake Nirzas) to 0.787 (Lake Riču), respectively. The individual locus tests (for each population) displayed that 20 cases out of 72 had significant deviations of genotype frequencies from Hardy–Weinberg equilibrium (HWE) before and after BFC. Table 4 shows the significant and non–significant deviations of HWE. No significant deviations from HWE were detected in Lake Nirzas only. A significant deviation from HWE was revealed at locus BWF1 for Lake Rāznas and Lake Alūksnes, at loci Cisco200 and Clatet6 for Lake Sventes and Lake Drīdzis, and at three loci for Lake Lejas (Cisco126, Clatet6, Clatet9), Lake Stirnu (Cisco200, Cisco126, Clatet6) and Lake Ežezers (Cisco200, Cisco157, BWF1). Significant deviations from HWE were detected at five out of eight loci in Lake Riču (Cisco200, Cisco157, BWF1, Clatet6, Clatet9). A heterozygote deficit was detected in 17 out of 20 cases of significant HWE deviations. The deficit
Animal Biodiversity and Conservation 45.2 (2022) 165
Table 3. Summary of genetic statistics of the studied European vendace populations: NA, total number of detected alleles; NRA, mean allelic richness; NRPA, private allelic richness; Ho, observed heterozygosity; He, expected heterozygosity.
Tabla 3. Resumen de los parámetros estadísticos genéticos de las poblaciones de corégono blanco estudiadas: NA, número total de alelos detectados; NRA, riqueza alélica media; NRPA, riqueza de alelos privados; Ho, heterocigosidad observada; He, heterocigosidad esperada.
Populations NA NRA NRPA Ho He
Lejas 59 5.14 0.39 0.554 0.700 Riču 84 6.22 0.87 0.758 0.787 Rāznas 46 5.75 0.6 0.536 0.647 Sventes 70 5.43 0.51 0.708 0.735
Drīdzis 78 5.56 0.47 0.615 0.715 Stirnu 53 4.24 0.29 0.532 0.614 Alūksnes 80 5.84 0.81 0.681 0.738 Ežezers 63 4.85 0.41 0.647 0.640 Nirzas 52 4.76 0.58 0.507 0.526
at 12 of these 17 cases was due to the presence of null alleles. Heterozygote excess was detected at three (Lake Riču, Cisco157; Lake Ežezers, Cisco157, BWF1) out of 20 cases of significant HWE deviations.
Population structure and spatial variation
Table 5 shows the pairwise FST estimates of genetic differentiation between the studied European vendace populations of Latvia. The pair Lake Sventes–Lake
Riču displayed the smallest differentiation (0.048, p ≤ 0.001), whereas the pair Lake Nirzas–Lake Alūksnes had the highest FST value (0.159, p ≤ 0.001). Little genetic differentiation was revealed for Lake Drīdzis–Lake Lejas and Lake Alūksnes–Lake Riču pairs (0.051, p ≤ 0.001). For all other pairs, moderate genetic differentiation was shown: the FST values varied from 0.062 to 0.133 (p ≤ 0.001) (table 5). The sequential BFC did not change the significance level (p value) from the pairwise FST comparisons.
Table 4. Significance of departure from Hardy–Weinberg equilibrium after sequential Bonferroni corrections: ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001).
Tabla 4. Significación de la desviación del equilibrio Hardy–Weinberg tras correcciones de Bonferroni sucesivas: ns, no significativa (*P < 0,05; **P < 0,01; ***P < 0,001).
Cisco200 Cisco126 Cisco157 BWF1 Cisco90 Clatet6 Clatet9 Clatet13
Lejas ns ** ns ns ns *** *** ns
Riču * ns *** * ns ** ** ns Rāznas ns ns ns * ns ns ns ns Sventes *** ns ns ns ns ** ns ns
Drīdzis *** ns ns ns ns *** ns ns Stirnu *** * ns ns ns *** ns ns Alūksnes ns ns ns *** ns ns ns ns Ežezers * ns *** ** ns ns ns ns Nirzas ns ns ns ns ns ns ns ns
166 Oreha
Škute
and
Table 5. FST values obtained during the pair comparison of European vendace samples from the studied lakes. The smallest and the highest FST–values are shown in bold; a value lying in the range between 0 and 0.05 indicates little genetic differentiation; a value between 0.05 and indicates 0.15, moderate differentiation; a value between 0.15 and 0.25, high differentiation; and values above 0.25, very high genetic differentiation (Wright, 1978; Hartl and Clark, 2007).
Tabla 5. Valores de FST obtenidos durante la comparación pareada de las muestras de corégono blanco de los lagos estudiados. Los valores mínimos y máximos de FST se muestran en negrita; un valor entre 0 y 0,05 indica poca diferenciación genética; un valor entre 0,05 y 0,15 indica diferenciación moderada; un valor entre 0,15 y 0,25 indica diferenciación elevada, y los valores superiores a 0,25 indican diferenciación genética muy elevada (Wright, 1978; Hartl y Clark, 2007).
Sample Lejas Riču Rāznas Sventes Drīdzis Stirnu Alūksnes Ežezers Nirzas Lejas 0.001 0.010 0.001 0.001 0.001 0.001 0.001 0.001 Riču 0.067 0.001 0.001 0.001 0.001 0.001 0.001 0.001 Rāznas 0.067 0.075 0.001 0.001 0.001 0.001 0.001 0.001 Sventes 0.079 0.048 0.093 0.001 0.001 0.001 0.001 0.001 Drīdzis 0.051 0.062 0.084 0.068 0.001 0.001 0,001 0.001 Stirnu 0.056 0.099 0.109 0.110 0.063 0.001 0.001 0.001 Alūksnes 0.067 0.051 0.103 0.067 0.077 0.117 0.001 0.001 Ežezers 0.115 0.071 0.133 0.115 0.100 0.133 0.080 0.001 Nirzas 0.132 0.119 0.114 0.141 0.129 0.152 0.159 0.107
The PCA plot by population (fig. 2) revealed four groups of populations. The first group combined specimens from Lakes Alūksnes, Sventes, and Riču. Populations from Lakes Stirnu, Lejas, Rāznas, and Drīdzis were included in the second group. Lakes Nirzas and Ežezers formed the third and fourth groups, respectively. Bayesian clustering partitioned populations into three genetic groups (K = 3; fig. 1s in supplementary material), placing lakes Alūksnes, Sventes and Riču in the first group; Lakes Stirnu, Lejas and Drīdzis in the second group; and Lakes Nirzas and Ežezers in the third group (instead of separated as in the PCA; fig. 2). Importantly, the European vendace population from Lake Rāznas showed a clear admixture of genotypes, and thus its genetic affiliation was unclear (fig. 3).
The NJ tree branched out into three main groups (fig. 4). Populations from Lakes Nirzas and Ežezers were separated as a single group as in Bayesian clustering (fig. 3). Those from Lake Rāznas branched out into a separate group. The populations from the remaining six lakes made up a third group, which had two main branches that separated lakes into two groups: Lakes Drīdzis, Lejas and Stirnu in the first group, and Lakes Riču, Sventes and Alūksnes in the second group.
Discussion
Translocation of freshwater fish of economic importance between rivers and lakes is a common procedure
in many countries. However, such transfer could represent a serious threat if the recipient river or lake has an indigenous or even endemic population. Mixing of gene pools may reduce fitness due to out–breeding depression and loss of local adaptations (Avise and Hamrick 1996). On the other hand, translocations are also widely used in conservation efforts (Rummel et al., 2016; Resende et al., 2020), and in such cases, genetic identification is of crucial importance both for the source populations and for the receiving community (Præbel et al., 2013a). Microsatellites are successfully used for genetic studies of different Coregonus species, as well as for the monitoring, protection and management of these species (Huuskonen et al., 2004; Schulz et al., 2006; Præbel et al., 2013a; Dierking et al., 2014; Delling and Palm, 2019). Here, genetic diversity and possible divergence in European vendace populations from nine Latvian lakes were analysed to determine the genetic diversity and differentiation of the investigated populations. The Latvian lake populations of European vendace showed a high level of He. Some loci showed a deficit of heterozygotes whereas others showed an excess (table 2). At the same time, the Ho and He in total at all loci in each of the lakes showed no significant difference (table 3). Disequilibrium may be caused mainly by the presence of null alleles. The use of microsatellite primers developed from a related species can result in non–amplification in the target species ( 'null' alleles; Rogers et al., 2007; Teterina et al., 2007), and the actual heterozygosity level may be underestimated in
Animal Biodiversity and Conservation 45.2 (2022) 167
%)
%)
Fig. 2. Principal component analysis (PCA) plot of the genetic structuring among the nine European vendace populations. PC1 and PC2 explain 31.06 % and 23.69 % of the total variation, respectively.
Fig. 2. Gráfico del análisis de componentes principales (PCA) de la estructura genética entre las nueve poblaciones de corégono blanco. PC1 y PC2 explican el 31,06 % y el 23,69 % de la variación total, respectivamente.
the studied populations (Ramstad, 2006). In our study, the level of heterozygosity was quite high, though the nine used microsatellite primers were developed for Coregonus artedi, Coregonus nasus and Coregonus lavaretus species (Patton et al., 1997; Turgeon et al., 1999; Winkler and Weiss, 2008). Similar quite high heterozygosity levels have been reported in whitefish populations (0.485–0.553, Fopp–Bajat et al., 2015; 0.433–0.455, Præbel et al., 2021). Relatively high levels of heterozygosity could also be recovered long after translocation (at least 16 years; Præbel et al., 2021) or may point to the existence of genetic drift effects rather than the loss of alleles in translocated populations.
A relatively large and stable indigenous population
tends to have a greater level of genetic variability than a transferred or stocked population (Huuskonen et al., 2004; Præbel et al., 2013a; Adams et al., 2016; Præbel et al., 2021). In the present study, the largest allele count was revealed at six loci for Lake Alūksnes and Lake Riču vendace populations and at three loci for Lake Drīdzis. The mean allelic richness and private allelic richness was higher in the European vendace population of Lake Riču than in any other populations studied, likely indicating that Lake Riču has an indigenous European vendace population (table 3). This assumption may be indirectly confirmed by the fact that data about any European vendace translocation in Lake Riču are lacking. A similar study on whitefish
Fig. 3. Bayesian clustering of all individuals using STRUCTURE (Hubisz et al., 2009).
Fig. 3. Agrupamiento bayesiano de todos los individuos utilizando STRUCTURE (Hubisz et al., 2009).
168 Oreha and Škute
Stirnu Drīdzis Lejas Sventes Riču Alūksnes Ežezers Nirzas Rāznas PC1 (31.06
PC2 (23.69
Lejas Riču Rāznas Sventes Drīdzis Stirnu Alūksnes Ežezers Nirzas 1.00 0.80 0.60 0.40 0.20 0.00
82 83 66 66 91 0.1
Drīdzis Lejas Riču Sventes Alūksnes Ežezers Nirzas
Stirnu
Fig. 4. Genetic differentiation of nine European vendace samples from Latvian lakes as revealed by a neighbour–joining tree using Nei et al. (1983) genetic distance.
Fig. 4. Diferenciación genética de las nueve muestras de corégono blanco procedentes de lagos de Letonia obtenida con un árbol elaborado mediante el método de unión de vecinos utilizando la distancia genética de Nei et al. (1983).
in Poland also recorded a higher number of private alleles in one of the studied populations, which was also assumed to have an indigenous population (Fopp–Bajat et al., 2015). A smaller private allelic richness was revealed in the European vendace population in Lake Alūksnes, but in this case, there are historical data on three cases of vendace and ripus release (table 1). A local population with either an indigenous gene pool or a mixture of indigenous and translocated gene pools could explain this pattern. The transferred fish could be genetically identical to the indigenous gene pool or introduce favourable traits for adaptation and survival. In addition, recent research pointed out that in translocated populations, new alleles can arise de novo, creating novel genotypes (Præbel et al., 2021). Therefore, the European vendace population of Lake Alūsknes most likely has an overall genetic mixture that fits within the general indigenous genofond. In comparison with recent European vendace population studies based on similar microsatellite loci (Delling and Palm, 2019, Præbel et al., 2021), the count of alleles in European vendace populations in Latvian lakes is not low, and it is at a level that is typical for both native and translocated populations.
The PCA plot, Bayesian clustering and NJ tree showed that individuals could be partitioned into three distinct genetic groups. All three methods grouped the populations of Lakes Riču, Alūksnes and Sventes together (fig. 2–4). As shown in table 1, translocation actions were conducted in lakes Alūksnes and Sventes at different times. It is known that vendace larvae from Lake Peipus were released in Lake Alūksnes whereas the origin of the material released in Lake Sventes is unknown (data were not provided in the
original publication; Andrušaitis, 1960). Therefore, it is possible that different gene pools were released in these two lakes. At the present time, it is most likely that populations in Lakes Alūksnes and Sventes have a mixture of indigenous and translocated gene pools, with most of the population having an indigenous gene pool similar to that of the Lake Riču population. Furthermore, similar moderate FST values were also reported for native whitefish populations in Scotland (Adams et al., 2016).
The second group combined populations from Lakes Drīdzis, Lejas and Stirnu. These lakes are located close to each other (the distances between them vary from 7 to 15 km) and they are connected by canals and small rivers. There are also data about European vendace translocations into these lakes (table 1). Moreover, these lakes have a smaller private allelic richness than that in Lakes Riču and Alūksnes. Lake Drīdzis was used as a source of material for vendace propagation between 1946 and1959 (Andrušaitis, 1960). Altogether, our results indicate that the populations from Lakes Drīdzis, Lejas and Stirnu have similar gene pools, formed as the result of mixing indigenous and translocated gene pools and possible random migrations. There is ample literature reporting small pairwise FST values between recently diverged populations. For example, FST = 0.011 was reported between two European vendace populations after recent invasion in water bodies in Finland (Præbel et al., 2013a). A low level of genetic difference (FST = 0.021) was also reported between two lakes in Finland after European vendace stock transfers (Huuskonen et al., 2004). The level of genetic difference between donor populations and
Animal Biodiversity and Conservation 45.2 (2022) 169
Rāznas
two translocated Coregonus lavaretus populations was also low (FST = 0.014 and 0.027; Præbel et al., 2021). Finally, very similar values were reported between whitefish populations from different localities within the same lake in Scotland (FST = 0.001–0.024; Adams et al., 2016). In the present study, pairwise FST values also show a moderate but somewhat higher genetic differentiation, most likely a result of translocation and mixing with the indigenous gene pool.
The third genetic group included populations from Lakes Nirzas and Ežezers. Although these lakes have witnessed similar historical translocations, their populations show a moderate to high level of genetic differentiation compared to all other investigated populations as well as one from each other. Such high FST levels are typical among native postglacial coregonid populations in northern Fenoscandia (Østbye et al., 2006; Saisa et al., 2008; Præbel et al., 2013a, 2013b). Thus, it can be considered that European vendace populations of Lake Nirzas and Lake Ežezers have indigenous gene pools.
The European vendace population from Lake Rāznas showed unclear genetic affiliation to any of the above–mentioned genetic groups as the three clustering methods rendered different results. One potential explanation for this pattern is that once translocated to Lake Rāznas, the introduced gene pool diverged enough to become quite distinct from the indigenous gene pool. However, it is most likely that the small sample size of the population from Lake Rāznas could explain the observed inconsistencies. Unfortunately, our data on past translocations are incomplete. As a result, we were unable to analyse historical samples of European vendace that could help conclude whether or not the genetic differentiation of the nine vendace populations analysed is the result of translocations alone. Other studies have rendered mixed results, as some show that transferred fish may have a minor effect on the indigenous European vendace population (Lake Höytiäinen in Finland; Huuskonen et al., 2004), whereas others show that the translocated gene pool can often displace native species, subspecies or populations. For example, the native whitefish (C. holsatus) of Lake Schaal in Germany disappeared from the lake, and the specimens currently found in Lake Schaal and in three adjacent lakes are identified as C. maraenoides introduced from Lake Peipsi (Mehner et al., 2018). Thus, we can only suppose that the vendace populations in the investigated Latvian lakes are a mix of several populations and may not therefore be fully indigenous. As the genetic differentiation among studied population shows quite a high value for local populations and migration is possibility limited, we consider that we have ecologically distinct populations in the studied lakes. This assumption can be supported by morphometric trait studies in European vendace populations in Latvian lakes, which showed that while morphological properties did not exceed the limits of variability, in almost every Latvian reservoir, due to their plastic (morphometric) variability, European vendace created a local form depending on the certain environmental conditions (Oreha, 2016).
The results of our study suggest each studied local population be considered a different management unit and may contribute to the development of an optimal strategy for their effective conservation and management, taking into account the peculiarity of each separate European vendace population. The European vendace populations with the highest genetic variability could serve as a potential genetic resource to enhance populations of the species in all Latvian lakes in the future.
Conclusions
The level of genetic variability differs among the populations studied. Such differences may be caused by translocations or a genetic drift, which influence the allele frequencies in different ways, and could be driven by environmental factors. Our present results could be useful in the design and monitoring of conservation programs of vendace populations in Latvian lakes.
Acknowledgements
The material was collected thanks to the help provided by specialists from the Latvian Fish Resources Agency.
References
Adams, C. E., Bean, C. W., Dodd, J. A., Down, A., Etheridge, E. C., Gowans, A. R. D., Hooker, O., Knudsen, R., Lyle, A. A., Winfield, I. J., Præbel, K., 2016. Inter and intra–population phenotypic and genotypic structuring in the European whitefish Coregonus lavaretus, a rare freshwater fish in Scotland. Journal of Fish Biology, 88: 580–594, Doi: 10.1111/jfb.12855
Aleksejevs, Ē., 2015. Latvian Lakes fishes. In: Latvian Fisheries Yearbook: 59–67 (N. Riekstiņš, Ed.). The Latvian Rural Advisory and Training Centre, Riga. [in Latvian].
– 2019. Latvian Lakes and reserviors fishes. In: Latvian Fisheries Yearbook: 60 (N. Riekstiņš, Ed.). The Latvian Rural Advisory and Training Centre, Riga. [in Latvian].
Aleksejevs, Ē., Birzaks, J., 2012. The current status of Coregonidae in the lakes of Latvia. Acta Biologica Universitatis Daugavpiliensis, 3: 3–13.
Aljanabi, S. M., Martinez, I., 1997. Universal and rapid salt extraction of high quality genomic DNA for PCR based techniques. Nucleic Acids Research, 25(22): 4692–4693, Doi: 10.1093/nar/25.22.4692
Andrušaitis, G., 1960. Zivju savairošana un aklimatizācija Latvijā (Fish propagation and acclimatization in Latvia). Latvijas PSR iekšējo ūdeņu zivsaimniecība, XVI: 41–70. [in Latvian].
Avise, J. C., Hamrick, J. L., 1996. Conservation genetics: case histories from nature. Chapman and Hall, New York.
Borovikova, E. A., Aleekseva, Y. I., Schreider, M. J.,
170 Oreha and Škute
Artamonova, V. S., Makhrov, A. A., 2013. Morphology and genetics of the ciscoes (Actinopterygii: Salmoniformes: Salmonidae: Coregoninae: Coregonus) from the Solovetsky Archipelago (White Sea) as a key to determination of the taxonomic position of ciscoes in northeastern Europe. Acta Ichthyologica et Piscatoria, 43: 183–194, http://www.ingentaconnect.com/content/wnars/ aiep/2013/00000043/00000003/art00002
Borovikova, E., Makhrov, A., 2012. Study of Coregonus Populations in the Zone of Intergradation between the Vendace and Least Cisco: the Role of the Environment in Speciation. Principles of the Ecology, 4: 5–20, Doi: 10.15393/j1.art.2012.1761
Burmakin, E. V., 1963. Acclimatization of freshwater fishes in USSR. The Articles of the State Scientific Research Institute of the Lake and River Fishery, LIII: 314. [in Russian].
Cornuet, J. M., Luikart, G., 1997. Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics, 144(4): 2001–2014, Doi: 10.1093/genetics/144.4.2001
Czerniejewski, P., Rybczyk, A., 2008. Variations in age and length growth rates of vendace, Coregonus albula (L.), from selected lakes in Western Pomerania. Archives of Polish Fisheries, 16(1): 63–74, Doi: 10.2478/s10086-008-0005-5
Delling, B., Palm, S., 2019. Evolution and disappearance of sympatric Coregonus albula in a changing environment – A case study of the only remaining population pair in Sweden. Ecology and Evolution, 9(22): 12727–12753, Doi: 10.1002/ece3.5745
Dierking, J., Phelps, L., Præbel, K., Ramm, G., Prigge, E., Borcherding, J., Brunke, M., Eizaguirre, C., 2014. Anthropogenic hybridization between endangered migratory and commercially harvested stationary whitefish taxa (Coregonus spp.). Evolutionary Applications, 7(9): 1068–1083, Doi: 10.1111/eva.12166
Earl, D. A., vonHoldt, B. M., 2012. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genetics Resources, 4: 359–361, Doi: 10.1007/s12686-011-9548-7
Evanno, G., Regnaut, S., Goudet, J., 2005. Detecting the number of clusters of individuals using the software structure: A simulation study. Molecular Ecology, 14: 2611–2620, Doi: 10.1111/j.1365294X.2005.02553.x
Fopp–Bayat, D., Kaczmarczyk, D., Szczepkowski, M., 2015. Genetic characteristics of Polish whitefish (Coregonus lavaretus maraena) broodstocks – recommendations for the conservation management. Czech Journal of Animal Science, 60(4): 171–177, Doi: 10.17221/8131-CJAS
Hartl, D. L., Clark, A. G., 2007. Principles of Population Genetics. Écoscience, 14(4): 544–545, Doi: 10.2980/1195-6860(2007)14[544b:POPG]2.0.CO;2
Heikinheimo, O., Mikkola, J., 2004. Effect of selective gill–net fishing on the length distribution of European whitefish (Coregonus lavaretus) in the Gulf of Finland. Annales Zoologici Fennici, 41: 357–366.
Hubisz, M. J., Falush, D., Stephens, M., Pritchard, A. J., 2009. Inferring weak population structure with the assistance of sample group information. Molecular Ecology Resources, 9(5): 1322–1332, Doi: 10.1111/j.1755-0998.2009.02591.x
Huuskonen, H., Haakana, H., Aho, T., 2004. Stock transfer in vendace: an evaluation using microsatellite markers. Annales Zoologici Fennici, 41: 69–74.
Kalinowski, S. T., 2005. HP–RARE: a computer program for performing rarefaction on measures of allelic richness. Molecular Ecology Notes, 5: 187–189, Doi: 10.1111/j.1471-8286.2004.00845.x
Kaupinis, A., Paulauskas A., Bukelskis, E., 2004. Isoenzyme Systems and Genotypes in Vendace (Coregonus albula (Linnaeus, 1758)) from Lithuanian Lakes. Acta Zoologica Lituanica, 14(4): 3–13, Doi: 10.1080/13921657.2004.10512594
Kottelat, M., Bogutskaya, N. G., Freyhof, J., 2005. On the migratory Black Sea lamprey and the nomenclature of the ludoga, Peipsi and ripus whitefishes (Agnatha: Petromyzontidae; Teleostei: Coregonidae). Zoosystematica Rossica, 14: 181–186.
Kotov, N. D., Nikanorova, E. A., Nikanorov, Y. I., 1958. Fishery Study of the Lakes of the Latvian SSR. Proceedings of the Biology Institute of the Latvian SSR Academy of Science, VII: 259–292. [In Russian]
Langella, O., 2005. Populations 1.2.32: Population genetic software (individuals or populations distances, phylogenetic trees). Accessible online at: http://bioinformatics.org/~tryphon/populations/ Mansfelds, V., 1936. Latvijas zivis (Fishes of Latvia). Latvijas zeme, daba un tauta, Rīga, II: 490–519. [In Latvian].
Mehner, T, Pohlmann, K., Bittner, D., Freyhof, J., 2018. Testing the devil’s impact on southern Baltic and North Sea basins whitefish (Coregonus spp.) diversity. BMC Evolutionary Biology, 18: 208, Doi: 10.1186/s12862-018-1339-2
Mehner, T., Pohlmann, K., Elkin, C., Monaghan, M. T., Nitz, B., Freyhof, J., 2010. Genetic population structure of sympatric and allopatric populations of Baltic ciscoes (Coregonus albula complex, Teleostei, Coregonidae). BMC Evolutionary Biology, 10: 85, Doi: 10.1186/1471-2148-10-85
Mosevich, N. A., Kumsare, A. Y., 1955. Modern Fishing Significance of the Latvian SSR Lakes and the Perspective of their Further Fishing Use. Riga: Fishery in the Inland Waters of the Latvian SSR, I: 5–15. [in Russian].
Nei, M., Tajima, F., Tateno, Y., 1983. Accuracy of estimated phylogenetic trees from molecular data. II. Gene frequency data. Journal of Molecular Evolution, 19: 153–170, Doi: 10.1007/BF02300753
Nikanorov, Y. I., Nikanorova, E. A., 1956. Artificial Breeding of Vendace in Latvian SSR. Scientific and Technical Bulletin of VNIORH (All–union Scientific Research Institute of the Lake and River Fishery), 3–4: 58–61. [In Russian].
Oreha, J., 2016. The genetic structure and morphological variability of vendace (Coregonus albula (L.)) populations in Latvian lakes. PhD thesis, Daugavpils University.
Animal Biodiversity and Conservation 45.2 (2022) 171
Oreha, J., Škute, N., 2009. Morphological characteristics of local populations of european vendace Coregonus albula (L.) in some lakes of Latvia during 50 years. Proceedings of the Latvian Academy of sciences, section B, 63(6)(665): 271–278, Doi: 10.2478/v10046-010-0003-z
Østbye, K., Amundsen, P. – A., Bernatchez, L., Klemetsen, A., Knudsen, R., Kristoffersen, R., Næsje, T. F., Hindar, K., 2006. Parallel evolution of ecomorphological traits in the European whitefish Coregonus lavaretus (L.) species complex during postglacial times. Molecular Ecology, 15: 3983–4001, Doi: 10.1111/j.1365-294X.2006.03062.x
Page, R. D., 1996. TreeView: an application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences, 12(4): 357–358, Doi: 10.1093/bioinformatics/12.4.357
Patton, J. C., Gallaway, B. J., Fechhelm, R. G., Cronin, M. A., 1997. Genetic variation of microsatellite and mitochondrial DNA markers in broad whitefish (Coregonus nasus) in the Colville and Sagavanirkrtok Rivers in northern Alaska. The Canadian Journal of Fisheries and Aquatic Sciences, 45: 1584–1556, Doi: 10.1139/f97-062
Peakall, R., Smouse, P. E., 2006. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes, 6(1): 288–295, Doi: 10.1111/j.14718286.2005.01155.x
Plikšs, M., Aleksejevs, Ē., 1998. Zivis (Fish) Gandrs, Rīga. [In Latvian].
Præbel, K., Bean, C. W., Dodd, J. A., Etheridge, E. C., Gowans, A. R. D., Knudsen, R., Lyle, A. A., Maitland, P. S., Winfield, I. J., Adams, C. E., 2021. Allelic losses and gains during translocations of a high conservation value fish, Coregonus lavaretus. Aquatic Conservation: Marine and Freshwater Ecosystems, 31(9): 2575–2585, Doi: 10.1002/aqc.3623
Præbel, K., Gjelland, K. Ø., Salonen, E., Amundsen, P.–A., 2013a. Invasion genetics of vendace (Coregonus albula (L.)) in the Inari–Pasvik watercourse: Revealing the origin and expansion pattern of a rapid colonization event. Ecology and Evolution, 3(5): 1400–1412, Doi: 10.1002/ece3.552
Præbel, K., Westgaard, J.–I., Amundsen, P.–A., Siwertsson, A., Knudsen, R., Kahilainen, K., Fevolden, S.–E., 2013b. A diagnostic tool for efficient analysis of population structure, hybridization and conservation status of European whitefish (Coregonus lavaretus (L.)) and vendace (C. albula (L.)). Advances in Limnology, 64: 247–255, Doi: 10.1127/1612-166X/2013/0064-0026
Ramstad, K., 2006. Colonization and local adaptation of sockeye salmon (Oncorhynchus nerka) in Lake Clark, Alaska. PhD thesis, the University of Montana.
Resende, P. S., Viana–Junior, A. B., Young, R. J., de Azevedo, C. S., 2020. A global review of animal translocation programs. Animal Biodiversity and Conservation, 43(2): 221–232, Doi: 10.32800/ abc.2020.43.0221
Rice, W. R., 1989. Analysing tabeles of statistical tests. Evolution, 43: 223–225, Doi: 10.1111/j.1558-
5646.1989.tb04220.x
Rogers, S. M., Isabel, N., Bernatchez, L., 2007. Linkage Maps of the dwarf and Normal Lake Whitefish (Coregonus clupeaformis) Species Complex and Their Hybrids Reveal the Genetic Architecture of Population Divergence. Genetics, 175: 375–398, Doi: 10.1534/genetics.106.061457
Rummel, L., Martínez–Abraín, A., Mayol, J., Ruiz–Olmo, J., Mañas, F., Jiménez, J., Gómez, J. A., Oro, D., 2016. Use of wild–caught individuals as a key factor for success in vertebrate translocations. Animal Biodiversity and Conservation, 39(2): 207–219, Doi: 10.32800/abc.2016.39.0207
Säisä, M., Rönn, J., Aho, T., Björklund, M., Pasanen, P., Koljonen, M.–L., 2008. Genetic differentiation among European whitefish ecotypes based on microsatellite data. Hereditas, 145: 69–83, Doi: 10.1111/j.0018-0661.2008.02050.x
Schulz, M., Freyhof, J., SaintLaurent, R., Østbye, K., Mehner, T., Bernatchez, L., 2006. Evidence for independent origin of two springspawning ciscoes (Salmoniformes: Coregonidae) in Germany. Journal of Fish Biology, 68(A): 119–135, Doi: 10.1111/j.0022-1112.2006.01039.x
Sendek, D. S., 2002. Electrophoretic studies of Coregonid fishes from across Russia. Archive of Hydrobiology: Special Issues Advanced Limnology, 57: 35–55.
Sendek, D. S., Ivanov, E. V., Khodulov, V. V., Novoselov, A. P., Matkovsky, A. K., Ljutikov, A. A., 2013. Genetic differentiation of coregonid populations in subarctic areas. Advances in Limnology, 64: 223–246.
Skrzypczak, A., Mamcarz, A., 2006. Occurrence of vendace, Coregonus albula (L.), and its habitats in Northeastern Poland in 1951–1994. Electronic Journal of Polish Agricultural Universities, 9(3): 1–10, http://www.ejpau.media.pl/volume9/issue3/ art-22.html
Škute, N., Oreha, J., 2016. Evaluation of Some Microsatellite Markers Variability in the Study of Genetic Structure of Vendace (Coregonus albula (L.)) Populations from Latvian Lakes. Contemporary Problems of Ecology, 9: 157–165, Doi: 10.1134/ S1995425516020074
Teterina, V. I., Sukhanova, L. V., Kirilchik, S. V., 2007. Microsatellite polymorphism within two Lake Baikal oilfish species (Comephorus lacepede, 1801). Ecological Genetics, 5: 50–57, Doi: 10.17816/ ecogen5250-57
Turgeon, J., Estoup, A., Bernatchez, L., 1999. Species flock in the North American Great Lakes: molecular ecology of Lake Nipigon ciscoes (Teleotei: Coregonidae: Coregonus). Evolution, 53(6): 1857–1871, Doi: 10.1111/j.1558-5646.1999.tb04568.x
Umbrasaite, V., Bukelskis, E., Kaupinis, A., 2012. Phenotypic changes of vendace (Coregonus albula (Linaeus, 1758)) in the lakes of Lithuania. Acta Biologica Universitatis Daugavpilensis, 3: 127–140.
Van Oosterhout, C., Hutchinson, W. F., Wills, D. P. M., Shipley, P., 2004. MICRO–CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Molecular Ecology Notes, 4(3):
172 Oreha
Škute
and
535–538, Doi: 10.1111/j.1471-8286.2004.00684.x
Vuorinen, J., 1984. Electrophoretic expression of genetic variation and duplicate gene activity in vendace, Coregonus albula (Salmonidae). Hereditas, 101(1): 85–96, Doi: 10.1111/j.1601-5223.1984. tb00453.x
Weir, B. S., Cockerham, C. C., 1984. Estimating F–Statistics for the Analysis of Population–Structure. Evolution, 38(6): 1358–1370, Doi: 10.2307/2408641
Winkler, K. A., Weiss, S., 2008. Eighteen new tetranu-
cleotide microsatellite DNA markers for Coregonus lavaretus cloned from an alpine lake population. Molecular Ecology Resources, 8(5): 1055–1058, Doi: 10.1111/j.1755-0998.2008.02153.x
Wright, S., 1978. Evolution and the Genetics of Population, Variability within and among Natural Populations. University of Chicago Press, Chicago. Yeh, F. C., Yang, R. C., Boyle, T., 1999. POPGENE version 1.31. Microsoft Windows–based Freeware for Population Genetics Software.
Animal Biodiversity and Conservation 45.2 (2022) 173
42 Muñoz and Farfán
Living under the risk of extinction: population status and conservation needs assessment of
tiger gecko in Vietnam
H. N. Ngo, H. Q. Nguyen, H. M. Tran, T. Q. Phan, T. T. Tran, L. R. Gewiss, D. Rödder, T. Q. Nguyen, T. Ziegler
Ngo, H. N., Nguyen, H. Q., Tran, H. M., Phan, T. Q., Tran, T. T., Gewiss, L. R., Rödder, D., Nguyen, T. Q., Ziegler, T., 2022. Living under the risk of extinction: population status and conservation needs assessment of a micro–endemic tiger gecko in Vietnam. Animal Biodiversity and Conservation, 45.2: 175–188, Doi: https://doi. org/10.32800/abc.2022.45.0175
Abstract
Living under the risk of extinction: population status and conservation needs assessment of a micro–endemic tiger gecko in Vietnam Human impact is considered the major threat to the global decline of biodiversity, especially for threatened endemic species in karst ecosystems. Studies assessing a species' demography based on temporal and spatial indicators of population size, density and structure are expected to evaluate the level of impact of threats and are therefore becoming increasingly important for species conservation efforts. Goniurosaurus huuliensis, an endemic species in Vietnam, is one of the most threatened reptiles in the world. This karst–adapted species is classified by the IUCN Red List as Critically Endangered and listed under CITES Appendix II due to habitat loss and over–exploitation for the international pet trade. Here we provide the first evaluation of the population status of G. huuliensis. We applied a 'capture mark–recapture' method to estimate the population size and identify the population density and structure. The total population size was estimated to comprise a maximum of 1,447 individuals in integrated suitable habitats, possibly reaching up to 2,855 individuals exclusively in karst habitats within the total extension of occurrence. This is exceedingly lower than the threshold for a minimum viable population. Furthermore, G. huuliensis is documented to occur in extremely small mean population densities of only 6.4 indiv./km and 2.5 indiv./km/day along the surveyed transects. Based on the demographic information, the ongoing severe human impact (e.g. wildlife exploitation and limestone quarrying) is driving G. huuliensis to the brink of extinction. In situ conservation measures are therefore urgently required. We recommend that in-situ actions should be increased, and a plan should be developed to establish a species and habitat conservation area for G. huuliensis
Key words: Density, Goniurosaurus huuliensis, Huu Lien Nature Reserve, Invisibility rate, Population size, Karst habitat
Resumen

Vivir bajo la amenaza de la extinción: estado de la población y evaluación de las necesidades de conservación de un gecko leopardo microendémico en Vietnam. La actividad humana se considera una de las principales causas de la disminución mundial de la biodiversidad, en especial de especies endémicas en peligro de extinción en ecosistemas kársticos. Se espera que los estudios realizados para evaluar la demografía de la especie a partir de indicadores temporales y espaciales del tamaño, la densidad y la estructura de la población permitan determinar la gravedad de las amenazas y que, por lo tanto, sean cada vez más importantes para las iniciativas de conservación de la especie. Goniurosaurus huuliensis, una especie endémica de Vietnam, es una de las especies de reptil más amenazadas del mundo. Esta especie adaptada al karst se considera en peligro crítico en la Lista Roja de Especies Amenazadas de la Unión Internacional para la Conservación de la Naturaleza, y se recoge en el Apéndice II de la Convención sobre el Comercio Internacional de Especies Amenazadas de Fauna y Flora Silvestres (CITES) debido a la pérdida de hábitat y a la sobreexplotación a la que se ve sometida por el comercio internacional de mascotas. En este artículo presentamos la primera evaluación de la situación demográfica de G. huuliensis. Se utilizó un sistema de captura, marcaje y recuperación
ISSN: 1578–665 X eISSN: 2014–928 X
© [2022] Copyright belongs to the authors, who license the journal Animal Biodiversity and Conservation to publish the paper under a Creative Commons Attribution 4.0 License
175 Animal Biodiversity
and Conservation 45.2 (2022)
a micro–endemic
de marcas para estimar el tamaño de la población y determinar su densidad y estructura. Como resultado, se estima que el tamaño total de la población de G. huuliensis es de 1.447 individuos como máximo en hábitats adecuados integrados y que puede llegar a 2.855 individuos exclusivamente en hábitat kársticos dentro de toda la extensión de presencia. Estas cifras son muy inferiores al límite que determina la viabilidad de una población. Además, se ha documentado que las poblaciones de G. huuliensis tienen una densidad media extraordinariamente baja, de solo 6,4 individuos/km y de 2,5 individuos/km/día a lo largo de los transectos estudiados. A partir de la información demográfica, los graves efectos constantes de la actividad humana (por ejemplo, la explotación de la flora y fauna silvestres y la excavación de canteras de piedra caliza) empujan a G. huuliensis al borde de la extinción. Por consiguiente, es urgente adoptar medidas de conservación in situ. En el presente estudio recomendamos mejorar la eficacia de las medidas in situ, incluida la elaboración de un plan para establecer un área de conservación de G. huuliensis y del hábitat kárstico.
Palabras clave: Densidad, Goniurosaurus huuliensis, Reserva Natural de Huu Lien, Tasa de invisibilidad, Tamaño de población, Hábitat kárstico
Received: 04 II 22; Conditional acceptance: 30 III 22; Final acceptance: 19 V 22
Hai Ngoc Ngo, Institute of Genome Research, Vietnam Academy of Science and Technology, Hanoi 10072, Vietnam.– Huy Quoc Nguyen, Vietnam National Museum of Nature, Vietnam Academy of Science and Technology, Hanoi, Vietnam.– Hieu Minh Tran, Truong Quang Nguyen, Tien Quang Phan, Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, Hanoi 10072, Vietnam.– Truong Quang Nguyen, Graduate University of Science and Technology, Vietnam Academy of Science and Technology, Hanoi 10072, Vietnam.– Tung Thanh Tran, Vinh Phuc College, Vinh Phuc Province, Vietnam.– Dennis Rödder, Herpetology Section, LIB, Museum Koenig, Bonn, Leibniz Institute for the Analysis of Biodiversity Change, Germany.– Hai Ngoc Ngo, Laurenz R. Gewiss, Thomas Ziegler, Cologne Zoo, Cologne, Germany and Institute of Zoology, University of Cologne, Cologne, Germany.– Laurenz R. Gewiss, Federal Agency for Nature Conservation, Bonn, Germany.
Corresponding authors: H. N. Ngo. E–mail: ngohai2709@gmail.com; T. Q. Nguyen. E–mail: nqt2@yahoo.com
ORCID ID: H. N. Ngo: 0000-0001-5471-965X; T. Q. Nguyen: 0000-0002-6601-0880; D. Rödder: 0000-0002-6108-1639
176 Ngo et al.
Introduction
The ongoing sixth mass extinction of wildlife is driven by severe anthropogenic impacts on a global scale (Gibbons et al., 2000; Ceballos et al., 2015; Marshall et al., 2020). The major factors accounting for 88 % of the decline in global biodiversity are exploitation of wildlife, habitat degradation, fragmentation and loss, and climate change (Monastersky, 2014). Reptiles are especially affected, with approximately 20 % of the total species number being threatened by extinction (Böhm et al., 2013). Marshall et al. (2020) reported the exploitation of 3,943 reptile species for wildlife trade, with more than 80 % of the species not being regulated by a listing in the appendices of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). A total of 540 of these exploited species were assessed as at least Vulnerable by the International Union for Conservation of Nature (IUCN) Red List of Threatened Species. However, most threatened reptile species were assessed following Criteria B and D2 relative to restricted geographic ranges, and very few data are available on population trends, needed for Criteria A and C (Böhm et al., 2013). Despite being globally threatened, these species have rarely been protected by conservation measures due to the lack of comprehensive knowledge concerning population status, ecology and threats. The gap in conservation assessments could bring threatened species to the brink of extinction (Gibbons et al., 2000; Böhm et al., 2013; Marshall et al., 2020).
Accounting for approximately only 10 % (460,000 km2) in Southeast Asia, karst ecosystems are still considered an 'ark' of biodiversity, containing an extraordinarily high level of endemism (Day and Urich, 2000; Clements et al., 2006; Luo et al., 2016; Tolentino et al., 2020). Giving an example of the gekkonid genus Cyrtodactylus, comprising approximately 300 species mostly native to South East Asia, the karstic habitats (which far out–number all others) are not simply refugia but rather a mosaic of unique micro–niches forcing speciation in disjunct outcrops (Clements et al., 2006; Grismer et al., 2020). However, unsustainable human activities (particularly limestone quarrying) are endangering the biodiversity of karst ecosystems in Southeast Asia (Clements et al., 2006; Luo et al., 2016; Grismer et al., 2020).
The Huulien Tiger gecko, Goniurosaurus huuliensis Orlov, Ryabov, Nguyen, Nguyen and Ho 2008, was originally described from the Huu Lien Nature Reserve (NR) in Lang Son Province, northern Vietnam (Orlov et al., 2008; Nguyen et al., 2009; Nguyen, 2011). Being a habitat specialist, this endemic tiger gecko is only found in evergreen forests on karst formations (Orlov et al., 2008). Thus, G. huuliensis is particularly susceptible to the increase of anthropogenic threats that lead to habitat fragmentation and degradation. Additionally, Ngo et al. (2019a, 2019b) documented that a large number of wild tiger geckos have been illegally harvested for both local and international pet trade. Climate change may also have potentially negative impacts on wild populations of G. huuliensis
in the future (Ngo et al., 2021a). Consequently, the species was recently listed as Critically Endangered (CR) in the IUCN Red List (Nguyen, 2018), and included in CITES Appendix II and the Vietnam Government's Decree No. 06/2019/ND–CP (Group IIB) in 2019 (Ngo et al., 2019b). Although the tiger gecko is dramatically affected by severe threats, a comprehensive assessment of population status of the target species is still lacking.
Research on the demography and viability of in–situ populations can quantify the impact level of anthropogenic threats and evaluate the explicit status of endangerment (Beissinger and Westphal, 1998; Coulson et al., 2001; Jones et al., 2017), and is therefore increasingly important for species conservation efforts (Selman and Jones, 2017; Maida et al., 2018). Based on this background, we aimed to provide a detailed description of the current demographic status of G. huuliensis by assessing the population size and density. Integrating results from previous studies on the species' ecological niche, we intended to estimate the global population size of G. huuliensis within the total extent of occurrence, covering suitable habitats in terms of elevation, karst formations and environmental characteristics (climate and vegetation cover). We further evaluated differences in the population structure between age and sex of G. huuliensis. Together with the evaluation of anthropogenic impacts, our main goal was to identify priorities and provide recommendations to improve the efficacy of in–situ conservation measures for the protection of G. huuliensis in the future.
Material and methods
Field surveys
The study site was selected within the known distribution range of G. huuliensis in the Huu Lien NR, Lang Son Province (S1) based on available literature (Orlov et al., 2008; Nguyen et al., 2009) and our direct observations, and in Thai Nguyen Province, northern Vietnam (S2) from interviews with local people (fig. 1). We carried out surveys along five transects (T1 to T5) in Lang Son Province and one transect (T6) in Thai Nguyen Province. Transects were set up along forest paths or patrol trails of forest rangers. These transects ranged from 0.4 to 2.6 km in length and were located at elevations from 176 up to 500 m a.s.l. (table 1). Their surrounding habitat is exclusively covered with the evergreen forest on karst formations, combining dominant characteristics of a high micro–vegetation coverage, high humidity, stable ambient temperature, dry–rock substrates and low height to forest ground (Orlov et al., 2008; Nguyen, 2011; Ngo et al., in press). We also checked rocks on two crop hills in close proximity to the survey transects (T1 and T5) for the presence of tiger geckos.
We conducted surveys during the non–hibernation season of Goniurosaurus in April and August 2019, June 2020 in Lang Son Province, and April 2021 in Thai Nguyen Province. To guarantee the highest
Animal Biodiversity and Conservation 45.2 (2022) 177
detection probability for the nocturnal species (Orlov et al., 2008; Nguyen, 2011) and to limit the bias of observers, excursions were carried out between 8 p.m. and 5 a.m. of the next day. Each survey in each transect was conducted with at least two researchers, together with a ranger and a local person.
Population assessment
Due to its small body size and distribution within remote areas (Orlov et al., 2008; Ngo et al., 2021a), estimating population size of G. huuliensis based on direct records is deemed problematic. Huang et al. (2008) used statistical data from a 'capture mark–recapture' approach, developing the 'invisibility rate' method to estimate the population size. This method has been applied for other lizards in Vietnam, namely Cnemaspis psychedelica, Cyrtodactylus gialaiensis, Goniurosaurus catbaensis, Physignathus cocincinus, and Shinisaurus crocodilurus (van Schingen et al., 2014, 2016; Ngo et al., 2016a, 2019a; Nguyen et al., 2018; Gewiss et al., 2020; Luu et al., 2020). Accordingly, all encountered individuals of G. huuliensis were captured and individually marked with passive integrated transponder (PIT) tags (ISO FDX–B Glastransponder, 1.4 x 9 mm, Germany). In newly captured individuals, a microchip was injected under the skin on the left side of the body behind the shoulder. All captured and recaptured individuals were identified with a transponder–reader (Breeder Reader lC, Planet ID GmbH, Germany) (van Schingen et al., 2014; Ngo et al., 2019b). Each transect was repeatedly surveyed in intervals of at least two days. Coordinates and elevations of all capture localities were recorded using a GPS device (Garmin GPSmap62s, WGS84 datum) and are available on request to the authors. Geckos were captured by hand and subsequently released at the same sites after taking measurements and marking.
The method of Huang et al. (2008) includes the calculation of the invisibility rate using the formula:
i = [S(bn – an)] / a n
where a n is the number of observed individuals along transect n during the first survey and b n is the total number of observed individuals along transect n. The invisibility rate is a compensation for the undetected but present individuals of the population along the surveyed transect. In this study, we calculated an average value of i for all transects and survey periods for each survey site. The average i was employed in the formula to estimate the population size:
Ň = S [m*(1 + (i/x))]
where Ň is the estimated population size within the survey site, m is the total number of observed individuals along the transect including all surveys and x is the number of conducted surveys along the transect (Huang et al., 2008).
However, the estimated population size only refers to the study site and does not encompass the entire
in–situ populations of the species, since it is difficult to detect and survey all occupied habitats within the extent of occurrence (EOO). By definition, the EOO range contains all the known occurrences of a taxon within the shortest continuous imaginary boundary (IUCN, 2019). The use of the species distribution modelling (SDM) method is expected to provide a reliable estimate of the total population size of a taxon within its suitable habitats. This technique is based on the hypothesis that the relationship between species abundance and environmental suitability is highly positive (Weber et al., 2016). In particular, environmental variables, to which a species has historically adapted to define the Grinnellian niche (Grinnell, 1917; Rödder and Engler, 2011), provide highly suitable conditions for larger populations within their optimal range with high birth and survival rates, and vice versa (Araújo et al., 2002; Morrison et al., 2006). Thus far, many organisms have been studied to test the relationship, such as 450 correlations of earthworms, mollusks, insects, reptiles, mammals, fishes, and plants in the research of Weber et al. (2016). Moreover, the projected habitat suitability has already been used to estimate the population size and density of some terrestrial species, such as Panthera onca (Tôrres et al., 2012); Mergus squamatus (Zeng et al., 2015), British mammals (Croft et al. 2017), and Nomascus annamensis in Vietnam (Tran and Vu, 2020).
For the extent of occurrence, we extracted the probability of environmental suitability. This varied from 0.27 to 0.88 in the climate–based model and from 0.08 to 0.90 in the vegetation–based model (fig. 1A, 1B). Values closer to 1 indicate higher suitability (Ngo et al., 2021a). However, the two probability values were not significantly different among survey routes, and they all provided conditions with very high suitability (from 0.66 to 0.90; table 1). A regression analysis was used to evaluate the relationship between species abundance and suitable probability per survey transects (Weber et al., 2016). However, the relationship was not statistically significant (Linear Regression Analysis, P > 0.05). Moreover, only six sample numbers for the statistics may lead to under– or over–fitting. In this study, we only employed the binary values of suitability and unsuitability to evaluate the presence and absence of the species, respectively. Thus, species abundance was considered equal among suitable sites within the extent of occurrence. The population size (ŇS) within the suitable area restricted in the extent of occurrence was estimated based on an equivalent rate:
ŇS / AS = Ň / AF
where AF is the area of the field survey site, covering visited transects in each survey; AS is the area of an overlapped layer of integrated suitable habitats. Given only in the karst layer within the EOO, the value:
Ň max = (Ň x AK) / AF
is considered a maximum that the population size can achieve (where AK is the area of karst habitats),
178 Ngo et al.
Fig. 1. Projected potential distribution of Goniurosaurus huuliensis in northern Vietnam: A, the climate model; B, the vegetation cover model, including histograms of suitable probability within the extent of occurrence (brown columns as unsuitable and blue columns as suitable); C, the suitable elevations within the selected buffer region (indicated by a black circle line); and D, the overlap of these three layers together with the karst layer within the extent of occurrence (marked with blue lines; teal stars, surveyed locations in Lang Son Province; yellow circle, surveyed location in Thai Nguyen Province; violet circles, other known occurrences). Projected data were extracted from the studies of Ngo et al. (2021a, in press).
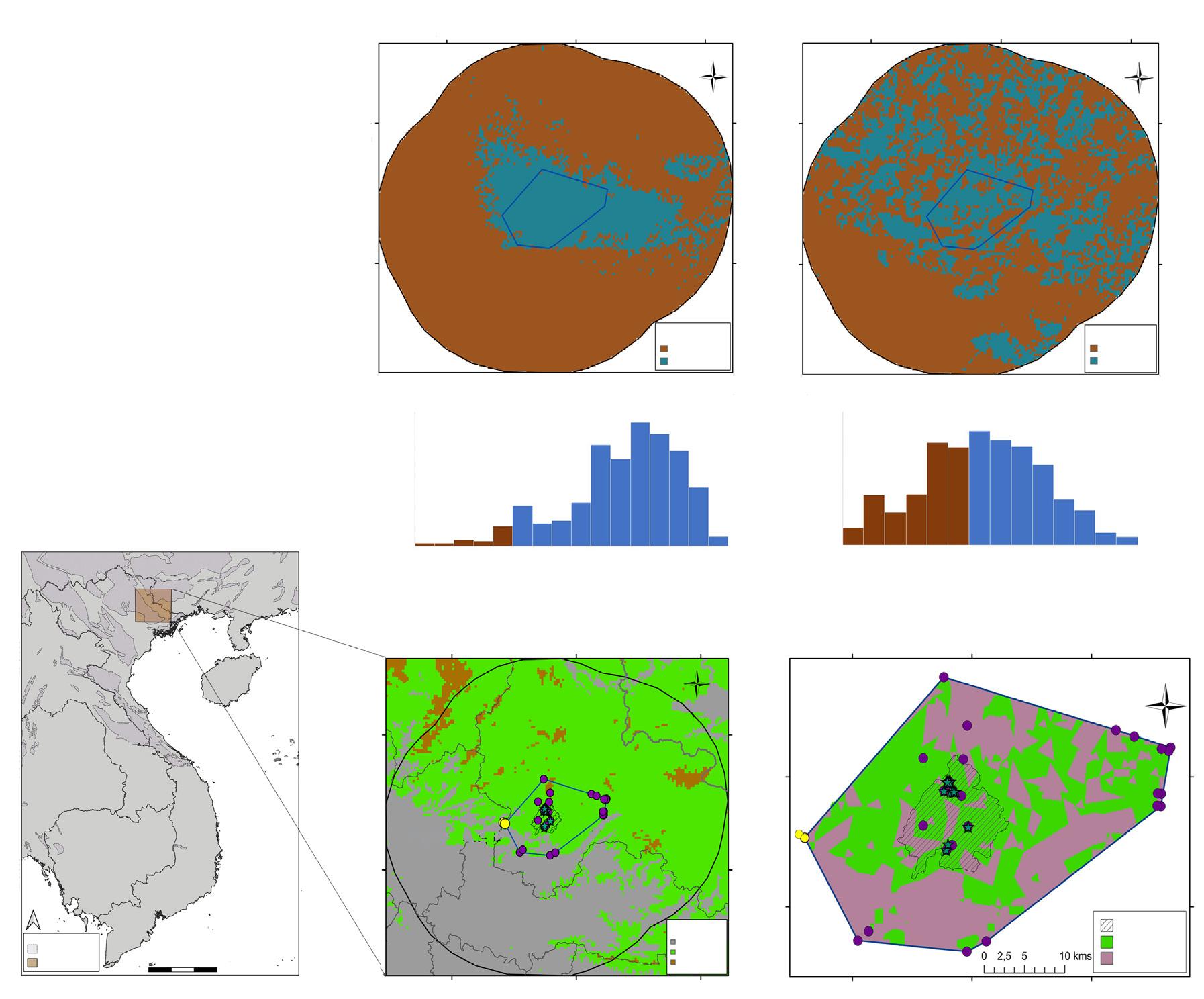
Fig. 1. Posible distribución prevista de Goniurosaurus huuliensis en el norte de Vietnam: A, el modelo climático; B, el modelo de la cobertura vegetal que incluye histogramas de la probabilidad adecuada dentro de la extensión de presencia (columnas marrones como no adecuada y columnas azules como adecuada); C, la elevación adecuada dentro de la región de transición seleccionada (indicada con un círculo negro), y D, la superposición de estas tres capas junto con la capa de karst dentro de la extensión de presencia (cubierta por líneas azules; estrellas azules, sitios estudiados en la provincia de Lang Son; círculo amarillo, sitio estudiado en la provincia de Thai Nguyen; círculos violeta, otros sitios con presencia conocida). Los datos previstos se extrajeron de los estudios de Ngo et al. (2021a, en prensa).
because the target species is exclusively found in karst habitats (Orlov et al., 2008; Grismer et al., 2021; Ngo et al., 2021b, in press).
In terms of environmentally suitable habitats, we used the structural data layers of climate and vegetation cover variables from previous species distribution models, projected by Ngo et al. (2021a) (fig. 1A, 1B). The suitability layer was extracted from each binary map (climate and vegetation cover) containing values
above the 10 % training presence Cloglog threshold within the selected buffer area (an area with a radius of 50 km enclosing all occurrence points; fig. 1A, 1B) (Ngo et al., 2021a). The localized elevation layer ranging from 176 m to 500 m a.s.l. (fig. 1C; Ngo et al., in press) and the karst layer from the World Karst Aquifer Map were extracted (downloaded from https://www.whymap.org/whymap/EN/Maps_Data/ Wokam/wokam_node_en.html [Accessed on May
Animal Biodiversity and Conservation 45.2 (2022) 179
Thailand Vietnam Laos China Cambodia Vietnam 106º0'0''E 106º30'0''E 107º0'0''E 106º0'0''E 106º30'0''E 107º0'0''E 21º30'0''N 22º0'0''N 21º30'0''N 22º0'0''N 21º30'0''N 22º0'0''N 21º30'0''N 22º0'0''N Vegetation Unsuitable Suitable Climates Unsuitable Suitable 106º0'0''E 106º30'0''E 107º0'0''E 106º16'0''E 106º24'0''E 106º32'0''E 106º0'0''E 106º30'0''E 107º0'0''E 106º16'0''E 106º24'0''E 106º32'0''E 21º30'0''N 22º0'0''N 21º30'0''N 22º0'0''N 21º36'0''N 21º44'0''N 21º36'0''N 21º44'0''N Elevation 6–176 76-–500 500–1,396 Huulien_NR Suitable Karst habitats 12º0' 16º0' 20º0' Karst regions Study site 104º0' 108º0' 112º0' 12º0' 16º0' 20º0' 104º0' 108º0' 112º0' 0.27, 0.31 0.31, 0.35 0.35, 0.39 0.39, 0.43 0.43, 0.47 0.47, 0.51 0.51, 0.55 0.55, 0.59 0.59, 0.63 0.63, 0.67 0.67, 0.71 0.71, 0.75 0.75, 0.79 0.79, 0.63 0.83, 0.87 0.87, 0.91 0.08, 0.14 0.14, 0.19 0.19, 0.25 0.25, 0.31 0.31, 0.37 0.37, 0.43 0.43, 0.49 0.49, 0.55 0.55, 0.61 0.61, 0.67 0.67, 0.73 0.73, 0.78 0.78, 0.84 0.84, 0.90 180 160 140 120 100 80 60 40 20 0 160 140 120 100 80 60 40 20 0 Frequency Frequency China N N A B C N N Lang Son (S1) Thai Nguyen (S2) Bac Giang Bac Kan 0 100 200 300 km Spatly Islands (Vietnam) Paracel Islands (Vietnam) Hainan Island (China) N 0 2.5 5 10 km
Table. 1. Number of individuals observed together with population densities and estimated population sizes (Ň) of Goniurosaurus huuliensis in Lang Son Province (S1) in April and August 2019, and June 2020, and in the Thai Nguyen Province (S2) in April 2021. Information on transect length, climate suitability, vegetation suitability and level of human impacts are also included. (* missing data).
Tabla. 1. Número de individuos observados, densidad de población y tamaño de la población estimado (Ň) de Goniurosaurus huuliensis en la provincia de Lang Son (S1) en abril y agosto 2019 y junio de 2020, y en la provincia de Thai Nguyen (S2) en abril de 2021, incluida información sobre la longitud de los transectos, la idoneidad climática, la idoneidad de la vegetación y el grado de afectación de la actividad humana. (* datos que faltan).
Transect T1 T2 T3 T4 T5 T6 Total Length (km) 0.9 2.6 1.5 0.4 0.9 1.5 Climate suitability 0.80 0.79 0.87 0.87 0.87 0.66 Vegetation suitability 0.81 0.74 0.83 0.83 0.9 0.77
Human impact level High High Medium Medium High Low April 2019 / Lang Son (S1): survey area 15 km2
Total obs 5 5 12 * * * 22
Density 5.6 1.9 8 * * * 5.2 Density/day 2.8 0.96 2.7 * * * 2.1
Ň 11 11 27 * * * 49
Ň Huu Lien NR (85 km2) 278 ŇS (408 km2) 1,333 Ň max (805 km2) 2,630
August 2019 / Lang Son (S1): survey area 21 km2
Total obs. 1 3 17 4 2 * 27
Density 1.1 1.15 11.3 10 2.2 * 6.5 Density/day 1.1 1.15 2.8 5 2.2 * 3.1 Ň 2 7 38 9 4 * 60
Ň Huu Lien NR (85 km2) 242 ŇS (408 km2) 1,166 Ň max (805 km2) 2,300
June 2020 / Lang Son (S1): survey area 12 km2
Total obs. 1 * 9 * 1 * 11 Density 1.1 * 6 * 1.1 * 2.7 Density/day 1.1 * 1.5 * 1.1 * 1.2 Ň 2 * 16 * 2 * 20
Ň Huu Lien NR (85 km2) 142
ŇS (408 km2) 680
Ň max (805 km2) 1,342
April 2021 / Thai Nguyen (S2): survey area 11 km2
Total obs. * * * * * 14 14 Density * * * * * 11.3 11.3 Density/day * * * * * 3.77 3.77 Ň * * * * * 39 39
ŇS (408 km2) 1,447 Ň max (805 km2) 2,855
180 Ngo et al.
Table. 1. (Cont.)
Transect
T1 T2 T3 T4 T5 T6 Total
Maximum abundance: survey area 32 km2
Total obs. 5 5 17 10 2 14 53 Ň 11 11 38 9 4 39 112
Ň Huu Lien NR (85 km2) 298 ŇS (408 km2) 1,428 Ň max (805 km2) 2,818
2022]; Goldscheider et al., 2020). These layers were geographically restricted within the extent of occurrence and later overlapped to calculate the intersected area of suitable habitats (fig. 1D). We collected the Huu Lien NR’s shape file from the website of https:// www.protectedplanet.net/ [Accessed on June 2019] (fig. 1D). The related maps were generated, and the areas (including all study sites, karst habitats, suitable habitats and the extent of occurrence) and the length of survey transects were calculated using Quantum GIS software (QGIS Version 3.12.0, Development Team. 2020. Available online at http://qgis.osgeo.org [Accessed on 25 March 2020].
Population densities of G. huuliensis were calculated per kilometer (indiv./km) with reference to each surveyed transect, and per day (indiv./km/day). To assess the age structure of the G. huuliensis population, geckos were categorized into two classes based on their snout–vent length (juveniles with SVL < 85 mm and adults with SVL ≥ 85 mm) (Ngo et al., 2021b). The sex of each captured individual was determined based on the presence of large swollen hemipenal bulges and 25–30 precloacal pores in males, and the lack of these characteristics in females (Orlov et al., 2008; Ngo et al., 2021b). To test for significant differences in the age and sex structures between surveyed periods, we used a Chi–square test. For the test, we applied α = 0.05. Statistical analyses were carried out in R v 3.1.2 (Rstudio Team, 2018).
Threat assessment
Day excursions were carried out to obtain evidence of anthropogenic activities within the Huu Lien NR and its surroundings, including impacts to the species habitat and hunting activities. We documented the information through our observations and interviews with local communities. The level of threats was classified for each surveyed transect depending on the frequency, extent and intensity of recorded human impacts (Ngo et al., 2019b). In particular, 'undisturbed', 'low', 'medium' and 'high' were respectively defined as never being observed, rarely observed, several times and frequently recorded in a high intensity (such as a large number of wild–caught individuals or extensive limestone quarrying).
Results
Population status
We observed a total of 74 individuals of G. huuliensis: 60 individuals in S1 (Lang Son Province) and 14 individuals in S2 (Thai Nguyen Province). None of the tiger geckos was observed in the two crop hills. We noted the highest number of individuals in August 2019 (27 indiv.), followed by April 2019 (22 indiv.), April 2021 (14 indiv.) and June 2020 (11 indiv.) (table 1). It is noteworthy that we observed only one juvenile in June 2020 and two other juveniles in April 2021 (fig. 2). We recorded a consistently imbalanced sex ratio among the four survey periods, with the number of observed females accounting for at least 54 % and at most 68 % (Chi–square test; x2 = 0.67, df = 3, P > 0.05; fig. 2).
The average invisibility rates were 1.27 in S1 and 1.8 in S2. The population size of G. huuliensis at the study site fluctuated significantly between survey periods, depending on the number of visited transects. In particular, the population was estimated to consist of a minimum of 20 indiv. along two transects in S1 in June 2020 and a maximum of 60 indiv. along five transects in S1 in August 2019 (table 1). For transect T3, we estimated 16 to 38 indiv., while the remaining transects in S1 had only a maximum estimated number of 11 indiv. (table 1). A total of 39 indiv. was estimated for the population along the transect T6 in S2.
To estimate the total population size of G. huuliensis, we extracted respective layers of the survey sites and calculated their areas according to the survey periods (15 km2 in April 2019; 21 km2 in August 2019; 12 km2 in June 2020 in S1; and 11 km2 in April 2021 in S2), the area of the Huu Lien NR (85 km2) and suitable habitats (408 km2) within the whole extent of occurrence (EOO, 805 km2) (table 1, fig. 1). The extracted karst area is equal to the EOO (table 1). The Huu Lien NR was estimated to harbor 142–289 indiv. (238 ± 67 indiv.). The population size (ŇS) within the suitable habitats was estimated to range from 680 to 1,447 indiv. (1,157 ± 293 indiv.), whereas the maximum population size (ŇMax) in the karst habitats can achieve up to 2,855 individuals (table 1). We further calculated a potential maximum population size based on the maximum number of animals within each
Animal Biodiversity and Conservation 45.2 (2022) 181
Fig. 2. Variations in the age and sex structures of G. huuliensis between survey periods in Lang Son (S1) and Thai Nguyen (S2) provinces, northern Vietnam (individuals observed but not caught were not included herein).
Fig. 2. Variaciones en las estructuras de edad y sexo de G. huuliensis en los períodos de estudio en las provincias de Lang Son (S1) y Thai Nguyen (S2) en el norte de Vietnam (no se incluyeron los individuos observados pero no capturados).
survey transect. Based on a maximum of 53 indiv. observed along all transects in the two study sites (S1 and S2), we estimated the potential total population size of 1,428 indiv. in the suitable habitats and 2,818 indiv. in the karst habitats (including 112 indiv. in the study site and 228 indiv. in the Huu Lien NR) (table 1).
The average population density of G. huuliensis was 6.4 indiv./km (1.1–11.3 indiv./km) along the surveyed transects. In particular, the lowest density was documented at T1 and T5 (1.1 indiv./km), whereas the highest density was recorded at T3 and T6 (11.3 ndiv./km) (table 1). The density per day was further calculated with an overall mean value of 2.5 indiv./km/day (max. 5 indiv./km/day at T4; table 1).
Anthropogenic impacts
We documented that human activities have severely degraded the habitats of G. huuliensis. More specifically, we observed many plastic products scattered on rocky shelters along survey transects (fig. 3A). Many large woody plants had been cut down, reducing forest cover (fig. 3B). Furthermore, evergreen of forests on karst formations have been gradually replaced by cultivated land or grassy hills; fig. 3C), and even completely destroyed so as to expand roads and for limestone quarrying (fig. 3D).
Regarding illegal collection activities, our interviews with local people and rangers revealed that wildlife dealers have paid 150,000–200,000 Vietnam Dong (7–9 USD) per adult of G. huuliensis to local hunters in the past. These geckos were transferred to Lang Son City, Vietnam, from where they were shipped to
other cities in Vietnam. However, according to local hunters, no dealers have contacted them to collect wild tiger geckos in the last two years.
Due to the over–exploitation in the past, the ongoing deforestation, and the low number of documented tiger geckos, the level of human impact in transects T1, T2 and T5 is considered 'high' (table 1). Transects T3 and T4 are close to ranger stations and have not experienced exploitation (T3) or deforestation (T4), which is why the overall impact level is classified as 'medium'. Transect T6 in S2 currently remains undisturbed but as it is not protected, its impact level is considered 'low' rather than 'undisturbed'.
Discussion
Population status
This is the first study to evaluate the population status of the endemic tiger gecko G. huuliensis in northern Vietnam. Basic knowledge of the population status is crucial to establish conservation plans to protect threatened species in their natural distribution range (Huang et al., 2008; IUCN, 2019; Ngo et al., 2019a). Being aware of this importance, a few studies were recently carried out on other lizard species in Vietnam (Ngo et al., 2016a, 2016b, 2019a; Nguyen et al., 2018; Gewiss et al., 2020; Luu et al., 2020). However, these studies only estimated the population size within small representative sites. To overcome this limitation, we conducted large–scale investigations to provide the full extent of occurrence of G. huuliensis (Ngo et
182 Ngo et al.
April 2019 August 2019 June 2020 April 2021 25 30 15 10 5 0 15 (68 %) 12 (60 %) 7 (32 %) 8 (40 %) 6 (67 %) 2 (22 %) 1 (11 %) 2 (16 %) 7 (54 %) 4 (30 %)
Individuals
Males Females Juveniles
Fig. 3. Efectos antrópicos en la zona del estudio: A, restos de plástico; B, tala de madera; C, colina cultivada; D, cantera (fotografías de Hai N. Ngo).
al., 2021a). Combined with the projected results of environmentally suitable habitats (climate and vegetation cover) (Ngo et al., 2021a), inhabited elevation ranges and karst formations (Ngo et al., in press), we were able to estimate the global population size of G. huuliensis. Similar to other tiger gecko species, the population size of G. huuliensis was predicted to be extremely small (Ngo et al., 2016b, 2019a, 2021b). In particular, we estimated that the global population size of G. huuliensis has a potential of 1,447 indiv. within the integrated suitable habitats. The maximum size can achieve up to 2,855 indiv. within the karst habitats in the whole extent of occurrence (table 1). However, the maximum population size seems to be much larger than the actual size since a large proportion of unsuitable habitats (such as crop hills, residential areas, roads) within the karst region were included in the estimate. Meanwhile, the minimum viable population was assessed to require at least 3,000–7,000 indiv. to ensure long–term persistence of a species (Reed et al., 2003; Traill et al., 2007).
The mean density of G. huuliensis (6.4 indiv./km) is slightly higher than that of an endangered relative (G. catbaensis in Cat Ba National Park, Hai Phong City with 1.2 indiv./km). However, it is considerably lower
than the density of G. catbaensis populations on islands in Ha Long Bay, Quang Ninh Province with more than 60 indiv./km (Ngo et al., 2016b, 2019a). Moreover, the population density of G. huuliensis is also significantly lower than that of other globally threatened lizards in Vietnam ( Cnemaspis psychedelica , Physignathus cocincinus) (Ngo et al., 2016a; Nguyen et al., 2018; Gewiss et al., 2020).

According to Ngo et al. (in press), the similar microhabitat selection among age and sex classes of G. huuliensis may be the main cause leading to competitive interactions among conspecifics. The occurrence in low densities likely mitigates the pressure of using shared resources within a restricted habitat (Irschick et al., 2005; Gilad, 2008; van Schingen et al., 2015). This is expected to occur in wild populations of G. huuliensis as well (Ngo et al., in press). Accordingly, we documented a small population density of G. huuliensis per day, with only 2.5 indiv./km/day (1.2–3.77 indiv./km/day, table 1).
Threats
The species has been illegally over–harvested by local hunters in the Huu Lien NR in the past (Ngo et
Animal Biodiversity and Conservation 45.2 (2022) 183
Fig. 3. Anthropogenic impacts in the study area: A, plastic trash; B, timber logging; C, cultivated hill; D, quarrying (Photographed by Hai N. Ngo).
A B
C D
al., 2019a, 2019b). Generally, the species abundance of in–situ populations is expected to be relatively balanced, with a large number of individuals under the same and eminently suitable environmental conditions (Araújo et al., 2002; Morrison et al., 2006). However, in this study, the abundance is uneven among survey transects. In particular, the number of estimated tiger geckos in each transect in the Huu Lien NR is relatively low and lower than that at the undisturbed transect (T6) in Thai Nguyen Province (table 1). We assume that the difference is due to the higher level of human impacts, especially exploitation, at the survey transects in S1 within the Huu Lien NR (Ngo et al., 2019a, 2019b). The decline of the population size due to the main anthropogenic impact of trade has been recorded in another insular Vietnamese tiger gecko, namely Goniurosaurus catbaensis (Ngo et al., 2019b). Long–term population assessments of Shinisaurus crocodilurus confirmed that its wild populations became extremely small in Vietnam and even declined up to 90 % in China due to the main threat of over–harvesting (Huang et al., 2008; van Schingen et al., 2014, 2016). Fetching alarmingly high prices of up to a thousand US dollars, the high commercial revenue provides a great incentive for poaching and excessive trade documented in other tiger gecko species (e.g. G. luii and G. araneus) and an insular Vietnamese gecko (Cnemaspis psychedelica) (Grismer et al., 1999; Stuart et al., 2006; Ngo et al., 2016a, 2019b). Due to the extremely small population size and occurrence in low densities, the exploitation severely imperils the existence of wild populations of G. huuliensis
Additionally, Ngo et al. (2021a) predicted that the potential distribution of G. huuliensis will significantly shrink and even likely vanish under climate change within the next decades. The potential impact of climate change was also documented for other Vietnamese tiger gecko species (G. catbaensis and G. lichtenfelderi) (Le et al., 2017; Ngo et al., 2022). Given the sex structure of viviparous G. huuliensis, the predominance of female individuals could be explained due to the rate of female offspring being more common in the warm temperature gradient (Cunningham et al., 2017). Under climate change, global warming temperatures may increase the current imbalance in the sex structure of in–situ G. huuliensis populations in the future.
Besides serving as preferred habitat and refugia for almost tiger gecko species under impacts of climate change, the karstic ecosystem containing complex topographies and unique micro–environmental conditions is considered the ancestral habitat of Goniurosaurus species (Grismer et al., 2021; Ngo et al., 2021b). Karst formations indeed are identified as the exclusive habitat of G. huuliensis (Ngo et al., in press); as such, its existence depends entirely on the integrity of karstic habitats. Similar to what is happening all over Southeast Asia, as documented by Clemens et al. (2006), the high rate of limestone quarrying is a primary threat to G. huuliensis, which has been extensively recorded in Lang Son and Thai Nguyen provinces, including habitats of the species in Huu Lien NR. As such, the newly undisturbed but
unprotected population of G. huuliensis in Thai Nguyen Province should be classified as at a low level of threat. To meet the local high demand for limestone products (e.g. cement), more cement factories could be built in Lang Son and Thai Nguyen provinces in the future, including the karst habitats of G. huuliensis and directly imperils untouched populations of G. huuliensis. Other human activities (namely logging activities, road construction, and pollution by plastic trash) have been observed within natural habitats of G. huuliensis, leading to forest fragmentation and degradation.
Conservation measures
In view of the ongoing severe human impacts, conservation measures to safeguard wild populations of the target species are urgently required. Recent studies on trade (Ngo et al., 2019b), taxonomy (Ngo et al., 2021b), ecology (Ngo et al., in press), impacts of climate change (Ngo et al., 2021a), and population status (this study) fill the gaps in basic knowledge on the biology of G. huuliensis. On the basis of findings from such research, in–situ and ex–situ conservation actions can be simultaneously implemented more effectively in the future. The target species was recently assessed as Critically Endangered (CR) in the IUCN Red List of Threatened Species (Nguyen, 2018), and was included in CITES Appendix II and the Vietnam Government's Decree No. 06/2019/ND–CP (Group IIB) in 2019 (Ngo et al., 2019b). As a result, any activity concerning domestic and international trade in wild individuals of G. huuliensis for commercial purposes is prohibited. Including the species in the international convention and the local decree may help to mitigate the illegal over–exploitation. Since the regulations came into effect, dealers have not yet contacted local hunters to collect wild animals of G. huuliensis To completely stop illegal trafficking in relation to the target species, we highly recommend implementing community education to enhance awareness of the biodiversity value in the local communities within the Huu Lien NR and surrounding areas. Furthermore, these areas need to be increasingly patrolled by local rangers to improve the effectiveness of forest protection and to stop the illegal activities of exploitation.
Covering a total area of 85 km2, the Huu Lien NR potentially harbors an estimated maximum number of 289 indiv. (accounting for approximately 15 % of the total population size). Based on this result, we strongly support a recommendation of Ngo et al. (2021a) that the Huu Lien NR should be considered as a 'center' for conservation programs to protect wild populations of G. huuliensis. However, approximately 85 % of the total wild individuals of G. huuliensis may be located outside the boundaries of the nature reserve. Without proper law enforcement and forest ranger patrolling in there, a plan to establish a species and karst habitat conservation area within the EOO is an adequate conservation solution to safeguard the wild populations of G. huuliensis. As such, priorities for the local economic development relative to karst formations (limestone quarrying) can be reassessed together with priorities
184 Ngo et al.
of biodiversity protection by relevant authorities. We highly recommend increasing research activities to promote the biodiversity value of karst ecosystems. Recently, many new species have been discovered from the karst habitats in the Indo–Burma biodiversity hotspot region, including tiger gecko species in China and Vietnam (Nguyen et al., 2009; CEPF, 2020; Ngo et al., 2021b). Globally threatened species, such as G. huuliensis should be highlighted as high–profile flagship species of karst ecosystems. The high value of biodiversity should be given with the priority of conservation policies to protect the karst landscape rather than favoring economic development.
Methodological limitations
The 'invisibility rate' method was developed by Huang et al. (2008) and it has been widely used to estimate the population size of lizards (van Schingen et al., 2014, 2016; Nguyen et al., 2018; Ngo et al., 2019a; Gewiss et al., 2020; Luu et al., 2020). Indeed, the advantage of this method is that the invisibility–rate index is calculated based on repeated surveys at one site and can then be employed for congeneric species or at other sites that are surveyed only once (Huang et al., 2008; Ngo et al., 2016b; Gewiss et al., 2020). However, since this method is based on direct records of observed individuals, the detection probability may have a strong influence on the observed and estimated number of individuals. In particular, the detectability of G. huuliensis is assumed to be rather low due to its small size, secretive lifestyle and association with habitats with high vegetation coverage in remote areas. In the present study, for example, based on one survey, the minimum population size was estimated at transect T1 in August 2019 and June 2020 (2 indiv.), while the value was significantly higher (9 indiv.) in April 2019 after the survey was repeated. These differences between surveys at each transect that mainly account for the variation in the total population size of G. huuliensis; a minimum of 1,342 indiv. compared to a maximum of 2,855 indiv. Further general impacts on the detectability are changes in environmental conditions, transect length, survey time, and human factors (Gewiss et al., 2020). For example, Ngo et al. (pers. obs.) recorded more than 40 indiv. of Goniurosaurus lichtenfelderi in Tay Yen Tu NR, northern Vietnam in August 2019, but failed to observe any individual at all after two repeated surveys in September 2019 due to temperature variations. We also recorded that the number of G. huuliensis individuals observed per day varied from 0.96 to 5 indiv./km/day. Therefore, to gain more complete knowledge of the real population status we highly recommend a long–term monitoring program in different habitat types. Furthermore, all selected sites should be surveyed at least three times rather than using representative invisibility–rate values of repeatedly surveyed sites. Thereby, other capture–mark–recapture methods such as Schnabel index could be applied to estimate the population size, which might improve the accuracy of the estimated values (Schlüpmann and Kupfer, 2009; Ngo et al., 2016a, 2016b).
As we mentioned above, the karst ecosystem is considered the prerequisite habitat of G. huuliensis However, the inclusion of karst formations to estimate the maximum population size (ŇMax) was limited by a low spatial resolution of the respective layer, including unsuitable habitats (crop hills, residential areas, roads). Given the total population size (ŇS) within the integrated suitable habitats, the species abundance is expected to be highly correlated with the probability of environmental suitability. A regression ratio from the correlation can be applied to estimate the total population size of G. huuliensis more properly. However, under– or over–fitting can take place due to using only representative data of the six survey transects, leading to inaccuracy in the interpolating estimation. In this study, the population size was equally estimated in all sites of environmental suitability, whereas all unsuitable sites indicated the species absence within the extent of occurrence. We recommend that further surveys should be carried out along other natural transects within occurrence areas of G. huuliensis with high probabilities of environmental suitability according to our models. Based on such data, it would be possible to correlate and interpolate the total size more precisely.
Acknowledgements
For supporting fieldwork and relevant permits (No. 105/2019/BTTNVN in 2019; No. 37/2020/BTTNVN in 2020; and No. 92/2021/CĐVP in 2021), we thank the Vietnam National Museum of Nature in Ha Noi, Vinh Phuc College in Vinh Phuc Province, and local authorities and forest protection departments of the Huu Lien Nature Reserve in Lang Son Province, Bac Giang and Thai Nguyen provinces. We are grateful to Theo Pagel and Christopher Landsberg (Cologne Zoo), Sinh V. Nguyen (IEBR, Hanoi), Lien V. Vu (VNMN, Hanoi) for their support of conservation–based biodiversity research in Vietnam. This research was supported by the Vietnam Academy of Science and Technology [Project code NVCC09.02/22–23] and Cologne Zoo. Cologne Zoo is the partner of the World Association of Zoos and Aquariums (WAZA): Herpetodiversity Research, Amphibian and Reptilian Breeding and Rescue Stations [Conservation Project 07011, 07012]. Research of Hai N. Ngo in Germany is funded by the German Academic Exchange Service (DAAD).
References
Araújo, M. B., Williams, P. H., Fuller, R. J., 2002. Dynamics of extinction and the selection of nature reserves. Proceedings of the Royal Society B: Biological Sciences, 269(1504): 1971–1980, Doi: 10.1098/rspb.2002.2121
Beissinger, S. R., Westphal, M. I., 1998. On the use of demographic models of population viability in endangered species management. Journal of Wildlife Management, 62(3): 821–841, Doi:
Animal Biodiversity and Conservation 45.2 (2022) 185
10.2307/3802534
Böhm, M., Collen, B., Baillie, J. E. M., Bowles, P., Chanson, J., Cox, N., Hammerson, G., Hoffmann, M., Livingstone, S. R., Ram, M., Rhodin, A. G. J., Stuart, S. N., van Dijk, P. P., Young, B. E. et al., 2013. The conservation status of the world's reptiles. Biological Conservation, 157: 372–385, Doi: 10.1016/j.biocon.2012.07.015
Ceballos, G., Ehrlich, P. R., Barnosky, A. D., García, A., Pringle, R. M., Palmer, T. M., 2015. Accerlerated modern human – induced species losses: Entering the sixth mass extinction. Science Advances, 1(5): e1400253, Doi: 10.1126/sciadv.1400253
CEPF (Critical Ecosystem Partnership Fund), 2020. Ecosystem Profile: Indo–Burma Biodiversity Hotspot, 2020 Update. Available online at: https:// www.cepf.net/sites/default/files/indo-burma-ecosystem-profile-2020-update.pdf [Accessed on May 2022].
Clements, R., Sodhi, N. S., Schilthuizen, M., Ng, P. K., 2006. Limestone karsts of Southeast Asia: imperiled arks of biodiversity. Bioscience, 56(9): 733–742, Doi: 10.1641/0006-3568(2006)56[733:LKOSAI]2.0.CO;2
Coulson, T. G., Mace, G. M., Hudson, E., Possingham, H., 2001. The use and abuse of population viability analysis. Trends in Ecology and Evolution, 16(5): 219–221, Doi: 10.1016/s0169-5347(01)02137-1
Croft, S., Chauvenet, A. L. M., Smith, G. C., 2017. A systematic approach to estimate the distribution and total abundance of British mammals. Plos One, 12: e0176339, Doi: 10.1371/journal.pone.0176339
Cunningham, G. D., While, G. M., Wapstra, E., 2017. Climate and sex ratio variation in a viviparous lizard. Biology Letters, 13(5): 201702182, Doi: 10.1098/ rsbl.2017.0218
Day, M. J., Urich, P. B., 2000. An assessment of protected karst landscapes in Southeast Asia. Cave and Karst Science, 27: 61–70.
Gewiss, R. L., Ngo, N. H., van Schingen–Khan, M., Bernardes, M., Rauhaus, A., Pham, T. C., Nguyen, Q. T., Ziegler, T., 2020. Population assessment and impact of trade on the Asian Water Dragon (Physignathus cocincinus Cuvier, 1829) in Vietnam. Global Ecology and Conservation, 23: e01193, Doi: 10.1016/j.gecco.2020.e01193
Gibbons, J. W., Scott, D. E., Ryan, T. J., Buhlmann, K. A., Tuberville, T. D., Metts, B. S., Greene, J. L., Mills, T., Leiden, Y., Poppy, S., Winne, C. T., 2000. The Global Decline of Reptiles, Déjà Vu Amphibians: Reptile species are declining on a global scale. Six significant threats to reptile populations are habitat loss and degradation, introduced invasive species, environmental pollution, disease, unsustainable use, and global climate change. BioScience, 50: 653–666, https://digitalcommons. butler.edu/cgi/viewcontent.cgi?article=1537&context=facsch_papers
Gilad, O., 2008. Competition and Competition models. In: Encyclopedia of Ecology: 707–712 (S. E. Jorgensen, B. Fath, Eds.). Oxford, UK.
Grinnell, J., 1917. The niche–relationships of the California thrasher. The Auk, 34: 427–433.
Goldscheider, N., Chen, Z., Auler, A.S., Bakalowicz, M., Broda, S., Drew, D., Hartmann, J., Jiang, G., Moosdorf, N., Stevanovic, Z., Veni, G., 2020. Global distribution of carbonate rocks and karst water resources. Hydrogeol Journal, 28: 1661–1677, Doi: 10.1007/s10040-020-02139-5
Grismer, L. L., Ngo, H. N., Qi, S., Wang, Y. Y., Le, M. D., Ziegler, T., 2021. Phylogeny and evolution of habitat preference in Goniurosaurus (Squamata: Eublepharidae) and their correlation with karst and granite–stream–adapted ecomorphologies in species groups from Vietnam. Vertebrate Zoology, 71: 335–352, Doi: 10.3897/vz.71.e65969
Grismer, L. L., Viets, B. E., Boyle, L. J., 1999. Two new continental species of Goniurosaurus (Squamata: Eublepharidae) with a phylogeny and evolutionary classification of the genus. Journal of Herpetology, 33(3): 382–393, Doi: 10.2307/1565635
Grismer, L. L., Wood Jr, P. L., Le, M. D., Quah, E. S., Grismer, J. L., 2020. Evolution of habitat preference in 243 species of Bent–toed geckos (Genus Cyrtodactylus Gray, 1827) with a discussion of karst habitat conservation. Ecology and Evolution, 10(24): 13717–13730, Doi: 10.1002/ece3.6961
Huang, C. M., Yu, H., Wu, Z. J., Li, Y., Wei, F. W., Gong, M. H., 2008. Population and conservation strategies for the Chinese crocodile lizard (Shinisaurus crocodilurus) in China. Animal Biodiversity and Conservation, 31(2): 63–70.
Irschick, D. J., Vanhooydonck, B., Herrel, A., Meyers, J. J., 2005. Intraspecific correlations among morphology, performance, and habitat use within a Green Anole Lizard (Anolis carolinensis) population. Biological Journal of the Linnean Society, 85(2): 211–221, Doi: 10.1111/j.1095-8312.2005.00486.x
IUCN Standards and Petitions Committee, 2019. Guidelines for Using the IUCN Red List Categories and Criteria. Version 14. Prepared by the Standards and Petitions Committee. Accesible online at: http:// www.iucnredlist.org/documents/RedListGuidelines. pdf [Accessed on May 2022]. ]
Jones, P. C., King, R. B., Sutton, S., 2017. Demographic analysis of imperiled Eastern Massasaugas (Sistrurus catenatus catenatus). Journal of Herpetology, 51(3): 383–387, Doi: 10.1670/15-058 Le, Q. T., Le, X. C., Le, M. H., Tran, A. T., Chu, T. H., Nguyen, Q. T., Ngo, N. H., 2017. Existing status and impact of climate change to the distribution of Goniurosaurus catbaensis. In: Proceedings of the 7th National Scientific Conference on Ecology and Biological Resources, 7: 1034–1040. The Institute of Ecology and Biological Resources, Ha Noi, Vietnam. Luo, Z., Tang, S., Jiang, Z., Chen, J., Fang, H., Li, C., 2016. Conservation of terrestrial vertebrates in a global hotspot of karst area in southwestern China. Scientific Reports, 6(1): 25717, Doi: 10.1038/ srep25717
Luu, V. Q., Van, O. L., Hoang, T. T., Van, T. P., Le, Do., Bordes, C., Luiselli, L., 2020. Ecological characteristics of a recently described, critically endangered gecko species, endemic to Central Highland, Vietnam. Tropical Zoology, 33(2): 53–62, Doi: https://doi.org/10.4081/tz.2020.71
186 Ngo et al.
Maida, R. J., Kirk, A. D., Mckibbin, O., Row, J. R., Larsen, K. W., Stringam, C., Bishop, C. A., 2018. Population estimate, survivorship, and generation time of the northern Pacific rattlesnake (Crotalus O. oreganus) at its northern–most range limits. Herpetological Conservation and Biology, 13(3): 662–672.
Marshall, B. M., Strine, C., Hughes, C. A., 2020. Thousands of reptile species threatened by under–regulated global trade. Nature Communications, 11: 4738, Doi: https://doi.org/10.1038/s41467-02018523-4
Monastersky, M., 2014. Life – a status report: species are disappearing quickly – but researchers are struggling to assess how bad the problem is. Nature, 516: 158, https://www.nature.com/news/ polopoly_fs/1.16523!/menu/main/topColumns/ topLeftColumn/pdf/516158a.pdf
Morrison, M. L., Bruce, G. M., Mannan R. W., 2006. Wildlife–habitat Relationships: Concepts and Applications. Third Edition. Island Press, Washington DC.
Ngo, N. H., Le, Q. T., Pham, L. M., Nguyen, Q. T., Le, D. M., van Schingen, M., Ziegler, T., 2019a. First record of the Cat Ba tiger gecko, Goniurosaurus catbaensis, from Ha Long Bay, Quang Ninh Province, Viet Nam: Microhabitat selection, potential distribution, and evidence of threats. Amphibian and Reptile Conservation, 13(2): 1–13 (e183).
Ngo, N. H., Nguyen, T. Q., Nguyen, T. V., Barsch, F., Ziegler, T., van Schingen, M., 2016a. First population assessment of the endemic insular Psychedelic Rock Gecko (Cnemaspis psychedelica) in southern Vietnam with implications for conservation. Amphibian and Reptile Conservation, 10(2): 18–26 (e124).
Ngo, N. H., Nguyen, Q. H., Phan, Q. T., Nguyen, Q. T., Gewiss, L. R., Rödder, D., Ziegler, T., 2022. Modeling the environmental refugia of the endangered Lichtenfelder’s Tiger Gecko (Goniurosaurus lichtenfelderi) towards implementation of transboundary conservation. Frontiers of Biogeography, 14(1): e51167, Doi: 10.21425/F5FBG51167
Ngo, N. H, Nguyen, Q. H., Phan, Q. T., Tran, M. H., Nguyen, Q. T., Rödder, D., Ziegler, T., 2021a. Vulnerability of an endemic Tiger Gecko (Goniurosaurus huuliensis) to climate change: modeling environmental refugia and implications for in–situ conservation. Salamandra, 57(4): 464–474.
Ngo, N. H., Nguyen, Q. H., Phan, Q. T., van Schingen–Khan, M., Nguyen, T. Q., Ziegler, T. (in press). Ecological niche overlap of two allopatric karst–adapted tiger geckos (Goniurosaurus) from northern Vietnam: Microhabitat use and implications for conservation. Journal of Natural History.
Ngo, N. H., Nguyen, T. Q., Phan, Q. T., van Schingen, M., Ziegler, T., 2019b. A case study on trade in threatened Tiger Geckos (Goniurosaurus) in Vietnam including updated information on the abundance of the Endangered G. catbaensis. Nature Conservation, 33: 1–19, Doi: 10.3897/ natureconservation.32.33590
Ngo, N. H., Nguyen, Q. H., Tran, M. H., Ngo, T. H., Le, D. M., Gewiss, L. R., van Schingen–Khan, M., Nguyen, T. Q., Ziegler, T., 2021b. A morphological
and molecular review of the genus Goniurosaurus, including an identification key. European Journal of Taxonomy, 751(1): 38–67, Doi: 10.5852/ ejt.2021.751.1379
Ngo, N. H., Ziegler, T., Nguyen, T. Q., Pham, T. C., Nguyen, T. T., Le, D. M., van Schingen, M., 2016b. First population assessment of two cryptic tiger geckos (Goniurosaurus) from Northern Vietnam: Implications for conservation. Amphibian and Reptile Conservation, 10(1): 34–45 (e120)..
Nguyen, T. Q., 2011. Systematics, ecology, and conservation of the lizard fauna in northeastern Vietnam, with special focus on Pseudocalotes (Agamidae), Goniurosaurus (Eublepharidae), Sphenomorphus and Tropidophorus (Scincidae) from this country. PhD thesis, University of Bonn, Germany.
– 2018. Goniurosaurus huuliensis. The IUCN Red List of Threatened Species 2018. International Union for Conservation of Nature. http://www.IUCN.org
Nguyen, V. S., Ho, C. T., Nguyen, T. Q., 2009. Herpetofauna of Vietnam. Edition Chimaira, Frankfurt am Main, Germany.
Nguyen, T. Q., Ngo, H. N., Pham, C. T., Nguyen, V. H., Ngo, D. C., van Schingen, M., Ziegler, T., 2018. First population assessment of the Asian water dragon (Physignathus cocincinus Cuvier, 1829) in Thua thien Hue province, Vietnam. Nature Conservation, 26: 1–14, Doi: 10.3897/natureconservation.26.21818
Orlov, N. L., Ryabov, S. A., Nguyen, T. T., Nguyen, T. Q., Ho, C. T., 2008. A new species of Goniurosaurus (Sauria: Gekkota: Eublepharidae) from north Vietnam. Russian Journal of Herpetology, 15(3): 229–244, Doi: 10.30906/1026-2296-2008-15-3229-244
Reed, D. H., O’Grady, J. J., Brook, B. W., Ballou, J. D., Frankham, R., 2003. Estimates of minimum viable population sizes for vertebrates and factors influencing those estimates. Biological Conservation, 113(1): 23–34, Doi: 10.1016/S00063207(02)00346-4
Rödder, D., Engler, J. O., 2011. Quantitative metrics of overlaps in overlaps in Grinnellian niches: advances and possible drawbacks. Global Ecology and Biogeography , 20(6): 915–927, Doi: h10.1111/j.1466-8238.2011.00659.x
RStudio Team., 2018. Rstudio: Integrated Development for R. Rstudio, Inc., Boston, MA, USA, http:// www.rstudio.com/
Schlüpmann, M., Kupfer, A., 2009. Methoden der Amphibienerfassung – eine Übersicht. Zeitschrift für Feldherpetologie, 15: 7–84, Doi: 10.13140/ RG.2.1.4038.1524
Selman, W., Jones, R. L., 2017. Population structure, status and conservation of two Graptemys species from the Pearl River, Mississippi. Journal of Herpetology, 51(1): 27–36, Doi: 10.1670/15-082
Stuart, B. L., Rhodin, A. G. J., Grismer, L. L., Hansell, T., 2006. Scientific description can imperil species. Science, 312(5777): 1137, Doi: 10.1126/ science.312.5777.1137b
The Government of Vietnam, 2019. Decree No.
Animal Biodiversity and Conservation 45.2 (2022) 187
06/2019/ND–CP of the Government of Vietnam on management of threatened and rare wild plants and animals, 22 January 2019, Hanoi.
Tolentino, P. J. S., Navidad, J. R. L., Angeles, M. D., Fernandez, D. A. P., Villanueva, E. L. C., Obeña, R. D. R., Bout Jr, I. E., 2020. Review: Biodiversity of forests over limestone in Southeast Asia with emphasis on the Philippines. Biodiversitas, 21(4), Doi: 10.13057/biodiv/d210441
Tôrres, N. M., Júnior, P. D. M., Santos, T., Silveira, L., Jácomo, A. T. A., Diniz–Filho, J. A. F., 2012. Can species distribution modelling provide estimates of population densities? A case study with jaguars in the Neotropics. Diversity and Distribution, 18(6): 615–627, Doi: 10.1111/j.1472-4642.2012.00892.x
Traill, L. W., Bradshaw, C. J. A., Brook, B. W., 2007. Minimum viable population size: A meta–analysis of 30 years of published estimates. Biological Conservation, 139(1): 159–166, Doi: 10.1016/j. biocon.2007.06.011
Tran, D. V., Vu, T. T., 2020. Combining species distribution modeling and distance sampling to assess wildlife population size: A case study with the northern yellow–cheeked gibbon (Nomascus annamensis). American Journal of Primatol, 82(9): e23169, Doi: 10.1002/ajp.23169
van Schingen, M., Ha, Q. Q., Pham, T. C., Le, Q. T., Nguyen, Q. T., Bonkowski, M., Ziegler, T., 2016. Discovery of a new crocodile lizard population
in Vietnam: Population trends, future prognoses and identification of key habitats for conservation. Revue Suisse de Zoologie, 123: 241–251, Doi: 10.1371/journal.pone.0091570
van Schingen, M., Pham, C. T., An, H. T., Bernardes, M., Hecht, V., Nguyen, T. Q., Bonkowski, M., Ziegler, T., 2014. Current status of the crocodile lizard Shinisaurus crocodilurus Ahl, 1930 in Vietnam with implications for conservation measures. Revue Suisse de Zoologie, 121(3): 425–440, https://kups. ub.uni-koeln.de/42994/
van Schingen, M., Pham, C. T., An, H. T., Nguyen, T. Q., Bernardes, M., Bonkowski, M., Ziegler, T., 2015. First ecological assessment of the endangered crocodile lizard Shinisaurus crocodilurus Ahl, 1930 in Vietnam: Microhabitat characterization and habitat selection. Herpetological Conservation and Biology, 10(3): 948–958.
Weber, M. M., Stevens, R. D., Diniz–Filho, J. A. F., Grelle, C. E. V., 2016. Is there a correlation between abundance and environmental suitability derived from ecological niche modelling? A meta–analysis. Ecography, 40(7): 817–828, Doi: 10.1111/ecog.02125
Zeng, Q., Zhang, Y., Sun, G., Duo, H., Wen, L., Lei, G., 2015. Using Species Distribution Model to Estimate the Wintering Population Size of the Endangered Scaly–Sided Merganser in China. Plos One, 10: e0117307, Doi: 10.1371/journal.pone.0117307
188 Ngo et al.
Biodiversity and Conservation 45.2 (2022)
Integrating multiple dimensions of biodiversity to inform global parrot conservation
Burgio, K. R., Davis, K. E., Dreiss, L. M., Cisneros, L. M., Klingbeil, B. T, Presley, S. J., van Rees, C. B., Willig, M. R., 2022. Integrating multiple dimensions of biodiversity to inform global parrot conservation. Animal Biodiversity and Conservation, 45.2: 189–202, Doi: https://doi.org/10.32800/abc.2022.45.0189
Abstact
Integrating multiple dimensions of biodiversity to inform global parrot conservation. In addition to changes associated with climate and land use, parrots are threatened by hunting and capture for the pet trade, making them one of the most at risk orders of birds for which conservation action is especially important. Species richness is often used to identify high priority areas for conserving biodiversity. By definition, richness considers all species to be equally different from one another. However, ongoing research emphasizes the importance of incorporating ecological functions (functional diversity) or evolutionary relationships (phylogenetic diversity) to more fully understand patterns of biodiversity, because (1) areas of high species richness do not always represent areas of high functional or phylogenetic diversity, and (2) functional or phylogenetic diversity may better predict ecosystem function and evolutionary potential, which are essential for effective long–term conservation policy and management. We created a framework for identifying areas of high species richness, functional diversity, and phylogenetic diversity within the global distribution of parrots. We combined species richness, functional diversity, and phylogenetic diversity into an Integrated Biodiversity Index (IBI) to identify global biodiversity hotspots for parrots. We found important spatial mismatches between dimensions, demonstrating species richness is not always an effective proxy for other dimensions of parrot biodiversity. The IBI is an integrative and flexible index that can incorporate multiple dimensions of biodiversity, resulting in an intuitive and direct way of assessing comprehensive goals in conservation planning.
Key words: Parrots, Biogeography, Phylogenetic diversity, Functional diversity, Conservation
Resumen

