Psychometric analysis of selected tools for pressure ulcer risk assessment – literature review
Dariusz Bazaliński ¹,2,3, Paulina Szymańska 4 , Anna Surmacz¹,2,3, Kamila Pytlak¹,2, Beata Barańska¹,2,3,
1 Father B. Markiewicz Podkarpackie Oncology Centre, Specialist Hospital in Brzozow, Poland
2 Department of Wound Prevention and Treatment, Institute of Nursing, Faculty of Health Sciences and Psychology, Collegium Medicum, University of Rzeszow, Poland
3 Laboratory for Innovative Research in Nursing, University Research and Development Centre in Health Sciences, Faculty of Health Sciences and Psychology, Collegium Medicum, University of Rzeszow, Poland
4 Radom School of Higher Education, Radom Specialist Hospital, Poland
Abstract
Introduction: Risk assessment tools have been utilised for several decades to systematically identify patients at risk of pressure ulcers.
Objective: To examine the psychometric properties of selected pressure ulcer risk assessment tools.
Material and methods: The present study employed a literature review method. A comprehensive review of the available literature from 2015 to 2025 was conducted, utilising the key words “tools for assessing pressure ulcer risk” and “pressure ulcer risk assessment scales”. The following databases were used: PubMed, EBSCO, and the national Termedia database. A comprehensive review of the existing literature was conducted, encompassing randomized studies, original studies, and meta-analyses. Following a thorough analysis of the available literature, the collected material was methodically categorized as follows: (a) universal pressure ulcer risk assessment tools, and (b) perioperative risk assessment tools. The present study focused on the analysis
Introduction
A pressure ulcer is defined as local damage to the skin and/or subcutaneous tissue resulting from pressure or pressure in combination with shear. Pressure-related wounds typically manifest on bony prominences; however, they may also be associated with medical devices or other objects [1]. Despite the considerable advances witnessed in the domains of medicine and health sciences, these complications persist in constituting a substantial challenge for healthcare
Address for correspondence: Anna Surmacz, Faculty of Health Sciences and Psychology, Collegium Medicum, University of Rzeszów, 16C Rejtana St., 35-310 Rzeszow, Poland, e-mail: annwojcik@ur.edu.pl
Nadesłano: 20.08.2025; Zaakceptowano: 29.09.2025
of 16 papers; the remaining cited manuscripts provide the necessary background for the discussion of the problem.
Results: The review did not confirm any domestic studies evaluating the psychometric properties of pressure ulcer risk assessment tools. The prevailing pressure ulcer risk scales are inadequate for consistent application in patients admitted to hospitals according to standardised criteria.
Conclusions: The persistent challenge of pressure ulcers continues to impose a significant burden on healthcare systems. The identification and implementation of preventive measures should be based on a comprehensive medical history, a physical examination, and the utilisation of standardised clinical tools. A review of the existing literature clearly indicates a deficit in current knowledge in the national literature. In the process of updating national guidelines, efforts should be made to prepare a psychometric evaluation of assessment tools and to prepare recommendations related to patient care in the perioperative period. Key words: pressure ulcer risk assessment, nurse, prevention.
systems. The aforementioned factors contribute to an exacerbation in patients’ overall health, thereby significantly affecting their dependence on the support of environment and health services in a manner that results in reduced autonomy, heightened feelings of insecurity, and a concomitant deterioration of mental well-being. The pressure ulcers are associated with considerable financial and individual costs and have a detrimental effect on the individual, affecting them physically, emotionally, and socially.
D. Bazaliński, P. Szymańska, A. Surmacz, et al.
Risk assessment and prophylactic measures are regarded as fundamental components of pressure ulcer prevention, as outlined in international and national practice guidelines [1, 2]. Risk assessment instruments (RAI) have been utilised for several decades to systematically identify patients at risk, as opposed to relying exclusively on clinical risk “assessment”. The identification of the environment in which preventive measures will be implemented (e.g. home environment or hospital ward) is a prerequisite for their effectiveness. This identification is followed by a clinical assessment of the patient’s condition. The assessment of the environment, individual risk, the availability of personnel, level of knowledge and skills, and the possibility of using pressure-relieving equipment and medical devices for preventive purposes form a uniform and coherent concept of pressure ulcer prevention. According to experts in the field, there are a number of highly specific situations that must be taken into consideration, including but not limited to operating room conditions, means of transport for patients, prostheses, and medical equipment [3, 4].
A reliable assessment of the risk of pressure ulcers should be based on a physical examination using questionnaires and clinical scales [5, 6].
Over the past 3 decades, the theoretical foundations of clinimetrics have evolved, resulting in the development and validation of a wide range of instruments designed to measure health status and quality of life. The gold standards for tool development emphasize the importance of ensuring content validity and conceptual frameworks. These standards include rigorous testing and evaluation processes to establish psychometric properties, such as content validity, discriminative validity, known group validity, criterion validity, and concurrent validity. Additionally, they encompass the assessment of inter-rater, intra-rater, and retest reliability. Risk assessment tools are combinations of individual risk factors used to assess the risk of tissue damage under pressure or shear forces. The majority of extant risk assessment instruments are scales that assign numerical values to various factors (e.g. mobility, nutrition, level of urinary and faecal continence), and the sum of these values forms a total score. The resulting score is used as an indicator of the risk of pressure ulcers. Risk assessment scales are utilised to stratify patients at risk of pressure ulcers into categories reflecting the degree of risk (e.g. low risk, medium risk, high risk). Risk assessment scales are evaluated for their ability to predict the development of pressure ulcers. However, preventive interventions are usually implemented immediately
after the risk is identified, and in some cases, the development of pressure ulcers is prevented [6].
The acceptability of the tool by users is equally important in clinical practice. Experts point to the availability of over 40 risk assessment scales, all of which address the issues of sensitivity (identification of actual risk) and specificity (identification of actual risk that is not risk). The complexity of the tools has been demonstrated to result in low reliability of results [7]. In the contemporary era, questionnaire-based assessment alone is inadequate and is susceptible to a high risk of error. Bearing in mind that it is the nurse’s responsibility to assess the patient’s health and perform a physical examination, each patient should be examined in accordance with the recommended algorithm, and the examination should be concluded with an assessment using selected (recommended) tools. Most available risk assessment tools encompass a range of factors, including activity, mobility, nutrition, moisture, sensory perception, friction and shear, and general health.
The objective of risk assessment is to identify individuals who are susceptible to developing pressure ulcers, with the aim of selecting and implementing recommended preventive measures. The utilisation of both risk assessment scales and clinical judgment is a pervasive component of routine practice, serving to identify individuals who are susceptible to developing pressure ulcers. The formulation of recommendations for action can be based on the assigned risk category. As a healthcare professional, nurses engage in a wide range of activities, including professional care, treatment, rehabilitation, and team management. They are obligated to make therapeutic and nursing decisions [8]. Moreover, the mounting demands on the nursing profession, stemming from the expansion of its competencies, underscores the necessity to delve more profoundly into a specialisation. The objective of individual professional development is to ensure the delivery of professional and effective care, in addition to meeting the needs of patients and their families [9]. These requirements have precipitated the implementation of evidence-based nursing practice (EBNP), which is defined as the utilisation of scientific evidence within the context of nursing practice. EBNP can be characterised as a clinical decision-making process that incorporates the most recent scientific research and therapeutic decisions, with these decisions being based on the patient’s condition [10].
A comprehensive review of the global literature reveals a marked increase in the involvement of nurses
in the prevention and treatment of wounds. The range of nursing competencies involved in the selection of methods and measures for treating slow-healing wounds is extensive and has achieved widespread consensus within the medical community. To provide effective and safe care for patients with wounds, it is essential that nursing staff possess a high level of qualification, extensive knowledge, and unquestionable social skills [11].
In a professional context, prevention is a meticulously formulated, acquired, guideline- and recommendation-based, widely comprehensible, holistic professional activity. The specific nature of the profession offers significant opportunities for the prevention and treatment of local wounds in individuals with self-care deficits who are at risk of developing pressure ulcers. The implementation of preventive measures in a timely manner, in conjunction with the utilisation of efficacious interventions, facilitates the deferral of the onset of pressure ulcers and the initiation of treatment during its early stages. The identification of individuals susceptible to developing pressure ulcers constitutes the initial phase in the prevention of such lesions [12]. It is imperative that procedures implemented as part of a prevention initiative consider the quality of nursing care in addition to the recommendations and scientific research results. Patients at risk of developing pressure ulcers should undergo continuous assessment by an interdisciplinary team. This team is responsible for defining the scope of activities for each team member, along with specific roles and tasks. The implementation of measures ought to be informed by the recommendations put forth by scientific societies and global guidelines [13].
A comprehensive literature review was conducted on selected tools for assessing the risk of pressure ulcers, taking into account the conditions of the healthcare system in the area of prevention.
The objective of this study is to examine the psychometric properties of selected pressure ulcer risk assessment tools.
Material and methods
The present study employed a literature review method. A comprehensive review of the available literature from 2015 to 2025 was conducted, using keywords such as “tools for assessing pressure ulcer risk” and “pressure ulcer risk assessment scales”. The following databases were utilised: PubMed, EBSCO, and the national Termedia database. Randomised studies, original studies, and meta-analyses were included. Following a thorough analysis of the existing
literature, the collected material was methodically categorised as follows: (a) universal pressure ulcer risk assessment tools, and (b) risk assessment tools in the perioperative period. The present study focused on the analysis of 16 papers; the remaining cited manuscripts provide the necessary background for the discussion of the problem. The process of searching for duplicates is illustrated in Figure 1.
Identification of records through database searching (PubMed, n = 519), (EBSCO, n = 42)
Identification of records through database searching (Termedia) (n = 76)
Excluded records (n = 585)
Papers rejected (n = 449);
• case studies,
• cost effectiveness of a system
• PI/U in newborns and children,
• historical studies,
• nutritional prevention
• mucous membranes
Papers on pressure injury/ulcer (PI/U) (n = 136)
Papers rejected (n = 120);
• nursing students,
• preventive dressing
• preventive repositioning
• PI/U treatment
Articles eligible for review that discuss tools for assessing the risk of pressure ulcers/injury PI/U (n = 16) INCLUDED
1. Data review process
Universal tools for assessing the risk of pressure ulcers
Pressure ulcers represent a grave health, social, and economic problem. It is widely accepted that most pressure injuries can be prevented through the implementation of fundamental preventive measures. The available assessment tools are based on an analysis of the most common risk factors [2, 14]. Despite the existence of a broad array of pressure ulcer risk assessment tools globally, the most prevalent and renowned instruments in Poland are the Norton, Braden, Waterlow, CBO, and Douglas scales. A review of the available Polish- and English-language literature failed to identify any studies that evaluated psychometric indicators for Polish conditions. The Douglas
Figure
D. Bazaliński, P. Szymańska, A. Surmacz, et al.
and Dutch Consensus Prevention of Bedsores (CBO) scales were not analysed due to the absence of studies evaluating these scales during the specified review period.
The Norton scale is among the initial instruments developed for the evaluation of the risk of pressure ulcers. Published in 1962, it was developed as an assessment instrument for geriatric patients, and it encompasses an evaluation of 5 variables: physical condition, level of consciousness, activity, and the ability to change position independently and control the sphincters of the anus and urethra [2].
The Waterlow scale was developed in 1985 by Judy Waterlow with a specific focus on elderly patients, particularly those undergoing surgical procedures who were over 60 years of age. The tool’s primary function is to assess the physiological condition and associated diseases in elderly patients, with a high degree of accuracy in predicting the risk of pressure ulcers [2].
The Braden Scale is a widely utilised instrument, considered the most prevalent tool globally. The development of this technology was initiated in 1987 in the United States by Barbara Braden and Nancy Bergstrom, who identified a pressing need for an effective tool to predict and prevent pressure ulcers. This instrument has the widest range of applications [15]. The utilisation of this instrument is appropriate for the purpose of evaluating the presence of pressure ulcers in elderly patients. The assessment encompasses a range of variables, including sensation, activity, mobility, nutritional status, friction, and shear force. The necessity to develop new research instruments remains salient because it is associated with the continuous advancement of health sciences and the pursuit of solutions for more precise identification of patients at risk of pressure ulcers [16]. Nonetheless, as indicated by the findings of certain studies, the system’s capacity to prognosticate the likelihood of skin injury across diverse populations and within varied clinical contexts appears to be constrained [17]. In a meta-analysis conducted by Park et al., the 3 most commonly used scales (Norton, Braden, Waterlow) were evaluated. The findings suggest a comparable scope with moderate precision for the scales examined, while heterogeneity exhibited over 80% variability across studies. The results obtained from the meta-analysis indicate that commonly used screening tools for assessing the risk of pressure ulcers have limitations in terms
of validity and accuracy in relation to older people due to heterogeneity in the studies [15, 18, 19]. The psychometric data and characteristics of the tools are presented in Table 1. A review of the existing literature on risk assessment tools (RAI) was conducted by Coleman et al., which included a systematic review of the content, development, and testing of 14 RAI tools included in the 2014 NICE (National Institute for Health and Care Excellence) review. The study identified limitations of existing RAI tools. The authors noted that numerous tools were developed decades ago, during a period characterised by a lack of evidence regarding risk factors for pressure ulcers and methodological guidelines for the development and evaluation of tools [3, 20]. A tool must demonstrate both sensitivity and specificity in its identification of at-risk and non-at-risk individuals, respectively. Assessing reliability and validity poses a significant challenge in clinical practice, as risk assessment scales are utilised to identify individuals who would develop pressure ulcers if no intervention were taken. Once the risk has been identified, various pressure ulcer prevention strategies are often implemented, which appear to alter the scale’s predictive ability. The lack of clear knowledge about the sensitivity and specificity of risk assessment tools has far-reaching implications for practice, because clinical decisions—such as whether or not to use pressure ulcer prevention strategies—are often made based on risk assessment results, although it has also been argued that nurses often rely solely on clinical judgment when deciding which preventive measures to use. To address the limitations of existing risk assessment tools and instruments, the Primary or Secondary Pressure Ulcer Risk Assessment Tool (PU) (PURPOSE-T) was developed [3, 7, 20]. This tool was developed using recommended research methods. The primary objective of this system is to identify adults at risk of developing pressure ulcers and to provide nurses with the necessary tools to make informed decisions aimed at reducing the incidence of these lesions (i.e. primary prevention). Additionally, the system is designed to identify individuals with existing or previous pressure ulcers, who require secondary prevention and treatment. The authors employed a color-coded system to denote the most significant risk factors and devised a three-step assessment procedure: Step 1: Screening – to quickly exclude those who are clearly not at risk. This includes an assessment of mobility and skin condition (including the use of
Table 1. Selected general tools for assessing pressure ulcers in research analysis
Scale Design
Braden
Norton
6 elements (perception, moisture, activity, mobility, nutrition, friction)
Clinical indications
Sensitivity according to studies Scoring (risk cutoff) Citation
Waterlow
5 elements (physical condition, mental condition, mobility, activity, incontinence)
Hospitals, intensive care units, long-term care
Simple assessment, alternative to other tools
Moderate predictive accuracy, more suitable for middle-aged patients under 60 years of age, hospitalised patients, and the Caucasian population; a cut-off point of 18 may be used to assess the risk of pressure injuries in clinical practice.
AUC for average age <60 years (0.87, 95% CI: 0.84–0.90),
Sensitivity between 62% and 83%, depending on the population and the cut-off point used.
Specificity between 45% and 67%
AUC ROC for Norton in studies 0.70–0.75
15–18 low risk, ≤ 9 points = very high risk
< 15 points = low or no risk, ≤ 11 very high risk
Huang et al. (2021) [16]
Wai et al. (2020) [15]
Šateková et al. [19] (2017)
Park et al. (2016) [18]
11+ elements (body composition, age, BMI, gender, disease, mobility, nutrition and dietary preferences, mobility and activity, specific factors, disability and incontinence)
Hospitals, palliative care, long-term
Sensitivity depends on the threshold adopted: at ≥ 10 points – up to 87.5% (range 82–90%), at ≥ 15 points – approx. 71.4%, at ≥ 20 points – up to 85.7%,
Specificity; at ≥ 10 points – approx. 28% (range 22–85%), at ≥ 15 points – approx. 94%, at ≥ 20 points – approx. 41% and variable predictive accuracy 0.54–0.90, with a median of approx. 0.60–0.61 in large population analyses. To achieve full effectiveness, it should be used in conjunction with clinical nursing assessment and ongoing staff training
A maximum of 64 points can be obtained. A score of 10–14 points indicates risk, 15–19 indicates high risk, and above 20 indicates very high risk
Charalambous et al. (2017) [21]
PURPOSE-T
3-stage: screening + assessment of 9 factors + colour-based decision Hospitals, home care, primary/secondary prevention
AUC = 0.83–0.87
Colour: green (none), orange (risk), pink (pressure ulcer)
Coleman et al. (2018) [20]
Sensitivity: the capacity of the instrument (scale) to discern subjects who are susceptible to developing pressure ulcers. Specificity: the capacity of the tool to discern individuals who are not at risk of developing pressure ulcers. Cut-off score: threshold score indicating the need to implement preventive measures, AUC (ROC): predictive accuracy of the scale (≥ 0.80 – very good), ICC (inter-rater): consistency of assessments by different users, CI (confidence interval), 95%
medical devices), and nurses are encouraged to use their clinical judgment to identify any other risk factors that are relevant to the individual patient.
Step 2: Full assessment – includes the following evidence-based elements: independent movement, detailed skin assessment, previous history of pressure ulcers, medical devices, perfusion, sensory perception, moisture, diabetes.
Step 3: Assessment decision based on Step 2 and supported by colour coding:
• green: no pressure ulcers – no current risk
• orange: no pressure ulcers, but risk present, requiring primary prevention
• red: pressure ulcer category 1 or higher, or scars from previous pressure ulcers requiring prevention/secondary treatment [20].
