






The Medical Research Institute of New Zealand (MRINZ) is Aotearoa New Zealand’s leading independent medical research institute. Our research is guided by a simple philosophy: it must challenge dogma, increase knowledge, and have the potential to improve clinical practice and outcomes, both in Aotearoa New Zealand, and internationally.
The MRINZ’s research teams are dedicated to investigating important public health problems, and delivering high quality evidence with which to improve the management of disease and patient care.
An internationally recognised academic institution, the MRINZ is a registered charitable trust, pursuing advances in clinical practice and providing a base for specialist training in medical research.

Our world-leading research spans ten key medical fields: Asthma, Cardiothoracic Surgery, Children’s Health, Complementary & Alternative Medicine, COVID-19, Emerging Therapeutics, Intensive Care Medicine, Māori & Pacific Peoples’ Health, Oxygen Therapy, and Stroke & Rehabilitation.
Committed to contributing to a more equitable society that celebrates Te Ao Māori and upholds Te Tiriti o Waitangi, the MRINZ is working to support tangata whenua-led processes, where Māori worldviews and values help shape our research.
It is with great enthusiasm that I introduce the 2024 Annual Report for the Medical Research Institute of New Zealand (MRINZ).
As the premier independent clinical research organisation in New Zealand, the MRINZ has achieved remarkable recognition on the global stage for its contributions to clinical medicine. Our accomplishments are underscored by independent metrics which demonstrate that our research impact and quality consistently exceeds those of New Zealand universities. This achievement is a testament to our relentless pursuit of excellence, as we strive to challenge dogma and contribute to advancements in medical knowledge and practice.
In the past year, MRINZ has continued to make significant strides in clinically relevant research aimed at enhancing treatment approaches for prevalent medical conditions. Our research initiatives cover the vital areas of Asthma, Cardiothoracic Surgery, Children’s Health, Complementary and Alternative Medicine, Infectious Disease, Intensive Care Medicine, Emerging Therapeutics, Māori and Pacific Peoples Health, Oxygen Therapy, and Stroke and Rehabilitation. The progress made in these domains over the past year has had a substantial impact on medical management and public health policy.
Throughout 2024, the MRINZ has maintained impressive research productivity with innovative scope, attracting major international interest in our findings. This outstanding output is a direct result of the unwavering commitment of our dedicated team, who have worked diligently to undertake and disseminate their research.
On behalf of the Board, I wish to express my sincere gratitude to our patient volunteers, our national and international partners, and our dedicated funders. Your invaluable support has played a crucial role in our achievements, and we are truly thankful for your contributions.
As we move forward, I am optimistic about MRINZ’s future as New Zealand’s leading independent medical research institute, continuing to advance our mission to improve health outcomes for everyone.
David Chamberlain BEc, FNZSA, FIAA, CMInstD Chair, MRINZ Board of Trustees
For over two decades, the MRINZ has steadfastly upheld its mission to undertake innovative research which has the potential to lead to improvements in clinical management and outcomes in Aotearoa New Zealand and internationally, and to provide a base for specialist training in medical research.
In 2024 we have built on this commitment and advanced our capability through some key strategic developments.
Firstly, we have established Ngā Kaitiaki o Te Rangahau, our Māori Advisory Board, an initiative aimed at advancing Māori health and cultural leadership. Comprising esteemed members and experts from Māori hauora communities, this group fosters collaboration, diversity, and cultural sensitivity in medical research, guiding the MRINZ to produce scientifically robust research that is socially and culturally relevant to diverse communities. Ngā Kaitiaki o Te Rangahau’s influence already runs deep within the MRINZ, fostering cultural understanding and diverse perspectives in every project.
Secondly, we have commissioned ‘The Pod’, an expanded workspace for the MRINZ workforce, which has grown over recent years. The ‘Pod’ provides our researchers with a dynamic environment that fosters collaboration and creativity. The design of the pod encourages interdisciplinary interactions, enabling our teams to work closely together.
Another key milestone was the submission of an application to become the first research institution in the Southern Hemisphere to obtain certification under the European Clinical Research Infrastructure Network (ECRIN) Data Centre Certification Programme. This affirms our commitment to maintaining high standards in clinical trials and data integrity, positions us as a leader in clinical research practice, and underpins our commitment to international benchmarks for quality and compliance.
The MRINZ continues to expand its leadership in large-scale, multicentre national and international randomised controlled trials. Our extensive systems and experience enable us to serve as the autonomous trial coordinating centre in New Zealand for pivotal clinical trials across a wide range of fields. A notable example is the MEGA-ROX study, which assesses optimal oxygen regimens in critically ill patients and involves the recruitment of 40,000 participants across over 140 sites in 14 countries, making it the largest intensive care clinical trial ever conducted.
The MRINZ has maintained extraordinary research productivity over recent years, as illustrated by the quality and quantity of its publications. In 2024 the Institute produced 79 publications, including 8 published by the world’s most prestigious medical journals: The Lancet, New England Journal of Medicine, and JAMA. This productivity has enabled the MRINZ to strengthen its position as the preeminent research-based organisation in New Zealand in terms of the quality and impact of its published research.
Finally, mention should be made of the leadership role of the MRINZ in health research workforce training. Together with our partners at Victoria University of Wellington—Te Herenga Waka we are expanding our capability to provide both academic and practical training across a range of health research disciplines and specialties. The MRINZ Doctorate of Medicine (MD) programme is thriving, and the Māori medical student internship programme is well established, with the first students now developing leadership roles in health research throughout Aotearoa New Zealand.
Looking forward, the MRINZ is confident it will continue to meet its goals to investigate the causes of important public health problems in New Zealand and internationally, andto use this knowledge to improve the prevention and treatment of diseases.

Richard Beasley
CNZM, DSc (Otago), DM (Southampton), MBChB, FRCP (London), FRACP, FAAAAI, FFOM (Hon), FAPSR (New Zealand), FERS, FThorSoc, FRSNZ
Since its establishment in December 2001, the MRINZ has been dedicated to investigating the causes of important public health problems, and using this knowledge to improve the prevention and treatment of a wide range of diseases.
The MRINZ has been at the forefront of many ground-breaking studies and clinical trials, providing valuable knowledge to the medical community both here at home in New Zealand, and globally. Beyond research, the MRINZ has played a crucial role in providing an established base for specialist training in medical research. Through collaborations with leading universities and institutions both nationally and internationally, the MRINZ has facilitated practical clinical research experience and training opportunities for aspiring researchers and healthcare professionals, furthering the advancement of medical knowledge and patient care.
The MRINZ's main offices and clinical research facility are located at Wellington Regional Hospital, in Te Whanganui-a-Tara, at Wellington Regional Hospital. These premises include a four-bed Te Whatu Ora Capital, Coast and Hutt Valley Clinical Trials Unit We partner with community health organisations, including the Maraeroa Marae Health Clinic, to ensure our research initiatives are culturally responsive, community-centred, and effectively address the health needs of diverse populations.
The MRINZ has established a growing database of over 7,500 adults who have participated in our clinical trials. Their involvement plays a crucial role in advancing our research initiatives and improving health outcomes for the communities we serve.
CLINICAL TRIALS GROUPS
The MRINZ is the New Zealand coordinating management centre for ANZICS CTG (the Australian and New Zealand Intensive Care Society Clinical Trials Group), the New Zealand Cardiothoracic Surgery Network, the New Zealand Respiratory Clinical Trials Group, the New Zealand Pharmacy Research Network, and the New Zealand Rehabilitation Research Group.
COLLABORATIVE PARTNERSHIPS
Collaborative partnerships of various types, whether local, national, or international, are vital to our medical research. The MRINZ engages with partners both locally and globally to enhance and promote our shared research efforts.
Clinical Research Units
Kapiti — P3 Research
Kirikiriroa / Hamilton — Lakeland Clinical Trials
Motueka — Greenwood Health
Ōtautahi / Christchurch — Christchurch Clinical Studies Trust, Southern Clinical Trials (Christchurch)
Ōtepoti / Dunedin — P3 Research
Rotorua — Lakeland Clinical Trials
Tāmaki Makaurau / Auckland — Optimal Clinical Trials, Papakura Marae Health Clinic, Southern Clinical Trials (Manukau, Totara, Waitemata), Total Healthcare PHO
Tauranga — Clinical Horizons NZ Ltd, Papamoa Pines Medical Centre
Te Matau-a-Māui / Hawkes Bay — Taradale Medical Centre
Te Papaioea / Palmerston North — P3 Research
Whakatū / Nelson — Southern Clinical Trials (Tasman)
General Practice Networks
Te Whanganui-a-Tara / Wellington — Brooklyn Central Health, Brooklyn Medical Centre, Capital Care Health Centre, Churton Park Medical Care, City GPs, City Medical Centre, Courtenay Medical Centre, Eastern Bays Health Centre, Island Bay Medical Centre, Johnsonville Medical Centre, Karori Medical Centre, Kelburn GPs, Kelburn Northland Medical, Khandallah Medical Centre, Kilbirnie Medical Centre, Miramar Medical Centre, Newlands Medical Centre, Newtown Medical Centre, Newtown Union Health Service, Ngaio Medical Centre, Onslow Medical Centre, Peninsula Medical Centre, Plimmer Steps Medical Centre, Plimmerton Medical Centre, Port Nicholson Medical Centre, Seatoun Medical Centre, Te Aro Medical Centre, Terrace Medical Centre, VUW Student Health Service, Wadestown Medical Practice
Heretaunga / Hutt Valley — Avalon Medical Centre, Gain Health, Hutt City Health Centre, Hutt Union and Community Health Service – Pomare, Petone and Taita, Kopata Medical Centre, Manuka Health Centre, Naenae Medical Centre, Petone Medical Centre, Pretoria Street Surgery, Queen Street Medical Centre, Ropata Medical Centre, Silverstream Medical Centre, Stokes Valley Medical Centre, Upper Hutt Health Centre, Waiwhetu Medical Centre, Whai Oranga O Te Iwi Health Centre
Kāpiti — Coastal Medical Rooms, Horo Te Pai Health Services, Ōtaki Medical Centre, Paraparaumu Medical Centre, Raumati Road Surgery, Team Medical, Waikanae Health Centre
Ōtautahi / Christchurch — Ilam Medical Centre, Linwood Medical Centre, Medical Corner
Doctors Rangiora, Village Health Medical Centre
Porirua — Mana Medical Centre, Ora Toa Practices (Pōneke, Mungavin, Takapūwāhia, Cannons Creek, Waitangirua), Plimmerton Medical Centre, Tawa Medical Centre and Linden Surgery, Tītahi Bay Surgery
Tāmaki Makaurau / Auckland — Allcare Family Medical Centre (Northcote), Birkenhead Medical Centre, Browns Bay Medical Centre, Green Bay Medical Centre, Greenwood Medical Centre, Henderson Medical Centre, Kumeū Village Medical Centre, MedPlus Family Medical Centre, OneHealth Remuera, Stoddart Road Medical Centre, Westview Medical Centre
Tauranga — Fifth Avenue Family Practice, Girven Family Practice, Pāpāmoa Pines Medical Centre
Te Matau-a-Māui / Hawkes Bay — Carlyle Medical Centre, Taradale Medical Centre
Pharmacy Research Network (PRN)
Ahuriri / Napier — Tamatea Pharmacy
Kiririroa / Hamilton — Campus Pharmacy Waikato
Ngāmotu / New Plymouth — Unichem New Plymouth
Manawatū Whanganui — Central Feilding Pharmacy, Vogel Street Pharmacy
Tauranga — My Pharmacy Te Puke, Life Pharmacy Te Puke, Unichem Cherrywood Pharmacy
Ōtautahi / Christchurch — Wigram Pharmacy
Ōtepoti / Dunedin — Anderson’s Exchange Pharmacy
Rotorua — Ranolf Pharmacy
Tāmaki Makaurau / Auckland — Newton Pharmacy Sport and Health, 7 Day Chemist Ōtāhuhu, Jaks Pharmacy
Te Whanganui-a-Tara / Wellington — Alexander Pharmacy, Waitangirua Pharmacy, Clive’s Chemist, Unichem Petone Pharmacy, Queen Street Pharmacy Upper Hutt
Waihōpi / Invercargill — Windsor Pharmacy
ICU Research Network Coordinating Centres
Australia — Australia and NZ Research Centre (Monash University), Melbourne; The George Institute for Global Health, Sydney
Brazil — Hcor, São Paulo
Canada — Alberta Health Services and University of Alberta
Ireland — St Vincent’s University Hospital, Dublin
Japan — The Jikei University, Tokyo
Sri Lanka — Critical Care Asia and Africa: National-Intensive Care Surveillance-M.O.R.U., Colombo
REMAP-Cap Collaborations
Canada — Unity Health, Toronto, Ontario
Europe — University Medical Center, Utrecht
Japan — St. Marianna University
Singapore — National University Hospital
United Kingdom — Intensive Care National Audit & Research Centre (ICNARC), London
United States of America — University of Pittsburgh Medical Centre, Pittsburgh, Pennsylvania
New Zealand
Ōtautahi/ Christchurch — Christchurch Hospital
Ōtepoti / Dunedin — Dunedin Hospital
Rotorua — Rotorua Hospital
Taranaki — Taranaki Base Hospital
Tauranga — Tauranga Hospital
Tāmaki Makaurau/ Auckland — Cardiothoracic and Vascular Intensive Care Unit (Auckland City Hospital), Department of Critical Care Medicine (Auckland City Hospital), Middlemore Hospital, North Shore Hospital
Te Matau-a-Māui/ Hawke’s Bay — Hawke's Bay Fallen Soldiers' Memorial Hospital
Te Whanganui-a-Tara / Wellington — Hutt Hospital, Wellington Regional Hospital
Waikato — Waikato Hospital
Whakatu / Nelson — Nelson Hospital
Whangarei — Whangarei Hospital
Australia
New South Wales — Dubbo Base Hospital, John Hunter Hospital, Nepean Hospital, Northern Beaches Hospital, Orange Health Service, Royal North Shore Hospital, Shoalhaven District
Memorial Hospital, St George Hospital, St Vincent's Hospital Sydney, Westmead Hospital, Wollongong Hospital, Wyong Hospital
Northern Territory — Alice Springs Hospital, Royal Darwin Hospital
Queensland — Caboolture Hospital, Cairns Hospital, Gold Coast University Hospital, Gosford Hospital, Ipswich Hospital, Mater Misericordiae Ltd, Princess Alexandra Hospital, Robina Hospital, Sunshine Coast University Hospital, Toowoomba Hospital
South Australia — Flinders Medical Centre, Lyell McEwin Hospital, Royal Adelaide Hospital, The Queen Elizabeth Hospital
Victoria — Alfred Health, Angliss Hospital – Eastern Health, Austin Health, Barwon Health, University Hospital Geelong, Bendigo Health, Box Hill Hospital – Eastern Health, Casey Hospital, Dandenong Hospital, Grampians Health, Maroondah Hospital – Eastern Health, Mercy Health
Werribee ICU, Monash Medical Centre, Northern Hospital, St Vincent's Hospital Melbourne, The Royal Melbourne Hospital, Victorian Heart Hospital
Western Australia — Bunbury Hospital, Fiona Stanley Hospital, Royal Perth Hospital
Brazil
Barbalha — Hospital Maternidade São Vicente de Paulo
Belo Horizonte — Centro de Estudos e Pesquisa em Saúde Coletiva, Santa Casa de Misericórdia
Brasilia — Hospital Santa Lucia, Hospital Universitário de Brasília
Caxias do Sul — Fundação Universidade de Caxias do Sul
Colatina — Hospital e Maternidade São José, Fundação Social Rural de Colatina
São Paulo — Mirante, Hcor, Hospital BP – A Beneficência Portuguesa de São Paulo, Hospital do Rim – Fundação Oswaldo Ramos, Hospital São Paulo – UNIFESP, Hospital SEPACO
Florianópolis — Hospital Nereu Ramos
Fortaleza — Hospital Oto Aldeota
Joinville — Centro Hospitalar Unimed Joinville
Juiz de Fora — Hospital Universitário – UFJF
Maringá — Hospital Universitário Regional de Maringá (HUM/UEM)
Salvador — Hospital Ana Nery
Ouro Preto — Irmandade da Santa Casa de Misericordia de Ouro Preto
Porto Alegre — Hospital Moinhos de Vento
Pouso Alegre — Hospital da Clínicas Samuel Libânio
Recife — Centro de Pesquisa Clínica do IMIP, Real Hospital Português de Beneficência em Pernambuco
Ribeirão Preto — Hospital das Clinicas de Ribeirão Preto
Rio de Janeiro — Hospital Naval Marcílio Dias, Instituto Estadual do Cerebro
Teresina — Centro de Pesquisa Clínica do Hospital Universitário do Piauí
Vitória da Conquista — Serviço de Assistência Médica e Urgência S.A. SAMUR
Canada
Edmonton, Alberta — Grey Nuns Hospital, University of Alberta Hospital
India
Chennai — Apollo Hospital, Apollo Cancer Centres Teynampet, Apollo Proton Cancer Centre, Dr Kamakshi Memorial Hospital
Maharashtra — Medicover Hospital
New Delhi — Fortis Escorts Hospital
Ireland
Dublin — St Vincent’s University Hospital
Italy
Milan — Ospedale San Raffaele
Japan
Hirakata — Kansai Medical University Hospital
Hiroshima — Fukuyama City Hospital
Nagoya — Nagoya University Hospital
Tokyo — Jikei University Hospital
Wakayama — Wakayama Medical University Hospital
Yokohama — Yokohama City University Hospital
Kuwait
Al-Amiri Hospital, Sheikh Jaber Al-Ahmad Al-Sabah Hospital Hospital
Malaysia
Kuala Lumpur — International Islamic University Malaysia, University Malaya Medical Center
Penang — Universiti Sains Malaysia
Nepal
B & B Hospital, Birat Nursing Home, Grande International Hospital, Hospital for Advanced Medicine & Surgery, Karuna Hospital, Nepal Mediciti Hospital, OM Hospital and Research Center
Oman
Sultan Qaboos Comprehensive Cancer Care and Research Centre
Pakistan
Karachi — Abbasi Shaheed Hospital, Darul Sehat Hospital, Indus Hospital & Health Network, Patel Hospital, SIUT Hospital, South City Hospital, Ziauddin University Hospital – Clifton Campus, Ziauddin University Hospital – North Nazimabad Campus Lahore — National Institute of Cardiovascular Diseases, National Hospital and Medical Center, Pakistan Kidney and Liver Institute and Research Center Peshawar — Lady Reading Hospital, Northwest General Hospital Rahim Yar Khan — Sheikh Zayed Medical College & Hospital
Kingdom of Saudi Arabia
Riyadh — King Abdullah International Medical Research Centre, King Faisal Specialist Hospital & Research Center