Integración de las múltiples dimensiones de la biodiversidad para fundamentar la estrategia mundial de conservación de los psitaciformes. Además de los cambios relacionados con el clima y el uso de la tierra, los psitaciformes están amenazados por la caza y la captura destinada al comercio de mascotas, lo que los convierte en uno de los órdenes de aves en mayor riesgo de extinción para el que las medidas de conservación son especialmente importantes. Para determinar las zonas de prioridad alta para la conservación de la biodiversidad, se suele utilizar la riqueza de especies. Por definición, la riqueza considera que todas las especies son igualmente diferentes entre sí. No obstante, los estudios que se están llevando a cabo en la actualidad hacen hincapié en la importancia de incorporar las funciones ecológicas (diversidad funcional) o las relaciones evolutivas (diversidad filogenética) para comprender mejor los patrones de la biodiversidad, ya que 1) las zonas con una elevada riqueza de especies no siempre son zonas con una elevada diversidad funcional o filogenética y 2) la diversidad funcional y la diversidad filogenética pueden predecir mejor la función de los ecosistemas y el potencial evolutivo, que son fundamentales para la elaboración y gestión de políticas de conservación eficaces a largo plazo. Hemos creado un marco para determinar las zonas dentro del área de distribución de los psitaciformes en las que la riqueza de especies, la diversidad funcional y la diversidad filogenética son elevadas. Asimismo, hemos combinado la riqueza de especies, la diversidad funcional y la diversidad filogenética en un índice integrado de biodiversidad (IBI por su sigla en inglés), que permite determinar puntos de biodiversidad críticos para los psitaciformes. Hemos observado importantes discrepan-
ISSN: 1578–665 X eISSN: 2014–928 X
© [2022] Copyright belongs to the authors, who license the journal Animal Biodiversity and Conservation to publish the paper under a Creative Commons Attribution 4.0 License
189
Animal
K. R. Burgio, K. E. Davis, L. M. Dreiss, L. M. Cisneros, B. T. Klingbeil, S. J. Presley, C. B. van Rees, M. R. Willig
Burgio et al.
cias entre las dimensiones, lo que pone de manifiesto que la riqueza de especies no siempre es una buena aproximación de las otras dimensiones de la biodiversidad de psitaciformes. El IBI es un índice integrador y flexible que puede incorporar múltiples dimensiones de la biodiversidad, lo que conlleva que sea una forma intuitiva y directa de evaluar los objetivos generales en la planificación de la conservación.
Palabras clave: Psitaciformes, Biogeografía, Diversidad filogenética, Diversidad funcional, Conservación
Received: 11 VIII 21; Conditional acceptance: 22 X 21; Final acceptance: 24 V 22
Kevin R. Burgio, Brian T. Klingbeil, Steven J. Presley, Michael R. Willig, Department of Ecology and Evolutionary Biology, University of Connecticut; 75 N. Eagleville Rd. U–3043, Storrs, CT 06269, USA.– Katie E. Davis, Department of Biology, University of York, Wentworth Way, York, YO10 5DD, UK.– Lindsay M. Dreiss, Center for Conservation Innovation, Defenders of Wildlife, 1130 17th Street NW, Washington DC 20036, USA.– Laura M. Cisneros, Department of Natural Resources and the Environment, University of Connecticut, 1376 Storrs Road, Storrs, CT 06269, USA.– Laura M. Cisneros, Steven J. Presley, Michael R. Willig, Institute of the Environment, University of Connecticut, 3107 Horsebarn Hill Road, Storrs, CT 06269, USA.– Brian T. Klingbeil, Steven J. Presley, Michael R. Willig, Environmental Sciences and Engineering, University of Connecticut, 3107 Horsebarn Hill Road, Storrs, CT 06269, USA.– Charles B. van Rees, River Basin Center and Odum School of Ecology, University of Georgia, 203 D. W. Brooks Drive, Athens, GA 30602, USA.
Corresponding author: Kevin R. Burgio. email: kevin.burgio@gmail.com
ORCID ID: 0000-0002-8375-2501
190
Introduction
As we enter the early stages of the 'Sixth Mass Extinction' (Ceballos et al., 2015), conservation agencies are struggling to face the challenges of a less certain future (Armsworth et al., 2015) as a consequence of habitat conversion and climate change (Urban, 2015). While the population–level responses of avian taxa to anthropogenic pressures are mixed (Radchuk et al., 2019), parrots (Psittaciformes) appear to be especially vulnerable, and are among the most threatened orders of birds (Butchart et al., 2004), with many species and populations subject to diverse and largely consistent threats across several continents (Martin et al., 2014; Berkunsky et al., 2017; Olah et al., 2018). Furthermore, 38 % of parrot populations in the Neotropics show declining trends in abundance (Berkunsky et al., 2017), while of 15 large parrot species researched in Africa and Madagascar, 12 (75 %) showed evidence of population declines in parts of their geographical range (Martin et al., 2014). However, estimates of population density exist for only 25 % of parrot species, and those estimates suggest that parrot density is higher inside of protected areas than outside of them (Marsden et al., 2015), demonstrating the critical importance of conservation action.
Some parrot species have been linked to important ecosystem functions, including invertebrate pest management, pollination, seed dispersal, and genetic–linking of plant communities, making these species 'keystone mutualists' (Tella et al., 2015, Blanco et al., 2015, 2016; Baños–Villalba et al., 2017). Some parrots also forage on plants that are toxic or poisonous to many vertebrate species (Gilardi and Toft, 2012; Blanco et al., 2015), allowing them to find food and persist in habitats where other frugivorous species cannot do so (Gilardi and Toft, 2012); moreover, this behavior may also confer benefits of reduced parasitism (Masello et al., 2018). Given the critical ecosystem services parrots provide, their loss may have detrimental effects on the persistence of many plant species, thereby contributing to ecosystem instability. Conversely, some parrot species have been spreading into new areas, primarily as a consequence of the pet trade, which has led to human–wildlife conflicts in Europe (White et al., 2019) and the introduction of 25 non–native species that now breed in the USA (Uehling et al., 2019).
In general, parrots have long generation times, and low population densities, both life history traits that are associated with increased extinction risk (Bennett and Owens 1997; Cardillo et al., 2005). Parrots are also disproportionately targeted for hunting and trapping, with > 68 % of populations in the Neotropics and 11 of 15 (> 73 %) of assessed large parrots in Africa and Madagascar affected by capture for the pet trade, and millions of parrots being exported for trade in recent decades (Martin et al., 2014, Berkunsky et al., 2017; Martin, 2018; Olah et al., 2018). With the exception of colony–nesting species (Wermundsen, 1998; Masello et al., 2006), parrot species that are larger–bodied and have longer generation times are generally found at relatively lower densities (Marsden et al., 2015) and
are more likely than are smaller parrot species to be obligate tree–cavity nesters (Renton et al., 2015). All of these factors are associated with elevated extinction risk in parrots (Jones et al., 2006, Olah et al., 2016). Despite this high level of threat, parrots have only recently been the subject of increasing research interest, and information on conservation–relevant parameters has been slow to accumulate (Masello and Quillfeldt, 2002; Brito and Orpea, 2009, Ducatez and Lefebvre, 2014; Martin et al., 2014; Olah et al., 2018). The previous lack of research may explain why density estimates, one of the most important factors in determining conservation status (Mace et al., 2008), are unreliable for most parrot species (Martin et al., 2014; Marsden et al., 2015).
The primary drivers of population decline and extinction risk in parrots include habitat loss or alteration (particularly for logging or agriculture), persecution as crop pests, capture for the global pet trade, and hunting for food (Martin et al., 2014). Of these, logging, agriculture, and capture for the pet trade simultaneously affect > 55 % of parrot populations in the Neotropics (Berkunsky et al., 2017), with apparently similar levels of incidence among large parrot species in Africa and Madagascar (Martin et al., 2014). In Oceania, logging affects the largest proportion of species (23%), followed by agriculture, hunting and trapping and invasive species (18 %, 17 %, 16 %, respectively); with invasive species including sugar gliders (Petaurus breviceps), fox (Vulpes vulpes), and feral cats (Felis catus) as a major threat primarily in New Zealand and Tasmania (Olah et al., 2018).
Over the past few decades, one of the most common methods for identifying areas of highest conservation priority has been based on the 'hotspot concept' (Reid, 1998; Myers et al., 2000), which uses existing species range maps to prioritize conservation efforts where species richness or richness of endemic species is highest. More recently, the multidimensional nature of biodiversity has emerged as a critical consideration for conservation prioritization and management (Mazel et al., 2014; Brum et al., 2017; Pollock et al., 2017) and considerations of irreplaceability and complementarity have supplemented the hotspot concept in spatial prioritization approaches (Andelman and Willig, 2003; Zonation and Marxan in Moilanen et al., 2009). Regions of high functional diversity (a measure of ecological trait diversity within an assemblage) or phylogenetic diversity (a measure of evolutionary diversity within an assemblage) may not coincide with areas with high species richness (for mammals; Safi et al., 2011; Mazel et al., 2014; Brum et al., 2017). Consequently, calls to incorporate phylogenetic or functional biodiversity into conservation planning have arisen in recent decades (Mace et al., 2003; Diaz et al., 2007). Phylogenetic or functional diversity may be better indicators of community resilience than is species richness (Naeem et al., 2012). Conserving functional diversity is critical for maintaining ecosystem functions (Naeem et al., 2012), and thus for maintaining critical ecosystem services, making it an important consideration in conservation (Chan et al., 2006; Diaz et al., 2007; Cimon–Morin et al., 2013; Kosman et al.,
Animal Biodiversity and Conservation 45.2 (2022) 191
2019). Nonetheless, cases that incorporate functional diversity in conservation research are rare (but see Devictor et al., 2010; Mazel et al., 2014; Kosman et al., 2019). Maintaining the capacity for future adaptation is an important consideration for communities undergoing rapid climatic and environmental changes, suggesting that phylogenetic diversity should be given consideration when determining conservation goals (Naeem and Li, 1997; Cardinale et al., 2012). Moreover, the loss of phylogenetic or functional diversity may be a better indicator than is the loss of species richness in quantifying ecosystem vulnerability (Srivastava et al., 2012).
The limited availability of data at appropriate scales, and the complex nature of metrics used to quantify functional or phylogenetic diversity, have previously inhibited incorporation of this information into spatial conservation planning for protected areas (Brum et al., 2017; Pollock et al., 2017). A phylogenetic supertree for the world’s parrots (Burgio et al., 2019) and an improved suite of functional diversity metrics that emphasize the summed functional uniqueness of species within parrot communities throughout the world (Kosman et al., 2019), are now available. These developments have provided data and methods that can complement the standard use of species richness to set parrot conservation goals (Kosman et al., 2019). However, a method to integrate taxonomic, phylogenetic, and functional information into a single comprehensive biodiversity metric to guide conservation decisions in general, or parrot conservation decisions in particular, has not been developed. Here, we create a framework to identify areas of high species richness, functional diversity, and phylogenetic diversity within the global distribution of parrots by combining these recent innovations in parrot biodiversity research with existing data on global species distributions. We separately calculated species richness, functional diversity, and phylogenetic diversity, and then combined them into an Integrated Biodiversity Index (IBI) to identify global biodiversity hotspots to aid in parrot conservation. In addition, we specifically searched for important spatial mismatches between the components of this integrated metric to identify situations in which species richness is not an effective proxy for other dimensions of parrot biodiversity.
Material and methods
Data collection
We used range maps for all 398 extant species of parrot (Birdlife International, 2015) following the taxonomy of del Hoyo et al. (2014) to inform spatially–explicit estimates of biodiversity at a global scale. We estimated functional diversity using two types of data: categorical (binary) and mensural traits (table 1). For each data type, we used a suite of traits that reflect particular niche axes and define functional components. Categorical traits included components of diet, foraging strategy, and foraging location, whereas mensural traits comprise body size
and range size. For each categorical trait, a species received a '1' if it exhibited the characteristic and a '0' if it did not. For each body size, we used the average value for each species based on measurements of multiple adults, when available. We obtained trait data for all parrot species from the literature (Burgio et al., 2019 for more details about this previously described and published dataset). We calculated phylogenetic diversity for each community using branch lengths from the phylogenetic supertree for all 398 extant parrots by Burgio et al. (2019).
Analyses
Biodiversity indices
We created a grid in ArcMap v.10.3 (ESRI, Redlands, CA, USA), using the Cylindrical Equal Area projection, with each grid cell measuring 50 x 50 km (hereafter 'grid cell'). For each grid cell (n = 21,078), we estimated species richness as the number of species with a range overlapping the cell. We estimated phylogenetic and functional diversity for each cell using Rao's quadratic entropy (Rao's Q: Botta–Dukát, 2005). Rao's Q measures the average difference between all pairs of species, thereby reflecting multivariate dispersion. We obtained the average phylogenetic or functional distances among species from pairwise dissimilarity matrices for the phylogenetic and functional components, as well as separately for each of the six functional categories. For the phylogenetic supertree, we populated a pairwise dissimilarity matrix via the 'cophenetic' function of the R package 'ape' (v.3.5; Paradis et al., 2004). We used the Gower metric from the R package 'cluster' (v.2.0.4; Maechler et al., 2012) to calculate pairwise functional dissimilarity matrices.
To allow meaningful comparisons among dimensions, we transformed each metric into its effective number of species or Hill number (hereafter numbers equivalent). The numbers equivalent is the number of maximally dissimilar species that is required to produce an empirical value of a diversity metric (Jost, 2006). This transformation facilitates intuitive interpretation of differences among assemblages and dimensions because indices are expressed in the same units (Jost, 2006, Chao et al., 2014). Species richness is its own numbers equivalent, because it is expressed in number of species. We transformed Rao's Q values into numbers equivalents using R functions developed by De Bello et al. (2010).
Integrated biodiversity index
The integrated biodiversity index (IBI) combines numbers–equivalent–transformations of Rao's Q for phylogenetic and functional diversity (all traits combined) with species richness. We scaled each dimension of biodiversity to a range from 0 to 1 so that each would have equal opportunity to contribute to the IBI value. Without such scaling, species richness would likely dominate spatial patterns of biodiversity. The IBI is the sum of the scaled representations of species richness (S), functional diversity (FD), and
192 Burgio et al.
Table 1. Functional attributes that reflect niche axes (functional components) were used to estimate functional biodiversity of parrot assemblages for each 500 km2 grid cell: Fc, functional component; At, Attribute; Tv, trait values.
Tabla 1. Atributos funcionales que reflejan las dimensiones (componentes funcionales) que empleamos para estimar la biodiversidad funcional de las comunidades de psitaciformes respecto de cada cuadrícula de 500 km2: Fc, componente funcional; At, Atributo; Tv, valores de los rasgos.
Type of data
Fc At Tv
Categorical
Diet Carrion 1.0 Invertebrates 1.0 Snails 1.0 Pollen 1.0 Nectar 1.0 Flower 1.0 Seed 1.0 Nut 1.0 Fruit 1.0 Plant matter 1.0 Roots 1.0 Fungi 1.0 Foraging strategy Glean 1.0 Dig 1.0 Scavenge 1.0 Graze 1.0 Flower probe 1.0 Excavate 1.0
phylogenetic diversity (PD) for a particular grid cell (i): Si – Smin FDi – FDmin PDi – PDmin IBI = S + + i S max – Smin FD max – FDmin PD max – PDmin
As a consequence of the numbers equivalent transformation and scaling functions, IBI values range from 0 to 3 and equally weight each dimension of diversity (a value of '0' would mean low combined biodiversity while a value of '3' would be highest in combined biodiversity).
Results
Species richness of parrots is highest in the Amazon Basin of South America, along the southeastern coast of Australia, and in the mountainous region of New
Type of data
Fc At Tv
Foraging location Water 1.0 Ground 1.0 Vegetation 1.0 Subcanopy 1.0 Canopy 1.0
Mensural
Body size Mass Mean (g) Lenght Mean (cm) Tarsus Mean (mm) Culmen Mean (mm) Wing Mean (mm) Tail Mean (mm) Range size Area km2
Guinea (fig. 1A). Functional diversity is highest in the dry Chaco of South America (fig. 1B). Our measure of functional diversity represents multivariate dispersion, which is greatest for assemblages that represent many functional types, but that have low redundancy in those functions. Dry Chaco parrot assemblages have low species richness (fig. 1A) and species that differ greatly from each other in functional traits associated with diet and foraging location. Phylogenetic diversity is highest in Australia, arising primarily from the diversification of multiple subfamilies within the Psittacidae, and the fact that cockatoos (Cacatuidae), which represent a deep split in the parrot phylogeny (fig. 2), are endemic to Australia and Oceania.
IBI is highest in Australia and New Guinea (fig. 3), and moderate in northern and central South America. For example, in South America, species richness is highest in the Amazon Basin (fig. 4A), phylogenetic diversity is fairly evenly distributed throughout the
Animal Biodiversity and Conservation 45.2 (2022) 193
B
S
32 1 1.36 1.00
Fig. 1. Global map of: A, parrot species richness (S); and B, functional diversity (FD: Rao's Q, based on Hill numbers). Functional traits in the analysis include: diet, foraging location, foraging strategy, body size and shape characteristics, and range size.
Fig. 1. Mapa mundial de: A, la riqueza de especies de psitaciformes (S); y B, la diversidad funcional (FD por su sigla en inglés: Q de Rao, basada en números de Hill). Los rasgos funcionales del análisis son: la dieta, el lugar de alimentación, la estrategia de alimentación, la talla corporal, las características de forma y el tamaño del área de distribución.
continent (fig. 4B), and functional diversity is highest in the dry Chaco (fig. 4C). Although IBI equally weights each of the three dimensions (fig. 4D), considerable spatial mismatches exist between hot spots of species richness and IBI (fig. 4E, 5).
Discussion
In general, most of Australia, the island of New Guinea, and to a lesser extent, the Amazon Basin, evince the highest values of IBI. This corroborates past findings from studies on species richness, and emphasizes that the most diverse hotspots for parrot conservation are understudied and in urgent need of future research (Brito and Orpea, 2009; Ducatez and Lefebvre, 2014; Wilson et al., 2016), In addition, research on functional singularity of parrot species (a measure of species–level functional uniqueness)
found that the parrot communities of these same regions comprise many species with highly unique and distinctive functional traits despite being communities with relatively low species richness (Kosman et al., 2019).
Aside from multiple dimensions of biodiversity, considerations of spatial scale are necessary for effective conservation planning from an applied perspective. For example, most natural resource management agencies and conservation initiatives are regional or local in their scope and capabilities, and cannot engage in global conservation planning. For instance, the Neotropics score rather low (fig. 2) in phylogenetic diversity compared to other regions because only one subfamily (Arinae) is endemic there. However, functional diversity is highest in the Chaco region of South America, likely because it is a harsh environment with low productivity; it likely cannot support multiple populations or taxa that perform
194 Burgio et al.
FD: Rao's Q A
Fig. 2. Global map of phylogenetic diversity (PD) of parrots (Rao's Q, based on Hill numbers) associated with a diagrammatic representation of the diversification of major clades and their biogeographic affinities. Images of Gondwanaland were redrawn from Li and Powell (2001), with paths of dispersal obtained from Schweizer et al. (2010). Subfamilial designations on the cladogram are: A, Arinae; B, Psittacinae; C, Coracopseinae; D, Agapornithinae; E, Loriinae; F, Platycercinae; and G, Psittaculinae. (All parrot images are Public Domain)
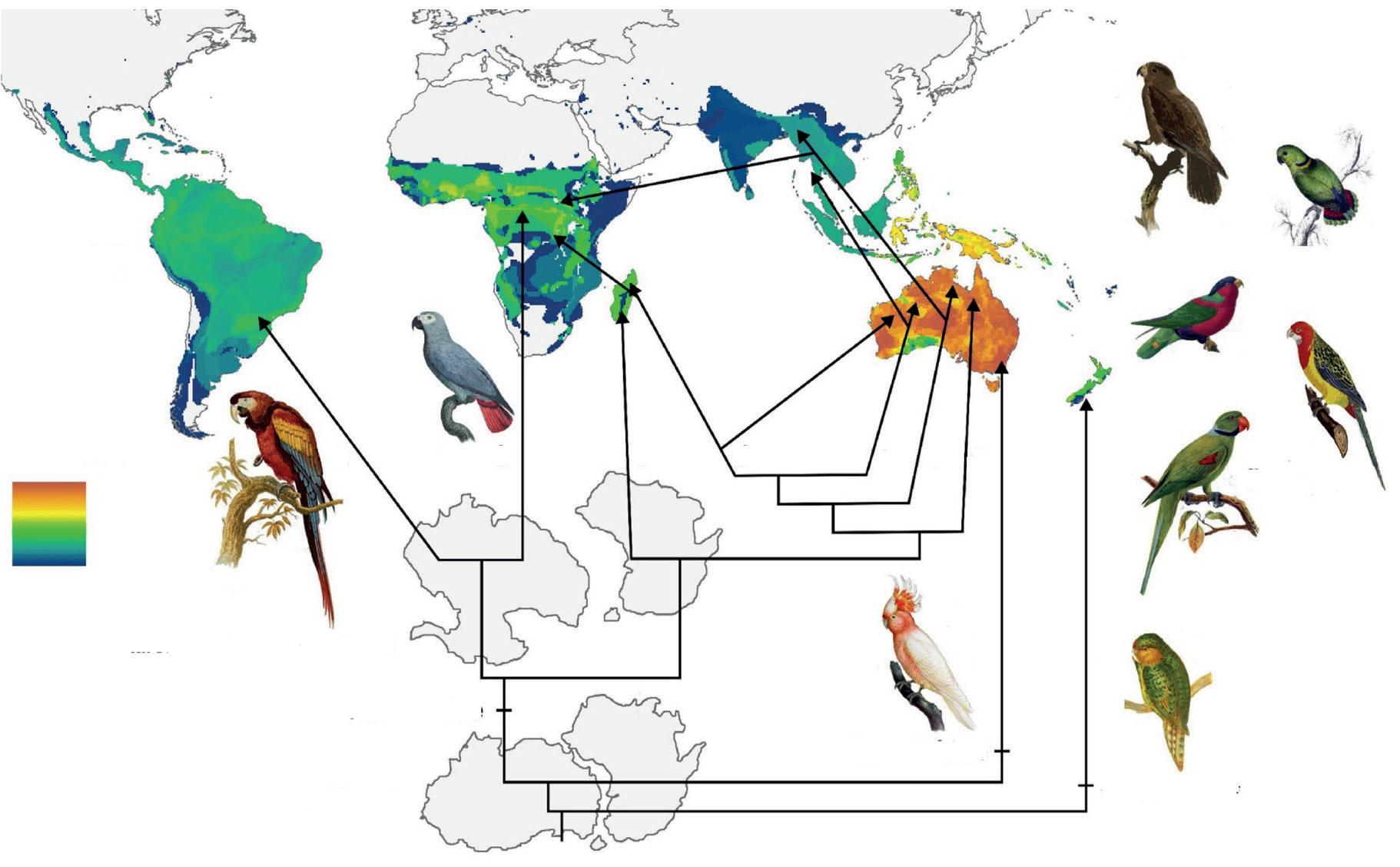
Fig. 2. Mapa mundial de la diversidad filogenética (PD por su sigla en inglés) de los psitaciformes (Q de Rao, basada en números de Hill) combinado con la representación de la diversificación de los principales clados y sus afinidades biogeográficas. Las imágenes de Gondwana se extrajeron de Li y Powell (2001) y los patrones de dispersión se obtuvieron de Schweizer et al. (2010). Las designaciones de las subfamilias en el cladograma son: A, Arinae; B, Psittacinae; C, Coracopseinae; D, Agapornithinae; E, Loriinae; F, Platycercinae; y G, Psittaculinae (todas las imágenes de psitaciformes son de dominio público).
similar functions. The Arinae subfamily diversified relatively quickly (Davies et al., 2007; Wright et al., 2008) and is the most species–rich subfamily in the parrot phylogeny, accounting for the discrepancies between dimensions of biodiversity in South America (fig. 4). Maps of functional and phylogenetic diversity generated using only species of parrots found in the Neotropics (i.e., the Arinae) likely would identify different areas of continental conservation concern than those presented here (fig. 2, 4).
The areas we identified as high priorities generally correspond with results from other global prioritization research, but with a few notable exceptions. For instance, Myers et al. (2000), who initiated the 'hotspots' concept, and included a wide variety of taxa, also identified Brazil's Cerrado and the southern expanse of the tropical Andes as areas of high priority; but did not prioritize central Australia. Research restricted to considerations of species–level functional distinctive-
ness of parrots (Kosman et al., 2019), also identified New Guinea, Australia, and the Cerrado as areas of high priority for parrot conservation. Recent research has incorporated spatial prioritization and multiple dimensions of diversity. For instance, high priority areas for birds (Pollock et al., 2017) and mammals (Brum et al., 2017) are the Andes, equatorial Africa, Indonesia, and New Guinea, which coincide well the patterns of high IBI for parrots, though our results also emphasize southeastern Australia and the Amazon Basin, likely due to the unique diversification pattern of parrots. The incorporation of socioeconomic data into conservation decisions can help anticipate new risks and adapt management targets accordingly (Armsworth et al., 2015). For example, high levels of urbanization correlate with an increased number of threatened parrot species, and a country's GDP (per capita) is associated with higher threat level (Butchart et al., 2015; Olah et al., 2016). Combining
Animal Biodiversity and Conservation 45.2 (2022) 195
PD:
3.67 1.00 A
C E G F D Antarctica ans Australia 45
Gondwana 96
Psittacidae Cacatuidae Strigopidae
Rao's Q
B C D E F G
mya
mya
Fig. 3. Global map of the integrated biodiversity index (IBI) for parrots, which is the sum of numbers equivalents of species richness, phylogenetic diversity, and functional diversity for each grid cell, with each of the three components scaled from 0 to 1.
Fig. 3. Mapa mundial del índice integrado de biodiversidad (IBI por su sigla en inglés) para los psitaciformes, que es la suma de los números equivalentes de la riqueza de especies, la diversidad filogenética y la diversidad funcional de cada cuadrícula, los tres van de 0 a 1.
the IBI approach with socioeconomic variables could identify areas within countries where high levels of these risk factors coincide with high biodiversity to facilitate conservation prioritization and early intervention before extinction debt is generated by the excess accumulation of population stressors (e.g. habitat loss and increased hunting).
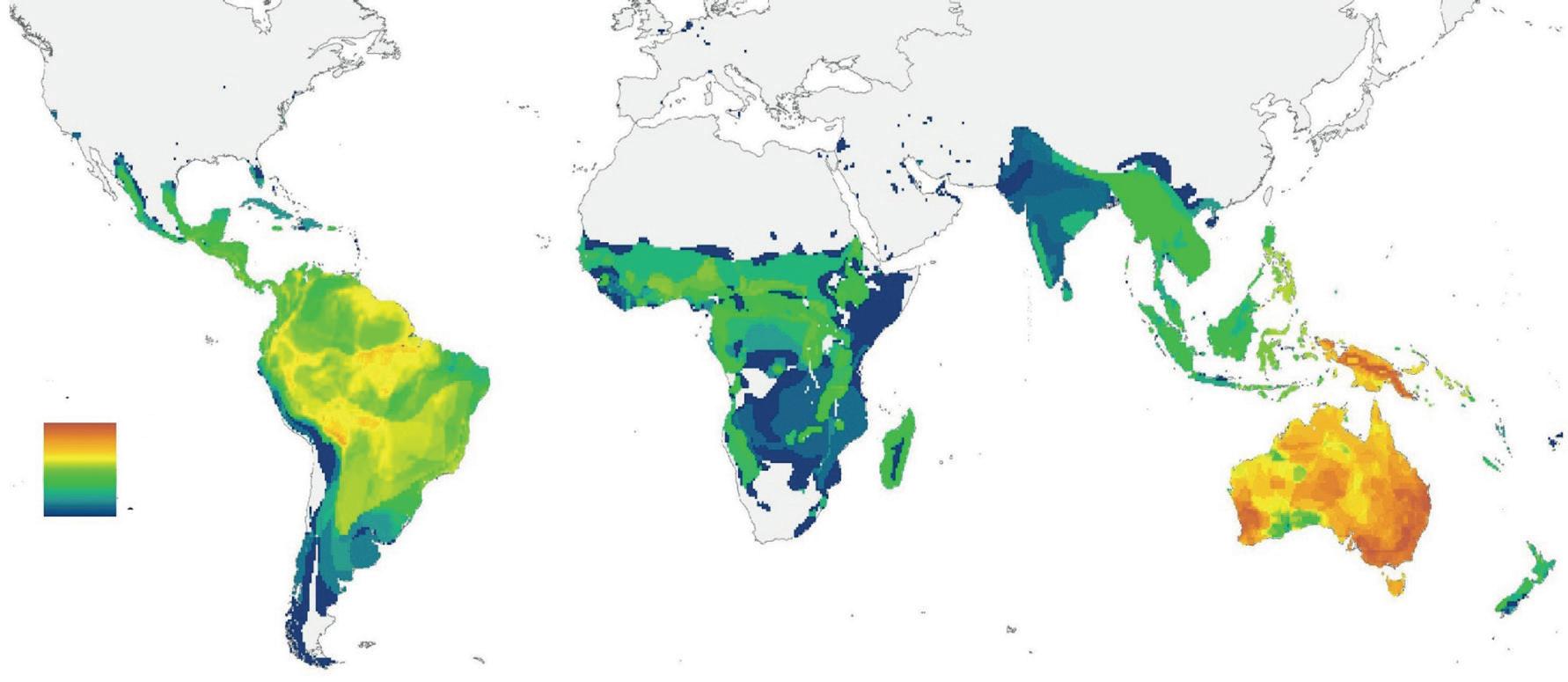
If a conservation practitioner were to use species richness as their only metric for spatial conservation prioritization of parrots in South America, they would focus on the Amazon Basin (fig. 4A), largely ignoring the high degree of functional diversity in the dry Chaco, which has the highest functional diversity of parrots in the world (fig. 4C). However, by incorporating multiple aspects of biodiversity in the IBI, these aspects of biodiversity are weighted equally (fig. 4D), allowing conservation agencies to make more informed decisions. Importantly, any particular dimension of biodiversity can be emphasized (or de–emphasized) within the IBI framework depending on the goals of a particular project. The mismatch between species richness and IBI (fig. 4E, 5) illustrates the importance of all aspects of biodiversity, and the problems with assumptions that protecting one dimension means that other dimensions are protected effectively. Spatial mismatches among hotspots of different dimensions have also been documented for mammals (Mazel et al., 2014). Conservation planners and practitioners should consider the scale and goals of conservation plans and should incorporate relevant information into an integrated framework to understand the relative value of particular policy options before taking action. Although we do not explore extinction risk spe-
cifically, our framework may be applied to examine specific correlates of extinction risk among communities across space. Specifically, some of the traits and datasets used for IBI calculation in this study are associated with elevated extinction risk. For instance, parrot species with larger bodies or that are more dependent on forest habitat may be at increased risk for extinction (Jones et al., 2006; Olah et al., 2016). Depending on the source of threat to a given species or region, conservation planners may find it useful to consider some functional components of our IBI approach separately. This is a complementary approach to an aggregated metric like the IBI, and could be a valuable exercise in certain circumstances.
For example, mapping the areas of relatively low diversity in traits such as body size (fig. 1s in supplementary material), location (fig. 3s in supplementary material), or range size (fig. 5s in supplementary material), may be a good first step in identifying assemblages that may be at greater risk for extinction. This step may be particularly important because the simultaneous consideration of all components of functional diversity can obscure important patterns that are relevant to particular conservation issues (Spasojevic and Suding, 2012; Lopez et al., 2016). Conservation agencies concerned primarily with protecting ecosystem functioning or ecosystem services may wish to focus on diet and foraging strategy diversity (fig. 2s, 4s in supplementary material, respectively), as opposed to broader summary indices of diversity.
As demonstrated in this study, the three components of IBI are given equal weight. This was done to demonstrate the extent of spatial mismatch between
196 Burgio et al.
2.31 0 IBI
Fig. 4. Graphical comparison of: A, species richness (S); B, phylogenetic diversity (PD); and C, functional diversity (FD) patterns as well as D, IBI of South America and E, the difference between species richness (S) and IBI (∆IBI), illustrating that correlation on a global level does not predict congruence of hotspots of each dimension at smaller spatial scales. To calculate ∆IBI, we scaled the results of panels A and D to 0–1 (to make them comparable) and subtracted S from IBI, resulting in ∆IBI, which can range from –1 to 1. Positive scores (purple) are areas more emphasized by IBI, whereas negative scores (green) are areas more emphasized by species richness. Yellow scores indicate approximately equal emphasis.
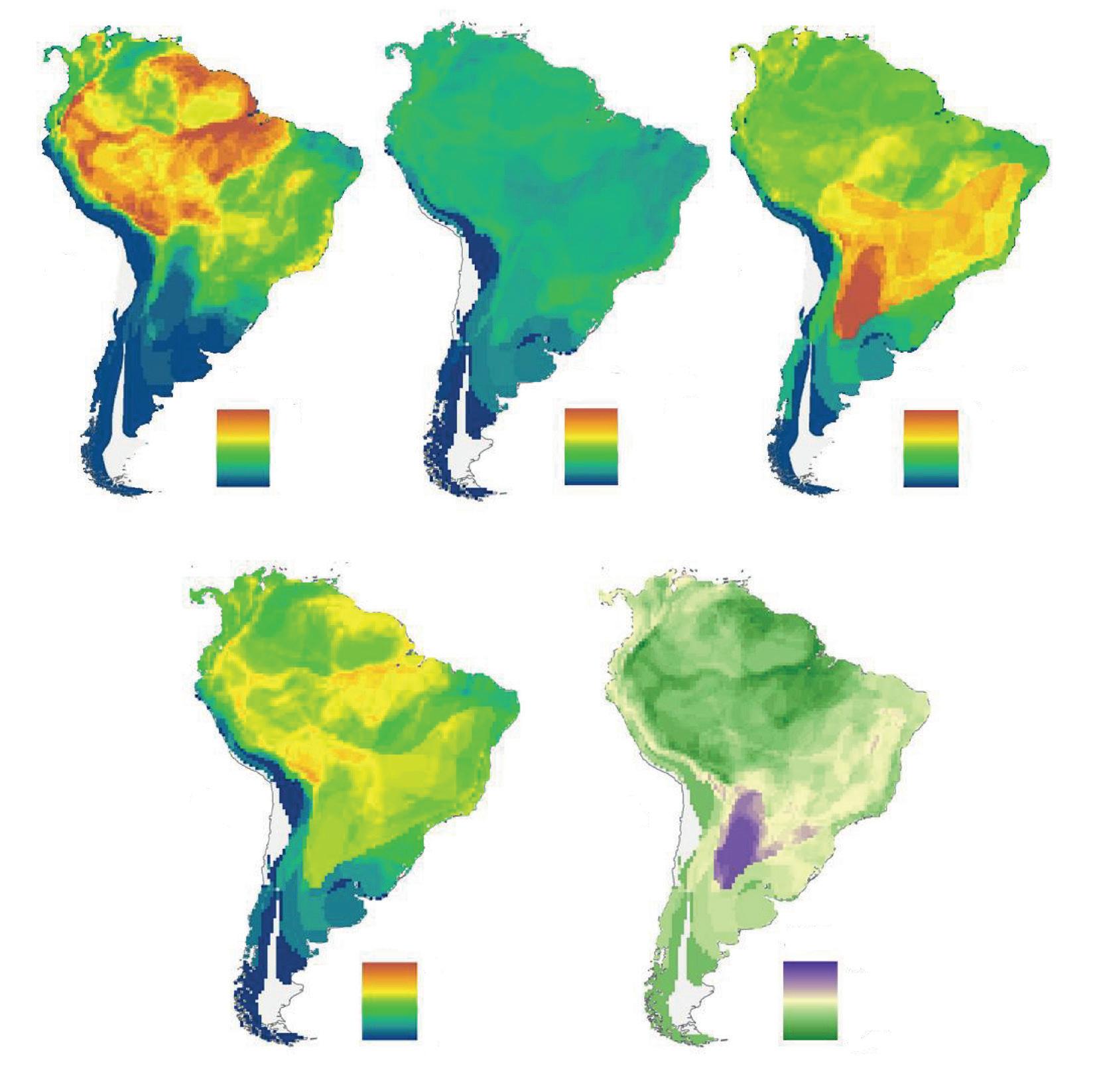
Fig. 4. Comparación gráfica de los patrones de: A, la riqueza de especies (S); B, la diversidad filogenética (PD por su sigla en inglés); y C, la diversidad funcional (FD por su sigla en inglés), así como D, el índice integrado de biodiversidad (IBI por su sigla en inglés) de Sudamérica y E, la diferencia entre la riqueza de especies (S) y el IBI (∆IBI), lo que muestra que la correlación a escala mundial no permite predecir la congruencia de los puntos críticos de cada dimensión de biodiversidad en escalas espaciales más reducidas. Para calcular ∆IBI, transformamos los resultados de A y D en una escala de 0 a 1 (para poderlos comparar) y restamos S del IBI, lo que dio lugar a ∆IBI, que puede valer entre –1 y 1. Las puntuaciones positivas (en color violeta) indican las zonas con un IBI elevado, mientras que las negativas (en color verde) indican las zonas con una elevada riqueza de especies. Las puntuaciones intermedias (en color amarillo) indican zonas en las que el IBI y la riqueza de especies tiene aproximadamente el mismo peso.
phylogenetic and species richness indicators for illustrative purposes. For conservation implementation, decision–makers can easily assign weights to these different components as needed for prioritization or gap analysis. For example, a potential extension of
our framework could evaluate how well particular dimensions of parrot biodiversity are protected, as a means of weighting the IBI equation to emphasize or de–emphasize particular dimensions when prioritizing areas to protect. The network of protected areas in
Animal Biodiversity and Conservation 45.2 (2022) 197
A B C
S PD: Rao's Q FD: Rao's Q 32 1 3.87 1.00 1.36 1.00
2.31 0 0.59 –0.19
D E
IBI ∆ IBI
∆ IBI 0.59 –0.19
Fig. 5. Global map of mismatch between integrated biodiversity index (IBI) and species richness. Results shown in figures 1A and 3 are scaled from 0 to 1 (to make them comparable), and subtracted species richness (S) from (IBI), resulting in ∆IBI, which can range from –1 to 1. Positive scores (purple) are areas more emphasized by IBI, whereas negative scores (green) are areas more emphasized by species richness. Yellow scores indicate similar conclusions based on IBI or species richness.
Fig. 5. Mapa mundial de la discrepancia entre el índice integrado de la biodiversidad (IBI) y la riqueza de especies. Los resultados que se muestran en las figuras 1A y 3 se han transformado en una escala del 0 al 1 (para poderlos comparar) y se ha restado la riqueza de especies (S) del IBI, lo que da como resultado ∆IBI, que puede valer entre –1 y 1. Las puntuaciones positivas (en color violeta) indican las zonas con un IBI elevado, mientras que las negativas (en color verde) indican las zonas con una elevada riqueza de especies. Las puntuaciones intermedias (en color amarillo) indican que se pueden extraer conclusiones parecidas tanto en función del IBI como de la riqueza de especies.
France provides different levels of protection for bird species richness, phylogenetic diversity, and functional diversity (Devictor et al., 2010). Similarly, measures such as 'ED' (Evolutionary Distinctiveness; Isaac et al., 2007), 'EDGE' (Evolutionary Distinctiveness/ Globally Endangered; Isaac et al., 2007), and 'EDR' (Evolutionary Distinctiveness Rarity; Jetz et al., 2014) can be added or can replace phylogenetic diversity to ensure that distinct clades of the parrot tree are given more weight when assessing conservation priorities.
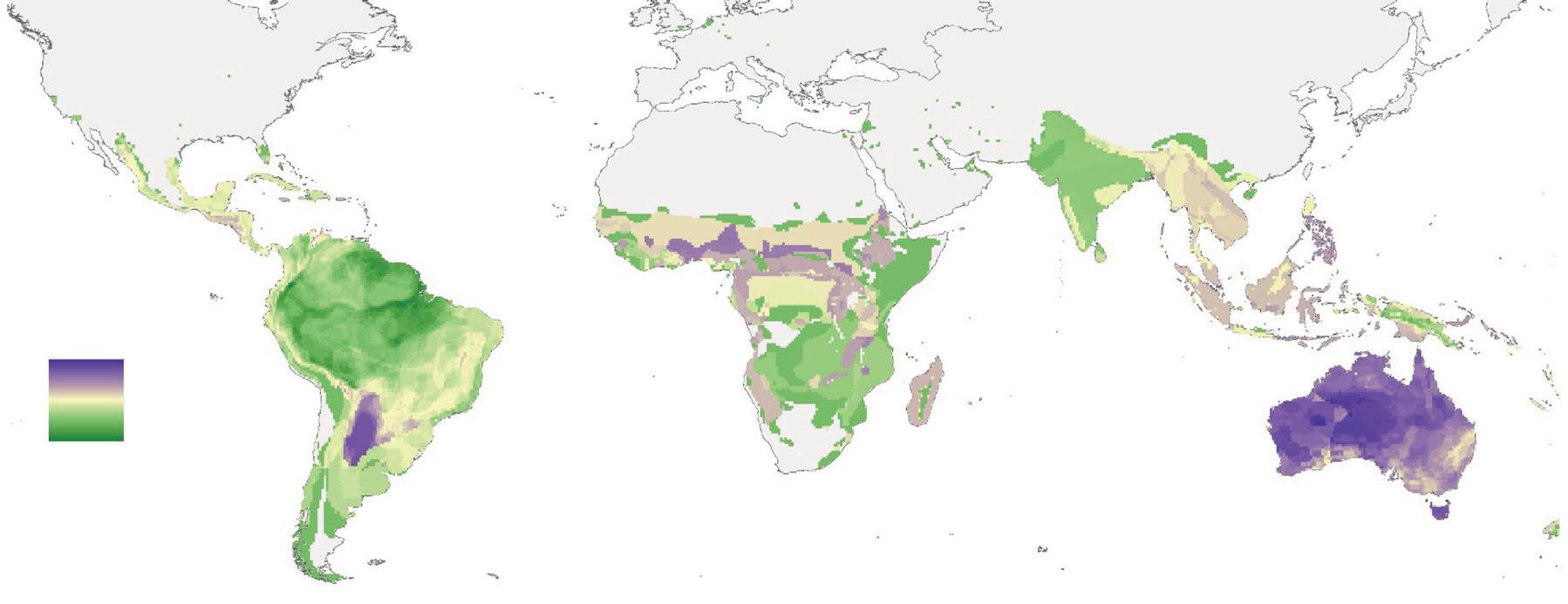
Climate change will have direct and indirect effects on species distributions (Jones et al., 2016). Direct effects are based on the physiological tolerances of species, which will track their climatic niches as spatial patterns of temperature and precipitation change. Indirectly, climate change will affect land–use patterns by humans (Turner et al., 2010), which may limit or form barriers against the dispersal of individuals (Faleiro et al., 2013). Indirect effects can also be mediated by interspecific interactions, for example changes in nesting or foraging habitat availability from changes in the plant community, or exposure to novel predators from climate range shifts (Porfirio et al., 2016; Hambuckers et al., 2021).
Preserving connectivity among habitat patches may be a key element of effective conservation strategies in the face of climate change (Schmitz et al., 2015), further supported here by the areas we identify as high diversity, including the belt across central
South America (fig. 4). The effects of recent climate change have been greater at high elevations and in tundra compared to tropical and subtropical lowlands (Seddon et al., 2016) that harbor most species of parrots. Nonetheless, many parrot species occur in areas that are sensitive to climate change. Based on a combination of species richness and the number of threatened species and endemic species, Indonesia, Brazil, Australia, Colombia, and Bolivia are the five highest priority countries for parrot conservation action (Olah et al., 2016), although several countries in the Neotropics (including Panama, Guyana, Surinam, French Guiana, Uruguay and the Greater and Lesser Antilles) represent data gaps and thus research priorities (Berkunsky et al., 2017). Olah et al. (2018)’s more recent analyses for Oceania also highlight New Caledonia, New Zealand (particularly the South Island), Tasmania, and the Moluccas as additional major priorities for parrot conservation.
Given the predicted extent and severity of effects of climate change, conservation agencies face a daunting task. Conservation planning must balance current protection needs with future expectations as species may become locally extinct, shift their ranges, or adapt to changing conditions, possibly leading to the production of novel assemblages. Additional complications for future conservation efforts include the push and pull between different scales of conservation prioritization (i.e. the 'actors' versus the 'stage'; Tingley
198 Burgio et al.
et al., 2014). Although some conservation agencies may opt to focus on particular species due to public and political values (Mace, 2004), IBI is an integrative and flexible index that can incorporate multiple dimensions of biodiversity, resulting in the intuitive way to assess more than just species richness in conservation planning.
Acknowledgements
We thank R. K. Colwell, C. Rittenhouse, M. Rubega, B. Walker, and an anonymous reviewer for providing valuable feedback. K. R. Burgio was supported by National Science Foundation (NSF) grant #DGE–0753455. Many of the methods in this paper were developed in part by participation of K. R. Burgio, L. M. Dreiss, L. M. Cisneros, B. T. Klingbeil, S. J. Presley, and M. R. Willig in an NSF–funded project entitled ‘The Dimensions of Biodiversity Distributed Graduate Seminar’ awarded to S. Andelman and J. Parrish (DEB–1050680). S. J. Presley and M. R. Willig were supported by the Center for Environmental Sciences and Engineering at the University of Connecticut, as well as by an NSF award (DEB–1546686 and DEB–1831952).
References
Andelman, S. J., Willig, M. R., 2003. Present patterns and future prospects for biodiversity in the Western Hemisphere. Ecology Letters, 6: 818–824, Doi: 10.1046/j.1461-0248.2003.00503.x
Armsworth, P. R., Larson, E. R., Jackson, S. T., Sax, D. F., Simonin, P., Blossey, B., Green, N., Klein, M. L., Lester, L., Ricketts, T. H., Runge, M. C., Shaw, M. R., 2015. Are conservation organizations configured for effective adaptation to global change? Frontiers in Ecology and the Environment, 13(3): 163–169, Doi: 10.1890/130352
Baños–Villalba, A., Blanco, G., Díaz–Luque, J. A. Denes, F. V., Hiraldo, F., Tella, J. L., 2017. Seed dispersal by macaws shapes the landscape of an Amazonian ecosystem. Scientific Reports, 7: 7373, Doi: 10.1038/s41598-017-07697-5
Bennett, P. M., Owens, I. P., 1997. Variation in extinction risk among birds: chance or evolutionary predisposition? Philosophical Transactions of the Royal Society B Biological Sciences, 264: 401–408.
Berkunsky, I., Quillfeldt, P., Brightsmith, D. J., Abbud, M. C., Aguilar, J. M. R. E., Alemán–Zelaya, U., Aramburú, R. M., Arias, A. A., McNab, R. B., Balsby, T. J., Barberena, J. B. et al., 2017. Current threats faced by Neotropical parrot populations. Biological Conservation, 214: 278–287, Doi: 10.1016/j. biocon.2017.08.016
BirdLife International, 2015. IUCN Red List for birds Available online at: http://www.birdlife.org
Blanco, G., Bravo, C., Pacifico, E. C., Chamorro, D., Speziale, K. L., Lambertucci, S. A., Hiraldo, F., Tella, J. L., 2016. Internal seed dispersal by parrots: an overview of a neglected mutualism. PeerJ, 4:
e1688, Doi: 10.7717/peerj.1688
Blanco, G., Hiraldo, F., Rojas, A., Dénes, F. V., Tella, J. L., 2015. Parrots as key multilinkers in ecosystem structure and functioning. Ecology and Evolution, 5(18): 4141–4160, Doi: 10.1002/ece3.1663
Botta–Dukát, Z., 2005. Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. Journal of Vegetation Science, 16(5): 533–540, Doi: 10.1111/j.1654-1103.2005.tb02393.x
Brito, D., Oprea, M., 2009. Mismatch of research effort and threat in avian conservation biology. Tropical Conservation Sciences, 2: 353–362, Doi: 10.1177/194008290900200305
Brum, F. T., Graham, C. H., Costa, G.C., Hedges, S.B., Penone, C., Radeloff, V. C., Rondinini, C., Loyola, R., Davidson, A. D., 2017. Global priorities for conservation across multiple dimensions of mammalian diversity. Proceedings of the National Academy of Sciences, 114(29): 7641–7646, Doi: 10.1073/pnas.1706461114
Burgio, K. R., Davis, K. E., Dreiss, L. M., Klingbeil, B. T., Cisneros, L. M., Presley, S. J., Willig, M. R., 2019. Phylogenetic supertree and functional trait database for all extant parrots. Data in Brief, 24: 103882, Doi: 10.1016/j.dib.2019.103882
Butchart, S., Clarke, M., Smith, R. J., Sykes, R., Scharlemann, J., Harfoot, M., Buchanan, G., Angulo, A., Balmford, A., Bertzky, B., 2015. Shortfalls and solutions for meeting national and global conservation area targets. Conservation Letters, 8: 329–337, Doi: 10.1111/conl.12158
Butchart, S. H. M., Stattersfield, A. J., Bennun, L. A., Shutes, S. M., Akçakaya, H. R., Baillie, J. E. M., Stuart, S. N., Hilton–Taylor, C., Mace, G. M., 2004. Measuring Global Trends in the Status of Biodiversity: Red List Indices for Birds. Plos Biology, 2(12): e383, Doi: 10.1371/journal.pbio.0020383
Cardillo, M., Mace, G. M., Jones, K. E., Bielby, J., Bininda–Emonds, O. R., Sechrest, W., Orme, C. D. L., Purvis, A., 2005. Multiple causes of high extinction risk in large mammal species. Science, 309(5738): 1239–1241, Doi: 10.1126/science.1116030
Cardinale, B. J., Duffy, E., Gonzalez, A., Hooper, D. U., Perrings, C., Venail, P., Narwani, A., Mace, G. M., Tilman, D., Wardle, D. A., Kinzig, A. P., Daily, G. C., Loreau, M., Grace, J. B., Larigauderie, A., Srivastava, D., Naeem, S., 2012. Biodiversity loss and its impact on humanity. Nature, 486(7401): 59–67, Doi: 10.1038/nature11148
Ceballos, G., Ehrlich, P. R., Barnosky, A. D., García, A., Pringle, R. M., Palmer, T. M., 2015. Accelerated modern human– induced species losses: Entering the sixth mass extinction. Science Advances, 1(5): e1400253, Doi: 10.1126/sciadv.1400253
Chan, K. M. A., Shaw, M. R., Cameron, D. R., Underwood, E.C., Daily, G. C., 2006. Conservation planning for ecosystem services. Plos Biology, 4: 2138, Doi: 10.1371/journal.pbio.0040379
Chao, A., Chiu, C., Jost, L., 2014. Unifying Species Diversity, Phylogenetic Diversity, Functional Diversity, and Related Similarity and Differentiation Measures Through Hill Numbers. Annual Review of Ecology, Evolution, and Systematics, 45: 297–324,
Animal Biodiversity and Conservation 45.2 (2022) 199
Doi: 10.1146/annurev-ecolsys-120213-091540
Cimon–Morin, J., Darveau, M., Poulin, M., 2013. Fostering synergies between ecosystem services and biodiversity in conservation planning: A review. Biological Conservation, 166: 144–154, Doi: 10.1016/j.biocon.2013.06.023
Davies, R. G., Orme, C. D. L., Webster, A. J., Jones, K. E., Blackburn, T. M., Gaston, K. J., 2007. Environmental predictors of global parrot (Aves: Psittaciformes) species richness and phylogenetic diversity. Global Ecology and Biogeography, 16(2): 220–233, Doi: 10.1111/j.1466-8238.2007.00282.x
De Bello, F., Lavergne, S., Meynard, C. N., Lepš, J., Thuiller, W., 2010. The partitioning of diversity: Showing Theseus a way out of the labyrinth. Journal of Vegetation Science, 21: 992–1000, Doi: 10.1111/j.1654-1103.2010.01195.x
del Hoyo, J., Collar, N. J., Christie, D. A., Elliott, A., Fishpool, L. D. C., 2014. HBW and BirdLife International Illustrated Checklist of the Birds of the World Vol. 1. Lynx Edicions, Barcelona.
Devictor, V., Mouillot, D., Meynard, C., Jiguet, F., Thuiller, W., Mouquet, N., 2010. Spatial mismatch and congruence between taxonomic, phylogenetic and functional diversity: The need for integrative conservation strategies in a changing world. Ecology Letters, 13(8): 1030–1040, Doi: 10.1111/j.14610248.2010.01493.x
Díaz, S., Lavorel, S., de Bello, F., Quétier, F., Grigulis, K., Robson, T. M., 2007. Incorporating plant functional diversity effects in ecosystem service assessments. Proceedings of the National Academy of Sciences of the United States of America, 104(52): 20684, Doi: 10.1073/pnas.0704716104
Ducatez, S., Lefebvre, L., 2014. Patterns of Research Effort in Birds. Plos One, 9: e89955, Doi: 10.1371/ journal.pone.0089955
Faleiro, F. V., Machado, R. B., Loyola, R. D., 2013. Defining spatial conservation priorities in the face of land–use and climate change. Biological Conservation, 158: 248–257, Doi: 10.1016/j.biocon.2012.09.020
Gilardi, J. D., Toft, C. A., 2012. Parrots eat nutritious foods despite toxins. Plos One, 7: e38293, Doi: 10.1371/journal.pone.0038293
Hambuckers, A., de Harenne, S., Ledezma, E. R., Zeballos, L. Z., Francois, L., 2021. Predicting the Future Distribution of Ara rubrogenys, an Endemic Endangered Bird Species of the Andes, Taking into Account Trophic Interactions. Diversity, 13: 94, Doi: 10.3390/d13020094
Isaac, N. J., Turvey, S. T., Collen, B., Waterman, C., Baillie, J. E., 2007. Mammals on the EDGE: conservation priorities based on threat and phylogeny. Plos One, 2: e296, Doi: 10.1371/journal. pone.0000296
Jetz, W., Thomas, G., Joy, J., Redding, D., Hartmann, K., Mooers, A., 2014. Global Distribution and Conservation of Evolutionary Distinctness in Birds. Current Biology, 24(9): 919–930, Doi: 10.1016/j. cub.2014.03.011
Jones, M. J., Fielding, A., Sullivan, M., 2006. Analysing extinction risk in parrots using decision trees.
Biodivers Conserv, 15: 1993–2007, Doi: 10.1007/ s10531-005-4316-1
Jones, K. R., Watson, J. E. M., Possingham, H. P., Klein, C. J., 2016. Incorporating climate change into spatial conservation prioritisation: A review. Biological Conservation, 194: 121–130, Doi: 10.1016/j. biocon.2015.12.008
Jost, L., 2006. Entropy and diversity. Oikos, 113(2): 363–375, Doi: 10.1111/j.2006.0030-1299.14714.x
Kosman, E., Burgio, K. R., Presley, S. J., Willig, M. R., Scheiner, S. M., 2019. Conservation prioritization based on trait–based metrics illustrated with global parrot distributions. Diversity and Distributions, 25(7): 1156–1165, Doi: 10.1111/ddi.12923
Li, Z., Powell, C. M., 2001. An outline of the palaeogeographic evolution of the Australasian region since the beginning of the Neoproterozoic. Earth Science Reviews, 53(3): 237–277, Doi: 10.1016/ S0012-8252(00)00021-0
Lopez, B., Burgio, K., Carlucci, M., Palmquist, K., Parada, A., Weinberger, V., Hurlbert, A., 2016. A new framework for inferring community assembly processes using phylogenetic information, relevant traits and environmental gradients. One Ecosystem, 1: e9501, Doi: 10.3897/oneeco.1.e9501
Mace, G. M., 2004. The role of taxonomy in species conservation. Philosophical Transactions of the Royal Society B: Biological Sciences, 359(1444): 711–719, Doi: 10.1098/rstb.2003.1454
Mace, G. M., Collar, N. J., Gaston, K. J., Hilton–Taylor, C., Akçakaya, H. R., Leader–Williams, N., Milner–Gulland, E. J., Stuart, S. N., 2008. Quantification of extinction risk: IUCN’s system for classifying threatened species. Conservation Biology, 22: 1424–1442.
Mace, G. M., Gittleman, J. L., Purvis, A., 2003. Preserving the tree of life. Science, 300(5626): 1707, Doi: 10.1126/science.1085510
Maechler, M., Rousseeuw, P., Struyf, A., Hubert, M., Hornik, K., 2012. Cluster: cluster analysis basics and extensions. R package version 1: 56.
Marsden, S. J., Royle, K., Downs, C., 2015. Abundance and abundance change in the world’s parrots. Ibis, 157: 219–229.
Martin, R. O., 2018. Grey areas: temporal and geographical dynamics of international trade of Grey and Timneh Parrots (Psittacus erithacus and P. timneh) under CITES. Emu–Austral Ornithology, 118: 113–125, Doi: 10.1080/01584197.2017.1369854
Martin, R. O., Perrin, M. R., Boyes, R. S., Abebe, Y. D., Annorbah, N. D., Asamoah, A., Bizimana, D., Bobo, K. S., Bunbury, N., Brouwer, J., Diop, M. S., Ewnetu, M., Fotso, R., Garteh, J., Maisels, F., 2014. Research and conservation of the larger parrots of Africa and Madagascar: a review of knowledge gaps and opportunities. Ostrich, 85: 205–233, Doi: 10.2989/00306525.2014.948943
Masello, J. F., Martínez, J., Calderón, L., Wink, M., Quillfeldt, P., Sanz, V., Theuerkauf, J., Ortiz–Catedral, L., Berkunsky, I., Brunton, D., Díaz–Luque, J. A., Hauber, M. E., Ojeda, V., Barnaud, A., Casalins, L., Jackson, B., Mijares, A., Rosales, R., Seixas, G., Serafini, P., Silva–Iturriza, A., Sipinski,
200 Burgio et al.
E., Vásquez, R. A., Widmann, P., Widmann, I., Merino, S., 2018. Can the intake of antiparasitic secondary metabolites explain the low prevalence of hemoparasites among wild Psittaciformes? Parasites and Vectors, 11: 357.
Masello, J. F., Pagnossin, M. L., Sommer, C., Quillfeldt, P., 2006. Population size, provisioning frequency, flock size and foraging range at the largest known colony of Psittaciformes: the Burrowing Parrots of the north–eastern Patagonian coastal cliffs. Emu–Austral Ornithology, 106: 69–79, Doi: 10.1071/MU04047
Masello, J. F., Quillfeldt, P., 2002. Chick growth and breeding success of the Burrowing Parrot. The Condor , 104(3): 574–586, Doi: 10.1093/ condor/104.3.574
Mazel, F., Guilhaumon, F., Mouquet, N., Devictor, V., Gravel, D., Renaud, J., Cianciaruso, M. V., Loyola, R., Diniz‐Filho, J. A. F., Mouillot, D., Thuiller, W., 2014. Multifaceted diversity–area relationships reveal global hotspots of mammalian species, trait and lineage diversity. Global Ecology and Biogeography, 23(8): 836–847, Doi: 10.1111/geb.12158
Moilanen A., Wilson K. A., Possingham, H., 2009. Spatial conservation prioritization: Quantitative methods and computational tools. Oxford University Press, Oxford, UK.
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Da Fonseca, G. A., Kent, J., 2000. Biodiversity hotspots for conservation priorities. Nature, 403: 853–858, Doi: 10.1038/35002501
Naeem, S., Duffy, J. E., Zavaleta, E., 2012. The Functions of Biological Diversity in an Age of Extinction. Science, 336(6087): 1401, Doi: 10.1126/ science.1215855
Naeem, S., Li, S., 1997. Biodiversity enhances ecosystem reliability. Nature, 390: 507–509, Doi: 10.1038/37348
Olah, G., Butchart, S. H., Symes, A., Guzmán, I. M., Cunningham, R., Brightsmith, D. J., Heinsohn, R., 2016. Ecological and socio–economic factors affecting extinction risk in parrots. Biodiversity and Conservation, 25(2): 205–223, Doi: 10.1007/ s10531-015-1036-z
Olah, G., Theuerkauf, J., Legault, A., Gula, R., Stein, J., Butchart, S., O’Brien, M., Heinsohn, R., 2018. Parrots of Oceania – a comparative study of extinction risk. Emu–Austral Ornithology, 118: 94–112, Doi: 10.1080/01584197.2017.1410066
Paradis, E., Claude, J., Strimmer, K., 2004. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics, 20(2): 289–290, Doi: 10.1093/bioinformatics/btg412
Pollock, L. J., Thuiller, W., Jetz, W., 2017. Large conservation gains possible for global biodiversity facets. Nature, 546: 141–144, Doi: 10.1038/ nature22368
Porfirio, L. L., Harris, R. M. B., Stojanovic, D., Webb, M. H., Mackey, B., 2016. Projected direct and indirect effects of climate change on the Swift Parrot, an endangered migratory species. Emu–Austral Ornithology, 116(3): 273, Doi: 10.1071/MU15094
Radchuk, V., Reed, T., Teplitsky, C., Van De Pol,
M., Charmantier, A., Hassall, C., Adamík, P., Adriaensen, F., Ahola, M. P., Arcese, P., Avilés, J. M., 2019. Adaptive responses of animals to climate change are most likely insufficient. Nature communications, 10: 3109, Doi: 10.1038/s41467019-10924-4
Reid, W. V., 1998. Biodiversity hotspots. Trends in Ecology and Evolution, 13(7): 275–280, Doi: 10.1016/s0169-5347(98)01363-9
Renton, K., Salinas–Melgoza, A., De Labra–Hernández, M. Á., de la Parra–Martínez, S. M., 2015. Resource requirements of parrots: nest site selectivity and dietary plasticity of Psittaciformes. J Ornithol, 156: 73–90, Doi: 10.1007/s10336-015-1255-9
Safi, K., Cianciaruso, M. V., Loyola, R. D., Brito, D., Armour–Marshall, K., Diniz–Filho, J. A. F., 2011. Understanding global patterns of mammalian functional and phylogenetic diversity. Philosophical Transactions of the Royal Society B Biological Sciences, 366(1577): 2536–2544, Doi: 10.1098/ rstb.2011.0024
Schmitz, O. J., Lawler, J. J., Beier, P., Groves, C., Knight, G., Boyce Jr, D. A., Bulluck, J., Johnston, K. M., Klein, M. L., Muller, K., Pierce, D. J., Singleton, W. R., Strittholt, J. R., Theobald, D. M., Trombulak, S. C., Trainor, A., 2015. Conserving biodiversity: practical guidance about climate change adaptation approaches in support of land–use planning. Natural Areas Journal, 35: 190–203, Doi: 10.3375/043.035.0120
Schweizer, M., Seehausen, O., Güntert, M., Hertwig, S. T., 2010. The evolutionary diversification of parrots supports a taxon pulse model with multiple trans–oceanic dispersal events and local radiations. Molecular Phylogenetics and Evolution, 54(3): 984–994, Doi: 10.1016/j.ympev.2009.08.021
Seddon, A. W., Macias–Fauria, M., Long, P. R., Benz, D., Willis, K. J., 2016. Sensitivity of global terrestrial ecosystems to climate variability. Nature, 531(7593): 229–232, Doi: 10.1038/nature16986
Spasojevic, M. J., Suding, K. N., 2012. Inferring community assembly mechanisms from functional diversity patterns: The importance of multiple assembly processes. Journal of Ecology, 100: 652–661, Doi: 10.1111/j.1365-2745.2011.01945.x
Srivastava, D. S., Cadotte, M. W., MacDonald, A. A. M., Marushia, R. G., Mirotchnick, N., 2012. Phylogenetic diversity and the functioning of ecosystems. Ecology Letters, 15(7): 637–648, Doi: 10.1111/j.1461-0248.2012.01795.x
Tella, J. L., Baños–Villalba, A., Hernández–Brito, D., Rojas, A., Pacífico, E., Díaz–Luque, J. A., Carrete, M., Blanco, G., Hiraldo, F., 2015. Parrots as overlooked seed dispersers. Frontiers in Ecology and the Environment, 13(6): 338–339, Doi: 10.1890/1540-9295-13.6.338
Tingley, M. W., Darling, E. S., Wilcove, D. S. 2014. Fine‐ and coarse‐ filter conservation strategies in a time of climate change. Annals of the New York Academy of Sciences, 13221: 92–109, Doi: 10.1111/nyas.12484
Turner, W. R., Bradley, B. A., Estes, L. D., Hole, D. G., Oppenheimer, M., Wilcove, D. S., 2010. Cli-
Animal Biodiversity and Conservation 45.2 (2022) 201
Burgio et al.
mate change: helping nature survive the human response. Conservation Letters, 3(5): 304–312, Doi: 10.1111/j.1755-263X.2010.00128.x
Uehling, J. J., Tallant, J., Pruett–Jones, S., 2019. Status of naturalized parrots in the United States. Journal of Ornithology, 160: 907–921, Doi: 10.1007/ s10336-019-01658-7
Urban, M. C., 2015. Accelerating extinction risk from climate change. Science, 348: 571–573, Doi: 10.1126/science.aaa4984
Wermundsen, T., 1998. Colony breeding of the Pacific Parakeet Aratinga strenua Ridgway 1915 in the Volcán Masaya National Park, Nicaragua. Tropical Zoology, 11(2): 241–248, Doi: 10.1080/03946975.1998.10539367
White, R. L., Strubbe, D., Dallimer, M., Davies, Z. G., Davis, A. J., Edelaar, P., Groombridge, J. J., Jackson, H., Menchetti, M., Mori, E., Nikolov, B. P., Pârâu, L. G., Pečnikar Živa, F., Pett, T. J., Reino,
L., Tollington, S., Turbé, A., Shwartz, A., 2019. Assessing the ecological and societal impacts of alien parrots in Europe using a transparent and inclusive evidence–mapping scheme. NeoBiota, 48: 45–69, Doi: 10.3897/neobiota.48.34222
Wilson, K. A., Auerbach, N. A., Sam, K., Magini, A. G., Moss, A. S. L., Langhans, S. D., Budiharta, S., Terzano, D., Meijaard, E., 2016. Conservation Research Is Not Happening Where It Is Most Needed. Plos Biology, 14: e1002413, Doi: 10.1371/ journal.pbio.1002413
Wright, T. F., Schirtzinger, E. E., Matsumoto, T., Eberhard, J. R., Graves, G. R., Sanchez, J. J., Capelli, S., Muller, H., Scharpegge, J., Chambers, G. K., Fleischer, R. C., 2008. A multilocus molecular phylogeny of the parrots (Psittaciformes): support for a Gondwanan origin during the cretaceous. Molecular Biology and Evolution, 25(10): 2141–2156, Doi: 10.1093/molbev/msn160
202
Assessment of endangered freshwater pearl mussel populations in
the Northern Iberian Plateau
in relation to non–native species: xenodiversity as a threat
J. Morales
Morales, J., 2022. Assessment of endangered freshwater pearl mussel populations in the Northern Iberian Plateau in relation to non–native species: xenodiversity as a threat. Animal Biodiversity and Conservation, 45.2: 203–215, Doi: https://doi.org/10.32800/abc.2022.45.0203
Abstract
Assessment of endangered freshwater pearl mussel populations in the Northern Iberian Plateau in relation to non–native species: xenodiversity as a threat. In the last two decades, small populations of freshwater pearl mussels, Margaritifera margaritifera, have been recorded in Mediterranean rivers of the Iberian Northern Plateau. A survey was carried out in Castilla and León in 2018 to assess the development of populations of this species in all the rivers of known distribution and to update the threat classification. Thirty sections in the rivers Negro, Tera, Alberche and Águeda were positive for its presence, and another 50 stretches of seven rivers were negative. The species is currently distributed over about 22.5 km. Águeda and Tera populations have decreased dramatically in the last 14 years and are on the threshold of extinction. The Negro river supports the largest population, although the species has now disappeared in at least 61 % of the stretches that were inhabited in 2004. All populations showed very low densities and an ageing population structure, with no recruitment for decades. The presence of non-native invasive alien species (NIS) was higher than in a previous regional survey, with the signal crayfish representing the greatest threat. We observed changes in benthic microhabitats and direct predation of adults and glochidia conglutinates. In the Alberche River, in strict syntopy with M. margaritifera and two other mussel species, 10 NIS were detected. The current hydrological and ecological conditions in the Duero watershed support the settlement of exotic species to the disadvantage of native mollusks, which are more demanding in terms of microhabitats.
Key words: Freshwater invasion, Pearl mussels, Survey, Declining population, Signal crayfish, Duero watershed
Resumen