D. Bazaliński, P. Szymańska, A. Surmacz, et al.
Selected tools for assessing the risk of pressure ulcers in the perioperative period
Clinical prevention of pressure injuries is based on professional and standard assessment of patients and ensuring adequate protection [22]. A comprehensive understanding of preoperative risk factors serves as the foundation for the implementation of preventive measures. Consequently, the nursing staff can implement preventive interventions prior to the occurrence of tissue injury. The primary preventive measure for pressure ulcers is the utilisation of recommended risk assessment tools for the purpose of conducting an early, systematic, accurate, dynamic, and effective assessment. A variety of preventive interventions are available, including the prophylactic use of multi-layer dressings, the utilisation of alternative pressure mattresses, and polymeric bladder-resistant-elastic (gel) on the operating table during surgery. Additionally, patient and caregiver education on nutrition and skin care, frequent position changes, and the use of positioning wedges are recommended [23]. Scales such as Norton, Waterlow, and Braden were not designed for risk assessment in surgical patients and therefore should not be recommended for use in this context. These scales do not take into account risk factors associated with surgery and have low predictive value and low sensitivity for perioperative pressure injuries. Wei et al. conducted a meta-analysis that indicated moderate predictive accuracy of Braden’s scale. The analysis demonstrated that the scale exhibited good sensitivity and low specificity in critically ill adult patients. The further development and modification of this tool, or the creation of a new tool with higher predictive power, is justified for use in critically ill patients [17]. The existing body of literature on hospital-acquired pressure ulcers (HAPIs) is characterised by a lack of reliable reports [24]. A global analysis of the literature and the 2019 recommendations indicates that significant emphasis should be placed on raising awareness of the risk of pressure ulcers in hospitalised patients [1]. In a systematic analysis conducted by Chen et al., the incidence of pressure injuries during surgery was reported to be 15%. Intraoperative pressure injuries (IAPI) are a prevalent cause of skin damage in the perioperative period. The necessity of anaesthesia and surgical intervention renders patients susceptible to acute, localised tissue injury associated with pressure. This injury manifests within 48 to 72 hours post-surgery and is associated with the specific surgical site [26]. Surgical interventions that extend
beyond 2 hours are recognised as a risk factor for the development of pressure ulcers. Aloveni et al. identified 8 significant risk factors associated with HAPI among the surgical population. These risk factors include age ≥ 75 years, female gender, American Society of Anesthesiologists (ASA) grade ≥ 3, low BMI, low preoperative Braden score ≤ 14, and comorbidities such as anaemia, respiratory diseases, and hypertension [23]. The development of a pressure ulcer can have numerous adverse consequences, including pain, the necessity for additional treatment, prolonged hospital stays, deformities, and scarring of the body. These consequences can lead to increased morbidity, elevated treatment costs, and an increase in the workload for nurses by more than 50%, resulting in a 3–4-fold increase in the cost per patient [1, 24].
Latimer et al. indicated a high risk of skin damage from pressure ulcers within the initial 36 hours of hospital admission. In a sample of 1047 participants, pressure ulcers were confirmed in 10.8% of the study population. Researchers’ observations indicate that older people with multiple comorbidities and those living in nursing homes are more likely to be admitted to hospital with existing pressure ulcers or to develop them shortly after admission [27, 28]. In the course of the analysis of selected studies, several tools were highlighted.
The Munro pressure ulcer risk assessment scale is a comprehensive tool that is utilised to evaluate the risk of developing pressure ulcers in the perioperative setting. This scale encompasses 3 distinct stages of assessment: preoperative, intraoperative, and postoperative. The preoperative stage involves a comprehensive evaluation of various factors, including mobility, nutritional status, BMI, recent weight loss, age, and comorbidities. The intraoperative stage focuses on the patient’s American Society of Anesthesiologists (ASA) status, the type of anaesthesia administered, temperature control, the presence of hypotension, humidity levels, the type of support and position used during surgery, and any movements. The postoperative stage is concerned with the duration of surgery and the amount of blood loss. The assessment yields a risk score for each stage, with the total score indicating the overall risk level [17]. The Association of Perioperative Registered Nurses (AORN) proposed and recommended this approach in 2016. The tool is employed to evaluate the primary perioperative risk factors and is extensively utilised for IAPI risk assessment. The EMINA scale was developed as an adaptation of the Norton scale and includes 5 key risk factors: mental status, mobility,
Table 2. Selected risk tools in perioperative care
Citation
Scoring (risk cutoff)
Sensitivity according to studies
Roca-Biosca et al. (2015) [28]
Score range: 0 to 15 points > 10 points = risk
Sensitivity: 94.3% (95% CI: 87.17–100%) Low specificity: 33.33% (95% CI: 25.01–41.66%) 4.3% (AUC 0.90)
Clinical indications
Lospitao-Gómez et al. (2017) [37] De Souza et al. (2023) [38]
≥ 10 points = risk
Sensitivity 80.43% (95% CI: 79.15–81.72%) Specificity 64.41% (95% CI: 63.68–65.14%) Has comparable predictive accuracy to the Braden scale (AUC 0.807) and may be more specific for ICU patients
Recommended for use as an initial screening tool, followed by more specific clinical assessment or another scale
Design
Intensive care units
Developed as an adaptation of the Norton scale and includes 5 key risk factors: mental status, mobility, urinary/faecal incontinence, nutrition, and activity level
Scale
EMINA
Park, et al . (2016) [18]
< 24 points = high risk
Adibelli et al. (2019) [32]
Mosher et al. (2025) [29]
≥ 2 points high risk
Sensitivity 87%, specificity 84% AUC 0.826–0.902 Valuable tool for assessing the risk of pressure ulcers in intensive care units, with advantages over classic scales such as Braden or Norton
Intensive care units, neurology, postoperative
Comprises 6 elements (mobility, perfusion, skin, consciousness, equipment, age)
EVARUCI
Includes 10 elements (including consciousness, haemodynamics, oxygen therapy, age, BMI)
Cubbin-Jackson
Points are calculated at each stage
Due to the small sample size, no statistical significance was achieved; the authors emphasise improved team communication, staff awareness, and potential for complication prevention
Hospital, operating room, surgical wards
Comprises 4 components (age, serum albumin level, estimated duration of surgery, and American Society of Anesthesiologists [ASA] score)
The Scott Triggers Tool
Lei et al. (2022) [17]
The points are added up to assess the risk in three levels: < 13 –low 14–24 –moderate ≥ 25 –high risk The assessment is performed dynamically at each of these stages (before, during, and after surgery)
specificity
Intraoperative sensitivity
AUC 0.874
Postoperative sensitivity 67.7%, specificity
AUC 0.774 (95% CI: 0.929–0.971)
Hospital, operating room, treatment wards
Cross-cultural adaptations: in China (2018), Turkey (2021), Brazil (2022)
Three-stage; assesses the risk of pressure ulcers in the perioperative period; preoperative (mobility, nutritional status, BMI, recent weight loss, age, comorbidities), intraoperative (ASA status, type of anaesthesia, temperature, hypotension, humidity, type of support/ position, movements) and postoperative (duration of surgery, blood loss). Each stage of the assessment gives a low, mediumor high-risk result, with a total result at the end.
Pressure Ulcer Risk Assessment Scale for Perioperative Patients MUNRO
D. Bazaliński, P. Szymańska, A. Surmacz, et al.
Citation
Scoring (risk cutoff)
Sensitivity according to studies
Sengul et al . 2023 [30]
Salvini et al. (2024) [31]
Sensitivity (85%), specificity (75%) predictive accuracy (AUC = 0.85) ≤ 15 points –low risk 16–28 points –moderate risk ≥ 29 points –high risk
Clinical indications
Konatake et al. (2025) [39]
urinary/faecal incontinence, nutrition, and activity level [29]. The manuscripts selected during the analysis, which included systematic reviews, meta-analyses, and original studies, were aimed at identifying tools for assessing the risk of pressure ulcers. A selection of these works is presented in Table 2.
The Scott Triggers Tool is a widely utilised instrument for the assessment of pressure ulcers in surgical patients. The model under consideration comprises 4 components: age, serum albumin level, estimated duration of surgery, and American Society of Anesthesiologists (ASA) score. This instrument evaluates various factors, including patient age, nutritional status, duration of surgery, and the nature of the procedure [30].
Table 2. Selected risk tools in perioperative care (cont.)
Design
Hospital, operating room, treatment wards
Consisted of a total of 7 elements, each containing 5 sub-elements. It is assessed on a scale from 1 to 5 in the Likert type. The total score ranges from 7 to 35. The risk of pressure ulcers increases in patients as the score increases
Scale
Surgical Positioning Risk Scale Surgical Positioning Risk Scale ELPO
No data on the sensitivity of the tool in the analysed literature, positive correlation between ELPO (Brazilian Perioperative Pressure Ulcer Risk Assessment Scale) and Munro
Hospital, operating room, surgical wards
(Surgery-Related Pressure Injury Risk Assessment Scale) SURPIRAS takes into account risk factors such as age, body mass index (BMI), ASA score, type of anaesthesia, duration of surgery, amount of bleeding, moisture in pressure areas, position changes, use of pressure relief/ accommodations
The ELPO pressure ulcer risk assessment scale for surgical patients provides more detailed descriptions of joint positioning during surgery as part of the assessment of limb positioning. It is employed to evaluate the likelihood of pressure ulcers that patients may develop as a result of surgical positioning during surgery [31, 32]. In a study by Sengul et al., a substantial accuracy rate of 0.944 was confirmed in a group of 186 patients who underwent surgical treatment. The sensitivity of the test was 60%, its specificity was 66%, and its accuracy was 66%. A negative, weak, statistically significant correlation was identified between the total scores on the risk assessment scale for the prevention of surgical positioning injuries and the Braden scale. The tool is recommended for the assessment of patients after surgery in Turkey [31].
The Cubbin Jackson scale was developed in 1991 for patients in intensive care units. The tool incorporates a comprehensive evaluation of general risk factors and those specific to ICU patients. It encompasses a range of parameters, including age, body weight, medical history, general skin condition, mobility, nutritional status, urinary incontinence, hygiene, mental status, haemodynamics, respiration, and oxygen requirements [18, 33]. However, the scientific evidence supporting the use of the Jackson/Cubbins scale to assess the risk of PI-induced complications in ICU patients remains limited and conflicting. As demonstrated by Higgins et al. [34] and Delawder et al. [35], the Jackson/Cubbins scale exhibits moderate predictive value. A meta-analysis conducted by Zhang et al. [36], demonstrated that previous studies were characterized by low quality of scientific evidence and significant heterogeneity, which requires verification in future studies.
The EVARUCI scale is a comprehensive assessment tool that evaluates 6 key domains: mobility, perfusion, skin, consciousness, devices, and age. It is a reliable instrument for evaluating the risk of pressure ulcers in the ICU. The model’s performance metrics, such as sensitivity and specificity, exhibit variations depending on the geographical location of the study (Spain vs. Brazil). However, its overall predictive accuracy (AUC ROC ~0.8) aligns closely with the Braden scale. Furthermore, its efficacy in cross-cultural adaptation has been substantiated in numerous studies [37, 38]. A study by de Souza et al. [38] confirmed the predictive ability of the EVARUCI scale, which was similar to
that of the Braden scale. However, the accuracy of both scales was found to be suboptimal in terms of accurately predicting patients at risk of PI [38].
The SURPIRAS (Surgery-Related Pressure Injury Risk Assessment Scale) is a novel instrument designed to evaluate the risk of pressure ulcers associated with surgical interventions. This scale was developed and validated in the Turkish population [39].
Discussion
The primary objective of care should be the prevention of pressure ulcers. Members of the treatment team should regularly assess the risk of pressure ulcers. The basis for prevention, therefore, must be strong scientific evidence. The efficacy of the implementation and application of clinical guidelines can be determined through well-designed studies and initiatives to enhance the quality of current practices. Clinical decisions should be based on current scientific evidence and present a range of options to aid decision-making between healthcare professionals, patients or their caregivers, and the interdisciplinary team in the prevention of pressure ulcers [2, 40].
The constantly growing number of patients with difficult-to-heal wounds determines the development of nurses’ competences and qualifications. The provision of professional care and the implementation of therapeutic procedures require an in-depth knowledge base, a high level of competence, and practical skills. It is imperative that nurses allocate particular attention to elderly patients, particularly during the perioperative period and during intensive care, and undergo specialised training related to clinical assessment and the utilisation of standardised tools [41]. In addition, other staff members responsible for fundamental care should be included in the care process, in accordance with established standards and procedures. A review of the literature did not reveal any national studies on the validation of pressure ulcer risk assessment tools or studies indicating tools for use in the perioperative period. According to Park et al. [18], a meta-analysis of 17 studies (5185 patients) revealed that the currently used pressure ulcer risk scales are not suitable for uniform practice in patients hospitalised according to standardised criteria. Consequently, to ensure the delivery of more effective nursing care for pressure ulcers, an in-depth analysis reflecting patient characteristics is necessary, as well as the development of a new or modified pressure ulcer risk scale to complement the strengths and weaknesses of existing tools [18]. Given the observed variability in the incidence of postoperative pressure ulcers,
which has been documented in various studies and ranges from 1.3% to 54.8%, depending on the surgical procedure and the demographic characteristics of the patient population, it is imperative to undertake comprehensive efforts to translate these instruments and prepare them for psychometric evaluation and subsequent implementation in clinical practice [25]. Consequently, the procedures implemented as part of prevention should be adapted to specific and well-defined risk factors. It is evident that the aforementioned subjects will manifest distinct behaviours in 3 distinct settings: the operating room, the intensive care unit, and the geriatric ward. Patients at risk of developing pressure ulcers should undergo continuous assessment by an interdisciplinary team, with a clearly delineated scope of responsibilities for each team member [41]. Recent research has led to significant advancements in our understanding of the risk factors associated with the development of pressure ulcers. A significant number of risk assessment instruments fail to incorporate these advancements in knowledge. A meta-analysis by Moore and Patton [42] was conducted to determine whether the use of risk assessment tools reduces the incidence of pressure ulcers. The authors identified only 2 studies that met the criteria [43, 44]. Despite the low certainty of the available scientific evidence related to the use of standardised pressure ulcer risk assessment tools, this area should be strengthened by developing tools based on methodological guidelines. Other variables that indicate a link between the development of pressure ulcers and staff shortages, low staffing levels, and low utilisation of qualifications and competencies in the prevention and treatment of pressure ulcers should also be taken into account [45, 46]. A meta-analysis was conducted by Lei et al. to evaluate the efficacy of the Munro pressure ulcer risk assessment scale in rapidly and accurately predicting the risk of pressure injuries in patients undergoing general anaesthesia. The results of this analysis suggest that further research is necessary to validate the tool for national conditions. A comparison of its reliability, validity, advantages, disadvantages, and predictive ability with the more commonly used Braden scale is indicated, as this would indicate the possibility of continuous use throughout the perioperative period and widespread use in operating rooms [17]. Another noteworthy instrument is PURPOSE-T, which was developed employing recommended research methodologies. It identifies adults at risk of developing pressure ulcers and supports nurses in making decisions to reduce them (primary prevention), as well
D. Bazaliński, P. Szymańska, A. Surmacz, et al.
as identifying individuals with existing and previous pressure ulcers who require secondary prevention and treatment. The aforementioned instruments are expected to facilitate a more comprehensive evaluation of the patient’s condition, particularly in light of the fact that the most elementary evaluation scale, the Norton scale, remains in use within the country, despite the emergence of novel global findings.
Conclusions
The persistent challenge of pressure ulcers imposes a substantial strain on healthcare systems. The identification and implementation of preventive measures should be based on a comprehensive medical history, a physical examination, and the utilisation of standardised clinical tools. A review of the available literature clearly indicates a deficit in current knowledge in the national literature. In the course of revising national guidelines, efforts should be made to prepare a psychometric evaluation of assessment tools and to prepare recommendations related to patient care in the perioperative period.
Acknowledgments
We would like to thank Anna Nowak for translating the text.
Disclosures
The authors declare no conflict of unterest. This research received no external funding. Approval of the Bioethics Committee was not required.
1. References
2. Kottner J, Cuddigan J, Carville K, et al. Prevention and treatment of pressure ulcers/injuries: The protocol for the second update of the international Clinical Practice Guideline 2019. J Tissue Viability 2019; 28: 51–58. DOI: 10.1016/j.jtv.2019.01.001.
3. Szewczyk M, Kózka M, Cierzniakowska K, et al. Prophylaxis of pressure ulcers – recommendations of the Polish Wound Management Association. Part I. Leczenie Ran 2020; 17: 113–146. DOI: 10.5114/lr.2020.101506.
4. Coleman S, Nixon J, Keen J, et al. Using cognitive pre-testing methods in the development of a new evidenced-based pressure ulcer risk assessment instrument. BMC Med Res Methodol 2016; 16: 158. DOI: 10.1186/s12874-016-0257-5.
5. Hotaling P, Black J. Ten top tips: honing your pressure injury risk assessment. Wounds International 2021; 12: 8–11.
6. Delmore BA, Ayello EA. Braden Scales for Pressure Injury Risk Assessment. Adv Skin Wound Care 2023; 36: 332–335. DOI: 10.1097/01. ASW.0000931808.23779.44.
7. Szewczyk M, Cwajda-Białasik J, Mościcka P, et al. Treatment of pressure ulcers – recommendations of the Polish Wound Management Association. Part II. Leczenie Ran 2020; 151–184. DOI: 10.5114/ lr.2020.103116.
8. Coleman S, Gorecki C, Nelson EA, et al. Patient risk factors for pressure ulcer development: systematic review. Int J Nurs Stud 2013; 50: 974–1003. DOI: 10.1016/j.ijnurstu.2012.11.019.
9. Ten Ham-Baloyi W. Nurses’ roles in changing practice through
implementing best practices: A systematic review. Health SA 2022; 27: 1776. DOI: 10.4102/hsag.v27i0.1776.
10. Wąsowska I., Kózka M. Nurses’ opinions on the use of scientific evidence in professional practice. Nursing Problems 2015; 23: 392–397.