The MRINZ has consistently delivered impactful research that addresses critical public health and clinical challenges, fostering improvements in disease management and patient outcomes. Our work spans diverse areas and is guided by a commitment to evidence-based solutions that benefit communities locally and globally.
Through national and international collaborations, the MRINZ has achieved a remarkable record of over 900 peer-reviewed publications, many of which have informed clinical guidelines and shaped global healthcare practices.
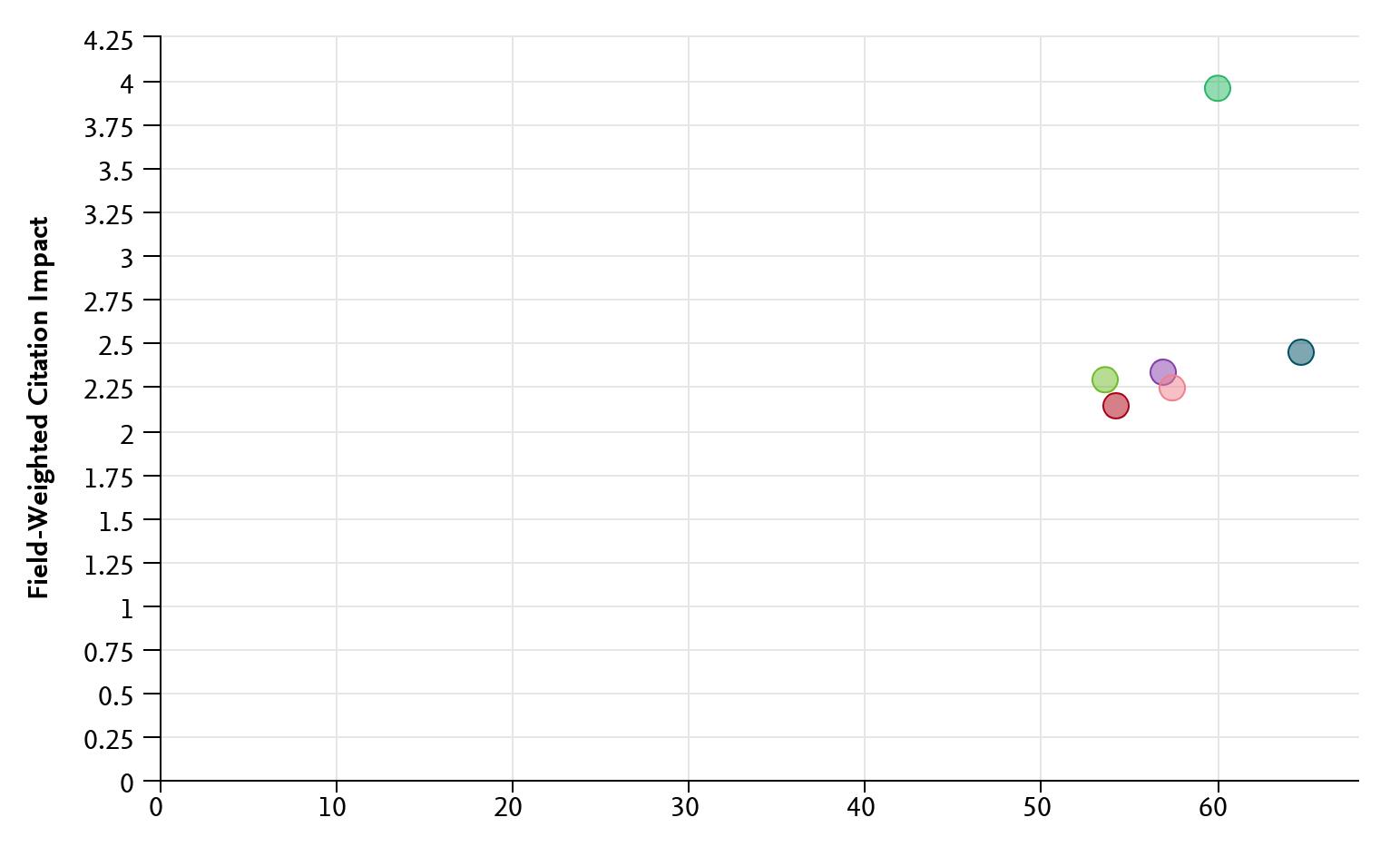
Recent analysis using SciVal, a global research metrics database, highlights the high calibre of the MRINZ’s contributions. One key measure, the Field-Weighted Citation Impact (FWCI), assesses how often an organisation’s research is cited compared to the global average. An FWCI value of 1.0 represents average citation rates, while values above 1.0 indicate greater influence. The MRINZ achieved a citation rate of 3.9, underscoring the international relevance and reach of our work.
Another indicator of our influence is the proportion of the MRINZ publications appearing in the top 10% of journals, ranked by SCImago Journal Rank (SJR), a respected metric of journal quality and scientific impact. The MRINZ figure of 60% highlights the rigorous standards of our research and its recognition within the global academic community. Together, these metrics provide a clear, standardised picture of the MRINZ’s performance.
In 2024, this commitment to excellence again placed the MRINZ ahead of all eight New Zealand universities in both citation impact and the proportion of publications in topranked journals. Additionally, our citation impact exceeded those of the top five universities globally as ranked by the Times in 2024.
Plot of field-weighted citation impact (y-axis) and publications in the top 10% of journal percentiles (x-axis) for the MRINZ and the New Zealand universities.




Plot of field-weighted citation impact (y-axis) and publications in the top 10% of journal percentiles (x-axis) for the MRINZ and the five highest ranked universities worldwide.



DIRECTOR AND FOUNDER
Professor Richard Beasley
DEPUTY DIRECTORS
Associate Professor Matire Harwood
Adjunct Professor Alex Semprini
Professor Paul Young
PRINCIPALS AND ADMINISTRATIVE STAFF
Mrs Marina Dzhelali
Ms Denise Fabian
Mr Mark Holliday
Dr Diane Mackle
Mr John Martindale
Ms Alison Pritchard
Mrs Joanna Read
PROGRAMME LEADS
Principal, Research Manager
Executive Assistant to Director
Principal, Clinical Operations
Principal, ICU Programme
Principal, Data and Quality
Senior Accounts Manager
Senior Administrator
Asthma — Professor Richard Beasley
Cardiothoracic Surgery — Dr Shay McGuinness and Associate Professor Rachael Parke
Children’s Health — Professor Stuart Dalziel
Complementary & Alternative Medicine — Adjunct Professor Alex Semprini
Education — Dr Harry McNaughton and Adjunct Professor Alex Semprini
Emerging Therapeutics — Adjunct Professor Alex Semprini
Infectious Diseases — Dr Thomas Hills and Associate Professor Colin McArthur
Intensive Care Medicine — Professor Paul Young
Māori Health — Associate Professor Matire Harwood
Oxygen Therapy — Professor Paul Young and Dr Louis Kirton
Pacific Peoples’ Health — Associate Professor Dianne Sika-Paotonu
Pharmacy — Adjunct Professor Alex Semprini
Stroke & Rehabilitation — Dr Harry McNaughton
Mr Augustus Anderson
Mr Jonathan Barrett
Dr Tasmin Barry
Dr Orlagh Bean
Mrs Nina Beehre
Dr Rowan Biggs
Ms Bianca Black
Ms Melissa Black
Mrs Maureen Blakemore
Informatics Manager
Study Coordinator
Medical Research Fellow
Medical Research Fellow
Project Manager
Medical Research Fellow
Clinical Research Associate
Senior Study Coordinator
Senior Study Coordinator
Mr Craig Boyd IT Specialist Fellow
Dr Pepa Bruce
Dr Julie Cook
Dr Amanda Clarke
Dr Atalie Colman
Ms Bianca Crichton
Dr Ryan Cullen
Mr Sarath Dayala
Mrs Luisa Diputado
Mrs Valentina Dzhelali
Ms Allie Eathorne
Ms Christina Elder
Mrs Trisha Falleni
Dr Richard Fuller
Mr James Gilchrist
Dr Lee Hatter
Mrs Anna Hunt
Ms Sally Hurford
Mr Zamir Joya
Ms Andrea Kerridge
Ms Kyley Kerse
Dr Louis Kirton
Ms Mary La Pine
Dr Rebekah Lamb
Ms Cassie Lawrence
Mr Helaman Luki
Dr Rob McLachlan
Mr Tony Mallon
Ms Nicola Marshall
Mr Alex Martin
Mrs Leanlove Navarra
Dr Jon Noble
Ms Shaanti Olatunji
Dr Karen Oldfield
Ms Melemafi Porter
Mrs Judith Riley
Dr Ross Sayers
Senior Medical Research Fellow
Clinical Research Fellow
Clinical Research Fellow
Clinical Research Fellow
Assistant ICU Research
Medical Research Fellow
Clinical Research Associate
Informatics Manager
Receptionist
Data Manager
Data Manager
Māori Advisor
Medical Research Fellow
Assistant Data and Quality Manager
Senior Medical Research Fellow
Project Manager
Senior Project Manager
Research Assistant
Quality and Project Manager
Senior Pharmacist
Senior Medical Research Fellow
Project Manager
Medical Research Fellow
Project Manager
Research Assistant
Specialist Medical Research Fellow
Facilities Manager
Communications and Engagement Lead
Junior Research Fellow
Project and Research Manager
Medical Research Fellow
Project Manager
Senior Medical Research Fellow
Research Fellow
Senior Study Coordinator
Medical Research Fellow
Dr Ruth Semprini
Dr Gabby Shortt
Mr Nick Shortt
Mr Jackson Smeed-Tauroa
Mrs Jenny Sparks
Dr Selwyn Te Paa
Dr Jordan Tewhaiti-Smith
Mrs Anne Turner
Ms Michaela Walton
Dr Samantha Warhurst
Ms Katja Zazulia
HONORARY APPOINTMENTS
Senior Medical Research Fellow
Research Fellow
Senior Informatics Manager
Assistant ICU Research/Māori Health
Project Manager
Medical Research Fellow
Medical Research Fellow
Project Manager
Study Coordinator
Clinical Research Fellow
Clinical Research Associate
Associate Professor Mike Armour, Western Sydney University, Australia
Professor Stuart Dalziel, Starship Hospital and Auckland University
Dr Ross Freebairn, ICU, Hawkes Bay Hospital
Dr Daniel Frei, Department of Anaesthesia, Wellington Regional Hospital
Mr Sean Galvin, Cardiothoracic Surgery, Wellington Regional Hospital
Dr Pooveshni Govender, Department of Anaesthesia, Wellington Regional Hospital
Dr Seton Henderson, ICU, Christchurch Hospital
Professor Anne La Flamme, Te Herenga Waka—Victoria University of Wellington
Associate Professor, Colin McArthur, Department of Critical Care Medicine,
Auckland City Hospital
Associate Professor Melanie McConnell, Te Herenga Waka—Victoria University of Wellington
Dr Shay McGuiness, Cardiothoraraic and Vascular ICU, Auckland City Hospital
Dr Robert Martynoga, ICU & Anaesthesia, Waikato Hospital
Dr James Moore, ICU, Wellington Hospital
Associate Professor Rachael Parke, Cardiovascular Intensive Care Unit,
Auckland City Hospital
Dr Alex Psirides, ICU, Wellington Regional Hospital
Associate Professor Marius Rademaker, Auckland University School of Medicine
Dr Louise Reiche, New Zealand Dermatology Research Trust
Dr Richard Steele, Awanui Labs
Professor Paul Teesdale-Spittle, Te Herenga Waka—Victoria University of Wellington
Dr Vivian Fu, University of Calgary, Canada
STUDENT INTERNSHIPS
Francesca Lynch, University of Otago, Ōtākou Whakaihu Waka
Abigail Kalontano, Te Herenga Waka—Victoria University of Wellington
Mia Kibiki, Te Herenga Waka—Victoria University of Wellington
Declan Murphy, University of Otago, Ōtākou Whakaihu Waka



David Chamberlain (Chair) Principal and Actuary, Melville Jessup Weaver, Wellington
Richard Beasley
Director, Medical Research Institute of New Zealand; Consultant Physician, Capital & Coast District Health Board; Visiting Professor, University of Southampton; Professor of Medicine, Te Herenga Waka—Victoria University of Wellington; Adjunct Professor, University of Otago
Matire Harwood
Associate Professor and Deputy Dean, Faculty of Medical and Health Science, University of Auckland






Shay McGuinness
Consultant Physician, Auckland District Health Board
Ian McIntosh
Professional Research Manager (retired)
Sean O’Sullivan Partner, Wotton Kearney, Wellington
Kyle Perrin Consultant Physician, Te Whatu Ora Health New Zealand Capital, Coast and Hutt Valley
Philippa Shirtcliffe Consultant Physician, Te Whatu Ora Health New Zealand Capital, Coast and Hutt Valley
Dianne Sika-Paotonu
Associate Dean (Pacific) and Associate Professor Biomedical / Health Sciences (Immunology), University of Otago

Trisha Falleni (Chair)
NGĀTI KUIA, NGĀTI KOATA, NGĀTI APA KE TE RĀ TŌ, RANGITĀNE O WAIRAU
Research Advisor Māori Health, MRINZ; Clinical Nursing and Cultural Competency Specialist.