Evaluación de las poblaciones de náyades en peligro de extinción en la meseta norte de la península ibérica en relación con las especies alóctonas: la xenodiversidad como amenaza. Hace dos décadas que se conocen pequeñas poblaciones de Margaritifera margaritifera en ríos mediterráneos de la meseta norte de la península ibérica. En 2018 se realizó un muestreo en Castilla y León para conocer el estado de las poblaciones en todos los ríos en los que se sabe que se encuentra esta especie y actualizar el inventario de amenazas. Resultaron positivos 30 tramos de los ríos Negro, Tera, Alberche y Águeda, mientras que otros 50 tramos de siete ríos fueron negativos. La especie se distribuye actualmente a lo largo de unos 22,5 km. Las poblaciones del Águeda y el Tera se han reducido de forma drástica en los últimos 14 años y están al borde de la extinción. El río Negro alberga la población más numerosa, aunque ha perdido al menos el 61 % de los tramos ocupados en 2004. Todas las poblaciones presentaron una densidad muy baja y la estructura poblacional envejecida por la ausencia de reclutamiento desde hace décadas. La presencia de especies exóticas invasoras fue más elevada que en el anterior muestreo regional. El cangrejo señal resultó ser la especie exótica invasora más extendida y peligrosa para las náyades. Se observaron cambios en los microhábitats bentónicos y predación directa de adultos y conglutinados de gloquidios. En el río Alberche se detectaron 10 especies exóticas invasoras en sintopía con M. margaritifera y otras dos especies de náyades. Las actuales condiciones hidrológicas y ecológicas en la cuenca del Duero favorecen el asentamiento de especies exóticas, que son menos exigentes con los microhábitats que los moluscos nativos.
ISSN: 1578–665 X eISSN: 2014–928 X
© [2022] Copyright belongs to the authors, who license the journal Animal Biodiversity and Conservation to publish the paper under a Creative Commons Attribution 4.0 License
203 Animal Biodiversity
and Conservation 45.2 (2022)
Palabras clave: Invasión dulceacuícola, Náyades, Muestreo, Reducción de la población, Cangrejo señal, Cuenca del Duero
Received: 14 II 22; Conditional acceptance: 04 IV 22; Final acceptance: 25 V 22
Javier Morales, Department of Animal Biology. Salamanca University, Campus Miguel de Unanumo, E–37007 Salamanca, Spain.
Corresponding author: Javier Morales. E-mail: mormarja@usal.es
ORCID ID: 0000-0002-7063-563X
204 Morales
Introduction
Freshwater pearl mussels (Margaritifera margaritifera Linnaeus, 1758) are a target species for the conservation of oligotrophic stream ecosystems throughout Europe (Geist, 2010). The species has been reported in the Spanish Duero River basin for two decades (Morales et al., 2004). During this period, while pearl mussels have been in decline, numerous aquatic species exotic to the Iberian Peninsula have become established in the same stretches of mountain rivers. According to Spanish legislation (Executive order 630/2013), many are considered invasive non–native species (NIS), and are included in the European Union strategy to combat NIS. Two exotic American crayfish (Pacifastacus leniusculus [Dana, 1852] and Procambarus clarkii [Girard, 1852]) are taxa of special concern (EU 1143/2014 and EU 1141/2016 Regulations) as reported by Vaeben and Hollert (2015). In the Iberian Peninsula M. margaritifera (Mm) has not been in contact with crayfish species and thus has no self–protective measures, either active or passive. In a review of 124 publications, Downing et al. (2010) showed that the presence of exotic species is the fourth most relevant cause of the decline of fresh water mussels.
These NIS have a negative impact on the conservation of this mussel, producing transformations in physical, chemical and/or ecological benthic microhabitats, typically through trophic networks (Dorn and Wojdak, 2004; Beisel and Lévêque, 2010) and interactions between species and synergy between pressures (fig. 1s in supplementary material). This negative effect is felt not only by native fauna communities through competition for food or substrate refuge, predation and territorial exclusion due to modifications in the food chain, but also pearl mussels, species with restricted mobility, and young brown trout (Salmo trutta fario) acting as hosts for glochidia larvae (Stenroth and Nyström, 2003). Some mollusks, such as the Asian clam Corbicula fluminea (O. F. Müller, 1774) or the mud snail Potamopyrgus antipodarum (Smith, 1889), can alter the oxygenated sand and gravel banks that juvenile mussels need for their growth, creating compacted and anoxic sediments (Sousa et al., 2008a, 2008b; Ferreira–Rodríguez et al., 2018). In addition, hypoxia increases their vulnerability to predators (Saloom and Duncan, 2005). This reduces the viability of mussels because of their high filtration rate, and effects them negatively due to the ammonium produced and excreted (Modesto et al., 2019). Furthermore, in sediments that are massively colonized by these exotic mollusks, the microbial communities –which control the biogeochemical cycling of nitrogen– undergo changes (Black et al , 2013), dominating communities as engineers (Sousa et al., 2009) by imposing their conditions on microhabitats, and reducing the recruitment of juvenile mussels that need highly specific epibenthic physicochemical conditions (Geist and Auerswald, 2007).
In the rivers studied, we found two exotic freshwater crayfish (NICs), the red swamp crayfish P. clarkii, and the signal crayfish P. leniusculus, both of which
have become widely dispersed within the Spanish hydrographic network since the 1960s. Both produce cumulative effects in terms of the physical alteration of the riverbed (by excavation of galleries, removal of sediments or herbivory on submerged plants) and ecological changes in underwater communities (Crawford et al., 2006).
Vaeben and Hollert (2015) showed that invasive crayfish in European rivers not only decimate populations of other benthic invertebrates, but also submerged plants, and epilithon, which are primary resources for trophic networks. They also showed how these NICs displace fish from their shelters and even prey on juveniles (Stenroth and Nyström, 2003; Peay et al., 2009; Gladman et al., 2012). In this way, they reduce the viability of host populations and cause the medium– and long–term decline of pearl mussels by changing the structure of the community and trophic networks (Vaughn et al., 2008; Inoue et al., 2017). They also prey on mussels (Machida and Akiyama, 2013; Meira et al., 2019; Dobler and Geist, 2022) and on the other benthic macroinvertebrate communities (Stenroth and Nyström, 2003; Ercoli et al., 2015), destabilizing trophic networks at various levels (Dorn and Wojdak, 2004). The signal crayfish can occur in high densities and its greater activity than that of native crayfish can further exacerbate problems for native species (Wutz and Geist, 2013).
In all cases, its presence is associated with increased turbidity and the formation of cyanobacterial blooms (Yamamoto, 2010; Turley et al., 2017) that are detrimental to epibenthic filter–feeding communities. In addition, Liräs et al. (1998) observed how crayfish accumulate cyanotoxins during toxic algae blooms (HAB) (Vasconcelos et al., 2001), moving them further up the food chain. Given these effects, signal crayfish are considered one of the most dangerous invaders in European waters (Gherardi et al., 2011) for several species, including freshwater mussels, as evidenced by Meira et al. (2019) in other Iberian rivers.
The aim of this study was to update the distribution of M. margaritifera in Mediterranean rivers of the Iberian Northern Plateau, locate populations of other mollusks in syntopy, and identify conservation problems related to the proliferation of aquatic exotic species, including the abundance of the signal crayfish in the Negro River. We also sought to identify the basic descriptors of the population of this endangered species.
Material and methods Study area
In summer 2018 we sampled eight rivers belonging to the Duero, Tajo and Miño–Sil watersheds in the northern plateau of the Iberian Peninsula. The sampling areas, approximately 80 km in length, were selected for survey within 15 UTM 10x10 km squares (cUTM) and are included in the management plan for species in the Autonomous Community of Castilla and León (Spain) in rivers of the Bibey, Tuela, Castro, Tera,
Animal Biodiversity and Conservation 45.2 (2022) 205
Negro, Águeda, Duero and Alberche sub–basins (Morales et al., 2007ab), all of which are included in the European Natura2000 network. In complementary terms, in 2015–2017, surveys were carried out to search for the possible presence of shells on the banks of seven other nearby rivers: rivers Tormes, Eria, Órbigo, Cea, Esla, Aliste and Manzanas. All these rivers have Mediterranean pluvial hydrological dynamics during cool summers (continental with intense low water levels and hot summers, Csb, Köppen and Geiger classification). In addition, these rivers have a certain Atlantic influence, with high amounts of autumn and spring precipitation but low winter snowfall. Precipitation in the valley areas ranges between 800 and 1,000 mm/y, and up to 1,500 mm/y in the headwaters (Morales and Lizana, 2014). The riverbeds are affected by high–summer low water levels and high–winter floods. All these watercourses have a perennial flow, with the exception of the River Águeda in August and September (Morales, 2020).
All the rivers studied have acidic and cold waters, with low–mineralization. They are oligotrophic in nature (Morales et al., 2004) and have a typical pool–riffle sequence to lotic hydrological conditions. The dominant substrate is composed of blocks, coarse gravel, and sand, except for silt areas with of lenitic conditions (caused by old traditional watermills and dams for livestock). The riparian vegetation is dominated by alluvial forests containing alder (Alnus glutinosa) and ash (Fraxinus excelsior and F. angustifolia) (corresponding to 91E0(*) N2000 code habitat: Alno–Padion, Alnion incanae, Salicion albae), poplar (Populus spp.) and willow (Salix spp.) trees. All populations are considered within the Natura2000 Special Areas of Conservation, with rivers that also hold other endangered species such as Macromia splendens, Oxygastra curtisii, Gomphus graslinii, Galemys pyrenaicus, Rana iberica and Cobitis calderoni. Other conservation values in midstream watercourses are present in two habitats of interest: 3250/constantly flowing Mediterranean river with Glaucium flavum and 3260/floating vegetation of Ranunculus of plain, submountainous rivers.
Survey methods
The surveys were carried out during the summer–autumn season during the seasonal drought and
when the rivers were at a low water phase. First, we searched for shells on the gravel banks of the riverbanks and the river channel. We then searched for adults in the wadeable areas using an opaque–walled bathyscope. In the 2018 survey, in each 10 x 10 km UTM grid (cUTM onwards), we sampled at least 1 km of watercourse in five stretches of varying length, following the recommendations of Leppänen (2018) for rivers with a low abundance of mollusks and the basic principles presented in the European CEN standard on monitoring of the fresh water pearl mussel (Boon et al., 2019). The riverbeds, in the wadeable areas, were thoroughly inspected (250–300 m in length) when the water level was at its lowest. We covered a total of 21.9 km in 81 georeferenced sections, using a sampling method that involved counting 50 m on the shoreline in 48 wadeable plots: totaling 17,200 m2. Mussel density is expressed in specimens per 100 m2. In the 2015–2017 shell survey, various distances (90–650 m) were covered in 37 transects of varying length on 29 cUTM in locations with environmental conditions for this species. The sampling locations and the minimum sampling area required were determined using the criteria established by experts.
We verified the presence of Mm juveniles in all stretches by sieving the sands and gravels inhabited by adults in sections using standard stainless steel sieves (1.0, 0.5, and 0.1–mm pore and 25 cm in diameter). In addition, when carrying out the underwater counts, we also quantified the presence of trout fry and other fish species, and signal crayfish. The abundance of crayfish (Pl) and brown trout (St) is expressed per sampled plot.
In the Negro River, we used traps baited with decomposing fish to determine the abundance of signal crayfish, and we placed groups of four closed pots (40 cm diameter) 50 m from the banks for 48 h in areas occupied by pearl mussels. All amounts captured are expressed as individuals per hour and trap (Pl/h–t). The mussel shells and crayfishes were measured 'in situ' using calipers (total length with 0.1 mm precision) and collected and stored in the laboratory for inspection for crayfish claw marks. Biometric data were grouped into intervals (using Sturges' rule) to construct size pyramids, and the comparison between sets was carried out using Welch's bilateral F–test for unequal variances.
Fig. 1. Location of the UTM grid including the range distribution of M. margaritifera in the Iberian Peninsula, study area and the distribution of P. leniusculus in the Castilla and León (CyL) (according to Spanish Biodiversity Database: MITECO, 2019). Hydrographical network are shown: RAG, River Águeda; RNE, River Negro; RTE, River Tera; RCA, River Castro; RDU, River Duero; RTU, River Tuela; RAL, River Alberche; RBI, River Bibey.
Fig. 1. Localización de la cuadrícula UTM que incluye el rango de distribución de M. margaritifera en la península ibérica, la zona de estudio y la distribución de P. leniusculus en Castilla y León (CyL) (según la Base de Datos de la Biodiversidad de España: MITECO, 2019). Se muestra la red hidrográfica: RAG, río Águeda; RNE, río Negro; RTE, río Tera; RCA, río Castro; RDU, río Duero; RTU, río Tuela; RAL, río Alberche; RBI, río Bibey.
206 Morales
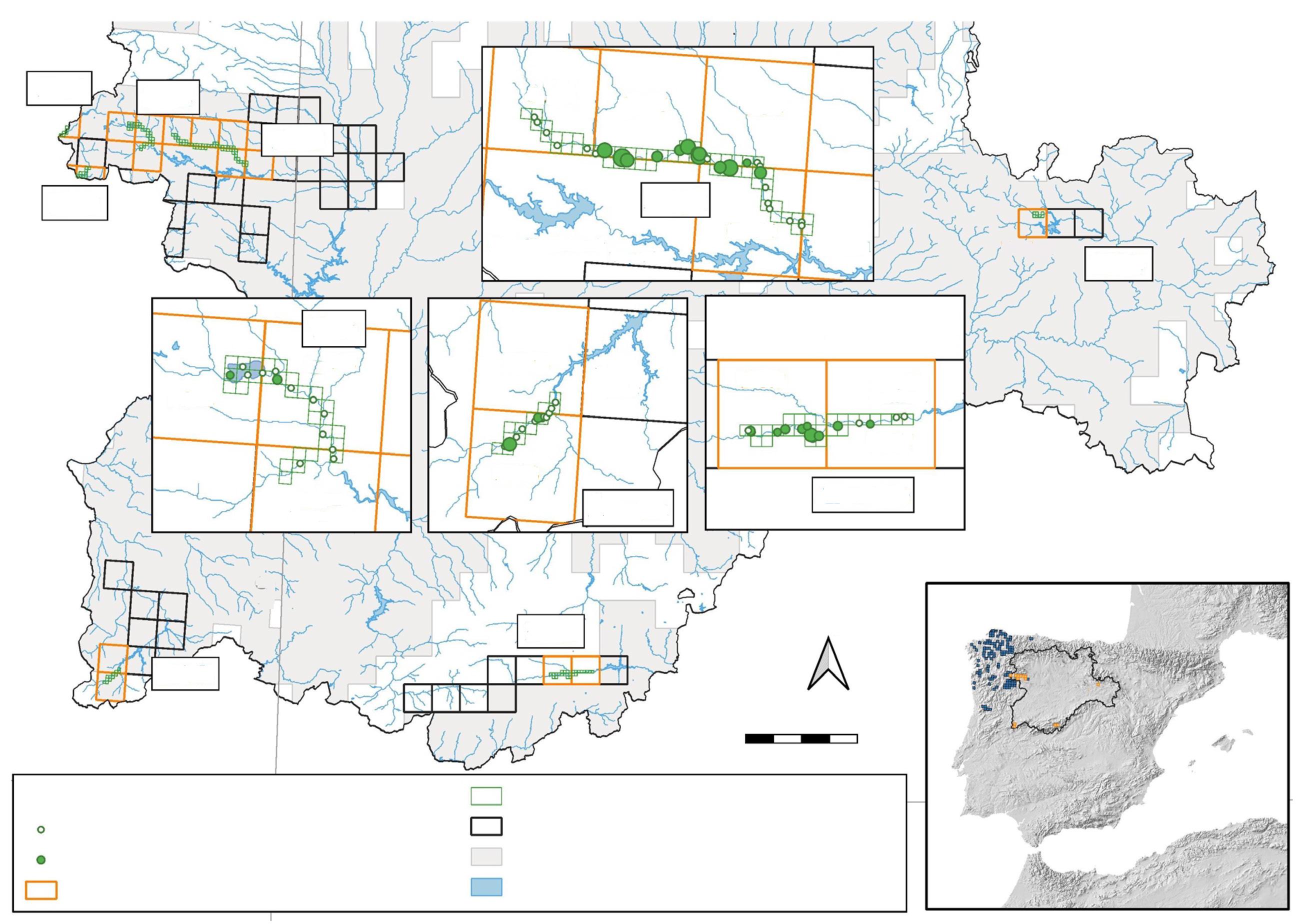
207 Animal
RAG RAL RDU Tera Águeda Alberche Negro QG06 QG16 QG26 QG25 PG86 PG96 PE97 PE96 UK47 UK57
N 0 10 20 30 40 km CyL 29T 30T Cernadilla Reservoir Irueña Reservoir Burguillo Reservoir Cernadilla Reservoir Valparaíso Agavanzal Margaritifera margaritifera survey 2018 Negative Positive (by population size) cUTM 10 x 10 (Morales et al., 2004, 2007) cUTM 1 x 1 (2018 survey) Negative cUTM 10x10 (2015-2018 survey) P. leniusculus distribution Hydrographic network
Biodiversity and Conservation 45.2 (2022) RBI RTE RNE RTU
WM13
Table 1. Results of the M. margaritifera survey and population status changes in the last fourteen years (2004–2018) in eight rivers of Castilla and León (for abbreviations of revers, see fig. 1): * the distribution of the species in this river is not natural, since specimens were rescued and transferred to Irueña reservoir and grouped in seven localities (Morales and Lizana, 2012); ** mussel (mu) population status according to Swedish standards (Degerman et al., 2009): 1, viable (> 20 % < 5 cm and > 0 % < 2 cm, > 500 mu); 2, viable? (> 20 % < 5 cm or >10 % < 5 cm and > 0 % < 2 cm, > 500 mu); 3, non–viable (20 % < 5 cm or > 20 % < 5 cm and < 500 mu); 4, dying out (all > 5 cm, many individuals, > 500 mu); 5, almost extinct (all > 5 cm, few individuals, < 500 mu); 6, extinct (documented presence that has disappeared); relative abundance of freshwater mollusk species (++ abundant; + presence; – absence; = unchanged).
Tabla 1. Resultados del último muestreo de M. margaritifera y los cambios demográficos en los últimos 14 años (2004–2018) en ocho ríos de Castilla y León (para las abreviaciones de los ríos, véase la fig. 1): * la distribución de la especie en este río no es natural, ya que los ejemplares fueron rescatados del embalse de Irueña y agrupados en siete localidades (Morales y Lizana, 2012); ** estatus de población de náyades (mu) según los tramos estandarizados en Suecia (Degerman et al., 2009): 1, viable (> 20 % < 5 cm ó >10 % < 5 cm y > 0 % < 2 cm, > 500 mu); 2, ¿viable? (> 20 % < 5 cm or > 10 % < 5 cm and > 0 % < 2 cm, > 500 mu); 3, no viable (< 20 % < 5 cm ó > 20 % < 5 cm, < 500 mu), 4, agonizante (todos > 5 cm, bastantes individuos, > 500 mu); 5, al borde de la extinción (todos > 5 cm, pocos ejemplares, < 500 mu); 6, extinta (presencia documentada de que ha desaparecido); abundancia relativa de especies de moluscos de agua dulce (++ abundante; + presencia; – ausencia; = sin cambios).
RAG RCA RNE RTE RTU RDU RAL RBI
Stretches
Number 12 1 28 7 5 5 15 4 Distance (km) 2.14 0.26 6.83 3.03 1.91 0.42 3.77 1.03
Positive survey
Frequency (%) 25 (*) 0 46 29 0 0 73 0
Density in plots (mu/100 m2)
Median 1.60 (*) 0 5.04 1.43 0 0 0.66 0 Maximum 7.41 (*) 0 20.65 2.45 0 0 1.87 0
Population changes 2004–2018
Estimated population decrease (%) > 95 40–54 81 0 100 = Estimated km occupied decrease (%) 93 61 90 0 100 = cUTM 1 x 1 km variation –9 –1 –16 –5 0 –3 –1 –1
Population status (**) 5 6 4 5 – 6 5 6
Other aquatic mollusk species in 2018
Unio delphinus + – – – – – + –Anodonta anatina – – + – – – ++ –Ancylus fluvitalis – + ++ ++ + – + + Radix balthica + – – + – – + –
Results
Mussel surveys
We found live pearl mussels (Mm: Margaritifera margaritifera) in three of the 15 rivers surveyed in 2018, and confirmed their absence in two rivers where they had been known to inhabit. A total of 30/81 stretches (37.5 %) along 22.5 km of the
Negro, Tera, Alberche and Águeda rivers showed positive signs in the 2018 survey. This indicated that the species inhabited 29.9 % of the potential area (fig. 1) initially established, but all samples in the upper Duero, Bibey, Tuela and Castro were negative (table 1). In addition, all epibenthic sediment points screening out for juvemiles (n = 30) were negative in 2018. The largest population (90.4 %) was found in the Negro River (fig. 2), at an altitudinal range
208 Morales
WM13
1 3 9 27 81 243 729
Number of pearl mussels
Mm
1 3 9 27 81 Number of trout/crayfish
Fig. 2. Number of specimens for the studied species: A, pearl mussels (Mm); B, brown trout (St) and signal crayfish (Pl) found in the 2018 survey (cUTM's 10 x 10 km plots).
Fig. 2. Número de ejemplares de las especies estudiadas: A, náyade (Mm); B, trucha (St) y cangrejo señal (Pl) encontrados en el muestreo de 2018 (parcelas cUTM 10 x 10 km).
of 802–913 m a.s.l. and consisting of about 2,000 mussels scattered over 15 km; total 23.5 km less than in the first survey. This was the only population for which we obtained counts of more than 100 animals per section. Moreover, this same location is where we found the most mussels in clumps, with up to 13 specimens in each cluster.
In the Alberche River, we found an estimated population of about 320 mussels in small clusters, scattered along 17.5 km of the riverbed. In the other two rivers that tested positive, the Tera and the Águeda, the number of mussels recorded barely reached 50 specimens in each river (fig. 2), representing a reduction of more than 90 % in a period of 14 and nine years, respectively. The average density in plots ranged between 0.66 Mm/100 m2 (max: 1.9) in the Alberche River and 5.04 Mm/100 m2 in the Negro (fig. 2). In the most favorable area of the Negro River, maximum density values of 20.6 Mm/100 m2 were detected. In 2 plots, the density exceeded 20 Mm/100 m2, in 34 plots it was less than 10, and in 14 plots it was less than 1 Mm/100 m2
We found four other species of freshwater mussels (table 1) in the stretches occupied by M. margaritifera Unio delphinus and Anodonta anatina were abundant, especially in the Alberche River. No specimens or shells of Potomida littoralis were found.
NIS Species in pearl mussel rivers
The pearl mussels were found to be in strict syntopy with 12 aquatic species exotic to Iberian fauna (table 2), and NIS were found in 55 % of the plots, with a species richness of 1–4 species per plot. The Alberche River showed the highest xenodiversity, with 10 aquatic NIS identified.
In the Negro River, we observed the presence of signal crayfish (Pl: Pacifastacus leniusculus) in 20 out of the 28 plots surveyed, with a maximum abundance of 13 Pl/plot. In 3 out of 5 cUTM (fig. 1) containing pearl mussel populations, there were indications of interactions with signal crayfish. In addition, in 17 shells (28.3 % of the measurements), we observed gnawed edges, and some also showed the mantel was gnawed. We also found larvae and subadults of long–legged frogs (Rana iberica) and partially mutilated fish fry. In the Alberche and Águeda rivers, mussels shared benthic habitats in syntopy with the red swamp crayfish (P. clarkii), although we observed no evidence of predation. In the initial surveys (2004–2006) we did not detect this exotic species in any of the rivers studied.
A statistically significant negative relationship was found between the abundance of mussels and signal crayfishes in relation to the counts and plot density
Animal Biodiversity and Conservation 45.2 (2022) 209
PE97 PE96 UK57 UK47 QG25 QG26 QG16 QG06 PG86 PG96 PG74 PG66 RBI RTU RTE RNE RAL RAG RDU
WM13 PE97 PE96 UK57 UK47 QG25 QG26 QG16 QG06 PG86 PG96 PG74 PG66 RBI RTU RTE RNE RAL RAG RDU Pl St
Table 2. Presence of the non–native aquatic species in nine rivers of Castilla and León with past and recent populations of M. margaritifera. The year in which the presence of the species was discovered in the area is indicated in brackets: * not in strict syntopy. (For abbreviations of rivers, see fig. 1).
Tabla 2. Presencia de especies acuáticas exóticas invasoras en nueve ríos de Castilla y León con poblaciones históricas y recientes de M. margaritifera. El año en que se descubrió la presencia de la especie en la zona se indica entre paréntesis: * no en estricta sintopía. (Para las abreviaturas de los ríos, véase la fig. 1).
Watersheds Duero Tajo Miño–Sil Lake Sanabria–
Rivers RAG RNE RTE RCA RTE RDU RTU RAL RBI (2002) (2001) (2000) (2000) (2014) (2012) (2000) (2006) (2007)
Natura 2000 ID code ES4150032 ES4190067 ES4190105 ES4170083 ES4190131 ES4110078 ES1130007 Altitudinal range (m.a.s.l.) 796–772 965–789 1,000–960 925–910 1,004 1,097–1,084 791–735 835–834 1,002–993 Diatoms
Didymosphenia geminata • Ferns
Azolla filiculoides • • Mollusks
Corbicula fluminea • (*) • (*)
Physella acuta • • (*) •
Potamopyrgus antipodarum • Crustaceans
Procambarus clarkii • •
Pacifastacus leniusculus • • • (*) • •
Fishes
Gambusia holbrooki • • (*)
Lepomis gibbosus • • (*) •
Micropterus salmoides • (*)
Alburnus alburnus • (*) • (*) •
Mammals
Neovison vison • • • • • (*) • • (*) • •
Total xenodiversity = 12 5 4 4 1 2 5 1 10 1
210 Morales
Shell length (mm)
Shell height (mm)
R2 = 0.038 F (1,193,65) = 23.72, p ≤ 0.0001 90.286 (11.18) 43.858 (2.282)
95.295 (5.557) 42.834 (4.231)
R2 = 0.011 F (1,177,13) = 6.32, p = 0.0128 2004 2018 2004 2018
Fig. 3. Biometric analysis (A, scatter and violin plot; B, histogram) of shell–size in the two surveys in Negro River separated by a 14–year period (average and standard deviation indicated in brackets).
Fig. 3. Análisis biométrico (A, diagrama de dispersión y violín; B, histograma) de tamaños de la concha en los dos muestreos del río Negro, realizados con 14 años de diferencia (media y desviación estándar indicadas entre paréntesis).
(Pl = 1.1883 exp [0.7263 x Mm]; r = 0.335, p = 0.02 / Pl = 1.7638 exp [0.0021 x Mm]; r = 0.296, p = 0.007 respectively), but not with the abundance of brown trout (St: Salmo trutta var fario) during the pearl mussel spawning season (St = 2.3012 exp [–0.021 x Mm]; r = 0.1364, p = 0.37].
Signal crayfish in the Negro River
Adult signal crayfish were caught in 5 out of 6 trapping sessions, over a course of 89 hours of surveying, with results of between three and 48 crayfish per session: the signal crayfish (n = 98) abundance fluctuated between 1.021 and 0.047 Pl/h–t. Some differences were found between the abundance of crayfish in the lower reaches of the river and upstream. The average size of females was 5 mm larger than that of males, which were less abundant in all stretches surveyed. The sex ratio was close to 1 male for every 2 females, with a maximum of 4:9. During crayfish trapping, 156 cyprinids and 0 trout were captured passively.
Mussel populations trend
In 2018, the population sizes found were smaller in all cases, with reductions of more than 80% in the
Tera and Águeda rivers (table 1), and less in the other rivers. This reduction equated to a loss of 71.7 % of the area occupied 14 years earlier, and currently only four rivers remain inhabited. The largest population was again found in the Negro River, and in 2018 a cluster of 617 pearl mussels, a higher count than in the previous survey, was located, despite an estimated 40 % reduction in the population and with an 8–km stretch of the riverbed being less inhabited.
Figure 3 compares the change in size (TL) of mussels found in the riverbed during both surveys of the Negro River that had died due to natural causes. The sizes considered medium were larger in 2018 (TLMe2004 = 92.0 vs TLMe2018 = 95.5 mm) and were mainly in the range of 90–105 mm (83,3 % in 2018 and 55,9 % in 2004). Comparing the size pyramids, we observed a statistically significant shift of 5 cm in the modal length frequencies, which could correspond to demographic changes over the 14–year period (F = 23.7, p < 0.001 for length (TL) and F = 6.3, p = 0.01 for shell height). The decades–long absence of juvenile recruitment –already detected in 2004–was confirmed, as was a reduction in the presence animals with an LT < 85 mm (table 3). This indicates a significant reduction in the number of younger animals and an increase of 27.4 % in older individuals.
Animal Biodiversity and Conservation 45.2 (2022) 211
A B 120 110 100 90 80 70 60 50 120 105 90 75 60 45 55 50 45 40 35 30 25 54 48 42 36 30 24
Table 3. Size distribution (by ranks) of M. margaritifera in the 2004 (229 shells) and 2018 (60 shells) in Negro River (RNE) surveys: TL, interval shell lenght (in mm); n, sample size; p, number positive plots.
Tabla 3. Distribución de la talla (por rangos) de las conchas de M. margaritifera en los muestreos del río Negro (RNE) de 2004 (229 conchas) y en 2018 (60 conchas): TL, longitud del caparazón; n, tamaño de muestra; p, número de parcelas positivas.
RNE 2004 survey 2018 survey
TL (mm) (p = 67) (p = 28) < 70 4.34% 0.0% (n = 10)
70–80 9.61% 1.67% (n = 22) (n = 1) 80–90 30.13% 15.00% (n = 69) (n = 9) 90–100 39.30% 66.67% (n = 90) (n = 40) 100–110 16.59% 16.67% (n = 38) (n = 10)
Discussion
Here we studied populations of M. margaritifera in the Northern Iberian Plateau. Small, non–functional groups of spatially isolated metapopulations had been discovered in these rivers over the last two decades (see review at Araujo et al., 2009; Lopes–Lima et al., 2017) but the numbers of this species have decreased, the distribution area has shrunk, and there is a total absence of recruitment (Morales et al., 2004). This species is typical of very shallow waters in turbulent rivers with very clear water and as the animals do not bury themselves in the summer they can be studied without using snorkeling or diving techniques, as is recommended for species that live in deeper waters (Degerman et al., 2009; Boon et al., 2019). In the Iberian Peninsula, these populations are the most threatened since according to the classification of the population status (table 1) in Sweden (Degerman et al., 2009), they are below the minimum viable population size, contrasting with Iberian populations in Galicia or Portugal (Lois et al , 2014; Sousa et al., 2015) and others in central Europe (Geist and Auerswald, 2007; Stoeckle et al., 2020).
In the Alberche River, the presence of aquatic NIS is higher than in the other rivers in the study, but there are better conditions in the sediment and more abun-
dant water flow in summer since more trout and fewer animals were found here than in the Negro River. Of particular concern is the presence of signal crayfish in the stretches observed in this study; they were found to live in strict syntopy with mussel species and are an increasing pressure (Morales et al., 2007ab). Added pressures include the lack of summer flow, warmer waters, and the dumping of untreated urban organic discharge into rivers and runoff that occurs during events linked to extreme climatic events (Diez et al., 2012; Morales and Lizana, 2014). NIS are greatly associated with the negative impact that influences the conservation of M. margaritifera, and act negatively on host species, water quality and alder conservation (more details in fig. 1s in supplementary material).
The conservation problems and pressures on the habitat responsible for this decline are not only local in nature within each stretch but also exist on a larger (sub–basin, regional, etc.) and more global scale, with multiple synergies and both spatial and temporal dimensions (fig. 1s in supplementary material). The problems derive mainly from a reduction in the quality of epibenthic microhabitats due to lack of water flow (reduction of precipitation, especially snow), the predominance of waters that are warmer than what is normally expected in trout spawning areas, and the extremely low water levels during extreme periodic droughts (Morales and Lizana, 2014; Garrido Nogueira et al., 2019) (more details are given in figures 1s and 2s in supplementary material).
Local anthropic pressures and climatic events are shared by all populations and affect the physicochemical conditions of the water through nutrient enrichment and warming, scarce longitudinal connectivity due to the succession of dams, and the frequent clouding produced by summer storms, which fall with great erosive power on areas without forest cover or on burned areas (Morales et al., 2007ab; more details in fig. 1s in supplementary material). As a result, sinkholes are lost due to siltation of gravels,clays and silts (Morales et al., 2004; Morales and Lizana, 2014), thereby increasing glochidia mortality (Ziuganov et al., 1994). Given the precarious demographic situation of this species, at or below the minimum viable level in all populations (table 1), each individual is important. During the breeding season, pearl mussels expel a mass of whitish–colored, dense–looking mucilage, the conglutinate. This mass attracts the fry that will then be infected by the glochidia when they are handled at the time of trying to eat them. We have found that crayfish consume the embryos and remove the adults by extracting them from the sandy substrate. While the adults are in the horizontal position they are vulnerable to the current dragging them along and are unable to find a vertical position in the riverbed. The resulting effect is unknown as they may eventually be flushed out during floods (Morales and Lizana, 2014).
The most negative effect on these almost sessile mollusks is direct predation, with crayfish being the only predator in the rivers studied, as occurs in other rivers (Meira et al., 2019; Sousa et al., 2019; Dobler and Geist, 2022). Colonization of P. leniusculus is positively related to winter temperature and positive connectivity of gravel beds (Nyström et al., 2006; Olsson,
212 Morales
2008); they also prefer this type of habit together with M. margaritifera. Degerman et al. (2009) report a similar scenario to the Negro River; they support the eradication of crayfish and activities to restore habitats in order to ensure the conservation of mussels.
The presence of P. leniusculus has been documented for no more than 10 years in the Negro and Alberche rivers, and the highest densities are found in the stretches where most mussels live. Nonetheless, during this period of expansion no control actions have been carried out to date. In both cases, the maximum trout density coincides with the absence of mussels. Vaeben and Hollert (2015) showed that the most negative effects of crayfish are related to their increasing density in the riverbed, indicating the need to initiate their immediate removal. Both U. delphinus and A. anatina have undergone an equally intense decline in the Negro River; while both species are frequent in the Alberche River, coinciding with a lower presence of crayfish in the mussel beds. In the first survey, no such NIS species were present in the stretches occupied by freshwater mussels (Morales et al., 2004, 2007b).
No conclusive relationship has been found between xenodiversity and the presence/absence or abundance of M. margaritifera, but there is a lower density of trout in both the mussel survey and crayfish plots, and this could be the first early symptom of a serious problem. Moorhouse and Macdonald (2011) have shown that it is possible to control crayfish in rivers through early and rigorous action, thus preventing their expansion along the riverbed and slowing down the simplification of communities and the disruption of trophic networks (Dorn and Wojdak, 2004; Machida and Akiyama, 2013; Meira et al , 2019) with the synergistic presence of NIS species.
Conclusion
This study shows urgent management measures are needed to eliminate or control the presence of exotic species and reduce their negative effects on the most Mediterranean populations of pearl mussels. There is a need to develop a specific conservation plan adapted to their special characteristics: low density, isolation, ageing and lack of recruitment, declining trout host populations, siltation of the beds and hydrological uncertainty accentuated by climate change. These effects are likely to be exacerbated in the very near future by the invasion of alien species.
Effective conservation requires prioritizing research and actions to identify extrinsic factors (environmental variables and threats) and mitigate current and potential direct impact on conservation status in a short timeframe. This can only be achieved by improving the environmental conditions (hydrological and biological) that mussels, trout and the rest of the native benthic community need. This is a priority for the conservation of these species at risk of extinction, especially considering the increasing hydrological pressure from the consequences of climate change (O'Briain, 2019) and the critical threat from the signal crayfish, as demonstrated in experimental and field studies in Iberian (Meira et
al., 2019; Sousa et al., 2019) and European rivers (Dobler and Geist, 2022). Over the past decades, the loss of integrity in the biological communities of these rivers and the consequent absence of trout fry when glochidia are present in the water has exacerbated the difficulty for M. margaritifera to complete its life cycle.
The presence of xenobiota endangers the survival of aging mussel adults, the only source of genetic material for implementing conservation plans in situ and ex situ in the immediate future. In conclusion, the current river conditions favor the settlement of exotic species of wide ecological valence and induce the decline of native mollusks that are more demanding in their naturally occurring microhabitats.
Acknowledgements
The 2018 survey was promoted by the Environmental Control Network of the Government of Castilla y León, with co–financing from FEADER financial resources, under 'Study for the updating of the knowledge of the population situation of Margaritifera margaritifera in Castilla and León, EN–09/2018' project. The mussel surveys were authorized and supervised by technical staff from the Junta de Castilla and León in Valladolid. Special thanks to R. Romero, E. Peñín, D. Fernández and F. Álvarez–Collado for help with the fieldwork. Ms. Emma–Jane Keck and PhD Miguel Lizana revised the English and enhanced the initial manuscript, and two anonymous reviewers improved the definitive version.
References
Araujo, R., Reis, J., Machordom, A., Toledo, C., Madeira, M. J., Gómez, I., Velasco, J. C., Morales, J., Barea, J. M., Ondina, P., Ayala, I., 2009. Las náyades de la Península Ibérica. Iberus, 27(2): 7–72.
Beisel, J. N., Lévêque, C., 2010. Introductions d’espèces dans les milieux aquatiques. Faut–il avoir peur des invasions biologiques? Editions Quae, Versalles, France. Black, E. M., Chimenti, M. S., Just, C. L., 2013. Effect of freshwater mussels on the vertical distribution of anaerobic ammonia oxidizers and other nitrogen transforming microorganisms in upper Mississippi river sediment. PeerJ, 5: e3536, Doi: 10.7717/peerj.3536
Boon, P. J., Cooksley, S. L., Geist, J., Killeen, I. J., Moorkens, E. A., Sime, I., 2019. Developing a standard approach for monitoring freshwater pearl mussel (Margaritifera margaritifera) populations in European rivers. Aquatic Conservation: Marine and Freshwater Ecosystems, 29(8): 1365–1379, Doi: 10.1002/aqc.3016
Crawford, L., Yeomans, W. E., Adams, C. E., 2006. The impact of introduced signal crayfish Pacifastacus leniusculus on stream invertebrate communities Aquatic Conservation: Marine and Freshwater Ecosystems, 16(6): 611–621, Doi: 10.1002/aqc.761
Degerman, E., Alexanderson, S., Bergengren, J., Henrikson, L., Johansson, B. E., Larsen, B. M., Söderberg, H., 2009. Restoration of freshwater pearl mussel streams. WWF Sweden, Solna.
Animal Biodiversity and Conservation 45.2 (2022) 213
Available online at: https://webgate.ec.europa.eu/ life/publicWebsite/index.cfm?fuseaction=search. dspPage&n_proj_id=2616
Diez, J. M., D'Antonio, C. M., Dukes, J. S., Grosholz, E. D., Olden, J. D., Sorte, C. J., Blumenthal, D. M., Bradley, B. A., Early, R., Ibáñez, I., 2012. Will extreme climatic events facilitate biological invasions? Frontiers in Ecology and the Environment, 10: 249–257, Doi: 10.1890/110137
Dobler, A. H., Geist, J., 2022. Impacts of native and invasive crayfish on three native and one invasive freshwater mussel species. Freshwater Biology, 67: 389–403, Doi: 10.1111/fwb.13849
Dorn, N. J., Wojdak, J. M., 2004. The role of omnivorous crayfish in littoral communities. Oecologia, 140: 150–159, Doi: 10.1007/s00442-004-1548-9
Downing, J. A., Van Meter, P., Woolnough, D. A., 2010. Suspects and evidence: a review of the causes of extirpation and decline in freshwater mussels. Animal Biodiversity and Conservation, 33(2): 151–185, Doi: 10.32800/abc.2010.33.0151
Ercoli, F., Ruokonen, T. J., Koistinen, S., Jones, R., Hämäläinen, H., 2015. The introduced signal crayfish and native noble crayfish have different effects on sublittoral macroinvertebrate assemblages in boreal lakes. Freshwater Biology, 60: 1688–1698, Doi: 10.1111/fwb.12601
Ferreira–Rodríguez, N., Sousa, R., Pardo, I., 2018. Negative effects of Corbicula fluminea over native freshwater mussels. Hydrobiologia, 810: 85–95, Doi: 10.1007/s10750-016-3059-1
Garrido–Noguiera, J., Lopes–Lima, M., Varandas, S., Teixeira, A., Sousa, R., 2019. Effects of an extreme drought on the endangered pearl mussel Margaritifera margaritifera: a before/after assessment. Hydrobiologia, 848: 3003–3013, Doi: 10.1007/ s10750-019-04103-1
Geist, J., 2010. Strategies for the conservation of endangered freshwater pearl mussels (Margaritifera margaritifera): A synthesis of Conservation Genetics and Ecology. Hydrobiologia, 644: 69–88, Doi: 10.1007/s10750-010-0190-2
Geist, J., Auerswald, K., 2007. Physicochemical stream bed characteristics and recruitment of the freshwater pearl mussel (Margaritifera margaritifera). Freshwater Biology, 52: 2299–2316, Doi: 10.1111/j.1365-2427.2007.01812.x
Gherardi, F., Aquiloni, L., Diéguez–Uribeondo, J., Tricarico, E., 2011. Managing invasive crayfish: is there a hope? Aquatic Science, 73: 185–200, Doi: 10.1007/s00027-011-0181-z
Gladman, Z. F., Adams, C. E., Bean, C. W., Long, J., Yeomans, W. E., 2012. Investigating the threat of non–native North American signal crayfish (Pacifastacus leniusculus) to salmon redds. Aquatic Conservation: Marine and Freshwater Ecosystems, 22: 134–137, Doi: 10.1002/aqc.1238
Inoue, K., Stoeckl, K., Geist, J., 2017. Joint species models reveal the effects of environment on community assemblage of freshwater mussels and fishes in European rivers. Diversity and Distributions, 23: 284–296, Doi: 10.1111/ddi.12520
Leppänen, J. J., 2018. Establishing minimum counts for
semiquantitative bank–to–bank river transect mussel studies in species–poor rivers. River Research and Applications: 1–6, Doi: 10.1002/rra.3394
Liräs, V., Lindberg, M., Nyström, P., Annodotter, H., Lawton, L. A., Graf, B., 1998. Can ingested cyanobacteria be harmful to the signal crayfish (Pacifastacus leniusculus)?. Freshwater Biology, 39: 233–242, Doi: 10.1046/j.1365-2427.1998.00278.x Lois, S., Ondina, P., Outeiro, A., Amaro, R., San Miguel, E., 2014. The north–west of the Iberian Peninsula is crucial for conservation of Margaritifera margaritifera (L.) in Europe. Aquatic Conservation: Marine and Freshwater Ecosystems, 24: 35–47, Doi: 10.1002/aqc.2352
Lopes–Lima, M., Sousa, R., Geist, J., Aldridge, D. C., Araujo, R., Bergengren, J., Bespalaya, Y., Bódis, E., Burlakova, L., Van Damme, D., Douda, K., Froufe, E., Georgiev, D., Gumpinger, C., Karatayev, A., Kebapçi, U., Killeen, I., Lajtner, J., Larsen, B. M., Lauceri, R., Legakis, A., Lois, S., Lundberg, S., Moorkens, E., Motte, G., Nagel, K. O., Ondina, P., Outeiro, A., Paunovic, M., Prié, V., von Proschwitzm T., Riccardim, N., Rudzīte, M., Rudzītis, M., Scheder, C., Seddon, M., Şereflişan, H., Simić, V., Sokolova, S., Stoeckl, K., Taskinen, J., Teixeira, A., Thielen, F., Trichkova, T., Varandas, S., Vicentini, H., Zajac, H., Zajac, T., Zogaris, S., 2017. Conservation status of freshwater mussels in Europe: state of the art and future challenges. Biological Reviews, 92: 572–607, Doi: 10.1111/brv.12244 Machida, Y., Akiyama, Y. B., 2013. Impacts of invasive crayfish (Pacifastacus leniusculus) on endangered freshwater pearl mussels (Margaritifera laevis and M. togakushiensis) in Japan. Hydrobiologia, 720: 145–151, Doi: 10.1007/s10750-013-1665-8 Meira, A., Lopes–Lima, M., Varandas, S., Teixeira, A., Arenas, F., Sousa, R., 2019. Invasive crayfishes as a threat to freshwater bivalves: Interspecific differences and conservation implications. Science of the Total Environment, 649: 938–948, Doi: 10.1016/j.scitotenv.2018.08.341
MITECO, 2019. Banco de Datos de la Naturaleza. Nature Database (in Spanish). Ministry for Ecological Transition, Government of Spain. Available online at: https://www.miteco.gob.es/es/biodiversidad/ servicios/banco-datos-naturaleza/default.aspx
Modesto, V., Castro, P., Lopes–Lima, M., Antunes, C., Ilarri, M., Sousa, R., 2019. Potential impacts of the invasive species Corbicula fluminea on the survival of glochidia. Science of the Total Environment, 673: 157–164, Doi: 10.1016/j.scitotenv.2019.04.043
Moorhouse, T. P., Macdonald, D. W., 2011. The effect of manual removal on movement distances in populations of signal crayfish (Pacifastacus leniusculus). Freshwater Biology, 56: 2370–2377, Doi: 10.1111/j.1365-2427.2011.02659.x
Morales, J., 2020. Notas ecológicas de esponjas dulceacuícolas parasitadas por larvas de Sisyra iridipennis Costa, 1884 (Neuroptera: Sisyridae) en el río Águeda (Salamanca, España). Nova Acta Científica Compostelana, 27: 29–34, https:// revistas.usc.gal/index.php/nacc/article/view/6589
Morales, J., Araujo, R., Santos, P. (Coords.), 2007b. Plan de Acción de Margaritifera margaritifera
214 Morales
en Castilla y León. LIFE NÁYADE. Junta de Castilla y León, https://webgate.ec.europa.eu/ life/publicWebsite/index.cfm?fuseaction=search. dspPage&n_proj_id=2481
Morales, J., Lizana, M., 2012. Rescate de una población de Margaritifera margaritifera L. (Bivalvia: Margaritiferidae), previo al llenado del embalse de Irueña (río Águeda, Salamanca). Lecciones aprendidas a posteriori. Ecología, 24: 45–60, https:// www.mapa.gob.es/ministerio/pags/Biblioteca/ Revistas/pdf_REPN%2FECO_2012_24_45_60.pdf
Morales, J., Lizana, M., 2014. Efectos negativos del cambio climático aceleran la extinción de la principal población de la náyade Margaritifera margaritifera L., 1758 en la cuenca del Duero. Munibe, 62: 103–127, http://www. aranzadi.eus/fileadmin/docs/Munibe/2014025049CN.pdf
Morales, J., Negro, A. I., Lizana, M., Martínez, A., Palacios, J., 2004. Preliminary study of the endangered populations of pearl mussel Margaritifera margaritifera (L.) in the River Tera (north–west Spain): habitat analysis and management considerations. Aquatic Conservation: Marine and Freshwater Ecosystems, 14: 587–596, Doi: 10.1002/aqc.624
Morales, J., Santos, P., Peñín, E., Palacios. J., 2007a. Incidencia negativa de los incendios forestales sobre una población de la náyade Margaritifera margaritifera L. (Bivalvia: Unionoida) en el río Negro (Zamora). Ecología, 21: 91–106, https://www. miteco.gob.es/ministerio/pags/Biblioteca/Revistas/ pdf_REPN%2FECO_2007_21_91_106.pdf
Nyström, P., Stenroth, P., Holmqvist, N., Berglund, O., Larsson, P., Graneli, W., 2006. Crayfish in lakes and streams: individual and population responses to predation, productivity and substratum availability. Freshwater Biology, 51(11): 2096–2113, Doi: 10.1111/j.1365-2427.2006.01641.x
O'Briain, R., 2019. Climate change and European rivers: An ecohydromorphological perspective. Ecohydrology, 2019: e2099, Doi: 10.1002/eco.2099
Olsson, K., 2008. Dynamics of omnivorous crayfish in freshwater ecosystem. Tryckeriet i E–huset, Lunds Universitet. Available online at: http://lup.lub.lu.se/ search/ws/files/5863429/1231075.pdf
Peay, S., Guthrie, N., Spees, J., Nilsson, E., Bradley, P., 2009. The impact of signal crayfish (Pacifastacus leniusculus) on the recruitment of salmonid fish in a headwater stream in Yorkshire, England. Knowledge and Management of Aquatic Ecosystems, 394–395: 12, Doi: 10.1051/kmae/2010003
Saloom, M. E., Duncan, R. S., 2005. Low dissolved oxygen levels reduce anti–predation behaviours of the freshwater clam Corbicula fluminea Freshwater Biology, 50: 1233–1238, Doi: 10.1111/j.13652427.2005.01396.x
Sousa, R., Antunes, C., Guilhermino, L., 2008b. Ecology of the invasive Asian clam Corbicula fluminea (Müller, 1774) in aquatic ecosystems: an overview. Annales de Limnologie–International Journal of Limnology, 44(2): 85–94, Doi: 10.1051/limn:2008017
Sousa, R., Amorim, A., Froufe, E., Varandas, S., Teixeira, A., Lopes–Lima, M., 2015. Conservation
status of the freshwater pearl mussel Margaritifera margaritifera in Portugal. Limnologica, 50: 4–10, Doi: 10.1016/j.limno.2014.07.004
Sousa, R., Garrido Nogueira, J., Ferreira, A., Carvalho, F., Lopes–Lima, M., Varandas, S., Teixeira, A., 2019. A tale of shells and claws: The signal crayfish as a threat to the pearl mussel Margaritifera margaritifera in Europe. Science of the Total Environment, 665: 329–337, Doi: 10.1016/j.scitotenv.2019.02.094
Sousa, R., Jorge, L., Gutiérrez, J. L., Aldridge, D. C., 2009. Non–indigenous invasive bivalves as ecosystem engineers. Biological Invasions, 11: 2367–2385, Doi: 10.1007/s10530-009-9422-7
Sousa, R., Rufino, M., Gaspar, M., Antunes, C., Guilhermino, L., 2008a. Abiotic impacts on spatial and temporal distribution of Corbicula fluminea (Müller, 1774) in the River Minho Estuary, Portugal. Aquatic Conservation: Marine and Freshwater Ecosystems, 18: 98–110, Doi: 10.1002/aqc.838
Stenroth, P., Nyström, P., 2003. Exotic crayfish in a brown water stream: effects on juvenile trout, invertebrates and algae. Freshwater Biology, 48: 466–475, Doi: 10.1046/j.1365-2427.2003.01020.x
Stoeckl, K., Denic. M., Geist, J., 2020. Conservation status of two endangered freshwater mussel species in Bavaria, Germany: Habitat quality, threats, and implications for conservation management. Aquatic Conservation: Marine and Freshwater Ecosystems, 30(4): 647–661, Doi: 10.1002/aqc.3310
Turley, M. D., Bilotta, G. S., Gasparrini, A., Sera, F., Mathers, K. L., Humpheryes, I., England, J., 2017. The effects of non–native signal crayfish (Pacifastacus leniusculus) on fine sediment and sediment–biomonitoring. Science of the Total Environment, 601–602: 186–193, Doi: 10.1016/j.scitotenv.2017.05.106
Vaeben, S., Hollert, H., 2015. Impacts of the North American signal crayfish (Pacifastacus leniusculus) on European ecosystems. Environmental Sciences Europe, 27: 33, Doi: 10.1186/s12302-015-0065-2
Vasconcelos, V., Oliveira, S., Oliva Teles, F., 2001. Impact of a toxic and a non–toxic strain of Microcystis aeruginosa on the crayfish Procambarus clarkii Toxicon, 39: 1461–1470, Doi: 10.1016/ S0041-0101(01)00105-2
Vaughn, C. C., Nichols, S. J., Spooner, D. E., 2008. Community and foodweb ecology of freshwater mussels. Journal of the North American Benthological Society, 27(2): 409–423, Doi: 10.1899/07-058.1
Wutz, S., Geist J., 2013. Sex– and size–specific migration patterns and habitat preferences of invasive signal crayfish (Pacifastacus leniusculus Dana). Limnologica, 43: 59–66, Doi: 10.1016/j. limno.2012.02.002
Yamamoto, Y., 2010. Contribution of bioturbation by the red swamp crayfish Procambarus clarkii to the recruitment of bloom–forming cyanobacteria from sediment. Journal of Limnology, 69(1): 102–11, Doi: 10.4081/jlimnol.2010.102
Ziuganov, V., Zotin, A., Tretiakov, V., 1994. Pearl mussels and their relationships with Salmonidae VNIRO Publ. House, Moscow.
Animal Biodiversity and Conservation 45.2 (2022) 215
42 Muñoz and Farfán
Biodiversity and Conservation 45.2 (2022)
Stable isotope measurements as analytical tools for the traceability of crocodile–derived products
J. Gamboa–Delgado, P. Ponce–Campos, S. G. Pérez–Martínez, J. M. Pacheco–Vega, D. Villarreal–Cavazos
Gamboa–Delgado, J., Ponce–Campos, P., Pérez–Martínez, S. G., Pacheco–Vega, J. M., Villarreal–Cavazos, D., 2022. Stable isotope measurements as analytical tools for the traceability of crocodile–derived products. Animal Biodiversity and Conservation, 45.2: 217–224, Doi: https://doi.org/10.32800/abc.2022.45.0217
Abstract
Stable isotope measurements as analytical tools for the traceability of crocodile–derived products. In this preliminary study we examined the application of dual stable isotope analysis (δ13C and δ15N) to identify the origin of skins and meat derived from wild and farmed crocodiles. Traceability protocols can benefit from analytical techniques that are able to distinguish farmed or wild organisms. Scutes and muscle samples were obtained from wild and farmed crocodiles Crocodylus acutus (n = 14) and C. moreletii (n = 9). Isotopic values in scutes differed significantly between wild and farmed organisms, this difference being higher for δ15N than for δ13C values. When both isotopic values were integrated using a discriminant analysis, we observed a significant categorization. The isotopic values of muscle samples were very similar to those measured in scutes from the same individuals. In addition, two specimens of C. acutus were kept on a constant diet for 97 days to obtain reference isotopic values and tissues were compared. We also estimated the isotopic discrimination factors between tissues and the supplied diet.
Key words: Crocodilians, Natural biomarkers, Skin trade, Wildlife
Resumen

Mediciones de isótopos estables como herramientas analíticas para la trazabilidad de productos derivados de cocodrilos. En el presente estudio preliminar se examinó la aplicación de análisis isotópicos duales (δ13C y δ15N) para determinar el origen de la piel y la carne de cocodrilos silvestres y criados en granjas. En algunos protocolos de trazabilidad, las técnicas analíticas que permiten distinguir los organismos silvestres de los criados pueden resultar de utilidad. Se recogieron muestras de escamas y músculo de cocodrilos silvestres y mantenidos en cautiverio. Las especies de las que se obtuvieron las muestras fueron Crocodylus acutus (n = 14) y C. moreletii (n = 9) Los valores isotópicos de las muestras de escamas fueron significativamente diferentes entre los animales silvestres y los criados en granjas, aunque la diferencia fue mayor con respecto a los valores de δ15N que a los de δ13C. Al integrar ambos valores isotópicos en un análisis discriminante, se observó una significativa categorización de ambos grupos. Los valores isotópicos relativos a las muestras de músculo fueron muy similares a los determinados en escamas provenientes de los mismos individuos. Asimismo, a dos de los ejemplares de C. acutus se les proporcionó una dieta constante durante 97 días a fin de obtener valores isotópicos de referencia y poder hacer comparaciones entre diferentes tejidos. También se calcularon los factores de discriminación isotópica entre los tejidos y la dieta suministrada.
Palabras clave: Cocodrilianos, Biomarcadores naturales, Comercio de pieles, Fauna silvestre
Received: 11 II 21; Conditional acceptance: 08 IX 21; Final acceptance: 16 VI 22
ISSN: 1578–665 X eISSN: 2014–928 X
© [2022] Copyright belongs to the authors, who license the journal Animal Biodiversity and Conservation to publish the paper under a Creative Commons Attribution 4.0 License
217 Animal
Gamboa–Delgado et al.
Programa Maricultura, Departamento de Ecología, Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, San Nicolás de los Garza, Nuevo León, México.– Paulino Ponce–Campos, Bosque Tropical, Investigación para la Conservación de la Naturaleza, Misión San Antonio, Torre 4a–2, Colonia Plaza Guadalupe, Zapopan, Jalisco, México.– Juan M. Pacheco–Vega, Escuela Nacional de Ingeniería Pesquera, Universidad Autónoma de Nayarit, Nayarit, México.
Corresponding author: J. M. Pacheco–Vega, E–mail: pachecovjm@yahoo.com.mx
ORCID ID: J. Gamboa–Delgado: 0000-0001-9041-1388; P. Ponce–Campos: 0000-0001-8202-0264; G. Pérez–Martínez: 0000-0002-4275-1764; J. M. Pacheco–Vega: 0000-0001-9443-6849; D. Villarreal–Cavazos: 0000-0001-5572-9710
218
Julián Gamboa–Delgado, Sandra G. Pérez–Martínez, David Villarreal–Cavazos,
Introduction
It has been estimated that up to 19 % of reptile species are threatened with extinction (Böhm et al., 2013). This figure includes Crocodilian species, in particular those subject to poaching and habitat loss. Given the high market value of reptile skins, illegal captures of wild individuals still represent a generalized problem in several tropical countries (IUCN SSC Crocodile Specialist Group, 2021). In the case of Mexico, the natural populations of American crocodile (Crocodylus acutus) and Morelet's crocodile (C. moreletii) were severely threatened during the 1970s due to intensive extraction. Populations successfully recovered after the implementation of new legislation that supported long–term protection programs and the re–establishment of authorized crocodile farms. The latter drove important changes in the CITES status of wild Morelet's crocodile, which was positioned at a lower threat status in 2012 (CONABIO, 2021). In Mexico, although crocodile species are still under special protection (NOM–059–SEMARNAT–2010), the trade of these animals and their derived products is legal, although strongly regulated (CITES, 2020). Crocodile farms have an important role in local economies as they foster ecotourism, repopulation programs and habitat conservation through pilot development projects. Commercialization of crocodile–derived products (skin, meat and fat) is currently increasing, and it is estimated that an average of 1,500 skins are sold from Mexico to the international markets every year (CONABIO, 2016). Skins and meat are produced in certified farms that follow both the local knowledge and recently developed ranching protocols (Barrios and Cremieux, 2018). Despite these protocols, however, poaching and illegal commercialization of crocodiles and their products continue in several areas of the country. Traceability methods have been implemented to guarantee the legal provenance of crocodiles and derived products. However, most of these traceability systems are paper–based and prone to falsification and deliberate mislabeling. The fraudulent mislabeling of animal–derived products is a recurrent illegal practice in the sale of unauthorized products.
Several analytical techniques can support and reinforce the traceability process of animal derived products (Van Eeden, 2021). One of the most reliable techniques consists of the analysis of stable isotopes at natural abundance levels. The underlying principle in the use of stable isotopes as biomarkers is that the carbon and nitrogen stable isotope values (δ13C and δ15N) of animal consumers reflect the isotopic values of the assimilated dietary components that contribute to tissue biosynthesis (Ramos and González–Solís, 2012). Under this assertion, stable isotope values can be applied to infer trophic relationships between diets and consumers. As nutrients flow within the organisms, the heavier isotopes of carbon and nitrogen (13C and 15N) tend to bioaccumulate in tissues due to a metabolic discrimination (Martínez del Rio and Wolf, 2005). The dietary nutrients and the metabolic pathways they take elicit distinctive isotopic values among animals and tissues.
Previous studies have employed isotopic measurements to infer the origin of animal–derived products. For example, Zhaxi et al. (2021) applied multi–element stable isotopes analysis to determine the geographical origin of chicken. In a similar approach, Liu et al. (2020) applied isotopic methods to discern the origin of wild, lake–farmed and pond–farmed carp.
Dietary attributes frequently confer specific isotopic values to wild and farmed animals and such values have been used as biomarkers to distinguish the origin of economically important animal species (Gamboa–Delgado et al., 2014; Wang et al., 2018). In the particular case of crocodilians, the nutrition of farmed crocodiles is based on the rather constant food regimes provided in contrast to wild animals, which have a more varied diet, such as crustaceans, insects, fish, birds, and mammals (Andreu and Quiroz, 2003). From an ecological point of view, farmed crocodiles occupy lower trophic positions than their wild counterparts due to the type of food they receive in captivity, frequently poultry and fish offal. As poultry, in turn, is fed on grain–based diets having characteristically less enriched δ13C and δ15N values, these isotopic values are reflected in poultry. It is therefore to be expected that the nutrients (and isotopes) composing the poultry tissue will be transferred to the crocodiles. The commercialized products derived from crocodiles are mainly skins and meat, both of which are composed of structural proteins with slow metabolic turnover rates. Their respective isotopic values can therefore reflect dietary characteristics over a long period of time (Rosenblatt and Heithaus, 2013).The aim of the present study was to determine the origin of crocodiles and their products by measuring the δ13C and δ15N values in samples of skin (scutes) and muscle obtained from two different crocodilian species, under the hypothesis that the natural δ13C and δ15N values in crocodile tissues differ between wild and farmed crocodiles. In addition, from the available data, we estimated discriminant functions in order to evaluate the use of isotopic values as discriminatory variables.
Material and methods
Species and sampling methods
In the present study, fourteen American crocodiles (Crocodylus acutus: 3 farmed, 11 wild) and nine Morelet's crocodiles (C. moreletii: 6 farmed, 3 wild) were sampled. The target tissues collected from both species were skin (caudal scutes) and muscle since skin and meat are the main commercialized products derived from crocodiles. Scutes were collected from wild crocodiles, from individuals held captive in rural households, and also from juvenile crocodiles raised on farms. In the present study, the latter two groups are referred to as farmed/captive crocodiles. After the animals were immobilized and total length was measured, two dorsal tail scutes were cut and preserved in 70 % ethanol solution. Sub–samples of preserved
Animal Biodiversity and Conservation 45.2 (2022) 219
Table 1. Mean carbon and nitrogen isotope values (‰) in scutes and muscle sampled from wild and farmed crocodiles (C. moreletii and C. acutus) (mean values ± SD): a, b significant differences in the mean isotopic values determined in wild and farmed crocodiles of the same species (horizontal comparisons).
Tabla 1. Promedio de valores isotópicos de carbono y nitrógeno (‰) en muestras de escamas y músculo de cocodrilos silvestres y criados (C. moreletii y C. acutus) (valores promedio ± DE): a, b diferencias significativas en los valores isotópicos medios determinados en cocodrilos salvajes y de granja de la misma especie (comparaciones horizontales).
Wild
Origin/species
Farmed/captive
Tissue Mean value C. moreletii C. acutus C. moreletii C. acutus Scutes δ13C –23.05 ± 0.92a –25.56 ± 1.31a –21.10 ± 1.31b –22.21 ± 2.71b
δ15N 13.68 ± 0.92a 12.59 ± 1.28a 7.80 ± 0.85b 8.20 ± 0.63b Muscle δ13C –24.66 ± 0.48a n/a –20.87 ± 1.64b –22.42 ± 0.67
δ15N 14.45 ± 0.60a n/a 7.48 ± 0.87b 7.84 ± 0.72
scutes from wild animals were also acquired from private and institutional herpetological collections. Muscle tissue was sampled from crocodile meat purchased from authorized farms, or retailers who had reliable information on the crocodile's origin (table 1s in supplementary material). In the case of wild animals, only three muscle samples were obtained, all three from preserved tissue of C. moreletii crocodiles.
Isotopic differences between crocodile tissues and isotopic discrimination factors
To further compare the isotopic values of scutes and muscle tissue and to determine the isotopic values of liver, dermis and whole blood, we kept two individuals of C. acutus (44 and 105 cm) under controlled conditions at a Wildlife Management Unit (SEMARNAT code: DGVS–PIMVS–CR–IN–1043). Crocodiles were donated by the crocodile farm 'La Palma' in San Blas, Nayarit Mexico. Upon arrival, they were fed a constant diet consisting of poultry meat and offal acquired from a supplier. We expected that the dietary isotopic values would eventually be reflected in the different tissues. This feeding period also allowed us to estimate the isotopic discrimination factors (isotopic difference between organism/ tissues and diet, Δ15N and Δ13C). After three months of rearing (97 days), animals were euthanized and dissected to obtain samples of representative organs and tissues (table 3). Dissected organs and tissues were rinsed in distilled water and preserved in 70 % ethanol until pre–treatment for stable isotope analysis. As sample preservation frequently includes solvent immersion (ethanol), tests were conducted to verify the conservation of stable isotope values in treated and untreated dry samples of scute derived from wild and farmed crocodiles.
Pretreatment of samples and stable isotope analysis
Small triplicate subsamples were oven dried (60 ºC, 24 h) and ground using mortar and pestle. As scutes are difficult to grind, dry scutes were frozen and cut into very small pieces before lipid extraction. Lipid extraction was performed following Beaudoin et al. (2001) by suspending the ground material in a 50:50 solution of chloroform–methanol for 12 h. Samples were then oven–dried, homogenized, and kept in desiccators. Tissue samples of 900 to 1,100 μg were packed in tin cups and organized in 96–well microplates. The procedures were carried out at the Escuela Nacional de Ingeniería Pesquera (Universidad Autónoma de Nayarit, México). Samples were analyzed at the Stable Isotope Facility (University of California–Davis) using a PDZ Europa Scientific Roboprep elemental analyzer coupled to a PDZ Europa Hydra 20/20 stable isotope ratio mass spectrometer. Repeated measurements of calibration standards indicated an instrument precision of 0.09 ‰ for δ15N and 0.12 ‰ for δ13C.
Statistical analysis
After analysis, the isotopic signatures of the scute samples were classified according to their origin and production method. A canonical discriminant analysis (CDA) was applied to determine the classification power of the variables δ15N and δ13C to categorize scute samples collected from wild animals and those from farmed/captive animals. The CDA standardizes values after applying discriminant functions and provides an indicator of how well the grouped variables are separated. Wilks' lambda indicated the magnitude at which the dual stable isotope values contributed to discriminating between groups (origin). Discriminant functions were estimated for each species. The isoto-
220 Gamboa–Delgado et al.
Table 2. Discriminant function coefficients estimated from the δ15N and δ13C values determined in wild and farmed/captive crocodile scutes.
Tabla 2. Coeficientes de las funciones discriminantes estimadas a partir de los valores isotópicos δ15N y δ13C determinados en escamas de cocodrilos silvestres y criados en granja/cautivos.
Discriminant functions
C. moreletii
C. acutus
Wild –6.9×δ15N – 17.5×δ13C − 156.7 18.9×δ15N – 13.6×δ13C – 261.4
Farmed/captive –18.4×δ15N – 24.8×δ13C – 192.1 13.8×δ15N – 11.3×δ13C – 184.4
pic values for each element (δ15N and δ13C) were also compared between wild and farmed/captive animals using Mann–Whitney tests. The isotopic values of newly hatched crocodiles were not included in the statistical analysis as these animals strongly reflected the isotopic values of the maternal nutrients. Moreover, as young animals have higher metabolic rates –which can in turn affect the isotopic values (Vander Zanden et al., 2015)– only juveniles and subadults were used for the statistical comparisons.
Results and discussion
δ13C and δ15N values in wild and farmed/captive animals
Table 1 shows mean δ13C and δ15N values of scute and muscle tissue from wild and farmed C. moreletii and C. acutus Scutes collected from wild crocodiles (both species) showed a mean δ13C value ± SD of –24.66 ± 1.88 ‰, while the value observed in farmed animals was –21.48 ± 1.79 ‰. Although the difference was small, it was statistically significant (p < 0.011). A higher difference was observed in scute δ15N values (p < 0.001) between wild (12.87 ± 1.36 ‰) and farmed animals (8.24 ± 0.62 ‰). Dual isotopic values measured in scutes were similar in both species from the same type of habitat. The differences of δ13C and δ15N values in wild and farmed crocodiles can be attributed to the isotopic influence of their respective diets (Phillips, 2012). Scutes from wild crocodiles had higher δ15N values than their farmed counterparts, while the opposite was observed for δ13C values.
Muscle tissue samples from wild crocodiles showed a mean δ13C value of –24.66 ± 0.48 ‰, while the respective estimated value observed in farmed animals was –21.32 ± 1.56 ‰. For δ15N values, the corresponding values were 14.45 ± 0.60 ‰ and 7.66 ± 0.69 ‰. A statistical comparison between muscle tissue from wild and farmed/captive crocodiles was not feasible as only three samples were acquired from wild animals. However, the isotopic similarity between muscle tissue and scutes in the same individuals indicates that a significant difference might also be detected between the isotopic values of muscle derived from wild and farmed/captive crocodiles.
Isotopic values as discriminant variables
After analyzing the dual isotopic values in a discriminant analysis (table 2), we detected a significant difference (Wilks' lambda = 0.131, p < 0.001) between the two groups of data representing wild and farmed/ captive crocodiles (fig. 1), indicating the significance of the independent variables δ13C and δ15N, values to the discriminant function. Results from the CDA indicated that the use of both isotopic values effectively separated the groups of wild and farmed/captive crocodiles. When applied separately for each species, δ15N values were more reliable (Wilks' lambda 0.088, p < 0.001 for C. moreletii; 0.167, p < 0.003 for C. acutus) than δ13C values (Wilks' lambda 0.541, p < 0.011 for C. moreletii; 0.575, p < 0.160 for C. acutus) in identifying the origin of crocodiles and their products. δ15N values were significantly higher in wild animals than in farmed animals. Such differences might be due to diet type given that farmed crocodiles derive most of their nutrients from poultry and fish offal (Barrios and Cremieux, 2018). In contrast, the natural diet of wild crocodiles is highly diverse (Perez–Higareda et al., 1989) and based on organisms belonging to higher trophic niches (Andreu and Quiroz, 2003). Such dietary characteristics were clearly reflected in the available isotopic data. We hypothesize that the isotopic values in tissue from farmed crocodiles differ from those measured in wild crocodiles because the δ13C and δ15N values of farmed animals are significantly influenced by the isotopic composition of the main feeding items they receive (poultry and fish offal). For example, poultry is fed on grain–based diets that have specific, rather depleted isotopic values that, in turn, are transferred to poultry. In a recent study, Woodborne et al. (2021) sampled different tissues from farmed Crocodylus niloticus fed exclusively on poultry. The isotopic values measured in poultry (δ13C = –17.2 and δ15N = 3.0 ‰) were similar to those determined in the poultry offal supplied in the present study (δ13C = –21.6 and δ15N = 4.1 ‰).
Isotopic differences among crocodile tissues and isotopic discrimination factors
After the 97 day feeding period, δ13C values measured in sampled scutes closely resembled the isotopic
Animal Biodiversity and Conservation 45.2 (2022) 221
δ 15 N values
16.0 14.0 12.0 10.0 8.0 6.0 4.0
Wilks' lambda = 0.131, p < 0.001 Wild
Farmed/captive
–28 –26 –24 –22 –20 –18 δ13C values
Fig. 1. Carbon and nitrogen stable isotope values of scutes sampled from wild and farmed crocodiles C. moreletii (triangles) and C. acutus (circles). Wilks' lambda indicates a significant categorization after applying a canonical discriminant analysis to the isotopic values.
Fig. 1. Valores isotópicos de carbono y nitrógeno en escamas obtenidas de cocodrilos C. moreletii (triángulos) y C. acutus (círculos) silvestres y criados. El valor lambda de Wilks indica una categorización significativa después de aplicar un análisis canónico discriminante a los valores isotópicos.
Table 3. δ13C and δ15N values (‰) measured in different tissues of C. acutus obtained from the crocodile farm 'La Palma' and kept in captivity for 97 days under a constant diet composed of poultry offal (mean values ± SD). Δ13C and Δ15N values (‰) indicate the isotopic differences between diet and tissues (isotopic discrimination factors).
Tabla 3. Valores δ13C y δ15N (‰) determinados en diferentes tejidos de C. acutus obtenidos de la granja de cocodrilos "La Palma" y mantenidos en cautiverio durante 97 días bajo una dieta constante compuesta por despojos de aves de corral (valores medios ± DE). Los valores Δ13C y Δ15N (‰) indican la diferencia isotópica entre tejidos y la dieta (factores de discriminación isotópica).
Tissue δ13C δ15N Δ13C Δ15N
Scutes –20.77 ± 0.62 7.49 ± 0.70 1.36 3.40
Muscle –22.59 ± 0.70 8.37 ± 0.73 –0.34 4.28
Liver –8.24 ± 0.87 8.42 ± 0.43 3.40 5.07
Blood –22.12 ± 0.31 8.06 ± 0.55 –0.48 3.97
Dermis –19.75 ± 1.34 9.24 ± 0.36 1.89 5.15 Intestine –21.12 ± 0.83 7.16 ± 0.30 0.52 3.07
Heart –20.79 ± 0.28 7.70 ± 0.74 0.85 3.61 Scat –18.39 ± 0.81 5.23 ± 0.92 3.25 1.14
Diet (poultry offal) –21.64 ± 0.17 4.09 ± 0.23 – –
222 Gamboa–Delgado et al.
values of diet (table 3). δ15N values between diet and scutes had a difference of 3.4 ‰. However, it is important to consider that in the present study, not all tissues types obtained from the two captive individuals might have reached full isotopic equilibrium with the diet, in particular tissues having slow turnover rates. Tissues with higher metabolic rates have a faster turnover rate, and thus tend to reflect the isotopic values of the assimilated dietary components faster (MacAvoy et al., 2005). Previous studies on crocodilians have reported that the t50 or half time (the time it takes for half of the existing tissue to resemble the dietary isotopic signature after a diet shift) for muscle tissue was 43 days for nitrogen and 31 days for carbon (Caut, 2013). In contrast, Rosenblatt and Heithaus (2013) reported t50 values of up to 103 days for nitrogen and 147 days for carbon in scutes of American alligator (Alligator mississippiensis). In the present study, and based on the available growth and isotopic data, we estimated that the t50 values of the constituent carbon and nitrogen in scutes from the two C. acutus kept in captivity, were 55 and 49 days, respectively
The immersion of scutes in ethanol (up to six months) had a negligible effect on the isotopic values as compared with the values of dry, untreated scute samples. The isotopic data determined in the different tissues showed that δ13C values were more isotopically enriched (13C) in liver, while muscle tissue was isotopically depleted. Tissues, ordered by decreasing 13C enrichment, were: liver > dermis > scutes > heart > intestine > blood > muscle. In contrast, the decreasing order of 15N enrichment in tissues was: dermis > liver > muscle > blood > heart > scutes > intestine. The isotopic differences between scutes and muscle obtained from the two captive individuals were small (2 ‰ for δ13C and 1 ‰ for δ15N values). On the other hand, the isotopic discrimination factors (Δ13C and Δ15N) between the supplied diet and the values found in the different tissues were smaller for δ13C (–0.48 to 3.40 ‰) and larger for δ15N (1.14 to 5.15 ‰). These values are similar to Δ13C and Δ15N values reported for scutes and blood in Alligator mississippiensis (Rosenblatt and Heithaus, 2013). Woodborne et al. (2021) conducted a study in Nile crocodiles (C. niloticus) fed on poultry and the isotopic discrimination factors between scutes (keratin) and diet were narrow (Δ13C = –0.9 and Δ15N = +1.4 ‰) but they are comparable to the values estimated in the present study (Δ13C = +1.3 and Δ15N = +3.4 ‰).
Conclusion
In this preliminary study, the isotopic values of scutes sampled from wild and farmed/captive crocodiles differed significantly. It can be inferred from these findings that crocodile skins having isotopic values in the range of –19.7 ‰ to –23.3 ‰ for δ13C values, and 7.5 to 9.0 ‰ for δ15N values, could be products derived from farmed animals. In Mexico, as most crocodile farms grow C. moreletii, the above–mentioned values and the obtained discriminant functions can be rele-
vant However, the number of available samples was limited and additional research is needed to collect and analyze samples from other representative locations. The effect of post–harvest treatments applied to skins (drying, tanning) on the isotopic values also requires further study. From the two captive crocodiles (C. acutus), referential isotopic values were obtained from different tissues, which allowed us to estimate the isotopic discrimination factors between diet and types of tissue. Isotopic measurements can be used as viable, natural chemical markers to differentiate the provenance of skins, supporting their potential for future use in traceability protocols designed to regulate the trade of products derived from crocodiles.
Acknowledgements
We thank Sara M. Huerta–Ortega for her assistance in sampling scutes from wild organisms. The present study was financially supported by the Universidad Autónoma de Nuevo León through Project PAICYT CN1210–20. We thank the crocodile farm 'La Palma' for providing access to their facilities and animals. Wild crocodiles were sampled under written permission from the Mexican Ministry of Environment and Natural Resources (SEMARNAT).
References
Andreu, G. C., Quiroz, G. B., 2003. Hábitos alimenticios de Crocodylus acutus (Reptilia: Crocodylidae) determinados por el análisis de sus excretas en la costa de Jalisco, México. Anales del Instituto de Biología, Serie Zoología, 74: 35–42.
Barrios, G., Cremieux, J., 2018. Protocolo de rancheo para cocodrilo de pantano (Crocodylus moreletii) en México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO), México. Available online at: https://biodiversidad.gob.mx/ planeta/cocodrilos_m/pdf/Prot_Ranch_v4_Web. pdf [Accessed on August 11, 2021].
Beaudoin, C. P., Prepas, E. E., Tonn, W. M., Wassenaar, L. I., Kotak, B. G., 2001. A stable carbon and nitrogen isotope study of lake food webs in Canada's Boreal Plain. Freshwater Biology, 46: 465–477, Doi: 10.1046/j.1365-2427.2001.00688.x
Böhm, M., Collen, B., Baillie, J. E., Bowles, P., Chanson, J., Cox, N., Hammerson, G., Hoffmann, M., Livingstone, S. R., Ram, M., Rhodin, A. G., 2013. The conservation status of the world's reptiles. Biological Conservation, 157: 372–385, Doi: 10.1016/j. biocon.2012.07.015
Caut, S., 2013. Isotope incorporation in broad–snouted caimans (crocodilians). Biology Open, 2: 629–634.
CITES, 2020. Convention on International Trade in Endangered Species of Wild Flora and Fauna. Appendices I, II and III. Available online at: https:// cites.org/eng/app/appendices.php [Accessed on April 7, 2021].
CONABIO, 2016. Comisión Nacional para el Cono-
Animal Biodiversity and Conservation 45.2 (2022) 223
cimiento y Uso de la Biodiversidad. Rancheo de cocodrilos. Available online at: https://www.gob.mx/ conabio/prensa/rancheo-de-cocodrilos [Accessed on June 1, 2021].
– 2021. Antecedentes sobre el estado de conservación de Crocodylus moreletii y listados nacionales e internacionales. Available online at: https://www. biodiversidad.gob.mx/planeta/cocodrilos_m/ [Accessed on February 27, 2021].
Gamboa–Delgado, J., Molina–Poveda, C., Godínez–Siordia, D. E., Villarreal–Cavazos, D., Ricque–Marie, D., Cruz–Suárez, L. E., 2014. Application of stable isotope analysis to differentiate shrimp extracted by industrial fishing or produced through aquaculture practices. Canadian Journal of Fisheries and Aquatic Sciences, 71: 1520–1528, Doi: 10.1139/cjfas-2014-0005
IUCN SSC Crocodile Specialist Group, 2021. Traceability in Crocodylian Conservation and Management. IUCN SSC Crocodile Specialist Group: Darwin, Australia, http://www.iucncsg.org/365_ docs/attachments/protarea/1798ca7fa30db1f2d9 ca905957288604.pdf
MacAvoy, S. E., Macko, S. A., Arneson, L. S., 2005. Growth versus metabolic tissue replacement in mouse tissues determined by stable carbon and nitrogen isotope analysis. Canadian Journal of Zoology, 83(5): 631–641, Doi: 10.1139/z05-038
Martínez del Rio, C., Wolf, B. O., 2005. Mass–balance models for animal isotopic ecology. In: Physiological and Ecological Adaptations to Feeding in Vertebrates: 141–174 (J. M. Starck, T. Wang, Eds.) Science Publishers, Enfield, NH.
Liu, Z., Yuan, Y., Zhao, Y., Zhang, Y., Nie, J., Shao, S., Rogers, K. M., 2020. Differentiating wild, lake–farmed and pond–farmed carp using stable isotope and multi–element analysis of fish scales with chemometrics. Food Chemistry, 328: 127115, Doi: 10.1016/j.foodchem.2020.127115
Perez–Higareda, G., Rangel–Rangel, A., Smith H.
A., Chizar, D., 1989. Comments on food and feeding habits of Morelet's crocodile. Copeia, 1989: 1039–1041, Doi: 10.2307/1445994
Phillips, D. L., 2012. Converting isotope values to diet composition: the use of mixing models. Journal of Mammalogy, 93(2): 342–352, Doi: 10.1644/11-MAMM-S-158.1
Ramos, R., González–Solís, J., 2012. Trace me if you can: the use of intrinsic biogeochemical markers in marine top predators. Frontiers in Ecology and the Environment, 10: 258–266, Doi: 10.1890/110140
Rosenblatt, A., Heithaus, M., 2013. Slow isotope turnover rates and low discrimination values in the American alligator: Implications for interpretation of ectotherm stable isotope data. Physiological and Biochemical Zoology: Ecological and Evolutionary Approaches, 86: 137–148, Doi: 10.1086/668295
Van Eeden, M., 2021. An over view of traceability in the livestock production chain. Stockfarm, 11(1): 33–33.
Vander Zanden, M. J., Clayton, M. K., Moody, E. K., Solomon, C. T., Weidel, B. C., 2015. Stable isotope turnover and half–life in animal tissues: a literature synthesis. Plos One, 10(1): e0116182, Doi: 10.1371/journal.pone.0116182
Wang, Y. V., Wan, A. H., Lock, E. J., Andersen, N., Winter–Schuh, C., Larsen, T., 2018. Know your fish: A novel compound–specific isotope approach for tracing wild and farmed salmon. Food Chemistry, 256: 380–389, Doi: 10.1016/j. foodchem.2018.02.095
Woodborne, S., Botha, H., Huchzermeyer, D., Myburgh, J., Hall, G., Myburgh, A., 2021. Ontogenetic dependence of Nile crocodile (Crocodylus niloticus) isotope diet‐to‐tissue discrimination factors. Rapid Communications in Mass Spectrometry, 35(18): e9159, Doi: 10.1002/rcm.9159
Zhaxi, C., Zhao, S., Zhang, T., Dong, H., Liu, H., Zhao, Y., 2021. Stable isotopes verify geographical origin of Tibetan chicken. Food Chemistry, 358: 129893, Doi: 10.1016/j.foodchem.2021.129893
224 Gamboa–Delgado
et al.
Biodiversity and Conservation 45.2 (2022)
Camera trap data reveal the habitat associations, activity patterns and population density of Indian pangolin (Manis crassicaudata) in Maduru Oya National Park, Sri Lanka
D. Jayasekara, W. D. S. C. Dharmarathne, U. K. G. K. Padmalal, W. A. D. Mahaulpatha
Jayasekara, D., Dharmarathne, W. D. S. C., Padmalal, U. K. G. K., Mahaulpatha, W. A. D., 2022. Camera trap data reveal the habitat associations, activity patterns and population density of Indian pangolin (Manis crassicaudata) in Maduru Oya National Park, Sri Lanka. Animal Biodiversity and Conservation, 45.2: 225–236, https://doi.org/10.32800/abc.2022.45.0225
Abstract
Camera trap data reveal the habitat associations, activity patterns and population density of Indian pangolin (Manis crassicaudata) in Maduru Oya National Park, Sri Lanka. The Indian pangolin (Manis crassicaudata) is a solitary, medium–sized mammal native to South Asia. In this study we used camera trap data recorded during a meso–mammal survey conducted from January 2019 to January 2021 to assess the occupancy, habitat associations, population density and activity patterns of Indian pangolins in Maduru Oya National Park (MONP), Sri Lanka. The preferred habitat of the species was dry–mixed forest with an occupancy probability of 0.42 ± 0.19. Occupancy modeling revealed the association of the species with the forested habitats of the park with rich canopy cover, high NDVI scores and abundant termite mounds. Activity of this pangolin was highly nocturnal, reaching a peak after midnight. We observed a considerable spatiotemporal overlap in Indian pangolin activity and human activity, possibly increasing hunting pressure on the species. We estimated occupancy and abundance–based population density (0.73 ± 0.21 indiv./km2) using the random encounter model for the first time in the study area. These findings could be useful for conservation and management decisions concerning the survival and vital habitats of one of the most trafficked mammals in the world, the Indian pangolin.
Key words: Indian pangolin, Activity patterns, Habitat associations, Population density, Distribution modeling, Occupancy modeling
Resumen

Los datos obtenidos con cámaras de trampeo revelan la asociación con los hábitats, las pautas de actividad y la densidad de población del pangolín indio (Manis crassicaudata) en parque nacional Maduru Oya, en Sri Lanka. El pangolín indio (Manis crassicaudata) es un mamífero solitario de talla media nativo de Asia meridional. En el presente estudio utilizamos datos obtenidos mediante cámaras de trampeo durante un estudio sobre mesomamíferos realizado entre enero de 2019 y enero de 2021, con la finalidad de evaluar la ocupación, la asociación con los hábitats, la densidad de población y las pautas de actividad del pangolín indio en el parque nacional Maduru Oya, en Sri Lanka. El hábitat preferido de la especie fue el bosque mixto–seco con una probabilidad de ocupación de 0,42 ± 0,19. Los modelos de ocupación revelaron la asociación de la especie con los hábitats forestales del parque dotados de una cubierta de dosel abundante, un elevado índice normalizado diferencial de la vegetación y gran cantidad de termiteros. La actividad del pangolín indio fue predominantemente nocturna y alcanzó su máximo después de la medianoche. Observamos una superposición espaciotemporal considerable de la actividad del pangolín indio con la actividad humana, lo que puede crear una cierta presión cinegética sobre la especie. La ocupación de la especie y su densidad de población basada en la abundancia (0,73 ± 0,21 indiv./km2) se obtuvieron siguiendo el modelo de encuentro aleatorio por primera vez en la zona de estudio. Los resultados de este estudio serán de utilidad para tomar decisiones relativas a la conservación y la gestión de uno de los mamíferos silvestres con los que más se trafica en el mundo (el pangolín indio) y los hábitats vitales para su supervivencia.
ISSN: 1578–665 X eISSN: 2014–928 X
© [2022] Copyright belongs to the authors, who license the journal Animal Biodiversity and Conservation to publish the paper under a Creative Commons Attribution 4.0 License
225
Animal
Jayasekara et al.
Palabras clave: Pangolín indio, Pautas de actividad, Asociación con los hábitats, Densidad de población, Modelos de distribución, Modelos de ocupación
Received: 10 I 22; Conditional acceptance: 28 IV 22; Final acceptance: 20 VI 22
D. Jayasekara, W. D. S. C. Dharmarathne, W. A. D. Mahaulpatha, Department of Zoology, Faculty of Applied Sciences, University of Sri Jayewardenepura, Sri Lanka.– D. Jayasekara, Faculty of Graduate Studies, University of Sri Jayewardenepura, Sri Lanka.– U. K. G. K. Padmalal, Open University of Sri Lanka, Nawala, Nugegoda, Sri Lanka.
Corresponding author: W. A. D. Mahaulpatha; E–mail: mahaulpatha@sjp.ac.lk
ORCID ID: W. A. D. Mahaulpatha: 0000-0002-6563-7462
226
Introduction
Pangolins, also known as scaly anteaters, are elongated amour–plated insectivores with a long tongue and no teeth (Atkins, 2004; Mahmood et al., 2020). Currently, eight extant pangolin species can be found throughout the world (Khwaja et al., 2019; Mahmood et al., 2019). The Indian pangolin, or thick–tailed pangolin (Manis crassicaudata) (fig. 1), is the only pangolin species recorded in Sri Lanka (Perera and Karawita, 2020); it is also native to South Asia. These pangolins are medium–sized, solitary, elusive, predominantly nocturnal mammals that belong to the family Manidae and order Pholidota (Mahmood et al., 2020; Perera and Karawita, 2020).
The Indian pangolin lives in a variety of natural environments, ranging from evergreen forests, deciduous forests, open scrublands, and grasslands to human–modified habitats such as urban cultivations and semi–arid areas (Mahmood et al., 2015, 2020, 2021a). Numbers of Indian pangolin, however, are thought to be falling across its range (Mahmood et al., 2021b). Pangolin scales, flesh, and other derivatives are in high demand in East Asian markets, making them one of the most trafficked wild mammals on the planet (Challender et al., 2015; Aditya et al., 2021). As a result, the Convention on International Trade in Endangered Species currently lists all eight pangolin species in Appendix I (CITES, 2021). In recognition of the threats and declining population trends of the species, the International Union for the Conservation of Nature (IUCN) has classified the Indian pangolin as globally Endangered (Mahmood et al., 2021b) and 'Near Threatened' in the National Red List of Sri Lanka (Weerakoon, 2012). The species has been strictly protected under the Flora and Fauna Protection Ordinance (amendment) Act No. 22 of 2009 of Sri Lanka (Perera and Karawita, 2020).
Recent studies on the species in Sri Lanka have investigated the island–wide distribution and threats based on primary (direct field data) and secondary (community science) data (Perera et al., 2017; Karawita and Perera, 2020; Perera and Karawita, 2020; Perera et al., 2022). However, research to generate primary data for this species has only been conducted in a few localities such as Yagirala, Wilpattu and Yala (Karawita et al., 2016, 2018; Perera and Karawita, 2020; Perera et al., 2022). Furthermore, except for Yagirala (wet lowland forest), long–term and continuous field–based data are lacking for the species, which in Sri Lanka is mostly distributed in the lowland forest habitats (Perera and Karawita, 2020). A few studies in the wet zone forests of Sri Lanka have been carried out to investigate the wild population densities, habitat associations and behavior of Indian pangolin in natural habitats (Perera et al., 2017; Karawita et al., 2018, 2020; Perera et al., 2022). The occurrence of this species in dry zone habitats of Sri Lanka has been identified (Karawita and Perera, 2020; Perera and Karawita, 2020). Hence, this study aimed to bridge the gap in knowledge regarding occupancy, habitat associations, population density and activity patterns of Indian pangolin in an important dry zone locality for the species; Maduru Oya National Park (MONP).
Wildlife crimes associated with the species are high in some dry zone areas, including the MONP (Perera and Karawita, 2020). A rising trend in such crimes has been observed in the forms of hunting for subsistence, live capture for sale as meat, and hunting for scales (Perera and Karawita, 2020). MONP is known for the occurrence of Indian pangolin and also for a considerable level of associated wildlife crimes (Karawita and Perera, 2020). Therefore, we also investigated the level of human presence associated with the occupancy of the Indian pangolin within MONP during our study.
28ºC TRAILCAM01 03/14/2019 04:49 a.m.
Fig. 1. An Indian pangolin captured on a camera trap.
Fig. 1. Un pangolín indio captado con una cámara de trampeo.

Animal Biodiversity and Conservation 45.2 (2022) 227
Sri Lanka Arid zone Arid zone Dry zone Wet zone Arid zone
Park boundary
5 7.5 10 km
Fig. 2. Map showing the location of Maduru Oya National Park within the climatic zone map of Sri Lanka (left), and habitat categorization of the park adapted from Jayasekara et al. (2021a).
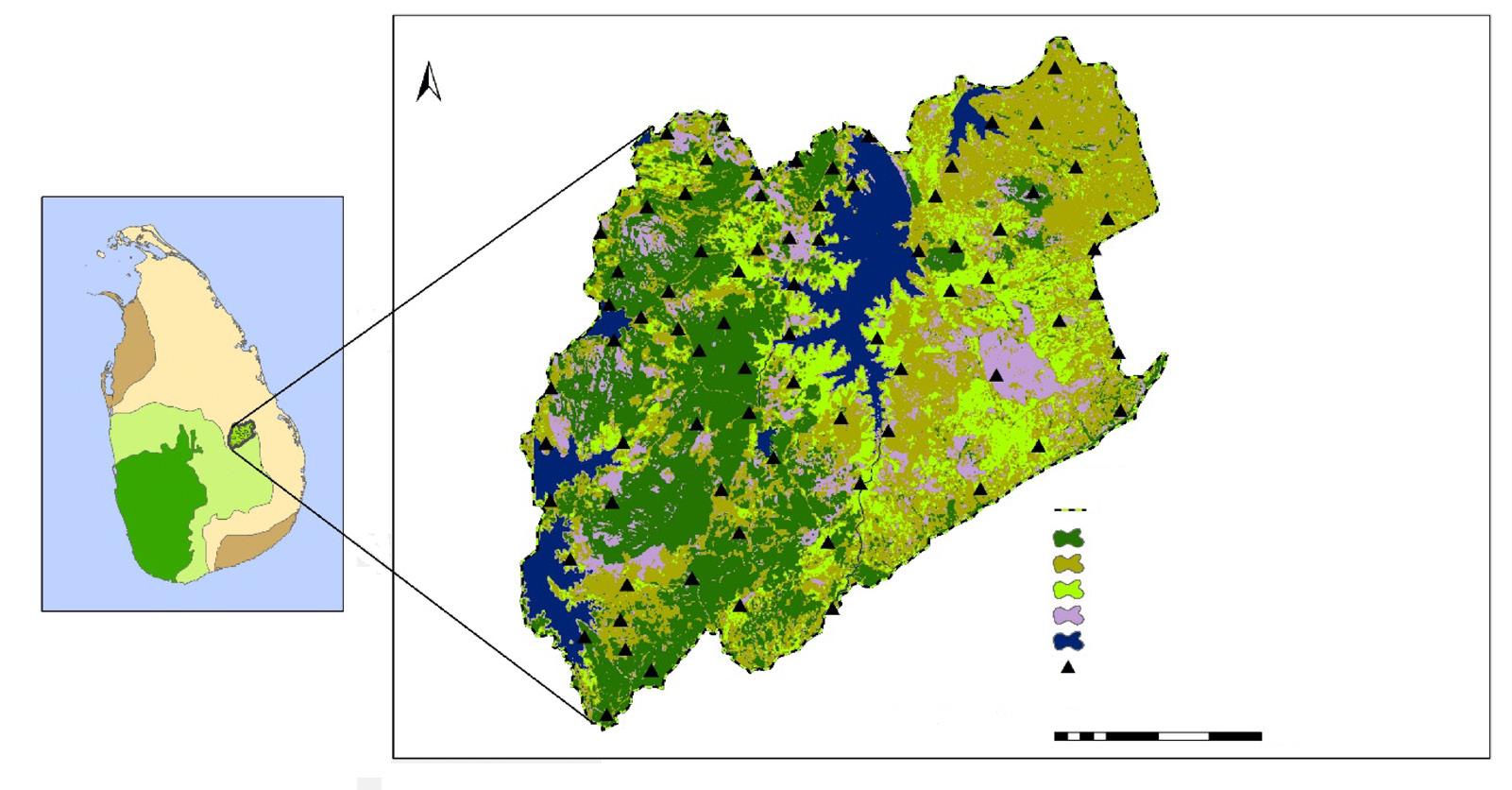
Being the only pangolin species found in Sri Lanka, the Indian pangolin has significant importance for conservation. Its threatened status, coupled with the increasing hunting pressure both locally and internationally, place it at the top of the list as an animal facing significant anthropogenic pressure. The traditional use of pangolins as a delicacy by villagers around forests is well known (Perera et al., 2017; Karawita and Perera, 2020). Furthermore, the accelerating international illegal trade of Indian pangolins (mainly pangolin scales) and the demand from the East–Asian markets (Challender et al., 2015; Perera et al., 2017; Mahmood et al., 2020; Perera and Karawita, 2020) have increased the exploitation rate of the species in its natural habitats in Sri Lanka. Habitat destruction and forest cover loss in the country as a result of numerous anthropogenic activities put this species in further peril. Despite the interest generated as a result of the revelations of trafficking data and CITES listing, little is known about ecology of the Indian pangolin in its natural habitats of Sri Lanka. Except for the work carried out in Yagirala forest reserve (a small forest reserve of in the lowland wet–zone of Sri Lanka) (Karawita et al., 2016; Perera et al., 2017; Karawita et al., 2018, 2020), the present study is one of the first to investigate the ecological aspects of this lesser studied species in the dry lowlands of the island. This is also one of the first efforts to estimate the population density of the species based on camera trap abundance data rather than on burrow counts. This method reduces bias due to heavy reliance on observer skills to locate burrows, issues regarding reproducibility of results, and false presence records by occupancy of other species (Perera et al., 2022). Altogether, determining
the spatiotemporal distribution of Indian pangolins, habitat associations, and population density estimation in MONP would facilitate the effective management and conservation planning for the species.
Material and methods Study area
In this study we used camera trap data, recorded during a meso–mammal survey conducted from January 2019 to January 2021 in MONP (fig. 2). MONP is situated mainly in the dry zone of Sri Lanka bordering the intermediate zone. The climatic conditions are dominated by the north–east monsoon, which persists from October to February. The mean annual rainfall is 1,650 mm and the mean annual temperature is about 27 ºC. Overall evapotranspiration rates usually exceed precipitation levels (Green, 1990). The park lies in the dry zone. However, the southern boundary of the park is near the intermediate zone, which is a narrow geographical area that separates the dry zone from the wet zone. The climax community of the area is tropical, dry mixed evergreen forest. The importance of the park's fauna is its richness, which includes several endemic species (Green, 1990).
Camera trapping
Browning Dark Ops and Browning Strike Force (Browning, USA) wildlife trail cameras were used for camera trapping. All cameras were equipped with IR motion and heat sensor triggered low/no glow
228 Jayasekara et al.
0 2.5
N
Dry–mixed
Shrubland Grassland/seasonal
Rocky–outcrops/bareland
Camera
Fig. 2. Mapa del parque nacional Maduru Oya y su localización en el mapa de zonas climáticas de Sri Lanka (a la izquierda) y clasificación de los hábitats del parque adaptada de Jayasekara et al. (2021a). Intermedial zone
evergreen forest
grasslands
Reservoirs
stations
Developed by: Dulan Jayasekara
Fig. 3.
flash, which generates minimal disturbance to the animals. Camera traps were placed representatively in all available habitat types in MONP using a grid of 2 × 2 km plots generated using ArcMap (ESRI, USA). A random camera placement was followed (Jayasekara et al., 2021b). The minimum distance between any two camera stations was greater than 1km. Cameras were placed at 25 cm above the ground and attached to a tree (fig. 3). Logs/metal poles were used to mount the cameras when large trunked trees were not available in the habitat. Camera traps were operating 24h/day with 1 second delay for a minimum of 30 consecutive days at each station. Capture records of Indian pangolins were obtained in the form of photos/videos. Additionally, the presence records of poachers captured in camera traps were used for the analysis. Since unauthorized entry is prohibited in the terrestrial areas of the MONP, we assumed that any humans captured on camera traps were poachers. The absence of uniforms (to differentiate poachers from rangers, fig. 3s in supplementary material) and carrying guns and other equipment were taken as clues of confirmation.