11. Melnyk BM, Tan A, Hsieh AP, Gallagher-Ford L. Evidence-Based Practice Culture and Mentorship Predict EBP Implementation, Nurse Job Satisfaction, and Intent to Stay: Support for the ARCC© Model. Worldviews Evid Based Nurs 2021; 18: 272–281. DOI: 10.1111/wvn.12524
12. Fukada M. Nursing Competency: Definition, Structure and Development. Yonago Acta Med. 2018; 61: 1–7. DOI: 10.33160/yam.2018.03.001
13. Szumska A. Legal conditions for wound treatment by nurses in Poland. Pielęg Chir Angiol 2020; 2: 47–52.
14. Głowacz J, Szwamel K. Nursing staff’s knowledge of chronic wounds and methods of their treatment. Surgical and Vascular Nursing. 2022; 16: 25–34.
15. Qaseem A, Humphrey LL, Forciea MA, Starkey M, Denberg TD; Clinical Guidelines Committee of the American College of Physicians. Treatment of pressure ulcers: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2015; 162: 370–379. DOI: 10.7326/M14-1568.
16. Huang C, Ma Y, Wang C, et al. Predictive validity of the braden scale for pressure injury risk assessment in adults: A systematic review and meta-analysis. Nurs Open 2021; 8: 2194–2207. DOI: 10.1002/ nop2.792.
17. Wei M, Wu L, Chen Y, Fu Q, Chen W, Yang D. Predictive Validity of the Braden Scale for Pressure Ulcer Risk in Critical Care: A Meta-Analysis. Nurs Crit Care 2020; 25: 165–170. DOI: 10.1111/nicc.12500.
18. Lei L, Zhou T, Xu X, Wang L. Munro Pressure Ulcer Risk Assessment Scale in Adult Patients Undergoing General Anesthesia in the Operating Room. J Healthc Eng 2022; 2022: 4157803. DOI: 10.1155/2022/4157803.
19. Park SH, Lee YS, Kwon YM. Predictive Validity of Pressure Ulcer Risk Assessment Tools for Elderly: A Meta-Analysis. West J Nurs Res 2016; 38: 459–483. DOI: 10.1177/0193945915602259.
20 Šateková L, Žiaková K, Zeleníková R. Predictive validity of the Braden Scale, Norton Scale, and Waterlow Scale in the Czech Republic. Int J Nurs Pract 2017; 23: 10.1111/ijn.12499. DOI: 10.1111/ ijn.12499.
21. Coleman S, Smith IL, McGinnis E, et al. Clinical evaluation of a new pressure ulcer risk assessment instrument, the Pressure Ulcer Risk Primary or Secondary Evaluation Tool (PURPOSE T). J Adv Nurs 2018; 74: 407–424. DOI: 10.1111/jan.13444.
22. Charalambous C, Koulori A, Vasilopoulos A, Roupa Z. Evaluation of the Validity and Reliability of the Waterlow Pressure Ulcer Risk Assessment Scale. Med Arch 2018; 72: 141–144. DOI: 10.5455/ medarh.2018.72.141-144
23. McEvoy N, Avsar P, Patton D, Curley G, Kearney CJ, Moore Z. The economic impact of pressure ulcers among patients in intensive care units. A systematic review. J Tissue Viability. 2021; 30: 168–177. DOI: 10.1016/j.jtv.2020.12.004.
24. Aloweni F, Ang SY, Fook-Chong S, et al. A prediction tool for hospital-acquired pressure ulcers among surgical patients: Surgical pressure ulcer risk score. Int Wound J 2019; 16: 164–175. DOI: 10.1111/ iwj.13007.
25. Popow A, Szewczyk M, Cierzniakowska K, Kozłowska E, Mościcka P, Cwajda-Białasik J. Risk factors for bedsore development among hospitalised patients. Surgical and Vascular Nursing 2018; 12: 152–158.
26. Chen HL, Chen XY, Wu J. The incidence of pressure ulcers in surgical patients of the last 5 years: a systematic review. Wounds 2012; 24: 234–241.
27. Shang Y, Wang F, Cai Y, et al. The accuracy of the risk assessment scale for pressure ulcers in adult surgical patients: a network meta-analysis. BMC Surg 2025; 25: 104.21. DOI: 10.1186/s12893-024-02739-y.
28. Latimer S, Chaboyer W, Thalib L, McInnes E, Bucknall T, Gillespie BM. Pressure injury prevalence and predictors among older adults in the first 36 hours of hospitalisation. J Clin Nurs 2019; 28: 4119–4127. DOI: 10.1111/jocn.14967.
29. Konateke S. A Major Risk in Operating Rooms: Pressure Injury.
Journal of Anatolia Nursing and Health Sciences 2021; 24, 3: 365–372.
30. Roca-Biosca A, Garcia-Fernandez FP, Chacon-Garcés S, et al. Validación de las escalas de valoración de riesgo de úlceras por presión EMINA y EVARUCI en pacientes críticos [Validation of EMINA and EVARUCI scales for assessing the risk of developing pressure ulcers in critical patients]. Enferm Intensiva 2015; 26: 15–23. DOI: 10.1016/j.enfi.2014.10.003.
31. Mosher R. Enhancing Perioperative Care: Implementing the Scott Triggers Tool to Prevent Hospital-Acquired Pressure Injuries in Vascular Surgery Patients. AORN J 2025; 121: 198–207. DOI: 10.1002/ aorn.14303.
32. Sengul T, Gul A, Yilmaz D, Gokduman T. Translation and validation of the ELPO for Turkish population: Risk assessment scale for the development of pressure injuries due to surgical positioning. J Tissue Viability 2022; 31: 358–364. DOI: 10.1016/j.jtv.2022.01.011.
33. Salvini A, Silva E, Passos C, et al. Validation of ELPO-PT: A Risk Assessment Scale for Surgical Positioning Injuries in the Portuguese Context. Nurs Rep 2024; 14: 3242–3263. DOI: 10.3390/ nursrep14040236.
34. Adibelli S, Korkmaz F. Pressure injury risk assessment in intensive care units: Comparison of the reliability and predictive validity of the Braden and Jackson/Cubbin scales. J Clin Nurs 2019; 28: 4595–4605. DOI: 10.1111/jocn.15054.
35. Higgins J, Casey S, Taylor E, Wilson R, Halcomb P. Comparing the Braden and Jackson/Cubbin Pressure Injury Risk Scales in Trauma-Surgery ICU Patients. Crit Care Nurse 2020; 40: 52–61. DOI: 10.4037/ ccn2020874
36. Delawder JM, Leontie SL, Maduro RS, Morgan MK, Zimbro KS. Predictive Validity of the Cubbin-Jackson and Braden Skin Risk Tools in Critical Care Patients: A Multisite Project. Am J Crit Care 2021; 30: 140–144. DOI: 10.4037/ajcc2021669.
37. Zhang Y, Zhuang Y, Shen J, et al. Value of pressure injury assessment scales for patients in the intensive care unit: Systematic review and diagnostic test accuracy meta-analysis. Intensive Crit Care Nurs 2021; 64: 103009. DOI: 10.1016/j.iccn.2020.103009
38. Lospitao-Gómez S, Sebastián-Viana T, González-Ruíz JM,
Álvarez-Rodríguez J. Validity of the current risk assessment scale for pressure ulcers in intensive care (EVARUCI) and the Norton-MI scale in critically ill patients. Appl Nurs Res 2017; 38: 76–82. DOI: 10.1016/j.apnr.2017.09.004.
39. de Souza MFC, Zanei SSV, Whitaker IY. Predictive validity of the EVARUCI scale to evaluate risk for pressure injury in critical care patients. J Wound Care 2023; 32 (Supl. 8): clxi-clxv. DOI: 10.12968/ jowc.2023.32.Sup8.clxi
40. Konateke S, Güner Şİ. Development of A Surgery-Related Pressure Injury Risk Assessment Scale (SURPIRAS): A Methodological Study. J Clin Nurs 2025; 34: 4841–4853. DOI: 10.1111/jocn.17765.
41. Tura İ, Arslan S, Türkmen A, Erden S. Assessment of the risk factors for intraoperative pressure injuries in patients. J Tissue Viability 2023; 32: 349–354. DOI: 10.1016/j.jtv.2023.04.006.
42. Chung ML, Widdel M, Kirchhoff J, et al. Risk factors for pressure ulcers in adult patients: A meta-analysis on sociodemographic factors and the Braden scale. J Clin Nurs 2023; 32: 1979–1992. DOI: 10.1111/jocn.16260,
43. Moore ZE, Patton D. Risk assessment tools for the prevention of pressure ulcers. Cochrane Database Syst Rev 2019; 1: CD006471. pub4. DOI: 10.1002/14651858.CD006471.pub4
44. Saleh M, Anthony D, Parboteeah S. The impact of pressure ulcer risk assessment on patient outcomes among hospitalised patients. J Clin Nurs 2009; 18: 1923–1929. DOI: 10.1111/j.1365-2702.2008.02717.x.
45. Webster J, Coleman K, Mudge A, et al. Pressure ulcers: effectiveness of risk-assessment tools. A randomised controlled trial (the ULCER trial). BMJ Qual Saf 2011; 20: 297–306. DOI: 10.1136/bmjqs.2010.043109.
46. Choi KR, Ragnoni JA, Bickmann JD, Saarinen HA, Gosselin AK. Health Behavior Theory for Pressure Ulcer Prevention: Root-Cause Analysis Project in Critical Care Nursing. J Nurs Care Qual 2016; 31: 68–74. DOI: 10.1097/NCQ.0000000000000137.
47. Kaba E, Kelesi M, Stavropoulou A, Moustakas D, Fasoi G. How Greek nurses perceive and overcome the barriers in implementing treatment for pressure ulcers: ‚against the odds’. J Wound Care 2017; 26 (Supl. 9): S20–S26. DOI: 10.12968/jowc.2017.26.Sup9.S20.
Opis przypadku | case report
LECZENIE RAN 2025; 22 (3): 104-114
DOI: https://doi.org/10.60075/lr.v22i3.118
Wykorzystanie rekomendowanych narzędzi do oceny stanu klinicznego i monitorowania leczenia chorych z przewlekłą niewydolnością żylną i owrzodzeniem żylnym – opis dwóch przypadków klinicznych
The use of recommended tools for assessing the clinical condition and monitoring the treatment of patients with chronic venous insufficiency and venous ulcers –a description of two clinical cases
Paulina Mościcka 1,2, Katarzyna Cierzniakowska 1,3 , Justyna Cwajda-Białasik 1,2, Arkadiusz Jawień 4 , Maria T. Szewczyk 1,2
1 Katedra Pielęgniarstwa Zabiegowego, Wydział Nauk o Zdrowiu, Collegium Medicum w Bydgoszczy, Uniwersytet Mikołaja Kopernika w Toruniu, Polska
2 Poradnia Leczenia Ran Przewlekłych, Szpital Uniwersytecki nr 1 im. dr. A. Jurasza w Bydgoszczy, Polska
3 Klinika Chirurgii Ogólnej i Małoinwazyjnej, Szpital Uniwersytecki nr 2 im. dr. J. Biziela w Bydgoszczy, Polska
Streszczenie
Wprowadzenie: Przewlekła choroba żylna (PChŻ) stanowi istotny problem kliniczny, społeczny i ekonomiczny, a jej najbardziej zaawansowaną manifestacją są owrzodzenia goleni. Cechują się one wysokim wskaźnikiem nawrotów i długim czasem gojenia, co wymaga kompleksowego podejścia diagnostyczno-terapeutycznego.
Cel pracy: Analiza przebiegu leczenia dwóch pacjentek z owrzodzeniem żylnym kończyn dolnych oraz ocena dynamiki zmian klinicznych w odniesieniu do trzech narzędzi: klasyfikacji CEAP, skali VCSS i skali Villalta.
Materiał i metody: W pracy zaprezentowano dwa opisy przypadków chorych z owrzodzeniem żylnym kończyny dolnej leczonych w poradni leczenia ran przewlekłych. Kryteria włączenia stanowiły potwierdzenie w badaniu dupleks scan przewlekłej niewydolności żylnej oraz prawidłowa wartość wskaźnika kostka–ramię. Kryterium wyłączenia było owrzodzenie o etiologii innej niż żylna oraz wynik wskaźnika kostka–ramię powyżej lub poniżej wartości referencyjnych. Dobór pacjentów miał charakter losowy.
Wyniki: W obu opisanych przypadkach zastosowano leczenie przyczynowe w postaci kompresjoterapii oraz terapię miejscową opartą na oczyszczaniu rany, stosowaniu opatrunków specjalistycznych, antyseptyków, a także edukację. Równoległe monitorowanie procesu gojenia
4 Katedra i Klinika Chirurgii Naczyniowej i Angiologii, Szpital Uniwersytecki nr 1 im. dr. A. Jurasza, Collegium Medicum w Bydgoszczy, Uniwersytet Mikołaja Kopernika w Toruniu, Polska
Adres do korespondencji
Paulina Mościcka, Katedra Pielęgniarstwa Zabiegowego, Wydział Nauk o Zdrowiu, Collegium Medicum, ul. Łukasiewicza 1, 85-821 Bydgoszcz, e-mail: moscicka76@op.pl
Nadesłano: 28.09.2025; Zaakceptowano: 27.10.2025
Abstract
Introduction: Chronic venous disease (CVD) is a significant clinical, social, and economic problem, and its most advanced manifestation is leg ulcers. They are characterized by a high recurrence rate and long healing times, requiring a comprehensive diagnostic and therapeutic approach.
The aim of the study: To analyze the treatment course of two patients with venous ulcers of the lower limbs and to assess the dynamics of clinical changes in relation to three tools: the CEAP classification, the VCSS scale, and the Villalta scale.
Material and methods: This study presents two case reports of patients with venous ulcers of the lower limbs. Inclusion criteria included confirmation of chronic venous insufficiency by duplex scanning and a normal ankle-brachial index. Exclusion criteria were ulcers of a non-venous etiology and an ankle-brachial index result above or below the reference values. Patient selection was random.
Results: In both cases, causal treatment was implemented in the form of compression therapy and local therapy based on wound cleansing, the use of specialized dressings, antiseptics, and education. Simultaneous monitoring of the healing process using scales allowed for an objective and subjective assessment of treatment progress. In both cases,
przy użyciu skal pozwoliło na obiektywną i subiektywną ocenę postępów leczenia. W obu przypadkach uzyskano całkowite wygojenie ran – w 9. i 10. tygodniu terapii.
Wnioski: Skale, w tym CEAP, umożliwiły precyzyjne określenie stanu wyjściowego i końcowego, VCSS odzwierciedlała zmiany morfologiczne i stopniową poprawę stanu miejscowego, natomiast skala Villalta uchwyciła szybkie ustąpienie objawów subiektywnych i poprawę jakości życia. Wykazano, że rekomendowane skale nie są zamienne, lecz komplementarne. Ich równoległe zastosowanie dostarcza pełniejszego obrazu klinicznego, wspiera proces monitorowania skuteczności leczenia i może przyczynić się do zmniejszenia ryzyka nawrotów owrzodzeń.
Słowa kluczowe: przewlekła niewydolność żylna, owrzodzenie żylne, skale kliniczne.
Wprowadzenie
Przewlekła choroba żylna (PChŻ) to postępujące i często niedoceniane schorzenie, charakteryzujące się wysoką częstością występowania w populacji ogólnej oraz znaczącym wpływem klinicznym, społecznym i ekonomicznym [1]. Obejmuje ona szerokie spektrum nieprawidłowości żylnych, w których powrót krwi jest poważnie upośledzony. W patofizjologii PChŻ wzajemne oddziaływanie czynników genetycznych i środowiskowych jest odpowiedzialne za wzrost ciśnienia żylnego, które prowadzi do istotnych zmian w całej strukturze i funkcjonowaniu układu żylnego [2]. Co istotne, termin przewlekła choroba żylna należy odróżnić od przewlekłej niewydolności żylnej (PNŻ). Przewlekła choroba żylna obejmuje morfologiczne i czynnościowe nieprawidłowości układu żylnego [3], natomiast PNŻ odnosi się do najcięższych postaci choroby, w których występują objawy kliniczne, takie jak żylaki, obrzęki, zmiany troficzne skóry, a w zaawansowanym stadium także owrzodzenia żylne. Chorobie tej towarzyszą również dolegliwości subiektywne, m.in. ból, kurcze mięśni, parestezje czy świąd [4, 5].
Owrzodzenia żylne stanowią 70–90% ran zlokalizowanych w okolicy kończyn dolnych [6]. Częstość ich występowania wzrasta wraz z wiekiem, a kobiety są trzykrotnie bardziej narażone na rozwój rany niż mężczyźni [7]. Duże znaczenie ma obecność czynników dziedzicznych, rasowych, a także nadwaga, przebyte ciąże, typ aktywności zawodowej, zaburzenia statyki stopy, nadmierna ekspozycja na działanie słońca, typ aktywności sportowej, dieta ubogoresztkowa i zaparcia [8, 9]. Trzymiesięczny wskaźnik gojenia owrzodzeń szacuje się na 30–60% [10–12], a mediana czasu ich trwania waha się od 6 miesięcy [13–15] do kilkudziesięciu lat [16]. Wskaźnik nawrotów jest wysoki – w niektórych krajach wynosi
complete wound healing was achieved – at weeks 9 and 10 of therapy.
Conclusions: The scales, including the CEAP, enabled precise determination of baseline and final status; the VCSS reflected morphological changes and gradual improvement in local conditions, and the Villalta scale captured the rapid resolution of subjective symptoms and improved quality of life. It was demonstrated that the recommended scales are not interchangeable, but complementary. Their simultaneous use provides a more comprehensive clinical picture, supports the process of monitoring treatment effectiveness, and may contribute to reducing the risk of ulcer recurrence.