Peter Jackson
TARANAKI, TE ATIAWA HAPŪ: NGĀTI HAUPOTO, TE MATEHOU
Kaumātua Capital Coast and Hutt Valley, Nursing Council, Physiotherapy Board; Former Member CCDHB and HVDHB Māori Partnership Boards; Independent Director HVDHB Māori Partnership Board.
Jane Patterson
NGĀ PUHI, TE MAHUREHURE
Senior Manager, Health Sector; Relationship Management and Diversity Specialist.
Associate Professor Clive Aspin
NGĀTI MARU, NGĀTI WHANAUNGA, NGĀTI TAMATERĀ
Associate Professor in Health; Te Herenga Waka–Victoria University of Wellington; Public Health Researcher; Former Executive Research Officer, Ngā Pae o te Māramatanga; Former Ministerial Appointee to Board of Health Research Council; Recipient, 2023 Te Rangi Hiroa Medal, Royal Society Te Apārangi.
Simon Phillips
TARANAKI, NGĀTI RUANUI
CEO, Maraeroa Marae Health Clinic; Psychiatric & Comprehensive Nurse.
The year 2024 at the MRINZ has been another period of focused research aimed at improving health outcomes. The selected updates that follow highlight the progress made in our key areas of focus and the ongoing collaborations dedicated to shaping effective, evidence-based health solutions.

MRINZ-led
research has changed
the way the world
manages asthma. Our research teams investigate novel approaches to the prevention and management of asthma,
to determine how to reduce the risk of developing this common disease, and how it is most effectively treated.
The stepwise approach to pharmacological treatment is a cornerstone of asthma guidelines, yet there is limited understanding of its outcomes when implemented in clinical practice. To address this evidence gap, the MRINZ conducted a clinical trial investigating the use of the ‘Track 1’ anti-inflammatory reliever (AIR) stepwise algorithm, as recommended in New Zealand and international asthma guidelines. The results, which are soon to be published, will provide guidance for its use in clinical practice.
This landmark study lays the foundation for a similar assessment of the short-acting beta-agonist (SABA) reliever-based ‘Track 2’ algorithm.
The MRINZ is part of an international collaborative group that has proposed a paradigm shift in the management of asthma and COPD. This 'treatable traits' approach is based on the concept that asthma and COPD represent a continuum of related diseases that share biological mechanisms and present with distinct clinical, pathophysiological, and psychosocial features requiring individualised treatment. Having advocated for the principle of treatable trait-based management of airway diseases, including through The Lancet Commission on Asthma, the MRINZ is now focusing on research into its implementation.
The MRINZ has completed an innovative Health Research Council of New Zealandfunded trial using a protocolised, treatable trait-based asthma management strategy that targets airflow obstruction and type 2 inflammation. While this targeted approach showed significant clinical benefits, it was insufficient to control asthma in most patients, even with high systemic corticosteroid exposure.
This novel study has provided crucial insights, suggesting that a protocolised algorithm aiming to 'normalise' key characteristics of airflow obstruction and type 2 inflammation is likely to lead to overtreatment and associated side effects.
Fostering discovery and innovation, our cardiac surgery research supports a multi-disciplinary consortium of cardiac surgeons, anaesthetists, intensivists, clinical perfusionists and researchers involved in the care of patients undergoing cardiac surgery at public hospitals across Aotearoa New Zealand.
Low blood pressure following heart surgery is a significant concern, often leading to complications such as acute kidney injury (AKI) and atrial fibrillation (AF), a type of abnormal heart rhythm. These complications can increase patient morbidity, mortality, and healthcare costs. Angiotensin 2, a naturally occurring vasoconstrictor peptide, has demonstrated efficacy in restoring blood pressure to normal levels, particularly in cases of severe septic shock caused by infection. Furthermore, studies have shown that patients with septic shock who developed AKI and received angiotensin 2 were more likely to recover kidney function after treatment.
However, the potential effects of angiotensin 2 in patients experiencing low blood pressure after heart surgery remain unclear. The Prospective angiOtensin vs. noRadrenaline Trial for Hypotension management to reduce length Of hospital stay in Cardiac Surgery (PORTHOS) study is set to be the first randomised trial designed to evaluate the impact of angiotensin 2 in this context.
This important study is led by investigators at Monash University in Melbourne and the MRINZ.
The PORTHOS trial will compare the effectiveness of angiotensin 2 with the usual care blood pressure medication, noradrenaline, in restoring blood pressure, reducing length of hospital stay, and minimizing kidney injury in patients with hypotension following cardiac surgery.
Committed to leading innovative studies in children’s health, the MRINZ brings together researchers from a range of specialist areas to improve the health and wellbeing
There is a global concern about the lack of research into innovative treatments for childhood asthma. While there is strong evidence supporting the use of the 2-in-1 combination inhaler as reliever therapy in adult asthma, comparable evidence to guide treatment in children is lacking.
To address this gap, the MRINZ conducted a Health Research Council-funded study comparing the 2-in-1 inhaler budesonide/ formoterol with salbutamol reliever therapy in 360 children aged 5 to 15 years with mild asthma. Despite COVID-19 disruptions, this randomised controlled trial has been successfully completed, with findings expected to be published in early 2025.
This is the first study to evaluate the safety and efficacy of as-needed budesonideformoterol in children with mild asthma, offering crucial evidence to inform paediatric asthma treatment.
If this trial demonstrates that as-needed budesonide-formoterol is as effective for childhood asthma as it has proven for adults, the findings could transform international guideline recommendations and its implementation significantly reduce asthma morbidity in children, both in New Zealand and worldwide.
Paediatric asthma is a significant public health issue in New Zealand, with childhood asthma prevalence rates among the highest in the world. There is an urgent need for research to develop evidence-based primary prevention strategies to reduce asthma prevalence. This has spurred investigation into novel risk factors that may increase susceptibility to asthma and could be targeted through simple public health interventions.
One such risk factor, with substantial evidence suggesting a potential causative role, is frequent paracetamol use. The first-ever randomised controlled trial examining whether paracetamol increases the risk of childhood asthma has now fully recruited 3,923 infants. These children will be followed for six years to assess the safety of paracetamol compared with ibuprofen for treating fever and pain, and to determine whether either regimen is associated with a higher risk of developing asthma and other allergic conditions such as eczema and hay fever. Led by Professor Stuart Dalziel from the University of Auckland in collaboration with the MRINZ, this study is funded by the Health Research Council of New Zealand.
This study not only has the potential not only to reveal if increased paracetamol use over recent decades has contributed to higher asthma rates, but also to pave the way for preventive intervention strategies.
Advanced airway procedures in children are crucial during complex shared-airway surgeries and emergency situations where intubation and oxygenation cannot be achieved. However, these procedures are performed infrequently, requiring anaesthetists and surgeons to rely on simulation training to maintain their skills. Unfortunately, there is a lack of high-quality training models for teaching these essential techniques.
To address this unmet need, investigators collaborating with the MRINZ are developing a novel 3D-printed, compressible, and anatomically accurate trachea. This innovative approach aims to enhance the training of healthcare professionals in advanced airway management for paediatric patients.
This project is in collaboration with the Wellington airway group, which brings over ten years of experience in creating 3D-printed airway models specifically designed for simulation skills training.

The MRINZ is committed to enhancing the evidence base for natural therapies and over the counter medications in Aotearoa New Zealand. Our research teams explore complementary and alternative therapies in the context of rigorous science, to identify products that are both safe and effective.
Building on a track record in both clinical studies for natural products in the Pharmacy Research Network and early phase studies in emerging asthma therapeutics, the MRINZ has developed capacity to undertake the first in-human, pharmacokinetic trials of botanical extracts. Working closely with Evithé Biotechnology, the MRINZ is assessing the safety and optimal dosage range for a ginger tincture extract in healthy adults. This extract has the potential to be a novel treatment for inflammatory disorders such as rheumatoid arthritis.
The MRINZ continues to focus on providing an accessible, high-quality capacity to undertake clinical trials for New Zealand biotechnology companies, including early phase human trials of botanical extracts.
A landmark study on endometriosis and pelvic pain has revealed a significant negative impact on women’s lives, affecting various aspects of their wellbeing. Involving 800 participants, this study is the first of its kind in Aotearoa and highlights alarming diagnostic delays—often exceeding eight years—demonstrating unmet needs for patients.
Researchers are currently studying the direct and indirect healthcare costs associated with endometriosis and chronic pelvic pain, marking another first in Aotearoa, with publication expected later this year. Additional projects include exploring the experiences of Māori and Pasifika patients with endometriosis, which indicate prolonged diagnostic delays of over 11 years and resulting inequities in healthcare experiences.
The MRINZ also contributed to an expert opinion paper on investigational treatments for dysmenorrhoea, emphasising the lack of advancements in new therapies and the need for a deeper understanding of menstrual physiology to identify potential therapeutic targets.
Endometriosis and chronic pelvic pain are crucial areas of medicine that remain understudied and under-resourced. Given the substantial impact of these conditions on women in Aotearoa, the MRINZ is committed to expanding this knowledge base.

Our research into infectious diseases aims to improve prevention, diagnosis, and treatment. The MRINZ's dedicated teams conduct comprehensive studies on various infectious diseases, including emerging pathogens. Through multicentre, multi-national clinical trials, we seek to advance the understanding of infectious diseases and contribute to better health outcomes here in New Zealand and worldwide.
Pneumonia is a common reason for hospital admission and is the most frequent cause of severe infection leading to organ failure and the need for intensive care. However, we lack definitive knowledge about which treatment approaches lead to the best outcomes. In collaboration with colleagues in Australia, Europe, the United Kingdom, North America, and Asia, we have developed a new research design that can simultaneously investigate the effect and safety of multiple treatment options across various ‘domains’ of care. This trial is known as the Randomised, Embedded, Multi-factorial, Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
The design is integrated into routine clinical processes, allowing patients to benefit from the knowledge gained as the trial progresses. Analysis using Bayesian statistics occurs at regular intervals, informing randomised treatment allocations for new participants. Each domain continues until a clear answer is found, then the best treatment becomes standard patient care. This approach contrasts with traditional trial designs that typically test only a single hypothesis, wait until a fixed sample size is reached for analysis, and often yield inconclusive clinical trial results.
In response to the COVID-19 pandemic, the study was significantly expanded to evaluate potential treatments for this viral respiratory tract infection, including corticosteroids, antivirals, immune modulation agents, antibody treatments, convalescent plasma, anticoagulation therapies, and more. This expansion demonstrates the potential of this novel trial design to respond swiftly to public health crises, particularly in managing newly emergent infectious diseases for which there is no evidence base.
The MRINZ plays a senior role in coordinating and managing REMAP-CAP, which is funded by the Health Research Council of New Zealand, Australia’s National Health and Medical Research Council, the Canadian Institute of Health Research, and a European Union FP7 grant. In 2024, the REMAP-CAP trial, led in New Zealand by Colin McArthur and Tom Hills, received funding for the next five years from a Health Research Council of New Zealand Programme Grant.
REMAP-CAP has included 14,106 patients and is active in 298 sites worldwide. In addition to optimising pneumonia treatment for critically ill patients, the REMAP-CAP trial provides a global research platform capable of adapting and efficiently evaluating multiple treatment options for patients facing critical illness due to a global respiratory pandemic.