A total of 75 camera trap locations were surveyed. Available habitat types were identified as dry–mixed evergreen forest (DEF) (1197 trap days), shrubland (SHB) (775 trap days), rocky outcrops (ROC) (282 trap days), grasslands (GSL) (423 trap days) and reservoirs (Jayasekara et al., 2021a) (fig. 2). All terrestrial habitats up to the edge of water bodies were considered for sampling.
Habitat/environmental covariates
Habitat type and habitat variables of each camera trap station were recorded. The variables used for the analysis included habitat type, canopy cover
(CN), stem density 1 (SD1), stem density 2 (SD2), litter cover (LC), litter depth (LD), ground vegetation (GV), normalized difference vegetation index (NDVI), Euclidean distance to water (EDW), elevation (EL), the abundance of termite mounds (TM) and the relative abundance index of humans (RAIH) (table 1s in supplementary material). Stem density was measured by the modified Point Centered Quarter (PCQ) method by Chen et al. (2009) from the original method of Higgins et al. (1996). Mean distance to the nearest woody plant (< 10 cm from the camera–trap) with a diameter at breast height (DBH) between 1 and 10 cm was measured for SD1, whereas SD2 included plants with a DBH > 10 cm (four distance measurements were taken in four different directions, having the camera as the center point). Stem density was calculated as 1/mean area [distance]2. Other parameters were measured using quadrates of 2 x 2 m and averaged for each camera location. Litter cover was determined by the photo–point method (Michel et al., 2010) and litter depth was measured using a metal ruler. The abundance of termite mounds was evaluated by visual observation of a 20 × 20 m area around the camera location and a rank of 0–2 was given (0 absent, 1 moderate availability, 2 high availability). At the end of the survey, the camera traps were tested to confirm that they were still operational; if not, the date on the last photograph/video was recorded as the last operational date. We kept the cameras active for an average of ~38 days and cameras were shifted to new locations following the moving survey method to increase the area coverage.
Macroscale parameters were obtained using Arc Map 10.4 (ESRI, Redlands, USA) based GIS techniques. Raster maps were prepared for Euclidean distance to water, normalized difference vegetation index, and elevation. Averaged values for each ca-
Animal Biodiversity and Conservation 45.2 (2022) 229
Fig. 3. A camera trap attached to a tree trunk to capture images/videos of Indian pangolins.
Una cámara de trampeo atada al tronco de un árbol para captar imágenes y vídeos del pangolín indio.
mera point were obtained using the zonal statistics tool in Arc GIS.
Occupancy modelling
Occupancy of Indian pangolins was estimated using the likelihood–based method (MacKenzie et al., 2002). Species detection history (e.g., 1100100) for each camera location (consisting of binary values with ‘1’ indicating species detection during the sampling occasion and '0’' indicating non–detection) was calculated by visually inspecting camera footage (Otis et al., 1978). It was assumed that each camera site was independent and no animal would move between sites during the survey period (Royle and Nichols, 2003). A survey of 30 days was divided into 10 sampling periods of three days each to increase the detection probability for each sampling period. Detection histories were pooled for two years and entered together as single–season models in PRESENCE v.4 (Proteus Wildlife Research Consultants, New Zealand; http:// www.proteus.co.nz). Logistic concerns, such as the low number of cameras and the field situation (high density of Asian elephants) restricted us from conducting seasonal sampling as two consecutive sampling seasons could not be conducted for each camera location. All continuous variables were standardized to z–scores prior to analysis (Cooch and White, 2005). To reduce the model over–fitting by having high correlations among covariates, all the covariates were tested using pair–wise analysis for high co–linearity using the Pearson correlation coefficient. Only independent variables were selected for each analysis by removing covariates with > 0.75 r values.
A number of candidate models were defined incorporating possible covariates for the species based on priori hypotheses and available literature regarding factors that could influence site occupancy and detection probability (Kalle, 2013). Models were used to understand how variables could influence species occupancy and habitat use in order to explore the power of covariates. The software program PRESENCE v.4 was used for the model set development.
The psi (ψ) value was interpreted as the overall proportion of a study area used by the Indian pangolins (MacKenzie et al., 2002). A global model that contained all potential covariates for occupancy was produced and detection probability (p) was allowed to vary by all covariates. Occupancy probability values obtained for each habitat type were averaged to determine the preferred habitat types.
Activity level and activity patterns
We analysed the time stamp data on camera videos and photo records to generate the activity pattern of Indian pangolins. To determine activity level (a), that is, the proportion of the day that the species is active, we used R version 4.0.3 (R Core Team, 2013) package ‘activity’ (Rowcliffe et al., 2014, 2016; Rowcliffe, 2019). The time stamp data of Indian pangolins captured on camera trap videos was converted to radian time. This
was analysed in R with 1,000 iterations. An activity graph was generated based on non–parametric von Mises kernel density (Ridout and Linkie, 2009).
To determine the activity overlap between Indian pangolins and poachers, activity time data were analysed following Ridout and Linkie (2009) using the R package 'overlap' (Meredith and Ridout, 2014) in R bootstrapping with 1000 iterations from the original data. The measure of overlap was calculated using the coefficient of overlapping (Δ1) (for sample size < 75); 0 (no overlap), 1 (complete overlap) (Ridout and Linkie, 2009; Hearn et al., 2018). Activity overlap graphs were generated based on non–parametric von Mises kernel density (Ridout and Linkie, 2009).
Population density estimation
We used the Random Encounter Model (REM) developed by Rowcliffe et al. (2008) to estimate the density (D, km–2) of Indian pangolin in MONP. The equation,
D = (y/t) x (o/v*r(2 + h)
was used for the calculation where: y, was the number of capture events; t, the survey effort (camera trapping days); v, the average daily distance travelled (km/day); r, the average distance to the first capture of animals (km); h, radians, the average angle to the capture animals.
To calculate the day range (v), we estimated the movement speed of Indian pangolins following the method of Rowcliffe et al. (2016). The distance travelled daily (v, day range) was derived using the movement speed (s) and activity level (a) of animals following the equation: v = s x a. We used the activity level calculated in the previous section. The movement speed (s) of each animal was derived from time taken by the animal to move in front of the camera (Pfeffer et al., 2018). We followed the procedure described by Rowcliffe et al. (2016) to calculate the average speed parameter by fitting probability distributions to samples of individual speed observations obtained from video captures. The distance from camera to the animal was measured following the distance grid method described in Jayasekara et al. (2021b). The R package 'fitdistrplus' (Delignette–Muller and Dutang, 2015) was used for model fitting and best fitting models were selected based on Akaike Information Criterion (AIC) values. Density calculations were performed in R bootstrapping with 1000 iterations from the original data. Goodman's (1960) variance of products formula was used to calculate the standard error when required. Coefficient of variation (CV) was obtained using the square root of the variance and the point estimates.
Results
Occupancy and habitat use of the Indian pangolin
Indian pangolins were detected in all four terrestrial habitat types. However, the highest occupancy probability of Indian pangolins was within the dry–mixed
230
Jayasekara et al.
Pangolin occupancy probability 0.4 0.3 0.2 0.1 0.0
Fig. 4. Habitat occupancy of Indian pangolin in Maduru Oya National Park: DEF, dry–mixed evergreen forest; GSL, grassland; ROC, rocky outcrop; SHB, shrubland
Fig. 4. Ocupación del hábitat del pangolín indio en el parque nacional Maduru Oya: DEF, bosque seco mixto perennifolio; GSL, pradera; ROC, promontorio rocoso; SHB, matorral.
evergreen forest habitat (0.41 ± 0.19) and it was significantly higher than in the other three habitat types (Kruskal–Wallis, z = 6.04, p = 0.0001) (fig. 4). The highest ranked occupancy model included canopy
cover and dry–mixed evergreen forest as the most associated covariates which positively influenced the overall occupancy probability of Indian pangolin. Euclidean distance to water, termite mounds and human
Table 1. Highest ranking models for factors influencing the site occupancy of Indian pangolin in Maduru Oya National Park (MONP): ΔAIC, delta AIC; AICw, AIC wiight; MLL, model likelihood; K, number of parameters in the model; Nocc, naïve occupancy; psi(SE), occupancy probability of the species ± standard error; p(SE), detection probability of species ± standard error; “(.)” indicates constant across all camera sites.
Tabla 1. Los mejores modelos para los factores que influyen en la ocupación del pangolín indio en el parque nacional Maduru Oya: ΔAIC, delta AIC; AICw, peso AIC; MLL, probabilidad de ocupación estimada con el modelo; K, número de parámetros en el modelo; Nocc., ocupación ingenua; psi(SE), probabilidad de ocupación de la especie ± error estándar; p(SE), probabilidad de detección de la especie ± error estándar; "(.)" indica constante en todas las cámaras.
Model AIC ΔAIC AICw MLL K Nocc. psi(SE) p(SE)
psi(CN + DEF), p(Edw) 222.37 0.00 0.215 1.000 5 0.187 0.219 ± 0.027 0.224 ± 0.053 psi(CN + DEF), 222.99 0.62 0.212 0.733 6 0.224 ± 0.027 0.203 ± 0.003 p(Edw + TM)
psi(CN + DEF), p(.) 223.14 0.77 0.197 0.681 4 0.197 ±0.024 0.257 ± 0.040 psi(CN + DEF), p(TM) 223.20 0.83 0.191 0.660 5 0.204 ± 0.025 0.230 ± 0.051 psi(CN + DEF), 224.31 1.94 0.110 0.379 6 0.222 ± 0.027 0.220 ± 0.004 p(Edw + RAIH)
Model averaged 0.213 ± 0.005 0.226 ± 0.008
Animal Biodiversity and Conservation 45.2 (2022) 231
DEF GSL ROC SHB Habitat type
Jayasekara et al.
Table 2. Untransformed estimates of beta coefficients and standard error (SE) for the covariates contained in the top ranked models of Indian pangolin occupancy.
Tabla 2. Estimaciones sin transformar de los coeficientes beta y el error estándar (SE) de las covariables contenidas en los mejores modelos de ocupación del pangolín indio.
Covariate Estimate ± SE
Occupancy probability
Intercept -2.523 ± 0.681
Canopy cover 1.010 ± 0.434
Dry-mixed evergreen forest 1.639 ± 0.812
Detection probability
Intercept -1.349 ± 0.267 Euclidean distance to water -0.762 ± 0.417
abundance were also present in the top ranked models that positively influenced the detection probability of pangolins (tables 1, 2).
Spatial distribution of Indian pangolin in MONP
The species was primarily distributed within the western flank of MONP where dry–mixed evergreen forest was prominent (fig. 5). The highest number of presence points for poachers was also recorded in this same region of the park where Indian pangolin occupancy probability was highest (fig. 5).
Temporal activity and activity patterns
Indian pangolin displayed a highly nocturnal activity pattern (nocturnal activity level, 0.633) with an overall activity level of 0.336 ± 0.039. The activity pattern was unimodal; peak activity density and the highest number of encounters were recorded after midnight. The activity level then decreased gradually towards dawn (fig. 6A). The earliest capture record during night was at 19:21 h. There was a single camera trap observation at 06:57 h, and this was the only recorded encounter after 06:00 h in the morning. Pangolins displayed a considerable overlap of activity with humans (poachers) at a Δ score of 0.442 (0.240–0.588). The activity level of poachers also peaked after midnight (fig. 6B)
Population density
The low movement speed of the species coupled with a moderate level of activity resulted in a modest day range of 8.70 ± 0.78 km/day compared to the day ranges recorded by Jayasekara et al. (2021b) for other
Table 3. Parameters associated with population density estimates of Indian pangolin based on REM: ADD, average detection distance; ADA, average detection angle; PD, population density.
Tabla 3. Parámetros asociados a la estimación de la densidad de población del pangolín indio basada en el modelo de encuentro aleatorio REM): ADD, distancia media de detección; ADA, ángulo medio de detección; PD, densidad de población.
Parameter
Estimate ± SE
Activity level 0.336 ± 0.0340 Speed 1.08 ± 0.09 km/h Day range 8.70 ± 0.78 km ADD 3.21 ± 0.26 m ADA 0.512 ± 0.05 radians PD 0.73 ± 0.21 indiv./km2 Coefficient of variation of PD 19.10 %
mammalian species of the park. The average detection distance of Indian pangolins was also relatively higher, indicating high detectability of the species when compared to other meso–mammals of MONP (Jayasekara et al., 2021b). The estimated population density for the species was 0.73 ± 0.21 indiv./km2 with a reasonable coefficient of variation value of 19.10 % (table 3).
Discussion
Our occupancy estimates for the species at MONP indicate that these dry zone lowlands provide habitat for the species, as was shown from the distribution studies of the Indian pangolin (Karawita and Perera, 2020; Perera and Karawita, 2020). Even though the species was observed in all available habitat types, there was a higher affinity for the dry–mixed evergreen forest habitats of MONP. The dry–mixed forest habitat can therefore be considered the preferred habitat of Indian pangolin in MONP. The occupancy modeling also revealed an association of Indian pangolin occupancy with canopy cover. Though not visible in the generated results, even in the dry–mixed forest, the availability of rocks was observed to some extent at locations of pangolin sightings. The species was more detectable in areas relatively closer to water bodies and where termite mounds were abundant. The high detection probability in areas closer to the water (low EDW) indicates the importance of water sources. Relatively higher productivity in alluvial habitats (Dittus, 2017) would have influenced this higher occupancy (as a result of increased prey availability). An impor-
232
0 2.5 5 10 15 km
Occupancy probability
0–0.1 0.1–0.2 0.2–0.3 0.3–0.4 0.4–0.5 0.5–0.6 0.6–0.7 0.7–0.8 0.8–0.9 0.9–1.0 Human presence
Fig. 5. Modeled distribution map of Indian pangolins in Maduru Oya National Park indicating the occupancy probability based on kernel density. Human presence points (poachers) are also indicated on the map.
Fig. 5. Modelo de la distribución del pangolín indio en el parque nacional Maduru Oya en el que se indica la probabilidad de ocupación basada en la densidad del kernel. En el mapa también se indican los puntos de presencia humana (cazadores furtivos).
tant observation was the higher detection probability recorded for the species in areas where unauthorized human activity was observed. Rather than a behavioral preference of Indian pangolin, this finding would likely have been generated as a consequence of high poaching activity in those areas and targeting hunting of the species. This was further visible in the generated spatial distribution map of the species. Unauthorized human activity should thus be prevented in order to stop hunting of Indian Pangolin and other species in MONP. In the recent past, there have been several raids by park officers that seized pangolins killed by the poachers entering the park. Within the dry–mixed forest habitat of MONP, we observed a number of Indian pangolin burrows dug mostly in association with old termite mounds, rock crevices, and decayed tree cavities near the ground surface (fig. 1s in supplementary material). Outside the protected areas in the dry zone farmlands, we observed man–made traps, called 'Habaka', which the villagers use to trap and kill Indian pangolins.
In a study in Pakistan, Waseem et al. (2020) reported similar habitat preference for the species for forested areas. In our study in MONP, the observations were similar; the Indian pangolins sought cover and protection in the denser forested areas of the park. However, the availability of such suitable habitat is limited. The forested habitats of the western flank of the park were especially identified as good habitats
for the species. The conservation and protection of such vital habitats is therefore highly recommended. Despite efforts by the park management, illegal hunting and man–made fires continue to occur in these critical habitats as we observed in our camera trap data and direct observations. We suggest staff numbers in the park should be increased and more ground patrols should be conducted to prevent such illegal activities.
The activity of Indian pangolin was exclusively nocturnal and peak activity after midnight overlapped with the activity of poachers, indicating the higher likelihood of the species being targeted by the local hunters. On several occasions, the camera traps showed the poachers on the same path as the pangolin (fig. 2s in supplementary material).
These data may help park authorities conduct more effective and more efficient raids in targeted areas and in specific time slots in order to apprehend wildlife criminals. This study is possibly the first published record where camera trap–based REM method (Rowcliffe et al., 2008) was applied to determine the population density of this pangolin. In the context of Sri Lanka, the supposed population density of the species (5.69 indiv./km2) could be overestimated, as recently suggested Mahmood et al. (2020). One very recent study conducted in the same small forest area (< 20 km2) of Yagirala based on N–mixture models predicted the density of the species as 0.037 ha–1 (3.7 indiv./km2) in the wet
Animal Biodiversity and Conservation 45.2 (2022) 233
N
B
Activity level
0.12 0.10 0.08 0.06 0.04 0.02 0.00
0.15 0.10 0.05 0.00 A
0:00 6:00 12:00 18:00 24:00 Time 12:00 18:00 0:00 6:00 12:00 Time
Fig. 6. A, activity pattern of Indian pangolin fitted with circular kernel distributions of radian time; B, activity overlap between Indian pangolin and humans (poachers), grey indicates overlapping activity.
Fig. 6. A, pauta de actividad del pangolín indio ajustada con las distribuciones de kernel circulares del tiempo en radianes; B, superposición de la actividad del pangolín indio y de los humanos (cazadores furtivos); el color gris indica la superposición de la actividad.
zone of Sri Lanka (Perera et al., 2022). However, the accuracy of results could not be compared due to the difference in methodology (N–mixture models) and the lack of CV values in the mentioned paper when compared to the present study which followed the REM method, as used in many other recent studies. The REM–based density estimation in the present study (0.73 indiv./km2 ~ 0.0073 ha–1) provides more supporting data to calculate the forms of activity level, movement speed, day range, and detection distance. Our density estimate is closer to but higher than most figures recorded elsewhere in the region and that range from 0.00044 to 1.5 indiv./km2 (Mahmood et al., 2014, 2020), most of which have used burrow counts rather than the actual abundance data. We consider our estimates of a coefficient of variation of 19.1% are accurate and we leave it to the research community to conduct a comparative study applying both the burrow count method and REM to
a single community of pangolins so as to investigate the most suitable method to estimate density of the species. Our findings also show the slow–moving nature of the Indian pangolin could be a disadvantage for the species in hostile habitat conditions where it could be hunted and also threatened by the seasonal man–made fires.
As indicated by Khwaja et al. (2019) and Perera et al. (2022) our results suggest that camera traps are an effective tool to monitor an elusive and nocturnal species like the Indian pangolin. The low–glow/no glow invisible flashed camera types used and low height of camera attachment allowed us to monitor the animal's movement and human movements without causing distractions to the behavior. Our observations regarding the ecological and habitat requirements could help the possible ex-situ conservation of this species, which is declining in its natural habitats (Perera et al., 2017; Perera and Karawita, 2020), and the population
234 Jayasekara et al.
Activity level
Human activity Indian pangolin activity
data generated may be useful to evaluate the status of Indian pangolin. In conclusion, to protect the species in this area there is a need to conserve the species’ natural habitat, provide more protection, reduce illegal hunting, and develop awareness programs to educate the local communities.
Acknowledgements
We appreciate the generous cooperation of the Maduru Oya National Park staff and the Department of Wildlife Conservation Sri Lanka during our field work. We also acknowledge the facilities provided by the University of Sri Jayewardenepura under the research grants and funding by the Rufford Foundation. We also thank 'Wildlife Circle' team, Mr. Suranga (MONP), Mr. M. R. Mohamed (former warden MONP) and Mr. Gunasinghe for the support provided.
References
Aditya, V., Komanduri, K. P., Subhedar, R., Ganesh, T., 2021. Integrating camera traps and community knowledge to assess the status of the Indian pangolin Manis crassicaudata in the Eastern Ghats, India. Oryx, 55: 677–683, Doi: 10.1017/ S0030605319001303
Atkins, W. A., 2004. Pholidota pangolins (Manidae). In: Grzimek's Animal Life Encyclopedia (B. Grzimek, D. G. Kleiman, V. Geist, M. C. McDade, Eds.). Thomson Gale, Detroit.
Challender, D. W., Harrop, S. R., MacMillan, D. C., 2015. Understanding markets to conserve trade–threatened species in CITES. Biological Conservation, 187: 249–59, Doi: 10.1016/j.biocon.2015.04.015
Chen, M. T., Tewes, M. E., Pei, K. J., Grassman, L. I., 2009. Activity patterns and habitat use of sympatric small carnivores in southern Taiwan. Mammalia, 73(1): 20–26, Doi: 10.1515/MAMM.2009.006
CITES, 2021. Convention on International Trade in Endangered Species of Wild Fauna and Flora. Appendices I, II and III. Available online at: https:// cites.org/eng/app/appendices.php [Accessed on 04 November 2021].
Delignette–Muller M. L., Dutang C., 2015. fitdistrplus: An R Package for Fitting Distributions. Journal of Statistical Software, 64(4): 1–34, Doi: 10.18637/ jss.v064.i04
Dittus, W. P. J., 2017. The biogeography and ecology of Sri Lankan mammals point to conservation priorities. Ceylon Journal of Science, 46(special issue): 33–64, Doi: 10.4038/cjs.v46i5.7453
Goodman, L. A., 1960. On the exact variance of products. Journal of the American statistical association , 55(292): 8–713, Doi: 10.1080/01621459.1960.10483369
Green, M. J. (Ed.), 1990. IUCN directory of South Asian protected areas. World Conservation Monitoring Centre, lUCN Commission on National Parks and Protected Areas.
Gunatilleke, C. V., Gunatilleke, I. A., 1986. Horton Plains: some aspects of its vegetation and ecology. Sri Lanka Wildlife, 3(4): 9–11.
Hearn, A. J., Cushman, S. A., Ross, J., Goossens, B., Hunter, L. T., Macdonald, D. W., 2018. Spatio–temporal ecology of sympatric felids on Borneo. Evidence for resource partitioning?. Plos One, 13: e0200828, Doi: 10.1371/journal.pone.0200828
Jayasekara, D., Kumara, P. K. P. M. P., Mahaulapatha, W. A. D., 2021a. Mapping the vegetation cover and habitat categorization of Maduru Oya and Horton Plains National Parks using Landsat 8 (OLI) imagery to assist the ecological studies. Wildlanka, 9(1): 122–135.
Jayasekara, D., Mahaulapatha, W. A. D., Miththapala, S., 2021b. Population density estimation of meso–mammal carnivores using camera traps without the individual recognition in Maduru Oya National Park, Sri Lanka. Hystrix, the Italian Journal of Mammalogy 32(2), Doi: 10.4404/hystrix-00452-2021
Kalle, R., 2013. Ecology of sympatric small carnivores in Mudumalai Tiger Reserve, Tamil Nadu. PhD thesis, Saurashtra University, Rajkot, India. Available online at: http://hdl.handle.net/10603/15963 [Accessed 18 June 2018].
Karawita, H., Perera, P., 2020. Dataset of distribution, habitats and conservation status of the Indian pangolin (Manis crassicaudata) in Sri Lanka. Data in Brief, 28: 104999, Doi: 10.1016/j.dib.2019.104999
Karawita, H., Perera, P. Dayawansa, N., Dias, S., 2020. Dietary composition and foraging habitats of the Indian Pangolin (Manis crassicaudata) in a tropical lowland forestassociated landscape in southwest Sri Lanka. Global Ecology and Conservation, 21: e00880, Doi: 10.1016/j.gecco.2019. e00880
Karawita, H., Perera, P., Gunawardane, P., Dayawansa, N., 2018. Habitat preference and den characterization of Indian Pangolin (Manis crassicaudata) in a tropical lowland forested landscape of southwest Sri Lanka. Plos One, 13: e020608, Doi: 10.1371/ journal.pone.0206082
Karawita, K. V. D. H. R., Perera, P. K. P., Pabasara, M. G. T., 2016. Indian Pangolin (Manis crassicaudata) in Yagirala Forest Reserve: Ethnozoology and Implications for Conservation. In: International Forestry and Environment Symposium, 34. Department of Forestry and Environmental Science, University of Sri Jayewardenepura, Sri Lanka.
Khwaja, H., Buchan, C., Wearn, O. R., Bantlin, D., Bernard, H., Bitariho, R., Bohm, T., Borah, J., Brodie, J., Chutipong, W., du Preez, B., 2019. Pangolins in global camera trap data: Implications for ecological monitoring. Global Ecology and Conservation, 20: e00769, Doi: 10.1016/j.gecco.2019.e00769
MacKenzie, D. I., Nichols, J. D. Lachman, G. B. Droege, S. Royle, A. J., Langtimm, C. A., 2002. Estimating site occupancy rates when detection probabilities are less than one. Ecology, 83: 2248–2255, Doi: 10.1890/0012-9658(2002)083[2248:ES ORWD]2.0.CO;2
Mahmood, T., Akrim, F., Irshad, N., Hussain, R., Fa-
Animal
235
Biodiversity and Conservation 45.2 (2022)
tima, H., Andleeb, S., Aihetasham, A. Y. E. S. H. A., 2019. Distribution and illegal killing of the Endangered Indian pangolin Manis crassicaudata on the Potohar Plateau, Pakistan. Oryx, 53: 159–164, Doi: 10.1017/S0030605317000023
Mahmood, T., Andleeb, S., Akrim, F., 2021a. Habitat preference of the Indian Pangolin Manis crassicaudata inhabitng Margalla Hills Natonal Park, Islamabad, Pakistan. Journal of Threatened Taxa, 13: 18148–18155, Doi: 10.11609/jott.5872.13.5.1814818155
Mahmood, T., Andleeb, M., Anwar, M., Rais, M., Nadeem, S., Akrim, F., Hussain, R., 2015. Distribution, abundance and vegetation analysis of the scaly anteater (Manis crassicaudata) in Margalla Hills National Park Islamabad, Pakistan. Journal of Animal and Plant Sciences, 25: 1311–1321.
Mahmood, T., Challender, D., Khatiwada, A., Andleeb, S., Perera, P., Trageser, S., Ghose, A., Mohapatra, R., 2021b. Manis crassicaudata. The IUCN Red List of Threatened Species 2019, IUCN. Available online at: https://www.iucnredlist.org/ species/12761/123583998 [Accessed on 4 November 2021]
Mahmood, T., Irshad, N., Hussain, R., 2014. Habitat Preference and Population Estimates of Indian Pangolin (Manis crassicaudata) in District Chakwal of Potohar Plateau, Pakistan. Russian Journal of Ecology, 45: 70–75, Doi: 10.1134/ S1067413614010081
Mahmood, T., Mohapatra, R. K., Perera, P., Irshad, N., Akrim, F., Andleeb, S., Waseem, M., Sharma, S., Panda, S., 2020. Indian Pangolin Manis crassicaudata (Geoffroy, 1803). Pangolins, 5: 71–88, Doi: 10.1016/B978-0-12-815507-3.00005-8
Meredith, M., Ridout, M., 2014. Overview of the overlap package In: R project 1–9.
Michel, P., Mathieu, R. Mark, A. F., 2010. Spatial analysis of oblique photo–point images for quantifying spatio–temporal changes in plant communities. Applied Vegetation Science, 13(2): 173–182, Doi: 10.1111/j.1654-109X.2009.01059.x
Otis, D. L., Burnham, K. P., White, G. C., Anderson, D. R., 1978. Statistical inference from capture data on closed animal populations. Wildlife Monographs, 62: 3–135.
Perera, P., Karawita, H., 2020. An update of distribution, habitats and conservation status of the Indian pangolin (Manis crassicaudata) in Sri Lanka. Global Ecology and Conservation, 20: e00799, Doi: 10.1016/j.gecco.2019.e00799
Perera, P., Karawita, H., Jayasinghe, C., 2022. The applicability of camera trap data to monitor the cryptic Indian pangolin (Manus crassicaudata) populations: A survey from a tropical lowland rainforest in Southwest Sri Lanka. Global Ecology and Conservation, 34: e02046, Doi: 10.1016/j.
gecco.2022.e02046
Perera, P. K. P., Karawita, K. V. D. H. R., Pabasara, M. G. T., 2017. Pangolins (Manis crassicaudata) in Sri Lanka: A Review of Current Knowledge, Threats and Research Priorities. Journal of Tropical Forestry and Environment, 7: 1–14, Doi: 10.31357/ jtfe.v7i1.3018
Pfeffer S. E., Spitzer R., Allen A. M., Hofmeester T. R., Ericsson G., Widemo F., Singh N. J., Cromsigt J. P., 2018. Pictures or pellets? Comparing camera trapping and dung counts as methods for estimating population densities of ungulates. Remote Sensing in Ecology and Conservation, 4(2): 173–183, Doi: 10.1002/rse2.67
R Core Team., 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria.
Ridout, M. S., Linkie, M., 2009. Estimating overlap of daily activity patterns from camera trap data. Journal of Agricultural, Biological, and Environmental Statistics, 14: 322–337, Doi: 10.1198/ jabes.2009.08038
Rowcliffe, J. M., 2019. Activity: Animal activity statistics. R package version 1.3.
Rowcliffe, J. M., Field, J., Turvey, S. T., Carbone, C., 2008. Estimating animal density using camera traps without the need for individual recognition. Journal of Applied Ecology, 45(4): 1228–1236, Doi: 10.1111/j.1365-2664.2008.01473.x
Rowcliffe, J. M., Jansen, P. A., Kays, R., Kranstauber, B., Carbone, C., 2016. Wildlife speed cameras: measuring animal travel speed and day range using camera traps. Remote Sensing in Ecology and Conservation, 2: 84–94, Doi: 10.1002/rse2.17
Rowcliffe, J. M., Kays, R., Kranstauber, B., Carbone, C., Jansen, P. A., 2014. Quantifying levels of animal activity using camera trap data. Methods in Ecology and Evolution, 5: 1170–1179, Doi: 10.1111/2041210X.12278
Royle, J. A., Nichols, J. D., 2003. Estimating abundance from repeated presence–absence data or point counts, Ecology , 84: 777–90, Doi: 10.1890/0012-9658(2003)084[0777:EAFRPA]2.0.CO;2
Waseem, M., Khan, B., Mahmood, T., Hussain, H. S., Aziz, R., Akrim, F., Ahmad, T., Nazir, R., Ali, M. W., Awan, M. N., 2020. Occupancy, habitat suitability and habitat preference of endangered indian pangolin (Manis crassicaudata) in Potohar Plateau and Azad Jammu and Kashmir, Pakistan. Global Ecology and Conservation, 23: e01135, Doi: 10.1016/j.gecco.2020.e01135
Weerakoon, D., 2012. The taxonomy and conservation status of mammals in Sri Lanka. In: The National Red List, 134–144 (D. K. Weerakoon, S. Wijesundara, Eds.). Ministry of Environment, Colombo, Sri Lanka, http://www.cea.lk/web/images/ pdf/redlist2012.pdf
236
Jayasekara et al.
Home range of pampas deer in a human–dominated agro–ecosystem
M. Cosse, J. M. B. Duarte, S. González
Cosse, M., Duarte, J. M. B., González, S., 2022. Home range of pampas deer in a human–dominated agro–ecosystem. Animal Biodiversity and Conservation, 45.2: 237–243, Doi: https://doi.org/10.32800/abc.2022.45.0237
Abstract
Home range of pampas deer in a human–dominated agro–ecosystem. The subspecies of pampas deer Ozotocerus bezoarticus uruguayensis is an endemic and endangered cervid from southeast Uruguay. However, knowledge regarding its home range requirements in agroecosystems is scarce. Our aim was to survey ten radio–collared pampas deer for two years to monitor their movements. The mean home–range size was 5.54 ± 3.18 km2. The core area size for all individuals monitored was 0.87 km2, which concurs with grazing crops for beef cattle. The population showed philopatric behavior and no significant differences were detected in the total area of occupation in respect to sex and season.
Key words: Ozotoceros bezoarticus uruguayensis , Neotropical deer, Spatial ecology, Habitat selection, Conservation, Uruguay
Resumen
El área de distribución del venado de campo en un ecosistema agrícola dominado por los seres humanos. La subespecie de venado de campo Ozotocerus bezoarticus uruguayensis es un cérvido endémico del sureste de Uruguay que se encuentra en peligro de extinción. A pesar de ello, se tienen pocos conocimientos sobre sus necesidades respecto del área de distribución en ecosistemas agrícolas. El objetivo de este trabajo fue rastrear los movimientos de 10 venados de campo marcados con collares transmisores por un período de dos años. La superficie media del área de distribución fue de 5,54 ± 3,18 km2. La superficie del área central para todos los individuos estudiados fue de 0,87 km2 y coincidió con parcelas de cultivo para el pastoreo de vacuno de carne. Esta población mostró un comportamiento filopátrico y no se detectaron diferencias significativas en la superficie total de ocupación en función del sexo o la estación.
Palabras clave: Ozotoceros bezoarticus uruguayensis, Venado neotropical, Ecología espacial, Selección de hábitat, Conservación, Uruguay
Received: 20 X 21; Conditional acceptance: 11 I 22; Final acceptance: 20 VI 22
Mariana Cosse, Susana González, Departamento de Biodiversidad y Genética, Instituto de Investigaciones Biológicas Clemente Estable (IIBCE), MEC, Montevideo, Uruguay.– José Mauricio Barbanti Duarte, Núcleo de Pesquisa e Conservação de Cervídeos, Departamento de Zootecnia, Universidade Estadual Paulista, Jaboticabal, Brazil.
Corresponding author: Susana González, E–mail: sgonzalez@iibce.edu.uy
ORCID ID: Susana González: 0000-0001-6470-6182; J. M. B. Duarte: 0000-0002-7805-0265

ISSN: 1578–665 X eISSN: 2014–928 X
© [2022] Copyright belongs to the authors, who license the journal Animal Biodiversity and Conservation to publish the paper under a Creative Commons Attribution 4.0 License
237 Animal Biodiversity and Conservation 45.2
(2022)
Introduction
Pampas deer, Ozotoceros bezoarticus, is a medium–size ungulate native to the open grassland habitats of South America from 1 ºS to 41 ºS (Cabrera et al., 1940; Jackson and Langguth, 1987; González et al., 2010; Rocha et al., 2019). Their natural habitat (Pampas, Cerrado, and grasslands of Argentina, Uruguay, Paraguay, Bolivia, and Brazil) has diminished drastically over the last two centuries, mainly due to modifications related to agricultural activities (Fonseca et al., 1999; González et al., 2010). The pampas deer has been cataloged by the Uruguayan government as a threatened species, and declared a living Uruguayan Natural Monument (Ministerial Decree 12/985). Nevertheless, management guidelines have not yet been established, and actions have not been taken to ensure the effective conservation of populations on private land (González et al., 2010). The subspecies O. b. uruguayensis (González et al., 2002) occurs at Sierra de Los Ajos, in the Rocha Department (southeast Uruguay) and is one of the most endangered subspecies; there is only one population, and th is consists of fewer than 350 individuals. The breeding season starts in September and continues through December. In 2002 this population occupied around 25 km2 on private ranches where various agricultural activities were conducted (Jackson et al., 1980; Cosse et al., 2009). In the same year, on some ranches in the Rocha Department, an outbreak of Brucella abortus was reported in livestock. In October 2002 the Ministerial Uruguayan Animal Sanitary Authority (MGAP) requested we perform a pampas deer capture to assess the role of pampas deer as a reservoir of B. abortus. This assessment provided the opportunity to radio–tag animals in order to evaluate this population's spatial patterns of use in an agro–ecosystem (González and Duarte, 2003).
Few studies have been conducted to determine the home range and habitat selection for Brazilian, Argentinian, and Uruguayan Pampas deer populations (Leeuwenberg et al., 1997; Pinder, 1997; Rodrigues and Monteiro–Filho, 2000; Moore, 2001; Lacerda, 2008; Vila et al., 2008). Understanding the ecological processes that determine the home range and patterns of use of space is crucial to develop and implement management and conservation plans (Gordon et al., 2004; Börger et al., 2006, 2008; Wingfield, 2009).
Our aim was to monitor the ten tagged pampas deer for two years to assess their home range size in an agro–ecosystem. This is the first report of the home range with radio–collared animals on Uruguayan populations.
Material and methods
Study area
The study area of the Los Ajos population is mainly located within an 80 km2 ranch (33º 50' 01'' S; 54º 01' 34'' W) located in the Bañados del Este
Biosphere Reserve in the southeastern Uruguayan department of Rocha. The landscape is characterized by low, rolling hills; the soils are predominantly gley. Altitudes range from –5 to 100 m a.s.l., the annual average rainfall is 1000 mm, and the average annual temperature is 16 ºC (Herzig, 1994). The pampas deer population size was estimated as 117 individuals, occupying an area of 25 km², the highest density recorded for the species with 11 animals/km² (Cosse and González, 2013). The main ranch activities at the study site are livestock (cattle and sheep ranching), rice crops for human consumption, and ryegrass for pasture of the livestock. Pampas deer and domestic animals in the area share grazing land. Cattle are fenced in various paddocks and managed by the ranchers. Rotation is performed in accordance with forage requirements. The pampas deer can move freely from one paddock to another by crossing under the bottom strand of wire.
Methods
In October 2002 pampas deer from the Los Ajos population were captured by ranch staff on horseback using fixed nets .The animals were herded under supervision of the Uruguayan animal health authorities (Duarte et al., 2010). The veterinarian staff restrained the deer immediately after capture and the animals were administered intravenous ketamine (150 mg) and xylazine (30 mg) (Pinho et al., 2010). All captured animals were tagged with Allflex ear tags and blood samples were taken to perform serological tests and genetic analysis. Ten animals were fitted with radio collars (Wildlife Material Inc., 150–151 Mhz; González and Duarte, 2003).
As the captured specimens were adults, this study does not take juvenile deer dispersal patterns into account. Radio–tagged animals were located monthly from December 2002 to February 2005.
Monitoring was carried out during the day, with two recording periods (7:30–13:30 h and 13:31–19:30 h). The geographic locations were obtained by triangulation using a hand–held four–element Yagi RA–2AK antenna and a TR–4 Telonics portable receiver. Three azimuth readings per fix were obtained with a compass. To minimize error due to the animals’ movements, the time between each recording was established at a maximum of 25 minutes. Each animal was located incorporating only the azimuths that differed from each other on 60–120º (White and Garrott, 1990). Whenever possible, location was verified by binoculars or telescope. These occasional sightings of radio–collared animals were geo–referenced using a Garmin eTrex H GPS (Olathe, KS, USA) and included in the analysis. In these cases we recorded the reproductive state and group composition.
Data points concerning the animals' location were obtained using the computer program LOCATE II (Nams, 2000). We rejected triangulation fixes with an error ellipse exceeding 0.5 km2 (Atwood et al., 2004). The locations were then loaded into an Arcview Gis 3.3 (ESRI, Redlands, CA, USA) project. The Animal Movement Analyst Extension 2.0 (Hooge and
238 Cosse et al.
Table 1. Radio–tracked Pampas deer in this study: EAY, estimated age in years at capture; RS, reproductive status at capture; RTTM, radio–tracked time in months; Fixes, number of fixes; GS, group size; and home ranges (km2) of pampas deer calculated using minimum convex polygon (MCP) and fixed Kernel estimators; DO, direct observation.
Tabla 1. Datos de individuos monitoreados en este estudio: EAY, edad estimada, en años, al momento de la captura; RS, estado reproductivo de las hembras al momento de la captura; RTTM, tiempo de monitoreo en meses; Fixes, número de registros; GS, tamaño de grupo; y áreas de distribución (km2) calculado utilizando los estimadores de mínimopolígono convexo (MCP) y Kernel; DO, observación directa.
Ind. Sex Sex EAY RS Mass (kg) RTTM Fixes MCP Kernel 95%
SG124 ♂ Male 3 – 35 5 5 1.27 5.28
SG125 ♂ 4 – 25 DO 2004 – – –
SG126 ♂ Male 4 – 35 20 29 7.97 9.28
SG129 ♂ Male No data – 31 21 30 4.19 4.39
SG130 ♂ Male 3 – no data 5 – – –
SG127 ♀ Female 4 Pregnant 25 18 15 6.26 8.68
SG131 ♀ Female 3 Pregnant 27 21 35 5.04 4.5
SG132 ♀ Female 2 – 29 21 37 5.66 3.3
SG133 ♀ Female 3 Lactating 25 21 25 6.62 5.5
SG137 ♀ Female 18 m. Pregnant 22 5 – – –
Male mean 31.5 4.5 6.3 Female mean 25.6 5.9 5.5
Eichenlaub, 2000) was used to estimate home range following the minimum convex polygon (Mohr, 1947) and Kernel density with 95 % of locations (Worton, 1989). We used Student's t–tests (Zar, 1999) to evaluate any significant differences between sexes and seasons regarding home–range size (Atwood et al., 2004; Shibuya et al., 2018).
Interactions between radio–collared animals were analyzed following the measure of static territorial interaction (S) (White and Garrott, 1990). This value represents the proportion of animali's home range overlapped by animalj’s home range. We also estimated the mean overlap area (Rij), which represents the size of the area of overlapping among deer i and j (Chaverri et al., 2007). In all cases, the home ranges included in each analysis were generated from locations taken at the same time (n = 6 from 2003; n = 5 from 2004). We did not test for statistical significance of home range overlap because of the low number of locations. To determine whether the radio–collared pampas deer interact in space, a complete linkage clustering method (Romesburg, 2004) was performed (StatSoft Inc., 2004) using Euclidian distance with the matrix data from the value of overlap between each deer pair. Additionally, we estimated the pampas deer densities in the paddocks where radio–collared animals were located following Cosse and González (2013).
Results
Sixteen adult deer were captured (8 males: 8 females); two deer (12.5 %) died in the capture procedure. Ten deer, 5 females and 5 males, were radio–tagged. Only one radio collar (deer identification number SG125) did not receive the VHS signal. However, the animal was seen on one occasion, and recognized by the ear tag number. We were able to determine that the radio–collar antenna was broken and not transmitting. Three deer were monitored until they died at less than six months and it was not possible to determine the cause of death in any of the three. Six individuals (two males and four females) were monitored for 18–21 months (table 1), during which time we obtained 184 locations. The estimated locations obtained with Locate II were confirmed by direct observation in 26 % of cases. The mean home range per individual was estimated as 5.54 ± 3.18 km2. The activity area and the core area sizes obtained for all the individuals monitored in this study were 16.83 km2 and 0.87 km2, respectively (fig. 1). The activity range area remained unchanged for the two consecutive years (MPC: 12.9 km2 and 13.5 km2).
The core area concurs with the capture zone and is part of the paddocks in which grazing crops were grown for beef cattle breeding. No statistically significant differences were detected with the Student's
Animal Biodiversity and Conservation 45.2 (2022) 239
4 0 4 km
Fig. 1. Monitored area showing details of the specimens' location points and the total area of occupation, obtained over the 26 months of monitoring. The fine black lines represent the paddocks with different forage types. The brown lines show level curves and blue lines represent water courses.
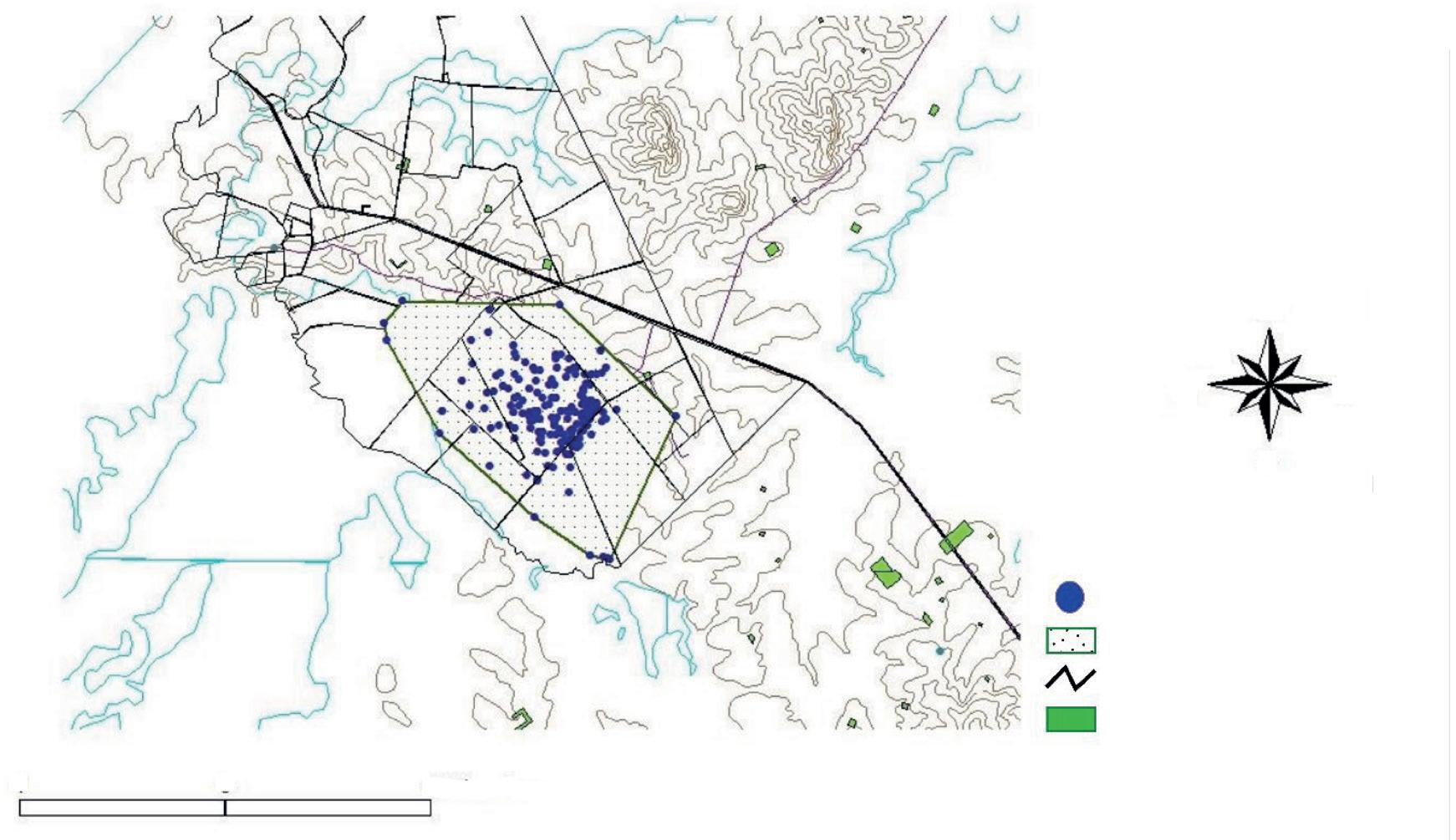
Fig. 1. Mapa del área estudiada donde se señala la localización de los individuos y la superficie total de ocupación en los 26 meses de rastreo. La línea negra continua delgada representa las parcelas con diferentes tipos de forraje. Las líneas marrones muestran las curvas de nivel y las líneas azules representan los cursos de agua.
t–test in the total area of occupation after discriminating by sex and season, including spring, which is the breeding season.
The pairwise percent overlap of home ranges showed an average value equal to 36.5 %. However, the overlap in home ranges of females was greater than that between mixed and male pairs (fig. 2). The results of cluster analysis of the overlapping matrix associations of radio–collared individuals (Rij) were consistent for the two years of the study (fig. 3). Both males evaluated were spatially highly associated with two females who, in turn, presented an association in the use of the environment (SG129♂ with SG131♀ and SG132♀ / SG126♂ with SG127♀ and SG133♀; see fig. 3).
Discussion
The home range sizes obtained in this study are in concordance with those observed for other pampas deer populations in Uruguay, Argentina, and Brazil, in which average values recorded ranged from 0.35 to 7.9 km2 (Leeuwenberg et al., 1997; Moore, 2001; Lacerda, 2008; Vila et al., 2008). These home range sizes and the low level of movement displayed by the individuals suggest the species has a philopatric
behavior. However, in a one–year radio–tracking study of six deer in an EMAS population (Cerrado biome, Rodrigues and Monteiro–Filho (2000) reported greater home ranges for the species, with a mean value of 82.3 km2 (min: 48; max: 146.8 km2). Based on the theory of optimal foraging (MacArthur and Pianka, 1966) tthey suggested that the animals at the Emas National Park limit their movements in the dry season (when forage is scarce) and stay in forage patches because it is energetically expensive to move to distant patches, but during the wet season, they move long distances so as to reach the areas with optimal forage, reaching the most distant home ranges described for pampas deer to date.
The species has been described as selective feeders or concentrate selectors (Jackson and Giulietti, 1988; Pinder, 1997; Rodrigues and Monteiro–Filho, 1999; Merino, 2003; Cosse et al., 2009). Pampas deer can also be considered mixed feeders, switchingbetween grazing and browsing throughout the year, mostly due to changes in environmental conditions. Pampas deer from Argentine and Uruguay occupy a wide variety of open grassland habitats in which temperate grasses are in general significantly more digestible than tropical grasses (from Cerrado and Pantanal in Brazil) (Demment and Van Soest, 1985; Pinder, 1997), and potentially more palatable.
240 Cosse et al.
N w E S
Fixed points Minimum convex polygon Route 14 Forest
Fig. 2. Home range overlap (mean and standard deviation) between pampas deer of the same sex and different sex: F, females; M, males.

Fig. 2. Solapamiento en el área de distribución (media y desviación estándar) entre venados de campo del mismo sexo y de diferente sexo: F, hembras; M, machos.
However, in the Pantanal, the pampas deer can find high quality forage on a local scale throughout the different seasons following the pulse of the floods (Lacerda, 2008). In contrast, in the Cerrado, where the scrub landscape is dominated by C4 rough grasses of reduced digestibility, the pampas deer may respond with a larger home range (Pinder, 1997; Lacerda, 2008).
The home ranges observed at Los Ajos population are in concordance with the opportunistic feeding behavior described by Cosse et al. (2009), where the pampas deer occupy a cattle–raising paddock with high nutritional temperate forage such as ryegrass (Lolium sp.). This population showed the highest densities of pampas deer (Cosse and González, 2013) in these large capacity paddocks. Chaverri et
Fig. 3. Cluster analysis results on the mean overlap matrix (S) for pampas deer pairs. Associations in the use of the environment were consistent over the two years (2003–2004) of monitoring.
Fig. 3. Resultados del análisis de conglomerados realizado a partir de la matriz del valor medio de solapamiento (S) para los pares de venados de campo. Las asociaciones en el uso del ambiente son las mismas en los dos años (2003 y 2004) de rastreo.
Animal Biodiversity and Conservation 45.2 (2022) 241
SG131♀ SG132♀ SG129♂ SG133♀ SG127♀ SG126♂ SG131♀ SG132♀ SG129♂ SG133♀ SG126♂
0.30
0.50 0.60 0.70 0.80
0.50 0.70 0.90 1.10 2003 2004 0.60 0.55 0.50 0.45 0.40 0.35 0.30 0.25 0.20 0.15 0.10 0.05
FF MM MF FM Pampas deer pair Home range overlap Mean ± SE
al. (2007) suggest that an extraordinary supply of resources favors high population density and this is a predominant force behind high social interaction, as observed in on the Los Ajos population. The nutritional quality of the Uruguayan temperate grassland allows the coexistence of pampas deer population and cattle ranching.
The individuals analyzed from the Los Ajos pampas deer population showed no statistically significant differences between home range size, sex, or season. These results are consistent with several other studies of pampas deer populations (Leeuwenberg et al., 1997; Rodrigues and Monteiro–Filho, 2000; Moore, 2001; Lacerda, 2008) and could be related to the gregarious behavior, with a group composition that includes both adult males and females, and the favorable nutritional conditions of the temperate grassland where pampas deer populations are mainly located.
At Los Ajos, we observed a mean value of overlap of 36.5 %, which is considerably lower than that for the Emas (around 80 %) and Samborombón (50%) populations (Rodrigues and Monteiro–Filho, 2000; Vila et al., 2008). However, when we analyzed the value of the mean overlapping area (Rij) each year separately, we found a permanent spatial association between the individuals. In turn, if the population has a stable group structure, we can expect to find different levels of overlap between individuals of the population, as observed at Los Ajos. Furthermore, the combined and independent effects of demography and social affinities could determine the overlapping in home range.
Conservation implications
As our findings show the pampas deer has a philopatric behavior, it is important to promote specific conservation policies to assure the long term viability of the remaining pampas deer populations in Uruguay. While biodiversity offsets are increasingly being used by governments and companies, an International Union for Conservation of Nature (IUCN) study found current efforts to mitigate impacts were proving insufficient to reduce the decline in biodiversity (Bull and Strange, 2018).
Acknowledgements
Special thanks to Dante Roibal and his wife Sonia, Clemente Tito Olivera, Raquel Álvarez, Alejandro Márquez, María Noel Merentiel and Susana Cardozo for their help in the fieldwork. We thank the Arrarte family of Los Ajos for providing permission to work on their property. This study was part of the PhD Project of M. Cosse and was funded by PEDECIBA and ANII. CSIC, Disney Worldwide Conservation Fund and Wildlife Trust also funded some of the activities.
References
Atwood, T. C., Weeks, H. P., Gehring, T. M., 2004. Spatial ecology of coyotes along a suburban–to–
rural gradient. The Journal of Wildlife Management, 68: 1000–1009, Doi: 10.2193/002-541X(2004)068 [1000:SEOCAA]2.0.CO;2
Börger, L., Dalziel, B. D., Fryxell, J. M., 2008. Are there general mechanisms of animal home range behaviour? A review and prospects for future research. Ecology Letters, 11: 637–650, Doi: 10.1111/j.1461-0248.2008.01182.x
Börger, L., Franconi, N., Ferretti, F., Meschi, F., Michele, Giampiero d., Gantz, A., Coulson, T., 2006. An Integrated Approach to Identify Spatiotemporal and Individual–Level Determinants of Animal Home Range Size. The American Naturalist, 168: 471–485, Doi: 10.1086/507883
Bull, J. W., Strange, N., 2018. The global extent of biodiversity offset implementation under no net loss policies. Nature Sustainability, 1: 790–798, Doi: 10.1038/s41893-018-0176-z
Cabrera, A., Yepes, J., Wiedner, C. C., 1940. Mamíferos sud–americanos: vida, costumbres y descripción. Compañia Argentina de Editores, Buenos Aires.
Cosse, M., González, S., 2013. Demographic characterization and social patterns of the Neotropical pampas deer. SpringerPlus, 2: 259, Doi: 10.1186/2193-1801-2-259
Cosse, M., González, S., Gimenez–Dixon, M., 2009. Feeding ecology of Ozotoceros bezoarticus : conservation implications in Uruguay. Iheringia. Série Zoologia, 99: 158–164, Doi: 10.1590/S007347212009000200007
Chaverri, G., Gamba–Rios, M., Kunz, T. H., 2007. Range overlap and association patterns in the tent–making bat Artibeus watsoni Animal Behaviour, 73: 157–164, Doi: 10.1016/j.anbehav.2006.06.003
Demment, M. W., Van Soest, P. J., 1985. A nutritional explanation for body–size patterns of ruminant and nonruminant herbivores. The American Naturalist, 125: 641–672, Doi: 10.1086/284369
Duarte, J., Uhart, M., Galvez, C., 2010. Capture and physical restraint. Neotropical Cervidology: Biology and Medicine of Latin American Deer, 218: 227.
Fonseca, G. a. B., Mittermeier, R. A., Cavalcanti, R. B., Mittermeier, C. G., 1999. Brazilian Cerrado. In: Hotspots: Earth's biologically richest and most endangered terrestrial ecoregions (R. A. Mittermeier, N. Myers, N., C. G. Mittermeier, R. Gil, Eds.) CEMEX, SA, Agrupación Sierra Madre, SC, Chicago.
González, S., Álvarez–Valin, F., Maldonado, J. E., 2002. Morphometric differentiation of endangered pampas deer (Ozotoceros bezoarticus), with description of new subspecies from Uruguay. Journal of Mammalogy, 83: 1127–1140, Doi: 10.1644/1545–1542(2002)083<1127:MDOEPD> 2.0.CO;2
González, S., Cosse, M., Góss Braga, F., Vila, A. R., Merino, M. L., Dellafiore, C., Cartes, J. L., Maffei, L., Gimenez Dixon, M., 2010. Pampas deer Ozotoceros bezoarticus (Linnaeus 1758). In: Neotropical Cervidology: Biology and Medicine of Latin American Deer (J. M. B. Duarte, S. González, Eds.) Funep/ IUCN, Jaboticabal.
González, S., Duarte, J. M. B., 2003. Emergency
242 Cosse et al.
Pampas deer capture in Uruguay. Deer Specialist Group News, 18: 16–17.
Gordon, I. J., Alison, J. H., Festa–Bianchet, M., 2004. The management of wild large herbivores to meet economic, conservation and environmental objectives. Journal of Applied Ecology, 41: 1021–1031, Doi: 10.1111/j.0021-8901.2004.00985.x
Herzig, M., 1994. Procedimiento de Monitoreo de la Convención de Ramsar. Informe No. 24 [vere 32]. Bañados del Este y Franja Costera, Uruguay. Gland, Ramsar, Suiza.
Hooge, P. N., Eichenlaub, B., 2000. Animal movement extension to Arcview. In: Alaska Science Center – Biological Science Office, 2.0 edition (U.S.G.S., Ed.). Anchorage, AK, USA.
Jackson, J., Landa, P., Langguth, A., 1980. Pampas Deer in Uruguay. Oryx, 15: 267–272, Doi: 10.1017/ S0030605300024674
Jackson, J. E., Giulietti, J. D., 1988. The food habits of Pampas deer Ozotoceros bezoarticus celer in relation to its conservation in a relict natural grassland in Argentina. Biological Conservation, 45: 1–10, Doi: 10.1016/0006-3207(88)90048-1
Jackson, J. E., Langguth, A., 1987. Ecology and status of the Pampas Deer in the Argentinian Pampas and Uruguay. In: Biology and management of the Cervidae (C. Wemmer, Ed.) Smithsonian Institute, Washingto DC.
Lacerda, A. C. R., 2008. Ecologia e estrutura social do veado–campeiro (Ozotoceros bezoarticus) no Pantanal. PhD dissertation, Universidade de Brasília.
Leeuwenberg, F. J., Lara Resende, S., Rodrigues, F. H. G., Bizerril, M. X. A., 1997. Home range, activity and habitat use of the Pampas deer Ozotoceros bezoarticus L., 1758 (Artiodactyla, Cervidae) in the Brazilian Cerrado. Mammalia, 6: 487–495.
Macarthur, R. H., Pianka, E. R., 1966. On optimal use of a patchy environment. The American Naturalist, 100: 603–609, Doi: 10.1086/282454
Merino, M. L., 2003. Dieta y uso de hábitat del venado de las pampas, Ozotoceros bezoarticus celer Cabrera 1943 (Mammalia–Cervidae) en la zona costera de Bahía Samborombón, Buenos Aires, Argentina. Implicancias para su conservación. PhD thesis, Universidad Nacional de la Plata.
Mohr, C., 1947. Table of equivalent populations of North American small mammals. American Midland Naturalist, Notre Dame, 37: 223–249, Doi: 10.2307/2421652
Moore, D., 2001. Aspects of the behavior, ecology and conservation of the Pampas Deer. PhD dissertation, State University of New York, New York.
Nams, V. O., 2000. LOCATE II. V. 2.81. Dalhousie University, Nova Scotia, Canada.
Pinder, L., 1997. Niche overlap among brown brocket deer, pampas deer, and cattle in the Pantanal of Brazil. PhD dissertation, University of Florida.
Pinho, M. P., Munerato, M. S., Nunes, A. L. V., 2010. Anesthesia and Chemical immobilization. In: Neotropical Cervidology: Biology and Medicine of Latin American Deer (J. M. B. Duarte, S. González, Eds.). Funep/IUCN, Jaboticabal.
Rocha, D. G., Vogliotti, A., Gräbin, D. M., Assunção, W. R., Cambraia, B. C., D’amico, A. R., Portela, A. E., Sollmann, R., 2019. New populations of pampas deer Ozotoceros bezoarticus discovered in threatened Amazonian savannah enclaves. Oryx, 53: 748–751, Doi: 10.1017/S0030605318001539
Rodrigues, F. H. G., Monteiro–Filho, E. L. A., 1999. Feeding behaviour of the pampas deer: a grazer or a browser? Deer Specialist Group News, 15: 12–13.
– 2000. Home range and activity patterns of pampas deer in Emas National Park, Brazil. Journal of Mammalogy , 81: 1136–1142, Doi: 10.1644/1545-1542(2000)081<1136:HRAAPO>2.0.CO;2 Romesburg, C., 2004. Cluster analysis for researchers. Lulu Press, North Carolina.
Shibuya, P. S., Melo, G. L., Cáceres, N. C., 2018. Determinants of home range size and spatial overlap of Gracilinanus agilis (Mammalia: Didelphidae) in central–western Brazil. Mammalia, 82: 328–337, Doi: 10.1515/mammalia-2016-0168
Vila, A. R., Beade, M. S., Barrios Lamunière, D., 2008. Home range and habitat selection of pampas deer. Journal of Zoology, 276: 95–102, Doi: 10.1111/j.1469-7998.2008.00468.x
White, G. C., Garrott, R. A., 1990. Analysis of wildlife radio–tracking data, San Diego, Academic Press. Wingfield, J. C., 2009. The Concept of Allostasis: Coping with a capricious environment. Journal of Mammalogy, 86: 248–254, Doi: 10.1644/BHE004.1
Worton, B. J., 1989. Kernel Methods for Estimating the Utilization Distribution in Home–Range Studies. Ecology, 70: 164–168, Doi: 10.2307/1938423
Zar, J. H., 1999. Biostatistical analysis, Upper Saddle River, New Jersey, Prentice–Hall.
Animal Biodiversity and Conservation 45.2 (2022) 243
244 Cosse et al.
¿Las diferencias ecomorfológicas predicen la coexistencia de murciélagos cavernícolas en Cuba?
Y. Ferrer–Sánchez, D. Denis Ávila
Ferrer–Sánchez, Y., Denis Ávila, D., 2022. ¿Las diferencias ecomorfológicas predicen la coexistencia de murciélagos cavernícolas en Cuba? Animal Biodiversity and Conservation, 45.2: 245–256, Doi: https://doi. org/10.32800/abc.2022.45.0245
Abstract
Do ecomorphological differences predict the co–existence of cave bats in Cuba? Identifying mechanisms that influence the coexistence of species is primordial to understanding patterns and processes in biodiversity. Here we aimed to assess the association between coexistence and morphology of cave bats in Cuba and differences in morphologic and dietary aspects. We assembled lists of species from 108 localities with at least three co–inhabiting species. Fourteen species of bats in at least three localities were included. Based on the literature we also obtained the mean values of five morphological variables and the basic composition of diet. We calculated coexistence patterns using EcoSim v7.72 based on the average number of checkerboard units that appeared between each pair of species. We evaluated the predictive capacity of the morphological variables and diet over the degree of coexistence between species using a neural regression network. The best neural network was accepted (correlation of 0.87, training error < 0.006). The prediction error was 13.5 % (± 1.1) of the value of the coexistence index. Our findings indicate that the composition of cave bat assemblages in Cuba has not come about by chance. Rather, we found that the most functionally different species tended to coexist. This finding is consistent with the effect of competitive relationships driving the composition of species of cave bats in Cuba.
Key words: Coexistence, Competitive exclusion, Chiroptera, Caves, Biodiversity Resumen
¿Las diferencias ecomorfológicas predicen la coexistencia de murciélagos cavernícolas en Cuba? Identificar los mecanismos que influyen en la coexistencia de las especies es primordial para comprender los patrones y procesos que rigen la biodiversidad. Este estudio tuvo como objetivo evaluar la asociación entre la coexistencia, la morfología y la dieta de los murciélagos cavernícolas de Cuba. Elaboramos listas de especies de 108 localidades, en las que había al menos tres especies cohabitantes. Se incluyeron 14 especies de murciélagos en al menos tres localidades. A partir de las publicaciones científicas, también obtuvimos los valores medios de cinco variables morfológicas y la composición básica de la dieta. Describimos los patrones de coexistencia por el número medio de unidades de tipo tablero de ajedrez que aparecen entre cada pareja de especies, calculado en el programa EcoSim v7.72. La capacidad predictiva de las variables morfológicas y la dieta respecto del grado de coexistencia entre las especies se evaluó entrenando una red neuronal de regresión. El desempeño de la mejor red neuronal obtenida fue aceptable (correlación de 0,87, error de entrenamiento < 0,006). El error de predicción fue el 13,5 % (± 1,1) del valor del índice de coexistencia. Según los resultados, la composición de los ensambles de murciélagos cavernícolas de Cuba no es aleatoria, ya que encontramos que las especies funcionalmente más distintas tienden a coexistir. Este resultado es coherente con que el efecto de las relaciones competitivas determine la composición de las especies de murciélagos cavernícolas en Cuba.
Palabras clave: Coexistencia, Exclusión competitiva, Chiroptera, Cuevas, Biodiversidad
Received: 15 XII 21; Conditional acceptance: 03 III 22; Final acceptance: 05 VII 22
Yarelys Ferrer–Sánchez, Universidad Técnica Estatal de Quevedo (UTEQ), Av. Quito km 1 1/2 vía a Santo Domingo de los Tsáchilas, Quevedo–Los Ríos, Ecuador.– Dennis Denis Ávila, Departamento de Biología Animal y Humana, Facultad de Biología, Universidad de La Habana, calle 25 entre J e I, Vedado, Ciudad de La Habana, Cuba.
Ccorresponding author: Y. Ferrer–Sánchez. E–mail: yferrer@uteq.edu.ec
ORCID ID: Y. Ferrer–Sánchez: 0000-0003-0623-1240; D. Denis Ávila: 0000-0003-4808-7195