Key words: chronic venous insufficiency, venous ulcer, clinical scales.
nawet 70% [17, 18]. U około 80% pacjentów nawrót występuje w ciągu 3 miesięcy od wygojenia [16], a około 26% owrzodzeń nawraca w ciągu pierwszych 12 miesięcy od zakończenia terapii [19]. Do oceny stanu chorego i planowania leczenia można wykorzystać dostępne narzędzia klasyfikacyjne, które pozwalają uwzględnić wiele czynników klinicznych oraz monitorować zmiany w czasie. W niniejszej pracy zastosowano trzy rekomendowane w literaturze narzędzia: klasyfikację CEAP (tab. 1), skalę Venous Clinical Severity Score (VCSS; tab. 2) oraz skalę Villalta (tab. 3) [20–24].
Celem pracy jest przedstawienie procesu leczenia dwóch losowo wybranych chorych z owrzodzeniem żylnym kończyny dolnej oraz ocena dynamiki zmian klinicznych w odniesieniu do zastosowanych skal.
Materiał i metody
W pracy zaprezentowano dwa opisy przypadków chorych z owrzodzeniem żylnym kończyny dolnej leczonych w Poradni Leczenia Ran Przewlekłych. Kryterium włączenia było potwierdzenie w badaniu duplex scan przewlekłej niewydolności żylnej oraz prawidłowa wartość wskaźnika kostka–ramię (WKR). Do badania nie zakwalifikowano chorych z owrzodzeniem o etiologii innej niż żylna oraz z wynikiem WKR powyżej lub poniżej wartości referencyjnych. Dobór pacjentów miał charakter losowy. W skład zespołu badawczego wchodził wykwalifikowany zespół pielęgniarski oraz lekarz, który pełnił funkcję konsultacyjną. Proces terapeutyczny, w tym diagnostyka i ocena wartości WKR, były wykonywane przez pielęgniarki posiadające wieloletnie doświadczenie kliniczne w leczeniu ran o etiologii naczyniowej oraz pracowników naukowo-dydaktycznych.
Tabela 1. Klasyfikacja CEAP [17, 25]
Komponent
C – Clinical (kliniczny)
E – Etiology (etiologia)
A – Anatomy (anatomia)
P – Pathophysiology (patofizjologia)
Kategorie
C 0 Brak objawów
Opis
C 1 Teleangiektazje, żyły siateczkowate
C 2 Żylaki
C 3 Obrzęk
C 4a Przebarwienia, wyprysk żylny
C4b Lipodermatoskleroza, atrophie blanch e
C 4c Corona phlebectatica (siateczka drobnych żyłek wokół kostki)
C 5 Wygojone owrzodzenie żylne
C 6 Czynne owrzodzenie żylne
C 6r Owrzodzenie nawrotowe
E p Pierwotna
Esi Wtórna (np. pozakrzepowa)
E c Wrodzona
E n Nieokreślona
A s Żyły powierzchowne (np. odpiszczelowa, odstrzałkowa)
Ad Żyły głębokie (np. udowa, podkolanowa)
A p Żyły przeszywające (perforatory)
A n Nieokreślone
P r Refluks
P r Niedrożność
P r,o
Refluks i niedrożność
P n Nieokreślone
Tabela 2. Zmieniona skala VCSS klinicznej ciężkości choroby żylnej [18, 19]
Brak: 0 Łagodny: 1 Umiarkowany: 2 Ciężki: 3
Ból
Żylaki
Obrzęk żylny
Przebarwienia
Nie występuje
Sporadycznie, nie ogranicza dziennej aktywności
Nie występują Pojedyncze żylaki
Nie występuje
Nie występują lub miejscowo
Zapalenie Nie występuje
Stwardnienie Nie występuje
Ograniczony do stopy i kostek
Ograniczone, okołokostkowe
Umiarkowany cellulitis, owrzodzenie okołokostkowe
Ograniczone do obszaru okolicy kostki
Codziennie, wpływa na aktywność, ale jej nie zaburza
Ograniczone do goleni lub uda
Powyżej kostek, ale ograniczony do goleni
Codziennie, ograniczający aktywność
Obejmujące goleń i udo
Dotyczący goleni i powyżej
Rozsiane, poniżej 1/3 goleni Rozlane, powyżej 1/3 goleni
Rozsiane poniżej 1/3 goleni Rozlane powyżej 1/3 goleni
Rozsiane poniżej 1/3 goleni Rozlane powyżej 1/3 goleni
Liczba owrzodzeń 0 1 2 3 i więcej
Czas trwania owrzodzenia – < 3 miesięcy > 3 i < 12 miesięcy Niewyleczone
Rozmiar owrzodzenia – < 2 cm 2–6 cm > 6 cm
Stosowanie kompresjoterapii Niestosowana Czasem Większość dni Cały czas
Tabela 3. Skala Villalta PTS [29, 32]
Objawy/oznaki
kliniczne Brak Łagodny Średni Ciężki
OBJAWY
Ból
Skurcze
Ciężkość
Parestezje
Świąd
OBJAWY KLINICZNE
Obrzęk
Stwardnienie skóry
Przebarwienia
Zaczerwienienie
Poszerzenia żył
Ból przy ucisku goleni 0 punktów
Owrzodzenie żylne Brak Obecne
Opis przypadku nr 1
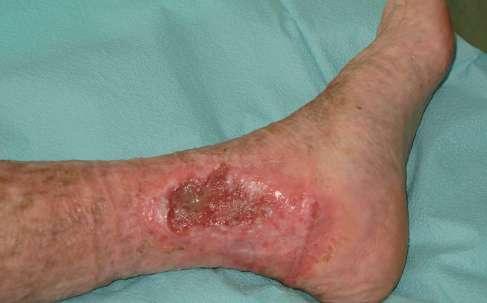
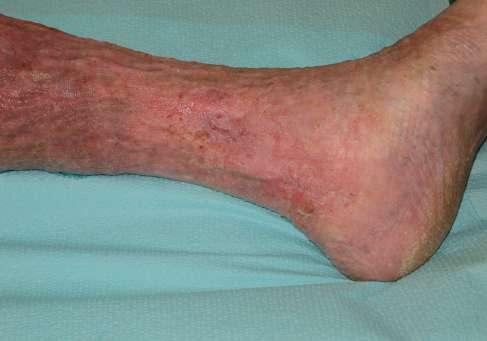
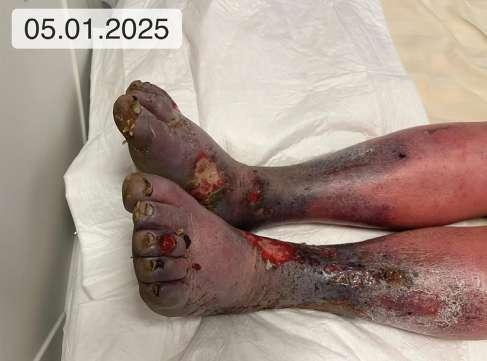
Pacjentka, lat 76, zgłosiła się do Poradni Leczenia Ran Przewlekłych Szpitala Uniwersyteckiego nr 1 im. dr. A. Jurasza w Bydgoszczy z powodu owrzodzenia kończyny dolnej lewej.
Badanie podmiotowe
• Rana powstała około 4 miesiące wcześniej.
• Owrzodzenie o charakterze nawrotowym, pierwsze powstało 35 lat temu, obecnie jest to 5. nawrót.
• Pacjentka do tej pory leczona doraźnie w różnych placówkach m.in.: POZ, poradni dermatologicznej, poradni chirurgicznej, punktach opieki farmaceutycznej.
• Miejscowo stosowano różne opatrunki specjalistyczne, maści, „przymoczki”, antybiotyki.
• Kompresjoterapię stosowano okazjonalnie.
• Choroby współistniejące – nadciśnienie tętnicze, około 45 lat temu przebyta zakrzepica żył głębokich (w okresie połogu) i od tego czasu chora zażywa leki antykoagulacyjne.
• Dolegliwości bólowe – 7/10 pkt w skali wizualno-analogowej (Visual Analogue Scale – VAS).
Badanie przedmiotowe
• Wskaźnik kostka–ramię: kończyna dolna prawa – 1,15, kończyna dolna lewa – 1,17.
• Wynik badania duplex scan: niewielki 1-sekundowy refluks w ujściu żyły udowej, poza tym żyła udowa podatna cienkościenna. W obrębie żyły podkolanowej widoczne zmiany pozakrzepowe w postaci pogrubienia i nieregularności ściany
naczynia, z obecnością zwłóknień i zniekształceń światła. Ujścia i pnie żył odpiszczelowych i odstrzałkowych wydolne. Na przyśrodkowej powierzchni 1/3 uda od żyły odpiszczelowej odchodzi żylakowato zmieniona obocznica, która podąża po stronie przyśrodkowej kończyny do wysokości 1/2 goleni, gdzie uchodzi do żył głębokich przez niewydolny perforator Cocketta III. Poza tym niewydolnych perforatorów nie wykazano.
• Ocena według klasyfikacji CEAP – C2,4a,b,6, Es, Asdp, Pr,o: C 2 – żylaki kończyn dolnych, C4a – pigmentacja lub egzema, C4b – lipodermatoskleroza lub biała atrofia, C6 – owrzodzenie, Esi – wtórne, np. pozakrzepowe, Asdp – układ powierzchowny, przeszywający i głęboki, Pr,o – refluks i niedrożność.
• Ocena według skali VCSS – 16 pkt: ból – 3, żylaki – 2, obrzęk – 1, pigmentacja skóry – 2, zapalenie – 1, stwardnienie – 1, liczba aktywnych owrzodzeń – 1, czas trwanie owrzodzenia – 2, rozmiar owrzodzenia – 3, stosowanie kompresjoterapii – 1.
• Ocena według skali Villalta – 17 pkt: ból – 3, skurcze – 2, ciężkość – 3, parestezje – 0, świąd – 1, obrzęk – 1, stwardnienie skóry – 1, zaczerwienienie – 1, poszerzenia żył – 2, ból przy ucisku goleni – 0, owrzodzenie żylne – 3.
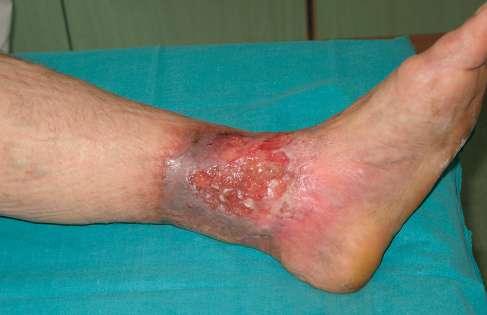
Opis owrzodzenia i otaczającej skóry
Rana zlokalizowana na kończynie dolnej lewej w okolicy kostki przyśrodkowej, o łącznej powierzchni 39,75 cm 2 i głębokości 0,3 cm. Łożysko
P. Mościcka, K. Cierzniakowska, J. Cwajda-Białasik i wsp.
rany pokryte w 70% żółtą martwicą, 20% stanowiła niepełnowartościowa ziarnina, a 10% powierzchni stanowił naskórek. Całą powierzchnię rany pokrywał biofilm. Brzeg owrzodzenia, zwłaszcza jego górna i przyśrodkowa krawędź, wyraźnie zaznaczone, miejscami przysłonięte włóknikiem, pozostałe krawędzie rany z cechami epitelizacji. Skóra w okolicy getrowej znacznie wysuszona, pergaminowa, z cechami hemosyderozy, lipodermatosklerozy, a wokół owrzodzenia zaczerwieniona, w dolnym biegunie rany zmacerowana.
Postępowanie pielęgnacyjno-lecznicze
Z powierzchni rany pobrano materiał do badania mikrobiologicznego, na podstawie którego wyizolowano Proteus mirabilis 104 , Staphylococcus aureus 102 Po przeanalizowaniu wyniku mikrobiologicznego oraz po ocenie stanu klinicznego podjęto decyzję o wyłącznie miejscowym leczeniu przeciwdrobnoustrojowym. Łożysko rany było systematycznie oczyszczane z tkanek martwych, resztek zrogowaciałego naskórka, pozostałości wysięku. Ranę opracowywano mechanicznie, sięgając 1–2 mm w głąb żywej tkanki, tak aby zwiększyć prawdopodobieństwo usunięcia wszystkich struktur ułatwiającym bakteriom wytwarzanie biofilmu. Jednocześnie monitorowano stan rany pod kątem ryzyka wystąpienia krwawienia. W celu eradykacji drobnoustrojów chorobotwórczych na powierzchnię rany stosowano antyseptyk o szerokim spektrum działania. Podczas pierwszej i drugiej wizyty, przed oczyszczeniem łożyska rany, choremu zalecono zażycie doustnego leku przeciwbólowego. W kolejnych etapach leczenia pacjent nie odczuwał dolegliwości bólowych.
Tabela 4. Przebieg procesu leczenia
W początkowym etapie stosowano opatrunki przeciwdrobnoustrojowe o właściwościach chłonnych, następnie postępowanie miejscowe było uzależnione od stanu klinicznego rany i modyfikowane podczas każdej wizyty. Częstotliwość zmian opatrunków ulegała stopniowej redukcji, w początkowym etapie konieczna zmiana co dwa dni, następnie dwa razy w tygodniu i w końcowym etapie raz na tydzień. Od początku leczenia u chorej włączono kompresjoterapię dwuwarstwową, aplikując ciśnienie w okolicy kostki w zakresie pomiędzy 41 a 50 mm Hg [25]. Pacjentkę poddawano systematycznej edukacji, m.in.: w zakresie stosowania terapii kompresyjnej, ćwiczeń usprawniających pracę stawu skokowego i pompy mięśniowej, odpowiedniej diety, zgodnej ze schematem postępowania dietetycznego w Poradni Leczenia Ran Przewlekłych i leczniczego w tym zakresie [26, 27].
Uzyskany efekt
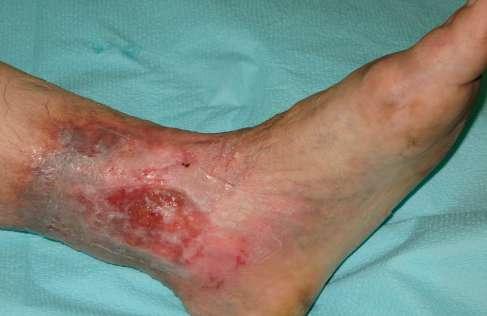
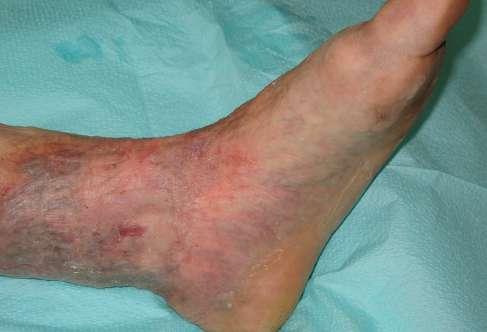
W trakcie prowadzonej terapii odnotowano istotny postęp procesu gojenia. W pierwszym etapie doszło do oczyszczenia łożyska rany z tkanek martwiczych, co zapoczątkowało kolejne fazy gojenia. Po 9 tygodniach intensywnej terapii uzyskano pełne wygojenie owrzodzenia. Skala VCSS dobrze odzwierciedliła proces gojenia – od stanu ostrego do utrwalonych następstw, z redukcją punktacji z 18 do 9. Skala Villalta była bardziej czuła na poprawę subiektywnych objawów i wygojenie rany, co przełożyło się na szybszy spadek wartości (z 21 do 5 punktów). Zgodnie z klasyfikacją CEAP po wygojeniu owrzodzenia kategoria C6 została zastąpiona kategorią C5 (tab. 4, tab. 5).
Tydzień terapii Powierzchnia w cm2 Skala CEAP Skala VCSS Skala Villalta
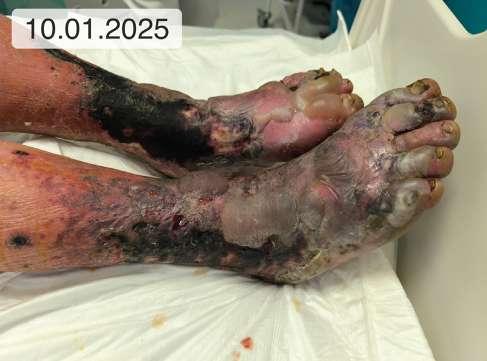
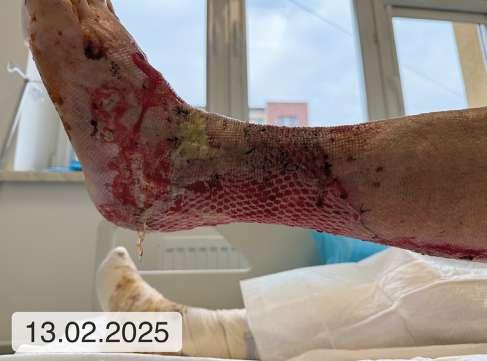
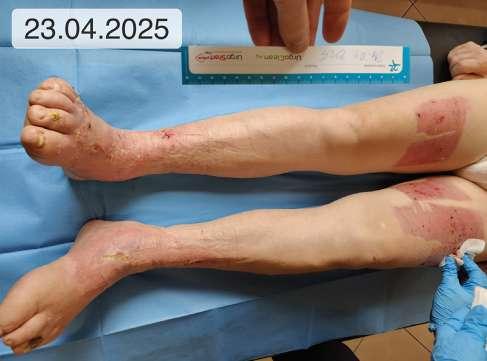
1. 39.75 (ryc. 1) C2,4a,b,6r,Esi,Asdp, Pr,o 18 pkt 21 pkt
5. 12 (ryc. 2) - 14 8
9. 0 (ryc. 3) C2, 4a,b,5, Esi,Asdp, Pr,o 9 5
5. Ocena dynamiki zmian na podstawie skal: VCSS i Villalta
tydzień 5. tydzień 9. tydzień Skala VCSS
Tabela
1.