COVID-19 vaccines are designed to be injected into the deltoid muscle of the arm. However, previous research at the MRINZ has shown that standard 25mm needles may not be long enough to ensure intramuscular injection in up to 45% of adults with obesity. Current vaccination guidelines provide non-specific advice about needle length selection, resulting in less than 2% of COVID-19 vaccinations in New Zealand being administered with a longer 38mm needle. As a result, a significant portion of the population may inadvertently receive their COVID-19 vaccines into the subcutaneous tissue.
It is currently unknown whether the delivery location of COVID-19 vaccines influences the immune response or the severity of adverse reactions following vaccination. The MRINZ's Te Niwha-funded study is a single-blind, parallel-group, randomised controlled trial conducted through the Pharmacy Research Network. Participants will receive a COVID-19 vaccine booster via either a 12.7mm or 38mm needle. This study will compare the immunogenicity and reactogenicity of subcutaneous versus intramuscular injection of the COVID-19 vaccine.
This study examines whether intramuscular COVID-19 vaccine delivery is essential for full therapeutic effect and if subcutaneous delivery increases reactogenicity. The findings will guide international vaccination guidelines and demonstrate the MRINZ's exceptional capacity for real-world clinical trials in community pharmacies.
The rapid expansion of viral surveillance activities during the COVID-19 response, combined with the ongoing challenges faced by General Practices in a resource-stretched health system, presents an opportunity to explore alternative community-based surveillance strategies. Funded by Te Niwha and in collaboration with ESR and the Pharmacy Guild of New Zealand, the MRINZ Pharmacy Research Network (PRN) is undertaking an observational feasibility study to investigate community pharmacy participation in New Zealand's influenza-like illnesses surveillance programme.
Pharmacy-based nasopharyngeal swabbing aims to survey a population with symptoms of influenza-like illness, not presenting to hospital or primary care services. This approach has the potential to contribute critical data on circulating viruses in the community, and establish an agile capacity to increase infectious disease surveillance in a future pandemic.
Integrating pharmacies into the existing respiratory infection surveillance programme can expand the demographic scope and improve early detection and response to circulating respiratory viruses in New Zealand.
The capability of the MRINZ to undertake early phase studies of the safety and efficacy of novel treatments is underpinned by our strong relationships with volunteers from the community and the shared use of the Capital & Coast District Health Board Clinical Trials Unit located at Wellington Regional Hospital.
The administration of platelets is a crucial treatment for managing major bleeding from various causes. In New Zealand, platelets currently have a shelf life of only seven days because they must be stored at room temperature. As a result, smaller hospitals have very limited in-house supplies, leading to significant wastage of donated platelets. To address this issue, the New Zealand Blood Service has developed a novel method for storing platelets at -80°C, along with a straightforward process for thawing and reconstituting them. This advancement should enable smaller hospitals to maintain a more adequate supply of platelets on-site and significantly reduce wastage.
Cardiac surgical patients are among the highest users of platelets, and the scheduled nature of their surgeries allows for easier recruitment into clinical trials. We are conducting a study (CLIP II NZ) on the safety of and efficacy of cryopreserved platelets in cardiac surgical patients at the five public hospitals that perform these procedures. If we can demonstrate that cryopreserved platelets are safe and effective for this patient group, they will be made available across New Zealand.
Access to platelets is significantly limited in smaller and more remote hospitals. Our Health Research Council of New Zealandfunded study will evaluate the safety and efficacy of cryopreserved platelets.
Bleeding is a leading cause of early deaths from major trauma, often worsened by abnormalities in blood clotting. Tranexamic acid (TXA) helps reduce abnormal clot breakdown and is known to diminish bleeding in other contexts, such as major surgery. Some evidence from low- and middle-income countries suggests that early prophylactic use of TXA improves outcomes after traumatic injuries. However, it remains uncertain whether the potential benefits outweigh the risks (such as dangerous blood clots) in advanced trauma care systems that have access to other rapid and effective treatments for controlling bleeding and enhancing blood clotting.
In this study, severely injured patients at risk of abnormal blood clotting were randomly assigned to receive either TXA or a placebo, administered by ambulance staff at the scene and continued in hospital alongside standard treatment. Patients were then assessed while in hospital and followed for six months to determine whether TXA treatment improved survival rates and/or reduced disability. This international trial was led in New Zealand by the MRINZ and funded by the Health Research Council of New Zealand, Australia’s National Health and Medical Research Council, and additional funding from Germany. The PATCH trial was published in the New England Journal of Medicine, revealing that TXA did not increase the proportion of major trauma patients who survived with a favourable functional outcome at six months. For every 100 patients assigned to receive TXA instead of placebo, approximately four additional patients were alive at six months; however, around four extra patients were also classified as having severe disability.
The PATCH trial provided crucial information to inform policy decisions regarding the implementation of TXA in major trauma systems in New Zealand
Severe injuries activate multiple chemical pathways that cause inflammation and damage vital organs, potentially leading to long-term disability and death. The kidney hormone erythropoietin, known for controlling red blood cell production and commonly used to treat anaemia, also has anti-inflammatory properties. Previous studies suggest that erythropoietin may reduce mortality and serious disability following severe injuries, without increasing side effects.
This study will involve 2,500 adults with severe injuries across New Zealand, Australia, the Republic of Ireland, France, and Finland, who will receive either erythropoietin or a placebo injection, in addition to standard treatment. Participants will be assessed after six months to determine their survival rates and levels of disability. The MRINZ is coordinating New Zealand's involvement in this trial, which is funded by the Health Research Council of New Zealand, Australia’s National Health and Medical Research Council, and the Irish Health Research Board.
If this simple treatment can effectively reduce death and disability after severe injury, it could lead to worldwide changes in clinical practice.
The choice of intravenous fluids administered to acutely unwell patients significantly influences their outcomes. For years, 0.9% sodium chloride (saline) has been the most commonly used fluid in intensive care units (ICUs) worldwide. However, recent concerns about saline potentially causing hyperchloremic acidosis and possibly increasing the risk of kidney failure and mortality have led to a rise in the use of balanced salt solutions—crystalloid solutions with a chloride concentration closer to that of human plasma. Despite this shift, it remained uncertain whether balanced solutions improve outcomes for ICU patients.
The Plasmalyte vs. Saline (PLUS) study tested the hypothesis that Plasmalyte would reduce mortality compared to saline and was published in the New England Journal of Medicine. This study represented the culmination of a decade of research evaluating intravenous fluid therapy for critically ill patients. We now understand that balanced crystalloid fluids reduce the risk of death compared to saline for most patients; however, saline may still reduce mortality compared to balanced crystalloids in patients with brain injuries.
Over the past decade, international collaborative research involving MRINZ researchers has yielded practice-changing results in the use of intravenous fluids in critically ill patients.
The Brain Oxygen Neuromonitoring in Australia and New Zealand Assessment (BONANZA) trial, focusing on severe traumatic brain injury (TBI), has recently received funding from the Health Research Council of New Zealand. With a global incidence rate of 17.3 per 100,000 population, severe TBI is a leading cause of death and disability among young adults, posing a significant international public health challenge. Many victims of severe TBI are young, and the prevalence of lifetime disability is high among this group. The social and economic costs associated with severe brain injuries are unacceptably high.
Given the long-term burden of TBI on individuals, whānau, and the community, developing additional effective management strategies is a high priority. Available data suggest that low brain oxygen levels are linked to poorer outcomes. This trial will assess whether treating low brain oxygen levels in TBI patients improves survival rates and neurological outcomes.
The BONANZA trial aims to change management practices for patients with severe TBI, potentially leading to significant improvements in the care provided to our most critically injured patients, resulting in better long-term outcomes and reduced impact on families and communities.
Up to 50% of antibiotic use is inappropriate, with excessive treatment duration being the primary contributor to antibiotic resistance. There has been a lack of highquality randomised trial evidence for treating patients with bloodstream infections, which affect 15% of critically ill patients. The MRINZ led the New Zealand component of the BALANCE trial, a large multicentre international study aimed at determining whether critically ill patients with bloodstream infections can be effectively treated with a shorter duration of antibiotic therapy instead of a longer one.
In addition to contributing to this international effort, local recruitment will yield specific data on New Zealand patients and facilitate the translation of results into clinical practice. The New Zealand contribution to the BALANCE trial was funded by the Health Research Council of New Zealand. The trial was completed in 2024 and published in the New England Journal of Medicine, demonstrating that shorter duration antibiotic therapy (7 days) is as effective as longer treatment in patients with bloodstream infections.
Antibiotic resistance is one of the major global threats to public health. Shorter antibiotic courses can reduce the risk of developing antibiotic resistance and minimise complications associated with prolonged treatment.
Patients who experience an out-of-hospital cardiac arrest frequently suffer brain damage due to oxygen deprivation, known as hypoxic ischaemic encephalopathy. This condition is the leading cause of death and disability among out-of-hospital cardiac arrest patients admitted to the ICU. Key components of supportive care for these patients include sedation, temperature control, and blood pressure support. However, significant 'evidence gaps' exist regarding the optimal application of these therapies.
The MRINZ is spearheading the STEPCARE trial in New Zealand to evaluate these supportive therapies aimed at preventing secondary brain injury after resuscitation from an out-of-hospital cardiac arrest. The goal is to generate knowledge that will improve survival rates, reduce lengths of hospital stays, enhance functional recovery, and address inequities among this patient population within a short timeframe. The STEPCARE trial is funded by the Health Research Council of New Zealand.
The burden of mortality and disability associated with cardiac arrest in New Zealand remains unacceptably high. This trial is highly likely to yield findings that will change practice and improve outcomes for this vulnerable patient group.
Patients in the ICU with severe acute brain injuries—resulting from conditions such as trauma, stroke, subarachnoid haemorrhage, or hypoxic brain damage—face a significantly elevated risk of death or disability. Lower respiratory tract infections that develop within the first week of hospitalisation for these patients occur during a period when the brain is particularly vulnerable. These infections can lead to fevers, hypotension, hypoxaemia, hypercapnia, and elevated intracranial pressure, all of which may exacerbate secondary brain injury.
It is highly plausible that administering antibiotic prophylaxis around the time of intubation in ICU patients with acute brain injuries could prevent early lower respiratory tract infections, thereby mitigating secondary brain damage and reducing mortality rates. In collaboration with investigators from the George Institute for Global Health, researchers from the MRINZ are conducting the PREVENT-NEURO trial—a large randomised clinical trial aimed at evaluating whether a dose of prophylactic antibiotics around the time of intubation can effectively reduce mortality in ICU patients. This study is funded by the Australian National Health and Medical Research Council.
If the hypothesis being tested in PREVENTNEURO is confirmed, it is estimated that 17 patients would need to receive antibiotic prophylaxis to prevent one death. With an average of 900 patients per year with acute brain injuries admitted to New Zealand ICUs, this could translate to approximately 53 lives saved annually through a low-cost treatment that is already readily available.
Metabolic acidosis is a prevalent acid–base disturbance that commonly affects the most critically ill patients in the ICU. There is an urgent need to enhance outcomes for ICU patients suffering from metabolic acidosis, as statistics reveal that currently, 1 in 4 of these patients require kidney dialysis, and 1 in 5 do not survive their hospital stay. Sodium bicarbonate, which increases blood pH, presents a logical and cost-effective therapy for ICU patients with metabolic acidosis. Despite its potential benefits, clinical guidelines do not recommend the routine use of sodium bicarbonate for this condition due to a lack of sufficiently powered double-blind studies.
Preliminary evidence from a multicentre unblinded phase 2 clinical trial (BICAR-ICU) indicates that sodium bicarbonate may reduce the need for kidney dialysis and decrease mortality rates among ICU patients with metabolic acidosis. To confirm these promising initial findings, we are conducting the SODa-BIC trial, a phase 3, double-blind, randomised clinical trial evaluating sodium bicarbonate for metabolic acidosis in the ICU.
The SODa-BIC trial is poised to definitively establish the efficacy of sodium bicarbonate treatment for metabolic acidosis, potentially transforming the management of this condition. Given that sodium bicarbonate is readily available and inexpensive, its use could not only prevent dialysis dependence and save lives but also result in significant savings for the healthcare system.
Fibrinogen is a crucial final component of the clotting cascade, playing a vital role in the formation of stable blood clots. In major trauma patients, low fibrinogen levels are independently linked to increased mortality. Early fibrinogen replacement may assist in haemorrhage control, improve coagulopathy, and reduce the need for transfusions, as well as lower mortality and disability rates. Recognising the significance of this issue, the NZ Trauma National Clinical Network has prioritised the FEISTY-II trial as a clinical research initiative.
Currently, access to fibrinogen replacement is inequitable; cryoprecipitate, the standard treatment for fibrinogen replacement, is only available in larger hospitals with direct access to a blood bank. Fibrinogen concentrate, an alternative product that could be distributed to all hospitals, is licensed for use, but its application has been limited by insufficient data supporting its safety and efficacy.
The FEISTY-II trial is a phase III, multi centre, randomised, controlled trial designed to assess whether early fibrinogen replacement using Fibrinogen concentrate is more effective than the current standard of care, cryoprecipitate, in increasing the number of days alive and out of the hospital (at home) at 90 days among severely injured trauma patients with major haemorrhage and hypofibrinogenaemia. This trial is being led in New Zealand by the MRINZ, and is funded by the Health Research Council of New Zealand.
The FEISTY-II trial will provide essential data on the safety and efficacy of Fibrinogen concentrate, paving the way for its implementation in New Zealand through the Trauma National Clinical Network.