ISSN: 1578–665 X eISSN: 2014–928 X
© [2022] Copyright belongs to the authors, who license the journal Animal Biodiversity and Conservation to publish the paper under a Creative Commons Attribution 4.0 License
245
Animal Biodiversity and Conservation 45.2 (2022)
Introducción
Los mecanismos que determinan la estructura de los ensambles y las comunidades de animales y plantas han sido objeto de intensos estudios y debates dentro de la ecología moderna. A medida que la ecología ha madurado, los estudios sobre la estructuración de las comunidades han dejado de centrase en los patrones observados para hacerlo en los mecanismos subyacentes, a fin de entender en mayor profundidad el funcionamiento de los sistemas ecológicos (Pickett et al., 1994). Identificar los factores que influyen en la coexistencia de las especies en lugares determinados es fundamental para comprender la biodiversidad como patrón y proceso. El debate sobre el determinismo o la estocasticidad de los procesos que producen los ensambles biológicos es antiguo (Polis, 1991) y los datos disponibles sobre su estado de equilibrio siguen siendo controvertidos (Siepielski y McPeek, 2010; Guillemot et al., 2011; Calatayud et al., 2020).
Poco se sabe acerca de la composición o estructura de los ensambles de murciélagos neotropicales y la mayoría de sus características se han deducido a partir de listas de especies y matrices de presencia–ausencia, que son herramientas fundamentales en los análisis biogeográficos y de las comunidades (Alurralde y Díaz, 2021). En este grupo se han observado similitudes entre la estructura de los ensambles reales y otros generados por selección aleatoria de especies (Willig y Moulton, 1989). Al ser sumamente vágiles, los murciélagos se mueven fácilmente entre localidades, hábitats e islas, lo cual los hace poco propensos al aislamiento que estabilizaría la estructura de los ensambles (Alurralde y Díaz, 2021). Según Willig (1986), hay tres preguntas básicas que se deben responder para comprender la estructuración de las comunidades de murciélagos sudamericanos: si realmente las locales tienen patrones no aleatorios en su estructura, si dichos patrones locales están o no extendidos y cuáles son sus factores determinantes.
El estudio de los patrones de coexistencia de las especies tuvo su auge a raíz del conflicto entre las reglas propuestas por Diamond (1975) y el descubrimiento de Connor y Simberloff (1979) de que estos mismos patrones no difieren significativamente de los obtenidos por azar. Independientemente de la polémica teórica y metodológica, el estudio de las frecuencias de coaparición de los pares de especies en muestras amplias de ensambles continúa siendo una herramienta más del estudio de las comunidades (Ulrich, 2004). Los patrones ecológicos de la estructura de los ensambles no solo se describen por medio de los índices de coexistencia de especies, sino también de la distribución de las abundancias proporcionales o empleando indicadores morfológicos poblacionales. La falta de información sobre la estructura precisa de los ensambles de murciélagos por lo que hace a las abundancias proporcionales se asocia a la dificultad de su medición, mientras que la composición y las mediciones morfométricas son mucho más simples de evaluar. Las restricciones fenotípicas que imponen los parámetros ambientales en los hábitats particulares influyen en la capacidad
reproductiva y generan respuestas morfológicas más fácilmente cuantificables que otros parámetros ecológicos directos como el poder reproductivo, el estrés o la superposición ecológica (MacArthur y Levins, 1967; Brown et al., 1996).
Se ha sugerido que un factor importante en la estructuración de los ensambles de murciélagos puede ser el tamaño corporal (Willig, 1986). El principio de la similitud limitante de MacArthur y Levins (1967) permite identificar a los competidores potenciales, coexistentes dentro del mismo gremio, cuando la diferencia entre la talla de los mismos es inferior a la razón de Hutchinson (1,26) (Hutchinson, 1959). Este valor ha sido sugerido como límite entre los pesos adyacentes de especies competidoras en un gremio para garantizar su coexistencia y, aunque ha sido cuestionado en términos teóricos, se ha utilizado como criterio en las matrices de gremios (Roth, 1981). La estructura de los ensambles de murciélagos también se ha descrito con frecuencia en términos de gremios tróficos (grupos de especies que consumen alimentos similares con técnicas parecidas de alimentación; sensu [Root, 1967]), en los que se cree que la competencia tiene un papel importante en su determinación (Salgado–Mejía et al., 2021). Las matrices de gremios y tallas, a falta de información ecológica detallada sobre las interacciones interespecíficas, han sido los instrumentos principales con los que se ha podido determinar la organización comunitaria en este grupo (McNab, 1971; Fleming et al., 1972; Alurralde y Díaz, 2021).
Los murciélagos del Caribe han sido bien estudiados (Baker y Genoways, 1978; Rodríguez–Durán y Kunz, 2001; Presley y Willig, 2008) y su distribución en las islas está bien delimitada (Fleming y Racey, 2010). Cuba posee la fauna de murciélagos más rica de la región, ya que posee más del 45 % de las especies, siete de ellas endémicas. El conocimiento de los murciélagos de Cuba es relativamente antiguo, pero su auge llegó con la influyente obra de Silva (1979). Puede decirse que, en la actualidad, se conoce en profundidad la taxonomía tanto de las especies vivientes como de las fósiles. Numerosos trabajos se han centrado en aspectos ecológicos básicos como el uso del hábitat, la conducta, la dieta, las relaciones con las plantas, la ecomorfología y la ecofisiología (Mancina y Sánchez, 2001; Casotti et al., 2006; Mancina et al., 2004, 2012). Su estado de conservación también ha sido estudiado (Mancina et al., 2007; Borroto–Páez y Mancina, 2017).
Las numerosas listas de especies y los materiales conservados en colecciones hacen que la distribución de las especies de murciélagos en Cuba sea relativamente bien conocida (Sánchez–Lozada et al., 2018). Sin embargo, los estudios sobre la estructura de los ensambles son notablemente escasos y no se han analizado los factores que pueden determinar la distribución y abundancia de sus poblaciones. Mancina (2011) advirtió de que en Cuba existen localidades donde se han registrado hasta más de 17 especies conviviendo. Específicamente, en ciertos refugios diurnos cavernícolas pueden coexistir hasta 13 especies, aunque se desconocen los mecanismos
246
Ferrer–Sánchez and Denis Ávila
Riqueza de especies
Species
Número total de refugios 108 Riqueza promedio: 5,2 especies/refugio 0 10 20 30 40 50 60
Artibeus jamaicensis Brachyphylla nana Chilonatalus macer Eptesicus fuscus Erophylla sezekorni Macrotus waterhousei Monophyllus redmani Mormoops blainvillei Nyctiellus lepidus Phyllonycteris poeyi Pteronotus macleayii Pteronotus parnelli Pteronotus quadridens Tadarida brasiliensis
Frecuencia de aparición
Fig. 1. Distribución de la riqueza de especies observada en la muestra de los ensambles de murciélagos en refugios diurnos empleada para evaluar los patrones de coexistencia entre las especies de murciélagos de Cuba: A, distribución geográfica de las cuevas estudiadas; B, frecuencia de aparición de cada especie en la muestra.
Fig. 1. Distribution of species richness observed in the sample of bat assemblages in diurnal roosts used to evaluate coexistence patterns among Cuban bat species: A, geographical distribution of caves studied; B, frequency of appearance of each species in the sample.
exactos que permiten esta coexistencia sin exclusión competitiva. Se cree que la distribución por gremios, las diferencias en los patrones de actividad nocturna (Mancina y Castro–Arellano, 2013), los patrones de vuelo y una amplia diversidad en la morfología craneal y alar son el reflejo de mecanismos de segregación con valor adaptativo en este sentido.
A pesar de lo anterior, los patrones de coexistencia entre estas especies aún no se han descrito directamente. Estos ordenamientos se han empleado para tratar de entender los mecanismos que pueden determinar la distribución de las especies y la estructura de muchas comunidades. Su análisis también puede ayudar a identificar las condiciones abióticas y bióticas que afectan a la fauna local (Peres–Neto, 2004). Si bien la morfología de las especies cubanas también ha sido extensamente descrita, no existen estudios previos que relacionen estas características con los patrones de distribución ni de coexistencia de los murciélagos.
El objetivo del presente trabajo fue validar la hipótesis de que los rasgos funcionales ecomorfológicos influyen en los patrones de coexistencia de los ensambles cavernícolas de murciélagos de Cuba, a través de la asociación de los índices de coexistencia con sus diferencias en términos de aspectos morfométricos y alimentarios. Este análisis permite establecer cuáles podrían ser los factores que determinan la estructuración por especies de estos ensambles.
Material y métodos
Se emplearon las listas de especies recogidas en fuentes bibliográficas, colecciones científicas y varias expediciones de campo, que abarcaron las 108 localidades en las que se habían observado más de tres especies de murciélagos cohabitando. En la mayor parte de los refugios se encontraron entre tres y cua-
Animal Biodiversity and Conservation 45.2 (2022) 247
3
4 5 6 7 8 9 10 11 12 13
40 35 30 25 20 15 10 5 0
Número de refugios A B
tro especies, pero en el 48 % de ellos se observaron más de cinco especies (fig. 1). El análisis se limitó a las especies que se habían registrado más de 10 veces en las cuevas estudiadas. Las 14 especies que cumplieron estos criterios y que formaron parte del análisis fueron: Artibeus jamaicensis, Brachyphylla nana, Chilonatalus macer, Eptesicus fuscus, Erophylla sezekorni, Macrotus waterhousei, Monophyllus redmani, Mormoops blainvillei, Nyctiellus lepidus, Phyllonycteris poeyi, Pteronotus macleayii, Pteronotus parnelli, Pteronotus quadridens y Tadarida brasiliensis
Los patrones de coexistencia se describieron en función del promedio de las unidades de tipo tablero de ajedrez que aparecen entre dos especies (como medida individual) de Stone y Roberts (1990). Una unidad de tipo tablero de ajedrez (UT) es cualquier submatriz de 2 x 2 celdas con la forma 1–0/0–1 o 0–1/1–0, en una matriz de presencias–ausencias por sitios o muestras. El número de unidades de tipo tablero de ajedrez para cada pareja de especies se calcula como: UT = (ri – S) (rj – S), donde S es el número de sitios compartidos por ambas especies y ri y rj son los totales por fila de las especies i y j. El cálculo de este índice se hizo en el programa EcoSim v7.72, de Gotelli y Entsminger (2001). Como este índice es una medida del grado de exclusión mutua, para facilitar un análisis más directo, se calculó su recíproco de forma que el valor resultante quedara entre 0 y 1; los valores más elevados indican las especies que más tienden a coexistir en los mismos refugios. La red de relaciones se representó en forma de gráfico de redes en el programa Gephi 0.9.2, con una distribución de Fruchterman Reingold; el grosor y tono de gris de las aristas son proporcionales al grado de asociación. La similitud entre los patrones de coexistencia de las especies se representó en un escalado multidimensional no métrico con la distancia euclidiana.
A partir de las publicaciones científicas se tomaron, además, los valores medios de cinco variables morfológicas (peso corporal [g], carga alar [g/cm2], expansión alar [mm], longitud del antebrazo [mm] y longitud del cráneo [longitud occipitopremaxilar, en mm]) y la composición cualitativa básica de la dieta (insectos, polen o néctar, frutas o pescado) (tabla 1). Con estas variables se confeccionaron matrices de distancias (euclidiana para las variables continuas y de Bray–Curtis para la composición de la dieta). Se comparó el agrupamiento obtenido mediante el método de Ward, empleando la matriz de índices de coexistencia, con el obtenido a partir de las variables morfológicas.
Para un enfoque unidimensional, se hicieron regresiones no paramétricas de Spearman entre los índices de coexistencia y las razones entre el peso de las especies, así como las diferencias alimentarias, dadas por el inverso de la similitud de Bray–Curtis.
Las matrices de coexistencia también se correlacionaron con las matrices de distancias morfológicas, de forma general, entre las especies (todas las variables simultáneamente y el índice de distancia euclidiana), así como con la matriz de similitud alimentaria (índice de Bray Curtis), con el objetivo de inferir el grado de asociación entre ambos procesos (coexistencia y
similitudes ecomorfológicas). Para ello se utilizaron pruebas de Mantel con 10.000 aleatorizaciones.
Por último, la capacidad predictiva de las variables morfológicas y de composición de la dieta respecto del grado de coexistencia entre las especies se evaluó por medio de una red neuronal de regresión obtenida con un procedimiento de búsqueda automatizada. Como el objetivo fue analizar un conjunto cerrado de especies, sin propósito predictivo, y como cada unidad muestral fue una pareja de especies diferentes, se utilizaron todos los datos en el entrenamiento de la red. Los indicadores de la capacidad predictiva fueron el coeficiente global de regresión y el valor medio de los errores cuadráticos. Los análisis fueron realizados en el programa PopTools v3.0, add–ins del MS Excel, y el programa Statistica v 8.0 (StatSoft, Tulsa, OK). Los datos utilizados, los análisis en extenso y la red neuronal entrenada se pueden consultar en la información suplementaria contenida en el repositorio Figshare (https://doi.org/10.6084/ m9.figshare.20161931.v1).
Resultados
Al ordenar las especies en un espacio euclidiano según su distancia al número de parejas tablero que promedian con las demás y representar en un dendrograma la estructura de estas relaciones de coexistencia, se pudieron identificar tres grupos de especies (fig. 2 y 3). Estos grupos no indican afinidad entre las especies, sino que identifican los tres grupos de especies con patrones de exclusión más marcados. El grupo mayor en el espacio de ordenamiento estuvo formado por seis especies: Monophyllus, Nyctiellus, Mormops y las tres especies de Pteronotus. El segundo grupo estuvo formado por Erophylla, Chilonatatus, Phyllonycteris y Brachyphylla y el tercero por Artibeus, Tadarida, Eptesicus y Macrotus
No solo la cantidad media de parejas tablero (checkerboard combination) de cada especie describe la estructura interna de los ensambles, sino que es relevante entre quienes tienden a aparecer. Por ello, el análisis de agrupamiento mostró una organización ligeramente diferente de las especies de murciélagos (fig. 3), también dividida en tres grupos, pero cuya composición solo es similar en el 63 % de las especies a la del ordenamiento anterior.
La estructura de las relaciones de coexistencia no tiene relación aparente con la que se obtiene a partir de las similitudes morfológicas, en la cual se pueden identificar dos grandes grupos que no coinciden con los descritos por las relaciones de coexistencia. La prueba de Mantel entre las matrices de coexistencia y la matriz de distancias morfológicas, teniendo en cuenta todas las variables, dio como resultado una correlación observada de solo 0,169 (p = 0,08), por lo que no se encontró dependencia entre ambos grupos de variables.
La cantidad de parejas tablero que tuvo cada especie con el resto, como promedio, fue máxima en el caso de Eptesicus y Tadarida y mínima en el de Chilonatalus y Natalus (fig. 4). Entre estas especies,
248
Ferrer–Sánchez and Denis Ávila
Tabla 1. Valores medios de las variables biológicas empleadas en el análisis de la relación de los patrones de coexistencia de los murciélagos cavernícolas de Cuba en los refugios diurnos y las características de las especies (Silva, 1979): Mc, masa corporal (gr); Ca, carga alar (g/cm2); Ea, expansión alar (mm); La; longitud antebrazo (mm); Lc, Longitud del cráneo (mm); Cd, composición de la dieta (P/n, polen/ néctar; F, frutas; I, insectos; S, semillas).
Table 1. Mean values of biological variables used in the analysis of the relationship between the coexistence of Cuban cave bats in diurnal roosts and characteristics of the various species (Silva, 1979): Mc, body mass (gr); Ca, wing loading (g/cm2); Ea, wing expansion (mm); La, forearm length (mm); Lc, Length of the skull (mm); Cd, diet composition (P/n, pollen/nectar; F, fruits; I, insects; S, seeds).
Cd
ID Especie Mc Ca Ea La Lc P/n F I S
1
Artibeus jamaicensis 37,3 0,148 392,5 56,5 26,9 0,5 0,5 0 0
2 Brachyphylla nana 34,1 0,139 416,5 59 28,3 0,33 0 0,33 0,33
3 Chilonatalus macer 3,0 0,03 232,5 32,5 14,6 0 0 1 0
4 Eptesicus fuscus 15,0 0,077 326,5 46 18,05 0 0 1 0
5 Erophylla sezekorni 17,0 0,6055 321,5 47 24,6 0,33 0 0,33 0,33
6 Macrotus waterhousei 15,0 0,078 349,00 51,50 23,9 0 0 1 0
7 Monophyllus redmani 11,0 0,098 288,50 39,50 21,8 0,5 0 0,5 0
8 Mormoops blainvillei 8,5 0,052 317,50 46,50 13,1 0 0 1 0
9 Nyctiellus lepidus 2,5 0,038 199,50 29,50 12,6 0 0 1 0
10 Phyllonycteris poeyi 20,8 0,13 322,00 47,00 25,2 0,5 0 0,5 0
11 Pteronotus macleayii 5,4 0,05 282,50 42,30 16 0 0 1 0
12 Pteronotus parnelli 11,2 0,061 347,50 52,50 20,3 0 0 1 0
13 Pteronotus quadridens 4,7 0,055 252,50 37,30 14,3 0 0 1 0
14 Tadarida brasiliensis 8,5 0,095 283,00 39,50 16,15 0 0 1 0
el índice tuvo valores decrecientes con un comportamiento similar al de su frecuencia de aparición en la matriz de datos primarios, lo cual pone de manifiesto un posible efecto confundido en el análisis de los índices de coexistencia. Se excluye de la tendencia a Artibeus que, a pesar de ser la especie más frecuente en la muestra, mostró valores intermedios de parejas tablero.
Una representación, tal vez más apropiada, de la complejidad de las relaciones de coexistencia entre estas especies es la red de interacciones, que se representa en la figura 5 y en la cual el grosor y la intensidad del tono de las líneas representan la fortaleza de las asociaciones de coexistencia. Algunas especies como P. poeyi, Chilonatalus macer, Lasiurus insularis, Mormopterus minutus, Nycticeius cubanus y las del género Pteronotus destacaron por poseer pocas y muy débiles relaciones de exclusión aparente (fig. 5).
Para analizar los posibles factores determinantes de esta red de asociaciones percibidas por presencias–ausencias conjuntas, se relacionó este patrón con las diferencias en otras variables biológicas de las especies, como es el caso del peso (fig. 6). La correlación por rangos de Spearman entre los índices de coexistencia y las razones entre pesos dio una
asociación significativa, aunque débil en intensidad (r s = 0,37; p < 0,05, al igual que con las diferencias en la alimentación (inverso de la similitud de Bray–Curtis, r s = –0,234; p < 0,05).
En la prueba de correlación de matrices de distancia, representada en la figura 6, se observó que cuando los pesos son similares, las intensidades de coexistencia pueden variar en todo el rango de valores, pero a medida que las razones entre pesos aumentan, los valores del índice C tienden a ser más elevados. No obstante, esta asociación con el peso en un modelo nulo no fue significativa (r m = 0,163, p = 0,07).
Ninguna de las variaciones en las variables predictivas estudiadas de forma individual pudo predecir el patrón de coexistencia en un nivel que no pudiera ser razonablemente atribuido al azar. Pero como en los fenómenos biológicos de alta variabilidad y relaciones débiles, la potencia de las pruebas estadísticas que se emplean en la detección de relaciones tiene una importancia fundamental, se empleó una red neuronal para evaluar la capacidad conjunta de las variables analizadas de estimar el nivel de coexistencia entre las especies. La red más eficiente estuvo formada por cuatro neuronas de entrada, correspondientes a las variables independientes, diez neuronas en la capa oculta y una neurona de salida, en ambos casos con
Animal Biodiversity
249
and Conservation 45.2 (2022)
Dimensión 2
1,0 0,8 0,6 0,4 0,2 0 –0,2 –0,4 –0,6 –0,8 –1,0 –1,2
2 10 3 12 1 7 11
Ferrer–Sánchez and Denis Ávila
Stress: 0,15
14 9 5
13 8
4
6
–2,0 –1,5 –1,0 –0,5 0 0,5 1,0 1,5 2,0 Dimensión 1
Fig. 2. Mapa de ordenamiento del escalado multidimensional no métrico a partir de las relaciones de coexistencia de 14 especies de murciélagos cavernícolas de Cuba en sus refugios diurnos. La distancia entre las especies (distancia euclidiana) corresponde a las similitudes en relación con la cantidad de unidades tableros entre ellas: 1, Artibeus jamaicensis; 2, Brachyphylla nana; 3, Chilonatalus macer; 4, Eptesicus fuscus; 5, Erophylla sezekorni; 6, Macrotus waterhousei; 7, Monophyllus redmani; 8, Mormoops blainvillei; 9, Nyctiellus lepidus; 10, Phyllonycteris poeyi; 11, Pteronotus macleayii; 12, Pteronotus parnelli; 13, Pteronotus quadridens; 14, Tadarida brasiliensis
Fig. 2. Non–metric multidimensional scaling ordering map from the coexistence relationships in 14 species of Cuban cave bats in their diurnal roosts. Distance between species (Euclidean distance) corresponds to the similarities in relation to the number of board units between them. (For the species' abbreviations, see above and table 1.)
A B
A. jamaicensis M. waterhousei E. fuscus T. brasiliensis B. nana C. macer P. poeyi E. sezekorni P. macleayii P. quadridens P. parnelli M. redmani M. blainvillei N. lepidus
A. jamaicensis B. nana E. fuscus E. sezekorni P. poeyi M. blainvillei M. waterhousei P. parnelli C. macer P. quadridens N. lepidus M. redmani P. macleayii T. brasiliensis
2.000 500 0 0 50 100 150 200 350 400
Distancia de agrupamiento Distancia de agrupamiento (método de Ward) (método de Ward)
Fig. 3. Relaciones de similitud entre los patrones de coexistencia en refugios diurnos (A) y la similitud morfométrica (B) de 14 especies de murciélagos cavernícolas de Cuba, obtenidas a partir de las cantidades de unidades de tipo tablero de ajedrez en la matriz de presencias–ausencias. La barra negra indica los grupos formados en el escalado multidimensional no métrico con la coexistencia y según las similitudes morfológicas.
Fig. 3. Similarity relationships between the coexistence patterns in diurnal roosts (A) and morphometric similarity (B) in 14 species of Cuban cave bats, based on the amount of board units in the presence–absence matrix. The black bars indicate the groups formed in the non–metric multidimensional scaling with coexistence and according to morphological similarities.
250
Puntaje C
900 800 700 600 500 400 300 200 100
Media ±ES LC 95 %
13 8 11 7 12 9 4 14 1 6 10 2 5 3 Especie
Fig. 4. Distribución de los puntajes C que aparecen en cada una de las 14 especies de murciélagos cavernícolas de Cuba en 106 refugios diurnos. (Para las abreviaturas de las especies, veáse fig. 2 y tabla 1).
Fig. 4. Distribution of the C scores that appear in each of the 14 species of Cuban cave bats in 106 diurnal roosts. (For the species' abbreviations, see fig. 2 and table 1).
una función de activación tangente. El desempeño de la red fue aceptable, ya que la correlación fue de 0,87 (fig. 7) y el error de entrenamiento inferior a 0,006. El error medio de predicción fue un 13,5 % (± 1,1) del valor del índice de coexistencia, lo cual puede considerarse muy bueno, teniendo en cuenta la débil asociación que mostraban de manera individual las variables predictoras. Eptesicus fuscus, Noctilio leporinus y Tadarida brasiliensis fueron las especies con mayores residuales y además tendieron a la sobreestimación de
la coexistencia. De manera inesperada, la red mostró mayor sensibilidad a las diferencias de la carga alar (51 %), seguidas por las razones entre pesos (30 %) y las similitudes en la alimentación (11 %).
Discusión
La estructuración por especies de los ensambles puede depender de múltiples factores, entre los que
Fig. 5. Representación gráfica de la red de relaciones de coexistencia entre las 14 especies de murciélagos cavernícolas de Cuba: A, relaciones totales; B, relaciones más frecuentes (índice > 0,148); C, relaciones menos frecuentes (índice < 0,066). (Para las abreviaturas de las especies, véase fig. 2 y tabla 1).
Fig. 5. Graphic representation of the network of coexistence relationships between the 14 species of Cuban cave bats. A, total relationships; B, most frequent relationships (index > 0.148); and C, less frequent relationships (Index < 0.066). (For the species' abbreviations, see fig. 2 and table 1).
Animal Biodiversity and Conservation 45.2 (2022) 251
6 14 14 14 13 13 13 12 12 12 11 11 11 10 10 10 9 9 9 8 8 8 7 7 7 6 6 5 5 5 4 4 4 3 3 3 2 2 2 1 1 1 A B C
1,00 0,90 0,80 0,70 0,60 0,50 0,40 0,30 0,20 0,10 0 Índice de coexistencias 0 1 2 3 4 5 6 7 8 9
Razón de pesos
Fig. 6. Relación entre el índice de coexistencia entre parejas de especies de murciélago y sus razones de peso corporal, independientemente del arreglo en gremios.
Fig. 6. Relationship between the coexistence index between pairs of bat species and their body weight ratios, regardless of the guild arrangement.
se encuentran las localidades físicas, las relaciones filogenéticas entre especies y algunos aspectos históricos y geográficos. En las últimas décadas, se ha descrito un marco conceptual multifacético sobre la biodiversidad que engloba múltiples formas de variación biológica, incluida la diversidad taxonómica, genética y fenotípica dentro de las comunidades (Purvis y Hector, 2000). Existe un marco común que vincula las teorías de coexistencia de especies con los patrones de diversidad funcional (Whittaker et al., 2001; Hillebrand, 2004; Bellwood et al., 2005). En el caso del análisis de la asociación entre los índices de coexistencia de las especies de murciélagos cavernícolas de Cuba y sus diferencias en cuanto a sus aspectos morfológicos y alimentarios, se esperaría que la coocurrencia de especies fuera menor, en caso de haber competencia, de la que se obtendría si las especies colonizaran el hábitat de forma independiente (si no existiera el efecto de la competencia, la coocurrencia de especies sería mayor, ya que las especies no se "evitarían").
La segregación en grupos de especies según sus patrones de coexistencia permite inferir la estructura interna de las relaciones interespecíficas en los ensambles de murciélagos. De esta forma, se pueden identificar los grupos de especies con un nivel de competencia o segregación más marcado. La clasificación de las especies basada en sus patrones de coexistencia también se ha empleado para desagregar la diversidad en grupos de especies que mejor reflejen sus similitudes y diferencias ecológicas, lo que da lugar a un marco para la identificación de especies que actúan como bioindicadores eficientes de riqueza (Azeria et al., 2009).
Los valores obtenidos en este trabajo concuerdan con el resultado del metaanálisis realizado por Gotelli
y McCabe (2002), que con la publicación de 96 matrices de presencia–ausencia de diferentes taxones y un modelo nulo demostraron la generalidad de los patrones predichos por las reglas de Diamond, así como diferencias específicas de los taxones en la organización comunitaria. Según estos autores, en la mayoría de las comunidades naturales hay menos coexistencia de especies de lo esperado por azar, de acuerdo con las predicciones de estas reglas y con la competencia como fuerza principal para determinar la estructura de los ensambles (Connell, 1980; Weiher y Keddy, 1999).
El patrón de coexistencia entre las especies de murciélagos analizadas debe reflejar la diversidad de factores que determinan su distribución. La teoría predice que si la competencia interespecífica entre especies semejantes que consumen recursos similares limitantes es intensa, se produce un desplazamiento de caracteres que, estadísticamente, se manifiesta en distancias ecomorfológicas entre especies mayores y más homogéneas de lo esperado por azar (patrones morfológicos hiperdispersos). La existencia de correlaciones significativas entre las tasas de exclusión y las diferencias de peso entre las especies de murciélagos estudiadas se ajustan a esto.
La morfología se ha usado como una aproximación de los patrones ecológicos subyacentes en muchas especies de murciélagos (McNab, 1971; Findley y Black, 1983; Willig, 1986; Willig y Moulton, 1989), a pesar de que este planteamiento se basa en numerosos supuestos simplificadores. Las características morfológicas de las especies permiten inferir indirectamente atributos ecológicos, ya que se supone que estos han evolucionado a consecuencia de la selección natural y, por tanto, las relaciones del nicho de las especies en la comunidad se reflejan en sus adaptaciones morfo-
252
Ferrer–Sánchez and Denis Ávila
A B
1,1 1,0 0,9 0,8 0,7 0,6 0,5 0,4 0,3 0,2 0,1 0 Índice de coexistencia (estimado) 4 9 14 1 8 5 6 12 13 10 7 2 11 3 Species
0,0 0,1 0,2 0,3 0,4 0,5 0,6 0,7 0,8 0,9 1,0 1,1 Índice de coexistencia (observado)
Residual de la predicción 0,08 0,06 0,04 0,02 0 –0,02 –0,04 –0,06
Fig. 7. A, predicción del índice de coexistencia en ensambles a partir de las distancias morfológicas, las razones de peso y las similitudes tróficas de 14 especies de murciélagos cavernícolas de Cuba por medio de una red neuronal de regresión; B, comportamiento de los residuales medios por especie. (Para las abreviaturas de las especies, véase fig. 2 y tabla 1).
Fig. 7. A, coexistence index prediction in assemblages from the morphological distances, weight ratios and trophic similarities of 14 Cuban cave bat species according to a regression neural network; B, behavior of the average residuals for each species. (For the species' abbreviations, see fig. 2 and table 1).
lógicas (Ricklefs, 1979). Las interacciones bióticas son causantes de la limitación en la riqueza de especies a escala local a través de la exclusión competitiva (Hardin, 1960) y los principios de similitud limitante (MacArthur y Levins, 1967; Statzner et al., 2004; Stubbs y Wilson, 2004). Por esta razón, también pueden existir efectos de prioridad, dados por el orden de llegada de las especies a la comunidad local (Fukami et al., 2005) o un filtrado ambiental, según el cual la presencia y abundancia de una especie en una comunidad también están en función de las propiedades de su nicho (MacArthur y Levins, 1967; van der Valk, 1981; Peres–Neto, 2004).
La similitud entre los patrones de ordenamiento basados en la coexistencia y en distancias morfo-
lógicas sugiere una relación funcional entre ambos fenómenos. A pesar de que los análisis estadísticos unidimensionales son insuficientes para predecir de forma sistemática el nivel de coexistencia, la red neuronal entrenada sí logra un nivel de asociación relativamente alto, normalmente superior al 86 % en el índice de coexistencia, pero con un comportamiento diferente entre las especies. Las variables morfológicas más relacionadas con la coexistencia fueron la carga alar y el peso, ambas relacionadas con el uso de los recursos tróficos.
La incertidumbre que se refleja en los resultados puede deberse a la posibilidad de que algunos factores ambientales como la heterogeneidad espacial, la
Animal Biodiversity and Conservation 45.2 (2022) 253
variabilidad climática o ciertos aspectos específicos de los taxones, como la movilidad, impidan ver con claridad los mecanismos asociados a procesos determinísticos e incluso que algún mecanismo particular opere con igual eficacia en todos los sitios y determinen la diferenciación morfológica de los murciélagos (Findley y Black, 1983; Patterson et al., 2003).
De cualquier forma, la interpretación de la coexistencia puede ser incorrecta debido a que la presencia conjunta de dos especies no es indicador suficiente de ausencia de competencia. La presencia de patrones no aleatorios de la coexistencia en ensambles naturales también puede ser originada por procesos azarosos (Ulrich, 2004). La evidencia circunstancial de un patrón observado puede ser insuficiente para inferir un proceso subyacente (Marjakangas et al., 2021), puesto que los procesos estocásticos pueden dar lugar a sistemas altamente organizados como ha quedado patente en el estudio del proceso de autoorganización. Por ello, la interpretación de estos patrones es compleja y requiere el empleo de toda la información disponible sobre la ecología de las especies. Los patrones observados en la naturaleza deben analizarse en su relación no determinante con los procesos que los originan, ya que pueden resultar de diferentes hipótesis alternativas.
Independientemente del papel de las similitudes ecomorfológicas de las especies, cabe esperar que otros factores como las características ecológicas del paisaje que rodea los refugios también influyan en la composición de los ensambles de murciélagos cavernícolas. La productividad, el grado de conservación y la propia estructura de la vegetación podrían influir en la presencia de determinadas especies (Alurralde y Díaz, 2021; Ocampo–Ariza et al. , 2022). No debe pasarse por alto la posible influencia de la competencia con los murciélagos no cavernícolas, que comprenden otras 12 especies, en las zonas de alimentación. También deben considerarse las características físicas de los propios refugios, ya que se ha demostrado la existencia de patrones específicos de selección de refugios por sus características estructurales, así como la utilización de diferentes estratos de las cuevas por las distintas especies.
Con posterioridad a las reglas de Diamond, se propusieron otras hipótesis para explicar la estructura de las comunidades, por ejemplo, los patrones de anidación entre especies (Patterson y Atmar, 1986), la proporcionalidad entre gremios (Wilson, 1989), la ley de los estados favorecidos (Fox y Brown, 1993), la regla de la razón de peso constante (Dayan y Simberloff, 1994) y las asociaciones entre ambiente y tendencias (Keddy y Weiher, 1999). Más recientemente, el discurso científico se ha centrado en parámetros multifacéticos para cuantificar las estructuras de ensamblaje, como la diversidad funcional y la diversidad filogenética, como complemento de la diversidad taxonómica (Gómez et al., 2010; Mouchet et al., 2010; Tucker et al., 2017). La dispersión de los murciélagos en las Antillas Mayores se combina con estructuras jerárquicas en los hábitats para producir, además, patrones anidados.
Conclusiones
El análisis de los índices de coexistencia entre 14 especies de murciélagos cavernícolas de Cuba mostró una estructura interna no aleatoria que de forma general se relaciona con la morfología y la dieta de las especies. Si bien ninguna variable morfológica muestra asociaciones suficientemente fuertes para hacer inferencias, el análisis conjunto sí mostró una asociación suficiente para poder predecir los índices de coexistencia con tasas de error relativamente bajas. La razón de peso, la carga alar y las diferencias de la dieta fueron las variables ecomorfológicas más influyentes en esas predicciones. Estos resultados confirman el papel de la competencia interespecífica en la determinación de la composición de los ensambles en los refugios cavernarios, pero dejan un grado suficiente de incertidumbre como para inferir la existencia de otros factores alineados, posiblemente relacionados con la disponibilidad local de recursos y las características físicas de los propios refugios.
Referencias
Alurralde, S. G., Díaz, M. M., 2021. Assemblage–level responses of Neotropical bats to forest loss and fragmentation. Basic and Applied Ecology, 50: 57–66. Doi: 10.1016/j.baae.2020.09.001
Azeria, E. T., Fortin, D., Hebert, C., Peres–Neto, P., Pothier, D., Ruel, J. C., 2009. Using null model analysis of species co–occurrences to deconstruct biodiversity patterns and select indicator species. Diversity and Distributions, 15: 958–971, Doi: 10.1111/j.1472-4642.2009.00613.x
Baker, R. J., Genoways, H. H., 1978. Zoogeography of Antillean bats. En: Zoogeography of the Caribbean: 53–98 (F. B. Gill, Ed.). Special Publications, Academy of Natural Sciences, Philadelphia.
Bellwood, D. R., Hughes, T. P., Connolly, S. R., Tanner, J., 2005. Environmental and geometric constraints on Indo–Pacific coral reef biodiversity. Ecology Letters, 8: 643–651, Doi: 10.1111/j.14610248.2005.00763.x
Borroto–Páez, R., Mancina, C. A., 2017. Biodiversity and conservation of Cuban mammals: past, present, and invasive species. Journal of Mammalogy, 98: 964–985, Doi: 10.1093/jmammal/gyx017
Brown, J. H., Tapa, M. L., Marquet, P. A., 1996. Darwinian fitness and reproductive power: reply to Kozlowski. The American Naturalist, 147: 1092–1097, Doi: 10.1086/285895
Calatayud, J., Andivia, E., Escudero, A., Melián, C. J., Bernardo–Madrid, R., Stoffel, M., Aponte, C., Medina, N. G., Molina–Venegas, R., Arnan, X., Rosvall, M., Neuman, M., Noriega, J. A., Alves–Martins, F., Draper, I., Luzuriaga, A., Ballesteros–Cánovas, J. A., Morales–Molino, C., Ferrandis, P., Herrero, A., Pataro, L., Juen, L., Cea, A., Madrigal–González, J., 2020. Positive associations among rare species and their persistence in ecological assemblages. Nature Ecology and Evolution, 4: 40–45, Doi: 10.6084/m9.figshare.9906092
254
Ferrer–Sánchez and Denis Ávila
Casotti, L. G., Herrera, M., Flores, J. J. Mancina, C. A., Braun, E., 2006. Relationships between renal morphology and diet in 26 species of new world bats (suborder microchiroptera). Zoology, 109: 196–207, Doi: 10.1016/j.zool.2006.03.003
Connell, J. H., 1980. Diversity and the coevolution of competitors, or the ghost of competition past. Oikos, 35: 131–138, Doi: 10.2307/3544421
Connor, E. F., Simberloff, D., 1979. The assembly of species communities: chance or competition? Ecology, 60: 1132–1140, Doi: 10.2307/1936961
Dayan, T., Simberloff, D., 1994. Morphological relationships among coexisting heteromyids: an incisive dental character. The American Naturalist, 143: 462–477, Doi: 10.1086/285613
Diamond, J., 1975. Assembly of species communities. En: Ecology and evolution of communities: 342–444 (M. L. Cody, J. M. Diamond, Eds.). Harvard University Press, Cambridge, Massachusetts.
Findley, J. S., Black, H. L., 1983. Morphological and dietary structuring of a Zambian insectivorous bat community. Ecology, 64: 625–630, Doi: 10.2307/1937180
Fleming, T. H., Hooper, E. T., Wilson, D. E., 1972. Three Central American bat communities: structure, reproductive cycles, and movement patterns. Ecology, 53: 555–569, Doi: 10.2307/1934771
Fleming, T. H., Racey, P. A. (Eds.), 2010. Island bats: evolution, ecology, and conservation. University of Chicago Press, Chicago.
Fox, B. J., Brown, J. H., 1993. Assembly rules for functional groups in North American rodent communities. Oikos, 67: 358–370, Doi: 10.2307/3545483
Fukami, T.; Bezemer, T. M., Mortimer, S. R., Van Der Putten, W. H., 2005. Species divergence and trait convergence in experimental plant community assembly. Ecol. Lett., 8: 1283–1290, Doi: 10.1111/j.1461-0248.2005.00829.x
Gómez, J. P., Bravo, G. A., Brumfield, R. T., Tello, J. G., Cadena, C. D., 2010. A phylogenetic approach to disentangling the role of competition and habitat filtering in community assembly of Neotropical forest birds. Journal of Animal Ecology, 79: 1181–1192, Doi: 10.1111/j.1365-2656.2010.01725.x
Gotelli, N. J., Entsminger, G. L., 2001. EcoSim: Null models software for ecology. Version 7.0. Acquired Intelligence Inc. & Kesey–Bear. Available online at: https://www.uvm.edu/~ngotelli/EcoSim/EcoSim.html
Gotelli, N. J., McCabe, D. J., 2002. Species co–occurrence: a meta–analysis of J. M. Diamond’s assembly rules model. Ecology, 83: 2091–2096, Doi: 10.1890/0012-9658(2002)083[2091:SCOAMA]2.0. CO;2
Guillemot, N., Kulbicki, M., Chabanet, P., Vigliola, L., 2011. Functional redundancy patterns reveal non–random assembly rules in a species–rich marine assemblage. Plos One, 6(10): e26735, Doi: 10.1371/journal.pone.0026735
Hardin, G., 1960. Competitive exclusion principle. Science, 131(3409): 1292–1297, Doi: 10.1126/ science.131.3409.1292
Hillebrand, H., 2004. Strength, slope and variability of marine latitudinal gradients. Marine Ecology
Progress Series, 273: 251–267, Doi: 10.3354/ meps273251
Hutchinson, G. E., 1959. Homage to Santa Rosalia, or why are there so many kinds of animals? The American Naturalist, 93: 145–159, Doi: 10.1086/282070 Keddy, P., Weiher, E., 1999. The scope and goals of research on assembly rules. En: Ecological assembly rules: perspectives, advances, retreats: 1–20 (E. Weiher, P. Keddy, Eds.). Cambridge University Press, Cambridge.
MacArthur, R. H., Levins, R., 1967. The limiting similarity, convergence and divergence of coexisting species. The American Naturalist, 101: 377–385, Doi: 10.1086/282505
Mancina, C. A., 2011. Introducción a los murciélagos. En: Mamíferos de Cuba: 122–134 (R. Borroto–Páez, C. A. Mancina, Eds.). UPC Print, Vaasa, Finlandia.
Mancina, C. A., Borroto, R., García, L., 2004. Tamaño relativo del cerebro en murciélagos cubanos. Orsis, 19: 7–19.
Mancina, C. A., Castro–Arellano, I., 2013. Unusual temporal niche overlap in a phytophagous bat ensemble of western Cuba. Journal of Tropical Ecology, 29: 511–521, Doi: 10.1017/ S0266467413000680
Mancina C. A., Echenique–Díaz, L. M., Tejedor, A., García, L., Daniel–Álvarez, A., Ortega–Huerta, M. A., 2007. Endemics under threat: an assessment of the conservation status of cuban bats. Hystrix the Italian Journal of Mammalogy, 18(1): 3–15, Doi: 10.4404/hystrix-18.1-4388
Mancina, C. A., García–Rivera, L., Miller, B. W., 2012. Wing morphology, echolocation, and resource partitioning in syntopic Cuban mormoopid bats. Journal of Mammalogy, 93: 1308–1317, Doi: 10.1644/11-MAMM-A-331.1
Mancina, C. A., Sánchez, J. A., 2001. Efecto de la actividad trófica de Artibeus jamaicensis (Mammalia: Chiroptera) sobre la dispersión de Andira inermis (Leguminosae). Revista Biología, 15(2): 81–85.
Marjakangas, E. L., Ovaskainen, O., Abrego, N., Grøtan, V., de Oliveira, A. A., Prado, P., de Lima, R. A. F., 2021. Co–occurrences of tropical tres in Eastern South America: disentangling abiotic and biotic forces. Plant Ecology, 222: 791–806, Doi: 10.1007/s11258-021-01143-3
McNab, B. K., 1971. The structure of tropical bat faunas. Ecology, 52: 352–358, Doi: 10.2307/1934596
Mouchet, M. A., Villeger, S., Mason, N. W. H., Mouillot, D., 2010. Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules. Functional Ecology, 24: 867–876, Doi: 10.1111/j.13652435.2010.01695.x
Ocampo–Ariza, C., Maas, B., Castro–Namuche, J. P., Thomas, E., Vansynghel, J., Steffan–Dewenter, I., Tscharntke, T., 2022. Trait–dependent responses of birds and bats to season and dry forest distance in tropical agroforestry. Agriculture, Ecosystems and Environment, 325: 107751, Doi: 10.1016/j. agee.2021.107751
Patterson, B. D., Atmar, W., 1986. Nested subsets
Animal Biodiversity
255
and Conservation 45.2 (2022)
and the structure of insular mammalian faunas and archipelagos. Biological Journal of the Linnean Society, 28: 65–82, Doi: 10.1111/j.1095-8312.1986. tb01749.x
Patterson, B. D., Willig, M. R., Stevens, R. D., 2003. Trophic strategies, niche partitioning, and patterns of ecological organization. En: Bat ecology: 536–579 (T. H. Kunz, M. B. Fenton, Eds). Univ. Chicago Press, Chicago.
Peres–Neto, P. R., 2004. Patterns in the co–occurrence of fish species in streams: the role of site suitability, morphology and phylogeny versus species interactions. Oecologia, 140: 352–360, Doi: 10.1007/s00442-004-1578-3
Pickett, S. T. A., Kolasa, J., Jones, C. G., 1994. Ecological understanding: the nature of theory and the theory of nature. Academic Press, San Diego, California.
Polis, G. A., 1991. Desert communities: an overview of pattern and processes. En: The Ecology of Desert Communities: 1–26 (G. A. Polis, Ed.). University of Arizona Press, Tucson, Arizona.
Presley, S. J., Willig, M. R., 2008. Composition and structure of Caribbean bat (Chiroptera) assemblages: effects of interisland distance, area, elevation and hurricane–induced disturbance. Global Ecology and Biogeography, 17: 747–757, Doi: 10.1111/j.1466-8238.2008.00412.x
Purvis, A., Hector, A., 2000. Getting the measure of biodiversity. Nature , 405: 212–219, Doi: 10.1038/35012221
Ricklefs, R. E., 1979. Ecology. Chiron Press, Concord, Massachusetts.
Rodríguez–Durán, A., Kunz, T. H., 2001. Biogeography of West Indian bats: an ecological perspective. En: Biogeography of the West Indies: patterns and perspectives: 355–368 (C. A. Woods, F. E. Sergile, Eds.). CRC Press, Boca Raton, Florida.
Root, R., 1967. The niche exploitation pattern of the blue–gray gnatcatcher. Ecological Monographs, 37: 317–350, Doi: 10.2307/1942327
Roth, L., 1981. Constancy in the size ratios of sympatric species. The American Naturalist, 118: 394–404, Doi: 10.1086/283831
Salgado–Mejia, F., López–Wilchis, R., Guevara–Chumacero, L. M., Valverde–Padilla, P. L., del Rio, P. C. M., Porto–Ramírez, S. L., Rojas–Martínez, I., Samano–Barbosa, G. A., 2021. Characterization of assemblages in neotropical cave dwelling bats based on their diet, wing morphology, and flight performance. Therya, 12: 435–447, Doi: 10.12933/ therya-21-1075
Sánchez–Lozada, M., Vela Rodríguez, H., Díaz Perdomo, H. M., Monzón, J., de la Cruz Mora, J. M., Hernández Muñoz, A., Longueira Loyola, A., Espinosa, Á., Rodríguez–Cabrera, T. M., Vidal
Bertucciolli, A., Mancina, C. A., 2018. Datos de distribución de murciélagos en Cuba: un acercamiento a través de inventarios biológicos rápidos. Poeyana, 507: 76–81. Siepielski, A. M., McPeek, M. A., 2010. On the evidence for species coexistence: a critique of the coexistence program. Ecology, 91: 3153–3164. Doi: 10.1890/10-0154.1
Silva, G., 1979. Los Murciélagos de Cuba. Editorial Academia, La Habana.
Statzner, B., Doledec, S., Hugueny, B., 2004. Biological trait composition of European stream invertebrate communities: assessing the effects of various trait filter types. Ecography, 27: 470–488. Doi: 10.1111/j.0906-7590.2004.03836.x
Stone, L., Roberts, A., 1990. The checkerboard score and species distributions. Oecologia, 85: 74–79, Doi: 10.1007/BF00317345
Stubbs, W. J., Wilson, J. B., 2004. Evidence for limiting similarity in a sand dune community. Journal of Ecology, 92: 557–567, Doi: 10.1111/j.00220477.2004.00898.x
Tucker, C. M., Cadotte, M. W., Carvalho, S. B., Davies, T. J., Ferrier, S., Fritz, S. A., Grenyer, R., Helmus, M. R., Jin, L. S., Mooers, A. O., Pavoine, S., Purschke, O., Redding, D. W., Rosauer, D. F., Winter, M., Mazel, F., 2017. A guide to phylogenetic metrics for conservation, community ecology and macroecology. Biological Reviews, 92: 698–715, Doi: 10.1111/brv.12252
Ulrich, W., 2004. Species co–occurrences and neutral models: reassessing J. M. Diamond's assembly rules. Oikos, 107: 603–609, Doi: 10.1111/j.00301299.2004.12981.x
van der Valk, A. G., 1981. Succession in wetlands: a Gleasonian approach. Ecology, 62: 688–696, Doi: 10.2307/1937737
Weiher, E., Keddy, P., 1999. Ecological assembly rules: perspectives, advances, retreats. Cambridge University Press, Cambridge.
Whittaker, R. J., Willis, K. J., Field, R., 2001. Scale and species richness: towards a general, hierarchical theory of species diversity. Journal of Biogeography, 28: 453–470, Doi: 10.1046/j.13652699.2001.00563.x
Willig, M. R., 1986. Bat community structure in South America: a tenacious chimera. Revista Chilena de Historia Natural, 59: 151–168.
Willig, M. R., Moulton, M. A., 1989. The role of stochastic and deterministic processes in the structure of Neotropical bat communities. Journal of Mammalogy, 70: 323–329, Doi: 10.2307/1381514
Wilson, J. B., 1989. A null model of guild proportionality, applied to stratification of a New Zealand temperate rain forest. Oecologia, 80: 263–267, Doi: 10.1007/ BF00380161
256
Ferrer–Sánchez and Denis Ávila
Diet of the bonnethead
Sphyrna tiburo) along
of Mexico
Branham, C. C., Frazier, B. S., Strange, J. B., Galloway, A. S., Adams, D. H., Drymon, J. M., Grubbs, R. D., Portnoy, D. S., Wells, R. J. D., Sancho, G., 2022. Diet of the bonnethead (Sphyrna tiburo) along the northern Gulf of Mexico and southeastern Atlantic coast of the United States. Animal Biodiversity and Conservation, 45.2: 257–267, Doi: https://doi.org/10.32800/abc.2022.45.0257

Abstract
Diet of the bonnethead (Sphyrna tiburo) along the northern Gulf of Mexico and southeastern Atlantic coast of the United States. The diet of a potentially omnivorous coastal shark species, the bonnethead (Sphyrna tiburo), was examined in the western Atlantic along the coast of the southeastern United States. A total of 423 stomachs collected from Texas, Alabama, Florida and South Carolina were analyzed using standardized stomach content analysis methods. The diet was dominated by crabs, primarily portunids (Callinectes spp.), across the geographical range analyzed, though the relative importance of crabs varied between regions. Ontogenetic shifts in diet were not observed throughout the region studied. Female and male bonnetheads in South Carolina displayed different diets, particularly in the amount of portunid crabs consumed, with a higher proportion ingested by females. Bonnetheads consumed limited amounts of seagrasses in all regions except in South Carolina, where they occupy habitats without seagrasses in marsh dominated bays and estuaries. This finding indicates that, at least seasonally, seagrasses are not an essential part of the diet of this shark species and may only occur in stomachs as accidental ingestion.
Key words: Elasmobranch diet, Feeding ecology, Sphyrna tiburo, Callinectes spp., Seagrasses
Resumen
La dieta de la cornuda de corona (Sphyrna tiburo) en el norte del golfo de México y la costa atlántica del sureste de los Estados Unidos de América. Se estudió la dieta de un tiburón costero potencialmente omnívoro, la cornuda de corona (Sphyrna tiburo), en el Atlántico occidental a lo largo de la costa suroriental de los Estados Unidos de América. Se procesaron los estómagos de 423 ejemplares capturados en Tejas, Alabama, Florida y Carolina del Sur utilizando métodos estandarizados de análisis de contenido estomacal. En la zona geográfica estudiada, predominaron los cangrejos, principalmente portúnidos (Callinectes spp.), aunque la proporción relativa de los cangrejos varió entre regiones. No se observaron cambios ontogenéticos en la dieta en la región estudiada. Se observaron diferencias en las dietas de las hembras y los machos de cornuda de corona en Carolina del Sur, sobre todo por la cantidad de cangrejos portúnidos consumidos, que fue mayor en las hembras. Las cornudas de corona consumieron cantidades limitadas de praderas submarinas en todas las regiones excepto en Carolina del Sur, donde los tiburones ocupan bahías y estuarios en marismas donde no existen tales praderas. Todo ello indica que, al menos temporalmente, las praderas submarinas no son una parte importante de la dieta de esta especie de tiburón y que su presencia en los estómagos podría ser tan solo el resultado de la ingestión accidental.
Palabras clave: Dieta elasmobranquios, Ecología alimentaria, Sphyrna tiburo, Callinectes spp., Praderas submarinas
ISSN: 1578–665 X eISSN: 2014–928 X
© [2022] Copyright belongs to the authors, who license the journal Animal Biodiversity and Conservation to publish the paper under a Creative Commons Attribution 4.0 License
257
Animal Biodiversity and Conservation 45.2 (2022)
(
the northern Gulf
and southeastern Atlantic coast of the United States C. C. Branham, B. S. Frazier, J. B. Strange, A. S. Galloway, D. H. Adams, J. M. Drymon, R. D. Grubbs, D. S. Portnoy, R. J. D. Wells, G. Sancho
Received: 29 I 22; Conditional acceptance: 20 V 22; Final acceptance: 14 VII 22
Creed C. Branham, James B. Strange, Gorka Sancho, Department of Biology, College of Charleston, Charleston, SC 29424 USA.– Bryan S. Frazier, Ashley S. Galloway, Marine Resources Resource Institute, South Carolina Department of Natural Resources, SC 29412 USA.– R. Dean Grubbs, Coastal and Marine Laboratory, Florida State University, 3618 Highway 98, Sta. Teresa, FL 32358 USA.– David S. Portnoy, Marine Genomics Laboratory, Texas A&M University–Corpus Christi, 6300 Ocean Dr Unit 5892, Corpus Christi, TX 78412 USA.–J. Marcus Drymon, Mississippi State University, Coastal Research and Extension Center, Biloxi, MS 39532 USA.– Douglas H. Adams, Florida Fish and Wildlife Conservation Commission, Fish and Wildlife Research Institute, 1220 Prospect Avenue, Melbourne, FL 32901 USA.– R. J. David Wells, Department of Marine Biology, Texas A&M University at Galveston, 1001 Texas Clipper Rd., Galveston, TX 77553 USA.
Corresponding author: G. Sancho. E–mail: sanchog@cofc.edu
ORCID ID: C. C. Branham: 0000-0003-1804-5955; B. S. Frazier: 0000-0002-7718-9492; A. S. Galloway: 0000-0001-9343-0365; D. H. Adams: 0000-0002-3539-6629; J. M. Drymon: 0000-0002-2104-004X; R. D. Grubbs: 0000-0002-2306-4731; D. S. Portnoy: 0000-0002-8178-1018; R. J. D. Wells: 0000-0002-1306-0614; G. Sancho: 0000-0002-8968-0625
258
et al.
Branham
Introduction
The bonnethead (Sphyrna tiburo L.) is a small coastal shark that inhabits the continental margins of the tropical and subtropical Atlantic waters of North and South America, from the Chesapeake Bay, United States to Brazil, including the Gulf of Mexico (GOM) (Ebert et al., 2021). Along coastlines of the GOM and the southeastern United States, bonnetheads are among the most abundant of coastal shark species and can tolerate high variation in salinities (16–38 ppt) (Ulrich et al., 2007; Bethea et al., 2008). The migratory habits of bonnetheads vary across their range and are poorly understood throughout most of the GOM. Bonnetheads in southwest Florida are thought to have limited migrations, and latitudinal variation in growth has been observed along the western Florida coastline (Parsons, 1993). On the U. S. Atlantic coast in the higher latitude waters of South Carolina, bonnetheads exhibit temperature driven migrations, spending summer months in South Carolina and winter months in warmer waters off the eastern coastline of Florida (Driggers et al., 2014). Sexual segregation has been observed in South Carolina with higher abundance of female bonnetheads in estuaries (Ulrich et al., 2007; Driggers et al., 2014). These individuals display intra–and interannual high site fidelity, with almost all tagged fish in South Carolina estuaries recaptured within and between years at the same estuary of initial capture (Driggers et al., 2014). Similar site fidelity has been found in Charlotte Harbor, Florida, and although migration likely occurs, migratory data are lacking (Heupel et al., 2006).
Population structure of bonnetheads along the southeastern and GOM coast of the U. S. is not fully understood. A recent study by Díaz–Jaimes et al. (2021) found significant population structure between the Atlantic and eastern GOM, as well as between these regions and the southern GOM; however, the study lacked samples from the northern and western GOM. Several other species of small coastal sharks have been found to have distinct population structure both between the Atlantic and GOM coasts of the United States as well as within the GOM (Keeney et al., 2003; Portnoy et al., 2014, 2016). Ongoing genetic work indicates an Atlantic population and two populations of bonnetheads within the U. S. GOM (eastern and western) with a geographic midpoint around 87.5 º W (Portnoy et al., unpublished data).
Bonnetheads are considered dietary specialists feeding mostly on crabs when in estuaries (Kroetz et al., 2017). Crabs in the genus Callinectes are especially important in bonnethead diet, comprising most crabs identified in stomach contents (Cortés et al., 1996; Lessa and Almeida, 1998; Plumlee and Wells, 2016). Blue crabs (Callinectes sapidus) are distributed in coastal and estuarine waters in the western Atlantic from Nova Scotia (Canada) to northern Argentina (Williams, 1974). The abundance of juvenile Callinectes sapidus in GOM, Florida and southeastern Atlantic coastline seem to follow density–independent patterns and are synchronized with environmental factors (Sanchez–Rubio et al., 2011; Colton et al.,
2014). Foraging opportunities may be one of the factors driving seasonal migration of bonnetheads along the east coast of North America. Driggers et al. (2014) suggested that high site fidelity observed in bonnetheads in South Carolina is driven by a seasonally abundant stock of blue crabs (Callinectes sapidus), with ovigerous female blue crabs providing the nutrients necessary for rapid (4.5 month, Gonzalez de Acevedo et al., 2020) embryo development in mature female bonnetheads.
Several studies have found and enumerated seagrasses (aquatic angiosperm plants) in the stomachs of bonnetheads but attributed them to accidental ingestion (Cortés et al., 1996; Kroetz et al., 2017). The presence of seagrass in bonnethead stomachs does not necessarily indicate omnivory; however, Leigh et al. (2018) found that bonnetheads fed seagrasses in captivity were able to assimilate seagrass nutrients into their tissue, suggesting omnivory is possible in the species. This finding, coupled with the high abundance of seagrasses in the diet of bonnetheads observed in some regions (Bethea et al., 2007), indicates that seagrasses may play a nutritional role in the species, at least during the immature life stage when seagrasses are more frequently found in stomach contents (Bethea et al., 2007; Kroetz et al., 2017). However, bonnetheads feed commonly on benthic prey found in proximity to seagrass beds, and other species of sharks with similar feeding strategies have been found to incidentally consume seagrass as well (Cortés and Gruber, 1990; Cortés et al., 1996; Bethea et al., 2007). Additionally, seagrasses are not ubiquitous throughout the range of bonnetheads, as no seagrasses occur in the turbid Spartina spp. marsh ecosystem on the Atlantic coastline of South Carolina (Dame et al., 2000; Green and Short, 2003).
The diet of bonnethead sharks has been well characterized in the GOM. In western Florida, bonnethead diet varies by season and habitat, but is nonetheless generally dominated by crabs, with seagrasses being the second most common observed stomach content (Cortés et al., 1996; Bethea et al., 2007). Bonnethead diet is also geographically variable in western Florida, with crabs generally being the most prevalent prey, although other crustaceans and cephalopods dominate bonnethead diet in southwestern Florida (Bethea et al., 2007). Juveniles in western Florida and the adjacent waters of Alabama show higher ingestion rates of seagrasses as compared to adults in the same locations (Bethea et al., 2007; Kroetz et al., 2017). Bonnetheads in Alabama have similar feeding habits to western Florida, but their diet also includes shrimp (Kroetz et al., 2017). The diet of bonnetheads in northern Brazil and the western GOM waters (Texas) is also dominated by crabs, with no seagrasses noted in stomach contents (Lessa and Almeida, 1998; Plumlee and Wells, 2016). Prior to the current study, diet of bonnetheads along the U. S. east coast had not been examined.
This study aims to complement previous dietary studies of bonnetheads and provide novel data on their diet from the seasonally migratory North Atlantic populations found in South Carolina and eastern Florida
Animal Biodiversity and Conservation 45.2 (2022) 259
Table 1. Number of mature and immature bonnethead (Sphyrna tiburo) stomachs analyzed, as well as mean length and size ranges (mm fork length, FL) for each location sampled.
Tabla 1. Numero de estómagos analizados de ejemplares adultos y juveniles de cornuda de corona (Sphyrna tiburo), longitud media y rango de talla (longitud de la horquilla en mm, FL) en cada localidad estudiada.
Location Maturity N Mean FL Max FL Min FL
Texas Immature 132 522 805 334 Mature 10 780 889 632
Alabama Immature 1 753 753 753 Mature 36 872 1,015 670
Northwest Florida Immature 45 520 750 390 Mature 50 665 900 560
West–central Florida Immature 18 490 600 360 Mature 12 753 810 595
East Florida Immature 10 494 845 265 Mature 8 796 994 620
South Carolina Immature 41 583 865 364 Mature 56 835 1,035 656
estuaries. By comparing the diet of sharks collected in the estuaries of South Carolina and eastern Florida with sharks collected from multiple locations within the GOM, we further characterize regional differences in the feeding ecology of bonnetheads.
Material and methods
Bonnetheads were collected during fishery–independent surveys from two Atlantic sites on the U. S. east coast: South Carolina (32.4º N –80.4º W to 33.0º N –79.5º W) and eastern Florida (27.9º N –80.6º W to 28.0º N –80.8º W), and four sites in the GOM: Texas (26.2º N –97.4º W to 29.3º N –95.2º W), Alabama (29.1º N –88.5º W to 30.3º N –87.5º W), northwest Florida (28.3º N –82.8º W to 30.1º N –84.1º W) and west central Florida (27.3º N –82.6º W to 27.4º N –82.6º W) (table 1). Seagrasses occur in all regions sampled except for South Carolina (Green and Short, 2003). Bonnetheads were collected primarily by gillnet from 2012 to 2019, however samples were also collected with other gears (longlines, seine, and trawl nets). Sharks were sexed and measured to the nearest centimeter (cm) for fork and total length upon capture, and maturity status was macroscopically determined when possible (table 1). If maturity was not determined at capture, maturity was assigned based on region–specific length at maturity estimates.
Female and male bonnetheads from South Carolina were considered mature at 819 mm and 618 mm fork length, respectively (Frazier et al., 2014). Female and male bonnetheads from the GOM were considered
mature at 944 mm and 830 mm total length, respectively (Lombardi–Carlson et al., 2003). Stomachs were excised from sharks, bagged, and frozen until analysis.
In the laboratory, stomachs were thawed, opened with scissors and contents were collected in a sieve (335 μm mesh). The sieve was lightly rinsed to remove excess mucus, contents were transferred to a sorting dish and identified to the lowest taxon possible under a dissecting scope. Each prey item was counted and weighed to the nearest 0.001 g (wet weight). From sharks collected using longlines, any stomach contents identified as bait were removed. Prey items were then grouped into one of eight categories for analysis: crabs, shrimp, stomatopods, cephalopods, teleost fishes, seagrasses, macroalgae, and other arthropods. Standard stomach content indices were calculated as the index of relative importance (Hyslop. 1980):
IRI = (%N + %W) x FO where FO is the frequency of occurrence, %N the percent number, %W the percent weight of contents. A percent index of relative importance (%IRI) was calculated according to the formula (Cortés, 1997):
%IRI = (IRICategory/ IRITotal) x 100
where IRICategory is the IRI for a prey category and IRITotal refers to the sum of IRIs from each category. Percent IRI of each prey category was calculated for mature and immature sharks of each site. In South Carolina, mature and immature sample %IRI was
260 Branham et al.
Fig. 1. %IRI contributions of each major prey group to both mature and immature bonnetheads (Sphyrna tiburo) from Texas (TX), Alabama (AL), northwest Florida (NW FL), west central Florida (WC FL), eastern Florida (E FL), and South Carolina (SC). Prey groups contributing to < 5 % of diet (cephalopods, macroalgae, and other arthropods) have been combined into 'Other' category.
Fig. 1. Contribuciones al porcentaje del indicador de importancia relativa (%IRI) de cada grupo de presas a los ejemplares maduros e inmaduros de las cornudas de corona (Sphyrna tiburo) de Tejas (TX), Alabama (AL), Florida nororiental (NW FL), Florida centrooccidental (WC FLl), Florida oriental (E FL), y Carolina del Sur (SC). Los grupos de presas que representan < 5 % de la dieta (cefalópodos, macroalgas y otros artrópodos) se han agrupado en la categoría "Other".
calculated for each unique taxon of prey to provide a detailed description of their diet. In addition, % IRI of each prey taxon was calculated for male and female bonnetheads, regardless of maturity, in South Carolina to examine potential differences in diet due to sexual segregation.
All statistical analyses were conducted in R Studio version 1.3.1093 (R Studio Team, 2020). For statistical analysis, samples were combined into three populations indicated by genetic analyses in the U.S. GOM and Atlantic (Portnoy et al., unpubl. data). These three groups are the western GOM (–97.5º to –88.0º longitude; Texas to Alabama; n = 179), the eastern GOM (–88.0º to –80.5º longitude; northwest and west central Florida ; n = 125), and the Atlantic, encompassing coastal waters from South Carolina to eastern Florida (n = 114). Species accumulation curves were generated for each regional group. These curves were used to assess whether the number of samples in each group was sufficient to accurately depict the diet of the region. Data were square root transformed and compiled into a Bray–Curtis dissimilarity matrix. A permutational multivariate analysis of variance (PERMANOVA) was used to test whether diets (%W) varied significantly (p < 0.05) between each combination of regions (western GOM with eastern GOM, western GOM with Atlantic, eastern GOM with Atlantic). Each PERMANOVA result was tested by the betadisper function followed by an analysis
of variance (ANOVA) to assess whether significant differences observed in the PERMANOVA were due to location (alpha diversity) or dispersion effects (beta diversity). A non–significant (p > 0.05) ANOVA result indicated that differences between groups were due to location effects. Finally, a similarity percentage (SIMPER) test was used to identify the contribution of individual prey groups to the differences observed in regional diets.
Results
In total, 423 bonnethead stomachs were analyzed. Of these 144 (two empty) were from Texas, 37 were from Alabama, 96 (one empty) were from northwest Florida, 18 were from east Florida, 30 were from west central Florida, and 97 (one empty) were from South Carolina (table 1). Empty stomachs (n = 4,1 % of total stomachs analyzed) were excluded from further diet analyses.
The diet of immature bonnetheads was similar across all areas sampled (fig. 1). Crabs, primarily the family Portunidae, dominated bonnethead diet in each region sampled but were most prevalent in the diet of immature individuals from Texas (89.2 %IRI). Shrimp were an abundant prey item only in juvenile bonnetheads from South Carolina (11.7 %IRI; fig. 1). Seagrasses, primarily Thalassia testudinum and Halodule wrightii, were found in stomachs of immature bon-
Animal Biodiversity and Conservation 45.2 (2022) 261
100 90 80 70 60 50 40 30 20 10 0 Crabs Shrimp Stomatopod Seagrass Other TX
Location
AL NWFL WCFL EFL SC TX AL NWFL WCFL EFL SC
Mature Immature %IRI
Table 2. Stomach contents from South Carolina bonnetheads (Sphyrna tiburo) reported to the lowest identified taxa as %IRI. Mature (n = 56), immature (n = 40), male (n = 36) and female (n = 60) individuals are shown.
Tabla 2. Contenido estomacal de los ejemplares de cornuda de corona (Sphyrna tiburo) en Carolina del Sur identificado hasta el menor nivel taxonómico como %IRI (porcentaje del índice de importancia relativa). Se presentan datos de individuos adultos (n = 56), inmaduros (n = 40), machos (n = 36) y hembras (n = 60).
Prey Mature Immature Male Female
Crabs
Callinectes sapidus 76.56 71.65 26.81 87.66
Callinectes similis – 0.16 – 0.03 Callinectes spp. 5.39 7.00 4.59 5.57 Portunus spp. – 0.04 0.06 –Unidentified Portunidae 0.09 2.16 1.27 0.28 Ovalipes oscellatus 0.27 – 1.02 0.01 Ovalipes spp. 0.01 0.09 0.04 0.02
Pagurus pollicaris 0.38 0.09 0.83 0.09 Unidentified Paguridae – 0.07 – 0.02 Menippe spp. 0.01 0.04 0.05 0.01 Libinia spp. 0.03 0.09 0.54 –
Ocypode quadrata – 0.03 0.04 –Hepatus epheliticus 0.01 – – 0.01
Unidentified Panopeidae 0.03 – 0.02 0.01 Unidentified Brachyuran 5.41 4.34 21.24 1.10 Shrimp
Litopenaeus setiferus 1.46 1.15 7.48 0.49 Farfantepenaeus aztecus 0.13 – 0.69 –Unidentified Penaeidae 8.13 8.88 25.35 3.77 Palaemonetes spp. 0.01 0.17 0.03 0.05 Upogebia affinis – 0.02 – 0.01 Alpheus spp. – 0.02 – 0.01
Unidentified shrimp 0.04 1.46 0.29 0.33 Stomatopods Squilla spp. 0.10 – 0.19 0.01 Unidentified Squillidae 0.13 0.34 0.89 0.05 Unidentified Stomatopod 0.11 0.07 0.85 –
Cephalopods
Unidentified Tuethida 0.26 – 0.90 –Teleost fishes Brevoortia spp. 0.08 0.05 0.70 0.02 Unidentified teleost 0.95 1.53 4.66 0.30
Other arthropods
Limulus polyphemus 0.12 0.03 – 0.15
Unidentified arthropod 0.28 0.22 1.12 0.01 Macroalgae Unidentified macroalgae 0.03 0.27 0.35 0.02
netheads from Texas (53.8 %FO), northwestern Florida (66.7 %FO), west–central Florida (77.7 %FO) and eastern Florida (50.0 %FO). Bonnetheads from west central Florida consumed the most seagrasses
(16.0 %IRI). Stomatopods, mostly Squilla spp., were present in the diet of individuals collected in Texas and west central Florida, although they comprised less than 3 %IRI in both regions. Teleost fishes made up
262 Branham et al.
Shrimp
Brachyuran crab Teleost fish Other
Fig. 2. % IRI contributions of each major prey group to both male and female bonnetheads (Sphyrna tiburo) from South Carolina. Prey groups contributing to < 5 % of diet (cephalopods, macroalgae, and other arthropods) have been combined into 'Other' category.
Fig. 2. Contribuciones al porcentaje del indicador de importancia relativa (%IRI) de cada grupo de presas a las cornudas de corona (Sphyrna tiburo) machos y hembras de Carolina del Sur. Los grupos de presas que representan < 5 % de la dieta (cefalópodos, macroalgas y otros artrópodos) se han agrupado en la categoría "Other".
1.6 %IRI of immature bonnethead diet in South Carolina. Bonnetheads collected in Texas and northwestern Florida consumed the most macroalgae (1.1 % and 1.5 %IRI, respectively). A notable portion of immature bonnethead diet in eastern Florida was other arthropods (3.3 %IRI), primarily Limulus polyphemus All other diet categories comprised less than 1 %IRI and were considered comparatively insignificant. No immature bonnetheads from Alabama were included in this study due to low sample size (n = 1).
The diet of mature bonnetheads was similar between regions (fig. 1). Texas, South Carolina, and northwestern Florida mature bonnetheads consumed a comparable quantity of crabs (85.9 %, 88.2 %, and 89.5 %IRI); however, the diet of mature sharks in Alabama and eastern Florida was nearly entirely composed of crabs (97.1 % and 97.3 %IRI, respectively). South Carolina was the only region where the adult bonnetheads consumption of shrimp was substantial (9.8 %IRI), predominately penaeid shrimp (table 2). Seagrasses, primarily Thalassia testudinum and Halodule wrightii, were found in mature bonnetheads from Alabama (11.1 %FO), northwestern Florida (70.0 %FO), west–central Florida (75 %FO), and eastern Florida (12.5 %FO). Bonnetheads from west central Florida consumed the most seagrasses (28.3%IRI) and this was the only region where mature sharks consumed a substantial amount of macroalgae (4.0 %IRI), although individuals in Texas and northwest Florida also consumed macroalgae (2.0 % and 1.3 %IRI, respectively). Stomatopods, largely Squilla spp., contributed to diet
of bonnetheads in Texas (5.4%IRI) and west central Florida (3.1%IRI; fig. 1). Teleost fishes were a minor contribution in the diet of bonnetheads from Texas, Alabama, northwestern Florida, and South Carolina (2.2 %, 2.4 %, 1.2 %, and 1.0 %IRI, respectively).
Similar to other regions studied, South Carolina bonnethead diet was dominated by crabs, especially in the family Portunidae. However, while shrimp were nearly absent from other regions, they comprised a substantial part of both immature and mature bonnethead diet (fig. 1). No seagrasses were found in the stomachs of South Carolina bonnetheads, nor were macroalgae found to be an important part of their diet (< 0.5 %IRI). Overall, no notable differences in mature and immature diet of South Carolina bonnetheads were observed (fig. 1, table 2). Diet was also compared between male (n = 36) and female (n = 60) bonnetheads in South Carolina to examine potential differences in diet due to sexual segregation. Broadly, female diet was almost entirely composed of crabs whereas males consumed much lower quantities of crabs (94.8 %IRI and 56.5 %IRI, respectively). Female bonnetheads also consumed less shrimp and teleost fishes than males (fig. 2). The species composition of diet varied between males and females. Callinectes spp accounted for the most identified crabs consumed by both sexes, however, they were more abundant in the diet of females than males (93.3 % and 31.3 %IRI, respectively). In male bonnetheads from South Carolina penaeid shrimp composed a larger portion of the diet than Callinectes spp. (table 2).
Animal Biodiversity and Conservation 45.2 (2022) 263
100
90
80
70
60
50
40
30
20
%
%
%
%
%
%
%
%
% 10 % 0 % Male Female Sex %IRI
Table 3. Results of statistical analysis comparing %W between the three distinct population structures of bonnetheads (Sphyrna tiburo): western Gulf of Mexico (WGOM), eastern Gulf of Mexico (EGOM), and Atlantic (ATL). Each column displays results of one of the three comparisons made. Percentages resulting from SIMPER test indicate contribution of each prey group to dietary differences between regions: Df, degreess of freedom.
Tabla 3. Resultados del análisis estadístico en el que se compara el porcentaje de peso de tres poblaciones distintas de cornuda de corona (Sphyrna tiburo): el golfo de México occidental (WGOM), el golfo de México oriental (EGOM) y el Atlántico (ATL). En cada columna se muestran los resultados de una de las tres comparaciones realizadas. Los porcentajes resultantes del análisis SIMPER indican la contribución de cada grupo de presas a las diferencias de las dietas entre regiones: Df, grados de libertad.
WGOM EGOM WGO EGOM ATL ATL
PERMANOVA
Df 1 1 1 F–statistic 21.296 8.079 12.47 p–value ≤ 0.001 ≤ 0.001 ≤ 0.001 Betadisper ANOVA p–value 0.5761 0.9418 0.6435
SIMPER
Crabs 43.13 % 41.71 % 43.12 % Stomatopod 20.63 % 13.31 % 15.76 % Seagrasses 12.40 % 9.90 % 4.03 % Shrimp 6.71 % 19.56 % 22.50 % Teleost 6.49 % 6.35 % 5.38 % Macroalgae 4.10 % 3.14 % 1.04 % Cephalopod 3.45 % 0.89 % 4.22 % Other arthropod 3.09 % 5.14 % 3.95 %
Species accumulation curves for each region approached an asymptote, indicating that sufficient samples had been processed from each region to accurately represent the diet (fig. 1s in supplementary material). The PERMANOVA tests found that each regional diet was significantly different from the others (table 3). The subsequent betadisper and ANOVA found that the differences observed by the PERMANOVA were due to location effects (table 3). The SIMPER test found that the primary prey group driving the differences in all comparisons were crabs.
Branham et al.
Between the western GOM and eastern GOM, the observed difference in %W was driven primarily by crabs, followed by stomatopods and seagrasses. Bonnetheads in the western GOM consumed more crabs and stomatopods, whereas bonnetheads in the eastern GOM consumed more seagrasses. The differences observed both between the western GOM and Atlantic and eastern GOM in the Atlantic were also primarily due to differences in crab consumption, followed by shrimp then stomatopods. Crabs were most prevalent in the diet of western GOM bonnetheads, followed by Atlantic bonnetheads. Shrimp were most prevalent in the diet of bonnetheads from the Atlantic while both GOM regions consumed more stomatopods than the Atlantic (table 3).
Discussion
Overall, bonnethead diet did not vary widely among locations investigated, and was universally dominated by crabs, confirming that crabs are the most important prey in the diet of bonnetheads across their range (Cortés et al., 1996; Lessa and Almeida, 1998; Bethea et al., 2007; Plumlee and Wells, 2016; Kroetz et al., 2017), with a few exceptions (Bethea et al., 2007). This study showed a preference for Callinectes spp. by bonnetheads in South Carolina that has been well documented in the GOM (Cortés et al., 1996; Lessa and Almeida, 1998). Bonnetheads in South Carolina consumed a proportion of shrimp that has only been previously observed in Alabama (Kroetz et al., 2017). However, the mature bonnetheads from Alabama in this study had almost no shrimp in their stomachs. Ontogenetic shifts in diet have been observed in bonnetheads in northwestern Florida (Bethea et al., 2007), but not elsewhere in their range (Cortés et al., 1996; Kroetz et al., 2017) and no clear ontogenetic dietary shifts were detected in our study. Regional dietary differences in prey weight detected were due to differences in the relative proportion of secondary prey categories, but the diet of all bonnethead populations was dominated by crabs across the analyzed range.
While immature and mature bonnethead diet in South Carolina was similar, consuming comparable quantities of Callinectes spp. and penaeid shrimp, males and females displayed significantly different diets. Females in South Carolina consumed almost exclusively Callinectes spp., whereas male bonnetheads had a more diverse diet that included a substantial amount of penaeid shrimp. Previous bonnethead trophic studies found sex–based differences in daily ration in the GOM (Bethea et al., 2007), but no significant dietary differences between females and males in western Florida (Cortés et al., 1996) and northern Brazil (Lessa and Almeida, 1998). Driggers et al. (2014) suggested that female adult bonnetheads in South Carolina feed heavily on Callinectes spp. in the estuary to support gestation. Our results support this hypothesis; ovigerous Callinectes sapidus migrate from low salinity waters to higher salinity waters near the mouths of estuar-
264
ies (Carr et al., 2004), and occur in South Carolina estuarine waters April through August, mainly in salinities above 15 ppt (Archambault et al., 1990). However, whether the observed difference in diet is due to female preference for Callinectes spp. or is simply a function of each sex occupying different habitats is unclear. Sexual differences in diet have been documented in sharks, yet are not universal, even in sexually segregated sharks (Simpfendorfer et al., 2001; McElroy et al., 2006). Habitat has been suggested to be the most important determinant of bonnethead diet (Cortés et al., 1996), and thus a spatially segregated population of males and females would be expected to have different diets. Previous research documented a 9:1 female to male sex ratio in in South Carolina estuarine waters (Ulrich et al., 2007). While the male bonnetheads examined in this study were captured in estuarine waters, they likely spend most of their time in nearshore coastal waters with lower Callinectes spp abundance (Ulrich et al., 2007), therefore stomach contents may be representative of nearshore foraging, or retention of a nearshore preferred prey when moving into estuarine waters.
Bonnetheads have been found to consume seagrasses in substantial quantities where seagrasses exist (Cortés et al., 1996; Bethea et al., 2007; Kroetz et al., 2017), and immature bonnetheads consume more seagrass than adults (Bethea et al., 2007; Kroetz et al., 2017). The results of this study confirm these observations. We found that bonnetheads living in seagrass ecosystems consume seagrasses, with immature sharks ingesting higher proportions of seagrass. The exception in our data was west central Florida, where seagrasses were nearly twice as important in mature bonnethead diet than in juveniles; however, this anomaly is likely an artifact of low sample size of mature fish from this study site (n = 12). Seagrass consumption by sharks is not unique to bonnetheads. Juvenile lemon sharks Negaprion brevirostris also ingest seagrasses (Cortés and Gruber, 1990), indicating seagrass consumption may be a relatively common occurrence in sharks that feed in seagrass beds. Bonnetheads feed mostly on benthic prey Callinectes spp., further increasing the likelihood of accidentally ingesting seagrasses. Generally, plant material found in shark stomachs has been considered incidental (Cortés and Gruber, 1990; Cortés et al., 1996; Kroetz et al., 2017). Originally, it was considered unlikely that bonnetheads had the digestive enzymes necessary to digest plant matter (Cortés et al., 1996; Bethea et al., 2007). Seagrasses were observed in 11.0 %–77.7 % of bonnethead stomachs that were collected in regions with seagrasses present, but these results do not directly prove that bonnetheads are omnivorous. However, our results support the concept proposed by Leigh et al. (2018) that bonnetheads may play a more substantial role as nutrient vectors in seagrass ecosystems than previously recognized.
Despite the potential benefits of inhabiting seagrass ecosystems, bonnetheads routinely occupy habitats devoid of seagrasses such as the estuaries
of South Carolina (Dame et al., 2000; Driggers et al., 2014). Bonnethead diet in South Carolina did not include seagrasses as they are not present in the lowland Spartina spp marsh environments in this region (Green and Short, 2003). The results of this study indicate that seagrass consumption is not a necessary component of bonnethead diet, at least during periods of up to 7–8 months when they occupy areas devoid of seagrasses (Driggers et al., 2014). If plant material is a necessary component of bonnethead diet, individuals in South Carolina could supplement their diet with the available macroalgae, however, almost none was found in stomach contents (0.03 %–0.27 %IRI). It is also possible that bonnetheads could consume seagrasses when they migrate south to the east coast of Florida (Driggers et al., 2014), though this study provides no evidence that this is the case.
In conclusion, our data indicate that bonnethead diet is dominated by crabs, primarily portunids, across the geographical range analyzed, though the relative importance of crabs varied between regions. Bonnetheads in South Carolina have a similar dietary reliance on crabs (especially Callinectes spp.) as has been found elsewhere in their range (Cortez et al., 1996; Lessa and Almeida, 1998; Bethea et al., 2007; Plumlee and Wells, 2016; Kroetz et al., 2017), though dietary differences can be found between populations. Diet of bonnetheads in South Carolina include a significant amount of shrimp, to an extent only previously observed in Alabama (Kroetz et al., 2017). Our results also suggest that male and female bonnetheads in South Carolina have different diet, a behavior that has not been observed elsewhere in their range (Cortés et al., 1996; Lessa and Almeida, 1998).
Acknowledgements
We thank Jeffrey D. Plumlee and Carly C. Burner, who helped with the statistical analyses. We also thank Cheston Peterson for collection of FSU samples from northwest Florida and the Center for Shark Research, Mote Marine Laboratory for samples from west central Florida. Funding for the FSU surveys was from the NOAA SEFSC as part of the GulfSPAN program. This study was funded by Summer Undergraduate Research Funds from the College of Charleston and the School of Science and Mathematics, and by NOAA/NMFS Saltonstall–Kennedy funds (NA16NMF4270225).This is contribution 857 of the South Carolina Marine Resources Division.
References
Archambault, J. A., Wenner, E. L., Whitaker, J. D., 1990. Life history and abundance of blue crab, Callinectes sapidus Rathbun, at Charleston Harbor, South Carolina. Bulletin of Marine Science, 46(1): 145–158.
Bethea, D. M., Hale, L., Carlson, J. K., Cortés, E.,
Animal Biodiversity and Conservation 45.2 (2022) 265
Manire, A., Gelsleichter, J., 2007. Geographic and ontogenetic variation in the diet and daily ration of the bonnethead shark, Sphyrna tiburo, from the eastern Gulf of Mexico. Marine Biology, 152: 1009–1020, Doi: 10.1007/s00227-007-0728-7
Bethea, D. M., Hollensead, L. D., Carlson, J. K., Ajemian, M. J., Grubbs, D., Hoffmayer, E. R., Del Rio, R., Peterson, G. W., Baltz, D. M., Romain, J., 2008. Shark nursery grounds and essential fish habitat studies. National Marine Fisheries Service Panama City Laboratory.
Carr, S. D., Tankersley, R. A., Hench, J. L., Forward, R. B., Luettich, R. A., 2004. Movement patterns and trajectories of ovigerous blue crabs Callinectes sapidus during the spawning migration. Estuarine and Coastal Shelf Science, 60: 567–579, Doi: 10.1016/j.ecss.2004.02.012
Colton A. R., Wilberg M. J., Coles V. J., Miller T. J., 2014. An evaluation of the synchronization in the dynamics of the blue crab (Callinectes sapidus) populations in the western Atlantic. Fisheries Oceanography, 23(2): 132–146, Doi: 10.1111/fog.12048
Cortés, E., 1997. A critical review of methods of studying fish feeding based on analysis of stomach contents: application to elasmobranch fishes. Canadian Journal of Fisheries and Aquatic Sciences, 54: 726–738, Doi: 10.1139/f96-31
Cortés, E., Gruber, S. H., 1990. Diet, Feeding Habits and Estimates of Daily Ration of Young Lemon Sharks, Negaprion brevirostris (Poey). Copeia, 1: 204–218, Doi: 10.2307/1445836
Cortés, E., Manire, C. A., Hueter, R. E., 1996. Diet, feeding habits, and diel feeding chronology of the bonnethead shark, Sphyrna tiburo, in southwest Florida. Bulletin of Marine Science, 58(2): 353–367.
Dame, R., Alber, M., Allen, D., Mallin M., Montague, C., Lewit, A., Chalmers, A., Gardner, R., Gilman, C., Kjerve, B., Pinkney, J., Smith, N., 2000. Estuaries of the South Atlantic Coast of North America: Their Geographical Signatures. Estuaries, 23(6): 793–819, Doi: 10.2307/1352999
Diaz–Jaimes, P., Bayona–Vásquez, N. J., Escatel–Luna, E., Uribe–Alcocer, M., Pecoraro, C., Adams, D. H., Frazier, B. S., Glenn, T. C., Babbucci, M., 2021. Population genetic divergence of bonnethead sharks Sphyrna tiburo in the western North Atlantic: Implications for conservation. Aquatic Conservation: Marine and Freshwater Ecosystems, 31: 83–98, Doi: 10.1002/aqc.3434
Driggers III, W. B., Frazier, B. S., Adams, D. H., Ulrich, G. F., Jones, C. M., Hoffmayer, E. R., Campbell, M. D., 2014. Site fidelity of bonnethead sharks Sphyrna tiburo (L. 1758) to specific estuaries in South Carolina, USA. Journal of Experimental Marine Biology and Ecology, 451: 61–69, Doi: 10.1016/j.jembe.2014.05.006
Ebert, D. A., Dando, M., Fowler, S., 2021. Sharks of the world: A complete guide. Princeton University Press, Princeton, New Jersey.
Frazier, B. S., Driggers III, W. B., Adams, D. H., Jones, C. M., Loefer, J. K., 2014. Validated age, growth and maturity of the bonnethead Sphyrna tiburo in the western North Atlantic Ocean. Journal of Fish
Biology, 85: 688–712, Doi: 10.1111/jfb.12450
Gonzalez De Acevedo, M., Frazier, B. S., Belcher, C., Gelsleichter, J., 2020. Reproductive cycle and fecundity of the bonnethead Sphyrna tiburo L. from the northwest Atlantic Ocean. Journal of Fish Biology, 97(6): 1733–1747, Doi: 10.1111/jfb.14537
Green, E. P., Short, F. T., 2003. World Atlas of Seagrasses. University of California Press, Berkeley and Los Angeles.
Heupel, M. R., Simpfendorfer, C. A., Collins, A. B., Tyminski, J. P., 2006. Residency and movement of bonnethead sharks, Spyrna tiburo, in a large Florida estuary. Environmental Biology of Fishes, 76: 47–67, Doi: 10.1007/s10641-006-9007-6
Hyslop, E. J., 1980. Stomach content analysis–a review of methods and their application. Journal of Fish Biology, 17: 411–429, Doi: 10.1111/j.10958649.1980.tb02775.x
Keeney, D. B., Heupel, M., Hueter, R. E., Heist E. J., 2003. Genetic heterogeneity among blacktip shark, Carcharhinus limbatus, continental nurseries along the U. S. Atlantic and Gulf of Mexico. Marine Biology, 143: 1039–1046, Doi: 10.1007/ s00227-003-1166-9
Kroetz, A. M., Drymon, M., Powers, S. P., 2017. Comparative dietary diversity and trophic ecology of two estuarine mesopredators. Estuaries and Coasts, 40: 1171–1182, Doi: 10.1007/s12237-016-0188-8
Leigh, S. C., Papastamatiou, Y. P., German, D. P., 2018. Seagrass digestion by a notorious 'carnivore'. roceedings of the Royal Society B: Biological Sciences, 285(1886): 20181583, Doi: 10.1098/ rspb.2018.1583
Lessa, R. P., Almeida, Z., 1998. Feeding habits of the bonnethead shark, Sphyrna tiburo, from northern Brazil. Cybium, 22(4): 383–394.
Lombardi–Carlson, L. A., Cortés, E., Parsons, G. R., Manire, C. A., 2003. Latitudinal variation in life history traits of bonnethead sharks, Spyrna tiburo, (Carcharhiniformes: Sphyrnidae) from the eastern Gulf of Mexico. Marine and Freshwater Research, 54: 875–883, Doi: 10.1071/MF03023
McElroy, W. D., Wetherbee, B. M., Mostello, C. S., Lowe, C. G., Crow, G. L., Wass, R. C., 2006. Food habits and ontogenetic changes in the diet of the sandbar shark, Carcharhinus plumbeus, in Hawaii. Environmental Biology of Fishes, 76: 81–92, Doi: 10.1007/s10641-006-9010-y
Parsons, G. R., 1993. Age determination and growth of the bonnethead shark Sphyrna tiburo: a comparison of two populations. Marine Biology, 117: 23–31, Doi: 10.1007/BF00346422
Plumlee, J. D., Wells, R. J. D., 2016. Feeding ecology of three coastal shark species in the northwest Gulf of Mexico. Marine Ecology Progress Series, 550: 163–174, Doi: 10.3354/meps11723
Portnoy, D. S., Hollenbeck, C. M., Belcher, C. N., Driggers III, W. B., Frazier, B. S., Gelsleichter, J., Grubbs, R. D., Gold, J. R., 2014. Contemporary population structure and post–glacial genetic demography in a migratory marine species, the blacknose shark, Carcharhinus acronotus Molecular Ecology, 23(22), 5480–5495, Doi: 10.1111/
266 Branham et al.
mec.12954
Portnoy, D. S., Hollenbeck, C. M., Bethea, D. M., Frazier, B. S., Gelsleichter, J., Grubbs, R. D., Gold, J. R., 2016. Population structure, gene flow, and historical demography of a small coastal shark (Carcharhinus isodon) in US waters of the Western Atlantic Ocean. ICES Journal of Marine Science, 73(9), 2322–2332, Doi: 10.1093/icesjms/fsw098
RStudio Team. (2020). RStudio: Integrated Development for R. RStudio, PBC, Boston. Accessible online at: http://www.rstudio.com/ Sanchez–Rubio, G., Perry, H. M., Biesiot, P. M., Johnson, D. R., Lipcius, R. M., 2011. Climate–related hydrological regime and their effects on the abundance of juvenile bluecrabs (Callinectes sapidus) in the northcentral Gulf of Mexico. Fishery Bulletin, 109(2): 139–146, https://scholarworks. wm.edu/vimsarticles/551
Simpfendorfer, C. A., Goodreid, A., McAuley, R. B., 2001. Diet of three commercially important shark species from Western Australia waters. Marine Freshwater Resources, 52: 975–985, Doi: 10.1071/ MF01017
Ulrich, G. F., Jones, C. M., Driggers III, W. B., Drymon, J. M., Oakley, D., Riley, C., 2007. Habitat utilization, relative abundance, and seasonality of sharks in the estuarine and nearshore waters of South Carolina. In: Shark Nursery Grounds of the Gulf of Mexico and the East Coast Waters of the United States, American Fisheries Society Symposium, 50: 125–139 (C. T. McCandless, N. E. Kohler, H. L. Pratt, Eds.). American Fisheries Society Bethesda, Maryland, USA. Williams, A. B., 1974. The swimming crabs of the genus Callinectes (Decapoda: Portunidae). Fishery Bulletin, 72: 685–798.
Animal Biodiversity
45.2
267
and Conservation
(2022)
42 Muñoz and Farfán
The effect of sex on home range in an urban population of European hedgehogs Erinaceus europaeus at the southern edge of the species distribution
J. Marco–Tresserras, G. M. López–Iborra
Marco–Tresserras, J., López–Iborra, G. M., 2022. The effect of sex on home range in an urban population of European hedgehogs Erinaceus europaeus at the southern edge of the species distribution Animal Biodiversity and Conservation, 45.2: 269–279, Doi: https://doi.org/10.32800/abc.2022.45.0269
Abstract
The effect of sex on home range in an urban population of European hedgehogs Erinaceus europaeus at the southern edge of the species distribution. As the transformation of natural habitats into urban environments increases, some species, such as hedgehogs, are able to adapt and thrive. Six hedgehogs, three males and three females, were tagged with radio–transmitters and tracked for three nights in the University of Alicante campus to study the effect of sex on their home range size, distance travelled per night, and night activity pattern. Time invested in several activities was also analyzed. Males showed larger home ranges than females (mean ± SD) (♂: 27.7 ha ± 19.2; ♀: 5.5 ha ± 3.4) and travelled longer distances per night (mean ♂: 1,077 m ± 251.18; ♀: 504 m ± 156.37). Activity rhythm through the night presented a bimodal pattern but differed between sexes. Males tended to be on the move significantly more often than females (♂: 38.7 %; ♀: 24.8 %) while females foraged more often than males (♂: 1.4 %; ♀: 9.2 %).
Key words: Hedgehog, Erinaceus europaeus, Radio–tracking, Home range, Activity pattern, Cat feeder Resumen
El efecto del sexo en el área de campeo de una población urbana de erizos europeos Erinaceus europaeus en el extremo sur de la distribución de la especie. En la creciente transformación de los hábitats naturales en entornos urbanos, algunas especies como el erizo pueden adaptarse y prosperar. Seis erizos, tres machos y tres hembras, fueron equipados con radiotransmisores y seguidos durante tres noches en el campus de la Universidad de Alicante con el fin de estudiar el efecto del sexo sobre el área de campeo, la distancia recorrida por noche y el patrón de actividad nocturna. También se analizó el tiempo invertido en diferentes actividades. Los machos presentaron áreas de campeo mayores que las hembras (media ± DE) (♂: 27,7 ha ± 19,2; ♀: 5,5 ha ± 3,4) y recorrieron distancias mayores por noche (media ♂: 1.077 m ± 251,18; ♀: 504 m ± 156,37). El ritmo de actividad durante la noche presentó un patrón bimodal, pero difirió entre sexos. Los machos tendieron a estar en movimiento con una frecuencia significativamente mayor que las hembras (♂: 38,7 %; ♀: 24,8 %), mientras que las hembras invirtieron más tiempo en forrajear (♂: 1,4 %; ♀: 9,2 %).
Palabras clave: Erizo, Erinaceus europaeus, Radioseguimiento, Área de campeo, Patrón de actividad, Comedero de gatos
Received: 22 XI 21; Conditional acceptance: 31 I 22; Final acceptance: 28 VII 22
Jana Marco Tresserras, Germán López Iborra, Departamento de Ecología, Universidad de Alicante, carretera de San Vicente del Raspeig s/n., 03690 San Vicente del Raspeig, Alicante, Spain.
Corresponding author: J. Marco. E–mail: janamarco13@gmail.com
ORCID ID: J. Marco–Tresserras: 0000-0002-4636-7647; G. M. López–Iborra: 0000-0003-3045-5498