Opis przypadku nr 2
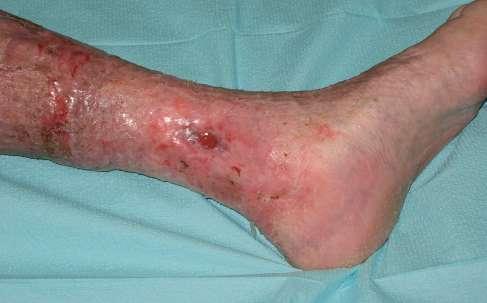
Chora, lat 67, została przyjęta do Poradni Leczenia Ran Przewlekłych Szpitala Uniwersyteckiego nr 1 im. dr. A. Jurasza w Bydgoszczy z powodu niegojącego owrzodzenia żylnego kończyny dolnej lewej.
Badanie podmiotowe
• Rana powstała 6 miesięcy wcześniej.
• Do tej pory stosowano wyłącznie terapię miejscową, niestandardową, w postaci maści z antybiotykami lub glikokortykosteroidami.
• Chorej wcześniej przed przyjęciem do Poradni
Ran Przewlekłych
Rycina 1. Stan rany u 76-letniej chorej w tygodniu 1. Rycina 2. Stan rany u 76-letniej chorej w tygodniu 5.
Rycina 3. Stan rany u 76-letniej chorej w tygodniu 9.
P. Mościcka, K. Cierzniakowska, J. Cwajda-Białasik i wsp.
zakazano mycia skóry (kończyna przez 6 miesięcy nie była umyta).
• Choroby współistniejące: nadciśnienie tętnicze.
• Brak stosowania kompresjoterapii.
• Dolegliwości bólowe na poziomie 4/10 pkt w skali VAS.
• Dokuczliwy świąd, zwłaszcza w okolicy getrowej
Badanie przedmiotowe
• Wskaźnik kostka–ramię: kończyna prawa – 1,2, kończyna lewa – 1,1.
• Wynik badania duplex scan: żyła głęboka uda i powierzchowna drożne, żyła podkolanowa i w żyłach goleni bez patologii. Niewydolne ujście żyły odpiszczelowej, refluks na całej długości. Żyła odstrzałkowa wydolna. Widoczny refluks w perforatorze Cocketta II i III, utrzymujący się powyżej 0,5 sekundy.
• Ocena według klasyfikacji CEAP – C2,4a,6, Ep, As,p, Pr: C 2 – żylaki kończyn dolnych, C4a – pigmentacja lub egzema, C6 – owrzodzenie, Ep – etiologia pierwotna, Asp – układ powierzchowny, przeszywający, Pr – refluks.
• Ocena według skali VCSS – 12 pkt: ból – 1, żylaki – 1, obrzęk – 1, przebarwienia – 1, zapalenie – 1, stwardnienie – 1, liczba owrzodzeń – 1, czas trwanie owrzodzenia – 2, rozmiar owrzodzenia – 3, stosowanie kompresjoterapii – 0.
• Ocena według skali Villetla – 15 pkt: ból – 1, skurcze – 1, ciężkość – 1, parestezje – 0, świąd – 3, obrzęk – 1, stwardnienie skóry – 1, zaczerwienienie – 1, poszerzenia żył – 1, ból przy ucisku łydki – 0, owrzodzenie żylne – 3.
Opis owrzodzenia i otaczającej skóry
Rana zlokalizowana na kończynie dolnej lewej po stronie przyśrodkowej. Owrzodzenie o powierzchni 52,25 cm2
Tabela 6. Przebieg procesu leczenia
pokryte w 75% żółtą martwicą, 20% powierzchni rany stanowiła ziarnina, a pozostałe łożysko rany wypełniał naskórek. Brzeg rany nieregularny i rozmyty, w górnym biegunie nieco wyraźniej zaznaczony. Rana obficie wydzielająca. Wysięk z rany miał charakter surowiczy i był bezwonny. Skóra otaczająca owrzodzenie przebarwiona, z obrzękiem okolicznych tkanek, z cechami maceracji zwłaszcza w części dystalnej.
Postępowanie pielęgnacyjno-lecznicze
Z powierzchni rany pobrany został materiał do badania mikrobiologicznego, z którego wyhodowano Streptococcus β-hemolizujacy grupy „C”2. Po przeanalizowaniu wyniku bakteriologicznego i ocenie całościowego stanu klinicznego, podjęto decyzję o zastosowaniu miejscowego leczenia przeciwdrobnoustrojowego. Podczas każdej wizyty rana i skóra otaczająca owrzodzenie, była dokładnie myta i oczyszczana w sposób mechaniczny. W celu eradykcji drobnoustrojów chorobotwórczych na powierzchnię rany aplikowano antyseptyk o szerokim spektrum działania. Podczas pierwszej wizyty choremu zalecono, aby przed oczyszczeniem powierzchni rany zażył doustny lek przeciwbólowy. W kolejnych etapach leczenia pacjent nie wymagał leczenia przeciwbólowego. W początkowym etapie stosowano opatrunki piankowe o właściwościach przeciwdrobnoustrojowych. Następnie, w zależności od stanu klinicznego rany – w tym fazy gojenia i ilości wysięku – modyfikowano postępowanie miejscowe. W końcowym etapie procesu gojenia stosowano opatrunki siatkowe. W pierwszym tygodniu terapii opatrunki zmieniano co 1–2 dni, natomiast w dalszym okresie – ze względu na poprawę stanu klinicznego – co 3 dni. Skórę wokół owrzodzenia zabezpieczano przeznaczonym do tego celu emolinetem. Od początku terapii włączono terapię uciskową w formie
Tydzień terapii Powierzchnia w cm2 Skala CEAP Skala VCSS Skala Villalta
1. 52,25 (ryc. 4) C2,4a,6, Ep, As,p, Pr 12 pkt 15 pkt
6. 18,75 (ryc. 5) - 11 7
10. 0 (ryc. 6) C2, 4a,b,5, Es, Asdp, Pr,o 7 3
Tabela 7. Ocena dynamiki zmian na podstawie skal: VCSS i Villalta
VCSS
specjalistycznych bandaży typu short-stretch, aplikując ciśnienie w okolicy kostki 31–40 mm Hg. Przebieg procesu leczenia przedstawiono w tabelach 6 i 7.
Uzyskany efekt
W trakcie terapii odnotowano systematyczny postęp procesu gojenia. Początkowo powierzchnia owrzodzenia wynosiła 52,25 cm², a pacjentka spełniała kryteria CEAP C6. W 6. tygodniu terapii powierzchnia rany zmniejszyła się do 18,75 cm², a w 10. tygodniu uzyskano całkowite wygojenie owrzodzenia. Skala VCSS potwierdziła
Rycina 6. Stan rany u 67-letniej chorej w tygodniu 10.
Rycina 5. Stan rany u 67-letniej chorej w tygodniu 6.
Rycina 4. Stan rany u 67-letniej chorej w tygodniu 1.
P. Mościcka, K. Cierzniakowska, J. Cwajda-Białasik i wsp.
się z 12 do 7, odzwierciedlając redukcję zarówno objawów, jak i zmian miejscowych. Skala Villalta wykazała jeszcze większą dynamikę zmian, z 15 punktów w 1. tygodniu do 3 punktów w 10. tygodniu, co wskazuje na szybkie ustąpienie objawów subiektywnych i znaczną poprawę komfortu chorej. W klasyfikacji CEAP końcowa ocena zmieniła się z C6 na C5, co dokumentuje przejście od fazy aktywnego owrzodzenia do fazy przebytego owrzodzenia żylnego.
Omówienie
Mimo upływu lat w ocenie PChŻ wciąż wykorzystuje się klasyczne narzędzia, takie jak klasyfikacja CEAP, skala VCSS oraz skala Villalta. Powstały w różnych okresach i dla odmiennych celów, jednak nadal są rekomendowane w literaturze i przydatne w codziennej praktyce klinicznej, ponieważ dostarczają uzupełniających informacji na temat stanu pacjenta. Każde z wymienionych narzędzi pełni odmienną funkcję i pozwala na analizę zarówno następstw morfologicznych, jak i subiektywnych objawów choroby, co ma istotne znaczenie w całościowej ocenie przebiegu PChŻ oraz skuteczności leczenia. Należy jednak podkreślić, że skale kliniczne mają charakter uzupełniający, podczas gdy podstawą diagnostyki przewlekłej choroby żylnej pozostaje badanie ultrasonograficzne metodą duplex scan [28]. Badanie obrazowe umożliwia m.in. identyfikację pacjentów z nadciśnieniem żylnym, którzy mogą odnieść korzyść z leczenia nieinwazyjnego lub inwazyjnego, zmniejszając tym samym ryzyko nawrotów owrzodzeń. Równie istotne jest wykluczenie współistniejącej choroby tętnic obwodowych, której obecność stwierdza się nawet u 26% pacjentów z owrzodzeniami kończyn dolnych [29–31].
Klasyfikacja CEAP stanowi od ponad dwóch dekad międzynarodowy standard w opisie PChŻ, umożliwiając dokładne określenie stopnia klinicznego (C), etiologii (E), lokalizacji anatomicznej (A) oraz mechanizmu patofizjologicznego (P). Po raz pierwszy została opublikowana w 1995 r. [20], następnie zaktualizowana w 2004 r. [32], a jej najnowsza rewizja pochodzi z 2020 r. [23]. W ostatniej aktualizacji dodano m.in. nowe kategorie dla corona phlebectatica (C4c), nawracających żylaków (C2r), nawracających owrzodzeń (C6r), a także wprowadzono rozróżnienie wtórnej etiologii na żylne (Esi) i pozażylne (Ese). Klasyfikacja CEAP znajduje zastosowanie głównie w diagnostyce i stratyfikacji pacjentów, natomiast jej użyteczność w monitorowaniu krótkoterminowych efektów terapii pozostaje ograniczona. W przedstawionych przypadkach klasyfikacja CEAP pozwoliła jednoznacznie określić
stan początkowy (C6) i końcowy (C5), stanowiąc punkt odniesienia w procesie leczenia. Skala VCSS została opracowana jako narzędzie uzupełniające CEAP, której statyczny charakter uniemożliwiał monitorowanie dynamiki choroby i efektów leczenia. Po raz pierwszy została zaproponowana w 2000 r. przez Rutherforda i wsp. jako element ujednoliconej oceny ciężkości PChŻ i reakcji na terapię [33]. W kolejnych latach Meissner i wsp. ocenili jej właściwości kliniczne, a Vasquez i wsp. zaproponowali rewizję i dalsze udoskonalenie [21, 34]. Skala obejmuje dziesięć parametrów klinicznych (m.in. ból, żylaki, obrzęk, przebarwienia, zapalenie, stwardnienie skóry, liczbę i czas trwania owrzodzeń, ich wielkość oraz stosowanie kompresjoterapii), z punktacją 0–3 dla każdego objawu. Skala VCSS pozwala na dynamiczną, okresową ocenę pacjenta – ustalenie stanu wyjściowego, obserwację progresji oraz analizę skuteczności leczenia (progresja/regresja/brak wpływu) [35]. W analizowanych przypadkach skala ta pozwoliła uchwycić stopniową poprawę stanu miejscowego – punktacja obniżyła się u pierwszej chorej z 18 do 9 i u drugiej z 12 do 7 – jednocześnie uwidaczniając utrwalone następstwa PChŻ, które pozostają mimo wygojenia rany.
Skala Villalta, opisana po raz pierwszy w 1994 r. [39], została opracowana jako narzędzie do diagnostyki i oceny ciężkości zespołu pozakrzepowego ( postthrombotic syndrome – PTS). Jej przydatność kliniczną potwierdzono w badaniach prospektywnych [40], a kolejne rekomendacje wskazywały ją jako standard w diagnostyce i monitorowaniu PTS [41]. W późniejszych analizach podkreślano jej zalety i ograniczenia w porównaniu z innymi systemami oceny PTS [39].
Skala obejmuje pięć objawów subiektywnych (ból, skurcze, ciężkość, parestezje, świąd) oraz sześć cech klinicznych (obrzęk przedgoleniowy, stwardnienie skóry, przebarwienia, zaczerwienienie, poszerzenia żył, ból przy ucisku goleni) ocenianych w skali 0–3. Dodatkowo obecność owrzodzenia żylnego automatycznie kwalifikuje chorobę jako ciężką. Wynik 5–9 punktów oznacza PTS łagodny, 10–14 umiarkowany, a ≥ 15 ciężki. W analizowanych przypadkach Villalta umożliwiła uchwycenie poprawy subiektywnej – punktacja zmieniła się z 21 do 5 i z 15 do 3 – w obu przypadkach głównie dzięki ustąpieniu bólu, ciężkości i obrzęku i wygojeniu owrzodzenia. W literaturze podkreśla się, że skala ta dobrze koreluje z oceną jakości życia pacjentów [40], co potwierdza jej przydatność jako uzupełnienia narzędzi skoncentrowanych na objawach obiektywnych.
Porównanie i ograniczenia
Skale VCSS i Villalta nie są zamienne, lecz komplementarne – pierwsza dostarcza informacji o morfologicznych następstwach choroby i postępie gojenia, druga odzwierciedla subiektywne objawy i poprawę komfortu pacjenta. Z kolei klasyfikacja CEAP stanowi fundament diagnostyczny i klasyfikacyjny. Należy jednak pamiętać, że każde z narzędzi ma swoje ograniczenia: klasyfikacja CEAP jest statyczna i nie rejestruje dynamiki zmian krótkoterminowych [20], VCSS obarczona częściową subiektywnością oceny [21], a skala Villalta może prowadzić do przeszacowania objawów u pacjentów bez epizodów zakrzepicy [44]. Z tego względu równoległe stosowanie różnych narzędzi wydaje się najbardziej uzasadnione klinicznie – umożliwia bowiem zarówno obiektywną ocenę następstw choroby, jak i analizę jakości życia pacjenta.
W praktyce pielęgniarskiej największą wartość mają skale łączące ocenę parametrów klinicznych z perspektywą pacjenta. Klasyfikacja CEAP stanowi solidne narzędzie diagnostyczne i punkt odniesienia w planowaniu opieki, jednak jej ograniczeniem jest brak możliwości bieżącego monitorowania efektów terapii. Skala VCSS jest szczególnie przydatna w codziennej pracy pielęgniarki, umożliwiając systematyczną ocenę skuteczności leczenia miejscowego i kompresyjnego. Skala Villalta wnosi natomiast istotną wartość w zakresie oceny jakości życia i odczuwanych dolegliwości, stanowiąc cenne uzupełnienie narzędzi koncentrujących się na zmianach morfologicznych. W praktyce klinicznej optymalne jest zatem równoległe stosowanie wszystkich trzech skal, co pozwala na pełniejszą, wielowymiarową ocenę pacjentów z PChŻ.
Podsumowanie
Przewlekła choroba żylna, a zwłaszcza owrzodzenia goleni, wymagają kompleksowego podejścia diagnostycznego i terapeutycznego. W przedstawionych przypadkach równoległe zastosowanie klasyfikacji CEAP, skali VCSS i skali Villalta pozwala na kompleksową ocenę przebiegu choroby – od obiektywnej charakterystyki zmian morfologicznych po subiektywne odczucia pacjenta. Klasyfikacja CEAP pełniła funkcję punktu odniesienia, VCSS pozwoliła uchwycić dynamikę zmian miejscowych, a skala Villalta odzwierciedliła poprawę jakości życia dzięki ustąpieniu objawów.
Wyniki wskazują, że skale te wzajemnie się uzupełniają, a ich łączne stosowanie dostarcza pełniejszego obrazu klinicznego i ułatwia monitorowanie
skuteczności terapii. Praktyka ta może przyczynić się do poprawy opieki nad pacjentami z owrzodzeniami żylnymi i ograniczenia ryzyka nawrotów.
Oświadczenia
Autorzy deklarują brak konfliktu interesów. Praca nie uzyskała finansowania zewnętrznego. Zgoda Komisji Bioetycznej nie była wymagana.
Piśmiennictwo
1. Nicolaides AN, Labropoulos N. Burden and Suffering in Chronic Venous Disease. Adv Ther 2019; 36 (Suppl 1): 1–4. DOI: 10.1007/ s12325-019-0882-6.
2. Ligi D, Croce L, Mannello F. Chronic Venous Disorders: The Dangerous, the Good, and the Diverse. Int J Mol Sci 2018; 19: 2544. DOI: 10.3390/ijms19092544.
3. Eklof B, Perrin M, Delis KT i wsp. Updated terminology of chronic venous disorders: the VEIN-TERM transatlantic interdisciplinary consensus document. J Vasc Surg 2009; 49: 498–501. DOI: 10.1016/ j.jvs.2008.09.014.
4. Hyder ON, Soukas PA. Chronic Venous Insufficiency: Novel Management Strategies for an Under-diagnosed Disease Process. R I Med J (2013) 2017; 100: 37–39.
5. Ortega MA, Fraile-Martínez O, García-Montero C i wsp. Understanding Chronic Venous Disease: A Critical Overview of Its Pathophysiology and Medical Management. J Clin Med 2021; 10: 3239. DOI: 10.3390/jcm10153239.
6. Nair HK, Mosti G, Atkin L i wsp. Leg ulceration in venous and arteriovenous insufficiency: assessment and management with compression therapy as part of a holistic wound-healing strategy. J Wound Care 2024; 33 (Sup10b): S1–S31. DOI: 10.12968/jowc.2024.33. Sup10b.S1.
7. Hellström A, Nilsson C, Nilsson A, Fagerström C. Leg ulcers in older people: a national study addressing variation in diagnosis, pain and sleep disturbance. BMC Geriatr 2016; 16: 25. DOI: 10.1186/ s12877-016-0198-1.
8. Dudziak S. Etiologia przewlekłej niewydolności żylnej. W: Jawień A, Szewczyk MT (red.). Kliniczne i pielęgnacyjne aspekty opieki pielęgniarskiej nad chorym z owrzodzeniem żylnym goleni. Termedia, Poznań 2008; 27–34.