The MRINZ is committed to Māori health and research workforce training, developing ways to weave tikanga into how we work within our organisation, and strengthening collaborations with Iwi and Māori partners across Aotearoa New Zealand.
The MRINZ has previously reported a widespread shift in Aotearoa New Zealand towards budesonide/formoterol maintenance and/or reliever regimens for asthma treatment in recent years. This shift follows landmark MRINZ studies and updated asthma guidelines from the Asthma and Respiratory Foundation of New Zealand, which recommend this approach. Importantly, these prescribing trends have been associated with reduced rates of asthma hospitalisations. However, it was previously unknown if these patterns also applied to Māori, who experience a disproportionate burden from asthma.
Using national data updated to December 2023, we examined these trends with respect to Māori ethnicity. Our analysis showed that the significant uptake of budesonide/ formoterol maintenance and/or reliever regimens among Māori was similar to that observed in non-Māori populations. Notably, this uptake has been associated with a greater absolute and relative reduction in asthma-related hospital discharges among Māori.
These findings suggest that the real-world clinical benefits of budesonide/formoterol maintenance and/or reliever therapy may be even greater for Māori and potentially other groups facing inequitable asthma burdens.
The greater reduction in hospital admissions among Māori illustrates the positive impact of translating research into clinical practice through guideline implementation. Reducing ethnicity-related health inequities in asthma remains the 'holy grail' of asthma research in Aotearoa New Zealand.
The MRINZ oxygen research team is internationally recognised for its landmark research of the optimal ways in which to administer oxygen therapy, and key role in the implementation of research findings into clinical practice through national and international guidelines.
Oxygen is a standard treatment for patients requiring care in an ICU. In partnership with investigators in Australia, we conducted a multicentre randomised controlled trial involving 1,000 participants to evaluate oxygen therapy in adults requiring life support (ICU-ROX). Funded by the Health Research Council of New Zealand, the trial was published in the New England Journal of Medicine in 2020. The study suggested no overall benefit from liberal oxygen therapy and confirmed the safety of conservative oxygen use. Notably, for patients who had suffered a cardiac arrest prior to ICU admission, conservative oxygen therapy was associated with improved outcomes.
The ICU-ROX study laid the groundwork for the Mega-ROX trial, a global study involving 40,000 participants examining oxygen therapy in ICU patients requiring life support. The MRINZ is leading this trial with funding from the Health Research Council of New Zealand and the Alpha Charitable Trust.
The Mega-ROX trial will be the largest clinical trial ever conducted in intensive care and will provide definitive insights that will shape clinical practice regarding oxygen therapy for patients on life support in the ICU. Approximately 1,000 patients are being recruited each month for the MegaROX trial, with recruitment expected to concludein 2025.
The Low OxyGen Intervention for Cardiac Arrest Injury Limitation (LOGICAL) randomised controlled trial (RCT) aims to improve outcomes for adults in a coma following resuscitation from a cardiac arrest, specifically targeting patients at risk of hypoxic ischaemic encephalopathy. This condition is a leading cause of death and disability in New Zealand. Determining whether conservative oxygen therapy increases survival rates with favourable neurological outcomes in these patients is a critical priority.
For Māori patients, 1 in 16 ICU admissions in NZ follows a cardiac arrest, compared to 1 in 22 for European patients. Māori account for 20.2% of all post-cardiac arrest admissions to NZ ICUs and experience lower survival rates than their European counterparts. If conservative oxygen therapy proves effective in enhancing favourable neurological outcomes in patients with hypoxic ischaemic encephalopathy, it would lead to significant changes in clinical practice, positively impacting Māori patients and their whānau.
The financial implications of cardiac arrest are considerable; for each patient resuscitated from cardiac arrest and admitted to ICU who survives to hospital discharge, admission costs alone exceed $120,000. The estimated ongoing communitybased cost for each patient who survives with moderately severe brain damage is $34,000 per year. Given that patients with hypoxic ischaemic encephalopathy who survive with severe neurological disability may require years of nursing home care, minimising significant neurological disability is crucial for healthcare delivery.
The LOGICAL RCT represents the culmination of a decade of work led by the MRINZ and supported by the Health Research Council of New Zealand to evaluate optimal oxygen therapy following cardiac arrest. Recruitment for the LOGICAL RCT has been completed, and the findings of this pivotal clinical trial are highly anticipated worldwide.
Postoperative death within 30 days of surgery ranks as the third most common cause of death globally, resulting in approximately 4.2 million fatalities annually. The complications most frequently associated with these deaths include localized infections, sepsis, myocardial injury, kidney injury, stroke, venous thromboembolism, heart failure, and atrial fibrillation. Even in cases where these complications do not lead to death, they can significantly delay recovery. Surgical site infections alone can double postoperative costs and adversely affect the wellbeing of both patients and their whānau.
Oxygen therapy has the potential to influence the risk of several postoperative complications, including surgical site infections, myocardial injury, and pulmonary complications. In New Zealand, over 300,000 individuals receive oxygen during surgery each year; however, the optimal perioperative oxygen regimen remains unknown. Given that oxygen is universally administered during general anaesthesia (GA), establishing any potential treatment effect of oxygen therapy would have profound implications for patient outcomes. This urgent need is underscored by the recent World Health Organization (WHO) call for evidence to address the optimal perioperative oxygen regimen.
The HOT-ROX trial aims to evaluate the efficacy and safety of liberal perioperative oxygen administration compared to intermediate or restrictive perioperative oxygen protocols for reducing the incidence of postoperative surgical site infections.
Each year, over 300,000 people undergo surgery under anaesthesia in Aotearoa New Zealand, yet the optimal oxygen regimen remains unclear.
It is widely recommended that oxygen be delivered to maintain a patient's oxygen saturation within a safe target range. The MRINZ and Fisher & Paykel Healthcare have undertaken a research programme investigating the use of an oxygen device that automatically adjusts the amount of oxygen it delivers to keep patients within a safe target oxygen saturation range.
In 2024, two randomised controlled trials were published, and two more are currently under peer review, broadening the clinical scenarios in which automated oxygen delivery has been studied. The automated oxygen device has consistently demonstrated an increased duration within a patient’s target range compared to standard care, which may improve patient outcomes by minimising the known harms associated with both over- and under-oxygenation.
Automated oxygen delivery represents an important advance in the safe and accurate administration of oxygen to critically unwell patients. As additional evidence emerges, the case for the widespread and routine use of automated oxygen delivery systems becomes increasingly compelling.
Our stroke research programme is home to innovation and advances in the development and implementation of novel interventions that are setting a global standard in person-centred rehabilitation. With a focus on ethnic disparities in outcome after stroke, our research seeks to improve the lives of stroke survivors in Aotearoa New Zealand and across the world.
The Take Charge intervention developed at the MRINZ has been elevated to a 'strong recommendation' for individuals discharged home after a stroke, as stated in the latest edition of the Australia and New Zealand Stroke Rehabilitation Guidelines. This recognition underscores the intervention's importance in improving recovery outcomes for stroke survivors.
Extending the original MRINZ studies in stroke care, two further randomised controlled trials evaluating the Take Charge intervention in non-stroke conditions have been successfully completed, with reports expected to be published within the next year. These trials, conducted in South Australia, focused on individuals diagnosed with mild cognitive impairment or early dementia and those with Long COVID. Should these trials demonstrate positive outcomes for Take Charge participants, following previous successes with individuals suffering from chronic obstructive pulmonary disease (COPD), they will strengthen the case for transforming rehabilitation practices across various conditions. This would position psychological interventions like Take Charge as essential components of rehabilitation.
Dr Vivian Fu, a former Research Fellow at the MRINZ, has also conducted a randomised controlled study of the Take Charge intervention delivered via telehealth to stroke patients in remote areas of Canada. While full results are pending, preliminary feedback indicates that telehealth delivery was feasible and well received by participants, as highlighted in a qualitative substudy.
To further enhance the intervention, we are collaborating with partners to develop smartphone-based apps that incorporate conversational agents (avatars) to simulate the role of the Take Charge facilitator. These innovative tools are currently being tested with stroke patients, and we anticipate broader testing opportunities over the next two years.
The Take Charge intervention represents a pivotal shift in rehabilitation practices, providing vital psychological support that can significantly enhance recovery for individuals across various health conditions.
Since its inception, the MRINZ has been at the forefront of addressing pressing public health challenges, providing highquality evidence that informs effective interventions to improve health outcomes. A core commitment to workforce development for future researchers, alongside a focus on recognising and addressing health inequities, has driven the MRINZ’s mission forward. The development, testing, and implementation of the Take Charge intervention exemplify these guiding principles in action. Take Charge is a simple, low-cost intervention—essentially a conversation between a trained facilitator and individuals facing health challenges. Initially designed for stroke patients, it has since expanded to include individuals with chronic obstructive pulmonary disease (COPD), early dementia, and Long COVID.
This groundbreaking work was initiated more than 25 years ago at the University of Otago Wellington School of Medicine, under the leadership of Dr Harry McNaughton and Professor Kathryn McPherson. The journey began with a pivotal question: “Why are 12-month outcomes for Māori and Pacific people with stroke significantly worse than those for European New Zealanders?” Harry’s observational study revealed alarming disparities in health outcomes, prompting further investigation into the community factors contributing to this inequality.
Dr Matire Harwood’s early exploratory research, including interviews with Māori stroke survivors and their caregivers, formed the basis for her PhD thesis and was critical
for understanding the specific challenges Māori stroke survivors faced. Kathryn and her colleagues further defined the role of Take Charge as a crucial element of recovery for stroke patients and others with chronic conditions. Small grants enabled the filming of inspiring stories featuring Māori and Pacific stroke survivors, which emphasised that recovery requires personal dedication.
In 2005, the MRINZ secured funding from the Health Research Council of New Zealand for the Māori and Pacific Stroke Study (MaPSS), a unique randomised clinical trial exclusively involving Māori and Pacific participants. This ambitious study, co-led by Matire and Harry, recruited nearly 200 participants from across seven centres in New Zealand, testing the effectiveness of two interventions: a filmed story DVD and a face-to-face Take Charge session. The study’s results showed that while the DVD was ineffective, the Take Charge intervention significantly improved outcomes and reduced caregiver stress. These promising results raised new questions: “Was this effect real? What made Take Charge successful?” To address these inquiries, MRINZ initiated the Taking Charge after Stroke (TaCAS) trial in 2015. Involving 400 participants, TaCAS aimed to extend the evidence base from MaPSS to a broader participant group. The study confirmed that Take Charge improved outcomes across all stroke population subsets, with two sessions proving more effective than one. This research was presented at an international conference in Milan in 2019 and later published, marking a major milestone in stroke rehabilitation.
Insights from the TaCAS study, along with related qualitative research, have deepened the MRINZ’s understanding of the Take Charge intervention. Its success lies in its ability to foster intrinsic motivation, empowering individuals to take charge of their own recovery. Participants in the intervention engage in goal-setting and learn strategies for self-management, leading to improvements in both physical and mental wellbeing. Notably, Take Charge has now expanded beyond its initial focus, with growing evidence of its benefits for individuals with various medical conditions. Its positive impact has garnered increasing global interest, with implementations reported in over ten countries, and materials translated into six languages. The Australasian Stroke Rehabilitation guidelines now strongly recommend Take Charge for stroke patients discharged home, and pilot programmes in New Zealand, including one by the Stroke Foundation, have further validated its effectiveness.
Recent studies on COPD and Long COVID have also highlighted Take Charge’s positive effects. In the COPD study, participants showed significant reductions in disease burden, allowing them to better manage their condition. In the Long COVID study, Take Charge has supported individuals grappling with the long-term effects of the virus. In addition to clinical outcomes, economic analyses of the intervention have shown it to be highly cost-effective. A study conducted on stroke patients indicated that Take Charge could yield a cost saving of approximately $2,000 per person treated in the first year post-stroke.
The MRINZ remains dedicated to advancing Take Charge. All related materials are made freely available online, ensuring broad access for both individuals and healthcare providers.
The MRINZ continues to train facilitators, assist with grant applications, and collaborate on new studies. One current initiative involves exploring a multi-country partnership to implement Take Charge in low-resource settings, where healthcare access is often limited. Additionally, the MRINZ has been selected to participate in a large UK platform trial aimed at enhancing psychosocial well-being after stroke. This trial represents a significant opportunity to further validate the impact of Take Charge and broaden its reach.
Beyond the clinical and economic successes, the MRINZ is proud of the broader societal impact that Take Charge has had. The initiative demonstrates how research can drive change by focusing on the needs of diverse communities and addressing social determinants of health. By empowering individuals to take charge of their health, Take Charge has not only improved physical outcomes but also helped people regain a sense of agency and control over their lives. The MRINZ continues to work closely with local communities, healthcare providers, and policymakers to ensure that the benefits of Take Charge reach as many people as possible, especially those in underrepresented and underserved groups.
Through its ongoing efforts, the MRINZ remains steadfast in its dedication to addressing health inequities and improving health outcomes for all New Zealanders and beyond. The Take Charge intervention stands as a testament to the MRINZ commitment to transformative public health research and represents a key component of the MRINZ’s broader mission to build a healthier future for communities both locally and globally. With continued support, collaboration, and innovation, Take Charge will undoubtedly continue to make a significant impact in the lives of individuals facing chronic health conditions.
Ki te wātea te hinengaro me te kaha o te rere o te wairua, ka taea ngā mea katoa. If the mind is free and the spirit is strong, anything is possible.
Established in early 2024, Ngā Kaitiaki o Te Rangahau, the MRINZ Māori Advisory Board, plays a vital role in the Institute’s commitment to Te Tiriti o Waitangi, health equity, and culturally attuned medical research. Chaired by MRINZ Māori Health Research Advisor Trisha Falleni, the board includes leaders and experts from Māori hauora (health) communities — Clive Aspin, Jane Patterson, Peter Jackson, and Simon Phillips. Together, they ensure that Māori perspectives are embedded in the MRINZ’s work, guiding research that is not only scientifically rigorous but also socially and culturally meaningful.
Ngā Kaitiaki o Te Rangahau’s influence alreadt runs deep within the MRINZ, fostering cultural understanding and diverse perspectives in every project. With strong connections to community services like Maraeroa Marae and Whānau Care at Te Whatu Ora Capital, Coast and Hutt Valley, the board plays a critical role in linking Māori communities to the institute’s research, and helps the MRINZ to understand and meet the needs of of these groups.
Quarterly meetings with MRINZ researchers allow the rōpū to provide insights, ask key questions, and ensure each study aligns with principles of health equity and cultural competence. These collaborative exchanges reflect the commitment of both the MRINZ and Ngā Kaitiaki o Te Rangahau to codevelop research that benefits Māori and all New Zealanders.
Trisha role of Māori Health Research Advisor role continues to deepen, aligning the MRINZ team with Māori health priorities. Increasingly, the MRINZ whānau seeks support and expertise from the Board across their mahi, recognising the value of Māori perspectives in shaping impactful research. Trisha’s evolving role underscores the MRINZ’s ongoing dedication to learning and improving its capacity to authentically serve Māori communities.
In 2024, the MRINZ warmly welcomed two young researchers, Bianca Crichton and Jackson Smeed-Tauroa. Their fresh perspectives and passion for enhancing Māori health are further strengthening the MRINZ's commitment to advancing indigenous representation in research.
A key initiative this year has been the promotion of te reo Māori and tikanga Māori within the MRINZ. The office now features Māori posters and language sheets, and staff are learning karakia and waiata, guided by Trisha Falleni, Bianca Crichton, and Christina Elder.
The wider MRINZ team have together visited He Tohu, a permanent exhibition of constitutional artefacts: the 1835 Declaration of Independence (He Whakaputanga o te Rangatiratanga o Nu Tireni) and the 1840 Treaty of Waitangi (Te Tiriti o Waitangi), and continue to reflect on how these documents shape Aotearoa New Zealand. All these efforts are fostering a culturally rich and inclusive environment that deepens respect and understanding of Te Ao Māori.
Through the collective guidance of Ngā Kaitiaki o Te Rangahau, the MRINZ continues to uphold the values of Te Tiriti o Waitangi, striving for health equity and research excellence. This partnership ensures that the MRINZ’s work is not only scientifically robust but also deeply connected to the cultural and spiritual values that sustain Māori health and wellbeing.

In 2024, the MRINZ reached a pivotal moment, outgrowing its existing facilities as a range of new, innovative research studies were planned. Recognising the need for a larger and more versatile workspace, the MRINZ worked closely with Hanita Shantilal and the Te Whatu Ora Capital, Coast and Hutt Valley Facilities Management team, who helped the Institute secure a valuable area within Wellington Hospital, creating a new footprint to support the MRINZ’s future growth.
Under the leadership of Deputy Director Alex Semprini, and with contributions from the wider MRINZ team, the Institute turned to CCM Architects—Adam Flowers and Rebecca Crookes—who had designed the original MRINZ fitout in 2010. With their expertise, what unfolded was a carefully planned reimagining of the MRINZ’s workspace, creatively utilising the open area in front of the Clinical Services Building (CSB) lift foyer and reconfiguring unused space in the Wellington Services Building (WSB) to create a functional, future-proof design.
With funding secured and plans in place, Hawkins Construction, led by Benjamin Solomona and an outstanding team of subcontractors, brought the vision to life with precision and professionalism. Despite the challenges of renovating an active work environment, the construction team ensured that the MRINZ remained fully operational throughout the project.
The ‘Pod’ has now been unveiled as a fresh, vibrant new space that energises the MRINZ’s physical environment. With modern décor, innovative glass panelling, and splashes of colour, the area offers an open and welcoming feel—designed to inspire both collaboration and focus.
Looking back on the journey, the MRINZ celebrates not only the expansion itself but the collaborative effort that made it possible. The ‘Pod’ is not just a workspace; it’s a symbol of the MRINZ’s progress and its dedication to pushing boundaries in research for the benefit of Aotearoa New Zealand and beyond.