ISSN: 1578–665 X eISSN: 2014–928 X
© [2022] Copyright belongs to the authors, who license the journal Animal Biodiversity and Conservation to publish the paper under a Creative Commons Attribution 4.0 License
269
Animal Biodiversity and Conservation 45.2 (2022)
Introduction
The increasing transformation of natural and semi–natural habitats into urban environments is a major cause of habitat loss (Pickett et al., 2001). Urbanization is generally viewed as deleterious for wildlife, affecting the ecosystem structure and function due to habitat fragmentation, pollution, human activity, and habitat change (Dickman, 1987; Foley et al., 2005). But although residential areas are often unfavorable for wildlife, some species can take advantage of their implicit characteristics (Luniak, 2004). Urban areas also mean low density or absence of potential natural predators (Gering and Blair, 1999; Moller 2012), abundance of alternative shelters, different micro–climatic and hydrological conditions and increase of food availability provided intentionally or accidentally by humans (Rebele, 1994; Cadenasso et al., 2007; Pautasso, 2007). Thus despite their potential disadvantages, urban environments can be successfully colonized, especially by species that exhibit high habitat and dietary flexibility and tolerance to human activity (Crooks, 2002; Bateman and Fleming, 2012). In such resource–rich habitats, species density and survival rates may even increase (Gloor et al., 2001; Prange et al., 2003).
The European hedgehog Erinaceus europaeus is a good example of an urban adapter (McKinney, 2006; Bateman and Fleming, 2012). A medium–sized solitary nocturnal insectivore, hedgehogs find semi–urban and urban places an ideal habitat as such areas are free of their main natural predators: eagle owls (Bubo bubo) and badgers (Meles meles) (Hof et al., 2012). Besides, they hold new food resources, such as pet food (Yalden, 1976; Morris, 1985; Hubert et al., 2011).
Studying a species home range is a basic step towards understanding its ecology because range is related to biological aspects such as number of mates, reproductive strategy, diet, food availability, body weight, and shelter ( Clutton–Brock and Harvey, 1978 ). These aspects can provide information about the quality of the habitat (Harestad and Bunnell, 1979 ). Hedgehogs' home range in Europe have been studied in environments ranging from arable landscapes to urban environments (Riber, 2006; Haigh et al., 2012; Rautio et al., 2013; Pettett et al., 2017), but data on the ecology of hedgehogs in southern Europe are scarce. Furthermore, little is known about their spatial ecology in these southernmost parts of its range. Only two studies are available about this species in Spain, and both are from the north of the country. One was performed in a peri–urban area with bush and open natural areas (García et al., 2009) while the other investigated hedgehogs released from a wildlife recovery center (Cahill et al., 2011). The aim of the present study was to analyse the effect of sex on the home range and distances travelled per night and to determine nocturnal patterns and activities of hedgehogs in an urban population in southern Spain.
Material and methods
Study area
The study was conducted in the campus of the University of Alicante (38º 23' 7.13'' N, 0º 30' 50.27'' W) in the southeastern Iberian Peninsula. The campus is a large gardened area (80 ha) located in the city of San Vicente del Raspeig (Alicante) (fig. 1). Half surrounded by buildings and roads, the campus is also connected to natural spaces through natural and man–made corridors.
Despite being located in a region with semi-arid Mediterranean climate with low annual rainfall (300 mm) and warm temperatures (annual mean 18 ºC), the campus itself has a micro–environment thanks to artificial irrigation. The landscape is dominated by wide open spaces, including pavement and grassy areas, with scattered vegetation such as bushes and garden plants, which provide good shelter. The development of vegetacion contrasts with the typical dry Mediterranean shurbland we find in the surroundings, with scattered Stipa tenacissima and Rhamnus lycioides as predominant species and large percentage of bare soil. Further information about the campus vegetation can be found in UVERDA (http://arbomapweb–accesociudadano.cloudapp.net/ UniversidadAlicante/indexes.html).
As in several public spaces in the cities, we also find several points with cat feeders (n = 14), intentionally placed and maintained by local people to feed stray cats. Cat feeders are permanently located (fig. 1) and food and water are replaced daily. The cat feeders consist of 5L plastic water bottles that have been remodeled for use as feeders through the creation of a lateral opening. This opening allows access of one individual at a time. In contrast with other studies, there is not shortage of food at these sites and they may be visited by several hedgehogs (besides other animals) throughout the night.
Radio tracking
Six adult hedgehogs (three males and three females) from a population that was monitored in the campus of Alicante University were equipped with radio–transmitters. Hedgehogs were captured by hand with the aid of spotlights and individually marked using a unique colour combination of heat shrink plastic tubes (Jones and Norbury, 2006; Young et al., 2006; Haigh et al., 2013). All hedgehogs caught in the campus were sexed according to the urogenital distance (Reeve, 1994; Morris, 2014) and classified as juvenile, subadult, or adult according to body measurements, weight and presence of growing spikes (Reeve, 1994). In order to study the effect of sex on home range, and to take into account the variability associated with body size, we selected for marking the smallest and the biggest individual of each sex known in the monitored population and one medium sized male and female.
The transmitters (Televilt TXH–2) were glued to the hedgehogs' spines with an epoxy putty (Ceys)
270 Marco–Tresserras and López–Iborra
Campus of the University of Alicante: area 80 ha (38º 23' 7.13'' N, 0º 30' 50.27'' W)
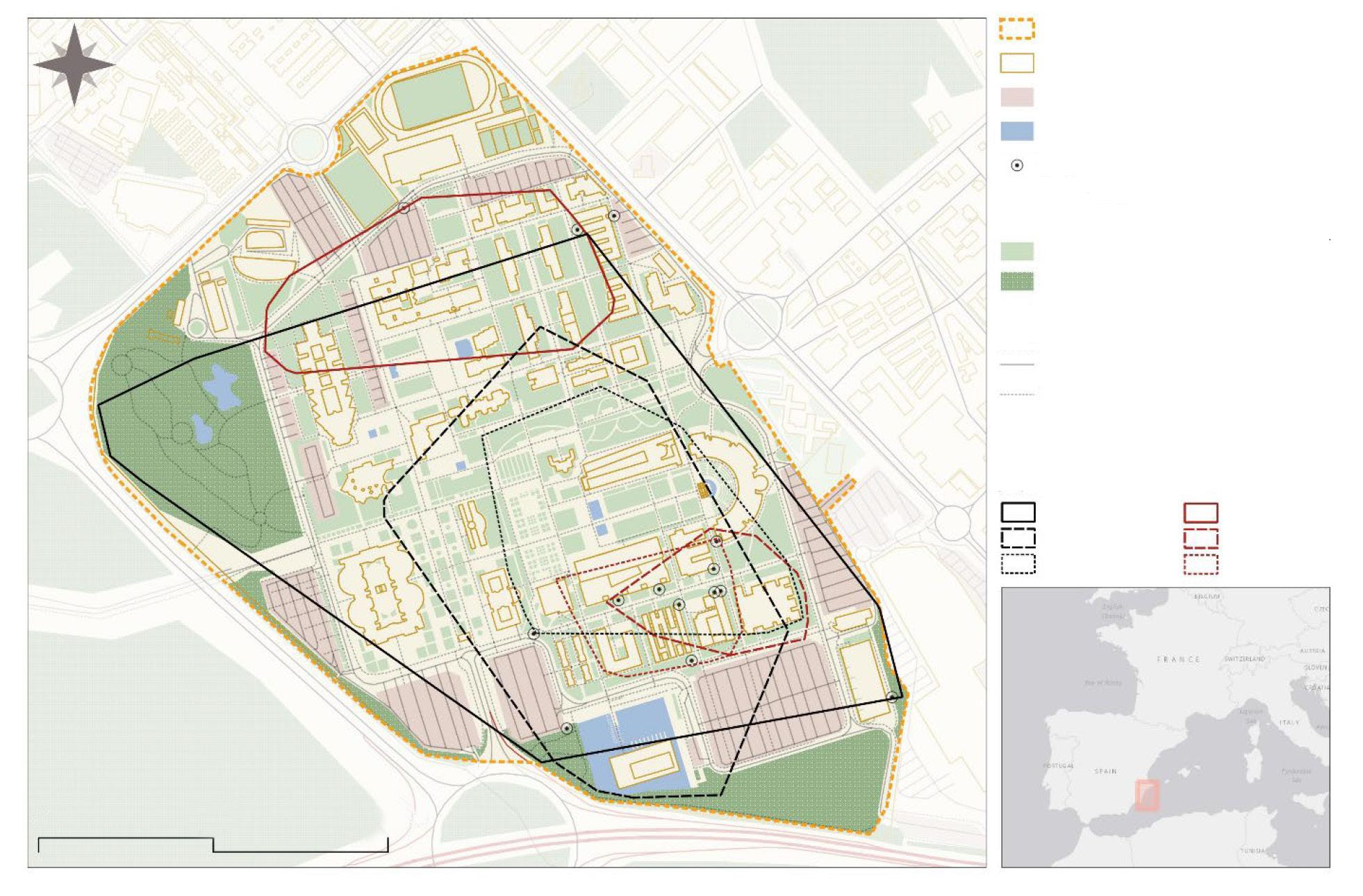
0 250 500 m
Fig. 1. Location of the study area (inset) and map of its habitat composition, showing cat feeder locations and individual MCP100 of the radio–tracked hedgehogs.
Fig. 1. Localización del área de estudio (cuadro pequeño) y mapa de los hábitats presentes donde se muestra también la localización de los comederos para gatos y los MPC100 de los erizos radio marcados.
directly attached to a mid–dorsal patch of clipped spines (Morris, 1988; Rautio et al., 2013). The six tagged hedgehogs had a minimum weight of 650 g, while the weight of the devices plus epoxy was 31.5 g and represented on average 3.5 % of the weight of marked hedgehogs, thus fulfilling the rule of being less than 5 % of the animals' weight (MITECO, 2015). All devices were removed at the end of the monitoring period by cutting off the tip of the spines below the epoxy putty (Barthel, 2019).
The radio tracking study was carried out during the mating season, when hedgehogs are most active (May–June 2014). They were tracked using radio receivers (RX98 Followit AB) and a four–element Yagi antenna (Y–4FL A11–0200; 163–165 MHZ) from a distance that allowed visual observation without disturbing the animals’ normal behavior (≤ 30 m) (Rautio et al., 2013). The hedgehogs in the study area are familiar with the presence of people and showed no signs of altered behaviour during the radio–tracking periods. Each hedgehog was followed for three nights with 1–28 days interval in between nights. Tracking was begun before the hedgehogs emerged in the evening and ended after the individual reached its nest the following morning (20:00–07:30 approximately) (GMT+2). When possible, locations were recorded every 15 minutes, and an activity category was assigned to each recording showing the hedgehogs' behavior: in motion (locomotion), resting or showing no movement (stationary), looking
for natural food (foraging), interacting with other hedgehogs (interaction), and feeding from a cat feeder (feeder). We analyzed the frequency of occurrence of the activity categories assigned to each recorded fix from radio tracking (n = 673). We assumed that the relative frequency with which any particular behavior was recorded reflected the proportion of time a hedgehog devotes to that behavior (Reeve, 1981; Wroot, 1984; Reeve, 1994).
Home range calculations
We calculated the home ranges for the six radio–tracked hedgehogs using radio tracking locations, and we used direct observations obtained during the general study of the university hedgehog population as reinforcement. We calculated three metrics related to home range: MCP100, Kernel 95 % and Kernel 50 %. The minimum convex polygon (MCP100) involves creating a convex polygon encompassing all locational points gathered for the individual (Gregory, 2017). It is prone to overestimating home range by including outliers and areas never visited by the animal (White and Garrot, 1990; Gregory, 2017), but despite this, it enables a better comparison with previous hedgehog studies in which MCP100 was often used (Reeve, 1982; Boitani and Reggiani, 1984; Kristiansson, 1984; Riber, 2006; Haigh, 2011; Rautio et al., 2013). Kernel methods describe the probability of finding an animal at any one place by centering a bivariate probability density
Animal Biodiversity and Conservation 45.2 (2022) 271
Green
Grassy
MCP100 Males ♂Big ♂Medium ♂Small Females ♀Big ♀Medium ♀Small
Study area Buildings Parking Water Cat feeders
areas
garden Grass, bushes and trees Tracks Car road Footway
Table 1. Home range sizes (ha) of all radio–tracked hedgehogs and average home range size of each sex using data from radio tracking plus direct observations: href, reference bandwidth estimated by the program; FBw, final bandwidth (bandwidth used to estimate home range after correction described in the text).
Tabla 1. Área de campeo (ha) de todos los erizos radiomarcados y área de campeo media de cada sexo según los datos obtenidos del radioseguimiento y las observaciones directas: href, ancho de banda de referencia estimado por el programa; FBw, ancho de banda final (ancho de banda usado para estimar las áreas de campeo tras la corrección descrita en el texto).
W MCP100 K95
Individual (g) (SD) (SD) K50 href FBw
Males Big 1,195 48.9 40.1 7 150.8 37.7
Medium 891 23.4 24.9 5.2 64.7 45.3
Small 794 11.4 12 2.2 43.3 28.2
Average 960 27.7 (19.22) 25.7 (14.05) 4.8 (2.45)
Females Big 1,236 9.4 12.7 3 64.2 35.3
Medium 764 3.4 4 0.9 29.8 14.9 Small 663 3.6 3.3 0.5 27.8 11.1
Average 888 5.5 (3.44) 6.7 (5.22) 1.5 (1.32)
function with unit volume over each recorded point (Rodgers and Kie, 2010). The 95 % kernel (K95: 95 % probability to be found in that area) was considered to represent the total home range of the hedgehog (Rautio et al., 2013) and kernel 50 % (K50) was used to describe the core area of the home range (areas of intensive use) where the animal spends 50 % of its time.
The minimum convex polygon (MCP100) was calculated using QGIS 2.18.25 (Quantum GIS Development Team, 2016), and the fixed kernel (FK) method (Worton, 1989) was used to estimate 95 % and 50 % isopleths, using Home Range Tools in ArcGIS 10.3 (ESRI Inc., Redlands, CA). The smoothing parameter for the kernels was estimated for each individual as href and was reduced in 5 % steps until the kernel 95 % line broke (Kie, 2013). The smoothing parameter value before the kernel 95 % line break was selected as the correct bandwidth.
Although successive locations using radio tracking methods may not have been independent, we used them to calculate home range because we had several tracking nights per home range (Smith et al., 1981) and the time interval between locations was relatively constant (De Solla et al., 1999). Repeated locations due to inactivity or resting periods (daylight nests, mid–night nap places) or continued use of cat feeders were not considered in the home range calculations.
Given that the number of days elapsed between radio–tracking nights varied between individuals (between 1 and 28 days in males, and between 1 and 15 days in females) we checked whether these differences affected home range estimates using all the metrics mentioned. To do so, we calculated the correlation between days elapsed and the increase
in home range (using the three metrics), obtained by adding the locations obtained on the latter day.
We estimated the possible effect of sex on home range size using the three metrics (MCP100, K95 and K50), analyzed using GLM with the Gaussian distribution. In these GLM, the response variable was the home range estimated for each individual. Sex (factor), weight and their interaction were included as predictors.
Night activity pattern
The distance between consecutive radio tracking locations was estimated for each individual. Speed was estimated by dividing this distance by elapsed time and average travel speed within each half–hour period was calculated for each hedgehog. The shape of the pattern of activity was estimated for each sex by averaging travel speeds in half–hour periods and fitting a polynomial regression to these averages. Polynomials of an increasing degree were fitted until the AIC value started to increase; in both sexes this led to the selection of a degree 4 polynomial.
The distance covered by each hedgehog each night was calculated as the sum of the distances of straight lines obtained by radio tracking. The possible effect of sex on distance covered was analyzed using a GLMM with Gaussian family distribution in which the response variable was the distance covered by each individual each night. Individual identity and date were included as random effects and sex and weight were included as fixed effects.
We checked the differences between sexes in the frequency of each type of activity by fitting a Poisson GLM with the number of cases of each activity re-
272 Marco–Tresserras
López–Iborra
and
Fig. 2. Home range metrics (MCP100, K95 and K50) and distance travelled per night for radio–tracked hedgehogs in relation to sex and weight: ● males; ● females.
Fig. 2. Estimas de áreas de campeo (MPC100, K95 y K50) y distancia recorrida por noche de los erizos radiomarcados en relación con el sexo y el peso: ● machos; ● hembras.
corded for each individual as response variable, and sex as predictor. The logarithm of the total number of activities recorded for each individual was included as an offset. Statistical analyses were performed using the R 3.6.1 program (R Core Team, 2019).
Results
Home range size
The number of locations recorded per hedgehog (mean ± SD and range) during the three nights of tracking was 112 ± 10 (99–124). Of these, an average of 69 ± 14 (41–79) were used to estimate the home range of the individuals after excluding repeated locations while resting or at cat feeders. In comparison, the number of direct observations obtained for each radio tracked hedgehog outside the radio tracking period was only 16 ± 8 (4–25). The correlation between the days elapsed between tracking nights and the increase in home range was low and non–significant for both sexes for all the metrics used (MCP 100: ♂ r = 0.132, p = 0.802; ♀ r = 0.043, p = 0.936; K95: ♂ r = 0.132, p = 0.803; ♀ r = 0.158, p = 0.765; K50: ♂ r = 0.427, p = 0.398; ♀ r = –0.173, p = 0.743).
GLM results showed a significant interaction between sex and weight for MCP100 (F1,2= 593.03; P = 0.001) and K95 (F1,2= 19.8; P = 0.047). The average home range was thus larger and increased faster with body weight in males (table 1; fig. 2). On the contrary, in the case of the core area (K50), interaction between sex and weight was not significant but the additive model showed sex had a significant effect (F1,2 = 10.2; P = 0.0495) and weight had a marginally significant effect (F1,2 = 9.07; P = 0.057) (fig. 2).
The GLMM for minimum distance travelled per night detected a significant effect of sex (z = 3.42, p = 0.0007) but not weight (z = 0.33 p = 0.739). Therefore, on average, males traveled larger distances per night (mean 1,077 m ± 251.18; range: 900–1,364 m) than females (mean: 504 m ± 156.37; range: 367–674 m). Within sex, variability in distance travelled per night was lower than variability in home range.
Night activity pattern
Figure 3 shows the average speed for each hedgehog in 30 minute periods. In general, males moved three times faster than females throughout most of the night (mean ± SD) (range), ♂ mean speed: 206 m/h ± 59.14 (146–265 m/h), ♀ mean speed:
Animal Biodiversity and Conservation 45.2 (2022) 273
60 50 40 30 20 10 0 45 40 35 30 25 20 15 10 5 0 8 7 6 5 4 3 2 1 0
1400 1200 1000 800 600 400 200
600 800
600
600
MCP100 Kernel 95 % Kernel 50 % Distance travelled 1600
0
1,000 1,200 1,400
800 1,000 1,200 1,400 Weight (g) Weight (g)
800 1,000 1,200 1,400 600 800 1,000 1,200 1,400 Weight (g) Weight (g) ha ha ha m
Fig. 3. Activity patterns calculated as the average travel speed within half–hour periods: A, males; B, females. Grey bars show the half–hour average for each radio–tracked hedgehog. The solid line shows the fit of a polynomial regression of degree 4. Black dots indicate the mean speed of the three hedgehogs of each sex for each half–hour period.
Fig. 3. Patrones de actividad calculados como el promedio de la velocidad en intervalos de media hora: A, machos; B, hembras. Las barras grises muestran el promedio de cada media hora para cada erizo radio marcado. La línea continua muestra el ajuste de una regresión polinómica de grado 4. Los puntos negros indican la velocidad media de los tres erizos de cada sexo por cada periodo de media hora.
70 m/h ± 21.20 (53–93 m/h). Both sexes showed a bimodal night activity but this differed in shape. Males appeared to show a speed peak in the first half of the night (22:00–23:00 h) and a minimum activity between 2:30–4:00 h, while females showed a slight trend to increase speed towards the end of the night (4:00–6:00 h) (fig. 3).
Nocturnal activities
Overall, hedgehogs invested 40.7 % of the night resting and 59.3 % active. Poisson GLMs showed that both sexes spent a similar amount of time interacting with other individuals and feeding at cat feeders (fig. 4). On the contrary, males tended to be found on the move significantly more often than females (males: 38.7 %; females: 24.8 %), while females were more often found foraging out of the feeders than males (males: 1.4 %;
females 9.2 %) (fig. 4). The distribution of activities also changed during the night, with males visiting the cat feeders more regularly but rarely foraging out of them, and females foraging more intensively before visiting the feeders and using them in the second half of the night (fig. 5). The frequency of use of the feeders and interactions throughout the night were highly correlated in females (r s = 0.960; p < 0.001) but not in males (r s = 0.432, p > 0.05), indicating that female interactions with other individuals are especially linked to the visits to the cat feeder sites.
Discussion
Home range sizes obtained in this study are smaller than those obtained in surveys of hedgehogs living in northern latitudes, but they are similar to those repor-
274 Marco–Tresserras and López–Iborra
A B 21:00 21:30 22:00 22:30 23:00 23:30 0:00 0:30 1:00 1:30 2:00 2:30 3:00 3:30 4:00 4:30 5:00 5:30 6:00 21:00 21:30 22:00 22:30 23:00 23:30 0:00 0:30 1:00 1:30 2:00 2:30
Big Medium Small 450 400 350 300 250 200 150 100 50 0 250 200 150 100 50 0 Speed
Speed
3:00 3:30 4:00 4:30 5:00 5:30 6:00
(m/h)
(m/h)
Forage Feeder
Fig. 4. Average frequency of activity categories recorded for European hedgehogs in an urban area, based on 346 behavioral records of three males, and 327 records of three females. Significance of sex effect on the percentage of each activity according to GLM is shown above the bars.
Fig. 4. Frecuencia media de las categorías de actividad registradas para erizos europeos en un área urbana, basada en 346 registros de comportamiento de los tres machos estudiados y 327 registros de las tres hembras estudiadas. En la parte superior de las barras se muestra el nivel de significación del sexo en el porcentaje de cada actividad según el modelo lineal generalizado.
ted by Garcia et al. (2009) further north in Spain, in Catalonia. Home ranges estimated in other European localities (table 2) are on average 26.1 ha (10–55.2) for females and 64.3 ha (32–97.9) for males, which are about four and twice times larger respectively than average home ranges found in both our study area and in Garcia et al. (2009). Our micro–environment with artificial irrigation and patchy habitat, together with the supplementary food supply for stray cats, may have contributed to the smaller home range. Such an environment likely alters natural conditions by providing greater shelter and more easily accessible food, possibly decreasing the need for hedgehogs to move long distances to cover basic needs.
In natural habitats, receptive females are widely dispersed because of patchy food resources (Kristiansson, 1984), making it difficult for males to predict the location of a female. On the contrary, because cat feeders make food predictable in some urban areas, female home range is reduced, and consequently, male home range was also smaller. The same could have happened in Garcia et al.'s (2009) survey because cat feeding stations are not uncommon in peri–urban areas. However, Rautio et al. (2013) found large home ranges for both sexes in an urban area in Finland with access to food supplies. The shorter breeding season in northern Europe could play a role here as males may try to maximize access to females by increasing their home ranges during a short mating season. This pressure is not a factor at Mediterranean latitudes because the climate is mild all year around, resulting in longer growth seasons (Hails, 1982; Huston and Wolverton, 2009). Furthermore, the
difference in climate not only allows the reduction, or even the elimination, of a hibernation period (own data) but also promotes faster sexual maturity and second litters for hedgehogs (Reeve, 1994).
The presence of physical barriers in urban habitats should not be ignored either. Buildings, roads, fences and other human constructions may restrict the movement of hedgehogs affecting spatial use in urban habitats. The campus of Alicante University is not fully fenced and has several corridors connecting the gardened area with its natural surroundings. We have data of some other hedgehogs monitored during the survey that moved in and out the campus, but none of the radio tracked individuals were recorded outside it. However this is a factor that potentially could also affect the spatial use of this campus by hedgehogs.
On average, the estimated home range size for males was larger than females, in line with findings in previous studies in Europe (table 2). As is common in a promiscuous mating system (Reeve, 1994; Jackson, 2006; Moran et al., 2009), male spatial organization is mostly affected by female space use, while female home ranges are expected to depend only on food and shelter availability. However, the difference between male and female home ranges was higher in our study than in previous studies. On average, in other European surveys, the male home range is 2.7 times greater than that of females (range: 1.8–3.7 times) but the difference was up to five times higher in our study. This finding could also be related to the high availability of predictable food at the cat feeding stations in our study area, which may have reduced more female than male home ranges, that still look
Animal Biodiversity and Conservation 45.2 (2022) 275
50 % 45 % 40 % 35 % 30 % 25 % 20 % 15 % 10 % 5 %
%
0
13.9
Locomotion Interaction Stationary Frequency 1.4 9.2
10.7 38.7 24.8 10.7 8.9 35.3 46.5 Male Female p = 0.00013 p = 0.2430 p = 0.0015 p = 0.450 p = 0.023
Frequency
100 % 90 % 80 % 70 % 60 % 50 % 40 % 30 % 20 % 10 % 0 %
20–21 21–22 22–23 23–24 00–01 01–02 02–03 03–04 04–05 05–06 06–07 07–08 (2) (12) (27) (43) (39) (48) (43) (41) (45) (37) (9)
Frequency
100 % 90 % 80 % 70 % 60 % 50 % 40 % 30 % 20 % 10 % 0 %
20–21 21–22 22–23 23–24 00–01 01–02 02–03 03–04 04–05 05–06 06–07 07–08 (3) (18) (34) (38) (36) (37) (35) (37) (43) (34) (11)
Feeder Forage Interaction Locomotion Stationary
Fig. 5. Percentage of each activity in one–hour periods throughout the night: A, males; B, females. (Sample size, number of locations, in parenthesis under each hour interval).
Fig. 5. Porcentaje de cada actividad en periodos de una hora a lo largo de la noche: A, machos; B, hembras. (Tamaño de la muestra, número de localizaciones, entre paréntesis debajo de cada intervalo de una hora).
out for as many females as possible (Morris, 1988). Although the presence of cat feeders may be common in habitats studied to date (e.g. suburban areas, golf courses), most studies provide no information about them, and only Rautio et al. (2013) provide some information about their presence. Variation in the abundance of cat feeders or in the amount and time that food is available at these feeders may have important consequences on hedgehogs' spatial use and home range (Cassini and Krebs, 1994). Therefore, more detailed information about the presence of cat feeders and their density should be provided in future studies to better understand hedgehog space use.
Despite the low sample size in our study, our analyses suggest the relation of body weight and home range size (Tucker et al., 2014) is stronger in males than in females. Weight correlated positively with the extension of the home range of males but had little influence on females' territories. The largest female in this study (1,236 g) was as heavy as the largest male (1,193 g), but its range was only slightly higher than the other females. The same occurred with
distances travelled per night, matching other studies where sexually active males ranged more widely and travelled further than females and non–rutting males (Morris, 1988; Reeve, 1994; Riber, 2006). Although hedgehogs have not been classified as a territorial species aggressive encounters between males have been documented (Reeve, 1994). Body mass can thus play an important role in dominance, with larger males being able to restrict smaller hedgehogs' use of space. Access to the feeders may also be restricted in order to avoid fighting with other conspecifics at the site. In such situations, lighter males likely invest more time in foraging in order to keep up with competition, and less time in other activities such as searching for females and wandering, thus accounting for their reduced home range.
Finally, our results showed that the intensity of nocturnal activity presented a bimodal pattern that was more marked in males than in females. This pattern has been described previously in captive (Campbell, 1975) and wild populations of European hedgehogs (Kristoffersson, 1964; Wroot, 1984; Garcia et al., 2009), but no difference between sexes has been
276 Marco–Tresserras and López–Iborra
A B
Biodiversity and Conservation 45.2 (2022)
Table 2. Summary of hedgehog home range area (HR, ha) estimated by the minimum convex polygon (MCP100) obtained in several studies using radio tracking. Besides our study, kernels were only calculated by Rautio et al. (2013) who found 81.6 ± 0.2 ha for males and 30.3 ± 7.0 ha for females, so only MCP100 will be used to compare studies: n, number of individuals; * Haigh (2012) estimates ± SE.
Tabla 2. Resumen de las áreas de campeo (HR, ha) de erizos europeos estimadas con el mínimo polígono convexo (MPC100) en varios estudios de radioseguimiento. Además de nuestro estudio, los kernels solo fueron calculados por Rautio et al. (2013), quienes estimaron un área de 81,6 ± 0,2 ha para los machos y 30,3 ± 7,0 ha para las hembras, por lo que solo se utilizará el MPC100 para comparar entre estudios. n, número de individuos; * Haigh (2012) estimas ± EE.
Sex HR ± SD Range n Country Latitude Habitat Study
♂ 97.9 ± 6.1 88.3–111.2 4 Finland 63ºN 29ºE Urban area Rautio et al. (2013)
♀ 55.2 ± 17.1 23.6–82.2 3
♂ 96 ± 24 4 Denmark 56º N 10º E Arable land, Riber (2006)
♀ 26 ± 15 4 forest and grassland
♂ 46.5 ± 15.8 25–67.7 5 Sweden 55º N 13º E Abandoned Kristiansson (1984)
♀ 19.7 ± 8.4 8.1–29.5 6 farmland
♂ 56 ± 0.7* 4 Ireland 51º N 8º W Rural area Haigh (2012)
♀ 16.5 ± 0.5* 3
♂ 32 ± 8.9 15.5–41.5 6 UK 51º N 0º W Golf course Reeve (1982) 10 ± 2.2 5.5–12 7
♂ 57.1 ± 36.6 5.5–102.5 9 Italy 42º N 11º E Mediterranean Boitani and Reggiani
♀ 29.1 ± 20.1 10–56.2 5 maquis region (1984)
♂ 23.8 ± 6.0 19.5–28.01 2 Spain 41º N 2º E Mediterranean García et al. (2009)
♀ 6.8 ± 3.2 3.47–9.89 3 forest near peri–urban area
♂ 27.7 ± 19.2 11.3–48.9 3 Spain 38º N 0º W Urban area Present study
♀ 5.5 ± 3.4 3.4–9.4 3
reported. Peaks of activity are usually associated with periods when hedgehogs are actively foraging and feeding (Reeve, 1994), so the resting pause necessary to digest their meals will only occur if they are able to fill their stomach quickly (Wroot, 1984). This will depend on the food supply and the individual characteristics, making bimodality not always obvious (Reeve, 1994). Presence of predictable food at cat feeders in the study area may have contributed to making the bimodality of the activity pattern clearer.
Cat feeders attract individuals and therefore increase the probability of encounters of both sexes. This may alter the pattern of activity of females. Males were found near the feeders during most part of the night until 4:00 a.m., after which they used these sites less frequently. On the other hand, females used the feeders infrequently before midnight and were found searching for prey in the lawn. Females increased their presence at cat feeders after 1:00 a.m. in the morning and used them most frequently between 4:00 and 5:00 a.m., the time when males were rarely
found there. The frequency of female interaction with other individuals was strongly correlated with the use of the feeders, while interactions were more uniformly distributed throughout the night in males. When several hedgehogs meet at a cat feeder, agonistic interactions are frequent and females are usually disturbed by males (pers. obs.). This limitation in access to easy food translates into females spending more time foraging throughout the night.
In summary, despite maintaining a bimodal pattern, male and female hedgehogs showed differences regarding activity peaks, with males being most active in the early hours after dark and females being more active towards dawn. The females' pattern of activity during the night appeared to be conditioned by the presence of males at cat feeders, as they seemed to avoid frequent interaction with them. In natural landscapes, without artificial food supplies, the probability of encounter of males and females is lower and the two sexes maintain more similar activity patterns (Campbell, 1975; Wroot, 1984).
277
Animal
Conclusion
Home ranges of hedgehogs in the Mediterranean tend to be smaller than those of hedgehogs in northern latitudes. Sex had a major effect on home range, with males having larger ranges than females. The presence of cat feeders altered females' spatial use, and also influenced activity patterns and social behavior. Despite this is a preliminary study with a small sample size, our observations may be useful to understanding how hedgehog populations will perform in northern latitudes in a changing environment with temperatures rising due to climate change. Our results also raise concern on how artificial food supplies in urban areas may alter the population dynamics of a species, the ecological consequences of which we are only starting to disentangle.
References
Barthel, L. M. F., Hofer, H., Berger, A., 2019. An easy, flexible solution to attach devices to hedgehogs (Erinaceus europaeus) enables long–term high–resolution studies. Ecology and Evolution, 9(1): 672–679, Doi: 10.1002/ece3.4794
Bateman, P. W., Fleming, P. A., 2012. Big city: carnivores in urban environments. Journal of Zoology, 287: 1–23, Doi: 10.1111/j.1469-7998.2011.00887.x
Boitani, L., Reggiani, G., 1984. Movements and activity patterns of hedgehog (Erinaceus europaeus) in Mediterranean coastal habitats. Zeitschrift für Säugetierkunde, 49: 193–206.
Cadenasso, M. L., Pickett, S. T. A., Schwarz, K., 2007. Spatial heterogeneity in urban ecosystems: reconceptualizing land cover. Frontiers in Ecology and the Environment, 5: 80–88, Doi: 10.1890/1540-9295(2007)5[80:SHIUER]2.0.CO;2
Cahill, S., Llimona, F., Tenés, A., Carles, S., Cabañeros, L., 2011. Radioseguimiento post recuperación de erizos europeos (Erinaceus europaeus Linnaeus, 1758) en el Parque Natural de la Sierra de Collserola (Barcelona). Galemys, 23: 63–72.
Campbell, P. A., 1975. Feeding rhythms of caged hedgehogs (Erinaceus europaeus L.). Proceedings of the New Zealand Ecological Society, 22: 14–18.
Cassini, M. H., Krebs, J. R., 1994. Behavioural responses to food addition by hedgehogs. Ecography, 17(4): 289–296, https://www.jstor.org/stable/3683356
Clutton–Brock, T. H., Harvey, P. H., 1978. Mammals, resources and reproductive strategies. Nature, 273(5659): 191–195, Doi: 10.1038/273191a0
Crooks, K. R., 2002. Relative sensitivities of mammalian carnivores to habitat fragmentation. Conservation Biology, 16: 488–502, Doi: 10.1046/j.15231739.2002.00386.x
De Solla, S. R., Bonduriansky, R., Brooks, R. J., 1999. Eliminating autocorrelation reduces biological relevance of home range estimates. Journal of Animal Ecology, 68: 221–234, https://www.jstor. org/stable/2647213
Dickman, C. R., 1987. Habitat fragmentation and vertebrate species richness in an urban environment. Journal of Applied Ecology, 24: 337–351, Doi:
10.2307/2403879
Foley, J. A., Defries, R., Asner, G. P., Barford, C., Bonan, G., Carpenter, S. R., Chapin, F. S., Coe, M. T., Daily, G. C., Gibbs, H. K., Helkowski, J. H., Holloway, T., Howard, E. A., Kucharik, C. J., Monfreda, C., Patzi, J. A., Prentice, I. C., Ramankutty, N., Snyder, P. K., 2005. Global consequences of land use. Science, 309: 570–574, Doi: 10.1126/science.1111772
García, S. Puig, X., Peris, A., 2009. Actividad y uso del hábitat por parte del erizo europeo Erinaceus europaeus Linnaeus, 1758 en el Parque Natural de la Serralada de Marina (Barcelona, Cataluña). Galemys, 21: 12–23.
Gering, J. C., Blair, R. B., 1999. Predation on artificial bird nest along an urban gradient: predatory risk or relaxation in urban environments? Ecography, 22(5): 532–541, https://www.jstor.org/ stable/3683146
Gloor, S., Bontadina, F., Hegglin, D., Deplazes, P., Breitenmoser, U., 2001. The rise of urban fox population in Switzerland. Mammalian Biology, 66: 155–164.
Gregory, T., 2017. Home Range Estimation. In: The International Encyclopedia of Primatology: 1–4 (J. Wiley and Sons, Eds.). Smithsonian Conservation Biology Institute, United States.
Haigh, A., 2011. The ecology of the European hedgehog (Erinaceus europaeus) in rural Ireland. PhD thesis, Cork University College.
Haigh, A., O'Riordan, R. M., Butler, F., 2012. Nesting behaviour and seasonal body mass changes in rural Irish population of the Western hedgehog (Erinaceus europaeus). Acta Theriologica, 57(4): 321–331.
– 2013. Habitat selection, philopatry and spatial segregation in rural Irish hedgehogs. Mammalia, 77(2): 163–172, Doi: 10.1515/mammalia-2012-0094
Hails, C. J., 1982. A comparison of tropical and temperate aerial insect abundance. Biotropica, 14(4): 310–313, Doi: 10.2307/2388092
Harestad, A. S., Bunnell, F. L., 1979. Home range and body weight a reevaluation. Ecology, 60: 389–402, http://www.jstor.org/stable/1937667
Hof, A. R., Snellenberg, J., Bright, P. W., 2012. Food or fear? Predation risk mediates edge refuging in an insectivorous mammal. Animal Behaviour, 83(4): 1099–1106, Doi: 10.1016/j.anbehav.2012.01.042
Hubert, P., Julliard, R. Biagianti, S., Poulle, M. L., 2011. Ecological factors driving the higher hedgehog (Erinaceus europaeus) density in an urban area compared to the adjacent rural area. Landscape and Urban Planning, 103(1): 34–43, Doi: 10.1016/j. landurbplan.2011.05.010
Huston, M. A., Wolverton, S., 2009. The global distribution of net primary production: resolving the paradox. Ecological Monographs, 79(3): 343–377, https://www.jstor.org/stable/40385214
Jackson, D. B., 2006. The breeding biology of introduced hedgehogs (Erinaceus europaeus) on a Scottish Island: lessons for population control and bird conservation. Journal of Zoology, 268(3): 303–314, Doi: 10.1111/j.1469-7998.2005.00035.x
Jones, C., Norbury, G., 2006. Habitat use as a predictor of nest raiding by individual hedgehogs Erinaceus europaeus in New Zealand. Pacific Con-
278 Marco–Tresserras and López–Iborra
servation Biology, 12(3): 180–188, Doi: 10.1071/ PC060180
Kie, J. G., 2013. A rule–based ad hoc method for a selecting a bandwidth in kernel home–range analyses. Animal Biotelemetry, 1: 13, Doi: 10.1186/2050-3385-1-13
Kristiansson, H., 1984. Ecology of a hedgehog Erinaceus europaeus population in southern Sweden. PhD thesis, Lund University.
Kristoffersson, R., 1964. An apparatus for recording general activity of hedgehogs. Annales Academiæ Scientiarum Fennicæ, A, IV, Biology, 79: 2–8.
Luniack, M., 2004. Synurbization – adaptation of animal wildlife to urban development. In: Proceedings 4th International Urban Wildlife Symposium: 50–55 (W. W. Shaw, L. K. Harris, L. VanDruff, Eds.). Arizona University, USA.
McKinney, M. L., 2006. Urbanization as a major cause of biotic homogenization. Biological Conservation, 127(3): 247–260, Doi: 10.1016/j.biocon.2005.09.005
MITECO (Ministerio para la Transición Ecológica y el Reto Demográfico), 2015. Buenas prácticas para la captura en vivo y marcaje de especímenes de fauna silvestre. Documento aprobado por la Comisión Estatal para el Patrimonio Natural y la Biodiversidad. Available online at: https:// www.miteco.gob.es/en/biodiversidad/temas/conservacion-de-especies/especies-proteccion-especial/2-5-1-ce-captura-marcaje.aspx
Moller, A. P., 2012. Urban areas as refuges from predators and flight distance of prey. Behavioral Ecology, 23(5): 1030–1035, Doi: 10.1093/beheco/ars067
Moran, S., Turner, P. D., O'Reilly, C., 2009. Multiple paternity in the European hedgehog. Journal of Zoology, 278: 349–353, Doi: 10.1111/j.1469-7998.2009.00583.x
Morris, P., 2014. Hedgehogs. Whittet Books Ltd., Stansted.
Morris, P. A., 1985. The effects of supplementary feeding on movements of hedgehogs (Erinaceus europaeus). Mammal Review, 15: 23–33, Doi: 10.1111/j.1365-2907.1985.tb00383.x
– 1988. A study of home range and movements in the hedgehog (Erinaceus europaeus). Journal of Zoology, 214: 433–449, Doi: 10.1111/j.14697998.1988.tb03751.x
Pautasso, M., 2007. Scale dependence of the correlation between human population presence and vertebrate and plant species richness. Ecology Letters, 10(1): 16–24, Doi: 10.1111/j.1461-0248.2006.00993.x
Pettett, C. E., Moorhouse, T. P., Johnson, P. J., Macdonald, D. W., 2017. Factors affecting hedgehog (Erinaceus europaeus) attraction to rural villages in arable landscapes. European Journal of Wildlife Research, 63: 54, Doi: 10.1007/s10344-017-1113-6
Picket, S. T. A., Cadenasso, M. L., Grove, J. M., Nikon, C. H., Pouyat, E. V., Zipperer, W. C., Constanza, B., 2001. Urban ecological systems: Linking terrestrial ecological, physical and economic component of metropolitan areas. Annual Review of Ecology and Systematics, 32: 127–157, Doi: 10.1146/annurev. ecolsys.32.081501.114012
Prange, S., Gehrt, S. D., Wiggers, E. P., 2003.
Demographic factors contributing to high raccoon densities in urban landscapes. The Journal of Wildlife Management, 67(2): 324–333, Doi: 10.2307/3802774
Quantum GIS Development Team 2016: Quantum GIS Geographic Information System – Open Source Geospatial Foundation Project. Available online at: http://qgis.osgeo.org
Rautio, A., Valtonen, A., Kunnasranta, M., 2013. The effect of the sex and season on home range in European hedgehogs at the northern edge of species range. Annales Zoological Fennici, 50(1–2): 107–12, Doi: 10.5735/086.050.0110
R Core Team, 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Rebele, F., 1994. Urban ecology and special features of urban ecosystems. Global Ecology and Biogeography Letters, 4(6): 173–187, Doi: https://doi. org/10.2307/2997649
Reeve, N., 1994. Hedghogs. T. A. D. Poyser Natural History, London.
Reeve, N. J., 1981. A field study of the hedgehog (Erinaceus europaeus) with particular reference to movements and behaviour. PhD thesis, London University.
– 1982. The home range of the hedgehog as revealed by a radio tracking study. Symposia of the Zoological Society of London, 49: 207–230, https:// eurekamag.com/research/021/938/021938658.php
Riber, A. B., 2006. Habitat use and behaviour of European hedgehog Erinaceus europaeus in a Danish rural area. Acta Theriologica, 51: 363–371, Doi: 10.1007/BF03195183
Rodgers, A. R., Kie, J. G., 2010. HRT: Home range Tools for ArcGIS, User's Manual. Centre of Northern Forest Ecosystem Research, Ontario Ministry of Natural Resources.
Smith, G. J., Cary, J. R., Rongstad, O. J., 1981. Sampling strategies for radio–tracking coyotes. Wildlife Society Bulletin, 9: 88–93.
Tucker, M. A., Ord, T. J., Rogers, T. L., 2014. Evolutionary predictors of mammalian home range size: body mass, diet and the environment. Global Ecology and Biogeography, 23(10): 1105–1114, Doi: 10.1111/geb.12194
White, G. C., Garrott, R. A., 1990. Analysis of radio tracking data. Academic Press, San Diego.
Worton, B. J., 1989. Kernel methods for estimating the utilization distribution in home range studies. Ecology, 70: 164–168, Doi: 10.2307/1938423
Wroot, A. J., 1984. Feeding ecology of the European hedgehog, Erinaceus europaeu. PhD thesis, London University.
Yalden, D. W., 1976. The food of the hedgehog in England. Acta Theriologica, 21: 401–424.
Young, R. P., Davison, J., Trewby, I. D. Wilson, G. J. Delahay, R. J., Doncaster, C. P., 2006. Abundance of hedgehogs (Erinaceus europaeus) in relation to the density and distribution of badgers (Meles meles). Journal of Zoology, 269(3): 349–356, Doi: 10.1111/j.1469-7998.2006.00078.x
Animal
279
Biodiversity and Conservation 45.2 (2022)
280 Marco–Tresserras and López–Iborra
Final instar larva of Neocordulia volxemi (Selys, 1874) (Odonata, Libelluloidea) from southeastern Brazil
F. Datto–Liberato, R. Cezário, R. Guillermo–Ferreira
Datto–Liberato, F., Cezário, R., Guillermo–Ferreira, R., 2022. Final instar larva of Neocordulia volxemi (Selys, 1874) (Odonata, Libelluloidea) from southeastern Brazil. Animal Biodiversity and Conservation, 45.2: 281–285, Doi: https://doi.org/10.32800/abc.2022.45.0281
Abstract
Final instar larva of Neocordulia volxemi (Selys, 1874) (Odonata, Libelluloidea) from southeastern Brazil. Neocordulia is a Neotropical genus with 16 species distributed in the South and Central Americas. To date, only seven larvae of this genus have been described. Here we describe the final instar larva of Neocordulia volxemi (Selys, 1874), collected in the Environmental Protection Area of the Uberaba River Basin in the State of Minas Gerais, Brazil. The metallic–green adults were found flying in a forested area in a Conservation Unit, while the larvae were found in a waterfall and surrounding rocky walls. The Cerrado remnant area is currently threatened by degraded pastures and increasing areas of monoculture agriculture.
Key words: Dragonfly, Damselfly, Savannah, Cerrado, Freshwater, Indicator
Resumen
Último estadio larvario de Neocordulia volxemi (Selys, 1874) (Odonata, Libelluloidea) del sureste del Brasil Neocordulia es un género neotropical que comprende 16 especies distribuidas en América del Sur y América Central. Hasta la fecha, solo se han descrito siete larvas de este género. En este artículo describimos el último estadio larvario de Neocordulia volxemi (Selys, 1874), recogida en la zona de protección ambiental de la cuenca del río Uberaba en el estado de Minas Gerais, en el Brasil. Se encontraron adultos de color verde metálico volando en una zona forestal de una unidad de conservación, mientras que la larva se encontró en una cascada rodeada de paredes rocosas. La zona, que es un vestigio de Cerrado, se encuentra actualmente amenazada por pastos degradados y el aumento de los monocultivos.
Palabras clave: Libélula, Caballito del diablo, Sabana, Cerrado, Agua dulce, Indicador
Received: 18 V 22; Conditional acceptance: 14 VI 22; Final acceptance: 17 VIII 22
Felipe Datto–Liberato, Rodrigo Roucourt Cezário, Rhainer Guillermo–Ferreira, Graduate Program in Entomology, Department of Biology, University of São Paulo–USP, Ribeirão Preto, Brazil and Lestes Laboratory, Federal University of Triângulo Mineiro, Av. Do Tutunas 490, Uberaba, 38061–500 MG, Brazil.
Corresponding author: R. Guillermo–Ferreira. E–mail: rhainer.ferreira@uftm.edu.rbr
ORCID ID: F. Datto–Liberato: 0000-0002-4622-1592; R. R. Cezário 0000-0002-0486-5867; R. Guillermo–Ferreira: 0000-0001-7774-5252

ISSN: 1578–665 X eISSN: 2014–928 X
© [2022] Copyright belongs to the authors, who license the journal Animal Biodiversity and Conservation to publish the paper under a Creative Commons Attribution 4.0 License.
281 Animal
Biodiversity and Conservation 45.2 (2022)
Introduction
Neocordulia is a Neotropical dragonfly genus represented by 16 species (Paulson et al., 2021). The genus is divided in two subgenera: (i) Mesocordulia; and (ii) Neocordulia (sensu stricto) (May, 1991). While Mesocordulia is predominantly found in Central America (but not exclusively, González Soriano, 1985), Neocordulia sensu stricto is exclusively found in South America. Neocordulia volxemi (Selys, 1874) was first described based on a female specimen (Selys–Longchamps, 1874), which Calvert (1909) further associated with a male (see also the drawings provided by Martin 1906 and Costa and Santos, 2000a). This species occurs in Minas Gerais, Mato Grosso and Goiás states in Brazil (Costa and Santos, 2000a; Vilela et al., 2020). Within Neocordulia sensu stricto, larvae have only been described for N. androgynis, N. setifera (Costa and Santos, 2000b), N. biancoi (De Marmels, 1990), N. machadoi (Carriço et al., 2011) and N. santacatarinensis (Costa et al., 2008). Here we describe the final instar larva of N. volxemi
Material and methods
The larva was collected emerging near the Vale Encantado Waterfall in the Vale Encantado Private Natural Heritage Reserve (RPPN–VE), Uberaba, Minas Gerais (19º 33' 13.8'' S 47º 54' 02.5'' W), in October 2021. The RPPN–VE, which was created in 2004, is a preserved area of 38.4 ha of Brazilian savannah remnants (i.e., Cerrado) located among large areas of pasture and plantations. The larva was placed in a plastic vial for a few hours until emergence was complete. The adult was then stored until sclerotization. The adult and exuvia were stored in 80 % ethanol. The
adult was identified based on Garrison et al. (2006). Measurements were made using a digital caliper. Larval morphology was studied and photos were made using a Leica MZ95 stereomicroscope. Photographs were taken with a Canon 70D digital camera. Images were vectorized and rendered using Adobe Illustrator CS6. Terminology for mandibular formula follows Watson (1956). Specimens are deposited in the collection of the Lestes Lab (LESTES) at the Federal University of Triângulo Mineiro (UFTM). Abbreviations: S1–S10: abdominal segments. Measurements are in mm.
Results
Neocordulia volxemi (Selys, 1874) Gomphomacromia volxemi Selys, 1874
Material examined
1 male exuvia (collected in field; fig. 1): Brazil, Uberaba, Vale Encantado waterfall (19º 33' 14'' S, 47º 54' 02'' W), 11 X 21, 878 m a.s.l., Guillermo–Ferreira leg.
Exuvia: general coloration dark brown; larva robust; rhomboid body; slender legs (fig. 2).
Head: about 1.5 x longer than wide, laterally elongated, oval in dorsal view; frons in a sharp transverse ridge; eyes small, rounded at the outer margin, occupying 1/3 of the head length (fig. 2). Posterior margin of occipital lobes covered with scale–like setae (fig. 2). Antennae 6 segmented, the first antennomere the longest (fig. 3). Submentum strongly constricted; distal margin of prementum bearing 11 setae preceding articulation (fig. 4); lateral margins of prementum with scale–like setae (fig. 4); distal margin of ligula covered with setae (fig. 4). Labial palp triangular with 7 crenulations, each bearing 5–6 spiniform setae
Fig. 1. Distribution of Neocordulia volxemi (Selys, 1874) (Odonata, Libelluloidea) in South America.
Fig. 1. Distribución de Neocordulia volxemi (Selys, 1874) (Odonata, Libelluloidea) en América del Sur.
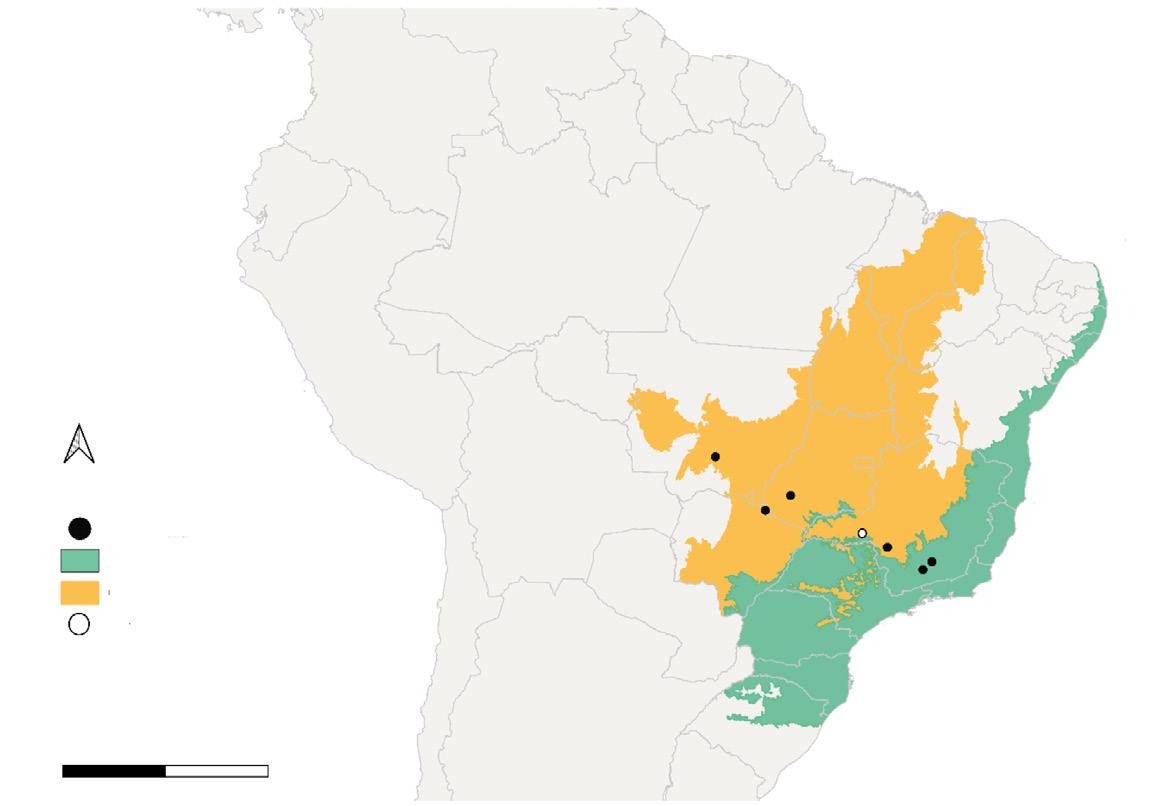
282 Datto–Liberato et al.
0
Neocordulia volxemi Atlantic forest Cerrado This study
250 500 km N
(fig. 5); distal margin with a conical movable hook (fig. 5); outer margin coated with small setae (fig. 5). Labrum trapezoidal, covered with long and slighted hairs; mandibular formula L1233'4 0 abd/ R1234 y abd (fig. 6). Mandibular palps each with 4 sclerotized terminal claws with numerous spine–like setae (fig. 7).
Thorax: pronotum rectangular, lateral margins straight, lateral border covered with scale–like setae (fig. 2); anterior and posterior angles of pronotum rounded (fig. 2); lateral margin sinuated (fig. 2). Wing bud reaching the 4th abdominal segment. Legs long, posterior leg the longest, longer than the abdomen (fig. 2).
Abdomen: cylindrical longer than wider with 2 lateral spines (S8–S9) (fig. 2). Anal appendages triangular and sharply pointed (fig. 8); epiproct longer and wider than cerci, slightly convergent, shorter than epiproct (fig. 7, 8); paraprocts longer than epiproct and cerci (fig. 8).
Measurements: total length (27.0 mm), labial palp (4.3 mm), prementum (6.8 mm), antennae (2.0 mm), head width (6.8 mm), head length (4.8 mm), thorax width (5.6 mm), thorax length (6.2 mm), abdomen max width (7.4 mm), abdomen length (16.0 mm), anterior leg (13.3 mm), midleg (16.8 mm), posterior leg (18.7 mm).
Habitat and ecology
The specimen was found emerging in a humid ravine covered with bryophytes and pteridophytes on the rocky walls, less than one meter from the stream border, ca. 10:30 h. Adults were collected in the adjacent semi–deciduous forest (1 male and 1 female), along a dirt path.
5 mm
Fig. 2. Neocordulia volxemi (Selys, 1874), habitus, dorsal view.
Fig. 2. Neocordulia volxemi (Selys, 1874), habitus, vista dorsal.
Discussion
The first antennomere of Neocordulia volxemi is the longest, distinguishing it from other known Neocordulia larvae, except N. androgynis (Costa et al., 2008)
3 4 5 6 7 8
1 mm 5 mm
Fig. 3–8. Neocordulia volxemi (Selys, 1874): 3, antenna; 4, prementum; 5, labial palp; 6, right (R) and left (L) mandibulae; 7, right (R) and left (L) mandibular palp; 8, caudal appendages.
Fig. 3–8. Neocordulia volxemi (Selys, 1874): 3, antena; 4, premento; 5, palpo labial; 6, mandíbulas derecha (R) e izquierda (L); 7, palpos mandibulares derecho (R) e izquierdo (L); 8, apéndices caudales.
Animal Biodiversity and Conservation 45.2 (2022) 283
Key to Neocordulia larvae.
Clave para larvas de Neocordulia
1 Presence of lateral spines on the abdomen 2
Lacking lateral spines on the abdomen Neocordulia batesi
2 First antennomere the longest 3 Not as above 5
3 Long lateral spines on S8–S9 4 Short lateral spines on S9–S10 Neocordulia santacatarinensis
4 Right mandible with 4 incisors Neocordulia volxemi Right mandible with 5 incisors Neocordulia androgynis
5 Apical blunted tooth on epiproct 6 Not as above Neocordulia setifera
6 Short or vestigial spines on S8–S9, cerci almost the same size as epiproct, sixth antennomere the longest 7 Long lateral spines on S8–S9, cerci smaller than epiproct, fourth antennomere the longest Neocordulia biancoi
7 Head rhomboidal, 8 palpal setae, 11 premental setae Neocordulia pedroi Head trapezoidal with anterior crest projected and crenulated, 7 palpal setae, 12 premental setae Neocordulia machadoi
(fig. 2; key to Neocordulia larvae). Neocordulia volxemi larva differs from N. androgynis by its mandibular formulae. The right mandible of N. androgynis exhibits 5 incisors and 3 molar teeth. The left mandible has 4 incisor teeth and 3 molar teeth. Neocordulia volxemi has 4 incisors in left and right mandible, and a smaller associated denticle in the third incisor of the left mandible (fig. 6; key to Neocordulia larvae). Prementum of N. volxemi bears 8 premental setae (fig. 3; key to Neocordulia larvae), more than N. batesi ( Novelo–Gutierrez and Ramírez, 1995) and N. biancoi; fewer than N. machadoi, N. pedroi (Costa et al., 2010), N. santacatarinensis, N. setifera Neocordulia volxemi palp bears 7 long setae on the inner surface and 28 smaller setae on the outer margin (fig. 4; key to Neocordulia larvae). Neocordulia androgynis palp bears 12 + 15 setae (key to Neocordulia larvae). Palpal crenulations are similar in all described species and should not be considered diagnostic traits.
The Brazilian Cerrado is a biodiversity hotspot but most of it is under threat of vast monocultures and cattle herding. The locality of this study is no different. The waterfall and the shallow stream are locally known as a refuge for wildlife (Fonseca et al., 2016). The Environmental Protection Area of Uberaba River Basin (EPA–UR), where the stream is located, is under high impact of degraded pastures despite recent conservation measures being taken recently conducted in the region (do Valle Júnior et al., 2019; Oliveira et al., 2019). Species like N. volxemi, whose adults were exclusively found inside forest remnants, therefore highlight the importance of such wildlife refuges in the Cerrado and the EPA–UR. Future studies should address whether this species and other rare aquatic insects may become good indicators of environmental health inside the EPA–UR.
Acknowledgements
FDL thanks CAPES (Proc. 88887.595419/2020–00). RRC thanks Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Proc. 88887.663621/2022–00). RGF thanks CNPq for a productivity grant (307836/2019–3). We thank Jordana Oliveira Borges and Vinicius Marques Lopez for field assistance. We also thank Rosângela Vilela and Leonardo Prata, owners of the Vale Encantado Private Natural Heritage Reserve.
References
Calvert, P. P,. 1909. Contributions to a knowledge of the Odonata of the neotropical region exclusive of Mexico and Central America. Annals of the Carnegie Museum, 6(1): 73–280.
Carriço, C., Costa, J. M., Santos, T. C., 2011. Description of the larva of Neocordulia machadoi Santos, Costa and Carriço, 2010 (Odonata: Corduliidae) from Brazil. Biota Neotropica, 11(2): 71–73, Doi: 10.1590/S1676-06032011000200008
Costa, J. M., Carriço, C., Santos, T. C., 2010. Neocordulia pedroi sp. nov. (Odonata: Corduliidae) from southeastern Brazil. Zootaxa, 2685: 51, Doi: 10.11646/zootaxa.2685.1.4
Costa, J. M., Ravanello, C. T., Souza–Franco, G. M., 2008. Description of a new species of Neocordulia Selys, 1882 (Odonata: Libellulidae, Corduliinae) from southern Brazil. Zootaxa, 1704: 64, Doi: 10.11646/zootaxa.1704.1.5
Costa, J. M., Santos, T. C., 2000a. Neocordulia mambucabensis spec. nov., a new dragonfly from Rio de Janeiro, Brazil (Anisoptera: Corduliidae). Odonatologica, 29(3): 247–253.
284 Datto–Liberato et al.
– 2000b. Two new species of Santosia Costa and Santos, 1992 with a description of five new corduliid larvae (Anisoptera: Corduliidae). Odonatologica, 29(2): 95–111.
do Valle Júnior, R. F., Siqueira, H. E., Valera, C. A., Oliveira, C. F., Fernandes, L. F. S., Moura, J. P., Pacheco, F. A. L., 2019. Diagnosis of degraded pastures using an improved NDVI–based remote sensing approach: an application to the Environmental Protection Area of Uberaba River Basin (Minas Gerais, Brazil). Remote Sensing Applications: Society and Environment, 14: 20–33, Doi: https://doi.org/10.1016/j.rsase.2019.02.001
Fonseca, P. H., Martins, G., Mota Da Silva, V. I., Martinelli, A. G., 2016. Levantamento dos mamíferos terrestres de médio e grande porte, na Reserva Particular do Patrimônio Natural–Vale Encantado, Uberaba, estado de Minas Gerais, Brasil. Acta Zoologica Lilloana, 60(1): 47–56, http://www.lillo. org.ar/journals/index.php/acta-zoologica-lilloana/ article/view/101
Garrison, R., von Ellenrieder, N., Louton, J., 2006. Dragonfly genera of the New World: an illustrated and annotated key to the Anisoptera. The Johns Hopkins University Press, Baltimore, Mariland.
González Soriano, E., 1985. Neocordulia longipollex Calv., a remarkable new record from Mexico (Anisoptera: Corduliidae). Notulae Odonatologicae, 2(6): 100–101.
De Marmels, J., 1990. Nine new Anisoptera larvae from Venezuela (Gomphidae, Aeshnidae, Corduliidae, Libellulidae). Odonatologica, 19: 1–15.
Martin, R., 1906. Cordulines. Collections Zoologiques du Baron Edmond de Selys–Longchamps, 17: 1–94.
May, M. L., 1991. A review of the genus Neocordulia, with a description of Mesocordulia subgen. nov. and of Neocordulia griphus spec. nov. from Central America, and a note on Lauromacromia (Odonata: Corduliidae). Folia Entomológica Mexicana, 82: 17–67.
Novelo–Gutierrez, R., Ramírez, A., 1995. The larva of Neocordulia batesi longipollex Calvert, 1909 (Odonata: Corduliidae). Journal of the New York Entomological Society, 103(2): 180–184.
Oliveira, C. F., do Valle Junior, R. F., Valera, C. A., Rodrigues, V. S., Fernandes, L. F. S., Pacheco, F. A. L., 2019. The modeling of pasture conservation and of its impact on stream water quality using Partial Least Squares–Path Modeling. Science of the Total Environment, 20(697): 134081, Doi: 10.1016/j.scitotenv.2019.134081
Paulson, D., Schorr, M., Deliry, C., 2021. World Odonata List. Available online at: https://www2. pugetsound.edu/academics/academic-resources/ slater-museum/biodiversity-resources/dragonflies/ world-odonata-list2/ [Accessed on July 7th, 2022]
Selys–Longchamps, E., 1874. Additions Synopsis des Cordulines. Bulletin de l’Acadmie royale de Belgique, 37: 16–34.
Vilela, D. S., Koroiva, R., Tosta, T. H. A., Novaes, M. C., Guillermo–Ferreira, R., 2020. Dragonflies and damselflies from the West of Minas Gerais, Brazil: checklist and new records. Biota Neotropica, 20(1), Doi: 10.1590/1676-0611-bn-2019-0851
Watson, M. C., 1956. The utilization of mandibular armature in taxonomic studies of Anisopterous nymphs. Transactions of the American Entomological Society, 81: 155–205.
Animal Biodiversity and Conservation 45.2 (2022) 285
286 Datto–Liberato et al.
Exploratory behaviour and foraging strategies in Mediterranean blue tits A. C. Velasco, E. S. Ferrer, J. J. Sanz
Velasco, A. C., Ferrer, E. S., Sanz J. J., 2022. Exploratory behaviour and foraging strategies in Mediterranean blue tits. Animal Biodiversity and Conservation, 45.2: 287–298, Doi: https://doi.org/10.32800/abc.2022.45.0287
Abstract
Exploratory behaviour and foraging strategies in Mediterranean blue tits. Animal behaviour is potentially a mechanism of individual diet specialization. To explore this possibility we assessed exploratory behaviour (EB) and foraging data for a population of blue tits Cyanistes caeruleus. Our results suggest that: 1) foraging differs between sexes; 2) the prey type selected by females depends on the provisioning rates of their mate, and adjustment of this prey-choice differs between EB phenotypes; and 3) foraging behaviour in males shows a trend towards linkage to their EB phenotype, with faster-exploring males obtaining marginally larger caterpillars but provisioning less frequently than slower-exploring males. Lastly, environmental variables contributed substantially to the diet composition of offspring. For this reason, we cannot suggest that EB dominates, but it may contribute to a diet specialization process in our study population.
Key words: Behaviour, Diet specialization, Prey choice, Intersexual differences
Resumen
Comportamiento exploratorio y estrategias de búsqueda de alimento en herrerillos Mediterráneos. El comportamiento animal tiene la capacidad de ser un mecanismo de especialización individual de la dieta. Para comprobarlo, se estudió el comportamiento exploratorio (EB por su sigla en inglés) y se obtuvieron datos sobre búsqueda de alimento en una población de herrerillo común, Cyanistes caeruleus. Los resultados muestran que 1) existen diferencias en la búsqueda de alimento entre sexos; 2) el tipo de presa elegido por las hembras depende de la frecuencia de aprovisionamiento de su pareja y este ajuste de la elección de la presa difiere entre los fenotipos de EB; y 3) existe una tendencia que relaciona el comportamiento de búsqueda de alimentos de los machos con su EB, por la que los machos con un comportamiento exploratorio más rápido obtienen orugas marginalmente más grandes, pero alimentan a sus pollos menos veces que los exploradores más lentos. Por último, las variables ambientales contribuyeron sustancialmente a la composición de la dieta de los pollos de herrerillo. Por ello, no podemos sugerir que el EB sea el principal factor determinante de los procesos de especialización de la dieta en la población estudiada, pero sí que puede contribuir a dichos procesos.
Palabras clave: Comportamiento, Especialización en dieta, Elección de presa, Diferencias intersexuales
Received: 21 VI 22; Conditional acceptance: 29 VII 22; Final acceptance: 25 VIII 22
Adara C. Velasco, Esperanza S. Ferrer, Juan José Sanz, Departamento de Ecología Evolutiva, Museo Nacional de Ciencias Naturales (MNCN-CSIC), c/ José Gutiérrez Abascal 2, 08028 Madrid, España (Spain).
Corresponding author: Adara C. Velasco. E-mail: ac.velasco@mncn.csic.es
ORCID ID: Adara C. Velasco: 0000-0001-9926-4820; Juan José Sanz: 0000-0003-2576-4050

ISSN: 1578–665 X eISSN: 2014–928 X
© [2022] Copyright belongs to the authors, who license the journal Animal Biodiversity and Conservation to publish the paper under a Creative Commons Attribution 4.0 License.
287 Animal
Biodiversity and Conservation 45.2 (2022)
Introduction
Animal behaviour may be a mechanism of individual diet specialization. A relationship between behavioural phenotypes and foraging strategies can imply that selection pressures differ among individuals within a population (Toscano et al., 2016). Studies on consistent behavioural differences between individuals (termed animal personality; Réale et al., 2010) have gained traction over the last two decades, steadily increasing our understanding of how behavioural traits in avian species relate to one another or to various fitness parameters. Diet studies in common passerine species have been fairly common since the 1970s, but most early studies are limited to descriptive statistics that provide rough information on prey proportions and often include broad categories of prey types (Eguchi, 1980; Blondel et al., 1991). In later diet studies, there has been a shift to a more thorough description in the offspring diet (García–Navas and Sanz, 2011; García–Navas et al., 2013), but to date, few studies have addressed in detail the intrapopulation variability in offspring diet composition (or parental prey preference).
Not only environmental variables but also social factors have been related to diet variability across taxa (Blondel et al., 1991; Naef–Daenzer et al., 2000; Sih and Christensen, 2001; Marshall et al., 2015). In passerines, possibly the most widely studied parameters associated with nestlings’ diet are those linked to the calendar date (Dias and Blondel, 1996). It is well known that insectivorous birds aim to synchronize the period in which their offspring have the greatest energetic demand with the phenological moment when their main caterpillar prey species peaks (Blondel et al., 1991; García–Navas and Sanz, 2011; but see Naef–Daenzer et al., 2000). Caterpillar phenology, their availability or their scarcity, and their size seem to have an effect in the diet of nestlings. García–Navas et al. (2013), for example, found that in blue tits Cyanistes caeruleus, a great abundance of noctuid caterpillars fosters the use of this resource, and for great tits Parus major, hairy caterpillars of the Malacosoma genus were fed to the offspring of late nests. In this latter example, the researchers also highlight that males were significantly more likely than females to feed their offspring hairy caterpillars, and they suggest that personality traits may be responsible for this difference.
Individual personality is a potential driver of dietary specialization because different behavioural phenotypes may contribute to creating differences in foraging behaviour. Along this line, Toscano et al. (2016) suggest a series of pathways relating personality traits to foraging behaviour, such as foraging activity (related to activity and prey search), risk–dependent foraging, social foraging, and physiological drivers (i.e. hormonal control). Other studies have assessed direct or indirect relationships between behavioural traits and provisioning rates (Mutzel et al., 2013; David et al., 2015; Serrano–Davies et al., 2017) or spatial foraging behaviour (Minderman et al., 2010; van Overveld and Matthysen, 2010). To date, however,
the relationship between avian behaviour and offspring diet composition remains understudied.
Exploratory behaviour (EB) refers to the way an individual interacts with a new object or environment. It has been widely used to study personality in passerines because it is a relatively easy trait to measure. Furthermore, it has proven to correlate with other personality traits (Verbeek et al., 1996; Mutzel et al., 2013) and to life–history traits (Dingemanse et al., 2004; Velasco et al., 2022). In blue tits, EB has been linked to local migration patterns (Nilsson et al., 2010), spatial aspects of foraging (Herborn et al., 2010), survival (Velasco et al., 2022), habitat selection and provisioning rate (Serrano–Davies et al., 2017).
In this study we assessed the phenotypic relationship between EB and provisioning strategies. We measured EB in situ in a population of blue tits in central Spain and we recorded the diet of their nestlings over two consecutive reproductive seasons. As intrapopulation differences in EB between sexes (Velasco et al., 2022) were observed in a previous study, we hypothesized that prey choice and provisioning effort would differ between males and females. Individuals with different behavioural phenotypes were expected to have different foraging strategies (Toscano et al., 2016). Therefore, we also hypothesized that the variability in EB between individuals would affect the diet composition of offspring. Because tortricid caterpillars (Tortrix spp. and Archips spp.) in our study area are superabundant, it could be expected that faster –and also shallower explorers (Verbeek et al., 1994) supply a higher proportion of tortricids to their offspring. In contrast, as noctuid and geometrid caterpillars are cryptic (but likely more caloric; García–Navas et al., 2013), slower and more thorough explorers are expected to supply higher proportions of these prey (Verbeek et al., 1994; García–Navas et al., 2013).
Material and methods
This research is part of an ongoing project in which two populations of blue tits have been monitored since 2005. These blue tits breed in the nest boxes situated in two study plots within the country estate of Quintos de Mora (Toledo, Spain). The study plots (Val: Arroyo de Valdeyernos 39º 26' 12'' N, 4º 05' 37'' W; and GG: Barranco de Gil García 39º 22' 43'' N, 4º 07' 31'' W) are located in forests dominated by Pyrenean and Portuguese oaks (Quercus pyrenaica and Q. faginea respectively). In each area, there are 100 nest boxes, and an average of 65 % of these boxes are occupied by blue tits each year. Data for this study are derived from the fieldwork of 2018 and 2019 breeding seasons. Laying dates for the blue tits vary between years, but monitoring tasks commonly begin in the second fortnight of March, and often extend into June. Nest box monitoring includes: detecting nests, determining laying and hatching dates, ringing adults and offspring, and taking biometric measures. The hatch day is defined as 'day 0' for each nest. Adults are captured on day 8 using spring traps inside the
288 Velasco et al.
nest boxes, and nestlings are ringed and measured on day 13. All the individuals are fitted with uniquely numbered aluminium rings (if not previously banded), and a EURING age class is assigned based on plumage characteristics (Demongin, 2016).
Novel environment exploratory tests
After the adults were banded and measured on day 8, 379 individuals were tested for exploratory behavior using an in situ Novel Environment (NE) test (Stuber et al., 2013). These tests are the same as those described by Velasco et al. (2022), but a brief summary is provided here. Tested individuals had access to a test box through a sliding pane that was connected to a small wooden box (holding room) to it. The individuals stayed in this holding room for 1 minute to homogenize their stress levels prior to the NE test. Once access to the test box was allowed, latency time was obtained (time to enter the test box). The maximum value for latency was two minutes, after which the bird was 'flushed' into the test box. The EB was then recorded for 5 minutes. After this time, the individuals were set free without further contact with the researchers. In 2018, all the exploratory tests included in this analysis took place between 14 IV and 28 V. In 2019, the exploratory tests took place between 19 III and 7 V.
During visualization, the test box was virtually (not physically) divided into six sections to determine the spatial distribution of EB. The first two minutes of the test were visualized to determine the exploratory variables: number of movements, number of area changes and number of areas explored. All the exploratory videos were observed by the same researcher (ACV). A principal components analysis (PCA) including the latency and the exploratory variables returned two axes or principal components (PC) with eigenvalues > 1. The PCs were centered and scaled prior to further analysis.
Provisioning behavior
The original nest box was replaced by a nest box adapted to hold a handycam (Sony HDR–XR550V; Sony Corp., Tokyo, Japan; see fig. 1s in supplementary material) one day prior to recording (day 10) to habituate birds to this setup. On day 11, the cameras were placed in the extra compartment of the adapted nest box, allowing us to film the entrance of the nest box in infrared light. A minimum of two–and a half hours was recorded per nest, but the first hour was discarded to avoid an artificially reduced provisioning rate due to nest manipulation disturbance (Hinde, 2006; García–Navas et al., 2013). No nest was deserted after recording. For the following hour, every time a bird entered the box, the following information was noted: time of entry, time of exit, sex of the bird (male or female), prey order (Arachnida, Lepidoptera, Other or Unidentified), prey family (only for caterpillars: Noctuidae, Geometridae, Tortricidae, Other or Unidentified) and prey length and width (only for caterpillars). All the provisioning videos were processed by the same researcher (ACV).
The sex of the visiting adult was easily identifiable from the rings. The leg on which the aluminum ring was fitted and an additional PVC ring on the opposite leg facilitated this. The PVC rings are also frequently readable in our recordings (see video 1s for an example in supplementary material). In this study we only used the videos that allowed identification of both provisioning individuals (N = 119 pairs). Caterpillars were measured using a pixel ruler (MB–ruler, MB–softwaresolutions). This measure was converted to centimeters using the nest box entrance diameter (3.5 cm) as a reference. We assumed a cylinder to be a relatively good approximation to the caterpillar volume and used the length and width of these prey to obtain their volume (cm3; Slagsvold and Wiebe, 2011).
The category 'Lepidoptera' included –from most common to least common– caterpillars, pupae and moths. The category 'Other' in prey order (6 % of the total identified observations) included Diptera, Coleoptera, Hemiptera, Hymenoptera or Orthoptera. Some items, especially if very small, were impossible to identify to Order level (approx. 7 % of the preys) or to Family level (approx. 10 % of the caterpillars). The category 'Other' in the case of caterpillar families (7 % of the identified caterpillars) almost entirely included Lycaenidae caterpillars. We took into account potential differences in diet composition due to prey phenology by including the laying date in the analyses (see 'Statistical analysis' section below).
Statistical analysis
All statistical analyses were performed in R 3.1.2 (R Core Team, 2020). Mann Whitney U–tests were used to explore differences in the response variables between sexes. For this test, we excluded individuals with only one provisioning event (N = 226 individuals). The descriptive statistics of the foraging behaviour for both sexes can be found in table 1s in supplementary material. The potential relationships between individual EB and the following individual provisioning behavior variables was assessed: provisioning frequency (number of visits per hour), proportion of lepidoptera over the total number of prey, proportion of arachnids over the total number of prey, proportion of noctuids over the total number of caterpillars, proportion of geometrids over the total number of caterpillars, proportion of tortricids over the total number of caterpillars, and average volume of the caterpillars. All the proportions were analyzed as logistic models with logit link function, while the error distribution of provisioning frequency and prey volume followed a Gamma distribution (log link function). An information–theoretic approach (Akaike Information Criterion (AIC); Zuur et al., 2009) was used to compare models. For each response variable, all models with ΔAIC ≤ 2 were considered equivalent (see tables 2s–7s in supplementary material). From these equivalent models, estimates were averaged and unconditional standard errors and confidence intervals were obtained (Burnham and Anderson, 2002).
Animal Biodiversity and Conservation 45.2 (2022) 289
Table 1. Results of the model averaging the equivalent best–ranked GLMs (ΔAIC ≤ 2) relating female prey choice at Order level to exploratory behaviour and extrinsic variables. Parameter estimates and their corresponding unconditional standard errors (USE) were obtained through model averaging of the best ranked equivalent models (ΔAIC ≤ 2; see table 2s in supplementary material): E, estimate; EB, exploratory behaviour; P.Freq., provisioning frequency; – indicate variables that were included in the analysis but that failed to appear within the best ranked equivalent models.
Tabla 1. Resultados de promediar los modelos lineales generalizados equivalentes al mejor modelo (ΔAIC ≤ 2) que relacionan la elección de presas a nivel de orden por las hembras con el comportamiento exploratorio de estas y con variables extrínsecas. Las estimaciones de los parámetros y sus correspondientes errores estándar no condicionales (USE) se obtuvieron calculando el promedio de los modelos equivalentes al mejor modelo (ΔAIC ≤ 2; véase la tabla 2s del material suplementario): E, estimación; EB, comportamiento exploratorio; P.Freq., frecuencia de aprovisionamiento; – indican variables que se incluyeron en el análisis, pero que no aparecieron en los modelos equivalentes al mejor modelo.
E ± USE Σω(AIC) 95 % CI E ± USE Σω(AIC) 95 % CI
♀ Order Lepidoptera (%)
♀ Order Arachnida (%)
Area: Val – – – –0.02 ± 0.17 0.019 –0.36, 0.32
Brood size 0.00 ± 0.03 0.024 –0.06, 0.07 – – –
♀ EB PC1 0.00 ± 0.06 0.020 –0.11, 0.11 –0.03 ± 0.11 0.048 –0.24, 0.19
♀ EB PC2 –0.06 ± 0.17 0.056 –0.39, 0.26 0.14 ± 0.26 0.130 –0.36, 0.65
♀ Age: > 1 year 0.08 ± 0.28 0.057 –0.48, 0.64 –0.05 ± 0.26 0.040 –0.56, 0.45
Relative lay date 0.00 ± 0.01 0.020 –0.01, 0.01 0.00 ± 0.01 0.039 –0.02, 0.02
♂ P.Freq. 0.00 ± 0.01 0.019 –0.02, 0.02 0.00 ± 0.01 0.019 –0.02, 0.02
Year: 2019 – – – 0.00 ± 0.16 0.019 –0.31, 0.31
The nest can be considered a working unit (Both et al., 2005; Hinde, 2006; Mutzel et al., 2013): individuals breeding together affect each other’s behavior. These pair–effects may not necessarily work in both directions (Schuett et al., 2010). Because of this, models were fitted separately for the sexes to include the parameters of the breeding partner in the models. Furthermore, only nests for which the EB of both individuals was known were used (N = 119 pairs). Because this study took place over two consecutive years, some individuals were repeated (15 females and 18 males), but only 4 couples remained the same in both years. Removing the repeated couples did not produce different results.
In addition to individual exploration scores (covariates, two principal components), all models included brood size (covariate), relative laying date (covariate, relativized to the average laying date of each area each year), individual age (factor with two levels: yearling or older), year (factor with two levels) and area (factor with two levels). The provisioning frequency of the breeding partner was also included in all analysis (covariate). Individuals were expected to adjust their provisioning frequency to the size of the available prey, so when analyzing this variable, the individuals’ average prey volume was included as a covariate. Initially, the EB score of the partner was included in all models, as was habitat effect (number of trees in a radius of 25 m), but these terms were non–significant in all cases and
were removed from the models. The variance inflation factor (VIF) was calculated for every model to test for collinearity between variables. Quadratic effects and potential two–way interactions between the explanatory variables were explored, but only those with P < 0.05 were included in the models. To facilitate the interpretation of our results, female EB PC1 was transformed to a categorical variable in the figures using the standard deviation (SD) so as to classify them into slower–explorers (EB < SD), faster–explorers (EB > SD) and intermediate explorers (EB within the SD).
Results
Exploratory PCA scores were the same as those obtained in a previous study (Velasco et al., 2022), but a brief summary is included here. Two principal components with eigenvalues > 1 were obtained and used in further analysis. Forty–seven percent of the variance in EB was explained by the first principal component (PC1) and 26 % of the variance was explained by the second component (PC2). PC1 conformed a slow–fast continuum, with higher values in individuals with a higher number of movements and area changes, and taking less time to explore a minimum of three areas. The PC2 axis was referred to as daring–timid as it reflects the latency to enter the test–box: higher values indicate longer latency (more timid EB).
290 Velasco et al.
Table 2. Results of model averaging the equivalent best–ranked GLMs (ΔAIC ≤ 2) relating male prey choice at Order level to exploratory behaviour and extrinsic variables. Parameter estimates and their corresponding unconditional standard errors (USE) were obtained through model averaging of the best ranked equivalent models (ΔAIC ≤ 2; see table 3s in supplementary material): E, estimate; EB, exploratory behaviour; P.Freq., provisioning frequency; – indicate variables that were included in the analysis but that failed to appear within the best ranked equivalent models.
Tabla 2. Resultados de promediar los modelos lineales generalizados equivalentes al mejor modelo (ΔAIC ≤ 2) que relacionan la elección de presas a nivel de orden por los machos con el comportamiento exploratorio de estos y con variables extrínsecas. Las estimaciones de los parámetros y sus correspondientes errores estándar (USE) no condicionales se obtuvieron calculando el promedio de los modelos equivalentes al mejor modelo (ΔAIC ≤ 2; véase la tabla 3s del material suplementario): E, estimación; EB, comportamiento exploratorio; P.Freq., frecuencia de aprovisionamiento; – indican variables que se incluyeron en el análisis, pero que no aparecieron en los modelos equivalentes al mejor modelo.
E ± USE Σω(AIC) 95 % CI
♂ Order Lepidoptera (%)
E ± USE Σω(AIC) 95 % CI
♂ Order Arachnida (%)
Area: Val 0.58 ± 0.55 0.166 –0.50, 1.66 – – –
Brood size 0.01 ± 0.04 0.024 –0.06, 0.07 – – –
♂ EB PC1 0.00 ± 0.05 0.015 –0.10, 0.09 0.01 ± 0.09 0.032 –0.18, 0.19
♂ EB PC2 0.00 ± 0.06 0.016 –0.12, 0.12 0.00 ± 0.12 0.032 –0.23, 0.24
♂ Age: > 1 year – – – – – –
Relative lay date 0.00 ± 0.01 0.042 –0.02, 0.02 – – –
♀ P.Freq. 0.01 ± 0.02 0.046 –0.03, 0.04 – – –
Year: 2019 0.02 ± 0.15 0.019 –0.28, 0.31 0.00 ± 0.24 0.032 –0.48, 0.48
Males in our study area brought a significantly higher proportion of spiders (W = 5,071; P < 0.001) and geometrid caterpillars (W = 5,530; P = 0.003), and had higher provisioning frequencies than females (W = 4,840; P < 0.001).
Offspring diet composition
For both sexes, EB was part of the best–fit models for all the response variables (tables 2s, 3s, 5s and 6s in supplementary material). None of the analysed variables had a strong relationship with the type of prey brought to the nest at an Order level (tables 1, 2). After model–averaging, only the first principal component of exploratory behaviour in females (female EB PC1) was significantly related to offspring diet composition. A significant interaction between female EB PC1 and the frequency of male visits revealed that when the male provisioning rate was high, faster–exploring females fed their nestlings more geometrids than slower–exploring ones (fig. 1; table 3). Similarly, a significant interaction between female EB PC1 and male provisioning frequency indicated that slower–exploring females brought a higher proportion of tortricids to the nest when they paired with males that fed their young at high frequencies (fig. 2; table 3). When females paired with a male with a low visit frequency,
slower–exploring females brought a lower proportion of tortricids than faster–exploring females (fig. 2). Although non–significant through model averaging, female EB PC2 had certain importance in the geometrid and tortricid proportion models. A trend indicated that more timid females (higher EB PC2) were more likely to bring geometrid caterpillars than their counterparts, which brought marginally higher proportions of tortricid caterpillars (table 3).
Consistently for both sexes, environmental variables related significantly (and in opposite directions) to the proportion of noctuid and tortricid caterpillars (tables 3, 4). Birds that bred relatively early brought higher proportions of tortricid caterpillars than later breeders, but later breeders brought a higher proportion of noctuids than early breeders (tables 3, 4). The proportion of tortricid caterpillars was significantly higher in 2019 than in 2018, and the opposite was true for the proportion of noctuids. For females, but not for males, the proportion of geometrids was significantly higher in 2019 than in 2018 (table 3).
Provisioning effort
Females' EB was unrelated to the provisioning effort in both regarding visits rate and prey volume (table 5). For males, although non–significant after model ave-
Animal Biodiversity and Conservation 45.2 (2022) 291
1.00 0.75 0.50 0.25 0.00
0 10 20 30 40
Male provisioning rate
Female PC1 Fast (> SD) Average Slow (< SD)
Fig. 1. Relationship between the proportion of geometrids obtained by females and the provisioning rate of males (/h), mediated by female exploratory behaviour (EB PC1): fast females, black circles, continuous line (EB > SD); slow females, grey triangles, short–dashed line (EB < SD); intermediate females, empty circles, long-dashed line; SD, standard deviation.
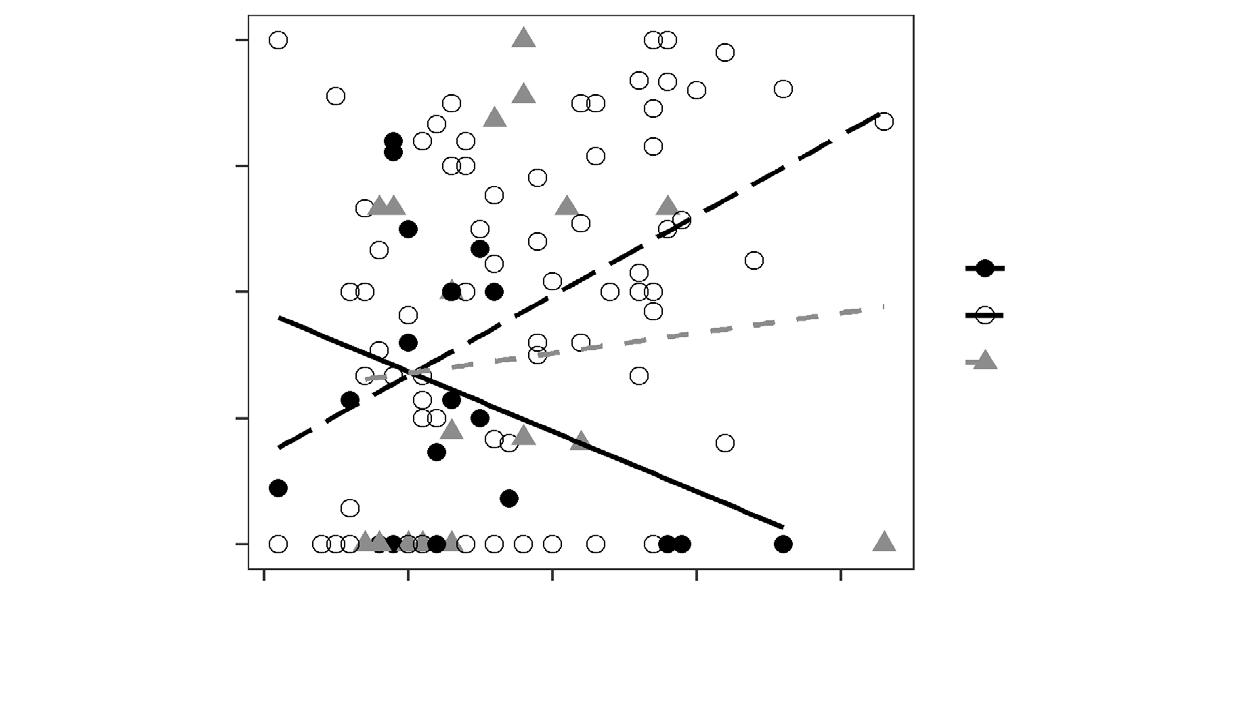
Fig. 1. Relación entre la proporción de geométridos obtenidos por las hembras y la frecuencia de aprovisionamiento de los machos (/h), mediada por el comportamiento exploratorio de las hembras (EB PC1: hembras rápidas, círculos negros, línea continua (EB > SD); hembras lentas, triángulos grises, línea discontinua con trazos cortos (EB < SD); hembras intermedias, círculos vacíos, línea discontinua con trazos largos; SD, desviación estándar.
Female Tortricids (%)
Female Geometrids (%) 1.00 0.75 0.50 0.25 0.00 0 10 20 30 40
Male provisioning rate
Female PC1 Fast (> SD) Average Slow (< SD)
Fig. 2. Relationship between the proportions of tortricids brought to the nest by females and the male provisioning frequency, mediated by female exploratory behaviour (EB PC1): fast females, black circles, continuous line (EB > SD); slow females, grey triangles, short–dashed line (EB < SD); intermediate females, empty circles, long–dashed line; SD, standrad deviation.
Fig. 2. Relación entre la proporción de tortrícidos llevados al nido por las hembras y la frecuencia de aprovisionamiento de los machos, mediada por el comportamiento exploratorio de las hembras (EB PC1): hembras rápidas, círculos negros, línea continua (EB > SD); hembras lentas, triángulos grises, línea discontinua con trazos cortos (EB < SD); hembras intermedias, círculos vacíos, línea discontinua con trazos largos; SD, desviación estándar.
292 Velasco et al.
Table 3. Results of model averaging the equivalent best–ranked GLMs (ΔAIC ≤ 2) relating female caterpillar prey choice to exploratory behaviour and extrinsic variables. Parameter estimates and their corresponding unconditional standard errors (USE) were obtained through model averaging of the best ranked equivalent models (ΔAIC ≤ 2; see table 4s in supplementary material): E, estimate; EB, exploratory behaviour; P.Freq., provisioning frequency; – indicate variables that were included in the analysis but that failed to appear within the best ranked equivalent models. Values in bold highlight significant variables (unconditional 95 % confidence interval (CI) does not include zero).
Tabla 3. Resultados de promediar los modelos lineales generalizados equivalentes al mejor modelo (ΔAIC ≤ 2) que relacionan la elección de orugas por las hembras con el comportamiento exploratorio de estas y con variables extrínsecas. Las estimaciones de los parámetros y sus correspondientes errores estándar (USE) no condicionales se obtuvieron calculando el promedio de los modelos equivalentes al mejor modelo (ΔAIC ≤ 2; véase la tabla 4s del material suplementario): E, estimación; EB, comportamiento exploratorio; P.Freq., frecuencia de aprovisionamiento; – indican variables que se incluyeron en el análisis pero que no aparecieron dentro de los modelos equivalentes mejor clasificados. Los valores en negrita resaltan las variables significativas (el intervalo de confianza (IC) incondicional del 95 % no incluye el cero).
E ± USE Σω(AIC) 95 % CI
E ± USE Σω(AIC) 95 % CI
♀ Noctuid caterpillars (%)
Area: Val 0.45 ± 0.47 0.303 –0.46, 1.37
Brood size –0.01 ± 0.04 0.051 –0.09, 0.08
♀ EB PC1 –0.01 ± 0.07 0.044 –0.16, 0.13
♀ EB PC2 – – –
♀ Age: > 1 year 0.03 ± 0.20 0.045 –0.36, 0.42
Relative lay date 0.07 ± 0.02 0.356 0.02, 0.11
♂ P.Freq. 0.00 ± 0.01 0.060 –0.03, 0.03
Year: 2019 –2.04 ± 0.49 0.356 –3.01, –1.08
♀ Geometrid caterpillars (%) ♀ Tortricid caterpillars (%)
Area: Val –0.19 ± 0.42 0.184 –1.01, 0.63 0.10 ± 0.41 0.000 –0.71, 0.91 Brood size 0.02 ± 0.07 0.081 –0.11, 0.15 – – –
♀ EB PC1 –0.64 ± 0.42 0.378 –1.51, 1.07 0.42 ± 0.34 0.193 –0.23, 1.11
♀ EB PC2 0.43 ± 0.29 0.127 –0.13, 0.99 –0.27 ± 0.26 0.162 –0.77, 0.23
♀ Age: > 1 year – – – –0.26 ± 0.48 0.050 –1.20, 0.67 Relative lay date – – – –0.05 ± 0.02 0.162 –0.10, –0.01
♂ P.Freq. –0.06 ± 0.04 0.378 –0.14, 0.02 0.04 ± 0.03 0.156 –0.02, 0.10
Year: 2019 1.73 ± 0.73 0.378 0.29, 3.16 1.35 ± 0.45 0.162 0.47, 2.23
♀ EB PC1*
♂ P.Freq. 0.06 ± 0.03 0.378 0.00, 0.11 0.02 ± 0.01 0.156 –0.04, –0.00
raging, EB PC1 had certain relevance in both models (table 6): (1) slower–exploring males had marginally higher provisioning rates than faster–exploring males, and (2) slower–exploring males tended to bring smaller caterpillars than faster–exploring ones.
Brood size related significantly to the provisioning rate in both sexes, indicating that birds with larger brood sizes had a higher number of visits per hour (tables 5, 6). Females with higher provisioning rates brought significantly smaller caterpillars (table 5). Caterpillar volume was significantly related consistently across sexes to the relative laying date and year of
the study. For both males and females, prey volume was larger in later broods than in earlier ones and in 2018 than in 2019 (tables 5, 6).
Discussion
This study presents evidence of how provisioning and foraging strategies in blue tits differ between sexes. Female with opposite EB phenotypes adapted differently to their partner’s provisioning behaviour by modifying their caterpillar prey choice. Males' EB was
Animal Biodiversity and Conservation 45.2 (2022) 293
Table 4. Results of model averaging the equivalent best–ranked GLMs (ΔAIC ≤ 2) relating male caterpillar prey choice to exploratory behaviour and extrinsic variables. Parameter estimates and their corresponding unconditional standard errors (USE) were obtained through model averaging of the best ranked equivalent models (ΔAIC ≤ 2; see table 5s in supplementary material): E, estimate; EB, exploratory behaviour; P.Freq., provisioning frequency; – indicate variables that were included in the analysis but that failed to appear within the best ranked equivalent models. Values in bold highlight significant variables (unconditional 95 % confidence interval (CI) does not include zero).
Tabla 4. Resultados de promediar los modelos lineales generalizados equivalentes al mejor modelo (ΔAIC ≤ 2) que relacionan la elección de presas a nivel de orden por los machos con el comportamiento exploratorio de estos y con variables extrínsecas. Las estimaciones de los parámetros y sus correspondientes errores estándar (USE) no condicionales se obtuvieron calculando el promedio de los modelos equivalentes al mejor modelo (ΔAIC ≤ 2; véase la tabla 5s del material suplementario): E, estimación; EB, comportamiento exploratorio; P.Freq., frecuencia de aprovisionamiento; – indican variables que se incluyeron en el análisis pero que no aparecieron dentro de los modelos equivalentes mejor clasificados. Los valores en negrita resaltan las variables significativas (el intervalo de confianza (IC) incondicional del 95 % no incluye el cero).
E ± USE Σω(AIC) 95 % CI
E ± USE Σω(AIC) 95 % CI
♂ Noctuid caterpillars (%)
Area: Val – – –
Brood size –0.01 ± 0.05 0.051 –0.10, 0.08
♂ EB PC1 – – –
♂ EB PC2 0.04 ± 0.13 0.062 –0.22, 0.30
♂ Age: >1 year 0.08 ± 0.30 0.072 –0.51, 0.67
Relative lay date 0.09 ± 0.02 0.349 0.04, 0.13
♀ P.Freq. 0.00 ± 0.01 0.047 –0.03, 0.02
Year: 2019 –1.95 ± 0.49 0.349 –2.90, –0.99
♂ Geometrid caterpillars (%)
♂ Tortricid caterpillars (%)
Area: Val –1.26 ± 0.50 0.335 –2.23, –0.28 0.51 ± 0.47 0.321 –0.40, 1.43
Brood size 0.07 ± 0.10 0.189 –0.13, 0.27 – – –
♂ EB PC1 0.01 ± 0.06 0.023 –0.11, 0.13 –0.01 ± 0.06 0.043 –0.14, 0.12
♂ EB PC2 –0.02 ± 0.10 0.052 –0.21, 0.18 –0.04 ± 0.13 0.104 –0.30, 0.23
♂ Age: > 1 year – – – – – –
Relative lay date 0.00 ± 0.01 0.023 –0.02, 0.01 –0.07 ± 0.02 0.376 –0.11, –0.02
♀ P.Freq. –0.01 ± 0.03 0.094 –0.06, 0.04 0.00 ± 0.02 0.040 –0.03, 0.04
Year: 2019 –0.22 ± 0.42 0.216 –1.05, 0.60 1.27 ± 3.53 0.376 1.27, 3.53
weakly related to prey choice, but gained importance in provisioning frequency and prey volume models. In addition to this, this study presents significant differences between the foraging habits of the sexes. These results contrast with previous research on blue tits that failed to detect differences in prey choice between males and females (Blondel et al., 1991; García–Navas et al., 2013). We found that males provisioned their offspring more frequently and brought overall a higher proportion of spiders and geometrid caterpillars than females. This can be due to differences in the exploratory behaviour between sexes described in previous research (Velasco et al.,
2022). A plausible explanation is that our slower–exploring males allocate less time to 'fast–associated' behaviours (such as male–male aggressiveness or nest defence; Schuett et al., 2010; Stuber et al., 2013), consequently increasing their parental care (Boon et al., 2007).
This study presents evidence of how EB links to offspring provisioning strategies and, consequently, to foraging strategies and prey choice. The results show that the relationship between EB and foraging behaviour differs between sexes, and in the case of females, this link was mediated by the provisioning frequency of their breeding male. Schuett et al. (2010)
294 Velasco et al.
Table 5. Results of model averaging the equivalent best–ranked GLMs (ΔAIC ≤ 2) relating female provisioning effort to exploratory behaviour and extrinsic variables. Parameter estimates and their corresponding unconditional standard errors (USE) were obtained through model averaging of the best ranked equivalent models (ΔAIC ≤ 2; see table 6s in supplementary material): E, estimate; EB, exploratory behaviour; P.Freq., provisioning frequency; – indicate variables that were included in the analysis but that failed to appear within the best ranked equivalent models. Values in bold highlight significant variables (unconditional 95 % confidence interval (CI) does not include zero).
Tabla 5. Resultados de promediar los modelos lineales generalizados equivalentes al mejor modelo (ΔAIC ≤ 2) que relacionan el esfuerzo de aprovisionamiento de las hembras con el comportamiento exploratorio de estos y con variables extrínsecas. Las estimaciones de los parámetros y sus correspondientes errores estándar (USE) no condicionales se obtuvieron calculando el promedio de los modelos equivalentes al mejor modelo (ΔAIC ≤ 2; véase la tabla 6s del material suplementario): E, estimación; EB, comportamiento exploratorio; P.Freq., frecuencia de aprovisionamiento; – indican variables que se incluyeron en el análisis pero que no aparecieron dentro de los modelos equivalentes mejor clasificados. Los valores en negrita resaltan las variables significativas (el intervalo de confianza (IC) incondicional del 95 % no incluye el cero).
E ± USE Σω(AIC) 95 % CI
♀ Provisioning rate (visits/h)
E ± USE Σω(AIC) 95 % CI
♀ Average caterpillar volume (cm3)
Area: Val 0.07 ± 0.10 0.128 –0.26, 0.12 0.06 ± 0.09 0.195 –0.24, 0.13 Brood size 0.12 ± 0.04 0.343 0.04, 0.19 0.02 ± 0.04 0.195 –0.06, 0.11
♀ EB PC1 – – – – – –
♀ EB PC2 0.05 ± 0.11 0.050 –0.17, 0.28 – – –
♀ Age: >1 year – – – – – –
Relative lay date –0.01 ± 0.01 0.102 –0.02, 0.01 0.02 ± 0.01 0.414 0.00, 0.03
Year: 2019 – – – –0.31 ± 0.10 0.414 0.11, 0.5
♀ Prey volume –2.21 ± 0.98 0.343 –4.13, –0.28
suggest that female mate choice promotes consistency in male behavioural traits (confirmed in our study population; Velasco et. al., 2022) because this facilitates females to adapt to the predictable behaviour of their mate (Sanz et al., 2000). This agrees with the results presented in this study: females selected their prey based on the provisioning frequency of their breeding pair, mediated by their own EB.
In our population, the frequency of male provisioning was unrelated to the rate of female provisioning, but it affected the female’s prey choice, mediated by female EB. When males had a low provisioning frequency, slower–exploring females brought a lower proportion of tortricids than faster–exploring females (fig. 2), but the opposite is observed for the proportion of geometrids (fig. 1). Faster–exploring females provided a higher proportion of geometrids and a lower proportion of tortricids when they paired with a high–frequency provisioning male than when their mate had a low–frequency of visits. Meanwhile, average– and slow–exploring females obtained higher proportions of tortricids when mating with a male with high provisioning rates compared to females with this same EB phenotype that pair with a low–frequency provisioning male. Thus, when mating with inattentive males, why do slower females provide a higher
proportion of geometrid caterpillars? and why do faster females do the opposite and provide a higher proportion of tortricids? Naef–Daenzer et al. (2000) and García–Navas et al. (2013) suggest different costs regarding search efforts, associated with different prey types and sizes. Without the interaction between the provisioning behaviour of the males, slower–exploring females bring a higher percentage of geometrid caterpillars to the nest, while faster–exploring females bring a higher proportion of tortricids. Thus, our results suggest that geometrids are the preferred prey of slower females, while tortricids are the preferred item of faster females. When offspring are underfed due to a poor–providing male, females will forage for caterpillars they find easier to find so as to reduce foraging costs (Naef–Daenzer et al., 2000). This difference between females of different behavioural phenotypes suggests a pathway for diet specialization regarding the EB. Agreeing with previous literature, our results indicate that faster– and slower–exploring females differ in foraging strategies (Drent and Marchetti, 1999), and these differences could lead to a disparity between the costs of foraging associated to different EB phenotypes. Exploring the spatial distribution of foraging activities could prove interesting to disentangle time–allocation aspects
Animal Biodiversity and Conservation 45.2 (2022) 295
Table 6. Results of model averaging the equivalent best–ranked GLMs (ΔAIC ≤ 2) relating male provisioning effort to exploration behaviour and extrinsic variables. Parameter estimates and their corresponding unconditional standard errors (USE) were obtained through model averaging of the best ranked equivalent models (ΔAIC ≤ 2; see table 7s in supplementary material): E, estimate; EB, exploratory behaviour; P.Freq., provisioning frequency; – indicate variables that were included in the analysis but that failed to appear within the best ranked equivalent models. Values in bold highlight significant variables (unconditional 95 % confidence interval (CI) does not include zero).
Tabla 6. Resultados de promediar los modelos lineales generalizados equivalentes al mejor modelo (ΔAIC ≤ 2) que relacionan el esfuerzo de aprovisionamiento de los machos con el comportamiento exploratorio de estos y con variables extrínsecas. Las estimaciones de los parámetros y sus correspondientes errores estándar (USE) no condicionales se obtuvieron calculando el promedio de los modelos equivalentes al mejor modelo (ΔAIC ≤ 2; véase la tabla 7s del material suplementario): E, estimación; EB, comportamiento exploratorio; P.Freq., frecuencia de aprovisionamiento; – indican variables que se incluyeron en el análisis pero que no aparecieron dentro de los modelos equivalentes mejor clasificados. Los valores en negrita resaltan las variables significativas (el intervalo de confianza (IC) incondicional del 95 % no incluye el cero).
E ± USE Σω(AIC) 95 % CI E ± USE Σω(AIC) 95 % CI
♂ Provisioning rate (visits/h)
♂ Average caterpillar volume (cm3)
Area: Val 0.10 ± 0.19 0.132 –0.27, 0.47 0.00 ± 0.19 0.043 –0.37, 0.37 Brood size 0.09 ± 0.04 0.440 0.01, 0.17 0.03 ± 0.04 0.477 –0.05, 0.1
♂ EB PC1 –0.04 ± 0.07 0.126 –0.18, 0.11 0.04 ± 0.07 0.347 –0.10, 0.18
♂ EB PC2 0.02 ± 0.10 0.035 –0.16, 0.21 –0.03 ± 0.10 0.111 –0.22, 0.15
♂ Age: >1 year 0.01 ± 0.24 0.031 –0.45, 0.47 –0.09 ± 0.24 0.129 –0.55, 0.37
Relative lay date –0.01 ± 0.01 0.210 –0.03, 0.01 0.02 ± 0.01 0.526 0.01, 0.04
Year: 2019 0.41 ± 0.24 0.440 –0.07, 0.89 –0.73 ± 0.20 0.526 –1.12, –0.34
♂ Prey volume –1.34 ± 1.32 0.440 –3.92, 1.25
related to differences in behavioural traits (Herborn et al., 2010; van Overveld and Matthysen, 2010).
In research performed in our study area a decade ago, blue tits were found to prefer noctuid and tortricid caterpillars (García–Navas and Sanz, 2011; García–Navas et al., 2013), the latter becoming the main prey type during tortricid outbreaks. García–Navas et al. (2013) found no intersexual differences regarding prey preference and provisioning frequency, and found that geometrids were overall underexploited by blue tits. Our results regarding prey choice suggest that, indeed, tortricid caterpillars are exploited in the years they are superabundant (see 2019 data in table 1s in supplementary material), and otherwise, noctuids are commonly preyed upon by both sexes. The difference between our study and previous studies is related to geometrid caterpillars. While females rarely exploit this prey, males obtain a substantial proportion of these caterpillars. We suggest that the shift in the EB described in our previous research (Velasco et al., 2022; males becoming slower explorers) is linked to the shift in their prey choice. A link between behavioural traits and foraging has already been described (Verbeek et al., 1994; Drent and Marchetti, 1999; van Overveld and Matthysen, 2010; Betini and Norris, 2012), and
slower explorers are observed to be more adaptable to changing environments, and to be more likely to explore alternative food resources. Nonetheless, we failed to find a direct relationship between male EB and the proportion of geometrid caterpillars brought to the nest. We consider that, while males in our area are indeed slower explorers than females (Velasco et al., 2022), there is not enough variability within male EB PC1 to conclude that slower–exploring males bring higher percentages of these caterpillars than faster ones. Nonetheless, the case of geometrid caterpillars in our study area is particularly interesting because –unlike tortricids and noctuids– we found that the proportion of preyed geometrid caterpillars was practically unrelated to phenological variables, suggesting an active choice for this type of prey.
Although male EB was part of various models predicting prey choice, the relevance of these terms remained weak throughout the analysis. In the case of males, environmental variables conditioned prey choice more relevantly than EB. Only in the case of tortricid caterpillars did male EB acquire certain relevance, and a tendency (although non–significant) seems to arise: daring males tended to bring higher proportions of tortricid caterpillars. This trend
296 Velasco et al.
also existed for females. It is possible that daring (potentially bolder) individuals tend to have more “straightforward” foraging strategies, and prey more often on caterpillars that are easily detectable and more readily available (Drent and Marchetti 1999; van Overveld and Matthysen, 2010). Lastly, in provisioning effort models (visit rates and prey volume), male EB variables also remained statistically non–significant through model averaging. However, the first principal component of male EB was relevant in the majority of the equivalent top–fit models for prey volume, suggesting that a trend exists in which faster–exploring males are more prone to obtain bigger caterpillars than slower–exploring ones. Slower–explorers are described to invest more in parental care than faster–explorers (Boon et al., 2007). Thus, we suggest that the tendency of faster–explorers provisioning bigger prey may be a compensation mechanism mitigating their decrease in parental attentiveness (lower visit rate), but further research is needed to confirm this.
In conclusion, exploratory behaviour seems to condition foraging strategies differently across sexes. The relationship between parental EB and offspring diet is complex and depends in some cases on the interaction between the members of the breeding pair. Regardless, in blue tits, environmental variables seem to contribute substantially to the diet composition of offspring. While interesting, we cannot suggest that EB is currently leading a diet specialization process in our studied species, although it may contribute to this.
Acknowledgements
Our warmest thanks to Carlos Rodríguez, Ángel Moreno and all the staff at Quintos de Mora. The projects CGL2016–79568–C3–1–P and PID2021–12217NB–I00 (Ministerio de Ciencia e Innovación) contributed to funding this study. Additionally, ACV was financially supported by a pre–doctoral FPI grant (BES–2017–079803; Ministerio de Ciencia e Innovación–European Social Fund). We thank three anonymous reviewers that contributed to improving this paper.
References
Betini, G. S., Norris, D. R., 2012. The relationship between personality and plasticity in tree swallow aggression and the consequences for reproductive success. Animal Behaviour, 83: 137–143, Doi: 10.1016/j.anbehav.2011.10.018
Blondel, J., Dervieux, A., Maistre, M., Perret, P., 1991. Feeding ecology and life history variation of Blue Tit in Mediterranean deciduous and sclerophyllous habitats. Oecologia, 88: 9–14, Doi: 10.1007/ BF00328397
Boon, A. K., Réale, D., Boutin, S., 2007. The interaction between personality, offspring fitness and food abundance in North American red squirrels. Ecology Letters, 10: 1094–1104, Doi: 10.1111/j.14610248.2007.01106.x
Both, C., Dingemanse, N. J., Drent, P. J., Tinbergen, J. M., 2005. Pairs of extreme avian personalities have highest reproductive success. Journal of Animal Ecology, 74: 667–674.
Burnham, K. P., Anderson, D. R., 2002. Model selection and multimodel inference: a practical information–theoretic approach. Springer, New York.
David, M., Pinxten, R., Martens, T., Eens, M., 2015. Exploration behavior and parental effort in wild great tits: partners matter. Behavioral Ecology and Sociobiology, 69: 1085–1095, Doi: 10.1007/ s00265-015-1921-1
Demongin, L., 2016. Identification guide to birds in the hand. Beauregard–Vendon, France.
Dias, P. C., Blondel, J., 1996. Breeding time, food supply and fitness components of Blue Tits Parus caeruleus in Mediterranean habitats. Ibis, 138: 644–649, Doi: 10.1111/j.1474-919X.1996.tb04766.x Dingemanse, N. J., Both, C., Drent, P. J., Tinbergen, J. M., 2004. Fitness consequences of avian personalities in a fluctuating environment. Proceedings of the Royal Society B: Biological Sciences, 271: 847–852, Doi: 10.1098/rspb.2004.2680
Drent, P. J., Marchetti, C., 1999. Individuality, exploration and foraging in hand raised juvenile great tits. In: Proceedings of the 22nd International Ornithological Congress, Durban: 896–914 (N. J. Adams, R. H. Slotow, Eds.). Birdlife South Africa, Johannesburg.
Eguchi, K., 1980. The feeding ecology of the nestling great tit, Parus major minor, in the temperate ever–green broadleaved forest II. With reference to breeding ecology. Researches on Population Ecology, 22: 284–300, Doi: 10.1007/BF02530852
García–Navas, V., Ferrer, E. S., Sanz, J. J., 2013. Prey choice, provisioning behaviour, and effects of early nutrition on nestling phenotype of titmice. Écoscience, 20(1): 9–1, Doi: 10.2980/20-1-3545
García–Navas, V., Sanz, J. J., 2011. The importance of a main dish: nestling diet and foraging behaviour in Mediterranean blue tits in relation to prey phenology. Oecologia, 165: 639–649, Doi: 10.1007/ s00442-010-1858-z
Herborn, K. A., Macleod, R., Miles, W. T. S., Schofield, A. N. B., Alexander, L., Arnold, K. E., 2010. Personality in captivity reflects personality in the wild. Animal Behavior, 79: 835–843, Doi: 10.1016/j. anbehav.2009.12.026
Hinde, C. A., 2006. Negotiation over offspring care? a positive response to partner–provisioning rate in great tits. Behavioral Ecology, 17: 6–12, Doi: 10.1093/beheco/ari092
Marshall, H. H., Carter, A. J., Ashford, A., Rowcliffe, J. M., Cowlishaw, G., 2015. Social effects on foraging behavior and success depend on local environmental conditions. Ecology and Evolution, 5: 475–92, Doi: 10.1002/ece3.1377
Martínez–Zurimendi, P., Rosa y Cubo, E., Domínguez–Domínguez, M., 2011. Defoliadores importantes del quejigo (Quercus faginea Lamck.) en Castilla y León (España). Boletín de Sanidad Vegetal – Plagas, 37: 145–161.
Minderman, J., Reid, J. M., Hughes, M., Denny, M.
Animal Biodiversity and Conservation 45.2 (2022) 297
J. H., Hogg, S., Evans, P. G. H., Whittingham, M. J., 2010. Novel environment exploration and home range in starlings Sturnus vulgaris Behavioral Ecology, 21: 1321–1329, Doi: 10.1093/beheco/arq151
Mutzel, A., Dingemanse, N. J., Araya–Ajoy, Y. G., Kempenaers, B., 2013. Parental provisioning behaviour plays a key role in linking personality with reproductive success. Proceedings of the Royal Society B: Biological Sciences, 280: 20131019, Doi: 10.1098/rspb.2013.1019
Naef–Daenzer, L., Naef–Daenzer, B., Nager, R. G., 2000. Prey selection and foraging performance of breeding Great Tits Parus major in relation to food availability. Journal of Avian Biology, 31: 206–214, Doi: 10.1034/j.1600-048X.2000.310212.x
Nilsson, A. L. K, Nilsson, J–Å, Alerstam, T., Bäckman, J., 2010. Migratory and resident blue tits Cyanistes caeruleus differ in their reaction to a novel object. Naturwissenschaften, 97: 981–985, Doi: 10.1007/ s00114-010-0714-7
R Core Team, 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Réale, D., Dingemanse, N. J., Kazem, A. J., Wright, J., 2010. Evolutionary and ecological approaches to the study of personality. Philosophical Transactions of the Royal Society B: Biological Sciences, 365, 3937–3946, Doi: 10.1098/rstb.2010.0222
Sanz, J. J., Kranenbarg, S., Tinbergen, J. M., 2000. Differential response by males and females to manipulation of partner contribution in the great tit (Parus major). Journal of Animal Ecology, 69: 74–84, Doi: 10.1046/j.1365-2656.2000.00373.x
Schuett, W., Tregenza, T., Dall, S. R. X., 2010. Sexual selection and animal personality. Biological Reviews, 85: 217–246, Doi: 10.1111/j.1469185X.2009.00101.x
Serrano–Davies, E., Araya–Ajoy, Y. G., Dingemanse, N. J., Sanz, J. J., 2017. Personality–related differences in response to habitat in Mediterranean blue tits. Ethology, 123: 861–869, Doi: 10.1111/ eth.12656
Sih, A., Christensen, B., 2001. Optimal diet theory. When does it work, and when and why does it
fail? Animal Behaviour, 61: 379–390, Doi: 10.1006/ anbe.2000.1592
Slagsvold, T., Wiebe, K. L., 2011: Social learning in birds and its role in shaping a foraging niche. Philosophical Transactions of the Royal Society B: Biological Sciences, 366: 969–977, Doi: 10.1098/ rstb.2010.0343
Soria, S., Toimil, F. J., 1983. Fuerte ataque de Erannis defoliaria Clerck. (Lep. Geometridae) en los Montes de Toledo y ensayos de lucha química para su combate. Boletín de Sanidad Vegetal –Plagas, 9: 61–75.
Stuber, E. F., Araya–Ajoy, Y. G., Mathot, K. J., Mutzel, A., Nicolaus, M., Wijmenga, J. J., Mueller, J. C., Dingemanse, N. J., 2013. Slow explorers take less risk: a problem of sampling bias in ecological studies. Behavioral Ecology, 24: 1092–1098, Doi: 10.1093/beheco/art035
Toscano, B. J., Gownaris, N. J., Heerhartz, S. M., Monaco, C. J., 2016. Personality, foraging behavior and specialization: integrating behavioral and food web ecology at the individual level. Oecologia, 182: 55–69, Doi: 10.1007/s00442-016-3648-8
van Overveld, T., Matthysen, E., 2010. Personality predicts spatial responses to food manipulations in free–ranging great tits (Parus major). Biology Letters, 6: 187–190, Doi: 10.1098/rsbl.2009.0764
Velasco, A. C., Ferrer, E. S., Sanz, J. J., 2022. Intersexual differences in the exploratory behaviour of blue tits ( Cyanistes caeruleus ). Behaviour (published online ahead of print 2022). Doi: 10.1163/1568539X-bja10171
Verbeek, M. E. M., Boon, A., Drent, P. J., 1996. Exploration, aggressive behaviour and dominance in pair–wise confrontations of juvenile male Great tits. Behaviour, 133: 945–963, Doi: 10.1163/156853996X00314
Verbeek, M. E. M., Drent, P. J., Wiepkema, P. R., 1994. Consistent individual differences in early exploratory behaviour of male great tits. Animal Behaviour, 48: 1113–1121, Doi: 10.1006/anbe.1994.1344
Zuur, A. F., Ieno, E. N., Walker, N. J., Saveliev, A. A., Smith, G. M., 2009. Mixed effects models and extensions in Ecology with R. Springer, New York.
298 Velasco et al.
The expansion process of the Iberian ibex in the Sierra de Guadarrama National Park, Madrid (Spain)
P. Refoyo, C. Olmedo, A. Murciano Cespedosa, B. Muñoz
Refoyo, P., Olmedo, C., Murciano Cespedosa, A., Muñoz, B. 2022. The expansion process of the Iberian ibex in the Sierra de Guadarrama National Park, Madrid (Spain). Animal Biodiversity and Conservation, 45.2: 299–313, Doi: https://doi.org/10.32800/abc.2022.45.0299
Abstract
The expansion process of the Iberian ibex in the Sierra de Guadarrama National Park, Madrid (Spain). In this paper we explore the usefulness of MaxEnt to predict the most suitable areas for a wildlife species, the Iberian ibex (Capra pyrenaica). For two decades (1990–2010), the species was established in a small part of the National Park Sierra de Guadarrama (Spain) and there has been a process of expansion to other areas of this protected area since 2010. However, almost two decades have elapsed since the modeling methods (MaxEnt) were proposed and no studies have tested their effectiveness using real distribution data, i.e. data from past predictions, to see if they fit the current distribution. We generated a model with presence–only data from 2007 and verified accuracy from 2017 data concerning real presence. Our results show a relationship between models and the species' current presence. The generated model can be useful to define the preferred locations of the species. We detected several differences between males and females of the species. This work not only shows the importance of selecting climatic and ecological variables for the construction of models but also indicates that they must be adjusted, at least for some species, to each sex and period of the year.
Key words: Capra pyrenaica, Iberian Ibex, Distribution area, MaxEnt, Spain
Resumen