9. De Maeseneer MG, Kakkos SK, Aherne T i wsp. Editor’s Choice – European Society for Vascular Surgery (ESVS) 2022 Clinical Practice Guidelines on the Management of Chronic Venous Disease of the Lower Limbs. Eur J Vasc Endovasc Surg 2022; 63: 184–267. DOI: 10.1016/j.ejvs.2021.12.024.
10. Parker CN, Finlayson KJ, Shuter P, Edwards HE. Risk factors for delayed healing in venous leg ulcers: a review of the literature. Int J Clin Pract 2015; 69: 967–977. DOI: 10.1111/ijcp.12635.
11. Rajhathy EM, Murray HD, Roberge VA, Woo KY. Healing Rates of Venous Leg Ulcers Managed With Compression Therapy: A Secondary Analysis of Data. J Wound Ostomy Continence Nurs 2020; 47: 477–483. DOI: 10.1097/WON.0000000000000693.
12. Berenguer Pérez M, López-Casanova P, Sarabia Lavín R, González de la Torre H, Verdú-Soriano J. Epidemiology of venous leg ulcers in primary health care: Incidence and prevalence in a health centre-A time series study (2010–2014). Int Wound J 2019; 16: 256–265. DOI: 10.1111/iwj.13026.
13. Moffatt CJ, Franks PJ, Doherty DC, Smithdale R, Martin R. Sociodemographic factors in chronic leg ulceration. Br J Dermatol 2006; 155: 307–312. DOI: 10.1111/j.1365-2133.2006.07265.x.
14. Szewczyk MT, Jawień A, Cierzniakowska K, Cwajda-Białasik J, Mościcka P. Comparison of the effectiveness of compression stockings and layer compression systems in venous ulceration treatment. Arch Med Sci 2010; 6: 793–799. DOI: 10.5114/aoms.2010.17097.
15. Mościcka P, Cwajda-Białasik J, Jawień A, Jaraczewski W, Szewczyk MT. Evaluation of factors affecting the healing process of venous
P. Mościcka, K. Cierzniakowska, J. Cwajda-Białasik
i wsp.
ulcers: A 12-week longitudinal study. Wound Repair Regen 2023; 31: 783–792. DOI: 10.1111/wrr.13140.
16. Abbade LP, Lastória S, de Almeida Rollo H, Stolf HO. A sociodemographic, clinical study of patients with venous ulcer. Int J Dermatol 2005; 44: 989–992. DOI: 10.1111/j.1365-4632.2004.02276.x.
17. Finlayson K, Edwards H, Courtney M. Factors associated with recurrence of venous leg ulcers: a survey and retrospective chart review. Int J Nurs Stud 2009; 46: 1071–1078. DOI: 10.1016/j.ijnurstu.2008.12.012.
18. Mościcka P, Szewczyk MT, Jawień A, Cierzniakowska K, Cwajda-Białasik J. Subjective and objective assessment of patients’ compression therapy skills as a predicator of ulcer recurrence. J Clin Nurs 2016; 25: 1969–1976. DOI: 10.1111/jocn.13218.
19. Nelson EA, Bell-Syer SE. Compression for preventing recurrence of venous ulcers. Cochrane Database Syst Rev 2014; 2014: CD002303. DOI: 10.1002/14651858.CD002303.pub3.
20. Porter JM, Moneta GL. Reporting standards in venous disease: an update. International Consensus Committee on Chronic Venous Disease. J Vasc Surg 1995; 21: 635–645. DOI: 10.1016/s0741-5214(95)70195-8.
21. Meissner MH, Natiello C, Nicholls SC. Performance characteristics of the venous clinical severity score. J Vasc Surg 2002; 36: 889–895. DOI: 10.1067/mva.2002.128637.
22. Villalta S, Bagatella P, Piccioli A, Lensing AW, Prins MH, Prandoni P. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis 1994; 24: 158a (abstract).
23. Lurie F, Passman M, Meisner M i wsp. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord 2020; 8: 342–352. DOI:10.1016/j.jvsv.2019.12.075.
24. Mościcka P. The most commonly used scales for assessing patients with chronic venous disease and ulcers. Pielęg Chir Angiol 2025; 19: 83–91. DOI: 10.5114/pchia.2025.154452.
25. Kudlová P, Kočvarová I, Pelikán A. Compression therapy in the prevention and treatment of venous diseases – a cross-sectional study. Pielęg Chir Angiol 2023; 17: 203–210. DOI: 10.5114/pchia.2023.134525.
26. Mościcka P, Cwajda-Białasik J, Jawień A, Szewczyk MT. Complex treatment of venous leg ulcers including the use of oral nutritional supplementation: results of 12-week prospective study. Postepy Dermatol Alergol 2022; 39: 336–346. DOI: 10.5114/ ada.2021.104730.
27. Mościcka P, Czaplicka A, Szewczyk MT. Żywienie w procesie gojenia ran – praktyczne algorytmy postępowania. W: Interdyscyplinarne algorytmy postępowania, w gabinecie lekarza specjalisty. Interna, neurologia, chirurgia, medycyna rodzinna, gastroenterologia. Wydawnictwo Lekarskie PZWL, Warszawa 2025; 61–86. DOI: 10.53271/ iap.2025.1.2.
28. Coleridge-Smith P, Labropoulos N, Partsch H, Myers K, Nicolaides A, Cavezzi A. Duplex ultrasound investigation of the veins in chronic venous disease of the lower limbs – UIP consensus document. Part I. Basic principles. Eur J Vasc Endovasc Surg 2006; 31: 83–92. DOI: 10.1016/j.ejvs.2005.07.019.
29. Hedayati N, Carson JG, Chi YW, Link D. Management of mixed arterial venous lower extremity ulceration: A review. Vasc Med 2015; 20: 479–486. DOI: 10.1177/1358863X15594683.
30. Hess CT. Venous Ulcer Assessment and Management: Using the Updated CEAP Classification System. Adv Skin Wound Care 2020; 33: 614–615. DOI: 10.1097/01.ASW.0000719052.33284.73.
31. Cierzniakowska K, Szewczyk MT, Kozłowska E i wsp. Wskaźnik kostka–ramię – efektywna diagnoza chorób tętnic obwodowych. Pielęg Chir Angiol 2016; 1: 26–33.
32. Eklöf B, Rutherford RB, Bergan JJ i wsp. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg 2004; 40: 1248–1252. DOI: 10.1016/j.jvs.2004.09.027.
33. Rutherford RB, Padberg FT Jr, Comerota AJ, Kistner RL, Meissner MH, Moneta GL. Venous severity scoring: An adjunct to venous outcome assessment. J Vasc Surg 2000; 31: 1307–1312. DOI: 10.1067/ mva.2000.107094.
34. Vasquez MA, Rabe E, McLafferty RB i wsp. Revision of the venous clinical severity score: venous outcomes consensus statement: special communication of the American Venous Forum Ad Hoc Outcomes Working Group. J Vasc Surg 2010; 52: 1387–1396. DOI: 10.1016/j.jvs.2010.06.161.
35. Vasquez MA, Munschauer CE. Venous Clinical Severity Score and quality-of-life assessment tools: application to vein practice. Phlebology 2008; 23: 259–275. DOI: 10.1258/phleb.2008.008018.
36. Villalta S, Bagatella P, Piccioli A, Lensing AW, Prins MH, Prandoni P. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis 1994; 24 (3): 158a (abstract).
37. Prandoni P, Villalta S, Bagatella P i wsp. The clinical course of deep-vein thrombosis. Prospective long-term follow-up of 528 symptomatic patients. Haematologica 1997; 82: 423–428.
38. Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of post-thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thromb Haemost 2009; 7: 879–883. DOI: 10.1111/j.1538-7836.2009.03294.x.
39. Soosainathan A, Moore HM, Gohel MS, Davies AH. Scoring systems for the post-thrombotic syndrome. J Vasc Surg 2013; 57: 254–261. DOI: 10.1016/j.jvs.2012.09.011.
40. Lee AJ, Robertson LA, Boghossian S, Allan PL, Ruckley CV, Fowkes FGR. Performance of two clinical scales to assess quality of life in chronic venous disease: correlation of the VCSS and Villalta with disease-specific quality-of-life measures. Phlebology 2021; 36: 580–588. DOI: 10.1177/0268355520955374.
41. Ning J, Ma W, Fish J, Trihn F, Lurie F. Biases of Villalta scale in classifying post-thrombotic syndrome in patients with pre-existing chronic venous disease. J Vasc Surg Venous Lymphat Disord 2020; 8: 1025–1030. DOI: 10.1016/j.jvsv.2020.01.018.
Opis przypadku | Case report
LECZENIE RAN 2025; 22 (3): 115-121
DOI: https://doi.org/10.60075/lr.v22i3.112
The use of split-thickness skin grafts and negative pressure wound therapy in the treatment of lower limb frostbite
Jakub Boryczko 1,2, Bogusław Strzałko1, Dariusz Bazaliński 1,3
1 Specialist Hospital Podkarpackie Oncology Center, Brzozow, Poland
2 Jan Grodek State University in Sanok, Poland
3 Faculty of Health Sciences and Psychology, University of Rzeszow, Poland
Abstract
Introduction: Soft tissue damage in the form of difficult-to-heal wounds is a common complication of frostbite of the distal parts of the body. Changes in the tissues impair microcirculation and disrupt regenerative processes. Selecting the appropriate treatment based on physical assessment and additional specialist tests improves the patient’s condition, reduces the number of complications, and speeds up recovery.
Aim of the study: To present the treatment process for frostbite of the lower limbs resulting from exposure to adverse weather conditions.
Material and methods: A case study method was used in this study. The study design was based on the CARE guidelines. A 64-year-old woman who was being treated for paranoid schizophrenia presented with third-degree frostbite on her shins and feet. Due to her refusal to undergo amputation, necrotic tissue was excised several times, free split-thickness skin grafts were performed, and negative pressure
Introduction
Frostbite is an injury to the soft tissues caused by exposure to low temperatures. Tissue damage can occur when exposed to temperatures below –0.5°C. Frostbite occurs when exposure is long enough for the water in the tissues to form ice crystals, which result in frostbite injuries [1]. Injuries that do not involve the formation of ice crystals (non-freezing cold injuries) are also classified. Functional changes that result in non-freezing tissue damage, both local and general, are caused by low ambient temperatures. Non-freezing injuries are referred to as chilblains, also known as frostbite, and hypothermia can occur when exposed to atmospheric conditions with temperatures between 0 and 16°C, usually in a cold, dry, or humid environment for several hours. Exposure to low temperatures can lead to systemic hypothermia,
Address for correspondence
Jakub Boryczko, Szpital Specjalistyczny Podkarpacki Ośrodek Onkologiczny, Brzozow, 18 Księdza Józefa Bielawskiego St., 36-200 Brzozow, Poland, e-mail: jakub.boryczko@icloud.com
Received: 11.08.2025 Accepted: 23.09.2025
wound therapy (NPWT) was applied. The treatment resulted in a good therapeutic effect and recovery.
Results: The treatment and care measures were aimed at controlling the developing infection, managing the wound professionally, restoring repair processes, restoring skin continuity, and minimising pain. The entire therapeutic process took place at the General Surgery Department in an inpatient setting. Controlling the infection through empirical and targeted antibiotic therapy, as well as excising the necrotic tissue that was the source of the infection, allowed the wound to be debrided and prepared for skin grafting. NPWT accelerated the healing process. Intensive rehabilitation and early mobilisation allowed the patient to regain function in her lower limbs.
Conclusions: The use of free skin grafts and NPWT in the treatment of frostbite wounds shortens the hospital stay and accelerates the healing process.
Key words: skin graft, negative pressure wound therapy, injury, wound treatment, frostbite
which poses a risk of serious health consequences [2]. The process of cooling the skin causes the blood vessels to constrict and then dilate periodically. When the skin is rewarmed, the injury leads to inflammation, constriction of blood vessels, thrombosis, vascular obstruction, blistering, and ultimately tissue damage and necrosis [3]. Most thermal injuries can be avoided by wearing appropriate clothing and limiting exposure to low temperatures. For people who have suffered tissue frostbite, early intervention to prevent tissue damage is essential. The treatment process is lengthy. Patients with extensive frostbite require long-term professional care. In some cases, amputation is necessary. Frostbite is associated with numerous risk factors. The most significant ones are homelessness and low socioeconomic status, alcohol abuse, smoking, mental disorders, and addiction to
J. Boryczko, B. Strzałko, D. Bazaliński
psychoactive substances. Personal factors have also been identified, including inadequate or inappropriate winter clothing, delay in seeking medical assistance, and lack of knowledge about how to behave in cold conditions [4]. Alcohol and other psychoactive substance abuse are among the factors that increase the risk of amputation [5]. Complications of frostbite place a significant burden on the healthcare system due to costly procedures and long hospital stays [6].
In Central Europe, low temperatures cause about 35 times fewer thermal injuries than burns. Their treatment is not standardised, and there is no algorithm for managing patients who have suffered frostbite. Therefore, it is largely based on case reports, clinical observations, and experience from other centres. The treatment of injuries caused by low temperatures depends on the type and severity of the injury, as well as the time of treatment initiation. In Europe, this group of patients is treated in burn units and surgical wards [7].
Prolonged healing of frostbite wounds resulting from progressive inflammation and tissue ischemia leads to extensive scarring and limited joint mobility. This causes significant physical impairment and reduces patients’ quality of life. Secondary malignant tumours may also develop as a result. Consequently, modern wound treatment methods are of great importance [8].
This study aims to present treatment methods for hard-to-heal wounds caused by low temperatures.
Admission to hospital
• Antithrombotic ptophylaxis
• Blood transfusion
• Emprical antibiotic therapy
• targeted antibiotic therapy
• Pain managment
• Specialists consultations
• Physical rehabilitation
• Local treatment of frostbite wounds (wound hygiene, specialized designs)
Hospital discharge
Outpatient treatment
Material and methods
The case study method was used. The study design was based on the CARE guidelines. The patient was a 64-year-old woman treated for paranoid schizophrenia, who also had third-degree frostbite of the shins and feet. In the absence of consent for amputation, necrotic tissue was excised several times, free split-thickness skin grafts were performed, and negative pressure wound therapy (NPWT) was applied. The treatment resulted in a positive therapeutic outcome and recovery. Observation, interview, and a nalysis of medical records were conducted in a hospital setting from January 2025 to April 2025. During this time, necrotic tissue was repeatedly removed, skin grafts were performed, and NPWT was administered. Systemic treatment was also used to control the developing infection, prevent thrombosis, and provide multimodal pain management. The head of the department authorised the study, and the patient consented to the publication of photos upon discharge. The entire research process was conducted in accordance with the guidelines of the Declaration of Helsinki [9]. The treatment process is presented in Figure 1.
Case description
A 64-year-old woman, previously independent in terms of self-care and self-support, diagnosed with paranoid schizophrenia, was admitted after being transferred by the Emergency Medical Services on 5 January 2025 to the Specialist Hospital in Brzozow,
Third-degree frostbite of the lower limbs
Diagnostic tests; conservative treatment
Necrectomy and fasciotomy
Necrectomy
Necrectomy
Necrectomy, sampling material for microbiological examination
Necrectomy
Split-thickness skin graft (left and right lower leg), Necrectomy (left lower leg and foot)
Split-thickness skin graft ( left and right lower leg)
Negative pressure wound therapy
Figure 1. Course of the patient’s treatment (own work)
HEALING OF FROSTBITE WOUNDS
Figure 2. Frostbite of the feet and lower legs on admission to hospital, tissue swelling and undermining, incomplete and full-thickness skin wounds, hyperalgesia, local treatment with PVP-I-soaked Hydrofiber dressing plus emollient
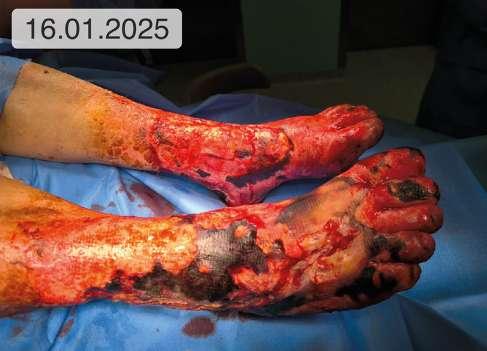
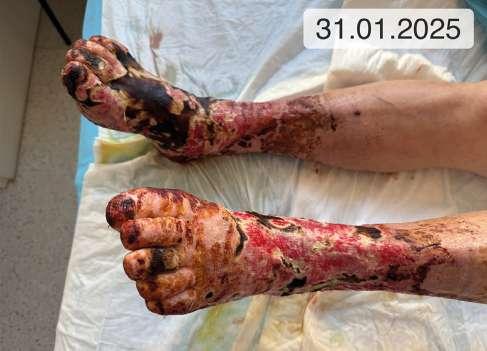
General Surgery Ward, due to third-degree frostbite of both shins and feet. The patient reported walking in the snow for several hours in low temperatures without shoes. Upon admission, she was alert, oriented to self, and allopsychic, exhibiting inappropriate affect and a depressed mood. A local examination revealed cold limbs with damaged skin on the feet and the lower parts of both shins. The tissue damage covered approximately 18% of the total body surface area. The pulse was weak, and sensation was impaired with signs of hyperalgesia. The tissue damage was initially classified as second- to third-degree frostbite (Fig. 2). There was a full-thickness skin wound on the right foot suggesting ulceration. Initial treatment was administered, and tissue biopsies were taken for microbiological evaluation. Laboratory tests showed signs of inflammation: C-reactive protein (CRP): 9.01 (0–5 mg/l), procalcitonin (PCT): 840 (150–400; 10³/
mm³), anaemia: hemoglobin (HGB): 7.4 g/dl (13.7–16.5 g/dl), and hematocrit (HCT): 22.2% (40.1–51.0%). The patient received 2 units of red blood cell concentrate, and the blood morphology normalised: HGB: 11.5 g/dl (13.7–16.5 g/dl), HCT: 35.4% (40.1–51.0%). The patient received observation, multimodal pain management, and local wound care. Over the next few days, conversion associated with deepening tissue damage, and spreading necrosis was observed. Doppler ultrasound of the lower extremities confirmed preserved vascular flow in the peripheral arteries. On the fifth day of hospitalisation, an increase in inflammatory parameters (CRP: 296 mg/l) was observed due to a developing infection originating from necrotic tissue in the lower legs and feet. Empirical antibiotic therapy was initiated, involving amoxicillin with clavulanic acid. Amputation of both lower limbs was proposed on life-saving grounds, pointing to critical skin
Figure 3. Conversion of frostbite changes on the 5th day of hospitalisation within the skin and subcutaneous tissue, and blisters associated with separation of the epidermis
Figure 4. Necrectomy in the operating room on day 11 of hospitalisation
Figure 5. Frostbite of the lower limbs – wounds on day 26 of hospitalisation after cleansing with antiseptic preparations
the patient maintained her position regarding her refusal to undergo surgery.