The MRINZ has officially applied to become the first research institution in the southern hemisphere to pursue certification under the European Clinical Research Infrastructure Network (ECRIN) Data Centre Certification Programme, marking a significant milestone. This certification reflects the MRINZ’s dedication to rigorous standards in data management, IT, and statistical programming in clinical research, ensuring that processes meet the international benchmark of Good Clinical Practice.
The ECRIN’s certification programme rigorously assesses data centres operating non-commercial clinical trials. The certification process mandates compliance with 126 specific requirements spanning data management, IT, statistical programming, and general clinical trial administration. For the MRINZ, the application process involved an exhaustive self-assessment survey, with nearly 300 documents compiled to demonstrate compliance across these standards. This extensive submission was completed in August 2024 after two months of focused effort by the MRINZ Data Management team.
In addition to detailed documentation, ECRIN certification involves an audit that verifies operational maturity, including training records, policies, and Standard Operating Procedures, as well as live demonstrations of processes. If successful, the MRINZ’s data management and IT functions will be recognised as meeting international quality standards.
Should the MRINZ’s application be successful, this certification would position the MRINZ to enhance the quality and integrity of clinical research throughout the region.
Through the development of novel clinical trial platforms using informatics integrations with the electronic health record, smartphone technology, and national pharmacy and general practice localities we are achieving Māori participation of over 13%. Harnessing this improved research engagement provides significant potential for both assessing and improving Māori health outcomes.
Over the past 15 years, the MRINZ, with funding from the Health Research Council of New Zealand, has conducted intensive care research that has led to transformative improvements in patient outcomes and healthcare delivery.
With a total investment of $16M, this research has not only saved lives and reduced complications but also delivered substantial financial savings to the New Zealand health system.
The findings have informed critical care practices, ensuring better patient recovery while alleviating financial pressures.
Intravenous Crystalloid Fluid Research: Implementing this research has resulted in 70 fewer deaths and 49 fewer cases of acute kidney dialysis annually in New Zealand, saving $60,000 per year.
Liberal Fluid Therapy After Major Surgery:
Annually, 360 fewer adults develop acute kidney injury, and 290 fewer patients experience surgical site infections, resulting in $6M in healthcare savings.
Restrictive Transfusion Research: Among heart surgery patients, 35 fewer people suffer heart attacks, strokes, kidney failure, or death each year, generating $2M in savings from reduced blood product use.
Pre-Hospital Tranexamic Acid Therapy: This intervention prevents 20 deaths per year from major trauma, at a cost of just $5.95 per patient.
Intravenous Starch Solutions Research:
Avoiding the use of toxic starch solutions prevents 221 cases of kidney dialysis annually, saving $2.5M in healthcare costs.
Vitamin C in Septic Shock Research:
Ceasing the use of vitamin C in septic shock treatments saves 46 lives annually and reduces costs by $1.2M.
Therapeutic Hypothermia
De-Implementation: Discontinuing the use of this therapy, which increased ICU support requirements without improving outcomes, has saved $3.25M per year.
Delayed Initiation of Dialysis in Acute Kidney Injury: 27 fewer patients require permanent dialysis each year, resulting in $3.34M in direct savings.
Hydrocortisone for Septic Shock: Using hydrocortisone to speed recovery in septic shock patients saves $14.5M annually in ICU costs.
DECRA Trial on Severe Brain Injury: Avoiding early decompressive craniectomy in patients with severe brain injury saves $20M annually, as this procedure increased the number of dependent survivors with high lifetime care costs.
COVID-19 Research: During the pandemic, the MRINZ contributed to research that established a range of life-saving therapies that likely saved over a million lives worldwide.
The total annual savings to the New Zealand health system from implementing these research findings amount to $51.59M.
For every dollar invested in MRINZ intensive care research, the New Zealand healthcare system receives a return of $48 in direct savings each year, with ongoing benefits that continue to generate these savings without further investment.
This research underscores the value of evidence-based clinical interventions in intensive care, providing improved patient outcomes and significant cost efficiency for New Zealand's healthcare system.
The lasting impact of the MRINZ’s intensive care research extends beyond impressive numbers—it represents a future where healthcare resources are optimised for the benefit of all New Zealanders. This transformative work, funded by the Health Research Council of New Zealand, exemplifies the profound return on investment that thoughtful, evidence-based medical research can deliver.
With each year, the knowledge gained from this research continues to save lives, prevent complications, and provide critical cost savings, ensuring a healthier and more resilient healthcare system for generations to come.
Publication in peer-reviewed scientific journals is the primary method for communicating research findings. This shared knowledge forms the scientific foundation for practicing evidence-based medicine, resulting in improved clinical outcomes.
1. Adler H, Lewis M, Ng CHM, Brooks C, Leonardi M, Mikocka-Walus A, Bush D, Semprini A, Wilkinson-Tomey J, Condous G, Patravali N, Abbott J, Armour M. Social media, endometriosis, and evidence-based information: an analysis of Instagram content. Healthcare 2024; 12: 121. https://doi.org/10.3390/ healthcare12010121
2. Barry T, Holliday M, Sparks J, Biggs R, Colman A, Lamb R, Oldfield K, Shortt N, Kerse K, Martindale J, Eathorne A, Walton M, Black B, Harwood M, Bruce P, Semprini R, Bush A, Fleming L, Byrnes CA, McNamara D, Hatter L, Dalziel SR, Weatherall M, Beasley R. START CARE: a protocol for a randomised controlled trial of step-wise budesonide–formoterol reliever-based treatment in children. ERJ Open Res 2024; 10: 00897-2023 [DOI: 10.1183/23120541.00897-2023].
3. Battaglini D, Schiavetti I, Ball L, Jakobsen JC, Lilja G, Friberg H, Wendel-Garcia PD, Young PJ, Eastwood G, Chew MS, Unden J, Thomas M, Joannidis M, Nichol A, Lundin A, Hollenberg J, Hammond N, Saxena M, Martin A, Solar M, Taccone FS, Dankiewicz J, Nielsen N, Morten Grejs A, Wise MP, Hängghi M, Smid O, Patroniti N, Robba C; TTM2 trial investigators. Association between early airway intervention in the pre-hospital setting and outcomes in out of hospital cardiac arrest patients: A post-hoc analysis of the Target Temperature Management-2 (TTM2) trial. Resuscitation 2024; 203: 110390. doi: 10.1016/j. resuscitation.2024.110390.
4. Beasley R. The low hanging fruit of anti-inflammatory reliever therapy. Respirol 2024; 29: 256-7.
5. Beasley R. Air pollution and global health: Need to prioritize alongside tobacco control. Respirol 2024; 29: 731-2. doi.org/10.1111/ resp.14779
6. Beasley R, Noble J. Time for the lens to focus on diagnostic algorithms in asthma. EClinicalMedicine 2024; 76: 102812. doi: 10.1016/j. eclinm.2024.102812.
7. Beasley R, Noble J, Weatherall M. Extending Personalized Evidence-Based Medicine in Severe Asthma. J Allergy Clin Immunol Pract. 2024 Sep;12(9):2362-2363. doi: 10.1016/j.jaip.2024.06.038.
8. Beasley R, Ferreira DS, Papi A. Extending Personalized Evidence-Based Medicine in Severe Asthma. J Allergy Clin Immunol Pract. 2024 Sep;12(9):2362-2363. doi: 10.1016/j.jaip.2024.06.038.
9. Beasley R, Hughes R, Agusti A, Calverley P, Chipps B, Del Olmo R, Papi A, Price D, Reddel H, Müllerová H, Rapsomaniki E. Prevalence, diagnostic utility and associated characteristics of bronchodilator responsiveness. Am J Respir Crit Care Med 2024; 209: 390-401.
10. Beasley R, Noble J, Weatherall M. The evidence base for ICS/formoterol maintenance and reliever therapy in severe asthma. Eur Respir J 2024; 63: 2400523; doi:10.1183/13993003.00523-2024
11. Bright FAS, Ibell-Roberts C, Featherstone K, Signal N, Wilson BJ, Collier A, Fu V. 'Physical well-being is our top priority': Healthcare professionals' challenges in supporting psychosocial well-being in stroke services. Health Expect 2024; 27: e14016. doi: 10.1111/hex.14016.
12. Buell KG, Spicer AB, Casey JD, Seitz KP, Qian ET, Graham Linck EJ, Self WH, Rice TW, Sinha P, Young PJ, Semler MW, Churpek MM. Individualized treatment effects of oxygen targets in mechanically ventilated critically ill adults. JAMA 2024: e242933. doi: 10.1001/ jama.2024.2933
13. Chan AHY, Te Ao B, Baggott C, Cavadino A, Eikholt AA, Harwood M, Hikaka J, Gibbs D, Hudson M, Mirza F, Naeem MA, Semprini R, Chang CL, Tsang KCH, Shah SA, Jeremiah A, Abeysinghe BN, Roy R, Wall C, Wood L, Dalziel S, Pinnock H, van Boven JFM, Roop P, Harrison J. DIGIPREDICT: physiological, behavioural and environmental predictors of asthma attacks-a prospective observational study using digital markers and artificial intelligence-study protocol. BMJ Open Respir Res 2024; 11(1): e002275. doi: 10.1136/bmjresp-2023-002275.
14. Chapple LS, Neuts A, O'Connor SN, Williams P, Hurford S, Young PJ, Hammond NE, Knowles S, Chapman MJ, Peake S; TARGET Investigators, The George Institute for Global Health and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Nutrition practices in Australia and New Zealand in response to evolving evidence: Results of three point-prevalence audits. Aust Crit Care 2024: S1036-7314(24)00208-X. doi: 10.1016/j.aucc.2024.07.079.
15. Cheung W, Naganathan V, Myburgh J, Saxena MK, Fiona B, Seppelt I, Parr M, Hooker C, Kerridge I, Nguyen N, Kelly S, Skowronski G, Hammond N, Attokaran A, Chalmers D, Gandhi K, Kol M, McGuinness S, Nair P, Nayyar V, Orford N, Parke R, Shah A, Wagh A. A survey of Australian public opinion on using comorbidity to triage intensive care patients in a pandemic. Aust Health Rev 2024; 48(4): 459-68. doi: 10.1071/AH23265.
16. Chipps BE, Papi A, Beasley R, Israel E, Panettieri RA Jr, Albers FC, Cooper M, Darken P, Gilbert I, Trudo F, Weinberg M, Cappelletti C. Albuterol–budesonide rescue reduces progression from asthma deterioration to severe exacerbation. J Allergy Clin Immunol Pract 2024; 12(10): 2847-51
17. Cook D, Deane A, Lauzier F, Zytaruk N, Guyatt G, Saunders L, Hardie M, Heels-Ansdell D, Alhazzani W, Marshall J, Muscedere J, Myburgh J, English S, Arabi YM, Ostermann M, Knowles S, Hammond N, Byrne KM, Chapman M, Venkatesh B, Young P, Rajbhandari D, Poole A, Al-Fares A, Reis G, Johnson D, Iqbal M, Hall R, Meade M, Hand L, Duan E, Clarke F, Dionne JC, Tsang JLY, Rochwerg B, Karachi T, Lamontagne F, D'Aragon F, St Arnaud C, Reeve B, Geagea A, Niven D, Vazquez-Grande G, Zarychanski R, Ovakim D, Wood G, Burns KEA, Goffi A, Wilcox ME, Henderson W, Forrest D, Fowler R, Adhikari NKJ, Ball I, Mele T, Binnie A, Trop S, Mehta S, Morgan I, Loubani O, Vanstone M, Fiest K, Charbonney E, Cavayas YA, Archambault P, Rewa OG, Lau V, Kristof AS, Khwaja K, Williamson D, Kanji S, Sy E, Dennis B, Reynolds S, Marquis F, Lellouche F, Rahman A, Hosek P, Barletta JF, Cirrone R,
Tutschka M, Xie F, Billot L, Thabane L, Finfer S; REVISE Investigators. Stress Ulcer Prophylaxis during Invasive Mechanical Ventilation. N Engl J Med 2024; doi: 10.1056/NEJMoa2404245.
18. Doppen M, Kearns C, Hills T, Weatherall M, Beasley R. Intramuscular vaccination needle length: a call to arms. Lancet 2024; 403: 528-9.
19. Dulhunty JM, Brett SJ, De Waele JJ, Rajbhandari D, Billot L, Cotta MO, Davis JS, Finfer S, Hammond NE, Knowles S, Liu X, McGuinness S, Mysore J, Paterson DL, Peake S, Rhodes A, Roberts JA, Roger C, Shirwadkar C, Starr T, Taylor C, Myburgh JA, Lipman J; BLING III Study Investigators. Continuous vs Intermittent β-Lactam Antibiotic Infusions in Critically Ill Patients With Sepsis: The BLING III Randomized Clinical Trial. JAMA 2024; e249779. doi: 10.1001/jama.2024.9779.
20. Eastwood GM, Bailey M, Nichol AD, Parke R, Nielsen N, Dankiewicz J, Bellomo R; TAME trial investigators. Impact of mild hypercapnia on renal function after outof-hospital cardiac arrest. Resuscitation 2024 Dec 30:110480. doi: 10.1016/j. resuscitation.2024.110480. Epub ahead of print. PMID: 39742940.
21. Eastwood GM, Bailey M, Nichol AD, Dankiewicz J, Nielsen N, Parke R, Cronberg T, Olasveengen T, Grejs AM, Iten M, Haenggi M, McGuigan P, Wagner F, Moseby-Knappe M, Lang M, Bellomo R; TAME trial investigators. Magnetic resonance imaging in comatose adults resuscitated after out-of-hospital cardiac arrest: A posthoc study of the Targeted Therapeutic Mild Hypercapnia after Resuscitated Cardiac Arrest trial. Aust Crit Care 2024; 101130. doi: 10.1016/j.aucc.2024.09.015.
22. Eathorne A, Noble J, Hatter L, Hills T, Te Paa S, Harwood M, Weatherall M, Beasley R. Reducing ethnic inequities: Patterns of asthma medication use and hospital discharges in Māori in Aotearoa New Zealand. Respirology 2024 Dec 11. doi: 10.1111/resp.14865. Epub ahead of print. PMID: 39662517.
23. Foot H, Beyene K, Horne R, Fingleton J, Harrison J, Chan AHY. Evaluating the feasibility of a community pharmacy-delivered behaviour change intervention to reduce reliever reliance in asthma. Patient Prefer Adherence 2024; 18: 361-71.
24. Frei DR, Moore MR, Bailey M, Beasley R, Campbell D, Leslie K, Myles PS, Short TG, Young PJ. Associations between the intraoperative fraction of inspired intraoperative oxygen administration and days alive and out of hospital after surgery. BJA Open 2024; 9: 100253.
25. Frei DR, Young PJ, Myles PS. Oxygen therapy during surgery: decisions need reliable data. Anesthesiology 2024; 141: 437-9. doi: 10.1097/ALN.0000000000005095
26. Hadley-Brown K, Hailstone L, Devane R, Chan T, Devaux A, Davis JS, Hammond N, Li MBioStat Q, Litton E, Myburgh J, Poole A, Santos JA, Seppelt I, Tong SYC, Udy A, Venkatesh B, Young PJ, Delaney AP. Prophylactic antibiotics in adults with acute brain injury who are invasively ventilated in the Intensive Care Unit: A systematic review and meta-analysis. Chest 2024; S0012-3692(24)05420-5. doi: 10.1016/j. chest.2024.10.031.
27. Harper B, Ranta S, McNaughton H, Ranta A. The impact of intensive blood pressure management in the post-thrombolysis setting: a real-world observational study. NZ Med J 2024; 137: 13-9.
28. Harris R, Li C, Stanley J, King PT, Priest N, Curtis E, Ameratunga S, Sorensen D, Tibble F, Tewhaiti-Smith J, Thatcher P, Araroa R, Pihema S, Lee-Kirk S, King SJR, Urlich T, Livingstone NZ, Kamau Brady S, Matehe C, Paine SJ. Racism and Health Among Aotearoa New Zealand Young People Aged 15-24 years: Analysis of Multiple National Surveys. J Adolesc Health 2024; S1054-139X(24)00230-1. doi: 10.1016/j. jadohealth.2024.04.021
29. Hatter L, Holliday M, Eathorne A, Bruce P, Pavord ID, Reddel HK, Hancox RJ, Papi A, Weatherall M, Beasley R. The carbon footprint of as-needed budesonide-formoterol in mild asthma: a post hoc analysis. Eur Respir J 2024; 64: 2301705. doi: 10.1183/13993003.01705-2023.
30. Hernández-Mitre MP, Morpeth SC, Venkatesh B, Hills TE, Davis J, Mahar RK, McPhee G, Jones M, Totterdell J, Tong SYC, Roberts JA. TMPRSS2 inhibitors for the treatment of COVID-19 in adults: a systematic review and meta-analysis of randomized clinical trials of nafamostat and camostat mesylate. Clin Microbiol Infect 2024; S1198743X(24)00055-7.
31. Hills T, Delaney A, Young PJ. Inhaled amikacin to prevent ventilator-associated pneumonia. N Engl J Med 2024; 390: 769.
32. Huemer M, Graca S, Bitsche S, Hofmann G, Armour M, Pichler M. Mapping the clinical practice of traditional, complementary and integrative medicine in oncology in Western countries: A multinational cross-sectional survey. J Integrative Med 2024; 22: 64-71.
33. Huemer M, Graca S, Bitsche S, Hofmann G, Armour M, Pichler M. Assessing the role and impact of research in clinical practice among acupuncturists in western countries: a multinational cross-sectional survey. Front Med (Lausanne) 2024; 11: 1331184. doi: 10.3389/ fmed.2024.1331184
34. Keijzers G, Donaghy M, Weatherall M, Beasley R, Ball EL, Simpson G, EgertonWarburton D, Lee YCG, Brown SGA. Primary Spontaneous Pneumothorax, does size matter? Eur Respir J 2024: 2400429. doi: 10.1183/13993003.00429-2024.
35. Kirton LW, Cruz RS, Navarra L, Eathorne A, Cook J, Beasley R, Young PJ. Effect of automated titration of oxygen on time spent in a prescribed oxygen saturation range in adults in the ICU after cardiac surgery. Crit Care Resusc 2024; 26: 64-70. doi: 10.1016/j.ccrj.2024.01.001
36. Kirton L, Kung S, Bird G, Black M, Semprini R, Eathorne A, Weatherall M, Semprini A, Beasley R. Automated oxygen titration with non-invasive ventilation in hypoxaemic adults with cardiorespiratory disease: a randomised cross-over trial. BMJ Open Respir Res 2024; 11(1): e002196. doi: 10.1136/ bmjresp-2023-002196.
37. Kivi N, Reiche L, Kingi T, Elder C, Semprini A. Improving access to dermatology specialist care: review of a dermatologist- and general practitioner-integrated clinic model. J Prim Health Care 2024; 16: 21-6.
38. Krings JG, Beasley R. The role of ICS-containing rescue therapy versus SABA alone in asthma management today. J Allergy Clin Immunol Pract 2024; 12: 870-9. S22132198(24)00063-1. doi: 10.1016/j.jaip.2024.01.011.
39. Kuek SL, Wong NX, Dalziel S, Hatter L, Fleming L, Bush A, Beasley R, Shanthikumar S. Dry powder inhaler use in primary school aged children with asthma: a systematic review. ERJ Open Res 2024; in press (https://doi. org/10.1183/23120541.00455-2024).
40. Kung S, Semprini A, Kirton L, Fogarin J, Zoellner S, Beasley R, Eathorne A, Semprini R. Efficacy of non-invasive ventilation with expiratory washout in stable COPD patients: a randomized cross-over pilot. Respir Care 2024; 11876. doi: 10.4187/respcare.
41. Lamb R, Kerse K, Kristono H, Oldfield K, Beasley R. A snapshot of SABA co-prescribing with ICS-formoterol maintenance and reliever therapy. Respirology 2024; https://doi. org/10.1111/resp.14815
42. Lawrence NJ, Laing BB, Tyro J, Babington S, Dzhelali M, Gautier A, Grant D, Hari Dass P, Jameson M, Lauren C, Maxwell J, Ngatai N, du Plessis R, Stratton C, Tan A, Williams M, Wilson M. Process of development of decentralised clinical trial methodology for cancer clinical trials in Aotearoa New Zealand. N Z Med J 2024 Dec 13;137(1607):12-21. doi: 10.26635/6965.6628. PMID: 39666881.
43. Levy ML, Beasley R, Bostock B, Capstick TG, Crooks MG, Fleming L, Freeman D, Marsh V, Rupani H, Whittamore A, Barnes PJ, Bush A. A simple and effective evidence-based approach to asthma management: ICS-formoterol reliever therapy. Br J Gen Pract. 2024; 74(739): 86-9. doi: 10.3399/bjgp24X736353. PMID: 38272684.
44. Mackle DM, Nelson K, Beasley RW, Eathorne A, Young PJ. The influence of participation in an intensive care trial on health practitioners' knowledge of the results-a selfreported survey. J Adv Nurs 2024; doi: 10.1111/ jan.16641.
45. Mak JN, Uzuner C, Espada M, Eathorne A, Reid S, Leonardi M, Armour M, Condous GS. Inter-observer reproducibility of the 2021 AAGL Endometriosis Classification. Aust N Z J Obstet Gynaecol 2024; doi: 10.1111/ajo.13851
46. Mardon AK, Whitaker L, Farooqi T, Girling J, Henry C, Ee C, Tewhaiti-Smith J, Armour M. Investigational drugs for the treatment of dysmenorrhea. Expert Opin Investig Drugs 2024; 33: 347-57. doi: 10.1080/13543784.2024.2326627
47. Mardon AK, White S, Howe D, O'Shea M, Eathorne A, Gannott M, Schott A, Armour M. Problematic Periods Costing Young Women-The Impact of Menstrual Symptoms on Work and Study. Aust N Z J Obstet Gynaecol 2024 Dec 19. doi: 10.1111/ajo.13926. Epub ahead of print. PMID: 39698797.
48. Martin DS, Shahid T, Gould DW, Richards-Belle A, Doidge JC, Camsooksai J, Charles WN, Davey M, Francis Johnson A, Garrett RM, Grocott MP, Jones J, Lampro L, Miller L, O'Driscoll BR, Rostron AJ, Sadique Z, Szakmany T, Young PJ, Rowan KM, Harrison DA, Mouncey PR. Evaluating the clinical and costeffectiveness of a conservative approach to oxygen therapy for invasively ventilated adults in intensive care: Protocol for the UK-ROX trial. J Intensive Care Soc 2024; 25: 223-30.
49. Maze MJ, Williman J, Anstey R, Best E, Bhally H, Bryce A, Chang CL, Chen K, Dummer J, Epton M, Good W, Goodson J, Grey C, Grimwade K, Hancox RJ, Hassan R, Hills T, Hotu S, McArthur C, Morpeth S, Murdoch DR, Pease F, Pylypchuk R, Raymond N, Ritchie S, Ryan D, Selak V, Storer M, Walls T, Webb R, Wong C, Wright K. Evaluation of risk prediction scores for adults hospitalised with COVID19 in a highly vaccinated population, Aotearoa New Zealand 2022. IJID Regions 2024; doi: https:// doi.org/10.1016/j.ijregi.2024.100424
50. Noble J, Hatter L, Eathorne A, Hills T, Bean O, Bruce P, Weatherall M, Beasley R. Patterns of asthma medication use and hospital discharges in New Zealand. J Allergy Clin Immunol Glob 2024; 3(3): 100258. doi: 10.1016/j. jacig.2024.100258
51. O'Driscoll BR, Kirton L, Weatherall M, Bakerly ND, Turkington P, Cook J, Beasley R. Effect of a lower target oxygen saturation range on the risk of hypoxaemia and elevated NEWS2 scores at a university hospital: a retrospective study. BMJ Open Respir Res 2024; 11(1): e002019
52. Opgenorth D, Duquette DJ, Tyre L, Auld R, Crowder K, Gilchrist P, Young PJ, Bagshaw SM. Public perception of participation in lowrisk clinical trials in critical care using waived consent: a Canadian national survey. Can J Anaesth 2024; doi: 10.1007/s12630-024-02723-3
53. Papi A, Chipps BR, Beasley R, Panettieri RA Jr, Israel E, Cooper M, Dunsire L, Jeynes-Ellis A, Rees R, Albers FC, Cappelletti C. Albuterol–budesonide fixed-dose combination rescue inhaler for asthma: a plain language summary of the MANDALA study. Ther Adv Respir Dis 2024; 18: 1–14
54. Parke RL, McGuinness SP, Cavadino A, Cowdrey KA, Bates S, Bihari S, Corley A, Gilder E, Hodgson C, Litton E, McArthur C, Nichol A, Parker J, Turner A, Webb S, Van Haren FM; SAGE-ANZ Study Investigators and the Australia and New Zealand Intensive Care Society Clinical Trials Group. Management of severe acute respiratory distress syndrome in Australia and New Zealand (SAGE-ANZ): An observational study. Crit Care Resusc 2024; 26(3): 161-8. doi: 10.1016/j.ccrj.2024.05.001.
55. Patanwala AE, Abu Sardaneh A, Alffenaar JC, Choo CL, Dey AL, Duffy EJ, Green SE, Hills TE, Howle LM, Joseph JA, Khuon MC, Koppen CS, Pang F, Park JY, Parlicki MA, Shah IS, Tran K, Tran P, Wills MA, Xu JH, Youssef M; ADEPT Study Investigators and Collaborators. Antibiotic de-escalation practices in the Intensive Care Unit: a multicenter observational study. Ann Pharmacother 2024; 10600280241271223. doi: 10.1177/10600280241271223.
56. Patanwala AE, Flannery AH, Mehta HB, Hills TE, McArthur CJ, Erstad BL. Comparative effectiveness of albumin versus no albumin on renal replacement therapy and mortality in patients with septic shock and renal impairment. Chest 2024; S0012-3692(24)053157. doi: 10.1016/j.chest.2024.10.012.
57. Patanwala AE, Nix DE, Hills TE, Erstad BL. A national retrospective cohort study comparing of cefepime versus piperacillintazobactam on the development of severe acute kidney injury in patients with septic shock. Clin Infect Dis 2024 Dec 5:ciae600. doi: 10.1093/cid/ ciae600. Epub ahead of print. PMID: 39657005.
58. Patanwala AE, Xiao X, Hills TE, Higgins AM, McArthur CJ, Alexander GC, Mehta HB; National Covid Cohort Collaborative (N3C) Consortium. Comparative Effectiveness of Baricitinib Versus Tocilizumab in Hospitalized Patients With COVID-19: A Retrospective Cohort Study of the National Covid Collaborative. Crit Care Med 2024; doi: 10.1097/ CCM.0000000000006444.
59. Pickkers P, Angus DC, Bass K, Bellomo R, van den Berg E, Bernholz J, Bestle MH, Doi K, Doig CJ, Ferrer R, Francois B, Gammelager H, Pedersen UG, Hoste E, Iversen S, Joannidis M, Kellum JA, Liu K, Meersch M, Mehta R, Millington S, Murray PT, Nichol A, Ostermann M, Pettilä V, Solling C, Winkel M, Young PJ, Zarbock A. REVIVAL investigators. Phase-3 trial of recombinant human alkaline phosphatase for patients with sepsis-associated acute kidney injury (REVIVAL). Intensive Care Med 2024; doi: 10.1007/s00134-023-07271-w.
60. Proudfoot A, Duffy S, Sinclair J, Abbott J, Armour M. A survey of cost, access and outcomes for cannabinoid-based medicinal product use by Australians with endometriosis. Aust N Z J Obstet Gynaecol 2024; doi: 10.1111/ ajo.13804
61. Sanfilippo F, Uryga A, Santonocito C, Jakobsen JC, Lilja G, Friberg H, Wendel-Garcia PD, Young PJ, Eastwood G, Chew MS, Unden J, Thomas M, Grejs AM, Wise MP, Lundin A, Hollenberg J, Hammond N, Saxena M, Martin A, Bánszky R, Taccone FS, Dankiewicz J, Nielsen N, Ebner F, BeloholaveK J, Hanggi M, Montagnani L, Patroniti N, Robba C; TTM-2 investigators. Effects of very early hyperoxemia on neurologic outcome after out-of-hospital cardiac arrest: A secondary analysis of the TTM-2 trial. Resuscitation 2024 Dec 7:110460. doi: 10.1016/j. resuscitation.2024.110460. Epub ahead of print. PMID: 39653237.
62. Santabarbara KL, Helms ER, Stewart TI, Armour MJ, Harris NK. Menstrual cycle patterns and their relationship with measures of wellbeing and perceived performance metrics in competitive and recreational resistance-trained athletes. J Sports Med Phys Fitness 2024; 64: 694-706. doi: 10.23736/S0022-4707.24.15752-
63. SantaBarbara KL, Helms ER, Stewart TI, Armour MJ, Harris NK. A Randomized Crossover Pilot Trial on the Influence of a Daily Mindfulness Yoga Practice on Menstrual Cycle Symptoms, Measures of Well-Being, and Training Perception in Athletic Women. Int J Yoga Therap 2024 Nov 1;34(2024):Article 17. doi: 10.17761/2024-D-23-00077. PMID: 39621508.
64. Serpa Neto A, Bailey M, Seller D, Agli A, Bellomo R, Brickell K, Broadley T, Buhr H, Gabbe BJ, Gould DW, Harrold M, Higgins AM, Hurford S, Iwashyna TJ, Nichol AD, Presneill JJ, Schaller SJ, Sivasuthan J, Tipping CJ, Poole A, Parke R, Bradley S, Webb S, Zoungas S, Young PJ, Hodgson CL. Impact of High Dose Early Mobilization on Outcomes for Patients with Diabetes: A Secondary Analysis of the TEAM Trial. Am J Respir Crit Care Med 2024;. doi: 10.1164/rccm.202312-2289OC.
65. Shortt G, Shortt N, Bird G, Kerse K, Lieffering N, Martin A, Eathorne A, Black B, Kim B, Rademaker M, Reiche L, Paa ST, Harding S, Armour M, Semprini A. Mānuka oil based ECMT-154 versus vehicle control for the topical treatment of eczema: study protocol for a randomised controlled trial in community pharmacies in Aotearoa New Zealand. BMC Complement Med Ther 2024; 24(1): 61. doi: 10.1186/s12906-024-04358-9. PMID: 38287323.
66. Showler L, Deane AM, Litton E, Ankravs MJ, Wibrow B, Barge D, Goldin J, Hammond N, Saxena MK, Young PJ, Venkatesh B, Finnis M, Abdelhamid YA. A multicentre point prevalence study of nocturnal hours awake and enteral pharmacological sleep aids in patients admitted to Australian and New Zealand intensive care units. Crit Care Resusc 2024; 26(3): 192-7. doi: 10.1016/j.ccrj.2024.06.009.
67. Sise A, Azzopardi P, Brown A, TewhaitiSmith J, Westhead S, Kurji J, McDonough D, Reilly R, Bingham B, Brown N, Cassidy-Matthews C, Clark TC, Elliott S, Finlay SM, Hansen KL, Harwood M, Knapp JMF, Kvernmo S, Lee C, Watts RL, Nadeau M, Pearson O, Reading J, Saewyc E, Seljenes A, Stoor JPA, Aubrey P, Crengle S. Health and well-being needs of Indigenous adolescents: a protocol for a scoping review of qualitative studies. BMJ Open 2024; 14(5): e079942. doi: 10.1136/bmjopen-2023-079942.
68. Sivapalan P, Ellekjaer KL, Perner A, Møller MH, Granholm A, Grønningsæter L, Ostermann M, Sweeney RM, Cronhjort M, Hästbacka J, Pfortmueller C, De Waele J, Nalos M, Jovaisa T, Reintam Blaser A, Cecconi M, Ergan B, Al-Fares A, Young PJ, Szczeklik W, Keus E, Alshamsi F, Khanna AK, Sigurdsson MI, Fujii T, Arabi YM, Meyhoff TS. Preferences for albumin use in adult intensive care unit patients with shock: An international survey. Acta Anaesthesiol Scand 2024; 68(9): 1234-43. doi: 10.1111/aas.14479.
69. Taccone FS, Cariou A, Zorzi S, Friberg H, Jakobsen JC, Nordberg P, Robba C, Belohlavek J, Hovdenes J, Haenggi M, Åneman A, Grejs A, Keeble TR, Annoni F, Young PJ, Wise MP, Cronberg T, Lilja G, Nielsen N, Dankiewicz J. Hypothermia versus normothermia in patients with cardiac arrest and shockable rhythm: a secondary analysis of the TTM-2 study. Crit Care 2024; 28(1): 335. doi: 10.1186/s13054-024-051193.
70. Taccone FS, Dankiewicz J, Cariou A, Lilja G, Asfar P, Belohlavek J, Boulain T, Colin G, Cronberg T, Frat JP, Friberg H, Grejs AM, Grillet G, Girardie P, Haenggi M, Hovdenes J, Jakobsen JC, Levin H, Merdji H, Njimi H, Pelosi P, Rylander C, Saxena M, Thomas M, Young PJ, Wise MP, Nielsen N, Lascarrou JB. Hypothermia vs normothermia in patients with cardiac arrest and nonshockable rhythm: a meta-analysis. JAMA Neurol 2024; 81: 126-33.
71. Taylor A, Best EJ, Walls T, Webb R, Bhally H, Bryce A, Chang CL, Chen K, Dummer J, Epton M, Good W, Goodson J, Grey C, Grimwade K, Hancox RJ, Hassan R, Hills T, Hotu S, McArthur C, Morpeth S, Murdoch DR, Pease F, Pylypchuk R, Raymond N, Ritchie S, Ryan D, Selak V, Storer M, Williman J, Wong C, Wright K, Maze MJ. COVID-19–related hospitalizations among Aotearoa, New Zealand children during the Omicron era of SARS-CoV-2. IJID Regions 2024; 12: 100408.
72. Wang Y, Parpia S, Ge L, Heels-Ansdell D, Lai H, Esfahani MA, Pan B, Alhazzani W, Schandelmaier S, Lauzier F, Arabi Y, Barletta J, Deane A, Finfer S, Williamson D, Kanji S, Møller MH, Perner A, Krag M, Young PJ, Dionne JC, Hammond N, Ye Z, Ibrahim Q, Cook D. ProtonPump Inhibitors to Prevent Gastrointestinal Bleeding - An Updated Meta-Analysis. NEJM Evid. 2024: EVIDoa2400134. doi: 10.1056/ EVIDoa2400134.
73. Young PJ. Less is best for oxygen therapy in adults in the intensive care unit with severe hypoxaemia due to COVID-19. Intensive Care Med 2024; doi: 10.1007/s00134-02407554-w
74. Young PJ, Al-Fares A, Aryal D, Arabi YM, Ashraf MS, Bagshaw SM, Beane A, de Oliveira Manoel AL, Dullawe L, Fazla F, Fujii T, Haniffa R, Hasan MS, Hodgson CL, Hunt A, Lawrence C, Maia IS, Mackle D, Monti G, Nichol AD, Olatunji S, Patodia S, Rashan A, Rashan S, Kasza J; Mega-ROX management committee; Australian and New Zealand Intensive Care Society Clinical Trials Group; Brazilian Research in Intensive Care Network; Critical Care Asia and Africa Network, and Irish Critical Care-Clinical Trials Group. Protocol and statistical analysis plan for the mega randomised registry trial comparing conservative vs. liberal oxygenation targets in adults in the intensive care unit with suspected hypoxic ischaemic encephalopathy following a cardiac arrest (Mega-ROX HIE). Crit Care Resusc 2024; 26: 87-94. doi: 10.1016/j.ccrj.2024.03.004. PMID: 39072241
75. Young PJ, Bailey M; ANZICS CORE Management Committee. Outcomes for Pacific and European patients admitted to New Zealand intensive care units from 2009 to 2018. Crit Care Resusc 2024; 26:100-7. doi: 10.1016/j. ccrj.2024.04.002
76. Young PJ, Cook DJ, Deane AM. Preventing stress ulcer bleeding. Intensive Care Med 2024; doi: 10.1007/s00134-024-07674-3.
77. Young PJ, Devaux A, Li Q, Billot L, Davis JS, Delaney A, Finfer SR, Hammond NE, Micallef S, Seppelt IM, Venkatesh B, Myburgh JA; SuDDICU Australia Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Selective digestive tract decontamination in critically ill adults with acute brain injuries: a post hoc analysis of a randomized clinical trial. Intensive Care Med 2024; 50(1): 56-67. doi: 10.1007/s00134-02307261-y.
78. Mackle, D. M., Nelson, K., Beasley, R. W., Eathorne, A., & Young, P. J. (2024). The Influence of Participation in an Intensive Care Trial on Health Practitioners’ Knowledge of the Results—A Self-Reported Survey. Journal of Advanced Nursing. https://doi.org/10.1111/ jan.16641
79. Zampieri FG, Cavalcanti AB, Di Tanna GL, Damiani LP, Hammond NE, Machado FR, Micallef S, Myburgh J, Ramanan M, Venkatesh B, Rice TW, Semler MW, Young PJ, Finfer S. Balanced crystalloids versus saline for critically ill patients (BEST-Living): a systematic review and individual patient data meta-analysis. Lancet Respir Med 2024; 12: 237-46.
Professor Richard Beasley, director and founder of the MRINZ, was awarded the 2024 Rutherford Medal, Aotearoa New Zealand’s highest scientific honour, for his pioneering contributions to asthma research and treatment.
Bestowed annually by the Royal Society Te Apārangi, the Rutherford Medal recognises exceptional achievements across science, mathematics, social science, and technology. Named in honour of Ernest Rutherford, the medal celebrates the legacy of New Zealand’s most iconic scientist and his groundbreaking advancements.
Accepting the Rutherford Medal at the 2024 Royal Society Te Apārangi Research Honours Aotearoa, Richard expressed deep gratitude to the collaborators, partners, funders, and tens of thousands of clinical trial participants who made these breakthroughs possible. “This honour reflects the collective efforts of multidisciplinary teams committed to improving health outcomes,” he noted.
The award also highlights the critical role Aotearoa New Zealand plays in addressing global health challenges through research excellence. Richard’s work exemplifies how locally driven research, supported by global collaboration, can yield solutions that resonate far beyond New Zealand’s borders, showcasing the country’s capacity to influence international healthcare practice.