El proceso de expansión de la cabra montés en el Parque Nacional de la Sierra de Guadarrama en Madrid (España). En este trabajo estudiamos la utilidad del programa MaxEnt para predecir las zonas más adecuadas para una especie silvestre, la cabra montés (Capra pyrenaica). Durante dos décadas (1990–2010), la especie estuvo establecida en una pequeña parte del Parque Nacional de la Sierra de Guadarrama (España) pero desde 2010 ha seguido un proceso de expansión a otras zonas de este espacio protegido. Sin embargo, ya han pasado casi dos décadas desde que se propuso el uso de estos modelos (MaxEnt) y no hay estudios que aborden su eficacia con valores reales de distribución, esto es, que comprueben si las predicciones del pasado se ajustan a la situación actual real. Hemos generado un modelo solo con los datos sobre presencia obtenidos en 2007 y hemos verificado su precisión a partir de estos datos reales de presencia de 2017. Nuestros resultados muestran una relación entre los modelos y la presencia actual de la especie. El modelo generado nos ha permitido determinar las localizaciones preferidas de la especie. Se han detectado algunas diferencias en función del sexo de los contactos. Este trabajo no solo muestra la importancia de la selección de variables climáticas y ecológicas para la realización de los modelos, sino también que estos modelos deben ajustarse, al menos para algunas especies, en función del sexo y el período del año.
Palabras clave: Capra pirenaica, Cabra montés, Área de distribución, MaxEnt, España
ISSN: 1578–665 X eISSN: 2014–928 X
© [2022] Copyright belongs to the authors, who license the journal Animal Biodiversity and Conservation to publish the paper under a Creative Commons Attribution 4.0 License.
299 Animal
Biodiversity and Conservation 45.2 (2022)
Received: 06 XII 21; Conditional acceptance: 17 I 22; Final acceptance: 21 IX 22
Pablo Refoyo Román, Cristina Olmedo Salinas, Antonio Murciano Cespedosa, Benito Muñoz Araújo, Department of Biodiversity, Ecology and Evolution, Faculty of Biological Sciences, Universidad Complutense de Madrid, c/ José Antonio Novais 12, E–28040 Madrid, España (Spain).– A. Murcian Cespedosa, Modeling, Data Analysis and Computational Tools for Biology Research Group, Complutense University of Madrid, Madrid, Spain. Neurocomputing and Neurorobotics Research Group, Complutense University of Madrid, Madrid, Spain. Brain Plasticity Group, Health Research Institute of the Hospital Clínico San Carlos (IdISSC), Madrid, Spain.
Corresponding author: Pablo Refoyo Román. E–mail: pa.refoyo@bio.ucm.es
ORCID ID: P. Refoyo Román: 0000-0002-9566-9297
300 Refoyo et al.
Introduction
The Iberian ibex (Capra pyrenaica, Schinz, 1838) is an endemic wild Artiodactylan of the Iberian Peninsula that frequents areas with outcropping rock associated with several types of natural vegetation and some isolation. It is associated with rocky areas with mountain and subalpine vegetation in the Iberian Peninsula, ranging from alpine meadows to wooded and scrubland areas (Granados et al., 2007; Refoyo, 2012).
In the mid–nineteenth century the species showed a strong decline, both nationally and locally (Alados and Escos, 1995; Soriguer et al., 1998), possibly due to competition with other ungulates, habitat destruction and human pressure (Pérez et al., 2002; Acevedo and Cassinello, 2009). However, in recent decades, its interest as a game species led to its reintroduction in other Spanish mountain ranges, facilitating its expansion (Fonseca et al., 2017).
These restockings, however, have not been conducted following international criteria. Nor have they been carried out using habitat suitability studies or specific tools to determine the adequacy of the environment for the species (Refoyo, 2012). Knowing parameters such as land cover, antrophic influence, flora and fauna diversity is essential as such factors affect the species' relationship with the environment (Odum, 1986; Hui, 2006).
A good method to characterize these ecological niches is to assess correlations between a dependent variable, defined by the distribution of the species (presence/absence), and to select independent variables using binomial logistic regression (Elith and Burgman, 2002; Anadón et al., 2007). Many studies have established the potential of a territory using presence and absence data (Moisen and Frescino, 2002; Segurado and Araújo, 2004; Higgins et al., 2017). This method has been used in many studies related to plants (Zimmermann and Kienast, 1999), birds (Suárez–Seoane et al., 2004), mammals (Jaberg and Guisan, 2001), reptiles (Guisan and Hofer, 2003), invertebrates (Maggini et al., 2002) and diseases (Wint et al., 2002).
However, conducting this type of analysis is often limited by a lack of information (absence data) (Broms et al., 2014), especially in large areas. As a result, the current modelling techniques often used (SDM) require presence–only data (data that are abundant in official databases) (Converse et al., 2013; Gedir et al., 2013; Weber et al., 2017) and MaxEnt is considered one of the best of these modelling techniques for developing distribution models (Hernández et al., 2006; Palialexis et al., 2011; Magarey et al., 2017). Such models make it possible to establish the suitability of the territory for a particular species on the basis of the known presence data (precise locations) and the values of variables that characterize this presence (predictor variables), and also to establish the places that, in other areas of the territory, show a certain degree of similarity to these conditions.
The use of the species distribution model (SDM) (Guisan and Zimmermann, 2000; Mateo et al., 2011) is an increasingly popular tool (Elith and Leathwick,
2009; Ferrer–Sánchez et al., 2017; Wu et al., 2018).
It is even used in game species (Vargas et al., 2007; Acevedo et al., 2010; Yongyut et al., 2012), as in the case of the Iberian ibex (Capra pyrenaica) (Acevedo et al., 2007; Refoyo et al., 2014).
The use of SDM provides the possibility to conduct studies in both conservation and basic science that are difficult, and sometimes impossible, to address with other methods (Warren and Seifert, 2011). Even so, previous studies have also shown that many factors could affect the performance of SDM, such as the size and spatial biases of sampling data, algorithms (Wisz et al., 2008; Phillips et al., 2009; Shcheglovitovaa and Andersona, 2013) and thresholds used (Liu et al., 2005; Nenzen and Araujo, 2011; Bean et al., 2012) and, in particular, over–adjustment (Wenger and Olden, 2012). One of the assumptions of the SDMs is that the data used for model calibration are free of bias. However, this is never the case, especially in data collection (Fourcade et al., 2014; Tessarolo et al., 2014). Although there are statistical techniques to correct these errors and determine the robustness of the model (Warren and Seifert, 2011; Liu et al., 2015) –and they are good statistical approximations– the best way to determine the efficiency of the models is to check with real data after a certain period of time. Although almost two decades have passed since the use of these models was proposed (Guisan and Zimmermann, 2000) no study has yet related the actual values of species occurrence with the proposed distribution models. The species' low speed of dispersion and the difficulty to perform these distribution studies in areas other than those of origin of the data make this work difficult (Tinoco–Torres et al., 2014; Fonseca et al., 2017). Nevertheless, Iberian ibex were reintroduced in the Sierra de Guadarrama National Park (Madrid, Central Spain) in the 1990s and their demographic trend and distribution has been monitored, allowing us to validate these models with real data. Until 2007 and for several decades, the population showed an increase in a limited area of the Park (reintroduction zone) and there was no dispersion to other areas of the Park. However, since then, the population has occupied other areas (Refoyo et al., 2015, 2016). Here we analysed whether the distribution models generated from data concerning the species in 2007 (based on data collected in a survey carried out in 2007) fit the current distribution of the species (2017) in this National Park since its expansion.
To achieve our goal, we: i) estimated the suitable areas for the distribution of Iberian Ibex using presence–only distribution data (2007); ii) tested the model with data from 2017; and iii) analyzed whether there are differences in the models generated due to different behaviour between the sexes during breeding.
Material and methods
Study area
The study was carried out in the Community of Madrid (Spain) where there is an Iberian ibex population in
Animal Biodiversity and Conservation 45.2 (2022) 301
the northern region, in the National Park of Sierra de Guadarrama (25,317 ha). The park has a continental climate with large temperature variations between seasons and very dry summers (fig. 1). The vegetation includes shrubs (Cytisus purgans, Juniperus communis nana) and grassland (Festuca indigesta, Nardus stricta, Festuca rubra) in highland areas, Mediterranean shrubs (Cistus ladanifer, Rosmarinus officinalis, Thymus vulgaris, Lavandula stoechas) in the steeply sloped areas, and forests (of Quercus ilex, Quercus pyrenaica, Pinus spp.) in the valleys, and on hillsides.
Although it would be of interest to study the presence of the mountain goat throughout the whole area of the Sierra de Guadarrama National Park, reliable data using systematic procedures are scarce and digital layers of the variables used (specifically vegetation and rock cover) for modelling on the northern slope (Castilla y León) are lacking, ruling out out the possibility to generalise the study with the necessary robustness for the entire park.
Generation of models
Dependent variable
Data used in this study consisted of 97 records from the sampling conducted in 2007 in 4,590 ha within the National Park of Sierra de Guadarrama (table 1, fig. 2.) where previous studies (2000, 2003, 2005 and 2007) determined the presence of the species (Refoyo, 2012). Eight people walked along 22 transects of an average length of 3.64 km between May 19th and June 12th to obtain the data by means of direct observation (Refoyo et al., 2015) using the distance sampling method (Buckland et al., 1993). For each contact, we recorded the habitat, the number of animals composing the group, sex, age of individuals (using 8 x 40 to 10 x 50 binoculars), and the perpendicular distance to the transect line using a laser distance meter (Bushnell Yardage Pro Sport). All transects were sampled on successive and climatically suitable days, either in the morning (2–3 hours after sunrise) or afternoon (2–3 hours before sunset) (Refoyo et al., 2015).
Predictor variables
The presence of rocky places and cliffs in association with the various types of natural vegetation and certain altitudes seem to be essential conditions for the species. Nevertheless, some populations were observed at sea level while others appeared to prefer to be far from areas with high densities of infrastructure and human activities (Refoyo, 2012). Although the Iberian ibex does not seem to have a preference for any specific vegetation, some variability in the trophic resources available seems necessary (Refoyo, 2012).
The selection of variables was based on the criterion established in Refoyo (2012) and Olmedo et al. (2016):
Altitude (continuous variable): this variable was obtained from a digital elevation model (DEM) produced by the Spanish National Geographic Institute.
Vegetation/stoniness (categorical variable): to determine the trophic availability and the availability of outcrops for the species, we used a digital layer designed by the Department of Environment and Spatial Planning of Madrid. This layer considers both the vegetation present at a place (land use and type of vegetation) and the percentage of existing rock outcropping.
Roughness (continuous variable): roughness is described as the variation in three–dimensional orientation of grid cells within a neighborhood. This method effectively captures variability in slope and aspect into a single measure. Roughness values in the output raster can range from 0 (no terrain variation) to 1 (complete terrain variation). This layer was made from a digital mapping of slope angles and orientation following the method of Felicísimo (1994), who defined it as: 'the uniformity of the unitary vectors perpendicular to the surface in each cell and in those of the environment, given by the value of the module of the vector sum of those'. Where xi, yi and zi are the rectangular coordinates of the unitary vector perpendicular to the surface at point i, their expressions as a function of slope and orientation, both in degrees, are:
xi = sin(Pi) x cos(Pi) yi = sin(Pi) x sin(Pi) zi = cos(Pi)
For a set of n vectors, the direction of the resulting vector coincides with the mean vector of the n vectors, a measure of dispersion between the different vectors being the value of the normalized modulus of its sum (R). R takes values between 0 and 1: a value of 1 indicates total dispersion and a value of 0 indicates null dispersion.
Anthropogenic influence (continuous variable): a new digital layer was created based on the isolation degree of the territory. The entire study area (Community of Madrid) was divided into grids of 20 x 20 m and the distance between each of these squares was calculated to the nearest linear infrastructure (digital linear infrastructure mapping of the Madrid government).
The values of these variables in all 97 presence data were obtained by the intersect point tools via GIS (Hawth's analysis tools).
Predictive modelling
The MaxEnt modelling approach is a discriminant technique that makes predictions or inferences from presence only data and estimates the probability of species presence. It also seeks a probability distribution that is as uniform as possible (maximum entropy) under the assumption that the expected value of each information layer must approach its empirical average (entropy) (Phillips and Shapire, 2004). Unlike the previous model, there is no need to enter the absence data into the program because it generates a number of randomly selected (pseudo–absences) observations (Mateo et al., 2011). To perform this analysis, we used the same predictor variables and 97 records from the sampling conducted in 2007 (Refoyo et al., 2015).
302 Refoyo
et al.
Fig. 1. Study area. The area of study in 2017 corresponds to the entire area of the National Park on its Madrid side while the 2007 census area corresponds to the area of presence of the species within the National Park in 2007.
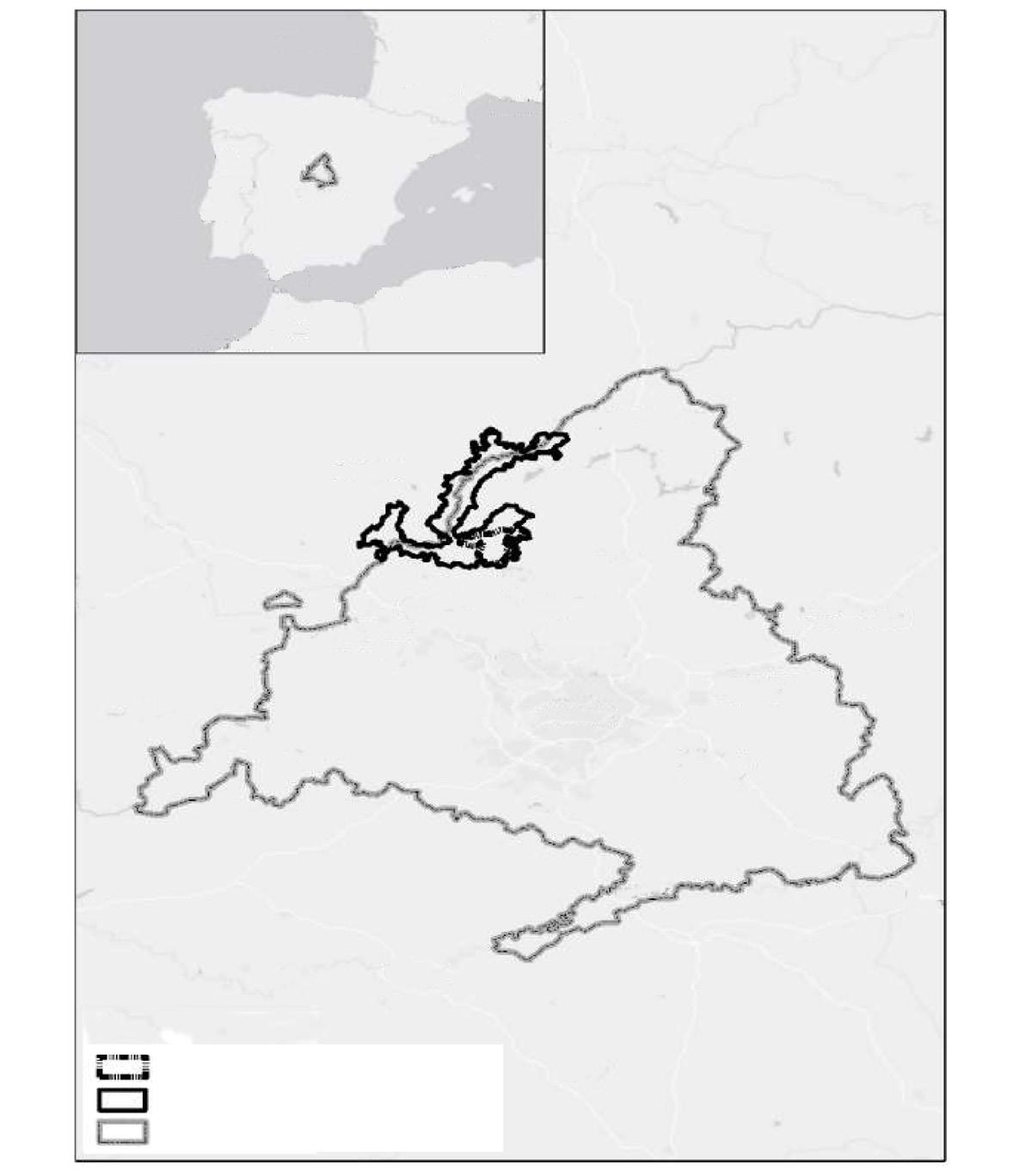
Fig. 1. Área de estudio. El área de estudio de 2017 corresponde a toda la superficie del Parque Nacional en su vertiente madrileña, mientras que el área de censo de 2007 corresponde a la zona de presencia de la especie dentro del Parque Nacional en 2007.
For running MaxEnt models (Version Maxent 3.4.2), the following default parameters were used: a maximum number of 500 iterations; a convergence–threshold limit of 0.00001 and 10,000 points as the number maximum of background points; and a regularization multiplier equal to 1 (Phillips et al., 2006). Ten replicates were run for each model to assess the influence of data selection on the randomization, and the final model was constructed with the average of these replicates. In all cases, 30 % of the records from the samples were randomly removed to be used as test points (i.e. a random sample was taken from the species presence localities in order to measure the quality of the model), and the remaining 70 % of records was used to build the model.
Additionally, the ROC–AUC technique was used to analyse the goodness of the MaxEnt analysis in contrast to other assessment models, since it avoids the problem of selecting threshold values (Lehmann et al., 2002) and is also capable of measuring the model’s
ability to discriminate between sites according to their species suitability (Fielding and Bell, 1997; Engler et al., 2004; Elith et al., 2010).
The data obtained were used to generate a suitable map of the territory with GIS tools (ArcGIS 10.0) (fig. 2).
Validation of the models
Obtainment of the current presence data: the monitoring studies were performed in an area of 25,317 ha (fig. 1). The Park has a marked difference in altitude, ranging from 900 m to 2,428 m, alternating very steep rocky areas with areas of gentle topography.
The population was monitored from the 6th to the 15th of June, 2017 (table 1) by direct observation of the animals along 48 transects with an average length of 4.5 km and a total length of 218.6 km using the distance sampling method (Buckland et al., 1993). All transects were sampled on successive and climatica-
Animal Biodiversity and Conservation 45.2 (2022) 303
Madrid Toledo
Guadalajara
Spain Portugal Study area 2007 National Park limit Madrid Region limit
Segovia
Table 1. Total contacts, number of individuals, and average group size both for the total population and by sex for 2007 and 2017 records: Nc, number of contacts; Av, average group size; Ns, number of specimens.
Tabla 1. Número de contactos, número de individuos y tamaño medio de grupo, tanto para el total poblacional como por sexo para los registros de 2007 y 2017.
2007 2017
Nc Av Ns Nc Av Ns Nc
Total 97 3.7 354 125 6.2 771 Males 19 7.7 144 54 8.7 474 Females 78 2.7 210 71 4.2 297
lly suitable days, either in the morning (2–3 h after sunrise) or afternoon (2–3 h before sunset). For each contact, we recorded the total number of ibex and the total number for each sex using 8 x 40 to 10 x 50 binoculars (Refoyo et al., 2015).
Analysis model: we obtained 120 records. Each record was georeferenced and included the total number of individuals, the total number of males, and the total number of males and females. We obtained the suitability values (MaxEnt) (model generated by 2007 records) using the intersect point tools (Hawth's tools). These tools can assign the values of digital layers (territory suitability generated with Maxet from the 2007 contacts) to a layer of points (Contacts, 2017) (fig. 2).
The group size for each contact was associated with the suitability models using a simple regression analysis for the total number of individuals and the total number by sex. With this procedure and for each point of presence of the species, we obtained four variables: the suitability value of the terrain, the total number of specimens, the total number of males, and total number of females. All four variables had a normal distribution. Linear regression revealed suitability of the territory as the dependent variable, and the total number of goats, the total number of males and the total number of females as the independent variables. Quantile regression (Koenker and Bassett, 1978) was performed to assess changes near the upper limit of the distribution (Huston, 2002; Carrascal et al., 2017) according to the group sizes detected. In addition, we calculated the Moran index to determine the possible spatial autocorrelation of the contacts. We also carried out the same analyses considering that values are appropriate areas (index > 0.7) and not adequate (index < 0.7). All statistical analyses were performed using Statistica 7.0 (StatSoft Inc., Tulsa, OK, USA) and STATA 15 (StataCorp LLC, USA).
Results
The ROC curve produced by MaxEnt indicates a high accuracy of the model since the data analysis generated an area under the curve of 0.976 (fig. 3), a value that is above the optimum threshold (0.8) (Phillips et al., 2006). The variables of altitude, anthropogenic influence, and vegetation/stoniness were relevant for the presence of the species (fig. 4).
The regression analyses showed a relative relationship between the model and the current presence of the species, (n = 133; r = 0.20; p = 0.02) (fig. 5A). If we consider the distribution of the records by sex, males showed a better relationship with the model (n = 67; r = –0.24; p = 0.004) (fig. 5B) than females (n = 78 r = –0.04; p = 0.59) (fig. 5C). Regression by quantiles shows that for the 95% quantile the relationship with the model improved considerably, especially for males (p = 0.018; R2 = 0.16) and total Ibex (p < 0.001; R2 = 0.18). For females however, it remained at low levels (p > 0.05; R2 = 0.03), although they were higher than in the previous analysis. Considering the 90% quantile, although the relationship improved for total Ibex (p = 0.007; R2 = 0.09), it did not improve for males and females (fig. 6). Although we did not detect any spatial autocorrelation between contacts, for female groups located in the 2017 samples this autocorrelation was marginally significant (table 2).
According to these data, when we consider suitability values higher than 0.5, the model is significant only for males (F(1;131) = 4.7929; p = 0.0303) while for females, the most suitable values are those with the lowest values (F(1;131) = 4.4351; p = 0.0371). As a result, the global value is not significant (total ibex: F(1;131) = 1.2467; p = 0.2662) (fig. 7).
The results are significant both for the total Ibex population (F(1;131) = 8.0127; p = 0.0054) and for the total male population (F(1;131) = 11.598; p = 0.0009) only when we consider suitability values higher than 0.7. They are not significant for the total female population (F(1;131) = 0.7502; p = 0.3880) (fig. 8).
Discussion
Here we verified that the distribution of Iberian ibex can be accurately assessed using presence–only data. Our findings emphasise the importance of considering not only environmental variables but also population variables, such as sex, as this allows us to generalize such studies regarding the reintroduction of a species.
MaxEnt allows working presence–only data and is considered one of the best methods for species distribution models (Hernández et al., 2006; Palialexis et al., 2011). It is useful for numerous works based on the use of occurrence data (bibliographic records, museums and herbaria databanks) (Suárez–Seoane et al., 2008; Cano et al., 2014; Wu et al., 2018).
Our results indicate that when no data are absent, MaxEnt can infer the distribution of the species in an acceptable way (Palialexis et al., 2011). The fact that the variables only explain 4 % of the variance (6 % in males and 2 % in females) may be due to the short
304
Refoyo et al.
Spain
Suitable index 0–0.14 0.15–0.5 0.51–0.7 0.71–1
Record 2017 Madrid region limit National Park limit
Fig. 2. Map of the suitability area obtained with MaxEnt (land suitability for the species is indicated in black).
Fig. 2. Mapa del área de idoneidad obtenido con MaxEnt (el color negro muestra la zona adecuada para la especie).
period of time elapsed (seven years), and it is foreseeable that in the near future, individuals will tend to occupy more suitable areas rather than areas close to the core area studied in 2007, thus increasing the R2. It is necessary to keep in mind that the expansion of the species in the National Park started in 2010.
When we analyzed the results according to sex we observed several differences. The model was better adjusted in the case of males than in females. For example, in the areas considered suitable (for suitability values of both 0.5 and 0.7), the male group size was larger than in the areas considered less suitable, indicating that the favourable conditions allow greater grouping of specimens. However, in the case of females, these differences did not appear. The reason for this could be related to the fact that in the breeding season females tend to form smaller family groups with the offspring (graphs 9 and 10).
As indicated by other authors (Huston, 2002; Carrascal et al., 2017), the model fit improves when assessing changes near the upper limit of the distribution according to the detected cluster sizes. When we analyse the 95 % quantile, the regression models
better explain the relationship with territory fitness. However, the fit remains low for females, possibly due to the spatial autocorrelation of contacts and lack of statistical power. The more gregarious behaviour of females, especially in the breeding season, by requiring areas suitable for breeding (Refoyo et al., 2015), causes some spatial autocorrelation for the 2017 data and possibly influences the results. These differences can be explained by the different behaviour of males and females, addressed by many authors in several species. Johnson et al. (2007) stated three reasons for male–biased dispersal and female philopatry in amphibians: avoidance of inbreeding, local mating competition, and local resource competition. Sex–biased migration has also been reported as widespread among vertebrates (Houlahan and Findlay, 2003) and Morelle and Lejeune (2015) established variables such as food resources and thermal and safety cover for the wild boar case.
In our case, the data for the verification of the model (2017) were obtained during the breedingseason when the differences in behaviour between males and females are greatest. While females usually limit
Animal Biodiversity and Conservation 45.2 (2022) 305
=
Fig. 3. ROC curve produced by MaxEnt.

Fig. 3. Curva ROC producida por MaxEnt.
Refoyo et al.
Fig. 4. Jackknife representation of each variable. In light grey the gain of the model without the variable, in black the contribution of the variable to the model, and in dark grey the gain of the models with all the variables.
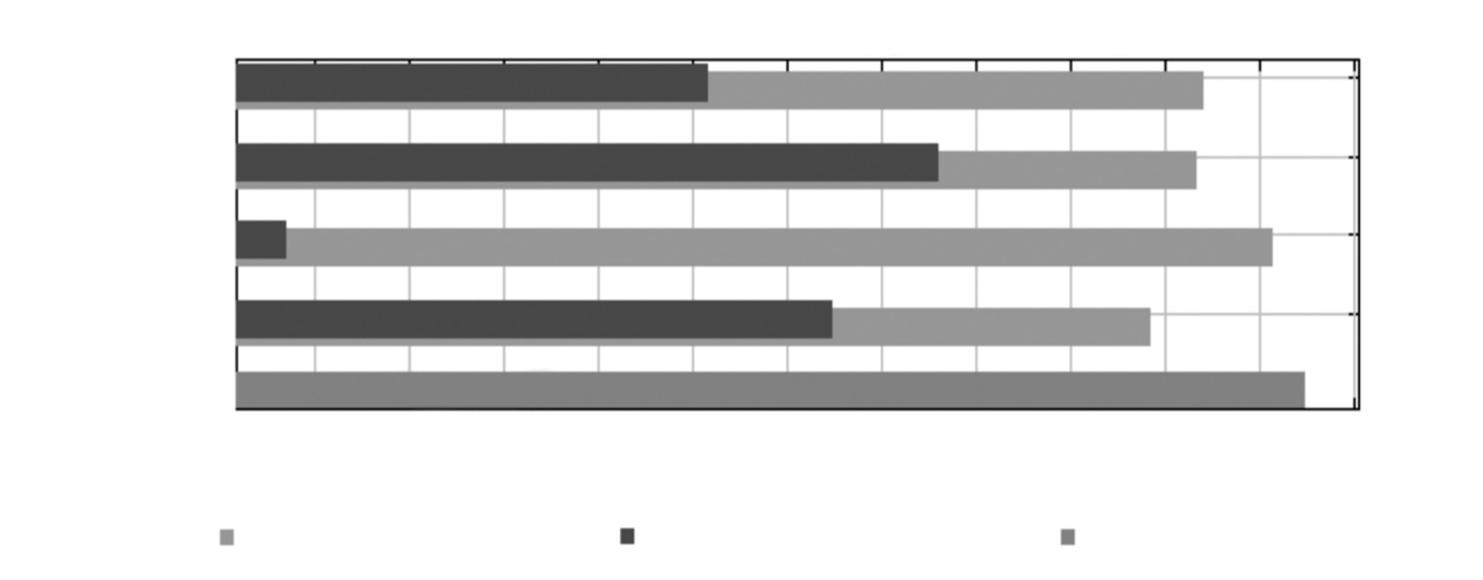
Fig. 4. Representación de Jackknife de cada variable. En gris claro se representa la ganancia del modelo sin la variable; en negro, la contribución de la variable al modelo, y en gris oscuro, la ganancia de los modelos con todas las variables.
their distribution to rocky areas –a variable that was little relevant in the models generated (fig. 4) where offspring protection is easier– we observed that males showed a greater dispersion between the available habitats (Refoyo et al., 2015, 2016).
This result matches those reported by Cao et al. (2013) that focus on habitat suitability rather than climate suitability that reported general over–predictions (Graham and Hijmans, 2006; Dubuis et al., 2011; Guisan and Rahbek, 2011). By including fea-
tures such as altitude, anthropogenic influence, land cover and roughness, our models can be considered better than other models that use climate–suitability only to describe suitable habitats, Nevertheless, the differences detected between sexes imply that models may still be underfitted, not only for the environmental requirements of the species under study but also for the specific characteristics of each sex and the differential behaviour that the species presents at different times of the year.
306
Altitude Roughness Vegetation 1.2 1.4 1.6 1.8 2.0 2.2
0.4
Jackknife of regularized training gain for cabra_madrid
Isolation
2.4 2.6 2.8 3.0 3.2 3.4 Regularized training gain Without variables With only variables With all variables Environmental variables 0.0 0.1 0.2 0.3
0.5 0.6 0.7 0.8 0.9 1.0 Specificity (fractional predicted area) Mean (AUC
0.976) Mean ± one stddev Random prediction Average specificity vs 1 – Specificity for cabra_madrid 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 Sensitivity (1 –omission rate)
A B C
Total ibex Total male
80 70 60 50 40 30 20 10 0 –10 80 70 60 50 40 30 20 10 0 –10
n = 133; r = 0.2006; p = 0.0206 y = 2.9556 + 7.2713 * x
–0.2 0.0 0.2 0.4 0.6 0.8 1.0 1.2 Suitability index
n = 67; r = 0.2484; p = 0.0039 y = –0.8054 + 7.9036* x 35 30 25 20 15 10 5 0 –5
–0.2 0.0 0.2 0.4 0.6 0.8 1.0 1.2 Suitability index
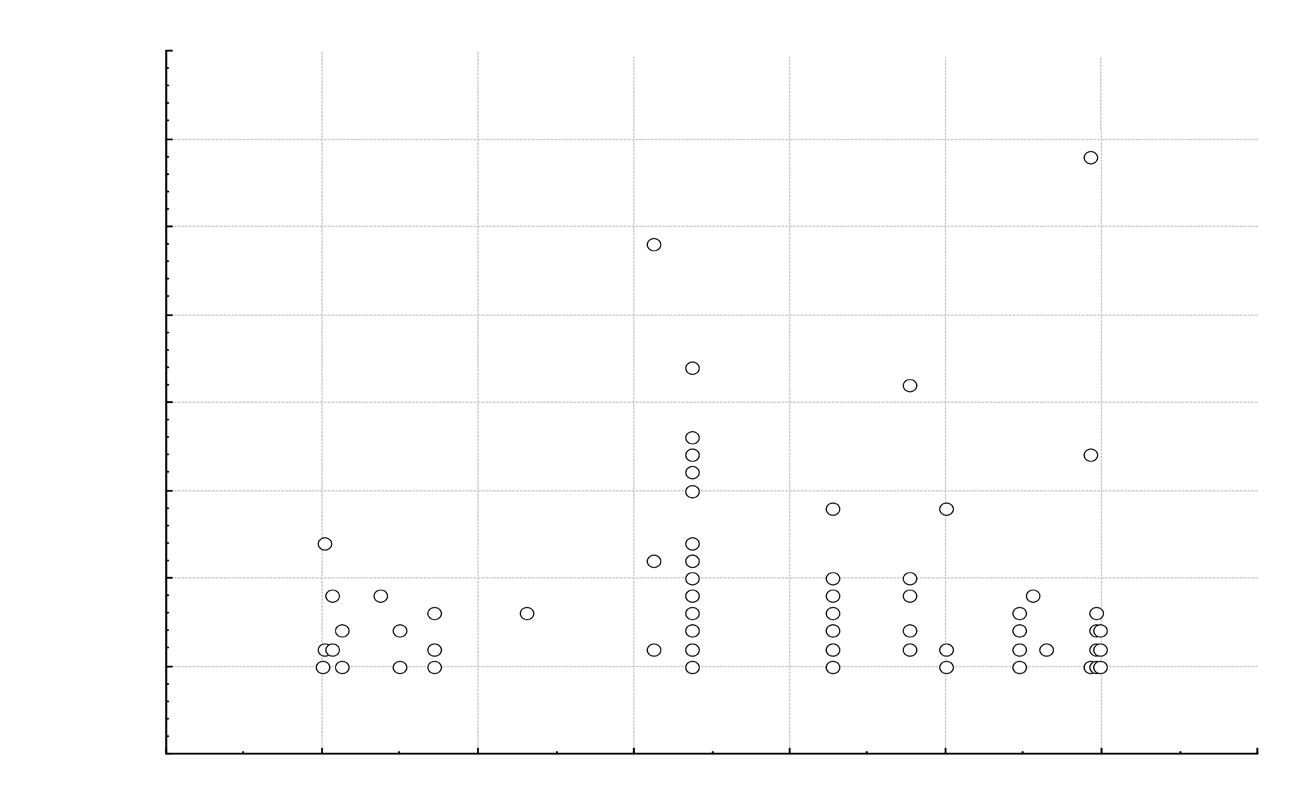
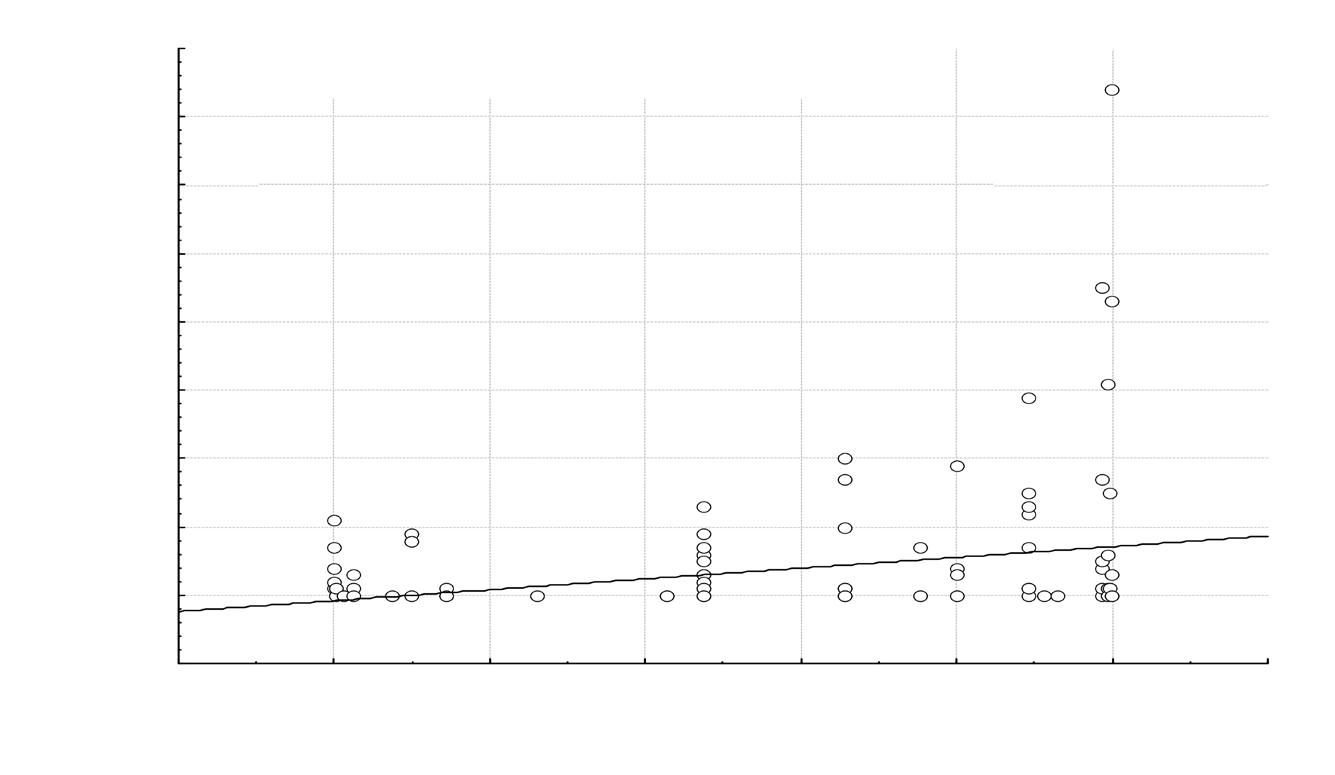

n = 78; r = –0.0467; p = 0.5934 y = 2.9999 + 0.7036* x
Total female –0.2 0.0 0.2 0.4 0.6 0.8 1.0 1.2 Suitability index
Fig. 5. Simple regression between suitable area (MaxEnt) and real presence of ibex (A), of males (B), and of females (C) in 2017.
Fig. 5. Regresión simple entre el área adecuada (MaxEnt) y la presencia real de cabras (A), de machos (B) y de hembres (C) en 2017.
Animal Biodiversity and Conservation 45.2 (2022) 307
Refoyo et al.
Table 2.
Autocorrelation spatial model using Moran’s index for total data and sex data for 2007 and 2017: MI, Moran index; V, variance; P, p–value.
Tabla 2. Autocorrelación espacial utilizando el índice de Moran para los datos totales y desglosados por sexo para 2007 y 2017: MI, índice Moran; V, varianza; P, valor p.
MI V P
Total ibex 2007 0.165 0.05 0.43
Total male 2007 0.165 0.10 0.46
Total female 2007 0.232 0.04 0.23
Total ibex 2017 –0.328 0.04 0.12
Total male 2017 0.157 0.04 0.34
Total female 2017 –0.421 0.04 0.04
It is also of note that the model generated with data of presence–only requires that the threshold
indexes for their consideration are higher than 0.7, as suggested by other works (Tellería et al., 2012; Shartell et al., 2013; Fernández–Marchán et al., 2015). In any case, the MaxEnt model is a powerful tool not only to understand the biology of the species but also to understand management aspects.
In this sense, having a predictive method based only on presence data is particularly useful for works related to the management of a species that is clearly increasing in number, as is the case of the Iberian ibex. Precise knowledge of the variables that characterize the presence of the species and the location of suitable areas is especially useful not only to determine natural processes of a species' expansion. Such knowledge is also often used for introductions of species, such as in hunting, in which case it is essential to know the most suitable areas for the species in order to optimize the available resources and carry out a specific analysis by sex and time of year.
Identifying areas where species are most abundant has been an invaluable tool for the design of biological reserves and for the reintroduction of game species (Araújo and Williams, 2000). Applying SDM to the systematic planning of conservation or management of wild fauna and flora can be effective in protecting populations, but besides environmental variables related to the suitability of the territory it is necessary to study 40 30 20 10 0 Total (ibex, male, female)
0.0 0.2 0.4 0.6 0.8 1.0
Suitability index
Total ibex q90
Total male q90
Total female q90
Total ibex q95
Total male q95
Total female q95
Fig. 6. Representation of the regression values for 90 and 95 % quantiles by sex and total contacts (statistically significant for males and total ibex in the 95th quantile and not significant for females in any quantile and males for quantiles at 90%) (total cabras q95 = 7.93 + 35.14 * suitability; total cabras q90 = 7 + 24.169 * suitability; total machos q90 = 7 + 10.142 * suitability; total machos q95 = 6.95 + 24.58 * suitability; total hembras q90 = 10.268 – 6.88 * suitability; total hembras q95 = 12 + 0 * suitability).
Fig. 6. Representación de los valores de regresión para los cuantiles 90 y 95 por sexo y total de contactos (estadísticamente significativa para machos y total de cabras en el cuantil 95 y no significativa para hembras en ningún cuantil y para machos en el cuantil 90 (idoneidad del total de cabras q95 = 7,93 + 35,14 * idoneidad; total de cabras q90 = 7 + 24,169 * idoneidad; total de machos q90 = 7 + 10,142 * idoneidad; total de machos q95 = 6,95 + 24,58 * idoneidad; total de hembras q90 = 10,268 – 6,88 * idoneidad; total de hembras q95 = 12 + 0 * idoneidad).
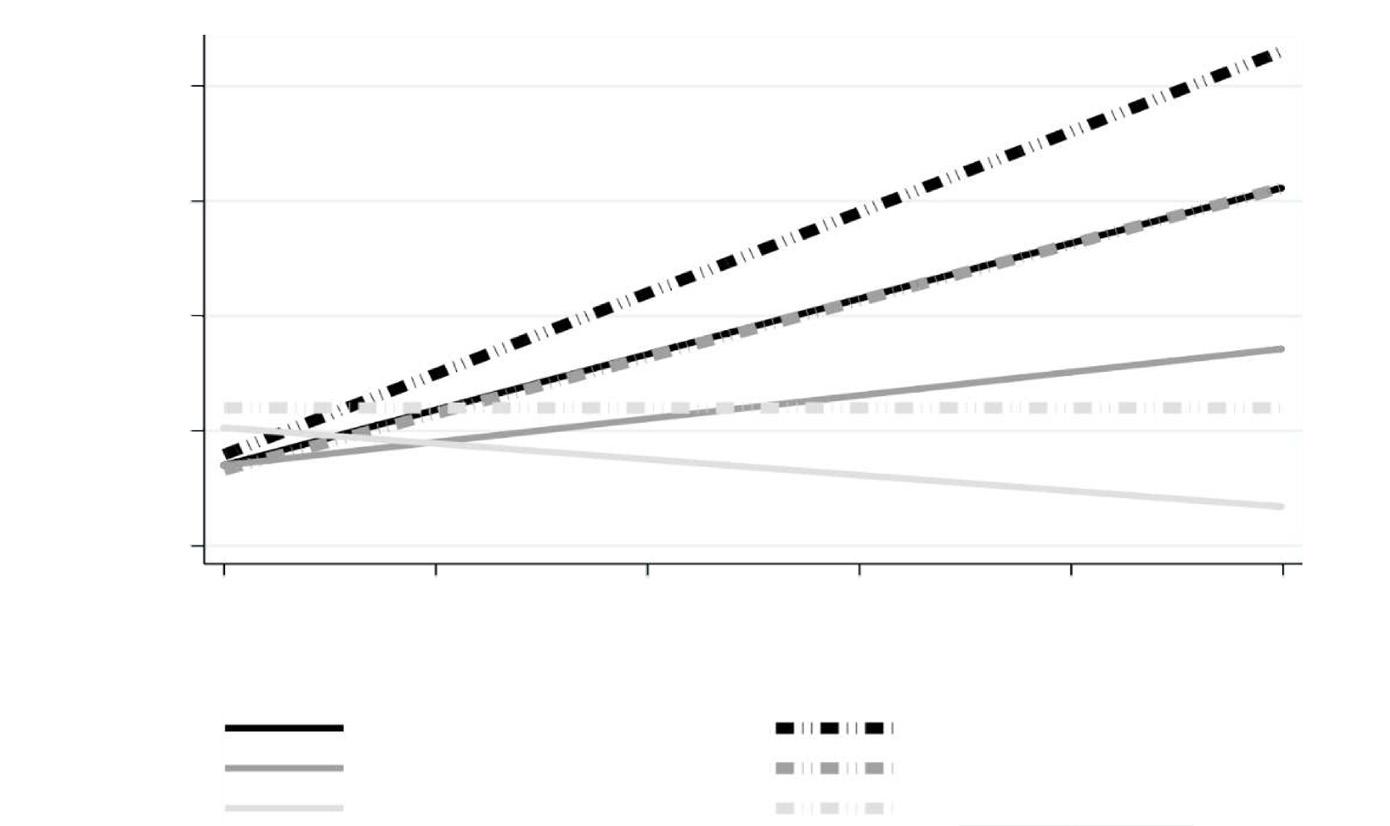
308
Total ibex: F(1; 131) = 1.246; p = 0.266
Total male: F(1; 131) = 4.792; p = 0.030
Total female: F(1; 131) = 4.435; p = 0.030
Total ibex Total male Total female
Fig. 7. Box–plot indicating the size of the total ibex group, the total male group, and the total female group in relation to the suitability of the area: N, suitable Index < 0.5; S, suitable Index > 0.5 (mean: box, mean ± SE; whisker, mean ± SD).
Fig. 7. Diagrama de cajas en el que se indica el tamaño de grupo: total cabras, machos y hembras en relación con la idoneidad de la zona: N, índice de idoneidad < 0,5; S, índice de idoneidad > 0,5 (media: caja, media ± EE; intervalo, media ± DE).
Suitability index S N Suitability index
Total ibex: F(1; 131) = 8.0127; p = 0.0054
Total male: F(1; 131) = 11.598; p = 0.0009
Total female: F(1; 131) = 0.7502; p = 0.3880
Total ibex Total male Total female
Fig. 8. Box–plot showing the size of the total group of ibex, and of males, and females in relation to the suitability of the area: N, suitable index < 0.7; S, suitable index > 0.7 (mean: box, mean ± SE; whisker, mean ± SD).
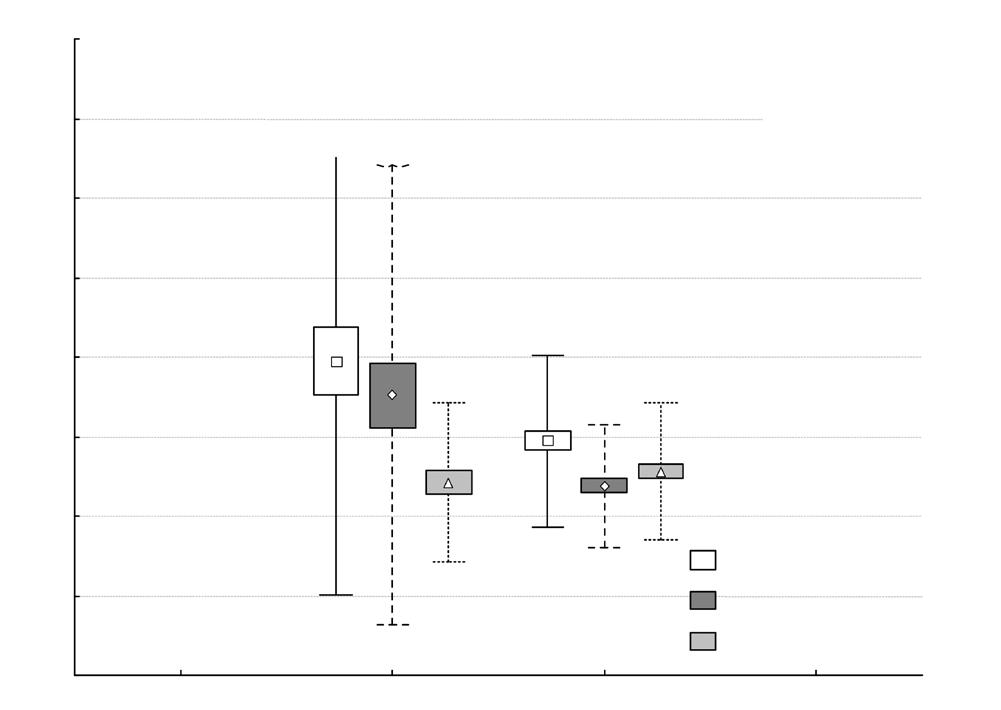
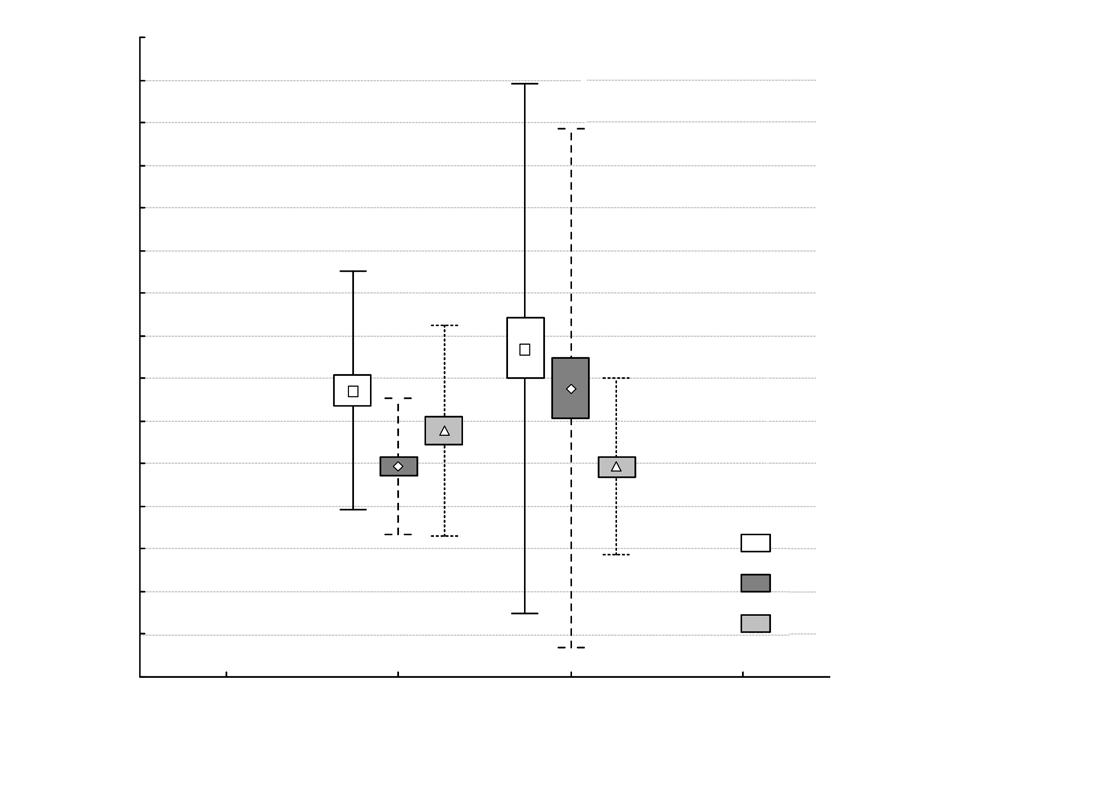
Fig. 8. Diagrama de cajas en el que se indica el tamaño de grupo: total cabras, machos y hembras en relación con la idoneidad de la zona: N, índice de idoneidad < 0,7; S, índice de idoneidad > 0,7 (media: caja: media ± EE; intervalo: media ± DE).
variables related to the ethology of the species itself, especially regarding taxa with differential behaviour between sexes, for example, or age. In our case, the different behaviours between male and female
Iberian ibex allows us to establish differences between the suitability of the territory for one or the other sex, and will facilitate the selection of the more suitable territories for reintroduction.
Animal Biodiversity and Conservation 45.2 (2022) 309
22 20 18 16 14 12 10 8 6 4 2
–2 –4
25 20 15
0
–6 –8 Size group Size group 30
10 5 0 –5 –10 N S
Refoyo et al.
Acknowledgements
This work has been possible thanks to the efforts of the working teams of ETISL and TRAGSA, those responsible for the Sierra de Guadarrama National Park, and especially Mr. Pablo Sanjuanbenito and Mr. Juan Vielva.
References
Acevedo, P., Cassinello, J., 2009. Biology, ecology and status of Iberian ibex Capra pyrenaica: a critical review and research prospectus. Mammal review, 39: 17–32, Doi: 10.1111/j.1365-2907.2008.00138.x
Acevedo, P., Cassinello, J., Gortazar, C., 2007. The Iberian Ibex is under an expansion trend but displacet to suboptimal habitats by the presence of extensive goat livestock in central Spain. Biodiversity and Conservation, 16: 3361–3376, Doi: 10.1007/s10531-006-9032-y
Acevedo, P., Cassinello, J., Hortal, J., Gortazar, C., 2007. Invasive exotic aoudad (Ammotragus lervia) as a major threat to native Iberian ibex (Capra pyrenaica): a habitat suitability model approach. Diversity and Distributions, 13: 587–597, Doi: 10.1111/j.1472-4642.2007.00374.x
Acevedo, P., Ward, A. I., Real, R., Smith, G. S., 2010. Assessing biogeographical relationships of ecologically related species using favourability functions: a case study on British deer. Diversity and Distributions, 16: 515–528, Doi: 10.1111/j.14724642.2010.00662.x
Alados, C., Escos, J., 1995. Ecología y comportamiento de la cabra montés. Consideraciones para su gestión. Monografías del Museo Nacional de Ciencias Naturales, nº 11. MNCN. Madrid
Anadón, L. D., Giménez, A., Martínez, M., Palazón, J. A., Esteve, M. A., 2007. Assessing changes in habitat quality due to land use changes in the spur–thighed tortoise Testudo graeca using hierarchical predictive habitat models. Diversity and Distributions, 13: 324–331, Doi: 10.1111/j.14724642.2007.00343.x
Araújo, M. B., Williams, P. H., 2000. Selecting areas for species persistence using occurrence data. Biological Conservation, 96: 331–345, Doi: https:// doi.org/10.1016/S0006-3207(00)00074-4
Bean, W. T., Stafford, R., Brashares, J. S., 2012. The effects of small sample size and sample bias on threshold selection and accuracy assessment of species distribution models. Ecography, 35: 250–258, Doi: https://doi.org/10.1111/j.16000587.2011.06545.x
Broms, K. M., Johnson, D. S., Altwegg, R. A., Conquest, L. L., 2014. Spatial occupancy models applied to atlas data show Southern Ground Hornbills strongly depend on protected areas. Ecological Applications, 24: 363–374, Doi: https:// doi.org/10.1890/12-2151.1
Buckland, S. T., Anderson, D. R., Burnham, K. P., Laake, J. L., 1993. Distance Sampling: Estimating Abundance of Biological Populations. Chapman
and Hall, London.
Cano, L. S., Pacheco, C., Refoyo, P., Tellería, J. L., 2014. Geographical and Environmental Factors affecting the Distribution of Wintering Black Storks Ciconia nigra in The Iberian Peninsula. Journal of Avian Biology, 45: 514–521, Doi: 10.1111/jav.00391
Carrascal, L. M., Moreno, A. C., Delgado, A., Suarez, V., Trujillo D., 2017. Habitat suitability–density relationship in an endangered woodland species: the case of the Blue Chaffinch (Fringilla polatzeki). PeerJ, 5: e3771, https://peerj.com/articles/3771/ Cao, Y., De Walt, E., Robinson, J. L., Tweddale, T., Hinz, L., Pessino, M., 2013. Using Maxent to model the historic distributions of stonefly species in Illinois streams: the effects of regularization and threshold selections. Ecological Modelling, 259: 30–39, Doi: 10.1016/j.ecolmodel.2013.03.012
Converse, S. J., Moore, C. T., Armstrong, D. P., 2013. Demographics of reintroduced populations: Estimation, modelling, and decision analysis. The Journal of Wildlife Management, 77: 1081–1093, Doi: 10.1002/jwmg.590
Dubuis, A., Pottier, J., Rion, V., Pellissier, L., Theurillat, J. P., Guisan, A., 2011 Predicting spatial patterns of plant species richness: a comparison of direct macroecological and species stacking modelling approaches. Diversity and Distributions , 17: 1122–1131, Doi: 10.1111/j.1472-4642.2011.00792.x
Elith, J., Burgman, M. A., 2002. Predictions and their validation: rare plants in the Central Highlands, Victoria, Australia. In: Predicting Species Occurrences: Issues of Accuracy and Scale: 303–314 (J. M. Scott, P. J. Heglund, M. L. Morrison, J. B. Haufler, M. G. Raphael, W. A. Wall, F. B. Samson, Eds.). Island Press, Washington DC.
Elith, J., Leathwick, J. R., 2009. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annual Review of Ecology Evolution and Systematics, 40: 677–697, Doi: 10.1146/annurev.ecolsys.110308.120159
Elith, J., Phillips, S. J., Hastie, T., Dudík, M., Chee, Y. E., Yates, C. J., 2010. A statistical explanation of MaxEnt for ecologists. Diversity and Distributions, 17: 43–57, Doi: https://doi.org/10.1111/j.14724642.2010.00725.x
Engler, R., Guisan, A., Rechsteiner, L., 2004. An improved approach for predicting the distribution of rare and endangered species from occurrence and pseudo–absence data. Journal of Applied Ecology, 41: 263–274, Doi: https://doi.org/10.1111/j.00218901.2004.00881.x
Felicísimo, A., 1994. Modelos digitales del terreno. Introducción y aplicaciones en las ciencias ambientales. Pentalfa, Oviedo.
Fernández–Marchán, D., Refoyo, P., Novo, M., Fernández, R., Trigo, D., Cosín, D., 2015. Predicting Soil micro–variables and the distribution of an endogeic earthworm species through a model based on large–scale variables. Soil Biology and Biochemistry, 81: 124–127, Doi: 10.1016/j.soilbio.2014.10.023
Ferrer–Sánchez, Y., Ruiz–Companioni, I., Abasolo–Pacheco, F., Plasencia–Vázquez, A. H., Denis
310
Ávila, D., Rodríguez Piña, E., 2017. Parámetros reproductivos y distribución geográfica potencial de las áreas de anidación de Grus canadensis nesiotes (Aves, Gruidae) en Cuba: implicaciones para su conservación. Animal Biodiversity and Conservation, 40(2): 175–186, Doi: 10.32800/ abc.2017.40.0175
Fielding, A. H., Bell, J. F., 1997. A review of methods for the assessement of prediction errors in conservation presence/absence models. Environmental Conservation, 24: 38–49, Doi: 10.1017/ S0376892997000088
Fonseca, C., Migueis, D., Fernandes, T., Carvalho, H., Loureiro, A., Carvalho, J., Tinoco–Torres, R., 2017. The return of the Iberian wild goat Capra pyrenaica to Portugal: from reintroduction to recolonization. Journal for Nature Conservation, 38: 56–61, Doi: 10.1016/j.jnc.2017.05.006
Fourcade, Y., Engler, J. O., Rödder, D., Secondi, J., 2014. Mapping species distributions with MAXENT using a geographically biased sample of presence data: a performance assessment of methods for correcting sampling bias. Plos One, 9(5): e97122, Doi: 10.1371/journal.pone.0097122
Gedir, J. V., Thorne, J. M., Brider, K., Armstrong, D. P., 2013. Using prior data to improve models for reintroduced populations: A case study with North Island Saddlebacks. The Journal of Wildlife Management, 77: 1114–1123, Doi: 10.1002/jwmg.544
Graham, C. H., Hijmans, R. J., 2006. A comparison of methods for mapping species ranges and species richness. Global Ecology and Biogeography, 15: 578–587, Doi: 10.1111/j.1466-8238.2006.00257.x
Granados, J. E., Soriguer, R. C., Pérez, J. M., Fandos, P., Garcia–Santiago, J., 2007. Capra pyrenaica, Schinz 1838. In: Atlas y Libro Rojo de los Mamíferos Terrestres de España: 366–368 (L. J. Palomo, J. Gisbert, J. C. Blanco, Eds.). Dirección General para la Biodiversidad–SECEM–SECEMU, Madrid.
Guisan, A., Rahbek, C., 2011. SeSAM–a new framework integrating macroecological and species distribution models for predicting spatio–temporal patterns of species assemblages. Journal of Biogeography, 38: 1433–1444, Doi: 10.1111/j.13652699.2011.02550.x
Guisan, A., Hofer, U., 2003. Predicting reptile distributions at mesoscale: relation to climate and topography. Journal of Biogeography, 30: 1233–1243, Doi: 10.1046/j.1365-2699.2003.00914.x
Guisan, A., Zimmermann, N. E., 2000. Predictive habitat distribution models in ecology. Ecological Modelling, 135: 147–186, Doi: 10.1016/S03043800(00)00354-9
Hernández, P. A., Graham, C. H., Master, L. L., Albert, D. L., 2006. The effect of sample size and species characteristics on performance of different species distribution modelling methods. Ecography, 29: 773–785, Doi: 10.1111/j.0906-7590.2006.04700.x
Higgins, R. J., Gillingham, M. G., Staffan Lindgren, B., 2017. Critical Habitat Elements, with an Emphasis on Coarse Woody Debris, Associated with Ant Presence or Absence in the Moist Cold Sub–Boreal Forests of the Interior of British Columbia. Forests,
8(4): 129, Doi: 10.3390/f8040129
Houlahan, J. E., Findlay, C. S., 2003. The effects of adjacent land use on wetland amphibian species richness and community composition. Canadian Journal of Fishesires and Aquatic Sciences, 60(9): 1078–1094, Doi: 10.1139/f03-095
Hui, C., 2006. Carrying capacity, population equilibrium, and envrionment's maximal load. Ecological Modelling, 19: 317–320, 10.1016/j.ecolmodel.2005.07.001
Huston, M. A., 2002. Introductory essay: critical issues for improving predictions. In: Predicting species occurrences: issues of accuracy and scale: 7–21 (J. M. Scott, J. H. Heglund, M. L. Morrison, J. B. Haufler, M. G. Raphael, W. A. Wall, F. B. Samson, Eds.). Island Press, Washington, DC.
Jaberg, C., Guisan, A., 2001. Modelling the distribution of bats in relation to landscape structure in a temperate mountain environment. Journal of Applied Ecology, 38: 1169–1181, Doi: 10.1046/j.00218901.2001.00668.x
Johnson, J. R., Knouftb, J. H., Semlitscha, R. D., 2007. Sex and seasonal differences in the spatial terrestrial distribution of gray treefrog (Hyla versicolor) populations. Biological Conservation, 140(3–4): 250–258, Doi: 10.1016/j.biocon.2007.08.010
Koenker, R., Bassett, G., 1978. Regression quantiles. Econometrica, 46: 33–50, Doi: 10.2307/1913643
Lehmann, A., Overton, J. M. C., Leathwick, J. R., 2002. GRASP: generalized regression analysis and spatial prediction. Ecological Modelling, 157: 189–207, Doi: 10.1016/S0304-3800(02)00195-3
Liu, C. R., Berry, P. M., Dawson, T. P., Pearson, R. G., 2005. Selecting thresholds of occurrence in the prediction of species distributions. Ecography, 28: 385–393, Doi: 10.1111/j.0906-7590.2005.03957.x
Liu, C. R., Newell, G., White, M., 2015. On the selection of thresholds for predicting species occurrence with presence–only data. Ecology and Evolution, 6(1): 337–348, Doi: 10.1002/ece3.1878
Magarey, R., Newton, L., Cheon Hong, S., Takeuchi, Y., Christie, D., Jarnevich, C. S., Kohl, L., Damus, M., Higgins, S. I., Millar, L., Castro, K., West, A., Hastings, J., Cook, G., Kartesz, J., Koop, A. L., 2017. Comparison of four modeling tools for the prediction of potential distribution for non–indigenous weeds in the United States. Biological Invasions, 20: 679–694, Doi: 10.1007/s10530-017-1567-1
Maggini, R., Guisan, A., Cherix, D., 2002. A stratified approach for modelling the distribution of a threatened ant species in the Swiss National Park. Biodiversity and Conservation, 11: 2117, Doi: 10.1023/A:1021338510860
Mateo, R. G., Felicísimo, A. M., Muñoz, J., 2011. Modelos de distribución de especies. Una revisión sintética. Revista Chilena de Historia Natural, 84: 217–240, Doi: 10.4067/S0716-078X2011000200008
Moisen, G. G., Frescino, T. S., 2002. Comparing five modeling techniques for predicting forest characteristics. Ecological Modelling, 157: 209–225, Doi: https://doi.org/10.1016/S0304-3800(02)00197-7
Morelle, K., Lejeune, P., 2015. Seasonal variations of wild boar Sus scrofa distribution in agricultural
Animal Biodiversity and Conservation 45.2 (2022) 311
landscapes: a species distribution modelling approach. European Journal of Wildlife Research, 61: 45–56, Doi: 10.1007/s10344-014-0872-6
Nenzen, H. K., Araujo, M. B., 2011. Choice of threshold alters projections of species range shifts under climate change. Ecological Modelling, 222: 3346–3354, Doi: 10.1016/j.ecolmodel.2011.07.011
Odum, E. P., 1986. Fundamentos de Ecología. Nueva Editorial Interamericana S.A. México, D.F.
Olmedo, C., Muñoz, B., Refoyo, P., 2016. A comparison of GLM and Maxent for modelling Iberian Ibex distribution in central Spain. 5th Internacional Conference on Biodiversidad. Madrid.
Palialexis, A., Georgakarakos, S., Karakassis, I., Lika, K., Valavanis, V. D., 2011. Prediction of marine species distribution from presence–absence acoustic data: comparing the fitting efficiency and the predictive capacity of conventional and novel distribution models. Hydrobiologia, 670: 241–266. Doi: 10.1007/s10750-011-0673-9
Pérez, J. M., Granados, J. E., Soriguer, R. C., Fandos, P., Márquez, F. J., Crampe, J. P., 2002. Distribution, status and conservation problems of the Spanish Ibex, Capra pyrenaica (Mammalia: Artiodactyla). Mammal Review, 32(1): 26–39, Doi: 10.1046/j.1365-2907.2002.00097.x
Phillips, S., Shapire, R., 2004. A maximum entropy approach to species distribution modeling. Proceedings of the 21st international Conference on Machine Learning: 655–662.
Phillips, S. J., Anderson, R. P., Shapire, R. E., 2006. A maximum entropy modelling of species geographic distributions. Ecological Modelling, 190: 231–259, Doi: 10.1016/j.ecolmodel.2005.03.026
Phillips, S. J., Dudik, M., Elith, J., Graham, C. H., Lehmann, A., Leathwick, J., Ferrier, S., 2009. Sample selection bias and presence–only distribution models: implications for background and pseudo–absence data. Ecological Applications, 19: 181–197, Doi: 10.1890/07-2153.1
Refoyo, P., 2012. La cabra montesa (Capra pyrenaica victoriae) en el Parque Regional de la Cuenca Alta del Manzanares (Sierra de Guadarrama). Proceso de reintroducción, parasitosis y modelización del nicho ecológico a nivel regional y peninsular. PhD thesis, Universidad Complutense de Madrid, Madrid, Spain.
Refoyo, P., Olmedo, C., Muñoz, B., 2014. La utilidad de los modelos de distribución de especies en la gestión cinegética de los ungulados silvestres. Animal Biodiversity and Conservation, 37: 165–176, Doi: 10.32800/abc.2014.37.0165
Refoyo, P., Olmedo, C., Polo, I., Fandos, P., Muñoz, B., 2015. Demographic trends of a re–introduced Iberian Ibex Capra pyreniaca victoriae population in central Spain. Mammalia, 79(2): 139–145, Doi: 10.1515/mammalia-2013-0141
Refoyo, P., Olmedo, C., Muñoz, B., 2016. Space use of a Reintroduction population of Iberian ibex (Capra pyrenaica) in a protected natural area. Canadian Journal of Zoology, 94: 181–189, Doi: 10.1139/cjz-2015-0166
Segurado, P., Araujo, M. B., 2004. An evaluation
of methods for modelling species distributions. Journal of Biogeography, 31: 1555–1568, Doi: 10.1111/j.1365–2699.2004.01076.x
Shartell, L. M., Lilleskov, E. A., Storer, A. J., 2013. Predicting exotic earthworm distribution in the northern Great Lakes Region. Biological Invasions, 15: 1665–1675, Doi: 10.1007/s10530-012-0399-2
Shcheglovitovaa, M., Andersona, R. P., 2013. Estimating optimal complexity for ecological niche models: a jackknife approach for species with small sample sizes. Ecological Modelling, 269: 9–17, Doi: 10.1016/j.ecolmodel.2013.08.011
Soriguer, R. C., Márquez, F. J., Pérez, J. M., 1998. Las translocaciones (introducciones y reintroduciones) de especies cinegéticas y sus efectos medioambientales. Galemys, 10(2): 19–35.
Suárez–Seoane, S., García de la Morena, E. L., Morales Prieto, M. B., Osborne, P. E., De Juana, E., 2008. Maximum entropy niche–based modelling of seasonal changes in little bustard (Tetrax tetrax) distribution. Ecological Modelling, 219: 17–29, Doi: 10.1016/j.ecolmodel.2008.07.035
Suárez–Seoane, S., Osborne, P. E., Rosema, A., 2004. Can climate data from METEOSAT improve wildlife distribution models? Ecography, 27: 629–636, Doi: 10.1111/j.0906-7590.2004.03939.x
Tellería, J. L., Santos, T., Refoyo, P. A., Muñoz, J., 2012. Use of ring recoveries to predict habitat suitability in small Passerines. Diversity and Distributions, 18(11): 1130–1138, Doi: 10.1111/j.14724642.2012.00900.x
Tessarolo, G., Rangel, T. F., Araujo, M. B., Hortal, J., 2014. Uncertainty associated with survey design in Species Distribution Models. Diversity and Distributions, 20: 1258–1269, Doi: https://doi. org/10.1111/ddi.12236
Tinoco–Torres, R., Herrero, J., Prada, C., Garcia–Serrano, A., Fernandez–Arberas, O., Garcia–Post, R., 2014. Estimating the population density of Iberan wild goat Capra pyrenaica and mouflon Ovis aries in a Mediterranean forest environment. Forest Systems , 23(1): 36–43, Doi: 10.5424/ fs/2014231-03322
Vargas, J. M., Farfán, M. A., Guerrero, J. C., Barbosa, A. M., Real, R., 2007. Geographical and environmental correlates of big and small game in Andalusia (southern Spain). Wildlife Research, 34(6): 498–506, Doi: 10.1071/WR06012
Warren, D. L., Seifert, S. N., 2011. Ecological niche modeling in Maxent: the importance of model complexity and the performance of model slection criteria. Ecological Applications, 21(2): 335–342, Doi: 10.1890/10-1171.1
Weber, M. M., Stevens, R. D., Diniz–Filho, J. A. F., Grelle, C. E. V., 2017. Is there a correlation between abundance and environmental suitability derived from ecological niche modelling? A meta–analysis. Ecography, 40: 817–828, Doi: 10.1111/ecog.02125
Wenger, S. J., Olden, J. D., 2012. Assessing transferability of ecological models: an underappreciated aspect of statistical validation. Methods. Ecology and Evolution, 3: 260–267, Doi: 10.1111/j.2041210X.2011.00170.x
312
Refoyo et al.
Wint, W. G. R., Robinson, T. P., Bourn, D. M., Durr, P. A., Hay, S. I., Radolph, S. E., Rogers, D. J., 2002. Mapping bovine tuberculosis in Great Britain using environmental data. Trends in Microbiology, 10: 441–444, Doi: 10.1016/S0966-842X(02)02444-7
Wisz, M. S., Hijmans, R. J., Li, J., Peterson, A. T., Graham, C. H., Guisan, A., 2008. Effects of sample size on the performance of species distribution models. Diversity and Distributions, 14: 763–773, Doi: 10.1111/j.1472-4642.2008.00482.x
Wu, M., Zhang, Q., Jing–yuan, S., Xi, W., Cai–xiang, X., Zhi–gang, H., 2018. Ecological Characteristics and suitability evaluation of Frintillaria Cirrhosa D
don Base don Maxent Model. African Journal of Traditional, Complementary and Alternative Medicines, 15(1); 158–167, Doi: 10.21010/ajtcam. v15i1.16
Yongyut, T., Bhumpakphan, N., Reed, D. H., Kanchanasaka, B., 2012. Using species distribution modeling to set management priorities for mammals in northern Thailand. Journal for Nature Conservation, 20: 264– 273, Doi: 10.1016/j.jnc.2012.05.002
Zimmermann, N. E., Kienast, F., 1999. Predictive mapping of alpine grasslands in Switzerland: species versus community approach. Journal of Vegetation Science, 10: 469–482, Doi: 10.2307/3237182
Animal Biodiversity and Conservation 45.2 (2022) 313
314 Refoyo et al.
Increases in avian diversity associated with COVID–19 lockdowns in urban Colombia
I. MacGregor–Fors, E. Arbeláez–Cortés, F. A. Estela, D. Ocampo, C. E. Sánchez–Sarria, M. García–Arroyo, G. K. Aguirre–Samboní, D. Cortés–Díaz, J. C. Franco Morales, C. D. Gaitán–García, S. Guerrero–Pelaez, Y. Gutiérrez Parodys, M. Holguín–Ruiz, E. Meza–Angulo, H. A. Vides, J. D. Wilches–Vega
MacGregor–Fors, I., Arbeláez–Cortés, E., Estela, F. A., Ocampo, D., Sánchez–Sarria, C. E., García–Arroyo, M., Aguirre–Samboní, G. K., Cortés–Díaz, D., Franco Morales, J. C., Gaitán–García, C. D., Guerrero–Pelaez, S., Gutiérrez Parodys, Y., Holguín–Ruiz, M., Meza–Angulo, E., Vides, H. A., Wilches–Vega, J. D., 2022. Increases in avian diversity associated with COVID–19 lockdowns in urban Colombia. Animal Biodiversity and Conservation, 45.2: 315–325, Doi: https://doi.org/10.32800/abc.2022.45.0315
Abstract
Increases in avian diversity associated with COVID–19 lockdowns in urban Colombia. Research on urban biodiversity has primarily addressed the effects of urbanization and human activity synergistically as it has been virtually impossible to dissociate their impact on city wildlife. However, the anthropause resulting from COVID–19 lockdowns provided an unprecedented scenario to study the relative role of human activity on avian communities. Here we provide evidence of the relationships between human activity and bird species richness in urban areas of Colombia during its strict and subsequent relaxed lockdowns. Once the strict lockdown was lifted and human activity increased, avian species richness decreased by 32 % in 46 % of our sampling sites. Although the strict lockdown lasted only six weeks, local assemblages (mainly from low–intensity urbanization peri–urban sites) swiftly became more diverse. Our findings highlight the importance of taking human activity into account when planning cities, with important focus on greenspaces, if our aim is to conserve and enhance urban biodiversity. Such plans will require not only the cooperation of local governments but also greater awareness among the local population regarding the importance of creating livable, healthy, biodiverse, and resilient cities.
Key words: Bird surveys, Coronavirus, Data–intensive science, Neotropic, Urban ecology
Resumen