The patient was referred to the operating room for a necrectomy and fasciotomy due to life-threatening fascial compartment syndrome in both lower limbs (Fig. 3). The necrotic tissue was removed, exposing the healthy muscle. A microbiological examination of the tissue biopsy revealed the presence of the following aerobic bacteria: Escherichia coli, Providencia rettgeri, Acinetobacter baumannii, and Enterococcus faecalis Based on the antibiogram, targeted treatment with cloxacillin and cotrimoxazole was initiated. In the days that followed, a drop in inflammatory markers was seen (CRP: 173 mg/l [0–5 mg/l] on the third day after surgery and 143 mg/l [0–5 mg/l] on the fifth day).
On day 11 of hospitalisation (Fig. 4), further excision of necrotic tissue was performed in the operating room, and material was collected again for microbiological examination. Follow-up laboratory tests performed on day 13 of hospitalisation revealed a decrease in CRP to 113 mg/l (normal range: 0-5 mg/l).
On day 15 of hospitalisation, further removal of necrotic tissue was performed in the operating room. Systematic removal of necrotic tissue and targeted antibiotic therapy resulted in a decrease in acute phase protein concentrations. Follow-up tests revealed a subsequent decrease in CRP levels, from 55 mg/l to 34 mg/l. Follow-up blood cultures were also performed with negative results. Additional tests were performed as part of further diagnostics, confirming a positive test result for Borrelia burgdorferi bacteria. A neurologist consulted the patient and additionally diagnosed neuroborreliosis.
On day 30 of hospitalisation (Fig. 5), a second necrectomy was performed in the operating room, and tissue samples were collected for microbiological examination. These samples yielded cultures of Staphylococcus aureus and Acinetobacter baumannii strains. Because both strains were sensitive to gentamicin, intravenous gentamicin was administered while monitoring the gentamicin concentration in the blood. The ward systematically changed polyhexanide and povidone-iodine dressings, as well as paraffin mesh dressings. Gradual cleansing of the frostbite wounds was achieved.
damage and the risk of sepsis. However, the patient did not consent to this procedure. The consulting psychiatrist found her to be fully conscious and capable of making decisions. Over the next few days,
On 10 February 2025 a free split-thickness skin graft was placed on the left shin and ankle area (Fig. 6) using a 1 : 3 mesh. The graft was secured with 3-0 absorbable sutures. Both the grafted mesh and the donor site were covered with a paraffin dressing. Another necrectomy was performed on the right lower limb and secured with an iodine-povidone dressing and
J. Boryczko, B. Strzałko, D. Bazaliński
Figure 6. Free split-thickness skin graft with 1 : 3 mesh placed on the left lower leg
Figure 7. Skin graft on the left lower leg on postoperative day
Figure 8. Healed wounds after frostbite of the lower limbs during an outpatient follow-up 5 weeks after the end of hospital treatment
The use of split-thickness skin grafts and negative pressure wound therapy in the treatment of lower limb frostbite – case study
antiseptic gel containing surfactant. There were no problems with wound healing at the donor site (thigh). During hospitalisation, the patient underwent physical rehabilitation. Due to anaemia (HGB: 8 g/dl [normal range: 13.7–16.5 g/dl]; HCT: 26.1% [normal range: 40.1–51.0%]), 2 more units of red blood cell concentrate were transfused.
On 25 February 2025, additional split-thickness skin grafts were performed (Fig. 7). A 0.3-mm-thick skin flap was harvested from the donor site on the left thigh and placed on the left foot. Two flaps from the donor site on the right thigh were placed on the dorsal part of the right foot and the right lower leg. The grafts and donor sites were secured with a paraffin-impregnated mesh dressing.
A follow-up microbiological examination (biopsy from the wound) was performed on 28 February 2025. The following microorganisms were cultured: E. coli, S. aureus, A. baumannii, E. faecalis, P. multocida , and B. fragilis. The patient continued to receive targeted antibiotic therapy, which led to a decrease in CRP concentration to 20.8 mg/l. Physical rehabilitation and verticalisation of the patient were also continued. A psychological consultation was also performed. Over the next few days, NPWT at 80–120 mmHg was administered, which resulted in the skin grafts healing satisfactorily.
On 20 March 2025, the patient was discharged to a nursing home in satisfactory general and local condition to receive further outpatient treatment at the hospital’s Wound Treatment Clinic (Fig. 8).
Discussion
The presented case of a patient with difficult-to-heal wounds resulting from thermal injury to the lower limbs demonstrates a multidirectional and time-consuming therapeutic process that is supervised by an interdisciplinary team of specialists. The need to take the risk of local treatment and provide holistic care created opportunities for radical measures, which resulted in therapeutic success. The measures taken prevented amputation, and local treatment was carried out in accordance with the Polish Wound Treatment Society’s current guidelines. In addition to measures aimed at restoring function, the treatment included the TIMERS strategy, wound hygiene, and infection prevention with minimal antibiotic therapy [10, 11].
Frostbite of the lower limbs is associated with a significant risk of amputation, which increases with the area and depth of tissue damage [12]. Additionally, soft tissue oedema resulting from inflammatory
mediators, vasomotor disorders, and thrombotic changes can cause compartment syndrome. This condition can lead to secondary acute ischaemia and amputation of the limb [13].
The patient refused to consent to the amputation of her limbs as proposed by the doctors on duty in the general surgery ward, so the initial course of treatment was determined by this refusal. This decision was made based on the condition of the limbs, but also on the potential difficulties associated with self-care and making rational decisions related to the course of mental illness. Given the situation, conservative treatment was initiated during the first days of hospitalisation to control the developing infection and treat the frostbite wounds. During this period, additional tests were performed to evaluate blood flow to the limbs. On day 5 of hospitalisation, with the support of a psychiatrist and basic psychotherapy, the patient consented to a necrectomy in the operating room. Due to the risk of fascial compartment syndrome caused by severe soft tissue oedema, fasciotomy was also performed.
During the subsequent days of hospitalisation, additional necrosectomies were performed in the operating room to gradually eliminate the source of infection. Targeted antibiotic therapy was also administered. Microbiological assessment of the wound is important for controlling the infection. Due to the colonisation of the wound with bacteria, it is recommended that the appearance of the wound be observed for signs of local infection. The use of moist therapy in conjunction with autolytic wound cleansing was avoided due to the potential risk of infection from non-demarcated dead tissue. The use of PVP-I preparations allowed the wound to be maintained without liquefied necrotic tissue and reduced the duration of antibiotic therapy.
Proper collection of materials for microbiological testing and analysis of the antibiogram are essential for microbiological diagnosis and selecting the appropriate antibiotic [10]. Targeted antibiotic therapy was used during treatment until markers of inflammation decreased to levels that did not pose a risk of systemic infection. This therapy was combined with local wound treatment, including irrigation with antiseptics and lavaseptics, scraping, and specialised dressings containing polyhexamethylene biguanide and iodopovidone, which penetrates deeply into tissues and exhibits effective antimicrobial activity [8].
The treatment significantly improved the local condition and decreased the concentration of acute
J. Boryczko, B. Strzałko, D. Bazaliński
phase proteins. Rapid repair processes and gradual formation of granulation tissue were observed in the wounds. In the next stage of treatment, free split-thickness skin grafts were used to cover the patient’s frostbite wounds. The grafts were taken from donor sites on the thighs. Before a skin graft can be performed, the wound must be properly prepared. In addition to preparing the granulating wound bed, which increases the chances of graft acceptance, it is important to treat the wound edges. One way to do this is to excise the wound edges before the graft, which causes the release of cytokines and inflammatory mediators. Additionally, the wound must be free of microorganisms and necrotic tissue that blocks the ingrowth of blood vessels into the graft. Skin grafts currently play a key role in modern wound treatment and tissue regeneration. Although autologous split-thickness skin grafts (STSGs) are considered the gold standard for treating chronic limb ulcers due to their safety and efficacy, their use may be limited in practice by patient factors such as fear of failure, larger wound formation, reluctance to be hospitalised, limited access to professional care, and insufficient knowledge about the method [13]. Proper wound preparation for skin grafting requires optimisation of all patient-related factors and allows for favourable outcomes [14].
The next stage of treatment involved the use of local NPWT. Negative pressure wound therapy is a safe and effective treatment method that promotes skin graft healing more effectively than traditional dressings [15]. Negative pressure achieves an 8.3% higher overall graft acceptance rate, and using the optimal value of –80 mmHg increases this rate by 10% [16]. Using controlled negative pressure reduces the risk of graft rejection and reoperation, as well as reducing treatment costs by shortening hospital stays and decreasing complications. Additionally, skin grafts are well reimbursed by insurers, and the overall hospitalisation cost is less than that of amputations [17].
In summary, the positive therapeutic outcome was achieved through the use of appropriately selected debridement methods, fasciotomy, multistage necrectomy, skin grafts, NPWT, and wound management concepts, as well as the selection of specialised dressings according to current guidelines and adaptation to the patient’s preferences and comfort. Thanks to the interdisciplinary team’s efforts, the patient regained her mobility.
Conclusions
Free split-thickness skin grafts significantly shorten the healing time of frostbite wounds in the lower extremities. The use of local NPWT accelerates the ingrowth of the grafted epidermis. The treatment in question has been shown to enhance patients’ quality of life and reduce wound healing time, leading to an overall reduction in treatment costs.
Disclosures
The authors declare no conflict of unterest. This research received no external funding. Approval of the Bioethics Committee was not required.
References
1. Loren Lorentzen AK, Davis C, Penninga L. Interventions for frostbite injuries. Cochrane Database Syst Rev 2020; 12: CD012980. DOI: 10.1002/14651858.CD012980.pub2.
2. Regli IB, Strapazzon G, Falla M, Oberhammer R, Brugger H. LongTerm Sequelae of Frostbite-A Scoping Review. Int J Environ Res Public Health 2021; 18: 9655. DOI: 10.3390/ijerph18189655.
3. Wroñski K, Bocian R. Treatment of frostbites. Pielęg Chir Angiol 2010; 4: 114–118.
4. Essien SK, Chireh B, Steinberg C, Omondi P, Zucker-Levin A. Psychosocial and personal predisposing factors of frostbite injury and associated amputation: a systematic review. Inj Epidemiol 2024; 11: 62. DOI: 10.1186/s40621-024-00546-w.
5. Dow J. Cold Injury. Emerg Med Clin North Am 2024; 42: 513–525. DOI: 10.1016/j.emc.2024.02.012.
6. Endorf FW, Alapati D, Xiong Y, et al. Biopsychosocial factors associated with complications in patients with frostbite. Medicine (Baltimore) 2022; 101: e30211. DOI: 10.1097/MD.0000000000030211.
7. Sachs C, Lehnhardt M, Daigeler A, Goertz O. The Triaging and Treatment of Cold-Induced Injuries. Dtsch Arztebl Int 2015; 112: 741–747. DOI: 10.3238/arztebl.2015.0741.
8. Wang W, Liu P, Zhu W, et al. Skin organoid transplantation promotes tissue repair with scarless in frostbite. Protein Cell 2025; 16: 240–259. DOI: 10.1093/procel/pwae055.
9. World Medical Association. Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. Available at: https://www.wma.net/policies-post/wma-declaration-of-hel-sinki-ethical-principles-for-medical-research-involving-human-subjects/ (Access: June 15, 2025).
10. Sopata M, Jawieñ A, Mrozikiewicz-Rakowska B, et al. Guidelines for local management of uninfected, infection-prone, and infected wounds – a review of available antimicrobial agents used in wound treatment. Recommendations of the Polish Wound Treatment Society. Leczenie Ran 2020; 17: 1–21.
11. Moœcicka P, Cwajda-Bia³asik J, Jawieñ A, et al. Wound hygiene in the care of patients with lower limb ulceration. A description of three clinical cases. Leczenie Ran 2021; 18: 57–64. DOI: 10.5114/ lr.2021.107150.
12. Menon V, Richards L, Walter C, et al. Patient and treatment characteristics that predict symptom resolution and limb amputation in patients with frostbite. Burns 2025; 51: 107547. DOI: 10.1016/j. burns.2025.107547.
13. Serra R, Rizzuto A, Rossi A, et al. Skin grafting for the treatment of chronic leg ulcers - a systematic review in evidence-based medicine. Int Wound J 2017; 14: 149–157. DOI: 10.1111/iwj.12575.
The use of split-thickness skin grafts and negative pressure wound therapy in the treatment of lower limb frostbite – case study
14. Brandão RA, St John JM, Langan TM, Schneekloth BJ, Burns PR. Acute Compartment Syndrome of the Foot Due To Frostbite: Literature Review and Case Report. J Foot Ankle Surg 2018; 57: 382–387. DOI: 10.1053/j.jfas.2017.07.005.
15. Gorka R. Preparation for a Successful Skin Grafting. In: Skin Grafts for Successful Wound Closure [Internet]. IntechOpen 2022.
16. Lee SC, Bayan L, Sato A, et al. Benefits of negative pressure wound therapy in skin grafts: A systematic review and meta-analysis of
randomised controlled trials. J Plast Reconstr Aesthet Surg 2025; 102: 204–217. DOI: 10.1016/j.bjps.2025.01.03.
17. Ker H, Al-Murrani A, Rolfe G, Martin R. WOUND Study: A CostUtility Analysis of Negative Pressure Wound Therapy After Split-Skin Grafting for Lower Limb Skin Cancer. J Surg Res 2019; 235: 308–314. DOI: 10.1016/j.jss.2018.10.016.
Case report | Opis przypadku
LECZENIE RAN 2025; 22 (3): 122-126
DOI: https://doi.org/10.60075/lr.v22i3.117
Klingsor syndrome as a rare case of self-amputation of the penis
Piotr Wojda
1 Psychiatric Clinic, Cologne, Germany
Abstract
Genital self-mutilation (GSM) is rare and a serious condition requiring multidisciplinary treatment. Life-saving measures (heavy bleeding) should be initiated first, followed by efforts to restore anatomical continuity and functionality of the damaged structures as much as possible. Concurrently, psychiatric disorders must be treated, wounds appropriately dressed, and support provided to the patient. Total penectomy is associated with long-term deterioration in the patient‘s quality of life.
This article presents the case of a patient who self-mutilated his genitals as a result of Klingsor syndrome. The aim of the study is to present the patient‘s health problems based
Introduction
Klingsor syndrome is the deliberate self-mutilation of the genital organs (GSM) in the course of a mental illness, especially psychosis, without suicidal intent [1].
The literature identifies three groups at risk for genital self-mutilation: patients with schizophrenia, transvestites, and men experiencing religious or cultural conflicts [2]. Over 90% of patients who self-mutilated their genital organs had a mental disorder, including 49% with schizophrenia spectrum disorders, substance use disorders such as marijuana or cocaine (19%), personality disorders (16%), gender dysphoria (15%), depression (9%), and bipolar disorder (1%) [1]. The incidence of psychiatric pathologies such as depression, anxiety, and mood disorders is also higher in amputees than in the general population [3]. Self-amputations in those without a psychiatric diagnosis (approximately 9–13%) are attributable to GSM, including sexual conflicts, guilt related to sexual desires, religious beliefs, and unconventional forms of sexual arousal [1,3].
In addition to the risk of infection and anatomical changes, GSM may be associated with an increased risk of suicide, repeated self-harm, and greater severity of psychopathology [1, 3]. It is estimated that
Address for correspondence
Psychiatric Clinic, Wilhelm Grisinger Str. 23, Cologne, Germany, e-mail: askot8wp.pl Received: 25.09.2025 Accepted: 12.11.2025
on the NANDA nursing diagnoses classification. Patient care should be focused on the patient‘s needs, and staff should be provided with current, evidence-based nursing knowledge. A holistic approach to the patient‘s problems and the implementation of nursing interventions in accordance with current knowledge prevented complications related to self-harm and positively influenced the improvement of his mental state. Proper wound care is one element of multidimensional care for patients with Klingsor syndrome, but nurses should also recognize other issues arising from the disease.
Key words: Klingsor syndrome, penile self-amputation, wound care, psychiatric nursing.
55–85% of people who self-harm attempt suicide [3]. One of problems may be difficulties in caring for wounds after genital amputation. Genital wounds are associated with an increased risk of infection, difficulty maintaining a wound dressing, and pain. Genital wounds heal rapidly, with significant swelling and absent or barely visible scarring, which is related to the anatomical and physiological differences in this region (lack of subcutaneous fat, high mobility of the skin relative to underlying tissues, abundant elastic fibers, persistent bacterial colonization, and rapid resolution of inflammation) [4]. Genital skin defects can be managed using a variety of techniques, including primary closure, local flaps, full-thickness skin grafts, and split-thickness skin grafts [5]. Perineal skin grafting carries the risk of graft rejection due to infection, difficulty in stabilizing the graft due to genital mobility, and fluid accumulation in the form of blood, serous, and purulent exudate. For penile reconstruction (if the amputated portion is inaccessible or not suitable for reimplantation), myoplasty (possible sexual satisfaction) or myocutaneous flap grafting with prosthesis implantation are used [5]. Donor penile transplantation with immunosuppressive therapy
is also an option. Another viable treatment option is allogeneic penile transplantation [6].