Associate Professor Matire Harwood (Ngāpuhi, Ngāti Hine, Ngāti Rangi ki Moerewa), deputy director of the MRINZ, has been made a Companion of the King’s Service Order, recognising her exceptional contributions to health equity and community well-being. This prestigious honour celebrates her leadership as MRINZ Māori & Pacific Peoples’ Health programme lead, Deputy Dean of the Faculty of Medical and Health Sciences at the University of Auckland, and as a primary care GP at Papakura Marae.
Matire’s groundbreaking work spans asthma, heart disease, diabetes, and Māori health equity. Her contributions during the COVID-19 pandemic were particularly notable, providing expert advice to the government and delivering vital care to South Auckland communities. She has also been a driving force behind initiatives that are Māori-led but offer broad benefits, including projects on stroke recovery, smoking cessation, and workforce development.
Reflecting on the recognition, Matire highlighted the collective spirit behind her achievements. “This award honours my whānau, who share me with my mahi, and the countless Māori health workers whose tireless advocacy often goes unseen,” she said. Guided by her tūpuna , who encouraged her to pursue medicine, Matire’s career is deeply rooted in a commitment to making Māori solutions central to healthcare advancements.
Despite the many demands of her roles, Matire remains focused on fostering environments where Māori communities can thrive. She advocates for solutions that address both acute health issues and the broader determinants of well-being, underscoring her vision for a society where health equity is not an aspiration but a reality. Her work is a reminder of the transformative power of culturally grounded, inclusive approaches to healthcare.
Matire's recognition is a powerful testament to her unwavering dedication to advancing health equity and inspiring systemic change across Aotearoa. The MRINZ celebrates this honour, which reflects Matire’s enduring impact on health outcomes for Māori and all New Zealanders.
Dr Tom Hills, Infectious Diseases programme lead at the MRINZ, was awarded the prestigious 2024 Sir Charles Hercus Fellowship from the Health Research Council of New Zealand (HRC). This fellowship builds on the achievements of Tom's research team during the COVID-19 pandemic, specifically through their success with the REMAP-CAP study, which investigated new treatments for COVID-19 and other severe respiratory infections.
Tom shared his gratitude for this HRC support, noting, “This fellowship offers significant backing as our team works to improve outcomes for people suffering from severe pneumonia.” As he takes over leadership of the REAMP-CAP project in New Zealand, Tom will expand his research to target severe influenza and pneumonia, aiming to address the urgent need for effective treatment options.
Tom's leadership in adaptive clinical trial design has already proven instrumental in driving the next phase of the REMAP-CAP study, which has identified treatments that improve both short- and long-term outcomes for COVID-19 patients worldwide. With a focus on innovative approaches, the study has established a collaborative platform that brings together researchers and healthcare professionals from multiple countries to share insights and resources.
The 2024 Sir Charles Hercus Fellowship recognises Tom's exceptional leadership in world-class research and his impressive track record, including a Rhodes Scholarship. His work, particularly in advancing pneumonia treatment, has the potential to significantly improve public health outcomes in Aotearoa New Zealand and beyond.
The fellowship not only marks a career milestone for Tom but also signals an important step for the future of infectious disease treatment. Over the next five years, Tom's work within the REMAPCAP framework will focus on leading pioneering research into therapies for severe pneumonia, with the aim of strengthening hospital care and saving lives both in New Zealand and internationally. By collaborating with healthcare institutions, policymakers, and community stakeholders, Tom intends to translate research findings into practical applications that enhance patient care and inform treatment guidelines.
As the MRINZ continues to tackle the challenges posed by respiratory infections, Tom’s fellowship-supported research represents a beacon of hope for patients and families affected by these diseases. His commitment to improving clinical outcomes reinforces the MRINZ’s mission to advance health research and ultimately contribute to a healthier future for all.