Aumento de la diversidad de aves asociada con los confinamientos impuestos a raíz de la COVID–19 en ciudades Colombianas. El estudio sobre la biodiversidad urbana ha abordado primordialmente los efectos combinados de la urbanización y las actividades humanas, ya que ha sido prácticamente imposible disociar el papel del cambio físico de la urbanización y el de las actividades diarias en relación con la fauna silvestre de las ciudades. La antropausa producida por los confinamientos impuestos a raíz de la COVID–19 generó una situación sin precedentes que permitió estudiar el papel relativo de la actividad humana en las comunidades de aves. En el presente estudio aportamos evidencia sobre las relaciones entre la actividad humana y la riqueza de especies de aves en zonas urbanas de Colombia durante el confinamiento estricto y el subsecuente confinamiento relajado. Una vez que el confinamiento estricto concluyó, la riqueza de especies decreció 32 % en 46 % de nuestros sitios de muestreo. A pesar de que el confinamiento estricto únicamente duró seis semanas, la diversidad de las aves, mayoritariamente en sitios periurbanos con una baja intensidad de urbanización, aumentó rápidamente. Nuestros resultados subrayan la importancia de los planes urbanos futuros en relación con la actividad humana, particularmente en espacios verdes si se desea conservar y mejorar la biodiversidad en las ciudades. Para llevar a cabo estos planes, será necesario que los gobiernos locales cooperen, pero también que se conciencie a la población local de la importancia de crear ciudades vivibles, saludables, biodiversas y resilientes.
Palabras clave: Muestreos de aves, Coronavirus, Ciencia con utilización intensiva de datos, Neotrópico, Ecología urbana
ISSN: 1578–665 X eISSN: 2014–928 X
© [2022] Copyright belongs to the authors, who license the journal Animal Biodiversity and Conservation to publish the paper under a Creative Commons Attribution 4.0 License.
315 Animal Biodiversity and Conservation 45.2 (2022)
MacGregor–Fors et al.
Received: 31 VIII 22; Conditional acceptance: 21 IX 22; Final acceptance: 24 X 22
Ian MacGregor–Fors, Michelle García–Arroyo, University of Helsinki, Lahti, Finland.– Enrique Arbeláez–Cortés, Universidad Industrial de Santander, Bucaramanga, Colombia.– Felipe A. Estela, Giann K. Aguirre–Samboní, Pontificia Universidad Javeriana–Cali, Colombia.– David Ocampo, Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, Villa de Leyva, Colombia and Princeton University, New Jersey, USA.– Camilo E. Sánchez–Sarria, Instituto de Ecología, A.C., Xalapa, México.– Daniela Cortés–Díaz, Universidad del Quindío, Armenia, Colombia.– Juan C. Franco Morales, Universidad Autónoma de Occidente, Cali, Colombia.– Cristhian D. Gaitán–García, Universidad del Tolima, Ibagué, Colombia.– Sebastian Guerrero–Pelaez, Pontificia Universidad Javeriana–Bogotá DC, Colombia.– Yossama Gutiérrez Parodys, Fundación Ecológica Los Besotes, Colombia.–Maira Holguín–Ruiz, Universidad de los Llanos, Colombia.– Enrick Meza–Angulo, Universidad de Caldas, Manizales, Colombia.– Hugo A. Vides, Universidad de Cartagena, Colombia.– Juan D. Wilches–Vega, Universidad de Santander, Campus Cúcuta, Colombia.
Corresponding author: I. MacGregor–Fors. E–mail: ian.macgregor@helsinki.fi
ORCID ID: Ian MacGregor–Fors 0000-0003-3198-7322; Enrique Arbeláez–Cortés 0000-0002-4350-1564; Felipe A. Estela 0000-0003-2090-1386; David Ocampo 0000-0003-1597-4038; Camilo E. Sánchez–Sarria 0000-0001-7990-7366; Michelle García–Arroyo 0000-0002-9167-4777; Giann K. Aguirre–Samboní 0000-0002-3526-7253; Daniela Cortés–Díaz 0000-0002-7363-5547; Juan C. Franco Morales 0000-0001-8363-8314; Cristhian D. Gaitán–García 0000-0002-3852-8615; Sebastian Guerrero–Pelaez 0000-0003-3008-0139; Maira Holguín–Ruiz 0000-0001-8360-3779; Enrick Meza–Angulo 0000-0002-0268-5029; Hugo A. Vides 0000-0002-3935-3319; Juan D. Wilches–Vega 0000-0003-1067-4079
316
Introduction
Beyond the profound and long–lasting shifts intrinsic to the urbanization process, the metabolism of urban areas is considered one of the major environmental threats to date (Maxwell et al., 2016). Cities drive environmental change at multiple spatiotemporal scales, altering hydro–systems, biogeochemical cycles, climate, and biodiversity (Grimm et al., 2008). The impact of cities on biodiversity is so extensive that urban development itself, not accounting for extrinsic urban metabolism, has been regarded as the third most influential cause of species endangerment worldwide (Maxwell et al., 2016). Evidence has shown that biodiversity plummets in cities when contrasted with non–urban counterparts (Aronson et al., 2014), with the remaining assemblages non–randomly filtered into cities (taxonomically/phylogenetically and functionally); a pattern that holds globally (La Sorte et al., 2018). However, wildlife species have been shown to respond differently to urbanization (MacGregor–Fors et al., 2022). While some species avoid cities, others use the resources and live therein, with intriguing behavioral adjustments (e.g., Quesada et al., 2022). The latter often represent sink populations of non–urban species, however, and few thrive within urban centers (Fischer et al., 2015).
Studies focused on biodiversity shifts in urban systems have mostly addressed the role of urbanization and human activity synergistically (Forman, 2014). Findings have shown general patterns such as decreases in overall species richness with urbanization and increases in total bird abundances (MacGregor–Fors and Escobar–Ibáñez, 2017; Pena et al., 2017). Except for some local experiments (e.g., Bötsch et al., 2017, 2018) it has been virtually impossible to separate the role of the physical change imposed to the landscape through urbanization and day–to–day human activity on wildlife inhabiting or seeking to inhabit cities (Magel et al., 2019; Zellmer et al., 2020). Although currently increasing, our understanding of the magnitude and the ways in which human activity drives urban biodiversity has been heavily biased by correlational studies and small–scale experiments. Previous studies have focused on human activity as a wildlife driver, mostly in the form of passing pedestrians, recreational activities, vehicle traffic, and other proxies (e.g., noise; Gil and Brumm, 2014). Findings have shown that human activity can have negative effects on several wildlife groups, with consequences that span from changes in behavior (habituation processes) to changes in health and fitness (Stankowich and Blumstein, 2005; Schlesinger et al., 2008; Corsini et al., 2017; Morelli et al., 2018). Interestingly, some species well–adapted to urban life not only tolerate but also profit from the conditions at sites where human activity peaks (Sol et al., 2002). Thus, on the one hand, species that do not adjust to urban habitats are often more specialist (rather than generalist) or species have basic needs that cannot be met in urban settings, and are incapable of making the necessary adaptations that confer tolerance to urban life (Sol et al., 2013). On the other hand, previous studies have
shown that some of the positive responses to urbanization are related to the capacity of some species to adjust their behavior, or to natural history traits, such as diet or nesting strata (Sol et al., 2013).
The COVID–19 anthropause (scenario resulting from COVID–19 country lockdowns; Rutz et al., 2020) altered the scenario of human activity at a global scale. With the majority of the population confined to their homes, cities suddenly became less active and quieter. Billions of people worldwide sheltered–in–place intermittently for months due to COVID–19 pandemic lockdowns and ordinances (Thomas et al., 2020). In Colombia, the government decreed a strict nationwide lockdown starting on March 23, 2020 (referred to as 'strict lockdown' hereafter) that lasted for six weeks (decrees 457 and 636, respectively; Consejería Presidencial para las Regiones, 2020), and affected approximately 80 % of the population (~40 million Colombians) (Thomas et al., 2020). On May 6, several economic sectors were reactivated with Colombians gradually, and partially, returning to the streets (referred to as ‘relaxed lockdown’ hereafter). The sharp reduction in human activities in cities during the strict COVID–19 lockdown period provided an unprecedented urban scenario in modern history, offering the opportunity to assess the role of human activity in relation to urban avian diversity.
After considering two windows of time during the anthropause, we here provide empirical evidence concerning the effect of human activity on bird diversity in urban areas in Colombia, the country with the richest avian diversity worldwide (Avendaño et al., 2017). We centered our study on birds as they are bioindicators and could feasibly be surveyed under lockdown conditions (Pollack et al., 2017). Birds have long been the most studied animal group in urban areas for a myriad of reasons, including their conformity to complex assemblages, their well–known natural history, and their rapid response to the changing dynamics in urban sites (Marzluff et al., 2001; Gil and Brumm, 2014; MacGregor–Fors and Escobar–Ibáñez, 2017).
To assess whether bird assemblages responded to this unique window of time in which cities were unprecedentedly calmer than usual, we used a standardized quantitative procedure to survey birds, starting a few days after the lockdown decree was put in place and continuously sampled throughout the strict lockdown and for several weeks after the first reactivation of human activities during the relaxed lockdown. We focused on species richness as it is a highly informative variable that relates to avian shifts in urban settings (Blair, 1996; Escobar–Ibáñez et al., 2020). Given that human activity has been shown to drive urban bird presence/absence and behavior (Sol et al., 2014; Spelt et al., 2021), and that a particular set of resources within the city are both abundant and predictable (Shochat, 2004), we predicted that the drop–off in outdoor human activity across urban Colombia would prompt the use of urban habitats by a larger number of bird species. We also expected to find a decrease in bird species richness as human activity increased after the strict lockdown in well–vegetated areas within Colombian cities (e.g., residential areas), where resources are more diverse than in heavily–built up areas (Faeth et al., 2005).
Animal Biodiversity and Conservation 45.2 (2022) 317
Material and methods
Study area
We conducted this study across urban Colombia, a mid–sized country (1.1 million km2) with at least 3,822 human settlements (cities, towns, villages) distributed across six major biomes and 29 ecological regions, but with an evident bias toward the Andes and the Caribbean (IGAC, 2008). We performed avian surveys in 13 locations within nine urban systems distributed across the country (fig. 1). Given the lockdown mobility restrictions, survey sites corresponded to the locations of observers’ homes or nearby areas. Health and safety regulations were met to prevent transmission of the coronavirus. The sprawl of the involved cities varies in size, ranging from 7 to 137 km2 Sprawl was quantified by freehand digitized polygons of built–up continuum using high–resolution satellite images provided by Google Earth Pro (google.com/ intl/en/earth) and following parameters of building aggregation and communication used by Lemoine–Rodríguez et al. (2019). The resulting polygons represented the urban fringe used to determine the survey site location in our study. This set of urban centers is located in a broad elevation range (9–1,298 m a.s.l.) across seven Colombian ecoregions (Olson et al., 2001). The distances between survey sites varied between 2 and 818 km.
Data collection
We retrieved data from a country–wide scale citizen–science monitoring scheme that aimed to compile bird records during the COVID–19 anthropause in Colombia (Arbeláez–Cortés et al., 2021). Data collection started on March 30, 2020, in response to the governmental lockdown decree enacted on March 23, which was continued until June 30, 2020 (Arbeláez–Cortés et al., 2021). Bird surveys consisted of 10 min fixed radius (50 m) circular or semi–circular point–counts between 06:00–09:00 h. All birds seen or heard during the time and space of the point–count were recorded; we only recorded individuals that were actively using the surveyed area (Bibby et al., 2000). The country–wide scheme included 45 survey sites from 22 urban systems (Arbeláez–Cortés et al., 2021). The information used in this study comprises only data collected through circular point–counts at ground level at sites where observers recorded observations at least 4 times per week (average 6.3 ± SD 2.4; range: 5–7 surveys/week) over the course of at least 12 weeks (average 12.9 ± SD 0.3; range: 12–13 weeks). This assessment thus includes information on the patterns found at 13 locations (located in nine cities), which were repeatedly surveyed in search of local patterns among replicates, for a total of 668 point–count repetitions. All observers were experienced in identifying bird species in the surveyed regions, with field experience ranging from 1.5–28 years (average 6.7 years). A few migrant species, austral and boreal, recorded during the first and last weeks of the survey were excluded from further analyses.
Survey sites traits
Given the relevance of the intensity of urbanization of sites within cities and their spatial location in relation to the core and periphery of the urban sprawl, we quantified built cover (% in 50 m radius) and classified the location of all survey sites. To quantify built cover, we freehand digitized all vegetation components from high–resolution satellite images (provided by Google Earth Pro) in the same 50 m radius circular plots where birds were surveyed and considered all of the remaining areas as built. We classified the location of survey sites following MacGregor–Fors (2010): peri–urban sites were those located along the urban–wildland ecotone that has been shown to represent an ecological barrier for birds, intra–urban areas were those located in the core of the city (inside the peri–urban belt), and extra–urban areas were those located outside the peri–urban belt, representing human settlements that are connected to larger urban centers.
Data analysis
We used a quantitative comparable dataset published by Google (2020) as a proxy for human activity in our study sites. Although we recognize that the information was only available to the regional (departmental) level, it was the best measurement of human activity available at a fine scale during and after the COVID–19 lockdown (Cot et al., 2021). Specifically, these data contrast mobile phone movement and activities after lockdowns began worldwide with baselines calculated using information of a five–week period before lockdowns began (i.e., January 3–February 6). Human mobility values were quantified as the difference between the baseline and the measured activity during the studied period. Thus, the more negative a value, the more it differed from human activity before the lockdowns. Given that the surveys analyzed here are mostly from weekdays, we excluded information provided by Google (2020) at weekends. It is notable that mobile phones, wearables (e.g., smartwatches), and other geolocated devices had reached most of the human population by 2014 (Blondel et al., 2015), allowing retrieval of highly reliable real time information concerning human movement with unprecedented spatial resolution (Meekan et al., 2017).
Given that our aim was to assess shifts in avian richness across time (the surveyed weeks represent two different scenarios of lockdown, and therefore of human activity: strict lockdown = March 23–May 5, 2020; relaxed lockdown = May 6–June 30, 2020), we calculated Pearson correlation coefficients between human activity and the weekly accumulated bird species richness recorded at each survey site (fig. 1s in supplementary material). The tendencies of such results, representing the pattern that species richness followed in the surveyed time, varied among the sampled sites. Thus, we considered coefficients > 0.35 and < –0.35 to represent moderate to strong correlations (Rubin, 2012).
We later related the Pearson correlation coefficients for the relationships between human activity and the
318 MacGregor–Fors et al.
250 500 km Caribbean Sea Villa del Rosario
Santa Marta Colombia
Villavicencio (90.5) Cali (42.9)
Turbaco
10º 0' 0'' N Number of sampling sites 1 5
Ibagué (82.4) Turbaco (79.1) Santa Marta (78.1) Villa del Rosario (72.7) Valledupar (58.8) Armenia (44.9) Pacific Ocean
Valledupar Armenia Ibagué Villavicencio Palmira Cali
79º 21' 0'' W 67º 9' 0'' W
2º 0' 0'' S
Built cover (%) Intra–urban Peri–urban Extra–urban Built Vegetation
Cali (42.5) Cali (18.9) Cali (0.9)
Fig. 1. Geographic location of survey sites and depictions of their built cover and location within the nine Colombian cities studied.
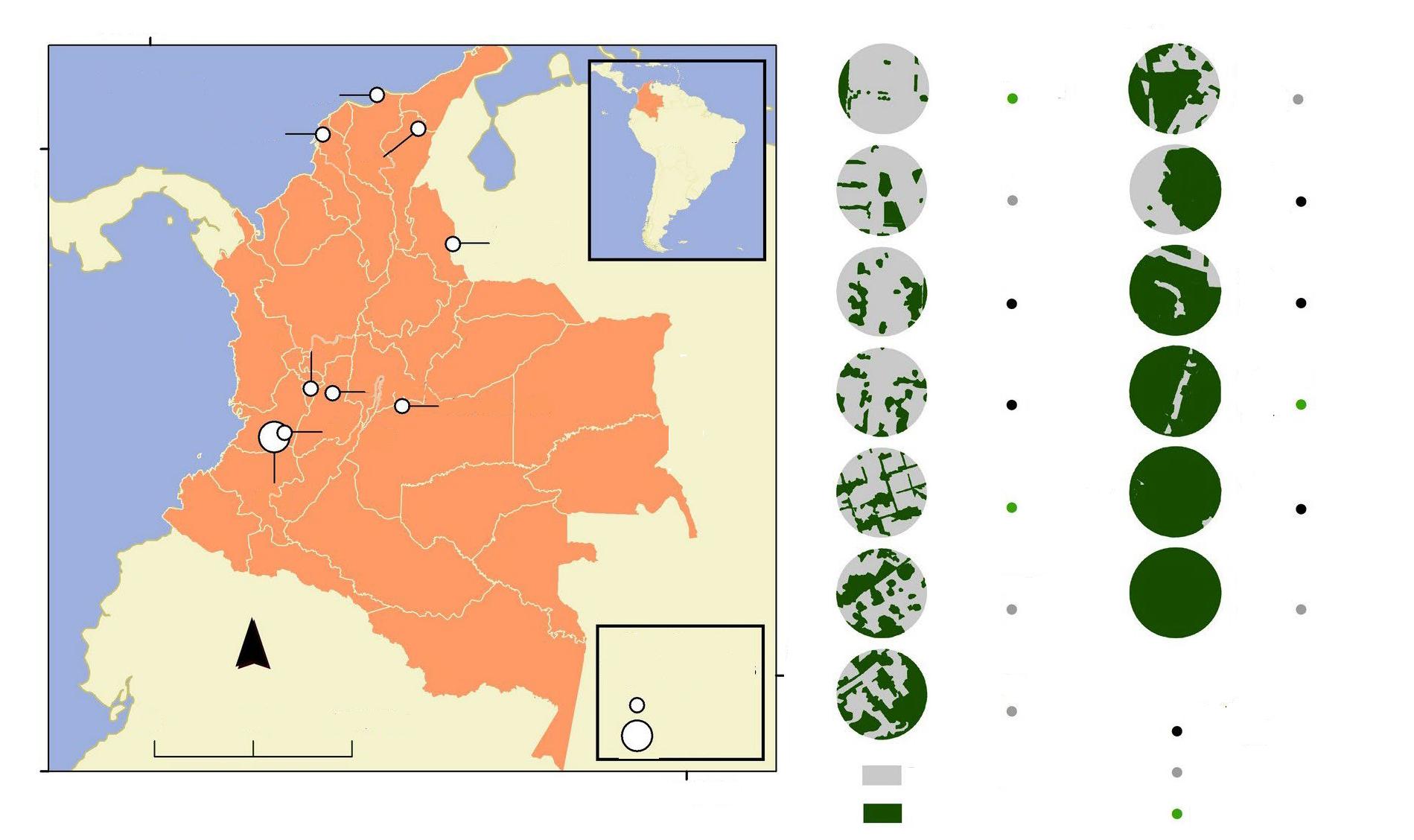
Fig. 1. Localización geográfica de los sitios de estudio y representación de la superficie construida y su localización dentro de las nueve ciudades colombianas estudiadas.
weekly accumulated bird species richness at each survey site to its built cover and spatial location using a generalized additive mixed model. We considered the correlation coefficients for the relationships between human activity and the weekly accumulated number of avian species recorded at each survey site as the dependent variable, built cover as the independent variable considering 'smoothers' for the three site location categories, and the identity of the studied cities and time (i.e., weeks since the strict lockdown decree) as random factors. Generalized additive models have different error structures and link functions able to provide a better fit for different types of variables. This allows non–parametric 'smoothers' to describe non–linear relationships by estimating the curves through the data and not based on predetermined equations (Crawley, 2013). All analyses were run in R (R Core Team, 2020).
Detectability
We acknowledge that ecological assessments focused on biodiversity changes can be biased by extrinsic factors that could affect detectability. Here, we assessed changes in bird species richness in relation to shifts in human activity as a result of the COVID–19 lockdown. However, such changes in human activity could also have decreased our ability to detect birds during the surveys, representing a con-
founding factor to take into account. Previous studies have shown that increasing human activity can also increase the amount and type of stressor stimuli for urban wildlife (Slabbekoorn and Ripmeester, 2008).
For instance, noisy scenarios can have important effects on the measurement of urban diversity, such as decreases in the ability to detect birds in field surveys (Ortega and Francis, 2012) and birds avoiding such conditions for a plethora of reasons (e.g., limitations in their vocal communication; Perillo et al., 2017). Although the response of birds to changes in human activity and its consequences was the central aim of this study, changes in detectability related to factors such as anthropogenic noise could represent a confounding factor.
We were unable to directly measure detectability because observers did not have rangefinders to calculate effective radial distances using distance sampling and estimations, for instance. We were also unable to quantify passing pedestrians and/or cars given the bio–sanitary restrictions in place. However, we performed a couple of ad hoc indirect assessments to evaluate whether our ability to detect birds changed with human activities.
Magnitude of human activity
The human activity data we retrieved from Google (Google, 2020) for the 6–7 weeks of the relaxed
Animal Biodiversity and Conservation 45.2 (2022) 319
0
Palmira (6.7) Cali (0) N
1.0 0.5 0.0 –0.5 –1.0
Intra–urban Peri–urban Extra–urban
Bird–richness ~ human activity coefficient of correlation ( r) 0 20 40 60 80 100 Built cover (%)
Fig. 2. Relationship between built cover and the location of survey sites within the nine studied Colombian cities and the correlation coefficients for the relationships between human activity and the weekly accumulated bird species richness at each survey site (left panel). Density plots for the coefficients of correlation of survey sites at each location within the studied cities (right panel).
Fig. 2. Relación entre la superficie construida y la ubicación de los sitios de estudio dentro de las nueve ciudades colombianas estudiadas y los coeficientes de correlación para las relaciones entre la actividad humana y la riqueza acumulada de especies de aves registrada en cada sitio de estudio (a la izquierda). Gráficos de densidad de los coeficientes de correlación de los centros del estudio en cada tipo de ubicación en las ciudades estudiadas (a la derecha).
lockdown remained negative (average –35.81 ± SE 0.72), showing that although human activity changed notably in relation to activity during the strict lockdown (average –50.96 ± SE 0.53), values were still lower than those of normal anthropogenic stimuli –mostly noise– that may affect the birds in the surveyed habitats. This was basically due to the type of reactivation allowed by the government, in which telecommuting and online education at all academic levels remained mandatory (decree 636, Consejería Presidencial para las Regiones, 2020).
Bird survey time and vehicle traffic
Vehicle traffic, even under regular conditions (i.e., before the COVID–19 lockdown), was relatively low during most of our surveys (07:36 h ± SD 49 min) compared to earlier and later periods. To address this, we analyzed the typical traffic in the surveyed cities using data provided through Google Maps. Specifically, we took screenshots of city–wide traffic in each city hourly from 0600–1000 h and at noon using the same zoom framing for each city. For practical reasons, we randomly chose one day a week (i.e., Friday) to measure vehicle traffic in the cities. We then quantified the number of pixels reflecting the ´'medium amount of traffic' (orange coded) and 'traffic delays' (red coded) categories using Gimp (a free and open–source image editor available at www.gimp.org). After deleting all visual elements of the screenshot with those colors to avoid miscalculations, we used a selection by color tool (Select–By color) using a threshold of 30 (which
allowed us to quantify as many pixels related to road and street traffic as possible without selecting other components of the map). We then added the amount of red– and orange–coded traffic pixels using a 2x factor for the red–coded pixels to denote the increase in traffic. Finally, we averaged the values in all the cities for the assessed times. As shown in figure 2s in supplementary material, the majority of surveys (75 %) were performed between 07:00 h and 09:00 h, hours that correspond with the averages of regular traffic values. The most frequent time of our surveys was around 07:00 h (06:45–07:15 h), when traffic conditions are lowest under normal circumstances in the studied cities.
Aural records and human activity
Both these data (assessed through mobile phones and vehicle movements) show that our surveys were not performed under relatively high human activity scenarios. However, we tested whether increasing human activity (and the related noise stimuli that could reduce our ability to detect birds) caused by the lifting of restrictions during the relaxed lockdown after the strict lockdown was associated with our ability to detect birds in the field. To do this we analyzed shifts in the proportion of aural records in relation to visual records, which we use here as a proxy of our ability to record birds in noisier scenarios. We calculated the weekly proportion of aural and visual bird records by survey site (to avoid observer biases), assuming that if aural records were negatively correlated with human activity, our detection ability could affect our
320 MacGregor–Fors et al.
Intra– Peri– Extra–urban urban urban
Table 1. Generalized additive mixed model showing the relationship between Pearson correlation coefficients for the relationships between human activity and bird species richness with built cover by survey location.
Tabla 1. Modelo aditivo generalizado mixto en el que se muestra la relación entre los coeficientes de correlación de Pearson para las relaciones entre la actividad humana y la riqueza de especies de aves con la superficie construida por tipo de ubicación en los sitios de estudio.
Variables Estimated df F P
Built cover × extra–urban 1.416 9.354 0.007
Built cover × peri–urban 1.938 6.233 0.021
Built cover × intra–urban 1.000 2.971 0.124
surveys in different human activity scenarios. To assess for a potential relationship between aural records and human activity, we correlated these variables per survey site and calculated the slope of the relationship (indicating its direction and magnitude). We then correlated the slopes of these relationships with the correlation coefficients for the relationships between human activity and the weekly accumulated bird species richness, assuming that if we found a positive relationship, then detectability could be an important confounding factor of our main results. This relationship was weak and non–significant (r = 0.143, P = 0.639).
We were particularly concerned about detectability at sites where we recorded negative correlation coefficients (r ≤ 0.35) for the relationships between human activity and weekly accumulated bird species richness. Thus, we performed a similar correlation between the slopes of the aural records–human activity relationships with the correlation coefficients for the relationships between human activity and weekly accumulated bird species richness that were ≤ –0.35. This correlation was also weak and non–significant (r = 0.180, P = 0.731). Two of the correlation coefficients ≤ –0.35 were related to positive slopes of the aural records–human activity relationships (i.e., a site in Cali and that of Valledupar). In the remaining four sites, the negative aural records–human activity relationships represented species richness losses of an average of 31.3 % (± SE 2.4 %). Thus, to provide conservative values for these sites, together with those observed, we also report these in our results to consider the loss in aural records as a correction factor of the decrease in species richness with increasing human activities. In doing so, we assume that decreases in aural records were caused by increases in the noise generated by human activity. For instance, for the peri–urban site of Cali that showed a 75 % decrease in bird species richness when contrasting the first two and last two survey weeks, we calculated a relative decrease in aural records of 26.5 %, and thus subtracted this proportion from the recorded decreases, as follows: 75 × [(100–26.5) / 100] = 55.12.
Results
We recorded 142 bird species across our survey sites in nine Colombian urban centers (table 1s in supplementary material). The average species richness per site was 36.4 species (± SD 10.0). Our analyses linking COVID–19 modifications in human activity to weekly accumulated bird species richness per site showed a negative relationship between human activity in 46 % of the surveyed sites (average correlation coefficient –0.54 ± SE 0.06), with the remaining 39 % being neutral (average correlation coefficient 0.09 ± SE 0.08), and 15 % being positive (average correlation coefficient 0.48 ± SE 0.09) (fig. 1s in supplementary material). Considering the aforementioned coefficients of correlation, results of the generalized additive mixed model showed that, when controlling for city identity and survey week as random factors, the coefficients decreased significantly in relation to human activity with increasing built cover in extra– and peri–urban sites, but not in intra–urban sites (table 1, fig. 2, 3s in supplementary material).
Negative coefficients (r ≤ –0.35) indicated a higher relationship between human activity and bird species richness in peri–urban areas, most of which were recorded at sites with less than 60% built cover. On the contrary, the two sites with the highest built cover (82.4 and 90.5 %) showed positive associations between human activity shifts and weekly accumulated bird species richness per survey site. To provide a perspective of the magnitude of our results, we calculated the average bird species richness in the first two weeks and in the last two weeks of the survey at sites that showed negative correlation coefficients. After the strict lockdown, the decrease in average species richness in these sites was 32 % (± SE 9.8 %), with one site having 75 % more species when contrasting the initial and final survey weeks, representing a ~55 % conservative increase after aural assessment correction.
Discussion
Evidence provided here shows that bird species richness in well–vegetated urban sites was more sensitive
Animal Biodiversity and Conservation 45.2 (2022) 321
to human activity than that in heavily–built conditions, which generally foster less diversity (Gil and Brumm, 2014). The rapid avian response to use well–vegetated survey sites during such an unprecedented reduction of human activity as result of the COVID–19 strict lockdown was probably due to the resources available at these sites, resources that are often scarce in heavily–built sites (Fischer et al., 2015). The finding that well–vegetated peri–urban sites were among those with the highest decreases in bird species richness as human activity increased highlights the ecological importance of urban peripheries, at least in landscapes where the human footprint is not as intense. There, in such circumstances, urban and non–urban systems interact, and a higher number of bird species inhabiting nearby non–urban habitats may facilitate the incursion into urban habitats (MacGregor–Fors, 2010). Our findings also show that well–vegetated intra–urban sites experienced a decrease in bird species richness, although smaller in magnitude, with increased human activity following the strict lockdown, underlining the importance of urban vegetation beyond greenspace networks (Wood and Esaian, 2020).
Many urban–related species, often human commensals, have been shown to use and even depend on people and the consequences of the modern urban lifestyle. Apparently, individuals of some of the recorded species started using well–vegetated sites in the absence of the resources and conditions they typically rely on in heavily–built sites (Rodewald and Shustack, 2008). Although little evidence is yet available, individuals of a species as dependent on urbanization as the rock pigeon (Columba livia) were only recorded at the site with the highest vegetation cover in this study during the strict lockdown. These observations of urban–related species add to the existing evidence of the dependency that some urban species have on human activity and its consequences, suggesting that this association could be tighter than previously thought (Murgui and Hedblom, 2017).
However, considering that our ability to detect birds could have decreased at some survey sites as human activity increased, a conservative average value for such a decrease is 25 % (± SE 7.6 %; see the methodological section for detectability). This indicates that the severe reduction in human activity during the COVID–19 lockdown in almost half of our survey sites was drastic, showing an increase of at least one–fourth in species richness during the strict lockdown period. It is noteworthy that the strict lockdown lasted only six weeks, and in this short window of time many bird species responded swiftly –as seen in other studies (Gordo et al., 2021, Sanderfoot et al., 2022)– and with a similar pattern for nocturnal species (Estela et al., 2021), making local assemblages more diverse, particularly those from low–intensity urbanization sites.
An example of the importance of human activity recorded in this study is that of a residential peri–urban location in the city of Cali where we recorded a 75 % decrease in bird species richness when comparing the accumulated richness from the first two weeks surveyed during the strict lockdown with
that of the last two surveyed weeks (~55 % when accounting for potential detectability issues; see the Detectability section in the Material and methods for further detail), that is, seven weeks after the initial reactivation of activities and the consequent increase in human activity during the relaxed lockdown. Such an increase in bird species richness was partially associated with the arrival of flycatcher species (family Tyrannidae) at the beginning of our surveys. Besides some very common generalist flycatchers (tropical kingbird Tyrannus melancholicus), we frequently recorded species such as the yellow–olive flatbill (Tolmomyias sulphurescens) and the piratic flycatcher (Legatus leucophaius), but only when human activity was low. This observation illustrates how species that are absent or scarce during regular human activity scenarios may still use urban habitats, yet seem to be driven away by our activity.
Given the unexpected nature of the pandemic itself, which molded the nature of our surveys (and thus the data), we recognize that many potential confounding factors, such as noise or seasonality, could play a role in our findings. We tried to consider the potential biases related to detectability in indirect ways, but were unable to measure them directly. Neither were we able to generate a comparable dataset from pre–pandemic times. However, having found the three types of relationships across our survey sites leads us to believe that a change in detectability in both our time windows (although not negligible) does not seem to be a key factor, and thus our results are reliable
Conclusions
Addressing the emerging mechanistic processes behind the patterns related to urbanization has become a major goal in urban ecology (McDonnell and Hahs, 2015). Our findings show that human activity was related to a considerable loss of bird species richness in urban systems. Such activity acts synergistically with urbanization, with profound effects on well–vegetated peri–urban sites. This highlights the importance of developing activity plans in urban greenspaces if our aim is not simply to maintain but to enhance biodiversity in cities. In doing so, human activity management plans in urban greenspaces will need to balance the positive and negative effects of visitation rates. Materializing such plans will require not only the cooperation of local governments but also increased awareness among the local population regarding the importance of creating livable, healthy, biodiverse, and resilient cities that can provide increasing ecosystem services to urbanites (McDonnell and MacGregor–Fors, 2016). Our findings may prompt future research on the response of avian communities and other wildlife groups for which information is available before, during, and/or after COVID–19 lockdowns. Did the COVID–19 lockdowns have only a momentary effect on bird diversity, or will they be sufficient to drive further behavioral or functional consequences? Together with our results, future studies focused on
322 MacGregor–Fors et al.
disentangling the effect of human activities from the rest of the urban–related environmental changes will increase our understanding of the consequences of our day–to–day activities in limiting the biodiversity that surrounds us urbanites.
Acknowledgements
We thank Roger Guevara, Christine C. Rega–Brodsky, Karl Evans, Pam J. Yeh, Eleanor S. Diamant, Juan Carlos Senar, and two anonymous reviewers for their helpful comments that enhanced the quality and clarity of our manuscript. We are also grateful to all the volunteers that participated in the citizen science initiative of urban birds in Colombia during COVID–19 lockdowns.
References
Arbeláez–Cortés, E., Sánchez–Sarria, C. E., Ocampo, D., Estela, F. A., García–Arroyo, M., MacGregor–Fors, I., 2021. Experiences of surveying urban birds during the anthropause in Colombia. Ornitología Neotropical, 32(2): 166–169.
Aronson, M. F. J., La Sorte, F. A., Nilon, C. H., Katti, M., Goddard, M. A., Lepczyk, C. A., Warren, P. S., Williams, N. S. G., Cilliers, S., Clarkson, B., Dobbs, C., Dolan, R., Hedblom, M., Klotz, S., Kooijmans, J. L., Kühn, I., MacGregor–Fors, I., McDonnell, M., Mörtberg, U., Pysek, P., Siebert, S., Sushinsky, J., Werner, P., Winter, M., 2014. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proceedings of the Royal Society B, 281(1780): 20133330, Doi: 10.1098/rspb.2013.3330
Avendaño, J. E., Bohórquez, C. I., Rosselli, L., Arzuza–Buelvas, D., Estela, F. A., Cuervo, A. M., Stiles, F. G., Renjifo, L. M., 2017. Lista de chequeo de las aves de Colombia: Una síntesis del estado del conocimiento desde Hilty & Brown (1986). Ornitología Colombiana, 16: eA01-83.
Bibby, C. J., Burges, N. D., Hill, D. A., Mustoe, S. H., 2000. Bird census techniques, 2nd edition. Academic Press Limited, London, UK. Blair, R. B., 1996. Land use and avian species diversity along an urban gradient. Ecological Applications, 6(2): 506–519, Doi: 10.2307/2269387
Blondel, V. D., Decuyper, A., Krings, G., 2015. A survey of results on mobile phone datasets analysis. EPJ Data Science, 4: 1–55, Doi: 10.1140/epjds/ s13688-015-0046-0
Bötsch, Y., Tablado, Z., Jenni, L., 2017. Experimental evidence of human recreational disturbance effects on bird–territory establishment. Proceedings of the Royal Society B: Biological Sciences, 284(1858): 20170846.20170846, Doi: 10.1098/rspb.2017.0846
Bötsch, Y., Tablado, Z., Scherl, D., Kéry, M., Graf, R. F., Jenni, L., 2018. Effect of recreational trails on forest birds: human presence matters. Frontiers in Ecology and Evolution, 6: 175, Doi: 10.3389/ fevo.2018.00175
Consejería Presidencial para las Regiones, 2020.
Coronavirus (COVID–19) Decretos y Lineamientos del Gobierno Nacional de Colombia. Available online at: https://www.minsalud.gov.co/salud/ publica/PET/Paginas/Documentos-Administrativoscovid-19.aspx
Corsini, M., Dubiec, A., Marrot, P., Szulkin, M., 2017. Humans and tits in the city: quantifying the effects of human presence on Great Tit and Blue Tit reproductive trait variation. Frontiers in Ecology and Evolution, 5: 82, Doi: 10.3389/fevo.2017.00082
Cot, C., Cacciapaglia, G., Sannino, F., 2021. Mining Google and Apple mobility data: temporal anatomy for COVID–19 social distancing. Scientific Reports, 11(1): 4150, Doi: 10.1038/s41598-021-83441-4
Crawley, M. J., 2013. The R book, 2nd edition. Wiley, West Sussex, UK.
Estela, F. A., Sánchez–Sarria, C. E., Arbeláez–Cortés, E., Ocampo, D., García–Arroyo, M., Perlaza–Gamboa, A., Wagner–Wagner, C. M., MacGregor–Fors, I., 2021. Changes in the nocturnal activity of birds during the COVID–19 pandemic lockdown in a neotropical city. Animal Biodiversity and Conservation, 44.2: 213–217, Doi: 10.32800/abc.2021.44.0213
Escobar–Ibáñez, J. F., Rueda–Hernández, R. A., MacGregor–Fors, I., 2020. The greener the better: Avian communities across a Neotropical gradient of urbanization densitity. Frontiers in Ecology and Evolution, 8: 500791, Doi: 10.3389/ fevo.2020.500791
Faeth, S. H., Warren, P. S., Shochat, E., Marussich, W. A., 2005. Trophic Dynamics in Urban Communities. BioScience, 55(5): 399–407, Doi: 10.1641/0006-3568(2005)055[0399:TDIUC]2.0.CO;2 Fischer, J. D., Schneider, S. C., Ahlers, A. A., Miller, J. R., 2015. Categorizing wildlife responses to urbanization and conservation implications of terminology. Conservation Biology, 29(4): 1246–1248, Doi: 10.1111/cobi.12451
Forman, R. T., 2014. Urban ecology: Science of cities Cambridge University Press, Cambridge, UK. Gil, D., Brumm, H., 2014. Avian urban ecology: behavioural and physiological adaptations. Oxford University Press, Oxford, USA
Google, 2020. See how your community moved differently due to COVID–19. Community Mobility Report. Available online at: google.com/covid19/ mobility?hl=en [Accessed on July 27th, 2020]
Gordo, O., Brotons, L., Herrando, S., Gargallo, G., 2021. Rapid behavioural response of urban birds to COVID–19 lockdown. Proceedings of the Royal Society B, 288: 20202513, Doi: 10.1098/ rspb.2020.2513
Grimm, N. B., Faeth, S. H., Golubiewski, N. E., Redman, C. L., Wu, J., Bai, X., Briggs, J. M., 2008. Global change and the ecology of cities. Science , 319(5864): 756–760, Doi: 10.1126/ science.1150195
IGAC (Instituto Geográfico Agustín Codazzi), 2008. Atlas Básico de Colombia, 7th edition. Imprenta Nacional de Colombia, Bogotá, Colombia.
La Sorte, F. A., Lepczyk, C. A., Aronson, M. F. J., Goddard, M., Hedblom, M., Katti, M., MacGregor–Fors, I., Mörtberg, U., Nilon, C. H., Warren, P. S.,
Animal Biodiversity and Conservation 45.2 (2022) 323
Williams, N. S. G., Yang, J., 2018. The phylogenetic and functional diversity of regional breeding bird assemblages is reduced and constrained through urbanization. Diversity and Distributions, 24(7): 928–938, Doi: 10.1111/ddi.12738
Lemoine–Rodríguez, R., MacGregor–Fors, I., Muñoz–Robles, C., 2019. Six decades of urban green change in a neotropical city: a case study of Xalapa, Veracruz, Mexico. Urban Ecosystems, 22: 609–618, Doi: 10.1007/s11252-019-00839-9
MacGregor–Fors, I., 2010. How to measure the urban–wildland ecotone: Redefining 'peri–urban' areas. Ecological Research, 25: 883–887, Doi: 10.1007/s11284-010-0717-z
MacGregor–Fors, I., Escobar–Ibáñez, J. F., 2017. Avian Ecology in Latin American Cityscapes Springer International Publishing, Cham.
MacGregor–Fors, I, García–Arroyo, M., Quesada, J., 2022. Keys to the city: an integrative conceptual framework on avian urban filtering. Journal of Urban Ecology, 8(1): juac026, Doi: 10.1093/jue/juac026
Marzluff, J. M., Bowman, R., Donnelly, R., Eds., 2001. Avian Ecology and Conservation in an Urbanizing World. Springer US, Boston, MA
Maxwell, S. L., Fuller, R. A., Brooks, T. M., Watson, J. E. M., 2016. Biodiversity: The ravages of guns, nets and bulldozers. Nature, 536: 143–145, Doi: 10.1038/536143a
McDonnell, M. J., Hahs, A. K., 2015. Adaptation and adaptedness of organisms to urban environments. Annual Review of Ecology, Evolution, and Systematics, 46: 261–280, Doi: 10.1146/annurevecolsys-112414-054258
McDonnell, M., MacGregor–Fors, I., 2016. The ecological future of the cities. Science, 352: 934–936, Doi: 10.1126/science.aaf3630
Meekan, M. G., Duarte, C. M., Fernández–Gracia, J., Thums, M., Sequeira, A. M. M., Harcourt, R., Eguíluz, V. M., 2017. The Ecology of Human Mobility. Trends in Ecology and Evolution, 32(3): 198–210, Doi: 10.1016/j.tree.2016.12.006
Morelli, F., Mikula, P., Benedetti, Y., Bussière, R., Jerzak, L., Tryjanowski, P., 2018. Escape behaviour of birds in urban parks and cemeteries across Europe: Evidence of behavioural adaptation to human activity. Science of the Total Environment, 631–632: 803–810, Doi: 10.1016/j. scitotenv.2018.03.118
Murgui, E., Hedblom, M., 2017. Ecology and Conservation of Birds in Urban Environments. Springer International Publishing, Cham, Switzerland. Olson, D. M., Dinerstein, E., Wikramanayake, E. D., Burgess, N. D., Powell, G. V. N., Underwood, E. C., D’amico, J. A., Itoua, I., Strand, H. E., Morrison, J. C., Loucks, C. J., Allnutt, T. F., Ricketts, T. H., Kura, Y., Lamoreux, J. F., Wettengel, W. W., Hedao, P., Kassem, K. R., 2001. Terrestrial ecoregions of the world: A new map of life on Earth. BioScience, 51(11): 933–938, Doi: 10.1641/0006-3568(2001)051[0933:TEOTWA ]2.0.CO;2
Ortega, C. P., Francis, C. D., 2012. Chapter 7: Effects of gas–well–compressor noise on the ability to detect birds during surveys in northwest New Mexico.
Ornithological Monographs, 74(1): 78–90, Doi: 10.1525/om.2012.74.1.78
Pollack, L., Ondrasek, N. R., Calisi, r., 2017. Urban health and ecology: the promise of an avian biomonitoring tool. Current Zoology, 63(2): 205–212, Doi: 10.1093/cz/zox011
Pena, J. C. de Castro, Martello, F., Ribeiro, M. C., Armitage, R. A., Young, R. J., Rodrigues, M., 2017. Street trees reduce the negative effects of urbanization on birds. Plos One, 12: e0174484, Doi: 10.1371/journal.pone.0174484
Perillo, A., Mazzoni, L. G., Passos, L. F., Goulart, V. D. L. R., Duca, C., Young, R. J., 2017. Anthropogenic noise reduces bird species richness and diversity in urban parks. Ibis, 159(3): 638–646, Doi: 10.1111/ibi.12481
Quesada, J., Chávez–Zichinelli, C. A., García–Arroyo, M., Yeh, P. J., Guevara, R., Izquierdo–Palma, J., MacGregor–Fors, I., 2022. Bold or shy? Examining the risk–taking behavior and neophobia of native and exotic house sparrows. Animal Biodiversity and Conservation, 45.1: 97–106, Doi: 10.32800/ abc.2022.45.0097
R Core Team, 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Rodewald, A. D., Shustack, D. P., 2008. Consumer resource matching in urbanizing landscapes: are synanthropic species over–matching. Ecology, 89(2): 515–521, Doi: 10.1890/07-0358.1
Rubin, A., 2012. Statistics for evidence–based practice and evaluation. Cengage Learning, Canada.
Rutz, C., Loretto, M. C., Bates, A. E., Davidson, S. C., Duarte, C. M., Jetz, W., Johnson, M., Kato, A., Kays, R., Mueller, T., Primack, R. B., Ropert–Coudert, Y., Tucker, M. A., Wikelski, M., Cagnacci, F., 2020. COVID–19 lockdown allows researchers to quantify the effects of human activity on wildlife. Nature Ecology and Evolution, 4(9): 1156–1159, Doi: 10.1038/s41559-020-1237-z
Sanderfoot, O. V., Kaufman, J. D., Gardner, B., 2022. Drivers of avian habitat use and detection of backyard birds in the Pacific Northwest during COVID–19 pandemic lockdowns. Scientific Reports, 12(1): 12655, Doi: 10.1038/s41598-022-16406-w
Schlesinger, M. D., Manley, P. N., Holyoak, M., 2008. Distinguishing stressors acting on land bird communities in an urbanizing environment. Ecology, 89(8): 2302–2314, Doi: 10.1890/07-0256.1
Shochat, E., 2004 Credit or debit? Resource input changes population dynamics of city–slicker birds. Oikos , 106(3): 622–626, Doi: 10.1111/j.00301299.2004.13159.x
Slabbekoorn, H., Ripmeester, E. A. P., 2008. Birdsong and anthropogenic noise: implications and applications for conservation. Molecular Ecology, 17(1): 72–83, Doi: 10.1111/j.1365-294X.2007.03487.x
Sol, D., González–Lagos, C., Moreira, D., Maspons, J., Lapiedra, O., 2014. Urbanisation tolerance and the loss of avian diversity. Ecology Letters, 17(8): 942–950, Doi: 10.1111/ele.12297
Sol, D., Lapiedra, O., González–Lagos, C., 2013. Behavioural adjustments for a life in the city. Ani-
324
MacGregor–Fors et al.
mal Behaviour, 85(5): 1101–1112, Doi: 10.1016/j. anbehav.2013.01.023
Sol, D., Timmermans, S., Lefebvre, L., 2002. Behavioural flexibility and invasion success in birds. Animal Behaviour, 63(3): 495–502, Doi: 10.1006/ anbe.2001.1953
Spelt, A., Soutar, O., Williamson, C., Memmott, J., Shamoun–Baranes, J., Rock, P., Windsor, S., 2021. Urban gulls adapt foraging schedule to human–activity patterns. Ibis, 163(1): 274–282, Doi: 10.1111/ibi.12892
Stankowich, T., Blumstein, D. T., 2005. Fear in animals: A meta–analysis and review of risk assessment. Proceedings of the Royal Society B, 272(1581): 2627–2634, Doi: 10.1098/rspb.2005.3251
Thomas, H., Webster, S., Petherick, A., Phillips, T.,
Kira, B., 2020. Oxford COVID–19 Government Response Tracker. Blavatnik School of Goverment, Oxford University. Available online at: https://www. bsg.ox.ac.uk/research/research-projects/coronavirus-government-response-tracker [Accessed on July 27th, 2020]
Wood, E. M., Esaian, S., 2020. The importance of street trees to urban avifauna. Ecological Applications, 30(7): e02149, Doi: 10.1002/eap.2149
Zellmer, A. J., Wood, E. M., Surasinghe, T., Putman, B. J., Pauly, G. B., Magle, S. B., Lewis, J. S., Kay, C. A. M., Fidino, M., 2020. What can we learn from wildlife sightings during the COVID–19 global shutdown? Ecosphere, 11(8): e03215, Doi: 10.1002/ecs2.3215
Animal Biodiversity and Conservation 45.2
325
(2022)
42 Muñoz and Farfán
Animal Biodiversity and Conservation 45.2 (2022) I

Animal Biodiversity and Conservation
Animal Biodiversity and Conservation és (abans Miscel·lània Zoològica) és una revista interdisciplinària publicada, des de 1958, pel Museu de Ciències Naturals de Barcelona. Inclou articles d'investigació empírica i teòrica en totes les àrees de la zoologia (sistemàtica, taxonomia, morfologia, biogeografia, ecologia, etologia, fisiologia i genètica) procedents de totes les regions del món. La revista presta una atenció especial als estudis que plantegen un problema nou o que introdueixen un nou tema, amb unes hipòtesis i prediccions clares i als treballs que d'una manera o altre tinguin rellevància en la biologia de la conservació. No es publicaran articles purament descriptius o articles faunístics o corològics que descriguin la distribució en l'espai o en el temps dels organismes zoològics. Aquests treballs s'han de redirigir a la nostra revista germana Arxius de Miscel·lània Zoològica (museucienciesjournals. cat/amz). Els estudis realitzats amb espècies rares o protegides poden no ser acceptats tret que els autors disposin dels permisos corresponents. Cada volum anual consta de dos fascicles.
Animal Biodiversity and Conservation es troba registrada en la majoria de les bases de dades més importants i està disponible gratuitament a internet a museucienciesjournals.cat/abc de manera que permet una difusió mundial dels seus articles.
Tots els manuscrits són revisats per l'editor executiu, un editor i dos revisors independents, triats d'una llista internacional, a fi de garantir–ne la qualitat. El procés de revisió és ràpid i constructiu. La publicació dels treballs acceptats es fa normalment dintre dels 12 mesos posteriors a la recepció.
Una vegada hagin estat acceptats passaran a ser propietat de la revista. Aquesta es reserva els drets d’autor, i cap part dels treballs no podrà ser reproduïda sense citar–ne la procedència.
Els drets d’autor queden reservats als autors, els qui autoritzen la revista a publicar l’article. Els articles es publiques amb una Llicència de Reconeixement 4.0 Internacional de Creative Commons: no es podrà reproduir ni reutilitzar cap part dels treballs publicats sense citar-ne la procedència.
Normes de publicació
Els treballs s'enviaran preferentment de forma electrònica ( abc@bcn.cat). El format preferit és un document Rich Text Format (RTF) o DOC que inclogui les figures i les taules. Les figures s'hauran d'enviar també en arxius apart en format TIFF, EPS o JPEG. Cal incloure, juntament amb l'article, una carta on es faci constar que el treball està basat en investigacions originals no publicades anteriorment i que està sotmès a Animal Biodiversity and Conservation en exclusiva. A la carta també ha de constar, per a aquells treballs en que calgui manipular animals, que els autors disposen dels permisos necessaris i que compleixen la normativa de protecció animal vigent. També es poden suggerir possibles assessors.
ISSN: 1578–665X eISSN: 2014–928X
Les proves d'impremta enviades a l'autor per a la correcció, seran retornades al Consell Editor en el termini de 10 dies. Publicar a Animal Biodiversity and Conservation es gratuït per als autors, tot i que les despeses degudes a modificacions substancials introduïdes per ells en el text original acceptat aniran a càrrec dels autors.
El primer autor rebrà una còpia electrònica del treball en format PDF.
Manuscrits
Els treballs seran presentats en format DIN A–4 (30 línies de 70 espais cada una) a doble espai i amb totes les pàgines numerades. Els manuscrits han de ser complets, amb taules i figures. No s'han d'enviar les figures originals fins que l'article no hagi estat acceptat.
El text es podrà redactar en anglès, castellà o català. Se suggereix als autors que enviïn els seus treballs en anglès. La revista els ofereix, sense cap càrrec, un servei de correcció per part d'una persona especialitzada en revistes científiques. En tots els casos, els textos hauran de ser redactats correctament i amb un llenguatge clar i concís.
Els caràcters cursius s’empraran per als noms científics de gèneres i d’espècies i per als neologismes intraduïbles; les cites textuals, independentment de la llengua, seran consignades en lletra rodona i entre cometes i els noms d’autor que segueixin un tàxon aniran en rodona. S'evitarà l'ús de termes extrangers (p. ex.: llatí, alemany,...).
Quan se citi una espècie per primera vegada en el text, es ressenyarà, sempre que sigui possible, el seu nom comú.
Els topònims s’escriuran o bé en la forma original o bé en la llengua en què estigui escrit el treball, seguint sempre el mateix criteri.
Els nombres de l’u al nou, sempre que estiguin en el text, s’escriuran amb lletres, excepte quan precedeixin una unitat de mesura. Els nombres més grans s'escriuran amb xifres excepte quan comencin una frase.
Les dates s’indicaran de la forma següent: 28 VI 99 (un únic dia); 28, 30 VI 99 (dies 28 i 30); 28–30 VI 99 (dies 28 a 30).
S’evitaran sempre les notes a peu de pàgina.
Format dels articles
Títol. Serà concís, però suficientment indicador del contingut. Els títols amb designacions de sèries numèriques (I, II, III, etc.) seran acceptats previ acord amb l'editor.
Nom de l’autor o els autors Abstract en anglès que no ultrapassi les 12 línies mecanografiades (860 espais) i que mostri l’essència del manuscrit (introducció, material, mètodes, resultats i discussió). S'evitaran les especulacions i les cites bibliogràfiques. Estarà encapçalat pel títol del treball en cursiva Key words en anglès (sis com a màxim), que orientin sobre el contingut del treball en ordre d’importància.
© 2022 Museu de Ciències Naturals de Barcelona Papers are published under a Creative Commons Attribution 4.0 International License
Resumen en castellà, traducció de l'Abstract. De la traducció se'n farà càrrec la revista per a aquells autors que no siguin castellanoparlants.
Palabras clave en castellà. Adreça postal de l’autor o autors, es publicaran tal i com s’indiqui en el manuscrit rebut.
Identificadors d’investigador (ORCID, ResearchID,…), al menys de l’investigador principal i de qui assumeixi la correspondència posterior.
(Títol, Nom dels autors, Abstract, Key words, Resumen, Palabras clave, Adreça postal e Identificadors d’investigador conformaran la primera pàgina.)
Introducción. S'hi donarà una idea dels antecedents del tema tractat, així com dels objectius del treball. Material y métodos Inclourà la informació pertinent de les espècies estudiades, aparells emprats, mètodes d’estudi i d’anàlisi de les dades i zona d’estudi.
Resultados. En aquesta secció es presentaran únicament les dades obtingudes que no hagin estat publicades prèviament.
Discusión Es discutiran els resultats i es compararan amb treballs relacionats. Els suggeriments de recerques futures es podran incloure al final d’aquest apartat.
Agradecimientos (optatiu).
Referencias Cada treball haurà d’anar acompanyat de les referències bibliogràfiques citades en el text.
Les referències han de presentar–se segons els models següents (mètode Harvard):
* Articles de revista: Conroy, M. J., Noon, B. R., 1996. Mapping of species richness for conservation of biological diversity: conceptual and methodological issues. Ecological Applications, 6: 763–773, Doi: 10.2307/2269481
* Llibres o altres publicacions no periòdiques: Seber, G. A. F., 1982. The estimation of animal abundance. C. Griffin and Company, London.
* Treballs de contribució en llibres: Macdonald, D. W., Johnson, D. P., 2001. Dispersal in theory and practice: consequences for conservation biology. In: Dispersal: 358–372 (T. J. Clober, E. Danchin, A. A. Dhondt, J. D. Nichols, Eds.). Oxford University Press, Oxford.
* Tesis doctorals: Merilä, J., 1996. Genetic and quantitative trait variation in natural bird populations. Tesis doctoral, Uppsala University.
* Els treballs en premsa només han d’ésser citats si han estat acceptats per a la publicació: Ripoll, M. (in press). The relevance of population studies to conservation biology: a review. Animal Biodiversity and Conservation. La relació de referències bibliogràfiques d’un tre -
ball serà establerta i s’ordenarà alfabèticament per autors i cronològicament per a un mateix autor, afegint les lletres a, b, c,... als treballs del mateix any. En el text, s’indi caran en la forma usual: "... segons Wemmer (1998)...", "...ha estat definit per Robinson i Redford (1991)...", "...les prospeccions realitzades (Begon et al. , 1999)...". Taules. Es numeraran 1, 2, 3, etc. i han de ser sempre ressenyades en el text. Les taules grans seran més estretes i llargues que amples i curtes ja que s'han d'encaixar en l'amplada de la caixa de la revista. Figures. Tota classe d’il·lustracions (gràfics, figures o fotografies) entraran amb el nom de figura i es numeraran 1, 2, 3, etc. i han de ser sempre ressenyades en el text. Es podran incloure fotografies si són imprescindibles. Si les fotografies són en color, el cost de la seva publicació anirà a càrrec dels autors. La mida màxima de les figures és de 15,5 cm d'amplada per 24 cm d'alçada. S'evitaran les figures tridimensionals. Tant els mapes com els dibuixos han d'incloure l'escala. Els ombreigs preferibles són blanc, negre o trama. S'evitaran els punteigs ja que no es reprodueixen bé.
Peus de figura i capçaleres de taula. Seran clars, concisos i bilingües en la llengua de l’article i en anglès.
Grans quantitats de dades o taules numèriques molt llargues es publicaran com a Material suplementari. Aquest material suplementari només acompanyarà a la versió online de l'article, en cap cas a la versió impresa.
Els títols dels apartats generals de l’article (Introducción, Material y métodos, Resultados, Discusión, Conclusiones, Agradecimientos y Referencias) no aniran numerats. No es poden utilitzar més de tres nivells de títols.
Els autors procuraran que els seus treballs originals no passin de 20 pàgines (incloent–hi figures i taules).
Si a l'article es descriuen nous tàxons, caldrà que els tipus estiguin dipositats en una institució pública.
Es recomana als autors la consulta de fascicles recents de la revista per tenir en compte les seves normes.
Comunicacions breus
Les comunicacions breus seguiran el mateix procediment que els articles i tindran el mateix procés de revisió. No excediran de 2.300 paraules incloent–hi títol, resum, capçaleres de taula, peus de figura, agraïments i referències. El resum no ha de passar de 100 paraules i el nombre de referències ha de ser de 15 com a màxim. Que el text tingui apartats és opcional i el nombre de taules i/o figures admeses serà de dos de cada com a màxim. En qualsevol cas, el treball maquetat no podrà excedir les quatre pàgines.
II
Animal Biodiversity and Conservation 45.2 (2022)
Animal Biodiversity and Conservation
Animal Biodiversity and Conservation (antes Miscel·lània Zoològica) es una revista interdisciplinar, publicada desde 1958 por el Museu de Ciències Naturals de Barcelona. Incluye artículos de investigación empírica y teórica en todas las áreas de la zoología (sistemática, taxonomía, morfología, biogeografía, ecología, etología, fisiología y genética) procedentes de todas las regiones del mundo. La revista presta especial interés a los estudios que planteen un problema nuevo o introduzcan un tema nuevo, con hipòtesis y prediccions claras, y a los trabajos que de una manera u otra tengan relevancia en la biología de la conservación. No se publicaran artículos puramente descriptivos, o artículos faunísticos o corológicos en los que se describa la distribución en el espacio o en el tiempo de los organismes zoológicos. Esos trabajos deben redirigirse a nuestra revista hemana Arxius de Miscel·lània Zoològica (museucienciesjournals.cat/amz). Los estudios realizados con especies raras o protegidas pueden no ser aceptados a no ser que los autores dispongan de los permisos correspondientes. Cada volumen anual consta de dos fascículos.
Animal Biodiversity and Conservation está registrada en todas las bases de datos importantes y además está disponible gratuitamente en internet en museucienciesjournals.cat/abc lo que permite una difusión mundial de sus artículos.
Todos los manuscritos son revisados por el editor ejecutivo, un editor y dos revisores independientes, elegidos de una lista internacional, a fin de garantizar su calidad. El proceso de revisión es rápido y constructivo, y se realiza vía correo electrónico siempre que es posible. La publicación de los trabajos aceptados se realiza con la mayor rapidez posible, normalmente dentro de los 12 meses siguientes a la recepción del trabajo.
Una vez aceptado, el trabajo pasará a ser propiedad de la revista. Ésta se reserva los derechos de autor, y ninguna parte del trabajo podrá ser reproducida sin citar su procedencia.
Los derechos de autor quedan reservados a los autores, quienes autorizan a la revista a publicar el artículo. Los artículos se publican con una Licencia Creative Commons Atribución 4.0 Internacional: no se podrá reproducir ni reutilizar ninguna de sus partes sin citar la procedencia.
Normas de publicación
Los trabajos se enviarán preferentemente de forma electrónica (abc@bcn.cat). El formato preferido es un documento Rich Text Format (RTF) o DOC, que incluya las figuras y las tablas. Las figuras deberán enviarse también en archivos separados en formato TIFF, EPS o JPEG. Debe incluirse, con el artículo, una carta donde conste que el trabajo versa sobre investigaciones originales no publicadas anteriormente y que se somete en exclusiva a Animal Biodiversity and Conservation. En dicha carta también debe constar, para trabajos donde sea necesaria la manipulación de animales, que los autores disponen de los permi-
ISSN: 1578–665X eISSN: 2014–928X

sos necesarios y que han cumplido la normativa de protección animal vigente. Los autores pueden enviar también sugerencias para asesores.
Las pruebas de imprenta enviadas a los autores deberán remitirse corregidas al Consejo Editor en el plazo máximo de 10 días. Publicar en Animal Biodiversity and Conservation es gratuito para los autores, sin embargo los gastos debidos a modificaciones sustanciales en las pruebas de imprenta, introducidas por los autores, irán a cargo de los mismos.
El primer autor recibirá una copia electrónica del trabajo en formato PDF.
Manuscritos
Los trabajos se presentarán en formato DIN A–4 (30 líneas de 70 espacios cada una) a doble espacio y con las páginas numeradas. Los manuscritos deben estar completos, con tablas y figuras. No enviar las figuras originales hasta que el artículo haya sido aceptado.
El texto podrá redactarse en inglés, castellano o catalán. Se sugiere a los autores que envíen sus trabajos en inglés. La revista ofrece, sin cargo ninguno, un servicio de corrección por parte de una persona especializada en revistas científicas. En cualquier caso debe presentarse siempre de forma correcta y con un lenguaje claro y conciso.
Los caracteres en cursiva se utilizarán para los nombres científicos de géneros y especies y para los neologismos que no tengan traducción; las citas textuales, independientemente de la lengua en que estén, irán en letra redonda y entre comillas; el nombre del autor que sigue a un taxón se escribirá también en redonda. Se evitará el uso de términos extranjeros (p. ej.: latín, aleman,...).
Al citar por primera vez una especie en el trabajo, deberá especificarse siempre que sea posible su nombre común.
Los topónimos se escribirán bien en su forma original o bien en la lengua en que esté redactado el trabajo, siguiendo el mismo criterio a lo largo de todo el artículo.
Los números del uno al nueve se escribirán con letras, a excepción de cuando precedan una unidad de medida. Los números mayores de nueve se escribirán con cifras excepto al empezar una frase.
Las fechas se indicarán de la siguiente forma: 28 VI 99 (un único día); 28, 30 VI 99 (días 28 y 30); 28–30 VI 99 (días 28 al 30).
Se evitarán siempre las notas a pie de página.
Formato de los artículos
Título. Será conciso pero suficientemente explicativo del contenido del trabajo. Los títulos con designaciones de series numéricas (I, II, III, etc.) serán aceptados excepcionalmente previo consentimiento del editor. Nombre del autor o autores Abstract en inglés de 12 líneas mecanografiadas (860 espacios como máximo) y que exprese la esencia del manuscrito (introducción, material, métodos, resultados y discusión). Se evitarán las especulaciones y las citas bibliográficas. Irá enca -
© 2022 Museu de Ciències Naturals de Barcelona Papers are published under a Creative Commons Attribution 4.0 International License
III
bezado por el título del trabajo en cursiva. Key words en inglés (un máximo de seis) que especifiquen el contenido del trabajo por orden de importancia.
Resumen en castellano, traducción del abstract. Su traducción puede ser solicitada a la revista en el caso de autores que no sean castellano hablantes. Palabras clave en castellano.
Direccion postal del autor o autores, se publicarán tal como se indique en el manuscrito recibido. Identificadores de investigador (ORCID, ResearchID…, al menos del investigador principal y de quien asuma la correspondencia posterior.
(Título, Nombre de los autores, Abstract, Key words, Resumen, Palabras clave, Direcciones postalo e Identificadores de investigador conformarán la primera página.)
Introducción. En ella se dará una idea de los antecedentes del tema tratado, así como de los objetivos del trabajo.
Material y métodos. Incluirá la información referente a las especies estudiadas, aparatos utilizados, metodología de estudio y análisis de los datos y zona de estudio.
Resultados. En esta sección se presentarán únicamente los datos obtenidos que no hayan sido publicados previamente.
Discusión. Se discutirán los resultados y se compararán con otros trabajos relacionados. Las sugerencias sobre investigaciones futuras se podrán incluir al final de este apartado.
Agradecimientos (optativo).
Referencias. Cada trabajo irá acompañado de una bibliografía que incluirá únicamente las publicaciones citadas en el texto.
Las referencias deben presentarse según los modelos siguientes (método Harvard):
* Artículos de revista: Conroy, M. J., Noon, B. R., 1996. Mapping of species richness for conservation of biological diversity: conceptual and methodological issues. Ecological Applications, 6: 763–773, Doi: 10.2307/2269481
* Libros y otras publicaciones no periódicas: Seber, G. A. F., 1982. The estimation of animal abundance. C. Griffin & Company, London.
* Trabajos de contribución en libros: Macdonald, D. W., Johnson, D. P., 2001. Dispersal in theory and practice: consequences for conservation biology. In: Dispersal: 358–372 (T. J. Clober, E. Danchin, A. A. Dhondt, J. D. Nichols, Eds.). Oxford University Press, Oxford.
* Tesis doctorales: Merilä, J., 1996. Genetic and quantitative trait variation in natural bird populations. Tesis doctoral, Uppsala University.
* Los trabajos en prensa sólo se citarán si han sido aceptados para su publicación: Ripoll, M. (in press). The relevance of population
studies to conservation biology: a review. Animal Biodiversity and Conservation.
Las referencias se ordenarán alfabéticamente por autores, cronológicamente para un mismo autor y con las letras a, b, c,... para los trabajos de un mismo autor y año. En el texto las referencias bibliográficas se indicarán en la forma usual: "...según Wemmer (1998)...", "...ha sido definido por Robinson y Redford (1991)...", "...las prospecciones realizadas (Begon et al., 1999)...". Tablas. Se numerarán 1, 2, 3, etc. y se reseñarán todas en el texto. Las tablas grandes deben ser más estrechas y largas que anchas y cortas ya que deben ajustarse a la caja de la revista. Figuras. Toda clase de ilustraciones (gráficas, figuras o fotografías) se considerarán figuras, se numerarán 1, 2, 3, etc. y se citarán todas en el texto. Pueden incluirse fotografías si son imprescindibles. Si las fotografías son en color, el coste de su publicación irá a cargo de los autores. El tamaño máximo de las figuras es de 15,5 cm de ancho y 24 cm de alto. Deben evitarse las figuras tridimensionales. Tanto los mapas como los dibujos deben incluir la escala. Los sombreados preferibles son blanco, negro o trama. Deben evitarse los punteados ya que no se reproducen bien. Pies de figura y cabeceras de tabla. Serán claros, concisos y bilingües en castellano e inglés.
Grandes cantidades de datos o tablas numéricas muy largas se publicarán como material suplementario. Este material suplementario sólo acompañará a la versión online del artículo, en ningún caso a la versión impresa.
Los títulos de los apartados generales del artículo (Introducción, Material y métodos, Resultados, Discusión, Agradecimientos y Referencias) no se numerarán. No utilizar más de tres niveles de títulos.
Los autores procurarán que sus trabajos originales no excedan las 20 páginas incluidas figuras y tablas.
Si en el artículo se describen nuevos taxones, es imprescindible que los tipos estén depositados en alguna institución pública.
Se recomienda a los autores la consulta de fascículos recientes de la revista para seguir sus directrices.
Comunicaciones breves
Las comunicaciones breves seguirán el mismo procedimiento que los artículos y serán sometidas al mismo proceso de revisión. No excederán las 2.300 palabras, incluidos título, resumen, cabeceras de tabla, pies de figura, agradecimientos y referencias. El resumen no debe sobrepasar las 100 palabras y el número de referencias será de 15 como máximo. Que el texto tenga apartados es opcional y el número de tablas y/o figuras admitidas será de dos de cada como máximo. En cualquier caso, el trabajo maquetado no podrá exceder las cuatro páginas.
IV
Animal Biodiversity and Conservation 45.2 (2022)
Animal Biodiversity and Conservation
Animal Biodiversity and Conservation (formerly Miscel·lània Zoològica) is an interdisciplinary journal published by the Museu de Ciències Naturals de Barcelona since 1958. It includes empirical and theoretical research from around the world that examines any aspect of Zoology (Systematics, Taxonomy, Morphology, Biogeography, Ecology, Ethology, Physiology and Genetics). It gives special emphasis to studies that expose a new problem or introduces a new topic, presenting clear hypotheses and predictions, and to studies related to Cconservation Biology. Papers purely descriptive or faunal or chorological describing the distribution in space or time of zoological organisms will not be published. These works should be redirected to our sister magazine Arxius de Miscel·lània Zoològica (museucienciesjournals.cat/amz). Studies concerning rare or protected species will not be accepted unless the authors have been granted the relevant permits or authorisation. Each annual volume consists of two issues.
Animal Biodiversity and Conservation is registered in all principal data bases and is freely available online at museucienciesjournals.cat/abc assuring world–wide access to articles published therein.
All manuscripts are screened by the Executive Editor, an Editor and two independent reviewers so as to guarantee the quality of the papers. The review process aims to be rapid and constructive. Once accepted, papers are published as soon as is practicable. This is usually within 12 months of initial submission.
Upon acceptance, manuscripts become the property of the journal, which reserves copyright, and no published material may be reproduced or cited without acknowledging the source of information.
All rights are reserved by the authors, who authorise the journal to publish the article. Papers are published under a Creative Commons Attribution 4.0 International License: no part of the published paper may be reproduced or reused unless the source is cited.
Information for authors
Electronic submission of papers is encouraged (abc@ bcn.cat). The preferred format is DOC or RTF. All figures must be readable by Word, embedded at the end of the manuscript and submitted together in a separate attachment in a TIFF, EPS or JPEG file. Tables should be placed at the end of the document. A cover letter stating that the article reports original research that has not been published elsewhere and has been submitted exclusively for consideration in Animal Biodiversity and Conservation is also necessary. When animal manipulation has been necessary, the cover letter should also specify that the authors follow current norms on the protection of animal species and that they have obtained all relevant permits and authorisations. Authors may suggest referees for their papers.
Proofs sent to the authors for correction should be returned to the Editorial Board within 10 days. Publishing in Animal Biodiversity and Conservation is free of charge, but expenses due to any substantial alterations of the proofs will be charged to the authors.
ISSN: 1578–665X eISSN: 2014–928X
The first author will receive electronic version of the article in PDF format.
Manuscripts
Manuscripts must be presented in DIN A–4 format, 30 lines, 70 keystrokes per page. Maintain double spacing throughout. Number all pages. Manuscripts should be complete with figures and tables. Do not send original figures until the paper has been accepted.
The text may be written in English, Spanish or Catalan, though English is preferred. The journal provides linguistic revision by an author’s editor. Care must be taken to use correct wording and the text should be written concisely and clearly.
Scientific names of genera and species as well as untranslatable neologisms must be in italics. Quotations in whatever language used must be typed in ordinary print between quotation marks. The name of the author following a taxon should also be written in lower case letters. Foreing terms (e.g. Latin, German,...) should not be used.
When referring to a species for the first time in the text, both common and scientific names should be given when possible. Do not capitalize common names of species unless they are proper nouns (e.g. Iberian rock lizard).
Place names may appear either in their original form or in the language of the manuscript, but care should be taken to use the same criteria throughout the text.
Numbers one to nine should be written in full within the text except when preceding a measure. Higher numbers should be written in numerals except at the beginning of a sentence.
Specify dates as follows: 28 VI 99 (for a single day); 28, 30 VI 99 (referring to two days, e.g. 28th and 30th), 28–30 VI 99 (for more than two consecutive days, e.g. 28th to 30th).
Footnotes should not be used.
Formatting of articles

Title. Must be concise but as informative as possible. Numbering of parts (I, II, III, etc.) should be avoided and will be subject to the Editor’s consent. Name of author or authors
Abstract in English, no longer than 12 typewritten lines (840 spaces), covering the contents of the article (introduction, material, methods, results and discussion). Speculation and literature citation should be avoided. The abstract should begin with the title in italics.
Key words in English (no more than six) should express the precise contents of the manuscript in order of relevance.
Resumen in Spanish, translation of the Abstract. Summaries of articles by non–Spanish speaking authors will be translated by the journal on request.
Palabras clave in Spanish.
Author’s address will be published as they appear in the manuscript file.
© 2022 Museu de Ciències Naturals de Barcelona Papers are published under a Creative Commons Attribution 4.0 International License
V
Researcher’s identifiers (ORCID, ResearchID,…), at least from the first and the corresponding authors.
(Title, Name, Abstract, Key words, Resumen, Palabras clave and Author’s address and Researcher’s identifiers must constitute the first page)
Introduction. Should include the historical background of the subject as well as the aims of the paper. Material and methods. This section should provide relevant information on the species studied, materials, methods for collecting and analysing data, and the study area.
Results. Report only previously unpublished results from the present study.
Discussion. The results and their comparison with related studies should be discussed. Suggestions for future research may be given at the end of this section.
Acknowledgements (optional).
References. All manuscripts must include a bibliography of the publications cited in the text. References should be presented as in the following examples (Harvard method):
* Journal articles: Conroy, M. J., Noon, B. R., 1996. Mapping of species richness for conservation of biological diversity: conceptual and methodological issues. Ecological Applications, 6: 763–773, Doi: 10.2307/2269481
* Books or other non–periodical publications: Seber, G. A. F., 1982. The estimation of animal abundance. C. Griffin & Company, London.
* Contributions or chapters of books: Macdonald, D. W., Johnson, D. P., 2001. Dispersal in theory and practice: consequences for conservation biology. In: Dispersal: 358–372 (T. J. Clober, E. Danchin, A. A. Dhondt, J. D. Nichols, Eds.). Oxford University Press, Oxford.
* PhD thesis: Merilä, J., 1996. Genetic and quantitative trait variation in natural bird populations. PhD thesis, Uppsala University.
* Works in press should only be cited if they have been accepted for publication: Ripoll, M. (in press). The relevance of population studies to conservation biology: a review. Animal Biodiversity and Conservation.
References must be set out in alphabetical and chronological order for each author, adding the letters a, b, c,... to papers of the same year. Bibliographic citations in the text must appear in the usual way: "...according to Wemmer (1998)...", "...has been defined by Robinson
and Redford (1991)...", "...the prospections that have been carried out (Begon et al., 1999)..." Tables. Must be numbered in Arabic numerals with reference in the text. Large tables should be narrow (across the page) and long (down the page) rather than wide and short, so that they can be fitted into the column width of the journal. Figures. All illustrations (graphs, drawings, photographs) should be termed as figures, and numbered consecutively in Arabic numerals (1, 2, 3, etc.) with reference in the text. Glossy print photographs, if essential, may be included. The Journal will publish colour photographs but the author will be charged for the cost. Figures have a maximum size of 15.5 cm wide by 24 cm long. Figures should not be tridimensional. Any maps or drawings should include a scale. Shadings should be kept to a minimum and preferably with black, white or bold hatching. Stippling should be avoided as it may be lost in reproduction. Legends of tables and figures. Legends of tables and figures should be clear, concise, and written both in English and Spanish.
Large amounts of data or long tables will be published as supplementary material. This supplementary material will accompany the online version of the article only, not the printed version.
Main headings (Introduction, Material and methods, Results, Discussion, Acknowledgements and References) should not be numbered. Do not use more than three levels of headings.
Manuscripts should not exceed 20 pages including figures and tables.
If the article describes new taxa, type material must be deposited in a public institution.
Authors are advised to consult recent issues of the journal and follow its conventions.
Brief communications
Brief communications should follow the same procedure as other articles and they will undergo the same review process. They should not exceed 2,300 words including title, abstract, figure and table legends, acknowledgements and references. The abstract should not exceed 100 words, and the number of references should be limited to 15. Section headings within the text are optional. Brief communications may have up to two figures and/or two tables but the whole paper should not exceed four published pages.
VI
Welcome to the electronic version of Animal Biodiversity and Conservation
Recommend this electronic access to your library!
www.museucienciesjournals.cat/abc
Animal Biodiversity and Conservation joins the worldwide Open Access Initiative of providing a permanent online version free of charge and access barriers

This is the result of the growing consensus that open access to research is essential for efficient and rapid scientific communication
ABC alert, a free alerting service, provides e–mail information on the latest issue
To sign on for this service, please send an e–mail to: abc@bcn.cat
Animal Biodiversity and Conservation 45.2 (2022) VII
VIII Animal Biodiversity and Conservation 45.2 (2022)
257–267
Branham, C. C., Frazier, B. S., Strange, J. B., Galloway, A. S., Adams, D. H., Drymon, J. M., Grubbs, R. D., Portnoy, D. S., Wells, R. J. D., Sancho, G. Diet of the bonnethead (Sphyrna tiburo) along the northern Gulf of Mexico and southeastern Atlantic coast of the United States
269–279
Marco–Tresserras, J., López–Iborra, G. M. The effect of sex on home range in an urban population of European hedgehogs Erinaceus europaeus at the southern edge of the species distribution
281–285
Datto–Liberato, F., Roucourt Cezário, R., Guillermo–Ferreira, R. Final instar larva of Neocordulia volxemi (Selys, 1874) (Odonata, Libelluloidea) from southeastern Brazil
287–298
Velasco, A. C., Ferrer, E. S., Sanz, J. J. Exploration behaviour and foraging strategies in Mediterranean blue tits 299–313
Refoyo, P., Olmedo, C., Murciano Cespedosa, A., Muñoz, B. The expansion process of the Iberian ibex in the Sierra de Guadarrama National Park, Madrid (Spain)
315–325
MacGregor–Fors, I., Arbeláez–Cortés, E., Estela, F. A., Ocampo, D., Sánchez–Sarria, C. E., García–Arroyo, M., Aguirre–Samboní, G. K., Cortés–Díaz, D., Franco Morales, J. C., Gaitán–García, C. D., Guerrero–Pelaez, S., Gutiérrez Parodys, Y., Holguín–Ruiz, M., Meza–Angulo, E., Vides, H. A., Wilches–Vega, J. D. Increases in avian diversity associated with COVID–19 lockdowns in urban Colombia
Les cites o els abstracts dels articles d'Animal Biodiversity and Conservation es resenyen a / Las citas o los abstracts de los artículos de Animal Biodiversity and Conservation se mencionan en / Animal Biodiversity and Conservation is cited or abstracted in:
Abstracts of Entomology, Agrindex, Academic Accelerator, Animal Behaviour Abstracts, Anthropos, Aquatic Sciences and Fisheries Abstracts, Behavioural Biology Abstracts, Biological Abstracts, Biological Abstracts, BIOSIS Previews, CiteFactor, Current Primate References, Current Contents/Agriculture, Biology & Environmental Sciences, Essential Science Indicators, Dialnet, DOAJ, DULCINEA, Ecological Abstracts, Ecology Abstracts, Entomology Abstracts, Environmental Abstracts, Environmental Periodical Bibliography, FECYT, Genetic Abstracts, Geographical Abstracts, Índice Español de Ciencia y Tecnología–ICYT, International Abstracts of Biological Sciences, International Bibliography of Periodical Literature, International Developmental Abstracts, Journals4Free, Latindex, Marine Sciences Contents Tables, MIAR, Oceanic Abstracts, RACO, Recent Ornithological Literature, REBIUN, REDIB, Referatirnyi Zhurnal, ResearchGate, Responsible Journals, Science Abstracts, Science Citation Index Expanded, Scientific Commons, SCI Journals, SCImago, SCOPUS, Serials Directory, SHERPA/RoMEO, Transpose, Ulrich's International Periodical Directory, WoS, Zoological Records
Consorci format per / Consorcio formado por / Consortium formed by:


Índex / Índice / Contents
Animal Biodiversity and Conservation 45.1 (2022) ISSN 1578–665 X eISSN 2014–928 X

131–144
González–Gallina, A., Equihua, M., Pérez–Garduza, F., Iglesias–Hernández, J. A., Oliveras de Ita, A., Chacón–Hernández, A., Vázquez–Zúñiga, O., Hidalgo–Mihart, M. G. Spatial ecology of jaguar (Panthera onca) outside protected areas in the Yucatan Peninsula, Mexico 145–160


Lavariega, M. C. , Briones–Salas, M. , Monroy–Gamboa, A. G., Ramos–Méndez, D. Population density and daily activity patterns of bobcat in its southernmost continental distribution 161–173
Oreha, J., Škute, N. Current genetic structure of European vendace Coregonus albula (L.) populations in Latvia after multiple past translocations 175–188
Ngo, H. N., Nguyen, H. Q., Tran, H. M., Phan, T. Q., Tran, T. T., Gewiss, L. R., Rödder, D., Nguyen, T. Q., Ziegler, T.
Living under the risk of extinction: population status and conservation needs assessment of a micro–endemic tiger gecko in Vietnam
189–202
Burgio, K. R., Davis, K. E., Dreiss, L. M., Cisneros, L. M., Klingbeil, B. T., Presely, S. J., van Rees, C. B., Willig, M. R.
Integrating multiple dimensions of biodiversity to inform global parrot conservation
203–215 Morales, J. Assessment of endangered freshwater pearl mussel populations in the Northern Iberian Plateau in relation to non–native species: xenodiversity as a threat 217–224 Gamboa–Delgado, J., Ponce–Campos, P., Pérez–Martínez, S. G., Pacheco–Vega, J. M., Villarreal–Cavazos, D. Stable isotope measurements as analytical tools for the traceability of crocodile–derived products 225–236 Jayasekara , D., Dharmarathne , W. D. C., Padmalal, U. K. K., Mahaulpatha, W. A. D. Camera trap data reveal the habitat associations, activity patterns and population density of Indian pangolin (Manis crassicaudata) in Maduru Oya National Park, Sri Lanka 237–243 Cosse, M., Duarte, J. M. B., González, S. Home range of pampas deer in a human–dominated agro–ecosystem 245–256
Amb el suport de / Con el apoyo de / With the support of:

Ferrer–Sánchez, Y., Denis, D. ¿Las diferencias ecomorfológicas predicen la coexistencia de murciélagos cavernícolas en Cuba?






































