The aim of the study is to present the patient‘s health problems based on the NANDA nursing diagnoses classification. For this purpose, the patient‘s condition was assessed, nursing diagnoses were made, nursing interventions were implemented, and their effectiveness was evaluated.
Case presentation
A 40-year-old patient, single, living with his family and unemployed, was admitted from the intensive care unit to a closed psychiatric ward after self-amputation of his penis during cannabinoid-induced psychosis (Fig. 1). The patient underwent urological treatment, which included dressing the injury and inserting a urinary catheter. Penile reconstruction following amputation was not possible. The testicles were preserved. By self-harming his genitals, the patient wanted to prevent a threat to his family from strangers in the home (he heard male and female voices). The patient was hospitalized numerous times for mental disorders caused by psychoactive substances (1 g of marijuana per day), which he had been taking since the age of 16 years. In 2007, the patient, under the influence of drugs, attempted suicide by self-injury to his wrists. Since 2017, the
patient has been under the care of a mental health clinic for mood disorders and obsessive-compulsive disorder. The patient currently complains of a depressed mood. He denies auditory hallucinations in the form of voices and distances himself from suicidal intentions. After a psychiatric examination, the diagnosis was: „mental and behavioral disorders caused by marijuana use”.
A physical examination revealed no abnormalities, except being slightly overweight (BMI = 25). Vital signs were normal, skin was pale, anemia was mild, and there were no allergies. Marijuana was detected in the urine. The following medications were taken venlafaxine 225 mg/day, quetiapine 50 mg/day, atorvastatin 10 mg/day, and ibuprofen 600 mg four times daily. Adjunctive treatment included individual psychotherapy, occupational therapy, and consultations with a sexologist.
Initial wound inspection: The amputation wound was oval (2.3 cm × 2 cm) and showed no signs of infection, and the scrotal postoperative wound was 8 cm long with visible surgical sutures, without signs of infection. The amputation wound had minimal exudate, and the wound edges were sunken, with visible surgical sutures securing the skin to the penile stump. The wounds were dressed in adhesive foam dressings, over which the patient wore normal underwear. Medical records from the previous hospitalization did not contain information about wound care or dressings. The patient was assessed for risk of wound infection (WAR scale), pain intensity (Numerical Rating Scale – NRS), nutritional status (Mini Nutritional Assessment – MNA), and willingness to participate in wound care. The patient did not complain of pain during dressing changes.
Wound care: The amputation wound was disinfected with a sterile gauze pad soaked in Octenisept disinfectant and left on the wound for 1 minute. The wound was dressed with a 5 × 7 cm non-adhesive Biatain foam dressing and secured with mesh underwear. This dressing was used for the entire patient‘s stay (5 days). Due to the risk of skin trauma, plasters were not used. The dressing was changed daily. The scrotal wound was disinfected with Octenisept and secured with sterile gauze pads. To optimize wound healing, increased caloric, protein, and trace element intake was introduced. After 5 days of stay, the patient was discharged at his request. He received a referral to the wound care outpatient clinic, along with information about his previous wound care
Fig. 1. Wound after self-amputation of the penis
and psychiatric outpatient clinic. It is unknown whether the patient complied with these recommendations after discharge.
Bioethical standards
The patient provided informed consent for publication. Institutional ethical review was not required in accordance with local case-control policy (Germany).
Discussion
A patient with a self-inflicted wound required multidisciplinary care. Initially, steps were taken to ensure the patient‘s safety and prevent further selfharm or suicide. The next challenge was to create optimal conditions for wound healing by preventing infection, providing appropriate wound care, and meeting the increased nutritional needs. Correctly securing and maintaining the dressing in place posed a challenge. The patient was active, and the anatomical location of the wound made it difficult to fit and adhere the dressing properly. The use of plasters was abandoned due to the risk of damaging the sensitive skin. The use of elastic mesh underwear, which conformed to the body‘s shape and stabilized the dressing, resolved this issue. The patient cooperated readily during dressing changes and adhered to wound care and hygiene recommendations. After the injury, the patient experienced psychosocial difficulties, manifesting as concerns about conforming to the cultural image of a man. This required the implementation of long-term individual therapy, sexological counseling, and rehabilitation, as well as tact and sensitivity in daily interactions. The patient refused some of the therapies offered to him (mirror therapy). He found relaxation therapy particularly helpful. Psychiatric treatment was also initiated with continued care in specialized outpatient clinics, depending on the specific problem. Penectomy-related complications reach 19.7%, with wound infections accounting for 3% and urinary tract infections (UTIs) 3% [13]. Wound infections, malnutrition, and failure to meet the increased demand for energy, macronutrients, micronutrients, and vitamins can lead to an increased risk of wound infection and delayed healing [14, 15]. Nursing interventions implemented in this case effectively prevented the development of a wound infection and supported the healing process.
The patient was catheterized for a long time to divert urine and reduce the risk of wound infection.
Conversely, bladder catheterization carries a higher risk of urinary tract infections. It is estimated that 20% of urinary tract obstructions are catheter-related [16]. Compliance with recommendations and patient education regarding catheter-related self-harm did not lead to urinary tract infections. Patients who self-harm are at increased risk of suicide. Evidence demonstrating the effectiveness of structured risk assessment, safety planning, and therapeutic communication underscores the crucial role of nursing in suicide prevention [17].
The loss of his penis through self-amputation impacted his psychological well-being and necessitated a redefinition of his male identity in a hegemonic culture. He feared he would not live up to the socially imposed male role and would not satisfy his sexual needs. The penis is culturally perceived as a symbol of masculinity, strength, and power [18]. However, research suggests that penis loss does not necessarily negatively impact relationships and, in some situations, can improve them, including the development of satisfying intimate relationships [19]. The patient feared he might not meet cultural expectations of masculinity, so he was recommended reality therapy, during which the patient learns to recognize his needs and make appropriate decisions, leading to increased self-confidence, gaining internal control, and taking responsibility for his own actions [20].
A phantom erection is a consequence of the severing or loss of sensory receptors in the penis following amputation. However, their counterparts remain in the cerebral cortex, where they are activated by sexual desire, leading to the sensation of an erection [21]. Using mirror therapy can create a visual illusion of penis movement or retraction, which can provide a sense of control or relief. By manipulating the visual experience, a person can potentially alter their perception and control this illusion [22]. The patient declined mirror therapy, citing „no clear purpose to the therapy.“ Alternatively, he was offered relaxation activities to distract him from the phantom erections, which yielded positive results.
In the nursing process, the NANDA classification was used, the interventions were developed ac cording to the Nursing Interventions Classification (NIC), and the results of care were rated according to the Nursing Outcomes Classification (NOC) [23].
The patient was diagnosed with the following nursing diagnoses (Table 1):
1. Suicide risk. The goal of the intervention was to ensure patient safety and prevent suicide attempts.
2. Increased risk of wound infection. Nursing interventions were aimed at minimizing the risk of wound infection and optimizing the healing process.
3. Risk of bladder infection. The goal of nursing interventions was to prevent urinary tract infections.
4. Reduced self-esteem. The goal of nursing care was to improve mood.
Table 1. Selected nursing diagnosis in a patient with Klingsor syndrome
Nursing diagnosis
1. Risk of suicide due to unstable mental status
2. Increased risk of wound infection due to self-harm, wound location and malnutrition
3. Risk of bladder infection due to long-term catheterizatio
Nursing interventions
1. The assessment of suicide risk using Nurses’ Global Assessment of Suicide Risk (NGASR) scale [7]
2. Monitoring factors that may influence the suicide attempt, changes in behavior, etc. using the Suicidal Patient Observation Chart (SPOC) [8]
3. Ensuring safety by collecting dangerous objects from the patient (cables, shavers, shoelaces, medicines)
4. Discreet observation of the patient, making a ward round at night, control of the oral cavity following drug administration
5. Conclusion of a therapeutic contract with the patient, obliging him to inform about any suicidal intentions
6. Constant readiness of the nurse to help the patient in crisis times (conversation, contact with another specialist, administration of tranquilizers)
7. Using active listening techniques, avoiding asking questions starting with “why”, not judging the patient’s behavior, which will allow him to speak openly about emotions
1. Assess the risk of wound infection using the WAR scale [9]
2. Observe the wound for signs of local and systemic infection (fever)
3. Use antiseptics in daily wound care as prescribed by the doctor.
4. Apply a polyurethane foam dressing (5 × 5 cm), non-adhesive
5. Properly secure the protective dressing using elastic mesh underwear
6. Proper care of intimate areas
7. Educating the patient on the symptoms of infection
8. Change of underwear and bed linen
9. Assessment of nutritional status using the MNA scale [10]
10. Support the wound healing process by providing the necessary amounts of calories, protein, vitamins, and trace elements as prescribed by the physician (2 g for each kg of body weight, supplementation of 1 g vit. C, 200 mg zinc,
7.5 mg vit. A, capsules with omega-3 and 6 acids twice a day, or at the request of the physician, administration of two bottles per day of Nutridrink Skin Repair®) [11]
11. Blood collection for laboratory tests
1. Change the catheter according to the manufacturer’s recommendations and antiseptic principles [12]
2. Assess the appearance of urine for infection (cloudiness, presence of pus, red color, odor)
3. Monitor the patient’s temperature (twice a day)
4. Ensure adequate fluid intake
5. Follow urinary catheter care guidelines (do not disconnect the system, disinfect the connecting points of the system)
6. Educate the patient on intimate hygiene (washing with warm soapy water)
Nursing outcomes
The patient scored 6 points on the NGARS scale, which corresponds to the risk of suicide. During his stay in the ward, the patient did not verbalize suicidal thoughts or plans and did not attempt suicide. In times of crisis, the undertaken actions turned out to be sufficient. During his stay, the patient was sad, quiet, and avoided contact with other patients. Initially, he refused to discuss the injury. During the morning care, the patient opened up more and verbalized his doubts and concerns about his ability to start a family
Wounds in the intimate area are associated with an increased risk of infection due to their anatomical proximity to the anus. The patient received a WAR score of 3, indicating an increased risk of infection, which required the use of antiseptics. The patient received 23 points on the MNA scale, which indicates a risk of malnutrition. The wound was covered with a non-adhesive pinacoat dressing. Adhesive dressings did not adhere to the wounds due to the greater moisture content of this area and the uneven anatomical contours. The dressing was immobilized with mesh underwear that conformed to the body’s contours. The patient did not complain of symptoms suggestive of infection. No local symptoms suggestive of infection were observed. No fiver. Laboratory test results were as follows: erythrocytes 4.0 million blood cells/µl, albumin 3.5 g/d, transferrin 205 mg/dl, total lymphocyte count 1500 mm3, iron 69 µg/dl, CRP 6 mg/dl
Educate the patient on intimate hygiene (washing with warm soapy water). The nursing interventions implemented proved effective in preventing urinary tract infections. No changes in the appearance of urine or systemic symptoms indicative of infection were observed. Temperature 36,8˚C
4. Decreased self-esteem caused by self-harm, aggravated by a phantom erection of the penis
Conclusions
1. Establishing contact with the patient and identifying the causes of low self-esteem
2. Rationally explaining the causes of their illness to the patient
3. Acquainting the patient with options for further treatment (presenting options for further therapy)
4. Showing understanding and respect for the feelings evoked by the current health condition, without criticizing or moralizing the patient’s behavior
5. Psychoeducation and indicating the possibility of further emotional support and sexological counseling.
6. Teaching the patient simple relaxation techniques
Penile self-harm is a rare but serious condition that carries long-term consequences for the patient. It requires the implementation of evidence-based nursing care and the involvement of multiple specialists, including surgeons, psychiatrists, and sexologists. The diversity of nursing problems poses a challenge for nurses caring for patients with Klingsor syndrome. It is important to consider the patient not only through the lens of wound care but also in the context of the psychosocial consequences of genital self-harm. Holistic patient care allows for the best outcomes and improved quality of life.
Disclosures
The author declares no conflict of interest. This research received no external funding. Approval of the Bioethics Committee was not required.
References
1. Veeder TA, Leo RJ. Male genital self-mutilation: a systematic review of psychiatric disorders and psychosocial factors. Gen Hosp Psychiatry 2017; 44: 43–50. DOI: 10.1016/j.genhosppsych.2016.09.003.
2. Roth K, Izard J, Beiko D. Self-performed glansectomy and surgical repair by a nonpsychotic patient on androgen replacement therapy. Can Urol Assoc J 2009; 3: 25–28. DOI: 10.5489/cuaj.1135.
3. Odeyemi PO, Idowu NA, Abiola OO, Okunade IA. Genital self-mutilation following cannabis-induced psychosis: Klingsor syndrome – a case report. African Urology 2024; 4: 36–39. DOI: 10.36303/ AUJ.0121.
4. Mirastschijski U, Jiang D, Rinkevich Y. Genital Wound Repair and Scarring. Med Sci (Basel) 2022; 10: 23. DOI: 10.3390/medsci10020023.
5. Mortada H, Alhablany T, Alkahtani D, et al. Meshed Versus Sheet Skin Graft for Scrotum and Perineal Skin Loss: A Retrospective Comparative Study. Cureus 2021; 13 (9). DOI: 10.7759/cureus.18348.
6. Van der Merwe A, Graewe F, Zühlke A, et al. Penile allotransplantation for penis amputation following ritual circumcision: a case report with 24 months of follow-up. Lancet 2017; 390: 1038–1047. DOI: 10.1016/S0140-6736(17)31807-X.
7. Grosse U, Pfster A, Zeyer A, Häusermann S. Klinisches Assessment Basiswissen für Pfegefachpersonen und Hebammen. Arbeitsheft Psychiatrie. Zürcher Hochschule für Angewandte Wissenschaften 2023.
8. Björkdahl A, Nyberg U, Runeson B, Omérov P. The development of the Suicidal Patient Observation Chart (SPOC): Delphi study. J Psych Ment Health Nurs 2011; 18: 558–561. DOI: 10.1111/j.1365-2850.2011.01758.x.
The feelings of low self-esteem, which were provoked by the phantom erections, were only partially alleviated. A rational explanation did not improve the patient’s well-being. The patient feared he would not meet cultural expectations of being a man. Long-term psychotherapy is necessary and mirror therapy should be considered
9. Sopata M, Jawień A, Mrozikiewicz-Rakowska B, et al. Wytyczne postępowania miejscowego w ranach niezakażonych, zagrożonych infekcją oraz zakażonych – przegląd dostępnych substancji przeciwdrobnoustrojowych stosowanych w leczeniu ran. Zalecenia Polskiego Towarzystwa Leczenia Ran. Leczenie Ran 2020; 1–21. DOI: 10.5114/lr.2020.96820.
10. Cierzniakowska K, Szewczyk M, Kozłowska E, et al. Nutrition state assessment of elder patients hospitalised in a surgical ward. Pielęg Chir Angiol 2017; 11: 61–67.
11. Wojda P. Chronic wound care in psychiatric nursing practice. A case study. Forum Leczenia Ran 2022; 3: 41–48. DOI: 10.15374/FLR2022026.
12. Prävention und Kontrolle Katheter-assoziierter Harnwegsinfektionen. Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robert Koch-Institut. Bundesgesundheitsbl 2015; 58: 641–650. DOI: 10.1007/s00103-015-2152-3
13. Velazquez N, Press B, Renson A, et al. Development of a Novel Prognostic Risk Score for Predicting Complications of Penectomy in the Surgical Management of Penile Cancer. Clin Genitourin Cancer 2019; 17: 123–129. DOI: 10.1016/j.clgc.2018.09.018.
14. Efron DT, Barbul A. Wounds in infection and sepsis – role of growth factors and mediators. In: Holzheimer RG, Mannick JA (eds.). Surgical Treatment: Evidence-Based and Problem-Oriented. Zuckschwerdt, Munich 2001; 1–10.
15. Myoungjean J, Yoonhong K, Kyung WS. Role of nutrition in wound healing and nutritional recommendations for promotion of wound healing: a narrative review. Ann Clin Nutr Metab 2023; 15: 67–71. DOI: 10.15747/ACNM.2023.15.3.6
16. Willson M, Wilde M, Webb ML, et al. Nursing interventions to reduce the risk of catheter-associated urinary tract infection: part 2: staff education, monitoring, and care techniques. J Wound Ostomy Continence Nurs 2009; 36: 137–154. DOI: 10.1097/01. WON.0000347655.56851.04
17. Alghamdi SA, Alahmari MA, Alenazy BA, et al. The Role of Nursing in Suicide Prevention: Evidence-Based Practices, Challenges, and Future Directions. Review of Contemporary Philosophy 2023; 22: 1044–1063.
18. Santos DD, Carneiro S, Corrêa RG. Nursing care and sexuality in Oncology for penectomized individuals. Revenferm UERJ, Rio de Janeiro 2023; 31. DOI: 10.12957/reuerj.2023.68807.
19. Sosnowski R, Kulpa M, Kosowicz M, et al. Quality of life in penile carcinoma patients – post-total penectomy. Cent European J Urol 2016; 69: 204–211. DOI: 10.5173/ceju.2016.828.
20. Karimyar Jahromi M, Mosallanejad L. The impact of reality therapy on metacognition, stress and hope in addicts. Glob J Health Sci 2014; 6: 281–287. DOI: 10.5539/gjhs.v6n6p281.
21. Namba Y, Sugiyama N, Yamashita S, Tokuyama E, Hasegawa K, Kimata Y. Phantom erectile penis after sex reassignment surgery. Acta Medica Okayama 2008; 62: 213–216. DOI: 10.18926/AMO/30981.
22. Ramachandran VS, McGeoch PD. Phantom penises in transsexuals: Evidence of an innate gender-specific body image in the brain. J Conscious Stud 2008; 15: 5–16.
23. Ackley BJ, Ladwig GB (eds.). Podręcznik diagnoz pielęgniarskich. Media House, Warszawa 2019.
LECZENIE RAN 2025; 22 (3): 127-128
Sprawozdanie | Report