Associate Professor Dianne Sika-Paotonu has been awarded the prestigious Callaghan Medal by the Royal Society Te Apārangi, recognising her exceptional contributions to evidence-based science communication across Aotearoa New Zealand and the Pacific region. A biomedical scientist and immunologist, Dianne's work spans critical areas like cancer, rheumatic fever, and infectious diseases, with a dedicated focus on addressing health inequities.
Dianne’s approach to science communication goes beyond sharing research findings. She champions a deeply inclusive model that fosters respectful, two-way engagement with communities disproportionately affected by health challenges. Her groundbreaking work on rheumatic fever and rheumatic heart disease exemplifies this commitment. Through partnerships with Pacific researchers and direct collaboration with communities, Dianne has pioneered approaches to drug design that honour Pacific and Māori voices, ensuring that solutions are culturally resonant and targeted at those most impacted.
Dianne blends expertise, empathy, and dynamic communication to engage diverse audiences. Her outreach extends to Pacific Fono and Talanoa gatherings, where she not only shares her research but actively listens to the needs and experiences of others.
Dianne’s work reflects her unwavering commitment to the principle that effective communication is as much about listening as it is about speaking. “Those who have the privilege to know, have a duty to act,” she says, embodying the ethos of using her scientific knowledge to serve communities.
Beyond her research, Dianne’s passion for mentoring the next generation of scientists has been a driving force in her career. She is dedicated to creating opportunities for younger Pacific and Māori scientists to develop their skills, empowering them to become future leaders in research and healthcare. By actively fostering diversity within the scientific community, Dianne ensures that the voices of those who have traditionally been underrepresented are included, promoting an environment where all perspectives are valued and heard. Through her work, she is paving the way for a more equitable future in science and health for generations to come.
Jackson Smeed-Tauroa (Ngāti KorokīKahukura) has been awarded a Te Niwha scholarship to lead a groundbreaking study at the MRINZ. This world-first research will explore the experiences of Māori whānau when a loved one is critically ill in an Intensive Care Unit (ICU). Jackson’s work, conducted under the mentorship of Associate Professor Clive Aspin at Te Herenga Waka— Victoria University of Wellington and Dr Diane Mackle, ICU Programme Manager at the MRINZ, aims to improve how healthcare providers support whānau through ICU care while respecting Māori cultural values.
This study is particularly timely in the wake of the COVID-19 pandemic, during which Māori were disproportionately affected by hospitalisation and visiting restrictions. Jackson’s research draws on the Te Whare Tapa Whā framework, a holistic Māori health model, to address the cultural, spiritual, and emotional needs of whānau during these difficult moments. Jackson’s work aims to ensure that Māori whānau feel heard, respected, and supported when navigating the ICU experience, with a focus on improving cultural safety in the healthcare environment.
“Receiving the Te Niwha scholarship has been a transformative opportunity to centre Māori voices in research that has the potential to make a lasting difference,” says Jackson. His study’s findings will inform ICU practices and guide future health policy, with the goal of creating an environment where Māori whānau can feel supported and culturally valued during healthcare crises.
Jackson’s work not only contributes to Māori health and wellbeing but has broader implications for healthcare systems in New Zealand and beyond. As one of the first studies to examine Māori whānau experiences specifically within an ICU context, Jackson’s research will fill a crucial gap in the literature and ensure that whānau are treated with manaakitanga and respect.
Through this research, Jackson hopes to drive meaningful change in how critical care is delivered to Māori families, contributing to a future where cultural safety is integrated into healthcare practices across the country.

The MRINZ provides practical clinical research experience and training opportunities which can contribute to or form the entirety of higher education study including PhDs, MDs, Master’s Degrees, and Diplomas.
PhDs and MDs AWARDED IN 2024

DANIEL FREI SENIOR CLINICAL RESEARCH FELLOW
Perioperative Oxygen Therapy Optimising Patient Safety and Outcomes
Daniel Frei’s PhD research investigated the optimal oxygen regimen during surgery, a critical issue in surgical care where over 300 million procedures occur globally each year. This work sought to establish guidelines that could enhance patient safety and outcomes during a pivotal time in their healthcare journey.
My PhD focused on addressing the question of the best approach to perioperative oxygen therapy—should it be liberal or conservative? I began by conducting a comprehensive review of existing literature on the topic, which revealed a significant lack of definitive evidence supporting either method of oxygen use.
Understanding the importance of clinical practice, I surveyed specialist anaesthetists and discovered a wide range of beliefs and practices in oxygen management. Most anaesthetists titrated inspired oxygen levels based on pulse oximetry readings they deemed safe, despite the absence of a consensus on the optimal approach.
To gain deeper insights, I conducted studies across ten metropolitan hospitals. I found that anaesthetists often adopted an intermediate strategy for oxygen administration. Through retrospective analyses of over 15,000 patient records, I uncovered valuable findings, including potential benefits and risks associated with liberal perioperative oxygen use. For example, I observed an association between low inspired oxygen levels and increased surgical site infection rates, while higher oxygen administration was linked to a greater risk of respiratory complications and a higher likelihood of intensive care unit admissions.
The results of my studies were not only statistically significant, but also pointed to the complexity of oxygen therapy and its varied impacts on patient outcomes. I began to realise that while oxygen administration is a routine part of surgical practice, the variations in its use suggest a broader issue within the field—there is a clear need for a more standardized and evidence-based approach to perioperative oxygen management. This understanding drove me to look deeper into the mechanisms behind oxygen therapy’s effects, particularly how it interacts with the body's immune system and respiratory functions during surgery.
Recognising the potential for a paradigm shift, I collaborated with experts from various disciplines, including surgeons, intensivists, and clinical researchers, to broaden the scope of my research. This multidisciplinary approach not only enriched the findings but also created a platform for collaborative discussions on how to refine and improve oxygen therapy practices.
My research culminated in a multicentre randomized feasibility trial, which laid the groundwork for a definitive investigation into perioperative oxygen therapy. This trial confirmed that further research could be conducted without requiring major protocol modifications, paving the way for future studies. By addressing a fundamental gap in the current clinical understanding, this trial opened the door to a deeper exploration of the risks and benefits of oxygen management in surgical settings.
The findings from my work underscore the urgent need for a definitive trial to address the ongoing uncertainties surrounding perioperative oxygen therapy. By shedding light on the complexities of oxygen management during surgery, my research aims to optimize patient outcomes and contribute to the evolution of best practices in anaesthesia and surgical care. Through continued research and collaboration, I hope to see the development of guidelines that will standardize perioperative oxygen therapy, ultimately improving recovery rates and reducing complications for patients undergoing surgery.

management—anti-inflammatory
with a focus on its clinical implications, adoption barriers, and environmental impact. This innovative treatment regimen, aimed at both relieving symptoms and reducing inflammation, was shown to have the potential to improve patient outcomes while also lessening the environmental footprint of asthma care.
My research explored several unresolved questions around anti-inflammatory reliever (AIR) therapy: its efficacy in patients with mild asthma, its uptake in everyday clinical practice, and how it compares against traditional inhalers in terms of environmental sustainability. I began with a systematic review of randomised controlled trials on the efficacy of AIR therapy in mild asthma, which led me to identify a strong evidence base supporting its use.
A key concern I had was that, despite the substantive evidence of benefit, it might not be possible to change the entrenched prescribing practices of doctors and achieve a switch to AIR therapy, even with the publication and dissemination of asthma guidelines recommending this approach.
To explore this further, I investigated the situation in New Zealand and observed a marked increase in the dispensing of budesonide/formoterol, the only AIR therapy available here. I found that these trends suggest that transitioning to budesonide-formoterol reliever with or without maintenance therapy regimens in clinical practice can be achieved if recommended and promoted as the preferred therapeutic approach in national asthma guidelines.
In addition to efficacy, I also focused on the environmental impact of asthma care. I conducted a comparative study examining the carbon footprint of inhalers, contrasting traditional metered-dose inhalers, which use potent greenhouse gas propellants, with eco-friendly alternatives like anti-inflammatory dry-powder inhalers. This study, the first of its kind in adults with mild asthma, showed that switching to as-needed ICS-formoterol substantially reduces the carbon footprint of asthma management while also improving treatment effectiveness.
Sustainability is a crucial consideration in modern healthcare. Not only does AIR therapy reduce the risk of severe asthma attacks, but it also offers a more environmentally friendly solution when administered via a dry powder inhaler. This could be a major step forward in reducing the healthcare sector’s carbon footprint.

We are deeply grateful to our generous funders, who come from diverse sectors and contribute in various ways. Our heartfelt thanks go to everyone who supported the MRINZ in 2024. We sincerely appreciate the enduring commitment of our funders and donors to our mission and purpose.
A M Smith donation in memory of E O'Donnell
AstraZeneca
Auckland Uniservices
Barbara Basham Medical Charitable Trust
Biovaleo
David and Cassie Anderson Medical Charitable Trust
Evithé Ltd
Fisher & Paykel Healthcare
Health Research Council of New Zealand
Kāpiti U3A Kaleidoscope donation
NICM Health Research Institute
Perpetual Guardian
PPD
S Penfold donation in memory of Maureen Stretch
Syneos Health
Te Niwha Infectious Disease Research Platform
Te Whatu Ora
University of Auckland
Victoria Link Limited
Victoria University of Wellington
Western Sydney University
There are many ways to support MRINZ philanthropic activities. Please contact us to discuss further: support@mrinz.ac.nz.

mrinz.ac.nz
info@mrinz.ac.nz
+64 4 805 0147
PHYSICAL ADDRESS
Level 7, CSB (Clinical Services Block) Building
Wellington Hospital
Riddiford St, Newtown
Wellington 6021
Aotearoa New Zealand
POSTAL ADDRESS
Private Bag 7902, Wellington 6242
Charity Registration: ID CC22439
mrinz.ac.nz
ClinResearchNZ the-mrinz

Published February 2025
Copies of the Research Report can be obtained by contacting info@mrinz.ac.nz